Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - GALECTIN THERAPEUTICS INC | d477296d8k.htm |
 Corporate Summary
February 5, 2013
NASDAQ: GALT
Exhibit 99.1 |
 Forward Looking Statements
This presentation contains, in addition to historical information,
forward-looking statements within the meaning of the Private Securities
Litigation Reform Act of 1995. These statements relate to future events or
future financial performance, and use words such as “may,”
“estimate,”
“could,”
“expect”
and others. They are based on our current expectations
and are subject to factors and uncertainties which could cause actual results to
differ materially from those described in the statements. Factors that could
cause our actual performance to differ materially from those discussed in
the forward-looking statements include, among others: incurrence of
operating losses since our inception, uncertainty as to adequate financing
of our operations, extensive and costly regulatory oversight that could
restrict or prevent product commercialization, inability to achieve commercial
product acceptance, inability to protect our intellectual property,
dependence on strategic partnerships, product competition, and others stated
in risk factors contained in our SEC filings. We cannot assure that we have
identified all risks or that others may emerge which we do not anticipate.
You should not place undue reliance on forward-looking statements.
Although subsequent events may cause our views to change, we disclaim any
obligation to update forward-looking statements.
2
©
2013 Galectin Therapeutics
NASDAQ:GALT |
 Proprietary
Compounds
•
First in class, proprietary compounds that inhibit galectin proteins
•
Complex carbohydrate drugs with highly favorable safety profile
•
GM-CT-01: Enhanced ability of immune cells to kill of cancer cells
•
GR-MD-02: Potential to treat non-alcoholic steatohepatitis (NASH)
and other causes of liver fibrosis
Validated Science
•
Pre-clinical models show galectins are critical targets for intended
diseases with mechanisms that would be novel in the market
Large Market
Opportunities
•
Enhancing the ability of immune system to kill cancer cells is
synergistic with many current and experimental therapies
•
NASH and liver fibrosis indications would be first therapies for
completely unmet medical needs, representing a multi-billion
dollar market
Intellectual Property
•
Sole ownership, no licenses granted
•
GM-CT-01: Matter and methods granted (expire 2023)
•
GR-MD-02: Use in liver fibrosis (expire 2026); Matter pending
(priority of 2011)
Experienced
Management Team
•
Management team has collective experiencer in multiple biotech
and Pharmaceutical companies and relevant scientific areas
3
Investment Highlights
©
2013 Galectin Therapeutics
NASDAQ:GALT |
 Experienced Management Team
4
Peter G. Traber, MD
President, CEO, CMO
•
Over 25 years experience in biomedicine and pharmaceutical industries in research
and development, clinical medicine, management and leadership, and business
development. Medical expertise in liver disease
•
GlaxoSmithKline (CMO), Un of Pennsylvania (CEO), Baylor College of Medicine
(CEO) Harold H. Shlevin, PhD
COO
•
Over
25
years
of
senior
management
experience
in
the
development
and
commercialization of pharmaceuticals, diagnostics and vaccines
•
Solvay Pharmaceuticals (CEO), CIBA Vision Ophthalmics (co-founder), Tikvah
Therapeutics (Founder, CEO), Georgia Institute of Technology’s
Advanced Technology Development Center, Altea Therapeutics
Corporation Eliezer Zomer, PhD
EVP, Product
Development
•
Over
30
years
experience
in
biotechnology
engineering
and
regulatory
in
pharmaceuticals and
diagnostics.
•
Koor Biotechnologies, Charm Sciences, Glycogenesis, HU Medical School
(Jerusalem), Harvard University
Thomas A. McGauley
CFO (acting)
•
Over 10 years in accounting and finance with life science and technology
companies •
PricewaterhouseCoopers, Pro-Pharmaceuticals, deCode Genetics
Anatole Klyosov, PhD
Chief Scientist
•
Over 30 years’
experience in business and operations management for public and
private scientific, and biotech corporations and startup companies
•
eHealthDirect, Signatron, ArsDigita and Thermo Fibergen
J. Rex Horton
Executive Director,
Regulatory Affairs and
Quality Assurance
•
Over 20 years of experience working in the biotech and life sciences industries,
regulatory affairs and manufacturing.
•
Solvay Pharmaceuticals, Chelsea Therapeutics, Georgia Institute of
Technology. ©
2013 Galectin Therapeutics
NASDAQ:GALT |
 •
Science of Galectins
•
Galectin Function
•
Galectin Inhibitors
•
Intellectual Property
•
Immune Enhancement in Cancer Therapy
•
Mechanism of Action
•
Regulatory and Clinical Plan
•
Competitive Positioning
•
Liver Fibrosis
•
Mechanism of Action
•
Regulatory and Clinical Plan
•
Competitive Positioning
•
Milestones
5
©
2013 Galectin Therapeutics
NASDAQ:GALT |
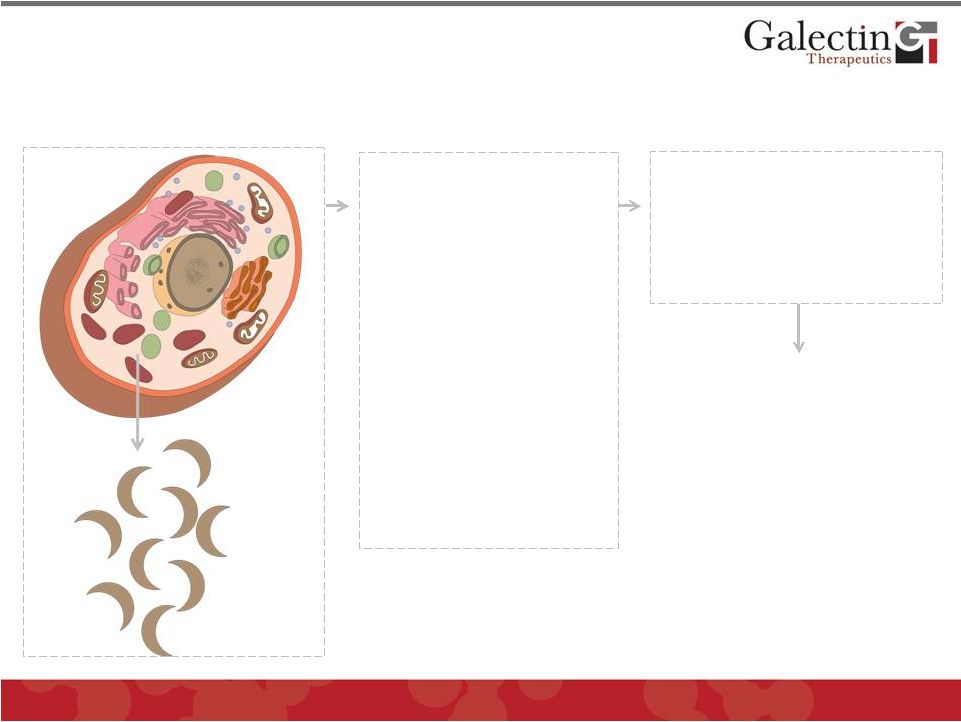 Galectin Proteins Are Critical Participants In
Pathogenesis of Many Fibrotic and Neoplastic
Diseases
6
Bind to cell surface
and matrix
glycoproteins
(galactose residues)
•
•
•
GALECTINS
PROMOTE
PATHOLOGY
Markedly Increased in:
1.
Inflammation
2.
Fibrosis
3.
Cancer
*Secreted in small amounts normally
by a number of cells, predominantly
macrophages
Galectin-3 is most
prominent galectin
secreted in disease
©
2013 Galectin Therapeutics
NASDAQ:GALT
Promote cell-
matrix interactions
Promote cell-cell
interactions
Modulate cell
signaling
Secreted
Galectin
Proteins* |
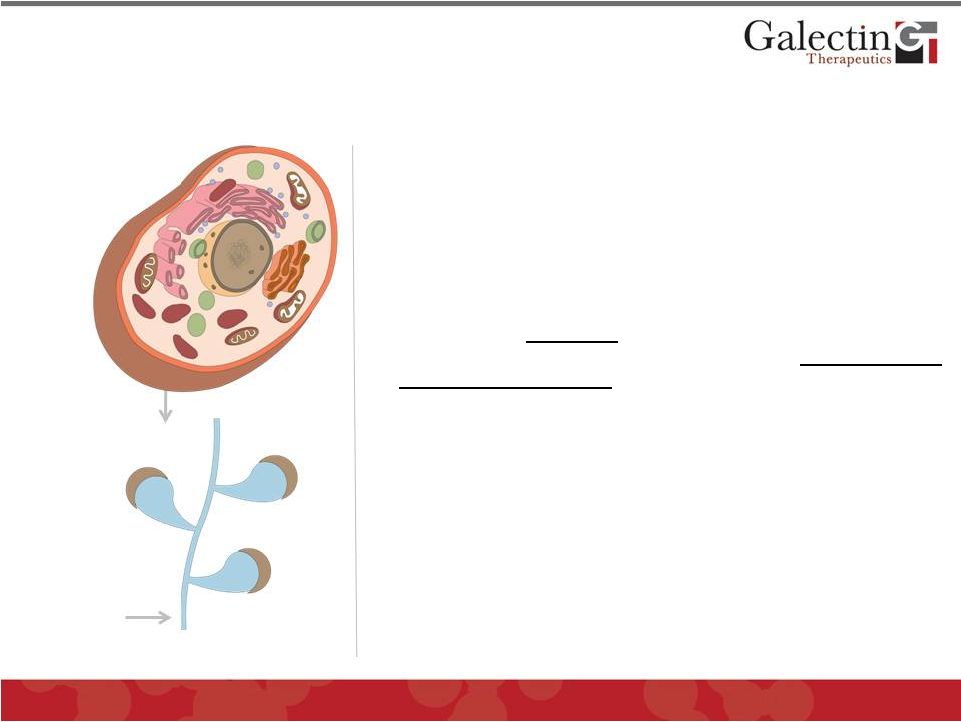 Galectin
Proteins
Galectin
Inhibitor
Galectin Inhibitors: A New Class of
Pathology Modulators
7
•
Novel complex carbohydrate drugs that target
secreted and membrane-associated galectins by
virtue of high molecular weight
•
Strongest binding to galectin-3, most prominent
galectin in disease processes
•
Binding to galectins disrupts function and
modulates multiple
cellular pathways in
pathology representing a potential new class of
therapeutic agents
•
Low toxicity potential as a carbohydrate
with no toxic metabolites
•
Two classes of compounds under development
•
GM-CT
•
GR-MD
•
Low manufacturing costs; abundant natural
plant product starting materials
©
2013 Galectin Therapeutics
NASDAQ:GALT |
 Intellectual Property
•
GM-CT Class (current NCE is GM-CT-01)
•
US Composition of matter patent Issued 2011 (expires 2023)
•
Five US issued method of use patents in combination with cancer therapy for
increased efficacy and reduced side effects
•
International Patents: 14 granted and 5 pending
•
Method of use in liver fibrosis issued 2012 (expires 2026)
•
Method of use in NASH patent pending (priority 2011)
•
GR-MD Class (current NCE is GR-MD-02)
•
Method of use in liver fibrosis patent issued (expires 2026)
•
Composition of matter patent pending (priority 2011)
•
Method of use in NASH patent pending (priority 2011)
•
All intellectual property generated in house with no encumbrances
•
No established generic pathway for such complex carbohydrate drugs
8
©
2013 Galectin Therapeutics
NASDAQ:GALT |
 •
Science of Galectins
•
Galectin Function
•
Galectin Inhibitors
•
Intellectual Property
•
Immune Enhancement in Cancer Therapy
•
Mechanism of Action
•
Regulatory and Clinical Plan
•
Competitive Positioning
•
Liver Fibrosis
•
Mechanism of Action
•
Regulatory and Clinical Plan
•
Competitive Positioning
•
Milestones
9
©
2013 Galectin Therapeutics
NASDAQ:GALT |
 The
Vast Majority of Cancers Secrete Large Amounts of Galectins Which Have Multiple
Roles in Tumor Pathogenesis
•
Tumor cell invasion:
extracellular matrix
adhesion & detachment
•
Metastasis:
cell invasion and migration
•
Angiogenesis
•
Tumor immunity has
recently been shown to be
critically affected by
galectins
10
The “Galectin Effect”
protects tumors from
immune system
©
2013 Galectin Therapeutics
NASDAQ:GALT |
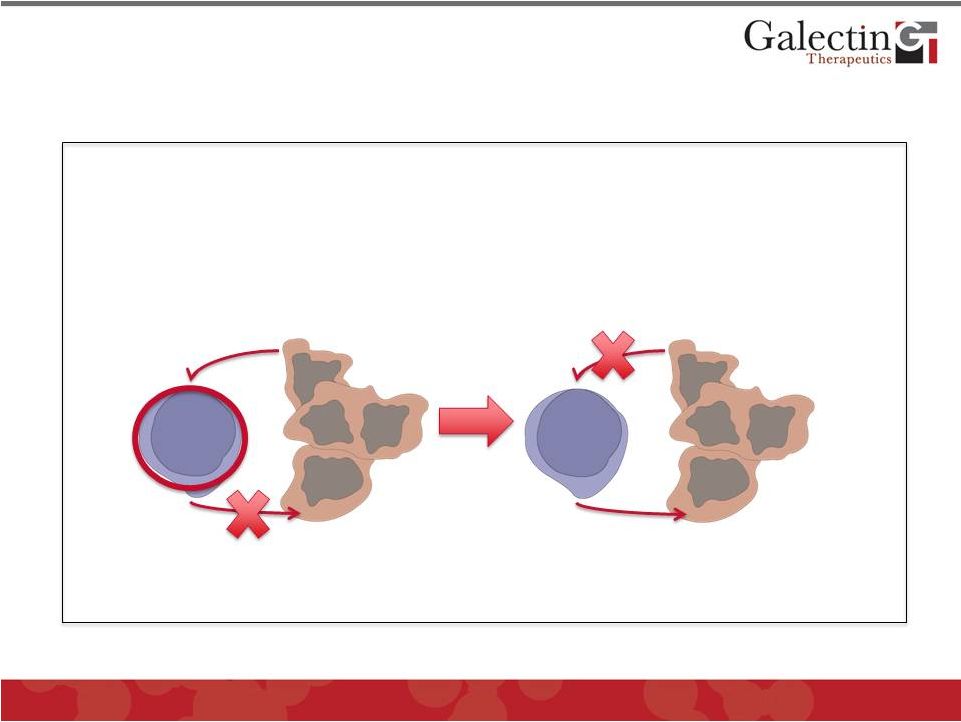 11
Cytokines
Galectin-3
Cytokines
Kill tumor cells
GM-CT-01
Galectin-3 secreted by
tumor cells binds to surface
of T-cells and inhibits
cytokine secretion
Treatment with GM-CT-01
blocks Galectin-3 and
restores T-cell cytokine
secretion and tumor killing
Experiments performed by Dr. Pierre van der Bruggen of the Ludwig Institute in
Brussels, Belgium in collaboration with Galectin Therapeutics
Tumors Evade the Immune System Using the
“Galectin Effect”
and GM-CT-01 Reverses This Effect
©
2013 Galectin Therapeutics
NASDAQ:GALT
Tumor
Cells
T-Cells
T-Cells
Tumor
Cells |
 GM-CT-01
Has
Demonstrated
Safety
in
over
100
Human Subjects in Phase I and Partially Completed
Phase II Clinical Trials with Some Evidence of Efficacy
•
Phase I trial (DAVFU-001) in 40 subjects with end stage cancer showed
GM- CT-01 was safe alone and in combination with the chemotherapy
5-FU •
Three Phase II trials were conducted, but only partially completed
•
One Phase II trial (DAVFU-001) of 5-FU plus GM-CT-01 in line 3/4
therapy of metastatic colorectal cancer showed 6.7 months median
survival. In similar patients, Erbitux
®*
had a 6.1 month survival compared to 4.6 months
with no therapy
•
When
the
data
from
the
three
partially
completed
Phase
II
trials
were
pooled, the serious adverse events associated with 5-FU
were reduced when compared to historical controls
•
Our preclinical efficacy and clinical safety data were strong enough to obtain an
IMPD for Phase I/II trial in metastatic melanoma with a combination of
a tumor vaccine and GM-CT-01 to test the efficacy of blocking the
“Galectin Effect” [Study not being conducted under FDA IND, but
there is open IND for GM-CT-01 12
*“Erbitux®
is a registered trademark of ImClone LLC, a wholly-owned subsidiary of Eli
Lilly and Company.“ ©
2013 Galectin Therapeutics
NASDAQ:GALT |
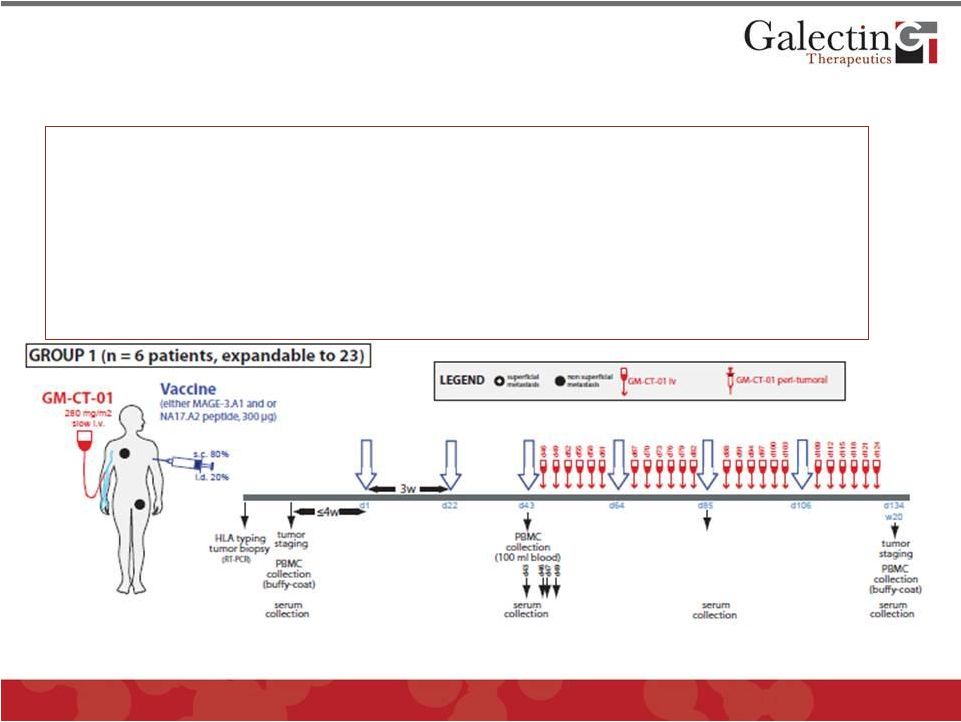 Preclinical efficacy and clinical safety data sufficient to
obtain an IMPD for treatment of metastatic melanoma to test
the efficacy of blocking the “Galectin Effect”
13
Melanoma “Proof of Concept”
Trial:
Patients:
Advanced metastatic melanoma
Design:
Two Stage (6 X 2 cohorts in stage 1 and 23 X 2
cohorts in stage 2)
Regimen:
Prime with melanoma specific peptide vaccine
Treat with GM-CT-01 to block “Galectin Effect”
Endpoint:
Partial or complete response by imaging
Group 2 patients have additional injection of GM-CT-01 in cutaneous
tumors ©
2013 Galectin Therapeutics
NASDAQ:GALT |
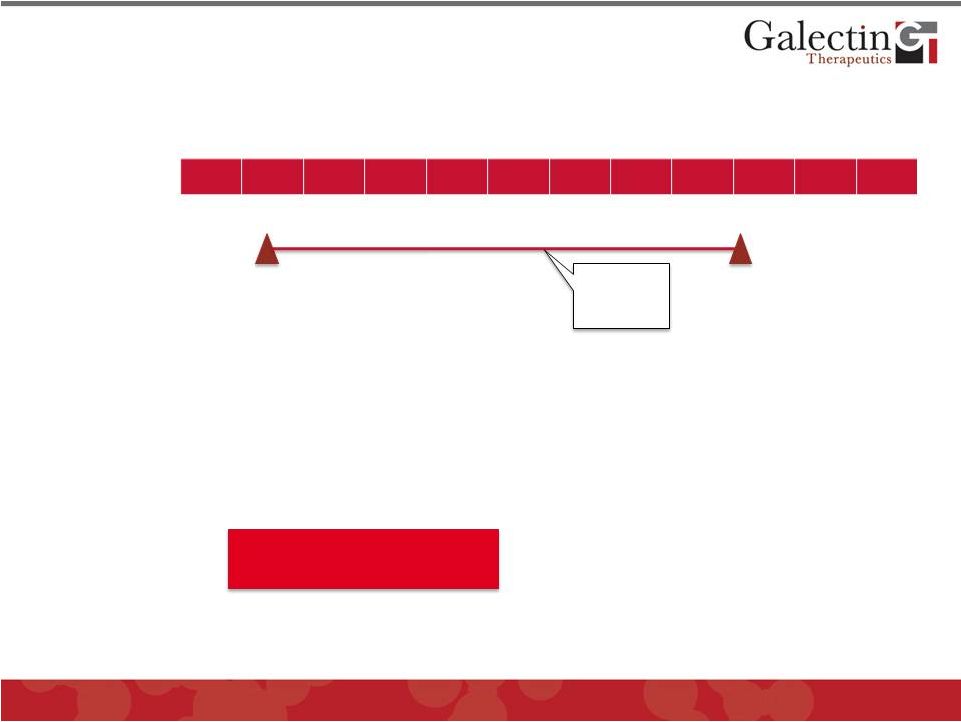 Tumor
Immune Enhancement Development Program 14
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
2012
2013
2014
GM-CT-01
Phase I/II Melanoma Trial*
Pursue Partnering
Discussions
*Conducted in Belgium under an IMPD. Not conducted under FDA IND, but there
is an open IND for GM-CT-01 At conclusion of Stage 1 of study:
•
•
•
•
©
2013 Galectin Therapeutics
NASDAQ:GALT
Stage 2 TBD
Top line
results in
Stage 1
Continue to Stage 2 with some
efficacy
Consider Phase IIb controlled trial
with strong efficacy
Seek partnership with company with
marketed or late stage cancer
immunotherapy
Stop for lack of efficacy |
 Immune Enhancement by Blocking “Galectin
Effect”
is Synergistic With Many Emerging
Cancer Immunotherapies
•
Enhancing
the
ability
of
the
immune
system
to
recognize
and
kill
tumor
cells is a very active area in the personalized approach to cancer therapy.
The “Galectin Effect”
inhibits the immune system
•
Two agents have been approved for use to date
•
Dendritic cell vaccine: Provenge®
(Dendreon)
•
T-cell activator (CTLA4 receptor mAb): Yervoy®
(Ipilimumab, BMS)
•
Many more vaccines and activators in development
•
Our drugs reverse the “Galectin Effect”
by which tumors inhibit the
immune system and may be synergistic
with all tumor immunotherapies.
•
While
tumor
vaccines
are
patient
and
tumor
specific,
reversal
of
the
“Galectin Effect”
appears to be universal. Initial Phase I/II clinical trial in
Belgium in combination with a vaccine to treat metastatic melanoma
•
The tumor vaccine market is forecast to be over $7 billion by 2015
15
©
2013 Galectin Therapeutics
NASDAQ:GALT
May be effective with unaltered immune system |
 Best
Positioned to Advance Tumor Immunotherapy with Galectin Inhibitor
•
Market for tumor vaccines is expected to grow to $7B by 2015. If
ipilimumab (Yervoy ®, BMS) is included, market is even larger
•
Blocking the “Galectin Effect”
would be synergistic with all types of tumor
vaccines or immune stimulatory approaches
•
In this regard, competition will come from other galectin-inhibitors
•
Galecto Biotech AG (Sweden): Discovery phase focusing on small molecule
inhibitors. Development program focused on lung fibrosis.
•
LaJolla Pharmaceuticals (CA): In Jan. 2012, they purchased GCS-100 from
Solana Therapeutics (formally Prospect Therapeutics, formally
Glycogenesis). GCS-100 is a natural product compound with claims
for binding galectins; focused on blood cancers; significant side effects
reported. Progressing in development for cancer and kidney fibrosis.
•
Mandel Med (Oakland, CA): Truncated galectin-3 protein. Not
progressed into human trials and no active program currently.
•
Galectin Therapeutics is best positioned with a human trial in cancer
immunotherapy; GM-CT-01 has proved safe in Phase I and three partially
completed Phase II trials.
16
©
2013 Galectin Therapeutics
NASDAQ:GALT |
 •
Science of Galectins
•
Galectin Function
•
Galectin Inhibitors
•
Intellectual Property
•
Immune Enhancement in Cancer Therapy
•
Mechanism of Action
•
Regulatory and Clinical Plan
•
Competitive Positioning
•
Liver Fibrosis
•
Mechanism of Action
•
Regulatory and Clinical Plan
•
Competitive Positioning
•
Milestones
17
©
2013 Galectin Therapeutics
NASDAQ:GALT |
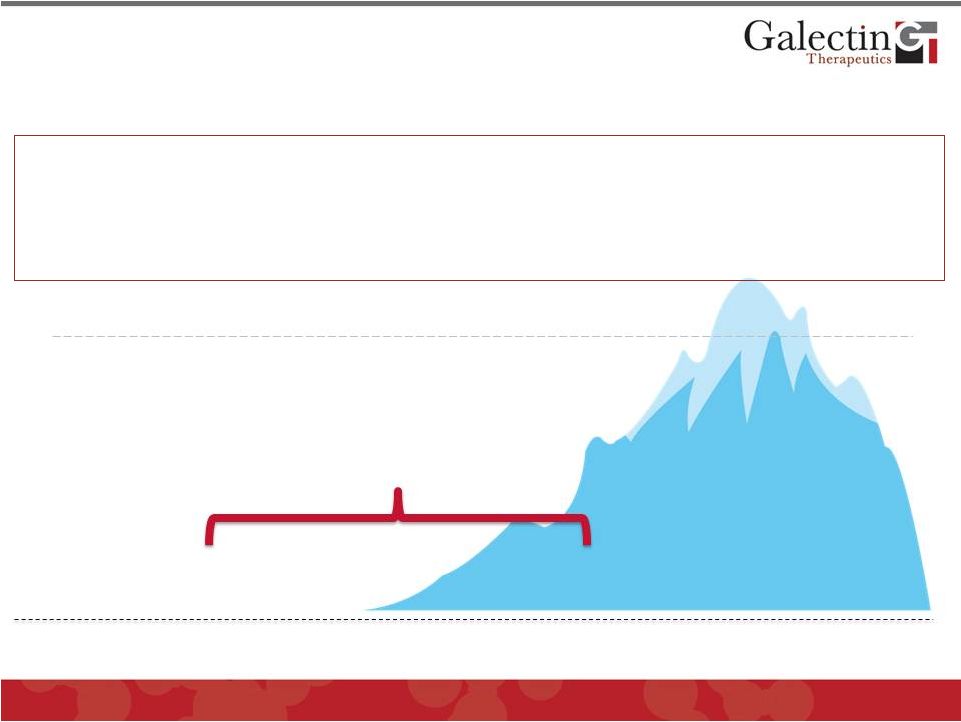 NASH
and Liver Fibrosis are Multi- Billion Dollar Markets In US Alone
Transplants
(6,291*)
Wait List
(17,000**)
Death From Cirrhosis
(44,677
#
)
Cirrhosis
(400,000
##
)
NASH: 9-15 Million
&
* Performed in US in 2010 (UNOS)
* * Prevalence in US 2010 (UNOS)
18
#
Deaths in 1998 (AASLD Workshop, 2001)
##
Prevalence in US 1976-1980 (NIDDK)
Hepatitis C, Hepatitis B, Alcohol
&
Prevalence in US 2011 (NIH)
•
The ONLY current therapy for advanced fibrosis (cirrhosis) is liver
transplantation •
No approved medical therapy for fibrosis
•
While there are treatments for some underlying etiologies (Hepatitis C and B), there
is no approved therapy for NASH
©
2013 Galectin Therapeutics
NASDAQ:GALT |
 Galectin-3 Is A Critical Protein Target
For Therapy of Liver Fibrosis
Galectin-3 is produced in large amounts by fibrotic liver (animal and
human)
Galectin-3 is essential in mice for the development of liver fibrosis
Fibrosis
due
to
toxin
exposure
or
fatty
liver
does
not
occur
in
mice that lack the galectin-3 gene
Galectin inhibitors reverse experimental fibrosis in rats induced by
both fibrosis and fatty liver
19
Key Evidence:
Key Evidence:
©
2013 Galectin Therapeutics
NASDAQ:GALT |
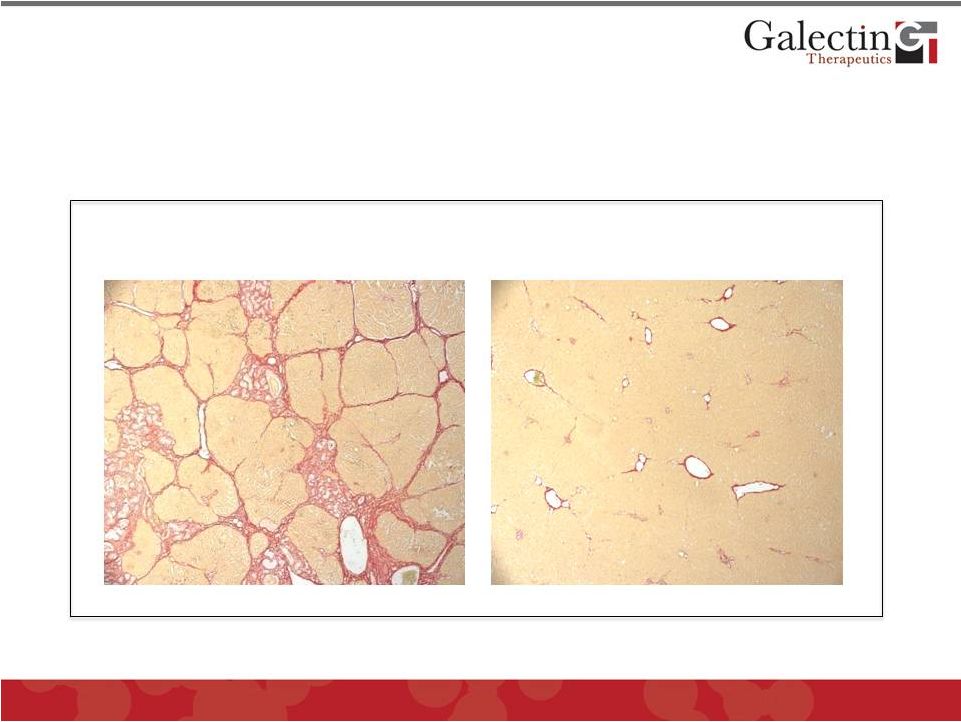 Galectin Inhibitor GR-MD-02 Effectively
Treats Toxin-Induced Liver Fibrosis in Rats
Treatment with vehicle alone for
four weeks (Control) shows
robust fibrosis
Treatment with GR-MD-02 for four
weeks shows dramatic regression
of fibrosis
20
Galectin Therapeutics Data
Liver fibrosis induced in all rats by injection of chemical
toxin (thioacetimide) for 8 weeks
©
2013 Galectin Therapeutics
NASDAQ:GALT |
 21
©
2013 Galectin Therapeutics
NASDAQ:GALT |
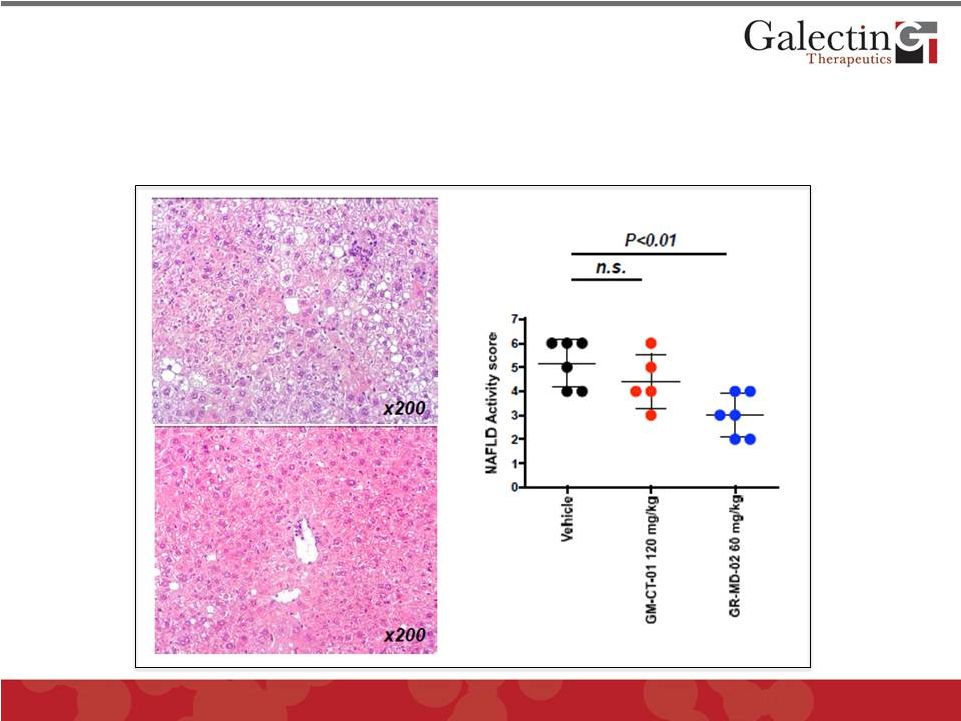 GR-MD-02 Markedly Improved NASH (Non-Alcoholic
Steatohepatitis) in a Mouse Model
Vehicle
GR-MD-02
NASH was induced in mice by rendering them diabetic and
feeding them a high fat diet
©
2013 Galectin Therapeutics
NASDAQ:GALT |
 GR-MD-02 Prevented and Completely Reversed
Fibrosis in NASH Mouse Model
23
Early Treatment
Late Treatment
normal
mouse 0.33
©
2013 Galectin Therapeutics
NASDAQ:GALT |
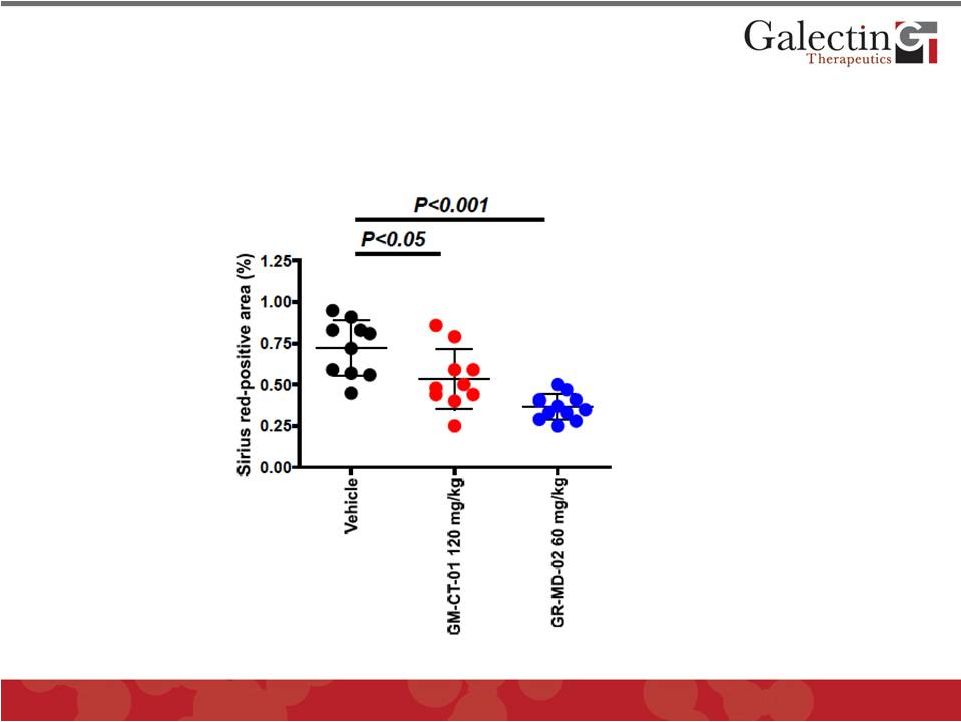 Early
and Late Therapy Compiled Data ©
2013 Galectin Therapeutics
NASDAQ:GALT
24 |
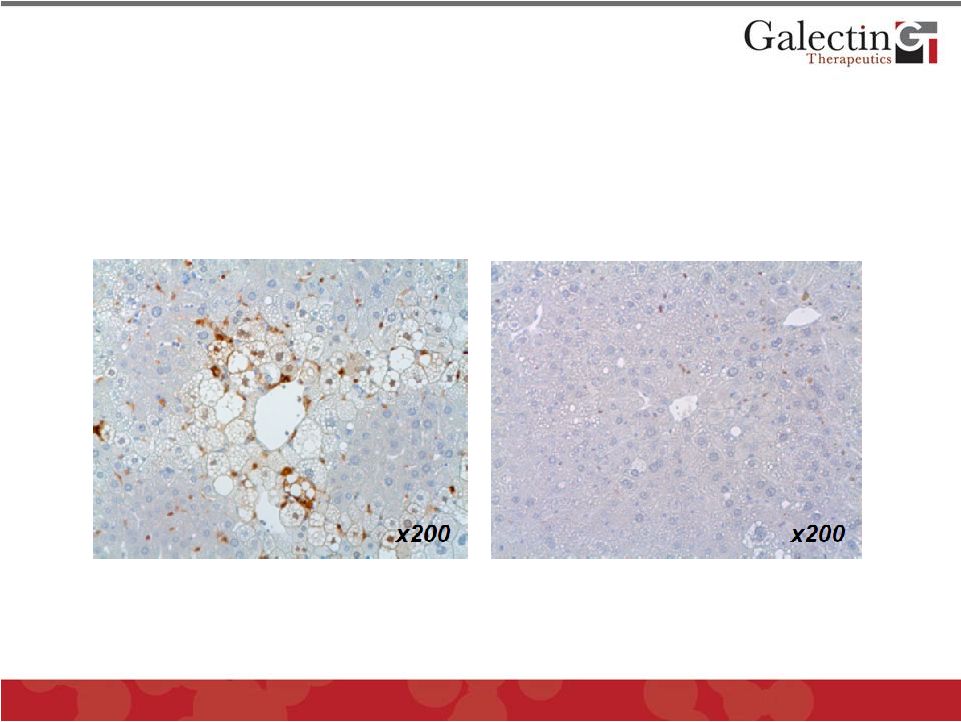 Treatment with GR-MD-02 Markedly
Reduces Galectin-3 in NASH Mice
25
Vehicle
GR-MD-02
Immunohistochemistry for detection of Galectin-3
©
2013 Galectin Therapeutics
NASDAQ:GALT |
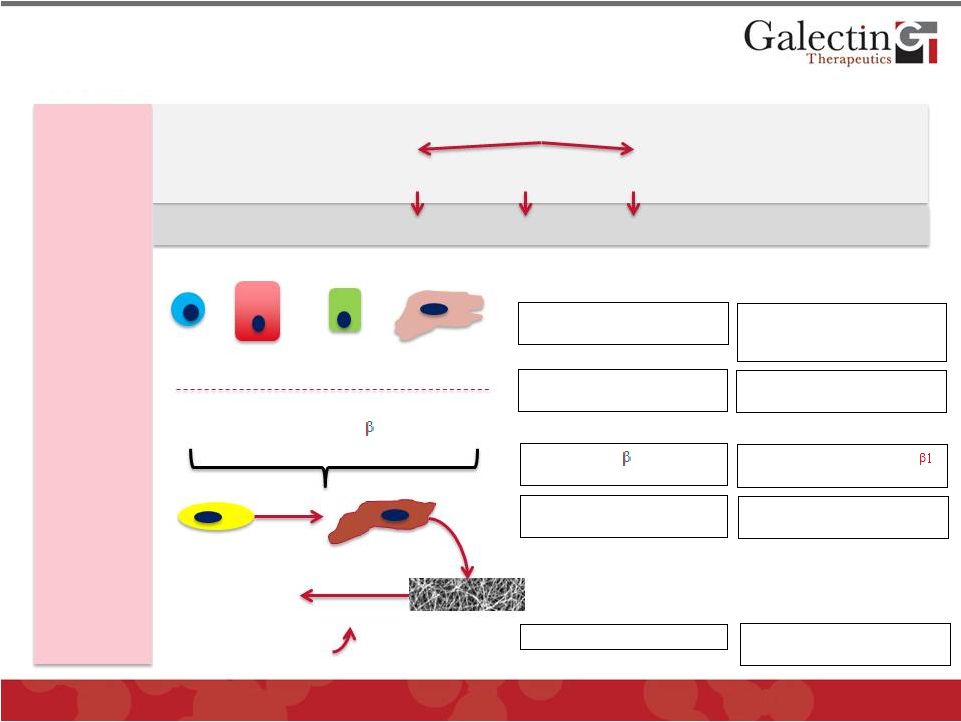 Inhibition of Gal-3 May Have Multiple Sites
of Action in Therapy of NASH
26
Extracellular
Matrix
Cytokines
ECM Dissolution
MMPs
Inflammation
ceases
Stellate Cells
Macrophages
Gal-3
Gal-3
Gal-3
Gal-3
Stellate Cell
Release of multiple inflammatory
cytokines,
including
TGF-
1,
a
critical
mediator of fibrosis
Quiescent
Activated
Hepatocyte
Macrophage
T-Cell
Bile Duct Cell
Lipo-peroxidation Products
Scavenger receptor increases
cellular uptake of toxic products
Inhibit scavenger mechanism
and reduce cellular toxicity
Galectin
GR-MD-02
Increases
TGF-
receptor
signaling
Reduce
cell
membrane
TGF-
receptor activity
Promotes activation of stellate
cells to myofibroblasts
Reduces activation of stellate
cells
? Effect on ECM dissolution;
Macrophages
©
2013 Galectin Therapeutics
NASDAQ:GALT
Gal-3 expression and activation
of macrophages
Reduces Gal-3 and activation of
macrophages; changes in
macrophage subtypes?
Hyperglycemia
Adipocytokines
Free Fatty Acids
Metabolic Syndrome
Glucose
Intolerance
Fat
Accumulation
Impaired Lipid
Metabolism
Cause of
Liver Injury
Mediators
Inflammation
Fibrosis
Resolution
1 |
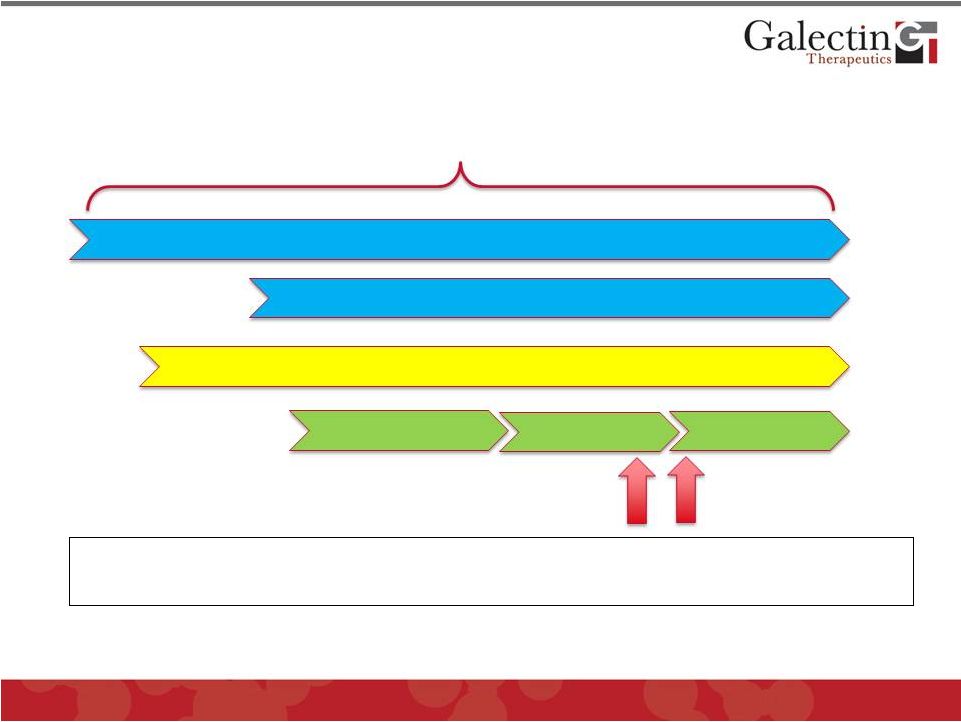 Targeting Anti-Galectin Therapy In The
Progression of NASH
27
Decades
Because of effect on inflammation in NASH and ability to reduce existing
fibrosis,
our
clinical
program
will
target
NASH
patients
with
advanced
fibrosis
©
2013 Galectin Therapeutics
NASDAQ:GALT
Development of Obesity
Insulin Resistance/Diabetes
Steatosis (fatty liver)
NASH
Fibrosis
Cirrhosis |
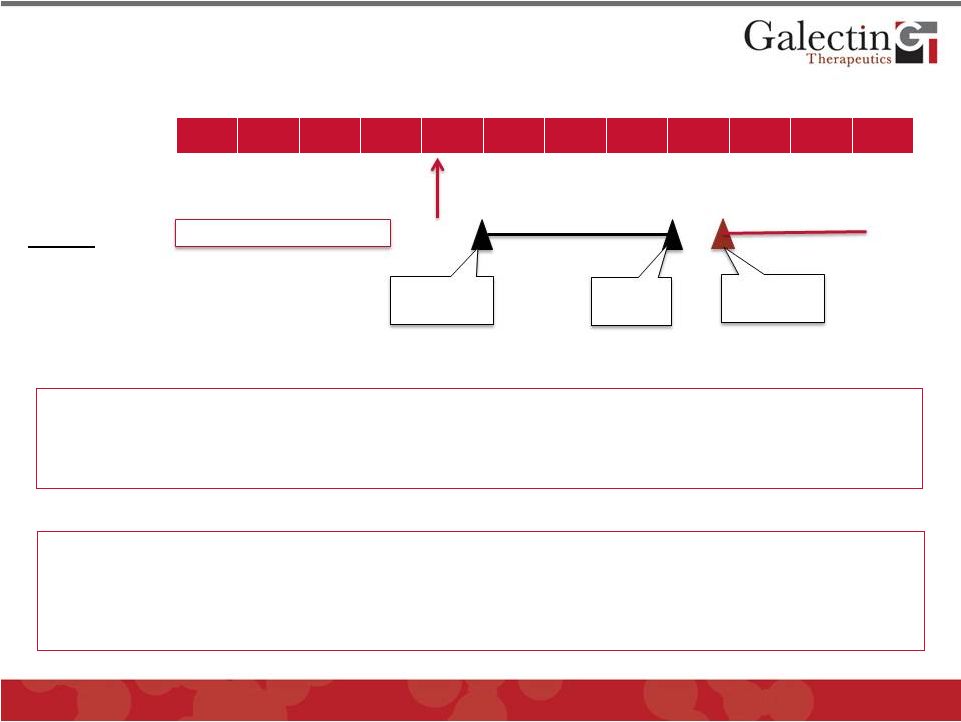 28
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
2012
2013
2014
Phase 1 NASH trial
NASH
NASH Development Program: GR-MD-02
Phase 2 NASH Trial
File Fast Track Designation to be filed; Breakthrough Designation following Phase
1 IND
Submitted
Phase 1 Trial:
Patient
Inclusion:
Phase 2 Trial:
Patient Inclusion:
Biopsy proven NASH with advanced fibrosis
©
2013 Galectin Therapeutics
NASDAQ:GALT
Timelines on final design
First patient
enrolled
Top line
results
First patient
enrolled
Pre-Clinical Completed
Serum
biomarkers
to
assess
for
pharmacodynamic
effect
Patient
safety
Four
weekly
IV
doses
per
cohort
with
escalation
to
target
dose
Biopsy
proven
NASH
with
advanced
fibrosis
Design
Primary
Endpoint
Secondary
Endpoints
Secondary
Endpoints
Safety;
Serum
biomarkers,
MR-fat
and
elastography
Primary Endpoint
Liver biopsy evaluated for percent area collagen
Design
Randomized, controlled, double blinded study with at least six
months of therapy
:
:
:
:
:
: |
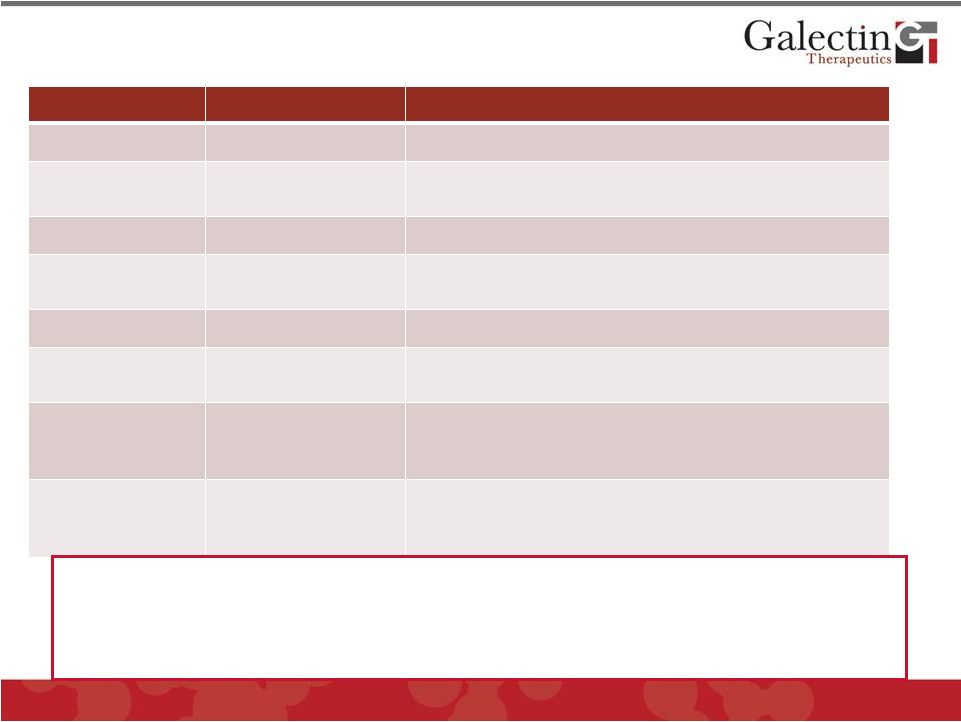 Examples of Therapeutic Approaches Under
Investigation for Therapy of NASH
General Mechanism
Examples
Comments
Obesity
•
Lifestyle, dieting
•
Can be beneficial; not effective in advanced NASH
Treat Diabetes and
Insulin Resistance
•
Pioglitazone
•
Failed to achieve significant endpoints in phase 2 and
phase 3 clinical trials, but some evidence of effect
Lipid Metabolism
•
Aramchol & others
•
Cholesterol inhibition, no clinical results
Modulate the
Immune System
•
EGS21 (Enzo)
•
Pentoxifylline
•
Abandoned after phase 2 trial
•
Non significant phase 3 results
Protease Inhibition
•
GS-9450 (Gilead)
•
Liver toxicity in phase 2: abandoned
Bile salt metabolism
•
Colesevelam
•
Obeticolic acid
•
Intestinal bile salt binder, Phase 2 trial failed
•
FXR agonist; Phase 2 coming to conclusion
Anti-Oxidant / toxin
metabolism
•
Vitamin E
•
MND-21 (Mochida)
•
Cysteamine
•
Effective in NASH score in phase 3, but not fibrosis
•
Omega-3 fatty acid (phase 2 trial)
•
Phase 2 trial in adolescents underway
Anti-collagen cross-
linking
•
Lysyl oxidase-like-2
mAb (GS-6624,
Gilead)
•
Initiated Phase 2 trials in 2012 in patients with NASH and
fibrosis, top line data Q3 2015.
29
•
GR-MD-02 is well positioned with respect to competition
•
Most attractive mechanism: multiple sites of action in disease
•
Independent of hyperglycemia or hyperlipidemia
•
May reverse established fibrosis
•
Low toxicity potential as a carbohydrate with no toxic metabolites
©
2013 Galectin Therapeutics
NASDAQ:GALT |
 Science of Galectins
Galectin Function
Galectin Inhibitors
Intellectual Property
Immune Enhancement in Cancer Therapy
Mechanism of Action
Regulatory and Clinical Plan
Competitive Positioning
Liver Fibrosis
Mechanism of Action
Regulatory and Clinical Plan
Competitive Positioning
Milestones
30
©
2013 Galectin Therapeutics
NASDAQ:GALT |
 Key
Company Milestones: 12 months •
Cancer
•
Phase I/II (IIa) metastatic melanoma trial, first patient infused 5/12
•
Five patients currently enrolled; stage 1 data likely available in first
half of 2013.
•
NASH with Advanced Fibrosis (Fatty Liver Disease)
•
1/30/13: IND Submitted to FDA
•
3-4/13: Initiate Phase 1 NASH trial
•
1/14: Phase 1 NASH trial results
•
Q1 2014: Initiate Phase 2 NASH trial
•
Top line data from Phase 2 trial TBD based on design
31
©
2013 Galectin Therapeutics
NASDAQ:GALT |
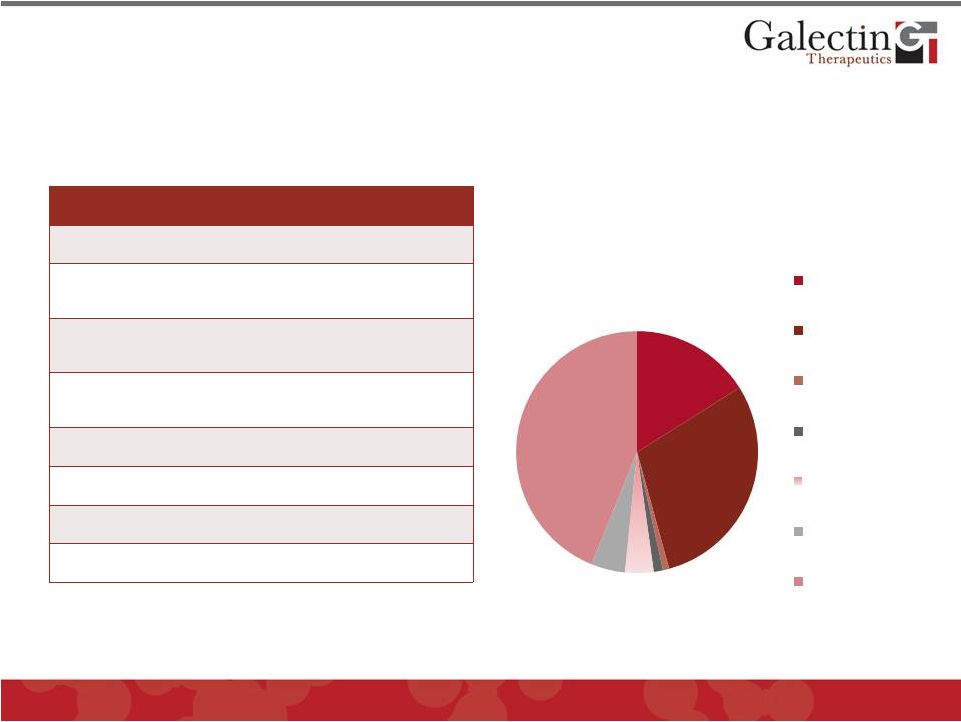 Finances & Capitalization
Common Stock
Shares (9/30/12)
Common Shares
15,966,437
Preferred Series A
(converted)
260,430
Preferred: Series B
(converted)
2,000,000
Preferred: Series C
(converted)
366,680
Warrants: Series B*
5,000,000
Other Warrants**
2,424,241
Options Outstanding***
3,541,630
Total Outstanding
29,559,418
32
* Exercise Price: $3.00 (all controlled by 10X Fund)
** Weighted Average Exercise Price: $4.97
Cash on Hand: $11.1 million (at 9/30/12)
Funded to Jan 2014 (based on current planned R&D)
*** Weighted Average Exercise Price: $5.88
©
2013 Galectin Therapeutics
NASDAQ:GALT
Total Outstanding
Management,
Officers and
Directors
10X Fund
(includes Series
B Shares)
Series A Shares
Series C Shares
Other Warrants
and Options
Publicly Traded
Warrants
Public Float |
 Proprietary
Compounds
•
First in class, proprietary compounds that inhibit galectin proteins
•
Complex carbohydrate drugs with highly favorable safety profile
•
GM-CT-01: Enhanced ability of immune cells to kill of cancer cells
•
GR-MD-02: Potential to treat non-alcoholic steatohepatitis (NASH)
and other causes of liver fibrosis
Validated Science
•
Pre-clinical models show galectins are critical targets for intended
diseases with mechanisms that would be novel in the market
Large Market
Opportunities
•
Enhancing the ability of immune system to kill cancer cells is
synergistic with many current and experimental therapies
•
NASH and liver fibrosis indications would be first therapies for
completely unmet medical needs, representing a multi-billion
dollar market
Intellectual Property
•
Sole ownership, no licenses granted
•
GM-CT-01: Matter and methods granted (expire 2023)
•
GR-MD-02: Use in liver fibrosis (expire 2026); Matter pending
(priority of 2011)
Experienced
Management Team
•
Management team has collective experiencer in multiple biotech
and Pharmaceutical companies and relevant scientific areas
33
Investment Highlights
©
2013 Galectin Therapeutics
NASDAQ:GALT |
