Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Sorrento Therapeutics, Inc. | d630332d8k.htm |
 November 2013
Next-Generation
Cancer Therapeutics
Sorrento Therapeutics
Exhibit 99.1 |
| 2
This presentation contains "forward-looking statements" as that term is
defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
including statements regarding expectations, beliefs or intentions regarding
our business, technologies and products strategies or prospects. Actual
results may differ from those projected due to a number of risks and
uncertainties, including, but not limited to, the possibility that some or all of
the pending matters and
transactions
being
considered
by
the
Company
may
not
proceed
as
contemplated,
and
by
all other matters specified in Company's filings with the Securities and Exchange
Commission, as well as risks inherent in funding, developing and obtaining
regulatory approvals of new, commercially-viable and competitive
products and product candidates. Sufficiency of the data for approval with
respect to Cynviloq™ will be a review issue after NDA filing.
These statements are made based upon current expectations that are subject
to risk and uncertainty and information available to the Company as of the
date of this presentation. The Company does not undertake to update
forward-looking statements in this presentation to reflect actual
results, changes in assumptions or changes in other factors affecting such
forward-looking information. Assumptions and other information that
could cause results to differ from those set forth in the
forward-looking information can be found in the Company's filings with the
Securities and Exchange Commission, including its most recent periodic report. We
intend that all forward-looking statements be subject to the
safe-harbor provisions of the PSLRA. Safe Harbor Statement
NASDAQ: SRNE |
 3
Management Team
Henry Ji, Ph.D.
President & CEO, Director
Vuong Trieu, Ph.D.
CSO and Director
George Uy
Chief Commercial Officer
David (Zhenwei) Miao, Ph.D.
CTO
Richard Vincent
EVP, CFO, Director
•
Inventor
of
G-MAB
®
Technology
•
President & CEO of Stratagene Genomics
•
VP of CombiMatrix and Stratagene
•
Founder and CEO of IgDraSol
•
Co-inventor of
IP
covering
Abraxane
®
•
Instrumental
in
the
approval
of
Abraxane
®
•
Celgene acquired Abraxis Biosciences for > $3 billion
•
CCO of IgDraSol
•
Directed
the
launches
of
Abraxane
®
,
Xeloda
®
&
Fusilev
®
•
Built commercial infrastructures and organizations in startup companies
•
President and CSO of Concortis Biosystems
•
Co-inventor of IP covering ADC technologies
•
Head of Chemistry at Ambrx
•
$430M sale of Elevation to Sunovion-Dainippon
•
Meritage
Pharma
option
agreement
with
ViroPharma
($90M
upfront
+
milestones)
•
$310M sale of Verus asthma program to AstraZeneca
•
Elan: various acquisitions and divestitures with aggregate values in excess of
$300M |
 4
Investment Highlights
Intractable Pain in Advanced Cancer
•
Ongoing Phase 1/2 study
•
Orphan drug status received
•
Two potential drug products from same API (Resiniferatoxin)
Targeted Drug Delivery (ADC)
•
G-MAB
®
antibody as specific targeting warhead
•
Proprietary toxins as potent tumor killing payload
•
Selective conjugation chemistry for homogenous ADC generation
Late Stage Oncology Drug with Exclusive US and EU Rights
•
Addresses multi-billion dollar paclitaxel market
•
Abbreviated regulatory pathway (“bioequivalence”) for approval
•
Bioequivalence registration trial in 2014 (study direct costs ~ $5M)
•
Product launch in 1H 2016
Therapeutic Antibody Engine
•
Antibody market >$50B in 2012
•
First antibody drug candidate in clinic 1H 2015
Cynviloq™
RTX
G-MAB
®
ADC |
 5
Sorrento’s Next-Generation Cancer Therapeutics
TUMOR
AfDC:
•
•
ADC:
•
•
Cynviloq™
•
•
•
G-MAB
®
•
•
•
G-MAB targets toxin to cancer cell
Programs include VEGFR2, c-Met
High-diversity human Ab library
Lead mAb programs include
PD-L1, PD-1, and CCR2
FTO and no stacking royalties
Bioequivalence (BE)
pathway for approval
Efficacy demonstrated
US and EU rights
G-MAB targets approved chemotherapeutics to the tumor
Effective against heterogeneous
tumors
Next-generation
Abraxane
®
ntibody
A
f
ormulated
D
rug
onjugate
C
ntibody
A
D
rug
onjugate
C |
 6
PHASE 1
PHASE 2
INDICATION
Cynviloq™
Metastatic Breast Cancer
Non-Small Cell Lung Cancer
Bladder Cancer (sNDA)
Ovarian Cancer (sNDA)
1H 2014
1H 2015
505(b)(2) Bioequivalence
}
Pancreatic Cancer (BE* or sNDA)
INDICATION > TARGET
G-MAB
®
ADC
Oncology > PD-L1 Oncology/Inflammation >
CCR2, CXCR3 Oncology > VEGFR2, c-Met, CXCR5, EGFR 1H
2015 2H 2015
2H 2015
RTX
Refractory Pain in Cancer Patients
In progress
1H 2015
Multiple
Strategic
Partnership
Opportunities
Pipeline
Oncology > PD-1 1H 2015
* Abraxane
®
orphan drug status (FDA approval, September 2013) |
 Lead
Product Opportunity Cynviloq™
Registration
Trial
7 |
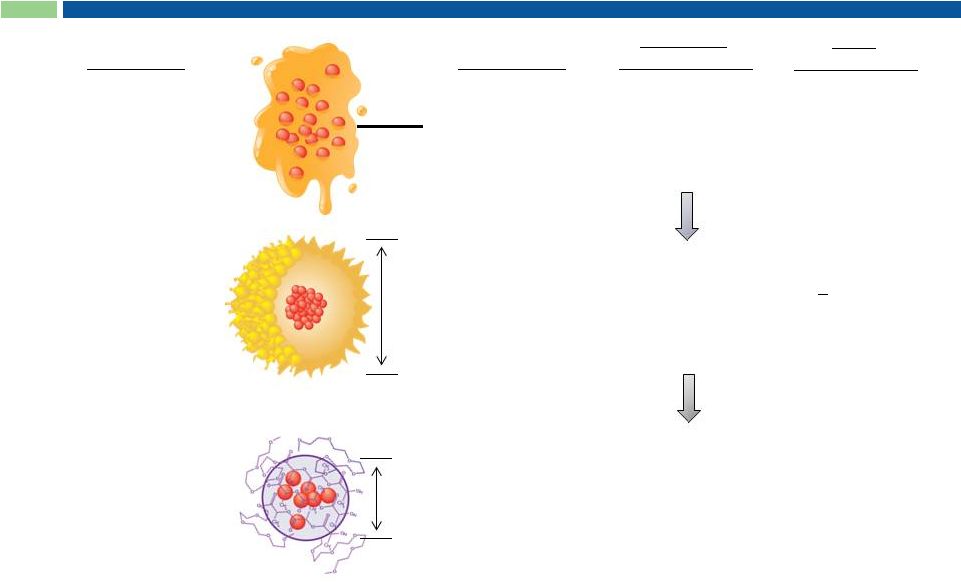 8
Cynviloq is the 3 Generation Paclitaxel Therapy
Abraxane
®
nab-paclitaxel
Mean size
130 nm
Biological
polymer:
Donor-derived human
serum albumin (HSA)
2
nd
260 mg/m
2
Taxol
®
paclitaxel
Cremophor EL
excipient:
Polyoxyethylated
castor oil
Formulation
Generation
1
st
175 mg/m
2
Maximum
Tolerated Dose
Peak
Product Sales
~ $1.6B (WW in 2000)
Est. >$1.7B* (US)
($430M in 2012)
Conversion of
Abraxane sales +
new indications
*Analyst projection ; in MBC + NSCLC + PC
Mean size
~25 nm
Cynviloq™
paclitaxel
polymeric micelle
Chemical
polymer:
Poly-lactide and
polyethylene glycol
diblock copolymer
3
rd
>300 mg/m
2
(up to 435 mg/m
2
)
rd |
| 9
Cynviloq is a High Value Proposition
1.
Exclusive US and EU Rights
2.
Cynviloq efficacy demonstrated in Phase 2 and Phase 3 studies
a.
Approved and marketed under brand name Genexol-PM in S. Korea
3.
FDA concurred
a.
Available data support pursuing 505(b)(2) regulatory pathway
b.
Bioequivalence (BE) study sufficient for approval of indications
in Abraxane
label (MBC and NSCLC)
4.
Large market opportunity
a.
Abraxis (Abraxane) sold to Celgene for > $3B
b.
$1.7B in projected US peak revenues for Abraxane |
 10
Total number of patients across all trials: 1,260
Phase 1:
Trials established higher MTD in US -
Dana Farber Cancer Inst, Russia, & S. Korea (total n=80)
>300 mg/m
2
(q3w) vs. 175
mg/m
2
(Taxol;
weekly)
and
275
mg/m
2
(Abraxane; q3w)
Phase 2:
Completed trials in MBC, NSCLC, PC, OC, BC; in US -
Yale Cancer Center, Russia, S. Korea
(total n=259)
Data suggestive of efficacy comparable to historic data for Abraxane and superior
to historic Taxol data or Standard-of-Care
Possible Phase 3 sNDA programs in these tumor types
Phase 3:
Ongoing trial for MBC in S. Korea (total n=209; Cynviloq n=105)
GPMBC301. An Open-label, Randomized, Parallel, Phase 3 Trial to Evaluate
the Efficacy and Safety of Cynviloq compared
to
Genexol
®
(Paclitaxel with Cremophor EL) in Subjects with Recurrent or Metastatic
Breast Cancer)
Interim analysis suggests ORR superior to Taxol and comparable to historic
Abraxane efficacy Efficacy and safety data
supportive of 505(b)(2) BE submission
PM-Safety:
Completed for MBC and NSCLC (total n=502)
Efficacy and safety data supportive of 505(b)(2) BE submission
Clinical Efficacy & Safety Summary
Phase 2b (IIS):
Chemo-naïve Stage IIIb/IV NSCLC vs Taxol in S. Korea (total n=276;
Cynviloq n=140) 230 mg/m
2
+ cis (q3w) vs. Taxol 175
mg/m
2
+ cis; non-inferiority established
Phase 2 (IIS):
1
st
line treatment of OC vs Taxol in S. Korea (total n=100; Cynviloq n=50)
260 mg/m
2
+ carbo (q3w) vs. Taxol 175
mg/m
2
+ carbo; non-inferiority established |
 11
Drug
HL (h)
T max (h)
AUCinf
(h*ng/mL)
Vz (mL/kg)
Cl (mL/h/kg)
Abraxane
2.99
0.08
61561.33
2103.71
487.32
Cynviloq
2.83
0.08
58151.31
2103.58
515.90
IV bolus at 30 mg/kg; n = 3
Equivalent Pharmacokinetics in Mice
Data on file
Cynviloq
Abraxane |
 12
Abraxane data from: Nurad K. Ibrahim, Phase I and Pharmacokinetic Study of
ABI-007, a Cremophor-free, Protein-stabilized, Nanoparticle Formulation of
Paclitaxel. Clinical Cancer Research 2002;8:1038-44
Data from 2 separate studies
(3 h infusion, 135 mg/m
2
dose, n=3)
Comparable Pharmacokinetics in Human |
 13
Simulated PK Parameters Supportive of BE:
Cynviloq vs. Abraxane
*
Gardner et all, 2008
•
PK BE was calculated as 95% confidence interval (CI)
•
Ratio
T/R
(Cmax
and
AUCinf)
within
80-125%
(FDA
Guidance
for
establishing
BE)
Comparison of mean non-compartmental pharmacokinetic parameters of
Cynviloq (T) and Abraxane (260 mg/m
2
) at 30 min infusion time:
Cmax
(ng/mL)
Ratio
Cmax(T)/Cmax(R)
AUCinf
(ng*h/mL)
Ratio
AUCinf(T)/AUCinf(R)
Cynviloq
(Simulated PK)
19486
99.6%
22198
109.2%
Abraxane
(Actual PK)*
19556
20324 |
 14
Bioequivalence = Efficient Pathway to Market
•
Bioequivalence registration study in breast cancer patients (2014)
-
12 months of duration (including patient recruitment)
-
Direct trial cost ~$5M
Abraxane
®
(50 patients)
Cynviloq™
(50 patients)
Cynviloq™
Abraxane
®
Key Parameters*:
•
•
•
•
Cycle 1
Cycle 2
Dose: 260 mg/m
2
Infusion time: 30 min
Duration: 3 weeks +
crossover for 3 weeks
Endpoints: AUC and
Cmax (90% CI)
*
Based on FDA “Draft Guidance on Paclitaxel” – September 2012
|
 15
Cynviloq Advantages
Cynviloq™
Abraxane
®
Taxol
®
Cynviloq
Advantage
Maximum Tolerated Dose
(mg/m
2
)
>300
260
175
Potential for
higher efficacy
Rapid reconstitution:
no foaming concerns
Convenience for busy
practices and pharmacies
No donor-derived human
serum albumin (HSA)
No viral / prion concerns
Convenient storage
conditions
No requirement for
controlled temp storage
No microbial growth
Chemical polymer
Cremophor-free
Reduced side effects
Dosing
q3w
q3w* &
weekly**
q3w & weekly
Exploits PK advantage
@ higher dose
* = MBC; ** = NSCLC & PC |
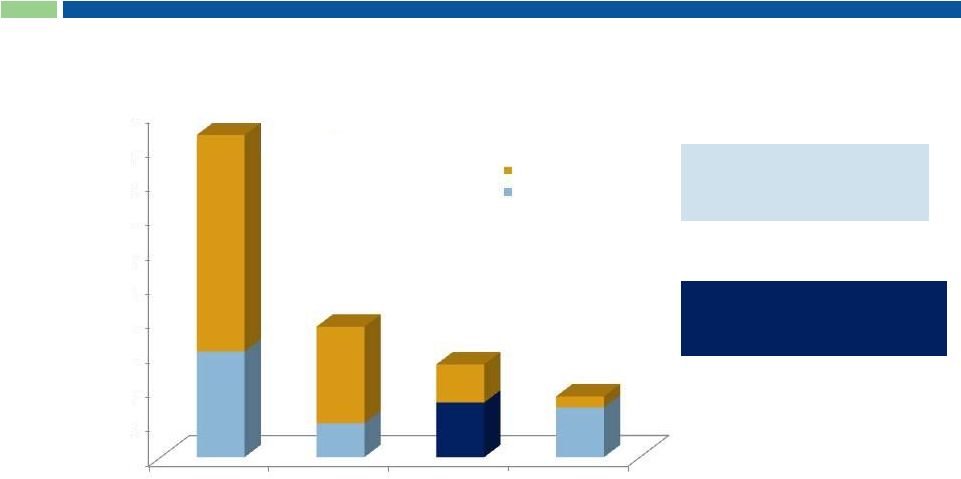 16
~55,000 patients with
paclitaxel
as
1
line
therapy
in MBC, NSCLC & OC
15,903 PC patients eligible for
treatment with Abraxane
®
+
Gemcitabine combination
Cynviloq Market Opportunity
Note: In Pancreatic Cancer, the blue portion
represents # patients treated with gem-based Rx in 2012 #
of Patients Treated in 1 line (US Only; 2012)
Sources: US information, SEER Annual Cancer Review 1975-2006; US Census;
Mattson Jack; UHC and Medicare Claims; IntrinsiQ; Synovate Tandem. WHO
mortality database 2008 http://www.who.int/whosis/whosis/. World Population Prospects. The 2008 Revision. UN Population
Division 2009. http://esa.un.org/unpp/. Roche-Genentech Clinical, Patient Chart
Audits. Total patient numbers represent treatable population. 1
~70,000
patients
treated
with
paclitaxel-based
regimen
in
1
st
line
NSCLC
n = 93,800
MBC
n = 38,000
Pancreatic Cancer
n = 27,000
Ovarian Cancer
n = 17,700
10,000
20,000
30,000
40,000
50,000
60,000
70,000
80,000
90,000
100,000
30,766
9,880
15,903
14,479
Other regimens
PAC+ treated
st
st
st
Line patient estimates from IntrinsiQ 2012 Monthly LOT Diag
Combo. |
 17
Potential to Expand Label Indications –
For
Example:
2
Line
Bladder
Cancer
Cynviloq
Phase 2 (Korea)*
260-300
mg/m²
q3w
n=34
#
Best Supportive Care
Phase 2 (Japan) **/ Phase 3 (EU)***
n=23** / n=108***
Overall Response
Rate (ORR)
21%
-
/ 0%***
Progression Free
Survival
2.7 M
-
/ 1.5 M***
Overall Survival
6.5 M
2.3 M** / 4.3 M***
*
Invest
New
Drugs
(2012)
30:1984–1990
# advanced urothelial carcinoma patients refractory to gemcitabine and
cisplatin **
AUA-
San
Diego
May
4th-8 ;
***
JCO
(2009):
4454-61
Summary:
•
High
unmet
need
-
no
FDA-approved
2
nd
line
drug
•
Demonstrated clinical Overall Response Rate (ORR)
•
Phase
3-ready
for
development
as
2
nd
line
chemotherapy
in
patients
refractory
to
platinum-based therapy
th
nd |
 18
Next Steps for Cynviloq
1.
Bioequivalence (BE) trial: 2014
2.
NDA filing: 2015
3.
Approval: 2016
a.
MBC and NSCLC
b.
Future Abraxane
indications (Pancreatic cancer and Melanoma)
4.
Product launch for MBC and NSCLC: 2016
5.
sNDA planning for label expansion into Bladder and Ovarian cancers
Bioequivalence
Trial
LAUNCH
2016
2016
2014
2015
NDA
Filing
FDA
Approval |
 19
Expanded Clinical Pipeline with Multiple Opportunities
RTX
Intractable
Pain in
Advanced
Cancer
19 |
 20
Resiniferatoxin (RTX):
Non-opiate Drug Candidate for Treating Cancer Pain
“Prickly Painkiller: An experimental plant extract may end intractable pain
with a single injection”*
* Arlene Weintraub; Sci Am. 2013 Jul;309(1):14. |
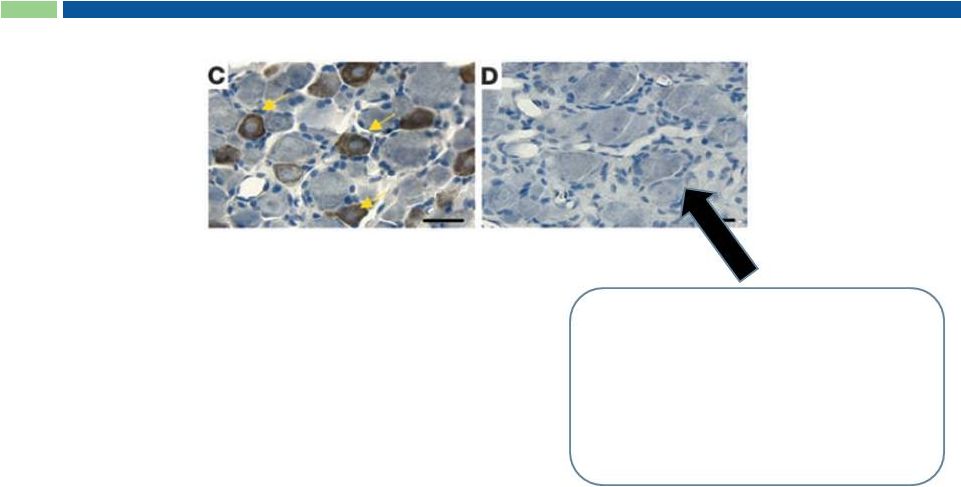 21
RTX Ultra Potent and Selective Toxin
* Adapted from Karai et al. 2004
Pharmacology:
Ultra potent agonist of the TRPV1 receptor
•
< 2 pM TRPV1 receptor binding
Binding in primate ex-vivo spinal cord
•
dorsal root ganglion
resiniferatoxin Ki = 309 pM
•
dorsal horn
resiniferatoxin Ki = 349 pM
RTX induces apoptosis of TRPV1-
expressing afferent nerves.
“ADC”-like killing, but no
antibody-mediated
targeting needed
Cross section of
spinal cord after
treatment* |
 22
Brown et al, Anesthesiology 2005; 103:1052–9
n=18
n=18
n=8
n=5
n=4
Weeks
(p < 0.0001 for all time points)
Single intrathecal injection
Permanent ablation of nerves
Attractive therapeutic effect
All subjects responded
Personality & mood of animals
visibly improved
No opioid-associated side effects
Single Injection Addresses Cancer Pain in Dogs
Veterinary Market: Strategic Partnership Opportunity
|
 23
Next Steps for Dual Pathway of RTX Development
2014
1.
2.
~3 years for clinical
development
Intractable cancer pain clinical trial (intrathecal injection); n~40
patients Phase 1/2 trial for intraganglionic injection
(osteosarcoma); n~15 patients |
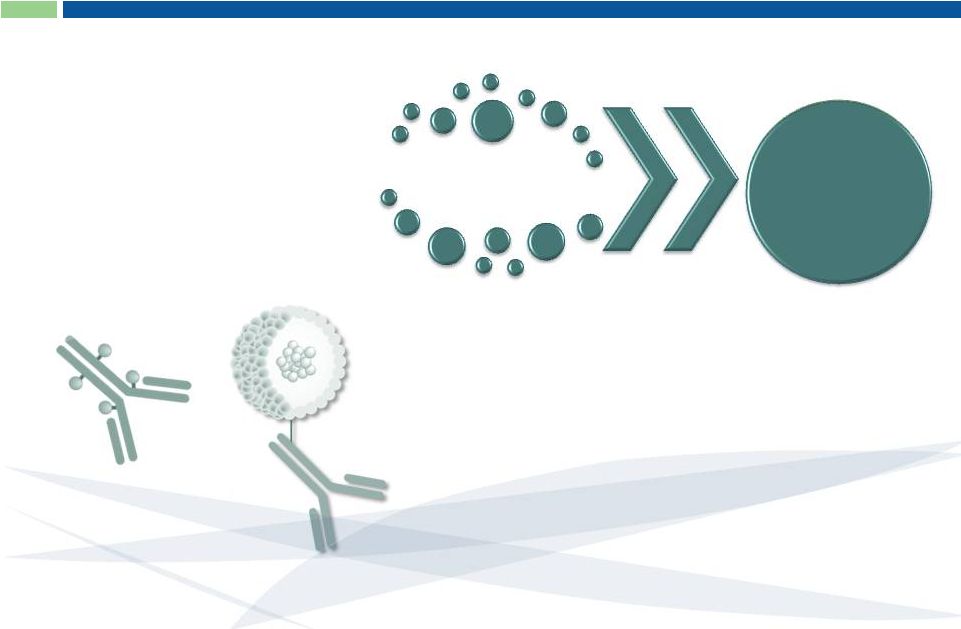 Pre-IND Immunotherapy Programs
Antibodies
+
Proprietary
Toxins
G-MAB
®
ADC
24 |
 25
G-MAB
®
: Library of Therapeutic Antibodies
High Value Oncology Targets:
Immunomodulation:
PD1 and PD-L1
Antibody Drug Conjugates: VEGFR2 and c-Met
Size of Target Antigen
Difficult Targets:
Small Peptides
Most Difficult Targets:
G Protein-Coupled Receptors
(GPCRs)
•
Proprietary technology
-
RNA amplification used for
library generation
-
Freedom-To-Operate
•
Very high library diversity:
2.1 x 10
16
distinct antibodies
•
Fully human antibodies
•
High successful screening hit rate
•
No stacking royalties |
 26
Competitor mAb
Sorrento mAb
Anti-PD-L1 mAbs Exhibit Potent Activity
Immune Modulation*
Tumor Mouse Model**
Day
* mAbs @ 0.05 mg/mL
** xenograft model using H1975 human NSCLC cells; % inhibition relative to control mAb
treatment ***
p<0.05, mean tumor volumes are significantly reduced in STI-A1010 group versus control groups as determined by Mann-Whitney u-test |
 27
Days After Disease Induction
Sorrento mAb
Untreated
Potent Antibody against Difficult GPCR Target*
mAb
Cell Binding
(EC
50
–
nM)
Sorrento
0.17
Competitor
21
0
0.5
1
1.5
2
2.5
3
3.5
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
* Sorrento mAb against C-C Chemokine Receptor 2 (CCR2) ** Experimental
Auto-immune Encephalomyelitis (EAE) = murine model of Multiple Sclerosis |
 28
Antibody Drug Conjugates (ADC)
Key Components:
1.
Target-specific internalizing
antibody
2.
Potent cytotoxic prodrugs
3.
Linker and conjugation
chemistries Drug released in CANCER CELL
|
 29
Proprietary ADC Screening and Optimization Panels
20-panel for discovery of lead ADCs
3 benchmark ADCs for rapid POC studies
20
ADCs
by
rationale
design
with
linker/conjugation
technologies
and
payload
pool
50-panel for generation of ADC candidates
Optimization of payloads
Optimization of linkers and conjugation methods
Expansion to new MOA payloads, if needed
Payload pool
20 panel
50 panel (up to)
Chemistry
•
linkers
•
conjugation |
 30
0
1
2
3
2
purified
K-lock Conjugation Enables Homogeneous ADCs
K-lock
Selective ADC chemistry
Fewer positional isomers
Better control of DAR |
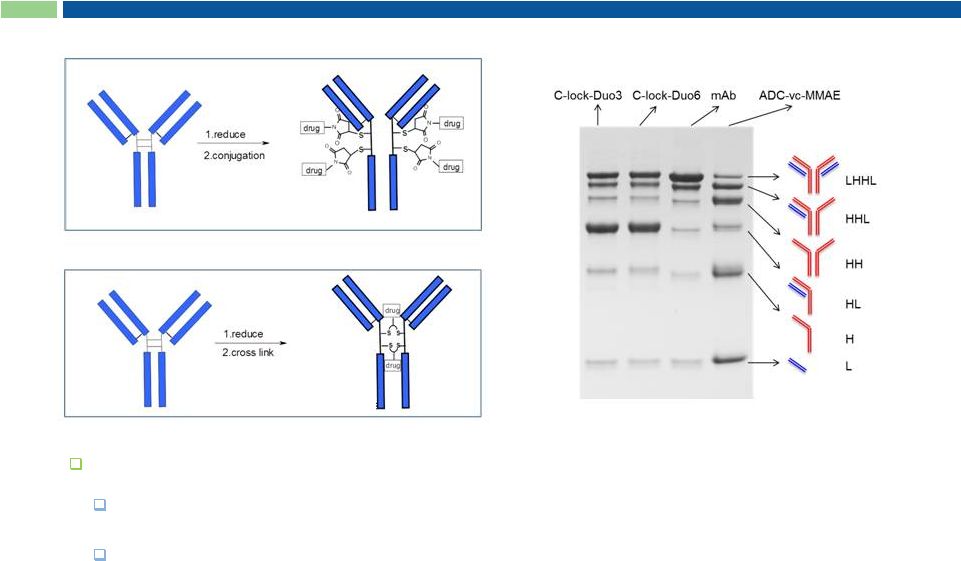 31
Irreversible C-lock-stabilized ADCs
C-lock enhances ADC stability leading to:
Prolonged PK profile
Reduced off-target effects |
 32
Proprietary Toxin Potency 30-fold Higher than DM1*
SKBR3
(Breast Cancer Cell Line)
HCC1954
(Breast Cancer Cell Line)
IC
50
(nM)
0.017
0.019
0.3
(0.24 nM reported)
0.025
0.035
Duo6 (K-lock)
DM1 (NHS)
MMAE (C-lock)
MMAE (maleimide)
cleavable MC-vc-PAB linker
IC
50
(nM)
0.035
0.052
0.25
(0.24 nM reported)
0.091
0.091
DM1 (NHS)
MMAE (C-lock)
MMAE (maleimide)
cleavable MC-vc-PAB linker
Duo3 (K-lock)
Duo6 (K-lock)
Duo3 (K-lock) |
 33
ONCOLOGY
PD-L1
PD-1
VEGFR2-ADC
EGFR
(STI-A100X)
(STI-A110X)
(STI-A020X)
(STI-A0168)
1H 2015
c-Met
(STI-A060X)
ADC
ADC
2H 2015
1H 2015
ONCOLOGY /
INFLAMMATION
(GPCR)
CCR2
CXCR3
CXCR5
(STI-B020X)
(STI-A120X)
(STI-B030X)
2H 2015
ADC
ADC
CCR2
(STI-B020X)
ADC
Multiple
Strategic
Partnership
Opportunities
G-MAB
®
and ADC Pipeline
Her2
ADC
(STI-A160X) |
 34
Positioned to Become Oncology Leader
G-MAB
®
ADC
RTX
Antibodies
+
Proprietary
Toxins
Intractable
Pain in
Advanced
Cancer
Registration
Trial
Cynviloq™
34 |
 35
Small Molecule Oncology Drug, Antibody Library
and ADC Company Valuations
Company
Small Molecule
Oncology Drug
Antibody
Platform
Targeted
Drug Delivery
Mkt Cap*
Puma:
PBYI
Pre-revenue
MBC (Phase 3)
~$1.2B
Clovis:
CLVS
Pre-revenue
NSCLC, MBC (Phase 1)
~$1.6B
MorphoSys:
MOR.DE
Pre-revenue
Antibody
Library
~$1.6B
CAT:
Acquired (2006)
Pre-revenue
Antibody
Library
~$1.4B
Domantis:
Acquired (2007)
Pre-revenue
Antibody
Library
~$450M
Seattle Genetics:
SGEN
Product sales + royalty
ADC
~$5.1B
ImmunoGen:
IMGN
Royalty only
ADC
~$1.3B
Sorrento: SRNE
NSCLC, MBC
(Ph3/Registration Trial)
Antibody
Library
ADC & AfDC
~$180M
* based on publicly-available information (11/14/13)
|
 36
Investment Highlights
Intractable Pain in Advanced Cancer
•
Clinical Phase 1/2 study ongoing at NIH
•
Potential patients for treatment: ~150,000 in the U.S. annually
•
Two potential drug products from same API
Targeted Drug Delivery (ADC)
•
G-MAB
®
antibody as specific targeting warhead
•
Proprietary toxins as potent tumor killing payload
•
Selective conjugation chemistry for homogenous ADC generation
Late Stage Oncology Drug with Exclusive US and EU Rights
•
Addresses multi-billion dollar paclitaxel market
•
Abbreviated regulatory pathway (“bioequivalence”) for approval
•
Bioequivalence registration trial in 2014 (study direct costs ~ $5M)
•
Product launch in 1H 2016
Therapeutic antibody engine
•
Antibody market >$50B in 2012
•
First antibody drug candidate in clinic 1H 2015
Cynviloq™
RTX
G-MAB
®
ADC |
 Sorrento Therapeutics
Next-Generation
Cancer Therapeutics
Contact:
Henry Ji
President and CEO
hji@sorrentotherapeutics.com
(858) 668-6923 |
