Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ZIOPHARM ONCOLOGY INC | d545839d8k.htm |
 Better Cancer Medicine
Deutsche Bank 38
th
Annual Healthcare Conference
Jonathan Lewis, MD, PhD
Chief Executive Officer
Exhibit 99.1
May 29, 2013 |
 Forward-Looking Statements
This presentation contains certain forward-looking information about ZIOPHARM Oncology
that is intended to be covered by the safe harbor for “forward-looking
statements” provided by the Private Securities Litigation Reform Act of 1995, as
amended. Forward-looking statements are statements that are not historical facts. Words such as “expect(s),”
“feel(s),” “believe(s),” “will,” “may,”
“anticipate(s)” and similar expressions are intended to identify forward-looking
statements. These statements include, but are not limited to, statements regarding our ability
to successfully develop and commercialize our therapeutic products; our ability to
expand our long-term business opportunities; financial projections and estimates
and their underlying assumptions; and future performance. All of such statements are
subject to certain risks and uncertainties, many of which are difficult to predict and
generally beyond the control of the Company, that could cause actual results to differ
materially from those expressed in, or implied or projected by, the forward-looking
information and statements. These risks and uncertainties include, but are not limited to: whether any
of our therapeutic candidates will advance further in the clinical trials process and whether
and when, if at all, they will receive final approval from the U.S. Food and Drug
Administration or equivalent foreign regulatory agencies and for which indications;
whether any of our therapeutic candidates will be successfully marketed if approved; whether our
DNA-based biotherapeutics discovery and development efforts will be successful; our
ability to achieve the results contemplated by our collaboration agreements; the
strength and enforceability of our intellectual property rights; competition from
pharmaceutical and biotechnology companies; the development of and our ability to take advantage
of the market for DNA-based biotherapeutics; our ability to raise additional capital to
fund our operations on terms acceptable to us; general economic conditions; and the
other risk factors contained in our periodic and interim reports filed with the SEC
including, but not limited to, our Annual Report on Form 10-K for the fiscal year ended December 31,
2012, and our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2013.
Our audience is cautioned not to place undue reliance on these forward-looking
statements that speak only as of the date hereof, and we do not undertake any
obligation to revise and disseminate forward-looking statements to reflect events or circumstances after
the date hereof, or to reflect the occurrence of or non-occurrence of any events.
May 29, 2013 |
 DNA Therapeutics
Using the power of DNA to treat
and prevent cancer
May 29, 2013 |
 May 29, 2013 •
Paradigm-shifting, synthetic biology technology for precise, controlled
delivery of therapeutic proteins in vivo
•
Engineered
approach to product design allows us to rapidly develop new
genetically-based treatments for cancer with multiple effectors
•
Focused, disciplined and iterative approach to development decreases risk
through:
–
Early “go/no go”
decisions
–
Fast proof-of-concept and preclinical validation
–
Creating a virtuous circle of product development and improvement
–
Minimizing expense
•
Lead candidate, Ad-RTS-IL-12 in Phase 2 clinical trials for melanoma
and breast cancer
DNA-Based Therapeutics |
 May 29, 2013 Cells
DC
T Cell
B Cell
Macrophage
NK cell
Treg
Other
immune
cells
Direct tumor lysis
ADCC
Complement cytotoxicity
Innate immunity stimulation
Adaptive immunity stimulation
Immune evasion inhibition
Pro-apoptosis
Necrosis
Anti-angiogenesis
Growth inhibition
Anti-tumor metabolism
EMT blockade
Anti-tumor function
MSC
Epithelial
Endothelial
Fibroblast and ECM
Tumor cell
Tumor and microenvironment
Effectors
Immune cells
Non-immune
Cytokines
mAb
scFv
scFv
toxin
Systemic
decoy
Intracellular
decoy
Metabolic
Enzyme
Protein
Ligand
RNA
Molecular and Cellular Oncology DNA-Coded Toolset |
 May 29, 2013 A Platform System for Rapidly Developing
Controllable DNA Therapies
Inventoried DNA modules
Monogenic
Multigenic
Viral delivery vector
RheoSwitch
Therapeutic System
®
3’
Target Gene(s)
for Expression
Intramuscular Cell Plasmid
REG |
 May 29, 2013 Using Natural Cell Biology to Regulate Proteins
Target Cell
Controlled
Therapeutic
proteins
Adaptable
Precision
mRNA
Oral ligand activates
DNA, protein
production begins
Translation |
 May 29, 2013 Ad IL-12
Melanoma
Breast
GBM
DC IL-12
Melanoma
GBM
Cell signal targeting
Multigenic platforms
Immunotherapy Programs
Palifosfamide
(SCLC)
Science-Driven Oncology Portfolio
IND
Compound
Pre Clinical
Phase 1
Phase 2 |
 IL-12 Program
Ad-RTS-IL-12
DC-RTS-IL-12
May 29, 2013 |
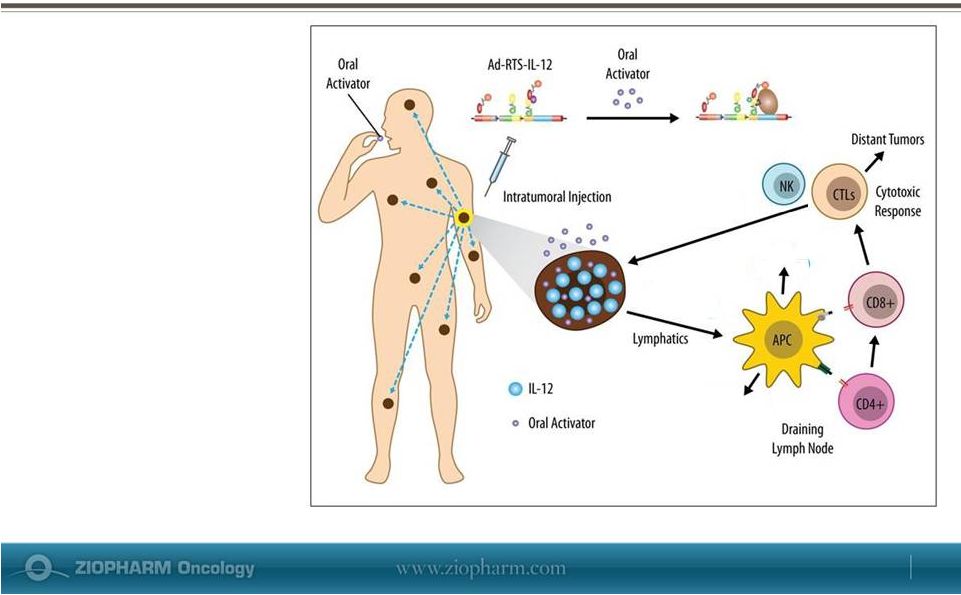 May 29, 2013 Ad-RTS-IL-12
•
Interleukin-12 (IL-12) is
a potent, naturally
occurring anti-cancer
protein central to
initiation and regulation
of cellular anti-cancer
immune responses
•
Regulated intratumoral
expression of IL-12
promotes activation of
TIL’s to drive a cytotoxic
immune response
against distant tumors |
 May 29, 2013 Increases CD8
+
TILs in the 4T1 Syngeneic Mouse Model
Vehicle
INXN-1001
150
mg/m
2
+
Ad-RTS-mIL-12
1x10
10
vp |
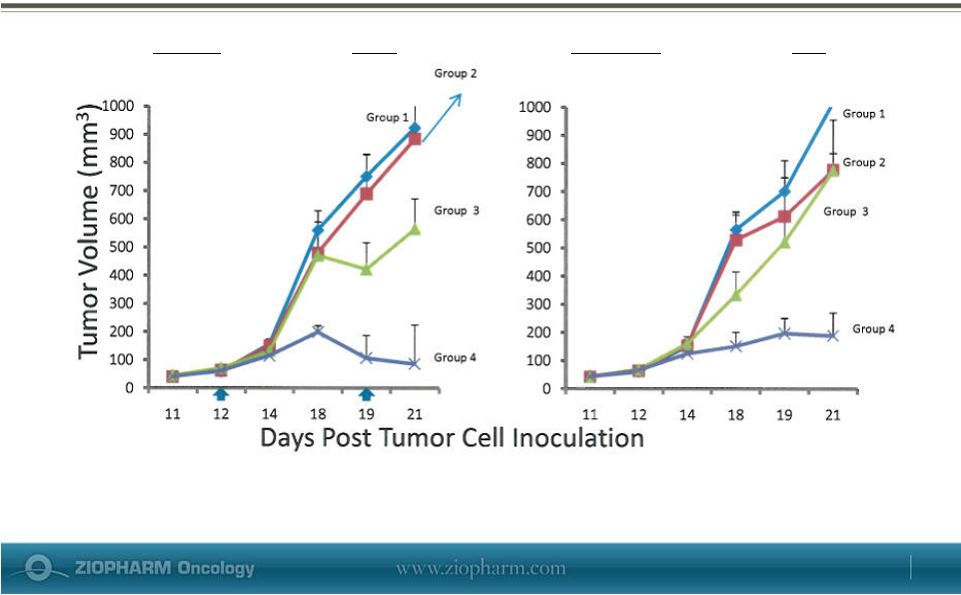 May 29, 2013 Systemic Tumor Response in B16 Melanoma Model
Group
1
untreated;
Group
2
AL
in
food
~675
mg/m
2
/day;
Group
3
Ad-RTS-mIL-12
1x10
10
vp
Days 12 & 19; Group 4 AL + Ad-RTS-mIL-12. Arrows =
administration of Ad-RTS-mIL-12 Treated
Tumor
on
the
Right
Flank
Untreated
Tumor
on
the
Left
Flank |
 May 29, 2013 A Phase I/II, Open Label Study of Ad-RTS-hIL-12 + AL in
Subjects with Unresectable Stage III or IV Melanoma
•
3 + 3, single-arm design
•
Primary endpoint –
Safety and tolerability of intratumoral injections of
1012 vp
Ad-RTS-hIL-12 in combination with escalating doses of
INXN-1001
•
Secondary endpoint –
Inform the selection of a dose of INXN-1001
•
Phase I dose escalation complete
•
Phase II ongoing (preliminary data 1H 2014)
5mg
(INXN-1001)
20mg
(INXN-1001)
100mg
(INXN-1001)
160mg
(INXN-1001)
160mg
(INXN-1001)
12 subjects in Phase I (dose escalation)
Up to 15 subjects in Phase 2 (expansion) |
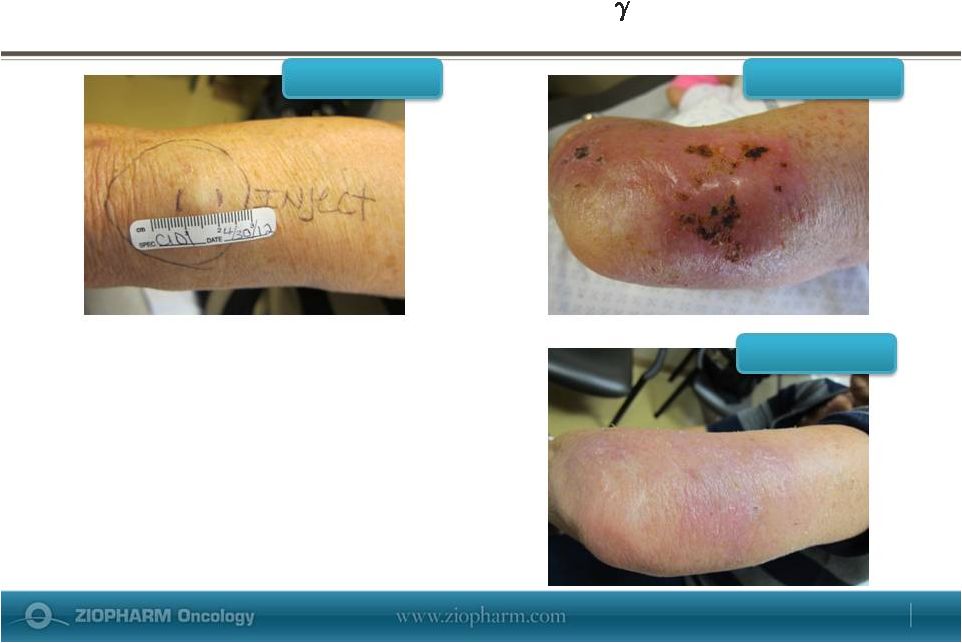 Prominent Inflammatory Response Correlates with
High levels of IFN-
•
Initial increase in lesion size due to
inflammatory response seen at Cycle 1
Day 16
•
Lesion was undetectable at Cycle 2 Day 1
•
Subject ultimately progressed and was
taken off study
C1D1
C1D1
C1D16
C1D16
C2D1
C2D1
May 29, 2013 |
 •
Clinical activity at 100 mg and 160 mg doses coincided with the highest
serum levels of IL-12 and IFN-
Summary of Immunological and Clinical Activity
–
Prominent inflammatory responses in injected and non-injected
lesions
–
Decrease in size of injected and non-injected lesions
•
Clinical activity at higher dose cohorts (5 of 7, 71%)
–
4-fold median increases from baseline at peak levels compared with
lower dose cohorts
•
Dose-dependent increase in serum levels of IL-12 and IFN-
•
Flow cytometric analyses of PBMCs revealed
–
7-fold and 4-fold median increase from baseline at peak levels in
absolute CD3+ and CD8+ T cell values, respectively, compared with
lower dose cohorts
May 29, 2013 |
 Summary of Safety
May 29, 2013
•
Controlled expression of IL-12 limits systemic toxicity while inducing
biological and clinical activity in a dose-dependent fashion
–
Toxicity reversible on stopping oral activator ligand
•
The
most
common
related
TEAEs
( 20%
of
subjects):
•
Chills and Pyrexia (73% each), Nausea (67%), Fatigue (60%),
Vomiting (33%), Anorexia (27%), Arthralgia and Diarrhea (20%
each)
•
Toxicity profile is consistent with the MOA of the drug
•
No DLT identified
•
One
unrelated
death
secondary
to
septicemia |
 •
Preclinical
–
Intratumoral administration of Ad-RTS-mIL-12 (Ad) in 4T1 BALB/c
mouse breast carcinoma model: dose-related decrease in tumor growth
rate. (AACR 2013)
–
Therapeutic strategy appears to be well tolerated
•
Phase 2
–
Multi-center, randomized, open-label
–
Ad-RTS IL-12 in combination w/palifosfamide
–
Non-resectable, recurrent or metastatic breast cancer
–
Enrolling up to 68 patients
–
Early data expected year end 2013
Advanced Breast Cancer
May 29, 2013 |
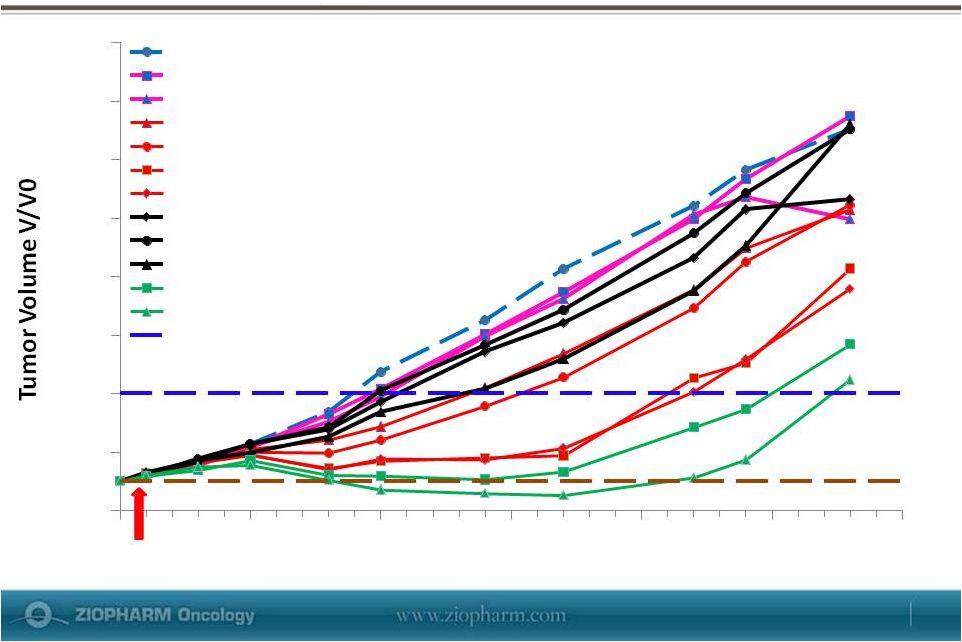 Dose-dependent Anti-Tumor Activity of in
Murine 4T1 (Breast Cancer) Model
4
2
0
6
8
10
12
14
16
5
10
15
Time (Days)
20
25
30
0
Vehicle Control
Ad-RTS-mIL12 (AD)
INXN-1001 (AL) 15 mg/m2
AD + AL 15 mg/m2
AD + AL 30 mg/m2
AD + AL 75 mg/m2
AD + AL 150
mg/m2 IPM 40
mg/m2
IPM 80 mg/m2
IPM 120 mg/m2
AD + AL 30 mg/m2 + IPM 40mg/m2
AD + AL 30 mg/m2 + IPM 120
mg/m2
Tumor Size Quadruple
Start of Treatment
May 29, 2013 |
 Glioblastoma Multiforme: Promising Preclinical Activity
100 % survival observed with Ad-RTS-IL-12 + AL or
DC-RTS-IL-12 + AL INXN-1001 dosing Day 4 to EOS at ~ 675
mg/m²/day in chow; DC –RTS-IL12 or Ad-RTS-IL12 on Day 5
Kaplan
Meier
Survival
in
GL261
Orthotopic
Syngeneic
Mouse
Glioma
Model
0
10
20
30
40
50
60
70
80
0
20
40
60
80
100
No Treatment
DC-no vector
DC-RTS-IL12 (MOI 10000)
Ad-RTS-IL12 (5x10
9
)
AL chow
Ad-RTS-IL12 (5x10
9
) + AL
DC-RTS-IL12 (MOI 10000) + AL
Time (Days)
May 29, 2013 |
 May 29, 2013
Preclinical and
Discovery
Programs |
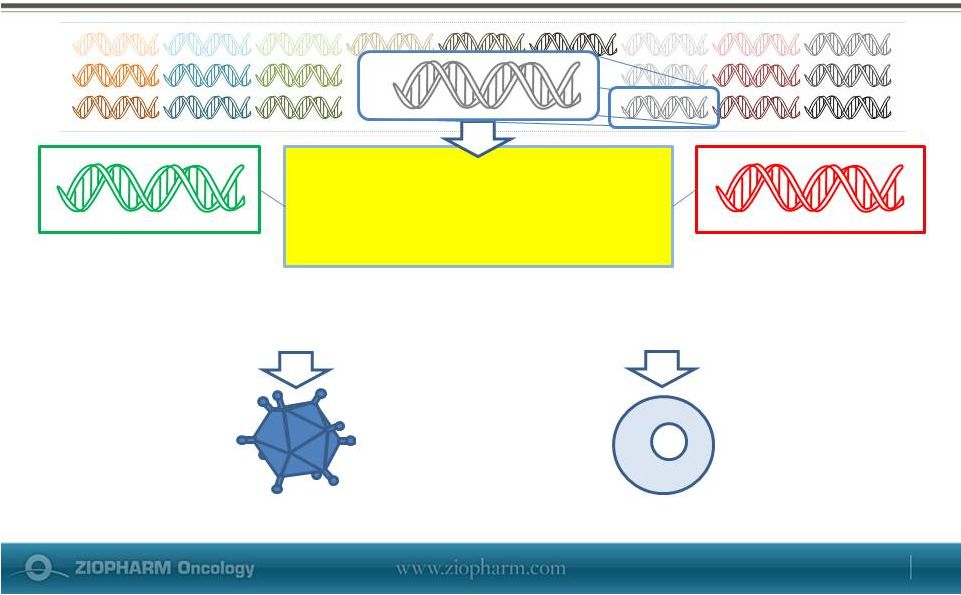 A
Platform System for Rapidly Developing Controllable DNA Therapies
Inventoried DNA modules
Monogenic
Multigenic
Viral delivery vector
RheoSwitch
Therapeutic System®
3’
REG
Target Gene(s)
for Expression
Intramuscular Cell Plasmid
May 29, 2013 |
 Using
Natural Cell Biology to Regulate Proteins May 29, 2013
Precision
Oral ligand activates
DNA, protein
production begins
Controlled
Therapeutic
proteins
Adaptable
Translation
mRNA
Target Cell |
 IP1:
LacZ
IP1:
LacZ
rLUC
rLUC
Stuffer
Stuffer
Stuffer
Stuffer
Stuffer
Stuffer
SP-RTS
SP-RTS
IP2:
fLuc
IP2:
fLuc
rLUC
rLUC
Stuffer
Stuffer
Stuffer
Stuffer
Stuffer
Stuffer
SP-RTS
SP-RTS
IP3:
SEAP
IP3:
SEAP
rLUC
rLUC
Stuffer
Stuffer
Stuffer
Stuffer
Stuffer
Stuffer
SP-RTS
SP-RTS
IP4:
GUS
IP4:
GUS
rLUC
rLUC
Stuffer
Stuffer
Stuffer
Stuffer
Stuffer
Stuffer
SP-RTS
SP-RTS
IP4:
GUS
IP4:
GUS
rLUC
rLUC
SP-RTS
SP-RTS
IP1:
LacZ
IP1:
LacZ
IP2:
fLuc
IP2:
fLuc
IP3:
SEAP
IP3:
SEAP
#: Not significantly different than
background (p>0.05)
*: Not significantly different than
the control (p>0.05)
Next Generation: Multigenic Approach
0.0
1.0
2.0
3.0
4.0
5.0
6.0
7.0
8.0
LacZ/rLUC Uninduced
SEAP/rLUC Uninduced
LacZ/rLUC Induced
SEAP/rLUC Induced
fLUC/rLUC Uninduced
GUS/rLUC Uninduced
fLUC/rLUC Induced
GUS/rLUC Induced
Conclusion:
4 Inducible gene programs can be placed in parallel on the
same vector without affecting gene program performance
May 29, 2013 |
 Small Molecule
Programs
May 29, 2013 |
 Small
Molecule Programs Palifosfamide
Bi-functional DNA alkylating agent that has activity in multiple tumors by
evading typical resistance pathways, less toxic and ease of
administration Phase
3
soft
tissue
sarcoma
program
terminated,
ongoing
adaptive
Phase
3
in
small
cell lung
Indibulin
Novel oral tubulin binding agent; expected low toxicity, neurotoxicity not
seen Ongoing Phase 1/2 study in metastatic breast cancer
Darinaparsin
Novel
mitochondrial-
and
hedgehog-targeted
agent
(organic
arsenic);
oral
and
IV
Ongoing studies in partnership with Solasia
May 29, 2013 |
 Expected Milestones
Program
Milestone
Timing
2013
IL-12
Melanoma preliminary Phase 2 data
4Q
Breast cancer preliminary Phase 2 data
4Q
GBM preclinical proof of concept
4Q
Multigenic platforms
Preclinical data
4Q
Immunotherapy
Programs
Preclinical data
4Q
2014
Palifosfamide
Interim SCLC data (MATISSE)
1H
IL-12
GBM Phase 1 / 2 study
initiation 1H
Melanoma Phase 2 data
1H
Breast Phase 2 data
2H
May 29, 2013 |
 •
Primary shares outstanding: approximately 82.9M
•
Cash: approximately $55.7M @ 3/31/13
•
Current cash resources expected to support operations
into 1Q 2014
Financial Highlights
May 29, 2013 |
 •
Paradigm-shifting, synthetic biology technology for precise,
controlled delivery of therapeutic proteins in vivo
•
Engineered approach to product design allows us to rapidly develop
new genetically-based treatments for cancer with multiple effectors
•
Focused, disciplined and iterative approach to development
•
Lead therapeutic Ad-RTS-IL-12 in Phase 2 melanoma and breast
cancer for early validation of target and platform
•
“Next-wave”
of therapeutic approaches in research pipeline
(antibody technology, protein-protein technology, immunotoxins,
etc.)
•
Well capitalized through data inflection points
Conclusion
May 29, 2013 |
 Better Cancer Medicine
Deutsche Bank 38
Annual Healthcare Conference
Jonathan Lewis, MD, PhD
Chief Executive Officer
th
May 29, 2013 |
