Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Mirati Therapeutics, Inc. | a2216897z8-k.htm |
QuickLinks -- Click here to rapidly navigate through this document
Overview
We are a clinical-stage biopharmaceutical company focused on developing a pipeline of targeted oncology products. We focus our development programs on drugs intended to treat specific subsets of cancer patients with unmet needs. Our pipeline consists of three product candidates: MGCD265, MGCD516 and mocetinostat. MGCD265 and MGCD516 are orally-bioavailable, multi-targeted kinase inhibitors with distinct target profiles that are in development to treat patients with non-small cell lung cancer, or NSCLC, and other solid tumors. MGCD265 is in Phase 1/2 clinical development and MGCD516 is in advanced preclinical development, with Phase 1 clinical development anticipated to begin in the first half of 2014. Mocetinostat is an orally-bioavailable, spectrum-selective histone deacetylase, or HDAC, inhibitor for the first line treatment of patients with myelodysplastic syndromes, or MDS. We are planning to initiate a Phase 3 clinical trial of mocetinostat in the second half of 2014.
We believe that an increased understanding of the genomic factors that drive tumor cell growth can lead to the development of cancer drugs with increased efficacy while reducing side effects. We are leveraging this knowledge to develop targeted cancer therapies to address unmet needs in selected cancer patient populations. Our novel kinase inhibitors target specific mutations present only in cancer cells, and mocetinostat acts through epigenetic mechanisms important in treating certain cancers. We plan to identify additional opportunities by leveraging our deep scientific understanding of molecular drug targets and mechanisms of resistance and potentially in-licensing promising, early-stage novel drug candidates.
Our three product candidates are as follows:
- •
- MGCD265 is an
orally-bioavailable, potent, small molecule multi-targeted kinase inhibitor of Met, Axl and VEGFRs. MGCD265 is in development for the treatment of solid tumors, with an initial focus on NSCLC and
squamous cell carcinoma of the head and neck, or HNSCC. We have conducted single agent and combination dose escalation trials in 252 patients, with acceptable tolerability and promising early signs of
clinical efficacy in patients with advanced solid tumors who have failed standard therapies. Our preclinical studies, in a variety of in vivo tumor models, have suggested that MGCD265 has relatively
low toxicity and appears more potent than some of the leading approved kinase inhibitors, including Nexavar, Sutent and Xalkori. We have developed new formulations of MGCD265 designed to increase
plasma exposure, improve the degree of target inhibition and increase the likelihood of seeing single agent clinical activity. Assuming one or more of the new formulations achieve sufficient patient
exposure in ongoing studies, we intend to select one of the new formulations for introduction into ongoing dose escalation trials with the goal of identifying the maximum tolerated dose, or MTD, by
early 2014. Following identification of the MTD, we plan to initiate dose expansion cohorts in patients selected for certain biomarkers.
- •
- MGCD516 is an
orally-bioavailable, potent, small molecule multi-targeted kinase inhibitor of RET, TRK, DDR and EphRs, as well as Met, Axl and VEGFRs, in development for the treatment of solid tumors. We plan to
focus on solid tumors expressing RET, TRK and DDR, initially in NSCLC, and we plan to evaluate other tumor types where the profile of MGCD516 would suggest activity. MGCD516 is in advanced preclinical
development. We plan to file an investigational new drug application, or IND, with the U.S. Food and Drug Administration, or FDA, and initiate a Phase 1 clinical trial of this product candidate
in the first half of 2014, and identify the MTD and initiate expansion cohorts in patients selected for certain biomarkers by the end of 2014.
- •
- Mocetinostat is an orally-bioavailable, spectrum-selective HDAC inhibitor for which we plan to conduct a dose confirmation trial starting in the fourth quarter of 2013, with the goal of initiating a Phase 3 clinical trial in the second half of 2014. We have completed 13 clinical trials which enrolled 437 patients with a variety of hematologic malignancies and solid tumors. We intend to seek a Special Protocol Assessment, or SPA, from the FDA prior to the initiation of our planned Phase 3
1
trial. This trial will evaluate mocetinostat for the first line treatment of patients with MDS in combination with Vidaza, a hypomethylating agent, or HMA. We believe that mocetinostat has the potential to be the first HDAC inhibitor to market for this indication.
Our management team has extensive experience in leading the discovery and development of targeted oncology therapies. Our President and Chief Executive Officer, Charles M. Baum, M.D., Ph.D., was Senior Vice President for Biotherapeutic Clinical Research within Pfizer Inc.'s Worldwide Research and Development division and previously Head of Oncology Development for Pfizer. Prior to Pfizer, he was also responsible for the development of several oncology compounds at Schering-Plough Corporation (acquired by Merck & Co., Inc., or Merck). Our Chief Medical and Development Officer, Isan Chen, M.D., was Chief Medical Officer of Aragon Pharmaceuticals, Inc., which was acquired by Johnson & Johnson in 2013. At Aragon Pharmaceuticals, Dr. Chen was responsible for the clinical development strategy of all of the company's programs, including prostate and breast cancer. Our Vice President of Research, James Christensen, Ph.D., was previously the Senior Director of Oncology Precision Medicine at Pfizer, where he was responsible for strategy and translational research for the entire Pfizer oncology portfolio. The collective experience of our research and development team includes direct involvement in the development and approval of a number of oncology drugs including Inlyta, Sutent, Temodar and Xalkori. In addition, our Executive Vice President and Chief Operations Officer, Mark J. Gergen, has experience in operations, finance, strategy and corporate development and was previously Senior Vice President of Corporate Development at Amylin Pharmaceuticals, Inc. until its acquisition by Bristol-Myers Squibb Inc. in 2012.
We have a collaboration agreement with Taiho Pharmaceutical Co. Ltd., or Taiho, covering mocetinostat in certain Asian territories and we own all rights to mocetinostat outside of those territories.
Our Strategy
Our goal is to be a leading developer of targeted cancer therapies for selected patient populations. The key components of our strategy include:
- •
- Develop a pipeline of targeted cancer
therapies. We believe that an increased understanding of the genomic factors that drive tumor cell growth can lead to the development of
cancer drugs with increased efficacy while reducing side effects. We are leveraging this knowledge to develop targeted cancer therapies to address unmet needs in specific cancer populations. Our
current pipeline is comprised of novel kinase inhibitors that target specific mutations present only in cancer cells and one of the most advanced epigenetic therapies in development. We plan to
identify additional targets by leveraging our deep scientific understanding of molecular drug targets and mechanisms of resistance and potentially in-licensing promising, early-stage novel drug
candidates.
- •
- Employ efficient and flexible approaches to
accelerate clinical development. We will pursue indications and select specific patient populations in which activity of our product
candidates can be assessed early in clinical development. When designing clinical trials, we structure our clinical development approach to test multiple clinical hypotheses in a single trial and
design trials with the flexibility to adapt quickly and accelerate once a signal of clinical activity is observed. We believe our approach may increase the likelihood of seeing results early in
clinical trials with fewer patients, reducing our clinical development risk and allowing us to potentially accelerate the development of our product pipeline.
- •
- Advance our two lead kinase inhibitors. Kinase inhibitors have significantly improved the care of many cancer patients and represent a commercially successful category of targeted cancer therapies with sales of over $29.1 billion in 2011, according to BCC Research. We have two internally discovered novel kinase inhibitors in development: MGCD265 and MGCD516. These product candidates target pathways of high scientific interest, including Met, Axl, TRK, RET, DDR, EphRs and VEGFRs, and are believed to be important in the regulation of tumor growth. We plan to initiate a Phase 2 clinical trial for MGCD265 and a Phase 1 clinical trial for MGCD516 in 2014.
2
- •
- Advance mocetinostat, our later-stage product
candidate. HDAC inhibitors have been shown to be effective in treating hematologic malignancies, as evidenced by the approval of Istodax
and Zolinza. We have completed 13 clinical trials in 437 patients which have shown promising signs of activity of mocetinostat in MDS and other hematologic malignancies. We believe that the
combination of the epigenetic mechanisms of mocetinostat and Vidaza may be effective in treating MDS. Subject to receipt of additional financing and successful completion of our planned dose
confirmation trial, we are planning to initiate a Phase 3 registration trial of mocetinostat in 2014 under an SPA to be agreed upon with the FDA for the first line treatment of patients with
MDS in combination with Vidaza.
- •
- Leverage partnerships to develop our product candidates. We plan to collaborate with third parties and partner certain rights to our product candidates as a means to accelerate their broader clinical development and maximize their therapeutic and market potential. We plan to retain certain key development and commercialization rights in our partnerships. We believe that retaining this strategic flexibility will enable us to maximize shareholder value.
Product Candidates
The following chart depicts the current state of our oncology development programs:
PRODUCT CANDIDATE |
INDICATION | TARGETS | COMMERCIAL RIGHTS |
STAGE OF DEVELOPMENT AND ANTICIPATED MILESTONES |
||||
|---|---|---|---|---|---|---|---|---|
| MGCD265 | Solid Tumors | Met, Axl, VEGFRs | Mirati: Global | • Initiate expansion cohorts Q1 2014 • Initiate Phase 2 Q4 2014 |
||||
MGCD516 |
Solid Tumors |
RET, TRK, DDR, EphRs, Met, Axl, VEGFRs |
Mirati: Global |
• Planned IND submission and initiate Phase 1 1H 2014 • Initiate expansion cohorts Q4 2014 |
||||
Mocetinostat |
MDS |
HDACs |
Taiho: Certain Asian Territories |
• Initiate dose confirmation trial Q4 2013 • Obtain SPA for Phase 3 1H 2014 • Initiate Phase 3 2H 2014 |
||||
Mirati: All Other Territories |
Our Targeted Kinase Programs
Targeted therapies selectively inhibit specific genes or pathways that are present in certain types of cancer cells and not in normal tissue. Receptor tyrosine kinases, or RTKs, are a family of kinases involved in the transmission of signals that regulate the expression of many genes, including those that control cell growth and cell division. RTKs may be inappropriately expressed in cancerous tissues resulting in uncontrolled tumor cell growth. Aberrant kinase function, caused by mutations or over-expression, underlies many cancer cell processes, making the kinome an important source for therapeutic targets in oncology. Discoveries of specific drivers of disease have led to the development of targeted therapies, or the tailoring of therapies to a particular tumor or disease profile. In some cases, these therapies have proven to be more efficacious while having fewer side effects than traditional non-targeted therapies, such as chemotherapy, which kill healthy cells along with cancer cells. Examples of successful development of oral targeted kinase inhibitors include Novartis AG's Gleevec, a BCR-ABL kinase inhibitor for the treatment of Philadelphia chromosome positive chronic myelogenous leukemia, and GlaxoSmithKline's Tykerb, a HER2 kinase inhibitor for the treatment of a subset of breast cancer patients over-expressing the HER2 kinase. Further examples of oral targeted kinase inhibitors include Pfizer's Xalkori and Bosulif and Bristol-Myers Squibb's Sprycel. We believe that therapies that target specific genetic abnormalities in subsets of cancer patients identified through diagnostic tests will result in streamlined clinical trials, stratified patient populations and improved patient outcomes and will be increasingly important in the continued evolution of the treatment of cancer.
3
We believe that by selecting patients whose tumors over-express specific genes, as well as patients with genetic mutations in the pathways that are critical for tumor growth and are potently inhibited by our drugs, we will increase the potential for clinical benefit. The greater clinical benefit in selected patients may increase the likelihood of seeing clinical activity earlier in development, potentially in Phase 1, which may allow us to move rapidly into registration trials. As a part of our ongoing development activities, we are using commercial diagnostic assays as well as assays developed internally for early clinical trials. We are working with external diagnostic providers to develop validated companion diagnostics for later stage clinical use and registration to ensure that the diagnostic is available for commercial use upon approval.
The clinical and commercial success of leading small molecule kinase inhibitors demonstrates the potential of new targeted treatments for cancer. BCC Research data indicates that the global kinase inhibitor market was $29.1 billion in 2011, and is expected to reach $40.2 billion by 2016. The following table lists retail sales figures for selected small molecule kinase inhibitors.
2012 Worldwide Retail Sales Figures of Selected Small Molecule Kinase Inhibitors
Brand Name
|
2012 Worldwide Sales(1) (in millions) | |||
|---|---|---|---|---|
Gleevec |
$ | 4,675 | ||
Tarceva |
$ | 1,401 | ||
Sutent |
$ | 1,236 | ||
Nexavar |
$ | 1,044 | (3) | |
Sprycel |
$ | 1,019 | ||
Tykerb |
$ | 380 | ||
Zelboraf(2) |
$ | 249 | ||
Xalkori(2) |
$ | 123 | ||
- (1)
- Source:
Thomson Pharma.
- (2)
- Launched
in 2011.
- (3)
- 792 euro converted into U.S. dollars based upon a published exchange rate of 0.7585 euro per U.S. dollar at December 31, 2012.
Our kinase inhibitor programs in clinical development include MGCD265 and MGCD516, which are multi-targeted kinase inhibitors with distinct target profiles. These new molecular entities are in development for the treatment of patients with NSCLC and other solid tumors. We own all global rights to MGCD265 and MGCD516.
MGCD265 — A Multi-targeted Kinase Inhibitor for Solid Tumors
Overview
MGCD265 is an orally-bioavailable, potent, small molecule multi-targeted kinase inhibitor of Met, Axl and VEGFRs. MGCD265 is in development for the treatment of solid tumors, with an initial focus on NSCLC and HNSCC. We have conducted single agent and combination dose escalation trials in 252 patients, with acceptable tolerability and signs of clinical efficacy in patients with advanced solid tumors who have failed standard therapies. Our preclinical studies, in a variety of in vivo tumor models, have suggested that MGCD265 has relatively low toxicity and appears more potent than some of the leading approved kinase inhibitors, including Nexavar, Sutent and Xalkori. We have developed new formulations of MGCD265 designed to increase plasma exposure, improve the degree of target inhibition and increase the likelihood of seeing single agent clinical activity. Assuming one or more of the new formulations achieve sufficient patient exposure in ongoing studies, we intend to select one of the new formulations for introduction into ongoing dose escalation trials with the goal of identifying the MTD by early 2014. Following identification of the MTD, we plan to initiate dose expansion cohorts in patients selected for certain biomarkers.
4
Our development strategy for MGCD265 is based on our understanding of the compound's target inhibition profile and, accordingly, our initial focus for this program will include NSCLC and HNSCC. Met and Axl are both over expressed in NSCLC and HNSCC, providing opportunities for targeted patient selection. In addition, we may target patients with certain mutations of Met and Axl that result in oncogenic activation of these targets and may be drivers of tumor growth.
The National Cancer Institute, or NCI, estimates that in 2013, approximately 228,200 patients in the United States will be diagnosed with lung cancer and 159,500 will die due to the disease. Approximately 85% of lung cancers are NSCLCs. Both Met and Axl are over-expressed in NSCLC tumors. Based on published literature, we believe Met to be over-expressed in 40% to 50% of NSCLC tumors, and Axl in over 40%. In addition, the potentially oncogenic mutations of Met and Axl that we are targeting may exist in up to 8% of NSCLC cases. In the United States, it is estimated that there will be approximately 41,400 new cases of head and neck cancer diagnosed and 7,900 deaths in 2013. Approximately 90% of head and neck cancers are HNSCC, and Met is overexpressed in 55% to 85% of HNSCC cases.
MGCD265 Market Overview
Although many tumor types may respond to treatment with MGCD265, NSCLC, HNSCC, hepatocellular carcinoma, or HCC, renal cell carcinoma, or RCC, and gastric cancers are of particular relevance to demonstrate the clinical activity of MGCD265. The selection of these indications is based on the expression or over-expression of markers such as Axl, Met and VEGFR. Key features of these markets are shown in the table below.
Estimated Market Size of Certain Cancer Therapies
Indication
|
Supporting Rationale |
Precedents for Targeted Therapy |
Estimated Market Size (United States, Europe and Japan) |
|||
|---|---|---|---|---|---|---|
| Lung Cancer | Over-expression of Axl, Met and VEGFR | Met inhibitor onartuzumab (MetMab) (Phase 2) (including patient selection) | $4.6B in 2011(1) $5.9B projected in 2021(1) |
|||
| Head & Neck Cancer | Over-expression of Axl and Met | EGFR inhibitor cetuximab | $700M in 2011(1) | |||
| Renal Cell Carcinoma | Over-expression of Axl, Met and VEGFR | VEGFR / RTK inhibitors: sunitinib, sorafenib, axitinib, bevacizumab/IFN | $1.6B in 2011(1) $2.0B projected in 2021(1) |
|||
| Liver Cancer | Over-expression of Axl, Met and VEGFR | Met inhibitor tivantinib (Phase 2) (including patient selection) RTK inhibitor sorafenib (Phase 3) |
$380M in 2009(3) $2.0B projected in 2015(2)(3) |
|||
| Gastric Cancer | Over-expression of Axl, Met and VEGFR | HGF inhibitor rilotumumab (Phase 2) (including patient selection) |
$1.1B in 2011(1) $2.3B projected in 2021(1) |
- (1)
- Source:
Decision Resources, 2012.
- (2)
- Source:
Global Industry Analysts Inc. 2010, Global Data 2010.
- (3)
- Worldwide market size.
Background
MGCD265 is a small molecule, multi-targeted kinase inhibitor that potently inhibits Axl, Met and VEGFR 1, 2 and 3. These targets have been shown to play key roles in tumor development, tumor survival, tumor escape and blood vessel formation, or angiogenesis. MGCD265 is highly specific for these five targets and shows little to no activity against a panel of over 400 other RTKs. We believe this profile provides the following potential advantages for MGCD265:
- •
- therapeutic action against a novel target (Axl);
- •
- high specificity reduces the risk of side effects from off-target activity;
5
- •
- an opportunity to identify patients that express specific markers allowing a
predictive and tailored therapeutic strategy using companion diagnostics; and
- •
- an opportunity to identify patients whose tumors express genetic alterations that may be drivers of tumor growth and the inhibition of which may demonstrate single agent clinical activity of MGCD265.
Axl is an RTK which has been shown to correlate with clinical-stage and lymph node status in NSCLC. Recent data has shown that Axl is involved in the mechanism of resistance to EGFR inhibitors such as Tarceva. Axl is expressed in other tumor types and may be a significant driver in RCC, ovarian, pancreatic and other tumors.
The Met receptor is a protein that is found on the cell's surface that, when not properly regulated, plays a key role in the growth, survival and metastasis of various types of cancers. The Met target has generated significant scientific and pharmaceutical interest because of its direct involvement in tumor cell survival and angiogenesis. Met expression is elevated in several major tumor types including NSCLC, gastric cancer, RCC and HCC and is associated with poor prognosis. Met activation may also be associated with resistance to EGFR inhibitors such as Tarceva and Iressa and resistance to VEGFR inhibitors such as Sutent. In tumors with Met over-expression, persistent activation of EGFR-dependent signals may be sustained constituting an escape mechanism leading to EGFR-inhibitor resistance. Inhibition of Met appears to block the Met-driven escape mechanism used by tumor cells when treated with other targeted cancer therapies. Similarly, VEGFR resistance may be overcome by inhibiting Met.
MGCD265 Preclinical Development
Our preclinical studies, in a variety of in vivo tumor models, have suggested that MGCD265 has relatively low toxicity and appears more potent than some of the leading kinase inhibitors which have recently been approved or are in clinical trials, including Nexavar, Sutent and Xalkori.
In preclinical studies, MGCD265 has demonstrated single agent activity as indicated in the figures below.
Anti-tumor Activity of MGCD265 Compared with Sutent in
a Met/HGF-Positive Glioblastoma Model
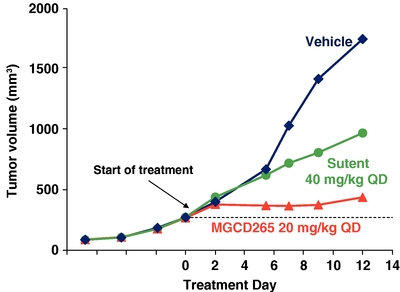
U87MG tumor cells were injected subcutaneously in immunocompromised mice. When tumor volume reached 50 mm3 mice were treated with MGCD265 or Sutent at the designated dose level or vehicle for 12 days. Tumor volume was measured at designated time points utilizing Vernier calipers.
6
Potent Cytoreductive Activity of MGCD265 in a Met
Amplified Gastric Cancer Model
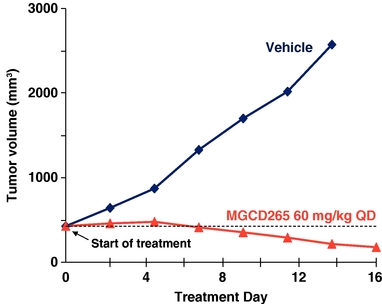
Met amplification positive MKN45 tumor cells (5 X106) were implanted subcutaneously in immunocompromised mice. When tumor volume reached 450 mm3 mice were treated with MGCD265 at the designated dose level or vehicle for 16 days. Tumor volume was measured at designated time points utilizing Vernier calipers.
MGCD265 Clinical Trials
Multiple Phase 1 clinical trials have been conducted with MGCD265 showing evidence of clinical activity as monotherapy as well as in combination studies. While MGCD265 showed some efficacy and selectively inhibited Met and Axl, it did not reach optimal plasma concentrations or sufficiently inhibit the targets. We are developing a new formulation of MGCD265 designed to increase plasma exposure, improve the degree of target inhibition and increase the likelihood of seeing single-agent clinical activity.
The original IND for MGCD265 was filed in December 2007 and became effective in January 2008. Three schedules of continuous dosing of MGCD265 were evaluated sequentially in the ongoing monotherapy and combination studies: once daily (QD), twice daily (BID) and three times daily (TID). MGCD265 has been generally well tolerated at all doses and schedules tested to date, both as monotherapy and in combination with either Taxotere or Tarceva.
Phase 1 Clinical Trial Evaluating MGCD265 in Solid Tumors (Ongoing)
This Phase 1, open-label, dose escalating clinical trial in patients with advanced solid tumors is evaluating MGCD265, administered orally every day in repeated 21-day cycles. Data is available for 79 patients who were treated with MGCD265 at doses escalating from 24 mg/m2 QD to a flat dose of 600 mg TID. Nine patients achieved stable disease for more than four months and up to nine months. One of these patients, who had squamous cell cancer, experienced a partial response after ten cycles of treatment based on one target axillary lesion. The non-target bone lesions remained stable. To date the safety profile continues to be favorable in this ongoing Phase 1 program. The most frequent treatment-related adverse events, observed in greater than 10% of patients, or grade 3 adverse events occurring in more than one patient, are summarized in the table below.
7
Adverse Events Observed in MGCD265
Monotherapy 265-101 (n=79)
| Most frequent treatment-related adverse events (>10%, all grades) |
Grade 3 adverse events occurring in > 1 patient | ||||||
|---|---|---|---|---|---|---|---|
Diarrhea |
52 | % | Diarrhea |
n=3 (DLT n=1) | |||
Fatigue |
30 | % | Fatigue |
n=3 (DLT n=1) | |||
Nausea |
33 | % | Lipase elevation |
n=2 (DLT n=1) | |||
Anorexia |
25 | % | Alk phosphatase elevation |
n=2 | |||
Vomiting |
20 | % | |||||
We expect to continue enrollment in this trial upon completion of ongoing MGCD265 formulation work.
Phase 1 Clinical Trial Evaluating MGCD265 in Solid Tumors (Complete)
In December 2011, we completed an open label Phase 1 clinical trial with dose-escalation of MGCD265 in patients with advanced solid tumors. We enrolled 47 patients with advanced solid tumors. Four patients (papillary renal cell, sarcomatoid bladder, neuroendocrine, and head and neck cancers) had prolonged stable disease with durations ranging from 4 to 12.9 months. The patient with sarcomatoid bladder cancer was stable for 7.5 months and exhibited decreases in Met and phospho-Met protein expression, as well as a change in intact vascular structures, in a post-treatment biopsy sample. The most frequent treatment-related adverse events, occurring in greater than 10% of patients, included diarrhea (30%), nausea (26%), and fatigue (26%). Most of these adverse events were reported as grade 1 or 2 in severity. The observed dose limiting toxicities, or DLTs, were grade 3 mood alteration (n=1) and grade 3 fatigue in the same patient and grade 3 hemoptysis (n=1) all at the dose of 170 mg/m2 BID (n=6). An additional grade 3 adverse event of increased lipase was also reported at a dose of 192 mg/m2 BID (n=1).
Phase 1/2 Clinical Trial Evaluating MGCD265 in Combination with Taxotere or Tarceva (Ongoing)
This dose-escalating Phase 2 clinical trial is evaluating MGCD265 in combination with Taxotere or Tarceva. Data is available for 124 patients treated with MGCD265 at doses of up to 700 mg BID taken with meals and administered in combination with full dose Tarceva or Taxotere. Overall, stable disease for 6 to 18 months was observed in nine patients: NSCLC (n=5), ovarian, prostate, pancreatic and head and neck cancer (n=1 each). Objective partial responses were observed in two out of sixteen patients with NSCLC, one out of four patients with prostate cancer, one out of two patients with head and neck cancer and the only patient with endometrial cancer.
Overall, the treatment was well tolerated and the adverse events observed are generally those associated with Taxotere or Tarceva treatment. The most common treatment-related non-hematologic adverse events observed to date have been constitutional or gastro-intestinal related and are summarized in the table below. Expected Taxotere associated adverse events of anemia (n=3, grade 3), leucopenia (n=5, grade 3-4) and neutropenia (n=31, grade 3 and 4) and one case of febrile neutropenia have also been observed. Grade 3 or higher adverse events occurring in more than one patient are summarized in the table below. In addition, one patient was reported to have a pulmonary embolism (grade 4) that was an incidental finding on CT scan. Another patient who had advanced NSCLC and was oxygen dependent at baseline, was diagnosed with fatal pneumonitis (inflammation of the lung tissue) (grade 5) in the context of worsening pleural effusion and increasing parenchymal consolidation. Additional cycle 1 DLTs reported in this study include grade 3 diarrhea (n=1), grade 3 lipase (n=1), grade 3 fatigue (n=1), elevated AST (n=1) and pancreatitis (n=1) in a patient with grade 3 lipase at baseline consistent with chronic pancreatitis.
8
Adverse Events Observed in MGCD265 Combination Therapy
Combination Therapy 265-103 with Taxotere (n=56)
| Most frequent treatment-related adverse events (>10%, all grades) |
Grade 3 adverse events or higher occurring in > 1 patient |
||||||
|---|---|---|---|---|---|---|---|
Fatigue |
53 | % | Neutropenia |
n=31 | |||
Alopecia |
45 | % | Leucopenia |
n=5 | |||
Diarrhea |
38 | % | Diarrhea |
n=3 (DLT n=1) | |||
Nausea |
32 | % | Elevated lipase |
n=3 (DLT n=2) | |||
Anorexia |
23 | % | Hypophosphatemia |
n=2 | |||
Constipation |
17 | % | |||||
Mucosal inflammation |
16 | % | |||||
Taste disturbance |
15 | % | |||||
Vomiting |
15 | % | |||||
Myalgia |
11 | % | |||||
Rash |
11 | % | |||||
As of the last data review in July 2013, 68 patients have been treated in the MGCD265-plus-Tarceva arm of our combination trial. Tarceva was started at a dose level of 100 mg (first dose level) and then escalated to 150 mg in combination with MGCD265.
Data is available for 68 patients treated with MGCD265 at doses of up to 700 mg BID taken with meals and administered in combination with Tarceva. Eight patients with a variety of tumors have experienced stable disease for six months or more. This includes two NSCLC patients, one of which had a partial response (also positive for EGFR activating mutation). Three out of nine patients with gastroesophageal cancer remained on study for approximately 11 to 34 months. Overall, the combination of MGCD265 with Tarceva has been well tolerated and the most common treatment-related adverse events are consistent with known Tarceva toxicity and include skin-cutaneous or gastro-intestinal related events. The most frequent treatment-related adverse events are summarized in the table below. No grade 4 or grade 5 toxicities have been reported in the Tarceva combination study.
Adverse Events Observed in MGCD265 Combination Therapy with Tarceva
Combination Therapy 265-103 with Tarceva (n=61)
| Most frequent treatment-related adverse events (>10%, all grades) |
Grade 3 adverse events occurring in > 1 patient |
||||||
|---|---|---|---|---|---|---|---|
Diarrhea |
75 | % | Diarrhea |
n=12 (DLT n=3) | |||
Fatigue |
39 | % | Hypokalemia |
n=3 | |||
Rash |
33 | % | Hypophosphatemia |
n=2 | |||
Anorexia |
21 | % | Fatigue |
n=1 (DLT n=1) | |||
Nausea |
18 | % | |||||
Dermatitis acneiform |
15 | % | |||||
Dry skin |
15 | % | |||||
9
Phase 1 Clinical Trial Evaluating the Pharmacokinetics of MGCD265 in Healthy Volunteers in a Fed versus Fasted State (Complete)
In 2012, a Phase 1 study in healthy volunteers (n=14) was conducted to compare the pharmacokinetics of a single 100 mg dose of MGCD265 under fed conditions versus those after a 10-hour overnight fast. Safety was evaluated in all subjects for seven days after each single dose. On average, the fed condition was associated with an approximately three-fold increase in exposure. All treatment-related adverse events were mild except for one patient who reported moderate diarrhea when dosed under fasting conditions. The study results indicated that exposures could significantly improve up to three-fold in the fed subjects and provided support for the formulation improvement work that was undertaken earlier this year.
MGCD265 Developmental Initiatives and Objectives
Since January 2013, we have developed new formulations of MGCD265 designed to increase plasma exposure, improve the degree of target inhibition and increase the likelihood of seeing single-agent clinical activity. Assuming one or more of the new formulations achieve sufficient patient exposure in ongoing studies, we intend to select one of the new formulations for introduction into ongoing dose escalation trials with the goal of identifying the MTD by early 2014. After the MTD is identified, we plan to initiate dose expansion cohorts in patients selected for Met and/or Axl over-expression as well as a cohort of patients that have genetic mutations of Met or Axl that we believe are drivers of tumor growth. Our initial focus for this program will include both NSCLC and HNSCC. Because the trial is open-label, we may see evidence of clinical activity from the expansion cohorts by mid-2014.
We believe that by selecting patients with over-expression of Met and/or Axl as well as patients with genetic mutations associated with pathways that are critical to tumor growth that are potently inhibited by MGCD265 may increase the likelihood of seeing clinical activity earlier in clinical development. We are currently using commercially available diagnostic assays as well as assays developed internally for early clinical uses. We are developing companion diagnostics in collaboration with third parties that we plan to use for later stage registration trials and commercialization, if approved.
MGCD516 — A Novel Multi-targeted Kinase Inhibitor for Solid Tumors
MGCD516 is an orally-bioavailable, potent, small molecule multi-targeted kinase inhibitor of RET, TRK, DDR and EphRs, as well as Met, Axl and VEGFRs, in development for the treatment of solid tumors. We plan to focus on solid tumors expressing RET, TRK and DDR, initially in NSCLC, and we plan to evaluate other tumor types where the profile of MGCD516 would suggest activity. MGCD516 is in advanced preclinical development. We plan to file an IND with the FDA, and initiate a Phase 1 clinical trial of this product candidate in the first half of 2014, and identify the MTD and initiate expansion cohorts in patients selected for certain biomarkers by the end of 2014.
MGCD516 has shown potent inhibition in vitro of cell proliferation, cell motility and angiogenesis. In preclinical animal studies, MGCD516 shows good oral bioavailability in mice, rats and dogs, and anti-tumor activities in multiple human xenograft tumor models in mice.
10
In preclinical lung cancer studies, MGCD516 demonstrated better tumor volume reduction activity than Nexavar as shown in the figure below.
Anti-tumor Activity of MGCD516 Compared with Nexavar
in the A549 Lung Cancer Model
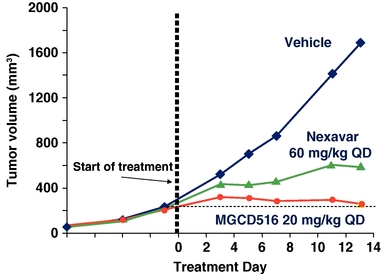
A549 tumor cells were injected subcutaneously in immunocompromised mice. When tumor volume reached 50 mm3 mice were treated with MGCD516 or Nexavar at the designated dose level or vehicle for 13 days. Tumor volume was measured at designated time points utilizing Vernier calipers.
MGCD516 also demonstrated significant activity in a RET fusion lung cancer preclinical model shown in the figure below.
Potent Cytoreductive Activity of MGCD516 in a RET Fusion-
Positive Lung Cancer Model
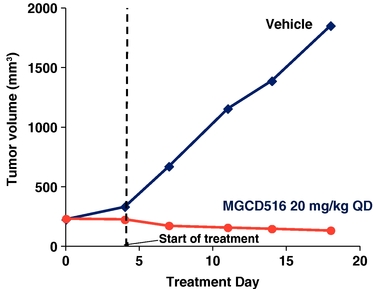
KIF5B-RET fusion positive primary tumors were implanted subcutaneously in immunocompromised mice. When tumor volume reached 200 mm3 mice were treated with MGCD516 at the designated dose level or vehicle for 16 days. Tumor volume was measured at designated time points utilizing Vernier calipers.
11
In addition, MGCD516 demonstrated activity in overcoming VEGFR resistance in a preclinical model treated with Sutent, as shown below.
Cytoreductive Anti-tumor Activity of MGCD516 in a Sutent-
Resistant Tumor Model
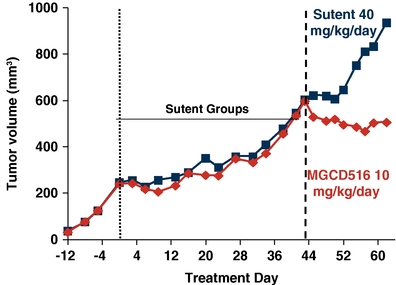
Primary tumors were implanted subcutaneously in immunocompromised mice. When tumor volume reached 250 mm3 mice were treated with Sutent for 44 days until they progressed to a volume of 600 mm3. Mice were randomized into two groups and treated with MGCD516 or Sutent at the designated dose level or vehicle for 18 additional days. Tumor volume was measured at designated time points utilizing Vernier calipers.
Mocetinostat — An Oral HDAC Inhibitor for MDS
Overview
Mocetinostat is an orally-bioavailable, spectrum-selective HDAC inhibitor for which we plan to conduct a dose confirmation trial starting in the fourth quarter of 2013, with the goal of initiating a Phase 3 clinical trial in the second half of 2014. We have completed 13 clinical trials which enrolled 437 patients with a variety of hematologic malignancies and solid tumors. We intend to seek an SPA from the FDA prior to the initiation of our planned Phase 3 trial. This trial will evaluate mocetinostat for the first line treatment of patients with MDS in combination with Vidaza, an HMA. We believe that mocetinostat has the potential to be the first HDAC inhibitor to market for this indication.
We believe that the epigenetic mechanisms of HDAC inhibitors and HMAs may be complementary in the treatment of MDS. Epigenetics is the regulation of gene expression and resulting cellular phenotypes through mechanisms other than primary DNA sequence alterations. The epigenetic regulation of gene expression involves the regulation of DNA methylation and modification of certain histones via modulation of acetylation or methylation of specific amino acid residues. Epigenetic pathways can become dysregulated during cancer progression through a variety of mechanisms, including the genetic alteration of molecules that participate in DNA methylation and histone modification. These alterations often result in silencing of selected tumor suppressor genes and uncontrolled tumor growth in certain malignancies including MDS and lymphomas. Because the epigenetic regulation of gene expression is controlled by DNA methylation and histone modification, we have focused on the development of mocetinostat for the treatment of MDS in combination with HMAs.
We partnered mocetinostat with Pharmion Corporation (predecessor to Celgene Corporation) in 2006. In 2008, Celgene voluntarily placed the mocetinostat program on clinical hold with the FDA following an
12
observation of pericarditis in clinical trials. Celgene subsequently terminated the collaboration in January 2009 and all rights to the mocetinostat program reverted to us. Following the termination and based upon a review of the safety data and discussion with the FDA, the clinical hold was removed in 2009. However, no further development was conducted by us under our prior management team. When our current management team joined us in late 2012, we began a detailed portfolio review and subsequently determined that further development of mocetinostat was warranted.
Mocetinostat Market Overview
The potential of HDAC inhibitors for the treatment of hematological malignancies has already been validated by the approval of two HDAC inhibitors, Zolinza and Istodax, for the treatment of T-cell lymphoma. Our clinical studies of mocetinostat indicate that this agent may have promising activity in MDS and other hematological malignancies such as Hodgkin's lymphoma, or HL, and non-Hodgkin's lymphomas, or NHL, including diffuse large B-cell lymphoma, or DLBCL, and follicular lymphoma, or FL.
Our primary focus for mocetinostat is on the first line treatment of patients with MDS. MDS consists of a group of heterogeneous, clonal hematopoietic stem cell disorders that are characterized by abnormal bone marrow and blood cell development. According to NCI, MDS will be diagnosed in more than 10,000 people in the United States in 2013. Utilizing Surveillance Epidemiology and End Results data from NCI, Decision Resources estimates the prevalence of MDS to be over 52,000 patients in the United States and over 49,000 patients in the European Union.
MDS is a complex and heterogeneous disease, divided into patient subgroups with differing therapy objectives. The International Prognostic Scoring System, or IPSS, for MDS was developed to assess patient prognosis and guide the course of treatment of MDS, and utilizes clinical variables such as bone marrow blast percentage, number of peripheral blood cytopenias and cytogenic risk group to categorize MDS patients and provide prognostic expectations. Approximately one-quarter of MDS patients are classified as high-risk (Intermediate-2 or high IPSS risk category). Prognosis for these high-risk MDS patients is generally poor and there is a significant medical need for therapeutic regimens that will improve clinical outcomes.
The standard of care, according to the National Comprehensive Cancer Network, for first line therapy for Intermediate-2 and high-risk patients is treatment with HMAs. The HMAs Vidaza and Dacogen are approved as first line agents for the treatment of high-risk MDS patients in the United States, and 2012 U.S. sales were approximately $327 million and $240 million, respectively. Although these therapies represent the standard of care for the treatment of high-risk MDS patients, only a minority of patients achieve an objective response. Almost all patients who initially respond to therapy eventually relapse, and the survival time of MDS patients who have failed HMAs is less than six months. Allogenic hematopoietic stem cell transplantation, or HSCT, is the only potential curative treatment for MDS; however, its use is restricted to a relatively small number of eligible patients and requires an appropriate donor.
NHL (including the aggressive DLBCL and FL) is the most common form of blood cancer. NCI estimates that 70,000 patients will be diagnosed with NHL in the United States in 2013 and that the incidence has grown annually over the past ten years. The prevalence of NHL in the United States is 509,000. Aggressive NHL is treated with rituximab (anti-CD20) plus chemotherapy, which is effective in about 67% of cases, but relapsed or refractory aggressive NHL has a poor outlook with limited therapeutic options.
NCI estimates that 9,300 patients will be diagnosed with HL in the United States in 2013 and that the prevalence of HL in the United States is 182,000. Treatments of HL typically include radiation, chemotherapy and HSCT. Chemotherapy followed by consolidation radiation therapy is the most effective treatment for early-stage HL. Current approaches seek to balance efficacy against the risk of long-term complications such as cardiac disease and other types of cancer. Patients with refractory HL currently have few therapeutic options such as high dose chemotherapy followed by stem cell transplant or brentuximab vedotin (anti-CD30 antibody conjugated to cytotoxin).
13
We believe that a significant unmet medical need remains for effective treatment that increases the response rates in patients with Intermediate-2 and high-risk MDS, HL and NHL.
Mocetinostat Background
Histones are protein components of the structural architecture of DNA known as chromatin (chromatin is the material that chromosomes are made of, and is comprised of DNA and protein). Local gene expression activity can be controlled through epigenetic mechanisms by inducing changes in chromatin conformation through chemical modifications of histones. Acetylated histones are associated with a more open configuration of chromatin that is receptive to gene expression signals. In contrast, HDAC leads to a more compact structure where gene expression is restricted or suppressed. Tumor suppressor genes serve to regulate cell growth and cell death, but during oncogenesis these tumor suppressor genes may become silenced by the action of HDACs leading to unrestricted growth of tumor cells. HDAC is a family of 11 enzymes (the individual HDAC enzymes are referred to as isoforms) that appear to act as a master regulator of genes affecting many diseases, including cancer. HDAC inhibitors modulate inappropriate deacetylation of histones to restore normal acetylation patterns as well as tumor suppressor gene expression. Inhibition of HDACs may result in multiple anti-cancer effects such as (1) the inhibition of cancer cell proliferation, (2) the induction of apoptosis of cancer cells, (3) improved cell cycle regulation, and (4) the induction of tumor suppressor genes.
We believe that a key differentiating feature of mocetinostat is its spectrum of activity, targeting HDAC isoforms 1, 2, 3 and 11. We believe that these isoforms, and particularly isoforms 1 and 2, are the most relevant HDAC isoforms in cancer therapy. Compared to other HDAC inhibitors that have a broader spectrum of activity, the profile of mocetinostat may allow us to inhibit the targets relevant to cancer more potently and thereby potentially demonstrate improved clinical efficacy and reduced side effects.
Mocetinostat Clinical Development
Our IND for mocetinostat was submitted in December 2003 and became effective in January 2004. To date, we have evaluated mocetinostat as a monotherapy and in combination with other anticancer agents in 437 patients in Phase 1 and Phase 2 clinical trials with various malignancies, including MDS, HL, NHL (including DLBCL or FL), AML, chronic lymphocytic leukemia and chronic myelogenous leukemia, as well as advanced solid tumors. Through these trials, the safety and tolerability of mocetinostat as a single agent and in combination has been well characterized. The clinical trials showed activity as a single agent in HL and NHL and in combination with Vidaza in MDS and AML.
14
The historical mocetinostat clinical trials are set forth in the following table.
CLINICAL TRIALS EVALUATING MOCETINOSTAT
| Phase 1 Clinical Trial | Daily dosing regimen (14 days on, 7 days off) | |
Three times weekly (14 days on, 7 days off) |
||
Three times weekly (continuously) |
||
Twice weekly (continuously) |
||
Phase 2 Monotherapy Clinical Trial |
AML/High-risk MDS |
|
Relapsed/Refractory NHL (DLBCL, FL) |
||
Refractory chronic lymphocytic leukemia |
||
Relapsed/Refractory HL |
||
Phase 1/2 Combination Clinical Trial with Vidaza |
AML and MDS |
|
Other Clinical Trials |
Phase 1/2 clinical trial of Mocetinostat in Combination with Gemcitabine |
|
Combination of mocetinostat with Vidaza and with Taxotere |
MDS
In late 2012 and early 2013, our new management team reviewed the data from our prior clinical trials of mocetinostat. As a result, our management team concluded that the combination of mocetinostat and Vidaza demonstrated clinically meaningful responses in MDS patients and demonstrated an improvement over published responses of Vidaza alone. The objective response rate of Vidaza, as shown in its product label, is 15.7%, with 5.6% of patients achieving a complete response, or CR, compared to CR's in 11% of the patients in the mocetinostat data as set forth in the table below. A complete response generally refers to the disappearance of all signs of cancer in response to treatment, while a partial response generally refers to a decrease in the size of the tumor or in the extent of cancer in the body.
In an open-label, Phase 1/2 trial of patients with MDS or AML that was conducted starting in 2006, we evaluated the activity of mocetinostat in combination with Vidaza in patients with MDS. A total of 66 subjects were enrolled, including 28 patients with MDS as assessed by independent analysis. Patients with MDS were treated with mocetinostat at starting doses of 35 to 135 mg three times weekly, with most patients starting at 90 mg, and continued treatment until disease progression or prohibitive toxicity. Among the 28 patients with MDS, the ORR (CR+CRi+HI) was 61% (17 of 28), and the disease control rate (CR+CRi+HI+SD) was 93% (26 of 28) in an independent assessment. A summary of this data is set forth in the table below.
15
MOCETINOSTAT COMBINED WITH VIDAZA PHASE 2 CLINCIAL DATA
MDS Best Response Rates (n=28) |
n (%) | |
CR+CRi |
14 (50) | |
CR |
3 (11) | |
CRi |
11 (39) | |
HI |
3 (11) | |
SD |
9 (32) | |
ORR (CR + CRi +HI) |
17 (61) | |
Disease Control (CR + CRi + HI + SD) |
26 (93) |
CR = complete response; CRi = complete marrow response but without normalization of peripheral counts; HI = hematologic improvement; SD = stable disease; ORR = objective response rate.
Mocetinostat Safety
In the mocetinostat plus Vidaza study, the most commonly reported adverse events are set forth in the tables below. These adverse events are generally consistent with those seen in MDS and AML patients treated with this class of agent.
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
| |
|
Treatment-related Adverse Events (Grades 3 and 4) Mocetinostat with Vidaza, MDS & AML Patients |
||||||
| Most Common Treatment-related Adverse Events (All Grades) Mocetinostat with Vidaza, MDS & AML Patients |
||||||||
| |
|
Grade 4 | ||||||
| EVENT | Patients, n(%) | EVENT | Grade 3(1) | |||||
Nausea |
44 (67) | Fatigue |
15 (23) | 0 (0) | ||||
Diarrhea |
43 (65) | Nausea |
14 (22) | 0 (0) | ||||
Fatigue |
32 (49) | Diarrhea |
11 (17) | 1 (2) | ||||
Anorexia |
30 (46) | Vomiting |
9 (14) | 0 (0) | ||||
Asthenia |
22 (33) | Anemia |
6 (9) | 1 (2) | ||||
Weight loss |
16 (24) | Anorexia |
6 (9) | 0 (0) | ||||
Thrombocytopenia |
13 (20) | Dehydration |
5 (8) | 0 (0) | ||||
Anemia |
10 (15) | Asthenia |
4 (6) | 0 (0) | ||||
Hypokalemia |
9 (14) | Thrombocytopenia |
3 (5) | 6 (9) | ||||
Constipation |
8 (12) | Leukopenia |
2 (3) | 2 (3) | ||||
Dysgeusia |
8 (12) | Neutropenia |
1 (2) | 4 (6) | ||||
Dehydration |
7 (11) | |||||||
Dizziness |
7 (11) | |||||||
- (1)
- Excludes Grade 3 adverse events with an incidence less than 5%.
Pericarditis Finding and Clinical Hold
In July 2008 Celgene instituted a voluntary clinical hold to new patient enrollment for mocetinostat, which was accepted by the FDA in August 2008. The voluntary clinical hold was put in place in response to an observation of pericarditis and pericardial effusion (inflammation of the pericardium, the fibrous sac surrounding the heart, and accumulation of fluid around the heart).
16
We provided the FDA with an integrated analysis of pericardial events identified in mocetinostat clinical studies. A causal association of mocetinostat with pericardial events was not established since the observed events could be related to the patient population and their prior therapy. Of the 437 patients treated with mocetinostat, there have been a total of 19 patients (4.3%) who had serious adverse events, or SAEs, where a pericardial adverse event was mentioned, and a total of 45 patients (10.3%) who had pericardial findings, which included the 19 SAE findings as well as 27 incidental findings identified through reviews of on-study CT scans, database searches and prospective echocardiogram monitoring, which did not have significant clinical sequellae. Only one pericardial SAE occurred among the 28 patients (3.6%) with MDS in the Phase 1/2 clinical trial. Based on literature reviews and other investigator-driven reviews, the rate of pericardial findings is approximately 10% of cancer patients, but rates have been reported to vary from 3% to approximately 40% for patients with advanced cancers who may have received multiple previous anticancer therapies. However, the potential exists for a relationship with treatment, and the possibility of mocetinostat being a contributing factor to the occurrence of pericardial events has not been excluded. We agreed with the FDA that the best way to assess the risk of pericarditis is in a sufficiently large randomized study of safety and efficacy.
Our complete response to the voluntary clinical hold was accepted by the FDA and the hold was lifted in September 2009. Our response included specific guidance for identifying patients at potential risk for, and guidance to manage patients who develop, pericarditis or pericardial effusions. As a result, new patient enrollment in mocetinostat clinical trials will include both the exclusion of patients who are diagnosed with cardiac abnormalities prior to starting mocetinostat therapy (i.e. myocardial infarction, congestive heart failure and pericardial disease) and patient monitoring by electrocardiogram and echocardiography at baseline and while on study. These diagnostic tests are non-invasive and relatively common procedures. The three patients with lymphoma who were enrolled after the voluntary clinical hold was lifted did not show signs of pericarditis or pericardial effusions.
Lymphoma
We tested the safety and efficacy of mocetinostat in patients with relapsed HL in a trial starting in 2006. Two doses were assessed (85 mg and 110 mg three times weekly), and patients were treated until disease progression or prohibitive toxicity. A total of 51 patients were enrolled. On the basis of intent-to-treat analysis, the disease control rate was 35% (8 of 23 patients) in the 110 mg group and 25% (7 of 28) in the 85 mg group. A total of 12 patients discontinued treatment because of adverse events, nine in the 85 mg cohort and three in the 110 mg cohort. The most frequent treatment-related grade 3 and 4 adverse events were neutropenia, fatigue and pneumonia. Four patients in the 110 mg cohort died during the study.
We also tested the safety and efficacy of mocetinostat in patients with relapsed/refractory DLBCL and FL in a trial starting in 2006. Patients continued treatment until disease progression or prohibitive toxicity. A total of 72 patients were enrolled. On the basis of intent-to-treat analysis, the objective response rate was 17% (7 of 41 patients) in patients with DLBCL and 10% (3 of 31) in patients with FL. Initially, 32 patients began treatment at 110 mg three times weekly (21 with DLBCL and 11 with FL), 37 additional patients were treated with a dose of 85 mg three times weekly (20 with DLBCL and 17 with FL) and 3 FL patients were treated with a dose of 70 mg three times weekly. The most commonly reported adverse events included myelosuppression and fatigue.
Mocetinostat Developmental Plans
Subject to successful completion of our planned dose confirmation trial, we are planning to initiate a Phase 3 registration trial for mocetinostat in the second half of 2014. The proposed randomized trial is intended to support regulatory approval of mocetinostat in combination with Vidaza for the treatment of patients with Intermediate or High-Risk MDS who have not previously received Vidaza, and for whom Vidaza is indicated.
In advance of the Phase 3 trial and subject to receipt of additional financing, we plan to conduct a dose confirmation trial to confirm the planned Phase 3 dose and to obtain further clinical data and test safety
17
monitoring protocols. The study is expected to be initiated in the fourth quarter of 2013 and to include approximately 30 patients, with 10 patients treated with mocetinostat at the 70 mg dose level and 20 patients treated at the 90 mg dose level, each in combination with Vidaza, in a single arm open-label study. The current proposed dose of mocetinostat for this Phase 3 clinical trial is 90 mg.
The proposed registration trial is expected to be a 1:1 randomized study comparing mocetinostat plus Vidaza with Vidaza alone in HMA naïve subjects who have been diagnosed with MDS and that have met criteria for the risk groups of Intermediate or high-risk according to IPSS. Although we have guidance from the FDA on certain key points, we intend to seek an SPA from the FDA on the design of the Phase 3 clinical trial. We intend to propose to the FDA an adaptive study design and intend to discuss both ORR and OS as potential endpoints for the basis for approval. While the detailed clinical and statistical plan are still under discussion and evaluation, the size range for the trial is currently estimated to be between 250 and 500.
We anticipate that the trial will include eligibility criteria to exclude patients with pre-existing pericardial effusion, on-study monitoring including echocardiograms and electrocardiograms in both treatment arms, and data monitoring safety committee oversight for potential adverse events including those specifically related to pericardial events.
We are also evaluating potential future development of mocetinostat in patients with NHL and HL.
Strategic Alliances and Commercial Agreements
Collaboration with Taiho
In October 2003, we entered into a license and research and development collaboration agreement with Taiho, a leading Japanese specialty oncology company, for mocetinostat and our small molecule HDAC inhibitor program for oncology for Japan, South Korea, Taiwan and China, or collectively the Taiho Territory. Under the terms of the agreement, we received an up-front license fee, equity investment and a contract research payment of $3.8 million. In addition, we may receive milestone payments based on successful development, regulatory approval, and commercialization of an HDAC oncology product totaling up to $16.2 million. We may also receive royalty payments in connection with commercial sales of HDAC oncology products as a percentage of annual net sales, which percentage is in the mid-single digit to mid-teen percent range, depending upon the total dollar amount of annual net sales. Such royalties may be reduced, subject to a mid-single digit floor, by (i) credits against recoupable development costs paid by Taiho to us and/or (ii) reduction by a percentage in the range of 20-30% in the event a generic competitor is introduced in a particular market, other than in China. Taiho provided us with contract research payments for scientists for two years at $2.0 million per year as well as funding for contract preclinical and contract clinical development costs in North America for mocetinostat, which totaled, in the aggregate, $5.4 million. In total, we have received $15.0 million from Taiho under the agreement, including a $1.5 million milestone payment relating to the start of the first Phase 2 trial with mocetinostat. However, upon the execution of our agreement with Celgene in 2008, Taiho's funding obligations for clinical trials in North America ceased. In addition, Taiho's collaboration entailed in-kind support in their research laboratories in order to select a next generation compound, and in some cases, will support a portion of preclinical development costs in North America. Currently, there are no efforts by either (i) Taiho to further advance mocetinostat in the Taiho Territory or (ii) Taiho or us to further advance other small molecule HDAC inhibitors that would be covered by this agreement. However, Taiho has retained rights in the Taiho Territory to certain sirtuin inhibitors for cancer. The term of the agreement will, on a country-by-country basis, continue until expiration of the last to expire issued patent, or ten years after the first commercial sale in Japan. Additionally, Taiho has a unilateral right to terminate the agreement for any reason with 30 days written notice, and we have a unilateral right to terminate the agreement if Taiho fails to make an undisputed payment. An arbitrator may terminate the agreement for a breach of obligations if such breach has remained uncured for 90 days. As long as the agreement continues, we are obligated to use reasonable efforts to contract with Taiho for our supply of the active bulk compounds for the sale of mocetinostat
18
outside of the Taiho Territory. In the event the parties wish to collaborate on the development of another HDAC inhibitor covered by this agreement or sirtuin inhibitor retained by Taiho, Taiho would be obligated to contribute to preclinical and clinical costs of such a compound. Such a compound would also be subject to potential development milestones and royalties. We are in preliminary discussions with Taiho to consider whether any amendments to the agreement should be made based upon our development plans for mocetinostat and their rights under the agreement.
Collaboration with Otsuka
In March 2008, we entered into a worldwide research collaboration and license agreement with Otsuka, a global Japanese pharmaceutical company, for the development of novel, small molecule, kinase inhibitors for local delivery and treatment of ocular diseases, excluding cancer. We were responsible for the design, characterization and initial screening of kinase inhibitors and control over determining which compounds to synthesize. Otsuka was responsible for funding efficacy and toxicity studies, as well as preclinical and clinical development of compounds. Otsuka is also responsible for the global commercialization of any resulting product. Under the terms of the agreement, we received an up-front license fee of $2.0 million. We may receive additional payments based on successful development, regulatory, commercialization and sales milestones that could total up to $50.5 million. We may also receive royalty payments in connection with commercial sales of licensed products under the agreement as a percentage of annual net sales, which percentage is in the mid-single digit to mid-teen percent range, depending upon the total dollar amount of annual net sales, subject to reduction by a percentage in the range of 40-50% in the event a generic competitor is introduced in a given market or intellectual property protection in a particular market does not exist or expires in a given market. We may receive aggregate milestone payments of up to $50.5 million under this agreement as follows: $7.5 million relates to development activities, $22.0 million relates to the completion of regulatory approvals and $21.0 million relates to the achievement of certain sale goals. Otsuka provided $1.9 million in research funding for the initial 18 months of the research collaboration, which was extended on three occasions: September, 2009; April 2010 and June 2010. The research component of the agreement ended on June 30, 2011. We received a total of $4.5 million in research funding from the research component of this agreement. In October 2009, Otsuka made, in connection to the terms of the agreement, a $1.5 million equity investment in our shares of common stock at a share price of CND$21.30 (or US$20.27, as converted), which was a 20% premium over the five-day volume-weighted average closing price at the date of the transaction. On June 30, 2010, the collaboration agreement was amended to, among certain other changes, provide Otsuka the rights to synthesize a limited number of compounds predetermined by us. A lead molecule was selected in June 2011 for further development. The research portion of the collaboration between us and Otsuka concluded on June 30, 2011; however, the term of the agreement will, on a country-by-country basis, continue until expiration of the last to expire issued patent, or if no patent has issued in such country, then 12 years after the first sale of a licensed product by Otsuka. Otsuka has a unilateral right to terminate the agreement for any reason with 90 days written notice and either party may terminate the agreement for a breach of obligations of the other party if such breach has remained uncured for 120 days (or 30 days for a breach of payment). Otsuka is currently advancing the lead compound through late preclinical development.
Collaboration with EnVivo
In March 2004, we entered into a proof of concept and option agreement with EnVivo, a private U.S. biotechnology company focusing on the treatment and prevention of certain neurodegenerative diseases, to exploit our HDAC inhibitors in diseases such as Huntington's disease, Parkinson's disease and Alzheimer's disease. In February 2005 we signed an exclusive research, collaboration and license agreement. Over the course of 2005, EnVivo paid us $0.6 million for research, plus a $0.5 million license fee, for a total of $1.1 million. As part of this agreement, EnVivo received a warrant to purchase 1,050 shares of common stock at an exercise price of CND$214.30 (or US$203.76, as converted). The warrant expired in March 2007. In February 2008, we exercised our right to opt-out of the program. As a result, we granted EnVivo exclusive rights to our HDAC inhibitors for neurodegenerative diseases and we ceased research and development funding for this program. We are prohibited under the surviving terms of the agreement with
19
EnVivo from developing or commercializing any HDAC products in the field of certain neurodegenerative diseases, including Huntington's disease, Parkinson's disease and Alzheimer's disease. We may receive royalty payments in an aggregate amount equal to a single digit percentage of net sales of any approved compound and will share in any sublicense income from future partnerships that EnVivo may enter into.
Intellectual Property
Patents and Proprietary Technology
Our goal is to obtain, maintain and enforce patent protection wherever appropriate for our product candidates, formulations, processes, methods and any other proprietary technologies and operate without infringing on the proprietary rights of other parties, both in the United States and in other countries. Our practice is to actively seek to obtain, where appropriate, intellectual property protection for our current product candidates and any future product candidates, proprietary information and proprietary technology through a combination of patents, protection of proprietary know-how and trade secrets, and contractual arrangements, both in the United States and abroad. However, patent protection may not afford us with complete protection against competitors who seek to circumvent our patents. We also depend upon the skills, knowledge, experience and know-how of our management and research and development personnel as well as that of our advisors, consultants and other contractors. To help protect our proprietary know-how that is not patentable, we seek to put in place appropriate internal policies for the management of confidential information, and require all of our employees, consultants, advisors and other contractors to enter into confidentiality agreements that prohibit the disclosure of confidential information and which require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business.
We typically file for patents in the United States with counterparts in certain countries in Europe and certain key market countries in the rest of the world, thereby covering the major pharmaceutical markets. As of September 30, 2013, we own or co-own 51 U.S. patents and patent applications and their foreign counterparts, including 25 issued U.S. patents as reflected in the following table:
Granted and Pending U.S. Patents
Program
|
Granted (United States) | Pending (United States) | |||||
|---|---|---|---|---|---|---|---|
Kinase |
8 | 10 | |||||
Hos 2 and HDAC |
10 | 15 | |||||
Beta-Lactamase |
6 | 1 | |||||
DNMT |
1 | 0 | |||||
TOTAL |
25 | 26 | |||||
Kinase — (8 granted U.S. patents; 10 pending U.S. patent applications)
As of September 30, 2013, we have eight issued patents and ten pending patent applications in the United States covering inhibitor compounds, including MGCD265 and MGCD516, and methods of use of these compounds. Of these issued patents, one covers multiple series of kinase inhibitors and protects MGCD265 generically. Another issued patent, which expires no earlier than 2026, protects a selection of compounds including MGCD265, as well as methods of inhibiting VEGF and HGF receptor signaling dual methods of treating angiogenesis-mediated cell proliferative disease or inhibiting solid tumor growth. Exclusivity arising from our issued patents for MGCD265 extends to at least 2026, including our patents covering the specific composition of matter of MGCD265 (expires 2026, prior to any legal or regulatory extensions, including any patent term extension, that may be available under the Hatch Waxman Act) and the generic class of compounds to which MGCD265 belongs (expires 2025, prior to legal or regulatory extensions, including any patent term extension, that may be available under the Hatch Waxman Act). Another four issued patents
20
cover several distinct classes of compounds. Such coverage includes specific claims to MGCD516, generic coverage of the class of compounds to which MGCD516 belongs, as well as patents covering methods of use of such compounds. Exclusivity arising from our patent protection for MGCD516 extends to at least 2029, prior to legal or regulatory extensions, including any patent term extension that may be available under the Hatch Waxman Act.
Our pending patent applications relating to our kinase inhibitors seek coverage of a broader scope of kinase inhibitors both for oncology and for the treatment of ophthalmic diseases. Methods of use of these inhibitors, such as methods of inhibiting VEGF and HGF receptor signaling, methods of treating angiogenesis-mediated cell proliferative disease or inhibiting solid tumor growth, as well as processes of manufacturing kinase inhibitors such as MGCD265 and synthetic intermediates required for the purpose are also being pursued.
Hos2 and HDAC Programs — (10 granted U.S. patents; 15 pending U.S. patent applications)
Our patent estate for our Hos2 and HDAC programs covers multiple series of HDAC inhibitors, including MGCD290 and mocetinostat. This group of patents includes 10 issued patents and 15 pending patent applications in the United States protecting composition of matter and method of use. One issued patent covers the Hos2 inhibitor MGCD290 both generically and specifically. Exclusivity arising from our patent protection for MGCD290 should extend to at least 2020, and exclusivity arising from our issued patents claiming the combination of MGCD290 with antifungal agents extends to 2026, prior to any legal or regulatory extensions that may be available to us. Exclusivity for mocetinostat extends to 2022 prior to legal or regulatory extensions, including any patent term extension that may be available under the Hatch Waxman Act.
In aggregate, these U.S. patents and patent applications cover the following inventions: novel HDAC inhibitors, including mocetinostat (eight issued patents and nine patent applications), methods of inhibiting HDACs, methods for treating cell proliferative disease or cancer, specific methods for treating colon, lung and pancreatic cancers, methods for treating polyglutamine expansion diseases (such as Huntington's disease) and methods for treating fungal infection. Three applications claim compositions of HDAC/Hos2 inhibitors with antifungal compounds, methods of enhancing the activity of the antifungal compounds with HDAC/Hos2 inhibitors, and methods of treating fungal infection. One pending application also seeks protection of the analogs of MGCD290 as well as prodrugs of HDAC/Hos2 inhibitors and their use, while another pending application claims methods for identifying/screening potentiators of antifungal compounds, the inhibitors of ergosterol biosynthesis. A provisional application is directed to novel HDAC/Hos2 inhibitors and their use.
Beta-Lactamase — (6 granted U.S. patents; 1 pending U.S. patent applications)
For our beta-lactamase inhibitor program, we co-filed two patent applications with Merck and Merck has since returned all rights to these patents to us. In line with our corporate objectives to promote the partnering and development of our lead beta-lactamase inhibitor, MG96077, we are currently supporting the prosecution of only one granted patent (coverage until 2027) that protects this molecule both specifically and generically. The majority of the other patents are in the process of abandonment.
DNMT Program — (1 granted U.S. patent)
In our DNA methyltransferase program, we own one U.S. patent specifically covering MG98. This U.S. patent covers MG98 and methods for inhibiting tumor growth with it. We may abandon this patent in the future as we are no longer pursing this program.
Licensing Agreements
We may enter into license or sub-license agreements when we believe such license is required to pursue a specific program.
21
Competition
Competitors in Oncology — Small Molecule Kinase Inhibitors
A large number of kinase inhibitors are currently in clinical trials, with many more in the early research stage. Biotechnology and pharmaceutical companies are also developing monoclonal antibodies to kinase targets and their ligands.
The Met kinase inhibitor field has recently generated intense scientific and industry interest. We believe that most of the biotechnology and pharmaceutical companies developing small molecule drugs for cancer have significant and active kinase inhibitor programs (including Met programs) that may be competitive with our own and these competitors are described below. Our MGCD265 program is attractively positioned in the pipeline of Met-targeted molecules and is characterized by potential advantages including: a unique kinase spectrum including the emerging RTK target Axl; a lack of activity against over 400 off-target kinases, supporting a favorable safety profile; and excellent tolerability to date with other anti-cancer agents (including chemotherapy), thus optimizing the potential for combination therapy approaches.
Companies with Met inhibitors believed to be in late preclinical or clinical development include, but are not limited to: Amgen Inc., ArQule Inc. and its partners Kyowa Hakko Kirin Pharma Inc. and Daiichi Sankyo Company Limited, Aveo Pharmaceuticals Inc., Bristol-Myers Squibb Company, Exelixis Inc., F. Hoffman-LaRoche Ltd., GlaxoSmithKline PLC, Novartis AG and Pfizer.
Axl is a newly emergent RTK target. However, a small number of RTK inhibitors that are launched in development are believed to inhibit Axl. These include foretinib (in Phase 2 development by Exelixis) and Xalkori.
Many companies have filed, and continue to file, patent applications which may or could affect our program if and when they issue, either because they protect a product that may compete with our product candidates, or because they protect intellectual property rights that are necessary for us to develop and commercialize our product candidates. These companies include, but are not limited to: Bristol-Myers Squibb, Compugen Limited, Exelixis, GlaxoSmithKline, Novartis and Pfizer. Since this area is competitive and of strong interest to pharmaceutical and biotechnology companies, we expect that these and other companies will continue to publish and file patent applications in this space in the future, as well as pursuing research and development programs in this area. We continue to monitor these and other companies in order to be aware of any third party products and/or intellectual property rights relevant to our products.
Competitors in Oncology — Mocetinostat Competitors
We believe that a key differentiating feature of mocetinostat is its spectrum of activity, covering only isoforms 1, 2, 3 and 11, the most relevant HDAC isoforms in human disease. Other companies that are developing spectrum-selective HDAC inhibitors in development include but are not limited to Acetylon Pharmaceuticals, Inc., Chroma Therapeutics Ltd., Shenzen Chipscreen Biosciences Ltd. and Syndax Pharmaceuticals Inc.
Companies with Pan-HDAC inhibitors, which are HDAC inhibitors that have an effect across a broader range of HDAC isoforms and therefore not as selective as molecules like mocetinostat, include but are not limited to: Celgene, Curis Inc., MEI Pharma Inc., Merck, Novartis, Pharmacyclics Inc. and others. We expect that these and other companies may continue to pursue research and development in relation to HDAC inhibitors. We continue to monitor these and other companies in order to be aware of any third party products and/or intellectual property rights relevant to our products.
Competitors in Oncology — General Competitors
In addition to companies that have HDAC inhibitors or kinase inhibitors addressing oncology indications, our competition also includes hundreds of private and publicly traded companies that operate in the area of oncology but have therapeutics with different mechanisms of action. The oncology market in general is highly competitive, with over 1,000 molecules currently in clinical development. Other important competitors, in addition to those mentioned above, include, but are not limited to: small and large
22
biotechnology companies, including but not limited to Amgen, Ariad Pharmaceuticals Inc., ArQule, Biogen Idec Inc, Celgene and Exelixis; and specialty and regional pharmaceutical companies and multinational pharmaceutical companies, including but not limited to, Abbott Laboratories Inc., Astellas Pharma Inc., AstraZeneca plc, Bayer-Schering Pharmaceutical, Boehringer Ingelheim AG, Bristol-Myers Squibb, Eisai Co. Ltd., Eli Lilly and Company, F. Hoffmann-LaRoche Ltd., GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Pfizer, Sanofi-Aventis, Taiho and Takeda Pharmaceutical Co.
Manufacturing
We do not own or operate manufacturing facilities for the production of MGCD265, mocetinostat or any of our other product candidates, nor do we plan to develop our own manufacturing operations in the foreseeable future. We currently depend on third-party contract manufacturers for all of our required raw materials, API and finished products for our preclinical and clinical trials.
Manufacturers of our products are required to comply with applicable FDA manufacturing requirements contained in the FDA's current good manufacturing practices, or cGMP, regulations. cGMP regulations require, among other things, quality control and quality assurance as well as corresponding maintenance of records and documentation. Pharmaceutical product manufacturers and other entities involved in the manufacture and distribution of approved pharmaceutical products are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance. Discovery of problems with a product after approval may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal of the product from the market. In addition, changes to the manufacturing process generally require prior FDA approval before being implemented.
Government Regulation
The Regulatory Process for Drug Development
The production and manufacture of our product candidates and our research and development activities are subject to regulation by various governmental authorities around the world. In the United States, drug products are subject to regulation by the FDA. There are other comparable agencies in Canada, Europe and other parts of the world. Regulations govern, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of products. Applicable legislation requires licensing of manufacturing and contract research facilities, carefully controlled research and testing of products, governmental review and/or approval of results prior to marketing therapeutic products. Additionally, adherence to good laboratory practices, or GLP, and good clinical practices, or GCP, during nonclinical and clinical testing and cGMP during production is required. The system of new drug approval in the United States is generally considered to be the most rigorous in the world and is described in further detail below under "U.S. Pharmaceutical Product Development Process." In Canada, these activities are regulated by the Food and Drug Act and the rules and regulations promulgated thereunder, which are enforced by the Therapeutic Products Directorate, or TPD of Health Canada.
U.S. Pharmaceutical Product Development Process
In the United States, the FDA regulates pharmaceutical products under the Federal Food, Drug and Cosmetic Act and implementing regulations. Pharmaceutical products are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. FDA sanctions could include refusal to approve
23
pending applications, withdrawal of an approval, a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production or distribution injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us.
It normally takes an average of 10 to 15 years for a typical experimental drug to proceed from concept to approval. The process required by the FDA before a pharmaceutical product may be marketed in the United States generally includes the following:
- •
- Completion of preclinical laboratory tests and animal studies. The latter often conducted according to GLPs or other applicable regulations, as well as synthesis and drug formulation development leading ultimately to clinical drug supplies manufactured according to cGMPs;
- •
- Submission to the FDA of an IND, which must become effective before human clinical trials may begin in the United States;
- •
- Performance of adequate and well-controlled human clinical trials according to the FDA's current GCPs, to establish the safety and efficacy of the proposed pharmaceutical product for its intended use;
- •
- Submission to the FDA of an NDA for a new pharmaceutical product;
- •
- Potential review by an external advisory committee to the FDA;
- •
- Satisfactory completion of an FDA inspection of the manufacturing facility or facilities where the pharmaceutical product is produced to assess compliance with the FDA's cGMP, to assure that the facilities, methods and controls are adequate to preserve the pharmaceutical product's identity, strength, quality and purity;
- •
- FDA audit of select preclinical and clinical trial sites that generated the data in support of the NDA; and
- •
- FDA review and approval of the NDA.
The lengthy process of seeking required approvals and the continuing need for compliance with applicable statutes and regulations require the expenditure of substantial resources, and approvals are inherently uncertain.
Preclinical Studies: Prior to clinical studies, a research phase takes place which involves demonstration of target and function, design, screening and synthesis of inhibitors. Preclinical studies include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies to evaluate efficacy and activity, toxic effects, pharmacokinetics and metabolism of the pharmaceutical product candidate and to provide evidence of the safety, bioavailability and activity of the pharmaceutical product candidate in animals. The conduct of the preclinical safety evaluations must comply with federal regulations and requirements including GLPs. The results of the formal IND-enabling preclinical studies, together with manufacturing information, analytical data, any available clinical data or literature as well as the comprehensive descriptions of proposed human clinical studies, are then submitted as part of the IND to the FDA.
The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA places the IND on a clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. The FDA may also impose clinical holds on a pharmaceutical product candidate at any time before or during clinical trials due to safety concerns or non-compliance. Accordingly, we cannot be certain that submission of an IND will result in the FDA allowing clinical trials to begin, or that, once begun, issues will not arise that suspend or terminate such clinical trial.
Clinical Trials: Clinical trials involve the administration of the pharmaceutical product candidate to healthy volunteers or patients under the supervision of qualified investigators, generally physicians not employed by the sponsor. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, and the parameters to be used
24
to monitor subject safety. Each protocol must be submitted to the FDA if conducted under a U.S. IND. Clinical trials must be conducted in accordance with the FDA's GCP requirements. Further, each clinical trial must be reviewed and approved by an independent institutional review board, or IRB, or ethics committee at or servicing each institution at which the clinical trial will be conducted. An IRB or ethics committee is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB or ethics committee also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
Phase 1 Clinical Trials: Phase 1 clinical trials are usually first-in-man trials, take approximately one to two years to complete and are generally conducted on a small number of healthy human subjects to evaluate the drug's activity, schedule and dose, pharmacokinetics and pharmacodynamics. However, in the case of life-threatening diseases, such as cancer, the initial Phase 1 testing may be done in patients with the disease. These trials typically take longer to complete and may provide insights into drug activity.
Phase 2 Clinical Trials: Phase 2 clinical trials can take approximately one to three years to complete and are carried out on a relatively small to moderate number of patients (as compared to Phase 3) in a specific indication. The pharmaceutical product is evaluated to preliminarily assess efficacy, to identify possible adverse effects and safety risks, and to determine optimal dose, regimens, pharmacokinetics, pharmacodynamics and dose response relationships. This phase also provides additional safety data and serves to identify possible common short-term side effects and risks in a larger group of patients. Phase 2 clinical trials sometimes include randomization of patients.
Phase 3 Clinical Trials: Phase 3 clinical trials take approximately two to five years to complete and involve tests on a much larger population of patients (several hundred to several thousand patients) suffering from the targeted condition or disease. These studies usually include randomization of patients and blinding of both patients and investigators at geographically dispersed test sites (multi-center trials). These trials are undertaken to further evaluate dosage, clinical efficacy and safety and are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labeling. Generally, two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of an NDA or foreign authorities for approval of marketing applications.
Special Protocol Assessment: A sponsor may be able to request an SPA, the purpose of which is to reach agreement with the FDA on the Phase 3 clinical trial protocol design and analysis that will form the primary basis of an efficacy claim. A sponsor meeting the regulatory criteria may make a specific request for an SPA and provide information regarding the design and size of the proposed clinical trial. An SPA request must be made before the proposed trial begins, and all open issues must be resolved before the trial begins. If a written agreement is reached, it will be documented and made part of the record. The agreement will be binding on the FDA and may not be changed by the sponsor or the FDA after the trial begins except with the written agreement of the sponsor and the FDA or if the FDA determines that a substantial scientific issue essential to determining the safety or efficacy of the product candidate was identified after the testing began. An SPA is not binding if new circumstances arise, and there is no guarantee that a study will ultimately be adequate to support an approval even if the study is subject to an SPA. Having an SPA does not guarantee that a product will receive FDA approval.
Post-Approval Studies: Phase 4 clinical trials may be conducted after initial marketing approval. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication and may be required by the FDA as a condition of approval.
Progress reports detailing the results of the clinical trial must be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events or for any finding from tests in laboratory animals that suggests a significant risk
25
for human subjects. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, if at all. The FDA or the sponsor or, if used, its data safety and monitoring board may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB or ethics committee can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB's or ethics committee's requirements or if the pharmaceutical product has been associated with unexpected serious harm to patients.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the pharmaceutical product, as well as finalize a process for manufacturing the product in commercial quantities in accordance with GMP requirements. The manufacturing process must be capable of consistently producing quality batches of the pharmaceutical product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final pharmaceutical product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the pharmaceutical product candidate does not undergo unacceptable deterioration over its shelf life.
U.S. Pharmaceutical Review and Approval Process
New Drug Application: Upon completion of pivotal Phase 3 clinical studies, the sponsor assembles all the product development, preclinical and clinical data along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the pharmaceutical product, proposed labeling and other relevant information, and submits it to the FDA as part of an NDA. If accepted by the FDA as substantially complete to permit substantive review, the submission or application is then reviewed by the regulatory body for approval to market the product. This process takes eight months to one year to complete, but in some cases may take longer. At the end of the review period the FDA may issue a Complete Response Letter, refusing to approve an NDA if the applicable regulatory criteria are not satisfied or requiring additional clinical data or other data and information. Even if such data and information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling.
Accelerated Approval
Accelerated Approval is a program that is intended to make promising products for life threatening diseases available on the basis of evidence of effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. Approvals of this kind typically include requirements for appropriate post-approval Phase 4 clinical trials to validate the surrogate endpoint or otherwise confirm the effect of the clinical endpoint. We currently intend to seek Accelerated Approval for mocetinostat in combination with Vidaza for MDS.
Post-Approval Requirements
Any pharmaceutical products for which we receive FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, complying with certain electronic records and signature requirements and complying with FDA promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, promoting pharmaceutical products for uses or in patient populations that are not described in the pharmaceutical product's approved labeling (known as "off-label use"), industry-sponsored scientific and educational activities and promotional activities involving the internet. Failure to comply with FDA requirements can have negative consequences, including adverse publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors and civil or criminal penalties.
26
The FDA also may require post-marketing testing, known as Phase 4 testing, risk evaluation and mitigation strategies and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product.
FDA Regulation of Companion Diagnostics
As part of our clinical development plans, we are exploring the use of companion diagnostics to identify patients most likely to respond to our product candidates. Companion diagnostics are classified as medical devices under the Federal Food, Drug, and Cosmetic Act in the United States. In the United States, the FDA regulates the medical device design and development, preclinical and clinical testing, premarket clearance or approval, registration and listing, manufacturing, labeling, storage, reporting, recordkeeping, advertising and promotion, export and import, sales and distribution, and post-market surveillance of medical devices. Unless an exemption applies, companion diagnostics require marketing clearance or approval from the FDA prior to commercial distribution. The two primary types of FDA marketing authorization applicable to a medical device are premarket notification, also called 510(k) clearance, and premarket approval, or PMA. According to a 2011 draft guidance issued by FDA officials, companion diagnostics ordinarily will be considered to be high risk and, therefore, will require PMA approval before they are marketed. Some companion diagnostics, however, could potentially be cleared through 510(k) clearance.
The 2011 draft guidance issued by the FDA, if finalized, would address issues critical to developing companion diagnostics, such as biomarker qualification, establishing clinical validity, the use of retrospective data, the appropriate patient population and when the FDA will require that the device and the drug be approved simultaneously. According to the draft guidance, if safe and effective use of a therapeutic product depends on a diagnostic, then the FDA generally will require approval or clearance of the diagnostic at the same time that the FDA approves the therapeutic product. The FDA has yet to issue further guidance, and it is unclear whether it will do so, or what the scope would be.
The FDA previously has required in vitro companion diagnostics intended to select the patients who will respond to the cancer treatment to obtain a PMA simultaneously with approval of the drug. Based on the draft guidance, and the FDA's past treatment of companion diagnostics, we believe that the FDA will require a PMA for one or more companion diagnostics to identify patient populations suitable for our product candidates. The review of these companion diagnostics in conjunction with the review of our product candidates involves coordination of review by the FDA's Center for Drug Evaluation and Research and by the FDA's Center for Devices and Radiological Health.
Other Healthcare Laws and Compliance Requirements
In the United States, our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services and other divisions of the U.S. government, including, the Department of Health and Human Services, the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, and state and local governments. For example, if a drug product is reimbursed by Medicare, Medicaid, or other federal or state healthcare programs, our company, including our sales, marketing and scientific/educational grant programs, must comply with the federal False Claims Act, the federal Anti-Kickback Statute, the federal Physician Payment Sunshine Act and similar state laws and regulations, each as amended from time to time. If a drug product is reimbursed by Medicare or Medicaid, pricing and rebate programs must comply with, as applicable, the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990, or OBRA, and the Medicare Prescription Drug Improvement and Modernization Act of 2003. Among other things, OBRA requires drug manufacturers to pay rebates on prescription drugs to state Medicaid programs and empowers states to negotiate rebates on pharmaceutical prices, which may result in prices for our future products that will likely be lower than the prices we might otherwise obtain. If our approved drug products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements may apply.
27
Pharmaceutical Coverage, Pricing and Reimbursement
In the United States and markets in other countries, sales of any products for which we receive regulatory approval for commercial sale will depend in part on the availability of coverage and adequate reimbursement from third-party payors, including government authorities, managed care providers, private health insurers and other organizations. In the United States, private health insurers and other third-party payors often provide reimbursement for products and services based on the level at which the government (through the Medicare or Medicaid programs) provides reimbursement for such products and services. Third-party payors are increasingly examining the medical necessity and cost-effectiveness of medical products and services in addition to their safety and efficacy and, accordingly, significant uncertainty exists as to the coverage and reimbursement status of newly approved therapeutics. In particular, in the United States, the European Union and other potentially significant markets for our product candidates, government authorities and third-party payors are increasingly attempting to limit or regulate the price of medical products and services, particularly for new and innovative products and therapies, which often has resulted in average selling prices that are lower than they would otherwise be. Further, the increased emphasis on managed healthcare in the United States and on country and regional pricing and reimbursement controls in the European Union will put additional pressure on product pricing, reimbursement and utilization, which may adversely affect our future product sales and results of operations. These pressures can arise from rules and practices of managed care groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and healthcare reform, pharmaceutical reimbursement policies and pricing in general. As a result, coverage and adequate third-party reimbursement may not be available for our product candidates to enable us realize an appropriate return on our investment in research and product development.
The market for our product candidates for which we may receive regulatory approval will depend significantly on access to third-party payors' drug formularies, or lists of medications for which third-party payors provide coverage and reimbursement. The industry competition to be included in such formularies often leads to downward pricing pressures on pharmaceutical companies. Also, third-party payors may refuse to include a particular branded drug in their formularies or may otherwise restrict patient access to a branded drug when a less costly generic equivalent or other alternative is available. In addition, because each third-party payor individually approves coverage and reimbursement levels, obtaining coverage and adequate reimbursement is a time-consuming and costly process. We would be required to provide scientific and clinical support for the use of any product to each third-party payor separately with no assurance that approval would be obtained, and we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our products. This process could delay the market acceptance of any of our product candidates for which we may receive approval and could have a negative effect on our future revenue and operating results. We cannot be certain that our product candidates will be considered cost-effective. If we are unable to obtain coverage and adequate payment levels for our product candidates from third-party payors, physicians may limit how much or under what circumstances they will prescribe or administer them and patients may decline to purchase them. This in turn could affect our ability to successfully commercialize our products and impact our profitability, results of operations, financial condition, and future success.
Additionally, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively, PPACA, substantially changes the way healthcare is financed by both governmental and private insurers. Among other cost containment measures, PPACA establishes: an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents; a new Medicare Part D coverage gap discount program; and a new formula that increases the rebates a manufacturer must pay under the Medicaid Drug Rebate Program. In the future, there may continue to be additional proposals relating to the reform of the U.S. healthcare system, some of which could further limit coverage and reimbursement of our drug products, once approved.
28
Employees
As of September 30, 2013, we had 38 employees including both permanent and contract employees, and we also utilize the services of consultants on a regular basis. Twenty-seven employees are engaged in product development activities and eleven are in support administration, including business development and finance. None of our employees are represented by labor unions or covered by collective bargaining agreements. On October 1, 2013 we announced that we would be winding down our operations in Montreal, Canada and Princeton, New Jersey. We intend to close operations in New Jersey by the end of October 2013. We expect that our operations in Montreal will transition to San Diego over the next three to six months.
Properties
Our Canadian office is currently located at 7150 Frederick Banting Street, Suite 200, Montreal, Québec, H4S 2A1, and we occupy approximately 10,000 square feet of office and laboratory space. Our U.S. subsidiary is currently located at 125 Village Boulevard, Princeton, New Jersey 08540 with 1,983 square feet of office space. Our corporate headquarters is located at 9363 Towne Centre Drive, San Diego, California 92121 where we occupy approximately 6,800 square feet of office space. The term of our lease at Frederick Banting Street, Montreal expires on August 31, 2014 with an option to extend the lease by six months. The term of our lease at Village Boulevard, Princeton expires on April 30, 2015 with an option to renew the lease for five years. The term of our sublease at Towne Centre Drive, San Diego expires on December 31, 2014. Rental payments are approximately $13,000 per month for our Montreal office, approximately $4,000 per month for our Princeton office and approximately $14,000 per month for our San Diego office. Following the closure of the Princeton and Montreal facilities, our sole location will be in San Diego.
Legal Proceedings
From time to time we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not presently a party to any legal proceedings that in the opinion of our management, if determined adversely to us, would have a material adverse effect on our business, financial condition, operating results or cash flows. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Corporate History
We were incorporated under the laws of the State of Delaware on April 29, 2013. We are a holding company and primarily conduct our operations through MethylGene Inc., a corporation incorporated under the Canada Business Corporations Act, or MethylGene Canada. On May 8, 2013, we entered into an Arrangement with MethylGene Canada. Subject to the terms and conditions of the Arrangement, the securityholders of MethylGene Canada received one share of our common stock in exchange for every 50 shares of MethylGene Canada pursuant to a court-approved plan of arrangement under the Canada Business Corporations Act. In addition, all outstanding options and warrants to purchase common shares of MethylGene Canada became exercisable on a 50-for-1 basis for shares of our common stock, and a proportionate increase was made to the exercise price or conversion price, as applicable. Upon consummation of the Arrangement on June 28, 2013, MethylGene Canada became our wholly-owned subsidiary. The primary motivations to enter into the Arrangement were to potentially increase trading liquidity and have better access to capital, while reducing the U.S. tax burdens of our significant stockholders. We were also motivated to enhance our U.S. profile with U.S. investors and to be better positioned to attract and retain key personnel.
9222-9129 Québec Inc., formerly MethylGene Inc., or Old MethylGene, was incorporated under Part IA of the Companies Act (Québec) by articles of incorporation dated December 13, 1995. Old MethylGene was party to a court approved arrangement, effective May 19, 2010, under the Companies Act (Québec) involving 1819400 Ontario Inc., 1815303 Ontario Limited, 7503466 Canada Inc. and 7503547 Canada Inc. As part of this arrangement, among other things, 7503466 Canada Inc. and 7503547 Canada Inc. were amalgamated to form MethylGene Canada, which carried on the business of Old MethylGene.
29
Risks Relating to Our Financial Position and Capital Requirements
We will require additional financing and may be unable to raise sufficient capital, which could lead us to delay, reduce or abandon development programs or commercialization.
Our operations have consumed substantial amounts of cash since inception. Our net research and development expenses were $10.0 million for the six months ended June 30, 2013, and $15.1 million and $8.9 million for 2012 and 2011, respectively. We believe that absent additional financing, our current cash and cash equivalents and marketable securities will sustain our operations into the second quarter of 2014. We have based these estimates on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect. We will require substantial additional capital to pursue additional clinical development for our lead clinical programs, including conducting late-stage clinical trials, manufacturing clinical supplies and potentially developing other assets in our pipeline, and, if we are successful, to commercialize any of our current product candidates. If the FDA or any foreign regulatory agency, such as the European Medicines Agency, or EMA, requires that we perform studies or trials in addition to those that we currently anticipate with respect to the development of our product candidates, or repeat studies or trials, our expenses would further increase beyond what we currently expect. Any delay resulting from such further or repeat studies or trials could also result in the need for additional financing. We may not be able to adequately finance our development programs, which could limit our ability to move our programs forward in a timely and satisfactory manner or require us to abandon the programs, any of which would harm our business, financial condition and results of operations. Because successful development of our product candidates is uncertain, we are unable to estimate the actual funds we will require to complete research and development and commercialize our product candidates.
If we are unable to obtain funding from equity offerings or debt financings on a timely basis, we may be required to (1) seek collaborators for one or more of our product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available; (2) relinquish or license on unfavorable terms our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves; or (3) significantly curtail one or more of our research or development programs or cease operations altogether.
Substantial doubt exists over our ability to continue as a going concern.
As of June 30, 2013, substantial doubt exists over our ability to continue as a going concern. We believe that our current cash and cash equivalents and marketable securities are sufficient to carry out our currently planned clinical development and operating plans into the second quarter of 2014. Our cash and cash equivalents and marketable securities decreased by $16.7 million in the six months ended June 30, 2013, reflecting an average rate of negative cash flow per month of approximately $2.8 million. Excluding non-recurring costs associated with recent management changes and costs associated with the previously described Arrangement agreement and listing on The NASDAQ Capital Market of $2.6 million, of which $1.4 million relates to the Arrangement and NASDAQ listing, our cash and cash equivalents and marketable securities decreased by $14.1 million in the six months ended June 30, 2013 reflecting an average rate of negative cash flow per month of approximately $2.4 million. At September 30, 2013, we estimate that we had approximately $15.0 million in cash, cash equivalents and marketable securities. Our future cash requirements could increase if we decide to expand our research and development efforts beyond our current plans.
30
We are a clinical-stage company with no approved products and no historical product revenue. Consequently, we expect that our financial and operating results will vary significantly from period to period.
We are a clinical-stage company that has incurred losses since its inception and expect to continue to incur substantial losses in the foreseeable future. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of uncertainty.
Our actual financial condition and operating results have varied significantly in the past and are expected to continue to fluctuate significantly from quarter-to-quarter or year-to-year due to a variety of factors, many of which are beyond our control. Factors relating to our business that may contribute to these fluctuations include:
- •
- the success of our clinical trials through all phases of clinical
development;
- •
- delays in the commencement, enrollment and timing of clinical trials;
- •
- our ability to secure and maintain collaborations, licensing or other
arrangements for the future development and/or commercialization of our product candidates, as well as the terms of those arrangements;
- •
- our ability to obtain, as well as the timeliness of obtaining, additional
funding to develop our product candidates;
- •
- the results of clinical trials or marketing applications for product
candidates that may compete with our product candidates;
- •
- competition from existing products or new products that may receive
marketing approval;
- •
- potential side effects of our product candidates that could delay or prevent
approval or cause an approved drug to be taken off the market;
- •
- any delays in regulatory review and approval of our product candidates;
- •
- our ability to identify and develop additional product candidates;
- •
- the ability of patients or healthcare providers to obtain coverage or
sufficient reimbursement for our products;
- •
- our ability, and the ability of third parties such as Clinical Research
Organizations, or CROs, to adhere to clinical study and other regulatory requirements;
- •
- the ability of third-party manufacturers to manufacture our product
candidates and key ingredients needed to conduct clinical trials and, if approved, successfully commercialize our products;
- •
- the costs to us, and our ability as well as the ability of any third-party
collaborators, to obtain, maintain and protect our intellectual property rights;
- •
- costs related to and outcomes of potential intellectual property litigation;
- •
- our ability to adequately support future growth;
- •
- our ability to attract and retain key personnel to manage our business
effectively; and
- •
- our ability to build our finance infrastructure and, to the extent required, improve our accounting systems and controls.
Accordingly, the likelihood of our success must be evaluated in light of many potential challenges and variables associated with a clinical-stage company, many of which are outside of our control, and past operating or financial results should not be relied on as an indication of future results. Fluctuations in our operating and financial results could cause our share price to decline. It is possible that in some future periods, our operating results will be above or below the expectations of securities analysts or investors, which could also cause our share price to decline.
31
We have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future. We have never generated any revenue from product sales and may never be profitable.
We have derived limited revenue from our research and licensing agreements which have not been sufficient to cover the substantial expenses we have incurred in our efforts to develop our product candidates. Consequently, we have accumulated net losses since inception in 1995. Our net loss for the six months ended June 30, 2013 was $12.2 million and for 2012 and 2011 it was $20.3 million and $9.8 million, respectively. As of June 30, 2013, we had an accumulated deficit of $157.8 million. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders' equity and working capital. Such losses are expected to increase in the future as we continue the development of our product candidates and seek regulatory approval and commercialization for our product candidates. We are unable to predict the extent of any future losses or when we will become profitable, if ever. Even if we do achieve profitability, we may not be able to sustain or increase profitability on an ongoing basis.
We do not anticipate generating revenue from sales of products for the foreseeable future, if ever. If any of our product candidates fail in clinical trials or do not gain regulatory approval, or if any of our product candidates, if approved, fail to achieve market acceptance, we may never become profitable. If one or more of our product candidates is approved for commercial sale and we retain commercial rights, we anticipate incurring significant costs associated with commercializing any such approved product candidate. Therefore, even if we are able to generate revenue from the sale of any approved product, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our ability to generate future revenue from product sales depends heavily on our success in:
- •
- completing development and clinical trial programs for our product
candidates;
- •
- entering into collaboration and license agreements;
- •
- seeking and obtaining marketing approvals for any product candidates that
successfully complete clinical trials;
- •
- establishing and maintaining supply and manufacturing relationships with
third parties;
- •
- successfully commercializing any product candidates for which marketing
approval is obtained; and
- •
- successfully establishing a sales force and marketing and distribution infrastructure.
Raising additional funds through debt or equity financing will be dilutive and raising funds through licensing agreements may be dilutive, restrict operations or relinquish proprietary rights.
To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of those securities could result in substantial dilution for our current stockholders and the terms may include liquidation or other preferences that adversely affect the rights of our current stockholders. Existing stockholders may not agree with our financing plans or the terms of such financings. Moreover, the incurrence of debt financing could result in a substantial portion of our operating cash flow being dedicated to the payment of principal and interest on such indebtedness and could impose restrictions on our operations. In addition, if we raise additional funds through collaboration and licensing arrangements, it may be necessary to relinquish potentially valuable rights to our products or proprietary technologies, or to grant licenses on terms that are not favorable to us. Additional funding may not be available to us on acceptable terms, or at all.
32
We may incur losses associated with foreign currency fluctuation.
Our headquarters were previously located in Canada and many of our material contracts were entered into in Canada. A significant portion of our expenditures are in foreign currencies, most notably in Canadian dollars; therefore, we are subject to foreign currency fluctuations which may, from time to time, impact (positively or negatively) our financial position and results of operations. Exchange rates can fluctuate significantly and cannot be easily predicted; thus, we may experience significant shifts in currency exchange variances in the future. We maintain bank accounts in both Canadian dollars and U.S. dollars and do not hedge our positions. Our functional currency at December 31, 2012 was the Canadian dollar and based on extensive analysis of projected expenses we changed our functional currency to the U.S. dollar effective January 1, 2013.
As a public company in the United States, we are subject to the Sarbanes-Oxley Act. We can provide no assurance that we will, at all times, in the future be able to report that our internal controls over financial reporting are effective.
Companies that file reports with the Securities and Exchange Commission, or the SEC, including us, are subject to the requirements of Section 404 of the Sarbanes-Oxley Act of 2002. Section 404 requires management to establish and maintain a system of internal control over financial reporting, and annual reports on Form 10-K filed under the Securities Exchange Act of 1934, as amended, or the Exchange Act, must contain a report from management assessing the effectiveness of a company's internal control over financial reporting.
As a "smaller reporting company" (as defined in the Exchange Act) we will be required to comply with Section 404 of the Sarbanes-Oxley Act although, as an "emerging growth company" (as defined in the JOBS Act) and a smaller reporting company, we are not required to comply with Section 404(b) which requires attestation from our external auditors on our internal control over financial reporting. We are subject to Section 404(a), which requires management to provide a report regarding the effectiveness of internal controls. We were previously listed on the Toronto Stock Exchange, or TSX, from June 2004 until July 2013 and were subject to similar governance requirements under Multi-lateral Instrument 52-109. We are required to review all of our control processes to align them to the SOX 404 requirements. Failure to provide assurance that our financial controls are effective could lead to lack of confidence by investors which could lead to a lower share price. When and if we become a "large accelerated filer" or an "accelerated filer" and are no longer an "emerging growth company" (each as defined in the Exchange Act or the Securities Act of 1933, as amended, or the Securities Act), our independent registered public accounting firm will be required to attest to the effectiveness of our internal control over financial reporting. The rules governing the standards that must be met for management to assess our internal control over financial reporting are complex and require significant documentation, testing and possible remediation. To comply with the requirements of being a reporting company under the Exchange Act, we may need to upgrade our systems including information technology, implement additional financial and management controls, reporting systems and procedures, and hire additional accounting and finance staff.
We will incur significant increased costs as a result of operating as a U.S. public company and continuing to be a Canadian "reporting issuer."
Although we de-listed from the TSX effective as of July 26, 2013, we will continue to be subject to Canadian reporting obligations. Our Canadian reporting obligations will continue until we meet certain prescribed thresholds which would allow us to apply to cease being a Canadian "reporting issuer." We may incur significant additional accounting, reporting and other expenses in order to maintain our listing on The NASDAQ Capital Market, and fulfill our obligations as a Canadian "reporting issuer." For example, we may incur additional expenses if we are required to continue to present our financial information according to International Financial Reporting Standards in Canada, as well as according to U.S. generally accepted accounting principles. In addition, as a U.S. listed public company, we will incur significant additional
33
legal, accounting and other expenses that we did not incur as a company listed on the TSX. Shareholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, any new regulations or disclosure obligations may increase our legal and financial compliance costs and will make some activities more time-consuming and costly. Incremental recurring external costs associated with being a publicly traded company in the United States are estimated to be approximately $0.5 million per year, consisting primarily of increased legal, accounting and insurance costs.
We are an emerging growth company and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an emerging growth company. Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
For as long as we continue to be an emerging growth company, we intend to take advantage of certain other exemptions from various reporting requirements that are applicable to other public companies including, but not limited to, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, exemptions from the requirements of holding a nonbinding advisory stockholder vote on executive compensation and any golden parachute payments not previously approved, exemption from the requirement of auditor attestation in the assessment of our internal control over financial reporting and exemption from any requirement that may be adopted by the Public Company Accounting Oversight Board. If we do, the information that we provide stockholders may be different than what is available with respect to other public companies. We cannot predict if investors will find our common stock less attractive because we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less-active trading market for our common stock and our stock price may be more volatile.
We will remain an emerging growth company until the earliest of (1) the end of the fiscal year in which the market value of our common stock that is held by non-affiliates exceeds $700 million as of the end of the second fiscal quarter, (2) the end of the fiscal year in which we have total annual gross revenue of $1 billion or more during such fiscal year, (3) the date on which we issue more than $1 billion in non-convertible debt in a three-year period or (4) the end of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement filed under the Securities Act.
Decreased disclosures in our SEC filings due to our status as an emerging growth company may make it harder for investors to analyze our results of operations and financial prospects.
We are a smaller reporting company and we cannot be certain if the reduced disclosure requirements applicable to smaller reporting companies will make our common stock less attractive to investors.
We are currently a "smaller reporting company," meaning that we are not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company and had a public float of less than $75 million and annual revenue of less than $50 million during the most recently completed fiscal year. In the event that we are still considered a smaller reporting company at such time as we cease being an emerging growth company, we will be required to provide additional disclosure in our SEC filings. However, similar to emerging growth companies, smaller reporting companies are able to provide simplified executive compensation disclosures in their filings, are exempt from the
34
provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting, and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports.
Decreased disclosures in our SEC filings due to our status as a smaller reporting company may make it harder for investors to analyze our results of operations and financial prospects.
Risks Relating to Our Business and Industry
Our research and development programs and product candidates are at an early stage of development. As a result we are unable to predict if or when we will successfully develop or commercialize our product candidates.
Our clinical-stage product candidates as well as our other pipeline assets are at an early stage of development and will require significant further investment and regulatory approvals prior to commercialization. We currently have no product candidates beyond Phase 2 clinical trials. MGCD265 is currently in Phase 1 and Phase 1/2 clinical trials, and MGCD516 is in advanced preclinical development. Each of our product candidates will require additional clinical development, management of clinical, preclinical and manufacturing activities, obtaining regulatory approval, obtaining manufacturing supply, building of a commercial organization, substantial investment and significant marketing efforts before we generate any revenues from product sales. We are not permitted to market or promote any of our product candidates before we receive regulatory approval from the FDA or comparable foreign regulatory authorities, and we may never receive such regulatory approval for any of our product candidates. In addition, some of our product development programs contemplate the development of companion diagnostics. Companion diagnostics are subject to regulation as medical devices and we may be required to obtain marketing approval for accompanying companion diagnostics before we may commercialize our product candidates. Subject to receipt of additional financing, we plan on conducting a dose confirmation trial and obtaining an SPA with the FDA prior to initiating Phase 3 trials with mocetinostat.
Even if we obtain the required financing or establish a collaboration to enable us to conduct late-stage clinical development of our product candidates and pipeline assets, we cannot be certain that such clinical development would be successful, or that we will obtain regulatory approval or be able to successfully commercialize any of our product candidates and generate revenue. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and the clinical trial process may fail to demonstrate that our product candidates are safe and effective for their proposed uses. Any such failure could cause us to abandon further development of any one or more of our product candidates and may delay development of other product candidates. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and initial clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials. Any delay in, or termination of, our clinical trials will delay and possibly preclude the filing of any new drug applications, or NDAs, with the FDA and, ultimately, our ability to commercialize our product candidates and generate product revenue.
We have not previously submitted an NDA to the FDA, or similar drug approval filings to comparable foreign authorities, for any product candidate, and we cannot be certain that any of our product candidates will receive regulatory approval. Further, our product candidates may not receive regulatory approval even if they are successful in clinical trials. If we do not receive regulatory approvals for our product candidates, we may not be able to continue our operations. Even if we successfully obtain regulatory approvals to market one or more of our product candidates, our revenues will be dependent, in part, upon our or our future collaborators' ability to obtain regulatory approval of the companion diagnostics to be used with our product candidates, if required, as well as the size of the markets in the territories for which we gain regulatory
35
approval and have commercial rights. If the markets for patient subsets that we are targeting are not as significant as we estimate, we may not generate significant revenues from sales of such products, if approved.
All of our product candidates are subject to extensive regulation, which can be costly and time consuming, cause delays or prevent approval of such product candidates for commercialization.
The clinical development of product candidates is subject to extensive regulation by the FDA in the United States and by comparable regulatory authorities in foreign markets. Product development is a very lengthy and expensive process, and its outcome is inherently uncertain. The product development timeline can vary significantly based upon the product candidate's novelty and complexity. Regulations are subject to change and regulatory agencies have significant discretion in the approval process.
Numerous statutes and regulations govern human testing and the manufacture and sale of human therapeutic products in the United States, Canada and other countries where we intend to market our products. Such legislation and regulation bears upon, among other things, the approval of protocols and human testing, the approval of manufacturing facilities, safety of the product candidates, testing procedures and controlled research, review and approval of manufacturing, preclinical and clinical data prior to marketing approval including adherence to good manufacturing practices, or GMP, during production and storage as well as regulation of marketing activities including advertising and labeling.
In order to obtain regulatory approval for the commercial sale of any of our product candidates, we must demonstrate through preclinical studies and clinical trials that the potential product is safe and effective for use in humans for each target indication. The failure to adequately demonstrate the safety and efficacy of a product under development could delay or prevent regulatory approval of our product candidates.
No assurance can be given that current regulations relating to regulatory approval will not change or become more stringent in the United States or foreign markets. Regulatory agencies may also require that additional trials be run in order to provide additional information regarding the safety or efficacy of any compound for which we seek regulatory approval. Moreover, any regulatory approval of a drug which is eventually obtained may entail limitations on the indicated uses for which that drug may be marketed. Furthermore, product approvals may be withdrawn or limited in some way if problems occur following initial marketing or if compliance with regulatory standards is not maintained. Regulatory agencies could become more risk adverse to any side effects or set higher standards of safety and efficacy prior to reviewing or approving a product. This could result in a product not being approved. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
We may not be successful in establishing development and commercialization collaborations which could adversely affect, and potentially prohibit, our ability to develop our product candidates.
Because developing pharmaceutical products, conducting clinical trials, obtaining regulatory approval, establishing manufacturing capabilities and marketing approved products are expensive, we may seek to enter into collaborations with companies that have more resources and experience in order to continue to develop and commercialize our product candidates. We also may be required due to financial or scientific constraints to enter into additional corporate collaboration agreements to research and/or to develop and commercialize our product candidates. The establishment and realization of such collaborations may be not be possible or may be problematic. There can be no assurance that we will be able to establish such additional collaborations on favorable terms, if at all, or that our current or future collaborative arrangements will be successful or maintained for any specific product candidate or indication. If we are unable to reach successful agreements with suitable partners for the ongoing development and commercialization of our product candidates, we may face increased costs, we may be forced to limit the scope and number of our product candidates we can commercially develop or the territories in which we commercialize such product candidates and we may be unable to commercialize products or programs for
36
which a suitable partner cannot be found. If we fail to achieve successful partnerships, our operating results and financial condition will be materially and adversely affected.
In addition, the terms of any collaboration agreements may place restrictions on our activities with respect to other products, including by limiting our ability to grant licenses or develop products with other third parties, or in different indications, diseases or geographical locations, or may place additional obligations on us with respect to development or commercialization of our product candidates. If we fail to comply with or breach any provision of a collaborative or license agreement, a collaborator may have the right to terminate, in whole or in part, such agreement or to seek damages.
Some of our collaboration agreements are complex and involve sharing or division of ownership of certain data, know-how and intellectual property rights among the various parties. Accordingly our collaborators could interpret certain provisions differently than we or our other partners which could lead to unexpected or inadvertent disputes with partners. In addition, these agreements might make additional partnering or mergers and acquisitions difficult.
There is no assurance that a collaborator who is acquired by a third party would not attempt to change certain contract provisions that could negatively affect our collaboration. The acquiring company may also not accept the terms or assignment of our contracts and may seek to terminate the agreements. Any one of our partners could breach covenants, restrictions and/or sub-license agreement provisions leading us into disputes and potential breaches of our agreements with other partners.
If we or third parties are unable to successfully develop companion diagnostics for our kinase inhibitor product candidates, or experience significant delays in doing so, we may not achieve marketing approval or realize the full commercial potential of such product candidates.
A key part of our strategy for our kinase inhibitor development program, including MGCD265 and MGCD516, is to identify patients or types of tumors that express specific genetic markers, which will require the use and development of companion diagnostics. We expect that the FDA and comparable foreign regulatory authorities will require the regulatory approval of a companion diagnostic as a condition to approving these product candidates. We do not have experience or capabilities in developing or commercializing diagnostics and plan to rely in large part on third parties to perform these functions. We do not currently have any long-term arrangements in place with any third party to develop or commercialize companion diagnostics for any of our therapeutic product candidates.
Companion diagnostics are subject to regulation by the FDA and comparable foreign regulatory authorities as medical devices and will likely require separate regulatory approval prior to commercialization.
If we or third parties are unable to successfully develop companion diagnostics for our kinase inhibitor product candidates, or experience delays in doing so:
- •
- the development of these product candidates may be adversely affected if we
are unable to appropriately select patients for enrollment in our clinical trials;
- •
- these product candidates may not receive marketing approval if their safe
and effective use depends on a companion diagnostic; and
- •
- we may not realize the full commercial potential of these product candidates that receive marketing approval if, among other reasons, we are unable to appropriately identify patients or types of tumors with the specific genetic alterations targeted by these product candidates.
Even if our kinase inhibitor product candidates and any associated companion diagnostics are approved for marketing, the need for companion diagnostics may slow or limit adoption of our product candidates. Although we believe genetic testing is becoming more prevalent in the diagnosis and treatment of cancer, our product candidates may be perceived negatively compared to alternative treatments that do not require
37
the use of companion diagnostics, either due to the additional cost of the companion diagnostic or the need to complete additional procedures to identify genetic markers prior to administering our product candidates.
If any of these events were to occur, our business and growth prospects would be harmed, possibly materially.
We may not be able to obtain an SPA prior to initiating Phase 3 clinical trials of mocetinostat. Even if obtained, an SPA would not guarantee any particular outcome from regulatory review.
We plan to submit an SPA to the FDA for the planned Phase 3 development of mocetinostat. The FDA's SPA process creates a written agreement between the sponsoring company and the FDA regarding clinical trial design and other clinical trial issues that can be used to support approval of a product candidate. The SPA is intended to provide assurance that if the agreed upon clinical trial protocols are followed and the clinical trial endpoints are achieved, the data may serve as the primary basis for an efficacy claim in support of an NDA. However, SPA agreements are not a guarantee of an approval of a product candidate or any permissible claims about the product candidate. In particular, SPAs are not binding on the FDA if previously unrecognized public health concerns arise during the performance of the clinical trial, if other new scientific concerns regarding product candidate safety or efficacy arise or if the sponsoring company fails to comply with the agreed upon clinical trial protocols. We cannot guarantee that we will obtain an SPA for the Phase 3 development of mocetinostat or that an SPA, if obtained, would ultimately aid in obtaining regulatory approval.
We rely upon third-party contractors and service providers for the execution of some aspects of our development programs. Failure of these collaborators to provide services of a suitable quality and within acceptable timeframes may cause the delay or failure of our development programs.
We outsource certain functions, tests and services to CROs, medical institutions and collaborators as well as outsourcing manufacturing to collaborators and/or contract manufacturers and we rely on third parties for quality assurance, clinical monitoring, clinical data management and regulatory expertise. In particular, we rely on CROs to run our clinical trials on our behalf. There is no assurance that such individuals or organizations will be able to provide the functions, tests, drug supply or services as agreed upon or to acceptable quality standards, and we could suffer significant delays in the development of our products or processes.
In some cases there may be only one or few providers of such services, including clinical data management or manufacturing services. In addition, the cost of such services could increase significantly over time. We rely on third parties as mentioned above to enroll qualified patients and conduct, supervise and monitor our clinical trials. Our reliance on these third parties and collaborators for clinical development activities reduces our control over these activities, but does not relieve us of our regulatory responsibilities, including ensuring that our clinical trials are conducted in accordance with good clinical practices, or GCP, regulations and the investigational plan and protocols contained in the regulatory agency applications. In addition, these third parties may not complete activities on schedule or may not manufacture compounds under GMP conditions. Preclinical studies may not be performed or completed in accordance with good laboratory practices, or GLP, regulatory requirements or our trial design. If we or our CROs fail to comply with GCP regulations, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, the EMA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving any marketing applications. If these third parties or collaborators do not successfully carry out their contractual duties or meet expected deadlines, obtaining regulatory approval for manufacturing and commercialization of our product candidates may be delayed or prevented. We rely substantially on third-party data managers for our clinical trial data. There is no assurance that these third parties will not make errors in the design, management or retention of our data or data systems. There is no assurance these third parties will pass FDA or regulatory audits, which could delay or prohibit regulatory approval.
38
Our CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials or other product development activities, which could harm our competitive position. If any of our relationships with these third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs or to do so on commercially reasonable terms. Further, switching or adding additional CROs involves additional cost and requires management time and attention. In addition, there is a natural transition period when a new CRO commences work. As a result, delays may occur, which could materially impact our ability to meet our desired clinical development timelines. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
The timelines of our clinical trials may be impacted by numerous factors and any delays may adversely affect our ability to execute our current business strategy.
Clinical testing is expensive, difficult to design and implement, can take many years to complete, and is uncertain as to outcome. We may experience delays in clinical trials at any stage of development and testing of our product candidates. Our planned clinical trials may not begin on time, have an effective design, enroll a sufficient number of subjects, or be completed on schedule, if at all.
Events which may result in a delay or unsuccessful completion of clinical trials include:
- •
- inability to raise funding necessary to initiate or continue a trial;
- •
- delays in obtaining regulatory approval to commence a trial;
- •
- delays in reaching agreement with the FDA on final trial design;
- •
- imposition of a clinical hold following an inspection of our clinical trial
operations or trial sites by the FDA or other regulatory authorities;
- •
- imposition of a clinical hold following an inspection of our clinical trial
operations or trial sites by the FDA or other regulatory authorities;
- •
- delays in reaching agreement on acceptable terms with prospective CROs and
clinical trial sites;
- •
- delays in obtaining required institutional review board approval at each
site;
- •
- delays in recruiting suitable patients to participate in a trial;
- •
- delays in having subjects complete participation in a trial or return for
post-treatment follow-up;
- •
- delays caused by subjects dropping out of a trial due to side effects or
otherwise;
- •
- clinical sites dropping out of a trial to the detriment of enrollment;
- •
- time required to add new clinical sites; and
- •
- delays by our contract manufacturers to produce and deliver a sufficient supply of clinical trial materials.
For example, due to the targeted indications and patient population we intend to focus on for development of our kinase inhibitor product candidates, the number of study sites and patient populations available to us may be relatively limited, and therefore enrollment of suitable patients to participate in clinical trials for these product candidates may take longer than would be the case if we were pursuing broader indications or patient populations.
If initiation or completion of any of our clinical trials for our product candidates are delayed for any of the above reasons, our development costs may increase, our approval process could be delayed, any periods after commercial launch and before expiration of patent protection may be reduced and our competitors may have more time to bring products to market before we do. Any of these events could impair the commercial potential of our product candidates and could have a material adverse effect on our business.
39
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any.
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign authorities. Results of our trials could reveal a high and unacceptable severity and prevalence of side effects. In such an event, our trials could be suspended or terminated and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product candidates for any or all targeted indications. Treatment-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial, or result in potential product liability claims. Any of these occurrences may harm our business, financial condition and prospects significantly.
Additionally if one or more of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by such products, a number of potentially significant negative consequences could result, including:
- •
- regulatory authorities may withdraw approvals of such product;
- •
- regulatory authorities may require additional warnings on the label;
- •
- we may be required to create a medication guide outlining the risks of such
side effects for distribution to patients;
- •
- we could be sued and held liable for harm caused to patients; and
- •
- our reputation may suffer.
Any of these events could prevent us from achieving or maintaining market acceptance of any product candidate, if approved, and could significantly harm our business, results of operations and prospects.
We are and continue to be subject to stringent government regulations concerning the clinical testing of our products. We will also continue to be subject to government regulation of any product that receives regulatory approval.
Numerous statutes and regulations govern human testing and the manufacture and sale of human therapeutic products in the United States and other countries where we intend to market our products. Such legislation and regulation bears upon, among other things, the approval of protocols and human testing, the approval of manufacturing facilities, testing procedures and controlled research, the review and approval of manufacturing, preclinical and clinical data prior to marketing approval, including adherence to GMP during production and storage, and marketing activities including advertising and labeling.
Clinical trials may be delayed or suspended at any time by us or by the FDA or other similar regulatory authorities if it is determined at any time that patients may be or are being exposed to unacceptable health risks, including the risk of death, or if compounds are not manufactured under acceptable GMP conditions or with acceptable quality. Current regulations relating to regulatory approval may change or become more stringent. The agencies may also require additional trials be run in order to provide additional information regarding the safety, efficacy or equivalency of any compound for which we seek regulatory approval.
Moreover, any regulatory approval of a drug which is eventually obtained may entail limitations on the indicated uses for which that drug may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate. In addition, if the FDA or a comparable foreign regulatory authority approves any of our product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include
40
submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with GMPs and GCPs for any clinical trials that we conduct post-approval. Furthermore, product approvals may be withdrawn or limited in some way if problems occur following initial marketing or if compliance with regulatory standards is not maintained. Similar restrictions are imposed in foreign markets. Regulatory agencies could become more risk adverse to any side effects or set higher standards of safety and efficacy prior to reviewing or approving a product. This could result in a product not being approved.
If we, or any future marketing collaborators or contract manufacturers, fail to comply with applicable regulatory requirements, we may be subject to sanctions including fines, product recalls or seizures and related publicity requirements, injunctions, total or partial suspension of production, civil penalties, suspension or withdrawals of previously granted regulatory approvals, warning or untitled letters, refusal to approve pending applications for marketing approval of new products or of supplements to approved applications, import or export bans or restrictions, and criminal prosecution and penalties. Any of these penalties could delay or prevent the promotion, marketing or sale of our products and product candidates.
The FDA's policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
We have no experience in commercial manufacturing and depend on others for the production of our product candidates at suitable levels of quality and quantity. Any problems or delays in the manufacture of our products would have a negative impact on our ability to successfully execute our development and commercialization strategies.
We do not currently have nor do we plan to acquire the infrastructure or capability internally to manufacture our clinical drug supplies for use in the conduct of our clinical trials, and we lack the resources and the capability to manufacture any of our product candidates on a clinical or commercial scale. We rely on collaborators and/or third parties for development, scale-up, formulation, optimization, management of clinical trial and commercial scale manufacturing and commercialization. There are no assurances we can scale-up, formulate or manufacture any product candidate in sufficient quantities with acceptable specifications for the conduct of our clinical trials or for the regulatory agencies to grant approval of such product candidate. We have not yet commercialized any products and have no commercial manufacturing experience. To be successful, our products must be properly formulated, scalable, stable and safely manufactured in clinical trial and commercial quantities in compliance with GMP and other regulatory requirements and at acceptable costs. Should any of our suppliers or our collaborators be unable to supply or be delayed in supplying us with sufficient supplies, no assurance can be given that we will be able to find alternative means of supply in a short period of time. Should such parties' operations suffer a material adverse effect, the manufacturing of our products would also be adversely affected. Furthermore, key raw materials could become scarce or unavailable. There may be a limited number of third parties who can manufacture our products. We may not be able to meet specifications previously established for compounds during scale-up and manufacturing.
Our reliance on third parties to manufacture our product candidates will expose us and our partners to risks including the following, any of which could delay or prevent the commercialization of our products, result in higher costs, or deprive us of potential product revenue:
- •
- Contract manufacturers can encounter difficulties in achieving the scale-up, optimization, formulation, volume production of a compound as well as maintaining quality control with appropriate quality assurance. They may also experience shortages of qualified personnel. Contract manufacturers are required to undergo a satisfactory GMP inspection prior to regulatory approval and are obliged to operate in accordance with FDA, International Conference on Harmonisation of Technical
41
- •
- For each of our current product candidates we will initially rely on a
limited number of contract manufacturers. Changing these or identifying future manufacturers may be difficult. Changing manufacturers requires re-validation of the manufacturing processes and
procedures in accordance with FDA, ICH, European and other mandated GMP regulations and/or guidelines. Such re-validation may be costly and time-consuming. It may be difficult or impossible for us to
quickly find replacement manufacturers on acceptable terms, if at all.
- •
- Our contract manufacturers may not perform as agreed or may not remain in the contract manufacturing business for the time required to produce, store and distribute our products successfully.
Requirements for Registration of Pharmaceuticals for Human Use, or ICH, European and other nationally mandated GMP regulations and/or guidelines governing manufacturing processes, stability testing, record keeping and quality standards. A failure of these contract manufacturers to follow GMP and to document their adherence to such practices or failure of an inspection by a regulatory agency may lead to significant delays in the availability of material for clinical study, leading to delays in our trials.
The successful commercialization of our product candidates, if approved, will depend on achieving market acceptance and we may not be able to gain sufficient acceptance to generate significant revenue.
Even if our product candidates are successfully developed and receive regulatory approval, they may not gain market acceptance among physicians, patients, healthcare payors such as private insurers or governments and other funding parties and the medical community. The degree of market acceptance for any of our products will depend on a number of factors, including:
- •
- demonstration of the clinical efficacy and safety of our products;
- •
- the prevalence and severity of any adverse side effects;
- •
- limitations or warnings contained in the product's approved labeling;
- •
- cost-effectiveness and availability of acceptable pricing;
- •
- competitive product profile versus alternative treatment methods and the
superiority of alternative treatment or therapeutics;
- •
- the effectiveness of marketing and distribution methods and support for the
products; and
- •
- coverage and reimbursement policies of government and third-party payors to the extent that our products could receive regulatory approval but not be approved for coverage by or receive adequate reimbursement from government and quasi-government agencies or other third-party payors.
Disease indications may be small subsets of a disease that could be parsed into smaller and smaller indications as different subsets of diseases are defined. This increasingly fine characterization of diseases could have negative consequences, including creating an approved indication that is so small as not to have a viable market for us. If future technology allows characterization of a disease in a way that is different from the characterization used for large pivotal studies, it may make those studies invalid or reduce their usefulness, and may require repeating all or a portion of the studies. Future technology may supply better prognostic ability which could reduce the portion of patients projected to need a new therapy. Even after being cleared by regulatory authorities, a product may later be shown to be unsafe or not to have its purported effect, thereby preventing its widespread use or requiring withdrawal from the market.
42
If we fail to obtain coverage and adequate reimbursement for our products, our revenue-generating ability will be diminished and there is no assurance that the anticipated market for our products will be sustained.
We believe that there will be many different applications for products successfully derived from our technologies and that the anticipated market for products under development will continue to expand. However, due to competition from existing or new products and the yet-to-be established commercial viability of our products, no assurance can be given that these beliefs will prove to be correct. Physicians, patients, formularies, payors or the medical community in general may not accept or utilize any products that we or our collaborative partners may develop. Other drugs may be approved during our clinical testing which could change the accepted treatments for the disease targeted and make our compound obsolete.
Our and our collaborators' ability to commercialize our products successfully will depend, in part, on the extent to which coverage and adequate reimbursement for such products and related treatments will be available from governmental health payor programs at the federal and state levels, including Medicare and Medicaid, private health insurers, managed care plans and other organizations. No assurance can be given that third-party coverage and adequate reimbursement will be available that will allow us to maintain price levels sufficient for the realization of an appropriate return on our investment in product development.
Coverage and adequate reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors is critical to new product acceptance. Coverage decisions may depend upon clinical and economic standards that disfavor new drug products when more established or lower cost therapeutic alternatives are already available or subsequently become available. Even if we obtain coverage for our product candidates, the resulting reimbursement payment rates might not be adequate or may require co-payments that patients find unacceptably high. Patients are unlikely to use our product candidates unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our product candidates.
In the United States, in Canada and in many other countries, pricing and/or profitability of some or all prescription pharmaceuticals and biopharmaceuticals are subject to varying degrees of government control. Healthcare reform and controls on healthcare spending may limit the price we charge for any products and the amounts thereof that we can sell. In particular, in the United States, the federal government and private insurers have changed and have considered ways to change, the manner in which healthcare services are provided. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, PPACA, became law in the United States. PPACA substantially changes the way healthcare is financed by both governmental and private insurers and significantly affects the healthcare industry. The provisions of PPACA of importance to our product candidates include the following:
- •
- an annual, nondeductible fee on any entity that manufactures or imports
specified branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs;
- •
- an increase in the statutory minimum rebates a manufacturer must pay under
the Medicaid Drug Rebate Program, to 23.1% and 13.0% of the average manufacturer price for most branded and generic drugs, respectively;
- •
- expansion of healthcare fraud and abuse laws, including the False Claims Act
and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance;
- •
- a new Medicare Part D coverage gap discount program, in which
manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for a
manufacturer's outpatient drugs to be covered under Medicare Part D;
- •
- extension of a manufacturer's Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations;
43
- •
- expansion of eligibility criteria for Medicaid programs by, among other
things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the federal
poverty level beginning in 2014, thereby potentially increasing a manufacturer's Medicaid rebate liability;
- •
- expansion of the entities eligible for discounts under the Public Health
Service pharmaceutical pricing program;
- •
- new requirements under the federal Open Payments program and its
implementing regulations (as described below);
- •
- a new requirement to annually report drug samples that manufacturers and
distributors provide to physicians; and
- •
- a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
In addition, other legislative changes have been proposed and adopted since PPACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect on April 1, 2013. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our customers and accordingly, our financial operations.
We anticipate that PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and additional downward pressure on the reimbursement we may receive for any approved product. Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, the Middle Class Tax Relief and Job Creation Act of 2012 requires the Centers for Medicare & Medicaid Services, or CMS, to reduce the Medicare clinical laboratory fee schedule by 2% in 2013, which in turn will serve as a base for 2014 and subsequent years. CMS also recently proposed to re-examine payment amounts for tests reimbursed under the Medicare clinical laboratory fee schedule due to changes in technology and, in addition, proposed to bundle the Medicare payments for certain laboratory tests ordered while a patient received services in a hospital outpatient setting. The proposals would replace the current methodology for certain tests and, if adopted, the changes would begin to go into effect January 1, 2014 for some codes. Levels of reimbursement may be impacted by current and future legislation, regulation or reimbursement policies of third-party payors in a manner that may harm the demand and reimbursement available for our products, including our companion diagnostic, which in turn, could harm our future product pricing and sales. Any reduction in reimbursement from Medicare and other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products.
Competition in our targeted market area is intense and this field is characterized by rapid technological change. Therefore developments by competitors may substantially alter the predicted market or render our product candidates uncompetitive.
There are several hundred drugs in clinical development today in the area of oncology therapeutics. We have competitors both in the United States and internationally, including major multinational pharmaceutical companies, biotechnology companies and universities and other research institutions. In the oncology market, our major competitors include, but are not limited to: Amgen Inc.; ArQule Inc. and its partners Kyowa Hakko Kirin Pharma Inc. and Daiichi Sankyo Company Limited; Aveo Pharmaceuticals Inc.; Bristol-Myers Squibb Company; Exelixis Inc.; F. Hoffman-LaRoche Ltd.; GlaxoSmithKline PLC; Novartis AG; and Pfizer, among others.
44
Many companies have filed, and continue to file, patent applications in oncology which may or could affect our program. Some of these patent applications may have already been allowed or issued, and others may issue in the future. These companies include, but are not limited to: Bristol-Myers Squibb; Compugen Limited; Exelixis; GlaxoSmithKline; Novartis; and Pfizer. Since this area is competitive and of strong interest to pharmaceutical and biotechnology companies, there will likely be additional patent applications filed, and additional patents granted, in the future, as well as additional research and development programs expected in the future.
In addition to companies that have HDAC inhibitors or kinase inhibitors addressing oncology indications, our competition also includes hundreds of private and publicly traded companies that operate in the area of oncology but have therapeutics with different mechanisms of action. The oncology market in general is highly competitive with over 1,000 molecules currently in clinical development.
Developments by others may render our products or technologies non-competitive or obsolete or we may not be able to keep pace with technological developments. Our competitors may have developed or may be developing technologies which may be the basis for competitive products. Some of these products may prove to be more effective and less costly than the products developed or being developed by us. Our competitors may obtain regulatory approval for their products more rapidly than we do which may change the standard of care in the indications we are targeting, rendering our technology or products non-competitive or obsolete. Others may develop treatments or cures superior to any therapy we are developing or will develop. Moreover, alternate, less toxic forms of medical treatment may be developed which may be competitive with our products.
Many of the organizations which could be considered to be our competitors have substantially more financial and technical resources, more extensive discovery research, preclinical research and development capabilities and greater manufacturing, marketing, distribution, production and human resources than we do. Many of our current or potential competitors have more experience than us in research, preclinical testing and clinical trials, drug commercialization, manufacturing and marketing, and in obtaining domestic and foreign regulatory approvals. In addition, failure, unacceptable toxicity, lack of sales or disappointing sales or other issues regarding competitors' products or processes could have a material adverse effect on our product candidates, including our clinical candidates or our lead compounds. Established pharmaceutical companies may invest heavily to accelerate discovery and development of novel compounds or to in-license novel compounds that could make our product candidates less competitive. In addition, any new product that competes with an approved product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to overcome price competition and brand recognition and to be commercially successful. Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA, EMA or other regulatory approval or discovering, developing and commercializing medicines before we do, which would have a material adverse impact on our business.
We will not be able to successfully commercialize our product candidates without establishing sales and marketing capabilities internally or through collaborators.
We currently have no sales and marketing staff. We may not be able to find suitable sales and marketing staff and collaborators for all of our product candidates. We have no prior experience in the marketing, sale and distribution of pharmaceutical products and there are significant risks involved in building and managing a sales organization, including our ability to hire, retain and incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel, and effectively manage a geographically dispersed sales and marketing team. Any collaborators may not be adequate or successful or could terminate or materially reduce the effort they direct to our products. The development of a marketing and sales capability will require significant expenditures, management resources and time. The cost of establishing such a sales force may exceed any potential product revenue, or our marketing and sales efforts may be unsuccessful. If we are unable to develop an internal marketing and sales capability in a timely fashion, or at all, or if we are unable to enter into a marketing and sales arrangement with a third
45
party on acceptable terms, we may be unable to successfully develop and seek regulatory approval for our product candidates and/or effectively market and sell approved products, if any.
We are subject to competition for our skilled personnel and may experience challenges in identifying and retaining key personnel that could impair our ability to conduct our operations effectively.
Our future success depends on our ability to retain our executive officers and to attract, retain and motivate qualified personnel. If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy. Although we have not experienced problems attracting and retaining highly qualified personnel in the recent past, our industry has experienced a high rate of turnover of management personnel in recent years. Our ability to compete in the highly competitive biotechnology and pharmaceuticals industries depends upon our ability to attract and retain highly qualified managerial, scientific and medical personnel. We are highly dependent on our management, scientific and medical personnel, especially Charles M. Baum, M.D., Ph.D., our President and Chief Executive Officer, Mark J. Gergen, our Executive Vice President and Chief Operations Officer, Isan Chen, M.D., our Executive Vice President and Chief Medical and Development Officer, James Christensen, Ph.D. our Vice President of Research, and Jamie A. Donadio, our Vice President of Finance, whose services are critical to the successful implementation of our product candidate acquisition, development and regulatory strategies, as well as the management of our financial operations. We are not aware of any present intention of any of these individuals to leave our Company. In order to induce valuable employees to continue their employment with us, we have provided stock options that vest over time. The value to employees of stock options that vest over time is significantly affected by movements in our stock price that are beyond our control, and may at any time be insufficient to counteract more lucrative offers from other companies.
Despite our efforts to retain valuable employees, members of our management, scientific and development teams may terminate their employment with us at any time, with or without notice. The loss of the services of any of our executive officers or other key employees and our inability to find suitable replacements could harm our business, financial condition and prospects. Our success also depends on our ability to continue to attract, retain and motivate highly skilled junior, mid-level and senior managers as well as junior, mid-level and senior scientific and medical personnel.
We may also experience growth in the number of our employees and the scope of our operations, especially in clinical development. This growth will place a significant strain on our management, operations and financial resources and we may have difficulty managing this future potential growth. No assurance can be provided that we will be able to attract new employees to assist in our growth. Many of the other pharmaceutical companies that we compete against for qualified personnel have greater financial and other resources, different risk profiles and a longer history in the industry than we do. We also may employ consultants or part-time and contract employees. There can be no assurance that these individuals are retainable. While we have been able to attract and retain skilled and experienced personnel and consultants in the past, no assurance can be given that we will be able to do so in the future.
Our recently announced closure of our Canadian and New Jersey operations and related reduction in employees may disrupt our business, and we may not be able to adequately replace lost functionality through planned additional hiring at our San Diego facility or use of third-party service providers.
In connection with the Arrangement completed on June 28, 2013, we relocated our corporate headquarters from Montreal, Canada to San Diego, California. Since relocating to San Diego, we have maintained operations in our Canadian office. In addition to the ongoing operations in our Canadian office, we also maintain facilities in Princeton, New Jersey. On October 1, 2013, we announced our intention to close our New Jersey operations as of October 31, 2013 and to transition our Canadian operations to our San Diego offices over the next three to six months. In connection with these efforts, there will be a reduction in force of approximately 27 employees in our Montreal and Princeton offices, or approximately 75% of our workforce. We plan to partially offset this reduction in force by hiring additional personnel in our San Diego
46
office and by engaging third-party service providers to perform certain functions. However, we may not be able to attract and retain the type and number of employees we desire in San Diego, or do so on our planned timeline. During this transition period, we may incur disruptions in our business, including from the loss of functionality we currently maintain in our Montreal and Princeton facilities. In addition, we may be unable to realize the efficiencies we are seeking by consolidating our operations in a single office in San Diego. If we are unable to realize such efficiencies or attract and retain qualified personnel in San Diego and effectively outsource certain other functions to third-party service providers, our operations and ability to execute our business plan would be adversely affected.
Our current and future relationships with customers and third-party payors in the United States and elsewhere may be subject, directly or indirectly, to applicable anti-kickback, fraud and abuse, false claims, transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, administrative burdens and diminished profits and future earnings.
As a pharmaceutical company, even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors, certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients' rights are and will be applicable to our business. Our current and future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act, which may constrain the business or financial arrangements and relationships through which we sell, market and distribute any drugs for which we obtain marketing approval. In addition, we may be subject to transparency laws and patient privacy regulation by U.S. federal and state governments and by governments in foreign jurisdictions in which we conduct our business. The laws that may affect our ability to operate include:
- •
- the federal Anti-Kickback Statute, which prohibits, among other things,
persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral
of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs, such as Medicare and Medicaid;
- •
- federal civil and criminal false claims laws and civil monetary penalty
laws, including the federal False Claims Act, which impose criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or
causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or
conceal an obligation to pay money to the federal government;
- •
- the federal Health Insurance Portability and Accountability Act of 1996, or
HIPAA, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
- •
- HIPAA, as amended by the Health Information Technology for Economic and
Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose obligations on covered healthcare providers, health plans, and healthcare clearinghouses, as well as
their business associates that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy,
security and transmission of individually identifiable health information;
- •
- the federal Open Payments program, which requires manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children's Health Insurance Program, with specific exceptions, to report annually to CMS information related to "payments or other transfers of value" made to physicians, which is defined to include doctors, dentists, optometrists, podiatrists and chiropractors, and teaching hospitals and applicable
47
- •
- analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
manufacturers and applicable group purchasing organizations to report annually to CMS ownership and investment interests held by the physicians and their immediate family members, with data collection beginning on August 1, 2013, requirements for manufacturers to submit reports to CMS by March 31, 2014 and the 90th day of each subsequent calendar year, and disclosure of such information to be made by CMS on a publicly available website beginning in September 2014; and
Because of the breadth of these laws and the narrowness of available statutory and regulatory exceptions, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent healthcare reform legislation has strengthened these laws. For example, PPACA, among other things, amends the intent requirement of the federal Anti-Kickback Statute such that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. Moreover, PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act. To the extent that any of our product candidates is ultimately sold in countries other than the United States, we may be subject to similar laws and regulations in those countries. If we or our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, imprisonment, exclusion from participation in government healthcare programs, and the curtailment or restructuring of our operations, any of which could have a material adverse effect on our business. If any of the physicians or other healthcare providers or entities with whom we expect to do business, including any of our collaborators, is found not to be in compliance with applicable laws, it may be subject to criminal, civil or administrative sanctions, including exclusion from participation in government healthcare programs, which could also materially affect our business.
We may become subject to the risk of product liability claims.
We face an inherent risk of product liability as a result of the clinical testing of our product candidates and will face an even greater risk if we commercialize any products. Human therapeutic products involve the risk of product liability claims and associated adverse publicity. Currently, the principal risks we face relate to patients in our clinical trials, who may suffer unintended consequences. Claims might be made by patients, healthcare providers, pharmaceutical companies or others. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates, if approved. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
- •
- decreased demand for our product candidates;
48
- •
- injury to our reputation;
- •
- withdrawal of clinical trial participants;
- •
- initiation of investigations by regulators;
- •
- costs to defend the related litigation;
- •
- a diversion of management's time and our resources;
- •
- substantial monetary awards to trial participants or patients;
- •
- product recalls, withdrawals or labeling, marketing or promotional
restrictions;
- •
- loss of revenue from product sales; and
- •
- the inability to commercialize any our product candidates, if approved.
We may not have or be able to obtain or maintain sufficient and affordable insurance coverage, and without sufficient coverage any claim brought against us could have a materially adverse effect on our business, financial condition or results of operations. We run clinical trials through investigators that could be negligent through no fault of our own and which could affect patients, cause potential liability claims against us and result in delayed or stopped clinical trials. We are required in many cases by contractual obligations to indemnify collaborators, partners, third-party contractors, clinical investigators and institutions. These indemnifications could result in a material impact due to product liability claims against us and/or these groups. We currently carry $10 million in product liability insurance, which we believe is appropriate for our clinical trials. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
Our business involves the controlled use of hazardous materials and as such we are subject to environmental and occupational safety laws. Continued compliance with these laws may incur substantial costs and failure to maintain compliance could result in liability for damages that may exceed our resources.
Our preclinical research, manufacturing and development processes involve the controlled use of hazardous and radioactive materials. We are subject to federal, provincial and local laws and regulations governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. The risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result, and any such liability could exceed our resources. We may not be adequately insured against this type of liability. We may be required to incur significant costs to comply with environmental laws and regulations in the future, and our operations, business or assets may be materially adversely affected by current or future environmental laws or regulations.
We may have to dedicate resources to the settlement of litigation.
Securities legislation in both the United States and Canada makes it relatively easy for stockholders to sue. This could lead to frivolous law suits which could take substantial time, money, resources and attention or force us to settle such claims rather than seek adequate judicial remedy or dismissal of such claims. we may
If we are required to defend patent infringement actions brought by third parties, or if we sue to protect our own patent rights or otherwise to protect our proprietary information and to prevent its disclosure, we may
49
be required to pay substantial litigation costs and managerial attention may be diverted from business operations even if the outcome is in our favor. If we are required to defend our patents or trademarks against infringement by third parties, we may be required to pay substantial litigation costs and managerial attention and financial resources may be diverted from our research and development operations even if the outcome is in our favor.
We may be vulnerable to disruption, damage and financial obligation as a result of system failures.
Despite the implementation of security measures, any of the internal computer systems belonging to us, our collaborators or our third party service providers are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failure. Any system failure, accident or security breach that causes interruptions in our own, in collaborators' or in third party service vendors' operations could result in a material disruption of our drug discovery programs. To the extent that any disruption or security breach results in a loss or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we may incur liability, our drug discovery programs may be adversely affected and the further development of our product candidates may be delayed. Furthermore, we may incur additional costs to remedy the damages caused by these disruptions or security breaches.
Risks Relating to Our Intellectual Property
We may not obtain adequate protection for our product candidates through patents and other intellectual property rights and as such our competitive advantage in the marketplace may be compromised.
Our success depends, in part, on our ability to secure and protect our patents, trade secrets, trademarks and other intellectual property rights and to operate without infringing on the proprietary rights of others or having third parties circumvent the rights that we own or license. We have filed and are actively pursuing patent applications in the United States, Canada, Japan, Europe and other major markets via the Patent Cooperation Treaty or directly in countries of interest. The patent positions of healthcare companies, universities and biopharmaceutical companies, including ours, are uncertain and involve complex questions of law and fact for which important legal issues may remain unresolved. Therefore, there is no assurance that our pending patent applications will result in the issuance of patents or that we will develop additional proprietary products which are patentable. Moreover, patents issued or to be issued to us may not provide us with any competitive advantage. Further, if the patent applications we hold or in-license with respect to our programs, product candidates and companion diagnostic fail to issue, if their breadth or strength of protection is threatened, or if they fail to provide meaningful exclusivity for our product candidates, it could dissuade companies from collaborating with us to develop product candidates, and threaten our ability to commercialize, future products.
Our patents may be challenged by third parties in patent litigation. In addition, it is possible that third parties with products that are very similar to ours will circumvent our patents by means of alternate designs or processes or file applications or be granted patents that would block or hurt our efforts. There are no assurances that our patent counsel, lawyers or advisors have given us correct advice or counsel. Opinions from such patent counsel or lawyers may not be correct or based on incomplete facts. We cannot be certain that we are the first to invent the inventions covered by pending patent applications and, if we are not, we may be subject to priority disputes. We may be required to disclaim part or all of the term of certain patents or all of the term of certain patent applications. There may be prior art of which we are not aware that may affect the validity or enforceability of a patent claim. There also may be prior art of which we are aware, but which we do not believe affects the validity or enforceability of a claim, which may, nonetheless, ultimately be found to affect the validity or enforceability of a claim. No assurance can be given that if challenged, our patents would be declared by a court to be valid or enforceable or that even if found valid and enforceable, a competitor's technology or product would be found by a court to infringe our patents. We may analyze patents or patent applications of our competitors that we believe are relevant to our activities, and consider
50
that we are free to operate in relation to our product candidates, but our competitors may achieve issued claims, including in patents we consider to be unrelated, which block our efforts or may potentially result in our product candidates or our activities infringing such claims. The possibility exists that others will develop products which have the same effect as our products on an independent basis which do not infringe our patents or other intellectual property rights, or will design around the claims of patents that we have had issued that cover our products. The steps we have taken to protect our intellectual property may not prevent the misappropriation of our proprietary information and technologies, particularly in foreign countries where laws or law enforcement practices may not protect proprietary rights as fully as in Canada, the United States or Europe. Unauthorized disclosure of our proprietary information could also harm our competitive position. We could also inadvertently use our collaborators' data inappropriately which could lead to liability. We may file patent applications but have claims restricted or we may not be able to supply sufficient data to satisfy a patent office to support our claims and, as a result, may not obtain the original claims desired or we may receive restricted claims. Alternatively, it is possible that we may not receive any patent protection from an application.
Maintaining our patents and applications requires timely payment of fees and other associated costs in the countries of filing, and we could inadvertently abandon a patent or patent application (or trademark or trademark application) due to non-payment of fees, or as a result of a failure to comply with filing deadlines or other requirements of the prosecution process, resulting in the loss of protection of certain intellectual property rights in a certain country. Alternatively, we, our collaborators or our patent counsel may take action resulting in a patent or patent application becoming abandoned which may not be able to be reinstated, or if reinstated, may suffer patent term adjustments. Any of these outcomes could hurt our ability to gain full patent protection for our products. Registered trademarks in Canada, the United States and other countries that belong to us are subject to the same risks as described above for patents and patent applications.
Many of our collaboration agreements are complex and may call for licensing or cross-licensing of potentially blocking patents, know-how or intellectual property. Due to the potential overlap of data, know-how and intellectual property rights there can be no assurance that one of our collaborators will not dispute our right to send data or know-how or other intellectual property rights to third parties and this may potentially lead to liability or termination of a program. There are no assurances that the actions of our collaborators would not lead to disputes or cause us to default with other collaborators. We cannot be certain that a collaborator will not challenge the validity of licensed patents.
We cannot be certain that any country's patent and/or trademark office will not implement new rules which could affect how we draft, file, prosecute and/or maintain patents and patent applications. We cannot be certain that increasing costs for drafting, filing, prosecuting and maintaining patent applications and patents will not restrict our ability to file for patent protection, or to prosecute applications through to grant. We may be forced to abandon or return the rights to specific patents due to a lack of financial resources. There is no assurance that we could enter into licensing arrangements at a reasonable cost, or develop or obtain alternative technology in respect of patents issued to third parties that incidentally cover our products. Any inability to secure licenses or alternative technology could result in delays in the introduction of some of our products or even lead to prohibition of the development, manufacture or sale of certain products by us.
We have filed applications for trademark registrations in connection with our product candidates in various jurisdictions, including the United States. We intend to file further applications for other possible trademarks for our product candidates. No assurance can be given that any of our trademark applications will be registered in the United States or elsewhere, or that the use of any registered or unregistered trademarks will confer a competitive advantage in the marketplace. Furthermore, even if we are successful in our trademark registrations, the FDA and regulatory authorities in other countries have their own process for drug nomenclature and their own views concerning appropriate proprietary names. No assurance can be
51
given that the FDA or any other regulatory authority will approve of any of our trademarks or will not request reconsideration of one of our trademarks at some time in the future. The loss, abandonment, or cancellation of any of our trademarks or trademark applications could negatively affect the success of the product candidates to which they relate.
Moreover, some of our know-how and technology which is not patented or not patentable may constitute trade secrets. Therefore, we require our consultants, advisors and collaborators to enter into confidentiality agreements and our employees to enter into invention, non-disclosure and non-compete agreements. However, no assurance can be given that such agreements will provide for a meaningful protection of our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure of information. Furthermore, we cannot provide assurance that any of our employees, consultants, contract personnel or collaborators, either accidentally or through willful misconduct, will not cause serious impact to our programs and/or our strategy. All of our employees have signed confidentiality agreements, but there can be no assurance that they will not inadvertently or through their misconduct give trade secrets away.
Third-party intellectual property infringement claims may result in a reduction in the scope of our patent protection and competitive exclusivity with respect to our product candidates. Patent litigation, including defense against third-party intellectual property claims, may result in us incurring substantial costs.
Patent applications which may relate to or affect our business may have been filed by others. Such patent applications or patents resulting therefrom may conflict with our technologies, patents or patent applications and reducing the scope of our patent protection. Such events could cause us to stop or change the course of our research and development or modify our intellectual property strategies. We could also become involved in interference proceedings in connection with one or more of our patents or patent applications to determine priority of invention. There can be no guarantees that an interference proceeding would be successful or that such an outcome could be reversed on appeal. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of such interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees.
No assurance can be given that our patents, once issued, would be declared by a court to be valid or enforceable, or that we would not be found to infringe a competitor's patent.
Third parties may assert that we are using their proprietary information without authorization. Third parties may also have or obtain patents and may claim that technologies licensed to or used by us infringe their patents. Because patent applications can take many years to issue, third parties may have currently pending patent applications which may later result in issued patents that our product candidates or companion diagnostic may infringe, or which such third parties claim are infringed by the use of our technologies. If any third-party patents are held by a court of competent jurisdiction to cover any aspect of our product candidates, including the formulation or method of use of such product candidate, the holders of any such patents may be able to block our ability to commercialize such product candidate unless we obtained a license under the applicable patents, or until such patents expire. In any such case, such a license may not be available on commercially reasonable terms or at all. In addition, any legal action that seeks damages or an injunction to stop us from carrying on our commercial activities relating to the affected technologies could subject us to monetary liability. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources.
Parties making claims against us for infringement of their intellectual property rights may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we could be required to redesign our
52
infringing products or obtain a license from such third party to continue developing and commercializing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms, or at all. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. It may be impossible to redesign our products and technology, or it may require substantial time and expense, which could force us to cease commercialization of one or more of our product candidates, or some of our business operations, which could materially harm our business. In addition, in any such proceeding, we may be required to pay substantial damages, including treble damages and attorneys' fees in the event we are found liable for willful infringement.
Our intellectual property may be infringed upon by a third party.
Third parties may infringe one or more of our issued patents or trademarks. We cannot predict if, when or where a third party may infringe one or more of our issued patents or trademarks. We may attempt to invalidate a competitor's patent. There is no assurance such action will ultimately be successful and, even if initially successful, it could be overturned upon appeal. There is no assurance that we would be successful in a court of law to prove that a third party is infringing one or more of our issued patents. Even if we are successful in proving in a court of law that a third party is infringing one or more of our issued patents there can be no assurance that we would be successful in halting their infringing activities, for example, through a permanent injunction, or that we would be fully or even partially financially compensated for any harm to our business. We may be forced to enter into a license or other agreement with the infringing third party at terms less profitable or otherwise less commercially acceptable to us than if the license or agreement were negotiated under conditions between those of a willing licensee and a willing licensor. We may not become aware of a third party infringer within legal timeframes that would enable us to seek adequate compensation, or at all, thereby possibly losing the ability to be compensated for any harm to our business. Such a third-party may be operating in a foreign country where the infringer is difficult to locate, where we do not have issued patents and/or the patent laws may be more difficult to enforce. Some third-party infringers may be able to sustain the costs of complex patent infringement litigation more effectively than we can because they have substantially greater resources. Any inability to stop third-party infringement could result in loss in market share of some of our products or even lead to a delay, reduction and/or inhibition of the development, manufacture or sale of certain products by us. There is no assurance that a product produced and sold by a third-party infringer would meet our or other regulatory standards or would be safe for use. Such third-party infringer products could irreparably harm the reputation of our products thereby resulting in substantial loss in market share and profits.
Risks Related to Our Shares of Common Stock
Our share price is volatile and may be influenced by numerous factors that are beyond our control.
A low share price and low market valuation may make it difficult to raise sufficient additional cash due to the significant dilution to current stockholders. Market prices for shares of biotechnology and biopharmaceutical companies such as ours are often volatile. Factors such as clinical and regulatory developments regarding our products or processes, developments regarding potential or future third-party collaborators, announcements of technological innovations, new commercial products, patents, the development of proprietary rights by us or by others or any litigation relating to these rights, regulatory actions, general conditions in the biotechnology and pharmaceutical industries, failure to meet analysts' expectations, publications, financial results or public concern over the safety of biopharmaceutical and biotechnological products, economic conditions in the United States, Canada or abroad, terrorism and other factors could have a significant effect on the share price for our shares of common stock. Any setback or delay in the clinical development of our programs could result in a significant decrease in our share price. In recent years the stock of other biotechnology and biopharmaceutical companies has experienced extreme price fluctuations that have been unrelated to the operating performance of the affected companies. There can be no assurance that the market price of our shares of common stock will not experience significant
53
fluctuations in the future, including fluctuations that are unrelated to our performance. These fluctuations may result due to macroeconomic and world events, national or local events, general perception of the biotechnology industry or to a lack of liquidity. In addition other biotechnology companies or our competitors' programs could have positive or negative results that impact their stock prices and their results, or stock fluctuations could have a positive or negative impact on our stock price regardless whether such impact is direct or not.
Stockholders may not agree with our business, scientific, clinical and financial strategy, including additional dilutive financings, and may decide to sell their shares or vote against such proposals. Such actions could materially impact our stock price. In addition, portfolio managers of funds or large investors can change or change their view on us and decide to sell our shares. These actions could have a material impact on our stock price. In order to complete a financing, or for other business reasons, we may elect to consolidate our shares of common stock. Investors may not agree with these actions and may sell our shares. We may have little or no ability to impact or alter such decisions.
Our principal stockholders control the majority of our shares, and their actions may significantly influence matters submitted to our stockholders for approval and our share price.
Based on the information available to us as of August 31, 2013 our stockholders and their affiliates, collectively who owned more than 5% of our oustanding common stock owned approximately 81% of our outstanding common stock. Baker Bros. Advisors, L.L.C., or Baker Brothers, and Tavistock Life Sciences Co., or Tavistock, and their affiliates collectively own approximately 40% of our outstanding common stock. In addition, in conjunction with certain financing transactions, we granted to Baker Brothers and Tavistock each the right to nominate a member of our Board of Directors and the right to appoint an observer on our Board of Directors. As a result, each of Baker Brothers and Tavistock has significant influence over matters submitted to our stockholders for approval, including the election and removal of directors and the approval of any merger, consolidation, or sale of all or substantially all of our assets. Furthermore, as a thinly traded stock, if Baker Brothers, Tavistock or any of other of our major stockholders determine to exit from the industry or from their holdings in us, for whatever reason, the impact on our share price could be detrimental over a prolonged period of time.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. To the extent we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. We may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by subsequent sales. These sales may also result in material dilution to our existing stockholders, and new investors could gain rights superior to our existing stockholders.
Pursuant to our 2013 Equity Incentive Plan, or the 2013 Plan, our management is authorized to grant stock options and other equity-based awards to our employees, directors and consultants. Any increase in the number of shares outstanding as a result of the exercise of outstanding options will cause our stockholders to experience additional dilution, which could cause our stock price to fall. Currently, we plan to register the increased number of shares available for issuance under the 2013 Plan each year.
54
Our ability to use our U.S. net operating loss carryforwards and certain other tax attributes may be limited.
Under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, if a corporation undergoes an "ownership change," generally defined as a greater than 50% change (by value) in its equity ownership over a three year period, the corporation's ability to use its pre-change U.S. net operating loss carryforwards, or NOLs, and other pre-change U.S. tax attributes (such as research tax credits) to offset its post-change income may be limited. We may experience ownership changes in the future as a result of subsequent shifts in our stock ownership. As a result, if we earn net taxable income, our ability to use our pre-change U.S. net operating loss carryforwards to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. In addition, at the state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.
Because we do not anticipate paying any cash dividends on our common stock in the foreseeable future, capital appreciation, if any, would be our stockholders' only source of gain.
We are a holding company with no material assets other than the stock of our wholly-owned subsidiary. Accordingly, all our operations are conducted by MethylGene Canada, our wholly-owned subsidiary (and its wholly-owned subsidiary, MethylGene US Inc.). MethylGene Canada has never declared or paid any cash dividends on its common shares, and we currently expect that the earnings and cash flow of MethylGene Canada will primarily be retained and used by it in its operations, including servicing any debt obligations it may have now or in the future. Accordingly, although we do not anticipate paying any dividends in the foreseeable future, our subsidiary may not be able to generate sufficient cash flow to distribute funds to us in order to allow us to pay future dividends on, or make any distributions with respect to our common stock. As a result, capital appreciation, if any, of our common stock would be our stockholders' sole source of gain on their investment in our common stock for the foreseeable future.
55
BUSINESS
Anti-tumor Activity of MGCD265 Compared with Sutent in a Met/HGF-Positive Glioblastoma Model
Potent Cytoreductive Activity of MGCD265 in a Met Amplified Gastric Cancer Model
Anti-tumor Activity of MGCD516 Compared with Nexavar in the A549 Lung Cancer Model
Potent Cytoreductive Activity of MGCD516 in a RET Fusion- Positive Lung Cancer Model
Cytoreductive Anti-tumor Activity of MGCD516 in a Sutent- Resistant Tumor Model
RISK FACTORS
