Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GALECTIN THERAPEUTICS INC | d656392d8k.htm |
| EX-99.1 - EX-99.1 - GALECTIN THERAPEUTICS INC | d656392dex991.htm |
Exhibit 99.2

Happy New Year!
2013 was a year of significant activity and noteworthy progress for Galectin Therapeutics. Over the past twelve months, we have achieved a number of major development milestones, including the submission of an investigational new drug (IND) application, the receipt of a Fast Track designation from the FDA, and the first patient dosed in a Phase 1 clinical trial.
With the addition of two new executives to our leadership team and renewed financial investment in the Company, we have continued to diligently and efficiently pursue development pathways to clinical enhancement and commercialization for our lead compounds.
We believe strongly that galectin inhibitors hold immense promise for the treatment of fibrosis and inflammation. Fatty liver disease affects as many as 15 million Americans, and there are no currently approved pharmaceutical therapies. Galectin Therapeutics perceives a substantial unmet medical need and the opportunity to provide a treatment to the millions suffering from this condition.
In this new year, I am excited for what is on the horizon for Galectin. Most notably, we expect clinical data from the first cohort of our Phase 1 clinical trial of GR-MD-02 in fatty liver disease early this year. Also in the first quarter, we will launch our updated website and hope that you take the time to visit to learn what is new with the Company.
2014 promises to be a busy year for us. We are at a key stage in the development of our novel compounds and remain optimistic in the advancement of our clinical programs. I am confident in our team and strategy for advancing our drug candidates in 2014 and beyond. Thank you for your continued support and interest in Galectin Therapeutics.
| Sincerely,
|
|
|
| Peter G. Traber, M.D. |
| Chief Executive Officer, President and Chief Medical Officer |
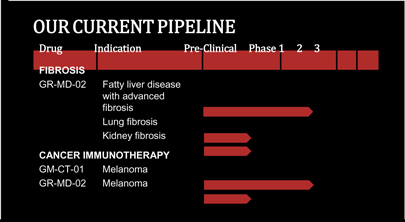
2013 DEVELOPMENT & REGULATORY MILESTONES
A key priority in 2013 was the GR-MD-02 development program. Preclinical data demonstrate that GR-MD-02 has a powerful therapeutic effect on liver fibrosis. Our milestones in 2013 included:
| • | January – Submitted IND application to the FDA for GR-MD-02 in fatty liver disease |
| • | March – Received notification from the FDA to proceed with a Phase 1 clinical trial for GR-MD-02 in fatty liver disease with advanced fibrosis |
| • | April – Investigators and sites announced for first- in-man Phase 1 clinical trial for GR-MD-02 in fatty liver disease |
| • | July – First patient enrolled in the first-in-man Phase 1 clinical trial for GR-MD-02 in fatty liver disease, currently taking place at six trial sites across the U.S. |
| • | August – Received Fast Track designation from the FDA for GR-MD-02 in fatty liver disease |
| • | September – Received US patent for GR-MD-02 in patients with fatty liver disease with or without fibrosis or cirrhosis |
| • | November – Reported that five of the eight patients were enrolled and had been infused in cohort 1 of the clinical trial of GR-MD-02 in fatty liver disease |
Looking ahead, completion of the enrollment of the first cohort will be an important milestone in the development of our proprietary, novel technology. Clinical data from the first cohort should be available early in 2014.
Outside of liver disease, the Company is working with Providence Portland Medical Center in planning for a Phase 1 clinical trial to evaluate the combination of Bristol-Myers Squibb’s Yervoy® (ipilimumab) and the Company’s GR-MD-02 in patients with metastatic melanoma. This trial is based on pre-clinical data obtained in collaboration with Dr. Will Redmond at the center which demonstrated that the combination of immune checkpoint inhibitors like ipilimumab with GR-MD-02 enhances the antitumor effect in syngeneic mouse cancer models.
Development programs for GR-MD-02 in other liver diseases, lung fibrosis and kidney fibrosis will require partnerships to initiate, which we are actively seeking.
4960 Peachtree Industrial Blvd, Ste 240 | Norcross, Georgia 30071 | (678) 620-3186 | galectintherapeutics.com

2013 SCIENTIFIC MILESTONES
The knowledge base for our lead compounds – GR-MD-02 and GM-CT-01 – was meaningfully enhanced in 2013. Our ongoing preclinical studies and newly-initiated Phase 1 trial revealed scientific data that further support our development programs particularly in the areas of fatty liver disease, lung fibrosis and cancer immunotherapy. Key scientific milestones in 2013 included:
| • | January – Preclinical data showed that treatment of diabetic mice with GR-MD-02 was found to reverse diabetic kidney disease including interstitial fibrosis. |
| • | February – Established a collaborative drug discovery program with Dr. Geert-Jan Boons’ laboratory located in the Complex Carbohydrate Research Center at the University of Georgia to focus on the discovery and synthesis of new carbohydrate molecules that inhibit galectin proteins. |
| • | February – Presented data at a Keystone Conference in a collaboration with Dr. William Redmond of the Robert W. Franz Cancer Research Center of the Providence Portland Medical Center. These data in part showed that inhibition of galectin-3 with GR-MD-02 in animals enhances CD8 T cell proliferation and activation in response to antigen. |
| • | April – Presented data, along with the Icahn School of Medicine at Mount Sinai, at the prestigious International Liver Congress 2013. Data showed that GR-MD-02 and GM-CT-01 were found to reverse cirrhosis, the most advanced stage of liver fibrosis, in experimental animals given toxin-induced cirrhosis. |
| • | June – Preclinical data found that combining GR-MD-02 with monoclonal antibodies that function as immune checkpoint blockade inhibitors enhance shrinkage of prostate and breast cancer tumors. |
| • | August – Preclinical data found that treatment with GR- MD-02 showed a robust effect in reducing lung fibrosis, with somewhat lesser effect of GM-CT-01. |
| • | October – Preclinical data published in PLOS ONE, demonstrated that GR-MD-02 and GM-CT-01 led to significantly reduced fibrosis, reversal of cirrhosis and a significant reduction in portal hypertension. |
| • | December – A second study published in PLOS ONE, revealed that treatment with GR-MD-02 significantly improved NASH activity and reduced fibrosis. Similar effects were seen with GM-CT-01 but with approximately four-fold lower potency than GR-MD-02. |
2013 CORPORATE & FINANCIAL MILESTONES
2013 was an active year throughout our business operations, as we continued to build a solid foundation for growing the Company. From a financial perspective, the company continued to gain confidence of investors as demonstrated by several key investments. Our 2013 corporate and financial milestones included:
| • | January – Galectin welcomed industry veteran Rex Horton as Executive Director of Regulatory Affairs and Quality Assurance. Mr. Horton is an experienced professional with 20 years of management and leadership experience in global regulatory affairs matters including drugs, biologics and vaccines. |
| • | July – Galectin welcomed Jack W. Callicutt to the position of Chief Financial Officer. Mr. Callicutt plays a key role in shaping corporate strategy as we advance key product assets in the clinic. Mr. Callicutt brings over 24 years of relevant experience including serving as CFO of publicly traded companies and raising capital. |
| • | August – All of the 710,834 common stock purchase warrants scheduled to expire on August 25, 2013 were exercised for total cash proceeds of $3 million. These proceeds added to the $3 million private placement of 500,000 shares of unregistered common stock. |
| • | October/November – 10X Fund exercised 500,000 common stock purchase warrants of Galectin Therapeutics at $3 per share for total cash proceeds of $1,500,000. Following this transaction, the Company’s total cash position was approximately $10.29 million and the total outstanding common shares were approximately 17.97 million. |
4960 Peachtree Industrial Blvd, Ste 240 | Norcross, Georgia 30071 | (678) 620-3186 | galectintherapeutics.com
Stock widget
Investing forum
GALECTIN THERAPEUTICS INC Reports
- 03/12/2010 - 10-K
- 03/15/2011 - 10-K
- 03/18/2015 - 10-K
- 03/15/2016 - 10-K
- 03/28/2017 - 10-K
- 03/29/2018 - 10-K
- 03/16/2020 - 10-K
- 03/31/2021 - 10-K
- 11/13/2009 - 10-Q
- 05/12/2010 - 10-Q
- 08/13/2010 - 10-Q
- 11/12/2010 - 10-Q
- 05/13/2011 - 10-Q
- 08/12/2011 - 10-Q
- 11/10/2011 - 10-Q
- 05/11/2012 - 10-Q
- 08/10/2012 - 10-Q
- 11/09/2012 - 10-Q
- 05/10/2013 - 10-Q
- 11/12/2013 - 10-Q
- 05/13/2014 - 10-Q
- 08/07/2014 - 10-Q
- 11/10/2014 - 10-Q
- 05/11/2015 - 10-Q
- 08/10/2015 - 10-Q
- 11/09/2015 - 10-Q
- 05/10/2016 - 10-Q
- 08/09/2016 - 10-Q
- 11/08/2016 - 10-Q
- 05/15/2017 - 10-Q
- 08/14/2017 - 10-Q
- 11/07/2017 - 10-Q
- 05/11/2018 - 10-Q
- 08/14/2018 - 10-Q
- 11/13/2018 - 10-Q
- 05/11/2020 - 10-Q
- 08/10/2020 - 10-Q
- 11/09/2020 - 10-Q
- 05/17/2021 - 10-Q
- 08/16/2021 - 10-Q

