Attached files
| file | filename |
|---|---|
| EX-31.1 - EXHIBIT 31.1 - CardioGenics Holdings Inc. | v333216_ex31-1.htm |
| EX-23.2 - EXHIBIT 23.2 - CardioGenics Holdings Inc. | v333216_ex23-2.htm |
| EX-23.3 - EXHIBIT 23.3 - CardioGenics Holdings Inc. | v333216_ex23-3.htm |
| EX-23.1 - EXHIBIT 23.1 - CardioGenics Holdings Inc. | v333216_ex23-1.htm |
| EX-32.1 - EXHIBIT 32.1 - CardioGenics Holdings Inc. | v333216_ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - CardioGenics Holdings Inc. | v333216_ex31-2.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________________
FORM 10-K
_________________________
| þ | Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 for the fiscal year ended October 31, 2012 |
OR
| ¨ | Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 for the transition period from __________ to __________. |
Commission file number: 000-28761
CARDIOGENICS
HOLDINGS INC.
(Exact name of registrant as specified in its charter)
| Nevada | 88-0380546 |
| (State or other jurisdiction of | (I.R.S. Employer |
| incorporation or organization) | Identification Number) |
| 6295 Northam Drive, Unit 8, Mississauga, Ontario L4V 1W8 |
| (Address of principal executive offices) (Zip code) |
| (905) 673-8501 |
| (Registrant’s telephone number, including area code) |
| Securities registered pursuant to Section 12(b) of the Act: None |
|
Securities registered pursuant to Section 12(g) of the Act: Common Stock—$0.00001 par value |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨.
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨.
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, non-accelerated filer or a small. See definition of “large accelerated filer, accelerated filer and smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company þ |
| (Do not check if smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No þ
The aggregate market value of the registrant’s voting and non-voting common stock held by non-affiliates on January 22, 2013 (based on the closing stock price on the OTC Bulletin Board) on such date was approximately $ 6,044,844.
As of January 22, 2013 the Registrant had the following number of shares of its capital stock outstanding: 32,499,239 shares of Common Stock and 1 share of Series 1 Preferred Voting Stock, par value $0.0001, representing 13 exchangeable shares of the Registrant’s subsidiary, CardioGenics ExchangeCo Inc., which are exchangeable into 24,176,927 shares of the Registrant’s Common Stock.
CARDIOGENICS HOLDINGS INC.
ANNUAL REPORT ON FORM 10-K
FOR THE FISCAL YEAR ENDED OCTOBER 31, 2012
TABLE OF CONTENTS
| Page | |||
| Part I | 1 | ||
| Item 1. | Business | 1 | |
| Item 1A | Risk Factors | 14 | |
| Item 1B | Unresolved Staff Comments | 21 | |
| Item 2. | Properties | 21 | |
| Item 3. | Legal Proceedings | 22 | |
| Item 4. | Mine Safety Disclosures | 23 | |
| Part II | 23 | ||
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Securities | 23 | |
| Item 6. | Selected Financial Data | 24 | |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 24 | |
| Item 7A | Quantitative and Qualitative Disclosures About Market Risk | 29 | |
| Item 8. | Financial Statements and Supplementary Data | 30 | |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 30 | |
| Item 9A | Controls and Procedures | 30 | |
| Item 9B | Other Information | 31 | |
| Part III | 31 | ||
| Item 10. | Directors, Executive Officers and Corporate Governance | 31 | |
| Item 11. | Executive Compensation | 33 | |
| Item 12. | Security Ownership of Certain Beneficial Owners and Related Stockholder Matters | 36 | |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 38 | |
| Item 14. | Principal Accounting Fees and Services | 38 | |
| Part IV | 39 | ||
| Item 15. | Exhibits, Financial Statement Schedules | 39 | |
| Signatures |
| i |
Part I
| ITEM 1. | BUSINESS |
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Such statements are based upon current expectations that involve risks and uncertainties. Any statements contained herein that are not statements of historical fact may be deemed to be forward-looking statements. Words such as “may,” “will,” “should,” “estimates,” “predicts,” “potential,” “continue,” “strategy,” “believes,” “anticipates,” “plans,” “expects,” “intends” and similar expressions are intended to identify forward-looking statements. Our discussions relating to our liquidity and capital resources, our business strategy, our competition, and the future of our market segment, our acquisition of CardioGenics Inc., an Ontario Canada corporation (“CardioGenics”), among others, contain such statements. Our actual results and the timing of certain events may differ significantly from the results discussed in the forward-looking statements.
Our forward-looking statements in this Annual Report on Form 10-K are based on management’s current views and assumptions regarding future events and speak only as of their dates. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by the federal securities laws. Unless the context requires otherwise, the terms “we,” “us” and “our” refer to CardioGenics Holdings Inc., our predecessors and subsidiaries. Our acquisition of CardioGenics as discussed in this Annual Report on Form 10-K is sometimes referred to as the “CardioGenics Acquisition.”
Overview
Prior to the CardioGenics Acquisition, our primary business was providing financial and investment information to the investment community which we have been doing since 1989. In May 1999, we began offering our services on a subscription fee basis to the general public for the first time through our website at jagnotes.com. Through our website and our traditional fax-based service, we offer timely financial data, reports and commentary.
In July 2009, we consummated the CardioGenics Acquisition and the main focus of our business switched from offering our customers fee-based financial information to the development of products targeting the immunoassay segment of the point-of-care in vitro diagnostic (“IVD”) testing market. See “—Our Strategy—Acquisition of CardioGenics.” In order to better reflect the new focus of our business, we changed our name to CardioGenics Holdings Inc. in October 2009.
We are a Nevada corporation. Our address is 6295 Northam Drive, Unit 8, Mississauga, Ontario, Canada L4V 1W8, and our telephone number is 905-673-8501.
Company Background
JagNotes, Inc.
We have been providing financial information to the investment community since 1989. In May 1999, we began offering our services on a subscription fee basis to the general public for the first time through our website at jagnotes.com. Through our website and our traditional fax-based service, we offer timely financial data, reports and commentary.
Our online services consisted of a subscription-based service that offered two specific products, the JAGNotes (Upgrade/Downgrade) Report and the Rumor Room, which provided timely market reports, including breaking news and potentially market moving information. We derived revenues primarily from the sale of subscriptions.
On November 24, 2004, through one of our subsidiaries, Pixaya (UK) Limited (“Pixaya”), we purchased certain development stage software products and related assets in the United Kingdom from TComm Limited, a company organized in the United Kingdom. We subsequently changed the name of our subsidiary, JAG Media LLC, to Pixaya LLC in order to better reflect its role as owner of Pixaya and primary provider of support for our Pixaya products in the United States. Due to cash constraints, we ceased financing development and marketing by Pixaya of our SurvayaCam product, a mobile surveillance system which streams live video in real time from the point of use back to a control center and, if desired, to other locations. We have only made minimal sales of SurvayaCam as part of our prior marketing and distribution efforts.
| 1 |
In light of the difficulties we encountered in growing our JAG Notes subscription business and Pixaya business, we began seeking merger and acquisition candidates, in related and unrelated lines of businesses, to augment our current business. On July 31, 2009, we completed the acquisition of CardioGenics, a developer of products targeting the immunoassay segment of the Point-of-Care IVD testing market, based in Ontario, Canada. See “—Our Acquisition of CardioGenics.” On February 11, 2010 we sold our Pixaya LLC subsidiary, and its related JAG Notes subscription and Pixaya businesses, since we believe it would be more beneficial for our resources to be devoted solely to the development and commercialization of our core CardioGenics products.
CardioGenics Inc.
CardioGenics was founded in Toronto, Canada in 1997 by Dr. Yahia Gawad to develop technology and products targeting the immunoassay segment of the IVD testing market. These include:
| ▪ | The QL Care Analyzer (the “QLCA”), a state-of-the-art proprietary Point-of-Care (“POC”) immunoassay analyzer; |
| ▪ | A series of immunoassay tests to detect cardiac markers (the “Cardiovascular Tests”); and, |
| ▪ | Paramagnetic beads developed through its proprietary method, which improves their light collection (the “Beads”). |
| 2 |
Our Industry
CardioGenics IVD POC Testing Markets
IVD Market
In vitro diagnostics (IVD) refers to testing that aims for the identification of disease states outside the body, using samples such as body fluids (blood, urine) and tissues (biopsies and tissue sections). The IVD is a well established market, offering essential products (tests, components and machinery) used by physicians and clinical chemistry personnel to assess disease conditions. The world market for IVD is estimated at $42 billion in 2007 and is expected to grow 6% annually to $56.3 billion by 20121. North America, Europe, Japan and Western Europe currently make up 81% of the total IVD market, and this is expected to decrease to 76% by 2012 as China and India become more significant players in the IVD market. Sales of IVD products in emerging economies in Latin America and Eastern Europe are expected to grow from 4% of the market in 2007 to 5% in 2012. Overall, sales growth of IVD products in emerging markets will account for 10-20% annual growth in the IVD market, while the developed world will see annual growth of 3-6%.2
The following table summarizes the market size and projections of the IVD market and the sub-sectors where our products will compete:
| Product | 2007 | 2008 | 2009 | 2010 | 2011 | |||||||||||||||
| IVD (billions) | 42.1 | 44.5 | 47.1 | 49.1 | 52.9 | |||||||||||||||
| Immunoassay Testing (billions) | 4.185 | 4.435 | 4.695 | 4.975 | 5.260 | |||||||||||||||
| POC Testing (billions) | 1.625 | 1.715 | 1.815 | 1.910 | 2.02 | |||||||||||||||
| Cardiac Marker Tests (millions) | 425 | 471.75 | 523.64 | 581.24 | 645.17 | |||||||||||||||
In 2007, 16 top tier IVD companies occupied 78% of the global market ($32 billion). Since 2005, there has been a trend toward consolidation at all levels of the IVD market. In 2007, three top tier companies, DPC, Dade Behring and Bayer Diagnostics, merged to become Siemens Medical Diagnostics.
Immunoassay Market
The 2007 world market for all immunoassays excluding infectious diseases is estimated at $4,185 million3, and by 2012 the market is projected to grow by 6% annually to reach $5,605 million worldwide. Immunoassays sales for cardiac markers were 785 million in 2007, or 12% of market, and this is expected to increase to 1,050 million (12%) by 20124. The following Table illustrates the relationships between the top IVD companies and sales of IVD products.
1 This includes all laboratory, hospital-based products and OTC products, according to Kalorama Information, The Worldwide Market for In Vitro Diagnostics Tests, 6th Edition, June 2008
2 Kalorama Information, The Worldwide Market for In Vitro Diagnostics Tests, 6th Edition, June 2008, p3
3 $6.685 million including infectious diseases
4 Kalorama Information, The Worldwide Market for In Vitro Diagnostics Tests, 6th Edition, June 2008, p401
| 3 |
Revenue History of Leading Immunoassay Vendors, $ million 2005-20075
| 2007 | 2006 | 2005 | ||||||||||
| Abbott Diagnostics | 2,100 | 1,900 | 1,800 | |||||||||
| Siemans/Dade Behring | 825 | 785 | 750 | |||||||||
| Siemens/Bayer | 750 | 714 | 680 | |||||||||
| Beckman Coulter | 596 | 484 | 402 | |||||||||
| Siemens/DPC | 595 | 517 | 473 | |||||||||
| Roche | 575 | 509 | 450 | |||||||||
| bioMérieux | 363 | 362 | 353 | |||||||||
| Fujirebio | 299 | 277 | 279 | |||||||||
| Ortho | 200 | 190 | 160 | |||||||||
| TOTAL | 6,303 | 5,738 | 5,347 | |||||||||
Immunoassay testing segment of the IVD market is characterized by:
Expanding opportunities after completion of the human genome project.
Demand for automated and sensitive POC immunoassay analyzers.
Search for an ideal POC platform.
Increased mergers and acquisition among top tier IVD companies to achieve more complete product lines
Greater cooperation between test developers and top tier IVD companies.
Over the next 5-10 years, the immunoassay business will see:
| The continued automation of routine immunoassays – thyroid, anemia, fertility, therapeutic drug monitoring and drugs of abuse; and |
| More new assays and test categories for disease risk evaluation.6 |
Point-Of-Care (POC) Testing Market
Point Of Care (POC) testing refers to a laboratory assay that can be performed outside of a centralized facility, with results available within minutes. POC testing is divided into personal use tests, such as pregnancy tests, and professional use tests, that are administered in a physician‘s office or hospital emergency ward. Our tests will compete in the professional use testing market sector.
The market for professional7 POC immunoassays is estimated at $1,625 million in 2007 and with the 14% projected growth, this market will reach $2,770 million in 2012. It is anticipated that most of the growth will come from increased use of cardiac markers and new assays for cancer markers and diabetes/cardiac disease markers. The market for professional POC tests for cardiac markers is estimated at $425 million in 2007 (11%) and this is expected to increase to $850 million (15%) by 2012.7
5 Estimated. Kalorama Information, The Worldwide Market for In Vitro Diagnostics Tests, 6th Edition, June 2008, p402
6 Kalorama Information, The Worldwide Market for In Vitro Diagnostics Tests, 6th Edition, June 2008
7 Administered in a professional setting, i.e. not home tests.
| 4 |
There is a wide perception that POC tests are more expensive than lab-based tests and that patient test results are lost to the historical record. There is also the perception that once the patient leaves the acute care area, the baseline POC tests done in that unit are of little value because the POC testing results do not correlate with lab-based systems.
Two critical characteristics are necessary for potential POC test products to become more prevalent; POC testing results must correlate with lab results and the POC devices must be more consistent and robust in delivering those results.
The impact of POC testing on improving patients’ care is clear and has been well documented. Further, the impact of POC testing on saving healthcare resources was also demonstrated by numerous agencies and institutions.
Cardiovascular Disease Testing Market
Cardiac markers are proteins released from heart muscle when it is damaged as a result of a heart attack (myocardial infarction), when the blood supply to part of the heart is interrupted. Physicians use cardiac markers in two ways – to diagnose a cardiac event in a hospital emergency room or within the hospital or to evaluate a risk of a cardiovascular event occurring. The routine markers of myocardial infarction – CK-MB, troponin and myoglobin and recently BNP are used in the acute care and tests such as cholesterol are used to evaluate risk.
The world market for cardiac markers is estimated at $740 million in 2007, and with projected annual growth of 5%, will reach $1,050 million in 2012.
Until recently, Troponin and CK-MB were the lead cardiac markers. Brain Natriuetic Pepetide (BNP) was recently introduced to differentiate between a myocardial infarction and heart failure. A number of companies are focused on developing new cardiac markers.
Magnetic Particles Market
Magnetic particles, or beads, are widely used as the solid phase for binding tests for automating and simplifying the methods for isolation and detection of biomolecules in both research and routine clinical laboratories. Eight of the top 10 IVD companies employ magnetic particles in their fully automated analyzers.
An independent 2006 market research report, prepared for CardioGenics by Adventus Research Inc. (the “Adventus Report”) and sponsored by the National Research Council of Canada (NRC), estimated the market for magnetic beads for immunoassays and molecular diagnostics to be approximately $900 million (between $833 million and $1.3 billion). The report of market size did not include magnetic beads produced in-house by some of the IVD test manufacturers or beads produced for research applications. The Adventus Report was conducted using several methods, including interviews with leading particle-manufacturers and the end-users, published industry reports and data from leading IVD manufacturers.
| 5 |
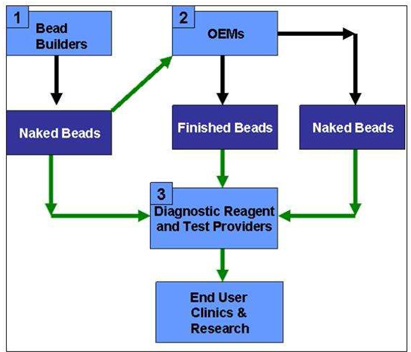
As stated in the Adventus Report, according to Dynal, a leading magnetic beads manufacturer, the largest part of its Molecular Systems’ business is OEM sales of magnetic beads to IVD companies. Dynal stated that “the IVD market is very large, and still growing. However, the magnetic bead-based part of this market is growing at an even higher rate per year”.8 According to Dynal, immunoassays make up more than USD 4 billion of the IVD market, and magnetic beads are now the gold standard for immunoassay testing, as opposed to older technologies such as microtitre plate based tests. Nucleic acid testing makes up a smaller portion of the IVD market, USD 2 billion, but is fast growing. Magnetic beads are also the most common solid phase employed in this market.
Furthermore, according to Dynal, as stated in the Adventus Report, end-user business rather than OEM business (referred to as functionalized and naked beads markets respectively) goes to research and routine laboratories within Genomics, Expression Profiling and Proteomics. The market size for Genomics, including DNA and RNA extraction and purification products was USD 300 million in 2001 while the market size of Pharmacogenomics was estimated to be USD 2.3 billion in 2001.
As stated in the Adventus Report, according to Gen-Probe, which is a leading DNA clinical testing company, other markets that are employing magnetic beads as a solid phase are growing also. Further, magnetic particles are used for Separation of Microorganisms in Food and Water Testing and also for HLA testing for organ transplantation.
8 Adventus Report
| 6 |
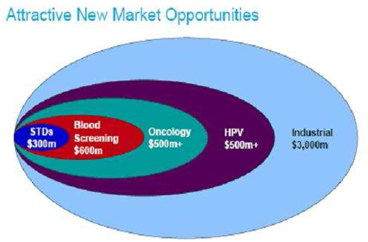
Source: Gen-Probe presentation- May 2006
Our Products
The CardioGenics Products
QL Care Analyzer

The QLCA represents a shift in the design of POC analyzers. The QLCA is a small, portable, stand-alone and completely automated point-of-care immunoassay analyzer. The QLCA has successfully miniaturized lab test technology, and combined it with a simplified mechanical design and proprietary triggering mechanism.
The QLCA uses a proprietary self-metering cartridge to perform immunoassay tests at the POC. Each cartridge is pre-loaded with our beads, which have been coated with specific bioluminescent proteins linked to the target marker. A drop of whole blood added to the Cartridge creates the chemiluminescent reaction needed to deliver sensitive and accurate test results. Operation of the QLCA does not require specialized training and testing can be completed in 15 minutes.
POC immunoassay analyzers are not new; however, none of the commercial analyzers can replicate the sensitivity and accuracy of a test done in a medical lab. The QLCA delivers the required laboratory sensitivity and accuracy. The QLCA employs chemical light generation or “chemiluminescence“ (“CL“), the same technology used in the medical labs. The QLCA uses a patented automated electronic process to trigger CL, which enhances light collection, speeds up marker binding and increases sensitivity.
| 7 |
We have rigorously tested the QLCA protocols and have compared our test results against medical laboratory test data. Based on these internal test results, we have consistently met or exceeded the sensitivity standards of medical laboratory immunoassay equipment.
Cardiovascular Tests
To support the use of the QLCA, we have developed four immunoassay tests designed to identify cardiac markers in the blood at the time of a heart attack.
| Test | Description | ||
| Troponin I (TnI) | ▪ | TnI testing is the current routine testing for a heart attack. | |
| ▪ | TnI is a heart muscle protein, released in the bloodstream shortly after a heart attack (myocardial infarction or MI). | ||
| ▪ | Current laboratory analyzers cannot detect TnI before 4-6 hours after the onset of symptoms, when TnI concentration in the blood reaches its detection threshold. | ||
| ▪ | Our test will take only 15 minutes to deliver quantitative results, allowing physicians to obtain much more rapid results and therefore accelerate patient triage. | ||
| ▪ | |||
| Plasminogen Activator Inhibitor Type-1 (PAI-1) | ▪ | This test will help to optimize the performance of a heart drug (“tPA” or tissue Plasminogen Activator), a clot buster used as the first line of therapy for MI patients. | |
| ▪ | This proprietary whole blood test will quantify PAI-1 levels within 15 minutes. | ||
| ▪ | Forty percent of patients do not respond to tPA, a fact recognized only after the “golden hour” (the time period in which permanent heart damage can be prevented) has passed. | ||
| ▪ | |||
| Heart Failure Risk Stratification (HFRS) | ▪ | We have discovered a family of related proteins that are released into the bloodstream during heart failure. | |
| ▪ | We are developing a proprietary test, the Heart Failure Risk Stratification or HFRS test to stratify the risk of death in patients with heart failure, thus permitting the initiation of appropriate therapy at an early stage. | ||
| ▪ | |||
| Heart Failure Genomics Risk (HFGR) | ▪ | We are developing a proprietary HFGR test that predicts the response of heart failure patients to routinely administered drugs. | |
| ▪ | The need to measure the precise response to these drugs in a timely manner would minimize the trial and error methods now used by doctors to optimize drugs best suited to each patient. | ||
These tests are designed to be administered in the diagnostic and management process of patients with heart disease. The full scope of our core technology, as well as the know-how we have developed respecting aspects of chemical entrapment in bioassays, are covered under our patent applications.
Upon receipt of FDA approval, we intend to market the QLCA and the Cardiovascular Tests through a major IVD distributor. We have initiated preliminary discussions with several of the Tier 1 IVD companies, and we anticipate that we will commence negotiations with one or more distribution partners before we receive FDA approval. In accordance with industry practice, we intend to enter into a license agreement with our distribution partner for the manufacture and distribution of our products.
| 8 |
Paramagnetic Beads
Medical laboratories widely use paramagnetic particles as a solid surface in heterogeneous immunoassay tests utilizing the process of phase separation done by eletromagnetic field. Such tests involve the measurement of light generated on the surface area of paramagnetic beads coated with bio-organic material.

Our Beads represent a significant product advance. Most paramagnetic beads are made of iron oxide, and all are traditionally black or brown. We have developed a proprietary process that coats the beads with a layer of silver, making them white, and more sensitive to light. Our production process is also significantly less expensive than those used by our competitors. We have internally tested our Beads against all commercially available beads, and have found our silver-coated Beads to be five times more sensitive than traditional black or brown magnetic particles.
On January 19, 2009 CardioGenics Inc., one of our Canadian subsidiaries, entered into a Supply, Development & Distribution Agreement with Merck Chimie S.A.S. (“Merck Chimie”) (the “Merck Agreement”), pursuant to which CardioGenics is required to furnish Merck Chimie with certain quantities of CardioGenics’ proprietary silver-coated paramagnetic beads (the “CardioGenics Test Samples”), which Merck Chimie is then required to encapsulate, on a test-basis, using Merck Chimie’s proprietary encapsulation process. After Merck Chimie selects the best encapsulation process, Merck Chimie agreed to then establish the manufacturing parameters for the final encapsulated beads (the “Merck Encapsulated Beads”) and thereafter scale-up production for commercial distribution of the Merck Encapsulated Beads. Currently, Merck Chimie is still in the process of refining its encapsulation of the CardioGenics Test Samples.
Pursuant to the Merck Agreement, Merck Chimie has the exclusive right, for ten (10) years, to distribute the Merck Encapsulated Beads on a worldwide basis, with CardioGenics receiving 30% of the net sales proceeds of the Merck Encapsulated Beads and Merck receiving 70% of such net sales proceeds. Merck is responsible for manufacturing and distributing the Merck Encapsulated Beads.
In addition to the agreement with Merck Chimie, we have also entered into Materials Transfer Agreements (“MTA”) with two other major international beads distributors with respect to our proprietary magnetic beads. Under the first MTA, CardioGenics will furnish the distributor with its silver-coated magnetic beads for polymer coating by the distributor. In addition, the distributor will provide CardioGenics with their magnetized bead prototypes, which CardioGenics will then silver-coat with its proprietary silver-coating technology. Under the second MTA, CardioGenics will furnish the distributor with its silver-coated magnetic beads for polymer coating and subsequent testing and evaluation by the distributor. Upon completion of the testing process, CardioGenics and the distributors will evaluate the test results and determine whether to further pursue commercialization of the resulting products.
Our Strategy
The success of our business depends on our ability to obtain the requisite financing and be able to:
| ● | complete the development of our QLCA and our cardiovascular tests; |
| ● | obtain FDA approval of our QLCA and the cardiovascular tests; |
| ● | develop further tests that can be run on our QLCA; |
| ● | commercialize our Beads. |
| 9 |
We will require additional funds in order to implement our full business strategy. Accordingly, we will need to raise additional funds through public or private financing, strategic relationships or other arrangements. We do not anticipate generating any significant revenue until after our first cardiovascular test has been approved by the FDA and our Beads are commercialized by Merck Chimie pursuant to our agreement with them.
Since our strength is product development and innovation, our strategy is focused on exploiting this strength. In terms of product development and innovation, we employ our internal resources to develop our products through the various phases of development. We also rely on external service providers to supplement our internal talents in product development.
We will outsource product manufacturing. In terms of the QLCA, both the cartridge assembly as well as the analyzer assembly will be contracted out to different OEM providers with the facilities and expertise to deliver quality products. We will maintain a quality control process to ensure that the products meet the predetermined specifications.
Product marketing and distribution will by achieved through partnerships with global companies with wide reach. As we have done with our magnetic beads, the QLCA will be marketed by a third party through licensing and distribution agreements. Notwithstanding this strategy, we also intend to evaluate the feasibility of directly marketing our magnetic beads and QLCA to appropriate end-users and may use such direct marketing efforts to supplement the efforts of our future distribution partner(s).
We are also focusing on protecting our intellectual property and know how though maintaining a patent filing process on a global basis as well as maintaining confidentiality agreements with our staff, employees and service providers under contractual agreements.
Although we believe in these strategies, goals and targets, we cannot guarantee that we will be successful in implementing them or that, even if implemented, they will be effective in creating a profitable business. In addition, we are dependent on having sufficient cash to carry out our strategies
Regulation
CardioGenics Products
Our QL Analyzer, Cartridge and Tests are classified as medical devices. Our beads are reagents of medical testing equipment. Accordingly, they are subject to a number of regulations in the jurisdictions where our products will be sold.
United States
The testing, production and sale of IVD products are subject to regulation by numerous state and federal government authorities, principally the FDA.
Pursuant to the U.S. Federal Food, Drug and Cosmetic Act (“FD&C Act”), the FDA regulates the preclinical and clinical testing, manufacture, labeling, distribution and promotion of medical devices.
Medical devices are classified into three categories, Class I, Class II or Class III. The classification of a device is based on the level of control necessary to assure the safety and effectiveness of the device. Generally, the complexity of the submission and the approval times are based on the regulatory class of the device. Device classification depends on the intended use and also the indications for use of the device. Classification is also based on the risk the device poses to the patient and/or the user. Class I devices include devices with the lowest risk, and Class III devices are those with the greatest risk. Class I devices are subject to general control, Class II devices are subject to general controls and special controls, and Class III devices are subject to general controls and must receive a Premarket Assessment or PMA by the FDA.
| 10 |
Before some Class I and most Class II devices can be introduced in the market, either the manufacturer or distributor of the device is required to follow the pre-market notification process described in section 510(k) of the FD&C Act. A 510(k) is a pre-marketing submission made to the FDA to demonstrate that the device to be marketed is as safe and effective, and is substantially equivalent to a legally marketed device. Applicants must compare their 510(k) device to one or more similar devices currently on the US market and support their claims for substantial equivalency. The FDA requires a rigorous demonstration of substantial equivalency. It generally takes three to six months from submission to obtain 510(k) clearance. If any device cleared through 510(k) is modified or enhanced, or if there is a change of use of the device, a new amended 510(k) application must be submitted. According to FDA regulations and our management team’s prior experiences with submissions of similar products, our QLCA and launch product (TnI) will be classified as a Class II device and will be subjected to the 510(K) process. Further, a second test product of ours (HFRS) will also be subjected to the same 510(K) process. As for both tests, predicate devices are commercially available. For other test products, depending on the claims and with a prior agreement with the FDA, the submissions would be either a PMA or 510(K). We have not yet approached the FDA for that purpose.
Canada
Health Canada sets out the requirements governing the sale, importation and advertisement of medical devices. These regulations are intended to ensure that medical devices distributed in Canada are both safe and effective. We are also required to comply with certain procedures for the disposal of waste products under the Canadian Code of Practice for the Management of Biological Waste (the “Code”). We believe we are currently in compliance with all required Code provisions.
Europe
Our products will be subject to registration under the EU Medical Device Directives for in-vitro diagnostic products.
Other countries
Our products will be subject to the regulations of any country where they are sold, and we will make the necessary applications for approval on a country-by-country basis.
Competition
CardioGenics Competitors
Numerous companies provide Point Of Care (POC) products, many with cardiovascular test products. However, in terms of quantitative POC products, few companies operate in this space with marketed devices. These include:
| ● | Biosite Diagnostics Incorporated; |
| ● | Response Biomedicals Corp.; |
| ● | Roche POC division; and |
| ● | i-Stat division of Abbott Diagnostics |
The first 3 companies employ fluorescence measurements in their platforms, while i-Stat employs electrochemical testing. We believe that our technology and products in development will offer superior products to the POC market. None of the above companies offer chemiluminescence in its platform, a technology that is well-recognized for its superiority as evidenced by its dominance in the laboratory testing market. We believe that harnessing chemiluminescence in our QLCA will fulfill the clinical demands for fast and accurate quantitative results at patient bedsides.
Research and Development
Our efforts are focused on the development of our QLCA and our cardiovascular tests and the commercialization of our beads. Over the years 2011 and 2012 we incurred expenses of $613,504 and $603,033 respectively on those efforts.
Website Technical Information
Our CardioGenics website (www.cardiogenics.com) and the website of our wholly owned subsidiary, LuxSpheres (www.luxspheres.com), are maintained by us internally and are hosted by DreamHost, which has hosting facilities located in Brea, California.
| 11 |
Employees
As of October 31, 2012, we had nine (9) employees. Of those employees, only Yahia Gawad, our Chief Executive Officer, has an employment agreement with the Company.
Acquisition of CardioGenics
On July 31, 2009 we completed the acquisition of CardioGenics by CardioGenics ExchangeCo Inc. (“ExchangeCo”), our Ontario, Canada subsidiary, pursuant to the terms of a Share Purchase Agreement dated May 22, 2009 among ExchangeCo, JAG Media Holdings, Inc., CardioGenics and CardioGenics’ principal stockholder, Yahia Gawad (the “Share Purchase Agreement”). CardioGenics is considered the acquirer in the transaction for accounting and financial reporting purposes.
In connection with the acquisition, ExchangeCo acquired all of the outstanding common shares of CardioGenics (the “CardioGenics Common Shares”), excluding 173,869 CardioGenics Common Shares in the aggregate owned by two (2) minority stockholders of CardioGenics (the “Dissenting Stockholders”), in consideration for the issuance of 422,183,610 shares of our common stock to the CardioGenics stockholders at the closing, as further described below (the “Share Consideration”). In consideration for the surrender of their CardioGenics Common Shares, the CardioGenics stockholders had the option to receive at the closing their pro-rata allocation of the Share Consideration in the form of (a) our common shares or (b) “Exchangeable Shares“ of ExchangeCo, which are exchangeable into our common shares in accordance with the terms of a Voting and Exchange Trust Agreement dated July 6, 2009 among JAG Media, ExchangeCo, and WeirFoulds LLP, as trustee and the rights and preferences of the Exchangeable Shares. Those CardioGenics stockholders who elected to receive directly our common shares were issued, in the aggregate, 145,528,195 common shares at the closing and those CardioGenics stockholders who elected to receive Exchangeable Shares were issued 16 Exchangeable Shares at the closing, which are exchangeable at any time into 276,655,415 of our common shares, in the aggregate. The Share Consideration issued at the closing provided the CardioGenics stockholders with direct and indirect ownership of approximately 85% of our outstanding common stock, on a fully diluted basis.
Immediately prior to the closing, all CardioGenics debenture holders converted their debentures into CardioGenics Common Shares in accordance with the terms of their respective debentures, as required by the terms of the Share Purchase Agreement. Accordingly, such former debenture holders became CardioGenics stockholders for purposes of the acquisition and received their pro-rata allotment of the Share Consideration in the form of JAG Common Shares and/or Exchangeable Shares at the closing in consideration for the surrender of the CardioGenics Common Shares they received upon conversion of their debentures.
Also prior to the closing, CardioGenics closed on an equity investment round of financing totaling $2,715,000. These equity investors in CardioGenics became CardioGenics stockholders for purposes of the acquisition and received their pro-rata allotment of the Share Consideration in the form of our common shares.
All of our common shares received by CardioGenics stockholders in exchange for their CardioGenics Common Shares may not be registered for resale and, therefore, shall remain subject to the rights and restrictions of Rule 144. All Exchangeable Shares received by CardioGenics stockholders in exchange for their CardioGenics Common Shares (and any of our common shares into which such Exchangeable Shares may be exchanged) also may not be registered for resale prior to six (6) months following the closing and, therefore shall remain subject to the rights and restrictions of Rule 144 prior to any such registration.
Also at the closing, all holders of CardioGenics warrants entitling the holders to purchase CardioGenics Common Shares at various prices exchanged their CardioGenics warrants for warrants to purchase, in the aggregate, 36,148,896 of our common shares at exercise prices of $0.047 per share, in accordance with the terms of the Share Purchase Agreement and the respective warrants. The terms of these newly issued warrants did not include any registration rights for the warrant holders. CardioGenics had no options to acquire CardioGenics Common Shares outstanding as of the closing.
| 12 |
At the closing, our then current directors resigned as directors of JAG Media and its subsidiaries after appointing their successors and our then current officers also resigned as officers and executives of JAG Media and its subsidiaries. After their resignation and the closing, our former directors entered into consulting agreements with the Company pursuant to which they are rendering various services to assist us in connection with certain transition matters. Each consulting agreement is for a term of 18 months, with each party receiving 500,000 shares of the Company’s common stock, issued pursuant to our 1999 Long-Term Incentive Plan, as compensation for their services under the consulting agreements.
Following the closing, a majority of our stockholders approved, by written consent, an amendment to our articles of incorporation, which provided for (a) a change in our corporate name from “JAG Media Holdings, Inc.“ to “CardioGenics Holdings Inc.” and (b) an increase in the number of our authorized JAG Common Shares from 500,000,000 to 650,000,000.
Financing Arrangements
None.
Increase in Authorized Shares
In October 2009 a majority of our stockholders approved, by written consent, an amendment to our articles of incorporation, which provided for, among other matters, an increase in the number of our authorized shares of common stock from 500,000,000 to 650,000,000. The 650,000,000 authorized shares of our common stock were subsequently reduced to 65,000,000 authorized shares of common stock pursuant to a reverse stock split implemented by the Company on June 18, 2010, as further described below.
On October 17, 2012 a majority of our stockholders approved an amendment to our articles of incorporation, which provided for, among other matters, an increase in the number of our authorized shares of common stock from 65,000,000 to 150,000,000.
Reverse Stock Split
As authorized by our Board of Directors, on June 18, 2010, we filed a “Certificate of Change” with the Nevada Secretary of State’s Office, which effected a 1:10 share consolidation of our outstanding and authorized shares of common stock. As a result of this share consolidation our authorized shares of common stock was reduced from 650,000,000 to 65,000,000 and our outstanding shares of common stock as of such date were consolidated in accordance with the 1:10 share consolidation ratio.
Facilities
See “Item 2.—Properties.”
Legal Proceedings
See “Item 3.—Legal Proceedings.”
Where You Can Find More Information About Us
We are required to file annual, quarterly and current reports, proxy statements and other information with the SEC. You can read and copy any of this information at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549 on official business days during the hours of 10:00 a.m. to 3:00 p.m. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. This information is also available from the SEC’s website at http://www.sec.gov. We will also gladly send any filing to you upon your written request to Dr. Yahia Gawad, our Chief Executive Officer, at 6295 Northam Drive, Unit 8, Mississauga, Ontario L4V 1W8.
| 13 |
ITEM 1A. RISK FACTORS
Risks Related to Our CardioGenics Business and Industry
The global financial crisis has had, and may continue to have, an impact on our business and financial condition.
The ongoing global financial crisis may limit our ability to access the capital markets at a time when we would like, or need, to raise capital, which could have an impact on our ability to react to changing economic and business conditions. Accordingly, if the global financial crisis and current economic downturn continue or worsen, our business, results of operations and financial condition could be materially and adversely affected.
The requirements of being a public company may strain our resources and distract our management
As a public company, we are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended and the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”). These requirements place a strain on our systems and resources. The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal controls for financial reporting. Management has identified the following material weaknesses in our internal controls over financial reporting: 1. lack of documented policies and procedures; 2. lack of resources to account for complex and unusual transactions; and, 3. there is no effective segregation of duties, which includes monitoring controls, between the members of management.
We are also required to document and test our internal control procedures in order to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act, which requires annual management assessments of the effectiveness of our internal controls over financial reporting. We may not be able to remediate these weaknesses in time to meet the deadlines imposed by the Sarbanes-Oxley Act. If we fail to achieve and maintain the adequacy of our internal controls, as such standards are modified, supplemented or amended from time to time, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with the Sarbanes-Oxley Act.
In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal control over financial reporting, significant resources and management oversight will be required. This may divert management’s attention from other business concerns, which could have a material adverse effect on our business, financial condition, results of operations and cash flows. In addition, we may need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge, and we cannot assure you that we will be able to do so in a timely fashion.
We have not earned any material revenues in our CardioGenics business unit since its incorporation and only have a limited operating history in its current business, which raise doubt about our ability to continue as a going concern.
Our CardioGenics business unit has a limited operating history in its current business and must be considered in the development stage. It has not generated any material revenues since its inception and we will, in all likelihood, continue to incur operating expenses without significant revenues until we complete development of our Cardiovascular Tests and commercialize our QLCA and the Cardiovascular Tests. The primary source of funds for our CardioGenics business unit has been the sale of common stock. We cannot assure that we will be able to generate any significant revenues or income. These circumstances make us dependent on additional financial support until profitability is achieved. There is no assurance that we will ever be profitable and we have not yet achieved profitable operations. These factors raise substantial doubt that we will be able to continue as a going concern.
| 14 |
We need to raise additional financing to support the research and development of our CardioGenics business but we cannot be sure that we will be able to obtain additional financing on terms favorable to us when needed. If we are unable to obtain additional financing to meet our needs, our operations may be adversely affected or terminated.
Our ability to develop new test products for our QLCA is dependent upon our ability to raise significant additional financing when needed. If we are unable to obtain such financing, we will not be able to fully develop and commercialize our platform and technology. Our future capital requirements will depend upon many factors, including:
| • | continued scientific progress in our research and development programs; |
| • | costs and timing of conducting clinical trials and seeking regulatory approvals and patent prosecutions; |
| • | competing technological and market developments; |
| • | our ability to establish additional collaborative relationships; and |
| • | the effect of commercialization activities and facility expansions if and as required. |
We have limited financial resources and to date, no material cash flow from the operations of our CardioGenics business unit and we are dependent for funds on our ability to sell our common stock, primarily on a private placement basis. There can be no assurance that we will be able to obtain financing on that basis in light of factors such as the market demand for our securities, the state of financial markets generally and other relevant factors. Any sale of our common stock in the future will result in dilution to existing stockholders. Furthermore, there is no assurance that we will not incur debt in the future, that we will have sufficient funds to repay any future indebtedness or that we will not default on our future debts, jeopardizing our business viability. Finally, we may not be able to borrow or raise additional capital in the future to meet our needs or to otherwise provide the capital necessary to continue the development of our technology, which might result in the loss of some or all of your investment in our common stock.
We may acquire other businesses, license rights to technologies or products, form alliances, or dispose of or spin-off businesses, which could cause us to incur significant expenses and could negatively affect profitability.
We may pursue acquisitions, technology licensing arrangements, and strategic alliances, or dispose of or spin-off some of our businesses, as part of our business strategy. We may not complete these transactions in a timely manner, on a cost-effective basis, or at all, and may not realize the expected benefits. If we are successful in making an acquisition, the products and technologies that are acquired may not be successful or may require significantly greater resources and investments than originally anticipated. We may not be able to integrate acquisitions successfully into our existing business and could incur or assume significant debt and unknown or contingent liabilities. We could also experience negative effects on our reported results of operations from acquisition or disposition-related charges, amortization of expenses related to intangibles and charges for impairment of long-term assets.
The expiration or loss of patent protection and licenses may affect our future revenues and operating income.
Much of our business relies on patent and trademark and other intellectual property protection. Although most of the challenges to our intellectual property would likely come from other businesses, governments may also challenge intellectual property protections. To the extent our intellectual property is successfully challenged, invalidated, or circumvented or to the extent it does not allow us to compete effectively, our business will suffer. To the extent that countries do not enforce our intellectual property rights or to the extent that countries require compulsory licensing of our intellectual property, our future revenues and operating income will be reduced. Our principal patents and trademarks are described in greater detail in the sections captioned, "Patents, Trademarks, and Licenses."
| 15 |
Competitors' intellectual property may prevent us from selling our products or have a material adverse effect on our future profitability and financial condition.
Competitors may claim that one or more of our products infringe upon their intellectual property. Resolving an intellectual property infringement claim can be costly and time consuming and may require us to enter into license agreements. We cannot guarantee that we would be able to obtain license agreements on commercially reasonable terms. A successful claim of patent or other intellectual property infringement could subject us to significant damages or an injunction preventing the manufacture, sale or use of our affected products. Any of these events could have a material adverse effect on our profitability and financial condition.
We may not be able to adequately protect our intellectual property
We believe the patents, trade secrets and other intellectual property we use are important to our business, and any unauthorized use of such intellectual property by third parties may adversely affect our business and reputation. We rely on the intellectual property laws and contractual arrangements with our employees, business partners and others to protect such intellectual property rights. Filing, prosecuting, defending and enforcing patents on all of our technologies and products throughout the world would be prohibitively expensive. Competitors may, without our authorization, use our intellectual property to develop their own competing technologies and products in jurisdictions where we have not obtained patent protection. These technologies and products may not be covered by any of our patent claims or other intellectual property rights. Furthermore, the validity, enforceability and scope of protection of intellectual property in some countries where we may conduct business is uncertain and still evolving, and these laws may not protect intellectual property rights to the same extent as the laws of the United States.
Many companies have encountered significant problems in protecting and defending their intellectual property rights in foreign jurisdictions. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties (for example, the patent owner has failed to “work” the invention in that country or the third party has patented improvements). In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. Moreover, litigation involving patent or other intellectual property matters in the United States or in foreign countries may be necessary in the future to enforce our intellectual property rights, which could result in substantial costs and diversion of our resources, and have a material adverse effect on our business, financial condition and results of operations.
We are subject to numerous governmental regulations and it can be costly to comply with these regulations and to develop compliant products and processes.
Our products are subject to regulation by the U.S. Food and Drug Administration (“FDA”), and numerous international, federal, and state authorities. The process of obtaining regulatory approvals to market a medical device can be costly and time-consuming, and approvals might not be granted for future products, or additional uses of existing products, on a timely basis, if at all. Delays in the receipt of, or failure to obtain approvals for, future products, or additional uses of existing products, could result in delayed realization of product revenues, reduction in revenues, and in substantial additional costs. In particular, in the United States our products are regulated under the 1976 Medical Device Amendments to the Food, Drug and Cosmetic Act, which is administered by the FDA. We believe that the FDA will classify our products as “Class II” devices, thus requiring us to submit to the FDA a pre-market notification form or 510(k). The FDA uses the 510(k) to substantiate product claims that are made by medical device manufacturers prior to marketing. In our 510(k) notification, we must, among other things, establish that the product we plan to market is “substantially equivalent” to (1) a product that was on the market prior to the adoption of the 1976 Medical Device Amendment or (2) a product that the FDA has previously cleared.
The FDA review process of a 510(k) notification can last anywhere from three to six months, and the FDA must issue a written order finding “substantial equivalence” before a company can market a medical device. We are currently developing a group of cardiovascular tests that we will have to clear with the FDA through the 510(k) notification procedures. These test products are crucial for our success and if we do not receive 510(k) clearance for a particular product, we will not be able to market these products in the United States, which will have a material adverse effect on our revenues, profitability and financial condition.
| 16 |
In addition, no assurance can be given that we will remain in compliance with applicable FDA and other regulatory requirements once clearance or approval has been obtained for a product. We must incur expense and spend time and effort to ensure compliance with these complex regulations. Possible regulatory actions could include warning letters, fines, damages, injunctions, civil penalties, recalls, seizures of our products and criminal prosecution. These actions could result in, among other things: substantial modifications to our business practices and operations; refunds, recalls, or seizures of our products; a total or partial shutdown of production while we or our suppliers remedy the alleged violation; the inability to obtain future pre-market clearances or approvals; and, withdrawals or suspensions of current products from the market. Any of these events could disrupt our business and have a material adverse effect on our revenues, profitability and financial condition.
Changes in third-party payor reimbursement regulations can negatively affect our business.
By regulating the maximum amount of reimbursement they will provide for blood testing services, third-party payors, such as HMOs, pay-per-service insurance plans, Medicare and Medicaid, can indirectly affect the pricing or the relative attractiveness of our diagnostic products. For example, the Centers for Medicare and Medicaid Services set the level of reimbursement of fees for blood testing services for Medicare beneficiaries. If third-party payors decrease the reimbursement amounts for blood testing services, it may decrease the amount that physicians and hospitals are able to charge patients for such services. Consequently, we would either need to charge less for our products or incur a reduction in our profit margins. If the government and third-party payors do not provide for adequate coverage and reimbursement levels to allow health care providers to use our products, the demand for our products will decrease.
Laws and regulations affecting government benefit programs could impose new obligations on us, require us to change our business practices, and restrict our operations in the future.
Our industry is also subject to various federal, state, and international laws and regulations pertaining to government benefit program reimbursement, price reporting and regulation, and health care fraud and abuse, including anti-kickback and false claims laws, the Medicaid Rebate Statute, the Veterans Health Care Act, and individual state laws relating to pricing and sales and marketing practices. Violations of these laws may be punishable by criminal and/or civil sanctions, including, in some instances, substantial fines, imprisonment, and exclusion from participation in federal and state health care programs, including Medicare, Medicaid, and Veterans Administration health programs. These laws and regulations are broad in scope and they are subject to evolving interpretations, which could require us to incur substantial costs associated with compliance or to alter one or more of our sales or marketing practices. In addition, violations of these laws, or allegations of such violations, could disrupt our business and result in a material adverse effect on our revenues, profitability, and financial condition.
Our research and development efforts may not succeed in developing commercially successful products and technologies, which may cause our revenue and profitability to decline.
To remain competitive, we must continue to launch new products and technologies. To accomplish this, we must commit substantial efforts, funds, and other resources to research and development. A high rate of failure is inherent in the research and development of new products and technologies. We must make ongoing substantial expenditures without any assurance that its efforts will be commercially successful. Failure can occur at any point in the process, including after significant funds have been invested.
Promising new product candidates may fail to reach the market or may only have limited commercial success because of efficacy or safety concerns, failure to achieve positive clinical outcomes, inability to obtain necessary regulatory approvals, limited scope of approved uses, excessive costs to manufacture, the failure to establish or maintain intellectual property rights, or infringement of the intellectual property rights of others. Even if we successfully develop new products or enhancements or new generations of our existing products, they may be quickly rendered obsolete by changing customer preferences, changing industry standards, or competitors' innovations. Innovations may not be accepted quickly in the marketplace because of, among other things, entrenched patterns of clinical practice or uncertainty over third-party reimbursement. We cannot state with certainty when or whether any of our products under development will be launched or whether any products will be commercially successful. Failure to launch successful new products or new uses for existing products may cause our products to become obsolete, causing our revenues and operating results to suffer.
| 17 |
New products and technological advances by our competitors may negatively affect our results of operations.
Our products face intense competition from our competitors' products. Competitors' products may be safer, more effective, more effectively marketed or sold, or have lower prices or superior performance features than our products. We cannot predict with certainty the timing or impact of the introduction of competitors' products.
We depend on key members of our management and scientific staff and, if we fail to retain and recruit qualified individuals, our ability to execute our business strategy and generate sales would be harmed.
We are highly dependent on the principal members of our management and scientific staff. The loss of any of these key personnel, including in particular Dr. Yahia Gawad, our Chief Executive Officer, might impede the achievement of our business objectives. We may not be able to continue to attract and retain skilled and experienced scientific, marketing and manufacturing personnel on acceptable terms in the future because numerous medical products and other high technology companies compete for the services of these qualified individuals. We currently do not maintain key man life insurance on any of our employees.
The manufacture of many of our products is a highly exacting and complex process, and if we or one of our suppliers encounter problems manufacturing products, our business could suffer.
The manufacture of many of our products is a highly exacting and complex process, due in part to strict regulatory requirements. Problems may arise during manufacturing for a variety of reasons, including equipment malfunction, failure to follow specific protocols and procedures, problems with raw materials, natural disasters, and environmental factors. In addition, we may use single suppliers for certain products and materials. If problems arise during the production of a batch of product, that batch of product may have to be discarded. This could, among other things, lead to increased costs, lost revenue, damage to customer relations, time and expense spent investigating the cause and, depending on the cause, similar losses with respect to other batches or products. If problems are not discovered before the product is released to the market, recall and product liability costs may also be incurred. To the extent we or one of our suppliers experience significant manufacturing problems, this could have a material adverse effect on our revenues and profitability.
Significant safety issues could arise for our products, which could have a material adverse effect on our revenues and financial condition.
All medical devices receive regulatory approval based on data obtained in controlled testing environments of limited duration. Following regulatory approval, these products will be used over longer periods of time with many patients. If new safety issues arise, we may be required to change the conditions of use for a product. For example, we may be required to provide additional warnings on a product's label or narrow its approved use, either of which could reduce the product's market acceptance. If serious safety issues with one of our products arise, sales of the product could be halted by us or by regulatory authorities. Safety issues affecting suppliers' or competitors' products also may reduce the market acceptance of our products.
In addition, in the ordinary course of business, we may be the subject of product liability claims and lawsuits alleging that our products or the products of other companies that we promote, or may be incorporated in our products, have resulted or could result in an unsafe condition for or injury to patients. Product liability claims and lawsuits and safety alerts or product recalls, regardless of their ultimate outcome, may have a material adverse effect on our business, reputation and financial condition, as well as on our ability to attract and retain customers. Product liability losses are self-insured.
The international nature of our business subjects us to additional business risks that may cause our revenue and profitability to decline.
Since we intend to market our products internationally, our business will be subject to risks associated with doing business internationally. The risks associated with any such operations outside the United States include:
| 18 |
| • | changes in foreign medical reimbursement policies and programs; |
| • | multiple foreign regulatory requirements that are subject to change and that could restrict our ability to manufacture, market, and sell our products; |
| • | differing local product preferences and product requirements; |
| • | trade protection measures and import or export licensing requirements; |
| • | difficulty in establishing, staffing, and managing foreign operations; |
| • | differing labor regulations; |
| • | potentially negative consequences from changes in or interpretations of tax laws; |
| • | political and economic instability; |
| • | inflation, recession and fluctuations in foreign currency exchange and interest rates; and, |
| • | compulsory licensing or diminished protection of intellectual property. |
These risks may, individually or in the aggregate, have a material adverse effect on our revenues and profitability.
Other factors can have a material adverse effect on our future profitability and financial condition.
Many other factors can affect our profitability and financial condition, including:
| • | Changes in or interpretations of laws and regulations including changes in accounting standards, taxation requirements and environmental laws in domestic or foreign jurisdictions. |
| • | Changes in the rate of inflation (including the cost of raw materials, commodities, and supplies), interest rates and the performance of investments held by us. |
| • | Changes in the creditworthiness of counterparties that transact business with or provide services to our distributors or us. |
| • | Changes in business, economic, and political conditions, including: war, political instability, terrorist attacks in the U.S. and other parts of the world, the threat of future terrorist activity in the U.S. and other parts of the world and related military action; natural disasters; the cost and availability of insurance due to any of the foregoing events; labor disputes, strikes, slow-downs, or other forms of labor or union activity; and, pressure from third-party interest groups. |
| • | Changes in our business units and investments and changes in the relative and absolute contribution of each to earnings and cash flow resulting from evolving business strategies, changing product mix, changes in tax rates both in the U.S. and abroad and opportunities existing now or in the future. |
| • | Changes in the buying patterns of a major distributor, retailer, or wholesale customer resulting from buyer purchasing decisions, pricing, seasonality, or other factors, or other problems with licensors, suppliers, distributors, and business partners. |
| • | Difficulties related to our information technology systems, any of which could adversely affect business operations, including any significant breakdown, invasion, destruction, or interruption of these systems. |
| • | Changes in credit markets impacting our ability to obtain financing for our business operations. |
| • | Legal difficulties, any of which could preclude or delay commercialization of products or adversely affect profitability, including claims asserting statutory or regulatory violations, adverse litigation decisions, and issues regarding compliance with any governmental consent decree. |
| 19 |
Risks Related to Our Capital Structure
Our stockholders may experience significant dilution from the exercise of warrants to purchase shares of our Common Stock.
The Company currently has outstanding warrants to purchase 6,287,085 shares of our Common Stock at exercise prices ranging from $0.10 to $1.00 per share. Accordingly, if such warrants are exercised, in whole or part, prior to their expiration dates, you may experience substantial dilution upon exercise of these warrants. In addition, the likelihood of such dilution may be accelerated if the price of our Common Stock increases to a level greater than the exercise price of these warrants.
Future Issuance of Our Common Stock Could Dilute Current Stockholder or Adversely Affect the Market.
Future issuances of our common stock could be at values substantially below the price paid by the current holders of our common stock. In addition, common stock could be issued to fend off unwanted tender offers or hostile takeovers without further stockholder approval. Sales of substantial amounts of our common stock in the public market, or even just the prospect of such sales, could depress the prevailing market price of our common stock and our ability to raise equity capital in the future.
The market for our common stock is limited.
Our common stock is traded on the OTC Bulletin Board. Trading activity in our stock has fluctuated and at times been limited. We cannot guarantee that a consistently active trading market for our stock will continue, especially while we remain on the OTC Bulletin Board.
Because our common stock currently trades below $5.00 per share and is quoted on the OTCBB, our common stock is considered by the SEC to be a “penny stock,” which adversely affects our liquidity.
Our common stock does not currently qualify for listing on any national securities exchange, and we do not anticipate that it will qualify for such a listing in the short-term future. If our common stock continues to be quoted on the OTC Bulletin Board or is traded on the Pink Sheets or other over-the-counter markets, and if the trading price of our common stock remains less than $5.00 per share, our common stock is considered a “penny stock,” and trading in our common stock is subject to the requirements of Rule 15g-9 under the Exchange Act. Under this rule, brokers or dealers who recommend low-priced securities to persons other than established customers and accredited investors must satisfy special sales practice requirements. The broker or dealer must make an individualized written suitability determination for the purchaser and receive the purchaser’s written consent prior to the transaction. SEC regulations also require additional disclosure in connection with any trades involving a penny stock, including the delivery, prior to any penny stock transaction, of a disclosure schedule explaining the penny stock market and its associated risks. These requirements could severely limit the liquidity of such securities in the secondary market because few brokers or dealers are likely to undertake these compliance activities. In addition to the applicability of the penny stock rules, another risk associated with trading in penny stocks may be large price fluctuations.
Our amended charter contains provisions that may discourage an unaffiliated party to take us over.
Without further stockholder action, our Board of Directors could authorize the issuance of additional shares of our common stock as well as preferred stock with special voting rights by class or with more than one vote per share, to a “white knight” in order to deter a potential buyer. This might have the effect of preventing or discouraging an attempt by a party unable to obtain the approval of our Board of Directors to take over or otherwise gain control of us.
Terms of subsequent financings may adversely impact your investment.
We may have to raise equity, debt or preferred stock financing in the future. Your rights and the value of your investment in our Common Shares could be reduced. For example, if we issue secured debt securities, the holders of the debt would have a claim against our assets that would be prior to the rights of stockholders until the debt is paid. Interest on these debt securities would increase costs and negatively impact operating results.
| 20 |
Preferred stock could be issued in series from time to time with such designations, rights, preferences, and limitations as needed to raise capital. The terms of preferred stock could be more advantageous to those investors than to the holders of our Common Shares.
Our articles of incorporation do not provide stockholders the pre-emptive right to buy shares from the company. As a result, you will not have the automatic ability to avoid dilution in your percentage ownership of the company.
Control of our stock is now held by the former CardioGenics shareholders.
The prior shareholders of CardioGenics, as of the closing of the CardioGenics Acquisition, owned, directly or indirectly, approximately 85% of our outstanding common stock. While their percentage would decline over time if and to the extent new shares of our common stock are issued, you should expect these persons to exert continuing influence over all matters requiring shareholder approval, including the election of directors. You may have little to no practical control over such matters.
It is not likely that we will pay dividends on the common stock or any other class of stock
We intend to retain any future earnings for the operation and expansion of our business. We do not anticipate paying cash dividends on our common stock, or any other class of stock, in the foreseeable future. Stockholders should look solely to appreciation in the market price of our Common Shares to obtain a return on investment.
Our stockholders ownership of our common stock may be in doubt due to possible naked short selling of our common stock.
We believe, but cannot confirm, that speculators may have engaged in a practice commonly known as a “naked short” sale of our common stock, which means that certain brokers may be permitting their short selling customers to sell shares of our common stock that their customers do not own and may have failed to borrow and therefore deliver the shares sold to the purchaser of the shares. We have from time to time been included by NASDAQ on the Regulation SHO Threshold Security List, which is indicative of a significant amount of naked shorting in the stock. Because naked shorting may result in an artificial depression of our stock price, our stockholders could lose all or part of their investment in our common stock. As a result of this naked short selling, there may be a substantial number of purchasers who believe they are our stockholders, but who in fact would not be stockholders since their brokers may never have received any shares of our common stock for their account. In addition, investors who believe they are our stockholders may not have received a stock dividend to which they are entitled or may have been deprived of the right to vote some or all of their shares.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not Applicable.
Item 2. Properties
Our executive and administrative headquarters are currently located at 6295 Northam Drive, Units 7 & 8, Mississauga, Ontario L4V 1W8 Canada. We rent this space at a cost of US$81,926 per year.
The servers for our websites are housed at separate locations as described above. See “Item 1.—Business—Website Technical Information.” We believe that our facilities are adequate for our current needs and that, if our lease is not renewed on commercially reasonable terms, we will be able to locate suitable new office space and obtain a suitable replacement for our executive and administrative headquarters.
| 21 |
Item 3. Legal Proceedings
On April 22, 2009, CardioGenics was served with a statement of claim in the Province of Ontario, Canada, from a prior contractor claiming compensation for wrongful dismissal and ancillary causes of action including payment of monies in realization of his investment in CardioGenics, with an aggregate claim of $514,000. The Company considers all the claims to be without any merit, has already delivered a statement of defence and intends to vigorously defend the action. If the matter eventually proceeds to trial, the Company does not expect to be found liable on any ground or for any cause of action.
On January 14, 2010, Flow Capital Advisors Inc. (“Flow Capital”) filed a lawsuit against JAG Media Holdings Inc. in the Circuit Court of the 17th Judicial Circuit In and For Broward County Florida (Case No. 10001713) (the “Flow Capital State Action”). Pursuant to this lawsuit, Flow Capital alleges that JAG Media Holdings breached a Non-Circumvention Agreement it had entered into with Flow Capital, dated January 1, 2004.
On January 15, 2010, Flow Capital filed a lawsuit against CardioGenics Inc., and another defendant in the United States District Court for the Southern District of Florida, Fort Lauderdale Division (Case No. 10-CV-60066-Martinez-Brown) (the “Flow Capital Federal Action”). This lawsuit alleges that CardioGenics (i) breached a Finder’s Fee Agreement in connection with the CardioGenics Acquisition; and (ii) breached a non-circumvention agreement. Flow Capital is claiming that it is entitled to the finder’s fee equal to eight percent (8%) of the JAG Media Holdings shares received by CardioGenics, or the equivalent monetary value of the stock. Plaintiff subsequently amended its complaint to add related tort claims.
Pursuant to applicable federal court rules, the parties to the Flow Capital Federal Action participated in a court mandated mediation session on August 17, 2011 where the parties attempted to settle their disputes. At the mediation, the parties agreed to a settlement of all claims as described below, subject to the approval of the Board of Directors of CardioGenics Holdings Inc., which approval was subsequently obtained. Pursuant to the settlement agreement, Flow Capital agreed to dismiss, with prejudice, the Flow Capital Federal Action and the Flow Capital State Action and CardioGenics agreed to issue Flow Capital 1,000,000 shares of restricted CardioGenics Holdings common stock and warrants to purchase restricted CardioGenics Holdings common stock as follows:
| Type of Warrant | Number of Shares | Exercise Price | Vesting Date | Term | ||||||||||
| Cash Exercise Only | 250,000 | $ | 0.30/share | immediate | 5 years | |||||||||
| Cash Exercise Only | 250,000 | $ | 0.50/share | immediate | 5 years | |||||||||
| Cash Exercise Only | 500,000 | $ | 0.75/share | immediate | 5 years | |||||||||
| Cash Exercise Only | 500,000 | $ | 1.00/share | immediate | 5 years | |||||||||
| Cash or Cashless Exercise | 500,000 | $ | 0.75/share | August 1, 2012 | 5 years | |||||||||
The restricted shares of common stock and the warrants are subject to the rights and restrictions of Rule 144 and do not have any registration rights. As part of the settlement, the parties also exchanged mutual general releases and CardioGenics Holdings agreed to pay Flow Capital, in three monthly installments, $100,000 for Flow Capital’s legal fees.
On August 23, 2011, the Company’s Board of Directors approved the settlement. As a result, the Company recorded a charge to the Consolidated Statement of Operations for the year ended October 31, 2011 of $1,753,800 for Cost of Settlement of Lawsuit.
On October 26, 2010, Karver International Inc. filed a lawsuit in the 11th Judicial Circuit in and for Miami-Dade County, Florida against CardioGenics Holdings Inc. and several other defendants including affiliates, officers and directors of CardioGenics Holdings, Inc. The Plaintiff generally alleges that the named defendants made certain alleged misrepresentations in connection with the purchase of shares of CardioGenics Holdings Inc. On December 20, 2010 CardioGenics Holdings Inc. and other defendants filed a motion to dismiss on the basis that the court lacks personal jurisdiction over most defendants, that an enforceable forum selection clause requires that the action be litigated in Ontario, Canada that the doctrine of forum non conveniens requires dismissal in favor of the Ontario forum, and that the complaint suffers from numerous other technical deficiencies warranting dismissal (e.g., failure to attach documents to the Complaint, failure to plead fraud with particularity, etc.). In addition, prior to the motion being heard, Karver’s attorney filed a motion to withdraw as counsel for Karver. The court granted Karver’s attorney’s motion to withdraw and Karver had until approximately April 26, 2011 to engage new counsel. On April 20, 2011, having not engaged new counsel as of that date, Karver filed with the court a Notice of Voluntary Dismissal Without Prejudice, which dismisses the lawsuit against the named defendants without prejudice to Karver’s rights to recommence the action.
| 22 |
While it is not feasible to predict with certainty the outcome and exposures of any of the above proceedings that are still pending, management believes that their ultimate disposition should not have a material adverse effect on the Company’s financial position, cash flows or results of operations.
| Item 4. | MINE SAFETY DISCLOSURES | |
| ||
| None |
Part II
| ITEM. 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Securities |
For the period covered below, our common stock (other than our class B common stock) is traded on the OTC Bulletin Board under the symbol CGNH. In October 2009, our symbol was changed from JAGH to CGNH as a result of the CardioGenics Acquisition. The following table based on Bloomberg L.P. reflects quarterly high and low bid prices of our common stock from October 31, 2011 through October 31, 2012. Such prices are inter-dealer quotations without retail mark-ups, mark-downs or commissions, and may not represent actual transactions.
On January 22, 2013 the closing bid price for our common stock was $0.21. A public trading market for our Series 2 and Series 3 Class B common stock, which were redeemed on April 4, 2011, never developed. Pursuant to an amendment to our Articles of Incorporation filed in Nevada on January 17, 2013 our Class B common stock was deauthorized and is no longer available for issuance.
As of January 22, 2013, there were 32,499,239 shares of our common stock outstanding. There was also outstanding 1 share of Series 1 Preferred Voting Stock, par value $0.0001, representing 13 Exchangeable Shares, which are exchangeable into 24,176,927 shares of our common stock.
In addition, there are 1,235 additional stockholders who did not turn in their shares of prior classes of our common stock in connection with our recapitalizations in 2002 and 2004. These stockholders, upon presentation of their shares, are entitled to receive shares of our common stock in exchange. As of January 29, 2013 17,265 Series 1 Class B common shares, 106,855 Class A common shares and 12,366 original JagNotes.com Inc. common shares remained unconverted.
| Fiscal Year Ending October 31, 2012 | ||||||||
| Quarter Ended | High $ | Low $ | ||||||
| October 31, 2012 | 0.27 | 0.17 | ||||||
| July 31, 2012 | 0.34 | 0.25 | ||||||
| April 30, 2012 | 0.23 | 0.16 | ||||||
| January 31, 2012 | 0.45 | 0.26 | ||||||
| Fiscal Year Ending October 31, 2011 | ||||||||
| Quarter Ended | High $ | Low $ | ||||||
| October 31, 2011 | 0.65 | 0.30 | ||||||
| July 31, 2011 | 0.80 | 0.55 | ||||||
| April 30, 2011 | 1.08 | 0.60 | ||||||
| January 31, 2011 | 1.39 | 0.87 |
| 23 |
Dividend Policy
We have never paid any cash dividends on our common stock and anticipate that, for the foreseeable future, no cash dividends will be paid on our common stock.
Equity Compensation Plans Information
See the information provided under “Item 12.—Security Ownership of Certain Beneficial Owners and Related Stockholder Matters—Equity Compensation Plan Information.”
Recent Sales of Unregistered Securities
During the year the Company sold 1,050,000 common shares for the sum of $262,500 under a private placement.
Purchases of Equity Securities
There were no repurchases made for any class or series of securities in a month within the fourth quarter of the fiscal year ended October 31, 2012.
| ITEM 6. | Selected Financial Data |
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide information under this item.
| ITEM 7. | Management’s Discussion and Analysis oF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
This annual report contains forward-looking statements relating to future events or our future financial performance. In some cases you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “estimates,” “predicts,” “potential,” “continue,” “strategy,” “believes,” “anticipates,” “plans,” “expects,” “intends” or the negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors which may cause our or our industry’s actual results, levels of activity or performance to be materially different from any future results, levels of activity or performance expressed or implied by these forward-looking statements.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity or performance. You should not place reliance on these statements, which speak only as of the date that they were made. These cautionary statements should be considered with any written or oral forward-looking statements that we may issue in the future. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward –looking statements to conform these statements to actual results, later events or circumstances or to reflect the occurrence of unanticipated events.
In this annual report unless otherwise specified, all dollar amounts are expressed in United States dollars and all references to “common shares” refer to the common shares of our capital stock.
The management’s discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
The financial statements contained herein include the results CardioGenics, Inc. and its subsidiaries and CardioGenics Holdings, Inc. and its subsidiaries (“CardioGenics Holdings, Inc.”) (the latter from July 31, 2009, date of acquisition) which are collectively referred to as the “Company.”
| 24 |
CardioGenics Holdings, Inc. was until February 11, 2010 a provider of Internet-based equities research and financial information that offered its subscribers a variety of stock market research, news and analysis, including "JAG Notes", CardioGenics Holdings, Inc.'s flagship early morning consolidated research product.
On July 31, 2009, JAG Media Holdings, Inc. completed a reverse acquisition of privately held CardioGenics Inc. (“CardioGenics”), an Ontario, Canada Corporation. The acquisition was effected pursuant to a Share Purchase Agreement dated May 22, 2009 by and among JAG Media Holdings, Inc., CardioGenics Inc. and CardioGenics ExchangeCo Inc., the Company’s wholly owned subsidiary (“ExchangeCo”). In accordance with the terms of the Share Purchase Agreement, 99% of the holders of common shares of CardioGenics Inc. (two (2) minority shareholders of CardioGenics holding in aggregate 17,387 common shares of CardioGenics Inc. did not participate) surrendered their CardioGenics Common Shares to ExchangeCo. ExchangeCo caused JAG Media Holdings, Inc. to issue to the CardioGenics shareholders 42,218,361 shares of the Company’s common stock, par value $0.00001 per share (the “Share Consideration”). The Share Consideration provides the former CardioGenics shareholders with direct and/or indirect ownership of approximately 85% of JAG Media Holdings, Inc.’s outstanding common stock (on a fully diluted basis) as of July 31, 2009.
On October 27, 2009, the name of the Company was changed from JAG Media Holdings, Inc. to CardioGenics Holdings, Inc.
CardioGenics develops technology and products targeting the immunoassay segment of the In-Vitro Diagnostic testing market. CardioGenics has developed the QL Care Analyzer, a proprietary Point Of Care immuno-analyzer, which will run a number of diagnostic tests under development by CardioGenics, the first of which will be a series of cardiovascular diagnostic tests. As part of its core proprietary technology, CardioGenics has also developed a proprietary method for silver coating paramagnetic microspheres (a fundamental platform component of immunoassay equipment), which improve instrument sensitivity to light. CardioGenics’ principal offices are located in Mississauga, Ontario, Canada.
With the acquisition of CardioGenics, the Company’s business is now refocused on developing technologies and products for the point-of-care In Vitro Diagnostics market.
On February 11, 2010, the Company entered into an LLC Membership Interest Purchase Agreement with Rothcove Partners LLS (“Rothcove”) pursuant to which the Company sold its interest in the Internet-based equities research and financial information business to Rothcove.
On April 23, 2010, the Company’s Board of Directors approved a reverse stock split of its issued and outstanding common shares. The total authorized shares of common stock was at the same time reduced to 65,000,000. The Board of Directors selected a ratio of one-for-ten and the reverse stock split was effective on June 20, 2010. Trading of the Company’s common stock on the Over-The-Counter Capital Market on a split adjusted basis began at the open of trading on June 21, 2010. The reverse stock split affected all shares of the Company’s common stock, as well as options to purchase the Company’s common stock and other equity incentive awards and warrants that were outstanding immediately prior to the effective date of the reverse stock split. All references to common shares and per-share data for prior periods have been retroactively restated to reflect the reverse stock split as if it had occurred at the beginning of the earliest period presented.
| 25 |
Results of Operations for the Years Ended October 31, 2012 and October 31, 2011
The following table sets forth the Company’s results of operations for the years ended October 31, 2012 and 2011:
| Years Ended October 31, | ||||||||
| 2012 | 2011 | |||||||
| Revenue | $ | 1,297 | $ | 8,876 | ||||
| Operating Expenses: | ||||||||
| Depreciation and amortization of property and equipment | 18,305 | 20,399 | ||||||
| Amortization of patent application costs | 6,882 | 5,207 | ||||||
| Write-off of patent application costs | 24,905 | 55,549 | ||||||
| General and administrative | 741,961 | 3,398,960 | ||||||
| Cost of settlement of lawsuit | — | 1,753,800 | ||||||
| Research and product development, net of investment tax credits | 522,953 | 426,007 | ||||||
| Total operating expenses | 1,315,006 | 5,659,922 | ||||||
| Operating loss | (1,313,709 | ) | (5,651,046 | ) | ||||
| Other Expenses: | ||||||||
| Interest expense and bank charges (net) | 21,672 | 20,135 | ||||||
| Loss (gain) on foreign exchange transactions | (18,922 | ) | 90,737 | |||||
| Total other expenses | 2,750 | 110,872 | ||||||
| Loss from Continuing Operations | (1,316,459 | ) | (5,761,918 | ) | ||||
| Net Loss | $ | (1,316,459 | ) | $ | (5,761,918 | ) | ||
Revenues
Revenues reflect sales of paramagnetic beads.
| 26 |
Operating expenses
General and administrative expenses
General and administrative expenses consist primarily of compensation to officers, occupancy costs, professional fees, listing costs and other office expenses. The change in general and administrative expenses is attributable primarily to a decrease in professional fees of approximately $250,000, a decrease in consulting related fees of approximately $339,000 and a decrease in stock compensation related expenses of approximately $2,068,000.
Research and product development costs, net of investment tax credits
Research and development expenses consist primarily of salaries and wages paid to officers and employees engaged in those activities and supplies consumed therefor. The change in research and development expenses is attributable primarily to a decrease in the amount of the Canadian refundable tax credit received over that of the previous year.
Cost of settlement of lawsuit
On August 17, 2011, the Company and Flow Capital Advisors Inc. participated in a court mandated mediation session wherein the parties agreed to a settlement of all claims. On August 23, 2011, the Company’s Board of Directors approved the settlement. As a result, the consolidated statements of operations for the year ended October 31, 2011 reflects a charge of $1,753,800 for the cost of settlement of lawsuit.
Other expenses
Loss (gain) on foreign exchange transactions
The Company conducts the majority of its transactions in Canadian dollars. The foreign exchange loss (gain) (2012-($18,922), 2011-$90,737) results from currency movements on transactions settled during the year.
Liquidity and Capital Resources
For the year ended October 31, 2012, the Company incurred a net loss of approximately $1,316,000 (2011-$5,762,000) and a cash flow deficiency from operating activities of approximately $971,000 (2011-$1,521,000). The Company has not yet established an ongoing source of revenues sufficient to cover our operating costs and allow us to continue as a going concern. The Company has funded its activities to date almost exclusively from debt and equity financings. These matters raise substantial doubt about the Company’s ability to continue as a going concern and our independent auditors included an explanatory paragraph to emphasize such doubt in their report on the audit of our financial statements.
The Company will continue to require substantial funds to continue research and development, including preclinical studies and clinical trials of our products, and to commence sales and marketing efforts. The Company’s plans include financing activities such as private placements of its common stock and issuances of convertible debt instruments. The Company is also actively pursuing industry collaboration activities including product licensing and specific project financing.
The Company believes that it will be successful in obtaining the necessary financing to fund its operations, meet revenue projections and manage costs; however, there are no assurances that such additional funding will be achieved and that the Company will succeed in its future operations.
Our current annual cash requirement is approximately $1,000,000. The cash balance at the year end was $27,009 meaning that we had sufficient cash reserves to cover less than 1 months operations, assuming no revenue over the period. We are in fact anticipating revenues during the next fiscal year.
| 27 |
On November 19, 2012, the Company entered into an agreement (“Line”) with JMJ Financial (“Lender”) whereby the Company may borrow up to $350,000 from the Lender in increments of $50,000. The Line is subject to an original issue discount of $50,000. Advances under the Line have a maturity date of one year from the date of the advance. If the advance is repaid within three months, the advance is interest free. If not repaid within three months, the advance may not be repaid before maturity and carries interest at 5%. The Lender has the right at any time to convert all or part of the outstanding principal and accrued interest (and any other fees) into shares of fully paid and non-assessable shares of common stock of the Company at a price equal to the lesser of $0.23 and 60% of the lowest trade price in the previous 25 trading days prior to the conversion. Unless agreed in writing by the parties, at no time will the Lender convert any amount owing under the Line into common stock that would result in the Lender owning more than 4.99% of the Company’s common stock outstanding.
Subsequent to the year end, two officer/director/shareholders advanced $200,000 to the Company.
Summary of Critical Accounting Policies and Estimates
The discussion and analysis of the Company’s financial condition and results of its operations are based upon its consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America for financial statements filed with the SEC.
| (a) | Research and Development Costs |
Expenditures for research and development are expensed as incurred and include, among other costs, those related to the production of prototype products, including payroll costs. Amounts expected to be received from governments under Scientific Research Tax Credit arrangements are offset against current expenses. The Company recognizes revenue from restricted grants in the period in which the Company has for refundable tax credits incurred the expenditures in compliance with the specific restrictions.
| (b) | Income Taxes |
The Company utilizes the liability method of accounting for income taxes as set forth in the authoritative guidance. Under the liability method, deferred taxes are determined based on the temporary differences between the financial statement and tax basis of assets and liabilities using tax rates expected to be in effect during the years in which the basis differences reverse. A valuation allowance is recorded when it is more likely than not that some of the deferred tax assets will not be realized. As there is no certainty that the Company will generate taxable income in the foreseeable future to utilize tax losses accumulated to date, no provision for ultimate tax reduction has been made in these financial statements.
On November 1, 2007, the Company adopted the guidance issued for accounting for uncertainty in income taxes which provides detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in an enterprise’s financial statements. Income tax positions must meet a more-likely-than-non recognition threshold at the effective date to be recognized upon the adoption of the guidance and in subsequent periods. The Company recognizes potential accrued interest and penalties related to unrecognized tax benefits within operations as income tax expense. Upon adoption, there were no adjustments required.
| 28 |
| (c) | Stock-Based Compensation |
The Company follows the authoritative guidance for stock-based compensation which requires that new, modified and unvested share-based payment transactions with employees, such as grants of stock options and restricted stock, be recognized in the financial statements based on their fair value at the grant date and recognized as compensation expense over their vesting periods. The Company has also considered the related guidance of the Securities and Exchange Commission (“SEC”). The Company estimates the fair value of stock options and shares issued as compensation to employees and directors as of the date of grant using the Black-Scholes pricing model and restricted stock based on the per share value. The Company also follows the guidance for equity instruments that are issued to other than employees for acquiring, or in conjunction with selling, goods or services for equity instruments issued to consultants which provides guidance on transactions in which (1) the fair value of the equity instruments is more reliably measurable than the fair value of the goods or services received and (2) the counterparty receives shares of stock, stock options, or other equity instruments in settlement of the entire transaction or, if the transaction is part cash and part equity instruments, in settlement of the portion of the transaction for which the equity instruments constitute the consideration. Options issued with a nominal exercise price in exchange for services rendered were measured at the fair value of the underlying services rendered on the date of grant. The expense was recorded to the statement of operations with a corresponding increase in share capital with no additional increase in the number of shares as they were legally not yet exercised.
| (d) | Foreign Currency Translation |
The Company maintains its accounting records for its Canadian operations in Canadian dollars. Transactions in United States dollars (“USD”) are translated into Canadian dollars at rates in effect at the date of the transaction and gains or losses on such transactions are recorded at the time of settlement in the statement of operations.
The Company’s reporting currency is the United States Dollar. Foreign denominated assets and liabilities of the Company are translated into USD at the prevailing exchange rates in effect at the end of the reporting period, the historical rate for stockholders’ equity (deficiency) and a weighted average of exchange rate in effect during the period for expenses, gains and losses. Adjustments that arise from translation into the reporting currency are recorded in the accumulated other comprehensive loss component of stockholders’ equity (deficiency).
| (e) | Non-controlling Interest in Consolidated Financial Statements |
The Company follows the authoritative guidance for accounting and reporting for minority interests which characterizes non-controlling interests as a component of equity within the consolidated balance sheets.
Recent Accounting Pronouncements
In December 2011, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2011-11, Balance Sheet (Topic 210): Disclosures about Offsetting Assets and Liabilities. ASU 2011-11 requires an entity to disclose information about offsetting and related arrangements to enable users of financial statements to understand the effect of those arrangements on its financial position, and to allow investors to better compare financial statements prepared under U.S. GAAP with financial statements prepared under IFRS. The new standards are effective for annual periods beginning January 1, 2013, and interim periods within those annual periods. Retrospective application is required. The Company will implement the provisions of ASU 2011-11 beginning in fiscal 2014.
Item 7A. QuantitaTive and Qualitative Disclosure about Market Risk
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide information under this item.
| 29 |
ITEM 8. Financial Statements and Supplementary Data
The consolidated financial statements and supplementary data required in this item are set forth beginning on Page F-1 of this Annual Report on Form 10-K.
ITEM 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
2012
None
2011
None
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
In connection with the preparation of this Annual Report on Form 10-K for the period ended October 31, 2012, our management, including our principal executive officer and principal financial officer, carried out an evaluation of the effectiveness of our disclosure controls and procedures, as such term is defined in Rules 13a-15(e) and 15d-15(e) promulgated under the Securities Exchange Act of 1934, as amended (“Exchange Act”). Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer’s management, including its principal executive officer and principal financial officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. There are inherent limitations to the effectiveness of any system of disclosure controls and procedures, including the possibility of human error and the circumvention or overriding of the controls and procedures. Accordingly, even effective disclosure controls and procedures can only provide reasonable assurance of achieving their control objectives. Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were not effective as of October 31, 2012.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate controls and procedures and internal control over financial reporting (as defined in the Exchange Act) to provide reasonable assurance regarding the reliability of our financial reporting and preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. A control system, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. Because of the inherent limitations in all control systems, internal controls over financial reporting may not prevent or detect misstatements. The design and operation of a control system must also reflect that there are resource constraints and management is necessarily required to apply its judgment in evaluating the cost-benefit relationship of possible controls.
Our management assessed the effectiveness of our disclosure controls and procedures and internal control over financial reporting for the fiscal year ended October 31, 2012 based on the criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on such assessment, our management concluded that during the period covered by this report, our disclosure controls and procedures and internal control over financial reporting were not effective. Management has identified the following material weaknesses in our disclosure controls and procedures and internal control over financial reporting:
| 30 |
| • lack of documented policies and procedures; | |
| • there is no effective separation of duties, which includes monitoring controls, between the members of management; and | |
| • lack of resources to account for complex and unusual transactions. |
Management is currently evaluating what steps, if any, can be taken in order to address these material weaknesses in light of our current management structure.
Changes in Internal Controls Over Financial Reporting
During the fiscal year ended October 31, 2012, there were no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
There are no items that required disclosure in a Form 8-K during the fourth quarter of the year covered by this Form 10-K that were not reported by the Company.
Part III
ITEM 10. Directors, Executive Officers and Corporate Governance
The following table sets forth the name, age and position of each of the members of our board of directors, executive officers, and certain significant employees as of the fiscal year ending October 31, 2010. All directors are elected to hold office until the next annual meeting of stockholders following election and until their successors are duly elected and qualified. Executive officers are appointed by the Board of Directors and serve at the discretion of the Board.
Board of Directors and Executive Officers
| Name | Age | Position | |||
| Yahia Gawad | 54 | Director & Chief Executive Officer | |||
| Alexander D.G. Reid | 74 | Director | |||
| J Neil Tabatznik | 62 | Director/Acting Chairman | |||
| Linda J. Sterling | 51 | Director & Secretary | |||
| James A. Essex | 64 | Chief Financial Officer | |||
| 31 |
Yahia Gawad, MB, Ch.B., MD, MSc. (age 54, Director and Chief Executive Officer of CardioGenics since 1997). Dr. Gawad is a Physician/Scientist with primary training in Cardiology, Biochemistry and Immunology. He received his medical education and post-graduate training at the University of Alexandria and the University of Toronto. Dr. Gawad's academic and commercial experience and expertise include many years of designing and managing cardiovascular disease research and product development.
Dr. Gawad was a co-founder of a division of Nanogen (NGEN) (formerly Syn X and Skye Pharmatech) where he held the position of Vice-President, Medical Affairs. Prior to that, he was Director of Clinical Research and Development at Spectral Diagnostics Inc. (now Nanogen).
For the past 17 years, he has been working extensively on cardiac diagnostic test products. He has prepared, submitted and obtained FDA regulatory approvals for several cardiac test products currently being marketed (including Cardiac Status Troponin I®, Myoglobin® and Myoglobin/CK-MB®, registered trademarks of Spectral Diagnostics Inc.). Through his expertise and contributions to an international committee, a new cardiac test, Troponin I, is now in routine clinical use.
In addition, Dr. Gawad has researched, developed and published several other tests. Dr. Gawad has received several awards and scholarships and was a member of both the Clinical Committee of the American Heart Association and the POC division of the American Association for Clinical Chemistry. He has served as a reviewer for the editorial board of the American Journal of Cardiology (1999-2003). Dr. Gawad published extensively and presented his research and clinical findings at national and international symposia.
J. Neil Tabatznik (age 62, Director of CardioGenics since 2005, Acting Chairman of CardioGenics since 2009). Mr. Tabatznik is the Chairman, CEO of Arrow Pharmaceuticals Inc. Arrow Pharmaceuticals is part of a global generic drug company established in 2000, and has seen rapid growth from $0 to $700 million in 8 years. The Arrow Group has sales operations in 5 continents and employs more than 1000 people worldwide. Prior to Arrow Pharmaceuticals, Mr. Tabatznik was the Chairman, CEO of Genpharm Inc. (1993-2000), which was acquired by MerckKGaA in 1994 and is now a part of Mylan Inc. the world's third largest generic and specialty pharmaceutical company. He was a Barrister-at-Law in London and was called to the Bar of England and Wales in 1978. He has extensive expertise in pharmaceutical manufacturing and negotiations of agreements with multinational companies.
Alexander D.G. Reid (age 74, Director of CardioGenics since 1998). Mr. Reid has been in the financial community with experience in public and private companies for over 30 years. He has held numerous positions and board memberships in various financial and non-financial corporations. For many years, Mr. Reid was the author of the market business column in the Financial Post. Through his writing, various business models have been analysed and critiqued. He has been involved with the Company as a shareholder since 1999;
Linda J. Sterling (age 51, Corporate Secretary of CardioGenics since 2003, Director of CardioGenics since 2009). Ms. Sterling has been in the legal community in the capacity as a Law Clerk with both Stikeman Elliott LLP and Davies Ward Phillips & Vineberg LLP since 1999. She developed expertise with both public and private company legal compliance and has been responsible for CardioGenics' compliance and maintenance of corporate governance since 2001. She is currently in the process of being licensed as a Legal Executive (F.Inst.L.C.O.), with the Institute of Law Clerks of Ontario, of which she is a member. She has held the position of CEO and director of Sterling Studios since 1989.
James A. Essex, CA, MBA (age 64, Chief Financial Officer of CardioGenics since 2001) Mr. Essex has been with CardioGenics since 1999. He founded J. Hunter & Associates Inc. in 1990, a private financial consulting firm. Previously, he was a co-owner, President and COO of Calais Investigations, Inc., a private company (from 1993 to 1998), a Vice President of Confederation Trust (1989) and a Vice President of Chemical Bank of Canada (now JP Morgan Chase Bank of Canada) from 1977 through 1987.
| 32 |
Family Relationships
There are no family relationships among the directors and executive officers.
Involvement in Legal Proceedings
We know of no pending proceedings to which any director, member of senior management, or affiliate is either a party adverse to us, or our subsidiaries, or has a material interest adverse to us or our subsidiaries.
None of our executive officers or directors have (i) been involved in any bankruptcy proceedings within the last five years, (ii) been convicted in or has pending any criminal proceedings, (iii) been subject to any order, judgment or decree enjoining, barring, suspending or otherwise limiting involvement in any type of business, securities or banking activity or (iv) been found to have violated any federal, state or provincial securities or commodities law and such finding has not been reversed, suspended or vacated.
Board Committees
Our Board of Directors does not have standing audit, nominating or compensation committees. Instead, the functions that might be delegated to such committees are carried out by our entire Board of Directors, to the extent required. Our Board of Directors anticipates that it will evaluate from time-to-time the appropriateness of forming one or more of such committees.
Nomination of Directors
There have been no material changes to the procedures by which our security holders may recommend nominees to our Board of Directors.
Section 16(a) Beneficial Ownership Reporting Compliance
Under the securities laws of the United States, our directors, executive officers and any person holding more than 10% of our common stock are required to file initial forms of ownership of our common stock and reports of changes in that ownership at the SEC. Specific due dates for these forms have been established, and we are required to disclose in this report any failure to file by these dates.
Based solely on our review of the copies of such forms received by it with respect to fiscal year 2010, or written representations from certain reporting persons, to the best of our knowledge, all reports were filed on a timely basis.
Code of Ethics
We have adopted a Code of Ethics (our “Code of Ethics”) that applies to our Chief Executive Officer and Chief Financial Officer. We will provide to any person without charge, upon request, a copy of our Code of Ethics by sending such request to the attention: Yahia Gawad, Chief Executive Officer, CardioGenics Holdings Inc., 6295 Northam Drive, Unit 8, Mississauga, Ontario L4V 1W8. The Company will promptly disclose any amendments or waivers to our Code of Ethics on Form 8-K.
ITEM 11. Executive Compensation
As a “smaller reporting company,” CardioGenics has elected to follow scaled disclosure requirements for smaller reporting companies with respect to Part III, Item 11 – Executive Compensation. Under the scaled disclosure obligations, CardioGenics is not required to provide Compensation Discussion and Analysis and certain other tabular and narrative disclosures relating to executive compensation. Nor is CardioGenics required to quantify payments due to the named executives upon termination of employment. Management believes that the scaled disclosure for the Company’s executive compensation policy and practices is appropriate because CardioGenics is small for a publicly-traded company, has only three named executives and has a relatively simple compensation policy and structure that has not changed in the last fiscal year.
| 33 |
Summary Compensation Table
The following table provides information concerning compensation of CardioGenics’ named executives for CardioGenics’ last two completed fiscal years ending October 31, 2011 and 2012.
| Name
& Principal Position |
Year | Salary |
Bonus |
Stock
|
Option |
Non-Equity
|
Non-Qualified
|
All
Other |
Total |
||||||||||
| Dr. Yahia Gawad, Chief Executive Officer |
2012
2011 |
150,465(1)
152,238(1)
|
—
— |
—
— |
—
— |
—
— |
—
— |
—
— |
150,465
152,238
|
||||||||||
| James A. Essex, Chief Financial Officer |
2012
2011 |
36,111(1)
36,537(1)
|
—
— |
—
— |
—
— |
—
— |
—
— |
—
— |
36,111
36,537
|
||||||||||
| Linda J. Sterling, Secretary | 2012
2011 |
25,078(1)
30,448(1) |
—
— |
—
— |
—
— |
—
— |
—
— |
—
— |
25,078
30,448 |
| (1) | Cash compensation is stated in the table in U.S. dollars. To the extent any cash compensation was paid in Canadian dollars, it has been converted into U.S. dollars based on the average Canadian/U.S. dollar exchange rate for the years ended October 31, 2012 and October 31, 2011. |
Employment Agreements
We currently do not have written employment agreements with any of our current officers or executive personnel, except for Dr. Yahia Gawad who has a 3 year employment agreement with CardioGenics Holdings Inc., which commenced on July 31, 2010, and provides for an annual salary of $150,000, heath and dental insurance coverage on terms not less favorable than the health insurance coverage to be offered by the Company to its employees, performance bonuses in the form of cash and stock options to be proposed to the Board of Directors on an annual basis, non-compete agreement for 24 months after effective takeover and 18 months full salary severance pay and benefit for firing without cause. Further, for each calendar year of the Term he will be entitled to five (5) weeks paid vacation. Also, he will be eligible for stock option incentives to the executives as approved by the Board of Directors.
| 34 |
Outstanding Equity Awards at October 31, 2012 Fiscal Year End
| Name | Number of Securities underlying unexercised options exercisable | Number of Securities underlying unexercised options unexercisable | Option exercise or base price per share ($/Share) | Option Expiration Date | ||||||||||||
| None |
|
| ||||||||||||||
Director Compensation
Non-Employee Directors' Compensation
In fiscal 2012 our policy for compensation of non-employee directors was as follows:
| 1. | Non-employee directors do not receive an annual cash base retainer. | |
| 2. | At the discretion of the full Board of Directors, nonemployee directors may receive shares of the Company’s common stock. The number and terms of such shares is within the discretion of the full Board of Directors. |
| 3. | Directors who are officers or employees of CardioGenics do not receive separate consideration for their service on the Board of Directors. |
Fiscal Year 2012 Director Compensation Table
| Name | Stock Award As Director $ | Stock Award (Other) $ | Total(1) $ | |||||||||
| J. Neil Tabatznik | 0 | 0 | 0 | |||||||||
| Alexander D.G. Reid | 0 | 0 | 0 | |||||||||
| (1) | As of October 31, 2012, the aggregate number of shares underlying stock awards granted to each non-employee director was as follows: Mr. Tabatznik (561,648) and Mr. Reid (52,393). |
Indemnification of Officers and Directors
Our amended and restated Articles of Incorporation provide that we shall indemnify our officers, directors, employees and agents to the full extent permitted by Nevada law. Our Bylaws include provisions to indemnify our officers and directors [and other persons] against expenses (including judgments, fines and amounts paid for settlement) incurred in connection with actions or proceedings brought against them by reason of their serving or having served as officers, directors or in other capacities. We do not, however, indemnify them in actions in which it is determined that they have not acted in good faith or have acted unlawfully or not in our best interest. In the case of an action brought by or in the right of us, we shall indemnify them only to the extent of expenses actually and reasonably incurred by them in connection with the defense or settlement of these actions and we shall not indemnify them in connection with any matter as to which they have been found to be liable to us, unless the deciding court determines that, notwithstanding such liability, that person is fairly entitled to indemnity in light of all the relevant circumstances.
| 35 |
We do not currently maintain director’s and officer’s liability insurance but we may do so in the future.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors and officers pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
ITEM 12. Security Ownership of Certain Beneficial Owners and Related Stockholder Matters
The following table sets forth certain information with respect to the beneficial ownership of our Common Stock and Series 1 Preferred Stock by: (i) each director, (ii) each of the executive officers of the Company, (iii) all current directors and executive officers as a group, and (iv) each stockholder known to the Company to be the beneficial owner of more than 5% of the outstanding shares of Common Stock or Series 1 Preferred Stock.
Unless otherwise indicated in the footnotes to the table, all information set forth in the table is as of January 22, 2013, and the address for each director and executive officer of the Company is: c/o CardioGenics Holdings Inc., 6295 Northam Drive, Unit 8, Mississauga, Ontario L4V 1W8. The addresses for the greater than 5% stockholders are set forth in the footnotes to this table.
| Common Stock | Series 1 Preferred | |||||||||||||||
Number of Shares Beneficially Owned(1) | Percentage of Class Outstanding(2) | Number of Shares Beneficially Owned(1) | Percentage of Class Outstanding | |||||||||||||
| Directors | ||||||||||||||||
| J. Neil Tabatznik | 2,325,356 | (3) | 4.1 | % | — | — | ||||||||||
| Alexander D. G. Reid | 523,196 | (4) | * | — | — | |||||||||||
| Named Executive Officers | ||||||||||||||||
| Yahia Gawad | 18,144,652 | (5) | 32. | % | — | — | ||||||||||
| Linda J. Sterling | 1,501,617 | (6) | 2.6 | % | — | — | ||||||||||
| James Essex | 398,183 | (7) | * | — | — | |||||||||||
| All directors and named executive officers as a group (5 persons) | 22,893,004 | (8) | 40 | % | — | — | ||||||||||
| 5% Stockholders | ||||||||||||||||
| Weirfoulds LLP | — | — | 1 | (9) | 100 | % | ||||||||||
| Paul H. Saunders | 4,998,000 | (10) | 8.8 | % | — | — | ||||||||||
| *Less than 1% |
| (1) The Company believes that each stockholder has sole voting and investment power with respect to the shares of Common Stock and Series 1 Preferred Stock listed, except as otherwise noted. The number of shares beneficially owned by each stockholder is determined under rules of the Securities and Exchange Commission, and the information is not necessarily indicative of ownership for any other purpose. Under these rules, beneficial ownership includes (i) any shares as to which the person has sole or shared voting power or investment power and (ii) any shares which the individual has the right to acquire within 60 days after January 22, 2013 through the exercise of any stock option, warrant, conversion of preferred stock or other right, but such shares are deemed to be outstanding only for the purposes of computing the percentage ownership of the person that beneficially owns such shares and not for any other person shown in the table. The inclusion herein of any shares of Common Stock or Series 1 Preferred Stock deemed beneficially owned does not constitute an admission by such stockholder of beneficial ownership of those shares of Common Stock or Series 1 Preferred Stock. |
| 36 |
| (2) Based on 56,676,166 shares of Common Stock outstanding as of January 22, 2013, which includes 24,176,927 shares of Common Stock into which all outstanding Exchangeable Shares are exchangeable at any time. |
| (3) J. Neil Tabatznik beneficially owns 2,325,356 shares of Common Stock, including (i) 1,725,356 shares of Common Stock issuable upon exchange of 1 Exchangeable Share. |
| (4) Alexander D.G. Reid beneficially owns 523,196 shares of Common Stock, including (i) 523,196 shares of Common Stock issuable upon exchange of 1 Exchangeable Share and (ii) -0- shares of Common Stock. |
| (5) Yahia Gawad beneficially owns 18,144,652 shares of Common Stock, including (i) 17,478,553 shares of Common Stock issuable upon exchange of 1 Exchangeable Share and (ii) 666,099 shares of Common Stock. Pursuant to the terms of a lock-up agreement dated March 15, 2010 entered into among Dr. Gawad, the Company, CardioGenics ExchangeCo Inc. and Weirfoulds LLP, Dr. Gawad has agreed to lock-up fifteen million (15,000,000) shares of his Exchangeable Share exchange rights until March 15, 2014, in accordance with the terms of the lock-up agreement. |
| (6) Linda J. Sterling beneficially owns 1,501,617 shares of Common Stock, including (i) 1,501,617 shares of Common Stock issuable upon exchange of 1 Exchangeable Share and (ii) -0- shares of Common Stock. |
| (7) James Essex beneficially owns 398,183 shares of Common Stock, including (i) 345,791 shares of Common Stock issuable upon exchange of 1 Exchangeable Shares and (ii) 52,392 shares of Common Stock. |
| (8) See notes 2 through 7 above. |
| (9) Weirfoulds LLP, as trustee pursuant to the Voting Trust Agreement (the “Trustee”), beneficially owns the 1 outstanding share of Series 1 Preferred Stock, which provides the Trustee voting power with respect to all outstanding Exchangeable Shares in accordance with the terms of the Voting Trust Agreement. The voting rights with respect to any Exchangeable Share may be exercised by the Trustee or, alternatively, the holder of the Exchangeable Share may exercise their voting rights directly. Under the Voting Trust Agreement, the Trustee may exercise the voting rights of the Exchangeable Shares only with the instruction of the holder of the Exchangeable Share. As of January 22, 2013, the Exchangeable Shares are exchangeable at any time into 24,176,931 shares of Common Stock. Weirfoulds LLP’s address is 1600-130 King Street, The Exchange Tower, Toronto, Ontario, M5X 1J5. |
| (10) Paul H. Saunders’ address is 2700 North Ocean Drive, 9A, Singer Island, Florida 33404. |
Equity Compensation Plan Information
The following table summarizes the shares of our common stock authorized for issuance under our equity compensation plans as of October 31, 2012.
| Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted average
exercise price of outstanding options, warrants and rights | Number of securities
remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | ||||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders | Not applicable | Not applicable | Not applicable | |||||||||
| Equity Compensation Plans not approved by security holders | __ | __ | 30,000 | (1) | ||||||||
| TOTAL | __ | __ | 30,000 | |||||||||
________________________
| (1) | The maximum number of shares that may be subject to outstanding awards under our 1999 Long-Term Incentive Plan is 600,000 shares of Common Stock. Because this limitation applies only to outstanding awards under the plan, as the outstanding options included in column (a) are either exercised, forfeited or expire pursuant to their terms, the number of shares remaining available for future issuance in column (c) shall be increased by the number of shares subject to such option so exercised, forfeited or expired. |
| 37 |
Our 1999 Long-Term Incentive Plan provides our directors, officers, employees and consultants with the opportunity to participate in our ownership. Our Board of Directors acts as the committee under the plan which administers the plan, addressing participation, the awards offered and any applicable conditions of exercise. In making these determinations, our Board of Directors will generally consider the participant’s position and record of service to us. The Board of Directors may issue options, stock appreciation rights, restricted stock, deferred stock, bonus stock, awards in lieu of cash obligations, dividend equivalents and other stock based awards, all subject to terms and conditions to be set by the Board of Directors. The plan also contains standard provisions dealing with matters such as adjustment of the number of shares subject to options and covered by the plan in addition to amendment and termination of the plan.
ITEM 13. Certain Relationships and Related Transactions, and Director Independence
As a smaller reporting company, we are required to follow the scaled disclosure requirements with respect to this Part III, Item 13 – Certain Relationships and Related Transactions, and Director Independence. The disclosures related to review of related person transactions are not applicable to smaller reporting companies.
Certain Relationships and Related Transactions
During the year ended October 31, 2012, an existing stockholder made advances to the company totaling S100,000 bearing interest at 10% per annum.
Director Independence
The Board of Directors currently consists of four members, two of whom are “independent” as defined under applicable rules of the SEC and The NASDAQ Stock Market LLC. The two independent members of the Board of Directors are Neil Tabatznik and Alexander D. G. Reid. However, since our stock trades on the OTC Bulletin Board, we are not required to have independent directors.
For a director to be considered independent, the Board must determine that the director has no relationship, which, in the opinion of the Board, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
ITEM 14. Principal Accounting Fees and Services
CohnReznick LLP has been appointed as our independent public accountants for the year ended October 31, 2012 by unanimous approval of our board of directors. CohnReznick LLP is the successor to our former independent auditors, KPMG LLP, who served as our independent auditor since February 2012.
The following table sets forth the aggregate fees paid by CardioGenics for the fiscal years ended October 31, 2012 and 2011 to our independent auditors:
| Fiscal Year Ended October 2012 | Fiscal Year Ended October 2011 | |||||||
| Audit Fees | $ | 60,000 | (1) | $ | 149,265 | |||
| Audit Related Fees | $ | 49,450 | (2) | $ | 53,173 | (2) | ||
| Tax Fees | $ | 0 | $ | 0 | ||||
| All Other Fees | $ | 0 | $ | 0 | ||||
| Total | $ | 109,450 | $ | 202,438 | ||||
(1) Represents estimated audit fees for the fiscal year ended October 31, 2012.
(2) Represents charges of CohnReznick LLP (formerly J.H. Cohn LLP) and KPMG LLP, CardioGenics’ auditors in fiscal years ended October 31, 2012 and October 31, 2011, for review of interim financial statements.
(3) CohnReznick LLP and KPMG LLP did not provide and did not bill for any tax services.
| 38 |
All Other Fees
There were no other fees billed by CohnReznick LLP or KPMG LLP in the years ended October 31, 2012 or 2011.
Pre-Approval Policies and Procedures
The Board of Directors is required to pre-approve the rendering by our independent auditor of audit or permitted non-audit services. The Board of Directors pre-approved all of the services rendered by CohnReznick LLP and KPMG LLP for the audits of the consolidated financial statements included in our Annual Reports on Form 10-K and reviews of consolidated financial statements included in our Quarterly Reports on Form 10-Q.
The services provided for 2012 were 55% audit services and 45% audit related fees. The services provided above for 2011 were 74% audit services and 26% audit related fees.
Part IV
ITEM 15. Exhibits, Financial Statement Schedules
Exhibits
The following Exhibits are filed as part of this Annual Report on Form 10-K or incorporated by reference.
| Exhibit No. | Description | |
| 3.1 | Amended and Restated Articles of Incorporation of Registrant. Incorporated by reference to the Registrant’s Form 10-QSB filed with the SEC on June 19, 2006. | |
| 3.2 | Bylaws of Registrant. Incorporated by reference to the Registrant’s Form SB-2 filed with the SEC on September 30, 1999. | |
| 3.3 | Certificate of Designation of Series 1 Preferred Stock of Registrant. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on July 24, 2009. | |
| 3.4 | Articles of Amendment of CardioGenics ExchangeCo Inc. effective July 14 2009 and Articles of Incorporation of CardioGenics ExchangeCo Inc. Effective May 22, 2009 | |
| 3.5 | Certificate of Amendment to Articles of Incorporation of Registrant. Incorporated by reference to the Registrant’s Form DEF 14C filed with the SEC on September 9, 2009. | |
| 4.1 | Form of Common Stock Certificate. Incorporated by reference to the Registrant’s Form 10-KSB filed with the SEC on November 8, 2005. | |
| 4.2 | Form of Series 2 Class B Stock Certificate. Incorporated by reference to the Registrant’s Form 10-KSB filed with the SEC on November 8, 2005. | |
| 4.3 | Securities Purchase Agreement, effective May 25, 2006, with YA Global. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on June 1, 2006. | |
| 4.4 | Letter Agreement, dated January 31, 2008, relating to the conversion of the remaining principal balance of the convertible secured debentures. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on February 6, 2008. |
| 39 |
| 4.5 | Warrant No. CCP-1 for 2,000,000 shares of common stock issued to YA Global, effective May 25, 2006. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on June 1, 2006. | |
| 4.6 | Warrant No. CCP-2 for 2,000,000 shares of common stock issued to YA Global, effective May 25, 2006. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on June 1, 2006. | |
| 4.7 | Warrant No. CCP-3 for 2,000,000 shares of common stock issued to YA Global, effective May 25, 2006. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on June 1, 2006. | |
| 4.8 | Warrant No. CCP-4 for 3,000,000 shares of common stock issued to YA Global, effective May 25, 2006. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on June 1, 2006. | |
| 4.9 | Warrant No. CCP-5 for 3,000,000 shares of common stock issued to YA Global, effective May 25, 2006. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on June 1, 2006. | |
| 4.10 | Letter Agreement, amending Warrant No. CCP-4. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on October 3, 2008. | |
| 4.11 | Investor Registration Rights Agreement, effective May 25, 2006, with YA Global. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on June 1, 2006. | |
| 10.1 | Non-Binding Letter of Intent, dated October 1, 2008, by and among the Registrant, BlueCreek, e2 Business and YA Global. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on October 3, 2008. | |
| 10.2 | 1999 Long-Term Incentive Plan, as amended. Incorporated by reference to Exhibit [ ] to the Registrant’s Form S-8 filed with the SEC on May 1, 2002. | |
| 10.3 | Amended and Restated Employment Agreement, dated August 31, 2001, between Thomas J. Mazzarisi and Registrant. Incorporated by reference to Exhibit 10.21 in Amendment No. 1 to the Registrant’s Form SB-2 filed with the SEC on September 26, 2001. | |
| 10.4 | Amended and Restated Employment Agreement, dated August 31, 2001, between Stephen J. Schoepfer and Registrant. Incorporated by reference to Exhibit 10.20 in Amendment No. 1 to the Registrant’s Form SB-2 filed with the SEC on September 26, 2001. | |
| 10.5 | Amendment to Amended and Restated Employment Agreement, dated as of November 3, 2005, between Registrant and Thomas J. Mazzarisi. Incorporated by reference to the Registrant’s Form 10-KSB filed with the SEC on November 8, 2005. | |
| 10.6 | Amendment to Amended and Restated Employment Agreement, dated as of November 3, 2005, between Registrant and Stephen J. Schoepfer. Incorporated by reference to the Registrant’s Form 10-KSB filed with the SEC on November 8, 2005. | |
| 10.7 | Amendment to Amended and Restated Employment Agreement, dated as of November 12, 2007, by and between Registrant and Thomas J. Mazzarisi. Incorporated by reference to Exhibit 10.6 of Registrant’s Form 10-K filed with the SEC on November 13, 2008. | |
| 10.8 | Amendment to Amended and Restated Employment Agreement, dated as of November 12, 2007, by and between Registrant and Stephen J. Schoepfer. Incorporated by reference to Exhibit 10.7 of the Registrant’s Form 10-K filed with the SEC on November 13, 2008 | |
| 10.9 | Extension of Amended and Restated Employment Agreement dated as of November 12, 2008 between registrant and Thomas J. Mazzarisi. Incorporated by reference to Exhibit 10.9 of the Registrant’s Form 10-K filed with the SEC on November 13, 2008. |
| 40 |
| 10.10 | Extension of Amended and Restated Employment Agreement dated as of November 12, 2008 between registrant and Stephen J. Schoepfer. Incorporated by reference to Exhibit 10.10 of the Registrant’s Form 10-K filed with the SEC on November 13, 2008. | |
| 10.11 | Consulting Agreement, dated November 12, 2007, between the Registrant and Walsh Organization, Inc. Incorporated by reference to Exhibit 10.31 of the Registrant’s Annual Report on Form 10-KSB filed November 13, 2003. | |
| 10.12 | Power of Attorney and Contingent Fee Contract, dated June 14, 2002, among the Registrant, Gary Valinoti and the Law Firm of O’Quinn, Laminack & Pirtle. Incorporated by reference to Exhibit 10.32 of the Registrant’s Annual Report on Form 10-KSB filed November 13, 2003. | |
| 10.13 | Subscription Agreement, dated December 10, 2002, between the Registrant and Bay Point Investment Partners LLC. Incorporated by reference to the Registrant’s Registration Statement on Form SB-2 filed on January 9, 2003. | |
| 10.14 | Placement Agent Agreement, dated December 10, 2002, between the Registrant and RMC 1 Capital Markets, Inc. Incorporated by reference to the Registrant’s Registration Statement on Form SB-2 filed on January 9, 2003. | |
| 10.15 | Placement Agent Agreement, dated as of June 19, 2003, between the Registrant and RMC 1 Capital Markets, Inc., as amended on August 12, 2003. Incorporated by reference to the Registrant’s Current Report on Form 8-K filed on August 13, 2003. | |
| 10.16 | Subscription Agreement, dated as of June 19, 2003, between the Registrant and Bay Point Investment Partners LLC, as amended on August 12, 2003. Incorporated by reference to the Registrant’s Current Report on Form 8-K filed on August 13, 2003. | |
| 10.17 | Subscription Agreement, dated as of September 25, 2003, between the Registrant and Kuekenhof Equity Fund L.P. Incorporated by reference to Exhibit 10.39 of the Registrant’s Form 10-KSB filed with the SEC on November 13, 2003. | |
| 10.18 | Non-Circumvention/Non-Disclosure Agreement, dated as of January 1, 2004 between Flow Capital Advisors Inc. and the Registrant. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on July 25, 2007. | |
| 10.19 | Finder’s Fee Agreement, dated as of January 5, 2004, between the Registrant and Flow Capital Advisors, Inc. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on January 20, 2004. | |
| 10.20 | Finder’s Fee Agreement, dated as of March 14, 2005, by and between the Registrant and Flow Capital Advisors, Inc. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on July 25, 2007. | |
| 10.21 | Irrevocable Transfer Agent Instructions, effective May 25, 2006, between the Registrant and YA Global. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on June 1, 2006. | |
| 10.22 | Letter, dated as of June 17, 2008, from Cryptometrics regarding termination of the agreement and plan of merger between the Registrant and Cryptometrics. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on June 18, 2008. | |
| 10.23 | Stand-By Equity Distribution Agreement dated March 12, 2009 between Registrant and YA Global Master SPV Ltd. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on March 13, 2009. | |
| 10.24 | Registration Rights Agreement dated March 12, 2009 between Registrant and YA Global Master SPV Ltd. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on March 13, 2009. |
| 41 |
| 10.23 | Share Purchase Agreement dated May 22, 2009 between Registrant, CardioGenics ExchnageCo Inc., CardioGenics Inc. And Yahia Gawad, Principal Shareholder of CardioGenics Inc. | |
| 10.24 | Voting and Exchange Trust Agreement dated July 6, 2009 among Registrant, CardioGenics ExchangeCo Inc. and Weirfoulds LLP. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on July 6, 2009. | |
| 10.25 | Support Agreement dated July 6, 2009 between Registrant and CardioGenics ExchangeCo Inc. Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on July 6, 2009. | |
| 10.26 | Agreement dated September 10, 2009 between Registrant and The Investor’s Relations Group, Inc. by reference to the Registrant’s Form 8-K filed with the SEC on September 11, 2009. | |
| 10.27 | Agreement dated September 28, 2009 between Registrant and Gilford Securities Incorporated by reference to the Registrant’s Form 8-K filed with the SEC on October 2, 2009. | |
| 10.28 | Retainer Agreement dated January 20, 2010 between Registrant and Wolf, Axelrod & Weinberger Associates LLC | |
| 10.29 | Letter of Agreement dated January 18, 2010 between Registrant and The Investor Relations Group, Inc. | |
| 10.30 | Employment agreement dated July 31, 2009 between Registrant and Dr. Yahia Gawad. | |
| 10.31 | LLC Membership Interest Purchase Agreement dated February 10, 2010 between Registrant and Rothcove Partners LLC. | |
| 14.1 | Code of Ethics. Incorporated by reference to the Registrant’s Form 10-KSB filed with the SEC on November 13, 2003. | |
| 21.1 | Subsidiaries of Registrant. Incorporated by reference to the Registrant’s Form 10-K filed with the SEC on January 31, 2011. | |
| 23.1 | Consent of CohnReznick LLP* | |
| 23.2 | Consent of KPMG LLP* | |
| 23.3 | Consent of BDO Canada LLP* | |
| 31.1 | Section 302 Certification of Chief Executive Officer* | |
| 31.2 | Section 302 Certification of Chief Financial Officer* | |
| 32.1 | Section 906 Certification of Chief Executive Officer and Chief Financial Officer* |
*Filed herewith
| 42 |
Consolidated Financial Statements
| Report of Independent Registered Public Accounting Firm | F-1 | |
| Report of Independent Registered Public Accounting Firm | F-2 | |
| Report of Independent Registered Public Accounting Firm | F-3 | |
| Consolidated Balance Sheets | F-4 | |
| Consolidated Statements of Operations | F-5 | |
| Consolidated Statements of Equity (Deficiency) | F-6-13 | |
| Consolidated Statements of Cash Flows | F-14 | |
| Notes to Consolidated Financial Statements | F-15-31 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Report of Independent Registered Public Accounting Firm |
To the Board of Directors and Stockholders
CardioGenics Holdings, Inc.
We have audited the accompanying consolidated balance sheet of CardioGenics Holdings Inc. (a development stage company) as of October 31, 2012, and the related consolidated statements of operations, changes in equity (deficiency) and cash flows for the year then ended and for the period from November 20, 1997 (date of inception) to October 31, 2012. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit. The financial statements of CardioGenics, Inc. for the period from November 20, 1997 to October 31, 2008 and the one year ended October 31, 2011 were audited by other auditors whose reports dated July 29, 2009 and April 13, 2012, respectively, expressed an unqualified opinion on those statements with explanatory paragraphs relating to the Company’s ability to continue as a going concern. Our opinion on the consolidated statements of operations, changes in equity (deficiency) and cash flows for the period from November 20, 1997 (date of inception) to October 31, 2012, insofar as it relates to amounts for prior periods through October 31, 2008 and the year ended October 31, 2011, is based solely on the report of the other auditors.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, based on our audits and the reports of other auditors, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of CardioGenics Holdings, Inc. as of October 31, 2012, and its results of operations and cash flows for the year then ended, and for the period from November 20, 1997 (date of inception) to October 31, 2012, in conformity with accounting principles generally accepted in the United States of America.
The consolidated financial statements referred to above have been prepared assuming that the Company will continue as a going concern. As further discussed in Note 3 to the consolidated financial statements, the Company's operations have generated recurring losses and negative cash flows from operating activities. Such matters raise substantial doubt about the Company's ability to continue as a going concern. Management's plans concerning these matters are also described in Note 3. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ CohnReznick LLP
Roseland, New Jersey
January 29, 2013
| F-1 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Report of Independent Registered Public Accounting Firm |
To the Directors and Shareholders of
CardioGenics Holdings Inc. (formerly CardioGenics Inc.)
(A Development Stage Company)
We have audited the accompanying consolidated balance sheet of CardioGenics Holdings Inc. (formerly CardioGenics Inc.) (a development stage company) as at October 31, 2008 and the related consolidated statements of operations, stockholders’ equity (deficit) and cash flows for the year ended October 31, 2008. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements and assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of CardioGenics Holdings Inc. (formerly CardioGenics Inc.) (a development stage company) as at October 31, 2008 and the results of its operations and its cash flows for the year ended October 31, 2008 in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company has suffered recurring net losses and negative cash flows from operations. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans regarding these matters are also described in Note 3. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
(Signed) “BDO Canada LLP”
Chartered Accountants, Licensed Public Accountants
Toronto, Ontario
July 29, 2009
| F-2 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Report of Independent Registered Public Accounting Firm |
To the Board of Directors and Stockholders of CardioGenics Holdings Inc.
We have audited the accompanying consolidated balance sheet of CardioGenics Holdings Inc. (a development stage company) as of October 31, 2011, and the related consolidated statements of operations, changes in equity (deficiency) and cash flows for the year then ended and for the period from November 20, 1997 (date of inception) to October 31, 2011. These consolidated financial statements are the responsibility of CardioGenics Holdings Inc.'s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit. The cumulative statements of operations, changes in equity (deficiency) and cash flows for the period from November 20, 1997 (date of inception) to October 31, 2011 include amounts for the period from November 20, 1997 (date of inception) to October 31, 2010, which were audited by other auditors whose unqualified reports, which contained an explanatory paragraph related to the Company’s ability to continue as a going concern, have been furnished to us, and our opinion, insofar as it relates to the amounts included for the period November 20, 1997 (date of inception) through October 31, 2010 is based solely on the reports of other auditors.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, based on our audit and the reports of other auditors, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of CardioGenics Holdings Inc. as of October 31, 2011, and the results of its operations and its cash flows for the years ended October 31, 2011, in conformity with U.S. generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in note 3 to the consolidated financial statements, the Company has incurred losses from operations and has experienced negative cash flows from operations since inception which raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in note 3. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
As discussed in note 2 to the consolidated financial statements, the 2011 consolidated financial statements have been restated to correct for misstatements.
/s/ KPMG LLP
Chartered Accountants, Licensed Public Accountants
Toronto, Canada
April 13, 2012
| F-3 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Consolidated Balance Sheets |
| October 31, | October 31, | |||||||
| 2012 | 2011 | |||||||
| Assets | ||||||||
| Current | ||||||||
| Cash and Cash Equivalents | $ | 27,009 | $ | 669,202 | ||||
| Accounts Receivable | 437 | 9,002 | ||||||
| Deposits and Prepaid Expenses | 51,422 | 51,541 | ||||||
| Refundable Taxes Receivable | 45,207 | 35,191 | ||||||
| Government Grants and Investment Tax Credits Receivable | 80,080 | 187,497 | ||||||
| 204,155 | 952,433 | |||||||
| Long-Term | ||||||||
| Property and Equipment, net | 67,827 | 82,308 | ||||||
| Patents, net | 110,031 | 130,732 | ||||||
| 177,858 | 213,040 | |||||||
| Total Assets | $ | 382,013 | $ | 1,165,473 | ||||
| Liabilities and Equity (Deficiency) | ||||||||
| Current Liabilities | ||||||||
| Accounts Payable and Accrued Expenses | $ | 786,135 | $ | 596,692 | ||||
| Due to Shareholders | 100,000 | — | ||||||
| Current Portion of Capital Lease Obligation | 2,627 | 25,711 | ||||||
| Funds Held in Trust for Redemption of Class B Common Shares | 4 | 4 | ||||||
| 888,766 | 622,407 | |||||||
| Long-Term Liabilities | ||||||||
| Capital Lease Obligation, net of current portion | — | 2,630 | ||||||
| — | 2,630 | |||||||
| Total Liabilities | 888,766 | 625,037 | ||||||
| Mandatorily redeemable Class B common stock; par value $.00001 per share: | ||||||||
| 400,000 shares designated as Series 2; 381,749 shares issued and outstanding | — | — | ||||||
| 40,000 shares designated as Series 3; 21,500 shares issued and outstanding | — | — | ||||||
| — | — | |||||||
| Commitments and contingencies | ||||||||
| Equity (Deficiency) | ||||||||
| Preferred stock; par value $.0001 per share, 5,000,000 shares authorized, none issued | — | — | ||||||
| Common stock; par value $.00001 per share; 65,000,000 shares authorized, 32,499,239 and 31,237,262 common shares and 24,176,927 and 24,388,904 exchangeable shares issued and outstanding as at October 31, 2012 and 2011, respectively | 543 | 540 | ||||||
| Additional paid-in capital | 42,036,498 | 41,774,001 | ||||||
| Deficit accumulated during development stage | (42,039,223 | ) | (40,731,174 | ) | ||||
| Accumulated other comprehensive loss | (166,637 | ) | (173,407 | ) | ||||
| Total Equity (Deficiency) Attributable to CardioGenics Holdings Inc. | (168,819 | ) | 869,960 | |||||
| Non-Controlling Interest | (337,934 | ) | (329,524 | ) | ||||
| Total Equity (Deficiency) | (506,753 | ) | 540,436 | |||||
| Total Liabilities and Equity (Deficiency) | $ | 382,013 | $ | 1,165,473 | ||||
See notes to consolidated financial statements.
| F-4 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Consolidated Statements of Operations |
| For the Years Ended October 31, 2012 and 2011 and |
| Cumulative from November 20, 1997 (Date of Inception) to October 31, 2012 |
| Cumulative | ||||||||||||
| From | ||||||||||||
| November 20, | ||||||||||||
| 1997 | ||||||||||||
| (Date of | ||||||||||||
| For the Years Ended | Inception) to | |||||||||||
| October 31, | October 31, | |||||||||||
| 2012 | 2011 | 2012 | ||||||||||
| Revenue | $ | 1,297 | $ | 8,876 | $ | 10,173 | ||||||
| Operating Expenses | ||||||||||||
| Depreciation and Amortization of Property and Equipment | 18,305 | 20,399 | 219,744 | |||||||||
| Amortization of Patent Application Costs | 6,882 | 5,207 | 19,293 | |||||||||
| Write-off of Patent Application Costs | 24,905 | 55,549 | 239,530 | |||||||||
| General and Administrative | 741,961 | 3,398,960 | 8,417,031 | |||||||||
| Write-off of Goodwill | — | — | 12,780,214 | |||||||||
| Research and Product Development, Net of Investment Tax Credits | 522,953 | 426,007 | 4,150,333 | |||||||||
| Cost of Settlement of Lawsuit | — | 1,753,800 | 1,753,800 | |||||||||
| Total operating expenses | 1,315,006 | 5,659,922 | 27,579,945 | |||||||||
| Operating Loss | (1,313,709 | ) | (5,651,046 | ) | (27,569,772 | ) | ||||||
| Other Expenses (Income) | ||||||||||||
| Interest Expense and Bank Charges (Net) | 21,672 | 20,135 | 2,158,308 | |||||||||
| Loss on Change in Value of Derivative Liability | — | — | 12,421,023 | |||||||||
| Loss (Gain) on Foreign Exchange Transactions | (18,922 | ) | 90,737 | 190,343 | ||||||||
| Total other expenses | 2,750 | 110,872 | 14,769,674 | |||||||||
| Loss from Continuing Operations | (1,316,459 | ) | (5,761,918 | ) | (42,339,446 | ) | ||||||
| Discontinued Operations | ||||||||||||
| Gain on Sale of Subsidiary | — | — | 90,051 | |||||||||
| Loss from Discontinued Operations | — | — | (127,762 | ) | ||||||||
| Net Loss | (1,316,459 | ) | (5,761,918 | ) | (42,377,157 | ) | ||||||
| Net Loss Attributed to Non-Controlling Interest | 8,410 | 37,302 | 337,934 | |||||||||
| Net Loss Attributed to CardioGenics Holdings Inc. | $ | (1,308,049 | ) | $ | (5,724,616 | ) | $ | (42,039,223 | ) | |||
| Basic and Fully Diluted Net Loss per Common Share | $ | (0.02 | ) | $ | (0.11 | ) | ||||||
| Weighted-average number of Common Shares | 55,629,034 | 54,167,687 | ||||||||||
See notes to consolidated financial statements.
| F-5 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Consolidated Statements of Changes in Equity (Deficiency) |
| For the year ended October 31, 2012 |
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | The | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Loss | Interest | (Deficiency) | ||||||||||||||||||||||
| Issuance of common shares for cash November 1997 | 1,592,732 | $ | 16 | $ | (15 | ) | $ | — | $ | — | $ | — | $ | 1 | ||||||||||||||
| Issuance of common shares for cash December 1997, $.00 | 796,366 | 8 | 35,028 | 35,036 | ||||||||||||||||||||||||
| Issuance of common shares for cash March 1998, $.00 | 551,611 | 6 | 24,442 | 24,448 | ||||||||||||||||||||||||
| Issuance of common shares for cash April 1998, $.00 | 12,986,611 | 130 | 5,573 | 5,703 | ||||||||||||||||||||||||
| Issuance of common shares for cash May 1998, $.01 | 210,249 | 2 | 17,297 | 17,299 | ||||||||||||||||||||||||
| Issuance of common shares for cash August 1998, $.00 | 2,787,281 | 28 | 200 | 228 | ||||||||||||||||||||||||
| Issuance of common shares for cash September 1998, $.01 | 84,100 | 1 | 6,570 | 6,571 | ||||||||||||||||||||||||
| Issuance of common shares for cash October 1998, $.01 | 31,949 | — | 2,500 | 2,500 | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (81,208 | ) | (81,208 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | (2,096 | ) | (2,096 | ) | ||||||||||||||||||||||||
| Total Comprehensive Loss | (81,208 | ) | (2,096 | ) | (83,304 | ) | ||||||||||||||||||||||
| Balance at October 31, 1998 | 19,040,899 | $ | 191 | $ | 91,595 | $ | (81,208 | ) | $ | (2,096 | ) | $ | — | $ | 8,482 | |||||||||||||
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Loss | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 1998 | 19,040,899 | $ | 191 | $ | 91,595 | $ | (81,208 | ) | $ | (2,096 | ) | $ | — | $ | 8,482 | |||||||||||||
| Issuance of common shares for cash November 1998, $.01 | 32,066 | — | 2,500 | 2,500 | ||||||||||||||||||||||||
| Issuance of common shares for cash February 1999, $.01 | 159,273 | 2 | 14,287 | 14,289 | ||||||||||||||||||||||||
| Commission paid on issuance of common stock for cash February 1999 | (935 | ) | (935 | ) | ||||||||||||||||||||||||
| Issuance of common shares for cash March 1999, $.01 | 278,728 | 3 | 24,707 | 24,710 | ||||||||||||||||||||||||
| Commission paid on issuance of common stock for cash March 1999 | (1,647 | ) | (1,647 | ) | ||||||||||||||||||||||||
| Issuance of common shares for cash to minority shareholders April 1999, $.01 | — | — | 10,707 | 10,707 | ||||||||||||||||||||||||
| Commission paid on issuance of common stock for cash April 1999 | (627 | ) | (627 | ) | ||||||||||||||||||||||||
| Issuance of common shares for cash April 1999, $.01 | 39,818 | — | 3,814 | 3,814 | ||||||||||||||||||||||||
| Commission paid on issuance of common stock for cash April 1999 | (314 | ) | (314 | ) | ||||||||||||||||||||||||
| Issuance of common shares for cash July 1999, $.01 | 119,455 | 1 | 10,073 | 10,074 | ||||||||||||||||||||||||
| Issuance of common shares for cash August 1999, $.01 | 119,455 | 1 | 10,045 | 10,046 | ||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (604 | ) | (604 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (100,141 | ) | (100,141 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | (3,489 | ) | (3,489 | ) | ||||||||||||||||||||||||
| Total Comprehensive Loss | (100,141 | ) | (3,489 | ) | (103,630 | ) | ||||||||||||||||||||||
| Balance at October 31, 1999 | 19,789,694 | $ | 198 | $ | 164,205 | $ | (181,349 | ) | $ | (5,585 | ) | $ | (604 | ) | $ | (23,135 | ) | |||||||||||
See notes to consolidated financial statements.
| F-6 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Consolidated Statements of Changes in Equity (Deficiency) |
| For the year ended October 31, 2012 |
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Income (Loss) | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 1999 | 19,789,694 | $ | 198 | $ | 164,205 | $ | (181,349 | ) | $ | (5,585 | ) | $ | (604 | ) | $ | (23,135 | ) | |||||||||||
| Issuance of common shares for cash November 1999, $.03 | 318,546 | 3 | 99,997 | 100,000 | ||||||||||||||||||||||||
| Issuance of common shares for minority shareholders as employee compensation December 1999, $.03 | — | — | 3,396 | 3,396 | ||||||||||||||||||||||||
| Issuance of common shares for cash March 2000, $.03 | 167,237 | 2 | 43,124 | 43,126 | ||||||||||||||||||||||||
| Issuance of common shares for minority shareholders for cash March, 2000, $.03 | — | — | 25,330 | 25,330 | ||||||||||||||||||||||||
| Issuance of common shares for cash April 2000, $.03 | 23,891 | — | 6,128 | 6,128 | ||||||||||||||||||||||||
| Loan Payable plus interest exchanged for shares July 2000, $.03 | 356,772 | 4 | 111,996 | 112,000 | ||||||||||||||||||||||||
| Issuance of common shares for minority shareholders as employee compensation October 2000, $.03 | — | — | 6,611 | 6,611 | ||||||||||||||||||||||||
| Issuance of stock options in exchange for services rendered October 2000 | — | 11,570 | 11,570 | |||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (1,840 | ) | (1,840 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (152,525 | ) | (152,525 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | 921 | 921 | ||||||||||||||||||||||||||
| Total Comprehensive Income (Loss) | (152,525 | ) | 921 | (151,604 | ) | |||||||||||||||||||||||
| Balance at October 31, 2000 | 20,656,140 | $ | 207 | $ | 472,357 | $ | (333,874 | ) | $ | (4,664 | ) | $ | (2,444 | ) | $ | 131,582 | ||||||||||||
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Loss | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2000 | 20,656,140 | $ | 207 | $ | 472,357 | $ | (333,874 | ) | $ | (4,664 | ) | $ | (2,444 | ) | $ | 131,582 | ||||||||||||
| Issuance of common shares as employee compensation October 2001, $.03 | 2,410 | — | 925 | 925 | ||||||||||||||||||||||||
| Issuance of common share for minority shareholders as employee compensation October 2001, $.03 | — | — | 6,169 | 6,169 | ||||||||||||||||||||||||
| Issuance of stock options in exchange for services rendered October 2001 | — | — | 22,269 | 22,269 | ||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (1,500 | ) | (1,500 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (114,761 | ) | (114,761 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | (10,528 | ) | (10,528 | ) | ||||||||||||||||||||||||
| Total Comprehensive Loss | (114,761 | ) | (10,528 | ) | (125,289 | ) | ||||||||||||||||||||||
| Balance at October 31, 2001 | 20,658,550 | $ | 207 | $ | 501,720 | $ | (448,635 | ) | $ | (15,192 | ) | $ | (3,944 | ) | $ | 34,156 | ||||||||||||
See notes to consolidated financial statements.
| F-7 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Consolidated Statements of Changes in Equity (Deficiency) |
| For the year ended October 31, 2012 |
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Loss | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2001 | 20,658,550 | $ | 207 | $ | 501,720 | $ | (448,635 | ) | $ | (15,192 | ) | $ | (3,944 | ) | $ | 34,156 | ||||||||||||
| Issuance of common shares for cash June 2002, $.03 | 1,051,211 | 11 | 319,011 | 319,022 | ||||||||||||||||||||||||
| Issuance of common shares to minority shareholders for cash July 2002, $.03 | — | — | 3,235 | 3,235 | ||||||||||||||||||||||||
| Issuance of common shares for cash September 2002, $.03 | 20,957 | — | 6,345 | 6,345 | ||||||||||||||||||||||||
| Issuance of common shares for minority shareholders as employee compensation October 2002, $.03 | — | — | 9,505 | 9,505 | ||||||||||||||||||||||||
| Issuance of stock options in exchange for services rendered October 2002 | — | — | 70,518 | 70,518 | ||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (2,243 | ) | (2,243 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (156,214 | ) | (156,214 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | (11,506 | ) | (11,506 | ) | ||||||||||||||||||||||||
| Total Comprehensive Income (Loss) | (156,214 | ) | (11,506 | ) | (167,720 | ) | ||||||||||||||||||||||
| Balance at October 31, 2002 | 21,730,718 | $ | 218 | $ | 910,334 | $ | (604,849 | ) | $ | (26,698 | ) | $ | (6,187 | ) | $ | 272,818 | ||||||||||||
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Income (Loss) | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2002 | 21,730,718 | $ | 218 | $ | 910,334 | $ | (604,849 | ) | $ | (26,698 | ) | $ | (6,187 | ) | $ | 272,818 | ||||||||||||
| Issuance of common shares for cash May 2003, $.03 | 28,292 | — | 9,871 | 9,871 | ||||||||||||||||||||||||
| Issuance of common shares for minority shareholders for cash May 2003 $.03 | — | — | 10,967 | 10,967 | ||||||||||||||||||||||||
| Issuance of warrants in conjunction with convertible debentures September 2003 | 358,406 | 358,406 | ||||||||||||||||||||||||||
| Issuance of common shares as employee compensation October 2003, $.04 | 56,584 | 1 | 20,421 | 20,422 | ||||||||||||||||||||||||
| Issuance of common shares for minority shareholders as employee compensation October 2003, $.04 | — | — | 7,564 | 7,564 | ||||||||||||||||||||||||
| Issuance of stock options in exchange for services rendered October 2003 | — | — | 23,580 | 23,580 | ||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (3,825 | ) | (3,825 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (228,993 | ) | (228,993 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | 42,957 | 42,957 | ||||||||||||||||||||||||||
| Total Comprehensive Income (Loss) | (228,993 | ) | 42,957 | (186,036 | ) | |||||||||||||||||||||||
| Balance at October 31, 2003 | 21,815,594 | $ | 219 | $ | 1,341,143 | $ | (833,842 | ) | $ | 16,259 | $ | (10,012 | ) | $ | 513,767 | |||||||||||||
See notes to consolidated financial statements.
| F-8 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Consolidated Statements of Changes in Equity (Deficiency) |
| For the year ended October 31, 2012 |
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Income (Loss) | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2003 | 21,815,594 | $ | 219 | $ | 1,341,143 | $ | (833,842 | ) | $ | 16,259 | $ | (10,012 | ) | $ | 513,767 | |||||||||||||
| Issuance of warrants in conjunction with convertible debentures September 2004 | 152,628 | 152,628 | ||||||||||||||||||||||||||
| Issuance of common shares as employee compensation October 2004, $.04 | 123,646 | 1 | 47,316 | 47,317 | ||||||||||||||||||||||||
| Issuance of common shares as directors' compensation October 2004, $.04 | 157,177 | 2 | 60,147 | 60,149 | ||||||||||||||||||||||||
| Issuance of stock options in exchange for services rendered October 2004 | — | — | 27,669 | 27,669 | ||||||||||||||||||||||||
| Issuance of options to directors and committee chairmen for services rendered in October 2004 | 54,582 | 54,582 | ||||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (9,774 | ) | (9,774 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (592,706 | ) | (592,706 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | (6,136 | ) | (6,136 | ) | ||||||||||||||||||||||||
| Total Comprehensive Income (Loss) | (592,706 | ) | (6,136 | ) | (598,842 | ) | ||||||||||||||||||||||
| Balance at October 31, 2004 | 22,096,417 | $ | 222 | $ | 1,683,485 | $ | (1,426,548 | ) | $ | 10,123 | $ | (19,786 | ) | $ | 247,496 | |||||||||||||
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Income (Loss) | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2004 | 22,096,417 | $ | 222 | $ | 1,683,485 | $ | (1,426,548 | ) | $ | 10,123 | $ | (19,786 | ) | $ | 247,496 | |||||||||||||
| Issuance of common shares as employee compensation November 2004, $.04 | 9,431 | — | 3,760 | 3,760 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation December 2004, $.04 | 9,431 | — | 3,692 | 3,692 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation January 2005, $.04 | 9,431 | — | 3,674 | 3,674 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation February 2005, $.04 | 9,431 | — | 3,629 | 3,629 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation March 2005, $.04 | 9,431 | — | 3,701 | 3,701 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation April 2005, $.04 | 9,431 | — | 3,641 | 3,641 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation May 2005, $.04 | 9,431 | — | 3,584 | 3,584 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation June 2005, $.04 | 9,431 | — | 3,628 | 3,628 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation July 2005, $.04 | 9,431 | — | 3,680 | 3,680 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation August 2005, $.04 | 9,431 | — | 3,737 | 3,737 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation September 2005, $.04 | 9,431 | — | 3,821 | 3,821 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation October 2005, $.04 | 9,431 | — | 3,822 | 3,822 | ||||||||||||||||||||||||
| Issuance of stock options in exchange for services rendered October 2005 | — | — | 33,973 | 33,973 | ||||||||||||||||||||||||
| Net loss attributtable to noncontolling interest | (11,195 | ) | (11,195 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (682,408 | ) | (682,408 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | (13,288 | ) | (13,288 | ) | ||||||||||||||||||||||||
| Total Comprehensive Income (Loss) | (682,408 | ) | (13,288 | ) | (695,696 | ) | ||||||||||||||||||||||
| Balance at October 31, 2005 | 22,209,589 | $ | 222 | $ | 1,761,827 | $ | (2,108,956 | ) | $ | (3,165 | ) | $ | (30,981 | ) | $ | (381,053 | ) | |||||||||||
See notes to consolidated financial statements.
| F-9 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Consolidated Statements of Changes in Equity (Deficiency) |
| For the year ended October 31, 2012 |
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Loss | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2005 | 22,209,589 | $ | 222 | $ | 1,761,827 | $ | (2,108,956 | ) | $ | (3,165 | ) | $ | (30,981 | ) | $ | (381,053 | ) | |||||||||||
| Issuance of common shares as employee compensation November 2005, $.04 | 10,478 | — | 4,232 | 4,232 | ||||||||||||||||||||||||
| Issuance of common shares in exchange for services rendered December 2005, $.04 | 10,478 | — | 4,305 | 4,305 | ||||||||||||||||||||||||
| Issuance of common shares in exchange for services rendered January 2006, $.04 | 10,478 | — | 4,321 | 4,321 | ||||||||||||||||||||||||
| Issuance of stock options in exchange for services rendered October 2006 | — | — | 2,658 | 2,658 | ||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (8,561 | ) | (8,561 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (522,532 | ) | (522,532 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | (25,688 | ) | (25,688 | ) | ||||||||||||||||||||||||
| Total Comprehensive Income (Loss) | (522,532 | ) | (25,688 | ) | (548,220 | ) | ||||||||||||||||||||||
| Balance at October 31, 2006 | 22,241,023 | $ | 222 | $ | 1,777,343 | $ | (2,631,488 | ) | $ | (28,853 | ) | $ | (39,542 | ) | $ | (922,318 | ) | |||||||||||
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Loss | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2006 | 22,241,023 | $ | 222 | $ | 1,777,343 | $ | (2,631,488 | ) | $ | (28,853 | ) | $ | (39,542 | ) | $ | (922,318 | ) | |||||||||||
| Incremental increase in fair value of warrants in conjunction with re-structuring of debentures, April 2007 | 44,096 | 44,096 | ||||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (4,972 | ) | (4,972 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (303,477 | ) | (303,477 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | (184,432 | ) | (184,432 | ) | ||||||||||||||||||||||||
| Total Comprehensive Income (Loss) | (303,477 | ) | (184,432 | ) | (487,909 | ) | ||||||||||||||||||||||
| Balance at October 31, 2007 | 22,241,023 | $ | 222 | $ | 1,821,439 | $ | (2,934,965 | ) | $ | (213,285 | ) | $ | (44,514 | ) | $ | (1,371,103 | ) | |||||||||||
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | income (Loss) | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2007 | 22,241,023 | $ | 222 | $ | 1,821,439 | $ | (2,934,965 | ) | $ | (213,285 | ) | $ | (44,514 | ) | $ | (1,371,103 | ) | |||||||||||
| Issuance of warrants in conjunction with re-structuring of debentures October 2008 | 231,580 | 231,580 | ||||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (21,813 | ) | (21,813 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (1,331,408 | ) | (1,331,408 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | 422,766 | 422,766 | ||||||||||||||||||||||||||
| Total Comprehensive Income (Loss) | (1,331,408 | ) | 422,766 | (908,642 | ) | |||||||||||||||||||||||
| Balance at October 31, 2008 | 22,241,023 | $ | 222 | $ | 2,053,019 | $ | (4,266,373 | ) | $ | 209,481 | $ | (66,327 | ) | $ | (2,069,978 | ) | ||||||||||||
See notes to consolidated financial statements.
| F-10 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Consolidated Statements of Changes in Equity (Deficiency) |
| For the year ended October 31, 2012 |
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Income (Loss) | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2008 | 22,241,023 | $ | 222 | $ | 2,053,019 | $ | (4,266,373 | ) | $ | 209,481 | $ | (66,327 | ) | $ | (2,069,978 | ) | ||||||||||||
| Issuance of common shares as payment of debenture interest, January 2009, $0.05 per share | 495,094 | 5 | 236,238 | 236,243 | ||||||||||||||||||||||||
| Issuance of common shares on exercise of options, April 2009 | 570,980 | 6 | 22 | 28 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation for the years 2001 to 2008, May 2009, $0.04 per share | 3,153,878 | 32 | 1,298,753 | 1,298,785 | ||||||||||||||||||||||||
| Issuance of common shares to directors, pursuant to debenture financing of January 2009, May 2009, $0.04 per share | 928,394 | 9 | 382,521 | 382,530 | ||||||||||||||||||||||||
| Issuance of common shares in exchange for services rendered, June 2009, $0.04 per share | 5,023 | — | 2,062 | \ | 2,062 | |||||||||||||||||||||||
| Issuance of common shares for cash June 2009, $0.04 per share | 24,090 | — | 8,602 | 8,602 | ||||||||||||||||||||||||
| Issuance of common shares in exchange for services rendered, July 2009, $0.04 per share | 47,153 | — | 20,250 | 20,250 | ||||||||||||||||||||||||
| Issuance of common shares as payment of director compensation, for the years 2004 through 2009, July 2009, $0.04 per share | 241,005 | 2 | 103,498 | 103,500 | ||||||||||||||||||||||||
| Issuance of common shares as employee compensation pursuant to reverse merger transaction, July 2009, $0.04 per share | 1,173,592 | 12 | 503,988 | 504,000 | ||||||||||||||||||||||||
| Issuance of common shares to retire debentures, July 2009, $0.03 per share | 3,346,028 | 34 | 997,538 | 997,572 | ||||||||||||||||||||||||
| Issuance of common shares as payment of debenture interest, January 2009, July 2009, $0.05 per share | 855,712 | 9 | 418,659 | 418,668 | ||||||||||||||||||||||||
| Issuance of common shares to retire director's loan, July 2009 $0.04 per share | 2,377,813 | 24 | 884,976 | 885,000 | ||||||||||||||||||||||||
| Issuance of common shares as payment of interest on director's loan, July 2009, $0.04 per share | 218,556 | 2 | 108,633 | 108,635 | ||||||||||||||||||||||||
| Issuance of common shares for cash, July 2009, $0.04 per share | 6,540,017 | 66 | 2,714,934 | 2,715,000 | ||||||||||||||||||||||||
| Issuance of common shares as compensation for consulting contract, July 2009, $0.38 per share | 100,000 | 1 | 379,999 | 380,000 | ||||||||||||||||||||||||
| Issuance of common shares on exercise of warrants by YA Global for cash August 2009 | 25,000 | — | 45,000 | 45,000 | ||||||||||||||||||||||||
| Beneficial conversion charge on 3rd debenture | — | 335,000 | 335,000 | |||||||||||||||||||||||||
| Beneficial conversion charge on director's loan | — | 117,109 | 117,109 | |||||||||||||||||||||||||
| Reclassification of warrants to derivative liability | (786,710 | ) | (786,710 | ) | ||||||||||||||||||||||||
| Assumption of options in reverse merger | 644,806 | 644,806 | ||||||||||||||||||||||||||
| Reclassification of derivative liability on increase of authorized shares | 13,501,360 | 13,501,360 | ||||||||||||||||||||||||||
| Effect of Reverse Merger | 7,089,282 | 71 | 11,573,465 | — | — | 11,573,536 | ||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (211,670 | ) | (211,670 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (28,715,913 | ) | (28,715,913 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | (529,296 | ) | (529,296 | ) | ||||||||||||||||||||||||
| Total Comprehensive Loss | (28,715,913 | ) | (529,296 | ) | (29,245,209 | ) | ||||||||||||||||||||||
| Balance October 31, 2009 | 49,432,640 | $ | 495 | $ | 35,543,722 | $ | (32,982,286 | ) | $ | (319,815 | ) | $ | (277,997 | ) | $ | 1,964,119 | ||||||||||||
See notes to consolidated financial statements.
| F-11 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Consolidated Statements of Changes in Equity (Deficiency) |
| For the year ended October 31, 2012 |
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | income (Loss) | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2009 | 49,432,640 | $ | 495 | $ | 35,543,722 | $ | (32,982,286 | ) | $ | (319,815 | ) | $ | (277,997 | ) | $ | 1,964,119 | ||||||||||||
| Issuance of common shares in exchange for services rendered January 2010, $.14 | 35,000 | — | 49,000 | 49,000 | ||||||||||||||||||||||||
| Common shares issued on exercise of Warrants, February 2010 | 75,000 | 1 | 35,249 | 35,250 | ||||||||||||||||||||||||
| Common shares issued for cash, February 2010 | 77,000 | 1 | 76,999 | 77,000 | ||||||||||||||||||||||||
| Common shares issued in exchange for services rendered, May 2010 | 78,371 | 1 | 88,199 | 88,200 | ||||||||||||||||||||||||
| Common shares issued in exchange for services rendered, June 2010 | 50,000 | — | 18,000 | 18,000 | ||||||||||||||||||||||||
| Common shares issued for cash, October 2010 | 3,031,150 | 15 | 1,515,558 | 1,515,573 | ||||||||||||||||||||||||
| Common shares issued for subscription receivable, October 2010 | 230,000 | 1 | 114,999 | 115,000 | ||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (14,225 | ) | (14,225 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Net Loss | (2,024,272 | ) | (2,024,272 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income (Loss) | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | 82,307 | 82,307 | ||||||||||||||||||||||||||
| Total Comprehensive Income (Loss) | (2,024,272 | ) | 82,307 | (1,941,965 | ) | |||||||||||||||||||||||
| Balance at October 31, 2010 | 53,009,161 | $ | 514 | $ | 37,441,728 | $ | (35,006,558 | ) | $ | (237,508 | ) | $ | (292,222 | ) | $ | 1,905,954 | ||||||||||||
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Income (Loss) | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2010 | 53,009,161 | $ | 514 | $ | 37,441,728 | $ | (35,006,558 | ) | $ | (237,508 | ) | $ | (292,222 | ) | $ | 1,905,954 | ||||||||||||
| Issuance of common shares in exchange for services rendered December 2010, $1.00 | 100,000 | 1 | 99,999 | 100,000 | ||||||||||||||||||||||||
| Issuance of common shares for cash December 2010, $.50 | 600,000 | 6 | 297,741 | 297,747 | ||||||||||||||||||||||||
| Issuance of common shares for cash February 2011, $.50 | 100,000 | 1 | 49,999 | 50,000 | ||||||||||||||||||||||||
| Issuance of common shares on exercise of warrants, February 2011, $.47 | 22,005 | 10,402 | 10,402 | |||||||||||||||||||||||||
| Refund of common shares subscribed for October 2010 in cash February 2011, $.50 | (30,000 | ) | (15,000 | ) | (15,000 | ) | ||||||||||||||||||||||
| Re-pricing of options in exchange for services rendered, February 2011 | 163,750 | 163,750 | ||||||||||||||||||||||||||
| Issuance of common shares on exercise of options, February 2011, $.01 | 275,000 | 3 | 2,747 | 2,750 | ||||||||||||||||||||||||
| Issuance of common shares on settlement of lawsuit, August 2011 | 1,000,000 | 10 | 599,990 | 600,000 | ||||||||||||||||||||||||
| Issuance of warrants on settlement of lawsuit, August 2011 | 1,053,800 | 1,053,800 | ||||||||||||||||||||||||||
| Issuance of common shares in exchange for services rendered September 2011 | 550,000 | 5 | 291,495 | 291,500 | ||||||||||||||||||||||||
| Issuance of warrants in exchange for services rendered, September 2011 | 1,777,350 | 1,777,350 | ||||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (37,302 | ) | (37,302 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss): | ||||||||||||||||||||||||||||
| Net Loss | (5,724,616 | ) | (5,724,616 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | 64,101 | 64,101 | ||||||||||||||||||||||||||
| Total Comprehensive Income (Loss) | (5,724,616 | ) | 64,101 | (5,660,515 | ) | |||||||||||||||||||||||
| Balance at October 31, 2011 | 55,626,166 | $ | 540 | $ | 41,774,001 | $ | (40,731,174 | ) | $ | (173,407 | ) | $ | (329,524 | ) | $ | 540,436 | ||||||||||||
See notes to consolidated financial statements.
| F-12 |
| CardioGenics Holdings Inc. |
| (A Development Stage Company) |
| Consolidated Statements of Changes in Equity (Deficiency) |
| For the year ended October 31, 2012 |
| Deficit | ||||||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||||||
| During | Accumulated | |||||||||||||||||||||||||||
| Additional | the | Other | Total | |||||||||||||||||||||||||
| Common Stock | Paid-in | Development | Comprehensive | Noncontrolling | Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Stage | Income (Loss) | Interest | (Deficiency) | ||||||||||||||||||||||
| Balance November 1, 2011 | 55,626,166 | $ | 540 | $ | 41,774,001 | $ | (40,731,174 | ) | $ | (173,407 | ) | $ | (329,524 | ) | $ | 540,436 | ||||||||||||
| Issuance of common shares for cash October 2012 | 1,050,000 | 3 | 262,497 | 262,500 | ||||||||||||||||||||||||
| Net loss attributable to noncontrolling interest | (8,410 | ) | (8,410 | ) | ||||||||||||||||||||||||
| Comprehensive Income (Loss): | ||||||||||||||||||||||||||||
| Net Loss | (1,308,049 | ) | (1,308,049 | ) | ||||||||||||||||||||||||
| Other Comprehensive Income | ||||||||||||||||||||||||||||
| Currency Translation Adjustment | 6,770 | 6,770 | ||||||||||||||||||||||||||
| Total Comprehensive Income (Loss) | (1,308,049 | ) | 6,770 | (1,301,279 | ) | |||||||||||||||||||||||
| Balance at October 31, 2012 | 56,676,166 | $ | 543 | $ | 42,036,498 | $ | (42,039,223 | ) | $ | (166,637 | ) | $ | (337,934 | ) | $ | (506,753 | ) | |||||||||||
See notes to consolidated financial statements.
| F-13 |
| CardioGenics Holdings Inc. |
| Consolidated Statements of Cash Flows |
| Years Ended October 31, 2012 and 2011 and |
| Cumulative from November 20, 1997 (Date of Inception) to October 31, 2012 |
| Cumulative from | ||||||||||||
| November 20, 1997 | ||||||||||||
| Years Ended | (Date of Inception) | |||||||||||
| October 31 | To October 31, | |||||||||||
| 2012 | 2011 | 2012 | ||||||||||
| Cash flows from operations activities | ||||||||||||
| Net Loss | $ | (1,316,459 | ) | $ | (5,761,918 | ) | $ | (42,377,157 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities | ||||||||||||
| Depreciation and Amortization of Property and Equipment | 18,305 | 20,399 | 219,744 | |||||||||
| Amortization of Patent Application Costs | 6,882 | 5,207 | 19,293 | |||||||||
| Write-off of Patent Application Costs | 24,905 | 55,549 | 239,530 | |||||||||
| Re-pricing of Options for Services Rendered | — | 163,750 | 163,750 | |||||||||
| Write-off of Goodwill | — | — | 12,780,214 | |||||||||
| Amortization of Deferred Debt Issuance Costs | — | — | 511,035 | |||||||||
| Loss on Extinguishment of Debt | — | — | 275,676 | |||||||||
| Loss on Change in Value of Derivative Liability | — | — | 12,421,023 | |||||||||
| Interest Accrued and Foreign Exchange Loss on Debt | — | — | 922,539 | |||||||||
| Unrealized Foreign Currency Exchange Gains | — | — | 25,094 | |||||||||
| Beneficial Conversion Charge included in | ||||||||||||
| Interest Expense | — | — | 452,109 | |||||||||
| Common Stock and Warrants Issued on Settlement of Lawsuit | — | 1,653,800 | 1,653,800 | |||||||||
| Common Stock Issued as Employee or | ||||||||||||
| Officer/Director Compensation | — | — | 2,508,282 | |||||||||
| Common Stock and Warrants Issued for Services Rendered | — | 2,168,750 | 2,726,262 | |||||||||
| Stock Options Issued for Services Rendered | — | — | 192,238 | |||||||||
| Stock Options Issued to Directors and Committee Chairman | — | — | 54,582 | |||||||||
| Changes in Operating Assets and Liabilities, Net of Acquisition | ||||||||||||
| Accounts Receivable | 8,565 | (9,002 | ) | (437 | ) | |||||||
| Share Subscriptions Receivable | — | 115,000 | — | |||||||||
| Deposits and Prepaid Expenses | 119 | 38,233 | (50,633 | ) | ||||||||
| Refundable Taxes Receivable | (10,016 | ) | (13,232 | ) | (44,343 | ) | ||||||
| Government Grants and Investment Tax Credits Receivable | 107,417 | (31,015 | ) | (60,018 | ) | |||||||
| Accounts Payable and Accrued Expenses | 189,443 | 73,537 | 18,223 | |||||||||
| Advances | — | — | 131 | |||||||||
| Net cash used in operating activities | (970,839 | ) | (1,520,942 | ) | (7,349,063 | ) | ||||||
| Cash flows from investing activities | ||||||||||||
| Cash Acquired from Acquisition | — | — | 195,885 | |||||||||
| Purchase of Property and Equipment | (3,824 | ) | (15,242 | ) | (223,490 | ) | ||||||
| Patent Application Costs | (4,204 | ) | (17,764 | ) | (318,774 | ) | ||||||
| Net cash used in investing activities | (8,028 | ) | (33,006 | ) | (346,379 | ) | ||||||
| Cash flows from financing activities | ||||||||||||
| Due to Shareholders | 100,000 | — | 100,000 | |||||||||
| Repayment of Capital Lease Obligation | (25,714 | ) | (13,532 | ) | (41,290 | ) | ||||||
| Due to Director | — | (15,149 | ) | 725,330 | ||||||||
| Issue of Debentures | — | — | 1,378,305 | |||||||||
| Issue of Common Shares on Exercise of Stock Options | — | 2,750 | 2,781 | |||||||||
| Issue of Common Shares on Exercise of Warrants | — | 10,402 | 45,652 | |||||||||
| Issue of Common Shares for Cash | 262,500 | 347,747 | 5,886,669 | |||||||||
| Refund of Share Subscription | — | (15,000 | ) | (15,000 | ) | |||||||
| Redemption of 10% Senior Convertible Debentures | — | — | (394,972 | ) | ||||||||
| Net cash provided by financing activities | 336,786 | 317,218 | 7,687,475 | |||||||||
| Effect of foreign exchange on cash and cash equivalents | (112 | ) | 61,180 | 34,976 | ||||||||
| Cash and Cash Equivalents | ||||||||||||
| Increase (Decrease) in cash and cash equivalents during the period | (642,193 | ) | (1,175,550 | ) | 27,009 | |||||||
| Beginning of Period | 669,202 | 1,844,752 | — | |||||||||
| End of Period | $ | 27,009 | $ | 669,202 | $ | 27,009 | ||||||
See notes to consolidated financial statements.
| F-14 |
| 1. | Nature of Business |
The accompanying audited consolidated financial statements have been prepared in accordance with the requirements of Form 10-K and Article 8 of Regulation S-X of the Securities and Exchange Commission (the “SEC”) and include the results of CardioGenics, Inc. and its subsidiaries and JAG Media Holdings, Inc and its subsidiaries (”JAG Media”) (from July 31, 2009, date of acquisition) which are collectively referred to as the “Company.”
CardioGenics Inc. (“CardioGenics”) was incorporated on November 20, 1997 in the Province of Ontario, Canada, and carries on the business of development and commercialization of diagnostic test products for the In Vitro Diagnostics testing market. CardioGenics has several test products that are in various stages of development. In the last quarter of 2011 CardioGenics commenced selling one of these products, but has generated no significant revenue therefrom.
On July 31, 2009, CardioGenics acquired the business of JAG Media Holdings, Inc. (“JAG Media”). The business acquired is that of gathering and compiling financial and investment information from various financial institutions and other Wall Street professionals. Revenues of the acquired business of JAG Media are generated by releasing such financial information to subscribers in a consolidated format on a timely basis through facsimile transmissions and a web site. Further, software focused on streaming video solutions was acquired through the acquisition of JAG Media by CardioGenics. Historically, further development of this software had been limited as a result of JAG Media’s lack of financial resources.
On February 11, 2010, the Company entered into an LLC Membership Interest Purchase Agreement with Rothcove Partners LLS (“Rothcove”) pursuant to which the Company sold its interest in JAG Media to Rothcove.
References herein to CardioGenics common shares has been retrospectively adjusted to reflect the exchange ratio of 20.957 established in the Share Purchase Agreement related to the acquisition of JAG Media Holdings, Inc. (“Holdings”).
On October 27, 2009, the name of the Company was changed from Jag Media Holdings, Inc. to CardioGenics Holdings, Inc.
On April 23, 2010, the Company’s Board of Directors approved a reverse stock split of its issued and outstanding common shares. The total authorized shares of common stock was at the same time reduced to 65,000,000. The Board of Directors selected a ratio of one-for-ten and the reverse stock split was effective on June 20, 2010. Trading of the Company’s common stock on the Over-The-Counter Capital Market on a split adjusted basis began at the open of trading on June 21, 2010. The reverse stock split affected all shares of the Company’s common stock, as well as options to purchase the Company’s common stock and other equity incentive awards and warrants that were outstanding immediately prior to the effective date of the reverse stock split. All references to common shares and per-share data for prior periods have been retroactively restated to reflect the reverse stock split as if it had occurred at the beginning of the earliest period presented.
| 2. | Restatement of Financial Statements |
During the preparation of the January 31, 2012 interim consolidated financial statements, the Company determined that the accounting for the issuance of common stock and warrants in the year ended October 31, 2011 required restatement. The Company also determined that the recording of certain tax related income and expenses required restatement.
| F-15 |
As a result of these restatements, amounts in our consolidated balance sheet, statement of operations, statement of cash flows and equity for the year ended October 31, 2011 have been corrected. This restatement also resulted in changes to Notes 8 and 9 to the consolidated financial statements. Statement of cash flows has been restated to reflect classification errors resulting in an increase in cash from operating activities of $111,927.
The restatement relates to the following:
| a) | In September 2011, we entered into a consulting contracts covering a period of eighteen months, compensation for which included the issuance of common shares stock and fully vested warrants. The Company determined that the fair value of the compensation was $2.1 million. Based on our initial evaluation of the relevant accounting guidance, the Company recorded the fair value as an asset as part of a prepaid expense in 2011 and began amortizing this balance to general and administrative expense over an eighteen month period. The offset to the prepaid expense credit was recorded as additional paid-in capital. The Company has subsequently determined that the entire fair value of the compensation for these contracts should have been expensed immediately rather than recorded as an asset. In order to correct this error, the Company reduced deposits and prepaid expenses by $1,309,296 and prepaid consulting contract asset by $567,015 as at October 31, 2011, expensed the fair value of these contracts of $1,869,660 as part of general and administrative expenses in the year ended October 31, 2011 and recorded a reduction in currency translation adjustment of $6,651; |
| b) | The Company determined that a government grant and investment tax credit receivable relating to investment tax credits under the scientific research and experimental development auspices of the Canadian Income Tax Act in the amount of approximately $187,497 had formerly not been recorded. In order to correct this error, the Company increased government grants and investment tax credits receivable and reduced general and administrative expenses as at and for the year ended October 31, 2011; and, |
| c) | The Company determined that potential tax penalties in the amount of $180,000 related to the late filing of certain tax returns with the Internal Revenue Services had formerly not been recorded. In order to correct this error, the Company increased accounts payable and accrued expenses and general and administrative expenses as at and for the year ended October 31, 2011. |
The following table summarizes the effects of the restated adjustments on our previously issued consolidated balance sheet for the year ended October 31, 2011:
| As Previously | ||||||||||||
| Reported | Adjustments | As Restated | ||||||||||
| Deposits and Prepaid Expenses | $ | 1,360,837 | $ | (1,309,296 | ) | $ | 51,541 | |||||
| Government Grants and Investment Tax Credits Receivable | — | 187,497 | 187,497 | |||||||||
| Prepaid Consulting Contract | 567,015 | (567,015 | ) | — | ||||||||
| Total Assets | 2,854,287 | (1,688,814 | ) | 1,165,473 | ||||||||
| Accounts Payable and Accrued Expenses | 416,692 | 180,000 | 596,692 | |||||||||
| Deficit Accumulated During Development Stage | (38,880,934 | ) | (1,850,240 | ) | (40,731,174 | ) | ||||||
| Accumulated Other Comprehensive Loss | (166,756 | ) | (6,651 | ) | (173,407 | ) | ||||||
| Total Equity Attributable To CardioGenics Holdings Inc. | 2,726,851 | (1,856,891 | ) | 869,960 | ||||||||
| Non-Controlling Interest | (317,601 | ) | (11,923 | ) | (329,524 | ) | ||||||
| Total Equity | 2,409,250 | (1,868,814 | ) | 540,436 | ||||||||
| Total Liabilities and Equity | 2,854,287 | (1,688,814 | ) | 1.165,473 | ||||||||
| F-16 |
The following table summarizes the effects of the restated adjustments on our previously issued statement of equity for the year ended October 31, 2011:
Changes in deficit accumulated during development stage
| As Previously | ||||||||||||
| Reported | Adjustments | As Restated | ||||||||||
| Deficit accumulated during development stage at October 31, 2010 | $ | (35,006,558 | ) | $ | — | $ | (35,006,558 | ) | ||||
| Net loss attributed to CardioGenics Holdings Inc. | (3,874,376 | ) | (1,850,240 | ) | (5,724,616 | ) | ||||||
| Deficit accumulated during development stage at October 31, 2011 | $ | (38,880,934 | ) | $ | (1,850,240 | ) | $ | (40,731,174 | ) | |||
Changes in accumulated other comprehensive loss
| As Previously | ||||||||||||
| Reported | Adjustments | As Restated | ||||||||||
| Accumulated other comprehensive loss October 31, 2010 | $ | (237,508 | ) | $ | — | $ | (237,508 | ) | ||||
| Currency translation Adjustment | 70,752 | (6,651 | ) | 64,101 | ||||||||
| Accumulated other comprehensive loss October 31, 2011 | $ | (166,756 | ) | $ | (6,651 | ) | $ | (173,407 | ) | |||
Changes in non-controlling interest
| As Previously | ||||||||||||
| Reported | Adjustments | As Restated | ||||||||||
| Accumulated non-controlling interest October 31, 2010 | $ | (292,222 | ) | $ | — | $ | (292,222 | ) | ||||
| Net loss attributable to Non-controlling interest | (25,379 | ) | (11,923 | ) | (37,302 | ) | ||||||
| Accumulated non-controlling interest October 31, 2011 | $ | (317,601 | ) | $ | (11,923 | ) | $ | (329,524 | ) | |||
Changes in total equity
| As Previously | ||||||||||||
| Reported | Adjustments | As Restated | ||||||||||
| Total equity October 31, 2010 | $ | 1,905,954 | $ | — | $ | 1,905,954 | ||||||
| Additions to common Stock | 26 | — | 26 | |||||||||
| Additions to APIC | 4,332,273 | — | 4,332,273 | |||||||||
| Net loss attributable to non-controlling interest | (25,379 | ) | (11,923 | ) | (37,302 | ) | ||||||
| Comprehensive loss | (3,803,624 | ) | (1,856,891 | ) | (5,660,515 | ) | ||||||
| Total equity October 31, 2011 | $ | 2,409,250 | $ | (1,868,814 | ) | $ | 540,436 | |||||
| F-17 |
The following table summarizes the effects of the restated adjustments on our previously issued statement of operations for the year ended October 31, 2011:
| As Previously | As | |||||||||||
| Reported | Adjustments | Restated | ||||||||||
| General and administrative expenses | $ | 1,349,300 | $ | 2,049,660 | $ | 3,398,960 | ||||||
| Research and product development, net of investment tax credits | 613,504 | (187,497 | ) | 426,007 | ||||||||
| Total operating expenses | 3,797,759 | 1,862,163 | 5,659,922 | |||||||||
| Operating loss | (3,788,883 | ) | (1,862,163 | ) | (5,651,046 | ) | ||||||
| Loss from continuing operations | (3,899,755 | ) | (1,862,163 | ) | (5,761,918 | ) | ||||||
| Net loss attributable to Non-controlling interest | 25,379 | 11,923 | 37,302 | |||||||||
| Net loss attributed to CardioGenics Holdings Inc. | (3,874,376 | ) | (1,850,240 | ) | (5,724,616 | ) | ||||||
| 3. | Basis of Presentation |
The accompanying consolidated financial statements have been prepared using the accounting principles generally accepted in the United States of America applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business.
The Company has incurred operating losses and has experienced negative cash flows from operations since inception. The Company has a deficit accumulated at October 31, 2012 of approximately $42.0 million. The Company has not yet established an ongoing source of revenues sufficient to cover its operating costs and to allow it to continue as a going concern. The Company has funded its activities to date almost exclusively from debt and equity financings. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
The Company will continue to require substantial funds to continue research and development, including preclinical studies and clinical trials of its products, and to commence sales and marketing efforts, if the FDA and other regulatory approvals are obtained. In order to meet its operating cash flow requirements Management’s plans include financing activities such as private placements of its common stock and issuances of convertible debt instruments. Management is also actively pursuing industry collaboration activities including product licensing and specific project financing.
While the Company believes it will be successful in obtaining the necessary financing to fund its operations, meet revenue projections and manage costs, there are no assurances that such additional funding will be achieved and that it will succeed in its future operations. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts of liabilities that might be necessary should the Company be unable to continue as a going concern.
| 4. | Summary of Significant Accounting Policies |
| (a) | Principles of Consolidation |
The consolidated financial statements include the accounts of the Company and its 100% owned subsidiaries. All significant intercompany transactions and balances have been eliminated in consolidation.
| F-18 |
| (b) | Development Stage Company |
The accompanying consolidated financial statements have been prepared in accordance with the provisions of the guidance for development stage enterprises.
| (c) | Cash and Cash Equivalents |
The Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents.
| (d) | Share Subscription Receivable |
Subscriptions for common shares received are recorded as receivable on the subscription date with the offsetting credit to capital on the same date.
| (e) | Government Grants and Investment Tax Credits Receivable |
The Company’s accounts include claims for investment tax credits relating to scientific research activities of the Company prior to the acquisition described in Note 1. The qualification and recording of this activity for investment tax credit purposes is established by Canadian Income Tax authorities when the income tax returns for the period are assessed. The credit has been recognized in the consolidated statement of operations in the year in which the expenses were incurred.
Subsequent to the acquisition described in Note 1, the Company no longer qualifies to receive substantial refunds of Investment Tax Credits (“ITCs”) resulting from scientific research. Currently, the majority of ITCs resulting from scientific research are carried forward to a time when the Company becomes tax paying at which time said ITCs are applicable against taxes payable.
| (f) | Property and Equipment |
Property under capital leases and the related obligation for future lease payments are initially recorded at an amount equal to the lesser of fair value of the property or equipment and the present value of those lease payments. Property and equipment is depreciated using methods and rates as follows:
| Furniture and Fixtures | 20% declining balance | |
| Lab Equipment | 20% declining balance | |
| Computer Equipment – Hardware | 30% declining balance | |
| Computer Equipment – Software | 50% declining balance | |
| Leasehold Improvements | Straight-line over the lesser of | |
| the life of the asset or the life of the lease |
| (g) | Patents |
Capitalized patent costs represent legal and application costs incurred to establish patents. Capitalized patent costs are amortized on a straight-line method over the related patent term. As patents are abandoned, the net book value of the patent is written off.
| (h) | Impairment or Disposal of Long-Lived Assets |
The Company assesses the impairment of long-lived assets under the guidance of standards for the impairment or disposal of long-lived assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable. For long-lived assets to be held and used, the Company recognizes an impairment loss only if its carrying amount is not recoverable and exceeds its fair value. The carrying amount of the long-lived asset is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposal of the asset.
| F-19 |
| (i) | Research and Development Costs |
Expenditures for research and development are expensed as incurred and include, among other costs, those related to the production of prototype products, including payroll costs. Amounts expected to be received from governments under Scientific Research Tax Credit arrangements are offset against current expenses. The Company recognizes revenue from restricted grants in the period in which the Company has incurred the expenditures in compliance with the specific restrictions.
| (j) | Income Taxes |
The Company utilizes the liability method of accounting for income taxes as set forth in the authoritative guidance. Under the liability method, deferred taxes are determined based on the temporary differences between the financial statement and tax bases of assets and liabilities using tax rates expected to be in effect during the years in which the bases differences reverse. A valuation allowance is recorded when it is more likely than not that some of the deferred tax assets will not be realized. As there is no certainty that the Company will generate taxable income in the foreseeable future to utilize tax losses accumulated to date, no provision for ultimate tax reduction has been made in these consolidated financial statements.
On November 1, 2007, the Company adopted the guidance issued for accounting for uncertainty in income taxes which provides detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in an enterprise’s financial statements. Income tax positions must meet a more-likely-than-not recognition threshold at the effective date to be recognized upon the adoption of the guidance and in subsequent periods. The Company recognizes potential accrued interest and penalties related to unrecognized tax benefits within operations as general and administrative expense.
| (k) | Stock-Based Compensation |
The Company follows the authoritative guidance for stock-based compensation which requires that new, modified and unvested share-based payment transactions with employees, such as grants of stock options and restricted stock, be recognized in the financial statements based on their fair value at the grant date and recognized as compensation expense over their vesting periods. The Company has also considered the related guidance of the SEC. The Company estimates the fair value of stock options and shares issued as compensation to employees and directors as of the date of grant using the Black-Scholes pricing model and restricted stock based on the per share value. The Company also follows the guidance for equity instruments that are issued to other than employees for acquiring, or in conjunction with selling, goods or services for equity instruments issued to consultants which provides guidance on transactions in which (1) the fair value of the equity instruments is more reliably measurable than the fair value of the goods or services received and (2) the counterparty receives shares of stock, stock options, or other equity instruments in settlement of the entire transaction or, if the transaction is part cash and part equity instruments, in settlement of the portion of the transaction for which the equity instruments constitute the consideration. Options issued with a nominal exercise price in exchange for services rendered were measured at the fair value of the underlying services rendered on the date of grant. The expense was recorded to the consolidated statement of operations with a corresponding increase in share capital with no additional increase in the number of shares as they were legally not yet exercised.
| (l) | Net Loss Per Common Share |
Basic loss per share is computed by dividing loss available to common stockholders by the weighted average number of common shares outstanding during the period. Diluted earnings (loss) per share gives effect to all dilutive potential common shares outstanding during the period. The computation of diluted earnings (loss) per share does not assume conversion, exercise or contingent exercise of securities that would have an anti-dilutive effect on earnings (loss) per share.
| F-20 |
| (m) | Comprehensive Income (Loss) |
Other comprehensive income (loss), which includes only foreign currency translation adjustments, is shown in the Consolidated Statements of Changes in Equity (Deficiency).
| (n) | Concentration of Credit Risk |
The Company maintains cash balances, at times, with financial institutions in excess of amounts insured by the Canada Deposit Insurance Corporation and the Federal Deposit Insurance Corporation. Management monitors the soundness of these institutions and has not experienced any collection losses with these financial institutions.
| (o) | Use of Estimates |
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates. By their nature, these estimates are subject to uncertainty and the effect on the consolidated financial statements of changes in such estimates in future periods could be material.
| (p) | Foreign Currency Translation |
The Company maintains its accounting records for its Canadian operations in Canadian dollars. Transactions in United States Dollars (“USD”) are translated into Canadian Dollars at rates in effect at the date of the transaction and gains or losses on such transactions are recorded at the time of settlement in the consolidated statement of operations.
The Company’s reporting currency is the United States Dollar. Foreign denominated assets and liabilities of the Company are translated into USD at the prevailing exchange rates in effect at the end of the reporting period, the historical rate for equity (deficiency) and a weighted average of exchange rate in effect during the period for expenses, gains and losses. Adjustments that arise from translation into the reporting currency are recorded in the accumulated other comprehensive income (loss) component of equity (deficiency).
| (q) | Financial Instruments |
The carrying values of cash and cash equivalents, other current assets, accounts payable and accrued expenses approximate their fair values due to their short-term nature.
| (r) | Revenue Recognition |
Revenue included in these consolidated financial statements is derived from sales of paramagnetic beads and is recognized on shipment to customers.
| (s) | Effects of Recent Accounting Pronouncements |
In December 2011, the Financial Accounting Standards Board issued Accounting Standards Update (“ASU”) No. 2011-11, Balance Sheet (Topic 210): Disclosures about Offsetting Assets and Liabilities. ASU 2011-11 requires an entity to disclose information about offsetting and related arrangements tp enable users of financial statements to understand the effect of those arrangements on its financial position, and to allow investors to better compare financial statements prepared under U.S. GAAP with financial statements prepared under International Financial Reporting Standards. The new standards are effective for annual periods beginning January 1, 2013, and interim periods within those annual periods. Retrospective application is required. The Company will implement the provisions of ASU 2011-11 beginning in fiscal 2014.
| F-21 |
| 5. | Property and Equipment |
The costs and accumulated depreciation and amortization of property and equipment are summarized as follows:
| October 31 | ||||||||
| 2012 | 2011 | |||||||
| Furniture and Fixtures | $ | 12,120 | $ | 12,120 | ||||
| Lab Equipment | 168,328 | 164,504 | ||||||
| Computer Hardware | 19,490 | 19,490 | ||||||
| Computer Software | 8,433 | 8,433 | ||||||
| Leasehold Improvements | 91,269 | 91,269 | ||||||
| Total Property and Equipment | 299,640 | 295,816 | ||||||
| Less Accumulated Depreciation and Amortization | 231,813 | 213,508 | ||||||
| Property and Equipment, Net | $ | 67,827 | $ | 82,308 | ||||
Depreciation and amortization expense amounted to $18,305 and $20,399 for the years ended October 31, 2012 and 2011, respectively.
| 6. | Patents |
The costs and accumulated amortization of patents are summarized as follows:
| October 31 | ||||||||
| 2012 | 2011 | |||||||
| Patents | $ | 128,336 | $ | 143,143 | ||||
| Less: Accumulated Amortization | (18,305 | ) | (12,411 | ) | ||||
| Patents, Net | $ | 110,031 | $ | 130,732 | ||||
| Weighted-Average Life | 17 Years | 17 Years | ||||||
| Amortization expense amounted to $6,882 and $5,207 for the years ended October 31, 2012 and 2011, respectively. Amortization expense is expected to be approximately $6,800 per year for the years ended October 31, 2013 through 2016. During the years ended October 31, 2012 and 2011, the Company wrote off approximately $24,905 and $55,529 of net book value of patents, respectively, for abandoned patents. |
| 7. | Due to Shareholders |
The amount due to shareholders is due on demand and carries interest at 10% per annum.
| 8. | Income Taxes |
The Company adopted the provisions of the guidance for uncertainty in income taxes on November 1, 2007. The guidance clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statement, and prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. It also provides guidance on derecognition classification, interest and penalties, accounting in interim periods, disclosure and transition.
| F-22 |
Based on the Company’s evaluation, management has concluded that there are no significant uncertain tax positions requiring recognition in the consolidated financial statements.
The Company has incurred losses in Canada since inception, which have generated net operating loss carryforwards for income tax purposes. The net operating loss carryforwards (“NOLs”) arising from Canadian sources as of October 31, 2012 were $6,319,184 (2011 - $5,819,492) which will expire from 2014 through 2032.
All fiscal years have been assessed; however, claims relating to research and development credits are open for review for the fiscal years ended October 31, 2012, 2011, 2010, 2009, 2008, 2007, and July 31, 2009.
As of October 31, 2012 and 2011, the Company had NOLs from US sources of approximately $40,713,000 and $40,476,000, respectively, available to reduce future Federal taxable income which will expire from 2019 through 2032.
Internal Revenue Code Section 382 (“Section 382”) imposes a limitation on a corporation’s ability to utilize NOLs if it experiences an ownership change. In general, an ownership change may occur from certain transactions that increase the ownership of 5% stockholders in the stock of a corporation by more than 50 percentage points over a three year period. If an ownership change occurs, utilization of the NOLs would be subject to an annual limitation. The annual limitation under Section 382 is calculated by multiplying the value of stock at the time of the ownership change by the applicable long- term tax exempt rate. Any unused annual limitation may be carried over to later years. The Company has historically been in a loss position and, therefore, the Section 382 limitation may not be relevant for the current period.
For the years ended October 31, 2012 and 2011, the Company’s effective tax rate differs from the statutory rate principally due to the NOLs for which no benefit was recorded.
As of October 31, 2012 and 2011, the Company’s deferred tax assets consisted of the effects of temporary differences attributable to the following:
| October 31 | ||||||||
| 2012 | 2011 | |||||||
| Temporary: | ||||||||
| Property and equipment | $ | (14,600 | ) | $ | (9,459 | ) | ||
| Net operating loss carryforwards | 15,589,530 | 15,221,715 | ||||||
| Unrealized foreign exchange | 16,601 | 11,287 | ||||||
| Investment tax credits | 381,836 | 359,436 | ||||||
| Transitional tax debits | (25,076 | ) | (25,076 | ) | ||||
| Total Deferred Tax Assets | 15,948,291 | 15,557,903 | ||||||
| Valuation Allowance | (15,948,291 | ) | (15,557,903 | ) | ||||
| Net Deferred Income Taxes | $ | — | $ | — | ||||
| F-23 |
A reconciliation of the Canadian combined statutory rate to the Company’s effective tax rate for the years ended October 31, 2012 and 2011 is as follows:
| October 31 | ||||||||
| 2012 | 2011 | |||||||
| Statutory rate | 34 | % | 34 | % | ||||
| Decrease in income tax rate resulting from: | ||||||||
| Rate differences between jurisdictions | (9.9 | )% | (1.2 | )% | ||||
| Changes in tax rate | — | (27.6 | )% | |||||
| Other | — | (8.5 | )% | |||||
| Permanent differences | — | (24.0 | )% | |||||
| Change in valuation allowance | 24.1 | % | 27.3 | % | ||||
| Effective tax rate | 0.0 | % | 0.0 | % | ||||
| 9. | Accounts Payable and Accrued Expenses |
| October 31 | ||||||||
| 2012 | 2011 | |||||||
| Accounts Payable | $ | 149,256 | $ | 103,544 | ||||
| Income Tax Reserve | 200,000 | 180,000 | ||||||
| Research and Development | 70,560 | 24,796 | ||||||
| Investor Relations | 21,165 | 7,924 | ||||||
| Patent Application Costs | 10,736 | 5,209 | ||||||
| Legal Fees | 263,703 | 274,616 | ||||||
| Accounting Fees | 70,715 | 603 | ||||||
| Total | $ | 786,135 | $ | 596,892 | ||||
| 10. | Stock-Based Compensation |
The Company follows the guidance for stock-based compensation. Stock-based employee compensation related to stock options for each of the years ended October 31, 2012 and 2011 amounted to $-0-.
The following is a summary of the common stock options granted, forfeited or expired and exercised under the plan:
| Weighted | ||||||||
| Average | ||||||||
| Exercise | ||||||||
| Options | Price | |||||||
| Outstanding – October 31, 2010 | 305,000 | $ | 2.34 | * | ||||
| Granted | — | — | ||||||
| Forfeited/expired | ||||||||
| Exercised | 275,000 | 0.01 | ||||||
| Outstanding – October 31, 2011 | 30,000 | $ | 0.90 | |||||
| Granted | — | — | ||||||
| Forfeited/expired | — | — | ||||||
| Exercised | — | — | ||||||
| Outstanding – October 31, 2012 | 30,000 | $ | 0.90 | |||||
| Exercisable | 30,000 | $ | 0.90 | |||||
| F-24 |
* of the 305,000 options outstanding as at October 31, 2010 at a weighted average exercise price of $2.34, 275,000 options were repriced during fiscal 2011 to $0.01.
Options typically vest immediately at the date of grant. As such, the Company does not have any unvested options or unrecognized compensation expense at October 31, 2012 and 2011.
| Options | ||||
| *Stock options formerly priced at $0.20 were repriced at $0.01 and extended to August 2011 | 75,000 | |||
| *Stock options formerly priced at $3.60 were repriced at $0.01 and extended to August 2011 | 200,000 | |||
| 275,000 | ||||
The fair value of each option granted is estimated on grant date using the Black-Scholes option pricing model which takes into account as of the grant date the exercise price and expected life of the option, the current price of the underlying stock and its expected volatility, expected dividends on the stock and the risk-free interest rate for the term of the option. The Company granted no stock options during the years ended October 31, 2012 and 2011.
The following table summarizes information on stock options outstanding at October 31, 2012:
| Options Outstanding and Exercisable | ||||||||||||||||||
| Weighted | ||||||||||||||||||
| Number | Weighted | Average | ||||||||||||||||
| Outstanding | Average | Remaining | Aggregate | |||||||||||||||
| Range of | at | Exercise | Life | Intrinsic | ||||||||||||||
| Exercise Price | October 31, 2012 | Price | (Years) | Value | ||||||||||||||
| $ | 0.90 | 30,000 | $ | 0.90 | 6.75 | |||||||||||||
| 30,000 | 6.75 | $ | 0 | |||||||||||||||
| For the Year Ended October 31, | ||||||||
| 2012 | 2011 | |||||||
| Weighted Average Fair Value of Options Granted | $ | — | $ | — | ||||
| Cash Received for Exercise of Stock Options | $ | — | $ | 2,750 | ||||
The intrinsic value is calculated as the difference between the market value as of October 31, 2012 and the exercise price of the shares. The market value as of October 31, 2012 was $0.23 as reported by the NASDAQ Stock Market.
| 11. | Capital Lease Obligations |
The Company finances certain equipment acquisitions through a capital lease agreement that expires in 2013. Future minimum rental payments under capital leases and related information in years subsequent to October 31, 2012 are presented in the table below:
| F-25 |
| October 31 | ||||||||
| 2012 | 2011 | |||||||
| Total amount payable in equal monthly installments of $500 | $ | 3,000 | $ | 9,000 | ||||
| Total amount payable in equal monthly installments of $2,090 | — | 22,440 | ||||||
| 3,000 | 31,440 | |||||||
| Less: Amount representing interest | 373 | 3,099 | ||||||
| Present value of minimum lease payments | 2,627 | 28,341 | ||||||
| Less: Current portion | 2,627 | 25,711 | ||||||
| $ | — | $ | 2,630 | |||||
The net book value of equipment under capital lease at October 31, 2012 is $7,554 and is included in property and equipment.
| 12. | Equity (Deficiency) |
Equity Instruments Issued for Services Rendered
During the years ended October 31, 2000 through 2006 CardioGenics Inc. issued stock options with a nominal exercise price in exchange for services rendered to CardioGenics Inc. The fair value of each stock option was measured at the fair value of the underlying services on the date of grant. The fair value of each grant was charged to the related expense in the consolidated statement of operations.
The Company assumed options outstanding at JAG Media entitling the employees to purchase 75,000 common shares of the Company’s stock at a price of $0.20 per share to August 31, 2011. The Company issued options to employees entitling the employees to purchase 200,000 common shares of the Company’s stock at a price of $3.60 per share to July 31, 2019, based upon change of control provisions in their employment agreements. All these options were immediately vested. The fair value of the 275,000 options was included in the purchase price. During 2011, these options were re-priced at $0.01 per share and subsequently exercised.
On August 1, 2009, the Company issued options to a consultant entitling the consultant to purchase 30,000 common shares of the Company’s stock at a price of $0.90 per share to July 31, 2019. These options were immediately vested.
| F-26 |
| October 31, | ||||||||
| 2012 | 2011 | |||||||
| Warrants | ||||||||
| Issued to subscribers to the debenture financing of 2003 and its related extension entitling the holder to purchase 1 common share of the Company at an exercise price of $0.47 per common share up to and including July 31, 2012 | — | 2,046,808 | ||||||
| Issued to subscribers to the debenture financing of 2004 and its related extension entitling the holder to purchase 1 common share of the Company at an exercise price of $0.47 per common share up to and including July 31, 2012 | — | 1,021,654 | ||||||
| Issued to agents for the debenture financings of 2003 and 2004 entitling the holder to purchase 1 common share of the Company at an exercise price of $0.47 per common share up to and including July 31, 2012 | — | 208,417 | ||||||
| Issued to former employee entitling the holder to purchase 1 common share of the Company at an exercise price of $0.47 per common share up to and including July 31, 2012 | — | 136,220 | ||||||
| Issued to consultants July 31, 2009, entitling the holder to purchase 1 common share of the Company at an exercise price of $0.90 per share up to and including July 31, 2012 | — | 104,785 | ||||||
| Issued to consultant August 1, 2009, entitling the holder to purchase 1 common share of the company at an exercise price of $0.90 per common share up to and including July 31, 2017 | 287,085 | 287,085 | ||||||
| Issued to Flow Capital Advisors Inc. on settlement of lawsuit in August 2011, entitling the holder to purchase 1 common share of the Company at an exercise price of $0.30 per common share up to and including August 23, 2016 | 250,000 | 250,000 | ||||||
| Issued to Flow Capital Advisors Inc. on settlement of lawsuit August 2011, entitling the holder to purchase 1 common share of the Company at an exercise price of $0.50 per common share up to and including August 23, 2016 | 250,000 | 250,000 | ||||||
| Issued to Flow Capital Advisors Inc. on settlement of lawsuit August 2011, entitling the holder to purchase 1 common share of the Company at an exercise price of $0.75 per common share up to and including August 23, 2016 | 500,000 | 500,000 | ||||||
| Issued to Flow Capital Advisors Inc. on settlement of lawsuit August 2011, entitling the holder to purchase 1 common share of the Company at an exercise price of $1.00 per common share up to and including August 23, 2016 | 500,000 | 500,000 | ||||||
| Issued to Flow Capital Advisors Inc. on settlement of lawsuit August 2011, entitling the holder to purchase 1 common share of the Company at an exercise price of $0.75 per common share up to and including August 23, 2016 | 500,000 | 500,000 | ||||||
| Issued to consultants in September 2011 entitling the holders to purchase 1 common share of the Company at an exercise price of $0.10 per common share up to and including March 20, 2013 | 1,500,000 | 1,500,000 | ||||||
| Issued to consultants in September 2011 entitling the holders to purchase 1 common share of the Company at an exercise price of $0.34 per common share up to and including March 20, 2013 | 1,500,000 | 1,500,000 | ||||||
| Issued to consultants in September 2011 entitling the holders to purchase 1 common share of the Company at an exercise price of $0.50 per common share up to and including March 20, 2013 | 1,000,000 | 1,000,000 | ||||||
| Total Warrants outstanding | 6,287,085 | 9,804,969 | ||||||
| 13. | Authorized Share Capital |
On September 30, 2009, the Company’s Articles of Incorporation were amended to increase the total number of common shares authorized for issuance from 500,000,000 shares to 650,000,000 shares of common stock, par value $0.00001 per share. On April 23, 2010, the Company’s Board of Directors approved a reverse stock split of its issued and outstanding common shares. The total authorized shares was at the same time reduced to 65,000,000. The Board of Directors selected a ratio of one-for-ten and the reverse split was effective June 20, 2010. As a result, the total number of shares of all classes of capital stock authorized for issuance by the Company decreased from 700,440,000 shares to 70,044,000 shares with a par value of $.00001 per share, of which 5,000,000 shares are authorized for issuance as preferred stock, 65,000,000 shares are authorized for issuance as common stock, 40,000 shares are authorized for issuance as Series 2 Class B common stock and 4,000 shares are authorized for issuance as Series 3 Class B common stock.
| F-27 |
| 14. | Issuance of Common Stock |
During the years ended October 31, 2012 and 2011, the Company issued the following common shares:
| Year Ended October 31, 2012 | Year Ended October 31, 2011 | |||||||||||||||
| # of shares | Amount | # of shares | Amount | |||||||||||||
| Issuance to third parties for services rendered | — | $ | — | 650,000 | $ | 391,500 | ||||||||||
| Issuance to a director for cash | — | $ | — | 600,000 | $ | 297,747 | ||||||||||
| Issuance to third parties for cash | 1,050,000 | $ | 262,500 | 70,000 | $ | 35,000 | ||||||||||
| Issuance to third parties on exercise of warrants | — | $ | — | 22,005 | $ | 10.402 | ||||||||||
| Issuance to third parties on exercise of options | — | $ | — | 275,000 | $ | 2,750 | ||||||||||
| Issuance to Flow Capital Advisors Inc. on Settlement of Lawsuit | — | $ | — | 1,000,000 | $ | 600,000 | ||||||||||
The fair value of shares issued services rendered were measured at the fair value of the services rendered on the date rendered.
| 15. | Redemption of Class B Common Stock |
On or about February 28, 2011, CardioGenics Holdings Inc. (“Holdings”) mailed notices to the holders of its outstanding Series 2 Class B Common Stock (the “Series 2 Shares”) and Series 3 Class B Common Stock (the “Series 3 Shares”), which notify such stockholders that Holdings has elected to redeem all outstanding Series 2 Shares and Series 3 Shares in accordance with their terms. The Redemption Date is April 4, 2011 and the Redemption Price is par value, $0.00001 per share.
Holdings has established a trust account with TD Bank Canada, which account will hold proceeds sufficient to redeem the issued and outstanding Series 2 Shares and Series 3 Shares. Accordingly, notwithstanding that any certificate for Series 2 Shares or Series 3 Shares called for redemption shall not have been surrendered for cancellation, all Series 2 Shares and Series 3 Shares called for redemption shall no longer be deemed outstanding, and all rights with respect to such Series 2 Shares and Series 3 Shares shall forthwith on the Redemption Date cease and terminate, except only the right of the holders thereof to receive the pro-rata amount payable of the Series 2 Shares and Series 3 Shares, without interest.
| F-28 |
| 16. | Net Loss per Share |
The following table sets forth the computation of weighted-average shares outstanding for calculating basic and diluted earnings per share:
| Years Ended October 31, | ||||||||
| 2012 | 2011 | |||||||
| Weighted-average shares - basic | 55,629,034 | 54,167,687 | ||||||
| Effect of dilutive securities | — | — | ||||||
| Weighted-average shares - diluted | 55,629,034 | 54,167,687 | ||||||
Basic earnings per share (“EPS”) and diluted EPS for the years ended October 31, 2012 and 2011 have been computed by dividing the net loss available to common stockholders for each respective period by the weighted average shares outstanding during that period. All outstanding options, warrants and shares to be issued upon the exercise of the outstanding options and warrants representing 6,317,085 and 9,834,969 incremental shares, respectively, have been excluded from the years ended October 31, 2012 and 2011, respectively, computation of diluted EPS as they are antidilutive given the net losses generated.
| 17. | Commitments and Contingent Liabilities |
Leases
The Company has entered into an operating lease agreement for the use of operating space.
Aggregate minimum annual lease commitments of the Company under the non-cancelable operating lease as of October 31, 2012 are as follows:
| Year | Amount | |||
| 2013 | $ | 81,926 | ||
| 2014 | 82,259 | |||
| 2015 | 83,924 | |||
| 2016 | 84,091 | |||
| Thereafter | 46,244 | |||
| Total Minimum Lease Payments | $ | 378,444 | ||
Lease expense amounted to $75,850 and $76,602 for the years ended October 31, 2012 and 2011, respectively.
The preceding data reflects existing leases and does not include replacements upon their expiration. In the normal course of business, operating leases are generally renewed or replaced by other leases.
Lawsuit
On April 22, 2009, the Company was served with a statement of claim from a former employee claiming compensation for wrongful dismissal and ancillary causes of action including payment of monies in realization of his investment in the Company, with an aggregate claim of $514,000. The Company considers all the claims to be without any merit, has already delivered a statement of defense and intends to vigorously defend the action. If the matter eventually proceeds to trial, the Company does not expect to be found liable on any ground or for any cause of action.
While it is not feasible to predict the outcome of the above proceeding and exposures with certainty, management believes that the ultimate disposition should not have a material adverse effect on the Company’s consolidated financial position, cash flows or results of operations.
| F-29 |
On October 26, 2010, Karver International Inc. (“Karver”) filed a lawsuit in the 11th Judicial Circuit in and for Miami-Dade County, Florida against Holdings Inc. and several other defendants including affiliates, officers and directors of Holdings. The Plaintiff generally alleges that the named defendants made certain alleged misrepresentations in connection with the purchase of shares of Holdings. On December 20, 2010, Holdings and other defendants filed a motion to dismiss on the basis that the court lacks personal jurisdiction over most defendants, that an enforceable forum selection clause requires that the action be litigated in Ontario, Canada that the doctrine of forum non conveniens requires dismissal in favour of the Ontario forum, and that the complaint suffers from numerous other technical deficiencies warranting dismissal (e.g., failure to attach documents to the Complaint, failure to plead fraud with particularity, etc.). The motion is currently pending. Should the motion be denied, Holdings will continue to pursue vigorous defenses to this action. In addition, Karver’s attorney recently filed a motion to withdraw as counsel for Karver. The courts have granted Karver’s attorney’s motion to withdraw and Karver had until approximately April 26, 2011 to engage new counsel. On April 20, 2011, having not engaged new counsel as of that date, Karver filed with the court a Notice of Voluntary Dismissal without Prejudice, which dismisses the lawsuit against the named defendants without prejudice to Karver’s rights to recommence the action.
On January 14, 2010, Flow Capital Advisors Inc. (“Flow Capital”) filed a lawsuit against JAG Media Holdings Inc. in the Circuit Court of the 17th Judicial Circuit In and For Broward County Florida (Case No. 10001713) (the “Flow Capital State Action”). Pursuant to this lawsuit, Flow Capital alleges that JAG Media Holdings Inc. breached a Non-Circumvention Agreement it had entered into with Flow Capital, dated January 1, 2004.
On January 15, 2010, Flow Capital filed a lawsuit against Holdings, and another defendant in the United States District Court for the Southern Distrcit of Florida, Fort Lauderdale Division (Case No. 10-CV-6006-Martinez-Brown) (the “Flow Capital Federal Action”). This lawsuit alleges that Holdings (i) breached a Finder’s Fee Agreement in connection with the CardioGenics Acquisition; and (ii) breached a non-circumvention agreement. Flow Capital is claiming that it is entitled to the finder’s fee equal to eight percent of the JAG Media Holdings shares received by Holdings, or the equivalent monetary value of the stock. Plaintiff subsequently amended its complaint to add related tort claims.
Pursuant to applicable Federal court rules, the parties to the Flow Capital Federal Action participated in a court mandated mediation session on August 17, 2011 where the parties attempted to settle their disputes. At the mediation, the parties agreed to a settlement of all claims as described below, subject to the approval of the Board of Directors of Holdings, which approval was subsequently obtained. Pursuant to the settlement agreement, Flow Capital agreed to dismiss, with prejudice, the Flow Capital Federal Action and the Flow Capital State Action and the Company agreed to issue Flow Capital 1,000,000 shares of restricted Company common stock and warrants to purchase restricted Company common stock as follow:
| Type of Warrant | Number of Shares | Exercise Price | Vesting Date | Term | ||||||||
| Cash Exercise Only | 250,000 | $ | 0.30/share | Immediate | 5 years | |||||||
| Cash Exercise Only | 250,000 | $ | 0.50/share | Immediate | 5 years | |||||||
| Cash Exercise Only | 500,000 | $ | 0.75/share | Immediate | 5 years | |||||||
| Cash Exercise Only | 500,000 | $ | 1.00/share | Immediate | 5 years | |||||||
| Cash or Cashless Exercise | 500,000 | $ | 0.75/share | Immediate | 5 years | |||||||
| F-30 |
The restricted shares of common stock and the warrants are subject to the rights and restrictions of Rule 144 and do not have any registration rights. As part of the settlement, the parties also exchanged mutual general releases and Holdings paid Flow Capital $100,000 for Flow Capital’s legal fees.
On August 23, 2011, the Company’s Board of Directors approved the settlement. As a result, the Company recorded a charge to the Consolidated Statement of Operations for the year ended October 31, 2011 of $1,753,800 for Cost of Settlement of Lawsuit.
| 18. | Supplemental Disclosure of Cash Flow Information |
| Years Ended | ||||||||
| October 31 | ||||||||
| 2012 | 2011 | |||||||
| Cash paid during the year for: | ||||||||
| Interest | $ | 18,221 | $ | 18,748 | ||||
| Non-cash financing activity: | ||||||||
| Fixed assets acquired through capital lease | $ | — | $ | 10,660 | ||||
| 19. | Subsequent Events |
| (a) | Equity Line of Credit |
On November 19, 2012, the Company entered into an agreement (the “Line”) with JMJ Financial (the “Lender”) whereby the Company may borrow up to $350,000 from the Lender in increments of $50,000. The Line is subject to an original issue discount of $50,000. Advances under the Line have a maturity date of one year from the date of the advance. If the advance is repaid within three months, the advance is interest free. If not repaid within three months, the advance may not be repaid before maturity and carries interest at 5%. The Lender has the right at any time to convert all or part of the outstanding principal and accrued interest (and any other fees) into shares of fully paid and non-assessable shares of common stock of the Company at a price equal to the lesser of $0.23 and 60% of the lowest trade price in the previous 25 trading days prior to the conversion. Unless agreed in writing by the parties, at no time will the Lender convert any amount owing under the Line into common stock that would result in the Lender owning more than 4.99% of the Company’s common stock outstanding.
| (b) | Shareholder Loans |
Subsequent to the year end, a shareholder who is also a director and an officer advanced $100,000 to the Company and another shareholder who is also a director advanced $100,000 to the Company.
| (c) | Authorized Capital |
| On January 17, 2013, the Company filed a Certificate of Amendment to the Company’s Certificate of Incorporation (the “Certificate of Amendment”) to (i) increase the number of shares of common stock authorized for issuance from 65,000,000 to 150,000,000 and (ii) de-authorize the Company’s Class B common stock. |
| F-31 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| CARDIOGENICS HOLDINGS INC. | |||
| By: | /s/ Yahia Gawad | ||
| Yahia Gawad | |||
| Chief Executive Officer | |||
Dated: January 29, 2013
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the date indicated.
| Signature | Title | DATE | ||
| /s/ Yahia Gawad | Chief Executive Officer | January 29, 2013 | ||
| Yahia Gawad | ||||
| /s/ James Essex | Chief Financial Officer | January 29, 2013 | ||
| James Essex |
