Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a13-15366_18k.htm |
Exhibit 99.1
Qualitative assessment of a two-strip dry powder inhaler
(ELLIPTA™) for COPD and asthma
Poster No. 1976
Woepse M(1), Dale P(2), Garrill K(3), Svedsater H(4), Walker R(5)
(1)Strategic Eye, Inc., Wayne, PA, USA; (2)HEOR Solutions, London, UK; (3)Medicine and Process Delivery, (4)Global Health Outcomes, (5)Product Development, GlaxoSmithKline, Uxbridge, UK
INTRODUCTION
· COPD and asthma are chronic respiratory disorders associated with significant morbidity(1),(2) commonly treated with inhaled therapies, usually delivered via a handheld inhaler.
· Poor adherence to inhaled therapies may be associated with inferior treatment outcomes in COPD and asthma.(3),(4) Guidelines recommend that patient preference, convenience and ability to use the device correctly should be considered when choosing an inhaler.(5)
· Fluticasone furoate (FF) is a novel corticosteroid in development as a once-daily monotherapy for asthma and in combination with vilanterol (VI), a novel long-acting beta2 agonist, as a once-daily therapy for COPD and asthma.
· In clinical trials, FF/VI and FF have been delivered via the ELLIPTA™ dry powder inhaler (DPI), which is in development for the delivery of these treatments.
OBJECTIVES
· To assess perception of the ELLIPTA DPI among patients who participated in phase 3 clinical trials of FF/VI and FF.
· To assess preference for the ELLIPTA DPI compared with other inhalers currently used by study participants.
· To understand any specific attributes of the ELLIPTA DPI associated with patient satisfaction and inhaler preference.
METHODS
· Patients (aged >18 years, recruited from US study sites, had English as their primary language) with COPD or asthma who completed one of six phase 3 studies of FF/VI or FF using the ELLIPTA DPI (Figure 1) participated in qualitative interviews.
Figure 1. Unlabelled (blank) ELLIPTA DPI used in clinical trials

· Telephone interviews were conducted <4 weeks after completion of the clinical study. Visual stimuli, including images of the ELLIPTA DPI, patient instructions and illustrations of ways in which users had been observed to open and grip the DPI, were made available to participants via a password-protected website and used as prompts.
· Interviews focused on the inhaler and followed a semi-structured format based upon a discussion guide:
· introduction: history of disease and inhaler use
· ELLIPTA inhaler discussion: patient perception of, and preference for the ELLIPTA DPI compared with other inhalers they were currently using (participant-reported); evaluation of ELLIPTA DPI on key performance measures
· follow-up queries: issues previously identified or recognised during the interview.
RESULTS
· See Table 1 for patient demographics; details of the studies the patients participated in are presented in Table 2.
Table 1. Patient demographics
|
|
|
Asthma |
|
COPD |
|
|
|
|
population |
|
population |
|
|
|
|
(n=33) |
|
(n=42) |
|
|
Age, years |
|
41 |
|
61 |
|
|
Duration of disease, years |
|
22.1 |
|
7.1 |
|
|
Self-reported disease severity* |
|
5.0 |
|
5.6 |
|
All data are mean values unless otherwise stated
*Qualitative 1–10 scale: 1 = least severe, 10 = most severe
Table 2. Participant involvement in clinical trials*
|
Study |
|
Study |
|
|
|
Duration |
|
|
number |
|
treatments |
|
n |
|
(weeks) |
|
|
COPD |
|
|
|
|
|
|
|
|
HZC102871/ |
|
FF/VI 50/25mcg, |
|
27 |
|
52 |
|
|
HZC112206 |
|
FF/VI 50/25mcg, FF/VI |
|
12 |
|
24 |
|
|
HZC112207 |
|
FF/VI 100/25mcg, FF/VI |
|
3 |
|
24 |
|
|
Asthma |
|
|
|
|
|
|
|
|
HZA106827 |
|
FF/VI 100/25mcg, FF 100mcg |
|
22 |
|
12 |
|
|
FFA114496 |
|
FF 200mcg and FF 100mcg |
|
11 |
|
24 |
|
*A two-strip configuration of the ELLIPTA DPI was used in all studies listed except for FFA114496, in which a single-strip ELLIPTA DPI was used
Figure 2. Overall* ELLIPTA DPI performance scores
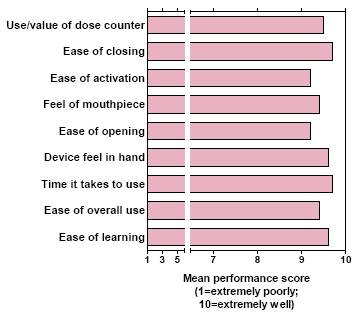
*Scores are combined for patients with COPD and asthma
Table 3. Patient inhaler preference (ELLIPTA DPI versus specified other)
|
Comparator |
|
No. patients |
|
No. (%) of patients |
|
Key features rated as positive attributes of the |
|
|
COPD |
|
|
|
|
|
|
|
|
HandiHaler |
|
20 |
|
19 (95) |
|
· Simple mode of action; no need to insert a capsule into device before inhalation · Increased confidence that a complete dose of medication was received |
|
|
DISKUS |
|
21 |
|
18 (86) |
|
· Ease of handling and operation · Ease of reading the dose counter · Mouthpiece provides a better seal with the lips |
|
|
MDI/HFA |
|
20 |
|
17 (85) |
|
· Ease of operation; requires less effort and fewer puffs · Co-ordination of activation of the DPI with the inhalation is not required · Readable dose counter |
|
|
Asthma |
|
|
|
|
|
|
|
|
DISKUS |
|
21 |
|
15 (71) |
|
· The shape of the mouthpiece · The larger size of the dose counter · Ease of handling and operation |
|
|
MDI/HFA |
|
10 |
|
6 (60) |
|
· Ease of operation; requires less effort and fewer puffs · Co-ordination of activation of the DPI with the inhalation is not required · Readable dose counter |
|
MDI/HFA = metered dose inhaler/hydrofluoroalkane (propellant)
· Participants reported high levels of satisfaction with, and a very positive experience of, using the ELLIPTA DPI.
· Ease of use, dose count awareness, comfortable and well-shaped mouthpiece design, confidence in delivery of the medication, small size and ergonomic shape, the easy to open and integrated cover, and ease of handling were cited as positive attributes of the ELLIPTA DPI.
· The majority of participants with asthma (>60%) and overwhelming majority of participants with COPD (>85%) preferred the ELLIPTA DPI to the inhaler used to deliver their current medication (as prescribed after the end of the clinical study) (Table 3).
· Average performance scores (on a 1–10 scale) were >9 on all attributes. This was observed for patients with asthma and with COPD, as well as when the COPD and asthma data were combined (Figure 2).
CONCLUSIONS
· The ease, simplicity and security of use of the ELLIPTA inhaler were its most frequently commended attributes.
· The ELLIPTA DPI was preferred over currently-used inhalers by the majority of COPD and asthma phase 3 trial patients who participated in the qualitative interviews.
· These findings suggest that patients appreciate and are satisfied with the ELLIPTA DPI, and that it meets the needs of patients with COPD and asthma.
REFERENCES
(1) Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention 2012. http://www.ginasthma.org/Guidelines/guidelines-resources.html (accessed 8 May 2013).
(2) Halbert RJ, et al. Eur Respir J 2006;28:523–32.
(3) Bender BG, et al. Curr Opin Allergy Clin Immunol 2004;3:191–5.
(4) Lareau SC, et al. Int J COPD 2010;5:401–6.
(5) Dolovich MB, et al. Chest 2005;127:335–71.
ACKNOWLEDGEMENTS
· The presenting author, Henrik Svedsater, has the following competing interests: is employed by and holds stock in GlaxoSmithKline.
· This research was sponsored by GlaxoSmithKline. GSK study codes (clinicaltrials.gov): HZC112206 (NCT01053988); HZC112207 (NCT01054885); HZC102871 (NCT01009463); HZC102970 (NCT01017952); HZA106827 (NCT01165138); FFA114496 (NCT01431950).
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Laura Maguire, MChem and David Cutler, PhD at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GlaxoSmithKline.
ELLIPTA™ is a trade mark of the
GlaxoSmithKline group of companies
|
|
Presented at the European Academy of Allergy and Clinical Immunology & World Allergy Organization World Allergy &Asthma Congress 2013, Milan, Italy, 22–26 June 2013
|

