Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a13-23869_18k.htm |
Exhibit 99.1
POSTER NO. PS108
Efficacy and safety of once-daily fluticasone furoate/vilanterol 200/25mcg compared with twice-daily fluticasone propionate 500mcg in asthma patients of Asian ancestry
Jiangtao L(1), Crawford J(2), Jacques L(3), Stone S(3)
(1)Department of Respiratory Diseases, China-Japan Friendship Hospital, Beijing 100029, China
(2)Quantitative Sciences Division, GlaxoSmithKline, Uxbridge, UK
(3)Respiratory Medicines Development Centre, GlaxoSmithKline, Uxbridge, UK
INTRODUCTION
· Fluticasone furoate (FF)/vilanterol (VI) is a novel once-daily ICS/long-acting beta2-agonist (LABA) combination therapy for the treatment of asthma.
· Fluticasone proprionate (FP) is an inhaled corticosteroid (ICS) taken twice daily via the DISKUSTM inhaler for the treatment of asthma.
· FF/VI delivered via the ELLIPTATM dry powder inhaler, has demonstrated 24h effectiveness in asthma patients in global studies(1),(2)
· The 200/25mcg strength significantly improved lung function versus FP 500mcg twice daily over 24 weeks.(2)
· Responses to pharmacotherapy can vary across ethnic groups,(3),(4) including in Asian patients.(4)
OBJECTIVES
· To evaluate the efficacy and safety of once-daily FF/VI 200/25mcg administered in the evening, compared with twice-daily FP 500mcg administered in the morning and evening, in asthma patients of Asian ancestry.
METHODS
· A randomised, double-blind, double-dummy, active-comparator, parallel-group, 12-week multicentre study.
· Inclusion criteria: aged >12 years; FEV1 40–90% predicted; reversibility of >12% and >200mL within 10–40 minutes following 2–4 inhalations of salbutamol; treated with stable high-dose ICS or mid-dose ICS/LABA therapy for >4 weeks prior to Screening.
· Patients were randomised (1:1) to receive FF/VI 200/25mcg once daily via the ELLIPTA device (equivalent to a delivered dose of FF/VI 184/22mcg) or FP 500mcg twice daily via the DISKUS inhaler for 12 weeks.
· Primary endpoint: mean change from baseline in daily evening peak expiratory flow (PEF) averaged over the 12-week treatment period.
· Secondary endpoints (Weeks 1–12, unless stated)
· Change from baseline in % rescue-free 24h periods
· Mean change from baseline in morning PEF (averaged over Weeks 1–12)
· Change from baseline in % symptom-free 24h periods
· Change from baseline in AQLQ+12 Total score (measured at Week 12).
· A step-down statistical hierarchy was applied to account for multiplicity across endpoints. Testing of each endpoint was dependent on the achievement of significance at the 5% level for the previous endpoint, in the following order
· Evening PEF > % rescue-free 24h periods > morning PEF > % symptom-free 24h periods > AQLQ score.
· Safety endpoints included incidence of adverse events (AEs), vital signs, electrocardiogram and laboratory evaluations.
RESULTS
Table 1. Demographic and baseline characteristics
(ITT population)
|
|
|
FF/VI 200/25 |
|
FP 500 |
|
|
|
|
|
OD |
|
BD |
|
Total |
|
Age (years) |
|
46.9 (12.93) |
|
48.8 (13.41) |
|
47.9 (13.19) |
|
Male, n (%) |
|
59 (38) |
|
68 (44) |
|
127 (41) |
|
Duration of asthma |
|
12.39 |
|
13.44 |
|
12.91 |
|
(years) |
|
(12.857) |
|
(13.551) |
|
(13.196) |
|
Lung function parameters |
|
|
|
|
|
|
|
FEV1 (L) |
|
1.78 (0.493) |
|
1.77 (0.552) |
|
1.77 (0.523) |
|
% predicted FEV1 |
|
67.51 |
|
67.55 |
|
67.53 |
|
|
(13.249) |
|
(13.432) |
|
(13.319) | |
|
% reversibility FEV1 |
|
27.31 |
|
26.98 |
|
27.14 |
|
at screening |
|
(14.570) |
|
(14.262) |
|
(14.395) |
Data are mean (SD) unless otherwise stated; OD=once daily; BD=twice daily
RESULTS
Efficacy
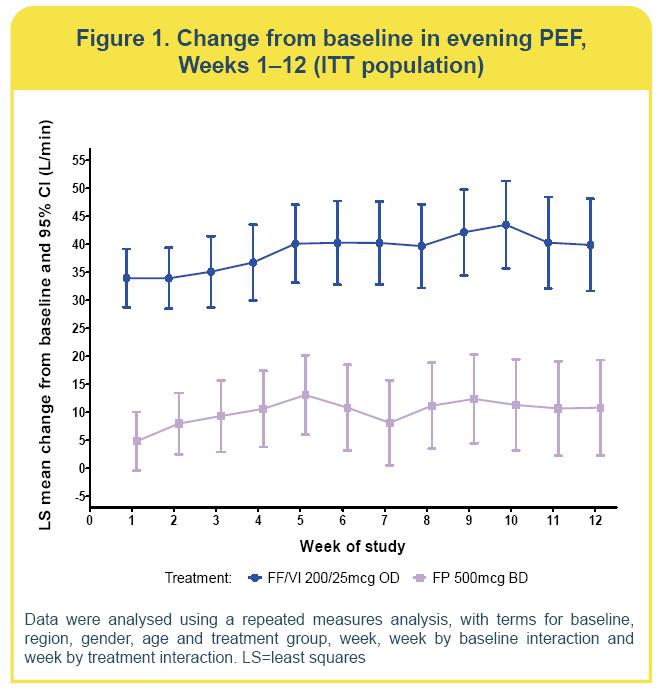
· FF/VI and FP improved evening PEF compared with baseline
(Figure 1)
· Change from baseline with FF/VI was 39.1L/min (standard error=3.01) and with FP was 10.5L/min (3.03)
· The effect was statistically significantly better (p<0.001) with FF/VI compared with FP (28.5L/min; 95% confidence interval [CI]: 20.1, 36.9).
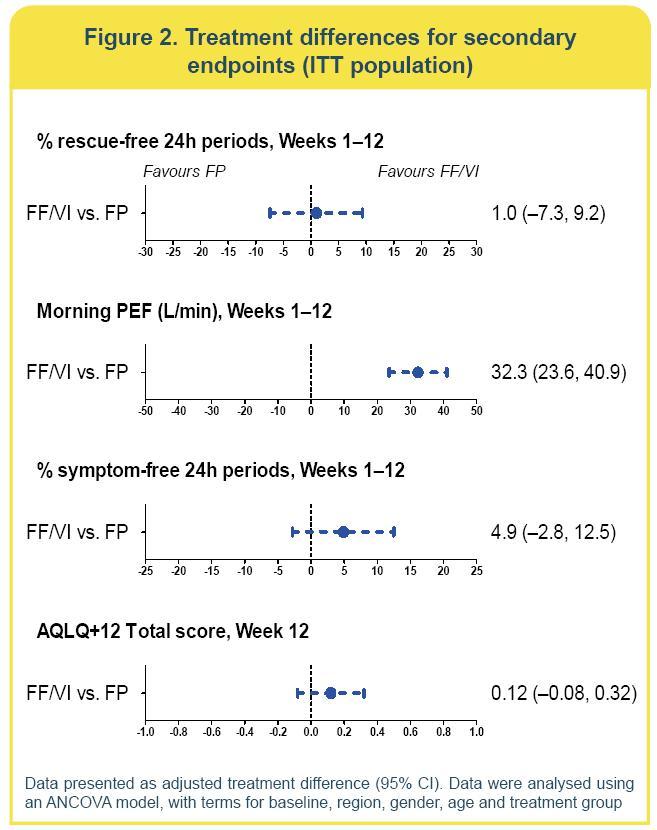
· Improvements in % rescue-free 24h periods were similar for FF/VI and FP
· The equivalent number of rescue-free days per week was 2.3 with FF/VI and 2.2 with FP
· The adjusted treatment difference (1.0%; 95% CI: —7.3, 9.2) was not statistically significant (p=0.821) (Figure 2).
· Due to the statistical hierarchy, significance for remaining endpoints could not be inferred.
· There were numerical improvements in the remaining secondary endpoints for FF/VI compared with FP (Figure 2).
RESULTS
Safety
· Incidence of AEs, treatment-related AEs and serious AEs was low and similar for FF/VI and FP (Table 2).
· There were no fatal AEs.
· The only treatment-related SAE was asthma (FP 500mcg).
· Pneumonia was reported by two patients (both with FF/VI 200/25mcg); neither required hospitalisation.
· There were no clinically significant changes in vital signs, electrocardiogram parameters or clinical laboratory evaluations.
Table 2. Summary of adverse events (ITT population)
|
|
|
FF/VI 200/25 |
|
FP 500 |
|
|
|
|
OD |
|
BD |
|
|
|
|
N=155 |
|
N=154 |
|
|
All AEs |
|
|
|
|
|
|
On-treatment |
|
40 (26) |
|
41 (27) |
|
|
On-treatment, treatment-related |
|
5 (3) |
|
5 (3) |
|
|
On-treatment leading to withdrawal |
|
2 (1) |
|
2 (1) |
|
|
Post-treatment |
|
0 |
|
1 (<1) |
|
|
SeriousAEs |
|
|
|
|
|
|
On-treatment |
|
1 (<1) |
|
2 (1) |
|
|
On-treatment, treatment-related |
|
0 |
|
1 (<1) |
|
|
Most frequent(a) on-treatment AEs |
|
|
|
|
|
|
Upper respiratory tract infection |
|
13 (8) |
|
18 (12) |
|
|
Nasopharyngitis |
|
6 (4) |
|
6 (4) |
|
|
Rhinitis allergic |
|
5 (3) |
|
2 (1) |
|
|
Oropharyngeal pain |
|
4 (3) |
|
1 (<1) |
|
(a)Occurring in >3% of patients in either treatment group
CONCLUSIONS
· FF/VI 200/25mcg once daily demonstrated clinically and statistically significant improvements in evening PEF compared with FP 500mcg twice daily in Asian asthma patients uncontrolled on high-dose ICS or mid-dose ICS/LABA.
· There were numerical improvements across the secondary endpoints with FF/VI 200/25mcg versus FP 500mcg.
· The safety profile of FF/VI 200/25mcg was broadly similar to that of FP 500mcg.
· The results are generally consistent with a previously published global study that compared FF/VI 200/25mcg with FP 500mcg.(2)
REFERENCES
(1) Woodcock A, et al. Chest 2013;144:1222–9.
(2) O’Byrne PM, et al. Eur Respir J 2013;Oct 17:ePub ahead of print.
(3) Bjornsson TD,et al. J Clin Pharmacol 2003;43:943–67.
(4) Huang SM, et al. Clin Pharmacol Ther 2008;84:287–94.
ACKNOWLEDGMENTS
· The presenting author, Dr Jiangtao Lin has received speaker’s honoraria from AstraZeneca, GlaxoSmithKline and MSD, and has been a member of global advisory boards for Boehringer Ingelheim.
· The study was funded by GlaxoSmithKline (GSK study code HZA113714 (clinicaltrials.gov registration number: NCT01498653).
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Laura Maguire, MChem at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GlaxoSmithKline.

DISKUS™ and ELLIPTA™ are trade marks of the GlaxoSmithKline group of companies
Presented at the 18th Congress of the Asian Pacific Society of Respirology, Yokohama, Japan, 11–14 November 2013
