Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a13-14319_18k.htm |
Exhibit 99.1
|
|
June 5, 2013 NASDAQ: THRX |
|
|
This presentation contains certain "forward-looking" statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives and future events. Theravance intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. Examples of such statements include statements relating to: plans for executing the separation of Theravance into two independent companies, the expected timing of the separation, expectations for the amount and estimated duration of the funding of Theravance Biopharma at the time of the separation, the strategies, plans and objectives of the two companies following the separation, expectations related to the staffing of the two companies, the expected timing of the Elan shareholder vote on the proposed royalty participation transaction and the outcome of such vote, the expected timing for consummating the royalty participation transaction if Elan shareholder approval is obtained, the effect of the royalty participation transaction if it is consummated on the strategies, plans and objectives of Theravance, the timing, manner and amount of anticipated potential returns of capital to stockholders if the royalty participation transaction and/or the separation is consummated, the possible tax effects of the royalty participation transaction and/or the separation, the status and timing of clinical studies, data analysis and communication of results, the potential benefits and mechanisms of action of product candidates, the enabling capabilities of Theravance’s approach to drug discovery and its proprietary insights, expectations for product candidates through development and commercialization, and the timing of seeking regulatory approval of product candidates. These statements are based on the current estimates and assumptions of the management of Theravance as of the date of this presentation and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance to be materially different from those reflected in the forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to: delays in preparing audited financial statements for Theravance Biopharma, difficulties in effecting the registration of Theravance Biopharma as a public company, failure to obtain necessary consents from third parties, changes in the development or operations of Theravance prior to the separation that could affect the plans for the separation or the cash available for the initial funding of the independent companies, delays encountered in obtaining, or the failure to obtain, a private letter ruling from the Internal Revenue Service (should Theravance seek to effect the separation on a tax-free basis) or approval of Elan’s shareholders for the royalty participation transaction, the possibility that intervening events could arise which could alter the timing, or the ability to consummate, the royalty participation transaction, the anticipated separation of Theravance into two independent companies or the intended return of capital to stockholders, the risk that third parties could challenge the royalty participation transaction, the risk that Theravance’s net operating loss may not be available to offset taxes from the royalty participation transaction, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective, our dependence on third parties to conduct our clinical studies, delays or failure to achieve regulatory approvals for product candidates, and risks of collaborating with third parties to discover, develop and commercialize products. Other risks affecting Theravance are described under the heading "Risk Factors" contained in Theravance’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on May 1, 2013 and the risks discussed in our other periodic filings with the SEC. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Theravance assumes no obligation to update its forward-looking statements. 2 |
|
|
BREO™ ELLIPTA™ (FF/VI 100/25 mcg) approved for COPD in the US RELVAR™ ELLIPTA™ regulatory applications in COPD and asthma filed ex-US GSK pays 15% royalty on first $3.0B of annual WW net sales; 5% thereafter ANORO™ ELLIPTA™ FDA PDUFA goal date December 18, 2013, regulatory applications in COPD filed globally GSK pays upward tiering 6.5% to 10% royalties on WW net sales Deep Pipeline Strong Financial Position: $558 million at March 31, 2013 Royalty Participation Agreement with Elan Acceleration of return of capital to stockholders 3 BREO™ ELLIPTA™ FDA Approved for COPD; BREO™ ELLIPTA™ is not indicated for the relief of acute bronchospasm or the treatment of asthma. RELVAR™ ELLIPTA™ (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) are investigational medicines and are not currently approved anywhere in the world. RELVAR™, BREO™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of the brand names ANORO™ and RELVAR™ has not yet been approved by any regulatory authority. Full US Prescribing Information, including BOXED WARNING and Medication Guide for BREO™ ELLIPTA™ is available at us.gsk.com |
|
|
4 *Source: 2012 market size based on an estimate derived from the use of information under license from the following IMS Health Inc. information service: MIDAS for the period ending December 2012. IMS expressly reserves all rights, including rights of copying, distribution and republication. Excludes solutions for nebulization. |
|
|
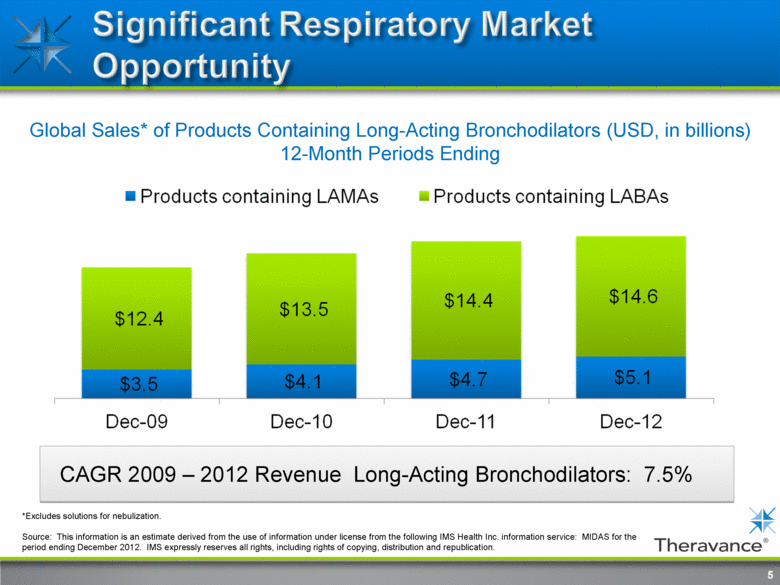
Global Sales* of Products Containing Long-Acting Bronchodilators (USD, in billions) 12-Month Periods Ending CAGR 2009 – 2012 Revenue Long-Acting Bronchodilators: 7.5% 5 *Excludes solutions for nebulization. Source: This information is an estimate derived from the use of information under license from the following IMS Health Inc. information service: MIDAS for the period ending December 2012. IMS expressly reserves all rights, including rights of copying, distribution and republication. |
|
|
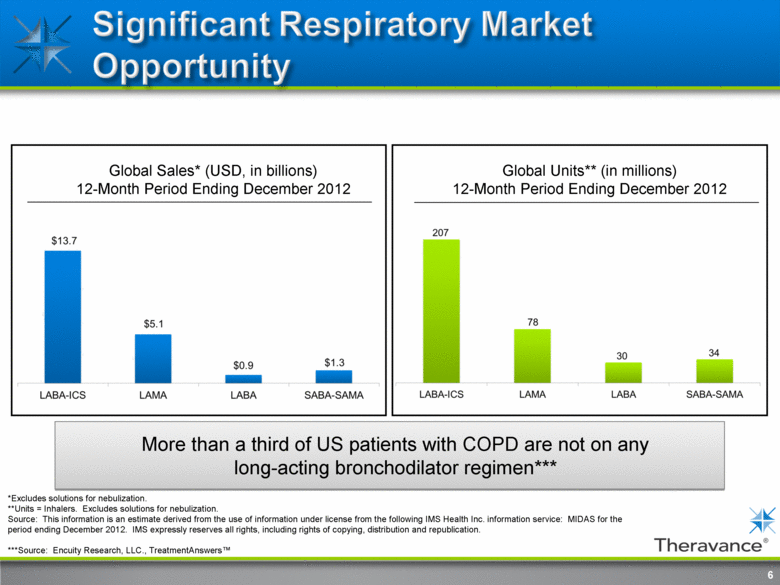
6 *Excludes solutions for nebulization. **Units = Inhalers. Excludes solutions for nebulization. Source: This information is an estimate derived from the use of information under license from the following IMS Health Inc. information service: MIDAS for the period ending December 2012. IMS expressly reserves all rights, including rights of copying, distribution and republication. ***Source: Encuity Research, LLC., TreatmentAnswers™ Global Sales* (USD, in billions) 12-Month Period Ending December 2012 Global Units** (in millions) 12-Month Period Ending December 2012 More than a third of US patients with COPD are not on any long-acting bronchodilator regimen*** |
|
|
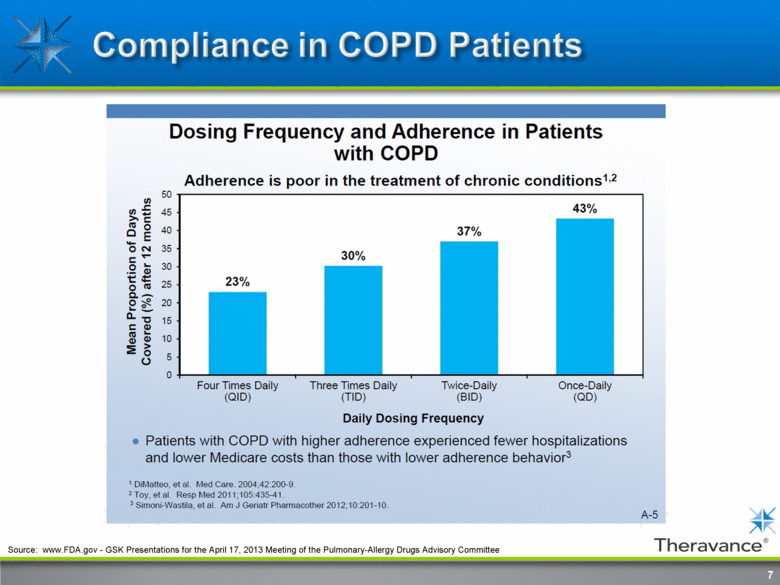
7 Source: www.FDA.gov - GSK Presentations for the April 17, 2013 Meeting of the Pulmonary-Allergy Drugs Advisory Committee |
|
|
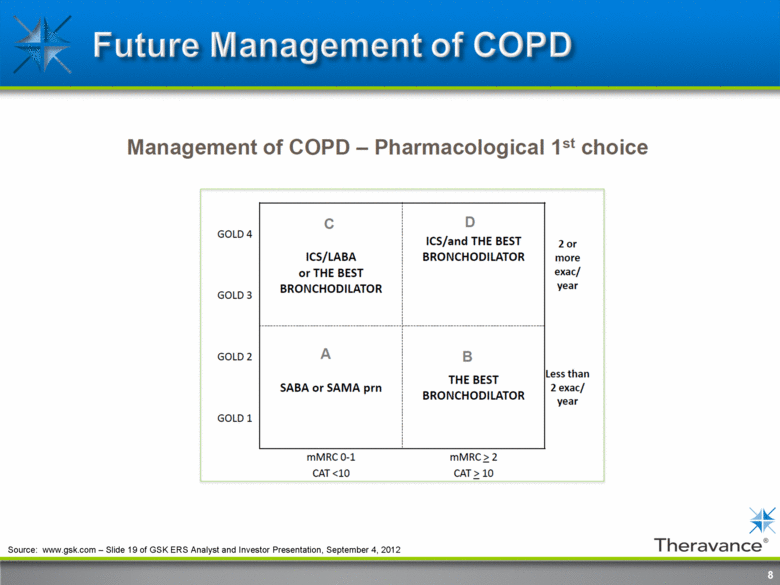
8 Source: www.gsk.com – Slide 19 of GSK ERS Analyst and Investor Presentation, September 4, 2012 |
|
|
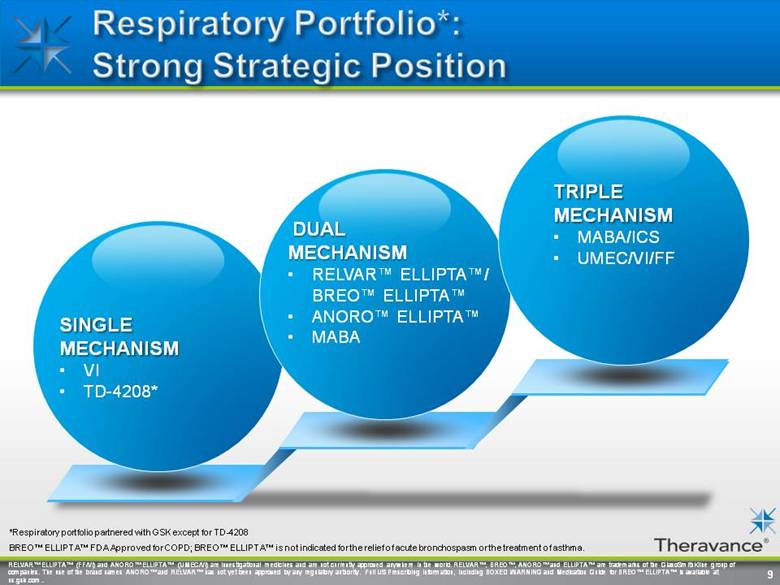
RELVAR™ ELLIPTA™ (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) are investigational medicines and are not currently approved anywhere in the world. RELVAR™, BREO™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of the brand names ANORO™ and RELVAR™ has not yet been approved by any regulatory authority. Full US Prescribing Information, including BOXED WARNING and Medication Guide for BREO™ ELLIPTA™ is available at us.gsk.com TRIPLE MECHANISM MABA/ICS UMEC/VI/FF 9 *Respiratory portfolio partnered with GSK except for TD-4208 BREO™ ELLIPTA™ FDA Approved for COPD; BREO™ ELLIPTA™ is not indicated for the relief of acute bronchospasm or the treatment of asthma. DUAL MECHANISM RELVAR™ ELLIPTA™/ BREO™ ELLIPTA™ ANORO™ ELLIPTA™ MABA |
|
|
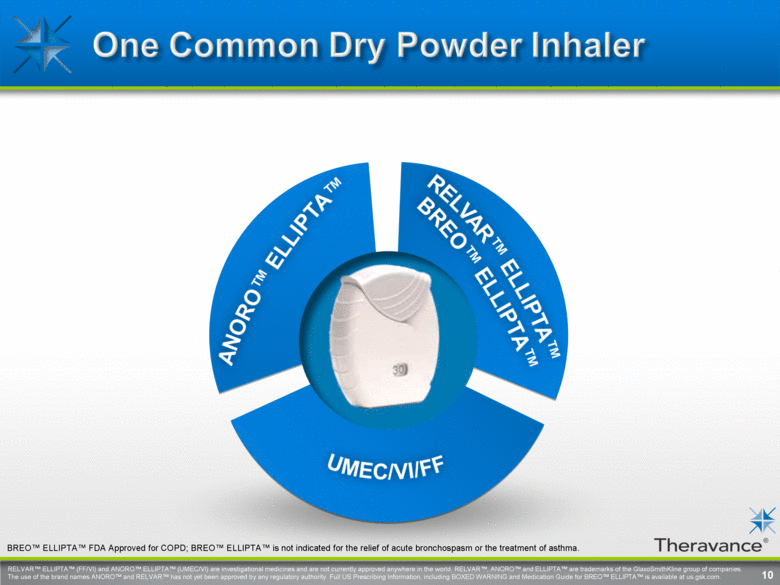
RELVAR™ ELLIPTA™ (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) are investigational medicines and are not currently approved anywhere in the world. RELVAR™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of the brand names ANORO™ and RELVAR™ has not yet been approved by any regulatory authority. Full US Prescribing Information, including BOXED WARNING and Medication Guide for BREO™ ELLIPTA™ is available at us.gsk.com. . 10 BREO™ ELLIPTA™ FDA Approved for COPD; BREO™ ELLIPTA™ is not indicated for the relief of acute bronchospasm or the treatment of asthma. |
|
|
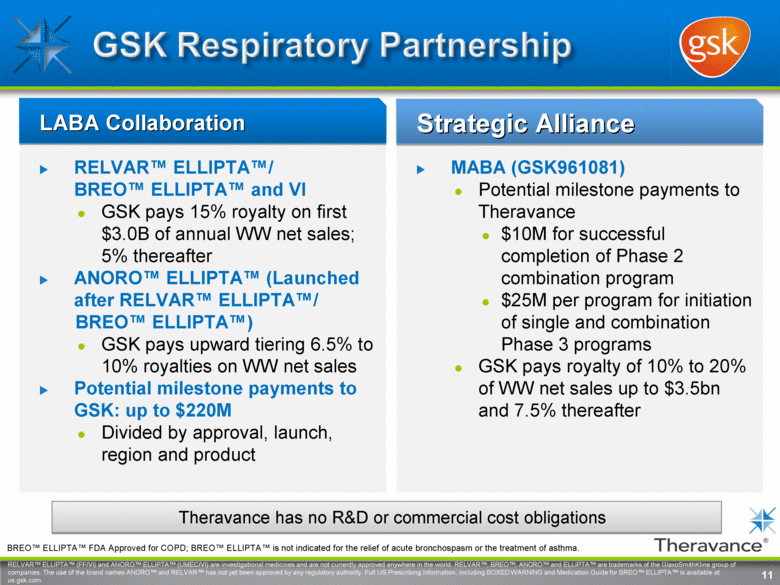
RELVAR™ ELLIPTA™/ BREO™ ELLIPTA™ and VI GSK pays 15% royalty on first $3.0B of annual WW net sales; 5% thereafter ANORO™ ELLIPTA™ (Launched after RELVAR™ ELLIPTA™/ BREO™ ELLIPTA™) GSK pays upward tiering 6.5% to 10% royalties on WW net sales Potential milestone payments to GSK: up to $220M Divided by approval, launch, region and product MABA (GSK961081) Potential milestone payments to Theravance $10M for successful completion of Phase 2 combination program $25M per program for initiation of single and combination Phase 3 programs GSK pays royalty of 10% to 20% of WW net sales up to $3.5bn and 7.5% thereafter LABA Collaboration Strategic Alliance Theravance has no R&D or commercial cost obligations 11 RELVAR™ ELLIPTA™ (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) are investigational medicines and are not currently approved anywhere in the world. RELVAR™, BREO™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of the brand names ANORO™ and RELVAR™ has not yet been approved by any regulatory authority. Full US Prescribing Information, including BOXED WARNING and Medication Guide for BREO™ ELLIPTA™ is available at us.gsk.com. BREO™ ELLIPTA™ FDA Approved for COPD; BREO™ ELLIPTA™ is not indicated for the relief of acute bronchospasm or the treatment of asthma. |
|
|
12 Theravance Biopharma Business Objectives Royalty Management Co Business Objectives RELVAR™ELLIPTA™/ BREO™ELLIPTA™ ANORO™ ELLIPTA™ ¥ VI monotherapy MABA* MABA /ICS* UMEC/VI/FF* VIBATIV® TD-1211: OIC TD-9855: ADHD and Fibromyalgia Programs Programs Two Companies with Distinct Business Objectives and Profiles 12 ¥This program will be held & managed by a limited liability company subsidiary of Royalty Management Co (the “LLC”) and all of the LLC’s economic interests in this program will accrue to Royalty Management Co. * These programs, partnered with GSK, will be held & managed by the LLC and 98% of the LLC’s economic interests in these programs will accrue to Theravance Biopharma and 2% will accrue to Royalty Management Co. RELVAR™ ELLIPTA™ (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) are investigational medicines and are not currently approved anywhere in the world. RELVAR™, BREO™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of the brand names ANORO™ and RELVAR™ has not yet been approved by any regulatory authority. Full US Prescribing Information, including BOXED WARNING and Medication Guide for BREO™ ELLIPTA™ is available at us.gsk.com. VIBATIV® is a registered trademark of Theravance, Inc. For full Prescribing Information and Medication Guide for VIBATIV® in the U.S. please visit www.VIBATIV.com. BREO™ ELLIPTA™ FDA Approved for COPD; BREO™ ELLIPTA™ is not indicated for the relief of acute bronchospasm or the treatment of asthma. |
|
|
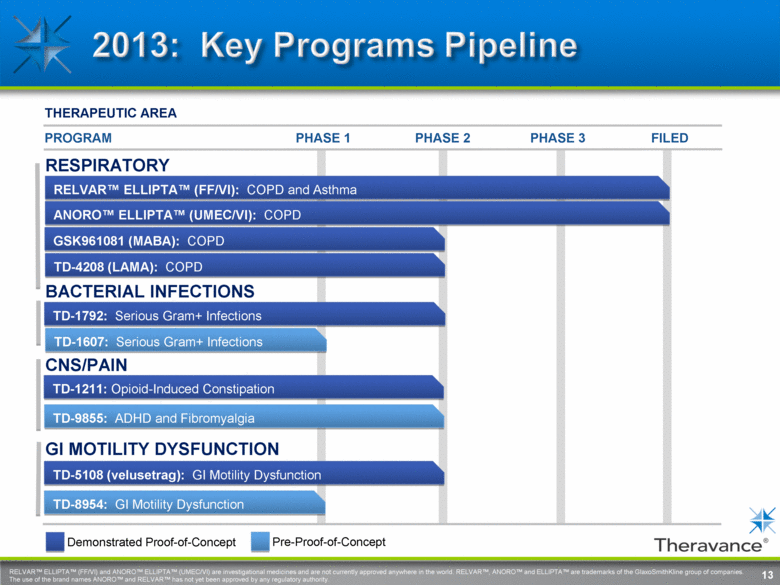
RELVAR™ ELLIPTA™ (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) are investigational medicines and are not currently approved anywhere in the world. RELVAR™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of the brand names ANORO™ and RELVAR™ has not yet been approved by any regulatory authority. PHASE 3 THERAPEUTIC AREA PHASE 1 PHASE 2 FILED BACTERIAL INFECTIONS TD-1792: Serious Gram+ Infections RESPIRATORY GI MOTILITY DYSFUNCTION TD-5108 (velusetrag): GI Motility Dysfunction TD-1211: Opioid-Induced Constipation GSK961081 (MABA): COPD ANORO™ ELLIPTA™ (UMEC/VI): COPD CNS/PAIN TD-9855: ADHD and Fibromyalgia PROGRAM RELVAR™ ELLIPTA™ (FF/VI): COPD and Asthma TD-4208 (LAMA): COPD TD-8954: GI Motility Dysfunction Demonstrated Proof-of-Concept Pre-Proof-of-Concept 13 TD-1607: Serious Gram+ Infections |
|
|
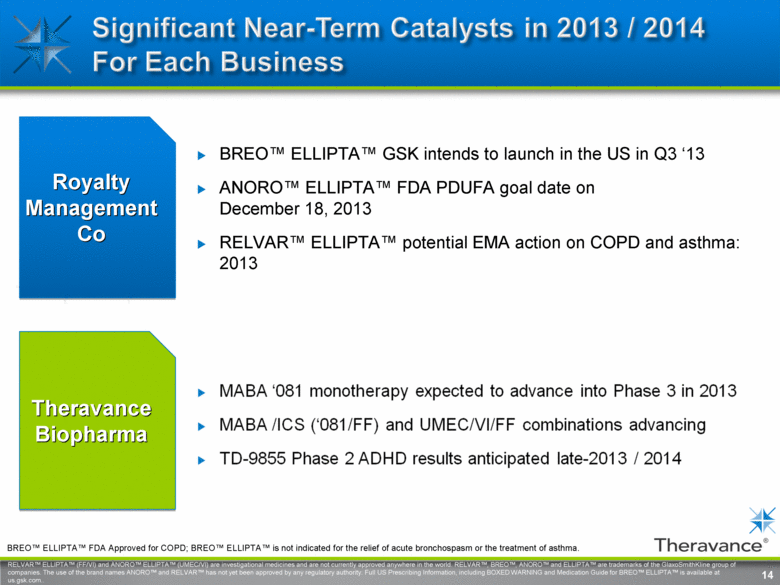
14 Theravance Biopharma Royalty Management Co BREO™ ELLIPTA™ GSK intends to launch in the US in Q3 ‘13 ANORO™ ELLIPTA™ FDA PDUFA goal date on December 18, 2013 RELVAR™ ELLIPTA™ potential EMA action on COPD and asthma: 2013 14 RELVAR™ ELLIPTA™ (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) are investigational medicines and are not currently approved anywhere in the world. RELVAR™, BREO™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of the brand names ANORO™ and RELVAR™ has not yet been approved by any regulatory authority. Full US Prescribing Information, including BOXED WARNING and Medication Guide for BREO™ ELLIPTA™ is available at us.gsk.com BREO™ ELLIPTA™ FDA Approved for COPD; BREO™ ELLIPTA™ is not indicated for the relief of acute bronchospasm or the treatment of asthma. |
|
|
NASDAQ: THRX |
|
|
16 BREO ELLIPTA is contraindicated in patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to either fluticasone furoate, vilanterol, or any of the excipients. BREO ELLIPTA should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD, or as rescue therapy for the treatment of acute episodes of bronchospasm, which should be treated with an inhaled, short-acting beta2-agonist. BREO ELLIPTA should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing LABAs, as an overdose may result. Oropharyngeal candidiasis has occurred in patients treated with BREO ELLIPTA. An increase in the incidence of pneumonia has been observed in subjects with COPD receiving the fluticasone furoate/vilanterol combination, including BREO ELLIPTA 100 mcg/25 mcg, in clinical trials. There was also an increased incidence of pneumonias resulting in hospitalization. In some incidences these pneumonia events were fatal. Patients who use corticosteroids are at risk for potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. A more serious or even fatal course of chickenpox or measles may occur in susceptible patients. Particular care is needed for patients who have been transferred from systemically active corticosteroids to inhaled corticosteroids because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. Hypercorticism and adrenal suppression may occur with very high dosages or at the regular dosage of inhaled corticosteroids in susceptible individuals. Caution should be exercised when considering the coadministration of BREO ELLIPTA with long-term ketoconazole and other known strong CYP3A4 inhibitors because increased systemic corticosteroid and cardiovascular adverse effects may occur. Inhaled medicines can produce paradoxical bronchospasm, which may be life-threatening. Vilanterol, the LABA in BREO ELLIPTA, can produce clinically significant cardiovascular effects in some patients. Decreases in bone mineral density have been observed with long-term administration of products containing inhaled corticosteroids, as have glaucoma, increased intraocular pressure, and cataracts. The most common adverse reactions (>3% and more common than in placebo) reported in two 6-month clinical trials with BREO ELLIPTA (and placebo) were nasopharyngitis, 9% (8%); upper respiratory tract infection, 7% (3%); headache, 7% (5%); and oral candidiasis, 5% (2%). In addition to the events reported in the 6-month studies, adverse reactions occurring in >3% of the subjects treated with BREO ELLIPTA in two 1-year studies included COPD, back pain, pneumonia, bronchitis, sinusitis, cough, oropharyngeal pain, arthralgia, hypertension, influenza, pharyngitis, diarrhea, peripheral edema, and pyrexia. BREO ELLIPTA is not indicated for the relief of acute bronchospasm or the treatment of asthma. The safety and efficacy of BREO ELLIPTA in patients with asthma have not been established. Long-acting beta2-adrenergic agonists (LABAs), such as vilanterol, one of the active ingredients in BREO ELLIPTA, increase the risk of asthma-related death. A placebo-controlled trial with another LABA (salmeterol) showed an increase in asthma-related deaths in subjects receiving salmeterol. This finding with salmeterol is considered a class effect of all LABAs, including vilanterol. Full US Prescribing Information, including BOXED WARNING and Medication Guide is available at us.gsk.com. BREO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. Full US Prescribing Information, including BOXED WARNING and Medication Guide for BREO™ ELLIPTA™ is available at us.gsk.com. |