Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Neurotrope, Inc. | v365194_8k.htm |

Targeting the Treatment of Alzheimer’s Disease and other Neurodegenerative Diseases www.neurotropebioscience.com January 2014

Safe Harbor Statement Cautionary language regarding forward - looking statements Certain statements in this presentation, particularly those pertaining to our strategy, constitute forward - looking statements. Such statements are based upon the current beliefs and expectations of management and are subject to significant risks and uncertainties. Actual results may differ from those set forth in the forward - looking statements. Any statements that are not statements of historical fact (including statements containing the words “ believes, ” “ plans, ” “ anticipates, ” “ expects, ” “ estimates ” and similar expressions) should also be considered to be forward - looking statements . There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements . These factors are contained in Neurotrope Inc . ’s filings with the SEC, including Neurotrope’s Form 8 - K filings on August 29 , August 30 , October 9 , October 24 and December 16 , 2013 and 10 - Q filing for quarter ended September 30 , 2013 . THESE MATERIALS DO NOT CONSTITUTE AN OFFER TO SELL, OR THE SOLICITATION OF ANY OFFER TO BUY, ANY SECURITIES OF THE COMPANY OR ANY ENTITY WHATSOEVER . ANY SUCH OFFER MAY ONLY BE MADE BY A PRIVATE PLACEMENT MEMORANDUM OR PROSPECTUS ISSUED BY THE COMPANY . ANY REPRESENTATION TO THE CONTRARY BY ANY PARTY SHOULD BE IGNORED . The full text of Neurotrope’s SEC filings can be found at the SEC ’ s website (http://www.sec.gov) 2

Neurotrope, Inc. (OTCQB: NTRP) • A clinical development stage, publicly - traded diagnostic and pharmaceutical company • Capital invested in technology to - date approaching $100 mm (by BRNI) over 13 years across ongoing R & D investment, facilities / infrastructure, and intellectual property (IP ). • License patents in the U.S. and international territories; preclinical & clinical data from BRNI and our subsidiary Neurotrope BioScience , Inc. • Proprietary technology with an exclusive IP position targeting a cardinal defect in AD and other neurodegenerative diseases • Highly promising preliminary human data on an AD diagnostic close to commercialization • Phase 2 clinical development to commence on AD pharmaceutical product » Compelling efficacy data in preclinical models of AD » Extensive in - human experience for our lead drug molecule • Seasoned Business and Scientific Management Team 3

Neurotrope BioScience , Inc. History • October 2012 - Company Formed • February 2013 – Technology and Product Licenses transfer from BRNI to Neurotrope ; hires Dr. James New as CEO; closes $10.4 million Series A preferred stock capital raise • February to August 2013 - Establishes business plans to develop both AD diagnostic and therapeutic products utilizing BRNI licensed technology • August 2013 - Raises $11.5 million issuing additional Series A preferred stock; merges with BlueFlash Communications, Inc. BlueFlash changes its name to Neurotrope, Inc. (OTCQB: NTRP) • September 2013 – Signs Statement of Work with BRNI to develop diagnostic product; hires Robert Weinstein as CFO; trading begins of its common stock • October 2013 – Adds Dr. Larry Altstiel, neurodegenerative disease researcher, to Scientific Advisory Board. Adds Paul Freiman, seasoned pharmaceutical and biotechnology executive, to Board of Directors. Completes $23 million Series A preferred financing • December 2013 – Adds James Gottlieb to Board of Directors, extensive interaction with FDA and other healthcare - related governmental agencies 4

Management Team • Dr. James New – Chief Executive Officer • Pfizer, Inc. - Senior Director of Licensing • Novartis - Director M&A and Head of Worldwide Business Development • Abrika Pharma., Lifecycle Pharma., AIKO Biotechnology – CEO • Dr. John Abeles – Chairman • Physician and pharmacologist • Founded several successful entrepreneurial ventures • Research Analyst – Kidder Peabody, first MD analyst • Dr. Dan Alkon – Chief Scientific Officer • Physician at Cornell University • 30 year career as medical director in US NIH health services specializing in memory disorders • 14 years as founding scientific director of BRNI • Robert Weinstein, CPA, MBA – Chief Financial Officer • Experienced healthcare industry CFO and consultant • Successful private equity fund manager and investment banker 5

Trading, Ownership & Capitalization Current Capitalization Shares Common Stock 21.7 MM Series A Convertible Preferred Stock (Convertible @ $1.00 / share) 23 .0 MM Options & Warrants Outstanding ( Wtd . Avg. E.P. $0.80 / share) 8.5 MM Total fully diluted shares 53.2 MM Trading, Ownership, Financials Information Ticker symbol (OTCQB) NTRP Current share price (1/10/14) $1.80 52 week range $1.32 to $2.25 Implied market capitalization $39 million Average daily trading volume NM Public Float 2,700,000 shares Management / Affiliate ownership 26.4% / 50.6% Cash @ September 30, 2013 plus October 2013 net funding $16.5 million Monthly operating burn rate quarter ended September 30, 2013 $500,000 6 6

Opportunity Target Alzheimer’s Disease Early with an Effective Drug and New Mechanism Delay the Disease Progression Estimated $15 - $20 BN in Annual Worldwide Sales 7
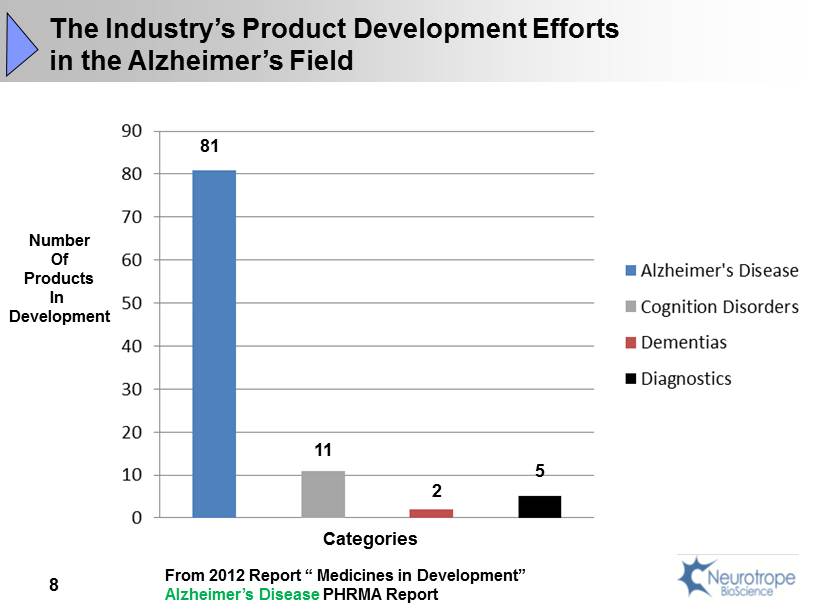
81 11 2 5 Number Of Products In Development Categories The Industry’s Product Development Efforts in the Alzheimer’s Field From 2012 Report “ Medicines in Development” Alzheimer’s Disease PHRMA Report 8

Past Efforts in Developing Drugs for AD have focused on Beta Amyloid 9

Current Themes Dominating AD Research Early Diagnosis Biomarker Analysis Disease Modifying Therapy Critical to early detection of AD pathology Allows treatment with a potentially wider range of current and future therapeutics Stemming disease progression would represent a breakthrough therapy Neurotrope R&D Program √ √ √ 10

Delayed Onset Current Trajectory Cost in Billions of Dollars Impact of a 5 - year delay in the Onset of AD on Costs Americans Age 65+ with Alzheimer’s Disease 2010 - 2050 Source: Alzheimer’s Association, “Changing the Trajectory of Alzheimer’s Disease: A National Imperative” May 2010 2010 2050 2020 2030 2040 $0 $200 $400 $600 $800 $1,000 5.1 mm 5.6 mm 7.8 mm 13.5 mm # of AD Patients 11
• New drug prototypes have been identified which activate Protein Kinase C (PKC ) in a highly selective manner - Bryostatin • “ Turning - on” PKC has been shown to “re - boot” neurons in various animal models • Neuronal regeneration occurs due to the fresh expression of proteins critical to the maintenance and function of the nerve cell • The results in animals are improved memory and cognition functions • Our most advanced clinical development program is focused on Alzheimer’s Disease, but our drug candidates will also be evaluated in stroke, traumatic brain injury, and mental retardation Neurotrope’s Core Technology is PKC Activation – Can Restore the Structure & Function of Nerve Cells 12

PKC Therapeutics provide Neuroprotection through a Multi - Modal Approach − Anti A β − Anti Ʈ - phosphorylation, and − Anti apoptosis/ or prevention of cell death through activation of Akt 13

Profile of Bryostatin vs. other AD Drugs Neurotrope’s drug prototype shows activity in a wide panel of test systems where other FDA approved drugs ( Donepezil ) or experimental drugs ( bapineuzumab ) have failed. 14

Competitive Advantage Two Lead Drug Candidates: Bryostatin and DHA - CP6 • Bryostatin – First lead drug candidate • Natural product isolated from a marine organism • Developed as an anticancer drug • Has been evaluated in 63 clinical studies; > 1,200 cancer patients • Well established safety, pharmacodynamics and toxicity information in cancer patients • Clinical drug supply could be available through the National Cancer Institute • The FDA has approved a Ph.2a clinical trial with this drug in AD • Estimated product launch ≈ 2018 • DHA - CP6 – Second lead drug candidate • Developed by BRNI for the treatment of Alzheimer’s Disease • Shows comparable efficacy to Bryostatin in pre - clinical studies • Expect to enter into Phase 1 in 2015 • Additional experimental drug prototypes offer fallback candidates to Bryostatin of DHA - CP6 15

Drug Development Pipeline 16

Neurotrope’s Alzheimer’s Diagnostic System ( proposed Brand Name for the Diagnostic Test ) 17

Physician’s Office Taking the Biopsy Sample Packaging & Mailing the sample to Neurotrope BioScience Neurotrope’s Testing Laboratory in Rockville, Maryland Three Steps to Processing the Biopsy Sample for Alzheimer’s Diagnostics 2 1 3 18

Three Different Peripheral Biomarkers are Monitored for AD Detection • Patient / Clinician provides a skin biopsy which is shipped to Neurotrope • The skin biopsy is used to: • Grow cultured fibroblasts in tissue culture • Analyzed for levels of PKC ε • Analyzed for the ratio of ERK 1/2 concentrations • Analysis is conducted in Neurotrope’s Rockville lab • 48 hour turnaround for the test results • Over 174 patients analyzed to - date; 64 Dx test results have been confirmed through autopsy • This is a state - of - the - art diagnostic test that offers accuracy equivalent to brain - imaging techniques, and superiority to other in - vitro test systems currently in development 19

1Q 2015 2Q 2014 2Q 2015 3Q 2015 3Q 2014 4Q2014 1Q 2014 Ongoing prospective clinical validation trial in 150 patients Technology Transfer to a 3 rd party Laboratory Study completes Near Term Milestones in the Development of our AD Dx Commercial Launch 20

Conclusions: The Power of Teaming an AD Diagnostic with an AD Therapeutic Potential for Early and Accurate Diagnosis Potential for Early Therapeutic Intervention Delaying AD Progression A Paradigm Shift in the Treatment and Management of AD • Near term monetization of AD Dx • Enrichment of clinical studies based on more narrow inclusion criteria • Library of bryo back - up candidates provides Pharma partnering opportunities 21

Contact Information Additional Information about Neurotrope , Inc. may be obtained by contacting: The Company: www.neurotropebioscience.com Dr. James New 954.632.6630 jnew@neurotropebioscience.com Robert Weinstein 914.295.2765 rweinstein@neurotropebioscience.com Investor Relations: Tony Schor Investor Awareness, Inc. Tony Schor , 847 - 945 - 2222 ext. 221 tony@investorawareness.com www.InvestorAwareness.com 22
