Attached files
| file | filename |
|---|---|
| EX-31.1 - EXHIBIT - LIPOSCIENCE INC | exhibit311certificationofp.htm |
| EX-10.35 - EXHIBIT - LIPOSCIENCE INC | exhibit1035amendedandresta.htm |
| EX-10.33 - EXHIBIT - LIPOSCIENCE INC | exhibit1033amendedandresta.htm |
| EX-10.32 - EXHIBIT - LIPOSCIENCE INC | exhibit1032amendedandresta.htm |
| EX-23.1 - EXHIBIT - LIPOSCIENCE INC | exhibit231consentofernstyo.htm |
| EX-10.34 - EXHIBIT - LIPOSCIENCE INC | exhibit1034amendedandresta.htm |
| EX-10.36 - EXHIBIT - LIPOSCIENCE INC | exhibit1036amendedandresta.htm |
| EX-10.38 - EXHIBIT - LIPOSCIENCE INC | exhibit10382012corporatego.htm |
| EX-31.2 - EXHIBIT - LIPOSCIENCE INC | exhibit312certificationofp.htm |
| EX-32 - EXHIBIT - LIPOSCIENCE INC | exhibit32certificationofpr.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF
THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2012 | Commission file number 001-35792 | |
LIPOSCIENCE, INC.
Incorporated under the Laws of the State of Delaware | I.R.S. Employer Identification No. 56-1879288 | |
2500 Sumner Boulevard
Raleigh, NC 27616
(919) 212-1999
Securities registered pursuant to Section 12(b) of the Exchange Act:
Title of each class | Name of each exchange on which registered | |
Common Stock, $0.001 par value | NASDAQ Global Market | |
Securities registered pursuant to Section 12(g) of the Exchange Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes o No ý
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulations S-K (§229.405 of this chapter) is not contained herein and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer o | Accelerated filer o | Non-accelerated filer x (Do not check if a smaller reporting company) | Smaller reporting company o | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of LipoScience, Inc. voting and non-voting common equity held by non-affiliates as of March 25, 2013, based on the closing sale price of $10.20 per share as reported on the NASDAQ Global Market on that date was $107,105,437. The registrant has provided this information as of March 25, 2013 because its common stock was not publicly traded as of the last business day of its most recently completed fiscal quarter.
At March 25, 2013, 14,668,203 shares of LipoScience, Inc. Common Stock, $0.001 par value, were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement, to be filed pursuant to Regulation 14A under the Securities Exchange Act of 1934, for its 2013 Annual Meeting of Stockholders are incorporated by reference in Part III of this Form 10-K.
1
SPECIAL NOTE REGARDING FORWARD‑LOOKING STATEMENTS
This Annual Report on Form 10-K (this “Annual Report”) contains “forward‑looking statements” within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, that involve substantial risks and uncertainties. The forward‑looking statements are contained principally in Part I, Item 1: “Business,” Part I, Item 1A: “Risk Factors,” and Part II, Item 7: “Management's Discussion and Analysis of Financial Condition and Results of Operations,” but are also contained elsewhere in this Annual Report. In some cases, you can identify forward‑looking statements by the words “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward‑looking statements. Although we believe that we have a reasonable basis for each forward‑looking statement contained in this Annual Report, we caution you that these statements are based on a combination of facts and factors currently known by us and our expectations of the future, about which we cannot be certain. All statements other than statements of historical fact could be deemed forward‑looking including but not limited to statements about:
• | our expectation that, for the foreseeable future, substantially all of our revenues will be derived from the NMR LipoProfile test; |
• | the future demand for our NMR LipoProfile test and future tests, if any, that we may develop; |
• | the factors that we believe drive demand for our NMR LipoProfile test and our ability to sustain such demand; |
• | the size of the market for our NMR LipoProfile test; |
• | our plans for the Vantera system and our expectations about deploying it on-site in third-party clinical diagnostic laboratories and the timing of its commercial availability; |
• | the potential clearance by the FDA of our HDL-P test pursuant to our 510(k) premarket notification and the timing thereof; |
• | the timing of our submissions of other non-cleared portions of our NMR LipoProfile test to the FDA for clearance; |
• | the potential impact resulting from any regulation of our NMR LipoProfile test or future tests, if any, that we may develop, by the FDA or any other regulation of our business or any regulatory proceedings to which we may be subject from time to time; |
• | our plans for pursuing coverage and reimbursement for our NMR LipoProfile test, and any changes in reimbursement affecting our business; |
• | the ability of our NMR LipoProfile test to impact treatment decisions; |
• | our plans for future diagnostic tests; |
• | the capacity of our laboratory to process our NMR LipoProfile test; |
• | our anticipated cash needs and our estimates regarding our capital requirements and our needs for additional financing; and |
• | the anticipated trends and challenges in our business and the market in which we operate. |
You should refer to “Item 1A. Risk Factors” in this Annual Report for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward‑looking statements. As a result of these factors, we cannot assure you that the forward‑looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward‑looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward‑looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. The forward-looking statements in this Annual Report represent our views as of the date of this Annual Report. Subsequent events and developments may cause our views to change. While we may elect to update these forward-looking statements at some point in the future, however, we undertake no obligation to publicly update any forward‑looking statements, whether as a result of new information, future events or otherwise, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this Annual Report.
2
TABLE OF CONTENTS
Page | |
PART I | |
PART II | |
PART III | |
PART IV | |
3
PART I
Item 1. Business
Overview
We are an in vitro diagnostic company pioneering a new field of personalized diagnostics based on nuclear magnetic resonance, or NMR, technology. Our first diagnostic test, the NMR LipoProfile test, directly measures the number of low density lipoprotein, or LDL, particles, also known as LDL-P, in a blood sample and provides physicians and their patients with actionable information to personalize management of risk for heart disease. To date, over 9 million NMR LipoProfile tests have been ordered, including nearly 2 million times during 2012. Our automated clinical analyzer, the Vantera system, was cleared by the U.S. Food and Drug Administration, or FDA, in August 2012 and became commercially available in December 2012. The Vantera system requires no previous knowledge of NMR technology to operate and has been designed to significantly simplify complex technology through ease of use and walk-away automation. We plan to selectively place the Vantera system on-site with national and regional clinical laboratories as well as leading medical centers and hospital outreach laboratories, which we believe will facilitate their ability to offer our NMR LipoProfile test and other diagnostic tests that we may develop. We are driving toward becoming a clinical standard of care by decentralizing our technology and expanding our menu of personalized diagnostic tests to address a broad range of cardiovascular, metabolic and other diseases.
Approximately 50% of people who suffer a heart attack have normal cholesterol levels. We believe that direct quantification of the number of LDL and other lipoprotein particles using our NMR-based technology platform addresses the deficiencies of traditional cholesterol testing and allows clinicians to more effectively manage their patients' risk of developing cardiovascular disease. We believe that the inherent analytical and clinical advantages of NMR-based technology, which can simultaneously analyze lipoproteins as well as hundreds of small molecule metabolites from blood serum, plasma and several other bodily fluids without time-consuming sample preparation, will also allow us to expand our diagnostic test menu. The scientific community is actively investigating our NMR-based technology for use in the prediction of diabetes, insulin resistance and other metabolic disorders, and we believe that our technology provides an attractive platform for potential expansion of the diagnostic tests we plan to offer into these areas.
Our strategy is to continue to advance patient care by converting clinicians, and the clinical diagnostic laboratories they use, from traditional cholesterol testing to our NMR LipoProfile test for the management of patients at risk for cardiovascular disease, with the goal of ultimately becoming a clinical standard of care. An increasing number of large clinical outcome studies, including the Multi-Ethnic Study of Atherosclerosis, or MESA, and the Framingham Offspring Study, indicate that a patient's number of LDL particles is more strongly associated with the risk of developing cardiovascular disease than is his or her level of LDL cholesterol when one of the measures suggests a higher risk and the other suggests a lower risk. LDL cholesterol, or LDL-C, is a measure of the amount of cholesterol contained in LDL particles and is used to estimate the patient's LDL level. LDL-P and LDL-C, both of which are alternative measures of LDL and its associated cardiovascular risk, are used clinically in the same manner to determine whether a patient has elevated LDL, potentially requiring treatment, and to monitor LDL-lowering treatment response over time. In the MESA and Framingham studies, which we believe clinically validate the performance of our test, participants' LDL-P levels were measured using our NMR LipoProfile test, while their LDL-C levels were measured using a traditional cholesterol test.
Because the NMR LipoProfile test provides direct quantification of the number of LDL particles, as well as additional measurements related to a patient's risk for developing cardiovascular disease, we believe that it has the potential to become a new paradigm by which clinicians evaluate key cardiovascular risk factors to provide better treatment recommendations and improve outcomes, even for patients considered to have normal levels of cholesterol. A 2008 joint consensus statement by the American Diabetes Association, or ADA, and the American College of Cardiology, or ACC, recognized that direct LDL particle measurement by NMR may be a more accurate way to capture the risk posed by LDL than is traditional LDL-C measurement. Additionally, in October 2011, the National Lipid Association, or NLA, convened an expert panel to evaluate the use of a number of biomarkers other than LDL-C, including LDL particle number, for initial clinical risk assessment of cardiovascular disease and ongoing management of cardiovascular disease risk in patients. The recommendations of this panel included:
• | for initial clinical risk assessment, the use of LDL particle number, as well as a number of the other non-LDL-C biomarkers, is reasonable for many patients considered to be at intermediate risk of coronary heart disease, patients with a family history of coronary heart disease and patients with recurrent cardiac events, and it should be considered for selected patients known to have coronary heart disease; and |
4
• | for ongoing management of risk, the use of LDL particle number, as well as some of the other biomarkers, is reasonable for many patients at intermediate risk, patients with known coronary heart disease and patients with recurrent cardiac events, and it should be considered for selected patients with a family history of coronary heart disease. |
The number of NMR LipoProfile tests ordered increased at a compound annual growth rate of approximately 28% from 2007 to 2012. The NMR LipoProfile test has its own dedicated Current Procedural Terminology, or CPT, code and is reimbursed by a number of governmental and private payors, which we believe collectively represent approximately 150 million covered lives. These payors include Medicare, TRICARE, WellPoint, United Healthcare and several Blue Cross Blue Shield affiliates.
In a number of states where we have targeted our sales and marketing efforts, we estimate that we have achieved market penetration rates of up to 11%. For example, in North Carolina, Alabama, West Virginia and Georgia we estimate that the number of NMR LipoProfile tests performed represented approximately 11%, 8%, 7% and 6%, respectively, of the total cholesterol tests performed in those states during 2012 for patient management purposes. We plan to significantly increase our geographic presence across the United States to expand market awareness and penetration of the NMR LipoProfile test, with the goal of ultimately becoming a clinical standard of care.
Our clinical laboratory, which is certified under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, allows us to fulfill current demand for our test and we believe serves as a strategic asset that will facilitate our ability to launch new personalized diagnostic tests that we plan to develop. We also intend to accelerate clinician and clinical diagnostic laboratory adoption of the NMR LipoProfile test and future clinical diagnostic tests by increasing access to our technology platform through the launch of the Vantera system. We have entered into agreements with some of our current clinical diagnostic laboratory customers to place the Vantera system in their laboratories, and we are also in discussions with additional laboratory customers that have indicated a similar interest in the placement of the Vantera system. In addition, we are placing the Vantera system with academic centers that are collaborating with us to develop additional high value diagnostic assays based on NMR technology. We will retain full ownership of any Vantera analyzers placed in third-party laboratories and be responsible for support and maintenance obligations. In general, we expect that the number of Vantera analyzers that will be placed in our clinical diagnostic laboratory customers' facilities will depend on their demonstrated annual production volume for the NMR LipoProfile test and their ability to increase demand for our tests.
Market Overview
Coronary Heart Disease
Cardiovascular disease is the leading cause of death in the United States and, according to a 2011 policy statement of the American Heart Association, or AHA, by 2030, 40.5% of the U.S. population is projected to have some form of cardiovascular disease. Coronary heart disease, or CHD, is the second most prevalent form of cardiovascular disease after hypertension and results from the failure of the heart to supply oxygenated blood to the body. According to the AHA, CHD accounted for over one-half of all cardiovascular disease deaths in 2009. CHD usually results from atherosclerosis, a hardening and narrowing of the arteries caused by a buildup of fatty plaque composed of cholesterol and other lipids, such as triglycerides, in the arterial wall. Heart attacks may result from reduced blood flow to the heart caused by progressive plaque buildup or by blood clots produced by plaque rupture.
Lipoproteins and Atherosclerosis
Lipoprotein particles are the “containers” that transport cholesterol, triglycerides and other lipids throughout the bloodstream. Lipoprotein particles span a range of sizes and densities and are grouped into three primary classes:
• | LDL, or low density lipoproteins, are intermediate-sized particles and carry cholesterol from the liver to the rest of the body; |
• | HDL, or high density lipoproteins, are the smallest particles and collect cholesterol from the body's tissues, bringing it back to the liver; and |
• | VLDL, or very low density lipoproteins, are the largest particles and are rich in triglycerides. |
Within each of these primary classes, there are several subclasses based on the size of the particles. For example, there are large, medium and small particles within each of the LDL, HDL and VLDL classes.
5
Atherosclerosis occurs when elevated numbers of LDL particles enter the arterial wall, become oxidized and are taken up by macrophages, a type of immune cell, and transformed into fatty plaque deposits. HDL particles, on the other hand, protect against atherosclerosis by, among other mechanisms, preventing oxidation of LDL particles and facilitating cholesterol removal from plaque. LDL particles are therefore considered to be “bad” because elevated levels of these particles in the blood promote atherosclerosis, and HDL particles are considered to be “good” because high levels in the blood help to prevent atherosclerosis.
Since the 1960s, the scientific community has recognized that LDL particles are a key causal factor for atherosclerosis. For many years, however, the only practical way to estimate the amount of LDL and HDL was to measure the level of cholesterol contained in these particles. Chemical measures of cholesterol concentration in lipoprotein particles, such as those reported by the lipid panel, have become the most commonly used clinical measurement not because they were shown to be better than alternative lipoprotein measurements for predicting cardiovascular outcomes, but because historically they were the simplest and easiest to perform in routine clinical laboratories. As a result, the terms “bad cholesterol” and “good cholesterol” have become synonymous with LDL and HDL in the minds of both patients and clinicians. With the medical community's initial focus on cholesterol, rather than on the lipoprotein particles carrying that cholesterol, statins, a widely prescribed class of LDL-lowering drugs, are perceived as cholesterol-lowering drugs, when in fact they function by lowering the number of LDL particles in the blood.
Clinical Uses and Market for Cholesterol Testing
The medical community seeks to more effectively manage patients' risk for developing CHD and atherosclerosis because of the serious health effects, mortality and high treatment cost associated with these conditions. Current clinical practice guidelines issued by the National Cholesterol Education Program, or NCEP, an influential authority on cholesterol management overseen by the National Heart, Lung, and Blood Institute, or NHLBI, part of the National Institutes of Health, recommend that the intensity of LDL-lowering therapy should be based on a person's risk for CHD. Accordingly, accurate measurement of a patient's CHD risk is critical to managing his or her ongoing treatment.
Due in large part to NCEP recommendations, cholesterol testing to help assess CHD risk and to manage LDL, and sometimes HDL, levels has become well-established in clinical practice. Drug companies have also contributed significantly to the awareness of the importance of cholesterol testing through physician education and direct-to-consumer advertising of LDL-lowering drugs, including statins. Cholesterol awareness programs, the aging of patient populations and the ongoing need to perform cholesterol tests for patient monitoring and management have all led to considerable growth in the cholesterol testing market.
Clinicians have historically assessed a patient's CHD risk and ongoing response to statins and other lipid-altering therapies by prescribing traditional cholesterol tests. These tests are a part of the conventional lipid panel, which has four components:
• | LDL-C, or the amount of cholesterol contained in LDL particles; |
• | HDL-C, or the amount of cholesterol contained in HDL particles; |
• | total cholesterol; and |
• | triglycerides. |
Lipid panels are among the most frequently ordered laboratory tests in the United States. According to Medicare billing data, the lipid panel was the fourth most frequently ordered test in clinical laboratories in 2009.
Limitations of Traditional Cholesterol Testing
While LDL and HDL testing is a generally well-accepted means to determine a patient's need for LDL-lowering or HDL-raising therapy and monitoring treatment response, there is increasing awareness that the traditional cholesterol measures of these key lipoprotein risk factors are deficient because they can overestimate or underestimate the actual levels of these lipoproteins in many patients and the CHD risk they confer. Many patients have disparities between their level of cholesterol and the number of lipoprotein particles in their blood, a state known as discordance. We believe that discordance leads directly to the under-treatment or over-treatment of millions of patients.
6
The cholesterol content of individual LDL and HDL particles can vary more than two-fold between patients and can change over time in the same patient. If the amounts of cholesterol per particle did not vary, LDL-C would always be an accurate measure of LDL. In practice, however, one person may have larger, more cholesterol-rich LDL particles, while a second person may have smaller, less cholesterol-rich LDL particles. As illustrated by the graphic below, a person with smaller LDL particles at a given level of LDL-C will always have more LDL particles, and consequently higher CHD risk, than a person with the same LDL-C carried in larger LDL particles.
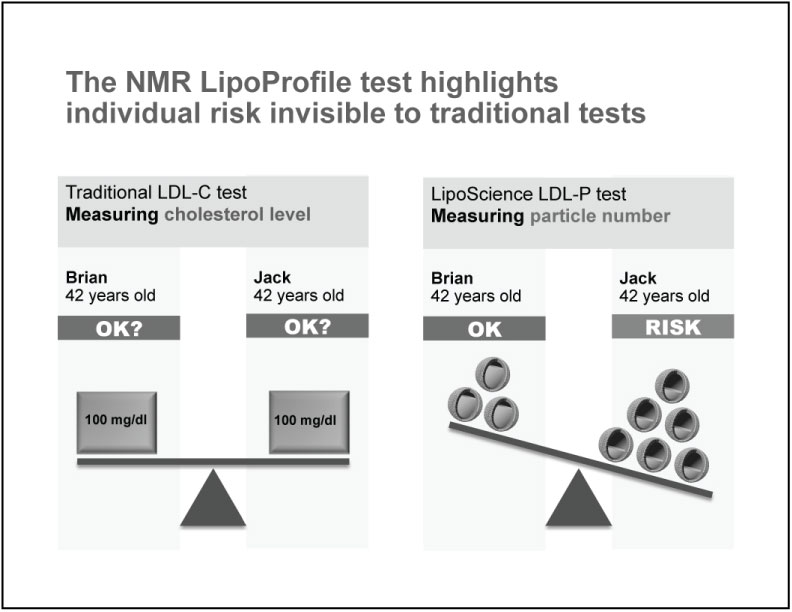
Research data have shown that LDL-P is more strongly correlated with CHD risk in discordant patients than is the level of LDL-C. In one large population study, over 30% of patients with “optimal,” or low, LDL-C had higher, less-than-optimal LDL-P levels. We believe these discordant patients may be at higher risk for developing CHD but would not be identified by traditional cholesterol testing as potentially needing LDL-lowering treatment. Such a patient's medical provider would be able to prescribe pharmaceutical therapies, such as statins, niacin or fibrates, as well as dietary and other lifestyle changes to benefit that patient. Conversely, discordant patients with low LDL-P but higher LDL-C might be expected to derive little clinical benefit from LDL-lowering treatments. Despite the increasing body of clinical evidence indicating the benefits of particle number measurement over cholesterol measurement, technological limitations and lack of clinician awareness have prevented LDL-P and HDL-P from becoming more well-accepted within the medical community.
The diagnostic limitations of traditional cholesterol testing were cited in the 2008 joint consensus statement of the ADA and ACC, which concluded that LDL-C may not accurately represent the quantity of atherosclerosis-causing LDL particles, especially in those patients with the typical lipid abnormalities of cardiometabolic risk, such as elevated triglycerides and low HDL-C. The consensus statement suggested that a more accurate way to capture the risk posed by LDL may be to measure LDL-P directly using NMR technology. The consensus statement recognized the need for more independent data confirming the accuracy of direct LDL particle measurement using NMR and whether its predictive power with respect to heart disease is consistent across various ethnicities, ages, and conditions that affect lipid metabolism. We believe that the subsequent publication of large clinical outcome studies, such as MESA, has helped to address these concerns and to confirm the accuracy and usefulness of our NMR-based technology.
Our Solution
Our NMR LipoProfile test has been cleared by the FDA for use in our clinical laboratory and directly measures LDL-P for use in managing cardiovascular risk. We believe that our test provides clinicians with more clinically relevant information about LDL and other classes and subclasses of lipoproteins than does the traditional cholesterol test for managing their patients' CHD risk.
The current NMR LipoProfile test report consists of two pages. The first page includes test results for the following measurements:
• | LDL-P, along with reference ranges to guide patient management decisions; |
• | HDL-C; and |
• | triglycerides. |
7
We have requested and received clearance from the FDA to provide the test results from our NMR LipoProfile test for each of these measurements, regardless of whether the test is performed using our standard clinical analyzer or using the Vantera system.
The second page of the NMR LipoProfile report includes test results for a number of additional lipoprotein measures that have been validated by us but have not been cleared by the FDA. These include:
• | measures related to cardiovascular risk, including HDL-P, the total number of small LDL particles, and LDL particle size; and |
• | measures associated with insulin resistance and diabetes risk, including numbers of large HDL particles, small LDL particles and large VLDL particles, as well as HDL, LDL and VLDL particle size. |
The second page includes a legend to the effect that these additional measurements, while validated by us, have not been cleared by the FDA and that the clinical utility of these measurements has not been fully established. When the Vantera system is placed in third-party laboratories, the laboratory will be able to process the blood sample in-house and produce the FDA-cleared results on the first page of the test report. At the option of the third-party laboratories, we will still make the second-page test results available to them at no additional charge for dissemination along with the first-page results. We generate these second-page results in our clinical laboratory using either NMR spectrum data digitally sent to us by the third-party laboratory or a portion of the original blood sample that they send to us. We send the second-page results back to the third-party laboratories for them to report the results to their customers along with the first-page results.
Clinical Validation of Lipoprotein Particle Quantification Using NMR
Our test was developed initially to provide a novel and efficient way to quantify, or count, the numbers of lipoprotein particles of different sizes in blood plasma or serum. Subsequently, clinical outcome studies were performed to compare the cardiovascular disease associations of our particle test results with those of traditional cholesterol tests. On the basis of the findings of those studies, we believe that our test provides clinicians with more clinically relevant information about LDL and other classes and subclasses of lipoproteins to aid in the management of the CHD risk of their patients.
The clinical utility of lipoprotein particle quantification has been supported by a number of scientific papers published in peer-reviewed journals, including Journal of the American Medical Association, New England Journal of Medicine, Circulation, American Journal of Cardiology, Atherosclerosis and Journal of Clinical Lipidology. To date, eleven cardiovascular disease outcome studies have specifically evaluated the link between LDL-P and CHD risk. In each case, LDL-P was associated significantly with atherosclerotic outcomes and, in ten of those studies, the strength of association was greater than for LDL-C. In each of the ten studies, the same blood samples were analyzed by traditional cholesterol testing and by our NMR LipoProfile test to enable a comparison of LDL-C and LDL-P in terms of their correlation with atherosclerosis and CHD risk.
We believe the following three studies are of particular note in showing the greater clinical relevance of LDL and HDL particle count as compared to LDL and HDL cholesterol measurement. Dr. James Otvos, our founder and Chief Scientific Officer, was an author of each of these published studies. Dr. Otvos collaborated with the studies' academic investigators to formulate the study hypotheses and data analysis plan, and he assisted with data interpretation and publication of the study results. We did not provide any funding for any of these studies. We performed all NMR lipoprotein testing in a fully blinded fashion, with us having no knowledge of the identity or clinical status of the participants who provided the blood samples being tested. In addition, Dr. Otvos's affiliation with us was disclosed in each publication. In each case, the person responsible for the scientific validity and integrity of the study was someone other than Dr. Otvos, and none of the investigators were affiliated with our company.
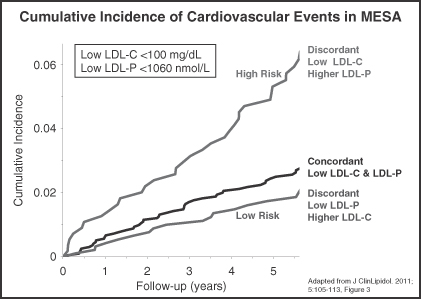
• | Multi-Ethnic Study of Atherosclerosis (MESA). In this observational study of frozen archived baseline blood samples from almost 5,600 ethnically diverse individuals conducted by the NHLBI, with a mean follow-up period of over five years, LDL-P was found to be more predictive of future cardiovascular events, such as heart attack, chest pain, stroke or death from CHD, among individuals with discordant LDL-P and LDL-C levels. Furthermore, the MESA data also indicated that individuals with “optimal,” or low, levels of LDL-C, but discordantly higher LDL-P, had significantly greater risk of cardiovascular events. In contrast, other patients with higher levels of LDL-C, but low LDL-P, did not have greater risk. The figure below illustrates these results. |
8
• | Framingham Offspring Study. In this long-running community-based observational study of over 3,000 men and women conducted by the NHLBI, blood samples from the same subjects at baseline were tested for both LDL-C by conventional cholesterol testing and for LDL-P by our NMR LipoProfile test. Data from this study indicated that LDL-P levels were more strongly associated with future cardiovascular events occurring during a median follow-up period of nearly 15 years than were either LDL-C or non-HDL cholesterol, defined as total cholesterol minus HDL cholesterol. Discrepancies between LDL-P and LDL-C were particularly prevalent at low LDL concentrations. When there was discordance between LDL-P and LDL-C levels above or below the median, cardiovascular disease risk was found to track more closely with LDL-P than LDL-C. |
• | Veterans Affairs HDL Intervention Trial (VA-HIT). This trial, in which over 2,500 men with existing CHD and low levels of HDL-C and LDL-C were treated for five years with gemfibrozil, a fibric acid derivative, demonstrated that reductions in new CHD events could be achieved by this HDL-raising drug. NMR LipoProfile analysis conducted in a subset of over 1,000 men from this trial showed that HDL-P was increased more than HDL-C by gemfibrozil, and that levels of HDL-P and LDL-P during the trial predicted future CHD events, whereas levels of HDL-C and LDL-C did not. |
In addition to the foregoing three studies, a recently published systematic literature review from the American Association of Clinical Chemistry, or AACC, Working Group on Best Practices reviewed 25 clinical studies that demonstrated the association of both apolipoprotein B, or apoB, a protein found on both LDL and VLDL particles, and LDL-P with specific patient outcomes such as CVD or metabolic conditions like type 2 diabetes. Although most of the comparisons showed equivalent strength of association with the outcome measures, in these 25 studies the measurement of LDL-P using NMR showed a significant association with a clinical outcome more often than using apoB alone, and the level of statistical significance, as indicated by p-value, and the strength of association, as indicated by the odds ratio, relative risk and hazard ratio, was more often higher for LDL-P than it was for apoB.
In addition, the discordance rate was reported to be 29.2% between the two measures, and in most of these discordant cases, the measurement of LDL-P using NMR was a stronger predictor of patient outcome than was apoB. The AACC Working Group also noted that NMR can measure other major lipoprotein classes, as compared to apoB, thus providing more information, and that NMR measurement of LDL-P appeared to be more precise than that of apoB in the reviewed studies.
The AACC Working Group formally recommended that the measurement of particle number, either as concentration of apoB or LDL-P, should be incorporated into the guidelines for assessment of CVD risk. The Working Group did indicate that in its opinion apoB appeared to be the preferred biomarker for guideline adoption because of its widespread availability, scalability, standardization and relatively low cost. It noted further that, as LDL-P is expected to become more readily available in the near future with potentially superior performance in direct comparison with apoB, LDL-P may become the preferred test as a cardiovascular marker. We believe that our strategy of decentralizing access through Vantera placements will allow for greater availability and scalability of measuring LDL-P using NMR.
9
Benefits of Our Test
We believe the NMR LipoProfile test provides the following benefits to clinicians and their patients, clinical diagnostic laboratories and healthcare payors:
Benefits to Clinicians and Patients
• | Improved patient management. We believe that the NMR LipoProfile test provides a more accurate picture of a patient's lipoprotein-related CHD risk and the patient's ongoing response to LDL-lowering and HDL-raising therapies than does LDL-C and HDL-C, as measured by traditional cholesterol tests. By providing clinicians with better information about the key lipoprotein risk factors, LDL and HDL, clinicians can design a personalized therapeutic and lifestyle management plan tailored to address the principal drivers of their patients' CHD risk. |
• | Strong clinical validation. Clinical research and numerous CHD outcome studies support the stronger predictive capacity of LDL-P for CHD patient management, compared to LDL-C. |
• | Reimbursement. The NMR LipoProfile test has a dedicated Category I CPT code. The American Medical Association, or AMA, assigns Category I CPT codes to procedures that are consistent with contemporary medical practice, are widely performed and meet other specified criteria. The NMR LipoProfile test is reimbursed by Medicare and other governmental payors, as well as by many private insurance carriers. |
• | Improved accessibility. We have commercial relationships with a number of national and regional clinical diagnostic laboratories, including Laboratory Corporation of America, or LabCorp. These arrangements allow clinicians to more easily order the NMR LipoProfile test through their laboratory providers instead of having to order it directly from us. |
• | Ease of Use. The NMR LipoProfile test requires only a standard blood draw and, unlike traditional cholesterol tests, does not require the patient to fast in order to measure LDL-P and HDL-P. |
Benefits to Laboratories
• | Driver of revenue and margin growth. Clinicians are increasingly recognizing the growing importance of LDL-P in measuring CHD risk, which we believe is evidenced by the approximately 28% compound annual growth in the number of NMR LipoProfile tests ordered from 2007 to 2012. Offering the NMR LipoProfile test allows laboratories to meet the medical community's growing demand for the test and expand their product offerings. Given the higher reimbursement rate our test enjoys as compared to traditional cholesterol testing, the NMR LipoProfile test also presents laboratories with an opportunity to increase their revenues and expand their margins. |
• | Simplicity, efficiency and cost-effectiveness. Our use of NMR does not require time-consuming physical sample separation procedures. |
• | Ability to leverage our sales and marketing efforts. Our NMR LipoProfile test is typically ordered by clinicians through clinical diagnostic laboratories. Our laboratory customers benefit from our direct sales force and our marketing programs and materials, which increase demand for our NMR LipoProfile test. |
• | Future integration into existing laboratory operations. We intend to selectively place the Vantera system on-site at our clinical diagnostic laboratory customers' locations. The Vantera system is designed to easily integrate into existing laboratory information systems and workflows, requiring limited technician attention and simple process management. |
• | Scalable platform. Using our NMR technology platform, multiple diagnostic tests can be performed simultaneously from a single sample. |
10
Benefits to Payors
• | More effective management of a costly disease. The NMR LipoProfile test is designed to help clinicians more effectively manage CHD risk, both in patients whose risk of CHD would have been underestimated by traditional cholesterol testing and in those patients who are being overtreated because traditional testing overstates their risk. Because our test provides information that allows physicians to make more informed therapeutic decisions, we believe it can lessen the financial burden of CHD on the payor community. |
• | Low relative cost. The price for our test, while higher than that of a traditional cholesterol test, is still relatively low compared to other advanced cardiovascular panels. Medicare generally reimburses our test at a rate of $43.36 per test. |
Our Strategy
Our strategy is to continue to advance patient care by converting clinicians, and the clinical diagnostic laboratories they use, from traditional cholesterol testing to our NMR LipoProfile test for the management of patients at risk for CHD, with the goal of ultimately becoming a clinical standard of care. The key elements of our strategy to achieve this goal include:
• | Expand our sales force nationally. We currently have sales representatives in 25 states who target clinicians, as well as dedicated representatives targeting clinical diagnostic laboratories and third-party payors. We intend to expand our investment in our sales force in order to penetrate all major markets in the United States and potentially in selected markets outside of the United States. |
• | Increase market awareness and educate clinicians about the clinical benefits of our test. To create awareness, encourage clinician evaluation and increase orders for our NMR LipoProfile test, we provide our sales force with peer-reviewed clinical outcome studies, medical society guidelines and other clinical evidence sources. Our direct sales force uses these sources in calls on high-prescribing cardiologists, primary care physicians, allied healthcare professionals and laboratory administrators. We plan to continue to increase our investment in medical education and marketing efforts to promote our test as the standard of care for the management of cardiovascular risk. |
• | Expand relationships with clinical diagnostic laboratories. Approximately 89% of the revenues derived from our NMR LipoProfile tests for the year ended December 31, 2012 were attributable to tests ordered through clinical diagnostic laboratories. We plan to expand our business with these existing laboratory customers and to develop relationships with additional national and regional laboratories. We intend to train laboratory customers' sales forces and partner with them to gain access to additional clinicians and educate them about the benefits of our test. Where appropriate, we intend to collaborate with our laboratory customers to encourage health plan administrators to support reimbursement for the test. |
• | Decentralize access to our technology platform with the Vantera system. We intend to selectively place our Vantera system, cleared by the FDA in August 2012, on-site with large clinical diagnostic laboratories, leading medical centers and hospital outreach laboratories. We intend to develop an expanded menu of additional personalized diagnostic tests that use our NMR-based technology, which we believe will increase the appeal of the Vantera system to laboratories. We expect that the availability of the Vantera system, if it receives the appropriate international regulatory approvals, would also facilitate our ability to expand internationally if we decide to do so. |
• | Broaden medical policy coverage. We have a dedicated team of managed care specialists who pursue the expansion of coverage for our test with third-party payors. Our clinical diagnostic laboratory customers, prescribing physicians and healthcare thought leaders also assist us in influencing payors to cover our test. We intend to further broaden coverage by leveraging the increasing weight of clinical data, expanding access to our test and increasing utilization to incentivize payors to cover our test and set adequate reimbursement levels. |
11
• | Pursue inclusion in treatment guidelines. We actively engage key opinion leaders and medical societies in an effort to have the NMR LipoProfile test included as a standard of care in clinical guidelines. The potential benefits of testing for LDL-P have been discussed in the ADA/ACC consensus statement, in a position statement published by the AACC Working Group and in recommendations by the panel of clinical lipidology experts convened by the NLA. Additionally, the 2013 Standards of Medical Care in Diabetes added lipoprotein particle measurement as an alternative to measurement of LDL cholesterol for purposes of treating patients with abnormal amounts of lipids in their blood, a condition known as dyslipidemia. The ADA standards of medical care now recognize each of non-HDL cholesterol, apolipoprotein B and lipoprotein particle measurements as a means to assess residual cardiovascular risk in patients being treated with statins. We also plan to pursue inclusion of our NMR LipoProfile test in other clinical guidelines, including those of the NCEP and the AHA. |
• | Develop and expand relationships with leading academic medical centers. We have collaborated with leading academic medical centers, including the Mayo Foundation, the Cleveland Clinic and Oxford University, on clinical validation studies and research for developing new applications using NMR technology. We intend to pursue additional collaborations to further validate our technology, investigate potential new diagnostic tests and provide clinical data for publications and regulatory submissions. |
• | Develop new personalized diagnostic tests using our NMR-based technology platform. Our NMR LipoProfile test exploits only a very small fraction of the available information contained within an NMR spectrum of a sample. We intend to exploit the inherent analytical advantages of NMR spectroscopy, including its ability to analyze bodily fluids other than blood serum and plasma, to expand our menu of available diagnostic tests. We are currently developing additional NMR-based diagnostic tests to predict a patient's risk for type 2 diabetes, and evaluating NMR technology for the detection and management of several different cancers as well as inflammatory, gastrointestinal and neurological diseases. |
Our Technology Platform
Our technology platform combines proprietary signal processing algorithms and NMR spectroscopic detection into a clinical analyzer to identify and quantify concentrations of lipoproteins and, potentially, small molecule metabolites. NMR detectors, or spectrometers, analyze a blood plasma or serum sample by subjecting it to a short pulse of radio frequency energy within a strong magnetic field. Each lipoprotein particle within a given diameter range simultaneously emits a distinctive radio frequency signal, similar to distinctive ringing sounds for bells of different sizes. The amplitude, or “volume,” of the NMR signal is directly proportional to the concentration of the particular subclass of lipoprotein particles emitting the signal. Our proprietary software then collects, records and analyzes the composite signals emitted by all of the particles in the sample in real time and separates the signals into distinct subclasses. Within minutes, we are able to quantify multiple subclasses of lipoprotein particles.
Our technology platform based on NMR offers the following advantages over conventional methods of quantifying lipoproteins and small molecule metabolites:
• | Information-rich detection. NMR can analyze lipoproteins as well as potentially hundreds of small molecule metabolites. |
• | Processing efficiency. Our technology does not require physical separation of the lipoprotein particles and does not require chemical reagents in order to evaluate a sample. |
• | Sample indifference. Our technology may be used to analyze multiple sample types, including plasma, serum, urine, cerebrospinal fluid and other biological fluids. |
• | Throughput. Simultaneous lipoprotein and metabolite quantification from a rapid NMR measurement makes the platform extremely efficient with high throughput. |
12
The Vantera System
The Vantera system is our next-generation automated clinical analyzer. In August 2012, we received FDA clearance to market the Vantera system commercially to laboratories. We intend to decentralize access to our technology through the Vantera system in order to drive both geographic expansion and the technology adoption necessary for successful execution of our market conversion strategy. We intend to place the Vantera system in select high-volume national and regional clinical diagnostic laboratories, as well as at leading medical centers and hospital outreach laboratories. We have entered into agreements with some of our current clinical diagnostic laboratory customers to place the Vantera system in their laboratories, and we are also in discussions with additional laboratory customers who have indicated a similar interest in the placement of the Vantera system. In addition, we are placing the Vantera system with academic centers that are collaborating with us to develop additional high value diagnostic assays based on NMR technology.
As with our existing clinical analyzers, the Vantera system uses NMR spectroscopy and proprietary signal processing algorithms to identify and quantify lipoproteins and metabolites from a single spectrum, or scan. We believe that the Vantera system provides the following strategic and technological benefits:
• | direct access to our technology on site, rather than relying on a “send-out” test; |
• | processing of samples at a rate that is approximately twice as fast as our current-generation analyzers; |
• | a reagent-less platform requiring no sample preparation for analysis; |
• | multiple NMR-test processing capabilities; and |
• | limited operator intervention, with no specialized NMR training required for operation. |
We believe the selective placement of our Vantera system directly in laboratories throughout the United States will further drive our market conversion strategy by decentralizing access to our technology. We expect that strong commercial relationships with clinical diagnostic laboratories will allow us to leverage the sales forces of these laboratories for additional access to prescribing clinicians, as well as third-party payors with whom the laboratories have existing contracts.
Sales, Marketing and Distribution
We currently market our NMR LipoProfile test through a direct sales force in 25 states. Our sales strategy involves the use of a combination of sales managers, sales representatives and medical science liaisons who target primary care physicians, cardiologists and key medical opinion leaders, as well as separate dedicated personnel targeting clinical diagnostic laboratories and third-party payors. As of December 31, 2012, we had 74 employees engaged in sales and marketing functions, including our sales managers and sales representatives, medical science liaisons, personnel focused on our sales efforts to clinical diagnostic laboratories and managed care specialists focused on third-party payors. We expect to increase our sales force as we seek to drive conversion of the market to our NMR LipoProfile test and expand into other parts of the United States.
The role of our sales force is to promote the NMR LipoProfile test and educate clinicians, laboratories and payors about its medical benefits over traditional cholesterol tests, as well as the potential economic benefits of providing patients with personalized cardiovascular risk management. Our sales and marketing activities are supported by the publication and presentation of relevant peer-reviewed medical studies and articles, educational programs, meetings and trade shows, print advertising in medical-related periodicals and customer support and service programs.
We intend to continue to distribute the NMR LipoProfile test directly through national and regional clinical diagnostic laboratories. During the year ended December 31, 2012, we generated approximately 89% of our revenues from our NMR LipoProfile tests through these laboratories.
Under our current agreement with LabCorp, which went into effect as of September 2012, LabCorp will make the NMR LipoProfile test available nationally to its clients. We also provide LabCorp with our sales materials, as well as access to the various medical education and other marketing programs that we sponsor and conduct, for their use in connection with the promotion of the NMR LipoProfile test.
13
Under our agreement with LabCorp, we will continue to fulfill all orders received from LabCorp for the NMR LipoProfile test and perform those tests at our own laboratory facilities, but we will also place Vantera analyzers directly in LabCorp laboratories, with the number of analyzers based on the annual volume of NMR LipoProfile tests performed in a particular facility. There is no minimum number of tests that LabCorp is required to order from us under the agreement. We will be providing service and support of the Vantera analyzers placed at LabCorp facilities.
Our agreement with LabCorp has a term that continues until September 2015 and is automatically renewable for additional two-year terms, unless either party provides 90 days written notice of its intent not to renew the agreement at the end of the initial term or any subsequent two-year term. Either we or LabCorp may terminate the agreement upon the occurrence of a breach of the agreement by the other party that is not cured within a specified number of days after notice thereof by the non-breaching party, or if the other party files for bankruptcy protection or enters into similar proceedings. LabCorp may also terminate the agreement upon 90 days written notice in specified circumstances.
Coverage and Reimbursement
Clinicians order the NMR LipoProfile test directly from us and indirectly through clinical diagnostic laboratories located throughout the United States. When we sell our NMR LipoProfile test to a laboratory customer, we receive a fixed fee per test at a level individually negotiated with each laboratory, and the laboratory takes responsibility for billing and collections from third parties, including Medicare and other governmental and commercial payors. Under our agreement with LabCorp, in the event that LabCorp is unable under applicable law or an existing agreement to bill and collect for the testing services from third parties, then LabCorp is not obligated to pay us the applicable fee, in which case we may bill the third parties directly for our tests performed. To date, this situation has not occurred, and we have billed LabCorp for all tests performed under our agreement with them.
When a clinician orders the test directly from us, we have the responsibility for securing reimbursement. Our managed care team seeks to establish coverage for our test with all payors, including Medicare, state Medicaid agencies and commercial insurance carriers, so that we and our laboratory customers can maximize reimbursement.
Laboratory tests, as with most other healthcare services, are classified for reimbursement purposes according to their respective CPT codes. In 2006, the AMA's CPT Editorial Board issued a Category I CPT code (83704) for our NMR LipoProfile test. With its own dedicated CPT code, our NMR LipoProfile test is reimbursed by a number of governmental and private payors, which we believe collectively represent approximately 150 million covered lives. These payors include Medicare, TRICARE, WellPoint, United Healthcare and several Blue Cross Blue Shield affiliates.
Medicare and Medicaid
The Centers for Medicare and Medicaid Services, or CMS, under the U.S. Department of Health and Human Services, which establishes reimbursement payment levels and coverage rules for Medicare, currently covers our NMR LipoProfile test. All NMR LipoProfile tests that are performed for Medicare patients and directly billed to Medicare are subject to Medicare's national coverage regulation. This applies to both our direct business as well as that of our laboratory customers. In order to obtain Medicare reimbursement under this policy, we and our laboratory customers are required to comply with all Medicare regulations. We believe that we have sufficient processes and procedures in place to comply with Medicare requirements and to directly seek reimbursement from Medicare for our NMR LipoProfile test.
Individual state agencies establish reimbursement levels for Medicaid. Our NMR LipoProfile test is currently reimbursed by several of these state Medicaid agencies, although it is not a significant portion of our business.
Commercial Insurance Carriers and Managed Care Organizations
In-network. Our laboratory customers have participating provider, or in-network, agreements with payors that we believe cover a majority of insured individuals in the United States and, as a result, our NMR LipoProfile test is frequently billed to an insurer as an in-network benefit. In-network agreements specify a fixed price for reimbursement over a fixed period of time. These in-network agreements between insurers and our laboratory customers allow for better and more consistent reimbursement to them, while we receive a fixed fee per test without assuming the risk of non-payment from an insurance company. We also have an in-network agreement with Blue Cross Blue Shield of North Carolina that covers NMR LipoProfile tests ordered directly from us.
14
Out-of-network. For our direct business that is not performed through a laboratory customer and not covered under our in-network agreement with Blue Cross Blue Shield of North Carolina, we are generally an out-of-network provider, meaning that we do not have a contractual agreement in place that specifies a fixed price for reimbursement. Instead, we bill these payors on a fee-for-service basis and then invoice the patient for the remainder of what the insurer does not pay, up to our total billed amount.
Competitive Products and Technologies
We compete primarily against the conventional lipid panel test as well as alternative methods of measuring cholesterol concentrations or lipoproteins.
The lipid panel test is widely ordered by physician offices and performed in substantially all clinical diagnostic laboratories. It is relatively inexpensive and reimbursed by virtually all payors. The market for lipid panel tests is highly fragmented, however, and there is no dominant provider for these tests.
We also compete against companies that offer other methods for measuring lipoproteins. Unlike our technology, however, these methods require lipoproteins to first be physically separated on the basis of differences in size or density or composition before being measured. These physical separation methods generally involve relatively labor-intensive steps to separate the sample and are more time-consuming and more costly to perform than the NMR LipoProfile test. Among the companies providing these tests are Berkeley HeartLab, Inc., now part of Quest Diagnostics, as well as Atherotech, Inc. and SpectraCell Laboratories.
There are also diagnostic tests available that measure other lipoprotein indicators of cardiovascular disease risk, including apoB. Plasma apoB levels provide a measure of the aggregate number of LDL plus VLDL particles. While an apoB test is generally less expensive and currently more widely available than our NMR LipoProfile test, it does not offer the breadth of information useful in the management of CHD risk provided by our test, including:
• | measures related to cardiovascular risk, including HDL-P, the total number of small LDL particles, and LDL particle size; and |
• | measures associated with insulin resistance and diabetes risk, including numbers of large HDL particles, small LDL particles and large VLDL particles, as well as HDL, LDL and VLDL particle size. |
The apoB test is a non-proprietary test offered by many clinical diagnostic laboratories. We believe that the lack of universal standardization of the apoB test has limited its use by physicians.
In order for us to successfully compete against these alternative tests and technologies, we will need to demonstrate that our products deliver superior results and value as a result of our key differentiators, including FDA clearance, clinical validation, improved patient outcomes, accessibility, ease of use, speed and efficiency, scalability and economic benefits.
Research and Development
With the recent FDA clearance of our Vantera system, our research and development efforts are focused on implementing improvements and enhancements to the Vantera system as well as on developing new personalized diagnostic tests using our NMR-based technology.
Each NMR analysis returns data that could be used to measure concentrations of hundreds of small molecule metabolites, and we believe that our technology is suited for the measurement of any number of those metabolites with minimal sample preparation in a rapid, easy-to-use and efficient manner. Our technology also supports the analysis of other bodily fluids in addition to plasma, such as urine and cerebrospinal fluid.
We are actively developing our test for use in assessing insulin resistance and risk for developing type 2 diabetes. We currently provide an insulin resistance score as a laboratory-developed test that is part of the NMR LipoProfile test. This test, which is not cleared or approved by the FDA at this time, uses a lipoprotein-based indicator to assess insulin resistance status, an early indicator of type 2 diabetes. We are developing enhancements to this assay to improve its utility and we intend to discuss the regulatory submission process with the FDA for this enhanced diagnostic test in 2013.
15
We are also investigating opportunities to develop a number of additional diagnostic tests, including:
• | Additional lipoprotein tests. These would utilize lipoprotein subclass and particle size information to address diagnosis or management of additional cardiovascular or metabolic disease states. |
• | Single-analyte tests. These would measure metabolites from a variety of sample types, such as plasma, urine, cerebrospinal fluid and other biological fluids. |
• | Multivariate-indexed tests. These would simultaneously measure multiple metabolites in order to evaluate risk for developing or to diagnose certain diseases. |
Research is under way to further explore the use of NMR technology for the detection and management of certain cancers, gastrointestinal, inflammatory and neurologic diseases.
We have also entered into a patent license agreement with Cleveland Clinic that will allow us to develop a diagnostic test using our NMR technology for cardiovascular disease risk based on a metabolite known as trimethylamine N-oxide, or TMAO, derived from an individual's intestinal microbes. A research team at Cleveland Clinic has discovered a link between this metabolite and cardiovascular disease risk, and we believe this discovery may lead to new diagnostic tests and therapeutic approaches to the treatment of heart disease.
As of December 31, 2012, we had 46 employees engaged in research and development functions. Our research and development expenses were $10.0 million, $7.8 million and $7.3 million for the years ended December 31, 2012, 2011 and 2010, respectively.
Testing and Laboratory Operations
We currently process all samples and perform all NMR LipoProfile tests at our laboratory, which occupies approximately 39,000 square feet at our headquarters in Raleigh, North Carolina. Our laboratory allows us to fulfill current demand for our test and serves as a strategic asset that we believe will facilitate our ability to launch new personalized diagnostic tests we plan to develop. Our laboratory is certified under CLIA, and is accredited by the College of American Pathologists, or CAP. We also satisfy the additional licensing requirements of a number of states, including New York.
Our NMR LipoProfile test begins with a standard blood sample taken at the direction of a clinician. The plasma from the blood sample is sent to our laboratory either directly by the referring clinician or by a clinical diagnostic laboratory. Our NMR LipoProfile test requires minimal preparation and the results are produced within minutes. We typically report results to clinicians or the referring laboratory within 24 hours of our receipt of a sample.
We operate both our current generation NMR analyzers and our Vantera system analyzers to perform our tests. We currently operate our laboratory six and one-half days per week, with multiple shifts each day. We will continue to operate our laboratory facility, even after the placement of our Vantera system analyzers at our laboratory customers' facilities, as an early launch platform for new NMR-based personalized diagnostic tests.
We source the components of the Vantera system, including the magnet and console, sample handler and shell, from Agilent Technologies and KMC Systems, Inc., original equipment manufacturers who will ship the components to us or at our direction directly to the laboratory or research center for assembly. As part of the assembly, we will install our proprietary signal processing software and diagnostic tests to complete the placement of the Vantera system.
16
Supply Agreements
Agilent Technologies
In July 2012, we entered into a supply agreement with Agilent Technologies pursuant to which we agreed to exclusively purchase from Agilent the magnet, console and probe used in the Vantera system. Under the supply agreement, subject to specified exceptions, Agilent agreed not to sell the NMR probes, components and controlling electronics and acquisition software to customers in the United States who intend to use such technology within a designated restricted field of use, which consists of the screening, detection, prognosis or monitoring of diseases in the cardiovascular, cancer, endocrine, central nervous system or autoimmune fields, or in connection with designated specimen types. Agilent retains all rights to sell such NMR technology to other customers for uses, applications and purposes outside of the specified field of use, including research use, investigative use and other specified in vitro diagnostic applications. If we fail to make specified progress in the development of a diagnostic application in the field of cancer within any 12-month period, then the designated restricted field of use would no longer include cancer, and the restrictions on Agilent's ability to sell such NMR technology to other customers would lapse as to the field of cancer, although we would continue to have the non-exclusive right to purchase the NMR technology from Agilent for use in that field. We are responsible for all regulatory filings and required approvals related to the commercial availability of the Vantera system.
Under the supply agreement, we agreed not to sell a product using NMR technology to customers in the United States for research use, and agreed not to develop in vitro diagnostic products using NMR technology outside the designated field of use. We are also obligated to purchase all of our requirements for the components to be supplied by Agilent under the supply agreement.
The initial term of the supply agreement continues until July 2022. The initial term may be renewed for additional five-year periods upon mutual agreement, unless either party provides one year written notice of its intent not to renew the agreement at the end of the initial term or any subsequent five-year term. Either we or Agilent may terminate the supply agreement upon the occurrence of a breach of a material term of the agreement by the other party that is not cured within a specified number of days after notice thereof by the non-breaching party, or if the other party files for bankruptcy protection or enters into similar proceedings. Agilent may terminate the supply agreement in the event that it discontinues the sale of all NMR flow cell probes, NMR components and NMR controlling electronics and acquisition software, and, in such event, Agilent is required to provide us with not less than two years' prior written notice of such termination.
KMC Systems
In 2007, we began collaborating with KMC Systems, Inc. on the design and manufacture of the Vantera hardware assembly, including the frame, the autosampler and the electronic interface. In 2009, we entered into a production agreement with KMC under which we have designated KMC as the exclusive manufacturer of the Vantera commercial production unit, subject to specified exceptions, and have agreed to purchase all of our Vantera units from KMC. The sales price for each Vantera unit is determined on an individual purchase order basis and is based on a pricing formula set forth in the production agreement.
The initial term of the production agreement continues until the later of three years from the first shipment of a Vantera analyzer or delivery of the 30th unit. The initial term will be automatically extended for additional one-year periods unless we or KMC provide the other with written notice of termination not less than 90 days prior the end of the term or an extension term. Either we or KMC may terminate the production agreement upon the occurrence of a material breach by the other party that is not cured within a specified number of days after notice thereof by the non-breaching party, if the other party files for bankruptcy protection or enters into similar proceedings, or upon a change of control of the other party, as defined in the agreement. KMC also has the right to terminate the production agreement if its production activities under a purchase order are interrupted or delayed due to our request or our failure to perform our obligations under the agreement, in each case for a 90-day continuous period. During the term of the agreement and for a specified period of time thereafter, neither we nor KMC may solicit for employment any employees of the other party.
17
Intellectual Property
In order to remain competitive, we must develop and maintain protection on the key aspects of our technology. We currently rely on a combination of patents, copyrights and trademarks and confidentiality, licenses and invention assignment agreements to protect our intellectual property rights. We also rely upon unpatented trade secrets and improvements, unpatented know-how and continuing technological innovation to develop and maintain our competitive position. Although the patent covering the measurement of lipoprotein classes and subclasses by NMR, which we license from a third party, expired in 2011, we believe that our other intellectual property and the know-how required to directly quantify lipoprotein particles and other metabolites using NMR-based technology, will provide sufficient barriers to entry that will not materially impact our competitive position.
Patents
We have implemented a strategy designed to optimize our intellectual property rights. For core intellectual property, we are pursuing patent coverage in the United States and those foreign countries that correspond to the majority of our current and anticipated customer base. We own, or co-own with exclusive license rights, eight issued U.S. patents and 12 pending U.S. patent applications, one of which has a pending counterpart Patent Cooperation Treaty, or PCT, application and three others of which have pending or issued counterpart foreign patents. We believe our patents and pending applications provide, or will provide, protection for our NMR-based systems and technologies that simultaneously analyze lipoproteins as well as hundreds of small molecule metabolites from blood serum, plasma, urine and other bodily fluids, and which will enable us to further expand our diagnostics test menu.
License from North Carolina State University
We license from North Carolina State University, or NCSU, on an exclusive basis, U.S. patent number 6,518,069, which expires in 2020. This patent, which we co-own, covers NMR measurements of lipoprotein subclasses for use in identifying patients at risk for type 2 diabetes and measurement of glucose levels.
Under the agreement, we paid an initial license fee of $25,000. We are required to pay NCSU a low single-digit royalty based on net sales of the licensed products and licensed tests, subject to a minimum annual royalty of $2,500. Dr. James Otvos, our founder and Chief Scientific Officer, is an adjunct professor of biochemistry at NCSU.
Under the license agreement, we are obligated to diligently pursue the development and commercialization of the licensed technologies, including manufacturing or producing a product for testing, development and sale and seeking required government approvals of the product. NCSU may terminate our license if we fail to perform our obligations under the license agreement, or if we engage in fraud, willful misconduct or illegal conduct. Unless earlier terminated, our license agreement with NCSU will terminate upon the expiration of the last-to-expire of the patents that are subjects of the license agreement.
License Agreement with Cleveland Clinic
In 2011, we entered into a license agreement with The Cleveland Clinic Foundation, under which we have received an exclusive license to one pending U.S. and one pending European patent application and know-how in order to develop and commercialize a diagnostic test for cardiovascular disease risk based on TMAO. Under the agreement, we are responsible for designing, developing, validating and registering any such test. Upon successfully developing a TMAO assay, we would work with the appropriate regulatory agencies to prepare for its commercialization. We are also responsible for all commercial aspects of the diagnostic test, including marketing, medical education and laboratory training. In addition, while our license is on an exclusive basis, the inventions claimed by the U.S. patent application were made pursuant to government-funded research and, consequently, are subject to statutory rights retained by the U.S. government.
We paid an initial license fee of $50,000 upon signing of the agreement and are obligated to pay annual minimum amounts of between $50,000 and $75,000 beginning in 2013. Additionally, beginning in 2014, we will be obligated to pay annual minimum amounts of between $25,000 and $50,000 for international rights. These annual payments continue for the term of the agreement, which lasts until the expiration of the last licensed patent that is the subject of the agreement. In addition, we are obligated to make payments to Cleveland Clinic of up to $100,000 in the aggregate upon the achievement of specified milestones set forth in the agreement, and any such milestone payments would be credited toward our annual minimum payment obligations for the period in which the milestone payment is made. We are also obligated to pay Cleveland Clinic a high-single digit royalty based on any net sales of a diagnostic test incorporating the licensed intellectual property.
18
For the first five years of the agreement, we and Cleveland Clinic have the option to convert the license from exclusive to co-exclusive with one other licensee, which would have the effect of reducing the minimum annual payment obligation and reducing the royalty rate to mid-single digits.
To date, no payments have been made under this agreement other than the initial license fee and reimbursed patent expenses of approximately $35,000.
Copyrights, Trademarks and Trade Secrets
We protect the software that we use to analyze the data from our NMR spectroscopic analysis through registered copyrights in the United States, common law copyrights and as trade secrets. We hold registered trademarks in the United States for our marks “LipoProfile,” “LipoScience,” “LipoTube,” “NMR LipoProfile,” and “Vantera”. We have pending U.S. trademark applications for the marks “The Particle Test,” “Valet,” “NMRDX” and “Vantera-Chek”.
We require all employees and technical consultants working for us to execute confidentiality agreements, which provide that all confidential information received by them during the course of the employment, consulting or business relationship shall be kept confidential, except in specified circumstances. Our agreements with our employees provide that all inventions, discoveries and other types of intellectual property, whether or not patentable or copyrightable, conceived by the individual while he or she is employed by us are assigned to us.
Government Regulation
Federal Food, Drug, and Cosmetic Act
In the United States, in vitro diagnostics are regulated by the FDA as medical devices under the Federal Food, Drug, and Cosmetic Act, or FDCA. We have previously received FDA clearance for our current NMR spectrometer together with the NMR LipoProfile test and specific portions of the report produced by the test for use in our clinical laboratory, and in August 2012, we received FDA clearance for the Vantera system. In the third quarter of 2011, we made a submission to the FDA seeking clearance of one additional test measurement, HDL-P, generated by the NMR LipoProfile test performed on our current NMR-based clinical analyzer platform. In March 2012, we voluntarily withdrew that submission and have since worked with the FDA outside of the formal review process to resolve issues with our submission that were identified by the FDA. We resubmitted the 510(k) premarket notification to the FDA, seeking clearance of the HDL-P test, in December 2012. In February 2013, the FDA provided comments on our submission and requested additional information from us. We are actively working with the FDA to provide them with the requested information, and to resolve the issues that they have identified. We have not yet sought FDA clearance of certain other portions of the report produced by our test, which may be considered to be laboratory-developed tests, or LDTs, as described below, but we plan to do so. We also plan to seek FDA clearance or approval for other diagnostic products currently under development. There are two regulatory pathways to receive authorization to market in vitro diagnostics: a 510(k) premarket notification and a premarket approval application, or PMA. The FDA makes a risk-based determination as to the pathway for which a particular in vitro diagnostic is eligible.
The information that must be submitted to the FDA in order to obtain clearance or approval to market a new medical device varies depending on how the medical device is classified by the FDA. Medical devices are classified into one of three classes on the basis of the controls deemed by the FDA to be necessary to reasonably ensure their safety and effectiveness. Class I devices are subject to general controls, including labeling and adherence to FDA's quality system regulation, which are device-specific good manufacturing practices. Class II devices are subject to general controls and special controls, including performance standards and postmarket surveillance. Class III devices are subject to most of these requirements, as well as to premarket approval. Most Class I devices are exempt from premarket submissions to the FDA; most Class II devices require the submission of a 510(k) premarket notification to the FDA; and Class III devices require submission of a PMA. Most in vitro diagnostic kits are regulated as Class I or II devices and are either exempt from premarket notification or require a 510(k) submission.
510(k) premarket notification. A 510(k) notification requires the sponsor to demonstrate that a medical device is substantially equivalent to another marketed device, termed a “predicate device,” that is legally marketed in the United States and for which a PMA was not required. A device is substantially equivalent to a predicate device if it has the same intended use and technological characteristics as the predicate; or has the same intended use but different technological characteristics, where the information submitted to the FDA does not raise new questions of safety and effectiveness and demonstrates that the device is at least as safe and effective as the legally marketed device. Under current FDA policy, if a predicate device does not exist, the FDA may make a risk-based determination based on the complexity and clinical utility of the device that the device is eligible for de novo 510(k) review instead of a requiring a PMA. The de novo 510(k) review process is similar to clearance of the 510(k) premarket notification, despite the lack of a suitable predicate device.
19
The FDA's performance goal review time for a 510(k) notification is 90 days from the date of receipt, however, in practice, the review often takes longer. In addition, the FDA may require information regarding clinical data in order to make a decision regarding the claims of substantial equivalence. Clinical studies of in vitro diagnostic products are typically designed with the primary objective of obtaining analytical or clinical performance data. If the FDA believes that the device is not substantially equivalent to a predicate device, it will issue a “Not Substantially Equivalent” letter and designate the device as a Class III device, which will require the submission and approval of a PMA before the new device may be marketed. Under certain circumstances, the sponsor may petition the FDA to make a risk-based determination of the new device and reclassify the new device as a Class I or Class II device. Any modifications made to a device, its labeling or its intended use after clearance may require a new 510(k) notification to be submitted and cleared by FDA. Some modifications may only require documentation to be kept by the manufacturer, but the manufacturer's determination of the absence of need for a new 510(k) notification remains subject to subsequent FDA disagreement and enforcement to cease marketing of the modified device.
The FDA has undertaken a systematic review of the 510(k) clearance process that includes both internal and independent recommendations for reform of the 510(k) system. The internal review, issued in August 2010, included a recommendation for development of a guidance document defining a subset of moderate risk (Class II) devices, called Class IIb, for which clinical or manufacturing data typically would be necessary to support a substantial equivalence determination. In the event that such new Class IIb sub-classification is adopted, we believe that most of the tests that we may pursue would be classified as Class IIb devices. In July 2011, the Institute of Medicine, or IOM, issued its independent recommendations for 510(k) reform. As the FDA receives public comment on the IOM recommendations and reconciles its plan of action to respond to both the internal and IOM recommendations, the availability of the 510(k) pathway for our diagnostic tests, and the timing and data burden required to obtain 510(k) clearance, could be adversely impacted. We cannot predict the impact of the 510(k) reform efforts on the development and clearance of our future diagnostic tests.
Premarket approval. The PMA process is more complex, costly and time consuming than the 510(k) process. A PMA must be supported by more detailed and comprehensive scientific evidence, including clinical data, to demonstrate the safety and efficacy of the medical device for its intended purpose. If the device is determined to present a “significant risk,” the sponsor may not begin a clinical trial until it submits an investigational device exemption, or IDE, to the FDA and obtains approval from the FDA to begin the trial.
After the PMA is submitted, the FDA has 45 days to make a threshold determination that the PMA is sufficiently complete to permit a substantive review. If the PMA is complete, the FDA will file the PMA. The FDA is subject to a performance goal review time for a PMA of 180 days from the date of filing, although in practice this review time is longer. Questions from the FDA, requests for additional data and referrals to advisory committees may delay the process considerably. Indeed, the total process may take several years and there is no guarantee that the PMA will ever be approved. Even if approved, the FDA may limit the indications for which the device may be marketed. The FDA may also request additional clinical data as a condition of approval or after the PMA is approved. Any changes to the medical device may require a supplemental PMA to be submitted and approved.
Laboratory-developed tests. There are some measurements reported by our NMR LipoProfile test that we have not submitted to the FDA for 510(k) clearance and which are therefore considered to be laboratory-developed tests. Although the FDA has stated that it has the regulatory authority to regulate laboratory-developed tests that are validated by the developing laboratory, it has generally exercised enforcement discretion and has not otherwise regulated most tests performed by laboratories that are certified under CLIA. When third-party laboratories using our Vantera system wish to deliver to their customers the LDT portion of our NMR LipoProfile test appearing on the second page of the report, we will still make the second-page test results available to them at no additional charge for dissemination along with the FDA-cleared first-page results. We will generate these second-page results in our clinical laboratory using either NMR spectrum data digitally sent to us by the third-party laboratory or a portion of the original blood sample that they send to us. We will then send the second-page results back to the third-party laboratories, which will report these results to their customer along with the first-page results. The second-page NMR LipoProfile test results cannot be performed at third-party laboratories. This division of LDT data collection and reporting of test results has not been endorsed or approved by the FDA or other regulatory agencies, and there can be no assurance that the FDA will continue to regard these as LDTs. Third-party laboratories utilizing or considering the utilization of the Vantera system may find the options for receiving the non-cleared portions of our test unacceptable, which may result in less use or adoption by third-party laboratories of the Vantera system, or less willingness to accept the placement of the Vantera system in their facilities in the first place.
20
In September 2007, the FDA published a draft guidance concerning laboratory-developed tests, or Draft Guidance, that is relevant to some of the tests we may develop in the future. The Draft Guidance describes the FDA's position regarding potential regulation of a type of laboratory developed test known as in vitro diagnostic multivariate index assays, or IVDMIAs, and the revision provided additional examples of the types of tests that would be subject to the Draft Guidance. An IVDMIA is a test system that employs data, derived in part from one or more in vitro assays, and an algorithm that usually, but not necessarily, runs on software, to generate a result that diagnoses a disease or condition or is used in the cure, mitigation, treatment, or prevention of disease.
Continuing FDA Regulation
Under the medical device regulations, the FDA regulates quality control and manufacturing procedures by requiring us to demonstrate and maintain compliance with the quality system regulation, which sets forth the FDA's current good manufacturing practices requirements for medical devices. The FDA monitors compliance with the quality system regulation and current good manufacturing practices requirements by conducting periodic inspections of manufacturing facilities. We could be subject to unannounced inspections by the FDA. Violations of applicable regulations noted by the FDA during inspections of our manufacturing facilities, or the manufacturing facilities of these third parties, could adversely affect the continued marketing of our tests.
The FDA also enforces post-marketing controls that include the requirement to submit medical device reports to the agency when a manufacturer becomes aware of information suggesting that any of its marketed products may have caused or contributed to a death, serious injury or serious illness or any of its products has malfunctioned and that a recurrence of a malfunction would likely cause or contribute to a death or serious injury or illness. The FDA relies on medical device reports to identify product problems and utilizes these reports to determine, among other things, whether it should exercise its enforcement powers. The FDA may also require postmarket surveillance studies for specified devices.
FDA regulations also govern, among other things, the preclinical and clinical testing, manufacture, distribution, labeling and promotion of medical devices. In addition to compliance with good manufacturing practices and medical device reporting requirements, we will be required to comply with the FDCA's general controls, including establishment registration, device listing and labeling requirements. If we fail to comply with any requirements under the FDCA, we could be subject to, among other things, fines, injunctions, civil penalties, recalls or product corrections, total or partial suspension of production, denial of premarket notification clearance or approval of products, rescission or withdrawal of clearances and approvals, and criminal prosecution. We cannot assure you that any final FDA policy, once issued, or future laws and regulations concerning the manufacture or marketing of medical devices will not increase the cost and time to market of new or existing tests. Furthermore, any current or future federal and state regulations also will apply to future tests developed by us.
If our promotional activities fail to comply with these FDA regulations or guidelines, we may be subject to warnings from, or enforcement action by, these authorities. In addition, our failure to follow FDA rules and guidelines relating to promotion and advertising may cause the FDA to issue warning letters or untitled letters, suspend or withdraw a product from the market, require a recall or institute fines or civil fines, or could result in disgorgement of money, operating restrictions, injunctions or criminal prosecution.
Advertising
Advertising of our tests is subject to regulation by the Federal Trade Commission, or FTC, under the FTC Act. The FTC Act prohibits unfair or deceptive acts or practices in or affecting commerce. Violations of the FTC Act, such as failure to have substantiation for product claims, would subject us to a variety of enforcement actions, including compulsory process, cease and desist orders and injunctions, which can require, among other things, limits on advertising, corrective advertising, consumer redress and restitution, as well as substantial fines or other penalties. Any enforcement actions by the FTC could have a material adverse effect our business.
Laboratory Certification, Accreditation and Licensing
We have obtained all federal and state licenses, certificates and permits necessary to conduct our diagnostic testing business. CLIA requires us and most clinical laboratories operating in the United States to maintain federal certification. The State of North Carolina also requires us to maintain a laboratory license. In addition, the laws of some states require licensure for our laboratory, even though we do not operate a laboratory in those states.
21
CLIA imposes requirements relating to test processes, personnel qualifications, facilities and equipment, record keeping, quality assurance and participation in proficiency testing, which involves comparing the results of tests on specimens that have been specifically prepared for our laboratory to the known results of the specimens. CLIA requirements also apply as a condition for participation by clinical laboratories under the Medicare program. Under CLIA regulations, the complexity of the tests performed determines the level of regulatory control. The U.S. Department of Health and Human Services, or HHS, classifies our NMR LipoProfile test as a high-complexity test. As a result, we must employ more experienced and highly educated personnel, as well as additional categories of employees.
HHS or an organization to which HHS delegates authority verifies compliance with CLIA standards through periodic on-site inspections. Sanctions for failure to meet these certification, accreditation and licensure requirements include suspension or revocation of the certification, accreditation or license, as well as imposition of plans to correct deficiencies, injunctive actions and civil monetary and criminal penalties. If HHS should remove or suspend our CLIA certificate, we would be forced to cease performing testing.
We are also accredited by CAP. The CAP Laboratory Accreditation Program is an internationally recognized program that utilizes teams of practicing laboratory professionals as inspectors, and accreditation by CAP can often be used to meet CLIA and state certification requirements.
HIPAA and Other Privacy Laws
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, established for the first time comprehensive protection for the privacy and security of health information. The HIPAA standards apply to three types of organizations, or “Covered Entities”: health plans, healthcare clearing houses, and healthcare providers that conduct certain healthcare transactions electronically. Covered Entities and their Business Associates must have in place administrative, physical, and technical standards to guard against the misuse of individually identifiable health information. Because we are a healthcare provider and we conduct certain healthcare transactions electronically, we are presently a Covered Entity, and we must have in place the administrative, physical, and technical safeguards required by HIPAA, HITECH and their implementing regulations. Additionally, some state laws impose privacy protections more stringent than HIPAA. Most of the institutions and physicians from which we obtain biological specimens that we use in our research and validation work are Covered Entities and must obtain proper authorization from their patients for the subsequent use of those samples and associated clinical information. We may perform future activities that may implicate HIPAA, such as providing clinical laboratory testing services or entering into specific kinds of relationships with a Covered Entity or a Business Associate of a Covered Entity.
If we or our operations are found to be in violation of HIPAA, HITECH or their implementing regulations, we may be subject to penalties, including civil and criminal penalties, fines, and exclusion from participation in U.S. federal or state health care programs, and the curtailment or restructuring of our operations. HITECH increased the civil and criminal penalties that may be imposed against Covered Entities, their Business Associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney's fees and costs associated with pursuing federal civil actions.
Our activities must also comply with other applicable privacy laws. For example, there are also international privacy laws that impose restrictions on the access, use, and disclosure of health information. All of these laws may impact our business. Our failure to comply with these privacy laws or significant changes in the laws restricting our ability to obtain tissue samples and associated patient information could significantly impact our business and our future business plans.
Federal and State Billing and Fraud and Abuse Laws
Antifraud Laws/Overpayments. As participants in federal and state healthcare programs, we are subject to numerous federal and state antifraud and abuse laws. Many of these antifraud laws are broad in scope, and neither the courts nor government agencies have extensively interpreted these laws. Prohibitions under some of these laws include:
• | the submission of false claims or false information to government programs; |
• | deceptive or fraudulent conduct; |
• | excessive or unnecessary services or services at excessive prices; and |
• | prohibitions in defrauding private sector health insurers. |
22
We could be subject to substantial penalties for violations of these laws, including denial of payment and refunds, suspension of payments from Medicare, Medicaid or other federal healthcare programs and exclusion from participation in the federal healthcare programs, as well as civil monetary and criminal penalties and imprisonment. One of these statutes, the False Claims Act, is a key enforcement tool used by the government to combat healthcare fraud. The False Claims Act imposes liability on any person who, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. In addition, violations of the federal physician self-referral laws, such as the Stark laws discussed below, may also violate false claims laws. Liability under the False Claims Act can result in treble damages and imposition of penalties. For example, we could be subject to penalties of $5,500 to $11,000 per false claim, and each use of our product could potentially be part of a different claim submitted to the government. Separately, the HHS office of the Office of Inspector General, or OIG, can exclude providers found liable under the False Claims Act from participating in federally funded healthcare programs, including Medicare. The steep penalties that may be imposed on laboratories and other providers under this statute may be disproportionate to the relatively small dollar amounts of the claims made by these providers for reimbursement. In addition, even the threat of being excluded from participation in federal healthcare programs can have significant financial consequences on a provider.
Numerous federal and state agencies enforce the antifraud and abuse laws. In addition, private insurers may also bring private actions. In some circumstances, private whistleblowers are authorized to bring fraud suits on behalf of the government against providers and are entitled to receive a portion of any final recovery.
Federal and State “Self-Referral” and “Antikickback” Restrictions
Self-Referral law. We are subject to a federal “self-referral” law, commonly referred to as the “Stark” law, which provides that physicians who, personally or through a family member, have ownership interests in or compensation arrangements with a laboratory are prohibited from making a referral to that laboratory for laboratory tests reimbursable by Medicare, and also prohibits laboratories from submitting a claim for Medicare payments for laboratory tests referred by physicians who, personally or through a family member, have ownership interests in or compensation arrangements with the testing laboratory. The Stark law contains a number of specific exceptions which, if met, permit physicians who have ownership or compensation arrangements with a testing laboratory to make referrals to that laboratory and permit the laboratory to submit claims for Medicare payments for laboratory tests performed pursuant to such referrals.
We are subject to comparable state laws, some of which apply to all payors regardless of source of payment, and do not contain identical exceptions to the Stark law. For example, we are subject to a North Carolina self-referral law that prohibits a physician investor from referring to us any patients covered by private, employer-funded or state and federal employee health plans. The North Carolina self-referral law contains few exceptions for physician investors in securities that have not been acquired through public trading, but will generally permit us to accept referrals from physician investors who buy their shares in the public market.
We have several stockholders who are physicians in a position to make referrals to us. We have included within our compliance plan procedures to identify requests for testing services from physician investors and we do not bill Medicare, or any other federal program, or seek reimbursement from other third-party payors, for these tests. The self-referral laws may cause some physicians who would otherwise use our laboratory to use other laboratories for their testing.
Providers are subject to sanctions for claims submitted for each service that is furnished based on a referral prohibited under the federal self-referral laws. These sanctions include denial of payment and refunds, civil monetary payments and exclusion from participation in federal healthcare programs and civil monetary penalties, and they may also include penalties for applicable violations of the False Claims Act, which may require payment of up to three times the actual damages sustained by the government, plus civil penalties of $5,500 to $11,000 for each separate false claim. Similarly, sanctions for violations under the North Carolina self-referral laws include refunds and monetary penalties.
23
Anti-Kickback Statute. The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce either the referral of an individual, or the furnishing, recommending, or arranging for a good or service, for which payment may be made under a federal healthcare program, such as the Medicare and Medicaid programs. The term “remuneration” is not defined in the federal Anti-Kickback Statute and has been broadly interpreted to include anything of value, including for example, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash, waivers of payment, ownership interests and providing anything at less than its fair market value. The reach of the Anti-Kickback Statute was also broadened by the Patient Protection and Affordable Care Act of 2010, or PPACA, which, among other things, amends the intent requirement of the federal Anti-Kickback Statute and certain criminal healthcare fraud statutes, effective March 23, 2010. Pursuant to the statutory amendment, a person or entity does not need to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. In addition, PPACA provides that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act or the civil monetary penalties statute, which imposes penalties against any person who is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. Sanctions for violations of the federal Anti-Kickback Statute may include imprisonment and other criminal penalties, civil monetary penalties and exclusion from participation in federal healthcare programs.
The OIG has criticized a number of the business practices in the clinical laboratory industry as potentially implicating the Anti-Kickback Statute, including compensation arrangements intended to induce referrals between laboratories and entities from which they receive, or to which they make, referrals. In addition, the OIG has indicated that “dual charge” billing practices that are intended to induce the referral of patients reimbursed by federal healthcare programs may violate the Anti-Kickback Statute.
Many states have also adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs, and do not contain identical safe harbors. For example, North Carolina has an anti-kickback statute that prohibits healthcare providers from paying any financial compensation for recommending or securing patient referrals. Penalties for violations of this statute include license suspension or revocation or other disciplinary action. Other states have similar anti-kickback prohibitions.
Both the federal Anti-Kickback Statute and the North Carolina anti-kickback law are broad in scope. The anti-kickback laws clearly prohibit payments for patient referrals. Under a broad interpretation, these laws could also prohibit a broad array of practices involving remuneration where one party is a potential source of referrals for the other.
If we or our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in U.S. federal or state health care programs, and the curtailment or restructuring of our operations. To the extent that any product we make is sold in a foreign country in the future, we may be subject to similar foreign laws and regulations, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws, and implementation of corporate compliance programs and reporting of payments or transfers of value to healthcare professionals. To reduce the risks associated with these various laws and governmental regulations, we have implemented a compliance plan. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management's attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal and state privacy, security and fraud laws may prove costly.
Sunshine Act
In 2010, Congress enacted a statute commonly known as the Sunshine Act, as part of PPACA. The Sunshine Act aims to promote transparency and requires manufacturers of drugs, devices, biologicals and medical supplies covered by Medicare, Medicaid or the Children's Health Insurance Program, or CHIP, to report annually to CMS any payments or other transfers of value made to physicians and teaching hospitals, with limited exceptions. Manufacturers must also disclose to CMS any physician ownership or investment interests. On February 8, 2013, CMS issued a final rule implementing the Sunshine Act. Entities covered by the Sunshine Act, including us, must begin reporting by March 31, 2014, and failure to comply with the reporting requirement may subject us to substantial penalties.
24
Other Laws
Occupational Safety and Health. The State of North Carolina has an OSHA-approved state occupational safety and health plan allowing it to impose stricter worker health and safety standards than those promulgated by the federal Occupational Safety and Health Administration, or OSHA. In addition to their comprehensive regulation of health and safety in the workplace in general, OSHA and the North Carolina Department of Labor Occupational Safety and Health Division have established extensive requirements aimed specifically at laboratories and other healthcare-related facilities. In particular, both agencies have implemented regulations intended to protect workers who may be exposed to bloodborne pathogens, such as HIV and hepatitis B, and other potentially infectious materials. In addition, because our operations require employees to use certain hazardous chemicals, we also must comply with regulations on hazard communication and hazardous chemicals in laboratories. These regulations require us, among other things, to develop written programs and plans, which must address methods for preventing and mitigating employee exposure, the use of personal protective equipment, and training.
Specimen Transportation. We also are subject to regulations of the Department of Transportation, the United States Postal Service and the CDC that apply to the surface and air transportation of clinical laboratory specimens.
Environmental Compliance. We handle and dispose of human fluids and medical waste, such as vials and needles, in connection with our operations. The fluids and waste are treated as biohazardous material. We must comply with numerous federal, state and local statutes and regulations, particularly, to the extent applicable, the Medical Waste Tracking Act of 1988 and the Resource Conservation and Recovery Act. The statutes with which we must comply relate to public health and the environment, including practices and procedures for labeling, handling and storage of, and public disclosure requirements regarding, medical waste, hazardous and toxic materials or other substances generated by operation of clinical laboratories. We must also comply with environmental protection requirements, such as standards relating to the discharge of pollutants into the air, water and land, emergency response and remediation or cleanup in connection with medical waste, hazardous and toxic materials or other substances.
Employees
As of December 31, 2012, we had 204 employees. None of our employees are represented by a labor union or covered under a collective bargaining agreement, nor have we experienced any work stoppages. We consider our employee relations to be good.
Available Information
Our internet website address is www.liposcience.com. In addition to the information about us and our subsidiaries contained in this Annual Report, information about us can be found on our website. Our website and information included in or linked to our website are not part of this Annual Report.
Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, are available free of charge through our website as soon as reasonably practicable after they are electronically filed with or furnished to the Securities and Exchange Commission, or SEC. The public may read and copy the materials we file with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Additionally the SEC maintains an internet site that contains reports, proxy and information statements and other information. The address of the SEC's website is www.sec.gov.
25
Item 1A. Risk Factors
Our business is subject to numerous risks. You should carefully consider the following risks and all other information contained in this Annual Report, as well as general economic and business risks, together with any other documents we file with the SEC. If any of the following events actually occur or risks actually materialize, it could have a material adverse effect on our business, operating results and financial condition and cause the trading price of our common stock to decline.
Risks Related to Our Business and Strategy
Our ability to successfully execute our strategy is dependent on our achieving greater market acceptance of the NMR LipoProfile test.
Our ability to generate revenue depends on our successful marketing of the NMR LipoProfile test. The NMR LipoProfile test accounted for 94% of our revenues for the year ended December 31, 2012, 93% of our revenues for the year ended December 31, 2011 and 87% of our revenues for the year ended December 31, 2010. We expect that our revenues and profitability will depend on sales of the NMR LipoProfile test for the foreseeable future.
There is not currently widespread awareness of the NMR LipoProfile test among clinicians, even though the test has been available since 1999. In order to achieve greater market acceptance of the NMR LipoProfile test, we must continue to demonstrate to clinicians, other healthcare professionals, clinical diagnostic laboratories, healthcare thought leaders and third-party payors that the test is a clinically useful and cost-effective diagnostic test and disease management tool for cardiovascular disease risk providing improved or additional benefits over traditional cholesterol testing, which has been widely accepted as effective for managing cardiovascular risk for many years.
When seeking testing and management recommendations for coronary heart disease, many physicians and other clinicians look to clinical guidelines published by influential organizations. Such organizations include the National Cholesterol Education Program, or NCEP, an authority on cholesterol management overseen by the National Heart, Lung and Blood Institute, or NHLBI, part of the National Institutes of Health, and the AHA. The NMR LipoProfile test is not currently included in guidelines published by NCEP or the AHA. If we are not successful in our strategy of gaining inclusion in the guidelines published by these or other organizations, it could ultimately limit market adoption of the NMR LipoProfile test.
Our industry is characterized by rapid technological changes, and current or future studies may question the utility of LDL-P testing or suggest new approaches or technologies that may be more beneficial to patient care than our current tests or the tests that we may develop in the future. In such cases, it could make it more difficult to persuade clinicians, payors and publishers of clinical guidelines of the utility of our NMR LipoProfile test and other tests that we may develop in the future compared to existing tests or new approaches or technologies.
A small number of clinical diagnostic laboratory customers account for most of the sales of our NMR LipoProfile test. If any of these laboratories orders fewer tests from us for any reason, our revenues could decline.
For the years ended December 31, 2012, 2011 and 2010, we generated 84%, 76% and 61% of our revenues, respectively, from clinical diagnostic laboratory customers. Sales to one of these laboratories, Laboratory Corporation of America Holdings, or LabCorp, accounted for 29% of our revenues for the year ended December 31, 2012, 33% of our revenues for the year ended December 31, 2011 and 33% of our revenues for the year ended December 31, 2010. Sales to a second laboratory customer, Health Diagnostics Laboratory, Inc., accounted for 32% of our revenues for the year ended December 31, 2012 and 21% of our revenues for the year ended December 31, 2011.
Our current agreements with our laboratory customers do not require them to purchase any minimum quantities of the NMR LipoProfile test. In addition, these customers generally have the right to terminate their respective agreements with us at any time. If any major customer were to terminate its relationship with us, or to substantially diminish its purchases of the NMR LipoProfile test, our revenues could significantly decline or it could adversely impact our revenue growth.
26
We expect to incur losses for the next several years as we increase expenses in our effort to increase market share for the NMR LipoProfile test, place the Vantera system in third-party clinical diagnostic laboratories, and develop new personalized diagnostic tests.
Although we generated net income for the year ended December 31, 2012, we have incurred significant losses since our inception. As of December 31, 2012, we had an accumulated deficit of $48.0 million. We anticipate experiencing losses for the next several years as we increase expenses in pursuit of our growth strategy and our efforts to increase market share for the NMR LipoProfile test, place the Vantera system in third-party clinical diagnostic laboratories, and develop new personalized diagnostic tests.
Historically, our losses have resulted principally from research and development programs, our sales and marketing efforts, and our general and administrative expenses. We expect to continue to incur significant operating expenses and anticipate that our expenses and losses will increase due to costs relating to, among other things:
• | expansion of our direct sales force and increasing our marketing capabilities to promote market awareness and acceptance of our NMR LipoProfile test; |
• | placement of the Vantera system in third-party clinical diagnostic laboratories; |
• | development of and, as necessary, pursuit of regulatory approvals for, new diagnostic tests; |
• | expansion of our operating capabilities; |
• | maintenance, expansion and protection of our intellectual property portfolio and trade secrets; |
• | employment of additional clinical, quality control, scientific and management personnel; and |
• | employment of operational, financial, accounting and information systems personnel, consistent with expanding our operations and our status as a newly public company. |
To become and remain profitable, we must succeed in increasing sales of our NMR LipoProfile test or develop and commercialize new tests with significant market potential, and place the Vantera system in third-party clinical diagnostic laboratories. We may never succeed in these activities and may never generate revenues that are sufficient to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain consistently profitable would likely depress the market price of our common stock and could significantly impair our ability to raise capital, expand our business or continue to pursue our growth strategy.
If we do not establish relationships with additional clinical diagnostic laboratories, we may not be able to increase the number of NMR LipoProfile tests we sell.
A significant element of our strategy is to leverage relationships with clinical diagnostic laboratories to increase market acceptance of the NMR LipoProfile test and gain market share. Most clinicians who request traditional cholesterol tests, our NMR LipoProfile test and other diagnostic tests to evaluate cardiovascular disease risk order these tests through clinical diagnostic laboratories.
If we are unable to establish relationships with additional clinical diagnostic laboratories, clinicians who order tests through these laboratories may be unwilling or unable to order our NMR LipoProfile test. In addition, we would not have the benefit of leveraging the sales, marketing and distribution capabilities of these laboratories, which we believe is important to our ability to increase awareness of and expand utilization of the NMR LipoProfile test. As a result, if we are unable to establish additional clinical laboratory relationships, our ability to increase sales of our NMR LipoProfile test and to successfully execute our strategy could be compromised.
27
We will need to expand our marketing and sales capabilities in order to increase demand for our NMR LipoProfile test, to expand geographically and to successfully commercialize any other personalized diagnostic tests we may develop.
We believe our current sales and marketing operations are not sufficient to achieve the level of market awareness and sales required for us to attain significant commercial success for our NMR LipoProfile test, to expand our geographic presence and to successfully commercialize any other diagnostic tests we may develop. In order to increase sales of our NMR LipoProfile test, we will need to:
• | expand our direct sales force in the United States by recruiting additional sales representatives in selected markets; |
• | educate clinicians, other healthcare professionals, clinical diagnostic laboratories, healthcare thought leaders and third-party payors regarding the clinical benefits and cost-effectiveness of our NMR LipoProfile test; |
• | expand our number of clinical diagnostic laboratory and hospital outreach laboratory customers; and |
• | establish, expand and manage sales and reimbursement arrangements with third parties, such as clinical diagnostic laboratories and insurance companies. |
We have limited experience in selling and marketing the NMR LipoProfile test nationally, and we have only limited experience in placing and servicing the Vantera system in third-party clinical diagnostic laboratories for commercial purposes. We intend to hire a significant number of additional sales and marketing personnel with experience in the diagnostic, medical device or pharmaceutical industries. We may face competition from other companies in these industries, some of whom are much larger than us and who can pay significantly greater compensation and benefits than we can, in seeking to attract and retain qualified sales and marketing employees. If we are unable to hire and retain qualified sales and marketing personnel, our business will suffer.
Furthermore, in order to successfully commercialize diagnostic tests that we may develop in the future, we may need to conduct lengthy, expensive clinical trials and develop dedicated sales and marketing operations to achieve market awareness and demand. If we are not able to successfully implement our marketing, sales and commercialization strategies, we may not be able to expand geographically, increase sales of our NMR LipoProfile test or successfully commercialize any future diagnostic tests that we may develop.
The diagnostic industry features rapidly changing technology that could make our current test or the tests we are developing obsolete or less competitive unless we continue to develop and manufacture new and improved tests and pursue new market opportunities.
Our industry is characterized by rapid technological changes, frequent new product introductions and enhancements and evolving industry standards. Our future success will depend on our ability to keep pace with the evolving needs of our customers on a timely and cost-effective basis and to pursue new market opportunities that develop as a result of technological and scientific advances. These new market opportunities may be outside the scope of our expertise or in areas that have unproven market demand, and the utility and value of new tests that we develop may not be accepted in the market. Our inability to gain market acceptance of new tests could harm our future operating results. Further, if new research or clinical evidence or economic comparative evidence arises that supports a different marker for coronary heart disease risk, demand for our test could decline.
Our NMR LipoProfile test competes with other diagnostic testing methods that may be more widely accepted than our test.
The clinical diagnostics market is highly competitive, and we must be able to compete effectively against existing and future competitors in order to be successful. In selling our NMR LipoProfile test, we compete primarily with existing diagnostic, detection and monitoring technologies, particularly the conventional lipid panel test, which is relatively inexpensive, widely reimbursed and broadly accepted as an effective test for managing the risk of developing cardiovascular disease. We also compete against companies that offer other methods for directly or indirectly measuring cholesterol concentrations, lipoproteins or lipoprotein particles. For example, measuring apolipoprotein B, or apoB, is an indirect way to approximate LDL-P. ApoB tests are offered by many clinical diagnostic laboratories and are generally less expensive than our tests. It is possible that apoB, or other competing tests, could be perceived by clinicians as more cost-effective than our test in providing information useful in managing CHD risk. In addition, some competitors offering these competing technologies may have longer operating histories, better name recognition and greater financial, technical, sales, marketing, distribution and public relations resources than we have. They may also have more experience in research and development, regulatory matters, manufacturing and marketing than we do, and may have established broad third-party reimbursement for their tests. If we do not compete successfully, we will not be able to increase our market share and our business will be seriously harmed.
28
Even though the Vantera system has received regulatory clearance in the United States, if laboratories are not receptive to placement of the Vantera system at their facilities, or if we do not receive regulatory clearance of the Vantera system in other jurisdictions, our growth strategy may not be successful.
A key element of our strategy is to place the Vantera system, our next-generation automated clinical NMR analyzer, on site with qualified clinical diagnostic laboratory customers to broaden access to our technology and increase demand for our NMR LipoProfile test and any future diagnostic tests that we may develop. Although we received clearance from the FDA to perform the FDA-cleared measurements of the NMR LipoProfile test using the Vantera system in August 2012, we have not applied for clearance from comparable regulatory agencies in other countries for the Vantera system, and we may not receive regulatory clearance for the commercial use of the Vantera system in other countries on a timely basis, or at all. Even though the Vantera system is cleared by the FDA, it may not gain significant acceptance by clinical diagnostic laboratories, or these laboratories may not be satisfied with the Vantera system after it is placed in their facilities. If clinical diagnostic laboratories do not accept the placement of the Vantera system in their facilities, our ability to grow our business by deploying the Vantera system could be compromised.
We rely on two key suppliers for the components used in the Vantera system. If we were to lose either of these suppliers, our ability to broadly place the Vantera system could be compromised.
We currently rely on a single supplier, Agilent Technologies, Inc., for the magnet, probe and console incorporated in the Vantera system. These are the key components of the analyzers necessary to perform our NMR LipoProfile test. We are party to a supply agreement with Agilent pursuant to which we have agreed to exclusively purchase all of our NMR-related components from them. We are also party to a production agreement with KMC Systems, Inc. under which KMC is our exclusive manufacturer of the sample handler and shell for the Vantera system.
We are currently aware of one other primary supplier of NMR spectrometers, Bruker BioSpin, part of Bruker Corporation. We have in the past acquired NMR spectrometers from Bruker BioSpin, and we use them in our current generation of NMR clinical analyzers, but we do not currently have an agreement or relationship with Bruker BioSpin. In the event it is necessary or desirable to acquire NMR spectrometers from Bruker BioSpin, we might not be able to obtain them on commercially reasonable terms, if at all. It could also require significant time and expense to redesign our current analyzers or the Vantera system to work with the spectrometers provided by another company.
If we are unable to obtain the NMR components we need at a reasonable price or on a timely basis, we may be unable to maintain the analyzers we use in our facility to perform our NMR LipoProfile test, which could compromise our ability to meet our customers' orders for the test. Likewise, if the components or any other part of the Vantera system are not available when needed, we may not be able to place the Vantera system broadly, which could impair our ability to pursue our growth strategy.
If we do not successfully develop or acquire and introduce new personalized diagnostic tests or other applications of our technology, we may not generate new sources of revenue and may not be able to successfully implement our growth strategy.
Our business strategy includes the acquisition, development and introduction of new clinical diagnostic applications of our NMR-based technology in addition to our NMR LipoProfile test. Additionally, we believe that for our Vantera system to be attractive to laboratories to place in their facilities, it may be necessary for us to offer additional tests for use on the Vantera system. All of our diagnostic tests under development will require significant additional research and development, a commitment of significant additional resources and possibly costly and time-consuming clinical testing prior to their commercialization. Our technology is complex, and we cannot be sure that any tests under development will be developed successfully, be proven to be effective, offer diagnostic or other improvements over currently available tests, meet applicable regulatory standards, be produced in commercial quantities at acceptable costs or be successfully marketed.
We may also in the future seek to acquire complementary products or technologies from third parties. Integrating any product or technology we acquire could be expensive and time-consuming, disrupt our ongoing business and distract our management. If we are unable to integrate any tests or technologies effectively, we may not be able to implement our business model. If we do not successfully develop new clinical diagnostic applications of our NMR-based technology or acquire complementary diagnostic products, we could lose interest from academic medical centers and could also lose revenue opportunities with existing or future clinical laboratory customers.
29
If we are not able to retain and recruit qualified management, sales and marketing, regulatory and research and development personnel, we may be unable to successfully execute our business strategy.
Our future success depends to a significant extent on the skills, experience and efforts of our senior management team, including Richard O. Brajer, our President and Chief Executive Officer; Lucy G. Martindale, our Chief Financial Officer; James D. Otvos, our Chief Scientific Officer and founder; Timothy J. Fischer, our Chief Operating Officer; and Thomas S. Clement, our Vice President of Regulatory and Quality Affairs. The loss of any or all of these individuals, or other management personnel, could harm our business and might significantly delay or prevent the achievement of our business objectives. We have entered into an employment agreement or offer letters with each of these individuals and with our other executives. The existence of an employment agreement or offer letter does not, however, guarantee retention of these employees, and we may not be able to retain those individuals for the duration of or beyond the end of their respective terms. We do not maintain key person life insurance on any of our management personnel.
Recruiting and retaining qualified sales and marketing, regulatory, scientific and laboratory personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms, given the competition among numerous diagnostic, medical device, pharmaceutical and biotechnology companies for similarly skilled personnel.
If we are unable to successfully manage our growth, our business will be harmed.
During the past several years, we have significantly expanded our operations, and we expect this expansion to continue to an even greater degree as we seek to expand nationally and explore potential expansion into international markets. Our growth has placed and will continue to place a significant strain on our management, operating and financial systems and our sales, marketing and administrative resources. As a result of our growth, our operating costs may escalate even faster than planned, and some of our internal systems may need to be enhanced or replaced. If we cannot effectively manage our expanding operations and our costs, we may not be able to continue to grow or we may grow at a slower pace and our business could be adversely affected.
We currently perform our tests exclusively in one laboratory facility. If this or any future facility or our equipment were damaged or destroyed, or if we experience a significant disruption in our operations for any reason, our ability to continue to operate our business could be materially harmed.
We currently perform our NMR LipoProfile tests exclusively in a single laboratory facility in Raleigh, North Carolina. If this or any future facility were to be damaged, destroyed or otherwise unable to operate, whether due to fire, floods, hurricanes, storms, tornadoes, other natural disasters, employee malfeasance, terrorist acts, power outages, or otherwise, or if performance of our analyzers is disrupted for any other reason, we may not be able to perform our tests or generate test reports as promptly as our customers expect, or possibly not at all. If we are unable to perform our tests or generate test reports within a timeframe that meets our customers' expectations, our business, financial results and reputation could be materially harmed.
Currently, we maintain insurance coverage totaling $12 million against damage to our property and equipment and an additional $10 million to cover business interruption and research and development restoration expenses, subject to deductibles and other limitations. If we have underestimated our insurance needs with respect to an interruption, or if an interruption is not subject to coverage under our insurance policies, we may not be able to cover our losses.
Failure in our information technology, storage systems or our analyzers could significantly disrupt our operations and our research and development efforts, which could adversely impact our revenues, as well as our research, development and commercialization efforts.
Our ability to execute our business strategy depends, in part, on the continued and uninterrupted performance of our information technology, or IT, systems, which support our operations and our research and development efforts, as well as our storage systems and our clinical analyzers, including the Vantera system analyzers. Due to the sophisticated nature of the NMR technology we use in our testing, we are substantially dependent on our IT systems. IT systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts and natural disasters. Moreover, despite network security and back-up measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptive problems. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our IT systems, sustained or repeated system failures that interrupt our ability to generate and maintain data, and in particular to operate our NMR analyzers, including any Vantera system analyzers placed in third-party clinical diagnostic laboratories, could adversely affect our ability to operate our business. Any interruption in the operation of our NMR analyzers, due to IT system failures, part failures or potential disruptions in the event we are required to relocate our analyzers within our facility or to another facility, or failures of the Vantera system analyzers within the facilities of third-party clinical diagnostic laboratories, could have an adverse effect on our operations.
30
We rely on courier delivery services to transport samples to our facility for analysis. If these delivery services are disrupted, our business and customer satisfaction could be negatively impacted.
Clinicians and clinical laboratories ship samples to us by air and ground express courier delivery service for analysis in our Raleigh, North Carolina facility. Disruptions in delivery service, whether due to bad weather, natural disaster, terrorist acts or threats, or for other reasons, can adversely affect specimen quality and our ability to provide our services on a timely basis to customers.
Our business involves the use of hazardous materials that could expose us to environmental and other liabilities.
Our laboratory facility is subject to various local, state and federal laws and regulations relating to safe working conditions, laboratory and manufacturing practices and the use and disposal of hazardous or potentially hazardous substances, including chemicals, biological materials and various compounds used in connection with our research and development activities. In the United States, these laws include the Occupational Safety and Health Act, the Toxic Test Substances Control Act and the Resource Conservation and Recovery Act. We cannot assure you that accidental contamination or injury to our employees and third parties from hazardous materials will not occur. We do not have insurance to cover claims arising from our use and disposal of these hazardous substances other than limited clean-up expense coverage for environmental contamination due to an otherwise insured peril, such as fire.
If product liability lawsuits are successfully brought against us, we may incur substantial liabilities that could have a significant negative effect on our financial condition or reputation.
Diagnostic testing entails the risk of product liability, and we may be exposed to liability claims arising from the use of our tests. We maintain product liability insurance that is subject to deductibles and coverage limitations and is in an amount that we believe to be reasonable. We cannot be certain, however, that our product liability insurance will be sufficient to protect us against losses due to liability. As a result, we may be required to pay all or a portion of any successfully asserted product liability claim out of our cash reserves. Furthermore, we cannot be certain that product liability insurance will continue to be available to us on commercially reasonable terms or in sufficient amounts. We can provide no assurance that we will be able to avoid significant product liability claims, which could hurt our reputation and our financial condition.
If we expand sales of our products or place the Vantera system outside of the United States, our business will be susceptible to costs and risks associated with international operations.
As part of our longer-term growth strategy, we may decide to target select international markets to grow our presence outside of the United States. Conducting international operations would subject us to new risks that, generally, we have not faced in the United States, including:
• | fluctuations in currency exchange rates; |
• | longer accounts receivable payment cycles and difficulties in collecting accounts receivable; |
• | uncertain regulatory registration and approval processes for seeking clearance of the Vantera system and our diagnostic tests; |
• | competition from companies located in the countries in which we offer our products, which may be a competitive disadvantage; |
• | difficulties in managing and staffing international operations and assuring compliance with foreign corrupt practices laws; |
• | the possibility of management distraction; |
• | potentially adverse tax consequences, including the complexities of foreign value added tax systems, tax inefficiencies related to our corporate structure and restrictions on the repatriation of earnings; |
• | increased financial accounting and reporting burdens and complexities; |
• | political, social and economic instability abroad, terrorist attacks and security concerns in general; and |
31
• | reduced or varied protection for intellectual property rights in some countries. |
The occurrence of any one of these risks could harm our business or results of operations. Additionally, operating internationally requires significant management attention and financial resources. We cannot be certain that the investment and additional resources required in establishing operations in other countries will produce desired levels of revenues or profitability.
We may use third-party collaborators to help us develop, validate or commercialize any new diagnostic tests, and our ability to commercialize such tests could be impaired or delayed if these collaborations are unsuccessful.
We may license or selectively pursue strategic collaborations for the development, validation and commercialization of any new diagnostic tests we may develop. In any third-party collaboration, we would be dependent upon the success of the collaborators in performing their responsibilities and their continued cooperation. Our collaborators may not cooperate with us or perform their obligations under our agreements with them. We cannot control the amount and timing of our collaborators' resources that will be devoted to performing their responsibilities under our agreements with them. Our collaborators may choose to pursue alternative technologies in preference to those being developed in collaboration with us. The development, validation and commercialization of our potential tests will be delayed if collaborators fail to conduct their responsibilities in a timely manner or in accordance with applicable regulatory requirements or if they breach or terminate their collaboration agreements with us. Disputes with our collaborators could also impair our reputation or result in development delays, decreased revenues and litigation expenses.
Failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could cause investors to lose confidence in our operating results and in the accuracy of our financial reports and could have a material adverse effect on our business and on the price of our common stock.
We are subject to the reporting requirements of the Securities Exchange Act of 1934, the Sarbanes‑Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and the rules and regulations of the NASDAQ Stock Market. The Sarbanes‑Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal controls over financial reporting. Commencing with our fiscal year ending December 31, 2013, we must perform system and process evaluation and testing of our internal controls over financial reporting to allow management to report on the effectiveness of our internal controls over financial reporting in our Form 10-K filing for that year, as required by Section 404 of the Sarbanes‑Oxley Act. This will require that we incur substantial additional professional fees and internal costs to expand our accounting and finance functions and that we expend significant management efforts.
The controls and other procedures are designed to ensure that information required to be disclosed by us in the reports that we file with the Securities and Exchange Commission, or SEC, is disclosed accurately and is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms. Prior to our initial public offering in January 2013, we were never required to test our internal controls within a specified period, and, as a result, we may experience difficulty in meeting these reporting requirements in a timely manner. We are in the early stages of conforming our internal control procedures to the requirements of Section 404 and we may not be able to complete our evaluation, testing and any required remediation needed to comply with Section 404 in a timely fashion. Our independent registered public accounting firm was not engaged to perform an audit of our internal control over financial reporting for the year ended December 31, 2012 or for any other period. Our independent registered public accounting firm's audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of our internal control over financial reporting. Accordingly, no such opinion was expressed. Even if we develop effective controls, these new controls may become inadequate because of changes in conditions or the degree of compliance with these policies or procedures may deteriorate.
Even after we develop new procedures, material weaknesses in our internal control over financial reporting may be discovered. Our internal control over financial reporting will not prevent or detect all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system's objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud will be detected.
In order to fully comply with Section 404, we will need to retain additional employees to supplement our current finance staff, and we may not be able to so in a timely manner, or at all. In addition, in the process of evaluating our internal control over financial reporting we expect that certain of our internal control practices will need to be updated to comply with the requirements of Section 404 and the regulations promulgated thereunder, and we may not be able to do so on a timely basis, or at all.
32
If we are not able to comply with the requirements of Section 404 of the Sarbanes‑Oxley Act in a timely manner, or if we are unable to maintain proper and effective internal controls, we may not be able to produce timely and accurate financial statements, and we may conclude that our internal controls over financial reporting are not effective. If that were to happen, we could be subject to sanctions or investigations by the NASDAQ Stock Market, the SEC or other regulatory authorities, and investors may lose confidence in our operating results and the price of our common stock could decline.
If we fail to comply with the covenants and other obligations under our credit facility, the lenders may be able to accelerate amounts owed under the facility and may foreclose upon the assets securing our obligations.
In December 2012, we entered into a credit facility with Oxford Finance, or Oxford, and Square 1 Bank, or Square 1. The facility consists of $10 million in term loans from Oxford, a $6 million term loan from Square 1 and a $6 million revolving line of credit from Square 1. The term loans are payable in monthly installments of interest only through January 2014 and then principal and interest thereafter in monthly installments through July 2016. The line of credit matures in December 2013. Borrowings under our credit facility are secured by substantially all of our tangible assets. The covenants set forth in the loan and security agreement require, among other things, that we maintain a specified liquidity ratio, measured monthly, that begins at 1.25 and is reduced to 1.0 over the term of the agreement, and that we achieve minimum three-month trailing revenue levels during the term of the agreement, which are based on 80% of our projected revenue levels. In addition, the loan and security agreement requires that our projections provided to the lenders include annual projected revenues of at least $55 million. If we fail to comply with the covenants and our other obligations under the credit facility, the lenders would be able to accelerate the required repayment of amounts due under the loan agreement and, if they are not repaid, could foreclose upon our assets securing our obligations under the credit facility.
We may need to raise additional capital to meet our financial obligations and to pursue our business objectives, and if we cannot raise additional capital when needed, we may have to curtail or cease operations.
We cannot assure you that the proceeds of our recent initial public offering will be sufficient to fully fund our business and growth strategy. We may need to raise additional funds through public or private equity or debt financing to continue to fund or expand our operations.
Our actual liquidity and capital funding requirements will depend on numerous factors, including:
• | the extent to which our tests, including the NMR LipoProfile test and other tests under development, are successfully developed, gain regulatory clearance and market acceptance and become and remain competitive; |
• | our ability to obtain more extensive medical policy coverage for our tests, and our ability to effectively manage and control the pricing we receive from third-party clinical laboratories for our tests; |
• | our ability to collect our accounts receivable; |
• | the costs and timing of further expansion of our sales and marketing activities and research and development activities; and |
• | the timing and results of any regulatory approvals that we are required to obtain for our diagnostic tests. |
If we seek to raise additional capital in order to meet various objectives, including developing future diagnostic tests, increasing working capital and responding to competitive pressures, that capital may not be available on favorable terms or may not be available at all. In addition, pursuant to the terms of our credit facility, we may be restricted from using the net proceeds of financing transactions for our operating objectives. Lack of sufficient capital resources could significantly limit our ability to take advantage of business and strategic opportunities. Furthermore, any additional capital raised through the sale of equity will dilute your ownership interest in us and may have an adverse effect on the price of our common stock. In addition, the terms of the financing may adversely affect your holdings or rights. Debt financing, if available, may include restrictive covenants.
If we are not able to obtain adequate funding when needed, we may have to delay development or commercialization of our diagnostic tests or license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize ourselves. We also may have to reduce research and development, sales and marketing, customer support or other expenses. Any of these outcomes could harm our business.
33
Our net operating loss carryforwards may expire unutilized or underutilized, which could prevent us from offsetting future taxable income.
We may be limited in the portion of net operating loss carryforwards that we can use in the future to offset taxable income for U.S. federal income tax purposes. As of December 31, 2012, our available federal net operating losses, or NOLs, and federal research and development tax credits totaled $34.9 million. In general, under Section 382 of the Internal Revenue Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change NOLs and tax credits to offset future taxable income. We believe that we have had one or more ownership changes, as a result of which our existing NOLs are currently subject to limitation. In addition, if we undergo an ownership change in connection with or after our initial public offering, our ability to utilize our NOLs could be further limited by Section 382. Future changes in our stock ownership, some of which are outside of our control, could result in additional ownership changes under Section 382. We are unable to predict the future ownership and other variables considered by, and elections available pursuant to, Section 382 for determining the usability of our net operating losses. We may not be able to utilize a material portion of our NOLs, even if we attain profitability.
We periodically assess the likelihood that we will be able to recover our net deferred tax assets. We consider all available evidence, both positive and negative, including historical levels of income, expectations and risks associated with estimates of future taxable income and ongoing prudent and feasible profits. As a result of this analysis of all available evidence, both positive and negative, we concluded that a valuation allowance against our net deferred tax assets should be applied as of December 31, 2012. To the extent we determine that all or a portion of our valuation allowance is no longer necessary, we will recognize an income tax benefit in the period such determination is made for the reversal of the valuation allowance. Once the valuation allowance is eliminated or reduced, its reversal will no longer be available to offset our current tax provision. These events could have a material impact on our reported results of operations.
We will incur costs and demands upon management as a result of complying with the laws and regulations affecting public companies in the United States, which may adversely affect our operating results.
As a public company listed in the United States, and particularly after we cease to be an “emerging growth company,” we will incur significant additional legal, accounting and other expenses. In addition, changing laws, regulations and standards relating to corporate governance and public disclosure, including regulations implemented by the SEC and NASDAQ, may increase legal and financial compliance costs and make some activities more time-consuming. These laws, regulations and standards are subject to varying interpretations and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management's time and attention from revenue-generating activities to compliance activities. If notwithstanding our efforts to comply with new laws, regulations and standards, we fail to comply, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
Failure to comply with these rules might also make it more difficult for us to obtain certain types of insurance, including director and officer liability insurance, and we might be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, on committees of our board of directors or as members of senior management.
Risks Related to Billing, Coverage and Reimbursement for Our Tests
Health insurers and other third-party payors may decide not to cover, or may discontinue reimbursing, our NMR LipoProfile test or any other diagnostic tests we may develop in the future, or may provide inadequate reimbursement, which could jeopardize our ability to expand our business and achieve profitability.
Our business is impacted by the level of reimbursement for our NMR LipoProfile test from third-party payors. In the United States, the regulatory process allows diagnostic tests to be marketed regardless of any coverage determinations made by payors. For new diagnostic tests, each third-party payor makes its own decision about which tests it will cover, how much it will pay and whether it will continue reimbursing the test. Clinicians may order diagnostic tests that are not reimbursed by third-party payors if the patient is willing to pay for the test without reimbursement, but coverage determinations and reimbursement levels and conditions are critical to the commercial success of a diagnostic product.
34
CMS, under the U.S. Department of Health and Human Services, or HHS, establishes reimbursement payment levels and coverage rules for Medicare. CMS currently covers our NMR LipoProfile test. State Medicaid plans and private payors establish rates and coverage rules independently. As a result, the coverage determination process is often a time-consuming and costly process that requires us to provide scientific and clinical support for the use of our tests to each payor separately, with no assurance that coverage or adequate reimbursement will be obtained. While our test is reimbursed by a number of governmental and private payors, which we believe collectively represent approximately 150 million covered lives, there are significant large private payors who do not currently cover our test. Additionally, certain employers may switch benefit design through their choice of payor, which may also negatively impact coverage for our tests. If CMS or other third-party payors decide not to cover our diagnostic tests, place significant restrictions on the use of our tests, or offer inadequate payment amounts, our ability to generate revenue from our diagnostic tests could be limited. It is possible that current or future studies may question the utility of LDL-P or other tests that we may develop in the future, which could make it more difficult for us to persuade third-party payors of the utility of our NMR LipoProfile test and other tests that we may develop in the future.
Even if one or more third-party payors decides to reimburse for our tests, that payor may reduce utilization or stop or lower payment at any time, which could reduce our revenues. For example, payment for diagnostic tests furnished to Medicare beneficiaries is made based on a fee schedule set by CMS. In recent years, payments under these fee schedules have decreased and may decrease more. We cannot predict whether or when third-party payors will cover our tests or offer adequate reimbursement to make them commercially attractive. Clinicians or patients may decide not to order our tests if third-party payments are inadequate, especially if ordering the test could result in financial liability for the patient.
Billing complexities associated with obtaining payment or reimbursement for our tests may negatively affect our revenues, cash flow and profitability.
Billing for clinical laboratory testing services is complex. In cases where we do not receive a fixed fee per test performed from a laboratory customer, we perform tests in advance of payment and without certainty as to the outcome of the billing process. In cases where we do receive a fixed fee per test from a laboratory customer, we may still have disputes over pricing and billing. We or our laboratory customers receive payment from individual patients and from a variety of payors, such as commercial insurance carriers, including managed care organizations and governmental programs, primarily Medicare. Each payor typically has different billing requirements, and the billing requirements of many payors have become increasingly stringent.
Among the factors complicating our billing of third-party payors are:
• | disputes among payors as to which party is responsible for payment; |
• | disparity in coverage among various payors; |
• | disparity in information and billing requirements among payors; and |
• | incorrect or missing billing information, which is required to be provided by the prescribing physician. |
These billing complexities, and the related uncertainty in obtaining payment for our tests, could negatively affect our revenues, cash flow and profitability.
Healthcare reform measures could hinder or prevent commercial success of our NMR LipoProfile test.
In March 2010, President Obama signed into law a legislative overhaul of the U.S. healthcare system, known as the Patient Protection and Affordable Care Act of 2010, as amended by the Healthcare and Education Affordability Reconciliation Act of 2010, or the PPACA, which may have far-reaching consequences for most healthcare companies, including diagnostic companies like us. As a result of this new legislation, substantial changes could be made to the current system for paying for healthcare in the United States, including changes made in order to extend medical benefits to those who currently lack insurance coverage. The mandatory purchase of insurance is strenuously opposed by a number of state governors, resulting in lawsuits challenging the constitutionality of these provisions. On June 28, 2012, the United States Supreme Court upheld the constitutionality of these provisions of the PPACA. Congress has also proposed a number of legislative initiatives, including possible repeal of the PPACA. At this time, it remains unclear whether there will be any changes made to the PPACA, whether in part or in its entirety.
Extending coverage to a large population could substantially change the structure of the health insurance system and the methodology for reimbursing medical services, drugs and devices. These structural changes could entail modifications to the existing system of private payors and government programs, such as Medicare and Medicaid, the creation of a government-sponsored healthcare insurance source, or some combination of both, as well as other changes.
35
Restructuring the coverage of medical care in the United States could impact the reimbursement for diagnostic tests like ours. If reimbursement for our diagnostic tests is substantially less than we or our clinical laboratory customers expect, or rebate obligations associated with them are substantially increased, our business could be materially and adversely impacted. In addition, certain members of Congress have declared their intentions to repeal some or all of the PPACA, adding further uncertainty to the law's future impact on us.
Regardless of the impact of the PPACA on us, the U.S. government and other governments have shown significant interest in pursuing healthcare reform and reducing healthcare costs. Any government-adopted reform measures could cause significant pressure on the pricing of healthcare products and services, including our NMR LipoProfile test, in the United States and internationally, as well as the amount of reimbursement available from governmental agencies or other third-party payors. The continuing efforts of the U.S. and foreign governments, insurance companies, managed care organizations and other payors to contain or reduce healthcare costs may compromise our ability to set prices at commercially attractive levels for the NMR LipoProfile test and other diagnostic tests that we may develop. Changes in healthcare policy, such as the creation of broad limits for diagnostic products, could substantially diminish the sale of or inhibit the utilization of future diagnostic tests, increase costs, divert management's attention and adversely affect our ability to generate revenues and achieve consistent profitability.
New laws, regulations and judicial decisions, or new interpretations of existing laws, regulations and decisions, relating to healthcare availability, methods of delivery or payment for diagnostic products and services, or sales, marketing or pricing, may also limit our potential revenues, and we may need to revise our research and development or commercialization programs. The pricing and reimbursement environment may change in the future and become more challenging for a number of reasons, including policies advanced by the U.S. government, new healthcare legislation or fiscal challenges faced by government health administration authorities. Specifically, in both the U.S. and some foreign jurisdictions, there have been a number of legislative and regulatory proposals and initiatives to change the healthcare system in ways that could affect our ability to sell our diagnostic tests profitably. Some of these proposed and implemented reforms could result in reduced utilization or reimbursement rates for our diagnostic products.
Risks Related to Our Proprietary Technology
Our NMR LipoProfile test and the Vantera system have limited patent protection, and future personalized diagnostic tests that we may develop may also have limited patent protection. As a result, our intellectual property position may not adequately protect us from competitors for sales of our NMR LipoProfile test, the Vantera system or any future diagnostic tests we may develop.
A significant amount of our technology, especially regarding algorithmic processes used in the NMR LipoProfile test, is unpatented. Additionally, the majority of the technology used in our Vantera system is unpatented. As a result, we are dependent to a significant degree upon unpatented trade secrets and improvements, unpatented know-how and continuing technological innovation to develop and maintain our competitive position. We also rely on copyrights and trademarks and confidentiality, licenses and invention assignment agreements to protect our intellectual property rights, as well as, to a more limited extent, patents.
In an effort to protect our trade secrets, we require our employees, consultants, collaborators and advisors to execute confidentiality agreements upon the commencement of their relationships with us. These agreements require that all confidential information developed by the individual or made known to the individual by us during the course of the individual's relationship with us be kept confidential and not disclosed to third parties. These agreements may not, however, provide us with adequate protection against improper use or disclosure of confidential information, and these agreements may be breached. Adequate remedies may not exist in the event of unauthorized use or disclosure of our confidential information. A breach of confidentiality could significantly affect our competitive position. In addition, in some situations, these agreements may conflict with, or be subject to, the rights of third parties with whom our employees, consultants, collaborators or advisors have previous employment or consulting relationships. Also, others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets.
36
The patent covering elements of our NMR LipoProfile test that we previously licensed from North Carolina State University, or NCSU, expired in August 2011. The U.S. patent we previously licensed from Siemens Medical Systems expired in 2008. We also own or co-own with exclusive license rights a number of U.S. patents and patent applications. The claims of the issued U.S. patents owned by or licensed to us, and the claims of any patents that may issue in the future and be owned by or licensed to us, may not confer on us significant commercial protection against competing diagnostic products. Third parties may challenge, narrow, invalidate or circumvent any patents we own or license currently or in the future. Also, our pending patent applications may not issue, and we may not receive any additional patents. Our patents might not contain claims that are sufficiently broad to prevent others from utilizing our technologies. Further, because of the extensive time required for development, testing and regulatory review of a potential diagnostic product, it is possible that any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantages of the patent. Similar considerations apply in any other country where we file for patent protection relating to our technology. The laws of foreign countries may preclude issuance of patents or may not protect our patent rights to the same extent as do laws of the United States.
We also hold copyrights, including copyright registrations, on documentation and software for our NMR LipoProfile test and have a number of registered and unregistered trademarks, including a trademark for Vantera. These copyrights and trademarks may not, however, provide competitive advantages for us, and our competitors may challenge or circumvent these copyrights and trademarks. Furthermore, others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our proprietary information. In addition, the laws of some foreign countries do not protect these types of proprietary rights to the same extent as the laws of the United States.
NMR spectroscopy technology, which we use in performing our NMR analyses, is not proprietary and is known in the scientific community generally, and it is possible to duplicate the methods we use to perform our diagnostic tests. Consequently, our competitors may independently develop competing diagnostic products that do not infringe our intellectual property.
If we infringe or are alleged to infringe intellectual property rights of third parties, our business could be harmed.
Our research, development and commercialization activities, including our current NMR LipoProfile test and our NMR-based technology platform, as well as any other diagnostic test resulting from these activities, may infringe or be claimed to infringe patents owned by other parties. There may also be patent applications that have been filed but not published that, when issued, could be asserted against us. These third parties could bring claims against us that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay research, development, manufacturing or sales of the diagnostic product or product candidate that is the subject of the suit.
As a result of patent infringement claims, or in order to avoid potential claims, we may choose or be required to seek licenses from third parties. These licenses may not be available on acceptable terms, or at all. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties or both, and the rights granted to us might be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the medical diagnostics industry. In addition to infringement claims against us, we may become a party to other patent litigation and other proceedings, including interference, derivation or post-grant proceedings declared or granted by the U.S. Patent and Trademark Office and similar proceedings in foreign countries, regarding intellectual property rights with respect to our current or future diagnostic tests or devices. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Patent litigation and other proceedings may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could impair our ability to compete in the marketplace.
37
We may become involved in lawsuits to protect or enforce our patents or other intellectual property or the patents of our licensors, which could be expensive and time-consuming.
Competitors may infringe our intellectual property, including our patents or the patents of our licensors. As a result, we may be required to file infringement claims to stop third-party infringement or unauthorized use. This can be expensive, particularly for a company of our size, and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patent claims do not cover its technology. An adverse determination of any litigation or other proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
Interference, derivation or other proceedings brought at the U.S. Patent and Trademark Office may be necessary to determine the priority of inventions with respect to our patent applications or those of our licensors or collaborators. Litigation or USPTO proceedings brought by us may fail or may be invoked against us by third parties. Even if we are successful, domestic or foreign litigation or USPTO or foreign patent office proceedings may result in substantial costs and distraction to our management. We may not be able, alone or with our licensors or collaborators, to prevent misappropriation of our proprietary rights, particularly in countries where the laws may not protect such rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or other proceedings, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation or proceedings. In addition, during the course of this kind of litigation or proceedings, there could be public announcements of the results of hearings, motions or other interim proceedings or developments or public access to related documents. If investors perceive these results to be negative, the market price for our common stock could be significantly harmed.
Risks Related to Government Regulation of Our Diagnostic Tests
If we are unable to comply with the requirements of the Clinical Laboratories Improvement Amendments of 1988 and state laws governing clinical laboratories or if we are required to expend significant additional resources to comply with these requirements, the success of our business could be threatened.
HHS has classified our NMR LipoProfile test as a high-complexity test under CLIA, which has implemented more stringent. Personnel requirements for laboratories conducting high-complexity tests are more stringent than those applicable to laboratories performing less complex tests. These personnel requirements require us to employ more experienced or more highly educated personnel and additional categories of employees, which increases our operating costs. If we fail to meet CLIA requirements, HHS or state agencies could require us to cease our NMR LipoProfile testing or other testing subject to CLIA that we may develop in the future. Even if it were possible for us to bring our laboratory back into compliance, we could incur significant expenses and potentially lose revenues in doing so. Moreover, new interpretations of current regulations or future changes in regulations under CLIA may make it difficult or impossible for us to comply with our CLIA classification, which would significantly harm our business.
Many states in which our physician and laboratory clients are located, such as New York, have laws and regulations governing clinical laboratories that are more stringent than federal law and may apply to us even if we are not located, and do not perform our NMR LipoProfile test, in that state. We may also be subject to additional licensing requirements as we expand our sales and operations into new geographic areas, which could impair our ability to pursue our growth strategy.
Portions of our NMR LipoProfile test are subject to the FDA's exercise of enforcement discretion, and any changes to the FDA's policies with respect to this exercise of enforcement discretion could hurt our business.
Clinical laboratory tests that are developed and validated by a laboratory for its own use are called laboratory-developed tests, or LDTs. The laws and regulations governing the marketing of diagnostic products for use as LDTs are extremely complex and in many instances there are no significant regulatory or judicial interpretations of these laws. For instance, while the FDA maintains that LDTs are subject to the FDA's authority as diagnostic medical devices under the Federal Food, Drug, and Cosmetic Act, or FDCA, the FDA has generally exercised enforcement discretion and not enforced applicable regulations with respect to most tests performed by CLIA-certified laboratories.
We have obtained, FDA clearance for several of the measurements we report as part of the NMR LipoProfile test, specifically LDL-P, HDL-C and triglycerides, in order to support our strategy of decentralizing access to the test, which will be helpful in order to make our test commercially available for other laboratories to perform and to report patient results. The remainder of the results reported as part of our NMR LipoProfile test, including HDL-P, small LDL-P and LDL size, as well as a number of lipoprotein markers associated with insulin resistance and diabetes risk, are LDTs and we include them in our report on this basis.
38
When the Vantera system is placed in third-party laboratories, if they elect to report these non-FDA cleared test results to their customers, we will generate the test results in our clinical laboratory using either NMR spectrum data digitally sent to us by the third-party laboratory or a portion of the original blood sample that they send to us. This division of LDT data collection and reporting of test results has not been endorsed or approved by the FDA or other regulatory agencies, and there can be no assurance that the FDA will continue to regard these as LDTs.
In the third quarter of 2011, we submitted a 510(k) premarket notification to the FDA for HDL-P. In March 2012, we voluntarily withdrew that submission and have since worked with the FDA outside of the formal review process to resolve issues identified by the FDA with respect to our submission. Specifically, the FDA raised concerns relating to two clinical studies from which we acquired specimens that we tested to produce data in support of our application. Both studies were conducted by other sponsors and involved large populations. In one case, the FDA expressed concern that the subjects of the study were initially evaluated in the early 1990s and, more specifically, that the specimens were too old and the criteria used to determine a cardiovascular event during that time period were no longer representative of current medical practice. In the second case, the FDA expressed the concern that, because we could only use specimens from the group of subjects who had previously given permission to use their results for commercial purposes, it was possible that this portion of the study population was not representative of the entire population and the results may therefore have been biased.
We resubmitted the 510(k) premarket notification to the FDA, seeking clearance of the HDL-P test, in December 2012. This submission was based on data derived from specimens both from a more recent large, third-party clinical study, as well as the complete data set from the study for which FDA had been concerned with bias. In February 2013, the FDA provided us with comments on our submission and asked for additional information from us. We are actively working with the FDA to provide them with the requested information, and to resolve the issues they have identified. There can be no assurance, however, that we will obtain clearance for this LDT. We have not yet sought FDA clearance for any other LDTs but currently intend to do so for some of these non-cleared portions of our test. In the event we were to not receive clearance for HDL-P or these other tests, we would plan to continue to offer them as LDTs.
The regulation of diagnostic tests classified as LDTs may become more stringent in the future. The FDA held a meeting in July 2010 during which it indicated that it intends to reconsider its current policy of enforcement discretion and to begin drafting an oversight framework for LDTs. We cannot predict the extent of the FDA's future regulation and policies with respect to LDTs and there can be no assurance that the FDA will not require us to obtain premarket clearance or approval for some or all of the non-FDA cleared portions of our NMR LipoProfile test report. If the FDA imposes significant changes to the regulation of LDTs, or if Congress were to pass legislation that more actively regulates LDTs and in vitro diagnostic tests, it could restrict our ability to provide the portions of our test that are not cleared by the FDA or potentially delay the launch of future tests.
While we believe that we are currently in material compliance with applicable laws and regulations relating to LDTs and we believe that our provision of these second-page results to the third-party laboratories utilizing the Vantera system will continue to be regarded as LDTs, we cannot assure you that the FDA or other regulatory agencies would agree with our determination, and a determination that we have violated these laws, or a public announcement that we are being investigated for possible violation of these laws, could hurt our business and our reputation. A significant change in any of these laws, or the FDA's interpretation of the scope of its enforcement discretion, may also require us to change our business model in order to maintain compliance with these laws.
If we are unable to obtain the required clearance of the currently non-cleared portions of our test from the FDA, third-party clinical diagnostic laboratories may be less willing to accept the Vantera system in their facilities.
In December 2011, we submitted a new 510(k) premarket notification for the Vantera system without a site restriction, and in August 2012 we received FDA clearance to market our Vantera system commercially to laboratories. Since then, we have entered into agreements with some of our current clinical diagnostic laboratory customers to place the Vantera system in their laboratories, and we are also in discussions with additional laboratory customers that have indicated a similar interest in the placement of the Vantera system. In addition, we are placing the Vantera system with academic centers that are collaborating with us to develop additional high value diagnostic assays based on NMR technology. We expect placement of the Vantera system in third-party clinical diagnostic laboratory facilities will facilitate their ability to offer our NMR LipoProfile test and other personalized diagnostic tests that we may develop.
39
In the third quarter of 2011, we submitted a 510(k) premarket notification to the FDA seeking clearance of the HDL-P test, as performed on our current NMR-based clinical analyzer platform. In March 2012, the FDA notified us that there were issues with our 510(k) submission that will need to be resolved prior to FDA clearance. We voluntarily withdrew our submission and have since worked with the FDA outside of the formal review process to resolve those issues. We resubmitted our 510(k) premarket notification to the FDA in December 2012, seeking clearance of the HDL-P test as performed on our current generation clinical analyzers or using the Vantera system, with a goal of having the HDL-P test cleared by the FDA in 2013. In February 2013, the FDA provided us with comments on our submission and requested additional information. We are actively working with the FDA to provide them with the requested information, and to resolve the issues that they have identified. We also intend to submit some of the other currently non-cleared test measurements to the FDA for 510(k) clearance.
If FDA clearance or approval of the non-cleared portions of our test is delayed or does not occur, clinical diagnostic laboratories may be less willing to accept the Vantera system in their facilities. Historically, many of our customers have valued the results from the non-FDA cleared portions of our test. Once the Vantera system is placed in third-party laboratories, at the option of the third-party laboratories, we will still make the second-page test results available to them at no additional charge for dissemination along with the first-page results. We will generate these second-page results in our clinical laboratory using either NMR spectrum data digitally sent to us by the third-party laboratory or a portion of the original blood sample that they send to us. We will then send the second-page results back to the third-party laboratories, which will report them to their customer along with the first-page results. Third-party laboratories utilizing the Vantera system may find the options for receiving the non-cleared portions of our test report unacceptable, which may result in less use or adoption of the Vantera system by third-party laboratories, or less willingness to accept the placement of the Vantera system in their facilities in the first place.
In addition, our NMR LipoProfile test report would continue to include a disclaimer that any non-cleared portions of tests had not been cleared by the FDA and that the clinical utility of such results had not been fully established. Furthermore, even if we do obtain FDA clearance for the currently non-cleared portions of our test, new premarket submissions for any modifications or enhancements we later make to such test, or to the Vantera system, that could significantly affect safety or effectiveness, or constitute a major change in the intended use of the test or the Vantera system, would be required. We cannot be sure that clearance of a new 510(k) premarket notification would be granted on a timely basis, or at all, or that FDA clearance processes will not involve costs and delays that could adversely affect our ability to pursue our growth strategy.
The NMR LipoProfile test is, and any other test for which we obtain marketing clearance or approval will be, subject to extensive ongoing regulatory requirements, and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products.
Any test or medical device for which we obtain marketing clearance or approval, including the Vantera system that received FDA clearance in August 2012, along with the manufacturing processes, labeling, advertising and promotional activities for such test or device, will be subject to continual requirements of, and review by, the FDA and comparable regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, requirements relating to quality control, quality assurance and corresponding maintenance of records and documents, requirements relating to product labeling, advertising and promotion, and recordkeeping. Even if regulatory approval of a test or device is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to other conditions of approval. In addition, approval may contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the test or device. Discovery after approval of previously unknown problems with our tests, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in actions such as:
• | restrictions on manufacturing processes; |
• | restrictions on marketing of a test; |
• | restrictions on distribution; |
• | warning letters; |
• | withdrawal of the test from the market; |
• | refusal to approve pending applications or supplements to approved applications that we submit; |
• | recall of tests; |
• | fines, restitution or disgorgement of profits or revenue; |
40
• | suspension or withdrawal of regulatory approvals; |
• | refusal to permit the import or export of our products; |
• | product seizure; |
• | injunctions; or |
• | imposition of civil or criminal penalties. |
Our business is subject to other complex and sometimes unpredictable government regulations. If we or any of our clinical diagnostic laboratory customers fail to comply with these regulations, we could incur significant fines and penalties.
As a provider of clinical diagnostic testing products and services, we are subject to extensive and frequently changing federal, state and local laws and regulations governing various aspects of our business. In particular, the clinical laboratory industry is subject to significant governmental certification and licensing regulations, as well as federal and state laws regarding:
• | test ordering and billing practices; |
• | marketing, sales and pricing practices; |
• | health information privacy and security, including the Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and comparable state laws; |
• | insurance; |
• | anti-markup legislation; and |
• | consumer protection. |
We are also required to comply with FDA regulation of our manufacturing practices and adverse event reporting activities, and regulation by the FDA of our labeling and promotion activities. In addition, advertising of our tests is subject to regulation by the Federal Trade Commission, or FTC, under the Federal Trade Commission Act, or FTC Act. Violation of any FDA requirement could result in enforcement actions, such as seizures, injunctions, civil penalties and criminal prosecutions, and violation of the FTC Act could result in injunctions and other associated remedies, all of which could have a material adverse effect on our business. Most states also have similar postmarket regulatory and enforcement authority for devices. Additionally, most foreign countries have authorities comparable to the FDA and processes for obtaining marketing approvals. Obtaining and maintaining these approvals, and complying with all laws and regulations, may subject us to similar risks and delays as those we could experience under FDA and FTC regulation. We incur various costs in complying and overseeing compliance with these laws and regulations.
We are unable to predict what additional federal or state legislation or regulatory initiatives may be enacted in the future regarding our business or the healthcare industry in general, or what effect such legislation or regulations may have on us. Federal or state governments may impose additional restrictions or adopt interpretations of existing laws that could have a material adverse effect on us. If we fail to comply with any existing or future regulations, restrictions or interpretations, we could incur significant fines and penalties.
If we or any of our clinical diagnostic laboratory customers are subject to an enforcement action involving false claims, kickbacks, physician self-referral or other federal or state fraud and abuse laws, we could incur significant civil and criminal sanctions and loss of reimbursement, which would hurt our business.
The government has made enforcement of the false claims, anti-kickback, physician self-referral and various other fraud and abuse laws a major priority. In many instances, private whistleblowers also are authorized to enforce these laws even if government authorities choose not to do so. Several clinical diagnostic laboratories and members of their management have been the subject of this enforcement scrutiny, which has resulted in very significant civil and criminal settlement payments. In most of these cases, private whistleblowers brought the allegations to the attention of federal enforcement agencies. The risk of our being found in violation of these laws and regulations is increased by the fact that some of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. These laws include:
41
• | the federal Anti-Kickback Statute, which regulates our marketing practices, educational programs, pricing policies, and relationships with health care providers or other entities, by prohibiting, among other things, soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal health care program, such as the Medicare and Medicaid programs; |
• | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent; |
• | federal physician self-referral laws, such as the Stark law, which prohibit a physician from making a referral to a provider of certain health services with which the physician or the physician's family member has a financial interest, and prohibit submission of a claim for reimbursement pursuant to a prohibited referral; and |
• | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payer, including commercial insurers, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
If we or our operations are found to be in violation of any of these laws and regulations, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in U.S. federal or state health care programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. We monitor our own compliance with federal and state fraud and abuse laws on an ongoing basis. We do not, however, monitor the compliance of our clinical diagnostic laboratory customers with federal and state fraud and abuse laws. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management's attention from the operation of our business and hurt our reputation. If we were excluded from participation in U.S. federal health care programs, we would not be able to receive, or to sell our tests to other parties who receive, reimbursement from Medicare, Medicaid and other federal programs. Any similar penalties imposed upon our laboratory customers could also materially harm our revenues and our reputation.
Our compliance program has not eliminated all risks related to these laws. In 2011, our general counsel became aware of a practice engaged in by our sales force that potentially implicated the fraud and abuse laws. The practice involved giving gift cards in small denominations, typically $25, to staff in doctors' offices or to employees of our laboratory partners. We do not believe that gift cards were given to the doctors actually ordering our test except in a single case. Since 2005, the total value of gift cards distributed by the sales force was approximately $100,000. After our general counsel learned of this practice, we stopped it. The audit committee of our board of directors later hired outside counsel to conduct an internal investigation of the matter. The investigation concluded that there was no evidence of willful wrongdoing by any of our employees, but did conclude that our internal policies and communications provided inconsistent guidance on the use of gift cards. We have subsequently revised our internal policies to eliminate any inconsistencies, and we have been taking and are continuing to take additional steps to strengthen our compliance activities. Among other things, we have adopted a new policy on interactions with healthcare professionals, which is based on the Code of Ethics on Interactions with Health Care Professionals promulgated by the Advanced Medical Technology Association, or AdvaMed, a leading medical technology association.
In late 2011, we decided to voluntarily disclose the gift card issue to the local office of the U.S. Attorney. In December 2011, our counsel disclosed this matter on our behalf to the U.S. Attorney's Office in Raleigh, North Carolina, or the USAO. After various meetings and communications between our counsel and the USAO, the USAO notified us in writing in March 2012 that, based on the information provided by our counsel, it had closed its file without investigation and did not intend to take further action in this matter.
Following our receipt of the notification from the USAO, we made a voluntary disclosure of this matter in April 2012 under the formal self-disclosure protocol established by the Office of Inspector General of HHS, or the OIG. Although we do not believe that we violated any laws, we recognized that our conduct potentially implicated the fraud and abuse laws and we decided to voluntarily make the OIG submission to resolve any potential liability. On July 5, 2012, we entered into a settlement agreement with the OIG to settle this matter for a payment of approximately $150,000. We neither admitted nor denied any wrongdoing in connection with this settlement.
42
In June 2012 we were informed by attorneys with the Civil Division of the U.S. Department of Justice and the U.S. Attorney's Office for the District of South Carolina, which we refer to collectively as the DOJ, that they were conducting a civil investigation of allegations that we defrauded federal healthcare programs, including allegations that we paid kickbacks to physicians and submitted claims to federal healthcare programs for medically unnecessary lab tests. We believe that this investigation also involves at least one other cardiovascular diagnostics company and likely arose out of a whistleblower action filed under the federal False Claims Act, which permits any individual who purports to have knowledge that false or fraudulent claims have been submitted for government funds to bring suit on behalf of the United States. Such matters are required to be filed under seal and typically are investigated by the DOJ to determine whether it will intervene in the case on behalf of the government.
We have cooperated with the DOJ's investigation, including by responding to an extensive document request and by initiating a detailed presentation to representatives of the DOJ on the full range of our financial relationships with health care professionals and on our test ordering forms and procedures. In correspondence to our counsel dated October 19, 2012, the Acting Director of the Fraud Section of DOJ's Civil Division, the office handling the DOJ's investigation, advised us that the Civil Division of the DOJ does not have any present intention, based on facts now known, to pursue further the investigation of our company and/or to file or join suit against us based on the allegations that initiated the investigation, and that we do not need to produce additional documents or information. The DOJ has further advised our counsel that additional action to close the matter publicly should not be expected in the near term, however, because the government's broader investigation, apparently of other parties, will continue for an indefinite period, which we believe is not uncommon in cases involving multiple parties.
Although we believe that we are in compliance in all material respects with applicable laws and regulations, there can be no assurance that new information will not come to light that would cause the DOJ to resume its investigation with respect to us. The DOJ letter did make clear that it does not preclude actions by agencies or agents of the United States, including the DOJ, to pursue overpayment or recoupment actions of any sort, contract actions, or any other type of legal action, which we are advised by our counsel is language typically included in letters of this type. In addition, if this matter was in fact initiated by a whistleblower under the False Claims Act, then even if the government ultimately declines to intervene and take over the case, the whistleblower has the right under the False Claims Act to conduct the action. Moreover, we cannot assure you that we will not become subject to similar government inquiries, investigations or actions in the future. Any finding of noncompliance by us with applicable laws and regulations could subject us to a variety of penalties and other sanctions as discussed above, the imposition of any of which could have a material adverse effect on us and our business. In addition, whether or not we are found to be in non-compliance with any applicable laws, we could incur significant expense in responding to or resolving any such inquiries, investigations or actions and we could be required to modify our business practices in a way that adversely affects our business.
Failure to obtain regulatory approval in international jurisdictions would prevent us from marketing products abroad, including our NMR LipoProfile test, the Vantera system and any new diagnostic tests we may develop.
We may in the future seek to market our NMR LipoProfile test, and potentially the Vantera system and any new diagnostic tests we may develop, outside the United States. In order to market these products in the European Union and many other jurisdictions, we must submit clinical data and comparative effectiveness data concerning our products and obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional clinical testing. The time required to obtain approval from foreign regulators may be longer than the time required to obtain FDA approval. The regulatory approval process outside the United States may include all of the risks associated with obtaining FDA approval.
In addition, in many countries outside the United States, it is required that our tests be approved for reimbursement before they can be approved for sale in that country. In some cases this may include approval of the price we intend to charge for our products, if approved. We may not obtain approvals from regulatory authorities outside the United States on a timely basis, or at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA, but a failure to obtain, or a delay in obtaining, regulatory approval in one country may negatively affect the regulatory process in other countries. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize any tests in any market and therefore may not be able to pursue these revenue opportunities.
43
Risks Related to Ownership of Our Common Stock
An active trading market for our common stock may not continue to develop or be sustained.
Prior to our initial public offering in January 2013, there was no public market for our common stock. The initial public offering price for our common stock was determined through negotiations with the underwriters and may bear no relationship to the price at which the common stock will trade. Although our common stock is listed on The NASDAQ Global Market, an active trading market for our shares may never develop or be sustained. If an active market for our common stock does not develop, it may be difficult for you to sell shares without depressing the market price for the common stock or to sell your shares at all.
The trading price of our common stock is likely to be volatile, and purchasers of our common stock could incur substantial losses.
Our stock price is likely to be volatile. The stock market in general and the market for diagnostic companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their common stock at or above the price paid for their shares. The market price for our common stock may be influenced by many factors, including:
• | sales of substantial amounts of our stock by insiders and large stockholders, or the expectation that such sales might occur; |
• | regulatory or legal developments in the United States and foreign countries; |
• | actual or anticipated variations in our financial results or those of companies that are perceived to be similar to us; |
• | changes in the structure of healthcare payment systems; |
• | announcements by us of significant acquisitions, licenses, strategic partnerships, joint ventures or capital commitments; |
• | market conditions in the diagnostic sector and issuance of securities analysts' reports or recommendations; |
• | general economic, industry and market conditions; |
• | additions or departures of key personnel; |
• | intellectual property, product liability or other litigation against us; |
• | expiration or termination of our potential relationships with customers and strategic partners; and |
• | the other factors described in this “Risk Factors” section. |
In addition, in the past, stockholders have initiated class action lawsuits against clinical diagnostics companies following periods of volatility in the market prices of these companies' stock. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management's attention and resources.
If securities or industry analysts do not publish research or publish unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will rely in part on the research and reports that equity research analysts publish about us and our business. As a newly public company, we have only limited research coverage by equity research analysts. Equity research analysts may elect not to initiate or to continue to provide research coverage of our common stock, and such lack of research coverage may adversely affect the market price of our common stock. Even if we do have equity research analyst coverage, we will not have any control over the analysts or the content and opinions included in their reports. The price of our stock could decline if one or more equity research analysts downgrade our stock or issue other unfavorable commentary or research. If one or more equity research analysts ceases coverage of our company or fails to publish reports on us regularly, demand for our stock could decrease, which in turn could cause our stock price or trading volume to decline.
44
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. If our stockholders sell, or the market perceives that our stockholders intend to sell, substantial amounts of our common stock in the public market, the market price of our common stock could decline significantly. A substantial majority of our outstanding shares of common stock will be available for sale in the public market beginning in July 2013 following the expiration of lock-up agreements between some of our stockholders and the underwriters for our initial public offering. The representatives of the underwriters may release these stockholders from their lock-up agreements with the underwriters at any time and without notice, which would allow for earlier sales of shares in the public market.
In addition, we have filed a registration statement on Form S-8 registering the issuance of shares of common stock subject to options or other equity awards issued or reserved for future issuance under our equity incentive plans. The shares registered under this registration statement will be available for sale in the public market subject to vesting arrangements and exercise of options, the lock-up agreements described above, our insider trading policy and the restrictions of Rule 144 under the Securities Act in the case of our affiliates.
Additionally, the holders of an aggregate of approximately 7.1 million shares of our common stock, including shares of our common stock issuable upon the exercise of outstanding warrants, or their transferees, have rights, subject to some conditions, to require us to file one or more registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. If we were to register the issuance of these shares, they could be freely sold in the public market. If these additional shares are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.
The issuance of additional stock in connection with financings, acquisitions, investments, our stock incentive plans or otherwise will dilute all other stockholders.
Our amended and restated certificate of incorporation authorizes us to issue up to 75,000,000 shares of common stock and up to 5,000,000 shares of preferred stock with such rights and preferences as may be determined by our board of directors. Subject to compliance with applicable rules and regulations, we may issue our shares of common stock or securities convertible into our common stock from time to time in connection with a financing, acquisition, investment, our stock incentive plans or otherwise. Any such issuance could result in substantial dilution to our existing stockholders and cause the trading price of our common stock to decline.
Provisions in our corporate charter documents and under Delaware law may prevent or frustrate attempts by our stockholders to change our management and hinder efforts to acquire a controlling interest in us, and the market price of our common stock may be lower as a result.
There are provisions in our amended and restated certificate of incorporation and amended and restated bylaws that may make it difficult for a third party to acquire, or attempt to acquire, control of our company, even if a change in control was considered favorable by you and other stockholders. For example, our board of directors has the authority to issue up to 5,000,000 shares of preferred stock. The board of directors can fix the price, rights, preferences, privileges, and restrictions of the preferred stock without any further vote or action by our stockholders. The issuance of shares of preferred stock may delay or prevent a change in control transaction. As a result, the market price of our common stock and the voting and other rights of our stockholders may be adversely affected. An issuance of shares of preferred stock may result in the loss of voting control to other stockholders.
Our charter documents also contain other provisions that could have an anti-takeover effect, including:
• | only one of our three classes of directors is elected each year; |
• | stockholders are not entitled to remove directors other than by a 66 2/3% vote and only for cause; |
• | stockholders are not permitted to take actions by written consent; |
• | stockholders cannot call a special meeting of stockholders; and |
• | stockholders must give advance notice to nominate directors or submit proposals for consideration at stockholder meetings. |
45
In addition, we are subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law, which regulates corporate acquisitions. These provisions could discourage potential acquisition proposals and could delay or prevent a change in control transaction. They could also have the effect of discouraging others from making tender offers for our common stock, including transactions that may be in your best interests. These provisions may also prevent changes in our management or limit the price that certain investors are willing to pay for our stock.
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware is the exclusive forum for substantially all disputes between us and our stockholders.
Concentration of ownership of our common stock among our existing executive officers, directors and principal stockholders may prevent new investors from influencing significant corporate decisions.
Our executive officers, directors and current beneficial owners of 5% or more of our common stock, in the aggregate, own approximately 34% of our outstanding common stock. These persons, acting together, are able to significantly influence all matters requiring stockholder approval, including the election and removal of directors and any merger or other significant corporate transactions. The interests of this group of stockholders may not coincide with the interests of other stockholders.
We do not anticipate paying any cash dividends on our common stock in the foreseeable future and our stock may not appreciate in value.
We have not declared or paid cash dividends on our common stock to date. We currently intend to retain our future earnings, if any, to fund the development and growth of our business. In addition, the terms of any existing or future debt agreements may preclude us from paying dividends. There is no guarantee that shares of our common stock will appreciate in value or that the price at which our stockholders have purchased their shares will be able to be maintained.
We are an “emerging growth company,” and if we decide to comply only with reduced disclosure requirements applicable to emerging growth companies, our common stock could be less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012, and, for as long as we continue to be an “emerging growth company,” we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies but not to “emerging growth companies,” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We could be an “emerging growth company” through 2018, although a variety of circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700 million as of any June 30 before that time, in which case we would no longer be an emerging growth company as of the following December 31. We cannot predict if investors will find our common stock less attractive if we choose to rely on these exemptions. If some investors find our common stock less attractive as a result of any choices to reduce future disclosure, there may be a less active trading market for our common stock and our stock price may be more volatile.
Under the JOBS Act, emerging growth companies that become public can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards following the completion of our initial public offering and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
46
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Our corporate headquarters and operations, including our laboratory facility, are located in Raleigh, North Carolina, where we currently lease approximately 83,000 square feet of office and lab space. The lease on this facility expires in September 2022. Our current rent under this lease is approximately $1.2 million annually, subject to annual increases.
Item 3. Legal Proceedings
We are not currently a party to any pending legal proceedings that we believe will have a material adverse effect on our business or financial condition. We may, however, be subject to various claims and legal actions arising in the ordinary course of business from time to time.
Item 4. Mine Safety Disclosures
Not applicable.
47
PART II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information for Common Stock
Our common stock has been publicly traded on the NASDAQ Global Market under the symbol “LPDX” since January 25, 2013. Prior to that time, there was no public market for our common stock. As a result, we have not set forth quarterly information with respect to the high and low prices for our common stock for the two most recent fiscal years.
On March 25, 2013, the closing price for our common stock as reported on the NASDAQ Global Market was $10.20 per share.
Dividend Policy
We have never declared or paid any dividends on our common stock. We anticipate that we will retain all of our future earnings, if any, for use in the operation and expansion of our business and do not anticipate paying cash dividends in the foreseeable future. Additionally, our ability to pay dividends on our common stock is limited by restrictions under the terms of the agreements governing our credit facility.
Stockholders
As of March 25, 2013, there were 303 registered stockholders of record for our common stock. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Use of Proceeds from Initial Public Offering of Common Stock
On January 24, 2013, our Registration Statement on Form S-1, as amended (Reg. No. 333-175102) was declared effective in connection with the IPO of our common stock, pursuant to which we sold 5,750,000 shares at a price to the public of $9.00 per share, including the full exercise of the underwriters' option to purchase additional shares. The offering closed on January 30, 2013, as a result of which we received net proceeds of approximately $44.4 million after underwriting discounts of approximately $3.4 million and offering-related expenses paid by us of approximately $4.0 million. We did not receive any proceeds from the shares sold by the selling stockholders. The joint managing underwriters of the offering were Barclays Capital Inc., UBS Securities LLC and Piper Jaffray & Co. No payments were made by us to directors, officers or persons owning ten percent or more of our common stock or to their associates, or to our affiliates, other than (i) payments of an aggregate of $3.9 million in respect of accrued dividends on shares of convertible preferred stock held by investors affiliated with our directors, which shares were converted into shares of common stock upon the closing of the IPO, and (ii) payments in the ordinary course of business to officers for salaries and to non-employee directors as compensation for board or board committee service.
There has been no material change in the planned use of proceeds from our initial public offering as described in our final prospectus filed with the SEC pursuant to Rule 424(b) under the Securities Act of 1933, as amended, on January 25, 2013.
Recent Sales of Unregistered Securities
During the year ended December 31, 2012, we granted options under our 2007 Stock Incentive Plan to purchase an aggregate of 379,414 shares of our common stock to a total of approximately 71 employees, consultants and directors, having exercise prices ranging from $11.11 to $12.81 per share. Of these, options to purchase an aggregate of 35,792 shares have been cancelled without being exercised and 343,622 remain outstanding. During the year ended December 31, 2012, an aggregate of 337,400 shares were issued upon the exercise of stock options, at a weighted-average exercise price of $2.52 per share, for aggregate cash proceeds of $0.2 million. Of these shares, 277,298 shares were issuable to two executive officers upon the net exercise of stock options, resulting in no cash proceeds to us. We withheld 138,611 shares in satisfaction of the exercise price and tax withholding obligations, and we issued the remaining 138,687 shares to the executive officers. The offers, sales and issuances of the securities described in this paragraph were exempt from registration under the Securities Act of 1933, as amended, pursuant to Rule 701 thereunder in that the transactions were under compensatory benefit plans. The recipients of such securities were our employees, directors or consultants and received the securities under our stock option plans.
48
Appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions had adequate access, through employment or business relationships, to information about us.
In December 2012, we issued warrants to purchase an aggregate of 73,564 shares of our Series E redeemable convertible preferred stock to two accredited investors. The warrants were issued in connection with our entering into a credit facility with commercial lenders. The offer and issuance of these securities were exempt from registration under Section 4(2) of the Securities Act as transactions by an issuer not involving a public offering. The recipients represented to us that they acquired the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities issued in these transactions.
In December 2012, we also issued 17,941 shares of our Series F redeemable convertible preferred stock to one accredited investor upon the net exercise of a warrant with an exercise price of $4.35 per share. The issuance of these securities was exempt from registration under Section 3(a)(9) of the Securities Act.
Purchases of Equity Securities by the Issuer and Affiliated Parties
None.
49
Item 6. Selected Financial Data
The following selected statement of operations data for the years ended December 31, 2012, 2011, 2010, 2009 and 2008 and the selected balance sheet data as of December 31, 2012, 2011, 2010, 2009 and 2008 are derived from our audited financial statements, which have been audited by Ernst & Young LLP, independent registered public accounting firm. Our historical results are not necessarily indicative of the results to be expected in the future. The selected financial data should be read together with “Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations” and in conjunction with the financial statements, related notes, and other financial information included elsewhere in this Annual Report.
Year Ended December 31, | |||||||||||||||||||
2012 | 2011 | 2010 | 2009 | 2008 | |||||||||||||||
(In thousands, except share and per share data) | |||||||||||||||||||
Statement of Operations Data: | |||||||||||||||||||
Revenues | $ | 54,798 | $ | 45,807 | $ | 39,368 | $ | 34,713 | $ | 28,954 | |||||||||
Cost of revenues | 10,060 | 8,529 | 8,139 | 7,792 | 7,354 | ||||||||||||||
Gross profit | 44,738 | 37,278 | 31,229 | 26,921 | 21,600 | ||||||||||||||
Operating expenses: | |||||||||||||||||||
Research and development | 9,974 | 7,808 | 7,276 | 6,156 | 7,245 | ||||||||||||||
Sales and marketing | 22,402 | 21,305 | 15,246 | 12,990 | 12,137 | ||||||||||||||
General and administrative | 10,373 | 8,550 | 7,331 | 7,020 | 5,964 | ||||||||||||||
Loss on disposal of long-lived assets | 30 | — | — | — | — | ||||||||||||||
Impairment of intangible assets | 13 | — | — | — | — | ||||||||||||||
Gain on extinguishment of other long-term liabilities | — | — | (2,700 | ) | — | — | |||||||||||||
Total operating expenses | 42,792 | 37,663 | 27,153 | 26,166 | 25,346 | ||||||||||||||
Income (loss) from operations | 1,946 | (385 | ) | 4,076 | 755 | (3,746 | ) | ||||||||||||
Total other (expense) income | (696 | ) | (163 | ) | 220 | (495 | ) | 112 | |||||||||||
Income (loss) before taxes | 1,250 | (548 | ) | 4,296 | 260 | (3,634 | ) | ||||||||||||
Income tax (benefit) expense | — | — | (16 | ) | 2 | — | |||||||||||||
Net income (loss) | 1,250 | (548 | ) | 4,312 | 258 | (3,634 | ) | ||||||||||||
Accrual of dividends on redeemable convertible preferred stock | — | (613 | ) | (1,040 | ) | (1,040 | ) | (1,040 | ) | ||||||||||
Undistributed earnings allocated to preferred stockholders | (1,004 | ) | — | (2,655 | ) | — | — | ||||||||||||
Net income (loss) attributable to common stockholders – basic | 246 | (1,161 | ) | 617 | (782 | ) | (4,674 | ) | |||||||||||
Undistributed earnings re-allocated to common stockholders | 113 | — | 303 | — | — | ||||||||||||||
Net income (loss) attributable to common stockholders – diluted | $ | 359 | $ | (1,161 | ) | $ | 920 | $ | (782 | ) | $ | (4,674 | ) | ||||||
Net income (loss) attributable to common stockholders per share – basic | $ | 0.14 | $ | (0.69 | ) | $ | 0.38 | $ | (0.49 | ) | $ | (2.93 | ) | ||||||
Net income (loss) attributable to common stockholders per share – diluted | $ | 0.13 | $ | (0.69 | ) | $ | 0.34 | $ | (0.49 | ) | $ | (2.93 | ) | ||||||
Weighted average shares of common stock outstanding used in computing net income (loss) per share – basic | 1,715,408 | 1,674,018 | 1,611,843 | 1,596,920 | 1,594,048 | ||||||||||||||
Weighted average shares of common stock outstanding used in computing net income (loss) per share – diluted | 2,814,323 | 1,674,018 | 2,713,770 | 1,596,920 | 1,594,048 | ||||||||||||||
50
As of December 31, | |||||||||||||||||||
2012 | 2011 | 2010 | 2009 | 2008 | |||||||||||||||
(in thousands) | |||||||||||||||||||
Balance Sheet Data: | |||||||||||||||||||
Cash and cash equivalents | $ | 24,768 | $ | 12,483 | $ | 11,058 | $ | 12,045 | $ | 9,889 | |||||||||
Accounts receivable, net | 5,149 | 5,626 | 4,194 | 3,363 | 3,076 | ||||||||||||||
Total assets | 47,679 | 28,117 | 20,141 | 19,509 | 17,348 | ||||||||||||||
Revolving line of credit | 5,000 | — | — | — | — | ||||||||||||||
Long-term debt, including current portion | 15,708 | 6,000 | 1,200 | 3,000 | 2,000 | ||||||||||||||
Preferred stock warrant liability | 412 | 229 | 597 | 1,104 | 901 | ||||||||||||||
Total liabilities | 29,934 | 12,025 | 4,929 | 10,296 | 8,978 | ||||||||||||||
Redeemable convertible preferred stock and convertible preferred stock | 57,301 | 57,163 | 55,845 | 53,599 | 52,362 | ||||||||||||||
Accumulated deficit | (47,993 | ) | (49,243 | ) | (48,695 | ) | (53,007 | ) | (53,265 | ) | |||||||||
Total stockholders’ deficit | (39,556 | ) | (41,071 | ) | (40,632 | ) | (44,385 | ) | (43,990 | ) | |||||||||
51
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with the financial statements and the related notes to those statements included later in this Annual Report. In addition to historical financial information, the following discussion contains forward‑looking statements that reflect our plans, estimates, beliefs and expectations that involve risks and uncertainties. Our actual results and the timing of events could differ materially from those discussed in these forward‑looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this Annual Report, particularly in “Item 1A. Risk Factors” and “Special Note Regarding Forward‑Looking Statements.”
Overview
We are an in vitro diagnostic company pioneering a new field of personalized diagnostics based on nuclear magnetic resonance, or NMR, technology. Our first diagnostic test, the NMR LipoProfile test, directly measures the number of low density lipoprotein, or LDL, particles in a blood sample and provides physicians and their patients with actionable information to personalize management of risk for heart disease. Our automated clinical analyzer, the Vantera system, was cleared by the FDA in August 2012 and became commercially available in December 2012. The Vantera system requires no previous knowledge of NMR technology to operate and has been designed to significantly simplify complex technology through ease of use and walk-away automation. We plan to selectively place the Vantera system on-site with national and regional clinical laboratories as well as leading medical centers and hospital outreach laboratories, which we believe will facilitate their ability to offer our NMR LipoProfile test and other diagnostic tests that we may develop. We are driving toward becoming a clinical standard of care by decentralizing our technology and expanding our menu of personalized diagnostic tests to address a broad range of cardiovascular, metabolic and other diseases.
To date, the NMR LipoProfile test has been ordered over 9 million times, including nearly 2 million times during 2012, and the number of tests ordered has grown at a compound annual growth rate of approximately 28% from 2007 to 2012. The NMR LipoProfile test is reimbursed by a number of governmental and private payors, which we believe collectively represent approximately 150 million covered lives.
We currently perform all NMR LipoProfile testing at our certified and accredited laboratory facilities in Raleigh, North Carolina. We intend to accelerate clinician and clinical diagnostic laboratory adoption of the NMR LipoProfile test and future clinical diagnostic tests by increasing access to our technology platform through the launch of the Vantera system. We have entered into agreements with some of our current clinical diagnostic laboratory customers to place the Vantera system in their laboratories, and we are also in discussions with additional laboratory customers who have indicated a similar interest in the placement of the Vantera system. In addition, we are placing the Vantera system with academic centers that are collaborating with us to develop additional high value diagnostic assays based on NMR technology. We will retain full ownership of any Vantera analyzers placed in third-party laboratories and will be responsible for support and maintenance obligations. In general, we expect that the number of Vantera analyzers that will be placed in our clinical diagnostic laboratory customers' facilities will depend on their demonstrated annual production volume for the NMR LipoProfile test and their ability to increase demand for our tests.
We believe that the inherent analytical advantages of NMR technology will also allow us to expand our diagnostic test menu. We are currently developing NMR-based diagnostic tests for use in the prediction of diabetes, including the assessment of insulin resistance, and we are investigating opportunities to develop new diagnostic tests for other diseases.
We have incurred significant losses since our inception. As of December 31, 2012, our accumulated deficit was $48.0 million. We expect to incur significant operating losses for the next several years as we seek to establish the NMR LipoProfile test as a clinical standard of care for managing a patient's risk of cardiovascular disease.
52
Financial Operations Overview
Revenues
Substantially all of our revenues are currently derived from sales of our NMR LipoProfile test to clinical diagnostic laboratories, physicians and other healthcare professionals for use in patient care. For the years ended December 31, 2012, 2011 and 2010, sales of the NMR LipoProfile test represented approximately 94%, 93% and 87%, respectively, of our total revenues. The remainder of our revenues is derived from sales of standard analytical chemistry tests, which we refer to as ancillary tests, requested by clinicians in conjunction with our NMR LipoProfile test, as well as revenue from research contracts. Ancillary tests are FDA-approved blood tests that any clinical laboratory can process but that may be ordered from us at the same time as the NMR LipoProfile test for convenience. These tests are not run on our NMR technology platform, but instead are run on a traditional chemistry analyzer. We anticipate that the proportion of our revenues represented by sales of the NMR LipoProfile test will continue to increase as we increase the number of these tests performed for our customers.
The following table presents our revenues by service offering and source:
Year Ended December 31, | |||||||||||
2012 | 2011 | 2010 | |||||||||
(in thousands) | |||||||||||
Revenues: | |||||||||||
NMR LipoProfile tests | $ | 51,759 | $ | 42,392 | $ | 34,394 | |||||
Ancillary tests | 1,712 | 2,178 | 3,425 | ||||||||
Research contracts | 1,327 | 1,237 | 1,549 | ||||||||
Total revenues | $ | 54,798 | $ | 45,807 | $ | 39,368 | |||||
Our revenues are driven by both test volume and the average selling price of our NMR LipoProfile test. We expect to increase the proportion of our business conducted on a wholesale basis through clinical diagnostic laboratories as compared to our direct distribution channel in which clinicians order the test directly from us. We expect this trend to continue as we decentralize access to our NMR LipoProfile test by placing the Vantera system directly in third-party laboratories. For direct sales, the price we ultimately receive depends upon the level of reimbursement from Medicare or commercial insurance carriers. Clinical diagnostic laboratories purchase our test at prices that we negotiate with them, which will continue to be the case for NMR LipoProfile tests performed using the Vantera system, whether the analyzer is located on-site at the customer's laboratory or at our own facility. These clinical diagnostic laboratories are responsible for obtaining reimbursement from third-party payors or directly from patients. The average selling price of our tests sold to these laboratories is less than that for tests we sell directly to clinicians. We expect that our overall average selling price will continue to decline in the near future, as we increase the proportion of our business conducted on a wholesale basis through clinical diagnostic laboratories. We expect this trend to continue as we place the Vantera system in third-party laboratories, as the price we receive for a test performed on-site at third-party laboratories using the Vantera system will generally be less than the price for the same test performed at our own facility. We do not, however, expect that our revenues, income from operations or liquidity will be materially affected by an erosion of average selling price due to changes in the channel mix, as we believe that the increase in test volumes through these laboratories and the number of laboratory customers offering our NMR LipoProfile tests will outweigh the impact of decreases in average selling price, especially if demand increases for Vantera placements.
During the initial rollout period for the Vantera system, we expect that most Vantera placements will be with our existing clinical diagnostic laboratory customers. As a result, we anticipate a gradual shift in the NMR LipoProfile tests performed from our existing laboratory facility to our customers' facilities. We expect that the reduced volume in the number of tests performed at our facility will be partially offset by growth in NMR LipoProfile test orders from new clinical diagnostic laboratory customers who may not initially meet our test volume criteria for a Vantera system placement.
Our revenues from ancillary tests, while a diminishing portion of our business, are similarly dependent upon our rates of reimbursement from various payor sources. For example, Medicare reimbursement rates are established by CMS each year. Changes in Medicare reimbursement rates are dependent on a number of factors that we cannot predict. Reductions in reimbursement rates for these ancillary tests would reduce our overall revenues from these tests.
53
Cost of Revenues and Operating Expenses
We allocate certain overhead expenses, such as rent, utilities, and depreciation of general office assets to cost of revenues and operating expense categories based on headcount and facility usage. As a result, an overhead expense allocation is reflected in cost of revenues and each operating expense category.
Cost of Revenues and Gross Margin
Cost of revenues consists of direct labor expenses, including employee benefits and stock-based compensation expenses, cost of laboratory supplies, freight costs, royalties paid under license agreements, depreciation of laboratory equipment, leasehold improvements and certain allocated overhead expenses. Once we launch the Vantera system and place the system on-site with third parties, the additional service and maintenance costs for these analyzers will also be included in cost of revenues. We expect these expenses to increase in absolute dollars as we support our customers' use of the Vantera system, although we expect these increased expenses to be offset by increased revenues from additional test volume. During the years ended December 31, 2012, 2011 and 2010, our cost of revenues represented approximately 18%, 19% and 21%, respectively, of our total revenues.
Our gross profit represents total revenues less the cost of revenues, and gross margin is gross profit expressed as a percentage of total revenues. Our gross margins were approximately 82%, 81% and 79%, respectively, for the years ended December 31, 2012, 2011 and 2010. We expect our overall cost of revenues to increase in absolute dollars as we continue to increase our volume of tests performed, including tests performed by laboratories using the decentralized Vantera system.
Research and Development Expenses
Our research and development expenses include those costs associated with performing research and development activities, such as personnel-related expenses, including stock-based compensation, fees for contractual and consulting services, travel costs, laboratory supplies and allocated overhead expenses. We expense all research and development costs as incurred.
During the years ended December 31, 2012, 2011 and 2010, our research and development expenses represented approximately 18%, 17% and 18%, respectively, of our total revenues. We expect that our overall research and development expenses will continue to increase in absolute dollars as we develop additional in vitro diagnostic assay candidates that can be performed using the Vantera system.
Sales and Marketing Expenses
Our sales and marketing expenses include costs associated with our sales organization, including our direct sales force and sales management, and our marketing, managed care and business development personnel. These expenses consist principally of salaries, commissions, bonuses and employee benefits for these personnel, including stock-based compensation, as well as travel costs related to sales and marketing activities, marketing and medical education activities and allocated overhead expenses. We expense all sales and marketing costs as incurred.
During the years ended December 31, 2012, 2011 and 2010, our sales and marketing expenses represented approximately 41%, 47% and 39%, respectively, of our total revenues. We expect our sales and marketing costs to increase, both in absolute dollars as well as a percentage of our total revenues, as we expand our sales force, increase our geographic presence, and increase marketing and medical education to drive awareness and adoption of the NMR LipoProfile test.
General and Administrative Expenses
Our general and administrative expenses include costs for our executive, accounting and finance, legal, and human resources functions. These expenses consist principally of salaries, bonuses and employee benefits for the personnel included in these functions, including stock-based compensation and professional services fees, such as consulting, audit, tax and legal fees, general corporate costs and allocated overhead expenses, and bad debt expense. We expense all general and administrative expenses as incurred.
Our general and administrative expenses represented approximately 19% of our total revenues for each of the years ended December 31, 2012, 2011 and 2010. We expect that our general and administrative expenses will increase, primarily due to the costs of operating as a public company, such as additional legal, accounting and corporate governance expenses, including expenses related to compliance with the Sarbanes-Oxley Act of 2002, directors' and officers' insurance premiums and investor relations expenses.
54
Medical Device Tax
The PPACA includes provisions that, among other things, require the medical device industry to subsidize healthcare reform in the form of a 2.3% excise tax on U.S. sales of most medical devices beginning in 2013. Regulations implementing the tax were finalized in December 2012, but the long-term impact to our company remains uncertain as some members of Congress are working to delay enactment of the tax. While we continue to evaluate the impact of this tax on our overall business, this tax is applicable to the sales of our NMR LipoProfile tests and could adversely affect our results of operations, cash flows and financial condition.
Other (Expense) Income
Interest income consists of interest earned on our cash and cash equivalents. During the years ended December 31, 2012, 2011 and 2010, this income has not been material, although we expect our interest income to increase as we invest the net proceeds from our initial public offering.
Interest expense consists primarily of interest expense on our loan balances and the amortization of debt discounts and debt issuance costs. We amortize debt issuance costs over the life of the loan and report them as interest expense in our statements of operations.
We had a term loan from Square 1 with an outstanding balance of $4.2 million as of September 30, 2012. This loan carried a variable annual interest rate equal to the greater of 7.25% or the prime rate plus 3.75%. We also had a revolving line of credit from Square 1 with an outstanding balance of $3.5 million as of September 30, 2012. Borrowings under this line of credit carried a variable annual interest rate equal to the greater of 6.25% or the prime rate plus 3.0%. In December 2012, we refinanced our existing indebtedness and paid off the foregoing loans. As part of the refinancing, we now have term loans from Oxford Finance with an outstanding balance of $9.8 million as of December 31, 2012, net of debt discount in the amount of $0.2 million, and a term loan from Square 1 with an outstanding balance of $5.9 million, net of debt discount in the amount of $86,000, as of December 31, 2012, as well as a new revolving line of credit with Square 1 with a maximum borrowing capacity of $6.0 million and an outstanding balance of $5.0 million as of December 31, 2012. Under the new credit facility, the term loans carry a fixed interest rate of 9.5%, while advances under the line of credit will continue to carry a variable interest rate equal to the greater of 6.25% or Square 1's prime rate plus 3.0%.
Other (expense) income primarily consists of costs incurred as a result of changes in the fair value of our preferred stock warrant liability and gains and losses on sale or disposal of assets. The fair value of preferred stock warrants was re-measured at the end of each reporting period prior to our initial public offering, and changes in fair value were recognized in other (expense) income. Upon completion of our initial public offering in January 2013, the preferred stock warrants automatically converted into warrants to purchase common stock and no further changes in fair value will be recognized in other (expense) income.
55
Results of Operations
Comparison of Years Ended December 31, 2012 and 2011
The following table sets forth, for the periods indicated, the amounts of certain components of our statements of operations and the percentage of total revenues represented by these items, showing period-to-period changes.
Year ended December 31, | Period-to-period change | |||||||||||||||||||
2012 | % of Revenues | 2011 | % of Revenues | Amount | Percentage | |||||||||||||||
(in thousands, except for percentages) | ||||||||||||||||||||
Revenues | $ | 54,798 | 100.0 | % | $ | 45,807 | 100.0 | % | $ | 8,991 | 19.6 | % | ||||||||
Cost of revenues | 10,060 | 18.4 | 8,529 | 18.6 | 1,531 | 17.9 | ||||||||||||||
Gross profit | 44,738 | 81.6 | 37,278 | 81.4 | 7,460 | 20.0 | ||||||||||||||
Operating expenses: | ||||||||||||||||||||
Research and development | 9,974 | 18.2 | 7,808 | 17.0 | (2,166 | ) | 27.7 | |||||||||||||
Sales and marketing | 22,402 | 40.9 | 21,305 | 46.5 | (1,097 | ) | 5.1 | |||||||||||||
General and administrative | 10,373 | 18.9 | 8,550 | 18.7 | (1,823 | ) | 21.3 | |||||||||||||
Loss on disposal of long-lived assets | 30 | 0.1 | — | — | (30 | ) | * | |||||||||||||
Impairment of intangible assets | 13 | 0.0 | — | — | (13 | ) | * | |||||||||||||
Total operating expenses | 42,792 | 78.1 | 37,663 | 82.2 | (5,129 | ) | 13.5 | |||||||||||||
Income (loss) from operations | 1,946 | 3.5 | (385 | ) | (0.8 | ) | (2,331 | ) | * | |||||||||||
Total other expense | (696 | ) | (1.2 | ) | (163 | ) | (0.4 | ) | 533 | * | ||||||||||
Income (loss) before taxes | 1,250 | 2.3 | (548 | ) | (1.2 | ) | (1,798 | ) | * | |||||||||||
Net income (loss) | $ | 1,250 | 2.3 | % | $ | (548 | ) | (1.2 | )% | $ | (1,798 | ) | * | |||||||
* Percentage not meaningful
Revenues
Total revenues increased by 19.6% to $54.8 million for the year ended December 31, 2012 from $45.8 million for the year ended December 31, 2011. Revenues from sales of our NMR LipoProfile test increased to $51.8 million for the year ended December 31, 2012 from $42.4 million for the year ended December 31, 2011, resulting from growth in the number of NMR LipoProfile tests sold, particularly to our clinical diagnostic laboratory customers. This growth reflected the impact of an increase in the number of our sales representatives and greater geographic coverage of our sales force, as well as increased market acceptance of our test.
The overall number of NMR LipoProfile tests increased by 29% to approximately 1,949,000 tests for the year ended December 31, 2012 from approximately 1,508,000 tests for the year ended December 31, 2011. The overall average selling price of NMR LipoProfile tests decreased 5.5%, to $26.56 for the year ended December 31, 2012 from $28.10 for the year ended December 31, 2011. This decrease in average selling price was primarily the result of a continuing shift in channel mix toward clinical laboratory customers. The percentage of our total NMR LipoProfile tests sold through direct distribution channels decreased from 8% for year ended December 31, 2011 to 5% for the year ended December 31, 2012. This continued shift reflects our current strategy of accelerating the adoption of our NMR LipoProfile test through clinical diagnostic laboratories, which we expect to result in fewer tests ordered through direct channels and an overall decrease in average selling price.
Revenues from sales of ancillary tests decreased from $2.2 million for the year ended December 31, 2011 to $1.7 million for the year ended December 31, 2012. The decrease in revenues from these ancillary tests was primarily driven by the shift in testing mix and an overall reduction of reimbursement rates from Medicare. Revenues from our clinical research clients were approximately $1.3 million and $1.2 million for the years ended December 31, 2012 and 2011, respectively.
56
Cost of Revenues and Gross Margin
Cost of revenues increased by 17.9%, to $10.1 million for the year ended December 31, 2012 from $8.5 million for the year ended December 31, 2011. This increase resulted primarily from the increase in the number of NMR LipoProfile tests sold to patient care clients during the year ended December 31, 2012. This additional testing volume resulted in increased required personnel, which increased our compensation, benefit and allocated costs. These increases were partially offset by lower material costs due to fewer ancillary tests being performed. Gross profit as a percentage of total revenues, or gross margin, increased to 81.6% for the year ended December 31, 2012 from 81.4% for the year ended December 31, 2011. The improvement we experienced in gross margin resulted primarily from increased sales volume coupled with operating efficiencies in our clinical laboratory.
Research and Development Expenses
Research and development expenses increased by 27.7% to $10.0 million for the year ended December 31, 2012 from $7.8 million for the year ended December 31, 2011. This increase was primarily the result of $1.5 million in higher salaries and benefits, including relocation and recruiting fees, stock-based compensation expense and higher travel-related expenses, as well as associated operational costs, due to increased headcount within our research and development function, and $0.4 million in higher depreciation expense associated with an immaterial correction of an error relating to prior periods during 2012. The total number of our research and development employees increased to 46 at December 31, 2012 from 42 at December 31, 2011. We also incurred $0.2 million in additional costs associated with contract services for conducting external research and development studies in support for our publication efforts. In addition, we experienced $0.1 million in higher allocated expenses due to increases in information technology and facility costs. As a percentage of total revenues, research and development expenses increased to 18.2% for the year ended December 31, 2012, as compared to 17.0% for the year ended December 31, 2011.
Sales and Marketing Expenses
Sales and marketing expenses increased by 5.1%, to $22.4 million for the year ended December 31, 2012 from $21.3 million for the year ended December 31, 2011. This increase reflected an increase of $0.8 million in compensation and benefit costs and travel and entertainment-related expenses as a result of the growth and expansion of our sales organization. The total number of our sales and marketing employees increased to 74 at December 31, 2012 from 70 at December 31, 2011. In addition, we experienced $0.3 million in higher allocated expenses due to increases in information technology and facility costs. While sales and marketing expenses increased in absolute dollar amounts, they decreased as a percentage of total revenues from 46.5% for the year ended December 31, 2011 to 40.9% for the year ended December 31, 2012.
General and Administrative Expenses
General and administrative expenses increased by 21.3%, to $10.4 million for the year ended December 31, 2012 from $8.6 million for the year ended December 31, 2011. This increase was primarily the result of $1.8 million in higher salaries and benefits, including stock-based compensation expense, from increased headcount within our general and administrative function, and $0.5 million in higher professional fees, largely from additional legal fees associated with the OIG gift card matter and the DOJ investigation and additional expenses associated with expanded compliance efforts. We incurred $0.4 million in legal and accounting expenses in 2012 in connection with the OIG gift card matter. The total number of our general and administrative employees increased to 34 at December 31, 2012 from 28 at December 31, 2011. In addition, we experienced $0.3 million in higher allocated expenses due to increases in information technology and facility costs. This increase was partially offset by $0.2 million in lower spending associated with contract services due to lower utilization of external consultants within our finance department and a $0.6 million reduction in bad debt expense.
Our bad debt expense was $0.8 million for the year ended December 31, 2012 and $1.4 million for the year ended December 31, 2011. As a percentage of total revenues, bad debt expense decreased to 1.5% for the year ended December 31, 2012 from 3.0% for the year ended December 31, 2011. The decrease in bad debt expense, both in absolute dollars and as a percentage of total revenues, resulted primarily from the shift of our customer base towards clinical diagnostic laboratories, from which we typically experience improved collection rates.
As a percentage of total revenues general and administrative expenses increased to 18.9% for the year ended December 31, 2012, compared to 18.7% for the year ended December 31, 2011.
57
Other Expense
Other expense increased to $0.7 million for the year ended December 31, 2012 from an expense of $0.2 million for the year ended December 31, 2011. The increase was primarily attributable to a $0.4 million increase in other expense as a result of changes in the fair value of our preferred stock warrant liability between the periods. We experienced a $0.2 million increase in interest expense compared to the prior year period, due to higher average principal amounts outstanding on our indebtedness during the later period. In addition, we incurred a $59,000 loss on the extinguishment of debt recognized during the first quarter of 2011 as a result of refinancing our credit facility with Square 1.
Comparison of Years Ended December 31, 2011 and 2010
The following table sets forth, for the periods indicated, the amounts of certain components of our statements of operations and the percentage of total revenues represented by these items, showing period-to-period changes.
Year ended December 31, | Period-to-Period Change | |||||||||||||||||||
2011 | % of Revenues | 2010 | % of Revenues | Amount | Percentage | |||||||||||||||
(in thousands, except for percentages) | ||||||||||||||||||||
Revenues | $ | 45,807 | 100.0 | % | $ | 39,368 | 100.0 | % | $ | 6,439 | 16.4 | % | ||||||||
Cost of revenues | 8,529 | 18.6 | 8,139 | 20.7 | 390 | 4.8 | ||||||||||||||
Gross profit | 37,278 | 81.4 | 31,229 | 79.3 | 6,049 | 19.4 | ||||||||||||||
Operating expenses: | ||||||||||||||||||||
Research and development | 7,808 | 17.0 | 7,276 | 18.5 | 532 | 7.3 | ||||||||||||||
Sales and marketing | 21,305 | 46.5 | 15,246 | 38.7 | 6,059 | 39.7 | ||||||||||||||
General and administrative | 8,550 | 18.7 | 7,331 | 18.6 | 1,219 | 16.6 | ||||||||||||||
Gain on extinguishment of other long-term Liabilities | — | — | (2,700 | ) | (6.9 | ) | 2,700 | * | ||||||||||||
Total operating expenses | 37,663 | 82.2 | 27,153 | 68.9 | 10,510 | 38.7 | ||||||||||||||
(Loss) income from operations | (385 | ) | (0.8 | ) | 4,076 | 10.4 | (4,461 | ) | * | |||||||||||
Total other (expense) income | (163 | ) | (0.4 | ) | 220 | 0.6 | (383 | ) | * | |||||||||||
(Loss) income before taxes | (548 | ) | (1.2 | ) | 4,296 | 11.0 | (4,844 | ) | * | |||||||||||
Income tax (benefit) | — | — | (16 | ) | 0.0 | 16 | * | |||||||||||||
Net (loss) income | $ | (548 | ) | (1.2 | )% | $ | 4,312 | 11.0 | % | $ | (4,860 | ) | * | |||||||
* Percentage not meaningful
Revenues
Total revenues increased by 16.4%, to $45.8 million for the year ended December 31, 2011 from $39.4 million for the year ended December 31, 2010. Revenues from sales of our NMR LipoProfile test increased to $42.4 million for the year ended December 31, 2011 from $34.4 million for the year ended December 31, 2010, resulting from growth in the number of NMR LipoProfile tests sold, particularly to our clinical diagnostic laboratory customers. This growth reflected the impact of an increase in the number of our sales representatives and greater geographic coverage of our sales force, and volume growth attributable to recently acquired clinical diagnostic laboratory customers such as Health Diagnostic Laboratory, Inc., as well as increased market acceptance of our test.
The overall number of NMR LipoProfile tests increased by 38.9% to approximately 1,508,000 tests for the year ended December 31, 2011 from approximately 1,086,000 tests for the year ended December 31, 2010. The overall average selling price of NMR LipoProfile tests decreased 11.3%, to $28.10 for the year ended December 31, 2011 from $31.67 for the year ended December 31, 2010. This decrease in average selling price was primarily the result of a continuing shift in channel mix toward clinical laboratory customers. The percentage of total NMR LipoProfile tests sold through our direct distribution channel decreased from 16% for the year ended December 31, 2010 to 8% for the year ended December 31, 2011. This decrease reflected our strategy of accelerating the adoption of our NMR LipoProfile test through clinical diagnostic laboratories.
58
Revenues from sales of ancillary tests decreased from $3.4 million for the year ended December 31, 2010 to $2.2 million for the year ended December 31, 2011. The decrease in revenues from ancillary tests was primarily driven by the shift in testing mix and an overall reduction of reimbursement rates from Medicare. Revenues from our clinical research clients were $1.2 million and $1.5 million for the years ended December 31, 2011 and 2010, respectively.
Cost of Revenues and Gross Margin
Cost of revenues increased by 4.8%, to $8.5 million for the year ended December 31, 2011 from $8.1 million for the year ended December 31, 2010. This increase resulted primarily from the increase in the number of NMR LipoProfile tests sold to patient care clients during the year ended December 31, 2011. This additional testing volume resulted in increased freight and material costs and repair and maintenance costs. We also experienced higher overhead costs due to increases in information technology and facility costs. Gross margin increased to 81.4% for the year ended December 31, 2011 from 79.3% for the year ended December 31, 2010. The improvement in gross margin resulted primarily from increased sales volume coupled with operating efficiencies in our clinical laboratory.
Research and Development Expenses
Research and development expenses increased by 7.3%, to $7.8 million for the year ended December 31, 2011 from $7.3 million for the year ended December 31, 2010. This increase was primarily the result of $1.5 million in higher salaries and benefits, including stock-based compensation expense, as well as associated operational costs from increased headcount within our research and development function. The total number of our research and development employees increased to 42 at December 31, 2011 from 32 at December 31, 2010. This increase was partially offset by $1.0 million in lower spending associated with contract services due to lower utilization of external consultants in the development of our Vantera system. As a percentage of total revenues, research and development expenses decreased to 17.0% for the year ended December 31, 2011, as compared to 18.5% for the year ended December 31, 2010.
Sales and Marketing Expenses
Sales and marketing expenses increased by 39.7%, to $21.3 million for the year ended December 31, 2011 from $15.2 million for the year ended December 31, 2010. This increase reflected $3.3 million in higher compensation and benefits costs as a result of the growth and expansion of our sales organization and $0.7 million in additional spending associated with travel and entertainment-related expenses, $1.9 million in higher marketing expenses associated with our market awareness efforts and medical education and $0.1 million in higher allocated expenses due to increases in information technology and facility costs. The total number of our sales and marketing employees increased to 70 at December 31, 2011 from 61 at December 31, 2010. As a percentage of total revenues, sales and marketing expenses increased to 46.5% for the year ended December 31, 2011 from 38.7% for the year ended December 31, 2010. The increased sales and marketing expenses as a percentage of total revenues resulted primarily from the expansion of our sales force as we increased our geographic presence.
General and Administrative Expenses
General and administrative expenses increased by 16.6%, to $8.6 million for the year ended December 31, 2011, from $7.3 million for the year ended December 31, 2010. This increase was attributable to a $0.9 million increase in legal and accounting expenses incurred in connection with our initial public offering that were not capitalized as deferred offering costs, as well as additional contracted labor costs within our finance department. We also experienced $0.6 million in higher compensation and benefits costs due mainly to higher bonus expense, as we met the overall 2011 corporate financial targets on which bonuses were based. In addition, we experienced $0.1 million in higher allocated expenses due to increases in information technology and facility costs. These increases were partially offset by $0.4 million in lower bad debt expense. As a percentage of total revenues, general and administrative expenses increased to 18.7% for the year ended December 31, 2011 from 18.6% for the year ended December 31, 2010.
Our bad debt expense was $1.4 million for the year ended December 31, 2011 and $1.7 million for the year ended December 31, 2010. As a percentage of total revenues, bad debt expense decreased to 3.0% for the year ended December 31, 2011 from 4.4% for the year ended December 31, 2010. The decrease in bad debt expense as a percentage of total revenues resulted primarily from the shift of our customer base towards clinical diagnostic laboratories, from which we typically experience improved collection rates.
59
Other (Expense) Income
Other (expense) income changed by $0.4 million, to an expense of $0.2 million for the year ended December 31, 2011 from income of $0.2 million for the year ended December 31, 2010. The change was primarily attributable to a $0.1 million decrease in other income as a result of changes in the fair value of our preferred stock warrant liability between the periods. We experienced a $0.2 million increase in interest expense compared to the prior year period, due to higher principal amounts outstanding on our indebtedness during the respective periods and a $0.1 million loss for extinguishment of debt during the first quarter of 2011 that we recognized as a result of refinancing our credit facility with Square 1.
Seasonality
Our financial results may vary significantly from quarter to quarter as a result of many factors, many of which are outside our control. For example, we expect that the volume of our NMR LipoProfile tests ordered will generally decline during the summer months and holiday periods, when patients are less likely to visit their healthcare providers. As a result, comparison of our results of operations for successive quarters may not accurately reflect trends or results for the full year. Our historical results should not be considered a reliable indicator of our future results of operations.
Inflation
We do not believe that inflation has had a material effect on our business, financial condition or results of operations. We continue to monitor the impact of inflation in order to minimize its effects through pricing strategies, productivity improvements and cost reductions. If our costs were to become subject to significant inflationary pressures, we may not be able to fully offset such higher costs through price increases. Our inability or failure to do so could harm our business, financial condition and results of operations.
Liquidity and Capital Resources
Sources of Liquidity
Prior to our IPO in January 2013, we funded our operations principally through private placements of our capital stock, bank borrowings and, since 2009, cash flows from operations. We have raised approximately $58.7 million from the sale of common stock and convertible preferred stock to third parties. The last of these equity financing transactions occurred in 2006.
In February 2008, we entered into a credit facility with Square 1 that provided for a term loan of $4.5 million and a revolving line of credit of up to $3.0 million. We have entered into a series of amendments to this credit facility with Square 1 to, among other things, increase the term loan to $6.0 million and the revolving line of credit capacity to $4.0 million. Interest on the term loan accrued at a variable annual rate equal to the greater of 7.25% or the prime rate plus 3.75%. Interest on amounts borrowed under the line of credit accrued at a variable annual rate equal to the greater of 6.25% or Square 1's prime rate plus 3.00%. We were required only to make interest payments on the term loan through December 31, 2011. Repayments of principal amounts due under the term loan commenced in January 2012 and were scheduled to continue in 30 monthly installments through June 2014. As of September 30, 2012, we owed $4.2 million under the term loan, and $3.5 million under the revolving line of credit, which was scheduled to mature in May 2013.
In December 2012, we entered into a new credit facility with Square 1 and Oxford Finance and repaid the foregoing loans in full. The new credit facility provides for term loans from Oxford Finance of $10.0 million, a term loan from Square 1 of $6.0 million and a new revolving line of credit of up to $6.0 million. Our borrowing capacity under the line of credit is subject to borrowing base limitations related to our eligible accounts receivable. Interest on the term loans accrues at a fixed annual rate of 9.5%, while advances under the line of credit will continue to carry a variable interest rate equal to the greater of 6.25% or the prime rate plus 3.0%. We are required only to make interest payments on the term loans through January 2014, and then repayments of principal and interest amounts due under the term loans will continue in monthly installments through July 2016. As of December 31, 2012, we had borrowed $5.0 million under the revolving line of credit and we had no additional available borrowing capacity. The revolving line of credit matures in December 2013.
60
Borrowings under the credit facility are secured by substantially all of our assets other than our intellectual property. The covenants set forth in the loan and security agreement require, among other things, that we maintain a specified liquidity ratio, measured monthly, that begins at 1.25 and is reduced to 1.0 over the term of the agreement, and that we achieve minimum three-month trailing revenue levels during the term of the agreement, which are based on 80% of our projected revenue levels. In addition, the loan and security agreement requires that our projections provided to the lenders include annual projected revenues of at least $55 million. As of December 31, 2012, our liquidity ratio was 2.45, and that ratio increased to 7.57 as of February 28, 2013 as a result of the receipt of proceeds from our initial public offering in January 2013. If we fail to comply with the covenants and our other obligations under the credit facility, the lenders would be able to accelerate the required repayment of amounts due under the loan agreement and, if they are not repaid, could foreclose upon our assets securing our obligations under the credit facility. We are currently in compliance with all required covenants.
In connection with the foregoing credit facilities, we issued warrants to Oxford Finance to purchase an aggregate of 45,978 shares of our Series E redeemable convertible preferred stock at an exercise price of $4.35 per share and warrants to Square 1 to purchase an aggregate of 27,586 shares of our Series E redeemable convertible preferred stock and 88,793 shares of our Series F redeemable convertible preferred stock, in each case at an exercise price of $4.35 per share. Upon the closing of our initial public offering, the warrants automatically became warrants to purchase an aggregate of 78,741 shares of common stock at an exercise price of $8.97 per share.
Initial Public Offering
On January 30, 2013, we completed our IPO in which we sold 5,750,000 shares of common stock and received net proceeds of approximately $44.4 million, after deducting underwriting discounts and offering-related expenses paid by us. Upon closing of the IPO, all of the redeemable convertible preferred stock then outstanding automatically converted into 6,994,559 shares of common stock. In addition, outstanding warrants to purchase redeemable convertible preferred stock automatically converted into warrants to purchase common stock, and our preferred stock warrant liability of $0.4 million as of January 30, 2013 was reclassified to additional paid-in capital.
Cash and Cash Equivalents
The following table summarizes our cash and cash equivalents, accounts receivable and cash flows for the periods indicated:
As of and for the Year Ended December 31, | |||||||||||
2012 | 2011 | 2010 | |||||||||
(in thousands) | |||||||||||
Cash and cash equivalents | $ | 24,768 | $ | 12,483 | $ | 11,058 | |||||
Accounts receivable, net | 5,149 | 5,626 | 4,194 | ||||||||
Operating activities | 7,597 | 96 | 1,143 | ||||||||
Investing activities | (8,127 | ) | (2,168 | ) | (1,209 | ) | |||||
Financing activities | 12,815 | 3,497 | (921 | ) | |||||||
Net increase (decrease) in cash and cash equivalents | $ | 12,285 | $ | 1,425 | $ | (987 | ) | ||||
Our cash and cash equivalents at December 31, 2012, 2011 and 2010 were held for working capital purposes. We do not enter into investments for trading or speculative purposes. Our policy is to invest any cash in excess of our immediate requirements in investments designed to preserve the principal balance and provide liquidity. Accordingly, our cash and cash equivalents are invested primarily in demand deposit accounts, certificates of deposit and money market funds that are currently providing only a minimal return.
Restricted cash, which totaled $1.5 million at December 31, 2012, 2011 and 2010 is not included in cash and cash equivalents, consists primarily of certificates of deposit that secure letters of credit related to operating leases for our office and laboratory space.
61
Cash Flows for the Years Ended December 31, 2012, 2011 and 2010
Operating Activities
Net cash provided by operating activities was $7.6 million during the year ended December 31, 2012, which included net income of $1.3 million and net non-cash items of $2.7 million. Non-cash items for the year ended December 31, 2012 consisted primarily of depreciation and amortization expense of $1.3 million and stock-based compensation expense of $1.2 million and $0.2 million in expense incurred due to changes in the fair value of our preferred stock warrant liability. We also had a net cash inflow from changes in operating assets and liabilities of $3.7 million during the year. The significant items in the changes in operating assets and liabilities included a decrease of $0.5 million in accounts receivable, an increase of $1.2 million in accounts payable, accrued liabilities and other current liabilities and an increase of $2.3 million in other long-term liabilities, offset by an increase of $0.1 million in inventories and $0.1 million in prepaid expenses. The decrease in accounts receivable was due primarily to the timing of collections. The increase in accrued expenses during 2012 was due to higher expected bonus and commission payouts based on performance against target goals and increased spending associated with our initial public offering. The increase in other current liabilities consisted primarily of deferred rent due to tenant allowances related to our facility lease.
Net cash provided by operating activities was $0.1 million during the year ended December 31, 2011, which included net loss of $0.5 million, partially offset by net non-cash items of $1.0 million. Non-cash items for the year ended December 31, 2011 consisted primarily of depreciation and amortization expense of $0.5 million and stock-based compensation expense of $0.7 million, a $0.1 million loss on extinguishment of debt, offset by a $0.3 million decrease in the fair value of our preferred stock warrant liability. We also had a net cash outflow from changes in operating assets and liabilities of $0.3 million during the year. The significant items in the changes in operating assets and liabilities included an increase of $1.4 million in accounts receivable, an increase of $0.1 million in prepaid expenses and a decrease of $0.1 million in other long-term liabilities, offset by an increase of $1.3 million in accounts payable, accrued liabilities and other current liabilities. The increase in accounts receivable was due primarily to the growth in our revenues. The increase in accounts payable during 2011 was due to higher expected bonus and commission payouts based on performance against target goals and increased spending associated with our initial public offering.
Net cash provided by operating activities was $1.1 million during the year ended December 31, 2010, which included net income of $4.3 million, partially offset by net non-cash items of $1.9 million. Non-cash items for the year ended December 31, 2010 consisted primarily of a $2.7 million gain on extinguishment of other long-term liabilities and a $0.4 million increase in the fair value of our preferred stock warrant liability, offset by depreciation and amortization expense of $0.6 million and stock-based compensation expense of $0.6 million. We also had a net cash outflow from changes in operating assets and liabilities of $1.3 million during the year. The significant items in the changes in operating assets and liabilities included an increase of $0.8 million in accounts receivable and an increase of $0.2 million in prepaid expenses, a decrease of $0.2 million in accounts payable and other current liabilities, and a decrease of $0.1 million in other long-term liabilities. The increase in accounts receivable was due primarily to the growth in our revenues.
Growth in the number of NMR LipoProfile tests we perform, the impact of other revenue and expenses and the timing and amount of future working capital changes will affect the future amount of cash used in or provided by operating activities.
Investing Activities
Net cash used in investing activities was $8.1 million, $2.2 million and $1.2 million for the years ended December 31, 2012, 2011 and 2010, respectively. The amounts related primarily to purchases of property and equipment and patent and trademark costs. The purchases of property and equipment during the years ended December 31, 2012 and 2011 were primarily for hardware components purchased from third-party manufacturers for the Vantera system not yet put into service, while the purchases of property and equipment during the year ended December 31, 2010 were primarily for computer and furniture for general office use due to increased headcount, leasehold improvements related to our facilities and equipment used in test production. The capitalized patent and trademark costs during the years ended December 31, 2012, 2011 and 2010 were primarily for pending domestic and international patent applications and prosecution.
62
Financing Activities
Net cash provided by financing activities was $12.8 million during the year ended December 31, 2012, consisting primarily of proceeds from borrowings under the revolving line of credit of $8.5 million and long-term debt of $16.0 million, and net proceeds from exercises of stock options and warrants of $0.2 million, partially offset by cash outlays of $1.1 million for tax withholding paid on behalf of certain employees in connection with the net exercise of stock options, deferred offering costs of $1.0 million, debt discount of $0.1 million, deferred issuance costs of $0.1 million and repayment of the revolving line of credit of $3.5 million and long-term debt of $6.0 million.
Net cash provided by financing activities was $3.5 million during the year ended December 31, 2011, consisting primarily of proceeds from long-term debt of $6.0 million and net proceeds from exercises of stock options and warrants of $0.6 million, partially offset by cash outlays for deferred offering costs of $1.9 million and repayment of long-term debt of $1.2 million.
Net cash used in financing activities was $0.9 million during the year ended December 31, 2010, consisting primarily of repayment of long-term debt of $1.8 million, partially offset by net proceeds from exercises of stock options and warrants of $0.9 million.
Operating and Capital Expenditure Requirements
We expect to incur substantial operating losses in the future and that our operating expenses will increase as we continue to expand our sales force and increase our marketing efforts to drive market adoption of the NMR LipoProfile test, commercially launch our Vantera system and develop additional diagnostic tests. Our liquidity requirements have historically consisted, and we expect that they will continue to consist, of sales and marketing expenses, research and development expenses, capital expenditures, working capital, debt service and general corporate expenses. As demand for placements of our Vantera system increases from our clinical diagnostic laboratory customers, we anticipate that our capital expenditure requirements will also increase in order to build additional analyzers for placement.
We believe the cash generated from operations and our current cash and cash equivalents balances, which include the net proceeds from our initial public offering, and interest income we earn on these balances, together with the available borrowing capacity under our credit facilities, will be sufficient to meet our anticipated cash requirements through at least the next 12 months. In the future, we expect our operating and capital expenditures to increase as we increase headcount, expand our sales and marketing activities and grow our customer base. As sales of our NMR LipoProfile test grow, we expect our accounts receivable balance to increase. Any such increase in accounts receivable may not be completely offset by increases in accounts payable and accrued expenses, which could result in greater working capital requirements.
If our cash balances and cash generated from operations are insufficient to satisfy our liquidity requirements, we may seek to sell common or preferred equity or convertible debt securities or enter into an additional credit facility or seek other debt financing. The sale of equity and convertible debt securities may result in dilution to our stockholders, and those securities may have rights senior to those of our common shares. If we raise additional funds through the issuance of preferred stock, convertible debt securities or other debt financing, these securities or other debt could contain covenants that would restrict our operations. We may require additional capital beyond our currently anticipated amounts. Additional capital may not be available on reasonable terms, or at all.
Our estimates of the period of time through which our financial resources will be adequate to support our operations and the costs to support research and development and our sales and marketing activities are forward-looking statements and involve risks and uncertainties and actual results could vary materially and negatively as a result of a number of factors, including the factors discussed in the section “Risk Factors” of this Annual Report. We have based our estimates on assumptions that may prove to be wrong and we could utilize our available capital resources sooner than we currently expect.
63
Our short- and long-term capital requirements will depend on many factors, including the following:
• | the cost of our selling and marketing efforts; |
• | the rate of adoption of the NMR LipoProfile test in the marketplace; |
• | our ability to generate cash from operations; |
• | the rate of our progress in establishing additional coverage and reimbursement with third-party payors; |
• | our ability to control our costs and implement operating efficiencies; |
• | demand from clinical diagnostic laboratories for placements of our Vantera system at their facilities; |
• | the emergence of competing or complementary technological developments; |
• | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights or participating in litigation-related activities; |
• | the economic and other terms and timing of any collaborations, licensing or other arrangements into which we may enter; and |
• | the acquisition of complementary tests or technologies that we may undertake. |
64
Contractual Commitments and Obligations
We have contractual obligations for non-cancelable office space and office equipment operating leases, as well as our credit facilities with Oxford Finance and Square 1. The following table discloses aggregate information about material contractual obligations and periods in which payments are due as of December 31, 2012. Future events could cause actual payments to differ from these estimates.
Payment due by period | |||||||||||||||||||
Contractual Obligations | Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||
(in thousands) | |||||||||||||||||||
Principal repayments on term loans | $ | 16,000 | $ | — | $ | 11,921 | $ | 4,079 | $ | — | |||||||||
Interest payments on term loans (1) | 4,165 | 1,440 | 2,035 | 690 | — | ||||||||||||||
Principal repayments on revolving line of credit | 5,000 | 5,000 | — | — | — | ||||||||||||||
Interest payments on revolving line of credit (2) | 302 | 302 | — | — | — | ||||||||||||||
Operating lease obligations | 12,398 | 1,188 | 2,381 | 2,202 | 6,627 | ||||||||||||||
Purchase obligations | 2,136 | 2,136 | — | — | — | ||||||||||||||
Total | $ | 40,001 | $ | 10,066 | $ | 16,337 | $ | 6,971 | $ | 6,627 | |||||||||
(1) | Assumes that there is no prepayment of principal balance during the term of the loan. |
(2) | Assumes that the variable rate is 6.25% and that there is no prepayment of principal balance during the term of the loan. |
Off-Balance Sheet Arrangements
As of December 31, 2012, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of SEC Regulation S-K, such as the use of unconsolidated subsidiaries, structured finance, special purpose entities or variable interest entities.
Critical Accounting Policies and Significant Judgments and Estimates
Our management's discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP. The preparation of these financial statements requires us to make estimates, assumptions and judgments that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reported period. In accordance with U.S. GAAP, we base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results could therefore differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 1 to our financial statements appearing elsewhere in this report, we believe the following accounting policies are critical to the process of making significant judgments and estimates in the preparation of our financial statements.
Revenue Recognition
We currently derive revenue from sales of our NMR LipoProfile test to clinical diagnostic laboratories and clinicians for use in patient care, from sales of ancillary tests for use in patient care requested in conjunction with the NMR LipoProfile test, and from research contracts.
Revenues from diagnostic tests for patient care, which consist of sales of the NMR LipoProfile test and sales of ancillary tests, are recognized on the accrual basis when the following revenue recognition criteria are met: (1) persuasive evidence that an arrangement exists; (2) services have been rendered or at the time final results are reported; (3) the fee is fixed or determinable; and (4) collectibility is reasonably assured. Testing services provided for patient care are covered by clinical diagnostic laboratories, programs with commercial insurance carriers, including managed care organizations, and various governmental programs, primarily Medicare.
65
Billings for diagnostic tests for patient care under governmental and physician-based programs are included in revenues net of contractual adjustments. These contractual adjustments represent the difference between the final settlement amount paid by the program and the estimated settlement amount based on either the list price for tests performed or the reimbursement rate set by commercial insurance carriers or governmental programs. Estimated contractual adjustments are updated either upon notification from payors as to changes in existing reimbursement rates, which are typically received prior to changes going into effect, or upon a material variance between the final settlement and the estimated contractual adjustment originally established when the revenues were recognized. To date, our final settlement adjustments have not been material.
Revenues from contract research arrangements are generally derived from studies conducted with academic institutions and pharmaceutical companies. The specific methodology for revenue recognition is determined on a case-by-case basis according to the facts and circumstances applicable to a given agreement. Our output, measured in terms of full-time equivalent level of effort or processing a set of diagnostic tests under a contractual protocol, typically triggers payment obligations under these agreements. Revenues are recognized as costs are incurred or diagnostic tests are processed. Contract research costs include all direct labor and material costs, equipment costs and fringe benefits. Advance payments received in excess of revenues recognized are classified as deferred revenue until such time as the revenue recognition criteria have been met.
Accounts Receivable
Accounts receivable are reported net of an allowance for uncollectible accounts. The process of estimating the collection of accounts receivable involves significant assumptions and judgments. Specifically, the accounts receivable allowance is based on management's analysis of current and past due accounts, collection experience in relation to amounts billed, channel mix, any specific customer collection issues that have been identified and other relevant information. Our provision for uncollectible accounts is recorded as bad debt expense and included in general and administrative expenses. Historically, we have not experienced significant credit loss related to our customers or payors. Although we believe amounts provided are adequate, the ultimate amounts of uncollectible accounts receivable could be in excess of the amounts provided.
Stock-Based Compensation Expense
We have included stock-based compensation as part of our cost of revenues and our operating expenses in our statements of operations as follows (in thousands):
Year Ended December 31, | |||||||||||
2012 | 2011 | 2010 | |||||||||
(in thousands) | |||||||||||
Cost of revenues | $ | 46 | $ | 8 | $ | 21 | |||||
Research and development expense | 454 | 155 | 45 | ||||||||
Sales and marketing expense | 255 | 217 | 137 | ||||||||
General and administrative expense | 435 | 271 | 447 | ||||||||
Total | $ | 1,190 | $ | 651 | $ | 650 | |||||
We account for stock-based compensation arrangements with our employees, consultants and non-employee directors using a fair value method, which requires us to recognize compensation expense for costs related to all stock-based payments. To date, our only stock-based awards have been grants of stock options. The fair value method requires us to estimate the fair value of stock-based awards on the date of grant using an option pricing model. The fair value is then recognized as stock-based compensation expense over the requisite service period, which is the vesting period, of the award.
Through December 31, 2012, our common stock was not publicly traded, and therefore we calculated the fair value of stock-based compensation awards using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the input of subjective assumptions, including stock price volatility and the expected life of stock options. As a new public company, we do not have sufficient history to estimate the volatility of our common stock price or the expected life of our options. We calculate expected volatility based on reported data for selected reasonably similar publicly traded companies within the diagnostic industry, or guideline peer group, for which the historical information is available. When selecting the public companies within the diagnostic industry, we selected companies with comparable characteristics to us, including enterprise value and financial leverage, and removed companies with significantly higher enterprise values, lower risk profiles or established positions within the industry. We also selected companies with historical share price volatility information sufficient to meet the expected life of our stock options. The historical volatility data was computed using the daily closing prices for the selected companies' shares during the equivalent period of the calculated expected term of our stock options.
66
We will continue to use the guideline peer group volatility information until the historical volatility of our common stock is relevant to measure expected volatility for future option grants.
We determine the average expected life of stock options according to the “simplified” method. Under this method, the expected term is calculated as the average of the time-to-vesting and the contractual life of the option. The assumed dividend yield is based on our expectation that we will not pay dividends in the foreseeable future, which is consistent with our history of not paying dividends. We determine the risk-free interest rate by using the weighted average assumption equivalent to the expected term based on the U.S. Treasury yield curve in effect as of the date of grant. We estimate forfeitures based on our historical analysis of actual stock option forfeitures. Although, we estimate forfeitures based on historical experience, actual forfeitures may differ. If actual results differ significantly from these estimates, stock-based compensation expense and our statements of operations could be materially impacted. We would record an adjustment for the difference in the period that the options vest.
For the years ended December 31, 2012, 2011 and 2010, we estimated the fair value of stock options at their grant dates using the following assumptions:
Year Ended December 31, | ||||||||
2012 | 2011 | 2010 | ||||||
Expected dividend yield | 0.0 | % | 0.0 | % | 0.0 | % | ||
Risk-free interest rate | 0.8 | % | 2.0 | % | 2.0 | % | ||
Expected volatility | 50.0 | % | 50.3 | % | 49.9 | % | ||
Expected life (in years) | 5.6 | 5.6 | 5.6 | |||||
There is a high degree of subjectivity involved when using option-pricing models to estimate stock-based compensation. There is currently no market-based mechanism or other practical application to verify the reliability and accuracy of the estimates stemming from these valuation models, nor is there a means to compare and adjust the estimates to actual values. Although the fair value of stock-based awards is determined using an option-pricing model, that value may not be indicative of the fair value that would be observed in a market transaction between a willing buyer and willing seller. If factors change and we employ different assumptions when valuing our options, the compensation expense that we record in the future may differ significantly from what we have historically reported.
Redeemable Convertible Preferred Stock Warrants
In connection with prior financing transactions, we issued freestanding warrants to purchase shares of our redeemable convertible preferred stock. These warrants were classified as liabilities on our balance sheets at their fair value, because the warrants conditionally obligated us to redeem the underlying convertible preferred stock. We adjusted the warrants to their current fair value at each balance sheet date, and we recognized any resulting change in fair value as a component of other income (expense) in the statements of operations.
We estimated the fair value of the warrants at each balance sheet date using a Black-Scholes option pricing model. At each valuation date, we estimated our enterprise value by allocating the value to the shares of our redeemable convertible preferred stock, convertible preferred stock, warrants to purchase shares of redeemable convertible preferred stock, and common stock, using a “hybrid” approach of the probability-weighted expected return method and the option pricing model. This approach treats the various components of our capital structure as a series of call options on the proceeds expected from the sale of our business or the liquidation of our assets in the future. This approach defines the securities' fair values as functions of the current fair value of our business and assumptions based on the securities' rights and preferences. These call options are then valued using the Black-Scholes option pricing model. As a result, the option-pricing model requires a number of assumptions to estimate the fair value, including the anticipated timing of a potential liquidity event, such as an initial public offering, remaining contractual terms of the warrant, risk-free interest rates, expected dividend yield and the estimated volatility of the price of the underlying securities. The fair value of the preferred stock issuable upon exercise of the warrants was also determined taking into account the relative dividend and liquidation preference rights of the various series of preferred stock. The anticipated timing of a liquidity event utilized in these valuations was based on then current plans and estimates of our board of directors and management regarding an initial public offering. These assumptions are highly judgmental and could differ significantly in the future.
Upon the closing of our initial public offering in January 2013 and the conversion of the underlying preferred stock to common stock, the preferred stock warrants automatically became warrants to purchase shares of our common stock. The then-current aggregate fair value of these warrants was reclassified from liabilities to additional paid-in capital, and we have ceased to record any further periodic fair value adjustments.
67
Income Taxes
We are subject to income taxes in the United States, and we use estimates in determining our provision for income taxes. We use the asset and liability method of accounting for income taxes. Under this method, deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect for the year in which the differences are expected to affect taxable income.
Recognition of deferred tax assets is appropriate when realization of such assets is more likely than not. We recognize a valuation allowance against our net deferred tax assets if it is more likely than not that some portion of the deferred tax assets will not be fully realizable. This assessment requires judgment as to the likelihood and amounts of future taxable income by tax jurisdiction. At December 31, 2012, we had a full valuation allowance against all of our deferred tax assets.
We have adopted authoritative guidance to account for uncertain tax positions. None of our currently unrecognized tax benefits would affect our effective income tax rate if recognized, due to the valuation allowance that currently offsets our deferred tax assets. We do not anticipate the total amount of unrecognized income tax benefits relating to tax positions existing at December 31, 2012 will significantly increase or decrease in the next 12 months.
We assess all material positions taken in any income tax return, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. Assessing an uncertain tax position begins with the initial determination of the position's sustainability and is measured at the largest amount of benefit that is greater than 50% likely to be realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed, and we will determine whether the factors underlying the sustainability assertion have changed and whether the amount of the recognized tax benefit is still appropriate.
The recognition and measurement of tax benefits requires significant judgment. Judgments concerning the recognition and measurement of a tax benefit might change as new information becomes available.
As of December 31, 2012, we had federal net operating loss carryforwards, state net operating loss carryforwards and research and development credit carryforwards of $32.9 million, $29.1 million, and $2.0 million, respectively. Our federal and state net operating loss carryforwards included $3.3 million of excess tax benefits related to deductions from the exercise of nonqualified stock options. The tax benefit of these deductions has not been recognized in deferred tax assets. The federal and state net operating loss carryforwards and the research and development credit carryforwards will continue to partially expire in 2015. We analyzed our filing positions in all significant federal, state and foreign jurisdictions where we are required to file income tax returns, as well as open tax years in these jurisdictions. With few exceptions, we are no longer subject to U.S. Federal and state and local tax examinations by tax authorities for years prior to 2009, although carryforward attributes that were generated prior to 2009 may still be adjusted upon examination by the Internal Revenue Service if they either have been or will be used in a future period.
Recent Accounting Pronouncements
In October 2012, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update, or ASU, No. 2012-04, Technical Corrections and Improvements. The amendments in this update cover a wide range of topics in the Accounting Standards Codification. These amendments include technical corrections and improvements to the Accounting Standards Codification and conforming amendments related to fair value measurements. For public companies, the amendments that are subject to the transition guidance will be effective for reporting periods beginning after December 15, 2012. The adoption of this new guidance is not expected to have a material impact on our financial position, results of operations or cash flows.
Under the JOBS Act, emerging growth companies that become public can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards following the completion of our initial public offering and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
68
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Market risk is the risk of loss to future earnings, to fair values or to future cash flows that may result from changes in price of a financial instrument. The value of a financial instrument may change as a result of changes in interest rates, exchange rates, commodity prices, equity prices and other market changes.
Interest Rate Sensitivity
We are exposed to market risk related to changes in interest rates as it impacts our interest income and expense.
Cash and Cash Equivalents. As of December 31, 2012, we had cash and cash equivalents of $24.8 million. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S. interest rates. Our cash equivalents are invested in interest-bearing certificates of deposit and money market funds. We do not enter into investments for trading or speculative purposes. Due to the conservative nature of our investment portfolio, which is predicated on capital preservation and mainly consists of investments with short maturities, we do not believe an immediate one percentage point change in interest rates would have a material effect on the fair market value of our portfolio, and therefore we do not expect our operating results or cash flows to be significantly affected by changes in market interest rates.
Term Loan and Line of Credit. As of December 31, 2012, we had debt obligations of $20.7 million under our credit facilities with Oxford Finance and Square 1. Our primary exposure to market risk is interest expense sensitivity, which is affected by changes in the general level of U.S. interest rates. Under the new credit facility, the term loans carry a fixed interest rate of 9.5%, while advances under the line of credit carry a variable interest rate equal to the greater of 6.25% or Square 1's prime rate plus 3.0%. If there is a rise in interest rates, our debt service obligations under the loan agreement would increase even though the amount borrowed remained the same, which would affect our results of operations, financial condition and liquidity. Assuming no changes in our variable rate debt obligations from the amount outstanding at December 31, 2012, a hypothetical one percentage point change in underlying variable rates would have changed our annual interest expense and cash flow from operations by approximately $48,000 without taking into account the effect of any hedging instruments. We have not entered into, and do not expect to enter into, hedging arrangements.
Foreign Currency Exchange Risk
We bill our customers and payors in U.S. dollars and receive payment in U.S. dollars. Accordingly, our results of operations and cash flows are not subject to fluctuations due to changes in foreign currency exchange rates. If we grow sales of our NMR LipoProfile test outside of the United States, or place the Vantera system in foreign jurisdictions, our contracts with foreign customers and payors may be denominated in foreign currency and may become subject to changes in currency exchange rates.
69
Item 8. Financial Statements and Supplementary Data
LipoScience, Inc.
INDEX TO FINANCIAL STATEMENTS
Page | |
70
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of LipoScience, Inc.
We have audited the accompanying balance sheets of LipoScience, Inc. as of December 31, 2012 and 2011, and the related statements of operations, redeemable convertible preferred stock and stockholders' deficit, and cash flows for each of the three years in the period ended December 31, 2012. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of LipoScience, Inc. at December 31, 2012 and 2011, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2012, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
Raleigh, North Carolina
March 27, 2013
71
LipoScience, Inc.
Balance Sheets
(in thousands, except share and per share data)
December 31, | |||||||
2012 | 2011 | ||||||
Assets | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 24,768 | $ | 12,483 | |||
Accounts receivable, net | 5,149 | 5,626 | |||||
Inventories | 125 | — | |||||
Prepaid expenses and other | 719 | 581 | |||||
Total current assets | 30,761 | 18,690 | |||||
Property and equipment, net | 11,493 | 5,292 | |||||
Other noncurrent assets: | |||||||
Restricted cash | 1,505 | 1,504 | |||||
Intangible assets, net of accumulated amortization of $147 and $128 at December 31, 2012 and 2011 | 637 | 553 | |||||
Deferred financing costs | 100 | — | |||||
Deferred offering costs | 3,151 | 2,046 | |||||
Other assets | 32 | 32 | |||||
Total other noncurrent assets | 5,425 | 4,135 | |||||
Total assets | $ | 47,679 | $ | 28,117 | |||
Liabilities, redeemable convertible preferred stock and stockholders’ deficit | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 1,003 | $ | 1,281 | |||
Accrued expenses | 5,348 | 4,413 | |||||
Revolving line of credit | 5,000 | — | |||||
Current maturities of long-term debt | — | 2,400 | |||||
Other current liabilities | 1 | 1 | |||||
Total current liabilities | 11,352 | 8,095 | |||||
Long-term liabilities: | |||||||
Long-term debt, less current maturities | 15,708 | 3,600 | |||||
Preferred stock warrant liability | 412 | 229 | |||||
Other long-term liabilities | 2,462 | 101 | |||||
Total liabilities | 29,934 | 12,025 | |||||
Redeemable Convertible Preferred Stock: | |||||||
Series D Redeemable Convertible Preferred Stock, par value $.001; 3,544,062 shares designated; 500,408 shares issued and outstanding at December 31, 2012 and 2011; aggregate liquidation preference of $2,612 | 2,612 | 2,612 | |||||
Series D-1 Redeemable Convertible Preferred Stock, par value $.001; 3,480,473 shares designated; 2,980,065 issued and outstanding at December 31, 2012 and 2011; aggregate liquidation preference of $15,556 | 15,556 | 15,556 | |||||
Series E Redeemable Convertible Preferred Stock, par value $.001; 5,059,330 shares designated; 4,718,752 issued and outstanding at December 31, 2012 and 2011; aggregate liquidation preference of $20,527 | 20,795 | 20,795 | |||||
Series F Redeemable Convertible Preferred Stock, par value $.001; 3,118,678 shares designated; 3,006,447 and 2,988,506 issued and outstanding at December 31, 2012 and 2011; aggregate liquidation preference of $13,000 | 18,338 | 18,200 | |||||
Total Redeemable Convertible Preferred Stock | 57,301 | 57,163 | |||||
Stockholders’ deficit: | |||||||
Series A Convertible Preferred Stock, par value $.001; 300,000 shares designated, 229,088 shares issued and outstanding at December 31, 2012 and 2011; aggregate liquidation preference of $1,291 | 0 | 0 | |||||
Series A-1 Convertible Preferred Stock, par value $.001; 252,700 shares designated, 23,612 shares issued and outstanding at December 31, 2012 and 2011; aggregate liquidation preference of $125 | 0 | 0 | |||||
Series B Convertible Preferred Stock, par value $.001; 166,667 shares designated, 154,536 shares issued and outstanding at December 31, 2012 and 2011; aggregate liquidation preference of $927 | 0 | 0 | |||||
Series B-1 Convertible Preferred Stock, par value $.001; 159,536 shares designated, 5,000 shares issued and outstanding at December 31, 2012 and 2011; aggregate liquidation preference of $30 | 0 | 0 | |||||
Series C Convertible Preferred Stock, par value $.001; 1,275,000 shares designated, 1,022,595 shares issued and outstanding at December 31, 2012 and 2011; aggregate liquidation preference of $4,090 | 1 | 1 | |||||
Series C-1 Convertible Preferred Stock, par value $.001; 1,274,774 shares designated, 252,179 shares issued and outstanding at December 31, 2012 and 2011; aggregate liquidation preference of $1,009 | 0 | 0 | |||||
Common stock, $.001 par value; 90,000,000 shares authorized, 1,894,277 and 1,695,485 shares issued and outstanding at December 31, 2012 and 2011 | 2 | 2 | |||||
Additional paid-in capital | 8,434 | 8,169 | |||||
Accumulated deficit | (47,993 | ) | (49,243 | ) | |||
Total stockholders’ deficit | (39,556 | ) | (41,071 | ) | |||
Total liabilities, redeemable convertible preferred stock and stockholders’ deficit | $ | 47,679 | $ | 28,117 | |||
See accompanying notes to financial statements.
72
LipoScience, Inc.
Statements of Operations
(in thousands, except share and per share data)
Year Ended December 31, | |||||||||||
2012 | 2011 | 2010 | |||||||||
Revenues | $ | 54,798 | $ | 45,807 | $ | 39,368 | |||||
Cost of revenues | 10,060 | 8,529 | 8,139 | ||||||||
Gross profit | 44,738 | 37,278 | 31,229 | ||||||||
Operating expenses: | |||||||||||
Research and development | 9,974 | 7,808 | 7,276 | ||||||||
Sales and marketing | 22,402 | 21,305 | 15,246 | ||||||||
General and administrative | 10,373 | 8,550 | 7,331 | ||||||||
Loss on disposal of long-lived assets | 30 | — | — | ||||||||
Impairment of intangible assets | 13 | — | — | ||||||||
Gain on extinguishment of other long-term liabilities (Note 9) | — | — | (2,700 | ) | |||||||
Total operating expenses | 42,792 | 37,663 | 27,153 | ||||||||
Income (loss) from operations | 1,946 | (385 | ) | 4,076 | |||||||
Other (expense) income: | |||||||||||
Interest income | 13 | 13 | 16 | ||||||||
Interest expense | (540 | ) | (350 | ) | (154 | ) | |||||
Loss on extinguishment of long-term debt | — | (59 | ) | — | |||||||
Other (expense) income | (169 | ) | 233 | 358 | |||||||
Total other (expense) income | (696 | ) | (163 | ) | 220 | ||||||
Income (loss) before taxes | 1,250 | (548 | ) | 4,296 | |||||||
Income tax (benefit) | — | — | (16 | ) | |||||||
Net income (loss) | 1,250 | (548 | ) | 4,312 | |||||||
Accrual of dividends on redeemable convertible preferred stock | — | (613 | ) | (1,040 | ) | ||||||
Undistributed earnings allocated to participating preferred stockholders | (1,004 | ) | — | (2,655 | ) | ||||||
Net income (loss) attributable to common stockholders—basic | 246 | (1,161 | ) | 617 | |||||||
Undistributed earnings re-allocated to common stockholders | 113 | — | 303 | ||||||||
Net income (loss) attributable to common stockholders—diluted | $ | 359 | $ | (1,161 | ) | $ | 920 | ||||
Net income (loss) per share attributable to common stockholders—basic | $ | 0.14 | $ | (0.69 | ) | $ | 0.38 | ||||
Net income (loss) per share attributable to common stockholders—diluted | $ | 0.13 | $ | (0.69 | ) | $ | 0.34 | ||||
Weighted average shares used to compute basic net income (loss) per share attributable to common stockholders | 1,715,408 | 1,674,018 | 1,611,843 | ||||||||
Weighted average shares used to compute diluted net income (loss) per share attributable to common stockholders | 2,814,323 | 1,674,018 | 2,713,770 | ||||||||
See accompanying notes to financial statements.
73
LipoScience, Inc.
Statements of Redeemable Convertible Preferred Stock and Stockholders’ Deficit
(in thousands)
Series D | Series D-1 | Series E | Series F | |||||||||||||||||||||||||||||||||||||||||||||||||||||
Redeemable | Redeemable | Redeemable | Redeemable | |||||||||||||||||||||||||||||||||||||||||||||||||||||
Convertible | Convertible | Convertible | Convertible | Convertible Preferred Stock | Common | Additional | Total | |||||||||||||||||||||||||||||||||||||||||||||||||
Preferred | Preferred | Preferred | Preferred | Stock | Paid-In | Accumulated | Stockholders’ | |||||||||||||||||||||||||||||||||||||||||||||||||
Stock | Stock | Stock | Stock | Series A | Series A-1 | Series B | Series B-1 | Series C | Series C-1 | Capital | Deficit | Deficit | ||||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2009 | $ | 2,612 | $ | 15,556 | $ | 19,195 | $ | 16,235 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 1 | $ | 0 | $ | 2 | $ | 8,619 | $ | (53,007 | ) | $ | (44,385 | ) | ||||||||||||||||||||||||||
Exercise of options and warrants | — | — | 891 | — | — | — | — | — | — | — | 0 | 28 | — | 28 | ||||||||||||||||||||||||||||||||||||||||||
Reclassification of preferred stock warrant liabilities to redeemable convertible preferred stock upon exercises | — | — | 117 | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
Accretion on redeemable convertible preferred stock | — | — | — | 197 | — | — | — | — | — | — | — | (197 | ) | — | (197 | ) | ||||||||||||||||||||||||||||||||||||||||
Accrual of Series F dividends | — | — | — | 1,040 | — | — | — | — | — | — | — | (1,040 | ) | — | (1,040 | ) | ||||||||||||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | — | 650 | — | 650 | ||||||||||||||||||||||||||||||||||||||||||
Net income | — | — | — | — | — | — | — | — | — | — | — | — | 4,312 | 4,312 | ||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2010 | 2,612 | 15,556 | 20,203 | 17,472 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 8,060 | (48,695 | ) | (40,632 | ) | ||||||||||||||||||||||||||||||||||||||||
Exercise of options and warrants | — | — | 440 | — | — | — | — | — | — | — | 0 | 186 | — | 186 | ||||||||||||||||||||||||||||||||||||||||||
Reclassification of preferred stock warrant liabilities to redeemable convertible preferred stock upon exercises | — | — | 152 | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
Accretion on redeemable convertible preferred stock | — | — | — | 115 | — | — | — | — | — | — | — | (115 | ) | — | (115 | ) | ||||||||||||||||||||||||||||||||||||||||
Accrual of Series F dividends | — | — | — | 613 | — | — | — | — | — | — | — | (613 | ) | — | (613 | ) | ||||||||||||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | — | 651 | — | 651 | ||||||||||||||||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | — | (548 | ) | (548 | ) | ||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2011 | 2,612 | 15,556 | 20,795 | 18,200 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 8,169 | (49,243 | ) | (41,071 | ) | ||||||||||||||||||||||||||||||||||||||||
Exercise of options and warrants, net of withholding for employee taxes | — | — | — | 138 | — | — | — | — | — | — | 0 | (925 | ) | — | (925 | ) | ||||||||||||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | — | 1,190 | — | 1,190 | ||||||||||||||||||||||||||||||||||||||||||
Net income | — | — | — | — | — | — | — | — | — | — | — | — | 1,250 | 1,250 | ||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2012 | $ | 2,612 | $ | 15,556 | $ | 20,795 | $ | 18,338 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 1 | $ | 0 | $ | 2 | $ | 8,434 | $ | (47,993 | ) | $ | (39,556 | ) | ||||||||||||||||||||||||||
See accompanying notes to financial statements.
74
LipoScience, Inc.
Statements of Cash Flows
(in thousands)
Year Ended December 31, | |||||||||||
2012 | 2011 | 2010 | |||||||||
Operating activities | |||||||||||
Net income (loss) | $ | 1,250 | $ | (548 | ) | $ | 4,312 | ||||
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | |||||||||||
Depreciation and amortization | 1,280 | 508 | 564 | ||||||||
Stock-based compensation expense | 1,190 | 651 | 650 | ||||||||
Fair value remeasurement of preferred stock warrant liability | 149 | (252 | ) | (391 | ) | ||||||
Gain on sale of property and equipment | — | — | (6 | ) | |||||||
Gain on extinguishment of other long-term liabilities | — | — | (2,700 | ) | |||||||
Loss on disposal of long-lived assets | 30 | — | — | ||||||||
Impairment of intangible assets | 13 | — | — | ||||||||
Loss on extinguishment of long-term debt | — | 59 | — | ||||||||
Changes in operating assets and liabilities: | |||||||||||
Accounts receivable, net | 477 | (1,432 | ) | (831 | ) | ||||||
Inventories | (125 | ) | — | — | |||||||
Prepaid expenses and other | (138 | ) | (100 | ) | (159 | ) | |||||
Accounts payable, accrued expenses and other current liabilities | 1,162 | 1,316 | (215 | ) | |||||||
Other long-term liabilities | 2,309 | (106 | ) | (81 | ) | ||||||
Net cash provided by operating activities | 7,597 | 96 | 1,143 | ||||||||
Investing activities | |||||||||||
Purchases of property and equipment | (8,015 | ) | (1,963 | ) | (1,138 | ) | |||||
Proceeds from sale of property and equipment | — | — | 6 | ||||||||
Capitalized patent and trademark costs | (112 | ) | (205 | ) | (77 | ) | |||||
Net cash used in investing activities | (8,127 | ) | (2,168 | ) | (1,209 | ) | |||||
Financing activities | |||||||||||
Proceeds from revolving line of credit | 8,500 | — | — | ||||||||
Payments on revolving line of credit | (3,500 | ) | — | — | |||||||
Proceeds from long-term debt | 16,000 | 6,000 | — | ||||||||
Payments on long-term debt | (6,000 | ) | (1,200 | ) | (1,800 | ) | |||||
Taxes paid on behalf of employees for net exercise of options | (1,083 | ) | — | — | |||||||
Payments on capital leases | — | (27 | ) | (63 | ) | ||||||
Changes in restricted cash for operating lease | (2 | ) | 4 | (2 | ) | ||||||
Changes in deferred financing costs | — | 3 | 24 | ||||||||
Debt discount | (120 | ) | — | — | |||||||
Debt issuance costs | (95 | ) | — | — | |||||||
Deferred offering costs | (1,044 | ) | (1,908 | ) | — | ||||||
Proceeds from exercise of stock options and warrants | 159 | 625 | 920 | ||||||||
Net cash provided by (used in) financing activities | 12,815 | 3,497 | (921 | ) | |||||||
Net increase (decrease) in cash and cash equivalents | 12,285 | 1,425 | (987 | ) | |||||||
Cash and cash equivalents at beginning of year | 12,483 | 11,058 | 12,045 | ||||||||
Cash and cash equivalents at end of year | $ | 24,768 | $ | 12,483 | $ | 11,058 | |||||
Supplemental disclosure of cash flow information | |||||||||||
Cash paid for interest | $ | 532 | $ | 354 | $ | 163 | |||||
Cash paid for income taxes | $ | — | $ | 2 | $ | 35 | |||||
Supplemental disclosure of non-cash financing activities | |||||||||||
Accrual of Series F Redeemable Convertible Preferred Stock dividends | $ | — | $ | 613 | $ | 1,040 | |||||
Accretion on Series F Redeemable Convertible Preferred Stock | $ | — | $ | 115 | $ | 197 | |||||
See accompanying notes to financial statements.
75
LipoScience, Inc.
Notes to Financial Statements
1. Description of Business and Significant Accounting Policies
Description of Business
LipoScience, Inc. (“LipoScience” or the “Company”) was incorporated under the laws of North Carolina in June 1994 under the name LipoMed, Inc. and reincorporated under the laws of Delaware in June 2000. In January 2002, the Company changed its corporate name to LipoScience, Inc. The Company is an in vitro diagnostic company pioneering a new field of personalized diagnostics based on nuclear magnetic resonance (“NMR”) technology. The Company's first diagnostic test, the NMR LipoProfile test, is cleared by the U.S. Food and Drug Administration, or FDA, and directly measures the number of low density lipoprotein, (“LDL”) particles in a blood sample and provides physicians and their patients with actionable information to personalize management of risk for heart disease. On January 30, 2013, the Company completed its initial public offering of common Stock (the “IPO”) pursuant to a registration statement that was declared effective on January 24, 2013.
Basis of Presentation and Use of Estimates
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) and include all adjustments necessary for the fair presentation of the Company's financial position, results of operations and cash flows for the periods presented. In preparing the financial statements, management must make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosures of contingent assets and liabilities as of the date of the financial statements and reported amounts of revenue and expenses during the reporting periods. Actual results could differ from those estimates and assumptions used.
Immaterial Correction of an Error
In the second quarter of 2012, the Company corrected an error in the amount of $0.3 million to increase its depreciation expense and accumulated depreciation as a result of an error discovered in the capitalization of laboratory equipment used for research and development activities. The adjustment to depreciation expense and accumulated depreciation should have been recognized during fiscal years 2011, 2010, 2009 and 2008 in the amounts of $90,000, $90,000, $90,000 and $25,000, respectively. The impact of the correction resulted in an increase in depreciation expense and accumulated depreciation, and a corresponding decrease in net income of $0.3 million during the second quarter of 2012.
The Company assessed the materiality of making these corrections in the current period under Staff Accounting Bulletin (“SAB”) No. 108, “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements,” (“SAB No. 108”) and has determined that this correction is immaterial to all of the affected financial statements. Accordingly, the correction has been reflected in the Company's financial statements for the year ended December 31, 2012.
Customers and Payors
The Company provides diagnostic tests to a broad range of customers. A majority of these tests are comprised of orders generated through clinical diagnostic laboratory customers and clinicians. The Company also receives requests from academic institutions as well as pharmaceutical companies. In most cases, the customer that orders the tests is not responsible for the payments for services. The Company considers a party that refers a test to it to be a “customer” and a party that reimburses the Company as a “payor”. Depending on the billing arrangement and applicable law, the payor may be (i) a clinical diagnostic laboratory, (ii) a third party responsible for providing health insurance coverage to patients, such as a health insurance plan or the traditional Medicare or Medicaid program, (iii) the physician or (iv) the patient or other party (such as academic institutions or pharmaceutical companies) who requested the test from the Company.
Revenue Recognition
The Company currently derives revenue from sales of its NMR LipoProfile test to clinical diagnostic laboratories and clinicians for use in patient care, from sales of ancillary tests for use in patient care requested in conjunction with the NMR LipoProfile test and from research contracts.
Revenues from diagnostic tests for patient care, which consist of sales of the NMR LipoProfile test and sales of ancillary tests, are recognized on the accrual basis when the following revenue recognition criteria are met: (1) persuasive evidence that an arrangement exists; (2) services have been rendered or at the time final results are reported; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. Testing services provided for patient care are covered by clinical diagnostic laboratories, programs with commercial insurance carriers (including managed care organizations) and various governmental programs, primarily Medicare.
76
Billings for diagnostic tests for patient care under governmental and physician-based programs are included in revenues net of contractual adjustments. These contractual adjustments represent the difference between the final settlement amount paid by the program and the estimated settlement amount based on either the list price for tests performed or the reimbursement rate set by commercial insurance carriers or governmental programs. Estimated contractual adjustments are updated either upon notification from payors as to changes in existing reimbursement rates, which are typically received prior to changes going into effect, or upon a material variance between the final settlement and the estimated contractual adjustment originally established when the revenues were recognized. To date, the Company's final settlement adjustments have not been material.
Revenues from contract research arrangements are generally derived from studies conducted with academic institutions and pharmaceutical companies. The specific methodology for revenue recognition is determined on a case-by-case basis according to the facts and circumstances applicable to a given agreement. The Company's output, measured in terms of full-time equivalent level of effort or processing a set of diagnostic tests under a contractual protocol, typically triggers payment obligations under these agreements. Revenues are recognized as costs are incurred or diagnostic tests are processed. Contract research costs include all direct material and labor costs, equipment costs and fringe benefits. Advance payments received in excess of revenues recognized are classified as deferred revenue until such time as the revenue recognition criteria have been met.
Billing for diagnostic testing services for patient care is complex. In some cases, tests are performed in advance of payment and without certainty as to the outcome of the billing process, which may negatively affect revenues, cash flow and profitability. Payments are received from a variety of payors, including clinical diagnostic laboratories, commercial insurance companies (including managed care organizations), governmental payors (primarily Medicare) and individual patients. Each payor typically has different billing requirements, and the billing requirements of many payors have become increasingly stringent.
The Company generally assumes the financial risk related to collection, including the potential uncollectibility of accounts and the other complex factors identified above. Delays in collection and uncollectible bills may negatively affect the Company's revenues, cash flow and profitability.
Shipping and Handling
The Company does not bill its customers for shipping and handling charges. All charges relating to inbound and outbound freight costs are incurred by the Company and recorded within cost of revenues. For the years ended December 31, 2012, 2011 and 2010, the Company incurred shipping and handling costs of approximately $1.5 million, $1.5 million and $1.3 million, respectively.
Fair Value of Financial Instruments
The Company measures certain financial assets and liabilities at fair value based on the price that would be received for an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. As of December 31, 2012 and 2011, the Company's financial instruments consisted principally of cash and cash equivalents, accounts receivable, prepaid expenses and other, other long-term assets, accounts payable, accrued expenses, revolving line of credit, long-term debt and preferred stock warrant liability. See Note 2, “Fair Value Measurement,” to the financial statements for further information on the fair value of the Company's financial instruments.
Cash and Cash Equivalents
The Company invests its available cash balances in cash, certificates of deposits and money market funds. The Company considers all highly liquid instruments purchased with original maturity of three months or less at the time of purchase to be cash equivalents. Cash equivalents are stated at cost and the carrying amounts approximate fair value. The Company maintains deposits in federally insured financial institutions in excess of federally insured limits. Management believes that the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. Additionally, the Company has established guidelines regarding approved investments and maturities of investments, which are designed to maintain safety and liquidity. See Note 6 for a discussion of Restricted Cash.
Accounts Receivable
Accounts receivable are primarily amounts due from clinical diagnostic laboratories, commercial insurance companies (including managed care organizations), governmental programs (primarily Medicare), physicians, and individual patients.
77
Accounts receivable are reported net of an allowance for uncollectible accounts. The process of estimating the collection of accounts receivable involves significant assumptions and judgments. Specifically, the accounts receivable allowance is based on management's analysis of current and past due accounts, collection experience in relation to amounts billed, channel mix, any specific customer collection issues that have been identified and other relevant information. The Company's provision for uncollectible accounts is recorded as bad debt expense and included in general and administrative expenses. Historically, the Company has not experienced significant credit loss related to its customers or payors. Although the Company believes amounts provided are adequate, the ultimate amounts of uncollectible accounts receivable could be in excess of the amounts provided.
Inventories
Inventories consist of materials and spare parts to service the Company's automated clinical analyzer, the Vantera system, which was cleared by the FDA in August 2012 and became commercially available in December 2012. Inventories are valued at the lower of cost or net realizable value. The cost of inventories is maintained using the average-cost method.
Property and Equipment
Property and equipment are stated at cost. Property and equipment financed under capital leases are initially recorded at the present value of minimum lease payments at the inception of the lease. Amortization of assets financed under capital leases is included with purchased property and equipment as part of depreciation and amortization.
Depreciation is calculated using the straight-line method over the estimated useful lives of the assets. Property and equipment under capital leases and leasehold improvements are amortized using the straight-line method over the shorter of the lease term or estimated useful life of the asset. Depreciable lives range from three to seven years for laboratory equipment, office equipment and furniture and fixtures and three years for software.
Intangible Assets
Intangible assets include patent costs, trademark costs and technology licenses that are capitalized and amortized over estimated useful lives (generally nine to twenty years) using the straight-line method. Patent costs are expensed if the patent is not granted. On an ongoing basis, the Company assesses the recoverability of its intangible assets by determining its ability to generate undiscounted future cash flows sufficient to recover the unamortized balances over the remaining useful lives. Intangible assets determined to be unrecoverable are expensed in the period in which the determination is made.
During the years ended December 31, 2012, 2011 and 2010, the Company recorded amortization expense on intangible assets of approximately $19,000, $23,000 and $13,000, respectively.
Impairment of Long-Lived Assets
The Company evaluates its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may be impaired and assesses their recoverability based upon anticipated future cash flows. If changes in circumstances lead the Company to believe that any of its long-lived assets may be impaired, the Company will (a) evaluate the extent to which the remaining book value of the asset is recoverable by comparing the future undiscounted cash flows estimated to be associated with the asset to the asset's carrying amount and (b) write-down the carrying amount to market value or discounted cash flow value to the extent necessary.
Deferred Offering Costs
Deferred offering costs represent legal, accounting and other direct costs related to the Company's efforts to raise capital through the IPO. Costs related to the Company's IPO activities were deferred until the completion of the IPO, at which time they were reclassified to additional paid-in capital as a reduction of the IPO proceeds. In connection with the IPO, the Company had recorded approximately $3.2 million of deferred offering costs as a non-current asset in the accompanying balance sheet as of December 31, 2012.
Redeemable Convertible Preferred Stock
The Company classifies its redeemable convertible preferred stock, for which the Company does not control the redemption, outside of permanent equity. The Company records redeemable convertible preferred stock at fair value upon issuance, net of any issuance costs or discounts, and the carrying value is increased by periodic accretion to its redemption value. These increases are effected through charges against additional paid-in capital.
78
Preferred Stock Warrant Liability
The Company accounted for its freestanding warrants to purchase the Company's Series E and Series F Redeemable Convertible Preferred Stock as liabilities at fair value on the accompanying balance sheets. The warrants were subject to re-measurement at each balance sheet date, and the change in fair value, if any, was recognized as other (expense) income. The Company estimated the fair value of these warrants at the respective balance sheet dates using the Black-Scholes option pricing model. The estimated fair value of the Company calculated at each valuation date (see Note 11) was allocated to the shares of redeemable convertible preferred stock, convertible preferred stock, warrants to purchase shares of redeemable convertible preferred stock, and common stock, using a “hybrid” approach of the probability-weighted expected return method and the option pricing model. This approach treats the various components of the Company's capital structure as a series of call options on the proceeds expected from the sale of the Company or the liquidation of the Company's assets in the future. This approach defines the securities' fair values as functions of the current fair value of the Company and assumptions based on the securities' rights and preferences. These call options were then valued using the Black-Scholes option pricing model. As a result, the option-pricing model requires a number of assumptions to estimate the fair value including the anticipated timing of a potential liquidity event, such as an initial public offering, remaining contractual terms of the warrant, risk-free interest rates, expected dividend yield and the estimated volatility of the price of the underlying securities. The anticipated timing of a liquidity event utilized in these valuations was based on then current plans and estimates of the Company's board of directors and management regarding the IPO. These assumptions were highly judgmental.
The fair values of outstanding Series E and Series F Redeemable Convertible Preferred Stock warrants were estimated using the Black-Scholes option-pricing model with the following assumptions:
Year Ended December 31, | ||||||||
2012 | 2011 | 2010 | ||||||
Expected term (in years) | 0.25-0.5 | 0.5-1.0 | 1.0-2.0 | |||||
Risk-free interest rate | 0.0%-0.1% | 0.1%-0.6% | 0.3%-0.6% | |||||
Expected volatility | 30 | % | 36 | % | 35%-42% | |||
Expected dividend rate | 0 | % | 0 | % | 0 | % | ||
The Company adjusted the liability for changes in fair value until the earlier of (i) exercise of the warrants, (ii) conversion of the warrants into warrants to purchase common stock upon an event such as the completion of the IPO or (iii) expiration of the warrants. Upon conversion of the warrants upon the IPO in January 2013, the preferred stock warrant liability was reclassified into additional paid-in capital.
A summary of warrant activity for the years ended December 31, 2012, 2011 and 2010 is presented below:
Number of Warrants | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (in years) | Aggregate Intrinsic Value | ||||||||||
Outstanding, December 31, 2009 | 776,896 | $ | 4.35 | 1.31 | $ | 3,059 | |||||||
Granted | — | — | — | — | |||||||||
Exercised | (204,996 | ) | 4.35 | — | — | ||||||||
Forfeited/Canceled | (340,578 | ) | 4.35 | — | — | ||||||||
Outstanding and Exercisable, December 31, 2010 | 231,322 | 4.35 | 2.36 | 846 | |||||||||
Granted | 13,793 | 4.35 | — | — | |||||||||
Exercised | (101,150 | ) | 4.35 | — | — | ||||||||
Forfeited/Canceled | — | — | — | — | |||||||||
Outstanding and Exercisable, December 31, 2011 | 143,965 | 4.35 | 2.86 | 802 | |||||||||
Granted | 73,564 | 4.35 | — | — | |||||||||
Exercised | (41,379 | ) | 4.35 | — | — | ||||||||
Forfeited/Canceled | — | — | — | — | |||||||||
Outstanding and Exercisable, December 31, 2012 | 176,150 | $ | 4.35 | 5.53 | $ | 932 | |||||||
79
The aggregate intrinsic value in the table above is before applicable income taxes and is calculated based on the difference between the exercise price of the warrants and the estimated fair market value of the Company's Series E and Series F Redeemable Convertible Preferred Stock as of the respective dates.
The following table summarizes additional information about warrants outstanding and exercisable at December 31, 2012 and 2011:
Warrants Outstanding and Exercisable as of December 31, | |||||||||||||||||||||||||||
Underlying Stock | Number of Shares | Weighted Average Remaining Life (Years) | Weighted Average Exercise Price | Weighted Average Fair Value per share | |||||||||||||||||||||||
2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | ||||||||||||||||||||
Series E Redeemable Convertible Preferred Stock | 73,564 | — | 9.95 | — | $ | 4.35 | $ | — | $ | 2.34 | $ | — | |||||||||||||||
Series F Redeemable Convertible Preferred Stock | 102,586 | 143,965 | 2.37 | 2.86 | 4.35 | 4.35 | 2.34 | 2.14 | |||||||||||||||||||
Concentration of Credit Risk and Other Risks
The Company derives its revenues from diagnostic testing services provided for patient care and contract research arrangements with institutional customers. The Company operates in one industry segment. Substantially all of the Company's historical revenues have been derived from the sale of the NMR LipoProfile test.
The Company's principal financial instruments subject to potential concentration of credit risk are cash equivalents and trade accounts receivable, which are unsecured. Through December 31, 2012, no material losses have been incurred.
Revenues from customers representing 10% or more of total revenues for the respective periods, are summarized as follows:
Year Ended December 31, | ||||||||
2012 | 2011 | 2010 | ||||||
LabCorp | 29 | % | 33 | % | 33 | % | ||
Health Diagnostics Laboratory | 32 | 21 | * | |||||
* Less than 10%.
The Company’s accounts receivable due from these significant customers as a percentage of total accounts receivable for the respective periods is summarized as follows:
As of December 31, | |||||
2012 | 2011 | ||||
LabCorp | 39 | % | 20 | % | |
Health Diagnostics Laboratory | * | 30 | |||
The Company depends on a limited number of suppliers, including single-source suppliers, of various critical components used in its NMR analyzers. The loss of these suppliers, or their failure to supply the Company with the necessary components on a timely basis, could cause delays in the diagnostic testing process and adversely affect the Company.
Research and Development Expenses
Research and development expenses include all costs associated with the development of nuclear magnetic resonance technology products and are charged to expense as incurred. Research and development expenses include direct costs and an allocation of indirect costs, including amortization, depreciation, telephone, rent, supplies, insurance and repairs and maintenance.
Financing Costs
The Company capitalizes direct financing costs, which are included as other noncurrent assets on the Company's balance sheets. The Company accretes debt discounts, which are included as a direct deduction from the face amount of the financing. Debt financing costs and debt discounts are deferred and amortized to interest expense using the effective interest method over the life of the related debt. The related expense is included in interest expense in the Company's statements of operations.
80
Income Taxes
The Company accounts for income taxes under the asset and liability method, which requires, among other things, that deferred income taxes be provided for temporary differences between the tax basis of the Company's assets and liabilities and their financial statement reported amounts. In addition, deferred tax assets are recorded for the future benefit of utilizing net operating losses and research and development credit carryforwards. A valuation allowance is established when necessary to reduce deferred tax assets to the amount expected to be realized.
The Company has adopted the accounting guidance for uncertainties in income taxes, which proscribes a recognition threshold and measurement process for recording uncertain tax positions taken, or expected to be taken, in a tax return in the financial statements. Additionally, the guidance also proscribes a new treatment for the derecognition, classification, accounting in interim periods and disclosure requirements for uncertain tax positions. The Company accrues for the estimated amount of taxes for uncertain tax positions if it is more likely than not that the Company would be required to pay such additional taxes. An uncertain tax position will not be recognized if it has less than a 50% likelihood of being sustained. As of the date of adoption of this guidance, the Company did not have any accrued interest or penalties associated with any unrecognized tax positions, and there were no such interest or penalties recognized during the years ended December 31, 2012, 2011 or 2010.
Advertising
Advertising costs, which are included in sales and marketing expenses, are expensed as incurred. Advertising expense was $0.8 million, $0.9 million and $0.2 million for the years ended December 31, 2012, 2011 and 2010, respectively.
Stock-Based Compensation
The Company accounts for stock-based compensation arrangements with employees and non-employee directors using a fair value method that requires the recognition of compensation expense for costs related to all stock-based payments, including stock options. The fair value method requires the Company to estimate the fair value of stock-based payment awards on the date of grant using an option pricing model.
Stock-based compensation costs are based on the fair value of the underlying option calculated using the Black-Scholes option-pricing model on the date of grant for stock options and recognized as expense on a straight-line basis over the requisite service period, which is the vesting period. Determining the appropriate fair value model and related assumptions requires judgment, including estimating stock price volatility, forfeiture rates and expected term. The expected volatility rates are estimated based on the actual volatility of comparable public companies over the expected term. The expected term for the years ended December 31, 2012, 2011 and 2010 represents the average time that options are expected to be outstanding based on the mid-point between the vesting date and the end of the contractual term of the award. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company has not paid dividends and does not anticipate paying a cash dividend in the foreseeable future and, accordingly, uses an expected dividend yield of zero. The risk-free interest rate is based on the rate of U.S. Treasury securities with maturities consistent with the estimated expected term of the awards. The measurement of nonemployee share-based compensation is subject to periodic adjustments as the underlying equity instruments vest and is recognized as an expense over the period over which services are received.
Segment Reporting
The Company operates in one operating segment. The Company's chief operating decision maker (the “CODM”), its chief executive officer, manages the Company's operations on an integrated basis for purposes of allocating resources. When evaluating the Company's financial performance, the CODM reviews separate revenue information for the Company's patient care testing and its research services, while all other financial information is reviewed on a combined basis. All of the Company's principal operations and decision-making functions are located in the United States. Accordingly, the Company has determined that it has a single reporting segment.
Off-Balance Sheet Arrangements
Through December 31, 2012, the Company has not entered into any off-balance sheet arrangements, other than the operating leases described in Note 16, and does not have any holdings in variable interest entities.
81
Net Income (Loss) Per Share
The Company computes net income (loss) per share in accordance with the accounting guidance regarding the computation of earnings or net loss per share by companies that have issued securities other than common stock that contractually entitle the holder to participate in earnings and dividends. The guidance requires earnings or net loss attributable to common stockholders for the period, after deduction of preferred stock preferences, to be allocated between the common and preferred stockholders based on their respective rights to receive dividends. The guidance does not require the presentation of basic and diluted net income (loss) per share for securities other than common stock; therefore, net income (loss) per share amounts only pertain to the Company's common stock. Since the Company's participating preferred stock was not contractually required to share in the Company's losses, in applying the two-class method to compute basic net income (loss) per share, no allocation was made to preferred stock if a net loss existed.
Basic net income (loss) per share is computed by dividing net income (loss) allocable to common stockholders, which is net income (loss) after deduction of any required returns to preferred stockholders prior to paying dividends to the common stock and assuming current income for the period had been distributed based on the respective rights of the common and preferred stockholders to receive dividends, by the weighted average number of shares of common stock outstanding during the period. Diluted net income (loss) per share is computed on the basis of the weighted average number of shares of common stock plus dilutive potential common shares outstanding during the period. Because of their anti-dilutive effect, the following common share equivalents, consisting of convertible preferred shares, redeemable convertible preferred shares, common stock options and warrants, have been excluded from the diluted loss per share calculations for the respective periods presented:
Anti-Dilutive Common Share Equivalents | Year Ended December 31, | |||||||
2012 | 2011 | 2010 | ||||||
Convertible Preferred Stock (Series A, A-1, B, B-1, C, and C-1) | 1,222,211 | 1,222,211 | 1,222,211 | |||||
Redeemable Convertible Preferred Stock (Series D, D-1, E and F) | 5,772,348 | 5,763,606 | 5,714,549 | |||||
Common Stock Options | 382,869 | 2,266,155 | 40,315 | |||||
Warrants | — | 69,821 | 112,189 | |||||
7,377,428 | 9,321,793 | 7,089,264 | ||||||
A reconciliation of the numerator and denominator used in the computation of basic and diluted net income (loss) per share allocable to common stockholders follows (in thousands, except share and per share data):
Year Ended December 31, | |||||||||||
2012 | 2011 | 2010 | |||||||||
Historical net (loss) income per share: | |||||||||||
Numerator: | |||||||||||
Net income (loss) | $ | 1,250 | $ | (548 | ) | $ | 4,312 | ||||
Less: Accrual of dividends on Series F redeemable convertible preferred stock | — | (613 | ) | (1,040 | ) | ||||||
Less: Undistributed earnings allocated to participating preferred shares | (1,004 | ) | — | (2,655 | ) | ||||||
Net income (loss) attributable to common stockholders – basic | 246 | (1,161 | ) | 617 | |||||||
Add: Undistributed earnings re-allocated to common stockholders | 113 | — | 303 | ||||||||
Net income (loss) attributable to common stockholders – diluted | $ | 359 | $ | (1,161 | ) | $ | 920 | ||||
Denominator: | |||||||||||
Weighted average common shares outstanding – basic | 1,715,408 | 1,674,018 | 1,611,843 | ||||||||
Dilutive effect of common stock equivalent shares resulting from common stock options and warrants | 1,098,915 | — | 1,101,927 | ||||||||
Weighted average common shares outstanding – diluted | 2,814,323 | 1,674,018 | 2,713,770 | ||||||||
Net income (loss) per common share: | |||||||||||
Net income (loss) per share attributable to common stockholders – basic | $ | 0.14 | $ | (0.69 | ) | $ | 0.38 | ||||
Net income (loss) per share attributable to common stockholders – diluted | $ | 0.13 | $ | (0.69 | ) | $ | 0.34 | ||||
82
Recent Accounting Pronouncements
In October 2012, the FASB issued ASU No. 2012-04, Technical Corrections and Improvements. The amendments in this update cover a wide range of topics in the Accounting Standards Codification. These amendments include technical corrections and improvements to the Accounting Standards Codification and conforming amendments related to fair value measurements. For public companies, the amendments that are subject to the transition guidance will be effective for reporting periods beginning after December 15, 2012. The adoption of this new guidance is not expected to have a material impact to the Company's financial position, results of operations or cash flows.
83
2. Fair Value Measurement
As a basis for determining the fair value of certain of the Company's financial instruments, the Company utilizes a three-tier value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
- | Level I - Observable inputs such as quoted prices in active markets for identical assets or liabilities. | |
- | Level II - Observable inputs, other than Level I prices, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. | |
- | Level III - Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. | |
This hierarchy requires the Company to use observable market data, when available, and to minimize the use of unobservable inputs when determining fair value. The carrying amount of certain of the Company's financial instruments, including accounts receivable, prepaid expenses and other, other long-term assets, accounts payable and accrued expenses, approximate fair value due to their short maturities. The carrying value of the Company's capital lease obligations approximate fair value because the interest rates under those obligations approximate market rates of interest available to the Company for similar instruments.
The fair value of the Company's revolving line of credit and long-term debt were estimated using the discounted cash flow method, which is based on the future expected cash flows, discounted to their present values, using a discount rate that approximates market rates. Such fair value measurements are categorized as Level II under the fair value hierarchy as of December 31, 2012.
The following table sets forth the carrying value and fair value of the Company's long-term debt as of December 31, 2012 and 2011 (in thousands):
December 31, 2012 | December 31, 2011 | ||||||||||||||
Fair Value | Carrying Value | Fair Value | Carrying Value | ||||||||||||
Revolving Line of Credit | $ | 4,990 | $ | 5,000 | $ | — | $ | — | |||||||
Long Term Debt | 16,389 | 15,708 | 5,789 | 6,000 | |||||||||||
Recurring Fair Value Measurements
The Company's financial instruments that are measured at fair value on a recurring basis consist only of cash equivalents and the preferred stock warrant liability. Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company's assessment of the significance of a particular input to the entire fair value measurement requires management to make judgments and consider factors specific to the asset or liability.
Cash equivalents
All of the Company's cash equivalents, which include certificates of deposits and money market funds, are classified within Level I of the fair value hierarchy because they are valued using quoted market prices.
Preferred stock warrant liability
The fair value determination of the Company's preferred stock warrant liability required a number of assumptions, including significant unobservable inputs that reflect the Company's own assumptions about the assumptions a market participant would use in valuing the liability, and therefore are classified within Level III of the fair value hierarchy. The significant unobservable inputs used in the fair value measurement of the Company's preferred stock warrant liability are (i) probability weighting of potential liquidity event outcomes, (ii) the implied value of the underlying preferred shares derived in the IPO scenario and (iii) the weighted average cost of capital (“WACC”). Significant increases (decreases) in the probability weighting of potential liquidity event outcomes and the implied value of the underlying preferred stock in isolation would result in a significantly higher (lower) fair value measurement. Conversely, significant increases (decreases) in the anticipated timing of a liquidity event and WACC inputs in isolation would result in a significantly lower (higher) fair value measurement.
84
The following table provides a summary of the significant unobservable inputs used to determine the fair value of the Company's preferred stock warrant liability as of December 31, 2012 (in thousands, except for per share data and percentages):
Fair Value at December 31, 2012 | Valuation Technique | Significant Unobservable Inputs | Input Range/ Value | ||||
Preferred Stock Warrant Liability | $412 | Black-Scholes Option Pricing Model* | Probability of potential liquidity event outcomes | 5% to 50% | |||
Implied equity value | $6.60-$7.15 | ||||||
Weighted average cost of capital | 19% | ||||||
* | Using a hybrid approach of the probability-weighted expected return method and the option pricing model |
The following table sets forth the Company's financial instruments that were measured at fair value as of December 31, 2012 and 2011 by level within the fair value hierarchy (in thousands):
December 31, 2012 | December 31, 2011 | ||||||||||||||||||||||
Level I | Level III | Total | Level I | Level III | Total | ||||||||||||||||||
Assets | |||||||||||||||||||||||
Certificates of deposits | $ | 5,253 | $ | — | $ | 5,253 | $ | 8,961 | $ | — | $ | 8,961 | |||||||||||
Money market funds | 21,016 | — | 21,016 | 4,962 | — | 4,962 | |||||||||||||||||
Total assets measured at fair value | $ | 26,269 | $ | — | $ | 26,269 | $ | 13,923 | $ | — | $ | 13,923 | |||||||||||
Liabilities | |||||||||||||||||||||||
Preferred stock warrants | $ | — | $ | 412 | $ | 412 | $ | — | $ | 229 | $ | 229 | |||||||||||
Total liabilities measured at fair value | $ | — | $ | 412 | $ | 412 | $ | — | $ | 229 | $ | 229 | |||||||||||
The change in the fair value of the Level III preferred stock warrant liability is summarized below:
December 31, | |||||||||||
2012 | 2011 | 2010 | |||||||||
Fair value at beginning of period | $ | 229 | $ | 597 | $ | 1,104 | |||||
Issuances | 172 | 36 | — | ||||||||
Exercises reclassified to additional paid in capital | (138 | ) | (152 | ) | (117 | ) | |||||
Change in fair value recorded in other income (expense) | 149 | (252 | ) | (390 | ) | ||||||
Fair value at end of period | $ | 412 | $ | 229 | $ | 597 | |||||
For the year ended December 31, 2012, there were no transfers between Level I and Level II within the fair value hierarchy.
3. Cash and Cash Equivalents
Cash and cash equivalents consist of the following as of (in thousands):
December 31, | |||||||
2012 | 2011 | ||||||
Cash | $ | 4 | $ | 63 | |||
Certificates of deposit | 5,253 | 8,962 | |||||
Money market funds | 19,511 | 3,458 | |||||
Total cash and cash equivalents | $ | 24,768 | $ | 12,483 | |||
85
The Company has an arrangement with its primary bank that excess cash balances are swept each night to an overnight sweep deposits account. At December 31, 2012 and 2011, the balance in the overnight sweep deposits account totaling $19.5 million and $3.5 million, respectively, was invested in shares of a money market fund.
4. Accounts Receivable and Revenues
Accounts receivable, net, consists of the following as of (in thousands):
December 31, | |||||||
2012 | 2011 | ||||||
Accounts receivable | $ | 6,526 | $ | 7,302 | |||
Less allowance for uncollectible accounts receivable | (1,377 | ) | (1,676 | ) | |||
Accounts receivable, net | $ | 5,149 | $ | 5,626 | |||
Activity for the allowance for uncollectible accounts receivable is as follows (in thousands):
Year Ended December 31, | |||||||
2012 | 2011 | ||||||
Balance at beginning of period | $ | 1,676 | $ | 1,704 | |||
Provision for bad debt expense | 818 | 1,384 | |||||
Write-off, net of recoveries | (1,117 | ) | (1,412 | ) | |||
Balance at end of period | $ | 1,377 | $ | 1,676 | |||
Revenues consist of the following (in thousands):
Year Ended December 31, | |||||||||||
2012 | 2011 | 2010 | |||||||||
Gross billings | $ | 56,603 | $ | 48,292 | $ | 43,128 | |||||
Less contractual adjustments | (1,805 | ) | (2,485 | ) | (3,760 | ) | |||||
Revenues | $ | 54,798 | $ | 45,807 | $ | 39,368 | |||||
5. Property and Equipment
Property and equipment consists of the following as of:
December 31, | |||||||
2012 | 2011 | ||||||
Laboratory equipment | $ | 9,415 | $ | 6,975 | |||
Office equipment, furniture and fixtures | 3,497 | 2,588 | |||||
Leasehold improvements | 2,652 | 1,338 | |||||
Software | 1,793 | 1,837 | |||||
Construction in progress | 5,778 | 4,338 | |||||
23,135 | 17,076 | ||||||
Less accumulated depreciation | (11,642 | ) | (11,784 | ) | |||
Property and equipment, net | $ | 11,493 | $ | 5,292 | |||
For the years ended December 31, 2012, 2011 and 2010, the Company recorded depreciation expense of $1.3 million, $0.5 million and $0.6 million, respectively.
For the year ended December 31, 2012, the Company disposed of $30,000 of damaged assets and retired $1.4 million of
86
fully depreciated assets. For the year ended December 31, 2011, the Company disposed of $0.1 million of fully depreciated assets. There was no such disposal or retirement for the year ended December 31, 2010.
6. Restricted Cash
As of December 31, 2012 and 2011, certificates of deposit totaling $1.5 million were pledged as security for the Company's office and laboratory space operating lease. These funds may be released by the bank upon the attainment of certain minimum benchmarks covering sales, cash flows and certain financial ratios.
7. Accrued Expenses
Accrued expenses consist of the following as of:
December 31, | |||||||
2012 | 2011 | ||||||
Accrued wages and benefits | $ | 1,541 | $ | 1,122 | |||
Accrued bonus | 2,200 | 1,375 | |||||
Other accrued liabilities | 1,607 | 1,916 | |||||
Total accrued expenses | $ | 5,348 | $ | 4,413 | |||
8. Long-Term Debt and Line of Credit
In February 2008, the Company executed a loan agreement with a new financial institution. This loan agreement is comprised of a $3 million revolving line of credit with a variable interest rate equal to the greater of 6.75% or the prime rate plus 3.25% and a $4.5 million loan with a variable interest rate equal to the greater of 7.25% or the prime rate plus 3.75%. Collateral for the line of credit and the loan is substantially all tangible assets of the Company. The Company may borrow up to 80% of all eligible domestic accounts receivable that are under 90 days old. Advances under the accounts receivable facility will require monthly payments of interest only with the principle due at maturity. Repayment of the term loan commenced March 2009 in thirty equal monthly payments of principal, plus interest due.
In connection with executing this loan agreement, the Company issued warrants to the financial institution to purchase 75,000 shares of Series F Redeemable Convertible Preferred Stock at $4.35 per share. These warrants are exercisable at any time and expire on the seventh anniversary of their issuance date. The fair value of these warrants at issuance is being amortized as interest expense over the term of the debt and accreted into the carrying value of Series F Redeemable Convertible Preferred Stock through January 2011. The warrants were valued under the level III hierarchy as there are significant unobservable inputs. The fair value of the warrants was determined with the assistance of a third-party consultant using a probability weighted valuation model. Values were determined for the warrants based on assumptions for each liquidity scenario using a Black-Scholes pricing model. These values were discounted back to February 2008 (the issuance date) while applying estimated probabilities to each scenario and associated value on a weighted average basis. These scenarios included a potential initial public offering or potential acquisition at different times throughout 2010 and 2012. Accordingly, the Company determined the fair value of the warrants at issuance to be $70,500, which was recorded as a preferred stock warrant liability and related debt discount. As of December 31, 2012 and 2011, the Company determined the fair value of these warrants to be $0.2 million and $0.1 million, respectively.
On May 7, 2010, the Company entered into the third amendment to this loan agreement with the financial institution, which included a waiver to the Company's violation of the stated milestone covenant as of April 30, 2010, and amendment to certain covenants on a prospective basis. Effective May 7, 2010, the amendment eliminates the stated milestone covenant and establishes a monthly revenue covenant based upon approved projected results, as approved by the Company's Board of Directors.
87
On March 31, 2011, the Company entered into a fourth amendment (the 4th amendment) to the loan agreement to refinance its existing term loan outstanding that was set to mature in August 2011. The 4th amendment is comprised of a $6.0 million term loan (Term Loan A) with a variable interest rate equal to the greater of 7.25% or the prime rate plus 3.75%, and a $4.0 million revolving line of credit with a variable interest rate equal to the greater of 6.25% or the prime rate plus 3.00%. Amounts borrowed pursuant to the 4th amendment are secured by substantially all of the Company's tangible assets. Under the 4th amendment, the Company must comply with specified financial covenants measured on a monthly basis and certain affirmative covenants, including a milestone covenant as it relates to the 510(k) filing for the final approval of the Vantera clinical analyzer with the United States Food and Drug Administration. In connection with the execution of the 4th Amendment, the Company drew down $6.0 million against Term Loan A and no amount was drawn on the line of credit. The proceeds from Term Loan A were used to repay the existing loan balance outstanding as of March 31, 2011 and to fund the Company's working capital. Term Loan A is payable initially in nine monthly installments of interest only followed by thirty monthly installments of principal plus interest. The Company also incurred direct financing costs and issued warrants to purchase 13,793 shares of Series F Redeemable Convertible Preferred Stock at $4.35 per share to the financial institution. The Company determined the fair value of the warrants at issuance to be approximately $36,000. Both the direct financing costs and the fair value of these warrants were recorded as part of the loss on extinguishment of debt in accordance with the accounting guidance for debt modifications and extinguishments.
On March 29, 2012, the Company entered into a fifth amendment to the loan agreement to extend the maturity date of the $4.0 million revolving line of credit from March 31, 2012 to May 1, 2012. On April 30, 2012, the Company entered into a sixth amendment to the loan agreement to extend the maturity date of the $4.0 million revolving line of credit from May 1, 2012 to May 1, 2013. On May 18, 2012, the Company borrowed $3.5 million under this line of credit.
On December 20, 2012, the Company entered into a new credit facility with its existing financial institution and a new financial institution and repaid the outstanding balance under the previous facility with the existing financial institution. The new facility consists of $16.0 million in term loans and a revolving line of credit with up to $6.0 million in borrowing capacity, subject to certain limitations relating to the Company's eligible accounts receivable. As of December 31, 2012, the Company had borrowed $5.0 million under the revolving line of credit. The revolving line of credit matures in December 2013.
The term loans carry interest at a fixed annual rate of 9.5% and are payable in monthly installments of interest only through January 2014 and then principal and interest thereafter in monthly installments through July 2016. Advances under the revolving line of credit, which matures in December 2013, carry a variable interest rate equal to the greater of 6.25% or the institution's prime rate plus 3.0%. Borrowings under the credit facility are secured by substantially all of the Company's tangible assets. The covenants set forth in the loan and security agreement require, among other things, that the Company maintain a specified liquidity ratio, measured monthly, that begins at 1.25 and is reduced to 1.0 over the term of the agreement. The Company is also required to achieve minimum three-month trailing revenue levels during the term of the agreement.
In connection with the new credit facility, the Company also incurred direct financing costs in the amount of $0.1 million and issued warrants to these financial institutions to purchase an aggregate of 73,564 shares of Series E redeemable convertible preferred stock at an exercise price of $4.35 per share. The Company determined the fair value of the warrants at issuance to be approximately $0.2 million.
Long-term debt consists of the following as of (in thousands):
December 31, | |||||||
2012 | 2011 | ||||||
Long-term debt to financial institutions | $ | 16,000 | $ | 6,000 | |||
Less unamortized debt discount | (292 | ) | — | ||||
Less current maturities of long-term debt | — | (2,400 | ) | ||||
Long-term debt, less current maturities and unamortized debt discount | $ | 15,708 | $ | 3,600 | |||
88
As of December 31, 2012, the annual principal payments on long-term debt and revolving line of credit are as follows (in thousands):
2013 | $ | 5,000 | |
2014 | 5,432 | ||
2015 | 6,489 | ||
2016 | 4,079 | ||
$ | 21,000 | ||
As of December 31, 2012 and 2011, the Company was in compliance with all financial and non-financial covenants under this loan agreement and related amendments.
89
9. Gain on Extinguishment of Other Long-Term Liabilities
On September 16, 2009, the Company and a third-party research and development contractor entered into an agreement whereby each party would, subject to certain circumstances, release the other from certain obligations set forth in two prior agreements between the parties. One of the obligations of the Company was to pay $2.7 million to such third party for prior research and development services which was reflected in other long-term liabilities in the Company's balance sheets. Based on events that occurred after September 16, 2009, the Company was released from the payment obligation of such long-term liabilities in September 2010. The Company recorded the release of these liabilities as a gain on extinguishment of other long-term liabilities within the operating expenses section of the statement of operations for the year ended December 31, 2010.
10. Redeemable Convertible Preferred Stock and Stockholders’ Deficit
Capital Structure
As of December 31, 2010, the Company was authorized to issue up to 90,000,000 shares of $.001 par value common stock and 18,631,220 shares of preferred stock, of which:
• | 300,000 shares were designated as $.001 par value Series A Convertible Preferred Stock (Series A), |
• | 252,700 shares were designated as $.001 par value Series A-1 Convertible Preferred Stock (Series A-1), |
• | 166,667 shares were designated as $.001 par value Series B Convertible Preferred Stock (Series B), |
• | 159,536 shares were designated as $.001 par value Series B-1 Convertible Preferred Stock (Series B-1), |
• | 1,275,000 shares were designated as $.001 par value Series C Convertible Preferred Stock (Series C), |
• | 1,274,774 shares were designated as $.001 par value Series C-1 Convertible Preferred Stock (Series C-1), |
• | 3,544,062 shares were designated as $.001 par value Series D Redeemable Convertible Preferred Stock (Series D), |
• | 3,480,473 shares were designated as $.001 par value Series D-1 Redeemable Convertible Preferred Stock (Series D-1), |
• | 5,059,330 shares were designated as $.001 par value, Series E Redeemable Convertible Preferred Stock (Series E) and |
• | 3,118,678 shares were designated as $.001 par value, Series F Redeemable Convertible Preferred Stock (Series F). |
On March 30, 2011, the Company amended its certificate of incorporation to increase the number of authorized shares of preferred stock, specifically as it relates to Series F, from 3,118,678 to 3,132,471 shares. As of December 31, 2012, the Company was authorized to issue up to 90,000,000 shares of $.001 par value common stock and 18,645,013 shares of preferred stock.
Series E Redeemable Convertible Preferred Stock
During 2003, the Company issued 3,493,066 shares of Series E for $15.2 million in cash and satisfaction of accrued interest on a bridge loan of approximately $15,000. The Company incurred related stock issuance costs paid in cash of $0.2 million. These stock issuance costs were accreted into the carrying value of Series E through July 2007.
During 2003, 2004 and 2005, the Company issued warrants to Series E investors to purchase an aggregate of 646,724 shares of Series E at an exercise price of $4.35 per share. These warrants are exercisable at any time and expire on the seventh anniversary of their respective issuance dates. The warrants were valued under the level III hierarchy as there are significant unobservable inputs. The fair value of the warrants was determined with the assistance of a third-party consultant using a probability weighted valuation model. Values were determined for the warrants based on assumptions for each liquidity scenario using a modified Black-Scholes pricing model. These values were discounted back to their respective issuance dates while applying estimated probabilities to each scenario and associated value on a weighted average basis. These scenarios included a potential initial public offering or potential acquisition at different times throughout 2010 and 2012. As of December 31, 2010 the Company determined the fair value of these warrants to be $0.3 million. The Company reclassified $0.1 million of Series E warrants to Series E Redeemable Convertible Preferred Stock in 2010 for 340,578 shares of expired warrants in 2010. During 2010, holders exercised Series E warrants for 204,996 shares, from which the Company received proceeds totaling $0.9 million. In 2011, holders exercised the remaining Series E warrants for 101,150 shares, from which the Company received proceeds totaling $0.4 million.
90
Series F Redeemable Convertible Preferred Stock
During 2006, the Company issued 2,988,506 shares of Series F for $13.0 million. The Company incurred related stock issuance costs paid in cash of $0.9 million. These stock issuance costs are being accreted into the carrying value of Series F through August 2011.
In connection with the Series F financing, the Company issued warrants to purchase an aggregate of 41,379 shares of Series F at an exercise price of $4.35 per share. The fair value of these warrants at issuance is being accreted into the carrying value of Series F through August 2011. These warrants are exercisable at any time and expire on the seventh anniversary of their issuance date. The warrants were valued under the level III hierarchy as there are significant unobservable inputs. The fair value of the warrants was determined with the assistance of a third-party consultant using a probability weighted valuation model. Values were determined for the warrants based on assumptions for each liquidity scenario using a modified Black-Scholes pricing model. These values were discounted back to their issuance dates while applying estimated probabilities to each scenario and associated value on a weighted average basis. These scenarios included a potential initial public offering or potential acquisition at different times throughout 2010 and 2012. As of December 31, 2011, the Company determined the fair value of these warrants to be $0.1 million. In December 2012, the holder of these warrants exercised its rights to exercise these warrants on a net basis and received 17,941 shares of Series F.
The following is a summary of the rights, preferences and terms of the Company’s outstanding series of preferred stock:
Dividends - The holders of Series F Preferred Stock are entitled to receive prior, and in preference to, the holders of any other class or series of capital stock of the Company, dividends at the rate of $0.348 per annum per share of the Series F Preferred Stock (subject to adjustment for any stock or share dividends, stock splits, combinations, reclassifications or any similar event affecting the shares of Series F Preferred Stock) (the Series F Dividend), payable in cash and out of funds legally available therefore. The Series F Dividend shall be cumulative and shall accrue on a daily basis for a period of five years from the original issue date of the Series F Preferred Stock, whether or not declared, from and including the most recent date to which dividends have been paid, or if no dividends have been paid, from the date of original issue thereof. On the fifth anniversary of the original issue date of the Series F Preferred Stock, which occurred on August 2, 2011, the Series F Dividend ceased accruing, provided, that each share of Series F Preferred shall remain entitled to all unpaid Series F Dividends that accrued during such five-year period. The right to dividends shall accrue during such five-year period regardless of whether there are profits, surplus, or other funds legally available for payment of dividends. Through December 31, 2012, the maximum of $5.2 million of Series F Dividends has been accrued by the Company and is reflected in the carrying amount of Series F on the balance sheet as of December 31, 2012. Subject to the rights of the Series F Preferred Stock set forth in this paragraph, if and when dividends are declared by the Board of Directors, holders of Series E, D, and D-1 are entitled to noncumulative dividends at a rate of 8% per annum of the original price per share, payable in cash out of legally available funds. The Series D, D-1 and E dividend shall be payable only when, as and if declared by the Board of Directors of the Corporation and shall be noncumulative. Through December 31, 2012, no cash dividends have been declared or paid by the Company.
Voting Rights – The holders of preferred stock are entitled to vote based on the number of common shares they would receive upon conversion.
Transfer Restrictions – The holders of each share of common and preferred stock are subject to transfer restrictions.
Liquidation – In the event of any liquidation, dissolution, or winding up of the Company (each, a Liquidity Event), the holders of Series F are entitled to receive, prior and in preference to any distribution of any assets or surplus funds to the other Preferred stockholders or holders of the common stock, $4.35 per share plus accrued dividends (the Series F Liquidation Preference). After payment of the Series F Liquidation Preference, the holders of Series E are entitled to receive, prior and in preference to any distribution of any assets or surplus funds to the other Preferred stockholders or holders of the common stock, $4.35 per share plus any declared but unpaid dividends (the Series E Liquidation Preference).
Any assets of the Company remaining after the payment to the holders of the Series F Preferred Stock and the holders of Series E Preferred Stock shall be distributed, on a pari passu basis, to the holders of Series D and D-1 (the Series D Preferred Stocks), in an amount equal to $5.22 per share plus any declared but unpaid dividends on such shares, if any (the D Preferred Stock Liquidation Preference). After payment of the D and D-1 Preferred Stock Liquidation Preference, the holders of Preferred Stock with preferences junior to the Series D and D-1 Preferred Stock shall be entitled to receive in preference to the common stock a per share amount equal to the original purchase price of each such series, plus any declared but unpaid dividends.
91
After the payment of the applicable liquidation preference to the holders of all of the Preferred Stock, the residual, if any, will be distributed pro rata to the holders of common stock, the holders of the Series D Preferred Stock, Series D-1 Preferred Stock, Series E Preferred Stock, and Series F Preferred Stock (on an as-converted basis) (the Participating Distribution); provided, however, that the rights of the holders of Series D Preferred Stock, Series D-1 Preferred Stock, Series E Preferred Stock, and Series F Preferred Stock to participate in such residual with the holders of common stock shall terminate if the per share price to be paid to the holders of each such series of Preferred Stock in a Liquidity Event exceeds four times the purchase price per share for such series of Preferred Stock (adjusted for stock splits, dividends, recapitalizations and similar events). Notwithstanding the foregoing, if the holders of the common stock would not receive in the Participating Distribution an aggregate amount equal to at least $4.0 million, then, in lieu of the payment of the Participating Distribution, 10% of the total assets available for distribution in such Liquidity Event (or such lesser amount as remains available for distribution) will be paid to the holders of the common stock on a pro rata basis and any remaining assets after payment of such amount will be distributed pro rata to the holders of common stock, the holders of the Series D Preferred Stock, Series E Preferred Stock, and Series F Preferred Stock (on an as-converted basis) (the Common Carve-Out); provided however, that in the event that the holders of common stock are entitled to payment of the Common Carve-Out, the proceeds to be paid to the holders of the common stock pursuant to the Common Carve-Out shall not exceed $4.0 million.
A consolidation or merger of the Company with or into any other corporation or corporations or other entity or the effectuation by the Company of a transaction or series of related transactions in which more than 50% of the voting power of the Company is disposed of (unless the stockholders of the Company immediately prior to any such event shall, immediately thereafter, hold as a group the right to cast at least a majority of the votes of all holders of voting securities of the resulting or surviving Company or entity on any matter on which any such holders of voting securities shall be entitled to vote), or a sale, conveyance, exclusive license or disposition of all or substantially all of the assets of the Company (except where such sale, conveyance, exclusive license or disposition is to a wholly owned subsidiary of the Company) (any such transaction, a Deemed Liquidity Event) shall be deemed to be a Liquidating Event, unless waived by the holders of a majority of the Series E Preferred Stock and the Series D Preferred Stock then outstanding, voting together as a single class, and the holders of a majority of the Series F Preferred Stock then outstanding. Upon the consummation of a Deemed Liquidity Event (including any sale, conveyance, exclusive license or disposition of all or substantially all of the assets of the Company) in which the proceeds of such Deemed Liquidity Event are received by the Company, the Board of Directors of the Company shall, to the extent such proceeds are legally available for distribution, promptly cause such proceeds to be distributed to the stockholders of the Company in accordance with liquidation preferences listed above.
Conversion – Each share of preferred stock shall be convertible, at the option of the holder at any time after the date of issuance and without payment of additional consideration, into such number of common stock as is determined by dividing the consideration received by the Company for the purchase of each share of preferred stock by the conversion price in effect at the time of conversion. The conversion price shall initially be the amount of consideration received for each share of preferred stock. The conversion price is subject to adjustment in the event of stock dividends, stock splits, combinations and similar adjustments to capitalization. The conversion prices of each series of preferred stock are also subject to adjustment in the event that the Company issues additional equity securities at a per share price less than the applicable conversion price for each such series of preferred stock. The conversion prices per share as of December 31, 2012 are as follows:
Series | Original Conversion Price | Conversion Price as Adjusted | |||||
A* | $ | 6.00 | $ | 5.16 | |||
A-1* | 6.00 | 5.16 | |||||
B | 6.00 | 5.16 | |||||
B-1 | 6.00 | 5.16 | |||||
C | 4.00 | 6.87 | |||||
C-1 | 4.00 | 6.87 | |||||
D | 5.22 | 8.97 | |||||
D-1 | 5.22 | 8.97 | |||||
E | 4.35 | 8.97 | |||||
F | 4.35 | 8.97 | |||||
* | except for 66,666 shares, as to which the original conversion price is $4.50 and the current conversion price is $3.87. |
92
Automatic Conversion – Each share of Series A, A-1, B, B-1, C and C-1 shall automatically be converted into common stock at the then effective conversion price upon the completion of an underwritten public offering involving the sale of the Company’s common stock. Each share of Series D, D-1, E and F shall automatically be converted into common stock at the then effective conversion price upon the completion of an underwritten public offering involving the sale of the Company’s common at a pre-money valuation of $132.0 million and gross proceeds of at least $25.0 million (a Qualified Public Offering). In January 2013, the Company’s certificate of incorporation was amended to remove the $132.0 million pre-money valuation condition from the definition of a Qualified Public Offering.
Preemptive Rights – The holders of preferred stock shall not be entitled to preemptive rights to acquire or subscribe for additional shares of securities that the Company authorizes to be issued. The holders of Series D, D-1, E and F have contractual rights of first refusal (which expire upon a Qualified Public Offering) to participate in certain future offerings of stock by the Company on a pro rata basis.
Antidilution Provision – The conversion price of Series F and Series E will be subject to a full-ratchet dilution adjustment in the event the Company issues additional securities at a purchase price less than the applicable conversion price of Series F and Series E. The conversion price will be subject to adjustment for stock splits, stock dividends, recapitalizations, and other such events.
In connection with the offering of Series E, the Company created several new series of preferred stock (Series A-1, B-1, C-1 and D-1) with rights and preferences identical to the existing respective series (Series A, B, C and D) except that the shares of the new series are entitled to a full-ratchet dilution adjustment in the event the Company issues additional securities at a purchase price less than the applicable conversion price for each series. Any holder of Series A, B, C or D who invested its pro-rata percentage in Series E was entitled to exchange its shares for Series A-1, B-1, C-1 or D-1, respectively.
The conversion price of Series D shall be adjusted on a weighted average basis if additional securities (as defined) are issued at a price less than the then existing conversion price.
Redemption – At any time on or after on August 2, 2011, the holders of at least 40% of the issued and outstanding shares of Series D, D-1, E and F (the Electing Holders) can request the Company redeem all of their shares of each Series at a price for each share equal to its original purchase price (subject to adjustment in the event of stock dividends, stock splits, combinations, etc.), plus any accrued but unpaid dividends. Upon election by the Electing Holders to redeem their shares of Series D, D-1, E or F, the Company shall pay cash to the holders for the redemption value in three equal installments over three years at an interest rate of 8% per annum from the date of the initial payment until the redemption amount is paid in full.
Common Stock Reserved for Future Issuance
The Company had reserved shares of common stock for future issuance as summarized in the table below.
December 31, 2012 | ||
For conversion of Series A Convertible Preferred Stock | 266,466 | |
For conversion of Series A-1 Convertible Preferred Stock | 27,448 | |
For conversion of Series B Convertible Preferred Stock | 179,867 | |
For conversion of Series B-1 Convertible Preferred Stock | 5,820 | |
For conversion of Series C Convertible Preferred Stock | 595,699 | |
For conversion of Series C-1 Convertible Preferred Stock | 146,911 | |
For conversion of Series D Redeemable Convertible Preferred Stock | 291,216 | |
For conversion of Series D-1 Redeemable Convertible Preferred Stock | 1,734,393 | |
For conversion of Series E Redeemable Convertible Preferred Stock | 2,288,579 | |
For conversion of Series F Redeemable Convertible Preferred Stock | 1,458,160 | |
Outstanding Series F Redeemable Convertible Preferred Stock Warrants | 85,430 | |
Outstanding employee stock options | 2,056,849 | |
Possible future issuance under stock option plan | 322,227 | |
Total shares reserved | 9,459,065 | |
93
11. Stock Option and Equity Incentive Plans
On September 12, 1997, the Board of Directors adopted the Stock Option Plan (the 1997 Plan) to create an additional incentive for key employees, directors and consultants or advisors of the Company. Both incentive stock options, which meet the requirements of Section 422 of the Internal Revenue Code, and nonqualified stock options, could be granted under the 1997 Plan. The exercise price of all options was determined by the Board of Directors, provided that such price for incentive stock options was not to be less than the estimated fair market value of the Company’s stock on the date of grant. The options vest based on terms in the stock option agreements (generally over four years). The 1997 Plan expired on September 12, 2007.
On October 25, 2007, the Board of Directors adopted the 2007 Stock Incentive Plan (the 2007 Plan) to replace the expired 1997 Plan. The terms of the 2007 Plan are consistent with the 1997 Plan.
Stock Option Repricing Program
On September 2, 2009, the Company’s board of directors approved a voluntary repricing program of certain common stock options with exercise prices above the fair market value of the Company’s common stock as of that date. In accordance with this repricing program, employees who had outstanding common stock options as of September 30, 2009 with an exercise price greater than the fair value of the Company’s common stock as of September 30, 2009, or $2.50 per share, were allowed to modify their original options to have an exercise price of $2.50 per share. In exchange for the lower exercise price, participating employees were required to relinquish 25% of the stock options that they elected to have repriced at $2.50 per share. The repricing program did not modify any other terms of the stock options. Options to purchase a total of 886,095 shares of common stock were repriced, resulting in additional compensation expense of approximately $37,000 during the year ended December 31, 2009, as all repriced options were fully vested at the time of repricing.
The following table summarizes activity under the Company’s 1997 Plan and 2007 Plan for the periods presented:
Shares Available for Grant | Total Stock Options Outstanding | Weighted Average Exercise Price | |||||||
Balance at December 31, 2009 | 353,960 | 2,154,461 | $ | 3.26 | |||||
Authorized | 436,500 | — | — | ||||||
Granted | (372,360 | ) | 372,360 | 5.51 | |||||
Exercised | — | (27,383 | ) | 1.04 | |||||
Forfeited under the expired 1997 Plan | — | (114,264 | ) | 1.72 | |||||
Forfeited | 80,232 | (80,232 | ) | 2.93 | |||||
Balance at December 31, 2010 | 498,332 | 2,304,942 | 3.74 | ||||||
Authorized | — | — | — | ||||||
Granted | (354,579 | ) | 354,579 | 7.86 | |||||
Exercised | — | (70,152 | ) | 2.64 | |||||
Forfeited under the expired 1997 Plan | — | (214,610 | ) | 4.75 | |||||
Forfeited | 108,604 | (108,604 | ) | 4.85 | |||||
Balance at December 31, 2011 | 252,357 | 2,266,155 | 4.25 | ||||||
Authorized | 291,000 | — | — | ||||||
Granted | (379,414 | ) | 379,414 | 11.72 | |||||
Exercised | — | (337,400 | ) | 2.52 | |||||
Forfeited under the expired 1997 Plan | — | (93,036 | ) | 10.27 | |||||
Forfeited | 158,284 | (158,284 | ) | 5.66 | |||||
Balance at December 31, 2012 | 322,227 | 2,056,849 | $ | 5.55 | |||||
94
The Company engaged a third-party consultant to assist in the determination of the estimated fair market value of the Company’s common stock. The dates of the Company’s contemporaneous valuations have not always coincided with the dates of its stock-based compensation grants. In such instances, management’s estimates have been based on the most recent contemporaneous valuation of the Company’s common stock and its assessment of additional objective and subjective factors it believed were relevant and that may have changed from the date of the most recent contemporaneous valuation through the date of the grant. In addition, the Company performed retrospective valuations as of September 30, 2009, June 30, 2010 and December 31, 2010 using similar methodologies as were used in the contemporaneous valuations. These retrospective valuations resulted in new estimates of fair value of the Company’s common stock at each respective period. Had the Company used these revised common stock fair values for financial reporting purposes, the effect would not have been material and, therefore, no adjustments were made to the Company’s financial statements.
In conducting the valuation of its common stock, the Company used a methodology that is consistent with the methods outlined in the AICPA Practice Aid Valuation of Privately-Held-Company Equity Securities Issued as Compensation. The methodology used derived equity values utilizing a probability-weighted expected return method, or PWERM, that weighs various potential liquidity outcomes with each outcome assigned a probability to arrive at a weighted equity value for all valuations conducted as of December 31, 2009 and thereafter.
For each of the possible events, a range of future equity values is estimated, based on the market and income approaches and over a range of possible event dates, all plus or minus a standard deviation for value and timing. The timing of these events is based on input from management. For each future equity value scenario, the rights and preferences of each stockholder class are considered in order to determine the appropriate allocation of value to common stock. The value of each share of common stock is then multiplied by a discount factor derived from the calculated discount rate and expected timing of the event (plus or minus a standard deviation of time). The value per share of common stock is then multiplied by an estimated probability for each of the possible events based on discussion with management. The calculated value per common share under each scenario is then discounted for a lack of marketability. A probability-weighted value per share of common stock is then determined. Under the PWERM, the value of the Company’s common stock is estimated based upon an analysis of values for the Company’s common stock assuming various possible future events for the Company including an initial public offering and acquisition scenarios.
The Company estimates the fair value of its stock options on the grant date, using the Black-Scholes option pricing model. The fair value of both employees and non-employee director options is being amortized on a straight-line basis over the requisite service period of the awards. The weighted average grant date fair value per share of options granted for the years ended December 31, 2012, 2011 and 2010 was $5.18, $3.78, $2.73, respectively. The fair value of these stock options was estimated using the following weighted average assumptions:
Year Ended December 31, | ||||||||
2012 | 2011 | 2010 | ||||||
Expected dividend yield | 0.0 | % | 0.0 | % | 0.0 | % | ||
Risk-free interest rate | 0.8 | % | 2.0 | % | 2.0 | % | ||
Expected volatility | 50.0 | % | 50.3 | % | 49.9 | % | ||
Expected life (in years) | 5.6 | 5.6 | 5.6 | |||||
Expected dividend yield: The Company has not paid and does not anticipate paying any dividends in the near future.
Risk –free interest rate: The Company determined the risk-free interest rate by using a weighted average assumption equivalent to the expected term based on the U.S. Treasury yield curve in effect as of the date of grant.
Expected volatility: The Company used an average historical stock price volatility based on an analysis of reported data for a peer group of comparable companies that have issued stock options with substantially similar terms, as the Company did not have any trading history for its common stock.
Expected term (in years): Expected term represents the period that the Company’s stock option grants are expected to be outstanding. The Company elected to utilize the “simplified” method to value stock option grants. Under this approach, the weighted-average expected life is presumed to be the average of the vesting term and the contractual term of the option.
Estimated forfeiture rate: Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from estimates. The Company estimates forfeitures based on its historical experience.
95
The Company recognized non-cash stock-based compensation expense to employees in its research and development, sales and marketing and general and administrative functions as follows (in thousands):
Year Ended December 31, | |||||||||||
2012 | 2011 | 2010 | |||||||||
Cost of revenues | $ | 46 | $ | 8 | $ | 20 | |||||
Research and development | 454 | 155 | 45 | ||||||||
Sales and marketing | 255 | 217 | 137 | ||||||||
General and administrative | 435 | 271 | 447 | ||||||||
Total: | $ | 1,190 | $ | 651 | $ | 649 | |||||
The following table summarizes information about stock options outstanding as of December 31, 2012:
Total Options Outstanding | Options Exercisable | ||||||||||||||||||
Range of Exercise Prices | Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Life in Years | Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Life in Years | |||||||||||||
$1.88 – $2.50 | 829,088 | $ | 2.44 | 826,489 | $ | 2.44 | |||||||||||||
$3.88 – $4.23 | 262,340 | 3.92 | 262,217 | 3.92 | |||||||||||||||
$5.16 | 69,576 | 5.16 | 69,088 | 5.16 | |||||||||||||||
$5.63 | 227,925 | 5.63 | 197,346 | 5.63 | |||||||||||||||
$6.89 | 161,553 | 6.89 | 93,319 | 6.89 | |||||||||||||||
$9.02 | 93,023 | 9.02 | 43,655 | 9.02 | |||||||||||||||
$9.84 | 44,017 | 9.84 | 23,681 | 9.84 | |||||||||||||||
$11.12 | 29,100 | 11.12 | 12,930 | 11.12 | |||||||||||||||
$11.45 | 245,361 | 11.45 | 88,033 | 11.45 | |||||||||||||||
$12.38 | 11,640 | 12.38 | 11,621 | 12.38 | |||||||||||||||
$12.81 | 83,226 | 12.81 | 17,765 | 12.81 | |||||||||||||||
2,056,849 | 5.56 | 6.2 | 1,646,144 | 4.44 | 5.5 | ||||||||||||||
The following table summarizes information about stock options outstanding as of December 31, 2011:
Total Options Outstanding | Options Exercisable | ||||||||||||||||||
Range of Exercise Prices | Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Life in Years | Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Life in Years | |||||||||||||
$1.88 – $2.50 | 1,271,684 | $ | 2.47 | 1,250,553 | $ | 2.47 | |||||||||||||
$3.88 – $4.23 | 276,429 | 3.96 | 276,429 | 3.96 | |||||||||||||||
$5.16 | 71,277 | 5.16 | 71,277 | 5.16 | |||||||||||||||
$5.63 | 280,667 | 5.63 | 187,954 | 5.63 | |||||||||||||||
$6.46 | 3,492 | 6.46 | 3,492 | 6.46 | |||||||||||||||
$6.89 | 188,225 | 6.89 | 51,795 | 6.89 | |||||||||||||||
$9.02 | 95,836 | 9.02 | 5,334 | 9.02 | |||||||||||||||
$9.84 | 45,977 | 9.84 | 4,041 | 9.84 | |||||||||||||||
$12.38 | 11,640 | 12.38 | 11,640 | 12.38 | |||||||||||||||
$34.80 | 20,915 | 34.80 | 20,915 | 34.80 | |||||||||||||||
2,266,142 | 4.27 | 5.8 | 1,883,430 | 3.69 | 5.1 | ||||||||||||||
96
The aggregate intrinsic value of options outstanding for the years ended December 31, 2012, 2011 and 2010 was $12.6 million, $11.4 million and $9.4 million, respectively.
The aggregate intrinsic value of options exercisable for the years ended December 31, 2012, 2011 and 2010 was $11.8 million, $10.6 million and $8.6 million, respectively.
The aggregate intrinsic value of options exercised for the years ended December 31, 2012, 2011 and 2010 was $3.4 million, $0.4 million and $0.1 million, respectively.
For the years ended December 31, 2012, 2011 and 2010, the Company recorded stock-based compensation expense in the amount of $1.2 million, $0.7 million and $0.6 million, respectively. Net cash provided by operating and financing activities was unchanged by stock option activity for the years ended December 31, 2012, 2011 and 2010, as there were no excess tax benefits from stock-based compensation plans.
The total fair value of shares vested for the years ended December 31, 2012, 2011 and 2010 was $1.2 million, $0.5 million and $0.5 million, respectively.
12. Related-Party Transactions
The Company licenses certain technology from North Carolina State University (North Carolina State) (see Note 14) based on certain prior research performed at North Carolina State by James Otvos, Ph.D. Dr. Otvos is a founder, Chief Scientific Officer and a principal stockholder of the Company. Dr. Otvos is an Adjunct Professor of Biochemistry at North Carolina State. There were no accrued license royalties due to North Carolina State as of December 31, 2012. As of December 31, 2011, the accrued license royalties due to North Carolina State totaled approximately $5,000.
13. Income Taxes
The Company did not record a provision for income taxes during the years ended December 31, 2012 and 2011. For the year ended December 31, 2010, the Company recognized income tax benefits of approximately $16,000.
Deferred income taxes reflect the net tax effect of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company's deferred tax assets are as follows at December 31 (in thousands):
2012 | 2011 | ||||||
Deferred tax assets: | |||||||
Net operating loss carryforwards | $ | 11,255 | $ | 12,865 | |||
Research and development credits | 1,963 | 1,978 | |||||
Stock-based compensation | 1,851 | 1,770 | |||||
Allowance for uncollectible accounts receivable | 471 | 537 | |||||
Deferred rent | 952 | — | |||||
Accrued compensation | 1,044 | — | |||||
Depreciation and amortization | (1,167 | ) | 234 | ||||
Other | 165 | 219 | |||||
Total deferred tax assets | 16,534 | 17,603 | |||||
Valuation allowance | (16,534 | ) | (17,603 | ) | |||
Net deferred tax asset | $ | — | $ | — | |||
97
At December 31, 2012 and 2011, the Company had federal net operating loss carryforwards of approximately $32.9 million and $34.0 million, respectively, state net economic loss carryforwards of approximately $29.1 million and $31.1 million, respectively, and research and development credit carryforwards of approximately $2.0 million and $2.0 million, respectively. The federal and state net operating loss carryforwards begin to expire in 2018 and 2015, respectively, and the research and development credit carryforwards begin to expire in 2018. The Company's federal and state net operating loss carryforwards include $3.3 million of excess tax benefits related to deductions from the exercise of nonqualified stock options. The tax benefit of these deductions has not been recognized in deferred tax assets. If utilized, the benefits from these deductions will be recorded as adjustments to income tax expense and additional paid-in capital. For financial reporting purposes, a valuation allowance has been recognized to offset the deferred tax assets related to the carryforwards. The total decrease in valuation allowance of $1.1 million was allocable to current operating activities. The utilization of the loss carryforwards to reduce future income taxes will depend on the Company's ability to generate sufficient taxable income prior to the expiration of the loss carryforwards. In addition, the maximum annual use of net operating loss and research credit carryforwards is limited in certain situations where changes occur in stock ownership. The recognized tax benefit related to net operating loss carryforwards was approximately $0.4 million, $38,000 and $1.0 million for the years ended December 31, 2012, 2011 and 2010, respectively.
The Small Business Jobs Act of 2010 was enacted on September 27, 2010. The new law allows research and development tax credits to offset both federal regular tax and federal alternative minimum tax of eligible small businesses. The provision is effective for any research and development credits generated during the tax year ended December 31, 2010 and to any carryback of such credits. The Company has determined that it qualifies as an eligible small business under these provisions and was able to offset its federal alternative minimum tax liability of approximately $0.1 million for the tax year ended December 31, 2010. The Company also carried forward excess 2010 tax credits generated to the tax year ended December 31, 2012 to offset federal alternative minimum tax.
Taxes computed at the statutory federal income tax rate of 34% are reconciled to the provision for (benefit from) income taxes for the years ended December 31, 2012, 2011 and 2010 are as follows (in thousands):
2012 | 2011 | 2010 | ||||||||||||||||||
Amount | % of Pretax Earnings | Amount | % of Pretax Earnings | Amount | % of Pretax Earnings | |||||||||||||||
United States federal tax at statutory rate | $ | 425 | 34.0 | % | $ | (186 | ) | 34.0 | % | $ | 1,461 | 34.0 | % | |||||||
State taxes (net of federal benefit) | 51 | 4.1 | (25 | ) | 4.5 | 196 | 4.6 | |||||||||||||
Research and development credits | 15 | 1.2 | (386 | ) | 70.4 | (381 | ) | (8.9 | ) | |||||||||||
Meals & entertainment | 271 | 21.7 | 272 | (49.7 | ) | 210 | 4.9 | |||||||||||||
Stock based compensation | 214 | 17.1 | 151 | (27.6 | ) | 152 | 3.5 | |||||||||||||
Change in preferred stock warrant liability | 57 | 4.5 | (97 | ) | 17.7 | (151 | ) | (3.5 | ) | |||||||||||
Other nondeductible expenses | — | — | 98 | (17.8 | ) | 155 | 3.6 | |||||||||||||
Provision to return true-ups | — | — | — | — | 58 | 1.4 | ||||||||||||||
Increase in unrecognized tax benefits | — | — | 51 | (9.3 | ) | 50 | 1.2 | |||||||||||||
Change in valuation allowance | (1,069 | ) | (85.5 | ) | 126 | (22.9 | ) | (1,759 | ) | (40.9 | ) | |||||||||
Other | 36 | 2.9 | (4 | ) | 0.7 | (7 | ) | (0.3 | ) | |||||||||||
Provision for (benefit from) income taxes | $ | — | — | % | $ | — | — | % | $ | (16 | ) | (0.4 | )% | |||||||
The Company had gross unrecognized tax benefits of approximately $0.3 million as of January 1, 2011. As of December 31, 2012, the total gross unrecognized tax benefits were approximately $0.3 million and of this total, none would reduce the Company’s effective tax rate if recognized. The Company does not anticipate a significant change in total unrecognized tax benefits or the Company’s effective tax rate due to the settlement of audits or the expiration of statutes of limitations within the next twelve months. Furthermore, the Company does not expect any cash settlement with the taxing authorities as a result of these unrecognized tax benefits as the Company has sufficient unutilized carryforward attributes to offset the tax impact of these adjustments.
98
The Company has analyzed its filing positions in all significant federal, state and foreign jurisdictions where it is required to file income tax returns, as well as open tax years in these jurisdictions. With few exceptions, the Company is no longer subject to US Federal and state and local tax examinations by tax authorities for years prior to 2009, although carryforward attributes that were generated prior to 2009 may still be adjusted upon examination by the IRS if they either have been or will be used in a future period. No income tax returns are currently under examination by taxing authorities.
The following is a tabular reconciliation of the Company's change in gross unrecognized tax positions (in thousands):
Year Ended December 31, | |||||||||||
2012 | 2011 | 2010 | |||||||||
Beginning balance | $ | 369 | $ | 327 | $ | 277 | |||||
Gross decreases for tax positions related to current periods | — | (9 | ) | — | |||||||
Gross increases for tax positions related to current periods | — | 51 | 50 | ||||||||
Ending balance | $ | 369 | $ | 369 | $ | 327 | |||||
14. Intellectual Property – License Agreements
On May 12, 1997, the Company entered into a license agreement with North Carolina State. Under the terms of the agreement, North Carolina State granted the Company an exclusive, worldwide license, including patent rights, to technology related to measuring lipoprotein levels using NMR spectroscopy. The Company paid an initial license fee and made a commitment to fund future collaborative research for $25,000. The Company is required to pay certain royalties based on net sales, subject to a minimum annual royalty. The agreement also requires the Company to commercialize the patent rights. The agreement shall remain in effect until the expiration of the last-to-expire patent covered by the agreement. Dr. Otvos, the Chief Scientific Officer of the Company, is an Adjunct Professor of Biochemistry at North Carolina State (see Note 12).
On June 15, 1997, the Company entered into a license agreement with Siemens Medical Systems, Inc. (Siemens). Under the terms of the agreement, Siemens granted to the Company an exclusive license to certain technology provided by patent rights. The license is based on an initial patent issued in June 1990 and subsequent patents. The Company is required to pay royalties based on net sales of each product covered by the patent, including the NMR LipoProfile test, subject to a minimum annual royalty, including sub-licensee sales. The agreement shall remain in effect until the expiration of the last-to-expire patent covered by the agreement.
Both licenses required reimbursement of patent costs and a commitment to fund future patent protection and maintenance costs. Royalty expense under these license agreements for the years ended December 31, 2012, 2011 and 2010 was $2,500, $0.3 million and $0.3 million, respectively, which is classified within cost of revenues in the accompanying statements of operations.
15. Employee Benefit Plan
The Company has adopted a defined contribution plan (the Employee Benefit Plan or the Plan) that qualifies under Section 401(k) of the Internal Revenue Code. All employees of the Company who have attained 21 years of age are eligible for participation in the Plan after ninety days of employment. The effective date of the Employee Benefit Plan is September 1, 1998.
Under the Plan, participating employees may defer up to the Internal Revenue Service annual contribution limit. Effective January 1, 2002, the Employee Benefit Plan includes an employer match of 25% of the first 6% an eligible participant contributes to the Plan. On July 1, 2012, the Company increased its employer match from 25% to 50%. During the years ended December 31, 2012, 2011 and 2010, the Company paid $0.4 million, $0.2 million and $0.2 million, respectively, in employer match contributions to the Plan.
16. Commitments and Contingencies
Leases
The Company leases an aggregate of approximately 83,000 square feet of office and laboratory space under an escalating lease agreement that expires September 30, 2022. In connection with the lease, the Company obtained a $1.5 million letter of credit that is secured by restricted cash (see Note 6).
The Company also leases office equipment and software license through operating leases.
99
Future minimum lease payments required under the non-cancelable operating leases in effect as of December 31, 2012 are as follows (in thousands):
Operating Leases | |||
2013 | $ | 1,188 | |
2014 | 1,175 | ||
2015 | 1,206 | ||
2016 | 1,242 | ||
2017 | 960 | ||
Thereafter | 6,627 | ||
Total minimum lease payments | $ | 12,398 | |
Rent expense is calculated on a straight-line basis over the term of the lease. Rent expense recognized under operating leases totaled $1.2 million for each of the years ended December 31, 2012, 2011 and 2010, respectively.
Property and equipment includes the following amounts financed under capital leases as of (in thousands):
December 31, | |||||||
2012 | 2011 | ||||||
Office equipment | $ | 182 | $ | 182 | |||
Less accumulated depreciation | (182 | ) | (182 | ) | |||
Property and equipment under capital leases, net | $ | — | $ | — | |||
Purchase Obligations
The Company has outstanding purchase obligations, relating to the purchase of hardware components from third-party manufacturers for the Vantera system, in the amount of $2.1 million as of December 31, 2012.
Letters of Credit
The Company has an outstanding letter of credit in the amount of $0.4 million that serves as security with one of its vendors.
Legal Contingencies
The Company is subject to claims and assessments from time to time in the ordinary course of business. The Company's management does not believe that any such matters, individually or in the aggregate, will have a material adverse effect on the Company's business, financial condition, results of operations or cash flows.
Indemnification
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnification. The Company's exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but that have not yet been made. To date, the Company has not paid any claims or been required to defend any action related to its indemnification obligations. The Company may, however, record charges in the future as a result of these indemnification obligations.
In accordance with its Certificate of Incorporation and bylaws, the Company has indemnification obligations to its officers and directors for certain events or occurrences, subject to certain limits, while they are serving at the Company's request in such capacity. There have been no claims to date, and the Company has director and officer insurance that enables it to recover a portion of any amounts paid for future potential claims.
In addition to the indemnification provided for in its certificate of incorporation and bylaws, the Company has also entered into separate indemnification agreements with each of its directors, which agreements provide such directors with broad indemnification rights under certain circumstances.
100
17. Quarterly Results of Operations
The following is a summary of the unaudited quarterly results of operations (in thousands):
Quarterly Period Ended | |||||||||||||||
March 31, 2012 | June 30, 2012 | September 30, 2012 | December 31, 2012 | ||||||||||||
Revenues | $ | 13,783 | $ | 13,894 | $ | 13,563 | $ | 13,558 | |||||||
Gross profit | 11,465 | 11,216 | 10,938 | 11,119 | |||||||||||
Income from operations | 1,503 | 371 | (213 | ) | 285 | ||||||||||
Other (expense) income | (234 | ) | (103 | ) | (268 | ) | (91 | ) | |||||||
Net income (loss) | 1,269 | 268 | (481 | ) | 194 | ||||||||||
Undistributed earnings allocated to preferred stockholders(1) | (1,021 | ) | (216 | ) | — | (155 | ) | ||||||||
Net income (loss) attributable to common stockholders – basic(1) | 248 | 52 | (481 | ) | 38 | ||||||||||
Undistributed earnings re-allocated to common stockholders(1) | 133 | 27 | — | 18 | |||||||||||
Net income (loss) attributable to common stockholders – diluted(1) | 381 | 80 | (481 | ) | 56 | ||||||||||
Net income (loss) per share attributable to common stockholders – basic(1) | 0.15 | 0.03 | (0.28 | ) | 0.02 | ||||||||||
Net income (loss) per share attributable to common stockholders – diluted(1) | 0.13 | 0.03 | (0.28 | ) | 0.02 | ||||||||||
Quarterly Period Ended | |||||||||||||||
March 31, 2011 | June 30, 2011 | September 30, 2011 | December 31, 2011 | ||||||||||||
Revenues | $ | 10,542 | $ | 11,162 | $ | 11,623 | $ | 12,480 | |||||||
Gross profit | 8,387 | 8,993 | 9,581 | 10,317 | |||||||||||
(Loss) income from operations | (406 | ) | (348 | ) | 317 | 52 | |||||||||
Other (expense) income | (78 | ) | 24 | (76 | ) | (33 | ) | ||||||||
Net (loss) income | (485 | ) | (324 | ) | 241 | 20 | |||||||||
Accrual of dividends on redeemable convertible preferred stock | (260 | ) | (260 | ) | (92 | ) | — | ||||||||
Undistributed earnings allocated to preferred stockholders(1) | — | — | (120 | ) | (16 | ) | |||||||||
Net (loss) income attributable to common stockholders – basic(1) | (745 | ) | (584 | ) | 29 | 4 | |||||||||
Undistributed earnings re-allocated to common stockholders(1) | — | — | 13 | 2 | |||||||||||
Net (loss) income attributable to common stockholders – diluted(1) | (745 | ) | (584 | ) | 42 | 6 | |||||||||
Net (loss) income per share attributable to common stockholders – basic(1) | (0.45 | ) | (0.35 | ) | (0.02 | ) | 0.00 | ||||||||
Net (loss) income per share attributable to common stockholders – diluted(1) | (0.45 | ) | (0.35 | ) | (0.03 | ) | 0.00 | ||||||||
(1) | These amounts are computed independently for each of the periods presented above and, therefore, may not add up to the total for the year presented in the statement of operations. |
101
18. Subsequent Events
Reverse Stock Split
On January 4, 2013, the Company's Board of Directors approved a 0.485-for-1 reverse stock split of the Company's outstanding common stock. The reverse stock split was effected on January 10, 2013, which resulted in an adjustment to the preferred stock conversion price to reflect a proportional decrease in the number of shares of common stock to be issued upon conversion. The accompanying financial statements and notes to financial statements give retroactive effect to the reverse stock split for all periods presented.
Amendment to Certificate of Incorporation
On January 8, 2013, the Company amended its certificate of incorporation to remove the $132.0 million pre-money valuation condition from the definition of a Qualified Public Offering.
Initial Public Offering
On January 30, 2013, the Company completed its IPO pursuant to a registration statement that was declared effective on January 24, 2013. The Company sold 5,750,000 shares of its common stock, at a price of $9.00 per share, for aggregate gross proceeds of $51.8 million, which included 750,000 shares of its common stock that the underwriters purchased pursuant to an over-allotment option granted to the underwriters. As a result of the IPO, the Company raised a total of $44.4 million in net proceeds after deducting underwriting discounts and commissions of approximately $3.4 million and offering expenses of approximately $4.0 million. Costs directly associated with the IPO were capitalized and recorded as deferred offering costs prior to the closing of the IPO. These costs were recorded as a reduction of the IPO proceeds received in arriving at the amount to be recorded in additional paid-in capital.
Upon the closing of the IPO, 12,892,682 shares of the Company's outstanding preferred stock automatically converted into a total of 6,994,559 shares of its common stock and the preferred stock warrant liability was reclassified to additional paid in capital upon the conversion of warrants to purchase preferred stock into warrants to purchase common stock.
102
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Under the supervision of and with the participation of our management, including our chief executive officer, who is our principal executive officer, and our chief financial officer, who is our principal financial officer, we conducted an evaluation of the effectiveness of our disclosure controls and procedures as of December 31, 2012, the end of the period covered by this Annual Report. The term “disclosure controls and procedures,” as set forth in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, means controls and other procedures of a company that are designed to provide reasonable assurance that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company's management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2012, our chief executive officer and chief financial officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting identified in connection with the evaluation required by Rule 13a-15(d) and 15d-15(d) of the Exchange Act that occurred during the quarter ended December 31, 2012 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management's Report on Internal Control Over Financial Reporting and Attestation Report of the Registered Public Accounting Firm
This Annual Report does not include a report of management's assessment regarding internal control over financial reporting or an attestation report of our independent registered public accounting firm due to a transition period established by the rules of the Securities and Exchange Commission for newly public companies.
Item 9B. Other Information
Not applicable.
103
PART III
We will file a definitive Proxy Statement for our 2013 Annual Meeting of Stockholders (the “2013 Proxy Statement”) with the SEC, pursuant to Regulation 14A, not later than 120 days after the end of our fiscal year. Accordingly, certain information required by Part III has been omitted under General Instruction G(3) to Form 10-K. Only those sections of the 2013 Proxy Statement that specifically address the items set forth herein are incorporated by reference.
Item 10. Directors, Executive Officers and Corporate Governance
The information required by Item 10 is hereby incorporated by reference to the sections of our 2013 Proxy Statement under the captions “Board of Directors and Committees,” “Election of Directors,” “Management” and “Section 16(a) Beneficial Ownership Reporting Compliance.”
Item 11. Executive Compensation
The information required by Item 11 is hereby incorporated by reference to the sections of our 2013 Proxy Statement under the captions “Executive Compensation” and “Director Compensation.”
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by Item 12 is hereby incorporated by reference to the sections of our 2013 Proxy Statement under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Securities Authorized for Issuance under Equity Compensation Plans.”
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by Item 13 is hereby incorporated by reference to the sections of our 2013 Proxy Statement under the captions “Transactions with Related Parties” and “Director Independence.”
Item 14. Principal Accountant Fees and Services
The information required by Item 14 is hereby incorporated by reference to the section of our 2013 Proxy Statement under the caption “Independent Registered Public Accounting Firm Fees.”
104
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a) Exhibits
Exhibit Number | Description of Document |
3.1 (1) | Amended and Restated Certificate of Incorporation. |
3.2 (2) | Amended and Restated Bylaws. |
4.1 (3) | Specimen stock certificate evidencing shares of Common Stock. |
10.1 (4) | Loan and Security Agreement, dated as of December 20, 2012, by and among the Registrant, Oxford Finance LLC and Square 1 Bank. |
10.2 (5) | Warrant to purchase Series E preferred stock issued to Square 1 Bank, dated December 20, 2012. |
10.3.1 (6) | Warrant No. 1 to purchase Series E preferred stock issued to Oxford Finance LLC, dated December 20, 2012. |
10.3.2 (7) | Warrant No. 2 to purchase Series E preferred stock issued to Oxford Finance LLC, dated December 20, 2012. |
10.4 (8) | Warrant to purchase Series F preferred stock issued to Silicon Valley Bank, dated December 13, 2006. |
10.5 (9) | Warrant to purchase Series F preferred stock issued to Square 1 Bank, dated February 7, 2008. |
10.6 (10) | Warrant to purchase Series F preferred stock issued to Square 1 Bank, dated March 31, 2011. |
10.7 (11) | Second Amended and Restated Investor Rights Agreement, dated as of August 2, 2006 and as amended to date, by and among the Registrant and certain of its stockholders. |
10.8 (12) | Standard Lease, dated as of October 4, 2001, as amended on March 5, 2002 and August 28, 2002, by and between the Registrant and Parker-Raleigh Development XXX, LLC. |
10.8.1 (13) | Lease Amendment No. 3, dated as of November 29, 2011, by and between the Registrant and Raleigh Portfolio JH, LLC, as successor to Parker-Raleigh Development XXX, LLC. |
10.9† (14) | Agreement, dated as of September 28, 2012, by and between the Registrant and Laboratory Corporation of America Holdings. |
10.10† (15) | Supply Agreement, dated as of July 16, 2012, by and between the Registrant and Agilent Technologies, Inc. |
10.11† (16) | Production Agreement, dated as of June 26, 2009, by and between the Registrant and KMC Systems, Inc. |
10.12† (17) | License Agreement, dated as of May 9, 1997, as amended on January 10, 2001 and October 11, 2010, by and between the Registrant and North Carolina State University. |
10.13+ (18) | 1997 Stock Option Plan, as amended to date. |
10.14+ (19) | Form of Incentive Stock Option Agreement under 1997 Stock Option Plan. |
10.15+ (20) | Form of Incentive Stock Option Agreement under 1997 Stock Option Plan with modified change of control provisions. |
10.16+ (21) | Form of Nonqualified Stock Option Agreement under 1997 Stock Option Plan. |
10.17+ (22) | 2007 Stock Incentive Plan, as amended through July 29, 2010. |
10.18+ (23) | Fourth Amendment to 2007 Stock Incentive Plan, effective as of August 27, 2012. |
10.19+ (24) | Form of Incentive Stock Option Agreement under 2007 Stock Incentive Plan. |
10.20+ (25) | Form of Nonqualified Stock Option Agreement under 2007 Stock Incentive Plan |
10.21+ (26) | Form of Nonqualified Stock Option Agreement under 2007 Stock Incentive Plan for initial grants to directors. |
10.22+ (27) | Form of Nonqualified Stock Option Agreement under 2007 Stock Incentive Plan for annual grants to directors. |
10.23+ (28) | Form of Nonqualified Stock Option Agreement under 2007 Stock Incentive Plan for grants to committee chairpersons. |
105
Exhibit Number | Description of Document |
10.24+ (29) | Form of Restricted Stock Purchase Agreement under 2007 Stock Incentive Plan. |
10.25+ (30) | 2012 Equity Incentive Plan. |
10.26+ (31) | Form of Stock Option Grant Notice and Stock Option Agreement under 2012 Equity Incentive Plan. |
10.27+ (32) | Form of Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement under 2012 Equity Incentive Plan. |
10.28+ (33) | Non-Employee Director Compensation Plan. |
10.29 (34) | Form of Indemnification Agreement between the Registrant and certain of its directors. |
10.30 (35) | Form of Indemnification Agreement between the Registrant and certain of its directors affiliated with stockholders. |
10.31+ (36) | 2012 Employee Stock Purchase Plan. |
10.32+ | Amended and Restated Employment Agreement, dated as of March 26, 2013, by and between the Registrant and Richard O. Brajer. |
10.33+ | Amended and Restated Employment Agreement, dated as of March 25, 2013, by and between the Registrant and Lucy G. Martindale. |
10.34+ | Employment Agreement, dated as of January 9, 2013, by and between the Registrant and Robert M. Honigberg. |
10.35+ | Amended and Restated Employment Agreement, dated as of March 25, 2013, by and between the Registrant and Timothy J. Fischer. |
10.36+ | Amended and Restated Employment Agreement, dated as of March 25, 2013, by and between the Registrant and Thomas S. Clement. |
10.37+ (37) | Executive Severance Benefit Plan. |
10.38+ | 2012 Corporate Goals for Strategic Leadership Team. |
23.1 | Consent of Ernst & Young LLP, independent registered public accounting firm. |
24.1 | Power of Attorney (contained on signature page hereto). |
31.1 | Certification of Principal Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as adopted pursuant to section 302 of the Sarbanes-Oxley Act of 2002. |
31.2 | Certification of Principal Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as adopted pursuant to section 302 of the Sarbanes-Oxley Act of 2002. |
32* | Certification of Principal Executive Officer and Principal Financial Officer pursuant to Rules 13a-14(b) and 15d-14(b) promulgated under the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350, as adopted pursuant to section 906 of The Sarbanes-Oxley Act of 2002. |
_______________________
* | These certifications are being furnished solely to accompany this report pursuant to 18 U.S.C. Section 1350, and are not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and are not to be incorporated by reference into any filing of the Registrant, whether made before or after the date hereof, regardless of any general incorporation language in such filing. |
+ | Indicates management contract or compensatory plan. |
† | Confidential treatment has been granted with respect to portions of this exhibit, indicated by asterisks, which have been filed separately with the Securities and Exchange Commission. |
(1) | Previously filed as Exhibit 3.1 to the Registrant's Current Report on Form 8-K (File No. 001-35792), filed with the Commission on January 30, 2013, and incorporated by reference herein. |
106
(2) | Previously filed as Exhibit 3.2 to the Registrant's Current Report on Form 8-K (File No. 001-35792), filed with the Commission on January 30, 2013, and incorporated by reference herein. |
(3) | Previously filed as Exhibit 4.2 to Amendment No. 7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 10, 2013, and incorporated by reference herein. |
(4) | Previously filed as Exhibit 10.1 to Amendment No. 7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 10, 2013, and incorporated by reference herein. |
(5) | Previously filed as Exhibit 10.2 to Amendment No. 7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 10, 2013, and incorporated by reference herein. |
(6) | Previously filed as Exhibit 10.3.1 to Amendment No. 7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 10, 2013, and incorporated by reference herein. |
(7) | Previously filed as Exhibit 10.3.2 to Amendment No. 7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 10, 2013, and incorporated by reference herein. |
(8) | Previously filed as Exhibit 10.4 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(9) | Previously filed as Exhibit 10.5 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(10) | Previously filed as Exhibit 10.6 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(11) | Previously filed as Exhibit 10.7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(12) | Previously filed as Exhibit 10.8 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(13) | Previously filed as Exhibit 10.8.1 to Amendment No. 4 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on April 27, 2012, and incorporated by reference herein. |
(14) | Previously filed as Exhibit 10.35 to Amendment No. 6 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on December 4, 2012, and incorporated by reference herein. |
(15) | Previously filed as Exhibit 10.9.2 to Amendment No. 8 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 24, 2013, and incorporated by reference herein. |
(16) | Previously filed as Exhibit 10.10 to Amendment No. 4 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on April 27, 2012, and incorporated by reference herein. |
(17) | Previously filed as Exhibit 10.11 to Amendment No. 1 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on August 1, 2011, and incorporated by reference herein. |
(18) | Previously filed as Exhibit 10.12 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(19) | Previously filed as Exhibit 10.13 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(20) | Previously filed as Exhibit 10.14 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(21) | Previously filed as Exhibit 10.15 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
107
(22) | Previously filed as Exhibit 10.16 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(23) | Previously filed as Exhibit 10.16.1 to Amendment No. 8 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 24, 2013, and incorporated by reference herein. |
(24) | Previously filed as Exhibit 10.17 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(25) | Previously filed as Exhibit 10.18 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(26) | Previously filed as Exhibit 10.19 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(27) | Previously filed as Exhibit 10.20 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(28) | Previously filed as Exhibit 10.21 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(29) | Previously filed as Exhibit 10.22 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(30) | Previously filed as Exhibit 4.16 to the Registrant's Registration Statement on Form S-8 (Registration No. 333-186545) filed with the Securities and Exchange Commission on February 8, 2013 and incorporated herein by reference. |
(31) | Previously filed as Exhibit 10.24 to Amendment No. 5 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on October 23, 2012, and incorporated by reference herein. |
(32) | Previously filed as Exhibit 10.25 to Amendment No. 5 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on October 23, 2012, and incorporated by reference herein. |
(33) | Previously filed as Exhibit 10.26.1 to Amendment No. 5 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on October 23, 2012, and incorporated by reference herein. |
(34) | Previously filed as Exhibit 10.28 to Amendment No. 1 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on August 1, 2011, and incorporated by reference herein. |
(35) | Previously filed as Exhibit 10.29 to Amendment No. 1 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on August 1, 2011, and incorporated by reference herein. |
(36) | Previously filed as Exhibit 4.19 to the Registrant's Registration Statement on Form S-8 (Registration No. 333-186545) filed with the Securities and Exchange Commission on February 8, 2013 and incorporated herein by reference. |
(37) | Previously filed as Exhibit 10.36 to Amendment No. 5 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on October 23, 2012, and incorporated by reference herein. |
(b) Financial Statement Schedules
No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or the notes thereto.
108
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
LIPOSCIENCE, INC. | ||
By: | /S/ RICHARD O. BRAJER | |
Richard O. Brajer President and Chief Executive Officer | ||
Date: March 27, 2013
109
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Lucy G. Martindale and Ashok D. Marin, jointly and severally, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign this Annual Report on Form 10-K of LipoScience, Inc., and any or all amendments (including post-effective amendments) thereto, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises hereby ratifying and confirming all that said attorneys-in-fact and agents, or his, her or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | Title | Date | ||
/S/ RICHARD O. BRAJER | President, Chief Executive Officer and Director (Principal Executive Officer) | March 27, 2013 | ||
Richard O. Brajer | ||||
/S/ LUCY G. MARTINDALE | Executive Vice President and Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | March 27, 2013 | ||
Lucy G. Martindale | ||||
/S/ BUZZ BENSON | Chairman of the Board of Directors | March 27, 2013 | ||
Buzz Benson | ||||
/S/ ROBERT J. GRECZYN JR. | Director | March 27, 2013 | ||
Robert J. Greczyn Jr. | ||||
/S/ CHRISTOPHER W. KERSEY | Director | March 27, 2013 | ||
Christopher W. Kersey | ||||
/S/ JOHN H. LANDON | Director | March 27, 2013 | ||
John H. Landon | ||||
/S/ DANIEL J. LEVANGIE | Director | March 27, 2013 | ||
Daniel J. Levangie | ||||
/S/ WOODROW A. MYERS, JR., M.D. | Director | March 27, 2013 | ||
Woodrow A. Myers, Jr., M.D. | ||||
/S/ CHARLES A. SANDERS, M.D. | Director and Chairman Emeritus | March 27, 2013 | ||
Charles A. Sanders, M.D. | ||||
/S/ RODERICK A. YOUNG | Director | March 27, 2013 | ||
Roderick A. Young | ||||
110
Exhibit Index
[copy Exhibit List here]
Exhibit Number | Description of Document |
3.1 (1) | Amended and Restated Certificate of Incorporation. |
3.2 (2) | Amended and Restated Bylaws. |
4.1 (3) | Specimen stock certificate evidencing shares of Common Stock. |
10.1 (4) | Loan and Security Agreement, dated as of December 20, 2012, by and among the Registrant, Oxford Finance LLC and Square 1 Bank. |
10.2 (5) | Warrant to purchase Series E preferred stock issued to Square 1 Bank, dated December 20, 2012. |
10.3.1 (6) | Warrant No. 1 to purchase Series E preferred stock issued to Oxford Finance LLC, dated December 20, 2012. |
10.3.2 (7) | Warrant No. 2 to purchase Series E preferred stock issued to Oxford Finance LLC, dated December 20, 2012. |
10.4 (8) | Warrant to purchase Series F preferred stock issued to Silicon Valley Bank, dated December 13, 2006. |
10.5 (9) | Warrant to purchase Series F preferred stock issued to Square 1 Bank, dated February 7, 2008. |
10.6 (10) | Warrant to purchase Series F preferred stock issued to Square 1 Bank, dated March 31, 2011. |
10.7 (11) | Second Amended and Restated Investor Rights Agreement, dated as of August 2, 2006 and as amended to date, by and among the Registrant and certain of its stockholders. |
10.8 (12) | Standard Lease, dated as of October 4, 2001, as amended on March 5, 2002 and August 28, 2002, by and between the Registrant and Parker-Raleigh Development XXX, LLC. |
10.8.1 (13) | Lease Amendment No. 3, dated as of November 29, 2011, by and between the Registrant and Raleigh Portfolio JH, LLC, as successor to Parker-Raleigh Development XXX, LLC. |
10.9† (14) | Agreement, dated as of September 28, 2012, by and between the Registrant and Laboratory Corporation of America Holdings. |
10.10† (15) | Supply Agreement, dated as of July 16, 2012, by and between the Registrant and Agilent Technologies, Inc. |
10.11† (16) | Production Agreement, dated as of June 26, 2009, by and between the Registrant and KMC Systems, Inc. |
10.12† (17) | License Agreement, dated as of May 9, 1997, as amended on January 10, 2001 and October 11, 2010, by and between the Registrant and North Carolina State University. |
10.13+ (18) | 1997 Stock Option Plan, as amended to date. |
10.14+ (19) | Form of Incentive Stock Option Agreement under 1997 Stock Option Plan. |
10.15+ (20) | Form of Incentive Stock Option Agreement under 1997 Stock Option Plan with modified change of control provisions. |
10.16+ (21) | Form of Nonqualified Stock Option Agreement under 1997 Stock Option Plan. |
10.17+ (22) | 2007 Stock Incentive Plan, as amended through July 29, 2010. |
10.18+ (23) | Fourth Amendment to 2007 Stock Incentive Plan, effective as of August 27, 2012. |
10.19+ (24) | Form of Incentive Stock Option Agreement under 2007 Stock Incentive Plan. |
10.20+ (25) | Form of Nonqualified Stock Option Agreement under 2007 Stock Incentive Plan |
10.21+ (26) | Form of Nonqualified Stock Option Agreement under 2007 Stock Incentive Plan for initial grants to directors. |
10.22+ (27) | Form of Nonqualified Stock Option Agreement under 2007 Stock Incentive Plan for annual grants to directors. |
10.23+ (28) | Form of Nonqualified Stock Option Agreement under 2007 Stock Incentive Plan for grants to committee chairpersons. |
111
Exhibit Number | Description of Document |
10.24+ (29) | Form of Restricted Stock Purchase Agreement under 2007 Stock Incentive Plan. |
10.25+ (30) | 2012 Equity Incentive Plan. |
10.26+ (31) | Form of Stock Option Grant Notice and Stock Option Agreement under 2012 Equity Incentive Plan. |
10.27+ (32) | Form of Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement under 2012 Equity Incentive Plan. |
10.28+ (33) | Non-Employee Director Compensation Plan. |
10.29 (34) | Form of Indemnification Agreement between the Registrant and certain of its directors. |
10.30 (35) | Form of Indemnification Agreement between the Registrant and certain of its directors affiliated with stockholders. |
10.31+ (36) | 2012 Employee Stock Purchase Plan. |
10.32+ | Amended and Restated Employment Agreement, dated as of March 26, 2013, by and between the Registrant and Richard O. Brajer. |
10.33+ | Amended and Restated Employment Agreement, dated as of March 25, 2013, by and between the Registrant and Lucy G. Martindale. |
10.34+ | Employment Agreement, dated as of January 9, 2013, by and between the Registrant and Robert M. Honigberg. |
10.35+ | Amended and Restated Employment Agreement, dated as of March 25, 2013, by and between the Registrant and Timothy J. Fischer. |
10.36+ | Amended and Restated Employment Agreement, dated as of March 25, 2013, by and between the Registrant and Thomas S. Clement. |
10.37+ (37) | Executive Severance Benefit Plan. |
10.38+ | 2012 Corporate Goals for Strategic Leadership Team. |
23.1 | Consent of Ernst & Young LLP, independent registered public accounting firm. |
24.1 | Power of Attorney (contained on signature page hereto). |
31.1 | Certification of Principal Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as adopted pursuant to section 302 of the Sarbanes-Oxley Act of 2002. |
31.2 | Certification of Principal Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as adopted pursuant to section 302 of the Sarbanes-Oxley Act of 2002. |
32* | Certification of Principal Executive Officer and Principal Financial Officer pursuant to Rules 13a-14(b) and 15d-14(b) promulgated under the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350, as adopted pursuant to section 906 of The Sarbanes-Oxley Act of 2002. |
_______________________
* | These certifications are being furnished solely to accompany this report pursuant to 18 U.S.C. Section 1350, and are not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and are not to be incorporated by reference into any filing of the Registrant, whether made before or after the date hereof, regardless of any general incorporation language in such filing. |
+ | Indicates management contract or compensatory plan. |
† | Confidential treatment has been granted with respect to portions of this exhibit, indicated by asterisks, which have been filed separately with the Securities and Exchange Commission. |
112
(1) | Previously filed as Exhibit 3.1 to the Registrant's Current Report on Form 8-K (File No. 001-35792), filed with the Commission on January 30, 2013, and incorporated by reference herein. |
(2) | Previously filed as Exhibit 3.2 to the Registrant's Current Report on Form 8-K (File No. 001-35792), filed with the Commission on January 30, 2013, and incorporated by reference herein. |
(3) | Previously filed as Exhibit 4.2 to Amendment No. 7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 10, 2013, and incorporated by reference herein. |
(4) | Previously filed as Exhibit 10.1 to Amendment No. 7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 10, 2013, and incorporated by reference herein. |
(5) | Previously filed as Exhibit 10.2 to Amendment No. 7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 10, 2013, and incorporated by reference herein. |
(6) | Previously filed as Exhibit 10.3.1 to Amendment No. 7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 10, 2013, and incorporated by reference herein. |
(7) | Previously filed as Exhibit 10.3.2 to Amendment No. 7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 10, 2013, and incorporated by reference herein. |
(8) | Previously filed as Exhibit 10.4 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(9) | Previously filed as Exhibit 10.5 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(10) | Previously filed as Exhibit 10.6 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(11) | Previously filed as Exhibit 10.7 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(12) | Previously filed as Exhibit 10.8 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(13) | Previously filed as Exhibit 10.8.1 to Amendment No. 4 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on April 27, 2012, and incorporated by reference herein. |
(14) | Previously filed as Exhibit 10.35 to Amendment No. 6 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on December 4, 2012, and incorporated by reference herein. |
(15) | Previously filed as Exhibit 10.9.2 to Amendment No. 8 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 24, 2013, and incorporated by reference herein. |
(16) | Previously filed as Exhibit 10.10 to Amendment No. 4 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on April 27, 2012, and incorporated by reference herein. |
(17) | Previously filed as Exhibit 10.11 to Amendment No. 1 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on August 1, 2011, and incorporated by reference herein. |
(18) | Previously filed as Exhibit 10.12 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(19) | Previously filed as Exhibit 10.13 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(20) | Previously filed as Exhibit 10.14 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
113
(21) | Previously filed as Exhibit 10.15 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(22) | Previously filed as Exhibit 10.16 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(23) | Previously filed as Exhibit 10.16.1 to Amendment No. 8 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on January 24, 2013, and incorporated by reference herein. |
(24) | Previously filed as Exhibit 10.17 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(25) | Previously filed as Exhibit 10.18 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(26) | Previously filed as Exhibit 10.19 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(27) | Previously filed as Exhibit 10.20 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(28) | Previously filed as Exhibit 10.21 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(29) | Previously filed as Exhibit 10.22 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on June 23, 2011, and incorporated by reference herein. |
(30) | Previously filed as Exhibit 4.16 to the Registrant's Registration Statement on Form S-8 (Registration No. 333-186545) filed with the Securities and Exchange Commission on February 8, 2013 and incorporated herein by reference. |
(31) | Previously filed as Exhibit 10.24 to Amendment No. 5 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on October 23, 2012, and incorporated by reference herein. |
(32) | Previously filed as Exhibit 10.25 to Amendment No. 5 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on October 23, 2012, and incorporated by reference herein. |
(33) | Previously filed as Exhibit 10.26.1 to Amendment No. 5 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on October 23, 2012, and incorporated by reference herein. |
(34) | Previously filed as Exhibit 10.28 to Amendment No. 1 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on August 1, 2011, and incorporated by reference herein. |
(35) | Previously filed as Exhibit 10.29 to Amendment No. 1 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on August 1, 2011, and incorporated by reference herein. |
(36) | Previously filed as Exhibit 4.19 to the Registrant's Registration Statement on Form S-8 (Registration No. 333-186545) filed with the Securities and Exchange Commission on February 8, 2013 and incorporated herein by reference. |
(37) | Previously filed as Exhibit 10.36 to Amendment No. 5 to the Registrant's Registration Statement on Form S-1 (File No. 333-175102), filed with the Commission on October 23, 2012, and incorporated by reference herein. |
114
