Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Inspyr Therapeutics, Inc. | v332718_8k.htm |

Precise targeting of a potent, unique prodrug directly to tumors OTCBB: GNSZ JANUARY 2013

GENSPERA DEFINED Highly differentiated , novel therapeutic agent with a unique mechanism of action GNSZ | JANUARY 2013 PAGE 2 • Thapsigargin has potential for “complete kill” – active against both slowly and rapidly dividing cells and cancer stem cells • IP acquired from Johns Hopkins University – no milestones or royalties due • Lead drug candidate, G - 202, currently in Phase II clinical trials in prostate cancer and hepatocellular carcinoma • Interim Phase II data expected in 2 indications in Q3 2013
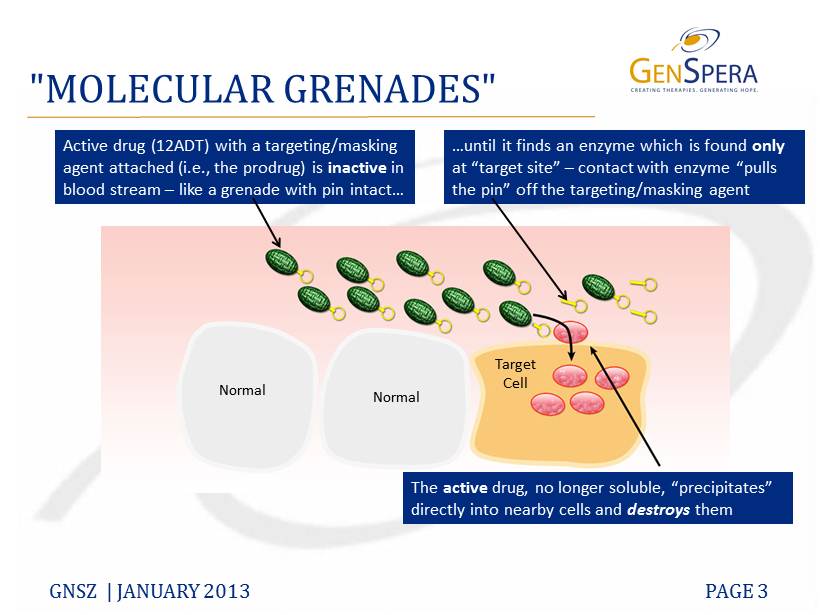
"MOLECULAR GRENADES" GNSZ | JANUARY 2013 PAGE 3 Active drug (12ADT) with a targeting/masking agent attached (i.e., the prodrug) is inactive in blood stream – like a grenade with pin intact… …until it finds an enzyme which is found only at “target site ” – contact with enzyme “pulls the pin” off the targeting/masking agent The active drug, no longer soluble, “precipitates” directly into nearby cells and destroys them Normal Normal Target Cell
G - 202 PHASE I RESULTS GNSZ | JANUARY 2013 PAGE 4 • Recommended dosing regimen: IV infusion, 40 mg/m 2 on Day 1, 66.8 mg/m 2 on Days 2 and 3 of 28 - day schedule with hydration and standard pre - meds • Side Effect Profile: Easily managed minimal side effects (fatigue, nausea on Day 1, rash). No apparent effect on liver, cardiovascular system or bone marrow.
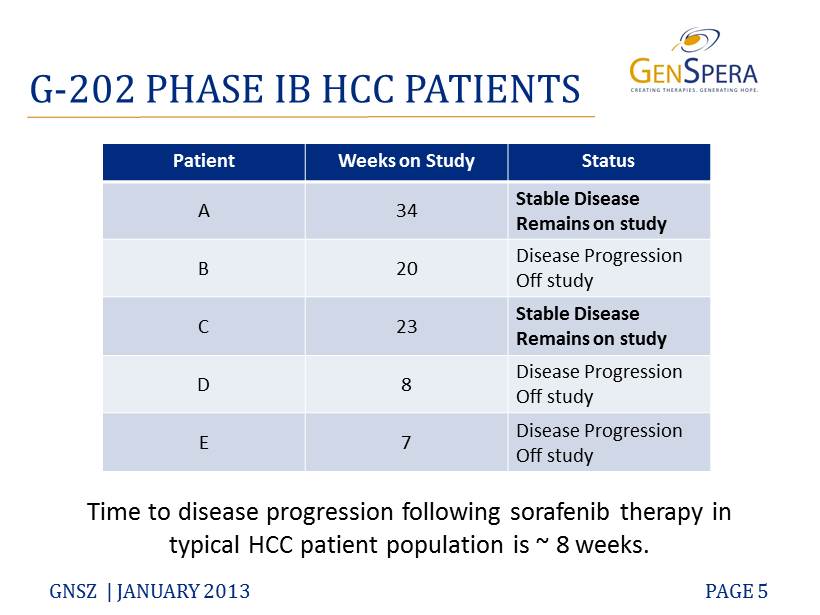
G - 202 PHASE IB HCC PATIENTS GNSZ | JANUARY 2013 PAGE 5 Time to disease progression following sorafenib therapy in typical HCC patient population is ~ 8 weeks. Patient Weeks on Study Status A 34 Stable Disease Remains on study B 20 Disease Progression Off study C 23 Stable Disease Remains on study D 8 Disease Progression Off study E 7 Disease Progression Off study

G - 202 PHASE II TRIALS GNSZ | JANUARY 2013 PAGE 6 • Hepatocellular Carcinoma • Population: Patients with progressive advanced hepatocellular cancer following sorafenib therapy • Number of Patients and Sites: 29 patients at 10 sites in Texas (relationship with CTNeT) • Prostate Cancer • Population: Patients with chemotherapy - naïve metastatic castrate - resistant prostate cancer • Number of Patients and Sites: 34 patients at 5 sites in the US and UK

STRATEGY & VALUE CREATION GNSZ | JANUARY 2013 PAGE 7 • Raised ~$ 18M since Nov 2007, majority from repeat investors • Trading symbol: OTCBB:GNSZ Financing History • ~22M common shares issued and outstanding • ~13M currently outstanding options and warrants • Management, inventors, and boards hold approximately 30% of issued shares Business Strategy and Value Creation • Operate with minimal infrastructure requirement • Develop drugs through Phase II clinical trials, then out - license, partner or sale in ~1 - 2 years • Individual drugs can be worth up to $1B • 2009 – J&J/Cougar and Astellas / Medivation • 2011 – Amgen/ Biovex Capital Structure
CORE DISTINCTIONS GNSZ | JANUARY 2013 PAGE 8 Highly differentiated , novel therapeutic agent with a unique mechanism of action • New approach to cancer therapy – not just different but intended to be better • Interim Phase II data expected in 2 indications in Q3 2013 • Out - license, partner or sale subsequent to Phase II • Focus is on rapidly achieving valuation milestones with minimal cost

CONTACT GNSZ | JANUARY 2013 PAGE 9 Craig A. Dionne, PhD President & CEO cdionne@genspera.com GenSpera, Inc. 2511 N Loop 1604 W, Suite 204 San Antonio, TX 78258 www.genspera.com Phone: (210) 479 - 8112 Fax: (210) 479 - 8113
