Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - InnoVision Labs, Inc | Financial_Report.xls |
| EX-5.1 - EXHIBIT 5.1 - InnoVision Labs, Inc | v358145_ex5-1.htm |
| EX-23.1 - EXHIBIT 23.1 - InnoVision Labs, Inc | v358145_ex23-1.htm |
| EX-21.1 - EXHIBIT 21.1 - InnoVision Labs, Inc | v358145_ex21-1.htm |
As filed with the Securities and Exchange Commission on October 28, 2013
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM S-1
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
GlassesOff Inc.
(Exact Name of Registrant as Specified in Its Charter)
| Nevada | 7372 | 26-4574088 | ||
| (State or Other Jurisdiction of Incorporation | (Primary Standard Industrial Classification | (I.R.S. Employer Identification | ||
| or Organization) | Code Number) | Number) |
|
GlassesOff Inc. 35 Jabotinski St. POB 126 Ramat Gan 52511 Israel (855) 393-7243 |
| (Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices) |
|
GlassesOff Inc. 35 Jabotinski St. POB 126 Ramat Gan 52511 Israel (855) 393-7243 |
| (Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service) |
|
Copies to : Robert L. Grossman, Esq. Greenberg Traurig, P.A. 333 Avenue of the Americas, Suite 4400 Miami, FL 33131 (305) 579-0500 (305) 579-0717 (facsimile) |
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| ¨ Large accelerated filer | ¨ Accelerated filer |
| ¨ Non-accelerated filer (Do not check if a smaller reporting company) | x Smaller reporting company |
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Amount to be Registered (1) | Proposed Maximum Offering Price Per Share (2) | Proposed Maximum Aggregate Offering Price (2) | Amount of Registration Fee (3) | ||||||||||||
| Common stock, par value $0.001 per share | 43,290,030 | $ | 1.24 | $ | 53,679,637.20 | $ | 6,913.94 | |||||||||
| Common stock, $0.001 par value per share, issuable upon exercise of warrants to purchase shares of common stock | 10,013,504 | $ | 1.24 | $ | 12,416,744.96 | $ | 1,599.28 | |||||||||
| Total | 53,303,534 | $ | 66,096,382.16 | $ | 8,513.22 | |||||||||||
| (1) | Pursuant to Rule 416 of the Securities Act of 1933, as amended, this Registration Statement also registers such additional shares of common stock as may become issuable to prevent dilution as a result of stock splits, stock dividends or similar transactions. |
| (2) | Based on the average of the reported asked price ($1.27) and bid price ($1.21) per share of the Registrant’s common stock on the OTCBB on October 22, 2013. |
| (3) | Estimated solely for the purpose of calculating the amount of the registration fee in accordance with Rule 457(o) under the Securities Act. |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. The selling stockholders may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
Subject to Completion, dated October 28, 2013
53,303,534 Shares

Common Stock
This prospectus relates to the resale by the selling stockholders named herein of up to an aggregate of 53,303,534 shares of common stock, par value $0.001 per share, of GlassesOff Inc., which we refer to as Common Stock, consisting of 43,290,030 shares that are currently issued and outstanding and 10,013,504 shares that are issuable upon the exercise of warrants held by the selling stockholders.
The shares of Common Stock described in this prospectus may be offered for sale from time to time by the selling stockholders named herein. The selling stockholders may offer and sell the shares in a variety of transactions as described under the heading “Plan of Distribution” beginning on page 50, including transactions on any stock exchange, market or facility on which our Common Stock may be traded, in privately negotiated transactions or otherwise at market prices prevailing at the time of sale, at prices related to such market prices or at negotiated prices. We have no basis for estimating either the number of shares of Common Stock that will ultimately be sold by the selling stockholders or the prices at which such shares will be sold.
All of the shares of Common Stock are being sold by the selling stockholders named in this prospectus. We will not receive any of the proceeds from the sale of the shares of Common Stock being sold by the selling stockholders. We are bearing all of the expenses in connection with the registration of the shares of Common Stock, but all selling and other expenses incurred by the selling stockholders, including commissions and discounts, if any, attributable to the sale or disposition of the shares will be borne by them.
Our Common Stock is quoted on the OTCBB under the symbol “GLSO”.
An investment in our Common Stock involves substantial risks. See “Risk Factors” beginning on page 7 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2013.
TABLE OF CONTENTS
Prospectus
| CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS | 1 |
| SUMMARY | 3 |
| RISK FACTORS | 7 |
| USE OF PROCEEDS | 20 |
| COMMON STOCK PRICE RANGE AND DIVIDENDS | 20 |
| CAPITALIZATION | 21 |
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 22 |
| BUSINESS | 30 |
| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS, DIRECTORS AND EXECUTIVE OFFICERS | 37 |
| SELLING STOCKHOLDERS | 39 |
| MANAGEMENT | 42 |
| CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS | 46 |
| DESCRIPTION OF SECURITIES | 48 |
| PLAN OF DISTRIBUTION | 50 |
| LEGAL MATTERS | 51 |
| EXPERTS | 51 |
| WHERE YOU CAN FIND MORE INFORMATION | 52 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains “forward-looking statements”, as that term is defined under the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements about our expectations, beliefs or intentions regarding our product offerings, business, financial condition, results of operations, strategies or prospects. You can identify forward-looking statements by the fact that these statements do not relate strictly to historical or current matters. Rather, forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause our actual results to differ materially from any future results expressed or implied by the forward-looking statements. Many factors could cause our actual activities or results to differ materially from the activities and results anticipated in forward-looking statements. We do not undertake any obligation to update forward-looking statements. These forward-looking statements are only predictions and reflect our views as of the date they are made with respect to future events and financial performance. We undertake no obligation to update, and we do not have a policy of updating or revising, these forward-looking statements.
Risks and uncertainties, the occurrence of which could adversely affect our business, include, without limitation, the following:
| · | We have a limited operating history, and we do not expect to become profitable in the near future. |
| · | We have not yet commercialized any products or technologies, and we may never become profitable. |
| · | Our future potential products in our application pipeline are in the early development stages and may never be commercially successful. |
| · | We do not control our expected distribution channel, and changes in such channel could impair our ability to distribute our products and adversely impact our financial performance. |
| · | We expect to initially distribute our first product, GlassesOff™ via Apple’s App Store, which requires that Apple accept GlassesOff™ for inclusion in the App Store, and Apple’s failure to accept GlassesOff™ for inclusion in the App Store, or Apple’s delay in including GlassesOff™ in the App Store, could adversely impact our financial performance and market acceptance of our product. |
| · | It is highly likely that we will need to raise additional capital to meet our business requirements in the future, and such capital raising may be costly or difficult to obtain and could dilute current stockholders’ ownership interests. |
| · | If we fail to obtain necessary funds for our operations, we will be unable to maintain and improve our technology, and we will be unable to develop and commercialize our products and technologies. |
| · | We depend on key members of our management and advisory team and will need to add and retain additional leading experts. |
| · | Under current U.S. and Israeli law, we may not be able to enforce employees’ covenants not to compete and therefore may be unable to prevent our competitors from benefiting from the expertise of some of our former employees. |
| · | We do not currently have sales, marketing or distribution capabilities, and we may be unable to effectively sell, market and distribute our products in the future, and the failure to do so would have an adverse effect on our business and results of operations. |
| · | We may suffer losses from product liability claims if our products cause harm to patients. |
| · | Failure by our customers to use our products correctly could result in limited efficacy and customer dissatisfaction, which could have a material adverse effect on our business and results of operations. |
| · | Regulatory requirements may have an adverse effect on our business and results of operations. |
| · | If we acquire or license additional technology or products, we may incur a number of costs, may have integration difficulties and may experience other risks that could harm our business and results of operations. |
| · | We may not be able to successfully grow and expand our business. |
| 1 |
| · | We may encounter difficulties in managing our growth, which could increase our losses. |
| · | If we are unable to obtain adequate insurance, our financial condition could be adversely affected in the event of uninsured or inadequately insured loss or damage. Our ability to effectively recruit and retain qualified officers and directors could also be adversely affected if we experience difficulty in obtaining adequate directors’ and officers’ liability insurance. |
| · | We are a holding company that depends on cash flows from our wholly owned subsidiaries to meet our obligations. |
| · | If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or detect fraud. Consequently, investors could lose confidence in our financial reporting and this may decrease the trading price of our Common Stock. |
| · | Potential political, economic and military instability in the State of Israel, where key members of our senior management and our research and development facilities are located, may adversely affect our results of operations. |
| · | Recent disruptions in the financial markets and economic conditions could affect our ability to raise capital and could disrupt or delay the performance of our third-party contractors and suppliers. |
| · | We license certain technology from RevitalVision LLC, and we could lose our rights to this license if a dispute with RevitalVision LLC arises or if we fail to comply with the financial and other terms of the license. |
| · | The failure to obtain or maintain patents, licensing agreements and other intellectual property could impact our ability to compete effectively. |
| · | Costly litigation may be necessary to protect our intellectual property rights and we may be subject to claims alleging the violation of the intellectual property rights of others. |
| · | We rely on confidentiality agreements that could be breached and may be difficult to enforce, which could result in third parties using our intellectual property to compete against us. |
| · | International patent protection is particularly uncertain, and if we are involved in opposition proceedings in foreign countries, we may have to expend substantial sums and management resources. |
| · | We may be unable to protect the intellectual property rights of the third parties from whom we license certain of our intellectual property or with whom we have entered into other strategic relationships. |
| · | We are potentially subject to government regulations, and we may experience delays in obtaining required regulatory approvals, if required, to market our proposed products. |
| · | We face significant competition and continuous technological change. |
| · | Our Common Stock has an extremely limited trading history, and prospective investors may not be able to sell their shares at their purchase price, if at all. |
| · | We cannot assure you that our Common Stock will become liquid or that it will be listed on a securities exchange. |
| · | Because our Common Stock may be a “penny stock,” it may be more difficult for investors to sell shares of our Common Stock, and the market price of our Common Stock may be adversely affected. |
| · | We do not expect to pay dividends on our Common Stock, and investors will be able to receive cash in respect of their shares of our Common Stock only upon the sale of the shares. |
| · | Securities analysts may not initiate coverage or continue to cover our Common Stock, and this may have a negative impact on its market price. |
| · | Stockholders may experience dilution of ownership interests because of the future issuance of additional shares of our Common Stock and our preferred stock. |
| · | A significant number of our shares will be eligible for sale, including under the Registration Statement, which could depress the market price of our stock. |
* * * * *
| 2 |
SUMMARY
This summary highlights information contained elsewhere in this prospectus. This summary is not complete and does not contain all of the information that you should consider before deciding to invest in our Common Stock. You should read this entire prospectus, including the section entitled “Risk Factors,” and our financial statements and the notes thereto contained herein by reference before deciding to invest in our Common Stock. Unless the context otherwise requires, the terms “we,” “us,” “our” or “GlassesOff” refer to the business of GlassesOff Inc. and its consolidated wholly owned subsidiaries, Ucansi Inc. and EYEKON E.R.D. LTD, which comprise our operating subsidiaries and all of our operations as of the date of this Registration Statement on Form S-1.
Our Company
Overview
We are a development stage healthcare software technology company, utilizing patented technology to develop and commercialize consumer-oriented software applications for improving near vision sharpness, by improving the image processing function in the visual cortex of the brain. We expect to deliver our products through a cloud-based client server architecture to hand-held devices, currently implemented on the Apple iOS platform (iPhone, iPod, iPad), and which are scheduled to be offered on Android and Windows Phone platforms.
Recent Developments
The Merger
On June 26, 2013, we (at the time known as Autovative Products, Inc.) entered into that certain Agreement and Plan of Merger, as amended by that certain First Amendment to Agreement and Plan of Merger, dated as of July 2, 2013, which we refer to collectively as the Merger Agreement, with Ucansi Acquisition Corp., a Delaware corporation and our wholly owned subsidiary, which we refer to as Merger Sub, and Ucansi Inc., a Delaware corporation, which we refer to as Ucansi. Pursuant to the Merger Agreement, on July 30, 2013, which we refer to as the Closing Date, Merger Sub merged with and into Ucansi, with Ucansi surviving the merger as our wholly owned subsidiary, which we refer to as the Merger. Upon consummation of the Merger, we changed our name from Autovative Products, Inc. to GlassesOff Inc.
Pursuant to the Merger Agreement, all shares of Ucansi’s common and preferred stock that were issued and outstanding immediately preceding the Merger were converted into the right to receive an aggregate of approximately 40,000,000 shares of Common Stock after giving effect to a 7.5-for-1 forward split of our Common Stock, which we refer to as the Forward Split. Following the Merger, Ucansi’s former stockholders hold approximately 80% of our issued and outstanding Common Stock. Additionally, pursuant to the Merger Agreement, upon consummation of the Merger, we assumed all of Ucansi’s options and warrants that were issued and outstanding immediately prior to the Merger and issued to the holders of such securities in exchange therefor options and warrants, which we refer to as Parent Warrants, to acquire approximately 9,019,872 shares and 7,523,504 shares of our Common Stock, respectively, in each case after giving effect to the Forward Split. None of the shares of our Common Stock, Parent Warrants, options or shares of our Common Stock issuable upon exercise of the Parent Warrants and options issued by us in connection with the Merger, which we refer to collectively as the Merger Securities, have been registered under the Securities Act of 1933, as amended, which we refer to as the Securities Act, or any state securities laws and may not be sold except in a transaction registered under, or exempt from, the registration provisions of the Securities Act, and applicable state securities laws. We issued the Merger Securities in reliance upon the exemption from registration contained in Section 4(a)(2) of the Securities Act. Persons acquiring Merger Securities represented to us that they were “accredited investors” as defined in Rule 501(a) under the Securities Act and that the Merger Securities were being acquired for investment purposes.
The consolidation effected by the Merger has been accounted for as a reverse acquisition wherein Ucansi will be treated as the acquirer for accounting purposes as it has acquired control of the combined enterprise.
Included in the shares of Common Stock offered hereby by the selling stockholders are the shares of Common Stock that were issued pursuant to the Merger and the shares of Common Stock issuable upon exercise of the Parent Warrants that we assumed in connection with the Merger.
The Spin-Off
On or about December 31, 2012, we contributed all of the operations, assets and liabilities of our historical business, which we refer to as the Legacy Business, generally comprising the distribution and development of automotive parts, to a newly-formed wholly owned subsidiary, Autovative Technologies, Inc. On May 23, 2013, we entered into a spin-off agreement, which we refer to as the Spin-Off Agreement, with our former Chairman of the Board and Chief Executive Officer, David Funderburk, pursuant to which, effective on the Closing Date, we sold, assigned, transferred and conveyed to Mr. Funderburk all of the issued and outstanding capital stock of Autovative Technologies, Inc. in exchange for which Mr. Funderburk has agreed to indemnify us for, and hold us harmless from, any and all losses and liabilities arising out of, or relating to, the Legacy Business.
| 3 |
Amended Articles and Bylaws; Trading Symbol
In connection with the Merger, our Board of Directors and holders of in excess of a majority of our issued and outstanding Common Stock voted to amend our Articles of Incorporation, which we refer to as our Amended and Restated Articles to (i) increase our capitalization to provide for the issuance of up to 200,000,000 shares of our Common Stock and up to 20,000,000 shares of “blank check” preferred stock, par value $0.001 per share, and (ii) change our name from Autovative Products, Inc. to “GlassesOff Inc.” In connection with our name change, our Common Stock, which was traded on the OTCBB under the symbol “ATVP” is now traded under the symbol “GLSO”. Also in connection with the Merger, our Board of Directors amended and restated our bylaws, which we refer to as our Amended and Restated Bylaws.
The Private Placement
Additionally, on the Closing Date, we consummated a private placement, which we refer to as the Private Placement, in connection with which we entered into subscription agreements, which we refer to as the Subscription Agreements, with certain private investors, which we refer to as the Investors, pursuant to which the Investors purchased an aggregate of 2,490,000 units for a purchase price in cash of $1.25 per unit, which we refer to as the Units, each of which comprised one share of Common Stock and one five-year warrant to purchase one share of Common Stock at an exercise price of $1.25 per share, which we refer to as the Warrants. Before expenses, we received an aggregate of approximately $3,112,500 in gross proceeds from the issuance of the Units. We issued the Units, including the underlying Common Stock, Warrants and shares of our Common Stock issuable upon exercise of the Warrants in reliance upon the exemption from registration contained in Section 4(a)(2) of the Securities Act. The Investors in the Private Placement represented to GlassesOff that they were “accredited investors” as defined in Rule 501(a) under the Securities Act and that the Units were being acquired for investment purposes. See “Description of Securities” on page 48 below for a description of the material terms of Common Stock.
In connection with the Private Placement, on the Closing Date, we entered into a registration rights agreement, which we refer to as the Registration Rights Agreement, with the Investors, pursuant to which we have agreed to use our reasonable efforts to file with the Securities and Exchange Commission, which we refer to as the SEC, on or prior to the 90th day immediately following the Closing Date, which we refer to as the Filing Deadline, a registration statement registering for resale all shares of our Common Stock issued in the Private Placement and all shares of our Common Stock issuable upon the exercise of the Warrants. We have agreed to use reasonable efforts to cause such registration statement to be declared effective by the SEC within 270 days subsequent to the filing thereof with the SEC, which we refer to as the Effectiveness Deadline.
We are filing this registration statement on Form S-1 pursuant to our obligations under the Registration Rights Agreement.
Plan of Operation
During 2013, we intend to complete the development of the first commercial version of our consumer-oriented software applications for improving near vision sharpness, for the iOS platform and start marketing the product to end consumers via Apple’s App Store. During 2013, we plan to launch marketing and public relations campaigns, aiming to increase awareness of our product and recruit customers. Additionally, we plan to participate in various conferences and conventions around the world to further expose our product and technology to professional audience and potentially form business partnerships.
In 2013, we intend to begin designing our product offering for new platforms and expect to start the development project for a new platform, currently expected to be Android.
Industry Background and Target Markets
As we age, we normally experience changes in reading abilities. These changes are influenced, among other factors, by our brain’s visual processing capabilities. The effect of these changes on most people results in slower, more difficult and less effective reading. According to a scientific review (Holden, B. A. et al., 2008), more than one billion people worldwide suffer from such age-related changes and this number is expected to rise significantly through 2050. At some point, natural age-related changes in reading abilities affect most people, who typically attempt to improve their reading abilities through the aid of magnifying devices, typically reading glasses. Such devices increase the size of words in reading material, making it easier for a person who uses them, whether that person is in his or her twenties or fifties, to read easily and quickly.
| 4 |
The GlassesOff product enhances the image processing capabilities of the brain and thus improves a person’s reading abilities. The use of the GlassesOff product results in faster, more efficient and more comfortable reading, thereby correcting reading vision without use of reading glasses.
Competition
Our product will compete in the market of solutions for better, faster and more effective reading. The natural changes in reading capabilities with age have typically been addressed by traditional products for improvement of reading capabilities, mainly magnification devices, such as reading glasses or contact lenses that support reading. Manufactures of such magnifier devices could therefore be considered as competition. We believe that our product, which is intended to eliminate the constant dependency on magnifiers, is therefore highly competitive with such devices.
In addition, there are several methods and products that claim to improve reading vision via various means, usually related to muscle exercises (e.g. the Bates method, the See Clearly Method, the Power Vision Program etc.). These methods and products typically attempt to correct the eye functionality through muscle exercises, whereas the GlassesOff solution does not involve any such corrective methods.
Finally, there are several competitors offering vision improvement solutions based on neuroscience methods, such as PositScience and RevitalVision (which obtained FDA clearance for a product that treats amblyopia, a condition typically referred to as “Lazy Eye”.) We believe that there is no other software solution for the improvement of reading capabilities that does not use magnifying devices, such as reading glasses, that has also demonstrated efficacy in controlled studies.
We believe that our technology platform and unique intellectual property assets present a high barrier to entry for others who would try to develop and sell software products for improvement of near vision sharpness and reading capabilities using hand-held devices.
Corporate Information
Our principal executive offices are located at GlassesOff Inc., 35 Jabotinski St. POB 126, Ramat Gan 52511, Israel and our telephone number is (855) 393-7243. Our website address is www.glassesoff.com. Information contained on our website or that can be accessed through our website does not constitute a part of this prospectus and is not incorporated herein by reference.
| 5 |
The Offering
| Common Stock offered by the selling stockholders: | 53,303,534 shares, consisting of 43,290,030 shares and 10,013,504 shares issuable upon the exercise of the Warrants and the Parent Warrants. |
| Common Stock outstanding: |
53,290,000 shares as of the date of this prospectus, excluding:
· 10,013,504 shares issuable upon the exercise of the Warrants and Parent Warrants; and
· 9,019,872 shares issuable upon the exercise of stock options. |
| Trading market: | Our Common Stock is quoted on the OTCBB under the symbol “GLSO”. |
| Use of proceeds: | The selling stockholders will receive the proceeds from the sale of shares of Common Stock offered hereby. We will not receive any proceeds from the sale of the shares of Common Stock, but will pay the expenses (other than any underwriting discounts and broker’s commissions and similar expenses) of this offering. |
| Risk factors: | We are subject to a number of risks that you should be aware of before you decide to purchase our Common Stock. These risks are discussed in the section captioned “Risk Factors,” beginning on page 7 of this prospectus. |
| 6 |
RISK FACTORS
An investment in our Common Stock involves a high degree of risk. Before making an investment decision, you should carefully consider the following risk factors. If any of these risks actually occur, our business, financial condition and results of operations could be materially harmed, and the trading price of our Common Stock could decline. In addition, risks and uncertainties not presently known to us or that we currently deem immaterial may also materially harm our business, financial condition and results of operations. If this were to happen, the value of our Common Stock could decline significantly and you could lose all or part of your investment.
Risks related to our Company and our Business
We have a limited operating history, and we do not expect to become profitable in the near future.
We are a development stage vision improvement software solutions company with a limited operating history. We are not profitable and have incurred losses since our inception. We have not generated any operating revenue since our inception, and we continue to incur research and development and general and administrative expenses related to our operations. We expect to incur losses for the foreseeable future. If our products do not achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Accordingly, it is difficult to evaluate our business prospects. Moreover, our prospects must be considered in light of the risks and uncertainties encountered by an early-stage company operating in a market with regulatory issues and where the need for market acceptance of our products is uncertain. There can be no assurance that our efforts will ultimately be successful or result in revenues or profits.
We have not yet commercialized any products or technologies, and we may never become profitable.
We have not yet commercialized any products or technologies. We will not be successful unless GlassesOff™ and any other products we develop, if any, gain market acceptance. The degree of market acceptance of our products will depend on a number of factors, including:
| · | the competitive landscape; and |
| · | the adequacy and success of distribution, sales and marketing efforts. |
Physicians, users, thirty-party payors or the medical community in general may be unwilling to accept, utilize or recommend any of our products or products incorporating our technologies. As a result, we are unable to predict the extent of future losses or the time required to achieve profitability, if at all. Even if we successfully develop one or more products that incorporate our technologies, we may not become profitable.
Our future potential products in our application pipeline are in the early development stages and may never be commercially successful.
Our future potential products in our application pipeline are either at very early stages of product development or pre-definition and may never be developed or commercialized. The progress and results of any future products are uncertain, and may result in a failure to develop additional effective products. Even if we successfully complete our products development they may not be commercially successful due to, among other things, low users’ acceptance or criticism by clinicians and other third-party opinion leaders. Third parties may develop superior products or have proprietary rights that preclude us from marketing our products.
We do not control our expected distribution channel, and changes in such channel could impair our ability to distribute our products and adversely impact our financial performance.
We expect that the main distribution channel for our applications will be via popular application stores, such as Apple’s App store and Google market. We do not control these application stores, and the owners of such stores can change various parameters, such as increasing fees or requiring additional product safeguards, any of which could have an adverse impact on our financial performance and our ability to distribute our products.
| 7 |
We expect to initially distribute our first product, GlassesOffTM via Apple’s App Store, which requires that Apple accept GlassesOffTM for inclusion in the App Store, and Apple’s failure to accept GlassesOffTM for inclusion in the App Store, or Apple’s delay in including GlassesOffTM in the App Store, could adversely impact our financial performance and market acceptance of our product.
Apple requires that developers, such as us, to comply with numerous guidelines and satisfy various conditions before Apple will accept an application for inclusion in its App Store. We cannot assure you that Apple will accept GlassesOffTM, or any of our other future products, for inclusion in the App Store, and Apple’s failure to accept GlassesOffTM for inclusion in the App Store, or Apple’s delay in including GlassesOffTM in the App Store, could materially and adversely affect or financial performance and market acceptance of our product.
It is highly likely that we will need to raise additional capital to meet our business requirements in the future, and such capital raising may be costly or difficult to obtain and could dilute current stockholders’ ownership interests.
Our future capital requirements will depend on many factors, including the progress and results of our product testing, the timing and outcome of regulatory review of our products (should some of our products require regulatory approval), the number and development requirements of other products that we pursue, and the costs of commercialization activities, including product marketing, sales, and distribution. Because of the numerous risks and uncertainties associated with the development and commercialization of our products, we are unable to reasonably estimate the amounts of increased capital outlays and operating expenditures that our business will require. It is likely that we will need to raise additional funds through public or private debt or equity financings to meet various objectives including, but not limited to:
| · | product testing; |
| · | research and development of new products; |
| · | pursuing growth opportunities, including more rapid expansion; |
| · | acquiring complementary businesses; |
| · | making capital improvements to improve our infrastructure; |
| · | hiring qualified management and key employees; |
| · | responding to competitive pressures; |
| · | complying with regulatory requirements; and |
| · | maintaining compliance with applicable laws. |
Any additional capital raised through the sale of equity or equity-linked securities may dilute our current stockholders’ ownership in us and could also result in a decrease in the market price of our Common Stock. The terms of those securities issued by us in future capital transactions may be more favorable to new investors and may include preferences, superior voting rights and the issuance of warrants or other derivative securities, which may have a further dilutive effect.
Furthermore, any debt or equity financing that we may need may not be available on terms favorable to us, or at all. If we are unable to obtain required additional capital, we may have to curtail our growth plans or cut back on existing business, and we may not be able to continue operating if we do not generate sufficient revenues from operations needed to stay in business.
We may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we issue, such as convertible notes and warrants, which may adversely impact our financial condition.
| 8 |
If we fail to obtain necessary funds for our operations, we will be unable to maintain and improve our technology, and we will be unable to develop and commercialize our products and technologies.
Our present and future capital requirements depend on many factors, including:
| · | future revenues and profits generated from the expected launch of our first product; |
| · | the level of research and development investment required to develop our products, and maintain and improve our patented technology position; |
| · | the costs of product development for research and at commercial scale; |
| · | the results of testing, which can be unpredictable; |
| · | changes in product development plans needed to address any difficulties that may arise in development or commercialization; |
| · | our ability and willingness to enter into new agreements with strategic partners and the terms of these agreements; |
| · | the costs of investigating patents that might block us from developing potential products; |
| · | the costs of recruiting and retaining qualified personnel; |
| · | the time and costs involved in obtaining regulatory approvals should such be required; |
| · | the costs of filing, prosecuting, defending, and enforcing patent claims and other intellectual property rights; and |
| · | our need or decision to acquire or license complementary technologies. |
If we are unable to obtain the funds necessary for our operations, we will be unable to maintain and improve our patented technology, and we will be unable to develop and commercialize our products and technologies, which would materially and adversely affect our business, liquidity and results of operations.
We depend on key members of our management and advisory team and will need to add and retain additional leading experts.
We are highly dependent on our executive officers and other key management and technical personnel. Our failure to retain our Chief Scientific Officer, Dr. Uri Polat, or any other key management and technical personnel could have a material adverse effect on our future operations. Our success is also dependent on our ability to attract, retain and motivate highly trained technical, marketing, sales and management personnel, among others, to market our products and to continue to develop enhanced releases of our products. We presently do not maintain “key person” life insurance policies on any of our personnel.
Our success also depends on our ability to attract, retain and motivate personnel required for the development, maintenance and expansion of our activities. There can be no assurance that we will be able to retain our existing personnel or attract additional qualified employees. The loss of key personnel or the inability to hire and retain additional qualified personnel in the future could have a material adverse effect on our business, financial condition and results of operation.
Under current U.S. and Israeli law, we may not be able to enforce employees’ covenants not to compete and therefore may be unable to prevent our competitors from benefiting from the expertise of some of our former employees.
We have entered into non-competition agreements with our key employees. These agreements prohibit our key employees, if they cease working for us, from competing directly with us or working for our competitors for a limited period. Under applicable U.S. and Israeli law, we may be unable to enforce these agreements. If we cannot enforce our non-competition agreements with our employees, then we may be unable to prevent our competitors from benefiting from the expertise of our former employees, which could materially adversely affect our business, results of operations and ability to capitalize on our proprietary information.
| 9 |
We do not currently have sales, marketing or distribution capabilities, and we may be unable to effectively sell, market and distribute our products in the future, and the failure to do so would have an adverse effect on our business and results of operations.
If we are unable to develop sales, marketing, customer relationship management and distribution capabilities or enter into agreements with third parties to perform these functions, we will not be able to successfully commercialize any of our products. We do not currently have internal sales, marketing or distribution capabilities. In order to successfully commercialize any of our products, we must either internally develop sales, marketing, customer relationship management and distribution capabilities or make arrangements with third parties to perform these services.
If we do not develop a skilled marketing and sales force and supporting customer relationship management, we will be unable to market any of our products directly. To promote any of our potential products through third parties, we will have to locate acceptable third parties for these functions and enter into agreements with them on acceptable terms, and we may not be able to do so. In addition, any third-party arrangements we are able to enter into may result in lower revenues than we could achieve by directly marketing and selling our potential products.
We may suffer losses from product liability claims if our products cause harm to patients.
Any of our products could potentially cause adverse events. These reactions may not be observed during testing, but may nonetheless occur after commercialization. If any of these reactions occur, they may render our products ineffective or harmful in some patients, and our sales would suffer, materially adversely affecting our business, financial condition and results of operations.
In addition, potential adverse events caused by our products could lead to product liability lawsuits. If professional liability and/or product liability lawsuits are successfully brought against us, we may incur substantial losses and may be required to limit commercialization of our products. Our business exposes us to potential product liability risks, which are inherent in the testing, marketing and sale of brain training software products. We may not be able to avoid product liability claims. Product liability insurance for our products and products in development is generally expensive, if available at all. We do not currently have sufficient product liability insurance. If we are unable to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims, we may be unable to commercialize our products. A successful product liability claim brought against us in excess of our insurance coverage, if any, may cause us to incur substantial liabilities, and, as a result, our business, liquidity and results of operations would be materially adversely affected.
Failure by our customers to use our products correctly could result in limited efficacy and customer dissatisfaction, which could have a material adverse effect on our business and results of operations.
Improper use of our products or failure by users to comply with the requirements for the correct use of our products could result in reduced product efficacy. The following, among other things, may result in reduced product efficacy:
| · | failure to comply with the required vision improvement protocol, such as session frequency and minimal number of sessions; |
| · | use of the application not in accordance with instructions; |
| · | use of the application by several users using the same user account on the same device; |
| · | use of the application by several users using the same user account on different devices; |
| · | use of the application by a single users on different devices; |
| · | use of the application under environmental conditions with extreme variation between training sessions; |
| · | use of the application without wearing the optical correction for far distance for users with significant refractive errors for far distance; and |
| 10 |
| · | use of the application while using medication, alcohol or any other chemical substance that affect vision, the ability to concentrate, attention, motor functions, etc. |
Reduced product efficacy could result in customer dissatisfaction and reduced product sales, which could have a material adverse effect on our business and results of operations.
Regulatory requirements may have an adverse effect on our business and results of operations.
Healthcare is heavily regulated by the federal government and by state and local governments. The federal laws and regulations affecting healthcare change constantly thereby increasing the uncertainty and risk associated with any healthcare-related venture, including our business.
The federal government regulates healthcare through various agencies, including but not limited to the following: (i) the Food and Drug Administration, which we refer to as the FDA, which administers the Food, Drug, and Cosmetic Act, which we refer to as the FD&C Act, and (ii) the Office of Civil Rights, which administers the privacy aspects of the Health Insurance Portability and Accountability Act of 1996, which we refer to as HIPAA. We do not envision seeking third-party coverage from any government healthcare program (e.g., Medicare, Medicaid, TriCare) or any private healthcare program, and therefore we believe that we will not be subject to regulation by certain other federal healthcare agencies such as the Centers for Medicare & Medicaid Services, Health and Human Services Office of Inspector General or the Department of Defense.
FDA
The FDA regulates medical devices. A “medical device”, which we refer to as a device, is as an article, including software associated with another medical device, which, among other things, is intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals. See FD&C Act § 201(h). We do not believe that our product is subject to FDA regulation as a medical device. However, there is a risk that the FDA may disagree with our assessment and conclude that it is in fact a device subject to FDA regulation. If such were to occur, the FDA may, among other things, order us to cease marketing the product until the product satisfies FDA’s regulatory regime and controls.
The regulatory requirements that would apply if our product were deemed to be a medical device would depend on the level of risk to the patient or user of the device. There are three categories of medical devices based on risk: Class I, which is the lowest risk, Class II, and Class III, which is the highest risk.
Generally speaking, companies that manufacture Class I medical devices must register with the FDA and list their product. They must also comply with quality system regulations. These regulations require companies manufacturing Class I medical devices to manufacture products and maintain documents in a prescribed manner with respect to design, manufacturing, testing and control activities. Further, such companies are required to comply with various FDA and other agency requirements for labeling and promotion and managing product complaints, which, in certain instances, requires notifying the FDA.
In addition to the Class I requirements, manufacturers of Class II products, generally, need to receive an FDA “clearance” which permits commercial distribution of that device for its intended use. The FDA will “clear” a product if it finds it is substantially equivalent to a similar legally marketed device. If clinical trial data are required to support the 510(k) submission, then these data must be gathered in compliance with investigational device exemption (IDE) regulations for investigations performed in the United States. The FDA review process for 510(k) submissions should take on average about 90 days, but it can and usually takes substantially longer and there is no guarantee that the agency will “clear” the device for marketing, in which case the device cannot be distributed in the United States. The agency could condition clearance on our narrowing the intended uses the device so that it may no longer be commercially feasible to market. Additionally, there may be special controls what apply to the development of a Class II product, including FDA guidance documents and recognized standards relevant to the product, which we refer to as Special Controls.
| 11 |
Class III manufacturers must comply with the Class I requirements and, in some instances, Special Controls. In addition, manufacturers of Class III medical devices need to get approval from the FDA before marketing their device, which we refer to as Pre-Market Approval or PMA. The PMA process is a more comprehensive approval process and requires the FDA to find there is reasonable assurance the device is safe and effective for its intended use. The manufacturer must submit a pre-market approval (PMA) application, which contains, among other things, clinical trial data, which was obtained in compliance with IDE regulations. Clinical trials associated with a Class III device are typically more complex than those of a Class II product. The PMA process also takes substantially longer than the 510(k) process.
The FDA may inspect the manufacturer’s facilities and, upon the occurrence of certain events, have the power to withdraw the clearance or require changes to a device, its manufacturing process, or its labeling or additional proof that regulatory requirements have been met. The FDA can also seize the device, ban its importation into the United States, or restrict is distribution, issue warning letters, untitled letters, impose civil or criminal penalties, fines, and injunctions, criminally prosecute a company for violations of the FD&C Act, decline to clear or approve modifications to the device, or take other regulatory action against such company.
Privacy Provisions of HIPAA
HIPAA, among other things, protects the privacy and security of individually identifiable health information by limiting its use and disclosure. HIPAA directly regulates “covered entities” (healthcare providers, insurers and clearinghouses) and indirectly regulates “business associates” with respect to the privacy of patients’ medical information. All entities that receive and process protected health information are required to adopt certain procedures to safeguard the security of that information. It is uncertain whether we would be deemed to be a covered entity under HIPAA, and it is unlikely that we, based on our current business model, would be a business associate. Nevertheless, we may be contractually required to physically safeguard the integrity and security of any patient information that we receive, store, create or transmit. If we fail to adhere to our contractual commitments, then our physician customers may be subject to civil monetary penalties and this could adversely affect our ability to market our device. If we are deemed to be a Vendor, under the HITECH Act, then we will be obligated to adopt various security measures. We may also be subject to state and foreign privacy laws under which breaches could lead to substantial fines and liability.
If we acquire or license additional technology or products, we may incur a number of costs, may have integration difficulties and may experience other risks that could harm our business and results of operations.
We may acquire or license additional products and technologies. Any product or technology we license or acquire will likely require additional development efforts prior to commercial sale, including extensive testing and, potentially, approval by the FDA and applicable foreign regulatory authorities, if any. All products are prone to risks of failure, including the possibility that the product or product developed based on licensed technology will not be shown to be sufficiently safe and effective for approval by regulatory authorities. In addition, we cannot assure you that any product that we develop based on acquired or licensed technology that is granted regulatory approval will be produced economically, successfully commercialized or widely accepted in the marketplace. Moreover, integrating any newly acquired products could be expensive and time-consuming. If we cannot effectively manage these aspects of our business strategy, our business may not succeed.
Furthermore, proposing, negotiating and implementing an economically viable acquisition or license can be a lengthy, costly and complex process. Other companies, including those with substantially greater financial, marketing and sales resources, may compete with us for the acquisition or license of products and/or technologies. We may not be able to acquire the rights to alternative products and/or technologies on terms that we find acceptable, or at all. Our failure to acquire or license alternative products and/or technologies could have a material adverse effect on our business, prospects and financial condition.
We may not be able to successfully grow and expand our business.
We may not be able to successfully expand. Successful implementation of our business plan will require management of growth, which will result in an increase in the level of responsibility for management personnel. To manage growth effectively, we will be required to continue to implement and improve our operating and financial systems and controls to expand, train and manage our employee base. The management, systems and controls currently in place or to be implemented may not be adequate for such growth, and the steps taken to hire personnel and to improve such systems and controls might not be sufficient. If we are unable to manage our growth effectively, it will have a material adverse effect on our business, results of operations and financial condition.
| 12 |
We may encounter difficulties in managing our growth, which could increase our losses.
We may experience rapid and substantial growth in order to achieve our operating plans, which will place a strain on our human and capital resources. If we are unable to manage this growth effectively, our losses could materially increase. Our ability to manage our operations and growth effectively requires us to continue to expend funds to enhance our operational, financial and management controls, reporting systems and procedures and to attract and retain sufficient numbers of talented employees. If we are unable to scale up and implement improvements to our control systems in an efficient or timely manner, or if we encounter deficiencies in existing systems and controls, then we will not be able to make available the products required to successfully commercialize our technology. Failure to attract and retain sufficient numbers of talented employees will further strain our human resources and could impede our growth or result in ineffective growth.
If we are unable to obtain adequate insurance, our financial condition could be adversely affected in the event of uninsured or inadequately insured loss or damage. Our ability to effectively recruit and retain qualified officers and directors could also be adversely affected if we experience difficulty in obtaining adequate directors’ and officers’ liability insurance.
We may not be able to obtain insurance policies on terms affordable to us that would adequately insure our business and property against damage, loss or claims by third parties. To the extent our business or property suffers any damages, losses or claims by third parties, which are not covered or adequately covered by insurance, our financial condition may be materially adversely affected.
We may be unable to maintain sufficient insurance as a public company to cover liability claims made against our officers and directors. If we are unable to adequately insure our officers and directors, we may not be able to retain or recruit qualified officers and directors.
We are a holding company that depends on cash flows from our wholly owned subsidiaries to meet our obligations.
We are a holding company with no material assets other than the stock of our wholly owned subsidiaries. Accordingly, all our operations are conducted by EYEKON E.R.D. LTD, our indirect wholly owned subsidiary. We currently expect that the earnings and cash flow of EYEKON E.R.D. LTD will primarily be retained and used by it in its operations, including servicing any debt obligations it may have now or in the future. Accordingly, although we do not anticipate paying any dividends in the foreseeable future, our subsidiary may not be able to generate sufficient cash flow to distribute funds to us in order to allow us to pay future dividends on, or make any distributions with respect to our Common Stock.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or detect fraud. Consequently, investors could lose confidence in our financial reporting and this may decrease the trading price of our Common Stock.
We must maintain effective internal controls to provide reliable financial reports and detect fraud. Our failure to properly maintain an effective system of internal controls could harm our operating results and cause investors to lose confidence in our reported financial information. In addition, such failure may cause us to suffer violations of the U.S. federal securities laws to the extent we are unable to maintain effective internal controls. Any such loss of confidence or violations would have a negative effect on the trading price of our Common Stock.
Potential political, economic and military instability in the State of Israel, where key members of our senior management and our research and development facilities are located, may adversely affect our results of operations.
We maintain office and research and development facilities in the State of Israel. Political, economic and military conditions in Israel may directly affect our ability to conduct business. Since the State of Israel was established in 1948, a number of armed conflicts have occurred between Israel and its Arab neighbors, as recently as November 2012. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners, or a significant downturn in the economic or financial condition of Israel, could affect adversely our operations. We believe that if conditions would require relocation of our offices to the U.S. we could do so within few weeks, ongoing and revived hostilities or other Israeli political or economic factors could potentially have a short-term negative effect on our operations and product development and may have a short-term negative effect to our revenues.
| 13 |
Recent disruptions in the financial markets and economic conditions could affect our ability to raise capital and could disrupt or delay the performance of our third-party contractors and suppliers.
In the past few years, the U.S. and global economies have taken a dramatic downturn as the result of the deterioration in the credit markets and related financial crisis as well as a variety of other factors including, among other things, extreme volatility in security prices, severely diminished liquidity and credit availability, ratings downgrades of certain investments and declining valuations of others. The United States and certain foreign governments have recently taken unprecedented actions in an attempt to address and rectify these extreme market and economic conditions by providing liquidity and stability to the financial markets. If the actions taken by these governments are not successful, the continued economic decline may cause a significant impact on our ability to raise capital, if needed, on a timely basis and on acceptable terms or at all. As a result of the current volatile and unpredictable global economic situation, our business could be severely adversely affected.
Risks Related to Our Intellectual Property
We license certain technology from RevitalVision LLC, and we could lose our rights to this license if a dispute with RevitalVision LLC arises or if we fail to comply with the financial and other terms of the license.
In July 2011, we licensed intellectual property from a RevitalVision LLC, referred to as the License Agreement. Pursuant to the License Agreement, we have obtained a worldwide, nonexclusive, irrevocable, transferable, sub licensable through multiple tiers, license to the field of vision improvement software applications limited to near-vision deficiencies and reading improvement. Our current product and future products are partly based on the intellectual property licensed under the License Agreement. RevitalVision may terminate the License Agreement if we are in material breach of its terms and cannot cure such breach, which termination would have a material adverse effect on our business, prospects and results of operations.
The failure to obtain or maintain patents, licensing agreements and other intellectual property could impact our ability to compete effectively.
To compete effectively, we need to develop and maintain a proprietary position with regard to our own technologies, intellectual property, licensing agreements, products and business. Legal standards relating to the validity and scope of claims in the field of brain training based vision improvement technologies may still evolve. Therefore, the degree of future protection for our proprietary rights in our core technologies and any products that might be made using these technologies is also uncertain. The risks and uncertainties that we face with respect to our existing and future patents and other proprietary rights include the following:
| · | while the patents we license have been issued, some are pending and may not result in issued patents or may take longer than we expect to result in issued patents; |
| · | we may be subject to interference proceedings; |
| · | we may be subject to opposition proceedings in foreign countries; |
| · | any patents that are issued may not provide meaningful protection; |
| · | we may not be able to develop additional proprietary technologies that are patentable; |
| · | other companies may challenge patents licensed or issued to us or our customers; |
| · | other companies may independently develop similar or alternative technologies, or duplicate our technologies; |
| · | other companies may design around technologies we have licensed or developed; and |
| · | enforcement of patents is complex, uncertain and expensive. |
| 14 |
We cannot be certain that patents will be issued as a result of any of our pending applications, and we cannot be certain that any of our issued patents, whether issued pursuant to our pending applications or licensed from a third party, will give us adequate protection from competing products. For example, issued patents, including the patents licensed from RevitalVision, may be circumvented or challenged, declared invalid or unenforceable, or narrowed in scope. In addition, since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we were the first to make our inventions or to file patent applications covering those inventions.
It is also possible that others may obtain issued patents that could prevent us from commercializing our products or require us to obtain licenses requiring the payment of significant fees or royalties in order to enable us to conduct our business. As to those patents that we have licensed, our rights depend on maintaining our obligations to the licensor under the applicable license agreement, and we may be unable to do so.
In addition to patents and patent applications, we depend upon trade secrets and proprietary know-how to protect our proprietary technology. We require our employees, consultants, advisors and collaborators to enter into confidentiality agreements that prohibit the disclosure of confidential information to any other parties. We require our employees and consultants to disclose and assign to us their ideas, developments, discoveries and inventions. These agreements may not, however, provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure.
Costly litigation may be necessary to protect our intellectual property rights, and we may be subject to claims alleging the violation of the intellectual property rights of others.
We may face significant expense and liability as a result of litigation or other proceedings relating to patents and other intellectual property rights of others. In the event that another party has also filed a patent application or been issued a patent relating to an invention or technology claimed by us in pending applications, we may be required to participate in an interference proceeding declared by the U.S. Patent and Trademark Office to determine priority of invention, which could result in substantial uncertainties and costs for us, even if the eventual outcome were favorable to us. We, or our licensors, also could be required to participate in interference proceedings involving issued patents and pending applications of another entity. An adverse outcome in an interference proceeding could require us to cease using the technology or to license rights from prevailing third parties.
The cost to us of any patent litigation or other proceeding relating to our licensed patents or patent applications, even if resolved in our favor, could be substantial. Our ability to enforce our patent protection could be limited by our financial resources, and may be subject to lengthy delays. If we are unable to effectively enforce our proprietary rights, or if we are found to infringe the rights of others, we may be in breach of our License Agreement.
A third party may claim that we are using inventions claimed by their patents and may go to court to stop us from engaging in our normal operations and activities, such as research, development and the sale of any future products. Such lawsuits are expensive and would consume time and other resources. There is a risk that the court will decide that we are infringing the third party’s patents and will order us to stop the activities claimed by the patents. In addition, there is a risk that a court will order us to pay the other party damages for having infringed their patents.
Moreover, there is no guarantee that any prevailing patent owner would offer us a license so that we could continue to engage in activities claimed by the patent, or that such a license, if made available to us, could be acquired on commercially acceptable terms. In addition, third parties may, in the future, assert other intellectual property infringement claims against us with respect to our products, technologies or other matters.
We rely on confidentiality agreements that could be breached and may be difficult to enforce, which could result in third parties using our intellectual property to compete against us.
Although we believe that we take reasonable steps to protect our intellectual property, including the use of agreements relating to the non-disclosure of confidential information to third parties, as well as agreements that purport to require the disclosure and assignment to us of the rights to the ideas, developments, discoveries and inventions of our employees and consultants while we employ them, the agreements can be difficult and costly to enforce. Although we seek to obtain these types of agreements from our contractors, consultants, advisors and research collaborators, to the extent that employees and consultants utilize or independently develop intellectual property in connection with any of our projects, disputes may arise as to the intellectual property rights associated with our products. If a dispute arises, a court may determine that the right belongs to a third party. In addition, enforcement of our rights can be costly and unpredictable. We also rely on trade secrets and proprietary know-how that we seek to protect in part by confidentiality agreements with our employees, contractors, consultants, advisors or others. Despite the protective measures we employ, we still face the risk that:
| 15 |
| · | these agreements may be breached; |
| · | these agreements may not provide adequate remedies for the applicable type of breach; |
| · | our trade secrets or proprietary know-how will otherwise become known; or |
| · | our competitors will independently develop similar technology or proprietary information. |
International patent protection is particularly uncertain, and if we are involved in opposition proceedings in foreign countries, we may have to expend substantial sums and management resources.
Patent law outside the United States is in some cases different than in the United States and is currently undergoing review and revision in many countries. Further, the laws of some foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States. For example, certain countries do not grant patent claims that are directed to the treatment of humans. We may participate in opposition proceedings to determine the validity of our foreign patents or our competitors’ foreign patents, which could result in substantial costs and diversion of our efforts.
We may be unable to protect the intellectual property rights of the third parties from whom we license certain of our intellectual property or with whom we have entered into other strategic relationships.
Certain of our intellectual property rights are currently licensed from a third party, and, in the future, we may license intellectual property from other key strategic partners. We are, and will continue to be, reliant upon such third parties to protect their intellectual property rights to any licensed technology. Such third parties may determine not to protect the intellectual property rights that we license from them and we may be unable to defend such intellectual property rights on our own or we may have to undertake costly litigation to defend the intellectual property rights of such third parties. There can be no assurances that we will continue to have proprietary rights to any of the intellectual property that we license from such third parties or otherwise have the right to use through similar strategic relationships. Any loss or limitations on use with respect to our right to use such intellectual property licensed from third parties or otherwise obtained from third parties with whom we have entered into strategic relationships could have a material adverse effect on our business, operating results and financial condition.
Risks Related to Our Industry
We are potentially subject to government regulations, and we may experience delays in obtaining required regulatory approvals, if required, to market our proposed products.
Various aspects of our operations are or may become subject to federal, state or local laws, rules and regulations, any of which may change from time to time. Costs arising out of any regulatory developments could be time-consuming, expensive and could divert management resources and attention and, consequently, could adversely affect our business operations and financial performance.
Delays in regulatory clearance, approval, limitations in regulatory approval and withdrawals of regulatory approval, if any are required, may have a negative impact on our results. If we experience significant delays in testing or approvals, our product development costs, or our ability to license products, will increase. If we are required or seek FDA regulatory clearance or approval for any of our products, then any such clearance or approval will be limited to those conditions for which the product has demonstrated, through testing, either substantially equivalent in terms of safety and effectiveness to another lawfully marketed and cleared device or to be safe and effective. Any product clearances or approvals that we receive in the future could also include significant restrictions on the use or marketing of our products. Product clearances, if issued, or approvals, if granted, can be withdrawn for failure to comply with regulatory requirements or upon the occurrence of adverse events following commercial introduction of the products. Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our products or us. If clearance or approval is withdrawn for a product, we would be unable to sell or license that product and our revenues would suffer. In addition, outside the United States, our ability to market any of our potential products may be contingent upon receiving market application authorizations from the appropriate regulatory authorities and these foreign regulatory approval processes include all of the risks associated with the FDA clearance or approval process described above.
| 16 |
We face significant competition and continuous technological change.
If our competitors develop and commercialize products faster than we do, or develop and commercialize products that are superior to our products, our commercial opportunities will be reduced or eliminated. The extent to which any of our products achieve market acceptance will depend on competitive factors, many of which are beyond our control. Competition in the vision care industry is intense. Our competitors include substitute products such as glasses manufactures, contact lens manufacturers, refractive surgery technology companies as well as developers of eye exercise methodologies and companies, universities, and public and private research institutions developing methods for vision improvement via brain training or alternative solutions for vision correction, including:
| · | new training techniques; |
| · | improved non-invasive optical techniques, such as monovision contact lenses, multifocal lenses, bifocal lenses and EDOF lenses; |
| · | new invasive or semi-invasive procedures, such as mono-vision refractive surgery, including LASIK and IOL-replacement surgery; and |
| · | medications or any chemical substances that can restore accommodation by affecting the eye muscles or eye lens elasticity. |
Many of these entities have substantially greater research and development capabilities and financial, scientific, manufacturing, marketing and sales resources than we do, as well as more experience in research and development, clinical trials, regulatory matters, marketing and sales.
Risks Relating to Our Common Stock
Our Common Stock has an extremely limited trading history, and prospective investors may not be able to sell their shares at their purchase price, if at all.
There is currently an extremely limited public market for our Common Stock. Our Common Stock is listed for quotation on the OTCBB under the symbol “GLSO”, but there is no assurance that a regular trading market will develop or, if developed, will be sustained. As a result, an investor may find it difficult to dispose of, or to obtain accurate quotations of the price of, our Common Stock.
We expect that the market price of our Common Stock will fluctuate significantly in response to factors, some of which are beyond our control, such as the announcement of new products or product enhancements by us or our competitors, developments concerning intellectual property rights and regulatory approvals, quarterly variations in our and our competitors’ results of operations, changes in earnings estimates or recommendations by securities analysts, developments in our industry, and general market conditions and other factors, including factors unrelated to our own operating performance or the condition or prospects of our industry.
We cannot assure you that our Common Stock will become liquid or that it will be listed on a securities exchange.
We may seek listing of our Common Stock on the NYSE MKT or the Nasdaq Capital Market; however, we cannot assure you that we will be able to meet the initial listing standards of either of those or of any other stock exchange, or that we will be able to maintain any such listing. Until our Common Stock is listed on an exchange, we expect to remain eligible for quotation on the OTCBB, on another over-the-counter quotation system or in the “pink sheets.” In those venues, however, an investor may find it difficult to obtain accurate quotations as to the market value of the Common Stock. In addition, if we fail to meet the criteria set forth in SEC regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling our Common Stock, which may further affect the liquidity of our Common Stock. This would also make it more difficult for us to raise additional capital or attract qualified employees or partners.
| 17 |
Additionally, the stock market in general, and securities of small-cap companies in particular, have historically experienced extreme price and volume fluctuations. Continued market fluctuations could result in extreme volatility in the price of our Common Stock, which could cause a decline in the value of our Common Stock. Price volatility might be worse if the trading volume of the Common Stock is low.
Because our Common Stock may be a “penny stock,” it may be more difficult for investors to sell shares of our Common Stock, and the market price of our Common Stock may be adversely affected.
Our Common Stock may be a “penny stock” if, among other things, the stock price is below $5.00 per share, it is not listed on a national securities exchange or it has not met certain net tangible asset or average revenue requirements. Broker-dealers who sell penny stocks must provide purchasers of these stocks with a standardized risk-disclosure document prepared by the SEC. This document provides information about penny stocks and the nature and level of risks involved in investing in the penny-stock market. A broker must also give a purchaser, orally or in writing, bid and offer quotations and information regarding broker and salesperson compensation, make a written determination that the penny stock is a suitable investment for the purchaser and obtain the purchaser’s written agreement to the purchase. Broker-dealers must also provide customers that hold penny stock in their accounts with such broker-dealer a monthly statement containing price and market information relating to the penny stock. If a penny stock is sold to an investor in violation of the penny stock rules, the investor may be able to cancel its purchase and get its money back.
If applicable, the penny stock rules may make it difficult for investors to sell their shares of our Common Stock. Because of the rules and restrictions applicable to a penny stock, there is less trading in penny stocks and the market price of our Common Stock may be adversely affected. Also, many brokers choose not to participate in penny stock transactions. Accordingly, investors may not always be able to resell their shares of our Common Stock publicly at times and prices that they feel are appropriate.
We do not expect to pay dividends on our Common Stock, and investors will be able to receive cash in respect of their shares of our Common Stock only upon the sale of the shares.
Cash dividends have never been declared or paid on our Common Stock, and we do not anticipate such a declaration or payment in the foreseeable future. We expect to use future earnings, if any, to fund business growth. Therefore, an investor in our Common Stock will obtain an economic benefit from the Common Stock only after an increase in its trading price and only by selling the Common Stock. We cannot assure stockholders of a positive return on their investment when they sell their shares, nor can we assure stockholders that they will not lose the entire amount of their investment.
Securities analysts may not initiate coverage or continue to cover our Common Stock, and this may have a negative impact on its market price.
The trading market for our Common Stock will depend in part on the research and reports that securities analysts publish about our business and us. We do not have any control over these analysts. There is no guarantee that securities analysts will cover our Common Stock. If securities analysts do not cover our Common Stock, the lack of research coverage may adversely affect its market price. If we are covered by securities analysts, and our stock is the subject of an unfavorable report, our stock price would likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, we could lose visibility in the financial markets, which could cause our stock price and/or trading volume to decline. In addition, because we will become public through a “reverse merger,” we may have additional difficulty attracting the coverage of securities analysts.
| 18 |
Stockholders may experience dilution of ownership interests because of the future issuance of additional shares of our Common Stock and our preferred stock.
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present stockholders. We are authorized to issue an aggregate of 220,000,000 shares of capital stock, consisting of 200,000,000 shares of Common Stock and 20,000,000 shares of preferred stock with preferences and rights to be determined by our Board of Directors. Additionally, we have 53,290,000 shares of our Common Stock outstanding and a total of 19,733,376 shares subject to outstanding options and warrants. We may also issue additional shares of our Common Stock or other securities that are convertible into or exercisable for Common Stock in connection with hiring or retaining employees, future acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of our Common Stock may create downward pressure on the trading price of the Common Stock. There can be no assurance that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with any capital raising efforts, including at a price (or exercise prices) below the price at which shares of our Common Stock are then traded on the OTCBB or otherwise.
A significant number of our shares will be eligible for sale, including under the Registration Statement, which could depress the market price of our stock.
Sales of a significant number of shares of our Common Stock in the public market could harm the market price of our stock. As additional shares of our Common Stock become available for resale in the public market, the supply of the Common Stock will increase, which could decrease its price. Further, shares may be offered by selling stockholders from time to time in the open market pursuant to Rule 144, and these sales may have a depressive effect on the market for the shares of our Common Stock.
| 19 |
USE OF PROCEEDS
The selling stockholders will receive the proceeds from the sale of shares of Common Stock offered hereby. We will not receive any proceeds from the sale of the shares of Common Stock but will pay the expenses (other than any underwriting discounts and broker’s commissions and similar expenses) of this offering.
COMMON STOCK PRICE RANGE AND DIVIDENDS
Our Common Stock is quoted on the OTCBB under the symbol “GLSO.” All OTCBB quotations reproduced herein reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
The following table sets forth, for each quarter during the period commencing May 18, 2012 through September 30, 2013, the reported high and low bid prices of our common stock on the OTCBB. Prior to May 18, 2012, our Common Stock was not quoted on the OTCBB or otherwise.
| Bid Price | ||||||||
| High | Low | |||||||
| 2013 | ||||||||
| First Quarter, ended March 31 | $ | 0.71 | $ | 0.55 | ||||
| Second Quarter, ended June 30 | $ | 0.55 | $ | 0.55 | ||||
| Third Quarter, ended September 30 | $ | 8.00 | $ | 0.55 | ||||
| 2012 | ||||||||
| Second Quarter, ended June 30 | $ | 0.71 | $ | 0.20 | ||||
| Third Quarter, ended September 30 | $ | 0.71 | $ | 0.71 | ||||
| Fourth Quarter, ended December 31 | $ | 0.71 | $ | 0.71 | ||||
As of the close of business on October 25, 2013, there were approximately 33 holders of record of our Common Stock.
We paid no cash dividends in respect of our Common Stock during our two most recent fiscal years, and we have no plans to pay any dividends or make any other distributions in the future.
Equity Compensation Plan Information
We had no equity compensation plans as of December 31, 2012.
| 20 |
CAPITALIZATION
The table below sets forth our cash and cash equivalents balances and our capitalization as of June 30, 2013:
You should read this table together with the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes thereto.
| As of June 30, 2013 (unaudited) | ||||
| Cash and cash equivalents | $ | 114,000 | ||
| Stockholders’ Equity (Deficit) | ||||
| Common Stock | $ | 28,000 | ||
| Preferred Stock | $ | 12,000 | ||
| Additional Paid-In Capital | $ | 9,006,000 | ||
| (Deficit) Accumulated during the Development Stage | $ | (9,319,000 | ) | |
| Total Stockholders’ Equity (Deficit) | $ | (273,000 | ) | |
| Total Capitalization | $ | (159,000 | ) | |
| 21 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Prospective investors should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the related notes and other financial information contained in this prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” section of this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Introduction
This management’s discussion and analysis of financial condition and results of operations is intended to provide investors with an understanding of GlassesOff’s financial condition, changes in financial condition and results of operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our historical consolidated financial statements and related notes thereto, which are contained in this prospectus. The discussion below contains forward-looking statements that are based upon our current expectations and are subject to uncertainty and changes in circumstances. Actual results may differ materially from these expectations due to inaccurate assumptions and known or unknown risks and uncertainties, including those identified in “Cautionary Note Regarding Forward-Looking Statements” and “Risk Factors” contained in this prospectus.
The discussion and analysis of GlassesOff’s financial condition and results of operations are based on GlassesOff’s financial statements, which GlassesOff has prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires GlassesOff to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, GlassesOff evaluates such estimates and judgments, including those described in greater detail below. GlassesOff bases its estimates on historical experience and on various other factors that GlassesOff believes are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Business
GlassesOff is a development stage healthcare software technology company, utilizing patented technology to develop and commercialize consumer-oriented software applications for improving near vision sharpness, by improving the image processing function in the visual cortex of the brain. GlassesOff expects to deliver its products through a cloud-based client server architecture to hand-held devices, currently implemented on the Apple iOS platform (iPhone, iPod, iPad), and which are planned to be offered on additional platforms, as Android and Windows Phone.
Plan of Operation
During 2013, GlassesOff intends to complete the development of the first commercial version of its consumer-oriented software applications for improving near vision sharpness, for the iOS platform and start marketing the product to end consumers via Apple’s App Store. During 2013, GlassesOff plans to launch marketing and public relations campaigns, aiming to increase the awareness to its product and recruit customers. Additionally, GlassesOff plans to participate in various conferences and conventions around the world to further expose its product and technology to professional audience and potentially form business partnerships.
| 22 |
In 2013, GlassesOff intends to begin designing its product offering for new platforms and expects to start the development project for a new platform, currently expected to be Android.
GlassesOff is not planning any major purchase or sale of equipment in 2013, but it expects that employee headcount will increase as GlassesOff prepares for the commercial launch of its first product.
Critical Accounting Estimates and Policies
The financial statements have been prepared in accordance with accounting principles generally accepted in the United States, which we refer to as GAAP. The significant accounting policies followed in the preparation of the financial statements, on a consistent basis are:
Use of estimates: The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. While management believes that such estimates are fair when considered in conjunction with the consolidated financial position and results of operations taken as a whole, actual results could differ from those estimates and such differences may be material to the financial statements.
Financial statements in U.S. dollars: The functional currency of GlassesOff is the U.S. dollar, as the U.S. dollar is the primary currency of the economic environment in which GlassesOff has operated and expects to continue to operate in the foreseeable future. The majority of Eyekon E.R.D. Ltd.’s operations are currently conducted in Israel, and most of the Israeli expenses are currently paid in new Israeli shekels, which we refer to as NIS; however, the subsidiary’s operations do not generate any positive cash flow to cover its expenses and is not able to exist without the parent company’s funding. Therefore the currency which is used in operating, financing and investing activities, including loans and equity transactions, is U.S. dollars.
Accordingly, the functional and reporting currency of GlassesOff is the U.S. dollar. Monetary accounts maintained in currencies other than the U.S. dollar are remeasured into U.S. dollars. All transaction gains and losses from the remeasurement of monetary balance sheet items are reflected in the statements of operations as financial income or expenses, as appropriate.
Principles of consolidation: The consolidated financial statements include the accounts of GlassesOff and its indirect, wholly owned subsidiary, Eyekon E.R.D. Ltd. Intercompany transactions and balances, have been eliminated upon consolidation.
Cash equivalents: For purposes of reporting within the statement of cash flows, GlassesOff considers all cash on hand, cash accounts not subject to withdrawal restrictions or penalties, and all highly liquid debt instruments purchased with a maturity of three months or less to be cash and cash equivalents.
Property and equipment: Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated by the straight-line method over the estimated useful lives of the assets. The annual depreciation rates are as follows:
| % | ||||
| Office furniture and equipment | 7-15 | |||
| Laboratory equipment | 7-15 | |||
| Computers and electronic equipment | 33 | |||
| Leasehold improvements | 10 | |||
Long lived assets: GlassesOff reviews the carrying value of its long-lived assets, including intangible assets subject to amortization, for impairment whenever events and circumstances indicate that the carrying value of the assets may not be recoverable. Recoverability of these assets is measured by comparing the carrying value of the assets to the undiscounted cash flows estimated to be generated by those assets over their remaining economic life. If the undiscounted cash flows are not sufficient to recover the carrying value of the assets, the assets are considered impaired. The impairment loss is measured by comparing the fair value of the assets to their carrying value.
| 23 |
Fair value is determined by either a quoted market price or a value determined by a discounted cash flow technique, whichever is more appropriate under the circumstances involved. No impairments were recognized from February 5, 2007 (inception date) through June 30, 2013.
Accounting for stock-based compensation: ASC 718 - “Compensation-stock Compensation”, which we refer to as “ASC 718”, requires companies to estimate the fair value of equity-based payment awards on the date of grant using an Option Pricing Model, or OPM. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in GlassesOff’s consolidated income statements.
GlassesOff estimates the fair value of stock options granted using the Black-Scholes Merton OPM. The OPM requires a number of assumptions, of which the most significant are the expected stock price volatility and the expected option term. There is no active external or internal market for GlassesOff’s common shares. Thus, it was not possible to estimate the expected volatility of GlassesOff’s share price in estimating fair value of options granted. Accordingly, as a substitute for such volatility, GlassesOff used the historical volatility of comparable companies in the industry. The expected term of options granted represents the period of time that options granted are expected to be outstanding, management uses a simplified method for estimating the expected term of options due to insufficient readily available historical exercise data. The risk-free interest rate is based on the yield rates of U.S. Government Treasury Bonds with an equivalent term. GlassesOff has historically not paid dividends and has no foreseeable plans to pay dividends.
GlassesOff applies ASC 505-50, “Equity Based Payments to Non Employees”, which we refer to as ASC 505-50”, with respect to options issued to non-employees.
Research and development costs: Research and development, or R&D, costs are expensed as they are incurred and consist of salaries, stock- based compensation benefits and other personnel related costs, fees paid to consultants, clinical trials and related clinical manufacturing costs, license and milestone fees, and facilities and overhead costs.
Income taxes: GlassesOff accounts for income taxes in accordance with the provisions of ASC 740-10, Income Taxes. ASC 740-10 requires companies to recognize deferred tax assets and liabilities based on the differences between financial reporting and tax bases of assets and liabilities. These differences are measured using the enacted tax rates and laws that are expected to be in effect when the temporary differences are expected to reverse. A valuation allowance is established against net deferred tax assets, if based on the weighted available evidence, it is more likely than not that all or a portion of the deferred tax assets will not be realized.
Concentrations of credit risk: Financial instruments that potentially subject GlassesOff and its subsidiaries to concentrations of credit risk consist principally of cash and cash equivalents. Cash and cash equivalents are invested in major banks in Israel and in the U.S. Such deposits in Israel and the U.S. are not insured. Management believes that the financial institutions that hold GlassesOff’s investments are financially sound and, accordingly, minimal credit risk exists with respect to these investments. GlassesOff has no off-balance-sheet concentration of credit risk such as foreign exchange contracts or any other hedging arrangements.
Fair value measurements: As defined in ASC 820-10, Fair Value Measurements and Disclosures, which we refer to as ASC 820-10, fair value is based on the price that would be received to sell an asset or pay to transfer a liability in an orderly transaction between market participants at the measurement date. In order to increase consistency and comparability in fair value measurements, ASC 820-10 establishes a fair value hierarchy that prioritizes observable and unobservable inputs used to measure fair value into three broad levels, which are described below:
Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs.
Level 2: Other inputs that are observable, directly or indirectly, such as quoted prices for similar assets and liabilities or market corroborated inputs.
| 24 |
Level 3: Unobservable inputs are used when little or no market data is available, which requires GlassesOff to develop its own assumptions about how market participants would value the assets or liabilities. The fair value hierarchy gives the lowest priority to Level 3 inputs. In determining fair value, GlassesOff utilizes valuation techniques in its assessment that maximize the use of observable inputs and minimize the use of unobservable inputs.
(Loss) per share: Basic and Diluted losses per share are presented in accordance with ASC 260-10 “Earnings per share”. Outstanding options and warrants have been excluded from the calculation of the diluted loss per share because all such securities are antidilutive.
Recent Accounting Pronouncements
On February 5, 2013, the FASB issued ASU No. 2013-02, Reporting of Amounts Reclassified Out of Accumulated Other Comprehensive Income, which adds additional disclosure requirements relating to the reclassification of items out of accumulated other comprehensive income. This ASU is effective for the first quarter of 2013 and affects disclosures only; it is not expected to have a material impact on GlassesOff’s consolidated results of operation and financial condition.
In December 2011, the FASB issued Accounting Standards Update No. 2011-11, “Disclosures about Offsetting Assets and Liabilities”, which we refer to as ASU 2011-11. The objective of ASU 2011-11 is to enhance disclosures by requiring improved information about financial instruments and derivative instruments in relation to netting arrangements. ASU 2011-11 is effective for interim and annual periods beginning on or after January 1, 2013. GlassesOff is currently evaluating the impact of this guidance; however, since this update affects disclosures only, it is not expected to have a material impact on GlassesOff’s consolidated financial statements.
There were various other updates recently issued. None of the updates are expected to a have a material impact on GlassesOff’s financial position, results of operations or cash flows.
Results of Operations
Six Months Ended June 30, 2013 Compared to the Six Months Ended June 30, 2012
Revenue
GlassesOff has not generated any revenue since its inception on February 5, 2007. To date, GlassesOff has funded its operations through the sale of equity securities. If GlassesOff successfully commercializes its products, then it could generate revenue from sales of its products.
Revenue, if ever generated, will be recognized in accordance with ASC 605-10-S99, “Revenue Recognition”. GlassesOff will recognize revenue when the significant risks and rewards of ownership have been transferred to the customer pursuant to applicable laws and regulations, including factors such as when there has been evidence of a sales arrangement, the performance has occurred, or service have been rendered, the price to the buyer is fixed or determinable, and collectability is reasonably assured.
Research and Development Expenses
GlassesOff expects its research and development expenses to increase as it continues to develop its products. Research and development expenses consist of:
| · | internal costs associated with research and development activities; |
| · | personnel-related expenses, including salaries and stock-based compensation expenses, benefits, travel, and related costs for the personnel involved in the research and development; |
| · | internal and external costs associated with scientific studies, to include payment to investigators and labs participating in the studies (potentially acquiring equipment required for such studies) and payment to subjects for participating in the subject; |
| · | outsource services associated with research and development activities; and |
| · | facilities and other expenses, which include expenses for rent and maintenance of facilities. |
| 25 |
GlassesOff expects its research and development expenditures to increase most significantly in the near future in connection with the development efforts for new devices, new operating systems and potentially new product applications. GlassesOff intends to continue to hire new employees in research and development in order to meet its operation goals and expedite the development of new product versions for the iOS platform, introduce a new product version for a new platform and potentially start working on new products. GlassesOff believes that significant investment in product development is a competitive necessity and plans to continue these investments in an effort to realize the potential of its product. For the six months ended June 30, 2013 and 2012 and for the period from February 5, 2007 (inception date) through June 30, 2013, GlassesOff incurred research and development expenses in the aggregate of $690,000, $1,683,000 and $5,633,000, respectively. The decrease in research and development expenses for the six month period ended June 30, 2013 as compared to the 2012 period was primarily due to stock options compensation expenses of $175,000 in the 2013 period as compared to $1,169,000 in 2012.
The successful development of GlassesOff’s products is subject to numerous risks, uncertainties, and other factors. Beyond the next twelve months, GlassesOff cannot reasonably estimate or know the nature, timing and costs of the efforts that will be necessary to complete the remainder of the development of, or the period, if any, in which material net cash inflows may commence from GlassesOff’s product and development efforts. This is due to the numerous risks and uncertainties associated with the launch of its first product and the lack of operational experience associated with it, GlassesOff’s dependency on a single product and a single distribution channel, as well as potential future regulatory requirements.
GlassesOff’s expenditures are subject to additional uncertainties, including the expense of filing, prosecuting, defending and enforcing any patent claims or other intellectual property rights.
Sales and Marketing Expenses
Sales and marketing expenses consist primarily of salaries and other related costs for employees of GlassesOff and external service providers for services, such as search engine optimization and public relations services. For the six month periods ended June 30, 2013 and 2012 and for the period from February 5, 2007 (inception date) through June 30, 2013, GlassesOff incurred sales and marketing expenses of $80,000, $0 and $80,000, respectively. The increase for the six month period ended June 30, 2013 as compared to the 2012 period resulted primarily from GlassesOff’s intent to market its products.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and other related costs, including stock-based compensation expenses, for persons serving in GlassesOff’s executive and administration functions. Other general and administrative expenses includes facility-related costs not otherwise included in research and development expenses, and professional fees for legal and accounting services, including those associated with reporting obligations applicable to public companies in the United States. GlassesOff expects that its general and administrative expenses will increase as it adds additional personnel. For the six month periods ended June 30, 2013 and 2012 and for the period from February 5, 2007 (inception date) through June 30, 2013, GlassesOff incurred general and administrative expenses of $428,000, $859,000 and $2,136,000, respectively. The decrease for the six month period ended June 30, 2013 as compared to the 2012 period resulted primarily due to stock based compensation on options granted to employees and directors of $215,000 in 2013 as compared to $722,000 in 2012.
Financial Expenses and Income
Financial expenses and income consists of the following:
| · | interest earned on the GlassesOff’s cash and cash equivalents; |
| · | bank fees and commissions; and |
| · | expenses or income resulting from fluctuations of the New Israeli Shekel, in which a portion of GlassesOff’s assets and liabilities is denominated, against the U.S. Dollar. |
For the six month periods ended June 30, 2013 and 2012 and for the period from February 5, 2007 (inception date) through June 30, 2013, GlassesOff incurred net financial (expense) income of $1,000, $16,000 and $(43,000), respectively. Financial income for the six month period ended June 30, 2013 decreased as compared to the 2012 period primarily due to currency fluctuations of the New Israeli Shekel.
| 26 |
Stock-based Compensation
GlassesOff’s stock-based compensation expenses are recorded according to ASC 718-10, “Compensation - Stock Compensation”, which requires the measurement and recognition of compensation expense for all stock-based payment awards made to employees and directors, including employee stock options under GlassesOff’s stock plans, based on estimated fair values.
ASC 718-10 requires companies to estimate the fair value of equity-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods in GlassesOff’s consolidated statement of operations.
GlassesOff applies ASC 505-50, “Equity Based Payments to Non Employees”, with respect to options issued to non-employees.
GlassesOff estimates the fair value of stock options granted using the Black-Scholes-Merton option-pricing model. For the six month periods ended June 30, 2013 and 2012 and for the period from February 5, 2007 (inception date) through June 30, 2013, GlassesOff’s stock-based compensation expenses were $390,000, $1,891,000 and $2,635,000, respectively. Stock-based compensation expenses for the six months ended June 30, 2013 decreased as compared to the 2012 period primarily due to 163,948 stock options granted to employees and non-employees in 2013, compared to 7,038,684 stock options granted to employees and non-employees in 2012.
Year Ended December 31, 2012 Compared to Year Ended December 31, 2011
Revenue
GlassesOff has not generated any revenue since its inception on February 5, 2007.
Research and Development Expenses
For the years ended December 31, 2012 and 2011 and for the period from February 5, 2007 (inception date) through December 31, 2012, GlassesOff incurred research and development expenses in the aggregate of $2,249,000, $1,035,000 and $4,943,000, respectively. The increase in research and development expenses in 2012 as compared to 2011 was primarily due to stock options granted to employees and directors and stock-based compensation to service providers of $1,241,000 in 2012, as compared to $30,000 in 2011.
General and Administrative Expenses
For the years ended December 31, 2012 and 2011 and for the period from February 5, 2007 (inception date) through December 31, 2012, GlassesOff incurred general and administrative expenses of $1,238,000, $284,000 and $1,708,000, respectively. The increase in 2012 as compared to 2011 resulted primarily from stock options granted to employees and directors of $751,000, marketing expenses of $68,000 and professional services of $125,000, as compared to $0, $0 and $66,000 in 2011, respectively.
Financial Expenses and Income
For the years ended December 31, 2012 and 2011 and for the period from February 5, 2007 (inception date) through December 31, 2012, GlassesOff recorded gross financial (expenses) income of $14,000, ($25,000) and ($44,000), respectively. The decrease in expenses for 2012 as compared to 2011 was primarily due to income of $16,000 resulting from fluctuations of the New Israeli Shekel as compared to expenses of ($23,000) in 2011.
Stock-based Compensation
For the years ended December 31, 2012 and 2011 and for the period from February 5, 2007 (inception date) through December 31, 2012, GlassesOff’s stock-based compensation expenses were $1,992,000, $30,000 and $2,245,000, respectively. The increase in expenses for 2012 as compared to 2011 resulted primarily due to 4,983,424 stock options granted to employees and 2,822,627 stock options granted to services providers during 2012 as compared to 0 and 111,130 granted in 2011, respectively.
| 27 |
Cash Flows
Six Months Ended June 30, 2013 Compared to the Six Months Ended June 30, 2012
For the six months ended June 30, 2013 and 2012 and for the period from February 5, 2007 (inception date) through June 30, 2013, net cash used in operations was $654,000, $650,000 and $4,727,000, respectively. Cash was used primarily for salaries, subcontractors and other software development expenses.
For the six months ended June 30, 2013 and 2012 and for the period from February 5, 2007 (inception date) through June 30, 2013, net cash (used in) provided by investing activities was $(11,000), $3,000 and $(144,000), respectively. The decrease in cash used in investing activities for the six month period ended June 30, 2013 as compared to 2012 period resulted primarily from the purchase of property and equipment in 2013 and the release of GlassesOff’s severance pay fund in 2012 in connection with a termination of employment.
For the six month periods ended June 30, 2013 and 2012 and for the period from February 5, 2007 (inception date) through June 30, 2013, net cash provided by financing activities was $0, $801,000 and $4,985,000, respectively. The decrease in cash provided by financing activities for the 2013 period as compared to the 2012 period resulted primarily from GlassesOff’s issuance and sale of no shares of preferred stock during the 2013 period as compared to the issuance and sale of 1,227,786 shares of preferred stock during 2012.
Year Ended December 31, 2012 Compared to Year Ended December 31, 2011
For the years ended December 31, 2012 and 2011 and for the period from February 5, 2007 (inception date) through December 31, 2012, net cash used in operations was $1,294,000, $1,328,000 and $4,073,000, respectively. Cash was used primarily for salaries, subcontractors and other software development expenses.
For the years ended December 31, 2012 and 2011 and for the period from February 5, 2007 (inception date) through December 31, 2012, net cash (used in) provided by investing activities was $2,000, ($39,000) and ($133,000), respectively. The decrease in cash used in investing activities for 2012 compared to 2011 resulted primarily from the purchase of property and equipment of $2,000 during 2012 as compared to the purchase of property and equipment of $33,000 in 2011.
For the years ended December 31, 2012 and 2011 and for the period from February 5, 2007 (inception date) through December 31, 2012, net cash provided by financing activities was $1,635,000, $680,000 and $4,985,000, respectively. The increase in cash provided by financing activities for 2012 as compared to 2011 resulted primarily from GlassesOff’s issuance and sale of 2,516,962 shares of preferred stock during 2012 as compared to the issuance and sale of 1,213,084 shares of preferred stock during 2011.
Liquidity and Capital Resources
GlassesOff expects to incur losses from operations for the foreseeable future. GlassesOff expects to incur increasing research and development expenses, including expenses related to the hiring of personnel and outsource services. GlassesOff expects that general and administrative expenses will also increase as it expands its finance and administrative staff and adds infrastructure. GlassesOff also expects that sales & marketing expenses will increase significantly in connection with its anticipated product launch. GlassesOff’s future capital requirements will depend on a number of factors, including the continued progress of its research and development of the current product and potential products and the total average cost of customer acquisition comprising of initial acquisition cost and customer retention cost.
| 28 |
GlassesOff believes that its existing cash and cash equivalents will be sufficient to enable it to fund operating expenses and capital expenditure requirements at least until June 30, 2014. GlassesOff’s future capital requirements will depend on many factors, including investment in developing new product versions for new territories (localization), new devices and new platforms, and potentially development of new product candidates. Furthermore, the ramp-up in sales and marketing activities will require significantly higher resources. GlassesOff does not anticipate that it will generate significant product revenues during 2013. In the absence of additional funding, GlassesOff expects continuing operating losses to result in increases in cash used in operations over the next 12 months. To the extent that GlassesOff’s capital resources are insufficient to meet its future capital requirements, GlassesOff will need to finance its future cash needs through public or private equity offerings, debt financings, or corporate collaboration and licensing arrangements. GlassesOff currently does not have any commitments for future external funding. GlassesOff may need to raise additional funds more quickly if one or more of its assumptions proves to be incorrect or if it chooses to expand product development efforts more rapidly than presently anticipated, and GlassesOff may decide to raise additional funds even before its needs such funds if the conditions for raising capital are favorable. GlassesOff may seek to issue equity or debt securities or obtain a credit facility from one or more financial institutions. The sale of equity or convertible debt securities may result in dilution to GlassesOff’s stockholders. The incurrence of indebtedness would result in increased fixed obligations and could also subject GlassesOff to covenants that restrict its operations. Additional equity or debt financing, or corporate collaboration and licensing arrangements may not be available on acceptable terms, or at all. If adequate funds are not available, GlassesOff may be required to delay, reduce the scope of or eliminate its research and development programs, reduce its planned commercialization efforts or obtain funds through arrangements with collaborators or others that may require it to relinquish rights to certain product candidates that it might otherwise seek to develop or commercialize independently.
Effects of Inflation and Currency Fluctuations
Inflation generally affects GlassesOff by increasing its cost of labor and other development costs. GlassesOff does not believe that inflation has had a material effect on its results of operations for the six month periods ended June 30, 2013 or 2012 or for the years ended December 31, 2012 or 2011, nor does it expect that inflation will have a material impact on its results of operations for the year ending December 31, 2013.
Currency fluctuations may affect GlassesOff by increasing or decreasing costs. Currency fluctuations had no material effect on its results of operations for the six month periods ended June 30, 2013 or 2012 or for the years ended December 31, 2012 or 2011. GlassesOff does not purchase forward contracts to secure foreign exchange potential profits and losses.
Off-Balance Sheet Arrangements
GlassesOff has no off-balance sheet arrangements that have had or are reasonably likely to have a current or future effect on its financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
| 29 |
BUSINESS
Except where the context otherwise requires, the terms, “we,” “us,” “our” or “GlassesOff” refer to the business of GlassesOff Inc. and its consolidated wholly owned subsidiaries, Ucansi Inc. and EYEKON E.R.D. LTD, which comprise our operating subsidiaries and all of our operations as of the date of this Registration Statement on Form S-1.
GlassesOff
Ucansi Inc., which we refer to as Ucansi, was originally incorporated on February 5, 2007, under the laws of the state of Delaware, under the name Eyekon Inc. and, in April 2008, Ucansi changed its name to Ucansi Inc. In July 2007, GlassesOff formed EYEKON BLUE WHITE LTD., a wholly owned Israeli subsidiary focusing on development and commercialization of next-generation software applications for reading improvement based on GlassesOff’s proprietary intellectual property. In August 2007, EYEKON BLUE WHITE LTD. changed its name to EYEKON E.R.D. LTD.
Overview
GlassesOff is a development stage healthcare software technology company, utilizing patented technology to develop and commercialize consumer-oriented software applications for improving near vision sharpness, by improving the image processing function in the visual cortex of the brain. GlassesOff expects to deliver its products through a cloud-based client server architecture to hand-held devices, currently implemented on the Apple iOS platform (iPhone, iPod, iPad), and which are scheduled to be offered on Android and Windows Phone platforms.
Industry Background and Target Markets
As we age, we normally experience changes in reading abilities. These changes are influenced, among other factors, by our brain’s visual processing capabilities. The effect of these changes on most people results in slower, more difficult and less effective reading. According to a scientific review (Holden, B. A. et al., 2008), more than one billion people worldwide suffer from such age-related changes and this number is expected to rise significantly through 2050. At some point, natural age-related changes in reading abilities affect most people, who typically attempt to improve their reading abilities through the aid of magnifying devices, typically reading glasses. Such devices increase the size of words in reading material, making it easier for a person who uses them, whether that person is in his or her twenties or fifties, to read easily and quickly.
The GlassesOff product enhances the image processing capabilities of the brain and thus improves a person’s reading abilities. The use of the GlassesOff product results in faster, more efficient and more comfortable reading, thereby correcting reading vision without use of reading glasses.
GlassesOff’s Technology
Human vision is limited by two main factors: (a) the quality of an image captured by the eyes; and (b) the image processing capabilities of the brain as it interprets an image captured by the eyes.
| 30 |
GlassesOff’s proprietary technology enables it to develop pure software product solutions that enhance the image processing abilities of the brain and therefore improve a person’s near vision sharpness and reading capabilities. GlassesOff’s unique, pure software approach provides an alternative to existing solutions, which generally rely on magnifying devices, typically reading glasses. GlassesOff’s technology platform is based on advanced scientific research of image processing functionality. GlassesOff’s solution utilizes the remarkable ability of the brain’s plasticity (the brain’s ability to change), which constitutes the neuronal basis for “perceptual learning”, that is, repeated practice on a demanding visual task. GlassesOff’s founder’s academic research into the area of image processing functions during the last 20 years has yielded a breakthrough in perceptual learning methodologies, which enhance visual skills by improving image processing speed and efficiency in the visual cortex of the brain without altering optical functions. The use of perceptual learning methodologies improves both the processing speed and the sensitivity through repetitive exercise of the brain’s image processing function, resulting in improved near vision sharpness and improved reading capabilities.
GlassesOff’s technology and methods are based on scientific research and achievements that have been published in leading scientific publications, such as Nature, Science, PNAS, Vision Research, Scientific Reports and others. GlassesOff’s first product application, GlassesOff™, which is designed to improve near vision sharpness and reading capabilities, has been tested in several studies, including a recent study conducted at the University Of California, Berkeley, whose results have been published in Nature’s Scientific Reports in February 2012.
The GlassesOff™ Application
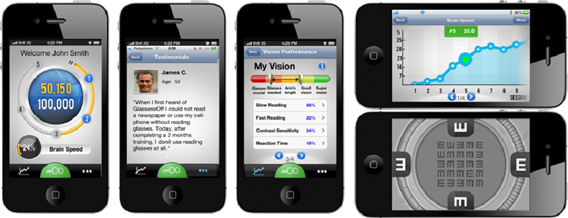
For those persons who experience the natural age-related changes in reading abilities, GlassesOff™, is designed to exercise the visual cortex of the brain to achieve comfortable reading without the use of magnifying devices such as reading glasses. According to a scientific review (Holden, B. A. et al., 2008), the target market for natural age-related changes in reading abilities is estimated to include one billion people worldwide, which is the market targeted by GlassesOff™. GlassesOff™ is a pure software visual cortex exercise solution to improve users’ reading abilities and maintain the ability to read comfortably, through enhancement of their image processing capabilities and near vision sharpness. The GlassesOff™ program will be delivered through cloud-based client server architecture to hand-held devices and is currently implemented on the Apple iOS platform, including iPhone, iPod and iPad. GlassesOff expects that users will initially download the application via Apple’s App Store, while their improved reading abilities program will be administrated through dedicated GlassesOff’s servers.
| 31 |
GlassesOff™ is a personalized application that delivers GlassesOff’s solution and monitors user performance and progress. The application automatically adjusts improved reading sessions based on each user’s ongoing progress.
The GlassesOff™ application is composed of two components:
| 1) | Basic Improvement Program – intensive visual stimulation tasks, composed of evaluations of reading abilities and approximately three 12-minute sessions per week over a period of approximately three months. At the end of this Basic Improvement Program, users who successfully pass an initial online evaluation of reading abilities are expected to achieve image processing capabilities that do not require the use of magnifying devices, such as reading glasses. |
| 2) | Ongoing Care – as needed ongoing maintenance of reading abilities, under which a user is prompted to use the product once every few weeks, according to that user’s then current state of reading abilities, in order for the person to maintain his or her improved brain processing state so he or she would not be required to use magnifying devices, such as reading glasses, for many years. |
GlassesOff™ Main Features
Simplicity. To facilitate its easy adoption, GlassesOff’s product does not require any third-party intervention, such as an optometrist or ophthalmologist examination. The product independently assesses each user’s reading abilities and compatibility with the program.
Ease of Use. GlassesOff™ product offers a user-friendly self-explanatory user interface, integrating interactive tutorials that allow users with no special technological or scientific background to complete the training program and follow their personal progress via easy to read charts.
Platform Compatibility. GlassesOff™ can be used on practically any device with a high quality display, including smart phones, tablets and personal computers. GlassesOff is currently developing its solution on Apple’s iOS platform (iPhone, iPad and iPod), and plans to offer its solution on a wide range of additional devices, including those running Android and Windows Phone operating systems. GlassesOff’s client-server architecture allows users to seamlessly migrate between devices while keeping their progress history and personal data.
Compliance. Working under a client-server architecture, each session a user performs is logged and stored in GlassesOff’s system, allowing GlassesOff to closely monitor the progress of its users and include automatic protocols that assist them throughout the reading improvement program, including such protocols as session reminders and personal improvement tips.
| 32 |
Product Architecture
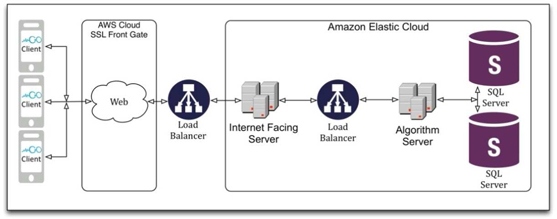
The client-server cloud-based architecture provides several key advantages for the security, scalability and redundancy of the infrastructure.
Client Security. GlassesOff™ is a “thin client”, meaning all algorithm and reading improvement logics reside on the server side. At the end of each session, the customer receives his or her personalized reading improvement session data from the server for the next session. All communications between the GlassesOff™ server and the customer are through an encrypted channel. Moreover, all temporary training data that are stored on the users’ client hard drive are also encrypted.
Server Scalability and Redundancy. All of GlassesOff’s web services are hosted on the Amazon EC2 cloud, which is a web service that provides resizable compute capacity in the cloud. GlassesOff’s current architecture allows it to further scale out (add more servers) and/or scale up (add more capacity to an existing server) within minutes. GlassesOff’s current architecture has been tested successfully under load and stress storms and was able to serve more than 500,000 requests over a period of five minutes, equivalent to more than ten million users. GlassesOff’s load balancer layers enable updates and maintenance without any downtime and all its data is backed up and mirrored between two SQL servers.
Server Security. GlassesOff™ web services on Amazon EC2 have gone through a hardening process to enhance their security according to known practices, and all algorithm and data servers are isolated from the internet. For example, only “sanitized” requests (those that comply with GlassesOff’s predefined request methodology) received by GlassesOff’s internet facing servers are delivered to GlassesOff’s algorithm and data servers, with all other connections logged and ignored.
Application Pipeline
GlassesOff plans to expand its product offerings to include additional suites of applications that would cater to other market segments related to enhancement of visual processing. Specifically, GlassesOff intends to develop additional applications for:
| · | Improvement of reading abilities for dyslexic children and adults; |
| · | Improvement of reading abilities for children and adults with attention deficit disorder; |
| · | Improvement of reading speed for children and adults; and |
| · | Sports packages for children and adults, designed to improve reaction time to visual stimulation, such as a baseball pitch, tennis and martial arts. |
| 33 |
Revenue Model
GlassesOff estimates that lifetime revenue per user will equal approximately $100 to $200. GlassesOff intends to offer various pricing schemes for both the Basic Improvement Program and Ongoing Care components. As with most subscription-based services, we intend to offer different subscription packages for the Ongoing Care component, such as an annual license and an unlimited Ongoing Care license. Following the initial launch of GlassesOff™, we intend to apply optimization techniques to determine the most effective pricing scheme.
As GlassesOff intends to offer a pure software solution its primary variable costs are customer acquisition and payment processing:
Customer Acquisition. GlassesOff expects that its customer acquisition costs will initially be significant, as it identifies the most effective marketing channels, following which it anticipates that such costs will decline as more people learn about its products through word-of-mouth advertising by existing users.
Payment Processing. Under the current policy of Apple’s App Store, which GlassesOff expects will be its first sales channel, a commission of 30% is charged per transaction. GlassesOff expects that its average per transaction fee will decline if it expands its sales channels to include other platforms, including those that use generic payment solutions, such as credit cards and PayPal, for which lower transaction fees (some as low as three percent) apply.
Sales, Marketing & Customer Support
Sales & Marketing
GlassesOff plans to initially offer GlassesOff™ directly to end users via Apple’s App Store, followed by other app stores, beginning with Android’s Google Play.
As GlassesOff believes the core target audience to initially adopt the GlassesOff™ solution consists of people between the ages of 40 to 60, GlassesOff intends to focus its marketing efforts on the most efficient marketing channels to reach this population, and intends to constantly monitor the efficacy of each marketing channel, to the best of its ability, to constantly optimize its marketing efforts and resources.
GlassesOff expects that its main marketing efforts will include the following:
| · | Public Relations: GlassesOff plans to generate both international and local media coverage for its GlassesOff™ application, including through media articles and interviews. To date GlassesOff has been approached by representatives from several international media companies that expressed interest in testing its product and writing an article about it. GlassesOff’s proactive efforts, supported by local PR agencies in key target markets, will target media channels and publications that target audiences between the ages of 40 to 60 or that focus on health and lifestyle issues. |
| · | Online Marketing: GlassesOff plans to utilize online campaigns that will direct potential customers to its application. GlassesOff has been investing in search engine optimization (SEO) aiming to achieve top ranks in Google for key search terms it deemed most appropriate for its marketing campaigns. |
| · | Social marketing: GlassesOff believes that its first application will generate strong viral distribution, or word-of–mouth, among its users, most of whom are expected to be within the same age group (40-60 years old) and, therefore, are most likely to go through the same natural age-related changes of near visual abilities. GlassesOff has integrated several social functions in the application in order to leverage the viral marketing potential, including: |
| o | Bring-A-Friend Campaign – GlassesOff created a personal invitation tool, encouraging users to send personal invitations to their friends via their list of friends on Facebook or their mobile device’s address book directly from the application. |
| o | Guest Reading Evaluation – GlassesOff also plans to offer a short online evaluation of reading abilities that users can share with their friends. This real-time evaluation tool quickly assesses the compatibility of potential users to the GlassesOff visual cortex training and generates download links for installing its application. |
| 34 |
| o | Post/Share Tool – An easy-to-use tool that allows users to share their experiences with their friends via their Facebook and Twitter accounts. |
| o | Partnerships and Affiliate Marketing – Once GlassesOff obtains a significant user base and will be able to provide statistically significant user acquisition and usage data, it plans to seek partnerships that will allow it to quickly grow its users customer base. |
GlassesOff anticipates that, following a short soft launch, the English version of its product will be launch targeting English-speaking countries (USA, Canada, UK, and Australia). This launch will be followed by additional localized versions for other major markets, based on market research prior to each launch. The main potential languages that GlassesOff currently considers include: Japanese, Chinese and Spanish.
Customer Support
To ensure customer satisfaction, GlassesOff’s planned customer support efforts include both proactive and responsive models.
| · | Proactive Model: GlassesOff has developed an event driven automatic communication plan that includes email messages, personal in-app notification screens, push notifications and interactive tutorials that are presented to each user according to that user’s particular training stages and personal progress. |
| · | Responsive Model: GlassesOff plans to offer online support via email, either directly from its application or through customer support representatives. |
Intellectual Property
GlassesOff has filed several patent applications to protect its core technology platform and products. GlassesOff has issued patents protecting its core technology platform and products in the United States (US 7,866,817 and US 8,403,485), Australia (AU 2005278771), Korea (KO 10-1016429), Japan (JP 5222556), Canada (CA 2578932) and Israel (IL 181660). GlassesOff’s pending patent applications are still in different evaluation phases in Europe, China and India.
GlassesOff has also filed a patent application for its vision evaluation method, which was recently issued by the United States Patent and Trademark Office (US 8,403,485).
In addition to GlassesOff’s patent estate, GlassesOff has entered into an intellectual property license agreement with RevitalVision LLC, a Kansas limited liability company, which is the owner of several patents in the area of perceptual learning systems and methods. Under this agreement, referred to as the “License Agreement”, GlassesOff has a non-exclusive license to certain patents for the field of near-vision solutions. The License Agreement does not assign any technology to GlassesOff, and, unless earlier terminated, the License Agreement terminates upon the expiration of the last of RevitalVision’s patents, which is expected to be in 2024. Under the License Agreement, GlassesOff is required to pay an initial fee of $75,000 in installments over a period of 12- 24 months following the effective date of the License Agreement (based on sales targets), of which $50,000 has been paid to date. In addition, GlassesOff is required to pay quarterly royalty payments of 5.5% of net sales up to aggregate net sales of $3 million and of 4.5% of net sales over $3 million (calculated annually).
Competition
GlassesOff’s product will compete in the market of solutions for better, faster and more effective reading. The natural changes in reading capabilities with age have typically been addressed by traditional products for improvement of reading capabilities, mainly magnification devices, such as reading glasses or contact lenses that support reading. Manufactures of such magnifier devices could therefore be considered as competition. GlassesOff believes that its product, which eliminates the constant dependency on magnifiers, is therefore highly competitive with such devices.
| 35 |
In addition, there are several methods and products that claim to improve reading vision via various means, usually related to muscle exercises (e.g. the Bates method, the See Clearly Method, the Power Vision Program etc.). These methods and products typically attempt to correct the eye functionality through muscle exercises, whereas the GlassesOff solution does not involve any such corrective methods.
Finally, there are several competitors offering vision improvement solutions based on neuroscience methods, such as PositScience and RevitalVision (which obtained FDA clearance for a product that treats amblyopia, a condition typically referred to as “Lazy Eye”.) GlassesOff believes that there is no other software solution for the improvement of reading capabilities to the extent that magnifying devices, such as reading glasses, are not required that has also demonstrated efficacy in controlled studies.
GlassesOff believes that its technology platform and unique intellectual property assets present a high barrier to entry for others who would try to develop and sell software products for improvement of near vision sharpness and reading capabilities using hand-held devices.
Employees
GlassesOff currently has one part-time employee in the United States and eighteen employees in Israel, of whom eight are full time employees, and ten are part time employees (including three part time employees with Ph.D. degrees, two of whom are certified optometrists, one part time employee with an MSc degree in neuroscience, and two additional part time employees who are also certified optometrists). All four neuroscientists focus on research and development. None of GlassesOff’s employees is represented by a labor union, and GlassesOff considers its employee relations to be good. GlassesOff also utilizes a number of consultants to assist with research and development and commercialization activities.
Immediately prior to the expected commercial launch of GlassesOff’s product, GlassesOff intends to recruit additional personnel to focus on marketing, customer support and tech support.
Properties
Our principal corporate office is located at 35 Jabotinski St., POB 126, Ramat Gan 52511, Israel.
GlassesOff leases approximately 1,600 square feet of office space in Ramat Gan, Israel. GlassesOff pays monthly rental and management fees of approximately $3,200, plus approximately $500 per month for taxes and utilities. GlassesOff’s facility lease will expire on March 2014.
Due to the expected commercial launch of GlassesOff’s product and an expected increase in the number of employees, GlassesOff intends to relocate to a new leased office.
Legal Proceedings
We are not a party to any legal proceedings nor is any of our property subject to any legal proceedings.
| 36 |
SECURITY
OWNERSHIP OF
CERTAIN BENEFICIAL OWNERS, DIRECTORS AND EXECUTIVE OFFICERS
As of October 25, 2013, the following table sets forth information regarding the beneficial ownership of our Common Stock by:
| · | each of our named executive officers; |
| · | each of our directors; |
| · | all of our directors and named executive officers as a group; and |
| · | each person who is known by us to beneficially own more than 5% of our Common Stock. |
Beneficial ownership is determined in accordance with the rules of the SEC. Except as indicated by footnote and subject to community property laws, where applicable, to our knowledge the persons named in the table below have sole voting and investment power with respect to all shares of our Common Stock that are shown as beneficially owned by them. In computing the number of shares of Common Stock by a person and the percentage ownership of that person, any such shares subject to options and warrants held by that person that are exercisable as of October 25, 2013 or that will become exercisable within 60 days thereafter are deemed outstanding for purposes of that person’s percentage ownership but not deemed outstanding for purposes of computing the percentage ownership of any other person. Unless otherwise indicated, the mailing address of each individual is c/o GlassesOff Inc. 35 Jabotinski St., POB 126, Ramat Gan, Israel 52511. The following information is based upon information provided to us or filed with the SEC by the stockholders.
| Name and Address of | Number of Shares | Percentage of Outstanding | ||||||
| Directors and Named Executive Officers: | ||||||||
| Uri Polat, Chief Scientific Officer and Director | 15,976,841 | 30.0 | % | |||||
| Shai Novik, Chairman of the Board | 3,305,550 | (2) | 6.2 | % | ||||
| Ram Shaffir, Chief Technology Officer and Director | 6,678,188 | (3) | 11.9 | % | ||||
| Nimrod Madar, President, Chief Executive Officer and Director | 3,378,811 | (4) | 6.0 | % | ||||
| Steve Schaeffer, Chief Financial Officer | - | * | ||||||
| All Executive Officers and Directors as a Group (5 Persons) | 29,339,390 | 54.1 | % | |||||
| Certain Beneficial Owners: | ||||||||
| Odysseus Ventures LLP (5) | 4,101,253 | 7.7 | % | |||||
| IGWT Global Services LTD. (6) | 4,007,898 | 7.5 | % | |||||
| * | Less than 1% |
| (1) | Based on 53,290,000 shares are issued and outstanding as of October 25, 2013. |
| (2) | Includes options to purchase 25,000 shares. |
| (3) | Includes Warrants to purchase 400,000 shares and options to purchase 2,597,638 shares. |
| (4) | Includes options to purchase 3,178,811 shares. |
| 37 |
| (5) | The address of Odysseus Ventures LLP is 28 Harakevet Street, Tel Aviv, Israel 67770. Ram Shaffir and Shai Novik have shared voting and investment control over the shares held by Odysseus Ventures LLP. |
| (6) | The address of IGWT Global Services LTD. is Craigmuir Chambers, P.O. Box 71, Road Town, Tortola VG1110 BVI. Kretzmer & Associates PLLC has voting and investment control over the shares held by IGWT Global Services LTD. |
| 38 |
SELLING STOCKHOLDERS
The following table sets forth the name of each selling stockholder and the number of shares of Common Stock that each selling stockholder may offer pursuant to this prospectus. The shares of Common Stock offered by the selling stockholders hereunder were acquired by the selling stockholders in private placements in connection with the Merger, which closed on July 30, 2013. The shares of Common Stock offered by the selling stockholders hereunder consist of 43,290,030 currently outstanding shares of Common Stock and 10,013,504 shares of Common Stock issuable upon the exercise of Warrants and Parent Warrants. Except as otherwise indicated, we believe that each of the beneficial owners and selling stockholders listed below has sole voting and investment power with respect to such shares, subject to community property laws, where applicable. Unless otherwise noted, the address of each stockholder is c/o GlassesOff Inc., 35 Jabotinski St. POB 126, Ramat Gan 52511, Israel.
Except as noted in the table below, none of the selling stockholders has had a material relationship with us other than as a stockholder at any time within the past three years or has ever been one of our or our affiliates’ officers or directors. Each of the selling stockholders has acquired its shares of our Common Stock to be resold hereunder in the ordinary course of business and, at the time of acquisition, none of the selling stockholders was a party to any agreement or understanding, directly or indirectly, with any person to distribute the shares of our Common Stock to be resold by such selling stockholder under this registration statement.
Because a selling stockholder may sell all, some or none of the shares of Common Stock it holds, and because the offering contemplated by this prospectus is not underwritten, no estimate can be given as to the number of shares of Common Stock that will be held by a selling stockholder upon termination of the offering. The information set forth in the following table regarding the beneficial ownership after resale of shares is based upon the hypothetical assumption that the selling stockholders will sell all of the shares of Common Stock owned by it and covered by this prospectus.
In accordance with the rules and regulations of the SEC, in computing the number of shares of common stock (as applicable) beneficially owned by a person and the percentage ownership of that person, shares issuable through the exercise of any option, warrant or right, through conversion of any security held by that person that are currently exercisable or that are exercisable within 60 days are included. These shares are not, however, deemed outstanding for the purpose of computing the percentage ownership of any other person.
| 39 |
| Shares Owned Prior to the Offering | Number of Shares Offered | Shares Owned After the Offering | ||||||||||||||||||
| Name | Number | Percent (1) | Shares | Number | Percent (1) | |||||||||||||||
| A.G. Babilon Ltd. (2) | 41,600 | * | 41,600 | 0 | * | |||||||||||||||
| Adi Babayoff | 34,107 | * | 34,107 | 0 | * | |||||||||||||||
| Yehoshua Abramovich | 77,320 | * | 77,320 | 0 | * | |||||||||||||||
| Zohar Bochan | 113,689 | * | 113,689 | 0 | * | |||||||||||||||
| Blue Bell Investors S.A. | 341,066 | * | 341,066 | 0 | * | |||||||||||||||
| Blue Fountain Commercial Business Limited (3) | 1,040,000 | 1.9 | % | 1,040,000 | 0 | * | ||||||||||||||
| Jose I. Canto (4) | 100,000 | * | 100,000 | 0 | * | |||||||||||||||
| Sasson Darwish (5) | 615,735 | 1.1 | % | 615,735 | 0 | * | ||||||||||||||
| Rakefet Dor | 19,330 | * | 19,330 | 0 | * | |||||||||||||||
| Shai Dotan | 107,664 | * | 107,664 | 0 | * | |||||||||||||||
| Nir Elias | 38,660 | * | 38,660 | 0 | * | |||||||||||||||
| Udi Eyal Fima | 46,392 | * | 46,392 | 0 | * | |||||||||||||||
| GAIA Ltd. (6) | 84,800 | * | 84,800 | 0 | * | |||||||||||||||
| Shmuel Geizler | 61,856 | * | 61,856 | 0 | * | |||||||||||||||
| Rivnay Gershon | 430,653 | * | 430,653 | 0 | * | |||||||||||||||
| G.H. Doron Consulting LTD (7) | 145,792 | * | 145,792 | 0 | * | |||||||||||||||
| Mor Meir Gluska | 30,928 | * | 30,928 | 0 | * | |||||||||||||||
| Emanuel Goldstein | 38,660 | * | 38,660 | 0 | * | |||||||||||||||
| Marian Gorecki | 77,320 | * | 77,320 | 0 | * | |||||||||||||||
| Ido Greenberg (8) | 456,812 | * | 456,812 | 0 | * | |||||||||||||||
| Shimon Hakim | 46,392 | * | 46,392 | 0 | * | |||||||||||||||
| Hanoch Peer LTD (9) | 96,360 | * | 96,360 | 0 | * | |||||||||||||||
| Abraham & Nitza Havron | 115,980 | * | 115,980 | 0 | * | |||||||||||||||
| IGWT Global Services | 4,007,898 | 7.5 | % | 4,007,898 | 0 | * | ||||||||||||||
| Interactive Agents Ltd | 34,107 | * | 34,107 | 0 | * | |||||||||||||||
| Steven Kantor | 165,802 | * | 165,802 | 0 | * | |||||||||||||||
| Ram Katzir | 92,784 | * | 92,784 | 0 | * | |||||||||||||||
| Assaf Kedem | 38,660 | * | 38,660 | 0 | * | |||||||||||||||
| Kishony Dalia & Yoav | 77,320 | * | 77,320 | 0 | * | |||||||||||||||
| David Kopelman (10) | 45,302 | * | 45,302 | 0 | * | |||||||||||||||
| Marion Kopelman (11) | 239,218 | * | 239,218 | 0 | * | |||||||||||||||
| David Kretzmer (12) | 78,660 | * | 78,660 | 0 | * | |||||||||||||||
| Israel Kuperman (13) | 194,287 | * | 194,287 | 0 | * | |||||||||||||||
| Eran Lasser (14) | 530,674 | 1.0 | % | 530,674 | 0 | * | ||||||||||||||
| Harel Lek | 38,660 | * | 38,660 | 0 | * | |||||||||||||||
| Nimrod Madar (15) | 3,378,811 | 6.0 | % | 200,000 | 3,178,811 | 5.6 | % | |||||||||||||
| Magenta International Partners Ltd. (16) | 1,600,000 | 3.0 | % | 1,600,000 | 0 | * | ||||||||||||||
| Haim Malka (17) | 181,014 | * | 181,014 | 0 | * | |||||||||||||||
| Mayoran Ltd. (18) | 160,000 | * | 160,000 | 0 | * | |||||||||||||||
| Raviv Menashe (19) | 1,230,697 | 2.3 | % | 1,230,697 | 0 | * | ||||||||||||||
| Tamir Migdal | 19,330 | * | 19,330 | 0 | * | |||||||||||||||
| Josef Mor | 139,176 | * | 139,176 | 0 | * | |||||||||||||||
| MyForm LTD (20) | 244,239 | * | 244,239 | 0 | * | |||||||||||||||
| NEWCALL LTD. (21) | 491,843 | * | 491,843 | 0 | * | |||||||||||||||
| Shai Novik (22) | 3,280,551 | 6.2 | % | 3,280,551 | 0 | * | ||||||||||||||
| Odysseus Ventures LLP | 4,101,253 | 7.7 | % | 4,101,253 | 0 | * | ||||||||||||||
| Odyssey Investments and Holdings, Ltd. (23) | 480,000 | * | 480,000 | 0 | * | |||||||||||||||
| Omri Ofer (24) | 691,846 | 1.3 | % | 691,846 | 0 | * | ||||||||||||||
| Neta Partuk | 38,660 | * | 38,660 | 0 | * | |||||||||||||||
| Harel Peleg | 129,749 | * | 129,749 | 0 | * | |||||||||||||||
| Uri polat (25) | 15,976,841 | 30.0 | % | 15,976,841 | 0 | * | ||||||||||||||
| Poltech Engineering Ltd (26) | 1,796,178 | 3.3 | % | 1,796,178 | 0 | * | ||||||||||||||
| Robin Igal (27) | 279,214 | * | 279,214 | 0 | * | |||||||||||||||
| Romira Investments AG. | 139,963 | * | 139,963 | 0 | * | |||||||||||||||
| The Sanitas Family Trust (28) | 1,163,890 | 2.1 | % | 1,163,890 | 0 | * | ||||||||||||||
| The Sanitas Trust (29) | 2,542,759 | 4.7 | % | 2,542,759 | 0 | * | ||||||||||||||
| Ram Shaffir (30) | 6,672,189 | 11.9 | % | 4,080,551 | 2,597,638 | 4.6 | % | |||||||||||||
| Shelly Shaffir (31) | 297,600 | * | 297,600 | 0 | * | |||||||||||||||
| S.L.A.R Kadima LTD (32) | 193,600 | * | 193,600 | 0 | * | |||||||||||||||
| Thomas F. Whayne (33) | 80,000 | * | 80,000 | 0 | * | |||||||||||||||
| The White Picket Fence Trust (34) | 2,342,759 | 4.4 | % | 2,342,759 | 0 | * | ||||||||||||||
| Amos Yanai (35) | 284,678 | * | 284,678 | 0 | * | |||||||||||||||
| Shmuel Yanai (36) | 246,018 | * | 246,018 | 0 | * | |||||||||||||||
| Smadar Zilberberg | 992,249 | 1.9 | % | 992,249 | 0 | * | ||||||||||||||
| Pritch Zvi (37) | 194,388 | * | 194,388 | 0 | * | |||||||||||||||
| * | Less than one percent. |
| (1) | Based on 53,290,000 shares issued and outstanding as of October 25, 2013. |
| (2) | Includes Warrants to purchase 20,800 shares. |
| (3) | Includes Warrants to purchase 520,000 shares. |
| 40 |
| (4) | Includes Warrants to purchase 50,000 shares. |
| (5) | Includes Warrants to purchase 341,801 shares and Parent Warrants to purchase 80,000 shares. |
| (6) | Includes Warrants to purchase 42,400 shares. |
| (7) | Includes Parent Warrants to purchase 109,344 shares. |
| (8) | Includes Parent Warrants to purchase 342,609 shares. |
| (9) | Includes Parent Warrants to purchase 72,270 shares. |
| (10) | Includes Parent Warrants to purchase 33,976 shares. |
| (11) | Includes Parent Warrants to purchase 179,413 shares. |
| (12) | Includes Warrants to purchase 20,000 shares. |
| (13) | Includes Parent Warrants to purchase 145,715 shares. |
| (14) | Includes Parent Warrants to purchase 398,005 shares. |
| (15) | Includes 200,000 shares of our restricted stock and options to purchase 3,178,811 shares. Nimrod Madar currently serves as the Company’s President and Chief Executive Officer and as a director. |
| (16) | Includes Warrants to purchase 800,000 shares. |
| (17) | Includes Parent Warrants to purchase 135,760 shares. |
| (18) | Includes Warrants to purchase 80,000 shares. |
| (19) | Includes Parent Warrants to purchase 865,032 shares. |
| (20) | Includes Parent Warrants to purchase 183,179 shares. |
| (21) | Includes Parent Warrants to purchase 368,882 shares. |
| (22) | Includes 300,000 shares of our restricted stock. Shai Novik currently serves as the Company’s Chairman of the Board. |
| (23) | Includes Warrants to purchase 240,000 shares. |
| (24) | Includes Parent Warrants to purchase 518,884 shares. |
| (25) | Uri Polat currently serves as the Company’s Chief Scientific Officer and as a director. |
| (26) | Includes Parent Warrants to purchase 1,289,143 shares. |
| (27) | Includes Parent Warrants to purchase 209,410 shares. |
| (28) | Includes Parent Warrants to purchase 871,074 shares. Adv. David Kretzmer, as trustee, has voting and investment control over the shares held by the Sanitas Family Trust. |
| (29) | Includes Warrants to purchase 100,000 shares. Adv. David Kretzmer, as trustee, has voting and investment control over the shares held by the Sanitas Trust. |
| (30) | Includes 300,000 shares of our restricted stock, Warrants to purchase 400,000 shares and options to purchase 2,597,638 shares. The options to purchase 2,597,638 shares were issued to Ambrosia Invesyments Ltd., a corporation formed under the laws of the State of Israel and wholly owned by Ram Shaffir, over which Mr. Shaffir has voting and investment control. Mr. Shaffir currently serves as the Company’s Chief Technology Officer and as a director. |
| 41 |
| (31) | Includes Parent Warrants to purchase 200,004 shares. |
| (32) | Includes Warrants to purchase 96,800 shares. |
| (33) | Includes Warrants to purchase 40,000 shares. |
| (34) | Yaron Biranbum, as trustee, has voting and investment control over the shares held by the White Picket Fence Trust. |
| (35) | Includes Parent Warrants to purchase 184,513shares. |
| (36) | Includes Parent Warrants to purchase 184,513 shares. |
| (37) | Includes Parent Warrants to purchase 145,791 shares. |
MANAGEMENT
Directors and Executive Officers
The following table sets forth information concerning our executive officers and directors, including their ages.
|
Name |
Age |
Title | ||
| Shai Novik, M.B.A. | 47 | Chairman of the Board | ||
| Nimrod Madar, M.B.A. | 40 | President, Chief Executive Officer and Director | ||
| Uri Polat, Ph.D. | 60 | Chief Scientific Officer and Director | ||
| Ram Shaffir | 43 | Chief Technology Officer and Director | ||
| Steve Schaeffer, CPA | 62 | Chief Financial Officer |
Shai Novik, M.B.A, Chairman of the Board. Mr. Novik serves as President and a director of PROLOR Biotech, Inc., a biopharmaceutical company developing long-acting therapeutic proteins, which was recently acquired by OPKO Health, Inc. Mr. Novik previously served as Chief Operating Officer and Head of Strategic Planning of THCG, a technology and life sciences investment company. THCG was a portfolio company of Greenwich Street Partners, one of the largest U.S. private equity funds. THCG’s own portfolio included several life sciences and medical devices companies. Prior to his position at THCG, Mr. Novik served as Chief Operating Officer and Chairman of Strategy Committee of RogersCasey, an investment advisory company serving Fortune 500 companies such as DuPont, Kodak, General Electric and others.
Nimrod Madar, M.B.A, President, Chief Executive Officer and Director. Mr. Madar previously founded and served as Chairman at iMind Networks, a cognitive training venture specializing in assessing and training cognitive capabilities, such as working memory, attention, visual search, etc. Prior to his position at iMind Networks, Mr. Madar served as VP Strategic Alliances at 888 Holdings Public Limited Company traded on the London Stock Exchange, one of the world’s most popular online gaming entertainment companies with both PC and mobile offering for end users (B2C) as well as white label customers (B2B). Prior to his position at 888 Holdings, Mr. Madar served as a Managing Partner at POC Management Consulting, a management consulting firm consulting to leading corporations in the Israeli market, to include: financial institutes, communication companies, leading manufacturers and retailers.
Prof. Uri Polat Ph.D., Chief Scientific Officer and Director. Dr. Polat is the director of the Visual and Clinical Neuroscience Laboratory at the Eye Research Institute at the Sheba Medical Center, Faculty of Medicine, Tel-Aviv University. He received a Ph.D. in Brain Research from the Weizmann Institute of Science, Rehovot, Israel. He was at the Smith-Kettlewell Eye Research Institute, San-Francisco, USA for several years as a Fellow and Associate Scientist. Dr. Polat was the Founder and Chief Scientific Officer of NeuroVision Inc. and developed the first FDA approved training for adult Amblyopia. Dr. Polat is a member of the Association for Research in Vision and Ophthalmology (ARVO), the Visual Sciences Society, The Israel Society for Neuroscience, and the International Brain Organization. His research and scientific publications received international recognition and are highly influential in the field of neuronal interaction, brain plasticity and perceptual learning in improving visual functions.
| 42 |
Ram Shaffir, Chief Technology Officer and Director. Mr. Shaffir previously served as Chief Executive Officer of Odysseus-Ventures, a private investment company specializing in early stage life science companies, and currently serves as a director at Stentomics, one of Odysseus Ventures’ portfolio companies. Prior to his position at Odysseus-Ventures, Mr. Shaffir founded and managed VbaTech, a specialized software house providing software architecture and development services to leading financial institutes in Israel. In additional to his extensive investment activities over the last 10 years, Mr. Shaffir consulted many of Israel’s leading insurance companies in IT issues.
Steve Schaeffer, CPA, Chief Financial Officer. Mr. Schaeffer has more than 30 years of accounting and tax experience. His specialty is corporate tax including reorganizations, acquisitions and dispositions. In 1993, he co-founded Cohen & Schaeffer, P.C., a full service CPA firm. Prior to founding Cohen & Schaeffer, Mr. Schaeffer was a tax partner at BDO Seidman and a principal at Laventhol & Horwath heading up the mergers and acquisitions department. Before entering public accounting, he worked for ten years at the Internal Revenue Service. In addition, he taught graduate level tax courses and presented seminars in corporate tax.
Director Compensation
Members of our Board of Directors who are also officers do not receive separate compensation for their respective service on our Board of Directors. Mr. Novik receives annual compensation of $75,000 for his service as Chairman of our Board of Directors. We reimburse each director for reasonable travel expenses related to such director’s attendance at Board of Directors and committee meetings. On July 30, 2013, we granted Mr. Novik 300,000 shares of restricted Common Stock, which vest in substantially equal monthly installments over a period of 12 months from the date of grant. In addition, we granted Mr. Novik an option to purchase 150,000 shares of Common Stock at an exercise price equal to $1.25 per share, which option vests in substantially equal monthly installments over a period of 12 months from the date of grant.
We did not compensate any of our directors for the year ended December 31, 2012.
Executive Compensation
The following table sets forth the 2012 and 2011 compensation for Mr. Madar, our principal executive officer, and our two other most highly compensated executive officers, for the years ended December 31, 2011 and 2012.
| Name and Principal Position | Year | Salary ($) | Option Awards ($) | All Other Compensation ($) | Total ($) | |||||||||||||
| Shai Novik | 2012 | $ | - | $ | - | $ | - | $ | - | |||||||||
| Chairman of the Board of Directors | 2011 | - | - | - | - | |||||||||||||
| Nimrod Madar | 2012 | $ | 110,789 | $ | 739,274 | (1) | $ | 37,104 | (2) | $ | 887,167 | |||||||
| President and Chief Executive Officer | 2011 | 100,935 | - | 21,458 | (3) | 122,393 | ||||||||||||
| Uri Polat | 2012 | $ | 102,018 | $ | - | $ | - | $ | - | |||||||||
| Chief Scientific Officer | 2011 | 159,510 | - | - | - | |||||||||||||
| Ram Shaffir | 2012 | $ | 122,581 | $ | 769,393 | (1) | $ | - | $ | 891,974 | ||||||||
| Chief Technology Officer | 2011 | 80,888 | - | - | 80,888 | |||||||||||||
| 43 |
| (1) | Represents the dollar amount recognized for financial statement reporting purposes with respect to the fiscal year in accordance with FASB ASC Topic 718 for stock options granted to the executive. |
| (2) | Includes $ 19,046 in automobile expenses paid by us, including leasing costs, insurance premiums gasoline and/or repairs incurred in connection with the executive’s automobile, $ 8,863 in pension costs related to the executive’s retirement plan and $ 9,195 in contributions to our severance pay fund as required by Israeli law. |
| (3) | Includes $ 5,005 in automobile expenses paid by us, including leasing costs, insurance premiums gasoline and/or repairs incurred in connection with the executive’s automobile, $ 8,075 in pension costs related to the executive’s retirement plan and $ 8,378 in contributions to our severance pay fund as required by Israeli law. |
All officers, whether executive or non-executive, serve at the discretion of our Board of Directors.
We have entered into an Employment Agreement with Mr. Madar, which we refer to as the Madar Employment Agreement, a part-time Consulting Agreement with Dr. Polat, Ph.D., which we refer to as the Polat Consulting Agreement, and a Consulting Agreement with Mr. Shaffir, which we refer to as the Shaffir Consulting Agreement, and a part-time Consulting Agreement, with Cohen & Schaeffer LLP, which we refer to as the Schaeffer Consulting Agreement, pursuant to which Steve Schaeffer acts as our Chief Financial Officer. Collectively the foregoing employment and consulting agreements are referred to as the Officer Agreements. The Officer Agreements each have an initial one-year term, which term, except under the Schaeffer Consulting Agreement, shall be automatically extended for additional successive one-year terms on each one-year anniversary, unless either party gives the other party written notice, no less than 60 days prior to the end of the then-current term, of an election not to renew the Officer Agreement. Under the Schaeffer Consulting Agreement, the term may be extended for subsequent one-year terms upon mutual agreement of the parties. Pursuant to their Officer Agreements, Dr. Polat and Messrs. Madar, Shaffir and Schaeffer will receive annual base salaries or compensation, as the case may be, of $100,000, $180,000, $50,000 and $48,000, respectively, provided, that Mr. Shaffir’s base compensation will increase to $120,000 following our consummation of a financing round in excess of $6,000,000. In addition, each of Dr. Polat and Messrs. Madar and Shaffir are entitled to an annual cash bonus of up to 25% of his base salary or compensation, as the case may be, based on corporate and personal milestones together with equity performance awards, each as determined by the compensation committee of our Board of Directors. Upon execution of the Officer Agreements on July 30, 2013, we granted Dr. Polat and Messrs. Madar and Shaffir options to purchase 350,000, 150,000 and 50,000 shares of Common Stock, respectively, at an exercise price of $1.25 per share, which options will vest in substantially equal amounts on the first, second and third anniversaries of the date of grant. In addition we granted Messrs. Madar and Shaffir 200,000 and 300,000 shares, respectively, of restricted Common Stock, vesting in substantially equal amounts on the first, second and third anniversaries of the date of grant.
Dr. Polat’s and Messrs. Madar’s and Shaffir’s Officer Agreements provide that if such person voluntarily terminates his employment or consultancy, as the case may be (other than in connection with a change of control of GlassesOff and certain other reasons), or we terminate his employment or consultancy, as the case may be, for “cause” (as defined in the agreement) then he will be entitled only to payment of his base salary or compensation, as the case may be, through the date of termination, and will not be entitled to any performance bonus for that year. However, if any such person terminates his Officer Agreement as the result of a material breach by us, he will be entitled to continued payment of his base salary for a period of six months following such termination, plus the value of any accrued benefits (if any), and all unvested options and shares of restricted Common Stock granted to him shall immediately vest and become exercisable. If we terminate any such person other than for cause or if the term expires and is not renewed by us, then, (i) in the case of Mr. Madar, he will be entitled to continued payment of his base salary or compensation, as the case may be, for a period of six months following such termination or non-renewal, and he would additionally receive the value of accrued benefits and a pro-rata portion of the current year’s performance bonus, and (ii) in the case of Dr. Polat and Mr. Shaffir, he will be entitled to receive an amount equal to his then-current base salary for a period of six months following such termination or non-renewal, and he would additionally receive the value of accrued benefits and a pro-rata portion of the current year’s performance bonus.
If either (a) Dr. Polat or Messrs. Madar or Shaffir terminates his employment or consultancy, as the case may be, for “good reason” (as defined in the agreement) or (b) we our its successor terminates his employment or consultancy, as the case may be, within 12 months following a change in control (as defined in the agreement), then he will be entitled to receive a lump-sum payment equal to the lesser of (i) his base salary or compensation, as the case may be, for six months and (ii) his base salary or compensation, as the case may be, for the remainder of the then unexpired term, and all unvested stock options and shares of Restricted Common Stock will immediately vest and become exercisable. This amount is subject to reduction so that the total amount of payments or benefits provided to him under his agreement or any benefit plans or agreements will not constitute an “excess parachute payment” under the Internal Revenue Code.
| 44 |
The Officer Agreements include covenants limiting the ability of each of Dr. Polat and Messrs. Madar and Shaffir to compete with us during the term of his employment or during the consulting period, as the case may be, and for a period of one year following his termination for cause by us, and includes a nonsolicitation provision that applies during the same period.
The Officer Agreements provide Mr. Madar and, to the extent not inconsistent with an independent contractor relationship, each of Dr. Polat and Mr. Shaffir, with such benefits as are provided generally to our executive officers, which includes the use of a company car for which he will be grossed-up for any tax liability incurred with respect to the car’s “value equivalent” (the value of the car usage) for tax purposes as updated from time to time, provided, that, the use of a company car for Dr. Polat and Mr. Shaffir is subject to approval of our Board of Directors.
Pursuant to their Officer Agreements, each of Dr. Polat and Mr. Shaffir, are entitled to assign all or a part of his rights and obligations thereunder to a controlled entity (a “Controlled Entity” is an entity wholly owned by such individual and/or members of such individual’s immediate family); provided, that (i) the Controlled Entity joins as a party to the Officer Agreement and agrees to be bound by the provisions thereof, (ii) the Controlled Entity shall at all times after such assignment employ or otherwise retain the services of such individual, (iii) such individual remains personally bound by and subject to the certain confidentiality and non-competition restrictions contained in the Officer Agreement, (iv) the management services and other duties required under the Officer Agreement shall be rendered by the Controlled Entity only through such individual and (v) we incur no additional cost due to such assignment.
The three primary components of compensation for our organization are salary, bonuses and equity incentives (stock options). Each is described in more detail below.
Salary
Salaries are initially negotiated and generally set forth in employment or consulting agreements between each of our executives and us. Thereafter, our Compensation Committee reviews the salaries of our executive management annually. Salaries are established by (a) reviewing the performance of the executive, (b) adjusting (upwards or downward) to reflect individual qualifications, job uniqueness and performance and (c) engaging in discussions between the CEO and the Compensation Committee in order to make revisions as needed.
Bonuses
We believe that bonuses based on both personal and corporate targets are important in order to incentivize employees and management to achieve our goals. We grant bonuses based on corporate and personal milestones, each as determined by the compensation committee of the Board of Directors.
Equity Compensation
We believe that equity ownership by executive management is important in order to align our long-term rewards program with the interests of our stockholders. Additionally, long-term awards are needed to attract and retain talented and success-driven employees.
Benefits and Perks
We do not currently have a written benefits and perks policy. We intend to provide our executive officers with benefits and perks that are customarily provided to executive officers of public companies of similar size, including, for example, the use of a company car for which we will gross-up the executive’s compensation for any tax liability incurred with respect to the car’s “value equivalent”.
| 45 |
Employment Agreements
We generally negotiate employment or consulting agreements with our named executive officers. The purpose of these arrangements is to secure qualified executives for leadership positions in our organization, as well as to protect our intellectual property by virtue of restrictive covenants contained in the agreements. As described above, we currently have an employment or consulting agreement with each of Dr. Polat, Mr. Madar and Mr. Shaffir.
Outstanding Equity Awards at Fiscal Year-End
As of December 31, 2012, Nimrod Madar, our President and Chief Executive Officer, had 2,538,713 unexercised options and 640,098 unvested options; and Ram Shaffir, our Chief Scientific Officer, had 2,597,638 unexercised options.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Transactions with Related Persons
On July 30, 2013, we consummated the Private Placement, in connection with which we entered into Subscription Agreements, with certain Investors, pursuant to which the Investors purchased an aggregate of 2,490,000 units for a purchase price in cash of $1.25 per Unit, each of which comprised one share of our Common Stock and one Warrant to purchase one share of our Common Stock at an exercise price of $1.25 per share. Before expenses, we received an aggregate of approximately $3,112,500 in gross proceeds from the issuance of the Units. Ram Shaffir, the Chief Technology Officer and a director, participated in the Private Placement as an Investor. Pursuant to the same terms and conditions as the other Investors, Mr. Shaffir purchased 400,000 Units for a total of $500,000.
Review, Approval or Ratification of Transactions with Related Persons
Our Board of Directors conducts an appropriate review of and oversees all related-party transactions. We have not yet adopted formal standards in respect of the review and approval or ratification of related-party transactions; however, our board has conformed to the following standards: (i) all related-party transactions must be fair and reasonable to us and on terms comparable to those reasonably expected to be agreed to with independent third parties for the same goods and/or services at the time authorized by the board, and (ii) all related-party transactions must be authorized, approved or ratified by the affirmative vote of a majority of the directors who have no interest, either directly or indirectly, in any such related party transaction.
Director Independence
We evaluate the independence of directors and the composition of the committees of the Board of Directors in accordance with the rules of the NYSE MKT, notwithstanding that our Common Stock is not listed for trading on the NYSE MKT or any other national securities exchange. Our board utilizes the definition of “independence”, including for Audit Committee purposes, as that term is defined by the applicable NYSE MKT rules. Our Board of Directors analyzes whether a director is independent by evaluating, among other factors, the following:
| · | whether the director has any material relationship that may impair his or her independent judgment; |
| · | whether the director is a current employee of GlassesOff or our subsidiaries or was an employee of GlassesOff or our subsidiaries within the three years preceding the determination date; |
| · | whether the director accepted or has an immediate family member who accepted any compensation from GlassesOff or our subsidiaries within the three years preceding the determination of independence, other than (a) compensation for board or board committee service, (b) compensation paid to an immediate family member who is our employee (other than an executive officer), (c) compensation received for former service as an interim executive officer (provided the interim employment did not last longer than one year), or (d) benefits under a tax-qualified retirement plan, or non-discretionary compensation; |
| 46 |
| · | whether the director is, or has an immediate family member who is, a partner in, or a controlling stockholder or an executive officer of, any organization to which we made, or from which we received, payments exceeding certain amounts in any of our most recent three fiscal years; |
| · | whether the director is, or has an immediate family member who is, employed as an executive officer of another entity where at any time during the most recent three fiscal years any of our executive officers serve on the compensation committee of such other entity; and |
| · | whether the director is, or has an immediate family member who is, a current partner of our outside auditor, or was a partner or employee of our outside auditor who worked on our audit at any time during any of the past three years. |
The above list is not exhaustive, and our board considers other factors when determining whether a director is independent.
Our board is composed of Shai Novik, M.B.A., Nimrod Madar, M.B.A., Uri Polat, Ph.D. and Ram Shaffir. Based on the independence standards discussed above, our board has affirmatively determined that Mr. Novik is independent. Mr. Madar, Dr. Polat and Mr. Shaffir are not independent because each serves as an executive officer.
| 47 |
DESCRIPTION OF SECURITIES
General
The following description of our capital stock and provisions of our Amended and Restated Articles of Incorporation and Amended and Restated Bylaws are summaries and are qualified by reference to our Amended and Restated Articles of Incorporation and Amended and Restated Bylaws.
Common Stock
We are authorized to issue up to 200,000,000 shares of our Common Stock, par value $0.001 per share, of which 53,290,000 shares are issued and outstanding as of October 25, 2013. Our outstanding Common Stock is held of record by approximately 33 holders as of October 25, 2013. Additionally, we are authorized to issue up to 20,000,000 shares of preferred stock, par value $0.001 per share, of which no shares are issued and outstanding.
Description of Common Stock
Preemptive Rights
The holders of Common Stock do not have preemptive rights to purchase or subscribe for any stock or other securities of ours.
Voting Rights
Each outstanding share of Common Stock is entitled to one vote per share with respect to each matter on which holders of Common Stock are entitled to vote.
Dividends
Holders of Common Stock are entitled to receive dividends or other distributions when and if declared by the Board, subject to any rights of the holders of other classes of our capital stock and the availability of sufficient funds under applicable law to pay dividends.
Liquidation Rights
In the event of the liquidation of GlassesOff, subject to the rights, if any, of the holders of other classes of our capital stock, the holders of Common Stock are entitled to receive any of our assets available for distribution to our stockholders ratably in proportion to the number of shares held by them.
Preferred Stock
The Board may issue shares of Preferred Stock in one or more series without stockholder approval, and each such series of Preferred Stock may have such preferences, liquidation, dividend, voting and other rights and limitations as the Board may determine, any of which could adversely affect the relative rights of the holders of Common Stock. Additionally, the Board could authorize and issue Preferred Stock as a method of discouraging, delaying or preventing a change in control of GlassesOff or making removal of management more difficult.
Description of Warrants
In connection with the Private Placement, we issued Warrants to purchase up to an aggregate of 2,490,000 shares of our Common Stock at any time and from time to time at an exercise price of $1.25 per share. The Warrants have a five year term, commencing on July 30, 2018 and expiring on July 30, 2018. The Warrants include customary adjustment provisions for stock splits, reorganizations and other similar transactions. As of the date of this registration statement, all such Warrants are outstanding.
| 48 |
Additionally, pursuant to the Merger Agreement, upon consummation of the Merger, we assumed all of Ucansi’s warrants that were issued and outstanding immediately prior to the Merger and issued to the holders of such securities in exchange therefor Parent Warrants to acquire approximately 7,523,504 shares of our Common Stock, respectively, in each case after giving effect to the Forward Split. Except to the extent required under the terms of the Ucansi warrants, all restrictions and limitations on transfer and vesting with respect thereto, to the extent that such restrictions or limitations had not already lapsed upon consummation of the Merger, remain in full force and effect with respect to the Parent Warrants issued in respect of such warrants upon consummation of the Merger.
Anti-Takeover Effects of Nevada Law and our Charter Documents
Nevada Law
As a corporation incorporated under the laws of the State of Nevada, we are subject to the Nevada Revised Statutes, or NRS, including Nevada’s “control share” and “business combination” statutes. Among other requirements, Nevada’s control share statute may apply to us if we have 200 or more stockholders of record, at least 100 of who have addresses in Nevada, and Nevada’s “business combination” statute may apply to us if we have 200 or more stockholders of record.
In general, the control share statute, under certain circumstances, prohibits a person from voting shares of a Nevada corporation’s voting stock after such person acquires an ownership percentage of such corporation in excess of certain thresholds, unless such corporation’s disinterested stockholders approve the granting of voting rights to such person’s shares.
In general, the business combination statute prohibits a publicly-held Nevada corporation from engaging in a business combination with an interested stockholder for a period of two years following the date on which the person became an interested stockholder, unless the business combination or the transaction pursuant to which the person became an interested stockholder was approved in a prescribed manner. Generally, a business combination includes a merger or consolidation with an interested stockholder, or any sale, lease, exchange, mortgage, pledge, transfer or other disposition, in one transaction or a series of transactions with: (i) an interested stockholder having direct or indirect beneficial ownership of 10% or more of outstanding voting stock of a Nevada corporation; or (ii) an affiliate or associate of a Nevada corporation who, at any time within two years before the date in question, was the direct or indirect owner of 10% or more the corporation’s outstanding voting stock. An interested stockholder cannot engage in specified business combinations with the corporation for a period of two years after the date on which the person became an interested stockholder.
Amended and Restated Articles of Incorporation and Bylaws
Our Amended and Restated Articles of Incorporation and Amended and Restated Bylaws contain material provisions that may make the acquisition of control of GlassesOff more difficult.
Blank Check Preferred Stock. We are authorized to issue up to 20,000,000 shares of preferred stock in one or more series without stockholder approval, and each such series of preferred stock may have such preferences, rights and limitations as our Board of Directors may determine.
Stockholder Action by Written Consent. Our Amended and Restated Bylaws provide that any action which our stockholders may take at a stockholders’ meeting can be taken by written consent in lieu of a meeting.
Indemnification. Our Amended and Restated Articles of Incorporation provide that we will indemnify each of our directors and officers to the fullest extent permitted by applicable law. Additionally, our Amended and Restated Bylaws permit us to purchase insurance on behalf of our directors, officers, employees and agents against liabilities that they may incur in those capacities, whether or not we would have the power to indemnify them against such liabilities.
Transfer Agent and Registrar
VStock Transfer, LLC serves as transfer agent and registrar for our Common Stock.
| 49 |
PLAN OF DISTRIBUTION
The selling stockholders will act independently of GlassesOff in making decisions with respect to the timing, manner and size of each and any sale. Each selling stockholder and any of their pledgees, donees, transferees or other successors-in-interest may, from time to time, sell any or all of the shares of Common Stock beneficially owned by them and offered hereby directly or through one or more broker-dealers or agents. Each of such selling stockholders will be responsible for commissions charged by such broker-dealers or agents. The Common Stock may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. The selling stockholders may use any one or more of the following methods when selling shares:
| · | through underwriters, brokers or dealers (who may act as agent or principal and who may receive compensation in the form of discounts, concessions or commissions from the selling stockholders, the purchaser or such other persons who may be effecting such sales) for resale to the public or to institutional investors at various times; |
| · | through negotiated transactions, including, but not limited to, block trades in which the broker or dealer so engaged will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| · | through purchases by a broker or dealer as principal and resale by that broker or dealer for its account; |
| · | on any national securities exchange or quotation service on which the shares may be listed or quoted at the time of sale at market prices prevailing at the time of sale, at prices related to such prevailing market prices, or at negotiated prices; |
| · | in private transactions other than exchange or quotation service transactions; |
| · | short sales, purchases or sales of put, call or other types of options, forward delivery contracts, swaps, offerings of structured equity-linked securities or other derivative transactions or securities; |
| · | hedging transactions, including, but not limited to: |
| o | transactions with a broker-dealer or its affiliate, whereby the broker-dealer or its affiliate will engage in short sales of shares and may use shares to close out its short position; |
| o | options or other types of transactions that require the delivery of shares to a broker-dealer or an affiliate thereof, who will then resell or transfer the shares; or |
| o | loans or pledges of shares to a broker-dealer or an affiliate, who may sell the loaned shares or, in an event of default in the case of a pledge, sell the pledged shares; |
| · | through offerings of securities exercisable, convertible or exchangeable for shares, including, without limitation, securities issued by trusts, investment companies or other entities; |
| · | offerings directly to one or more purchasers, including institutional investors; |
| · | through ordinary brokerage transactions and transactions in which a broker solicits purchasers; |
| · | through distribution to the securityholders of the selling stockholders; |
| · | by pledge to secure debts and other obligations; |
| · | through a combination of any such methods of sale; or |
| · | through any other method permitted under applicable law. |
| 50 |
Additionally, each selling stockholder may resell all or a portion of its shares in open market transactions in reliance upon Rule 144 under the Securities Act provided it meets the criteria and conforms to the requirements of Rule 144.
The selling stockholders may be deemed to be statutory underwriters under the Securities Act. In addition, any broker-dealers who act in connection with the sale of the shares hereunder may be deemed to be “underwriters” within the meaning of Section 2(11) of the Securities Act, and any commissions received by them and profit on any resale of the shares as principal may be deemed to be underwriting discounts and commissions under the Securities Act. The selling stockholders have acknowledged that they understand their obligations to comply with the provisions of the Securities Exchange Act of 1934, as amended, and the rules thereunder relating to stock manipulation, particularly Regulation M.
Broker-dealers engaged by the selling stockholders may arrange for other brokers-dealers to participate in sales. Such broker-dealers and any other participating broker-dealers may, in connection with such sales, be deemed to be underwriters within the meaning of the Securities Act. If the selling stockholders effect such transactions through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling stockholders or commissions from purchasers of the shares of Common Stock for whom they may act as agent or to whom they may sell as principal, or both (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be less than or in excess of those customary in the types of transactions involved). Any discounts or commissions received by any such broker-dealers may be deemed to be underwriting discounts and commissions under the Securities Act.
There can be no assurance that the selling stockholders will sell any or all of the shares of Common Stock registered pursuant to the registration statement of which this prospectus supplement and the accompanying prospectus form a part.
We are not aware of any plans, arrangements or understandings between the selling stockholders and any underwriter, broker-dealer or agent regarding the sale of shares of Common Stock by the selling stockholders.
We will pay all expenses incident to the filing of this registration statement, estimated to be $70,513.22. These expenses include accounting and legal fees in connection with the preparation of the registration statement of which this prospectus supplement and the accompanying prospectus form a part, legal and other fees in connection with the qualification of the sale of the shares under the laws of certain states (if any), registration and filing fees and other expenses. We have agreed to keep the registration of the shares offered hereby effective for one (1) year following the date on which it is declared effective by the SEC or for such shorter period ending on the earlier to occur of (i) the date on which all Investors may sell all of their securities registered for resale thereunder without restriction pursuant to Rule 144 or (ii) the date when all of the securities registered thereunder have been sold.
LEGAL MATTERS
The legality of the securities offered by this prospectus has been passed upon by Greenberg Traurig, LLP, Las Vegas, Nevada.
EXPERTS
The audited consolidated financial statements of GlassesOff Inc. contained in this prospectus have been so included in reliance upon the report of Yarel + Partners CPA (ISR), independent registered public accountants, upon the authority of said firm as experts in accounting and auditing in giving said report.
| 51 |
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the Common Stock. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement, some items of which are contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and our Common Stock, we refer you to the registration statement, including the exhibits and the consolidated financial statements and notes that are filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract or any other document are not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please see the copy of the contract or document that has been filed. Each statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit. The exhibits to the registration statement should be reviewed for the complete contents of these contracts and documents.
We also file annual, quarterly and current reports, proxy statements and other information with the SEC. Any document we file with the SEC (including the registration statement of which this prospectus is a part) may be inspected without charge at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549, and copies of all or any part of any such document may be obtained from the SEC upon the payment of fees prescribed by it. You may call the SEC at 1-800-SEC-0330 for more information on the operation of the public reference facilities. The SEC maintains a website at http://www.sec.gov that contains reports, proxy and information statements and other information regarding companies that file electronically with it.
| 52 |
GLASSESOFF INC.

Common Stock
PROSPECTUS
, 2013
PART II - INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth an estimate of the fees and expenses payable by us in connection with the registration of our securities offered hereby. The selling stockholders named in the prospectus comprising a part of this registration statement will not bear any portion of such fees and expenses. All of such fees and expenses, except for the SEC Registration Fee, are estimated:
| SEC Registration and Filing Fee | $ | 8,513.22 | ||
| Legal Fees and Expenses | $ | 50,000.00 | ||
| Accounting Fees and Expenses | $ | 5,000.00 | ||
| Printing Fees and Expenses | $ | 5,000.00 | ||
| Miscellaneous | $ | 2,000.00 | ||
| TOTAL | $ | 70,513.22 |
Item 14. Indemnification of Directors and Officers.
As a corporation incorporated under the laws of the State of Nevada, we are subject to the Nevada Revised Statutes, which provide that a director or officer is not individually liable to the corporation or its stockholders or creditors for any damages as a result of any act or failure to act in his capacity as a director or officer unless it is proven that his act or failure to act constituted a breach of his fiduciary duties as a director or officer and his breach of those duties involved intentional misconduct, fraud or a knowing violation of law. The Articles of Incorporation or an amendment thereto may, however, provide for greater individual liability. Furthermore, directors may be jointly and severally liable for the payment of certain distributions in violation of Chapter 78 of the Nevada Revised Statutes.
The Nevada Revised Statutes also provide that under certain circumstances, a corporation may indemnify any person for amounts incurred in connection with a pending, threatened or completed action, suit or proceeding in which he is, or is threatened to be made, a party by reason of his being a director, officer, employee or agent of the corporation or serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, if such person (a) is not liable for a breach of fiduciary duty involving intentional misconduct, fraud or a knowing violation of law or such greater standard imposed by the corporation’s articles of incorporation; or (b) acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. Additionally, a corporation may indemnify a director, officer, employee or agent with respect to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor, if such person (a) is not liable for a breach of fiduciary duty involving intentional misconduct, fraud or a knowing violation of law or such greater standard imposed by the corporation’s articles of incorporation; or (b) acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, however, indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court to be liable to the corporation or for amounts paid in settlement to the corporation, unless the court determines that the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper. To the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to above, or in defense of any claim, issue or matter therein, the corporation shall indemnify him against expenses, including attorneys’ fees, actually and reasonably incurred by him in connection with the defense.
Subject to authorization by our Board of Directors upon its determination that an Indemnified Party (as defined below) has met the applicable standard of conduct, our bylaws require that we indemnify any person who was or is a party or is threatened to be made a party to any proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of GlassesOff) by reason of the fact that such person is or was our director, trustee, officer, employee or agent, or is or was serving at our request as a director, trustee, officer, employee or agent of another entity (any such person, an “Indemnified Person”), against expenses, including attorneys’ fees, judgment, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to our best interests, and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. Further, we shall indemnify each Indemnified Person in respect of actions by or in the right of GlassesOff; provided, however, that, with limited, court-determined exceptions, we shall not indemnify any such Indemnified Person in any such action if such person is adjudged liable for gross negligence or willful misconduct in the performance of such person’s duty to us.
| II-1 |
Additionally, subject to our Board of Directors’ authorization as described above, expenses incurred by an Indemnified Person shall be paid by us at any time or from time to time in advance of the final disposition of any above-described action, suit or proceeding upon our receipt of an undertaking by or on behalf of such Indemnified Person to repay such amount unless it shall ultimately be found that we are authorized under the bylaws to pay such amount.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us under the provisions that we describe above or otherwise, we have been informed that in the opinion of the SEC, this indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Item 15. Recent Sales of Unregistered Securities
We effected the following sales of unregistered securities, each of which we believe was exempt from registration under the Securities Act pursuant to Section 4(a)(2) therein:
The Merger
On June 26, 2013, we (at the time known as Autovative Products, Inc.) entered into that certain Merger Agreement with Merger Sub, and Ucansi. Pursuant to the Merger Agreement, on July 30, 2013, we effected the Merger, with Ucansi surviving the Merger as our wholly owned subsidiary.
Pursuant to the Merger Agreement, all shares of Ucansi’s common and preferred stock that were issued and outstanding immediately preceding the Merger were converted into the right to receive an aggregate of approximately 40,000,000 shares of Common Stock after giving effect to the Forward Split. Additionally, pursuant to the Merger Agreement, upon consummation of the Merger, we assumed all of Ucansi’s options and warrants that were issued and outstanding immediately prior to the Merger and issued to the holders of such securities in exchange therefor options and Parent Warrants to acquire approximately 9,019,872 shares and 7,523,504 shares of our Common Stock, respectively, in each case after giving effect to the Forward Split. The consolidation effected by the Merger has been accounted for as a reverse acquisition wherein Ucansi will be treated as the acquirer for accounting purposes as it has acquired control of the combined enterprise.
Included in the shares of Common Stock offered hereby by the selling stockholders are the shares of Common Stock that are issued pursuant to the Merger and the shares of Common Stock issuable upon exercise of the Parent Warrants that we assumed in connection with the Merger.
The Private Placement
Additionally, on July 30, 2013, we consummated the Private Placement, pursuant to which the Investors purchased an aggregate of 2,490,000 Units for a purchase price in cash of $1.25 per Unit, each of which comprised one share of Common Stock and one Warrant. Before expenses, we received an aggregate of approximately $3,112,500 in gross proceeds from the issuance of the Units.
| II-2 |
Item 16. Exhibits
|
Exhibit
|
Description | |
| 2.1 | Agreement and Plan of Merger, dated as of June 26, 2013, by and among Autovative Products, Inc., Ucansi Acquisition Corp. and Ucansi Inc., filed as Exhibit 2.1 to our Current Report on Form 8-K, filed with the SEC on July 2, 2013 and incorporated herein by reference. | |
| 2.2 | First Amendment to Agreement and Plan of Merger, dated as of July 2, 2013, by and among Autovative Products, Inc. and Ucansi Inc., filed as Exhibit 2.2 to our Current Report on Form 8-K, filed with the SEC on July 2, 2013 and incorporated herein by reference. | |
| 2.3 | Spin-Off Agreement, dated as of May 23, 2013, by and between Autovative Products, Inc. and David Funderburk. (1) | |
| 3.1 | Amended and Restated Articles of Incorporation of GlassesOff Inc. (1) | |
| 3.2 | Amended and Restated Bylaws of GlassesOff Inc. (1) | |
| 4.1 | Form of Warrant (1) | |
| 4.2 | Form of Subscription Agreement (1) | |
| 5.1 | Opinion of Greenberg Traurig, LLP* | |
| 10.1 | Registration Rights Agreement, dated as of July 30, 2013, by and among GlassesOff and the Investors party thereto. (1) | |
| 10.2 | License Agreement, dated July 15, 2011, by and between RevitalVision LLC and Ucansi Inc. (1) | |
| 10.3+ | Employment Agreement, dated July 30, 2013, by and between Eyekon E.R.D Ltd. and Nimrod Madar. (1) | |
| 10.4+ | Consulting Agreement, dated July 30, 2013, by and between Eyekon E.R.D Ltd. and Uri Polat, Ph.D. (1) | |
| 10.5+ | Consulting Agreement, dated July 30, 2013, by and between Eyekon E.R.D Ltd. and Ram Shaffir. (1) | |
| 10.6+ | Consulting Agreement, dated July 30, 2013, by and between GlassesOff and Cohen & Schaeffer LLP. (1) | |
| 10.7+ | GlassesOff Inc. 2013 Incentive Compensation Plan (1) | |
| 21.1 | SUBSIDIARIES* | |
| 23.1 | Consent of Yarel + Partners CPA (ISR)* | |
| 23.2 | Consent of Greenberg Traurig, LLP (included in Exhibit 5.1)* | |
| 24.1 | Power of Attorney (included on the signature page to this registration statement)* | |
| 99.1 | Audited Financial Statements of Ucansi Inc. (1) | |
| 99.2 | Unaudited Interim Financial Statements of Ucansi Inc. (1) |
| II-3 |
|
Exhibit
|
Description | |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Schema Document | |
| 101.CAL | XBRL Calculation Linkbase Document | |
| 101.LAB | XBRL Label Linkbase Document | |
| 101.PRE | XBRL Presentation Linkbase Document |
| + | Management contract or compensation plan arrangement. |
| * | Filed herewith. |
| (1) | Previously filed as an exhibit to our Current Report on Form 8-K filed with the SEC on August 5, 2013. |
Item 17. Undertakings
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
| II-4 |
(b) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
| II-5 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-1 and has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Ramat Gan, State of Israel, on October 28, 2013.
| GLASSESOFF INC. | ||
| (Registrant) | ||
| By: | /s/ Nimrod Madar | |
| Name: | Nimrod Madar | |
| Title: | President and Chief Executive Officer | |
POWER OF ATTORNEY
Each individual whose signature appears below constitutes and appoints each of Nimrod Madar and Steve Schaeffer, such person’s true and lawful attorney-in-fact and agent with full power of substitution and resubstitution, for such person and in such person’s name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and all documents in connection therewith, with the Securities and Exchange Commission, granting unto each said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as such person might or could do in person, hereby ratifying and confirming all that any said attorney-in-fact and agent, or any substitute or substitutes of any of them, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed below by the following persons in the capacities and on the dates indicated.
|
Signature |
Title |
Date | ||
| /s/ Nimrod Madar | President, Chief Executive Officer and Director | October 28, 2013 | ||
| Nimrod Madar | (Principal Executive Officer) | |||
| /s/ Shai Novik | Chairman of the Board | October 28, 2013 | ||
| Shai Novik | ||||
| /s/ Uri Polat | Chief Scientific Officer and Director | October 28, 2013 | ||
| Uri Polat | ||||
| /s/ Steve Schaeffer | Chief Financial Officer | October 28, 2013 | ||
| Steve Schaeffer | (Principal Financial Officer and Principal Accounting Officer) | |||
| /s/ Ram Shaffir | Chief Technology Officer and Director | October 28, 2013 | ||
| Ram Shaffir |
| II-6 |
|
Exhibit
|
Description | |
| 2.1 | Agreement and Plan of Merger, dated as of June 26, 2013, by and among Autovative Products, Inc., Ucansi Acquisition Corp. and Ucansi Inc., filed as Exhibit 2.1 to our Current Report on Form 8-K, filed with the SEC on July 2, 2013 and incorporated herein by reference. | |
| 2.2 | First Amendment to Agreement and Plan of Merger, dated as of July 2, 2013, by and among Autovative Products, Inc. and Ucansi Inc., filed as Exhibit 2.2 to our Current Report on Form 8-K, filed with the SEC on July 2, 2013 and incorporated herein by reference. | |
| 2.3 | Spin-Off Agreement, dated as of May 23, 2013, by and between Autovative Products, Inc. and David Funderburk. (1) | |
| 3.1 | Amended and Restated Articles of Incorporation of GlassesOff Inc. (1) | |
| 3.2 | Amended and Restated Bylaws of GlassesOff Inc. (1) | |
| 4.1 | Form of Warrant (1) | |
| 4.2 | Form of Subscription Agreement (1) | |
| 5.1 | Opinion of Greenberg Traurig, LLP* | |
| 10.1 | Registration Rights Agreement, dated as of July 30, 2013, by and among GlassesOff and the Investors party thereto. (1) | |
| 10.2 | License Agreement, dated July 15, 2011, by and between RevitalVision LLC and Ucansi Inc. (1) | |
| 10.3+ | Employment Agreement, dated July 30, 2013, by and between Eyekon E.R.D Ltd. and Nimrod Madar. (1) | |
| 10.4+ | Consulting Agreement, dated July 30, 2013, by and between Eyekon E.R.D Ltd. and Uri Polat, Ph.D. (1) | |
| 10.5+ | Consulting Agreement, dated July 30, 2013, by and between Eyekon E.R.D Ltd. and Ram Shaffir. (1) | |
| 10.6+ | Consulting Agreement, dated July 30, 2013, by and between GlassesOff and Cohen & Schaeffer LLP. (1) | |
| 10.7+ | GlassesOff Inc. 2013 Incentive Compensation Plan (1) | |
| 21.1 | Subsidiaries* | |
| 23.1 | Consent of Yarel + Partners CPA (ISR)* | |
| 23.2 | Consent of Greenberg Traurig, LLP (included in Exhibit 5.1)* | |
| 24.1 | Power of Attorney (included on the signature page to this registration statement)* | |
| 99.1 | Audited Financial Statements of Ucansi Inc. (1) | |
| 99.2 | Unaudited Interim Financial Statements of Ucansi Inc. (1) |
| II-7 |
|
Exhibit
|
Description | |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Schema Document | |
| 101.CAL | XBRL Calculation Linkbase Document | |
| 101.LAB | XBRL Label Linkbase Document | |
| 101.PRE | XBRL Presentation Linkbase Document |
| + | Management contract or compensation plan arrangement. |
| * | Filed herewith. |
| (1) | Previously filed as an exhibit to our Current Report on Form 8-K filed with the SEC on August 5, 2013. |
| II-8 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
U.S. DOLLARS
IN THOUSANDS
(Except shares and per share amounts)
CONSOLIDATED BALANCE SHEETS
| June 30, | ||||||||
| 2013 | December 31, | |||||||
| (Unaudited) | 2012 | |||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
| Cash and cash equivalents | $ | 114 | $ | 779 | ||||
| Accounts receivable | 20 | 11 | ||||||
| Total Current Assets | 134 | 790 | ||||||
| Long-term Assets: | ||||||||
| Property and equipment, net | 41 | 46 | ||||||
| Long term prepaid expenses | 2 | 2 | ||||||
| Restricted cash | 21 | 20 | ||||||
| Total Long Term Assets | 64 | 68 | ||||||
| Total Assets | $ | 198 | $ | 858 | ||||
| LIABILITIES AND SHAREHOLDERS' EQUITY (DEFICIT) | ||||||||
| Current Liabilities: | ||||||||
| Trade payables | $ | 39 | $ | 48 | ||||
| Related parties payable | 275 | 168 | ||||||
| Accrued expenses and other liabilities | 157 | 108 | ||||||
| Total Current Liabilities | 471 | 324 | ||||||
| Commitments and Contingent Liabilities | ||||||||
| Shareholders' Equity (deficit): | ||||||||
| Stock capital - Common shares of $0.001 par value per share 200,000,000 shares of common stock authorized; 28,136,775 and 28,499,993 shares issued and outstanding at June 30, 2013 and December 31, 2012, respectively | 28 | 28 | ||||||
| Preferred stock of $0.001 par value per share 20,000,000 shares of preferred stock authorized;11,782,997 issued and outstanding at June 30, 2013 and December 31, 2012 | 12 | 12 | ||||||
| Additional paid-in capital | 9,006 | 8,616 | ||||||
| (Deficit) accumulated during the development stage | (9,319 | ) | (8,122 | ) | ||||
| Total Shareholders' Equity (deficit) | (273 | ) | 534 | |||||
| Total Liabilities and Shareholders' Equity | $ | 198 | $ | 858 | ||||
The accompanying notes are an integral part of the unaudited consolidated financial statements.
| 1 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
U.S. DOLLARS
IN THOUSANDS
(Except shares and per share amounts)
CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
| Period of February 5, 2007 | ||||||||||||||||||||
| For the three months ended | For the six months ended | (date of inception) | ||||||||||||||||||
| June 30, | June 30, | through June 30, | ||||||||||||||||||
| 2013 | 2012 | 2013 | 2012 | 2013 | ||||||||||||||||
| Revenues | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
| Operating expenses: | ||||||||||||||||||||
| In-process research and development | - | - | - | - | (1,427 | ) | ||||||||||||||
| Research and development | (417 | ) | (1,439 | ) | (690 | ) | (1,683 | ) | (5,633 | ) | ||||||||||
| Sales and Marketing | (80 | ) | - | (80 | ) | - | (80 | ) | ||||||||||||
| General and administrative | (268 | ) | (768 | ) | (428 | ) | (859 | ) | (2,136 | ) | ||||||||||
| Total operating expenses | (765 | ) | (2,207 | ) | (1,198 | ) | (2,542 | ) | (9,276 | ) | ||||||||||
| Operating (loss) | (765 | ) | (2,207 | ) | (1,198 | ) | (2,542 | ) | (9,276 | ) | ||||||||||
| Financial (expense), income net | 7 | 22 | 1 | 16 | (43 | ) | ||||||||||||||
| Net (loss) | $ | (758 | ) | $ | (2,185 | ) | $ | (1,197 | ) | $ | (2,526 | ) | $ | (9,319 | ) | |||||
| (Loss) per share (basic & diluted) | $ | (0.02 | ) | $ | (0.06 | ) | $ | (0.03 | ) | $ | (0.07 | ) | ||||||||
| Weighted average number of shares outstanding | 39,919,772 | 36,906,280 | 39,919,772 | 36,568,808 | ||||||||||||||||
The accompanying notes are an integral part of the unaudited consolidated financial statements.
| 2 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
U.S. DOLLARS
IN THOUSANDS
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
| For the six months ended June 30, | Period of February 5, 2007 (date of inception) through June 30, | |||||||||||
| 2013 | 2012 | 2013 | ||||||||||
| Cash flows from operating activities | ||||||||||||
| Net (loss) | $ | (1,197 | ) | $ | (2,526 | ) | $ | (9,319 | ) | |||
| Adjustments to reconcile net (loss) to net cash (used in) Operating activities: | ||||||||||||
| Depreciation | 11 | 7 | 75 | |||||||||
| In-process research and development | - | - | 1,427 | |||||||||
| Stock based compensation related to employees and nonemployees | 390 | 1,891 | 2,635 | |||||||||
| Liability in respect of employees severance payment | - | (14 | ) | - | ||||||||
| Decrease (increase) in accounts receivable and prepaid expenses | (9 | ) | 3 | (20 | ) | |||||||
| Increase (decrease) in trade payables | (9 | ) | 2 | 39 | ||||||||
| Increase in related parties payables | 107 | 20 | 275 | |||||||||
| Decrease (increase) in restricted cash | 4 | - | 4 | |||||||||
| Increase (decrease) in accrued expenses and other liabilities | 49 | (33 | ) | 157 | ||||||||
| Net cash (used in) operating activities | (654 | ) | (650 | ) | (4,727 | ) | ||||||
| Cash flows from investing activities | ||||||||||||
| Purchase of property and equipment | (6 | ) | - | (116 | ) | |||||||
| Assets held for employees’ severance payment | - | 4 | - | |||||||||
| Investment in deposits | (5 | ) | - | (26 | ) | |||||||
| Prepaid expenses long term | - | (1 | ) | (2 | ) | |||||||
| Net cash (used in) provided by investing activities | (11 | ) | 3 | (144 | ) | |||||||
| Cash flows from financing activities | ||||||||||||
| Proceeds from issuance of preferred stock | - | 800 | 4,631 | |||||||||
| Proceeds from issuance of stock warrants | - | - | 286 | |||||||||
| Proceeds from issuance of common stock, net | - | 1 | 68 | |||||||||
| Net cash provided by financing activities | - | 801 | 4,985 | |||||||||
| Increase (decrease) in cash and cash equivalents | (665 | ) | 154 | 114 | ||||||||
| Cash and cash equivalents at the beginning of the period | 779 | 436 | - | |||||||||
| Cash and cash equivalents at the end of the period | $ | 114 | $ | 590 | $ | 114 | ||||||
The accompanying notes are an integral part of the unaudited consolidated financial statements.
| 3 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
U.S. DOLLARS
IN THOUSANDS
CONSOLIDATED STATEMENTS
OF CASH FLOWS
(Unaudited)
| For the six months ended June 30, | Period of February 5, 2007 (date of inception) through June 30, | |||||||||||
| 2013 | 2012 | 2013 | ||||||||||
| Non-cash transactions: | ||||||||||||
| Issuance of common stock in acquisition of in-process research and development | $ | - | $ | - | $ | 1,427 | ||||||
| Exchange of redeemable preferred stock with non-redeemable preferred stock | $ | - | $ | - | $ | 1,117 | ||||||
| Additional information: | ||||||||||||
| Cash paid for income taxes | $ | - | $ | - | $ | - | ||||||
| Cash paid for interest expense | $ | - | $ | - | $ | - | ||||||
The accompanying notes are an integral part of the unaudited consolidated financial statements.
| 4 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
JUNE 30, 2013
U.S. DOLLARS
IN THOUSANDS
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 1 - GENERAL
| a. | Ucansi Inc. (the “Company”) formerly named "Eyekon Inc." was incorporated on February 5, 2007 under the laws of the State of Delaware. Ucansi is a development stage healthcare software technology company, utilizing patented technology to develop consumer-oriented software applications for improving near vision sharpness, by improving the image processing function in the visual cortex of the brain. The Company’s development efforts are conducted through its wholly-owned Israeli subsidiary, Eyekon E.R.D Ltd formerly named Eyekon Blue White Ltd. |
| b. | The Company devotes substantially all of its efforts toward research and development activities. In the course of such activities, the Company has sustained operating losses and expects such losses to continue for the foreseeable future. The Company has not generated any revenues or product sales and has not achieved profitable operations or positive cash flow from operations. The Company’s deficit accumulated during the development stage aggregated $9,319 through June 30, 2013. There is no assurance that profitable operations, if ever achieved, could be sustained on a continuing basis. The Company plans to continue to finance its operations with a combination of stock issuances and private placements and, in the longer term, revenues. There are no assurances, however, that the Company will be successful in obtaining an adequate level of financing needed for the long-term development. | |
| As discussed in Note 10, on July 30, 2013 the Company consummated a private placement in which the Company received an aggregate of approximately $3,113 in gross proceeds. The Company believes that its current cash sources will enable the continuance of the Company’s activities for at least twelve months with no need for additional fundraising. |
| c. | Starting Q4 2010 and throughout 2011 a scientific study testing the efficacy of the Company's technology was conducted at the University of California, Berkeley USA. In February 2012 positive results from a scientific study testing the efficacy of the product were published in Nature’s Scientific Reports, a leading scientific publication. |
| d. | The Company’s product for reading improvement is currently in beta testing phase. |
| e. | As discussed in Note 10, Autovative Products, Inc., a Nevada corporation (“Autovative”), entered into an Agreement and Plan of Merger by and among Autovative, Ucansi Acquisition Corp., a Delaware corporation and wholly owned subsidiary of Autovative (“Merger Sub”), and the Company. Pursuant to the Merger Agreement, on July 30, 2013 (the “Closing Date”), Merger Sub merged with and into the Company (the “Merger”), with the Company surviving the merger as Autovative's wholly owned subsidiary. Upon consummation of the Merger, Autovative changed its name from Autovative Products, Inc. to GlassesOff Inc. |
The merger between Autovative and the Company has been accounted for as a reverse acquisition under the purchase method of accounting in accordance with ASC Topic 805 “Business Combinations”. The combination of the two companies is recorded as a recapitalization pursuant to which the Company is treated as the continuing entity.
These Consolidated Financial Statements following the reverse acquisition are under the name of the legal parent Glassesoff Inc. (formerly Autovative) but reflect the financial statements of the Company retroactively adjusted to reflect Glassesoff Inc.'s legal capital.
| 5 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
U.S. DOLLARS
IN THOUSANDS
(Except shares and per share amounts)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES
| a. | Basis of presentation: The accompanying unaudited financial statements of the Company are presented in accordance with the requirements of Form 10-Q and Article 10 of Regulation S-X. Certain information and footnote disclosures normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”) have been condensed or omitted pursuant to such U.S. Securities and Exchange Commission (“SEC”) rules and regulations. In the opinion of management, all adjustments (consisting of normal recurring adjustments) considered necessary for a fair presentation have been made. The results for these interim periods are not necessarily indicative of the results for the entire year. The accompanying financial statements should be read in conjunction with the Company’s audited financial statements for the year ended December 31, 2012 and the notes thereto included in the Company’s Current Report on Form 8-K filed with the SEC on August 5, 2013, with the accompanying financial statements. |
| b. | Principles of consolidation: The consolidated financial statements include the accounts of the Company and its wholly owned subsidiary, Eyekon E.R.D. Ltd. Intercompany transactions and balances have been eliminated upon consolidation. |
| c. | Loss per share: |
Basic and diluted losses per share are presented in accordance with ASC No. 260 “Earnings per share”. Outstanding share options and warrants have been excluded from the calculation of the diluted loss per share because all such securities are antidilutive. The number of shares of the Company’s common stock, par value $0.001 per share (“Common Stock”), issuable upon exercise or conversion of the foregoing securities that have been excluded from calculations for the three and six months ended June 30, 2013 and 2012 were 16,603,409, 9,996,942, 16,590,470, and 8,034,968 respectively.
The following data show the amounts used in computing (losses) per share and the effect on income and the weighted average number of shares of dilutive potential common stock (in thousands, except share amounts and per share amounts):
| For the three months ended | For the six months ended | |||||||||||||||
| June 30, | June 30, | |||||||||||||||
| 2013 | 2012 | 2013 | 2012 | |||||||||||||
| Basic & Diluted net (loss) per share: | ||||||||||||||||
| (Loss) from continuing operations | $ | (758 | ) | $ | (2,185 | ) | $ | (1,197 | ) | $ | (2,526 | ) | ||||
| Less: accumulating dividend on preferred stock | - | (32 | ) | - | (62 | ) | ||||||||||
| (758 | ) | (2,217 | ) | (1,197 | ) | (2,588 | ) | |||||||||
| Number of shares used in per share computation: | ||||||||||||||||
| Common Stock | 28,136,775 | 28,136,775 | 28,136,775 | 28,136,775 | ||||||||||||
| Series A Preferred Stock | 11,782,997 | 8,767,170 | 11,782,997 | 8,429,699 | ||||||||||||
| 39,919,772 | 36,903,945 | 39,919,772 | 36,566,474 | |||||||||||||
| Basic & Diluted net (loss) per share | $ | (0.02 | ) | $ | (0.06 | ) | $ | (0.03 | ) | $ | (0.07 | ) | ||||
Series A Preferred Stock participates in dividends with common stock. Therefore, it is included in the computation of basic EPS.
| 6 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
JUNE 30, 2013
U.S. DOLLARS
IN THOUSANDS
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (continued)
| d. | Fair value measurements: As defined in ASC 820-10, Fair Value Measurements and Disclosures (“ASC 820-10”), fair value is based on the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. ASC 820-10 establishes a fair value hierarchy that prioritizes observable and unobservable inputs used to measure fair value into three broad levels, which are described below: |
- Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs.
- Level 2: Other inputs that are observable, directly or indirectly, such as quoted prices for similar assets and liabilities or market corroborated inputs.
- Level 3: Unobservable inputs are used when little or no market data is available, which requires the Company to develop its own assumptions about how market participants would value the assets or liabilities. The fair value hierarchy gives the lowest priority to Level 3 inputs.
In determining fair value, the
Company utilizes valuation techniques in its assessment that maximize the use of observable inputs and minimize the use of unobservable
inputs.
The following table presents the Company’s financial assets and liabilities that are carried at fair value, classified according to the three categories described above:
| Fair Value Measurements at June 30, 2013 | ||||||||||||||||
| Total | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
| Cash and cash equivalents | $ | 114 | $ | 114 | $ | - | $ | - | ||||||||
| Restricted cash | 21 | 21 | - | - | ||||||||||||
| Total assets at fair value, net | $ | 135 | $ | 135 | $ | - | $ | - | ||||||||
| Fair Value Measurements at December 31, 2012 | ||||||||||||||||
| Total | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
| Cash and cash equivalents | $ | 779 | $ | 779 | $ | - | $ | - | ||||||||
| Restricted cash | 20 | 20 | - | - | ||||||||||||
| Total assets at fair value, net | $ | 799 | $ | 799 | $ | - | $ | - | ||||||||
| e. | Recent accounting pronouncements: In December 2011, the FASB issued Accounting Standards Update No. 2011-11, "Disclosures about Offsetting Assets and Liabilities" ("ASU 2011-11"). The objective of ASU 2011-11 is to enhance disclosures by requiring improved information about financial instruments and derivative instruments in relation to netting arrangements. ASU 2011-11 is effective for interim and annual periods beginning on or after January 1, 2013. The adoption of ASU 2011-11 did not have a material impact on its consolidated results of operation and financial condition. |
On February 5, 2013, the FASB issued Accounting Standards Update No. 2013-02, “Reporting of Amounts Reclassified Out of Accumulated Other Comprehensive Income” (“ASU 2013-02”), which adds additional disclosure requirements relating to the reclassification of items out of accumulated other comprehensive income. ASU 2013-02 is effective for the first quarter of 2013 and affects disclosures only. The adoption of ASU 2013-02 did not have a material impact on its consolidated results of operation and financial condition.
There were various other updates recently issued. None of the updates are expected to a have a material impact on the Company's financial position, results of operations or cash flows.
| 7 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
JUNE 30, 2013
U.S. DOLLARS
IN THOUSANDS
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 - ACOUNTS RECEIVABLE
| June 30, | December 31 | |||||||
| 2013 | 2012 | |||||||
| Israeli government authorities | $ | 16 | $ | 11 | ||||
| Prepaid expenses | 4 | - | ||||||
| $ | 20 | $ | 11 | |||||
NOTE 4 - PROPERTY AND EQUIPMENT, NET
| June 30, | December 31 | |||||||
| 2013 | 2012 | |||||||
| Cost: | ||||||||
| Office furniture and equipment | $ | 19 | $ | 18 | ||||
| Computers and electronic equipment | 59 | 54 | ||||||
| Laboratory equipment | 31 | 31 | ||||||
| Leasehold improvements | 7 | 7 | ||||||
| 116 | 110 | |||||||
| Accumulated depreciation: | ||||||||
| Office furniture and equipment | 6 | 6 | ||||||
| Computers and electronic equipment | 47 | 41 | ||||||
| Laboratory equipment | 20 | 15 | ||||||
| Leasehold improvements | 2 | 2 | ||||||
| 75 | 64 | |||||||
| Depreciated cost | $ | 41 | $ | 46 | ||||
Depreciation expenses for the three and six months ended June 30, 2013 and 2012 and for the period of February 5, 2007 (inception date) through June 30, 2013 were $7, $3, $11, $7 and $75, respectively.
| 8 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
JUNE 30, 2013
U.S. DOLLARS
IN THOUSANDS
(Except options stock amounts and option exercise price)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 5 - ACCRUED EXPENSES AND OTHER LIABILITIES
| June 30, | December 31, | |||||||
| 2013 | 2012 | |||||||
| Employees and payroll accruals | $ | 111 | $ | 77 | ||||
| Accrued expenses | 44 | 29 | ||||||
| Other | 2 | 2 | ||||||
| $ | 157 | $ | 108 | |||||
NOTE 6 - STOCK OPTION PLANS
| a. | The following table summarizes all share-based compensation expenses related to grants under the Equity Incentive Plans to employees, directors and consultants included in the consolidated statements of operations: |
| Period of February 5, 2007 | ||||||||||||||||||||
| For the three months | For the six months | (date of inception) | ||||||||||||||||||
| ended June 30, | ended June 30, | through June 30, | ||||||||||||||||||
| 2013 | 2012 | 2013 | 2012 | 2013 | ||||||||||||||||
| Research & development | $ | 134 | $ | 1,163 | $ | 176 | $ | 1,169 | $ | 1,661 | ||||||||||
| General & administrative | 156 | 722 | 214 | 722 | 974 | |||||||||||||||
| Total | $ | 290 | $ | 1,885 | $ | 390 | $ | 1,891 | $ | 2,635 | ||||||||||
| b. | The following is a summary of the stock options granted to employees under the 2008 Plan and the 2010 Plan: |
| For the six months ended June 30, 2013 | ||||||||||||||||
| 2010 Plan | 2008 Plan | |||||||||||||||
| Number of Options | Weighted Average Exercise Price | Number of Options | Weighted Average Exercise Price | |||||||||||||
| Outstanding at the beginning of the period | 4,983,424 | $ | 0.00 | 117,660 | $ | 0.00 | ||||||||||
| Issued | - | - | - | - | ||||||||||||
| Outstanding at the end of the period | 4,983,424 | $ | 0.00 | 117,660 | $ | 0.00 | ||||||||||
| Options exercisable | 4,294,421 | $ | 0.00 | 117,660 | $ | 0.00 | ||||||||||
| 9 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
JUNE 30, 2013
U.S. DOLLARS
IN THOUSANDS
(Except options stock amounts and option exercise price)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 6 - STOCK OPTION PLANS (continued)
| For the six months ended June 30, 2012 | ||||||||||||||||
| 2010 Plan | 2008 Plan | |||||||||||||||
| Number of Options | Weighted Average Exercise Price | Number of Options | Weighted Average Exercise Price | |||||||||||||
| Outstanding at the beginning of the period | - | - | 117,660 | $ | 0.00 | |||||||||||
| Issued | 4,216,057 | $ | 0.00 | - | - | |||||||||||
| Outstanding at the end of the period | 4,216,057 | $ | 0.00 | 117,660 | $ | 0.00 | ||||||||||
| Options exercisable | 3,467,522 | $ | 0.00 | 117,660 | $ | 0.00 | ||||||||||
The total unrecognized estimated compensation cost related to employees’ non-vested stock options granted through June 30, 2013 was $153, which is expected to be recognized over a weighted average period of 1.43 years.
| c. | The following is a summary of the stock options granted to non-employees under the 2010 Plan: |
| For the six months ended June 30, 2013 | ||||||||
| Number of Options | Weighted Average Exercise Price | |||||||
| Outstanding at the beginning of the period | 3,872,953 | $ | 0.04 | |||||
| Issued | 163,948 | $ | 0.00 | |||||
| Forfeited | (118,113 | ) | $ | 0.00 | ||||
| Outstanding at the end of the period | 3,918,788 | $ | 0.04 | |||||
| Options exercisable | 3,774,906 | $ | 0.03 | |||||
| For the six months ended June 30, 2012 | ||||||||
| Number of Options | Weighted Average Exercise Price | |||||||
| Outstanding at the beginning of the period | 1,050,325 | $ | 0.13 | |||||
| Issued | 2,822,627 | $ | 0.00 | |||||
| Outstanding at the end of the period | 3,872,953 | $ | 0.04 | |||||
| Options exercisable | 3,585,190 | $ | 0.02 | |||||
The total unrecognized estimated compensation cost related to non-employees’ non-vested stock options granted through June 30, 2013 was $25, which is expected to be recognized over a weighted average period of 1 year.
| 10 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
JUNE 30, 2013
U.S. DOLLARS
IN THOUSANDS
(Except shares, options stock amounts, option exercise price and par value amount)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 6 - STOCK OPTION PLANS (continued)
| d. | The options outstanding as of June 30, 2013 have been separated by exercise prices, as follows: |
| Exercise Price | # of Options Outstanding | Average Remaining Contractual Life (years) | # of Options Exercisable | |||||||||||
| $ | 0.00 | 8,444,347 | 9.17 | 7,755,344 | ||||||||||
| $ | 0.24 | 575,525 | 6.99 | 431,644 | ||||||||||
| 9,019,872 | 8,186,988 | |||||||||||||
NOTE 7 - COMMITMENTS AND CONTINGENT LIABILITIES
Aggregate minimum rental commitments under non-cancelable leases, as of June 30, 2013, are as follows:
| Period ended June 30, | ||||
| 2014 | 34 | |||
| 2015 | 1 | |||
| Total | $ | 35 | ||
NOTE 8 - STOCK CAPITAL
Due to the Merger as described in Note 10 - Subsequent Events, the capital structure of the Company has changed. Although the Merger occurred after the date of the interim financial statements, the change has been given retroactive treatment in the Company's balance sheets. Pursuant to the Merger Agreement, all shares of the Company’s common and preferred stock that were issued and outstanding immediately preceding the Merger were converted into the right to receive an aggregate of approximately 40,000,000 shares of common stock of the post-Merger company, par value $0.001 per share, after giving effect to a 7.5-for-1 forward split.
NOTE 9 - FINANCIAL (EXPENSES) INCOME, NET
| Period from February 5, 2007 | ||||||||||||
| For the six months ended | (date of inception) | |||||||||||
| June 30, | June 30, | |||||||||||
| 2013 | 2012 | 2013 | ||||||||||
| Financial income | $ | 2 | $ | - | $ | 8 | ||||||
| Financial (expenses) and bank fees | - | - | (10 | ) | ||||||||
| Exchange rate differences gain (loss) | (1 | ) | 16 | (41 | ) | |||||||
| $ | 1 | $ | 16 | $ | (43 | ) | ||||||
| 11 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
JUNE 30, 2013
U.S. DOLLARS
IN THOUSANDS
(Except shares, per and par share value amounts)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 10 - SUBSEQUENT EVENTS
In accordance with ASC 855-10, the Company has analyzed its operations subsequent to June 30, 2013 through the date these financial statements were issued and has determined that the following events that are material subsequent events necessitate disclosure:
On June 26, 2013, Autovative Products, Inc., a Nevada corporation (“Autovative”), entered into that certain Agreement and Plan of Merger (the “Original Merger Agreement”), as amended by that certain First Amendment to the Original Merger Agreement, dated as of July 2, 2013 (the “Amendment” and, together with the Original Merger Agreement, the “Merger Agreement”), by and among Autovative, Ucansi Acquisition Corp., a Delaware corporation and wholly owned subsidiary of Autovative (“Merger Sub”), and the Company. Pursuant to the Merger Agreement, on July 30, 2013 (the “Closing Date”), Merger Sub merged with and into the Company (the “Merger”), with the Company surviving the merger as Autovative's wholly owned subsidiary. Upon consummation of the Merger, Autovative changed its name from Autovative Products, Inc. to GlassesOff Inc.
Pursuant to the Merger Agreement, all shares of the Company’s common and preferred stock that were issued and outstanding immediately preceding the Merger were converted into the right to receive an aggregate of approximately 40,000,000 shares of Autovative's common stock, par value $0.001 per share (“Common Stock”), after giving effect to a 7.5-for-1 forward split of Autovative's Common Stock (the “Forward Split”). Following the Merger, the Company’s former stockholders hold approximately 80% of Autovative's issued and outstanding Common Stock. Additionally, pursuant to the Merger Agreement, upon consummation of the Merger, Autovative assumed all of the Company’s options and warrants that were issued and outstanding immediately prior to the Merger and issued to the holders of such securities in exchange therefor options and warrants to acquire approximately 9,019,872 shares and 7,523,504 shares of Common Stock, respectively, in each case after giving effect to the Forward Split. None of the shares of Common Stock, warrants, options or shares of Common Stock issuable upon exercise of such warrants and options issued by Autovative in connection with the Merger (collectively, the “Merger Securities”) have been registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state securities laws and may not be sold except in a transaction registered under, or exempt from, the registration provisions of the Securities Act and applicable state securities laws. Autovative issued the Merger Securities in reliance upon the exemption from registration contained in Section 4(2) of the Securities Act. Persons acquiring Merger Securities represented to Autovative that they were “accredited investors” as defined in Rule 501(a) under the Securities Act and that the Merger Securities were being acquired for investment purposes.
Additionally, on the Closing Date, Autovative consummated a private placement (the “Private Placement”) in connection with which Autovative entered into subscription agreements (each, a “Subscription Agreement”) with certain private investors (the “Investors”), pursuant to which the Investors purchased an aggregate of 2,490,000 units (each, a “Unit”), for a purchase price in cash of $1.25 per Unit, each of which comprised one share of Common Stock and one five-year warrant (each, a “Warrant”) to purchase one share of Common Stock at an exercise price of $1.25 per share (each, a “Warrant Share”). Before expenses, Autovative received an aggregate of approximately $3,113 in gross proceeds from the issuance of the Units. Autovative issued the Units, including the underlying Common Stock, Warrants and Warrant Shares (collectively, the “Private Placement Securities”) in reliance upon the exemption from registration contained in Section 4(2) of the Securities Act. The Investors represented to Autovative that they were “accredited investors” as defined in Rule 501(a) under the Act and that the Shares were being acquired for investment purposes.
| 12 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
JUNE 30, 2013
U.S. DOLLARS
IN THOUSANDS
(Except shares, per and par share value amounts)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 10 - SUBSEQUENT EVENTS (continued)
Both the Company and Autovative have incurred customary transaction-related costs in connection with the Merger. The consolidation effected by the Merger will be accounted for as a reverse acquisition wherein the Company will be treated as the acquirer for accounting purposes as it has acquired control of the combined enterprise.
On or about December 31, 2012, Autovative contributed all of the operations, assets and liabilities of its historical business, generally comprising the distribution and development of automotive parts (the “Legacy Business”), to a newly-formed wholly owned subsidiary, Autovative Technologies, Inc. (“Autovative Tech”). On May 23, 2013, Autovative entered into a spin-off agreement (the “Spin-Off Agreement”) with its former Chairman of the Board and Chief Executive Officer, David Funderburk, pursuant to which, effective on the Closing Date, Autovative sold, assigned, transferred and conveyed to Mr. Funderburk all of the issued and outstanding capital stock of Autovative Tech, in exchange for which Mr. Funderburk has agreed to indemnify Autovative for, and hold Autovative harmless from, any and all losses and liabilities arising out of, or relating to, the Legacy Business. We refer to the foregoing as the “Split-Off”.
In connection with the Merger, Autovative's Board of Directors and holders of in excess of a majority of its issued and outstanding Common Stock (the “Majority Holders”) voted to amend its Articles of Incorporation (the “Amended and Restated Articles”) to (i) increase its capitalization to provide for the issuance of up to 200,000,000 shares of its Common Stock and up to 20,000,000 shares of “blank check” preferred stock, par value $0.001 per share, and (ii) change its name from Autovative Products, Inc. to “GlassesOff Inc.” In connection with the name change, Autovative expect that its Common Stock, which is currently traded on the OTCBB and the OTCQB under the symbol “ATVP”, will soon trade under a new symbol. Also in connection with the Merger, Autovative's Board of Directors amended and restated its bylaws (the “Amended and Restated Bylaws”).
The Majority Holders approved the Amended and Restated Articles pursuant to a written consent. An aggregate of 7,080,555 votes were cast “for” the Amended and Restated Articles, no votes were cast “against” the Amended and Restated Articles, and there were no abstentions or broker non-votes.
Effective upon consummation of the Merger: (i) the board of Autovative increased the number of seats on the board from one to four; (ii) in accordance with its bylaws, Mr. Qasim Husain, MD, the sole director, appointed Messrs. Novik, Madar and Shaffir and Prof. Polat to fill the vacancies created by the increase in number of seats on our board; and (iii) Dr. Husain resigned as a director. Each of the new directors will hold office until the earlier of the next annual meeting of stockholders and the election and qualification of their successors or their earlier death, resignation or removal. Additionally, effective upon consummation of the Merger, Dr. Qasim resigned as Chief Executive Officer and the Board of Directors appointed the following persons to serve in the offices set forth across from their names:
| Name | Title | |
| Nimrod Madar, M.B.A. | President and Chief Executive Officer | |
| Uri Polat, Ph.D. | Chief Scientific Officer | |
| Ram Shaffir | Chief Technology Officer | |
| Steve Schaeffer, CPA | Chief Financial Officer |
| 13 |
UCANSI INC. AND SUBSIDIARY
(A DEVELOPMENT STAGE COMPANY)
JUNE 30, 2013
U.S. DOLLARS
IN THOUSANDS
(Except shares, per and par share value amounts)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 10 - SUBSEQUENT EVENTS (continued)
Upon execution of the new officer agreements on the Closing Date, Autovative granted Dr. Polat and Messrs. Madar and Shaffir options to purchase 350,000, 150,000 and 50,000 shares of Common Stock, respectively, at an exercise price of $1.25 per share, which options will vest in substantially equal amounts on the first, second and third anniversaries of the date of grant. In addition Autovative granted Messrs. Madar and Shaffir 200,000 and 300,000 shares, respectively, of restricted Common Stock, vesting in substantially equal amounts on the first, second and third anniversaries of the date of grant.
Upon appointment as Chairman of the Board at the Closing Date, Autovative granted Mr. Novik 300,000 shares of restricted Common Stock, which vest in substantially equal monthly installments over a period of 12 months from the date of grant. In addition, Autovative granted Mr. Novik an option to purchase 150,000 shares of Common Stock at an exercise price equal to $1.25 per share, which option vests in substantially equal monthly installments over a period of 12 months from the date of grant.
Pursuant to the Merger Agreement, upon consummation of the Merger, the Company has adopted a new incentive compensation plan – the GlassesOff Inc. 2013 Incentive Compensation Plan (the “Plan”). According to the plan the total number of shares initially reserved and available for delivery under the Plan shall be 14,000,000.
| 14 |
