Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Fibrocell Science, Inc. | d462689d8k.htm |
Exhibit 99.1

| OneMedForum January 9, 2013 |

| 2 Forward Looking Statement This presentation includes statements that are "forward-looking statements." Forward-looking statements include, without limitation, our ability (i) to obtain medical reimbursement for new non-aesthetic indications, (ii) to timely commence our clinical programs in 2013 to treat patients with restrictive burn scars and vocal cord scars, (iii) to successfully genetically modify the fibroblast cell to treat patients who have collagen deficient diseases, (iv) to successful leverage our relationships with UCLA and MIT to develop new applications, and (v) to adopt technologies that will dramatically reduce manufacturing time, complexity, and labor costs for our cell therapies. While management has based any forward-looking statements contained in the presentation on its current expectations, the information on which such expectations were based may change. These forward-looking statements rely on a number of assumptions concerning future events and are subject to a number of risks, uncertainties, and other factors, many of which are outside of Fibrocell Science's control, that could cause actual results to materially differ from such statements. Such risks, uncertainties, and other factors include, but are not necessarily limited to, those set forth under the caption "Item 1A. Risk Factors" in Fibrocell Science's most recent Form 10-K filing, as updated in "Item 1A. Risk Factors" in Fibrocell Science's most recent Form 10-Q filing. In addition, Fibrocell Science operates in a highly competitive and rapidly changing environment, and new risks may arise. Accordingly, you should not place any reliance on forward-looking statements as a prediction of actual results. Fibrocell Science disclaims any intention to, and undertakes no obligation to, update or revise any forward-looking statement. You are also urged to carefully review and consider the various disclosures in Fibrocell Science's most recent annual report on Form 10-K, our most recent Form 10-Q as well as other public filings with the SEC since the filing of Fibrocell Science's most recent annual report. |

| Unlocking the Potential of the Autologous Fibroblast |

| Fibrocell - Uniquely Positioned in Regenerative Medicine & Cell Based Therapies The first and only cell based product in aesthetics laViv(r) - one of only 8 cell based products approved by the FDA laViv(r) - evaluated in over 1,000 patients in well controlled clinical trials Robust pipeline and clinical programs based on the autologous fibroblast cell Strong base of knowledge and expertise in cell based product development, manufacturing and seeking regulatory approvals |

| Role of the Fibroblast Cell Role of the Fibroblast Cell A fibroblast is a type of cell that synthesizes the extracellular matrix and collagen, the structural framework for animal tissues, and plays a critical role in wound healing Fibroblasts are the most common cells of connective tissue in animals Produces an array of growth factors and other cytokines to aid in cell signaling and wound repair |

| Dermal fibroblasts produce: Collagen type I Collagen type III Collagen type VII Elastin Hyaluronic acid (hyaluronin) Matrix metalloproteinases (MMPs) Dermal Fibroblasts Synthesize and Organize Extracellular Matrix Artist's interpretation |

| Fibroblast Location in Skin |
| Key Focus Areas Aesthetic Dermatology: Launched the first and only FDA approved, cell based therapy in aesthetics Developed a personalized topical cosmetic Markets where scars are unacceptable, value per cell is increased, and COGS is less of an issue: Restrictive Burn Scars (RBS) 45,000 people hospitalized each year with severe burns (1) Vocal Cord Scars (VCS) ~176,778 - 589,260 patients with vocal cord scarring (2) Pricing per RBS and VCS patient will be multiples of the aesthetic patient Reimbursement is likely for RBS and VCS Acne Scarring 20 million Americans have acne badly enough to cause scars (3) In end of Phase II discussions with the FDA and evaluating a path forward American Burn Association. www.ameriburn.org; Goodis. J and E.d. Schraga. Burns, thermal. eMedicine Journal Cohen, (2010); Dailey et al. (2007); Roy et al. (2005); Poels et al. (2003); Koufman and Isaacson (1991); Painter (1990); Bouchayer et al. (1985); Laguaite (1972) Acne Resource Center. www.acne-resource.org; Lead Programs |
| Preclinical Programs Rare and ultra rare markets where GM (genetically modified) fibroblasts may significantly improve patients well being Initial target is recessive dystrophic epidermolysis bullosa (RDEB) a rare devastating disease Epidermolysis bullosa is a group of genetic conditions that cause the skin to be very fragile and to blister easily Prevalence is 6.5 per million newborns (US) (1) Mutations in the COL7A1 gene cause all three major forms of dystrophic epidermolysis bullosa This gene provides instructions for making a protein that is used to assemble type VII collagen Type VII collagen plays an important role in strengthening and stabilizing the skin It is the main component of structures called anchoring fibrils, which anchor the epidermis to the dermis Currently at least 12 products for rare diseases have a wholesale cost of $200,000 plus (2) National Institutes of Health (2008) http:\\ghr.nlm.nih.gov/condition/dystrophic-epidermolysis-bullosa.Genetics Home Reference. (2)Herper, Matthew. "How A $440,000 Drug Is Turning Alexion Into Biotech's New Innovation Powerhouse." Forbes. 5 September 2012. http://www.forbes.com/sites/matthewherper/2012/09/05/how-a-440000-drug-is-turningalexion-into-biotechs-new-innovation-powerhouse |

| Aesthetics Cell Therapies for Niche Medical Indications (Scars) Cell Therapies for Rare and Ultra Rare Diseases Higher Value Per Cell 45,000 people hospitalized each year with severe burns(1) ~176,778 - 589,260 patients with vocal cord scarring (2) 20 million Americans have acne badly enough to cause scars (3) Pricing per patient will be multiples of the aesthetic patient Unlike LAVIV(r), reimbursement is expected. Currently there are no drugs approved for VCS, RBS, or acne scars. 7.6 million non-surgical cosmetic procedures in 2011 (4) Aesthetic Indication is a Beachhead for Higher Value Indications 10 Higher Value Per Cell Prevalence of RDEB is 6.5 per million newborns in the US (5) Currently at least 12 products for other rare diseases have a wholesale cost of $200,000 plus (6) Unlike LAVIV(r), reimbursement is expected. Currently there are no drugs approved for treatment of RDEB. www.ameriburn.org; Goodis. J and E.d. Schraga. Burns, thermal. eMedicine Journal Cohen, (2010); Dailey et al. (2007); Roy et al. (2005); Poels et al. (2003); Koufman and Isaacson (1991); Painter (1990); Bouchayer et al. (1985); Laguaite (1972) Acne Resource Center . www.acne-resource.org; American Society for Aesthetic Plastic Surgery. National Institutes of Health (2008) http:\\ghr.nlm.nih.gov/condition/dystrophic-epidermolysis-bullosa.Genetics Home Reference. (6)Herper, Matthew. "How A $440,000 Drug Is Turning Alexion Into Biotech's New Innovation Powerhouse." Forbes. 5 September 2012. http://www.forbes.com/sites/matthewherper/2012/09/05/how-a-440000-drug-is-turningalexion-into-biotechs-new-innovation-powerhouse |

| Product Pipeline Status Aesthetic (Nasolabial Lines and Other) Approved by the FDA in June 2011 for moderate to severe nasolabial fold wrinkles Acne Scarring In end of Phase II discussions with the FDA and evaluating a path forward Restrictive Burn Scars Phase II study protocol accepted by FDA, study expected to begin first half 2013 Vocal Cord Scarring Phase II protocol expected to be submitted to FDA first half 2013 Personalized Skin Care Cosmetic Cream Clinical trial is fully enrolled and ongoing Intrexon Collaboration The ECC (Joint Fibrocell/Intrexon Team) is evaluating a number of targets for additional research programs using GM-fibroblasts for rare and ultra rare diseases i.e. Recessive Dystrophic Epidermolysis Bullosa RDEB laViv (r) Cell Based Initiatives |

| Autologous fibroblast cell therapy offers potential functional and aesthetic improvements for burn scar victims Restrictive burn scars are a potential blockbuster indication 45,000 yearly hospitalizations for severe burns (1) Open-label case study for autologous fibroblast therapy in the management of burn scars produced promising results Currently there are no approved drugs for restrictive burn scars Pre-Treatment 12 Months Post Treatment Full range-clench 14 Months Post Treatment Fine finger movement Example Case Study of Autologous Fibroblast Therapy Example Case Study of Autologous Fibroblast Therapy Situation Patient Treated with Fibroblasts 63 year-old woman Burned 10 years previously Full thickness burns Originally treated with skin grafts Scar tissue progressively became hardened, contracted and inflexible Constant pain with severe restriction and abduction Wearing cervical collar, taking strong analgesics Plastic surgeon reluctant to intervene Reviewed 5 months post second treatment Improvement on both sides of neck Stopped all analgesics Discarded cervical collar Full range of motion and pain free www.ameriburn.org; Goodis. J and E.d. Schraga. Burns, thermal. eMedicine Journal Overview of Burn Scarring Potential functional and aesthetic solution Description Note: Photos from Fibrocell clinical studies. |

| Other Case Studies In Burn Victims Note: Photos from LAVIV prescribers. Not from clinical studies. Case 1 Case 2 |
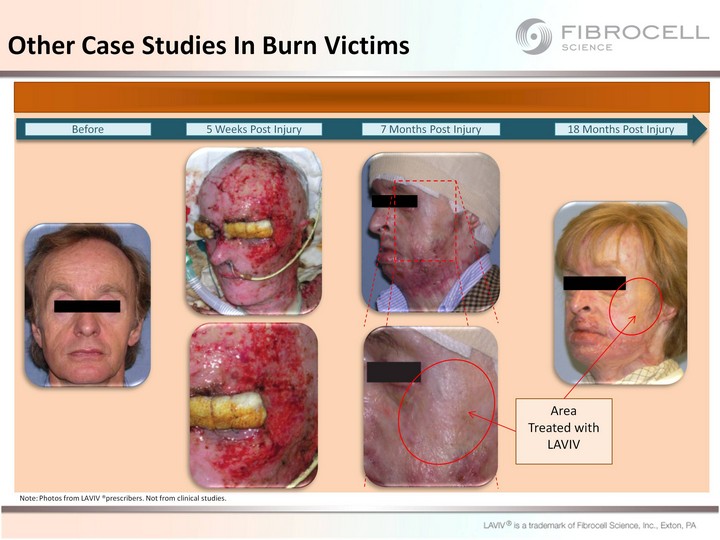
| Other Case Studies In Burn Victims Before 5 Weeks Post Injury 7 Months Post Injury 18 Months Post Injury Area Treated with LAVIV Note: Photos from LAVIV (r)prescribers. Not from clinical studies. |

| Phase II Trial Design: Single site, placebo-controlled, 20 subjects Enrollment: Subjects who have unilateral restrictive burn scars of a jointed area (i.e., wrist, elbow, shoulder, knee) with > 20% decrease in normal ROM with no additional improvement after physical therapy Measurement Scales: Range of Motion (ROM) Measurement Brief Pain Index Scar Appearance Targeting first half 2013 for initiating of Phase II Clinical Study Restrictive Burn Scar Status |
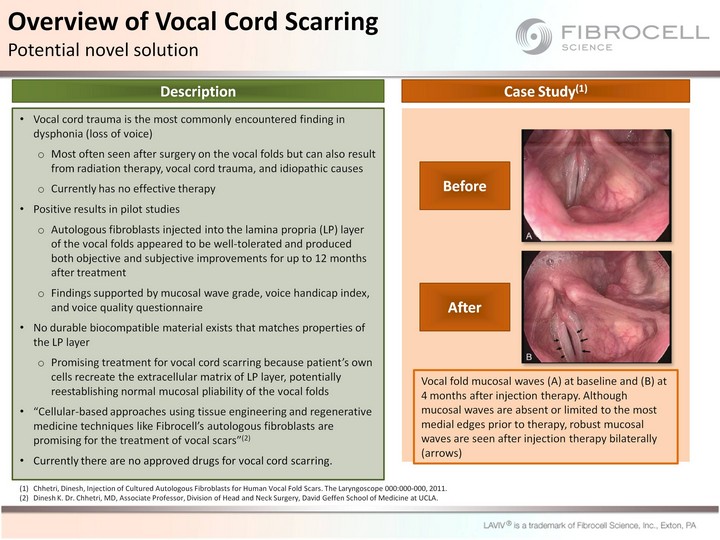
| Vocal cord trauma is the most commonly encountered finding in dysphonia (loss of voice) Most often seen after surgery on the vocal folds but can also result from radiation therapy, vocal cord trauma, and idiopathic causes Currently has no effective therapy Positive results in pilot studies Autologous fibroblasts injected into the lamina propria (LP) layer of the vocal folds appeared to be well-tolerated and produced both objective and subjective improvements for up to 12 months after treatment Findings supported by mucosal wave grade, voice handicap index, and voice quality questionnaire No durable biocompatible material exists that matches properties of the LP layer Promising treatment for vocal cord scarring because patient's own cells recreate the extracellular matrix of LP layer, potentially reestablishing normal mucosal pliability of the vocal folds "Cellular-based approaches using tissue engineering and regenerative medicine techniques like Fibrocell's autologous fibroblasts are promising for the treatment of vocal scars"(2) Currently there are no approved drugs for vocal cord scarring. Vocal fold mucosal waves (A) at baseline and (B) at 4 months after injection therapy. Although mucosal waves are absent or limited to the most medial edges prior to therapy, robust mucosal waves are seen after injection therapy bilaterally (arrows) Chhetri, Dinesh, Injection of Cultured Autologous Fibroblasts for Human Vocal Fold Scars. The Laryngoscope 000:000-000, 2011. Dinesh K. Dr. Chhetri, MD, Associate Professor, Division of Head and Neck Surgery, David Geffen School of Medicine at UCLA. Overview of Vocal Cord Scarring Potential novel solution Description Case Study(1) Before After |

| Phase II protocol drafted and under review internally expected to submit to the FDA first half 2013 Proposed Endpoints: Subject's impression of improvement in voice quality (VAS) Change from baseline in the noise-to-harmonic ratio (dB) as measured from acoustic voice analysis Absolute and percentage change from baseline in the Voice Handicap Index (VHI) Proposed trial design: Multi site, placebo-controlled, 20-40 subjects Vocal Cord Scarring Status |
| Overview of Recessive Dystrophic Epidermolysis Bullosa (RDEB) Epidermolysis bullosa is a group of genetic conditions that cause the skin to be very fragile and to blister easily Blisters and skin erosions form in response to minor injury or friction, such as rubbing or scratching RDEB: In mild cases, blistering may primarily affect the hands, feet, knees, and elbows Severe cases of this condition involve widespread blistering that can lead to vision loss, disfigurement, and other serious medical problems Prevalence is 6.5 per million newborns (US) (1) Mutations in the COL7A1 gene cause all three major forms of dystrophic epidermolysis bullosa This gene provides instructions for making a protein that is used to assemble type VII collagen Type VII collagen plays an important role in strengthening and stabilizing the skin It is the main component of structures called anchoring fibrils, which anchor the epidermis to the dermis (1)National Institutes of Health (2008) http:\\ghr.nlm.nih.gov/condition/dystrophic-epidermolysis-bullosa.Genetics Home Reference. |
| Recessive Dystrophic Epidermolysis Bullosa (RDEB) Proposed new therapeutic product: A genetically modified (GM) fibroblast up-regulated to produce Collagen VII in a controlled manner for localized or systemic treatment of RDEB Employ Intrexon's synthetic biology platforms for optimal gene expression from GM fibroblasts Development and characterization of GM fibroblast with Intrexon |

| UCLA Collaboration: Stem Cell Program |
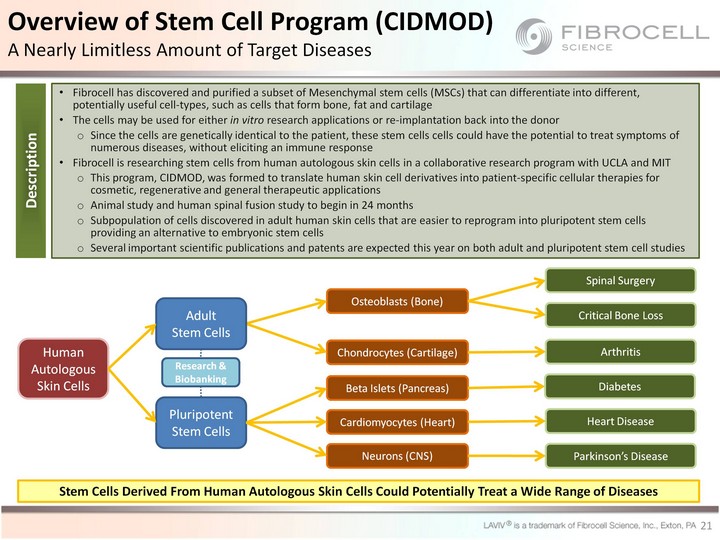
| 21 Fibrocell has discovered and purified a subset of Mesenchymal stem cells (MSCs) that can differentiate into different, potentially useful cell-types, such as cells that form bone, fat and cartilage The cells may be used for either in vitro research applications or re-implantation back into the donor Since the cells are genetically identical to the patient, these stem cells cells could have the potential to treat symptoms of numerous diseases, without eliciting an immune response Fibrocell is researching stem cells from human autologous skin cells in a collaborative research program with UCLA and MIT This program, CIDMOD, was formed to translate human skin cell derivatives into patient-specific cellular therapies for cosmetic, regenerative and general therapeutic applications Animal study and human spinal fusion study to begin in 24 months Subpopulation of cells discovered in adult human skin cells that are easier to reprogram into pluripotent stem cells providing an alternative to embryonic stem cells Several important scientific publications and patents are expected this year on both adult and pluripotent stem cell studies Human Autologous Skin Cells Pluripotent Stem Cells Osteoblasts (Bone) Beta Islets (Pancreas) Cardiomyocytes (Heart) Spinal Surgery Heart Disease Chondrocytes (Cartilage) Critical Bone Loss Arthritis Diabetes Stem Cells Derived From Human Autologous Skin Cells Could Potentially Treat a Wide Range of Diseases Overview of Stem Cell Program (CIDMOD) A Nearly Limitless Amount of Target Diseases Description Neurons (CNS) Parkinson's Disease Adult Stem Cells Research & Biobanking |

| Stem Cell Collaboration Expansion of Isolated MSCs and IPSCs Background After characterizing UCLA MSC and IPSC dermal fibroblast subpopulations, scientists determined a commercially viable method for maintenance and expansion of the specialized cells is required Fibrocell has entered into a collaborative agreement with UCLA and MIT to develop a culture method Project Title Combinatorial development of plastics for growth and maintenance of human dermal primary cell subpopulations Laboratory Work is being conducted at the David H. Koch Institute for Integrative Cancer Research at MIT, in the laboratory of Principle Investigator Dr. Daniel G. Anderson Goal Develop culture systems and supportive media requirements to maintain target cell subpopulations defined by viable cell surface biomarkers General Experimental Approach Fluorescence Activated Cell Sorting (FACS) purified cells expressing these biomarkers will be cultured under different spatially defined culture systems and the cell surface biomarker expression analyzed using both FACS and immunocytochemistry Overview |

| Personalized Cosmetic Development |

| Product Overview A novel, high quality moisturizer Conditioned complete growth media from the azficel-T production process is collected, concentrated and used to formulate a personalized topical cosmetic Blend with an appropriate vehicle cream Specially formulated for Fibrocell Science Contains botanicals Paraben-free |

| Clinical Study Status Cosmetic clinical trial evaluating impact of product on photo damaged skin Single center, 20 subjects Apply product twice a day Evaluated with expert graded scale assessment and photography after two months Enrollment Completed |
| Manufacturing Capacity Update |
| Manufacturing Capacity Update Current autologous cell manufacturing process: Complex and intricate Labor intensive Requires comprehensive quality control checks Requires comprehensive quality assurance practices As cellular therapies grow in use, significant progress is being made in supportive technologies. |

| Manufacturing Capacity Update Fibrocell has identified technologies that may dramatically reduce manufacturing time, complexity, and labor costs for our cell therapies, both laViv(r) and those in clinical development Pending a full assessment of these technologies we are deferring the previously announced expansion of our manufacturing facility We will continue at our current capacity of 1,000 processed biopsies per year until further notice When the evaluation is complete, we will provide an update on our timetable for increasing our capacity |

| Financials / Capitalization Table Strong cash position Large private capital raise completed in October 2012, investor syndicate led by Third Security, an investment firm led by RJ Kirk No debt on the balance sheet Company's capital structure greatly simplified All outstanding preferred stock was converted into common stock All outstanding convertible debt was extinguished Approximately 99% of warrants conceded anti-dilution provisions |
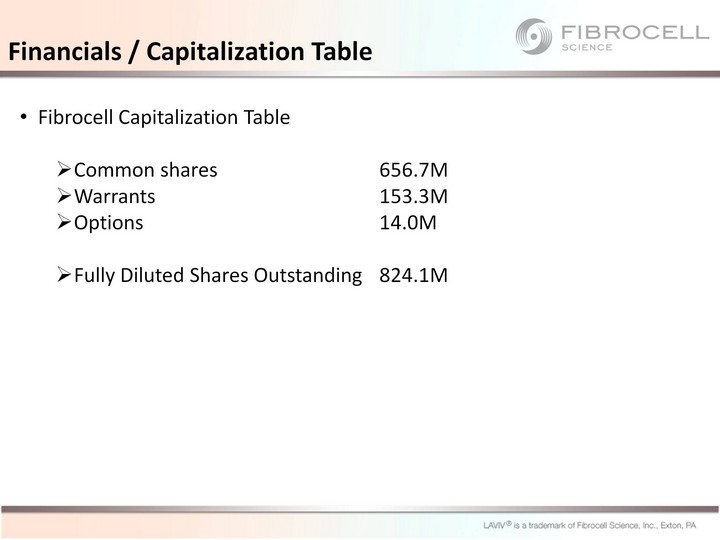
| Financials / Capitalization Table Fibrocell Capitalization Table Common shares 656.7M Warrants 153.3M Options 14.0M Fully Diluted Shares Outstanding 824.1M |

| Summary Fibrocell is uniquely positioned in regenerative medicine & cell based therapies Aesthetic applications are a beachhead for higher value indications Pursuing higher value per cell opportunities: Currently there are no drugs approved for Vocal Cord Scarring, Restrictive Burn Scars and Acne Scarring Pricing per patient are expected to be multiples of the aesthetic patient Unlike LAVIV(r) reimbursement is expected for Vocal Cord Scarring & Restrictive Burn Scars |

| Summary Exploring higher value per cell in rare and ultra rare diseases Prevalence of RDEB is 6.5 per million newborns in the US (1) Currently at least 12 products for other rare diseases have a wholesale cost of $200,000 plus (2) Unlike laViv(r), reimbursement is expected. Currently there are no drugs approved for treatment of RDEB Progressing UCLA Stem Cell Program Developing personalized topical daily moisturizing cosmetic Fibrocell has identified technologies that may dramatically reduce manufacturing time, complexity, and labor costs for our cell therapies, both laViv(r) and those in clinical development National Institutes of Health (2008) http:\\ghr.nlm.nih.gov/condition/dystrophic-epidermolysis-bullosa.Genetics Home Reference. (2)Herper, Matthew. "How A $440,000 Drug Is Turning Alexion Into Biotech's New Innovation Powerhouse." Forbes. 5 September 2012. http://www.forbes.com/sites/matthewherper/2012/09/05/how-a-440000-drug-is-turningalexion-into-biotechs-new-innovation-powerhouse |
