Attached files
| file | filename |
|---|---|
| 8-K - 8-K - TESARO, Inc. | a14-3137_18k.htm |
Exhibit 99.1
|
|
Corporate Presentation January 2014 |
|
|
Safe Harbor Statement Statements made in this presentation about TESARO, Inc. that are not descriptions of historical facts are forward-looking statements reflecting the current beliefs and expectations of management. Forward-looking statements are sometimes identified by words such as “plan,” “may,” “will,” “expect,” and similar expressions referencing future events, conditions or circumstances. These forward-looking statements involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Actual results could differ materially from those expressed or implied by these forward-looking statements as a result of various factors, including, without limitation, the various risks described in our Annual Report on Form 10-K for the year ended December 31, 2012 and our subsequent filings with the SEC. TESARO, Inc. undertakes no obligation to update or revise any forward-looking statement for any reason. Rolapitant, niraparib and TSR-011 are investigational products that have not been approved by any regulatory agency. The most frequently observed adverse events in the two completed rolapitant Phase 3 studies were balanced across treatment arms and included fatigue, alopecia and loss of appetite. Phase 1 data indicate that the most frequently observed adverse events for niraparib at a 300 milligram dose included grade 1/2 anemia, fatigue and nausea. Phase 1 data indicate that TSR-011 was well tolerated at therapeutic dose levels, and the most frequently occurring dose limiting toxicities included ECG changes and dysaethesia, both of which were reversible. |
|
|
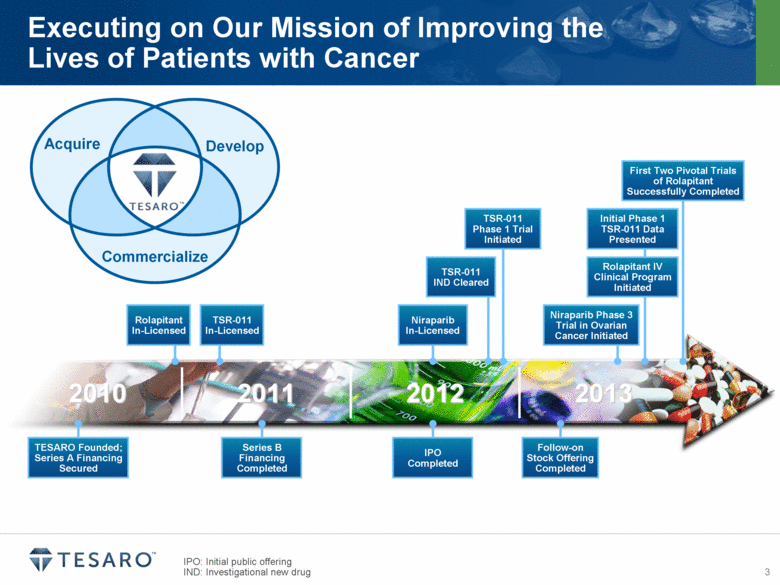
Executing on Our Mission of Improving the Lives of Patients with Cancer Develop Acquire Commercialize IPO: Initial public offering IND: Investigational new drug 2010 2011 2012 2013 Series B Financing Completed IPO Completed Follow-on Stock Offering Completed TESARO Founded; Series A Financing Secured Rolapitant In-Licensed Niraparib In-Licensed TSR-011 IND Cleared TSR-011 Phase 1 Trial Initiated First Two Pivotal Trials of Rolapitant Successfully Completed Niraparib Phase 3 Trial in Ovarian Cancer Initiated Initial Phase 1 TSR-011 Data Presented Rolapitant IV Clinical Program Initiated TSR-011 In-Licensed |
|
|
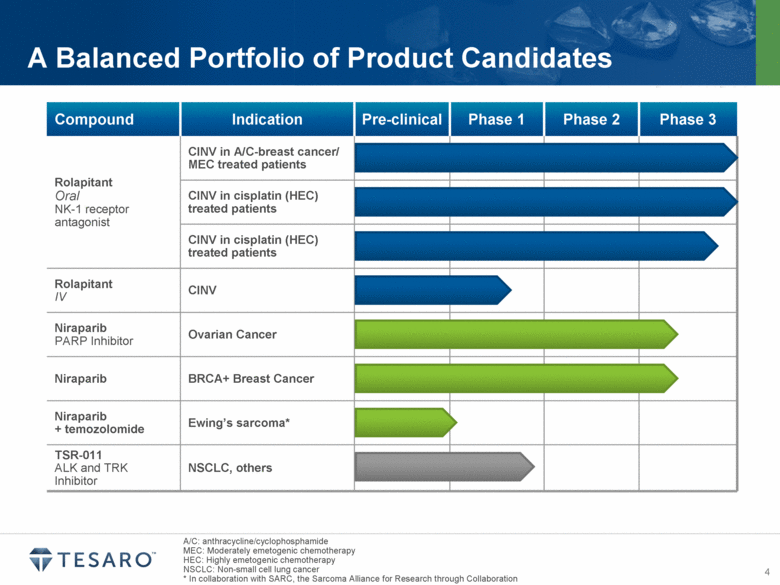
A/C: anthracycline/cyclophosphamide MEC: Moderately emetogenic chemotherapy HEC: Highly emetogenic chemotherapy NSCLC: Non-small cell lung cancer * In collaboration with SARC, the Sarcoma Alliance for Research through Collaboration A Balanced Portfolio of Product Candidates Compound Indication Pre-clinical Phase 1 Phase 2 Phase 3 Rolapitant Oral NK-1 receptor antagonist CINV in A/C-breast cancer/ MEC treated patients CINV in cisplatin (HEC) treated patients CINV in cisplatin (HEC) treated patients Rolapitant IV CINV Niraparib PARP Inhibitor Ovarian Cancer Niraparib BRCA+ Breast Cancer Niraparib + temozolomide Ewing’s sarcoma* TSR-011 ALK and TRK Inhibitor NSCLC, others |
|
|
A Potent and Selective NK-1 Receptor Antagonist (RA) with an Extended Half-Life Completing Phase 3 for the Prevention of Chemotherapy-Induced Nausea & Vomiting (CINV) |
|
|
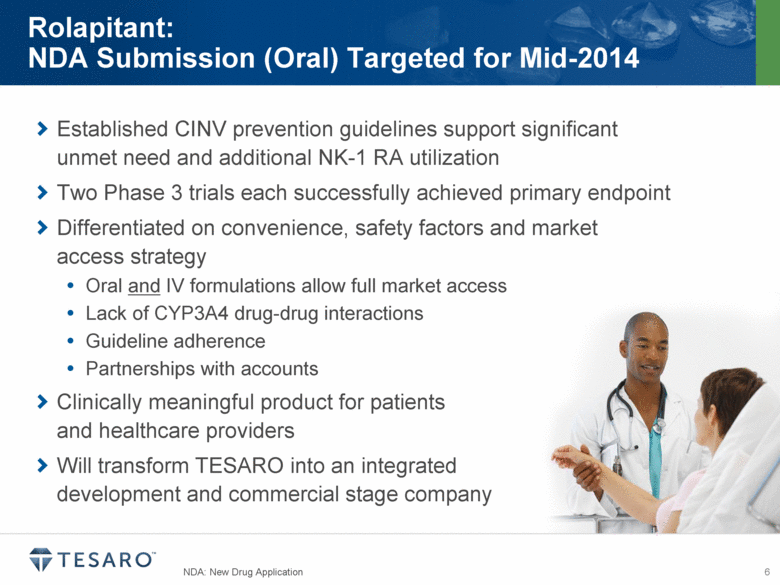
Established CINV prevention guidelines support significant unmet need and additional NK-1 RA utilization Two Phase 3 trials each successfully achieved primary endpoint Differentiated on convenience, safety factors and market access strategy Oral and IV formulations allow full market access Lack of CYP3A4 drug-drug interactions Guideline adherence Partnerships with accounts Clinically meaningful product for patients and healthcare providers Will transform TESARO into an integrated development and commercial stage company NDA: New Drug Application Rolapitant: NDA Submission (Oral) Targeted for Mid-2014 |
|
|
1For selected patients as appropriate 2J Support Oncol 2004;2(suppl 1):1-12 HCPs: Healthcare Providers Despite Available Therapies, Significant Unmet Need Remains Start before chemotherapy Serotonin (5-HT3) receptor antagonist AND Steroid AND Neurokinin 1 antagonist NCCN Guidelines® Version 1.2013 Antiemesis Opportunity to Educate HCPs Regarding Antiemesis Guidelines and Actual Patient CINV Experience Perception vs. Reality2 Agent AC combination defined as either doxorubicin or epirubicin with cyclophosphamide s Cisplatin Carboplatin1 Cyclophosphamide >1,500mg/m2 Doxorubicin >60mg/m2 Dacarbazine Epirubicin >90mg/m2 Ifosfamide >2g/m per2 dose Mechlorethamine Streptozocin |
|
|
Favorable Pharmacokinetics and Pharmacodynamics: Extended Half-Life and Brain Receptor Occupancy Plasma half-life of ~180 hours Nearly 100% bioavailable High affinity for human NK-1 receptor (Ki = 0.7 nM) Greater than 1,000-fold more selective for the human NK-1 receptor vs. other targets PET scan 5 days post-dose showed >90% NK-1 receptor occupancy following single 200mg oral dose + PET tracer Scale: 0.0 – 3.0 kBq/ml MRI Baseline PET (t00729) Blocked PET (t00739) 10 20 30 40 50 60 70 80 90 100 1 10 100 50 500 5 EC90 = 348 ng/mL EC50 = 15 ng/mL Concentration (ng/mL) NK1 Receptor Occupancy (%) 200 mg |
|
|
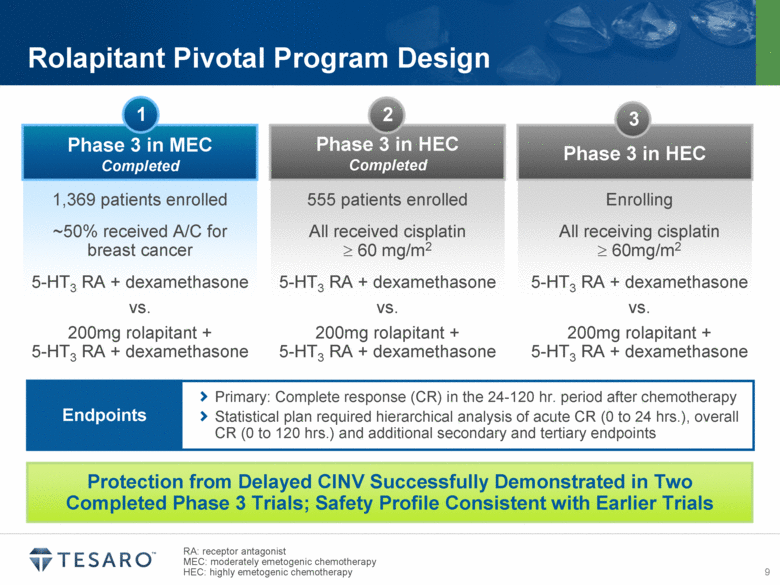
Rolapitant Pivotal Program Design Protection from Delayed CINV Successfully Demonstrated in Two Completed Phase 3 Trials; Safety Profile Consistent with Earlier Trials Phase 3 in MEC Completed 1,369 patients enrolled ~50% received A/C for breast cancer 5-HT3 RA + dexamethasone vs. 200mg rolapitant + 5-HT3 RA + dexamethasone Phase 3 in HEC Completed Phase 3 in HEC 1 2 3 555 patients enrolled All received cisplatin o 60 mg/m2 5-HT3 RA + dexamethasone vs. 200mg rolapitant + 5-HT3 RA + dexamethasone Enrolling All receiving cisplatin o 60mg/m2 5-HT3 RA + dexamethasone vs. 200mg rolapitant + 5-HT3 RA + dexamethasone RA: receptor antagonist MEC: moderately emetogenic chemotherapy HEC: highly emetogenic chemotherapy Primary: Complete response (CR) in the 24-120 hr. period after chemotherapy Statistical plan required hierarchical analysis of acute CR (0 to 24 hrs.), overall CR (0 to 120 hrs.) and additional secondary and tertiary endpoints Endpoints |
|
|
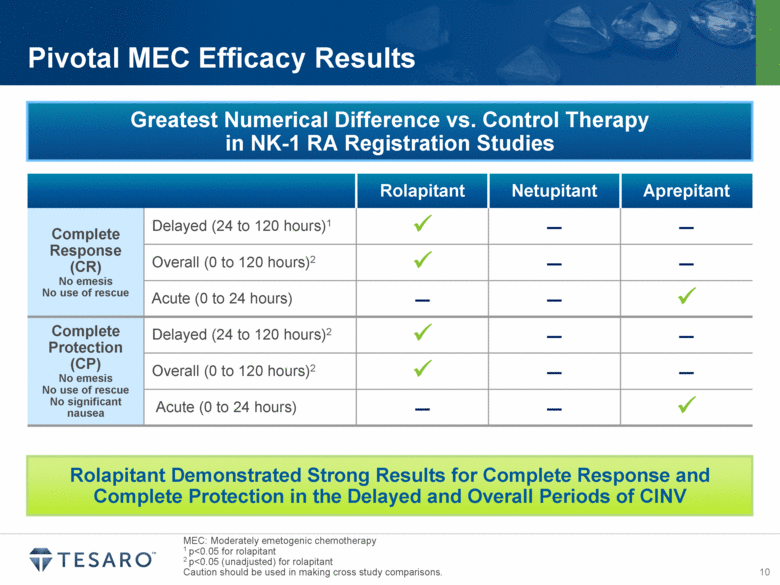
MEC: Moderately emetogenic chemotherapy 1 p<0.05 for rolapitant 2 p<0.05 (unadjusted) for rolapitant Caution should be used in making cross study comparisons. Pivotal MEC Efficacy Results Rolapitant Netupitant Aprepitant Complete Response (CR) No emesis No use of rescue Delayed (24 to 120 hours)1 – – Overall (0 to 120 hours)2 – – Acute (0 to 24 hours) – – Complete Protection (CP) No emesis No use of rescue No significant nausea Delayed (24 to 120 hours)2 – – Overall (0 to 120 hours)2 – – Acute (0 to 24 hours) – – Rolapitant Demonstrated Strong Results for Complete Response and Complete Protection in the Delayed and Overall Periods of CINV Greatest Numerical Difference vs. Control Therapy in NK-1 RA Registration Studies |
|
|
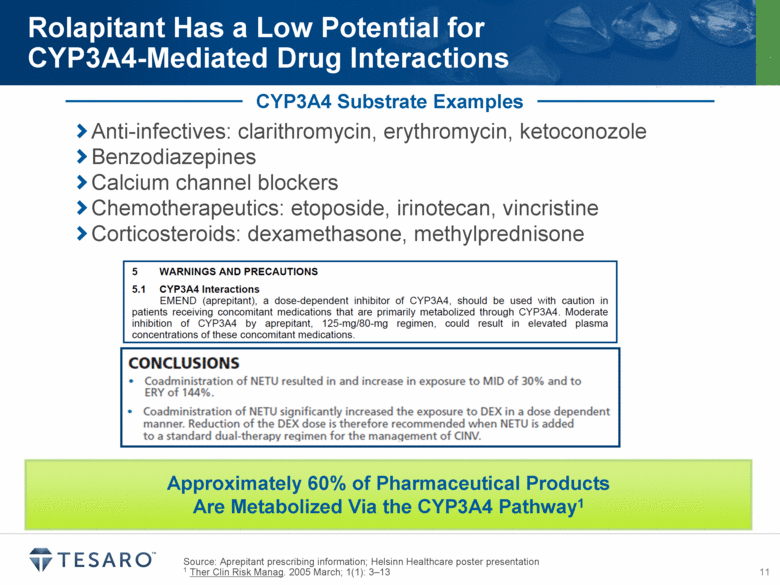
Source: Aprepitant prescribing information; Helsinn Healthcare poster presentation 1 Ther Clin Risk Manag. 2005 March; 1(1): 3–13 Rolapitant Has a Low Potential for CYP3A4-Mediated Drug Interactions CYP3A4 Substrate Examples Anti-infectives: clarithromycin, erythromycin, ketoconozole Benzodiazepines Calcium channel blockers Chemotherapeutics: etoposide, irinotecan, vincristine Corticosteroids: dexamethasone, methylprednisone Approximately 60% of Pharmaceutical Products Are Metabolized Via the CYP3A4 Pathway1 |
|
|
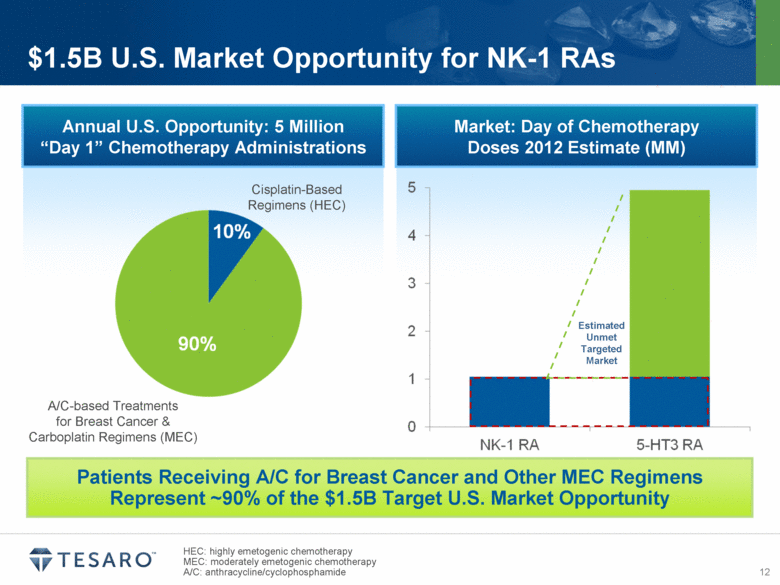
HEC: highly emetogenic chemotherapy MEC: moderately emetogenic chemotherapy A/C: anthracycline/cyclophosphamide $1.5B U.S. Market Opportunity for NK-1 RAs Annual U.S. Opportunity: 5 Million “Day 1” Chemotherapy Administrations Cisplatin-Based Regimens (HEC) A/C-based Treatments for Breast Cancer & Carboplatin Regimens (MEC) Market: Day of Chemotherapy Doses 2012 Estimate (MM) Estimated Unmet Targeted Market Patients Receiving A/C for Breast Cancer and Other MEC Regimens Represent ~90% of the $1.5B Target U.S. Market Opportunity |
|
|
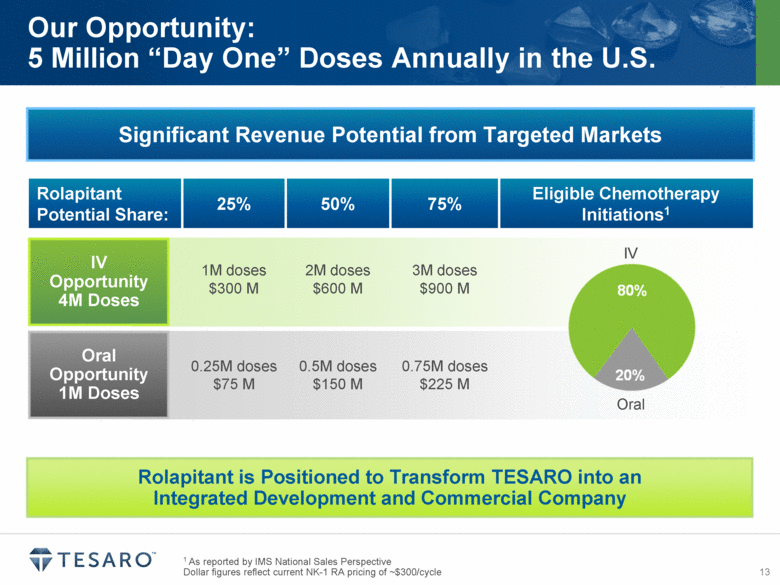
1 As reported by IMS National Sales Perspective Dollar figures reflect current NK-1 RA pricing of ~$300/cycle Our Opportunity: 5 Million “Day One” Doses Annually in the U.S. Significant Revenue Potential from Targeted Markets Rolapitant Potential Share: 25% 50% 75% Eligible Chemotherapy Initiations1 1M doses $300 M 2M doses $600 M 3M doses $900 M 0.25M doses $75 M 0.5M doses $150 M 0.75M doses $225 M Rolapitant is Positioned to Transform TESARO into an Integrated Development and Commercial Company IV Opportunity 4M Doses Oral Opportunity 1M Doses IV Oral |
|
|
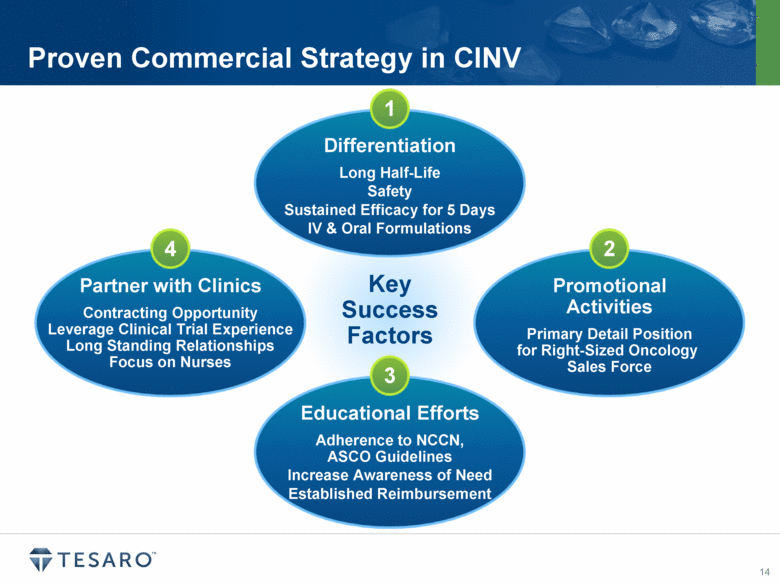
Proven Commercial Strategy in CINV Key Success Factors 1 3 2 4 |
|
|
A Potentially First and Best-in-Class PARP Inhibitor in Phase 3 for Ovarian and Breast Cancers |
|
|
Phase 3 dose of 300mg daily was well characterized in Phase 1 Compelling response rates and durations of response in highly pre-treated populations Clinical and genetic enrichment allow for targeted development Two Phase 3 studies ongoing in patients with ovarian and breast cancer Partnerships in place with BIG and ENGOT to accelerate enrollment Evaluating potential to combine with chemotherapy via partnership with SARC1 for Ewing’s sarcoma 1Sarcoma Alliance for Research through Collaboration Niraparib: Overview |
|
|
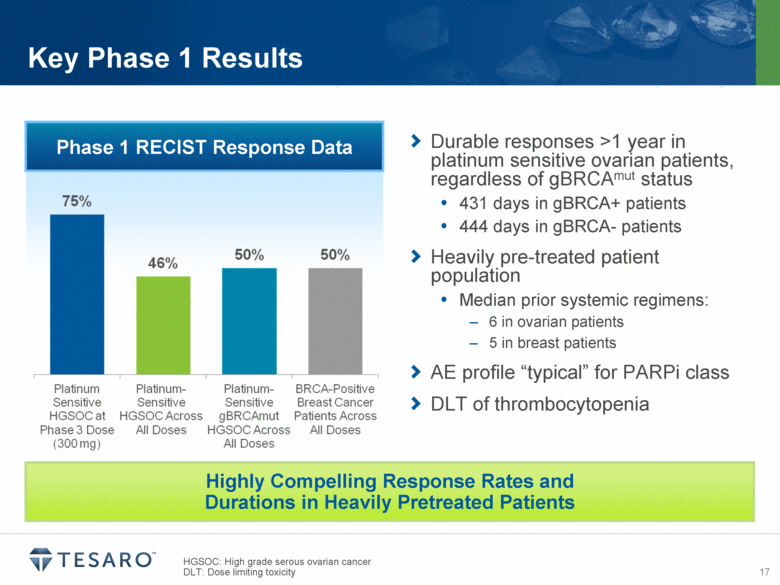
HGSOC: High grade serous ovarian cancer DLT: Dose limiting toxicity Key Phase 1 Results Phase 1 RECIST Response Data Durable responses >1 year in platinum sensitive ovarian patients, regardless of gBRCAmut status 431 days in gBRCA+ patients 444 days in gBRCA- patients Heavily pre-treated patient population Median prior systemic regimens: 6 in ovarian patients 5 in breast patients AE profile “typical” for PARPi class DLT of thrombocytopenia Highly Compelling Response Rates and Durations in Heavily Pretreated Patients |
|
|
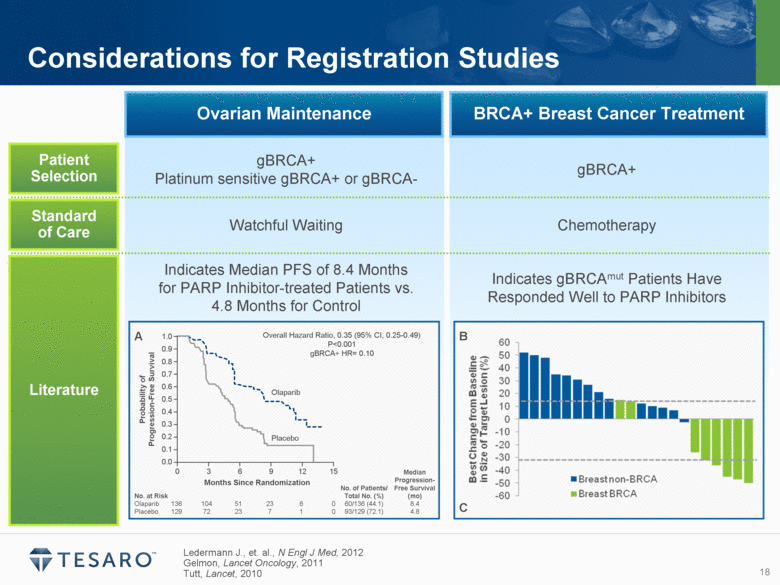
Patient Selection Standard of Care Literature Ledermann J., et. al., N Engl J Med, 2012 Gelmon, Lancet Oncology, 2011 Tutt, Lancet, 2010 Considerations for Registration Studies Ovarian Maintenance BRCA+ Breast Cancer Treatment gBRCA+ Platinum sensitive gBRCA+ or gBRCA- gBRCA+ Watchful Waiting Chemotherapy Indicates Median PFS of 8.4 Months for PARP Inhibitor-treated Patients vs. 4.8 Months for Control Indicates gBRCAmut Patients Have Responded Well to PARP Inhibitors A B C 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 0 3 6 9 12 15 Months Since Randomization Probability of Progression-Free Survival Placebo Olaparib Overall Hazard Ratio, 0.35 (95% CI, 0.25-0.49) P<0.001 gBRCA+ HR= 0.10 No. at Risk No. of Patients/ Total No. (%) Median Progression-Free Survival (mo) Olaparib 136 104 51 23 6 0 60/136 (44.1) 8.4 Placebo 129 72 23 7 1 0 93/129 (72.1) 4.8 |
|
|
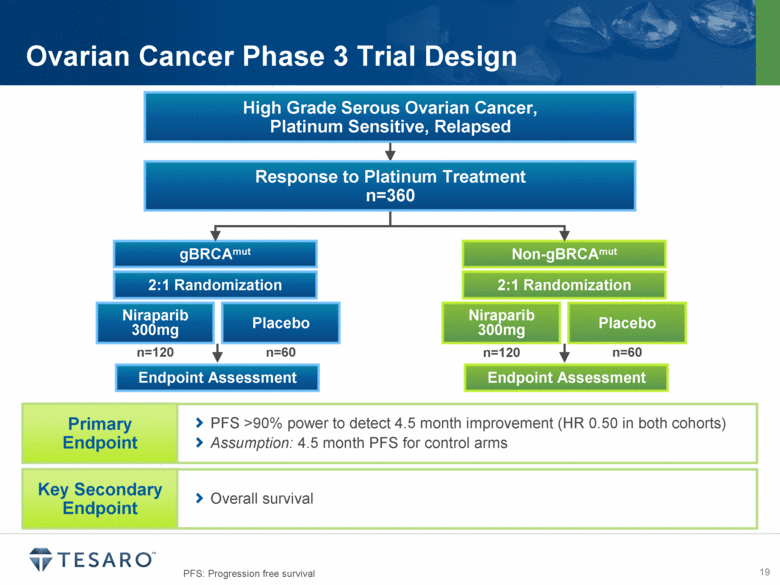
Ovarian Cancer Phase 3 Trial Design gBRCAmut Endpoint Assessment Niraparib 300mg Placebo Non-gBRCAmut Endpoint Assessment Niraparib 300mg Placebo Response to Platinum Treatment n=360 High Grade Serous Ovarian Cancer, Platinum Sensitive, Relapsed 2:1 Randomization 2:1 Randomization n=120 n=60 n=120 n=60 PFS >90% power to detect 4.5 month improvement (HR 0.50 in both cohorts) Assumption: 4.5 month PFS for control arms Primary Endpoint Overall survival Key Secondary Endpoint PFS: Progression free survival |
|
|
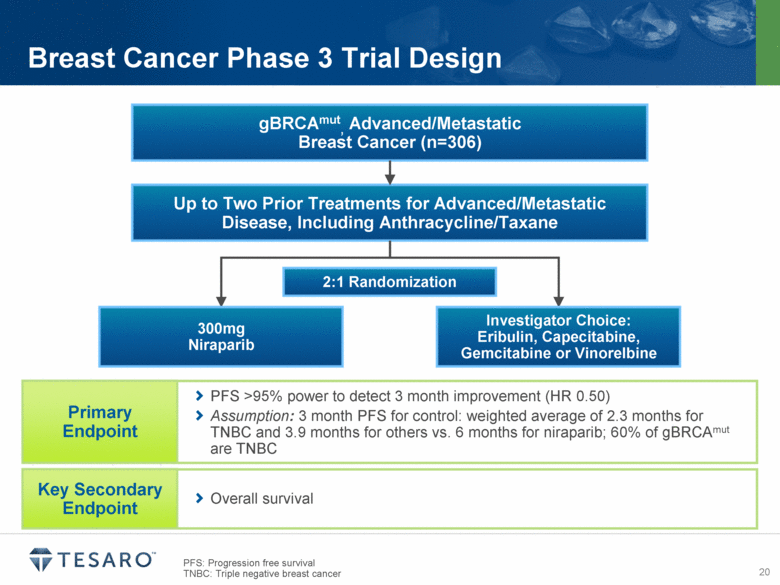
Breast Cancer Phase 3 Trial Design gBRCAmut, Advanced/Metastatic Breast Cancer (n=306) Up to Two Prior Treatments for Advanced/Metastatic Disease, Including Anthracycline/Taxane 2:1 Randomization 300mg Niraparib Investigator Choice: Eribulin, Capecitabine, Gemcitabine or Vinorelbine PFS >95% power to detect 3 month improvement (HR 0.50) Assumption: 3 month PFS for control: weighted average of 2.3 months for TNBC and 3.9 months for others vs. 6 months for niraparib; 60% of gBRCAmut are TNBC Primary Endpoint Overall survival Key Secondary Endpoint PFS: Progression free survival TNBC: Triple negative breast cancer |
|
|
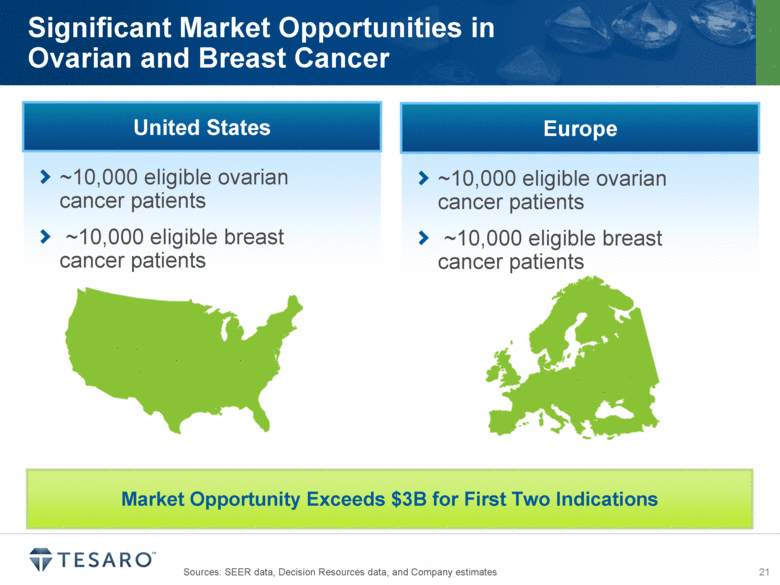
Significant Market Opportunities in Ovarian and Breast Cancer United States ~10,000 eligible ovarian cancer patients ~10,000 eligible breast cancer patients Europe ~10,000 eligible ovarian cancer patients ~10,000 eligible breast cancer patients Market Opportunity Exceeds $3B for First Two Indications Sources: SEER data, Decision Resources data, and Company estimates |
|
|
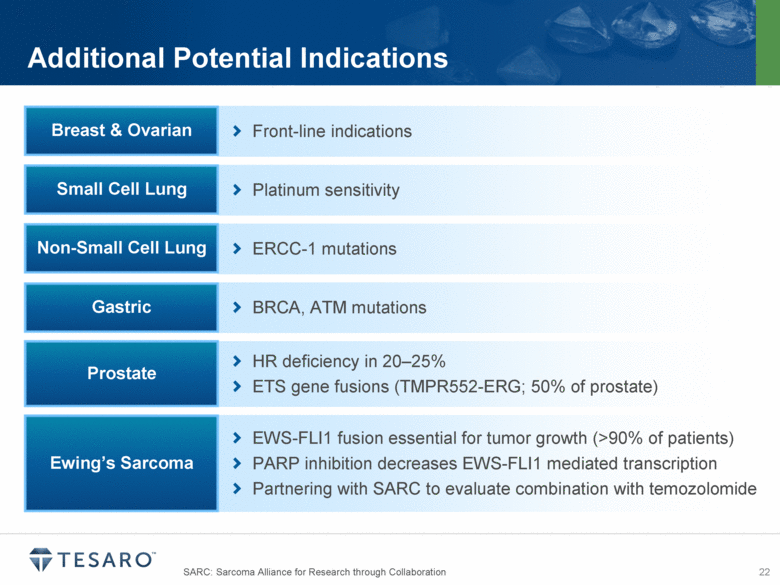
SARC: Sarcoma Alliance for Research through Collaboration Additional Potential Indications Platinum sensitivity Small Cell Lung BRCA, ATM mutations Gastric ERCC-1 mutations Non-Small Cell Lung Front-line indications Breast & Ovarian HR deficiency in 20–25% ETS gene fusions (TMPR552-ERG; 50% of prostate) Prostate EWS-FLI1 fusion essential for tumor growth (>90% of patients) PARP inhibition decreases EWS-FLI1 mediated transcription Partnering with SARC to evaluate combination with temozolomide Ewing’s Sarcoma |
|
|
A Novel and Potential Best-in-Class ALK and TRK Inhibitor; NSCLC and Other Cancers |
|
|
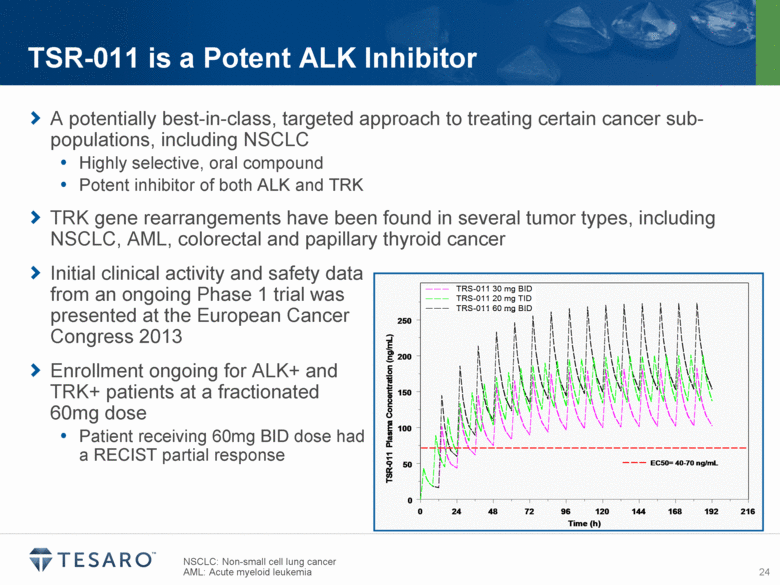
z A potentially best-in-class, targeted approach to treating certain cancer sub-populations, including NSCLC Highly selective, oral compound Potent inhibitor of both ALK and TRK TRK gene rearrangements have been found in several tumor types, including NSCLC, AML, colorectal and papillary thyroid cancer Initial clinical activity and safety data from an ongoing Phase 1 trial was presented at the European Cancer Congress 2013 Enrollment ongoing for ALK+ and TRK+ patients at a fractionated 60mg dose Patient receiving 60mg BID dose had a RECIST partial response NSCLC: Non-small cell lung cancer AML: Acute myeloid leukemia TSR-011 is a Potent ALK Inhibitor |
|
|
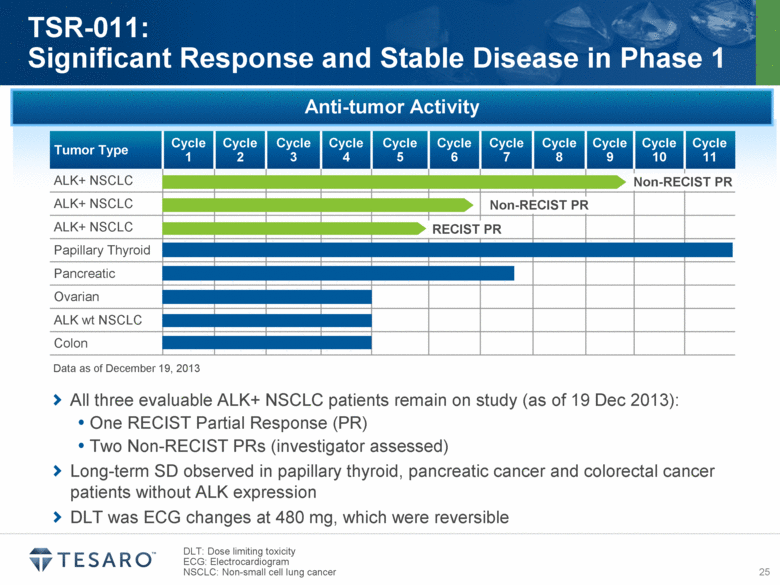
All three evaluable ALK+ NSCLC patients remain on study (as of 19 Dec 2013): One RECIST Partial Response (PR) Two Non-RECIST PRs (investigator assessed) Long-term SD observed in papillary thyroid, pancreatic cancer and colorectal cancer patients without ALK expression DLT was ECG changes at 480 mg, which were reversible DLT: Dose limiting toxicity ECG: Electrocardiogram NSCLC: Non-small cell lung cancer TSR-011: Significant Response and Stable Disease in Phase 1 Anti-tumor Activity Tumor Type Cycle 1 Cycle 2 Cycle 3 Cycle 4 Cycle 5 Cycle 6 Cycle 7 Cycle 8 Cycle 9 Cycle 10 Cycle 11 ALK+ NSCLC ALK+ NSCLC ALK+ NSCLC Papillary Thyroid Pancreatic Ovarian ALK wt NSCLC Colon Non-RECIST PR RECIST PR Non-RECIST PR Data as of December 19, 2013 |
|
|
Example of a Non-RECIST Response to TSR-011 Screening Visit: ALK+ NSCLC, progressed after 7.5 months on crizotinib Extensive tumor involvement of the right lung: lung consolidated with tumor After 4 Cycles of TSR-011: Significant reduction of tumor burden with less consolidation Clinical improvement noted Remains on study as of 19 December after 6 cycles of TSR-011 Continued improvement and significantly reduced use of oxygen |
|
|
WW: Worldwide ALKi: ALK-inhibitor KOL: key opinion leader TESARO is Positioned for Success Cash and equivalents of $156M as of 9/30/2013 Average quarterly cash utilization of $20M to $25M Well Capitalized >30 collective years of commercial, development and regulatory experience in oncology Relationships with KOLs and national oncology networks Experienced Team Promising Pipeline Rolapitant: Differentiated profile; unmet market need that can be addressed via education and adherence to existing guidelines Niraparib: Two Phase 3 trials; Phase 1 data demonstrated high response rates among heavily pre-treated patients TSR-011: Compelling activity in ALKi-resistant patients Significant Revenue Opportunities Rolapitant: $1.5B U.S. CINV market Niraparib: >$3B WW initial indications |
|
|
Complete final Phase 3 trial of rolapitant Advance rolapitant IV clinical program Present data at ASCO, MASCC, and ESMO Present full rolapitant pivotal data set at a 2014 medical meeting Submit NDA for rolapitant to the U.S. FDA in mid-2014 Continue to enroll niraparib Phase 3 NOVA and BRAVO trials Expand the niraparib clinical development program and initiate the trial in Ewing’s sarcoma Identify optimal dosing schedule for TSR-011 and continue to evaluate activity in ALK+ and TRK+ patients ASCO: American Society of Clinical Oncology MASCC: Multinational Association of Supportive Care in Cancer ESMO: European Society for Medical Oncology Anticipated Near-term Milestones |
|
|
Corporate Presentation January 2014 |