Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CymaBay Therapeutics, Inc. | d659718d8k.htm |
 Corporate Presentation
January 2014
Exhibit 99.1 |
 2
Safe Harbor Statements
This
presentation
contains
"forward-looking“
statements
that
involve
risks,
uncertainties
and
assumptions.
The
opinions,
forecasts,
projections,
or
other
statements
about
future
events
or
results,
are
forward-looking
statements.
If
the
risks
or
uncertainties
ever
materialize
or
the
assumptions
prove
incorrect,
CymaBay's
results
may
differ
materially
from
those
expressed
or
implied
by
such
forward-looking
statements.
Forward-looking
statements
include,
but
are
not
limited
to:
any
projections
of
financial
information;
any
statements
about
historical
results
that
may
suggest
trends
for
CymaBay's
business
and
results
of
operations;
any
statements
concerning
CymaBay's
plans,
strategies
or
objectives;
any
statements
of
expectation
or
belief
regarding
future
events;
any
statement
of
projected
sales
forecasts,
revenues
or
anticipated
or
projected
markets;
and
any
statements
of
assumptions
underlying
any
of
the
foregoing.
These
statements
are
based
on
estimates
and
information
available
to
CymaBay
at
the
time
of
this
presentation
and
are
not
guarantees
of
future
performance.
Actual
results
could
differ
materially
from
CymaBay's
current
expectations
as
a
result
of
many
factors,
including
but
not
limited
to:
CymaBay's
ability
to
obtain
additional
financing
to
fund
its
operations
as
it
currently
expects;
unexpected
delays
or
results
in
clinical
trials;
uncertainties
regarding
obtaining
regulatory
approvals;
uncertainties
regarding
the
ability
to
protect
CymaBay's
intellectual
property;
uncertainties
regarding
market
acceptance
of
any
products
for
which
CymaBay
is
able
to
obtain
market
acceptance;
the
effects
of
competition;
and
market
factors
and
general
economic
conditions.
You
should
read
CymaBay's
Form
10
and
S-1
registration
statements,
which
are
available
on
the
SEC
web
site
at
http://www.sec.gov,
including
the
Risk
Factors
set
forth
therein,
completely
and
with
the
understanding
that
our
actual
future
results
may
be
materially
different
from
what
we
expect.
CymaBay
assumes
no
obligation
for
and
does
not
intend
to
update
these
forward-looking
statements.
Nothing
contained
herein
is,
or
should
be
relied
on
as,
a
promise
or
representation
as
to
the
future
performance
of
CymaBay. |
 3
CymaBay Investment Highlights
•
Restructured company retaining the prior Metabolex pipeline and core
management team
–
Became public through Form 10 self registration route
–
$38M financing completed to fund arhalofenate development
–
Application to FINRA to trade on the OTCBB under review
–
Application for NASDAQ listing under review
•
Arhalofenate is a potentially game changing dual-acting treatment for gout
–
Anti-flare activity of Colcrys (URL, acquired by Takeda for $800M)
–
Uricosuric activity of lesinurad (Ardea, acquired by AZ for $1.3B)
–
Large (>$500M peak sales) market opportunity
–
Highly de-risked asset with large clinical safety database
•
Other pipeline projects
–
MBX-2982 and MBX-8025 in Phase 2 |
 4
CymaBay Leadership
Averages >20 years of drug development experience
Name
Title
Experience
Harold Van Wart
President,
CEO
Syntex, Roche
Sujal Shah
CFO
Credit Suisse, Citi
Charles McWherter
CSO
Pfizer, Sugen
Mary Jean Stempien
Interim CMO
Roche, Tularik
Robert Martin
VP Project Management
Syntex, Roche
Patrick O’Mara
VP Business Dev.
Metabolex
Diana Petty
VP Human Resources
SmithKline, 3M |
 5
Preclinical
Phase 1
Phase 3
Phase 2
Lead Optimization
Arhalofenate
MBX-8025
MBX-2982
Gout
Diabetes
Diabetes
High Unmet Need/
Orphan Disease
Diabetes
Target
Novel Target
Diabetes
Targets
CymaBay
Pipeline, January 2014
Multiple partnered and unpartnered programs |
 6
Key Features of Gout
Hyperuricemia, urate crystal deposits and flares
Hyperuricemia
Serum Uric
Acid (sUA)
Mono Sodium
Urate (MSU)
crystal deposits
Inflammatory
Response
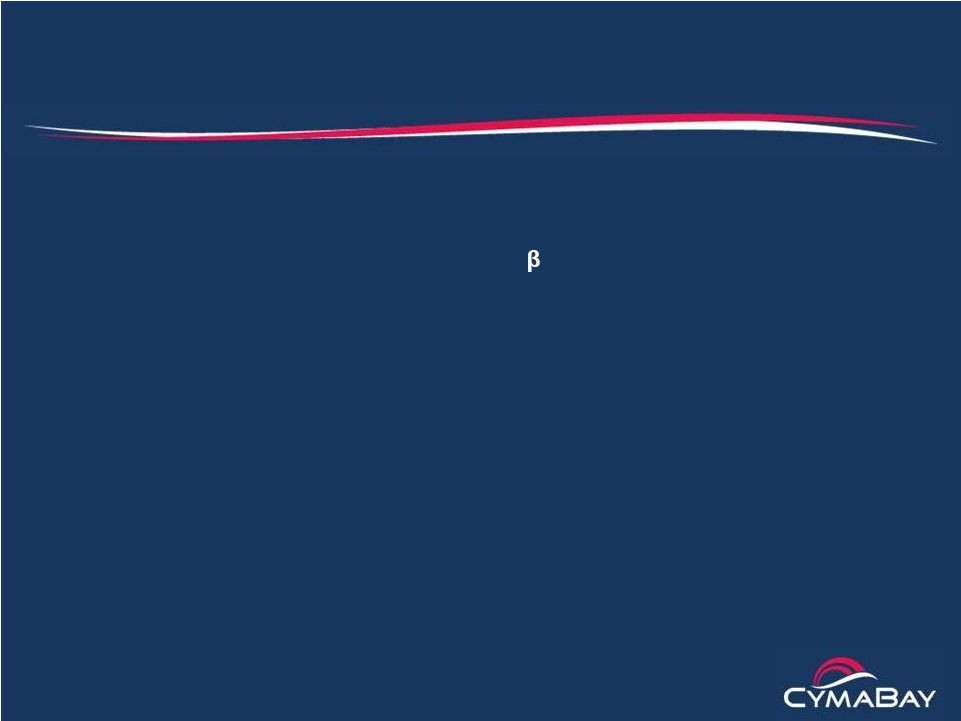
IL-1ß
Painful flare
Therapeutic
targets
Joint
erosion |
 7
Current Treatment of Gout
Uric acid Lowering Therapies (ULTs) and anti-inflammatories
–
Treatment paradigm (ACR Guidelines)
–
Anti-inflammatory to treat the flare
–
ULT is initiated to address the hyperuricemia with a goal of sUA
< 6
mg/dL to debulk offending MSU burden
–
Initiation of ULT increases flare risk, requiring Colcrys prophylaxis
–
Anti-inflammatory drugs
–
Colchicine (Colcrys)
–
NSAIDs, steroids
–
Ilaris (anti-IL-1
biologic) approved in EU
•
ULTs
–
Xanthine oxidase (XO) inhibitors (allopurinol, febuxostat)
–
Uricosurics (probenecid, lesinurad)
–
Pegloticase (for severe treatment failure gout) |
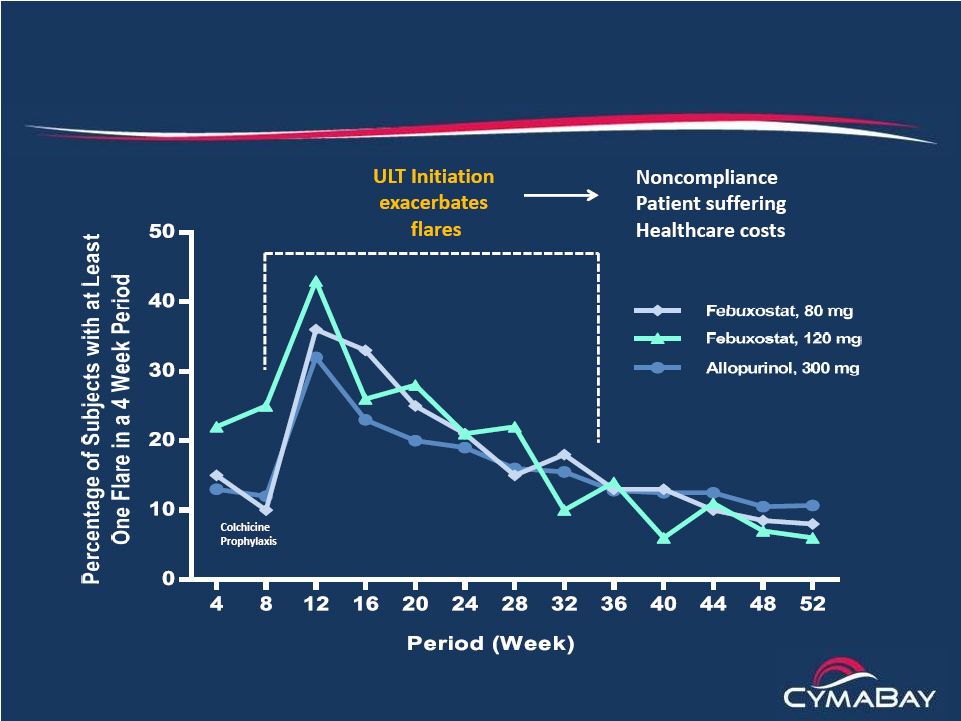 8
The Big Paradox
Currently marketed ULTs increase flares |
 9
Gout Patients Are Poorly Served by Available Drugs
Need for better control of flares and sUA
•
Patients care about the pain, suffering and medical costs due to
flares
–
Despite ULT, at least 1 million patients flare
3 times/year
–
More than 50% of patients on ULT do not reach the sUA goal of < 6
mg/dL and are unable to debulk their MSU burden
–
Poor performance of available therapies leads to non-compliance
•
Current anti-inflammatory drugs have limitations for gout patients
–
Colcrys ($496M sales in 2012) has GI side effects, drug interactions
and is difficult to use in patients with comorbidities (CKD, CVD)
–
Steroids and NSAIDs are also problematic
–
Ilaris is an expensive injectable with risk of infections
•
Unmet needs
–
Better flare control
–
Additional sUA lowering, but not if it causes more flares
|
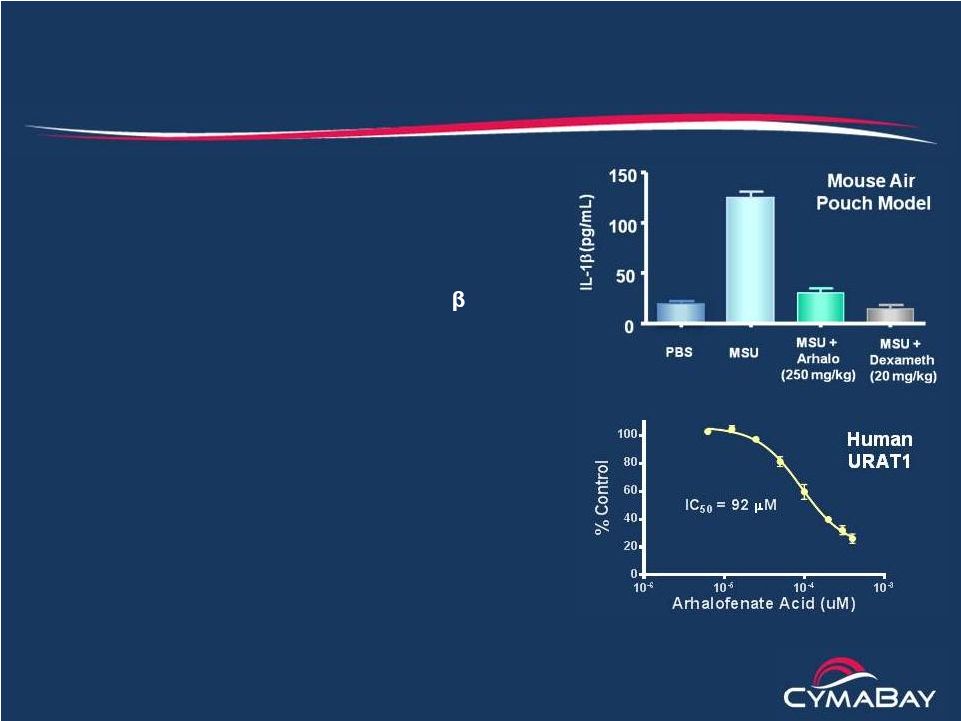 10
The Arhalofenate Solution
The only therapy that reduces flares while lowering sUA
•
Reduces flares through
anti-inflammatory
properties and long plasma
half-life
–
Suppresses MSU crystal-induced
IL-1ß
in gouty joints
–
No systemic suppression of IL-1
and no infection risk
–
50 hour half-life “buffers”
sUA levels
to minimize intraday fluctuations
•
Lowers sUA by improving uric acid excretion
in the kidney
–
Blocks urate reabsorption by URAT1
–
Same mechanism for lowering sUA as
lesinurad
–
Retains uricosuric activity in CKD patients |
 11
Allopurinol
Intolerant
~300 K
Diagnosed
Gout*
8.3 M
ULT Treated**
~3.3 M
Mild & Moderate
~581K
Severe
~282K
Arhalofenate
Arhalofenate
+ Febuxostat
Arhalofenate
* NHANES, 2008
** Source Healthcare 2012
*** BioTrends
Target
Population
~ 1M patients
3 flares/yr***
Arhalofenate Target Population
Three or more flares a year or allopurinol intolerant
CymaBay/Biotrends
Market Research:
PCPs cite 3 flares/year
as the threshold for
trying a new therapy |
 12
Switch Strategy is Made Possible by Patient Presentations
Repeat visits to PCPs due to flare recurrence
Allopurinol
Intolerant
~300K
Steady State
XO-Treated
3.3M
Untreated Gout
Patients
5.3-5.8M
Discontinuations
~1.2M/year*
Restarts
~0.75M/year*
Newly
Diagnosed
New Starts
~200K/year**
* Sarawate et al. (2006)
** NHANES data
~20K/year |
 13
Market Opportunity
Arhalofenate’s unique profile creates large opportunity
•
Arhalofenate offers what patients care about –
relief from flares and pain
–
US Colcrys sales ($496M) in 2012 validate value of flare reduction
–
Pharmacoeconomic argument for payers on reduced healthcare costs
•
25% of patients with
3 flares/yr need an ER visit or hospitalization*
•
Hospitalizations have a mean length of stay of 4 days**
•
Arhalofenate US Sales forecast (Sage Path Partners)
–
Peak sales >$500M
•
Pure ULTs that lack anti-flare activity have not been commercially successful
–
Allopurinol is an inexpensive entrenched generic (>90% market share)
–
Febuxostat sales of only ~$216M/year is due to minimal differentiation
–
Lesinurad may face the same challenges
* BioTrends, 2012
** Mandell BF et al |
 14
Discovery and History of Arhalofenate
Single enantiomer of halofenate
•
Halofenate
–
Racemic drug studies by Merck in late 1970s
–
1200 patient year clinical database with effective reduction in sUA
and triglycerides and good overall safety (studies up to 4 years)
–
Lowered glucose in diabetics
–
Discontinued due to GI side effects associated with S-enantiomer
•
Arhalofenate
–
R
-enantiomer
of
halofenate
partnered
with
JnJ
for
type
2
diabetes
–
Eight Phase 1 and four Phase 2 studies (3-6 months) completed
–
Total of 873 patients studied giving ~165 patient-years of exposure
–
Decreases in HbA1c fell short of commercial target
–
Now being repurposed for gout |
 15
Phase 2 Gout Studies Conducted with Arhalofenate
Strong support for monotherapy and febuxostat combination
•
Monotherapy study (64 patients, 4-week treatment)
–
Arhalofenate (400 or 600 mg) or placebo
–
Reductions in sUA lowering and flare parameters
•
Febuxostat combination study (11 patients; up-titration over 5 weeks)
–
80 mg febuxostat plus arhalofenate (400 or 600 mg)
–
Percentage of patients that reach sUA goals (< 6, < 5, < 4 and
< 3
mg/dL) and decrease in flare parameters
•
Allopurinol combination study (95 patients, 4 weeks of treatment)
–
Patients on allopurinol (300 mg) not reaching sUA < 6 mg/dL
received arhalofenate (400 or 600 mg) or placebo
–
Reductions in flare parameters
–
Effect on sUA partially offset by drug interaction with oxypurinol
|
 16
Arhalofenate Phase 2 Monotherapy Study
Gradual dose-dependent reductions in sUA
Weeks of treatment
Baseline
sUA 9.7
9.4 9.8
No. of
patients 22
20 22 |
 17
Incidence (%)
Duration (Days)
Combined Score
(Incidence x Duration)
All patients received Colcrys for flare prophylaxis
Arhalofenate Phase 2 Monotherapy Study
Decrease in flare incidence, duration and combined score
Pbo 400
mg 600 mg
p = 0.08
p = 0.16 |
 18
Arhalofenate Phase 2 Monotherapy Study
Decreases in flare severity
Placebo
400 mg
600 mg
Mild
Moderate |
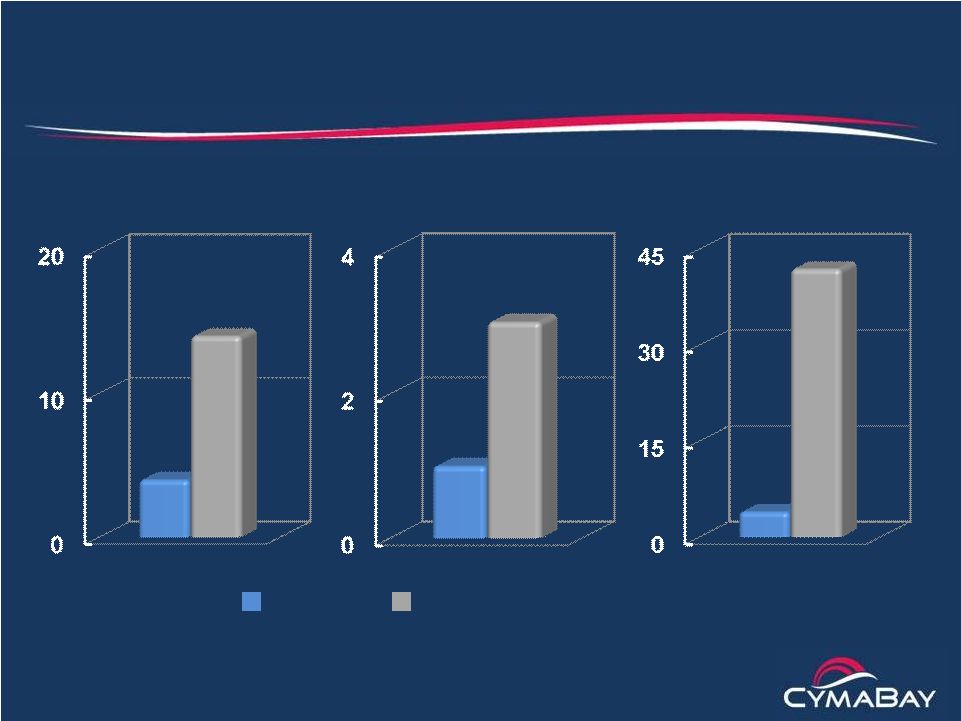 19
Lesinurad Phase 2 Monotherapy Study (Ardea*)
Increases in flare incidence, duration and combined score
* 28-day study
up-titration of lesinurad from 200 to 400 to 600 mg
Incidence (%)
Duration (Days)
Combined Score
(Incidence x Duration)
Placebo Lesinurad
All patients received Colcrys for flare prophylaxis |
 20
Arhalofenate Phase 2 Febuxostat Combination Study
Best-in-class sUA responder rate
6.0
mg/dL 5.0 mg/dL
4.0
mg/dL
3.0 mg/dL
Febuxostat (80 mg)
Febuxostat (80 mg) +
Arhalofenate (400 mg)
Febuxostat (80 mg) +
Arhalofenate (600 mg)
~10-fold
increase vs.
febuxostat
(p = 0.013)
Baseline sUA = 9 mg/dL
Week 1
Week
2-3
Week
4-5 |
 21
Arhalofenate Phase 2 Febuxostat Combination Study
Decrease in flare incidence and duration
Febuxostat Plus 400 mg Plus 600
mg Post-
Alone Arhalofenate
Arhalofenate Treatment Week 1
Weeks 2-3
Weeks 4-5
Weeks 6-7
All patients received
Colcrys for flare
prophylaxis through
post-treatment phase |
 22
Arhalofenate Clinical Studies
Efficacy summary
•
Consistent reductions in flare parameters in all three studies
–
Incidence, severity and duration
–
Effects comparable to the anti-IL-1 biologics
–
Effects achieved without need for dose titration
•
Consistent reductions in sUA
–
Up to 27% with monotherapy
–
Up to 60% in combination with febuxostat
–
Subset analyses show that sUA reductions are retained in:
•
Patients with Stage 2 and 3 CKD
•
Patients taking diuretics and aspirin
•
Favorable effects on metabolic comorbidities
–
Lowers triglycerides and reverses insulin resistance |
 23
Arhalofenate Clinical Studies
Safety summary
•
Completed 15 clinical studies
–
Nearly 1000 subjects exposed to arhalofenate for up to 6 months
•
General safety
–
Adverse events similar to placebo and balanced across dose groups
–
Low incidence of asymptomatic liver transaminase elevations
–
No increase in infections, no changes in neutrophils
•
Renal safety
–
No kidney stones, decrease in urine pH or effect on eGFR
–
No creatinine signal (no grade 3 or 4 elevations)
•
No dose-limiting toxicity has been identified |
 24
Arhalofenate Clinical Studies
Development status
•
Drug materials
–
Economical, proprietary synthesis
•
200 kg of drug substance in hand
–
Commercial tablet formulation developed
•
200, 300, 400 and 600 mg strengths
•
Completed preclinical safety package
–
Sub-chronic and chronic toxicology in rat and monkey
–
Safety pharmacology and reproductive toxicology
–
Two-year carcinogenicity studies in rodents
–
Carcinogenicity and CV safety review by FDA completed
–
All studies satisfactorily completed and support further development
•
Additional Phase 2/3 study to refine product profile |
 25
•
12-week study in gout patients experiencing
3 flares in the prior year
•
Goals for arhalofenate
–
Prevent flares without colchicine prophylaxis
–
800 mg lowers sUA comparable to allopurinol (300 mg)
–
Generate safety data with 800 mg dose
•
Primary endpoint
–
Mean flares/patient for arhalofenate (800 mg) vs. allopurinol (300 mg)
–
>80% power to detect a 50% decrease in flares
•
Secondary endpoint
–
sUA responder rate (<6 mg/dL) for arhalofenate (800 mg) vs. placebo
–
>90% power to detect a responder rate of 40%
Replace
allopurinol -
Colcrys
Arhalofenate Phase 2/3 Study |
 26
Arhalofenate Phase 2/3 Study Design
Screening
Run-in
3 Month Treatment Phase
1 Month
Run-in
Flare Rescue
Arhalofenate 600 mg
Arhalofenate 800 mg
Allopurinol 300 mg + Colchicine
Allopurinol 300 mg
Placebo
n = 25
n = 50
n = 50
n = 50
n = 50
1 Month
Follow-up |
 27
CymaBay Milestones for Arhalofenate
•
Dose first patient in Phase 2/3 study
1H 2014
•
Phase 2/3 headline data
1H 2015
•
End-of-phase 2 meeting
2H 2015
•
Start Phase 3
1H 2016 |
