Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - CorMedix Inc. | crmd_8k.htm |
EXHIBIT 99.1

DRIVES EVERYTHING WE DO
Company Overview
NYSE MKT: CRMD
December 2013
© 2013 CorMedix

2
Forward Looking Statements
This presentation contains certain statements that constitute forward-looking statements
within the meaning of the federal securities laws. Statements that are not historical
facts, including statements about our beliefs and expectations, are forward-looking
statements. These statements are not guarantees of future performance and involve
risks, uncertainties and assumptions that are difficult to predict. The forward looking
statements in this presentation include statements about our business, including
commercialization plans and potential markets for our products and product candidates,
clinical trials, potential indications for our product candidates, development timelines,
regulatory timelines and future events that have not yet occurred. Pharmaceutical and
medical device development inherently involves significant risks and uncertainties,
including the risks outlined in “Risk Factors” in our Annual Report on Form 10-K filed
with the Securities and Exchange Commission on March 27, 2013 and in “Risk Factors”
in our Quarterly Reports on Form 10-Q filed with the Securities and Exchange
Commission on May 15 and August 14, 2013. Our actual results may differ materially
from our expectations due to these risks and uncertainties, including, but not limited to,
our dependence on the success of our lead product candidate, and factors relating to
commercialization and regulatory approval thereof, ability to raise sufficient capital,
retaining our stock’s listing on the NYSE MKT, research and development activities,
intellectual property protection, competition, industry environment, and other matters.
Any forward-looking statements included in this presentation are based on information
available to us on the date of this presentation. We undertake no obligation to update or
revise any forward-looking statement, whether as a result of new information, future
events or otherwise.
within the meaning of the federal securities laws. Statements that are not historical
facts, including statements about our beliefs and expectations, are forward-looking
statements. These statements are not guarantees of future performance and involve
risks, uncertainties and assumptions that are difficult to predict. The forward looking
statements in this presentation include statements about our business, including
commercialization plans and potential markets for our products and product candidates,
clinical trials, potential indications for our product candidates, development timelines,
regulatory timelines and future events that have not yet occurred. Pharmaceutical and
medical device development inherently involves significant risks and uncertainties,
including the risks outlined in “Risk Factors” in our Annual Report on Form 10-K filed
with the Securities and Exchange Commission on March 27, 2013 and in “Risk Factors”
in our Quarterly Reports on Form 10-Q filed with the Securities and Exchange
Commission on May 15 and August 14, 2013. Our actual results may differ materially
from our expectations due to these risks and uncertainties, including, but not limited to,
our dependence on the success of our lead product candidate, and factors relating to
commercialization and regulatory approval thereof, ability to raise sufficient capital,
retaining our stock’s listing on the NYSE MKT, research and development activities,
intellectual property protection, competition, industry environment, and other matters.
Any forward-looking statements included in this presentation are based on information
available to us on the date of this presentation. We undertake no obligation to update or
revise any forward-looking statement, whether as a result of new information, future
events or otherwise.
© 2013 CorMedix

© 2013 CorMedix
Investment Highlights
CorMedix (NYSE MKT: CRMD) develops and markets
products to treat renal, cardiac and infectious diseases
products to treat renal, cardiac and infectious diseases
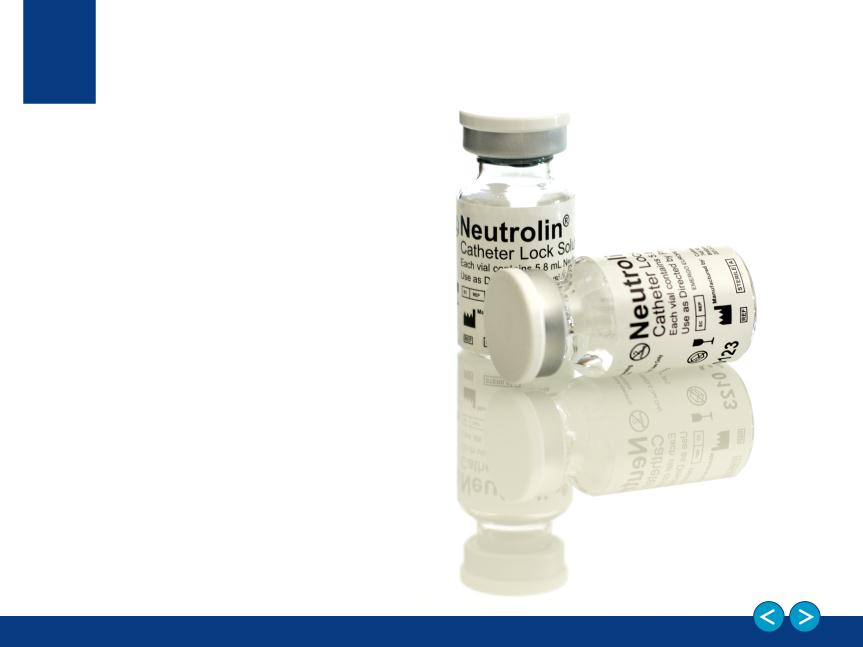
Lead product Neutrolin®
Class III CE Mark approved (drug-device) in July 2013
Catheter-related bloodstream infections (CRBSI)
cost approx. $25,000-40,000/hospitalization, or $6+ B/year, in the US
Clear European commercialization strategy
specialized and highly experienced EU sales force
Using a catheter lock solution (CLS) such as Neutrolin can reduce
occurrence of CRBSI by as much as 60-90 percent
occurrence of CRBSI by as much as 60-90 percent
$500M worldwide market potential
Solid Global IP portfolio
17 issued U.S. patents
4
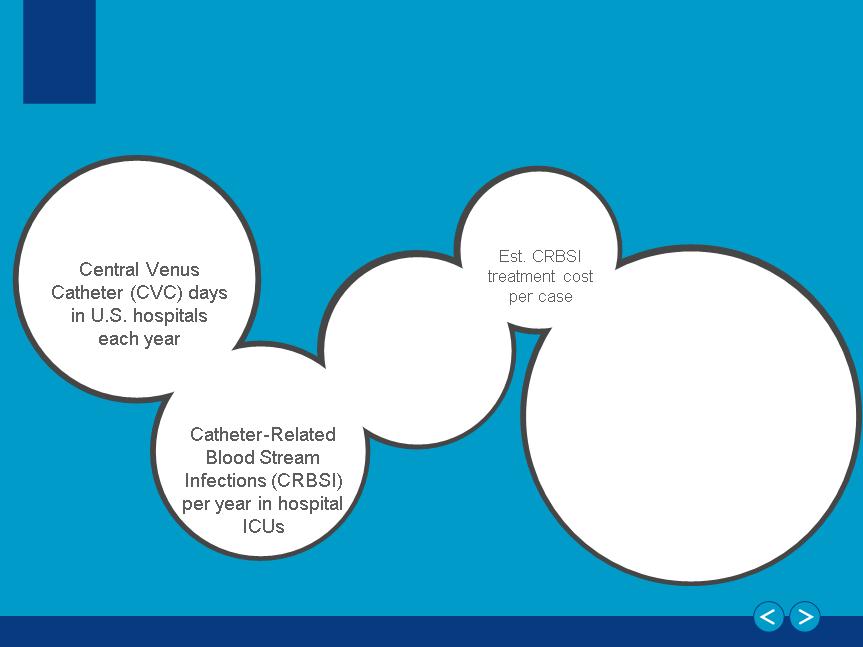
Catheter Safety and Efficacy:
15 Million
80,000
$25,000-
40,000
40,000
$6+ Billion
Total CRBSI cost to the
U.S. healthcare system
U.S. healthcare system
per year
By the Numbers
250,000
Estimated CRBSI
across all
across all
hospitals
© 2013 CorMedix
Sources: U.S. Centers for Disease Control and Prevention. Trish Perl, M.D., professor, medicine and pathology, Johns Hopkins School
of Public Health, Baltimore; Eyal Zimlichman, M.D., research associate, The Center for Patient Safety, Brigham and Women's Hospital,
Boston; Sept. 2, 2013, JAMA Internal Medicine, online
of Public Health, Baltimore; Eyal Zimlichman, M.D., research associate, The Center for Patient Safety, Brigham and Women's Hospital,
Boston; Sept. 2, 2013, JAMA Internal Medicine, online

What could save the US Healthcare system
Billions per year?
© 2013 CorMedix

6
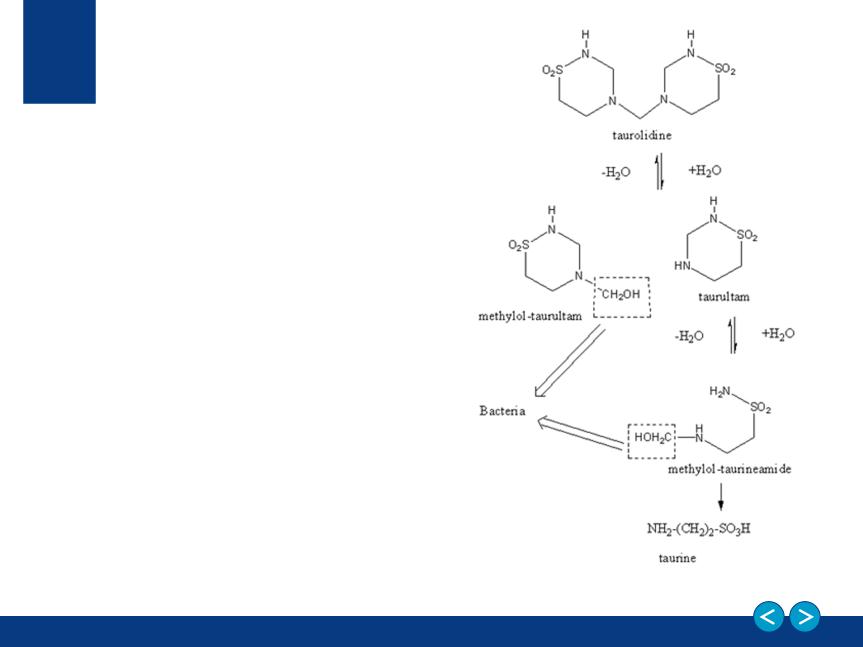
Neutrolin®
A Catheter Lock Solution (CLS)
that fills the catheter to:
that fills the catheter to:
• Significantly decrease CRBSI
• Decrease catheter blood clotting
episodes
episodes
Neutrolin® contains:
• Taurolidine, an anti-infective
• Citrate, a buffering compound
• Heparin, an anticoagulant
© 2013 CorMedix
7
Neutrolin®
Mechanism of Action
Mechanism of Action
Taurolidine: a taurine amino acid
derivative
derivative
• Shows broad antibacterial action
against gram-positive and gram-
negative bacteria, mycobacteria and
some clinically relevant fungi
against gram-positive and gram-
negative bacteria, mycobacteria and
some clinically relevant fungi
• Shows a higher affinity than
mannose (the natural substrate) for
bacterial fimbriae protein responsible
for bacterial adhesion to human cells
mannose (the natural substrate) for
bacterial fimbriae protein responsible
for bacterial adhesion to human cells
© 2013 CorMedix
Source: Caruso F, Darnowski JW, Opazo C, Goldberg A, Kishore N, et al. (2010) Taurolidine Antiadhesive Properties on Interaction with E. coli; Its
Transformation in Biological Environment and Interaction with Bacteria Cell Wall. PLoS ONE 5(1): e8927. doi:10.1371/journal.pone.0008927
Transformation in Biological Environment and Interaction with Bacteria Cell Wall. PLoS ONE 5(1): e8927. doi:10.1371/journal.pone.0008927

8
Why is Antimicrobial Resistance a Problem?
© 2013 CorMedix
• Antibiotic/antimicrobial resistant infections cause:
– prolonged illness
– greater risk of death
– higher treatment costs
• A high percentage of hospital-acquired infections are
caused by highly resistant microorganisms
caused by highly resistant microorganisms
– Dialysis patients with catheters are susceptible to biofilm, which
nurtures and protects bacterial and fungal cell growth
nurtures and protects bacterial and fungal cell growth
– Neutrolin® controls bacteria growth in biofilm
Source: World Health Organization
9
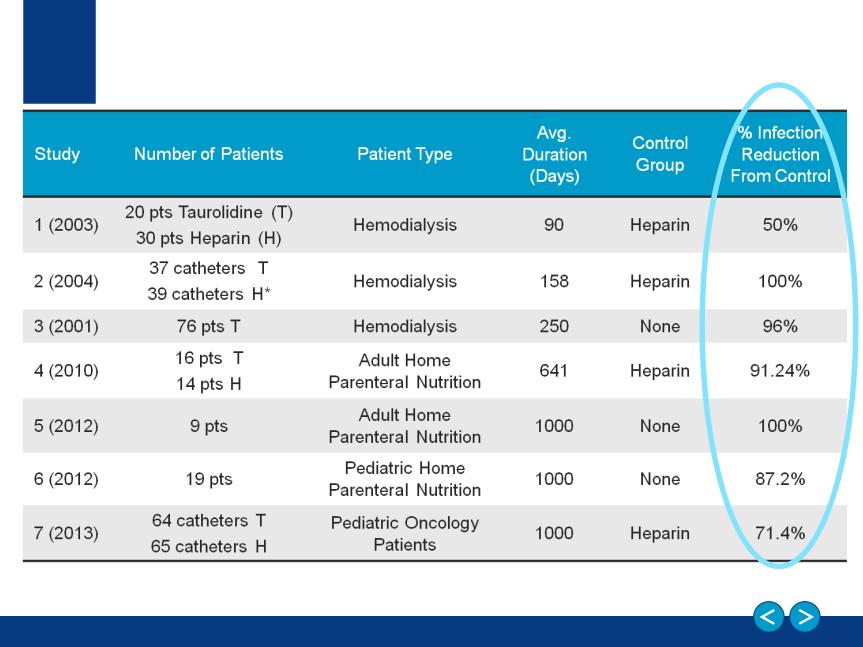
Taurolidine-Based Catheter Lock Solution:
Demonstrated Effectiveness
Demonstrated Effectiveness
*From 58 patients
1. Allon M Clin Infect Dis (2003) 36 (12):1539-44, 2. Betjes Nephrol Dial Transplant (2004) 19:1546-1551, 3. Sodemann K et al Poster: ASN 2001, 4. Bisseling, T. M., M. C. Willems, et al. (2010)
Clin Nutr 29(4): 464-8 , 5.Al-Amin, A. H., J. Sarveswaran, et al. (2013). J Vasc Access 0(0): 0., 6.Chu, H. P., J. Brind, et al. (2012). J Pediatr Gastroenterol Nutr 55(4): 403-7, 7Handrup, M. M.,
J. K. Moller, et al. (2013). Pediatr Blood Cancer 60(8): 1292-8
Clin Nutr 29(4): 464-8 , 5.Al-Amin, A. H., J. Sarveswaran, et al. (2013). J Vasc Access 0(0): 0., 6.Chu, H. P., J. Brind, et al. (2012). J Pediatr Gastroenterol Nutr 55(4): 403-7, 7Handrup, M. M.,
J. K. Moller, et al. (2013). Pediatr Blood Cancer 60(8): 1292-8
© 2013 CorMedix
Successful EU Launch for Hemodialysis
Label Expansion in EU
Rollout to Other Key Countries
Neutrolin Growth Strategy
© 2013 CorMedix
1
2
3
Entry into US Market for Hemodialysis
4
Initial Neutrolin Indication:
Hemodialysis
Possible expanded Indications:
Oncology, ICU, Parenteral Nutrition, Peritoneal Dialysis

11
Strategy:
Successful EU Launch for Hemodialysis
Successful EU Launch for Hemodialysis
• Successful launch in Germany and Austria
– Experienced sales team
– Increase healthcare provider awareness
– Sales being booked: not yet shipped
• Negotiate partner arrangements
• Position Neutrolin® strategically
– Pricing
– Understand payer reimbursement policies
© 2013 CorMedix

13
Strategy:
Rollout to Other Key Countries
Rollout to Other Key Countries
• Identify best sales partners in
key countries
key countries
• Complete agreements
• Monitor product positioning
and coordinate manufacturing
capacity
and coordinate manufacturing
capacity
© 2013 CorMedix
14
Strategy:
Entry Into US Market
Entry Into US Market
• U.S. FDA discussions (pre-IND meeting, timelines)
– FDA clinical trial requirements
• Duration
• Clinical trial execution decision
• Strategic partner
© 2013 CorMedix
15
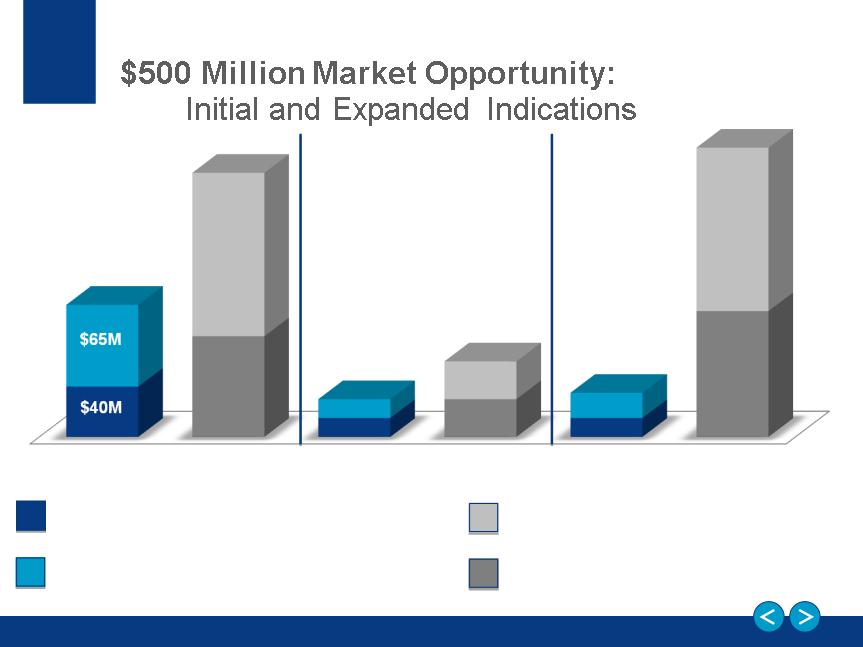
Neutrolin Growth Strategy
Phase I: Europe for Hemodialysis and label expansions
Phase II: Other key countries for Hemodialysis
Phase III: Hemodialysis in the U.S.
© 2013 CorMedix
16
$130M
© 2013 CorMedix
Hemodialysis - Defined Market
Label Expansions - Defined Market
(oncology, ICU, total parenteral nutrition and
peritoneal dialysis)
(oncology, ICU, total parenteral nutrition and
peritoneal dialysis)
$130M
$80M
$15M
$15M
$30M
$30M
$20M
$15M
$100M
$130M
Hemodialysis - Upside Potential
Label Expansions - Upside Potential
(oncology, ICU, total parenteral nutrition and
peritoneal dialysis)
(oncology, ICU, total parenteral nutrition and
peritoneal dialysis)
European Union
Rest of World (Ex. US)
United States

17
IP Status Update
© 2013 CorMedix
• Global Patent Portfolio - 17 issued
patents provide protection through 2019-
2025
patents provide protection through 2019-
2025
– Freedom to Operate in Europe
– Proprietary
• Planned additional filings
18
Financial Information
© 2013 CorMedix
• Total Pro Forma cash of
approximately $3.3 million
as of September 30, 2013
approximately $3.3 million
as of September 30, 2013
• Total Pro Forma debt of
approximately $0.3 million
as of September 30, 2013
approximately $0.3 million
as of September 30, 2013
• Capitalized to fund
operations through the
second quarter of 2014
assuming no sales and/or
strategic partnerships
operations through the
second quarter of 2014
assuming no sales and/or
strategic partnerships
• Approximately 16 million
common shares
outstanding as of
September 30, 2013
common shares
outstanding as of
September 30, 2013

© 2013 CorMedix
Investment Highlights
CorMedix (NYSE MKT: CRMD) develops and markets
products to treat renal, cardiac and infectious diseases
products to treat renal, cardiac and infectious diseases
Lead product Neutrolin®
Class III CE Mark approved (drug-device) in July 2013
Catheter-related bloodstream infections (CRBSI)
cost approx. $25,000-40,000/hospitalization, or $6+ B/year, in the US
Clear European commercialization strategy
specialized and highly experienced EU sales force
Using a catheter lock solution (CLS) such as Neutrolin can reduce
occurrence of CRBSI by as much as 60-90 percent
occurrence of CRBSI by as much as 60-90 percent
$500M worldwide market potential
Solid Global IP portfolio
17 issued U.S. patents
745 Route 202-206
Suite 303
Suite 303
Bridgewater, NJ 08807
908.517.9500 (ph)
908.429.4307 (fax)
NYSE MKT: CRMD
@CorMedix
rmilby@cormedix.com
Thank You
November 2013
November 2013
© 2013 CorMedix
