Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a13-10598_18k.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a13-10598_1ex99d2.htm |
Exhibit 99.1
|
|
April 25, 2013 NASDAQ: THRX Theravance Medicines that Make a Difference® Investor Presentation THERAVANCE®, the Theravance logo, and MEDICINES THAT MAKE A DIFFERENCE® are registered trademarks of Theravance, Inc. © 2013 Theravance, Inc. All rights reserved. |
|
|
Safe Harbor This presentation contains certain “forward-looking” statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives and future events. Theravance intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. The words “may”, “will”, “should”, “could”, “would”, “plan”, “anticipate”, “believe”, “estimate”, “intend”, “goal,” “project”, “potential”, “designed,” “expect”, “consistent”, “support”, “target” and “promising” and similar expressions are intended to identify such forward-looking statements. Examples of such statements include statements relating to plans for executing the separation, the expected timing of the separation, expectations for the funding of Theravance Biopharma at the time of the separation, the possible tax effects of the separation, the strategies, plans and objectives of the two companies following the separation, the anticipated potential distributions by Royalty Management Co following the separation, expectations related to the staffing of the two companies, the status and timing of clinical studies, data analysis and communication of results, statements regarding the potential benefits and mechanisms of action of drug candidates, statements concerning the timing of seeking regulatory approval of our product candidates, statements concerning the enabling capabilities of Theravance's approach to drug discovery and its proprietary insights, statements concerning expectations for the discovery, development and commercialization of product candidates and projections of revenue, expenses and other financial items. These statements are based on the current estimates and assumptions of the management of Theravance as of the date of this presentation and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance to be materially different from those reflected in its forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to delays in preparing audited financial statements for Theravance Biopharma, difficulties in effecting the registration of Theravance Biopharma as a public company, failure to obtain necessary consents from third parties, changes in the development or operations of Theravance prior to the separation that could affect the plans for the separation or the cash available for the initial funding of the independent companies, the receipt of a private letter ruling from the Internal Revenue Service (should Theravance seek to effect the transaction on a tax-free basis), the possibility that alternative transactions or opportunities could arise or be pursued which would alter the timing or advisability of, or the ability to consummate, the anticipated separation transaction, the potential that results of clinical or non-clinical studies indicate product candidates are unsafe or ineffective, delays or failure to achieve regulatory approvals for product candidates, and risks of collaborating with third parties to discover, develop and commercialize products. These and other risks are described in greater detail under the heading "Risk Factors" contained in Theravance’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (SEC) on February 26, 2013 and the risks discussed in our other period filings with SEC. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Theravance assumes no obligation to update its forward-looking statements. 2 |
|
|
Theravance Today Track record of success exploiting insights into multivalency 20+ development candidates discovered to-date, a number of which have advanced to mid-/late-stage development and one approved product Strong Financial Position: $558 million at March 31, 2013 Opportunity to unlock potential additional value 3 Three Late-Stage Respiratory Programs Partnered with GSK RELVAR™ or BREO™ ELLIPTA™, ANORO™ ELLIPTA™ and VI monotherapy Potential to enter large markets: COPD and Asthma Two programs filed with regulatory agencies: U.S. action dates expected in 2013 BREO™ ELLIPTA™ (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) are investigational medicines and are not currently approved anywhere in the world. RELVAR™, BREO™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of these brand names has not yet been approved by any regulatory authority. Global Commercialization resource Deep pipeline MABA, MABA/ICS, UMEC/VI/FF partnered with GSK |
|
|
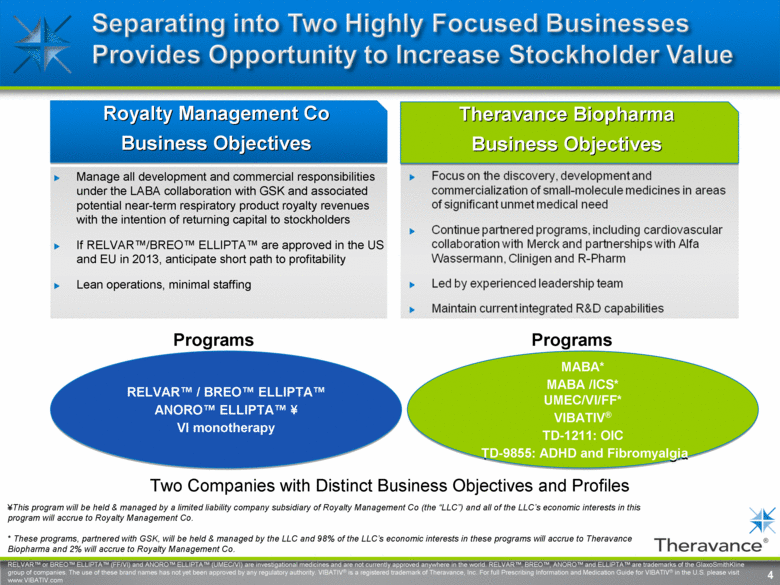
4 Theravance Biopharma Business Objectives Focus on the discovery, development and commercialization of small-molecule medicines in areas of significant unmet medical need Continue partnered programs, including cardiovascular collaboration with Merck and partnerships with Alfa Wassermann, Clinigen and R-Pharm Led by experienced leadership team Maintain current integrated R&D capabilities Royalty Management Co Business Objectives Manage all development and commercial responsibilities under the LABA collaboration with GSK and associated potential near-term respiratory product royalty revenues with the intention of returning capital to stockholders If RELVAR™/BREO™ ELLIPTA™ are approved in the US and EU in 2013, anticipate short path to profitability Lean operations, minimal staffing RELVAR™ / BREO™ ELLIPTA™ ANORO™ ELLIPTA™ ¥ VI monotherapy MABA* MABA /ICS* UMEC/VI/FF* VIBATIV® TD-1211: OIC TD-9855: ADHD and Fibromyalgia Programs Programs Two Companies with Distinct Business Objectives and Profiles 4 ¥This program will be held & managed by a limited liability company subsidiary of Royalty Management Co (the “LLC”) and all of the LLC’s economic interests in this program will accrue to Royalty Management Co. * These programs, partnered with GSK, will be held & managed by the LLC and 98% of the LLC’s economic interests in these programs will accrue to Theravance Biopharma and 2% will accrue to Royalty Management Co. RELVAR™ or BREO™ ELLIPTA™ (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) are investigational medicines and are not currently approved anywhere in the world. RELVAR™, BREO™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of these brand names has not yet been approved by any regulatory authority. VIBATIV® is a registered trademark of Theravance, Inc. For full Prescribing Information and Medication Guide for VIBATIV® in the U.S. please visit www.VIBATIV.com Separating into two highly focused businesses provided opportunity to increase stockholder value |
|
|
5 Objectives Continue to manage all development and commercial responsibilities under the LABA collaboration with GSK and associated potential near-term respiratory product royalty revenues Intend to distribute to stockholders a significant portion of any future royalty revenues less opex, debt service and taxes Efficient corporate structure: GSK pays all commercialization and R&D expenses related to partnered respiratory programs RELVAR™ or BREO™ ELLIPTA™ (FF/VI) GSK pays 15% royalty on first $3.0bn of annual WW net sales; 5% thereafter FDA PDUFA goal date of May 12, 2013 for COPD indication Potential EMA action in 2013 for asthma and COPD ANORO™ ELLIPTA™ (UMEC/VI) GSK pays upward tiering 6.5% to 10% royalties on WW net sales FDA PDUFA goal date of December 18, 2013 VI monotherapy GSK pays all commercialization and R&D expenses WW net sales of VI monotherapy are added to WW net sales of RELVAR™/BREO™ ELLIPTA™ for purposes of royalty calculations RELVAR™ or BREO™ ELLIPTA™ (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) are investigational medicines and are not currently approved anywhere in the world. RELVAR™, BREO™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of these brand names has not yet been approved by any regulatory authority. royalty managements co highlights potential regulatory events in 2013 and lean expense profile regulatory review underway in the EU |
|
|
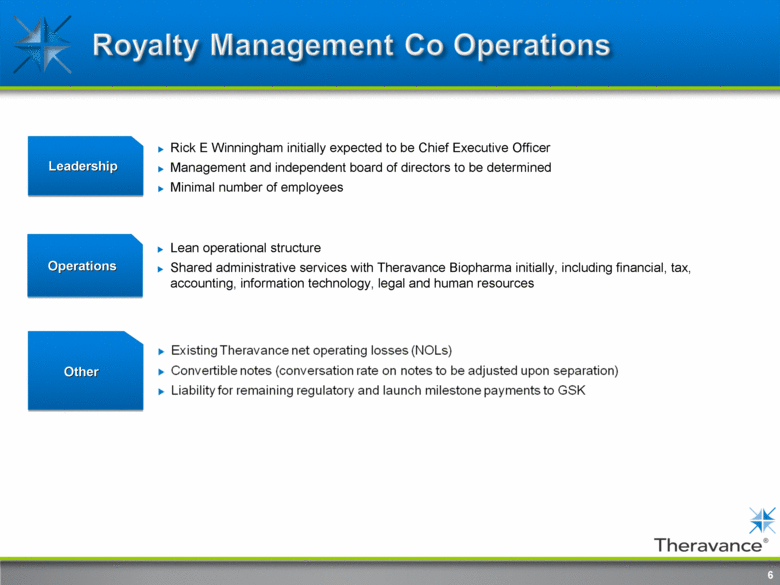
Royalty Management Co Operations 6 Leadership Rick E Winningham initially expected to be Chief Executive Officer Management and independent board of directors to be determined Minimal number of employees Operations Lean operational structure shared administrative services with Theravance Biopharma initially, including financial, tax, accounting, information technology, legal and human resources Other 6 Existing Theravance net operating losses (NOLs) Convertible notes (conversation rate on notes to be adjusted upon separation) Liability for remaining regulatory and launch milestone payments to GSK |
|
|
7 Objectives Economic Interest in MABA Dual bronchodilator muscarinic antagonist and beta2 agonist in a single molecule MABA ‘081 monotherapy expected to advance into Phase 3 in 2013 GSK pays royalty of 10% to 20% of WW net sales up to $3.5bn and 7.5% thereafter GSK pays all commercialization and R&D expenses Economic Interest in Triple Mechanism Combos MABA /ICS (‘081/FF) combination expected to advance to Phase 3-enabling studies in 2013 UMEC/VI/FF: Currently in Phase 1 safety (PK) study GSK pays all commercialization and R&D expenses TD-1211 Once-daily oral compound for opioid-induced constipation (OIC) with positive Phase 2b results Currently evaluating Phase 3 strategy TD-9855 Norepinephrine and serotonin reuptake inhibitor (NSRI) with long half-life and reduced serotonergic side effects Ongoing Phase 2 studies in ADHD and Fibromyalgia Additional Programs VIBATIV® approved in US for cSSSI due to Gram-positive pathogens, including MRSA Additional other programs including velusetrag in Phase 2 for gastroparesis Continue partnered programs, including cardiovascular collaboration with Merck and partnerships with Alfa Wassermann, Clinigen and R-Pharm 7 VIBATIV® is a registered trademark of Theravance, Inc. For full Prescribing Information and Medication Guide for VIBATIV® in the U.S. please visit www.VIBATIV.com Focus on mix of research- & development-stage programs and partnered partnerships for late-stage development candidates. Theravance Biopharma proven R&D capabilities and significant near-term catalysts |
|
|
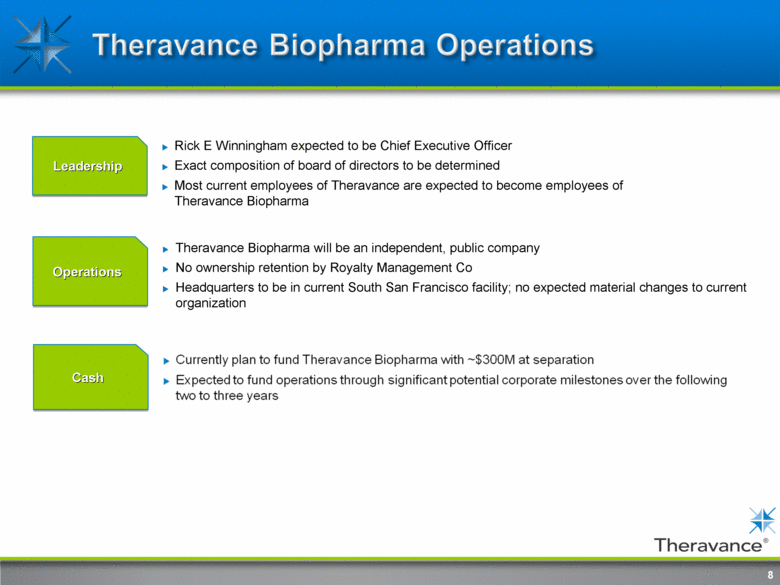
Theravance Biopharma operations 8 Leadership Rick E Winningham initially expected to be Chief Executive Officer Most current employees of Theravance are expected to become employees of Theravance Biopharma Cash Currently Plan to fund Theravance Biopharma with ~$300M at separation Expected to fund operations through significant potential corporate milestones over the following two to three years Operations Theravance Biopharma will be an independent, public company No ownership retention by Royalty Management Co Headquarters to be in current South San Francisco facility; no expected material changes to current organization 8 exact composition of board of directors to be determined |
|
|
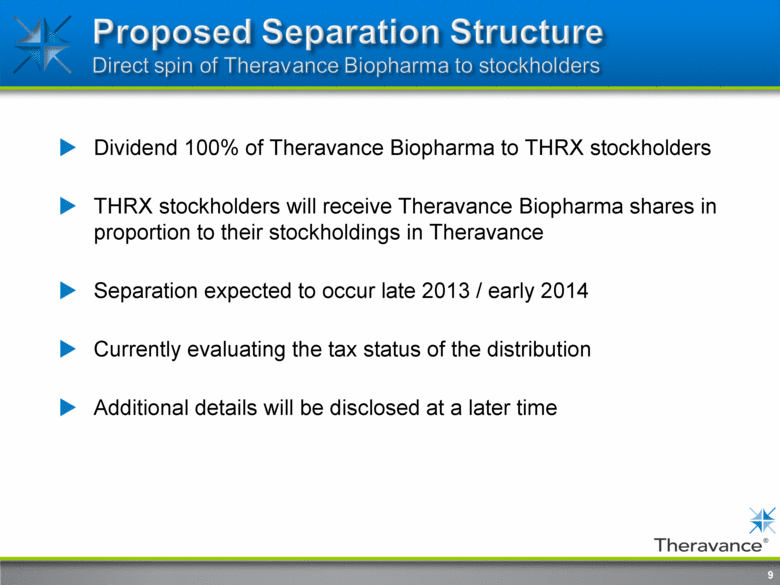
Proposed Separation Structure Direct spin of Theravance Biopharma to stockholders dividend 100% of Theravance Biopharma to THRX stockholders will receive Theravance Biopharma shares in proportion to their stockholdings in Theravance Separation expected to occur late 2013 / early 2014 Currently evaluating tax status of the distribution Additional details will be disclosed at a later time 9 |
|
|
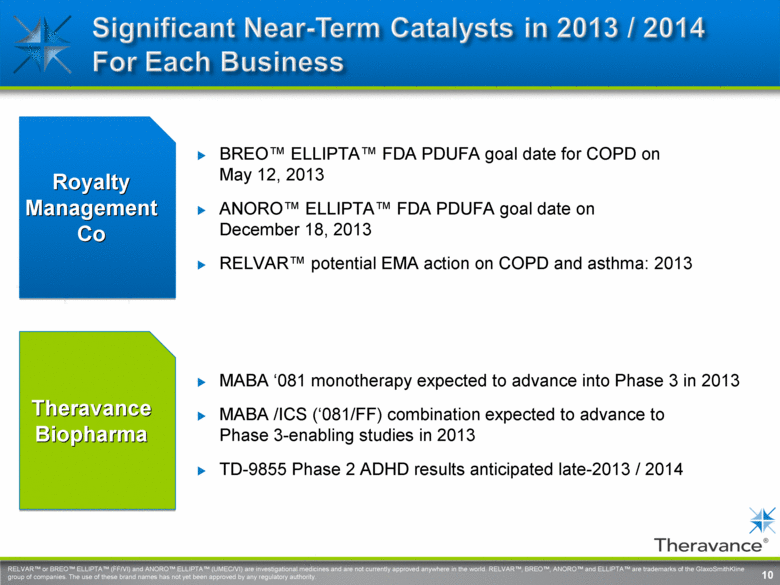
10 Theravance Biopharma MABA ‘081 monotherapy expected to advance into Phase 3 in 2013 MABA /ICS (‘081/FF) combination expected to advance to Phase 3-enabling studies in 2013 TD-9855 Phase 2 ADHD results anticipated late-2013 / 2014 Royalty Management Co BREO™ ELLIPTA™ FDA PDUFA goal date for COPD on May 12, 2013 ANORO™ ELLIPTA™ FDA PDUFA goal date on December 18, 2013 RELVAR™ potential EMA action on COPD and asthma: 2013 10 RELVAR™ or BREO™ ELLIPTA™ (FF/VI) and ANORO™ ELLIPTA™ (UMEC/VI) are investigational medicines and are not currently approved anywhere in the world. RELVAR™, BREO™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of these brand names has not yet been approved by any regulatory authority. Significant near-term catalysts in 2013 / 2014 for each business |
|
|
Theravance Medicines That make a Difference® THANK YOU NASDAQ: THRX THERAVANCE®, the Theravance logo, and MEDICINES THAT MAKE A DIFFERENCE® are registered trademarks of Theravance, Inc. © 2013 Theravance, Inc. All rights reserved. |