Attached files
| file | filename |
|---|---|
| 8-K - 8-K - INTERMUNE INC | d659710d8k.htm |
| EX-99.1 - EX-99.1 - INTERMUNE INC | d659710dex991.htm |
 J.P.
Morgan 2014 Healthcare Conference
January 13, 2014
Exhibit 99.2 |
 Forward-looking Statements
This
presentation
contains
forward-looking
statements
made
pursuant
to
the
"safe
harbor"
provisions
of
the
Private
Securities Litigation Reform Act of 1995. Investors are cautioned that, without
limitation, statements in this presentation regarding InterMune's plans and
expectations; anticipated availability of top-line results from the ASCEND trial; the
estimated patient populations suffering from IPF and market potential for Esbriet;
anticipated timing of pricing and reimbursement discussions and/or
initiating commercial launches for Esbriet; intellectual property protection for Esbriet;
and expectations regarding the ASCEND trial and prospects for success thereof are
forward-looking statements. All forward-looking statements
included in this presentation are based on information available to InterMune as of the date
hereof, and InterMune assumes no obligation to update any such forward-looking
statements. Actual results could differ materially from those described in
the forward-looking statements. Factors that could cause or contribute to such
differences
include,
but
are
not
limited
to,
those
discussed
in
detail
under
the
heading
“Risk
Factors”
in
InterMune’s
periodic reports filed with the SEC, including but not limited to the following:
(i) the risks related to the uncertain, lengthy and expensive clinical
development process for the company’s product candidates; (ii) risks related to the regulatory
process for Esbriet, including that the results of the ASCEND trial may not be
satisfactory to the FDA to receive regulatory approval; (iii) risks related
to unexpected regulatory actions or delays or government regulation generally; (iv) risks related
to the company’s manufacturing strategy; (v) government, industry and general
public pricing pressures; (vi) risks related to the company’s ability
to successfully launch and commercialize Esbriet; and (vii) the company’s ability to maintain
intellectual property protection. The risks and other factors discussed above
should be considered only in connection with the risks and other factors
discussed in detail in InterMune’s Form 10-K, Form 10-Q and its other periodic reports filed
with the SEC, which are also available at www.intermune.com.
1 |
 Our
Company: A Fully Integrated Global Biotech Company with Significant Growth
Potential 2
Demonstrated
Research
Capability
–
first
in
HCV,
now
delivering
in
fibrosis
Development and Regulatory expertise in Specialty Diseases
–
Multiple, high-quality, multi-national clinical trials in Phases
1-4 –
Esbriet approved by EMA and Health Canada
A Successful Commercial Organization
–
Built high-performing EU and Canadian organizations
–
Achieved
attractive
pricing
and
reimbursement
in
13
countries
in
Europe
–
Strong year-on-year revenue growth in 2013 and more expected in 2014
($115-$135M) Poised to Launch in the United States
–
ASCEND results early Q2 2014
U.S. launch in 1H 2015
–
ITMN is building its U.S. capability
ITMN is the Leader in IPF and expanding via R&D and BD into new growth areas
focused primarily on fibrosis |
 Our
Franchise: Idiopathic Pulmonary Fibrosis (IPF) A Large, Lethal Orphan Disease
Progressive scarring of the
lungs with no known cause
–
Median survival: 2-5
years*
Large market in North
America + European Top-15:
–
118K to 158K prevalence
–
28K to 37K incidence
Esbriet
®
is the only approved
IPF medicine in EU or
Canada
–
None approved in U.S.
% Patients Surviving at 5 Years
100
0
80
60
40
20
Lung
Cancer
IPF
Ovarian
Cancer
PAH
Colo-
rectal
Cancer
Breast
Cancer
*
Bjoraker JA, Am J Respir Crit Care Med. 1998 Jan; 157(1):199-203.
3 |
 Our
Strategy: Maximize the Value of Esbriet and Grow Beyond Esbriet and IPF
Grow Beyond
Esbriet and IPF
Expand
Esbriet in IPF
New formulations
Phase 4
ISTs
Registries
Leader in IPF:
Commercialize Esbriet
Key launch and
commercialization
activities
Vision: Leader
in Specialty
Fibrotic Diseases
4
New
indications
Pirfenidone
Analog
LPA-1
Biomarkers
Business
Development
A
B
C |
 InterMune Is a First Mover in an Emerging Growth Area
with Significant Unmet Need: Fibrotic Diseases
Lung, kidney, liver,
bone, heart, GI tract,
others
~40M patients
in all fibrotic diseases
High Unmet
Need and
Severity
Affecting
Multiple
Organ
Systems
Fibrotic
Diseases
5
~120 assets in
development
(50 from top biopharma)
InterMune, AbbVie, AstraZeneca,
BI, BMS, Celgene, Gilead, GSK,
Incyte, J&J, Lilly, Merck, Novartis,
Pfizer, Roche, Sanofi
~$15-$30B in R&D
investment in fibrotic
diseases
Growing
R&D
Investment
Major
Players
Growing
Pipeline
Emerging growth
area
InterMune is well-
positioned
–
Research
–
Development
capabilities
–
Esbriet
commercialization |
 Commercializing
Esbriet
®
(pirfenidone)
in IPF |
 Esbriet is now available for prescription for 85%
of the IPF patients in the Top 15 EU countries
7
Focus First on
Top 5 Countries:
Germany, France,
Italy, UK, Spain
Population of 314
million
~ 70-75% of EU
market value
Esbriet has
launched in
4 of 5 Top 5
Countries
Invest in 10 Mid-Sized
Countries & Regions:
Austria, Nordics (4),
Netherlands, Belgium
Ireland, Iceland and
Luxembourg
~ 10% of EU market
value
Esbriet has
launched in
9 of 10 Mid-
Sized
Countries
Successful EU Commercialization Strategy:
Focus First on Top 15 |
 Esbriet Revenue –
EU and Canada
Nine Consecutive Quarters of Growth
8
2.7
4.9
5.5
7.5
8.2
10.5
14.4
19.7
25.6
0
2
4
6
8
10
12
14
16
18
20
22
24
26
Q4’11
Q1’12
Q2’12
Q3’12
Q4’12
Q1’13
Q2’13
Q3’13
Q4’13 |
 The
U.S. IPF Market |
 The
U.S. IPF Market Is Very Attractive Very Large Orphan
Disease –
Incidence ~ 15-20K/year
–
Prevalence ~ 30-50K mild/moderate
Desperate Unmet Need
–
No FDA-approved IPF medicines
Attractive Business Model
–
Value based, Orphan Disease pricing
–
Small molecule margins
–
No third-party royalties
–
Specialty target audience: 3,000 MDs
–
Targeted
salesforce
of
~
75
-
100
reps
10
U.S. IPF Prevalence
Mild to Moderate
(30,000-50,000)
Severe
(~20,000) |
 Esbriet
®
EU: $33K to $45K
Canada: $42K
Benchmark Products in the U.S. Have
a Broad Range of Prices ($000’s/year)*
11
*
Wholesale Acquisition Cost (WAC). Source: AnalySource (12/17/13)
(PAH)
(PAH)
(mCRPC)
(cystic
fibrosis)
29
(severe
epilepsy)
41
(multiple
sclerosis)
49
(multiple
sclerosis)
58
(multiple
sclerosis)
55
(cataplexy)
63
78
83
(multiple
sclerosis)
60
Oral or inhaled administration
Infusion or injection administration |
 ASCEND Phase 3 Study
for U.S. Registration |
 Three
Phase 3 Trials Support Clinically Meaningful Effect on Lung Function, with
Generally Consistent Results at One Year 13
PIPF-004: FVC Change Week 72*
PIPF-006: FVC Change Week 72*
0
72
48
36
24
12
60
Weeks
0
-15
-10
-5
0
72
48
36
24
12
60
Weeks
0
-15
-10
-5
Week 48
52% reduction
P<0.001
Week 48
27% reduction
P=0.005
*
Pre-specified primary endpoint
Pirfenidone
2403 mg/d
Placebo
Pirfenidone
2403 mg/d
Placebo
SP3: VC Change at Week 52*
0
52
24
Weeks
0
-140
-40
-20
-60
-80
-100
-120
Week 52
44% reduction
P=0.042
36
12
Pirfenidone
1800 mg/d
Placebo
P=0.042
P=0.501
P=0.001 |
 Rigorously Analyzed our IPF Databases to Determine “Predictors of
Progression” 14
Variables*
2 CAPACITY Trials
ASCEND Trial
Time Since Diagnosis
No time limitation
6 to 48 months
Baseline % Predicted FVC
>50%, no upper limit
50% -
90%
% DLco
>35%, no upper limit
30% -
90%
FEV1 / FVC Ratio
(lower is Indicative of
emphysema)
>0.70
0.80
*Variables
consistently
associated
with
greater
FVC
progression
in
placebo
patients
(CAPACITY
and
INSPIRE
data
bases)
ASCEND Design Based on Key Learnings from Prior IPF
Trials to Maximize Probability of Success (1)
Red font
indicates change from CAPACITY Phase 3 Studies |
 ASCEND Design Based on Key Learnings from Prior IPF
Trials to Maximize Probability of Success (2)
15
Duration
A 52-Week Study –
All three Phase 3 studies of pirfenidone
have “won”
at 1 year
Primary
Endpoint
FVC Change Versus Placebo (difference versus placebo)
Categorical FVC for treatment effect size
Key
Secondary
Endpoints
6MWT
Distance,
Progression-Free
Survival*
Power
A large study (n=555) –
the
largest pirfenidone Phase 3 study
Centralized
Procedures
HRCT Review –
for high level of confidence in IPF diagnosis
Pulmonary
Function
Testing
–
to
minimize
variability
in
primary
endpoint metric
Red
font
indicates
change
from
CAPACITY
Phase
3
Studies
*DLco component replaced with 6MWT |
 Clinically Meaningful Changes in FVC in IPF Studies (1)
The Clinical Reality:
–
~2/3 of IPF patients have little decline in FVC in clinical studies of ~ one
year –
~1/3 of IPF patients have significant decline in FVC
–
Therefore MEAN decline in FVC of a study population is not the best measure
of treatment effect size
What is the minimal clinically important difference (MCID) in FVC in IPF patients?
–
MCID is the difference to an individual patient
that is meaningful
–
MCID for FVC in an IPF patient is a 2-6% decline*
–
FVC declines of 5-10% in an IPF patient translate to 2-5X risk of
death* Categorical Change in FVC is the clinically meaningful metric:
16
*
du Bois RM Am J Respir Crit Care Med 184: 1382-9, 2011
Percent of patients with a 10% FVC Decline
|
 17
Why is Categorical Change in FVC the best metric of clinical
meaningfulness?
–
Measures
changes
in
an
individual
patient
–
Addresses the statistical problem of the population mean
–
Can be easily tied to the MCID (a population mean cannot)
–
Relevant to clinicians and regulators
Esbriet label in Europe and Canada only reference Categorical FVC
Change
Clinically Meaningful Changes in FVC in IPF Studies (2)
Measure of Clinical Meaningfulness in ASCEND:
Categorical
FVC
Change
Percent
of
patients
with
a
10%
FVC
Decline |
 Categorical FVC Results at One Year From CAPACITY ITT
18
*Rank ANCOVA
p < 0.001*
Pirfenidone
Placebo
40% Reduction
36% Increase
0
5
10
15
20
25
30
35
40
45
10% Decline/Death/LT
No Decline (>0%)
Patients
(%)
Categorical
FVC:
Patients
with
10%
FVC
Decline/Death/Lung
Transplant
(LT) |
 P<0.0001
P=0.0002
P<0.0001
P=0.002
Patients
(%)
Pirfenidone
2403 mg/d
Placebo
CAPACITY ITT Patients
40% Reduction
Proportion of patients with No FVC decline was 36% greater relative to
placebo
19
0
5
10
15
20
25
30
35
3 Months
6 Months
9 Months
12 Months
Clinically
Meaningful
Outcome:
Proportion
of
Patients
with
10%
FVC Decline/Death/LT Reduced by 40% Relative to Placebo |
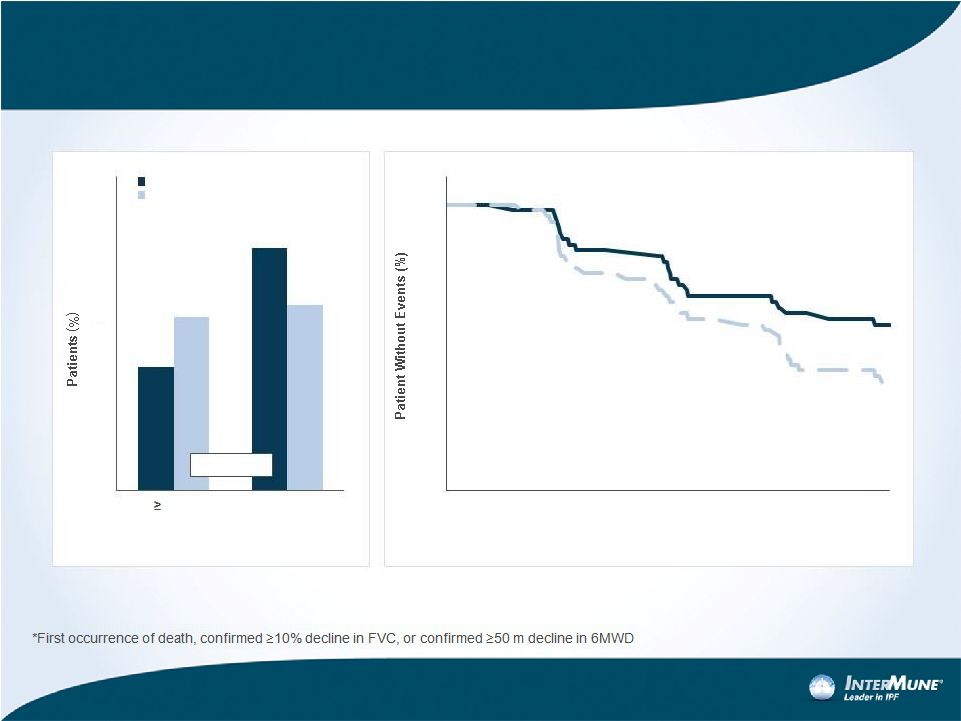 Two
Other Key Secondary Endpoints In ASCEND Showed Clinically Meaningful Change in
CAPACITY at One Year 29%
Reduction
31%
Increase
HR 0.62 (0.46 –
0.84)
P-value = 0.0015
20
Pirfenidone
2403 mg/d
Placebo
0
10
20
30
40
50
60
50 m
Decrement or
Death
No Decline
p = 0.0037
50
60
70
80
90
100
0
1
2
3
4
5
6
7
8
9
10
11
12
Months
Pirfenidone
Placebo
6MWD Change
Progression-Free Survival |
 ASCEND Summary and Timeline
ASCEND
is
designed
to
win
—
built
on
our
decade
of
IPF
experience
–
Large, one-year study
–
Over 95% power using CAPACITY ITT results
–
Centralized procedures for more homogeneity
–
Enrichment Strategy for patients with more FVC progression
Excellent Study Conduct
–
High retention rate (>90% of patients remain in study)
–
High persistence rate (>90% of patients are on study medicine)
–
High roll-over rate to open-label “RECAP”
study (>95%)
Top-line data expected in early Q2 2014
–
Presentation at ATS (May 2014)
21 |
 Financial Outlook |
 Worldwide Esbriet Revenue
Nine consecutive quarters of
growth since launch
168% revenue growth in 2013
–
New countries –
one-time effects
due to NPP conversion and pent-
up demand
–
Launched countries –
greater
penetration and persistence
2014 guidance range of $115 to
$135 million represents ~65% to
90% projected growth
Spain and the Netherlands
included
in
the
range
–
update
in
1H 2014
23
Guidance
Range
$115 -
$135
168%
65%-
90%
$70.2
$26.2
$2.8
2011
2012
2013
2014 |
 Total
Operating Expenses – 2014: Building a
Commercial Company with an R&D Pipeline
24
$320 -
$345
$255 -
$265
2014
2013
R&D
SG&A
SG&A
R&D
Guidance
Important SG&A increase
–
U.S. launch preparations to ensure
successful launch of Esbriet
–
Expanding commercial presence in
Europe to maximize Esbriet growth
Sustained R&D investment to fund our
growing pipeline |
 Research & Development Expenses —
2013 to 2014
Increased investment —
research
and early stage programs:
–
New formulation
–
Pirfenidone analog
–
LPA-1 inhibitor
–
Biomarkers program
–
New antifibrotic compounds
Stable
—
Worldwide
regulatory,
quality, and medical affairs expense
Increased
investment
—
NDA
preparation, RECAP and new
Esbriet clinical studies
Decreased
investment
—
ASCEND
as study completes in Q2
25
$110 -
$120
$110 -
$115
2014
2013
Regulatory,
quality and
medical
affairs support
Research and
early stage
programs
Regulatory,
quality and
medical
affairs
support
Research and
early stage
programs
NDA Prep,
RECAP and
New Clinical
ASCEND
ASCEND
NDA Prep,
RECAP and
New Clinical
Guidance |
 30%
60%
10%
Selling, General & Administrative
Expenses —
2013 to 2014
26
$210 -
$225
$145 -
$150
2014
2013
EU/Canada
SG&A
U.S. Launch
Preparation
EU/Canada
SG&A
Corporate/US
G&A
Corporate/US
G&A
U.S. Launch
Preparation
Increase driven primarily by U.S.
commercial launch preparations
(“S”)
–
Weighted to H2 (post ASCEND)
Modest increase in Corporate/U.S.
G&A
Modest increase for EU/Canada
SG&A:
–
Full-year effect of commercial
organizations created mid-2013 (Italy
and UK)
–
Expansion beyond current 13
countries
in Europe
Guidance |
 InterMune Showing Strong Momentum
and Poised to Enter the U.S. Market
With EU reimbursement now resolved, Esbriet is growing very well
–
Nine consecutive quarters of revenue growth since first launch
–
2013 revenue growth +168% vs. 2012
–
2014 guidance range: $115-$135 million (+65-90%)
–
Expect cash flows from European sales to fully support European operations
in late 2014
Phase 3 ASCEND study for U.S. Registration
–
High confidence: designed from our lessons of a decade in IPF
–
Excellent study conduct: extremely high retention and roll-over rates
–
Results expected in early Q2 2014 (previously Q2 2014)
Momentum
in
our
R&D
efforts
to
be
a
leader
in
specialty
fibrotic
diseases
27 |
 |
