Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Anacor Pharmaceuticals, Inc. | a13-3922_18k.htm |
| EX-99.1 - EX-99.1 - Anacor Pharmaceuticals, Inc. | a13-3922_1ex99d1.htm |
Exhibit 99.2
|
|
Results from the Phase 3 Study (Study 301) of Tavaborole to Evaluate Efficacy and Safety in the Treatment of Onychomycosis |
|
|
Safe Harbor Statement Certain items in this presentation and other matters discussed today or answers that may be given to questions asked could constitute forward-looking statements, including statements regarding the potential for approval based on the 301 study results, the potential for similar or more favorable results from the 302 study, whether our current arbitration with Valeant may result in a favorable outcome, the potential competition from IDP-108, the progress and timing of clinical trials and NDA filing, the safety and efficacy of our product candidates, our collaborators, and estimates of the potential markets for our product candidates. Additional risks and uncertainties are described more fully in Anacor’s 10-Q for the quarter ended September 30, 2012 filed with the Securities and Exchange Commission. These statements are subject to risks and uncertainties relating to Anacor’s future financial or business performance. Anacor’s actual results or achievements could differ materially from those anticipated in these forward-looking statements. Please note that Anacor is under no obligation to update any of the forward-looking statements discussed today. |
|
|
Tavaborole Phase 3 Study Design – Special Protocol Assessment (SPA) on All Major Parameters Including Endpoints Size and Location Study 301 ~600 patients in US and Mexico Study 302 ~600 patients in US and Canada Study Treatment Tavaborole, 5% solution Dosed daily for 48 weeks Patients randomized 2:1 active vs. vehicle Inclusion Criteria Aged 18+ years (no upper limit) 20% - 60% nail involvement Positive culture and positive potassium hydroxide (“KOH”) examinations Primary Endpoint, at 52 Weeks Completely clear nail and mycological cure (defined as negative culture and negative KOH) of target great toenail Secondary Endpoints, at 52 Weeks Completely clear or almost clear (≤10% clinical involvement) target great toenail Treatment success (completely clear or almost clear nail + mycological cure) of target great toenail Mycological cure of target great toenail Safety Frequency and severity of adverse events and local tolerability signs/symptoms |
|
|
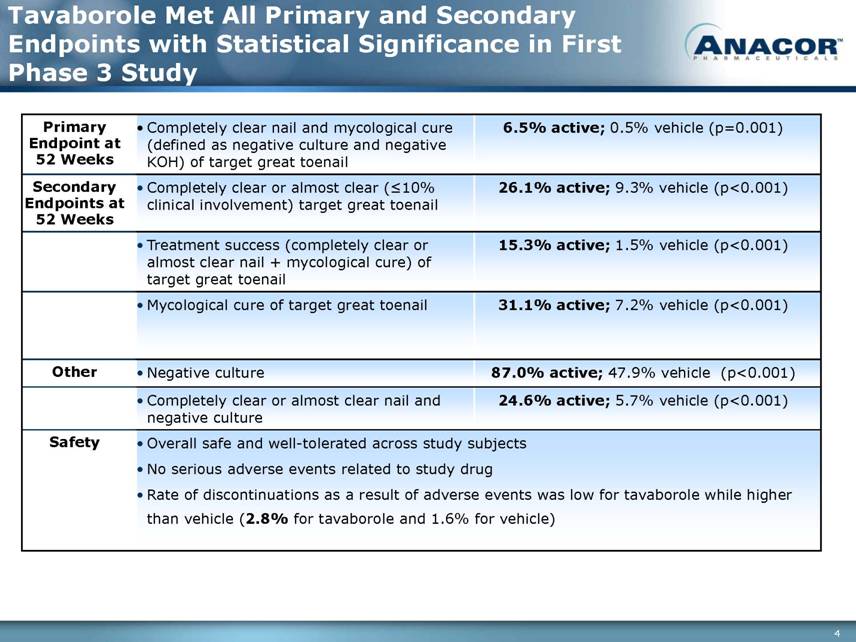
Tavaborole Met All Primary and Secondary Endpoints with Statistical Significance in First Phase 3 Study Primary Endpoint at 52 Weeks Completely clear nail and mycological cure (defined as negative culture and negative KOH) of target great toenail 6.5% active; 0.5% vehicle (p=0.001) Secondary Endpoints at 52 Weeks Completely clear or almost clear (≤10% clinical involvement) target great toenail 26.1% active; 9.3% vehicle (p<0.001) Treatment success (completely clear or almost clear nail + mycological cure) of target great toenail 15.3% active; 1.5% vehicle (p<0.001) Mycological cure of target great toenail 31.1% active; 7.2% vehicle (p<0.001) Other Negative culture 87.0% active; 47.9% vehicle (p<0.001) Completely clear or almost clear nail and negative culture 24.6% active; 5.7% vehicle (p<0.001) Safety Overall safe and well-tolerated across study subjects No serious adverse events related to study drug Rate of discontinuations as a result of adverse events was low for tavaborole while higher than vehicle (2.8% for tavaborole and 1.6% for vehicle) |
|
|
Tavaborole vs. Penlac, the Only Approved Topical Treatment for Onychomycosis Tavaborole Penlac (1) Adjunctive Treatment Required None Nail debridement, as often as monthly Dosing / Application Solution applied once daily Lacquer painted on nail daily Weekly removal of Penlac with alcohol Phase 3 Efficacy Results (Active / Vehicle) (Active / Vehicle) Completely clear nail and mycological cure (defined as negative culture and negative KOH) of target great toenail 6.5% / 0.5% (Study 301) (Study 302 pending) 5.5% / 1.0% (Study 312) 8.5% / 0.0% (Study 313) Mycological cure of target great toenail 31.1% / 7.2% (Study 301) (Study 302 pending) 29.0% / 11.0% (Study 312) 36.0% / 9.0% (Study 313) Completely clear or almost clear (≤10% clinical involvement) target great toenail 26.1% / 9.3% (Study 301) (Study 302 pending) N/A Treatment success (completely clear or almost clear nail + mycological cure) of target great toenail 15.3% / 1.5% (Study 301) (Study 302 pending) 6.5% / 0.9% (Study 312) 12.0% / 0.9% (Study 313) (1) Penlac label |
|
|
Topical therapy Little or no detectable systemic exposure Safety lab monitoring not expected All preclinical toxicology completed Not an inhibitor of CYP450 enzymes Anticipate pregnancy category B Safety Novel mechanism of action - targets LeuRS to kill fungus Potent against broad spectrum of fungi (T. mentagrophytes , T. rubrum, C. albicans, etc) and yeast Activity in the presence of keratin Statistically significant efficacy in the first of two Phase 3 studies Efficacy Tavaborole: A Potential Novel Topical Treatment for Onychomycosis Apply with dropper once daily Dries in about one minute. Does not need to be removed. No special cleansing or preparation prior to application Ease of Use Small molecular weight 152 Da compared to >300 Da for most antifungals Balanced preference for oil and water (logP = 1.24) Water soluble (0.8 mg/mL) Physical Properties Enable Nail Penetration |
|
|
Potential FDA Approval Mid-2014 January - Phase 3 Study 301 data March - Phase 3 Study 302 data FDA Review 1H13 2H13 1H14 PDUFA date Mid-2014 Note: timeline based on Anacor’s current best estimates for NDA filing and FDA review dates Mid-2013 – Submit NDA |
|
|
Next Steps and Considerations Data from the next Phase 3 study (known as Study 302) is expected in March and will complete tavaborole’s clinical profile Based on the results from Study 301, and depending on the results from Study 302, tavaborole may have a more compelling efficacy profile than any currently approved topical drugs for onychomycosis Based on our completed clinical and pre-clinical studies, tavaborole has demonstrated an excellent safety profile Dermatologists and podiatrists continue to show strong interest in a novel compound with tavaborole’s profile to treat their patients with onychomycosis While Valeant has published Phase 3 efficacy data for IDP-108, we don’t know when or if that drug will be approved or what its label will contain. We note that differences in inclusion criteria and trial methodology make it difficult to reliably compare the results at this time As has been previously disclosed, we have an ongoing arbitration claim against Valeant which seeks both monetary damages and preliminary and permanent injunctive relief, which may impact IDP-108’s market launch if approved |