Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - CorMedix Inc. | crmd_8k.htm |
EXHIBIT 99.1

NYSE MKT: CRMD
August 2013
Company Overview

© 2013 CorMedix Inc
2
Forward Looking Statements
This presentation contains certain statements that constitute forward-looking statements within the
meaning of the federal securities laws. Statements that are not historical facts, including statements
about our beliefs and expectations, are forward-looking statements. These statements are not
guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to
predict. The forward looking statements in this presentation include statements about our business,
including commercialization plans and potential markets for our products and product candidates,
clinical trials, potential indications for our product candidates, development timelines, regulatory
timelines and future events that have not yet occurred. Pharmaceutical and medical device
development inherently involves significant risks and uncertainties, including the risks outlined in “Risk
Factors” in our Annual Report on Form 10-K filed with the Securities and Exchange Commission on
March 27, 2013 and in “Risk Factors” in our Quarterly Reports on Form 10-Q filed with the Securities
and Exchange Commission on May 15 and August 14, 2013. Our actual results may differ materially
from our expectations due to these risks and uncertainties, including, but not limited to, our
dependence on the success of our lead product candidate, and factors relating to commercialization
and regulatory approval thereof, ability to raise sufficient capital, retaining our stock’s listing on the
NYSE MKT, research and development activities, intellectual property protection, competition,
industry environment, and other matters. Any forward-looking statements included in this presentation
are based on information available to us on the date of this presentation. We undertake no obligation
to update or revise any forward-looking statement, whether as a result of new information, future
events or otherwise.
meaning of the federal securities laws. Statements that are not historical facts, including statements
about our beliefs and expectations, are forward-looking statements. These statements are not
guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to
predict. The forward looking statements in this presentation include statements about our business,
including commercialization plans and potential markets for our products and product candidates,
clinical trials, potential indications for our product candidates, development timelines, regulatory
timelines and future events that have not yet occurred. Pharmaceutical and medical device
development inherently involves significant risks and uncertainties, including the risks outlined in “Risk
Factors” in our Annual Report on Form 10-K filed with the Securities and Exchange Commission on
March 27, 2013 and in “Risk Factors” in our Quarterly Reports on Form 10-Q filed with the Securities
and Exchange Commission on May 15 and August 14, 2013. Our actual results may differ materially
from our expectations due to these risks and uncertainties, including, but not limited to, our
dependence on the success of our lead product candidate, and factors relating to commercialization
and regulatory approval thereof, ability to raise sufficient capital, retaining our stock’s listing on the
NYSE MKT, research and development activities, intellectual property protection, competition,
industry environment, and other matters. Any forward-looking statements included in this presentation
are based on information available to us on the date of this presentation. We undertake no obligation
to update or revise any forward-looking statement, whether as a result of new information, future
events or otherwise.

© 2013 CorMedix Inc
3
CorMedix: A Catheter Care Company
© 2013 CorMedix Inc
4
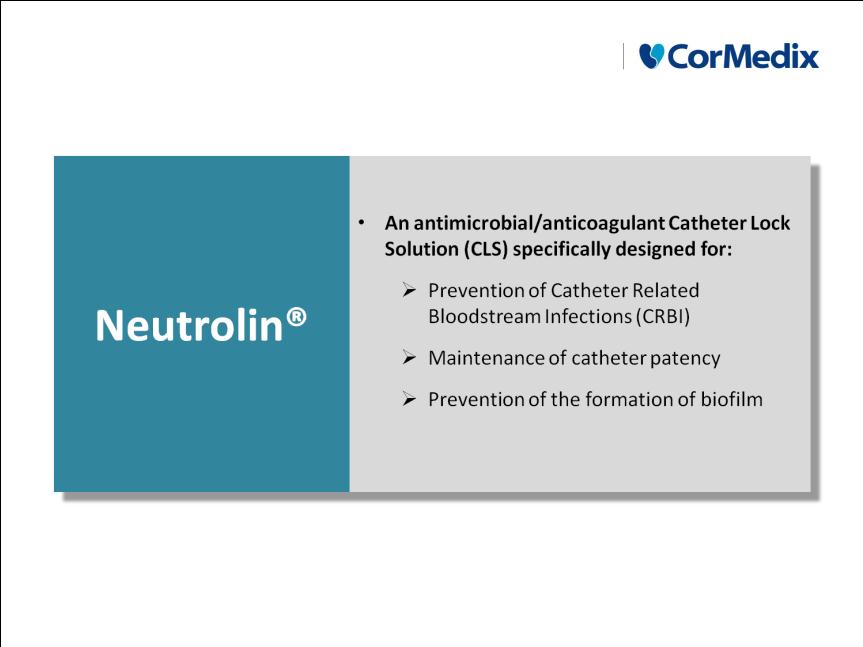
What is Neutrolin®?

© 2013 CorMedix Inc
5
Why Neutrolin®?

© 2013 CorMedix Inc
6
Neutrolin®
Strategic Pillars
© 2013 CorMedix Inc
Key Activities:
•Successful launch in Germany and Austria
• Experienced sales team targets early
adopters
adopters
• Increase healthcare provider awareness
•Negotiate Partner Arrangements
• Position Neutrolin® strategically
§ Pricing
§ Understand payer reimbursement policies
Neutrolin®
Strategic Pillars


© 2013 CorMedix Inc
9
Key Activities:
•Expansion into oncology, ICU, Total
Parenteral Nutrition, and peritoneal
dialysis fields
Parenteral Nutrition, and peritoneal
dialysis fields
•Submission of required data to TUV
•Develop target list of physicians and
expand the sales force
expand the sales force
Neutrolin®
Strategic Pillars

© 2013 CorMedix Inc
10
Key Activities:
•Identify and prioritize key countries
•Identify best sales partners in key
countries
countries
§ Screening/due diligence / deal
negotiations conducted with
country and regional specific
advisors
negotiations conducted with
country and regional specific
advisors
•Complete agreements
•Monitor product positioning and
coordinate manufacturing capacity
coordinate manufacturing capacity
Neutrolin®
Strategic Pillars

© 2013 CorMedix Inc
11
Potential Opportunity for Consideration:
• U.S. FDA discussions (pre-IND meeting, timelines)
• Clinical trial requirements by FDA
§ Patient population size
§ Duration
• Clinical trial execution decision
§ Strategic partner
§ Go alone if sufficient internal funding
Neutrolin®
Strategic Pillars

© 2013 CorMedix Inc
12
Business Development to Target Countries with
High Catheter Use and Favorable Reimbursement
High Catheter Use and Favorable Reimbursement
• Major Countries: U.S., Japan,
China, Brazil, Germany, Canada
China, Brazil, Germany, Canada
Source: Derived from USRDS, 2011, and Dialysis Outcomes and Practice Pattern Study (DOPPS) Annual
Report 2010, and Canadian Institute for Health Information, Canadian Organ Replacement Registry
Report 2010, and Canadian Institute for Health Information, Canadian Organ Replacement Registry

© 2013 CorMedix Inc
13
1
Process in Place to Select Best Sales Partner

© 2013 CorMedix Inc

© 2013 CorMedix Inc

© 2013 CorMedix Inc
© 2013 CorMedix Inc
17
Large Worldwide Hemodialysis Opportunity
European Union Sales Partnerships:
• 91,000 hemodialysis catheter patients
• 28 countries
U.S. Clinical Development Funding and Sales Partnerships
• 104,000 hemodialysis catheter patients
Top 5 Hemodialysis markets: U.S., Japan, China, Brazil, Germany.
Source: Derived from USRDS, 2011, and Grassman, Neprol. Dial. Transplant (Dec 2005) 20 (12):25-87-2593, and
Dialysis Outcomes and Practice Pattern Study (DOPPS) Annual Report 2010, CorMedix estimates
Asia/Middle East/North and South America Sales Partnerships
• 106,000 hemodialysis catheter patients
• China, Japan, Korea, Saudi Arabia, Brazil, Canada

© 2013 CorMedix Inc
18
Opportunity to Grow with Label Expansion into other
Catheter Applications
European Union Sales Partnerships:
• 145,600 catheter patients
• 28 countries
U.S. Clinical Development Funding and Sales Partnerships
• 124,800 catheter patients
Other catheter applications: chemotherapy, intensive care, total parenteral nutrition.
Source: CorMedix estimates based on hemodialysis catheter patients derived from USRDS, 2011, and Grassman,
Neprol. Dial. Transplant (Dec 2005) 20 (12):25-87-2593, and Dialysis Outcomes and Practice Pattern Study (DOPPS)
Asia/Middle East/North and South America Sales Partnerships
• 106,000 catheter patients
• China, Japan, Korea, Saudi Arabia, Brazil, Canada

© 2013 CorMedix Inc
19
Significant Near Term Hemodialysis Opportunity in E.U.
Initially CorMedix will target the Hemodialysis market:
• E.U. : 91,000 hemodialysis catheter patients
These patients represent ~14.2 million HD sessions per year
(91,000 HD catheter patients * 3 weekly dialysis sessions/week * 52 weeks per year = 14.2 million)
Potential Hemodialysis Market Size in E.U. :
USD $40 - $80 million
Source: Derived from USRDS, 2011, and Grassman, Neprol. Dial. Transplant (Dec 2005) 20 (12):25-87-2593, and
Dialysis Outcomes and Practice Pattern Study (DOPPS)

© 2013 CorMedix Inc
20
…..and Even Greater Opportunity in the Long Term
with Other Key Countries and an Expanded Label
with Other Key Countries and an Expanded Label
Hemodialysis and Expanded Label market:
• E.U.: 236,600 catheter patients
• Other Key Countries: 212,000 catheter patients
Long Term Potential Market Size in E.U. and Other Key Countries
for Hemodialysis and Expanded Label:
for Hemodialysis and Expanded Label:
USD $180 - $340 million
Top 5 Hemodialysis markets: U.S., Japan, China, Brazil, Germany.
Source: Derived from USRDS, 2011, and Grassman, Neprol. Dial. Transplant (Dec 2005) 20 (12):25-87-2593, and
Dialysis Outcomes and Practice Pattern Study (DOPPS)
© 2013 CorMedix Inc
21
Option to Enter the United States with Strategic
Partnerships or Internal Funding
Partnerships or Internal Funding
U.S. Hemodialysis and Expanded Label market:
• 228,800 catheter patients
Long Term Potential Market Size in U.S. for Hemodialysis and
Expanded Label:
Expanded Label:
USD $140 - $270 million
Top 5 Hemodialysis markets: U.S., Japan, China, Brazil, Germany.
Source: Derived from USRDS, 2011, and Grassman, Neprol. Dial. Transplant (Dec 2005) 20 (12):25-87-2593, and
Dialysis Outcomes and Practice Pattern Study (DOPPS)

© 2013 CorMedix Inc
22
IP Status Update
• Global Patent Estate - 17 issued patents provide protection
through 2019-2025
through 2019-2025
§ Freedom to Operate
§ Proprietary
Ø Sodemann: A method of inhibiting or preventing infection and blood
coagulation as a medical prosthetic device using a pharmaceutical
composition comprising taurolidine and citric acid
coagulation as a medical prosthetic device using a pharmaceutical
composition comprising taurolidine and citric acid
Ø Prosl: A locking solution comprising a taurinamide derivative, a
biologically acceptable acid and low concentration heparin
biologically acceptable acid and low concentration heparin
Ø Polaschegg: gel technology with taurolidine
• Planned additional filings under prosecution to extend
protection
protection

© 2013 CorMedix Inc
23
Neutrolin®
Strategic Pillars

http://www.CorMedix.com/
NYSE MKT: CRMD
August 2013
Thank You!
