Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Receptos, Inc. | a13-14386_18k.htm |
Exhibit 99.1
|
|
Jefferies 2013 Global Healthcare Conference Faheem Hasnain President and Chief Executive Officer June 5, 2013 |
|
|
Forward-Looking Statements All statements in this presentation other than those of historical fact, including statements regarding our clinical development plans for RPC1063 and RPC4046, our research and other development programs, projected timelines for our research and development activities and possible regulatory approvals, if any, our expectations regarding the relative benefits of our product candidates versus competitive therapies, our expectations regarding the possibility of licensing or collaborating with third parties regarding our product candidates or research, and our expectations regarding the therapeutic and commercial potential of our product candidates, research, technologies and intellectual property, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “design,” “intend,” “expect,” “potential” and similar expressions, as well as the negative version of these words and similar expressions, are intended to identify forward-looking statements. Our forward-looking statements do not constitute guarantees of future performance, and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those anticipated or implied in such statements. Our forward-looking statements are based upon our current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated as a result of various risks and uncertainties which include, without limitation, risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics and risks inherent in the effort to build a business around such drugs. Although we believe our expectations are reasonable, we do not in any way guarantee future results, level of activity, performance or achievements. In addition, neither we nor any other person assumes responsibility for the accuracy and completeness of any forward-looking statements. Our forward-looking statements in this presentation speak only as of the date this presentation is actually delivered by us in person. We assume no obligation or undertaking to update or revise any statements to reflect any changes in our expectations or any change in events, conditions or circumstances on which any such statement is based. You should review disclosure that further describes risks and uncertainties relevant to us in additional detail in our filings with the Securities and Exchange Commission, including our registration statement on Form S-1 filed in respect of our initial public offering which was declared effective on May 8, 2013. You may get these documents for free by visiting EDGAR on the SEC web site at http://www.sec.gov. |
|
|
% Experienced Executive Team with History of Success Faheem Hasnain President & CEO Sheila Gujrathi MD, CMO Robert Peach PhD, CSO, Co-Founder Chrysa Mineo VP, Corporate Development Graham Cooper CFO daclizumab ocrelizumab IMMUNOLOGY & ONCOLOGY Marcus Boehm PhD, CTO, Co-Founder Bill Rastetter, Chairman Former Chairman Biogen-Idec |
|
|
RPC1063: Promising new therapy in relapsing MS (RMS) and ulcerative colitis (UC) RMS: Oral S1P1 receptor modulator with potential best-in-class profile UC: Opportunity for first- and best-in-class oral profile Expected patent coverage through 2029 with potential for patent term extension Key inflection points expected in near term Phase 2 data expected in mid-2014 in both RMS and UC Accelerated Phase 3 RMS trial (under SPA) expected to start in late 2013/early 2014 Building innovative pipeline to create long term value Monoclonal antibody targeting IL-13 to treat Eosinophilic Esophagitis (EoE) ready to start randomized Phase 2 trial following IND Preclinical oral GLP-1 receptor targeted program for treatment of T2D based on proprietary GPCR discovery platform Receptos Key Investment Highlights |
|
|
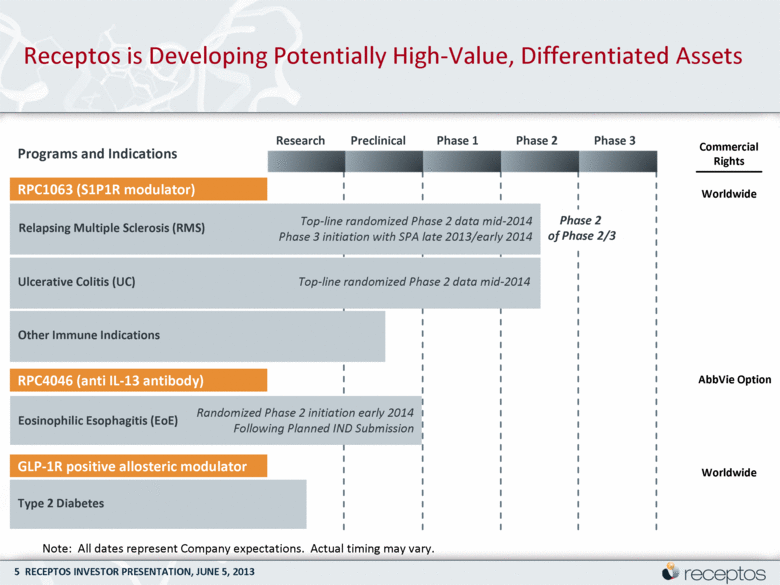
Receptos is Developing Potentially High-Value, Differentiated Assets Note: All dates represent Company expectations. Actual timing may vary. Programs and Indications Relapsing Multiple Sclerosis (RMS) Randomized Phase 2 initiation early 2014 Following Planned IND Submission Top-line randomized Phase 2 data mid-2014 RPC1063 (S1P1R modulator) Phase 2 of Phase 2/3 RPC4046 (anti IL-13 antibody) GLP-1R positive allosteric modulator Ulcerative Colitis (UC) Other Immune Indications Eosinophilic Esophagitis (EoE) Type 2 Diabetes Research Preclinical Phase 1 Phase 2 Phase 3 Commercial Rights Worldwide AbbVie Option Worldwide Top-line randomized Phase 2 data mid-2014 Phase 3 initiation with SPA late 2013/early 2014 |
|
|
RPC1063 Program RMS Indication |
|
|
Oral Therapy Expected to Expand Usage as New Mechanisms Become Available RMS Market Valued at $13B and Growing Trend Toward Oral Therapy Transforming RMS Market *Current company expectation for therapeutic applicability; subject to FDA approval Newly Diagnosed Other 1st Line Treatment Cycling New therapies expected to be introduced* 2nd Line Treatment 1st line failures Tolerability Issues Last Line Treatment Salvage treatment Beta Interferons / Copaxone® $10B sales (2011) RPC1063* Tecfidera™ (BG-12) Gilenya® $1.2B sales (2012) Aubagio® , Laquinimod* Daclizumab* Lemtrada™* Ocrelizumab* Tysabri® $1.1B sales (2011) JC virus negative patients |
|
|
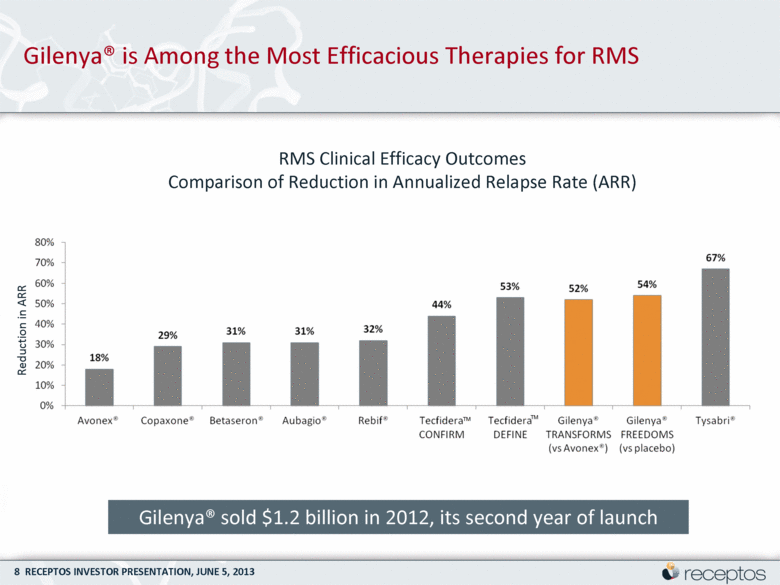
Gilenya® is Among the Most Efficacious Therapies for RMS Reduction in ARR RMS Clinical Efficacy Outcomes Comparison of Reduction in Annualized Relapse Rate (ARR) TM TM Gilenya® sold $1.2 billion in 2012, its second year of launch |
|
|
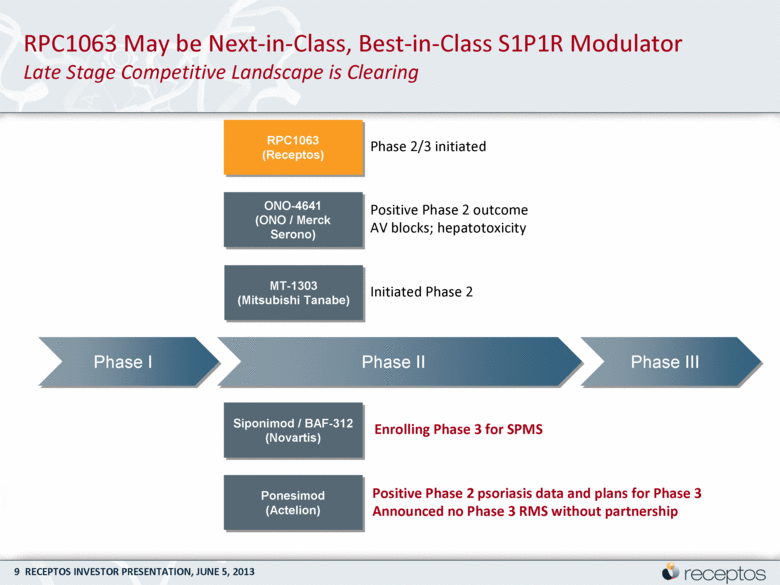
Phase I Phase II Phase III ONO-4641 (ONO / Merck Serono) Positive Phase 2 outcome AV blocks; hepatotoxicity Siponimod / BAF-312 (Novartis) Enrolling Phase 3 for SPMS RPC1063 (Receptos) Phase 2/3 initiated MT-1303 (Mitsubishi Tanabe) Initiated Phase 2 Ponesimod (Actelion) Positive Phase 2 psoriasis data and plans for Phase 3 Announced no Phase 3 RMS without partnership RPC1063 May be Next-in-Class, Best-in-Class S1P1R Modulator Late Stage Competitive Landscape is Clearing |
|
|
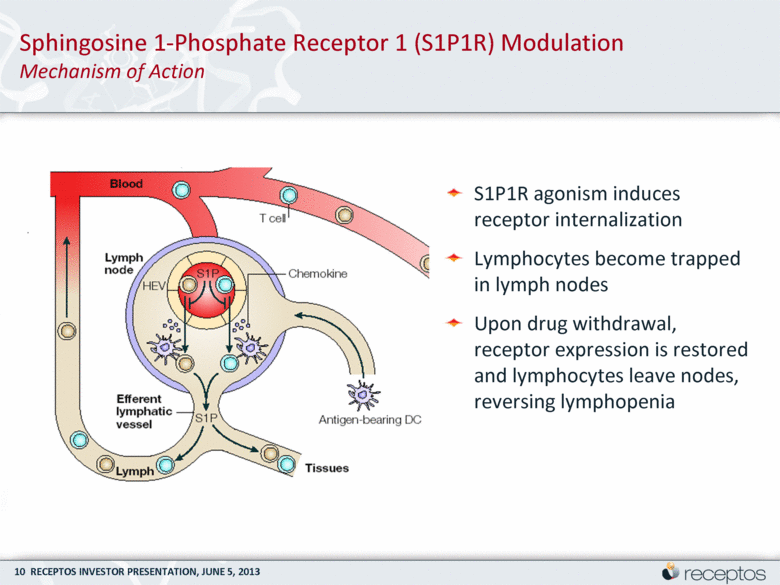
S1P1R agonism induces receptor internalization Lymphocytes become trapped in lymph nodes Upon drug withdrawal, receptor expression is restored and lymphocytes leave nodes, reversing lymphopenia Sphingosine 1-Phosphate Receptor 1 (S1P1R) Modulation Mechanism of Action |
|
|
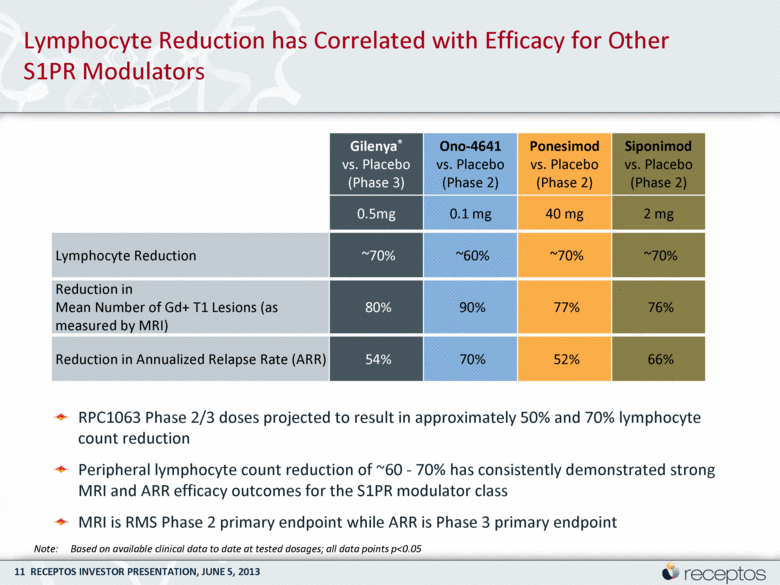
Lymphocyte Reduction has Correlated with Efficacy for Other S1PR Modulators Gilenya® vs. Placebo (Phase 3) Ono-4641 vs. Placebo (Phase 2) Ponesimod vs. Placebo (Phase 2) Siponimod vs. Placebo (Phase 2) 0.5mg 0.1 mg 40 mg 2 mg Lymphocyte Reduction ~70% ~60% ~70% ~70% Reduction in Mean Number of Gd+ T1 Lesions (as measured by MRI) 80% 90% 77% 76% Reduction in Annualized Relapse Rate (ARR) 54% 70% 52% 66% Note: Based on available clinical data to date at tested dosages; all data points p<0.05 RPC1063 Phase 2/3 doses projected to result in approximately 50% and 70% lymphocyte count reduction Peripheral lymphocyte count reduction of ~60 - 70% has consistently demonstrated strong MRI and ARR efficacy outcomes for the S1PR modulator class MRI is RMS Phase 2 primary endpoint while ARR is Phase 3 primary endpoint |
|
|
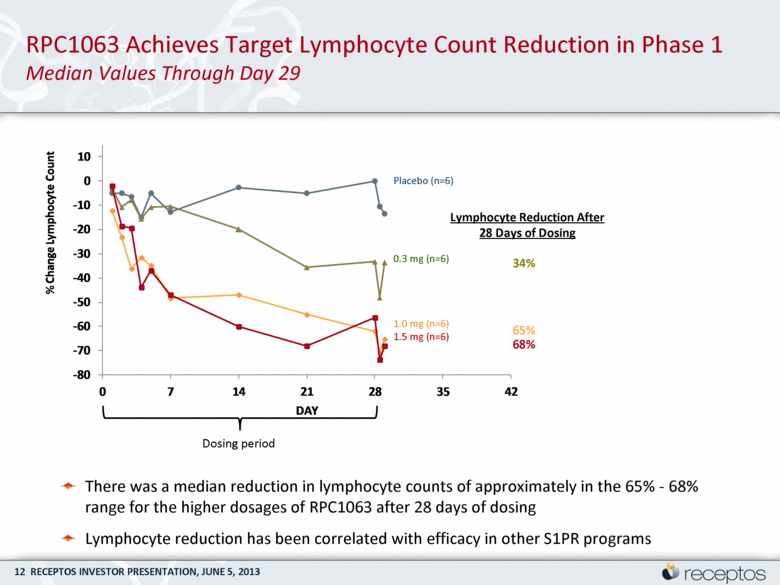
RPC1063 Achieves Target Lymphocyte Count Reduction in Phase 1 Median Values Through Day 29 Dosing period There was a median reduction in lymphocyte counts of approximately in the 65% - 68% range for the higher dosages of RPC1063 after 28 days of dosing Lymphocyte reduction has been correlated with efficacy in other S1PR programs 65% 34% Lymphocyte Reduction After 28 Days of Dosing 68% Placebo (n=6) 0.3 mg (n=6) 1.0 mg (n=6) 1.5 mg (n=6) - 80 - 70 - 60 - 50 - 40 - 30 - 20 - 10 0 10 0 7 14 21 28 35 42 % Change Lymphocyte Count DAY |
|
|
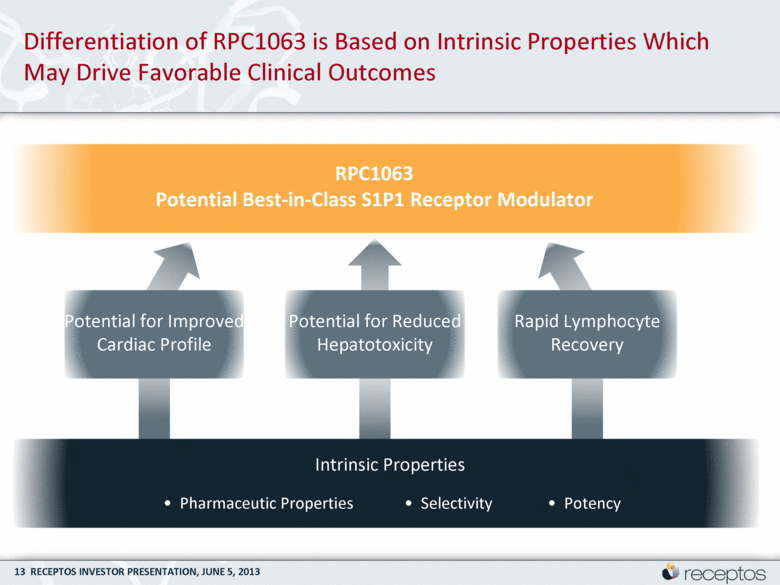
Differentiation of RPC1063 is Based on Intrinsic Properties Which May Drive Favorable Clinical Outcomes Intrinsic Properties • Pharmaceutic Properties • Selectivity • Potency RPC1063 Potential Best-in-Class S1P1 Receptor Modulator Potential for Improved Cardiac Profile Rapid Lymphocyte Recovery Potential for Reduced Hepatotoxicity |
|
|
RPC1063 Has Multiple Points of Cardiac Differentiation Which Can Lead to Label Differences Recently completed Thorough QT/QTc study ruled out QTc effect for RPC1063* Dose titration appears to improve the cardiac profile, both by attenuating first dose heart rate effects and reducing the risk of cardiac adverse events No significant differences in cardiac adverse events between RPC1063 treated and placebo groups have been observed with dose titration * TQT study of Gilenya® at supra-therapeutic doses at steady state, when a negative effect on heart rate was still present, resulted in a prolongation of QTc (upper bound of 90% CI was 14.0ms). RPC1063 QTc Effect Supratherapeutic dose (2 mg) Estimate (msec) Time point (h) 2-sided 90% confidence intervals (CIs) are shown RECEPTOS INVESTOR PRESENTATION, JUNE 2013 14 |
|
|
Higher Exposure Drives First Dose Heart Rate Changes RECEPTOS INVESTOR PRESENTATION, JUNE 5, 2013 14 Cmax(nM) at therapeutic or expected therapeutic dosesRPC1063 First Dose HR Decrease (BPM) 15 |
|
|
S1PR Modulators have Demonstrated Hepatotoxicity in Clinical Trials Hepatotoxicity* (elevated ALT/AST >3x ULN) No elevated liver transaminases observed to date with RPC1063 either pre-clinically (9-month dosing) or in Phase 1 healthy volunteer study (28-day dosing) Phase 2 Outcomes Phase 3 Outcomes ® * Based on available clinical data to date at tested dosages. Gilenya® had a rate of 8% at a dose of 0.5 mg; ONO-4641 had rates of 11.9%, 5.9% and 14.2% at doses of 0.05 mg, 0.1 mg and 0.15 mg, respectively; ponesimod had rates of 2.8%, 4.5% and 4.2% at doses of 10 mg, 20 mg and 40 mg, respectively; and siponimod had a rate of 4.3% at doses of 2 mg and 10 mg 8.0% |
|
|
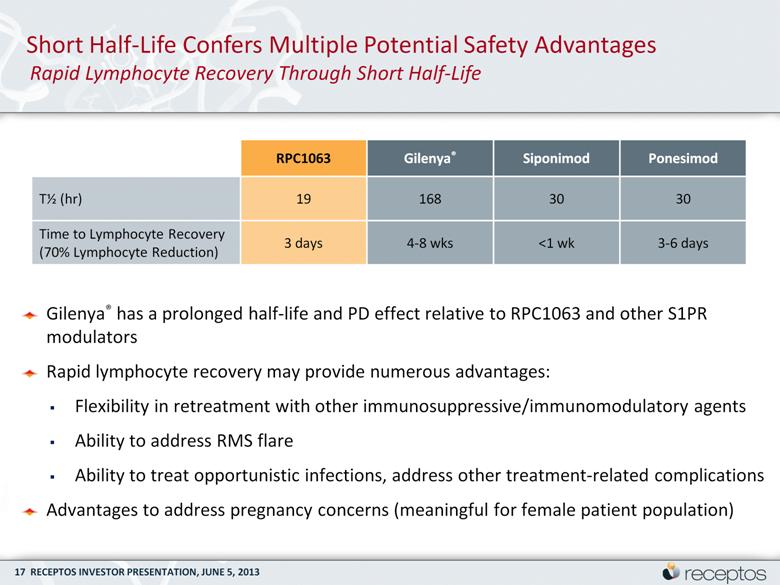
Short Half-Life Confers Multiple Potential Safety Advantages Rapid Lymphocyte Recovery Through Short Half-Life Gilenya® has a prolonged half-life and PD effect relative to RPC1063 and other S1PR modulators Rapid lymphocyte recovery may provide numerous advantages: Flexibility in retreatment with other immunosuppressive/immunomodulatory agents Ability to address RMS flare Ability to treat opportunistic infections, address other treatment-related complications Advantages to address pregnancy concerns (meaningful for female patient population) RPC1063 Gilenya® Siponimod Ponesimod T½ (hr) 19 168 30 30 Time to Lymphocyte Recovery (70% Lymphocyte Reduction) 3 days 4-8 wks <1 wk 3-6 days |
|
|
Potential Underlying Causes of Differentiation RPC1063 Potential for Label Differentiation vs. Gilenya Rapid Lymphocyte Recovery Short Half Life Starting Dose Heart Rate Effects Favorable PK Properties, Titration, Minimal Efficacious Dose Selectivity for S1P1 Potential for Reduced Hepatotoxicity Compound Differences, |
|
|
RADIANCE Randomized Phase 2/3 Study in RMS Phase 2 Initiated October 2012; Top-line Results Anticipated Mid-2014 Phase 2 Primary objective: To demonstrate superior efficacy of RPC1063 compared to placebo by showing a reduction in the cumulative number of total gadolinium enhancing (GdE) lesions by MRI from Week 12 to Week 24 (~210 patients total) Phase 3 – Planned initiation late 2013/early 2014 Primary objective: To assess whether the efficacy of RPC1063 is superior to interferon (IFN) β-1a (Avonex®) in reducing the Annualized Rate of Relapse (ARR) at the end of Month 24 (~900 patients total) Interim Phase 2 safety assessment will trigger Phase 3 enrollment Additional pivotal trial to be conducted as part of Phase 3 program Robust Phase 3 program with SPA agreements reached with FDA for two pivotal Phase 3 clinical trials |
|
|
RPC1063 Program IBD Indication |
|
|
Rationale for RPC1063 in Inflammatory Bowel Disease RPC1063 is a novel lymphocyte trafficking agent for IBD Preclinical efficacy across multiple disease models (acute, chronic, preventive, therapeutic) Clinical POC in IBD has been demonstrated with other lymphocyte trafficking agents such as Tysabri® (Crohn’s disease) and vedolizumab (Crohn’s disease and ulcerative colitis) RPC1063 could have “oral vedolizumab” efficacy profile, since the 2 agents impact largely the same lymphocyte populations Naïve CD4+ T cell Naïve CD8+ T cells Central memory CD4+ T cells Central memory CD8+ T cells B cells Vedolizumab RPC1063 Chronic, transmural colitis responsive to immunotherapy Histopathology Score (Mean +/- SEM) |
|
|
TOUCHSTONE Randomized Phase 2 Study in Ulcerative Colitis (UC) Initiated December 2012, Top-line Results Anticipated Mid-2014 Primary Objective: Compare the efficacy of RPC1063 vs. placebo for induction of clinical remission at Week 8 in patients with moderately to severely active ulcerative colitis (UC) (~180 patients total) Secondary Objectives: Compare the efficacy of RPC1063 vs. placebo at weeks 8 and 32 as measured by clinical response, clinical remission, and mucosal healing Compare the overall safety and tolerability of RPC1063 vs. placebo for the duration of the study FDA agreement that Phase 2 could be considered pivotal Phase 3 trial if results are compelling Efficacy in UC expected to predict efficacy in Crohn’s Disease with expanded reach of a future RPC1063 partnership |
|
|
RPC4046 Program Eosinophilic Esophagitis |
|
|
Endoscopic Image Histologic Image Eosinophilic Esophagitis (EoE) is an Orphan Disease with Significant Unmet Need Disease Overview EoE is a chronic, immune-mediated atopic GI-related disease ~160K patients in the US, ~145K in EU with growing prevalence Disease presents in children and adults and is marked by eosinophilic infiltration and structural changes in esophagus No FDA approved drugs for treatment; standard-of-care swallowed steroids are not effective in controlling disease Scientific Rationale for targeting anti-IL13 Preclinical and human tissue studies point to a central role for IL-13 cytokine driving disease pathogenesis RPC4046 targets anti-inflammatory and anti-fibrotic pathways Anti-IL-13 validated in Asthma with lebrikizumab (Genentech) Biomarker/diagnostic strategy exists for selecting responders |
|
|
2014 2013 Significant Potential Milestones Through 2015 Q1 Q2 Q3 Q4 Q1 Q3 Q4 Q1 Q2 Q3 Q4 2015 Q2 RPC 1063: RMS P3 Initiation w/ SPA Late 2013/early 2014 RPC4046: EoE P2 Initiation 1H 2014 RPC1063: RMS P2 Data Mid-2014 RPC1063: UC P2 Data Mid-2014 RPC4046: EoE P2 Top-line Data 2H 2015 |