Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - JUNIPER PHARMACEUTICALS INC | d657050d8k.htm |
| EX-99.1 - EX-99.1 - JUNIPER PHARMACEUTICALS INC | d657050dex991.htm |
 Frank
Condella President & CEO
Columbia Laboratories
Columbia Laboratories, Inc.
(Nasdaq: CBRX)
Molecular Profiles, Ltd.
(a wholly-owned subsidiary of Columbia Laboratories)
Jonathan Lloyd Jones
VP & CFO
Columbia Laboratories
Dr. Nikin Patel
CEO
Molecular Profiles
*
*
*
*
*
*
Exhibit 99.2 |
 Safe
Harbor 2
9 January 2014
This
presentation
contains
forward-looking
statements,
which
statements
are
indicated
by
the
words
“may,”
“will,”
“plans,”
“believes,”
“expects,”
“anticipates,”
“potential,”
“should,”
and
similar
expressions.
These
include
all
statements
relating
to
expected
financial
performance,
including,
without
limitation,
statements
involving
projections
of
revenue,
margin,
and
the
Company’s
future
growth
prospects.
Such
forward-looking
statements
involve
known
and
unknown
risks,
uncertainties,
and
other
factors
that
may
cause
actual
results
to
differ
materially
from
those
projected
in
the
forward-looking
statements.
Readers
are
cautioned
not
to
place
undue
reliance
on
these
forward-looking
statements,
which
speak
only
as
of
the
date
on
which
they
are
made.
Factors
that
might
cause
future
results
to
differ
include,
but
are
not
limited
to,
the
following:
completing
the
financial
reporting
closing
process
and
financial
audit,
which
could
necessitate
changes
to
these
preliminary
results;
the
effect
of
converting
Molecular
Profiles’
financial
statements
from
U.K.
to
U.S.
GAAP;
Actavis'
and
Merck
Serono's
success
in
marketing
CRINONE
for
use
in
infertility
in
their
respective
markets;
the
successful
launch
by
Actavis
of
the
next-generation
vaginal
progesterone
product
for
the
U.S.
market;
Molecular
Profiles’
ongoing
ability
to
retain
current
and
attract
new
customers;
difficulties
or
delays
in
manufacturing;
the
availability
and
pricing
of
third-party
sourced
products
and
materials;
successful
compliance
with
FDA,
MHRA
and
other
governmental
regulations
applicable
to
manufacturing
facilities,
products
and/or
businesses;
changes
in
the
laws
and
regulations;
the
ability
to
obtain
and
enforce
patents
and
other
intellectual
property
rights;
the
impact
of
patent
expiration;
the
impact
of
competitive
products
and
pricing;
the
strength
of
the
United
States
dollar
relative
to
international
currencies,
particularly
the
euro,
British
pound
and
the
Swiss
franc;
competitive,
economic,
and
regulatory
factors
in
the
pharmaceutical
and
healthcare
industry;
general
economic
conditions;
and
other
risks
and
uncertainties
that
may
be
detailed,
from
time-to-time,
in
Columbia's
reports
filed
with
the
SEC,
including,
but
not
limited
to,
its
Annual
Report
on
Form
10-K
for
the
period
ended
December
31,
2012.
Columbia
does
not
undertake
any
responsibility
to
revise
or
update
any
forward-looking
statements
contained
herein,
except
as
expressly
required
by
law. |
 Columbia
Laboratories Overview •
World-class pharmaceutical development company providing to
multinational pharmaceutical companies:
–
Formulation development
–
Clinical trial manufacturing
–
High-end Analytical / IP support
•
Growing & diversified revenue streams
–
Molecular Profiles service contracts
–
CRINONE
®
(progesterone gel) sales to Merck Serono + royalty from Actavis
•
Potential for proprietary product pipeline
–
Leverages CBRX IP & MP expertise
–
Focus: New Therapeutic Entity (NTE) regulatory pathway
•
505(b)(2) in U.S.
–
Plan: outlicense upon proof-of-concept
•
Profitable & cash-flow positive with solid balance sheet: $20m cash
3
9 January 2014 |
 Experienced
Management Team •
Frank Condella, BS Pharm, MBA –
President & CEO
–
Over 30 years’
experience in life sciences industry
–
Skyepharma plc, IVAX Corp, Faulding Pharma, Roche
•
Dr. Nikin Patel, MRPharmS –
CEO, Molecular Profiles
–
Molecular Profiles co-founder
–
15+ years’
pharmaceutical industry experience
•
Jonathan Lloyd Jones, ACA, MBA –
Vice President & CFO
–
25+ years of corporate development & finance experience
–
TetraLogic Pharma, TransMolecular, Genzyme, Deloitte
•
Dr. Shen Luk, BSc –
CSO, Molecular Profiles
–
25+ years of technical expertise in pharmaceutical analysis & formulations
–
Courtaulds Corporate Research Laboratories
4
9 January 2014 |
 Leveraging
Synergies Across the Business 5
Columbia Laboratories
Molecular Profiles
Development & Manufacturing Service Business
•
U.S. presence
•
Strong industry relationships
•
Capital to invest in business to support &
drive future growth
•
Contract formulation & development
•
Clinical trial manufacturing
•
Analytical & consulting
Over 100 clients
CRINONE
®
(progesterone gel) Global Franchise
•
Long-standing relationships
•
Product sales revenues from Merck
Serono: 60+ countries
•
Royalty from Actavis: U.S.
•
Quality Assurance
•
Supply Chain Operations
•
Life-cycle management
Potential
Proprietary Product Development
•
Intellectual Property
•
Nascent development programs
•
Successful history of transmucosal drug
delivery
•
Broad development expertise including
topical delivery
•
Analytical / IP Support
•
Clinical trial manufacturing
9 January 2014 |
 Molecular
Profiles, Ltd. (a wholly-owned subsidiary of Columbia Laboratories)
6
*
*
*
*
*
9 January 2014 |
 World Class
Pharmaceutical Provider 7
9 January 2014 |
 Facilities and
Capabilities •
Flexible contract services
–
8 cGMP process rooms, expandable to 14
–
State-of-the-art Analytical, Development & Characterisation suites
–
Facilities
& processes to handle potent molecules
•
Highly
skilled & diversified team
–
60+ FTEs, over 25% with advanced scientific degrees
•
Solution-driven & science-led
–
Expertise & growing range of enabling technologies for difficult
to formulate
molecules
•
Trusted ability to deliver
–
Clients range from emerging biotech to top 10 Pharma
8
9 January 2014 |
 Molecular
Profiles’ Growth Drivers
•
Favorable market dynamics
–
Addressable outsourced pharmaceutical development market
segment projected at $21.5 billion
(1)
–
Increasing trend toward pharmaceutical outsourcing
•
New state-of the-art facility can accommodate growth
•
Broadening service offerings to meet client needs
–
Specialty processing for challenging molecules
•
Expanding customer base
–
Virtual to top 10 Pharma
–
Leverage U.S. presence
•
Unsurpassed
specialty expertise
9
(1)
Source: Jeffries equity research report, 18 June 2013
9 January 2014 |
 CRINONE®
(progesterone gel)
Franchise
10
*
*
*
*
9 January 2014 |
 U.S. TRx Growth
(1)
CRINONE Continued Growth Globally
Ex-U.S. Opportunity
•
Ex-U.S. revenue CAGR was 13.4%
from 2009 -
2012
•
Key growth drivers of
ex-U.S.
in-market sales:
11
Most Major Markets Covered
(1)
Source: IMS data
•
Continued growth in emerging markets
•
Entry into new markets
9 January 2014 |
 12
CRINONE Revenue Streams
U.S.
Pays us a 10% royalty on U.S.
net sales
Royalty continues with newly
launched next-gen CRINONE
Ex-U.S.
Markets CRINONE in 60+ countries
outside U.S.
CBRX is exclusive CRINONE supplier
to Merck Serono through May 2020
* Unaudited, preliminary estimates as announced on 09 January 2014
FY 2013 Royalty Revenues:
~$3.3-$3.6 million*
FY 2013 Product Revenues:
~$21.2-$21.6 million*
9 January 2014 |
 Capabilities to
Support & Grow CRINONE Revenues 13
9 January 2014 |
 14
*
*
*
**
*
*
*
*
9 January 2014 |
 Product
Pipeline Strategy 15
•
Selectively develop product candidates
-
Commercially viable; relative low risk
-
Address an unmet patient need (women’s health)
-
Add value by applying our expertise in
formulation & manufacturing
-
Focus on New Therapeutic Entities
•
505(b)(2) regulatory path (US)
•
Reference safety & efficacy data of original molecule
•
Solid Intellectual Property coverage
•
Develop through Phase IIb
with
low-level capital investment
•
Partner for late stage
development
& commercialization
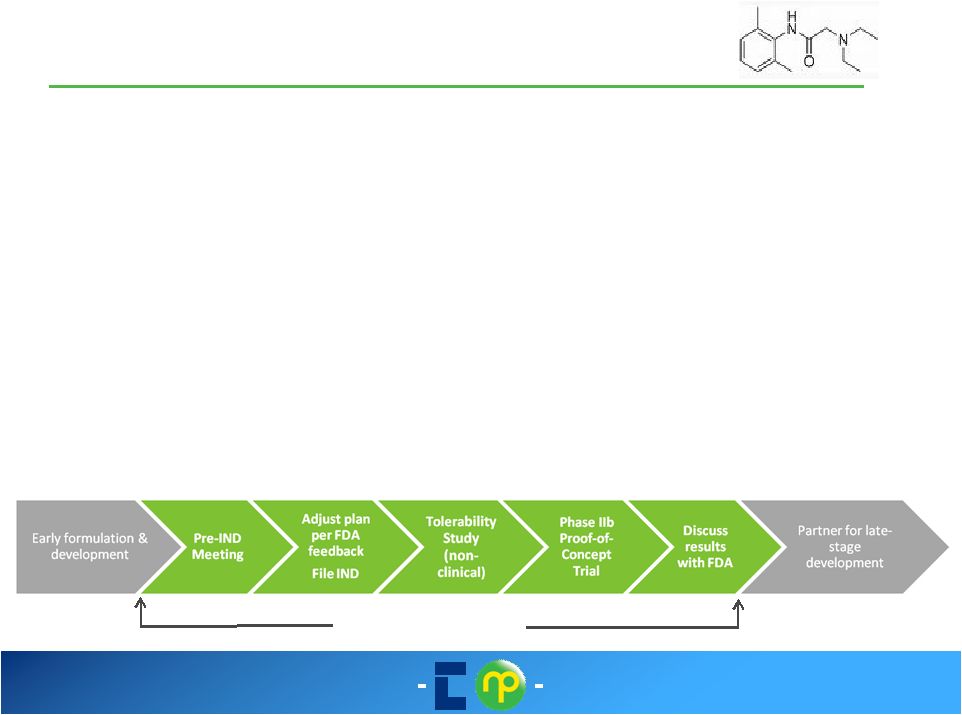
9 January 2014 |
 Early-stage development
candidates
Intellectual Property
Proven Expertise in Product Development
Strong Internal Development Capability
16
Columbia Laboratories
Molecular Profiles
State-of-the-Art Characterisation
Enabling Technologies
Clinical Trial Manufacturing
Analytical Development & Support
9 January 2014 |
 17
COL -1077: Potential Pipeline Product
•
Target indication: prevention of pain associated with gynecological
procedures (e.g. IUD insertion, endometrial biopsy)
–
Significant unmet medical need; currently no marketed products indicated
–
Peak sales projected at approx. $100m
•
Leverage clinical development work previously completed (Ph. I &
II)
•
Proprietary Formulation: extended-release lidocaine in bioadhesive gel
•
Exclusivity
–
Two approved patents; 1 pending
–
Hatch Waxman 3-year exclusivity
–
Other barriers to generic entry
•
Currently re-assessing commercial opportunity & development plan
Est. $2.5-$3.0m Investment
9 January 2014 |
 Financials
18
*
*
*
*
**
*
*
*
*
9 January 2014 |
 19
Nine-months Growth
Total Revenues by Type
(in millions)
Services (Molecular Profiles)
Other revenues
Royalties
CRINONE sales to Actavis
CRINONE sales to Merck Serono
Non-GAAP Adjusted Net Income
(1)
(in millions)
Nine months
ended
Reconciliation of
Nine months ended
GAAP to Non-GAAP Results
30 Sept. 2013
30 Sept. 2012
Net Income
$ 744,202
$ 7,285,932
Transaction costs
1,439,946
-
Severance and relocation costs
627,794
483,337
Change in fair value of stock
warrants
4,394,778
(5,399,569)
Total Non-GAAP adjustments to net
income
6,462,518
(4,916,232)
Non-GAAP Income from Operations
$ 7,206,720
$ 2,369,700
9 January 2014
Non-GAAP figures exclude transaction-related costs, severance & relocation costs
associated with workforce reduction & relocation, and the change in the fair
value of stock warrants.
(1) |
 (1)
Includes Q4 2013 preliminary and unaudited estimated data as reported 09 January
2014 (2)
Total Revenues for 2011 of $43M included additional Other Revenues of $22.1M as
follows: $17M in amortization of the $34 million gain on the sale of the
progesterone assets to Actavis (concluded Q2 2011) The recognition of the
$5M milestone payment from Actavis for the filing of NDA 22-139 Core
Revenues (in millions)
Annual Revenue Growth
20
9 January 2014 |
 Q4 2012
Q4
2013*
$7.1
$7.4 -
$8.0
FY 2012
FY 2013*
$25.8
$28.8 –
$29.4
Services (Molecular Profiles)
Other revenues
Royalties
CRINONE sales to Actavis
CRINONE sales to Merck Serono
Increasingly Diverse Revenue Stream
* Unaudited, preliminary estimates as reported on 09 January 2014
21
Revenues by Type
(in millions)
9 January 2014 |
 •
Strong cash-flow + solid balance sheet
•
World-class pharmaceutical development services
business
–
Double digit year-over-year revenue growth
–
Market opportunity: $21.5 billion and growing
•
Strong ongoing revenue from CRINONE franchise
•
Potential product pipeline provides additional
upside opportunities
–
Leverages synergies across the business
–
Would be funded from free cash flow
CBRX: Positioned for Growth
22
9 January 2014 |
 Jonathan Lloyd
Jones Chief Financial Officer
Columbia Laboratories, Inc.
T: 617.639.1522
jlloydjones@columbialabs.com
Investor Relations Contacts
Tricia Truehart
Senior Associate
The Trout Group
T: 646.378.2953
ttruehart@troutgroup.com
23
9 January 2014 |
 9/30/2013
12/31/2012
Cash & Short Term Investments
$ 18.82
$ 28.64
Accounts Receivable
7.75
3.35
Inventories
1.99
2.63
Prepaid Expenses
2.81
1.28
Total Current Assets
$ 31.37
$ 35.90
Plant Property & Equipment
12.73
0.93
Goodwill and Intangibles, net
15.56
-
Other Assets
0.12
0.04
TOTAL ASSETS
$ 59.78
$ 36.87
Total Current Liabilities
$ 6.56
$
3.75 Deferred Revenues
2.32
0.03
Note Payable
3.73
-
Stock Warrant
5.57
1.17
TOTAL LIABILITIES
$ 18.18
$ 4.95
Series C Preferred
0.55
0.55
Total Shareholders Equity
41.05
31.37
TOTAL LIABILITIES & EQUITY
$ 59.78
$ 36.87
R-1
Balance Sheet (US $, millions)
9 January 2014 |
 R-2
Cash Flow Statement (US $, millions)
9 Months Ended
9/30/2013
9/30/2012
Net Income
$
0.74
$
7.29 Non-Cash Items (net)
5.10
(3.67)
Cash Flow before movements in Working Capital
5.85
3.62
Movements in Working Capital (net)
(0.93)
(2.36)
Net Cash from Operations
4.92
1.25
Purchase of Plant & Equipment
(0.25)
(0.99)
Sale of Short Term Investments
15.35
(0.18)
Acquisitions
(14.52)
-
Investing Activities
0.59
(1.17)
Financing Activities (net)
(0.02)
(0.05)
Foreign Exchange
0.12
0.04
TOTAL INCREASE IN CASH
$
5.61
$
0.07 Cash Beginning
$
13.20
$
10.11 Cash End
$
18.82
$
10.19 9 January 2014 |
 Nasdaq:
CBRX Shares Outstanding
12.15 M
Average
Preferred Shares
@
$ 6.70
0.1
Warrants*
@
$11.34
1.2
Options
@
$11.09
0.3
Fully Diluted Shares
(approximate)**
13.75 M
*
All warrants have “cashless”
exercise option
** At 12/31/13
CBRX Capitalization Table
R-3
9 January 2014 |
