Attached files
| file | filename |
|---|---|
| EX-5.1 - EXHIBIT 5.1 - AutoGenomics, Inc. | d402968dex51.htm |
| EX-1.1 - EXHIBIT 1.1 - AutoGenomics, Inc. | d402968dex11.htm |
| EX-3.5 - EXHIBIT 3.5 - AutoGenomics, Inc. | d402968dex35.htm |
| EX-23.1 - EXHIBIT 23.1 - AutoGenomics, Inc. | d402968dex231.htm |
| EX-10.24 - EXHIBIT 10.24 - AutoGenomics, Inc. | d402968dex1024.htm |
| EX-10.26 - EXHIBIT 10.26 - AutoGenomics, Inc. | d402968dex1026.htm |
| EX-10.25 - EXHIBIT 10.25 - AutoGenomics, Inc. | d402968dex1025.htm |
Table of Contents
As filed with the Securities and Exchange Commission on January 23, 2013
Registration No. 333-184121
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 4
to
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
AutoGenomics, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 3826 | 80-0252299 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
2980 Scott Street
Vista, California 92081
(760) 477-2248
(Address, including zip code and telephone number, including area code, of registrant’s principal executive offices)
Fareed Kureshy
President and Chief Executive Officer
AutoGenomics, Inc.
2980 Scott Street
Vista, California 92081
(760) 477-2248
(Name, address, including zip code and telephone number, including area code, of agent for service)
Copy to:
| Todd A. Hentges Timothy R. Rupp Bingham McCutchen LLP 600 Anton Boulevard, 18th Floor Costa Mesa, CA 92626-7653 (714) 830-0600 |
Charles S. Kim Sean M. Clayton Cooley LLP 4401 Eastgate Mall San Diego, CA 92121-1909 (858) 550-6000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||||
| Non-accelerated filer | x | (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | ||||
CALCULATION OF REGISTRATION FEE
|
| ||||
| Title of Each Class of Securities to be Registered |
Proposed Maximum Aggregate Offering Price (1) (2) |
Amount of Registration Fee (3) | ||
| Common Stock, $0.01 par value |
$75,900,000 | $8,936 | ||
|
| ||||
|
| ||||
| (1) | Estimated solely for the purpose of computing the amount of the registration fee, in accordance with Rule 457(o) promulgated under the Securities Act of 1933. |
| (2) | Includes offering price of shares that the underwriters have the option to purchase to cover overallotments, if any. |
| (3) | Calculated pursuant to Rule 457(o) under the Securities Act of 1933, as amended, based on an estimate of the proposed maximum aggregate offering price. $7,449 of this amount was previously paid. |
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(a) OF THE SECURITIES ACT OF 1933, OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE COMMISSION, ACTING PURSUANT TO SAID SECTION 8(a), MAY DETERMINE.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any jurisdiction where such offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JANUARY 22, 2013
Preliminary Prospectus
6,000,000 Shares

Common Stock
We are offering 6,000,000 shares of our common stock. This is our initial public offering, and no public market currently exists for our common stock. We expect the initial public offering price to be between $9.00 and $11.00 per share. We have applied to list our common stock on the NASDAQ Global Market under the symbol “AGMX.”
We are an “emerging growth company,” as defined under federal securities laws, and have elected to comply with certain reduced public company reporting requirements available to such companies.
Investing in our common stock involves a high degree of risk. Please read “Risk Factors” beginning on page 20.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions |
$ | $ | ||||||
| Proceeds, before expenses, to us |
$ | $ | ||||||
Delivery of the shares of common stock is expected to be made on or about , 2013. We have granted the underwriters an option for a period of 30 days to purchase, on the same terms and conditions set forth above, up to an additional 900,000 shares of our common stock to cover overallotments. If the underwriters exercise the option in full, the total underwriting discounts and commissions payable by us will be $ and the total proceeds to us, before expenses, will be $ .
Leerink Swann
| Stephens Inc. | Mizuho Securities |
Cantor Fitzgerald & Co.
The date of this prospectus is , 2013.
Table of Contents

Not all products have received all necessary domestic or international regulatory approvals or clearances for commercial sale. The vast majority of products sold by AutoGenomics are offered for sale to allow for the collection of research data, and may only be used for clinical purposes by laboratories certified under the Clinical Laboratory Improvements Amendments of 1988 and that have incorporated the products into laboratory-developed tests pursuant to guidelines issued by the College of American Pathologists. The FDA has not adopted these guidelines and AutoGenomics is not permitted to represent these products as in vitro diagnostic products.
Table of Contents
| 1 | ||||
| 20 | ||||
| 46 | ||||
| 47 | ||||
| 48 | ||||
| 49 | ||||
| 50 | ||||
| 52 | ||||
| 54 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
56 | |||
| 87 | ||||
| 122 | ||||
| 128 | ||||
| 144 | ||||
| 146 | ||||
| 150 | ||||
| 154 | ||||
| Material United States Federal Income Tax Consequences to Non-U.S. Holders |
157 | |||
| 161 | ||||
| 167 | ||||
| 167 | ||||
| 167 | ||||
| F-1 |
INFINITI, BioFilmChip, Intellipac and QMatic are our trademarks. All other service marks, trademarks and trade names referred to in this prospectus are the property of their respective owners.
Neither we, nor the underwriters, have authorized anyone to provide you with additional or different information than that contained in this prospectus and any free writing prospectus prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of the common stock.
Until , 2013 (25 days after the date of this prospectus), all dealers that buy, sell or trade in our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Table of Contents
This summary highlights certain information contained in this prospectus. Because this is only a summary, it does not contain all of the information that may be important to you. For a more complete understanding of the information that you may consider important in making your investment decision, we encourage you to read this entire prospectus. Among the other information in this prospectus, you should carefully consider the information set forth under the heading “Risk Factors” and our financial statements and accompanying notes included elsewhere in this prospectus. Unless the context requires otherwise, the words “we,” “us,” “our,” “Company” and “AutoGenomics” refer to AutoGenomics, Inc.
Our Company
We design, develop, manufacture and market the INFINITI molecular diagnostics system. The system includes an extensive and expanding menu of genetic tests and a family of highly automated analyzers. Our products are sold to reference laboratories, hospital laboratories and specialty clinics that produce genetic test results in a broad range of market segments, including personalized medicine, women’s health, oncology and infectious disease. Genetic tests are performed on our INFINITI analyzer utilizing our high-margin and test-specific consumables, which include our proprietary BioFilmChip microarrays and our proprietary Intellipac Reagent Management Modules. In the United States, we market and sell our genetic tests that have been cleared by the U.S. Food and Drug Administration, or FDA, for use in the indications specified in those clearances. The remainder of our genetic tests are marketed and sold on a research use only, or RUO, basis. Our RUO tests may be used for clinical purposes in the United States only by customers that are certified under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, and that have incorporated our test into the customer’s laboratory-developed tests, or LDTs, pursuant to guidelines issued by the College of American Pathologists. Our INFINITI analyzers are capable of both mid- to high-volume testing and generating many different laboratory results from one patient sample at the same time, which is commonly referred to as multiplexing, while providing a high level of accuracy and reproducibility. Our INFINITI system is easy to use, as it eliminates the need for multiple, specialized instruments and automates many of the discrete processes of traditional genetic testing.
As of December 31, 2012, we offered 53 tests for use on our INFINITI analyzers, and had more than 15 additional tests in development. Our current and in-development tests are focused on the areas of personalized medicine (including pain management, mental health and cardiovascular health assessment), women’s health, oncology, infectious disease, genetic disorders, newborn screening and blood banking, which we believe represent large and growing market opportunities in genetic testing. We believe the depth and breadth of our test menu is a significant competitive advantage that will allow laboratories to utilize laboratory space, labor and capital investment more efficiently to conduct additional molecular diagnostic tests. The proprietary design of our INFINITI system allows us to introduce new and enhanced tests to our genetic test menu without the need to modify our INFINITI analyzers. We intend to increase the number of tests offered in each of our target market segments, which will further increase the utility of our INFINITI system to our customers. Our internal test development efforts are generally driven by our customers’ current and anticipated needs, our analysis and projections for the molecular diagnostics market, and our ability to leverage our core competencies such as automation and multiplexing. We have entered into, and expect to continue to enter into, collaborative relationships with leading research and academic institutions for the development of additional or enhanced tests to further increase the depth and breadth of our genetic test menu. Since the initial launch of our INFINITI system in 2007 we have introduced more than five new or enhanced tests per year.
We have received FDA 510(k) clearance for our INFINITI Analyzer and five of our genetic tests, and we have submitted an additional notification to the FDA for 510(k) clearance of our UGT1A1 test. We are finalizing the protocol for a clinical trial necessary to support a premarket approval application, or PMA, for our HPV-HR tests and intend to commence this clinical trial in 2013. The balance of our tests are currently offered for sale in
1
Table of Contents
the United States under the RUO designation. These RUO tests are labeled “For Research Use Only. Not for use in diagnostic procedures.” as required by FDA regulations. We are not permitted to market these products for in vitro diagnostic use, and must maintain distribution controls to assure that these products are not used for diagnostic purposes. We therefore train our personnel to only market these products to laboratories for research or investigational use in the collection of research data, and to not promote any off-label uses of our products. Internationally, we have obtained a Conformité Européenne, or CE, mark for our INFINITI Analyzer, our INFINITI Plus Analyzer and a total of 22 of our tests. This designation is supported by completed clinical and validation studies that demonstrate the analytical performance of each CE marked test. The CE mark facilitates the marketing and sale of our CE marked analyzers and tests in the European Union and the European Economic Area as well as certain other international markets.
Sales of products for which we have received 510(k) clearance accounted for 13%, 8% and 4% of our net revenue for 2010, 2011 and the first nine months of 2012, respectively. The balance of our product sales for those same periods, representing 87%, 92% and 96% of our net revenue for 2010, 2011 and the first nine months of 2012, respectively, were derived from sales of RUO products. These products are offered for sale to allow for the collection of research data, and may only be used in the United States for clinical purposes by laboratories and other facilities certified under CLIA that have incorporated these products into their LDTs pursuant to guidelines issued by the College of American Pathologists. We believe that nearly all of our RUO product sales are incorporated into LDTs. In order to develop an LDT utilizing our products, these certified laboratories and other facilities must develop and validate a test protocol that includes specimen collection, DNA extraction, PCR amplification, hybridization and detection, and data analysis, interpretation and reporting. Our products provide components that can be used by these certified laboratories and other facilities for the PCR amplification, hybridization and detection portions of these LDTs. We sell each of these components individually, as ordered by the customer in its discretion, and not as a kit or system. The validation process engaged in by these certified laboratories and other facilities can involve validation of the sample collection and extraction process, establishing limits of detection and analytical sensitivity, testing for specificity and cross-reactivity, including interfering substances, validation for assay accuracy, precision and reproducibility, and establishing reportable ranges of test results for the test system and reference values that will be measured against as controls. This validation process also requires verifying the result from the LDT against known standard samples or the results of a high-standard laboratory testing method such as sequencing, and can involve the testing of a large number of patient samples. Depending on the availability of patient samples, this process may take from several weeks to several months or more to complete, and thus requires a significant investment by the customer. This validation process must be completed for each of our RUO genetic test components that a customer wishes to incorporate into one of its LDTs.
We believe that all sales of our RUO products in the United States are to customers that are either certified in the manner described above and have incorporated our products into their LDT’s, or that use such products for research only. The FDA has not adopted these guidelines and we are not permitted to represent our RUO products as in vitro diagnostic products. Our decision to seek FDA approvals or clearances domestically, and CE marking internationally, for our genetic tests is made on a test-by-test basis, and is based on a variety of factors, including:
| • | the regulatory environment for the use of genetic tests, in particular the FDA’s requirements and limitations on marketing RUO tests, which may not be marketed as in vitro diagnostic products; |
| • | the demand by our existing and target customers, as expressed to us, for particular genetic tests that have received regulatory approvals or clearances; |
| • | the competitive environment for the use of genetic tests that have received regulatory approvals or clearances versus similar tests that have not; for example, certain of our competitors provide FDA cleared or approved tests in the area of HPV testing, and to compete with those competitors we intend to obtain FDA clearance or approval for certain of our HPV tests as well; and |
2
Table of Contents
| • | the size of the available market for the particular test, given the relatively significant expense and time required to obtain regulatory approvals or clearances. |
For those of our tests for which we have not obtained FDA regulatory clearance or approval, we experience delays of anywhere from a few weeks to several months or more in obtaining revenue from customers that desire to utilize our RUO test consumables during the period of time that the customer is developing its own LDT that incorporates our RUO test.
We believe that we are in compliance with existing FDA rules and regulations governing our business, including those governing the marketing and sale of RUO tests; however, a significant change in existing laws, or their enforcement, may require us to change our business model or our business practices to maintain compliance with these laws. For instance, in June 2011 the FDA issued a Draft Guidance entitled “Commercially Distributed In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only: Frequently Asked Questions,” which, if enforced, would limit our marketing of RUO tests to general discovery laboratories and would require us to halt sales to clinical laboratories that validate and use our RUO tests as part of their LDTs. The FDA has generally exercised its enforcement discretion to not enforce applicable regulations with respect to LDTs. However, the FDA has indicated, since 2010, that it intends to reconsider its policy regarding enforcement and to begin drafting an oversight framework for such tests. If the FDA imposes significant changes to the regulation or enforcement of LDTs, including our RUO tests that are used as LDTs, it may require us to suspend sales of our RUO tests, which together represented 96% of our net revenues for the nine months ended September 30, 2012, and it would require us to seek FDA clearance or approval for our RUO tests, which would in turn require significant time and capital investments on our part and reduce our revenues or increase our costs and adversely affect our business, prospects, results of operations or financial condition. We have not undertaken any specific efforts to comply with this draft guidance. If we were to voluntarily elect to comply with this draft guidance, we would be required to seek regulatory approval for all of our RUO tests that are sold in the United States, which would require significant time and capital investments on our part, significantly reduce our revenues until we obtain regulatory approvals, and significantly increase our capital costs, which could in turn adversely affect our business, prospects, results of operations or financial condition during the affected fiscal periods. Our management currently believes that finalization or enforcement of this draft guidance in its present form is unlikely given the significant adverse effect it would have on a variety of industry participants, and on the ability of physicians to provide effective healthcare.
We believe that compared to traditional genetic testing methods our INFINITI system can significantly improve laboratory productivity, workflow and throughput while reducing the cost per reportable result. We believe that these and other attributes of our INFINITI system decrease the cost and complexity of genetic testing and reduce the need for specialized laboratory personnel, training, equipment and facilities. Our INFINITI system has been designed to enable our customers to start performing, or to more cost-effectively perform, molecular diagnostic tests, which we believe will facilitate acceptance and adoption of our INFINITI system.
We experienced meaningful revenue growth in the first nine months of 2012, with net revenue of $14.5 million during this period, $13.2 million of which was derived from consumables sales, as compared to net revenue of $7.5 million and $8.0 million for fiscal years 2010 and 2011, respectively, of which $3.9 million and $6.8 million was derived from consumables sales, respectively. We expect to continue to generate the substantial majority of our net revenue through the sale of our genetic test consumables for the foreseeable future. As of December 31, 2012, we had 191 INFINITI analyzers placed with customers.
Market Opportunity
Industry Background
Molecular diagnostic testing is used to measure or detect genetic biomarkers associated with a predisposition to, or the presence of, a particular disease or condition, or other genetic variance such as drug
3
Table of Contents
response. The information provided by molecular diagnostic testing may enable physicians to achieve better patient outcomes and better contain health care costs through, for example, earlier diagnosis of disease, improved monitoring of disease progression and more personalized treatment. According to Kalorama Information, an independent market research firm, the global molecular diagnostics market is expected to grow from an estimated $4.8 billion in 2010 to $8.1 billion in 2015, which represents a compound annual growth rate of approximately 11%, a rate we believe will be greater than the growth of the overall diagnostics market.
Current practices in developing and running molecular diagnostic tests typically involve manual and complex procedures that require significant expertise, time and expense. We believe the resource and time constraints of traditional testing methods have limited the growth of the molecular diagnostics market, and that the recent availability of more automated and integrated testing methods will result in accelerated use of molecular diagnostic testing. The growth of the molecular diagnostics market will also depend on increasing physician education regarding the use of genetic testing and greater coverage of tests by insurance carriers. Growing understanding of the utility of genetic information for the diagnosis and treatment of disease, as well the increase in identification of new biomarkers, may lead to increased growth in the molecular diagnostics market.
Our Target Markets
We believe there are additional factors that will continue to drive growth in the molecular diagnostics market segments we target, including:
| • | Personalized Medicine and Companion Diagnostics. The matching of treatment options to a patient’s specific genetic profile has emerged as an important trend in medicine because the efficacy and side-effect profile of certain treatments can be predicted by the presence or absence of specific genetic markers in a particular patient. Better targeted and more effective pharmacogenomic-based treatments have the potential to improve healthcare outcomes and lower healthcare costs. As of December 31, 2012, we offered 23 genetic tests for use in the area of personalized medicine and companion diagnostics, three of which have received FDA 510(k) clearance. |
| • | Pain Management. Pharmacogenomics is playing an integral role in the administration and management of pain medication, as gene mutations can be key factors in determining whether specific pain medications will be effective, or will otherwise result in adverse side effects. As of December 31, 2012, we offered five RUO genetic tests for use in the area of pain management. |
| • | Mental Health. Mental health represents a major component of overall pharmaceutical sales. According to the Centers for Disease Control and Prevention, or CDC, as much as 11% of the U.S. population is taking antidepressants at a given time, while as many as 23% of women between the age of 40 and 59 are on psychiatric medication. As of December 31, 2012, we offered 11 genetic tests for use in the area of mental health, two of which have received FDA 510(k) clearance. |
| • | Cardiovascular Health Assessment. According to the World Health Organization, or WHO, cardiovascular diseases were responsible for 30% of global deaths in 2008. It is estimated that by 2030, 23.6 million people will die from some form of cardiovascular disease. In addition to the increasing prevalence of cardiovascular diseases, the information generated by molecular diagnostic testing is becoming increasingly important for determining the predisposition and treatment of cardiovascular diseases. As of December 31, 2012, we offered 16 genetic tests for use in the area of cardiovascular health assessment, five of which have received FDA 510(k) clearance. |
| • | Women’s Health. We believe the women’s health diagnostics market will continue to grow and represent a substantial market opportunity. Non-molecular tests are commonly employed in this market, but the use of molecular diagnostics is expanding significantly due to increased applications, |
4
Table of Contents
| better performance and better clinical discriminatory capabilities. As of December 31, 2012, we offered 15 RUO genetic tests for use in the area of women’s health. |
| • | Cancer and Companion Diagnostics. Because of the high cost of many cancer therapeutics, and the varied levels of efficacy and toxicity across different patients, tests to diagnose or direct the treatment of, or to determine the predisposition to, various forms of cancer are becoming increasingly important. As of December 31, 2012, we offered 23 RUO genetic tests for use in the area of cancer and companion diagnostics. |
| • | Infectious Diseases. According to the WHO, infectious diseases caused approximately 20% of all recorded deaths in 2008. Within this group, HIV, tuberculosis, or TB, and respiratory infections were the top three contributors to overall mortality in adults aged 15-59, at 35%, 21% and 10%, respectively. Molecular diagnostic testing offers advantages in identifying infectious disease pathogens compared to traditional testing methods. As of December 31, 2012, we offered 15 RUO genetic tests for use in the area of infectious diseases. |
| • | Genetic Disorders. Genetic and inherited disease testing is a cornerstone of molecular diagnostic testing. Molecular diagnostic tests offer significant advantages over prior, often subjective, forms of diagnosis. As of December 31, 2012, we offered six RUO genetic tests for use in the area of genetic disorders; however, we did not have any material sales or net revenue from sales of genetic tests in the area of genetic disorders during the year ended December 31, 2012. |
| • | Newborn Screening. Many common newborn screening panels require the identification of multiple (often five or more) genetic markers which makes traditional testing impractical. Classic testing algorithms are limited in that they utilize subjective analysis of a newborn’s parental health history with little to no genetic evaluation. Targeted genetic evaluation can inform the clinician if the newborn is at risk for developing the disease in question. As of December 31, 2012, we offered four RUO genetic tests for use in the area of newborn screening; however, we did not have any material sales or net revenue from sales of genetic tests in the area of newborn screening during the year ended December 31, 2012. |
| • | Blood Banking. As genetic testing products have become more prevalent, more accurate and more cost-effective, the use of genetic tests in screening in the blood banking market has grown, and is expected to continue to grow. As of December 31, 2012, we did not offer any tests for use in the area of blood banking, and did not have any sales or net revenue in the blood banking market during the year ended December 31, 2012. |
As is noted in the tables appearing in “Business — Our Current Test Menu”, many of our 53 genetic tests may be utilized in more than one of our target markets, resulting in particular genetic tests being counted multiple times in the information presented above.
The Limitations of Traditional Testing Methods
Traditional testing platforms to detect genetic biomarkers have a number of drawbacks, which we believe have significantly limited their use, including:
| • | High cost per reportable result; |
| • | Impractical for use by smaller reference labs, hospitals and specialty clinics; |
| • | Limited testing menu; |
| • | Inability to multiplex; |
| • | Limited automation and throughput capability; |
| • | Need for specialized labor; and |
5
Table of Contents
| • | Inaccurate results and challenges with reproducibility. |
These limitations have created the need in the molecular diagnostics market for a highly integrated system to perform a large menu of automated, cost-effective and easy to use tests with a high degree of accuracy and reproducibility.
The AutoGenomics Solution
Our INFINITI system has been designed to enable a broad range of reference laboratories, hospital laboratories and specialty clinics to start performing, or to more cost-effectively perform, molecular diagnostic testing, which we believe will drive adoption and use of our INFINITI system as well as expand the potential of the molecular diagnostic testing market.
To use our system, an operator loads the prepared test samples into an INFINITI analyzer, along with the specific BioFilmChip and Intellipac Reagent Management Module, for the desired test. Once the INFINITI analyzer is loaded and the test is initiated, no further action by the operator is required. After the test is completed, the system generates an electronic report that can be transmitted directly to a laboratory information system.
Our INFINITI system has a number of key advantages, including:
| • | Enhanced cost-efficiency. Our system eliminates the need for complex protocols and manual intervention once a test is initiated, which is intended to reduce the laboratory’s cost of testing by simplifying workflow and reducing the need for highly skilled technicians. |
| • | Ability to decentralize molecular diagnostics. We believe that medium-sized reference laboratories, hospital laboratories and specialty clinics are increasingly seeking to add or expand molecular diagnostics capabilities to treat patients more efficiently and provide a more comprehensive offering, lower the cost of providing healthcare, and participate in the value provided by diagnostic testing. We believe that this trend is being facilitated in part by new technologies like ours that are more automated, easier to use, more cost-effective and require less bench space in a laboratory than traditional genetic testing methods. |
| • | Broad menu of tests. As of December 31, 2012, we offered 53 tests as part of our INFINITI system, of which five have received FDA clearance. The balance of our tests are sold on an RUO basis. We believe that this represents one of the broadest available test menus on a single system. We believe that the depth and breadth of our test menu is a strong competitive advantage that will allow our customers to utilize laboratory space, labor and capital investment more efficiently. We also believe that our offering of five FDA cleared tests is comparable to similar testing system offerings of our competitors, which we believe based on publicly available information ranges from zero to seven FDA-cleared tests. As we increase the number of tests available for use on our INFINITI system, laboratories using our system will be able to broaden their molecular diagnostics offerings without significant additional capital investment or operator training. |
| • | Ability to multiplex. Many diseases and patient responses to therapy are caused by multiple genetic mutations that necessitate testing for multiple biomarkers to diagnose those diseases or to predict and/or monitor therapy response. Our INFINITI system is able to detect up to 1,024 individual features of biochemical sensors within a single microarray, which reduces the amount of sample needed, reduces the time required to run the test, and often reduces the need for multiple tests. |
| • | Multiple patient array technology. Our proprietary multiple patient array, or MPA, technology is designed to test up to eight patient samples on a single microarray. This significantly enhances throughput by up to |
6
Table of Contents
| 300% while reducing cost per sample by up to 75% as compared to our single patient microarrays. Our MPA technology is particularly well suited for addressing high volume test markets such as HPV and TB. |
| • | Better workflow. Our broad offering of INFINITI analyzers combined with the integrated, “load and go” design of the INFINITI system is designed to address our target customers’ varied throughput and workflow requirements. We believe that we can substantially increase a laboratory’s workflow by enabling them to perform their tests on our highly integrated and automated system that has the ability to multiplex and run high volume MPAs. The INFINITI system can run multiple different tests simultaneously which reduces or eliminates the need for laboratories to run tests in batches. |
| • | Increased accuracy of results. Human handling of samples is the most common cause of contamination in existing technologies. By reducing the risk of human error and contamination, we believe that our INFINITI system can provide more accurate and more reproducible test results compared to other, less automated systems. In addition, where certain systems only use target or signal amplification (e.g., polymerase chain reaction, or PCR, amplification), we believe that our combined target and signal amplification technologies can increase the sensitivity and specificity over these widely used stand-alone amplification methods. |
Our Products
Our INFINITI Analyzers
Our INFINITI system includes a family of analyzers, each of which is designed to address customer-specific needs based on the customer’s productivity, workflow and throughput requirements. Our INFINITI analyzers integrate and automate the discrete processes of sample handling, reagent management, hybridization, detection, results analysis and reporting in a self-contained system. They have been designed to operate on a “load and go” basis, which means that to run a genetic test, an operator loads prepared samples into the INFINITI analyzer along with the BioFilmChip microarrays and the Intellipac Reagent Management Modules specific to that test. From the perspective of the operator, the test protocols are substantially identical for all of our genetic tests, which eliminates the need to retrain operators when additional tests are added. After the test is completed, the INFINITI analyzer generates an electronic report that can be transmitted directly to a laboratory information system. Our BioFilmChips and Intellipac Reagent Management Modules are test-specific, but are not analyzer-specific, so all of our INFINITI analyzers use substantially the same consumables.
The following table illustrates the test capabilities of our different INFINITI analyzers:
| Analyzer |
Capacity per run |
Patient results * | ||
| INFINITI |
24 samples | up to 96 per day | ||
| INFINITI PLUS |
48 samples | up to 192 per day | ||
| INFINITI PLUS 96 (in beta testing) |
96 samples | up to 288 per day | ||
| INFINITI HTS (in beta testing) |
384 samples | up to 1,152 per day |
| * | numbers presented are based on average time to complete an HPV-HR Quad test run |
Selected Key Tests
We have a demonstrated track record of successfully developing genetic tests that leverage our core competencies and that address current and anticipated customer needs. We believe that by offering a wide variety of tests for each of our target market segments, we will provide significant value to our customers in these areas
7
Table of Contents
by allowing them to consolidate multiple testing platforms, expand their testing capabilities, increase workflow, reduce costs, and limit additional investment in equipment, personnel and training. Some of our key test offerings in our focus market segments currently include:
| • | HPV. We have developed four HPV tests, which are designed for screening and/or genotyping and each of which addresses a different segment of the HPV testing market. Our tests include our HPV-HR Quad and HPV-HR Hex tests, designed to screen for and genotype 14 high-risk types of HPV simultaneously, our HPV Quad test, designed to screen for 13 high-risk and two low-risk types of HPV and our HPV Genotyping test, designed to identify 26 types of HPV. Our HPV-HR Quad, HPV-HR Hex and HPV Quad tests are designed to allow a laboratory to test samples from four to six different patients simultaneously on a single BioFilmChip. We believe our tests offer several competitive advantages: consolidation of multiple testing steps, better automation, reduction of sample requirements and enhanced accuracy and reproducibility. We plan to seek a PMA for our HPV-HR tests and 510(k) clearance for our HPV Genotyping test. Our HPV-HR Quad, HPV Quad and HPV Genotyping tests have been CE marked. |
| • | Other STDs. We have launched a variety of panels consisting of tests that our customers use to identify numerous organisms associated with sexually transmitted diseases, or STDs. Our panels are designed to screen for multiple STDs in a single sample. |
| • | Breast Cancer. We have developed tests such as our CHEK-2 and the Breast Cancer Panel-AJ tests, which are designed to identify individuals at greater risk for breast cancer, and our CYP450 2D6T test, which is designed to determine if a woman will benefit from tamoxifen, a frequently prescribed drug for the prevention of breast cancer recurrence. |
| • | Colorectal Cancer. We have developed KRAS and KRAS-BRAF tests, which enable laboratories to identify certain genetic mutations associated with poor response to anti-epidermal growth factor receptor, or EGFR, therapies. |
| • | Personalized Medicine. One of our leading personalized medicine offerings is our CYP450 2C19 Panel test, which is designed to enable laboratories to identify certain gene variants that affect the metabolism and efficacy of the anticoagulant drug Plavix (clopidogrel). Our other leading tests in this area currently include our CYP450 2C19 Plus, Warfarin and CYP450 2D6I tests, which are designed to enable laboratories to identify certain gene variants associated with responsiveness to certain medications for psychiatric disorders, the oral anticoagulant Warfarin and certain antidepressants and cancer drugs, respectively. |
| • | Infectious Disease. Our Multidrug Resistance Tuberculosis (MDR-TB) test is a multiplexed test that can identify Mycobacterium tuberculosis infections (as opposed to nontuberculous mycobacterium infections) while simultaneously determining resistance to the three front-line TB treatments: rifampin, isoniazid and pyrazinamide. |
| • | Genetic Disorders. Our Familial Mediterranean Fever (FMF) panel multiplexes multiple markers into a single test that allows simultaneous identification of the five most common FMF variations as well as eight other variations spanning 14 ethnicities. |
| • | Newborn Screening. Our Ashkenazi Jewish Panel is capable of simultaneously detecting 31 genetic variants that account for eight diseases commonly found among those of Ashkenazi Jewish descent. |
| • | Blood Banking. We have several tests for blood typing and infectious disease screening currently in development that are intended to take advantage of our multiplexed automation capabilities. |
8
Table of Contents
BioFilmChip
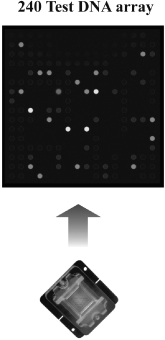
Our proprietary BioFilmChip microarrays consist of multiple layers of a hydro-gel matrix coated on polyester film sandwiched between a plastic base and a reaction body to form a microarray. Our microarrays are printed with 36 to 1,024 individual features of biochemical sensors depending on the requirements of the test. These spotted microarrays are then packaged into specifically designed magazines and are used on all of our INFINITI analyzers.
Intellipac Reagent Management Module
Our proprietary Intellipac Reagent Management Modules hold the reagent needed to run our specific genetic tests. Our reagent module is designed to communicate all relevant information about a test to the INFINITI analyzer without any intervention from the operator, saving time and reducing errors. A read-write memory chip embedded in the module saves test-specific information on the module, including reagent identification, expiration dates, lot number, amount of reagent remaining for future tests, specific instructions for test processing, the time last used and the serial number for the instrument.
Our Strategy
Our objective is to become a leading provider of genetic tests to a broad array of customers within our target market segments. We believe our INFINITI system will allow us to achieve this objective by facilitating molecular diagnostic testing by reference laboratories, hospital laboratories and specialty clinics. To achieve our objective, we intend to:
| • | Capitalize on the capabilities of our INFINITI system to increase penetration within our target market segments. We believe that our INFINITI system’s high level of automation, ability to multiplex and broad test menu are attractive to our target customers in our target market segments as our genetic tests provide an easy to use solution with greater breadth of diagnostic information at a lower cost per reported result than many competing systems. |
| • | Develop and launch new and enhanced tests. We believe that developing a broad menu of genetic tests to run on our system will increase the value of our INFINITI system, drive additional placements of our INFINITI analyzers and increase our consumables sales. We believe that the depth and breadth of our test menu is a significant competitive advantage that will allow customers to increase their ability to conduct molecular testing and utilize laboratory space, labor and capital investment more efficiently, as well as generate supplementary revenue. In addition, the depth and breadth of our test menu diversifies our revenue so that we are not dependent on the performance of any single test. The majority of tests that we offer, are developing and intend to develop have established market demand and reimbursement by public and private payors. |
| • | Target molecular diagnostic laboratories with high potential utilization of our INFINITI system. We believe that our INFINITI system’s automation, broad genetic test menu and family of analyzers designed to address various throughput requirements will generate demand from both larger reference laboratories seeking a more flexible and efficient molecular diagnostic platform and from smaller reference laboratories, hospital laboratories and specialty clinics for whom it has not previously been cost-effective to develop their own tests. |
| • | Expand our domestic sales force and international distribution of our products. We are marketing and selling the INFINITI system in the United States through our own sales and marketing organization and believe there is a meaningful opportunity to further penetrate existing markets and customers as well as enter new markets by expanding our U.S. sales force. We also plan to expand our global distribution networks to address increasing international demand in addition to driving increased utilization with our existing distributors. |
9
Table of Contents
| • | Pursue regulatory clearances, approvals and certifications for products and facilities, as necessary. We have received FDA 510(k) clearance for our INFINITI Analyzer and five of our genetic tests, and we have submitted an additional notification to the FDA for 510(k) clearance of our UGT1A1 test. We are finalizing the protocol for a clinical trial necessary to support a PMA application to the FDA for our HPV-HR tests and intend to commence the clinical trial in 2013. We intend to seek regulatory clearance or approval, as necessary, for our tests. |
| • | Align with key opinion leaders at leading institutions and increase scientific awareness of our products. We align with key opinion leaders at leading institutions and clinical research laboratories to help to increase awareness of our system, to demonstrate its benefits relative to existing technologies and to accelerate its adoption in the molecular diagnostics market. We also seek to increase awareness of our products through participation at trade shows, academic conferences, online webinars and hospital-based grand, or teaching, rounds. In addition, our INFINITI system has been discussed in several published peer review articles. |
Risks Affecting Us
Our business is subject to numerous risks, as more fully described in the section entitled “Risk Factors” elsewhere in this prospectus, including the following:
| • | There is limited information available to evaluate our business, as we have a limited operating history and limited current revenue; |
| • | We have a long history of losses and negative cash flows and may not be able to maintain profitability in the current fiscal year or in future fiscal periods; |
| • | The vast majority of our net revenue (96% for the nine month period ended September 30, 2012) is derived from the sale of products designated for research use only; changes in RUO regulations or the FDA’s enforcement discretion with respect to RUO regulations, or violations of these regulations by us, could significantly limit our ability to sell our products to our target customers, or otherwise require us to obtain regulatory approvals for our products at considerable time and expense; |
| • | Some of our existing indebtedness will come due and be payable in the immediate future, and we do not have the resources to satisfy this indebtedness absent the completion of this offering; |
| • | After completing this offering, we may not be able to meet our cash requirements without obtaining additional capital, and if we are unable to do so, we may have to curtail or cease operations; |
| • | Our financial results depend on commercial acceptance of the INFINITI system and its tests and the development of additional tests; |
| • | We currently derive a significant portion of our revenue from a few customers; |
| • | Many of our competitors are large and well capitalized, and we face significant competition; |
| • | The molecular research use and diagnostic market may fail to fully develop, or we may fail to capture a significant share of that market; |
| • | If the medical relevance of the biomarkers targeted by our tests is not demonstrated or is not recognized by others, we may experience reduced demand for our products; |
| • | We have identified material weaknesses and significant deficiencies in our internal controls; and |
| • | Our success will depend in part on our ability to operate without infringing or misappropriating the proprietary rights of others, on our ability to own or license patents that are adequate to reduce competition and on our ability to license intellectual property from third parties for certain tests and manufacturing processes needed for our business. |
10
Table of Contents
Corporate Information
We were incorporated as Neuron Technologies, Incorporated in California in April 1999, and changed our name to AutoGenomics, Incorporated in August 2000. We subsequently changed our name to AutoGenomics, Inc. in October 2002. We reincorporated in Delaware in November 2008. Our principal executive offices are located at 2980 Scott Street, Vista, California, 92081. Our telephone number is (760) 477-2248. Our website address is www.autogenomics.com. Information contained in or that can be accessed through our website is not incorporated by reference into this prospectus and should not be considered to be part of this prospectus.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012. As an emerging growth company, we are eligible to comply with less stringent disclosure requirements than those applicable to larger, more established companies. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of this offering, (b) in which we have total annual gross revenue of at least $1.0 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700.0 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period. We refer to the Jumpstart Our Business Startups Act of 2012 herein as the “JOBS Act,” and references herein to “emerging growth company” shall have the meaning associated with it in the JOBS Act.
Recent Financial Results
We are finalizing our financial results as of and for the year ended December 31, 2012. Complete financial information and operating data as of and for such period are not available, and we believe that this data will not be available prior to the completion of this offering; however, based on the preliminary information and data available, our management estimates that for the three months ended December 31, 2012, our net revenue will range between $4.0 million and $4.2 million, as compared to net revenue of $2.8 million for the three months ended December 31, 2011, and as compared to net revenue of $5.8 million for the three months ended September 30, 2012; and our loss from operations will range between $1.3 million and $1.6 million, as compared to a loss from operations of $0.8 million for the three months ended December 31, 2011, and as compared to income from operations of $1.5 million for the three months ended September 30, 2012. Taken together with the statement of operations financial data for the nine months ended September 30, 2012 included elsewhere in this prospectus, these estimates would result in income from operations for the year ended December 31, 2012 to range between $1.7 million and $2.0 million, as compared to losses from operations of $6.8 million and $16.5 million for the years ended December 31, 2011 and 2010, respectively.
Net revenue during the three months ended December 31, 2012 was impacted positively as compared to the three months ended December 31, 2011 primarily due to increases in INFINITI analyzer and consumables sales, and was impacted negatively as compared to the three months ended September 30, 2012 by decreased sales of consumables to a key customer, partially offset by increased sales of INFINITI analyzers. We believe the decrease in sales of consumables to this key customer was the result of this customer having increased its purchases of equivalent products from one of our competitors in October and November 2012. We have taken steps to recapture purchases by this key customer and we believe that the recent volume decrease was temporary, as consumables sales to this customer began to increase in December 2012; however, we can provide no assurance that either sales volume or net revenue from this key customer will return to levels we observed in the third quarter of 2012, or that either or both will not decrease further, in future quarters.
Income/(loss) from operations during the three months ended December 31, 2012 was impacted positively as compared to the three months ended December 31, 2011 primarily due to increases in INFINITI analyzer and consumables sales and higher gross margins resulting from a shift in product sales mix that reflected increased
11
Table of Contents
sales of consumables as compared to sales of INFINITI analyzers, and was impacted negatively as compared to the three months ended September 30, 2012 primarily as a result of significantly lower gross profit from decreased consumable sales to a key customer, a significant increase in operating expenses associated with the estimated accounts receivable reserve described below, and lower gross margins resulting from a shift in product sales mix that reflected increased sales of INFINITI analyzers as compared to sales of consumables.
We are unable to estimate with reasonable specificity our net income/(loss) and net income/(loss) per share attributable to common stockholders (basic and diluted) for the three months, and for the year, ended December 31, 2012, primarily due to the impact on such financial measures of certain non-cash charges that we expect to take during these periods, the determination of which have not yet been made. These determinations include the impact on our financial results for the three months, and for the year, ended December 31, 2012 of:
| • | Our exchange in November 2012 of subordinated promissory notes for 4,287,074 shares of Series NC convertible preferred stock, which we anticipate will be treated as an exchange of debt for what we currently estimate to be equity of equal value based on the fair value of the debt and equity on the exchange date. If we were to determine in connection with finalizing our financial results for the three months, and for the year, ended December 31, 2012, that the Series NC convertible preferred stock was not issued at fair value on the issue date, but rather at a discount, then we would be required to recognize a non-cash charge for the three months, and for the year, ended December 31, 2012 in an amount equal to the amount of that discount, which would increase our net loss for that period. For example, if we were to determine that the fair value of the Series NC convertible preferred stock at the date of issue was $2.00 per share (rather than the $1.75 per share that we currently estimate), then the discount on the date of issue would be $0.25 per share ($1.1 million in the aggregate), and if we were to determine that the fair value of the Series NC convertible preferred stock at the date of issue was $2.50 per share (rather than the $1.75 per share that we currently estimate), then the discount on the date of issue would be $0.75 per share ($3.2 million in the aggregate). This discount would then increase our net loss for the three months, and for the year, ended December 31, 2012. |
| • | The issuance in November 2012 of common stock warrants in connection with the exchange of debt for Series NC convertible preferred stock, the fair value of which we anticipate will be recognized as a charge against the proceeds of the Series NC convertible preferred stock, with an offsetting charge against additional paid in capital, and which will not affect our income statement. The fair value of these issued common stock warrants will be determined using the Black-Scholes valuation model. We estimate that this charge against proceeds and against additional paid in capital will range between $1.7 million and $2.2 million. |
| • | Our exchange in November 2012 of subordinated promissory notes and warrants to purchase common stock for new subordinated promissory notes with extended maturity dates and warrants to purchase common stock, which we anticipate will be recorded in our financial statements for the three months, and the year, ended December 31, 2012 as an extinguishment of debt in accordance with FASB ASC 470-50, and which we anticipate will be recorded at fair value on the exchange date as calculated using future discounted cash flows, and the issuance in November 2012 of common stock warrants in connection with the exchange of debt for new debt with extended maturity dates, the fair value of which we anticipate will be recorded as a discount to the new debt and will be amortized over the three year term of the new debt using the effective interest method. The fair value of these issued common stock warrants will be determined using the Black-Scholes valuation model. We estimate that the result of this exchange and accounting treatment will result in a non-cash charge in a range between $0.2 and $0.5 million for the three months, and for the year, ended December 31, 2012. This non-cash charge, once determined, will be recognized in each of our fiscal quarters going forward for the entire three-year term of the new debt. If the offering referred to in this prospectus is consummated and our outstanding promissory note indebtedness is paid in full from the proceeds of this offering, as is |
12
Table of Contents
| expected, then the remaining unamortized portion of this non-cash charge will be recognized in the same fiscal quarter that our outstanding promissory note indebtedness is paid in full. |
In addition, we expect to recognize the deferred costs we incurred in connection with our November 2012 debt-to-equity exchange transaction, which we estimate were approximately $0.3 million, as a non-cash charge for the three months, and for the year, ended December 31, 2012, and we expect to recognize the deferred costs we incurred in connection with our November 2012 debt-for-debt exchange transaction, which we estimate were approximately $0.7 million, as a non-cash charge over the three year term of the new debt, which we estimate will result in a non-cash charge of $0.1 million for the three months, and for the year, ended December 31, 2012. If the offering referred to in this prospectus is consummated and our outstanding promissory note indebtedness is paid in full from the proceeds of this offering, as is expected, then the remaining unamortized portion of these deferred costs will be recognized as a non-cash charge in the same fiscal quarter that our outstanding promissory note indebtedness is paid in full.
Each of these above non-cash items, when determined, will impact our net income/(loss) and net income/(loss) per share attributable to common stockholders (basic and diluted) for the three months, and for the year, ended December 31, 2012. If we were to determine that each of the above non-cash items were to be at the low end of the estimated ranges and examples presented above, based on the preliminary information and data available, and using our estimated range of loss from operations for the same period described above, our management estimates that for the three months ended December 31, 2012, our net loss would range between $1.9 million and $2.2 million, as compared to a net loss of $1.9 million for the three months ended December 31, 2011, and as compared to net income of $0.7 million for the three months ended September 30, 2012. Taken together with the statement of operations financial data for the nine months ended September 30, 2012 included elsewhere in this prospectus, these estimates would result in net income for the year ended December 31, 2012 to range between $1.1 million and $1.4 million, as compared to net losses of $10.0 million and $19.7 million for the years ended December 31, 2011 and 2010, respectively. If we were to determine that each of the above non-cash items were to be at the top end of the estimated ranges and examples presented above, based on the preliminary information and data available, and using our estimated range of loss from operations for the same period described above, our management estimates that for the three months ended December 31, 2012, our net loss would range between $5.4 million and $5.7 million, as compared to a net loss of $1.9 million for the three months ended December 31, 2011, and as compared to net income of $0.7 million for the three months ended September 30, 2012. Taken together with the statement of operations financial data for the nine months ended September 30, 2012 included elsewhere in this prospectus, these estimates would result in a net loss for the year ended December 31, 2012 to range between $2.4 million and $2.7 million, as compared to net losses of $10.0 million and $19.7 million for the years ended December 31, 2011 and 2010, respectively.
As of December 31, 2012, approximately $1.1 million in accounts receivable from a key customer were past due 90 days or more. We have not finalized our assessment of the collectability of these receivables; however, for the purposes of the estimates provided above, we have reserved this entire past due amount. If we determine that we are able to reduce this reserved amount, it would have a positive impact on our income/(loss) from operations, as well as our net income/(loss) and our net income/(loss) per share attributable to common stockholders (basic and diluted), for the three months, and the year, ended December 31, 2012.
The preliminary financial data and accounting treatment information above has been prepared by, and is the responsibility of, our management. Our independent registered public accounting firm has not audited, reviewed, compiled, or performed any procedures with respect to this preliminary financial data and accounting treatment information and does not express an opinion or any other form of assurance with respect thereto. Because the three months ended December 31, 2012 has recently ended, the unaudited net revenue, income/(loss) from operations and net income/(loss) information presented above for the three months and year ended December 31, 2012 has not yet been subject to our normal quarterly financial closing processes, reflects estimates based only
13
Table of Contents
upon preliminary information available to us as of the date of this prospectus, and is not a comprehensive statement of our financial results for the three months, or for the year, ended December 31, 2012. We believe that our financial statements and operating data as of and for the three months and the year ended December 31, 2012 will not be available until after this offering is completed and may differ from the unaudited net revenue, income/(loss) from operations and net income/(loss) information we have provided. Such differences may be material. Accordingly, the net revenue, income/(loss) from operations and net income/(loss) information and accounting treatment information presented should not be viewed as a substitute for full interim financial statements prepared in accordance with GAAP, and undue reliance should not be placed on these preliminary estimates. These preliminary estimates are not necessarily indicative of any future period and should be read together with “Risk Factors,” “Special Note Regarding Forward-looking Statements,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Selected Financial Data” and our financial statements and related notes included elsewhere in this prospectus.
14
Table of Contents
The Offering
| Common stock to be offered by us |
6,000,000 shares |
| Common shares to be outstanding immediately after this offering |
16,746,791 shares |
| Overallotment option |
We have granted the underwriters an option for 30 days from the date of this prospectus to purchase up to 900,000 additional shares of our common stock at the initial public offering price to cover overallotments. |
| Use of proceeds |
We anticipate that we will use the net proceeds from this offering to (i) repay the principal and interest under our outstanding promissory notes, (ii) fund the expansion of our sales force, enhance our international distributor network, and increase our marketing and promotional activity and business development efforts, (iii) support a PMA for our HPV-HR tests and 510(k) and CE mark studies and submissions for several tests associated with women’s health and personalized medicine, (iv) fund research and development activities to add new or enhanced tests to our menu, (v) fund the expansion of our manufacturing capacity and efficiency, including purchasing automation equipment, (vi) satisfy outstanding accounts payable to advisors and service providers incurred in connection with certain of our prior capital raising activities conducted from 2008 through 2010, and (vii) make payment of past due amounts owed to our landlord. We anticipate that we will use the remainder of the net proceeds from this offering for additional working capital and general corporate purposes. See “Use of Proceeds.” |
| NASDAQ Global Market listing |
We have applied to list our common stock on the NASDAQ Global Market under the symbol “AGMX.” |
| Risk factors |
Investing in our common stock involves a high degree of risk. You should carefully read and consider the information set forth under the heading “Risk Factors” and all other information set forth in this prospectus before deciding to invest in our common stock. |
The number of shares of common stock outstanding after this offering is based on the following as of December 31, 2012: 2,855,771 shares of common stock, and 1,579,227 shares of Series A Convertible Preferred Stock, 4,468,369 shares of Series B Convertible Preferred Stock, 6,417,680 shares of Series C Convertible Preferred Stock, 3,423,258 shares of Series D Convertible Preferred Stock, 732,555 shares of Series E Convertible Preferred Stock and 4,287,074 shares of Series NC Convertible Preferred Stock which will convert into an aggregate of 7,891,020 shares of common stock in connection with the completion of this offering, and excludes as of that date:
| • | 1,549,720 shares of common stock issuable upon exercise of options outstanding at a weighted average exercise price of $5.16 per share; |
| • | 465,328 and 82,500 shares of common stock reserved for future issuance under our 2008 Equity Incentive Plan and 2008 Employee Stock Purchase Plan, respectively; and |
| • | warrants to purchase 3,372,711 shares of common stock at a weighted average exercise price of $6.08 per share and warrants to purchase 371,300 shares of our preferred stock which will become warrants to purchase 133,488 shares of common stock at a weighted average exercise price of $10.78 per share in connection with the completion of this offering. |
15
Table of Contents
Unless otherwise indicated, all information in this prospectus assumes an initial public offering price of $10.00 per share, the midpoint of the range on the cover page of this prospectus, and gives effect to the 1-for-0.33 reverse split of our common stock that we effected in January 2013.
Effective immediately prior to the completion of this offering, each outstanding share of our Series A Convertible Preferred Stock will convert into 0.6600 shares of our common stock, each outstanding share of our Series B, Series C and Series E Convertible Preferred Stock will convert into 0.3595 shares of our common stock, each outstanding share of our Series D Convertible Preferred Stock will convert into 0.3674 shares of our common stock and each outstanding share of our Series NC Convertible Preferred Stock will convert into 0.3300 shares of our common stock. The conversion into common stock of our Series C, Series E and Series NC Convertible Preferred Stock is predicated on the offering referred to in this prospectus resulting in net proceeds to us of $25 million or more. Our outstanding warrants to purchase our convertible preferred stock will automatically become exercisable for shares of our common stock in connection with the completion of this offering.
Except as otherwise indicated, all information in this prospectus assumes:
| • | no issuance of any options under our 2008 Equity Incentive Award Plan after December 31, 2012 and no exercise of any outstanding warrants or options after December 31, 2012; and |
| • | no exercise by the underwriters of their overallotment option. |
As of December 31, 2012, we had outstanding total indebtedness and accrued interest under promissory notes of approximately $17.5 million, of which approximately $2.3 million in principal amount was in payment default. In November 2012, the holders of $7.5 million in aggregate principal amount of and accrued interest on our outstanding subordinated promissory notes with rates of interest ranging from six percent to 12% that were then past due or scheduled to come due in the immediate future surrendered their right to payment of those promissory notes in exchange for an aggregate of 4,287,074 shares of our Series NC Convertible Preferred Stock. In connection with this surrender and issuance, these noteholders surrendered warrants for the purchase of an aggregate of 637,813 shares of our common stock with exercise prices ranging from $5.24 per share to $15.15 per share in exchange for warrants of a like tenor for the purchase of an aggregate of 637,813 shares of our common stock with exercise prices of $4.55 per share that are exercisable through November 2017. Also in November 2012, the holders of $14.4 million in aggregate principal amount of and accrued interest on our outstanding subordinated promissory notes with rates of interest ranging from six percent to 13% that were then past due or scheduled to come due in the immediate future surrendered their right to payment of those promissory notes in exchange for new promissory notes in aggregate principal amount of $14.4 million with eight and one-half percent rates of interest and November 2015 maturity dates. In connection with this surrender and issuance, these noteholders surrendered warrants for the purchase of an aggregate of 1,156,013 shares of our common stock with exercise prices ranging from $5.24 per share to $15.15 per share in exchange for warrants of a like tenor for the purchase of an aggregate of 1,734,020 shares of our common stock with exercise prices of $5.30 per share that are exercisable through November 2017. As of November 27, 2012, after giving effect to these actions, we had outstanding total indebtedness and accrued interest under promissory notes of approximately $17.4 million, of which approximately $2.9 million was past due or scheduled to come due in the immediate future, and outstanding warrants to purchase an aggregate of 3,372,711 shares of our common stock.
On December 28, 2012, Tregale Group Ltd, the holder of approximately $2.2 million in principal amount under our promissory notes that had been in default beginning in the first quarter of 2012, filed a request for judicial intervention and motion for summary judgment in lieu of complaint, demanding payment of the principal and interest outstanding under its promissory notes as well as reimbursement of certain legal and other expenses. See “Business—Legal Proceedings” elsewhere in this prospectus. In January 2013, we issued and sold a
16
Table of Contents
subordinated promissory note in the amount of $2.4 million to one of our existing investors, who is a holder of more than 5% of our capital stock. This promissory note has a maturity date of March 31, 2013, an annual interest rate of 8.5%, and is prepayable at any time without premium or penalty. We used the proceeds of this sale to retire the outstanding principal and interest owed to Tregale Group Ltd under our outstanding promissory notes, and are negotiating with Tregale Group Ltd with respect to the reimbursement of its legal and other expenses, and the dismissal of the lawsuit.
17
Table of Contents
Summary Financial Data
The following tables provide our summary financial data and should be read in conjunction with our audited financial statements, the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus. The summary statement of operations data for each of the years ended December 31, 2010 and 2011 were derived from our audited financial statements appearing elsewhere in this prospectus. The summary statement of operations data for the nine months ended September 30, 2011 and September 30, 2012 and the summary balance sheet data as of September 30, 2012 were derived from our unaudited financial statements. The unaudited financial data, in management’s opinion, have been prepared on the same basis as the audited financial statements and related notes included elsewhere in this prospectus, and include all adjustments, consisting only of normal recurring adjustments, that our management considers necessary for a fair presentation of the information for the periods presented. The results of operations for the nine months ended September 30, 2012 are not necessarily indicative of the results that may be expected for the year ended December 31, 2012 or any other period.
| Years ended December 31, | Nine months ended September 30, | |||||||||||||||
| 2010 | 2011 | 2011 | 2012 | |||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands, except share and per share data) | ||||||||||||||||
| Statement of operations data: |
||||||||||||||||
| Net revenue |
$ | 7,504 | $ | 8,005 | $ | 5,166 | $ | 14,473 | ||||||||
| Cost of sales |
7,666 | 6,019 | 4,378 | 5,035 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit/(loss) |
(162 | ) | 1,986 | 788 | 9,438 | |||||||||||
| Operating expenses |
||||||||||||||||
| Research and development |
3,560 | 2,768 | 2,146 | 1,724 | ||||||||||||
| General and administrative |
5,376 | 3,360 | 2,587 | 2,775 | ||||||||||||
| Sales and marketing |
4,717 | 2,672 | 2,023 | 1,655 | ||||||||||||
| Impairment of film coating equipment |
981 | — | — | — | ||||||||||||
| Initial public offering costs - terminated offering |
1,752 | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
16,386 | 8,800 | 6,756 | 6,154 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income/(loss) from operations |
(16,548 | ) | (6,814 | ) | (5,968 | ) | 3,284 | |||||||||
| Interest expense, net |
(4,109 | ) | (3,626 | ) | (2,598 | ) | (1,913 | ) | ||||||||
| Other income/(expense), net |
36 | (2 | ) | — | 5 | |||||||||||
| Change in fair value of warrant liabilities |
926 | 445 | 434 | (170 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income/(loss) |
(19,695 | ) | (9,997 | ) | (8,132 | ) | 1,206 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income/(loss) per share attributable to common stockholders |
||||||||||||||||
| Basic |
$ | (7.63 | ) | $ | (3.79 | ) | $ | (3.09 | ) | $ | 0.45 | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
$ | 0.13 | ||||||||||||||
|
|
|
|||||||||||||||
| Shares used to compute net income/(loss) per share attributable to common stockholders |
||||||||||||||||
| Basic |
2,581,708 | 2,634,385 | 2,633,929 | 2,651,833 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
9,148,420 | |||||||||||||||
|
|
|
|||||||||||||||
18
Table of Contents
| As of September 30, 2012 | ||||||||||||
| Actual | Pro forma (1) | Pro forma as adjusted (2) |
||||||||||
| (unaudited) | ||||||||||||
| (in thousands) | ||||||||||||
| Balance sheet data: |
||||||||||||
| Cash and cash equivalents |
$ | 294 | $ | 294 | $ | 36,727 | ||||||
| Current assets |
7,104 | 7,104 | 43,537 | |||||||||
| Total assets |
9,826 | 9,826 | 46,259 | |||||||||
| Total debt (3) |
20,784 | 17,057 | — | |||||||||
| Convertible preferred stock (4) |
47,432 | — | — | |||||||||
| Total stockholders’ equity/(deficit) |
(77,080 | ) | (22,140 | ) | 31,510 | |||||||
| (1) | On a pro forma basis after giving effect to the restructuring of debt described in note (3) below, which occured in November 2012, and the conversion of all outstanding shares of convertible preferred stock into common stock, including our Series NC Convertible Preferred Stock, which will occur immediately prior to the completion of this offering. |
| (2) | On a pro forma as adjusted basis after giving effect to (i) the conversion of all outstanding shares of convertible preferred stock into common stock, (ii) the sale of 6,000,000 shares of our common stock in this offering at an assumed initial offering price of $10.00 per share, the midpoint of the range on the cover page of this prospectus, and after deducting underwriting discounts and commissions and our estimated offering expenses and (iii) the application of $17.7 million of the net proceeds of this offering to repay the principal and interest under our outstanding promissory notes. A $1.00 increase or decrease in the assumed initial public offering price of $10.00 per share would increase or decrease, as applicable, the net proceeds to us from this offering by approximately $5.6 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and our estimated offering expenses. |
| (3) | Amounts include principal only of promissory notes payable. The summary unaudited pro forma, and pro forma as adjusted, balance sheet data as of September 30, 2012 give effect to the exchange, in November 2012, of $22.0 million in aggregate principal amount of and accrued interest on our outstanding subordinated promissory notes for an aggregate of 4,287,074 shares of our Series NC Convertible Preferred Stock and $14.4 million in aggregate principal amount of new promissory notes due November 2015. |
| (4) | Our convertible preferred stock has been classified as temporary equity on our balance sheets instead of in stockholders’ deficit due to the possibility of the occurrence of certain change in control events that are outside of our control, including our sale or transfer of control, which trigger the rights of holders of the convertible preferred stock to force redemption. Accordingly, these shares are considered contingently redeemable. We have adjusted the carrying values of the convertible preferred stock to their liquidation values at each period end. |
19
Table of Contents
Before deciding to invest in our common stock, you should carefully consider each of the following risk factors and all of the other information set forth in this prospectus. The following risks and the risks described elsewhere in this prospectus, including in the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” could materially harm our business, financial condition, future results and cash flow. If that occurs, the trading price of our common stock could decline, and you could lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business.
Risks Relating to Our Business
Because we have a limited operating history and limited current revenue, there is limited information available to evaluate our business.
We have a limited operating history upon which one can evaluate our business and our products. As an early commercial-stage company in the rapidly evolving market for molecular diagnostics, we face numerous risks and uncertainties. Based on our limited operating experience, we may not be able to effectively:
| • | drive adoption of our system; |
| • | attract and retain customers for our products; |
| • | demonstrate the commercial viability of our tests; |
| • | comply with evolving regulatory requirements applicable to products that are intended for research use only; |
| • | obtain regulatory approvals to market and sell our products; |
| • | anticipate and adapt to changes in the molecular diagnostics market; |
| • | focus our research and development efforts in areas that generate appropriate returns on these efforts; |
| • | maintain and develop strategic relationships with vendors and manufacturers to acquire necessary materials and the production of our products; |
| • | implement an effective marketing strategy to promote awareness of our products; |
| • | scale our manufacturing capacity to meet potential demand; |
| • | avoid infringement and misappropriation of third-party intellectual property; |
| • | obtain licenses on commercially reasonable terms to third-party intellectual property; |
| • | obtain valid and enforceable patents that give us a competitive advantage; |
| • | protect our proprietary technology; |
| • | provide appropriate levels of customer support for our products; |
| • | protect our operations from any equipment- or software-related system failures; |
| • | develop and operate computer systems and related infrastructure that are adequate to manage our growth and provide our services effectively; and |
| • | attract, retain and motivate qualified personnel. |
Our operations are subject to many of the risks inherent in the growth of a new business. The likelihood of our success must be evaluated in light of the challenges, expenses, difficulties, complications and delays frequently encountered in the operation of a new business. We cannot assure you that we will achieve anticipated revenue growth and maintain profitability in the current or future fiscal years. Our failure to meet any of these goals could have a material adverse effect on us and may force us to reduce or cease our operations.
20
Table of Contents
We have a long history of operating losses and negative cash flows and we may not be able to maintain profitability.
We have incurred substantial costs to develop our technology. As of September 30, 2012, we had an accumulated deficit of $92.4 million. We expect to continue to spend substantial financial and other resources on conducting clinical studies and trials, seeking regulatory approvals, servicing our existing indebtedness, introducing new products, expanding our sales and marketing activities, developing our technology and manufacturing capabilities, engaging in laboratory testing and manufacturing new products. As a result, we will need to generate significant revenue to maintain profitability.
Our ability to generate significant revenue will depend on our ability to successfully implement our business strategies and address the risks and uncertainties facing us, and we cannot assure you that we will be successful in these efforts. Even if we do address these risks successfully and implement our business strategies, we may not generate sufficient revenue to maintain profitability. We cannot assure you that we would be able to increase profitability on a quarterly or annual basis in the future.
Our operating results may be variable and unpredictable.
Due to the nature of the molecular diagnostic testing market and facets of our business, our revenue and operating results may be difficult to predict and may vary significantly from period to period. The sales cycles for our products may be lengthy, which may make it difficult for us to accurately forecast revenue in a given period. Specifically, initial sales of our consumable products are often dependent on completing customer validation processes that may be time-consuming and unique to each customer. In addition to its length, the sales cycle associated with our products is subject to a number of significant risks, including the budgetary constraints of our customers, their inventory management practices and possibly internal acceptance reviews and the timing of FDA approval and review, all of which are beyond our control. Sales of our products will also involve the purchasing decisions of reference laboratories, hospital laboratories and specialty clinics, which can require many levels of pre-approvals, further lengthening sales time. These purchasing decisions are subject to a number of significant risk factors beyond their and our control and are difficult for us to predict. For example, reference laboratories, hospitals and specialty clinics may purchase fewer of our consumable products due to a decline in the volumes of tests needed at these facilities. As a result, we may expend considerable resources on unsuccessful sales efforts or we may not be able to complete sales as anticipated. In addition, a significant percentage of our revenue is generated from a few customers. Changes in ordering volume from one or more of these customers could materially impact our operating results. For example, in the fourth quarter of 2012, one of our key customers decreased its ordering volumes, which resulted in substantially lower net revenues compared to the prior quarter. Our international sales are also difficult to predict because they depend upon the activities of our distributors, over which we have limited control.
Our revenue and operating results may also vary due to the evolving mix between sales of our INFINITI analyzers and consumables and the introduction of new or enhanced tests. Changes in the relative mix of our INFINITI analyzers and consumables sales can have a significant impact on our gross margin, as consumable sales typically have significantly higher margins than those of INFINITI analyzer sales. Further, our revenue and operating results are difficult to predict because many of our tests have only recently been launched and we do not have sufficient history to forecast revenue reliably for those tests. The difficulty in accurately forecasting, and any period-to-period variations in, our revenue and operating results may cause our stock price to fluctuate significantly in the future.
Our independent registered public accounting firm has included an explanatory paragraph relating to our ability to continue as a going concern in its report on our audited financial statements included in this prospectus.
As of December 31, 2011 and September 30, 2012, we did not have sufficient working capital to fund our planned operations through December 31, 2012 without additional financing. These conditions raise substantial doubt about our ability to continue as a going concern. Accordingly, the report of our independent registered
21
Table of Contents
public accounting firm on our audited financial statements included in this prospectus contains an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern; however, the audited financial statements have been prepared assuming that we will continue as a going concern, which contemplates the recovery of our assets on rental or loan to our customers and the satisfaction of our liabilities in the normal course of business.
After completion of this offering, we may not be able to meet our cash requirements without obtaining additional capital from external sources, and if we are unable to do so, we may have to curtail or cease operations.
We anticipate that our current cash and cash equivalents and cash provided by this offering and our operating activities will be sufficient to meet our currently estimated cash requirements for at least the next 12 months; however, we expect capital outlays and operating expenditures to increase over the next several years as we expand our infrastructure, commercialization, training and support, manufacturing and research and development activities. We operate in a market that makes our prospects difficult to evaluate, and we may need additional financing to execute on our current or future business strategies. The amount of additional capital we may need to raise depends on many factors, including:
| • | the level of research and development investment required to maintain and improve our technology, including efforts to expand our molecular diagnostic test menu, to fund clinical studies and clinical trials of our tests and to invest in the development of new products; |
| • | the amount of future cash provided by or used in operating activities; |
| • | the costs of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; |
| • | our need or decision to acquire or license complementary technologies or acquire complementary businesses; and |
| • | changes in regulatory policies or laws that affect our operations. |
We have a history of operating losses and negative cash flows since our inception and we may not be able to maintain profitability. We cannot be certain that additional capital will be available when and as needed or that our actual cash requirements will not be greater than anticipated. If we require additional capital at a time when investment in diagnostics companies, or in the marketplace in general, is limited due to the then-prevailing market or other conditions, we may not be able to raise such funds at the time that we desire or any time thereafter. If we are unable to raise additional capital, we may be required to curtail some or all of our operations, including commercialization and research and development efforts, and forced to forego otherwise valuable business opportunities. Any failure to raise additional capital when needed could have a material adverse effect on us. In addition, if we raise additional funds through the issuance of common stock, preferred stock or convertible securities, the percentage ownership of our stockholders could be significantly diluted, and any preferred stock or convertible securities may have rights, preferences or privileges senior to those of common stockholders. If we obtain additional debt financing, a substantial portion of our operating cash flow or other cash resources may be dedicated to the payment of principal and interest on such indebtedness, and the terms of the debt securities issued could impose significant restrictions on our operations. If we raise additional funds through collaborations and licensing arrangements, we may be required to relinquish significant rights to our technologies or products, or grant licenses on terms that are not favorable to us.
We conduct business in a heavily regulated industry, and any changes in regulations or the FDA’s enforcement discretion, or violations of regulations by us, could adversely affect our business, prospects, results of operations or financial condition.
The clinical laboratory testing industry is highly regulated, and we cannot assure you that the regulatory environment in which we operate will not change significantly and adversely in the future. In particular, the laws and regulations governing the marketing of molecular research use or diagnostic products for use as laboratory-
22
Table of Contents
developed tests are extremely complex and in many instances there are no significant regulatory or judicial interpretations of these laws and regulations. While we believe that we are currently in material compliance with applicable laws and regulations as historically enforced by the FDA, we cannot assure you that the FDA or other regulatory agencies would agree with our determination, and a determination that we have violated these laws, or a public announcement that we are being investigated for possible violations of these laws, could adversely affect our business, prospects, results of operations or financial condition.
In addition, a significant change in any of these laws may require us to change our business model in order to maintain compliance with these laws. For instance, in June 2011 the FDA issued a Draft Guidance entitled “Commercially Distributed In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only: Frequently Asked Questions,” which, if enforced, would limit our marketing of research use only, or RUO, tests to general discovery laboratories and would require us to halt sales to clinical laboratories that validate and incorporate our RUO tests as laboratory developed tests, or LDTs. The FDA has generally exercised its enforcement discretion to not enforce applicable regulations with respect to LDTs. However, the FDA has indicated, since 2010, that it intends to reconsider its policy regarding enforcement and to begin drafting an oversight framework for such tests. Sales of RUO products accounted for 87%, 92% and 96%, respectively, of our net revenue for 2010, 2011 and the first nine months of 2012, respectively. Accordingly, if the FDA imposes significant changes to the regulation or enforcement of LDTs, including our RUO tests that are incorporated into LDTs, it could reduce our revenues or increase our costs and adversely affect our business, prospects, results of operations or financial condition.
Our existing indebtedness could adversely affect our ability to continue operations and to raise additional capital to fund our operations, and could prevent us from meeting our obligations.
As of December 31, 2012, the principal amount of our total indebtedness was approximately $17.0 million and there was approximately $0.5 million in accrued and unpaid interest on this principal amount. As of December 31, 2012, we were in default on approximately $2.3 million in aggregate principal amount of and accrued interest on our indebtedness. Absent the completion of this offering, we do not have sufficient cash to satisfy our debt obligations. We are required pursuant to the terms of substantially all of our indebtedness to use at least 50% of the net proceeds of this offering to repay that indebtedness.
Our outstanding debt and related debt service obligations that remain after this offering, if any, could have important adverse consequences to us, including:
| • | heightening our vulnerability to downturns in our business, our industry or the general economy and restricting us from making improvements to our products, acquiring complementary technology or businesses, or exploring other business opportunities; |
| • | requiring a substantial portion of our cash flow from operations to be dedicated to the payment of principal and interest on our indebtedness, therefore reducing our ability to use our cash flow to fund our operations, capital expenditures and future business opportunities; |
| • | limiting our ability to obtain additional financing for working capital, capital expenditures, debt service requirements, acquisitions and general corporate or other purposes; |
| • | limiting our ability to adjust to changing market conditions and placing us at a competitive disadvantage compared to our competitors who have greater capital resources; and |
| • | subjecting us to financial and other restrictive covenants in our indebtedness, the failure with which to comply could result in an event of default. |
If our cash flows and capital resources continue to be insufficient to fund our existing and future debt service obligations, we may be forced to reduce or delay capital expenditures, sell assets, seek additional capital
23
Table of Contents
or restructure or refinance our indebtedness. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations. Failure to pay our indebtedness on time would constitute an event of default under the agreements governing such indebtedness, and could constitute an event of default under the agreements governing our other indebtedness. Future events of default under such agreements would give rise to these creditors’ ability to accelerate the applicable debt obligations and/or seek other remedies against us, which could have a material adverse effect on us. We intend to use a portion of the proceeds of this offering to pay down our outstanding indebtedness. See “Use of Proceeds”.
Our past inability to pay our indebtedness and trade payables when due could adversely affect our reputation and business prospects.
We have in the past been in default on a substantial amount of indebtedness owed to various persons due to our inability to repay our indebtedness by their applicable maturity dates. We have also periodically been unable to timely pay various trade payables, including amounts owed to our suppliers, services providers and landlord. On November 1, 2012, we went into default on certain of our payment obligations to our landlord. In December 2012, we entered into an amendment to our lease with our landlord which provides that our past due amounts owed to the landlord, which total $1.1 million, must be paid by February 4, 2013. If we do not complete the offering referred to in this prospectus prior to February 4, 2013, we will be unable to satisfy this payment obligation, we will be in default under the lease, and our landlord will have the right to elect any or all of the remedies available to it under the lease and applicable law, including the termination of the lease and our eviction from our Vista, CA facility. In December 2012, we were sued for repayment by the holder of $2.2 million in past due principal under our outstanding promissory notes. See “Business—Legal Proceedings”. These events may have a negative impact on our reputation and perceived credit-worthiness, and make it more difficult for us to maintain existing relationships and enter into new relationships with suppliers and service providers, or to obtain future financing, particularly debt financing. In addition, concerns about our long-term ability to continue operations may deter potential customers from investing the time and resources necessary to install our INFINITI analyzers and use our system to conduct genetic testing. To the extent that our reputation has been harmed from our past failure to timely pay our debt and trade payables, it may take us many years to repair our reputation. In the meantime, we may receive less favorable terms from our suppliers and service providers, find it more difficult to obtain financing, and face challenges convincing customers to invest in the long-term use of the INFINITI system.
Our financial results depend on commercial acceptance of the INFINITI system, its menu of tests and the development of additional tests and other products.
Our future depends on the success of the INFINITI system, which depends primarily on its acceptance by reference laboratories, hospitals and specialty clinics as a reliable, accurate and cost-effective replacement for traditional molecular diagnostic testing methods. Many reference laboratories and hospitals already use molecular diagnostics testing instruments in which they have made substantial investments, and may be reluctant to change their current procedures for performing such analyses. In addition, many laboratories currently rely on their own LDTs. These laboratories, hospitals and specialty clinics may be reluctant to discontinue using their own LDTs due to the time and expense already invested in developing and validating these tests and their familiarity with these tests compared to the INFINITI system. If we are unable to displace molecular diagnostic testing instruments and LDTs currently in use, our market opportunity will be limited and we may not be able to achieve significant sales of our products.
We continue to seek to develop additional tests for our INFINITI system as well as additional products (such as our INFINITI PLUS 96 and INFINITI HTS analyzers, both of which are currently in the beta testing phase) to respond to what we perceive to be the needs of reference laboratories, hospital laboratories and specialty clinics; however, we cannot guarantee that we will be able to develop enough additional tests or other products in a timely manner or in a manner that is cost-effective or at all. The development of new or enhanced tests and other products is a complex and uncertain process requiring the accurate anticipation of technological and market trends, as well as precise technological execution. We are currently not able to estimate when or if we will be able to develop, obtain
24
Table of Contents
licenses to third-party intellectual property on commercially acceptable terms for, obtain regulatory approval or clearance for, commercialize or sell, additional tests or enhance existing products. If we are unable to increase sales of our INFINITI system or to successfully develop, obtain licenses to third-party intellectual property on commercially acceptable terms for, obtain regulatory approval or clearance for and commercialize, additional tests or other products, our revenue, prospects and ability to maintain profitability would be impaired.
Because we derive a significant portion of our revenue from a few customers, our business, financial condition and results of operations could be materially adversely affected by the loss of one or more of these customers, a significant decline in sales to one or more of these customers or a delay in collection of payments from one or more of these customers.
We have in the past derived, and expect to continue to derive, a significant portion of our revenue from a few major customers. For fiscal years 2010 and 2011, we had two and three customers, respectively, that each represented between 12% and 14% of our revenue in each year. For the nine months ended September 30, 2012, we had two customers that each represented more than 17% of our revenue, one of whom represented 47% of our revenue during such period. Our continued business relationship with these major customers, and the amount of purchases and timing of payment by these major customers, may be impacted by several factors beyond our control, including product offerings by our competitors, pricing pressures or the financial health of these customers. The loss of any of these major customers, or a significant decline in sales to or a delay in collection of payments from any of these customers, would materially adversely affect our business, financial condition and results of operations. For example, in the fourth quarter of 2012, one of our key customers decreased its ordering volumes, which resulted in substantially lower net revenues compared to the prior quarter.
Our business may suffer if we have difficulty acquiring and retaining customers.
A large part of our business strategy depends on our ability to place our INFINITI analyzers with customers that are high-volume users of genetic tests in our areas of focus to drive sales of our consumable products. We may not succeed in acquiring and retaining a sufficiently large customer base for our INFINITI system in order to execute on our strategy. If we are unable to establish a sufficient installed base of INFINITI analyzers or if customers with which we place INFINITI analyzers do not use them, we may not be able to generate revenue or successfully implement our business strategy. In addition, if we are unable to bring our products to market as quickly as anticipated or to acquire and develop our customer base as quickly as we have projected, our ability to generate revenue could be materially adversely impacted.
Failure by our customers or distributors to pay for products we have sold to them could have a material adverse effect on our financial condition and results of operations.
In certain cases, we may not be able to recognize revenue on sales even though we may have delivered products to our customers or distributors. For example, we have in the past sold, and may continue to sell, our INFINITI analyzers to certain of our international distributors where our ability to collect payment was not reasonably assured. Although our customers and distributors are required to pay us for the products that we sell to them, from time to time certain of our customers and distributors have not paid us or have delayed payment. In particular, some of our distributors experience long delays in receiving reimbursement from foreign government payors and healthcare providers, which in turn causes them to withhold payment to us. As of December 31, 2011 and September 30, 2012, approximately $0.2 million of our accounts receivable were 90 days or more past due. As of December 31, 2012, approximately $1.1 million of our accounts receivable were 90 days or more past due. In the past we have deferred, and in the future we may be required to defer, the recognition of revenue where the collection of payment is not reasonably assured until such payment is received. Deferring the recognition of revenue could negatively impact our results of operations for any period in which the related sales occurred. Although we have reserved for or deferred the recognition of revenue related to the amount of all late payments on our balance sheets, we also may incur unexpected costs or losses resulting from these sales in the event of any delays or failures to make payment for the related products. If we are unable to collect payment from our customers or distributors for
25
Table of Contents
products we have sold to them, we may never be able to recover our costs to manufacture and sell such products, which could have a material adverse effect on our financial condition and results of operations.
If our customers are unwilling or unable to validate the INFINITI system or follow required protocols, our business could be harmed.
In most cases, we are dependent on our customers to validate our INFINITI system and the associated tests we offer. These test validation processes are done on a test-by-test basis and can take anywhere from several weeks to several months or more to complete, and we do not generate revenue from these customers for sales of the tests being validated during these processes. Individual laboratories have their own validation procedures and protocols that our system and tests must pass and we cannot predict how long each validation process will take or whether laboratories will be willing or able to validate our INFINITI system. To the extent that any laboratories are unwilling or unable to validate our INFINITI system, we will not be able to generate meaningful revenue from sales to these laboratories.
Proper use of the INFINITI system also requires our customers to follow certain sample collection and other protocols. If we are unable to develop these protocols or if, despite our efforts, customers do not follow the required protocols, the INFINITI system may not generate a result or a correct result. With respect to international customers, we are generally dependent on our distributors to provide support services. Failure by our customers to follow required protocols may lead to a perception, even if unfounded, that the INFINITI system does not function properly. Such perceptions could damage our reputation and brand and reduce the frequency that our customers use the INFINITI system and purchase our consumable products.
Many of our competitors are large and well capitalized, and we face significant competition.
We face, and will continue to face, significant competition from organizations such as large in vitro diagnostics companies that compete directly or indirectly with us in the general molecular diagnostics market. We compete in an industry characterized by: (i) rapid technological change, (ii) evolving industry standards and regulatory requirements, (iii) emerging competition and (iv) new product introductions. Our competitors may develop and commercialize products and technologies that may mitigate any advantages our products may currently have and that may compete successfully with our products and technologies. Since several potentially competing companies and institutions have greater financial resources, more established brand names and larger existing customer bases than we do, they may be able to: (a) provide broader services and product lines, (b) make greater investments in research and development, (c) undertake more extensive sales efforts and marketing campaigns and (d) adopt more aggressive pricing policies than we are able. Some of our competitors are also our suppliers, and there can be no assurance that these suppliers will continue to provide us products at competitive prices or at all. Many of our competitors have also been able to enter into long-term, exclusive agreements with major potential customers, such as large reference labs, oftentimes by offering favorable pricing and other terms. Until these agreements expire, our ability to place our analyzers with and sell our testing consumables to these customers will be limited. Even after exclusive agreements expire, we may not be able to compete with the terms offered by our competitors in their efforts to extend exclusive relationships with these major potential customers. In addition, we compete against companies on the basis of the relative regulatory status of their and our products. Some of our competitors may have already received regulatory approval and conducted extensive clinical trials for tests we seek to sell. For example, other companies have received approval of PMAs from the FDA for HPV screening tests. We expect that it may take us several years to receive approval of a PMA for our HPV-HR tests, if at all. Any tests that we offer on an RUO basis may be at a competitive disadvantage to similar tests offered by others that have received FDA clearances or approvals.
We face competition from enhanced or alternative technologies and products.
We expect to continue to face competition from enhanced or alternative technologies and products. One or more of our competitors could develop a product that is superior to a product we offer or intend to offer or our technology and products may be rendered obsolete or uneconomical by advances in existing technologies. At least two of our competitors currently offer on a commercial basis self-contained, integrated systems for molecular
26
Table of Contents
diagnostic testing that are similar in many ways to our products. If we are unable to keep pace with technological advances in the molecular diagnostics market, our business, financial condition and results of operations may suffer.
Our business may be materially adversely affected if the molecular research use and diagnostics market fails to develop or if we fail to capture a significant share of the market.
Our business may suffer if the market for research use and molecular diagnostics products fails to develop or develops more slowly than expected. Our revenue is dependent on the acceptance of molecular research use and diagnostics products by laboratories, hospitals and specialty clinics and on the expanded use of molecular diagnostic testing by physicians and hospitals. Even if we are successful in gaining acceptance of our products in laboratories, hospitals and specialty clinics, our revenue may be limited if physicians and hospitals curtail or do not expand the use of molecular diagnostic testing. Barriers to the adoption of widespread genetic testing include the need to increase physician education on molecular research use and diagnostic testing and to keep physicians up-to-date on new and emerging technologies, as well as overcoming coverage denials from insurance companies generally. Moreover, we rely in part on reference laboratories to drive demand for molecular diagnostic testing with physicians and hospitals and to provide this education. Reference laboratories are private, commercial facilities that conduct high-volume routine and specialty testing and which are relied upon for testing by many physicians and hospitals. If these laboratories are unsuccessful in their efforts to drive demand for testing, or if laboratories that use our INFINITI system lose market share to other laboratories that use competing molecular diagnostics products, our growth potential will be limited. Even if the market does develop, we cannot assure you that we will be successful in capturing a significant share of it by expanding the sale of our products at the prices we currently project. In the event that the market in general, or our market share in particular, does not grow as we expect, our business may be materially adversely affected.
We have limited experience pricing and marketing our products and we may not be able to appropriately adjust our pricing and marketing efforts in response to changes in the market.
We intend to generate revenue from one-time sales of our INFINITI analyzers and recurring sales of consumables, including our BioFilmChips and Intellipac Reagent Management Modules. We have limited experience marketing and pricing molecular research use and diagnostics products. As such, we cannot assure you that our expectations as to pricing and marketing of these products are appropriate. The molecular research use and diagnostics market is also rapidly evolving due to, among other things, regulatory changes and the introduction of new technologies. If we are unable to appropriately adjust our pricing and marketing efforts in response to this changing environment, we may lose market share by pricing our products too high or lose potential revenue by pricing our products lower than required to maintain or grow our market share. Our failure to price and market our products appropriately could impact our ability to attract and retain customers in the molecular research use and diagnostics market, or to establish and maintain recurring revenue streams on acceptable terms. In light of these factors, we cannot assure you that we will be able to attract a sufficient number of customers or generate sufficient revenue to support our operations.
We are subject to risks relating to the placement of INFINITI analyzers under our domestic placement plans.
In the United States, we offer the INFINITI analyzer through direct sale, monthly rental plans and our domestic placement plan. As of December 31, 2012, we had 191 INFINITI analyzers placed with customers, 48 of which were placed with customers under domestic placement plans. Under our domestic placement plan, an INFINITI analyzer is placed at the customer’s location at no direct cost to the customer with the intent of generating recurring demand for our high-margin testing consumables, including our test-specific BioFilmChips and Intellipac Reagent Management Modules. The customers under our domestic placement plans are required by the terms of the plans to purchase minimum amounts of consumables; however, in some cases, customers have not met this requirement. We have the right to reclaim an INFINITI analyzer placed with a customer pursuant to a domestic placement plan if the customer does not purchase the minimum amount of consumables. During the years ended December 31, 2010, 2011 and 2012, we reclaimed 27, 18, and 16 INFINITI analyzers,
27
Table of Contents
respectively, either because the customer failed to purchase the minimum amount of consumables or otherwise elected not to retain the INFINITI analyzer at the end of the term of the applicable domestic placement plan. We bear the costs of producing these INFINITI analyzers and rely on the revenue from the sale of consumables to recoup our costs. As a result, we could expend significant resources to produce INFINITI analyzers for our domestic placement plans and not generate enough revenue from the sale of consumables to recoup our costs, in which case our business could be materially adversely affected.
The spending policies of our customers have a significant effect on the demand for our products.
Our targeted customers include reference laboratories, hospital laboratories and specialty clinics, and their spending policies can have a significant effect on the demand for our products. These policies are based on a wide variety of factors, including governmental regulation or price controls, reimbursement policies, the resources available for purchasing diagnostic tests, the spending priorities among various types of diagnostic tests and the policies regarding capital expenditures during recessionary periods. Any decrease in spending by reference laboratories, hospital laboratories or specialty clinics could cause our revenue to decline. As a result, we are subject to significant volatility in revenue. Therefore, our operating results can be materially affected by the spending policies and priorities of our customers, over which we have little control.
We rely on the innovation and resources of larger industry participants and public programs to advance genetic research, establish the medical relevance of biomarkers, and educate physicians and clinicians on molecular diagnostics.
The link between the genetic variations that our products detect and the underlying disease states or response to medications is not always fully medically validated, and our current and future products may involve biomarkers whose medical relevance is unproven. Additionally, the availability of validated biomarkers is dependent on significant investment in genetic research, often funded through public programs for which there are no assurances of ongoing support. Should any government limit patent rights to specific genetic information, private investment in this area could also be significantly curtailed. In addition, the adoption of molecular diagnostics is dependent to a great extent on the education and training of physicians and clinicians. We do not have the resources to undertake such training, and we are relying on larger industry participants and professional medical colleges to establish, communicate and educate physicians and clinicians regarding the use and application of molecular diagnostics.
We have identified material weaknesses and significant deficiencies in our internal controls, and we cannot provide assurances that these weaknesses and deficiencies will be effectively remediated or that additional material weaknesses and significant deficiencies will not occur in the future. If our internal control over financial reporting or our disclosure controls and procedures are not effective, we may not be able to accurately report our financial results, prevent fraud, or file our periodic reports in a timely manner, which may cause investors to lose confidence in our reported financial information and may lead to a decline in our stock price.
We have historically operated as a private company and the number and qualifications of our finance and accounting staff have not been consistent with those of a public company. We also currently do not have an internal audit function. Further, we have in the past been required to expend significant time and resources to improve our internal controls over financial reporting. For example, in the first and second quarter of 2010, we identified material weaknesses and significant deficiencies in our internal controls under the standards established by the Public Company Accounting Oversight Board, or PCAOB, with respect to (i) revenue recognition as it relates to properly recording negotiated terms and conditions in customer agreements that deviate from our standard terms and conditions, (ii) the misapplication of generally accepted accounting principles, or GAAP, with respect to the timing of the recognition of revenue for product sales characterized by multiple deliverables or additional elements, and (iii) our financial statement closing process and errors in calculating and allocating the fair value of the warrants and the amortization of the related interest expense related to our accounting for warrants issued with our promissory notes. In response to the identification of these material weaknesses, we terminated one of our sales
28
Table of Contents
personnel that had contributed to the failure to communicate negotiated terms and conditions, revised our sales and revenue recognition policies and procedures and trained our personnel with respect to the revised policies and procedures, and amended our code of ethics and required all of our employees to certify compliance with this code.
Furthermore, in connection with the audits of our financial statements for the years ended December 31, 2010 and 2011, and the review of our financial statements for the period ended September 30, 2012, both our independent registered public accounting firm and our management discovered several conditions that we deemed to again be material weaknesses and significant deficiencies in our internal controls, as follows:
| • | We noted several issues related to effective oversight by management primarily attributed to lack of significant prior audit experience and expertise relating to complex accounting issues (such as debt and equity accounting and valuation). A lack of sufficient controls over debt/equity accounting and recordkeeping of equity and debt holders’ transactions resulted in material adjustments. |
| • | We lacked a comprehensive, consistent and supportable methodology to capture the cost of manufacturing our inventory, specifically as it relates to labor and overhead charges and inventory reserves. In addition, updates to our costing system were not performed timely to ensure accurate recordkeeping. |
| • | A lack of qualified accounting and finance resources as well as effective oversight by those in charge of governance resulted in insufficient controls over timely financial statement preparation and review as well as the inability to timely and accurately account for complex transactions. A lack of technical knowledge and expertise attributed to non-compliance with certain GAAP accounting disclosure requirements. |
| • | The design of monitoring controls used to assess the design and operating effectiveness of our internal controls is inadequate. We also do not have an adequate internal process to report deficiencies in internal control to management on a timely basis. |
We believe that we have taken actions that have substantially remediated the material weaknesses and significant deficiencies identified in 2010 other than with respect to our financial statement closing process, with respect to which we have continued to experience material weaknesses. In an attempt to remediate our closing process weaknesses, and in response to the identification of our more recent material weaknesses and significant deficiencies, we (i) have hired two new employees to augment our accounting staff, and intend to hire additional finance and accounting personnel, in addition to providing more resources and/or assistance on equity accounting and other complex GAAP accounting matters, and have engaged a third party finance specialist to perform certain accounting functions for us, (ii) have established a series of internal controls over critical financial processes designed to ensure the proper oversight, accuracy and timeliness of entries made by our finance personnel and to ensure that periodic financial reporting is completed within requisite timeframes, and (iii) will seek to establish better operating controls and involve our board of directors in our internal controls process, which will involve establishing formal procedures to communicate deficiencies in internal controls on a timely basis, and encourage our board of directors to more actively participate in guiding management as it relates to internal controls matters. However, we cannot assure you that our internal controls over financial reporting, as modified, will enable us to identify or avoid material weaknesses or significant deficiencies in the future. Regardless, following the completion of this offering we will be required to expend significant time and resources to further improve our internal controls over financial reporting, including by further expanding our finance and accounting staff.
Growth in our business could strain our managerial, operational, manufacturing, customer support, sales, financial and information systems resources.
The anticipated future growth necessary to expand our operations will place a significant strain on our managerial, operational, manufacturing, sales, financial and information systems resources. These increased demands could cause us to operate our business less effectively, which in turn could cause deterioration in the financial performance of our business. To increase revenues and achieve growth, we will need to expand our sales efforts and continue to improve and develop our products. The future growth of our business will also
29
Table of Contents
require us to expand our customer support resources, including adding additional personnel for customer training and technical product support. If our customer support infrastructure does not keep pace with future growth of our customer base, we may lose customers and our reputation and future sales potential may be harmed. In addition, we expect that we will need to improve our financial and managerial controls, reporting systems and procedures, and that we also will need to expand, train and manage our workforce. We may be unable to hire, train and retain a sufficient number of qualified personnel or successfully manage our growth. This growth may also place increased burdens on our international distributors, increase the complexities we face related to international sales and increase our inventory-related risk. Future growth will also make it difficult for us to adequately predict the expenditures we will need to make in the future.
If we do not make the necessary capital or other expenditures to accommodate our anticipated growth, or if we are unable to manage our growth effectively, our business, financial condition and results of operations will suffer. We cannot anticipate all of the demands that our expanding operations will impose on our business, personnel, systems and controls and procedures, and our failure to appropriately address such demands could have a material adverse effect on us.
The loss of the services of one or more of our key personnel or failure to attract and retain other highly qualified personnel in the future could adversely affect operations and result in a loss of revenue.
We are dependent on the continued services and performance of senior management and educated, technically-trained personnel. We currently do not have employment or severance agreements with any of these persons. Certain of the members of our senior management, including certain of our named executive officers, are at or close to traditional retirement age in the United States. Our business depends and will depend in the future on the ability to identify, attract, hire, train, retain and motivate senior management and other highly skilled technical, managerial, marketing and customer service personnel. Competition for such personnel is intense, and we cannot assure you that we will be able to successfully attract and retain sufficiently qualified personnel in the future. While we do not currently expect any of our senior management or other technically-trained personnel to retire or otherwise cease employment with us in the near-term, we will need to effectively plan for management succession to maintain effective leadership and continuity in our business. The failure to attract and retain necessary senior management and technically-trained personnel and to successfully plan for management succession could adversely affect our business and result in a loss of revenue.
Our failure to comply with regulatory requirements or receive any required regulatory clearance or approval for our products or operations in the United States or abroad would adversely affect our revenue and potential for future growth.
As a manufacturer of molecular research use and diagnostics products, our products are medical devices that are subject to extensive regulation in the United States by the FDA, and by respective authorities in foreign countries where we do business. The FDA regulates virtually all aspects of a medical device’s design and testing, manufacture, safety, labeling, storage, recordkeeping, reporting, clearance and approval, promotion and distribution. The FDA also regulates the export of medical devices to foreign countries. Failure to comply with the regulatory requirements of the FDA and other applicable U.S. regulatory requirements may subject a company to administrative or judicially imposed sanctions ranging from warning letters to criminal penalties or product withdrawal or recall. Unless an exemption applies, a medical device must receive either clearance or premarket approval from the FDA before it can be marketed in the United States. The FDA’s 510(k) clearance process for Class II devices usually takes from three to twelve months, but may take significantly longer. The premarket approval process for Class III devices generally takes from one to three years from the time the application is filed with the FDA, but also can be significantly longer. Premarket approval typically requires extensive clinical data and can be significantly longer, more expensive and more uncertain than the 510(k) clearance process. Despite the time, effort and expense expended, there can be no assurance that a particular device ultimately will be cleared or approved by the FDA through either the 510(k) clearance process or the premarket approval process.
30
Table of Contents
Medical devices may only be marketed for the indications for which they are approved or cleared. We may be required to obtain additional new premarket approvals, premarket approval supplements or 510(k) clearances to market additional products or for new indications for or modifications to our existing products. We cannot be certain that we would obtain additional premarket approvals or 510(k) clearances in a timely manner or at all. We have modified various aspects of our devices in the past and we determined that new approvals, supplements or clearances were not required. The FDA may not agree with our decisions not to seek approvals, supplements or clearances for particular device modifications. If the FDA requires us to obtain premarket approvals, supplement approvals, or 510(k) clearances for any modification to a previously cleared or approved device, we may be required to cease manufacturing and marketing the modified device or to recall such modified device until we obtain FDA clearance or approval and we may be subject to significant regulatory fines or penalties. In addition, we cannot assure you that the FDA will clear or approve such submissions in a timely manner, if at all.
The vast majority of the tests that we currently sell, which together represented 87%, 92% and 96% of our net revenue for 2010, 2011 and the first nine months of 2012, respectively, have not been cleared or approved for clinical use by the FDA. These tests are labeled “For Research Use Only. Not for use in diagnostic procedures.” as required by FDA regulations. These RUO tests are marketed to allow for the collection of research data and may only be used for clinical purposes by laboratories certified under the Clinical Laboratory Improvements Amendments of 1988, or CLIA, that have incorporated our RUO tests into their own laboratory-developed tests pursuant to guidelines issued by the College of American Pathologists. In order to develop an LDT utilizing our products, these certified laboratories and other facilities must develop and validate a test protocol that includes specimen collection, DNA extraction, PCR amplification, hybridization and detection, and data analysis, interpretation and reporting. We provide components that can be used by these certified laboratories and other facilities for the PCR amplification, hybridization and detection portions of these LDTs. We sell each of these components individually, as ordered by the customer in its discretion, and not as a kit or system. The validation process engaged in by these certified laboratories and other facilities can involve validation of the sample collection and extraction process, establishing limits of detection and analytical sensitivity, testing for specificity and cross-reactivity, including interfering substances, validation for assay accuracy, precision and reproducibility, and establishing reportable ranges of test results for the test system and reference values that will be measured against as controls. This validation process also requires verifying the result from the LDT versus known standard samples or the results of a high-standard laboratory testing method such as sequencing, and can involve the testing of a large number of patient samples. Depending on the availability of patient samples, this process may take from several weeks to several months or more to complete. The FDA has not adopted these guidelines and we are not permitted to represent our RUO products as effective in vitro diagnostic products. On December 4, 2009, the FDA issued a letter to us indicating that certain of our promotional materials for our RUO tests may include diagnostic/clinical claims which may cause these products to be in vitro diagnostic tests for clinical use for which FDA clearance or approval is required. In addition, the FDA noted that the allegations in its letter may not constitute an exhaustive list of potential violations and noted that we are required to comply with the RUO requirements for all of our products that have not received FDA clearance or approval. We responded to the FDA’s allegations in a letter dated December 14, 2009 and believe that we have taken appropriate action to modify and revise the promotional materials in light of the FDA’s concerns. However, the FDA has not responded to our submission and has not confirmed its agreement with our approach. If the FDA does not agree with our approach, it could require that we take additional steps, initiate enforcement action or require that we recall or withdraw certain or all of our RUO products from the market. Our failure to comply with the FDA’s regulatory requirements, obtain FDA clearance or approvals of new or existing products or product modifications or enhancements that we develop in the future, any limitations imposed by the FDA on such products’ development or use, or the costs of obtaining FDA clearance or approvals could have a material adverse effect on our business, particularly given the proportion of our business that is reliant upon RUO products.
In many of the foreign countries in which we market our products, including those countries in the European Union, we are subject to extensive regulations similar to those of the FDA. As discussed under “Business — Government Regulation,” there are additional regulatory requirements outside of the United States, including certifications, registrations or approvals that may require third-party certification of compliance with ISO
31
Table of Contents
13485:2003, a quality management system standard developed by the International Organization for Standardization, or ISO. This certification must be satisfied in order for us to market our products in those jurisdictions. If we fail to satisfy such requirements or obtain the requisite certifications, registrations or approvals in these countries, it could have a material adverse effect on our international operations.
If the FDA or another regulatory agency determines that we have promoted off-label use of our products, or are not in compliance with restrictions on the marketing of products labeled for research use only, we may be subject to various penalties, including civil or criminal penalties.
The FDA and other regulatory agencies actively enforce regulations prohibiting the promotion of a medical device for a use that has not been cleared or approved by the FDA. Use of a device outside its cleared or approved indications is known as “off-label” use. If the FDA or another regulatory agency determines that our promotional materials or training constitutes promotion of an off-label use, it could require us to modify our training or promotional materials or subject us to regulatory enforcement actions, including the issuance of a warning letter, injunction, seizure, civil fine and criminal penalties.
As required by the FDA, we have a policy in place requiring that our labeling and marketing practices comply with FDA regulations for products labeled for research use only. We also have a policy in place to refrain from making statements that could be considered off-label promotion of our products. Although our policy is to refrain from making statements that could be considered off-label promotion of our products or outside of the restrictions for products labeled for research use only, the FDA or another regulatory agency could conclude that we have engaged in off-label promotion or marketing of products labeled for research use only. If the FDA disagrees with the marketing of these products, we may be subject to a variety of enforcement actions ranging from public warning letters, fines, injunctions, consent decrees and civil penalties to suspension or delayed issuance of approvals, seizure or recall of our products, total or partial shutdown of production, withdrawal of approvals or clearances already granted, and criminal prosecution. In addition, the off-label use of our products or the use of our products labeled for research use only for clinical purposes may increase the risk of injury to patients, and, in turn, the risk of product liability claims. Product liability claims are expensive to defend and could divert our management’s attention and result in substantial damage awards against us.
We may fail to receive favorable clinical results from the diagnostic tests currently in development that require clinical trials, and even if we receive favorable clinical results, we may still fail to receive the necessary clearances or approvals to market these tests.
We are investing in the research and development of new products to expand the menu of testing options for the INFINITI system. To commercialize our products, we are required to undertake time-consuming and costly development activities, sometimes including clinical trials for which the outcome is uncertain. We are in the process of finalizing the protocol for a clinical trial necessary to support a PMA application to the FDA for our HPV-HR tests and intend to commence the clinical trial in 2013 once the protocol is complete. As discussed under “Use of Proceeds,” we intend to use a portion of the net proceeds of this offering to fund the clinical trial. We do not know whether clinical trials of our HPV-HR tests or any other pre-clinical or clinical trials will begin on time or be completed on schedule, if at all. The commencement and completion of clinical trials can be delayed for a number of reasons, including delays related to:
| • | obtaining sufficient funding for the clinical trial; |
| • | satisfying regulatory requirements to commence a clinical trial; |
| • | reaching agreement on acceptable terms with prospective clinical research organizations, or CROs, clinical investigators and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs, clinical investigators and trial sites; |
| • | obtaining institutional review board approval to initiate and conduct a clinical trial at a prospective site; |
| • | identifying, recruiting and training suitable clinical investigators; and |
| • | identifying, recruiting and enrolling subjects to participate in clinical trials. |
32
Table of Contents
Clinical trials may also be delayed, halted or repeated as a result of ambiguous or negative interim results or unforeseen complications in testing. In addition, a clinical trial may be suspended or terminated by us, the FDA, the institutional review board overseeing the clinical trial at issue, any of our clinical trial sites with respect to that site, or other regulatory authorities due to a number of factors, including:
| • | failure by us, our employees, our CROs or their employees to conduct the clinical trial in accordance with all applicable FDA or other regulatory requirements or our clinical protocols; |
| • | inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities resulting in the imposition of a clinical hold; |
| • | lack of adequate funding to continue the clinical trial, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional trials and studies and increased expenses associated with the services of our CROs and other third parties; |
| • | lack of effectiveness of any product candidate during clinical trials; |
| • | slower than expected rates of subject recruitment and enrollment rates in clinical trials; |
| • | failure of our CROs or other third-party contractors to comply with all contractual requirements or to perform their services in a timely or acceptable manner; or |
| • | unfavorable results from ongoing clinical trials and pre-clinical studies. |
Clinical investigations of in vitro diagnostic tests, including our products and product candidates, typically are exempt from the investigational device exemption, or IDE, requirements. Thus, we do not need the FDA’s prior approval to conduct clinical trials of our products, provided the testing is non-invasive, does not require an invasive sampling procedure that presents a significant risk, does not intentionally introduce energy into the subject and is not used as a diagnostic procedure without confirmation of the diagnosis by another, medically established diagnostic device or procedure. The FDA requires each sponsor of a clinical trial to make this determination in the first instance, but the FDA can review any decision. If the FDA disagrees with our decision not to submit an IDE for the clinical trials we are currently conducting, the agency may retroactively require us to submit an IDE and halt the clinical trial until the IDE is approved.
Products that appear promising during early development and preclinical studies may, nonetheless, fail to demonstrate the results needed to support regulatory approval or commercial viability. Even if we receive favorable clinical results, we may still fail to obtain the necessary FDA clearance and approvals. Any such failure, or any material delay in obtaining the necessary clearance or approvals, could materially adversely affect our business, financial condition and results of operations.
Our products are subject to recalls even after receiving FDA clearance or approval, or after affixing the CE marking, which would harm our reputation and business.
We are subject to medical device reporting regulations (e.g., MDR, vigilance reporting) that require us to report to the FDA or governmental authorities in other countries if our products cause or contribute to a death or serious injury, or malfunction in a way that would be reasonably likely to contribute to death or serious injury if the malfunction were to recur. The FDA and similar governmental authorities in other countries have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacturing. To date, we have not conducted a product recall. A government mandated, or voluntary, recall by us could occur as a result of component failures, manufacturing errors or design defects, including defects in labeling. Any recall would divert managerial and financial resources and could harm our reputation with customers. There can be no assurance that there will not be product recalls in the future or that such recalls would not have a material adverse effect on our business.
If we fail to comply with the FDA’s Quality System regulation and similar requirements in other jurisdictions, our manufacturing could be delayed, and our product sales and profitability could suffer.
Our manufacturing processes are required to comply with the FDA’s Quality System regulation, which covers the procedures concerning (and documentation of) the design, testing, production processes, controls,
33
Table of Contents
quality assurance, labeling, packaging, storage and shipping of our devices. We also are subject to similar requirements in other jurisdictions and to state requirements and licenses applicable to manufacturers of medical devices. In addition, we must engage in extensive recordkeeping and reporting and must make available our manufacturing facilities and records for periodic unscheduled inspections by governmental agencies, including the FDA, state authorities and comparable agencies in other countries. Moreover, failure to pass a Quality System regulation inspection or to comply with these and other applicable regulatory requirements could result in disruption of our operations and manufacturing delays. Failure to take adequate corrective action could result in, among other things, significant fines, suspension of approvals, seizures or recalls of products, operating restrictions and criminal prosecutions. Any failure to comply with applicable requirements could adversely affect our product sales and profitability.
The California Department of Health Services, Food and Drug Branch audited our quality system in 2007. That audit resulted in two observations related to CAPA documentation and failure to make instructions available to employers to define how an activity is to be carried out. We responded to those observations on October 16, 2007 and have not received any further correspondence. The FDA also audited our quality system in April 2009. This audit resulted in a Form 483 notice of inspectional observations that identified eight deficiencies relating primarily to CAPA documentation. On April 30, 2009, we formally addressed the observations from the audit. On September 22, 2009, we received a copy of the establishment inspection report, or EIR, from the FDA District Director for the audit conducted in April 2009. Although we have addressed the audit observations from this audit, we cannot assure you that the FDA will not raise issues relating to our activities as a result of this or any other audit.
We may not be able to compete effectively if we are unable to protect our proprietary rights.
Our success depends in part on our ability to protect our intellectual property rights, which involve complex legal, scientific, and factual questions and uncertainties. We rely upon patent, trade secret, copyright and contract laws to protect proprietary technology and trademark law to protect brand identities. We cannot be certain that we have taken adequate steps to prevent misappropriation of our technology or that our competitors will not independently develop technologies that are substantially equivalent or superior to ours. We also cannot be certain that we have taken adequate steps to protect our inventions through patents or our brand identities through trademarks. To date we have not sought trademark protection for our brand identities in countries outside of the United States, and this may adversely affect our ability to prevent unauthorized use of these brand identities after we, or our distributors, have expended resources to increase their recognition. Our success depends in part on our ability to obtain and maintain patent protection for our products and their use, maintain trade secret protection and operate without infringing upon the proprietary rights of others. Our policy is to protect our proprietary technology through patents, where appropriate, and in other cases, through trade secrets. In addition, we rely on the licenses of patents of third parties. We cannot assure you that any patent applications filed by, assigned to, or licensed to us will be granted, that any patents issued to or licensed by us will provide us with any competitive advantages or adequate protection for inventions, that any patents issued to or licensed by us will not be challenged, invalidated, held to be unenforceable, or circumvented by others or that issued patents, or patents that may be issued, will provide protection against competitive products or otherwise be commercially valuable.
If we fail to obtain and/or maintain sufficient patent protection for our products, our ability to realize a competitive advantage for these products could be materially affected, which may have a material adverse effect on our results of operations. In particular, if we fail to obtain and/or maintain patent protection, competitors may be able to produce products that would be directly competitive with our products using similar or identical processes with impunity. Moreover, should any government or court limit or alter patent rights relating to specific genetic information or diagnostic methods, our business could be materially and adversely affected.
Patent law relating to the scope of claims in the fields of healthcare and biosciences generally, and molecular diagnostics in particular, is evolving and our patent rights are subject to this uncertainty. For example, on March 29, 2010, the U.S. District Court in New York invalidated seven patents held by Myriad Genetics
34
Table of Contents
related to the genes BRCA1 and BRCA2. Mutations in these genes have been associated with breast cancer, indicating that such patents “are directed to a law of nature and were therefore improperly granted”; however, that decision was overruled by the Federal Circuit, Association for Molecular Pathology v. USPTO, Fed. Cir., No. 2010-1406, 8/16/2012, which held that isolated BRCA1/2 DNA molecules used for diagnosing increased breast and ovarian cancer risk are eligible for patent protection under 35 U.S.C. §101. If patents covering naturally occurring genes are invalidated, it could adversely impact the scope of our patent protection relating to naturally occurring genes and certain aspects of their diagnostic methods.
Our patent rights on our products might conflict with the patent rights of others, whether existing now or in the future. We cannot assure you that the inventors of the patents and applications that we own and license were the first to invent or the first to file on the claimed inventions, and if others were determined to be the first inventors, such determination could materially reduce or even eliminate the value of such patents. We also cannot assure you that a third party does not have or will not obtain patents that dominate the patents we own or license now or in the future. Consequently, we could be subject to charges of patent infringement. The defense and prosecution of patent claims are both costly and time-consuming even if the outcome is ultimately in our favor. An adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease selling the affected products. As our products are distributed in the marketplace, we expect to face opposition to our intellectual property by competitors. If we face opposition to our intellectual property, we likely will incur substantial costs defending our patents.
Additionally, we rely on trade secrets and proprietary know-how, which we seek to protect, in part, by confidentiality agreements with our collaborators, employees and consultants. We cannot assure you that these agreements will not be breached, that we will have adequate remedies for any such breach or that our trade secrets will not otherwise become known or be independently developed by competitors.
Our success will depend partly on our ability to operate without infringing or misappropriating the proprietary rights of others.
The in vitro diagnostics industry has a history of patent litigation and likely will continue to experience patent litigation suits. A third party could claim that we are infringing a patent, which could prevent us from selling our INFINITI analyzers and tests, or from developing new technologies we expect to utilize or products we expect to sell. We may be sued for infringing or misappropriating the proprietary rights of others. We may have to pay substantial damages, including treble damages and the opposing party’s attorney fees, for past infringement if it is determined that our products or their use infringe on a third party’s proprietary rights. In addition, in the future we may need to obtain one or more licenses to the intellectual property of others, and the failure to obtain such licenses could prevent us from manufacturing or selling the affected products.
Our INFINITI system utilizes a wide variety of technologies and we cannot assure you that we have identified or can identify all inventions and patents that may be infringed by the development, manufacture, sale and use of our INFINITI system, including its various components. If relevant patents are upheld as valid and enforceable and we are found to infringe, we could be prevented from selling our system unless we can obtain a license to use technology or ideas covered by such patents or are able to redesign our INFINITI system to avoid infringement. A license may not be available at all or on commercially reasonable terms, and we may not be able to redesign our INFINITI system to avoid infringement.
The landscape for intellectual property rights in the field of genetics is complex. Although a significant amount of intellectual property in the field of genetics is already in the public domain, some of our existing and future tests for the INFINITI system could require modification, or the acquisition of certain rights from third parties related to those tests which we have not yet obtained, to avoid infringement. We may not be able to make such modifications or obtain such rights at all or on terms that are commercially acceptable. If we sell an otherwise infringing test without such modifications or such rights, a third party patent holder may sue us for patent infringement. If a court finds that we infringed the third party’s patent and that the infringement was
35
Table of Contents
willful, we could be ordered to pay treble damages and the patent holder’s attorneys’ fees, and we may be enjoined from further selling our tests, which may materially impact our business, financial condition or results of operations.
We also enter into collaborative agreements with research and academic institutions for the development of additional or enhanced tests to be run on our system. Under these agreements, the institution typically retains the intellectual property rights to any inventions or discoveries made solely by the institution, while we receive a right to negotiate a license to these intellectual property rights. If these institutions independently develop intellectual property that is necessary for us to sell new tests that arise from the collaboration, there can be no assurance that we will be able to obtain a license through our negotiation rights. Acquisition of any necessary intellectual property rights from third parties related to any of our existing or future tests, including licenses to patents, could also be time-consuming and expensive, and could require us to pay significant royalties to third parties. And likewise any such rights may not be available on commercially reasonable terms or at all.
We cannot assure you that any third party intellectual property rights to use technology or biomarkers that we determine are necessary to our business and the development of new tests for the INFINITI system can be acquired on commercially reasonable terms or at all. Resulting limitations on our ability to offer certain tests or expand our menu of tests could have a material adverse effect on our business, financial condition and results of operations and negatively impact a key basis upon which we compete.
We have hired and will continue to hire individuals who have experience in molecular diagnostics and these individuals may have confidential trade secret or proprietary information of third parties. We cannot assure you that these individuals will not use this third-party information in connection with performing services for us or otherwise reveal this third-party information to us. Thus, we could be sued for misappropriation of proprietary information and trade secrets. Such claims are expensive to defend and could divert our attention and result in substantial damage awards and injunctions that could have a material adverse effect on our business, financial condition or results of operations.
We may need to initiate lawsuits to protect or enforce our patents or other proprietary rights, which would be expensive and, if unsuccessful, may cause us to lose some of our intellectual property rights.
To protect or enforce our patent rights, it may be necessary for us to initiate patent litigation proceedings against third parties, such as infringement suits or interference proceedings. These lawsuits would be expensive, take significant time and would divert management’s attention from other business concerns. In the event that we seek to enforce any of our patents against an infringing party, it is likely that the party defending the claim will seek to invalidate the patents we assert, which could put our patents at risk of being invalidated, held unenforceable, or interpreted narrowly, and our patent applications at risk of not being issued. Further, these lawsuits may provoke the defendants to assert claims against us. The patent position of in vitro diagnostics firms is highly uncertain, involves complex legal and factual questions and recently has been the subject of much litigation. We cannot assure you that we will prevail in any of such suits or proceedings or that the damages or other remedies awarded to us, if any, will be commercially valuable.
We are subject to litigation, which, if adversely determined, could cause us to incur substantial losses.
We are, from time to time, during the normal course of operating our businesses, subject to various litigation claims and legal disputes. Some of the litigation claims may not be covered under our insurance policies or our insurance carriers may seek to deny coverage. As a result, we might be required to incur significant legal fees, which may have a material adverse impact on our financial position. In addition, because we cannot predict the outcome of any action, it is possible that, as a result of current and/or future litigation, we will be subject to adverse judgments or settlements that could significantly reduce our earnings or result in losses.
36
Table of Contents
If our products contain undetected defects or errors or do not operate as expected, we could incur significant unexpected expenses, experience product returns and lost sales, suffer damage to our brand and reputation and be subject to product liability or other claims and product recalls and other field or regulatory actions.
Our products are complex and may contain undetected defects, errors or failures, particularly when first introduced or when new versions are released. Some errors and defects may be discovered only after a product has been installed and used by the customer. To the extent our products incorporate technologies of third parties, we will be dependent on the reliability of these technologies. If our products contain undetected defects or errors or do not operate as expected, we could experience decreased sales and increased product returns, loss of customers and market share and increased service, warranty and insurance costs. In addition, our reputation and brand could be damaged, and we could face potential legal claims regarding our products and be subject to product recalls or corrections and other field or regulatory actions. A successful product liability or other claim or product recall could result in negative publicity and further harm our reputation, result in unexpected expenses and materially adversely impact our business, financial condition and results of operations. In addition, we may be subject to claims resulting from false identification of genetic variations or other misdiagnoses made in connection with our tests. Litigating any such claims could be costly. We could expend significant funds during any litigation proceeding brought against us. Further, if a court were to require us to pay damages to a plaintiff or if we agree to pay damages in settlement of a claim, the amount of such damages could materially adversely affect our business, financial condition and results of operations.
The imposition of new healthcare legislation could significantly impact our earnings and negatively impact our results of operations and cash flows.
In March 2010, significant reforms to the healthcare system in the form of the Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act of 2010 were adopted as law in the United States. This legislation includes provisions that, among other things, reduce or limit Medicare reimbursement, require all individuals to have health insurance (with limited exceptions) and impose increased taxes. Specifically, this legislation requires the medical device industry to subsidize healthcare reform in the form of a 2.3% excise tax on U.S. sales of most medical devices, including our products, beginning in January 2013. This significant increase in the tax burden on our industry could have a material, negative impact on our results of operations and our cash flows, and directly and adversely impact our gross profit. Other elements of this legislation, such as comparative effectiveness research, new requirements to report certain financial arrangements with physicians and teaching hospitals, an independent payment advisory board, payment system reforms, including shared savings pilots, and other provisions, could meaningfully change the way health care is developed and delivered, and may materially impact numerous aspects of our business. Because many parts of this legislation remain subject to implementation, the long-term impact on us is uncertain. However, this new legislation, or any future legislation, could reduce medical procedure volumes, lower reimbursement for our products, and impact the demand for our products or the prices at which we sell our products. In addition, various healthcare reform proposals have also emerged at the state level. We cannot predict with certainty what healthcare initiatives, if any, will be implemented at the state level, or what the ultimate effect of federal healthcare reform or any future legislation or regulation may have on us or on our customers’ purchasing decisions regarding our products and services.
We are subject, directly or indirectly, to federal and state healthcare fraud and abuse and other laws and regulations and could face substantial penalties if we are unable to fully comply with such laws.
While we do not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors, many healthcare laws and regulations apply to our business. For example, we are subject to healthcare fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business. These healthcare laws and regulations include, for example:
| • | the federal Anti-Kickback Law, which prohibits, among other things, persons or entities from soliciting, receiving, offering or providing remuneration, directly or indirectly, in return for or to induce either the referral of an individual for, or the purchase order or recommendation of, any item or services for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs; |
37
Table of Contents
| • | federal false claims laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, and which may apply to entities like us to the extent that our interactions with customers may affect their billing or coding practices; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, which established new federal crimes for knowingly and willfully executing a scheme to defraud any healthcare benefit program or making false statements in connection with the delivery of or payment for healthcare benefits, items or services, and which imposed certain requirements relating to privacy, security, and transmission of individually identifiable health information; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor, including commercial insurers. |
If our operations, or our sales techniques or product placement strategies, are found to be in violation of, or to encourage or assist the violation by third parties of, any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs, and the curtailment or restructuring of our operations. We currently do not have a comprehensive compliance program concerning our relationships with healthcare providers and we may need to implement such a program as our operations grow. Any penalties, damages, fines, exclusions, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that many of these laws are broad and their provisions are open to a variety of interpretations. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
If third-party payors do not reimburse our customers for the use of our clinical diagnostic products or if they reduce reimbursement levels, our ability to sell our products will be harmed.
We sell our products primarily to reference laboratories and hospital laboratories, substantially all of which receive reimbursement for the healthcare services they provide to their patients from third-party payors, such as Medicare and Medicaid, private insurance plans and managed care programs. These third-party payors may deny reimbursement if they determine that a medical product was not used in accordance with cost-effective treatment methods, as determined by the third-party payor, or was used for an unapproved indication. Third-party payors also may refuse to reimburse for procedures and devices deemed to be experimental, or medically unnecessary.
In the United States, the American Medical Association assigns specific Current Procedural Terminology, or CPT, codes, which are necessary for reimbursement of diagnostic tests. Once the CPT code is established, the Centers for Medicare and Medicaid Services establish reimbursement payment levels and coverage rules under Medicaid and Medicare, and private payors independently establish rates and coverage rules. Although there are previously assigned CPT codes for the tests that we offer and our tests are therefore approved for reimbursement by Medicare and Medicaid as well as most third-party payors, certain of our future products may not be approved for reimbursement.
Third-party payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for medical products and services. Increasingly, Medicare, Medicaid and other third-party payors are challenging the prices charged for medical services, including clinical diagnostic tests. Levels of reimbursement may decrease in the future, and future legislation, regulation or reimbursement policies of third-party payors may adversely affect the demand for and price levels of our products. If our customers are not reimbursed for our products, they may reduce or discontinue purchases of our products, which would cause our revenue to decline.
38
Table of Contents
Our ability to use our net operating loss and research and development credit carryforwards may be subject to limitations.
As of September 30, 2012, we had approximately $66.1 million of net operating loss carryforwards and $1.1 million or research and development credit carryforwards for U.S. federal tax purposes. As of December 31, 2011, we had approximately $67.9 million of net operating loss carryforwards and $1.0 million of research and development credit carryforwards for U.S. federal income tax purposes. Realization of any tax benefit from our carryforwards is dependent on: (i) our ability to generate future taxable income, and (ii) the absence of certain “ownership changes” of our common stock. An “ownership change,” as defined in the applicable federal income tax rules, could place significant limitations, on an annual basis, on the amount of our future taxable income that may be offset by our carryforwards. Such limitations, in conjunction with the net operating loss expiration provisions, could effectively eliminate our ability to utilize a substantial portion of our carryforwards.
Although we have not conducted a study to make this determination, it is possible that we have incurred one or more “ownership changes” in the past, in which case our ability to use our carryforwards may be limited. In addition, the issuance of shares of our common stock (including due to this offering) could cause an “ownership change” which could also limit our ability to use our carryforwards. Other issuances of shares of our common stock which could cause an “ownership change” include the issuance of shares of common stock upon future conversion or exercise of outstanding options and warrants.
We may be unsuccessful in our long-term goal of expanding our product offerings outside the United States.
For the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2011 and 2012, 52%, 22%, 32% and 6%, respectively, of our net revenue was from customers in countries outside the United States, including countries in Europe, Asia, the Middle East and South America. We are dependent on third-party distributor relationships in these countries to market, sell and provide customer and technical support for our products. Distributors may not commit the necessary resources to market, sell and support our products to the level of our expectations. If distributors do not perform adequately, or we are unable to locate distributors in particular geographic areas, our ability to realize long-term international revenue growth would be materially adversely affected.
Furthermore, there are risks inherent in doing business internationally, including:
| • | imposition of governmental controls and changes in laws, regulations or policies; |
| • | political and economic instability; |
| • | changes in U.S. and other national government trade policies affecting the markets for our products; |
| • | changes in regulatory practices, tariffs and taxes; and |
| • | currency exchange rate fluctuations, devaluations and other conversion restrictions. |
Any of these factors could have a material adverse effect on our business, results of operations or financial condition.
Manufacturing risks, shortages and inefficiencies may adversely affect our ability to produce products.
We must manufacture or engage third parties to manufacture components of our products in sufficient quantities and on a timely basis, while maintaining product quality and acceptable manufacturing costs and complying with regulatory requirements. In determining the required quantities of our products and the manufacturing schedule, we must make significant judgments and estimates based on historical experience, inventory levels, current market trends and other related factors. Because of the inherent nature of estimates, there could be significant differences between our estimates and the actual amounts of products we require.
39
Table of Contents
We may also experience unforeseen technical complications in the processes we use to develop, manufacture, customize or receive orders for our products. Such complications could materially delay or limit the use of products we attempt to commercialize, substantially increase the anticipated cost of our products or prevent us from implementing our processes at appropriate quality and scale levels, thereby causing our business to suffer.
We have relationships with suppliers who are sources of raw materials and components for our product offerings. We do not have long-term agreements with our suppliers and have not arranged for alternate suppliers. It may be difficult to find alternate suppliers in a timely manner and on terms acceptable to us. Additionally, the availability of some components of the INFINITI system may be limited as only a few outside vendors produce them. In the event that we become subject to a shortage of components or raw materials, our business, financial condition and results of operations could be materially adversely affected.
We have limited experience with the specialized film coating process needed to produce our BioFilmChip microarrays, and there are limited alternative sources for this aspect of our manufacturing process.
Our BioFilmChip microarrays are formed by coating polyester film with several layers of emulsion. To date, we have outsourced the special film coating process needed to produce our BioFilmChip microarrays. In connection with the establishment of manufacturing operations at our Vista, California facility, we purchased the film coating equipment required to conduct this process ourselves. We have not yet installed this equipment and have no experience as a company conducting the film coating process. If we are unable to successfully operate and maintain this equipment and produce the coated film necessary for our BioFilmChip microarrays, we will have to continue to outsource this operation to a third party. Film coating capacity is limited and is becoming less available in the marketplace as film vendors move away from analog formats and focus increasingly on digital formats. Although we believe we have adequate coated film for the foreseeable future, we cannot guarantee that we would be able to obtain an alternative supply if we are unable to manufacture the coated film ourselves.
We will need to expand manufacturing capacity by ourselves or with third parties.
We will need either to continue to build internal manufacturing capacity or contract with one or more manufacturing partners, or both. We currently rely solely on internal manufacturing of our end products. Due to the complexity of our manufacturing process and the advanced technologies we employ, we may encounter difficulties in manufacturing our end products. We may not be able to build manufacturing capacity internally or find one or more suitable manufacturing partners, or both, to meet the volume, regulatory and quality requirements necessary to be successful in the market. If our products do not consistently meet our customers’ performance expectations or the requirements of the FDA and authorities in other countries, we may be unable to generate sufficient revenue to become profitable. Significant additional resources, implementation of additional manufacturing equipment and changes in our manufacturing processes and organization may be required for the scale-up of each new product prior to commercialization or to meet increasing customer demand once commercialization begins, and this work may not be successfully or efficiently completed. Any delay in establishing or inability to expand our manufacturing capacity could delay our ability to develop or sell our products, which would result in lost revenue and materially adversely harm our business, financial condition and results of operations.
Our business and future operating results may be adversely affected by events outside of our control.
We develop and manufacture the INFINITI system in our facility located in Vista, California. Any damage to our facilities or the manufacturing equipment we use would be costly and could require substantial lead-time to repair or replace. Our business and operating results may be harmed due to interruption of our manufacturing by events outside of our control, including earthquakes and fires. Other possible disruptions may include power loss and telecommunications failures. In the event of a disruption, we may lose customers and we may be unable to regain those customers thereafter. Our insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all.
40
Table of Contents
We use hazardous chemicals and biological materials in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our research and development and manufacturing processes involve the controlled use of hazardous materials, including chemicals, and biological materials. Our operations produce hazardous waste products. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, and our liability may exceed our insurance coverage and our total assets. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of these hazardous materials and specified waste products, as well as the discharge of pollutants into the environment and human health and safety matters. Compliance with environmental laws and regulations may be expensive, and may impair our research, development and production efforts. If we fail to comply with these requirements, we could incur substantial costs, including civil or criminal fines and penalties, cleanup costs, or capital expenditures for control equipment or operational changes necessary to achieve and maintain compliance. In addition, we cannot predict the impact on our business of new or amended environmental laws or regulations, or any changes in the way existing and future laws and regulations are interpreted and enforced.
We will incur increased costs as a result of being a public company, and our management will be required to devote substantial time to new compliance matters.
We will incur significant legal, accounting, and other expenses as a public company, including costs resulting from public company reporting obligations under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the rules and regulations regarding corporate governance practices, including those under the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, and the listing requirements of the NASDAQ Global Market.
Our management and other personnel will need to devote a substantial amount of time to ensure that we comply with all of these requirements. Moreover, despite recent reforms made possible by the JOBS Act, the reporting requirements, rules, and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly, particularly after we are no longer an “emerging growth company.” Any changes that we make to comply with these obligations may not be sufficient to allow us to satisfy our obligations as a public company on a timely basis, or at all.
We also expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. These factors could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, particularly to serve on our audit and compensation committees, or as executive officers.
As a public company, we will be subject to the requirements related to Section 404 of the Sarbanes-Oxley Act. If we are unable to comply with these requirements in a timely manner, it may affect the reliability of our internal control over financial reporting and negatively impact our business and stock price.
Beginning with our annual report on Form 10-K for the year ending December 31, 2013, we will be required to furnish a report by management on the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. In addition, our independent registered public accounting firm will be required to attest to the effectiveness of our internal controls over financial reporting beginning with our annual report on Form 10-K following the later of the year following our first annual report required by the Securities and Exchange Commission, or the SEC, and the date on which we are no longer an “emerging growth company.” We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of this offering, (b) in which we have total annual gross revenue of at least $1.0 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our
41
Table of Contents
common stock that is held by non-affiliates exceeds $700.0 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
The process of designing and implementing effective internal controls and procedures is a continuous effort that requires us to anticipate and react to changes in our business and economic and regulatory environments. We cannot assure you that our efforts will be adequate to satisfy our reporting obligations as a public company or that we will be able to successfully complete the procedures and certification and attestation requirements related to Section 404 of the Sarbanes-Oxley Act or that we or our independent registered public accounting firm will not identify additional material weaknesses or significant deficiencies in our internal control over financial reporting. If we identify any reason that we cannot certify as to the effectiveness of our internal control over financial reporting, we could incur additional costs remedying the cause of our failure to certify as to the effectiveness of our internal control over financial reporting, and our reputation, investor perceptions of us and the price of our common stock could be materially adversely affected and you may not be able to rely on our financial statements.
Our international operations are subject to trade and anti-corruption laws and regulations.
Due to the international scope of our operations, we are subject to a complex system of import- and export-related laws and regulations, including U.S. regulations issued by the Bureau of Industry and Security and the Office of Foreign Assets Control, as well as the counterparts of these agencies in other countries. Any alleged or actual violations of these U.S. and foreign regulations may subject us to government scrutiny, investigation and civil and criminal penalties, and may limit our ability to import or export our products or to provide services outside the United States. We cannot predict the nature, scope or effect of future regulatory requirements to which our operations might be subject or the manner in which existing laws might be administered or interpreted.
In addition, the U.S. Foreign Corrupt Practices Act and similar foreign anti-corruption laws generally prohibit companies and their intermediaries from making improper payments or providing anything of value to improperly influence foreign government officials for the purpose of obtaining or retaining business, or obtaining an unfair advantage. Recent years have seen a substantial increase in the global enforcement of anti-corruption laws. Our international distributors are domiciled in areas of the world with laws, rules and business practices that differ from those in the United States. As a result, we face the reputational and legal risk that our international distributors will violate applicable laws, rules and business practices. If we or our international distributors are determined to be in violation of these laws, rules and business practices, it may result in severe criminal or civil sanctions for us or our distributors, could disrupt our business, and could result in an adverse effect on our reputation, business and results of operations or financial condition.
Risks Relating to this Offering and our Common Stock
There has been no prior market for our common stock, and an active trading market may not develop.
Prior to this offering, there has been no public market for our common stock. An active trading market may not develop following the closing of this offering or, if developed, may not be sustained. The lack of an active market may impair your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value and increase the volatility of your shares. An inactive market may also impair our ability to raise capital by selling shares and may impair our ability to acquire other companies or technologies by using our shares as consideration.
We are an “emerging growth company” and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, our common stock may be less attractive to investors.
We are an “emerging growth company” as defined in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not
42
Table of Contents
emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an emerging growth company. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of this offering, (b) in which we have total annual gross revenue of at least $1.0 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700.0 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
Under Section 107(b) of the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
The price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for investors purchasing shares in this offering.
The initial public offering price for the shares of our common stock sold in this offering will be determined by negotiation between the representatives of the underwriters and us. This price may not reflect the market price of our common stock following this offering. In addition, the market price of our common stock is likely to be highly volatile and may fluctuate substantially due to many factors, including:
| • | actual or anticipated fluctuations in our results of operations; |
| • | variance in our financial performance from the expectations of market analysts; |
| • | conditions and trends in the markets we serve; |
| • | changes in our pricing policies or the pricing policies of our competitors; |
| • | legislation or regulatory policies, practices or actions; |
| • | the announcements of significant new products, contracts, acquisitions or strategic alliances by us or our competitors; |
| • | the commencement or outcome of litigation; |
| • | our ability to repay remaining indebtedness after completion of this offering; |
| • | our sale of common stock or other securities in the future or sales of our common stock by our significant stockholders; |
| • | changes in market valuation or earnings of our competitors; |
| • | the trading volume of our common stock; |
| • | the loss of key personnel; |
| • | changes in the estimated future size and growth rate of our markets; |
| • | general economic conditions; and |
| • | general volatility of the stock market. |
43
Table of Contents
These broad market and industry factors may materially harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted against that company. Such litigation, if instituted against us, could result in substantial costs and a diversion of management’s attention and resources, which could materially harm our financial condition and results of operations.
If equity research analysts do not publish research or reports about our business or if they issue unfavorable commentary or downgrade our common stock, the price of our common stock could decline.
The trading market for our common stock will rely in part on the research and reports that equity research analysts publish about our business and us. The price of our stock could decline if one or more equity research analysts downgrade our stock or if those analysts issue other unfavorable commentary or cease publishing reports about our business or us.
We currently do not intend to pay dividends on our common stock and, consequently, your only opportunity to achieve a return on your investment is if the price of our common stock appreciates.
We currently do not plan to declare dividends on shares of our common stock for the foreseeable future. See “Dividend Policy” for more information. Consequently, your only opportunity to achieve a return on your investment in our company will be if the market price of our common stock appreciates and you sell your shares at a profit. There is no guarantee that the price of our common stock that will prevail in the market after this offering will ever exceed the price that you pay. In addition, the lack of dividends may limit the number of potential buyers of our common stock.
Future sales of our common stock may depress our share price.
After this offering, we will have 16,746,791 shares of common stock outstanding, based upon our outstanding shares as of December 31, 2012. Sales of substantial amounts of our common stock in the public market following this offering, or the perception that these sales may occur, could cause the market price of our common stock to decline. After the lock-up agreements pertaining to this offering expire, additional stockholders will be able to sell their shares in the public market, subject to legal restrictions on transfer. As described in “Shares Eligible for Future Sale — Registration Rights,” we also have granted certain of our security holders registration rights which, subject to certain limitations, will permit them to cause us to file a registration statement with respect to the registrable securities held by them. As soon as practicable upon completion of this offering, we also intend to file a registration statement covering shares of our common stock issuable upon exercise of outstanding stock options or reserved for future issuance under our equity incentive and stock purchase plans. We may also sell additional shares of common stock in subsequent public offerings, which may materially adversely affect market prices for our common stock. See “Shares Eligible for Future Sale” for more information.
We have broad discretion in the use of the net proceeds from this offering, and our investment of these proceeds may not yield a favorable return.
Our management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described in “Use of Proceeds.” Accordingly, you will have to rely upon the judgment of our management with respect to the use of the proceeds. Our management may spend a portion or all of the net proceeds from this offering in ways that our stockholders may not desire or that may not yield a significant return or any return at all. Of the net proceeds from this offering, we intend to use approximately $5.7 million to satisfy outstanding accounts payable to advisers and service providers incurred in connection with our prior capital raising activities conducted from 2008 through 2010, approximately $1.1 million to pay past due amounts owed to our landlord, and approximately $17.7 million to retire existing debt obligations. The payment of these amounts will not enhance our business operations or increase our revenues. The failure by our management to effectively apply the remaining net proceeds from this offering could harm our business. Pending their use, we may also invest the net proceeds from this offering in a manner that does not produce income or that loses value.
44
Table of Contents
As a new investor, you will experience immediate and substantial dilution as a result of this offering.
The initial public offering price of our common stock will be considerably more than the net tangible book value per share of our outstanding common stock. Accordingly, investors purchasing shares of common stock in this offering will:
| • | pay a price per share that substantially exceeds the value of our assets after subtracting liabilities; and |
| • | contribute 48.4% of the total amount invested to fund our company, but will own only 35.8% of the shares of common stock outstanding after this offering and the use of proceeds therefrom. |
To the extent that outstanding stock options and warrants are exercised, there will be further dilution to new investors. See “Dilution” for more information.
Certain provisions of our corporate governing documents and Delaware law could make an acquisition of our company more difficult.
Our certificate of incorporation and bylaws contain provisions that may make the acquisition of our company more difficult without the approval of our board of directors. For example, our certificate of incorporation and bylaws:
| • | authorize the issuance of preferred stock that can be created and issued by our board of directors without prior stockholder approval, commonly referred to as “blank check” preferred stock, with rights senior to those of our common stock; |
| • | limit the persons who can call special stockholder meetings; |
| • | provide that a supermajority vote of our stockholders is required to amend our bylaws and some portions of our certificate of incorporation; |
| • | establish advance notice requirements to nominate persons for election to our board of directors or to propose matters that can be acted on by stockholders at stockholder meetings; |
| • | do not provide for cumulative voting in the election of directors; and |
| • | provide for the filling of vacancies on our board of directors between annual meetings by action of a majority of the directors and not by the stockholders. |
These and other provisions in our organizational documents could allow our board of directors to affect your rights as a stockholder in a number of ways, including making it more difficult for stockholders to replace members of the board of directors. Because our board of directors is responsible for approving the appointment of members of our management team, these provisions could in turn affect any attempt to replace the current management team. These provisions could also limit the price that investors would be willing to pay in the future for shares of our common stock.
In addition, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, or DGCL, which, subject to certain exceptions, impose certain restrictions on mergers and other business combinations between any holder of 15% or more of our common stock and us. See “Description of Capital Stock — Provisions of our Certificate of Incorporation and Bylaws and Delaware Law that May Have an Anti-Takeover Effect.”
45
Table of Contents
Special Note Regarding Forward-Looking Statements
This prospectus contains forward-looking statements that reflect our current views with respect to, among other things, future events or our future financial performance. All statements in this prospectus other than statements of historical fact, including statements regarding future events, our future financial performance, business strategy and plans, predictions about our markets, and objectives of management for future operations, are forward-looking statements.
In some cases, you can identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “might,” “plans,” “potential,” “predicts,” “should,” “will” or “would,” or the negative of these terms or other comparable terminology. Forward-looking statements used in this prospectus address topics such as, among other things:
| • | our business strategy; |
| • | our development of new products; |
| • | our anticipated growth strategies; |
| • | anticipated trends in our business, trends in the market for our products and trends in the adoption of new technologies; |
| • | competition and competitive factors or trends in our market; |
| • | uncertainty regarding our future operating results, including our projected sales, net sales, financial results, cash flows, pricing, and similar items; |
| • | our intended use of proceeds; |
| • | our ability to continue to control costs and maintain quality; |
| • | anticipated changes in our expenses over future periods; |
| • | the impact of U.S. health care reform legislation and other health care policy changes, including the imposition of significant new taxes on medical device makers in the form of a 2.3% excise tax on U.S. medical device sales beginning in January 2013; |
| • | the protection or acquisition of, investment in, or legal proceedings regarding, our intellectual property; |
| • | our ability to obtain FDA or other regulatory clearances or approvals; |
| • | the regulatory environment for our products and our business, and our ability to comply with and adapt to such environment; and |
| • | the plans, objectives, expectations and intentions expressed in this prospectus that are not historical facts. |
These statements are only predictions. Actual events or results may differ materially. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors.” We cannot guarantee future results, levels of activity, performance or achievements. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, or to conform these statements to actual results or changes in our expectations.
Moreover, we operate in a competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
46
Table of Contents
Market, Industry and Other Data
Unless otherwise indicated, information contained in this prospectus concerning our industry and the market segments in which we operate, including our general expectations and market position, market opportunity and market size, is based on information from various sources, on assumptions that we have made that are based on that information and other similar sources and on our knowledge of the markets for our products. This information involves a number of assumptions and limitations, and you are cautioned not to give undue weight to or reliance on such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the industry in which we operate is necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors” and elsewhere in this prospectus. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
47
Table of Contents
We estimate that our net proceeds (after deducting underwriting discounts and commissions payable to the underwriters and our estimated offering expenses) from this offering will be approximately $53.7 million ($62.0 million if the underwriters exercise their overallotment option in full), based upon an assumed initial public offering price of $10.00 per share, which is the midpoint of the range listed on the cover page of this prospectus. A $1.00 increase or decrease in the assumed initial public offering price of $10.00 per share would increase or decrease, as applicable, the net proceeds to us from this offering by approximately $5.6 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions and our estimated offering expenses. Similarly, an increase or decrease in the number of shares we sell in the offering will increase or decrease, as applicable, our net proceeds by an amount equal to such number of shares multiplied by the public offering price, less underwriting discounts and commissions.
From the anticipated net proceeds from this offering, we anticipate that we will use (i) approximately $17.7 million to repay the principal and interest under our outstanding promissory notes, (ii) approximately $10.0 million to fund the expansion of our sales force, enhance our international distributor network, and increase our marketing and promotional activity and business development efforts, (iii) approximately $10.0 million in 2013 and 2014 in support of a PMA for our HPV-HR tests and of 510(k) and CE mark studies and submissions for several tests associated with womens’ health and personalized medicine, (iv) approximately $3.0 million to fund research and development activities to add new or enhanced tests to our menu, (v) approximately $3.0 million to fund the expansion of our manufacturing capacity and efficiency, including purchasing automation equipment, (vi) approximately $5.7 million to satisfy outstanding accounts payable to advisors and service providers incurred in connection with certain of our prior capital raising activities conducted from 2008 through 2010, and (vii) approximately $1.1 million to satisfy past due amounts owed to our landlord. We anticipate that we will use the remainder of the net proceeds from this offering for additional working capital and general corporate purposes.
As of December 31, 2012, there was an aggregate of $17.5 million of principal and accrued interest under outstanding promissory notes of which approximately $2.2 million in principal amount was in default. For each additional day that the notes remain outstanding, we were required to pay approximately $4,300 of additional interest under the notes. See “Note 3. Promissory Notes” included in our financial statements included as part of this prospectus. As of December 31, 2012, there was an aggregate of $1.1 million of principal and accrued interest outstanding under the notes we have issued to our directors, executive officers and holders of more than 5% of our common stock. See “Certain Relationships and Related Persons Transactions — Promissory Notes Financings.” In November 2012, the holders of $22.0 million in aggregate principal amount of and accrued interest on our outstanding subordinated promissory notes that were then past due or scheduled to come due in the immediate future exchanged these promissory notes for an aggregate of 4,287,074 shares of our Series NC Convertible Preferred Stock and $14.4 million in aggregate principal amount of new promissory notes due November 2015. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources — Promissory Notes”. We do not have sufficient cash to satisfy our indebtedness obligations absent the completion of this offering. Under the terms of our November 2012 promissory notes, we are required to use at least 50% of the net proceeds from this offering to repay our indebtedness. As a result, we intend to use $17.7 million of the net proceeds of this offering to repay all of the indebtedness owed under our outstanding promissory notes.
The expected use of the net proceeds of this offering represents our current intentions based upon our present plans and business condition. The amounts and timing of our actual expenditures may vary significantly and will depend upon numerous factors, such as the level of research and development investment required by our business (including for clinical studies and trials), cash flows from operations, the anticipated growth of our business and other factors. Pending the allocation of the net proceeds of this offering, we intend to invest the net proceeds in short-term, interest-bearing obligations, investment grade instruments, and certificates of deposit or guaranteed obligations of the U.S. government.
48
Table of Contents
We do not anticipate paying any cash dividends after this offering and for the foreseeable future. Instead, we anticipate that all of our earnings on our common stock will be used to provide working capital, to support our operations and to finance the growth and development of our business. Any future determination relating to dividend policy will be made at the discretion of our board of directors and will depend on our future earnings, capital requirements, financial condition, future prospects, financing arrangements, applicable Delaware law, which provides that dividends are only payable out of surplus or current net profits, and other factors our board of directors deems relevant.
49
Table of Contents
The following table sets forth our capitalization as of September 30, 2012 (i) on an actual basis, (ii) on a pro forma basis to reflect the conversion of all outstanding shares of our Series A, Series B, Series C, Series D, Series E and Series NC Convertible Preferred Stock into 7,891,020 shares of our common stock and (iii) on a pro forma as adjusted basis to additionally reflect the sale of 6,000,000 shares of our common stock in this offering at an assumed initial offering price of $10.00 per share, the midpoint of the range on the cover page of this prospectus, and after deducting underwriting discounts and commissions and our estimated offering expenses, and the application of approximately $17.7 million of the net proceeds of this offering to repay principal and interest under certain of our outstanding promissory notes, including notes to related parties.
The pro forma and pro forma as adjusted information below is illustrative only and our capitalization following the completion of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this table together with “Use of Proceeds,” “Selected Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Description of Capital Stock” and our financial statements and the related notes appearing elsewhere in this prospectus.
| As of September 30, 2012 | ||||||||||||
| Actual | Pro forma | Pro
forma as adjusted (1) |
||||||||||
| (unaudited) | ||||||||||||
| (in thousands, except share and per share data) | ||||||||||||
| Senior and subordinated promissory notes (2) |
$ | 20,784 | $ | 17,057 | $ | — | ||||||
| Convertible preferred stock, $0.01 par value, 30,000,000 authorized (3) |
— | — | — | |||||||||
| Series A Convertible Preferred Stock, $0.01 par value, 1,589,600 authorized, 1,579,227 issued and outstanding, actual, 0 issued and outstanding, pro forma and pro forma as adjusted |
4,990 | — | — | |||||||||
| Series B Convertible Preferred Stock, $0.01 par value, 4,515,858 authorized, 4,468,369 issued and outstanding, actual, 0 issued and outstanding, pro forma and pro forma as adjusted |
12,288 | — | — | |||||||||
| Series C Convertible Preferred Stock, $0.01 par value, 6,850,000 authorized, 6,417,680 issued and outstanding, actual, 0 issued and outstanding, pro forma and pro forma as adjusted |
17,647 | — | — | |||||||||
| Series D Convertible Preferred Stock, $0.01 par value, 3,423,258 authorized, 3,423,258 issued and outstanding, actual, 0 issued and outstanding, pro forma and pro forma as adjusted |
11,126 | — | — | |||||||||
| Series E Convertible Preferred Stock, $0.01 par value, 5,500,000 authorized, 732,555 issued and outstanding, actual, 0 issued and outstanding, pro forma and pro forma as adjusted |
1,381 | — | — | |||||||||
| Series NC Convertible Preferred Stock, $0.01 par value, 4,318,363 authorized, 0 issued and outstanding, actual, pro forma and pro forma as adjusted |
— | — | — | |||||||||
| Stockholders’ equity (deficit): |
||||||||||||
| Common stock, $0.01 par value, 220,000,000 authorized, 2,673,358 issued and outstanding, actual, 10,564,378 issued and outstanding, pro forma and 16,564,378 issued and outstanding, pro forma as adjusted |
81 | 326 | 386 | |||||||||
| Additional paid-in capital (4) |
15,251 | 69,946 | 123,536 | |||||||||
| Accumulated deficit |
(92,412 | ) | (92,412 | ) | (92,412 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ equity/(deficit) |
(77,080 | ) | (22,140 | ) | 31,510 | |||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | (8,864 | ) | $ | (5,083 | ) | $ | 31,510 | ||||
|
|
|
|
|
|
|
|||||||
50
Table of Contents
| (1) | A $1.00 increase or decrease in the assumed initial public offering price of $10.00 per share of our common stock in this offering would increase or decrease, as applicable, each of additional paid-in capital, total stockholders’ equity (deficit) and total capitalization by $5.6 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and our estimated offering expenses. Similarly, an increase or decrease in the number of shares we sell in the offering will increase or decrease, as applicable, our net proceeds by an amount equal to such increased or decreased number of shares multiplied by the public offering price, less underwriting discounts and commissions and our estimated offering expenses. |
| (2) | Amounts include principal only. As of September 30, 2012, approximately $14.8 million in aggregate principal amount of our outstanding promissory notes payable was in payment default. The pro forma and pro forma as adjusted information presented as of September 30, 2012 gives effect to the exchange, in November 2012, of $22.0 million in aggregate principal amount of and accrued interest on our outstanding subordinated promissory notes that were then past due or scheduled to come due in the immediate future for an aggregate of 4,287,074 shares of our Series NC Convertible Preferred Stock and $14.4 million in aggregate principal amount of new promissory notes due November 2015. |
| (3) | Our convertible preferred stock has been classified as temporary equity on our balance sheets instead of stockholders’ deficit due to the possibility of the occurrence of certain change in control events that are outside of our control, including our sale or transfer of control, which trigger the rights of holders of the convertible preferred stock to cause its redemption. Accordingly, these shares are considered contingently redeemable. |
| (4) | Excludes, as of September 30, 2012, 1,769,712 shares of common stock issuable upon exercise of options outstanding at a weighted average exercise price of $4.82 per share, 2,794,696 shares of common stock issuable upon exercise of warrants outstanding at a weighted average exercise price of $10.73 per share and 381,300 shares of our preferred stock issuable upon exercise of warrants outstanding at a weighted average exercise price of $3.56 per share. |
51
Table of Contents
As of September 30, 2012, we had a net tangible book deficit of $77.1 million, or approximately $9.51 per share of common stock, not taking into account the conversion of all outstanding series of convertible preferred stock into common stock. Net tangible book deficit per share represents the amount of our total tangible assets less total liabilities, divided by the number of common shares outstanding.
Included in our total liabilities as of September 30, 2012 is approximately $24.7 million of indebtedness and accrued interest under promissory notes, of which approximately $14.8 million in principal amount was in payment default. In November 2012, the holders of $22.0 million in aggregate principal amount of and accrued interest on our outstanding subordinated promissory notes that were then past due or due to come owing in the immediate future exchanged these promissory notes for an aggregate of 4,287,074 shares of our Series NC Convertible Preferred Stock and $14.4 million in aggregate principal amount of new promissory notes due November 2015. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Promissory Notes”.
After giving effect to the indebtedness restructuring described immediately above, and to the conversion of all outstanding series of convertible preferred stock, including our Series NC Convertible Preferred Stock, into common stock as of September 30, 2012, we would have had a net tangible book deficit of $22.1 million, or $2.06 per share of common stock. Pro forma net tangible book deficit per share represents the amount of our total tangible assets less our total liabilities, after giving effect to the indebtedness restructuring described above, divided by the number of shares of common stock outstanding as of September 30, 2012 after giving effect to our reverse stock split effected in January 2013 and the conversion of all outstanding series of convertible preferred stock into shares of common stock, which will occur immediately prior to the completion of this offering.
After giving effect to the sale by us of shares of common stock in this offering at an assumed initial public offering price of $10.00 per share, which is the midpoint of the range listed on the cover page of this prospectus, and after deducting underwriting discounts and commissions and our estimated offering expenses, our pro forma net tangible book value as of September 30, 2012 would have been approximately $31.5 million, or approximately $1.88 per share. This amount represents an immediate increase in pro forma net tangible book value of $3.94 per share to our existing stockholders and an immediate dilution in pro forma net tangible book value of approximately $8.12 per share to new investors purchasing shares of common stock in this offering at the assumed initial public offering price. We determine dilution to new investors participating in this offering by subtracting the pro forma net tangible book value per share after this offering from the amount of cash that a new investor paid for a share of common stock in this offering.
Assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and our estimated offering expenses, a $1.00 increase or decrease in the assumed initial public offering price of $10.00 per share would increase or decrease, as applicable, the pro forma net tangible book value by $0.34 per share to our existing stockholders, and the dilution in pro forma net tangible book value by $0.34 per share to new investors participating in this offering.
The following table illustrates this dilution on a per share basis:
| Assumed initial public offering price per share |
$ | 10.00 | ||||||
| Pro forma net tangible book value per share as of September 30, 2012 |
$ | (2.06 | ) | |||||
| Increase in pro forma net tangible book value per share attributable to this offering |
$ | 3.94 | ||||||
| Pro forma net tangible book value per share after this offering |
$ | 1.88 | ||||||
|
|
|
|||||||
| Dilution in pro forma net tangible book value per share to new stockholders |
$ | 8.12 | ||||||
|
|
|
The following table summarizes, as of September 30, 2012, the differences for our existing stockholders and new investors in this offering, with respect to the number of shares of common stock purchased from us, the total
52
Table of Contents
consideration paid and the average price per share paid at an assumed initial public offering price of $10.00 per share, which is the midpoint of the range listed on the cover page of this prospectus, after deducting underwriting discounts and commissions and our estimated offering expenses. The following table assumes the conversion of all series of convertible preferred stock, including our Series NC Convertible Preferred Stock, into common stock, which will be effected immediately prior to the completion of this offering.
| Shares purchased | Total consideration | Average price per share |
||||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||||
| Existing stockholders |
10,746,791 | 64.2 | % | $ | 57,259,713 | 51.6 | % | $ | 5.33 | |||||||||||
| New stockholders in this offering |
6,000,000 | 35.8 | % | $ | 53,650,000 | 48.4 | % | $ | 8.94 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
16,746,791 | 100 | % | $ | 110,909,713 | 100.0 | % | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
The discussion and tables above assume no exercise of the underwriters’ overallotment option. If the underwriters’ overallotment option is exercised in full:
| • | the number of shares of common stock held by existing stockholders will represent 60.9% of the total number of shares of common stock to be outstanding after this offering and the number of shares of common stock held by investors participating in this offering will represent 39.1% of the total number of shares of common stock to be outstanding after this offering; and |
| • | our adjusted pro forma net tangible book value at September 30, 2012 would have been $40.5 million, or $2.30 per share of common stock, representing an immediate increase in pro forma net tangible book value of $4.36 per share of common stock to our existing stockholders and an immediate dilution of $7.70 per share to investors participating in this offering. |
In addition, the above discussion and tables assume no exercise of stock options or warrants to purchase common stock or preferred stock. As of September 30, 2012, we had outstanding options to purchase a total of 1,769,383 shares of common stock, warrants to purchase a total of 2,794,697 shares of common stock and warrants to purchase 381,300 shares of our preferred stock convertible into 137,063 shares of common stock at a weighted average exercise price of $4.82 per share, $10.75 per share and $10.78 per share, respectively.
If all of the outstanding options and warrants as of September 30, 2012 with exercise prices at or below $10.00 per share, which is the midpoint of the range listed on the cover page of this prospectus, had been exercised (and the convertible preferred stock issuable upon the exercise of the preferred stock purchase warrants was converted into common stock), our pro forma net tangible book deficit would have been $2.79 per share of common stock, representing an immediate increase in pro forma net tangible book value of $4.85 per share of common stock and dilution in pro forma net tangible book value to investors in this offering would be $7.21 per share of common stock.
In addition, if all of the outstanding options and warrants were so exercised (and the convertible preferred stock issuable upon the exercise of the preferred stock purchase warrants was so converted) as of September 30, 2012, before deducting underwriting discounts and commissions and our estimated offering expenses, (i) existing stockholders would have purchased shares representing 70.3% of the total shares for $79.2 million, or approximately 59.6% of the total consideration paid, with an average price per share of $5.58 and (ii) using an assumed public offering price of $10.00 per share, 6,000,000 shares purchased by new stockholders in this offering would represent approximately 29.7% of the total shares for approximately $53.7 million, or approximately 40.4% of the total consideration paid.
53
Table of Contents
The selected financial data set forth below is derived in part from and should be read in conjunction with our financial statements, the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus. The statement of operations data for each of the years ended December 31, 2010, and 2011 and the statement of condition data as of December 31, 2010 and 2011 were derived from our audited financial statements appearing elsewhere in this prospectus. The statement of operations data for the nine months ended September 30, 2011 and 2012 and the statement of condition data as of September 30, 2012 were derived from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited financial data, in management’s opinion, has been prepared on the same basis as the audited financial statements and related notes included elsewhere in this prospectus, and include all adjustments, consisting only of normal recurring adjustments, that our management considers necessary for a fair presentation of the information for the periods presented. The results of operations for the years ended December 2010 and 2011 and for the nine months ended September 30, 2011 and 2012 are not necessarily indicative of the results that may be expected for the year ended December 31, 2012 or any other period.
| Years ended December 31, | Nine months ended September 30, | |||||||||||||||
| 2010 | 2011 | 2011 | 2012 | |||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands, except share and per share data) | ||||||||||||||||
| Statement of operations data: |
||||||||||||||||
| Net revenue |
$ | 7,504 | $ | 8,005 | $ | 5,166 | $ | 14,473 | ||||||||
| Cost of sales |
7,666 | 6,019 | 4,378 | 5,035 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit/(loss) |
(162 | ) | 1,986 | 788 | 9,438 | |||||||||||
| Operating expenses |
||||||||||||||||
| Research and development |
3,560 | 2,768 | 2,146 | 1,724 | ||||||||||||
| General and administrative |
5,376 | 3,360 | 2,587 | 2,775 | ||||||||||||
| Sales and marketing |
4,717 | 2,672 | 2,023 | 1,665 | ||||||||||||
| Impairment of film coating equipment |
981 | — | — | — | ||||||||||||
| Initial public offering costs — terminated offering |
1,752 | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
16,386 | 8,800 | 6,756 | 6,154 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income/(loss) from operations |
(16,548 | ) | (6,814 | ) | (5,958 | ) | 3,284 | |||||||||
| Interest expense, net |
(4,109 | ) | (3,626 | ) | (2,598 | ) | (1,913 | ) | ||||||||
| Other income/(expense), net |
36 | (2 | ) | — | 5 | |||||||||||
| Change in fair value of warrant liabilities |
926 | 445 | 434 | (170 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income/(loss) |
(19,695 | ) | (9,997 | ) | (8,132 | ) | 1,206 | |||||||||
| Net income/(loss) per share attributable to common stockholders |
||||||||||||||||
| Basic |
$ | (7.63 | ) | $ | (3.79 | ) | $ | (3.09 | ) | $ | 0.45 | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
$ | 0.13 | ||||||||||||||
|
|
|
|||||||||||||||
| Shares used to compute net income/(loss) per share attributable to common stockholders |
||||||||||||||||
| Basic |
2,581,708 | 2,634,385 | 2,633,929 | 2,651,833 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
9,148,420 | |||||||||||||||
|
|
|
|||||||||||||||
54
Table of Contents
| As of December 31, | As of September 30, | |||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| (unaudited) | ||||||||||||
| (in thousands) | ||||||||||||
| Balance sheet data: |
||||||||||||
| Cash and cash equivalents |
$ | 129 | $ | 61 | $ | 294 | ||||||
| Current assets |
3,559 | 3,749 | 7,104 | |||||||||
| Total assets |
5,924 | 5,412 | 9,826 | |||||||||
| Total debt (1) |
19,181 | 20,914 | 20,784 | |||||||||
| Convertible preferred stock (2) |
46,044 | 47,343 | 47,432 | |||||||||
| Total stockholders’ deficit |
(70,895 | ) | (79,250 | ) | (77,080 | ) | ||||||
| (1) | Amounts include principal only. As of September 30, 2012, approximately $14.8 million in aggregate principal amount of our outstanding promissory notes payable was in payment default. In November 2012, the holders of $22.0 million in aggregate principal amount of and accrued interest on our outstanding subordinated promissory notes that were then past due or scheduled to come due in the immediate future exchanged these promissory notes for an aggregate of 4,287,074 shares of our Series NC Convertible Preferred Stock and $14.4 million in aggregate principal amount of new promissory notes due November 2015. After giving effect to these actions, as of November 27, 2012 we had outstanding total indebtedness and accrued interest under promissory notes of approximately $17.4 million, of which approximately $2.9 million was due and scheduled to come due in the immediate future. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Promissory Notes”. The information presented speaks as of the dates indicated and does not reflect this restructuring of indebtedness. |
| (2) | Our convertible preferred stock had been classified as temporary equity on our balance sheets instead of in stockholders’ deficit due to the possibility of the occurrence of certain change in control events that are outside of our control, including our sale or transfer of control, which trigger the rights of holders of the convertible preferred stock to force its redemption. Accordingly, these shares are considered contingently redeemable. |
55
Table of Contents
Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and the related notes appearing elsewhere in this prospectus. This discussion may contain forward-looking statements that are based upon current expectations that involve risks and uncertainties. Our actual results and the timing of many events could differ materially from those anticipated in forward-looking statements as a result of many factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
We design, develop, manufacture and market the INFINITI molecular diagnostics system. The system includes an extensive and expanding menu of genetic tests and a family of highly automated analyzers. Our tests provide reference laboratories, hospitals and specialty clinics with genetic test results in a broad range of market segments, including personalized medicine, women’s health, oncology and infectious disease. As of December 31, 2012, our genetic test offering included 53 tests for use on our INFINITI analyzers, with more than 15 additional tests in development. We intend to continue increasing the number of tests offered in each of our target market segments, which we believe will further increase the utility of our INFINITI system to our customers.
In the United States, we offer our INFINITI analyzers for placement with customers through direct sales, monthly rental plans and our domestic placement plan. Under the domestic placement plan, an INFINITI analyzer is placed at the customer’s location at no initial cost to the customer. We expect to recover the cost of the instrument over time by generating significant recurring demand for our high-margin testing consumables. Outside the United States, we offer our INFINITI analyzers solely on a direct sale basis through our network of distributors. We plan to use a portion of the net proceeds of this offering to expand our domestic sales force and our international sales presence.
Our BioFilmChips and Intellipac Reagent Management Modules and other consumable sales are made primarily pursuant to standard purchase orders. We are able to manufacture our consumables to satisfy demand for new or rescheduled purchase orders on relatively short notice. Thus, we do not have a significant backlog.
We have in the past derived, and expect to continue to derive, a significant portion of our revenue from a few major customers. For fiscal years 2010 and 2011, we had two and three customers, respectively, that each represented between 12% and 14% of our revenue. For the nine months ended September 30, 2012, we had two customers that each represented more than 17% of our revenue, including one that represented 47% of our revenue during the period. Sales outside the United States accounted for 52%, 22% and 6% of product sales for the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2012, respectively. As of December 31, 2010, one customer accounted for 22% of our aggregate accounts receivable of $1.3 million. As of December 31, 2011, three customers accounted for 43%, 14%, and 10%, respectively, of our aggregate accounts receivable of $1.9 million. As of September 30, 2012, two customers accounted for 39% and 31%, respectively, of our aggregate accounts receivable of $3.9 million. Our concentration of net revenue with a single customer in our most recent fiscal periods is in part due to this customer being an early adopter of our INFINITI system and related technologies. We intend to take steps to attempt to alleviate our customer concentration following the completion of this offering, including by hiring additional sales and marketing personnel, which we believe will allow us to acquire additional customers and distributors and increase sales to existing customers. Our continued business relationship with these or any other major customers, and the amount of purchases and timing of payment by these or any other major customers, may be impacted by several factors beyond our control, including product offerings by our competitors, pricing pressures or the financial health of these or any other major customers. The loss of any of our major customers, or a significant decline in sales to or a delay in collection of payments from any of our major customers, could materially adversely affect our business, financial condition and results of operations. A key customer of our personalized medicine diagnostic testing products reduced its purchase volume with us during the
56
Table of Contents
three months ended December 31, 2012, which resulted in a substantial decrease in our net revenues as compared to the three months ended September 30, 2012. We believe this decrease in purchases was due to this customer having increased its purchases of equivalent products from one of our competitors. We have taken steps to recapture purchases by this key customer and we believe that the recent volume decrease was temporary, as consumables sales to this customer began to increase in December 2012; however, we can provide no assurance that either sales volume or net revenue from this key customer will return to levels we observed in the third quarter of 2012, or that either or both will not decrease, in future quarters. We also experienced a delay in the collection of payments from another key customer for the three months ended December 31, 2012, which resulted in a significant increase in our past due accounts receivable. We are in negotiations with this key customer to collect on the full amount of this past due amount; however, we do not know whether the receivable is collectable in full and how much, if any, will need to be reserved.
From our inception in April 1999 through our fiscal year ended March 31, 2005, we focused on the design and development of our microarray film technology, an initial offering of genetic tests and the INFINITI Analyzer. In 2005, we launched the INFINITI Analyzer and our first four tests. Our revenue has grown from $7.5 million in fiscal year 2010 to $8.0 million in 2011, and was $14.5 million for the nine months ended September 30, 2012. Since our inception, we have incurred significant operating losses, including net losses of $19.7 million and $10.0 million in the years ended December 31, 2010 and 2011, respectively. As of September 30, 2012, we had an accumulated deficit of $92.4 million. We may incur net operating losses for the immediate future as we seek to further expand our test menu and develop other products, conduct clinical studies and trials for certain of our tests, and invest in the continued commercialization of our INFINITI system. We also will incur substantial costs associated with the transition to operating as a public company. We are unable to predict the extent of any future losses or if we will be able to achieve sustained profitability.
Key Factors Affecting Performance
Genetic Test Consumables Sales. We generate most of our revenue through the sale of our genetic test consumables, and we expect this trend to continue for the foreseeable future. We closely track our genetic test consumables sales and the average revenue per microarray. For purposes of calculating average revenue per microarray, we consider a “microarray” to consist of a BioFilmChip and the portion of the associated Intellipac Reagent Management Module and related amplification mixture used with that BioFilmChip to perform a genetic test. We believe that taken together these metrics are key indicators of our performance. Our total genetic test consumables sales for fiscal years 2010 and 2011, and for the nine months ended September 30, 2012, were $3.9 million, $6.8 million and $13.2 million, respectively. Our average revenue per microarray for the same periods was $84, $83 and $84, respectively. Because our tests are sold at various prices, the average revenue per microarray is impacted by the mix of tests sold during each period.
We believe that while instrument placements play a role in the generation of revenue for our business, in particular in driving related test consumable sales, they do not provide a direct correlation to future revenue growth, and thus we do not view it as a key metric for our business. This is in part due to the wide variation in levels of customer utilization of our INFINITI system and in part due to the variability in throughput capability of the various models of our INFINITI analyzers. As of December 31, 2010, 2011 and 2012, we had a total of 168, 179 and 191 INFINITI analyzers placed with customers, respectively. We measure INFINITI analyzer placements based on the number of INFINITI analyzers located at our customers’ facilities at a particular measurement time.
Investment in Infrastructure and Growth. Our ability to increase our sales of genetic tests and to further penetrate our target market segments are dependent in large part on our ability to invest in our infrastructure and in our sales and marketing efforts. In order to drive further growth, we plan to hire additional sales personnel and invest in marketing our products to our target customers both in the United States and internationally. This will lead to corresponding increases in our operating expenses, and thus may negatively impact our operating results in the short term, although we anticipate that these investments will grow and improve our business over the long term. Marketing and selling to our target customers involves a substantial amount of time and effort, as it generally involves physical site visits and an investment in educating potential customers regarding the capabilities and benefits of molecular diagnostic testing in general and of our INFINITI system specifically. There can be a significant amount of time invested in marketing to a customer before that customer makes a
57
Table of Contents
purchasing decision. Once a purchasing decision is made by the customer, there can be a material amount of time invested by the customer in validating our INFINITI system. As a result, it will be difficult for us to determine whether and when our investments in sales and infrastructure will be recovered through sales to new customers or additional sales to existing customers. We will also incur substantial operating costs in connection with our transition to operating as a public company.
Utilization of our Products. Our ability to achieve sales growth is substantially dependent on the adoption of our INFINITI analyzers and utilization of our genetic tests. In order for us to continue to grow our business, it is important to acquire new customers, add new and enhanced tests and generate additional revenue from existing customers. We believe there is significant opportunity to increase the number and type of genetic tests we sell as awareness of opportunities in molecular diagnostic testing becomes more apparent to our targeted customer base. We have invested, and intend to continue to invest, significant time and effort to develop detailed marketing aids to be used by, and to educate, our customers’ sales and marketing networks. We believe that this will assist our customers in taking advantage of meaningful growth opportunities in molecular diagnostic testing in our target market segments, which may result in increased demand for our products. We have experienced some sales growth attributable to these co-marketing efforts, but the correlation is not direct either in volume or in timing of receipt of revenue. These investments in co-marketing increase our operating expenses, and it is uncertain as to whether these investments will positively impact our results going forward. We intend to invest in our sales and marketing organization and to pursue new customers in order to alleviate our current customer concentration. We believe that as more of our present and future customers realize the potential of molecular diagnostic testing generally, and pharmacogenomics specifically, our sales will continue to grow and diversify.
Regulatory Developments. Our INFINITI Analyzer and five of our genetic tests have been cleared by the U.S. Food and Drug Administration, or FDA, for sale in the United States under 510(k) clearance procedures. We have obtained Conformité Européenne, or CE, mark of conformity for 22 of our tests, which facilitates the marketing and sale of our INFINITI Analyzer, INFINITI Plus Analyzer and CE marked tests in the European Union and the European Economic Area as well as certain other international markets. The vast majority of the genetic tests that we currently sell, which together represented 87%, 92% and 96% of our net revenue for fiscal years 2010 and 2011 and the nine months ended September 30, 2012, respectively, have not been cleared or approved for clinical use in the United States by the FDA. These tests are labeled “For Research Use Only. Not for use in diagnostic procedures.” as required by FDA regulations. We refer to these tests as RUO tests.
Our decision to seek FDA approvals or clearances domestically, and CE marking internationally, for our genetic tests is made on a test-by-test basis, and is based on a variety of factors, including the regulatory environment for the use of genetic tests, the demand by our existing and target customers for particular genetic tests that have received regulatory approvals or clearances, the competitive environment for the use of genetic tests that have received regulatory approvals or clearances versus similar tests that have not, and the expense and time required to obtain regulatory approvals or clearances for particular genetic tests. If we decide to seek PMA approvals for a particular test, the time and expense involved could vary widely based on the type of test involved, although we would expect to spend on average approximately two to three years and approximately $10 to $15 million or more in seeking and obtaining such approval. If we decide to seek 510(k) clearance for a particular test, we expect to spend on average approximately six to 12 months and approximately $300,000 to $500,000 in seeking and obtaining such clearance. If we decide to seek CE marking for a particular test, we expect to spend on average approximately two to three months and approximately $100,000 in seeking and obtaining such clearance. For example, we have decided to seek a pre-market approval, or PMA, for our HPV-HR genetic tests, and we expect to spend approximately $8 million across 2013 and 2014 to conduct clinical trials and make related submissions to the FDA in connection with seeking a PMA for these tests. If the regulatory environment for the sale of RUO tests changes, we may be required to seek additional clearances or approvals from the FDA for the sale of our tests. In addition, if we determine that the diagnostic marketplace generally prefers, or requires, FDA clearance or approval of genetic tests as a condition for adoption of our INFINITI system or the purchase of our genetic tests, then we may decide to seek additional clearances or approvals from the FDA, beyond those that we currently intend to pursue in the near-term. This would require substantial time and investment, and could restrict or delay our ability to realize
58
Table of Contents
sales opportunities and/or maintain profitability. These expenditures are estimates based on information currently available to management and actual expenditures may vary from or exceed these estimates. If we invest capital to seek regulatory clearances or approvals, and do not receive these clearances or approvals, or do not realize sufficient returns on these investments in the form of additional sales, it would negatively impact our financial results and results of operations.
For a discussion of additional factors affecting our performance, see “Risk Factors.”
Financial Operations Overview
Revenue
We generate revenue from the sale of our products, which include our INFINITI analyzers and our high-margin consumables used to run tests on our analyzers, including our BioFilmChips, Intellipac Reagent Management Modules and various other disposable products consumed in the testing process. Generally, the pricing of our consumables for customers that participate in our domestic placement plans is higher than for our customers who own INFINITI analyzers; however, we may discount the price of consumables depending on the volume and nature of the products purchased by our customers. We expect that revenue generated through sales of our products in both domestic and international markets will be driven largely by the growth of the overall molecular diagnostic market, the breadth of our genetic test menu, increased utilization of our products by our existing customers, the expansion of our domestic sales force and international distribution network, the placement of additional INFINITI analyzers with new and with existing customers, and our development of additional and enhanced tests for sale in connection with our INFINITI system.
The variability of our revenue from period to period depends in part on our product sales mix of INFINITI analyzers and consumables. We anticipate that our revenue from the sale of consumables will become a larger portion of our total revenue over time as we increase our customer base and our customers increase their utilization of our INFINITI system. In the United States, we believe that for the foreseeable future our domestic placement plan will continue to account for more placements of INFINITI analyzers than direct sales.
Cost of Sales and Gross Profit
Cost of sales includes the expenses related to the production and estimated warranty costs of our various products. Included in these costs are materials, labor, general manufacturing overhead and other costs. Our general manufacturing overhead and other costs include indirect labor, facilities costs, and depreciation of capitalized manufacturing equipment. Cost of sales also includes depreciation (over a three year period) of capitalized INFINITI analyzers placed under our domestic placement plans, since we place the analyzers at the customers’ facility at no direct cost to the customer.
Research and Development
Research and development expenses include the cost of the personnel and consultants related to our activities in developing and improving our INFINITI analyzers and the development and expansion of our test menu. Also included in research and development costs are expenditures related to clinical studies and trials undertaken to validate and obtain FDA and other regulatory clearances for our tests. We expense all research and development costs in the period in which they are incurred. Continued development and improvement of our INFINITI analyzers and expansion of our test menu are key aspects of our business strategy and we expect that our research and development expenditures will increase for the foreseeable future.
We plan to incur significant expenditures in 2013 and 2014 for a clinical trial to support the submission of a PMA application to the FDA for our HPV-HR tests. We intend to submit a 510(k) notification to the FDA for our cystic fibrosis and our HPV genotyping tests in 2013. We are also conducting studies to support, and are preparing to submit, a pre-investigational device exemption, or pre-IDE, application for our CFTR and Flu A-sH1N1 tests. In addition, we have initiated clinical studies to support future submissions to the FDA for clearance of several of our other tests, including our 2D6, 3A4, 3A5, ureaplasma urealyticum, mycoplasma
59
Table of Contents
genitalium, mycoplasma hominis (Urogen Quad), bacterial vaginosis, or BV, candida vaginitis, or CV, chlymadia, gonorrhea and KRAS-BRAF genetic tests.
General and Administrative
General and administrative expenses include the costs of personnel and consultants charged to administration, finance and accounting, human resources and regulatory affairs. General and administrative expenses also include corporate, administration, accounting, patent, facility and other costs and depreciation. We expect general and administrative expenses to increase for the foreseeable future as a result of the need to hire additional personnel to support the anticipated growth of our business and as a result of additional legal and accounting expenses related to compliance with Securities and Exchange Commission and exchange listing requirements, directors’ and officers’ insurance premiums and investor relations expenses.
Sales and Marketing
Sales and marketing expenses include the costs of personnel related to selling and marketing the INFINITI system, customer support, advertising, trade show participation and other expenses. We expect our sales and marketing expenses to increase significantly for the foreseeable future as we expand our sales and marketing organization to drive the growth in sales of our consumable products, and as we increase our technical support personnel to support this additional growth.
Interest Income
Interest income includes interest earned on our cash balances. These balances are invested primarily in money market accounts.
Interest Expense
Interest expense relates to our outstanding promissory notes, which are more fully described in this prospectus under the heading “Use of Proceeds” and in “Note 3. Promissory Notes” of our financial statements that are included elsewhere in this prospectus.
Promissory Notes
We have issued promissory notes pursuant to a number of private placements since 1999. At times these promissory notes were issued with separate warrants to purchase shares of our common stock. The fair value of these warrants is allocated as a debt discount using the relative fair value allocation method determined using the Black-Scholes valuation model. The discount is amortized using the effective interest method over the term of the related promissory notes.
Change in the Fair Value of Warrant Liabilities
One of our outstanding warrants to purchase shares of our common stock provides for adjustment of the exercise price based upon subsequent issuances at prices per share that are below the exercise price of the warrant, and is classified as a liability and recorded at fair value regardless of the timing of the redemption feature or the redemption price or the likelihood of redemption. Our outstanding warrants to purchase shares of our convertible preferred stock are also classified as a liability and recorded at fair value regardless of the timing of the redemption feature or the redemption price or the likelihood of redemption. These warrants are subject to re-measurement at each balance sheet date and any change in fair value is recognized as a component of other interest income (expense), net. We will continue to adjust the liability for changes in fair value until the earlier of the conversion, exercise or expiration of the warrants.
All of our other outstanding warrants to purchase shares of our common stock that do not provide for exercise price adjustments were determined to be equity instruments and thus included in stockholders deficit upon issuance and are not subject to re-measurement.
60
Table of Contents
Results of Operations
The following tables set forth selected items in our statements of operations for the periods presented, together with the difference in amounts over such periods in dollars and in percentages. The financial data set forth below is derived in part from and should be read in conjunction with our financial statements and the related notes included elsewhere in this prospectus.
| Nine months ended September 30, |
Change | |||||||||||||||
| 2011 | 2012 | $ | % | |||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands, except for percentages) | ||||||||||||||||
| Net revenue |
$ | 5,166 | $ | 14,473 | $ | 9,307 | 180.2 | % | ||||||||
| Cost of sales |
4,378 | 5,035 | 657 | 15.0 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
788 | 9,438 | 8,650 | 1,097.7 | % | |||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
2,146 | 1,724 | (422 | ) | (19.7 | )% | ||||||||||
| General and administrative |
2,587 | 2,775 | 188 | 7.3 | % | |||||||||||
| Sales and marketing |
2,023 | 1,655 | (368 | ) | (18.2 | )% | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
6,756 | 6,154 | (602 | ) | (8.9 | )% | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Income/(loss) from operations |
$ | (5,968 | ) | $ | 3,284 | $ | 9,252 | * | ||||||||
| Interest expense, net |
(2,598 | ) | (1,913 | ) | 685 | (26.4 | )% | |||||||||
| Other income/(expense), net |
— | 5 | 5 | * | ||||||||||||
| Change in fair value of warrant liabilities |
434 | (170 | ) | (604 | ) | * | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net income/(loss) |
$ | (8,132 | ) | $ | 1,206 | $ | 9,338 | * | ||||||||
|
|
|
|
|
|
|
|||||||||||
| * | Percentage change is not meaningful. |
| Years ended December 31, |
Change | |||||||||||||||
| 2010 | 2011 | $ | % | |||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands, except for percentages) | ||||||||||||||||
| Net revenue |
$ | 7,504 | $ | 8,005 | $ | 501 | 6.7 | % | ||||||||
| Cost of sales |
7,666 | 6,019 | (1,647 | ) | (21.5 | )% | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Gross profit/(loss) |
(162 | ) | 1,986 | 2,148 | * | |||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
3,560 | 2,768 | (792 | ) | (22.2 | )% | ||||||||||
| General and administrative |
5,376 | 3,360 | (2,016 | ) | (37.5 | )% | ||||||||||
| Sales and marketing |
4,717 | 2,672 | (2,045 | ) | (43.4 | )% | ||||||||||
| Impairment of film equipment |
981 | — | (981 | ) | * | |||||||||||
| Initial public offering costs — terminated offering |
1,752 | — | (1,752 | ) | * | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
16,386 | 8,800 | (7,586 | ) | (46.3 | )% | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
$ | (16,548 | ) | $ | (6,814 | ) | $ | 9,734 | 58.8 | % | ||||||
| Interest expense |
(4,109 | ) | (3,626 | ) | 483 | 11.8 | % | |||||||||
| Other income/(expense), net |
36 | (2 | ) | (38 | ) | * | ||||||||||
| Change in fair value of warrant liabilities |
926 | 445 | (481 | ) | (51.9 | )% | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (19,695 | ) | $ | (9,997 | ) | $ | 9,698 | 49.2 | % | ||||||
|
|
|
|
|
|
|
|||||||||||
| * | Percentage change is not meaningful. |
61
Table of Contents
Comparison of the Nine Months Ended September 30, 2012 and 2011
Net revenue
Net revenue for the nine months ended September 30, 2012 was $14.5 million compared to $5.2 million for the nine months ended September 30, 2011. Net revenue for the nine months ended September 30, 2012 consisted of $1.1 million from the sale of INFINITI analyzers, $13.2 million from the sale of consumables, including sales to one customer of $6.4 million, and $0.2 million in non-product revenue. Net revenue for the nine months ended September 30, 2011 consisted of $0.7 million from the sale of INFINITI analyzers, $4.3 million from the sale of consumables, and $0.2 million in non-product revenue. Sales of RUO products to laboratories operating under CLIA guidelines comprised 96% and 92% of our total net revenues in the nine months ended September 30, 2012 and 2011, respectively. The increase in net revenue is primarily attributable to increased utilization of the INFINITI system by our customers in general and in additional high-volume sales to a few customers of our personalized medicine and women’s health diagnostic testing products, specifically, which are for the most part sold on an RUO basis.
As a percentage of total net revenues, revenue from markets outside the United States continued to decline, and was 6% for the nine months ended September 30, 2012, as compared to 32% for the nine months ended September 30, 2011. This ongoing and increasing weakness in international sales is a result of tumultuous economic conditions in international markets and of our shift to a focus on domestic activities due to constraints in our resources that limited our ability to service international markets.
Cost of Sales and Gross Profit
Cost of sales for the nine months ended September 30, 2012 was $5.0 million compared to $4.4 million for the nine months ended September 30, 2011. The increase in cost of sales is due primarily to an increase in consumables sold. The increase in gross profit is due primarily to an increase in gross margins from the sale of high margin testing consumables.
Operating Expenses
Research and Development
Research and development expenses consisting primarily of personnel costs and development materials for the nine months ended September 30, 2012 were $1.7 million compared to $2.1 million for the nine months ended September 30, 2011. The decrease is attributable to reduced headcount as we focused on growing sales of existing products during a period of resource constraints. We expect our research and development expenses to grow in future periods following the completion of this offering, as we seek to further expand and enhance our test menu and conduct studies to support regulatory approvals for our products.
General and Administrative
General and administrative expenses consisting of personnel, professional services and other administrative expenses for the nine months ended September 30, 2012 were $2.8 million compared to $2.6 million for the nine months ended September 30, 2011. The increase is primarily attributable to increased professional fees incurred in 2012 related to annual financial audits.
Sales and Marketing
Sales and marketing expenses for the nine months ended September 30, 2012 were $1.7 million compared to $2.0 million for the nine months ended September 30, 2011. The decrease in sales and marketing costs is due primarily to reduced headcount as we more narrowly focused our sales and marketing efforts on a targeted group of customers. We expect our sales and marketing expenses to grow in future periods following the completion of this offering, as we hire additional sales representatives and expand our international sales and marketing presence.
62
Table of Contents
Profit/(Loss) from Operations and Net Profit/(Loss)
Profit from operations for the nine months ended September 30, 2012 was $3.3 million compared to a loss of $6.0 million for the nine months ended September 30, 2011. The profit from operations for the nine months ended September 30, 2012 was comprised of net sales revenue of $14.5 million less cost of sales of $5.0 million and aggregate operating expenses of $6.2 million. The loss from operations for the nine months ended September 30, 2011 was comprised of net sales revenue of $5.2 million less cost of sales of $4.4 million and aggregate operating expenses of $6.8 million.
Net profit for the nine months ended September 30, 2012 was $1.2 million compared to a net loss of $8.1 million for the nine months ended September 30, 2011. The net profit for the nine months ended September 30, 2012 was comprised of a profit from operations of $3.3 million less interest expense from debt financing of $1.9 million and changes in the fair value of preferred and common stock warrant liabilities of $0.2 million. The net loss for the nine months ended September 30, 2011 was comprised of a loss from operations of $6.0 million and interest expense from debt financing of $2.6 million offset by changes in the fair value of preferred and common stock warrant liabilities of $0.4 million.
Interest Income
We had no meaningful interest income for the nine months ended September 30, 2012 and September 30, 2011.
Interest Expense
Interest expense for the nine months ended September 30, 2012 was $1.9 million compared to interest expense of $2.6 million for the nine months ended September 30, 2011. The decrease in interest expense is due primarily to the repayment of interest-bearing debt and the fair value associated with common stock warrants recorded as debt discount and amortized as interest expense under the effective interest method.
Other Income (Expense), Net
Other income for the nine months ended September 30, 2012 was $5,000 compared to no other income or expense for the nine months ended September 30, 2011. The increase in other income is due primarily to non-operating activities.
Change in the Fair Value of Warrant Liabilities
The change in the fair value of warrant liabilities decreased our net income by $0.2 million for the nine months ended September 30, 2012 and decreased our net loss by $0.4 million for the nine months ended September 30, 2011. The increase in the fair value of warrant liabilities is due to the increase in the fair value of our common stock and underlying preferred stock and to the extension of the term of certain preferred stock warrants resulting in an increase to the contractual life over which the associated warrant liabilities are marked to market.
Comparison of the Years Ended December 31, 2011 and 2010
Net revenue
Net revenue for the year ended December 31, 2011 was $8.0 million compared to $7.5 million for the year ended December 31, 2010. Net revenue for the year ended December 31, 2011 consisted of $0.9 million from the sale of INFINITI analyzers and $7.1 million from the sale of consumables. Net revenue for the year ended December 31, 2010 consisted of $3.4 million from the sale of INFINITI analyzers and $4.1 million from the sale of consumables. Sales of RUO products to laboratories operating under CLIA guidelines comprised 92% and
63
Table of Contents
87% of our total net revenues in the years ended December 31, 2011 and 2010, respectively. The decrease in revenue from sales of INFINITI analyzers year-over-year is due primarily to a weakening in capital sales in the industry in general and in our international distributor network, specifically. The increase in consumable revenue year-over-year is primarily due to our increased installed base and in additional high-volume sales to a few customers of our personalized medicine and women’s health diagnostic testing products which are for the most part sold on an RUO basis.
As a percentage of total net sales, revenue from markets outside the United States were 22% and 52% for the years ended December 31, 2011 and 2010, respectively. The decrease in net revenues from sales outside the United States was due primarily to sales in 2010 of the INFINITI analyzer to our distributors in European and Asian markets not repeated in 2011 as a result of a weakening of economic conditions in the European region and a focusing of Company resources on domestic sales generation due to constrained resources.
Cost of Sales and Gross Profit
Cost of sales for the year ended December 31, 2011 was $6.0 million compared to $7.7 million for the year ended December 31, 2010. The decrease in cost of sales and increase in gross margin percentage year-over-year is due primarily to an increase in the sales mix of higher margin testing consumables over analyzers.
Operating Expenses
Research and Development
Research and development expenses consisting of personnel costs and costs to develop our diagnostic testing products and future generations of testing platforms for the year ended December 31, 2011 were $2.8 million compared to $3.6 million for the year ended December 31, 2010. The decrease in research and development expenses is due primarily to reduced headcount as we focused on growing sales of existing products during a period of resource constraints. We expect our research and development expenses to grow in future periods following the completion of this offering.
General and Administrative
General and administrative expenses for the year ended December 31, 2011 were $3.4 million compared to $5.4 million for the year ended December 31, 2010. The decrease primarily was driven by a reduction of management salaries and other general operating expenses in response to constrained resources, and legal, consulting and audit fees not incurred in 2011. In 2010, we incurred $0.9 million in legal and audit fees in connection with our then-planned initial public offering that was subsequently terminated.
Sales and Marketing
Sales and marketing expenses for the year ended December 31, 2011 were $2.7 million compared to $4.7 million for the year ended December 31, 2010. The decrease was driven primarily by reduced headcount as we more narrowly focused our sales and marketing efforts on a targeted group of customers, and by extraordinary stock compensation costs not incurred in 2011.
Impairment of Film Coating Equipment
During the year ended December 31, 2010, we recognized $1.0 million of impairment cost related to film coating equipment that had not been installed as of the end of the fiscal year.
Initial Public Offering — Terminated Offering
During the year ended December 31, 2010, we recognized $1.8 million in legal and accounting costs associated with a proposed initial public offering that was not completed in 2010.
64
Table of Contents
Loss from Operations and Net Loss
Loss from operations for the year ended December 31, 2011 was $6.8 million compared to a loss of $16.5 million for the year ended December 31, 2010. The loss from operations for the year ended December 31, 2011 was comprised of net sales revenue of $8.0 million less cost of sales of $6.0 million and aggregate operating expenses of $8.8 million. The loss in operations for the year ended December 31, 2010 was comprised of net sales revenue of $7.5 million less cost of sales of $7.6 million and aggregate operating expense of $16.4 million.
Net loss for the year ended December 31, 2011 was $10.0 million compared to a net loss of $19.7 million for the year ended December 31, 2010. The net loss for the year ended December 31, 2011 was comprised of a loss from operations of $6.8 million and interest expense from debt financing of $3.6 million offset by changes in the fair value of preferred and common stock warrant liabilities of $0.4 million. Net loss for the year ended December 31, 2010 was comprised of a loss from operations of $16.5 million and interest expense from debt financing of $4.1 million, offset by changes in the fair value of preferred and common stock warrant liabilities of $0.9 million.
Interest Expense
Net interest expense for the year ended December 31, 2011 was $3.6 million compared to $4.1 million for the year ended December 31, 2010. The decrease was attributable primarily to the issuance of senior promissory notes during 2011 and associated common stock warrants recorded as a debt premium and amortized as a credit interest expense under the effective interest method.
Other Income (Expense), Net
Other expenses net for the year ended December 31, 2011 were $2,000 compared to other income of $36,000 in non-operating revenue for the year ended December 31, 2010. This change is primarily due to non-operating activities.
Change in the Fair Value of Warrant Liabilities
The change in the fair value of warrant liabilities decreased our net loss by $0.4 million for the year ended December 31, 2011 and decreased our net loss by $0.9 million for the year ended December 31, 2010. The change is attributable to a decrease in the fair value of our common stock and in the contractual life over which the associated preferred stock warrant liabilities are marked to market.
Liquidity and Capital Resources
Historical Cash Flows
From our inception in April 1999 through September 30, 2012, we have financed our operations primarily through sales of privately placed shares of convertible preferred stock and promissory notes.
Our primary uses of cash are to fund operating expenses, inventory purchases and the acquisition of machinery and equipment. Cash used to fund operating expenses excludes the impact of non-cash items such as the provision for excess and obsolete inventory, write-off of equipment depreciation, stock-based compensation and non-cash interest expense and is impacted by the timing of when we pay these expenses as reflected in the change in our outstanding accounts payable and accrued expenses. Acquisitions of machinery and equipment primarily consist of our cost to manufacture INFINITI analyzers utilized in our domestic placement plans, purchases of laboratory equipment, computer hardware and software and facility improvements.
As of September 30, 2012, we had cash and cash equivalents of $0.3 million compared to $0.1 million and $61,000 as of September 30, 2011 and December 31, 2011, respectively.
65
Table of Contents
The following table summarizes our cash flows for each of the periods indicated:
| Years ended December 31, |
Nine months ended September 30, | |||||||||||||||
| 2010 | 2011 | 2011 | 2012 | |||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands) | ||||||||||||||||
| Net cash provided by/(used in) operating activities |
$ | (6,736 | ) | $ | (2,846 | ) | $ | (2,617 | ) | $ | 608 | |||||
| Net cash provided by/(used in) investing activities |
(260 | ) | (5 | ) | (3 | ) | (136 | ) | ||||||||
| Net cash provided by/(used in) financing activities |
6,503 | 2,783 | 2,600 | (239 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | (493 | ) | $ | (68 | ) | $ | (20 | ) | $ | 233 | ||||||
|
|
|
|
|
|
|
|
|
|||||||||
Operating Activities
Net cash used in operating activities for the year ended December 31, 2010 of $6.7 million consisted of a net loss of $19.7 million, a decrease of $0.9 million related to the change in the fair value of preferred and common stock warrant liabilities and a decrease of $0.3 million in the reserve for doubtful accounts offset by $4.2 million of changes in working capital, $3.8 million of non-cash interest expense and amortization of debt discount/premium related to our promissory notes, $2.9 million in stock compensation expense, $1.0 million in impairment costs related to film coating equipment, $0.8 million of depreciation and amortization, $0.9 million in deferred rent expense and tenant improvement allowances related to our corporate headquarters, $0.4 million of amortization of deferred financing costs and an increase of $0.3 million in reserves for excess and obsolete inventory. The primary changes in working capital were increases of $0.7 million of prepaid and other current assets, $0.4 million in accounts receivable and decreases of $1.9 million in other current liabilities and accrued expenses offset by increases of $5.7 million in accounts payable and decreases of $1.4 million in inventory.
Net cash used in operating activities for the year ended December 31, 2011 of $2.8 million consisted of a net loss of $10.0 million and a decrease of $0.4 million related to changes in the fair value of preferred and common stock warrant liabilities offset by $3.3 million of non-cash interest expense and amortization of debt discount/premium related to our promissory notes, $1.7 million in stock compensation expense, $1.3 million of changes in working capital, $0.7 million of depreciation and amortization, $0.4 million of amortization of deferred financing costs and $0.3 million in deferred rent expense related to our corporate headquarters. The primary changes in working capital were an increase of $2.0 million in accounts payable offset by increases of $0.6 million in accounts receivable and decreases of $0.1 million in other current liabilities and accrued expenses.
Net cash used in operating activities for the nine months ended September 30, 2011 of $2.6 million consisted of a net loss of $8.1 million, a decrease of $0.4 million related to changes in the fair value of preferred and common stock warrant liabilities and a decrease of $0.1 million in reserves for excess and obsolete inventory offset by $2.3 million of non-cash interest expense and amortization of debt discount/premium related to our promissory notes, $1.3 million of changes in working capital, $1.3 million in stock compensation expense, $0.6 million of depreciation and amortization, $0.3 million of amortization of deferred financing costs and $0.2 million in deferred rent expense related to our corporate headquarters. The primary changes in working capital consisted of increases of $1.2 million in accounts payable and decreases of $0.4 million in accounts receivable offset by decreases of $0.2 million in inventory and other current assets.
Net cash provided by operating activities for the nine months ended September 30, 2012 of $0.6 million consisted of a net profit of $1.2 million, $1.8 million of non-cash interest expense and amortization of debt discount/premium related to our promissory notes, $0.7 million in stock compensation expense, $0.5 million of depreciation and amortization, $0.3 million in deferred rent expense related to our corporate headquarters, $0.2
66
Table of Contents
million related to changes in the fair value of preferred and common warrant liabilities and $0.1 million in amortization of deferred financing costs offset by $4.2 million of changes in working capital. The primary changes in working capital consisted of increases of $2.0 million in accounts receivable, $1.6 million in prepaid and other assets, and $1.0 million in inventory offset by increases of $0.4 million in other current liabilities and accrued expenses and $0.1 million in accounts payable.
Investing Activities
Net cash used in investing activities for all periods noted above consisted primarily of invested capital and facility investment to manufacture INFINITI analyzers utilized in our domestic placement plans and purchases of machinery and equipment, including furniture, computer equipment and software, in support of all functional areas of the business.
Financing Activities
For the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2011 and 2012, net cash provided by financing activities consisted primarily of net proceeds from the sale of promissory notes and shares of Series E Convertible Preferred Stock issued in private placement transactions of $6.1 million, $2.8 million, $2.6 million and $1.8 million, respectively. We also made payments on promissory notes and accrued interest in the amount of $2.1 million in the nine months ended September 30, 2012. We used the proceeds from the issuances of the notes for general corporate purposes. Certain of these promissory notes limit our ability to incur additional indebtedness.
Promissory Notes
As of September 30, 2012, we had an aggregate of $24.7 million of principal and accrued interest under outstanding promissory notes, including those described above, of which approximately $14.8 million in aggregate principal amount was in default. For each additional day that the notes remain outstanding, we were required to pay approximately $4,600 additional interest under the notes. As of September 30, 2012, there was an aggregate of $4.0 million of principal and accrued interest outstanding under promissory notes we have issued to our directors, executive officers and holders of more than 5% of our common stock. We do not have sufficient cash to satisfy our indebtedness obligations absent the completion of this offering.
The following table describes the components of our outstanding indebtedness as of the following dates:
| December 31, | September 30, 2012 |
|||||||||||
| 2010 | 2011 | |||||||||||
| (unaudited) | ||||||||||||
| (in thousands) | ||||||||||||
| Subordinated Promissory Notes, 13% interest, due July 2011 |
$ | 5,071 | $ | — | $ | — | ||||||
| Subordinated Promissory Notes, 6% interest, due November to December 2011 |
595 | — | — | |||||||||
| Senior Promissory Notes, 13% interest, due December 2011 |
— | 400 | — | |||||||||
| Senior Promissory Note, 13% interest, due February 2012 |
— | 3,500 | 2,100 | |||||||||
| Short Term Notes Payable, 12% interest, due April & May 2012 |
— | 175 | 695 | |||||||||
| Short Term Notes Payable, 10% interest, due June 2012 |
— | — | 1,150 | |||||||||
| Subordinated Promissory Notes, 6% interest, due February to June 2012 |
10,715 | 10,794 | 10,794 | |||||||||
| Subordinated Promissory Notes, 6% interest, due December 2012 |
2,000 | 2,345 | 2,345 | |||||||||
| Subordinated Promissory Notes, 7% interest, due December 2012 |
300 | 1,200 | 1,200 | |||||||||
| Subordinated Promissory Notes, 8% interest, due December 2012 |
500 | 500 | 500 | |||||||||
| Subordinated Promissory Notes, 13% interest, due December 2012 |
$ | — | $ | 2,000 | $ | 2,000 | ||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 19,181 | $ | 20,914 | $ | 20,784 | ||||||
| Unamortized premium/(discount) |
(2,522 | ) | (281 | ) | 78 | |||||||
| Accrued interest |
2,120 | 2,882 | 3,863 | |||||||||
|
|
|
|
|
|
|
|||||||
| Outstanding balance |
$ | 18,779 | $ | 23,515 | $ | 24,725 | ||||||
|
|
|
|
|
|
|
|||||||
67
Table of Contents
In February and March 2010, we issued $525,000 aggregate principal amount of subordinated promissory notes to certain accredited investors. The notes bear interest at the rate of 6.0% per annum, with the principal and accrued interest balances generally due two years after the date of issuance, depending on the provisions of the note. In connection with the issuance of these notes, we also issued warrants to purchase 49,203 shares of our common stock with an exercise price of $12.12 per share. The warrants are now fully exercisable and have a term of five years from their date of issue.
In March 2010, we issued $5,250,000 aggregate principal amount of subordinated notes in a private placement to certain accredited investors. The notes bear interest at the rate of 6.0% per annum, with the principal and accrued interest balances due two years after the date of issuance. In the private placement, we also issued warrants to purchase 519,750 shares of our common stock with an exercise price of $15.15 per share. The warrants are now fully exercisable and have a term of five years from their date of issue.
In April 2010, we and the applicable note holder amended a subordinated promissory note with a principal amount of $1,000,000 set to mature in August 2010 by extending the maturity date to April 2011. In connection with this amendment, we cancelled a warrant to purchase 93,720 shares of our common stock at an exercise price of $21.21 per share held by the note holder which was to expire five years after the issuance date, and reissued a warrant to purchase 93,720 shares of our common stock at an exercise price of $15.15 per share to the note holder which will expire six and one-half years after the original issuance date. Also in April 2010, we and the applicable note holders amended certain subordinated promissory notes with an aggregate principal amount of $5,040,000 set to mature between August 2010 and December 2011 by extending the maturity dates to April 2012. In connection with these amendments, we cancelled warrants to purchase 472,349 shares of our common stock at an exercise price of $21.21 per share held by the note holders which were to expire five years after the issuance date and reissued warrants to purchase 472,349 shares of our common stock at an exercise price of $15.15 per share to the note holders which will expire six and one-half years after the original issuance date.
In November 2010, we and the applicable note holder amended certain promissory notes with an aggregate principal amount of $2,500,000 set to mature in April 2010, February 2011, May 2011 and November 2011, by extending the maturity dates to December 2012. In connection with these amendments, we cancelled warrants to purchase 234,300 shares of our common stock at exercise prices of $12.12, $15.15 and $21.21 per share held by the note holder and reissued warrants to purchase 234,300 shares of our common stock at an exercise price of $9.09 per share to the note holder which will expire in January 2016.
From December 2010 through May 2011, we issued $1,300,000 aggregate principal amount of promissory notes to certain accredited investors. The notes bear interest at the rate of 7.0% per annum, and generally mature in December 2012. In connection with this offering, we also issued warrants to purchase 128,700 shares of our common stock with an exercise price of $9.09 per share. The warrants have a term of five years from their date of issue.
In June 2011, we and the applicable note holder amended a certain promissory note with an aggregate principal amount of $2,000,000 set to mature in July 2011, by extending the maturity date to December 2012. In connection with this amendment, we cancelled a warrant to purchase 187,440 shares of our common stock at an exercise price of $12.12 per share held by the note holder and reissued a warrant to purchase 187,440 shares of our common stock at an exercise price of $9.09 per share to the note holder which will expire in August 2014.
In August 2011, we and the applicable note holder exchanged promissory notes with an aggregate principal amount of $3,071,000 that matured in July 2011, for promissory notes with an aggregate principal amount of $3,900,000, set to expire December 2011 and February 2012. In connection with this exchange, we cancelled warrants to purchase 281,160 shares of our common stock at an exercise price of $12.12 per share held by the note holder and reissued a warrant to purchase 429,000 shares of our common stock at an exercise price of $9.09 to the note holder which will expire in August 2016.
68
Table of Contents
In October and December 2011, we and the applicable note holders amended promissory notes with an aggregate principal amount of $595,000 that were set to expire in November and December 2011, by extending the maturity dates to dates in 2012. In connection with this amendment, we amended warrants to purchase 55,763 shares of our common stock by reducing the exercise price from $12.12 per share to $9.09 per share.
From November 2011 through January 2012, we issued $695,000 aggregate principal amount of promissory notes to certain accredited investors. The notes bear interest at the rate of 12.0% per annum, and generally matured in April 2012. In connection with the issuance of these notes, we issued warrants to purchase 114,675 shares of our common stock at an exercise price of $5.24 per share to the note holders which will expire five years from their date of issue.
In February 2012, we and the applicable note holder amended a certain promissory note with a principal amount of $250,000 set to mature in April 2012, by extending the maturity date to June 2012. In connection with this amendment, we amended a warrant to purchase 23,430 shares of our common stock at an exercise price of $15.15 per share held by the note holder, by reducing the exercise price to $9.09 per share and extending the term of the warrant until June 2017.
In February 2012, we and the applicable note holders amended certain promissory notes with an aggregate principal amount of $400,000 set to mature in February 2012, by extending the maturity dates to April 2012. In connection with these amendments, we amended warrants to purchase 37,488 shares of our common stock at an exercise price of $12.12 per share held by the note holder, by reducing the exercise price to $9.09 per share and extending the term of the warrant until April 2017.
In March 2012, we issued $1,150,000 aggregate principal amount of promissory notes to certain accredited investors. The notes bear interest at the rate of 10.0% per annum, with the principal and accrued interest balances matured in June 2012. In connection with the issuance of these notes, we issued warrants to purchase 113,850 shares of our common stock with an exercise price of $5.24 per share which will expire five years from their date of issue.
In November 2012, the holders of $7.5 million in aggregate principal amount of and accrued interest on our outstanding subordinated promissory notes with rates of interest ranging from six percent to 12% that were then past due or scheduled to come due in the immediate future surrendered their right to payment of those promissory notes in exchange for an aggregate of 4,287,074 shares of our Series NC Convertible Preferred Stock. In connection with this surrender and issuance, these noteholders surrendered warrants for the purchase of an aggregate of 637,813 shares of our common stock with exercise prices ranging from $5.24 per share to $15.15 per share in exchange for warrants of a like tenor for the purchase of an aggregate of 637,813 shares of our common stock with exercise prices of $4.55 per share. Also in November 2012, the holders of $14.4 million in aggregate principal amount of and accrued interest on our outstanding subordinated promissory notes with rates of interest ranging from six percent to 13% that were then past due or scheduled to come due in the immediate future surrendered their right to payment of those promissory notes in exchange for new promissory notes in aggregate principal amount of $14.4 million with eight and one-half percent rates of interest and November 2015 maturity dates. In connection with this surrender and issuance, these noteholders surrendered warrants for the purchase of an aggregate of 1,156,013 shares of our common stock with exercise prices ranging from $5.24 per share to $15.15 per share in exchange for warrants of a like tenor for the purchase of an aggregate of 1,734,020 shares of our common stock with exercise prices of $5.30 per share. As of November 27, 2012, after giving effect to these actions, we had outstanding total indebtedness and accrued interest under promissory notes of approximately $17.4 million, of which approximately $2.9 million was due and scheduled to come due in the immediate future, and outstanding warrants to purchase an aggregate of 3,372,711 shares of our common stock.
Our outstanding subordinated promissory notes are secured by the grant of a security interest in our trade receivables. We are required to use up to 50% of the net proceeds to us from the offering referred to in this prospectus to pay down our indebtedness under these subordinated promissory notes, and as a result we expect to retire all of our existing promissory note indebtedness in connection with the completion of this offering. If, after
69
Table of Contents
completion of this offering, we have any indebtedness outstanding under our November 2012 notes, we will be required to use 80% of the free cash that we have at the end of each fiscal quarter to pay down these notes pro rata until they are paid in full. For this purpose, “free cash” means our available cash after (i) payment or accrual of operating expenses (including tax liabilities) and all scheduled or required payments on our senior indebtedness (including amounts owed to our landlord) for that fiscal quarter just ending and (ii) establishing reserves for operating expenses and scheduled or required payments on our indebtedness (including amounts owed to our landlord) for the immediately following fiscal quarter as determined by us in good faith. These subordinated promissory notes restrict our ability to incur additional senior or pari passu indebtedness unless we use 50% of the net proceeds to us from the incurrence of that senior or pari passu indebtedness to pay down our indebtedness under these subordinated promissory notes. These subordinated promissory notes also restrict our ability to make cash dividends during the period of time that they remain outstanding.
In January 2013, we issued and sold a subordinated promissory note in the amount of $2.4 million to one of our existing investors, who is a holder of more than 5% of our capital stock. This promissory note has a maturity date of March 31, 2013, an annual interest rate of 8.5%, and is prepayable at any time without premium or penalty. We used the proceeds of this sale to retire the outstanding principal and interest owed under our outstanding promissory notes with Tregale Group Ltd. On December 28, 2012, Tregale Group Ltd filed a request for judicial intervention and motion for summary judgment in lieu of complaint, demanding payment of the principal and interest outstanding under promissory notes held by it, as well as reimbursement of certain legal and other expenses. See “Business — Legal Proceedings” elsewhere in this prospectus.
See the description of our outstanding indebtedness described elsewhere in this prospectus under the heading “Use of Proceeds” and “Note 3. Subordinated Promissory Notes” and “Note 12. Subsequent Events (unaudited)” of our financial statements that are included elsewhere in this prospectus.
Contractual Obligations
As of September 30, 2012, the annual amounts of future minimum payments under certain of our contractual obligations were:
| Payments due by period | ||||||||||||||||||||
| Total | Less than 1 year |
1-3 years | 3-5 years | More than 5 years |
||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Contractual obligations: |
||||||||||||||||||||
| Subordinated promissory notes (1) |
$ | 24,647 | $ | 24,647 | $ | — | $ | — | $ | — | ||||||||||
| Operating lease (2) |
41,995 | 1,590 | 3,585 | 3,966 | 32,854 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| $ | 66,642 | $ | 26,237 | $ | 3,585 | $ | 3,966 | $ | 32,854 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | From September 2008 through March 2012, we issued $24.3 million aggregate principal amount of promissory notes in private placements to certain accredited investors of which $4.0 million in aggregate principal amount was issued to directors, executive officers, and holders of more than 5% of our common stock. The notes bear a weighted average annual interest rate of 6.8%, with the principal and accrued interest (shown above accrued through September 30, 2012). These notes were exchanged and their maturities extended in November 2012. See “Note 12. Subsequent Events (unaudited)” of our financial statements that are included elsewhere in this prospectus. We may elect to prepay our outstanding promissory notes at any time without penalty. |
| (2) | Our lease for our corporate offices in Vista, California commenced on February 1, 2009 and will expire in December 2029. This facility houses our research and development, manufacturing and warehousing operations and our administrative offices. We are required to make a payment to the landlord in the amount of $1.1 million by February 4, 2013. We will not be able to satisfy this obligation absent the completion of this offering. See “Rick Factors” and “Note 12. Subsequent Events (unaudited)” of our financial statements that are included elsewhere in this prospectus. |
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements or any special-purpose entities.
70
Table of Contents
Working Capital Commitments and Liquidity
We have a history of operating losses and negative cash flows since our inception. We may not be able to achieve profitability on an annual basis or maintain profitability over any periods. If we do not consummate this offering or obtain additional capital from other external sources, we do not have sufficient working capital to fund our planned operations, and repay the current portion of our promissory notes, through the immediate future. As a result, our independent registered public accounting firm included an explanatory paragraph relating to our ability to continue as a going concern in its report on our audited financial statements for the year ended December 31, 2011.
We anticipate that our current cash and cash equivalents and cash provided by this offering and our operating activities will be sufficient to meet our currently estimated cash requirements for at least the next 12 months. We anticipate that we will use the net proceeds from this offering to (i) repay the principal and interest under our outstanding promissory notes, (ii) fund the expansion of our sales force, enhance our international distributor support network, and increase our marketing and promotional activity and business development efforts, (iii) support a PMA for our HPV-HR test and 510(k) and CE mark studies and submissions for several tests associated with womens’ health and personalized medicine, (iv) fund research and development activities to add new or enhanced tests to our menu, (v) fund the expansion of our manufacturing capacity and efficiency, including purchasing automation equipment, (vi) satisfy outstanding accounts payable to advisors and service providers incurred in connection with certain of our prior capital raising activities conducted from 2008 through 2010, and (vii) make payment of past due amounts owed to our landlord. We anticipate that we will use the remainder of the net proceeds from this offering for additional working capital and general corporate purposes. See “Use of Proceeds.”
We expect capital outlays and operating expenditures to increase over the next several years as we expand our infrastructure, commercialization, manufacturing and research and development activities, and as a result we may need additional capital financing. The amount of additional capital we may need to raise depends on many factors, including:
| • | the level of research and development investment required to maintain and improve our technology, including efforts to expand our molecular diagnostic test menu, to fund clinical studies and trials of our tests and to invest in the development of new analyzers; |
| • | the amount of future cash provided by or used in operating activities; |
| • | whether or not we are able to pay down our outstanding promissory notes from the proceeds of this offering or from our quarterly free cash flow, as required by the terms of the notes; |
| • | the costs of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; |
| • | our need or decision to acquire or license complementary technologies or acquire complementary businesses; and |
| • | changes in regulatory policies or laws that affect our operations. |
We cannot be certain that additional capital will be available when and as needed or that our actual cash requirements will not be greater than anticipated. If we require additional capital at a time when investment in diagnostics companies or in the marketplace in general is limited by the then prevailing market or other conditions, we may not be able to raise such funds at the time that we desire or any time thereafter. In addition, if we raise additional funds through the issuance of common stock or securities convertible into shares of common stock, the percentage ownership of our stockholders could be significantly diluted, and these newly issued securities may have rights, preferences or privileges senior to those of existing stockholders. If we obtain additional debt financing, a substantial portion of our operating cash flow may be dedicated to the payment of principal and interest on such indebtedness, and the terms of the debt securities issued could impose significant restrictions on our operations. If we raise additional funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or products, or grant licenses on terms that are not favorable to us.
71
Table of Contents
In the United States, market and economic conditions continue to be challenging with relatively tight credit conditions and slow economic growth. Continued concerns regarding economic growth, recessionary conditions in certain international economies, healthcare reforms, including changes in reimbursement policies and the scope of health insurance coverage, the levels of sovereign debt in the United States and Europe, energy costs, geopolitical issues, the availability and cost of credit, the U.S. mortgage and real estate market and recent tax increases have contributed to relatively low expectations for the U.S. economy. These conditions, combined with relatively low business and consumer confidence and high levels of unemployment could continue to contribute to adverse market and economic conditions. As a result of these conditions, the cost and availability of credit may continue to be adversely affected by illiquid credit markets and wider credit spreads. Continued uncertainty with respect to these conditions may adversely affect our liquidity and financial condition, and the liquidity and financial condition of our customers. If these market conditions continue, they may limit our ability to timely satisfy maturing liabilities, and access the capital markets to meet liquidity needs and invest in new technologies and products, resulting in adverse effects on our financial condition and results of operations.
Critical Accounting Policies, Significant Judgments and Estimates
Our financial statements and related notes included elsewhere in this prospectus have been prepared in accordance with United States generally accepted accounting policies, or GAAP. The preparation of these statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, costs, and expenses and related disclosures. We base such estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Changes in accounting estimates are reasonably likely to occur from period to period. Accordingly, actual results could differ significantly from the estimates made by company management. We evaluate our estimates and assumptions on an ongoing basis. To the extent that there are material differences between these estimates and actual results, our future financial statement preparation, financial condition, results of operations and cash flows will be affected.
The following accounting policies involve a greater degree of judgment and complexity than our other accounting policies. Accordingly, these are the policies we believe to be most critical to understanding and evaluating our financial condition and results of operations.
Revenue Recognition
Our revenue is primarily derived from sales of our INFINITI analyzers and the BioFilmChip, Intellipac Reagent Management Module, and various disposable items consumed by our diagnostic testing process, collectively referred to as consumables. Our INFINITI analyzers and all consumables are offered through direct sale in domestic markets and through distributors in international markets. Our INFINITI analyzers also are available to domestic customers through our domestic placement plan. In such arrangements, customers receive an analyzer at no direct cost in return for a commitment to purchase minimum quantities of consumables based on an agreed pricing schedule over the contract period, which is usually two years, and which may be extended at the option of the customer or us. We reserve the option to cancel the arrangement and repossess the analyzer if minimum quantities of consumables are not purchased. These analyzers are recorded in our financial records as capitalized assets and are depreciated over an estimated useful life of, typically, three years. When the analyzer is returned to us it is placed back into inventory at its then net book value. Analyzers that are returned are subject to a review and refurbishment process designed to enable the return of the analyzer to the field as soon as practicable. Any costs associated with the refurbishment of an analyzer are capitalized and added to our cost basis in the analyzer. The new basis, including refurbishment costs, either is amortized to cost of sales over the useful life of the analyzer once the refurbished unit is placed with a new customer under the domestic placement plan, or is charged to cost of sales upon the sale of the refurbished analyzer. Throughout the term of the placement agreement, we retain title to the analyzer.
72
Table of Contents
We recognize revenue when persuasive evidence of an arrangement exists, the price to the buyer is fixed or determinable, collectability is reasonably assured and risk of loss transfers, normally upon shipment. For sales that include customer specific acceptance criteria, revenue is recognized when the acceptance criteria have been met. Credit is extended based upon the evaluation of the customer’s financial condition. Collectability is assessed based on a number of factors, including payment history and the creditworthiness of a customer. If we determine that collection is not reasonably assured, we do not recognize revenue until collection becomes reasonably assured, which is generally upon receipt of cash. We do not request collateral from our customers. We recognize revenue when the products have been shipped and the title and risk of loss have been transferred.
In instances where final acceptance of the product or system is required, we defer revenue until all the acceptance criteria have been met. The rights granted in the domestic placement plan in exchange for a minimum annual purchase commitment constitutes a leasing arrangement. When a customer enters into a domestic placement plan agreement, the lease revenue is dependent on purchases of consumables, which is not measurable at the inception of the lease, and thus is accounted for as contingent rentals in their entirety. Accordingly, lease revenues are excluded from minimum lease payments but included in revenue as the consumables are purchased. The cost of the leased equipment is depreciated over its useful life and the expense included in cost of sales.
Arrangements to sell products to customers frequently include multiple deliverables, including the sale of the INFINITI analyzer combined with the sale of consumables. Sales to foreign distributors include training of their field agents whom will be responsible for installation, training, and maintenance for their customers internationally. We do not provide any price protection, rights of return, or extended payment terms to the distributors. Prior to January 1, 2011 we recognized revenue upon completion of training as we could not establish vendor-specific objective evidence (“VSOE”) of the training. Beginning on January 1, 2011, we adopted new authoritative guidance on multiple-element arrangements, using the prospective method for all arrangements entered into or materially modified from the date of adoption. Under this new guidance, we allocate arrangement consideration in multiple-element revenue arrangements at the inception of an arrangement to all deliverables or those packages in which all components of the package are delivered at the same time, based on the relative selling price method in accordance with the selling price hierarchy, which includes: (i) VSOE if available; (ii) third-party evidence (“TPE”) if VSOE is not available, and (iii) best estimate of selling price (“BESP”) if neither VSOE nor TPE is available. In 2011, we established selling price using management’s best estimate, considering internal factors such as historical selling prices, pricing practices and controls, and customer segment pricing strategies.
Implementation of this new authoritative guidance had an insignificant impact on reported revenue as compared to revenue under previous guidance.
Warranty
We provide a one-year warranty on our INFINITI analyzers. Accruals are provided for the estimated warranty expense at the time the associated revenue is recognized. The estimates of warranty expense are based on actual expenses incurred historically to service our INFINITI analyzers. We do not accrue warranty expense for international sales because our international distributors are responsible for servicing any INFINITI analyzers they sell to customers. Because we do not recognize revenue on the placement of our INFINITI analyzers through our domestic placement plans, we also do not accrue any associated warranty expense. Warranty expense accruals inherently require the use of management judgment. If we were to experience an increase in warranty claims or if our cost of servicing these warranties increased, our gross margins would be affected adversely. We periodically review our actual warranty expense to ensure that it is not materially different than actual results.
Income Taxes
We account for income taxes utilizing the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and for tax loss
73
Table of Contents
carryforwards. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income or expense in the period that includes the enactment date. Future tax benefits are recognized to the extent that realization of such benefit is more likely than not.
At September 30, 2012, we had federal tax net operating loss carryforwards of $66.1 million and state tax net operating loss carryforwards of $55.2 million. At December 31, 2011, we had federal tax net operating loss carryforwards of $67.9 million and state tax net operating loss carryforwards of $56.8 million. The federal tax net operating loss carryforwards will begin to expire in 2020. The state tax net operating loss carryforwards began to expire in 2012. At September 30, 2012 and at December 31, 2011 we had federal research and development credit carryforwards of $1.1 million and state research credit carryforwards of $1.3 million. The federal research and development credit carryforwards will begin to expire in 2022. The state research credit carryforwards do not expire.
Utilization of net operating loss carryforwards, credit carryforwards and certain deductions may be subject to substantial annual limitation as a result of ownership change limitations provided by the Internal Revenue Code of 1986, as amended, or the Internal Revenue Code, and similar state provisions. The tax benefits related to future utilization of federal and state net operating loss carryforwards, credit carryforwards and other deferred tax assets may be limited or lost if cumulative changes in ownership exceed 50% within any three-year period. We have not completed a study to assess whether an ownership change has occurred or whether there have been multiple ownership changes since our formation as a result of the complexity and cost associated with such a study, and the fact that there may be additional ownership changes in the future. Such a limitation may occur as a result of a change in ownership resulting from this offering. If we have experienced an ownership change at any time since our formation, utilization of the net operating loss or credit carryforwards to offset future taxable income and taxes, respectively, would be subject to an annual limitation under Section 382 of the Internal Revenue Code, which is determined by first multiplying the value of our stock at the time of the ownership change by the applicable long-term, tax-exempt rate, and then could be subject to additional adjustments, as required. Any limitation may result in expiration of all or a portion of the net operating loss or credit carryforwards before utilization. Until a study is completed and any limitation known, all domestic net operating losses and tax credit carryforwards are being considered as an uncertain tax position and disclosed as unrecognized tax benefits under current guidance. No tax benefits from our domestic net operating losses and tax credit carryforwards have been realized to date.
Convertible Preferred Stock Warrant Liabilities
We use the Black-Scholes valuation model to calculate the fair value of our preferred stock warrants. The inputs utilized in this valuation model are discussed below. Each of these inputs is subjective and generally requires significant judgment to determine.
| • | Fair Value of Our Preferred Stock. Because our stock is not publicly traded, we must estimate the fair value of our preferred stock, as discussed below. |
| • | Expected Term. The expected term is the remaining contractual life of the applicable preferred stock warrant. |
| • | Volatility. As we do not have a trading history for our preferred stock, the expected stock price volatility for our preferred stock is estimated by taking the average historic price volatility for industry peers based on daily price observations over a period equivalent to the expected term of the preferred stock warrants. Industry peers consist of several public companies similar in size, stage of life cycle and financial leverage. We do not rely on implied volatilities of traded common stock in our industry peers’ common stock because the volume of activity has been relatively low. |
| • | Risk-free Interest Rate. The risk-free interest rate is based on the yields of U.S. Treasury securities with maturities similar to the contractual term of the preferred stock warrants. |
| • | Dividend Yield. We have not historically paid cash dividends or distributions and do not intend to pay cash dividends or distributions in the foreseeable future. Consequently, we used an expected dividend yield of zero. |
74
Table of Contents
The fair value of the preferred stock warrants was estimated at the balance sheet dates using the following assumptions (fair value and exercise price are presented on a common stock equivalents basis):
| December 31, | September 30, 2012 | |||||
| 2010 | 2011 | |||||
| (unaudited) | ||||||
| Fair value |
$8.65 | $11.13 | $13.63 | |||
| Exercise Price |
$7.65 - $10.57 | $9.04 - $10.57 | $9.74 - $11.27 | |||
| Expected Term (in years) |
0.08 - 0.99 | 0.55 - 0.63 | 0.2 - 0.9 | |||
| Stock Price Volatility |
76.84 | 60.17 | 53.95 | |||
| Risk-free interest rate |
0.29% | 0.12% | 0.18% | |||
| Dividend yield |
— | — | — | |||
The fair value of the preferred stock underlying the warrants has historically been determined by our board of directors. Because there has been no public market for our stock, our board of directors has determined the fair value of the preferred stock at the time of issuance by considering a number of objective and subjective factors including valuations performed by unrelated third-party specialists, which included valuations of comparable companies, operating and financial performance, lack of liquidity of capital stock and general and industry-specific economic outlook, among other factors. For each period in which the fair value of preferred stock warrant liabilities was asssessed, the our business enterprise value was first determined. This enterprise value then was allocated across our capital structure using a Probability Weighted Expected Return Method (PWERM) model as the primary approach and an Option Pricing Model (OPM) analysis as a confirming approach. Under the PWERM model, various future potential liquidity scenarios were forecasted and the allocable value to each class of security in our capital structure in each respective scenario was determined. The probability that each potential liquidity event would occur was then assessed. The future proceeds were then discounted to determine the present value of proceeds in each scenario for each security class. The probability weighted present value of each security class was then determined to conclude the value of the preferred stock. Under the confirming OPM analysis, the Black Scholes option pricing framework was used to determine the proceeds allocable to each security class. A series of option tranches was established whereby each tranche represented the value allocable to various security classes between computed breakpoints. Breakpoints are the value inflection points, or indifference points, whereby the allocation to securities change based on the underlying economic rights of each security class. The breakpoints served as the strike prices in the Black Scholes option calculations and tranche values were determined based on the difference between option values in each tranche. The proceeds of the value of each option tranche were then allocated to each respective security class based on the economic rights to the security class between breakpoints. The value allocable to each respective security class was then totaled across all option tranches to conclude the fair value of each security class.
We have classified our convertible preferred stock warrants as a liability and have re-measured the liability to estimated fair value at December 31, 2011 and 2010 and September 30, 2012 using the Black-Scholes valuation model.
Stock-Based Compensation
Compensation expense related to stock-based transactions, including employee stock options, is measured and recognized in the financial statements based on fair value.
The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service period and classified in the statement of operations based on the department to which the related employee reports. Determining the fair value of stock-based awards at the grant date requires judgment. We use the Black-Scholes valuation model to determine the fair value of grants. The determination of the grant date fair value of grants using a valuation model is affected by assumptions regarding a number of complex and subjective variables. These variables include the fair value of our common stock, the expected term of our options, our
75
Table of Contents
expected stock price volatility over the expected term of the options, stock option exercise and cancellation behaviors, risk-free interest rates, and expected dividends, which are estimated as follows:
| • | Fair Value of Our Common Stock. Because our stock is not publicly traded, we must estimate the fair value of our common stock, as discussed in “Common Stock Valuations” below. |
| • | Expected Term. We estimate the expected term of our options using the simplified method allowed under SEC guidance. |
| • | Volatility. As we do not have a trading history for our common stock, the expected stock price volatility for our common stock is estimated by taking the average historic price volatility for industry peers based on daily price observations over a period equivalent to the expected term of the stock options. Industry peers consist of several public companies similar in size, stage of life cycle and financial leverage. We do not rely on implied volatilities of traded options in our industry peers’ common stock because the volume of activity has been relatively low. We intend to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our own common stock price becomes available, or until circumstances change such that the identified companies are no longer similar to us, in which case more suitable companies whose share prices are publicly available would be utilized in the calculation. |
| • | Risk-free Interest Rate. The risk-free interest rate is based on the yields of U.S. Treasury securities with maturities similar to the expected term of the options for each option group. |
| • | Dividend Yield. We have not historically paid cash dividends or distributions and do not intend to pay cash dividends or distributions in the foreseeable future. Consequently, we used an expected dividend yield of zero. |
The following table presents the weighted average assumptions used to estimate the fair value of grants during 2010 and 2011 and for the nine months ended September 30, 2012:
| Years ended December 31, | Nine months ended September 30, 2012 |
|||||||||||
| 2010 | 2011 | |||||||||||
| (unaudited) | ||||||||||||
| Fair value |
$6.09 - $8.30 | $5.24 | $5.24 | |||||||||
| Risk-free interest rate |
1.35% - 2.93% | 0.80% - 1.23% | 0.90% | |||||||||
| Dividend yield |
— | — | — | |||||||||
| Expected life of options (years) |
5.00 - 6.25 | 5.08 - 6.25 | 5.50 | |||||||||
| Volatility |
68.65% - 71.59% | 69.89% - 75.00% | 69.89% | |||||||||
Information for the first six months of fiscal year 2011 is not included in the above chart as there were no option grants during that period. We estimate forfeitures at the time of grant and revise, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We utilized our historical forfeitures to estimate our future forfeiture rate at 4.0% for the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2011 and 2012.
The weighted average exercise price per share of employee stock options granted during the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2012 was $5.48, $5.24 and $5.24, respectively.
Common Stock Valuations
We are required to estimate the fair value of the common stock underlying our stock-based awards when performing the fair value calculations with the Black-Scholes valuation model. The fair value of the common stock underlying our stock-based awards was determined on each grant date by our board of directors, with input from management. This fair value has been calculated on an as-converted basis for each period, assuming for such
76
Table of Contents
purposes that all outstanding convertible preferred stock has been converted into common stock without taking into account any dividend or liquidation preferences. All options to purchase shares of our common stock granted under our incentive stock option plans are intended to be granted with an exercise price per share no less than the fair value per share of our common stock underlying those options on the date of grant, based on the information known to us on the date of grant. In the absence of a public trading market for our common stock, on each grant date, we develop an estimate of the fair value of our common stock in order to determine an exercise price for the option grants. We have determined the fair value of our common stock using methodologies, approaches and assumptions consistent with the American Institute of Certified Public Accountants, or AICPA, Audit and Accounting Practice Aid Series: Valuation of Privately Held Company Equity Securities Issued as Compensation, or the AICPA Practice Guide. In addition, our board of directors considered various objective and subjective factors, along with input from management to determine the fair value of our common stock, including: the prices at which we sold shares of preferred stock, the superior rights and preferences of the preferred stock relative to our common stock at the time of each grant, our results of operations, financial position and status of our research and development efforts, our stage of development and business strategy, the lack of an active public market for our common and our preferred stock, and the likelihood of achieving a liquidity event such as an initial public offering, or IPO, or sale of our company in light of prevailing market conditions. The determination of fair value of our common stock also involves complex and subjective judgments including estimates of revenue, earnings, assumed market growth rates and estimated costs, as well as appropriate discount rates. At the time of each of the below-referenced valuations, the significant estimates used in the discounted cash flow approach included our best estimates of our revenue and revenue growth rates for several years into the future. Although each time we prepared such forecasts we did so based on assumptions that we believed to be reasonable and appropriate at that time, there can be no assurance that any such estimates for earlier periods or for future periods will prove to be accurate.
Our board of directors utilizes the methodologies described above to calculate our implied enterprise value as of each stock option grant date, taking into account our ability or inability to achieve certain milestones, principally among which was the completion of an initial public offering. A probability-weighted expected return methodology, or PWERM, analysis is then used to allocate a portion of this implied enterprise value based on six possible scenarios: (i) an initial public offering of our common stock in the immediate future at a price per share of our common stock based on an implied enterprise value equal to a multiple of our management’s estimate of our then current-year revenue; (ii) an initial public offering of our common stock at the end of the subsequent fiscal year at a price per share of our common stock based on an implied enterprise value equal to a multiple of our management’s estimate of our next fiscal year’s revenue; (iii) a merger or sale of our company at a date that is approximately two and one-half years in the future at a price per share of our common stock based on an implied enterprise value equal to a multiple of our management’s estimate of that future fiscal year’s revenue; (iv) a merger or sale of our company at the end of the second future fiscal year ending after the then-current fiscal year at a price per share of our common stock based on an implied enterprise value equal to a multiple of our management’s estimate of that future fiscal year’s revenue; (v) a dissolution of our company with no value available to be distributed to the holders of our common stock; and (vi) remaining a private company. For the initial public offering scenarios, the value assigned to our common stock was determined using the number of outstanding shares of our common stock assuming the conversion of all outstanding shares of our preferred stock into shares of our common stock. For the merger or sale scenarios, a break point analysis was used to determine the various enterprise values at which holders of each series of our outstanding shares of preferred stock would elect to convert their shares of our preferred stock into shares of our common stock and the points at which the holders of outstanding options and warrants to acquire shares of our common stock would exercise their options or warrants. The values allocated to our common stock as of a future date under each scenario by the PWERM analysis are then discounted using a weighted average cost of capital to calculate an implied present value of the value allocated to our common stock under the PWERM analysis.
There is inherent uncertainty in these estimates and if we had made different assumptions than those described above, the fair value of the underlying common stock and amount of our stock-based compensation expense, net income (loss), and net income (loss) per share amounts would have differed.
77
Table of Contents
The following is a summary of our stock option activity since January 1, 2011:
| Grant date (1) |
Number of options granted |
Exercise price ($ per share) |
Fair market value ($ per share) (2) |
|||||||||
| June 22, 2011 |
29,370 | 5.24 | 5.24 | |||||||||
| August 3, 2011 |
198,072 | 5.24 | 5.24 | |||||||||
| May 17, 2012 |
90,750 | 5.24 | 5.24 | |||||||||
| January 14, 2013 |
232,617 | 9.85 | 9.85 | |||||||||
| (1) | The grant date for options represented in the above table is considered to be the date the key terms and conditions of the award are approved by our board of directors or compensation committee, as applicable. |
| (2) | Represents the fair market value of our common stock at the date of grant as estimated by our board of directors on the date of grant. |
Significant factors considered by our board of directors in determining the fair value of our common stock at these grant dates include:
June 2011 Grant. On June 22, 2011, our board of directors determined that the fair value of our common stock was $5.24 per share. As part of this determination, our board of directors considered a retrospective valuation analysis as at December 31, 2010 that was finalized in May 2011. This valuation analysis used the following valuation methodologies to calculate our enterprise value: (i) a selected companies analysis using publicly available information regarding historical and projected future financial performance, including last twelve months, or LTM, and projected next fiscal year revenues for 14 companies in the molecular diagnostics industry with publicly traded equity securities, (ii) a selected transactions analysis using publicly available information regarding the historical financial performance, including LTM revenues, for 27 companies in the molecular diagnostics industry that had been acquired and (iii) a discounted cash flow analysis to determine the net present value of our future cash flows based on our management’s best estimates of our future financial performance from January 2012 to December 2016. We then utilized a PWERM analysis to allocate a portion of this implied enterprise value to our common stock on the same principles described above. For purposes of this PWERM analysis, the present values calculated for our common stock under each of the possible outcomes were weighted based on management’s estimates of the probability of each scenario occurring (initial public offering - early: 15.0%, initial public offering - late: 20.0%, merger/sale - high: 5.0%; merger/sale - low: 10.0%; dissolution: 25.0%; and staying private: 25%).
Finally, because our stock was not publicly traded, the implied present value of the value allocated to our common stock under the PWERM analysis was discounted 15% for lack of marketability based on the Black-Scholes valuation model to take into account variables including an expected time to liquidity under the six possible scenarios described above, the potential share price volatility of our common stock, and a risk-free rate of return. The foregoing analysis resulted in an estimate of the fair market value of our common stock as of December 31, 2010 of $5.24 per share. We believe that this estimate, which is lower than the fair value determined by our board of director for our 2010 stock option grants, is reasonable in light of the fact that between the dates of the 2010 stock option grants and December 31, 2010, the Company abandoned its former initial public company offering, was faced with large accounts payable and then-current notes payable obligations, and was not operating on a profitable basis, all of which significantly negatively affected the value of the Company.
Our board of directors then considered whether there were any significant internal or external positive or negative value-changing events since the date of the December 2010 valuation. During that six-month period, we continued to experience significant capital constraints due to the withdrawal of our initial public offering in the fourth quarter of 2010 and the slowdown in the economy generally, both of which contributed to our being in default at the June 2011 measurement date under a significant amount of our indebtedness. Our ability to continue as a going concern continued to be in question as we did not have cash on hand, or expected net profits in the foreseeable future, sufficient to satisfy our indebtedness that was then in default or that was then expected
78
Table of Contents
to come due in the immediate future and there was no material change in our sales or net revenues during that period of time. As a result, our board of directors concluded that no significant net change in value had taken place between the December 31, 2010 valuation and the date of this stock option grant.
August 2011 Grant. On August 3, 2011, our board of directors determined that the fair value of our common stock was still $5.24 per share. As part of this determination, our board of directors concluded that no significant internal or external value-changing events had taken place since the June 2011 grant date. Between June 22 and August 3, 2011 we continued to experience significant capital constraints due to outstanding promissory note obligations then in default and expected to come due in the immediate future and due to and the continued economic slowdown, with no material change in our sales or net revenues during that intervening period.
May 2012 Grant. On May 17, 2012, our board of directors determined that the fair value of our common stock was still $5.24 per share. As part of this determination, our board of directors considered whether there were any significant internal or external positive or negative value-changing events since the August 2011 grant date. Between August 3, 2011 and May 17, 2012, we had experienced increased acceptance and utilization of our INFINITI system and meaningful growth in our sales and net revenue. These positive results were significantly offset, however, by the continued worldwide economic slowdown, a lack of available capital resources and significant working capital and cash constraints, the incurrence of continued and increasing defaults under outstanding indebtedness, which amounts in default totaled more than $14.9 million as of this May 2012 measurement date, our then existing default of our payment obligations under our real property lease at our Vista facility, and accounts payable balances that were in excess of ordinary course working capital commitments, the payment of which were in substantial question as of this May 2012 measurement date. Our ability to continue as a going concern as of this measurement date was therefore in question as we did not have cash on hand, or expected net profits in the foreseeable future, sufficient to satisfy our then current obligations, including our indebtedness that was then in default or that was then expected to come due in the immediate future. As a result, our board of directors concluded that no significant net change in value had taken place since the August 2011 grant date.
January 2013 Grant. On January 14, 2013, our board of directors determined that the fair value of our common stock had increased to $9.85 per share. As part of this determination, our board of directors considered whether there were any significant internal or external positive or negative value-changing events since the May 2012 grant date, when our board of directors determined that the fair value of our common stock was $5.24 per share, and since the September 30, 2012 valuation date discussed below, which resulted in an estimated fair market value of our common stock of $5.58 per share.
As is described under “—June 2011 Grant” above, our determinations of fair value per share of common stock at June 2011, August 2011 and May 2012 utilized, among other things, a retrospective analysis of the fair value per share of our common stock as of December 31, 2010 that gave a probability estimate for an initial public offering - early of 15% and for an initial public offering - late of 20%, as well as a 15% discount for lack of marketability. A subsequent retrospective analysis of the fair value per share of our common stock as of September 30, 2012 that was conducted in October 2012 gave a probability estimate for an initial public offering - early of 10% and for an initial public offering - late of 20%, and again used a 15% discount for lack of marketability. These probability estimates reflected the fact that we had just initiated the initial public offering process reflected in the offering referred to in this prospectus, and did not yet have substantial confidence in the offering being able to be completed, in part due to our past experience with having withdrawn our 2008 through 2010 initial public offering activity. The September 2012 retrospective analysis determined that the fair value per share of our common stock as of September 30, 2012 was $5.58 per share. See “—Subsequent Retrospective Valuations” below. The fair value of our common stock was significantly depressed at that time due to a variety of factors, perhaps most significantly of which were the continuing (and increasing amount of) payment defaults under our then outstanding indebtedness during the period and our ability to continue as a going concern being in question.
79
Table of Contents
Our board of directors considered several significant positive value changing events that occurred between the September 2012 retrospective valuation date and January 14, 2013. These included the initial filing in September 2012 of a registration statement in support of the initial public offering referred to in this prospectus, which advanced significantly during the fourth quarter of 2012. Most significantly, our board of directors analyzed the effect of the exchange in November 2012 of our outstanding and in-default indebtedness for equity and/or new indebtedness with extended maturity dates. Prior to the exchange, we were in payment default under a substantial portion of our debt obligations and did not have sufficient cash resources to pay the defaulted obligations. As a result, we faced a substantial risk of bankruptcy or the seizure of our assets and suspension of our operating activities. This risk had created significant downward pressure on the fair market value of our common stock prior to the exchange. In addition to lessening our aggregate outstanding indebtedness by $7.5 million, another effect of this exchange was to extend the maturity date of our outstanding indebtedness that participated in the exchange from being past due or coming due in the immediate future to being due in November 2015. Our board of directors considered these factors to have added significant positive value to the fair value of our common stock as of January 2013.
Additionally, in January 2013, we had received quantitative input from the underwriters as to our valuation in an initial public offering, with a potential offering range of $9.00 to $11.00 per share of common stock. Our board of directors also determined that the probability of completing the offering referred to in this prospectus was much higher at the January 14, 2013 grant date than it had been at the September 30, 2012 retrospective valuation date or the May 17, 2012 grant date. As a result, our board of directors considered the likelihood of an initial public offering - early exit scenario to have increased significantly, and the likelihood of a dissolution or staying private scenario to have decreased significantly. In addition, our board of directors considered that the lack of marketability discount on the fair value of our common stock needed to be decreased due to the belief that an initial public offering was imminent. This change in probability weighting, and decrease in the discount for lack of marketability, was determined to have added significant positive value to the fair value of our common stock as of January 2013 compared to September 30, 2012.
Our board of directors also considered negative factors affecting the fair market value of our common stock since September 30, 2012, including the apparent downturn in our net revenue during the fourth quarter of 2012, due primarily to decreased sales to one of our key customers (which was offset in part by increased sales of INFINITI analyzers), and a significant increase in our accounts receivable past due more than 90 days due, the collectability of which had not yet been assessed. Taking into account these positive and negative factors, our board of directors concluded that a significant and material positive net change in value had taken place since September 30, 2012, driven primarily by the substantial reduction of bankruptcy risk effected through our November 2012 debt exchange and the perceived likelihood of a near-term initial public offering with a price to the public of between $9.00 and $11.00 per share.
Subsequent Retrospective Valuations
In August 2012, we conducted a retrospective valuation of our common stock as of December 31, 2011, and used the following valuation methodologies to calculate our enterprise value: (i) a selected companies analysis using publicly available information regarding historical and projected future financial performance, including LTM and projected next fiscal year revenues for 14 companies in the molecular diagnostics industry with publicly traded equity securities, (ii) a selected transactions analysis using publicly available information regarding the historical financial performance, including LTM revenues, for 30 companies in the molecular diagnostics industry that had been acquired and (iii) a discounted cash flow analysis to determine the net present value of our future cash flows based on our management’s best estimates of our future financial performance from January 2012 to December 2018, utilizing a discount rate based on our weighted average cost of capital.
A PWERM analysis then was used to allocate a portion of the implied enterprise value for our company based on those analyses to our common stock utilizing the same principles described above. For purposes of this PWERM analysis, the present values calculated for our common stock under each of the possible outcomes were
80
Table of Contents
weighted based on management’s estimates of the probability of each scenario occurring (initial public offering - early: 5.0%, initial public offering - late: 15.0%, merger/sale - high: 10.0%; merger/sale - low: 20.0%; dissolution: 30.0%; and staying private: 20%).
Finally, because our stock was not publicly traded, the implied present value of the value allocated to our common stock under the PWERM analysis was discounted 15% for lack of marketability based on the Black-Scholes put option valuation model to take into account variables, including an expected time to liquidity under the six possible scenarios described above, the potential share price volatility of our common stock, and a risk-free rate of return. The foregoing analysis resulted in an estimate of the fair market value of our common stock as of December 31, 2011 of $3.82 per share which was lower than the $5.24 per share exercise price at which we granted stock options in June 2011, August 2011 and May 2012. In light of this new information from an independent party, we decided to give more weight to the identified offsetting negative factors than was initially given to such factors, and determined, for stock compensation expense purposes, to value the Company’s common stock at $3.82 as of the date of the May 2012 stock option grants. Our Black-Scholes calculations for purposes of stock-based compensation expense as presented above therefore utilized a $3.82 fair value in calculating this expense.
Subsequently, in September 2012, we commissioned another independent retrospective valuation of our common stock as of June 30, 2012, and used the following valuation methodologies to calculate our enterprise value: (i) a selected companies analysis using publicly available information regarding historical and projected future financial performance, including LTM and projected next fiscal year revenues for 13 companies in the molecular diagnostics industry with publicly traded equity securities, (ii) a selected transactions analysis using publicly available information regarding the historical financial performance, including LTM revenues, for 31 companies in the molecular diagnostics industry that had been acquired and (iii) a discounted cash flow analysis to determine the net present value of our future cash flows based on our management’s best estimates of our future financial performance from June 30, 2012 to December 2018, utilizing a discount rate based on our weighted average cost of capital.
A PWERM analysis then was used to allocate a portion of the implied enterprise value for our company based on those analyses to our common stock utilizing the same principles described above. For purposes of this PWERM analysis, the present values calculated for our common stock under each of the possible outcomes were weighted based on management’s estimates of the probability of each scenario occurring (initial public offering - early: 10.0%, initial public offering - late: 20.0%, merger/sale - high: 10.0%; merger/sale - low: 20.0%; dissolution: 20.0%; and staying private: 20%).
Finally, because our stock was not publicly traded, the implied present value of the value allocated to our common stock under the PWERM analysis was discounted 15% for lack of marketability based on the Black-Scholes valuation model to take into account variables including an expected time to liquidity under the six possible scenarios described above, the potential share price volatility of our common stock, and a risk-free rate of return. The foregoing analysis resulted in an estimate of the fair market value of our common stock as of June 30, 2012 of $5.18 per share, which was again lower than the $5.24 per share exercise price at which we granted stock options in June 2011, August 2011 and May 2012. While we did not downward adjust our prior fair value determinations for stock-based compensation purposes based on these retrospective valuations, we believe they confirm the conclusion that our stock option grants discussed above were all granted at exercise prices that were equal to or greater than the fair values as of their respective grant dates.
Subsequently, in October 2012, we commissioned another independent retrospective valuation of our common stock as of September 30, 2012, and used the following valuation methodologies to calculate our enterprise value: (i) a selected companies analysis using publicly available information regarding historical and projected future financial performance, including LTM and projected next fiscal year revenues for 13 companies in the molecular diagnostics industry with publicly traded equity securities, (ii) a selected transactions analysis using publicly available information regarding the historical financial performance, including LTM revenues, for 31 companies in the molecular diagnostics industry that had been acquired and (iii) a discounted cash flow
81
Table of Contents
analysis to determine the net present value of our future cash flows based on our management’s best estimates of our future financial performance from September 30, 2012 to December 2018, utilizing a discount rate based on our weighted average cost of capital.
A PWERM analysis then was used to allocate a portion of the implied enterprise value for our company based on those analyses to our common stock utilizing the same principles described above. For purposes of this PWERM analysis, the present values calculated for our common stock under each of the possible outcomes were weighted based on management’s estimates of the probability of each scenario occurring (initial public offering - early: 10.0%, initial public offering - late: 20.0%, merger/sale - high: 10.0%; merger/sale - low: 20.0%; dissolution: 20.0%; and staying private: 20%).
Finally, because our stock was not publicly traded, the implied present value of the value allocated to our common stock under the PWERM analysis was discounted 15% for lack of marketability based on the Black-Scholes valuation model to take into account variables including an expected time to liquidity under the six possible scenarios described above, the potential share price volatility of our common stock, and a risk-free rate of return. The foregoing analysis resulted in an estimate of the fair market value of our common stock as of September 30, 2012 of $5.58 per share.
We recognized employee stock-based compensation in the statements of operations as follows:
| Years ended December 31, |
Nine months ended September 30, |
|||||||||||||||
| 2010 | 2011 | 2011 | 2012 | |||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands) | ||||||||||||||||
| Cost of sales |
$ | 227 | $ | 236 | $ | 195 | $ | 90 | ||||||||
| Research and development |
432 | 395 | 358 | 126 | ||||||||||||
| General and administrative |
901 | 780 | 661 | 282 | ||||||||||||
| Sales and marketing |
1,203 | 166 | 150 | 52 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 2,763 | $ | 1,577 | $ | 1,364 | $ | 550 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The total compensation cost related to unvested stock option grants not yet recognized as of September 30, 2012 was $1.9 million, and the weighted average period over which these grants are expected to vest was 1.2 years.
Based on the initial public offering price of $10.00 per share, the intrinsic value of stock options outstanding at September 30, 2012 would have been $9.2 million, of which $8.5 million and $0.7 million would have been related to stock options that were vested and unvested, respectively, at that date.
In most cases, the fair value of the equity securities granted is more reliably determinable than the fair value of the goods or services received. The fair value of an equity award granted to a non-employee generally is determined in the same manner as an equity award granted to an employee. Stock-based compensation expense related to non-employee consultants totaled $0.2 million and $0.1 million in the years ended December 31, 2010 and December 31, 2011, respectively, and $0 for each of the nine months ended September 30, 2011 and 2012.
Inventory Valuation
We report our inventories net of our reserve for slow-moving and obsolete parts. Inventory is valued using the lower of cost or market value and includes direct labor, materials and manufacturing overhead. Our inventory includes raw materials, work-in-process and finished goods in instrument and reagent production. We periodically review inventory for evidence of slow-moving or obsolete parts and expired reagents, and a reserve is recorded based on management’s reviews of inventories on hand, compared to estimated future usage and sales, and assumptions about the likelihood of obsolescence.
82
Table of Contents
Recently Issued Accounting Standards Not Yet Adopted
In May 2011, the Financial Accounting Standards Board, or FASB, issued an amendment to the accounting guidance on fair value measurements to ensure that GAAP and International Financial Reporting Standards have common requirements for fair value measurement and disclosures, including a consistent definition of fair value. The guidance is effective for interim and annual periods beginning on or after December 15, 2011. We adopted this guidance and it did not have a material impact on our financial statements for the period ended September 30, 2012.
In June 2011, the FASB issued an amendment to the accounting guidance on the presentation of comprehensive income. The guidance eliminates the option to present components of other comprehensive income as part of the statement of changes in stockholders’ equity, and instead requires that all nonowner changes in stockholders’ equity be presented in either a single continuous statement of comprehensive income or in two separate but consecutive statements. The guidance is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. We adopted this guidance and it did not have a material impact on our financial statements for the period ended September 30, 2012.
In December 2011, the FASB issued an amendment to the accounting guidance on disclosures about offsetting assets and liabilities. The guidance requires an entity to disclose both gross and net information about financial instruments and derivative instruments that are eligible for offset in the balance sheet or subject to an enforceable master netting arrangement or similar agreement. The guidance is effective for annual reporting periods beginning on or after January 1, 2013, and interim periods within those annual periods. We do not expect the adoption of this guidance will have a material impact on our financial statements.
Under Section 107(b) of the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Ernst & Young LLP resigned from acting as our independent registered public accounting firm in August, 2010, and so was not asked to report on our financial statements for our fiscal year 2010. We did not have any disagreements with Ernst & Young LLP on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure that was not resolved to the satisfaction of Ernst & Young LLP or that would have caused Ernst & Young LLP to make reference to the subject matter of the disagreement(s) in connection with its reports on our financial statements.
Prior to its resignation, Ernst & Young LLP had identified the following issues in its audit of our balance sheets as of December 31, 2008 and 2009, and the related statements of operations, convertible preferred stock and stockholders’ deficit, and cash flows for the year ended December 31, 2007 and for each of the two years in the period ended December 31, 2009:
| • | we had incurred recurring operating losses and had insufficient working capital to support our then planned operations, which raised substantial doubt about our ability to continue as a going concern; |
| • | we had material weaknesses in our internal control over financial reporting that resulted from two different internal control deficiencies. The first involved the failure to appropriately document negotiated terms and conditions that deviated from our standard terms and conditions, and to consistently and accurately communicate the negotiated terms so that the transactions could be properly recorded in the accounting records. The second related to the misapplication of generally accepted accounting principles with respect to the timing of recognition of revenue for product sales characterized by multiple deliverables or additional elements; and |
| • | we had a material weakness in our internal control over financial reporting related to our financial statement closing process, which resulted from having identified numerous post-closing accounting |
83
Table of Contents
| adjustments that we were required to record in connection with the preparation of our 2009 financial statements, and we discovered in the first quarter of 2010 certain errors related to our accounting for warrants issued with our subordinated promissory notes. These errors involved calculating and allocating the fair value of the warrants and calculating the amortization of the related interest expense. |
In response to the identification of these material weaknesses, we terminated one of our sales employees that had contributed to the failure to communicate negotiated terms and conditions, substantially revised our sales and revenue recognition policies and procedures and trained our personnel with respect to these revised policies and procedures, and amended our code of ethics and have since required all of our employees to certify compliance with this code. We believe that these actions substantially remediated the material weaknesses and significant deficiencies identified in 2010 other than our financial statement closing process, with respect to which we continue to experience material weaknesses. See also “—Internal Control over Financial Reporting” below.
We engaged Marcum LLP as our independent registered public accounting firm in December 2010. Our audit committee of our board of directors approved this engagement. Prior to Ernst & Young LLP’s resignation and prior to the appointment of Marcum LLP, we did not consult with Marcum LLP regarding the application of accounting principles to a specific completed or contemplated transaction or any matter that was either the subject of a disagreement or a reportable event, or regarding the type of audit opinion that might be rendered on our financial statements.
Qualitative and Quantitative Disclosures about Market Risk
Our exposure to market risk is currently confined to our cash and cash equivalents. We have not used derivative financial instruments for speculation or trading purposes. In addition, we have not invested in auction rate securities. Our subordinated promissory notes have a weighted average fixed annual interest rate of 6.8%.
The primary objective of our investment activities is to preserve our capital for the purpose of funding operations while at the same time maximizing the income we receive from our investments without significantly increasing risk. To achieve these objectives, we invest our cash primarily in liquid money market funds. As a result, we believe we have minimal interest rate risk. Due to our limited funds invested in interest bearing accounts, a one percentage point change in the average interest rate on invested funds would have had a minimal effect on interest income for the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2012.
Our international sales are all denominated and paid in U.S. dollars and as a result we believe we are not exposed to foreign exchange currency risk.
Internal Control over Financial Reporting
Overview
In connection with the audits of our financial statements for the years ended December 31, 2010 and 2011, and the review of our financial statements for the period ended September 30, 2012, both our independent registered public accounting firm and our management discovered several conditions that we deemed to be material weaknesses and significant deficiencies in our internal controls under the standards established by the Public Company Accounting Oversight Board, or PCAOB.
According to the PCAOB’s definitions, a deficiency in internal control over financial reporting exists when the design or operation of a control does not allow management or employees, in the normal course of performing their assigned functions, to prevent or detect misstatements on a timely basis. A deficiency in design exists when (a) a control necessary to meet the control objective is missing or (b) an existing control is not properly designed so that, even if the control operates as designed, the control objective would not be met. A deficiency in operation exists when a properly designed control does not operate as designed or when the person
84
Table of Contents
performing the control does not possess the necessary authority or competence to perform the control effectively. A significant deficiency is defined as a deficiency, or a combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness, yet important enough to merit attention by those responsible for oversight of a company’s financial reporting. A material weakness is defined as a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a company’s annual or interim financial statements will not be prevented or detected on a timely basis.
We are committed to maintaining effective internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with GAAP. We believe that the actions we have taken to date and that we expect to take in the future, as set forth in greater detail below, to enhance the reliability and effectiveness of our internal control over financial reporting will substantially remediate our material weaknesses and significant deficiencies in our internal control over financial reporting. We also intend to continue to enhance our internal control over financial reporting following the completion of this offering. However, our independent registered public accounting firm has not evaluated the measures we have taken to address our material weaknesses, and will not be able to confirm to us that these material weaknesses have been remediated until our independent registered public accounting firm has completed its next audit of our financial statements. There can be no assurance that our internal control over financial reporting, as modified or remediated, will allow us to identify or avoid future material weaknesses or significant deficiencies. The material weaknesses described below are primarily related to the lack of personnel with the appropriate skills and experience, particularly with respect to technical accounting matters, and the lack of policies and procedures related to the financial reporting and period closing processes.
Material Weaknesses
| • | We noted several issues related to effective oversight by management primarily attributed to lack of significant prior audit experience and expertise relating to complex accounting issues (such as debt and equity accounting and valuation). A lack of sufficient controls over debt/equity accounting and recordkeeping of equity and debt holders’ transactions resulted in material adjustments. |
| • | We lack a comprehensive, consistent and supportable methodology to capture the cost of manufacturing our inventory, specifically as it relates to labor and overhead charges and inventory reserves. In addition, updates to our costing system were not performed timely to ensure accurate recordkeeping. |
| • | A lack of qualified accounting and finance resources as well as effective oversight by those in charge of governance resulted in insufficient controls over timely financial statement preparation and review as well as the inability to timely and accurately account for complex transactions. A lack of technical knowledge and expertise attributed to non-compliance with certain GAAP accounting disclosure requirements. |
| • | Improper revenue recognition as it relates to properly recording negotiated terms and conditions in customer agreements that deviate from standard terms and conditions and the related misapplication of the proper guidance related to the timing of revenue recognition for product sales characterized by multiple deliverables or additional elements. |
In response to the identification of these material weaknesses, we (i) have hired two new employees to augment our accounting staff and intend to hire additional finance and accounting personnel in addition to providing more assistance on equity accounting, and have engaged a third party finance specialist to perform certain accounting functions for us, (ii) have established a series of internal controls over critical financial processes designed to ensure the proper oversight, accuracy and timeliness of entries made by our finance personnel and to ensure that periodic financial reporting is completed within requisite timeframes, and (iii) terminated, in 2010, one of our sales employees who had contributed to the failure to communicate negotiated
85
Table of Contents
terms and conditions, revised our sales and revenue recognition policies and procedures and trained our personnel with respect to the revised policies and procedures, and amended our code of ethics and have since required all employees to certify compliance with this code.
Significant Deficiencies
We have noted that our design of monitoring controls used to assess the design and operating effectiveness of our internal controls is inadequate and constitutes a significant deficiency. We also do not have an adequate internal process to report deficiencies in internal control to management on a timely basis. We intend to address our significant deficiency in the design of monitoring controls by establishing better operating controls and involve our board of directors in this process, which will involve establishing formal procedures to communicate deficiencies in internal controls on a timely basis, and request that our board of directors more actively participate in guiding management as it relates to such matters.
Conclusion
Although we plan to take steps to substantially remediate, and have identified the means by which we intend to substantially remediate, the material weaknesses and significant deficiencies that have been identified to date, all as further described above, our remediation efforts may not be successful, or we may in the future have additional material weaknesses or significant deficiencies in our internal control over financial reporting. Failure to implement and maintain effective internal control over financial reporting could result in material misstatements in our financial statements. For more information, see the section titled “Risk Factors — We have identified material weaknesses and significant deficiencies in our internal controls, and we cannot provide assurances that these weaknesses and deficiencies will be effectively remediated or that additional material weaknesses and significant deficiencies will not occur in the future. If our internal control over financial reporting or our disclosure controls and procedures are not effective, we may not be able to accurately report our financial results, prevent fraud, or file our periodic reports in a timely manner, which may cause investors to lose confidence in our reported financial information and may lead to a decline in our stock price,” included elsewhere in this prospectus above.
86
Table of Contents
Overview
We design, develop, manufacture and market the INFINITI molecular diagnostics system. The system includes an extensive and expanding menu of genetic tests and a family of highly automated analyzers. Our products are sold to reference laboratories, hospital laboratories and specialty clinics that produce genetic test results in a broad range of markets, including personalized medicine, women’s health, oncology and infectious disease. Genetic tests are performed on our INFINITI analyzer utilizing our high-margin and test-specific consumables, which include our proprietary BioFilmChip microarrays and our proprietary Intellipac Reagent Management Modules. In the United States, we market and sell our genetic tests that have been cleared by the U.S. Food and Drug Administration, or FDA, for use in the indications specified in those clearances. The remainder of our genetic tests are marketed and sold on a research use only, or RUO, basis. Our RUO tests may be used for clinical purposes in the United States only by customers that are certified under the Clinical Laboratory Improvements Amendments of 1988, or CLIA, and that have incorporated our test into the customer’s laboratory-developed tests, or LDTs, pursuant to guidelines issued by the College of American Pathologists.
Our INFINITI analyzers are capable of both mid- to high-volume testing and generating many different laboratory results from one patient sample at the same time, which is commonly referred to as multiplexing, while providing a high level of accuracy and reproducibility. Our INFINITI system is easy to use, as it eliminates the need for multiple, specialized instruments and automates many of the traditionally discrete processes of genetic testing.
As of December 31, 2012, we offered 53 tests for use on our INFINITI analyzers and had more than 15 additional tests in development. Our current and in-development tests are focused on the areas of personalized medicine (including pain management, mental health and cardiovascular health assessment), women’s health, oncology, infectious disease, genetic disorders, newborn screening and blood banking, which we believe represent large and growing market opportunities in genetic testing. We believe the depth and breadth of our test menu is a significant competitive advantage that will allow laboratories to utilize laboratory space, labor and capital investment more efficiently and to conduct additional molecular diagnostic tests. The proprietary design of our INFINITI system allows us to introduce new and enhanced tests to our genetic test menu without the need to modify our INFINITI analyzers. We intend to increase the number of tests offered in each of our target market segments, which will further increase the utility of our INFINITI system to our customers. Our internal test development efforts are generally driven by our customers’ current and anticipated needs, our analysis and projections for the molecular diagnostics market, and our ability to leverage our core competencies such as automation and multiplexing. We have entered into, and expect to continue to enter into, collaborative relationships with leading research and academic institutions for the development of additional or enhanced tests to further increase the depth and breadth of our genetic test menu. Since the initial launch of our INFINITI system in 2007 we have introduced more than five new or enhanced tests per year.
In February 2007, we received 510(k) clearance from the FDA for the commercial sale of our INFINITI Analyzer. We have also received 510(k) clearance for our Warfarin sensitivity test, our CYP450 2C19 test, which is designed to determine the varied efficacy and toxicity of certain highly prescribed drugs based on certain genetic variants, and our Factor II, Factor V and Factor II-V panel tests, which aid in the determination of an individual’s risk for the development of blood clots. The balance of our tests are currently offered for sale in the United States under the RUO designation. These RUO tests are labeled “For Research Use Only. Not for use in diagnostic procedures.” as required by FDA regulations. We are not permitted to market these products for in vitro diagnostic use, and must maintain distribution controls to assure that these products are not used for diagnostic purposes. We therefore train our personnel to only market these products to laboratories for research or investigational use in the collection of research data, and to not promote any off-label uses of our products.
87
Table of Contents
We intend to seek regulatory clearance or approval, as necessary, for our tests. For example:
| • | we have submitted notification to the FDA for 510(k) clearance for our uridine diphosphate glucuronosyltransferaseisoform 1A1, or UGT1A1, test, which is designed to help determine the initial dosing of irinotecan, a leading drug for a broad variety of cancers; |
| • | we intend to submit a 510(k) notification to the FDA for our cystic fibrosis and our human papillomavirus, or HPV, genotyping tests in 2013; |
| • | we are conducting studies in support of and are preparing to submit a pre-investigational device exemption, or pre-IDE, application for our CFTR and Flu A-sH1N1 tests; |
| • | we have initiated clinical studies to support future submissions to the FDA for clearance of several of our other tests, including our 2D6, 3A4, 3A5, ureaplasma urealyticum, mycoplasma genitalium, mycoplasma hominis (Urogen Quad), bacterial vaginosis, or BV, candida vaginitis, or CV, chlamydia, gonorrhea and KRAS-BRAF genetic tests; and |
| • | we are finalizing the protocol for a clinical trial necessary to support a premarket approval, or PMA, application to the FDA for our HPV-HR tests and intend to commence the clinical trial in 2013; |
We obtained the Conformité Européenne, or CE, mark for our INFINITI Analyzer in June 2008, our INFINITI Plus Analyzer in September 2012, our Factor II, Factor V, Factor II-V panel, MTHFR and FII-FV-MTHFR panel tests in August 2008, our HPV Quad test in October 2008 and our CFTR-31 assay in November 2008. In total, 22 of our tests are CE marked, with the marking supported by completed clinical and validation studies that demonstrated the analytical performance of each CE marked test. The CE mark facilitates the marketing and sale of our INFINITI Analyzer and CE marked tests in the European Union and the European Economic Area as well as other international markets.
Sales of products for which we have received 510(k) clearance accounted for 13%, 8% and 4% of our net revenue for 2010, 2011 and the first nine months of 2012, respectively. The balance of our product sales for those same periods, representing 87%, 92% and 96% of our net revenue for 2010, 2011 and the first nine months of 2012, respectively, were derived from sales of RUO products. These products are offered for sale to allow for the collection of research data, and may only be used in the United States for clinical purposes by laboratories certified under CLIA that have incorporated these products into their LDTs pursuant to guidelines issued by the College of American Pathologists. We believe that nearly all of our RUO product sales are incorporated into LDT’s. In order to develop an LDT utilizing our products, these certified laboratories and other facilities must develop and validate a test protocol that includes specimen collection, DNA extraction, PCR amplification, hybridization and detection, and data analysis, interpretation and reporting. Our products provide components that can be used by these certified laboratories and other facilities for the PCR amplification, hybridization and detection portions of these LDTs. We sell each of these components individually, as ordered by the customer in its discretion, and not as a kit or system. The validation process engaged in by these certified laboratories and other facilities can involve validation of the sample collection and extraction process, establishing limits of detection and analytical sensitivity, testing for specificity and cross-reactivity, including interfering substances, validation for assay accuracy, precision and reproducibility, and establishing reportable ranges of test results for the test system and reference values that will be measured against as controls. This validation process also requires verifying the result from the LDT against known standard samples or the results of a high-standard laboratory testing method such as sequencing, and can involve the testing of a large number of patient samples. Depending on the availability of patient samples, this process may take from several weeks to several months or more to complete and this requires a significant investment by the customer. This validation process must be completed for each of our RUO genetic test components that a customer wishes to incorporate into one of its LDTs.
We believe that all sales of our RUO products in the United States are to customers that are either certified in the manner described above and have incorporated our products into their LDTs or that use such products for research only. The FDA has not adopted these guidelines and we are not permitted to represent our RUO
88
Table of Contents
products as effective in vitro diagnostic products. Our decision to seek FDA approvals or clearances domestically, and CE marking internationally, on our genetic tests is made on a test-by-test basis, and is based on a variety of factors, including:
| • | the regulatory environment for the use of genetic tests, in particular the FDA’s requirements and limitations on marketing RUO tests, which may not be marketed as in vitro diagnostic products; |
| • | the demand by our existing and target customers, as expressed to us, for particular genetic tests that have received regulatory approvals or clearances; |
| • | the competitive environment for the use of genetic tests that have received regulatory approvals or clearances versus similar tests that have not; for example, certain of our competitors provide FDA cleared or approved tests in the area of HPV testing, and to compete with those competitors we intend to obtain FDA clearance or approval for certain of our HPV tests as well; and |
| • | the size of the available market for the particular test, given the relatively significant expense and time required to obtain regulatory approvals or clearances. |
For those of our tests for which we have not obtained FDA regulatory clearance or approval, we experience delays of anywhere from a few weeks to several months or more in obtaining revenue from customers that desire to utilize our RUO test consumables during the period of time that the customer is developing its own LDT that incorporates our RUO test.
We believe that we are in compliance with existing FDA rules and regulations governing our business, including those governing the marketing and sale of RUO tests; however a significant change in existing laws, or their enforcement, may require us to change our business model or our business practices to maintain compliance with these laws. For instance, in June 2011 the FDA issued a Draft Guidance entitled “Commercially Distributed In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only: Frequently Asked Questions,” which, if enforced, would limit our marketing of RUO tests to general discovery laboratories and would require us to halt sales to clinical laboratories that validate and use our RUO tests as part of their LDTs. The FDA has generally exercised its enforcement discretion to not enforce applicable regulations with respect to LDTs. However, the FDA has indicated, since 2010, that it intends to reconsider its policy regarding enforcement and to begin drafting an oversight framework for such tests. If the FDA imposes significant changes to the regulation or enforcement of LDTs, including our RUO tests that are used as LDTs, it may require us to suspend sales of our RUO tests, which together represented 96% of our net revenues for the nine months ended September 30, 2012, and it would require us to seek FDA clearance or approval for our RUO tests, which would in turn require significant time and capital investments on our part and reduce our revenues or increase our costs and adversely affect our business, prospects, results of operations or financial condition. We have not undertaken any specific efforts to comply with this draft guidance. If we were to voluntarily elect to comply with this draft guidance, we would be required to seek regulatory approval for all of our RUO tests that are sold in the United States, which would require significant time and capital investments on our part, significantly reduce our revenues until we obtained regulatory approvals, and significantly increase our capital costs, which could in turn adversely affect our business, prospects, results of operations or financial condition during the affected fiscal periods. Our management currently believes that finalization or enforcement of this draft guidance in its present form is unlikely given the significant adverse effect it would have on a variety of industry participants, and on the ability of physicians to provide effective healthcare.
If we decide to seek PMA approvals for a particular test, the time and expense involved could vary widely based on the type of test involved, although we would expect to spend on average approximately two to three years and approximately $10 to $15 million or more in seeking and obtaining such approval. If we decide to seek 510(k) clearance for a particular test, we expect to spend on average approximately six to 12 months and approximately $300,000 to $500,000 in seeking and obtaining such clearance. If we decide to seek CE marking for a particular test, we expect to spend on average approximately two to three months and approximately
89
Table of Contents
$100,000 in seeking and obtaining such clearance. These expenditures are estimates based on information currently available to management and actual expenditures may vary from or exceed these estimates.
We believe that compared to traditional genetic testing methods, our INFINITI system can significantly improve laboratory productivity, workflow and throughput while reducing the cost per reportable result. We believe that these and other attributes of our INFINITI system decrease the cost and complexity of genetic testing and reduce the need for specialized laboratory personnel, training, equipment and facilities. Our INFINITI system has been designed to enable a broad range of reference laboratories, hospital laboratories and specialty clinics to start performing, or to more cost-effectively perform, molecular diagnostic tests, which we believe will facilitate the adoption of our INFINITI analyzers and the utilization of our genetic tests.
We experienced meaningful revenue growth in the first nine months of 2012, with revenue of $14.5 million during this period, $13.2 million of which was derived from sales of our genetic test consumables, as compared to revenue of $7.5 million and $8.0 million for fiscal years 2010 and 2011, respectively, of which $3.9 million and $6.8 million was derived from sales of our genetic test consumables, respectively. We expect to continue to generate the substantial majority of our revenue through the sale of our genetic test consumables for the foreseeable future. As of December 31, 2012, we had 191 INFINITI analyzers placed with customers.
Market Opportunity
Industry Background
Molecular diagnostic testing is used to measure or detect genetic biomarkers associated with a predisposition to, or the presence of, a particular disease or condition, or other genetic variance such as drug response. The information provided by molecular diagnostic testing may enable physicians to achieve better patient outcomes and better contain health care costs through, for example, earlier diagnosis of disease, improved monitoring of disease progression and more personalized treatment. According to Kalorama Information, an independent market research firm, the global molecular diagnostics market is expected to grow from an estimated $4.8 billion in 2010 to $8.1 billion in 2015, which represents a compound annual growth rate of approximately 11%, which we believe will exceed the growth of the overall diagnostics market.
Current practices in developing and running molecular diagnostic tests typically involve manual and complex procedures that require significant expertise, time and expense. We believe the resource and time constraints of traditional testing methods have limited the growth of the molecular diagnostics market and that the recent availability of more automated and integrated testing methods will result in accelerated use of molecular diagnostic testing. The growth of the molecular diagnostics market will also depend on increasing physician education on the use of genetic testing and greater coverage of tests by insurance carriers. Growing understanding of the utility of genetic information for the diagnosis and treatment of disease, as well the increase in identification of new biomarkers, may lead to increased growth in the molecular diagnostics market.
Our Target Markets
We believe there are additional factors that will continue to drive growth in the molecular diagnostics market segments we target including:
| • | Personalized medicine and companion diagnostics. The matching of treatment options to a patient’s specific genetic profile has emerged as an important trend in medicine. Better targeted and more effective pharmacogenomic-based treatments have the potential to improve healthcare outcomes and lower healthcare costs, which may lead to increased use of molecular diagnostic testing. |
| • | Healthcare providers are able to better treat patients by optimizing potential efficacy while minimizing the potential for adverse side effects by conducting molecular diagnostic tests prior to prescribing drugs. |
| • | Many pharmaceutical researchers, companies, and pharmacy benefit companies are screening drugs for differences in efficacy and toxicity among individuals with varied genetic profiles. |
90
Table of Contents
| • | Regulatory agencies have revised drug labels to improve safety and efficacy based on information provided by molecular diagnostic testing. |
| • | Pain Management. Pharmacogenomics is playing an integral role in the administration and management of pain medication, as gene mutations can be key factors in determining the most appropriate drug to determine efficacy and safety. |
| • | At least 116 million adult Americans suffer from acute or chronic pain each year. Drugs are the “first line” of treatment for most forms of pain. The goal of successful pain management is to effectively control patient pain without causing excess side effects from the medication prescribed. However only 58% of patients taking prescription medication reported pain relief and fewer than 41% of patients taking over-the-counter pain medication reported relief. |
| • | In order to improve clinical outcomes physicians are using genetic markers to enhance pain management and optimize dosing strategy. Opioids are the most frequently prescribed class of pain management drugs in the United States. These drugs, including Hydrocodone, Codeine, Oxycodone, and Morphine, are often indicated for the relief of chronic and moderate to severe post-surgical pain, in addition to pain experienced by cancer patients. According to the IMS Health National Prescription Audit, hydrocodone alone accounted for more than 136.7 million prescriptions in 2011. |
| • | We believe key factors accounting for the rising use of these drugs include the expanding population of cancer patients and patients undergoing surgical, including orthopedic, procedures. |
| • | Mental Health. Mental health represents a major component of overall pharmaceutical sales. According to the Centers for Disease Control and Prevention, or CDC, as much as 11% of the U.S. population is taking antidepressants at a given time, while as many as 23% of women between the age of 40 and 59 are on psychiatric medication. Improving care for mental health patients by tailoring treatment options to their specific genetic profile may continue to drive the expansion of the molecular diagnostics industry. |
| • | An increase in the diagnoses of autism and attention deficit hyperactivity disorder has further expanded the need for treatment to children and adolescents. Because of potential developmental issues that could result from psychiatric drug treatment for minors, doctors must take precautions in choosing medications for young patients. |
| • | One of the biggest challenges in treating depression is the lack of reliable efficacy and potential adverse effects of various therapeutic options. Approximately 40% of patients don’t respond to the first medication prescribed. The resulting trial and error process delays effective treatment for patients and increases healthcare costs. |
| • | Cancer and Companion Diagnostics. Because of the high cost of many cancer therapeutics, and the varied levels of efficacy and toxicity across different patients, tests to direct cancer treatment are becoming increasingly important. In addition, tests to diagnose or determine the predisposition to various forms of cancer are becoming more common. We believe molecular diagnostic testing to determine a particular patient’s expected response to various cancer therapeutics will continue to drive expanded demand for molecular diagnostics. |
| • | The FDA has required or recommended that molecular diagnostic tests be performed before the administration of certain drugs, including Herceptin and Erbitux. |
| • | Tests such as Colorectal KRAS-BRAF, KRAS, BRAF, BRAF XP, and EGFR have experienced meaningful clinical adoption due to their ability to improve patient outcomes and decrease costs. For example, Erbitux, a leading treatment for colorectal cancer, costs approximately $61,000 per course of treatment. There are approximately 28,700 colorectal cancer patients in the United States and KRAS mutations occur in up to 45% of colorectal carcinomas. The presence of KRAS mutations suggest that anti-EGFR therapies (Erbitux/Vectibix) will not be effective. If all of these patients had KRAS testing over $600 million in unnecessary treatment expense could be avoided. |
91
Table of Contents
| • | Cardiovascular Health Assessment. According to the World Health Organization, or WHO, cardiovascular diseases were responsible for 30% of global deaths in 2008. It is estimated that by 2030, 23.6 million people will die from some form of cardiovascular disease. Due to the increasing prevalence of cardiovascular diseases and the information generated by molecular diagnostic testing, risk assessment for and the treatment of cardiovascular diseases is an area of growth within molecular diagnostics. |
| • | Based on recent research, it is now widely accepted that the efficacy and tolerability of two of the most commonly used drugs in the prevention of cardiovascular disease (Lipitor (atorvastatin) for cholesterol regulation and prevention of cardiovascular disease and Plavix (clopidogrel) for prevention of stent thrombosis and cardiovascular death following acute coronary syndrome) are heavily influenced by a patient’s genetics. Lipitor (atorvastatin) is the top selling drug of its class and was dispensed in more than 40.0 million prescriptions in the United States in 2011. Plavix (clopidogrel) was dispensed in more than 25.0 million prescriptions in the United States in 2010. |
| • | Women’s Health. We believe the women’s health diagnostics market will continue to grow and represent a substantial market opportunity. Non-molecular tests are commonly employed in this market, but the use of molecular diagnostics is expanding significantly due to increased applications, better performance and better clinical discriminatory capabilities. |
| • | Key tests that have seen rapid adoption of molecular methodologies include HPV testing and sexually transmitted disease, or STD, testing. Current regulations from the U.S. Preventive Services Task Force recommend that molecular HPV tests be utilized in conjunction with cytology (Pap test) in standard 5 year intervals. |
| • | Infectious Diseases. According to the WHO, infectious diseases caused approximately 20% of all recorded deaths in 2008. Within this group, HIV, tuberculosis, or TB, and respiratory infections were the top three contributors to overall mortality in adults aged 15-59, at 35%, 21% and 10%, respectively. |
| • | Historically, many of these infections required 2 stages of testing: a rapid screening test to confirm the presence of a general infection, followed by a lengthy type-specific culture to assess other clinically significant attributes such as antimicrobial susceptibility. The new paradigm offered by molecular diagnostics, via multiplexing capabilities and gene specific differentiation, allows for simultaneous detection of each infection and the identification of subtypes and drug resistances, all within a clinically acceptable turnaround time. |
| • | Genetic Disorders. Genetic and inherited disease testing is a cornerstone of molecular diagnostic testing. Molecular diagnostic tests offer significant advantages over prior, often subjective, forms of diagnosis. |
| • | We believe that growth in this area has been, and will continue to be, focused on large markets for testing such as Familial Mediterranean Fever, Thalassemia and sickle-cell anemia, where large-scale populations exhibiting these genetic traits provide an opportunity for high-volume testing. |
| • | The adaptability of molecular technologies to the detection of genetic disorders is aided by the ability to simultaneously identify multiple pertinent genetic markers to form a more complete diagnosis. |
| • | Newborn Screening. Many common newborn screening panels such as cystic fibrosis and various ethnically dependent ailments require the identification of multiple (often five or more) genetic markers which makes traditional testing impractical. Classic testing algorithms are limited in that they utilize subjective analysis of a newborn’s parental health history with little to no genetic evaluation. |
| • | For example, because various Ashkenazi Jewish inherited disorders (such as Tay Sachs) are autosomal recessive, meaning not all parents who carry the necessary genes to produce the disease exhibit symptoms, a targeted genetic evaluation of the affected genes would inform the clinician if the newborn is at risk for developing the disease in question. |
| • | Blood Banking. As genetic testing products have become more prevalent, more accurate and more cost-effective, the use of genetic tests in screening in the blood banking market has grown, and is expected to continue to grow. |
92
Table of Contents
| • | Traditional testing algorithms follow a tiered procedure where tests are performed in stages, with prominent blood bank “disqualifier tests” being performed first. While the tiered approach reduces unnecessary procedures and eases the overall testing burden, these tests are still being performed individually during each tier. Genetic test multiplexing allows for multiple typing and screening tests within the same run, which can reduce the time and expense involved in satisfying each tier of blood banking tests. |
| • | Key tests in this segment include blood typing/grouping and infectious disease screening (e.g., hepatitis B virus/hepatitis C virus, syphilis, and HIV 1/2). |
As of December 31, 2012, we offered for use on our INFINITI system 23 genetic tests for use in the area of personalized medicine and companion diagnostics, three of which have received FDA 510(k) clearance, five RUO genetic tests for use in the area of pain management, 11 genetic tests for use in the area of mental health, two of which have received FDA 510(k) clearance, 16 genetic tests for use in the area of cardiovascular health assessment, five of which have received FDA 510(k) clearance, 15 RUO genetic tests for use in the area of women’s health, 23 RUO genetic tests for use in the area of cancer and companion diagnostics, 15 RUO genetic tests for use in the area of infectious diseases, six RUO genetic tests for use in the area of genetic disorders, four RUO genetic tests for use in the area of newborn screening, and no tests for use in the area of blood banking. Although we have genetic tests available for use in the areas of genetic disorders and newborn screening, we did not have any material sales or net revenue from sales of genetic tests in these target markets, and no sales in the blood banking market, during the year ended December 31, 2012. As is noted in the tables appearing in “Business—Our Current Test Menu”, many of our 53 genetic tests may be utilized in more than one of our target markets, resulting in particular genetic tests being counted multiple times in the information presented above.
The Limitations of Current Testing Methods
Scientists have developed a variety of genetic analysis methods, including DNA sequencing, gene expression and genotyping, to detect genetic biomarkers. These analytical methods are performed using various genetic testing technologies, the most common being real time polymerase chain reaction, or RT-PCR, which involves amplifying the DNA sequence in question and then detecting the DNA with the use of fluorescent dyes.
These existing testing methods have a number of drawbacks, which we believe have significantly limited their use, including:
| • | High cost per reportable result. Because many existing systems require specialized personnel and/or specialized training to complete complex and extensive protocols, the tests can be time-consuming and result in high labor costs. These processes may also use a significant amount of reagent, which can be costly. The use of supplementary, discrete instrumentation to perform semi-manual tests also increases costs, as compared to a system that automates the discrete processes of genetic testing. |
| • | Centralization of molecular diagnostics market. There may be a reluctance on the part of providers to take advantage of molecular diagnostic testing in those instances where it is not readily available in the geographic region in which the provider is situated. The molecular diagnostic testing market is dominated by large reference laboratories and large hospital laboratories. As a result, healthcare providers may be required to ship patient samples to laboratories not locally situated, which can lead to delays in receiving test results. |
| • | Limited testing menu. Many existing diagnostic systems have limited test menus. As a result, a laboratory may need to purchase many different systems to satisfy its testing needs. This requires separate training of operators on the use and maintenance of each system and may require a significant amount of laboratory bench space. The combination of these factors often makes molecular diagnostic testing in-house impractical. |
| • | Inability to multiplex. In many cases, the predisposition to a genetic disorder, or the presence of a particular disease, condition or genetic variance affecting therapy, is caused by multiple genetic |
93
Table of Contents
| mutations that necessitate testing for multiple biomarkers to diagnose those diseases, conditions or variances. Many existing technologies are only able to examine one biomarker at a time, and, in order to make a diagnosis, the laboratory must perform repeated tests on a sample. Serial testing is expensive, significantly increases the amount of workflow and requires higher sample volumes. |
| • | Limited automation and throughput capability. While a number of companies offer systems that can perform molecular diagnostic tests, these systems tend to automate only certain steps in the testing process. Some of these systems require sequential processing through multiple instruments prior to generating results. In addition, most of these systems do not allow for the testing of multiple patient samples in a single microarray, which does not allow for the cost-benefits of higher throughput. Consequently, utilizing these systems requires a significant amount of time, capital and specialized labor. |
| • | Specialized labor. Specialized laboratory technicians and in some cases specialized training are required to properly perform and evaluate the quality and accuracy of the results of most existing molecular diagnostics technologies. We believe that there is a labor shortage of specialized laboratory technicians in the clinical laboratory market, which, in addition to the cost of this labor, has limited the availability of molecular diagnostic testing and has restrained the growth of the market. |
| • | Inaccurate results and challenges with reproducibility. The additional handling of samples required to operate manual or semi-automated systems can lead to an increased risk of sample contamination and human error. The lack of automation also can lead to problems related to reproducibility of results. In addition, many existing testing systems are not capable of simultaneous target and signal amplification (methods for increasing the detectability of genes) when running a sample, which limits the sensitivity and specificity of these systems and could result in the need for a larger sample size. |
These limitations have created the need in the molecular diagnostics market for a highly integrated system to perform a large menu of automated, cost-effective and easy to use tests with a high degree of accuracy and reproducibility.
The AutoGenomics Solution
We believe our INFINITI system addresses many of the limitations of current molecular diagnostic technologies. Our INFINITI system has been designed to enable a broad range of reference laboratories, hospital laboratories and specialty clinics to start performing, or to more cost-effectively perform, molecular diagnostic testing, which we believe will drive adoption and use of our INFINITI system as well as expand the potential of the molecular diagnostic testing market.
To use our system, an operator only needs to load prepared test samples into an INFINITI analyzer, along with the specific BioFilmChip and Intellipac Reagent Management Module, for the desired test. Once the INFINITI analyzer is loaded and the test is initiated, no further action by the operator is required. After the test is completed, the system generates an electronic report that can be transmitted directly to a laboratory information system.
Our INFINITI system has a number of key advantages, including:
| • | Enhanced cost-efficiency. Our system eliminates the need for complex protocols and manual intervention once a test is initiated, which is intended to reduce the laboratory’s cost of testing by simplifying workflow and reducing the need for highly skilled technicians. |
| • | Ability to decentralize molecular diagnostics. We believe that medium-sized reference laboratories, hospital laboratories and specialty clinics are increasingly seeking to add or expand molecular diagnostics capabilities to treat patients more efficiently and provide a more comprehensive offering, lower the cost of providing healthcare, and participate in the value provided by diagnostic testing. We believe that this trend is being facilitated in part by new technologies like ours that are more automated, |
94
Table of Contents
| easier to use, more cost effective and require less bench space in a laboratory than traditional genetic testing methods. |
| • | Broad menu of tests. As of December 31, 2012, we offered 53 tests as part of our INFINITI system, of which five have received FDA clearance. The balance of our tests are sold on an RUO basis. We believe that this represents one of the broadest available test menus of on a single system. Our INFINITI system is designed to allow for the development of new and enhanced tests without modification to our platform or our INFINITI analyzers. As we increase the number of tests available for use on our INFINITI system, our customers will be able to broaden their molecular diagnostics offerings without additional capital investment or operator training. We believe the depth and breadth of our test menu is a meaningful competitive advantage that will allow laboratories to utilize laboratory space, labor and capital investment more efficiently. We also believe that our offering of five FDA cleared tests is comparable to similar testing system offerings of our competitors, which we believe based on publicly available information ranges from zero to seven FDA-cleared tests. |
| • | Ability to multiplex. Many diseases and patient responses to therapy are caused by multiple genetic mutations that necessitate testing for multiple biomarkers to diagnose those diseases or to predict and/or monitor therapy response. Our INFINITI system is able to detect up to 1,024 individual features of biochemical sensors within a single microarray, which reduces the amount of sample needed, reduces the time required to run the test, and often reduces the need for multiple tests. This capability is advantageous in genetic testing, for example, in HPV screening, which requires testing for numerous biomarkers and is often performed at the same time and using the same patient sample as the Pap test. |
| • | Multiple patient array technology. Our proprietary multiple patient array, or MPA, technology is designed to test up to eight patient samples on a single microarray. This significantly enhances throughput by up to 300% while reducing cost per sample by up to 75% as compared to our single patient microarrays. This MPA technology is particularly well suited for addressing high volume test markets such as those for HPV and TB, and affords us the flexibility to continue to offer competitive pricing while maintaining established margins. |
| • | Better workflow. Our broad offering of INFINITI analyzers combined with the integrated, “load and go” design of the INFINITI system is designed to address our target customers’ varied throughput and workflow requirements. We believe that we can substantially increase a laboratory’s throughput over existing laboratory-developed and other manual and semi-automated tests by enabling them to perform their tests on our highly integrated and automated system that has the ability to multiplex and run high volume MPAs, resulting in better workflow. The INFINITI system can run multiple different tests simultaneously which reduces or eliminates the need for laboratories to run tests in batches. It also eliminates the need for complex protocols and manual intervention once a test is initiated. |
| • | Increased accuracy of results. Human handling of samples is the most common cause of contamination and error in existing technologies. By reducing the risk of human error and contamination, we believe our INFINITI system can provide more accurate and more reproducible test results compared to other, less automated systems. In addition, where certain systems only use target or signal amplification (e.g., PCR), we believe our combined target and signal amplification technologies can increase the sensitivity and specificity over these widely-used stand-alone amplification methods. |
Our Strategy
Our objective is to become a leading provider of genetic tests to a broad array of customers within our target
market segments. We believe our INFINITI system will allow us to achieve this objective by facilitating molecular diagnostic testing by reference laboratories, hospital laboratories and specialty clinics. To achieve our
objective, we intend to:
| • | Capitalize on the capabilities of our INFINITI system to increase penetration within our target market segments. We believe that our INFINITI system’s high level of automation, ability to multiplex and |
95
Table of Contents
| broad test menu are attractive to our target customers in our target market segments as our genetic tests provide an easy to use solution with greater breadth of diagnostic information at a lower cost per reported result than many competing systems. |
| • | Develop and launch new and enhanced tests. We believe that developing a broad menu of genetic tests to run on our system will increase the value of our INFINITI system, drive additional placements of our INFINITI analyzers and increase our consumables sales. We launched our INFINITI system in 2007 with an introductory panel of four tests. As of December 31, 2012, we offered 53 genetic tests and had more than 15 additional tests in development. We believe that the depth and breadth of our test menu is a significant competitive advantage that will allow customers to increase their ability to conduct molecular testing and utilize laboratory space, labor and capital investment more efficiently, as well as generate supplementary revenue. In addition, the depth and breadth of our test menu diversifies our revenue so that we are not dependent on the performance of any single test. The majority of tests that we offer, are developing and intend to develop have established market demand and reimbursement by public and private payors. |
| • | Target molecular diagnostic laboratories with high potential utilization of our INFINITI system. We believe that our INFINITI system’s automation, broad genetic test menu and family of analyzers designed to address various throughput requirements will generate demand from both larger reference laboratories seeking a more flexible and efficient molecular diagnostic platform and from smaller reference laboratories, hospital laboratories and specialty clinics for whom it has not previously been cost-effective to develop their own tests. We offer our family of INFINITI analyzers to our customers through direct sales and rentals, and through our domestic placement plans, in which the customer’s choice of our INFINITI analyzers is placed at the customer’s location at no direct cost to the customer. We believe that our targeted customer base will generate significant recurring demand for our high margin BioFilmChips and Intellipac Reagent Management Modules consumables. |
| • | Expand our domestic sales force and international distribution of our products. We are marketing and selling the INFINITI system in the United States through our own sales and marketing organization, which, as of December 31, 2012, was comprised of ten sales persons located in key metropolitan cities in the United States. We believe there is a meaningful opportunity to further penetrate existing markets and customers as well as enter new markets by adding to the size and scope of our US salesforce. We have established distributor relationships for the marketing of our INFINITI system in 21 countries outside the United States, including Brazil, Canada, China, Mexico and countries in Europe and the Middle East. We also plan to expand our global distribution networks to address increasing international demand in addition to driving increased utilization with our existing distributors. |
| • | Pursue regulatory clearances, approvals and certifications for products and facilities, as necessary. We have received FDA 510(k) clearance for our INFINITI Analyzer and five of our genetic tests and we have obtained a CE mark for our INFINITI Analyzer, our INFINITI Plus Analyzer and 22 of our tests. These CE markings are supported by completed clinical and validation studies that demonstrated the analytical performance of the respective test. We believe that most of the tests we submit to the FDA will require 510(k) clearance rather than the more time-consuming and costly process of obtaining a PMA. |
We intend to seek regulatory clearance or approval, as necessary, for our tests. Currently:
| • | we have submitted notification to the FDA for 510(k) clearance for our UGT1A1 test which is designed to help determine the initial dosing of irinotecan, a leading drug for a broad variety of cancers; |
| • | we are finalizing the protocol for a clinical trial necessary to support a PMA application to the FDA for our HPV-HR tests and intend to commence the clinical trial in 2013; |
| • | we intend to submit a 510(k) notification to the FDA for our CF and our HPV genotyping tests in 2013; |
96
Table of Contents
| • | we are conducting studies in support of and are preparing to submit a pre-IDE application for our CFTR and Flu A-sH1N1 tests; and |
| • | we have initiated clinical studies to support future submissions to the FDA for clearance of several of our other tests, including our 2D6, 3A4, 3A5, ureaplasma urealyticum, mycoplasma genitalium, mycoplasma hominis (Urogen Quad), bacterial vaginosis (BV), candida vaginitis (CV), chlamydia, gonorrhea and KRAS-BRAF genetic tests. |
| • | Align with key opinion leaders at leading institutions and increase scientific awareness of our products. We align with key opinion leaders at leading institutions and clinical research laboratories, including ARUP Laboratories, Cleveland Clinic, Montreal Heart Institute, New York Presbyterian Hospital and San Francisco General Hospital. As either a customer or a collaborative partner, each of these opinion leaders has helped to increase awareness of our system, to demonstrate its benefits relative to existing technologies and to accelerate its adoption in the molecular diagnostics market. We also seek to increase awareness of our products through participation at trade shows and academic conferences where we sponsor lectures by leading scientific figures and through strategic placements of advertisements in various industry publications. We also conduct online webinars and hospital-based grand, or teaching, rounds in laboratories and hospitals to promote the utility of our products to our potential customers. In addition, our INFINITI system has been discussed in several published peer review articles. |
Our Products
Our INFINITI Analyzers
Our INFINITI system includes a family of analyzers that we have developed and are marketing under the names INFINITI and INFINITI PLUS, and that we are developing and intend to market under the names INFINITI PLUS 96 and INFINITI HTS. These analyzers are the core of our INFINITI system. Each model is designed to address customer-specific needs based on the customer’s productivity, workflow and throughput requirements. Our INFINITI analyzers integrate and automate the discrete processes of sample handling, reagent management, hybridization, detection, results analysis and reporting in a self-contained system. They have been designed to operate on a “load and go” basis, which means that to run a genetic test, an operator loads prepared samples into the INFINITI analyzer along with the BioFilmChip microarrays and the Intellipac Reagent Management Modules specific to that test. Once an INFINITI analyzer is loaded and the tests are initiated, no supervision is required. From the perspective of the operator, the test protocols are identical for all of our genetic tests, which eliminates the need to repeatedly train operators when additional tests are added. After the test is completed, the INFINITI analyzer generates an electronic report that can be transmitted directly to a laboratory information system. Our BioFilmChips and Intellipac Reagent Management Modules are test-specific, but are not analyzer-specific so each of our INFINITI analyzers use substantially the same consumables.
The following table illustrates the test capabilities of our different INFINITI analyzers:
| Analyzer |
Capacity per run |
Patient results * | ||
| INFINITI |
24 samples | up to 96 per day | ||
| INFINITI PLUS |
48 samples | up to 192 per day | ||
| INFINITI PLUS 96 (in beta testing) |
96 samples | up to 288 per day | ||
| INFINITI HTS (in beta testing) |
384 samples | up to 1152 per day |
| * | numbers presented are based on average time to complete an HPV-HR Quad test run |
97
Table of Contents
Our Tests
In developing our menu of genetic tests, we are focused on a broad range of markets, with a particular emphasis on the areas of personalized medicine (including pain management, mental health and cardiovascular health assessment), women’s health, oncology, infectious disease, genetic disorders, newborn screening and blood banking, which we believe represent large and growing market opportunities in genetic testing. We believe that by offering a wide variety of tests for each of these markets, each of which can be run on all of our INFINITI analyzers, we will provide significant value to reference and hospital laboratories and specialty clinics in these areas by allowing them to consolidate multiple testing platforms, expand their testing capabilities, increase workflow and reduce costs, and limit additional investment in equipment, personnel and training.
Selected Key Tests in our Focus Market Segments
| • | HPV. We have developed four HPV tests, which are designed for screening and/or genotyping and each of which addresses a different segment of the HPV testing market. Our HPV genetic test portfolio includes our HPV-HR Quad and HPV-HR Hex tests, designed to screen for and genotype 14 high-risk types of HPV simultaneously, our HPV Quad test, designed to screen for 13 high-risk and two low-risk types of HPV, and our HPV Genotyping test, designed to identify 26 high-and low-risk types of HPV. Importantly, our HPV tests identify and separately report on the type of HPV present, as opposed to reporting just on the existence of HPV generally, which assists in determining whether the HPV is a new, repeated or persistent infection. Our HPV-HR Quad, HPV-HR Hex and HPV Quad tests are designed to allow a laboratory to test samples from four to six different patients simultaneously on a single BioFilmChip. We believe our tests offer several competitive advantages over existing HPV screening and genotyping tests, such as the consolidation of multiple testing steps required in screening and genotyping, increased automation, reduction of sample requirements and enhanced accuracy and reproducibility. We plan to seek a PMA for our HPV-HR tests and 510(k) clearance for our HPV Genotyping test. Our HPV-HR Quad, HPV Quad and HPV Genotyping tests have been CE marked in accordance with applicable law. |
| • | Other STDs. We intend to capitalize on the significant market opportunity in the area of STD testing by providing solutions to address some of the inadequacies and inefficiencies of many current testing methods. Our panels are designed to screen for multiple STDs in a single sample and are well suited to test for these infections in a cost-effective manner. We have launched a variety of panels consisting of tests that our customers use to identify numerous organisms associated with STDs. |
| • | Breast Cancer. Breast cancer is the second most-common form of cancer in women. An estimated 227,000 women will be diagnosed with, and approximately 40,000 women will die of, breast cancer in 2012. We have developed tests such as our CHEK-2 and the Breast Cancer Panel-AJ tests, which are designed to determine individuals at greater risk for breast cancer, and our CYP450 2D6T test, which is designed to determine if a woman will benefit from tamoxifen, a frequently prescribed drug for the prevention of breast cancer recurrence. We believe that these tests could play an important role in improving the screening, early diagnosis and effective treatment of certain individuals with, or potentially at risk of developing, breast cancer. |
| • | Colorectal Cancer. The American Cancer Society estimated that, in 2012, there will be over 100,000 new cases of colon cancer and over 40,000 new cases of rectal cancer, and that these diseases will cause more than 50,000 deaths, making it the second leading cause of cancer-related deaths in the United States. Anti-EGFR drugs have emerged as prevalent anticancer therapeutics as they help neutralize EGFR over-activity that has been linked to several cancers, including colorectal cancer. However, certain genetic mutations are associated with poor response to anti-EGFR therapies. We have developed KRAS and KRAS-BRAF tests, which enable laboratories to identify these mutations. We believe that these tests could assist in reducing healthcare costs while providing better patient outcomes. |
| • | Personalized Medicine. We offer 21 tests that may be used by laboratories in the area of personalized medicine. One of our leading personalized medicine offerings is our CYP450 2C19 panel test, which is |
98
Table of Contents
| designed to enable laboratories to identify certain gene variants that affect the metabolism and efficacy of the anticoagulant drug Plavix (clopidogrel). Plavix is the most commonly prescribed anti-platelet drug in the United States, with more than 28 million total prescriptions filled in the United States in 2011, including more than 8 million new prescriptions, based on data from the IMS Health National Prescription Audit. As of March 2010, the FDA now requires the clopidogrel label to include language that informs doctors that patients with CYP450 2C19 mutations have a diminished response to the drug, and thus, such patients may be susceptible to a heart attack, stroke or cardiovascular death due to the ineffectiveness of the drug. This labeling requirement has increased, and future FDA requirements may increase, the demand for genetic tests such as ours. Our other leading tests in this area currently include our CYP450 2C19 Plus, Warfarin and CYP450 2D6I tests, which are designed to enable laboratories to identify certain gene variants associated with responsiveness to certain medications for psychiatric disorders, the oral anticoagulant Warfarin and certain antidepressants and cancer drugs, respectively. Warfarin is the most commonly prescribed anticoagulant in the United States, with more than 33 million total prescriptions filled in the United States in 2011, including more than 15 million new prescriptions, based on data from the IMS Health National Prescription Audit. We believe that our tests could improve patient care and reduce healthcare costs. |
| • | Infectious Disease. Infectious disease is a significant category of the molecular diagnostics market. This market segment continues to increase due to the greater necessity for multiplexed tests that can not only identify the presence of an infection but also provide guidance on therapy, namely subtyping to assist in patient management. Our Multidrug Resistance Tuberculosis (MDR-TB) test is one such application. Tuberculosis is the second most deadly infectious disease in the world and resulted in the death of 1.4 million people in 2011. Our MDR-TB product is designed to enable laboratories to identify Mycobacterium tuberculosis infections (as opposed to nontuberculous mycobacterium infections) while simultaneously determining resistance to the three front-line treatments: rifampin, isoniazid and pyrazinamide. |
| • | Genetic Disorders. The global genetic disorders testing market is expected to reach $2.2 billion by 2017. A key driver in the rapid adoption of molecular diagnostics in genetic and inherited diseases testing is the ability to multiplex multiple markers into a single test. One such test is our Familial Mediterranean Fever (FMF) panel. FMF is an autoinflammatory disease mutation that is carried by significant portions of populations of selected Mediterranean countries. Some countries, such as Turkey, have started testing broad segments of their populations to identify recessive carriers of the mutation and to better inform these individuals’ reproductive choices. FMF disease can cause recurrent fever and renal failure and is often misdiagnosed. In the United States, as many as 1 in 249 are carriers of this disease. The development of this disease has been attributed to mutations in the MEFV gene. Our FMF panel allows laboratories to simultaneously identify 13 common MEFV mutations, covering the five most common variations as well as eight other variations spanning 14 ethnicities. |
| • | Newborn Screening. Neonatal screening programs are increasingly available across the United States and internationally. However, traditional methods for newborn screening allow the testing of only one gene of a particular disease at a time, increasing the burden of testing and adversely affecting patient care by delaying the delivery of critical information. In some cases diagnosis is being performed via subjective evaluation of parental medical histories due to a lack of practical genetic evaluation techniques. As an example, our Ashkenazi Jewish Panel is capable of simultaneously detecting 31 genetic variants that account for eight diseases commonly found among those of Ashkenazi Jewish descent, which greatly facilitates newborn screening by shortening the testing period for these eight diseases from days to hours. |
| • | Blood Banking. Blood banking is a rapidly growing market segment where molecular diagnostic technologies are increasingly utilized to optimize current testing algorithms. We have several tests for blood typing and infectious disease screening currently in development that are intended to take advantage of our multiplexed automation capabilities take advantage of our key strength of multiplexed automation. |
99
Table of Contents
Our Current Test Menu
As of December 31, 2012, we offered the following 53 genetic tests for use with our family of INFINITI analyzers:
| Product (1) |
Application |
Market Segments | ||||||||||||||||||
| Pain Management (5 tests) |
Mental Health (11 tests) |
Cardiovascular risk assessment (16 tests) |
Other Personalized medicine (23 tests) |
Women’s health (15 tests) |
Oncology (23 tests) |
Infectious disease (15 tests) |
Genetic disorders (6 tests) |
Newborn screening (4 tests) | ||||||||||||
| 5-FU (Fluorouracil) |
Metabolism of leading cancer drug/toxicity assessment | ¿ | ¿ | |||||||||||||||||
| ApoE |
Drug metabolism | ¿ | ¿ | ¿ | ||||||||||||||||
| Ashkenazi Jewish Panel |
Diseases more prevalent in the Ashkenazi Jewish population | ¿ | ¿ | |||||||||||||||||
| Bacterial Vaginosis |
Bacterial infection - Sexually transmitted disease | ¿ | ¿ | |||||||||||||||||
| BRAF |
KRAS and BRAF amino acid changes - Metastatic Colorectal Cancer | ¿ | ¿ | |||||||||||||||||
| BRAF XP |
KRAS and BRAF amino acid changes - Metastatic Colorectal Cancer | ¿ | ¿ | |||||||||||||||||
| Breast Cancer Panel - AJ |
Breast cancer risk | ¿ | ¿ | ¿ | ||||||||||||||||
| Candida Vaginitis |
Fungal infection - Sexually transmitted disease | ¿ | ¿ | |||||||||||||||||
| CFTR-15 |
Cystic fibrosis gene mutation | ¿ | ¿ | |||||||||||||||||
| CFTR-31 |
Cystic fibrosis gene mutation | ¿ | ¿ | |||||||||||||||||
| CHEK-2 |
Familial breast cancer risk | ¿ | ¿ | ¿ | ||||||||||||||||
| CODX2 |
Drug metabolism | ¿ | ¿ | ¿ | ¿ | |||||||||||||||
| CT-NG Quad |
Gonorrhea, chlamydia | ¿ | ¿ | |||||||||||||||||
| CYP450 2C19 (2) |
Drug metabolism | ¿ | ¿ | ¿ | ||||||||||||||||
| CYP450 2C19 Plus |
Drug metabolism | ¿ | ¿ | ¿ | ||||||||||||||||
| CYP450 2C19 Plus Duplex |
Drug metabolism | ¿ | ¿ | ¿ | ||||||||||||||||
| CYP450 2C19 QUAD (2) |
Drug metabolism | ¿ | ¿ | ¿ | ||||||||||||||||
| CYP450 2C9 - VKORC1 |
Sensitivity to Warfarin | ¿ | ¿ | ¿ | ||||||||||||||||
| CYP450 2D6I |
Drug metabolism | ¿ | ¿ | ¿ | ¿ | |||||||||||||||
| CYP450 2D6T |
Efficacy of leading breast cancer drug | ¿ | ¿ | ¿ | ||||||||||||||||
100
Table of Contents
| Product (1) |
Application |
Market Segments | ||||||||||||||||||
| Pain Management (5 tests) |
Mental Health (11 tests) |
Cardiovascular risk assessment (16 tests) |
Other Personalized medicine (23 tests) |
Women’s health (15 tests) |
Oncology (23 tests) |
Infectious disease (15 tests) |
Genetic disorders (6 tests) |
Newborn screening (4 tests) | ||||||||||||
| CYP450 3A4 |
Drug metabolism | ¿ | ¿ | ¿ | ¿ | |||||||||||||||
| CYP450 3A5 |
Drug metabolism | ¿ | ¿ | ¿ | ¿ | |||||||||||||||
| CYP450 3A4-3A5 |
Drug metabolism | ¿ | ¿ | ¿ | ¿ | |||||||||||||||
| EGFR |
Lung cancer efficacy | ¿ | ¿ | |||||||||||||||||
| Factor II (FII) (2) |
Increased risk of blood clots | ¿ | ||||||||||||||||||
| Factor II Plus |
Increased risk of blood clots | ¿ | ||||||||||||||||||
| Factor II Plus - V Panel |
Increased risk of blood clots | ¿ | ||||||||||||||||||
| Factor II/V Panel (2) |
Increased risk of blood clots | ¿ | ||||||||||||||||||
| Factor V (FV) (2) |
Increased risk of blood clots | ¿ | ||||||||||||||||||
| Factor V Genotyping |
Increased risk of blood clots | ¿ | ||||||||||||||||||
| Familial Mediterranean Fever Panel |
Familial Mediterranean Fever | ¿ | ¿ | |||||||||||||||||
| FII - FV - MTHFR Panel |
Increased risk of blood clots | ¿ | ||||||||||||||||||
| FII Plus - FV - MTHFR Panel |
Increased risk of blood clots | ¿ | ||||||||||||||||||
| Flu A-sH1N1 |
Flu A-sH1N1 | ¿ | ||||||||||||||||||
| HPV - HR HEX |
Cervical cancer risk | ¿ | ¿ | ¿ | ||||||||||||||||
| HPV - HR Quad |
Cervical cancer risk | ¿ | ¿ | ¿ | ||||||||||||||||
| HPV Genotyping |
Cervical cancer risk | ¿ | ¿ | ¿ | ||||||||||||||||
| HPV Quad |
Cervical cancer risk | ¿ | ¿ | ¿ | ||||||||||||||||
| KRAS |
KRAS amino acid changes | ¿ | ¿ | |||||||||||||||||
| KRAS-BRAF |
KRAS and BRAF amino acid changes - Metastatic Colorectal Cancer | ¿ | ¿ | |||||||||||||||||
| Leuko Quad |
Gonorrhea, chlamydia, trichomonas vaginalis | ¿ | ¿ | |||||||||||||||||
| MDR-1 |
Drug metabolism | ¿ | ¿ | |||||||||||||||||
| MDR-TB |
Drug resistance to leading treatments for tuberculosis | ¿ | ||||||||||||||||||
| Mobi Quad |
Mobiluncus infection | ¿ | ¿ | |||||||||||||||||
| MTHFR |
Increased risk of blood clots | ¿ | ¿ | ¿ | ||||||||||||||||
| NAT-2 |
Bladder and other types of cancer risk | ¿ | ¿ | |||||||||||||||||
| NTM |
Nontuberculous mycobacterium infection | ¿ | ||||||||||||||||||
101
Table of Contents
| Product (1) |
Application |
Market Segments | ||||||||||||||||||
| Pain Management (5 tests) |
Mental Health (11 tests) |
Cardiovascular risk assessment (16 tests) |
Other Personalized medicine (23 tests) |
Women’s health (15 tests) |
Oncology (23 tests) |
Infectious disease (15 tests) |
Genetic disorders (6 tests) |
Newborn screening (4 tests) | ||||||||||||
| Respiratory Virus Panel |
Respiratory virus infection | ¿ | ||||||||||||||||||
| STD-6 Quad |
Gonorrhea, chlamydia, trichomonas and others | ¿ | ¿ | |||||||||||||||||
| TAMX3 |
Drug metabolism | ¿ | ¿ | |||||||||||||||||
| UGT1A1 |
Initial dosing of leading cancer drug | ¿ | ¿ | |||||||||||||||||
| Urogen Quad |
Ureaplasma urealyticum, mycoplasma genitalium, mycoplasma hominis | ¿ | ¿ | |||||||||||||||||
| Warfarin Assay (2) |
Sensitivity to Warfarin | ¿ | ¿ | |||||||||||||||||
| (1) | Unless otherwise indicated, the tests listed in this table have not been cleared or approved for diagnostic use by the FDA and are sold on an RUO basis. |
| (2) | We have received 510(k) clearance for our Warfarin test, our CYP450 2C19 test and our FII, FV, and FII-FV Panel tests. |
Descriptions of Our Tests
The following is a description of our tests that are available for sale.
5-FU (Fluorouracil). Our Fluorouracil, or 5-FU, test is designed to detect and identify point mutations in the Dihydropyrimidine Dehyrogenase, MTHFR and Thymidylate Synthase genes that affect the toxicity and efficacy of 5-FU, a leading chemotherapy drug. 5-FU is given as a treatment for several types of cancer, including colon, rectal, breast, stomach and pancreatic cancers.
ApoE. Our ApoE test is designed to identify 2 mutations on the apolipoprotein E gene that affects the function of ApoE.
Ashkenazi Jewish Panel. Our Ashkenazi Jewish Panel test is designed to detect 31 genetic mutations associated with susceptibility to eight genetic diseases that occur more frequently in the Ashkenazi Jewish population, including Tay-Sachs disease, Canavan disease, Familial Dysautonomia, Gaucher’s disease, Fanconi Anemia, Niemann-Pick disease and Bloom Syndrome.
Bacterial Vaginosis Panel. Our Bacterial Vaginosis Panel is designed to detect the presence of the organisms that can cause six sexually transmitted bacterial infections: Bacteroides fragilis, Gardnerella vaginalis, Mobiluncus mulieris, Mobiluncus curtisii, Atopobium vaginae and Prevotella bivia.
BRAF Panel/BRAF XP. Our BRAF and BRAF XP Panels are designed to identify select variants in regions important for BRAF activity, which we believe could assist in developing proper therapeutic treatment of certain cancers.
Breast Cancer Panel-AJ. Our Breast Cancer Panel-AJ test is designed to identify three genetic mutations common in the Ashkenazi Jewish population in the breast cancer 1, or BRCA1, and breast cancer 2, or BRCA2, genes that are associated with an increased risk of developing breast cancer.
102
Table of Contents
Candida Vaginitis Panel. Our Candida Vaginitis Panel is designed to detect the presence of the organisms that can cause five sexually transmitted fungal infections: Candida albicans, Candida parapsilosis, Candida tropicalis, Candida glabrata and Candida krusei.
CFTR-31. Our CFTR-31 test is designed to detect the presence of 31 mutations in the cystic fibrosis transmembrane conductance regulator, or CFTR, gene which are associated with an increased risk of developing cystic fibrosis.
CFTR-15. Our CFTR-15 test is designed to detect the presence of 15 mutations in the cystic fibrosis transmembrane conductance regulator, or CFTR, gene which are associated with an increased risk of developing cystic fibrosis. This panel of variants are targeted for the international market.
CHEK-2. Our CHEK-2 test is designed to identify select variants of the CHEK-2 gene that are associated with an increased risk of developing breast cancer. Mutations in the CHEK-2 gene also have been associated with other hereditary and somatic (not inherited) cancers, including prostate, lung, colon, kidney, thyroid and ovarian cancers.
CODX2. Our Codeine test is designed to identify select variants of the CYP450 2D6 and UGT2B7 genes, which determine an individual’s ability to metabolize codeine. Certain individuals exhibiting these variants transform codeine into morphine, potentially resulting in toxicity to nursing infants whose mothers have been prescribed the drug.
CT-NG Quad. Our CT-NG Quad test is designed to detect the presence of Chlamydia trachomatis and Neisseria gonorrhea in four different patient samples simultaneously on a single BioFilmChip.
CYP2C19 Panel / CYP2C19 QUAD. Our CYP2C19 single test Panel and four test Panel are designed to identify select variants of the CYP450 2C19 gene that affect the metabolism and efficacy of the anticoagulant drug Plavix (clopidogrel).
CYP450 2C19 Plus/CYP450 2C19 Plus Duplex. Our single test panel and two test panel CYP450 2C19 Plus tests are designed to identify select variants of the CYP450 2C19 gene that affect the efficacy of many drugs, including diazepam (an antiepileptic drug) and pantoprazole (a proton pump inhibitor).
CYP450 2C9-VKORC1. Our CYP450 2C9-VKORC1 test is designed to identify select variants of the CYP450 2C9 and VKORC1 genes that affect the metabolism of warfarin. The test is designed to detect expanded numbers of CYP450 2C9/VKORC1 variants found in certain ethnic groups as compared to our Warfarin test.
CYP450 2D6I. Our CYP450 2D6I test is designed to identify select variants of the CYP450 2D6 gene that affect the efficacy of many drugs, including the antidepressants Paroxetine and Fluoxetine, and the cancer drug Tamoxifen.
CYP450 2D6T. Our CYP450 2D6T test is designed to identify select variants of the CYP450 2D6 gene that affect the efficacy of Tamoxifen, a breast cancer drug.
CYP450 3A4 / CYP450 3A5/CYP450 3A4-3A5. Our CYP450 3A4, CYP450 3A5 and CYP450 3A4-3A5 tests are designed to identify select variants of the CYP450 3A4 and CYP450 3A5 genes that affect the metabolism of many drugs, including most calcium channel blockers, most benzodiazepines and human immunodeficiency virus, or HIV, protease inhibitors. The presence of these variants may be used to determine the cause of amplified side effects to a drug and unresponsiveness to the therapeutic effects of a drug.
EGFR. Our EGFR test is designed to identify receptors that may indicate whether cancer patients will respond to drugs that inhibit EGFR expression, such as Tarceva and Gefitinib.
Factor II (FII) / Factor V (FV) / FII/V Panel. Our Factor II, Factor V and FII-V Panel tests are designed to identify select variants of the Factor II and Factor V genes associated with an increased risk of developing blood clots. Each of these tests received 510(k) clearance from the FDA in February 2007.
103
Table of Contents
Factor II Plus / Factor II Plus–V Panel / FII-FV-MTHFR Panel / FII Plus-FV-MTHFR Panel. Our Factor II Plus, Factor II Plus-V Panel, FII-FV-MTHFR Panel, and FII Plus-FV-MTHFR Panel thrombophilia tests are designed to identify select variants of the Factor II, Factor V and Methylenetetrahydrofolate reductase, or MTHFR, genes associated with an increased risk of developing blood clots.
Factor V Genotyping. Our Factor V genotyping test is designed to detect 6 select variants of the Factor V gene common to multiple ethnicities.
Familial Mediterranean Fever Panel. Our Familial Mediterranean Fever, or FMF, panel is designed to detect 13 genetic mutations in the MEFV gene, with intent to cover the most prominent disease-causing mutations in all afflicted ethnicities.
Flu A-sH1N1. Our Flu A-sH1N1 test is designed to detect the presence of Flu A and 2009 H1N1, common respiratory viruses found in the human respiratory tract.
HPV HR Hex. Our HPV HR Hex test is designed to screen six different patient samples for 14 types of HPV simultaneously on a single BioFilmChip.
HPV-HR Quad. Our HPV–HR Quad test is designed to genotype 14 high-risk types of HPV in four different patient samples simultaneously on a single BioFilmChip.
HPV Genotyping. Our HPV Genotyping test is designed to identify 26 high and low-risk HPV types and help identify women at high risk for the development of cervical disease and cervical cancer.
HPV Quad Screening. Our HPV Quad Screening test is designed to screen four different patient samples for 13 high-risk and two low-risk types of HPV simultaneously on a single BioFilmChip.
KRAS. Our KRAS test is designed to identify select mutations of the KRAS gene in colorectal cancer patients, who may not benefit from anti-EGFR therapies, such as Cetuximab and Panitumumab.
KRAS-BRAF. Our KRAS-BRAF test is designed to identify select mutations of the BRAF gene and the KRAS gene in metastatic colorectal cancer patients who have been found to not respond to certain anti-EGFR therapies, such as Cetuximab and Panitumumab.
Leuko Quad. Our Leuko Quad test is designed to detect Chlamydia trachomatis, Neisseria gonorrhea and Trichomonas vaginalis in four patient samples simultaneously on a single BioFilmChip.
MDR-1. Our MDR-1 test is designed to identify select variants of the MDR-1 gene, which is helpful in predicting how certain drugs will penetrate through different pharmacological barriers in the body and how new anti-cancer or anti-parasite agents will interact with MDR-1 and potential liver toxicity.
MDR-TB. Our multidrug-resistant tuberculosis, or MDR-TB, test is designed to detect the presence of tuberculosis and assess drug resistance to the tuberculosis treatments rifampin, isoniazid and pyrazinamide.
Mobi Quad. Our Mobi Quad test is designed to detect the presence of the organisms that can cause two sexually transmitted bacterial infections: Mobiluncus mulieris and Mobiluncus curtisii.
MTHFR. Our MTHFR Panel thrombophilia test is designed to identify select variants of the Methylenetetrahydrofolate reductase, or MTHFR, gene associated with an increased risk of developing blood clots.
NAT-2. Our N-acetyltransferase 2, or NAT-2, test is designed to identify select variants of the NAT-2 gene which are associated with an increased risk of developing bladder and other types of cancer.
NTM. Our nontuberculous mycobacterium, or NTM, test is designed to detect the presence of 12 of the most common pathogenic NTMs from a single sample.
104
Table of Contents
Respiratory Virus Panel. Our Respiratory Virus Panel test is designed to detect the presence of 24 common respiratory viruses found in the human respiratory tract.
STD-6 QUAD. Our STD-6 QUAD test is designed to detect the presence of six common microorganisms that cause sexually transmitted microorganisms (Chlamydia trachomatis, Neisseria gonorrhea, Trichomonas vaginalis, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium) in four different patient samples simultaneously on a single BioFilmChip.
TAMX3. Our TAMX3 test is designed to identify select variants of the CYP450 2D6, CYP450 3A5 and SULT1A1 genes, which affect the metabolism of certain drugs, including the cancer drug Tamoxifen.
UGT1A1. Our UGT1A1 test is designed to identify a select variant of the UGT1A1 gene that affects the toxicity of Irinotecan, a leading cancer drug.
Urogen Quad. Our Urogen Quad test is designed to detect Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in four patient samples simultaneously on a single BioFilmChip.
Warfarin Assay. Our Warfarin test is designed to identify select variants of the CYP450 2C9 and Vitamin K epoxide reductase, or VKORC1, genes that affect the metabolism of the oral anticoagulant warfarin. This test received 510(k) clearance from the FDA in January 2008.
105
Table of Contents
Tests In Development
We have a demonstrated track record of successfully developing genetic tests to address current and anticipated customer needs. We are currently developing more than 15 genetic tests for use on our family of INFINITI analyzers. Historically, we have introduced greater than five new or enhanced tests per year. Our tests under development include the following:
| Product in Development | Application | Market Segments | ||||||||||||||||||
| Pain Management | Mental Health | Cardiovascular risk assessment |
Other Personalized medicine |
Women’s health | Oncology | Infectious disease | Genetic disorders | Newborn screening | ||||||||||||
| Alpha-1-antitrypsin |
Alpha-1-antitrypsin deficiency | ¿ | ¿ | |||||||||||||||||
| ALS |
Lou Gehrig’s Disease | ¿ | ||||||||||||||||||
| APO-E QUAD |
Drug metabolism; 4 patient format | ¿ | ¿ | ¿ | ||||||||||||||||
| C. diff |
Clostridium difficile, which can cause intestinal disease | ¿ | ||||||||||||||||||
| Carbamazepine |
Management of anti-epilepsy drug | ¿ | ¿ | ¿ | ||||||||||||||||
| HSV 1/2 |
Detects Herpes Simplex Virus | ¿ | ¿ | |||||||||||||||||
| IL-28B |
HCV anti-viral response | ¿ | ¿ | |||||||||||||||||
| MRSA-HAI |
Hospital acquired infection | ¿ | ||||||||||||||||||
| PIK3CA |
PIK3CA gene, which is linked to ovarian cancer | ¿ | ||||||||||||||||||
| Resolve |
Detects bacterial, yeast and trichomonas causes of vaginosis/ vaginitis | ¿ | ¿ | |||||||||||||||||
| RVP—10 |
Respiratory virus infection | ¿ | ||||||||||||||||||
| TB-MDR-8 |
Drug resistance tuberculosis; 8 patient format | ¿ | ||||||||||||||||||
| Thalassemia |
Genetic Blood Disorder | ¿ | ||||||||||||||||||
| TPMT |
Thiopurine metabolism | ¿ | ¿ | |||||||||||||||||
| Urogen Plus QUAD |
Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma genitalium, Mycoplasma hominis | ¿ | ¿ | |||||||||||||||||
| Warfarin Resistance Panel |
Warfarin Resistance Panel | ¿ | ¿ | |||||||||||||||||
Descriptions of Our Tests in Development
Alpha-1-antitrypsin. We are developing our alpha-1-antitrypsin (AAT) test to identify 2 mutations on the SERPINA1 gene that confer AAT deficiency, causing COPD and liver disease.
ALS. We are developing our amyotrophic lateral sclerosis, or ALS, test to identify select genetic variants associated with the development of the inherited form of ALS. ALS, which is often referred to as “Lou Gehrig’s Disease,” is a progressive neurodegenerative disease that affects nerve cells in the brain and the spinal cord.
106
Table of Contents
ApoE QUAD. We are developing our ApoE QUAD test to identify 2 mutations on the apolipoprotein E gene that affects the function of ApoE in four patient samples simultaneously on a single BioFilmChip.
C. diff. We are developing our C. diff. test to detect the presence of Clostridium difficile, or C. diff., which is a bacterium that can cause symptoms ranging from diarrhea to life-threatening inflammation of the colon.
Carbamazepine. We are developing our carbamazepine test to identify a select variant of the human leukocyte antigen, or HLA, gene that is associated with an increased risk of serious dermal toxicities to carbamazepine in individuals with Asian ancestry. Carbamazepine is a drug used to treat epilepsy, bipolar disorder and neuropathic pain.
HSV1/2. We are developing our HSV1/2 test to detect and differentiate the herpes simplex virus 1 and 2, which cause cold sores and genital ulcers, respectively.
IL-28B. We are developing our IL-28B test to identify a mutation in the interleukin-28 gene, which can predict HCV anti-viral response.
MRSA-HAI. We are developing our MRSA-HAI test to detect the presence of certain microorganisms that may be associated with hospital acquired infections, or HAIs, including MRSA.
PIK3CA. We are developing our PIK3CA test to identify a select variant of the PIK3CA gene which is linked to gynecological malignancy, including ovarian cancer.
Resolve. We are developing our Resolve test to detect and differentiate causes for bacterial vaginosis, candida vaginitis, and trichomoniasis in a single assay.
RVP-10. We are developing our RVP-10 test to detect the presence of 10 common respiratory viruses found in the human respiratory tract.
TB-MDR-8. We are developing our TB-MDR-8 test to detect the presence of tuberculosis and assess drug resistance to the tuberculosis treatments rifampin, isoniazid and pyrazinamide in eight patient samples simultaneously on a single BioFilmChip.
Thalassemia. We are developing our Thalassemia test to identify select variants of the beta globin genes associated with beta thalassemia. Beta thalassemia is a group of blood disorders that affect the production of normal hemoglobin and causes different forms of anemia.
TPMT. We are developing our TPMT test to identify mutations in the TPMT gene that affect the metabolism of thiopurine drugs.
Urogen Plus QUAD. We are developing our Urogen Plus Quad test to detect Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis and Mycoplasma genitalium in four patient samples simultaneously on a single BioFilmChip.
Warfarin Resistance Panel. We are developing our Warfarin Resistance Panel to identify with a single test select variants of the CYP450 2C9, VKORC1, Factor II and Factor V genes that play a role in resistance to warfarin.
The development of the tests described above are subject to a number of factors, some of which are beyond our control, including factors impacting the cost of development, changes in customer demand and the results of our research and development activities. For these or other reasons, we may decide to cease development of any of these tests at any time. As a result, we cannot assure you that we will develop the tests described above on a
107
Table of Contents
timely basis or at all. In addition, we do not currently have any plans to seek regulatory approvals or clearances for any of our tests in development. Our decision to seek FDA approvals or clearances domestically, and CE marking internationally, for our genetic tests is made on a test-by-test basis, and is based on a variety of factors, including the regulatory environment for the use of genetic tests, the demand by our existing and target customers for particular genetic tests that have received regulatory approvals or clearances of particular genetic tests, the competitive environment for the use of genetic tests that have received regulatory approvals or clearances versus similar tests that have not, and the expense and time required to obtain regulatory approvals or clearances for particular genetic tests.
Our Technology
Our INFINITI system uses four key technological innovations that integrate and automate the discrete processes of sample handling, reagent management, hybridization, detection and results analysis of genes into a fully integrated, self-contained molecular diagnostics system. These four innovations are: (1) our INFINITI analyzers; (2) our BioFilmChip microarrays; (3) our Intellipac Reagent Management Module; and (4) our multiplexing test format (the ability to generate many different laboratory results from one patient sample at one time).
| INFINITI Analyzers
Our family of INFINITI analyzers are a line of instruments offering different test throughput using the same BiofilmChip, reagents, disposables and process to generate a test result. Our INFINITI analyzers are an automated, multiplexing, continuous flow, random access microarray instrument that are designed to integrate all the discrete processes of genetic testing, including sample handling, reagent management and detection for the analyses of DNA sequences, into a self-contained system. Our INFINITI analyzers feature a built-in confocal microscope and a temperature cycler for target and signal amplification, and are |
| |
| designed to operate in a random access mode. Our INFINITI analyzers have been designed to operate on a “load and go” basis, which means that to run a test, an operator need only load prepared samples into the applicable INFINITI analyzer along with the test-specific BioFilmChips, which are the multilayer microarrays on which tests are run, and the test-specific Intellipac Reagent Management Modules, which are the reagent-holding conduit designed to communicate all relevant information about a test to the INFINITI analyzers without any intervention from the operator. Once the INFINITI analyzer is loaded and the tests are initiated, no supervision is required. From the perspective of the operator, the test protocols are identical for each of our genetic tests, which eliminates the need to repeatedly train operators when additional tests are added. After the test is completed, the INFINITI analyzer generates an electronic report that can be transmitted directly to a laboratory information system. | ||
The hardware in each of our INFINITI analyzers is controlled by our proprietary QMatic scheduling software, which is embedded within the on-board computer. The QMatic software has a schedule manager that is designed to control all operations of the INFINITI analyzer, including test protocol, fluid handling, robotics, optical detection, and results analysis. Some key features include: (1) processing of multiple patient samples simultaneously; (2) running multiple samples and multiple tests at the same time; (3) test protocol monitoring; and (4) multiplexing different patient samples on the single microarray for increased throughput and reduced cost.
108
Table of Contents
Tests are processed automatically and read by the built-in confocal microscope. Results are analyzed and presented in numerical and graphical format. The data generated is analyzed and formatted as a result report. The electronic results report can be reviewed via the liquid crystal display, or LCD, screen or can be transmitted directly to a laboratory information system.
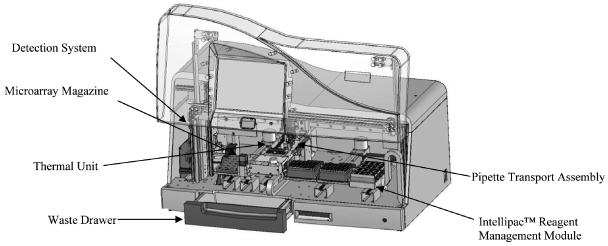
INFINITI Analyzer functional layout
| BioFilmChip
Our proprietary BioFilmChip microarrays consist of multiple layers of a hydro-gel matrix coated on polyester film sandwiched between a plastic base and a reaction body to form a microarray. Polyester film, with length of up to 10,000 feet and width of up to 18 inches, is coated with four different layers of |
BioFilm cross-sectional view | |||
| emulsions and then cut into sections for each BioFilmChip. Each of the emulsion layers has a unique formulation designed to address a specific function necessary for the film to act as the base of the microarray. The microarrays are printed with 36 to 1,024 individual features of biochemical sensors depending on the requirements of the test. These spotted microarrays are then packaged into specifically designed magazines and are used on all INFINITI analyzers. | ||||
|
Microarray assembly Components |

Microarray |
Microarray magazine | ||
109
Table of Contents
| Intellipac Reagent Management Module
Our proprietary Intellipac Reagent Management Modules hold the reagent needed to run our specific genetic tests. The reagent module is designed to communicate all relevant information about a test to the INFINITI analyzer without any intervention from the operator, saving time and preventing errors. A read-write memory chip embedded in the module saves test-specific information on the module, including reagent identification, expiration dates, lot number, amount of reagent remaining for future tests, specific instructions for test processing, the time last used and the serial number for the instrument. The INFINITI analyzer opens the reagent module and breaks the seal, reads all the needed information and prompts the operator for further action if needed. |

Intellipac Reagent module |
Microarray Test Methods
The BioFilmChip has a set of unique universal immobilized capture probes. A target gene-specific detection primer is hybridized (the reaction of complementary DNA sequences) to the PCR amplicon of the sample. (PCR, or Polymerase Chain Reaction, is an enzymatic reaction that makes millions of copies of DNA. The PCR-copied products are known as PCR amplicons.) The hybridized detection primer is designed to extend in the presence of a fluorescence precursor to produce a fluorescent extension product that hybridizes to the microarray (the process increases the signal so that the detection system can read it). The detection primer has two complementary parts: one target specific and the other immobilized capture probe specific. After hybridization and washing of the excess unbound probes, the chip is scanned for fluorescence and the data is analyzed. Where certain systems only use target or signal amplification, we believe our combined target and signal amplification technologies can increase the sensitivity and specificity over these widely-used stand-alone amplification methods. For example, we have conducted internal studies that have demonstrated that our system has achieved sensitivity to the level of detection of 10 copies of mycobacterium tuberculosis and five copies of HPV, which we believe is more sensitive than many other available tests.
Microarray test methods are subject, however, to the possibility of false negative test results due to unexpected gene sequence variations. Nearly all genetic tests are designed to detect a sequence in the given region on the targeted genome. Because genetic test methods are premised upon identifying these specific gene sequences, the phenomenon of sequence variance in a gene away from the expected, mapped sequence being measured will result in the sample not being detected and the result being reported as a negative test. This is common to all genetic tests, and as a result laboratory technicians and molecular biologists are generally aware of this possibility. Although unexpected gene sequence variations tend to be rare occurrences, when we or other industry participants identify that unexpected gene sequence variations are recurring, and that there is a market demand to test for this sequence variance, we may be inclined to modify our tests or develop new tests, to the extent commercially feasible, to capture the newly identified variations. These test modifications or new test developments go through our standard development process, and can involve significant time and expense.
Multiple Patient Microarray
Our proprietary multiple patient array, or MPA, technology is designed to test up to eight patient samples on a single microarray. This significantly enhances throughput by up to 300% while reducing cost per sample by up to 75% as compared to our single patient microarrays. We are also developing an application to produce an MPA that permits samples from up to ten patients to be processed simultaneously on a single microarray.
Our MPA technology, when implemented in a microarray, first processes the patient samples individually. During this process, each patient sample is assigned a separate set of detection primer tags. The detection primer tags correspond to one of four separate zones on the microarray (one zone for each patient sample). After the
110
Table of Contents
dye-labeled probes bind to the specific site on the microarray that corresponds to its detection primer tag, the INFINITI analyzers scan for the presence of the dye-labeled probes at the appropriate sites to determine whether the genetic variant being tested for is present in each of the patient samples.
Microarray scanning and result interpretation
The optics module in our INFINITI analyzers is a lightproof assembly comprised of a camera, a laser and a photomultiplier tube. Using an excitation wavelength from the laser light source, the camera takes micron-level pictures of reference fluorescent dye spots on the BioFilmChip. The integrated software uses that data to calculate the location of the spots on the specific microarray, and the optics module scans those calculated locations. The optics module scans and analyzes the microspots on the microarray. We have demonstrated capability to increase the number of spots on a BioFilmChip to up to 1,024.
|
|

|
Images of low- to medium-density spotted microarrays
Research and Development
Our research and development efforts focus on developing additional genetic tests and INFINITI analyzers, improving or enhancing current tests and supporting clinical studies and trials for certain genetic tests. We spent $3.6 million, $2.8 million, $2.1 million and $1.7 million on research and development in the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2011 and 2012, respectively. We believe that developing a broad menu of tests should increase the value of our system, drive additional placements and increase consumable sales. We plan to address the areas of women’s health, cancer, personalized medicine (including pain management, mental health and cardiac risk assessment), infectious disease, genetic disorders, newborn screening and blood banking. We also focus our research and development efforts on improving existing tests as new genetic research becomes available. For example, we conducted various studies to detect alleles involved with warfarin metabolism that are more common among certain ethnic groups. This resulted in the development of our CYP450 2C9 / VKORC1 test, which detects Warfarin sensitivity amongst diverse ethnic groups. We also have conducted, and intend to conduct, clinical studies and trials to support our FDA and other
111
Table of Contents
regulatory clearances or approvals, as necessary, and to explore opportunities to enhance the INFINITI analyzer. We have entered into, and expect to continue to enter into collaborative relationships with leading research and academic institutions for the development of additional or enhanced tests to further increase the depth and breadth of our genetic test menu. Where appropriate, we enter into royalty agreements with our collaborative partners to license intellectual property for use in our tests.
Customers, Sales and Marketing
Our sales and marketing strategy is to focus on key customers and market segments to establish a large base of customers that provide genetic analysis services for clinical information to guide and manage therapy and medical treatment. We focus our sales efforts on both clinical laboratories seeking a more flexible and efficient molecular diagnostic platform, and from smaller reference laboratories, hospital laboratories and specialty clinics for whom it has previously not been cost-effective for them to develop their own tests. Our marketing efforts emphasize the workflow efficiency, low cost, ease of use, bench-top convenience, high quality and consistent results of our system, as well as the potential versatility afforded by a broad genetic test menu on a single system. Our goal is to place a large base of INFINITI analyzers in the market to generate sales of our high-margin testing consumables, including our BioFilmChips and Intellipac Reagent Management Modules.
In the United States, we offer our family of INFINITI analyzers for placement with customers through direct sales, monthly rental plans and our domestic placement plan. Under the domestic placement plan, an INFINITI analyzer is placed at the customer’s location at no direct cost to the customer to generate significant recurring demand for our high-margin testing consumables, including our test-specific BioFilmChips and Intellipac Reagent Management Modules. In the event that certain consumable sales thresholds are not met we have the right to reclaim our INFINITI analyzer from the customer, which we may exercise at our discretion. To execute our marketing strategy, we have established a direct sales force, which, as of December 31, 2012, was comprised of ten sales persons located in key metropolitan cities in the United States. To supplement our sales and marketing effort, we maintain a customer support team that is comprised of training specialists, hotline staff, field service engineers and test specialists. Our technical service line is staffed by experienced molecular biologists and engineers who are well trained on the functioning of the INFINITI system and are able to provide assistance rapidly to domestic customers. We also have assigned product managers who focus on the major molecular diagnostics categories of infectious disease, cancer, genetic disorders and pharmacogenetics to develop product specific strategies to identify market opportunities and to position the product against competitors to maximize market penetration.
Our INFINITI analyzer is offered on a direct sale basis internationally through our network of distributors. We have established distributor relationships for the marketing of our INFINITI system in 21 countries outside the United States, including Canada, China, Mexico, and countries in Europe and the Middle East. We plan to expand our global distribution networks to increase international sales, with an emphasis on additional countries in Europe, Asia, the Middle East and South America.
Our consumables sales are made primarily pursuant to standard purchase orders that may be manufactured, scheduled, rescheduled or canceled on relatively short notice. Thus, we do not have a significant backlog. We have derived, and expect to continue to derive, a significant portion of our revenue from a few major customers. For fiscal year 2010 we had two customers, CDK and Bostwick Laboratories, and for fiscal year 2011 we had three customers, American International Biotechnology, or AIB, Bostwick Laboratories and Natural Molecular Testing Corporation, or NMTC, that each represented between 12% and 14% of our net revenue in each year. For the nine months ended September 30, 2012, we had two customers, AIB and NMTC, that represented 17% and 47% of our net revenue, respectively. Our continued business relationship with these customers, and the amount of purchases and timing of payment by these customers, may be impacted by several factors beyond our control, including product offerings by our competitors, pricing pressures or the financial health of these customers. The loss of any of these customers, or a significant decline in sales to or a delay in collection of payments from any of these customers, would materially adversely affect our business, financial condition and results of operations.
112
Table of Contents
Our recent concentration of net revenue with a single customer is in part due to this customer’s being an early adopter of our INFINITI system and related technologies, which has led to increased sales to this customer. We do not have any firm commitments with this customer and our sales to this customer can decrease at any time. For example, in the three months ended December 31, 2012, we experienced a substantial decrease in consumable sales revenue to this customer, we believe as a result of this customer increasing its purchases of equivalent products from one of our competitors. We also intend to take steps to attempt to alleviate our customer concentration following the completion of this offering, including by hiring additional sales and marketing personnel which we believe will allow us to acquire additional customers and distributors and increase sales to existing customers.
Our distributor agreements allow our distributors to purchase our products from us at a discount to market price and to resell them to customers in the territories covered by the respective agreements. Under these distributor agreements, the distributors generally are responsible for all customer sales, support and service activities relating to our products in the applicable countries. The distributor agreements generally also provide the distributors with the exclusive right to sell products in the applicable country or countries, so long as they meet minimum purchase requirements. For those distributor agreements that do not include minimum purchase requirements, the distributor is required to use best efforts to meet specified sales objectives. Our distributor agreements are generally for between three and five year terms and either provide for automatic renewal or require the parties to negotiate extensions in good faith. Either party generally may terminate the distributor agreements (subject to applicable notice and cure periods) if the other party materially breaches the agreement. We also may terminate the distributor agreements in the event that (i) minimum purchase requirements (if applicable) are not met, (ii) in the event the distributor experiences certain insolvency or bankruptcy events or (iii) upon other specified events. Under certain of the distributor agreements, the distributor may terminate the agreement after a specified notice period has expired if it does not have appropriate regulatory approvals to market and sell our products.
To increase company and product awareness, we attend major trade shows for the diagnostic industry and actively participate in scientific abstract presentations by key customers. We also collaborate with research partners in preparing publications for leading journals, advertise our products and sponsor molecular diagnostics events. In addition, we conduct online seminars and grand, or teaching, rounds to promote the utility of our products to our potential customers. Our system also has been discussed in several published peer review articles.
Manufacturing
We operate within an approximately 125,000 square foot building in Vista, California, that houses our research and development, manufacturing and warehousing operations and our administrative offices, under a lease that expires in December 2029.
We manufacture our family of INFINITI analyzers, BioFilmChips and Intellipac Reagent Management Modules in-house at our Vista facility. We have implemented a fully integrated enterprise resource planning software system developed specifically for medical manufacturing companies. We have relationships with vendors who are sources of raw materials and components for our product offerings, including reagents, oligonucleotides used for microarray spotting and film coating for our microarrays. Film coating capacity is becoming less available in the marketplace as film vendors move away from analog formats and focus increasingly on digital formats; however, we believe we have sufficient coating capacity for the foreseeable future, and we are in the process of developing the ability to coat film in-house.
Our Vista facility is registered with the FDA as a Medical Device Manufacturing Establishment. We have established a quality system that we believe is in compliance with the FDA’s Quality Systems Regulations and we have obtained ISO 13485:2003 certification for our Vista facility. We manufacture all of our products at the Vista facility under established quality system guidelines. Our quality system covers all phases of product design and development, the manufacturing operations from purchasing to shipping, product distribution, installation and servicing.
113
Table of Contents
Competition
We face competition in the molecular testing markets primarily from LDTs and products developed by companies such as Abbott Laboratories Inc., Becton, Dickinson and Company, Cepheid, GenMark Diagnostics, Inc., Hologic, Inc. and its recently acquired subsidiary, Gen-Probe Incorporated, Luminex Corporation, Nanosphere, Inc., Qiagen N.V., Roche Holding Ltd. and Sequenom, Inc. We believe that the INFINITI system competes with products offered by these companies on the basis of test menu, cost-effectiveness, ease of use, accuracy of results, throughput and multiplexing capability and, with respect to some of our tests, FDA clearance. We believe that only two of the competitors listed above currently offer on a commercial basis self-contained, integrated systems for molecular diagnostic testing, and neither of these two competitive systems allow for mutliplexing.
Many of our competitors have significantly greater financial, technical, research and other resources and larger, more established marketing, sales and distribution services organizations than we do. Many of our competitors also offer broader product lines outside of the molecular research use or diagnostic testing market, and many have greater brand recognition than we do. Moreover, our competitors may make rapid technological developments that may result in our technologies and products becoming obsolete before we recover the expenses incurred to develop them or before they generate significant revenue. Our success will depend, in part, on our ability to establish successful marketing, sales and distribution efforts.
Intellectual Property and Trade Secrets
As of December 31, 2012, we had 13 issued patents, including seven in the United States and six foreign counterparts, and four pending patent applications in the United States. Our issued patents expire between 2018 and 2023. Our patent and patent applications cover certain of the technologies relating to the method and design of the BioFilmChip and its film technology, the design of the INFINITI analyzer, the Intellipac Reagent Management Module, and the system’s chemistry methods, system robotics and signal enhancements.
In addition to our own genetic test development efforts, we are currently using, and intend to use in the future, certain tests and biomarkers in the INFINITI system that have been developed by third parties. While a significant amount of intellectual property in the field of genetics is already in the public domain, one of our tests requires, and some of the future tests developed by us, or by third parties on our behalf for use in our system, may require, that we license the right to use certain intellectual property from third parties and pay customary royalties or make one time payments. We currently license certain patents used in our UGT1A1 test. The license requires that we pay $75,000 and a royalty equal to six percent of net sales of our tests that incorporate the patented technology from the Mayo Foundation for Medical Education and Research, or Mayo Laboratories. The patents covered by the license expire between July 5, 2015 and May 28, 2024. The license expires when the last patent covered by the license expires. The license is terminable by us upon 90 days notice or by the licensor upon certain bankruptcy or liquidation events involving us, or 30 days after licensor’s written notice of our failure to observe a material obligation of the license. We have granted a license to one of our patents relating to oligonucleotides of CYP450 1A1, CYP450 3A4, CYP450 2D6 and NAT-2 to Mayo Laboratories. The license requires the licensee to pay, among other amounts, a royalty equal to six percent of net sales of any products that incorporate the patented technology. The license is terminable by the licensee upon 30 days notice or by us upon the licensee’s failure to cure a payment default within 45 days or its failure to cure a material breach of the license other than a payment default within 90 days.
Government Regulation
The healthcare industry, and thus our business, is subject to extensive federal, state, local and foreign regulation. Both federal and state governmental agencies continue to subject the healthcare industry to intense regulatory scrutiny, including heightened civil and criminal enforcement efforts. We believe that we have structured our business operations and relationships with our customers to comply with applicable legal
114
Table of Contents
requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise. We discuss below the statutes and regulations that are most relevant to our business and most frequently cited in enforcement actions.
U.S. Food and Drug Administration Regulation
The Federal Food, Drug and Cosmetic Act, or FFDCA, includes within the definition of a medical device any instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory, intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals. Our in vitro diagnostic products are considered by the FDA to be medical devices. Among other things, pursuant to the FFDCA and its implementing regulations, the FDA regulates the research, testing, manufacturing, safety, labeling, storage, recordkeeping, pre-market clearance or approval, marketing and promotion, and sales and distribution of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. In addition, the FDA regulates the export of medical devices manufactured in the United States to international markets.
FDA’s Pre-Market Clearance and Approval Requirements
Unless an exemption applies, each medical device that we wish to market in the United States must first receive either clearance of a 510(k) pre-market notification or approval of a PMA from the FDA. The FDA’s 510(k) clearance process usually takes from three to twelve months, but it can take significantly longer and clearance is never guaranteed. The process of obtaining PMA approval is much more costly, lengthy and uncertain. It generally takes from one to three years or even longer and approval is not guaranteed. Our INFINITI Analyzer, as well as our Factor II, Factor V, and Factor II-V Panel tests received 510(k) clearances in February 2007. Our Warfarin test received 510(k) clearance in January 2008 and our CYP2C19 test received 510(k) clearance in October 2010.
Whether a device must undergo either the 510(k) clearance or PMA approval process is based upon statutory criteria. These criteria include the level of risk that the agency determines is associated with the device and a determination whether the product is a type of device that is similar to devices that are already legally marketed. Devices deemed to pose relatively less risk are placed in either Class I or II, which requires the manufacturer to submit a pre-market notification requesting 510(k) clearance, unless an exemption applies. The pre-market notification must demonstrate that the proposed device is “substantially equivalent” in intended use and in safety and effectiveness to a legally marketed “predicate device” that is either in Class I or Class II or is a Class III device that was in commercial distribution before May 28, 1976, for which the FDA has not yet called for submission of a PMA application. We believe that most of our tests are Class II devices for which the 510(k) clearance standard is available, and we believe our HPV-HR Quad test is a Class III device for which the more stringent PMA requirements are applicable.
Class I devices are those for which safety and effectiveness can be assured by adherence to the FDA’s general regulatory controls for medical devices, which include compliance with the applicable portions of the FDA’s Quality System Regulations, facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials, or General Controls. Many Class I (and certain Class II) devices are exempt from pre-market regulation, however, some Class I devices require pre-market clearance by the FDA through the 510(k) pre-market notification process described below.
Class II devices are subject to the FDA’s General Controls, and any other special controls as deemed necessary by the FDA to establish the safety and effectiveness of the device. Pre-market review and clearance by the FDA for Class II devices is generally accomplished through the 510(k) pre-market notification procedure. 510(k) pre-market notification submissions are subject to user fees, unless a specific exemption applies.
115
Table of Contents
Class III devices are those devices which are deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed “predicate device”. These devices are required to undergo the PMA approval process in which the manufacturer must demonstrate the safety and effectiveness of the device to the FDA’s satisfaction. These devices almost always require formal clinical trials to demonstrate safety and effectiveness. A PMA application must provide extensive preclinical and clinical trial data and also information about the device and its components regarding, among other things, device design, manufacturing and labeling. Pre-market approval applications (and supplemental pre-market approval applications) are subject to significantly higher user fees than are 510(k) pre-market notifications. After approval of a PMA, a new PMA or PMA supplement is required in the event of a modification to the device, its labeling or its manufacturing process.
Medical devices can be marketed only for the indications for which they are cleared or approved. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require a PMA approval. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any decision. If the FDA disagrees with a manufacturer’s decision not to pursue a new 510(k) clearance or a PMA approval, the agency may retroactively require the manufacturer to pursue 510(k) clearance or PMA approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or PMA approval is obtained. We have made modifications to our 510(k) cleared system and tests, but have determined that, in our view, based on FDA guidance as to when to submit a 510(k) notification for changes to a cleared device, new 510(k) clearances or PMA approvals are not required. We cannot assure you that the FDA would agree with any of our decisions not to pursue 510(k) clearance or PMA approval. If the FDA requires us to pursue 510(k) clearance or PMA approval for any modification, we also may be required to cease marketing and/or recall the modified device until we obtain a new 510(k) clearance or PMA approval.
A clinical trial may be required in support of a 510(k) submission and generally is required for a PMA application. These trials generally require an effective Investigational Device Exemption, or IDE, from the FDA for a specified number of patients, unless the product is exempt from IDE requirements or deemed a non significant risk device eligible for more abbreviated IDE requirements. The IDE application must be supported by appropriate data, such as animal and laboratory testing results. Clinical trials may begin 30 days after the submission of the IDE application unless the FDA or the appropriate institutional review boards at the clinical trial sites place the trial on clinical hold. In the future, we expect to submit additional 510(k) notifications or PMA applications in order to market new claims, uses or products. Clinical investigations of in vitro diagnostic tests, including our products and product candidates, typically are exempt from the IDE requirements. Thus, we do not need FDA’s prior approval to conduct clinical trials on our products, provided the testing is non-invasive, does not require an invasive sampling procedure that presents a significant risk, does not intentionally introduce energy into the subject and is not used as a diagnostic procedure without confirmation of the diagnosis by another, medically established diagnostic device or procedure. Our devices that are undergoing clinical testing to support FDA clearance or approval can not be used for clinical diagnosis unless the diagnosis is confirmed by another medically established test or procedure.
The vast majority of the tests that we currently sell, which together represent 87%, 92% and 96% of our net revenue for 2010, 2011 and the first nine months of 2012, respectively, have not been cleared or approved for clinical use by the FDA. These tests are labeled “For Research Use Only. Not for use in diagnostic procedures.” as required by FDA regulations. We are not permitted to market these products for in vitro diagnostic use, and must establish distribution controls to assure that these products are not used for diagnostic purposes. We therefore market these products to laboratories for research or investigational use in the collection of research data. In some cases, laboratories certified under CLIA may use our products in their own laboratory-developed clinical tests when no FDA regulated analyte specific reagents or in vitro diagnostic products are available pursuant to guidelines recently issued by the College of American Pathologists. These guidelines have not been adopted or approved by FDA. While we believe that the sale of our products that have not been cleared or approved by the FDA for research purposes complies with FDA’s laws and regulations, the FDA may determine
116
Table of Contents
otherwise. If the FDA disagrees with the marketing of these products, we may be subject to a variety of enforcement actions ranging from public warning letters, fines, injunctions, consent decrees and civil penalties to suspension or delayed issuance of approvals, seizure or recall of our products, total or partial shutdown of production, withdrawal of approvals or clearances already granted, and criminal prosecution. In June 2011 the FDA issued a Draft Guidance entitled “Commercially Distributed In Vitro Diagnostic Products Labeled for Research Use only or Investigational Use Only: Frequently Asked Questions,” which, if enforced, would limit our marketing of RUO tests to general discovery laboratories and would require us to halt sales to clinical laboratories that validate and use our RUO tests as LDTs.
Pervasive and Continuing FDA Regulation
After a device is placed on the market, regardless of the classification or pre-market pathway, it remains subject to significant regulatory requirements. Even if regulatory approval or clearance of a medical device is granted, the FDA may impose limitations or restrictions on the uses and indications for which the device may be labeled and promoted. Medical devices may be marketed only for the uses and indications for which they are cleared or approved. FDA regulations prohibit a manufacturer from promoting a device for an unapproved, or “off-label” use. Manufacturers that sell products to laboratories for research or investigational use in the collection of research data are similarly prohibited from promoting such products for clinical or diagnostic tests. While we have policies in place to restrict the promotion of our products sold on an RUO basis, we cannot assure you that the FDA would agree that our practices do not constitute the promotion of these products for a clinical or diagnostic use without clearance or approval.
Device manufacturers must establish registration and device listings with the FDA. Our manufacturing processes and those of our suppliers are required to comply with the applicable portions of the Quality Systems Regulations, which cover the methods and documentation of the design, testing, production, processes, controls, quality assurance, labeling, packaging and shipping of our products. Domestic facility records and manufacturing processes are subject to periodic unscheduled inspections by the FDA. The FDA also may inspect foreign facilities that export products to the United States.
We are required to report to the FDA if our products cause or contribute to a death or serious injury or malfunction in a way that would likely cause or contribute to death or serious injury were the malfunction to recur. We are also subject to correction and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FFDCA that may present a risk to health. We have never submitted a medical device report for our products, nor conducted a recall or FDA-reportable correction or removal. The FDA and authorities in other countries can require the recall of products in the event of material defects or deficiencies in design or manufacturing. The FDA can also withdraw or limit our product approvals or clearances in the event of serious, unanticipated health or safety concerns.
The FDA has broad regulatory and enforcement powers. If the FDA determines that we have failed to comply with applicable regulatory requirements, it can impose a variety of enforcement actions ranging from public warning letters, fines, injunctions, consent decrees and civil penalties to suspension or delayed issuance of approvals, seizure or recall of our products, total or partial shutdown of production, withdrawal of approvals or clearances already granted, and criminal prosecution. The FDA can also require us to repair, replace or refund the cost of devices that we manufactured or distributed. In the event that one of our suppliers fails to maintain compliance with our quality requirements or FDA’s applicable regulatory requirements, we may have to qualify a new supplier and could experience manufacturing bottlenecks or delays as a result. Furthermore, the regulation and enforcement of in vitro reagents and equipment by the FDA is an evolving area that is subject to change. While we believe that we are in compliance with the current regulatory requirements and policies of the FDA, the FDA may impose more rigorous regulations or policies that may expose us to enforcement actions or require a change in our business practices. If any of these events were to occur, it could materially adversely affect us.
117
Table of Contents
Fraud and Abuse Laws
Because of the significant federal funding involved in Medicare and Medicaid, Congress and the states have enacted, and actively enforce, a number of laws whose purpose is to eliminate fraud and abuse in federal healthcare programs. Our business is subject to compliance with these laws, which include the following:
Anti-Kickback Statutes and Federal False Claims Act
The federal healthcare program’s Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. The definition of “remuneration” has been broadly interpreted to include anything of value, including, for example, gifts, discounts, the furnishing of free supplies, equipment or services, credit arrangements, payments of cash and waivers of payments. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. In addition, some kickback allegations have been claimed to violate the Federal False Claims Act, discussed in more detail below.
The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress authorized the OIG to issue a series of regulations, known as “safe harbors.” These safe harbors set forth requirements that, if met in their entirety, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal, or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by government enforcement authorities, such as the OIG.
Many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to referral of patients for healthcare items or services reimbursed by any payor, not only the Medicare and Medicaid programs, and do not contain identical safe harbors.
Government officials have focused their enforcement efforts on marketing of healthcare services and products, among other activities, and have brought cases against numerous pharmaceutical and medical device companies, and certain sales and marketing personnel for allegedly offering unlawful inducements to potential or existing customers in an attempt to procure their business.
Another development affecting the healthcare industry is the increased use of the federal civil False Claims Act and, in particular, actions brought pursuant to the False Claims Act’s “whistleblower” or “qui tam” provisions. The False Claims Act imposes liability on any person or entity who, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The qui tam provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government, and to share in any monetary recovery. In recent years, the number of suits brought by private individuals has increased dramatically. In addition, various states have enacted false claim laws analogous to the False Claims Act. Many of these state laws apply where a claim is submitted to any third-party payor and not merely a federal healthcare program.
When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of $5,500 to $11,000 for each separate false claim. There are many potential bases for liability under the False Claims Act. Liability arises, primarily,
118
Table of Contents
when an entity knowingly submits, or causes another to submit, a false claim for reimbursement to the federal government. The False Claims Act has been used to assert liability on the basis of inadequate care, kickbacks and other improper referrals, improper use of Medicare numbers when detailing the provider of services, and allegations as to misrepresentations with respect to the services rendered. Our future activities relating to the reporting of discount and rebate information and other information affecting federal, state and third party reimbursement of our products, and the sale and marketing of our products, may be subject to scrutiny under these laws. We are unable to predict whether we would be subject to actions under the False Claims Act or a similar state law, or the impact of such actions. However, the costs of defending such claims, as well as any sanctions imposed, could significantly adversely affect our financial performance.
HIPAA and Other Fraud and Privacy Regulations
Among other things, the Health Insurance Portability and Accountability Act of 1996 (as amended by the Health Information Technology for Economic and Clinical Health Act), or HIPAA, created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The HIPAA healthcare fraud statute prohibits, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment and/or exclusion from government-sponsored programs. The HIPAA false statements statute prohibits, among other things, knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines and/or imprisonment.
In addition to creating two new federal healthcare crimes, regulations implementing HIPAA also establish uniform standards governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of individually identifiable health information maintained or transmitted by healthcare providers, health plans and healthcare clearinghouses, which are referred to as “covered entities.” Three standards have been promulgated under HIPAA’s regulations: the Standards for Privacy of Individually Identifiable Health Information, which restrict the use and disclosure of certain individually identifiable health information; the Standards for Electronic Transactions, which establish standards for common healthcare transactions, such as claims information, plan eligibility, payment information and the use of electronic signatures; and the Security Standards, which require covered entities to implement and maintain certain security measures to safeguard certain electronic health information. Although we are not a covered entity, we expect that our customers generally will be covered entities and may ask us to contractually comply with certain aspects of these standards. Recent amendments to HIPAA make its requirements applicable to such business associates of covered entities. While the government intended this legislation to reduce administrative expenses and burdens for the healthcare industry, our compliance with certain provisions of these standards entails significant costs for us, and our failure to comply could lead to enforcement action that could have a material adverse effect on our business. If we or our operations are found to be in violation of HIPAA or its implementing regulations, we may be subject to penalties, including civil and criminal penalties, damages, and fines.
In addition to federal regulations issued under HIPAA, some states have enacted privacy and security statutes or regulations that, in some cases, are more stringent than those issued under HIPAA. In those cases, it may be necessary to modify our planned operations and procedures to comply with the more stringent state laws. If we fail to comply with applicable state laws and regulations, we could be subject to additional sanctions.
Third Party Coverage and Reimbursement
Our primary customers are clinical laboratories that bill many different payor groups. The majority of reimbursement dollars for traditional laboratory services are provided by health maintenance organizations, or HMOs, and other managed care plans, as well as government healthcare programs, such as Medicare and
119
Table of Contents
Medicaid. HMOs and other managed care plans typically contract with a limited number of clinical laboratories and then designate the laboratory or laboratories to be used for tests ordered by participating physicians.
There are a number of factors that influence coverage and reimbursement for diagnostic tests. In the United States, the American Medical Association assigns specific CPT codes, which are necessary for reimbursement of diagnostic tests. Once the CPT code is established, the Centers for Medicare and Medicaid Services establish reimbursement payment levels and coverage rules under Medicaid and Medicare, and private payors establish rates and coverage rules independently.
United States and other government regulations governing coverage and reimbursement for diagnostic testing may affect directly or indirectly the design of our products and the potential market for their use. The availability of third-party reimbursement for our products and services may be limited or uncertain. Third-party payors may deny coverage if they determine that the prescribed product or service has not received appropriate FDA or other government regulatory clearances, is not used in accordance with cost-effective treatment methods as determined by the payor, or is deemed by the third-party payor to be experimental, unnecessary or inappropriate. Furthermore, third-party payors are increasingly challenging the prices charged for healthcare products and services.
Foreign Regulation
In order for us to market our products in other countries, we must obtain regulatory approvals or registrations and comply with extensive safety and quality regulations in other countries. These regulations, including the requirements for approvals or registrations and the time required for regulatory review, if required, vary from country to country. Failure to obtain required regulatory approval or registration in any foreign country in which we plan to market our products may harm our ability to generate revenue and harm our business.
Many foreign countries in which we market or may market our products have regulatory bodies and restrictions similar to those of the FDA. International sales are subject to foreign government regulation, the requirements of which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval and the requirements may differ.
European Countries. In the EEA, a medical device can only be placed on the market if it is in conformity with the essential requirements set out in the European Medical Devices Directives (Directive 93/42/EEC on Medical Devices, Directive 90/385/EEC on Active Implantable Medical Devices and the IVDD Directive). In vitro diagnostic medical devices that comply with the requirements of the IVDD Directive are entitled to bear the CE conformity mark, indicating that the device meets minimum standards of performance, safety and quality (i.e., the essential requirements), and can be commercially distributed throughout the EEA and other countries outside the EEA that have accepted the CE marking as an acceptable certification of efficiency and safety of medical devices. For certain classes of devices, certification by a recognized European Notified Body may be required to permit the manufacturer to affix the CE marking on its products and commercially distribute those products throughout the EEA.
The CE marking of conformity was affixed to the INFINITI Analyzer in accordance with the IVDD Directive in June 2008 and to the INFINITI Plus Analyzer in September 2012. We also have CE marked 22 of our tests. The CE marking allows the INFINITI Analyzer to be marketed in the EEA. Our tests that are not CE marked are offered to European laboratories on an RUO basis to conduct research projects and other pre-market activities. We are working with our European distributors to prioritize and fulfill requirements to affix CE marking as required by applicable law. We cannot assure you that we will be able to obtain necessary foreign government approvals or successfully comply with foreign regulations. Our failure to do so could hurt our business, results of operations and financial condition.
Corporate Headquarters
Our corporate offices are located at 2980 Scott Street, Vista, California, in approximately 125,000 square feet occupied under a lease that commenced on February 1, 2009 and will expire on December 31, 2029. This facility houses our research and development, manufacturing and warehousing operations and our administrative offices.
120
Table of Contents
Employees
As of December 31, 2012, we had 100 full-time employees and one part-time employee. None of our employees are represented by a labor union and we consider our employee relations to be good.
Legal Proceedings
We are from time to time subject to various claims and legal actions during the ordinary course of business.
On December 28, 2012, Tregale Group Ltd, the holder of approximately $2.2 million in principal amount under our outstanding promissory notes that had been in default beginning in the first quarter of 2012, filed a request for judicial intervention and motion for summary judgment in lieu of complaint with the Supreme Court of the State of New York, County of New York, demanding payment of the principal and interest outstanding under its promissory notes as well as reimbursement of certain legal and other expenses, in the aggregate amount of $2.3 million plus interest, attorneys’ fees and costs.
In January 2013, we made payment of the outstanding principal and interest owed to Tregale Group Ltd under our outstanding promissory notes, and are negotiating with Tregale Group Ltd with respect to the reimbursement of its legal and other expenses, and the dismissal of the lawsuit.
We believe that there are currently no claims or legal actions that would, in management’s judgment based on information currently available, have a material adverse effect on our results of operations or financial condition.
121
Table of Contents
The following table sets forth information about our directors and executive officers as of December 31, 2012. The biographies for Mr. Kureshy and our other directors, below, include information on the directors’ experience, qualifications, attributes or skills that have led our board to conclude that they should serve on our board.
| Name |
Age | Position | ||||
| Fareed Kureshy |
69 | Founder, Chairman, President and Chief Executive Officer and Director | ||||
| Thomas V. Hennessey, Jr. |
64 | Chief Operating Officer, Chief Financial Officer, Secretary and Director | ||||
| Patrick Dillon, Ph.D. |
51 | Chief Scientific Officer | ||||
| Shailendra Singh, Ph.D. |
73 | Co-Founder, Vice President, System Development | ||||
| Ramanath Vairavan |
64 | Co-Founder, Senior Vice President, Sales and Marketing | ||||
| Stephen Allison |
67 | Director | ||||
| Charles Birmingham |
62 | Director | ||||
| Laurence M. Demers, Ph.D. |
74 | Director | ||||
| Donald E Pogorzelski |
62 | Director | ||||
| Peter Wilding, Ph.D. |
78 | Director | ||||
| Eugene J. Zurlo |
75 | Director | ||||
Executive Officers
Fareed Kureshy: Mr. Kureshy, the Company’s Founder, has been Chairman, President and Chief Executive Officer of AutoGenomics since its founding in April 1999. Mr. Kureshy has over 30 years of entrepreneurial leadership experience in the healthcare industry. Mr. Kureshy served as President and Chief Executive Officer of Sequenom, Inc. from 1996 to 1997, and as President of Behring Diagnostics, Inc. and PB Diagnostics Systems, Inc. from 1992 to 1996, where he set up the infrastructure to design, develop, manufacture and market platform technologies in the areas of chemistry, immunoassays and DNA analysis. Mr. Kureshy also spent 12 years at Abbott Laboratories from 1976 to 1987 where he held various management positions. Mr. Kureshy holds undergraduate degrees in physics, chemistry and mathematics from Karachi University, an undergraduate degree in engineering from Northrop University and has completed graduate engineering studies. He was awarded an M.B.A. from Southern Methodist University. Our board of directors has determined that Mr. Kureshy brings to the board knowledge of our business and his historical understanding of our operations combined with his extensive entrepreneurial leadership, operations, strategic planning and marketing experience in the healthcare industry in which we operate.
Thomas V. Hennessey, Jr.: Mr. Hennessey joined AutoGenomics in January 2008 as Chief Operating Officer and Chief Financial Officer and has served as a director of AutoGenomics since 2001. Mr. Hennessey served as AutoGenomics’ Chief Financial Officer in his capacity as an independent contractor in December 2007 and in his capacity as a non-employee director from January 2007 through November 2007. Mr. Hennessey has 35 years of experience in the medical and technology industries. Mr. Hennessey was an independent consultant to AutoGenomics from January 2003 until December 2007. From 2001 until 2003, he was Chief Operating Officer and Chief Financial Officer of Photovac, Inc. He has also served as Chief Operating Officer and Chief Financial Officer for several public and private start-up companies, including Behring Diagnostics, Inc., Autoimmune, Inc., Medical Diagnostics, Inc., Cambridge Heart, Inc. and Sonamed Corporation. Mr. Hennessey received a S.B. and a S.M. degree in mechanical engineering from Massachusetts Institute of Technology and an M.B.A. from Harvard Business School. Our board of directors had determined that Mr. Hennessey brings to the board his knowledge of our operations as well as management and financial experience, having served as Chief Operating Officer and Chief Financial Officer for several public and private start-up companies in the medical and technology industries in which we operate.
122
Table of Contents
Patrick Dillon, Ph.D.: Dr. Dillon joined AutoGenomics in January 2009 and became our Chief Scientific Officer in December 2011. Prior to that he served as our Vice President of R&D Chemistry. He has over 20 years of research and management experience related to the discovery, development and commercialization of bioscience related products and technologies. Dr. Dillon has held senior executive positions at Human Genome Sciences, Inc., Nanogen, Invitrogen, and ArtisOptimus, Inc. Dr. Dillon is an inventor or co-inventor of 56 U.S. issued patents and numerous pending U.S. and international patents. He is an author or co-author of 25 scientific publications in peer-reviewed journals. Dr. Dillon received a B.S. in Biology from the University of Illinois and a Ph.D. in Immunology from Rush University in Chicago.
Shailendra Singh, Ph.D.: Dr. Singh has been Vice President, System Development of AutoGenomics since its founding in April 1999. Dr. Singh has 34 years of experience in software engineering, systems development and integration, the last 29 of which have been spent in the healthcare industry and participating in the invention and development of eight medical instruments. Dr. Singh has held the position of Vice President of Systems Development at Sequenom, Inc., PB Diagnostics Systems, Inc. and Behring Diagnostics, Inc. Dr. Singh received a B.S. in electrical engineering, an M.E. in electrical engineering and a Ph.D. in electrical engineering from Southern Methodist University.
Ramanath Vairavan: Mr. Vairavan has been Senior Vice President, Sales and Marketing of AutoGenomics since January 2007 and has been an executive officer of AutoGenomics since its founding in April 1999. Mr. Vairavan has over 30 years of experience in the healthcare industry in the areas of research, product development, manufacturing, sales, marketing and business development. He began his career with Hoechst AG / Behring Diagnostics, Inc. where he held various management positions in research, development, operations, international sales and marketing in the United States, Singapore and Germany. Mr. Vairavan received a B. Tech. in chemical engineering from University of Madras, India, an M.S. in biomedical engineering from Washington University and an M.B.A. from Fairleigh Dickinson University.
Directors
Stephen Allison: Mr. Allison joined our board of directors in April 2011. Mr. Allison has over 25 years of experience advising and working for organizations in the healthcare and technology sectors and is currently an independent business consultant. From 2006 to 2007, Mr. Allison was Vice President, Chief Financial Officer of Gensym Corporation, a leading provider of software and services for mission-critical solutions in real time. Prior to Gensym, he was CFO for Webhire, Inc. from 2000 - 2003, PRI Automation from 1997 to 2000 and Helix Technology from 1995 to 1997. From 1991 until 1995, Mr. Allison was Vice President, Finance for Behring Diagnostics, Inc. an international company engaged in the development, manufacture and sale of immunoassay systems. Mr. Allison holds a B.A. degree in Economics from Columbia University and an M.B.A. from the Amos Tuck School of Business Administration at Dartmouth College. Our board of directors has determined that Mr. Allison brings to our board his knowledge of public accounting, his public company management experience, having served as chief financial officer of several public companies, and knowledge of the healthcare sectors in which we operate.
Charles Birmingham: Mr. Birmingham joined our board of directors in August 2012. He has over 30 years of experience in leading growth-oriented companies in the health care services area. Since October 2010 he has served as Vice President of Corporate Development of CareMore Medical Enterprises and General Manager of CareMore Touch. From February 2010 to October 2010 Mr. Birmingham was a Principal at White Branch Partners, where he provided consulting services to health care and early-stage companies. From October 2009 to February 2010 Mr. Birmingham was a board member and Vice President of Operations at Initiate Healthcare, and was the Executive Vice President and Chief Operating Officer of its predecessor-by-acquisition, Accenx Technologies, from 2007 to October 2009. Mr. Birmingham was a Senior Consultant at XLHealth Corporation from 2006 to 2007 and at PacifiCare Health Systems from 2004 to 2006. From 1987 to 1994 Mr. Birmingham was the co-founder and Senior Vice President of Operations of First Mental Health, Inc. Mr. Birmingham is a board member of National Healthcare Services, a health system-based venture capital fund that specializes in
123
Table of Contents
health care technology. Mr. Birmingham holds a B.S. in psychology from St. Joseph’s University and an M.B.A from Vanderbilt University. Our board of directors has determined that Mr. Birmingham brings to our board an extensive knowledge of the healthcare industry in general and the reimbursement environment in which our customers operate.
Laurence M. Demers, Ph.D.: Dr. Demers, DABCC, FACB, has served as a director of AutoGenomics since March 2008. He is a Distinguished Professor Emeritus of Pathology and Medicine at the M. S. Hershey Medical Center of The Pennsylvania State University, where he has taught since 1973. Dr. Demers is a diplomat of the American Board of Clinical Chemistry, a fellow of the National Academy of Clinical Biochemistry, a past president of the American Association for Clinical Chemistry as well as of the National Academy of Clinical Biochemistry, the founding director of Clinical Chemistry at the M.S. Hershey Medical Center, a director of the Core Endocrine Laboratory in the Pennsylvania State University Hospital and a former director of the Pennsylvania State Clinical Research Center Core Laboratory. His primary research interests have been in the areas of molecular diagnostics, laboratory automation, thyroid disease, metabolic bone disease and breast cancer. Dr. Demers has had extensive experience as principal investigator of clinical trials for both the pharmaceutical and in vitro diagnostic industry. Dr. Demers holds an A.B. in biology from Merrimack College and a Ph.D. in biochemistry from State University of New York. Dr. Demers recently served as Chairman of the Board of Trustees of Merrimack College, North Andover, MA from 2007-2010 and was awarded an honorary Doctor of Science by Merrimack College in 2011. Our board of directors has determined that Dr. Demers brings to our board his scientific knowledge and understanding of our product offerings and knowledge of the pharmaceutical industry and the diagnostic industry in which we operate.
Donald E. Pogorzelski: Mr. Pogorzelski joined our board of directors in August 2011. Mr. Pogorzelski has over 30 years of experience in the medical diagnostics industry, with the last 23 years at Genzyme Corporation. Mr. Pogorzelski joined Genzyme in 1988 in a domestic sales and marketing role in the Diagnostics Division when annual revenue was approximately $4 million. As president in 1986, he assumed global leadership of the division and became an Officer of the Corporation in 2001. Revenue when the business was sold in February 2011 to longtime partner Sekisui Chemical approximated $175 million. Mr. Pogorzelski began his career in diagnostics with Abbott Laboratories in 1976 and experienced several assignments of increasing responsibility prior to leaving Abbott to join Genzyme in Boston at the start of 1988. He was elected to the New England Baptist Hospital Board of Trustees in February 2007 and is currently serving in the capacity of Vice-Chair. He was elected to the Wentworth Institute of Technology Board of Trustees in 2007 and received an Honorary Degree of Doctor of Engineering Technology from Wentworth in 2006. Mr. Pogorzelski earned his undergraduate degree at Loyola University, Chicago and his M.B.A. from the University of Chicago. Our board of directors has determined that Mr. Pogorzelski brings to our board his extensive knowledge of marketing and the market environment in the medical diagnostics industry and sectors in which we operate.
Peter Wilding, Ph.D.: Dr. Wilding, FRSC, joined our board of directors in October 2012. He has been professor emeritus in the department of pathology and laboratory medicine at the University of Pennsylvania Medical Center in Philadelphia since 2003, where he was director of clinical chemistry from 1986 to 2003. For the past 20 years, he has concentrated on the creation of new technology involving microfabricated devices now known as microchips for clinical analyses. Dr. Wilding has held many offices in organizations associated with laboratory medicine, including that of president of the American Association of Clinical Chemistry, and has served as an adviser or consultant to the U.K. Department of Health, World Health Organization, International Federation of Clinical Chemistry and Laboratory Medicine, National Institutes of Health, and National Academy of Sciences. He is also an Honorary Professor of the West China University of Medical Sciences in Chengdu, China. Dr. Wilding is a Fellow of the Royal College of Pathologists (United Kingdom), the Royal Society of Chemistry (United Kingdom), and the National Academy of Clinical Biochemistry. He was certified by the American Board of Bioanalysis as a High Complexity Clinical Laboratory Director. Dr. Wilding received his B.Sc. with Honors in medical biochemistry in 1961 and his Ph.D. in clinical chemistry in 1965, both from the University of Birmingham, UK. Dr. Wilding was elected to our board of directors in October 2012 pursuant to
124
Table of Contents
the right granted to the holders of our Series C Convertible Preferred Stock in our certificate of incorporation, acting by majority vote, to elect one director to our board of directors.
Eugene J. Zurlo: Mr. Zurlo has served as a director of AutoGenomics since January 2005. Mr. Zurlo has over 50 years of experience in the healthcare industry as a manager, executive and investor. Mr. Zurlo has been managing director of Zurlo Investment Trust, an active investor in real estate, pharmaceutical and early-stage healthcare companies, since 2000 and is a director of private companies Penny Creek Associates LLC and Plasma Technologies, LLC. He was founder of Hemasure, Inc. and served in the positions of Chairman from 1996 to 1997 and President and Chief Executive Officer from 1993 to 1996. Prior to Hemasure, Mr. Zurlo held senior executive positions at Millipore Corporation, where he served as Senior Vice President of Operations, Baxter International, Inc., where he served as Senior Vice President of several divisions, including Laboratory Diagnostics, and the New York Blood Center, where he served as Chief Operating Officer. He holds a B.S. in pharmacy from Fordham University and an M.S. in economics from Long Island University. Our board of directors has determined that Mr. Zurlo contributes to our board extensive management and operations experience, as well as scientific knowledge and knowledge of the healthcare industry and diagnostic industry in which we operate.
Corporate Governance and Board Composition
Our board of directors is composed of eight directors with one vacancy. All directors hold office until the next annual meeting of stockholders and until a successor has been duly elected and qualified. A resolution of the board of directors may change the authorized number of directors; however, our certificate of incorporation and bylaws currently limit the number to not more than 15 nor fewer than five directors. Our board of directors has determined that each of our non-employee directors is independent within the meaning of NASDAQ rules.
Board Structure and Committees Composition
Our board of directors directs the management of our business and affairs, as provided by Delaware law, and conducts its business through meetings of the board of directors and standing committees. Our board of directors currently has an audit committee, compensation committee and nominating committee. Our board of directors may establish other committees to facilitate the management of our business. The membership and function of each of the committees are described below.
Audit Committee. The audit committee of our board of directors consists of Stephen Allison (Chairman), Charles Birmingham and Laurence M. Demers. The audit committee, which is composed solely of independent directors, has sole authority for hiring the company’s independent auditors, oversees the results and scope of the audit and other services provided by our independent auditors, oversees our audit and control functions and reviews all related persons transactions. Our board of directors has determined that Mr. Allison qualifies as an “audit committee financial expert” as defined by the rules under the Exchange Act. The background and experience of each of our audit committee members are set forth above.
Compensation Committee. The compensation committee of our board of directors consists of Laurence M. Demers (Chairman), Donald E. Pogorzelski and Stephen Allison. The compensation committee, comprised solely of independent directors, oversees our compensation plans and is responsible for setting the compensation of our chief executive officer. Such oversight includes reviewing and, as it deems appropriate, recommending to our board of directors policies, practices and procedures relating to the compensation of our directors, officers and other managerial employees and exercising authority under our equity incentive plans.
Nominating and Corporate Governance Committee. The nominating and corporate governance committee of our board of directors consists of Donald E. Pogorzelski (Chairman), Peter Wilding, Ph.D., and Fareed Kureshy. The nominating and corporate governance committee is responsible for recruiting and retaining qualified persons to serve on our board of directors, including proposing such individuals to the board of
125
Table of Contents
directors for nomination for election as directors, evaluating the performance, size and composition of the board of directors and overseeing our corporate governance programs and compliance activities. The nominating and corporate governance committee considers written suggestions from stockholders, including potential nominees for election as directors.
Code of Business Conduct and Ethics
Prior to completing this offering, we will adopt a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. The code of business conduct and ethics will be available on our website at www.autogenomics.com. Information contained in or that can be accessed through our website is not incorporated by reference into this prospectus and should not be considered to be part of this prospectus. Any amendments to the code, or any waivers of its requirements with respect to our principal executive officer, principal financial officer, principal accounting officer or controller or persons performing similar functions will be promptly disclosed on our website.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee has at any time during our last completed fiscal year been one of our officers or employees. None of our executive officers currently serves, or during our last completed fiscal year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers on our board of directors or compensation committee.
Director Compensation
The non-employee members of our board of directors are reimbursed for travel, lodging and other reasonable expenses incurred in attending board of directors or committee meetings. We do not currently provide any cash compensation to our non-employee directors. From time to time, we have granted stock options to our non-employee directors as compensation for their services, although there has been no formal policy in place with respect to such awards.
Following the completion of this offering, we will provide cash compensation in the form of an annual retainer of $35,000 for each non-employee director. We will also pay an additional annual retainer of $10,000 to the lead non-employee director, $10,000 to the chairman of our audit committee, $7,500 to each other non-employee member of our audit committee, $7,000 to the chairman of our compensation committee, $5,000 to each other non-employee member of our compensation committee, $5,000 to the chairman of our nominating and corporate governance committee and $3,000 to each other non-employee member of our nominating and corporate governance committee. In lieu of receiving this cash compensation, the directors will be able to elect to receive restricted stock units with an equal value on their date of grant. We will continue to reimburse our non-employee directors for their reasonable expenses incurred in attending meetings of our board of directors and committees of the board of directors.
Following the completion of this offering, any non-employee director who is first elected to the board of directors will be eligible to receive a restricted stock unit covering 3,300 shares of our common stock in connection with his or her initial election to the board of directors. In addition, on the date of each annual meeting of our stockholders following this offering, each non-employee director will be eligible to receive a restricted stock unit covering 1,650 shares of our common stock.
The restricted stock units granted to non-employee directors described above will vest one year after they were granted, subject to the director’s continuing service on our board of directors on that date. The terms of these restricted stock units are described in more detail under “Executive Compensation — Employee Plans — 2008 Equity Incentive Award Plan.”
126
Table of Contents
The following table summarizes the compensation received by our non-employee directors during the year ended December 31, 2012.
| Name (1) |
Option awards ($) (3) |
|||
| Stephen Allison |
20,600 | |||
| William H. Davidson (2) |
20,600 | |||
| Laurence M. Demers |
20,600 | |||
| Donald E Pogorzelski |
20,600 | |||
| Eugene J. Zurlo |
20,600 | |||
| (1) | Mr. Birmingham and Mr. Wilding are not represented in the above table as they joined our board of directors during 2012 and did not receive any compensation during fiscal year 2012. |
| (2) | Mr. Davidson left our board of directors in October 2012. |
| (3) | Represents the full grant date fair value of the stock option grants awarded during fiscal year 2012, as computed in accordance with FASB Accounting Standards Codification, or ASC, Topic 718. For a discussion of the valuation assumptions, see Note 8 to our financial statements included elsewhere in this prospectus. |
The aggregate number of shares subject to stock options outstanding at the end of fiscal year 2012 for each individual that was a non-employee director during fiscal year 2012 is as follows:
| Name (1) |
Shares underlying options outstanding at December 31, 2012 |
|||
| Stephen Allison |
14,850 | |||
| William H. Davidson (2) |
70,950 | |||
| Laurence M. Demers |
41,250 | |||
| Donald E. Pogorzelski |
6,600 | |||
| Eugene J. Zurlo |
33,000 | |||
| (1) | Mr. Birmingham and Mr. Wilding are not represented in the above table as they joined our board of directors during 2012 and did not receive any compensation during fiscal year 2012. |
| (2) | Mr. Davidson left our board of directors in October 2012. |
127
Table of Contents
Summary Compensation Table
The following table summarizes the compensation that we paid to our named executive officers during the year ended December 31, 2012.
| Name and principal position |
Year | Salary ($) | Option awards ($) (1) |
Other compensation ($) (2) |
Total ($) | |||||||||||||||
| Fareed Kureshy |
2012 | 255,769 | 86,500 | 10,096 | 352,365 | |||||||||||||||
| President and Chief Executive Officer |
2011 | 201,923 | 55,650 | 13,430 | 271,003 | |||||||||||||||
| Thomas V. Hennessey, Jr. (3) |
2012 | 138,461 | 173,000 | — | 311,461 | |||||||||||||||
| Chief Operating Officer, Chief Financial Officer and Secretary |
||||||||||||||||||||
| Shailendra Singh, Ph.D. |
2012 | 165,769 | — | 8,400 | 174,169 | |||||||||||||||
| Vice President, System Development |
2011 | 126,923 | 34,980 | 11,258 | 173,161 | |||||||||||||||
| Ramanath Vairavan |
2012 | 133,076 | — | 10,096 | 147,172 | |||||||||||||||
| Senior Vice President, Sales and Marketing |
2011 | 121,154 | 39,750 | 13,430 | 174,334 | |||||||||||||||
| (1) | Represents the grant date fair value for 2012 of stock options granted to our named executive officers, computed in accordance with FASB ASC Topic 718, Stock Compensation. For information regarding assumptions made in connection with this valuation, please see Note 1 to our financial statements included elsewhere in this prospectus. |
| (2) | The amounts presented consist of healthcare insurance premiums paid by us. |
| (3) | Mr. Hennessey was not a named executive officer for the year ended December 31, 2011. |
Base Salary and Bonuses
Our compensation committee of our board of directors reviews and recommends to our board of directors the base salary and bonus compensation of our named executive officers. Our board of directors, without our named executive officers present, ratifies and approves all compensation matters for our named executive officers. Compensation for our named executive officers has historically been highly individualized, resulted from arm’s length negotiations and been based on a variety of informal factors including our financial condition and available resources, our need for that particular position to be filled and the compensation levels of our other executive officers, each as of the time of the applicable compensation decision. As an early commercial-stage company, we have historically operated with limited cash resources. Consequently, our board of directors and compensation committee have been limited in the ability to consider significant increases in cash compensation for our named executive officers.
Our board of directors and our compensation committee has not undertaken a formal review of relevant market compensation data in setting named executive officer compensation. Instead, our board of directors and our compensation committee rely primarily upon the judgment of their members in making compensation decisions, after reviewing our performance and financial condition and carefully evaluating a named executive officer’s performance during the year against established goals, leadership qualities, operational performance, business responsibilities, career with us, current compensation arrangements and long-term potential to enhance stockholder value. To date, base salary and bonuses for our named executive officers have been determined and paid on a discretionary basis in those instances where the compensation committee and the board of directors desire to reward outstanding performance during the fiscal year by our named executive officers. Bonuses are not based upon the achievement of specified performance goals.
In 2011, our named executive officers took substantially reduced salaries, and no bonuses, as an economic concession during a period of significant financial constraint due to the withdrawal of our planned initial public
128
Table of Contents
offering in 2010 and general economic conditions. Similarly, no cash bonuses were awarded to our named executive officers in 2011. In 2012, base salary for our named executive officers was increased as a result of the lessening of economic constraints and the achievement of significant financial performance by the company during the last quarter of 2011 and the 2012 year. We expect base salary and cash bonuses for our named executive officers to increase, in some cases significantly, following the completion of this offering, as we align our named executive officer compensation with general market practices and if our financial and operating performance increases in future periods. For example, we recently signed an employment agreement with Fareed Kureshy, our President, Chief Executive Officer and Chairman of our Board of Directors, that provides for an annual base salary of $450,000 for Mr. Kureshy following the completion of this offering, with annual increases of at least five percent, which represents a substantial increase to his 2012 base salary and reflects the fact that Mr. Kureshy has in recent past periods, including during 2010, 2011 and 2012, accepted significantly reduced pay due to significant financial constraints. See “—Executive Employment Agreements” below. Decisions regarding salary increases in future periods may take into account the executive officer’s current salary, equity ownership, and the amounts paid to an executive officer’s peers inside our company by conducting an internal analysis, which compares the pay of each executive officer to other members of the management team. Other than for Mr. Kureshy as described above, no formulaic base salary increases are provided to our named executive officers. This strategy is consistent with our intent of offering compensation that is both cost-effective, competitive and consistent with our financial resources.
Long-Term Equity Incentives
The goals of our long-term, equity-based incentive awards are to align the interests of our named executive officers with the interests of our stockholders. Because vesting is based on continued employment, our equity-based incentives also encourage the retention of our named executive officers through the vesting period of the awards. In determining the size of the long-term equity incentives to be awarded to our named executive officers, we take into account a number of internal factors, such as the relative job scope, the value of existing long-term incentive awards, individual performance history, prior contributions to us, the size of prior grants, competitive considerations and our limited cash resources. Our compensation committee does not refer to formal competitive market data in determining long-term equity incentive awards. Based upon these factors, the compensation committee determines the size of the long-term equity incentives at levels it considers appropriate to create a meaningful opportunity for reward predicated on the creation of long-term stockholder value.
To reward and retain our named executive officers in a manner that best aligns employees’ interests with stockholders’ interests, we use stock options as the primary incentive vehicles for long-term compensation. We believe that stock options are an effective tool for meeting our compensation goal of increasing long-term stockholder value by tying the value of the stock options to our future performance. Because employees are able to profit from stock options only if our stock price increases relative to the stock option’s exercise price, we believe stock options provide meaningful incentives to employees to achieve increases in the value of our stock over time.
We use stock options to compensate our named executive officers both in the form of initial grants in connection with the commencement of employment and additional or “refresher” grants. To date there has been no set program for the award of additional or “refresher” grants, and the compensation committee retains discretion to make stock option awards to employees at any time, including in connection with the promotion of an employee, to reward an employee, for retention purposes or for other circumstances recommended by management or the compensation committee.
The exercise price of each stock option granted under our incentive stock option plans is the fair market value of our common stock on the grant date, as determined by our board of directors. Stock option awards to our named executive officers typically vest over a four-year period as follows: 25% of the shares underlying the option vest on each of the first four anniversaries of the vesting commencement date established at the time of grant. For new hire awards, the vesting commencement date is typically the new employee’s date of hire. For
129
Table of Contents
awards to existing employees or service providers the vesting commencement date is typically the same as the grant date. Some of the option awards granted to our named executive officers in the past were fully vested on the date of grant. The board of directors granted these awards in consideration of the officer’s past service to our company. We do not have any security ownership requirements for our named executive officers.
In 2012, we granted Mr. Kureshy and Mr. Hennessey options to purchase 16,500 and 33,000, respectively, shares of our common stock at an exercise price per share of $5.24. In January 2013, we granted Mr. Kureshy fully vested options to purchase 99,000 shares of our common stock at an exercise price of $9.85 per share. These grants were made pursuant to our 2008 Equity Incentive Award Plan, as further described below under “—Employee Plans — 2008 Equity Incentive Award Plan.” The compensation committee granted these awards based on its subjective review of each executive’s performance during the previous year, its review and consideration of the named executive officers’ respective unvested equity holdings, its desire to reward the executives for service to the company, its recognition of the executives having received significantly reduced base salary in 2011 and 2012, and its determination that such awards were advisable in order to continue to retain and incentivize such officers.
In 2011, we granted Mr. Kureshy, Mr. Singh and Mr. Vairavan options to purchase 17,325, 10,890 and 12,375 shares of our common stock, respectively, all at an exercise price per share of $5.24 under our 2008 Equity Incentive Award Plan, as further described below under “— Employee Plans — 2008 Equity Incentive Award Plan.” The compensation committee granted these awards based on its subjective review of each executive’s performance during the previous year, its review and consideration of the named executive officers’ respective unvested equity holdings, its desire to reward the executives for service to the company and its determination that such awards were advisable in order to continue to retain and incentivize such officers.
As a privately owned company, there has been no active market for our common stock. Accordingly, we have had no program, plan or practice pertaining to the timing of stock option grants to named executive officers coinciding with the release of material non-public information.
Retirement Savings
Our employees, including our named executive officers, are eligible to participate in our 401(k) plan. Pursuant to our 401(k) plan, employees may elect to reduce their eligible compensation by up to the statutorily prescribed annual limit of $17,500 in 2013 (additional elective deferrals not to exceed $5,500 are available to those employees 50 years of age or older) and to have the amount of this reduction contributed to our 401(k) plan. We do not make matching or profit sharing contributions under our 401(k) plan.
Perquisites, Health and Welfare Benefits and Other Compensation
The establishment of competitive benefit packages for our employees is an important factor in attracting and retaining highly qualified personnel.
Health and Welfare Benefits
Our named executive officers are eligible to participate in all of our employee benefit plans, including our medical, dental, vision, group life, and disability insurance plans, in each case on the same basis as other employees. We believe that these health and welfare benefits help ensure that we have a productive and focused workforce through reliable and competitive health and other benefits.
Perquisites
We do not provide significant perquisites or personal benefits to our named executive officers.
130
Table of Contents
Post Termination and Change in Control Benefits
We do not have any employment or severance agreements with our named executive officers.
As described above, we routinely grant our named executive officers stock options under our equity incentive plans. For a description of the change in control provisions in such equity incentive plans applicable to these stock options, see “— Employee Plans — 2008 Equity Incentive Award Plan” and “— Employee Plans — 2000 Equity Incentive Plan” below.
Executive Employment Agreements
We entered into an employment agreement with Mr. Kureshy on January 3, 2013. The employment agreement has an initial term of 3 years, ending January 3, 2016, which can be renewed for additional one year terms upon the mutual consent of us and Mr. Kureshy. Mr. Kureshy will serve as our President and Chief Executive Officer and the Chairman of our board of directors. Under the terms of the employment agreement, Mr. Kureshy will be paid an annual base salary of $450,000 per year following the completion of this offering. Beginning on the first anniversary of the employment agreement, Mr. Kureshy’s then current base salary will be increased by five percent per year. Our board of directors may authorize an increase greater than five percent based on the board’s evaluation of Mr. Kureshy’s annual performance, our financial condition and our ability to pay Mr. Kureshy an increased base salary. Mr. Kureshy is also entitled to an annual bonus in the target amount of at least fifty percent of his then current base salary; provided, however that any bonus is determined in the discretion of the compensation committee of our board of directors and is based on our performance and Mr. Kureshy’s achievement of the quantitative and qualitative goals to be set by the compensation committee.
Upon a change in control (as defined in the employment agreement) of the Company, Mr. Kureshy will earn a cash bonus in an amount equal to one percent of the net proceeds of the transaction constituting the change of control, which will be paid at the same time and in the same form as the proceeds of the transaction that are payable to us or our stockholders in connection with the consummation of the transaction. We have agreed to take all actions necessary to ensure that immediately prior to any change in control of the Company any stock options, restricted stock awards or other rights to acquire any of our capital stock granted to Mr. Kureshy, which are subject to vesting or a right of repurchase, will immediately vest in full and all rights to repurchase will lapse in their entirety, and the stock options will become exercisable for all the shares of capital stock subject to the option.
Pursuant to the employment agreement, we granted a fully vested option to purchase 99,000 shares of our common stock to Mr. Kureshy on January 14, 2013 with an exercise price of $9.85 per share.
Under the terms of the employment agreement, Mr. Kureshyy earns vacation time in accordance with our vacation benefit policy set forth in our employee handbook; provided that (i) in no event will Mr. Kureshy accrue less than three weeks of vacation each calendar year and (ii) Mr. Kureshy is entitled to accrue up to the greater of (A) sixteen weeks’ of vacation time or (B) the aggregate amount of vacation allowed to be accrued under our vacation benefit policy. Mr. Kureshy is eligible to participate in all benefit plans that are generally available to other employees of similar employment positions, subject to meeting the applicable eligibility requirements.
Mr. Kureshy’s employment agreement grants him rights in the event of certain terminations of his employment:
| • | If we terminate his employment for cause (as defined in the employment agreement), or Mr. Kureshy terminates his employment without good reason (as defined in the employment agreement), Mr. Kureshy will be entitled only to (A) the salary and other benefits accrued under the employment agreement through the effective date of his termination and (B) the reimbursement of certain expenses incurred in performing services under the employment agreement. |
| • | If we terminate his employment without cause, or Mr. Kureshy terminates his employment for good reason, Mr. Kureshy will be entitled to receive: (A) all base salary and other benefits accrued under the |
131
Table of Contents
| employment agreement through the date of such separation from service (as defined in the employment agreement); (B) reimbursement of certain expenses incurred in performing services under the employment agreement; and (C) a lump sum severance benefit (as defined in the employment agreement) equal to two and one half times his base salary as in effect immediately prior to the date of such separation from service. Mr. Kureshy will only be entitled to the severance benefit if he executes and delivers to us a general release within fifty days after the date of such separation from service and he does not revoke the release. Additionally, all stock options, restricted stock awards and other rights to acquire any of our capital stock granted to Mr. Kureshy that are subject to vesting or any right of repurchase will immediately vest in full and all of our rights to repurchase such shares will lapse in their entirety. |
| • | In the event of his termination due to death or disability (as defined in the employment agreement), Mr. Kureshy (or his estate in the event of his death) is entitled to receive: (A) all base salary and other benefits accrued under the employment agreement through the date of termination; (B) reimbursement for certain expenses incurred in performing services under the employment agreement; and (C) a lump sum severance amount equal to two and one half times his base salary as in effect immediately prior to the date of such termination. Additionally, all stock options, restricted stock awards and other rights to acquire any of our capital stock granted to Mr. Kureshy that are subject to vesting or any right of repurchase will immediately vest in full and all of our rights to repurchase such shares will lapse in their entirety. |
We have agreed to ensure that, with respect to any stock options granted to Mr. Kureshy after January 3, 2013, that Mr. Kureshy, or his estate or other people entitled to exercise such stock options following Mr. Kureshy’s death, will be entitled to exercise such stock option for not less than three years after the date of termination (but not after the expiration of the original term of the stock option). We have also agreed to reimburse Mr. Kureshy in the event certain excise taxes are imposed on any portion of the compensation or benefits payable to Mr. Kureshy under the employment agreement.
Tax Deductibility of Executive Compensation
The compensation committee and our board of directors have considered the potential future effects of Section 162(m) of the Internal Revenue Code on the compensation paid to our executive officers. Section 162(m) disallows a tax deduction for any publicly held corporation for individual compensation exceeding $1.0 million in any taxable year for our President and Chief Executive Officer and each of the other named executive officers (other than our Chief Financial Officer), unless compensation is performance based. As we are not currently publicly traded, our board of directors has not taken the deductibility limit imposed by Section 162(m) into consideration in setting compensation. Our compensation committee, however, has adopted a policy that, where reasonably practicable, we will seek to qualify the variable compensation paid to our executive officers for an exemption from the deductibility limitations of Section 162(m).
In approving the amount and form of compensation for our executive officers, the compensation committee will continue to consider all elements of the cost to our company of providing such compensation, including the potential impact of Section 162(m).
Accounting for Stock-Based Compensation
We account for stock-based payments in accordance with the requirements of stock-based compensation guidance.
132
Table of Contents
Outstanding Stock Options at Fiscal Year End
The following table shows grants of stock options outstanding on December 31, 2012 to each of our named executive officers.
| Name |
Number of securities underlying option awards (exercisable) (1) |
Number of securities underlying option awards (unexercisable) |
Option exercise price ($) |
Option expiration date |
||||||||||||||||
| Fareed Kureshy |
57,042 | — | (4 | ) | 0.379 | 3/31/2013 | ||||||||||||||
| 24,750 | — | 0.758 | 10/16/2013 | |||||||||||||||||
| 99,000 | — | (2 | ) | 2.152 | 1/11/2018 | |||||||||||||||
| 49,500 | 49,500 | (2 | ) | 8.303 | 2/23/2020 | |||||||||||||||
| 8,646 | — | (5 | ) | 6.091 | 9/29/2020 | |||||||||||||||
| 17,325 | — | (6 | ) | 5.242 | 8/3/2021 | |||||||||||||||
| — | 16,500 | (7 | ) | 5.242 | 5/16/2022 | |||||||||||||||
| Thomas V Hennessey, Jr |
51,150 | — | (2 | ) | 8.333 | 6/13/2018 | ||||||||||||||
| 8,663 | 2,888 | 7.970 | 5/27/2019 | |||||||||||||||||
| 6,600 | 6,600 | 8.303 | 3/30/2020 | |||||||||||||||||
| 15,477 | — | (5 | ) | 6.091 | 9/29/2020 | |||||||||||||||
| 30,938 | — | (6 | ) | 5.242 | 8/3/2021 | |||||||||||||||
| 33,000 | (7 | ) | 5.242 | 5/16/2022 | ||||||||||||||||
| Shailendra Singh, Ph.D. |
4,455 | 1,485 | 7.970 | 5/27/2019 | ||||||||||||||||
| 1,650 | 1,650 | 8.303 | 3/30/2020 | |||||||||||||||||
| 5,445 | — | (5 | ) | 6.091 | 9/29/2020 | |||||||||||||||
| 10,890 | — | (6 | ) | 5.242 | 8/3/2021 | |||||||||||||||
| Ramanath Vairavan |
— | 85,800 | (3 | ) | 0.379 | 12/23/2013 | ||||||||||||||
| 14,108 | 0.379 | 3/31/2013 | ||||||||||||||||||
| 8,663 | 2,888 | 7.970 | 5/27/2019 | |||||||||||||||||
| 1,650 | 1,650 | 8.303 | 3/30/2020 | |||||||||||||||||
| 6,270 | — | (5 | ) | 6.091 | 9/29/2020 | |||||||||||||||
| 12,375 | — | (6 | ) | 5.242 | 8/3/2021 | |||||||||||||||
| (1) | Except as described below, each option grant has a ten year term from the date of grant and vests as follows: 25% of the total shares subject to the option vest on each of the first four anniversaries of the date of grant. |
| (2) | This option was not granted under our 2000 Equity Incentive Plan or our 2008 Equity Incentive Award Plan. The terms of these options are substantially the same as those for our 2000 Equity Incentive Plan. |
| (3) | This option was not granted under our 2000 Equity Incentive Plan or our 2008 Equity Incentive Award Plan. The terms of this option are substantially the same as those for our 2000 Equity Incentive Plan except that it is exercisable only in calendar year 2013. |
| (4) | All of the shares subject to this grant were vested at grant. |
| (5) | All of the shares subject to this grant were vested on December 31, 2010. |
| (6) | All of the shares subject to this grant were vested on September 30, 2011. |
| (7) | 50% of the total shares subject to this grant vest on each of the first two anniversaries of the date of grant. |
Risk Assessment of Compensation Program
In June 2010, management assessed our compensation program for the purpose of reviewing and considering any risks presented by our compensation policies and practices that are reasonably likely to have a material adverse effect on us.
As part of that assessment, management reviewed the primary elements of our compensation program, including base salary, short-term incentive compensation and long-term incentive compensation. Management’s risk assessment included a review of the overall design of each primary element of our compensation program, and an analysis of the
133
Table of Contents
various design features, controls and approval rights in place with respect to compensation paid to management and other employees that mitigate potential risks to us that could arise from our compensation program.
Following the assessment, management determined that our compensation policies and practices did not create risks that were reasonably likely to have a material adverse effect on us and reported the results of the assessment to our compensation committee.
Employee Plans
2008 Equity Incentive Award Plan
Our board of directors has adopted, and our stockholders have approved, a 2008 Equity Incentive Award Plan, or the “2008 Plan.” The 2008 Plan became effective upon its approval by our stockholders on November 2, 2008. The purpose of the 2008 Plan is to promote the success and enhance the value of our company by continuing to link the personal interests of participants to those of our stockholders and by providing participants with an incentive for outstanding performance.
A summary of the principal provisions of the 2008 Plan is set forth below. This summary is qualified in its entirety by reference to the 2008 Plan itself. The form of the 2008 Plan is filed as an exhibit to the registration statement of which this prospectus is a part.
Shares Available for Awards. We reserved initially 660,000 shares of our common stock for issuance under the 2008 Plan. The number of shares reserved under the 2008 Plan will be increased by (1) the number of shares of common stock available for issuance and not subject to awards granted under our 2000 Equity Incentive Plan as of the effective date of the 2008 Plan and (2) the number of shares of common stock related to awards granted under our 2000 Equity Incentive Plan on or before the effective date of the 2008 Plan that are repurchased, forfeited, expired or are cancelled on or after the effective date of the 2008 Plan. As of December 31, 2012, the aggregate number of shares available for issuance under the 2008 Plan as a result of grants that were repurchased, forfeited, expired, or cancelled in the 2000 Plan was 519,219 shares. The maximum number of shares of common stock that may be added to the share reserve under the 2008 Plan pursuant to the preceding two sentences will not exceed 2,178,000 shares. The 2008 Plan contains an “evergreen provision” that mandates an annual increase in the number of shares available for issuance under the 2008 Plan on January 1 of each year, beginning on the first January 1 following the completion of this offering. The annual increase in the number of shares shall be equal to the lesser of:
| • | 3.5% of our outstanding common stock on the applicable January 1st of the applicable year; |
| • | 495,000 shares; and |
| • | a lesser number of shares as determined by our board of directors. |
As of December 31, 2012, options to purchase 932,223 shares of our common stock had been issued under the 2008 Plan, 2,145 of the options granted had been exercised and 218,331 of the options granted had been forfeited, resulting in 711,747 options outstanding.
The 2008 Plan also provides for an aggregate limit on the number of shares of common stock that may be issued under the 2008 Plan over the course of its ten-year term of 7,788,000 shares, subject to changes in our capital structure as set forth in the 2008 Plan.
To the extent that an award terminates, expires, lapses for any reason or is settled in cash, any shares of common stock subject to the award will again be available for the grant of an award pursuant to the 2008 Plan. Any shares of common stock tendered or withheld to satisfy the grant or exercise price or tax withholding obligation with respect to any award and any shares forfeited by a participant or repurchased by us will be available for subsequent grant under the 2008 Plan.
134
Table of Contents
Administration. The compensation committee of our board of directors administers the 2008 Plan (except with respect to any award granted to non-employee directors, which is administered by our full board of directors). Following the completion of this offering, to administer the 2008 Plan, our compensation committee must consist of at least two members of our board of directors, each of whom is a “non-employee director” for purposes of Rule 16b-3 under the Exchange Act, and, with respect to awards that are intended to be performance-based awards, an “outside director” for purposes of Section 162(m) of the Internal Revenue Code. Subject to the terms and conditions of the 2008 Plan, our compensation committee has the authority to select the persons to whom awards are to be made, to determine the type or types of awards to be granted to each person, the number of shares to be subject to such awards and the terms and conditions of such awards, and to make all other determinations and decisions and to take all other actions necessary or advisable for the administration of the 2008 Plan. Our compensation committee is also authorized to establish, adopt or revise rules relating to administration of the 2008 Plan, subject to certain restrictions. Our board of directors may at any time revert to itself the authority to administer the 2008 Plan. The board of directors and any committee exercising discretion under the 2008 Plan from time to time are referred to in this section as the plan administrator.
Eligibility. Options, stock appreciation rights, or SARs, restricted stock and other awards under the 2008 Plan may be granted to individuals who are our officers or employees or are the officers or employees of our parent or subsidiary companies, if any, on the date of grant. Such awards may also be granted to our non-employee directors and consultants but only employees may be granted incentive stock options, or ISOs. As of December 31, 2012, there were six non-employee directors and approximately 100 employees who are eligible for awards under the 2008 Plan. Certain individuals who are consultants covered by active consulting agreements are also eligible for awards.
Awards. The 2008 Plan provides that the plan administrator may grant or issue stock options, SARs, restricted stock, restricted stock units, dividend equivalents, stock payments and performance bonus awards, or any combination thereof. The plan administrator will consider each award grant subjectively, considering factors such as the individual performance of the recipient and the anticipated contribution of the recipient to the attainment of our long-term goals.
At such time after the offering date when the Company is subject to the requirements of Section 162(m) of the Internal Revenue Code, the maximum number of shares of our common stock that may be subject to awards granted to any one participant during any calendar year is 660,000 shares and the maximum amount that may be paid to a participant in cash during any calendar year with respect to one or more awards payable in cash is $2,000,000.
Awards under the 2008 Plan will be evidenced by a written award agreement that sets forth the terms, conditions and limitations for each award, as determined by the plan administrator.
| • | Stock Options. Stock options, including incentive stock options, as defined under Section 422 of the Internal Revenue Code, and nonqualified stock options, may be granted pursuant to the 2008 Plan. The option exercise price of all incentive stock options granted pursuant to the plan will not be less than 100% of the fair market value of our common stock on the date of grant. No incentive stock option may be granted to a grantee who owns more than 10% of the total combined voting power of all classes of our capital stock on the date of grant unless the exercise price is at least 110% of the fair market value at the time of grant. |
Notwithstanding whether an option is designated as an incentive stock option, to the extent that the aggregate fair market value of the shares with respect to which such option (and other options designated as incentive stock options) is exercisable for the first time by any optionee during any calendar year exceeds $100,000, such excess will be treated as a nonqualified stock option.
The plan administrator will determine the methods of payment of the exercise price of an option, including, without limitation, cash, shares of our common stock with a fair market value on the date of
135
Table of Contents
delivery equal to the exercise price of the option or exercised portion thereof (including shares issuable upon exercise of the option) or other property acceptable to the plan administrator (including the delivery of a notice that the participant has placed a market sell order with a broker with respect to shares then issuable upon exercise of the option, and that the broker has been directed to pay a sufficient portion of the net proceeds of the sale to us in satisfaction of the option exercise price, provided that payment of such proceeds is then made to us not later than settlement of such sale). However, no participant who is a director or an executive officer of our company within the meaning of Section 13(k) of the Exchange Act will be permitted to pay the exercise price of an option in any method that would violate Section 13(k) of the Exchange Act.
Stock options shall vest and may be exercised as determined by the plan administrator, but in no event may incentive stock options be exercised after the tenth anniversary of the date of grant. However, in the case of an incentive stock option granted to a person who owns more than 10% of the total combined voting power of all classes of our capital stock on the date of grant, such term will not exceed 5 years.
| • | Restricted Stock. Eligible employees, consultants and directors may be issued restricted stock in such amounts and on such terms and conditions as determined by the plan administrator. Restricted stock will be evidenced by a written restricted stock agreement, which will contain restrictions on transferability and other such restrictions as the plan administrator may determine, including, without limitation, limitations on the right to vote restricted stock or the right to receive dividends on the restricted stock. These restrictions may lapse separately or in combination at such times, pursuant to such circumstances, in such installments, or otherwise, as the plan administrator determines at the time of grant of the award or thereafter. Typically, restricted stock may be repurchased by us at the original purchase price or forfeited for no consideration if the conditions or restrictions are not met. |
| • | Stock Appreciation Rights. A stock appreciation right, or a SAR, is the right to receive payment of an amount equal to the excess of the fair market value of a share of our common stock on the date of exercise of the SAR over the per share exercise price of the SAR. The plan administrator may issue SARs in such amounts and on such terms and conditions as it may determine, consistent with the terms of the 2008 Plan. SARs shall vest and may be exercised as determined by the plan administrator. The plan administrator may elect to pay SARs in cash, in our common stock or in a combination of cash and our common stock. |
| • | Restricted Stock Units. Restricted stock units may be granted to any eligible employee, consultant or director in such amounts and subject to such terms and conditions as are determined by the plan administrator. At the time of grant, the plan administrator will specify the date or dates on which the restricted stock units will become fully vested and nonforfeitable, and may specify such conditions to vesting as it deems appropriate. At the time of grant, the plan administrator will specify the distribution date or event applicable to each grant of restricted stock units which will be no earlier than the vesting date or dates of the award. On the distribution date or event, we will transfer to the participant one unrestricted, fully transferable share of our common stock for each restricted stock unit scheduled to be paid out on such date and not previously forfeited. The plan administrator will specify the purchase price, if any, to be paid by the participant to us for such shares of our common stock. |
| • | Dividend Equivalents. Dividend equivalents are rights to receive the equivalent value (in cash or our common stock) of dividends paid on our common stock. They represent the value of the dividends per share paid by us, calculated with reference to the number of shares that are subject to any award held by the participant, other than options or SARs. |
| • | Stock Payments. Stock payments include payments in the form of our common stock or options or other rights to purchase our common stock, in each case made in lieu of all or any portion of the compensation that would otherwise be paid to the participant. The number of shares will be determined by the plan administrator and may be based upon specific performance criteria determined appropriate by the plan administrator. |
136
Table of Contents
| • | Performance Bonus Awards. Any eligible employee, consultant or director selected by the plan administrator may be granted one or more performance-based awards in the form of a cash bonus payable upon the attainment of performance goals that are established by the plan administrator and relate to any one or more performance criteria determined appropriate by the plan administrator on a specified date or dates or over any period or periods determined by the plan administrator. Any such cash bonus paid to a “covered employee” within the meaning of Section 162(m) of the Internal Revenue Code may be, but need not be, qualified performance-based compensation as described below. |
Qualified Performance-Based Compensation. The plan administrator may grant awards to employees who are or may be “covered employees,” as defined in Section 162(m) of the Internal Revenue Code, that are intended to be “qualified performance-based compensation” within the meaning of Section 162(m) of the Internal Revenue Code in order to preserve the deductibility of these awards for federal income tax purposes. Participants are only entitled to receive payment for “qualified performance-based compensation” for any given performance period to the extent that pre-established performance goals set by the plan administrator for the period are satisfied. These pre-established performance goals must be based on one or more of the following performance criteria: net earnings (either before or after interest, taxes, depreciation and amortization), sales or revenue, net income (either before or after taxes), operating earnings, cash flow (including, but not limited to, operating cash flow and free cash flow), return on net assets, return on stockholders’ equity, return on assets, return on capital, return on sales, gross or net profit margin, working capital, earnings per share and price per share. These performance criteria may be measured in absolute terms or as compared to performance in an earlier period or as compared to any incremental increase or as compared to results of a peer group. With regard to a particular performance period, the plan administrator will have the discretion to select the length of the performance period, the type of performance-based awards to be granted, and the goals that will be used to measure the performance for the period. In determining the actual size of an individual performance-based award for a performance period, the plan administrator may reduce or eliminate (but not increase) the award to take into account additional factors the plan administrator deems relevant to the performance for the period. Generally, a participant will have to be employed by us or a parent or subsidiary company of ours, if any, throughout the performance period to be eligible for a performance-based award for any period.
Adjustments. If there is any stock dividend, stock split, combination or exchange of shares, merger, consolidation or other distribution (other than normal cash dividends) of our assets to stockholders, or any other change affecting the shares of our common stock or the share price of our common stock other than an equity restructuring (as defined in the 2008 Plan), the plan administrator will make such equitable adjustments, if any, as the plan administrator in its discretion may deem appropriate to reflect such change with respect to (1) the aggregate number and type of shares that may be issued under the 2008 Plan (including, but not limited to, adjustments of the number of shares available under the plan and the maximum number of shares which may be subject to one or more awards to a participant pursuant to the plan during any calendar year), (2) the terms and conditions of any outstanding awards (including, without limitation, any applicable performance targets or criteria with respect thereto), and (3) the grant or exercise price per share for any outstanding awards under the plan. If there is any equity restructuring, (1) the number and type of securities subject to each outstanding award and the grant or exercise price per share for each outstanding award, if applicable, will be proportionately adjusted, and (2) the plan administrator will make proportionate adjustments to reflect such equity restructuring with respect to the aggregate number and type of shares that may be issued under the 2008 Plan (including, but not limited to, adjustments of the number of shares available under the plan and the maximum number of shares which may be subject to one or more awards to a participant pursuant to the plan during any calendar year). Adjustments in the event of an equity restructuring will not be discretionary. Any adjustment affecting an award intended as “qualified performance-based compensation” will be made consistent with the requirements of Section 162(m) of the Internal Revenue Code. The plan administrator also has the authority under the 2008 Plan to take certain other actions with respect to outstanding awards in the event of a corporate transaction, including provision for the cash-out, termination, assumption or substitution of such awards.
137
Table of Contents
Repricing. The plan administrator may, without stockholder approval, (1) amend any award to reduce the per share exercise price of such an award below the per share exercise price as of the date the award is granted and (2) grant an award in exchange for, or in connection with, the cancellation or surrender of an award having a higher per share exercise price.
Corporate Transactions. In the event of a change of control where the acquirer does not continue, convert, assume or replace awards granted under the 2008 Plan, awards issued under the 2008 Plan will be subject to accelerated vesting such that 100% of the awards will become vested and exercisable or payable, as applicable, immediately prior to a change of control. Under the 2008 Plan, a change of control is generally defined as:
| • | a transaction or series of related transactions (other than an offering of our stock to the general public through a registration statement filed with the SEC) whereby any person or entity or related group of persons or entities (other than us, our subsidiaries, if any, an employee benefit plan maintained by us or our subsidiaries, if any, or a person or entity that, prior to such transaction, directly or indirectly controls, is controlled by, or is under common control with, us) directly or indirectly acquires beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act) of more than 50% of the total combined voting power of our securities outstanding immediately after such acquisition; |
| • | during any two-year period, individuals who, at the beginning of such period, constitute our board of directors together with any new director(s) whose election by our board of directors or nomination for election by our stockholders was approved by a vote of at least two-thirds of the directors then still in office who either were directors at the beginning of the two-year period or whose election or nomination for election was previously so approved, cease for any reason to constitute a majority of our board of directors; |
| • | our consummation (whether we are directly or indirectly involved through one or more intermediaries) of (x) a merger, consolidation, reorganization, or business combination, (y) the sale or other disposition of all or substantially all of our assets in any single transaction or series of transactions or (z) the acquisition of assets or stock of another entity, in each case other than a transaction: |
| • | which results in our voting securities outstanding immediately before such transaction continuing to represent (either by remaining outstanding or by being converted into voting securities of the company or the person that, as a result of the transaction, controls, directly or indirectly, the company or owns, directly or indirectly, all or substantially all of the company’s assets or otherwise succeeds to our business (the “successor entity”)), directly or indirectly, at least a majority of the combined voting power of the successor entity’s outstanding voting securities immediately after the transaction; and |
| • | after which no person or group beneficially owns voting securities representing 50% or more of the combined voting power of the successor entity (but no person or group will be treated as beneficially owning 50% or more of combined voting power of the successor entity solely as a result of the voting power held in the company prior to the transaction). |
Amendment and Termination of the 2008 Plan. Our board of directors may terminate, amend or modify the 2008 Plan. However, stockholder approval of any amendment to the 2008 Plan will be obtained to the extent necessary and desirable to comply with any applicable law, regulation or stock exchange rule, or for any amendment to the 2008 Plan that increases the number of shares available under the 2008 Plan. If not terminated earlier by the board of directors, the 2008 Plan will terminate in 2018.
2000 Equity Incentive Plan
Our board of directors initially adopted our 2000 Equity Incentive Plan, or the “2000 Plan,” on December 13, 2000. We terminated the 2000 Plan in connection with the approval of the 2008 Plan, and, therefore, we will not make any further awards of options or stock purchase rights under the 2000 Plan.
138
Table of Contents
Outstanding stock options granted under the 2000 Plan will continue to be governed by the 2000 Plan until they are exercised, expire or otherwise terminate.
The purpose of the 2000 Plan was to further our growth, development and financial success by providing incentives to certain key persons by assisting them in acquiring shares of common stock so that they may benefit directly from our growth, development and financial success.
A summary of the principal provisions of the 2000 Plan is set forth below. This summary is qualified in its entirety by reference to the 2000 Plan itself. The 2000 Plan has been filed as an exhibit to the registration statement of which this prospectus is a part.
Shares Available for Awards. Subject to certain adjustments set forth in the 2000 Plan, the maximum number of shares of our common stock that could have been issued or awarded under the 2000 Plan was 2,178,000 shares. As of December 31, 2012, options to purchase 466,117 shares of our common stock were outstanding and 105,600 shares of our common stock had been issued pursuant to stock purchase rights under the 2000 Plan.
To the extent that an award expires, terminates or is not exercised, any shares of common stock subject to such award that have not been issued will again be available for the grant of an award pursuant to the 2008 Plan. Any shares of our common stock acquired by a participant pursuant the 2000 Plan that are reacquired by us will also again be available for the grant of an award pursuant to the 2008 Plan.
Administration. Either the board of directors or a committee of the board of directors will administer the 2000 Plan. The board of directors and any committee exercising discretion under the 2000 Plan from time to time are referred to in this section as the plan administrator. Subject to the terms of the 2000 Plan, the plan administrator had express authority to determine the eligible persons who will receive awards, the number of shares of common stock to be covered by each award and the terms and conditions of awards. The plan administrator has broad discretion to prescribe, amend and rescind rules relating to the 2000 Plan and its administration, to interpret the 2000 Plan and the terms of all award agreements, to determine the rights and obligations of participants of the 2000 Plan, to authorize the amendment of the terms of any outstanding awards under the plan.
Eligibility. Stock options and stock purchase rights under the 2000 Plan were granted to individuals who were then our employees, consultants, advisors and members of our board of directors or who were employees, consultants, advisors or members of the board of directors of our subsidiaries, if any. Only employees may be granted ISOs.
Awards. The 2000 Plan provides for the grant of stock options and stock purchase rights. The options and stock purchase rights are evidenced by option agreements and restricted stock purchase agreements, respectively, which set forth the terms, conditions and limitations for each award, as determined by the plan administrator. Such terms and conditions include certain rights of repurchase, rights of first offer, rights of first refusal and call rights in our favor.
Subject to certain adjustments set forth in the plan, the maximum number of shares of common stock that could be subject to awards granted to any one participant pursuant to the 2000 Plan was 264,000 shares.
| • | Stock Options. Stock options, including incentive stock options, as defined under Section 422 of the Internal Revenue Code, and nonqualified stock options, could be granted pursuant to the 2000 Plan. The option exercise price of all stock options granted pursuant to the plan could not be less than 85% of the fair market value of our common stock on the date of grant. No stock option could be granted to a grantee who owned more than 10% of the total combined voting power of all classes of our capital |
139
Table of Contents
| stock on the date of grant unless the exercise price was at least 110% of the fair market value at the time of grant. |
Notwithstanding whether an option is designated as an incentive stock option, to the extent that the aggregate fair market value of the shares with respect to which such option is exercisable for the first time by any optionee during any calendar year exceeds $100,000, such excess will be treated as a nonqualified stock option.
The plan administrator will determine the methods of payment of the exercise price of an option, including, without limitation, cash or other consideration acceptable to the plan administrator.
Stock options may be exercised as determined by the plan administrator, but no options were granted that could be exercised after the tenth anniversary of the date of grant. However, in accordance with tax rules governing incentive tax options, no incentive stock options were granted to a person who owned more than 10% of the total combined voting power of all classes of our capital stock on the date of grant with a term exceeding five years.
| • | Stock Purchase Rights. Stock purchase rights could have been granted pursuant to the 2000 Plan. The exercise price of a stock purchase right granted pursuant to the plan could not have been less than 85% of the fair market value of our common stock on the date of grant or at the time the participant purchases shares of common stock pursuant thereto, and the exercise price of any stock purchase right granted pursuant to the plan to any person who owns stock possessing more than 10% of the total combined voting power of all classes of our stock must have been not less than 100% of the fair market value on the date of grant or at the time the participant purchases shares of common stock pursuant thereto. |
Adjustments. If the outstanding shares of our common stock are increased, decreased or exchanged for different securities through a stock split, stock dividend, recapitalization, reorganization or similar capital adjustment other than in connection with a merger or consolidation (as described in the 2000 Plan), appropriate adjustments will be made in (1) the aggregate number of shares of common stock covered by the plan and/or the kind of shares subject to the plan, (2) the number and kind of shares of stock which may be purchased pursuant to the exercise of outstanding awards granted under the plan and (3) the exercise price or purchase price of outstanding awards granted under the plan.
Corporate Transactions. Except as otherwise provided in any option agreement between the participant and us, in the event of a corporate transaction (as defined in the 2000 Plan) involving our company in which options are not assumed or substituted with an equivalent option by the surviving corporation or its parent or subsidiary, if any, such options will become exercisable in full immediately prior to the consummation of such corporate transaction and will terminate upon the consummation of such corporate transaction to the extent not exercised prior to such consummation. At least ten days prior to the consummation of any corporate transaction, we will give each participant written notice that states whether (1) the outstanding options will be expressly assumed or substituted with equivalent options, or (2) such participant will have the right to exercise such awards immediately prior to such consummation of the corporate transaction. Any shares of our common stock issued pursuant to any stock purchase right pursuant to the 2000 Plan shall, except as otherwise provided in the applicable restricted stock purchase agreement, become fully vested upon the consummation of a corporate transaction (as defined in the 2000 Plan).
The 2000 Plan defines a corporate transaction as any of the following stockholder approved transactions to which our company is a party:
| • | a merger or consolidation in which our company is not the surviving entity; |
| • | the sale, transfer or other disposition of all or substantially all of the assets of our company (including the capital stock of our company’s subsidiary corporations, if any) in connection with the complete liquidation or dissolution of our company; |
140
Table of Contents
| • | any merger in which our company is the surviving entity but in which the shares of our company’s capital stock outstanding immediately prior to such transaction are converted into the right to receive cash, debt securities and/or equity securities of another corporation; or |
| • | the sale by the holders of more than 90% of the outstanding shares of our company’s capital stock in a single transaction or a series of related transactions. |
Amendment and Termination of the 2000 Plan. Our board of directors may amend or modify the 2000 Plan. The 2000 Plan was terminated in connection with our adoption of the 2008 Plan and as of the effective date of the 2008 Plan, no further awards will be granted under the 2000 Plan.
Options issued outside of the 2000 Plan and the 2008 Plan
We have issued options to purchase our common stock to certain of our directors, executive officers and key employees that are not subject to either the 2008 Plan or the 2000 Plan. The terms of these options are substantially the same as those issued under the 2000 Plan. As of December 31, 2012, there were outstanding options to purchase 371,828 shares of our common stock that were not issued under either the 2008 Plan or the 2000 Plan.
2008 Employee Stock Purchase Plan
Our board of directors adopted, and our stockholders have approved, our 2008 Employee Stock Purchase Plan, or the Purchase Plan. The compensation committee of the board of directors administers the Purchase Plan. The Purchase Plan is designed to allow our eligible employees and the eligible employees of our designated subsidiaries, if any, to purchase shares of common stock with accumulated payroll deductions. The Purchase Plan will become effective on the date prior to the date on which the registration statement filed with respect to the Purchase Plan becomes effective, but we expect that the first offering period under the Purchase Plan will not commence until the first May 15 or November 15 following the date on which the registration statement filed by us with respect the Purchase Plan becomes effective. We have initially reserved a total of 82,500 shares of our common stock for issuance under the Purchase Plan. The Purchase Plan contains an “evergreen provision” that mandates an annual increase in the number of shares available for issuance under the Purchase Plan on January 1 of each year, beginning on the first January 1 following the completion of this offering and continuing through, and including, January 1, 2018. The annual increase shall be equal to the lesser of:
| • | 1% of our outstanding common stock on January 1st of the applicable year; |
| • | 66,000 shares; and |
| • | a lesser number of shares determined by our board of directors. |
The Purchase Plan also provides for an aggregate limit on the number of shares of common stock that may be issued under the Purchase Plan over the course of its ten-year term of 742,500 shares. The material terms of the Purchase Plan are summarized below. The Purchase Plan has been filed as an exhibit to the registration statement of which this prospectus is a part.
The Purchase Plan will have consecutive six-month offering periods. Under the Purchase Plan, purchases will be made on the last day of each offering period. We expect the first offering period under the Purchase Plan will commence on the first May 15 or November 15 following the date on which the registration statement filed by us with respect to the Purchase Plan becomes effective. A new six-month offering period will commence on each May 15 and November 15 thereafter during the term of the Purchase Plan. Our compensation committee or board of directors may change the frequency and duration of offering periods under the Purchase Plan.
Individuals scheduled to work more than 20 hours per week for greater than five calendar months per year, may join an offering period on the first day of the offering period to the extent such individual does not,
141
Table of Contents
immediately after any rights under the Purchase Plan are granted, own (directly or through attribution) stock possessing 5% or more of the total combined voting power or value of all classes of our common or other stock of ours or a parent or subsidiary of ours, if any. As of December 31, 2012, substantially all of our 100 full-time employees would have been eligible to participate in the Purchase Plan if it were in effect.
Participants may contribute up to 20% of their cash earnings through payroll deductions, and the accumulated deductions will be applied to the purchase of shares on each purchase date. Unless otherwise designated by the compensation committee, the purchase price per share will be equal to 85% of the fair market value per share on the first day of the offering period or, if lower, 85% of the fair market value per share on the purchase date. In each calendar year, no employee is permitted to purchase shares under the Purchase Plan at a rate in excess of $25,000 worth of shares, based on the fair market value per share determined as of the first day of the offering period, for each year such a purchase right is outstanding.
In the event there is a specified type of change in our capital structure, such as a stock dividend or stock split, the compensation committee may make appropriate adjustments to (a) the number and type of shares that may be issued under the Purchase Plan, including the maximum number of shares by which the share reserve may increase automatically each year, (b) the class(es) and number of shares and price per share of stock subject to outstanding rights and (c) the purchase price with respect to any outstanding rights.
In the event of certain significant corporate transactions and other events or a change in control (as defined in the Purchase Plan), the compensation committee may provide for (a) the replacement or termination of outstanding rights in exchange for cash or other rights or property, (b) the assumption or substitution of outstanding rights by the successor or survivor corporation or parent or subsidiary thereof, if any, (c) the adjustment in the number and type of shares of stock subject to outstanding rights or in the terms and conditions of outstanding rights and rights which may be granted in the future, (d) the use of participants’ accumulated payroll deductions to purchase stock on a new purchase date prior to the next purchase date and termination of any rights under ongoing offering periods or (e) the termination of all outstanding rights.
Under the Purchase Plan, a change in control has the same definition as given to such term in the 2008 Plan.
The compensation committee may amend, suspend or terminate the Purchase Plan. However, stockholder approval of any amendment to the Purchase Plan will be obtained for any amendment which changes the aggregate number of shares that may be sold pursuant to rights under the Purchase Plan, changes the corporations or classes of corporations whose employees are eligible to participate in the Purchase Plan or changes the Purchase Plan in any manner that would cause the Purchase Plan to no longer be an “employee stock purchase plan” within the meaning of Section 423(b) of the Internal Revenue Code. The Purchase Plan will terminate no later than the tenth anniversary of the Purchase Plan’s initial adoption by our board of directors.
401(k) Plan
We provide a basic savings plan, or 401(k) plan, which is intended to qualify under Section 401(a) of the Internal Revenue Code. Pre-tax contributions by our employees or any contributions by us to the 401(k) plan, and the investment earnings thereon, are not taxable to employees until withdrawn from our 401(k) plan. Roth contributions by our employees to the 401(k) plan are made on an after-tax basis and neither the investment earnings thereon, nor the withdrawals, subject to certain holding requirements, are taxable to employees. If our 401(a) plan qualifies under Section 401(k) of the Internal Revenue Code, contributions by us, if any, will be deductible by us when made.
Our full-time employees are eligible to participate in our 401(k) plan. Pursuant to our 401(k) plan, employees may elect to reduce their eligible compensation by up to the statutorily prescribed annual limit of $17,500 in 2013 and to have the amount of this reduction contributed to our 401(k) plan. Participants who are 50 years or older also may make “catch-up” contributions, which in calendar year 2013 may be up to an additional
142
Table of Contents
$5,500 above the statutory limit. Under the 401(k) plan, each participant is fully vested in his or her elective deferred contributions when contributed. Participant contributions are held and invested, pursuant to the participant’s instructions, by the plan’s trustee. We have the ability to make matching or profit sharing contributions to our 401(k) plan in our discretion. We have not historically made such contributions, and do not currently have any plans to do so in the immediate future. The 401(k) plan does not offer the ability to invest in our securities.
Indemnification of Directors and Officers and Limitation of Liability
We are subject to the DGCL. Section 145 of the DGCL authorizes a corporation’s board of directors to grant indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities, including reimbursement for expenses incurred, arising under the Securities Act.
Certificate of Incorporation and Bylaws
Our certificate of incorporation and bylaws require us to indemnify our directors, officers and other persons serving at our request as a director, officer or agent of another entity to the fullest extent permitted by the DGCL. We are required to advance expenses, as incurred, to the covered persons in connection with defending a legal proceeding if we have received an undertaking by that person to repay all such amounts if it is determined that he or she is not entitled to be indemnified by us.
Our certificate of incorporation eliminates the personal liability of our directors for monetary damages for breach of fiduciary duty as a director, except for liability for:
| • | any breach of the director’s duty of loyalty to the corporation or its stockholders; |
| • | acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
| • | unlawful payments of dividends or unlawful stock repurchases, redemptions or other distributions, as provided in Section 174 of the DGCL; or |
| • | any transaction from which the director derived an improper personal benefit. |
Indemnification Agreements
We have entered into indemnification agreements with each of our directors and executive officers. These agreements provide indemnification to our directors and executive officers under certain circumstances for acts or omissions which may not be covered by directors’ and officers’ liability insurance, and may, in some cases, be broader than the specific indemnification provisions contained under the DGCL.
Indemnification for Securities Act Liability
Insofar as indemnification for liabilities arising under the Securities Act may be permitted for directors, officers or persons controlling us pursuant to the foregoing, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Insurance Policies
We maintain an insurance policy covering our directors and officers with respect to certain liabilities, including liabilities arising under the Securities Act or otherwise.
143
Table of Contents
The following table sets forth information with respect to the beneficial ownership of our common stock as of December 31, 2012 after giving effect to our debt restructuring described elsewhere in this prospectus:
| • | each person or group of affiliated persons known by us to own beneficially more than 5% of our outstanding common stock; |
| • | each of our directors; |
| • | each of our named executive officers; and |
| • | all of our executive officers and directors as a group. |
We have determined beneficial ownership in accordance with the rules of the SEC. Except as otherwise indicated in the footnotes below, all of the shares reflected in the table are shares of common stock and we believe, based on the information furnished to us, that all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws.
Percentage ownership calculations are based on 10,746,791 shares outstanding as of December 31, 2012, and assumes the conversion of all outstanding series of convertible preferred stock, including our Series NC Convertible Preferred Stock, into common stock. In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we have deemed outstanding shares of common stock subject to options or warrants held by that person that are exercisable within 60 days of December 31, 2012. We have not deemed these shares outstanding for the purpose of computing the percentage ownership of any other person. Beneficial ownership representing less than one percent is denoted with an asterisk “*.”
Unless otherwise indicated below, the address for each of the beneficial owners in the table below is c/o AutoGenomics, Inc., 2980 Scott Street, Vista, California, 92081.
| Shares beneficially owned | ||||||||
| Name and address of beneficial owner |
Number | Percent | ||||||
| 5% stockholders (other than directors and named executive officers) |
||||||||
| Dennis A. Repp (1) |
1,318,581 | 11.97 | % | |||||
| Named executive officers and directors |
||||||||
| Fareed Kureshy (2) |
1,285,278 | 11.66 | % | |||||
| Thomas V. Hennessey, Jr. (3) |
248,229 | 2.29 | % | |||||
| Shailendra Singh, Ph.D. (4) |
638,880 | 5.93 | % | |||||
| Ramanath Vairavan (5) |
252,615 | 2.32 | % | |||||
| Stephen Allison (6) |
31,350 | * | ||||||
| Charles Birmingham (7) |
9,803 | * | ||||||
| Laurence M. Demers, Ph.D (8) |
125,847 | 1.16 | % | |||||
| Donald E. Pogorzelski (9) |
315,559 | 2.87 | % | |||||
| Peter Wilding, Ph.D |
|
— |
|
* | ||||
| Eugene J. Zurlo (10) |
140,462 | 1.30 | % | |||||
| All executive officers and directors as a group (11 persons) |
3,084,323 | 26.28 | % | |||||
| * | Represents less than 1% of the outstanding shares of common stock. |
| (1) | Includes (i) warrants exercisable for 36,300 shares of common stock, (ii) 891,280 shares of Series B Convertible Preferred Stock convertible into 320,380 shares of common stock, 527,273 shares of Series C Convertible Preferred Stock convertible into 189,534 shares of common stock, 61,539 shares of Series D Convertible Preferred Stock convertible into 22,609 shares of common stock, 1,503,011 shares of Series NC Convertible Preferred Stock convertible into 495,993 shares of common stock, warrants exercisable for |
144
Table of Contents
| 222,876 shares of common stock and warrants exercisable for 17,818 shares of Series C Convertible Preferred Stock convertible into 6,404 shares of common stock held by A R Properties, of which Mr. Repp is the General Partner, and (iii) 36,363 shares of Series C Convertible Preferred Stock convertible into 13,071 shares of common stock held by ValueTech Investors, of which Mr. Repp is the General Partner. The business address of Mr. Repp is 3419 Via Lido #262, Newport Beach, California 92663. |
| (2) | Includes options to purchase 281,013 shares of common stock and 4,915 shares of Series A Convertible Preferred Stock convertible into 3,243 shares of common stock. The shares of Series A Convertible Preferred Stock are held jointly by Fareed Kureshy and Dawn Beth Kureshy. |
| (3) | Includes (i) warrants to purchase 3,300 shares of common stock, options to purchase 112,827 shares of common stock, and 12,658 shares of Series NC Convertible Preferred Stock convertible into 4,177 shares of common stock, and (ii) 44,668 shares of Series A Convertible Preferred Stock convertible into 29,480 shares of common stock, 26,000 shares of Series C Convertible Preferred Stock convertible into 9,345 shares of common stock, and 49,500 shares of common stock held by the Mary D. and Thomas V. Hennessey, Jr. Irrevocable Trust. |
| (4) | Includes options to purchase 22,440 shares of common stock. |
| (5) | Includes options to purchase 128,865 shares of common stock. |
| (6) | Includes warrants exercisable for 3,300 shares of common stock and options to purchase 8,250 shares of common stock. |
| (7) | Consists of 27,274 shares of Series B Convertible Preferred Stock convertible into 9,803 shares of common stock held by ABHV Partnership, of which Mr. Birmingham is a partner. Mr. Birmingham disclaims beneficial ownership of the shares held by ABHV Partners. |
| (8) | Includes 58,763 shares of Series E Convertible Preferred Stock convertible into 21,123 shares of common stock, options to purchase 34,650 shares of common stock and warrants exercisable for 66,774 shares of common stock. |
| (9) | Includes warrants exercisable for 219,150 shares of common stock, 72,727 shares of Series E Convertible Preferred Stock convertible into 26,142 shares of common stock and 137,928 shares of series NC Convertible Preferred Stock convertible into 45,516 shares of common stock held by the Donald E. Pogorzelski Trust UA May 6, 1998, of which Mr. Pogorzelski is a trustee. |
| (10) | Includes warrants exercisable for 14,978 shares of common stock held by the Zurlo Investment Trust, options to purchase 26,400 shares of common stock, 64,000 shares of Series A Convertible Preferred Stock convertible into 42,240 shares of common stock, 36,364 shares of Series B Convertible Preferred Stock convertible into 13,071 shares of common stock, and 20,791 shares of Series E Convertible Preferred Stock convertible into 7,473 shares of common stock. |
145
Table of Contents
Certain Relationships and Related Persons Transactions
In addition to the director and executive compensation arrangements discussed above in “Management” and “Executive Compensation,” we have been a party to the following transactions since January 1, 2009, in which any director, executive officer or holder of more than 5% of any class of our voting stock, or any member of the immediate family of or entities affiliated with any of them, had or will have a material interest.
Warrant Issuances
As of December 31, 2012 we had outstanding warrants to purchase an aggregate of 3,372,711 shares of our common stock and 371,300 shares of our Series C Convertible Preferred Stock. These warrants were issued in connection with promissory note financings and in connection with services rendered to us. The participants in these warrant issuances included the following directors, executive officers and holders of more than 5% of our capital stock. The following table presents the number of shares issued to these related parties in these financings:
| Participants (1) |
Warrants to purchase common stock |
|||
| 5% Stockholders (other than directors and executive officers) |
||||
| Dennis A. Repp |
259,175 | (2) | ||
| Directors and Executive Officers |
||||
| Stephen Allison, Director |
3,300 | (3) | ||
| William H. Davidson, D.B.A., Director |
36,300 | (4) | ||
| Laurence M. Demers, Ph.D, Director |
62,649 | (5) | ||
| Thomas V. Hennessey, Jr., COO and CFO |
3,300 | (6) | ||
| Donald E Pogorzelski, Director |
219,150 | (7) | ||
| Eugene J. Zurlo, Director |
14,978 | (8) | ||
| (1) | Additional detail regarding these stockholders and their equity holdings is provided in “Principal Stockholders.” |
| (2) | In March 2009 and in December 2009, we issued warrants to purchase 93,720 shares of our common stock at an exercise price of $21.21 per share and 8,904 shares of our common stock at an exercise price of $12.12 per share, respectively, to A R Properties in connection with our promissory notes financings. In December 2009, we issued a warrant to purchase 23,430 shares of our common stock at an exercise price of $12.12 per share to A R Properties in connection with our promissory notes financings. In exchange for A R Properties’ agreement to extend the maturity date of a promissory note, we amended this warrant to have an exercise price of $9.09 per share. In January 2010, we issued a warrant to purchase 33,000 shares of our common stock at an exercise price of $8.30 per share to Mr. Repp as consideration for services performed for us. In February 2010, we issued a warrant to purchase 9,372 shares of our common stock at an exercise price of $12.12 per share to A R Properties in connection with our promissory notes financings. In March 2010, we issued a warrant to purchase 12,375 shares of our common stock at an exercise price of $15.15 per share to A R Properties in connection with our promissory notes financings. In April 2010, in exchange for A R Properties’ agreement to extend the maturity date of a promissory note, we cancelled the March 2009 warrant to purchase 93,720 shares of our common stock and replaced it with a new warrant to purchase 93,720 shares of our common stock at an exercise price of $15.15 per share. In January 2011 and May 2011, we issued warrants to A R Properties in connection with our promissory notes financings to purchase 9,900 shares of our common stock and 19,800 shares of our common stock, each at an exercise price of $9.09 per share, respectively. In August 2011, we issued a warrant to purchase 3,300 shares of our common stock at an exercise price of $5.24 per share as consideration for services provided to us. In November 2011 and March 2012, we issued warrants to A R Properties in connection with our promissory notes financings to purchase 20,625 shares of our common stock and 24,750 shares of our common stock, each at an exercise price of $5.24 per share, respectively. In November 2012, in connection with an exchange of notes payable aggregating $2.6 million for 1,498,637 shares of Series NC convertible preferred stock, we cancelled |
146
Table of Contents
| warrants to purchase 8,903, 21,300, 9,372, 12,375, 93,720, 9,900, 19,800, 20,625, and 24,750 shares of our common stock at exercise prices of $21.21, $21.21, $12.12, $12.12, $15.15, $15.15, $9.09, $9.09, $5.24 and $5.24, respectively, and issued an aggregate of 222,876 warrants to purchase shares of our common stock at an exercise price of $4.55. See “Promissory Notes Financings” below. |
| (3) | In May 2012, we issued a warrant to Mr. Allison to purchase 3,300 shares of common stock at an exercise price of $5.24 in connection with his service as Chairman of the Audit Committee of the Board of Directors of AutoGenomics, Inc. |
| (4) | Dr. Davidson left our board of directors in October 2012. In September 2008, we issued a warrant to purchase 4,686 shares of our common stock at an exercise price of $21.21 per share to Dr. Davidson in connection with our promissory notes financings. In December 2009, we cancelled this warrant and replaced it with a warrant to purchase 9,372 shares of our common stock at an exercise price of $21.21 per share. This warrant expired in September 2012 without exercise. In December 2010 we issued a warrant to purchase 9,900 shares of our common stock at an exercise price of $9.09 per share to Dr. Davidson in connection with our promissory notes financings. In August 2011, we issued a warrant to purchase 23,100 shares of our common stock at an exercise price of $5.24 per share to Dr. Davidson as consideration for services provided to us and in connection with his service on our board of directors. In May 2012, we issued a warrant to Dr. Davidson to purchase 3,300 shares of common stock at an exercise price of $5.24 in connection with his service as chairman of the governance committee of our board of directors. In November 2012, in connection with an exchange of notes payable aggregating $0.1 million for 64,793 shares of Series NC convertible preferred stock, we cancelled a warrant to purchase 9,900 shares of our common stock at an exercise price of $9.09 and issued a warrant to purchase 9,900 shares of our common stock at an exercise price of $4.55. See “Promissory Notes Financings” below. Dr. Davidson ceased to be a member of our board of directors in October 2012. |
| (5) | In March 2009, we issued a warrant to purchase 4,686 shares of our common stock at an exercise price of $21.21 per share to Dr. Demers in connection with our promissory notes financings. In April 2010, in exchange for Dr. Demers’ agreement to extend the maturity date of a promissory note, we cancelled this warrant and replaced it with a warrant to purchase 4,686 shares of our common stock at an exercise price of $15.15 per share. In December 2010, we issued to Dr. Demers a warrant to purchase 9,900 shares of our common stock at an exercise price of $9.09 per share in connection with our promissory notes financings. In August 2011, we issued a warrant to purchase 3,300 shares of our common stock at an exercise price of $5.24 per share to Dr. Demers in connection with his service on our board of directors. In November 2011, in connection with our sale of Series E Convertible Preferred Stock, we issued to Dr. Demers a warrant to purchase 29,088 shares of our common stock at an exercise price of $8.33 per share. In December 2011, we issued a warrant to purchase 8,250 shares of our common stock at an exercise price of $5.24 per share to Dr. Demers in connection with our promissory notes financings. In May 2012, we issued a warrant to Dr. Demers to purchase 3,300 shares of common stock at an exercise price of $5.24 in connection with his service as chairman of the compensation committee of our board of directors. In November 2012, in connection with a refinancing of a note payable in the amount of $55,000 including accrued interest into a note payable of $55,000, we cancelled a warrant to purchase 8,250 shares of our common stock at an exercise price of $5.24 and issued a warrant to purchase 12,375 shares of our common stock at an exercise price of $5.30. See “Promissory Notes Financings” below. |
| (6) | In January 2012, we issued to Mr. Hennessey a warrant to purchase 3,300 shares of our common stock at an exercise price of $5.24 per share in connection with our promissory notes financings. In November 2012, in connection with an exchange of notes payable aggregating $20,000 for 12,658 shares of Series NC convertible preferred stock, we cancelled a warrant to purchase 3,300 shares of our common stock at an exercise price of $5.24 and issued a warrant to purchase 3,300 shares of our common stock at an exercise price of $4.55. See “Promissory Notes Financings” below. |
| (7) | In November 2011, in connection with our sale of Series E Convertible Preferred Stock, we issued to Mr. Pogorzelski a warrant to purchase 36,000 shares of our common stock at an exercise price of $8.33 per share. In January and March 2012, we issued warrants to purchase 41,250 shares and 89,100 shares of our common stock, respectively, each at an exercise price of $5.24 per share to Mr. Pogorzelski in connection with our promissory notes financings. In November 2012, in connection with an exchange of notes payable |
147
Table of Contents
| aggregating $1.2 million for 137,928 shares of Series NC convertible preferred stock and a new note in the amount of $1.0 million, we cancelled warrants to purchase 41,250 and 89,100 shares of our common stock each at an exercise price of $5.24 and issued a warrant to purchase 24,750 shares of our common stock at an exercise price of $4.55 and warrants to purchase an aggregate of 158,400 shares of our common stock at an exercise price of $5.30. See “Promissory Notes Financings” below. |
| (8) | In March 2009, we issued a warrant to purchase 4,686 shares of our common stock at an exercise price of $21.21 per share to Mr. Zurlo, as trustee of the Zurlo Investment Trust, in connection with our promissory notes financings. In April 2010, in exchange for Mr. Zurlo’s agreement, as trustee of the Zurlo Investment Trust, to extend the maturity date of a promissory note, we cancelled this warrant and we replaced it with a warrant to purchase 4,686 shares of our common stock at an exercise price of $15.15 per share. See “Promissory Notes Financings” below. In November 2011, in connection with our sale of Series E Convertible Preferred Stock, we issued to Mr. Zurlo, as trustee of the Zurlo Investment Trust, a warrant to purchase 10,292 shares of our common stock at an exercise price of $8.33 per share. |
Promissory Notes Financings
As of September 30, 2012, we had an aggregate of $24.7 million in principal and accrued interest outstanding under promissory notes. These promissory notes had a weighted average annual interest rate of 6.8%, with the principal and accrued interest balances generally due two years after the date of issuance. We may elect to prepay the notes at any time without penalty. In the private placements, we also issued warrants to purchase an aggregate of 2,314,402 shares of our common stock with a weighted average exercise price of $7.09 per share. The warrants become exercisable one year after issuance and have terms of five years. Dennis A. Repp, William Davidson, Laurence M. Demers, Thomas V. Hennessey, Jr., Donald E. Pogorzelski and Eugene J. Zurlo participated in the financings and purchased $2,245,000, $200,000, $200,000, $20,000, $1,250,000 and $50,000 aggregate principal amount of notes and received warrants to purchase 222,876, 19,272, 22,836, 3,300, 130,350 and 4,686 shares of our common stock, respectively. In December 2009, we cancelled warrants to purchase 4,686 shares issued in 2008 and reissued warrants to purchase 9,372 to William H. Davidson. In addition, in April 2010, we extended to April 2012 the maturity dates of notes with aggregate principal amounts of $1,000,000, $50,000, and $50,000 held by Mr. Repp, Dr. Demers, and Mr. Zurlo, respectively. In connection with those extensions, we cancelled warrants and issued new warrants to purchase 93,720, 4,686, and 4,686 shares of our common stock to each of these individuals, respectively. Each of the new warrants has an exercise price of $15.15 per share and expires six and one-half years after the issuance date of the original warrants. As of September 30, 2012, there was an aggregate of $321,482, $10,510, $3,008, $1,164, $42,712 and $0 of accrued interest outstanding under the notes issued to these persons, respectively. For each additional day that these notes remain outstanding, we were required to pay approximately $0.16 of additional interest under each $1,000 principal amount of the notes. None of these notes are subject to any redemption premium. In November 2012, Dennis A Repp, William Davidson, Thomas V Hennessey, Jr., and Donald E Pogorzelski surrendered outstanding promissory notes payable of $2,245,000, $100,000, $20,000 and $150,000 aggregate principal and received 1,498,637, 64,595, 12,590, and 134,545 shares, respectively, of Series NC Convertible Preferred Stock. Also in November 2012, Donald E Pogorzelski and Laurence M. Demers exchanged outstanding promissory notes payable of $1,000,000 and $50,000 aggregate principal and received new subordinated promissory notes bearing rates of interest of 8.5% and maturity dates in November 2015 aggregating $1,000,000 and $55,000, respectively. Our directors, executive officers and holders of more than 5% of our common stock that participated in the private placements and the extensions, cancellations and reissuances described above received warrants to purchase a number of shares of our common stock in the same proportion to their respective note investments as all other investors participating in the same private placement, extension, cancellation or reissuance. In January 2013, Dennis A. Repp purchased a $2.4 million promissory note from us in a private placement. This note has an annual interest rate of 8.5%, a maturity date of March 31, 2013, and is prepayable at any time without premium or penalty.
148
Table of Contents
Employment Relationships
Saeed Kureshy, our Vice President of Operations, and Shafie Kureshy, our Director of Engineering, are the brother and son, respectively, of Fareed Kureshy, our Founder, President, Chief Executive Officer and Chairman of the Board. Mr. Saeed Kureshy received employment compensation in the amounts of $134,038, $144,025 and $197,790 for the fiscal years 2012, 2011 and 2010, respectively, which included stock-based compensation in the amount of $60,482 for fiscal year 2010 associated with the grant of options to purchase 12,573 shares of our common stock at a weighted average exercise price of $7.54 per share, and stock-based compensation in the amount of $39,025 for fiscal year 2011 associated with the grant of options to purchase 11,963 shares of our common stock at an exercise price of $5.24 per share. Mr. Shafie Kureshy received employment compensation in the amounts of $95,717, $90,609 and $183,509 for the fiscal years 2012, 2011 and 2010, respectively, which included stock-based compensation in the amount of $88,194 for fiscal year 2010 associated with the grant of options to purchase 15,741 shares of our common stock at a weighted average exercise price of $1.53 per share, and stock-based compensation in the amount of $5,665 for fiscal year 2011 associated with the grant of options to purchase 1,764 shares of our common stock at an exercise price of $5.24 per share.
Indemnification Agreements
We have entered into indemnification agreements with each of our directors and executive officers, as described in “Executive Compensation — Indemnification of Directors and Officers and Limitation of Liability.”
Series C Registration Rights Agreement
We have entered into a registration rights agreement with the holders of our Series C Convertible Preferred Stock and warrants to purchase our Series C Convertible Preferred Stock, including certain of our directors and beneficial owners of more than 5% of our outstanding common stock, as described in “Shares Eligible for Future Sale — Registration Rights.”
Review, Approval or Ratification of Transactions with Related Persons
Pursuant to its written charter, our audit committee must review and approve all related person transactions, which includes any transactions with an executive officer, director, beneficial owner of more than 5% of our outstanding common stock, or any of such persons’ immediate family members in which the amount involved exceeds $120,000, and in which any such persons had or will have a direct or indirect material interest. For a discussion of the composition and responsibilities of our audit committee see “Management — Board Structure and Committees Composition — Audit Committee.” In determining whether to approve a related person transaction, our audit committee will consider such matters as it deems appropriate under the circumstances. After considering these factors, our audit committee will decide whether the related person transaction is in our best interests and will approve or reject the transaction accordingly.
149
Table of Contents
The following is a description of our capital stock, after giving effect to the automatic conversion of our outstanding preferred stock in connection with the completion of this offering, and the material provisions of our certificate of incorporation and bylaws. The following is only a summary and is qualified by the provisions of our certificate of incorporation and bylaws, copies of which are available as set forth under “Where You Can Find More Information.”
Our authorized capital stock consists of 220 million shares of common stock, par value $0.01 per share, and 30 million shares of preferred stock, par value $0.01 per share.
Common Stock
As of December 31, 2012, there were 2,855,771 shares of common stock outstanding, and we had 398 stockholders (including holders of our convertible preferred stock). The number of shares of common stock outstanding as of December 31, 2012 does not include (i) 7,891,020 shares of common stock issuable upon the conversion of outstanding shares of convertible preferred stock, (ii) 3,372,711 shares of common stock issuable upon the exercise of outstanding warrants to purchase common stock, (iii) 133,488 shares of common stock issued upon conversion of shares of convertible preferred stock that are issuable upon the exercise of outstanding warrants to purchase preferred stock and (iv) 1,549,692 shares of common stock issuable upon the exercise of outstanding options to purchase common stock. The holders of our common stock are entitled to the following rights:
Voting Rights
Each share of our common stock entitles its holder to one vote per share on all matters to be voted upon by the stockholders. There is no cumulative voting, which means that a holder or group of holders of more than 50% of the shares of our common stock can elect all of our directors.
Dividend Rights
The holders of our common stock are entitled to receive dividends when and as declared by our board of directors from legally available sources, subject to any restrictions in our certificate of incorporation or prior rights of the holders of our preferred stock. See “Dividend Policy.”
Liquidation Rights
In the event of our liquidation or dissolution, the holders of our common stock are entitled to share ratably in the assets available for distribution after the payment of all of our debts and other liabilities, subject to the prior rights of the holders of our preferred stock.
Other Matters
The holders of our common stock have no subscription, redemption or conversion privileges. There are no sinking fund provisions applicable to our common stock. Our common stock does not entitle its holder to preemptive rights. All of the outstanding shares of our common stock are fully paid and nonassessable. The rights, preferences and privileges of the holders of our common stock are subject to the rights of the holders of shares of any series of preferred stock that we may issue in the future.
Preferred Stock
As discussed under “Prospectus Summary — The Offering,” effective immediately prior to the completion of this offering, each outstanding share of our Series A Convertible Preferred Stock will convert into 0.6600
150
Table of Contents
shares of our common stock, each outstanding share of our Series B, Series C and Series E Convertible Preferred Stock will convert into 0.3595 shares of our common stock, each outstanding share of our Series D Convertible Preferred Stock will convert into 0.3674 shares of our common stock and each outstanding share of our Series NC Convertible Preferred Stock will convert into 0.3300 shares of our common stock. Effective upon the completion of this offering, each warrant exercisable for preferred stock will become exercisable for a number of shares of common stock based on the same conversion ratios.
Following completion of this offering our board of directors, without further vote or action by our stockholders, may fix the designations, powers, preferences and rights, and the qualifications, limitations or restrictions thereof including dividend rights, dividend rates, conversion rights, voting rights, terms of redemption, redemption prices, liquidation preferences and the number of shares constituting any class or series, of up to an aggregate of 9.1 million shares of our preferred stock, and may authorize the issuance of the same. The issuance of our preferred stock may have the effect of delaying, deferring, or preventing a change in control of our company without further action by the stockholders and may adversely affect the voting and other rights, including the right to dividends and to payments upon liquidation, of the holders of our common stock. Upon the completion of this offering, no shares of our preferred stock will be outstanding, and we have no present plans to issue any shares of preferred stock.
Provisions of Our Certificate of Incorporation and Bylaws and Delaware Law That May Have an Anti-Takeover Effect
Certificate of Incorporation and Bylaws
Certain provisions of Delaware law, our certificate of incorporation and our bylaws may be deemed to have an anti-takeover effect and may delay, deter or prevent a tender offer or takeover attempt that a stockholder might consider to be in its best interests, including attempts that might result in a premium being paid over the market price for the shares held by stockholders.
Our certificate of incorporation and bylaws contain provisions that could have the effect of discouraging potential acquisition proposals or tender offers or delaying or preventing a change of control of our company. In particular, our certificate of incorporation and bylaws, as applicable, among other things:
| • | provide that special meetings of the stockholders may be called only by the chairman of our board, chief executive officer or a majority of the members of our board of directors; |
| • | provide that any action required or permitted to be taken by the stockholders of our company must be effected at a duly called annual or special meeting of the stockholders and the taking of action by written consent of the stockholders in lieu of a meeting of the stockholders is specifically denied; |
| • | establish procedures with respect to stockholder proposals and stockholder nominations, including requiring that advance written notice of a stockholder proposal or director nomination generally must be received at our principal executive offices not less than 90 nor more than 120 days prior to the first anniversary date of the previous year’s annual meeting of stockholders; |
| • | do not include any provision for cumulative voting in the election of directors or for any other matters. Under cumulative voting, a minority stockholder holding a sufficient number of shares may be able to ensure the election of one or more directors. The absence of cumulative voting may have the effect of limiting the ability of minority stockholders to effect changes in the board of directors and, as a result, may have the effect of deterring a hostile takeover or delaying or preventing changes in control or management of our company; |
| • | provide that the exact number of directors on our board may be fixed from time to time exclusively by resolution of our board of directors within a range of five to 15 directors and that newly-created directorships and vacancies on our board of directors may be filled by a majority of directors in office, although less than a quorum; |
151
Table of Contents
| • | provide that the board of directors may determine the number of committee members on each board committee at any time, and may remove any individual board committee member at any time for any reason. Any vacancy or newly-created committee memberships may be filled by our board of directors; |
| • | provide that a director may be removed from office by the affirmative vote of at least 66-2/3% of the total voting power of the outstanding shares entitled to vote generally in the election of directors, voting together as a single class; |
| • | provide that our board of directors has the power to alter, amend or repeal the bylaws without stockholder approval; |
| • | require that the stockholders may alter, amend or repeal our bylaws only with the vote of holders of 66-2/3% of the voting power of the outstanding shares entitled to vote generally in the election of directors; and |
| • | require that the vote of holders of 66-2/3% of the voting power of the outstanding shares entitled to vote generally in the election of directors is required to amend various provisions of our certificate of incorporation, including provisions relating to: |
| • | the number of directors on our board of directors; |
| • | the election, qualification and term of office of our directors; |
| • | filling vacancies on our board of directors; |
| • | removal of members of our board of directors; and |
| • | amendments to our certificate of incorporation. |
Our certificate of incorporation authorizes our board of directors, without further vote or action by the stockholders, to issue, after giving effect to the completion of this offering and the automatic conversion of all of our shares of outstanding Series A, Series B, Series C, Series D, Series E and Series NC Convertible Preferred Stock into shares of common stock, up to an additional 9.1 million shares of preferred stock, par value $0.01 per share, in one or more classes or series, and to fix or alter:
| • | the number of shares constituting any class or series; |
| • | the designations, powers and preferences of each class or series; |
| • | the relative, participating, optional and other special rights of each class or series; and |
| • | any qualifications, limitations or restrictions on each class or series. |
The above provisions are intended to promote continuity and stability in the composition of our board of directors and in the policies formulated by the board and to discourage certain types of transactions that may involve an actual or threatened change of control. These provisions are expected to reduce our vulnerability to unsolicited acquisition attempts as well as discourage certain tactics that may be used in proxy fights. Such provisions, however, could discourage others from making tender offers for our shares and, as a consequence, may inhibit fluctuations in the market price of our common stock that could result from actual or rumored takeover attempts. These provisions also could operate to prevent changes in our management.
Delaware Takeover Statute
We are subject to the provisions of Section 203 of the DGCL. Subject to certain exceptions, Section 203 prohibits a Delaware corporation from engaging, under certain circumstances, in a “business combination” with an “interested stockholder” for a period of three years following the date that the stockholder became an interested stockholder, unless:
| • | prior to the date of the business combination, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
152
Table of Contents
| • | on consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock of the interested stockholder) those shares owned: |
| • | by persons who are directors and also officers, and |
| • | by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| • | at or subsequent to such time, the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66-2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
A “business combination” includes:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| • | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| • | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; or |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
Subject to various exceptions, an “interested stockholder” is an entity or person who, together with affiliates and associates, owns (or within three years from the date of determination, did own) 15% or more of the corporation’s outstanding voting stock. This statute could delay, defer or prohibit a merger or other takeover or a change of control of our company. We also anticipate that this statute may discourage business combinations or other attempts that might result in a premium over the market price for the shares of common stock held by our stockholders.
NASDAQ
We have applied to list our common stock on the NASDAQ Global Market under the symbol “AGMX.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, LLC.
153
Table of Contents
Shares Eligible for Future Sale
Prior to this offering, there has been no public market for our common stock, and a significant public market for our common stock may not develop or be sustained after this offering. Future sales of significant amounts of our common stock, including shares of our outstanding common stock and shares of our common stock issued upon exercise of outstanding options, in the public market after this offering could adversely affect the prevailing market price of our common stock and could impair our future ability to raise capital through the sale of our equity securities.
Sale of Restricted Shares and Lock-Up Agreements
Upon the closing of this offering, 16,746,791 shares of common stock will be outstanding assuming no exercise of the underwriters’ overallotment option and no exercise of currently outstanding options or warrants. Of these shares, the shares of common stock sold in this offering, plus any additional shares sold upon exercise of the underwriters’ overallotment option, will be freely tradable without restriction under the Securities Act, unless they are held by our affiliates, as that term is defined in Rule 144 under the Securities Act and the rules and regulations promulgated thereunder.
The remaining shares of common stock held by existing stockholders are restricted securities as that term is defined in Rule 144 under the Securities Act or are subject to the contractual restrictions described below. Of these remaining securities:
| • | 1,905,899 shares may be sold as of the date of this prospectus; |
| • | 0 additional shares may be sold beginning 90 days after the date of this prospectus; and |
| • | 8,840,892 additional shares may be sold upon expiration of the lock-up period described below, although a portion of those shares will be subject to volume limitations pursuant to Rule 144. |
Restricted securities may be sold in the public market only if registered or if they qualify for an exemption from registration under Rule 144 or 701 under the Securities Act, which rules are summarized below.
Lock-Up Agreements
Our officers, directors and other stockholders owning approximately 82% of our common stock on an as-converted basis have agreed, subject to certain exceptions, not to offer, pledge, announce the intention to sell, sell, contract to sell, transfer or dispose of, directly or indirectly, any shares of our common stock, or any securities convertible into or exercisable or exchangeable for shares of our common stock, for a period of 180 days after the date of this prospectus, without the prior written consent of Leerink Swann LLC. Leerink Swann LLC currently does not anticipate shortening or waiving any of the lock-up agreements or other contractual obligations restricting the sale of securities and does not have any pre-established conditions for such modifications or waivers; however, Leerink Swann LLC may, in its sole discretion, at any time, and without notice, release for sale in the public market all or any portion of the shares subject to the lock-up agreement. See “Underwriting—Lock-Up Agreements.”
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to public company reporting requirements for at least 90 days, a person who is not one of our affiliates and who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than an affiliate, is entitled to sell those shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to our compliance with the public information
154
Table of Contents
requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then that person is entitled to sell those shares without complying with any of the requirements of Rule 144.
In general, under Rule 144 as currently in effect, our affiliates or persons selling shares on behalf of our affiliates are entitled to sell upon expiration of the lock-up agreements described above, within any three-month period, a number of shares that does not exceed the greater of:
| • | 1% of the number of shares of common stock then outstanding, which will equal approximately 167,468 shares immediately after this offering; or |
| • | the average weekly trading volume of the common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to that sale. |
Such sales by affiliates may commence beginning 90 days after the date of this prospectus, subject to continued availability of current public information about us. Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of our company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information or holding period provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this prospectus before selling such shares pursuant to Rule 701.
Options
In addition to the shares of common stock outstanding immediately after this offering, as of December 31, 2012, there were outstanding options to purchase 1,549,720 shares of our common stock. As soon as practicable upon completion of this offering, we intend to file a registration statement on Form S-8 under the Securities Act covering shares of our common stock issued or reserved for issuance under our stock plans and other eligible shares of our common stock. Accordingly, shares of our common stock registered under such registration statement will be available for sale in the open market upon exercise of related options by the holders, subject to vesting restrictions with us, contractual lock-up restrictions, and/or market stand-off provisions applicable to each option agreement that prohibit the sale or other disposition of the shares of common stock underlying the options for a period of 180 days after the date of this prospectus without the prior written consent from us or our underwriters.
Registration Rights
We have entered into a registration rights agreement with the holders of our Series C Convertible Preferred Stock and warrants to purchase our Series C Convertible Preferred Stock. Pursuant to the agreement, these holders are entitled to cause us to register the shares of common stock or other securities issuable upon conversion or exercise of such preferred stock and warrants (referred to as “registrable securities”), subject to the terms and conditions of the agreement. Pursuant to the lock-up agreements described above, holders of a majority of the registrable securities have agreed, on behalf of all such holders, not to exercise their registration rights until 180 days after the completion of this offering. However, following this 180-day period, subject to certain limitations, these holders will be entitled to cause us to include their registrable securities in certain registrations by us of our common stock under the Securities Act. If, in connection with any underwritten registration of our
155
Table of Contents
common stock, the managing underwriter in its judgment imposes a limitation on the number of shares to be registered, the number of a holder’s registrable securities to be included in the registration may be limited on a pro rata basis. In addition, at any time after we become eligible to register securities for resale on Form S-3, subject to certain limitations, the holders will be entitled to cause us to prepare and file a Form S-3 for their registrable securities. In the event that a holder causes us to file a Form S-3, we will be obligated to maintain its effectiveness for a period of 180 days, subject to certain exceptions. However, notwithstanding the provisions described above, we will not be required to effect a registration to the extent that all of a holder’s registrable securities may be sold under Rule 144 during any 90-day period.
Pursuant to the terms of certain of the warrants we issued in our March 2010 private placement, we will be required to prepare and file a registration statement for the resale of the warrants and the shares of common stock issuable upon exercise of the warrants as soon as is practicable following the later of (a) the first anniversary of the closing dates of the private placement and (b) the date when we are required to comply with the periodic reporting requirements under the Exchange Act. We will be required to maintain the effectiveness of this registration statement for a period of up to 180 days. If we intend to file a registration statement on our behalf after the later of the times referenced in (a) and (b) above, the investors in the private placement will have the right, subject to certain limitations, to cause us to include in the registration the warrants and shares of common stock issuable upon exercise of the warrants. These registration rights will terminate as to any investor when all of such investor’s warrants and shares of common stock underlying the warrants may be sold pursuant to Rule 144 under the Securities Act during any 90-day period.
156
Table of Contents
Material United States Federal Income Tax Consequences to Non-U.S. Holders
The following is a summary of the material U.S. federal income tax consequences to non-U.S. holders (as defined below) of the acquisition, ownership and disposition of our common stock issued for cash pursuant to this offering. This summary is not a complete analysis of all of the potential U.S. federal income tax consequences relating to the acquisition, ownership and disposition of our common stock, nor does it address any U.S. federal estate or gift tax consequences or any tax consequences arising under any state, local or foreign tax laws, or under the unearned income Medicare contribution tax pursuant to the Health Care and Education Reconciliation Act of 2010 or any other U.S. federal tax laws. This discussion is based on the Internal Revenue Code of 1986, as amended, or the Code, Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative procedures of the Internal Revenue Service, or IRS, all as in effect as of the date of this offering. These authorities may change, possibly retroactively, resulting in U.S. federal income tax consequences different from those summarized below. No ruling has been or will be sought from the IRS with respect to the matters summarized below, and there can be no assurance that the IRS will not take a contrary position regarding the tax consequences of the acquisition, ownership or disposition of our common stock, or that any such contrary position would not be sustained by a court.
This summary is limited to non-U.S. holders who purchase our common stock issued pursuant to this offering for cash and who hold our common stock as a “capital asset” within the meaning of the Code (generally, property held for investment). This summary does not address all of the U.S. federal income tax consequences that may be relevant to a particular holder in light of the holder’s particular circumstances. Special rules under the U.S. federal income tax laws, which are not addressed in this summary, may apply to certain non-U.S. holders, including foreign governments or entities they control; U.S. expatriates or former long-term residents of the United States; partnerships, other pass-through entities, or beneficial owners of interests in those entities; “controlled foreign corporations” and their shareholders; “passive foreign investment companies” and their shareholders; corporations that accumulate earnings to avoid U.S. federal income tax; banks; financial institutions; insurance companies; brokers, dealers or traders in securities, commodities or currencies; tax-exempt pension funds or other tax-exempt organizations; tax-qualified retirement plans; persons subject to the alternative minimum tax; persons that own, or have owned, actually or constructively, more than 5% of our common stock; and persons holding our common stock as part of a hedging or conversion transaction, straddle, constructive sale, or other risk reduction strategy.
PROSPECTIVE INVESTORS SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES TO THEM OF ACQUIRING, OWNING AND DISPOSING OF OUR COMMON STOCK, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL OR FOREIGN TAX LAWS AND ANY OTHER U.S. FEDERAL TAX LAWS. PROSPECTIVE INVESTORS SHOULD ALSO CONSULT THEIR TAX ADVISORS REGARDING THE POTENTIAL IMPACT OF ANY APPLICABLE INCOME TAX TREATY.
Definition of Non-U.S. Holder
For purposes of this discussion, a “non-U.S. holder” means a beneficial owner of our common stock that is, for U.S. federal income tax purposes:
| • | a nonresident alien individual; |
| • | a foreign corporation (or an entity treated as a foreign corporation for U.S. federal income tax purposes); |
| • | a foreign estate; or |
| • | a foreign trust. |
In the case of a holder that is classified as a partnership for U.S. federal income tax purposes, the tax treatment of a partner in that partnership generally will depend upon the status of the partner and the activities of the partner and the partnership.
157
Table of Contents
Distributions on Our Common Stock
If we make cash or other property distributions (other than certain stock distributions) on our common stock, or effect one of certain redemptions that are treated as distributions on our common stock, those distributions or redemptions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will be allocated ratably among the shareholder’s shares of common stock with respect to which the distribution is paid, will constitute a return of capital, and will first be applied against and reduce a shareholder’s adjusted tax basis in those shares of common stock, but not below zero. Distributions in excess of our current and accumulated earnings and profits and in excess of the shareholder’s tax basis in its shares will constitute capital gain realized on the sale or other disposition of the common stock and will be treated as described under “— Gain on Disposition of Our Common Stock” below. A non-U.S. holder’s adjusted tax basis in a share of common stock is generally the purchase price of the share, reduced by the amount of any distributions constituting a return of capital with respect to that share.
Any dividends paid to a non-U.S. holder of our common stock generally will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends, or such lower rate as is specified by an applicable income tax treaty. To receive the benefit of a reduced treaty rate, a non-U.S. holder must furnish to us or our paying agent a valid IRS Form W-8BEN (or other applicable form) certifying under penalties of perjury the holder’s qualification for the reduced rate. This certification must be provided to us or our paying agent prior to the payment of dividends and must be updated periodically. Non-U.S. holders that do not timely provide us or our paying agent with the required certification, but that qualify for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
If a non-U.S. holder holds our common stock in connection with the conduct of a trade or business in the U.S., and dividends paid on the common stock are effectively connected with the holder’s U.S. trade or business (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base maintained by the non-U.S. holder in the United States, as defined under the applicable treaty), the non-U.S. holder will be exempt from U.S. federal withholding tax on the dividends. To claim the exemption, the non-U.S. holder must furnish to us or our paying agent a properly executed IRS Form W-8ECI (or other applicable form) prior to the payment of the dividends.
Any dividends paid on our common stock that are effectively connected with a non-U.S. holder’s U.S. trade or business (and if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base maintained by the non-U.S. holder in the U.S., as defined under the applicable treaty) generally will be subject to U.S. federal income tax on a net income basis at the regular graduated U.S. federal income tax rates generally applicable to U.S. persons or at such lower rate as may be specified by an applicable income tax treaty. A non-U.S. holder that is a foreign corporation also may be subject to an additional branch profits tax equal to 30% (or such lower rate as is specified by an applicable income tax treaty) of a portion of its effectively connected earnings and profits for the taxable year, as adjusted for certain items.
Gain on Disposition of Our Common Stock
Subject to the discussion below regarding backup withholding and foreign accounts, a non-U.S. holder generally will not be subject to U.S. federal income tax on any gain realized upon the sale, exchange or other taxable disposition of our common stock, unless:
| • | the gain is effectively connected with the non-U.S. holder’s conduct of a trade or business in the United States, and, if required by an applicable income tax treaty, is attributable to a permanent establishment or fixed base, as defined under the applicable treaty, maintained by the non-U.S. holder in the United States; |
| • | the non-U.S. holder is a nonresident alien individual present in the United States for 183 days or more during the taxable year of the sale, exchange or other taxable disposition, and certain other requirements are met; or |
158
Table of Contents
| • | our common stock constitutes a “United States real property interest” by reason of our status as a United States real property holding corporation, or USRPHC, for U.S. federal income tax purposes at any time within the shorter of the five-year period preceding the disposition or the non-U.S. holder’s holding period for our common stock, and, in the case where shares of our common stock are regularly traded on an established securities market, the non-U.S. holder has owned, directly or indirectly, more than 5% of our common stock at any time within the shorter of the five-year period preceding the disposition or the non-U.S. holder’s holding period for our common stock. There can be no assurance that our common stock will be treated as regularly traded on an established securities market for this purpose. |
We believe we are not currently, and we do not anticipate becoming, a USRPHC for U.S. federal income tax purposes. Generally, we would be a USRPHC if the fair market value of our U.S. real property interests were to equal or exceed fifty percent of the sum of the fair market values of our worldwide real property interests and other assets used or held for use in a trade or business, all as determined for U.S. federal income tax purposes.
Gain described in the first or third bullet point above will be subject to U.S. federal income tax on a net income basis at the regular graduated U.S. federal income tax rates generally applicable to U.S. persons or at such lower rate as is specified by an applicable income tax treaty. A non-U.S. holder that is a foreign corporation also may be subject to an additional branch profits tax on gain described in the first bullet point equal to 30% (or such lower rate as is specified by an applicable income tax treaty) of a portion of its effectively connected earnings and profits for the taxable year, as adjusted for certain items.
Gain described in the second bullet point above will be subject to U.S. federal income tax at a flat 30% rate (or such lower rate as is specified by an applicable income tax treaty), but may be offset by U.S. source capital losses (even though the individual is not considered a resident of the United States) provided that the non-U.S. holder has timely filed U.S. federal income tax returns with respect to those losses.
The gross proceeds from transactions that generate gains described in the third bullet point above will generally be subject to a 10% withholding tax, which a non-U.S. holder may claim as a credit against the non-U.S. holder’s federal income tax liability.
Information Reporting and Backup Withholding
We must report annually to the IRS and to each non-U.S. holder the amount of dividends and other distributions paid to the non-U.S. holder and the amount of tax, if any, withheld with respect to those distributions. The IRS may make this information available to the tax authorities in the country in which the non-U.S. holder is resident.
In addition, a non-U.S. holder may be subject to information reporting requirements and backup withholding with respect to dividends paid on, and the proceeds of disposition of, shares of our common stock, unless, generally, the non-U.S. holder certifies under penalties of perjury (usually on IRS Form W-8BEN) that the non-U.S. holder is not a U.S. person or otherwise establishes an exemption. The backup withholding rate is 28%. Additional rules relating to information reporting requirements and backup withholding with respect to payments of the proceeds from the disposition of shares of our common stock are as follows:
| • | If the proceeds are paid to or through the U.S. office of a broker, the proceeds generally will be subject to backup withholding and information reporting, unless the non-U.S. holder certifies under penalties of perjury (usually on IRS Form W-8BEN) that the non-U.S. holder is not a U.S. person or otherwise establishes an exemption. |
| • | If the proceeds are paid to or through a non-U.S. office of a broker that is not a U.S. person and is not a foreign person with certain specified U.S. connections, which we refer to below as a “U.S.-related person,” information reporting and backup withholding generally will not apply. |
159
Table of Contents
| • | If the proceeds are paid to or through a non-U.S. office of a broker that is a U.S. person or a U.S.-related person, the proceeds generally will be subject to information reporting (but not to backup withholding), unless the non-U.S. holder certifies under penalties of perjury (usually on IRS Form W-8BEN) that the non-U.S. holder is not a U.S. person. |
Backup withholding is not a tax. Any amounts withheld from a non-U.S. holder under the backup withholding rules may be allowed as a refund or a credit against the non-U.S. holder’s U.S. federal income tax liability, provided that the non-U.S. holder timely furnishes the required information to the IRS.
Foreign Accounts
Legislation enacted in 2010 will impose withholding taxes on certain types of payments made to “foreign financial institutions” and other non-U.S. entities after December 31, 2013 (or, in certain cases, after later dates) unless those institutions and entities meet additional certification, information reporting and other requirements. The legislation will generally impose a 30% withholding tax on dividends on, or gross proceeds from the sale or other disposition of, our common stock paid to a foreign financial institution unless the foreign financial institution enters into an agreement with the U.S. Treasury to, among other things, (i) undertake to identify accounts held by certain U.S. persons (including certain equity and debt holders of such institution) or by U.S.-owned foreign entities, (ii) annually report certain information about such accounts, and (iii) withhold 30% on payments to account holders whose actions prevent it from complying with these reporting and other requirements. In addition, subject to certain exceptions, the legislation will impose a 30% withholding tax on the same types of payments to a foreign entity that is not a foreign financial institution unless the entity certifies that it does not have any substantial U.S. owners (which generally include any U.S. persons who directly or indirectly own more than 10% of the entity) or furnishes identifying information regarding each such substantial U.S. owner. These withholding taxes would be imposed on dividends paid on our common stock after December 31, 2013 (or, in certain cases, after later dates), and on gross proceeds from sales or other dispositions of our common stock after December 31, 2016. A non-U.S. holder may be exempt from the withholding described in this paragraph under an applicable intergovernmental agreement between the U.S. and a foreign government, provided that the non-U.S. holder and the applicable foreign government comply with the terms of the agreement. We will not pay any additional amounts to non-U.S. holders in respect of any amounts withheld. Prospective investors should consult their tax advisors regarding this legislation.
160
Table of Contents
Subject to the terms and conditions set forth in an underwriting agreement dated the date of this prospectus among us and the underwriters named below, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase from us, the number of shares of common stock listed next to its name in the following table. Leerink Swann LLC is acting as the sole book-running manager for the offering and as the representative of the underwriters.
| Name |
Number of Shares |
|||
| Leerink Swann LLC |
||||
| Stephens Inc. |
||||
| Mizuho Securities USA Inc. |
||||
| Cantor Fitzgerald & Co. |
||||
| Total |
6,000,000 | |||
|
|
|
|||
The underwriters are committed to purchase all the shares of common stock offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated. The underwriters are not obligated to purchase the shares of our common stock covered by the underwriters’ over-allotment option described below.
The underwriters are offering our shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Discounts and Commissions
The underwriters propose initially to offer our shares to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. After the initial offering of our shares, the public offering price and other selling terms may be changed by the representatives.
The following table shows the public offering price, underwriting discounts and commissions and proceeds before expenses to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Share | Without Option | With Option | ||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discounts and commissions |
$ | $ | $ | |||||||||
| Proceeds, before expenses, to us |
$ | $ | $ | |||||||||
The total estimated expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts and commissions, are approximately $ million and are payable by us.
Over-Allotment Option
We have granted the underwriters an option to purchase up to 900,000 additional shares of our common stock at the public offering price, less underwriting discounts and commissions. The underwriters may exercise this option for 30 days from the date of this prospectus solely to cover sales of shares of common stock by the
161
Table of Contents
underwriters in excess of the total number of shares set forth in the table above. If any shares are purchased pursuant to this over-allotment option, the underwriters will purchase the additional shares in approximately the same proportion as shown in the table above. If any of these additional shares are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered. We will pay the expenses associated with the exercise of the over-allotment option.
Initial Public Offering Pricing
Prior to this offering, there has been no public market for our common stock. The initial public offering price will be negotiated between us and the underwriters. Among the factors considered in these negotiations are:
| • | the prospects for our company and the industry in which we operate; |
| • | our past and present financial and operating performance; |
| • | financial and operating information and market valuations of publicly traded companies engaged in activities similar to ours; |
| • | the prevailing conditions of U.S. securities markets at the time of this offering; and |
| • | other factors deemed relevant. |
The estimated initial public offering price range set forth on the cover of this preliminary prospectus is subject to change as a result of market conditions and other factors.
Lock-Up Agreements
We, our officers and directors and holders of substantially all of our outstanding stock, options and warrants have entered into lock-up agreements with the underwriters. Under these agreements, we and these other individuals have agreed, subject to specified exceptions, not to sell or transfer any common stock or securities convertible into, or exchangeable or exercisable for, our common stock, during a period ending 180 days after the date of this prospectus, without first obtaining the written consent of Leerink Swann LLC. Specifically, we and these other individuals have agreed not to:
| • | offer, sell, contract to sell, pledge, grant any option to purchase, make any short sale or otherwise dispose of any shares of our common stock (including any shares issued in this offering or other issuer-directed shares), or any options or warrants to purchase any shares of our common stock, or any other securities convertible into, exchangeable for or that represent the right to receive shares of our common stock; or |
| • | engage in any hedging or other transactions, including, without limitation, any short sale or any purchase, sale or grant of any right (including without limitation any put or call option), or exercise any registration rights, with respect to any shares of our common stock or with respect to any security that includes, relates to, or derives any significant part of its value from shares of our common stock; |
whether any such transaction described above is to be settled by delivery of common stock or other securities, in cash or otherwise. In addition, we have agreed with the underwriters not to file a registration statement under the Securities Act relating to the offering of any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock, or publicly disclose the intention to file such a registration statement.
The restrictions described above, applicable to us, do not apply to:
| • | the sale of shares of common stock to the underwriters pursuant to the underwriting agreement; |
162
Table of Contents
| • | the issuance by us of shares of common stock upon the exercise of an option or warrant or the conversion of a security outstanding on the date of this prospectus of which the underwriters have been advised in writing or that is described in this prospectus; |
| • | the grant by us of stock options or other stock-based awards, or the issuance of shares of our common stock upon exercise thereof, to eligible participants pursuant to employee benefit or equity incentive plans described in this prospectus, provided that, prior to the grant of any such stock options or other stock-based awards that vest within the restricted period, each recipient of such grant shall sign and deliver a lock-up agreement agreeing to be subject to the restrictions on transfer described above; |
| • | the issuance of shares of our common stock (or options, warrants or convertible securities relating to shares of our common stock) in connection with bona fide mergers or acquisitions, joint ventures, commercial relationships or other strategic transactions; and |
| • | the filing by us of a registration statement on Form S-8 or any successor form thereto with respect to the registration of securities to be offered under any employee benefit or equity incentive plans described in this prospectus. |
The restrictions described above, applicable to our officers and directors and holders of substantially all of our outstanding stock, options and warrants, do not apply to transfers:
| • | representing repurchases of securities by us pursuant to pre-existing contractual arrangements that provide for such repurchases upon the separation from service of any such security holder; |
| • | to which Leerink Swann LLC has previously consented in writing; |
| • | representing a bona fide gift; |
| • | upon death by will or intestacy; |
| • | to any such security holder’s immediate family or to any trust for the benefit of such security holder or the immediate family of such security holder; |
| • | to any affiliate, limited partner, general partner, limited liability company member, stockholder or wholly-owned subsidiary of any such security holder; or |
| • | by operation of law, including a merger or a qualified domestic order; |
provided that in the case of each of the preceding six types of transactions, each transferee signs and delivers a lock-up agreement agreeing to be subject to the restrictions on transfer described above and no public report, including but not limited to a report pursuant to Rule 144 of the Securities Act, or pursuant to Section 16 of the Exchange Act, is required or is voluntarily made during the lock-up period (other than, in the case of a disposition for no value, required reports on Form 5).
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
The NASDAQ Global Market Listing
We have applied to list our common stock on the NASDAQ Global Market under the symbol “AGMX.”
Price Stabilization, Short Positions and Penalty Bids
In order to facilitate the offering of our common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock. In connection with the offering, the
163
Table of Contents
underwriters may purchase and sell our common stock in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares of common stock in the offering. The underwriters may close out any covered short position by either exercising their over-allotment option or purchasing shares of common stock in the open market. In determining the source of shares of common stock to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. “Naked” short sales are sales in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares of common stock in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of shares of common stock made by the underwriters in the open market prior to the completion of the offering.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of our common stock, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase common stock in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
The underwriters do not expect sales to discretionary accounts to exceed five percent of the total number of shares offered.
The underwriters make no representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that these transactions, once commenced, will not be discontinued at any time without notice.
Electronic Offer, Sale and Distribution of Shares
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares of common stock to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters’ websites and any information contained in any other website maintained by the underwriters is not part of this prospectus or the registration statement of which this prospectus forms a part.
Notice to Prospective Investors in the European Economic Area
In relation to each member state of the European Economic Area that has implemented the Prospectus Directive, each of which we refer to as a relevant member state, with effect from and including the date on which
164
Table of Contents
the Prospectus Directive is implemented in that relevant member state, or the relevant implementation date, an offer of securities described in this prospectus may not be made to the public in that relevant member state other than:
| • | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| • | to any legal entity that has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; |
| • | to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) subject to obtaining the prior consent of representatives for any such offer; or |
| • | in any other circumstances that do not require the publication of a prospectus pursuant to Article 3 of the Prospectus Directive; |
provided that no such offer of securities shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares of common stock in any relevant member state means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe for the shares, as the same may be varied in that member state by any measure implementing the Prospectus Directive in that member state and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each relevant member state.
Notice to Prospective Investors in Hong Kong
The securities may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the securities may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to securities which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Notice to Prospective Investors in Singapore
This document has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this document and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the securities may not be circulated or distributed, nor may the securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the securities are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust
165
Table of Contents
(where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation or that trust has acquired the securities under Section 275 except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law.
Other Relationships
From time to time, certain of the underwriters and their affiliates have provided, and may provide in the future, various advisory, investment and commercial banking and other services to us in the ordinary course of business, for which they have received and may continue to receive customary fees and commissions.
166
Table of Contents
The validity of the shares of common stock offered by us under this prospectus will be passed upon for us by Bingham McCutchen LLP, Costa Mesa, California. As of the date of this prospectus, an investment partnership that includes certain Bingham McCutchen LLP attorneys holds 87,754 shares of our preferred stock that will be converted into an aggregate of 40,903 shares of our common stock upon the completion of this offering. This investment partnership also holds warrants for the purchase of 4,686 shares of our common stock. The underwriters are represented by Cooley LLP, San Diego, California.
Marcum LLP, independent registered public accounting firm, has audited our financial statements at and for the years ending December 31, 2010 and 2011, as set forth in their report (which contains an explanatory paragraph describing conditions that raise substantial doubt about our ability to continue as a going concern as described in Note 1 to our financial statements included in this prospectus). We have included our financial statements in the prospectus and elsewhere in the registration statement in reliance on Marcum LLP’s report, given their authority as experts in accounting and auditing.
Where You Can Find More Information
We have filed with the SEC a registration statement on Form S-1 (including the exhibits, schedules and amendments to the registration statement) under the Securities Act with respect to the shares of common stock offered by this prospectus. This prospectus does not contain all of the information set forth in the registration statement. For further information about us and the new shares of common stock to be sold in this offering, we refer you to the registration statement. Statements contained in this prospectus as to the contents of any contract, agreement or other document to which we make reference are not necessarily complete. In each instance, we refer you to the copy of such contract, agreement or other document filed as an exhibit to the registration statement, each such statement being qualified in all respects by the more complete description of the matter involved.
Upon completion of this offering, we will become subject to the reporting and information requirements of the Exchange Act, and, as a result, will file periodic and current reports, proxy statements, and other information with the SEC. You may read and copy this information at the Public Reference Room of the SEC located at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the Public Reference Room. Copies of all or any part of the registration statement may be obtained from the SEC’s offices upon payment of fees prescribed by the SEC. The SEC maintains an Internet site that contains periodic and current reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of the SEC’s website is http://www.sec.gov.
167
Table of Contents
| Page | ||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| Statements of Convertible Preferred Stock and Stockholders’ Deficit |
F-5 | |||
| F-7 | ||||
| F-8 | ||||
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee of the
Board of Directors and Stockholders
of AutoGenomics, Inc.
We have audited the accompanying balance sheets of AutoGenomics, Inc. (the “Company”) as of December 31, 2010 and 2011, and the related statements of operations and comprehensive loss, changes in convertible preferred stock and stockholders’ deficit and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of AutoGenomics, Inc., as of December 31, 2010 and 2011, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company does not have sufficient working capital to fund its planned operations through December 31, 2012 without additional financing, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The unaudited pro forma balance sheet information contained in the financial statements, which is the responsibility of management, is presented for purposes of additional analysis and is not a required part of the financial statements. Such information has not been subjected to the auditing procedures applied in the audit of the financial statements, and, accordingly, we do not express an opinion or provide any assurance on it.
/s/ Marcum LLP
Marcum LLP
IRVINE, CALIFORNIA
September 26, 2012, except for the last paragraph of Note 1, as to which the date is January 22, 2013
F-2
Table of Contents
Balance Sheets
| December 31, | September
30, 2012 |
Pro-forma September 30, 2012 |
||||||||||||||
| 2010 | 2011 | |||||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands, except per share and par value amounts) |
||||||||||||||||
| Assets |
||||||||||||||||
| Current assets: |
||||||||||||||||
| Cash and cash equivalents |
$ | 129 | $ | 61 | $ | 294 | $ | 294 | ||||||||
| Accounts receivable (net of allowance of $38, $52, and $93 at December 31, 2010 and 2011 and September 30, 2012, respectively) |
1,262 | 1,865 | 3,777 | 3,777 | ||||||||||||
| Inventory, net |
2,124 | 1,743 | 2,714 | 2,714 | ||||||||||||
| Prepaid and other current assets |
44 | 80 | 319 | 319 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total current assets |
3,559 | 3,749 | 7,104 | 7,104 | ||||||||||||
| Property, equipment and improvements, net |
1,684 | 1,417 | 1,145 | 1,145 | ||||||||||||
| Intangible assets, net |
46 | 14 | — | — | ||||||||||||
| Other long-term assets |
635 | 232 | 1,577 | 1,577 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets |
$ | 5,924 | $ | 5,412 | $ | 9,826 | $ | 9,826 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities and stockholders’ deficit |
||||||||||||||||
| Current liabilities: |
||||||||||||||||
| Accounts payable |
$ | 7,162 | $ | 9,183 | $ | 9,263 | $ | 9,263 | ||||||||
| Accrued expenses |
1,389 | 1,215 | 1,840 | 1,840 | ||||||||||||
| Related party notes, current portion, net |
366 | 2,401 | 4,017 | 1,055 | ||||||||||||
| Senior promissory notes, net |
— | 4,407 | 2,276 | 2,276 | ||||||||||||
| Subordinated promissory notes, current portion, net |
5,744 | 16,707 | 18,432 | 13,886 | ||||||||||||
| Preferred stock warrant liabilities |
354 | 412 | 629 | 629 | ||||||||||||
| Common stock warrant liabilities |
718 | 215 | 168 | 168 | ||||||||||||
| Other current liabilities |
397 | 527 | 317 | 317 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total current liabilities |
16,130 | 35,067 | 36,942 | 29,434 | ||||||||||||
| Deferred rent and other long-term liabilities |
1,976 | 2,252 | 2,532 | 2,532 | ||||||||||||
| Related party notes, long-term portion, net |
1,653 | — | — | — | ||||||||||||
| Subordinated promissory notes, long term portion, net |
11,016 | — | — | — | ||||||||||||
| Commitments and contingencies: |
||||||||||||||||
| Convertible preferred stock, $0.01 par value; 30,000,000 shares authorized: |
||||||||||||||||
| Series C Convertible Preferred Stock, 6,850,000 shares authorized; 6,414,689 shares issued and outstanding at December 31, 2010 and 6,417,680 shares issued and outstanding at December 31, 2011 and September 30, 2012; liquidation preference in the aggregate of $17,640 at December 31, 2010 and $17,647 at December 31, 2011 and September 30, 2012; 0 shares issued and outstanding, pro forma (unaudited) |
17,640 | 17,647 | 17,647 | — | ||||||||||||
| Series A Convertible Preferred Stock, 1,589,600 shares authorized; 1,579,227 shares issued and outstanding at December 31, 2010 and 2011 and September 30, 2012; liquidation preference in the aggregate of $4,990 at December 31, 2010 and 2011 and September 30, 2012; 0 shares issued and outstanding, pro forma (unaudited) |
4,990 | 4,990 | 4,990 | — | ||||||||||||
| Series B Convertible Preferred Stock, 4,515,858 shares authorized; 4,468,369 shares issued and outstanding at December 31, 2010 and 2011 and September 30, 2012; liquidation preference in the aggregate of $12,288 at December 31, 2010 and 2011 and September 30, 2012; 0 shares issued and outstanding, pro forma (unaudited) |
12,288 | 12,288 | 12,288 | — | ||||||||||||
| Series D Convertible Preferred Stock, 3,423,258 shares authorized; 3,423,258 shares issued and outstanding at December 31, 2010 and 2011 and September 30, 2012; liquidation preference in the aggregate of $11,126 at December 31, 2010 and 2011 and September 30, 2012; 0 shares issued and outstanding, pro forma (unaudited) |
11,126 | 11,126 | 11,126 | — | ||||||||||||
| Series E Convertible Preferred Stock, 5,500,000 shares authorized; 0, 685,555 and 732,555 shares issued and outstanding at December 31, 2010, December 31, 2011 and September 30, 2012, respectively; liquidation preference in the aggregate of $0 and $2,828 at December 31, 2010 and 2011, respectively, and $3,022 at September 30, 2012; 0 shares issued and outstanding, pro forma (unaudited) |
— | 1,292 | 1,381 | — | ||||||||||||
| Stockholders’ deficit: |
||||||||||||||||
| Common stock, $0.01 par value, 220,000,000 shares authorized; 2,631,695, 2,635,738 and 2,673,358 shares issued and outstanding at December 31, 2010 and 2011 and September 30, 2012, respectively; 10,564,378 outstanding, pro forma (unaudited) |
80 | 80 | 81 | 326 | ||||||||||||
| Additional paid-in-capital |
12,646 | 14,288 | 15,251 | 69,946 | ||||||||||||
| Accumulated deficit |
(83,621 | ) | (93,618 | ) | (92,412 | ) | (92,412 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total stockholders’ deficit |
(70,895 | ) | (79,250 | ) | (77,080 | ) | (22,140 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities and stockholders’ deficit |
$ | 5,924 | $ | 5,412 | $ | 9,826 | $ | 9,826 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
See accompanying notes to financial statements.
F-3
Table of Contents
Statements of Operations and Comprehensive Income/(Loss)
| Years ended December 31, | Nine months ended September 30, | |||||||||||||||
| 2010 | 2011 | 2011 | 2012 | |||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands, except per share amounts) | ||||||||||||||||
| Net revenue |
$ | 7,504 | $ | 8,005 | $ | 5,166 | $ | 14,473 | ||||||||
| Cost of sales |
7,666 | 6,019 | 4,378 | 5,035 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit/(loss) |
(162 | ) | 1,986 | 788 | 9,438 | |||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
3,560 | 2,768 | 2,146 | 1,724 | ||||||||||||
| General and administrative |
5,376 | 3,360 | 2,587 | 2,775 | ||||||||||||
| Sales and marketing |
4,717 | 2,672 | 2,023 | 1,655 | ||||||||||||
| Impairment of film coating equipment |
981 | — | — | — | ||||||||||||
| Initial public offering costs — terminated offering |
1,752 | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
16,386 | 8,800 | 6,756 | 6,154 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income/(loss) from operations |
(16,548 | ) | (6,814 | ) | (5,968 | ) | 3,284 | |||||||||
| Interest expense, net |
(4,109 | ) | (3,626 | ) | (2,598 | ) | (1,913 | ) | ||||||||
| Other income/(expense), net |
36 | (2 | ) | — | 5 | |||||||||||
| Change in fair value of warrant liabilities |
926 | 445 | 434 | (170 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income/(loss) |
$ | (19,695 | ) | $ | (9,997 | ) | $ | (8,132 | ) | $ | 1,206 | |||||
| Net income/(loss) per share attributable to common stockholders |
||||||||||||||||
| Basic |
$ | (7.63 | ) | $ | (3.79 | ) | $ | (3.09 | ) | $ | 0.45 | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
$ | 0.13 | ||||||||||||||
|
|
|
|||||||||||||||
| Shares used to compute net income/(loss) per share attributable to common stockholders |
||||||||||||||||
| Basic |
2,581,708 | 2,634,385 | 2,633,929 | 2,651,833 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted |
9,148,420 | |||||||||||||||
|
|
|
|||||||||||||||
See accompanying notes to financial statements.
F-4
Table of Contents
Statements of Convertible Preferred Stock and Stockholders’ Deficit
(in thousands, except for share and per share data)
| Series A Convertible Preferred Stock |
Series B Convertible Preferred Stock |
Series C Convertible Preferred Stock |
Series D Convertible Preferred Stock |
Series
E Convertible Preferred Stock |
Common Stock | Additional paid-in capital |
Accumulated Deficit |
Total Stockholders’ Deficit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Issued Amount |
Shares | Issued Amount |
Shares | Issued Amount |
Shares | Issued Amount |
Shares | Issued Amount |
Shares | Issued Amount |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at January 1, 2010 |
1,579,227 | $ | 4,990 | 4,302,040 | $ | 11,831 | 6,411,089 | $ | 17,630 | 3,423,258 | $ | 11,126 | — | $ | — | 2,557,148 | $ | 78 | $ | 6,459 | $ | (63,926 | ) | $ | (57,389 | ) | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock for exercise of stock options |
— | — | — | — | — | — | — | — | — | — | 74,547 | 2 | 172 | — | 174 | |||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation related to issuance of stock options to employees |
— | — | — | — | — | — | — | — | — | — | — | — | 2,763 | — | 2,763 | |||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation related to issuance of stock options to consultants |
— | — | — | — | — | — | — | — | — | — | — | — | 5 | — | 5 | |||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of stock in connection with the exercise of warrants |
— | — | 166,329 | 457 | 3,600 | 10 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock warrants in connection with subordinated notes |
— | — | — | — | — | — | — | — | — | — | — | — | 3,082 | — | 3,082 | |||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock warrants in lieu of services |
— | — | — | — | — | — | — | — | — | — | — | — | 165 | — | 165 | |||||||||||||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | (19,695 | ) | (19,695 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2010 |
1,579,227 | $ | 4,990 | 4,468,369 | $ | 12,288 | 6,414,689 | $ | 17,640 | 3,423,258 | $ | 11,126 | — | $ | — | 2,631,695 | $ | 80 | $ | 12,646 | $ | (83,621 | ) | $ | (70,895 | ) | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||||||
| Issuance of common stock for exercise of stock options |
— | — | — | — | — | — | — | — | — | — | 4,043 | — | 6 | — | 6 | |||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation related to issuance of stock options to employees |
— | — | — | — | — | — | — | — | — | — | — | — | 1,577 | — | 1,577 | |||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation related to issuance of stock options to consultants |
— | — | — | — | — | — | — | — | — | — | — | — | 113 | — | 113 | |||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of Series E convertible preferred stock at $2.75 per share in conjunction with conversion of debt, net of issuance costs of $12,186 and fair value of common stock warrants of $593,526 |
— | — | — | — | — | — | — | — | 685,555 | 1,292 | — | — | 594 | — | 594 | |||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of stock in connection with the exercise of warrants |
— | — | — | — | 2,991 | 7 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||
| Modification of common stock warrants in connection with subordinated notes |
(648 | ) | (648 | ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | (9,997 | ) | (9,997 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2011 |
1,579,227 | $ | 4,990 | 4,468,369 | $ | 12,288 | 6,417,680 | $ | 17,647 | 3,423,258 | $ | 11,126 | 685,555 | $ | 1,292 | 2,635,738 | $ | 80 | $ | 14,288 | $ | (93,618 | ) | $ | (79,250 | ) | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||||||
See accompanying notes to financial statements.
F-5
Table of Contents
Statements of Convertible Preferred Stock and Stockholders’ Deficit
(in thousands, except for share and per share data)
(continued)
| Series A Convertible Preferred Stock |
Series B Convertible Preferred Stock |
Series C Convertible Preferred Stock |
Series D Convertible Preferred Stock |
Series
E Convertible Preferred Stock |
Common Stock | Additional paid-in capital |
Accumulated Deficit |
Total Stockholders’ Deficit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Issued Amount |
Shares | Issued Amount |
Shares | Issued Amount |
Shares | Issued Amount |
Shares | Issued Amount |
Shares | Issued Amount |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for exercise of stock options |
— | — | — | — | — | — | — | — | — | — | 37,620 | 1 | 38 | — | 39 | |||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation related to issuance of stock options to employees |
— | — | — | — | — | — | — | — | — | — | — | — | 551 | — | 551 | |||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of Series E convertible preferred stock at $2.75 in conjunction with surrender of accounts payable |
— | — | — | — | — | — | — | — | 47,000 | 89 | — | — | 40 | — | 40 | |||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock warrants in connection with subordinated notes |
— | — | — | — | — | — | — | — | — | — | 231 | 231 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock warrants as compensation to non-employee directors |
— | — | — | — | — | — | — | — | — | — | — | — | 103 | — | 103 | |||||||||||||||||||||||||||||||||||||||||||||||
| Net income |
— | — | — | — | — | — | — | — | — | — | — | — | — | 1,206 | 1,206 | |||||||||||||||||||||||||||||||||||||||||||||||
| Balance at September 30, 2012 (unaudited) |
1,579,227 | $ | 4,990 | 4,468,369 | $ | 12,288 | 6,417,680 | $ | 17,647 | 3,423,258 | $ | 11,126 | 732,555 | $ | 1,381 | 2,673,358 | $ | 81 | $ | 15,251 | $ | (92,412 | ) | $ | (77,080 | ) | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||
See accompanying notes to financial statements.
F-6
Table of Contents
Statements of Cash Flows
| Years ended December 31, | Nine months ended September 30, | |||||||||||||||
| 2010 | 2011 | 2011 | 2012 | |||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands) | ||||||||||||||||
| Operating activities |
||||||||||||||||
| Net income/(loss) |
$ | (19,695 | ) | $ | (9,997 | ) | $ | (8,132 | ) | $ | 1,206 | |||||
| Adjustments to reconcile to changes in cash and cash equivalents: |
||||||||||||||||
| Depreciation and amortization |
823 | 675 | 580 | 475 | ||||||||||||
| Amortization of debt discount/premium |
2,322 | 1,592 | 1,091 | 591 | ||||||||||||
| Amortization of deferred financing costs |
365 | 403 | 313 | 60 | ||||||||||||
| Impairment of film coating equipment |
981 | — | — | — | ||||||||||||
| Provision for doubtful accounts |
(345 | ) | 14 | (2 | ) | 41 | ||||||||||
| Reserve for excess and obsolete inventory |
314 | (16 | ) | (109 | ) | 20 | ||||||||||
| Employee and nonemployee stock-based compensation |
2,933 | 1,690 | 1,364 | 654 | ||||||||||||
| Change in fair value of stock warrant liabilities |
(926 | ) | (445 | ) | (434 | ) | 170 | |||||||||
| Non-cash interest expense |
1,447 | 1,611 | 1,186 | 1,258 | ||||||||||||
| Deferred rent |
715 | 311 | 209 | 304 | ||||||||||||
| Changes in operating assets/liabilities |
||||||||||||||||
| Accounts receivable |
(405 | ) | (617 | ) | 415 | (1,953 | ) | |||||||||
| Inventory |
1,379 | 25 | (200 | ) | (1,044 | ) | ||||||||||
| Prepaid and other current assets |
(657 | ) | (36 | ) | (54 | ) | (1,643 | ) | ||||||||
| Accounts payable |
5,720 | 2,021 | 1,157 | 79 | ||||||||||||
| Other current liabilities and accrued expenses |
(1,857 | ) | (77 | ) | (1 | ) | 390 | |||||||||
| Other long term liabilities |
150 | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash provided by/(used in) operating activities |
(6,736 | ) | (2,846 | ) | (2,617 | ) | 608 | |||||||||
| Investing activities |
||||||||||||||||
| Purchase of property and equipment |
(260 | ) | (5 | ) | (3 | ) | (136 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash used in investing activities |
(260 | ) | (5 | ) | (3 | ) | (136 | ) | ||||||||
| Financing activities |
||||||||||||||||
| Proceeds from the issuance of common stock |
174 | 6 | 6 | 39 | ||||||||||||
| Proceeds from the issuance of Series B preferred stock |
457 | — | — | — | ||||||||||||
| Proceeds from the issuance of Series C preferred stock |
10 | 7 | 10 | — | ||||||||||||
| Proceeds from the issuance of Series E preferred stock |
— | 1,595 | 1,584 | 129 | ||||||||||||
| Proceeds from issuance of promissory notes |
6,075 | 1,175 | 1,000 | 1,670 | ||||||||||||
| Payment of promissory notes and accrued interest |
(213 | ) | — | — | (2,077 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash provided by/(used in) financing activities |
6,503 | 2,783 | 2,600 | (239 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Increase/(decrease) in cash and cash equivalents |
(493 | ) | (68 | ) | (20 | ) | 233 | |||||||||
| Cash and cash equivalents at the beginning of the period |
622 | 129 | 129 | 61 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash and cash equivalents at the end of the period |
$ | 129 | $ | 61 | $ | 109 | $ | 294 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Supplemental disclosure of cash flow information: |
||||||||||||||||
| Interest paid |
$ | 20 | $ | 11 | $ | 6 | $ | 280 | ||||||||
| Income taxes paid |
$ | 9 | $ | 29 | $ | — | $ | — | ||||||||
| Non-cash investing and financing activities |
||||||||||||||||
| Property and equipment acquired and included in accounts payable |
$ | 60 | $ | — | $ | — | $ | — | ||||||||
| Change in classification of domestic placement plan analyzers to/from inventory and property and equipment |
$ | 48 | $ | 372 | $ | 401 | $ | 55 | ||||||||
| Modifications to warrants and notes payable |
$ | 1,369 | $ | 905 | $ | — | $ | 85 | ||||||||
| Issuance of common stock warrants in connection with notes payable |
$ | 1,713 | $ | 257 | $ | 198 | $ | 316 | ||||||||
| Surrender of notes payable and related interest in exchange for Series E preferred stock |
$ | — | $ | 290 | $ | — | $ | — | ||||||||
| Surrender of accounts payable in exchange for Series E preferred stock |
$ | — | $ | — | $ | — | $ | 129 | ||||||||
| Issuance of common stock warrants in connection with issuance of Series E preferred stock |
$ | — | $ | 594 | $ | — | $ | 40 | ||||||||
See accompanying notes to financial statements.
F-7
Table of Contents
Notes to Financial Statements
1. Organization, Liquidity and Management’s Plan, and Summary of Significant Accounting Policies
(Information as of September 30, 2012 and thereafter and for the nine months ended September 30, 2011 and 2012 is unaudited.)
Organization and business
AutoGenomics, Inc., or the Company, incorporated in California and commenced its operations on April 7, 1999. In October 2008, the Company reincorporated in Delaware. The Company designs, develops, manufactures and markets a fully integrated molecular diagnostics system called the INFINITI system that includes its highly automated bench-top INFINITI Analyzer, which has the versatility to run a large menu of genetic tests. The Company markets its products in the United States and internationally.
Liquidity and management’s plan
As of September 30, 2012, the Company did not have sufficient working capital to fund its planned operations through December 31, 2012 without additional financing. The Company is currently in default on approximately $14.8 million in principal amount of its outstanding promissory notes. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. This basis of accounting contemplates the recovery of the Company’s assets and the satisfaction of its liabilities in the normal course of business. A successful transition to attaining profitable operations is dependent upon achieving a level of positive cash flows adequate to support the Company’s cost structure. Until that time, the Company may need to raise additional financing. While the Company actively seeks additional financing to fund its development efforts, to commercialize its technologies and to fund its other business activities, there is no assurance such financing will be available to the Company when needed or that such financing would be available under favorable terms. If the Company is unable to obtain sufficient funding or otherwise cure its defaults on its outstanding promissory notes, it may be required to significantly curtail its planned operations, which may have a material, adverse impact on its ability to continue as a going concern. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be forced to take any such actions.
Unaudited interim financial statements
The financial statements as of September 30, 2012 and for the nine months ended September 30, 2011 and 2012 are unaudited. The unaudited financial statements have been prepared on the same basis as the audited financial statements and, in the opinion of management, include all adjustments, consisting of normal recurring adjustments, considered necessary to state fairly the financial information set forth therein, in accordance with generally accepted accounting principles, or GAAP.
The results of operations for the audited years ended December 31, 2010 and 2011 and the unaudited interim period ended September 30, 2012 are not necessarily indicative of the results which may be reported for any other interim period or for the year ending December 31, 2012.
Unaudited Pro Forma Balance Sheet Information
The unaudited pro forma stockholders’ deficit information in the accompanying balance sheet assumes the conversion of all outstanding shares of convertible preferred stock, including the Series NC convertible preferred stock issued in November 2012, into 7,891,020 shares of common stock in connection with the completion of the offering referred to in this prospectus, which in turn assumes a completed offering that results in proceeds to the Company of greater than $25 million. There can be no assurance that this offering will be completed.
F-8
Table of Contents
Use of estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and disclosures made to the accompanying notes to the financial statements. The Company’s critical accounting policies that involve significant judgment and estimates include revenue recognition, accounts receivable regarding the same, inventory reserve, warranty reserve, stock-based compensation, valuation of debt and equity instruments, valuation of deferred taxes, and useful lives of property and equipment, including the potential impairment of capital assets. Actual results could differ from those estimates and assumptions.
Cash and cash equivalents
The Company considers all highly liquid investments with an original maturity of three months or less when purchased to be cash equivalents.
Concentration of credit risk
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist primarily of cash and cash equivalents and accounts receivable. The Company limits its exposure to credit loss by placing its cash with high credit-quality financial institutions. At times, such deposits may be in excess of insured limits. The Company has not experienced any losses on its deposits of cash and cash equivalents.
For the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2011 and 2012, two customers, three customers, three customers and two customers accounted for 27%, 39%, 35% and 64% of product sales, respectively.
As of December 31, 2010 and 2011 and September 30, 2012, one customer, three customers and two customers accounted for 22%, 67% and 70% of accounts receivable, respectively.
Sales outside the United States accounted for 52%, 22%, 32% and 6% of product sales for the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2011 and 2012, respectively.
Fair value of financial instruments
The carrying amounts shown for the Company’s cash and cash equivalents, accounts receivable, prepaid expenses, accounts payable and accrued expenses approximate their fair value due to the short-term nature of these items. The carrying amount of common stock and convertible preferred stock warrant liabilities represents their respective fair values (see Note 5).
The carrying value of the notes payable approximates fair value principally because of their short-term nature. Fair value is defined as the exchange price that would be received for an asset or an exit price paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The current accounting guidance for fair value measurements defines a three-level valuation hierarchy for disclosures as follows:
Level I — Unadjusted quoted prices in active markets for identical assets or liabilities;
Level II — Inputs other than quoted prices included within Level I that are observable, unadjusted quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data; and
F-9
Table of Contents
Level III — Unobservable inputs that are supported by little or no market activity, which requires the Company to develop its own assumptions.
The categorization of a financial instrument within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The Company’s financial instruments consist of Level I assets and Level III liabilities. Level I assets include money market funds. Level III liabilities consist of the convertible preferred and common stock warrant liabilities. The fair values of the outstanding convertible preferred and common stock warrants are measured using a Black-Scholes valuation model. The Company determined that using alternative valuation models, such as a Monte Carlo simulation model, would result in de minimus differences.
A company may elect to use fair value to measure accounts and loans receivable, available-for-sale and held-to-maturity securities, equity method investments, accounts payable, guarantees and issued debt. Other eligible items include firm commitments for financial instruments that otherwise would not be recognized at inception and non-cash warranty obligations where a warrantor is permitted to pay a third party to provide the warranty goods or services. If the use of fair value is elected, any upfront costs and fees related to the item such as debt issuance costs must be recognized in earnings and cannot be deferred. The fair value election is irrevocable and generally made on an instrument-by-instrument basis, even if a company has similar instruments that it elects not to measure based on fair value. Unrealized gains and losses on existing items for which fair value has been elected are reported as a cumulative adjustment to beginning retained earnings and any changes in fair value are recognized in earnings. The Company has elected not to apply the fair value option to its financial assets and liabilities.
Segment
The Company’s business is considered as operating in one segment based upon the Company’s organizational structure, the way in which the operations are managed and evaluated and the manner in which the Company records its financial information. All of the Company’s assets are located, and all of its sales originate, in the United States.
Accounts receivable and allowance for doubtful accounts
The Company performs ongoing credit evaluations of its customers. Accounts receivable are recorded at invoiced amounts, net of the Company’s estimated allowances for doubtful accounts. The allowance for doubtful accounts is based on an assessment of the Company’s ability to collect on customer accounts receivable. The Company regularly reviews the allowance by considering certain factors such as historical experience, industry data, credit quality, age of accounts receivable balances and current economic conditions that may affect a customer’s ability to pay. In cases where the Company is aware of circumstances that may impair a customer’s ability to meet their financial obligations, the Company records a specific allowance against amounts due from the customer and thereby reduces the net recognized receivable to the amount the Company reasonably believes will be collected. The Company writes-off accounts receivable against the allowance when it determines the balance is uncollectible and no longer actively pursues collection of the receivable.
The following table summarizes the activity in the allowance for doubtful accounts during the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2012:
| Balance at beginning of period |
Charged to costs and expenses |
Allowance reductions/ write-offs |
Balance at end of period |
|||||||||||||
| (in thousands) | ||||||||||||||||
| For the year ended December 31, 2010 |
$ | 382 | $ | 38 | $ | (382 | ) | $ | 38 | |||||||
| For the year ended December 31, 2011 |
$ | 38 | $ | 38 | $ | (24 | ) | $ | 52 | |||||||
| For the nine months ended September 30, 2012 (unaudited) |
$ | 52 | $ | 41 | $ | — | $ | 93 | ||||||||
F-10
Table of Contents
Inventory, net
The Company reports its inventories net of its reserve for slow-moving and obsolete parts. Inventories are valued at the lower of cost or market value using the first-in first-out method and include direct labor, materials, and manufacturing overhead. The Company periodically reviews inventory for evidence of slow-moving or obsolete parts, and the estimated reserve is based on management’s reviews of inventories on hand, compared to estimates of future usage and sales, and assumptions about the likelihood of obsolescence.
Deferred offering costs
Deferred offering costs, which consist of direct incremental legal and accounting fees relating to planned financing activities, are capitalized. The deferred offering costs will be offset against the Company’s initial public offering proceeds upon the consummation of the offering. In the event the offering is terminated, deferred offering costs will be expensed. The Company terminated a planned initial public offering in 2010, and accordingly wrote-off $1.8 million of offering costs and recorded them as expenses. In connection with the current offering, as of the nine months ended September 30, 2012, the Company has deferred $0.9 million in offering costs.
Property, equipment and improvements
Property, equipment and improvements includes manufacturing, laboratory, office and computer hardware and software, and is stated at cost and depreciated over the estimated useful lives of the assets (three to seven years) using the straight-line method. Leasehold improvements are stated at cost and amortized over the lesser of the expected remaining lease term or the remaining useful life of the asset.
Equipment placed with customers under the Company’s “domestic placement plan” is recorded at the Company’s cost to manufacture and the related depreciation expense is charged to cost of sales over the equipment’s expected life of three years while in service.
Long-lived assets
The Company periodically re-evaluates the original assumptions and rationale utilized in the establishment of the carrying value and estimated lives of all of its long-lived assets including property and equipment. The determinants used for this evaluation include management’s estimate of each such asset’s ability to generate positive income from operations and positive cash flow in future periods as well as the strategic significance of such assets to the Company’s business objectives. Included in operating costs in 2010 is an impairment loss of $1.0 million related to film coating equipment acquired in 2008. The fair value of assets is estimated using discounted cash flows based on unobservable inputs.
Intangible assets
Intangible assets are comprised of acquired licenses or sublicenses to technology covered by patents owned by third parties, and are amortized over the expected useful lives of these assets, generally five years. No conditions of impairment of these intangible assets were noted as of December 31, 2010 and 2011 and September 30, 2012.
Warranty reserves
The Company provides a standard twelve month warranty on all INFINITI analyzers sold domestically. The cost of warranties is recognized in cost of sales at the time revenue is earned based on the estimated cost to repair over its warranty period. Cost of sales reflects not only estimated warranty expense for equipment sold in the current period, but also any changes in estimated warranty expense for the portion of the aggregate installed base that is under warranty. Estimated warranty expense is based on a variety of inputs, including historical performance in the customers’ environment, historical costs incurred in servicing equipment and projected
F-11
Table of Contents
equipment reliability and service costs. Should actual service rates or costs differ from our estimates, which are based on historical data and projections of future costs, revisions to the estimated warranty liability would be required. The Company reviews these inputs, at a minimum, on an annual basis. The liability for warranties is included in other liabilities in the accompanying balance sheets.
Promissory notes
The Company has issued promissory notes pursuant to a number of private placements since 1999. The promissory note agreements permit the Company to issue up to $4.0 million of senior debt. At times these promissory notes were issued with separate warrants to purchase the Company’s common stock. These warrants are treated as equity instruments. The fair value of such warrants was determined using the Black-Scholes valuation model and was allocated as a debt discount using the relative fair value allocation method. The discount has been amortized using the effective interest method over the term of the related notes payable.
Common stock and convertible preferred stock warrant liabilities
The Company has issued a freestanding warrant to purchase shares of its common stock that includes a provision that provides for the adjustment of the exercise price upon the occurrence of certain events. The Company has classified the fair value of this warrant as a liability on the balance sheet. The Company will continue to adjust the liability for changes in fair value until the earlier of the expiration of this warrant or its exercise, at which time the liability will be reclassified into stockholders’ deficit. The Company records any change in fair value as a component of other income or expense.
The Company has issued freestanding warrants to purchase shares of its convertible preferred stock. The Company has classified the fair value of these warrants as liabilities on the balance sheet as they correspond to the treatment of the preferred stock as temporary equity. The Company will continue to adjust the liability for changes in fair value until the earlier of the expiration of the warrants or their exercise, at which time the liability will be reclassified into stockholders’ deficit. The Company records any change in fair value as a component of other income or expense.
Revenue recognition
The Company’s revenue is primarily derived from sales of its INFINITI analyzers and the BioFilmChip, Intellipac Reagent Management Module, and various disposable items consumed by the diagnostic testing process, collectively referred to as consumables. The INFINITI analyzers and all consumables are offered through direct sale in domestic markets and through distributors in international markets. The INFINITI analyzers also are available to domestic customers through the Company’s domestic placement plan. In such arrangements, customers receive an analyzer at no direct cost in return for a commitment to purchase minimum quantities of consumables based on an agreed to pricing schedule over the contract period, which is usually two years, and which may be extended at the option of the customer or the Company. The Company reserves the option to cancel the arrangement and repossess the analyzer if minimum quantities of consumables are not purchased. These analyzers are recorded in the Company’s financial records as capitalized assets and are depreciated over an estimated useful life of, typically, three years. When the analyzer is returned to the Company it is placed back into inventory at its then net book value. Analyzers that are returned are subject to a review and refurbishment process designed to enable the return of the analyzer to the field as soon as practicable. Any costs associated with the refurbishment of an analyzer are capitalized and added to the Company’s cost basis in the analyzer. The new basis, including refurbishment costs, either is amortized to cost of sales over the useful life of the analyzer once the refurbished unit is placed with a new customer under the domestic placement plan, or is charged to cost of sales upon the sale of the refurbished analyzer. Throughout the term of the placement agreement, the Company retains title to the analyzer.
F-12
Table of Contents
Revenue is recognized when persuasive evidence of an arrangement exists, the price to the buyer is fixed or determinable, collectability is reasonably assured and risk of loss transfers, which is normally upon shipment. For sales that include customer specific acceptance criteria, revenue is recognized when the acceptance criteria have been met. Credit is extended based upon the evaluation of the customer’s financial condition. Collectability is assessed based on a number of factors, including payment history and the creditworthiness of a customer. If it is determined that collection is not reasonably assured, revenue is not recognized until collection becomes reasonably assured, which is generally upon receipt of cash. The Company does not request collateral from its customers. The Company recognizes revenue when the products have been shipped and the title and risk of loss have been transferred.
In instances where final acceptance of the product or system is required, revenue is deferred until all the acceptance criteria have been met. The rights granted in the domestic placement plan in exchange for a minimum annual purchase commitment constitutes a leasing arrangement. When a customer enters into a domestic placement plan agreement, the lease revenue is dependent on purchases of consumables, which is not measurable at the inception of the lease, and thus is accounted for as contingent rentals in their entirety. Accordingly, lease revenues are excluded from minimum lease payments but included in revenue as the consumables are purchased. The cost of the leased equipment is depreciated over its useful life and the expense included in cost of sales.
Arrangements to sell products to customers frequently include multiple deliverables, including the sale of the INFINITI analyzer combined with the sale of consumables. Sales to foreign distributors include training of their field agents whom will be responsible for installation, training, and maintenance for their customers internationally. The Company does not provide any price protection, rights of return, or extended payment terms to the distributors. Prior to January 1, 2011 revenue was recognized upon completion of training as the Company could not establish vendor-specific objective evidence (“VSOE”) of the training. Beginning on January 1, 2011, the Company adopted new authoritative guidance on multiple-element arrangements, using the prospective method for all arrangements entered into or materially modified from the date of adoption. Under this new guidance, the Company allocates arrangement consideration in multiple-element revenue arrangements at the inception of an arrangement to all deliverables or those packages in which all components of the package are delivered at the same time, based on the relative selling price method in accordance with the selling price hierarchy, which includes: (i) VSOE if available; (ii) third-party evidence (“TPE”) if VSOE is not available, and (iii) best estimate of selling price (“BESP”) if neither VSOE nor TPE is available. In 2011, the Company established selling price using management’s best estimate, considering internal factors such as historical selling prices, pricing practices and controls, and customer segment pricing strategies.
Implementation of this new authoritative guidance had an insignificant impact on reported revenue as compared to revenue under previous guidance.
Shipping and handling fees and costs
The Company records shipping and handling costs in cost of sales. No significant expense was incurred for the years ended December 31, 2010 and 2011 or for the periods ended September 30, 2011 and 2012.
Research and development costs
Research and development costs consist primarily of salaries and related personnel costs, stock-based compensation, fees paid to outside parties, facilities costs, travel costs, depreciation and materials used in research and development. All research and development costs are charged to expense as incurred.
Advertising costs
All advertising costs are charged to expense as incurred. No significant expense was incurred for the years ended December 31, 2010 and 2011 or for the periods ended September 30, 2011 and 2012.
F-13
Table of Contents
Income taxes
The Company has adopted the provisions of ASC 740, Accounting for Uncertainty in Income Taxes. Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. The Company measures tax assets and liabilities using the enacted tax rates expected to apply to taxable income in the years in which the Company expects to recover or settle those temporary differences. The Company recognizes the effect of a change in tax rates on deferred tax assets and liabilities in income in the period that includes the enactment date. The Company provides a valuation allowance against net deferred tax assets unless, based upon the available evidence, it is more likely than not that the deferred tax assets will be realized.
Stock-based compensation
The Company recognizes compensation expense related to stock-based transactions, including employee stock options, based on their fair value. The Company recognizes compensation expense over the vesting period using the straight-line method and classifies these amounts in the statements of operations and comprehensive income (loss) based on the department to which the related employee reports. The Company uses the Black-Scholes valuation model to calculate the fair value of stock options.
The Company accounts for stock options issued to non-employees based on the fair value of the awards determined using the Black-Scholes valuation model. The fair value of stock options is recognized over the vesting period. Grants are made to non-employees after services are provided by the non-employee.
Net income (loss) per share
Basic net income (loss) per share attributable to common stockholders is calculated by dividing the net income (loss) attributable to common stockholders by the weighted average number of common shares outstanding for the period, without consideration for common stock equivalents. Diluted net income (loss) per share attributable to common stockholders is computed by dividing the net income (loss) attributable to common stockholders by the weighted average number of common share equivalents outstanding for the period determined using the treasury-stock method or the if-converted method, if applicable. For purposes of this calculation, convertible preferred stock, stock options, and warrants are considered to be common stock equivalents and are only included in the calculation of diluted net income (loss) per share attributable to common stockholders when their effect is dilutive.
F-14
Table of Contents
The unaudited pro forma basic and diluted net income (loss) per share is calculated by dividing the pro forma net income (loss) by the weighted average number of common shares outstanding for the period plus the weighted average number of common shares resulting from the assumed conversion of the outstanding shares of convertible preferred stock. The assumed conversion is calculated using the if-converted method, as if such conversion had occurred as of the beginning of each period presented or the original issuance date, if later.
| Years ended December 31, | Nine months ended September 30, | |||||||||||||||
| 2010 | 2011 | 2011 | 2012 | |||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands, except for shares and per share price) | ||||||||||||||||
| Historical |
||||||||||||||||
| Numerator: |
||||||||||||||||
| Net income/(loss) |
$ | (19,695 | ) | $ | (9,997 | ) | $ | (8,132 | ) | $ | 1,206 | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Denominator: |
||||||||||||||||
| Weighted average shares used to compute net income/(loss) per share, basic |
2,581,708 | 2,634,385 | 2,633,929 | 2,651,833 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average shares used to compute net income/(loss), diluted |
2,581,708 | 2,634,385 | 2,633,929 | 9,148,420 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic net income/(loss) per share attributable to common stockholders |
$ | (7.63 | ) | $ | (3.79 | ) | $ | (3.09 | ) | $ | 0.45 | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Diluted net income/(loss) per share attributable to common stockholders |
$ | (7.63 | ) | $ | (3.79 | ) | $ | (3.09 | ) | $ | 0.13 | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Historical outstanding anti-dilutive securities not included in diluted net loss per share calculation |
||||||||||||||||
| Preferred stock |
5,734,096 | 5,795,244 | 5,764,199 | — | ||||||||||||
| Preferred stock warrants |
143,652 | 130,914 | 136,455 | 126,362 | ||||||||||||
| Common stock warrants |
1,649,974 | 2,322,723 | 2,227,777 | 2,749,676 | ||||||||||||
| Common stock options |
1,752,533 | 1,892,961 | 1,902,300 | 1,347,757 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
9,280,254 | 10,141,842 | 10,030,731 | 4,223,795 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma (unaudited) |
||||||||||||||||
| Net income/(loss) |
$ | (19,695 | ) | $ | (9,997 | ) | $ | (8,132 | ) | $ | 1,206 | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma weighted average common shares outstanding |
2,581,708 | 2,634,385 | 2,633,929 | 2,651,833 | ||||||||||||
| Pro forma adjustments to reflect assumed weighted average effect of conversion of preferred stock |
5,734,096 | 5,795,244 | 5,763,983 | 6,027,959 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma shares used to compute net income/(loss) per share, basic |
8,315,803 | 8,429,629 | 8,397,912 | 8,679,791 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma shares used to compute net income/(loss) per share, diluted |
8,315,803 | 8,429,629 | 8,397,912 | 9,148,420 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Comprehensive income (loss)
All components of comprehensive income (loss), including net income (loss), are reported in the statements of operations and comprehensive income (loss) in the period in which they are recognized. Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources, including unrealized gains and losses on investments. The Company does not have any components of comprehensive income other than net income (loss) for any of the periods presented.
F-15
Table of Contents
Recently Issued Accounting Standards
In May 2011, the Financial Accounting Standards Board (FASB) issued an amendment to the accounting guidance on fair value measurements to ensure that GAAP and International Financial Reporting Standards have common requirements for fair value measurement and disclosures, including a consistent definition of fair value. The guidance is effective for interim and annual periods beginning on or after December 15, 2011. The Company adopted this guidance and it did not have a material impact on its financial statements for the period ended September 30, 2012.
In June 2011, the FASB issued an amendment to the accounting guidance on the presentation of comprehensive income. The guidance eliminates the option to present components of other comprehensive income as part of the statement of changes in stockholders’ equity, and instead requires that all nonowner changes in stockholders’ equity be presented in either a single continuous statement of comprehensive income or in two separate but consecutive statements. The guidance is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. The Company adopted this guidance and it did not have a material impact on its financial statements for the period ended September 30, 2012.
In December 2011, the FASB issued an amendment to the accounting guidance on disclosures about offsetting assets and liabilities. The guidance requires an entity to disclose both gross and net information about financial instruments and derivative instruments that are eligible for offset in the balance sheet or subject to an enforceable master netting arrangement or similar agreement. The guidance is effective for annual reporting periods beginning on or after January 1, 2013, and interim periods within those annual periods. The Company does not expect the adoption of this guidance will have a material impact on its financial statements.
Reverse Stock Split
The Company duly authorized and approved, and effected in January 2013, a 1-for-0.33 reverse stock split of the Company’s issued and outstanding shares of common stock. The par value of the common and convertible preferred stock was not adjusted as a result of the reverse stock split. All issued and outstanding common stock, warrants for common stock, options for common stock, contingently issuable shares of common stock and per share amounts contained in the Company’s financial statements have been retroactively adjusted to reflect this reverse stock split for all periods presented.
2. Balance Sheet Details
Inventory consisted of the following at:
| December 31, | September 30, 2012 |
|||||||||||
| 2010 | 2011 | |||||||||||
| (unaudited) | ||||||||||||
| (in thousands) | ||||||||||||
| Raw materials |
$ | 767 | $ | 716 | $ | 1,882 | ||||||
| Work in process |
646 | 601 | 618 | |||||||||
| Finished goods |
711 | 426 | 214 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 2,124 | $ | 1,743 | $ | 2,714 | ||||||
|
|
|
|
|
|
|
|||||||
F-16
Table of Contents
The following table summarizes the activity in the reserve for excess and obsolete inventory during the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2012. Increases to the reserve are reflected as “Charged to costs and expenses.” “Allowance reductions/write-offs” is comprised primarily of inventory that has been scrapped.
| Balance at beginning of period |
Charged to costs and expenses |
Allowance reductions/ write-offs |
Balance at end of period |
|||||||||||||
| (in thousands) | ||||||||||||||||
| For the year ended December 31, 2010 |
$ | 381 | $ | 412 | $ | (98 | ) | $ | 695 | |||||||
| For the year ended December 31, 2011 |
$ | 695 | $ | 94 | $ | (110 | ) | $ | 679 | |||||||
| For the nine months ended September 30, 2012 (unaudited) |
$ | 679 | $ | 263 | $ | (243 | ) | $ | 699 | |||||||
Property, equipment and improvements consisted of the following at:
| Estimated life | December 31, | September 30, 2012 |
||||||||||||||
| 2010 | 2011 | |||||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands) | ||||||||||||||||
| Domestic placement plan assets |
3 years | $ | 1,861 | $ | 1,824 | $ | 1,570 | |||||||||
| Equipment |
5 - 7 years | 1,829 | 1,831 | 1,907 | ||||||||||||
| Computer equipment and software |
3 years | 166 | 168 | 228 | ||||||||||||
| Leasehold improvements |
various | 159 | 159 | 159 | ||||||||||||
| Furniture |
5 years | 155 | 156 | 156 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| $ | 4,170 | $ | 4,138 | $ | 4,020 | |||||||||||
| Less accumulated depreciation and amortization |
2,486 | 2,721 | 2,875 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 1,684 | $ | 1,417 | $ | 1,145 | ||||||||||
|
|
|
|
|
|
|
|||||||||||
Depreciation and amortization of property, equipment and improvements, including capitalized INFINITI analyzers in domestic placement plans, was $779,415, $642,875, $554,634 and $461,172 for the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2011 and 2012, respectively.
Intangible assets consisted of the following at:
| Estimated life | December 31, | September 30, 2012 |
||||||||||||||
| 2010 | 2011 | |||||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands) | ||||||||||||||||
| Licenses |
5 years | $ | 216 | $ | 216 | $ | 216 | |||||||||
| Less accumulated amortization |
170 | 202 | 216 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 46 | $ | 14 | $ | — | ||||||||||
|
|
|
|
|
|
|
|||||||||||
Amortization of licenses was $43,200, $31,950, $24,900 and $14,100 for the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2011 and 2012, respectively.
F-17
Table of Contents
Accrued expenses consisted of the following at:
| December 31, | September 30, 2012 |
|||||||||||
| 2010 | 2011 | |||||||||||
| (unaudited) | ||||||||||||
| (in thousands) | ||||||||||||
| Payroll and related expenses |
$ | 632 | $ | 618 | $ | 714 | ||||||
| Professional fees |
25 | 79 | 46 | |||||||||
| Initial public offering costs — terminated offering |
395 | 159 | 159 | |||||||||
| Initial public offering costs |
— | — | 339 | |||||||||
| Other |
337 | 359 | 582 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 1,389 | $ | 1,215 | $ | 1,840 | ||||||
|
|
|
|
|
|
|
|||||||
The following table summarizes the activity in accrued warranty costs included in accrued expenses in the table above during the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2012:
| Balance at beginning of period |
Charged to costs and expenses |
Costs incurred |
Balance at end of period |
|||||||||||||
| (in thousands) | ||||||||||||||||
| For the year ended December 31, 2010 |
$ | 41 | $ | 61 | $ | (49 | ) | $ | 53 | |||||||
| For the year ended December 31, 2011 |
$ | 53 | $ | 33 | $ | (56 | ) | $ | 30 | |||||||
| For the nine months ended September 30, 2012 (unaudited) |
$ | 30 | $ | 41 | $ | (40 | ) | $ | 31 | |||||||
3. Promissory Notes
During the year ended December 31, 2010, the Company issued $6.1 million in subordinated notes bearing interest rates between 6% and 7% per annum with two year maturities, $0.1 million of which was subsequently paid in full in June 2010. In connection with these notes, the Company issued freestanding warrants to purchase 598,653 shares of the Company’s common stock at exercise prices ranging from $9.09 to $15.15 per share. The warrants are immediately exercisable and expire five years after the date of issuance. The fair value of the warrants was determined using the Black-Scholes valuation model and was used to calculate the debt discount of $1.7 million. This amount is being amortized to interest expense using the effective interest method over the two-year term of the notes.
During the year ended December 31, 2011, the Company issued $1.0 million in subordinated notes bearing interest at the rate of 7% per annum with a maturity date of December 31, 2011. In connection with these notes, the Company issued freestanding warrants to purchase 99,000 shares of the Company’s common stock at an exercise price of $9.09 per share. The warrants are immediately exercisable and expire five years after the date of issuance. The fair value of the warrants was determined using the Black-Scholes valuation model and was used to calculate the debt discount of $0.2 million. This amount was amortized to interest expense using the effective interest method over the term of the notes. The Company also issued $0.2 million of short term notes during the year ended December 31, 2011. These subordinated notes bear an interest at rate of 12% per annum with a maturity date of up to six months after issuance. In connection with these notes, the Company issued freestanding warrants to purchase 28,875 shares of the Company’s common stock at an exercise price of $5.24 per share. The warrants are immediately exercisable and expire five years after the date of issuance. The fair value of the warrants was determined using the Black-Scholes valuation model and was used to calculate the debt discount of $0.1 million. This amount was amortized to interest expense using the effective interest method over the six month term of the notes. The Company also accepted the surrender of $0.3 million in aggregate principal amount and accrued and unpaid interest on outstanding promissory notes from the holders thereof as consideration for the issuance of an equivalent value of 105,554 shares of the Company’s Series E Convertible Preferred Stock at a price of $2.75 per share.
F-18
Table of Contents
During the nine months ended September 30, 2012, the Company issued $1.7 million in short term notes payable bearing interest at rates between 10% and 12% per annum with maturity dates up to six months after issuance. In connection with these notes, the Company issued freestanding warrants to purchase 199,650 shares of the Company’s common stock at an exercise price of $5.24 per share. The warrants are immediately exercisable and expire five years after the date of issuance. The fair value of the warrants was determined using the Black-Scholes valuation model and was used to calculate the debt discount of $316,000. This amount is being amortized to interest expense using the effective interest method over the term of the notes.
In August 2011 the Company restructured two previously issued subordinated notes to senior unsecured promissory notes with a maturity date in February 2012. These notes are senior in terms of repayment and have a total outstanding principal of $3.9 million and bear interest rate at 13% per annum. These notes were originally issued with freestanding warrants to purchase 429,000 shares of the Company’s common stock at an exercise price of $9.09 per share. The warrants are immediately exercisable and expire five years after the date of issuance.
Refinanced subordinated promissory notes where the present value of the future cash flows, under the modified notes, did not exceed the present value of the future cash flows under the original notes by more than 10%, were treated as a modification and the increase in the fair value of the warrants resulting from the modification was recorded as a discount to the notes and is being amortized over the new term of the notes using the effective interest method. For notes where the present value of future cash flows changed by more than 10% under modified terms as compared to original terms, the Company accounted for the modification as a debt extinguishment, and accordingly recorded the notes at fair value as calculated using future discounted cash flows. The gain or loss on extinguishment is recorded in the statement of operations and comprehensive income and loss as interest expense in the period in which the extinguishment occurred. The gain or loss on extinguishment was determined by calculating the difference between the net carrying amount of the extinguished debt (which includes principal, accrued interest, original fair value of the associated warrants originally recorded in equity, unamortized discount and unamortized deferred financing cost) and the fair value of the modified debt (which includes fair value of modified debt, fair value of modified warrants and any amendment related fees). The refinancings of the subordinated promissory notes are summarized as follows:
April 2010 refinancing to modify the terms of the applicable subordinated promissory notes and related warrants:
| Modification | ||||
| Original notes and warrants |
||||
| Note principal amount |
$ | 6,040,000 | ||
| Original maturity date |
|
August 2010- November 2011 |
| |
| Common stock warrants shares |
566,069 | |||
| Warrant exercise price |
$ | 21.21 | ||
| Warrant expiration date |
|
5 years from original issuance |
| |
| Modified notes and warrants |
||||
| Note principal amount |
$ | 6,040,000 | ||
| Extended maturity date |
April 2012 | |||
| Common stock warrants shares |
566,069 | |||
| Warrant exercise price |
$ | 15.15 | ||
| Warrant expiration date |
|
6.5 years from original issuance |
| |
| Increase in fair value of warrant(s) |
$ | 304,000 | ||
| Gain on extinguishment |
N/A | |||
F-19
Table of Contents
November 2010, refinancing to modify the terms of the applicable subordinated promissory notes and related warrants:
| Extinguishment | ||||
| Original notes and warrants |
||||
| Note principal amount |
$ | 2,640,000 | ||
| Original maturity date |
|
August 2010- November 2011 |
| |
| Common stock warrants shares |
234,300 | |||
| Warrant exercise price |
$ | 12.12 and 21.21 | ||
| Warrant expiration date |
|
5 years from original issuance |
| |
| Modified notes and warrants |
||||
| Note principal amount |
$ | 2,640,000 | ||
| Extended maturity date |
December 2012 | |||
| Common stock warrants shares |
234,300 | |||
| Warrant exercise price |
$ | 9.09 | ||
| Warrant expiration date |
|
6.5 years from original issuance |
| |
| Increase in fair value of warrant(s) |
$ | 336,000 | ||
| Gain on extinguishment |
$ | 136,000 | ||
Refinancings in 2011 to modify the terms of the applicable subordinated promissory notes and related warrants:
| Modification | Extinguishment | |||||||
| Original notes and warrants |
||||||||
| Note principal amount |
$ | 3,250,000 | $ | 2,345,000 | ||||
| Original maturity date |
|
July 2011- December 2011 |
|
July 2011- | ||||
| November 2011 | ||||||||
| Common stock warrants shares |
234,300 | 219,773 | ||||||
| Warrant exercise price |
$ | 12.12 | $ | 12.12 and 21.21 | ||||
| Warrant expiration date |
5 years from | 5 years from | ||||||
| original issuance | original issuance | |||||||
| Modified notes and warrants |
||||||||
| Note principal amount |
$ | 3,250,000 | $ | 2,345,000 | ||||
| Extended maturity date |
|
February-April 2012 |
|
|
February- December 2012 |
| ||
| Common stock warrants shares |
452,430 | 219,773 | ||||||
| Warrant exercise price |
$ | 9.09 | $ | 9.09 | ||||
| Warrant expiration date |
6.5 years from | 6.5 years from | ||||||
| original issuance | original issuance | |||||||
| Increase in fair value of warrants |
$ | 853,000 | $ | 75,000 | ||||
| Gain on extinguishment |
NA | $ | 124,000 | |||||
F-20
Table of Contents
February 2012 refinancing to modify the terms of the applicable subordinated promissory notes and related warrants:
| Modification | Extinguishment | |||||||
| Original notes and warrants |
||||||||
| Note Principal Amount |
$ | 250,000 | $ | 400,000 | ||||
| Original Maturity Date |
Apr-12 | Feb-12 | ||||||
| Common Stock Warrants Shares |
23,430 | 37,488 | ||||||
| Warrant Exercise Price |
$ | 21.21 | $ | 12.12 | ||||
| Warrant Expiration Date |
5 years from | 5 years from | ||||||
| original issuance | original issuance | |||||||
| Modified notes and warrants |
||||||||
| Note Principal Amount |
$ | 250,000 | $ | 400,000 | ||||
| Extended Maturity Date |
Jun-12 | Apr-12 | ||||||
| Common Stock Warrants Shares |
23,430 | 37,488 | ||||||
| Warrant Exercise Price |
$ | 9.09 | $ | 9.09 | ||||
| Warrant Expiration Date |
6.5 years from | 6.5 years from | ||||||
| original issuance | original issuance | |||||||
| Increase in Fair Value of Warrant(s) |
$ | 36,000 | $ | 47,000 | ||||
| Gain on Extinguishment |
NA | $ | 78,000 | |||||
The warrant cancellations and reissuances, summarized above, include the cancellation of original warrants and reissuance of new warrants to purchase 145,735 shares of the Company’s common stock beneficially held by members of the board of directors of the Company, officers of the Company, and a significant stockholder.
The components of the Company’s promissory notes payable consisted of:
| December 31, | September 30, 2012 |
|||||||||||
| 2010 | 2011 | |||||||||||
| (unaudited) | ||||||||||||
| (in thousands) | ||||||||||||
| Subordinated Promissory Notes, 13% interest, due July 2011 |
$ | 5,071 | $ | — | $ | — | ||||||
| Subordinated Promissory Notes, 6% interest, due November to December 2011 |
595 | — | — | |||||||||
| Senior Promissory Notes, 13% interest, due December 2011 |
— | 400 | — | |||||||||
| Senior Promissory Note, 13% interest, due February 2012 |
— | 3,500 | 2,100 | |||||||||
| Subordinated Short Term Notes Payable, 12% interest, due April & May 2012 |
— | 175 | 695 | |||||||||
| Subordinated Short Term Notes Payable, 10% interest, due June 2012 |
— | — | 1,150 | |||||||||
| Subordinated Promissory Notes, 6% interest, due February to June 2012 |
10,715 | 10,794 | 10,794 | |||||||||
| Subordinated Promissory Notes, 6% interest, due December 2012 |
2,000 | 2,345 | 2,345 | |||||||||
| Subordinated Promissory Notes, 7% interest, due December 2012 |
300 | 1,200 | 1,200 | |||||||||
| Subordinated Promissory Notes, 8% interest, due December 2012 |
500 | 500 | 500 | |||||||||
| Subordinated Promissory Notes, 13% interest, due December 2012 |
— | 2,000 | 2,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 19,181 | $ | 20,914 | $ | 20,784 | ||||||
| Unamortized premium/(discount) |
(2,522 | ) | (281 | ) | 78 | |||||||
| Accrued Interest |
2,120 | 2,882 | 3,863 | |||||||||
|
|
|
|
|
|
|
|||||||
| Outstanding Balance |
$ | 18,779 | $ | 23,515 | $ | 24,725 | ||||||
|
|
|
|
|
|
|
|||||||
The Company’s outstanding promissory notes have matured or will mature on or before December 31, 2012. As of September 30, 2012, the Company was in default on $14.8 million in principal amount of outstanding promissory notes. Of the total notes payable listed above, $2.0 million, $2.4 million, and $4.0 million were issued
F-21
Table of Contents
to related parties and outstanding as of December 31, 2010 and 2011 and September 30, 2012, respectively, with interest rates ranging from 6.0% to 12.0%. As of September 30, 2012, the Company had outstanding total indebtedness and accrued interest under promissory notes of approximately $24.7 million, of which approximately $14.8 million in principal amount was in payment default. The Company does not have sufficient cash to satisfy its indebtedness obligations, and its lenders can demand repayment in full of, or sue to collect on, these past due obligations at any time. The Company restructured a significant portion of this indebtedness in November 2012. (See Note 12.)
4. Commitments
Operating lease
The Company leases its corporate headquarters and manufacturing facility under a non-cancelable operating lease agreement expiring in December 2029. Rent expense under the lease was $1.6 million, $1.8 million, $1.3 million and $1.7 million for the years ended December 31, 2010 and 2011 and for the nine months ended September 30, 2011 and 2012, respectively. The terms of the facility lease provide for rental payments on a monthly basis with periodic rent escalations over the term of the lease as well as penalties of 11% of the monthly lease payment for late payment.
The Company recognizes rent expense on a straight-line basis over the lease period and has accrued for rent expense incurred but not paid. Certain incentives, including discounted rental payments and tenant improvement allowances, were granted when the original lease was executed.
Accumulated discounted rental payments recorded to deferred rent totaled $1.8 million, $2.1 million and $2.4 million as of December 31, 2010 and 2011 and September 30, 2012, respectively, and the balance of the tenant improvement allowance stayed consistent at $0.2 million. The Company amortizes the allowance on a straight-line basis over the term of the lease.
The Company has at times been unable to remit timely lease payments and the remediation of these defaults has resulted in amendments to the original lease that have included rent escalations and extensions of the original leasehold expiration date.
The amounts of future minimum lease commitments under the operating lease are as follows:
| Years ending December 31, |
Amount | |||
| (in thousands) | ||||
| 2012 |
$ | 1,590 | ||
| 2013 |
1,751 | |||
| 2014 |
1,834 | |||
| 2015 |
1,924 | |||
| 2016 and thereafter |
34,896 | |||
|
|
|
|||
| Total |
$ | 41,995 | ||
|
|
|
|||
License agreement
Included in intangible assets are amounts paid for various license agreements obligating the Company to pay royalties of six to ten percent of certain product sales. These license agreements expire at various times through May 2024.
F-22
Table of Contents
5. Common and Convertible Preferred Stock Warrant Liabilities
Common stock warrant liability
One of the Company’s common stock warrants provides for the adjustment of its exercise price on a broad-based weighted average basis if the Company issues certain types of securities at a price per share below the then-current exercise price per share of the warrant. The Company uses the Black-Scholes valuation model to calculate the fair value of its common stock warrant liability. The Company determined that using alternative valuation models, such as a Monte Carlo simulation model, would result in de minimus differences. The fair value of the common stock warrant liability was estimated at the balance sheet dates using the following assumptions:
| December 31 | September 30, 2012 | |||||
| 2010 | 2011 | |||||
| (unaudited) | ||||||
| Fair value |
$8.30 | $5.24 | $5.58 | |||
| Exercise price |
$12.06 | $9.00 | $8.91 - $9.09 | |||
| Contractual Term (yrs) |
3.60 | 2.60 | 1.90 - 3.60 | |||
| Stock price volatility |
76.84% | 60.17% | 52.71% - 72.97% | |||
| Risk-free interest rate |
1.42% | 0.39% | 0.26% - 0.34% | |||
| Dividend yield |
— | — | — | |||
At December 31, 2010, outstanding common stock warrants consisted of:
| Issuance Date |
Term | Exercise price per share |
Number of shares outstanding underlying warrant |
Fair value at December 31, 2010 |
||||||||||||
| (in thousands) | ||||||||||||||||
| August 2009 |
5 years | $ | 12.06 | 187,440 | $ | 718 | ||||||||||
|
|
|
|
|
|||||||||||||
| 187,440 | $ | 718 | ||||||||||||||
|
|
|
|
|
|||||||||||||
At December 31, 2011, outstanding common stock warrants consisted of:
| Issuance Date |
Term | Exercise price per share |
Number of shares outstanding underlying warrant |
Fair value at December 31, 2011 |
||||||||||||
| (in thousands) | ||||||||||||||||
| August 2009 |
5 years | $ | 9.00 | 187,440 | $ | 215 | ||||||||||
|
|
|
|
|
|||||||||||||
| 187,440 | $ | 215 | ||||||||||||||
|
|
|
|
|
|||||||||||||
In June 2011, the Company and the applicable note holder amended a certain promissory note with an aggregate principal amount of $2,000,000 set to mature in July 2011, by extending the maturity date to December 2012. In connection with this amendment, the Company cancelled a warrant to purchase 187,440 shares of its common stock at an exercise price of $12.12 per share held by the note holder and reissued a warrant to purchase 187,440 shares of common stock at an exercise price of $9.09 per share to the note holder which will expire in August 2014.
F-23
Table of Contents
At September 30, 2012, outstanding common stock warrants consisted of:
| Issuance date |
Term | Exercise price per share |
Number of shares outstanding underlying warrant |
Fair value at September 30, 2012 |
||||||||||||
| (in thousands) (unaudited) |
||||||||||||||||
| August 2009 |
5 years | $ | 8.91 | 187,440 | $ | 168 | ||||||||||
|
|
|
|
|
|||||||||||||
| 187,440 | $ | 168 | ||||||||||||||
|
|
|
|
|
|||||||||||||
Convertible preferred stock warrant liabilities
The Company uses the Black-Scholes valuation model to calculate the fair value of its preferred stock warrants.
The inputs utilized in this valuation model are discussed below. Each of these inputs is subjective and generally requires significant judgment to determine.
| • | Fair Value of Preferred Stock. Because the Company’s stock is not publicly traded, the Company must estimate the fair value of its preferred stock, as discussed below. |
| • | Expected Term. The expected term is the remaining contractual life of the applicable preferred stock warrant. |
| • | Volatility. As the Company does not have a trading history for its preferred stock, the expected stock price volatility for its preferred stock is estimated by taking the average historic price volatility for industry peers based on daily price observations over a period equivalent to the expected term of the preferred stock warrants. Industry peers consist of several public companies similar in size, stage of life cycle and financial leverage. The Company does not rely on implied volatilities of traded common stock in its industry peers’ common stock because the volume of activity has been relatively low. |
| • | Risk-free Interest Rate. The risk-free interest rate is based on the yields of U.S. Treasury securities with maturities similar to the contractual term of the preferred stock warrants. |
| • | Dividend Yield. The Company has not historically paid cash dividends or distributions and does not intend to pay cash dividends or distributions in the foreseeable future. Consequently, the Company used an expected dividend yield of zero. |
The fair value of the preferred stock warrants was estimated at the balance sheet dates using the following assumptions (fair value and exercise price are presented on a common stock equivalents basis):
| December 31, | September 30, 2012 | |||||
| 2010 | 2011 | |||||
| (unaudited) | ||||||
| Fair value |
$8.65 | $11.13 | $13.63 | |||
| Exercise Price |
$7.65 - $10.57 | $9.04 - $10.57 | $9.74 - $11.27 | |||
| Expected Term (in years) |
0.08 - 0.99 | 0.55 - 0.63 | 0.2 - 0.9 | |||
| Stock Price Volatility |
76.84 | 60.17 | 53.95 | |||
| Risk-free interest rate |
0.29% | 0.12% | 0.18% | |||
| Dividend yield |
— | — | — | |||
The fair value of the preferred stock underlying the warrants has historically been determined by the Company’s board of directors. Because there has been no public market for the Company’s stock, the board of directors has determined the fair value of the preferred stock at the time of issuance by considering a number of objective and subjective factors including valuations performed by unrelated third-party specialists, which included valuations of comparable companies, operating and financial performance, lack of liquidity of capital stock and general and
F-24
Table of Contents
industry-specific economic outlook, among other factors. For each period in which the fair value of preferred stock warrant liabilities was assessed, the business enterprise value of the Company was first determined. This enterprise value then was allocated across the Company’s capital structure using a Probability Weighted Expected Return Method (PWERM) model as the primary approach and an Option Pricing Model (OPM) analysis as a confirming approach. Under the PWERM model, various future potential liquidity scenarios were forecasted and the allocable value to each class of security in the Company’s capital structure in each respective scenario was determined. The probability that each potential liquidity event would occur was then assessed. The future proceeds were then discounted to determine the present value of proceeds in each scenario for each security class. The probability weighted present value of each security class was then determined to conclude the value of the preferred stock. Under the confirming OPM analysis, the Black Scholes option pricing framework was used to determine the proceeds allocable to each security class. A series of option tranches was established whereby each tranche represented the value allocable to various security classes between computed breakpoints. Breakpoints are the value inflection points, or indifference points, whereby the allocation to securities change based on the underlying economic rights of each security class. The breakpoints served as the strike prices in the Black Scholes option calculations and tranche values were determined based on the difference between option values in each tranche. The proceeds of the value of each option tranche were then allocated to each respective security class based on the economic rights to the security class between breakpoints. The value allocable to each respective security class was then totaled across all option tranches to conclude the fair value of each security class.
The Company’s convertible preferred stock has historically been issued by the Company at a purchase price that reflects a premium to the fair market value of the Company’s common stock. These premiums have been negotiated at arms’-length between the Company and the various participating investors in the offering and sale of such convertible preferred stock, and generally result from the preferential position that the Company’s convertible preferred stock maintains in relation to the Company’s common stock with respect to matters such as preferences upon dividends and preferences upon liquidation. (See Note 6.)
As of December 31, 2010, outstanding convertible preferred stock warrants consisted of (share and exercise price information is presented on a common stock equivalents basis):
| Issuance date |
Term | Exercise price per share |
Number of shares outstanding underlying warrant |
Fair value at December 31, 2010 |
||||||||||||
| (in thousands) | ||||||||||||||||
| December 2010 |
5 years | $ | 10.57 | 5,644 | $ | 11 | ||||||||||
| January 2006 |
5 years | $ | 9.18 | 863 | — | |||||||||||
| May 2006 |
5 years | $ | 7.65 | 6,327 | 8 | |||||||||||
| July and August 2006 |
5 years | $ | 9.18 | 15,481 | 27 | |||||||||||
| July and August 2006 |
5 years | $ | 7.65 | 124,583 | 297 | |||||||||||
| December 2006 |
5 years | $ | 7.65 | 3,595 | 11 | |||||||||||
|
|
|
|
|
|||||||||||||
| Total |
156,494 | $ | 354 | |||||||||||||
|
|
|
|
|
|||||||||||||
During the year ended December 31, 2010, 166,329 and 3,600 shares of Series B Convertible Preferred Stock and Series C Convertible Preferred Stock, which represent 59,795 and 1,294 shares of common stock on an as-converted basis, respectively, were issued pursuant to the exercise of preferred stock warrants at exercise prices of $2.75 and $3.30, respectively.
F-25
Table of Contents
As of December 31, 2011, outstanding convertible preferred stock warrants consisted of (share and exercise price information is presented on a common stock equivalents basis):
| Issuance/amendment date |
Term | Exercise price per share |
Number of shares outstanding underlying warrant |
Fair value at December 31, 2011 |
||||||||||||
| (in thousands) | ||||||||||||||||
| May 2011 |
1 year | $ | 10.57 | 5,932 | $ | 11 | ||||||||||
| July and August 2011 |
1 year | $ | 10.57 | 14,716 | 33 | |||||||||||
| July and August 2011 |
1 year | $ | 9.04 | 121,969 | 368 | |||||||||||
|
|
|
|
|
|||||||||||||
| Total |
|
142,617 | $ | 412 | ||||||||||||
|
|
|
|
|
|||||||||||||
During the year ended December 31, 2011, 2,991 shares of Series B Convertible Preferred Stock, representing 1,075 shares of common stock on an as-converted basis, were issued pursuant to the exercise of preferred stock warrants at an exercise price of $3.30. In addition, warrants for the purchase of 35,609 shares of preferred stock representing 12,801 shares of common stock on an as-converted basis, expired.
As of September 30, 2012, outstanding convertible preferred stock warrants consisted of (share and exercise price information is presented on a common stock equivalents basis):
| Issuance/amendment date |
Term | Exercise price per share |
Number of shares outstanding underlying warrant |
Fair value at September 30, 2012 |
||||||||||||
| (unaudited) | ||||||||||||||||
| (in thousands) | ||||||||||||||||
| December 2011 |
1 year | $ | 9.74 | 3,595 | $ | 14 | ||||||||||
| July and August 2011 |
1 year | $ | 11.27 | 14,128 | 53 | |||||||||||
| July and August 2011 |
1 year | $ | 9.74 | 119,354 | 562 | |||||||||||
|
|
|
|
|
|||||||||||||
| Total |
|
137,077 | $ | 629 | ||||||||||||
|
|
|
|
|
|||||||||||||
During the period ended September 30, 2012, warrants for the purchase of 15,409 shares of preferred stock, representing 5,540 shares on common stock on an as-converted basis, expired.
During 2010, 2011 and the nine months ended September 30, 2012, the Company agreed with various warrant holders to increase the exercise price and extend the maturity one year for convertible preferred stock warrants exercisable for 15,700, 399,200, and 381,300 preferred shares, respectively, that were expiring or expired, representing 5,644, 143,512, and 137,077 shares of common stock on an as-converted basis, respectively. The Company has classified these warrants as a liability and has re-measured the liability to estimated fair value at December 31, 2011 and 2010 and September 30, 2012 using the Black-Scholes valuation model.
6. Convertible Preferred Stock
Upon the occurrence of certain change in control events that are outside of the control of the Company, including sale or transfer of control of the Company, holders of the convertible preferred stock can force the Company to redeem these shares. Accordingly, these shares are considered contingently redeemable and are classified as temporary equity on the accompanying balance sheets.
The rights, preferences and privileges of the convertible preferred stock are as follows:
Dividends
The holders of Series A, Series B, Series C, Series D and Series E Convertible Preferred Stock are entitled to receive noncumulative dividends, in preference and in priority to any dividends on common stock, at a rate of
F-26
Table of Contents
$0.19, $0.17, $0.17, $0.20 and $0.165 per share, respectively, when and if declared by the board of directors. As of September 30, 2012, the board of directors had not declared any dividends.
Conversion
All preferred stock will automatically convert to common stock immediately prior to the closing of an underwritten public offering of common stock under the Securities Act of 1933, as amended, except for Series C Convertible Preferred Stock and Series E Convertible Preferred Stock, each of which automatically converts only if such public offering results in proceeds of $25,000,000 or more. In addition, each individual series of convertible preferred stock will convert automatically to common stock upon the vote of a majority of the outstanding shares of each series. The conversion price of each series of the Company’s convertible preferred stock is adjusted on a broad-based weighted average basis if the Company issues certain types of securities at a price per share below the then current conversion price of the applicable series of convertible preferred stock. This conversion feature was evaluated and determined not to be subject to derivative accounting. As of September 30, 2012, the shares of Series A Convertible Preferred Stock are convertible on a 0.66-for-1 basis into an aggregate of 1,042,290 shares of common stock, the shares of Series B, Series C and Series E Convertible Preferred Stock are convertible on a 0.3314-for-1 basis into an aggregate of 1,480,805, 2,126,639 and 242,759 shares of common stock, respectively, and the shares of Series D Convertible Preferred Stock are convertible on a 0.3356-for-1 basis into an aggregate of 1,148,761 shares of common stock.
Liquidation preference
Upon a liquidation, dissolution or winding up of the Company, the holders of the Series C Convertible Preferred Stock are entitled to receive liquidation preferences at the rate of $2.75 per share (subject to appropriate adjustments for stock splits, stock dividends and other similar reclassifications) plus any declared but unpaid dividends. Liquidation payments to the holders of the Series C Convertible Preferred Stock have priority over the holders of the Series A, Series B, Series D and Series E Convertible Preferred Stock and are made in preference to any payments to the holders of common stock. Upon completion of the required distributions to the Series C Convertible Preferred stockholders, the Series A, Series B, Series D and Series E Convertible Preferred stockholders, prior and in preference to any distribution to the holders of common stock, are entitled to receive liquidation preferences at the rate of $3.16, $2.75, $3.25 and $4.125 per share, respectively. The remaining assets of the Company are to be distributed to the Series C Convertible Preferred and common stockholders, pro rata based on the number of shares of stock held on an if-converted to common stock basis by each stockholder.
Voting
The holders of convertible preferred stock vote on an equivalent basis with common stockholders on an as-converted basis.
7. Stockholders’ Deficit
Common stock warrant issuances
Included in the Company’s outstanding warrants as of September 30, 2012 are warrants exercisable for 2,299,459, 362,616 and 84,150 shares of its common stock in connection with the issuance of promissory notes, the sale of Series E Convertible Preferred Stock and the provision of services rendered to the Company by non-employee consultants as compensation for those services, respectively.
F-27
Table of Contents
The following summarizes transactions related to common stock warrants:
| Number of shares |
Weighted-average exercise price per share |
Weighted-average contractual life (years) |
||||||||||
| Outstanding—January 1, 2010 |
1,326,184 | $ | 16.03 | 3.76 | ||||||||
| Issued |
1,441,922 | 13.36 | 5.73 | |||||||||
| Cancelled |
(800,369 | ) | 20.61 | — | ||||||||
| Exercised |
(19,800 | ) | 1.52 | — | ||||||||
| Expired |
— | — | — | |||||||||
| Outstanding—December 31, 2010 |
1,947,937 | $ | 12.85 | 5.07 | ||||||||
| Issued |
1,141,416 | 9.09 | 4.66 | |||||||||
| Cancelled |
(468,600 | ) | 12.12 | — | ||||||||
| Exercised |
— | — | — | |||||||||
| Expired |
— | — | — | |||||||||
| Outstanding—December 31, 2011 |
2,620,753 | $ | 11.06 | 5.22 | ||||||||
| Issued |
232,815 | 5.70 | 5.00 | |||||||||
| Cancelled |
— | — | — | |||||||||
| Exercised |
— | — | — | |||||||||
| Expired |
(58,872 | ) | 1.97 | — | ||||||||
| Outstanding—September 30, 2012 (unaudited) |
2,794,696 | $ | 10.73 | 5.21 | ||||||||
The Company had 10,029,785, 10,927,890 and 11,312,410 shares of common stock reserved for future issuance at December 31, 2010 and 2011 and September 30, 2012, respectively, upon the conversion or exercise, as applicable, of its outstanding common stock options, common stock and preferred stock warrants (assuming conversion of the preferred stock warrants into common stock), and convertible preferred stock.
8. Stock-Based Compensation
In December 2000, the Company adopted the 2000 Equity Incentive Plan, or the 2000 Plan, which now has expired. The 2000 Plan allowed for the grant of stock options and the right to acquire restricted stock to employees, directors and consultants of the Company. The terms and conditions of specific awards were set at the discretion of the Company’s board of directors although generally options under the 2000 Plan vest in four annual installments of 25% and are generally immediately exercisable. The exercise price of incentive stock options was required to be not less than 100% of the fair market value of the Company’s common stock on the date of grant and the exercise price of any option granted to a 10% stockholder was required to be not less than 110% of the fair market value of the Company’s common stock on the date of grant. Options granted under the 2000 Plan expire no later than 10 years from the date of grant. Unvested common shares obtained upon early exercise of options are subject to repurchase by the Company at the original issue price. As of September 30, 2012, there were no unvested common shares outstanding that had originally been issued upon early exercise of options. The 2000 Plan expired in 2010 and no shares remain available for grant under the 2000 Plan.
As of December 31, 2010 there were outstanding vested and unvested options of 524,323 and 198,909, respectively, to purchase 723,230 shares of the Company’s common stock pursuant to the 2000 Plan.
As of December 31, 2011, there were outstanding vested and unvested options of 575,743 and 89,925, respectively, to purchase 665,668 shares of the Company’s common stock pursuant to the 2000 Plan.
As of September 30, 2012, there were outstanding vested and unvested options of 526,177 and 0, respectively, to purchase a total of 526,177 shares of the Company’s common stock pursuant to the 2000 Plan.
In October 2008, the Company adopted the 2008 Equity Incentive Award Plan, or the 2008 Plan. The 2008 Plan allows for the grant of stock options and the right to acquire restricted stock to employees, directors and
F-28
Table of Contents
consultants of the Company. The terms and conditions of specific awards are set at the discretion of the Company’s board of directors although generally options vest in four annual installments of 25%. The exercise price of incentive stock options shall not be less than 100% of the fair market value of the Company’s common stock on the date of grant and the exercise price of any option granted to a 10% stockholder may be no less than 110% of the fair market value of the Company’s common stock on the date of grant. Options granted under the 2008 Plan expire no later than 10 years from the date of grant. Unvested common shares obtained upon early exercise of options are subject to repurchase by the Company at the original issue price. The 2008 Plan reserved 660,000 shares of common stock pursuant to awards under the 2008 Plan. In addition, shares available for issuance and not subject to awards and shares forfeited under the 2000 Plan, aggregating 519,219 shares, transferred to and were made available for granting pursuant to the 2008 Plan. In aggregate, 398,098 shares remained available for grant under the 2008 Plan at September 30, 2012.
As of December 31, 2010 there were outstanding vested and unvested options of 185,031 and 394,812, respectively, to purchase a total of 579,843 shares of the Company’s common stock pursuant to the 2008 Plan.
As of December 31, 2011, there were outstanding vested and unvested options of 371,718 and 350,526, respectively, to purchase a total of 722,244 shares of the Company’s common stock pursuant to the 2008 Plan.
As of September 30, 2012, there were outstanding vested and unvested options of 361,626 and 417,681, respectively, to purchase a total of 779,307 shares of the Company’s common stock pursuant to the 2008 Plan.
The following table summarizes stock option transactions from January 1, 2010 through September 30, 2012:
| Options | Weighted-average exercise price per share |
Weighted-average remaining contractual term (years) |
Intrinsic value |
|||||||||||||
| (in thousands) | ||||||||||||||||
| Outstanding—January 1, 2010 |
1,444,144 | $ | 3.97 | |||||||||||||
| Granted |
637,632 | $ | 5.48 | |||||||||||||
| Exercised |
(37,208 | ) | $ | 1.97 | $ | 235 | ||||||||||
| Forfeited |
(305,048 | ) | $ | 3.18 | ||||||||||||
| Outstanding—December 31, 2010 |
1,739,251 | $ | 4.70 | |||||||||||||
| Granted |
227,442 | $ | 5.24 | |||||||||||||
| Exercised |
(4,043 | ) | $ | 1.52 | $ | 15 | ||||||||||
| Forfeited |
(103,910 | ) | $ | 6.33 | ||||||||||||
| Outstanding—December 31, 2011 |
1,858,740 | $ | 4.70 | |||||||||||||
| Granted |
90,750 | $ | 5.24 | |||||||||||||
| Exercised |
(37,620 | ) | $ | 1.03 | $ | 156 | ||||||||||
| Forfeited |
(142,158 | ) | $ | 4.39 | ||||||||||||
| Outstanding—September 30, 2012 (unaudited) |
1,769,712 | $ | 4.82 | |||||||||||||
| Vested and expected to vest, exercisable as of September 30, 2012 (unaudited) |
1,546,640 | $ | 4.55 | 5.9 | $ | 1,581 | ||||||||||
| Non–vested as of September 30, 2012 (unaudited) |
223,072 | $ | 6.67 | 8.3 | $ | — | ||||||||||
F-29
Table of Contents
Determining Fair Value of Stock Options
The fair value of each grant of stock options was determined by the Company and its board of directors using the methods and assumptions discussed below. Each of these inputs is subjective and generally requires significant judgment to determine.
Valuation Method—The Company estimates the fair value of its stock options using the Black-Scholes valuation model.
Expected Term—The expected life of employee and non-employee director stock options represents the average of the contractual term of the options and the weighted-average vesting period, as permitted under the simplified method. The Company has elected to use the simplified method, as the Company does not have enough historical exercise experience to provide a reasonable basis upon which to estimate the expected term and the stock option grants are considered “plain vanilla” options.
Expected Volatility—The expected volatility is derived from the historical stock volatilities of several comparable publicly listed peer companies over a period approximately equal to the expected term of the options because the Company has limited information on the volatility of its common stock since the Company has no trading history. When making the selections of the comparable industry peers to be used in the volatility calculation, the Company considered the size, operational and economic similarities to its principle business operations.
Fair Value of Common Stock—The fair value of the common stock underlying the stock options has historically been determined by the Company’s board of directors. Because there has been no public market for the Company’s common stock, the board of directors has determined the fair value of the common stock at the time of the option grant by considering a number of objective and subjective factors including valuations performed by unrelated third-party specialists, which included valuations of comparable companies, operating and financial performance, lack of liquidity of capital stock and general and industry-specific economic outlook, amongst other factors. This fair value has been calculated on an as-converted basis for each period, assuming for such purposes that all outstanding convertible preferred stock has been converted into common stock without taking into account any dividend or liquidation preferences. Once the Company’s common stock is listed on an established stock exchange or national market system, the fair value will be based on the publicly traded share price.
Risk-Free Interest Rate—The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for zero coupon U.S. Treasury notes with maturities approximately equal to the expected term of the options.
Expected Dividend—The expected dividend has been zero as the Company has never paid dividends and has no expectations to do so.
Forfeiture Rate—The Company estimated its forfeiture rate at 4% based on an analysis of its actual forfeitures and will continue to evaluate the adequacy of the forfeiture rate based on actual forfeiture experience, analysis of employee turnover behavior, and other factors. The impact from a forfeiture rate adjustment will be recognized in full in the period of adjustment, and if the actual number of future forfeitures differs from that estimated, the Company may be required to record adjustments to stock-based compensation expense in future periods.
F-30
Table of Contents
The fair value of employee stock options was estimated at the grant date using the following assumptions:
| Years ended December 31, | Nine months ended September 30, | |||||||||||||||
| 2010 | 2011 | 2011 | 2012 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Fair value |
$ | 6.09 - 8.30 | $ | 5.24 | $ |
5.24 |
|
$ |
5.24 |
| ||||||
| Risk-free interest rate |
1.35% - 2.93% | 0.80% - 1.23% | |
0.90% - 1.23% |
|
0.90% | ||||||||||
| Dividend yield |
— | — | |
— |
|
— | ||||||||||
| Expected life of options (years) |
5.00 - 6.25 | 5.08 - 6.25 | |
5.08 - 6.25 |
|
5.50 | ||||||||||
| Volatility |
68.65% - 71.59% | 69.89% - 75.00% | 72.00% - 75.00 | % | 69.89% | |||||||||||
The Company recognized employee stock-based compensation in the statements of operations and comprehensive income (loss) as follows:
| Years ended December 31, | Nine months ended September 30, | |||||||||||||||
| 2010 | 2011 | 2011 | 2012 | |||||||||||||
| (in thousands) | ||||||||||||||||
| (unaudited) | ||||||||||||||||
| Cost of sales |
$ | 227 | $ | 236 | $ | 195 | $ | 90 | ||||||||
| Research and development |
432 | 395 | 358 | 126 | ||||||||||||
| General and administrative |
901 | 780 | 661 | 282 | ||||||||||||
| Sales and marketing |
1,203 | 166 | 150 | 52 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 2,763 | $ | 1,577 | $ | 1,364 | $ | 550 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
No income tax benefit has been recognized in the statement of operations for the periods presented. The total compensation cost related to unvested stock option grants not yet recognized as of September 30, 2012 was $1.9 million, and the weighted average period over which these grants was expected to vest is 1.2 years.
Stock based compensation expense related to non-employee consultants totaled $0.2 million, $0.1 million, $0 and $0 for the years ended December 31, 2010 and 2011 and the nine months ended September 30, 2011 and 2012, respectively.
The Company has issued options to purchase shares of the Company’s common stock to certain of the Company’s directors, executive officers and key employees that are not subject to either the 2008 Plan or the 2000 Plan. The terms of these options are substantially the same as those issued under the 2000 Plan. As of September 30, 2012, there were outstanding options to purchase 464,228 shares of our common stock that were not issued under either the 2008 Plan or the 2000 Plan.
The Company has adopted an Employee Stock Purchase Plan which contains an “evergreen provision” that allows for an annual increase in the number of shares available for issuance. This plan provides for an aggregate limit on the number of shares of common stock that may be issued over the course of its ten-year term of 742,500 shares. The Company has not issued any shares or awards under this plan as it is not yet effective.
9. Fair Value Measurements
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following table summarizes the Company’s assets and liabilities that require fair value measurements on a recurring basis and their respective input levels based on the fair value hierarchy:
| Fair value at December 31, 2010 |
Fair value measurements
at December 31, 2010 using: |
|||||||||||
| Quoted market prices for identical assets (Level 1) |
Significant unobservable inputs (Level 3) |
|||||||||||
| (in thousands) | ||||||||||||
| Money market funds |
$ | 92 | $ | 92 | $ | — | ||||||
| Common stock warrant liabilities |
718 | — | 718 | |||||||||
| Preferred stock warrant liabilities |
354 | — | 354 | |||||||||
|
|
|
|
|
|||||||||
| Total |
$ | 92 | $ | 1,072 | ||||||||
|
|
|
|
|
|||||||||
F-31
Table of Contents
| Fair value at December 31, 2011 |
Fair value measurements at December 31, 2011 using: |
|||||||||||
| Quoted market prices for identical assets (Level 1) |
Significant unobservable inputs (Level 3) |
|||||||||||
| (in thousands) | ||||||||||||
| Money market funds |
$ | 1 | $ | 1 | $ | — | ||||||
| Common stock warrant liabilities |
215 | — | 215 | |||||||||
| Preferred stock warrant liabilities |
412 | — | 412 | |||||||||
|
|
|
|
|
|||||||||
| Total |
$ | 1 | $ | 627 | ||||||||
|
|
|
|
|
|||||||||
| Fair value at September 30, 2012 |
Fair value measurements
at September 30, 2012 (unaudited) using: |
|||||||||||
| Quoted market prices for identical assets (Level 1) |
Significant unobservable inputs (Level 3) |
|||||||||||
| (in thousands) | ||||||||||||
| Money market funds |
$ | 1 | $ | 1 | $ | — | ||||||
| Common stock warrant liabilities |
168 | — | 168 | |||||||||
| Preferred stock warrant liabilities |
629 | — | 629 | |||||||||
|
|
|
|
|
|||||||||
| Total |
$ | 1 | $ | 783 | ||||||||
|
|
|
|
|
|||||||||
Preferred and common stock warrant liabilities
The following table summarizes the activity in the common stock and preferred stock warrant liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3):
| Preferred stock warrant liabilities |
Common stock warrant liabilities |
|||||||
| (in thousands) | ||||||||
| Balance at January 1, 2010 |
$ | 1,212 | $ | 786 | ||||
| Change in fair value of warrant liabilities |
(599 | ) | (68 | ) | ||||
| Issuances and settlements |
(259 | ) | — | |||||
| Transfers in and out of Level III |
— | — | ||||||
|
|
|
|
|
|||||
| Balance at December 31, 2010 |
354 | 718 | ||||||
| Change in fair value of warrant liabilities |
77 | (503 | ) | |||||
| Issuances and settlements |
(19 | ) | — | |||||
| Transfers in and out of Level III |
— | — | ||||||
|
|
|
|
|
|||||
| Balance at December 31, 2011 |
412 | 215 | ||||||
| Change in fair value of warrant liabilities |
228 | (47 | ) | |||||
| Issuances and settlements |
(11 | ) | — | |||||
| Transfers in and out of Level III |
— | — | ||||||
|
|
|
|
|
|||||
| Balance at September 30, 2012 (unaudited) |
$ | 629 | $ | 168 | ||||
|
|
|
|
|
|||||
Certain assets and liabilities are measured at fair value on a nonrecurring basis and therefore are not included in the tables above. These include certain long-lived assets measured at cost that were written down to fair value during 2010 as a result of an impairment. In addition, modified notes payable determined to be extinguished were measured and recognized at fair value on the extinguishment date. The fair values of these assets and notes were calculated using discounted cash flow models that utilized certain unobservable inputs (Level III).
F-32
Table of Contents
10. Income Taxes
The Company had a taxable loss for all periods presented with the exception of the September 30, 2012 period for which previously incurred net operating losses will be utilized to offset taxable income. Additionally, management has determined that a full valuation allowance should be maintained against net deferred tax assets (discussed further below). As such, no income tax provision has been recorded for any of the periods presented.
The effective tax rate on income taxes is reconciled to the statutory federal income tax rate as follows:
| For the years ended December 31, |
For the nine months ended September 30, |
|||||||||||||||
| 2010 | 2011 | 2011 | 2012 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Tax computed at the federal statutory rate |
(35.0 | )% | (35.0 | )% | (35.0 | )% | (35.0 | )% | ||||||||
| State income taxes, net of federal benefit |
(4.8 | )% | (4.8 | )% | (4.8 | )% | (4.8 | )% | ||||||||
| Permanent differences and other |
3.1 | % | 1.3 | % | 2.0 | % | (11.9 | )% | ||||||||
| Changes in valuation allowance |
36.7 | % | 38.5 | % | 37.8 | % | 51.7 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Actual rate |
0.0 | % | 0.0 | % | 0.0 | % | 0.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities, and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Significant components of the deferred tax assets are as follows:
| December 31, | September 30, 2012 |
|||||||||||
| 2010 | 2011 | |||||||||||
| (unaudited) | ||||||||||||
| (in thousands) | ||||||||||||
| Deferred tax assets: |
||||||||||||
| Net operating losses |
$ | 23,018 | $ | 26,456 | $ | 25,745 | ||||||
| Credits |
1,687 | 1,856 | 1,976 | |||||||||
| Accrued expenses |
1,343 | 1,407 | 1,701 | |||||||||
| Intangible assets |
5,264 | 5,936 | 6,419 | |||||||||
| Stock-based compensation |
1,053 | 1,179 | 1,195 | |||||||||
| Other |
349 | 344 | 352 | |||||||||
|
|
|
|
|
|
|
|||||||
| 32,714 | 37,178 | 37,388 | ||||||||||
| Valuation allowance |
(32,661 | ) | (37,023 | ) | (37,122 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total deferred tax assets |
53 | 155 | 266 | |||||||||
|
|
|
|
|
|
|
|||||||
| Deferred tax liabilities: |
||||||||||||
| Fixed assets |
(53 | ) | (155 | ) | (266 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total deferred tax liabilities |
(53 | ) | (155 | ) | (266 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total deferred tax assets, net of valuation allowance |
$ | — | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
A valuation allowance of $32.7 million, $37.0 million and $37.1 million at December 31, 2010 and 2011 and September 30, 2012, respectively, has been recorded to offset net deferred tax assets as the Company is unable to conclude that it is more likely than not that such deferred tax assets will be realized.
At December 31, 2011, the Company had federal tax net operating loss carryforwards of $67.9 million and state tax net operating loss carryforwards of $56.8 million. The federal and state tax net operating loss carryforwards will begin to expire in 2020 and 2012, respectively. At December 31, 2011, the Company had federal research and development credit carryforwards of $1.0 million and state research credit carryforwards of $1.3 million. The
F-33
Table of Contents
federal research and development credit carryforwards begins to expire in 2022. The state research credit carryforwards do not expire. At September 30, 2012, the Company had federal tax net operating loss carryforwards of $66.1 million and state tax net operating loss carryforwards of $55.2 million. The federal and state tax net operating loss carryforwards will begin to expire in 2020 and 2012, respectively. At September 30, 2012, the Company had federal research and development credit carryforwards of $1.1 million and state research credit carryforwards of $1.3 million. The federal research and development credit carryforwards begins to expire in 2022. The state research credit carryforwards do not expire.
The Company recognizes uncertain tax positions in accordance with ASC 740, Income Taxes. The Company adopted the uncertain tax position standard on January 1, 2008. As of the date of adoption, the Company had no unrecognized tax benefits. The adoption did not result in an adjustment to accumulated deficit. The Company had no unrecognized tax benefits at September 30, 2012. The Company will recognize interest and penalties related to unrecognized tax benefits as a component of income tax expense. The Company recognized no interest or penalties upon the adoption of the standard or at September 30, 2012. The Company does not expect any significant increases or decreases to its unrecognized tax benefits within 12 months of this reporting date.
The Company is subject to U.S. federal and state income taxes. The Company is no longer subject to U.S. federal or state income tax examinations for years ended before December 31, 2007 and December 31, 2006, respectively. However, to the extent allowed by law, the tax authorities may have the right to examine prior periods where net operating losses or tax credits were granted.
Deferred tax assets pertaining to windfall tax benefits on exercise of share awards and the corresponding credit to additional paid-in-capital are recorded if the related tax deduction reduces tax payable and the Company has elected the “with and without” approach regarding windfall tax benefits.
Utilization of net operating loss (NOL) carryforwards, credit carryforwards, and certain deductions may be subject to substantial annual limitation due to ownership change limitations provided by the Internal Revenue Code of 1986, as amended, and similar state provisions. The tax benefits related to future utilization of federal and state net operating loss carryforwards, credit carryforwards, and other deferred tax assets may be limited or lost if cumulative changes in ownership exceeds 50% within any three-year period. The Company has not completed a study to assess whether an ownership change has occurred or whether there have been multiple ownership changes since the Company’s formation due to the complexity and cost associated with such a study, and the fact that there may be additional such ownership changes in the future. Such a limitation may occur as a result of a change in ownership upon the planned initial public offering. If the Company has experienced an ownership change at any time since its formation, utilization of the NOL or credit carryforwards to offset future taxable income and taxes, respectively, would be subject to an annual limitation under Section 382 of the Code, which is determined by first multiplying the value of the Company’s stock at the time of the ownership change by the applicable long-term, tax-exempt rate, and then could be subject to additional adjustments, as required. Any limitation may result in expiration of all or a portion of the NOL or credit carryforwards before utilization. Until a study is completed and any limitation known, no amounts of domestic NOL or credit carryforwards are being considered as an uncertain tax position or disclosed as unrecognized tax benefits under FIN 48 since no benefits have been realized to date.
11. Employee Savings Plan
The Company maintains a defined contribution 401(k) plan available to eligible employees. Employee contributions are voluntary and are determined on an individual basis, limited to the maximum amount allowable under federal tax regulations. The Company, at its discretion, may make certain contributions to the 401(k) plan. No contributions were approved or funded by the Company through September 30, 2012.
12. Subsequent events (unaudited)
The Company evaluated subsequent events through January 22, 2013, the date of the issuance of these financial statements. In July 2012 the Company entered into a new lease amendment for the Company’s facilities. This
F-34
Table of Contents
amendment extended the lease until 2029 and increased lease payments. The amendment also required that the past due rent of approximately $1.1 million be paid by November 1, 2012. The Company went into default under the terms of the lease of its corporate headquarters in November 2012. In December 2012, the Company and its landlord entered into a new lease amendment pursuant to which the Company issued the landlord 29,700 shares of the Company’s common stock in exchange for continued forbearance by the landlord. In addition to this issuance, the Company must remit its current month lease payment each month in accordance with the terms of the lease. The terms of the lease amendment require the Company to remit all past due lease payments, property tax payments, and associated penalties not later than February 4, 2013. If the Company does not complete the offering referred to in this prospectus prior to February 4, 2013, it will be unable to satisfy this payment obligation, the Company will be in default under the lease, and the landlord will have the right to elect any or all of the remedies available to it under the lease and applicable law, including the termination of the lease and the eviction of the Company from the Vista, CA facility.
In November 2012, the holders of $7.5 million in aggregate principal amount of and accrued interest on the Company’s outstanding subordinated promissory notes with rates of interest ranging from six percent to 12% that were then past due or scheduled to come due in the immediate future surrendered their right to payment of those promissory notes in exchange for an aggregate of 4,287,084 shares of the Company’s Series NC Convertible Preferred Stock. In connection with this surrender and issuance, these noteholders surrendered warrants for the purchase of an aggregate of 637,812 shares of the Company’s common stock with exercise prices ranging from $5.24 per share to $15.15 per share in exchange for warrants of substantially like tenor for the purchase of an aggregate of 637,812 shares of the Company’s common stock with exercise prices of $4.55 per share. Also in November 2012, the holders of $14.4 million in aggregate principal amount of and accrued interest on the Company’s outstanding subordinated promissory notes with rates of interest ranging from six percent to 13% that were then past due or scheduled to come due in the immediate future surrendered their right to payment of those promissory notes in exchange for new promissory notes in aggregate principal amount of $14.4 million with eight and one-half percent rates of interest and November 2015 maturity dates. In connection with this surrender and issuance, these noteholders surrendered warrants for the purchase of an aggregate of 1,156,013 shares of the Company’s common stock with exercise prices ranging from $5.24 per share to $15.15 per share in exchange for warrants of substantially like tenor for the purchase of an aggregate of 1,734,020 shares of the Company’s common stock with exercise prices of $5.30 per share that are exercisable through November 2017. As of November 27, 2012, after giving effect to these actions, the Company had outstanding total indebtedness and accrued interest under promissory notes of approximately $17.4 million, of which approximately $2.9 million was past due or scheduled to come due in the immediate future, and outstanding warrants to purchase an aggregate of 3,372,711 shares of the Company’s common stock that are exercisable through November 2017.
On December 28, 2012, Tregale Group Ltd, the holder of our approximately $2.2 million in principal amount under promissory notes that had been in default beginning in the first quarter of 2012, filed a request for judicial intervention and motion for summary judgment in lieu of complaint, demanding payment of the principal and interest outstanding under its promissory notes as well as reimbursement of certain legal and other expenses. See “Business—Legal Proceedings” elsewhere in this prospectus. In January 2013, the Company issued and sold a subordinated promissory note in the amount of $2.4 million to one of its existing investors, who is a holder of more than 5% of the Company’s capital stock. This promissory note has a maturity date of March 31, 2013, an annual interest rate of 8.5%, and may be prepaid at any time without premium or penalty. The Company used the proceeds of this sale to retire the outstanding principal and interest owed to Tregale Group Ltd under its outstanding promissory notes, and is negotiating with Tregale Group Ltd with respect to the reimbursement of its legal and other expenses, and the dismissal of the lawsuit.
F-35
Table of Contents
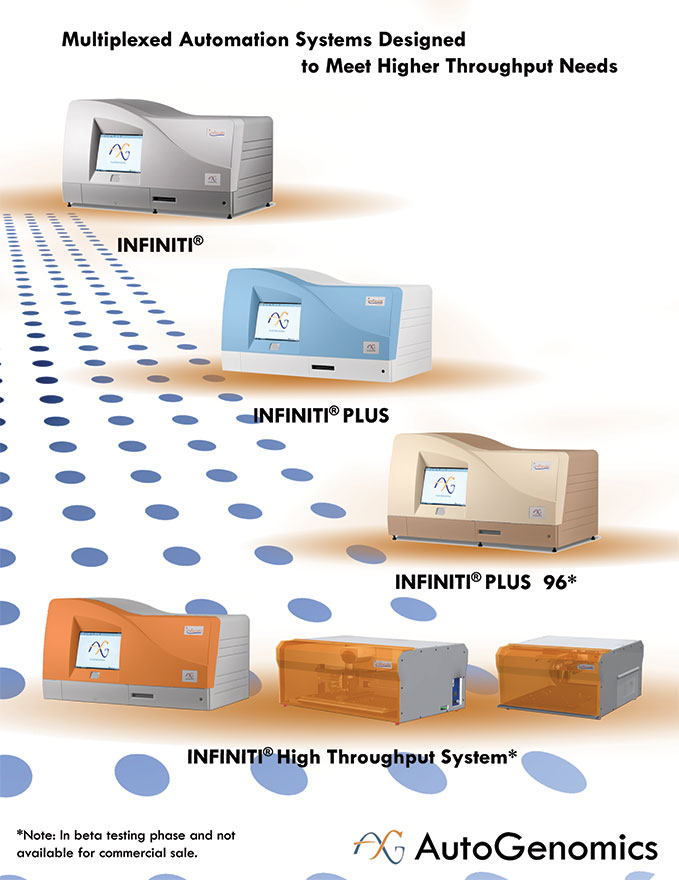
Table of Contents
6,000,000 Shares

Common Stock
PROSPECTUS
Leerink Swann
| Stephens Inc. | Mizuho Securities |
Cantor Fitzgerald & Co.
Table of Contents
Part II
Information Not Required in Prospectus
Item 13. Other expenses of issuance and distribution.
The table below lists various expenses, other than underwriting discounts and commissions, we expect to incur in connection with the sale and distribution of the securities being registered hereby. All the expenses are estimates, except the Securities and Exchange Commission registration fee, the FINRA filing fee and the NASDAQ listing fee.
| Type |
Amount | |||
| Securities and Exchange Commission Registration Fee |
$ | 8,936 | ||
| FINRA Filing Fee |
$ | 10,250 | ||
| NASDAQ Fee |
$ | 150,000 | ||
| Legal Fees and Expenses |
$ | 1,250,000 | ||
| Accounting Fees and Expenses |
$ | 332,000 | ||
| Printing and Engraving Expenses |
$ | 385,000 | ||
| Transfer Agent and Registrar Fees |
$ | 7,500 | ||
| Miscellaneous Expenses |
$ | 6,314 | ||
|
|
|
|||
| Total |
$ | 2,150,000 | ||
|
|
|
|||
Item 14. Indemnification of Directors and Officers.
Section 102 of the Delaware General Corporation Law, or DGCL, as amended, allows a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except where the director breached the duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our certificate of incorporation eliminates this liability.
Section 145 of the DGCL provides for the indemnification of officers, directors and other corporate agents in terms sufficiently broad to indemnify such persons under circumstances for liabilities (including reimbursement for expenses incurred) arising under the Securities Act. Our certificate of incorporation and bylaws provide for indemnification of our officers, directors, employees and agents to the extent and under the circumstances permitted under the DGCL.
The Underwriting Agreement (Exhibit 1.1) provides for indemnification by the underwriters of us, our directors and officers, and by us of the underwriters, for some liabilities arising under the Securities Act, and affords some rights of contributions with respect thereto.
We have executed indemnification agreements with each of our directors and each of our executive officers. These agreements provide indemnification to our directors and executive officers under certain circumstances for acts or omissions that may be sufficiently broad to indemnify such persons for liabilities arising under the Securities Act.
II-1
Table of Contents
Item 15. Recent Sales of Unregistered Securities.
Since June 30, 2009, we have issued the following securities that were not registered under the Securities Act of 1933, as amended:
(a) Stock Option Grants
From January 1, 2009 through December 31, 2012, we granted stock options to our employees, directors and consultants pursuant to which the optionees may purchase up to an aggregate of 1,250,673 shares of our common stock at a weighted average exercise price of $6.00 per share. Of the options we granted during this period, options to purchase a total of 157,702 shares have been forfeited or cancelled. In January 2013, we granted stock options to our employees, directors and consultants pursuant to which the optionees may purchase up to an aggregate of 232,617 shares of our common stock at an exercise price of $9.85 per share. The sale and issuance of the stock options were exempt from registration under Section 4(2) of the Securities Act or Rule 701 under the Securities Act.
(b) Issuances of Common Stock
From January 1, 2009 through December 31, 2012, we issued and sold 246,480 shares of our common stock to our employees, directors and consultants upon the exercise of stock options at a weighted average exercise price of $1.14 per share for an aggregate exercise price equal to approximately $278,795. In January 2013 we issued and sold 11,220 shares of common stock to employees upon the exercise of stock options at an exercise price of $0.38 per share. These underlying stock options were granted between December 2000 and August 2011 pursuant to our equity incentive compensation plans and agreements.
In August 2010 we issued and sold 19,800 shares of our common stock to one of our board members at an exercise price of $1.52 per share, for an aggregate exercise price of $30,000, pursuant to the exercise by such board member of a then outstanding warrant to purchase such shares that was issued in August 2005.
In December 2012, we issued 29,700 shares of our common stock to the landlord for our Vista, CA facility in connection with an amendment and forbearance agreement that we entered into with the landlord. This amendment and forbearance agreement provides for an extension of the period of time in which we have to satisfy our past due amounts owed to our landlord, which totaled $1.1 million as of the date of the agreement.
No underwriters were involved in the foregoing sales of securities. The sale and issuance of the securities described in this Item 15(b) above were exempt from registration under Section 4(2) of the Securities Act or Rule 701 under the Securities Act.
(c) Issuances of Preferred Stock and related Warrants
In July 2010, we issued 166,329 shares of our Series B Convertible Preferred Stock to three existing holders at an exercise price of $2.75 per share, for an aggregate exercise price of $457,000, pursuant to the exercise by such holders of then outstanding warrants to purchase such shares that were originally issued in connection with the sale and issuance of our Series B Convertible Preferred Stock in 2005.
From December 2010 through July 2012, we issued 6,591 shares of our Series C Convertible Preferred Stock to four existing holders at a weighted average exercise price of $3.34 per share for an aggregate exercise price of $22,000, pursuant to the exercise by such holders of then outstanding warrants to purchase such shares that were originally issued in connection with the sale and issuance of our Series C Convertible Preferred Stock in 2007.
In May, July and August 2011, warrants to purchase 59,072 shares of our Series C Convertible Preferred Stock at an exercise price of $3.30 per share were due to expire. These warrants were originally issued in connection with the sale and issuance of our Series C Convertible Preferred Stock in 2007. We offered to extend the expiration date of these warrants to May 2012, July 2012 and August 2012, respectively, in exchange for an increase in the exercise price of such warrants from $3.30 per share to $3.80 per share. Holders of warrants for the purchase of 51,800 of these warrants accepted the offer. The remaining warrants, for the purchase of 7,272 shares of our Series C Convertible Preferred Stock, expired in accordance with their respective terms.
In July and August 2011, warrants to purchase 348,910 shares of our Series C Convertible Preferred Stock at an exercise price of $2.75 per share were due to expire. These warrants were originally issued in connection with the sale and issuance of our Series C Convertible Preferred Stock in 2007. We offered to extend the expiration date of these warrants to July 2013 and August 2013, respectively, in exchange for an increase in the exercise price of such warrants from $2.75 per share to $3.25 per share. Holders of warrants for the purchase of 334,364 shares of our
II-2
Table of Contents
Series C Convertible Preferred Stock accepted the offer. The remaining warrants, for the purchase of 14,546 shares of our Series C Convertible Preferred Stock, expired in accordance with their respective terms.
In November 2011, we issued and sold an aggregate of 685,555 shares of our Series E Convertible Preferred Stock to eight investors at a purchase price of $2.75 per share, for an aggregate purchase price of $1.9 million. As part of this offering, we also issued to these investors five-year warrants for the purchase of 339,351 shares of our common stock at an exercise price of $8.33 per share.
In July and August 2012, warrants to purchase 42,300 shares of our Series C Convertible Preferred Stock at an exercise price of $3.80 per share were due to expire. These warrants were originally issued in connection with the sale and issuance of our Series C Convertible Preferred Stock in 2007 (and, as described above, were amended in 2011). We offered to extend the expiration date of these warrants to July 2013 and August 2013, respectively, in exchange for an increase in the exercise price of such warrants from $3.80 per share to $4.05 per share. Holders of warrants for the purchase of 34,064 of these warrants accepted the offer. The remaining warrants, for the purchase of 8,236 shares of our Series C Convertible Preferred Stock, expired in accordance with their respective terms.
In July and August 2012, warrants to purchase 342,000 shares of our Series C Convertible Preferred Stock at an exercise price of $3.25 per share were due to expire. These warrants were originally issued in connection with the sale and issuance of our Series C Convertible Preferred Stock in 2007 (and, as described above, were amended in 2011). We offered to extend the expiration date of these warrants to July 2013 and August 2013, respectively, in exchange for an increase in the exercise price of such warrants from $3.25 per share to $3.50 per share. Holders of warrants for the purchase of 308,727 of these warrants accepted the offer. The remaining warrants, for the purchase of 33,273 shares of our Series C Convertible Preferred Stock, expired in accordance with their respective terms.
In August 2012, we issued and sold 47,000 shares of our Series E Convertible Preferred Stock at a purchase price of $2.75 per share, for an aggregate purchase price in the form of surrender of accounts payable of $129,250. In connection with this sale, we also issued to this investor a five year warrant for the purchase of 23,265 shares of our common stock at an exercise price of $8.33 per share.
In November 2012, the applicable holders of $7.5 million in aggregate principal amount of and accrued interest on our outstanding subordinated promissory notes with rates of interest ranging from six percent to 12% that were then past due or scheduled to come due in the immediate future surrendered their right to payment of those promissory notes in exchange for an aggregate of 4,290,136 shares of our Series NC Convertible Preferred Stock. In connection with this surrender and issuance, these noteholders surrendered warrants for the purchase of an aggregate of 637,813 shares of our common stock with exercise prices ranging from $5.24 per share to $15.15 per share in exchange for warrants of a like tenor for the purchase of an aggregate of 637,813 shares of our common stock with exercise prices of $4.55 per share that are exercisable through November 2017.
No underwriters were involved in the foregoing sales of securities. The securities described above were sold and issued to accredited investors in reliance upon the exemption from the registration requirements of the Securities Act, as set forth in Section 3(a)(9) and Section 4(2) of the Securities Act and Rule 506 of Regulation D under the Securities Act.
(d) Issuances of Subordinated Promissory Notes and related Warrants
From March 2009 through December 2009, we issued $5,035,000 aggregate principal amount of subordinated promissory notes in a private placement to certain accredited investors. The notes bear a weighted average annual interest rate of 6.2%, with the principal and accrued interest balances due two years after the date of issuance. We may elect to prepay the notes at any time without penalty. In the private placement, we also issued warrants to purchase 471,880 shares of our common stock with exercise prices ranging from $12.12 to $21.21 per share. The warrants are now fully exercisable and have a term of five years from their date of issue.
II-3
Table of Contents
In July 2009 and August 2009, we issued $5,071,000 in subordinated promissory notes in a private placement to certain accredited investors. Of this amount, $1,571,000 was used to repay the principal and accrued interest on a subordinated promissory note we issued in September 2008. The new notes bear interest at the rate of 13% per annum, with the principal and accrued interest balances due two years after the date of issuance. We may elect to prepay the notes at any time without penalty. In the private placement, we also issued warrants to purchase 468,600 shares of our common stock with an exercise price of $12.12 per share. The warrants are now fully exercisable and have a term of five years from their date of issue. In connection with this issuance of subordinated promissory notes, warrants to purchase 70,290 shares of our common stock with an exercise price of $21.21 per share associated with the subordinated promissory note issued in September 2008 and repaid in July 2009 were cancelled and replaced by warrants to purchase 140,580 shares of our common stock with an exercise price of $12.12 per share.
In February and March 2010, we issued $525,000 aggregate principal amount of subordinated promissory notes to certain accredited investors. The notes bear interest at the rate of 6.0% per annum, with the principal and accrued interest balances generally due two years after the date of issuance, depending on the provisions of the note. We may elect to prepay the notes at any time without penalty. In connection with this offering, we also issued warrants to purchase 49,203 shares of our common stock with an exercise price of $12.12 per share. The warrants are now fully exercisable and have a term of five years from their date of issue.
In March 2010, we issued $5,250,000 aggregate principal amount of subordinated notes in a private placement to certain accredited investors. The notes bear interest at the rate of 6% per annum, with the principal and accrued interest balances due two years after the date of issuance. We may elect to prepay the notes at any time without penalty. In the private placement, we also issued warrants to purchase 519,750 shares of our common stock with an exercise price of $15.15 per share. The warrants are now fully exercisable and have a term of five years from their date of issue.
In April 2010, we and the applicable note holder amended a subordinated promissory note with a principal amount of $1,000,000 set to mature in August 2010 by extending the maturity date to April 2011. In connection with this amendment, we cancelled a warrant to purchase 93,720 shares of our common stock at an exercise price of $21.21 per share held by the note holder which was to expire five years after the issuance date, and reissued a warrant to purchase 93,720 shares of our common stock at an exercise price of $15.15 per share to the note holder which will expire six and one-half years after the original issuance date. Also in April 2010, we and the applicable note holders amended certain subordinated promissory notes with an aggregate principal amount of $5,040,000 set to mature between August 2010 and December 2011 by extending the maturity dates to April 2012. These notes are now due and owing. In connection with these amendments, we cancelled warrants to purchase 472,349 shares of our common stock at an exercise price of $21.21 per share held by the note holders which were to expire five years after the issuance date and reissued warrants to purchase 472,349 shares of our common stock at an exercise price of $15.15 per share to the note holders which will expire six and one-half years after the original issuance date.
In November 2010, we and the applicable note holder amended certain promissory notes with an aggregate principal amount of $2,500,000 set to mature in April 2010, February 2011, May 2011 and November 2011, by extending the maturity dates to December 2012. In connection with these amendments, we cancelled warrants to purchase 234,300 shares of our common stock at exercise prices of $12.12, $15.15 and $21.21 per share held by the note holder and reissued warrants to purchase 234,300 shares of our common stock at an exercise price of $9.09 per share to the note holder which will expire in January 2016.
From December 2010 through May 2011, we issued $1,300,000 aggregate principal amount of promissory notes to certain accredited investors. The notes bear interest at the rate of 7.0% per annum, and generally mature in December 2012. In connection with the issuance of these notes, we also issued warrants to purchase 128,700 shares of our common stock with an exercise price of $9.09 per share. The warrants have a term of five years from their date of issue.
II-4
Table of Contents
In June 2011, we and the applicable note holder amended a certain promissory note with an aggregate principal amount of $2,000,000 set to mature in July 2011, by extending the maturity date to December 2012. In connection with this amendment, we cancelled a warrant to purchase 187,440 shares of our common stock at an exercise price of $12.12 per share held by the note holder and reissued a warrant to purchase 187,440 shares of our common stock at an exercise price of $9.09 per share to the note holder which will expire in August 2014.
In August 2011, we and the applicable note holder exchanged promissory notes with an aggregate principal amount of $3,071,000 that were originally set to mature in July 2011 for promissory notes with an aggregate principal amount of $3,900,000, set to expire December 2011 and February 2012. In connection with this exchange, we cancelled warrants to purchase 281,160 shares of our common stock at an exercise price of $12.12 per share held by the note holder and reissued a warrant to purchase 429,000 shares of our common stock at an exercise price of $9.09 to the note holder which will expire in August 2016.
In October and December 2011, we and the applicable note holders amended promissory notes with an aggregate principal amount of $595,000 that were set to expire in November and December 2011, by extending the maturity dates to dates in 2012. In connection with this amendment, we amended warrants to purchase 55,764 shares of our common stock by reducing the exercise price from $12.12 per share to $9.09 per share.
From November 2011 through January 2012, we issued $695,000 aggregate principal amount of promissory notes to certain accredited investors. The notes bear interest at the rate of 12.0% per annum, and matured in April 2012. In connection with this offering, we issued warrants to purchase 114,675 shares of our common stock at an exercise price of $5.24 per share to the note holders which will expire five years from their date of issue.
In February 2012, we and the applicable note holder amended a certain promissory note with a principal amount of $250,000 set to mature in April 2012, by extending the maturity date to June 2012. In connection with this amendment, we amended a warrant to purchase 23,430 shares of our common stock at an exercise price of $15.15 per share held by the note holder, by reducing the exercise price to $9.09 per share and extending the term of the warrant until June 2017.
In February 2012, we and the applicable note holders amended certain promissory notes with an aggregate principal amount of $400,000 set to mature in February 2012, by extending the maturity dates to April 2012. In connection with these amendments, we amended warrants to purchase 37,488 shares of our common stock at an exercise price of $12.12 per share held by the note holder, by reducing the exercise price to $9.09 per share and extending the term of the warrant until April 2017.
In March 2012, we issued $1,150,000 aggregate principal amount of promissory notes to certain accredited investors. The notes bear interest at the rate of 10.0% per annum, with the principal and accrued interest balances generally due in June 2012. In connection with the issuance of these notes, we issued warrants to purchase 113,850 shares of our common stock with an exercise price of $5.24 per share which will expire five years from their date of issue.
In November 2012, the applicable holders of $14.4 million in aggregate principal amount of and accrued interest on our outstanding subordinated promissory notes with rates of interest ranging from six percent to 13% that were then past due or scheduled to come due in the immediate future surrendered their right to payment of those promissory notes in exchange for new promissory notes in aggregate principal amount of $14.4 million with eight and one-half percent rates of interest and November 2015 maturity dates. In connection with this surrender and issuance, these noteholders surrendered warrants for the purchase of an aggregate of 1,156,013 shares of our common stock with exercise prices ranging from $5.24 per share to $15.15 per share in exchange for warrants of a like tenor for the purchase of an aggregate of 1,734,020 shares of our common stock with exercise prices of $5.30 per share that are exerciseable through November 2017.
No underwriters were involved in the foregoing sales of securities. The sale and issuance of the securities described in this Item 15(d) above were exempt from registration under Section 3(a)(9) and Section 4(2) of the Securities Act or Rule 506 of Regulation D under the Securities Act.
II-5
Table of Contents
(e) Issuances of Other Warrants
In October 2010, we issued a consultant a warrant to purchase 9,900 shares of our common stock at an exercise price of $6.09 per share in connection with services provided to us. The warrant is set to expire in September 2015.
In August 2011, we issued to certain non-employee members of our board of directors warrants to purchase an aggregate of 33,000 shares of our common stock at an exercise price of $5.24 per share. These warrants will expire five years from their date of issue.
In October 2011, we issued a consultant a warrant to purchase 24,750 shares of our common stock at an exercise price of $0.76 per share in connection with services provided to us. The warrant is set to expire in October 2014.
In May 2012, we issued to certain non-employee members of our board of directors warrants to purchase an aggregate of 9,900 shares of our common stock at an exercise price of $5.24 per share. These warrants will expire five years from their date of issue.
No underwriters were involved in the foregoing sales of securities. The securities described in this Item 15(c) above were sold and issued to accredited investors in reliance upon the exemption from the registration requirements of the Securities Act, as set forth in Section 4(2) of the Securities Act or Rule 506 of Regulation D under the Securities Act.
Item 16. Exhibits and Financial Statement Schedules.
See “Exhibit Index.”
Item 17. Undertakings.
The undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933, as amended, and will be governed by the final adjudication of such issue.
The registrant hereby undertakes that:
| (a) | For purposes of determining any liability under the Securities Act of 1933, as amended, the information omitted from a form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in the form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933, as amended, shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (b) | For the purpose of determining any liability under the Securities Act of 1933, as amended, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-6
Table of Contents
Signatures
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Vista, State of California, on this day of January 22, 2013.
| AUTOGENOMICS, INC. | ||
| By: |
/s/ Fareed Kureshy | |
| Fareed Kureshy | ||
| Chairman, President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities indicated below on the dates indicated below.
| Name |
Title |
Date | ||
| /s/ Fareed Kureshy Fareed Kureshy |
Chairman, President, Chief Executive Officer and Director (principal executive officer) | January 22, 2013 | ||
| /s/ Thomas V. Hennessey, Jr. Thomas V. Hennessey, Jr. |
Chief Operating Officer, Chief Financial Officer and Director (principal financial officer and principal accounting officer) | January 22, 2013 | ||
| /s/ Stephen Allison* Stephen Allison |
Director | January 22, 2013 | ||
| /s/ Charles Birmingham* Charles Birmingham |
Director | January 22, 2013 | ||
| /s/ Laurence M. Demers, Ph.D.* Laurence M. Demers, Ph.D. |
Director | January 22, 2013 | ||
| /s/ Donald E. Pogorzelski* Donald E. Pogorzelski |
Director | January 22, 2013 | ||
| /s/ Peter Wilding, Ph.D.* Peter Wilding, Ph.D. |
Director |
January 22, 2013 | ||
| /s/ Eugene J. Zurlo* Eugene J. Zurlo |
Director | January 22, 2013 | ||
| *By |
/s/ Fareed Kureshy | |
| Fareed Kureshy | ||
| Attorney-in-fact |
II-7
Table of Contents
Exhibit Index
| Exhibit |
Exhibit Description | |
| 1.1 | Form of Underwriting Agreement | |
| 3.1* | Certificate of Incorporation of the Registrant | |
| 3.2* | Certificate of Designation of Series E Preferred Stock of the Registrant | |
| 3.3* | Amended and Restated Bylaws of the Registrant | |
| 3.4* | Certificate of Designation of Series NC Preferred Stock of the Registrant | |
| 3.5 | Certificate of Amendment of Certificate of Incorporation of the Registrant | |
| 4.1** | Form of Common Stock Certificate | |
| 4.2* | Subordinated Promissory Note (No. 200907-001), issued by the Registrant to Tregale Group Ltd in the principal amount of $1,500,000 | |
| 4.3* | Subordinated Promissory Note (No. 200907-002), issued by the Registrant to Tregale Group Ltd in the principal amount of $1,571,014 | |
| 4.4* | Subordinated Promissory Note (No. 200908-001), issued by the Registrant to Scott GRAT No. 5 in the principal amount of $2,000,000 | |
| 4.5* | Agreement to Furnish Debt Instruments | |
| 5.1 | Opinion of Bingham McCutchen LLP, related to the shares of common stock being sold in the initial public offering. | |
| 10.1* | Indemnification Agreement dated April 28, 2010, by and between the Registrant and Ram Vairavan (all other Indemnification Agreements, which are substantially identical in all material respects, except as to the parties thereto and the dates of execution, are omitted pursuant to Instruction 2 to Item 601 of Regulation S-K) | |
| 10.2*f | 2000 Equity Incentive Plan and forms of agreements relating thereto | |
| 10.3*f | Form of Common Stock Option Agreement used for certain grants outside of the 2000 Equity Incentive Plan | |
| 10.4*f | 2008 Equity Incentive Award Plan and form of stock option agreement relating thereto | |
| 10.5*f | 2008 Employee Stock Purchase Plan | |
| 10.6* | Director Compensation Policy | |
| 10.7* | Registration Rights Agreement dated July 19, 2006, by and among the Registrant, MESA Development Inc. of Nevada and the purchasers of the Registrant’s Series C Convertible Preferred Stock | |
| 10.8* | License Agreement dated April 14, 2006, between the Registrant and Mayo Foundation for Medical Education and Research | |
| 10.9* | Nonexclusive Patent License Agreement dated April 14, 2006, between the Registrant and the Mayo Foundation for Medical Education and Research | |
| 10.10* | Standard Industrial/Commercial Single Tenant Lease dated February 12, 2009, by and between PCCP DJ Ortho, LLC (“PCCP”) and the Registrant (the “Vista Lease”) | |
| 10.11* | First Amendment to Vista Lease dated August 6, 2009 by and between PCCP and the Registrant; Second Amendment to Vista Lease dated August 13, 2010 by and between PCCP and the Registrant; Third Amendment to Vista Lease dated March 14, 2011 by and between PCCP and the Registrant; Fourth Amendment to Vista Lease dated May 20, 2011 by and between PCCP and the Registrant; Fifth Amendment to Vista Lease dated July 26, 2011 by and between PCCP and the Registrant; Sixth Amendment to Vista Lease dated December 1, 2011 by and between PCCP and the Registrant; Seventh Amendment to Vista Lease dated March 30, 2012 by and between PCCP and the Registrant; and Eighth Amendment to Vista Lease dated July 1, 2012 by and between PCCP and the Registrant | |
Table of Contents
| Exhibit |
Exhibit Description | |
| 10.12* | Subordinated Promissory Note (No. 200809-010), issued by the Registrant to William Davidson in the principal amount of $100,000 (all other Subordinated Promissory Notes which are substantially identical in all material respects to this Subordinated Promissory Note, except as to the parties thereto, dates of issuance, principal amounts, interest rates and maturity dates, are omitted pursuant to Instruction 2 to Item 601 of Regulation S-K) | |
| 10.13* | Subordinated Promissory Note (No. 201003-001), issued by the Registrant to Elissa Kenna Trust in the principal amount of $500,000 (all other Subordinated Promissory Notes which are substantially identical in all material respects to this Subordinated Promissory Note, except as to the parties thereto, dates of issuance, principal amounts and maturity dates, are omitted pursuant to Instruction 2 of Item 601 of Regulation S-K) | |
| 10.14* | Warrant to Purchase Common Stock (No. CS-05), dated March 21, 2007, issued to AR Properties (all other Warrants to Purchase Common Stock which are substantially identical in all material respects to this Warrant to Purchase Common Stock, except as to the parties thereto, dates of issuance, number of warrant shares, exercise prices, vesting periods and expiration dates, are omitted pursuant to Instruction 2 of Item 601 of Regulation S-K) | |
| 10.15* | Warrant to Purchase Common Stock (No. 201004-010), dated April 15, 2010, issued to Terrance A Noonan (all other Warrants to Purchase Common Stock which are substantially identical in all material respects to this Warrant to Purchase Common Stock, except as to the parties thereto, dates of issuance, number of warrant shares, exercise prices and expiration dates, are omitted pursuant to Instruction 2 of Item 601 of Regulation S-K) | |
| 10.16* | Warrant to Purchase Common Stock (No. 200908-001), dated August 19, 2009, issued to Scott GRAT No. 5 | |
| 10.17* | Warrant to Purchase Common Stock (No. 201003-001), dated March 9, 2010, issued to Elissa Kenna Trust (all other Warrants to Purchase Common Stock which are substantially identical in all material respects to this Warrant to Purchase Common Stock, except as to the parties thereto, dates of issuance, number of warrant shares and expiration dates, are omitted pursuant to Instruction 2 of Item 601 of Regulation S-K) | |
| 10.18* | Warrant to Purchase Preferred Stock (No. C-01), dated December 30, 2005, issued to The Rue Family Trust dtd 2-10-89, Michael M. Rue, Trustee (all other Warrants to Purchase Preferred stock which are substantially identical in all material respects to this Warrant to Purchase Preferred Stock, except as to the parties thereto, dates of issuance, number of warrant shares and expiration dates, are omitted pursuant to Instruction 2 of Item 601 of Regulation S-K) | |
| 10.19* | Warrant to Purchase Series C Convertible Preferred Stock (No. C2-01), dated July 19, 2006, issued to Robert A. Levin (all other Warrants to Purchase Series C Convertible Preferred Stock which are substantially identical in all material respects to this Warrant to Purchase Series C Convertible Preferred Stock, except as to the parties thereto, dates of issuance, number of warrant shares and expiration dates, are omitted pursuant to Instruction 2 of Item 601 of Regulation S-K) | |
| 10.20* | Warrant to Purchase Common Stock (No. E-01), dated November 29, 2011, issued to Roland F. Smith (all other Warrants to Purchase Common Stock that are substantially identical in all material respects to this Warrant to Purchase Common Stock, except as to number of warrant shares, are omitted pursuant to Instruction 2 of Item 601 of Regulation S-K) | |
| 10.21* | 8-1/2% Subordinated Note (No. 201211-001), issued by the Registrant to Argus Reinsurance, Ltd in the principal amount of $312,671.23 (all other 8-1/2% Subordinated Notes which are substantially identical in all material respects to this 8-1/2% Subordinated Note, except as to the parties thereto and principal amounts, are omitted pursuant to Instruction 2 of Item 601 of Regulation S-K) | |
| 10.22* | Security Agreement by and between the Registrant and Citibank, N.A., acting through its Citibank Agency & Trust Division, as collateral agent for the holders of the New Notes (as defined therein) | |
Table of Contents
| Exhibit |
Exhibit Description | |
| 10.23* | Warrant to Purchase Common Stock (No. 201211-050) issued to AR Properties (all other Warrants to Purchase Common Stock which are substantially identical in all material respects to this Warrant to Purchase Common Stock, except as to the parties thereto, number of warrant shares and exercise prices, are omitted pursuant to Instruction 2 of Item 601 of Regulation S-K) | |
| 10.24 | Promissory Note (No. 201301-001), issued by the Registrant to A R Properties in the principal amount of $2,400,000 | |
| 10.25f | Employment Agreement dated January 3, 2013, by and between the Registrant and Fareed Kureshy | |
| 10.26 | Ninth Amendment to Vista Lease dated December 27, 2012 by and between PCCP and the Registrant and associated Issuance Letter of even date therewith | |
| 16.1* | Letter regarding change in certifying accountant | |
| 23.1 | Consent of Independent Registered Public Accounting Firm | |
| 23.2 | Consent of Bingham McCutchen LLP (included in Exhibit 5.1) | |
| 24.1* | Power of Attorney | |
| * | Previously filed. |
| ** | To be filed by amendment. |
| f | Indicates a management contract or compensatory plan or arrangement. |