Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Inspyr Therapeutics, Inc. | v356987_8k.htm |

Specific targeting of tumors using a potent, unique prodrug GNSZ OCTOBER 2013

SAFE HARBOR STATEMENT GNSZ | OCTOBER 2013 PAGE 2 Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995: Any statements that are not historical facts are forward - looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward - looking statements, which may include, but are not limited to, factors related to GenSpera's anticipated growth strategies, the outcome of its clinical trials, future business development, ability to develop new products, expand to other related industries or markets in other geographical locations, and other information detailed from time to time in the filings and future filings with the United States Securities and Exchange Commission. Readers are advised that this information is intended for the use of investment professionals. Anyone interested in obtaining information on GenSpera should contact GenSpera to receive GenSpera's most recent financial reports. This presentation was developed by GenSpera and is intended solely for informational purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy the Company's stock. This presentation is based upon information available to the public, as well as other information from sources which management believes to be reliable, but is not guaranteed by the Company as being accurate nor does it purport to be complete. Opinions expressed herein are those of management as of the date of presentation and are subject to change without notice.

GENSPERA OVERVIEW GNSZ | OCTOBER 2013 PAGE 3 Highly differentiated, targeted therapeutic agent with encouraging clinical data • Platform technology developed at Johns Hopkins • Lead drug candidate, G - 202, is designed to be activated only within tumor • Novel mechanism of tumor killing, benign side effect profile • G - 202 is expected to be active in a wide range of tumor types • Completed Phase I with encouraging data in liver cancer • Phase II ongoing in liver cancer patients - preliminary data expected Q2 2014
A NOVEL CYTOTOXIN GNSZ | OCTOBER 2013 PAGE 4 • Active toxic ingredient from Thapsia garganica – a Mediterranean plant used for centuries in folk remedies • Unique molecular mechanism of action • Potent inhibitor of the intracellular SERCA pump - causes Ca2 + (calcium) to rise significantly and trigger apoptosis (cell death) • Kills cancer cells independent of growth rate • Completely indiscriminate – must be delivered directly to tumor Thapsigargin
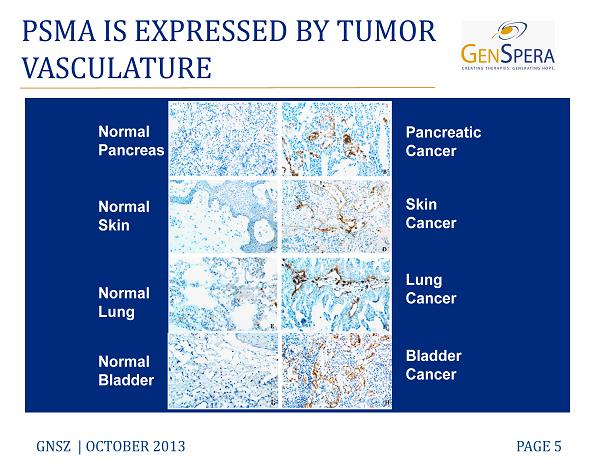
PSMA IS EXPRESSED BY TUMOR VASCULATURE GNSZ | OCTOBER 2013 PAGE 5 Normal Pancreas Normal Skin Normal Lung Normal Bladder Pancreatic Cancer Bladder Cancer Lung Cancer Skin Cancer
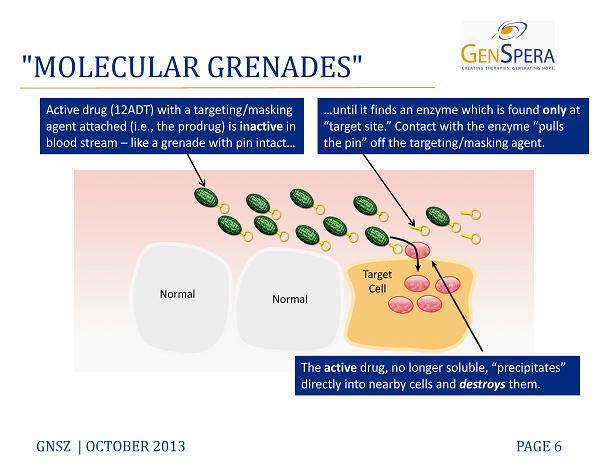
"MOLECULAR GRENADES" GNSZ | OCTOBER 2013 PAGE 6 Active drug (12ADT) with a targeting/masking agent attached (i.e., the prodrug) is inactive in blood stream – like a grenade with pin intact… …until it finds an enzyme which is found only at “target site.” Contact with the enzyme “pulls the pin” off the targeting/masking agent. The active drug, no longer soluble, “precipitates” directly into nearby cells and destroys them. Normal Normal Target Cell

G - 202 PHASE I RESULTS GNSZ | OCTOBER 2013 PAGE 7 • Recommended dosing regimen: IV infusion, 40 mg/m 2 on Day 1, 66.8 mg/m 2 on Days 2 and 3 of 28 - day schedule with hydration and standard pre - medications • Side Effect Profile: Easily managed minimal side effects (fatigue, nausea on Day 1, rash, transient small elevation in serum creatinine). No significant effect on liver, bone marrow or cardiovascular system.

HEPATOCELLULAR CARCINOMA GNSZ | OCTOBER 2013 PAGE 8 • Hepatocellular carcinoma (HCC) is the most common type of liver cancer • 6 th most common type of cancer worldwide and 3 rd most common cause of cancer deaths worldwide • 75% of HCC patients are in Asia - Pacific Rim with ~360,000 deaths annually • Only one approved drug (Nexavar®) – gives ~ 2 - 3 month increase in median survival
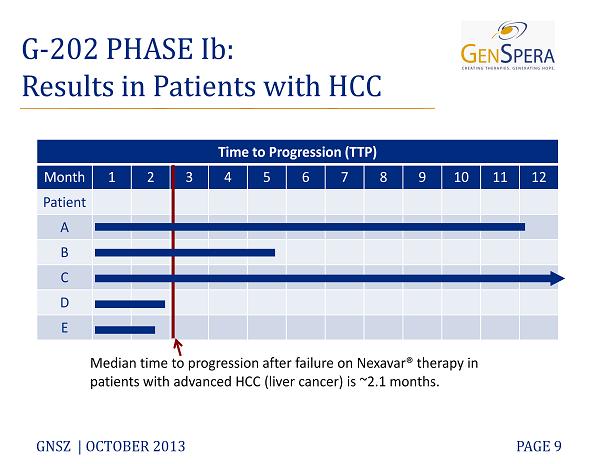
G - 202 PHASE Ib : Results in Patients with HCC GNSZ | OCTOBER 2013 PAGE 9 Time to Progression (TTP) Month 1 2 3 4 5 6 7 8 9 10 11 12 Patient A B C D E Median time to progression after failure on Nexavar® therapy in patients with advanced HCC (liver cancer) is ~2.1 months.
G - 202 PHASE II LIVER CANCER GNSZ | OCTOBER 2013 PAGE 10 • Population: Patients with progressive advanced hepatocellular cancer (HCC) following Nexavar® therapy • Dosing Regimen: 40 mg/m 2 on Day 1, 66.8 mg/m 2 on Days 2 and 3 of 28 - day schedule • Primary Endpoint: Time to disease progression (TTP) • Number of Patients: up to 29 patients (9 enrolled to date) • Interim Data Available: Expected Q2 2014

FINANCIAL OVERVIEW GNSZ | OCTOBER 2013 PAGE 11 • Raised $24M since November 2007 – majority of capital from repeat investors • ~$5M on hand – burn rate ~ $1M per quarter • ~27M common issued and outstanding • ~17M warrants and options • Management & Scientific Advisory Board own ~30% Cash Stock

SUMMARY AND MILESTONES GNSZ | OCTOBER 2013 PAGE 12 • G - 202 is a targeted agent with a unique mechanism of action and benign side effect profile • Encouraging Phase I human clinical data in liver cancer • Phase II liver cancer trial underway - preliminary data expected in Q2 2014 • Pipeline of additional solid tumor indications • Anticipated out - license or partnering subsequent to Phase II

Specific targeting of tumors using a potent, unique prodrug GNSZ OCTOBER 2013
