Attached files
| file | filename |
|---|---|
| 8-K - OPEXA THERAPEUTICS, INC. 8-K - Acer Therapeutics Inc. | a50559650.htm |
Exhibit 99.1

Opexa Therapeutics, Inc.
Precision Medicine February 2013Neil WarmaPresident & CEO

Forward-Looking Statements This presentation contains forward-looking statements which are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. The words “expects,”“believes,” “may,” “could,” “potential” and similar expressions are intended to identify forward-looking statements.These forward-looking statements do not constitute guarantees of future performance. Investors are cautioned that statements in this presentation which are not strictly historical statements, including, without limitation, statements regarding development of the Company’s technologies, including its product candidate Tcelna (imilecleucel-T), constitute forward-looking statements. Such forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated, including, without limitation, risks associated with: our ability to finance our ongoing Phase IIb trial for Tcelna in secondary progressive multiple
sclerosis (MS), as well as any other development programs; the efficacy and safety profile of Tcelna, including for any particular indication such as relapsing remitting MS or secondary progressive MS; the success of our
development efforts, including the Phase IIb trial for Tcelna in secondary progressive MS which, depending upon results, may determine whether Merck Serono elects to exercise its option pursuant to an option and development
agreement dated February 5, 2013 to acquire control of Tcelna in MS; whether Merck Serono exercises such option and, if so, whether we receive any development or commercialization milestone payments or royalties from Merck Serono pursuant to such option and development agreement; our dependence, if Merck Serono exercises its option, on Merck Serono for the further development of Tcelna; whether we are able to establish, obtain financing for and otherwise initiate and/or continue development programs beyond Tcelna in MS; our compliance with all Food and Drug Administration regulations; our ability to obtain, maintain and protect intellectual property rights (including for Tcelna); and other risks detailed in our filings with the Securities and Exchange Commission. These forwardlooking statements speak only as of the date made. We assume no obligation or undertaking to update any forwardlooking statements to reflect any changes in expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. You should, however, review additional disclosures we make in our reports filed with the Securities and Exchange Commission, including our Annual Report on Form 10-K for the year ended December 31, 2011 and our Quarterly Report on Form 10-Q for the quarter ended September 30, 2012.

Opexa Highlights Opexa is one of the leading cellular therapy companies – Focused on developing TcelnaTM, a T-cell immunotherapy for Multiple Sclerosis (MS) Currently conducting a Phase IIb clinical trial in Secondary Progressive MS (SPMS) – Fast Track designation granted by FDA February 5, 2013 announcement of option and license agreement with Merck Serono for Tcelna in MS – Up to $225 million in payments + royalties Opexa platform capable of generating multiple disease treatment opportunitiesPharma agreement is supportive of platform’s potential Publicly traded on NASDAQ Capital Markets (NASDAQ : OPXA)
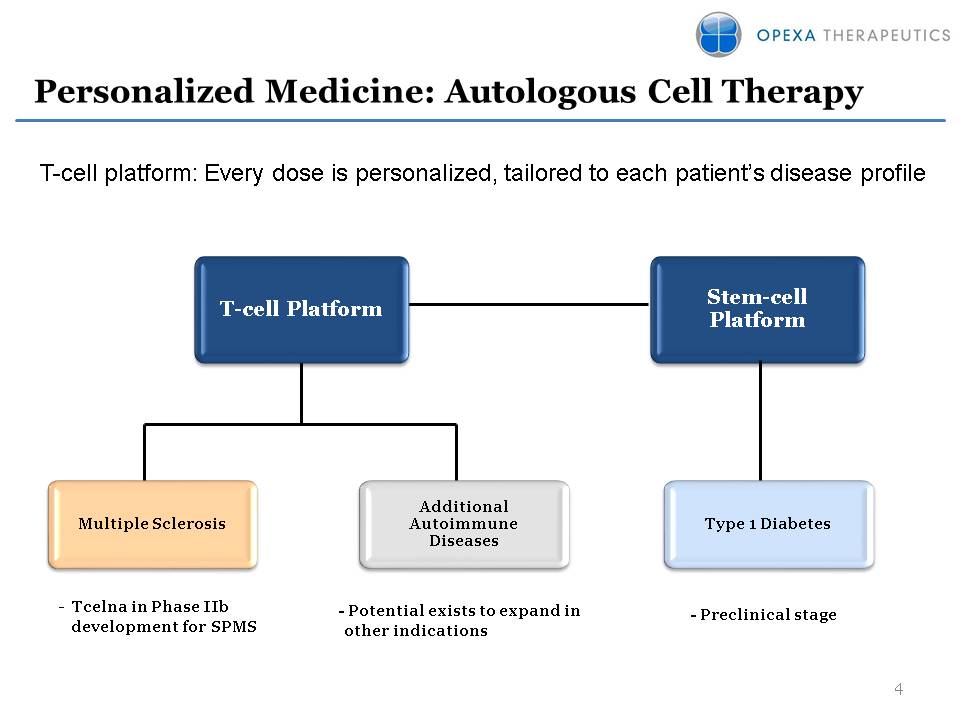
Personalized Medicine: Autologous Cell Therapy Stem-cell Platform Multiple Sclerosis Additional Autoimmune Diseases - Preclinical stage Tcelna in Phase IIb development for SPMS T-cell Platform Type 1 Diabetes - Potential exists to expand in other indications T-cell platform: Every dose is personalized, tailored to each patient’s disease profile
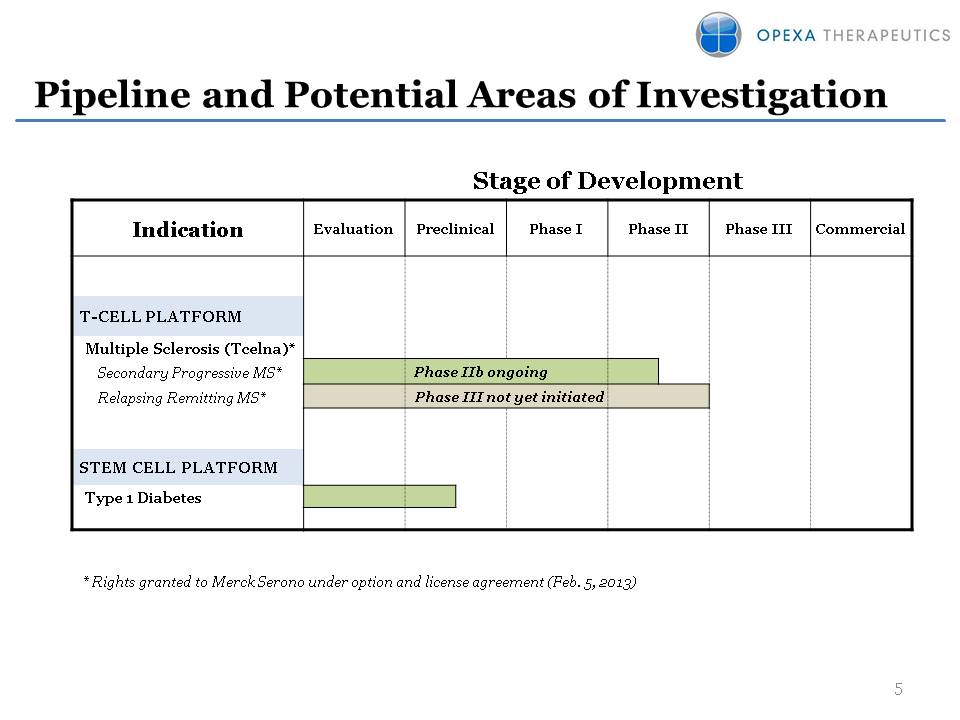
Pipeline and Potential Areas of Investigation Stage of Development * Rights granted to Merck Serono under option and license agreement (Feb. 5, 2013)
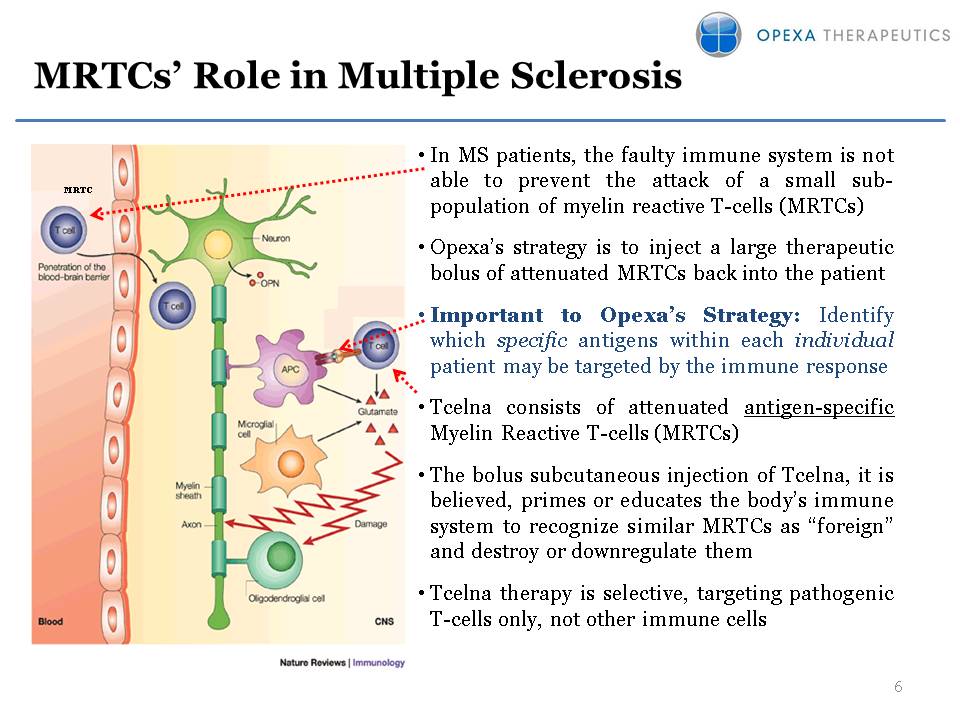
MRTCs’ Role in Multiple Sclerosis MRTC In MS patients, the faulty immune system is not able to prevent the attack of a small sub-population of myelin reactive T-cells (MRTCs)Opexa’s strategy is to inject a large therapeutic bolus of attenuated MRTCs back into the patientImportant to Opexa’s Strategy: Identify which specific antigens within each individual patient may be targeted by the immune response Tcelna consists of attenuated antigen-specific Myelin Reactive T-cells (MRTCs)The bolus subcutaneous injection of Tcelna, it is believed, primes or educates the body’s immune system to recognize similar MRTCs as “foreign” and destroy or downregulate them Tcelna therapy is selective, targeting pathogenic T-cells only, not other immune cells
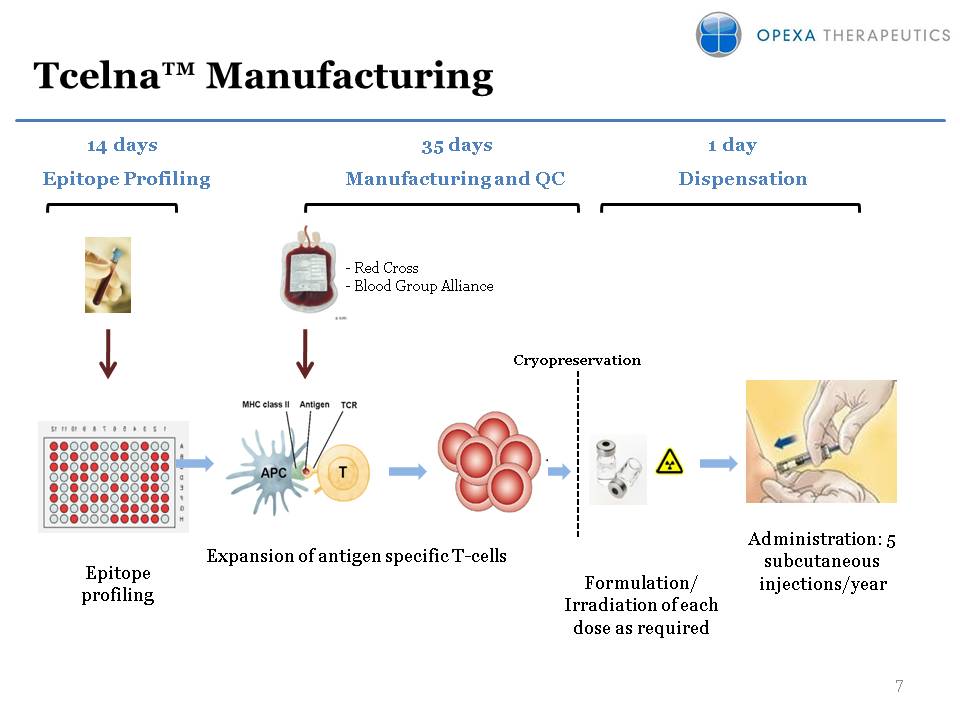
Tcelna™ Manufacturing (Gp:) Expansion of antigen specific T-cells (Gp:) Cryopreservation (Gp:) Formulation/Irradiation of each dose as required (Gp:) Epitope profiling (Gp:) Administration: 5 subcutaneous injections/year (Gp:) Manufacturing and QC (Gp:) Dispensation (Gp:) 35 days (Gp:) Epitope Profiling (Gp:) 1 day (Gp:) 14 days (Gp:) - Red Cross- Blood Group Alliance
Safety and Tolerability Overview To date, Tcelna has demonstrated superior safety to MS drugs marketed and in developmentTcelna is very well tolerated with favorable safety profileNo Serious Adverse Events (SAE) associated with treatment in previous Phase IIb Relapsing Remitting MS (RRMS) clinical trial in 150 patients (100 on active therapy)Most common side effect – mild and transient injection site reactions

Tcelna Clinical and Regulatory Overview Five Phase I and Phase II clinical trials have been conducted to date with Tcelna in both RRMS and SPMS patientsOver 300 subjects have been enrolled in these trials with over 140 being treated with TcelnaEncouraging signs of clinical efficacy observed in both MS patient populations treated with TcelnaDose range studies have been conducted confirming dose and treatment regimen30-45 million cells per dose, 5 subcutaneous injections (doses) administered/yearRRMS - Opexa received FDA endorsement to initiate Phase III pivotal studies but deferred in favor of SPMS trialSPMS – Opexa received Fast Track designation from FDA
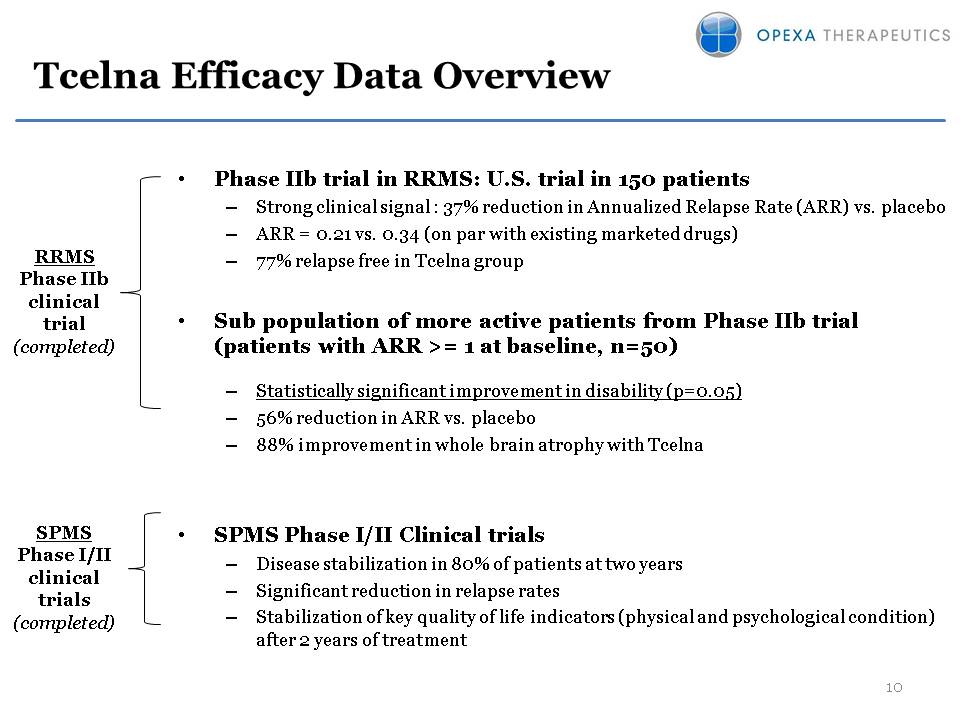
Tcelna Efficacy Data Overview Phase IIb trial in RRMS: U.S. trial in 150 patients Strong clinical signal : 37% reduction in Annualized Relapse Rate (ARR) vs. placeboARR = 0.21 vs. 0.34 (on par with existing marketed drugs)77% relapse free in Tcelna groupSub population of more active patients from Phase IIb trial (patients with ARR >= 1 at baseline, n=50)Statistically significant improvement in disability (p=0.05)56% reduction in ARR vs. placebo88% improvement in whole brain atrophy with TcelnaSPMS Phase I/II Clinical trialsDisease stabilization in 80% of patients at two yearsSignificant reduction in relapse ratesStabilization of key quality of life indicators (physical and psychological condition) after 2 years of treatment
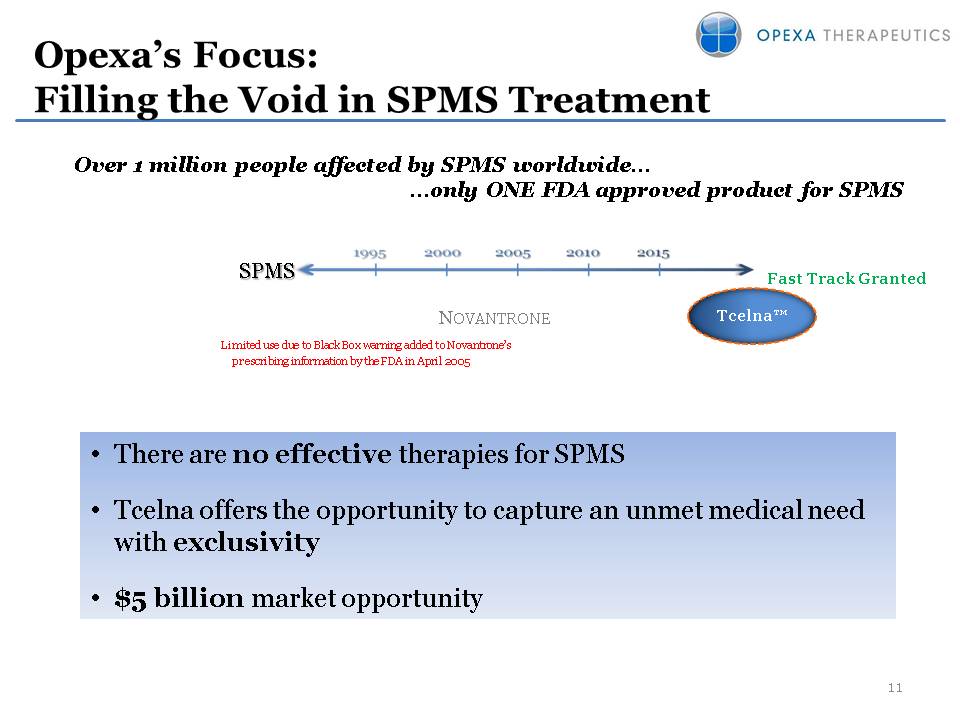
Opexa’s Focus:Filling the Void in SPMS Treatment Over 1 million people affected by SPMS worldwide… …only ONE FDA approved product for SPMS (Gp:) SPMS (Gp:) Tcelna™ (Gp:) Novantrone Limited use due to Black Box warning added to Novantrone’s prescribing information by the FDA in April 2005 There are no effective therapies for SPMSTcelna offers the opportunity to capture an unmet medical need with exclusivity$5 billion market opportunity Fast Track Granted

Abili-T TM : Landmark trial in SPMS Abili-T Phase IIb clinical trial in SPMS is ongoingDouble-blind, 1:1 randomized, placebo-controlledEnthusiasm among MS community (patients and sites) is highApproval by Health Canada enables expansion into Canada180 PatientsSPMS population30 sites in USA and CanadaKey Efficacy Endpoints:Whole-brain atrophySustained progression measured by EDSS2 annual courses of personalized therapy2 years of assessment
Opexa’s Immune Monitoring Program OverviewOpexa is undertaking a comprehensive Immune Monitoring Program to capture valuable information from the Abili-T trial in SPMS patientsThe Immune Monitoring Program will harvest multiple samples from 180 SPMS patients over two years and will be analyzed in real timeThe longitudinal assessment of both placebo and active patient samples will provide valuable first time information for a key underserved patient populationPurposeIdentify potential biomarkers for SPMS Investigate the impact of Tcelna treatment on systemic immunity Further define the Mechanism of Action of TcelnaFurther elucidate the Immunopathology of SPMS

Opexa secures option and license agreement with Merck Serono for MS indication Option and License agreement jointly announced February 5, 2013Total potential payments to Opexa up to $225 million$5 million upfront option grant fee$25 million, or $15 million, option exercise feeUp to $195 in development and commercial milestone paymentsRoyalties: Tiered, based on net sales, from upper single digit – mid teensOpexa maintains key rights:Development and commercialization rights to Tcelna in JapanCertain manufacturing rightsCo-development funding option in exchange for increased royaltiesRights to all other disease indications

Value for Opexa If option is exercised, potential payments to Opexa up to $225 million over next several years and beyond plus tiered royalties based on net salesAlliance with one of the leading global Pharma companies with commitment, franchise and expertise in MSPath to commercialization of Tcelna potentially streamlinedMerck Serono to fully fund clinical, regulatory and commercial development (subject to Opexa’s co-development rights)Endorsement and support for Opexa’s platformPotential to expand pipeline to other autoimmune diseasesValuable rights retained by Opexa
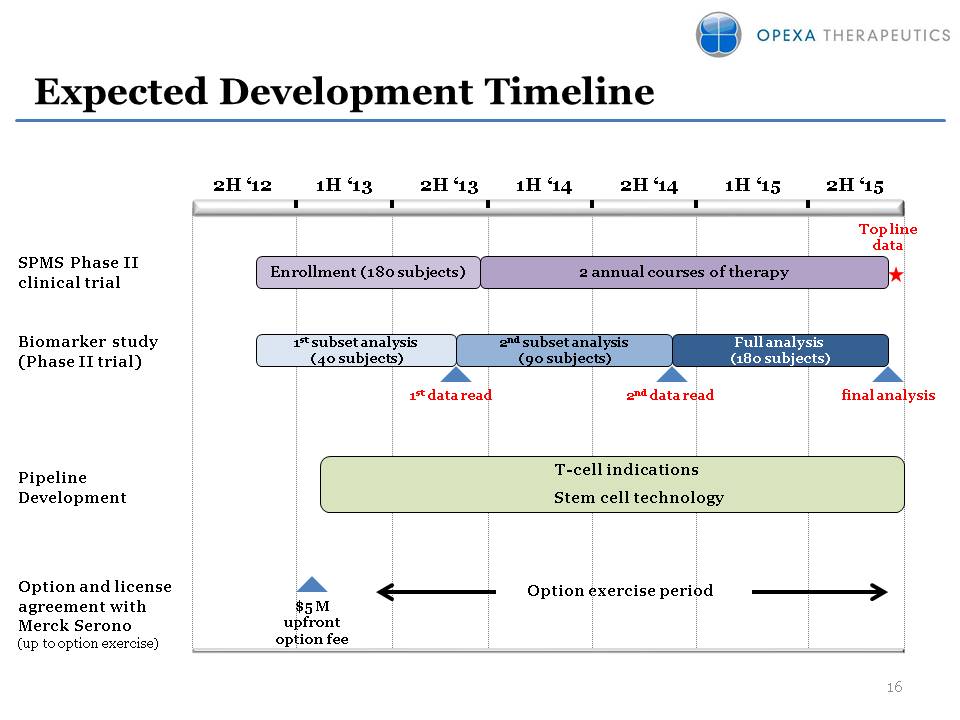
Expected Development Timeline Option and license agreement with Merck Serono(up to option exercise) SPMS Phase II clinical trial 1H ‘13 2H ‘13 1H ‘14 2H ‘14 1H ‘15 2H ‘15 2H ‘12 $5 M upfront option fee Option exercise period Top line data Full analysis (180 subjects) Pipeline Development Biomarker study(Phase II trial) 2nd data read 1st data read final analysis T-cell indications Stem cell technology 2 annual courses of therapy Enrollment (180 subjects) 1st subset analysis (40 subjects) 2nd subset analysis (90 subjects)

Milestones and Goals Obtained FDA Fast Track approval for Tcelna™ in SPMS (Q4’11 )Initiated “Abili-T” 180 patient Phase IIb SPMS clinical trial in U.S. (Q3’12)Closed option and license agreement with Merck Serono, global leader in MS (Q1’13) Secured $3.25 million in private offering (Q1’13)Evaluate T-cell platform to possibly advance in other development opportunitiesSecure additional resources to fund Phase IIb SPMS trialEvaluate potential of stem cell platform
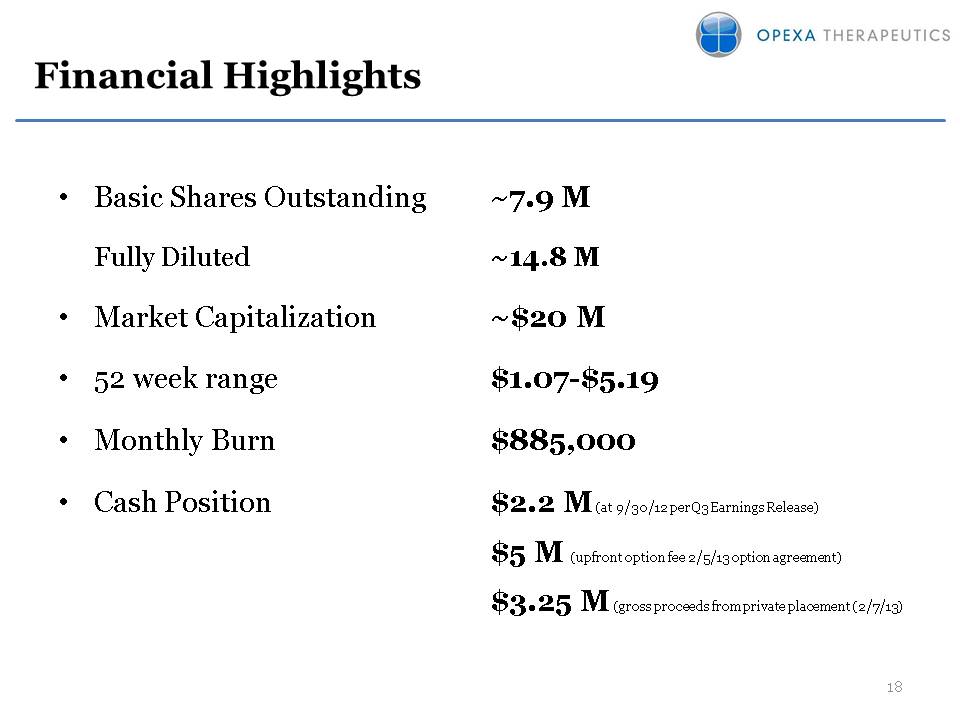
Financial Highlights Basic Shares Outstanding ~7.9 M Fully Diluted ~14.8 MMarket Capitalization ~$20 M52 week range $1.07-$5.19Monthly Burn $885,000Cash Position $2.2 M (at 9/30/12 per Q3 Earnings Release) $5 M (upfront option fee 2/5/13 option agreement) $3.25 M (gross proceeds from private placement (2/7/13)

Opexa Highlights Developing one of most promising therapies for Multiple SclerosisPersonalized, patient specific, with encouraging clinical efficacy and superior safety profileSecured option agreement for Tcelna in MS with leading global Pharma company (potential payments up to $225 M + royalties)Key rights retained by Opexa could generate future valueEndorsement highlights potential of Opexa’s platform in other disease areasOwnership of manufacturing platform enables tight control of costs and quality assurance

Opexa: Developing Novel Treatments for Cell Therapy
