Attached files
| file | filename |
|---|---|
| 8-K/A - 8-K/A - Anacor Pharmaceuticals, Inc. | a13-9098_18ka.htm |
| EX-99.1 - EX-99.1 - Anacor Pharmaceuticals, Inc. | a13-9098_1ex99d1.htm |
Exhibit 99.2
|
|
Preliminary Results of Phase 2 Dose-Ranging Study of AN2728 in Adolescents with Mild-to-Moderate Atopic Dermatitis (AN2728-AD-204) March 21, 2013 |
|
|
Safe Harbor Statement Certain items in this presentation and other matters discussed today or answers that may be given to questions asked could constitute forward-looking statements, including statements regarding the progress and timing of clinical trials, the safety and efficacy of our product candidates, and estimates of the potential markets for our product candidates. Additional risks and uncertainties are described more fully in Anacor’s 10-K for the year ended December 31, 2012 filed with the Securities and Exchange Commission. These statements are subject to risks and uncertainties relating to Anacor’s future financial or business performance. Anacor’s actual results or achievements could differ materially from those anticipated in these forward-looking statements. Please note that Anacor is under no obligation to update any of the forward-looking statements discussed today, except as required by law. |
|
|
Atopic Dermatitis Overview David Perry CEO |
|
|
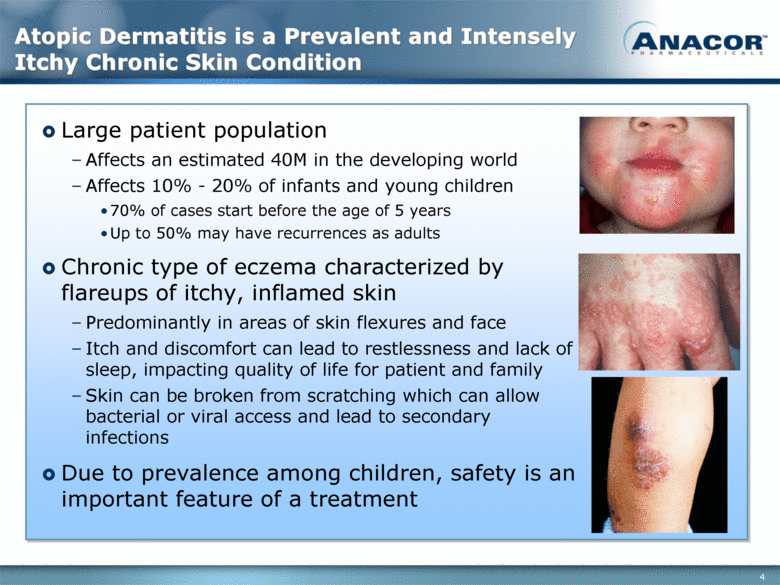
Atopic Dermatitis is a Prevalent and Intensely Itchy Chronic Skin Condition Large patient population Affects an estimated 40M in the developing world Affects 10% - 20% of infants and young children 70% of cases start before the age of 5 years Up to 50% may have recurrences as adults Chronic type of eczema characterized by flareups of itchy, inflamed skin Predominantly in areas of skin flexures and face Itch and discomfort can lead to restlessness and lack of sleep, impacting quality of life for patient and family Skin can be broken from scratching which can allow bacterial or viral access and lead to secondary infections Due to prevalence among children, safety is an important feature of a treatment 4 |
|
|
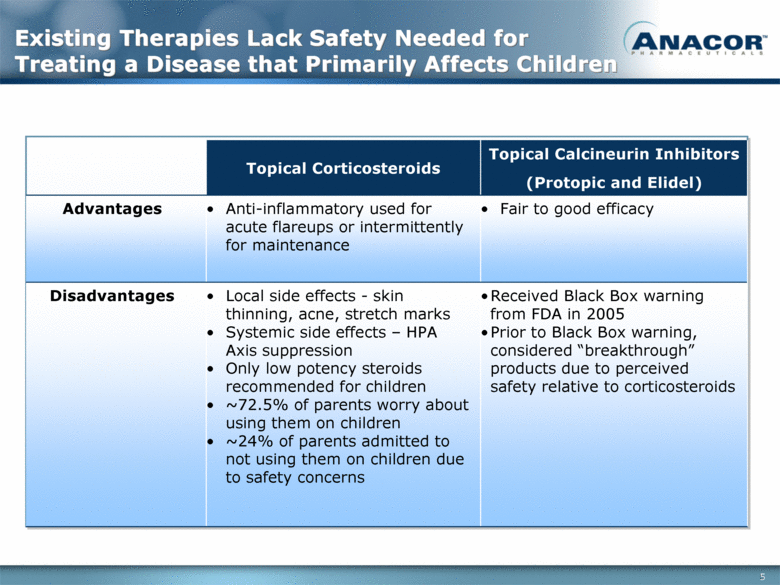
Existing Therapies Lack Safety Needed for Treating a Disease that Primarily Affects Children Topical Corticosteroids Topical Calcineurin Inhibitors (Protopic and Elidel) Advantages Anti-inflammatory used for acute flareups or intermittently for maintenance Fair to good efficacy Disadvantages Local side effects - skin thinning, acne, stretch marks Systemic side effects – HPA Axis suppression Only low potency steroids recommended for children ~72.5% of parents worry about using them on children ~24% of parents admitted to not using them on children due to safety concerns Received Black Box warning from FDA in 2005 Prior to Black Box warning, considered “breakthrough” products due to perceived safety relative to corticosteroids 5 |
|
|
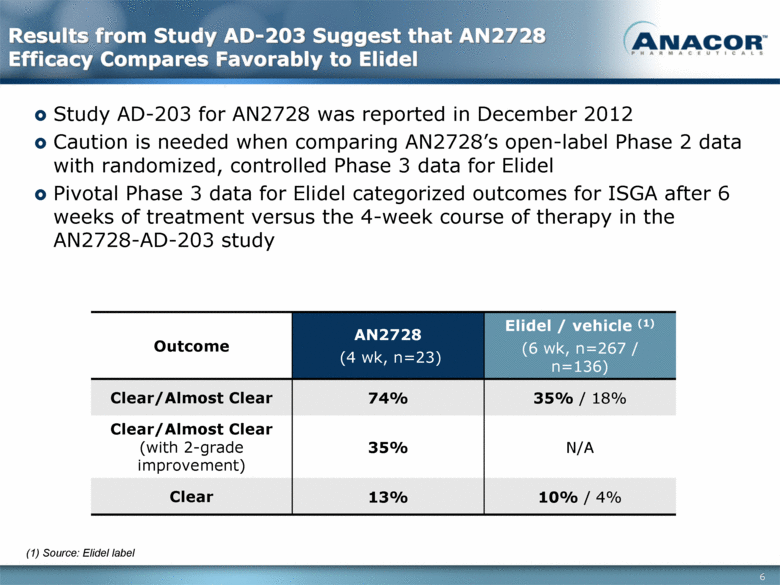
Results from Study AD-203 Suggest that AN2728 Efficacy Compares Favorably to Elidel Study AD-203 for AN2728 was reported in December 2012 Caution is needed when comparing AN2728’s open-label Phase 2 data with randomized, controlled Phase 3 data for Elidel Pivotal Phase 3 data for Elidel categorized outcomes for ISGA after 6 weeks of treatment versus the 4-week course of therapy in the AN2728-AD-203 study Outcome AN2728 (4 wk, n=23) Elidel / vehicle (1) (6 wk, n=267 / n=136) Clear/Almost Clear 74% 35% / 18% Clear/Almost Clear (with 2-grade improvement) 35% N/A Clear 13% 10% / 4% (1) Source: Elidel label 6 |
|
|
Summary of Preliminary Data for AN2728 Phase 2 Dose-Ranging Study in Atopic Dermatitis (AN2728-AD-204) Lee Zane, MD, MAS Vice-President, Clinical Development 7 |
|
|
Previously Completed Phase 2 Studies of AN2728 in Atopic Dermatitis AD-202 Completed in December 2011 Human proof of concept trial in adults Randomized, double-blind, vehicle controlled study Bilateral design treating one atopic dermatitis lesion with AN2728 ointment, 2% and treating a comparable lesion with ointment vehicle BID for 6 weeks AN2728 demonstrated statistically significant efficacy when compared to vehicle in this trial AD-203 Completed in December 2012 Open-label study in adolescents (ages 12-17) with mild-to-moderate atopic dermatitis involving 10% - 35% of treatable body surface area Patients applied AN2728 ointment, 2% to all treatable areas of atopic dermatitis AN2728 was generally safe and well-tolerated and led to substantial improvements in global disease severity as well as the individual signs and symptoms of atopic dermatitis 8 |
|
|
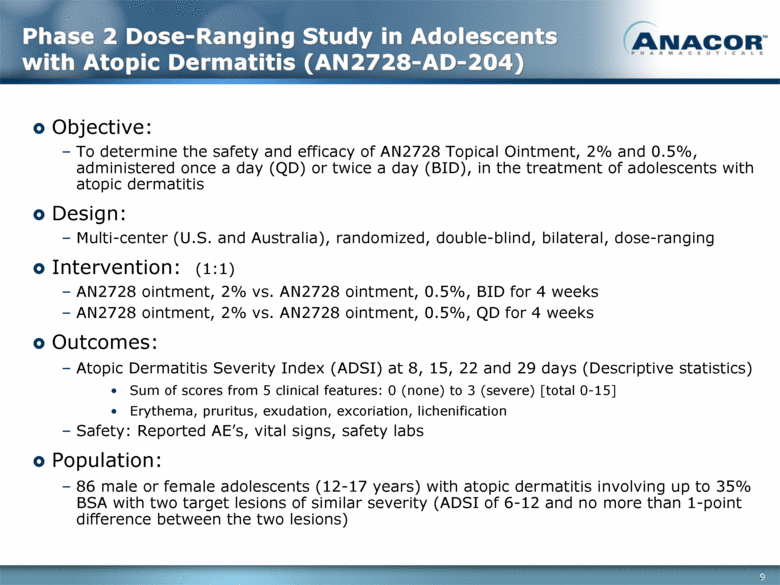
Phase 2 Dose-Ranging Study in Adolescents with Atopic Dermatitis (AN2728-AD-204) Objective: To determine the safety and efficacy of AN2728 Topical Ointment, 2% and 0.5%, administered once a day (QD) or twice a day (BID), in the treatment of adolescents with atopic dermatitis Design: Multi-center (U.S. and Australia), randomized, double-blind, bilateral, dose-ranging Intervention: (1:1) AN2728 ointment, 2% vs. AN2728 ointment, 0.5%, BID for 4 weeks AN2728 ointment, 2% vs. AN2728 ointment, 0.5%, QD for 4 weeks Outcomes: Atopic Dermatitis Severity Index (ADSI) at 8, 15, 22 and 29 days (Descriptive statistics) Sum of scores from 5 clinical features: 0 (none) to 3 (severe) [total 0-15] Erythema, pruritus, exudation, excoriation, lichenification Safety: Reported AE’s, vital signs, safety labs Population: 86 male or female adolescents (12-17 years) with atopic dermatitis involving up to 35% BSA with two target lesions of similar severity (ADSI of 6-12 and no more than 1-point difference between the two lesions) 9 |
|
|
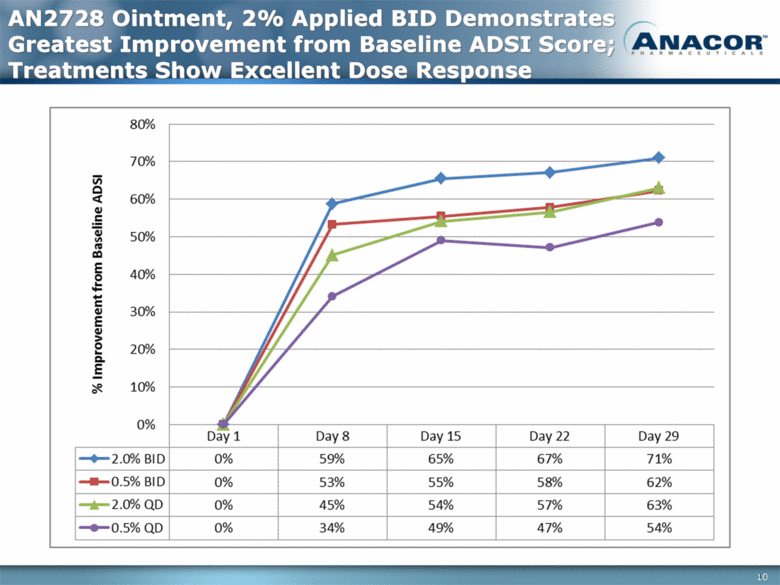
AN2728 Ointment, 2% Applied BID Demonstrates Greatest Improvement from Baseline ADSI Score; Treatments Show Excellent Dose Response 10 |
|
|
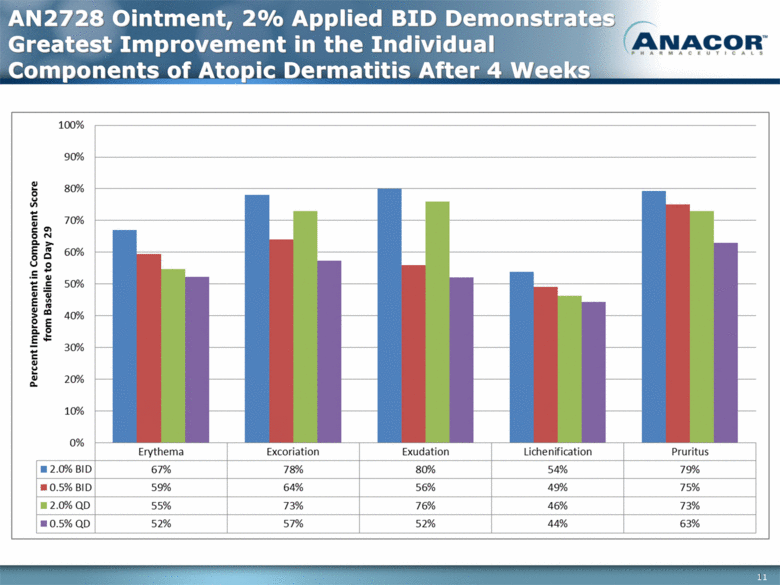
AN2728 Ointment, 2% Applied BID Demonstrates Greatest Improvement in the Individual Components of Atopic Dermatitis After 4 Weeks 11 |
|
|
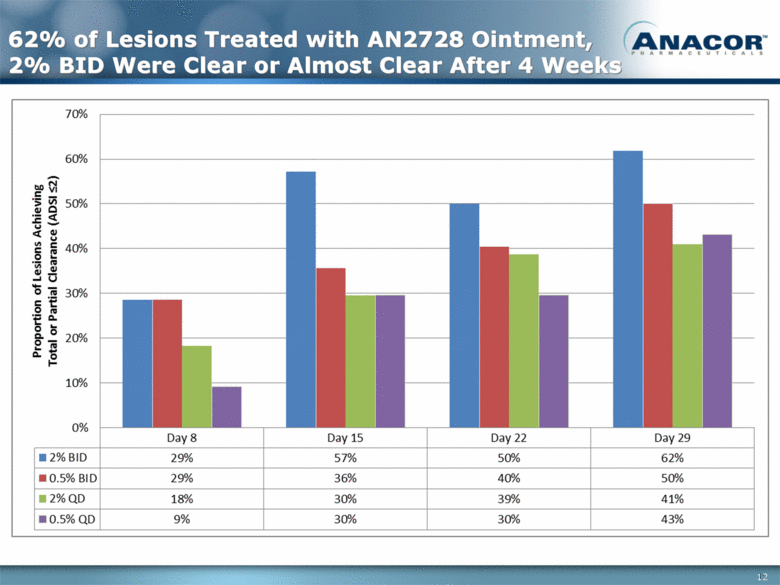
62% of Lesions Treated with AN2728 Ointment, 2% BID Were Clear or Almost Clear After 4 Weeks 12 |
|
|
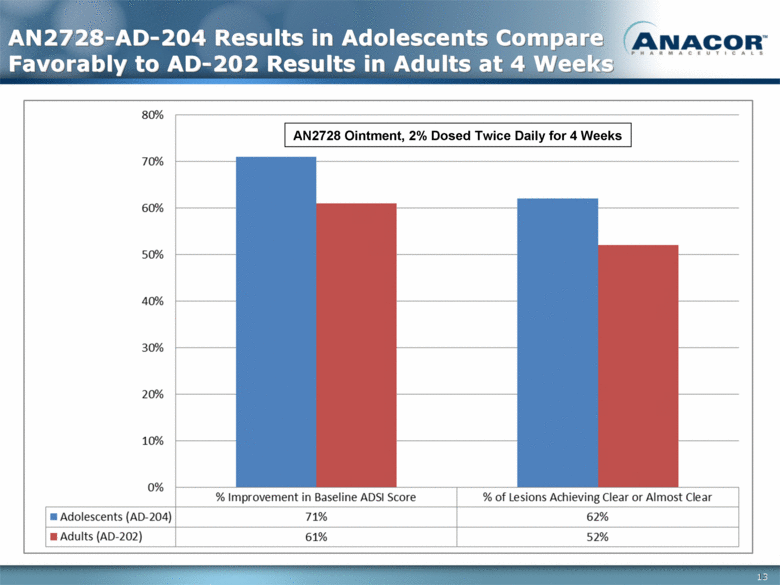
AN2728-AD-204 Results in Adolescents Compare Favorably to AD-202 Results in Adults at 4 Weeks AN2728 Ointment, 2% Dosed Twice Daily for 4 Weeks 13 |
|
|
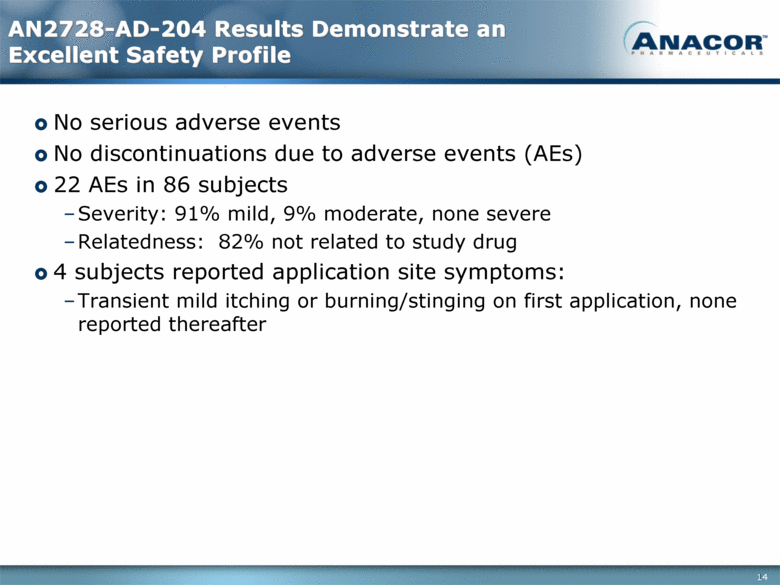
AN2728-AD-204 Results Demonstrate an Excellent Safety Profile No serious adverse events No discontinuations due to adverse events (AEs) 22 AEs in 86 subjects Severity: 91% mild, 9% moderate, none severe Relatedness: 82% not related to study drug 4 subjects reported application site symptoms: Transient mild itching or burning/stinging on first application, none reported thereafter 14 |
|
|
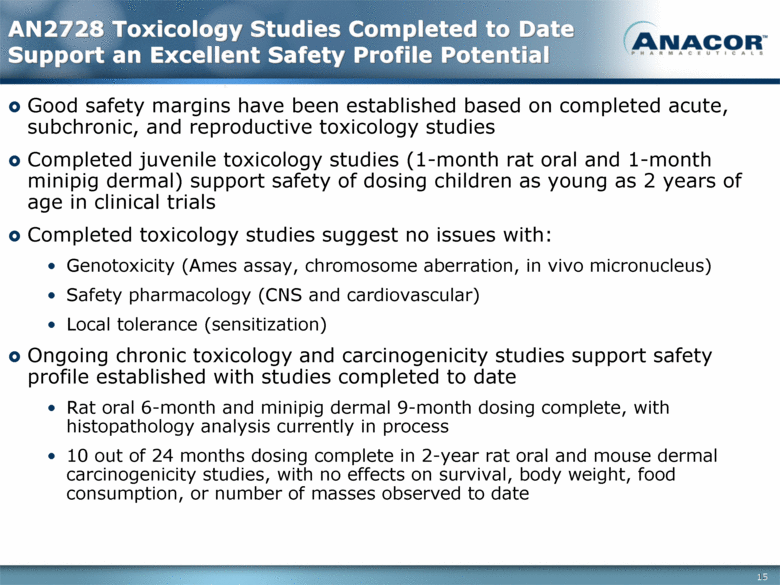
AN2728 Toxicology Studies Completed to Date Support an Excellent Safety Profile Potential Good safety margins have been established based on completed acute, subchronic, and reproductive toxicology studies Completed juvenile toxicology studies (1-month rat oral and 1-month minipig dermal) support safety of dosing children as young as 2 years of age in clinical trials Completed toxicology studies suggest no issues with: Genotoxicity (Ames assay, chromosome aberration, in vivo micronucleus) Safety pharmacology (CNS and cardiovascular) Local tolerance (sensitization) Ongoing chronic toxicology and carcinogenicity studies support safety profile established with studies completed to date Rat oral 6-month and minipig dermal 9-month dosing complete, with histopathology analysis currently in process 10 out of 24 months dosing complete in 2-year rat oral and mouse dermal carcinogenicity studies, with no effects on survival, body weight, food consumption, or number of masses observed to date 15 |
|
|
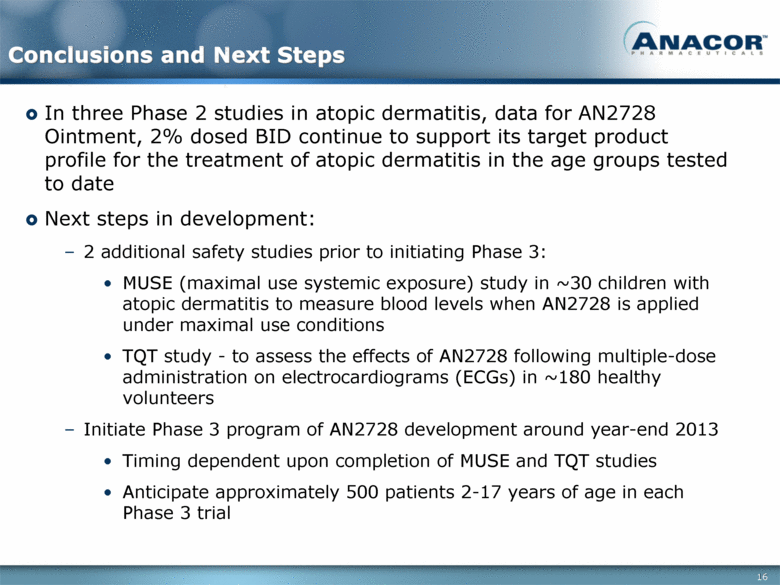
Conclusions and Next Steps In three Phase 2 studies in atopic dermatitis, data for AN2728 Ointment, 2% dosed BID continue to support its target product profile for the treatment of atopic dermatitis in the age groups tested to date Next steps in development: 2 additional safety studies prior to initiating Phase 3: MUSE (maximal use systemic exposure) study in ~30 children with atopic dermatitis to measure blood levels when AN2728 is applied under maximal use conditions TQT study - to assess the effects of AN2728 following multiple-dose administration on electrocardiograms (ECGs) in ~180 healthy volunteers Initiate Phase 3 program of AN2728 development around year-end 2013 Timing dependent upon completion of MUSE and TQT studies Anticipate approximately 500 patients 2-17 years of age in each Phase 3 trial 16 |
|
|
Q&A |