Attached files
| file | filename |
|---|---|
| EX-10.1 - LICENSE AGREEMENT - Jubilant Flame International, Ltd | jfil_ex101.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
________________
FORM 8-K
________________
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(D) OF
THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported)
September 30, 2013
Jiu Feng Investment Hong Kong Ltd
(Exact name of registrant as specified in its charter)
|
Nevada
|
333-173456
|
27-2775885
|
||
|
(State or other jurisdiction of incorporation)
|
(Commission File Number)
|
(IRS Employer of Identification No.)
|
2293 Hong Qiao Rd, Shanghai China, 200336
(Address of principal executive offices)
+86 21 64748888
Registrant’s telephone number, including area code
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
¨
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
|
¨
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
|
|
¨
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
|
|
¨
|
Item 1.01 Entry into a Material Definitive Agreement.
On September 30, 2013 Jiu Feng Investment Hong Kong Ltd., a Nevada corporation (the “Company”) entered into a License Agreement (the “Agreement”) with BioMark Technologies (Asia) Limited, a limited liability company incorporated in Hong Kong under the Companies Ordinance ("BioMark") whereby the Company is licensed, worldwide, for a period of five years, to sell, market, and/or distribute certain products pertaining to the health care industry (the “Licensed Products”); and to conduct research and development of BioMark’s cancer detection scanning technology. In the event that the research and development of BioMark’s cancer detection scanning technology provides marketable technology, the Company shall have the right of first refusal to a license to market, sell and distribute such cancer detection scanning technology. The Company’s president, Ms. Yan Li, is also president of BioMark. The Company does not have any policies or procedures in place for the review, approval or ratification of transactions with related persons.
2
Licensed Products:
The primary Licensed Products include the following Bone-Induction Artificial Bone (BIAB) products and Vacuum Sealing Drainage (VSD) products:
Product List
|
Name
|
Description
|
|
VSD 1
|
Negative pressure drainage special bolster
|
|
VSD 2
|
Negative pressure drainage special bolster
|
|
VSD 3
|
Medical Operation Film
|
|
VSD 4
|
Medical Operation Film
|
|
VSD 5
|
Negative pressure drainage device
|
|
VSD 6
|
Negative pressure drainage device
|
|
Bone induction Artificial bone A1
|
Bone induction to tissue regeneration membrane
|
|
Artificial bone A1
|
Artificial bone to tissue regeneration membrane
|
|
Bone induction Artificial bone A2
|
Bone induction to albumin layer
|
|
Artificial bone A2
|
Artificial bone to collagen layer
|
|
Bone induction Artificial bone A3
|
Bone induction to regeneration microporous membrane
|
|
Artificial bone A3
|
Artificial bone to regeneration microporous membrane
|
|
Bone induction Artificial bone A4
|
Bone induction to microporous albumin layer
|
|
Artificial bone A4
|
Artificial bone to microporous albumin layer
|
|
Xishu Qing
|
Gynecological antibacterial care dressing
|
|
Microcyn Skin and Wound Hydrogel
|
Gel dressing
|
|
Incision protection sleeve
|
Incision protection sleeve
|
|
Kangfu Shengyuan
|
Collagen antimicrobial dressing
|
3
I. Bone-Induction Artificial Bone
BIAB has completed over 200 animal tests, 5000 clinical trail tests, and was approved by the State Food and Drug Administration of China (“SFDA”) in 2006. The BIAB won the second prize of 2007 China National Natural Science. VSD also has been approved by SFDA in 2006.
BIAB is a bionic porous repairing bone material which is made of calcium phosphate through a special process. Its composition and structure is similar with the natural mineral of human bone, which stands for its predominant bio-compatibility, biological activity and biological safety. It helps to absorb human self’s BMP growth factor; it also regulates gene function to induct bone regeneration, shorter the convalescence, and meet the target of repairing bone defect permanently. The advanced artificial bone is used: (i) in repairing traumatic bone defects; (ii) in repairing bone defect after complete removal of bone tissue as required in the treatment of certain diseases including bone tumor, bone tuberculosis, chronic osteomyelitis, osteofibrous dysplasia, delayed union, nonunion, and false joint fracture; (iii) for treatment of bone loss or bone defects caused by congenital malformation; (iv) as a filling material for spinal fusion, joint fusion, and orthopedic bone grafting; and (v) as a filling material for bone grafting fusion and decompressive laminectomy.
Product Characteristics:
The BIAB provides three-dimensional support structure and the physical and chemical composition which is similar to the body's natural bone mineral. They reassembled in human body’s environment. It can help lead the fibrous tissue and bone marrow stromal stem cells to grow into the porous of the material, thus obtains the essential multipotent mesenchymal cells for bone formation and provide the growth support of cells.
The human body fluid contains BMP and other growth factors, but the content is too low, and is not enough to cause induction phenomena happen. The specific composition and structure of BIAB provides the growth factor with binding sites. The material implanted could selectively enrich and adsorb the bone growth factors in the blood and fluid of human body. The implantation of growth factor in the microenvironment will induce mesenchymal cells to the osteoblasts differentiation and new bone growth threshold. Under the synergistic effect of bone induction of signaling molecules and biological environment, BIAB can promote bone gene up-regulation, enhance down-stream gene function, and regulate cell movement in the direction of bone differentiation.
As the cells and nutrients transfer through the porous structure, the BMP growth factors cause the formation and maturation of new bone within the Bone-induction artificial bone. The implanted materials are thus gradually replaced with new bone, and the new bone finishes growth and ossification.
4
This innovative material provides several benefits:
|
1.
|
Optimizes bone conduction performance
|
|
2.
|
Precise osteo-induction
|
|
3.
|
Rapid bone formation
|
|
4.
|
Suitable biodegradation absorption and ossification
|
|
5.
|
Long-term safety of implantation.
|
5
Comparison with other products
|
Category
|
|
Advantage and Disadvantage
|
|
|
Autogenous bone graft material
|
l Bone conduction and bone induction property
l None immunological rejection
l May damage healthy tissue, cause secondary vulnus to patients
l Source of bone is limited; operation lasts longer, higher risk of intra-operative bleeding and infection
l May cause injury and pain around the bone
|
||
|
Allogenic bone transplantation material and Xenogeneic bone transplantation material
|
l Only bone conduction property, no bone induction property
l Limit Source
l Potential of immunological rejection and spreading underlying diseases
l May cause over reaction with large numbers of applications
|
||
|
Traditional artificial synthetic material
|
l Good biocompatibility and bone conduction property
l No bone induction; absorptivity does not match the speed of bone growth
l Only for filing material, not for bone tissue regeneration
|
||
|
External growth factor and bone matrix removal protein
|
l Bone induction
l External source
l No mechanical strength, need support material in practices
l Potential risk of immunological rejection and spreading underlying diseases
l High requirements for storage and transportation
l Not fully mature technology
|
||
|
BioMark’s Bone-induction artificial bone
|
l Both bone conduction and safe bone induction properties
l Replicates normal process of osteogenesis and bone formation
l Sufficient and safe sources
l Avoids immunological rejection and spreading underlying diseases, is an ideal material for bone repairing
|
6
Comparison with similar products
|
Biological safety
|
Absorption
|
Bone induction
|
|
|
HA、Silicate
|
+
|
-
|
-
|
|
ß-TCP、Caso4
|
+
|
Too fast
|
-
|
|
Allogeneic bone
|
-
|
+
|
-
|
|
Allogeneic bone + BMP/DBM
|
?
|
+
|
+
|
|
BioMark’s Bone-induction artificial bone
|
+
|
Moderate
|
+
|
7
II. Vacuum Sealing Drainage
VSD was approved by the SFDA in 2006. It is made of polyvinyl alcohol aqueous gelatin foam: a three-dimensional porous structure, which is non ciliated, and exhibits strong water absorption characteristics. It is hydrophilic and has excellent thermal insulation capabilities as compared with other vacuum sealing drainage specialty foams. VSD has good histocompatibility and will not adhere to a wound. VSD aids skin creation around a wound bed with minimal vulnus. The dressing material acts as a drug carrier with strong bactericidal characteristics, and the gelatin protein promotes the growth of granulation, accelerating wound healing. It can be used in the surgery of burns, orthopedics, trauma repair, plastic, and general surgery.
Product Characteristics:
Advantages:
|
1.
|
Good treatment effect. VSD allows an individualized complete treatment plan, which fully ensures the effect of clinical treatment. VSD basically eliminates adverse events such as clinical wound blowing and drainage tube blocking, leading to excellent treatment reliability;
|
|
2.
|
Easy to operate. Using VSD is as simple as changing a fresh dressing for the wound; the material does not adhere with the wound, which avoids secondary vulnus;
|
|
3.
|
Large range of indications; innovation of operation, especially for large size wound treatments.
|
8
Comparison with previous technology
|
Category
|
Using Method
|
Requirements for the surrounding skin
|
Product properties
|
Clinical effect
|
Adverse events happening %
|
Indication
|
|
Old technology
|
Need certain conditions, experience and technology.
Difficult to seal the wound;
operation time long;
huge nursing work
|
High
|
Single function;
can not clean the wound
|
Common
|
Drainage tube blocking >70%
Wound blowing 100%
Material becomes dry and hard >90%
|
Suitable for in- patients
|
|
BioMark’s
VSD
technology
|
No certain conditions, experience and technology required.
Easy to seal the wound;
operation time low;
small nursing work
|
No special requirements.
|
Functions of wound cleaning and vacuuming
|
Good
|
All very seldom
|
Suitable for out-patient and in-patient
|
9
Comparison with other products
|
Category
|
Working principals
|
|
Using method
|
Products properties
|
Clinical Effect
|
Adverse events happening %
|
|
BioMark’s
VSD
Products
|
Cleaning the wound through the inlay drainage tube which transmits the vacuum
|
|
Easy
|
Functions of wound cleaning and vacuuming
|
Good
|
Very seldom
|
|
Other VSD/VAC with suckers
|
Drainage tube is connected with the foam material through the suckers.
Transmitting Vacuum effect is poor;
Draining effect is poor.
Potential problem for drainage tube blocking.
|
Need open the sealing membrane to clean the suckers.
Hard to use the suckers since the different sizes of wound .
|
Single function
|
Poor
|
Very high, Drainage tube blocking happens upto 70% after a 3-days usage
|
10
III. Cancer Detection Scanning Technology
The Company is also licensed to conduct research and development of BioMark’s cancer detection scanning technology. The technology uses biomarkers for the early detection of cancers. In the event that the research and development of BioMark’s cancer detection scanning technology provides marketable technology, the Company shall have the right of first refusal to a license to market, sell and distribute such cancer detection scanning technology.
BioMark’s cancer detection scanning technology provides innovative techniques and assay analysis to increases early detection of tumors in the latent growth phase.

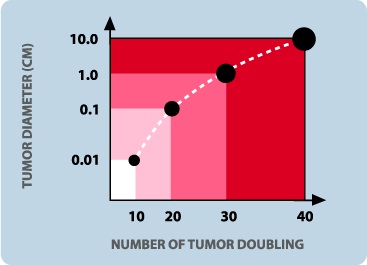
Poor prognosis associated with late diagnosis = large tumour size
The graph above indicates the current limit of clinical detection for most tumours. A good 70% of the natural history of the tumour has already existed by time it is detected.
11
Facts About Cancer
The leading cause of premature mortality
1 in 3 individuals will develop cancer
70% of those will die as a result of the disease
7.6 million deaths a year or 20,000 per day
Poor prognosis due to poor therapy and, poor detection
Cancer Prevalence
|
CANCER SITE
|
NEW CASES
|
|
Lung and Bronchus
|
1.6 million
|
|
Colon and Rectum
|
1.12 million
|
|
Stomach
|
1.1 million
|
|
Esophagus
|
0.56 million
|
|
Liver
|
0.7 million
|
|
Breast
|
1.3 million
|
|
Prostrate
|
0.8 million
|
|
Cervix
|
0.6 million
|
Demographics
750,000 cases of breast, lung and prostate cancer diagnosed annually in the U.S. alone
Those who are most aware of the dangers of specific cancers are also those most able and likely to pay for early screening, detection and treatment
High awareness of these diseases among health care professionals and among the general Population
Cancer has become one of the most significant causes of morbidity and mortality in the world, and recently overtook heart disease as the leading cause of death for Americans
Close to 20 million people in Europe and the U.S. live with cancer today and approximately 2.6 million new cases are diagnosed each year
The number of new cases diagnosed each year is increasing mainly as a result of demographics, because most types of solid cancer are typically diseases of the elderly
More than 6 million people around the world die of cancer every year, and one of two men and one of three women will develop cancer in their lifetimes. The overall annual costs associated with malignancies currently amount to $107 billion (Source: Biomarkers in Oncology, June 29, 2004)
12
Characterics of an Ideal Cancer Biomarker
Can be detected in the early stages of disease
Accurately detected
Highly specific
Detected with high sensitivity
Low cost
Reliable
Non-invasive method
Applications of Biomarkers
Early disease identification
Identification of potential drug targets
Predicting the response of patients to treatments
Acceleration of clinical trials
Personalized medicine
Industry Trends
Rapid rise in specific cancers - breast, lung, and prostate cancer cases in U.S. have doubled over past 20 years
Currently, diagnostic findings influence 60–70% of healthcare decision-making (source: Lewin Grp)
More health services delivered out of hospital — need for technology that is portable and compact
Increased popularity of wellness centers throughout the world — interest and demand for preventative medicine
13
Market for Diagnostic Equipment
Worldwide market for diagnostics was estimated to be $28.6 billion in 2005. U.S. accounted for $11.2 billion.
Diagnostic testing in hospitals accounts for 60% of revenue from diagnostics; reference labs account for 32%
Low compliance with diagnostic-based quality measures was linked to up to 34,000 avoidable deaths and $900 million in avoidable healthcare costs in the U.S., according to the National Committee for Quality Assurance
Item9.01Financial Statement and Exhibits.
(d) Exhibits.
| EXHIBIT 10.1 | LICENSE AGREEMENT |
14
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
Jiu Feng Investment Hong Kong Ltd
|
||
|
|
|
||
|
Date: October 3, 2013
|
By:
|
/s/ Yan Li
|
|
|
|
|
Yan Li
|
|
|
|
President and Director
|
||
15
