Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a14-3052_28k.htm |
| EX-99.2 - EX-99.2 - AMICUS THERAPEUTICS, INC. | a14-3052_2ex99d2.htm |
|
|
Safe Harbor This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business, operations and financial conditions of Amicus including but not limited to preclinical and clinical development of Amicus’ candidate drug products, cash runway, ongoing collaborations and the timing and reporting of results from clinical trials evaluating Amicus’ candidate drug products. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Although Amicus believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in Amicus’ forward-looking statements due to numerous known and unknown risks and uncertainties, including the “Risk Factors” described in our Annual Report on Form 10-K for the year ended December 31, 2012. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. |
|
|
Company Mission Amicus Therapeutics is a biopharmaceutical company at the forefront of developing next-generation medicines to treat a range of rare and orphan diseases, with a focus on improved therapies for Lysosomal Storage Disorders |
|
|
2014 Highlights Amicus Strongly Positioned to Advance Next-Generation Enzyme Replacement Therapies Platform Technologies Experienced Leadership Team Next-Generation ERTs for Fabry, Pompe and MPS I $82M Cash Position (12/31/13) |
|
|
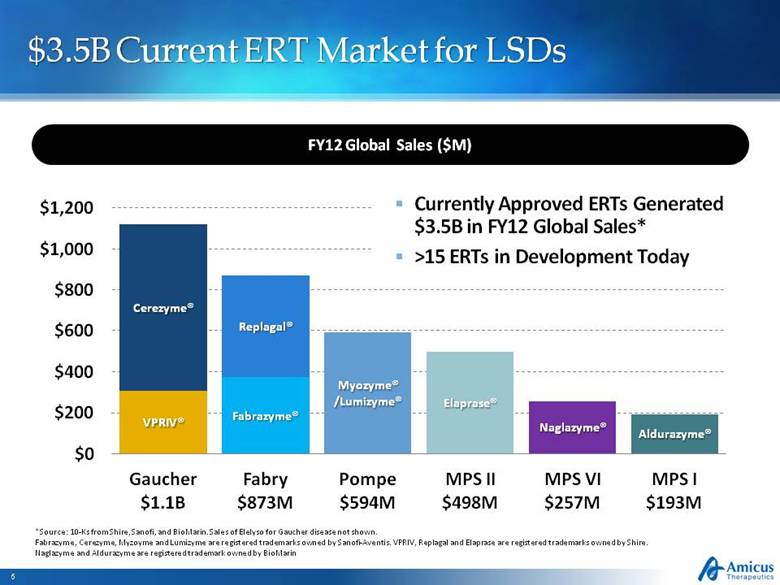
$3.5B Current ERT Market for LSDs *Source: 10-Ks from Shire, Sanofi, and BioMarin. Sales of Elelyso for Gaucher disease not shown. Fabrazyme, Cerezyme, Myzoyme and Lumizyme are registered trademarks owned by Sanofi-Aventis. VPRIV, Replagal and Elaprase are registered trademarks owned by Shire. Naglazyme and Aldurazyme are registered trademark owned by BioMarin FY12 Global Sales ($M) Currently Approved ERTs Generated $3.5B in FY12 Global Sales* >15 ERTs in Development Today |
|
|
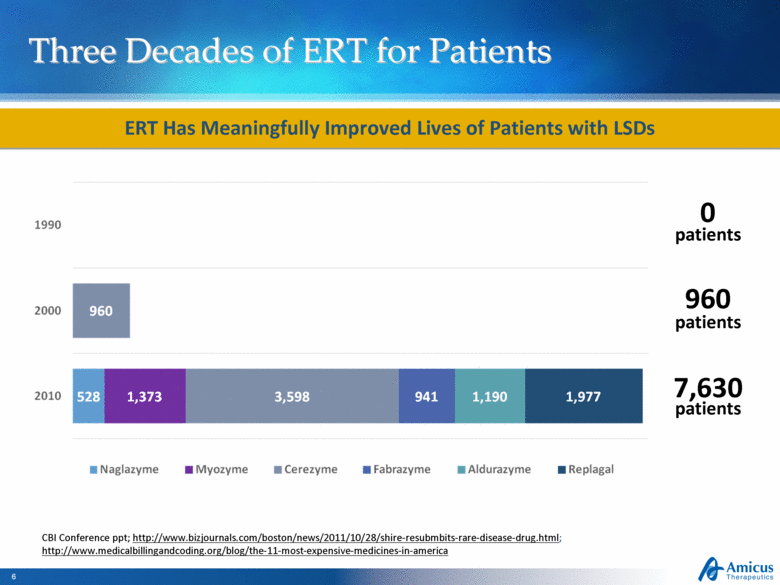
Three Decades of ERT for Patients ERT Has Meaningfully Improved Lives of Patients with LSDs CBI Conference ppt; http://www.bizjournals.com/boston/news/2011/10/28/shire-resubmbits-rare-disease-drug.html; http://www.medicalbillingandcoding.org/blog/the-11-most-expensive-medicines-in-america 7,630 960 0 patients patients patients |
|
|
Unmet Needs in LSDs Today Significant Unmet Needs Remain Due to Limitations of First-Generation ERTs “All 18 patients who enrolled in the initial [infantile-onset Pompe] study survived significantly longer and with fewer ventilation events However, morbidity and mortality remain substantial, with a 28% mortality rate and a 51% invasive ventilation rate at age 36 months.” - Kishnani, et al. 2009 Lumizyme statistically improves overall motor and pulmonary function14% of pts on treatment have declining 6-minute walk test and 36% have declining forced vital capacity. - van der Ploeg, et al. 2010 “one of the major obstacles to enzyme replacement with α-Gal is the instability of the enzyme.” - Beutler and Mayes, 1977 ORPHANET JOURNAL OF RARE DISEASES “Long term ERT does not prevent [Fabry] disease progression, but the risk of developing a first or second complication declines with increasing treatment duration.” - Rombach, et al. 2013 |
|
|
LSD Products Today: Three Fundamental Challenges Enzyme Activity and Stability Tissue Uptake and Targeting Tolerability and Immunogenicity |
|
|
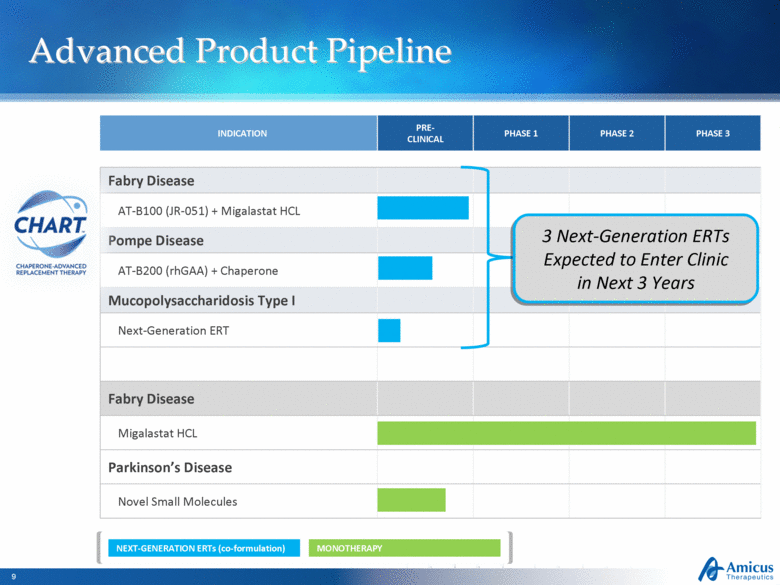
Advanced Product Pipeline INDICATION PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 Fabry Disease AT-B100 (JR-051) + Migalastat HCL Pompe Disease AT-B200 (rhGAA) + Chaperone Mucopolysaccharidosis Type I Next-Generation ERT Fabry Disease Migalastat HCL Parkinson’s Disease Novel Small Molecules MONOTHERAPY NEXT-GENERATION ERTs (co-formulation) 3 Next-Generation ERTs Expected to Enter Clinic in Next 3 Years |
|
|
Next-Generation ERT for Pompe Disease |
|
|
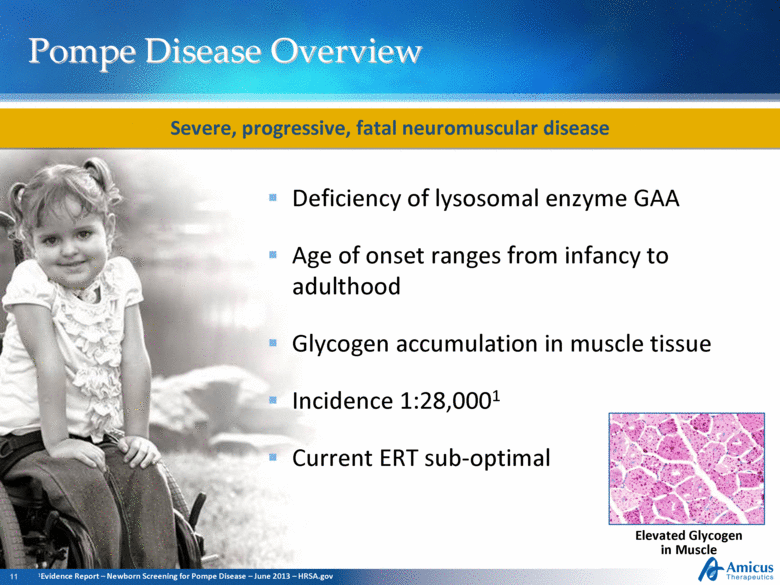
Pompe Disease Overview Deficiency of lysosomal enzyme GAA Age of onset ranges from infancy to adulthood Glycogen accumulation in muscle tissue Incidence 1:28,0001 Current ERT sub-optimal Elevated Glycogen in Muscle Severe, progressive, fatal neuromuscular disease 1Evidence Report – Newborn Screening for Pompe Disease – June 2013 – HRSA.gov |
|
|
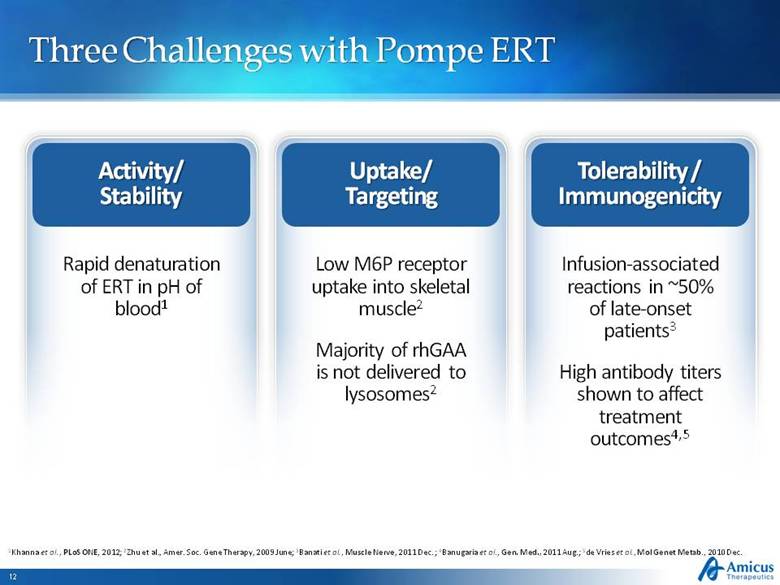
Three Challenges with Pompe ERT Activity/ Stability Tolerability / Immunogenicity Uptake/ Targeting Infusion-associated reactions in ~50% of late-onset patients3 High antibody titers shown to affect treatment outcomes4,5 Rapid denaturation of ERT in pH of blood1 Low M6P receptor uptake into skeletal muscle2 Majority of rhGAA is not delivered to lysosomes2 1Khanna et al., PLoS ONE, 2012; 2Zhu et al., Amer. Soc. Gene Therapy, 2009 June; 3Banati et al., Muscle Nerve, 2011 Dec. ; 4Banugaria et al., Gen. Med., 2011 Aug.; 5de Vries et al., Mol Genet Metab., 2010 Dec. |
|
|
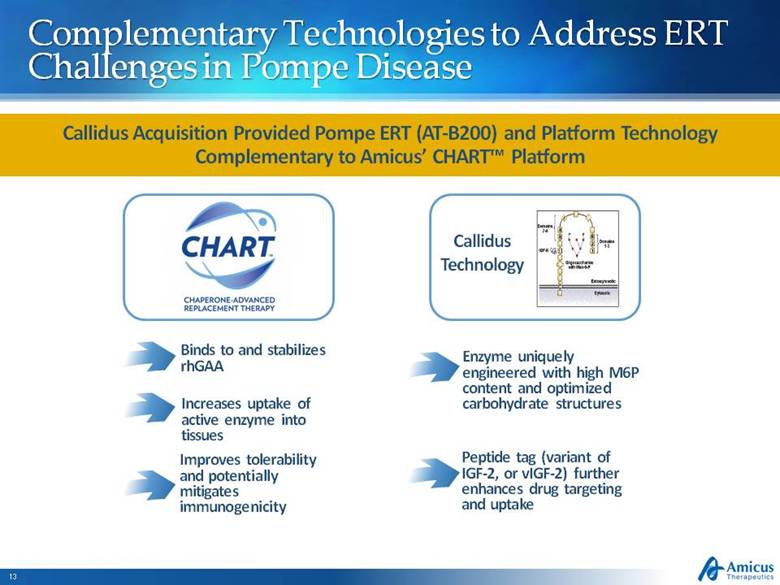
Complementary Technologies to Address ERT Challenges in Pompe Disease Binds to and stabilizes rhGAA Increases uptake of active enzyme into tissues Improves tolerability and potentially mitigates immunogenicity Enzyme uniquely engineered with high M6P content and optimized carbohydrate structures Peptide tag (variant of IGF-2, or vIGF-2) further enhances drug targeting and uptake Callidus Acquisition Provided Pompe ERT (AT-B200) and Platform Technology Complementary to Amicus’ CHART™ Platform Callidus Technology |
|
|
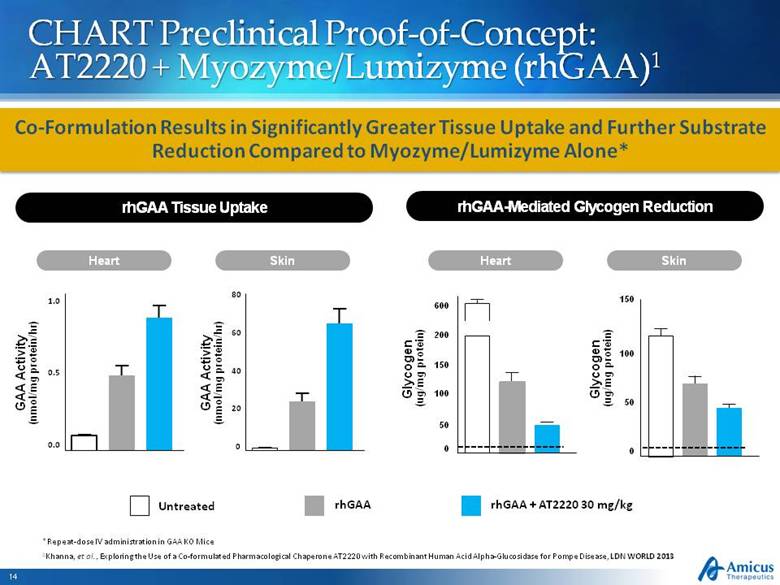
CHART Preclinical Proof-of-Concept: AT2220 + Myozyme/Lumizyme (rhGAA)1 1Khanna, et al., Exploring the Use of a Co-formulated Pharmacological Chaperone AT2220 with Recombinant Human Acid Alpha-Glucosidase for Pompe Disease, LDN WORLD 2013 Skin 0 100 200 50 150 600 Heart Glycogen (ug/mg protein) 150 0 50 100 Untreated rhGAA rhGAA + AT2220 30 mg/kg Co-Formulation Results in Significantly Greater Tissue Uptake and Further Substrate Reduction Compared to Myozyme/Lumizyme Alone* rhGAA-Mediated Glycogen Reduction rhGAA Tissue Uptake Glycogen (ug/mg protein) Skin Heart 0.0 0.5 1.0 0 20 40 60 80 GAA Activity (nmol/mg protein/hr) GAA Activity (nmol/mg protein/hr) *Repeat-dose IV administration in GAA KO Mice |
|
|
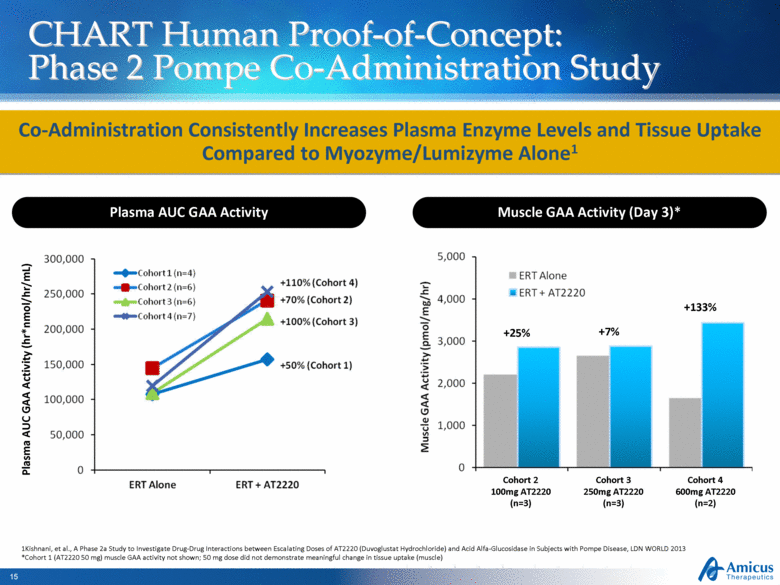
CHART Human Proof-of-Concept: Phase 2 Pompe Co-Administration Study +133% +7% +25% Co-Administration Consistently Increases Plasma Enzyme Levels and Tissue Uptake Compared to Myozyme/Lumizyme Alone1 1Kishnani, et al., A Phase 2a Study to Investigate Drug-Drug Interactions between Escalating Doses of AT2220 (Duvoglustat Hydrochloride) and Acid Alfa-Glucosidase in Subjects with Pompe Disease, LDN WORLD 2013 *Cohort 1 (AT2220 50 mg) muscle GAA activity not shown; 50 mg dose did not demonstrate meaningful change in tissue uptake (muscle) Plasma AUC GAA Activity Muscle GAA Activity (Day 3)* Plasma AUC GAA Activity (hr*nmol/hr/mL) Cohort 2 100mg AT2220 (n=3) Cohort 3 250mg AT2220 (n=3) Cohort 4 600mg AT2220 (n=2) |
|
|
Callidus Biopharma Acquisition Strengthens Amicus’ Pompe Program and Advances Time to Clinic Pompe rhGAA ERT Product Novel vIGF-2 Technology Platform Synergies with CHART Biologics Experience and Leadership Manufacturing Relationships and Contracts |
|
|
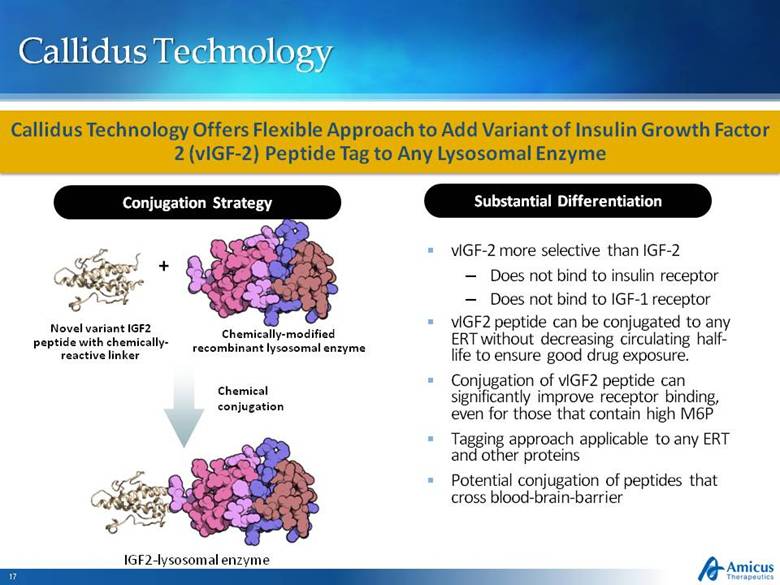
Callidus Technology Conjugation Strategy Callidus Technology Offers Flexible Approach to Add Variant of Insulin Growth Factor 2 (vIGF-2) Peptide Tag to Any Lysosomal Enzyme Substantial Differentiation vIGF-2 more selective than IGF-2 Does not bind to insulin receptor Does not bind to IGF-1 receptor vIGF2 peptide can be conjugated to any ERT without decreasing circulating half-life to ensure good drug exposure. Conjugation of vIGF2 peptide can significantly improve receptor binding, even for those that contain high M6P Tagging approach applicable to any ERT and other proteins Potential conjugation of peptides that cross blood-brain-barrier Novel variant IGF2 peptide with chemically-reactive linker + Chemically-modified recombinant lysosomal enzyme Chemical conjugation IGF2-lysosomal enzyme |
|
|
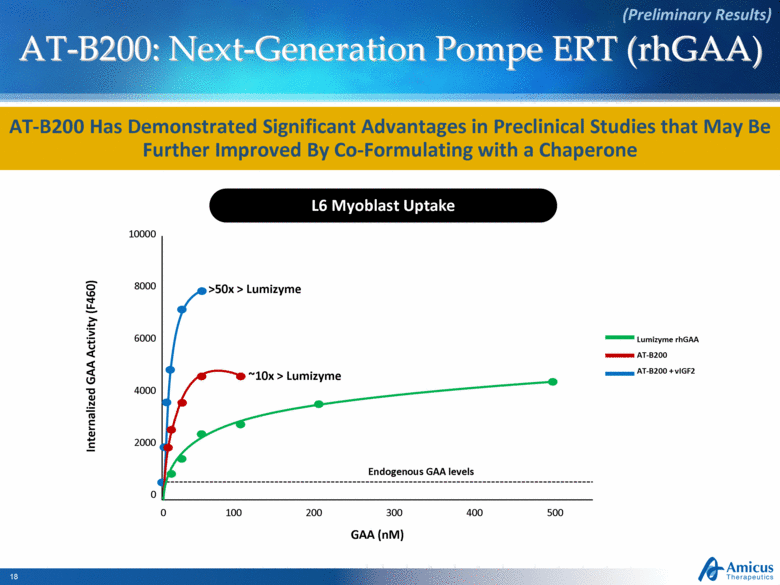
AT-B200: Next-Generation Pompe ERT (rhGAA) AT-B200 Has Demonstrated Significant Advantages in Preclinical Studies that May Be Further Improved By Co-Formulating with a Chaperone L6 Myoblast Uptake (Preliminary Results) 10000 8000 6000 4000 0 0 100 200 300 400 500 2000 Endogenous GAA levels Internalized GAA Activity (F460) GAA (nM) Lumizyme rhGAA AT-B200 AT-B200 + vIGF2 ~10x > Lumizyme >50x > Lumizyme |
|
|
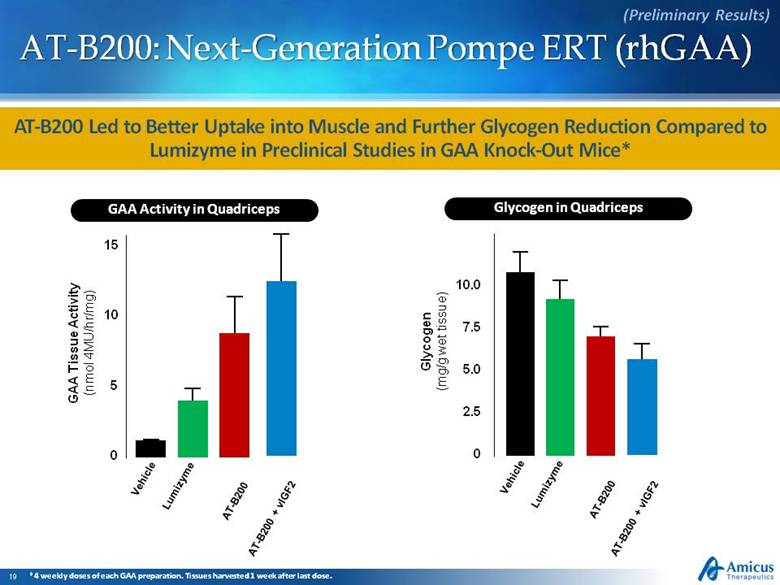
AT-B200: Next-Generation Pompe ERT (rhGAA) AT-B200 Led to Better Uptake into Muscle and Further Glycogen Reduction Compared to Lumizyme in Preclinical Studies in GAA Knock-Out Mice* (Preliminary Results) Glycogen (mg/g wet tissue) GAA Tissue Activity (nmol 4MU/hr/mg) Vehicle Lumizyme AT-B200 15 10 5 0 10.0 7.5 5.0 2.5 0 GAA Activity in Quadriceps Glycogen in Quadriceps Vehicle Lumizyme AT-B200 AT-B200 + vIGF2 AT-B200 + vIGF2 *4 weekly doses of each GAA preparation. Tissues harvested 1 week after last dose. |
|
|
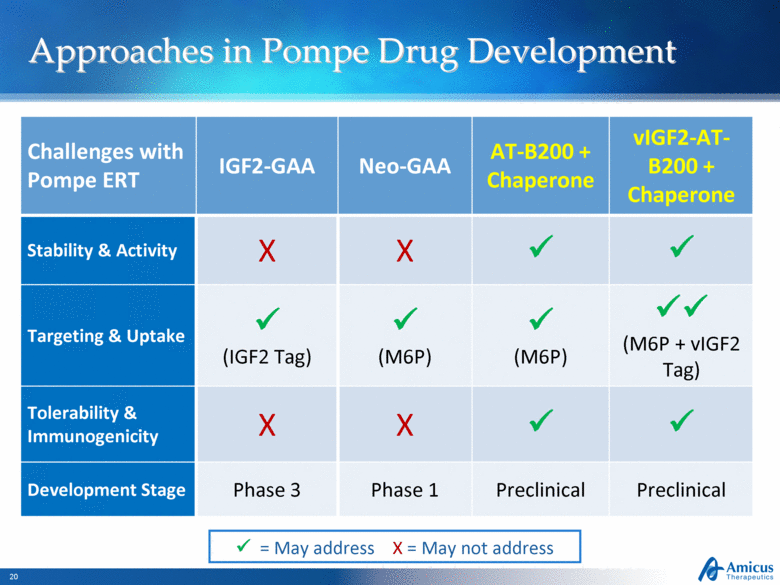
Approaches in Pompe Drug Development Slide 20 Challenges with Pompe ERT IGF2-GAA Neo-GAA AT-B200 + Chaperone vIGF2-AT-B200 + Chaperone Stability & Activity X X Targeting & Uptake (IGF2 Tag) (M6P) (M6P) (M6P + vIGF2 Tag) Tolerability & Immunogenicity X X Development Stage Phase 3 Phase 1 Preclinical Preclinical = May address X = May not address |
|
|
Next-Generation ERT for Fabry Disease |
|
|
Fabry Disease Overview Deficiency of lysosomal enzyme GLA GL-3 accumulation Renal failure, cardiac failure, stroke 5-10K diagnosed WW (51% female/49% male1) Newborn screening suggests prevalence of 1:3000 or more2,3 Current ERT suboptimal Kidney GL-3 1Fabry Registry 2011; 2 Spada et al., Am J Human Genetics 2006; 3Lin, et al., Circulation 2009 4Rombach et al., PLoS ONE, 2012 Proprietary ERT (AT-B100) Co-Formulated with Migalastat HCl Expected to Enter Clinic in 2014 |
|
|
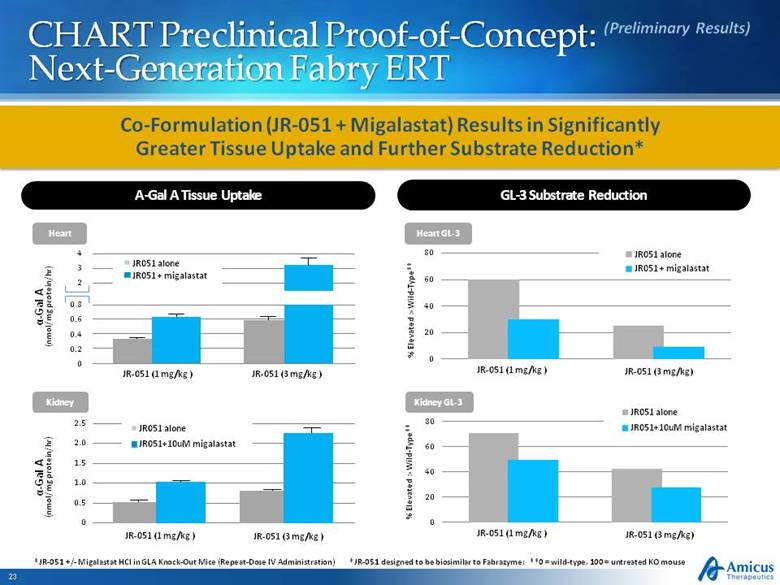
CHART Preclinical Proof-of-Concept: Next-Generation Fabry ERT Heart GL-3 *JR-051 designed to be biosimilar to Fabrazyme; **0 = wild-type, 100 = untreated KO mouse % Elevated > Wild-Type** % Elevated > Wild-Type** Kidney GL-3 A-Gal A Tissue Uptake GL-3 Substrate Reduction (Preliminary Results) *JR-051 +/- Migalastat HCl in GLA Knock-Out Mice (Repeat-Dose IV Administration) Co-Formulation (JR-051 + Migalastat) Results in Significantly Greater Tissue Uptake and Further Substrate Reduction* JR-051 (1 mg/kg ) JR-051 (3 mg/kg) JR-051 (1 mg/kg ) JR-051 (3 mg/kg) Heart Kidney 4 3 2 0.8 0.6 0.4 0.2 0 α-Gal A (nmol/mg protein/hr) JR-051 (1 mg/kg ) JR-051 (3 mg/kg ) JR051 alone JR051 + migalastat 2.5 2.0 1.5 1.0 0.5 0 α-Gal A (nmol/mg protein/hr) JR-051 (1 mg/kg ) JR-051 (3 mg/kg ) JR051 alone JR051+10uM migalastat |
|
|
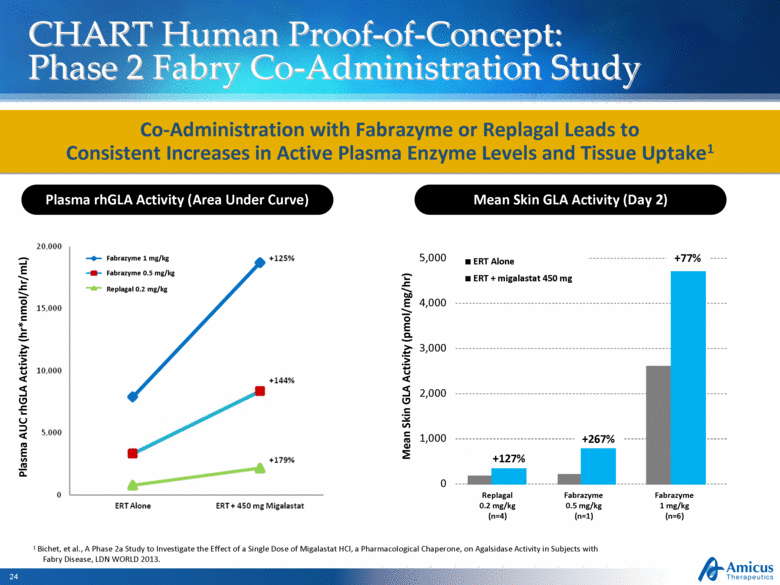
CHART Human Proof-of-Concept: Phase 2 Fabry Co-Administration Study Co-Administration with Fabrazyme or Replagal Leads to Consistent Increases in Active Plasma Enzyme Levels and Tissue Uptake1 1 Bichet, et al., A Phase 2a Study to Investigate the Effect of a Single Dose of Migalastat HCl, a Pharmacological Chaperone, on Agalsidase Activity in Subjects with Fabry Disease, LDN WORLD 2013. Plasma rhGLA Activity (Area Under Curve) Mean Skin GLA Activity (Day 2) Fabrazyme 1 mg/kg Fabrazyme 0.5 mg/kg Replagal 0.2 mg/kg Plasma AUC rhGLA Activity (hr*nmol/hr/mL) Mean Skin GLA Activity (pmol/mg/hr) Replagal 0.2 mg/kg (n=4) Fabrazyme 0.5 mg/kg (n=1) Fabrazyme 1 mg/kg (n=6) 0 1,000 2,000 3,000 4,000 5,000 ERT Alone ERT + migalastat 450 mg +127% +267% +77% |
|
|
Pathway to Clinic in 2014 (Preliminary Results) Timing Milestone 1Q12 Initial preclinical studies for next-generation Fabry ERT 3Q12 Preclinical proof-of-concept 1H13 Scale up to 2,000 L Ongoing IND-enabling studies 2H13 Preclinical data presented at SSIEM and ASHG 1H14 Phase 1 study initiation of IV migalastat in healthy volunteers 2H14 Pre-IND meeting 2H14 Next-generation ERT Phase 2 study initiation |
|
|
Migalastat HCl Monotherapy for Fabry Disease |
|
|
Global Registration Studies Placebo-controlled (6 months) 67 patients naïve to ERT 6-month surrogate primary endpoint: kidney GL-3 (reported 4Q12) 12-month biopsy and 24-month clinical data (expected 2Q14) ERT switch study 60 patients (1.5:1 randomization) 18 Month clinical endpoint: kidney function (GFR) Data expected 3Q14 Assembling Robust Dataset to Maximize Chances for Global Approvals of Migalastat HCl Monotherapy for Fabry Patients with Amenable Mutations |
|
|
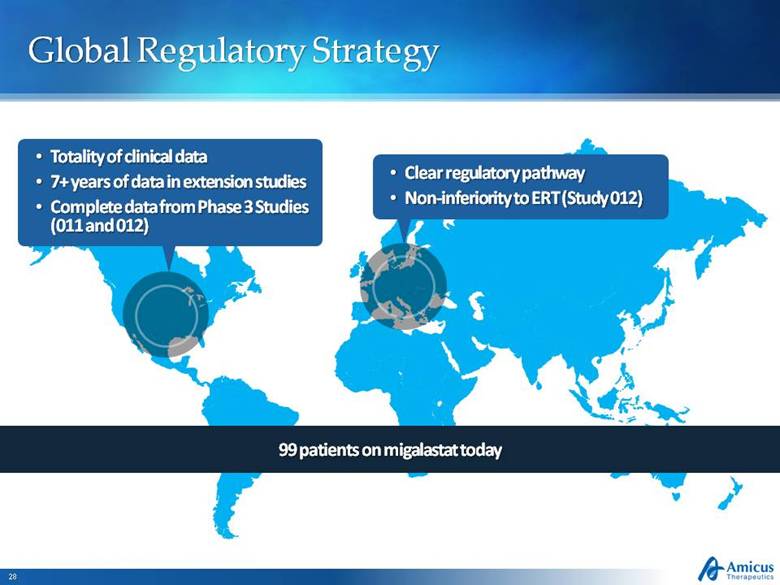
Global Regulatory Strategy Totality of clinical data 7+ years of data in extension studies Complete data from Phase 3 Studies (011 and 012) Clear regulatory pathway Non-inferiority to ERT (Study 012) 99 patients on migalastat today |
|
|
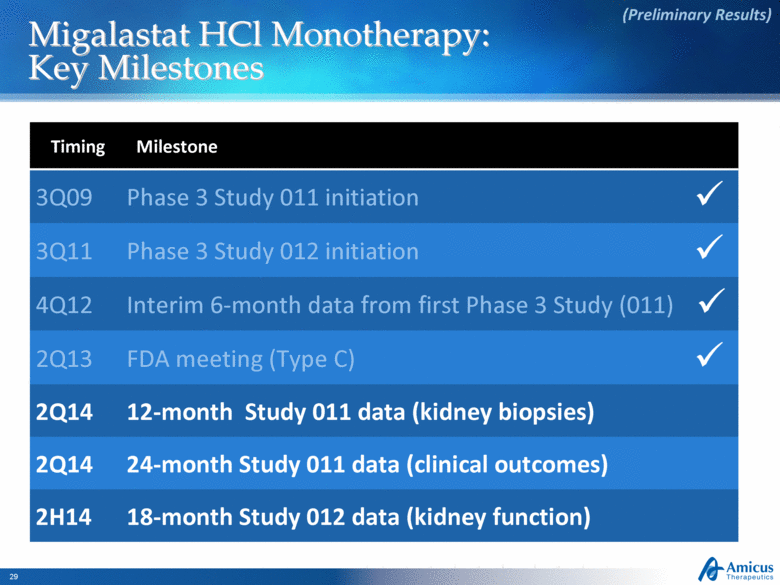
Migalastat HCl Monotherapy: Key Milestones (Preliminary Results) Timing Milestone 3Q09 Phase 3 Study 011 initiation 3Q11 Phase 3 Study 012 initiation 4Q12 Interim 6-month data from first Phase 3 Study (011) 2Q13 FDA meeting (Type C) 2Q14 12-month Study 011 data (kidney biopsies) 2Q14 24-month Study 011 data (clinical outcomes) 2H14 18-month Study 012 data (kidney function) |
|
|
Financial Overview and Upcoming Milestones |
|
|
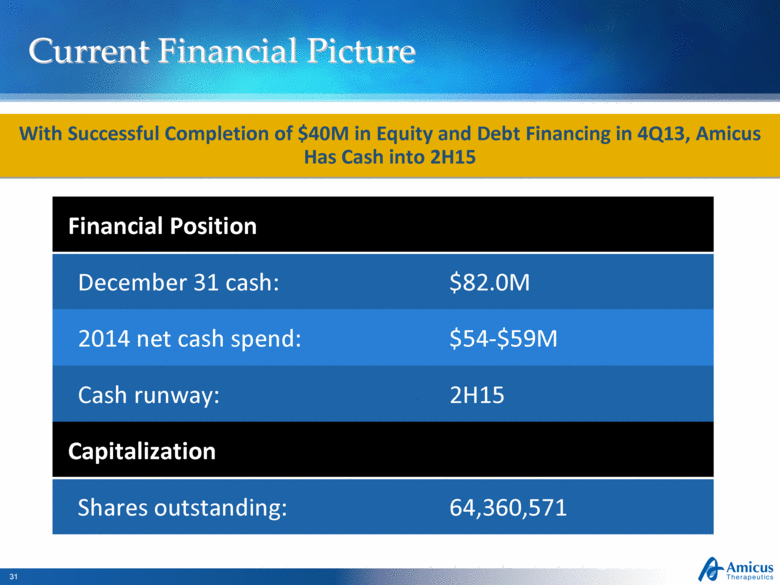
Current Financial Picture Slide 31 With Successful Completion of $40M in Equity and Debt Financing in 4Q13, Amicus Has Cash into 2H15 Financial Position December 31 cash: $82.0M 2014 net cash spend: $54-$59M Cash runway: 2H15 Capitalization Shares outstanding: 64,360,571 |
|
|
2014 Highlights Amicus Strongly Positioned to Advance Next-Generation Enzyme Replacement Therapies Platform Technologies Experienced Leadership Team Next-Generation ERTs for Fabry, Pompe and MPS I $82M Cash Position (12/31/13) |
|
|
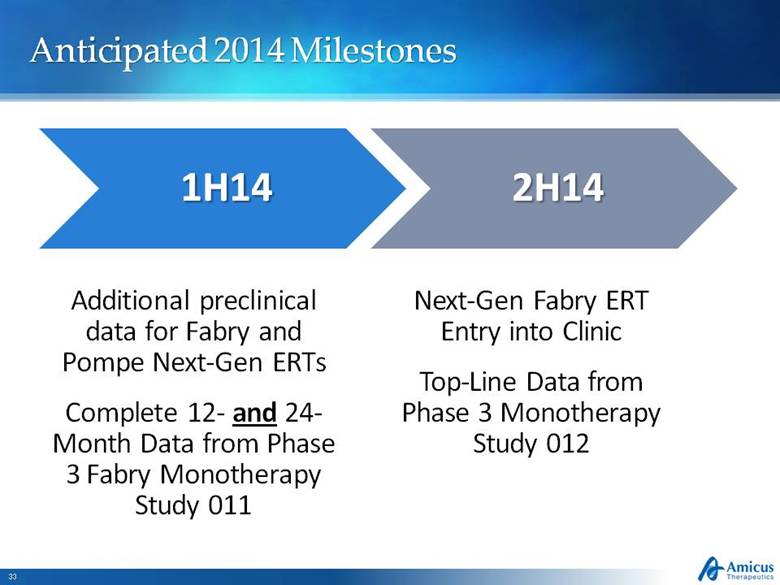
Anticipated 2014 Milestones Additional preclinical data for Fabry and Pompe Next-Gen ERTs Complete 12- and 24-Month Data from Phase 3 Fabry Monotherapy Study 011 Next-Gen Fabry ERT Entry into Clinic Top-Line Data from Phase 3 Monotherapy Study 012 |
|
|
32nd Annual J.P. Morgan Healthcare Conference January 16, 2014 |