Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a13-12528_38k.htm |
| EX-99.4 - EX-99.4 - Innoviva, Inc. | a13-12528_3ex99d4.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a13-12528_3ex99d1.htm |
| EX-99.3 - EX-99.3 - Innoviva, Inc. | a13-12528_3ex99d3.htm |
Exhibit 99.2
POSTER NO. G38
Efficacy of fluticasone furoate (FF)/vilanterol (VI) on lung function in COPD: a pre-specified subgroup analysis
Martinez F(1), Midwinter DA(2), Lettis S(2), Scott-Wilson C(3), Crim CJ(3), Kerwin EM(4)
(1)University of Michigan, Ann Arbor, MI, USA; (2)GlaxoSmithKline, Uxbridge, UK; (3)GlaxoSmithKline, Research Triangle Park, NC, USA; (4)Clinical Research Institute of Southern Oregon, Medford, OR, USA
INTRODUCTION
· FF/VI is a novel ICS/LABA therapy demonstrated to improve lung function in COPD patients when administered once daily at various strengths.(1),(2)
OBJECTIVE
· This pre-specified analysis investigated the effect of FF/VI on lung function from two 24-week studies(1),(2) in seven pre-specified subgroups.
METHODS
· Data from two phase III, multi-center, randomized, double-blind, parallel-group, 24-week studies were pooled.
· FF/VI at strengths of 50/25, 100/25 and 200/25mcg or placebo was dosed once daily in the morning via ELLIPTA™ dry powder inhaler. Other treatment arms (VI 25mcg, FF 100 and 200mcg) are not shown.
· Patients: >40 years of age; smoking history >10 pack-years; post-bronchodilator FEV1/FVC ratio <0.70; post-bronchodilator FEV1 <70% predicted; score of >2 on the modified Medical Research Council Dyspnea scale. Patients were not utilizing ICS or ICS/LABA medications within 4 weeks of study entry, and were not utilizing any LAMA within 1 week and/or any LABA within 48 hours of study entry.
· The present analysis describes the co-primary endpoint of trough FEV1 (23–24h post-dose) on Day 169; weighted mean FEV1 (0–4h) on Day 168 was the other co-primary endpoint but is not described here.
· The pre-specified subgroups were: gender; age; reversibility (>12% and 200mL increase in FEV1 post-albuterol/salbutamol); smoking status (former vs. current); patient-reported cardiovascular (CV) history/risk (current or past medical history of >1 of the following: arrhythmia, cerebrovascular accident, congestive heart failure, coronary artery disease, diabetes mellitus, hypercholesterolemia, hypertension, myocardial infarction); geographic region; race.
RESULTS
Study population and demographics
· 2254 patients were randomized and received at least one dose of study medication (intent-to-treat [ITT] population); 1647 patients completed the studies. Data for 1233 patients are reported here (data for patients in FF and VI monotherapy arms not shown).
Table 1. Patient demographics and screening characteristics (pooled ITT population)
|
|
|
|
|
FF/VI |
|
FF/VI |
|
FF/VI |
|
|
|
|
Placebo |
|
50/25 |
|
100/25 |
|
200/25 |
|
|
|
|
(N=412) |
|
(N=206) |
|
(N=410) |
|
(N=205) |
|
|
Age, years |
|
62.0 (8.47) |
|
62.8 (9.13) |
|
62.1 (8.63) |
|
61.1 (8.67) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Male sex, n (%) |
|
293 (71) |
|
135 (66) |
|
281 (69) |
|
137 (67) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current smoker, n (%) |
|
220 (53) |
|
111 (54) |
|
220 (54) |
|
112 (55) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Smoking history, pack-years |
|
45.7 (25.4) |
|
44.2 (25.4) |
|
44.7 (24.6) |
|
41.5 (23.4) |
|
|
|
|
|
|
|
|
|
|
|
|
|
CV risk subgroup: yes, n (%) |
|
257 (62) |
|
127 (62) |
|
239 (58) |
|
126 (61) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Reversible subgroup: yes, n (%) |
|
138 (34) |
|
73 (36) |
|
124 (31) |
|
54 (27) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Pre-bronchodilator FEV1, L |
|
1.31 (0.45) |
|
1.23 (0.47) |
|
1.30 (0.51) |
|
1.33 (0.50) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Post-bronchodilator FEV1, L |
|
1.48 (0.47) |
|
1.41 (0.50) |
|
1.45 (0.51) |
|
1.46 (0.51) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Percent predicted* post-bronchodilator FEV1, % |
|
48.4 (12.6) |
|
48.4 (12.7) |
|
48.0 (12.6) |
|
47.1 (12.8) |
|
Values are mean (SD) unless otherwise stated
*Reference values were those of NHANES III
RESULTS (cont.)
Efficacy
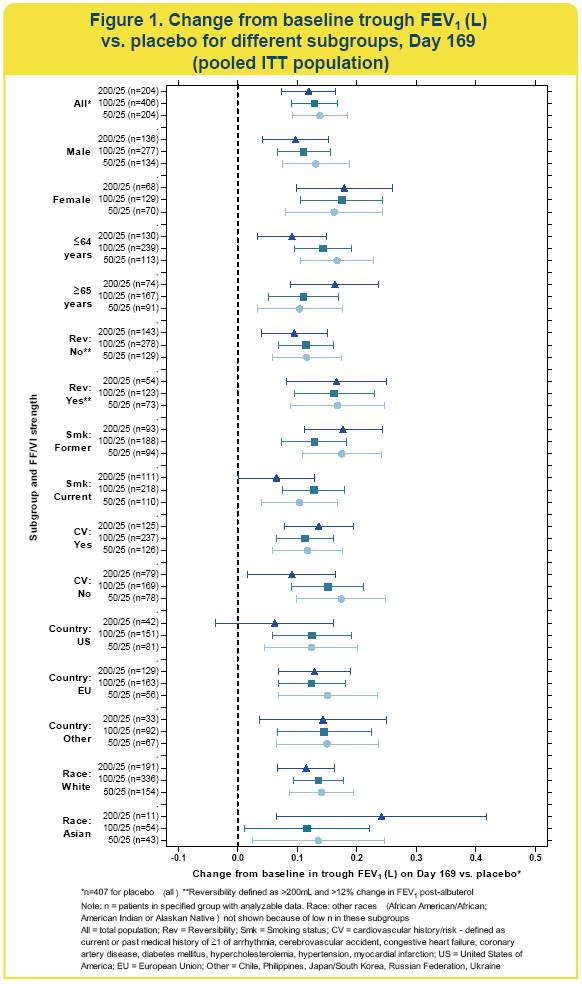
· FF/VI significantly improved trough FEV1 compared with placebo in most subgroups (Figure 1)
· because of the small sample size in the Asian race subgroup, the confidence intervals for treatment comparisons in this subgroup were very wide.
· In all subgroups, FF/VI strengths of 50/25 and 100/25 improved mean trough FEV1 by 100mL or greater compared with placebo.
· Compared with the placebo group, there were numerically larger improvements in Day 169 trough FEV1 in reversible patients (~160mL) compared with non-reversible patients (~100mL).
· In most subgroups, FF/VI 200/25 showed no additional numerical improvement in trough FEV1 compared with FF/VI 100/25. Improvements with FF/VI 50/25 were similar to those with FF/VI 100/25.
Safety
· The most frequently reported adverse events (AEs) were nasopharyngitis, headache, upper respiratory tract infections and candidiasis.
· Candidiasis (including candidiasis, oral candidiasis, oropharyngeal candidiasis and oropharyngitis fungal preferred terms) occurred in 4–10% of patients receiving active treatment compared with 2% receiving placebo.
· Incidence of nasopharyngitis (6–9%) and headache (5–7%) was similar across all treatment groups.
· Upper respiratory tract infections (preferred term) were less frequent in patients receiving FF/VI 200/25mcg or placebo (3%) than in those receiving FF/VI 50/25 or 100/25mcg (7–8%).
· Lower respiratory tract infections (preferred term) were less frequent with active treatment (<1–1%) than placebo (3%).
· On-treatment AEs deemed to be drug related were reported in 12%, 10%, 9% and 8% of patients receiving FF/VI 50/25mcg, 100/25mcg, 200/25mcg and placebo, respectively.
· The AE profile did not differ markedly between the ITT population and any specific subgroup (data not shown).
CONCLUSIONS
· FF/VI once daily at strengths of 50/25 and 100/25mcg improved Day 169 mean trough FEV1 by 100mL or greater compared with placebo, in all subgroups shown. No further benefit was apparent with FF/VI 200/25mcg.
· None of the baseline characteristics had a notable effect on the efficacy of FF/VI over placebo with respect to trough FEV1.
· All treatment groups exhibited a broadly similar AE profile.
REFERENCES
(1) Kerwin EM et al. Respir Med 2013;107:560–9.
(2) Martinez F et al. Respir Med 2013;107:550–9.
ACKNOWLEDGMENTS
· The presenting author, Fernando Martinez, declares the following real or perceived conflicts of interest during the last 3 years in relation to this presentation: Dr. Martinez has participated in advisory boards covering COPD or IPF topics for Able Associates, Actelion, Almirall, Bayer, GSK, Ikaria, Janssen, Medlmmune, Merck, Pearl, Pfizer and Vertex. He has consulted on COPD or IPF topics for American Institute for Research, AstraZeneca, Bayer, Carden Jennings, Cardiomems, Grey Healthcare, HealthCare Research and Consulting, Janssen, Merion, Nycomed/Takeda, and Sudler and Hennessey. He has been a member of steering committees for studies sponsored by Actelion, Centocor, Forest, GlaxoSmithKline, Gilead, Mpex, Nycomed/Takeda. He has participated in Food and Drug Administration mock panels for Boehringer Ingelheim, Forest and GSK. The University of Michigan received funds from the National Institutes of Health for COPD and IPF studies. He has served on speaker’s bureaus or in continuing medical education activities sponsored by American College of Chest Physicians, American Lung Association, Astra Zeneca, Bayer, William Beaumont Hospital, Boehringer Ingelheim, Center for Health Care Education, CME Incite, Forest, France Foundation, GlaxoSmithKline, Lovelace, MedEd, MedScape/WebMD, National Association for Continuing Education, Network for Continuing Medical Education, Nycomed/Takeda, Projects in Knowledge, St Luke’s Hospital, the University of Illinois Chicago, University of Texas Southwestern, University of Virginia, UpToDate. He has served on DSMBs for Biogen and Novartis. He has received royalties from Castle Connolly and Informa. Both studies were funded by GlaxoSmithKline; GSK study codes HZC112206 and HZC112207; Clinicaltrials.gov NCT01053988 and NCT01054885, respectively.
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Ian Grieve, PhD and Vikas Sharma, PhD at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GlaxoSmithKline.
ELLIPTA™ is a trade mark of GlaxoSmithKline

Presented at the American Thoracic Society Annual Congress, Philadelphia, PA, USA, 17–22 May 2013

