Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Sonoma Pharmaceuticals, Inc. | oculus_8k-053013.htm |
Exhibit 99.1


The Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995 This presentation includes forward - looking statements that are made pursuant to the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995 . While these statements are made to convey to the public the company’s progress, business opportunities and growth prospects, readers and listeners are cautioned that such forward - looking statements represent management's opinion . Whereas management believes such representation to be true and accurate, based on information and data available to the Company at this time, actual results may differ materially from those described . The company's operations and business prospects are always subject to risk and uncertainties . Important factors that may cause actual results to differ are set forth in the Company’s periodic filings with the U . S . Securities and Exchange Commission . Nasdaq : OCLS

Era of Antibiotics - Penicillin Fleming 1928 Florey & Chain 1940

“ The time has come to close the book on infectious diseases. We have basically wiped out infection in the United States. ” William Stewart, U.S. Surgeon General, 1967
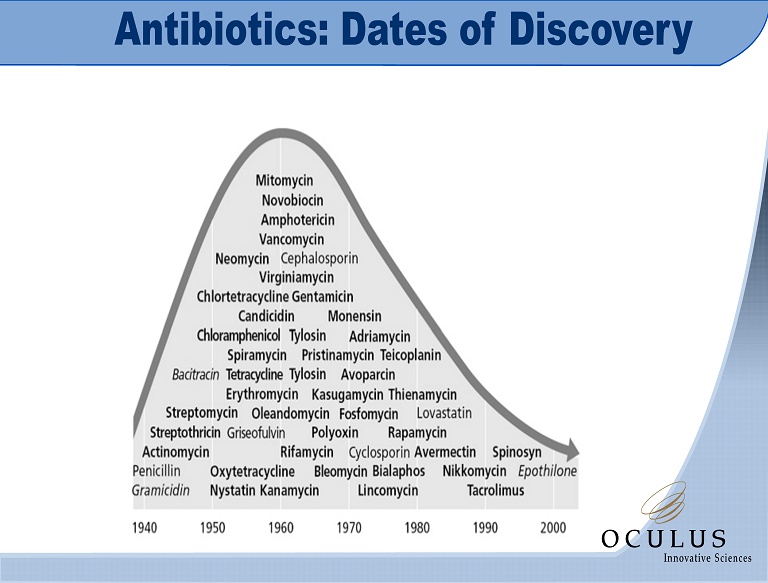
Antibiotics: Dates of Discovery

U.S. Antimicrobial Use

“The danger posed by growing resistance to antibiotics should be ranked along with terrorism on a list of threats to the nation.” UK’s Chief M edical O fficer, Professor Dame Sally Davies Mar 2013

Studying Microbes in Frozen Tundra

Human Immune System

Neutrophil Mode of Action

The Breakthrough – Non - Antibiotic Antimicrobial Kills broad spectrum of: • bacteria , viruses, spores and fungi • antibiotic - resistant strains (MRSA, VRE) 4 million patients without any significant adverse events Proven efficacy in over 30 studies, including randomized/controlled Phase II FDA study against levofloxacin Due to a different MOA, no facilitation of pathogenic resistance

Dr. Adam Landsman Assistant Professor Harvard Medical School “Microcyn is one of the few topicals that’s effective for patients that are affected with MRSA ...”

Dr. Tom Wolvos Chief of General Surgery Scottsdale Healthcare Scottsdale, Arizona Editor of the Scottsdale Wound Management Guide “Microcyn seems to decrease inflammation ... I changed to Microcyn gel and saw not only healing of the wound, but a dramatic decrease in the inflammatory reaction that my patient experienced when treated with an antibiotic ointment.”

Acute Care/Hospital Products U.S. Partner : Eloquest Healthcare® is focused specifically on serving hospitals, their healthcare practitioners, and patients. Eloquest Healthcare® provides value within the acute care market by helping to improve patient outcomes.

Dermatology Products U.S. Partner: Quinnova Pharmaceuticals is a specialty pharmaceutical company founded on innovative, patent - protected dermal delivery technologies

Dr. Rebecca Smith “I see a lot of colonization of MRSA. I see kids with chronic atopic dermatitis who end up with the infection over again and again and again. And they’re often colonized in their nose, so I can take the solution or the gel even and have them apply in their noses a couple of times a day . This cuts down on the colonization so I can prevent chronic recurrence of superficial MRSA.” Dermatologist Fort Mill Dermatology South Carolina

Animal Healthcare Products Partner: Innovacyn Inc. is a privately held company that provides premier human and animal healthcare products based on advanced scientific research

Cesar Millan The Dog Whisperer “Vetericyn will help your dog heal faster from a wound or infection. That means fewer costly and stressful visits to the vet .” “Vetericyn is great to use on hot spots, rashes and skin irritations . It is safe as water and contains no steroids, antibiotics, alcohol or other harmful ingredients.”
Ruthigen -- New Drug Timeline RUT58 - 60 Timeline for Surgical & Traumatic Wounds 2013 2014 2015 2016 2017 Pre - IND Meeting Phase IIb EOPII meeting Phase III Pre - NDA meeting NDA review and Approval


Summary Income Statement
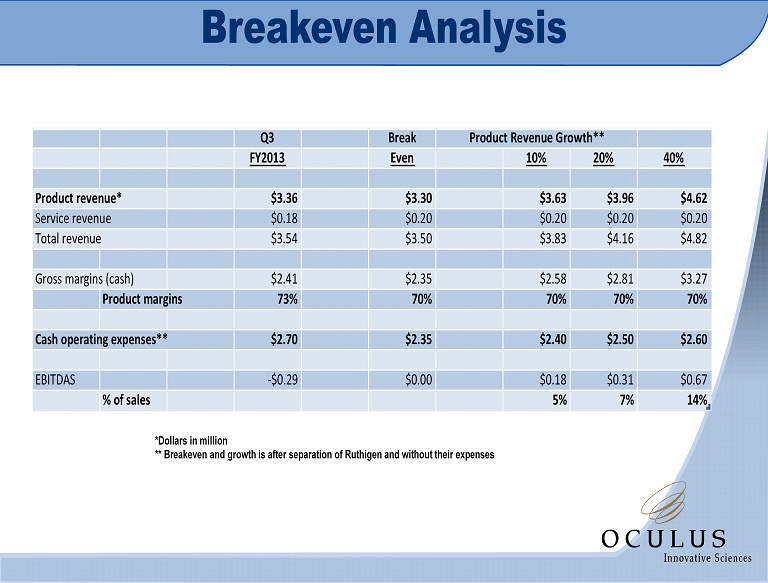
Breakeven Analysis *Dollars in million ** Breakeven and growth is after separation of Ruthigen and without their expenses

Current Revenue Sources Partners Approvals SKUs Commercialized Markets Innovacyn FDA 510K 40+ 2009 US/Int’l: Animal health AmDerma FDA 510K 3 2012 US: Derm Rx Eloquest FDA 510K 7 2012 US : Acute care More Pharma MOH Drug 4 2005 Mexico: Pharmacy, hospital, derm Alkem Drug 3 India: Hospital and pharmacy Shanghai Sunvic Device 3 2013 China: Hospital and pharmacy EU Distributors Device 2 2005 EU: Acute care
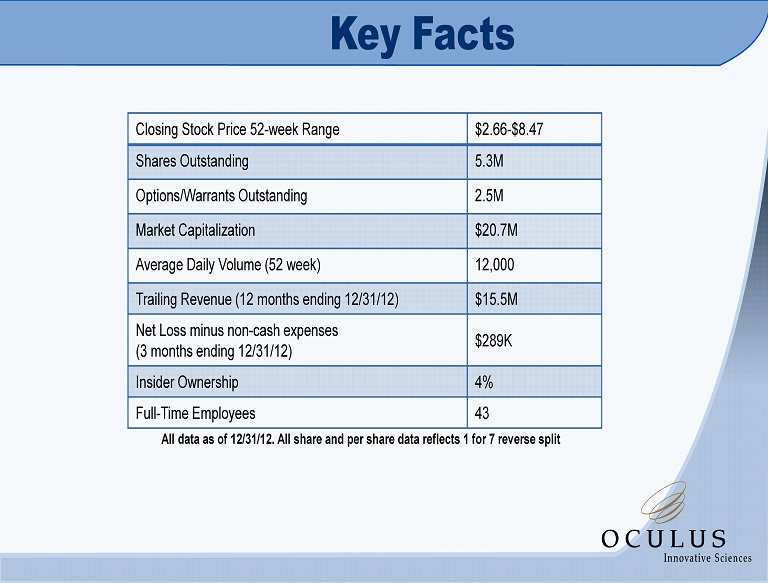
Key Facts Closing Stock Price 52 - week Range $2.66 - $8.47 Shares Outstanding 5.3M Options/Warrants Outstanding 2.5M Market Capitalization $20.7M Average Daily Volume (52 week) 12,000 Trailing Revenue (12 months ending 12/31/12) $15.5M Net Loss minus non - cash expenses (3 months ending 12/31/12) $289K Insider Ownership 4% Full - Time Employees 43 All data as of 12/31/12. All share and per share data reflects 1 for 7 reverse split

Intellectual Property 35 Issued Patents • 5 issued US • 12 issued EU • 18 issued International Pending Applications • 109 U.S. and foreign Claims Covering: • Chemical composition • Apparatus • Method of manufacturing • Therapeutic uses IP ruled enforceable by U.S. Federal Court in 2009

Preliminary Scar Data % Improvement of Microcyn over Market Leader: (based on % reduction from base line) Investigator Global Assessment: Microcyn Market Leader % Very good/ good at Day 84 55% 38% Percentage Microcyn Leader DifferenceImprovement P values Vascularity -42% -28% -14% 50% 25% Pliability -47% -34% -13% 38% 22% Height -30% -11% -19% 173% 7% Vancouver -40% -26% -14% 55% 8% Pain -100% -72% -28% 39% 12% Itch -78% -70% -8% 11% 65% Day 84

“De - Risked Company” Microcyn Technology marketed in more than 20 countries Last three years => average revenue growth = 35% Robust product pipeline in US, Europe, Mexico/Latin America, Middle East and Asia Expanding global footprint Ruthigen = > internally used antimicrobial drug focused on prevention of surgically acquired infections

More Information Jim Schutz , Oculus CEO jschutz@oculusis.com Bob Miller , Oculus CFO bmiller@oculusis.com Dan McFadden , Oculus VP of IR/PR dmcfadden@ oculusis.com Oculus Innovative Sciences 1129 North McDowell Blvd. Petaluma, California 94954 Telephone: +1 (707) 283 - 0550 www.oculusis.com Nasdaq : OCLS
Addendum Slides
