Attached files
| file | filename |
|---|---|
| 8-K - SUCAMPO PHARMACEUTICALS, INC. 8-K - Sucampo Pharmaceuticals, Inc. | a50590197.htm |
| EX-99.1 - EXHIBIT 99.1 - Sucampo Pharmaceuticals, Inc. | a50590197-ex991.htm |
| EX-99.3 - EXHIBIT 99.3 - Sucampo Pharmaceuticals, Inc. | a50590197ex99_3.htm |
Exhibit 99.2

Fourth Quarter and Full Year 2012 Results

Introductions and Forward-Looking Statements Silvia TaylorSenior Vice President, Investor Relations, Public Relations and Corporate Communications
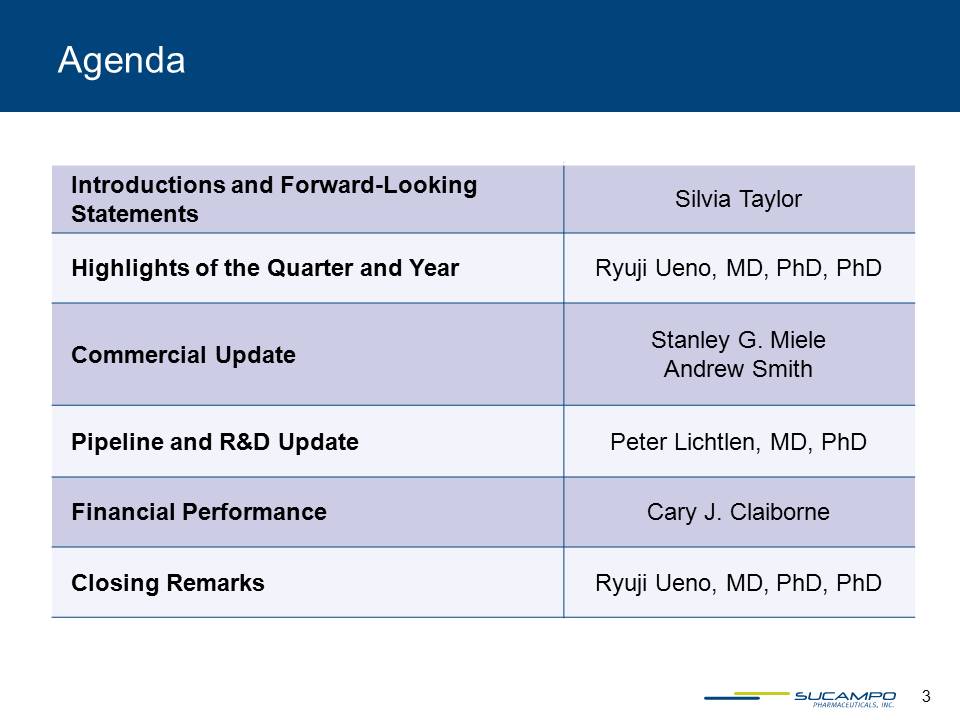
Introductions and Forward-Looking Statements Silvia Taylor Highlights of the Quarter and Year Ryuji Ueno, MD, PhD, PhD Commercial Update Stanley G. Miele Andrew Smith Pipeline and R&D Update Peter Lichtlen, MD, PhD Financial Performance Cary J. Claiborne Closing Remarks Ryuji Ueno, MD, PhD, PhD Agenda

This presentation contains "forward-looking statements" as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements are based on management's current expectations and involve risks and uncertainties, which may cause results to differ materially from those set forth in the statements. The forward-looking statements may include statements regarding product development, product potential, future financial and operating results, and other statements that are not historical facts. The following factors, among others, could cause actual results to differ from those set forth in the forward-looking statements: the impact of pharmaceutical industry regulation and health care legislation; Sucampo's ability to accurately predict future market conditions; dependence on the effectiveness of Sucampo's patents and other protections for innovative products; the risk of new and changing regulation and health policies in the US and internationally and the exposure to litigation and/or regulatory actions.No forward-looking statement can be guaranteed and actual results may differ materially from those projected. Sucampo undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise. Forward-looking statements in this presentation should be evaluated together with the many uncertainties that affect Sucampo's business, particularly those mentioned in the risk factors and cautionary statements in Sucampo's most recent Form 8-K and 10-K, which Sucampo incorporates by reference. Forward-Looking Statements

Q4 and FY 2012 Highlights Ryuji Ueno, MD, PhD, PhDChairman, Chief Executive Officer, Chief Scientific Officer, and Co-founder
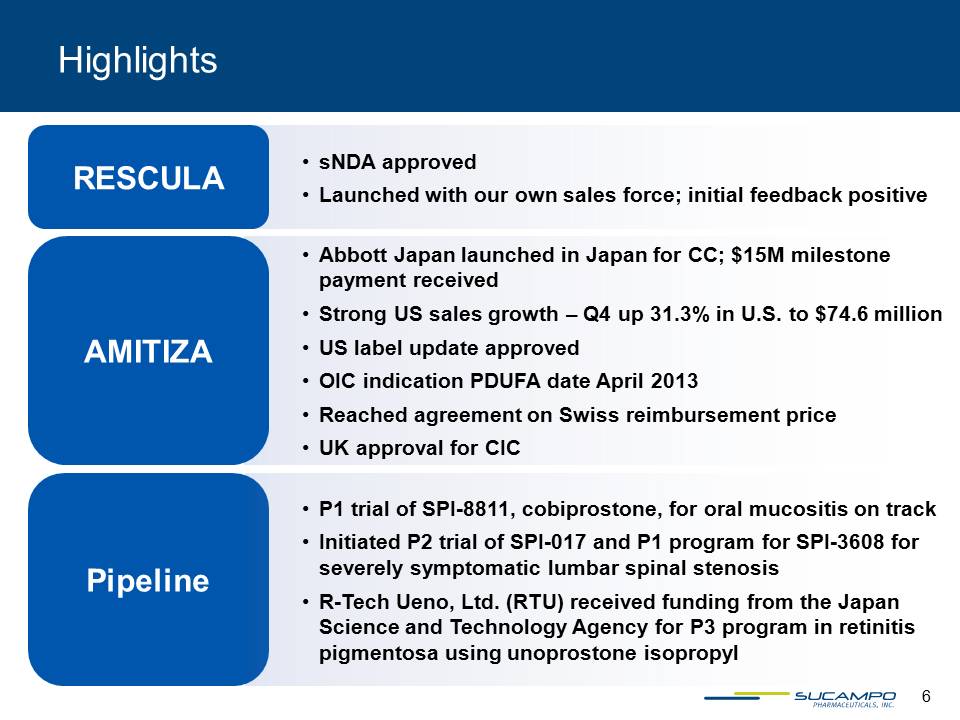
Highlights Abbott Japan launched in Japan for CC; $15M milestone payment receivedStrong US sales growth – Q4 up 31.3% in U.S. to $74.6 millionUS label update approvedOIC indication PDUFA date April 2013Reached agreement on Swiss reimbursement priceUK approval for CICAMITIZA sNDA approvedLaunched with our own sales force; initial feedback positive RESCULA Pipeline P1 trial of SPI-8811, cobiprostone, for oral mucositis on trackInitiated P2 trial of SPI-017 and P1 program for SPI-3608 for severely symptomatic lumbar spinal stenosisR-Tech Ueno, Ltd. (RTU) received funding from the Japan Science and Technology Agency for P3 program in retinitis pigmentosa using unoprostone isopropyl

Commercial Update Andrew SmithVice President of Operations and Finance Stanley G. MielePresident, Sucampo Pharma Americas and SVP, Sales and Marketing

RESCULA US Other Placeholder: sNDA approved December 2012Patients with open-angle glaucoma or ocular hypertensionIn the US: 2 million US glaucoma patients1, additional 3-6 million with ocular hypertension2Unique mechanism of action (BK channel activator) and well-tolerated safety profileLaunched with our own sales forceExpect 11% share of voicePositive feedback and significant progressMore than 5,000 face-to-face callsOver 50,000 samples shipped47 face-to-face meetings with plans and PBMs See References 1-2
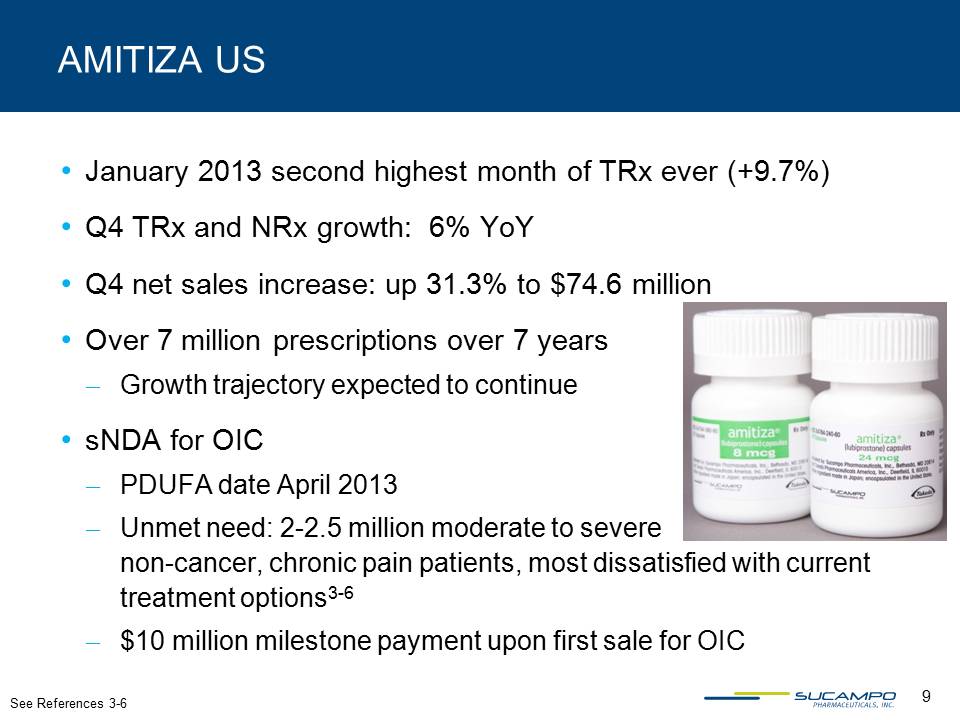
Other Placeholder: January 2013 second highest month of TRx ever (+9.7%)Q4 TRx and NRx growth: 6% YoYQ4 net sales increase: up 31.3% to $74.6 millionOver 7 million prescriptions over 7 yearsGrowth trajectory expected to continuesNDA for OICPDUFA date April 2013Unmet need: 2-2.5 million moderate to severe non-cancer, chronic pain patients, most dissatisfied with current treatment options3-6$10 million milestone payment upon first sale for OIC AMITIZA US See References 3-6
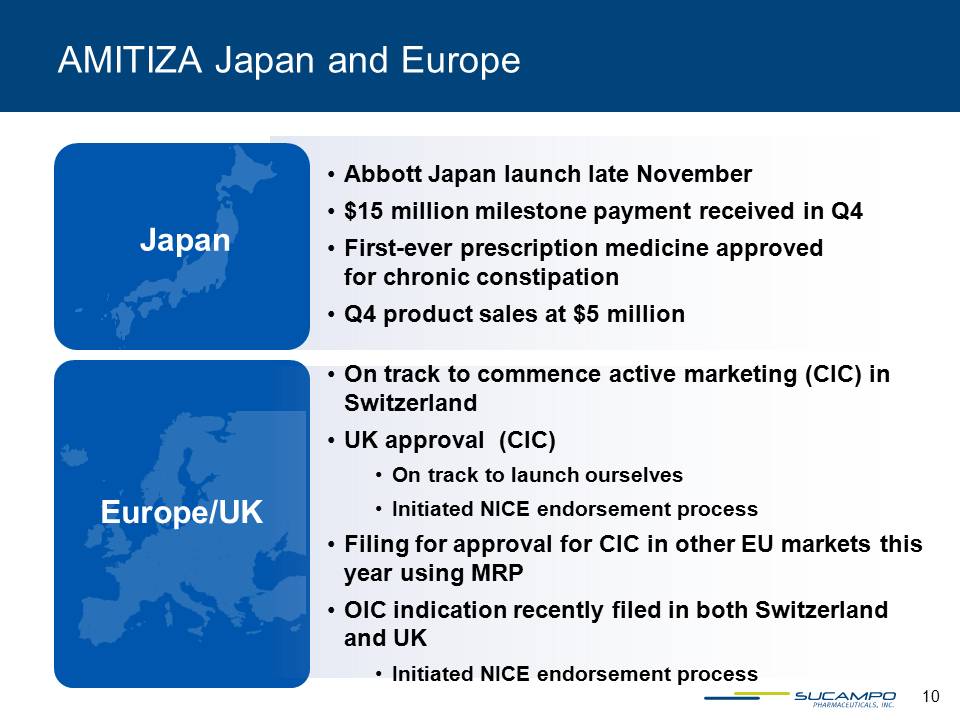
AMITIZA Japan and Europe Abbott Japan launch late November$15 million milestone payment received in Q4First-ever prescription medicine approved for chronic constipationQ4 product sales at $5 million On track to commence active marketing (CIC) in SwitzerlandUK approval (CIC)On track to launch ourselves Initiated NICE endorsement processFiling for approval for CIC in other EU markets this year using MRP OIC indication recently filed in both Switzerland and UK Initiated NICE endorsement process Japan Europe/UK

Pipeline and R&D Update Peter Lichtlen, MD, PhDSenior Medical Officer and Vice President, European Operations
Development of new liquid dosage formSome patients cannot swallow gel capsPediatric Geriatric100% of development costs to be reimbursed by TakedaInitiate P3 pediatric trials in Q3 2013Pediatric functional constipation indicationTakeda to fund significant amount of developmental costs AMITIZA
Other Placeholder: Oral mucositis is a severely painful inflammation of the oral cavity 100% incidence rate in certain cancersUnmet medical needP1 trial in healthy volunteers initiatedNew oral spray formulationExpect to complete P1 in Q2 2013 SPI-8811 (cobiprostone) for Oral Mucositis See Reference 7; photos from Silverman. Diagnosis and management of oral mucositis. J Support Oncol 2007; 5 (2 Suppl 1):13-21.
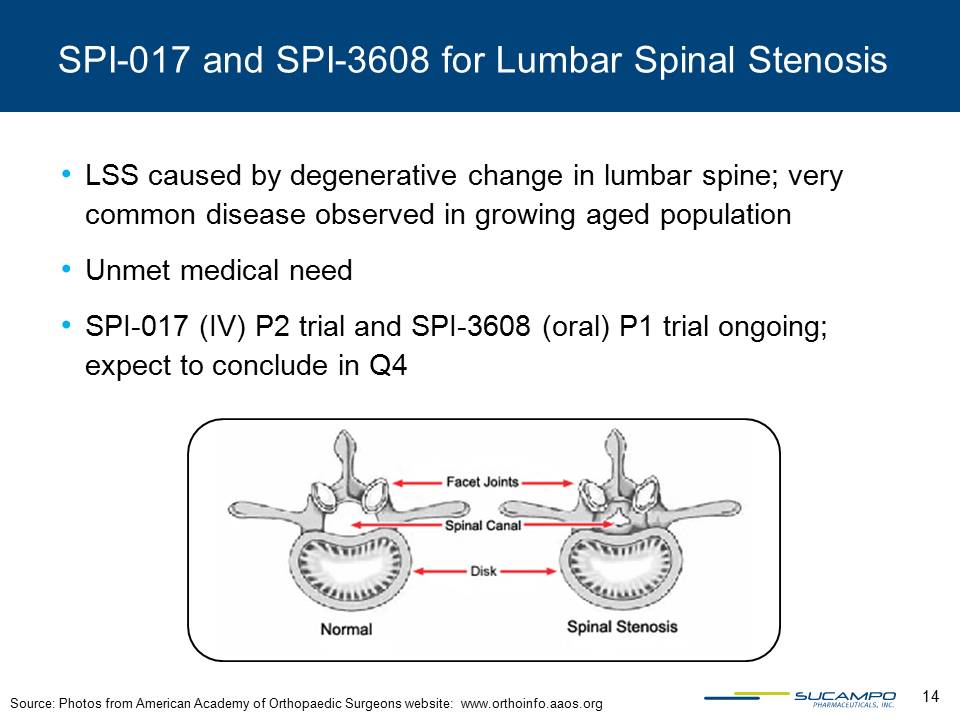
Other Placeholder: LSS caused by degenerative change in lumbar spine; very common disease observed in growing aged populationUnmet medical needSPI-017 (IV) P2 trial and SPI-3608 (oral) P1 trial ongoing; expect to conclude in Q4 SPI-017 and SPI-3608 for Lumbar Spinal Stenosis Source: Photos from American Academy of Orthopaedic Surgeons website: www.orthoinfo.aaos.org
Japan Science and Technology Agency to provide RTU with the majority of funding for P3 clinical developmental costsCo-developing with RTURetinitis pigmentosa (RP) begins with degeneration of rods, followed by progressive and irreversible death of cones leading to blindnessCurrently no drugs or treatments approved for RPSucampo received orphan drug designation for unoprostone isopropyl in US Unoprostone Isopropyl for Retinitis Pigmentosa Source: Photos from Foundation Fighting Blindness website: www.blindness.org

Financial Performance Cary J. ClaiborneChief Financial Officer
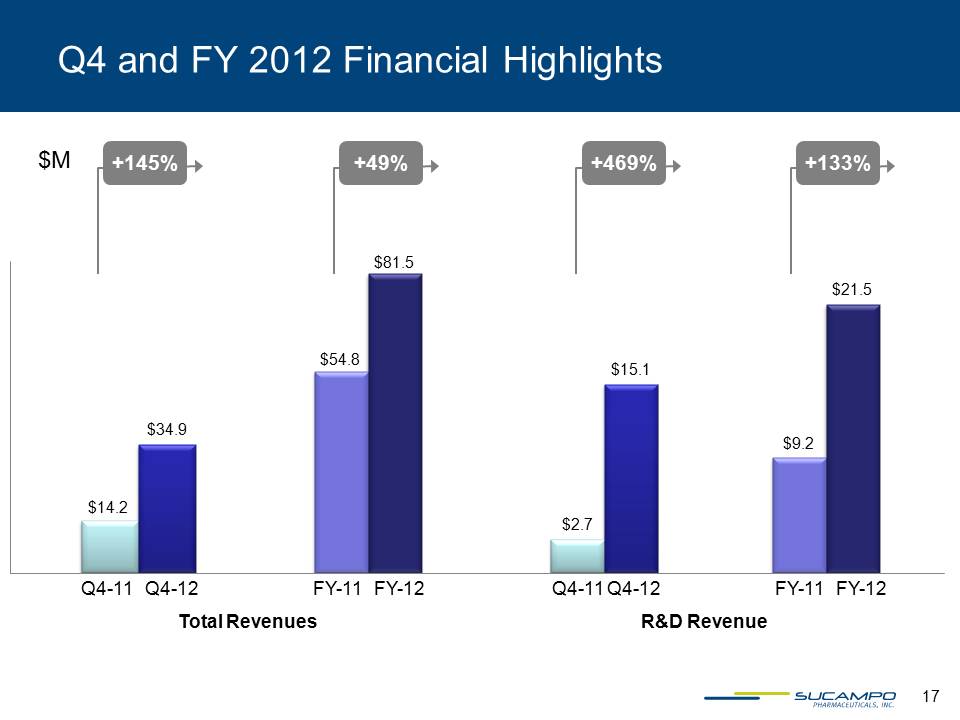
Q4 and FY 2012 Financial Highlights +49% +145% Q4-11 Q4-12 FY-11 FY-12 Total Revenues $M +469% Q4-11 Q4-12 +133% FY-11 FY-12 R&D Revenue $14.2 $34.9 $54.8 $2.7 $15.1 $9.2 $21.5 $81.5
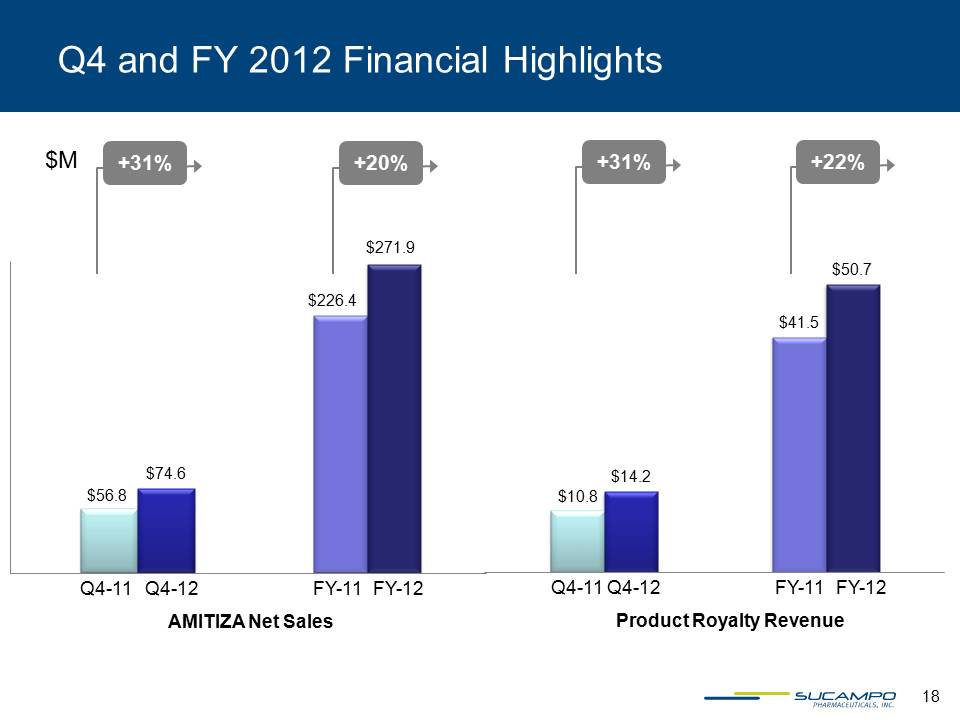
Q4 and FY 2012 Financial Highlights $M +20% +31% Q4-11 Q4-12 FY-11 FY-12 AMITIZA Net Sales $226.4 $271.9 +31% +22% Q4-11 Q4-12 Product Royalty Revenue FY-11 FY-12 $56.8 $74.6 $10.8 $14.2 $41.5 $50.7
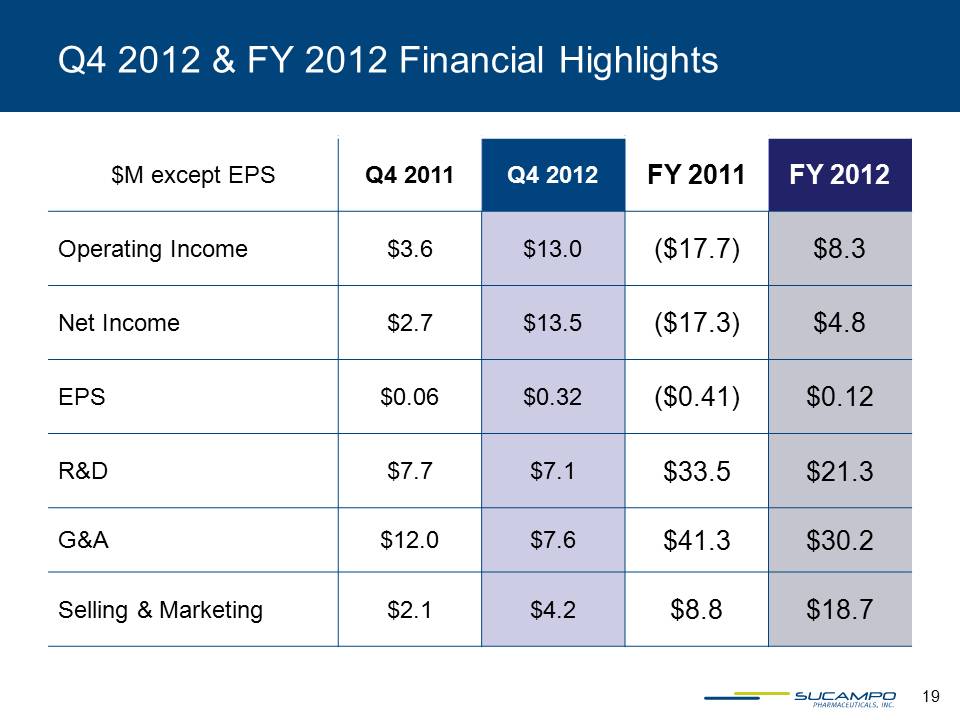
$M except EPS Q4 2011 Q4 2012 FY 2011 FY 2012 Operating Income $3.6 $13.0 ($17.7) $8.3 Net Income $2.7 $13.5 ($17.3) $4.8 EPS $0.06 $0.32 ($0.41) $0.12 R&D $7.7 $7.1 $33.5 $21.3 G&A $12.0 $7.6 $41.3 $30.2 Selling & Marketing $2.1 $4.2 $8.8 $18.7 19

Cash position $91.4 million as of December 31, 2012$15 million milestone payment received from Abbott Japan Repurchased 146,908 shares during quarterRecently raised authorized amount to $5 millionOne class of common stockOperating cash flow of $17.2 million for Q4 and $12 million for FY 2012 Q4 and FY 2012 Financial Highlights

Closing Remarks Ryuji Ueno, MD, PhD, PhDChairman, Chief Executive Officer, Chief Scientific Officer, and Co-founder
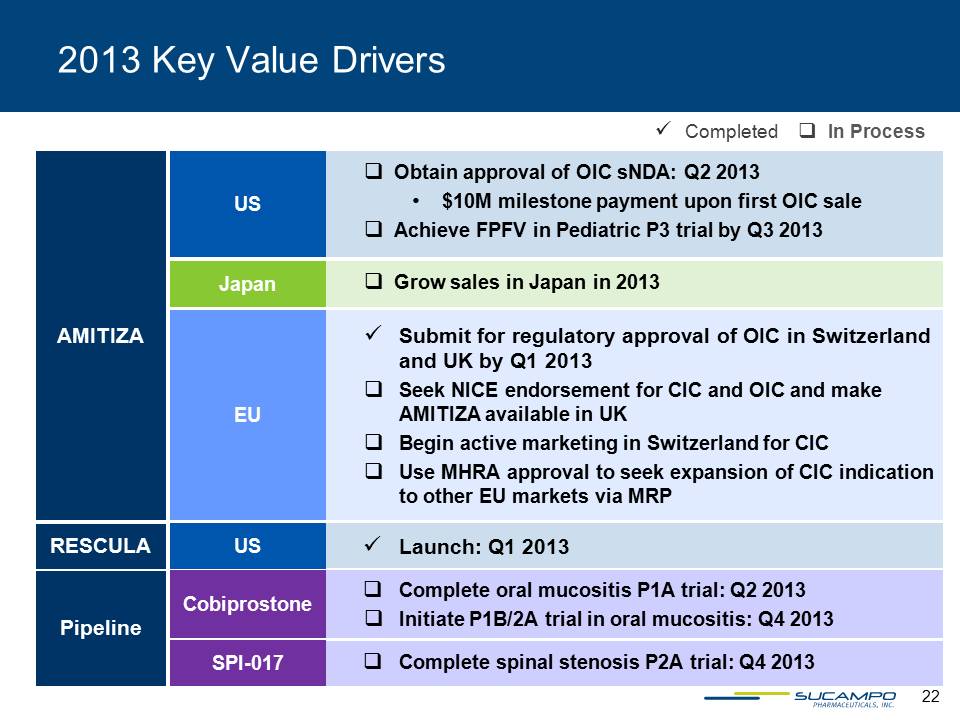
2013 Key Value Drivers Completed In Process Obtain approval of OIC sNDA: Q2 2013$10M milestone payment upon first OIC saleAchieve FPFV in Pediatric P3 trial by Q3 2013 US AMITIZA Grow sales in Japan in 2013 Japan Submit for regulatory approval of OIC in Switzerland and UK by Q1 2013Seek NICE endorsement for CIC and OIC and make AMITIZA available in UKBegin active marketing in Switzerland for CIC Use MHRA approval to seek expansion of CIC indication to other EU markets via MRP EU RESCULA Launch: Q1 2013 US Pipeline Complete oral mucositis P1A trial: Q2 2013 Initiate P1B/2A trial in oral mucositis: Q4 2013 Cobiprostone Complete spinal stenosis P2A trial: Q4 2013 SPI-017

Sucampo Pharmaceuticals, Inc.

References American Academy of Ophthalmology Glaucoma Panel. Preferred Practice Pattern® guideline: Primary open-angle glaucoma. 2010Kass MA et al. Arch Ophthalmol. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. 2002 Jun;120(6):701-13; discussion 829-30.IMS HealthVerispan PDDAPhysician Interviews ClearView AnalysisTrotti A et al. Radiother Oncol. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. 2003 Mar;66(3):253-62.
