Attached files
| file | filename |
|---|---|
| EX-23.1 - CONSENT - HARROW HEALTH, INC. | immy_ex231.htm |
As filed with the Securities and Exchange Commission on February 4, 2013
No. 333-182846
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
___________________
Amendment No. 5
Amendment No. 5
to
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
___________________________
IMPRIMIS PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
___________________________
|
Delaware
|
2834
|
45-0567010
|
||
|
(State or other jurisdiction of
incorporation or organization)
|
(Primary Standard Industrial
Classification Code Number)
|
(I.R.S. Employer
Identification Number)
|
437 S. Hwy 101, Suite 209
Solana Beach, CA 92075
(858) 433-2800
(Address, including zip code, and telephone number, including
area code, of registrant’s principal executive offices)
___________________________
Mark L. Baum
Chief Executive Officer
437 S. Hwy 101, Suite 209
Solana Beach, CA 92075
(858) 433-2800
(Name, address, including zip code, and telephone number, including
area code, of agent for service)
___________________________Copies to:
|
Steven G. Rowles, Esq.
Jeannette V. Filippone, Esq.
Morrison & Foerster LLP
12531 High Bluff Drive, Suite 100
San Diego, California 92130
Tel: (858) 720-5100
Fax: (858) 720-5125
|
Kevin Friedmann, Esq.
|
|
Marc A. Jones, Esq.
|
|
|
Richardson & Patel LLP
|
|
|
750 Third Avenue, 9th Floor
|
|
|
New York, New York 10017
|
|
|
Tel: (212) 561-5559
|
|
|
Fax: (917) 591-6898
|
Approximate date of commencement of proposed sale to the public: As soon as possible after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer o
|
Accelerated filer o
|
|
Non-accelerated filer o (Do not check if a smaller reporting company)
|
Smaller reporting company þ
|
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED FEBRUARY 4, 2013
IMPRIMIS PHARMACEUTICALS, INC.
PRELIMINARY PROSPECTUS
1,619,047 Shares of Common Stock
___________________
We are offering 1,619,047 shares of our common stock in a firm commitment offering, which share number reflects our proposed one-for-five reverse stock split described further in this prospectus. After the effectiveness of the registration statement of which this prospectus is a part and concurrently with the pricing of this offering, we will effect a one-for-five reverse stock split.
Our common stock is quoted on the OTC Markets Group’s OTCQB tier under the symbol “IMMY”. On February 1, 2013, the closing price of our common stock on the OTCQB was $8.25 per share (assuming a one-for-five reverse stock split). We have applied for listing of our common stock on The NASDAQ Capital Market, which listing we expect to occur at the consummation of this offering. No assurance can be given that our application will be approved. If the application is not approved, we will not commence this offering and the shares of our common stock will continue to be traded on the OTC Markets Group’s OTCQB tier.
___________________
Investing in our common stock involves a high degree of risk. Before making any investment in our common stock, you should read and carefully consider the risks described in this prospectus under “Risk Factors” beginning on page 8 of this prospectus.
You should rely only on the information contained in this prospectus or any prospectus supplement or amendment thereto. We have not authorized anyone to provide you with different information.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
Per Share
|
Total
|
|||||||
|
Initial price to public
|
$ | 5.25 | $ | 8,499,997 | ||||
|
Underwriting discount
|
$ | 0.4463 | $ | 722,500 | ||||
|
Proceeds, before expenses, to Imprimis Pharmaceuticals(1)
|
$ | 4.8037 | $ | 7,777,497 | ||||
| (1) | Does not include a non-accountable expense amount of $150,000 payable to the underwriters and up to $10,000 in accountable expenses of the underwriters. See "Underwriting" for a description of compensation payable to the underwriters. |
To the extent that the underwriters sell more than 1,619,047 shares of common stock, the underwriters have the option to purchase up to an additional 242,857 shares from us at the initial price to the public less the underwriting discount (assuming a one-for-five reverse stock split).
The underwriters expect to deliver the shares on or about , 2013.
___________________
| MDB Capital Group LLC | Aegis Capital Corp |
This prospectus is dated , 2013
TABLE OF CONTENTS
|
Page
|
||||
|
PROSPECTUS SUMMARY
|
1 | |||
|
RISK FACTORS
|
8 | |||
|
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
20 | |||
|
USE OF PROCEEDS
|
21 | |||
|
DESCRIPTION OF CAPITAL STOCK
|
22 | |||
|
CAPITALIZATION
|
25 | |||
|
DILUTION
|
27 | |||
| UNDERWRITING | 29 | |||
|
MARKET PRICE OF AND DIVIDENDS ON COMMON STOCK AND RELATED MATTERS
|
33 | |||
| SHARES AVAILABLE FOR FUTURE SALE | 34 | |||
| MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 37 | |||
|
DESCRIPTION OF THE BUSINESS
|
48 | |||
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
58 | |||
|
EXECUTIVE COMPENSATION
|
61 | |||
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
|
67 | |||
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
68 | |||
|
LEGAL MATTERS
|
69 | |||
|
EXPERTS
|
69 | |||
|
WHERE YOU CAN FIND MORE INFORMATION
|
69 | |||
|
FINANCIAL STATEMENTS
|
F-1 | |||
About This Prospectus
You should rely only on the information that we have provided or incorporated by reference in this prospectus or any prospectus supplement. We have not authorized anyone to provide you with different information. No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus or any prospectus supplement. You must not rely on any unauthorized information or representation. This prospectus is an offer to sell only the securities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. You should assume that the information in this prospectus or any prospectus supplement is accurate only as of the date on the front of the document and that any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus or any prospectus supplement, or any sale of a security.
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find Additional Information.”
We have pending trademark applications for Imprimis Pharmaceuticals, Accudel, Impracor and Generecycle. All other trademarks, tradenames and service marks included in this prospectus or any related prospectus supplement, are the property of their respective owners.
- i -
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus and does not contain all of the information that should be considered before investing in our common stock. Investors should read the entire prospectus carefully, including the more detailed information regarding our business, the risks of purchasing our common stock discussed in this prospectus under “Risk Factors” beginning on page 8 of this prospectus, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” beginning on page 37 of this prospectus, and our consolidated financial statements and the accompanying notes beginning on page F-1 of this prospectus.
As used in this prospectus, unless the context requires otherwise, the “Company”, “we”, “us”, and “our” refer to Imprimis Pharmaceuticals, Inc., a Delaware corporation.
Our Company
We are a specialty pharmaceutical company developing non-invasive, topically delivered products. Our innovative patented Accudel cream formulation technology is designed to enable highly targeted site specific treatment. Impracor, our lead pain product candidate, utilizes the Accudel platform technology to deliver the active drug, ketoprofen, a non-steroidal anti-inflammatory drug, through the skin directly into the underlying tissues where the drug exerts its localized anti-inflammatory and analgesic effects.
Through our strategic relationship with Professional Compounding Centers of America, Inc. (“PCCA”), one of the largest drug compounding organizations in the world, we expect to facilitate our future selection, formulation and development of potential product candidates. Our relationship with PCCA is exclusive and provides us with the opportunity to develop new products using PCCA’s proprietary drug formulations and drug delivery technologies, as well as access to an extensive database of market-oriented information related to specific drug development candidates. We plan to use our proprietary Accudel drug delivery technology, coupled with these licensed technologies, formulations and market data, to identify pharmaceutical development opportunities where there is a significant unmet need for a new drug product. We are currently considering potential product candidates in the muscle relaxant and neuropathic pain fields, and we expect those areas may be our next avenues for additional product development.
To better enable us to analyze the myriad of PCCA and PCCA-related development opportunities, we have designed a comprehensive drug development selection methodology. In determining the viability of a potential opportunity, we group all development candidates according to delivery modality and health category. We review intellectual property related to a respective opportunity, as well as manufacturing considerations, various market-related criterion and other clinical development issues. We believe that our review process will aid us in identifying viable development candidates and assist us in determining whether to internally develop a program or seek an appropriate commercialization partner.
Our common stock has been quoted in the over-the-counter market since March 2007 and is currently quoted on OTC Markets Group’s OTCQB tier under the symbol “IMMY”. Our executive offices are located at 437 S. Hwy 101, Suite 209, Solana Beach, CA 92075 and our telephone number is (858) 433-2800. Our website address is www.imprimispharma.com. Information contained on our website is not deemed part of this prospectus.
Reverse Stock Split and NASDAQ Listing Application
On April 25, 2012, our Board of Directors (the “Board of Directors” or the “Board”) and stockholders holding a majority of our outstanding voting power approved a resolution authorizing our Board of Directors to effect a reverse split of our common stock at an exchange ratio of (i) one-for-three, (ii) one-for-four, (iii) one-for-five, or (iv) one-for-six, with our Board of Directors retaining the discretion as to whether to implement the reverse split and which exchange ratio to implement. The action by written consent of the stockholders became effective on May 31, 2012, following our compliance with certain notice requirements under the Securities Exchange Act of 1934 (the “Exchange Act”). We anticipate that immediately following the effectiveness of the registration statement of which this prospectus forms a part, and prior to the closing of this offering, our Board of Directors will effect a reverse stock split at a ratio of one-for-five (the “reverse stock split”).
The reverse stock split is intended to allow us to meet the minimum share price requirement of The NASDAQ Capital Market. We have applied for listing of our common stock on The NASDAQ Capital Market, which listing we expect to occur at the closing of this offering. If the application is not approved, we will not complete this offering or effect the reverse stock split, and the shares of our common stock will continue to be traded on the OTC Markets Group’s OTCQB tier.
Other than as otherwise indicated and except in our consolidated financial statements, all information regarding share amounts of common stock and prices per share of common stock contained in this prospectus assume the consummation of the one-for-five reverse stock split to be effected following effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of this offering.
Impracor
Impracor, our lead drug candidate, is comprised of a transdermal formulation of ketoprofen, a non-steroidal anti-inflammatory drug (NSAID). Impracor is formulated using our proprietary Accudel drug delivery system and is being developed for the treatment of acute musculoskeletal pain. Impracor penetrates the skin barrier to reach the targeted underlying tissues where it exerts its localized anti-inflammatory and analgesic effect. The topical delivery of the drug may minimize systemic exposure, which may in turn lead to fewer concerns pertaining to gastrointestinal, hepatic, cardiovascular and other adverse systemic effects, which are associated with orally administered NSAIDs. We believe that this product may be considered for patients with site specific localized pain and who also (i) have a history of gastrointestinal, cardiovascular, kidney or liver problems, (ii) are geriatric or pediatric and/or (iii) are at risk for drug interactions.
1
Completed Clinical Studies for Impracor
In June 2008, we initiated a Phase 3 clinical trial designed as a randomized, double-blind, placebo-controlled, multi-center study that enrolled a total of 364 patients with acute soft tissue injuries of the upper or lower extremities in 26 centers in the United States. As we reported in October 2009, the top-line results showed that the study demonstrated statistical significance in its primary endpoint in the per protocol analysis and was favorable for Impracor in the Intent-To Treat ("ITT") analysis. Impracor also demonstrated a safety and tolerability profile similar to the placebo used in the study. Of the over 180 patients treated with Impracor, there were no treatment related gastrointestinal, cardiovascular, hepatic or other clinically relevant adverse events reported. Furthermore, Impracor was observed to be well absorbed through the skin and only minimal blood concentrations of ketoprofen were detected in a subset of patients who underwent blood sampling for pharmacokinetic analyses following repeated topical applications.
In January 2010, we reported on further in-depth analyses of the ITT data from the Impracor Phase 3 study. For the modified ITT analysis we identified 35 patients who did not meet study entry criteria at the time of randomization. Excluding the data from these patients who should not have been randomized into the study based on information that was not known at the time of enrollment, the study demonstrated statistical significance (p<0.038) on the primary efficacy endpoint. This post-hoc analysis was confirmed by a third-party statistical expert.
In February 2012, our management conducted an additional analysis of the ITT data and a body weight adjusted modified per protocol (“mPP”) analysis of those participants who completely complied with the Phase 3 Study protocol, including taking the recommended therapeutic quantity of the study drug. This analysis excluded 52 participants from the ITT group who did not take a minimum therapeutic quantity of the study drug, and 20 patients who did not have a valid Day 3 primary endpoint assessment and 4 patients who were misdiagnosed. This mPP analysis on 250 patients demonstrated statistical significance of the primary endpoint (p=0.034).
We believe that the weight of evidence of a treatment effect in this study is further strengthened by a key secondary endpoint (pain intensity recorded three times daily on patient diary cards) that supports the primary endpoint. The patient diary data which yield pain curves over time show consistent separation between treatment groups reaching statistical significance in favor of Impracor using both the original and modified ITT population. Furthermore, the physician's global assessment, using a 7 Point Likert Scale, on day 3 produced statistically significant results (p=0.037), and a later exploratory analysis of the patient's global assessment of treatment satisfaction, using a binomial method, produced statistically significant results (p=0.023).
Proposed Clinical Program for Impracor
For Impracor to be approved by the U.S. Food and Drug Administration ("FDA"), two confirmatory Phase 3 trials with exposure of at least 300 to 500 patients and supportive dermal safety studies are required. We plan to commence two adequate and well-controlled Phase 3 trials of Impracor in patients experiencing pain from osteoarthritis flare in their knees. We plan to discuss the clinical development program for those trials, which has been endorsed by our scientific and regulatory advisory board, with the FDA at a Type C meeting scheduled to occur in April 2013.
Also as required by the FDA, we recently completed a clinical study that measured the amount of ketoprofen found in the bloodstream following topical application of two different doses of the anti-inflammatory cream under different conditions, including normal activities, heat exposure to the application site and standardized exercise, as well as the amount of the drug in the bloodstream after taking an oral dose of ketoprofen (the relative oral bioavailability). The study included a total of 40 healthy volunteers (36 of which completed the study) assigned to one of two cohorts (2g or 4g applications). Subjects were dosed according to a four-sequence, four-treatment randomization schedule in which they received topical Impracor applications under each of the three conditions and an oral ketoprofen dose in weekly intervals. Overall the pharmacokinetic parameters were observed to be consistent between the two different dose cohorts. The application of an occlusive knee bandage with either heat or exercise following topical administration showed faster initial, but lower overall plasma exposure of ketoprofen relative to non-occluded topical administration with no heat or exercise. The extent of bioavailability over 48 hours as measured by the area under the concentration curve from time zero to the time of last measurable concentration (AUC0-t) was 2% or less in cohort 1 (2 g single dose applied to one knee) and 4% or less in cohort 2 (2 g single dose applied to each knee) for the topical treatments relative to the oral treatment. All treatments were observed to be well tolerated. We also expect to initiate a routine supportive trial in healthy subjects related to the potential of contact sensitization. We expect that all of our planned clinical studies for Impracor will be executed with the professional help of clinical research organizations (“CROs”) with experience in clinical trials of similar design. We are in the process of selecting and negotiating arrangements with potential CROs and other third parties in order to initiate our Phase 3 clinical trials.
Following completion of our clinical trials, we expect to file a New Drug Application for marketing authorization for Impracor under Section 505(b)(2) of the Hatch-Waxman Act of 1984, a regulatory route towards U.S. approval that leverages previously established safety and/or effectiveness of already approved ketoprofen products in other dosage forms. This regulatory path also improves the chances of success over a completely new drug chemical entity.
The timing of Phase 3 trials and the other supportive studies will be dependent on obtaining adequate financing to support the execution of these activities and for other working capital expenditures. Upon receipt of such financing, we anticipate initiating the supportive studies and Phase 3 trials in mid and late 2013, respectively. Assuming successful timely completion and outcome of the additional Phase 3 trials, we would expect to file the New Drug Application for Impracor in the second half of 2014.
We expect that Impracor, if approved by the FDA, could become one of the first NSAID cream products available by prescription in the United States for the topical treatment of acute musculoskeletal pain.
The Accudel Technology
Accudel is our proprietary transdermal cream drug delivery platform which can facilitate the transdermal penetration of drugs, thus enabling the avoidance of first pass metabolism by the liver and minimizing systemic exposure. The following diagram provides a schematic of the Accudel drug delivery system:
2
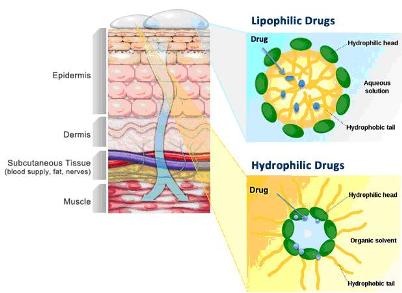
Accudel has the following properties, which make it a highly versatile vehicle for topical drug administration:
|
●
|
utilizes a pluronic lecithin organogel based matrix which is known to penetrate the stratum corneum and aid in the diffusion of active ingredients through the skin;
|
|
●
|
helps solubilize various types of drugs and its components (lipophilic, hydrophilic and amphiphilic);
|
|
●
|
uses penetration enhancers in a synergistic combination;
|
|
●
|
can incorporate compounds of various molecular sizes;
|
|
●
|
contains biocompatible components which are generally regarded as safe by the FDA;
|
|
●
|
is thermodynamically stable, insensitive to moisture and resistant to microbial contamination;
|
|
●
|
potentially results in decreased safety concerns associated with oral or intravenous drugs;
|
|
●
|
avoids certain limitations associated with transdermal patches;
|
|
●
|
is easy to apply, aesthetically acceptable and odorless; and
|
|
●
|
potentially produces patentable new products when combined with established or new drugs.
|
3
Product Development Program
We believe that the clinical success of Impracor will facilitate the use of the Accudel delivery technology in other products. We have identified development opportunities for potential products in pain management and other therapeutic areas utilizing the Accudel platform technology and we are exploring potential commercial relationships for these identified product candidates. In particular, we are currently considering potential new drug candidates in the muscle relaxant and neuropathic pain fields, and we expect those to be our next avenues for new product development. We estimate that pre-Phase 3 clinical studies for these two potential product candidates could each be completed approximately 18 to 24 months after their commencement, and that costs for such development would range from approximately $2 million to $2.5 million for each proposed drug candidate.
In addition, we expect our new relationship with PCCA to facilitate our future selection, development and formulation of potential product candidates. We plan to use our proprietary Accudel drug delivery technology, coupled with these licensed technologies, formulations and market data, to identify pharmaceutical development opportunities where there is a significant unmet need for a new drug product. We are currently considering potential product candidates in the muscle relaxant and neuropathic pain fields, and we expect those areas may be our next avenues for additional product development.
In the past our product development program has included cosmetic and cosmeceutical products utilizing our patented transdermal delivery system technology, Accudel. Our lead product candidate was an anti-cellulite formulation, for which we have initial clinical information supporting the beneficial effects of this cosmetic product on skin appearance. Our potential pipeline of cosmetic products includes hyperpigmentation and anti-aging formulations. We remain interested in pursuing this business opportunity and continue to consider entering into new relationships with third parties. We may also pursue the out-licensing of our Accudel drug delivery technology for the development and commercialization of additional innovative drug and cosmeceutical products.
Market and Opportunity
According to Wolters-Kluwer PHAST, the U.S. pain market was approximately $39.8 billion in 2011. Of that total, the NSAID market made up approximately $13.5 billion from approximately 155 million written prescriptions. The topical NSAID market in 2011 was over $500 million, averaging an approximately 28% compound annual growth rate since 2007.
According to the Archives of Internal Medicine, NSAIDs are regularly used by more than 60 million Americans. Approximately 70% of people aged 65 or older take NSAIDs weekly. As a result of the widespread usage of oral NSAIDs, according to Bandolier, there are over 100,000 hospitalizations annually and 16,500 deaths in the U.S. due to gastro-intestinal complications annually. In the United Kingdom, there are approximately 12,000 hospitalizations and an estimated 2,600 deaths annually related to GI complications following oral NSAID use per year. One study published in 1998 in the American Journal of Medicine found that NSAID-related gastro-intestinal side effects cause almost as many deaths as asthma, cervical cancer and malignant melanona combined, and another 1999 study published in the Journal of Rheumatology found that death resulting from gastro-intestinal complications was the 15th most common cause of death in the U.S. According to Singh G, Triadafilopoulos G., Epidemiology of NSAID induced gastrointestinal complications, J Rheumatol. 1999, the hospitalizations and deaths related to systemic NSAID use has a financial impact of more than $2 billion per year in the U.S. Therefore, we believe there is a significant demand from physicians and patients for topical pain management products such as Impracor, especially with respect to the treatment of localized, acute musculoskeletal pain, which we believe is driven primarily by the concern of possible negative systemic effects of orally administered NSAIDs.
For more information regarding our business, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” included elsewhere in this prospectus.
Recent Developments
All information regarding share amounts of common stock and prices per share of common stock described below assume the consummation of the one-for-five reverse stock split to be effected following the effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of this offering.
PCCA Transaction
On August 30, 2012, we entered into a License Agreement (the “PCCA License Agreement") and a Stock Purchase Agreement (the “PCCA Purchase Agreement”) in a strategic transaction with PCCA (the “PCCA Transaction”).
Pursuant to the terms of the PCCA License Agreement, effective August 30, 2012, PCCA has granted to us and our affiliates certain exclusive rights under PCCA’s proprietary formulations, other technologies and data, and we have agreed to pay to PCCA certain royalties on net sales relating to the sale of certain future products, which royalties range from 4.5% to 9% for each product, subject to certain minimum royalty payments. PCCA may terminate the PCCA License Agreement if we fail to commence efforts to research and develop future products within certain time periods.
Pursuant to the terms of the PCCA Purchase Agreement, closed on August 31, 2012, we issued and sold to PCCA 832,683 shares of our common stock at a per share purchase price of $4.8038, for aggregate gross proceeds to us of $4,000,000. The PCCA Purchase Agreement does not grant to PCCA any registration rights with respect to the shares purchased and sold thereunder. The shares sold to PCCA were sold in reliance on the exemption from the registration requirements of the Securities Act of 1933 (the “Securities Act”) afforded by Section 4(2) thereof.
April Private Placement
On April 20, 2012, we entered into a Securities Purchase Agreement with certain accredited investors relating to the sale and issuance of an aggregate of 2,011,691 shares of our common stock and warrants to purchase up to 502,928 shares of common stock at an exercise price of $5.925 per share, for an aggregate purchase price of approximately $7.95 million (the “April Private Placement”). We closed the April Private Placement on April 25, 2012. The securities sold in the April Private Placement were sold in reliance on the exemption from the registration requirements of the Securities Act afforded by Section 4(2) thereof and Rule 506 of Regulation D.
The investors are not entitled to any registration rights with respect to the common stock and warrants issued in the April Private Placement. The warrants have a term of three years and are exercisable any time after April 25, 2012. We may require that the investors exercise the warrants in whole, but not in part, at any time within 20 business days after all of the following conditions have been satisfied: (i) the volume weighted average price of the our common stock for 10 consecutive trading days is equal to or greater than the exercise price of the warrants; (ii) we have received a Filing Review Notification from the FDA regarding the status of Impracor; and (iii) sufficient shares of common stock are authorized and reserved for issuance upon full exercise of the warrants.
4
Conversion of Convertible Note and Balance Under Line of Credit
Effective immediately following the effective time of the Certificate of Amendment to our Certificate of Incorporation increasing the number of authorized shares of our common stock on February 28, 2012, the entire outstanding balance and all accrued but unpaid interest owing under our $1,000,000 7.5% Convertible Promissory Note issued on April 5, 2010, as well as certain outstanding accounts payable held by DermaStar International, LLC (“DermaStar”), were converted into 1,835,830 shares of common stock, and the convertible promissory note was terminated. DermaStar was the holder of 80% of the convertible promissory note.
On April 25, 2012, the entire outstanding principal balance and all accrued and unpaid interest under our line of credit with DermaStar, an aggregate of $762,534, was converted into 193,047 shares of common stock and warrants to purchase 48,262 shares of our common stock pursuant to a conversion agreement we entered into with DermaStar on April 20, 2012. The warrants have substantially the same terms as the warrants issued in the April Private Placement. The line of credit was terminated upon the completion of the conversion. Director and Chief Executive Officer Mark L. Baum and the Chairman of our Board of Directors, Robert J. Kammer, were the Managing Members of DermaStar prior to its dissolution in July 2012. The conversion agreement was unanimously approved by the Company’s disinterested directors, with Mr. Baum and Dr. Kammer abstaining.
Written Consent of the Stockholders Approving Increase in Option Plan Reserve
On June 29, 2012, stockholders holding a majority of our outstanding voting power approved an amendment to our 2007 Incentive Stock and Awards Plan (the “2007 Plan”) to increase the number of shares available for issuance under the 2007 Plan from 750,000 to 2,400,000. The stockholder approval of the increase became effective upon our compliance with certain notice requirements under the Exchange Act.
Going Concern
Our independent registered public accounting firm issued an unqualified opinion with an explanatory paragraph to the effect that there is substantial doubt about our ability to continue as a going concern in its report included in our consolidated financial statments for the fiscal year ended December 31, 2011. This unqualified opinion with an explanatory paragraph could have a material adverse effect on our business, financial condition, results of operations and cash flows. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources” and Note 2 to our consolidated financial statements for the fiscal year ended December 31, 2011 included elsewhere in this prospectus. We experienced net losses of $(953,936) and $(2,531,228) for the years ended December 31, 2011 and 2010, respectively. As of September 30, 2012, our accumulated deficit was $(22,651,240).
Unless and until we execute an underwriting agreement with the underwriters in connection with this offering, we have no committed sources of capital and do not know whether additional financing will be available when needed on terms that are acceptable, if at all. The going concern statement from our independent registered public accounting firm may discourage some investors from purchasing our stock or from providing alternative capital financing to us. The failure to satisfy our capital requirements would adversely affect our business, financial condition, results of operations and prospects.
Unless we raise additional funds, either through the sale of equity securities such as through this offering or one or more collaborative arrangements, we will not have sufficient funds to continue operations. Even if we take these actions, the funds we raise may be insufficient, particularly if our costs are higher than projected or unforeseen expenses arise.
Risks Related to Our Business
Our business is subject to a number of risks. You should understand these risks before making an investment decision. If any of these risks actually occurs, our business, financial condition or results of operations would likely be materially adversely affected. In such case, the trading price of our common stock would likely decline, and you may lose all or part of your investment. Below is a summary of some of the principal risks we face. The risks are discussed more fully in the section of this prospectus below entitled “Risk Factors.”
|
●
|
We have a limited operating history since the dismissal of our voluntary petition for reorganization relief under Chapter 11 of the Bankruptcy Code in December 2011, and we may be unable to successfully resume our operations and implement our business plan.
|
5
|
●
|
The report of our independent registered public accounting firm on our 2011 consolidated financial statements contains a going concern modification.
|
|
●
|
We have incurred losses in the research and development of Impracor and our Accudel technology since inception. We may never generate revenue or become profitable.
|
|
●
|
Timing and results of clinical trials to demonstrate the safety and efficacy of products as well as FDA approval of products are uncertain.
|
|
●
|
Delays in the conduct or completion of our clinical and non-clinical trials for Impracor or the analysis of the data from our clinical or non-clinical trials may result in delays in our planned filings for regulatory approvals, and may adversely affect our business.
|
|
●
|
If we are not successful in introducing our products or if the market does not accept our products, or if our proposed product identification and development strategy is not successful, our business, financial position and results of operations may be materially adversely affected and the market price for our common stock would decline.
|
|
●
|
If our patents are determined to be unenforceable or expire, or if we are unable to obtain new patents based on current patent applications or for future inventions, we may not be able to prevent others from using our intellectual property.
|
|
●
|
Our principal stockholders have the ability to exert significant control in matters requiring a stockholder vote and could delay, deter or prevent a change in control of our company.
|
6
THE OFFERING
The following summary contains basic information about the offering and our common stock and is not intended to be complete. It does not contain all the information that is important to you. For a more complete understanding of our common stock, please refer to the section of this prospectus entitled “Description of Capital Stock.”
All information regarding share amounts of common stock and prices per share of common stock described below assume the consummation of the one-for-five reverse stock split to be effected following the effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of this offering.
|
Issuer
|
Imprimis Pharmaceuticals, Inc.
|
|
Securities offered
|
Up to 1,619,047 shares of common stock
|
|
Common stock outstanding prior to offering
|
6,772,125 (1)
|
|
Common stock outstanding after the offering
|
8,391,172 (1)(2)
|
|
Use of Proceeds
|
We expect to use the net proceeds received from the offering, to fund our clinical trials and for working capital and general corporate purposes. See “Use of Proceeds” for more information.
|
|
OTC Markets Group Symbol
|
“IMMY”
|
|
Proposed NASDAQ Symbol
|
“IMMY”
|
|
Risk Factors
|
See “Risk Factors” beginning on page 8 and other information in this prospectus for a discussion of the factors you should consider before you decide to invest in our common stock.
|
|
Underwriters common stock purchase warrant
|
In connection with this offering, we have also agreed to sell to the underwriters and their respective designees a warrant to purchase up to 8.5% of the shares of common stock sold in this offering. If the warrants are exercised, each share may be purchased by the holder thereof at $5.25 per share (100% of the price of the shares sold in this offering.)
|
|
Lock-Up Agreements
|
Each of our officers, directors and shareholders beneficially owning 5% or more of our common stock have agreed that for a period of 180 days from the effective date of this offering, they will be subject to a lockup prohibiting any sales, transfers or hedging transactions in our securities held by them. See section titled “Lock-Up Agreements” in this prospectus.
|
(1) Excludes (i) 556,872 shares of common stock issuable upon the exercise of outstanding warrants with exercise prices ranging from $5.925 to $176.00 per share, (ii) 917,235 shares of our common stock issuable upon exercise of outstanding options with a weighted average exercise price of $5.30 per share outstanding under the 2007 Plan, and (iii) 200,000 shares of common stock underlying restricted stock units granted outside the 2007 Plan.
(2) Excludes any shares of common stock issuable pursuant to the exercise of the underwriters' over-allotment option and any shares of common stock issuable upon the exercise of warrants issuable to the underwriters upon the closing of this offering.
7
RISK FACTORS
Investing in our common stock involves a high degree of risk. You should carefully consider the following risk factors in addition to the other information contained in this prospectus. This prospectus contains forward-looking statements. Our business, financial condition, results of operations and stock price could be materially adversely affected by any of these risks. Additional risks not presently known to us or that we currently deem immaterial may also impair our business financial condition, results of operations and stock price.
Risks Related to Our Business
We have a limited operating history since the dismissal of our voluntary petition for reorganization relief under Chapter 11 of the Bankruptcy Code in December 2011, and we may be unable to successfully resume our operations and implement our business plan.
On June 26, 2011, we suspended our operations and filed a voluntary petition for reorganization relief under Chapter 11 of the United States Bankruptcy Code in the United States Bankruptcy Court for the Southern District of California (the “Bankruptcy Court”), Case No. 11-10497-11 (the “Chapter 11 Case”). On November 21, 2011, in connection with our entry into a line of credit agreement and securities purchase agreement with DermaStar International, LLC (“DermaStar”), we requested that the Bankruptcy Court dismiss the Chapter 11 Case. On December 8, 2011, the Bankruptcy Court entered an order dismissing the Chapter 11 Case, and since that date we have engaged a new management team, appointed new directors to fill certain vacancies on our Board and worked towards re-initiating our Phase 3 clinical trials for Impracor. However, we have a limited operating history since the dismissal of the Chapter 11 Case, and we may not be successful in our efforts to resume our operations. We did not receive any type of discharge of debts, claims or obligations in the Chapter 11 Case, and prior unknown or contingent liabilities could have a material adverse effect on our financial condition. Prior to the filing of the Chapter 11 Case, we were unable to successfully pursue our business plan due to a lack of funding. We will require additional capital to pursue our clinical trials and maintain our operations. We may be unable to obtain such funds when necessary. In addition, by September 2011 we employed no full-time employees and had retained the consulting services of one former employee in order to manage any matters related to the Chapter 11 Case. We have had to re-assemble an executive management team and a research and development team, and other employees to assist with our general operations. We currently have five employees, a number of whom are former employees, and we will need to hire additional employees in order to execute our business plan. Given our operating history, we may be unable to maintain an effective management team, or hire and retain the additional qualified individuals we will need. As a result, we may be unable to successfully pursue our business plan.
We must raise additional capital in order to continue operating our business, and such additional funds may not be available on acceptable terms or at all.
We do not generate any cash from operations and, although we believe we have sufficient cash reserves to execute our business plan for the next twelve months, we must raise additional funds in order to continue operating our business and fully execute our business plan. Even following our recent issuance of securities to PCCA in the PCCA Transaction and the completion of this offering, based on our use of proceeds from the PCCA Transaction and our proposed use of proceeds from this offering, we will likely need significant additional capital, which we may seek to raise through, among other things, public and private equity offerings and debt financings. If we are not able to generate significant revenues and attain profitable operations, we will need to continue to seek further additional financing. In addition, estimates of our operating expenses and working capital requirements could be incorrect, and we could be required to seek additional financing earlier than we anticipate. We expect to continue to fund our operations primarily through equity and debt financings in the future, and could also pursue funding from corporate partnerships or licensing arrangements (as we did with the PCCA Transaction) or similar financings. If additional capital is not available when necessary, we may not be able to continue to operate our business pursuant to our business plan or we may have to discontinue our operations entirely.
We expect our total expenditures over the next 12 months to be approximately $9.3 million. However, our estimate of total expenditures could increase if we encounter unanticipated difficulties. In addition, our estimates of the amount of cash necessary to fund our business may prove to be wrong and we could spend our available financial resources much faster than we currently expect. If we do not have sufficient funds to continue to develop our business, we will be forced to delay, scale back or eliminate some or all of our proposed operations. If any of these events were to occur, there is a substantial risk that our business would fail. Sources of additional funds may not be available on acceptable terms or at all. Weak economic and capital market conditions could result in increased difficulties in raising capital for our operations. We may not be able to raise money through the sale of our equity securities or through borrowing funds on terms we find acceptable, or at all. If we cannot raise the funds that we need, we will be unable to continue our operations, and our stockholders could lose their entire investment in our company.
8
If we issue equity or convertible debt securities to raise additional funds, our existing stockholders may experience substantial dilution, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, if we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish potentially valuable rights to our product candidates or proprietary technologies, or grant licenses on terms that are not favorable to us. If we incur additional debt, it may increase our leverage relative to our earnings or to our equity capitalization, requiring us to pay additional interest expenses. Obtaining commercial loans, assuming those loans would be available, would increase our liabilities and future cash commitments. Further, we may incur substantial costs in pursuing future capital and/or financing, including investment banking fees, legal fees, accounting fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we may issue, such as options, convertible notes and warrants, which would adversely impact our financial results.
The report of our independent registered public accounting firm on our 2011 consolidated financial statements contains a going concern modification, and we will need additional financing to execute our business plan, fund our operations and to continue as a going concern.
We have limited remaining funds to support our operations. We have prepared our consolidated financial statements for the fiscal year ended December 31, 2011 and the nine months ended September 30, 2012 on a going-concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The report of our independent registered public accounting firm included in our December 31, 2011 consolidated financial statements includes an explanatory paragraph stating that the recurring losses incurred from operations and a working capital deficiency raise substantial doubt about our ability to continue as a going concern. In the nine months ended September 30, 2012, we received proceeds, net of related costs, of approximately $7.93 million in the April Private Placement and $3.98 million in connection with the PCCA Transaction. We expect to use those funds and any funds we may receive from this offering to pursue obtaining regulatory approval to market Impracor and pursue our business plan, but we will need to secure additional funds in order to complete our clinical trials and pursue other product development opportunities. As a result of our recent capital raising transactions and in light of our current cash and cash equivalents position as of the date of this prospectus, we expect to have adequate resources in order to operate our business through the next twelve months. However, our auditors may have doubt about our ability to continue as a going concern in future periods, and our financial statements relating to those periods may not be prepared on a going-concern basis based on any such doubts. Further, even with our current cash position as a result of our recent financings, we will need to secure additional funds in order to complete our clinical trials and pursue other product development opportunities. If adequate financing is not available, we will not be able to meet FDA requirements to obtain regulatory approval to market Impracor. In addition, if one or more of the risks discussed in these risk factors occur or our expenses exceed our expectations, we may be required to raise further additional funds sooner than anticipated. The inclusion of a going concern modification in our independent registered public accounting firm’s report for the year ended December 31, 2011, or in any future report, may materially and adversely affect our stock price or our ability to raise new capital.
We have incurred losses in the research and development of Impracor and our Accudel technology since inception. We may never generate revenue or become profitable.
We have incurred losses in every year of our operations, including net losses of $(953,936) and $(2,531,228) for the years ended December 31, 2011 and 2010, respectively. As of September 30, 2012, our accumulated deficit was $(22,651,240). In addition, we expect to incur increasing operating losses for the foreseeable future as we continue to incur costs for research and development and clinical trials, and in other development activities. Our ability to generate revenue and achieve profitability depends upon our ability, alone or with others, to complete the development of our proposed products, obtain the required regulatory approvals and manufacture, market and sell our proposed products. Development is costly and requires significant investment. In addition, we may choose to in-license rights to particular drugs or active ingredients for use in cosmetic products. The license fees for such drugs or active ingredients may increase our costs.
9
As we continue to engage in the development of Impracor and develop other products, we may never be able to achieve or sustain market acceptance, profitability or positive cash flow. Our ultimate success will depend on many factors, including whether Impracor receives FDA approval. We cannot be certain that we will receive FDA approval for Impracor, or that we will reach the level of sales and revenues necessary to achieve and sustain profitability. Unless we raise additional capital, we will not be able to execute our business plan or fund business operations. Furthermore, we will be forced to reduce our expenses and cash expenditures to a material extent, which would impair or delay our ability to execute our business plan.
We may not be able to correctly estimate our future operating expenses, which could lead to cash shortfalls.
Our operating expenses may fluctuate significantly in the future as a result of a variety of factors, many of which are outside of our control. These factors include:
|
●
|
the time and resources required to develop, conduct clinical trials and obtain regulatory approvals for our drug candidates;
|
|
●
|
the costs to rebuild our management team following the dismissal of the Chapter 11 Case, including attracting and retaining personnel with the skills required for effective operations; and
|
|
●
|
the costs of preparing, filing, prosecuting, defending and enforcing patent claims and other patent related costs, including litigation costs and the results of such litigation.
|
If our estimates of our operating expenses prove to be wrong, we could spend our available financial resources much faster than we currently expect. If we do not have sufficient funds to continue to develop our business, we will be forced to delay, scale back or eliminate some or all of our proposed operations.
Our clinical trials may not demonstrate the safety and efficacy of our product candidates.
We are subject to extensive government regulations. The process of obtaining FDA approval is costly, time consuming, uncertain and subject to unanticipated delays. Before obtaining regulatory approvals for the sale of any of our product candidates, we must demonstrate through preclinical studies and clinical trials that the product candidate is safe and effective for each intended use. Preclinical and clinical studies may fail to demonstrate the safety and effectiveness of our product candidates. Even promising results from preclinical and early clinical studies do not always accurately predict results in later, large scale trials. A failure to demonstrate safety and efficacy would result in our failure to obtain regulatory approvals. Moreover, if the FDA grants regulatory approval of a product candidate, the approval may be limited to specific indications or limited with respect to its distribution, which could limit revenues.
The FDA or other regulatory agencies may not approve any product candidates developed by us on a timely basis or at all, and, if granted, such approval may subject the marketing of our product candidates to certain limits on indicated use. In particular, the outcome of the final analyses of the data from the Phase 3 clinical trials for Impracor may vary from our initial conclusions or the FDA may not agree with our interpretation of such results or may challenge the adequacy of our clinical trial design or the execution of the clinical trial. The FDA has required two adequate and well controlled Phase 3 clinical trials for Impracor before we can submit a New Drug Application under Section 505(b)(2) of the Hatch-Waxman Act of 1984. We have not yet initiated these Phase 3 clinical trials, although in September 2012 we commenced certain supportive studies relating to Impracor that are also required by the FDA. The results of any future clinical trials or studies may not be favorable and we may never receive regulatory approval for Impracor. Any limitation on use imposed by the FDA or delay in or failure to obtain FDA approvals of product candidates developed by us would adversely affect our ability to generate product revenue, as well as the price of our common stock.
Delays in the conduct or completion of our clinical and non-clinical trials for Impracor or the analysis of the data from our clinical or non-clinical trials may adversely affect our business.
We cannot predict whether we will encounter problems with any of our completed or planned clinical or non-clinical studies that will cause us or regulatory authorities to delay or suspend planned clinical and non-clinical studies. Any of the following could delay the completion of our planned clinical studies:
|
●
|
failure of the FDA to approve the scope or design of our clinical or non-clinical trials or manufacturing plans;
|
|
●
|
delays in enrolling volunteers in clinical trials;
|
|
●
|
insufficient supply or deficient quality of materials necessary for the performance of clinical or non-clinical trials;
|
|
●
|
negative results of clinical or non-clinical studies; and
|
|
●
|
adverse side effects experienced by study participants in clinical trials relating to a specific product.
|
10
There may be other circumstances other than the ones described above, over which we may have no control that could materially delay the successful completion of our clinical and non-clinical studies.Furthermore, we expect to rely on CROs to ensure the proper and timely conduct of our clinical trials, and while we expect to enter into agreements governing their committed activities, we have limited influence over their actual performance.
If our patents are determined to be unenforceable or expire, or if we are unable to obtain new patents based on current patent applications or for future inventions, we may not be able to prevent others from using our intellectual property.
Our success will depend in part on our ability to:
|
●
|
obtain and maintain patent protection with respect to our products;
|
|
●
|
prevent third parties from infringing upon our proprietary rights;
|
|
●
|
maintain trade secrets;
|
|
●
|
operate without infringing upon the patents and proprietary rights of others; and
|
|
●
|
obtain appropriate licenses to patents or proprietary rights held by third parties if infringement would otherwise occur.
|
We obtained a patent from the United States Patent and Trademark Office on our Accudel technology in 1998, which affords protection of Accudel through 2016 in the United States. We may not be successful in our efforts to extend the date of our patent protection beyond 2016. Failure to maintain or extend the patent could adversely affect our business. We will only be able to protect our drug candidates and our technologies from unauthorized use by third parties to the extent that valid and enforceable patents cover them
The patent and intellectual property positions of specialty pharmaceutical companies, including ours, are uncertain and involve complex legal and factual questions. There is no guarantee that we have or will develop or obtain the rights to products or processes that are patentable, that patents will issue from any pending applications or that claims allowed will be sufficient to protect the technology we develop or have developed or that is used by us, our contract manufacturing organizations or our other service providers. In addition, we cannot be certain that patents issued to us will not be challenged, invalidated, infringed or circumvented, including by our competitors, or that the rights granted thereunder will provide competitive advantages to us.
Furthermore, patent applications in the U.S. are confidential for a period of time until they are published, and publication of discoveries in scientific or patent literature typically lags actual discoveries by several months. As a result, we cannot be certain that the inventors listed in any patent or patent application owned by us were the first to conceive of the inventions covered by such patents and patent applications or that such inventors were the first to file patent applications for such inventions.
We also may rely on unpatented trade secrets and know-how and continuing technological innovation to develop and maintain our competitive position, which we seek to protect, in part, by confidentiality agreements with current employees, consultants, collaborators and others. We also have invention or patent assignment agreements with our current employees and certain consultants. There can be no assurance, however, that binding agreements will not be breached, that we will have adequate remedies for any breach, or that trade secrets will not otherwise become known or be independently discovered by competitors. In addition, there can be no assurance that inventions relevant to us will not be developed by a person not bound by an invention assignment agreement with us.
Our product development program may not be successful.
In addition to the development of Impracor, we expect to pursue development of potential products in pain management and other therapeutic areas. In particular, we are currently considering potential new product candidates in the muscle relaxant and neuropathic pain fields. We also expect to utilize our relationship with PCCA to identify development opportunities where we perceive an unmet need for a new drug product, and thereby facilitate our future selection, formulation and development of potential product candidates. In addition, our product development program has included cosmetic products, which utilizes the basis of our patented transdermal delivery system technology, Accudel. Since our primary focus will remain seeking FDA approval for Impracor, we currently expect to use limited resources on our other development programs.
None of our potential pharmaceutical product candidates have commenced any clinical trials and there are a number of FDA requirements that we must satisfy in order to commence clinical trials. These requirements will require substantial time, effort and financial resources. We may never satisfy these requirements. In addition, prior to commencing any trials of a drug candidate, we must evaluate whether a market exists for the drug candidate. This is costly and time consuming, and any market studies we rely on may not be accurate. We may expend significant capital and other resources on a drug candidate and find that no commercial market exists for the drug. Further, our relationship with PCCA, on which we intend to rely to facilitate our evaluation of the potential market for future products we may develop, is terminable if we fail to commence efforts to research and develop future products within certain time periods, as set forth in the PCCA License Agreement. We may not be able to meet such requirements within the required time periods or at all, and our relationship with PCCA could be terminated. If we do commence clinical trials of our other potential product candidates, such product candidates may never be approved by the FDA. Even if we are not required to obtain FDA pre-market approval for our potential cosmeceutical product candidates, we will still be subject to a number of federal and state regulations, including regulation by the FDA and the Federal Trade Commission on any marketing claims we make, and we may be unable to satisfy these requirements. Any cosmeceutical products we develop may cause undesirable side effects that could limit their use, require their removal from the market and subject us to adverse regulatory action and product liability claims. As a result, we may never successfully develop and obtain approval to market and sell any of our potential product candidates. Even if we do develop and obtain approval to market and sell such product candidates, we may be unable to compete against the many products and treatments currently being offered or under development by other established, well known and well-financed cosmetic, health care and pharmaceutical companies.
11
If approved, failure to comply with continuing federal and state regulations could result in the loss of approvals to market our drugs.
Following initial regulatory approval of any drugs we may develop, we will be subject to continuing regulatory review, including review of adverse drug experiences and clinical results that are reported after our drug products become commercially available. This would include results from any post-marketing tests or continued actions required as a condition of approval. The manufacturer and manufacturing facilities we use to make any of our drug candidates will be subject to periodic review and inspection by the FDA. If a previously unknown problem or problems with a product or a manufacturing and laboratory facility used by us is discovered, the FDA may impose restrictions on that product or on the manufacturing facility, including requiring us to withdraw the product from the market. Any changes to an approved product, including the way it is manufactured or promoted, often requires FDA approval before the product, as modified, can be marketed. In addition, we and our contract manufacturers will be subject to ongoing FDA requirements for submission of safety and other post-market information. If we or our contract manufacturers fail to comply with applicable regulatory requirements, a regulatory agency may:
|
●
|
issue warning letters;
|
|
●
|
impose civil or criminal penalties;
|
|
●
|
suspend or withdraw our regulatory approval;
|
|
●
|
suspend or terminate any of our ongoing clinical trials;
|
|
●
|
refuse to approve pending applications or supplements to approved applications filed by us;
|
|
●
|
impose restrictions on our operations;
|
|
●
|
close the facilities of our contract manufacturers; or
|
|
●
|
seize or detain products or require a product recall.
|
Regulatory review also covers a company’s activities in the promotion of its drugs, with significant potential penalties and restrictions for promotion of drugs for an unapproved use. Sales and marketing programs are under scrutiny for compliance with various mandated requirements, such as illegal promotions to health care professionals. We are also required to submit information on our open and completed clinical trials to public registries and databases. Failure to comply with these requirements could expose us to negative publicity, fines and penalties that could harm our business.
If we violate regulatory requirements at any stage, whether before or after marketing approval is obtained, we may be fined, be forced to remove a product from the market or experience other adverse consequences, including delay, which would materially harm our financial results. We may not be able to obtain the labeling claims necessary or desirable for product promotion.
If approved, there is no guarantee that the market will accept our products. If we are not successful in introducing our products or if the market does not accept our products, our business, financial position and results of operations may be materially adversely affected and the market price for our common stock would decline.
Even if we obtain regulatory approvals, uncertainty exists as to whether the market will accept our products or if the market for our products is as large as we anticipate. A number of factors may limit the market acceptance of our products, including the timing of regulatory approvals and market entry relative to competitive products, the availability of alternative products, the price of our products relative to alternative products, the availability of third party reimbursement and the extent of marketing efforts by third party distributors or agents that we retain. We cannot assure you that our products will receive market acceptance in a commercially viable period of time, if at all. We cannot be certain that any investment made in developing products will be recovered, even if we are successful in commercialization. To the extent that we expend significant resources on research and development efforts and are not able, ultimately, to introduce successful new products as a result of those efforts, our business, financial position and results of operations may be materially adversely affected, and the market value of our common stock could decline.
12
We may be subject to product liability claims.
The development, manufacture, and sale of pharmaceutical and cosmetic products expose us to the risk of significant losses resulting from product liability claims. Although we have obtained and intend to maintain product liability insurance to offset some of this risk, we may be unable to maintain such insurance or it may not cover certain potential claims against us.
In the future, we may not be able to afford to obtain insurance due to rising costs in insurance premiums in recent years. Currently we have been able to secure insurance coverage; however, we may be faced with a successful claim against us in excess of our product liability coverage that could result in a material adverse impact on our business. If insurance coverage is too expensive or is unavailable to us in the future, we may be forced to self-insure against product-related claims. Without insurance coverage, a successful claim against us and any defense costs incurred in defending ourselves may have a material adverse impact on our operations.
We may not be successful in receiving additional patents based on our intellectual property strategy.
We have undertaken an effort to examine our intellectual property assets and have or shall file certain patents in certain jurisdictions, with the goal of attaining additional protections for our technologies and any related future products. The applications we have filed or we expect to file may never yield patents that protect our inventions and intellectual property assets. Failure to obtain additional patents may limit our protection against generic drug manufacturers and other parties who may seek to copy or otherwise produce products substantially similar to ours using technologies that may be substantially similar to those we own.
The use of our technologies could potentially conflict with the rights of others.
The manufacture, use or sale of our proprietary products may infringe on the patent rights of others. If we are unable to avoid infringement of the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court. Patent litigation is costly and time consuming and may divert management’s attention and our resources. We may not have sufficient resources to bring these actions to a successful conclusion. In such case, we may be required to alter our products, pay licensing fees or cease activities. If our products conflict with patent rights of others, third parties could bring legal actions against us claiming damages and seeking to enjoin manufacturing and marketing of affected products. If these legal actions are successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to manufacture or market the affected products. We may not prevail in any legal action and a required license under the patent may not available on acceptable terms, if at all.
We will be dependent on outside manufacturers in the event that we successfully develop our product candidates into commercial products; therefore, we will have limited control of the manufacturing process, access to raw materials, timing for delivery of finished products and costs. One manufacturer may constitute the sole source of one or more of our products.
We expect that third party manufacturers will manufacture all of our products, in the event that we successfully develop our product candidates into commercial products. Currently, certain of our contract manufacturers constitute the sole source of one or more of our products. If any of our existing or future manufacturers cease to manufacture or are otherwise unable to deliver any of our products or any of the components of our products, we may need to engage additional manufacturing partners. Because of contractual restraints and the lead-time necessary to obtain FDA approval of a new manufacturer, replacement of any of these manufacturers may be expensive and time consuming and may disrupt or delay our ability to supply our products and reduce our revenues.
Because all of our products, in the event that we successfully develop our product candidates into commercial products, will be manufactured by third parties, we have a limited ability to control the manufacturing process, access to raw materials, the timing for delivery of finished products or costs related to this process. There can be no assurance that our contract manufacturers will be able to produce finished products in quantities that are sufficient to meet demand or at all, in a timely manner, which could result in decreased revenues and loss of market share. There may be delays in the manufacturing process over which we will have no control, including shortages of raw materials, labor disputes, backlog or failure to meet FDA standards. Increases in the prices we pay our manufacturers, interruptions in our supply of products or lapses in quality could adversely impact our financial condition. We are reliant on our third-party manufacturers to maintain their manufacturing facilities in compliance with FDA and other federal, state and/or local regulations including health, safety and environmental standards. If they fail to maintain compliance with FDA or other critical regulations, they could be ordered to curtail operations, which would have a material adverse impact on our business, results of operations and financial condition.
13
We also rely on our outside manufacturers to assist us in the preparation of key documents such as drug master files and other relevant documents that are required by the FDA as part of the drug approval process and post-approval oversight. Failure by our outside manufacturers to properly prepare and retain these documents could cause delays in obtaining FDA approval of our drug candidates.
We are dependent on third parties to conduct clinical trials and non-clinical studies of our drug candidates and to provide services for certain core aspects of our business. Any interruption or failure by these third parties to meet their obligations pursuant to various agreements with us could have a material adverse effect on our business, results of operations and financial condition.
We do not employ personnel or possess the facilities necessary to conduct many of the activities associated with our programs. We expect to engage consultants, advisors, CROs and others to design, conduct, analyze and interpret the results of studies in connection with the research and development of our product candidates. As a result, many important aspects of our product candidates’ development are outside our direct control. Such third parties may not perform all of their obligations under arrangements with us or may not perform those obligations satisfactorily.
The CROs with whom we expect to contract for execution of our clinical studies will play a significant role in the conduct of our anticipated clinical studies or assist with our analysis of completed studies and to develop corresponding regulatory strategies. Individuals working at the CROs with whom we expect to contract, as well as investigators at the sites at which our studies are conducted, are not our employees, and we cannot control the amount or timing of resources that they devote to our programs. If these CROs fail to devote sufficient time and resources to our studies, or if their performance is substandard, it would delay the approval of our applications to regulatory agencies and the introduction of our products. Failure of these CROs to meet their obligations could adversely affect development of our product candidates and as a result could have a material adverse effect on our business, financial condition and results of operations. Moreover, these CROs may have relationships with other commercial entities, some of which may compete with us. If they assist our competitors at our expense, it could harm our competitive position.
We currently have no internal sales and marketing resources and may have to rely on third parties in the event that we successfully commercialize our product.
In order to market any of our products in the United States or elsewhere, we must develop internally or obtain access to sales and marketing forces with technical expertise and with supporting distribution capability in the relevant geographic territory. We may not be able to enter into marketing and distribution arrangements or find a corporate partner to market our drug candidates, and we currently do not have the resources or expertise to market and distribute our products ourselves. If we are not able to enter into marketing or distribution arrangements or find a corporate partner who can provide support for commercialization of our products, we may not be able to successfully commercialize our products. Moreover, any new marketer or distributor or corporate partner for our specific combinations with whom we choose to contract may not establish adequate sales and distribution capabilities or gain market acceptance for our products.
14
If we are unable to retain our key personnel or attract additional professional staff, we may be unable to maintain or expand our business.
As we described elsewhere in this prospectus, we terminated all of our employees following our filing of the Chapter 11 Case. Since the dismissal of the Chapter 11 Case in December 2011, we have focused on rebuilding our management team and engaging consultants in order to begin operating our business. However, because of this history, we may have significant difficulty attracting and retaining necessary employees. In addition, because of the specialized scientific nature of our business, our ability to develop products and to compete will remain highly dependent, in large part, upon our ability to attract and retain qualified scientific, technical and commercial personnel. The loss of key scientific, technical and commercial personnel or the failure to recruit key scientific, technical and commercial personnel could have a material adverse effect on our business. While we have consulting agreements with certain key individuals and institutions, we may not succeed in retaining personnel or their services under existing agreements or otherwise. There is intense competition for qualified personnel in the pharmaceutical industry, and we may be unable to continue to attract and retain the qualified personnel necessary for the development of our business.
Risks Relating to Our Industry
If we are unable to compete with other companies that develop rival products to our products, then we may never gain market share or achieve profitability.
The pharmaceutical industry is intensely competitive, and we face competition across the full range of our activities. If we fail to compete successfully, our business, results of operations and financial condition could be adversely affected. Our competitors include brand name and generic manufacturers of pharmaceuticals specializing in transdermal drug delivery, especially those doing business in the United States. In the market for pain management products, our competitors include manufacturers of over-the-counter and prescription pain relievers. Because we are smaller than many of our national competitors, we may lack the financial and other resources needed to compete for market share in the pain management sector. Our other potential drug candidates will also face intense competition from larger and better established pharmaceutical and biotechnology companies. Many of these competitors have significantly greater financial, technical and scientific resources than we do. In addition to product safety, development and efficacy, other competitive factors in the pharmaceutical market include product quality and price, reputation, service and access to scientific and technical information. If our products are unable to compete with the products of our competitors, we may never gain market share or achieve profitability.
We may not be able to keep up with the rapid technological change in the biotechnology and pharmaceutical industries, which could make our products obsolete and reduce our potential revenues.
Biotechnology and related pharmaceutical technologies have undergone and continue to be subject to rapid and significant change. Our future will depend in large part on our ability to maintain a competitive position with respect to these technologies. It is possible that developments by our competitors will render our products and technologies obsolete or unable to compete. Any products that we develop may become obsolete before we recover expenses incurred in developing those products, which may require that we raise additional funds to continue our operations.
Our ability to generate revenues will be diminished if we fail to obtain acceptable prices or an adequate level of reimbursement from third-party payors.
If we succeed in bringing a specific product to market, we cannot be certain that the products will be considered cost effective and that reimbursement from insurance companies and other third-party payors will be available or, if available, will be sufficient to allow us to sell the products on a competitive basis.
Significant uncertainty exists as to the reimbursement status of newly approved health care products. Third-party payors, including Medicare, are challenging the prices charged for medical products and services. Government and other third-party payors increasingly are attempting to contain health care costs by limiting both coverage and the level of reimbursement for new drugs and by refusing, in some cases, to provide coverage for uses of approved products for disease indications for which the FDA has not granted labeling approval. Third-party insurance coverage may not be available to patients for any products we discover and develop, alone or with collaborators. If government and other third-party payors do not provide adequate coverage and reimbursement levels for our products, the market acceptance of these products may be reduced.
15
Changes in the healthcare industry that are beyond our control may be detrimental to our business.
The healthcare industry is changing rapidly as consumers, governments, medical professionals and the pharmaceutical industry examine ways to broaden medical coverage while controlling the increase in healthcare costs. In 2009 and 2010, the U.S. Congress adopted legislation regarding health insurance, which has been signed into law. As a result of this new legislation, substantial changes could be made to the current system of paying for healthcare in the United States, including changes made in order to extend medical benefits to those who currently lack insurance coverage. Extending coverage to a large population could substantially change the structure of the health insurance system and the methodology for reimbursing medical services, drugs and devices. These structural changes could entail modifications to the existing system of private payers and government programs, such as Medicare, Medicaid and State Children’s Health Insurance Program, creation of a government-sponsored healthcare insurance source, or some combination of both, as well as other changes. Restructuring the coverage of medical care in the United States could impact the reimbursement for prescribed drugs, biopharmaceuticals, medical devices, or our product candidates and could put pressure on the prices of pharmaceutical products, which could adversely affect our business or products.
Risks Relating to the Offering and Ownership of our Common Stock
You will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future.
You will incur immediate and substantial dilution as a result of this offering. After giving effect to the sale by us of 1,619,047 shares of common stock (assumes that the underwriters do not exercise its over-allotment option), and after deducting underwriting fees and estimated offering expenses payable by us, investors in this offering can expect an immediate dilution of $3.12 per share, or 59.4%, at the public offering price.
Since inception we have funded our operations primarily through equity and debt financings and we expect to continue to do so in the future. Any additional equity or convertible debt offerings we may effect in the future will further dilute your ownership interest in the Company. To the extent any of the warrants and options we have issued is ultimately exercised you will sustain future dilution. In addition to the issuance of equity to PCCA in connection with the recent PCCA Tranaction, we may acquire or license other technologies or finance strategic alliances by issuing additional equity, which may result in additional dilution to our stockholders.
We may allocate the net proceeds from this offering in ways that differ from our estimates based on our current plans and assumptions discussed in the section titled “Use of Proceeds” and with which you may not agree.
The allocation of net proceeds of the offering set forth in the “Use of Proceeds” section below represents our estimates based upon our current plans and assumptions regarding industry and general economic conditions, our future revenues and expenditures. The amounts and timing of our actual expenditures will depend on numerous factors, including market conditions, cash generated by our operations, business developments and related rate of growth. We may find it necessary or advisable to use portions of the proceeds from this offering for other purposes. Circumstances that may give rise to a change in the use of proceeds and the alternate purposes for which the proceeds may be used are discussed in the section entitled “Use of Proceeds” below. You may not have an opportunity to evaluate the economic, financial or other information on which we base our decisions on how to use our proceeds. As a result, you and other stockholders may not agree with our decisions. See "Use of Proceeds" for additional information.
Sales of common stock by our stockholders, or the perception that such sales may occur, could depress our stock price.
Sales of our common stock in the public market following this offering could lower the market price of our common stock. Sales may also make it more difficult for us to sell equity securities or equity-related securities in the future at a time and price that our management deems acceptable or at all.
In addition, the market price of our common stock could decline as a result of sales by, or the perceived possibility of sales by, our existing stockholders. We have completed a number of private placements of our common stock and other securities over the last year. Future sales of common stock by significant stockholders, including those who acquired their shares in private placements or who are affiliates, or the perception that such sales may occur, could depress the price of our common stock.
16
An active trading market for shares of our common stock may not develop or be sustained.
Trading in our common stock is sporadic and volatile. We cannot predict the extent to which an active public market for our common stock will develop or be sustained. It is a condition of this offering that we be listed on The NASDAQ Capital Market and we have filed an application for such listing. However, approval of our application is contingent upon a number of factors and events, including the final terms of this offering. Further, even if our common stock begins to trade on The NASDAQ Capital Market in the future, we may not be able to meet the requirements for continued listing going forward. Our common stock has historically been sporadically or “thinly-traded” in the over-the-counter market. As a consequence, there may be extended periods when trading activity in our shares is minimal, as compared to a seasoned issuer with a large and steady volume of trading activity. The market for our common shares is also characterized by significant price volatility compared to seasoned issuers, and we expect that such volatility will continue. As a result of this lack of liquidity, the trading of relatively small quantities of shares may disproportionately influence the price of those shares in either direction. It is possible that a broader or more active public trading market for our common stock will not develop or be sustained.
There may not be any broker interested in making a market for our stock. Therefore, it may be difficult to sell your shares of common stock if you desire or need to sell them. Our underwriters are not obligated to make a market in our securities, and even if they choose to do so they can discontinue at any time without notice. It is possible that an active and liquid trading market in our securities may never develop or, if one does develop, that the market will not continue.
Because of their significant stock ownership, some of our existing stockholders will be able to exert control over us and our significant corporate decisions, and sales by management and the Board of Directors from time to time could have an adverse effect on our stock price.
Our executive officers and directors own or have the right to acquire within 60 days, in the aggregate, approximately 24% of the shares of common stock outstanding following such issuance to them. In addition, four individual stockholders hold an additional approximately 45% of our common stock. The sale of even a portion of these shares will likely have a material adverse effect on our stock price. In addition, these persons, acting together, have the ability to exercise significant influence over the outcome of all matters submitted to our stockholders for approval, including the election and removal of directors and any significant transaction involving us, as well as control our management and affairs. Since our stock ownership is concentrated among a limited number of holders and our Amended and Restated Certificate of Incorporation and Bylaws permit our stockholders to act by written consent, a limited number of stockholders may approve stockholder actions without holding a meeting of stockholders and could control the outcome of actions requiring stockholder approval. This concentration of ownership may harm the market price of our common stock by, among other things:
|
●
|
delaying, deferring, or preventing a change in control of our company;
|
|
●
|
impeding a merger, consolidation, takeover, or other business combination involving our company;
|
|
●
|
causing us to enter into transactions or agreements that are not in the best interests of all stockholders; or
|
|
●
|
discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of our company.
|
We have identified material weaknesses in our internal control over financial reporting. If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or prevent fraud.
As described in our periodic reports filed with the SEC, including Item 4 of Part I of our Quarterly Report on Form 10-Q for the period ended September 30, 2012 and our Annual Report on Form 10-K for the fiscal year ended December 31, 2011, we have identified material weaknesses in our internal controls and procedures. As a result, we have concluded that our disclosure controls and procedures were not effective as of the end of the period covered by these reports. We have implemented, and continue to implement, actions to address these weaknesses and to enhance the reliability and effectiveness of our internal controls and operations; however, the measures we have taken to date and any future measures may not remediate the material weaknesses discussed in our periodic reports. In addition, we may not be able to maintain adequate controls over our financial processes and reporting in the future. We may discover additional material weaknesses, which we may not successfully remediate on a timely basis or at all. Any failure to remediate any material weaknesses identified by us or to implement required new or improved controls, or difficulties encountered in their implementation, could cause us to fail to meet our reporting obligations or result in material misstatements in our consolidated financial statements. Inadequate internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative impact on the trading price of our stock. Moreover, we will be required to expend significant resources to design, implement and maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company. The costs associated with external consultants, as well as internal resources are significant and difficult to predict. As a result, our business, results of operations, financial condition and cash flows could be adversely affected.
17
Our stock price may be volatile.
The market price of our common stock is likely to be highly volatile and could fluctuate widely in response to various factors, many of which are beyond our control, including the following:
|
●
|
changes in the pharmaceutical industry and markets;
|
|
●
|
competitive pricing pressures;
|
|
●
|
our ability to obtain working capital financing;
|
|
●
|
new competitors in our market;
|
|
●
|
additions or departures of key personnel;
|
|
●
|
limited “public float” in the hands of a small number of persons whose sales or lack of sales could result in positive or negative pricing pressure on the market price for our common stock;
|
|
●
|
sales of our common stock;
|
|
●
|
our ability to execute our business plan;
|
|
●
|
operating results that fall below expectations;
|
|
●
|
loss of any strategic relationship with our contract manufacturers or with other third parties (including PCCA) and clinical and non-clinical research organizations;
|
|
●
|
industry or regulatory developments; or
|
|
●
|
economic and other external factors.
|
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
We have the right to issue shares of preferred stock. If we were to issue preferred stock, it is likely to have rights, preferences and privileges superior to those of our common stock.
We are authorized to issue 5,000,000 shares of “blank check” preferred stock, with such rights, preferences and privileges as may be determined from time-to-time by our board of directors. Following the conversion of our Series A Preferred Stock on June 29, 2012, we have no shares of preferred stock issued and outstanding. Our board of directors is empowered, without stockholder approval, to issue preferred stock in one or more series, and to fix for any series the dividend rights, dissolution or liquidation preferences, redemption prices, conversion rights, voting rights, and other rights, preferences and privileges for the preferred stock. We have no immediate plans to issue shares of preferred stock. The issuance of shares of preferred stock, depending on the rights, preferences and privileges attributable to the preferred stock, could adversely reduce the voting rights and powers of the common stock and the portion of our assets allocated for distribution to common stock holders in a liquidation event, and could also result in dilution in the book value per share of the common stock we are offering. The preferred stock could also be utilized, under certain circumstances, as a method for raising additional capital or discouraging, delaying or preventing a change in control of the company.
18
We have not paid dividends in the past and do not expect to pay dividends in the future. Any return on investment may be limited to the value of our common stock.
We have never paid cash dividends on our common stock and do not anticipate doing so in the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition and other business and economic factors affecting us at such time as our board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
The sale by our stockholders of substantial amounts of our common stock in the public market or upon the expiration of any statutory holding period, under Rule 144, or upon expiration of lock-up periods applicable to outstanding shares, or issued upon the exercise of outstanding options or warrants, could create a circumstance commonly referred to as an “overhang” and in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether or not sales have occurred or are occurring, also could make more difficult our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
19
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Information contained in this prospectus contains forward-looking statements. This information may involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from future results, performance or achievements expressed or implied by any forward-looking statements. Forward-looking statements, which involve assumptions and describe our future plans, strategies and expectations, are generally identifiable by use of the words “may,” “will,” “should,” “expect,” “anticipate,” “estimate,” “believe,” “intend” or “project” or the negative of these words or other variations on these words or comparable terminology. In addition to the risks and uncertainties described in “Risk Factors” above and elsewhere in this prospectus, these risks and uncertainties may include risks related to:
|
●
|
our ability to successfully implement our business plan following dismissal of the Chapter 11 Case;
|
|
●
|
the success of our proposed clinical trials;
|
|
●
|
our ability to research and successfully develop our product candidates;
|
|
●
|
general economic and business conditions;
|
|
●
|
our ability to continue as a going concern;
|
|
●
|
our ability to obtain financing necessary to operate our business;
|
|
●
|
our limited operating history;
|
|
●
|
our ability to recruit and retain qualified personnel;
|
|
●
|
our ability to manage future growth;
|
|
●
|
our ability to successfully complete and realize the benefits of potential acquisitions and collaborative arrangements (including our arrangement with PCCA); and
|
|
●
|
other factors discussed under the section entitled “Risk Factors”.
|
Forward-looking statements are based on assumptions that may be incorrect, and there can be no assurance that any projections or other expectations included in any forward-looking statements will come to pass. Moreover, we operate in a competitive and rapidly changing environment in which new risks emerge from time to time. Our actual results could differ materially from those expressed or implied by the forward-looking statements as a result of various factors. Except as required by applicable laws, we undertake no obligation to update publicly any forward-looking statements for any reason, even if new information becomes available or other events occur in the future.
20
USE OF PROCEEDS
Based on an assumed offering price of $5.25 per share, we estimate the gross proceeds from the sale of 1,619,047 shares of common stock, prior to deducting underwriting discounts and commissions and the estimated offering expenses payable by us, will be approximately $8.5 million (approximately $9.775 million if the over-allotment option granted to the underwriters is exercised in full).
We estimate that we will receive net proceeds of approximately $7.1 million, after deducting underwriting discounts and commissions and our underwriters' expense allowance and estimated expenses of approximately $0.7 million, which includes legal, accounting, printing costs and various fees associated with the registration and listing of our shares. If the underwriters exercise their right to purchase an additional 242,857 shares of common stock to cover over-allotments, we will receive approximately an additional $1.275 million, after deducting approximately $0.83 million for underwriting discounts and commissions.
We currently expect to use approximately $7.1 million of the funds received in this offering to fund our Phase 3 clinical trials for Impracor and pre-Phase 3 clinical studies for potential drug candidates in the muscle relaxant and neuropathic pain fields. We also expect to use any funds we receive from the underwriters in connection with its exercise of the over-allotment option, along with the $7.95 million raised in our April Private Placement and the $4 million raised in connection with the PCCA Transaction, to further fund these efforts, and fund additional IP protection and other Accudel related research and development expenses. We may also use a portion of these proceeds for the potential acquisition of, or investment in, product candidates, technologies, formulations or companies that complement our business, although other than as provided in the PCCA License Agreement, we have no current understandings, commitments, or agreements to do so. The following table summarizes our intent regarding use of such proceeds:
|
Over-Allotment
|
||||||||||||||||
|
Clinical:
|
Use of Proceeds
|
Option
|
||||||||||||||
|
Phase 3 Impracor Trials
|
$4,000,000 | 57% | $4,000,000 | 49% | ||||||||||||
|
Pre-Phase 3 Trials - Other Drug Candidates
|
3,068,414 | 43% | 4,035,038 | 49% | ||||||||||||
|
IP Protection, Research and Development
|
- | 0% | 200,000 | 2% | ||||||||||||
|
Total
|
$7,068,414 | 100% | $8,235,038 | 100% | ||||||||||||
The amounts that will actually be spent by us for any specific purpose may vary significantly, and will depend on a number of factors including the pace of progress of our Phase 3 Trials, development and actual needs with respect to product testing, development and research, market conditions, and changes in or revisions to our marketing and/or licensing strategies. In addition, we may allocate capital from the above referenced net proceeds to uses other than those described above in the event one or more opportunities become available to invest in near term drug development projects that are as advanced as our Impracor program is or that we believe have a greater chance for positive clinical results with at least as large of a market opportunity as Impracor has; however, we do not have any commitments for any opportunities of this nature at this time.
The amounts and timing of our actual expenditures will depend upon numerous factors, including market conditions, cash generated by our operations, business developments and related rate of growth, sales and marketing activities and competition. Accordingly, our management will have broad discretion in the application of the net proceeds, and investors will be relying on the judgment of our management regarding the application of the proceeds from this offering. We may find it necessary or advisable to use portions of the proceeds from this offering for other purposes.
21
Circumstances that may give rise to a change in the use of proceeds and the alternate purposes for which the proceeds may be used include:
|
●
|
the existence of other opportunities or the need to take advantage of changes in timing of our existing activities;
|
|
●
|
the need or desire on our part to accelerate, increase or eliminate existing initiatives due to, among other things, changing market conditions and competitive developments; and/or
|
|
●
|
if strategic opportunities of which we are not currently aware present themselves (including acquisitions, joint ventures, licensing and other similar transactions).
|
From time to time, we evaluate these and other factors and we anticipate continuing to make such evaluations to determine if the existing allocation of resources, including the proceeds of this offering, is being optimized.
Pending its use, we intend to invest the net proceeds of this offering in direct and guaranteed obligations of the United States, interest-bearing, investment-grade instruments or certificates of deposit.
DESCRIPTION OF CAPITAL STOCK
The following is a summary of the rights of our common and preferred stock and of certain provisions of our Amended and Restated Certificate of Incorporation and Bylaws. For more detailed information, please see our Amended and Restated Certificate of Incorporation and Bylaws, which are filed as exhibits to the registration statement of which this prospectus forms a part.
Our Amended and Restated Certificate of Incorporation provides for one class of common stock and authorizes the issuance of undesignated preferred stock, the rights, preferences and privileges of which may be designated from time to time by our Board of Directors. Our Board has designated 10 shares of preferred stock as Series A Preferred Stock.
Reverse Stock Split
On April 25, 2012, our Board of Directors and stockholders holding a majority of our outstanding voting power approved a resolution authorizing our Board of Directors to effect a reverse split of our common stock at an exchange ratio of (i) one-for-three, (ii) one-for-four, (iii) one-for-five, or (iv) one-for-six, with our Board of Directors retaining the discretion as to whether to implement the reverse split and which exchange ratio to implement. The action by written consent of the stockholders became effective on May 31, 2012, following our compliance with certain notice requirements under the Exchange Act. We anticipate that immediately following the effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of this offering, our Board of Directors will effect a reverse stock split at a ratio of one-for-five. The anticipated reverse split of our common stock will not affect the number of shares of authorized common stock or the par value of our shares of common stock.
Unless otherwise stated below, all information regarding share amounts of common stock and prices per share of common stock described below assume the consummation of the one-for-five reverse stock split to be effected following the effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of this offering.
Authorized Capital Stock
On February 28, 2012, we amended our Amended and Restated Certificate of Incorporation to, among other things, (i) increase the number of authorized shares of our capital stock to 400,000,000 and the number of authorized shares of common stock to 395,000,000 and (ii) effect a one for eight reverse stock split of our authorized, issued and outstanding common stock. Our authorized capital stock now consists of 400,000,000 shares, 395,000,000 of which are designated as common stock, par value $0.001 per share, and 5,000,000 of which are designated as preferred stock, par value $0.001 per share.
Capital Stock Issued and Outstanding
As of February 1, 2013, we had outstanding 6,772,125 shares of common stock held by 175 stockholders of record. In addition, as of February 1, 2013, we had outstanding (i) options to acquire 917,235 shares of our common stock with a weighted average exercise price of $5.30 per share, all of which are held by current or former employees, directors and consultants, (ii) warrants to purchase 556,872 shares of common stock with exercise prices ranging from $5.925 to $176.00 per share, and (iii) 200,000 shares of common stock underlying restricted stock units granted outside the 2007 Plan.
22
Description of Common Stock
We are authorized to issue 395,000,000 shares of common stock, par value $0.001 per share. The holders of our common stock are entitled to one vote per share on all matters submitted to a vote of the stockholders, including the election of directors. Our Amended and Restated Certificate of Incorporation does not provide for cumulative voting in the election of directors. Subject to any preferential rights of any outstanding series of preferred stock created by our Board of Directors from time to time the holders of our common stock will be entitled to cash dividends as may be declared, if any, by our Board of Directors from funds available. Subject to any preferential rights of any outstanding series of preferred stock that we may issue, upon liquidation, dissolution or winding up of our company, the holders of our common stock will be entitled to receive pro rata all assets available for distribution to the holders. There are no outstanding shares of our common stock that we have agreed to register under the Securities Act for sale by stockholders.
Description of Preferred Stock
Undesignated Preferred Stock
Our Board of Directors has the authority, without further action by our stockholders, to issue up to 5,000,000 shares of preferred stock, par value $0.001 per share, in one or more series. Our Board of Directors may designate the rights, preferences, privileges and restrictions of the preferred stock, including dividend rights, conversion rights, voting rights, terms of redemption, liquidation preference, sinking fund terms, and number of shares constituting any series and the designation of any series. The issuance of preferred stock could have the effect of restricting dividends on our common stock, diluting the voting power of our common stock, impairing the liquidation rights of our common stock, or delaying or preventing a change in control. The ability to issue preferred stock could delay or impede a change in control. As of the date of this prospectus and following the completion of this offering, no shares of preferred stock are outstanding and we currently have no plan to issue any shares of preferred stock.
Series A Preferred Stock
On December 12, 2011, we issued 10 shares of Series A Preferred Stock to DermaStar in a private placement. The Series A Preferred Stock has the rights and preferences identified in the Certificate of Designation to our Amended and Restated Certificate of Incorporation filed with the Delaware Secretary of State on December 9, 2011. Among other things, the Certificate of Designation (i) authorizes 10 shares of the Company’s preferred stock to be designated as “Series A Convertible Preferred Stock”, (ii) grants the holders of the Series A Preferred Stock the right to convert into our common stock at a conversion price of approximately $0.06668, as adjusted, (iii) grants a liquidation preference of $10,000 per share of Series A Preferred Stock, (iv) provides that the holders of Series A Preferred Stock shall vote with the holders of our common stock on an “as converted basis”, and (v) provides that the affirmative vote of a majority of the outstanding shares of the Series A Preferred Stock is required to approve certain other corporate matters including, among other things, changes to the rights of the holders of the Series A Preferred Stock, amendments to our Amended and Restated Certificate of Incorporation or Bylaws, issuance of priority or parity securities, issuance of debt securities, entry into certain fundamental transactions and increase or decrease in the size of our Board of Directors. On June 29, 2012, DermaStar converted the 10 shares of Series A Preferred Stock held by it into 1,499,700 shares of common stock. Immediately following the conversion of the Series A Preferred Stock, all 10 shares were retired to our treasury and cancelled.
Anti-Takeover Provisions
We are subject to the provisions of Section 203 of the Delaware General Corporation Law, or DGCL, an anti-takeover law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a ‘‘business combination’’ with an ‘‘interested stockholder’’ for a period of three years after the date of the transaction in which such stockholder became an interested stockholder, unless the business combination is approved in a prescribed manner. For purposes of Section 203, a ‘‘business combination’’ includes a merger, asset sale or other transaction resulting in a financial benefit to the interested stockholder, and an ‘‘interested stockholder’’ is a stockholder who, together with affiliates and associates, owns, or within three years prior, did own, 15% or more of the voting stock.
23
Liability and Indemnification of Directors and Officers
Section 145 of the DGCL provides, in general, that a corporation incorporated under the laws of the State of Delaware, such as us, may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action, a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
Our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws provide that we will indemnify our directors, officers, employees and agents to the extent and in the manner permitted by the provisions of the DGCL, as amended from time to time, subject to any permissible expansion or limitation of such indemnification, as may be set forth in any stockholders’ or directors’ resolution or by contract.
We also have director and officer indemnification agreements with each of our executive officers and directors that provide, among other things, for the indemnification to the fullest extent permitted or required by Delaware law, provided that such indemnitee shall not be entitled to indemnification in connection with any proceedings or claims initiated or brought voluntarily by the indemnitee and not by way of defense, unless (i) such indemnification is expressly required to be made by law, (ii) the proceeding was authorized by our Board of Directors, (iii) indemnification is provided by us, in our sole discretion, pursuant to powers vested in us under the DGCL, or (iv) the proceeding is brought to establish or enforce a right to indemnification under the indemnification agreement or any other statute or law or otherwise as required under Section 145 of the DGCL. We are not required to indemnify the indemnitee for any amounts paid in settlement of a proceeding unless we consent to such settlement.
Any repeal or modification of these provisions approved by our stockholders shall be prospective only, and shall not adversely affect any limitation on the liability of a director or officer existing as of the time of such repeal or modification.
We have purchased and intend to maintain insurance on our behalf and on behalf of any person who is or was a director or officer against any loss arising from any claim asserted against him or her and incurred by him or her in that capacity, subject to certain exclusions and limits of the amount of coverage.
Transfer Agent
The transfer agent and registrar for our common stock is Action Stock Transfer Corporation, 2469 E. Fort Union Blvd., Suite 214, Salt Lake City, UT 84121.
24
CAPITALIZATION
The following table sets forth our cash and cash equivalents and our capitalization as of September 30, 2012, in each case after giving effect to the one-for-five reverse stock split of our common stock to be effected following the effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of the offering:
|
●
|
on an actual basis;
|
|
●
|
on an as adjusted basis, giving effect to the sale by us of 1,619,047 shares of common stock in this offering at an assumed public offering price of $5.25 per share, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
|
You should read this table together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes appearing elsewhere in this prospectus.
|
September 30, 2012
|
||||||||
|
Actual
|
As Adjusted
|
|||||||
|
(unaudited)
|
(unaudited)
|
|||||||
|
Cash and cash equivalents
|
$ | 10,990,871 | $ | 18,059,285 | ||||
|
Total debt
|
$ | - | $ | - | ||||
|
Stockholders' equity:
|
||||||||
|
Preferred stock, $0.001 par value; 5,000,000 shares authorized,
|
||||||||
|
no shares issued and outstanding, actual and as adjusted
|
- | - | ||||||
|
Common stock, $0.001 par value; 395,000,000 shares authorized,
|
||||||||
|
6,771,926 shares issued and outstanding, actual;
|
||||||||
|
8,390,973 shares issued and outstanding, as adjusted
|
6,772 | 8,391 | ||||||
|
Additional paid-in capital
|
33,438,601 | 40,505,396 | ||||||
|
Deficit accumulated during the development stage
|
(22,651,240 | ) | (22,651,240 | ) | ||||
|
Total stockholders' equity
|
10,794,133 | 17,862,547 | ||||||
|
Total capitalization
|
$ | 10,794,133 | $ | 17,862,547 | ||||
25
The number of shares of our common stock to be outstanding after this offering is based on 6,771,926 shares outstanding as of September 30, 2012, and excludes:
|
●
|
898,404 shares of common stock issuable upon the exercise of options outstanding as of September 30, 2012 with exercise prices ranging from $2.40 to $80.00 and a weighted average exercise price of $5.20 per share;
|
|
●
|
557,341 shares of common stock underlying warrants issuable upon the exercise of warrants outstanding as of September 30, 2012 with exercise prices ranging from $5.925 to $176.00 and a weighted average exercise price of $7.80 per share, of which warrants to acquire up to 469 shares of common stock with an exercise price of $160.00 per share expired subsequent to September 30, 2012;
|
|
●
|
200,000 shares of common stock issuable underlying restricted stock units granted outside of our 2007 Plan;
|
|
●
|
warrants to purchase up to 136,000 shares of our common stock issuable to the underwriters in connection with the completion of this offering;
|
|
●
|
1,485,491 shares of common stock available for future grant as of September 30, 2012 under our 2007 Plan.
|
26
DILUTION
If you invest in the securities offered in this offering, your interest will be diluted immediately to the extent of the difference between the assumed public offering price per share of our common stock and the net tangible book value per share of our common stock after this offering. As of September 30, 2012, our net tangible book value was $10,794,133, or $1.59 share of common stock (after giving effect to the one-for-five reverse stock split). Net tangible book value per share represents the total tangible assets of the Company, less all liabilities, divided by the number of shares of common stock outstanding.
Net tangible book value dilution per share represents the difference between the amount per share of common stock paid by the new investors who purchase securities in this offering and the net tangible book value per share of common stock immediately after completion of this offering. After giving effect to our sale of 1,619,047 shares of common stock at a public offering price of $5.25 per share, and after underwriting fees and commissions and estimated offering expenses payable by us, our net tangible book value as of September 30, 2012 would have been $17,862,547, or $2.13 per share. This represents an immediate increase of net tangible book value of $0.54 per share to our existing stockholders and an immediate dilution in net tangible book value of $3.12 per share to purchasers of our common stock in this offering. The following table illustrates this per share dilution:
| Adjusted | ||||||||
|
Assumed public offering price per unit
|
$ | 5.25 | ||||||
|
Net tangible book value per share as of September 30, 2012
|
$ | 1.59 | ||||||
|
Increase in net tangible book value per share attributable to new investors
|
0.54 | |||||||
|
Adjusted net tangible book value per share after this offering
|
2.13 | |||||||
|
Dilution per share to new investors purchasing common stock in this offering
|
$ | 3.12 | ||||||
27
The above discussion and table do not include the following:
|
●
|
898,404 shares of common stock issuable upon the exercise of options outstanding as of September 30, 2012 with exercise prices ranging from $2.40 to $80.00 and a weighted average exercise price of $5.20 per share;
|
|
●
|
557,341 shares of common stock underlying warrants issuable upon the exercise of warrants outstanding as of September 30, 2012 with exercise prices ranging from $5.925 to $176.00 and a weighted average exercise price of $7.80 per share, of which warrants to acquire up to 469 shares of common stock with an exercise price of $160.00 per share expired subsequent to September 30, 2012;
|
|
●
|
200,000 shares of common stock issuable underlying restricted stock units granted outside of our 2007 Plan;
|
|
●
|
warrants to purchase up to 136,000 shares of our common stock issuable to the underwriters in connection with the completion of this offering;
|
|
●
|
1,485,491 shares of common stock available for future grant as of September 30, 2012 under our 2007 Plan.
|
28
UNDERWRITING
This is an underwritten offering of our common stock. MDB Capital Group, LLC is the sole book-running manager of the offering and is acting as the representative of the underwriters named below. We have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to each of the underwriters, and each of the underwriters has agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of shares of common stock set forth opposite such underwriter’s name below (not including shares that may be issued pursuant to the underwriters’ over-allotment option). The underwriters are committed to purchase all the common shares offered by us, other than those covered by the option to purchase additional shares described below, if they purchase any shares. A copy of the underwriting agreement has been filed as an exhibit to the registration statement of which this prospectus forms a part.
|
Underwriters
|
Number of
Shares
|
||
|
MDB Capital Group, LLC
|
|||
| Aegis Capital Corp. | |||
|
Total
|
1,619,047 | ||
|
|
The underwriters have advised us that they propose to offer the shares of our common stock directly to the public at the public offering price set forth on the cover page of this prospectus and to certain dealers that are members of the Financial Industry Regulatory Authority (FINRA). Any securities sold by the underwriter to such securities dealers will be sold at the public offering price less a selling concession not in excess of $0.26775 per share. After the public offering of the shares this figure may be changed by the underwriters.
The underwriting agreement provides that the underwriters’ obligation to purchase shares of our common stock is subject to conditions contained in the underwriting agreement.
None of our securities included in this offering may be offered or sold, directly or indirectly, nor may this prospectus and any other offering material or advertisements in connection with the offer and sales of any of our common stock be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who receive this prospectus are advised to inform themselves about and to observe any restrictions relating to this offering of our common stock and the distribution of this prospectus. This prospectus is neither an offer to sell nor a solicitation of any offer to buy any of our common stock included in this offering in any jurisdiction where that would not be permitted or legal.
The underwriters have advised us that they do not intend to confirm sales to any accounts over which they exercise discretionary authority.
Underwriting Discount and Expenses
The following table summarizes the underwriting discount and commission to be paid to the underwriters by us.
|
Without
Over-Allotment
|
With
Over-Allotment
|
|||||||
|
Public offering price per share
|
$
|
5.25
|
$
|
5.25
|
||||
|
Underwriting discount per share to be paid to the underwriter by us for the common stock
|
$
|
0.4463
|
$
|
0.4463
|
||||
|
Non-accountable expense allowance
|
$
|
150,000
|
$
|
150,000
|
||||
|
Proceeds, before expenses, to us
|
$
|
7,777,497
|
$
|
8,944,121
|
||||
We estimate the expenses payable by us for this offering to be $1.4 million, including the underwriting discount, or $1.5 million if the over-allotment option is exercised in full, and reimbursement of up to $160,000 in expenses of the underwriters as follows: (i) non-accountable expense amount of $150,000, of which $30,000 we advanced prior to the commencement of this offering, and (ii) up to $10,000 of accountable expenses, which we advanced prior to the commencement of this offering. We will not reimburse the underwriters more than a total of $160,000 in expenses, including all of the underwriters' legal, road show and out of pocket expenses in connection with this offering.
29
Over-allotment Option
We have granted to the underwriters an option, exercisable not later than 45 days after the date of this prospectus, to purchase up to an additional 242,857 shares of our common stock (up to 15% of the shares firmly committed in this offering) at the public offering price, less the underwriting discount, set forth on the cover page of this prospectus. The underwriters may exercise the option solely to cover over-allotments, if any, made in connection with this offering. If any additional shares of our common stock are purchased pursuant to the over-allotment option, the underwriters will offer these additional shares of our common stock on the same terms as those on which the other shares of common stock are being offered hereby.
Determination of Offering Price
The public offering price of the common stock was negotiated between us and the underwriters, based on the trading price of the common stock prior to the offering, among other things. Other factors considered in determining the price of the common stock include the history and prospects of our company, the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment of our management, general conditions of the financial markets at the time of the offering and such other factors as were deemed relevant.
Underwriter Warrants
We have agreed to issue to the underwriters warrants to purchase up to 8.5% of the shares of common stock sold in this offering. These warrants are exercisable at $5.25 per share (100% of the price of the common stock sold in this offering), commencing on the effective date of this offering and expiring five years from the effective date of this offering. The warrants and the shares of common stock underlying the warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of the FINRA. The underwriters (or permitted assignees under the Rule) will not sell, transfer, assign, pledge or hypothecate the warrants or the securities underlying the warrants, nor will they engage in any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the warrants or the underlying securities for a period of 180 days from the effective date of the registration statement of which this prospectus is a part.
Lock-Up Agreements
All of our officers and directors and stockholders beneficially owning 5% or more of our common stock have agreed that, for a period of 180 days from the date of this prospectus, they will not sell, contract to sell, grant any option for the sale or otherwise dispose of any of our equity securities, or any securities convertible into or exercisable or exchangeable for our equity securities, without the consent of MDB Capital Group, LLC, except for exercise or conversion of currently outstanding warrants and options, as applicable, and exercise of options under an acceptable stock incentive plan. MDB Capital Group, LLC may consent to an early release from the lock-up period if, in its opinion, the market for the common stock would not be adversely impacted by sales and in cases of a financial emergency of an officer, director or other stockholder. We are unaware of any officer, director or stockholder who intends to ask for consent to dispose of any of our equity securities during the relevant lock-up period.
Indemnification
We will agree to indemnify the underwriters against certain liabilities, including certain liabilities arising under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
30
Stabilization, Short Positions and Penalty Bids
The underwriters may engage in over-allotment, stabilizing transactions, syndicate covering transactions, and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of the common stock, in accordance with Regulation M under the Exchange Act.
Over-allotment involves sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriters may close out any short position by either exercising the over-allotment option and/or purchasing shares in the open market.
Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum.
Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. If the underwriters sell more shares than could be covered by the over-allotment option, a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering.
Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the shares originally sold by the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions.
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result, the price of the common stock may be higher than the price that might otherwise exist in the open market. These transactions, if commenced, may be discontinued at any time.
Neither we nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of the common stock. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these stabilizing transactions or that any transaction, once commenced, will not be discontinued without notice.
Passive Market Making
In connection with the offering, the underwriters may engage in passive market making transactions in the common stock in accordance with Rule 103 of Regulation M under the Exchange Act during the period before the commencement of offers or sales of common stock and extending through the completion of distribution. A passive market maker must display its bids at a price not in excess of the highest independent bid of the security. However, if all independent bids are lowered below the passive market maker’s bid, that bid must be lowered when specified purchase limits are exceeded.
Electronic Distribution
A prospectus in electronic format may be made available on the Internet sites or through other online services maintained by the underwriters, or by their affiliates. In those cases, prospective investors may view offering terms online and, depending upon the underwriter, prospective investors may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of shares for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriters on the same basis as other allocations.
31
Other than the prospectus in electronic format, the information on the underwriters' websites and any information contained in any other websites maintained by the underwriters is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the underwriters in their capacity as underwriters and should not be relied upon by investors.
The underwriters' compensation in connection with this offering is limited to the fees and expenses described in this section under “Underwriting Discount and Expenses.”
United Kingdom
This document is only being distributed to and is only directed at (i) persons who are outside the United Kingdom or (ii) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”), or (iii) high net worth entities, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (e) of the Order (all such persons together being referred to as “relevant persons”). The shares of common stock are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such common stock will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
The underwriters have represented and agreed that:
(a) they have only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 or FSMA) received by them in connection with the issue or sale of the shares in circumstances in which Section 21(1) of the FSMA does not apply to us, and
(b) they have complied with, and will comply with all applicable provisions of FSMA with respect to anything done by them in relation to the shares in, from or otherwise involving the United Kingdom.
European Economic Area
To the extent that the offer of the common stock is made in any Member State of the European Economic Area that has implemented the Prospectus Directive before the date of publication of a prospectus in relation to the common stock which has been approved by the competent authority in the Member State in accordance with the Prospectus Directive (or, where appropriate, published in accordance with the Prospectus Directive and notified to the competent authority in the Member State in accordance with the Prospectus Directive), the offer (including any offer pursuant to this document) is only addressed to qualified investors in that Member State within the meaning of the Prospectus Directive or has been or will be made otherwise in circumstances that do not require us to publish a prospectus pursuant to the Prospectus Directive.
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”), the underwriters have represented and agreed that with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State (the “Relevant Implementation Date”) they have not made and will not make an offer of shares to the public in that Relevant Member State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the Prospectus Directive, except that it may, with effect from and including the Relevant Implementation Date, make an offer of shares to the public in that Relevant Member State at any time:
32
(a) to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities,
(b) to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000, and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts, or in any other circumstances which do not require the publication by us of a prospectus pursuant to Article 3 of the Prospectus Directive. For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
The EEA selling restriction is in addition to any other selling restrictions set out below. In relation to each Relevant Member State, each purchaser of shares of common stock (other than the underwriters) will be deemed to have represented, acknowledged and agreed that it will not make an offer of shares of common stock to the public in any Relevant Member State, except that it may, with effect from and including the date on which the Prospectus Directive is implemented in the Relevant Member State, make an offer of shares of common stock to the public in that Relevant Member State at any time in any circumstances which do not require the publication by us of a prospectus pursuant to Article 3 of the Prospectus Directive, provided that such purchaser agrees that it has not and will not make an offer of any shares of common stock in reliance or purported reliance on Article 3(2)(b) of the Prospectus Directive. For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares of common stock in any Relevant Member State has the same meaning as in the preceding paragraph.
MARKET PRICE OF AND DIVIDENDS ON COMMON STOCK AND RELATED MATTERS
Our common stock has been quoted on the over-the-counter market beginning on March 14, 2007 and is currently quoted on the OTC Markets Group QB tier, or OTCQB, under the symbol IMMY. Effective September 21, 2007, our common shares began quotation on the Over-the-Counter Bulletin Board, or OTCBB, under the symbol TDLP. Due to our failure to comply with SEC filing requirements and following our entry into bankruptcy proceedings (as described in more detail elsewhere in this prospectus), effective May 20, 2011, our common stock ceased being quoted on the OTCBB and, effective on June 28, 2011, began quotation under the symbol TDLPQ.PK on the OTC Markets Group Pink tier, or OTC Pink. On February 24, 2012, our common stock began quotation on the OTCQB under the symbol TDLPD, in connection with the one-for-eight reverse split of our authorized, issued and outstanding common stock effected on February 28, 2012. On March 23, 2012, our common stock began trading under the symbol IMMY in connection with our name change to Imprimis Pharmaceuticals, Inc. The liquidity of our shares on the OTCBB, OTC Pink and OTCQB markets has been extremely limited and any prices quoted may not be a reliable indication of the value of our common stock. There is no established trading market for our common stock.
The following table sets forth the high and low last-bid prices for our common stock for the periods indicated, as reported by the OTCBB, OTC Pink or the OTCQB, as applicable, after giving effect to the expected one-for-five reverse stock split. The quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not represent actual transactions.
|
Fiscal Year 2013
|
High
|
Low
|
||||||
| First Quarter (through February 1, 2013) | $ | 8.75 | $ | 7.20 | ||||
|
Fiscal Year 2012
|
High
|
Low
|
||||||
|
First Quarter
|
$ | 3.75 | $ | 0.50 | ||||
|
Second Quarter
|
$ | 4.98 | $ | 2.55 | ||||
|
Third Quarter
|
$ | 9.00 | $ | 3.00 | ||||
| Fourth Quarter | $ | 15.25 | $ | 4.75 | ||||
|
Fiscal Year 2011
|
High
|
Low
|
||||||
|
First Quarter
|
$ | 26.00 | $ | 6.00 | ||||
|
Second Quarter
|
$ | 10.40 | $ | 1.08 | ||||
|
Third Quarter
|
$ | 5.64 | $ | 1.24 | ||||
|
Fourth Quarter
|
$ | 9.64 | $ | 1.20 | ||||
|
Fiscal Year 2010
|
High
|
Low
|
||||||
|
First Quarter
|
$ | 50.00 | $ | 22.40 | ||||
|
Second Quarter
|
$ | 44.00 | $ | 22.40 | ||||
|
Third Quarter
|
$ | 48.40 | $ | 21.20 | ||||
|
Fourth Quarter
|
$ | 24.00 | $ | 12.00 | ||||
33
Holders
As of February 1, 2013 we had approximately 6,772,125 shares of common stock issued and outstanding (after giving effect to the one-for-five reverse stock split) held by 175 holders of record (excluding an indeterminable number of stockholders whose shares are held in street or “nominee” name).
Dividends
We have never paid any dividends on our common stock and do not expect to pay dividends on our common stock in the foreseeable future.
SHARES ELIGIBLE FOR FUTURE SALE
Since the filing of our Chapter 11 Case with the Bankruptcy Court in June 2011, there has been a limited public market for our common stock. Future sales of substantial amounts of our common stock in the public market, or the perception that such sales may occur, could adversely affect the prevailing market price of our common stock. No prediction can be made as to the effect, if any, future sales of shares, or the availability of shares for future sales, will have on the market price of our common stock prevailing from time to time.
Unless otherwise stated below, all information regarding share amounts of common stock and prices per share of common stock described below assume the consummation of the one-for-five reverse stock split to be effected following the effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of this offering.
Sale of Restricted Shares
Upon completion of this offering, we will have 8,391,172 shares of common stock and no shares of preferred stock outstanding, assuming the underwriters do not exercise their over-allotment option. Of these shares of common stock, the shares being sold in this offering will be freely tradable without restriction under the Securities Act, except for any such shares which may be held or acquired by an “affiliate” of ours, as that term is defined in Rule 144 promulgated under the Securities Act, which shares will be subject to the volume limitations and other restrictions of Rule 144 as described below. Of the remaining shares of common stock held by our existing stockholders upon completion of this offering, approximately 6,480,484 shares will be “restricted securities,” as that phrase is defined in Rule 144, and may be resold only after registration under the Securities Act or pursuant to an exemption from such registration, including, among others, the exemptions provided by Rule 144 and 701 under the Securities Act, which rules are summarized below. Included in these restricted shares are approximately 4,752,499 shares of common stock held by our existing stockholders, which upon completion of this offering will be available for sale in the public market after the expiration of the lock-up agreements described in “Underwriting” and under the heading “Lock Up Agreements” below, taking into account the provisions of Rules 144 and 701 under the Securities Act. We do not have any outstanding obligations to register any shares of capital stock.
34
Rule 144
Under Rule 144, persons who became the beneficial owner of shares of our common stock prior to the completion of this offering may not sell their shares until the earlier of (1) the expiration of a six-month holding period, if we have been subject to the reporting requirements of the Exchange Act and have filed all required reports for at least 90 days prior to the date of the sale, or (2) a one-year holding period.
At the expiration of the six-month holding period, a person who was not one of our affiliates at any time during the three months preceding a sale would be entitled to sell an unlimited number of shares of our common stock provided current public information about us is available, and a person who was one of our affiliates at any time during the three months preceding a sale would be entitled to sell within any three-month period only a number of shares of common stock that does not exceed the greater of either of the following:
|
●
|
1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering, based on the number of shares of our common stock outstanding after completion of this offering; or
|
|
●
|
the average weekly trading volume of our common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale.
|
At the expiration of the one-year holding period, a person who was not one of our affiliates at any time during the three months preceding a sale would be entitled to sell an unlimited number of shares of our common stock without restriction as long as we maintain current public information. A person who was one of our affiliates at any time during the three months preceding a sale would remain subject to the volume restrictions described above.
Sales under Rule 144 by our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
Stock Incentive Plans
We have filed registration statements on Form S-8 under the Securities Act to register all of the shares of our common stock issued or reserved for issuance under the 2007 Plan, as well as the shares underlying the restricted stock units granted outside of the 2007 Plan as described below. Accordingly, shares registered under such registration statement are available for sale in the open market, unless such shares are subject to vesting restrictions with us, Rule 144 restrictions applicable to our affiliates or the lock-up restrictions described below.
On July 18, 2012, the Board of Directors granted to Mr. Baum, in connection with his services as Chief Executive Officer, 160,000 restricted stock units (RSUs) outside of the 2007 Plan. The RSUs are subject to certain performance-based vesting criteria, such that 40,000 RSUs will vest upon the satisfaction of each of the following events: (i) successful completion of a financing that results in aggregate cash proceeds to the Company of at least $5,000,000 at any time following the effective date of the grant; (ii) the Company meets the primary endpoints of its Phase III clinical studies for Impracor; (iii) the Company submits a New Drug Application for Impracor to the U.S. Food and Drug Administration; and (iv) the Company enters into a definitive license, collaboration or similar agreement for Impracor that would reasonably be expected to generate cash flow for the Company. The RSUs vest in full upon a change in control of the Company.
On July 18, 2012, the Board of Directors granted to Dr. Kammer 40,000 RSUs outside of the 2007 Plan in connection with his services as a consultant and advisor to the Company. The RSUs are subject to certain performance-based vesting criteria, such that all 40,000 RSUs will vest at such time as the Company meets the primary endpoints of its Phase III clinical studies for Impracor.
35
Lock-Up Agreements
We, our executive officers and directors, and other existing security holders have agreed, subject to certain limited exceptions, not to sell or transfer any common stock or securities convertible into, exchangeable for, exercisable for, or repayable with common stock, for 180 days after the date of this prospectus without first obtaining the written consent of MDB Capital Group LLC. See “Underwriting.”
On June 27, 2012, DermaStar and certain other individuals entered into an agreement whereby all of the equity securities held by such parties (including the 1,499,700 shares of common stock underlying the then-outstanding 10 shares of Series A Preferred Stock) would be restricted from resale until the earlier of (i) 15 months from June 27, 2012 or (ii) the date we publicly announce we have filed a new drug application with the FDA for Impracor. The restrictions continue to apply to all members of DermaStar following DermaStar’s dissolution in July 2012 and the related distribution in kind of the shares of our common stock and warrants held by it. Holders of approximately 51% of our outstanding stock as of February 1, 2013 are bound by these lock-up restrictions, including Mr. Baum and Dr. Kammer. The restrictions terminate upon our entry into a definitive agreement for certain change in control transactions.
36
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with the consolidated financial statements and the related notes contained elsewhere in this prospectus. In addition to historical information, the following discussion contains forward looking statements based upon current expectations that are subject to risks and uncertainties. Actual results may differ substantially from those referred to herein due to a number of factors, including but not limited to risks described in the section entitled “Risk Factors” and elsewhere in this prospectus.
Unless otherwise stated below, all information regarding share amounts of common stock and prices per share of common stock described under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations” assume the consummation of the one-for-five reverse stock split to be effected following the effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of the offering.
Overview
We are a specialty pharmaceutical company developing non-invasive, topically delivered product candidates. Our patented Accudel cream formulation technology is designed to enable highly targeted site specific treatment. Impracor, our lead pain product candidate, utilizes the Accudel platform technology to deliver the active drug, ketoprofen, a non-steroidal anti-inflammatory drug, through the skin directly into the underlying tissues where the drug exerts its localized anti-inflammatory and analgesic effects.
Through our strategic relationship with Professional Compounding Centers of America, Inc. (“PCCA”), one of the largest drug compounding organizations in the world, we expect to facilitate our future selection, formulation and development of potential product candidates. Our relationship with PCCA is exclusive and provides us with the opportunity to develop new products using PCCA’s proprietary drug formulations and drug delivery technologies, as well as access to an extensive database of market-oriented information related to drug development opportunities. We plan to use our proprietary Accudel drug delivery technology, coupled with these licensed technologies, formulations and market data, to identify pharmaceutical development opportunities where we perceive a significant unmet need for a new drug product. We are currently considering potential product candidates in the muscle relaxant and neuropathic pain fields, and we expect those areas may be our next avenues for additional product development.
On February 28, 2012, we changed our name from Transdel Pharmaceuticals, Inc. to Imprimis Pharmaceuticals, Inc. All prior references to Transdel Pharmaceuticals, Inc. have been changed to Imprimis to reflect our current name. Unless the context otherwise requires, all references in this Report to “we,” “us,” “our,” “the Company,” or “Imprimis” refers to Imprimis Pharmaceuticals, Inc. and its subsidiaries.
On February 28, 2012, we effected a one-for-eight reverse split of our authorized, issued and outstanding common stock. The information in this prospectus and the accompanying consolidated financial statements for interim and annual prior periods presented have been retroactively adjusted to reflect the effects of the one-for-eight reverse stock split.
We have incurred recurring operating losses, have had negative operating cash flows and have not recognized any significant revenues since July 24, 1998 (inception). In addition, we have a deficit accumulated during the development stage of approximately $22.7 million at September 30, 2012. We have not yet generated commercial sales revenue from any of our product candidates and we will incur further losses through the 2012 fiscal year and beyond as we continue the clinical development of our drug candidates, including Impracor, and conduct preclinical studies on other programs. Our research and development activities are budgeted to expand over time, and we will require further capital resources to fund the continued operation of our business model for a long enough period to achieve profitable operations.
Plan of Operations
For the next twelve months, our current operating plan is focused on the development of our lead product candidate, Impracor, for the indication of acute musculoskeletal pain, inflammation and swelling associated with soft tissue injuries, and limited development of other potential product candidates and pursuit of co-development opportunities in other therapeutic areas, in each case utilizing our Accudel platform technology.
On June 26, 2011 we filed a voluntary petition for reorganization relief under Chapter 11 of the United States Bankruptcy Code in the United States Bankruptcy Court for the Southern District of California (the" Bankruptcy Court"), Case No. 11-10497-11 (the "Chapter 11 Case"). Following the filing of the Chapter 11 Case with the Bankruptcy Court, we suspended our operations and terminated nearly all of our employees. Since the dismissal of the Chapter 11 Case in December 2011, as further described below, we have engaged a new management team, appointed new directors to fill certain vacancies on our Board and worked towards re-initiating our Phase 3 clinical trials for Impracor. However, we have a limited operating history since the dismissal of the Chapter 11 Case, and we may not be successful in our efforts to resume our operations. Prior to the filing of the Chapter 11 Case, we were unable to successfully pursue our business plan and continue our clinical trials due to a lack of funding. Given our operating history, we may be unable to obtain additional funds when necessary, maintain an effective management team, or hire and retain further qualified individuals. As a result, we may be unable to successfully pursue our business plan.
37
Recent Developments
Bankruptcy Petition and Dismissal
On June 26, 2011 we filed the Chapter 11 Case with the Bankruptcy Court. In connection with the Chapter 11 Case, we, as seller, and Cardium Healthcare, Inc., a wholly-owned subsidiary of Cardium Therapeutics, Inc., as purchaser (“Cardium”), entered into an Asset Purchase Agreement dated June 23, 2011 (the “Asset Purchase Agreement”) pursuant to which we agreed to sell substantially all of our assets pursuant to Sections 105, 363 and 365 of the Bankruptcy Code, subject to court approval and the satisfaction of certain conditions set forth in the Asset Purchase Agreement. Consummation of the sale to Cardium was subject to a number of conditions, including, among others, the approval by the Bankruptcy Court of the transactions contemplated by the Asset Purchase Agreement and compliance with certain specified deadlines for actions in connection with the Chapter 11 Case. The Asset Purchase Agreement was terminable by the parties under a number of circumstances, including failure to obtain certain Bankruptcy Court orders by agreed dates.
On July 26, 2011, the Bankruptcy Court denied our motion to sell our assets pursuant to the Asset Purchase Agreement. On October 7, 2011, we terminated the Asset Purchase Agreement pursuant to its terms. On November 21, 2011, in connection with the transactions described below, we requested that the Bankruptcy Court dismiss the Chapter 11 Case and retain jurisdiction to decide matters related to claims brought in the Chapter 11 Case by Cardium. On December 8, 2011, the Bankruptcy Court entered an order dismissing the Chapter 11 Case. In connection with the dismissal of the Chapter 11 Case, the Bankruptcy Court, among other things, declined to retain jurisdiction over claim objection proceedings and found moot our objection to certain claims of Cardium. The dismissal of the Chapter 11 Case was based upon the provisions of both 11 U.S.C. Sections 305(a) and 1112(b).
Secured Line of Credit
On November 21, 2011, we entered into a Secured Line of Credit Letter Agreement (the “Line of Credit Agreement”) with DermaStar International, LLC (“DermaStar”), pursuant to which DermaStar agreed to lend us funds under a line of credit upon certain conditions, including the dismissal of the Chapter 11 Case by the Bankruptcy Court. The Line of Credit Agreement became effective on December 9, 2011, in connection with the dismissal of the Chapter 11 Case by the Bankruptcy Court. The Line of Credit Agreement provided for advances of up to an aggregate of $750,000, subject to the satisfaction by us of certain conditions in connection with the initial advance and each subsequent advance.
On April 25, 2012, the entire outstanding principal balance and all accrued and unpaid interest under the line of credit, an aggregate of $762,534, was converted into 193,047 shares of common stock and warrants to purchase 48,262 shares of common stock at the offering price and on the terms of the April Private Placement described below, pursuant to the terms of a conversion agreement we entered into with DermaStar on April 20, 2012. The warrants have substantially the same terms as the warrants issued in the April Private Placement. The line of credit was terminated upon the completion of the conversion.
Change in Control – Issuance of Preferred Stock
In partial consideration for and in connection with the Line of Credit Agreement, on November 21, 2011 we executed a Securities Purchase Agreement (the “Series A Purchase Agreement”) with DermaStar, pursuant to which we agreed to issue 10 shares of newly-designated Series A Convertible Preferred Stock (the “Series A Preferred Stock”) to DermaStar for an aggregate purchase price of $100,000. The Series A Purchase Agreement, as amended, became effective on December 9, 2011, in connection with the dismissal of the Chapter 11 Case by the Bankruptcy Court. On December 12, 2011, we and DermaStar consummated the transactions contemplated by the Series A Purchase Agreement. The shares of Series A Preferred Stock issued to DermaStar in the offering were convertible into 1,499,700 shares of our common stock. Upon issuance of the Series A Preferred Stock, DermaStar, and its members individually, became control persons of the Company. We appointed DermaStar Managing Members Mark L. Baum and Robert J. Kammer to our Board of Directors in December 2011.
38
On June 29, 2012, DermaStar converted the 10 shares of Series A Preferred Stock held by it into 1,499,700 shares of our common stock. In connection with the conversion, we paid to DermaStar $200,000 as partial consideration for the conversion pursuant to a conversion agreement. Immediately following the conversion of the Series A Preferred Stock, all 10 shares were retired to our treasury and cancelled. The conversion agreement was unanimously approved by the Company’s disinterested directors, with Mr. Baum and Dr. Kammer abstaining.
Settlement with the Holders of the Company’s 7.5% Convertible Promissory Note
On April 5, 2010, we issued a $1,000,000 7.5% Convertible Promissory Note (the “Convertible Note”) to Alexej Ladonnikov. During January 2012, Mr. Ladonnikov sold 80% of the Convertible Note to DermaStar in a private transaction. Effective as of January 25, 2012, we entered into separate waiver and settlement agreements with DermaStar and Mr. Ladonnikov. Under each of the waiver and settlement agreements, the holders of the Convertible Note agreed to forever waive (i) their rights to accelerate the entire unpaid principal sum of the Convertible Note and all accrued interest pursuant to Section 1 of the Convertible Note, (ii) their rights under Section 7 of the Senior Convertible Note Purchase Agreement dated April 5, 2010, and (iii) certain conversion rights pursuant to Section 3 of the Convertible Note. Under the terms of the waiver and settlement agreement with DermaStar, we and DermaStar agreed to the mandatory conversion of the principal and accrued and unpaid interest of the Convertible Note and $56,087 in current accounts payable of the Company held by DermaStar into our common stock at a conversion price of approximately $0.06668 per share at such time as we had a sufficient number of shares of authorized common stock to effect such conversion. Under the terms of the waiver and settlement agreement with Mr. Ladonnikov, we and Mr. Ladonnikov agreed to the mandatory conversion of the 20% of the principal and accrued and unpaid interest of the Convertible Note held by Mr. Ladonnikov, at such time as we had a sufficient number of authorized common shares to effect such a conversion, into our common stock at a conversion price of $0.60. Mr. Ladonnikov also agreed to make a one-time payment of $50,000 to us at such time as the Convertible Note was converted into common stock.
On February 28, 2012, effective immediately following the effective time of our Certificate of Amendment to our Certificate of Incorporation increasing the number of authorized shares of common stock and implementing the one-for-eight reverse split of our common stock, the entire outstanding balance and all accrued but unpaid interest owing under the Convertible Note and the accounts payable held by DermaStar were converted into 1,835,830 shares of common stock, and the Convertible Note was terminated. Mr. Ladonnikov made the required one-time payment of $50,000 to us at the time of the conversion.
Changes in Management and Board of Directors
As a result of the Chapter 11 Case, our management team has undergone significant changes. The Board accepted the resignation of John N. Bonfiglio, Ph.D. as our Chief Executive Officer and President, effective May 13, 2011. On the same date, the Board appointed John T. Lomoro to serve as the Company’s Principal Executive Officer. Effective September 16, 2011, the Board accepted the resignation of John T. Lomoro as Principal Executive Officer, Chief Financial Officer and Treasurer of the Company. On the same date, the Board appointed Terry Nida, the Company’s Chief Business Officer, to serve as the Company’s Principal Executive Officer and Principal Financial Officer. Effective December 16, 2011, Terry Nida resigned as Principal Executive Officer and Principal Financial Officer of the Company.
In January 2012, we began assembling a new management team. Effective January 1, 2012, the Board appointed Balbir Brar, D.V.M., Ph.D. as President of the Company. Effective February 1, 2012, the Board appointed Andrew R. Boll as Vice-President of Accounting and Public Reporting and Principal Accounting and Financial Officer of the Company. Effective February 15, 2012, the Board appointed Joachim Schupp, M.D. as Chief Medical Officer of the Company. Dr. Schupp had previously served as our Chief Medical Officer and Dr. Brar had previously served as our Vice President of Research and Development. Mr. Baum served as our Chairman of the Board of Directors and principal executive officer beginning in December 2011. On April 1, 2012, the Board appointed Mr. Baum as our Chief Executive Officer and Mr. Baum stepped down as our Chairman of the Board. He continues to serve as a director.
Our Board of Directors has also undergone significant change. Effective December 16, 2011, Anthony S. Thornley resigned from our Board of Directors, and Mr. Baum and Dr. Kammer, managing members of DermaStar, joined the Board of Directors. Effective February 15, 2012, Paul Finnegan, M.D., and Dr. Brar, our President, were appointed as directors of the Company. On April 1, 2012, Dr. Kammer began serving as the Chairman of the Board of Directors. On July 26, 2012, Stephen G. Austin, CPA, was appointed as a director on our Board of Directors and Dr. Brar resigned as a director (Dr. Brar continues to serve as our President). Additionally, on December 14, 2012, Mr. August S. Bassani, Pharm.D., was appointed as a director on our Board of Directors. We currently have the following six directors: Jeffrey Abrams, M.D., Mr. Bassani, Mr. Baum, Dr. Kammer, Dr. Finnegan and Mr. Austin.
39
April Private Placement
On April 20, 2012, we entered into a Securities Purchase Agreement with certain accredited investors relating to the sale and issuance of an aggregate of 2,011,691 shares of our common stock and warrants to purchase up to 502,928 shares of common stock at an exercise price of $5.925 per share, for an aggregate gross purchase price of approximately $7.95 million (the “April Private Placement”). We closed the April Private Placement on April 25, 2012. The securities sold in the April Private Placement were sold in reliance on the exemption from the registration requirements of the Securities Act of 1933 (the “Securities Act”) afforded by Section 4(2) of the Securities Act and Rule 506 of Regulation D.
The investors are not entitled to any registration rights with respect to the common stock and warrants issued in the April Private Placement. The warrants have a term of three years and are exercisable any time after April 25, 2012. We may require that the investors exercise the warrants in whole, but not in part, at any time within 20 business days after all of the following conditions have been satisfied: (i) the volume weighted average price of the our common stock for 10 consecutive trading days is equal to or greater than the exercise price of the warrants; (ii) we have received a Filing Review Notification from the U.S. Food and Drug Administration ("FDA") regarding the status of Impracor; and (iii) sufficient shares of common stock are authorized and reserved for issuance upon full exercise of the warrants.
PCCA Transaction
On August 30, 2012, we entered into a License Agreement (the “PCCA License Agreement") and a Stock Purchase Agreement (the “PCCA Purchase Agreement”) in a strategic transaction with PCCA (the “PCCA Transaction”).
Pursuant to the terms of the PCCA License Agreement, effective August 30, 2012, PCCA has granted to us and our affiliates certain exclusive rights under PCCA’s proprietary formulations, other technologies and data, and we have agreed to pay to PCCA certain royalties on net sales relating to the sale of certain future products, which royalties range from 4.5% to 9% for each product, subject to certain minimum royalty payments. PCCA may terminate the PCCA License Agreement if we fail to commence efforts to research and develop future products within certain time periods.
Pursuant to the terms of the PCCA Purchase Agreement, closed on August 31, 2012, we issued and sold to PCCA 832,683 shares of our common stock at a per share purchase price of $4.8038, for aggregate gross proceeds to us of $4,000,000. The PCCA Purchase Agreement does not grant to PCCA any registration rights with respect to the shares purchased and sold thereunder. The shares sold to PCCA were sold in reliance on the exemption from the registration requirements of the Securities Act afforded by Section 4(2) thereof.
Critical Accounting Policies
We rely on the use of estimates and make assumptions that impact our financial condition and results. These estimates and assumptions are based on historical results and trends as well as our forecasts as to how results and trends might change in the future. Although we believe that the estimates we use are reasonable, actual results could differ from those estimates.
We believe that the accounting policies described below are critical to understanding our business, results of operations and financial condition because they involve more significant judgments and estimates used in the preparation of our consolidated financial statements. An accounting policy is deemed to be critical if it requires an accounting estimate to be made based on assumptions about matters that are highly uncertain at the time the estimate is made, and any changes in the different estimates that could have been used in the accounting estimates that are reasonably likely to occur periodically could materially impact our condensed consolidated financial statements.
Our most critical accounting policies and estimates that may materially impact our results of operations include:
Stock-Based Compensation. All share-based payments to employees, including grants of employee stock options and restricted stock grants, to be recognized in the consolidated financial statements are based upon their fair values. We use the Black-Scholes-Merton option pricing model to estimate the grant-date fair value of share-based awards. Fair value is determined at the date of grant. The financial statement effect of forfeitures is estimated at the time of grant and revised, if necessary, if the actual effect differs from those estimates.
Our accounting policy for equity instruments issued to consultants and vendors in exchange for goods and services follows Financial Accounting Standards Board (“FASB”) guidance. As such, the value of the applicable stock-based compensation is periodically remeasured and income or expense is recognized during the vesting terms. The measurement date for the fair value of the equity instruments issued is determined at the earlier of (i) the date at which a commitment for performance by the consultant or vendor is reached or (ii) the date at which the consultant or vendor’s performance is complete. In the case of equity instruments issued to consultants, the fair value of the equity instrument is recognized over the term of the consulting agreement. An asset acquired in exchange for the issuance of fully vested, nonforfeitable equity instruments should not be presented or classified as an offset to equity on the grantor’s balance sheet once the equity instrument is granted for accounting purposes. Accordingly, we record the fair value of nonforfeitable equity instruments issued for future consulting services as prepaid consulting fees in our condensed consolidated balance sheets.
40
Income Taxes. As part of the process of preparing our consolidated financial statements, we must estimate our actual current tax liabilities together with assessing temporary differences resulting from differing treatment of items for tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within the balance sheet. We must assess the likelihood that the deferred tax assets will be recovered from future taxable income and, to the extent we believe that recovery is not likely, a valuation allowance must be established. To the extent we establish a valuation allowance or increase or decrease this allowance in a period, the impact will be included in the tax provision in the statement of operations.
Research and Development. We expense all costs related to research and development as they are incurred.
Results of Operations
The following period to period comparisons of our financial results and our interim results are not necessarily indicative of future results.
For the Three and Nine Months Ended September 30, 2012, Compared to the Three and Nine Months Ended September 30, 2011
Revenues
No revenues were recognized during the three months ended September 30, 2012 and 2011. For the nine months ended September 30, 2012 we recognized $100,000 in revenues, compared to no revenues recognized during the same period in the prior year. These revenues were non-refundable royalty advances, unrelated to product sales, paid to the Company in December 2010 and April 2011. The revenues stem from our terminated license agreement which had provided JH Direct rights to our anti-cellulite cosmetic product. This agreement was terminated in January 2012, and we do not expect any other revenues to be recognized from it.
Selling, General and Administrative Expenses
Our selling, general and administrative expenses include personnel costs including wages and stock-based compensation, corporate facility expenses, investor relations, consulting, insurance, legal and accounting expenses.
The table below provides information regarding selling, general and administrative expenses.
|
Three months ended September 30,
|
$ |
Nine months ended September 30,
|
$ | |||||||||||||||||||||
|
2012
|
2011
|
Variance
|
2012 | 2011 |
Variance
|
|||||||||||||||||||
|
Selling, general and administrative
|
$ | 946,381 | $ | 66,496 | $ | 879,885 | $ | 2,240,004 | $ | 514,529 | $ | 1,725,475 | ||||||||||||
For the three and nine months ended September 30, 2012, there was an increase of $879,885 and $1,725,475, respectively, in selling, general and administrative expenses, as compared to the same periods in the prior year. The increase in selling, general and administrative expenses is largely attributable to the resumption of our operations in December 2011, following the winding down and ceasing of operations during these periods in 2011, including the suspension of payroll beginning in March 2011. Selling, general and administrative expenses during the three and nine months ended September 30, 2012 were primarily due to the hiring of new personnel, consultants and management, legal and accounting fees associated with complying with our SEC reporting obligations and fees and expenses related to financing activities. A significant portion of the increase in personnel costs is associated with stock-based compensation for the three and nine months ended September 30, 2012, which increased $194,685 and $1,246,961, respectively, as compared to the same periods in the prior year.
Research and Development Expenses
Our research and development expenses primarily include expenses related to the Impracor clinical program. These costs are comprised of expenses for our first Phase 3 study, including costs for our contract research organization and investigator payments to the clinical sites participating in the study. Other expenses are personnel costs including wages and stock-based compensation, contract manufacturing, non-clinical studies, consulting and other costs related to the clinical program.
The table below provides information regarding research and development expenses.
|
Three months ended September 30,
|
$ |
Nine months ended September 30,
|
$ | |||||||||||||||||||||
|
2012
|
2011
|
Variance
|
2012 | 2011 |
Variance
|
|||||||||||||||||||
|
Research and development
|
$ | 303,666 | $ | 0 | $ | 303,666 | $ | 580,240 | $ | 111,554 | $ | 468,686 | ||||||||||||
For the three and nine months ended September 30, 2012, there was an increase of $303,666 and $468,686, respectively, in research and development expense as compared to the same periods in the prior year. The increase was primarily related to the hiring of new personnel and consultants in 2012 for the planning and development of additional Phase 3 studies of our Impracor clinical program, and costs related to supportive safety studies for Impracor, which began in September 2012. A significant portion of the increase in research and development personnel costs is associated with stock-based compensation for the three and nine months ended September 30, 2012, which increased $61,299 and $105,957, respectively, as compared to the same periods in the prior year.
41
Interest Expense
Interest expense was $0 and $24,658 for the three and nine months ended September 30, 2012, respectively, compared to $18,904 and $56,095 for the three and nine months ended September 30, 2011, respectively. The 10% promissory notes issued under our Line of Credit Agreement with DermaStar accounted for $0 and $12,535 of interest expense during the three and nine months ended September 30, 2012, respectively, and $0 during the same periods in the prior year. The 7.5% Convertible Note with a principal balance of $1,000,000, issued in April 2010 (and converted to shares of our common stock in February 2012) accounted for $0 and $12,123 of interest expense during the three and nine months ended September 30, 2012, respectively, and $18,904 and $56,095 during the three and nine months ended September 30, 2011, respectively.
Loss on Extinguishment of Debt
Loss from extinguishment of debt was $0 and $1,195,410 for the three and nine months ended September 30, 2012, respectively. As further described above under the heading “Recent Developments”, effective as of January 25, 2012, we entered into separate waiver and settlement agreements with DermaStar and Alexej Ladonnikov, the two holders of the Convertible Note. Pursuant to the waiver and settlement agreements, on February 28, 2012, the entire outstanding balance and all accrued but unpaid interest owing under the Convertible Note and the accounts payable held by DermaStar were converted into an aggregate of 1,835,830 shares of our common stock, and the Convertible Note was terminated. On February 28, 2012, we received payment from Mr. Ladonnikov of $50,000 and issued 380,868 shares of common stock to Mr. Ladonnikov as payment in full for his 20% ownership of the Convertible Note ($200,000) and its related accrued interest ($28,521). We determined this to be a substantial modification to the debt instrument and applied debt extinguishment accounting to record a loss on extinguishment of debt of $150,000 ($200,000 Convertible Note principal balance less $50,000 cash payment) for the nine months ended September 30, 2012. On February 28, 2012, we issued 1,454,962 shares of our common stock to DermaStar as payment in full for its 80% ownership of the Convertible Note ($800,000), its related accrued interest ($114,082) and $56,087 in accounts payable. We determined this to be a substantial modification to the debt instrument and applied debt extinguishment accounting to record a loss on extinguishment of debt of $856,087 for the nine months ended September 30, 2012.
As further described above under the heading “Recent Developments”, on April 20, 2012, DermaStar agreed to convert the promissory notes issued under the Line of Credit Agreement and their related accrued interest, totaling $762,534, into 193,047 shares of our common stock and a related warrant to purchase up to an additional 48,262 shares of our common stock at an exercise price of $5.925 per share. We determined this to be a substantial modification to the debt instrument and applied debt extinguishment accounting to record a loss on extinguishment of debt of $189,323 for the nine months ended September 30, 2012.
Net Loss
Net losses attributable to common stockholders for the three and nine months ended September 30, 2012, were $1,245,826 and $4,130,507, respectively, or $(0.20) and $(1.11), respectively, per basic and diluted share, compared to net losses attributable to common stockholders for the three and nine months ended September 30, 2011 of $85,400 and $682,178, respectively, or $(0.21) and $(1.71), respectively, per basic and diluted share.
42
Fiscal Year Ended December 31, 2011 Compared to Fiscal Year Ended December 31, 2010
Selling, General and Administrative Expenses
Our selling, general and administrative expenses include personnel costs including wages and stock-based compensation, corporate facility expenses, investor relations, consulting, insurance, legal and accounting expenses.
The table below provides information regarding selling, general and administrative expenses:
|
Year ended December 31,
|
$ | |||||||||||
|
2011
|
2010
|
Variance
|
||||||||||
|
Selling, general and administrative
|
$ | 827,674 | $ | 2,307,972 | $ | (1,480,298 | ) | |||||
For the fiscal year ended December 31, 2011, the decrease of $1,480,298 in selling, general and administrative expense, as compared to the prior year, was primarily a result of the suspension of payroll and primary business operations at and about March 1, 2011. In addition, we recognized an aggregate one-time expense of approximately $416,000 during the same period in 2010 related to the separation agreement with our former Chief Executive Officer. This amount was comprised of approximately $242,000 related to the accrual of continued salary and medical benefits to be provided for a period of one year after the separation date of February 17, 2010 and approximately $174,000 of stock-based compensation expense related to the modification of terms for the former Chief Executive Officer’s stock options.
Research and Development Expenses
Our research and development expenses primarily include costs for the Accudel clinical program. These costs are comprised of expenses for our current Phase 3 study, including costs for our contract research organization and investigator payments to the clinical sites participating in the study. Other expenses are personnel costs including wages and stock-based compensation, contract manufacturing, non-clinical studies, consulting and other costs related to the clinical program.
The table below provides information regarding research and development expenses:
| Year ended December 31, | $ | |||||||||||
|
2011
|
2010
|
Variance
|
||||||||||
|
Research and development
|
$ | 111,554 | $ | 194,588 | $ | (83,034 | ) | |||||
For the fiscal year ended December 31, 2011, the decrease of $83,034 in research and development expense, as compared to the prior year, was primarily related to a decrease of activities for the Phase 3 study, clinical trials and staff/consulting expenses as a result of the suspension of our operations in March 2011. In November 2010, we received a Federal grant amount of $244,479 under the Qualifying Therapeutic Discovery Project that is part of the Patient Protection and Affordable Care Act and was accounted for as a reduction to research and development expenses during the year ended December 31, 2010. The funds were awarded in support of Impracor, our late-stage topical NSAID for the treatment of acute soft tissue injuries.
43
Interest Income
Interest income was $0 and $512, for the years ended December 31, 2011 and 2010, respectively. The decrease was due to a lower average cash balance and lower interest rates during fiscal year 2011 as compared to fiscal year 2010
Interest Expense
In April 2010, we issued a senior convertible promissory note to an existing investor through a private placement. The note accrues interest at an annual interest rate of 7.5%. Interest expense on the note was $75,000 and $55,479 for the years ended December 31, 2011 and 2010, respectively.
Forgiveness of Liabilities
On October 5, 2011, priority claims of former employees (the “Priority Claimants”) in the amount of $119,667 originating as a result of the Chapter 11 Case, were settled and paid by the Company. These amounts consisted of accrued and owed payroll amounts, accrued vacation and any other claims held against the Company at October 5, 2011. The Priority Claimants were given cash in the amount $47,975 and 60,000 stock options valued at $11,400 (using the Black-Scholes option pricing model to estimate the grant-date fair value) and the difference of $60,292 was recognized as a gain on forgiveness of liabilities during the year ended December 31, 2011.
On October 2, 2008, we entered into a payment agreement with a vendor, settling a balance of $52,598, pursuant to which we agreed that 50% of the amount owed, or $26,299 would be forgiven and the remainder would be paid in two installments of $13,150 upon execution of the payment agreement and $13,149 upon an infusion of capital into the Company. Since the inception of the payment agreement, the amount to be forgiven, $26,299, continued to be recorded as an accounts payable up until the infusion of $1,000,000 from the issuance of the convertible note in April 2010. When the note was issued, the final installment payment of $13,149 was paid and the $26,299 was recognized as a gain by the Company during the year ended December 31, 2010.
Liquidity and Capital Resources
Our cash on hand at September 30, 2012 was $10,990,871 as compared to $2,154 at September 30, 2011. The increase in cash on hand is primarily attributable to aggregate net proceeds of approximately $11,916,000 received from our issuance of securities in the April Private Placement and the PCCA Transaction during the nine months ended September 30, 2012, and the $750,000 drawn under our Line of Credit Agreement with DermaStar between December 2011 and April 2012. From inception through September 30, 2012, we have incurred aggregate losses of approximately $(22,351,000). These losses are primarily due to selling, general and administrative and research and development expenses incurred in connection with developing and seeking regulatory approval for our lead drug, Impracor. Historically, our operations have been financed through capital contributions and debt and equity financings.
As we described in more detail above under the heading “Recent Developments,” on June 26, 2011 we filed a voluntary petition for reorganization relief under Chapter 11 of the U.S. Bankruptcy Code. Thereafter, we suspended our operations and terminated almost all of our employees. After receiving certain commitments from DermaStar to provide funding to us under a secured line of credit (as further described above under the heading "Recent Developments" and below), on November 21, 2011 we requested that the Bankruptcy Court dismiss the Chapter 11 Case. The Bankruptcy Court entered an order dismissing the Chapter 11 Case on December 8, 2011. Since December 9, 2011, we have focused on resuming the operation of our business, including assembling a management team and hiring employees.
44
Convertible Note
As we described in more detail above under the heading “Recent Developments,” on April 5, 2010 we issued a $1,000,000 7.5% Convertible Promissory Note. Effective as of January 25, 2012, we entered into separate waiver and settlement agreements with DermaStar and Alexej Ladonnikov, the two holders of the Convertible Note. Pursuant to the waiver and settlement agreements, on February 28, 2012, the entire outstanding balance and all accrued but unpaid interest owing under the Convertible Note and $56,087 in accounts payable held by DermaStar were converted into 1,835,830 shares of common stock, and the Convertible Note was terminated. In addition, Mr. Ladonnikov made a one-time payment of $50,000 to us at the time of the conversion.
Line of Credit
As further described above under the heading “Recent Developments,” on November 21, 2011 we entered into the Line of Credit Agreement with DermaStar. The Line of Credit Agreement provided for advances of up to an aggregate of $750,000, subject to the satisfaction by us of certain conditions in connection with each advance. Interest under the line of credit accrued at 10% per annum. As of December 31, 2011 and up to April 25, 2012 (the date of the conversion thereof), we had requested advances of $300,000 and $750,000, respectively, under the line of credit. On April 25, 2012, the entire outstanding principal balance and all accrued and unpaid interest under the line of credit, an aggregate of $762,534, was converted into 193,047 shares of common stock and warrants to purchase 48,262 shares of our common stock. The line of credit was terminated upon the completion of the conversion.
April Private Placement
As further described above under the heading “Recent Developments,” on April 25, 2012 we closed a private placement of securities with certain accredited investors for the sale and issuance of 2,011,691 shares of common stock and warrants to purchase up to 502,928 shares of common stock at an exercise price of $5.925 per share, for aggregate proceeds, net of offering costs, to us of approximately $7,930,000.
PCCA Transaction
Pursuant to the terms of the PCCA Purchase Agreement that we entered into with PCCA in connection with the PCCA Transaction, on August 31, 2012, we issued and sold to PCCA 832,683 shares of our common stock at a per share purchase price of $4.8038, for aggregate net proceeds to us of approximately $3,980,000.
Net Cash Flow
The following table provides detailed information about our net cash flow for the nine months ended September 30, 2012 and 2011.
|
Cash Flow
|
Nine Months Ended
September 30,
|
|||||||
|
2012
|
2011
|
|||||||
|
Net cash used in operating activities
|
$ | (1,287,127 | ) | $ | (316,845 | ) | ||
|
Net cash used in investing activities
|
(15,308 | ) | - | |||||
|
Net cash provided by financing activities
|
12,147,146 | 27,537 | ||||||
|
Net Increase (Decrease) in Cash and Cash Equivalents
|
10,844,711 | (289,308 | ) | |||||
|
Cash and Cash Equivalents at Beginning of the Period
|
146,160 | 291,462 | ||||||
|
Cash and Cash Equivalents at End of the Period
|
$ | 10,990,871 | $ | 2,154 | ||||
45
The following table provides detailed information about our net cash flow for the fiscal years ended December 31, 2011 and 2010:
|
Cash Flow (All amounts in U.S. dollars)
|
For The Years Ended
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Net cash used in operating activities
|
$
|
(291,160
|
)
|
$
|
(2,298,311
|
)
|
||
|
Net cash used in investing activities
|
-
|
-
|
||||||
|
Net cash provided by financing activities
|
145,858
|
1,000,000
|
||||||
|
Net Decrease in Cash and Cash Equivalents
|
(145,302
|
)
|
(1,298,311
|
)
|
||||
|
Cash and Cash Equivalents at Beginning of the Year
|
291,462
|
1,589,773
|
||||||
|
Cash and Cash Equivalents at End of the Year
|
$
|
146,160
|
$
|
291,462
|
||||
Operating Activities
Net cash used in operating activities was $1,287,127 for the nine months ended September 30, 2012, as compared to $316,845 used in operating activities during the same period for the prior year. The increase in net cash used in operating activities was mainly due to resuming the operation of our business, including assembling a management team and hiring employees, planning and development of additional Phase 3 studies, and the reduction of our historical working capital debt.
Net cash used in operating activities was $291,160 for the year ended December 31, 2011, as compared to $2,298,311 used in operating activities during 2010. The decrease in net cash used in operating activities was mainly due to the suspension of operations in fiscal 2011, and related matters including minimizing certain administrative expenses, suspension of payroll at March 1, 2011, and lengthening our accounts payable payment process.
Investing Activities
Net cash used in investing activities for the nine months ended September 30, 2012 and 2011 was $15,308 and $0, respectively. Net cash used in investing activities for the year ended December 31, 2011 and 2010 was $0. The increase in investing activities during the nine months ended September 30, 2012 was due primarily to our move into our new office space and our acquisition of furniture and office equipment to furnish that office space.
Financing Activities
Net cash provided by financing activities for the nine months ended September 30, 2012 and 2011 was $12,147,146 and $27,537, respectively. The increase in cash is primarily attributable to aggregate proceeds, net of offering costs, of approximately $7,930,000 received from the April Private Placement, $3,980,000 from the PCCA Purchase Agreement, and the $450,000 drawn under our Line of Credit Agreement with DermaStar between January 2012 and April 2012.
Net cash provided by financing activities for the year ended December 31, 2011 was $145,858, as compared to $1,000,000 net cash provided by financing activities for the year ended December 31, 2010. The decrease in net cash provided by financing activities was attributable to the issuance of the Convertible Note with a principal balance of $1,000,000 issued in exchange for cash of the same amount during April 2010. During the year ended December 31, 2011, we received $100,000 from the sale of Series A Preferred Stock and $300,000 in advances under our line of credit. This was offset by amounts reimbursed DermaStar for its payment of certain of our expenses incurred prior to the dismissal of the Chapter 11 Case, which totaled $254,142.
46
We expect to use our current cash position to begin executing on our business plan, including starting additional clinical studies related to our Accudel technology, and otherwise fund our operations. Management believes we have sufficient cash reserves to execute our business plan for the next twelve months. If we are not able to generate significant revenues and attain profitable operations, we will need to seek additional financing, including equity or debt financing, funding from a corporate partnership or licensing arrangement or any similar financing. In addition, estimates of our operating expenses and working capital requirements could be incorrect, and we could be required to seek additional financing earlier than we anticipate.
We will require additional funds in order to fully conduct our planned clinical trials and any other studies that may be required to obtain regulatory approval to market Impracor, to pursue additional pharmaceutical development programs and to explore other co-development opportunities. If adequate financing is not available, we may not be able to obtain regulatory approval to market Impracor or develop any additional products.
We may seek funds from equity or debt financings, a corporate partnership, or licensing arrangements (as we did with the PCCA transaction), or any other similar financing. Any future financings through equity investments are likely to be dilutive to existing stockholders. Also, the terms of securities we may issue in future capital transactions may be more favorable for our new investors. Newly issued securities may include preferences, superior voting rights and the issuance of warrants or other derivative securities, which may have additional dilutive effects. In addition, if we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish potentially valuable rights to our product candidates or proprietary technologies, or grant licenses on terms that are not favorable to us. Further, we may incur substantial costs in pursuing future capital and/or financing, including investment banking fees, legal fees, accounting fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we may issue, such as convertible notes and warrants, which will adversely impact our financial results.
We may be unable to obtain financing when necessary as a result of, among other things, general economic conditions, conditions in the pharmaceuticals industry or as a result of our operating history, including our recent bankruptcy proceedings. In addition, the fact that we are not and have never been profitable and will require additional funds to complete our planned clinical trials could further impact the availability or cost of future financings. As a result, there is no assurance that sufficient funds will be available when needed from any source or, if available, will be available on terms that are acceptable to us. If we are unable to raise funds to satisfy our capital needs on a timely basis, then we may not be able to obtain regulatory approval to market Impracor or develop any additional products or otherwise pursue our business plan and we may be required to cease operations.
As reported in the Report of Independent Registered Public Accounting Firm on our December 31, 2011 consolidated financial statements, we have incurred recurring losses from operations and have an accumulated deficit that raised substantial doubt about our ability to continue as a going concern. At September 30, 2012, we had increased our cash position by approximately $11 million as compared to our cash position at December 31, 2011. As of the date of this prospectus, management believes we have sufficient cash reserves to support our operating plan and fund operating cash flow requirements through the next twelve months.
Off-Balance Sheet Arrangements
Since our inception, except for standard operating leases, we have not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities. We have no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to stockholders.
There are no recent accounting pronouncements issued by the FASB that management believes have had or are reasonably likely to have a material impact on our present or future consolidated financial statements.
47
DESCRIPTION OF THE BUSINESS
General
We are a specialty pharmaceutical company developing non-invasive, topically delivered products. Our innovative patented Accudel cream formulation technology is designed to enable highly targeted site specific treatment. Impracor, our lead pain product candidate, utilizes the Accudel platform technology to deliver the active drug, ketoprofen, a non-steroidal anti-inflammatory drug, through the skin directly into the underlying tissues where the drug exerts its localized anti-inflammatory and analgesic effects.
Through our strategic relationship with Professional Compounding Centers of America, Inc. (“PCCA”), one of the largest drug compounding organizations in the world, we expect to facilitate our future selection, formulation and development of potential product candidates. Our relationship with PCCA is exclusive and provides us with the opportunity to develop new products using PCCA’s proprietary drug formulations and drug delivery technologies, as well as access to an extensive database of market-oriented information related to specific drug development candidates. We plan to use our proprietary Accudel drug delivery technology, coupled with these licensed technologies, formulations and market data, to identify pharmaceutical development opportunities where there is a significant unmet need for a new drug product. We are currently considering potential product candidates in the muscle relaxant and neuropathic pain fields, and we expect those areas may be our next avenues for additional product development.
To better enable us to analyze the myriad of PCCA and PCCA-related development opportunities, we have designed a comprehensive drug development selection methodology. In determining the viability of a potential opportunity, we group all development candidates according to delivery modality and health category. We review intellectual property related to a respective opportunity, as well as manufacturing considerations, various market-related criterion and other clinical development issues. We believe that our review process will aid us in identifying viable development candidates and assist us in determining whether to internally develop a program or seek an appropriate commercialization partner.
We were incorporated in Delaware in January 2006 as Bywater Resources, Inc. in order to conduct mineral exploration activities. We changed our name to Transdel Pharmaceuticals, Inc. on September 11, 2007. On September 17, 2007, Transdel Pharmaceuticals, Inc. entered into an Agreement of Merger and Plan of Reorganization by and among Transdel Pharmaceuticals, Inc., Transdel Pharmaceuticals Holdings, Inc., a privately held Nevada corporation (“Transdel Holdings”), and Trans-Pharma Acquisition Corp., a newly formed, wholly-owned Delaware subsidiary of Transdel (“Acquisition Sub”). Upon closing of the merger transaction contemplated under the merger agreement, Acquisition Sub merged with and into Transdel Holdings, Transdel Holdings, as the surviving corporation, became our wholly-owned subsidiary, and the former owners of Transdel Holdings became our controlling stockholders. Upon completion of the merger, we began our operations as a specialty pharmaceutical company.
On February 28, 2012, we changed our name to Imprimis Pharmaceuticals, Inc. and effected a one-for-eight reverse split of our authorized, issued and outstanding common stock. The information in this prospectus and the accompanying consolidated financial statements for interim and annual prior periods presented have been retroactively adjusted to reflect the effects of that reverse stock split.
On April 25, 2012, our Board of Directors and stockholders holding a majority of our outstanding voting power approved a resolution authorizing our Board of Directors to effect a reverse split of our common stock at an exchange ratio of (i) one-for-three, (ii) one-for-four, (iii) one-for-five, or (iv) one-for-six, with our Board of Directors retaining the discretion as to whether to implement the reverse split and which exchange ratio to implement. The action by written consent of the stockholders became effective on May 31, 2012, following our compliance with certain notice requirements under the Exchange Act. We anticipate that immediately following the effectiveness of the registration statement of which this prospectus forms a part, and prior to the closing of this offering, our Board of Directors will effect a reverse stock split at a ratio of one-for-five.
The reverse stock split is intended to allow us to meet the minimum share price requirement of The NASDAQ Capital Market. We have applied for listing of our common stock on The NASDAQ Capital Market, which listing we expect to occur at the closing of this offering. If the application is not approved, we will not complete this offering or effect the reverse stock split, and the shares of our common stock will continue to be traded on the OTC Markets Group’s OTCQB tier.
Our common stock has been quoted in the over-the-counter market since March 14, 2007 and is currently quoted on OTC Markets Group’s OTCQB tier under the symbol IMMY. Our executive offices are located at 437 S. Hwy 101, Suite 209, Solana Beach, CA 92075 and our telephone number at such office is (858) 433-2800. Our website address is www.imprimispharma.com. Information contained on our website is not deemed part of this prospectus.
48
Impracor
Impracor, our lead drug candidate, is comprised of a transdermal formulation of ketoprofen, a NSAID. Impracor is formulated using our proprietary Accudel drug delivery system and is being developed for the treatment of acute musculoskeletal pain. Impracor penetrates the skin barrier to reach the targeted underlying tissues where it exerts its localized anti-inflammatory and analgesic effect. The topical delivery of the drug may minimize systemic exposure, which may in turn lead to fewer concerns pertaining to gastrointestinal, hepatic, cardiovascular and other adverse systemic effects, which are associated with orally administered NSAIDs. We believe that this product may be considered for patients with site specific localized pain and who also (i) have a history of gastrointestinal, cardiovascular, kidney or liver problems, (ii) are geriatric or pediatric and/or (iii) are at risk for drug interactions.
Completed Clinical Studies for Impracor
In June 2008, we initiated a Phase 3 clinical trial designed as a randomized, double-blind, placebo-controlled, multi-center study that enrolled a total of 364 patients with acute soft tissue injuries of the upper or lower extremities in 26 centers in the United States. As we reported in October 2009, the top-line results showed that the study demonstrated statistical significance in its primary endpoint in the per protocol analysis and was favorable for Impracor in the Intent-To Treat ("ITT") analysis. Impracor also demonstrated a safety and tolerability profile similar to the placebo used in the study. Of the over 180 patients treated with Impracor, there were no treatment related gastrointestinal, cardiovascular, hepatic or other clinically relevant adverse events reported. Furthermore, Impracor was observed to be well absorbed through the skin and only minimal blood concentrations of ketoprofen were detected in a subset of patients who underwent blood sampling for pharmacokinetic analyses following repeated topical applications.
In January 2010, we reported on further in-depth analyses of the ITT data from the Impracor Phase 3 study. For the modified ITT analysis we identified 35 patients who did not meet study entry criteria at the time of randomization. Excluding the data from these patients who should not have been randomized into the study based on information that was not known at the time of enrollment, the study demonstrated statistical significance (p<0.038) on the primary efficacy endpoint. This post-hoc analysis was confirmed by a third-party statistical expert.
In February 2012, our management conducted an additional analysis of the ITT data and a body weight adjusted modified per protocol (“mPP”) analysis of those participants who completely complied with the Phase 3 Study protocol, including taking the recommended therapeutic quantity of the study drug. This analysis excluded 52 participants from the ITT group who did not take a minimum therapeutic quantity of the study drug, and 20 patients who did not have a valid Day 3 primary end point assessment and 4 patients who were misdiagnosed. This mPP analysis on 250 patients demonstrated statistical significance of the primary endpoint (p=0.034).
We believe that the weight of evidence of a treatment effect in this study is further strengthened by a key secondary endpoint (pain intensity recorded three times daily on patient diary cards) that supports the primary endpoint. The patient diary data which yield pain curves over time show consistent separation between treatment groups reaching statistical significance in favor of Impracor using both the original and modified ITT population. Furthermore, the physician's global assessment, using a 7 Point Likert Scale on day 3, produced statistically significant results (p=0.037), and a later exploratory analysis of the patient's global assessment of treatment satisfaction, using a binomial method, produced statistically significant results (p=0.023).
Proposed Clinical Program for Impracor
For Impracor to be approved by the FDA, two confirmatory Phase 3 trials with exposure of at least 300 to 500 patients and supportive dermal safety studies are required. We plan to commence two adequate and well-controlled Phase 3 trials of Impracor in patients experiencing pain from osteoarthritis flare in their knees. We plan to discuss the clinical development program for those trials, which has been endorsed by our scientific and regulatory advisory board, with the FDA at a Type C meeting scheduled to occur in April 2013.
Also as required by the FDA, we recently completed a clinical study that measured the amount of ketoprofen found in the bloodstream following topical application of two different doses of the anti-inflammatory cream under different conditions, including normal activities, heat exposure to the application site and standardized exercise, as well as the amount of the drug in the bloodstream after taking an oral dose of ketoprofen (the relative oral bioavailability). The study included a total of 40 healthy volunteers (36 of which completed the study) assigned to one of two cohorts (2g or 4g applications). Subjects were dosed according to a four-sequence, four-treatment randomization schedule in which they received topical Impracor applications under each of the three conditions and an oral ketoprofen dose in weekly intervals. Overall the pharmacokinetic parameters were observed to be consistent between the two different dose cohorts. The application of an occlusive knee bandage with either heat or exercise following topical administration showed faster initial, but lower overall plasma exposure of ketoprofen relative to non-occluded topical administration with no heat or exercise. The extent of bioavailability over 48 hours as measured by the area under the concentration curve from time zero to the time of last measurable concentration (AUC0-t) was 2% or less in cohort 1 (2 g single dose applied to one knee) and 4% or less in cohort 2 (2 g single dose applied to each knee) for the topical treatments relative to the oral treatment. All treatments were observed to be well tolerated. We also expect to initiate a routine supportive trial in healthy subjects related to the potential of contact sensitization. We expect that all of our planned clinical studies for Impracor will be executed with the professional help of CROs with experience in clinical trials of similar design. We are in the process of selecting and negotiating arrangements with potential CROs and other third parties in order to initiate our Phase 3 clinical trials.
Following completion of our clinical trials, we expect to file a New Drug Application for marketing authorization for Impracor under Section 505(b)(2) of the Hatch-Waxman Act of 1984, a regulatory route towards U.S. approval that leverages previously established safety and/or effectiveness of already approved ketoprofen products in other dosage forms. This regulatory path also improves the chances of success over a completely new drug chemical entity.
The timing of Phase 3 trials and the other supportive studies will be dependent on obtaining adequate financing to support the execution of these activities and for other working capital expenditures. Upon receipt of such financing, we anticipate initiating the supportive studies and Phase 3 trials in mid and late 2013, respectively. Assuming successful timely completion and outcome of the additional Phase 3 trials, we would expect to file the New Drug Application for Impracor in the second half of 2014.
We expect that Impracor, if approved by the FDA, could become one of the first NSAID cream products available by prescription in the United States for the topical treatment of acute musculoskeletal pain.
49
The Accudel Technology
Accudel is our proprietary transdermal cream drug delivery platform which can facilitate the transdermal penetration of drugs, thus enabling the avoidance of first pass metabolism by the liver and minimizing systemic exposure. The following diagram provides a schematic of the Accudel drug delivery system:

Accudel has the following properties, which make it a highly versatile vehicle for topical drug administration:
|
●
|
utilizes a pluronic lecithin organogel based matrix which is known to penetrate the stratum corneum and aid in the diffusion of active ingredients through the skin;
|
|
●
|
helps solubilize various types of drugs and its components (lipophilic, hydrophilic and amphiphilic);
|
|
●
|
uses penetration enhancers in a synergistic combination;
|
|
●
|
can incorporate compounds of various molecular sizes;
|
|
●
|
contains biocompatible components which are generally regarded as safe by the FDA;
|
|
●
|
is thermodynamically stable, insensitive to moisture and resistant to microbial contamination;
|
|
●
|
potentially results in decreased safety concerns associated with oral or intravenous drugs;
|
|
●
|
avoids certain limitations associated with transdermal patches;
|
|
●
|
is easy to apply, aesthetically acceptable and odorless; and
|
|
●
|
potentially produces patentable new products when combined with established or new drugs.
|
50
Product Development Program
We believe that the clinical success of Impracor will facilitate the use of the Accudel delivery technology in other products. We have identified development opportunities for potential products in pain management and other therapeutic areas utilizing the Accudel platform technology and we are exploring potential commercial relationships for these identified product candidates. In particular, we are currently considering potential new drug candidates in the muscle relaxant and neuropathic pain fields, and we expect those to be our next avenues for new product development. We estimate that pre-Phase 3 clinical studies for these two potential product candidates could each be completed approximately 18 to 24 months after their commencement, and that costs for such development would range from approximately $2 million to $2.5 million for each proposed drug candidate.
In addition, we expect our new relationship with PCCA to facilitate our future selection, development and formulation of potential product candidates. We plan to use our proprietary Accudel drug delivery technology, coupled with these licensed technologies, formulations and market data, to identify pharmaceutical development opportunities where there is a significant unmet need for a new drug product. We are currently considering potential product candidates in the muscle relaxant and neuropathic pain fields, and we expect those areas may be our next avenues for additional product development.
In the past our product development program has included cosmetic and cosmeceutical products utilizing our patented transdermal delivery system technology, Accudel. Our lead product candidate was an anti-cellulite formulation, for which we have initial clinical information supporting the beneficial effects of this cosmetic product on skin appearance. Our potential pipeline of cosmetic products includes hyperpigmentation and anti-aging formulations. We remain interested in pursuing this business opportunity and continue to consider entering into new relationships with third parties. We may also pursue the out-licensing of our Accudel drug delivery technology for the development and commercialization of additional innovative drug and cosmeceutical products.
51
Market and Opportunity
According to Wolters-Kluwer PHAST, the U.S. pain market was approximately $39.8 billion in 2011. Of that total, the NSAID market made up approximately $13.5 billion from approximately 155 million written prescriptions. The topical NSAID market in 2011 over $500 million, averaging an approximately 28% compound annual growth rate since 2007.
According to the Archives of Internal Medicine, NSAIDs are regularly used by more than 60 million Americans. Approximately 70% of people aged 65 or older take NSAIDs weekly. As a result of the widespread usage of oral NSAIDs, according to Bandolier, there are over 100,000 hospitalizations annually and 16,500 deaths in the U.S. due to gastro-intestinal complications annually. In the United Kingdom, there are approximately 12,000 hospitalizations and an estimated 2,600 deaths annually related to GI complications following oral NSAID use per year. One study published in 1998 in the American Journal of Medicine found that NSAID-related gastro-intestinal side effects caused almost as many deaths as asthma, cervical cancer and malignant melanoma combined, and another 1999 sutdy publinshed in the Journal of Rheumatology found that death resulting from gastro-intestinal complications was the 15th most common cause of death in the U.S., higher than cervical cancer, asthma and malignant melanoma. According to Singh G, Triadafilopoulos G., Epidemiology of NSAID induced gastrointestinal complications, J Rheumatol. 1999, the hospitalizations and deaths related to sytemic NSAID use has a financial impact of more than $2 billion per year in the U.S. Therefore, we believe there is a significant demand from physicians and patients for topical pain management products such as Impracor, especially with respect to the treatment of localized, acute musculoskeletal pain, which we believe is driven primarily by the concern of possible negative systemic effects of orally administered NSAIDs.
We believe there is a large and growing need for Impracor, and specifically, a non-liquid topical NSAID. Recent prescription data from Wolters-Kluwer PHAST through May 2012 showed that following a production disruption with Voltaren® gel, the leading topical NSAID in prescription volume, and a corresponding spike in prescription volume for Pennsaid (a liquid) and Flector (a patch), once the Voltaren® gel supply issues were resolved in April 2012, prescription volumes for Voltaren® gel dramatically increased, nearly to pre-failure volumes. We believe that this data shows that there is a market preference for gels over liquids and patches, and we further believe there may also be a market preference for creams such as Impracor as well.
Assuming that we can show positive efficacy and strong safety data, and assuming FDA approval of Impracor, we believe we will be able to enter into an agreement on reasonable terms with a suitable marketing partner to distribute Impracor. We also intend to assess alternative options, in parallel, to invest in the distribution of Impracor alone or in partnership with a more established sales organization. We believe that finding a marketing partner with a sales force that will call on physicians who would potentially prescribe Impracor is of critical importance. Given the growth in the use of topical NSAIDs we believe that interest in bringing Impracor to market by strong partners under acceptable terms should be significant.
Competition
The pharmaceutical industry is highly competitive. There are competitors in the United States that are currently selling FDA-approved topical NSAID products that our products would compete with, if our products are approved by the FDA. Also, we are aware of companies developing patch products, topical NSAIDs and other pain formulations.
In the topical NSAID category, since 2008, three diclofenac-based topical NSAID products have been introduced in the US market: Endo Pharmaceutical’s Voltaren® gel (licensed from Novartis), Alpharma’s (now subsidiary of Pfizer) Flector® patch and Covidien’s Pennsaid® Topical Solution (licensed from Nuvo). While Voltaren Gel and Pennsaid are indicated for osteoarthritis of the knee, Flector Patch is indicated for acute sprains and strains. The three FDA approved topical NSAID products currently in the US market are all diclofenac-based. Currently, there are no FDA approved non-diclofenac-based topical NSAID products in the US market. We believe that additional transdermal NSAID products such as our ketoprofen-based Impracor would be well received by the FDA and patients by providing safe and effective treatment options to address pain in addition to diclofenac-based products.
52
According to Wolters-Kluwer PHAST, as of December 2011, Voltaren® gel dominated the topical NSAID market with approximately 74% of the U.S. monthly prescription volume. Flector® patch held the number two position with approximately 16% of the U.S. monthly prescription volume. Solaraze® Gel held the number three position in the market with approximately 6% of the U.S. monthly prescription volume. Pennsaid® Topical Solution makes up the remaining 4% of topical NSAID prescriptions
In addition to product safety, development and efficacy, other competitive factors in the pharmaceutical market include product quality and price, reputation, service and access to scientific and technical information. It is possible that developments by our competitors will make our products or technologies uncompetitive or obsolete. In addition, the intensely competitive environment for pain management products requires an ongoing, extensive search for medical and technological innovations and the ability to market products effectively, including the ability to communicate the effectiveness, safety and value of branded products for their intended uses to healthcare professionals in private practice, group practices and managed care organizations. Because we are significantly smaller than our primary competitors, we may lack the financial and other resources needed to develop, produce, distribute, market and commercialize any of our drug candidates or compete for market share in the pain management sector.
At this time, no generic version of any of the three currently marketed topical NSAID drugs have been approved by the FDA. Additionally, the Office of Generic Drugs, or OGD, recently issued draft guidance representing the FDA’s opinion on the requirements for approval of a generic version of the currently marketed topical NSAIDs, Voltaren Gel and Flector Patch. OGD recommends a bioequivalence study with clinical endpoints and/or a bioequivalence study with pharmacokinetic endpoints and/or a skin irritation and sensitization study to determine bioequivalence between the products, i.e. demonstrating that there is no difference between the original drug and the generic. We believe that the cost to develop a generic topical NSAID is comparable to the cost of a Phase 3 new drug application and thereby discourages companies from genericizing the topical NSAID category of drugs.
Governmental Regulation
Our ongoing product development activities are subject to extensive and rigorous regulation at both the federal and state levels. Post development, the manufacture, testing, packaging, labeling, distribution, sales and marketing of our products is also subject to extensive regulation. The Federal Food, Drug and Cosmetic Act of 1983, as amended, and other federal and state statutes and regulations govern or influence the testing, manufacture, safety, packaging, labeling, storage, record keeping, approval, advertising, promotion, sale and distribution of pharmaceutical products. Noncompliance with applicable requirements can result in fines, recall or seizure of products, total or partial suspension of production and/or distribution, refusal of the government to approve New Drug Applications, or NDAs, civil sanctions and criminal prosecution.
FDA approval is typically required before each dosage form or strength of any new drug can be marketed. Applications for FDA approval must contain information relating to efficacy, safety, toxicity, pharmacokinetics, product formulation, raw material suppliers, stability, manufacturing processes, packaging, labeling, and quality control. The FDA also has the authority to revoke previously granted drug approvals. Product development and approval within this regulatory framework requires a number of years and involves the expenditure of substantial resources.
Current FDA standards for approving new pharmaceutical products are more stringent than those that were applied in the past. As a result, labeling revisions, formulation or manufacturing changes and/or product modifications may be necessary. For example, due to an increased understanding of the cardiovascular and gastrointestinal risks associated with NSAIDs, the FDA approved new rules requiring that professional labeling for all prescription and over-the-counter NSAIDs include information on such risks. We cannot determine what effect changes in regulations or legal interpretations, when and if promulgated, may have on our business in the future. Changes could, among other things, require expanded or different labeling, the recall or discontinuance of certain products, additional record keeping and expanded documentation of the properties of certain products and scientific substantiation. Such regulatory changes, or new legislation, could have a material adverse effect on our business, financial condition and results of operations. The evolving and complex nature of regulatory requirements, the broad authority and discretion of the FDA and the generally high level of regulatory oversight results in a continuing possibility that from time to time, we will be adversely affected by regulatory actions despite ongoing efforts and commitment to achieve and maintain full compliance with all regulatory requirements.
53
FDA Approval Process
To obtain approval of a new product from the FDA, we must, among other requirements, submit data supporting safety and efficacy, as well as detailed information on the manufacture and composition of the product and proposed labeling. The testing and collection of data and the preparation of necessary applications are expensive and time-consuming. The FDA may not act quickly or favorably in reviewing these applications, and we may encounter significant difficulties or costs in our efforts to obtain FDA approvals that could delay or preclude us from marketing our products.
The process required by the FDA before a new drug may be marketed in the U.S. generally involves the following: (i) completion of nonclinical laboratory and animal testing in compliance with FDA regulations; (ii) submission of an investigational new drug application, which must become effective before human clinical trials may begin; (iii) performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug for its intended use; and (iv) submission and approval of an NDA by the FDA.
The sponsor typically conducts human clinical trials in the following three sequential phases, but the phases may overlap
|
●
|
Phase 1 clinical studies frequently begin with the initial introduction of the compound into healthy human subjects prior to introduction into patients, involves testing the product for safety, adverse effects, dosage, tolerance, absorption, metabolism, excretion and other elements of clinical pharmacology.
|
|
●
|
Phase 2 clinical studies typically involve studies in a small sample of the intended patient population to assess the efficacy of the compound for a specific indication, to determine dose tolerance and the optimal dose range as well as to gather additional information relating to safety and potential adverse effects.
|
|
●
|
Phase 3 clinical studies are undertaken to further evaluate clinical safety and efficacy in an expanded patient population at typically dispersed study sites, in order to determine the overall risk-benefit ratio of the compound and to provide an adequate basis for product labeling.
|
As a product candidate moves through the clinical phases, manufacturing processes are further defined, refined, controlled and validated. The level of control and validation required by the FDA in the conduct of clinical trials increases as clinical studies progress.
Clinical trials must be conducted in accordance with the FDA’s good clinical practices requirements. The FDA may order the temporary or permanent discontinuation of a clinical trial at any time or impose other sanctions if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. An institutional review board, or IRB, generally must approve the clinical trial design and patient informed consent at each clinical site and may also require the clinical trial at that site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
The applicant must submit to the FDA the results of the nonclinical studies and clinical trials, together with, among other things, detailed information on the manufacture and composition of the product and proposed labeling, in the form of an NDA, including payment of a user fee, unless waived. The FDA reviews all NDAs submitted before it accepts them for filing and may request additional information rather than accepting an NDA for filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. Under the Prescription Drug User Fee Act, or PDUFA, the FDA ordinarily has 10 months in which to complete its initial review of the NDA and respond to the applicant. However, the PDUFA goal dates are not legal mandates and the FDA response often occurs several months beyond the original PDUFA goal date. The review process and the target response date under PDUFA may be extended if the FDA requests or the NDA sponsor otherwise provides additional information or clarification regarding information already provided in the NDA submission. Following completion of the FDA’s initial review of the NDA and the clinical and manufacturing procedures and facilities, the FDA will issue a complete response or action letter, which will either include an approval authorizing commercial marketing of the drug for certain indications or contain the conditions that must be met in order to secure final approval of the NDA. If the FDA’s evaluation of the NDA submission and the clinical and manufacturing procedures and facilities is not favorable, the FDA may refuse to approve the NDA.
54
Section 505(b)(2) New Drug Applications
Since the active pharmaceutical ingredient in Impracor is ketoprofen, which has already been approved by the FDA, we are able to file a NDA under section 505(b)(2) of the Hatch-Waxman Act of 1984 for this product as well as other products that we may develop including approved active pharmaceutical ingredients. This is an alternate path to FDA approval for new formulations of previously approved products. Section 505(b)(2) was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984, otherwise known as the Hatch-Waxman Act. Section 505(b)(2) permits the submission of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The Hatch-Waxman Act permits the applicant to rely upon certain published nonclinical or clinical studies conducted for an approved product or the FDA’s conclusions from prior review of such studies. The FDA may also require companies to perform additional studies or measurements to support any changes from the approved product. The FDA may then approve the new product for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant. While references to nonclinical and clinical data not generated by the applicant or for which the applicant does not have a right of reference are allowed, all development, process, stability, qualification and validation data related to the manufacturing and quality of the new product must be included in an NDA submitted under Section 505(b)(2).
Each study is conducted in accordance with certain standards under protocols that detail the objectives of the study, the parameters to be used to monitor safety, and efficacy criteria to be evaluated. Each protocol must be submitted to the FDA. In some cases, the FDA allows a company to rely on data developed in foreign countries or previously published data, which eliminates the need to independently repeat some or all of the studies.
To the extent that the Section 505(b)(2) applicant is relying on the FDA’s conclusions regarding studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book publication. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. A certification that the new product will not infringe the already approved product’s listed patents or that such patents are invalid is called a paragraph IV certification. If the applicant does not challenge the listed patents, the Section 505(b)(2) application will not be approved until all the listed patents claiming the referenced product have expired. The Section 505(b)(2) application also will not be approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired. As of July 24, 2012, there were 14 ketoprofen based prescription drugs approved by the FDA in the Orange Book. All of the approved applications are for oral capsules and oral extended release capsules, with dosage strengths ranging from 25 milligrams to 200 milligrams.
As a condition of approval, the FDA or other regulatory authorities may require further studies, including Phase 4 post-marketing studies to provide additional data. Other post-marketing studies may be required to gain approval for the use of a product as a treatment for clinical indications other than those for which the product was initially tested. Also, the FDA or other regulatory authorities require post-marketing reporting to monitor the adverse effects of the drug. Results of post-marketing programs may limit or expand the further marketing of the products.
The FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the Internet. A company can make only those claims relating to safety and efficacy that are approved by the FDA. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available drugs for uses that are not described in the drug's labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, impose stringent restrictions on manufacturers’ communications regarding off-label use.
55
The FDA enforces regulations to ensure that the methods used in, and facilities and controls used for, the manufacture, processing, packing and holding of drugs conform to current good manufacturing practices, or cGMP. The cGMP regulations the FDA enforces are comprehensive and cover all aspects of operations, from receipt of raw materials to finished product distribution, insofar as they bear upon whether drugs meet all the identity, strength, quality, purity and safety characteristics required of them. To assure compliance requires a continuous commitment of time, money and effort in all operational areas.
The FDA conducts pre-approval inspections of facilities engaged in the development, manufacture, processing, packing, testing and holding of the drugs subject to NDAs. If the FDA concludes that the facilities to be used do not meet cGMP, good laboratory practices or good clinical practices requirements, it will not approve the NDA. Corrective actions to remedy the deficiencies must be performed and verified in a subsequent inspection. In addition, manufacturers of both pharmaceutical products and active pharmaceutical ingredients used to formulate the drug also ordinarily undergo a pre-approval inspection, although the inspection can be waived when the manufacturer has had a passing cGMP inspection in the immediate past. Failure of any facility to pass a pre-approval inspection will result in delayed approval and would have a material adverse effect on our business, results of operations and financial condition.
The FDA also conducts periodic inspections of facilities to assess their cGMP status. If the FDA were to find serious cGMP non-compliance during such an inspection, it could take regulatory actions that could adversely affect our business, results of operations and financial condition. The FDA could initiate product seizures, request product recalls and seek to enjoin a product’s manufacture and distribution. In certain circumstances, violations could lead to civil penalties and criminal prosecutions. In addition, if the FDA concludes that a company is not in compliance with cGMP requirements, sanctions may be imposed that include preventing the company from receiving the necessary licenses to export its products and classifying the company as an “unacceptable supplier,” thereby disqualifying the company from selling products to federal agencies. Imported active pharmaceutical ingredients and other components needed to manufacture our products could be rejected by United States Customs.
We believe that we and our suppliers and outside manufacturers are currently in compliance with all FDA requirements.
Impracor is manufactured by a large contract manufacturer in the United States that specializes in topical products. We believe that this supplier has sufficient capability to manufacture Impracor if it is approved for sale. We are currently assessing alternative suppliers for Impracor in the event there are problems associated with the manufacturing of Impracor by our current contract supplier, although we do not expect any such problems to occur. Our active pharmaceutical ingredients (APIs), including ketoprofen, are manufactured by well-known and established chemical and pharmaceutical companies. We are currently assessing alternative suppliers for our ketoprofen API. Our preferred vendors for our non-API inactive raw materials suppliers are established companies.
Other FDA Matters
If there are any modifications to an approved drug, including changes in indication, manufacturing process or labeling or a change in a manufacturing facility, an applicant must notify the FDA, and in many cases, approval for such changes must be submitted to the FDA or other regulatory authority. The FDA also regulates post-approval promotional labeling and advertising activities to assure that such activities are being conducted in conformity with statutory and regulatory requirements. Failure to adhere to such requirements can result in regulatory actions that could have a material adverse effect on our business, results of operations and financial condition.
56
Research and Development
Our research and development expenses primarily include costs for the Impracor clinical program. These expenses have included costs related to our Phase 3 clinical studies, including costs for our contract research organizations and investigator payments to the clinical sites participating in the study. Other expenses are personnel costs including wages and stock-based compensation, contract manufacturing, non-clinical studies, consulting and other costs related to the clinical program.
During the year ended December 31, 2011, we incurred $111,554 on research and development expenses, as compared to $194,588 during the year ended December 31, 2010. During the nine months ended September 30, 2012, we incurred $580,240 on research and development expenses, as compared to $111,554 during the nine months ended September 30, 2011. We expect research and development activities will increase significantly as we execute on our business plan and begin Phase 3 studies.
Intellectual Property
We obtained a patent from the United States Patent and Trademark Office on our Accudel technology in 1998, which affords protection of Accudel through 2016 in the United States. This patent specifically lists over 500 different drugs in over 60 therapeutic areas, including both approved and established drugs. The Accudel technology may also have an application to deliver drugs not listed in its patent, including novel drugs. It also covers composition of matter, methods of use and methods of manufacture. We have engaged counsel and consultants who have specific expertise in topical drug delivery to assist us in executing on an intellectual property strategy with the aim of extending the life of the technology derived from our existing patent beyond 2016. We have also been granted a patent related to our Accudel technology in Canada. We have filed additional patent applications in various jurisdictions. We have pending trademark applications for Imprimis Pharmaceuticals, Accudel, Impracor and Generecycle.
Employees
As of February 1, 2013, we have four full-time and one part-time employees. Our employees are responsible for financial accounting and investor relations, business and corporate development, research and development management, and general administration. We believe that our current staff is sufficient to carry out our business plan in the coming twelve months; however, if our operations in the future require it, we will consider the employment of additional staff or the use of additional consultants. We are not party to any collective bargaining agreements with any of our employees. We have never experienced a work stoppage, and we believe our employee relations are good. We hire independent contractor labor and consultants on an as needed basis and have entered into consulting arrangements with certain directors in exchange for stock options and/or cash payments.
Scientific and Regulatory Advisors
On October 25, 2012, the Board approved the establishment of a scientific and regulatory advisory board to provide guidance to our management team relating to clinical trial procedures and product development. The members of our advisory board are not members of our Board of Directors and do not otherwise hold management roles with the Company.
Our advisory board currently has six members as follows: Dr. Gerald J. Yakatan, Dr. Lee S. Simon, Dr. Allan Green, Dr. Marc Hochberg, Dr. Roy Altman and Dr. Roland Moskowitz. Dr. Yakatan has served in both academic and industrial environments in connection with pharmaceutical product development efforts, and we believe that his experience with the drug development and FDA approval process for various notable drug products will be valuable for our business. Dr. Simon, who has served as a division director and on advisory committees for the FDA and as a funded investigator and a member of the Steering Committee for the National Institutes of Health, brings important FDA expertise to the advisory board. Dr. Green is a physician, attorney, research scientist and inventor on several US patents and has significant operating and management experience with a number of biomedical companies, who we believe can provide invaluable advice to our management in the fields in which we operate. Dr. Altman has over 35 years of clinical experience in osteoarthritis and rheumatology and has been internationally recognized for his life work in the area of rheumatology and immunology. Dr. Hochberg serves in academic surroundings in connection with rheumatology and clinical immunology, where his research focuses on the clinical epidemiology of musculoskeletal diseases, particularly osteoarthritis and osteoporosis. Dr. Moskowitz has over 30 years of experience, where he has conducted extensive research in the pathophysiology and genetics of osteoarthritis. We believe that the experience and know-how of Dr. Altman, Dr. Moskowitz, and Dr. Hochberg, particularly in the areas of rheumatology and osteoarthritis, will provide our management with vital knowledge in order to serve our needs in those clinical areas. We have also entered into consulting agreements with the members of our advisory board. On August 28, 2012, we entered into an independent contractor services agreement with SDG, LLC (“SDG”), of which Dr. Simon and Dr. Green are principals, pursuant to which SDG will provide consulting services for us relating to our clinical development strategy for clinical trial design and management and regulatory affairs. In 2012, we entered into separate consulting agreements with each of Dr. Yakatan, Dr. Moskowitz, Dr. Hochberg and Dr. Altman, pursuant to which each has agreed to provide services for us relating to our regulatory and development strategy in connection with the FDA approval process.
Properties
We lease approximately 1,486 square feet of office space in Solana Beach, California. The current lease term expires on February 28, 2014. This facility serves as our corporate headquarters.
We believe our current facility is adequate for our immediate and near-term needs. Additional space may be required as we expand our activities. We do not currently foresee any significant difficulties in obtaining any required additional facilities.
57
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Our directors hold office for one-year terms until the earlier of their death, resignation or removal or until their successors have been elected and qualified. Our officers are elected annually by the board of directors and serve at the discretion of the board. Set forth below is certain information regarding our directors and executive officers as of the date of this prospectus:
|
Name
|
Age
|
Position
|
||
|
Joachim Schupp, M.D.
|
59
|
Chief Medical Officer
|
||
|
Balbir Brar D.V.M., Ph.D.
|
76
|
President
|
||
|
Andrew R. Boll
|
30
|
Vice President of Accounting and Public Reporting
|
||
|
Mark L. Baum, Esq.
|
40
|
Chief Executive Officer and Director
|
||
|
Paul Finnegan, M.D., M.B.A.
|
52
|
Director
|
||
|
Jeffrey J. Abrams, M.D.
|
65
|
Director
|
||
|
Robert Kammer, D.D.S.
|
63
|
Chairman of the Board of Directors
|
||
| Stephen G. Austin, C.P.A. | 60 | Director | ||
| August S. Bassani, Pharm.D. | 40 | Director |
Business Experience
The following is a brief account of the education and business experience of our current directors and executive officers:
Joachim Schupp, M.D. has been the Chief Medical Officer of the Company since February 2012. Dr. Schupp has more than 25 years of leadership experience in the pharmaceutical industry. He has achieved the professional distinction of leading international project teams that have brought several drugs through the development and the regulatory process and on to the market globally. Most recently, Dr. Schupp has worked as an executive consultant for pharmaceutical and biotechnology companies. He held positions as Vice-President of Clinical Development at Apricus Biosciences, Inc. from April 2011 to February 2012, Senior Consultant to and Chief Medical Officer at Transdel Pharmaceuticals, Inc. from April 2009 to April 2011, Vice President of Medical Affairs at Adventrx Pharmaceuticals from 2006 to 2008 and Vice President of Clinical Data Services at ProSanos Corporation from 2004 to 2006. In addition, Dr. Schupp spent 19 years with Novartis Pharmaceuticals in Switzerland where he held various positions in clinical development and global project management. Dr. Schupp began his pharmaceutical career at Ciba-Geigy, now Novartis, in 1985 where he was appointed to lead international clinical project teams to discover new NSAIDs with improved gastrointestinal tolerability. Dr. Schupp received several prestigious awards at Ciba-Geigy and Novartis for his team leadership contributions. Dr. Schupp received his M.D. and his research doctorate (Dr.med.) from the Free University of Berlin in Germany, and he served on the faculty at the University of Pretoria, South Africa, in Internal Medicine and Rheumatology.
Balbir Brar, D.V.M., Ph.D., has been President of the Company since January 2012 and served as a director from February 15, 2012 until July 25, 2012. Dr. Brar served as our Vice President of Research and Development from December 2007 until April 2008. Dr. Brar has over 25 years of experience in drug and device development and worldwide registration of eight major drugs, including Botox. He has significant experience in research and development, conducting clinical trials, implementation of product development plans and working with U.S. and international regulators. Dr. Brar has also served as a consultant to numerous biotechnology companies since June 2002 including AtheroNova Inc., Aciont, Inc., Altheos, Inc., Aciex Therapeutics, Inc. Dr. Brar has worked with major pharmaceutical companies, including Lederle Laboratories (acquired by Wyeth, then by Pfizer, Inc. (NYSE: PFE), and served as Senior Director of Drug Safety at SmithKline Beckman, now GlaxoSmithKline plc (NYSE: GSK). In addition, he served as Vice President Drug Safety, Research & Development at Allergan, Inc. (NYSE: AGN), where he was responsible for regulatory submission of 50 IND’s/510K’s and worldwide approval of six New Drug Applications. Dr. Brar is listed as the inventor of numerous patents. He has a Ph.D. in Toxicology/Pathology from Rutgers University and D.V.M. from India with finance training from Harvard Business School. Dr. Brar is a recipient of numerous achievements awards for excellence belongs to a number of scientific organizations and is the author/coauthor of over 55 scientific publications. Dr. Brar’s significant and specifically relevant research and development background brings an important technical perspective to our board.
58
Andrew R. Boll has been our Vice President of Accounting and Financial Reporting since February 2012 and was a consultant to the Company from December 2011 to February 2012. Mr. Boll has several years of experience in financial reporting and accounting, including four years of experience working with small publicly traded companies, with a particular focus on restructured and reorganized businesses. From November 2007 to November 2011, Mr. Boll was an accountant for BCGU, LLC, a privately held fund manager that specializes in capital venture investment opportunities. There he provided consulting services to public company clients, compiled SEC financial reports, and accounted for numerous public company restructurings, financings and private to public mergers. From December 2004 to November 2007, Mr. Boll was an accountant for Welsh Companies, LLC, a privately held commercial real estate company, its fund and its other subsidiaries. Mr. Boll received his B.S. degree in Corporate and Public Finance, summa cum laude, from Huron University.
Mark L. Baum, Esq. has served as a director since December 2011 and as our Chairman of our Board of Directors from December 16, 2011 through April 1, 2012. Mr. Baum has also served as our principal executive officer since December 2011, and was appointed our Chief Executive Officer effective April 1, 2012. Mr. Baum has served as the principal of The Baum Law Firm, P.C. (now TBLF, LLC) since 1998, and has more than 15 years of experience in financing, operating and advising small capitalization publicly traded enterprises, with a particular focus on restructured or reorganized businesses. As a manager of capital, he has completed more than 125 rounds of financing for more than 40 publicly traded companies. As a securities attorney, Mr. Baum has focused his practice on US securities laws, reporting requirements and public company finance-related issues that affect small capitalization public companies. Mr. Baum has actively participated in numerous public company spin-offs, restructurings and recapitalizations, venture fundings, private-to-public mergers, asset acquisitions and divestitures. In addition to his fund management and legal experience, Mr. Baum has operational experience in the following industries: life science and diagnostics, closed door pharmacies, cleaner and renewable energy and retail home furnishings. Mr. Baum has served on numerous boards of directors, including Chembio Diagnostic Systems, Inc. (CEMI), Applied Natural Gas Fuels, Inc. (formerly AGAS), Shrink Nanotechnologies, Inc. (INKN), You on Demand, Inc. (CBBD) and CoConnect, Inc. (CCON), as well as boards of advisors for domestic and international private and public companies. Mr. Baum founded and capitalized the Mark L. Baum Scholarship which has funded tuition grants to college students in Texas. Mr. Baum is a published inventor and a licensed attorney in California and Texas. Mr. Baum was a Managing Member of DermaStar International, LLC, our former majority stockholder. Mr. Baum brings to our board years of public company executive experience, including knowledge of securities laws, reporting requirements and public company finance-related issues.
Paul Finnegan, M.D., M.B.A. has served as a director since February 15, 2012 and currently serves as the Chair of our Compensation Committee and our Nomination and Corporate Governance Committee and as a member of our Audit Committee. Dr. Finnegan brings to the Company experience as a board member and a global senior executive in the pharmaceutical and biotechnology industries. His expertise involves development, commercialization, and product launches of multiple novel drugs, both blockbusters and ultra-orphan therapeutics, which encompassed various clinical indications. He has served in leadership roles in commercial, clinical, medical affairs and business development functions of public and private companies. Most recently, from November 2008 to January 2012, Dr. Finnegan has been an entrepreneur in residence with Avalon Ventures, serving as President, Chief Executive Officer and Board Director of Avelas BioSciences and InCode Pharmaceutics, as well as a member of the biotechnology investment team, leading the clinical, commercial and regulatory due diligence efforts for over three years. Dr. Finnegan served as our Chief Operating Officer and Chief Medical Officer from April 2008 to November 2008. From October 2007 to April 2008, Dr. Finnegan served as the President and Chief Executive Officer of Cecoura Therapeutics, a private drug development company. From April 2001 to September 2007, Dr. Finnegan served as Vice President of Global Strategic Marketing and Development and other senior management positions at Alexion Pharmaceuticals. Prior to joining Alexion in 2001, Dr. Finnegan served as Senior Director, Global Medical Marketing for Pharmacia Corporation and G.D. Searle & Co., providing medical affairs leadership for all therapeutic areas for the Asia-Pacific, Japan, Latin America and Canadian business regions. Dr. Finnegan served as a board observer at AnaptysBio, Inc., a privately held therapeutic antibody company, from 2008 to 2011, and as a member of the boards of directors of Avelas Biosciences, Inc. from November 2008 to January 2011, and InCode BioPharmaceuticals, Inc. from April 2009 to the present. Dr. Finnegan earned his MBA with Honors, in Finance and Strategy, from the University of Chicago, Graduate School of Business, and the degrees of MD, CM from McGill University, Faculty of Medicine, in Montreal. He is a Fellow of the Royal College of Physicians, Canada (FRCPC), Member of the American Society of Hematology and practiced as an interventional radiologist specializing in oncology and vascular diseases prior to transitioning to industry. Dr. Finnegan was appointed to our Board of Directors in accordance with the terms of the senior advisory agreement dated January 17, 2012 with the Company. Dr. Finnegan terminated his senior advisory agreement on May 9, 2012, but remains as an independent member of our Board of Directors. Dr. Finnegan’s extensive leadership, marketing, investment and financial expertise and international business knowledge provides valuable guidance to our management and board.
59
Jeffrey J. Abrams, M.D., MPH, has served as a director since September 2007 and served as Chairman of the Board from February 2010 until December 2011. Currently, Dr. Abrams serves as a member of our Audit Committee, Compensation Committee, and Nomination and Corporate Governance Committee. Prior to 2007, Dr. Abrams was a practicing primary care clinician for over twenty years. Dr. Abrams received a B.A. from the State University of New York at Buffalo, an M.D. from the Albert Einstein College of Medicine and an M.P.H. from San Diego State University. Dr. Abrams was one of the co-founders of our company, and we believe that his qualifications to sit on our Board include his scientific and technical knowledge of our Accudel technology and our lead product candidate, Impracor, as well as his years and his years of experience as a practicing primary care clinician.
Robert Kammer, D.D.S., has served as a director since December 2011 and as Chairman of the Board of Directors since April 1, 2012. Dr. Kammer received his Bachelor of Science Degree in 1971 from Xavier University, Cincinnati, Ohio. He received his Doctor of Dental Surgery Degree from the University of Iowa in 1974. Dr. Kammer is a Diplomat of The American Board of Orofacial Pain and a Founding Charter Member of The Academy for Sports Dentistry and Colorado Osseointegration Study Club. From 1979 to 1996, Dr. Kammer was an Associate Professor and Course Director of Orofacial Pain Section in the Department of Restorative Dentistry at The University of Colorado Health Science Center. From 1982 through 1993, he served on the Sports Medicine Advisory Committee at The University of Colorado Intercollegiate Athletics and was the Team Dentist for Football and Basketball. From 1983 to 1990, Dr. Kammer was a consultant to the Boulder-Denver Pain Control Center and from 1988 through 1991, he served as a Referee and Editorial Staff Consultant of the Journal of Orofacial Pain. Dr. Kammer recently contributed a chapter to the groundbreaking text Osteoperiosteal Flap, is consulting for Clear Choice Dental Implant Centers, co-authoring scientific papers and is a co-investigator for a landmark study of Titanium Implant Prostheses at the Mayo Institute. Dr. Kammer was a Managing Member of DermaStar International, LLC, our former majority stockholder. Dr. Kammer brings to our Board of Directors over 30 years of practical experience treating patients for orofacial pain as well as a history of success in leadership positions he has been associated with.
Stephen G. Austin, CPA, has served as a director since July 2012 and currently serves as the Chair of our Audit Committee and as a member of our Compensation Committee and Nomination and Corporate Governance Committee. He has been a Partner in Swenson Advisors, LLP, a regional accounting firm (registered with the PCAOB), since May 1998 and has served as Managing Partner since October 2006. At Swenson Advisors, Mr. Austin manages audit, SEC, Sarbanes-Oxley and business consulting engagements with a focus on technology, manufacturing, service, real estate, social media and non-profit organizations. Prior to joining Swenson Advisors, Mr. Austin accumulated over 22 years of experience as an audit partner with Price Waterhouse LLP, where he worked from 1976 to 1996, and with McGladrey & Pullen, LLP, where he worked from 1996 to 1998, serving both public and private companies. While at Price Waterhouse, Mr. Austin worked in their national office in New York, where he addressed complex accounting and reporting issues for publicly-traded companies and worked with various members of the FASB and EITF staffs. Mr. Austin is licensed as a CPA in California and Georgia. He serves as a board member or advisory board member for various not-for-profit foundations, associations and public service organizations in the United States, serves on the Global board of directors of Integra International, an international association of accounting firms, and serves as a director on the board of Avanir Pharmaceuticals, Inc. (NASDAQ: AVNR). In 2004, Mr. Austin published a book on business ethics entitled “Rise of the New Ethics Class,” and in 2005 and 2006 he published articles in Asia discussing The Sarbanes-Oxley Act of 2002. In 2010 and 2011, Mr. Austin authored articles for the AICPA. Mr. Austin holds a B.S. degree in accounting from Bob Jones University and an M.B.A. degree from the University of Georgia. Mr. Austin brings to our Board financial and accounting expertise and extensive experience serving as a director of other companies.
August (“Gus”) S. Bassani, Pharm.D., has served as a director since December 2012. He currently serves as Vice-President of Consulting, R&D and Formulations at PCCA and has been with PCCA since September 2002. Prior to joining PCCA, Mr. Bassani was a formulation pharmacist in the Product Development Lab of a veterinary pharmaceutical company. He has worked in multiple pharmacy practice settings, located in Alaska, Iowa and Kansas, and has taught extemporaneous compounding principles to pharmacy students in Drake University’s Pharmaceutics Laboratory course. Mr. Bassani received his Doctor of Pharmacy degree from Drake University College of Pharmacy and Health Sciences. He is a member of the 2010 – 2015 United States Pharmacopeia (USP) Council of Experts – Compounding Expert Committee, and is serving on the 2012 – 2014 Drake University College of Pharmacy and Health Sciences National Advisory Council. He is a member of the American Pharmacists Association (APhA), International Academy of Compounding Pharmacists (IACP), American Society of Health Systems Pharmacists (ASHP) and the American Association of Pharmaceutical Scientists (AAPS). Mr. Bassani's widespread experience in the pharmaceutical industry and his formulation expertise provides valued guidance to our management and board.
There are no family relationships among our directors and executive officers.
Committees of the Board of Directors
On August 7, 2012, the Board established the following committees: the Audit Committee, the Compensation Committee and the Nomination and Corporate Governance Committee.
Audit Committee
Mr. Austin, Dr. Abrams, and Dr. Finnegan have been appointed to serve on our Audit Committee, and Mr. Austin has been appointed as the Chair of the Audit Committee. Our Board of Directors has determined that each current member of our Audit Committee is “independent” within the meaning of Section 10A(m)(3) of the Exchange Act and Rule 10A-3(b)(1) thereunder and satisfies the requirements for membership in the Audit Committee as set forth in Rule 5605(c)(2)(A) of the Rules of The NASDAQ Stock Market (“NASDAQ Rules”). Our Board of Directors has also determined that Mr. Austin qualifies as an “audit committee financial expert” as defined in applicable rules of the Securities and Exchange Commission.
Our Board of Directors has adopted a written charter for our Audit Committee, which sets forth the specific duties and responsibilities of the Audit Committee, including: (i) overseeing our accounting and financial reporting processes and our financial reporting legal and regulatory compliance, (ii) overseeing and evaluating management’s assessment of the effectiveness of our internal controls over financial reporting, (iii) reviewing any transactions by us with related parties, (iv) appointing our independent registered public accounting firm, (v) monitoring the independence and performance of our independent registered public accounting firm, (vi) pre-approving all audit and permissible non-audit services to be provided to us by our independent registered public accounting firm, subject to a “de minimus” exception, (vii) meeting separately, periodically, with management and with our independent registered public accounting firm, and (viii) establishing procedures for our receipt, retention and treatment of information provided by our employees regarding accounting, internal accounting controls or audit matters. The Audit Committee’s charter is provided on our website, http://www.imprimispharma.com.
Compensation Committee
Dr. Finnegan, Dr. Abrams and Mr. Austin have been appointed to serve on our Compensation Committee, and Dr. Finnegan has been appointed as the Chair of the Compensation Committee. Each member of our Compensation Committee is independent within the meaning of applicable NASDAQ Rules and is a “non-employee director” as defined under Rule 16b-3 of the Exchange Act and an “outside director” as that term is defined in applicable rules under the Internal Revenue Code.
Our Board of Directors has adopted a written charter for our Compensation Committee, which sets forth the specific duties and responsibilities of the Compensation Committee, including: (i) reviewing and approving our executive officer compensation programs and arrangements, (ii) determining the specific objectives of our compensation programs and structuring such programs to effectively attract and retain qualified personnel, (iii) reviewing and establishing goals and objectives relevant to the compensation of our Chief Executive Officer, and evaluating the Chief Executive Officer’s performance in light of those goals and objectives, (iv) administering our equity and incentive compensation plans, and (v) reviewing and approving director compensation and benefits. Pursuant to its charter, the Compensation Committee may select and engage such advisors and consultants as it deems necessary or desirable in its sole discretion to carry out its duties, and has the authority to approve the fees and retention terms relating to such advisors and/or consultants. The Compensation Committee’s charter is provided on our website, http://www.imprimispharma.com.
60
Nomination and Corporate Governance Committee
Dr. Finnegan, Dr. Abrams and Mr. Austin have been appointed to serve on our Nomination and Corporate Governance Committee, and Dr. Finnegan has been appointed as the Chair of that committee. Each member of our Nomination and Corporate Governance Committee is independent within the meaning of applicable NASDAQ Rules.
Our Board of Directors has adopted a written charter for our Nomination and Corporate Governance Committee, which sets forth the specific duties and responsibilities of that committee, including: (i) assisting in identifying and recruiting qualified candidates for our Board and our management team, (ii) advising the Board regarding the membership and chairs of the committees of our Board, (iii) overseeing and evaluating the performance of our Board and our management team, including assessing the independence of our directors, (iv) recommending to the Board and overseeing our corporate governance principles, and (vii) recommending to the Board and monitoring compliance with our code of business conduct and ethics. A copy of the Nomination and Corporate Governance Committee’s charter is provided on our website, http://www.imprimispharma.com.
Director Independence
Current Directors
We are not currently listed on any national securities exchange that has a requirement that the Board of Directors be independent. However, Mr. Austin, Dr. Abrams, Mr. Bassani and Dr. Finnegan would each be considered an “independent director” as defined in Rule 5605(a)(2) of the NASDAQ Rules. Mr. Baum would not be considered independent because he currently serves as our Chief Executive Officer, and Dr. Kammer is not independent because of certain ongoing advisory relationships.
Former Directors
Commencing on February 15, 2012, Dr. Balbir Brar, our President, served as a director on our Board. Dr. Brar resigned as a director on July 25, 2012, but continues in his capacity as our President. Dr. Brar was not considered independent because of his position as our President.
During the fiscal year ended December 31, 2011, the following individuals served as our directors: John N. Bonfliglio, Lynn C. Swann, Anthony S. Thornley, Jeffrey J. Abrams, Mark L. Baum and Robert J. Kammer. Under NASDAQ Rules, during the fiscal year ended December 31, 2011, Mr. Swann, Mr. Thornley and Dr. Abrams would have been considered independent. Mr. Bonfiglio, Mr. Baum and Dr. Kammer would not be considered independent because of their employment or consulting relationships with the Company.
EXECUTIVE COMPENSATION
All information regarding share amounts of common stock and prices per share of common stock contained under the heading “Executive Compensation” assumes the consummation of the one-for-five reverse stock split to be effected following effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of this offering.
Summary Compensation Table
The following table summarizes compensation earned by or awarded or paid to our principal executive officer and our other two most highly compensated executive officers (our “named executive officers”). Also included is compensation information for our Vice President, Accounting and Public Reporting (our Principal Accounting and Financial Officer).
|
Name and
Principal Position
|
Year
|
Salary
|
Stock Awards (1)
|
Option Awards (2)
|
All Other Compensation
|
Total
|
|||||||||||||||
|
Mark L. Baum, Esq.
|
2012
|
$ | 150,300 | $ | 520,000 | (3) | $ | 655,773 | (4) | - | $ | 1,326,073 | |||||||||
|
Chief Executive Officer
|
2011
|
- | - | - | - | - | |||||||||||||||
|
Joachim P.H. Schupp, M.D.
|
2012
|
$ | 178,500 | - | $ | 260,100 | $ | 29,134 | (5) | $ | 467,734 | ||||||||||
|
Chief Medical Officer
|
2011
|
$ | 38,800 | - | $ | 2,192 | - | $ | 40,992 | ||||||||||||
|
Balbir Brar, D.V.M., Ph.D.
|
2012
|
$ | 84,000 | - | $ | 669,300 | (6) | - | $ | 753,300 | |||||||||||
|
President
|
2011
|
- | - | - | - | - | |||||||||||||||
|
Andrew R. Boll
|
2012
|
$ | 64,500 | - | $ | 51,780 | $ | 7,524 | (7) | $ | 123,804 | ||||||||||
|
Vice-President, Accounting and Public Reporting
|
2011
|
- | - | - | - | - | |||||||||||||||
|
(1)
|
Represents the dollar value of the restricted stock awards calculated on the basis of the fair value of the underlying shares of our common stock on the respective grant dates in accordance with FASB ASC Topic 718 and without any adjustment for estimated forfeitures. The actual value that an executive will realize on each restricted stock award will depend on the price per share of our common stock at the time shares underlying the restricted stock awards are sold. The actual value realized by an executive may not be at or near the grant date fair value of the restricted stock awarded.
|
|
(2)
|
Reflects the dollar amount of the grant date fair value of awards granted during the respective fiscal years, measured in accordance with Accounting Standards Codification Topic 718 and without adjustment for estimated forfeitures. For a discussion of the assumptions used to calculate the value of option awards, refer to Note 7 "Stock Option Plan" of Notes to Consolidated Financial Statements for the fiscal year ended December 31, 2011 included in this prospectus. For a discussion of the material terms of each stock option award, see the table entitled "Outstanding Equity Awards at Fiscal Year End."
|
|
(3)
|
Represents restricted stock units granted to Mr. Baum outside the 2007 Plan in connection with his services as our Chief Executive Officer, the vesting of which is subject to certain performance conditions. The value of the award at the grant date assuming that the highest level of the performance conditions will be achieved is the same as reflected in the above table.
|
61
|
(4)
|
Represents (i) an option to purchase up to 125,000 shares of common stock under the 2007 Plan granted on January 25, 2012 for his uncompensated services as Chairman of the Board of Directors and significant ongoing services related, but not limited, to the Company’s emergence from Chapter 11 bankruptcy protection, negotiation with creditors, pursuit of additional financing opportunities and hiring of executive officers, (ii) an option to purchase up to 25,000 shares of common stock under the 2007 Plan, which was granted to all of the Company’s directors on April 1, 2012 for their service as directors and which vests in equal installments of 6,250 shares on each of June 30, 2012, September 30, 2012, December 31, 2012 and March 31, 2013 subject to continued service as a director on each such date, and (iii) an option to purchase up to 60,000 shares of common stock under the 2007 Plan granted on April 1, 2012 in connection with his appointment as our Chief Executive Officer.
|
|
(5)
|
Consists of (i) $23,500 paid to an entity beneficially owned by Mr. Schupp for consulting services performed during the fiscal year 2012 prior to his hire as our Chief Medical Officer, and (ii) $5,634 paid for medical and dental insurance premiums.
|
|
(6)
|
Represents (i) an option to purchase up to 225,000 shares of common stock under the 2007 Plan granted on January 25, 2012 in connection with his appointment as our President, and (ii) an option to purchase up to 25,000 shares of common stock under the 2007 Plan, which was granted to all of the Company’s directors on April 1, 2012 for their service as directors and which vests in equal installments of 6,250 shares on each of June 30, 2012, September 30, 2012, December 31, 2012 and March 31, 2013 subject to continued service as a director on each such date. On July 25, 2012, Dr. Brar resigned as a director (but continues in his capacity as our President). As a result of such resignation, the 18,750 unvested shares under Dr. Brar’s April 1, 2012 option grant were forfeited, and the 6,250 vested shares under such option remain exercisable until March 22, 2013.
|
|
(7)
|
Consists of (i) $5,000 paid to Mr. Boll for consulting services performed during the fiscal year 2012 prior to his hire as our Vice President, Accounting and Public Reporting, and (ii) $2,524 paid for medical and dental insurance premiums.
|
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth certain information concerning outstanding stock awards held by our named executive officers and our Vice President, Accounting and Public Reporting serving during the fiscal year ended December 31, 2012.
|
Option Awards
|
Stock Awards
|
|||||||||||||||||||||||
|
Number of
|
Number of
|
Market Value
|
||||||||||||||||||||||
|
Securities
|
Securities
|
Number
|
of Shares or
|
|||||||||||||||||||||
|
Underlying
|
Underlying
|
of Shares
|
Units of
|
|||||||||||||||||||||
|
Unexercised
|
Unexercised
|
Option
|
Option
|
or Units
|
Stock that
|
|||||||||||||||||||
|
Options (#)
|
Options (#)
|
Exercise
|
Expiration
|
of Stock that
|
Have Not
|
|||||||||||||||||||
|
Name
|
Exercisable
|
Unexercisable
|
Price ($)
|
Date
|
Have Not Vested
|
Vested(1)
|
||||||||||||||||||
|
Mark L. Baum, Esq.
|
114,583 | (2) | 10,417 | (2) | $ | 2.40 |
1/25/2022
|
- | - | |||||||||||||||
| 31,875 | (3) | 28,125 | (3) | $ | 4.50 |
3/31/2017
|
- | - | ||||||||||||||||
| 18,750 | (4) | 6,250 | (4) | $ | 4.50 |
3/31/2017
|
- | - | ||||||||||||||||
| - | - | $ | - | - | 160,000 | (5) | $ | 1,560,000 | ||||||||||||||||
|
Balbir Brar, D.V.M., Ph.D.
|
68,750 | (6) | 156,250 | (6) | $ | 3.68 |
1/25/2016
|
- | - | |||||||||||||||
| 6,250 | (7) | - | $ | 4.50 | - | - | - | |||||||||||||||||
|
Joachim P.H. Schupp, M.D.
|
22,917 | (8) | 52,083 | (8) | $ | 3.60 |
2/15/2016
|
- | - | |||||||||||||||
| 493 | (9) | - | $ | 4.00 |
10/5/2014
|
- | - | |||||||||||||||||
|
Andrew R. Boll
|
4,583 | (10) | 10,417 | (10) | $ | 3.68 |
2/1/2016
|
- | - | |||||||||||||||
62
|
(1)
|
Calculated by multiplying the number of unvested shares by $9.75, the closing price per share of our common stock on December 31, 2012 (which was the last business day of the fiscal year).
|
|
(2)
|
Represents an option granted to Mr. Baum on April 1, 2012 under the 2007 Plan for his uncompensated services as Chairman of the Board of Directors and significant ongoing services related, but not limited, to the Company’s emergence from Chapter 11 bankruptcy protection, negotiation with creditors, pursuit of additional financing opportunities and hiring of executive officers. The option vests in 12 equal monthly installments of 10,417 shares commencing on January 25, 2012 and ending on January 25, 2013.
|
|
(3)
|
Represents an option granted to Mr. Baum an April 1, 2012 under the 2007 Plan in connection with his appointment as our Chief Executive Officer. The option vests over a two-year period, with 15,000 shares vesting immediately upon issuance and an additional 1,875 shares vesting monthly for the 24 months thereafter.
|
|
(4)
|
Represents an option granted to Mr. Baum an April 1, 2012 under the 2007 Plan in connection with his services as a director. The option vests in four equal quarterly installments of 6,250 shares commencing on June 30, 2012.
|
|
(5)
|
Represents restricted stock units granted to Mr. Baum outside the 2007 Plan in connection with his services as our Chief Executive Officer. The total award vests as follows: (i) 25% vests on successful completion of a financing that results in aggregate cash proceeds to the Company of at least $5,000,000 at any time following the effective date of the grant; (ii) 25% vests on the Company meeting the primary endpoints of its Phase 3 clinical studies for its drug candidate, Impracor; (iii) 25% vests on the Company submitting a New Drug Application for Impracor to the U.S. Food and Drug Administration; and (iv) 25% vests on the Company entering into a definitive license, collaboration or similar agreement for Impracor that would reasonably be expected to generate cash flow for the Company.
|
|
(6)
|
Represents an option granted to Dr. Brar on January 25, 2012 under the 2007 Plan in connection with his appointment as our President. The option vests in equal monthly installments over the 36 month period following the date of grant.
|
|
(7)
|
Represents an option granted to Dr. Brar on April 1, 2012 under the 2007 Plan in connection with his former services as a director. The option vests in equal installments of 6,250 shares on each of June 30, 2012, September 30, 2012, December 31, 2012 and March 31, 2013 subject to continued service as a director on each such date. On July 25, 2012, Dr. Brar resigned as a director (but continues in his capacity as our President). As a result of such resignation, the 18,750 unvested shares under the option were forfeited, and the 6,250 vested shares under the option remain exercisable until March 22, 2013.
|
|
(8)
|
Represents an option granted to Dr. Schupp on February 15, 2012 under the 2007 Plan in connection with his appointment as our Chief Medical Officer. The option vests in equal monthly installments over the 36 month period following the date of grant.
|
|
(9)
|
Represents an option granted to Dr. Schupp on December 15, 2011 under the 2007 Plan in connection with a release given by Dr. Schupp upon DermaStar’s investment in the Company. The option was 100% vested upon its grant.
|
|
(10)
|
Represents an option granted to Mr. Boll on February 1, 2012 under the 2007 Plan in connection with his appointment as our Vice President, Accounting and Public Reporting. The option vests in equal monthly installments over the 36 month period following the date of grant.
|
Employment Agreements
Mark Baum
On April 1, 2012, the Board of Directors appointed Mr. Mark L. Baum, Esq. as our Chief Executive Officer. Mr. Baum had served as our Chairman of the Board of Directors and principal executive officer and Secretary since December 17, 2011. Concurrently with Mr. Baum’s appointment to Chief Executive Officer, Mr. Baum resigned from his position as Chairman of the Board. Mr. Baum continues to serve as a member of the Board of Directors and as Secretary. Concurrent with his appointment as Chief Executive Officer, we entered into an employment agreement with Mr. Baum, effective as of April 1, 2012, which was subsequently amended and restated on July 24, 2012 (as amended, the “Baum Employment Agreement”). Under the terms of the Baum Employment Agreement, Mr. Baum’s initial base annual salary is $200,400, with a minimum salary increase of no less than 15% annually. Mr. Baum may be eligible, at the sole discretion of the Board, to receive an annual cash bonus of up to 30% of his annual base salary beginning in the fiscal year ending 2013 contingent upon his satisfaction of certain company and individual performance criteria. Mr. Baum may be terminated by us at any time. Upon the closing of a financing transaction that results in aggregate cash proceeds to the Company of over $5,000,000 at any time after July 24, 2012, Mr. Baum will automatically become entitled to receive a severance package of one year’s base salary and annual bonus in effect at the time of termination and continued Company paid healthcare expenses for one year upon the Company’s termination of Mr. Baum’s employment without cause.
Also on April 1, 2012, the Company granted to Mr. Baum an option to purchase up to 60,000 shares of common stock at an exercise price of $4.50 per share under the 2007 Plan . The option terminates on March 31, 2017 and vests over a two year period, with 15,000 options vesting immediately upon issuance and an additional 1,875 options vesting monthly for the next twenty-four months thereafter. The option vests immediately upon the involuntary termination of Mr. Baum’s employment within 12 months following a change in control, as defined in the 2007 Plan.
63
On January 25, 2012, the Board approved an option grant to Mr. Baum to purchase up to 125,000 shares pursuant to the 2007 Plan. The options were issued to Mr. Baum for his uncompensated services as Chairman of the Board of Directors and significant ongoing services related, but not limited to, the Company’s emergence from Chapter 11 bankruptcy protection, negotiation with creditors, pursuit of additional financing opportunities and hiring of executive officers. The option vests in twelve equal monthly installments commencing on January 25, 2012 and ending on January 25, 2013, and has an exercise price of $2.40.
On July 18, 2012, the Board granted to Mr. Baum, in connection with his services as the Chief Executive Officer of the Company, 160,000 restricted stock units (RSUs) outside of the 2007 Plan. The restricted stock units granted to Mr. Baum are subject to certain performance-based vesting criteria, such that 40,000 RSUs will vest upon the satisfaction of each of the following events: (i) successful completion of a financing that results in aggregate cash proceeds to the Company of at least $5,000,000 at any time following the effective date of the grant; (ii) the Company meets the primary endpoints of its Phase III clinical studies for Impracor; (iii) the Company submits a New Drug Application for Impracor to the U.S. Food and Drug Administration; and (iv) the Company enters into a definitive license, collaboration or similar agreement for Impracor that would reasonably be expected to generate cash flow for the Company. The RSUs vest in full upon a change in control of the Company.
Dr. Balbir Brar
On January 17, 2012, we entered into an Employment Agreement with Dr. Balbir Brar in connection with his appointment as our President, effective January 1, 2012. Under the agreement, Dr. Brar must commit 20 hours each week to the Company and will receive an initial base salary of $84,000 per year. On January 25, 2012, the Board granted Dr. Brar an option to purchase 225,000 shares of common stock with an exercise price of $3.68 under the 2007 Plan. The option has a four year term and vests monthly over a 36 month period following the date of grant and vests in full upon a change of control, as defined in the 2007 Plan. Dr. Brar has agreed to not sell more than 5% of the shares of the Company’s common stock acquired through the exercise of his stock options in any monthly period without the approval of the Board of Directors. We may terminate Dr. Brar’s employment without notice for cause, and upon 60 days’ notice without cause. Dr. Brar’s employment will also terminate upon his death or disability, or Dr. Brar may terminate his employment upon 60 days’ notice.
Dr. Joachim Schupp
On February 15, 2012, we entered into an Employment Agreement with Dr. Joachim Schupp in connection with his appointment as our Chief Medical Officer. Under the terms of his Employment Agreement, Dr. Schupp will receive an initial base salary of $204,000 per year. Also on February 15, 2012, Dr. Schupp was issued an option to purchase 75,000 shares of common stock with an exercise price of $3.60 per share under the 2007 Plan. The option has a four year term and vests monthly over a 36 month period following the date of grant. The option vests in full upon a change of control as defined in the 2007 Plan. Dr. Schupp has agreed to not sell more than 5% of the shares of the Company’s common stock acquired through the exercise of his stock options in any monthly period without the approval of the Board of Directors. We may terminate Dr. Schupp’s employment without notice for cause, and upon 60 days’ notice without cause. Dr. Schupp’s employment will also terminate upon his death or disability, or Dr. Schupp may terminate his employment upon 60 days’ notice.
Andrew R. Boll
On January 25, 2012, the Company entered into an Employment Agreement with Mr. Boll, effective as of February 1, 2012. Under the terms of the Employment Agreement, Mr. Boll will receive an initial base salary of $60,000 per year. On January 25, 2012, the Board approved the issuance of an option to purchase 15,000 shares of common stock under the 2007 Plan to Mr. Boll, which was granted on February 1, 2012, the date of his employment with the Company. The option has an exercise price of $3.68 per share, has a four year term and vests monthly over a 36 month period following the date of grant. The option vests in full upon a change of control as defined in the 2007 Plan. Mr. Boll has agreed to not sell more than 5% of the shares of the Company’s common stock acquired through the exercise of his stock option in any monthly period without the approval of the Board of Directors. We may terminate Mr. Boll’s employment without notice for cause, and upon 60 days’ notice without cause. Mr. Boll’s employment will also terminate upon his death or disability, or Mr. Boll may terminate his employment upon 60 days’ notice. On October 1, 2012, Mr. Boll's salary was increased to $90,000 per year.
64
2007 Incentive Stock and Awards Plan
On September 17, 2007, our Board of Directors and stockholders adopted the 2007 Incentive Stock and Awards Plan (the “2007 Plan”). The purpose of the 2007 Plan is to provide an incentive to attract and retain directors, officers, consultants, advisors and employees whose services are considered valuable, to encourage a sense of proprietorship and to stimulate an active interest of such persons in our development and financial success. Under the 2007 Plan, we are authorized to issue incentive stock options intended to qualify under Section 422 of the Internal Revenue Code of 1986, as amended, non-qualified stock options, and restricted stock. The 2007 Plan is administered by our Board of Directors until such time as such authority has been delegated to a committee of the Board of Directors. Effective November 5, 2008, our stockholders approved an amendment to the 2007 Plan to increase the number of authorized shares to 75,000 from 37,500. On January 25, 2012, our Board of Directors and stockholders approved an amendment to, among other things, increase the maximum number of shares issuable under the 2007 Plan to 750,000 shares. The amendment became effective following our compliance with certain information requirements of the SEC. Effective as of July 18, 2012, our Board of Directors and stockholders approved a further amendment to increase the maximum number of shares to 2,400,000 shares and to increase the number of shares that may be granted to an individual in a calendar year. The stockholder approval of the amendment became effective upon our compliance with certain information requirements of the SEC.
DIRECTOR COMPENSATION
Narrative Disclosure Regarding Director Compensation Practices
We do not currently have a standard director compensation program in place; however, the Board of Directors approved the following compensation to our directors for their service as directors in 2012:
· On April 1, 2012, the Board of Directors approved the grant to each of our directors on that date, including our employee and non-employee directors, of an option to purchase up to 25,000 shares of our common stock under the 2007 Plan (each, a “2012 Director Option”). The directors who received such an option were as follows: (i) employee directors Dr. Brar (whose option has been forfeited as a result of his resignation as a director but remains exercisable until March 22, 2013) and Mr. Baum, and (ii) non-employee directors Dr. Kammer, Dr. Finnegan and Dr. Abrams. Each of the options has an exercise price of $4.50 per share and a term of five years, and vests quarterly over a one year period such that options to purchase 6,250 shares vest on each of June 30, 2012, September 30, 2012, December 31, 2012 and March 31, 2013 (subject to continued service as a director on each such date).
· On April 1, 2012, the Board of Directors approved the grant to director Dr. Jeffrey Abrams, in consideration of his service as a director of the Company during 2011 and 2012, of an option to purchase up to 60,000 shares of common stock under the 2007 Plan. The option has an exercise price of $4.50 per share and a term of ten years, and vests in equal monthly installments over a one year period.
· On July 26, 2012, in connection with his appointment as a director, the Board of Directors granted to Mr. Austin an option to purchase up to 17,123 shares of our common stock under the 2007 Plan. That option has an exercise price of $4.50 per share and a term of five years, and vests in equal monthly installments over a period of one year commencing on January 1, 2013. In addition, the Board of Directors approved our payment to Mr. Austin of a quarterly cash payment of $5,000 for his services as a director and a quarterly cash payment of $1,250 for his services as the chair of the Audit Committee.
· On December 14, 2012, in connection with his appointment as a director, the Board of Directors granted to Mr. Bassani an option to purchase up to 7,603 shares of our common stock under the 2007 Plan. That option has an exercise price of $10.75 per share and a term of five years, and vests in equal monthly installments over a period of one year commencing on January 1, 2013. In addition, the Board of Directors approved the payment to Mr. Bassani of a quarterly cash payment of $5,000 for his services as a director.
Director Compensation Table
The following table shows the compensation paid in fiscal 2012 to our non-employee directors. All compensation received by directors Dr. Brar and Mr. Baum, including compensation received by them for services as a director, is disclosed in the Summary Compensation Table.
|
Name
|
Fees Earned or Paid in Cash
|
Stock Awards(1)(3)
|
Option Awards (2)(3)
|
Total
|
||||||||||||
|
Robert J. Kammer, D.D.S.
|
$ | - | $ | 269,444 | (4) | $ | 381,578 | (5) | $ | 651,022 | ||||||
|
Paul Finnegan, M.D.
|
$ | 18,000 | (6) | $ | - | $ | 450,325 | (7) | $ | 468,325 | ||||||
|
Jeffrey J. Abrams, M.D.
|
$ | - | $ | - | $ | 381,480 | (8) | $ | 381,480 | |||||||
|
Stephen G. Austin, CPA
|
$ | 12,500 | $ | - | $ | 58,099 | (9) | $ | 70,599 | |||||||
|
August S. Bassani, Pharm.D.
|
$ | - | $ | - | $ | 81,627 | (10) | $ | 81,627 | |||||||
65
|
(1)
|
Represents the dollar value of the restricted stock awards calculated on the basis of the fair value of the underlying shares of our common stock on the respective grant dates in accordance with FASB ASC Topic 718 and without any adjustment for estimated forfeitures. The actual value that an executive will realize on each restricted stock award will depend on the price per share of our common stock at the time shares underlying the restricted stock awards are sold. The actual value realized by an executive may not be at or near the grant date fair value of the restricted stock awarded.
|
|
(2)
|
Reflects the dollar amount of the grant date fair value of awards granted during the respective fiscal years, measured in accordance with Accounting Standards Codification Topic 718 and without adjustment for estimated forfeitures. For a discussion of the assumptions used to calculate the value of option awards, refer to Note 7 "Stock Option Plan" of Notes to Consolidated Financial Statements for the fiscal year ended December 31, 2011 included in this prospectus.
|
|
(3)
|
The aggregate number of stock and option awards outstanding as of December 31, 2012 for each non-employee director are as follows:
|
|
Name
|
Shares Underlying Options Awards
|
Shares Underlying Stock Awards
|
Total
|
|||||||||
|
Robert J. Kammer, D.D.S.
|
85,000 | 60,000 | 145,000 | |||||||||
|
Paul Finnegan, M.D.
|
150,000 | - | 150,000 | |||||||||
|
Jeffrey J. Abrams, M.D.
|
87,250 | - | 87,250 | |||||||||
|
Stephen G. Austin, CPA
|
17,123 | - | 17,123 | |||||||||
|
August S. Bassani, Pharm.D.
|
7,603 | - | 7,603 | |||||||||
|
( 4)
|
Represents (i) 20,000 shares of common stock earned by but not yet issued to Dr. Kammer under his advisory agreement entered into with the Company on April 1, 2012, pursuant to which Dr. Kammer provides certain consultant and advisory services in addition to his services as a director and, among other compensation, earns $10,000 per month in the form of common stock based on a price per share of $4.50, and (ii) 40,000 RSUs granted to Dr. Kammer on July 18, 2012 outside the 2007 Plan in connection with his services as a consultant and advisor to the Company, which RSUs are subject to certain performance-based vesting criteria such that all 40,000 RSUs will vest at such time as the Company meets the primary endpoints of its Phase III clinical studies for Impracor.
|
|
(5)
|
Represents (i) a 2012 Director Option granted to Dr. Kammer, and (ii) an option to purchase up to 60,000 shares of common stock granted to Dr. Kammer on April 1, 2012 under the 2007 Plan pursuant to the terms of his advisory agreement with the Company, which agreement provides for, in addition to certain other compensation provided to Dr. Kammer under that agreement for his consulting and advisory services that is described in footnote (4) above, the grant to Dr. Kammer of this non-qualified stock option with an exercise price of $4.50 per share, an expiration date of March 31, 2017, and a vesting schedule as follows: 15,000 shares vest on the date of grant and the remaining shares vest in equal monthly installments over a two year period beginning on May 1, 2012.
|
|
(6)
|
Reflects the total amount paid to Dr. Finnegan under a senior advisory agreement entered into with the Company on January 17, 2012 and terminated on May 9, 2012. Such amount was paid in April 2012 prior to the termination of the agreement in exchange for services rendered under the agreement in the first quarter of 2012.
|
|
(7)
|
Represents (i) a 2012 Director Option granted to Dr. Finnegan, and (ii) an option to purchase 125,000 shares of common stock at an exercise price of $3.20 per share granted to Dr. Finnegan on January 25, 2012 under the 2007 Plan in connection with a senior advisory agreement entered with the Company on January 17, 2012, which agreement was terminated on May 9, 2012. Also effective May 9, 2012, we entered into an amendment to Dr. Finnegan’s option agreement which modifies the vesting schedule of the option to provide that the option to purchase 40% of the shares covered by the grant will vest on September 30, 2012, 40% will vest on March 31, 2013 and 20% will vest on September 30, 2013, provided that Dr. Finnegan is serving as a director, employee or consultant at the time of such vesting.
|
|
(8)
|
Represents (i) a 2012 Director Option granted to Dr. Abrams, and (ii) an option to purchase 60,000 shares of common stock granted to Dr. Abrams on April 1, 2012 under the 2007 Plan in consideration of his service as a director of the Company during 2011 and 2012, which option has an exercise price of $4.50 per share, a term of ten years, and vests in equal monthly installments over a one year period.
|
|
(9)
|
Represents an option to purchase up to 17,123 shares of our common stock granted to Mr. Austin on August 26, 2012 under the 2007 Plan, as consideration for his service as a director. That option has an exercise price of $4.50 per share, a term of five years, and vests in equal monthly installments over a period of one year commencing on January 1, 2013.
|
|
(10)
|
Represents an option to purchase up to 7,603 shares of our common stock granted to Mr. Bassani on December 14, 2012 under the 2007 Plan, as consideration for his services as a director. That option has an exercise price of $10.75 per share, has a term of five years, and vests monthly over a period of one year commencing on January 1, 2013.
|
66
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Transactions with Related Persons
During the fiscal years ended December 31, 2012, 2011 and 2010, and through the date of this prospectus, other than as described below, there have been no transactions, and there are no currently proposed transactions, in which we were or are to be a participant and the amount involved exceeds the lesser of $120,000 or one percent of the average of our total assets at year end for the last two completed fiscal years and in which any related person had or will have a direct or indirect material interest.
Our Chief Executive Officer and director, Mr. Mark L. Baum, and the Chairman of our Board of Directors, Robert J. Kammer, served as Managing Members of DermaStar prior to DermaStar’s conversion of all of the outstanding shares of Series A Preferred Stock into common stock and the distribution of all shares of capital stock and warrants held by it to its members in July 2012. Mr. Baum and Dr. Kammer were appointed to our Board on December 16, 2011, following the closing of the Line of Credit Agreement and the purchase of the Series A Preferred Stock by DermaStar described below and elsewhere in this prospectus.
All information regarding share amounts of common stock and prices per share of common stock contained under the heading “Certain Relationship and Related Transactions” assumes the consummation of the one-for-five reverse stock split to be effected following effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of this offering.
Secured Line of Credit
On November 21, 2011, we entered into a Secured Line of Credit Letter Agreement (the “Line of Credit Agreement”) with DermaStar, pursuant to which DermaStar agreed to lend us funds under a line of credit upon certain conditions, including the dismissal of the Chapter 11 Case by the Bankruptcy Court. The Line of Credit Agreement became effective on December 9, 2011, in connection with the dismissal of the Chapter 11 Case by the Bankruptcy Court. The Line of Credit Agreement provided for advances of up to an aggregate of $750,000, subject to the satisfaction by us of certain conditions in connection with the initial advance and each subsequent advance. The largest outstanding principal balance under the line of credit at any time was $750,000. Interest accrued at 10% per annum. No interest payments were made by us during the period other than in connection with the conversion of the line of credit described below.
On April 25, 2012, the entire outstanding principal balance and all accrued and unpaid interest under the line of credit, an aggregate of $762,534, was converted into 193,047 shares of common stock and warrants to purchase 48,262 shares of common stock at the offering price and on the terms of the April Private Placement described below, pursuant to the terms of a conversion agreement we entered into with DermaStar on April 20, 2012. The warrants have substantially the same terms as the warrants issued in the April Private Placement. The line of credit was terminated upon the completion of the conversion.
Series A Preferred Stock Purchase
In partial consideration for and in connection with the Line of Credit Agreement, on November 21, 2011 we executed a Securities Purchase Agreement with DermaStar, pursuant to which we agreed to issue 10 shares of newly-designated Series A Convertible Preferred Stock (the “Series A Preferred Stock”) to DermaStar for an aggregate purchase price of $100,000. The Securities Purchase Agreement, as amended, became effective on December 9, 2011, in connection with the dismissal of the Chapter 11 Case by the Bankruptcy Court. On December 12, 2011, we and DermaStar consummated the transactions contemplated by the Securities Purchase Agreement. The shares of Series A Preferred Stock issued to DermaStar in the offering are convertible into 1,499,700 shares of our common stock.
On June 29, 2012, DermaStar converted the 10 shares of Series A Preferred Stock held by it into 1,499,700 shares of our common stock. In connection with the conversion, we paid to DermaStar $200,000 as partial consideration for the conversion pursuant to a conversion agreement. Immediately following the conversion of the Series A Preferred Stock, all 10 shares were retired to our treasury and cancelled. The conversion agreement was unanimously approved by the Company’s disinterested directors, with Mr. Baum and Dr. Kammer abstaining.
7.5% Convertible Promissory Note
On April 5, 2010, we issued a $1,000,000 7.5% Convertible Promissory Note (the “Convertible Note”) to Alexej Ladonnikov, an existing stockholder of the Company. The Convertible Note had an annual interest rate of 7.5% and all principal and interest were due and payable on its maturity date, April 5, 2012.
During January 2012, Mr. Ladonnikov sold 80% of the Convertible Note to DermaStar in a private transaction. Effective as of January 25, 2012, we entered into separate waiver and settlement agreements with DermaStar and Mr. Ladonnikov. Under each of the waiver and settlement agreements, the holders of the Convertible Note agreed to forever waive (i) their rights to accelerate the entire unpaid principal sum of the Convertible Note and all accrued interest pursuant to Section 1 of the Convertible Note, (ii) their rights under Section 7 of the Senior Convertible Note Purchase Agreement dated April 5, 2010, and (iii) certain conversion rights pursuant to Section 3 of the Convertible Note. Under the terms of the waiver and settlement agreement with DermaStar, we and DermaStar agreed to the mandatory conversion of the principal and accrued and unpaid interest of the Convertible Note and $56,087 in current accounts payable of the Company held by DermaStar into our common stock at a conversion price of approximately $0.06668 per share at such time as we had a sufficient number of shares of authorized common stock to effect such conversion. Under the terms of the waiver and settlement agreement with Mr. Ladonnikov, we and Mr. Ladonnikov agreed to the mandatory conversion of the 20% of the principal and accrued and unpaid interest of the Convertible Note held by Mr. Ladonnikov, at such time as we had a sufficient number of authorized common shares to effect such a conversion, into our common stock at a conversion price of $0.60. Mr. Ladonnikov also agreed to make a one-time payment of $50,000 to us at such time as the Convertible Note was converted into common stock.
On February 28, 2012, effective immediately following the effective time of our Certificate of Amendment to our Certificate of Incorporation increasing the number of authorized shares of common stock and implementing the one-for-eight reverse split of our common stock, the entire outstanding balance and all accrued but unpaid interest owing under the Convertible Note and the accounts payable held by DermaStar were converted into 1,835,830 shares of common stock, and the Convertible Note was terminated. At the time of conversion, there was approximately $142,603 in accrued and unpaid interest due under the Convertible Note. Mr. Ladonnikov made the required one-time payment of $50,000 to us at the time of the conversion.
Company Policy Regarding Related Party Transactions
The charter of the Audit Committee of our Board tasks the Audit Committee with reviewing and overseeing all related party transactions for potential conflict of interest situations on an ongoing basis. In accordance with that policy, the Audit Committee’s general practice is to review and oversee those transactions that are reportable as related party transactions under the Financial Accounting Standards Board and Securities and Exchange Commission rules and regulations. Management advises the Board of Directors on a regular basis of any such transaction that is proposed to be entered into or continued and seeks approval.
67
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth the shares of our common stock beneficially owned by (1) each of our directors, (2) the named executive officers, (3) all of our directors and executive officers as a group, and (4) all persons known by us to beneficially own more than 5% of our outstanding voting stock. We have determined the beneficial ownership shown on this table in accordance with the rules of the Securities and Exchange Commission. Under those rules, shares are considered beneficially owned if held by the person indicated, or if such person, directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise has or shares the power to vote, to direct the voting of and/or to dispose of or to direct the disposition of such security. Except as otherwise indicated in the accompanying footnotes, beneficial ownership is shown as of January 31, 2013. Unless otherwise indicated in the footnotes to the following table, each person named in the table has sole voting and investment power with respect to shares of common stock and the address is c/o Imprimis Pharmaceuticals, Inc. 437 S. Hwy 101, Suite 209, Solana Beach, CA 92075. All information regarding share amounts assumes the consummation of the one-for-five reverse stock split to be effected following effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of this offering.
|
Amount and Nature of Beneficial Ownership
|
||||||||
|
Beneficial Owner
|
Number of Shares
|
Percentage (1)
|
||||||
|
5% + Stockholders
|
||||||||
|
Alexej Ladonnikov (2)
|
396,831 | 5.86 | % | |||||
|
John W. Fish, Jr. (3)
|
603,171 | 8.89 | % | |||||
|
Don Miloni (4)
|
1,243,513 | 18.13 | % | |||||
|
Professional Compounding Centers of America, Inc. (5)
|
832,683 | 12.30 | % | |||||
|
Directors and Officers
|
||||||||
|
Jeffery J. Abrams, M.D. (6)
|
126,013 | 1.84 | % | |||||
|
Mark L. Baum, Esq. (7)
|
359,299 | 5.16 | % | |||||
|
Andrew R. Boll (8)
|
5,833 | * | ||||||
|
John Bonfigilio
|
469 | * | ||||||
|
Balbir Brar, D.V.M., Ph.D. (9)
|
106,954 | 1.56 | % | |||||
|
Paul Finnegan, M.D. (10)
|
125,000 | 1.81 | % | |||||
|
Robert J. Kammer, D.D.S. (11)
|
991,496 | 14.43 | % | |||||
|
Stephen G. Austin, CPA (12)
|
5,708 | * | ||||||
|
August S. Bassani, Pharm.D. (14)
|
2,534 | * | ||||||
|
John Lomoro
|
1,241 | * | ||||||
|
Terry Nida
|
3,639 | * | ||||||
|
Joachim Schupp, M.D. (13)
|
27,576 | * | ||||||
|
All current executives and directors as a group (9 persons)
|
1,750,413 | 23.62 | % | |||||
68
|
*
|
Represents less than 1%.
|
|
|
(1)
|
Applicable percentage ownership is based on 6,772,125 shares of our common stock outstanding as of January 31, 2013. Shares of common stock subject to options or warrants and convertible notes subject to conversion into shares of our common stock currently exercisable or convertible, or exercisable or convertible within 60 days after January 31, 2013 are deemed outstanding for the purpose of computing the percentage ownership of the person holding such options, warrants or convertible notes, but are not deemed outstanding for computing the percentage ownership of any other person.
|
|
|
(2)
|
The address for Mr. Ladonnikov is 13388 Surrey Lane, Saratoga, CA 95070.
|
|
|
(3)
|
Includes 10,190 shares of common stock issuable upon the exercise of warrants exercisable within 60 days of January 31, 2013.
|
|
|
(4)
|
Includes 878,576 shares held in his name, 25,316 shares held by Mr. Miloni’s spouse, 151,899 shares held by 1425 Greenwood Lane, LLC, of which Mr. Miloni is the beneficial owner, 102,766 shares held by RCHER Financial, LLC, of which Mr. Miloni is a beneficial owner and 84,956 shares of common stock issuable upon the exercise of warrants exercisable within 60 days of January 31, 2013 (of which Mr. Miloni holds warrants to acquire 15,282 shares, Mr. Miloni’s spouse holds warrants to acquire 6,329 shares, 1425 Greenwood Lane, LLC holds warrants to acquire 37,975 shares, and RCHER Financial, LLC holds warrants to acquire 25,730 shares).
|
|
|
(5)
|
The address for Professional Compounding Centers of America, Inc. is 9901 South Wilcrest Dr., Houston, TX 77099.
|
|
|
(6)
|
Jeffrey J. Abrams, M.D., a director, is a trustee of the Abrams Family Trust, which owns 39,063 shares of our common stock. Dr. Abrams has sole voting and investment control with respect to the shares of common stock owned by the Abrams Family Trust. Includes 86,950 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of January 31, 2013.
|
|
|
(7)
|
Includes 187,500 shares of common stock issuable upon the exercise of stock options and 2,413 shares of common stock issuable upon the exercise of warrants exercisable within 60 days of January 31, 2013.
|
|
|
(8)
|
Includes 5,833 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of January 31, 2013.
|
|
|
(9)
|
Includes 93,750 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of January 31, 2013.
|
|
|
(10)
|
Includes 125,000 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of January 31, 2013.
|
|
|
(11)
|
Includes 20,000 shares of common stock to which Dr. Kammer is entitled for services performed under his advisory agreement, and 62,500 shares of common stock issuable upon the exercise of stock options and 15,282 shares of common stock issuable upon the exercise of warrants exercisable within 60 days of January 31, 2013.
|
|
|
(12)
|
Includes 5,708 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of January 31, 2013.
|
|
|
(13)
|
Includes 27,576 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of January 31, 2013.
|
|
|
(14)
|
Includes 2,534 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of January 31, 2013.
|
|
LEGAL MATTERS
The validity of the common stock being offered hereby will be passed upon for us by Morrison & Foerster LLP, San Diego, California.
EXPERTS
KMJ Corbin & Company LLP, an independent registered public accounting firm, has audited our consolidated financial statements for the fiscal years ended December 31, 2011 and 2010, as stated in its report appearing herein, and such audited consolidated financial statements have been so included in reliance upon the report of such firm given upon its authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file annual reports, quarterly reports, current reports, proxy statements and other information with the Securities and Exchange Commission (“SEC”). You may read or obtain a copy of these reports at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549, on official business days during the hours of 10:00 am to 3:00 pm. You may obtain information on the operation of the public reference room and its copy charges by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains registration statements, reports, proxy information statements and other information regarding registrants that file electronically with the SEC, which are available free of charge. The address of the website is http://www.sec.gov.
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock being offered by this prospectus. This prospectus is part of that registration statement. This prospectus does not contain all of the information set forth in the registration statement or the exhibits to the registration statement. For further information with respect to us and the shares we are offering pursuant to this prospectus, you should refer to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract, agreement or other document referred to are not necessarily complete, and you should refer to the copy of that contract or other documents filed as an exhibit to the registration statement. You may read or obtain a copy of the registration statement at the SEC’s public reference room and website referred to above.
69
IMPRIMIS PHARMACEUTICALS, INC.
(A Development Stage Company)
Unaudited Condensed Consolidated Financial Statements
|
Page
|
||||
|
Condensed Consolidated Balance Sheets – September 30, 2012 (Unaudited) and December 31, 2011
|
F-2 | |||
|
Unaudited Condensed Consolidated Statements of Operations for the three and nine months ended September 30, 2012 and 2011 and for the Period from July 24, 1998 (Inception) Through September 30, 2012
|
F-3 | |||
|
Unaudited Condensed Consolidated Statements of Cash Flows for the nine months ended September 30, 2012 and 2011 and for the Period from July 24, 1998 (Inception) Through September 30, 2012
|
F-4 | |||
|
Notes to the Unaudited Condensed Consolidated Financial Statements
|
F-5 | |||
IMPRIMIS PHARMACEUTICALS, INC.
(A Development Stage Company)
Audited Consolidated Financial Statements
|
Report of Independent Registered Public Accounting Firm
|
F-17 | |||
|
Consolidated Balance Sheets at December 31, 2011 and 2010
|
F-18 | |||
|
Consolidated Statements of Operations for the Years Ended December 31, 2011 and 2010 and for the Period from July 24, 1998 (Inception) Through December 31, 2011
|
F-19 | |||
|
Consolidated Statements of Stockholders’ Deficit for the Years Ended December 31, 2011 and 2010 and for the Period from July 24, 1998 (Inception) Through December 31, 2011
|
F-20 | |||
|
Consolidated Statements of Cash Flows for the Years Ended December 31, 2011 and 2010 and for the Period from July 24, 1998 (Inception) Through December 31, 2011
|
F-21 | |||
|
Notes to the Consolidated Financial Statements
|
F-22 |
F-1
IMPRIMIS PHARMACEUTICALS, INC.
(A Development Stage Company)
CONDENSED CONSOLIDATED BALANCE SHEETS
|
September 30,
2012
(unaudited)
|
December 31,
2011
|
Pro Forma Stockholder's Equity as of September 30,
2012
(unaudited)
|
||||||||||
|
ASSETS
|
||||||||||||
|
Current assets
|
||||||||||||
|
Cash and cash equivalents
|
$ | 10,990,871 | $ | 146,160 | ||||||||
|
Prepaid expenses and other current assets
|
76,141 | 14,797 | ||||||||||
|
Deferred offering costs
|
383,746 | - | ||||||||||
|
Total current assets
|
11,450,758 | 160,957 | ||||||||||
|
Furniture and equipment, net
|
13,218 | - | ||||||||||
|
TOTAL ASSETS
|
$ | 11,463,976 | $ | 160,957 | ||||||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT)
|
||||||||||||
|
Current liabilities
|
||||||||||||
|
Accounts payable and accrued expenses
|
$ | 594,944 | $ | 218,612 | ||||||||
|
Accounts payable - related party
|
- | 56,087 | ||||||||||
|
Accrued Phase 3 expenses
|
55,784 | 55,784 | ||||||||||
|
Accrued payroll
|
19,115 | - | ||||||||||
|
Deferred revenue
|
- | 100,000 | ||||||||||
|
Notes payable and accrued interest - related party
|
- | 300,000 | ||||||||||
|
Convertible note payable and accrued interest
|
- | 1,130,479 | ||||||||||
|
Total current liabilities
|
669,843 | 1,860,962 | ||||||||||
|
Commitments and contingencies
|
||||||||||||
|
STOCKHOLDERS' EQUITY (DEFICIT)
|
||||||||||||
|
Series A convertible preferred stock, $0.001 par value, 10 shares authorized,
|
||||||||||||
|
none and 10 shares issued and outstanding
|
||||||||||||
|
at September 30, 2012 and December 31, 2011, respectively
|
- | - | - | |||||||||
|
Common stock, $0.001 par value, 395,000,000 shares authorized,
|
||||||||||||
|
33,859,627 and 1,987,601 shares issued and outstanding
|
||||||||||||
|
at September 30, 2012 and December 31, 2011, respectively
|
33,860 | 1,988 | 6,772 | |||||||||
|
Additional paid-in capital
|
33,411,513 | 16,818,740 | 33,438,601 | |||||||||
|
Deficit accumulated during the development stage
|
(22,651,240 | ) | (18,520,733 | ) | (22,651,240 | ) | ||||||
|
TOTAL STOCKHOLDERS' EQUITY (DEFICIT)
|
10,794,133 | (1,700,005 | ) | 10,794,133 | ||||||||
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT)
|
$ | 11,463,976 | $ | 160,957 | ||||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
F-2
IMPRIMIS PHARMACEUTICALS, INC.
(A Development Stage Company)
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
|
For The
Three MonthsEnded September 30,
|
For The
Three MonthsEnded September 30,
|
For The
Nine MonthsEnded September 30,
|
For The
Nine MonthsEnded September 30,
|
For the Period From July 24, 1998 (Inception) through
September 30, |
||||||||||||||||
|
2012
|
2011
|
2012
|
2011
|
2012
|
||||||||||||||||
|
Revenues:
|
||||||||||||||||||||
|
License revenues
|
$ | - | $ | - | $ | 100,000 | $ | - | $ | 100,000 | ||||||||||
|
Operating Expenses:
|
||||||||||||||||||||
|
Selling, general and administrative
|
946,381 | 66,496 | 2,240,004 | 514,529 | 11,813,331 | |||||||||||||||
|
Research and development
|
303,666 | - | 580,240 | 111,554 | 8,400,498 | |||||||||||||||
|
Loss from operations
|
(1,250,047 | ) | (66,496 | ) | (2,720,244 | ) | (626,083 | ) | (20,113,829 | ) | ||||||||||
|
Other income (expense)
|
||||||||||||||||||||
|
Interest expense
|
- | (18,904 | ) | (24,658 | ) | (56,095 | ) | (1,730,892 | ) | |||||||||||
|
Interest income
|
4,221 | - | 9,805 | - | 137,386 | |||||||||||||||
|
Loss on extinguishment of debt
|
- | - | (1,195,410 | ) | - | (1,195,410 | ) | |||||||||||||
|
Gain on settlement
|
- | - | - | - | 375,000 | |||||||||||||||
|
Gain on forgiveness of liabilities
|
- | - | - | - | 176,505 | |||||||||||||||
|
Total other income (expense), net
|
4,221 | (18,904 | ) | (1,210,263 | ) | (56,095 | ) | (2,237,411 | ) | |||||||||||
|
Net loss
|
(1,245,826 | ) | (85,400 | ) | (3,930,507 | ) | (682,178 | ) | (22,351,240 | ) | ||||||||||
|
Deemed dividend to preferred stockholders
|
- | - | (200,000 | ) | - | (300,000 | ) | |||||||||||||
|
Net loss attributable to common stockholders
|
$ | (1,245,826 | ) | $ | (85,400 | ) | $ | (4,130,507 | ) | $ | (682,178 | ) | $ | (22,651,240 | ) | |||||
|
Net loss attributable to common stockholders per common share, basic and diluted
|
$ | (0.04 | ) | $ | (0.04 | ) | $ | (0.22 | ) | $ | (0.34 | ) | ||||||||
|
Weighted average common shares outstanding,
|
||||||||||||||||||||
|
basic and diluted
|
31,099,103 | 1,987,601 | 18,642,566 | 1,989,490 | ||||||||||||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
F-3
IMPRIMIS PHARMACEUTICALS, INC.
(A Development Stage Company)
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
|
For The
Nine Months Ended |
For The
Nine Months Ended |
For the Period From
July 24, 1998 (Inception) |
||||||||||
|
2012
|
2011
|
2012
|
||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||||||
|
Net loss
|
$ | (3,930,507 | ) | $ | (682,178 | ) | $ | (22,351,240 | ) | |||
|
Adjustments to reconcile net loss to net cash used in
|
||||||||||||
|
operating activities:
|
||||||||||||
|
Estimated fair value of contributed services
|
- | - | 2,475,000 | |||||||||
|
Gain on forgiveness of liabilities
|
- | - | (176,505 | ) | ||||||||
|
Amortization of prepaid consulting fees
|
- | - | 807,608 | |||||||||
|
Depreciation
|
2,090 | 338 | 5,244 | |||||||||
|
Loss on extinguishment of debt
|
1,195,410 | - | 1,195,410 | |||||||||
|
Non-cash interest on notes payable
|
24,658 | 56,095 | 1,730,892 | |||||||||
|
Stock-based compensation
|
1,502,080 | 149,162 | 3,630,896 | |||||||||
|
Payments made on behalf of Company by related party
|
- | - | 254,142 | |||||||||
|
Changes in assets and liabilities:
|
||||||||||||
|
Prepaid consulting costs
|
- | - | (140,000 | ) | ||||||||
|
Prepaid expenses and other current assets
|
(61,344 | ) | (13,876 | ) | (76,141 | ) | ||||||
|
Accounts payable and accrued expenses
|
61,371 | 104,779 | 369,897 | |||||||||
|
Accrued Phase 3 expenses
|
- | - | 111,871 | |||||||||
|
Accrued payroll
|
19,115 | 48,835 | 105,706 | |||||||||
|
Deferred revenue
|
(100,000 | ) | 20,000 | - | ||||||||
|
NET CASH USED IN OPERATING ACTIVITIES
|
(1,287,127 | ) | (316,845 | ) | (12,057,220 | ) | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
||||||||||||
|
Purchases of fixed assets
|
(15,308 | ) | - | (18,462 | ) | |||||||
|
NET CASH USED IN INVESTING ACTIVITIES
|
(15,308 | ) | - | (18,462 | ) | |||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
||||||||||||
|
Proceeds from issuance of notes payable to a related party
|
450,000 | - | 976,300 | |||||||||
|
Proceeds received in connection with debt modification
|
50,000 | - | 50,000 | |||||||||
|
Proceeds from issuance of preferred stock
|
- | - | 100,000 | |||||||||
|
Proceeds from notes payable
|
- | - | 2,500,000 | |||||||||
|
Preferred stock deemed dividend paid at conversion
|
(200,000 | ) | - | (200,000 | ) | |||||||
|
Cash advances from related party
|
- | 27,537 | 27,537 | |||||||||
|
Repayment of advances from related party
|
- | - | (281,679 | ) | ||||||||
|
Capital contributions
|
- | - | 168,707 | |||||||||
|
Net proceeds from purchase of common stock and
|
||||||||||||
|
exercise of warrants and stock options
|
- | - | 99,450 | |||||||||
|
Proceeds from issuance of common stock and warrants
|
||||||||||||
|
for cash, net of offering costs
|
11,915,931 | - | 19,695,023 | |||||||||
|
Deferred offering costs
|
(68,785 | ) | - | (68,785 | ) | |||||||
|
NET CASH PROVIDED BY FINANCING ACTIVITIES
|
12,147,146 | 27,537 | 23,066,553 | |||||||||
|
NET CHANGE IN CASH AND CASH EQUIVALENTS
|
10,844,711 | (289,308 | ) | 10,990,871 | ||||||||
|
CASH AND CASH EQUIVALENTS, beginning of period
|
146,160 | 291,462 | - | |||||||||
|
CASH AND CASH EQUIVALENTS, end of period
|
$ | 10,990,871 | $ | 2,154 | $ | 10,990,871 | ||||||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION:
|
||||||||||||
| Issuance of and adjustment to common stock and warrants to | ||||||||||||
|
consulting firms for prepaid consulting fees
|
$ | - | $ | - | $ | 432,007 | ||||||
|
Deferred offering costs in connection with equity
|
||||||||||||
|
offering recorded in accounts payable
|
$ | 314,961 | $ | - | $ | 314,961 | ||||||
|
Conversion of related party accounts payable into common stock
|
$ | 56,087 | $ | - | $ | 56,087 | ||||||
|
Conversion of notes payable and accrued interest into
|
||||||||||||
|
common stock
|
$ | 1,905,137 | $ | - | $ | 3,435,314 | ||||||
|
Forgiveness of notes payable and accrued interest to
|
||||||||||||
|
shareholders
|
$ | - | $ | - | $ | 241,701 | ||||||
|
Conversion of advances to notes payable to shareholders
|
$ | - | $ | - | $ | 196,300 | ||||||
|
Accretion of preferred stock discount
|
$ | - | $ | - | $ | 100,000 | ||||||
|
Related party acquisition of Phase 3 liabilities
|
$ | - | $ | - | $ | 56,087 | ||||||
|
Conversion of preferred stock into common stock
|
$ | 7,499 | $ | - | $ | 7,499 | ||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
F-4
IMPRIMIS PHARMACEUTICALS, INC.
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the three and nine months ended September 30, 2012 and 2011 and the period from July 24, 1998 (Inception) through September 30, 2012
NOTE 1. OVERVIEW AND BASIS OF PRESENTATION
Company and Background
Imprimis Pharmaceuticals, Inc. (“Imprimis”, the “Company”, “we”, “us”, or “our”) is a specialty pharmaceutical company developing non-invasive, topically delivered products. Our innovative patented Accudel cream formulation technology is designed to enable highly targeted site specific treatment. Impracor, our lead pain product candidate, utilizes the Accudel platform technology to deliver the active drug, ketoprofen, a non-steroidal anti-inflammatory drug, through the skin directly into the underlying tissues where the drug exerts its localized anti-inflammatory and analgesic effects.
Through our strategic relationship with Professional Compounding Centers of America, Inc. (“PCCA”) (see Note 4 and Note 6), one of the largest drug compounding organizations in the world, we expect to facilitate our future selection, formulation and development of potential product candidates. Our relationship with PCCA is exclusive and provides us with the opportunity to develop new products using PCCA’s proprietary drug formulations and drug delivery technologies, as well as access to an extensive database of market-oriented information related to a specific drug development opportunity. We plan to use our proprietary Accudel drug delivery technology, coupled with these licensed technologies, formulations and market data, to identify pharmaceutical development opportunities we perceive a significant unmet need for a new drug product. We are currently considering potential product candidates in the muscle relaxant and neuropathic pain fields, and we expect those areas may be our next avenues for additional product development.
Basis of Presentation
On February 28, 2012, the Company changed its name from Transdel Pharmaceuticals, Inc. to Imprimis Pharmaceuticals, Inc. All prior references to Transdel Pharmaceuticals, Inc. have been changed to Imprimis Pharmaceuticals, Inc. to reflect the change. On February 28, 2012, the Company effected a one-for-eight reverse stock split. All share and per share amounts and calculations in this report reflect the effects of that reverse stock split.
Imprimis has prepared the accompanying interim condensed unaudited consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (“GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 8 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by GAAP for complete financial statements. In the opinion of management, all adjustments (consisting of only normal recurring accruals) considered necessary for a fair presentation have been included. Operating results for the three and nine months ended September 30, 2012 are not necessarily indicative of the results that may be expected for the year ending December 31, 2012. For further information, refer to the Company’s audited consolidated financial statements and footnotes thereto included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2011.
Principles of Consolidation
On September 17, 2007, Imprimis entered into an Agreement of Merger and Plan of Reorganization (the “Merger Agreement”) by and among Imprimis, Transdel Pharmaceuticals Holdings, Inc., a privately held Nevada corporation (“Transdel Holdings”), and Trans-Pharma Acquisition Corp., a newly formed, wholly-owned Delaware subsidiary of Imprimis (“Acquisition Sub”). Upon closing of the merger transaction contemplated under the Merger Agreement (the “Merger”), Acquisition Sub merged with and into Transdel Holdings, and Transdel Holdings, as the surviving corporation, became a wholly-owned subsidiary of Imprimis. As a result of the Merger, the former owners of Transdel Holdings became the controlling stockholders of Imprimis. Accordingly, the merger of Transdel Holdings and Imprimis is a reverse merger that has been accounted for as a recapitalization of Transdel Holdings.
F-5
Effective on September 17, 2007, and for all reporting periods thereafter, Imprimis’ operating activities, including any prior comparative period, include only those of Transdel Holdings. All references to share and per share amounts in the accompanying consolidated financial statements and footnotes have been restated to reflect the aforementioned share exchange. All significant intercompany accounts and transactions have been eliminated in consolidation.
On June 20, 2011, Transdel Holdings was merged with Imprimis Pharmaceuticals, Inc., at which time Transdel Holdings ceased as a corporation, and Imprimis Pharmaceuticals, Inc. remains as the sole surviving corporation.
Development Stage Enterprise
The Company is a development stage company as defined under Financial Accounting Standards Board (“FASB”) guidance. The Company is devoting substantially all of its present efforts to establish a new business, and its planned principal operations have not yet commenced. All losses accumulated since inception have been considered as part of the Company’s development stage activities.
These condensed consolidated financial statements contemplate the realization of assets and the satisfaction of liabilities in the normal course of business. The Company is a development state enterprise and has incurred recurring operating losses, has had negative operating cash flows and has not recognized any significant revenues since July 24, 1998 (inception). In addition, the Company has a deficit accumulated during the development stage of approximately $22.7 million at September 30, 2012, and anticipates incurring further losses through the year 2012 and beyond. The Company has not yet generated commercial sales revenue and has funded its operating losses to date through debt and equity offerings and borrowings under its line of credit. The Company believes that its existing cash and cash equivalents will be sufficient to cover its cash flow requirements through the next twelve months.
Research and Development
The Company expenses all costs related to research and development as they are incurred.
Revenue Recognition and Deferred Revenue
The Company will recognize revenues in accordance with FASB guidance, which requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred; (3) the selling price is fixed and determinable; and (4) collectibility is reasonably assured. Determination of criteria (3) and (4) will be based on management’s judgments regarding the fixed nature of the selling prices of the products delivered and the collectibility of those amounts. Provisions for discounts and rebates to customers, estimated returns and allowances, and other adjustments will be provided for in the same period the related sales are recorded. The Company will defer any revenue for which the product has not been delivered or for which services have not been rendered or are subject to refund until such time that the Company and the customer jointly determine that the product has been delivered or services have been rendered or no refund will be required.
For the nine months ended September 30, 2012, the Company recorded $100,000 in revenues for non-refundable royalty advances, which were previously deferred. The Company does not anticipate that it will generate any significant revenues until one or more of its drug candidates are approved by the U.S. Food and Drug Administration (“FDA”) and the Company is able to commercialize one or more of its product candidates. Also, effective sales and marketing support must be in place for either the drug candidates or any other products the Company may develop in order to generate any revenues. The FDA approval process is highly uncertain and the Company cannot estimate when it will generate revenues at this time from sales of its products.
F-6
Income Taxes
Income tax expense is provided for the tax effects of transactions reported in the financial statements and consists of taxes currently due, plus deferred taxes. Deferred taxes are recognized for differences between the basis of assets and liabilities for financial statement and income tax purposes. The differences relate primarily to the effects of net operating loss carry forwards and differing basis, depreciation methods, and lives of depreciable assets. The deferred tax assets represent the future tax return consequences of those differences, which will be deductible when the assets are recovered.
No income tax benefit (expense) was recognized for the nine months ended September 30, 2012 as a result of tax losses in this period and because deferred tax benefits, derived from the Company’s prior net operating losses, were previously fully reserved. At September 30, 2012, the Company had federal and California net operating loss carryforwards of approximately $14.1 million and $13.6 million, respectively. The use of our net operating losses may be restricted in future years due to the limitations pursuant to IRC Section 382 on changes in ownership.
The Company is subject to taxation in the United States and California. The Company’s tax years for 2000 and forward are subject to examination by the United States and state tax authorities due to the carry forward of unutilized net operating losses.
Cash and Cash Equivalents
Cash equivalents include short-term, highly liquid investments with maturities of three months or less at the time of acquisition.
Concentrations of Credit Risk
The Company places its cash with financial institutions deemed by management to be of high credit quality. The Federal Deposit Insurance Corporation (“FDIC”) provides basic deposit coverage with limits to $250,000 per owner. In addition to the basic insurance deposit coverage, the FDIC is providing temporary unlimited coverage for noninterest-bearing transaction accounts from December 31, 2010 to December 31, 2012. At September 30, 2012, the Company had approximately $10.1 million in cash deposits in excess of FDIC limits.
Furniture and Equipment
Furniture and equipment is stated at cost less accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of three to five years.
Furniture and equipment, net, as of September 30, 2012 and December 31, 2011 consisted of the following:
|
September 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Furniture and Equipment, net:
|
||||||||
|
Computer Software and Hardware
|
$ | 6,341 | $ | - | ||||
|
Furniture and Equipment
|
8,967 | - | ||||||
| 15,308 | - | |||||||
|
Accumulated Depreciation
|
(2,090 | ) | - | |||||
| $ | 13,218 | $ | - | |||||
F-7
During the three and nine months ended September 30, 2012, the Company recorded depreciation expenses of $843 and $2,090, respectively, and during the three and nine months ended September 30, 2011, the Company recorded depreciation expenses of $0 and $338, respectively.
Deferred Offering Costs
On July 25, 2012, the Company filed with the Securities and Exchange Commission a registration statement in connection with a proposed offering of its common stock. At September 30, 2012, the Company had deferred offering costs of $383,746 for legal, accounting and other expenses directly related to the proposed offering. Any cash proceeds the Company may receive from the proposed offering will be netted against these deferred offering costs and any future costs directly associated with the offering. There is no obligation to consummate an offering and an offering may not occur.
Deferred Rent
The Company accounts for rent expense related to its operating leases by determining total minimum rent payments on the leases over their respective periods and recognizing the rent expense on a straight-line basis. The difference between the actual amount paid and the amount recorded as rent expense in each fiscal year is recorded as an adjustment to deferred rent. The deferred rent balance at September 30, 2012 was $1,734 and is included in accounts payable and accrued expenses in the accompanying unaudited condensed consolidated balance sheet.
Fair Value Measurements
Fair value measurements are determined based on the assumptions that market participants would use in pricing an asset or liability. GAAP establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. The established fair value hierarchy prioritizes the use of inputs used in valuation methodologies into the following three levels:
|
●
|
Level 1: Quoted prices (unadjusted) for identical assets or liabilities in active markets. A quoted price in an active market provides the most reliable evidence of fair value and must be used to measure fair value whenever available.
|
|
●
|
Level 2: Significant other observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data.
|
|
●
|
Level 3: Significant unobservable inputs that reflect a reporting entity’s own assumptions about the assumptions that market participants would use in pricing an asset or liability. For example, level 3 inputs would relate to forecasts of future earnings and cash flows used in a discounted future cash flows method.
|
The fair values of the Company’s cash and cash equivalents, accounts payable, and accrued expenses approximate carrying values due to their short term maturities.
Beneficial Conversion Features and Debt Discounts
The convertible features of debt provide for a rate of conversion that is below market value. Such feature is normally characterized as a “beneficial conversion feature” (“BCF”). The relative fair values of the BCF were recorded as discounts from the face amount of the respective debt instrument. The Company amortized the discount using the effective interest method through maturity of such instruments.
Stock-Based Compensation
All share-based payments to employees, including grants of stock options to employees, directors and consultants and restricted stock grants, are recognized in the financial statements based upon their fair values.
The Company's accounting policy for equity instruments issued to consultants and vendors in exchange for goods and services follows FASB guidance. As such, the value of the applicable stock-based compensation is periodically remeasured and income or expense is recognized during their vesting terms. The measurement date for the fair value of the equity instruments issued is determined at the earlier of (i) the date at which a commitment for performance by the consultant or vendor is reached or (ii) the date at which the consultant or vendor's performance is complete. In the case of equity instruments issued to consultants, the fair value of the equity instrument is primarily recognized over the term of the consulting agreement. In accordance with FASB guidance, an asset acquired in exchange for the issuance of fully vested, nonforfeitable equity instruments should not be presented or classified as an offset to equity on the grantor's balance sheet once the equity instrument is granted for accounting purposes. Accordingly, the Company records the fair value of nonforfeitable equity instruments issued for future consulting services as prepaid consulting fees in its consolidated balance sheets.
The Company recorded stock-based compensation related to equity instruments granted to employees, directors and consultants as follows:
|
For The Three
|
For The Three
|
For The Nine
|
For The Nine
|
|||||||||||||
|
Months
Ended
|
Months
Ended
|
Months Ended
|
Months Ended
|
|||||||||||||
|
September 30, 2012
|
September 30, 2011
|
September 30, 2012
|
September 30, 2011
|
|||||||||||||
|
Employees - selling, general and administrative
|
$ | 180,630 | $ | 32,757 | $ | 298,854 | $ | 104,675 | ||||||||
|
Employees - research and development
|
61,299 | - | 143,711 | 37,754 | ||||||||||||
|
Directors - selling, general and administrative
|
331,264 | 4,041 | 898,679 | 6,733 | ||||||||||||
|
Consultants - selling, general and administrative
|
80,849 | - | 160,836 | - | ||||||||||||
|
Total
|
$ | 654,042 | $ | 36,798 | $ | 1,502,080 | $ | 149,162 | ||||||||
Basic and Diluted Loss per Common Share
Basic net loss per common share is computed by dividing net loss attributable to common stockholders for the period by the weighted average number of common shares outstanding during the period. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders for the period by the weighted average number of common and common equivalent shares, such as stock options and warrants outstanding during the period.
Basic and diluted net loss applicable to common stock per share is computed using the weighted average number of common shares outstanding during the period. Common stock equivalents (prior to application of the treasury stock or, “if converted” method) from convertible notes, preferred stock, stock options, unvested restricted stock units (“RSUs”) and warrants were 8,345,388 and 395,846 at September 30, 2012 and 2011, respectively, and are excluded from the calculation of diluted net loss per share for all periods presented because the effect is anti-dilutive.
The following table shows the computation of basic and diluted earnings per common share for the three- and nine-month periods ended September 30, 2012 and September 30, 2011:
|
For the three months ended
|
For the three months ended
|
For the nine months ended
|
For the nine months ended
|
|||||||||||||
|
September 30, 2012
|
September 30, 2011
|
September 30, 2012
|
September 30, 2011
|
|||||||||||||
|
Net loss
|
$ | (1,245,826 | ) | $ | (85,400 | ) | $ | (3,930,507 | ) | $ | (682,178 | ) | ||||
|
Deemed dividend to preferred stockholders
|
- | - | (200,000 | ) | - | |||||||||||
|
Numerator – loss attributable to common stockholders
|
$ | (1,245,826 | ) | $ | (85,400 | ) | $ | (4,130,507 | ) | $ | (682,178 | ) | ||||
|
Denominator – weighted average
|
||||||||||||||||
|
number of shares outstanding, basic and diluted
|
31,099,103 | 1,987,601 | 18,642,566 | 1,989,490 | ||||||||||||
|
Loss per share, basic and diluted
|
$ | (0.04 | ) | $ | (0.04 | ) | $ | (0.22 | ) | $ | (0.34 | ) | ||||
F-8
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and judgments that affect the reported amounts of assets, liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting periods. Significant estimates made by management are, among others, the valuation of contributed services, deferred taxes and stock-based compensation issued to employees and non-employees. Actual results could differ from those estimates.
Unaudited Pro Forma Stockholders’ Equity
The Company has filed a registration statement on Form S-1 with the U.S. Securities and Exchange Commission in connection with a proposed public offering of its securities. If the offering contemplated by the registration statement is consummated and the Company raises sufficient equity to meet the listing requirements of The NASDAQ Capital Market, the Company will effect a 1-for-5 reverse stock split of its common stock after the effectiveness of the registration statement and prior to the closing of the offering. The unaudited pro forma consolidated balance sheet as of September 30, 2012 gives effect to the assumed 1-for-5 reverse stock split.
Since the 1-for-5 reverse stock split is to be effected after the effectiveness of the registration statement, the historical share information included in the accompanying consolidated financial statements and notes hereto does not assume the 1-for-5 reverse stock split, and accordingly has not been adjusted therefor.
NOTE 2. BANKRUPTCY PETITION AND ASSET PURCHASE AGREEMENT
On June 26, 2011, the Company filed a voluntary petition for reorganization relief under Chapter 11 of the United States Bankruptcy Code in the United States Bankruptcy Court for the Southern District of California (the “Bankruptcy Court”), Case No. 11-10497-11 (the “Chapter 11 Case”). In connection with the Chapter 11 Case, the Company, as seller, and Cardium Healthcare, Inc., a wholly-owned subsidiary of Cardium Therapeutics, Inc., as purchaser (“Cardium”), entered into an Asset Purchase Agreement dated June 23, 2011 (the “Asset Purchase Agreement”) pursuant to which the Company agreed to sell substantially all of its assets pursuant to Sections 105, 363 and 365 of the Bankruptcy Code, subject to court approval and the satisfaction of certain conditions set forth in the Asset Purchase Agreement.
Consummation of the sale to Cardium was subject to a number of conditions, including, among others, the approval by the Bankruptcy Court of the transactions contemplated by the Asset Purchase Agreement and compliance with certain specified deadlines for actions in connection with the Bankruptcy Case. The Asset Purchase Agreement was terminable by the parties under a number of circumstances, including failure to obtain certain Bankruptcy Court orders by agreed dates.
On July 26, 2011, the Bankruptcy Court denied the Company’s motion to sell its assets pursuant to the Asset Purchase Agreement. On October 7, 2011, the Company terminated the Asset Purchase Agreement pursuant to its terms. On November 21, 2011, in connection with certain transactions with DermaStar International, LLC (“DermaStar”) described in Notes 4 and 5 below, the Company requested that the Bankruptcy Court dismiss the Chapter 11 Case and retain jurisdiction to decide matters related to claims brought in the Bankruptcy Case by the Purchaser. On December 8, 2011, the Bankruptcy Court entered an order dismissing the Chapter 11 Case. In connection with the dismissal of the Chapter 11 Case, the Bankruptcy Court, among other things, declined to retain jurisdiction over claim objection proceedings and found moot the Company’s objection to certain claims to receive a break-up fee pursuant to the Asset Purchase Agreement of Cardium Therapeutics, Inc. and Cardium Healthcare, Inc., a wholly owned subsidiary of Cardium. The dismissal of the Chapter 11 Case was based upon the provisions of both 11 U.S.C. Sections 305 (a) and 1112(b).
F-9
NOTE 3. NOTES PAYABLE – RELATED PARTY
DermaStar is a former control person of the Company and had the ability to direct or cause direction of management and policies of the Company through its ownership of the Company’s capital stock. Also, Dr. Robert Kammer, a director and the Chairman of the Board of the Company, and Mark L. Baum, Chief Executive Officer and a director of the Company, were managing members and partial owners of DermaStar. In July 2012, the Company was informed by DermaStar that it had dissolved and distributed all of its shares of the Company’s capital stock held by it to its members. As a result of that dissolution and distribution, DermaStar is no longer a control person of the Company.
Convertible Note – April 2010
On April 5, 2010, the Company issued a Senior Convertible Promissory Note (the “Note”) to Alexej Ladonnikov in a private placement. The Note included an annual interest rate of 7.5% and (unless converted or prepaid, as noted below) all principal and interest was due and payable on its maturity date of April 5, 2012 (“Maturity Date”). At any time prior to the Maturity Date, the investor had the right to convert all or a portion of the outstanding principal and accrued interest at a conversion ratio of one share of the Company’s common stock for each $8 (the fair market value of the Company’s common stock on April 5, 2010) owed. Also, at any time prior to the Maturity Date, the Company had the option to prepay the outstanding principal and accrued interest. The Company received gross proceeds from the issuance of the Note in the aggregate amount of $1,000,000. There were no discounts or commissions paid in connection with this private placement. Accrued interest on the Note was $0 and $130,479 at September 30, 2012 and December 31, 2011, respectively, and interest expense on the Note for the three and nine months ended September 30, 2012 was $0 and $12,123, respectively, and for the three and nine months ended September 30, 2011 was $18,904 and $56,095, respectively. Following the Company’s bankruptcy petition filed June 26, 2011, as well as the change in ownership control following the issuance of Series A Convertible Preferred Stock to DermaStar, the entire unpaid principal sum of this Note, together with its accrued and unpaid interest became immediately due and payable.
In January 2012, DermaStar acquired 80% of the Note in a private transaction with Mr. Ladonnikov. On January 25, 2012, the Board of Directors of the Company approved, and the Company entered into, separate waiver and settlement agreements with DermaStar and Mr. Ladonnikov, the two parties holding the Note.
In connection with each of the waiver and settlement agreements, the holders of the Note each agreed to forever waive their rights to (i) accelerate the entire unpaid principal sum of the Note and all accrued interest pursuant to Section 1 of the Note related to the Company’s bankruptcy petition filed June 26, 2011, (ii) Section 7 of the Senior Convertible Note Purchase Agreement dated April 5, 2010, regarding the designation and creation of the Series A Convertible Preferred Stock (“Series A Preferred Stock”) and (iii) certain conversion rights pursuant to Section 3 of the Note related to the change of control that resulted from the sale of the Series A Preferred Stock.
Pursuant to the terms of the waiver and settlement agreement by and between the Company and DermaStar, DermaStar and the Company agreed to the mandatory conversion of the eighty percent (80%) of the principal and accrued and unpaid interest of the Note held by DermaStar, at such time as (and not until) the Company has a sufficient number of authorized common shares to effect such a conversion, into common stock of the Company at a conversion price of approximately $0.13336 (“DermaStar Conversion Price”). Additionally, DermaStar agreed to a mandatory conversion of an additional $56,087 current accounts payable of the Company (“AP Conversion”) held by DermaStar, at such time as (and not until) the Company had a sufficient number of authorized common shares for such conversion. The AP Conversion was made at the DermaStar Conversion Price.
On February 28, 2012, the Company issued 7,274,812 common shares to DermaStar as payment in full for its 80% ownership of the Note ($800,000), its related accrued interest ($114,082) and $56,087 in the Company’s accounts payable. The Company has determined this to be a substantial modification to the debt instruments and has applied debt extinguishment accounting to record a loss on extinguishment of debt of $856,087 for the nine months ended September 30, 2012.
Pursuant to the terms of the waiver and settlement agreement by and between the Company and Mr. Ladonnikov, Mr. Ladonnikov and the Company agreed to the mandatory conversion of the twenty percent (20%) of the principal and accrued and unpaid interest of the Note held by Mr. Ladonnikov, at such time as (and not until) the Company had a sufficient number of authorized common shares to effect such a conversion, into common stock of the Company at a conversion price of $0.12. Additionally, Mr. Ladonnikov agreed to make a one-time payment to the Company, at such time as the Note is converted into Company common stock, of $50,000.
On February 28, 2012, the Company received payment from Mr. Ladonnikov of $50,000 and issued 1,904,338 common shares to Mr. Ladonnikov as payment in full for his 20% ownership of the Note ($200,000) and its related accrued interest ($28,521). The Company has determined this to be a substantial modification to the debt instrument and has applied debt extinguishment accounting to record a loss on extinguishment of debt of $150,000 ($200,000 Note principal balance less $50,000 cash payment received) for the nine months ended September 30, 2012.
F-10
Secured Line of Credit
On November 21, 2011, the Company entered into a Secured Line of Credit Letter Agreement (the “Line of Credit Agreement”) with DermaStar. The Line of Credit Agreement became effective on December 10, 2011, in connection with the dismissal of the Chapter 11 Case by the Bankruptcy Court. The line of credit was secured by a blanket security interest in all of the Company’s assets, including its intellectual property. The Line of Credit Agreement provided for advances to the Company of up to an aggregate of $750,000 (each an “Advance” and collectively the “Loan”), subject to the satisfaction by the Company of certain conditions in connection with the initial Advance and each subsequent Advance. Each Advance was made pursuant to a promissory note in favor of DermaStar. The Company had received advances totaling $750,000 and $300,000 up to April 25, 2012 (the date of the conversion thereof) and December 31, 2011, respectively. The promissory notes accrued interest at 10% annually and had a maturity of one year after the effective dates of the applicable Advance. There was no accrued interest on the promissory notes at September 30, 2012 and interest expense for the three and nine months ended September 30, 2012 was $0 and $12,534, respectively.
As of April 20, 2012, the aggregate principal balance owing under the Line of Credit was $750,000. Effective April 20, 2012, the Company and DermaStar entered into a Promissory Note Conversion Agreement (the “Conversion Agreement”) wherein the parties agreed that the entire outstanding principal balance of the promissory notes issued in favor of DermaStar pursuant to the Line of Credit Agreement and all related accrued interest, totaling $762,534, would be converted into shares of the Company’s common stock and warrants to purchase the Company’s common stock on the same terms as the issuance of such securities in the April Private Placement (as described in Note 4). Pursuant to the Conversion Agreement, on April 25, 2012 and upon conversion of the outstanding principal balance and unpaid interest under the Line of Credit Agreement, DermaStar was issued a total of 965,233 shares of the Company’s common stock and a related warrant to purchase up to an additional 241,308 shares of the Company’s common stock. The warrant has an exercise price of $1.185 per share and a three year term. The Line of Credit Agreement has been terminated.
The addition of a conversion feature to the Line of Credit Agreement resulted in terms that were substantially different from the terms of the original agreement, and therefore, the conversion resulted in an extinguishment of debt. The relative fair value of the warrant issued to DermaStar was determined to be $137,383 using the Black-Scholes-Merton valuation model. The variables used in this pricing model included: (1) discount rate of 0.4% (2) expected warrant life of 3 years, (3) expected volatility of 350% and (4) zero expected dividends. In addition, the value of the effective BCF resulting from the Conversion Agreement was determined to be $51,940. The value of the debt discount was recorded as additional paid-in capital and as the Line of Credit is immediately convertible, the debt discount of $189,323 was immediately expensed as a loss on extinguishment of debt.
Notes payable consist of the following:
|
September 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
10% convertible notes
|
$ | - | $ | 300,000 | ||||
|
7.5% convertible note
|
- | 1,000,000 | ||||||
|
Total convertible notes payable
|
$ | - | $ | 1,300,000 | ||||
|
Less: Current portion
|
- | (1,300,000 | ) | |||||
|
Long-term portion
|
$ | - | $ | - | ||||
NOTE 4. STOCKHOLDERS’ EQUITY AND STOCK-BASED COMPENSATION
Common Stock
On February 28, 2012, the Company increased the number of authorized shares of capital stock to 400,000,000, and the number of authorized shares of common stock to 395,000,000 and effected a one-for-eight reverse stock split. All share and per share amounts and calculations in this report reflect the one-for-eight reverse stock split.
On February 28, 2012, the Company issued 1,904,338 common shares to Alexej Ladonnikov as payment in full for his 20% ownership of the Note ($200,000) and its related accrued interest ($28,521).
On February 28, 2012, the Company issued 7,274,812 common shares to DermaStar as payment in full for its 80% ownership of the Note ($800,000), its related accrued interest ($114,082) and $56,087 in the Company’s accounts payable.
On April 20, 2012, the Company entered into a Securities Purchase Agreement with certain accredited investors relating to the sale and issuance of an aggregate of 10,058,455 shares of its common stock and warrants to purchase up to 2,514,642 shares of its common stock at an exercise price of $1.185 per share, for an aggregate purchase price of approximately $7,950,000 (the “April Private Placement”). The April Private Placement closed on April 25, 2012, and the Company received proceeds, net of offering costs, of approximately $7,930,000.
On April 25, 2012, the Company converted debt totaling $762,534 (including accrued interest of $12,534) owed to DermaStar, a related party, into 965,233 shares of the Company’s common stock and a related warrant to purchase 241,308 shares of the Company’s common stock at an exercise price of $1.185 per share on the same terms as the issuance of such securities in the April Private Placement (see Note 3).
On August 30, 2012, the Company entered into a License Agreement (the “PCCA License Agreement") and a Stock Purchase Agreement (the “PCCA Purchase Agreement”) in a strategic transaction with PCCA (the “PCCA Transaction”). Pursuant to the terms of the PCCA Purchase Agreement, on August 31, 2012, the Company issued and sold to PCCA 4,163,414 shares of its common stock at a per share purchase price of $0.96075, for aggregate proceeds, net of offering costs, of approximately $3,980,000.
F-11
Preferred Stock
At September 30, 2012, the Company had 5,000,000 shares of preferred stock, $0.001 par value, authorized and no shares of preferred stock issued and outstanding.
Series A Preferred Stock - Converted
On December 12, 2011, the Company issued 10 shares of Series A Preferred Stock to DermaStar, a related party, in a private placement. The Series A Preferred Stock has the rights and preferences identified in the Certificate of Designation to our Amended and Restated Certificate of Incorporation filed with the Delaware Secretary of State on December 9, 2011. Among other things, the Certificate of Designation (i) authorizes 10 shares of the Company’s preferred stock to be designated as “Series A Convertible Preferred Stock”; (ii) grants the holders of the Series A Preferred Stock the right to convert into our common stock at a conversion price of approximately $0.013336, as adjusted; (iii) grants a liquidation preference of $10,000 per share of Series A Preferred Stock; (iv) provides that the holders of Series A Preferred Stock shall vote with the holders of our common stock on an “as converted basis”; and (v) provides that the affirmative vote of a majority of the outstanding shares of the Series A Preferred Stock is required to approve certain corporate matters including, among other things, changes to the rights of the holders of the Series A Preferred Stock, amendments to our Amended and Restated Certificate of Incorporation or Bylaws, issuance of priority or parity securities, issuance of debt securities, entry into certain fundamental transactions and increase or decrease in the size of our Board of Directors.
On June 29, 2012, DermaStar converted the 10 shares of Series A Preferred Stock held by it into 7,498,500 shares of the Company’s common stock. In connection with the conversion, the Company paid to DermaStar $200,000 as partial consideration for the conversion pursuant to a conversion agreement. Immediately following the conversion of the Series A Preferred Stock, all 10 shares were retired to our treasury and cancelled. The Company recognized the $200,000 payment as additional consideration transferred in the transaction in excess of the fair value of the consideration issuable in accordance with the original conversion terms. As a result, the cash payment to DermaStar was recorded as a deemed preferred stock dividend. Accordingly, the Company recorded a deemed preferred stock dividend at the date of conversion, June 29, 2012, totaling $200,000, which represents an increase to reported net loss in arriving at net loss attributable to common stockholders.
Restricted Stock Units (“RSUs”)
RSU awards are granted subject to certain restrictions, including performance based conditions. The grant-date fair value of the RSUs, which has been determined based upon the market value of the Company’s shares on the grant date, is expensed over the vesting period.
On July 18, 2012, the Company granted to Mr. Baum, in connection with his services as the Chief Executive Officer, 800,000 RSUs pursuant to a Stand-alone Restricted Stock Unit Agreement (the “Baum RSUs”) outside of the Company’s 2007 Incentive Stock and Awards Plan, as amended on November 5, 2008, January 25, 2012 and July 18, 2012 (the “Plan”). The Baum RSUs are subject to certain performance-based vesting criteria, such that 200,000 RSUs will vest upon the satisfaction of each of the following events: (i) successful completion of a financing that results in aggregate cash proceeds to the Company of at least $5,000,000 at any time following the effective date of the grant; (ii) the Company meets the primary endpoints of its Phase 3 clinical studies for its drug candidate, Impracor; (iii) the Company submits a New Drug Application for Impracor to the U.S. Food and Drug Administration; and (iv) the Company enters into a definitive license, collaboration or similar agreement for Impracor that would reasonably be expected to generate cash flow for the Company. The Baum RSUs vest in full upon a change in control of the Company. The Company has accounted for the Baum RSUs based on an assumption of the full satisfaction of its performance-based vesting criteria.
On July 18, 2012, the Company granted to Dr. Kammer, in connection with his services as a consultant and advisor to the Company, 200,000 RSUs, pursuant to a Stand-alone Restricted Stock Unit Agreement (the “Kammer RSUs”) outside of the Plan. The Kammer RSUs are subject to certain performance-based vesting criteria, such that all 200,000 RSUs will vest when the Company meets the primary endpoints of its Phase 3 clinical studies for its drug candidate, Impracor. The Kammer RSUs vest in full upon a change in control of the Company. The Company has accounted for the Kammer RSUs based on an assumption of the full satisfaction of its performance-based vesting criteria. In accordance with accounting guidance for share-based compensation to consultants, the unvested portion of the Kammer RSUs will be revalued on an interim basis until the performance-based vesting criteria is met. Once the performance-based vesting criteria is met, the fair value and total expense amount of the Kammer RSUs will be calculated based on the market value of the Company’s common stock on that day. On the date of issuance, July 18, 2012, the Kammer RSUs were valued at $130,000, and as of September 30, 2012, the revalued estimated fair value of the Kammer RSUs was $380,000.
A summary of the Company’s RSU activity and related information for the nine months ended September 30, 2012 is as follows:
|
Number of Shares
|
Weighted Average Grant Date Fair Value
|
|||||||
|
Unvested – January 1, 2012
|
- | $ | - | |||||
|
RSUs granted
|
1,000,000 | 0.65 | ||||||
|
RSUs vested
|
- | - | ||||||
|
RSUs cancelled
|
- | - | ||||||
|
Unvested - September 30, 2012
|
1,000,000 | $ | 0.65 | |||||
The grant-date fair value of RSUs granted during the nine month period ended September 30, 2012 was approximately $650,000. As of September 30, 2012, the total unrecognized compensation expense related to unvested RSUs was approximately $685,000 (including recognized and unrecognized expenses of the revalued fair value of the Kammer RSUs) which is expected to be recognized over a weighted-average period of 1 year, based on estimated vesting schedules.
F-12
Stock Option Plan
On September 17, 2007, the Company’s Board of Directors and stockholders adopted the Plan, which, as of September 30, 2012, provided for the issuance of a maximum of an aggregate of 12,000,000 shares of the Company’s common stock. The purpose of the Plan is to provide an incentive to attract and retain directors, officers, consultants, advisors and employees whose services are considered valuable, to encourage a sense of proprietorship and to stimulate an active interest of such persons in the Company’s development and financial success. Under the Plan, the Company is authorized to issue incentive stock options intended to qualify under Section 422 of the Internal Revenue Code, non-qualified stock options and restricted stock. The Plan will be administered by the Company’s Board of Directors until such time as such authority has been delegated to a committee of the Board of Directors. On January 25, 2012, our stockholders approved an amendment to the Plan to increase the number of shares available for issuance under the Plan from 375,000 to 3,750,000 and to modify the definition of “fair market value” under the Plan, among other things. The approval became effective on February 26, 2012. Effective as of July 18, 2012, our board of directors and stockholders holding a majority of the Company’s outstanding voting power approved a further amendment to the Plan to increase the number of shares available for issuance under the Plan from 3,750,000 to 12,000,000 and to increase the per person limit on the maximum number of shares of the Company’s common stock that may be granted to an individual under the Plan in a calendar year.
A summary of the Plan activity for the nine months ended September 30, 2012 is as follows:
|
Number of shares
|
Weighted Avg. Exercise Price
|
Weighted Avg. Remaining Contractual Life
|
Aggregate Intrinsic Value
|
|||||||||||||
|
Options outstanding - December 31, 2011
|
150,152 | $ | 9.68 | |||||||||||||
|
Options granted
|
5,060,616 | 0.73 | ||||||||||||||
|
Options exercised
|
- | |||||||||||||||
|
Options cancelled
|
(718,750 | ) | 0.67 | |||||||||||||
|
Options outstanding - September 30, 2012
|
4,492,018 | $ | 1.04 | 5.01 | $ | 5,072,416 | ||||||||||
|
Options exercisable - September 30, 2012
|
1,847,652 | $ | 1.45 | 5.59 | $ | 2,038,108 | ||||||||||
|
Options vested and expected to vest - September 30, 2012
|
4,227,582 | $ | 1.06 | 5.04 | $ | 4,768,985 | ||||||||||
The aggregate intrinsic value in the table above represents the total pre-tax amount of the proceeds, net of exercise price, which would have been received by option holders if all option holders had exercised and immediately sold all options with an exercise price lower than the market price on September 28, 2012, based on the closing price of the Company’s common stock of $1.90 on that date.
On January 25, 2012, the Board approved the grant to Dr. Balbir Brar, the Company’s President, of an option to purchase 1,125,000 shares of common stock under the Plan. The exercise price of the option is $0.736 and the option vests as follows: 1/36th of the unvested shares will vest on each of the 36 monthly periods following the date of the grant provided Dr. Brar continues to be employed by the Company as of the applicable vesting date.
F-13
On January 25, 2012, in connection with a senior advisory agreement, the Board approved the grant to Dr. Paul Finnegan, a director, an option to purchase up to 625,000 shares of common stock under the Plan. The exercise price of the option is $0.64. The option was originally scheduled to vest as follows: 250,000 shares on January 6, 2013, 250,000 shares on January 6, 2014 and 125,000 on January 6, 2015. On May 9, 2012, the Company entered into a termination agreement to terminate its senior advisory agreement with Dr. Finnegan, and, in connection therewith, entered into an amendment to Dr. Finnegan’s option agreement. The amendment to the option agreement modifies the vesting schedule of the option to provide that 40% of the shares covered by the grant will vest on September 30, 2012, 40% will vest on March 31, 2013 and 20% will vest on September 30, 2013, provided that Dr. Finnegan is serving as a director, employee or consultant at the time of such vesting. In connection with the termination of the senior advisory agreement, the option agreement was also modified to provide for the issuance of the option as compensation for Dr. Finnegan’s services as a director rather than a consultant. This option is accounted for as an employee stock option agreement, the final valuation of the option was determined at the date of modification and the remaining expense of the option agreement will be recognized ratably over the remaining vesting periods in accordance with the modified terms.
On January 25, 2012, the Board approved a one-time stock option grant to Mr. Baum, the Company’s current Chief Executive Officer and a director, to purchase up to 625,000 shares of the Company’s common stock under the Plan. The option was issued to Mr. Baum for his uncompensated services as Chairman of the Board and significant ongoing services related, but not limited to, the Company’s emergence from Chapter 11 bankruptcy protection, negotiation with creditors, pursuit of additional financing opportunities and hiring of executive officers. The option vests in twelve equal monthly periods, commencing on January 25, 2012 and ending on January 25, 2013 and has an exercise price of $0.48.
On January 25, 2012 the Board approved the grant to Andrew R. Boll, the Company’s Vice-President of Accounting and Public Reporting, of an option to purchase up to 75,000 shares of common stock under the Plan, which option was granted on February 1, 2012, the commencement date of Mr. Boll’s employment with the Company. The exercise price of the option is $0.736 and the option vests as follows: 1/36th of the unvested shares will vest on each of the 36 monthly periods following the date of the grant provided Mr. Boll continues to be employed by the Company as of the applicable vesting date.
On January 25, 2012 the Board approved the grant to Dr. Joachim Schupp, the Company’s Chief Medical Officer, of an option to purchase up to 375,000 shares of common stock under the Plan, which option was granted on February 15, 2012, the commencement date of Dr. Schupp’s employment with the Company. The exercise price of the option is $0.72 and the option vests as follows: 1/36th of the unvested shares will vest on each of the 36 monthly periods following the date of the grant provided Dr. Schupp continues to be employed by the Company as of the applicable vesting date.
On April 1, 2012, the Board of Directors approved the issuance of options to purchase 125,000 shares of the Company’s common stock to each of the Company’s directors, including the Company’s employee and non-employee directors, under the Plan. Each of the options has an exercise price of $0.90 per share. The options have a term of five years and vest quarterly over a one year period, such that the option to purchase 31,250 shares vests on each of June 30, 2012, September 30, 2012, December 31, 2012 and March 31, 2013.
On April 1, 2012, in recognition and consideration for his services as a director to the Company during 2010 and 2011, the Board approved the issuance to Dr. Jeff Abrams of an additional option to purchase 300,000 shares of the Company’s common stock with an exercise price of $0.90 per share under the Plan. The option has a ten year term and vests monthly over a one year period.
On April 1, 2012, the Company granted to Mr. Baum an option to purchase up to 300,000 shares of the Company’s common stock at an exercise price of $0.90 per share under the Plan. The option terminates on March 31, 2017 and vests over a two year period, with 75,000 options vesting immediately upon issuance and an additional 9,375 options vesting monthly for the next twenty four months thereafter.
Effective as of the close of business on July 25, 2012, Dr. Brar submitted his resignation as a director on the Board of Directors of the Company. Dr. Brar continues in his capacity as the President of the Company. At the time of his resignation as a director, options to purchase 31,250 shares had vested under Dr. Brar’s April 1, 2012 option grant related to his Board service, and all unvested shares subject to the option were forfeited. Dr. Brar was granted an extension of 240 days from his resignation date to exercise the 31,250 vested shares.
On July 26, 2012, the Board of Directors of the Company appointed Stephen G. Austin, CPA, as a new director on the Board of Directors of the Company. In connection with his appointment as a director, the Board approved the issuance to Mr. Austin of an option to purchase up to 85,616 shares of the Company’s common stock under the Plan. Such option has an exercise price of $0.90 per share, has a term of five years, and vests monthly over a period of one year commencing on January 1, 2013.
F-14
The table below illustrates the fair value per share determined by the Black-Scholes-Merton option pricing model with the following assumptions used for the grants issued to employees and directors during the nine months ended September 30, 2012:
|
Nine Months Ended September 30, 2012
|
||||
|
Weighted-average fair value of options granted
|
$ | 0.65 | ||
|
Expected terms (in years)
|
2.5-5.5 | |||
|
Expected volatility
|
219-360 | % | ||
|
Risk-free interest rate
|
0.31-1.03 | % | ||
|
Dividend yield
|
- | |||
Effective April 1, 2012, the Company entered into an advisory agreement with director Dr. Robert Kammer (the “Advisory Agreement”) pursuant to which Dr. Kammer will provide certain services to the Company in addition to his services as a director, including, but not limited to, providing management and advice regarding the operations of the registration clinical trials including start-up and on-going clinical operational and development activities, manufacturing and quality control of the clinical and commercial supplies, project and operational management, assistance in the identification of new drug delivery technologies that may be available for acquisition or license and assistance in the development of the Company’s intellectual property. As part of Dr. Kammer’s compensation under the Advisory Agreement, the Company granted to Dr. Kammer on April 1, 2012 an option to purchase up to 300,000 shares of the Company’s common stock at an exercise price of $0.90 per share under the Plan. The option terminates on March 31, 2017 and vests over a two year period, with 75,000 options vesting immediately upon issuance and an additional 9,375 options vesting monthly for the next twenty four months thereafter. In accordance with accounting guidance for share-based compensation to consultants, the unvested portion of the option will be revalued on an interim basis until the termination of the Advisory Agreement. The Advisory Agreement is to terminate on the earlier of the completion of the services or the second anniversary of the date of the agreement. As of September 30, 2012, the revalued aggregate estimated fair value of the stock option, based on the Black-Scholes-Merton pricing model, was $338,420.
The table below illustrates the fair value per share determined by the Black-Scholes-Merton option pricing model with the following assumptions used for the grants issued to consultants during the nine months ended September 30, 2012:
|
Nine Months Ended September 30, 2012
|
||||
|
Weighted-average fair value of options granted
|
$ | 1.46 | ||
|
Expected terms (in years)
|
4.50-5.00 | |||
|
Expected volatility
|
306% - 361 | % | ||
|
Risk-free interest rate
|
0.62%-1.03 | % | ||
|
Dividend yield
|
- | |||
The Company’s outstanding options have been granted to the employees, directors and consultants at exercise prices that range from $0.48 to $16.00, the estimated fair market value of the common stock on the dates of issuance. These options have expiration dates that range from 4 – 10 years from their grant date and vest immediately, monthly, quarterly, or on an annual basis for a period of up to five years. The Company uses the Black-Scholes-Merton option pricing model to estimate the grant-date fair value of share-based awards. The Black-Scholes-Merton model requires subjective assumptions regarding future stock price volatility and expected time to exercise, along with assumptions about the risk-free interest rate and expected dividends, which affect the estimated fair values of the Company’s stock-based awards. The expected term of options granted was determined in accordance with the “simplified approach” as the Company has limited historical data on employee exercises and post-vesting employment termination behavior. The expected volatility is based on the historical volatilities of the common stock of the Company. The risk-free rate selected to value any particular grant is based on the U.S. Treasury rate that corresponds to the expected term of the grant effective as of the date of the grant. The Company used 0% as an expected dividend yield assumption. These factors could change in the future, affecting the determination of stock-based compensation expense in future periods. Utilizing these assumptions, the fair value is determined at the date of grant.
The Company issued options to purchase up to 5,060,616 shares of the Company’s common stock during the nine months ended September 30, 2012. The weighted average fair value per share of grants issued during the nine months ended September 30, 2012 was $0.65.
As of September 30, 2012, there was approximately $1,800,000 of total unrecognized compensation expense related to unvested stock options under the Plan. That expense is expected to be recognized over the weighted-average remaining vesting period of 1.42 years.
Other Stock Based Compensation
As additional compensation to Dr. Kammer under his Advisory Agreement, Dr. Kammer is to be compensated $10,000 per month in the form of common stock issued outside of the Plan, based on a $0.90 price per share being allocated to each dollar of payment due to Dr. Kammer. Upon the completion of a financing transaction yielding not less than $15 million to the Company, Dr. Kammer may unilaterally choose to be paid in either cash or common stock, based on the same $0.90 price per share. Dr. Kammer and the Company have agreed that the common stock issuable to Dr. Kammer as compensation under his advisory agreement is to be accrued and issued on a quarterly or annual basis; accordingly, as of the date hereof no such shares have been issued to Dr. Kammer. The balance due to Dr. Kammer at September 30, 2012 under the Advisory Agreement was $71,667 (share equivalent of 66,667 common shares) and is included in accounts payable and accrued expenses in the accompanying unaudited condensed consolidated balance sheet.
F-15
NOTE 5. WARRANTS
On April 25, 2012, at the closing of the April Private Placement (see Note 4), the Company issued warrants to certain accredited investors to purchase up to an aggregate amount of 2,514,642 shares of common stock with an exercise price of $1.185. The warrants have an initial exercise date of April 25, 2012 and a three-year term. Also on April 25, 2012, in connection with the Conversion Agreement (see Note 3) between the Company and DermaStar, a related party, the Company issued to DermaStar a warrant to purchase up to 241,308 shares of the Company’s common stock with an exercise price of $1.185 per share. The warrant has an initial exercise date of April 25, 2012 and a three-year term.
The warrants issued as part of the April Private Placement and to DermaStar have mandatory exercise provisions providing that the Company may require the holders of the warrants to exercise the warrants in full but not in part within twenty (20) business days after the date of a written notice delivered by the Company to each holder of a warrant; provided that (i) the value weighted average price of the Company’s common stock for ten (10) consecutive trading days is equal to or greater than the exercise price, (ii) the Company has received a Filing Review Notification (commonly referred to as a “74 Day Letter”) from the U.S. Food and Drug Administration regarding the status of the Company’s Impracor topical non-steroidal anti-inflammatory drug, and (iii) sufficient shares of the Company’s common stock are authorized and reserved for issuance upon full exercise of the warrants.
A summary of the activity of the warrants for the nine months ended September 30, 2012 is as follows:
|
Number of Shares Subject to Warrants Outstanding
|
Weighted Avg.
Exercise Price |
|||||||
|
Warrants outstanding – January 1, 2012
|
95,498 | $ | 33.16 | |||||
|
Granted
|
2,755,950 | 1.19 | ||||||
|
Exercised
|
- | |||||||
|
Expired
|
(64,745 | ) | 32.00 | |||||
|
Warrants outstanding and exercisable - September 30, 2012
|
2,786,703 | $ | 1.56 | |||||
|
Weighted average remaining contractual life of the outstanding warrants in years - September 30, 2012
|
2.55 | |||||||
NOTE 6. COMMITMENTS AND CONTINGENCIES
Commitments
The Company leases its office facilities under a noncancelable operating lease, which expires on February 28, 2014, with a monthly amount due of $2,972 for the first 12 months beginning March 1, 2012, and $3,715 due monthly for the next 12 months. For the remaining fiscal year 2012, the Company’s lease commitment is approximately $8,900.
Indemnities and Guarantees
In addition to the indemnification provisions contained in the Company’s charter documents, the Company generally enters into separate indemnification agreements with the Company’s directors and officers. These agreements require the Company, among other things, to indemnify the director or officer against specified expenses and liabilities, such as attorneys’ fees, judgments, fines and settlements, paid by the individual in connection with any action, suit or proceeding arising out of the individual’s status or service as the Company’s director or officer, other than liabilities arising from willful misconduct or conduct that is knowingly fraudulent or deliberately dishonest, and to advance expenses incurred by the individual in connection with any proceeding against the individual with respect to which the individual may be entitled to indemnification by the Company. These guarantees and indemnities do not provide for any limitation of the maximum potential future payments the Company could be obligated to make. Historically, the Company has not been obligated nor incurred any payments for these obligations and, therefore, no liabilities have been recorded for these indemnities and guarantees in the accompanying condensed consolidated balance sheets.
PCCA License Agreement
Pursuant to the terms of the PCCA License Agreement entered in connection with the PCCA Transaction (Note 4), effective August 30, 2012, PCCA has granted to the Company and its affiliates certain exclusive rights under PCCA’s proprietary formulations, other technologies and data, and the Company has agreed to pay to PCCA certain royalties on net sales relating to the sale of certain future products, which royalties range from 4.5% to 9% for each product, subject to certain minimum royalty payments. PCCA may terminate the PCCA License Agreement if the Company fails to commence efforts to research and develop future products within certain time periods, as set forth in the PCCA License Agreement.
Cosmetic License Agreements - Terminated
On May 20, 2009, the Company and JH Direct, LLC (“JH Direct”) entered into a licensing agreement providing JH Direct with the exclusive worldwide rights to the Company’s anti-cellulite cosmetic product which utilizes the Company’s patented transdermal delivery system technology, Accudel. Under the terms of the agreement, JH Direct must pay the Company initial royalty advances and a continuing licensing royalty on the worldwide sales of the anti-cellulite product. The Company received non-refundable royalty advances totaling $100,000 from JH Direct. During the nine months ended September 30, 2012, management of the Company concluded that JH Direct had abandoned its efforts to commercialize the anti-cellulite cream and the Company exercised its rights to terminate the agreement in January 2012, at which time all revenues from this agreement were recognized in full. The Company does not expect to receive any additional funds from JH Direct under this contract.
F-16
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Imprimis Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheets of Imprimis Pharmaceuticals, Inc. (formerly Transdel Pharmaceuticals, Inc.) and subsidiary (a development stage company) (the “Company”) as of December 31, 2011 and 2010, and the related consolidated statements of operations, stockholders’ deficit and cash flows for each of the two years in the period ended December 31, 2011 and for the period from July 24, 1998 (date of inception) through December 31, 2011. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit on its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Imprimis Pharmaceuticals, Inc. (formerly Transdel Pharmaceuticals, Inc.) and subsidiary as of December 31, 2011 and 2010, and the consolidated results of their operations and their cash flows for each of the two years in the period ended December 31, 2011 and for the period from July 24, 1998 (date of inception) through December 31, 2011 in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As more fully described in Note 2 to the consolidated financial statements, the Company has incurred significant operating losses, had negative cash flows from operations, has not recognized any revenues since inception and has a deficit accumulated during the development stage. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amount and classification of liabilities that may result from the outcome of this uncertainty.
/s/ KMJ Corbin & Company LLP
Costa Mesa, California
February 23, 2012
Except for Note 3 and the effects of
the retrospective application of the
reverse stock split as described in
Note 3, as to which the date is
February 28, 2012
F-17
|
(A Development Stage Company)
|
||||||
|
CONSOLIDATED BALANCE SHEETS
|
||||||
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
ASSETS
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$ | 146,160 | $ | 291,462 | ||||
|
Prepaid expenses and other current assets
|
14,797 | 60,492 | ||||||
|
Total current assets
|
160,957 | 351,954 | ||||||
|
Computer equipment, net
|
- | 338 | ||||||
|
TOTAL ASSETS
|
$ | 160,957 | $ | 352,292 | ||||
|
LIABILITIES AND STOCKHOLDERS' (DEFICIT)
|
||||||||
|
Current liabilities
|
||||||||
|
Accounts payable
|
$ | 218,612 | $ | 73,632 | ||||
|
Accounts payable - related party
|
56,087 | - | ||||||
|
Accrued Phase 3 expenses
|
55,784 | 111,871 | ||||||
|
Accrued expenses and payroll liabilities
|
- | 69,532 | ||||||
|
Deferred revenue
|
100,000 | 80,000 | ||||||
|
Notes payable - related party
|
300,000 | - | ||||||
|
Convertible note payable and accrued interest
|
1,130,479 | - | ||||||
|
Total current liabilities
|
1,860,962 | 335,035 | ||||||
|
Convertible note payable and accrued interest
|
- | 1,055,479 | ||||||
|
TOTAL LIABILITIES
|
1,860,962 | 1,390,514 | ||||||
|
Commitments and contingencies
|
||||||||
|
STOCKHOLDERS' DEFICIT
|
||||||||
|
Series A Convertible preferred stock, $0.001 par value, 10 shares authorized,
|
||||||||
|
10 and 0 shares issued and outstanding
|
||||||||
|
at December 31, 2011 and 2010, respectively
|
- | - | ||||||
|
Common stock, $0.001 par value, 50,000,000 shares authorized,
|
||||||||
|
1,987,601 and 1,991,508 issued and outstanding
|
||||||||
|
at December 31, 2011 and 2010, respectively
|
1,988 | 1,992 | ||||||
|
Additional paid in capital
|
16,818,740 | 16,426,583 | ||||||
|
Deficit accumulated during the development stage
|
(18,520,733 | ) | (17,466,797 | ) | ||||
|
TOTAL STOCKHOLDERS' DEFICIT)
|
(1,700,005 | ) | (1,038,222 | ) | ||||
|
TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT
|
$ | 160,957 | $ | 352,292 | ||||
The accompanying notes are an integral part of these consolidated financial statements
F-18
|
IMPRIMIS PHARMACEUTICALS, INC.
|
|||||||
|
(A Development Stage Company)
|
|||||||
|
CONSOLIDATED STATEMENTS OF OPERATIONS
|
|
For The
|
For The
|
For the Period From
|
||||||||||
|
Year Ended
|
Year Ended
|
July 24, 1998 (Inception)
|
||||||||||
|
December 31,
|
December 31,
|
through December 31,
|
||||||||||
|
2011
|
2010
|
2011
|
||||||||||
|
Operating Expenses:
|
||||||||||||
|
Selling, general and administrative
|
827,674 | 2,307,972 | 9,573,327 | |||||||||
|
Research and development
|
111,554 | 194,588 | 7,820,258 | |||||||||
|
Loss from operations
|
(939,228 | ) | (2,502,560 | ) | (17,393,585 | ) | ||||||
|
Other income (expense)
|
||||||||||||
|
Interest expense
|
(75,000 | ) | (55,479 | ) | (1,706,234 | ) | ||||||
|
Interest income
|
- | 512 | 127,581 | |||||||||
|
Gain on settlement
|
- | - | 375,000 | |||||||||
|
Gain on forgiveness of liabilities
|
60,292 | 26,299 | 176,505 | |||||||||
|
Total other expense, net
|
(14,708 | ) | (28,668 | ) | (1,027,148 | ) | ||||||
|
Net loss
|
(953,936 | ) | (2,531,228 | ) | (18,420,733 | ) | ||||||
|
Deemed dividend to preferred stockholders
|
(100,000 | ) | - | (100,000 | ) | |||||||
|
Net loss attributable to common stockholders
|
$ | (1,053,936 | ) | $ | (2,531,228 | ) | $ | (18,520,733 | ) | |||
|
Net loss per common share, basic and diluted:
|
$ | (0.53 | ) | $ | (1.28 | ) | ||||||
|
Weighted average common shares outstanding, basic and diluted
|
1,989,014 | 1,973,155 | ||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-19
|
IMPRIMIS PHARMACEUTICALS, INC.
|
||||||||||||
|
(Development Stage Company)
|
||||||||||||
|
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (DEFICIT)
|
||||||||||||
|
For the years ended December 31, 2011 and 2010 and for the period from June 24, 1998 (Inception) through December 31, 2011
|
|
Preferred Stock
|
Common Stock
|
Additional
|
Deficit accumulated
|
Total
|
||||||||||||||||||||||||
|
Par
|
Par
|
Paid-in
|
during the
|
Stockholders'
|
||||||||||||||||||||||||
|
Shares
|
Value
|
Shares
|
Value
|
Capital
|
development stage
|
Equity (Deficit)
|
||||||||||||||||||||||
|
Balance at June 24, 1998 (Inception)
|
- | $ | - | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||
|
Estimated fair value of services contributed by
|
||||||||||||||||||||||||||||
|
stockholders
|
- | - | - | - | 100,000 | - | 100,000 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (100,000 | ) | (100,000 | ) | |||||||||||||||||||
|
Balance at December 31, 1998
|
- | - | - | - | 100,000 | (100,000 | ) | - | ||||||||||||||||||||
|
Estimated fair value of services contributed by
|
||||||||||||||||||||||||||||
|
stockholders
|
- | - | - | - | 200,000 | - | 200,000 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (204,000 | ) | (204,000 | ) | |||||||||||||||||||
|
Balance at December 31, 1999
|
- | - | - | - | 300,000 | (304,000 | ) | (4,000 | ) | |||||||||||||||||||
|
Issuance of common stock at $0.0512 per share in
|
||||||||||||||||||||||||||||
|
May and June 2000
|
- | - | 117,188 | 117 | 5,883 | - | 6,000 | |||||||||||||||||||||
|
Estimated fair value of services contributed by
|
||||||||||||||||||||||||||||
|
stockholders
|
- | - | - | - | 200,000 | - | 200,000 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (213,092 | ) | (213,092 | ) | |||||||||||||||||||
|
Balance at December 31, 2000
|
- | - | 117,188 | 117 | 505,883 | (517,092 | ) | (11,092 | ) | |||||||||||||||||||
|
Estimated fair value of services contributed by
|
||||||||||||||||||||||||||||
|
stockholders
|
- | - | - | - | 200,000 | - | 200,000 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (208,420 | ) | (208,420 | ) | |||||||||||||||||||
|
Balance at December 31, 2001
|
- | - | 117,188 | 117 | 705,883 | (725,512 | ) | (19,512 | ) | |||||||||||||||||||
|
Estimated fair value of services contributed by
|
||||||||||||||||||||||||||||
|
stockholders
|
- | - | - | - | 200,000 | - | 200,000 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (228,217 | ) | (228,217 | ) | |||||||||||||||||||
|
Balance at December 31, 2002
|
- | - | 117,188 | 117 | 905,883 | (953,729 | ) | (47,729 | ) | |||||||||||||||||||
|
Estimated fair value of services contributed by
|
||||||||||||||||||||||||||||
|
stockholders
|
- | - | - | - | 200,000 | - | 200,000 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (207,196 | ) | (207,196 | ) | |||||||||||||||||||
|
Balance at December 31, 2003
|
- | - | 117,188 | 117 | 1,105,883 | (1,160,925 | ) | (54,925 | ) | |||||||||||||||||||
|
Estimated fair value of services contributed by
|
||||||||||||||||||||||||||||
|
stockholders
|
- | - | - | - | 400,000 | - | 400,000 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (508,226 | ) | (508,226 | ) | |||||||||||||||||||
|
Balance at December 31, 2004
|
- | - | 117,188 | 117 | 1,505,883 | (1,669,151 | ) | (163,151 | ) | |||||||||||||||||||
|
Capital contributions
|
- | - | - | - | 14,200 | - | 14,200 | |||||||||||||||||||||
|
Issuance of common stock at $0.0512 per share in
|
||||||||||||||||||||||||||||
|
August 2005
|
- | - | 306,641 | 307 | 15,393 | - | 15,700 | |||||||||||||||||||||
|
Exercise of stock options at $0.0512 per share in
|
||||||||||||||||||||||||||||
|
August 2005
|
- | - | 1,953 | 2 | 98 | - | 100 | |||||||||||||||||||||
|
Estimated fair value of services contributed by
|
||||||||||||||||||||||||||||
|
stockholders
|
- | - | - | - | 400,000 | - | 400,000 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (539,622 | ) | (539,622 | ) | |||||||||||||||||||
|
Balance at December 31, 2005
|
- | - | 425,782 | 426 | 1,935,574 | (2,208,773 | ) | (272,773 | ) | |||||||||||||||||||
|
Capital contributions
|
- | - | - | - | 48,600 | - | 48,600 | |||||||||||||||||||||
|
Exercise of stock options at $0.0512 per share in June
|
||||||||||||||||||||||||||||
|
and July 2006
|
- | - | 46,875 | 47 | 2,353 | - | 2,400 | |||||||||||||||||||||
|
Estimated fair value of services contributed by
|
||||||||||||||||||||||||||||
|
stockholders
|
- | - | - | - | 400,000 | - | 400,000 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (584,232 | ) | (584,232 | ) | |||||||||||||||||||
|
Balance at December 31, 2006
|
- | - | 472,657 | 473 | 2,386,527 | (2,793,005 | ) | (406,005 | ) | |||||||||||||||||||
|
Issuance of common stock at $0.0512 per share
|
||||||||||||||||||||||||||||
|
during January and March 2007
|
- | - | 498,047 | 498 | 25,002 | - | 25,500 | |||||||||||||||||||||
|
Exercise of stock options and warrants at $0.0512
|
||||||||||||||||||||||||||||
|
per share in April and August 2007
|
- | - | 4,883 | 5 | 245 | - | 250 | |||||||||||||||||||||
|
Estimated fair value of services contributed by
|
||||||||||||||||||||||||||||
|
stockholders
|
- | - | - | - | 175,000 | - | 175,000 | |||||||||||||||||||||
|
Capital contributions
|
- | - | - | 105,907 | - | 105,907 | ||||||||||||||||||||||
|
Forgiveness of notes payable and interest
|
- | - | - | - | 241,701 | - | 241,701 | |||||||||||||||||||||
|
Issuance of restricted common stock at $16.00 per share
|
||||||||||||||||||||||||||||
|
in August 2007
|
- | - | 24,414 | 25 | (25 | ) | - | - | ||||||||||||||||||||
|
Issuance of common stock in connection with merger
|
||||||||||||||||||||||||||||
|
on September 17, 2007
|
- | - | 231,249 | 231 | (231 | ) | - | - | ||||||||||||||||||||
|
Net proceeds from private placement offering issued
|
||||||||||||||||||||||||||||
|
at $100,000 per unit in September and October 2007
|
- | - | 258,979 | 259 | 3,837,532 | - | 3,837,791 | |||||||||||||||||||||
|
Issuance of common stock related to conversion of
|
||||||||||||||||||||||||||||
|
senior convertible notes payable and accrued interest
|
- | - | 191,272 | 191 | 1,529,986 | - | 1,530,177 | |||||||||||||||||||||
|
Beneficial conversion feature upon conversion of
|
||||||||||||||||||||||||||||
|
senior convertible notes payable
|
- | - | - | - | 1,530,177 | - | 1,530,177 | |||||||||||||||||||||
|
Issuance of common stock and warrants for consulting
|
||||||||||||||||||||||||||||
|
services in September 2007 at a value of $16.00 per
|
||||||||||||||||||||||||||||
|
share for stock transaction and $100,000 per unit
|
||||||||||||||||||||||||||||
|
for stock and warrant transaction
|
- | - | 34,375 | 34 | 549,966 | - | 550,000 | |||||||||||||||||||||
|
Stock-based compensation
|
- | - | - | - | 184,522 | - | 184,522 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (4,284,540 | ) | (4,284,540 | ) | |||||||||||||||||||
|
Balance at December 31, 2007
|
- | - | 1,715,876 | 1,716 | 10,566,309 | (7,077,545 | ) | 3,490,480 | ||||||||||||||||||||
|
Net proceeds from private placement offering issued
|
||||||||||||||||||||||||||||
|
at $110,000 per unit in May 2008 and final costs of
|
||||||||||||||||||||||||||||
|
2007 private placement offering
|
- | - | 227,273 | 228 | 3,941,073 | - | 3,941,301 | |||||||||||||||||||||
|
Adjustment and issuance of common stock, warrant and
|
||||||||||||||||||||||||||||
|
stock options related to consulting services agreement
|
- | - | (1,738 | ) | (2 | ) | (117,991 | ) | - | (117,993 | ) | |||||||||||||||||
|
Issuance of restricted stock at $5.60 per share
|
||||||||||||||||||||||||||||
|
in November 2008
|
- | - | 3,125 | 3 | (3 | ) | - | - | ||||||||||||||||||||
|
Stock-based compensation
|
- | - | - | - | 562,442 | - | 562,442 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (3,304,388 | ) | (3,304,388 | ) | |||||||||||||||||||
|
Balance at December 31, 2008
|
- | - | 1,944,535 | 1,945 | 14,951,830 | (10,381,933 | ) | 4,571,842 | ||||||||||||||||||||
|
Issuance of common stock and stock options related
|
||||||||||||||||||||||||||||
|
consulting agreements
|
- | - | 5,722 | 6 | 121,449 | - | 121,455 | |||||||||||||||||||||
|
Exercise of stock options at $7.92 per share August 2009
|
- | - | 6,250 | 6 | 49,494 | - | 49,500 | |||||||||||||||||||||
|
Stock-based compensation
|
- | - | - | - | 388,050 | - | 388,050 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (4,553,636 | ) | (4,553,636 | ) | |||||||||||||||||||
|
Balance at December 31, 2009
|
- | - | 1,956,508 | 1,957 | 15,510,823 | (14,935,569 | ) | 577,211 | ||||||||||||||||||||
|
Issuance of common stock and stock options related
|
||||||||||||||||||||||||||||
|
to consulting agreements
|
- | - | 28,750 | 29 | 367,871 | - | 367,900 | |||||||||||||||||||||
|
Issuance of restricted stock at $6.40 per share
|
||||||||||||||||||||||||||||
|
in October 2010
|
- | - | 6,250 | 6 | 12,077 | - | 12,083 | |||||||||||||||||||||
|
Stock-based compensation
|
- | - | - | - | 535,812 | - | 535,812 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (2,531,228 | ) | (2,531,228 | ) | |||||||||||||||||||
|
Balance at December 31, 2010
|
- | - | 1,991,508 | 1,992 | 16,426,583 | (17,466,797 | ) | (1,038,222 | ) | |||||||||||||||||||
|
Forfeiture of unvested restricted stock in May 2011
|
- | - | (3,906 | ) | (4 | ) | 3,336 | - | 3,332 | |||||||||||||||||||
|
Issuance of Series A Preferred Stock at $10,000 per
|
||||||||||||||||||||||||||||
|
share in December 2011
|
10 | - | - | - | 100,000 | - | 100,000 | |||||||||||||||||||||
|
Preferred stock beneficial conversion feature
|
- | - | - | - | 100,000 | - | 100,000 | |||||||||||||||||||||
|
Accretion of preferred stock discount
|
- | - | - | - | - | (100,000 | ) | (100,000 | ) | |||||||||||||||||||
|
Estimated fair value of stock options granted to former
|
||||||||||||||||||||||||||||
|
employees in forgiveness of liabilities
|
- | - | - | - | 11,400 | - | 11,400 | |||||||||||||||||||||
|
Stock-based compensation
|
- | - | - | - | 177,421 | - | 177,421 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (953,936 | ) | (953,936 | ) | |||||||||||||||||||
|
Balance at December 31, 2011
|
10 | $ | - | 1,987,601 | $ | 1,988 | $ | 16,818,740 | $ | (18,520,733 | ) | $ | (1,700,005 | ) | ||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-20
|
IMPRIMIS PHARMACEUTICALS, INC.
|
|||||||
|
(A Development Stage Company)
|
|||||||
|
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
For The
|
For The
|
For the Period From
|
||||||||||
|
Year Ended
|
Year Ended
|
July 24, 1998 (Inception)
|
||||||||||
|
December 31,
|
December 31,
|
through December 31,
|
||||||||||
|
2011
|
2010
|
2011
|
||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||||||
|
Net loss
|
$ | (953,936 | ) | $ | (2,531,228 | ) | $ | (18,420,733 | ) | |||
|
Adjustments to reconcile net loss to net cash used in
|
||||||||||||
|
operating activities:
|
||||||||||||
|
Estimated fair value of contributed services
|
- | - | 2,475,000 | |||||||||
|
Gain on forgiveness of liabilities
|
(60,292 | ) | (26,299 | ) | (176,505 | ) | ||||||
|
Amortization of prepaid consulting fees
|
- | 235,600 | 807,608 | |||||||||
|
Depreciation
|
338 | 1,056 | 3,154 | |||||||||
|
Non-cash interest on notes payable
|
75,000 | 55,479 | 1,706,234 | |||||||||
|
Stock-based compensation
|
192,153 | 680,195 | 2,128,816 | |||||||||
|
Payments made on behalf of Company by related party
|
254,142 | - | 254,142 | |||||||||
|
Changes in assets and liabilities:
|
||||||||||||
|
Prepaid consulting costs
|
- | - | (140,000 | ) | ||||||||
|
Prepaid expenses and other current assets
|
45,695 | 20,425 | (14,797 | ) | ||||||||
|
Accounts payable
|
144,980 | (607,382 | ) | 308,526 | ||||||||
|
Accrued Phase 3 expenses
|
- | (231,762 | ) | 111,871 | ||||||||
|
Accrued expenses and payroll liabilities
|
(9,240 | ) | 25,605 | 86,591 | ||||||||
|
Deferred revenue
|
20,000 | 80,000 | 100,000 | |||||||||
|
NET CASH USED IN OPERATING ACTIVITIES
|
(291,160 | ) | (2,298,311 | ) | (10,770,093 | ) | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
||||||||||||
|
Purchases of fixed assets
|
- | - | (3,154 | ) | ||||||||
|
NET CASH USED IN INVESTING ACTIVITIES
|
- | - | (3,154 | ) | ||||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
||||||||||||
|
Proceeds from issuance of notes payable to stockholders
|
300,000 | - | 526,300 | |||||||||
|
Proceeds from issuance of preferred stock
|
100,000 | - | 100,000 | |||||||||
|
Proceeds from notes payable
|
- | 1,000,000 | 2,500,000 | |||||||||
|
Cash advances from related party
|
27,537 | - | 27,537 | |||||||||
|
Repayment of advances from related party
|
(281,679 | ) | - | (281,679 | ) | |||||||
|
Capital contributions
|
- | - | 168,707 | |||||||||
|
Net proceeds from purchase of common stock and exercise
|
||||||||||||
|
of warrants and stock options
|
- | - | 99,450 | |||||||||
|
Proceeds from issuance of common stock for cash, net of offering costs
|
- | - | 7,779,092 | |||||||||
|
NET CASH PROVIDED BY FINANCING ACTIVITIES
|
145,858 | 1,000,000 | 10,919,407 | |||||||||
|
NET CHANGE IN CASH AND CASH EQUIVALENTS
|
(145,302 | ) | (1,298,311 | ) | 146,160 | |||||||
|
CASH AND CASH EQUIVALENTS, beginning of period
|
291,462 | 1,589,773 | - | |||||||||
|
CASH AND CASH EQUIVALENTS, end of period
|
$ | 146,160 | $ | 291,462 | $ | 146,160 | ||||||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION:
|
||||||||||||
|
Issuance of and adjustment to common stock and warrants to
|
||||||||||||
|
consulting firms for prepaid consulting fees
|
$ | - | $ | - | $ | 432,007 | ||||||
|
Conversion of notes payable and accrued interest into common stock
|
$ | - | $ | - | $ | 1,530,177 | ||||||
|
Forgiveness of notes payable and accrued interest to shareholders
|
$ | - | $ | - | $ | 241,701 | ||||||
|
Conversion of advances to notes payable to shareholders
|
$ | - | $ | - | $ | 196,300 | ||||||
|
Accretion of preferred stock discount
|
$ | 100,000 | $ | - | $ | 100,000 | ||||||
|
Payment of Phase 3 Liabilities by related party
|
$ | 56,087 | $ | - | $ | 56,087 | ||||||
The accompanying notes are an integral part of these consolidated financial statements
F-21
IMPRIMIS PHARMACEUTICALS, INC.
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended December 31, 2011 and 2010 and the period from July 24, 1998 (Inception) through December 31, 2011
NOTE 1. ORGANIZATION
On February 28, 2012, the Company changed its name from Transdel Pharmaceuticals, Inc. to Imprimis Pharmaceuticals, Inc. All prior references to Transdel Pharmaceuticals, Inc. have been changed to Imprimis Pharmaceuticals, Inc. to reflect the change.
Imprimis Pharmaceuticals, Inc. (“Imprimis” or “Company”) is a specialty pharmaceutical company developing non-invasive, topically delivered products. The Company’s innovative patented Accudel cream formulation technology is designed to facilitate the effective penetration of a variety of products through the skin barrier. Impracor , the Company’s lead pain product, utilizes the Accudel platform technology to deliver the active drug, ketoprofen, a non-steroidal anti-inflammatory drug (“NSAID”), through the skin directly into the underlying musculoskeletal and soft tissues where the drug exerts its anti-inflammatory and analgesic effects. The Company intends to leverage its Accudel platform technology to expand and create a portfolio of topical products for a variety of indications.
As described in Note 4, the Company, on June 26, 2011, filed a voluntary petition for reorganization relief under Chapter 11 of the United States Bankruptcy Code in the United States Bankruptcy Court for the Southern District of California (the “Bankruptcy Court”), Case No. 11-10497-11 (the “Chapter 11 Case”). On November 21, 2011, in connection with the transactions described throughout these notes to the consolidated financial statements, the Company requested that the Bankruptcy Court dismiss the Chapter 11 Case, and on December 8, 2011, the Bankruptcy Court entered an order dismissing the Chapter 11 Case.
NOTE 2. GOING CONCERN
The accompanying consolidated financial statements have been prepared on a going-concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has incurred recurring operating losses, had negative operating cash flows and has not recognized any revenues since July 24, 1998 (Inception). In addition, the Company had a deficit accumulated during the development stage of $18.5 million at December 31, 2011. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
The Company’s continuation as a going concern is dependent on its ability to obtain additional financing to fund operations, implement its business model, and ultimately, to attain profitable operations. In order to execute the second Phase 3 clinical trial and other supportive safety studies for Impracor , which are required by the U.S. Food and Drug Administration (“FDA”) to obtain final regulatory approval for Impracor , the Company will need to secure additional funds through various means, including equity and debt financing, funding from a corporate partnership or licensing arrangement or any similar financing. There can be no assurance that the Company will be able to obtain additional debt or equity financing, if and when needed, on terms acceptable to the Company. Any additional equity or debt financing may involve substantial dilution to the Company’s stockholders, restrictive covenants or high interest costs. The failure to raise needed funds on sufficiently favorable terms could have a material adverse effect on the execution of the Company’s business plan, operating results and financial condition. The Company’s long term liquidity also depends upon its ability to generate revenues from the sale of its products and achieve profitability. The failure to achieve these goals could have a material adverse effect on the execution of the Company’s business plan, operating results and financial condition. The Company intends to raise additional financing to fund its operations through various means, including equity or debt financing, funding from a corporate partnership or licensing arrangement or any similar financing. However, there is no assurance that sufficient financing will be available or, if available, on terms that would be acceptable to the Company.
The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
F-22
NOTE 3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”), and with the rules and regulations of the Securities and Exchange Commission (“SEC”) related to an annual report on Form 10-K. The consolidated financial statements include the accounts of Imprimis Pharmaceuticals Inc. and its wholly-owned subsidiary, Transdel Pharmaceuticals Holdings, Inc. (collectively, the “Company”). All significant intercompany balances and transactions have been eliminated in consolidation. The Company has evaluated subsequent events through the filing date of this Form 10-K, and determined that no subsequent events have occurred that would require recognition in the consolidated financial statements or disclosure in the notes thereto other than as disclosed in the accompanying notes.
Principles of Consolidation
On September 17, 2007, Imprimis entered into an Agreement of Merger and Plan of Reorganization (the “Merger Agreement”) by and among Imprimis, Transdel Pharmaceuticals Holdings, Inc., a privately held Nevada corporation (“Transdel Holdings”), and Trans-Pharma Acquisition Corp., a newly formed, wholly-owned Delaware subsidiary of Imprimis (“Acquisition Sub”). Upon closing of the merger transaction contemplated under the Merger Agreement (the “Merger”), Acquisition Sub merged with and into Transdel Holdings, and Transdel Holdings, as the surviving corporation, became a wholly-owned subsidiary of Imprimis.
In connection with the merger, 231,242 of Imprimis common shares remain outstanding and all other outstanding shares of Imprimis were cancelled. Also, at the closing of the Merger, each share of Transdel Holdings common stock issued and outstanding immediately prior to the closing of the Merger was exchanged for the right to receive 1.25 of one share of Imprimis’ common stock. An aggregate of 1,000,000 shares of Imprimis’ common stock, which includes 24,414 shares of restricted stock which were subject to forfeiture, were issued to the holders of Transdel Holdings’ common stock. As a result of the transaction, the former owners of Transdel Holdings became the controlling stockholders of Imprimis. Accordingly, the merger of Transdel Holdings and Imprimis is a reverse merger that has been accounted for as a recapitalization of Transdel Holdings.
Effective on September 17, 2007, and for all reporting periods thereafter, Imprimis’ operating activities, including any prior comparative period, include only those of Transdel Holdings. All references to share and per share amounts in the accompanying consolidated financial statements and footnotes have been restated to reflect the aforementioned share exchange. All significant intercompany accounts and transactions have been eliminated in consolidation.
On June 20, 2011, Transdel Holdings was merged with Imprimis Pharmaceuticals, Inc., at which time Transdel Holdings ceased as a corporation, and Imprimis Pharmaceuticals, Inc. remains as the sole surviving corporation.
Development Stage Enterprise
The Company is a development stage company as defined by the Financial Accounting Standards Board (the “FASB”). The Company is devoting substantially all of its present efforts to establish a new business, and its planned principal operations have not yet commenced. All losses accumulated since inception have been considered as part of the Company’s development stage activities.
These consolidated financial statements contemplate the realization of assets and the satisfaction of liabilities in the normal course of business. The Company is a development stage enterprise and has sustained significant losses since inception and expects to continue to incur losses through 2012.
F-23
Research and Development
The Company expenses all costs related to research and development as they are incurred.
Revenue Recognition and Deferred Revenue
The Company will recognize revenues in accordance with FASB guidance, which requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred; (3) the selling price is fixed and determinable; and (4) collectibility is reasonably assured. Determination of criteria (3) and (4) will be based on management’s judgments regarding the fixed nature of the selling prices of the products delivered and the collectibility of those amounts. Provisions for discounts and rebates to customers, estimated returns and allowances, and other adjustments will be provided for in the same period the related sales are recorded. The Company will defer any revenue for which the product has not been delivered or for which services have not been rendered or are subject to refund until such time that the Company and the customer jointly determine that the product has been delivered or services have been rendered or no refund will be required.
As of December 31, 2011, the Company had not generated any revenues and the Company does not anticipate that it will generate any significant revenues until one or more of its drug candidates are approved by the FDA or until the Company is able to commercialize one or more of its cosmetic products. Also, effective sales and marketing support must be in place for either the drug candidates or the cosmetic products in order to generate any revenues. The FDA approval process is highly uncertain and the Company cannot estimate when it will generate revenues at this time from sales of its products.
Cash and Cash Equivalents
Cash equivalents include short-term, highly liquid investments with maturities of three months or less at the time of acquisition.
Concentrations of Credit Risk
A financial instrument which potentially subjects the Company to concentrations of credit risk is cash. The Company places its cash with financial institutions deemed by management to be of high credit quality. The Federal Deposit Insurance Corporation (“FDIC”) provides basic deposit coverage with limits to $250,000 per owner. In addition to the basic insurance deposit coverage, the FDIC is providing temporary unlimited coverage for noninterest-bearing transaction accounts from December 31, 2010 to December 31, 2012. At December 31, 2011, there were no uninsured deposits.
Computer Equipment
Computer equipment is stated at cost less accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful life of three years.
During the years ended December 31, 2011 and 2010, the Company recorded $338 and $1,056, respectively, in depreciation expense.
F-24
Fair Value Measurements
Fair value measurements are determined based on the assumptions that market participants would use in pricing an asset or liability. US GAAP establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. The established fair value hierarchy prioritizes the use of inputs used in valuation methodologies into the following three levels:
|
●
|
Level 1: Quoted prices (unadjusted) for identical assets or liabilities in active markets. A quoted price in an active market provides the most reliable evidence of fair value and must be used to measure fair value whenever available.
|
|
●
|
Level 2: Significant other observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data.
|
|
●
|
Level 3: Significant unobservable inputs that reflect a reporting entity’s own assumptions about the assumptions that market participants would use in pricing an asset or liability. For example, level 3 inputs would relate to forecasts of future earnings and cash flows used in a discounted future cash flows method.
|
The fair values of the Company’s cash and cash equivalents, accounts payable, accounts payable due to related parties, accrued expenses and notes payable approximate carrying values due to their short term maturities.
Stock-Based Compensation
All share-based payments to employees, including grants of stock options to employees, directors and consultants and restricted stock grants, are recognized in the consolidated financial statements based upon their fair values.
The Company's accounting policy for equity instruments issued to consultants and vendors in exchange for goods and services follows FASB guidance. As such, the value of the applicable stock-based compensation is periodically remeasured and income or expense is recognized during their vesting terms. The measurement date for the fair value of the equity instruments issued is determined at the earlier of (i) the date at which a commitment for performance by the consultant or vendor is reached or (ii) the date at which the consultant or vendor's performance is complete. In the case of equity instruments issued to consultants, the fair value of the equity instrument is primarily recognized over the term of the consulting agreement. In accordance with FASB guidance, an asset acquired in exchange for the issuance of fully vested, nonforfeitable equity instruments should not be presented or classified as an offset to equity on the grantor's balance sheet once the equity instrument is granted for accounting purposes. Accordingly, the Company records the fair value of nonforfeitable equity instruments issued for future consulting services as prepaid consulting fees in its consolidated balance sheets.
Income Taxes
We account for income taxes under the provision of Accounting Standards Codification 740, “Income Taxes”, or ASC 740. As of December 31, 2011 and 2010, there was no unrecognized tax benefits included in the consolidated balance sheets that would, if recognized, affect the effective tax rate. Our practice is to recognize interest and/or penalties related to income tax matters in income tax expense. We had no accrual for interest or penalties on our consolidated balance sheets at December 31, 2011 and 2010, respectively and have not recognized interest and/or penalties in the consolidated statement of operations for the years ended December 31, 2011 and 2010. We are subject to taxation in the United States and California.
F-25
Basic and Diluted Loss per Common Share
Basic net loss per common share is computed by dividing net loss attributable to common stockholders for the period by the weighted average number of common shares outstanding during the period. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders for the period by the weighted average number of common and common equivalent shares, such as stock options and warrants outstanding during the period.
Basic and diluted net loss applicable to common stock per share is computed using the weighted average number of common shares outstanding during the period. Common stock equivalents (prior to application of the treasury stock, if converted method) from convertible notes, preferred stock, stock options and warrants were 4,136,10 and 541,335 for the years ended December 31, 2011 and 2010, respectively, are excluded from the calculation of diluted net loss per share for all periods presented because the effect is anti-dilutive.
|
For the year ended
|
For the year ended
|
|||||||
|
December 31, 2011
|
December 31, 2010
|
|||||||
|
Net loss
|
$ | (953,936 | ) | $ | (2,531,228 | ) | ||
|
Deemed dividend to preferred stockholders
|
(100,000 | ) | - | |||||
|
Numerator – loss attributable to common stockholders
|
(1,053,936 | ) | (2,531,228 | ) | ||||
|
Denominator – weighted average
|
||||||||
|
number of shares outstanding, basic and diluted
|
1,989,014 | 1,973,155 | ||||||
|
Loss per share, basic and diluted
|
$ | (0.53 | ) | $ | (1.28 | ) | ||
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and judgments that affect the reported amounts of assets, liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting periods. Significant estimates made by management are, among others, the valuation of contributed services, stock options, deferred taxes and stock-based compensation issued to employees and non-employees. Actual results could differ from those estimates.
Recent Accounting Pronouncements
In May 2011, the FASB issued ASU 2011-04, Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs. The update contains the results of the work of the FASB and the International Accounting Standards Board to develop common requirements for measuring fair value and for disclosing fair value measurements in accordance with U.S. GAAP and IFRSs. The amendments in this update are effective for periods beginning after December 15, 2011 and as a result are not yet applicable to the Company. The Company is evaluating the impact of the update on its consolidated financial statements.
F-26
Reverse Stock Split
On February 28, 2012, the Company effected a one-for-8 reverse stock split of its common stock. The reverse stock split did not affect the Company’s Series A convertible preferred stock. The accompanying consolidated financial statements and footnotes have been retroactively adjusted to reflect the effects of the reverse stock split. The reverse stock split did not affect the amount of the Company’s equity or its market capitalization.
NOTE 4. BANKRUPTCY PETITION AND ASSET PURCHASE AGREEMENT
On June 26, 2011 we filed a voluntary petition for reorganization relief under Chapter 11 of the United States Bankruptcy Code in the United States Bankruptcy Court for the Southern District of California (the “Bankruptcy Court”), Case No. 11-10497-11 (the “Chapter 11 Case”). In connection with the Chapter 11 Case, we, as seller, and Cardium Healthcare, Inc., a wholly-owned subsidiary of Cardium Therapeutics, Inc., as purchaser (“Cardium”), entered into an Asset Purchase Agreement dated June 26, 2011 (the “Asset Purchase Agreement”) pursuant to which we agreed to sell substantially all of our assets pursuant to Sections 105, 363 and 365 of the Bankruptcy Code, subject to court approval and the satisfaction of certain conditions set forth in the Asset Purchase Agreement.
Consummation of the sale to Cardium was subject to a number of conditions, including, among others, the approval by the Bankruptcy Court of the transactions contemplated by the Asset Purchase Agreement and compliance with certain specified deadlines for actions in connection with the Bankruptcy Case. The Asset Purchase Agreement was terminable by the parties under a number of circumstances, including failure to obtain certain Bankruptcy Court orders by agreed dates.
On July 26, 2011, the Bankruptcy Court denied our motion to sell our assets pursuant to the Asset Purchase Agreement. On October 7, 2011, we terminated the Asset Purchase Agreement pursuant to its terms. On November 21, 2011, in connection with the transactions described below, we requested that the Bankruptcy Court dismiss the Chapter 11 Case and retain jurisdiction to decide matters related to claims brought in the Bankruptcy Case by Cardium. On December 8, 2011, the Bankruptcy Court entered an order dismissing the Chapter 11 Case. In connection with the dismissal of the Chapter 11 Case, the Bankruptcy Court, among other things, declined to retain jurisdiction over claim objection proceedings and found moot our objection to certain claims to receive a break-up fee pursuant to the Asset Purchase Agreement of Cardium Therapeutics, Inc. and Cardium Healthcare, Inc., a wholly owned subsidiary of Cardium. The dismissal of the Chapter 11 Case was based upon the provisions of both 11 U.S.C. Sections 305(a) and 1112(b).
NOTE 5. NOTES PAYABLE
Convertible Notes – August 2005
In August 2005, the Company issued seven convertible promissory notes in the aggregate amount of $226,300 to various stockholders (collectively, the “Stockholders’ Notes”). The Stockholders’ Notes bore interest at 4% per annum and were to mature on August 25, 2010. In connection with the issuance of the Stockholders’ Notes, the Company granted warrants that were exercisable into an aggregate of 4,443 shares of the Company’s common stock. The warrants were determined to have an insignificant fair value at the time of the grant.
In May 2007, the holders of the Stockholders’ Notes and related warrants forgave the amounts due and forfeited the related warrants. In connection with the forgiveness, the Company recorded additional paid-in capital of $241,701 equal to the value of the Stockholders’ Notes and related accrued interest. Interest expense on the Stockholders’ Notes was $15,401 for the period from Inception through December 31, 2007.
F-27
Convertible Notes – May and June 2007
In May and June 2007, the Company issued convertible notes payable to various lenders for an aggregate amount of $1,500,000 (collectively, the “2007 Notes”). Each of the 2007 Notes included interest at 7% per annum and was to mature on December 16, 2007 (“Maturity Date”). However, as a result of the Merger and Private Placement (see Note 6), the entire outstanding principal amount and accrued interest was converted into the Company’s common stock at a conversion price equal to $8.00 per share, which resulted in the issuance of 191,272 shares. Also, the Company recorded a debt discount of $1,530,177, which was amortized immediately to interest expense upon the conversion of the 2007 Notes. Excluding the debt discount, interest expense on the 2007 Notes was $30,177 for the period from Inception through December 31, 2008.
Convertible Note – April 2010
On April 5, 2010, the Company issued a Senior Convertible Promissory Note (the “Note”) to an existing investor through a private placement. The Note includes an annual interest rate of 7.5 percent and (unless converted or prepaid, as noted below) all principal and interest are due and payable on its maturity date April 5, 2012 (“Maturity Date”). At any time prior to the Maturity Date, the investor may convert all or a portion of the outstanding principal and accrued interest at a conversion ratio of one share of Imprimis’ common stock for each $1 (the fair market value of the Company’s common stock on April 5, 2010) owed. Also, at any time prior to the Maturity Date, the Company has the option to prepay the outstanding principal and accrued interest. The Company received gross proceeds from the issuance of the Note in the aggregate amount of $1,000,000. There were no discounts or commissions paid in connection with this private placement. Accrued interest on the Note was $130,479 and $55,479 at December 31, 2011 and 2010, respectively, and interest expense on the Note was $75,000 and $55,479 for the years ended December 31, 2011 and 2010, respectively. Following the Company’s bankruptcy petition filed on June 26, 2011, and the change in ownership control following the issuance of preferred stock, the entire unpaid principal sum of this Note, together with its accrued and unpaid interest became immediately due and payable. Subsequent to the year ended December 31, 2011, the Company, the noteholder and its assignee entered into a waiver and settlement agreement described in Note 14.
Secured Line of Credit – Related Party
On November 21, 2011, the Company entered into a Secured Line of Credit Letter Agreement (the “Line of Credit Agreement”) with DermaStar International, LLC (“DermaStar”). The Line of Credit Agreement became effective on December 9, 2011, in connection with the dismissal of the Chapter 11 Case by the Bankruptcy Court. On December 9, 2011, as required by the Line of Credit Agreement, the Company entered into a Security Agreement and an Intellectual Property Security Agreement with DermaStar, pursuant to which the Company granted to DermaStar a blanket security interest in all of its assets, including its intellectual property. The Line of Credit Agreement provides for advances to the Company of up to an aggregate of $750,000 (each an “Advance” and collectively the “Loan”), subject to the satisfaction by the Company of certain conditions in connection with the initial Advance and each subsequent Advance. Each Advance will be made pursuant to a Promissory Note in favor of DermaStar. The Company has received advances totaling $300,000 at December 31, 2011. The Promissory Notes accrue interest at 10% annually and mature one year after the effective dates of the respective advance.
DermaStar, and its members individually, are control persons of the Company, as they have the ability to direct or cause direction of management and policies of the Company through their ownership. Also Dr. Robert J. Kammer, a director of the Company, and Mark L. Baum, Esq., Executive Chairman of the Company, are managing members and partial owners of DermaStar.
F-28
Notes Payable consists of the following
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
10% note payable due December 2012
|
$ | 300,000 | $ | - | ||||
|
7.5% convertible note
|
1,000,000 | 1,000,000 | ||||||
|
Total convertible notes payable
|
$ | 1,300,000 | $ | 1,000,000 | ||||
|
Less: Current portion
|
(1,300,000 | ) | - | |||||
|
Long-term portion
|
$ | - | $ | 1,000,000 | ||||
NOTE 6. STOCKHOLDERS’ EQUITY
Preferred Stock
At December 31, 2011, the Company had 5,000,000 shares of preferred stock, $0.001 par value, authorized and 10 shares issued and outstanding.
On December 9, 2011, the Company filed a Certificate of Designation to its Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware (the “Certificate of Designation”), setting forth the rights and preferences of the Series A Preferred Stock. Among other things, the Certificate of Designation (i) authorizes 10 shares of the Company’s preferred stock to be designated as “Series A Convertible Preferred Stock” (“Series A Preferred Stock”); (ii) grants the holders of the Series A Preferred Stock the right to convert into the Company’s Common Stock at a conversion price of approximately $0.013336, as adjusted; (iii) grants a liquidation preference of $10,000 per share of Series A Preferred Stock; (iv) provides that the holders of Series A Preferred Stock shall vote with the holders of the Company’s common stock on an “as converted basis”; and (v) provides that the affirmative vote of a majority of the outstanding shares of the Series A Preferred Stock is required to approve certain other corporate matters including, among other things, changes to the rights of the holders of the Series A Preferred Stock, amendments to the Company’s Certificate of Incorporation or Bylaws, issuance of priority or parity securities, issuance of debt securities, entry into certain fundamental transactions and increase or decrease the size of the Board of Directors of the Company.
In partial consideration for and in connection with the Line of Credit Agreement described in Note 5, on November 21, 2011, the Company entered into a Securities Purchase Agreement (the “Purchase Agreement”) with DermaStar, pursuant to which the Company agreed to issue 10 shares of newly-designated Series A Preferred Stock to DermaStar for an aggregate purchase price of $100,000. The Purchase Agreement, as amended, became effective on December 9, 2011, in connection with the dismissal of the Chapter 11 Case by the Bankruptcy Court. On December 12, 2011, the Company and DermaStar consummated the transactions contemplated by the Purchase Agreement. On December 31, 2011 and made effective November 21, 2011, the Company entered into a First Amendment to Securities Purchase Agreement (the “Amendment”). Pursuant to the terms of the Amendment, DermaStar agreed not to convert more than 5 shares of Series A Preferred Stock into common stock until such time as the Company has a sufficient number of authorized shares of common stock to enable the conversion of all ten shares of Series A Preferred Stock held by DermaStar. The five shares of preferred stock can be converted into 7,498,500 shares of common stock, which represents approximately 65% of the capital stock of the Company on an as-converted basis.
The Company recorded a beneficial conversion feature of $100,000 to the preferred share purchase and recorded a preferred stock discount. As the preferred shares do not have a stated redemption date, the associated discount was amortized from the date of issuance to the earliest possible conversion date, which is the date of issuance and recognized as a deemed dividend to the preferred stockholders using the effective yield method. Accordingly, the Company recorded non-cash accretion of preferred stock deemed dividend totaling $100,000 in 2011, which represents an increase to reported net loss in arriving at net loss attributable to common stockholders and additional paid-in capital by a corresponding $100,000. The non-cash accretion of the preferred stock deemed dividend does have an effect on net loss or cash flows for the year ended December 31, 2011.
Upon issuance of the Series A Preferred Stock, DermaStar, and its members individually, became control persons of the Company. Also Dr. Robert J. Kammer, a director of the Company, and Mark L. Baum, Esq., Executive Chairman of the Company, are managing members and partial owners of DermaStar.
F-29
Common Stock
The following is a summary of common stock and capital contribution transactions from inception through December 31, 2011:
|
-
|
In fiscal year 1998, the Company recorded capital contributions of $100,000 (the estimated fair value of the services contributed) in connection with services contributed by stockholders, which is recorded respectively in selling, general and administrative and research and development expenses in the accompanying consolidated statements of operations.
|
|
-
|
In fiscal year 1999, the Company recorded capital contributions of $200,000 (the estimated fair value of the services contributed) in connection with services contributed by stockholders, which is recorded respectively in selling, general and administrative and research and development expenses in the accompanying consolidated statements of operations.
|
|
-
|
In fiscal year 2000, the Company issued 117,188 shares of common stock at a price of $0.0512 per share for proceeds of $6,000. Also, recorded capital contributions of $200,000 (the estimated fair value of the services contributed) in connection with services contributed by stockholders, which is recorded respectively in selling, general and administrative and research and development expenses in the accompanying consolidated statements of operations.
|
|
-
|
In fiscal year 2001, the Company recorded capital contributions of $200,000 (the estimated fair value of the services contributed) in connection with services contributed by stockholders, which is recorded respectively in selling, general and administrative and research and development expenses in the accompanying consolidated statements of operations.
|
|
-
|
In fiscal year 2002, the Company recorded capital contributions of $200,000 (the estimated fair value of the services contributed) in connection with services contributed by stockholders, which is recorded respectively in selling, general and administrative and research and development expenses in the accompanying consolidated statements of operations.
|
|
-
|
In fiscal year 2003, the Company recorded capital contributions of $200,000 (the estimated fair value of the services contributed) in connection with services contributed by stockholders, which is recorded respectively in selling, general and administrative and research and development expenses in the accompanying consolidated statements of operations.
|
|
-
|
In fiscal year 2004, the Company recorded capital contributions of $400,000 (the estimated fair value of the services contributed) in connection with services contributed by stockholders, which is recorded respectively in selling, general and administrative and research and development expenses in the accompanying consolidated statements of operations.
|
|
-
|
In fiscal year 2005, the Company issued 308,594 shares of common stock at a price of $0.0512 per share for gross proceeds of $15,800 for common stock purchases and stock option exercises. The Company received additional capital contributions in cash of $14,200 from the Company’s stockholders and recorded capital contributions of $400,000 (the estimated fair value of the services contributed) in connection with services contributed by stockholders, which is recorded respectively in selling, general and administrative and research and development expenses in the accompanying consolidated statements of operations.
|
|
-
|
In fiscal year 2006, the Company issued 46,875 shares of common stock at a price of $0.0512 per share for gross proceeds of $2,400. The Company received additional capital contributions in cash of $48,600 from the Company’s stockholders and recorded capital contributions of $400,000 (the estimated fair value of the services contributed) in connection with services contributed by stockholders, which is recorded respectively in selling, general and administrative and research and development expenses in the accompanying consolidated statements of operations.
|
|
-
|
Prior to the Merger during fiscal year 2007, the Company issued 498,047 shares of its common stock at a price of $0.0512 per share for proceeds of $25,750, which includes the issuance of 31,250 shares upon the exercise of a warrant and 7,813 shares upon exercise of stock options. Also, prior to the Merger, the Company received capital contributions of $105,907 from the Company’s stockholders and recorded capital contributions of $175,000 (the estimated fair value of the services contributed) in connection with services contributed by stockholders, which is recorded respectively in selling, general and administrative and research and development expenses in the accompanying consolidated statements of operations.
|
F-30
|
-
|
Prior to the Merger during fiscal year 2007, the Company recorded additional paid-in capital of $241,701 related to the forgiveness of Stockholders’ Notes (see Note 5).
|
|
-
|
In August 2007, the Company issued a restricted stock grant to an executive of the Company for 195,313 shares of the Company’s common stock.
|
|
-
|
In connection with the Merger in 2007, 231,249 of Imprimis common shares remained outstanding (see Note 3).
|
|
-
|
Concurrent with the Merger in 2007, the Company sold 258,979 shares of common stock for net proceeds of $3,837,791 ($4,143,667 gross) through a private placement (the “Private Placement”). In addition, the investors received warrants to purchase 64,745 shares of common stock for a period of five years at a cash and cashless exercise price of $32.00 and $40.00 per share, respectively. In connection with the Private Placement, the Company incurred placement agent fees and other related expenses totaling $342,105 (of which $36,229 was paid in fiscal year 2008 and netted with the 2008 private placement discussed below) and issued warrants to purchase up to 33,750 shares of common stock for a period of three years at cash and cashless exercise price of $32.00 and $40.00 per share, respectively.
|
|
-
|
Concurrent with the Merger in 2007, the Company issued 191,272 shares of common stock related to the conversion of the 2007 Notes and accrued interest of $1,530,177 (see Note 5). Also, the Company recorded a debt discount of $1,530,177 related to the 2007 Notes (see Note 5).
|
|
-
|
In September 2007, the Company entered into three, one-year consulting agreements with three separate firms to provide services related to investor communications. In the aggregate, 34,375 shares of common stock were issued in accordance with the terms of the agreements along with a warrant to purchase 2,344 shares of common stock for a period of five years at a cash and cashless exercise price of $32.00 and $40.00, respectively. The fair value of the stock and warrants were valued at $550,000. The estimated costs of the consulting agreements, including the stock, warrants and non-refundable fee were amortized over the one-year terms.
|
|
-
|
On May 12, 2008, the Company sold 227,273 shares of common stock for net proceeds of $3,941,301 ($4,000,000 gross) through a follow-on private placement (the “Follow-on Private Placement”) to accredited investors. In addition, the investors received warrants to purchase 28,409 shares of common stock for a period of five years at a cash and cashless exercise price of $35.20 and $44.00 per share, respectively. In connection with the Follow-On Private Placement, the Company incurred expenses of $22,470, which was recorded as a reduction of additional paid-in capital, and the gross proceeds were also netted with $36,229 related to the 2007 private placement that was paid in 2008.
|
|
-
|
In 2008, in connection with the termination of certain consulting agreements entered into in 2007 and 2008, 10,321 shares of common stock were forfeited at a value that was reversed of $135,136. The Company also decreased additional paid-in capital and consulting expense by $70,000 because of the remeasurement of certain consulting agreements. Additionally, during 2008, the Company entered into an agreement with an investor relations firm (“IR Firm”). Pursuant to the agreement with the IR Firm, the Company issued 8,583 shares of common stock during 2008 at a value of $85,833. In a separate agreement, the Company entered into a consulting agreement in which the Company issued a three-year warrant to purchase 625 shares of the Company’s common stock at a cash and cashless price of $16.00 per share. The fair value of the warrant, determined based on the Black-Scholes pricing model, was valued at $1,310. The net amount of shares forfeited during 2008 from consulting agreements and the IR Firm was (13,901) and the net expense reversed and charged to additional paid-in capital was ($117,993).
|
|
-
|
On November 21, 2008, the Company issued a restricted stock grant to a director of the Company for 3,125 shares of the Company’s common stock. The restricted stock grant vested over a one-year period.
|
F-31
|
-
|
During 2009, in connection with the agreement with the IR Firm, the Company issued 5,722 shares of common stock valued at $50,356. In a separate agreement, the Company entered into a consulting agreement in which the Company issued a stock option to purchase 6,250 shares of the Company’s common stock at an exercise price of $7.92 per share. The fair value of the option, determined based on the Black-Scholes pricing model, was recorded as $14,434. In another agreement, the Company entered into a consulting agreement in which the Company issued stock options to purchase 5,938 shares of the Company’s common stock at an exercise price of $12.80 per share. The fair value of the options, determined based on the Black-Scholes pricing model, was recorded at $56,665. The total value of common stock, warrants and options recorded during 2009 was $121,455.
|
|
-
|
In August 2009, the Company issued 6,250 shares of common stock at a price of $7.92 per share for gross proceeds of $49,500 for stock option exercises.
|
|
-
|
In June 2010, the Company entered into two separate agreements with an investor relations firm and a financial advisory services firm (collectively “the firms”) in order to provide certain investor relations and advisory services to the Company for a period of one year. In exchange for such services, the Company issued 25,000 shares, in the aggregate, of its unregistered common stock, of which all shares were nonforfeitable (valued at $208,000 and recorded as prepaid consulting fees upon issuance) to the firms as a prepayment of services to be received over a three-month period. The Company agreed to suspend the services related to these agreements, therefore, at this time no additional shares of common stock will be issued to the firms. For the year ended December 31, 2010, the Company recorded stock-based compensation related to the stock of $208,000. On August 13, 2010, the Company entered into a consulting agreement in which the Company issued stock options to purchase 201,217 shares of the Company’s common stock at an exercise price of $8.56 per share (see Note 7). The fair value of the options, determined based on the Black-Scholes pricing model, was recorded at $132,300. In September 2010, the Company entered an agreement with an investor relations firm in order to provide certain investor relations services to the Company for a period of six months. In exchange for such services, the Company issued 3,750 shares, in the aggregate, of its unregistered common stock, of which all shares were nonforfeitable (valued at $27,600 and recorded as prepaid consulting fees upon issuance) to the investor relations firm as a prepayment of services to be received for the initial three-month period of the agreement. The agreement was terminated by the Company during November 2010. For the year ended December 31, 2010, the Company recorded stock-based compensation related to the restricted stock of $27,600. The total number of shares issued to consultants during 2010 was 230,000 and the total value of common stock and options issued to consultants during 2010 was $367,900.
|
|
-
|
On October 20, 2010, the Company appointed John N. Bonfiglio, Ph.D. as Chief Executive Officer and President of the Company. Dr. Bonfiglio was also appointed as a director on the Company’s Board. The Board granted Dr. Bonfiglio a stock option for 50,000 shares of common stock and issued 6,250 shares of restricted common stock in accordance with the Company’s 2007 Incentive Stock and Awards Plan. The stock option and the restricted common stock vested as follows: 25% of the option shares and the restricted stock vested immediately upon grant, with the balance of the option shares and the restricted stock vesting in equal monthly installments over the next 36 months beginning 30 days after the grant date. The restricted stock was valued at $6.40 per share, the reported closing price of the Company’s common stock on October 20, 2010. For the year ended December 31, 2010, the Company recorded stock-based compensation expense related to the issuance and partial vesting of the restricted stock award of $12,083.
|
|
-
|
On May 13, 2011, the Board accepted the resignation of Dr. Bonfiglio, Ph.D. as Chief Executive Officer and President of the Company and as a director on the Board. As a result of Dr. Bonfiglio’s resignation, of the 6,250 shares of restricted stock awarded to him, 2,344 shares had vested and 3,906 shares were returned to treasury and cancelled effective his date of resignation. For the year ended December 31, 2011, the Company recorded stock-based compensation expense related to the issuance and partial vesting of the restricted stock award of $3,332.
|
|
-
|
On October 5, 2011, the Company issued 37,500 stock options to former employees, valued at $11,400 (see Note 7).
|
F-32
For the year ended December 31, 2011, the Company recorded $177,421 of stock-based compensation expense for employee options, $11,400 of compensation expense to employees paid in stock options in lieu of cash and $3,332 of stock-based compensation expense related to the vesting of restricted stock (total expense of $192,153). For the year ended December 31, 2010, the Company recorded $535,812 of stock-based compensation expense for employee options, $12,083 of stock-based compensation expense related to the vesting of restricted stock, $132,300 of expense for stock options issued for consulting services (total expense of $680,195 related to stock options and restricted stock for employees and consultants) and $235,600 of expense for stock issued for consulting services. For the period from Inception through December 31, 2011, the Company recorded stock-based compensation expense for employees, directors and consultants of $2,128,816, respectively, for options and restricted stock granted and vested. The expense for options and restricted stock issued to employees and consultants included in selling, general and administrative expenses and research and development expenses for the years ended December 31, 2011 and 2010 and the period from inception through December 31, 2011 is $154,399 and $37,754, $579,592 and $100,603, and $1,514,145, and $614,671, respectively. For the years ended December 31, 2011 and 2010 and for the period from Inception through December 31, 2011, the Company amortized $0, $235,600 and $807,608, respectively, of prepaid consulting fees which is included as part of selling, general and administrative expenses.
NOTE 7. STOCK OPTION PLAN
On September 17, 2007, the Company’s Board of Directors and stockholders adopted the 2007 Incentive Stock and Awards Plan (the “Plan”), which provides for the issuance of a maximum of an aggregate of 3,000,000 (as amended on November 5, 2008) shares of Common Stock. The purpose of the Plan is to provide an incentive to attract and retain directors, officers, consultants, advisors and employees whose services are considered valuable, to encourage a sense of proprietorship and to stimulate an active interest of such persons into the Company’s development and financial success. Under the Plan, the Company is authorized to issue incentive stock options intended to qualify under Section 422 of the Code, non-qualified stock options and restricted stock. The Plan will be administered by the Company’s Board of Directors until such time as such authority has been delegated to a committee of the board of directors.
A summary of the Plan for the year ended December 31, 2011 is as follows:
|
Number of shares
|
Weighted Avg. Exercise Price
|
Weighted Avg. Remaining Contractual Life
|
Aggregate Intrinsic Value
|
|||||||||||||
|
Outstanding - January 1, 2011
|
313,277 | $ | 10.96 | |||||||||||||
|
Granted
|
37,500 | 0.80 | ||||||||||||||
|
Exercised
|
- | - | ||||||||||||||
|
Cancelled/Forfeited
|
(200,625 | ) | 10.08 | |||||||||||||
|
Outstanding - December 31, 2011
|
150,152 | $ | 9.68 | 3.28 | $ | 6,000 | ||||||||||
|
Exercisable - December 31, 2011
|
146,152 | $ | 9.76 | 3.18 | $ | 6,000 | ||||||||||
|
Vested and expected to vest - December 31, 2011
|
149,752 | $ | 9.68 | 3.27 | $ | 6,000 | ||||||||||
The aggregate intrinsic value in the table above represents the total pre-tax amount of the proceeds, net of exercise price, which would have been received by option holders if all option holders had exercised and immediately sold all options with an exercise price lower than the market price on December 31, 2011, based on the closing price of the Company’s common stock of $0.96 on that date.
F-33
The options were granted to the employees, directors and consultants at exercise prices that ranged from $0.80 to $20.96, the estimated fair market value of the common stock on the dates of issuance. All options granted prior to 2011 expire on the ten year anniversary of the issuance date and were vested immediately or on a quarterly basis up to five years. The Company uses the Black-Scholes option pricing model to estimate the grant-date fair value of share-based awards. The Black-Scholes model requires subjective assumptions regarding future stock price volatility and expected time to exercise, along with assumptions about the risk-free interest rate and expected dividends, which affect the estimated fair values of the Company’s stock-based awards. The expected term of options granted was determined in accordance with the “simplified approach” as the Company has very limited historical data on employee exercises and post-vesting employment termination behavior. The expected volatility is based on the historical volatilities of the common stock of comparable publicly traded companies based on the Company’s belief that it currently has limited historical data regarding the volatility of its stock price on which to base a meaningful estimate of expected volatility. The risk-free rate selected to value any particular grant is based on the U.S. Treasury rate that corresponds to the expected term of the grant effective as of the date of the grant. The Company used 0% as an expected dividend yield assumption. These factors could change in the future, affecting the determination of stock-based compensation expense in future periods. Utilizing these assumptions, the fair value is determined at the date of grant. The weighted fair value of the stock options granted during 2011 is $0.32. For the years ended December 31, 2011 and 2010 and for the period from inception through December 31, 2011, the Company recorded stock-based compensation related to stock options and restricted stock for employees and directors of $192,153, $547,895 and $1,461,165, respectively which is included in selling, general and administrative expenses and research and development expenses in the amount of $154,399 and $37,754, $447,292 and $100,603, and $1,024,102 and $437,063, respectively. The Company cancelled 200,625 stock options during the year ended December 31, 2011. These options were cancelled due to the resignation of the optionees during the fiscal year.
The financial statement effect of forfeitures is estimated at the time of grant and revised, if necessary, if the actual effect differs from those estimates. For option grants to employees and directors in 2010, the Company assigned a forfeiture factor of 10%. This percentage was determined based on consideration of actual forfeitures realized to date and estimated forfeitures to potentially occur in the future. All option grants during 2011 were immediately exercisable; therefore, there was no forfeiture factor assigned.
As of December 31, 2011, there was approximately $14,094 of total unrecognized compensation expense related to unvested stock options under the Plan. That expense is expected to be recognized over the weighted-average period of 1.89 years.
Effective February 17, 2010, the Board of Directors of the Company accepted the resignation of Dr. Juliet Singh as Chief Executive Officer of the Company and as a director on the Board. In connection with Dr. Singh’s resignation, the Company and Dr. Singh entered into a separation agreement that provided Dr. Singh with one year of continued salary in accordance with the terms of her existing employment agreement as well as the accelerated vesting of 37,500 stock options previously granted. In addition, the term in which Dr. Singh may exercise the vested options (which included 76,250 options in total, comprised of 38,750 stock options that were vested as of the separation date as well as the 37,500 stock options subject to the accelerated vesting) was modified and extended to three years from the date of her resignation. In accordance with accounting guidance, since these stock options were modified, the value of the modification for each stock option was determined. For the stock options vested as of the separation date, the modified value was equal to the number of options multiplied by the difference in value (per the Black-Scholes option pricing model) between the original and modified terms of the stock options utilizing current values for market stock price, interest rate and volatility. For the stock options in which the vesting was accelerated, the new value for these stock options was calculated as of the separation date using the Black-Scholes option pricing model. In total, the additional stock based compensation expense recognized for the modified stock options was approximately $174,000 and was recorded in stock-based compensation in additional paid-in capital and general and administrative expenses in the accompanying consolidated balance sheets and consolidated statement of operations as of and for the year ended December 31, 2010, respectively.
On February 26, 2010, the Company’s Board of Directors granted 37,500 stock options to an executive officer of the Company under the Company’s 2007 Incentive Stock and Awards Plan. All of the options were granted with an exercise price of $7.20 and have a ten year life. Also, the options vest one-twelfth per quarter commencing on the first full quarter after the initial grant date of February 26, 2010.
F-34
On August 13, 2010, the Company entered into a consulting agreement (previously approved by the Board of Directors) with a retained search firm to provide the Company with executive recruitment services. In accordance with the agreement, the Company had the option to pay for such services in cash or by issuing stock options of an equivalent value. Per the agreement, 50% of the fee (deemed non-refundable) was due upon execution of the agreement and the remaining 50% was due if and when the retained search firm placed a candidate with the Company. The total fee ultimately owed to the retained search firm would not be finalized until an executive was hired as it will be based on the total compensation for the executive in the first year of employment. It was agreed between the retained search firm and the Company that the value of the stock option as of the execution of the agreement would be the basis for determining the number of stock options to be issued for the initial fee as well as in the total fee due to the retained search firm. The option value was determined to be $5.26 based on the Black-Scholes pricing model using an exercise price of $8.56. Using an estimated first year salary (including bonus) of $350,000, the total fee was estimated to be $105,000. As noted above, the Company was obligated to pay 50% of the estimated total fee, or $52,500, upon execution of the agreement, which the Company opted to issue a non-qualified stock option in lieu of cash. Therefore, the Company issued a non-qualified stock option, under the Plan, to purchase up to 10,000 shares of common stock in payment of this initial fee. The stock option is non-refundable and therefore, fully vested upon issuance. As a result, the total value of the fee/option was recognized in August 2010. Effective October 20, 2010, the Board of Directors appointed a new president and chief executive officer that was a candidate referred to the Company from the retained search firm (see below). The total first year compensation for the executive was estimated to be $441,000, therefore, the final fee due to the retained search firm was $132,300. The Company opted to pay the remainder of the fee due with a non-qualified stock option. Considering the option issued in August 2010 for the purchase of up to 10,000 shares of common stock, the final stock option issued in October 2010 was to purchase an additional 15,152 shares of common stock. The value of this stock option representing the remainder of the fee, $79,800, was recognized in October 2010. For the years ended December 31, 2011 and 2010 and the period from Inception through December 31, 2011, the Company recorded stock-based compensation related to these stock options of $0, $132,300 and $132,300, respectively.
Effective October 20, 2010, the Company appointed John N. Bonfiglio, Ph.D. as Chief Executive Officer and President of the Company. Dr. Bonfiglio was also appointed as a director on the Company’s Board. The Board granted Dr. Bonfiglio a stock option for 50,000 shares of common stock and issued 6,250 shares of restricted common stock in accordance with the Company’s 2007 Incentive Stock and Awards Plan. The stock option and the restricted common stock will vest as follows: 25% of the option shares and the restricted stock shall vest immediately upon grant, with the balance of the option shares and the restricted stock vesting in equal monthly installments over the next 36 months beginning 30 days after the grant date; provided, however, Dr. Bonfiglio shall gain a vested interest in an additional 10% of the option shares and the restricted stock upon the closing of a Qualified Transaction. The exercise price of the stock option will be $6.40 per share, the reported closing price of the Company’s common stock on October 20, 2010. The vesting of all options will fully accelerate upon an involuntary termination of Dr. Bonfiglio’s employment within twelve months following a change of control (as such terms are defined in the Employment Agreement). Effective May 13, 2011, this individual resigned and all options granted to the employee have been cancelled.
On October 5, 2011, priority claims of former employees in the amount of $119,667 originating as a result of the Company’s Bankruptcy petition filed June 26, 2010 (the “Priority Claimants”), were settled and paid by the Company. These amounts consisted of accrued and owed payroll amounts, accrued vacation and any other claims held against the Company at October 5, 2011. The Priority Claimants were given cash in the amount $47,975 and 37,500 stock options valued at $11,400 and the difference of $60,292 was recognized as a gain on forgiveness of liabilities during the year ended December 31, 2011. These options have an exercise price of $0.80, vested immediately upon issuance, and have a three year life from the date of issuance.
F-35
The table below illustrates the fair value per share and Black-Scholes option pricing model with the following assumptions used for the grants issued to the employees and directors during the years ended December 31, 2011 and 2010
| 2011 | 2010 | |||||||
|
Weighted-average fair value of options granted
|
$ | 0.32 | $ | 4.48 | ||||
|
Expected terms (in years)
|
3.0 | 6.0 | ||||||
|
Expected volatility
|
85 | % | 75 | % | ||||
|
Risk-free interest rate
|
0.46 | % | 2.02 | % | ||||
|
Dividend yield
|
- | - | ||||||
No options were issued to consultants during the year ended December 31, 2011.
The table below illustrates the fair value per share and Black-Scholes option pricing model with the following assumptions used for the grants issued to the consultants during the year ended December 31, 2010:
|
2010
|
||||
|
Weighted-average fair value of options granted
|
$ | 5.28 | ||
|
Expected terms (in years)
|
5.0 | |||
|
Expected volatility
|
75 | % | ||
|
Risk-free interest rate
|
1.63 | % | ||
|
Dividend yield
|
- | |||
NOTE 8. WARRANTS
A summary of the status of the warrants for the year ended December 31, 2011 is as follows:
|
Number of Shares Subject to Warrants Outstanding
|
Weighted Avg. Exercise Price
|
|||||||
|
Warrants outstanding - January 1, 2011
|
96,123 | $ | 32.80 | |||||
|
Granted
|
- | |||||||
|
Exercised
|
- | |||||||
|
Expired
|
(625 | ) | 16.00 | |||||
|
Warrants outstanding and exercisable - December 31, 2011
|
95,498 | $ | 33.16 | |||||
|
Weighted average remaining contractual life of the outstanding warrants in years - December 31, 2011
|
0.91 | |||||||
F-36
The expiration of the outstanding warrants at December 31, 2011 occurs through May 2013 at various dates.
On April 24, 2008, the Company entered into a one-year consulting agreement with a firm to provide the Company with financial advisory services. As compensation for the services, the Company issued a three-year warrant to purchase 625 shares of the Company’s common stock at a cash and cashless price of $16.00 per share. The fair value of the warrant, determined based on the Black-Scholes pricing model, this warrant expired during the year ended December 31, 2011.
NOTE 9. INCOME TAXES
The Company is subject to taxation in the United States and California. The Company does not have any income tax provision for the years ended December 31, 2011 and 2010 due to current and historical losses.
The provision for income taxes using the statutory federal income tax rate of 34% as compared to the company’s effective tax rate is summarized as follows:
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Federal tax benefit at statutory rate
|
$ | (319,489 | ) | $ | (842,257 | ) | ||
|
State tax benefit, net
|
(58,881 | ) | (161,372 | ) | ||||
|
Research and development credits
|
(10,123 | ) | (39,517 | ) | ||||
|
Employee stock-based compensation
|
- | - | ||||||
|
Other differences
|
- | 46 | ||||||
|
Change in valuation allowance
|
388,493 | 1,043,100 | ||||||
|
Provision for income taxes
|
$ | - | $ | - | ||||
At December 31, 2011 and 2010, the Company had deferred tax assets of $6,167,481 and $5,778,988, respectively. Due to uncertainties surrounding the Company’s ability to generate future taxable income to realize these assets, a full valuation allowance has been established to offset the net deferred tax asset. Additionally, the future utilization of the Company’s net operating loss to offset future taxable income may be subject to an annual limitation, pursuant to Internal Revenue Code Section 382, as a result of ownership changes that may have occurred previously or that could occur in the future. The Company has not performed a Section 382 analysis to determine the limitation of the net operating loss and research and development credit carry forwards.
Significant components of the company’s deferred tax assets are as follows:
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Deferred tax assets
|
||||||||
|
Federal and state net operating loss carryforwards
|
$ | 4,886,429 | $ | 4,565,466 | ||||
|
Stock-based compensation
|
743,789 | 671,940 | ||||||
|
Tax credits
|
532,278 | 522,155 | ||||||
|
Other
|
4,985 | 19,427 | ||||||
|
Total deferred tax assets
|
6,167,481 | 5,778,988 | ||||||
|
Less: Valuation allowance
|
(6,167,481 | ) | (5,778,988 | ) | ||||
|
Net deferred income tax asset
|
$ | - | $ | - | ||||
F-37
Realization of the deferred tax assets is dependent upon the generation of future taxable income, the amount and timing of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by approximately $388,000 and $1.0 million in 2011 and 2010, respectively.
As of December 31, 2011, the Company had federal and California net operating loss carryforwards of approximately $12.3 million and $12.2 million, respectively. The federal and California tax loss carry forwards will begin to expire in 2020 and 2015, respectively, unless previously utilized. The Company estimates its federal and California research and development tax credit carryforwards of approximately $315,000 and $330,000, respectively, which begin to expire in 2027 unless previously utilized.
A portion of the net operating loss carry forwards as of December 31, 2011 and 2010 include amounts related to stock option deductions. Excess tax benefits, if any, from share-based compensation are only realized when income taxes payable is reduced, with the corresponding credit posted to Additional Paid-in Capital.
The impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has 50% or less likelihood of being sustained upon examination. The Company believes that its income tax filing positions and deductions will be sustained on audit and does not anticipate any adjustments that will result in a material change to its financial position. Therefore, no reserves for uncertain income tax positions have been recorded.
The Company’s practice is to recognize interest and/or penalties related to income tax matters in income tax expense. The Company had no accrual for interest or penalties at December 31, 2011 and 2010, and has not recognized interest and/or penalties in the consolidated statements of operations for the years ended December 31, 2011 and 2010. The Company’s tax years for 2000 and forward are subject to examination by the United States and state tax authorities due to the carry forward of unutilized net operating losses
NOTE 10. COMMITMENTS AND CONTINGENCIES
Commitments
The Company leased its office facilities under a noncancelable operating lease, which expired December 31, 2010. The Company renewed the lease from January 1, 2011 to June 30, 2011, with a monthly amount due of $3,835. Rent expense for the years ended December 31, 2011, 2010 and the period from Inception through December 31, 2011 was $18,299, $54,821 and $243,955, respectively. The Company entered into a new lease agreement for office facilities from February 15, 2012 to February 28, 2014. Monthly rent begins on March 1, 2012 in the amount of $2,972 for the first 12 months, and $3,715 is due monthly for the next 12 months.
Indemnities and Guarantees
In addition to the indemnification provisions contained in the Company’s charter documents, the Company will generally enter into separate indemnification agreements with the Company’s directors and officers. These agreements require the Company, among other things, to indemnify the director or officer against specified expenses and liabilities, such as attorneys’ fees, judgments, fines and settlements, paid by the individual in connection with any action, suit or proceeding arising out of the individual’s status or service as the Company’s director or officer, other than liabilities arising from willful misconduct or conduct that is knowingly fraudulent or deliberately dishonest, and to advance expenses incurred by the individual in connection with any proceeding against the individual with respect to which the individual may be entitled to indemnification by the Company. The Company has also entered into an indemnification agreement with DermaStar as a secured lender. This agreement requires the Company, among other things, to indemnify DermaStar, and any of its directors or officers as individuals, against specified expenses and liabilities, such as attorneys’ fees in connection with the preparation, amendment, appraisal, audit, modification, waiver, of the Line of Credit Agreement and enforcement of any rights/interest under the Line of Credit Agreement. These guarantees and indemnities do not provide for any limitation of the maximum potential future payments the Company could be obligated to make. Historically, the Company has not been obligated nor incurred any payments for these obligations and, therefore, no liabilities have been recorded for these indemnities and guarantees in the accompanying consolidated balance sheets.
F-38
Cato Research Ltd. Agreement
In accordance with the Master Services Agreement, dated April 10, 2007, between the Company and Cato Research Ltd. (“Cato”), a contract research and development organization, the Company entered into a clinical trial services agreement (“Agreement”) with Cato on June 10, 2008. Under the Agreement, Cato served as the Company’s strategic partner and contract research organization in conducting the Company’s Phase 3 clinical trial for Impracor . As of December 31, 2009, the Company incurred approximately $3.2 million (original estimate of costs was $3.3 million) related to Cato’s fees as well as pass-through costs incurred by Cato or payable to the clinical sites for patients enrolled in the study. The Company does not anticipate incurring any additional costs related to this Agreement.
Cosmetic Products Consulting Agreement
On August 25, 2008, the Company entered into an agreement with RIL-NA, LLC (“RIL-NA”) in order to enter into business relationships with third parties for certain of the Company’s cosmetic product formulations. RIL-NA was to be paid a commission equal to approximately 20% of the adjusted gross revenues realized from transactions related to this agreement. This agreement was terminable with 60 days written notice by either RIL-NA or the Company. On June 12, 2011, the Company entered into another agreement with RIL-NA whereby RIL-NA paid approximately $5,000 in related legal filing fees to acquire exclusive marketing rights for the Company’s anti-cellulite product formulation from June 13, 2011 through August 11, 2011. This agreement automatically terminated on August 12, 2011, and no revenues or amounts were paid to or on behalf of the Company.
Cosmetic Product License Agreements
On May 20, 2009, the Company and JH Direct, LLC (“JH Direct”) entered into a licensing agreement providing JH Direct with the exclusive worldwide rights to the Company’s anti-cellulite cosmetic product which utilizes the Company’s patented transdermal delivery system technology, Accudel. Under the terms of the agreement, JH Direct will pay the Company initial royalty advances and a continuing licensing royalty on the worldwide sales of the anti-cellulite product. The Company retained the exclusive rights to seek pharmaceutical/dermatological partners for the anti-cellulite product for an initial period of one year following the launch of the product, thereafter JH Direct will be allowed to expand in this channel. The expiration date for this agreement is May 31, 2013. In accordance with the cosmetic products consulting agreement, the consulting firm will receive a percentage of the operating profits paid to the Company.
As of December 31, 2011, the Company had received non-refundable royalty advances totaling $100,000 from JH Direct, and has deferred all of these revenues. Management believes JH Direct has abandoned its efforts to commercialize the anti-cellulite cream and the Company has exercised its rights to terminate the agreement in 2012, at which time all revenues from this agreement will be recognized in full. Management believes no other monies will come from this contract.
In June 2010, the Company and Jan Marini Skin Research, Inc. (“JMSR”) entered into a licensing agreement providing JMSR with the exclusive U.S. rights to Imprimis’ transdermal delivery technology for use in an anti-cellulite cosmetic product for the dermatological market. Under the terms of the agreement, JMSR will pay Imprimis a licensing royalty on the U.S. and worldwide sales of an anti-cellulite product using Imprimis’ delivery technology. JMSR obtained an exclusive right to promote and sell a product in the U.S. dermatological market for approximately one year after which time they have a non-exclusive right. Also, JMSR obtained a non-exclusive right to promote and sell the product in the ex-U.S. dermatological market. In accordance with the cosmetic products consulting agreement, the cosmetic consultants will receive a percentage of the royalties paid to the Company. Management believes JMSR has abandoned its efforts to commercialize the anti-cellulite cream and the Company will look to terminate this agreement in 2012. No revenues or amounts were paid to or on behalf of the Company related to this agreement.
Separation Agreement
Effective February 17, 2010, the Board of Directors of the Company accepted the resignation of Dr. Juliet Singh as Chief Executive Officer of the Company and as a director on the Board. In connection with Dr. Singh’s resignation, the Company and Dr. Singh entered into a separation agreement that provides Dr. Singh with one year of continued salary in accordance with the terms of her existing employment agreement as well as the accelerated vesting of 37,500 stock options previously granted. The separation agreement also includes a mutual release of claims. In accordance with this agreement, the Company recorded a one-time accrual, in the year ended December 31, 2010, of $242,000 for the one year of continued salary (including the related employer payroll taxes) and medical benefits. Also, the Company recorded a total expense of approximately $174,000 for the value of the modifications to the stock options. As of December 31, 2011, no amounts are due under the separation agreement.
F-39
NOTE 11. OTHER RELATED PARTY TRANSACTIONS
During the year ended December 31, 2011, the Company received cash advances from its Board member Jeffery Abrams and former Board member Anthony Thornley in the amount of $27,537 to extend insurance policies of the Company. Following the dismissal of the Chapter 11 Case by the Bankruptcy Court on December 8, 2011, $27,537 was paid back by the Company in cash to Mr. Thornley and Mr. Abrams. There are currently no amounts due to Mr. Thornley and/or Dr. Abrams related to this or any other transaction.
During the year ended December 31, 2011, DermaStar purchased trade debt from third party vendors totaling $56,087. The amount owed to DermaStar related to this debt is included in the accounts payable – related party line item on the consolidated balance sheet. No amounts were paid to DermaStar related to this debt. DermaStar also made cash payments on behalf of the Company during the year ended December 31, 2011 in the amount of $254,142. On December 31, 2011, the Company made a payment to DermaStar totaling $254,142, as reimbursement for DermaStar’s cash payments made on behalf of the Company. DermaStar, and its members individually, are control persons of the Company. Also Dr. Robert J. Kammer, a director of the Company, and Mark L. Baum, Esq., Executive Chairman of the Company, are managing members and partial owners of DermaStar.
NOTE 12. RESEARCH AND DEVELOPMENT CREDIT
In November 2010, the Company received a Federal grant amount of $244,479 under the Qualifying Therapeutic Discovery Project that is part of the Patient Protection and Affordable Care Act. The funds were awarded in support of Impracor, the Company’s late-stage topical NSAID product candidate for the treatment of acute soft tissue injuries. The proceeds from this grant were recorded as a reduction in research and development expenses in the accompanying consolidated statement of operations.
NOTE 13. GAIN ON FORGIVENESS OF LIABILITIES
On October 2, 2008, the Company entered into a payment agreement with a vendor, settling a balance of $52,598. It was agreed between the Company and the vendor that 50% of the amount owed, or $26,299, would be forgiven and the remainder would be paid in two installments, which were, 50%, or $13,150, upon execution of the payment agreement and $13,149 upon an infusion of capital into the Company. Since the inception of the payment agreement, the amount to be forgiven, $26,299, continued to be recorded as an accounts payable up until the infusion of $1 million from the issuance of the Note in April 2010. When the Note was issued, the final installment payment of $13,149 was paid and the $26,299 was recognized as a gain on forgiveness of liabilities by the Company during the year ended December 31, 2010.
On October 5, 2011, priority claims of former employees in the amount of $119,667 originating as a result of the Company’s Bankruptcy petition filed June 26, 2010 (the “Priority Claimants”), were settled and paid by the Company. These amounts consisted of accrued and owed payroll amounts, accrued vacation and any other claims held against the Company at October 5, 2011. The Priority Claimants were given cash in the amount $47,975 and 300,000 stock options valued at $11,400 (using the Black-Scholes option pricing model to estimate the grant-date fair value) and the difference of $60,292 was recognized as a gain on forgiveness of liabilities during the year ended December 31, 2011.
F-40
IMPRIMIS PHARMACEUTICALS, INC.
PROSPECTUS
| Up to 1,619,047 Shares of Common Stock | ||
| MDB Capital Group LLC | Aegis Capital Corp |
Prospectus dated , 2013
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The following table sets forth the costs and expenses, other than the underwriting discount, payable by the registrant in connection with the sale and distribution of the securities being registered. All amounts are estimated except the SEC registration fee and the FINRA filing fee.
|
Amount
|
||||
|
SEC Registration Fee
|
$
|
1,333
|
||
|
FINRA Filing Fee
|
2,750
|
|||
|
NASDAQ fee
|
50,000
|
|||
|
Legal Fees and Expenses
|
550,000
|
|||
|
Accounting Fees and Expenses
|
70,000
|
|||
|
Printing and Related Expenses
|
20,000
|
|||
|
Transfer Agent and Registrar Fees
|
5,000
|
|||
|
Miscellaneous Expenses
|
10,000
|
|||
| Total |
$
|
709,083
|
||
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Section 145 of the Delaware General Corporation Law permits a corporation to include in its charter documents, and in agreements between the corporation and its directors and officers, provisions expanding the scope of indemnification beyond that specifically provided by the current law. Our Amended and Restated Certificate of Incorporation provides for the indemnification of directors and officers to the fullest extent permissible under Delaware law. Our Bylaws provide for the indemnification of officers and directors to the fullest extent permissible under Delaware law, except that we are only obligated to indemnify such officer or director in connection with a proceeding initiated by such person if the proceeding was authorized by our Board of Directors.
We have entered into indemnification agreements with our directors and certain executive officers, in addition to indemnification provided for in our charter documents, and we intend to enter into indemnification agreements with any new directors and our other executive officers in the future.
The underwriting agreement (Exhibit 1.1 hereto) provides for the underwriters' indemnification of us for certain liabilities, including liabilities arising under the Securities Act, in connection with matters specifically provided in writing by the underwriters for inclusion in the registration statement, and our indemnification of the underwriters for certain liabilities as set forth in the underwriting agreement.
We have purchased and intend to maintain insurance on our behalf and on behalf of any person who is or was a director or officer against any loss arising from any claim asserted against him or her and incurred by him or her in that capacity, subject to certain exclusions and limits of the amount of coverage.
II-1
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
All information regarding share amounts of common stock and prices per share of common stock contained below assumes the consummation of the one-for-five reverse stock split to be effected following effectiveness of the registration statement of which this prospectus forms a part and prior to the closing of this offering.
Issuance to Consultants and Executive Officers
In June 2010, the Company entered into two separate agreements with an investor relations firm and a financial advisory services firm (collectively “the firms”) in order to provide certain investor relations and advisory services to the Company for a period of one year. In exchange for such services, the Company issued 5,000 shares, in the aggregate, of its unregistered common stock, of which all shares were nonforfeitable (valued at $208,000 and recorded as prepaid consulting fees upon issuance) to the firms as a prepayment of services to be received over a three-month period.
In determining that the issuance of the securities in such transactions qualified for an exemption under Section 4(2), we relied on the following facts: each recipient represented that it was an accredited investor; the Company did not use general solicitation or advertising to market the securities; in each case the securities were issued to only one investor; and the securities issued were restricted securities.
On July 18, 2012, the Board granted to Mr. Baum 160,000 restricted stock units (RSUs) outside of the 2007 Plan. The restricted stock units granted to Mr. Baum are subject to certain performance-based vesting criteria, such that 40,000 RSUs will vest upon the satisfaction of each of the following events: (i) successful completion of a financing that results in aggregate cash proceeds to the Company of at least $5,000,000 at any time following the effective date of the grant; (ii) the Company meets the primary endpoints of its Phase III clinical studies for Impracor; (iii) the Company submits a New Drug Application for Impracor to the U.S. Food and Drug Administration; and (iv) the Company enters into a definitive license, collaboration or similar agreement for Impracor that would reasonably be expected to generate cash flow for the Company. The RSUs vest in full upon a change in control of the Company.
On July 18, 2012, the Board granted to Dr. Kammer 40,000 RSUs outside of the 2007 Plan in connection with his services as a consultant and advisor to the Company. The RSU is subject to certain performance-based vesting criteria, such that all 40,000 RSUs will vest at such time as the Company meets the primary endpoints of its Phase III clinical studies for Impracor.
The RSUs described in the two immediately preceding paragraphs have been registered under the Securities Act pursuant to a registration statement on Form S-8, filed by the Company with the SEC on August 22, 2012.
Convertible Note
On April 5, 2010, we issued a $1,000,000 Senior Convertible Promissory Note (the “Convertible Note”) to an existing stockholder, Alexej Ladonnikov, in a private placement. The Convertible Note had a two-year term and an annual interest rate of 7.5% percent. We had a right to prepay the Convertible Note at any time upon providing written notice to the holder. At the time of issuance, the Convertible Note provided that at any time prior to the Company’s repayment of the Convertible Note, the holder may convert all or any part of the outstanding principal and accrued interest on the Convertible Note into shares of our common stock at a conversion rate of $40.00 per share. We received gross proceeds from the issuance of the Convertible Note in the aggregate amount of $1,000,000.
II-2
There were no discounts or commissions paid in connection with this private placement. The proceeds were used for working capital purposes. The private placement was made solely to a single “accredited investor,” as that term is defined in Regulation D under the Securities Act. The securities issuable upon conversion of all or a portion of the Convertible Note were not registered under the Securities Act or the securities laws of any state, and were offered and sold in reliance on the exemption from registration afforded by Section 4(2) and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving any public offering.
During January 2012, Mr. Ladonnikov sold 80% of the Convertible Note to DermaStar in a private transaction. Effective as of January 25, 2012, we entered into separate waiver and settlement agreements with DermaStar and Mr. Ladonnikov. Under each of the waiver and settlement agreements, the holders of the Convertible Note agreed to forever waive (i) their rights to accelerate the entire unpaid principal sum of the Convertible Note and all accrued interest pursuant to Section 1 of the Convertible Note, (ii) their rights under Section 7 of the Senior Convertible Note Purchase Agreement dated April 5, 2010, and (iii) certain conversion rights pursuant to Section 3 of the Convertible Note. Under the terms of the waiver and settlement agreement with DermaStar, we and DermaStar agreed to the mandatory conversion of the principal and accrued and unpaid interest of the Convertible Note and $56,087 in current accounts payable of the Company held by DermaStar into our common stock at a conversion price of approximately $0.06668 per share at such time as we had a sufficient number of shares of authorized common stock to effect such conversion. Under the terms of the waiver and settlement agreement with Mr. Ladonnikov, we and Mr. Ladonnikov agreed to the mandatory conversion of the 20% of the principal and accrued and unpaid interest of the Convertible Note held by Mr. Ladonnikov, at such time as we had a sufficient number of authorized common shares to effect such a conversion, into our common stock at a conversion price of $0.60. Mr. Ladonnikov also agreed to make a one-time payment of $50,000 to us at such time as the Convertible Note was converted into common stock.
On February 28, 2012, effective immediately following the effective time of our Certificate of Amendment to our Certificate of Incorporation increasing the number of authorized shares of common stock and implementing the one-for-eight reverse split of our common stock, the entire outstanding balance and all accrued but unpaid interest owing under the Convertible Note and the accounts payable held by DermaStar were converted into 1,835,830 shares of common stock, and the Convertible Note was terminated. Mr. Ladonnikov made the required one-time payment of $50,000 to us at the time of the conversion.
Secured Line of Credit
On November 21, 2011, we entered into a Secured Line of Credit Letter Agreement (the “Line of Credit Agreement”) with DermaStar, pursuant to which DermaStar agreed to lend us funds under a line of credit upon certain conditions, including the dismissal of the Chapter 11 Case by the Bankruptcy Court. The Line of Credit Agreement became effective on December 9, 2011, in connection with the dismissal of the Chapter 11 Case by the Bankruptcy Court. The Line of Credit Agreement provided for advances of up to an aggregate of $750,000, subject to the satisfaction by us of certain conditions in connection with the initial advance and each subsequent advance.
On April 25, 2012, the entire outstanding principal balance and all accrued and unpaid interest under the line of credit, an aggregate of $762,534, was converted into 193,047 shares of common stock and warrants to purchase 48,262 shares of common stock at the offering price and on the terms of the April Private Placement described below, pursuant to the terms of a Conversion Agreement we entered into with DermaStar on April 20, 2012. The warrants have substantially the same terms as the warrants issued in the April Private Placement. The line of credit was terminated upon the completion of the conversion.
The securities issued upon conversion of the outstanding principal and interest owing under the line of credit pursuant to the Conversion Agreement were issued in reliance on the exemption from the registration requirements of Section 4(2) of the Securities Act. In determining that the issuance of the securities pursuant to the Conversion Agreement qualified for an exemption under Section 4(2), we relied on the following facts: DermaStar is an accredited investor and the Company’s major stockholder; the Company did not use general solicitation or advertising to market the securities; DermaStar represented that it was purchasing the securities for its own account and not with a view to distribute them; and the securities issued were restricted securities.
II-3
Series A Preferred Stock
In partial consideration for and in connection with the Line of Credit Agreement, on November 21, 2011 we executed a Securities Purchase Agreement (the “Series A Purchase Agreement”) with DermaStar, pursuant to which we agreed to issue ten (10) shares of newly-designated Series A Convertible Preferred Stock (the “Series A Preferred Stock”) to DermaStar for an aggregate purchase price of $100,000. The Series A Purchase Agreement, as amended, became effective on December 9, 2011, in connection with the dismissal of the Chapter 11 Case by the Bankruptcy Court. On December 12, 2011, we and DermaStar consummated the transactions contemplated by the Series A Purchase Agreement. The shares of Series A Preferred Stock issued to DermaStar in the offering are convertible into 1,499,700 shares of our common stock. Upon issuance of the Series A Preferred Stock, DermaStar, and its members individually, became control persons of the Company. We appointed DermaStar Managing Members Mark L. Baum and Robert J. Kammer to our Board of Directors in December 2011.
The Series A Preferred Stock issued to DermaStar in the offering were issued in reliance on the exemption from the registration requirements of Section 4(2) of the Securities Act. In determining that the issuance of the securities pursuant to the Series A Purchase Agreement qualified for an exemption under Section 4(2), we relied on the following facts: DermaStar is an accredited investor; the Company did not use general solicitation or advertising to market the securities; DermaStar represented that it was purchasing the securities for its own account and not with a view to distribute them; and the securities issued were restricted securities.
On December 9, 2011, we filed a Certificate of Designation to our Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware setting forth the rights and preferences of the Series A Preferred Stock. Among other things, the Certificate of Designation (i) authorizes 10 shares of the Company’s preferred stock to be designated as “Series A Convertible Preferred Stock”; (ii) grants the holders of the Series A Preferred Stock the right to convert into the Company’s Common Stock at a conversion price of $0.06668, as adjusted; (iii) grants a liquidation preference of $10,000 per share of Series A Preferred Stock; (iv) provides that the holders of Series A Preferred Stock shall vote with the holders of the Company’s common stock on an “as converted basis”; and (v) provides that the affirmative vote of a majority of the outstanding shares of the Series A Preferred Stock is required to approve certain other corporate matters including, among other things, changes to the rights of the holders of the Series A Preferred Stock, amendments to our Certificate of Incorporation or Bylaws, issuance of priority or parity securities, issuance of debt securities, entry into certain fundamental transactions and increase or decrease the size of the Board of Directors of the Company.
On June 29, 2012, DermaStar converted the 10 shares of Series A Preferred Stock held by it into 1,499,700 shares of our common stock. In connection with the conversion, we paid to DermaStar $200,000 as partial consideration for the conversion pursuant to a conversion agreement. Immediately following the conversion of the Series A Preferred Stock, all 10 shares were retired to our treasury and cancelled. The conversion agreement was unanimously approved by the Company’s disinterested directors, with Mr. Baum and Dr. Kammer abstaining.
April Private Placement
On April 20, 2012, we entered into a Securities Purchase Agreement with certain accredited investors relating to the sale and issuance of an aggregate of 2,011,691 shares of our common stock and warrants to purchase up to 502,928 shares of common stock at an exercise price of $5.925 per share, for an aggregate purchase price of approximately $7.95 million (the “April Private Placement”). We closed the April Private Placement on April 25, 2012.
The investors are not entitled to any registration rights with respect to the common stock and warrants issued in the April Private Placement. The warrants have a term of three years and are exercisable any time after April 25, 2012. We may require that the investors exercise the warrants in whole, but not in part, at any time within 20 business days after all of the following conditions have been satisfied: (i) the volume weighted average price of the our common stock for 10 consecutive trading days is equal to or greater than the exercise price of the warrants; (ii) we have received a Filing Review Notification from the FDA regarding the status of Impracor; and (iii) sufficient shares of common stock are authorized and reserved for issuance upon full exercise of the warrants.
The securities sold in the April Private Placement were sold in reliance on the exemption from the registration requirements of Section 4(2) of the Securities Act and Rule 506 of Regulation D promulgated thereunder. In determining that the issuance of the securities in the April Private Placement qualified for an exemption under Section 4(2) and Rule 506 of Regulation D, we relied on the following facts: the Company did not use general solicitation or advertising to market the securities; each investor in the April Private Placement represented to the Company that it was an accredited investor (as that term is defined in Rule 501 of Regulation D) and that it was purchasing the securities for its own account and not with a view to distribute them; and the securities issued are restricted securities.
PCCA Transaction
On August 30, 2012, we entered into a Stock Purchase Agreement (the “PCCA Purchase Agreement”) with PCCA in connection with the PCCA Transaction. Pursuant to the terms of the PCCA Purchase Agreement, on August 31, 2012, we issued and sold to PCCA 832,683 shares of our common stock at a per share purchase price of $4.8038, for aggregate gross proceeds to us of $4,000,000. The PCCA Purchase Agreement does not grant to PCCA any registration rights with respect to the shares purchased and sold thereunder. The shares sold to PCCA were sold in reliance on the exemption from the registration requirements of the Securities Act afforded by Section 4(2) thereof. In determining that the issuance of such shares qualified for an exemption under Section 4(2) of the Securities Act, we relied on the following facts: PCCA represented that it was an accredited investor (as that term is defined in Rule 501 of Regulation D) and that it was purchasing such shares for its own account and not with a view to distribute them; the shares were sold to only one purchaser in connection with a strategic transaction; and the securities issued are restricted securities.
II-4
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
Financial Statement Schedules
All financial statement schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto.
Exhibits
The following exhibits are being filed with this registration statement on Form S-1.
|
Exhibit No.
|
Description
|
|
|
1.1**
|
Form of Underwriting Agreement
|
|
|
2.1
|
Agreement and Plan of Merger, dated as of September 17, 2007, by and among Transdel Pharmaceuticals, Inc., Transdel Pharmaceuticals Holdings, Inc. and Trans-Pharma Acquisition Corp. Incorporation (incorporated herein by reference to Exhibit 2.1 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation (incorporated herein by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission September 13, 2007)
|
|
|
3.2
|
Amended and Restated Bylaws (incorporated herein by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission September 13, 2007)
|
|
|
3.3
|
Certificate of Designation of Series A Convertible Preferred Stock of Transdel Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit 3.1 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
3.4
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
5.1**
|
Opinion of Morrison & Foerster LLP
|
|
|
10.1
|
Form of Warrant to purchase Common Stock (incorporated herein by reference to Exhibit 10.2 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
10.2
|
Form of Directors and Officers Indemnification Agreement (incorporated herein by reference to Exhibit 10.8 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
10.3#
|
Transdel Pharmaceuticals, Inc. 2007 Incentive Stock and Awards Plan (incorporated herein by reference to Exhibit 10.11 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
10.4#
|
Transdel Pharmaceuticals, Inc. 2007 Incentive Stock and Awards Plan — Amendment No. 1 (incorporated herein by reference to Exhibit A to the Definitive Proxy Statement on Schedule 14A of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on October 1, 2008)
|
|
|
10.5#
|
Amendment No. 2 to Transdel Pharmaceuticals, Inc. 2007 Incentive Stock and Awards Plan (incorporated herein by reference to Annex B to the Information Statement on Schedule 14C of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on February 6, 2012)
|
|
|
10.6#**
|
Amendment No. 3 to Imprimis Pharmaceuticals, Inc. 2007 Incentive Stock and Awards Plan
|
|
|
10.7#
|
Form of 2007 Incentive Stock Option Agreement (incorporated herein by reference to Exhibit 10.12 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
10.8#
|
Form of 2007 Non-Qualified Stock Option Agreement (incorporated herein by reference to Exhibit 10.13 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
10.9
|
Agreement of Conveyance, Transfer and Assignment of Assets and Assumption of Obligations, dated as of September 17, 2007, by and between Transdel Pharmaceuticals, Inc. and Bywater Resources Holdings Inc. (incorporated herein by reference to Exhibit 10.15 to the Registration Statement on Form SB-2 of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 7, 2007)
|
|
|
10.10
|
Research and Development Services Agreement, dated as of October 11, 2007, by and between DPT Laboratories, Ltd. And Transdel Pharmaceuticals Holdings, Inc. (incorporated herein by reference to Exhibit 10.17 to the Registration Statement on Form SB-2 of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 7, 2007) (portions of this exhibit have been omitted pursuant to a request for confidential treatment)
|
|
|
10.11
|
Project Scope Document, effective as of May 30, 2007, by and between DPT Laboratories, Ltd. and Transdel Pharmaceuticals Holdings, Inc. (incorporated herein by reference to Exhibit 10.18 to the Registration Statement on Form SB-2 of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 27, 2007) (portions of this exhibit have been omitted pursuant to a request for confidential treatment)
|
|
|
10.12
|
Form of Warrant to purchase Common Stock (incorporated herein by reference to Exhibit 10.2 the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on May 15, 2008).
|
II-5
|
10.13#
|
Employment Agreement, dated as of October 18, 2010, between Transdel Pharmaceuticals, Inc. and John Bonfiglio, Ph.D. (incorporated herein by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on November 14, 2010)
|
|
|
10.14#
|
Nonqualified Stock Option Agreement, dated as of October 20, 2010, between Transdel Pharmaceuticals, Inc., and Dr. John Bonfiglio (incorporated herein by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on November 14, 2010)
|
|
|
10.15#
|
Restricted Stock Agreement, dated as of October 20, 2010, between Transdel Pharmaceuticals, Inc., and Dr. John Bonfiglio (incorporated herein by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on November 14, 2010)
|
|
|
10.16
|
Form of Senior Convertible Note Purchase Agreement (incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on April 8, 2011)
|
|
|
10.17
|
Form of Senior Convertible Promissory Note (incorporated herein by reference to Exhibit 10.2 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on April 8, 2011)
|
|
|
10.18
|
Asset Purchase Agreement, dated as of June 23, 2011, by and among Transdel Pharmaceuticals, Inc. and Cardium Healthcare, Inc. (incorporated herein by reference to Exhibit 2.1 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on June 26, 2011)
|
|
|
10.19
|
Secured Line of Credit Letter Agreement, dated November 21, 2011 and effective as of December 9, 2011, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC. (incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
10.20
|
Security Agreement, dated as of December 9, 2011, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC. (incorporated herein by reference to Exhibit 10.2 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
10.21
|
Intellectual Property Security Agreement, dated as of December 9, 2011, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC. (incorporated herein by reference to Exhibit 10.3 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
10.22
|
Securities Purchase Agreement, dated November 21, 2011 and effective as of December 9, 2011, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC. (incorporated herein by reference to Exhibit 10.3 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
10.23**
|
First Amendment to Securities Purchase Agreement, effective as of December 31, 2011, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC
|
|
|
10.24
|
Mutual General Release Agreement, dated as of December 13, 2011, by and between Transdel Pharmaceuticals, Inc. and the other signatories thereto. (incorporated herein by reference to Exhibit 10.4 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
10.25
|
Waiver and Settlement Agreement, effective as of January 25, 2012, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC (incorporated by reference to Exhibit 10.11 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
10.26
|
Waiver and Settlement Agreement, effective as of January 25, 2012, by and between Transdel Pharmaceuticals, Inc. and Alexej Ladonnikov (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
10.27#
|
Employment Agreement, effective as of January as of 1, 2012, by and between Transdel Pharmaceuticals, Inc. and Balbir Brar, D.V.M., Ph.D. (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
10.28#
|
Employment Agreement, effective as of February 1, 2012, by and between Transdel Pharmaceuticals, Inc. and Andrew Boll (incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
II-6
|
10.29#
|
Employment Agreement, effective as of February 15, 2012, by and between Transdel Pharmaceuticals, Inc. and Joachim Schupp, M.D. (incorporated by reference to Exhibit 10.5 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
10.30#**
|
Amended and Restated Employment Agreement, dated July 24, 2012, by and between Imprimis Pharmaceuticals, Inc. and Mark L. Baum, Esq.
|
|
|
10.31#
|
Advisory Agreement, effective as of April 1, 2012, by and between Imprimis Pharmaceuticals, Inc. and Dr. Robert Kammer (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on 8-K filed with the Securities and Exchange Commission on April 27, 2012)
|
|
|
10.32#**
|
Amendment to Advisory Agreement, dated July 24, 2012, by and between Imprimis Pharmaceuticals, Inc. and Dr. Robert Kammer
|
|
|
10.33#
|
Senior Advisory Agreement, effective as of January 17, 2012, by and between Transdel Pharmaceuticals, Inc. and Paul Finnegan, M.D. (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
10.34#**
|
Termination Agreement, effective as of May 9, 2012, by and between Imprimis Pharmaceuticals, Inc. and Paul Finnegan, M.D.
|
|
|
10.35
|
Promissory Note Conversion Agreement, dated as of April 20, 2012, by and between Imprimis Pharmaceuticals, Inc. and DermaStar International, LLC (incorporated herein by reference to the Current Report on Form 8-K of Imprimis Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on April 27, 2012)
|
|
|
10.36
|
Securities Purchase Agreement, dated as of April 20, 2012, by and between Imprimis Pharmaceuticals, Inc. and the investors signatory thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on 8-K filed with the Securities and Exchange Commission on April 27, 2012)
|
|
|
10.37
|
Form of Warrant dated as of April 25, 2012 (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on 8-K filed with the Securities and Exchange Commission on April 27, 2012)
|
|
|
10.39**
|
Conversion Agreement, dated June 29, 2012, by and between Imprimis Pharmaceuticals, Inc. and DermaStar, LLC
|
|
|
10.40#**
|
Stand-alone Restricted Stock Unit Agreement, dated July 18, 2012, granted by Imprimis Pharmaceuticals, Inc. to Mark L. Baum
|
|
| 10.41#** |
Stand-alone Restricted Stock Unit Agreement, dated July 18, 2012, granted by Imprimis Pharmaceuticals, Inc. to Robert J. Kammer
|
|
| 10.42 |
License Agreement, dated as of August 30, 2012, by and between Imprimis Pharmaceuticals, Inc. and Professional Compounding Centers of America, Inc. (incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K of Imprimis Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on August 31, 2012)
|
|
| 10.43 |
Stock Purchase Agreement, dated as of August 30, 2012, by and between Imprimis Pharmaceuticals, Inc. and Professional Compounding Centers of America, Inc. (incorporated herein by reference to Exhibit 10.2 to the Current Report on Form 8-K of Imprimis Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on August 31, 2012)
|
|
| 10.44** |
Form of Underwriter’s Warrant
|
|
|
23.1*
|
Consent of KMJ Corbin & Company LLP
|
|
|
23.2**
|
Consent of Morrison & Foerster LLP (contained in Exhibit 5.1)
|
|
|
24.1**
|
Power of Attorney (contained on the signature page)
|
|
101.INS++**
|
XBRL Instant Document
|
|
|
101.SCH++**
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL++**
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
101.DEF++**
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
101.LAB++**
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
|
101.PRE++**
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
*
|
Filed herewith.
|
|
|
#
|
Management contract or compensatory plan or arrangement.
|
|
|
++
|
XBRL (Extensible Business Reporting Language) information is furnished and not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is not otherwise subject to liability under these Sections.
|
|
| ** | Previously filed. | |
II-7
ITEM 17. UNDERTAKINGS.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-8
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Solana Beach, State of California, on February 4, 2013.
| IMPRIMIS PHARMACEUTICALS, INC. | |||
|
By:
|
/s/ Mark L. Baum
|
||
|
Date: February 4, 2013
|
Mark L. Baum, Chief Executive Officer
(Principal Executive Officer)
|
||
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
SIGNATURE
|
TITLE
|
DATE
|
|||
|
/s/ Mark L. Baum
|
Chief Executive Officer and Director
|
February 4, 2013
|
|||
|
Mark L. Baum, Esq.
|
(Principal Executive Officer) | ||||
|
/s/ Andrew R. Boll
|
Vice President of Accounting and Public Reporting
|
February 4, 2013
|
|||
|
Andrew R. Boll
|
(Principal Accounting & Financial Officer) | ||||
|
/s/ Jeffrey J. Abrams*
|
Director
|
February 4, 2013
|
|||
|
Jeffrey J. Abrams, M.D.
|
|||||
|
/s/ Paul Finnegan*
|
Director
|
February 4, 2013
|
|||
|
Paul Finnegan, M.D., M.B.A.
|
|||||
|
/s/ Robert J. Kammer*
|
Chairman of the Board of Directors
|
February 4, 2013
|
|||
|
Robert J. Kammer, D.D.S.
|
|||||
| *By: | /s/ Mark L. Baum | ||||
| Mark L. Baum | |||||
| Attorney-in-Fact | |||||
II-9
EXHIBIT INDEX
|
Exhibit No.
|
Description
|
|
|
1.1**
|
Form of Underwriting Agreement
|
|
|
2.1
|
Agreement and Plan of Merger, dated as of September 17, 2007, by and among Transdel Pharmaceuticals, Inc., Transdel Pharmaceuticals Holdings, Inc. and Trans-Pharma Acquisition Corp. Incorporation (incorporated herein by reference to Exhibit 2.1 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation (incorporated herein by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission September 13, 2007)
|
|
|
3.2
|
Amended and Restated Bylaws (incorporated herein by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission September 13, 2007)
|
|
|
3.3
|
Certificate of Designation of Series A Convertible Preferred Stock of Transdel Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit 3.1 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
3.4
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
5.1**
|
Opinion of Morrison & Foerster LLP
|
|
|
10.1
|
Form of Warrant to purchase Common Stock (incorporated herein by reference to Exhibit 10.2 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
10.2
|
Form of Directors and Officers Indemnification Agreement (incorporated herein by reference to Exhibit 10.8 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
10.3#
|
Transdel Pharmaceuticals, Inc. 2007 Incentive Stock and Awards Plan (incorporated herein by reference to Exhibit 10.11 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
10.4#
|
Transdel Pharmaceuticals, Inc. 2007 Incentive Stock and Awards Plan — Amendment No. 1 (incorporated herein by reference to Exhibit A to the Definitive Proxy Statement on Schedule 14A of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on October 1, 2008)
|
|
|
10.5#
|
Amendment No. 2 to Transdel Pharmaceuticals, Inc. 2007 Incentive Stock and Awards Plan (incorporated herein by reference to Annex B to the Information Statement on Schedule 14C of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on February 6, 2012)
|
|
|
10.6#**
|
Amendment No. 3 to Imprimis Pharmaceuticals, Inc. 2007 Incentive Stock and Awards Plan
|
|
|
10.7#
|
Form of 2007 Incentive Stock Option Agreement (incorporated herein by reference to Exhibit 10.12 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
10.8#
|
Form of 2007 Non-Qualified Stock Option Agreement (incorporated herein by reference to Exhibit 10.13 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on September 21, 2007)
|
|
|
10.9
|
Agreement of Conveyance, Transfer and Assignment of Assets and Assumption of Obligations, dated as of September 17, 2007, by and between Transdel Pharmaceuticals, Inc. and Bywater Resources Holdings Inc. (incorporated herein by reference to Exhibit 10.15 to the Registration Statement on Form SB-2 of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 7, 2007)
|
|
|
10.10
|
Research and Development Services Agreement, dated as of October 11, 2007, by and between DPT Laboratories, Ltd. And Transdel Pharmaceuticals Holdings, Inc. (incorporated herein by reference to Exhibit 10.17 to the Registration Statement on Form SB-2 of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 7, 2007) (portions of this exhibit have been omitted pursuant to a request for confidential treatment)
|
|
|
10.11
|
Project Scope Document, effective as of May 30, 2007, by and between DPT Laboratories, Ltd. and Transdel Pharmaceuticals Holdings, Inc. (incorporated herein by reference to Exhibit 10.18 to the Registration Statement on Form SB-2 of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 27, 2007) (portions of this exhibit have been omitted pursuant to a request for confidential treatment)
|
|
|
10.12
|
Form of Warrant to purchase Common Stock (incorporated herein by reference to Exhibit 10.2 the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on May 15, 2008).
|
II-10
|
10.13#
|
Employment Agreement, dated as of October 18, 2010, between Transdel Pharmaceuticals, Inc. and John Bonfiglio, Ph.D. (incorporated herein by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on November 14, 2010)
|
|
|
10.14#
|
Nonqualified Stock Option Agreement, dated as of October 20, 2010, between Transdel Pharmaceuticals, Inc., and Dr. John Bonfiglio (incorporated herein by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on November 14, 2010)
|
|
|
10.15#
|
Restricted Stock Agreement, dated as of October 20, 2010, between Transdel Pharmaceuticals, Inc., and Dr. John Bonfiglio (incorporated herein by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on November 14, 2010)
|
|
|
10.16
|
Form of Senior Convertible Note Purchase Agreement (incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on April 8, 2011)
|
|
|
10.17
|
Form of Senior Convertible Promissory Note (incorporated herein by reference to Exhibit 10.2 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on April 8, 2011)
|
|
|
10.18
|
Asset Purchase Agreement, dated as of June 23, 2011, by and among Transdel Pharmaceuticals, Inc. and Cardium Healthcare, Inc. (incorporated herein by reference to Exhibit 2.1 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on June 26, 2011)
|
|
|
10.19
|
Secured Line of Credit Letter Agreement, dated November 21, 2011 and effective as of December 9, 2011, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC. (incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
10.20
|
Security Agreement, dated as of December 9, 2011, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC. (incorporated herein by reference to Exhibit 10.2 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
10.21
|
Intellectual Property Security Agreement, dated as of December 9, 2011, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC. (incorporated herein by reference to Exhibit 10.3 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
10.22
|
Securities Purchase Agreement, dated November 21, 2011 and effective as of December 9, 2011, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC. (incorporated herein by reference to Exhibit 10.3 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
10.23**
|
First Amendment to Securities Purchase Agreement, effective as of December 31, 2011, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC
|
|
|
10.24
|
Mutual General Release Agreement, dated as of December 13, 2011, by and between Transdel Pharmaceuticals, Inc. and the other signatories thereto. (incorporated herein by reference to Exhibit 10.4 to the Current Report on Form 8-K of Transdel Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on December 20, 2011)
|
|
|
10.25
|
Waiver and Settlement Agreement, effective as of January 25, 2012, by and between Transdel Pharmaceuticals, Inc. and DermaStar International, LLC (incorporated by reference to Exhibit 10.11 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
10.26
|
Waiver and Settlement Agreement, effective as of January 25, 2012, by and between Transdel Pharmaceuticals, Inc. and Alexej Ladonnikov (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
10.27#
|
Employment Agreement, effective as of January as of 1, 2012, by and between Transdel Pharmaceuticals, Inc. and Balbir Brar, D.V.M., Ph.D. (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
10.28#
|
Employment Agreement, effective as of February 1, 2012, by and between Transdel Pharmaceuticals, Inc. and Andrew Boll (incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
II-11
|
10.29#
|
Employment Agreement, effective as of February 15, 2012, by and between Transdel Pharmaceuticals, Inc. and Joachim Schupp, M.D. (incorporated by reference to Exhibit 10.5 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
10.30#**
|
Amended and Restated Employment Agreement, dated July 24, 2012, by and between Imprimis Pharmaceuticals, Inc. and Mark L. Baum, Esq.
|
|
|
10.31#
|
Advisory Agreement, effective as of April 1, 2012, by and between Imprimis Pharmaceuticals, Inc. and Dr. Robert Kammer (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on 8-K filed with the Securities and Exchange Commission on April 27, 2012)
|
|
|
10.32#**
|
Amendment to Advisory Agreement, dated July 24, 2012, by and between Imprimis Pharmaceuticals, Inc. and Dr. Robert Kammer (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on 8-K filed with the Securities and Exchange Commission on July 24, 2012)
|
|
|
10.33#
|
Senior Advisory Agreement, effective as of January 17, 2012, by and between Transdel Pharmaceuticals, Inc. and Paul Finnegan, M.D. (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on 10-Q filed with the Securities and Exchange Commission on May 10, 2012)
|
|
|
10.34#**
|
Termination Agreement, effective as of May 9, 2012, by and between Imprimis Pharmaceuticals, Inc. and Paul Finnegan, M.D.
|
|
|
10.35
|
Promissory Note Conversion Agreement, dated as of April 20, 2012, by and between Imprimis Pharmaceuticals, Inc. and DermaStar International, LLC (incorporated herein by reference to the Current Report on Form 8-K of Imprimis Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on April 27, 2012)
|
|
|
10.36
|
Securities Purchase Agreement, dated as of April 20, 2012, by and between Imprimis Pharmaceuticals, Inc. and the investors signatory thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on 8-K filed with the Securities and Exchange Commission on April 27, 2012)
|
|
|
10.37
|
Form of Warrant dated as of April 25, 2012 (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on 8-K filed with the Securities and Exchange Commission on April 27, 2012)
|
|
|
10.39**
|
Conversion Agreement, dated June 29, 2012, by and between Imprimis Pharmaceuticals, Inc. and DermaStar International, LLC
|
|
|
10.40#**
|
Stand-alone Restricted Stock Unit Agreement, dated July 18, 2012, granted by Imprimis Pharmaceuticals, Inc. to Mark L. Baum
|
|
| 10.41#** |
Stand-alone Restricted Stock Unit Agreement, dated July 18, 2012, granted by Imprimis Pharmaceuticals, Inc. to Robert J. Kammer
|
|
| 10.42 |
License Agreement, dated as of August 30, 2012, by and between Imprimis Pharmaceuticals, Inc. and Professional Compounding Centers of America, Inc. (incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8-K of Imprimis Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on August 31, 2012)
|
|
| 10.43 |
Stock Purchase Agreement, dated as of August 30, 2012, by and between Imprimis Pharmaceuticals, Inc. and Professional Compounding Centers of America, Inc. (incorporated herein by reference to Exhibit 10.2 to the Current Report on Form 8-K of Imprimis Pharmaceuticals, Inc. filed with the Securities and Exchange Commission on August 31, 2012)
|
|
| 10.44** |
Form of Underwriter’s Warrant
|
|
|
23.1*
|
Consent of KMJ Corbin & Company LLP
|
|
|
23.2**
|
Consent of Morrison & Foerster LLP (contained in Exhibit 5.1)
|
|
|
24.1**
|
Power of Attorney (contained on the signature page)
|
|
101.INS++**
|
XBRL Instant Document
|
|
|
101.SCH++**
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL++**
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
101.DEF++**
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
101.LAB++**
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
|
101.PRE++**
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
*
|
Filed herewith.
|
|
|
#
|
Management contract or compensatory plan or arrangement.
|
|
|
++
|
XBRL (Extensible Business Reporting Language) information is furnished and not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is not otherwise subject to liability under these Sections.
|
|
| ** | Previously filed. | |
II-12
