Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Anacor Pharmaceuticals, Inc. | a13-6303_18k.htm |
| EX-99.1 - EX-99.1 - Anacor Pharmaceuticals, Inc. | a13-6303_1ex99d1.htm |
Exhibit 99.2
|
|
Results from the Phase 3 Study (Study 302) of Tavaborole to Evaluate Efficacy and Safety in the Treatment of Onychomycosis February 28, 2013 |
|
|
Safe Harbor Statement Certain items in this presentation and other matters discussed today or answers that may be given to questions asked could constitute forward-looking statements, including statements regarding the potential for approval of tavaborole based on the Phase 3 study results, whether our current arbitration with Valeant may result in a favorable outcome and the timing and results of the arbitration, the potential competition from efinaconazole, the potential commercial opportunity for tavaborole, the potential and timing for us to raise additional capital on acceptable terms, if at all, whether or not we are able to partner tavaborole, the progress and timing of NDA filing, the safety and efficacy of our product candidates, other estimates of the potential markets for our product candidates, and the potential use of tavaborole and efinaconazole, if approved. These statements are subject to risks and uncertainties relating to Anacor’s future financial or business performance. Additional risks and uncertainties are described more fully in Anacor’s 10-Q for the quarter ended September 30, 2012 filed with the Securities and Exchange Commission. Anacor’s actual results or achievements could differ materially from those anticipated in these forward-looking statements. Please note that Anacor is under no obligation to update any of the forward-looking statements discussed today, except as required by law. |
|
|
David Perry Chief Executive Officer |
|
|
Lee Zane, MD Vice President, Clinical Development |
|
|
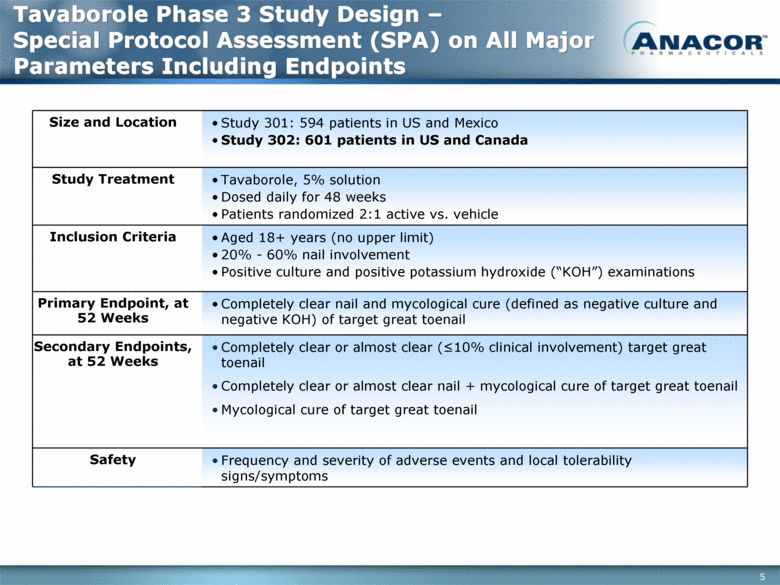
Tavaborole Phase 3 Study Design – Special Protocol Assessment (SPA) on All Major Parameters Including Endpoints Size and Location Study 301: 594 patients in US and Mexico Study 302: 601 patients in US and Canada Study Treatment Tavaborole, 5% solution Dosed daily for 48 weeks Patients randomized 2:1 active vs. vehicle Inclusion Criteria Aged 18+ years (no upper limit) 20% - 60% nail involvement Positive culture and positive potassium hydroxide (“KOH”) examinations Primary Endpoint, at 52 Weeks Completely clear nail and mycological cure (defined as negative culture and negative KOH) of target great toenail Secondary Endpoints, at 52 Weeks Completely clear or almost clear (<10% clinical involvement) target great toenail Completely clear or almost clear nail + mycological cure of target great toenail Mycological cure of target great toenail Safety Frequency and severity of adverse events and local tolerability signs/symptoms |
|
|
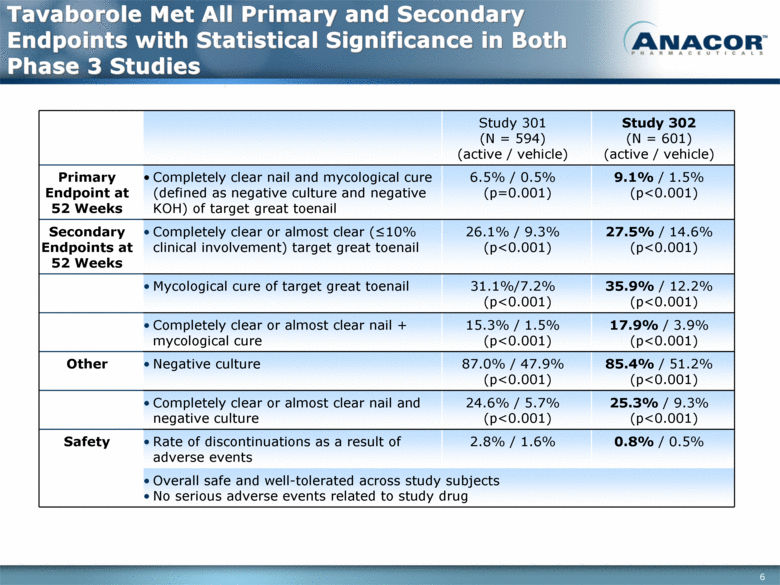
Tavaborole Met All Primary and Secondary Endpoints with Statistical Significance in Both Phase 3 Studies Study 301 (N = 594) (active / vehicle) Study 302 (N = 601) (active / vehicle) Primary Endpoint at 52 Weeks Completely clear nail and mycological cure (defined as negative culture and negative KOH) of target great toenail 6.5% / 0.5% (p=0.001) 9.1% / 1.5% (p<0.001) Secondary Endpoints at 52 Weeks Completely clear or almost clear (<10% clinical involvement) target great toenail 26.1% / 9.3% (p<0.001) 27.5% / 14.6% (p<0.001) Mycological cure of target great toenail 31.1%/7.2% (p<0.001) 35.9% / 12.2% (p<0.001) Completely clear or almost clear nail + mycological cure 15.3% / 1.5% (p<0.001) 17.9% / 3.9% (p<0.001) Other Negative culture 87.0% / 47.9% (p<0.001) 85.4% / 51.2% (p<0.001) Completely clear or almost clear nail and negative culture 24.6% / 5.7% (p<0.001) 25.3% / 9.3% (p<0.001) Safety Rate of discontinuations as a result of adverse events 2.8% / 1.6% 0.8% / 0.5% Overall safe and well-tolerated across study subjects No serious adverse events related to study drug |
|
|
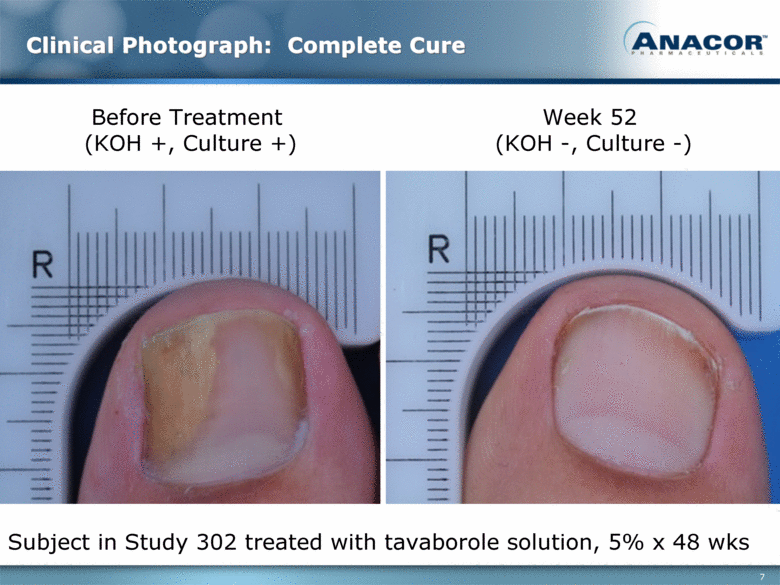
Clinical Photograph: Complete Cure Before Treatment (KOH +, Culture +) Week 52 (KOH -, Culture -) Subject in Study 302 treated with tavaborole solution, 5% x 48 wks |
|
|
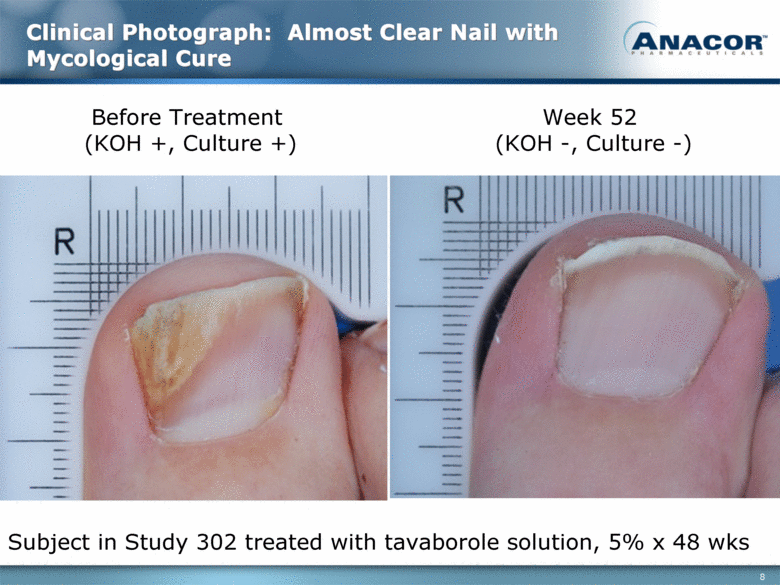
Clinical Photograph: Almost Clear Nail with Mycological Cure Before Treatment (KOH +, Culture +) Week 52 (KOH -, Culture -) Subject in Study 302 treated with tavaborole solution, 5% x 48 wks |
|
|
David Perry Chief Executive Officer |
|
|
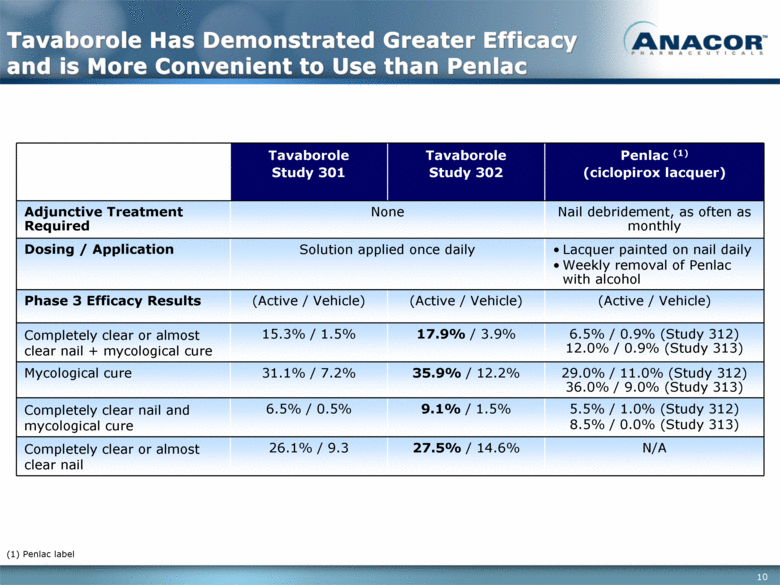
Tavaborole Has Demonstrated Greater Efficacy and is More Convenient to Use than Penlac Tavaborole Study 301 Tavaborole Study 302 Penlac (1) (ciclopirox lacquer) Adjunctive Treatment Required None Nail debridement, as often as monthly Dosing / Application Solution applied once daily Lacquer painted on nail daily Weekly removal of Penlac with alcohol Phase 3 Efficacy Results (Active / Vehicle) (Active / Vehicle) (Active / Vehicle) Completely clear or almost clear nail + mycological cure 15.3% / 1.5% 17.9% / 3.9% 6.5% / 0.9% (Study 312) 12.0% / 0.9% (Study 313) Mycological cure 31.1% / 7.2% 35.9% / 12.2% 29.0% / 11.0% (Study 312) 36.0% / 9.0% (Study 313) Completely clear nail and mycological cure 6.5% / 0.5% 9.1% / 1.5% 5.5% / 1.0% (Study 312) 8.5% / 0.0% (Study 313) Completely clear or almost clear nail 26.1% / 9.3 27.5% / 14.6% N/A (1) Penlac label |
|
|
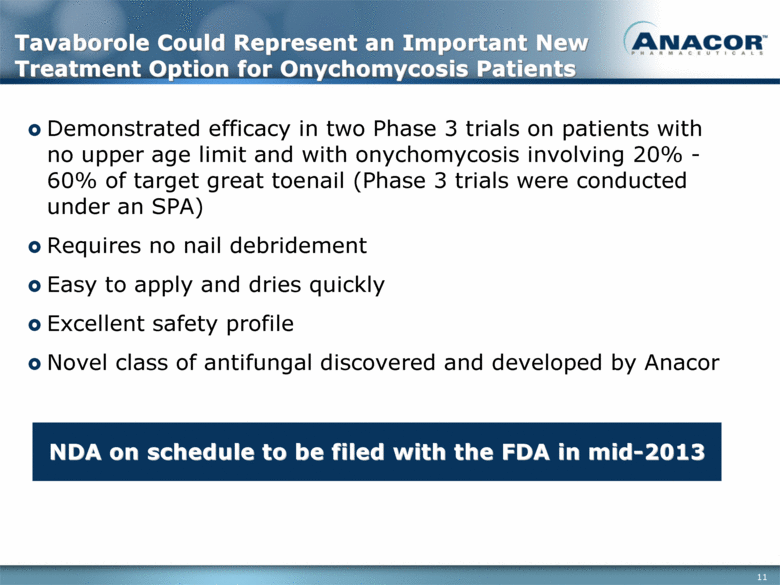
Tavaborole Could Represent an Important New Treatment Option for Onychomycosis Patients Demonstrated efficacy in two Phase 3 trials on patients with no upper age limit and with onychomycosis involving 20% - 60% of target great toenail (Phase 3 trials were conducted under an SPA) Requires no nail debridement Easy to apply and dries quickly Excellent safety profile Novel class of antifungal discovered and developed by Anacor NDA on schedule to be filed with the FDA in mid-2013 |
|
|
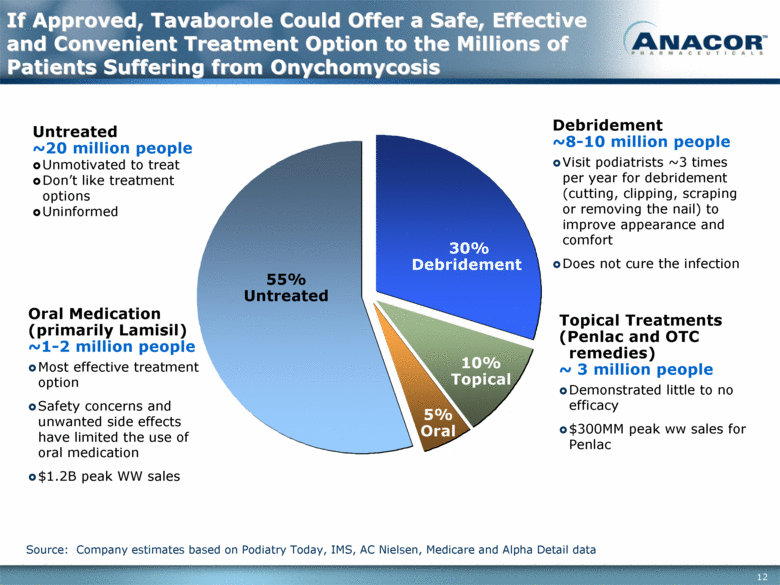
Source: Company estimates based on Podiatry Today, IMS, AC Nielsen, Medicare and Alpha Detail data If Approved, Tavaborole Could Offer a Safe, Effective and Convenient Treatment Option to the Millions of Patients Suffering from Onychomycosis 10% Topical 55% Untreated 30% Debridement 5% Oral Debridement ~8-10 million people Visit podiatrists ~3 times per year for debridement (cutting, clipping, scraping or removing the nail) to improve appearance and comfort Does not cure the infection Topical Treatments (Penlac and OTC remedies) ~ 3 million people Demonstrated little to no efficacy $300MM peak ww sales for Penlac Untreated ~20 million people Unmotivated to treat Don’t like treatment options Uninformed Oral Medication (primarily Lamisil) ~1-2 million people Most effective treatment option Safety concerns and unwanted side effects have limited the use of oral medication $1.2B peak WW sales |
|
|
Geoff Parker Chief Financial Officer |
|
|
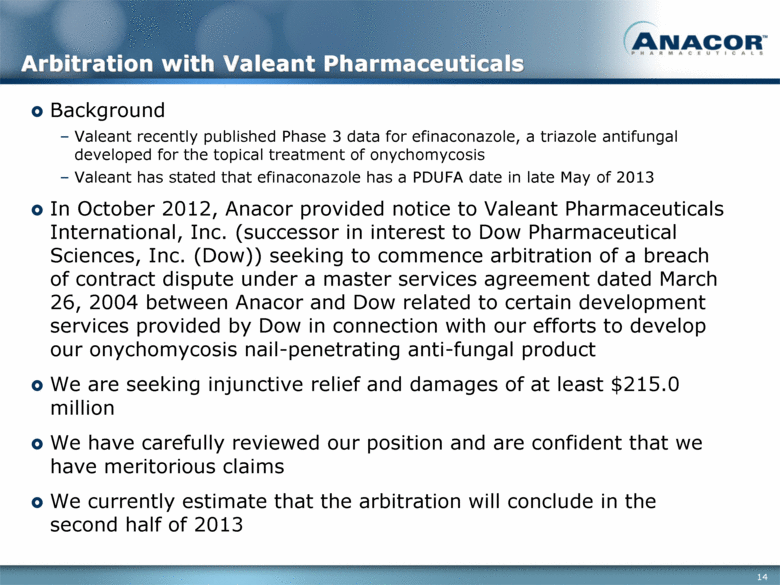
Arbitration with Valeant Pharmaceuticals Background Valeant recently published Phase 3 data for efinaconazole, a triazole antifungal developed for the topical treatment of onychomycosis Valeant has stated that efinaconazole has a PDUFA date in late May of 2013 In October 2012, Anacor provided notice to Valeant Pharmaceuticals International, Inc. (successor in interest to Dow Pharmaceutical Sciences, Inc. (Dow)) seeking to commence arbitration of a breach of contract dispute under a master services agreement dated March 26, 2004 between Anacor and Dow related to certain development services provided by Dow in connection with our efforts to develop our onychomycosis nail-penetrating anti-fungal product We are seeking injunctive relief and damages of at least $215.0 million We have carefully reviewed our position and are confident that we have meritorious claims We currently estimate that the arbitration will conclude in the second half of 2013 |
|
|
Current Cash Position and Upcoming Milestones Ended 2012 with $45.5M cash Current capital resources are sufficient to support operations through NDA filing for tavaborole in mid-2013 Net cash burn estimated to be approximately $15M / quarter Specific upcoming milestones of note include: Results from AN2728 Phase 2 dose-ranging study in adolescents with atopic dermatitis (March 2013) Labeling details for Valeant’s efinaconazole, if approved (May 2013) NDA filing for tavaborole (Mid-2013) Continued evaluation of commercial partnering options (2H13) Conclusion of arbitration with Valeant regarding breach of contract (2H13) Continue to explore capital raising opportunities from a number of potential sources: A commercial partnership for tavaborole A commercial partnership for AN2728 Royalty financing related to tavaborole Grants and contracts that support research Debt or equity financing |
|
|
Q&A David Perry, CEO Geoff Parker, CFO Lee Zane, MD, Vice President, Clinical Development |