Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REGENERON PHARMACEUTICALS, INC. | d463788d8k.htm |
 J.P.
Morgan Healthcare Conference January 2013
Exhibit 99.1 |
 2
SAFE
HARBOR
STATEMENT
Except for historical information, the matters contained in this presentation may constitute
forward-looking statements that involve risks and uncertainties, including risks
and uncertainties related to product development and clinical trials, unforeseen safety
issues resulting from the administration of products and product candidates in patients,
uncertainties related to the need for regulatory and other government approvals,
government regulations, risks related to third party patents and proprietary technology,
litigation, the need for additional capital, uncertainty of market acceptance of Regeneron’s
products and product candidates, the ability of the Company to meet any of its sales or
other financial projections, the receipt of future payments, the continuation of business
partnerships, and additional risks detailed from time to time in Regeneron’s filings with
the Securities and Exchange Commission (SEC). Please refer to Regeneron’s Form 10-K
for the year ended December 31, 2011 and its Form 10-Q for the quarter ended September
30, 2012 for additional information on these risks and uncertainties and for other
information related to our business.
Because forward-looking statements involve risks and uncertainties, actual results may
differ materially from current results expected by Regeneron. Regeneron is providing this
information as of the original date of this presentation and expressly disclaims any duty to
update any information contained in these materials, including without limitation any sales
forecasts and any other forward-looking statements.
|
 |
 4
REGENERON TRANSFORMED
One of the top 5 biopharmaceutical launches of all time
EYLEA
®
LAUNCH HAS EXCEEDED EXPECTATIONS
EYLEA US, EU, Japan, Australia, and other countries; ZALTRAP US
MULTIPLE REGULATORY APPROVALS
GAAP & Non-GAAP FOUR FULL
QUARTERS OF PROFITABILITY Year end cash and receivables of ~$1.2B
STRONG FINANCIAL POSITION
Science
magazine
annual
poll;
“Biotechnology
Company
of
the
Year”
by
Scrip
Intelligence
WORLD’S #1 BIOPHARMACEUTICAL EMPLOYER |
 5
REGENERON LOOKING FORWARD
RESEARCH & DEVELOPMENT
Expanding late stage pipeline with REGN727 (PCSK9), Sarilumab (IL6R), & REGN668
(IL4R) Continuing to invest in early stage pipeline and innovative
technologies EYLEA
Increasing market share, expanding
geographies and indications, securing future
ZALTRAP
Global launch; Novel combinations
FINANCIAL
Continuing growth in sales and profits |
 6
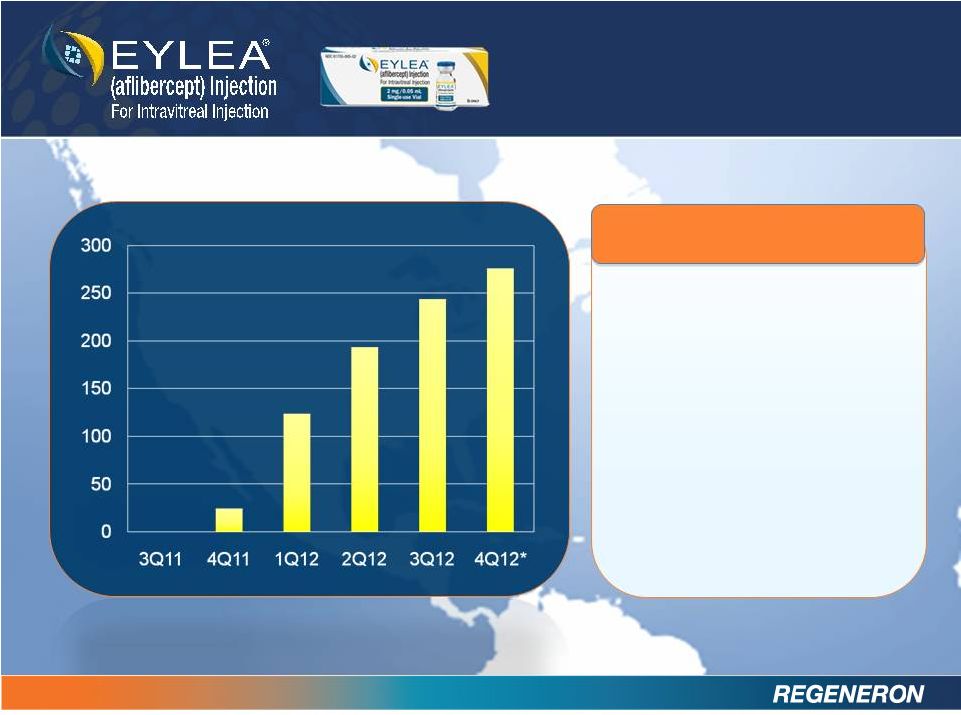
EYLEA
net product
sales of $276 million
in 4Q12*
$838 million in full year
2012*
2013 EYLEA U.S. net sales
guidance: ~50% growth
(i.e. ~$1.2B to $1.3B)
U.S. LAUNCH
* 4Q12 Sales Estimate
EXCEEDING
EXPECTATIONS |
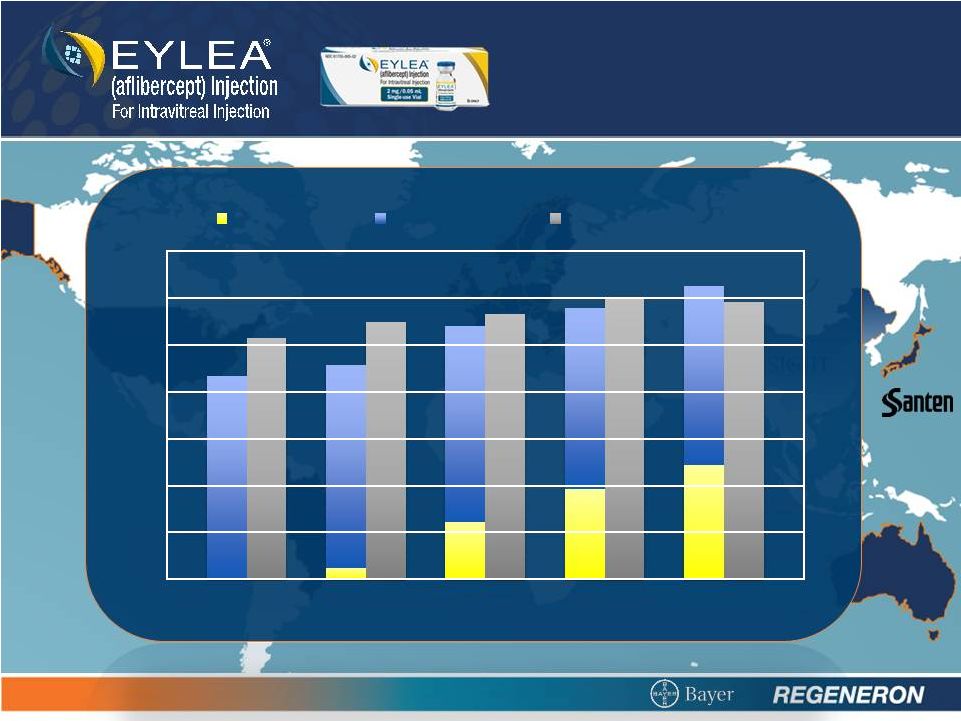
 7
* 4Q12 Sales Estimate
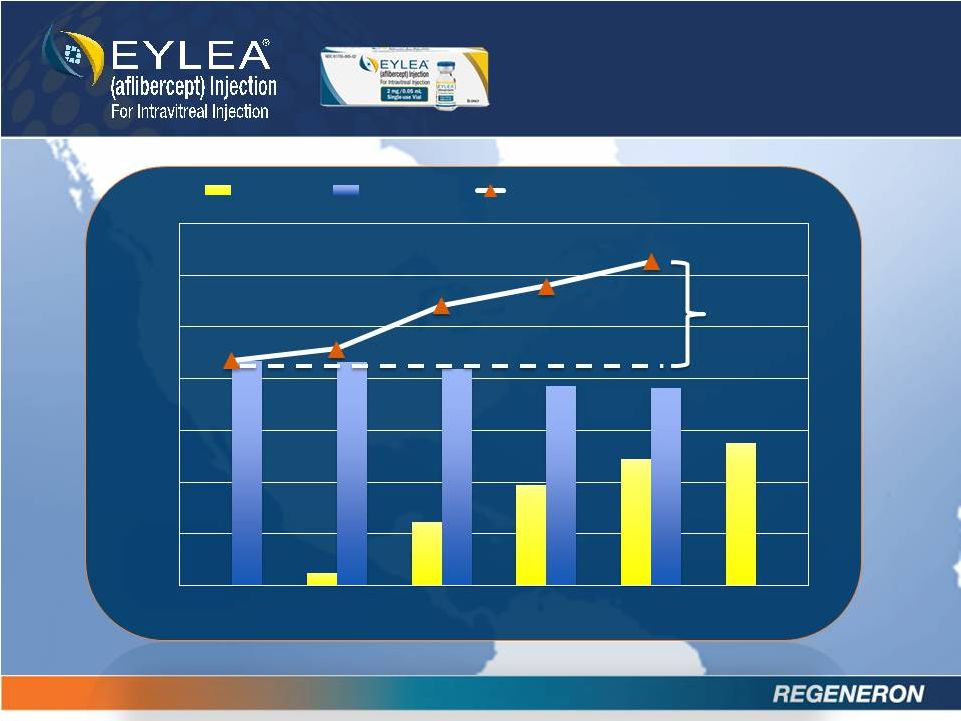
EXPANDING
BRANDED MARKET
0
100
200
300
400
500
600
700
3Q11
4Q11
1Q12
2Q12
3Q12
4Q12
EYLEA
Lucentis
EYLEA & Lucentis Total
+47
% |
 8
EXPANDING
GEOGRAPHY
3Q11
4Q11
1Q12
2Q12
3Q12
0
100
200
300
400
500
600
700
US: EYLEA
US: Lucentis
exUS: Lucentis |
 9
Trials fully enrolled
Diabetic Macular Edema (DME)
BROADENING
INDICATIONS
Myopic CNV
Trial fully enrolled in Asia
Trial enrolling
Branch Retinal Vein Occlusion (BRVO)
Approved in U.S.; Filings globally
Central Retinal Vein Occlusion (CRVO) |
 10
SECURING
THE FUTURE
High affinity, fully-human
antibody to PDGFR
Goal to enter clinic with
co-formulated product in 2H13
PDGFR
High affinity, fully human
antibody to ang2
Goal to enter into ophthalmology
clinical development in 2013
ANG2
Novel formulation and delivery of EYLEA as
well as evaluation of new technologies
Novel Targets in Development for Retinal Disease |
 11
OPPORTUNITIES
IN CANCER
Approved and launched in U.S.
Discovered by Regeneron and
co-developed with Sanofi
Regeneron has worldwide
co-commercialization rights
Approved in the US
Positive recommendation for
approval by European CHMP
EU approval expected 1Q13
Regeneron has worldwide
co-commercialization rights
Pending Approval in the EU
ZALTRAP is the only agent that has demonstrated a statistically significant
improvement in overall survival in combination with FOLFIRI versus FOLFIRI
alone in patients who progressed on a prior oxaliplatin-containing
regimen. |
 12
EXPANDING LATE
STAGE PIPELINE
IL6R antibody for
rheumatoid arthritis
Sarilumab
PCSK9 antibody for
elevated cholesterol
REGN727
IL4R antibody for Asthma
and Atopic Dermatitis
REGN668 |
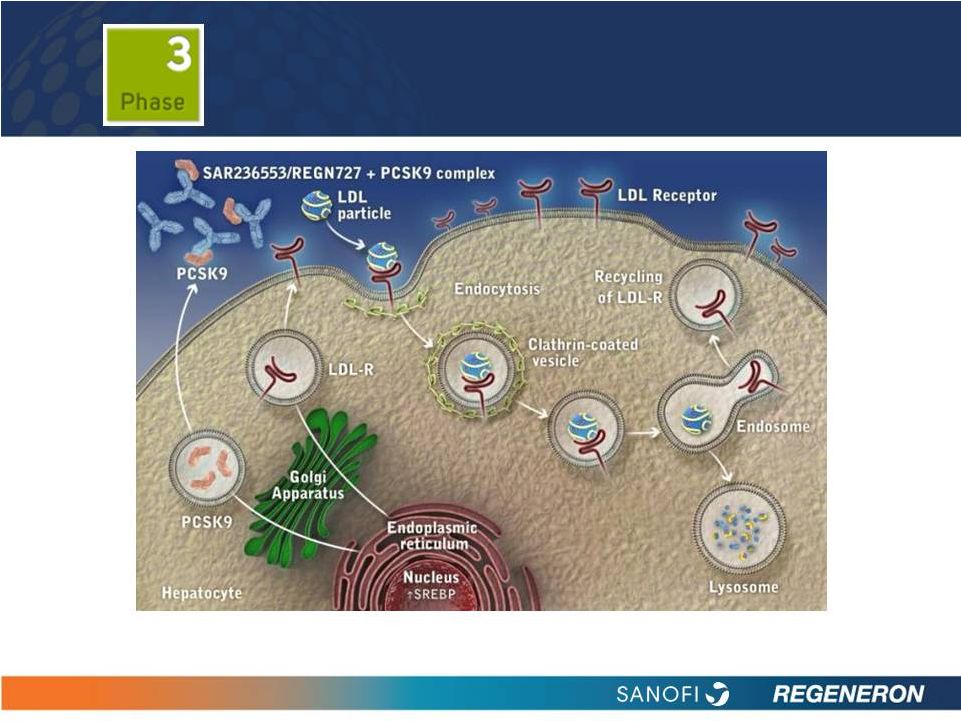 13
(1) Tibolla G et al. Nutr Metab Cardiovasc Dis 2011;21:835-43. (2) Akram ON et
al. Arterioscler Thromb Vasc Biol 2010;30:1279-81. (3) Duff CJ et al.
Expert Opin Ther Targets 2011;15:157-68. (4) Horton JD et al. J Lipid Res 2009;50 Suppl:S172-7. (5) Cariou B et al.
Atherosclerosis 2011;216:258-65.
PCSK9: BLOCKING PCSK9 CAN
POTENTIALLY
LOWER
LDL-C
LEVELS
1-5 |
 14
Phase 2 data published in The
Lancet, Journal of the American
College of Cardiology, and NEJM
Significantly reduced mean LDL-C
by 40% to 72% over 8 to 12 weeks
in patients on stable dose of statins
Most common adverse event
was injection site reaction
Mean percentage change in calculated LDL-C from baseline to weeks 2, 4, 6, 8,
10, and 12 in the modified intent-to-treat (mITT) population, by
treatment group. Week 12 estimation using LOCF method. *Data from Phase 2 ,
dose-ranging study. Patients were on background atorvastatin therapy of 10, 20, or 40 mg.
Decrease in LDL-C shown is at week 12.
REGN727:
LDL CHOLESTEROL REDUCTION
Change in Calculated LDL-C at Bi-Weekly Intervals from Baseline to Week 12 in
Patients With Primary Hypercholesterolemia. |
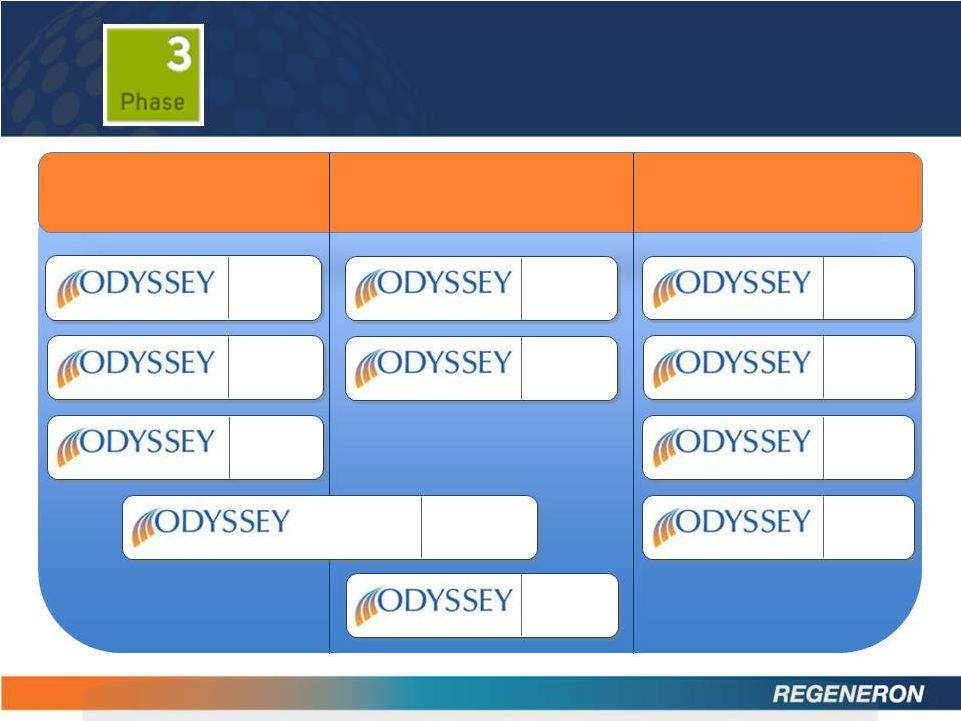 15
FH I
HIGH FH
FH II
OPTIONS I
OPTIONS II
MONO
ALTERNATIVE
LONG TERM
COMBO I
COMBO II
N=105
18 months
N=471
18 months
N=250
18 months
N=2,100
18 months
N=306
12 months
N=660
24 months
N=250
6 months
N=300
6 months
N=100
6 months
N=350
6 months
N=18,000
(Event
Driven)
REGN727:
LDL CHOLESTEROL REDUCTION
OUTCOMES
Heterozygous Familial
Hypercholesterolemia
High Cholesterol at High
Cardiovascular Risk
Additional
populations/studies |
 SARILUMAB:
RHEUMATOID ARTHRITIS
Sarilumab is a fully human, high
affinity, interleukin-6 receptor (IL-6R)
antibody
Positive Phase 2 study in rheumatoid
arthritis
Types and incidence of adverse
events consistent with those previously
reported with IL-6 inhibition
Phase 3 MOBILITY trial fully enrolled
Phase 3 TARGET trial enrolling
16
*p<0.01 versus placebo (only unadjusted p-values
<0.01 are considered statistically significant)
Significant improvements in signs and symptoms of moderate-to-severe RA in
patients receiving sarilumab in combination with methotrexate in Phase 2
study. ACR20
ACR50
ACR70
46.2
66.7
65.4
15.4
35.3
40.4
1.9
11.8
17.3
0
10
20
30
40
50
60
70
80
Placebo
150 mg q2w
200mg q2w |
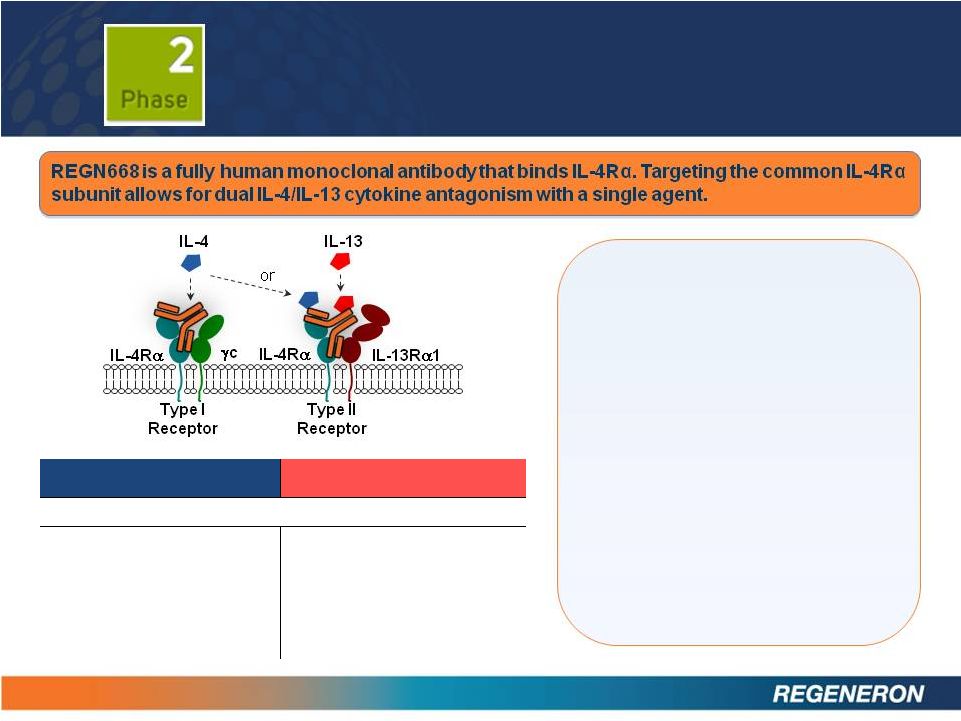 REGN668:
ASTHMA AND ATOPIC DERMATITIS
17
Positive proof of concept data
for atopic dermatitis to be
submitted for presentation at
a medical conference in 2013
Positive proof of concept data
for asthma to be submitted for
presentation at a medical
conference in 2013
Phase 2b initiation in both
indications expected mid-year
IL-4
IL-13
Dominant (some overlapping) functions in :
•
Initiation
and
drive
of
T
H
2
differentiation
•
Activation and growth of B
cells
•
Class switching to IgE and
IgG1a
•
Recruitment of eosinophils
•
Airway hyper responsiveness
(AHR)
•
Goblet cell hyperplasia
•
Tissue remodeling
•
Fibrosis
•
Regulation of gastrointestinal
parasite expulsion |
 BROAD ANTIBODY PIPELINE
18
Inflammation
Therapeutic Focus Legend:
Metabolism
Oncology
Ophthalmology
Pain
REGN421 (DII4 Antibody)
Advanced malignancies
REGN910 (ANG2 Antibody)
Advanced malignancies
REGN1033 (GDF8 Antibody)
Metabolic disorders
REGN1400 (ERBB3 Antibody)
Advanced malignancies
REGN846
(undisclosed target)
Atopic dermatitis
REGN1154
(undisclosed target)
REGN1500
(undisclosed target)
Phase 1
REGN668 (IL-4R Antibody)
Eosinophilic asthma
REGN668 (IL-4R Antibody)
Atopic dermatitis
REGN475* (NGF Antibody)
Osteoarthritis
Phase 2
REGN727 (PCSK9 Antibody)
LDL cholesterol reduction
Sarilumab (IL-6R Antibody)
Rheumatoid arthritis
EYLEA
Diabetic macular edema
EYLEA
Branch Retinal Vein Occlusion
Phase 3
Novel Antibody Technologies in Preclincal
Development
Bispecific Antibodies
Long Acting Technologies
Antibody Drug Conjugates
*Remains on clinical hold by the FDA |
 SANOFI ANTIBODY COLLABORATION
19
*100% development funding by Sanofi for all opted-in antibodies except 80% of
an antibody’s Phase 3 costs incurred after receipt of the first
positive results in a Phase 3 trial for that antibody.
**Regeneron repays Sanofi for 50% of development costs out of profits.
Repayment capped in any year at 10% of Regeneron share of total antibody
profits Discovery
Discovery
Development
Development
Commercialization
Commercialization
$160 million of annual
funding through 2017
(plus possible
tail period through 2020)
Sanofi funds
approximately 100% of
clinical development cost*
Current antibodies
include:
•
REGN727 (Phase 3)
•
Sarilumab (Phase 3)
•
REGN668 (Phase 2)
•
3 additional in Phase 1
Regeneron retains
50% of profits in US**
Regeneron retains 35%
to 45% of profits ex-US**
Co-promotion rights
in US and other major
market countries |
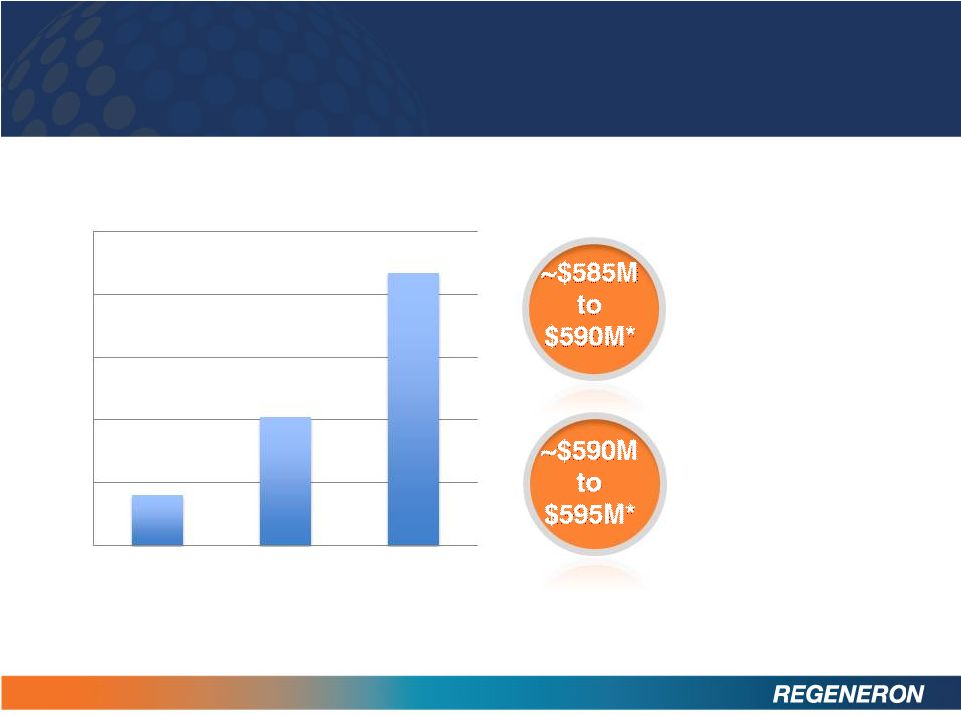 FINANCIALS
20
Year-end 2012
Year-end 2012
cash and
cash and
securities
securities
Year-end 2012
Year-end 2012
trade
receivables
receivables
Non-GAAP Net Income
* Unaudited Estimate
$0
$50
$100
$150
$200
$250
1Q12
2Q12
3Q12 |
 THE YEAR AHEAD
21
•
EYLEA US sales guidance of ~50% growth (i.e. $1.2B to $1.3B)
•
EYLEA ex-US launch in multiple additional countries
COMMERCIAL MILESTONES
•
EYLEA approval in CRVO outside the U.S.
•
ZALTRAP approval in metastatic colorectal cancer outside the U.S.
EXPECTED REGULATORY MILESTONES
•
Top-line 1-year Phase 3 EYLEA results in DME by year end
•
REGN668
Phase
1b
data
in
Atopic
Dermatitis
to
be
presented
at
a
medical
conference
•
REGN668 Phase 2a data in Asthma to be presented at a medical conference
•
Sarilumab additional Phase 3 trials in RA to start
•
Enrollment updates for REGN727 in hypercholesterolemia
EXPECTED CLINICAL MILESTONES |
