Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Paratek Pharmaceuticals, Inc. | d461871d8k.htm |
 January 2013
January 2013
A specialty pharmaceutical company focused on the development
and commercialization of proprietary products to address important
therapeutic needs in the field of neuroscience
Exhibit 99.1 |
 This
presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other
than statements of historical facts, included in this presentation are forward-looking
statements. Examples of such statements include our expectations regarding the
potential market for Intermezzo® and the potential market size for a middle of
the night sleep aid; our expectations regarding an ideal therapeutic; Purdue plans to
invest approximately $100 million to support sales and marketing over the first 12
months of commercialization of Intermezzo in the U.S., including the size, timing and
nature of such sales and marketing plans; the success of the commercial launch of Intermezzo
by Purdue in the U.S., including continued growth in the number of Intermezzo
prescriptions; our eligibility for and the receipt and size of royalty payments from
Purdue pursuant to our Collaboration Agreement, and our expectations regarding future co-promote and
royalty opportunities; intellectual property protection for Intermezzo being obtained and
maintained; plans for the Phase 2 study of TO-2061, including the expected timing
of receipt of final clinical trial results; and our strategy to build a specialty
sales and marketing organization, in-license or acquire additional product
candidates, and determine the future development path for TO-2061. All of these
forward-looking statements are based on estimates and assumptions by our
management that,
although we believe to be reasonable, are inherently uncertain. Forward-looking
statements involve risks and uncertainties, including, but not limited to, achieving
acceptance of Intermezzo by physicians, patients and third-party payors; the impact of
competitive products and the market for Intermezzo generally; our dependence on
Purdue’s commercialization efforts, including our reliance on Purdue to set the
future pricing of Intermezzo and on our Collaboration Agreement with Purdue;
obtaining, maintaining and protecting marketing and other regulatory exclusivity and
intellectual property protection for Intermezzo and TO-2061, as well as
protection from generic versions of our products; competitive product commercialization;
manufacturing and supply risks for Intermezzo; adverse results from our clinical trial
of TO-2061; adverse patent decisions at the USPTO or in court; our intent
and ability to carry out plans to promote Intermezzo to psychiatrists in the United
States through our co-promotion option; our intent and ability to successfully
in-license or acquire additional product candidates; and variability in the
business of Transcept generally. These and other risks are described in greater detail in the "Risk Factors"
section of Transcept periodic reports filed with the Securities and Exchange Commission.
Forward-looking statements do not reflect the potential impact of any future
in-licensing, collaborations, acquisitions, mergers, dispositions, joint ventures, or
investments Transcept may enter into or make. Transcept does not
assume any obligation to update any forward-looking
statements, except as may be required by law.
Forward looking statements
Forward looking statements
2 |
 Dec 31
2012: ~$85M cash & equivalents, net of $10M
Intermezzo DTC investment 3
Neuroscience / psychiatry
First and only Rx sleep aid approved for middle-of-
the-night dosing
U.S. primary care partnership: Purdue Pharma
$29M 6-month DTC campaign: TV launch Jan 2013
600+ Purdue sales reps presenting Intermezzo
TSPT option to co-promote to psychiatrists
Transcept corporate overview
Transcept corporate overview
Strong balance
sheet
Therapeutic focus |
 Intermezzo:
the first and only prescription sleep aid approved for middle-of-the-night
dosing Intermezzo: the first and only prescription sleep aid
approved for middle-of-the-night dosing
Indication statement as approved by FDA:
–
Intermezzo
is indicated for use as needed for the
treatment of insomnia when a middle-of-the-night
awakening is followed by difficulty returning to sleep.
–
Intermezzo
is not indicated for the treatment of middle-
of-the-night insomnia when the patient has fewer than 4
hours of bedtime remaining before the planned time of
waking.
4 |
 Intermezzo
unit dose bedside packaging Intermezzo unit dose bedside packaging
5
“Intermezzo is to be taken in bed when a patient wakes in the middle
of the night and has difficulty returning to sleep. Intermezzo should
only be taken if the patient has at least 4 hours of bedtime remaining
before the planned time of waking.” |
 Middle of the
night (MOTN) awakening: a major unmet medical need in the insomnia category
Middle of the night (MOTN) awakening:
a major unmet medical need in the insomnia category
Large U.S. insomnia market
–
79
million
new
and
refill
prescriptions
(1)
Insomnia
is
an
under-treated
condition
(2)
–
11
million
patients
receive
Rx
(3)
–
4x
to
6x
more
are
not
diagnosed
or
treated
by
a
physician
(2,3)
MOTN
awakening:
the
most
common
insomnia
symptom
(4)
–
35%
of
Americans
suffer
from
MOTN
awakenings
at
least
3x
/
week
(4)
–
>90% report awakenings persist more than six months;
50%
report
awakenings
persist
more
than
five
years
(5)
6
(1)
IMS
Oct
2010
to
Sep
2011;
(2)
Institute
of
Medicine
-
Sleep
disorders
and
sleep
deprivation
Apr.
2006;
(3)
BluePrint
Research
Group;
(4) Ohayon,
Nocturnal awakenings and comorbid disorders in the American general population. J of Psych
Research (2009); (5) Ohayon, Difficulty in
resuming
or
inability
to
resume
sleep
and
the
links
to
daytime
impairment,
J
of
Psych
Research
(2009). |
 Commonly
prescribed sleep aids are indicated only for bedtime use
Commonly prescribed sleep aids are indicated
only for bedtime use
MOTN awakenings typically do not occur every night
7-8 hr sleep aids (Ambien
®
, Ambien CR
®
, Lunesta
®
) require
bedtime prophylactic dosing to prevent awakenings
An ideal therapeutic would:
–
Be used only at the time patients need help returning to sleep,
not every night in advance of a problem that may not occur
–
Return patients to sleep rapidly
–
Be effective despite the low dose necessary to avoid next day
residual effects when used in the middle of the night
7 |
 Intermezzo:
the first and only sleep aid approved for middle-of-the night dosing
Intermezzo: the first and only sleep aid approved for
middle-of-the night dosing
Novel zolpidem formulation
–
Sublingual tablet
–
Bicarbonate-carbonate buffers
Approved dose
–
1.75 mg in women & patients > 65 years
–
3.5 mg in men < 65 years
Rapidly absorbed in both men and women
Effective vs. placebo in sleep laboratory & outpatient studies
Instructions to patients
–
Take Intermezzo “while in bed”
–
“When you wake up in the morning, be sure that at least 4 hours have passed
since you have taken Intermezzo
and you feel fully awake before driving. Do
not do dangerous activities until you know how
Intermezzo affects you.”
8 |
 Purdue
commercialization agreement: key Transcept benefits
Purdue commercialization agreement:
key Transcept benefits
Base royalty: mid-teens up to mid 20% level on net sales
Milestone payments
–
Received: $25M up front + $20M for IP-related milestones
–
Potential: Up to an additional $70M related to net sales targets
Co-promote option: foundation for a commercial future
–
Option to exercise co-promote extends through August 2015
–
After option exercise, Transcept must wait 8 to 15 months to begin
promotion, depending on when in the calendar year the exercise is made
–
Co-promote royalty
•
Ranges from 40% (if option had been exercised by Apr 2012) to
22% (if option is exercised in Aug 2015) of psychiatrist net sales
•
Net sales qualifying for this additional co-promote royalty is capped
at 15% of total Intermezzo annual net U.S. sales
9 |
 10
0
200
400
600
800
1000
1200
1400
1600
1800
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
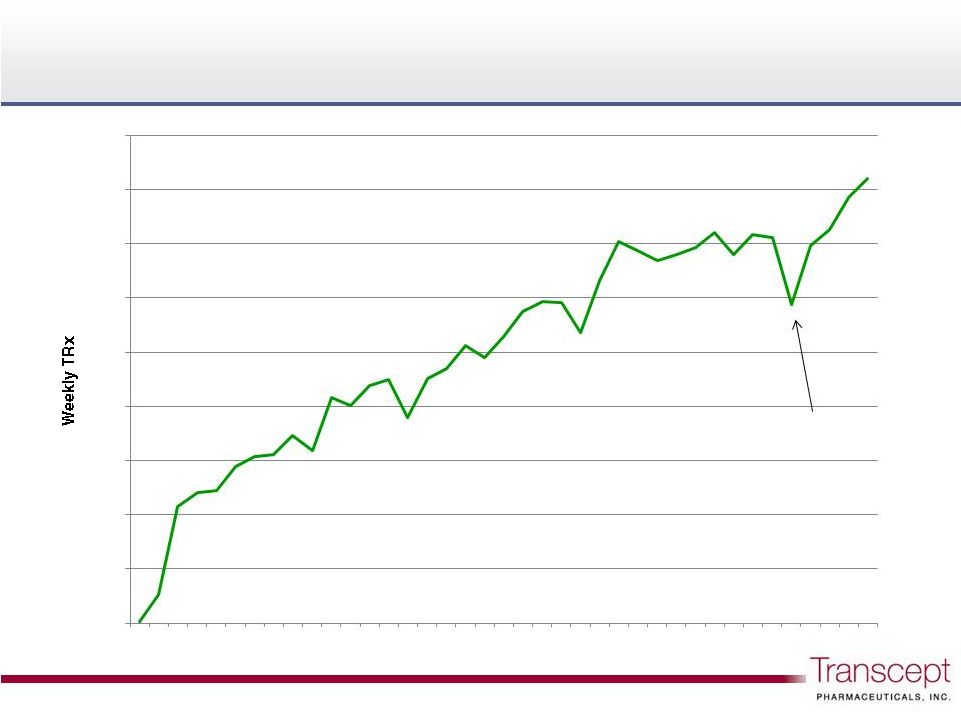
Week post-launch
Intermezzo
Thanksgiving holiday week
Intermezzo weekly TRx as of 12/21/12 |
 Purdue DTC
campaign launch January 2013 Purdue DTC campaign launch January 2013
11 |
 Intermezzo
ad placement on leading TV networks Intermezzo ad placement on leading TV
networks |
 $29 M
six-month DTC advertising campaign –
Purdue contribution $19M; Transcept contribution $10M
–
Television ads to commence January 2013
New sales force commitment with 600+ reps
–
Purdue analgesic sales force of 525 sales
representatives will present Intermezzo
–
Dedicated Intermezzo contract sales force of 90 sales
representatives to continue to call on top prescribers of
prescription sleep aids
DTC launch and broadened Intermezzo
commercialization effort
DTC launch and broadened Intermezzo
commercialization effort
12 |
 Intermezzo:
intellectual property
Intermezzo:
intellectual property |
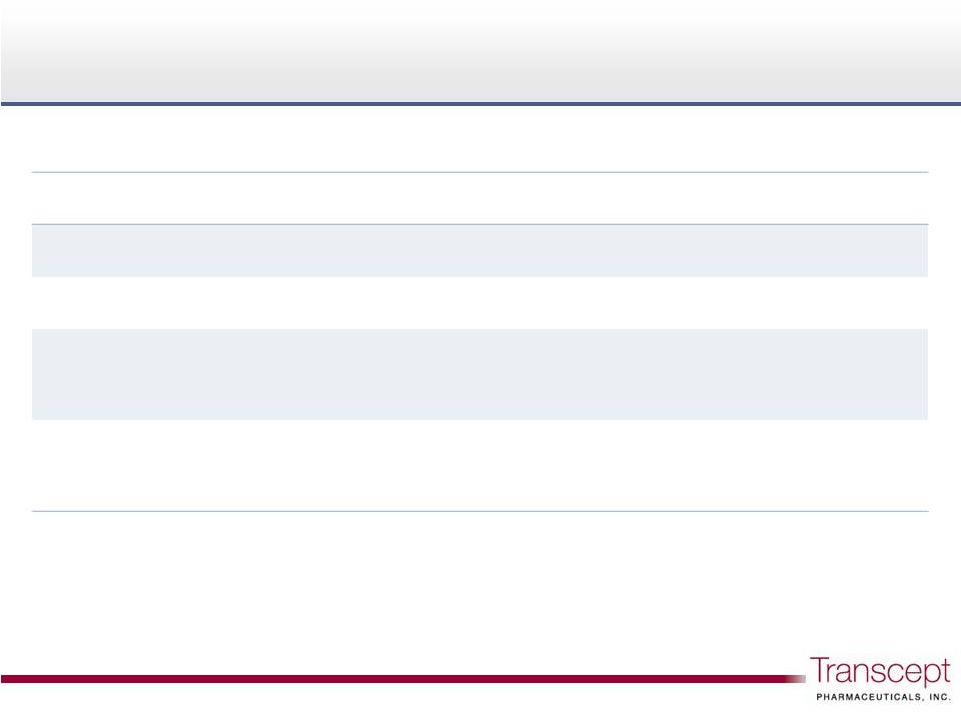 Four
Intermezzo U.S. patents issued Four Intermezzo U.S. patents issued
14
Methods for Treating Middle
of-
the
Night Insomnia
8,242,131
2029
Compositions for Delivering Hypnotic Agents across
the Oral Mucosa and Methods of Use thereof
7,682,628
2025
-
-
U.S. patent title
Patent no.
Expiration
Compositions
for Treating Insomnia
8,252,809
2025
Compositions for Delivering Hypnotic Agents across
the Oral Mucosa and Methods of Use thereof
7,658,945
2027 |
 Financial
overview Financial overview |
 Financial
position (1)
Financial position
(1)
December 31, 2012
Cash, equivalents & investments: ~$85 M
(2)
Shares outstanding
19 M
Options / warrants / other:
3 M
Total:
22 M
Employees:
21
(3)
(1)
All numbers are unaudited.
(2)
Net of $10M Intermezzo DTC investment.
(3)
On Jan 4, 2013, Transcept announced a reduction in force of 8 employees
to be implemented throughout the first quarter of 2013.
16 |
 Intermezzo
launched April 2012 –
First approval for middle of the night awakening
–
Large potential market
–
Significant royalty and co-promote opportunities
–
600+ sales reps
–
DTC launch Jan 2013
Strong balance sheet
Key investment highlights
Key investment highlights
17 |
 Intermezzo
®
is a registered trademark of Purdue Pharmaceuticals L.P.
Ambien
®
and Ambien CR
®
are registered trademarks of sanofi-aventis
Lunesta
®
is a registered trademark of Sunovion Pharmaceuticals Inc. |
