Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a13-24088_18k.htm |
Exhibit 99.1
|
|
OBESITY WEEK Preconference Session ©2013 VIVUS Inc. All rights reserved | www.vivus.com November 12, 2013 Barbara Troupin, MD, MBA VP, Scientific Communication & Risk Management |
|
|
FORWARD LOOKING STATEMENT ©2013 VIVUS Inc. All rights reserved | www.vivus.com 1 Certain statements in this presentation are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as "anticipate," "believe," "forecast," "estimate," "expect," "intend," "likely," "may," "plan," "potential," "predict," "opportunity" and "should," among others. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. VIVUS does not undertake an obligation to update or revise any forward-looking statements. Investors should read the risk factors set forth in VIVUS's Form 10-K for the year ending December 31, 2012, as amended by the Form 10-K/A filed on April 30, 2013 and by the Form 10-K/A filed on June 12, 2013, and periodic reports filed with the Securities and Exchange Commission. |
|
|
QSYMIA PRESCRIBING INFORMATION About Qsymia Qsymia is approved in the U.S. and is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with an initial body mass index (BMI) of 30 kg/m2 or greater (obese) or 27 kg/m2 or greater (overweight) in the presence of at least one weight-related medical condition such as high blood pressure, type 2 diabetes, or high cholesterol. Important Safety Information Qsymia® (phentermine and topiramate extended-release) capsules CIV is contraindicated in pregnancy; in patients with glaucoma; in hyperthyroidism; in patients receiving treatment or within 14 days following treatment with monoamine oxidase inhibitors (MAOIs); or in patients with hypersensitivity or idiosyncrasy to sympathomimetic amines, topiramate, or any of the inactive ingredients in Qsymia. Qsymia can cause fetal harm. Females of reproductive potential should have a negative pregnancy test before treatment and monthly thereafter and use effective contraception consistently during Qsymia therapy. If a patient becomes pregnant while taking Qsymia, treatment should be discontinued immediately, and the patient should be informed of the potential hazard to the fetus. The most commonly observed side effects in controlled clinical studies, 5% or greater and at least 1.5 times placebo, include paraesthesia, dizziness, dysgeusia, insomnia, constipation, and dry mouth. 2 |
|
|
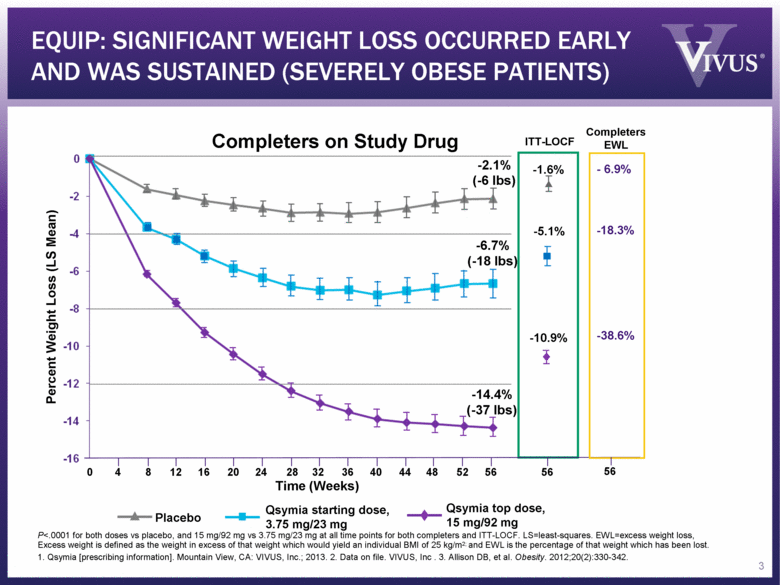
EQUIP: SIGNIFICANT WEIGHT LOSS OCCURRED EARLY AND WAS SUSTAINED (SEVERELY OBESE PATIENTS) P<.0001 for both doses vs placebo, and 15 mg/92 mg vs 3.75 mg/23 mg at all time points for both completers and ITT-LOCF. LS=least-squares. EWL=excess weight loss, Excess weight is defined as the weight in excess of that weight which would yield an individual BMI of 25 kg/m2; and EWL is the percentage of that weight which has been lost. 1. Qsymia [prescribing information]. Mountain View, CA: VIVUS, Inc.; 2013. 2. Data on file. VIVUS, Inc . 3. Allison DB, et al. Obesity. 2012;20(2):330-342. Placebo Qsymia top dose, 15 mg/92 mg -2.1% (-6 lbs) -6.7% (-18 lbs) -14.4% (-37 lbs) ITT-LOCF Completers on Study Drug Percent Weight Loss (LS Mean) Qsymia starting dose, 3.75 mg/23 mg -1.6% -5.1% -10.9% Time (Weeks) 8 16 24 32 40 48 56 56 0 4 12 20 28 36 44 52 3 - 6.9% -18.3% -38.6% Completers EWL 56 |
|
|
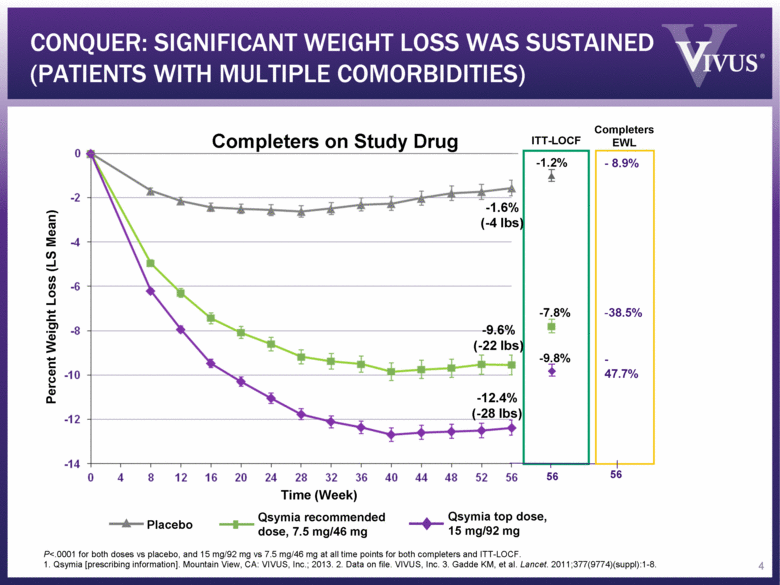
ITT-LOCF CONQUER: SIGNIFICANT WEIGHT LOSS WAS SUSTAINED (PATIENTS WITH MULTIPLE COMORBIDITIES) P<.0001 for both doses vs placebo, and 15 mg/92 mg vs 7.5 mg/46 mg at all time points for both completers and ITT-LOCF. 1. Qsymia [prescribing information]. Mountain View, CA: VIVUS, Inc.; 2013. 2. Data on file. VIVUS, Inc. 3. Gadde KM, et al. Lancet. 2011;377(9774)(suppl):1-8. -1.2% -7.8% -9.8% Placebo Qsymia top dose, 15 mg/92 mg Qsymia recommended dose, 7.5 mg/46 mg 56 Percent Weight Loss (LS Mean) -1.6% (-4 lbs) -9.6% (-22 lbs) -12.4% (-28 lbs) Time (Week) Completers on Study Drug 4 - 8.9% 38.5% - 47.7% 56 Completers EWL |
|
|
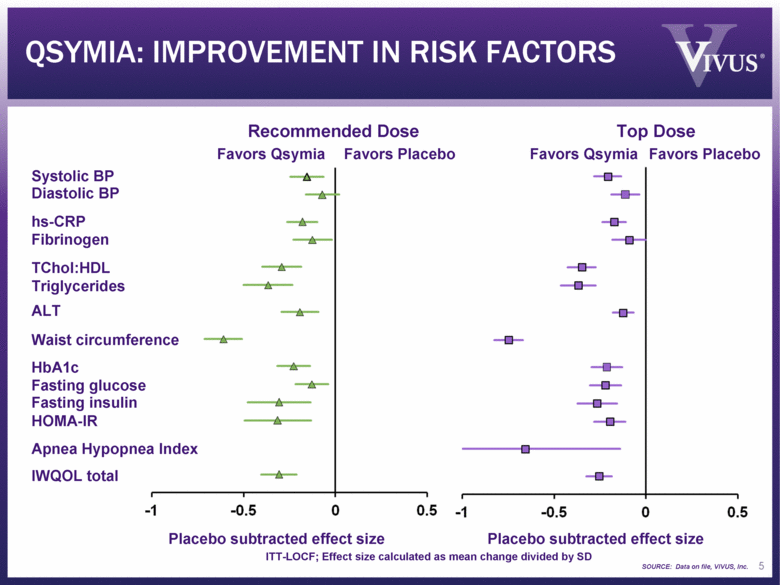
QSYMIA: IMPROVEMENT IN RISK FACTORS Diastolic BP Systolic BP Fibrinogen Triglycerides TChol:HDL IWQOL total Fasting glucose HOMA-IR Fasting insulin HbA1c hs-CRP ALT Apnea Hypopnea Index Placebo subtracted effect size Placebo subtracted effect size Favors Qsymia Favors Placebo Favors Qsymia Favors Placebo Waist circumference ITT-LOCF; Effect size calculated as mean change divided by SD SOURCE: Data on file, VIVUS, Inc. Recommended Dose Top Dose |
|
|
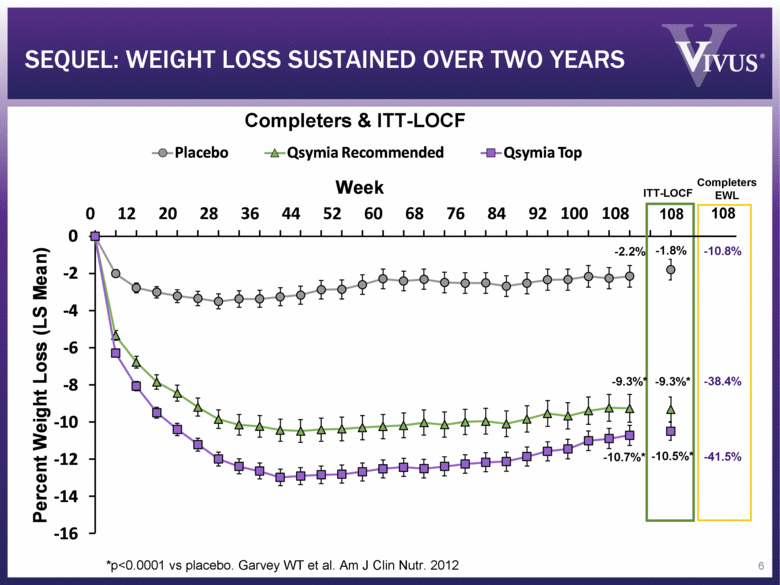
SEQUEL: WEIGHT LOSS SUSTAINED OVER TWO YEARS *p<0.0001 vs placebo. Garvey WT et al. Am J Clin Nutr. 2012 ITT-LOCF Completers & ITT-LOCF -10.8% -38.4% -41.5% Completers EWL 108 108 -1.8% -9.3%* -10.5%* -9.3%* -10.7%* -2.2% 6 - 16 - 14 - 12 - 10 - 8 - 6 - 4 - 2 0 Percent Weight Loss (LS Mean) Week Placebo Qsymia Recommended Qsymia Top |
|
|
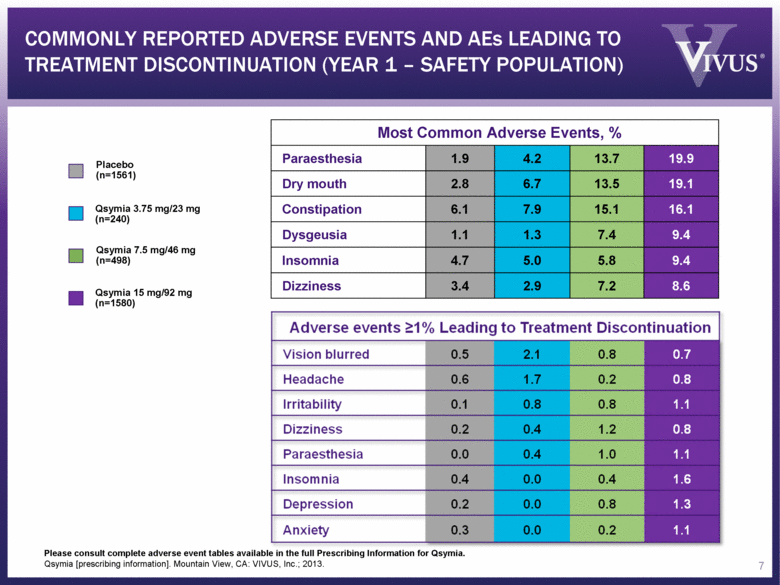
Please consult complete adverse event tables available in the full Prescribing Information for Qsymia. Qsymia [prescribing information]. Mountain View, CA: VIVUS, Inc.; 2013. Most Common Adverse Events, % Paraesthesia 1.9 4.2 13.7 19.9 Dry mouth 2.8 6.7 13.5 19.1 Constipation 6.1 7.9 15.1 16.1 Dysgeusia 1.1 1.3 7.4 9.4 Insomnia 4.7 5.0 5.8 9.4 Dizziness 3.4 2.9 7.2 8.6 Placebo (n=1561) Qsymia 3.75 mg/23 mg (n=240) Qsymia 15 mg/92 mg (n=1580) Qsymia 7.5 mg/46 mg (n=498) COMMONLY REPORTED ADVERSE EVENTS AND AEs LEADING TO TREATMENT DISCONTINUATION (YEAR 1 – SAFETY POPULATION) 7 |
|
|
OBESITY MARKET EVOLUTION 8 |
|
|
NEW AACE TREATMENT ALGORITHM (MAY-2013): MEDICAL THERAPY IS NOW RECOMMENDED 9 Obesity management and FDA-approved anti-obesity medications now recommended for: Prediabetes Diabetes Dyslipidemia Hypertension 1st ever inclusion of drug therapy in treatment algorithm Primary Care Physicians refer to AACE algorithm |
|
|
GREATER RECOGNITION OF OBESITY AS A DISEASE Jun-2013 - For the first time, obesity is recognized as a disease by the leading medical association in the U.S. AMA recognition is important support for policy makers, healthcare providers and payers 10 |
|
|
TREAT AND REDUCE OBESITY ACT OF 2013 Introduced into House and Senate Jun-2013 Among other provisions, the Act would remove the Medicare Part D exclusion of obesity medications Bi-partisan support Senators Carper (D-DE), Murkowski (R-AK) Representatives Cassidy (R-LA), Kind (D-WI) 11 |
|
|
QSYMIA RECENT HIGHLIGHTS 12 |
|
|
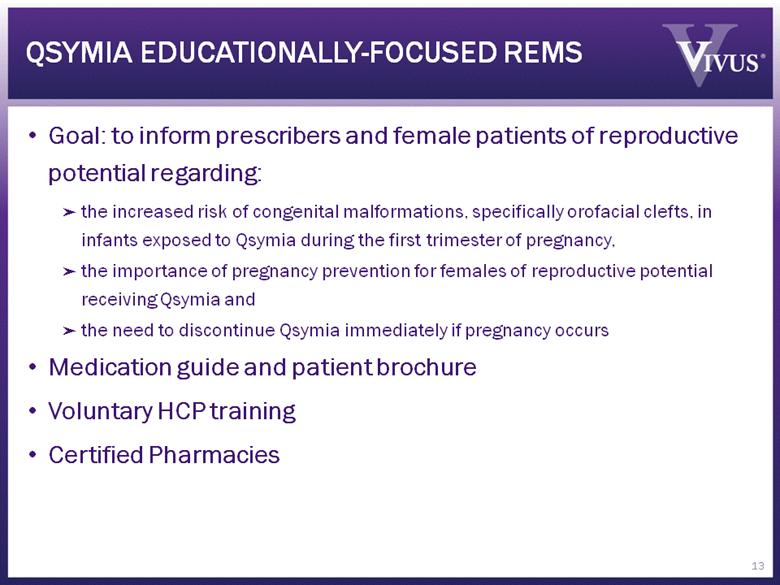
QSYMIA EDUCATIONALLY-FOCUSED REMS Goal: to inform prescribers and female patients of reproductive potential regarding: the increased risk of congenital malformations, specifically orofacial clefts, in infants exposed to Qsymia during the first trimester of pregnancy, the importance of pregnancy prevention for females of reproductive potential receiving Qsymia and the need to discontinue Qsymia immediately if pregnancy occurs Medication guide and patient brochure Voluntary HCP training Certified Pharmacies 13 |
|
|
QSYMIA RETAIL ROLL-OUT SUCCESS Certified Retail Pharmacy Status (31-Oct-2013) >31,000 pharmacies now certified to dispense Qsymia Chains include: A&P, Albertsons, Costco, CVS, Kmart, Kroger, Publix, Rite Aid, Safeway, Walgreens/Duane Reade, Walmart, Wegmans, Winn-Dixie, many others Prescriber and patient experience vastly simplified Independent pharmacies being certified 4Q2013 14 |
|
|
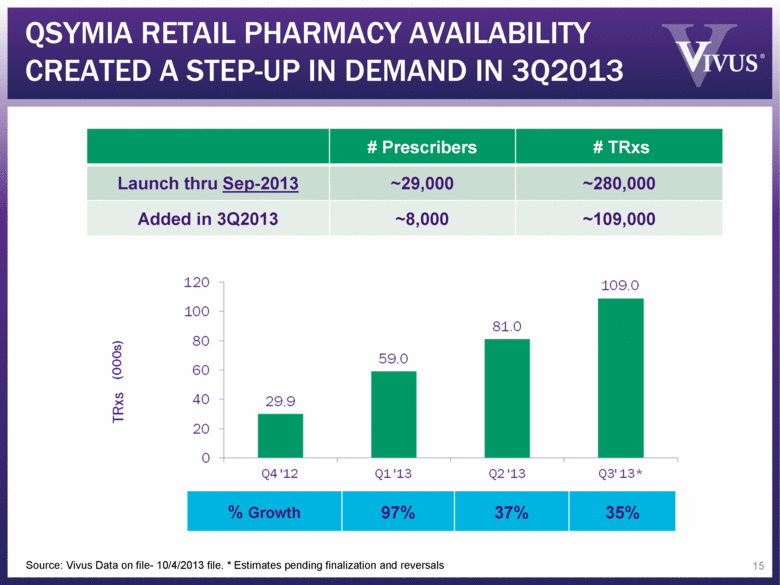
# Prescribers # TRxs Launch thru Sep-2013 ~29,000 ~280,000 Added in 3Q2013 ~8,000 ~109,000 Source: Vivus Data on file- 10/4/2013 file. * Estimates pending finalization and reversals % Growth 97% 37% 35% QSYMIA RETAIL PHARMACY AVAILABILITY CREATED A STEP-UP IN DEMAND IN 3Q2013 15 |
|
|
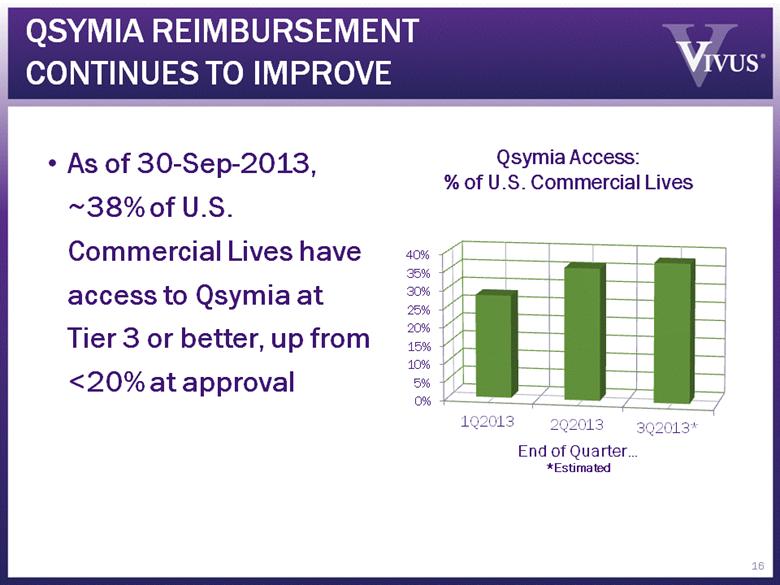
QSYMIA REIMBURSEMENT CONTINUES TO IMPROVE As of 30-Sep-2013, ~38% of U.S. Commercial Lives have access to Qsymia at Tier 3 or better, up from <20% at approval 16 Qsymia Access: % of U.S. Commercial Lives |
|
|
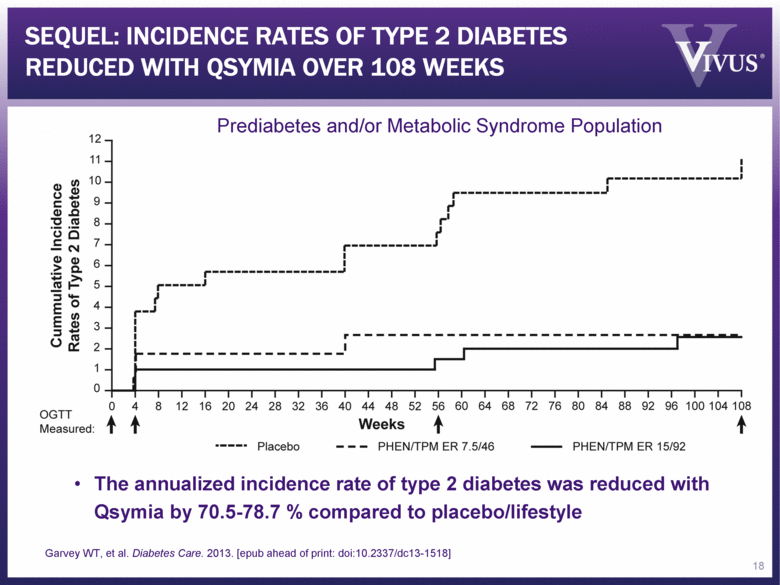
RECENT PUBLICATION REPORTS IMPACT OF QSYMIA ON TYPE 2 DIABETES “Prevention of Type 2 Diabetes in Subjects With Prediabetes and Metabolic Syndrome Treated With Phentermine and Topiramate Extended-Release” W. Timothy Garvey, MD, et al. Diabetes Care (epub Oct 8, 2013) Patients on the Recommended (7.5/46) and Top (15/92) doses experienced reduced annualized incidence rates of progression to type 2 diabetes of 70.5% and 78.7%, respectively, compared to placebo, driven by magnitude of weight lost (10.9% and 12.1%, respectively vs. placebo; P<0.0001; ITT-MI) Qsymia therapy was well tolerated by this subgroup over two years Data further supports Qsymia reimbursement discussions 17 Garvey WT, et al. Diabetes Care. 2013. [epub ahead of print: doi:10.2337/dc13-1518] |
|
|
SEQUEL: INCIDENCE RATES OF TYPE 2 DIABETES REDUCED WITH QSYMIA OVER 108 WEEKS 18 The annualized incidence rate of type 2 diabetes was reduced with Qsymia by 70.5-78.7 % compared to placebo/lifestyle Prediabetes and/or Metabolic Syndrome Population Garvey WT, et al. Diabetes Care. 2013. [epub ahead of print: doi:10.2337/dc13-1518] |
|
|
QSYMIA EXPERIENCE ON MARKET 19 |
|
|
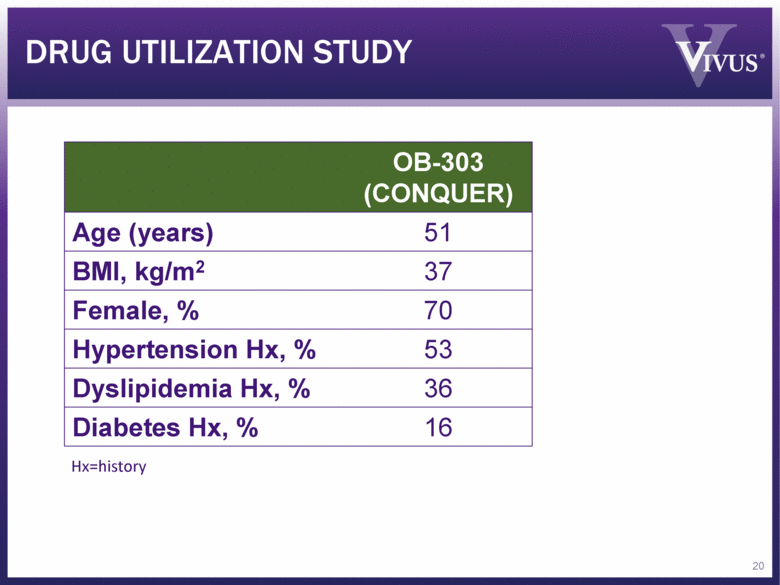
DRUG UTILIZATION STUDY OB-303 (CONQUER) Age (years) 51 BMI, kg/m2 37 Female, % 70 Hypertension Hx, % 53 Dyslipidemia Hx, % 36 Diabetes Hx, % 16 Hx=history 20 |
|
|
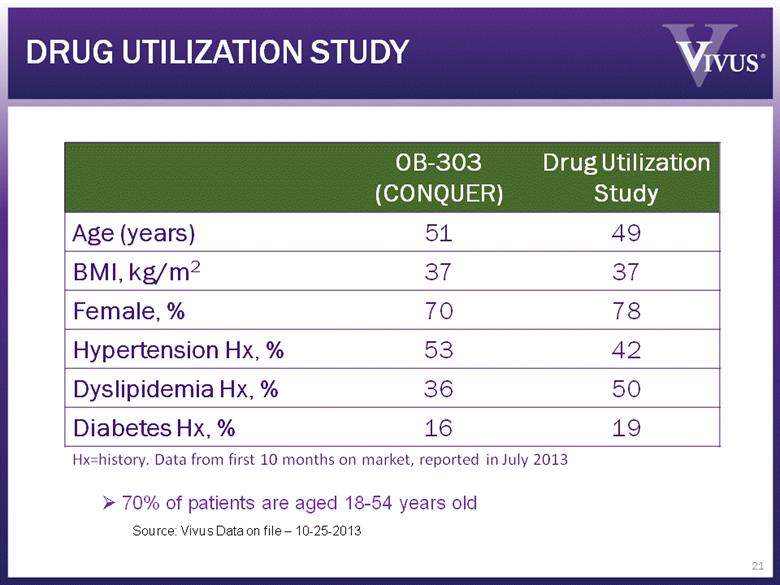
DRUG UTILIZATION STUDY OB-303 (CONQUER) Drug Utilization Study Age (years) 51 49 BMI, kg/m2 37 37 Female, % 70 78 Hypertension Hx, % 53 42 Dyslipidemia Hx, % 36 50 Diabetes Hx, % 16 19 Hx=history. Data from first 10 months on market, reported in July 2013 70% of patients are aged 18-54 years old Source: Vivus Data on file – 10-25-2013 21 |
|
|
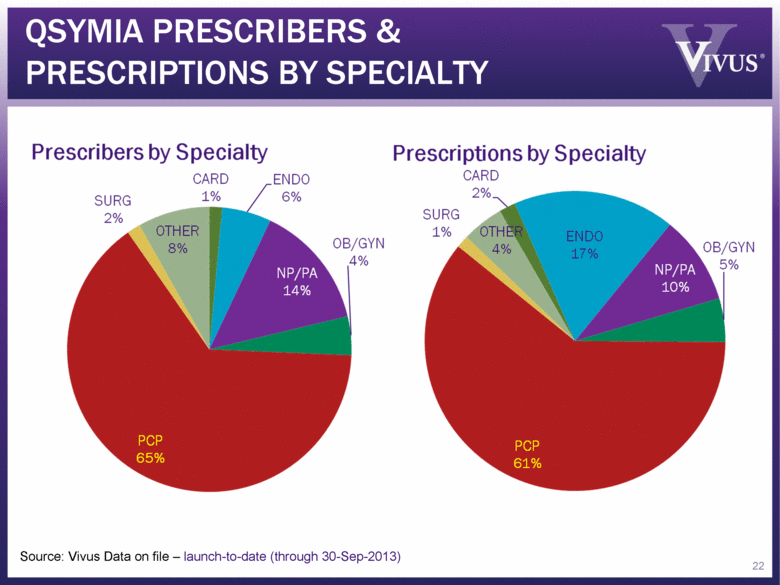
Source: Vivus Data on file – launch-to-date (through 30-Sep-2013) QSYMIA PRESCRIBERS & PRESCRIPTIONS BY SPECIALTY 22 |
|
|
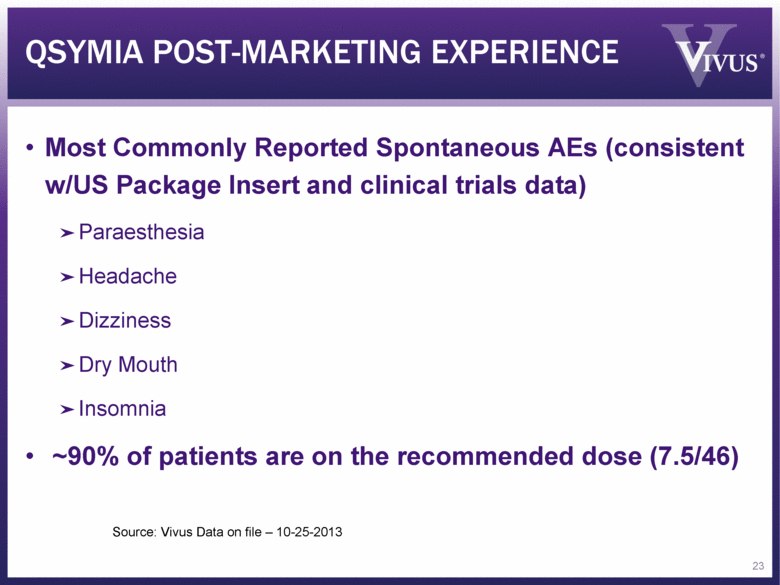
Most Commonly Reported Spontaneous AEs (consistent w/US Package Insert and clinical trials data) Paraesthesia Headache Dizziness Dry Mouth Insomnia ~90% of patients are on the recommended dose (7.5/46) QSYMIA POST-MARKETING EXPERIENCE 23 Source: Vivus Data on file – 10-25-2013 |
|
|
QSYMIA SUMMARY Qsymia was approved by FDA in Jul-2012; launched Sep-2012 First and only medication that provides sustained, best-in-class weight loss efficacy in excess of 10% Clinically relevant improvements in multiple cardiometabolic and other risk factors Well tolerated; known risks are appropriately managed through labeling and educational REMS Market experience very consistent with clinical program experience 24 |
|
|
SUMMARY Obesity treatment landscape evolving Treatment being used in appropriate patient population Access and coverage slowly but steadily improving This market is starting to build, and will only benefit from additional new entrants and investment 25 |