Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a13-23869_2ex99d2.htm |
| 8-K - 8-K - Innoviva, Inc. | a13-23869_28k.htm |
Exhibit 99.1
|
|
The efficacy and safety of inhaled fluticasone furoate (FF)/vilanterol (VI) in Asian patients with COPD Jinping Zheng1; Teresita de Guia2; Jie Wang-Jairaj3; Amy H Newlands3; Changzheng Wang4; Chin-chou Wang5; Courtney Crim6; Nanshan Zhong1 11st Affiliated Hospital of Guangzhou Medical University, Guangzhou, China 2Philippine Heart Center, Quezon City, Philippines 3GlaxoSmithKline, Uxbridge, UK 4Xinqiao Hospital, Chongqing, China 5Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan 6GlaxoSmithKline, North Carolina, USA |
|
|
STATEMENT OF INTEREST DISCLOSURE Jinping Zheng has the following statement of interest served as a consultant for a local advisory board, and received lecture fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Takeda and Zambon ACKNOWLEDGEMENTS Patients, investigators and staff at the 33 study centers in mainland China, Taiwan, the Philippines and South Korea Janet Flemming (data management); Eva Gomez and Farshid Hamayoun-Valiani (study management); Rehan Ali and QSI, Bangalore (data analysis and programming) Funded by GlaxoSmithKline (HZC113684; NCT01376245) Editorial support by Gardiner-Caldwell Communications, funded by GlaxoSmithKline |
|
|
Background and objectives Fluticasone furoate/vilanterol (FF/VI) combination Inhaled corticosteroid/long-acting beta2-agonist Once-daily combination treatment for COPD and asthma Delivered via the ELLIPTATM dry powder inhaler 100/25mcg approved in the USA and Canada for COPD Objectives To investigate efficacy and safety of three strengths of FF/VI in Asian COPD patients over 24 weeks 50/25mcg; 100/25mcg; 200/25mcg To evaluate suitability of FF/VI 100/25mcg as the therapeutic strength in Asian COPD patients ELLIPTATM is a trademark of the GlaxoSmithKline group of companies |
|
|
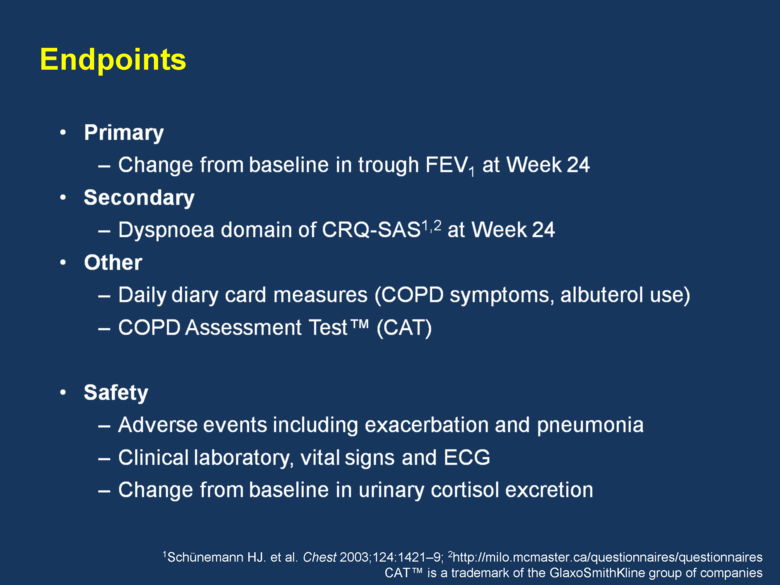
Study design FF/VI 200/25mcg FF/VI 100/25mcg FF/VI 50/25mcg PLACEBO Run-in –2 weeks Follow-up 1 week Study population Male or female Asian outpatients aged >40 years Clinical diagnosis of COPD (ATS/ERS1) Current or prior smoking history of >10 pack-years Post-salbutamol FEV1/FVC ratio of <0.70 Post-salbutamol FEV1 <70% of predicted Modified Medical Research Council Dyspnoea Scale >2 24 weeks R 1Celli BR, MacMee W. Eur Respir J 2004;23:932–46 S F Placebo-controlled, double-blind, randomised, parallel-group, multicentre |
|
|
Endpoints Primary Change from baseline in trough FEV1 at Week 24 Secondary Dyspnoea domain of CRQ-SAS 1,2 AT Week 24 Other Daily diary card measures (COPD symptoms, albuterol use) COPD Assessment Test TM (CAT) Safety Adverse events including exacerbation and pneumonia Clinical laboratory, vital signs and ECG Change from baseline in urinary cortisol excretion 1 Schunemann HJ. et al. Chest 2003; 124: 1421-9; 2 http://milo.mcmaster.ca/questionnaires/questionnaires CAT TM is a trademark of the GlaxoSmithKline group of companies |
|
|
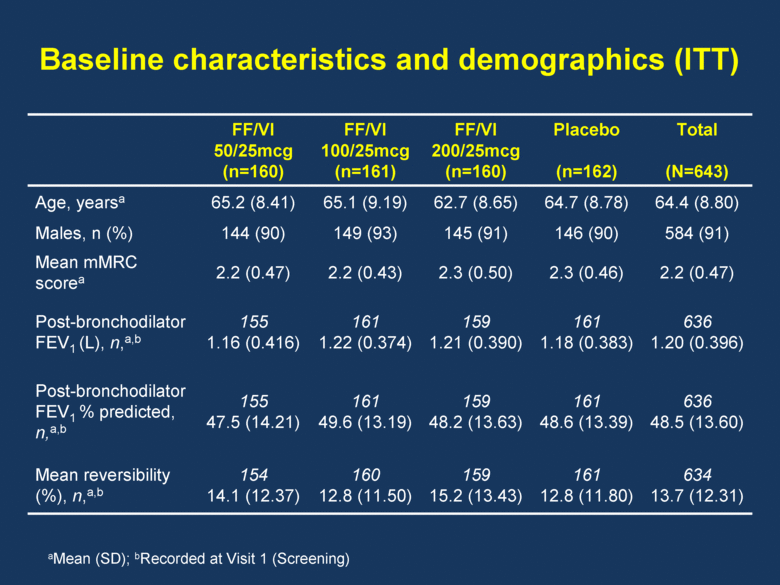
Baseline characteristics and demographics (ITT) FF/VI 50/25mcg (n=160) FF/VI 100/25mcg (n=161) FF/VI 200/25mcg (n=160) Placebo (n=162) Total (N=643) Age, yearsa 65.2 (8.41) 65.1 (9.19) 62.7 (8.65) 64.7 (8.78) 64.4 (8.80) Males, n (%) 144 (90) 149 (93) 145 (91) 146 (90) 584 (91) Mean mMRC scorea 2.2 (0.47) 2.2 (0.43) 2.3 (0.50) 2.3 (0.46) 2.2 (0.47) Post-bronchodilator FEV1 (L), n,a,b 155 1.16 (0.416) 161 1.22 (0.374) 159 1.21 (0.390) 161 1.18 (0.383) 636 1.20 (0.396) Post-bronchodilator FEV1 % predicted, n,a,b 155 47.5 (14.21) 161 49.6 (13.19) 159 48.2 (13.63) 161 48.6 (13.39) 636 48.5 (13.60) Mean reversibility (%), n,a,b 154 14.1 (12.37) 160 12.8 (11.50) 159 15.2 (13.43) 161 12.8 (11.80) 634 13.7 (12.31) aMean (SD); bRecorded at Visit 1 (Screening) |
|
|
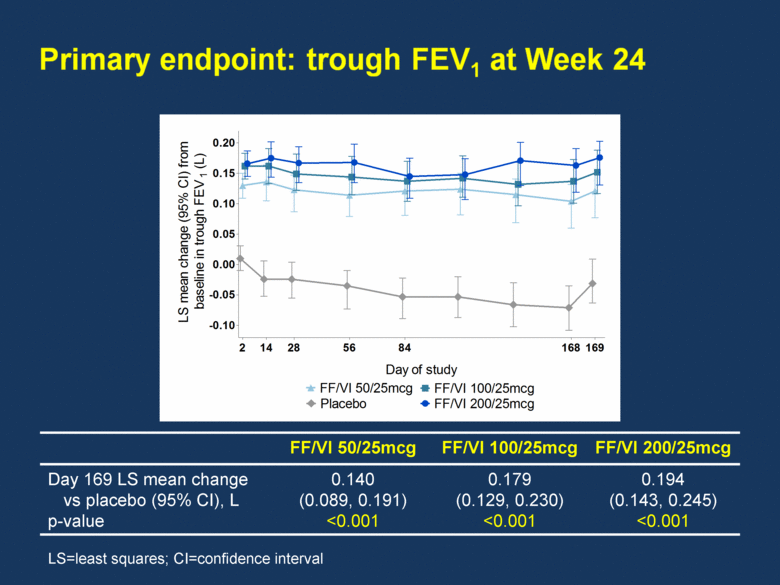
FF/VI 50/25mcg FF/VI 100/25mcg FF/VI 200/25mcg Day 169 LS mean change vs placebo (95% CI), L p-value 0.140 (0.089, 0.191) <0.001 0.179 (0.129, 0.230) <0.001 0.194 (0.143, 0.245) <0.001 LS=least squares; CI=confidence interval Day of study LS mean change (95% CI) from baseline in CRQ-SAS dyspnoea domain score 0.0 0.2 0.4 0.6 0.8 Placebo FF/VI 50/25mcg 28 FF/VI 100/25mcg FF/VI 200/25mcg 56 84 168 Day of study LS mean change (95% CI) from baseline in trough FEV 1 (L) -0.10 -0.05 0.00 0.05 0.10 0.15 0.20 Placebo FF/VI 50/25mcg FF/VI 100/25mcg FF/VI 200/25mcg 28 56 84 168 169 2 14 |
|
|
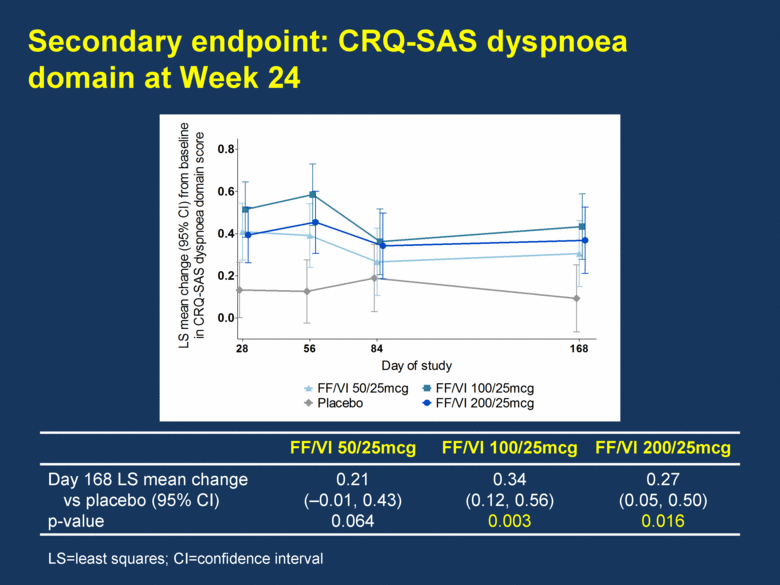
Secondary endpoint: CRQ-SAS dyspnoea domain at Week 24 FF/VI 50/25mcg FF/VI 100/25mcg FF/VI 200/25mcg Day 168 LS mean change vs placebo (95% CI) p-value 0.21 (–0.01, 0.43) 0.064 0.34 (0.12, 0.56) 0.003 0.27 (0.05, 0.50) 0.016 LS=least squares; CI=confidence interval Day of study LS mean change (95% CI) from baseline in CRQ-SAS dyspnoea domain score 0.0 0.2 0.4 0.6 0.8 Placebo FF/VI 50/25mcg 28 FF/VI 100/25mcg FF/VI 200/25mcg 56 84 168 Day of study LS mean change (95% CI) from baseline in trough FEV 1 (L) -0.10 -0.05 0.00 0.05 0.10 0.15 0.20 Placebo FF/VI 50/25mcg FF/VI 100/25mcg FF/VI 200/25mcg 28 56 84 168 169 2 14 |
|
|
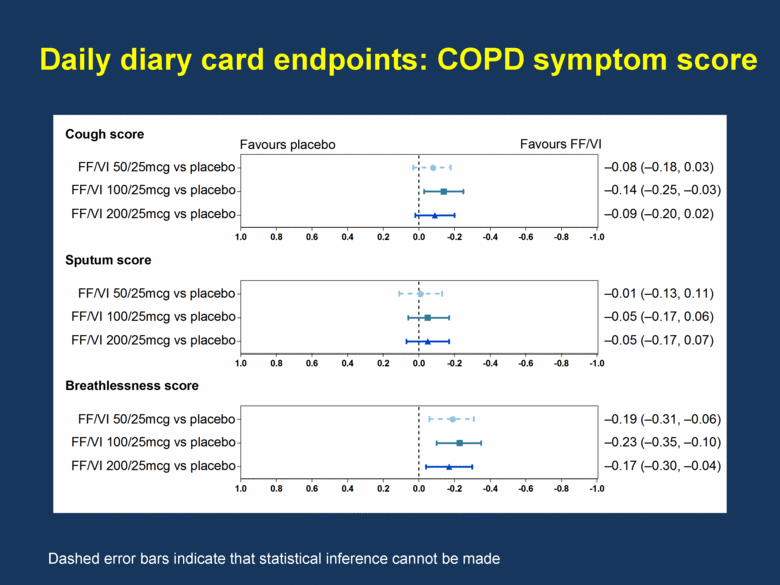
Daily diary card endpoints: COPD symptom score Dashed error bars indicate that statistical inference cannot be made -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0.2 0.4 0.6 0.8 1.0 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0.2 0.4 0.6 0.8 1.0 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0.2 0.4 0.6 0.8 1.0 Cough score Sputum score Breathlessness score FF/VI 50/25mcg vs placebo FF/VI 100/25mcg vs placebo FF/VI 200/25mcg vs placebo FF/VI 50/25mcg vs placebo FF/VI 100/25mcg vs placebo FF/VI 200/25mcg vs placebo FF/VI 50/25mcg vs placebo FF/VI 100/25mcg vs placebo FF/VI 200/25mcg vs placebo –0.08 (–0.18, 0.03) –0.14 (–0.25, –0.03) –0.09 (–0.20, 0.02) –0.01 (–0.13, 0.11) –0.05 (–0.17, 0.06) –0.05 (–0.17, 0.07) –0.19 (–0.31, –0.06) –0.23 (–0.35, –0.10) –0.17 (–0.30, –0.04) Favours placebo Favours FF/VI |
|
|
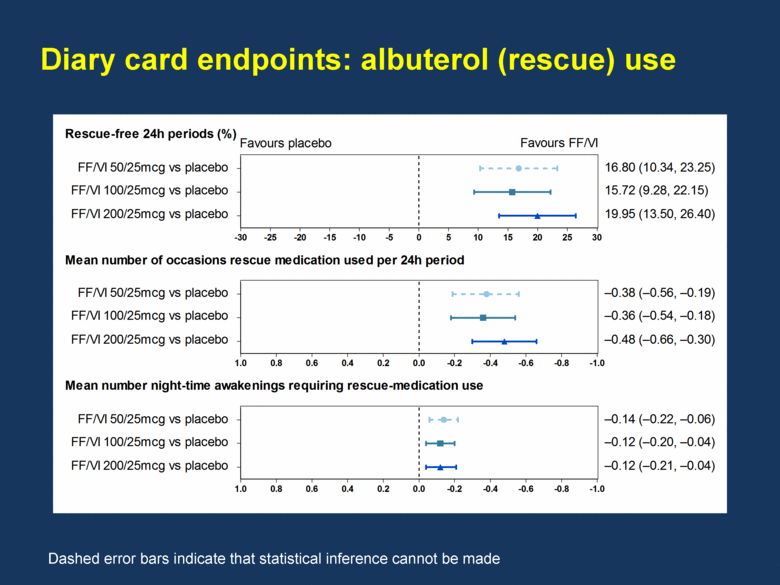
Diary card endpoints: albuterol (rescue) use Dashed error bars indicate that statistical inference cannot be made -30 -25 -20 -15 -10 -5 0 5 10 15 20 25 30 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0.2 0.4 0.6 0.8 1.0 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0.2 0.4 0.6 0.8 1.0 Rescue-free 24h periods (%) Mean number of occasions rescue medication used per 24h period Mean number night-time awakenings requiring rescue-medication use FF/VI 50/25mcg vs placebo FF/VI 100/25mcg vs placebo FF/VI 200/25mcg vs placebo FF/VI 50/25mcg vs placebo FF/VI 100/25mcg vs placebo FF/VI 200/25mcg vs placebo FF/VI 50/25mcg vs placebo FF/VI 100/25mcg vs placebo FF/VI 200/25mcg vs placebo 16.80 (10.34, 23.25) 15.72 (9.28, 22.15) 19.95 (13.50, 26.40) –0.38 (–0.56, –0.19) –0.36 (–0.54, –0.18) –0.48 (–0.66, –0.30) –0.14 (–0.22, –0.06) –0.12 (–0.20, –0.04) –0.12 (–0.21, –0.04) Favours placebo Favours FF/VI |
|
|
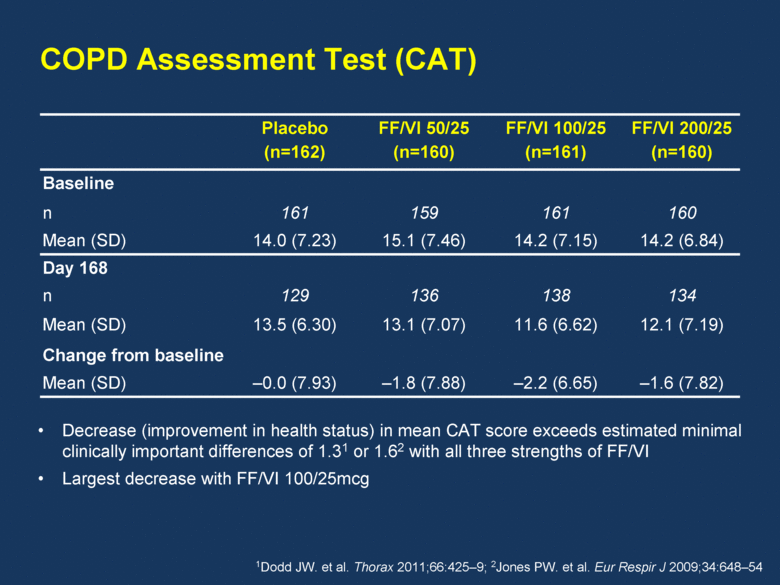
Placebo (n=162) FF/VI 50/25 (n=160) FF/VI 100/25 (n=161) FF/VI 200/25 (n=160) Baseline n 161 159 161 160 Mean (SD) 14.0 (7.23) 15.1 (7.46) 14.2 (7.15) 14.2 (6.84) Day 168 n 129 136 138 134 Mean (SD) 13.5 (6.30) 13.1 (7.07) 11.6 (6.62) 12.1 (7.19) Change from baseline Mean (SD) –0.0 (7.93) –1.8 (7.88) –2.2 (6.65) –1.6 (7.82) Decrease (improvement in health status) in mean CAT score exceeds estimated minimal clinically important differences of 1.31 or 1.62 with all three strengths of FF/VI Largest decrease with FF/VI 100/25mcg 1Dodd JW. et al. Thorax 2011;66:425–9; 2Jones PW. et al. Eur Respir J 2009;34:648–54 COPD Assessment Test (CAT) |
|
|
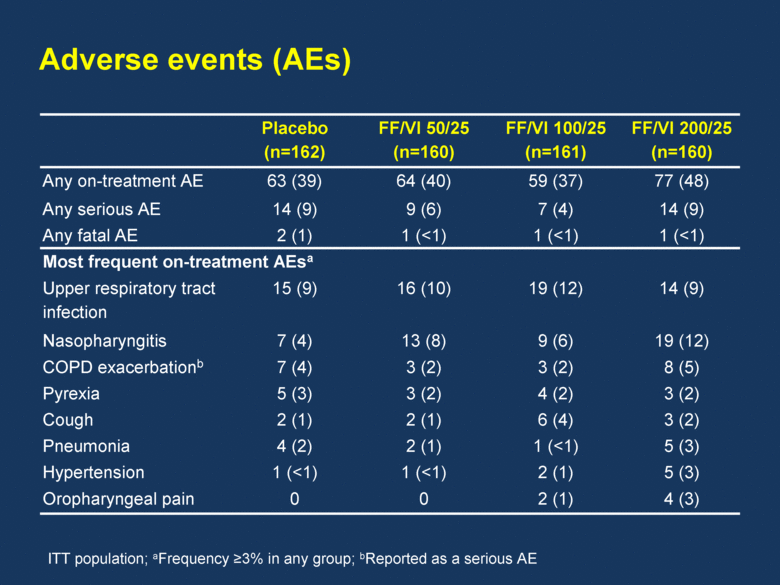
Adverse events (AEs) Placebo (n=162) FF/VI 50/25 (n=160) FF/VI 100/25 (n=161) FF/VI 200/25 (n=160) Any on-treatment AE 63 (39) 64 (40) 59 (37) 77 (48) Any serious AE 14 (9) 9 (6) 7 (4) 14 (9) Any fatal AE 2 (1) 1 (<1) 1 (<1) 1 (<1) Most frequent on-treatment AEsa Upper respiratory tract infection 15 (9) 16 (10) 19 (12) 14 (9) Nasopharyngitis 7 (4) 13 (8) 9 (6) 19 (12) COPD exacerbationb 7 (4) 3 (2) 3 (2) 8 (5) Pyrexia 5 (3) 3 (2) 4 (2) 3 (2) Cough 2 (1) 2 (1) 6 (4) 3 (2) Pneumonia 4 (2) 2 (1) 1 (<1) 5 (3) Hypertension 1 (<1) 1 (<1) 2 (1) 5 (3) Oropharyngeal pain 0 0 2 (1) 4 (3) ITT population; aFrequency >3% in any group; bReported as a serious AE |
|
|
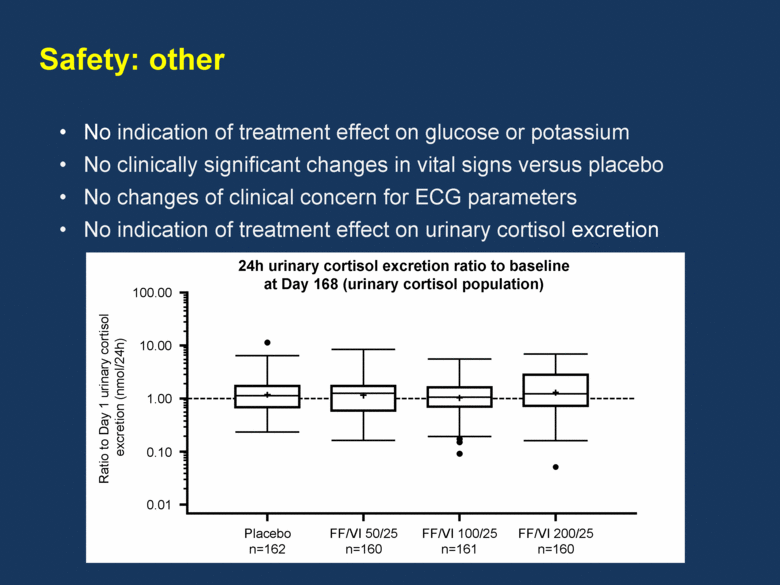
Safety: other No indication of treatment effect on glucose or potassium No clinically significant changes in vital signs versus placebo No changes of clinical concern for ECG parameters No indication of treatment effect on urinary cortisol excretion 24h urinary cortisol excretion ratio to baseline at Day 168 (urinary cortisol population) 100.00 10.00 1.00 0.10 0.01 Placebo n=162 FF/VI 50/25 n=160 FF/VI 100/25 n=161 FF/VI 200/25 n=160 Ratio to Day 1 urinary cortisol excretion (nmol/24h) |
|
|
Summary All three strengths of FF/VI improved trough FEV1 Improvements observed on CRQ-SAS Dyspnoea domain but not clinically important Improvements observed on diary card measures Treatment benefit perceived by patients was also demonstrated by CAT scores All three strengths of FF/VI displayed acceptable safety profiles consistent with historical data 1,2 1 Kerwin EM. et al. Respir Med 2013;107:560-9; 2 Martinez FJ. et al. Respair Med 2013; 107:550-9 |
|
|
FF/VI 100/25mcg is an appropriate therapeutic strength for the treatment of COPD in Asian patients based on the totality of data from this study. Conclusions |