Attached files
| file | filename |
|---|---|
| 8-K - 8-K - TESARO, Inc. | a13-2256_18k.htm |
Exhibit 99.1
|
|
JANUARY 2013 CORPORATE PRESENTATION |
|
|
COMPANY SUMMARY Novel cancer therapeutics Differentiated oncology supportive care agents Currently advancing three pipeline products Identify and in-license or acquire Fully develop and commercialize products in-house Commercial presence in core strategic markets Management team with highly relevant experience Proven ability to execute product transactions, develop and commercialize Strong investor backing $121M raised privately since 2010 founding $86M raised in recent IPO $138.6M of cash and cash equivalents as of September 30, 2012 Leadership Capital Opportunity Strategy 2 |
|
|
Assemble and advance a balanced1 portfolio of cancer therapeutics and oncology supportive care agents, including: 1Balanced by stage, resource requirements and development risk PRODUCT PORTFOLIO STRATEGY 3 Compound Indication Pre-Clinical Phase 1 Phase 2 Phase 3 Rolapitant Oral (NK-1 receptor antagonist) CINV in HEC treated patients CINV in MEC treated patients Niraparib (PARP Inhibitor) Monotherapy – Solid Tumors TSR-011 (ALK inhibitor) NSCLC, others |
|
|
Commercialize a portfolio of cancer therapeutics and supportive care products: 1. Deploy fully integrated sales, marketing and medical affairs organizations in core strategic markets: North America: United States, Canada Europe: Germany, France, UK, Italy, Spain Asia: China 2. Establish a comprehensive distributor / licensee network in non-core markets: Japan Europe: Non-core countries, Russia South/Central America Asia/Pacific: Australia, New Zealand, S. Korea, Taiwan COMMERCIAL STRATEGY 4 |
|
|
ROLAPITANT OVERVIEW CHEMOTHERAPY-INDUCED NAUSEA & VOMITING TESARO, INC. |
|
|
ROLAPITANT OVERVIEW Acquired in December 2010 from OPKO Health Schering-Plough (S-P) divested to OPKO Health as an FTC condition for its merger with Merck Potentially a Best-in-Class cancer supportive care product candidate Potent and long-acting neurokinin-1(NK-1) receptor antagonist for chemotherapy-induced nausea and vomiting (CINV) Differentiated profile compared to other NK-1 receptor antagonists Global rights and strong intellectual property Phase 3 oral program initiated following positive Phase 2 results Over 1,000 subjects evaluated in Phase 1 and 2 studies by S-P Efficacy and dose identified in a randomized, placebo controlled 454-patient Phase 2 trial Well accepted regulatory endpoints utilized in Phase 3 program Phase 3 top-line results expected 2H:13 Conduct dose finding study for the IV formulation 6 |
|
|
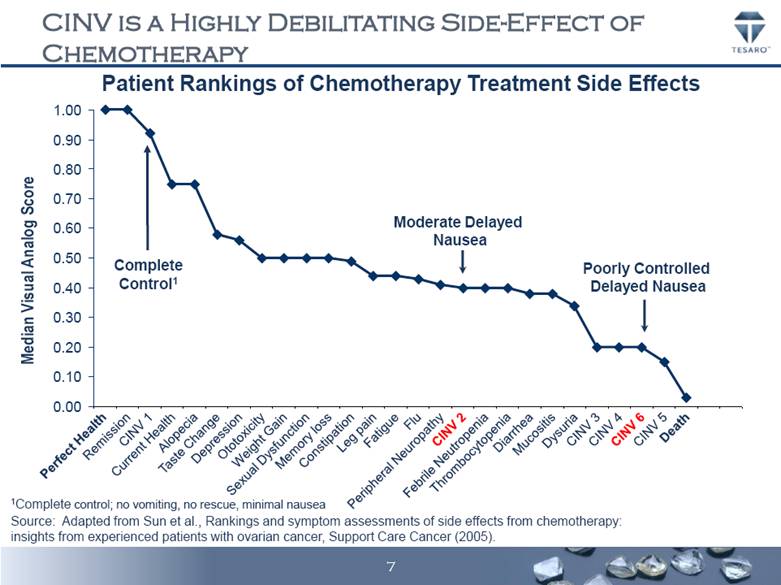
CINV IS A HIGHLY DEBILITATING SIDE-EFFECT OF CHEMOTHERAPY Taste Change Patient Rankings of Chemotherapy Treatment Side Effects Source: Adapted from Sun et al., Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer, Support Care Cancer (2005). Thrombocytopenia Median Visual Analog Score Remission CINV 1 Current Health Alopecia Depression Ototoxicity Weight Gain Sexual Dysfunction Memory loss Constipation Leg pain Fatigue Flu Peripheral Neuropathy Diarrhea Dysuria CINV 4 CINV 6 CINV 5 Death Perfect Health CINV 2 Mucositis CINV 3 Febrile Neutropenia Complete Control1 Moderate Delayed Nausea Poorly Controlled Delayed Nausea 1Complete control; no vomiting, no rescue, minimal nausea 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 7 |
|
|
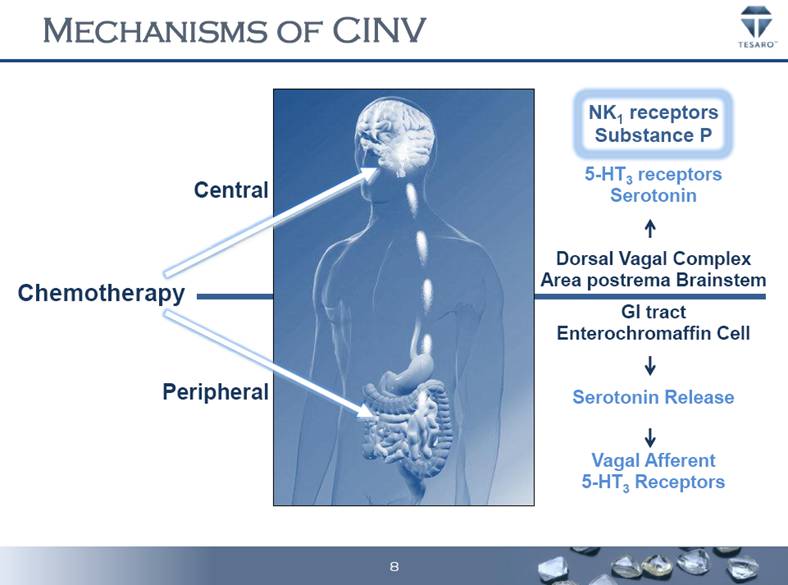
MECHANISMS OF CINV Chemotherapy NK1 receptors Substance P 5-HT3 receptors Serotonin Dorsal Vagal Complex Area postrema Brainstem GI tract Enterochromaffin Cell Serotonin Release Vagal Afferent 5-HT3 Receptors Peripheral Central 8 |
|
|
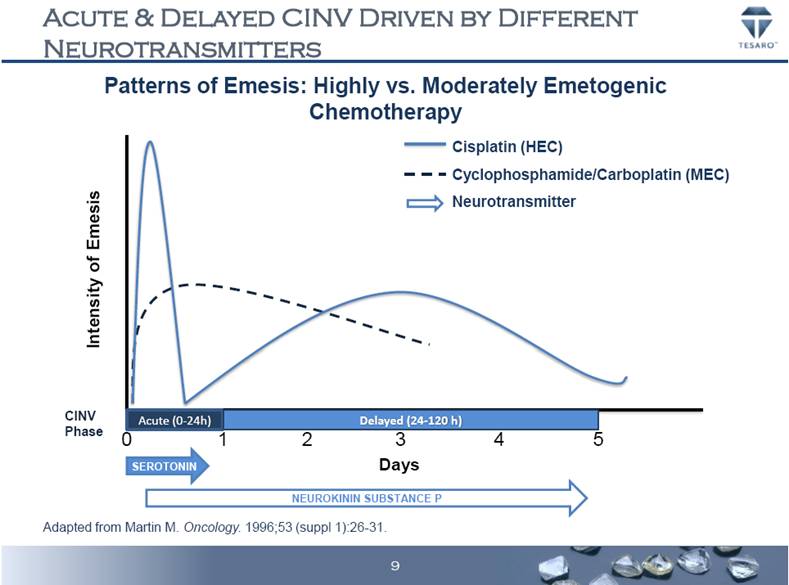
ACUTE & DELAYED CINV DRIVEN BY DIFFERENT NEUROTRANSMITTERS Intensity of Emesis Cisplatin (HEC) Cyclophosphamide/Carboplatin (MEC) Neurotransmitter 0 1 2 3 4 5 Days Adapted from Martin M. Oncology. 1996;53 (suppl 1):26-31. Patterns of Emesis: Highly vs. Moderately Emetogenic Chemotherapy SEROTONIN NEUROKININ SUBSTANCE P Acute (0-24h) Delayed (24-120 h) CINV Phase 9 |
|
|
PHASE 2 DESIGN AND OBJECTIVES Overview Trial Design Objectives Schering-Plough evaluated the safety and efficacy of rolapitant for the prevention of CINV in 454 patients receiving HEC for up to 6 cycles of chemotherapy Multicenter, randomized, double blind, placebo controlled clinical trial Compare rolapitant to Standard of Care (SOC): 5-HT3 receptor antagonist + corticosteroid SOC + rolapitant 10mg, 25mg, 100mg or 200mg SOC + placebo Primary Outcome: Complete Response (CR) for the “overall phase” (0 -120 hours) of CINV CR is defined as no vomiting and no use of rescue medication Safety and tolerability Secondary Outcome: Relevant CINV measures including CR for the “delayed phase” (24-120 hrs.) and “acute phase” (0-24 hrs.), and the incidence and intensity of nausea 10 |
|
|
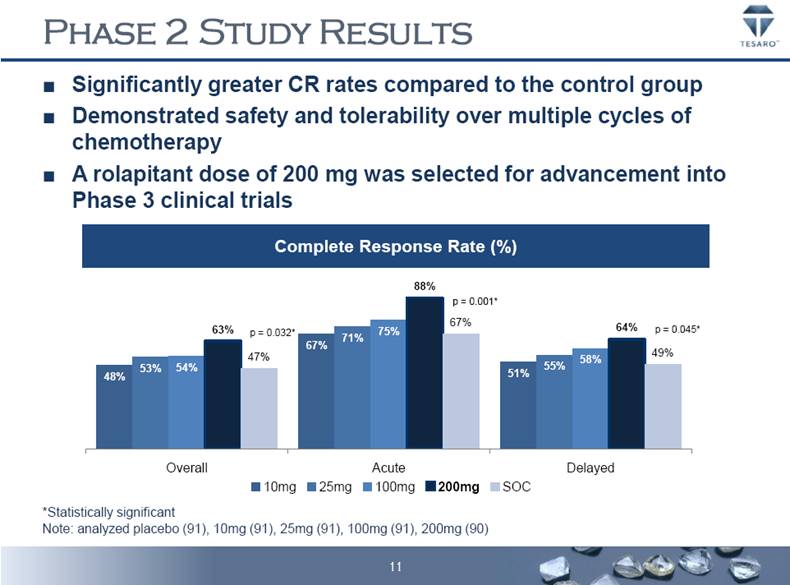
PHASE 2 STUDY RESULTS Significantly greater CR rates compared to the control group Demonstrated safety and tolerability over multiple cycles of chemotherapy A rolapitant dose of 200 mg was selected for advancement into Phase 3 clinical trials Complete Response Rate (%) *Statistically significant Note: analyzed placebo (91), 10mg (91), 25mg (91), 100mg (91), 200mg (90) p = 0.032* p = 0.001* p = 0.045* 48% 67% 51% 53% 71% 55% 54% 75% 58% 63% 88% 64% 47% 67% 49% Overall Acute Delayed 10mg 25mg 100mg 200mg SOC 11 |
|
|
PHASE 2 STUDY RESULTS Demonstrated superior treatment effect for no significant nausea versus the control group in all CINV phases Validated questionnaire (FLIE1) demonstrated statistically significant better quality of life scores for the treatment group versus the control group *Statistically significant 1Functional Living Index-Emesis Note: analyzed placebo (91) and 200mg (90) Patients Experiencing No Significant Nausea (%) p = 0.005* p = 0.029* p = 0.026* 63% 87% 64% 42% 73% 48% Overall Acute Delayed 200mg SOC 12 |
|
|
ONGOING PHASE 3 & IV FORMULATION Overview Trial Design Objectives Global Phase 3 program initiated February 2012 Three studies to enroll 2,400 patients: 2 HEC (n=530) and 1 MEC (n=1,350) Multicenter, randomized, double blind, placebo controlled clinical trial SOC + rolapitant 200mg SOC + placebo Primary Outcome: CR in the “delayed phase” Secondary Outcome: CR in the overall and acute phases of CINV Relevant measures of CINV including the incidence and intensity of nausea Safety and tolerability IV Formulation Plans Conduct dose finding study Followed by bridging safety study to support regulatory strategy 13 |
|
|
ROLAPITANT: A POTENTIALLY DIFFERENTIATED NK-1 RA1 Product / Product Candidate Status Single Dose Oral Single Dose IV No CYP 3A4 DDI Potential Potential to Prevent Nausea2 Rolapitant Phase 3 (oral) EMEND® (aprepitant Oral & fosaprepitant IV)3 Oral 3-Day; 2003 IV 150 mg; 2010 + - - Netupitant -palonosetron combo4 Phase 3 + - - Unknown 3-day oral 1 No head-to-head clinical trials have been conducted 2 Based upon the secondary endpoint of “No Significant Nausea”; P3 data and the product label (aprepitant, fosaprepitant) and P2 data (rolapitant) 3 Merck 4 Helsinn (US- Eisai) + + + + 14 |
|
|
ROLAPITANT HIGHLIGHTS Highly potent, long acting NK-1 receptor antagonist Differentiation includes: Long half-life Reduced risk of CYP 3A4 drug interactions Rapid onset of activity Potential for reduction in significant nausea Phase 3 on-going and results anticipated 2H:13 Significant growth potential for the NK-1 RA market 15 |
|
|
NIRAPARIB (PARP INHIBITOR) SOLID TUMORS TESARO, INC. |
|
|
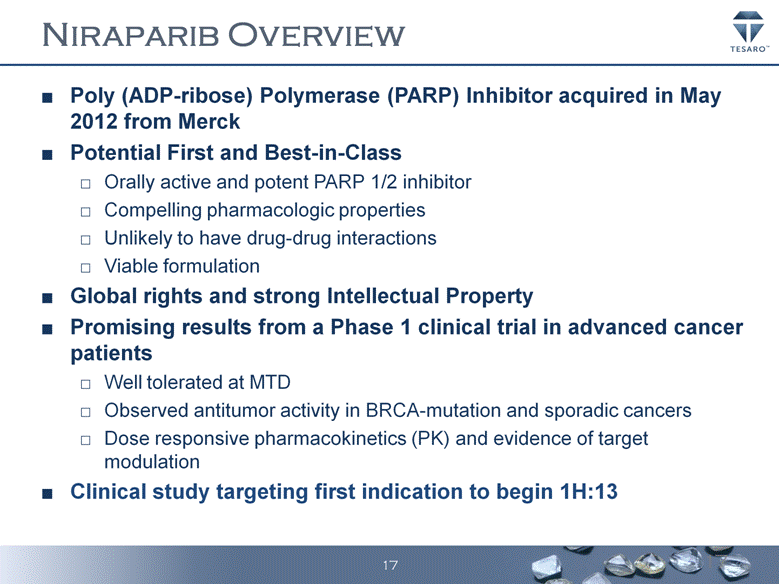
NIRAPARIB OVERVIEW Poly (ADP-ribose) Polymerase (PARP) Inhibitor acquired in May 2012 from Merck Potential First and Best-in-Class Orally active and potent PARP 1/2 inhibitor Compelling pharmacologic properties Unlikely to have drug-drug interactions Viable formulation Global rights and strong Intellectual Property Promising results from a Phase 1 clinical trial in advanced cancer patients Well tolerated at MTD Observed antitumor activity in BRCA-mutation and sporadic cancers Dose responsive pharmacokinetics (PK) and evidence of target modulation Clinical study targeting first indication to begin 1H:13 17 |
|
|
NIRAPARIB PRE-CLINICAL OVERVIEW Potent PARP inhibition on isolated target (IC50 4nM) and in cells (20-30nM) Active in BRCA xenograft models Prolonged PARP inhibition evident in tumors following treatment with niraparib (MK-4827) MDA-MB-436 (BRCA1-mut) Tumor Volume (mm3) Residual PARP Activity in MDA-MB-436 (BRCA1-mut) Tumors Following MK-4827 Treatment 18 |
|
|
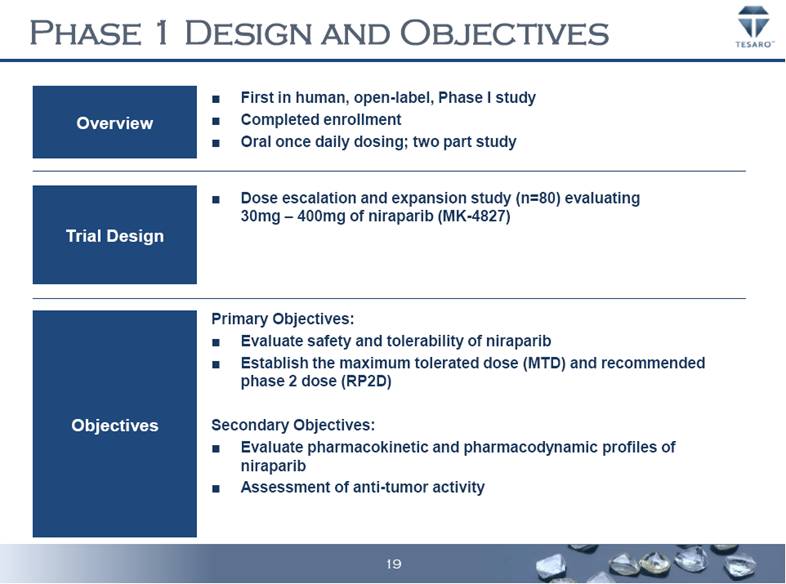
PHASE 1 DESIGN AND OBJECTIVES Overview Trial Design Objectives First in human, open-label, Phase I study Completed enrollment Oral once daily dosing; two part study Dose escalation and expansion study (n=80) evaluating 30mg – 400mg of niraparib (MK-4827) Primary Objectives: Evaluate safety and tolerability of niraparib Establish the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) Secondary Objectives: Evaluate pharmacokinetic and pharmacodynamic profiles of niraparib Assessment of anti-tumor activity 19 |
|
|
PHASE 1 STUDY RESULTS Summary of activity overall in Phase 1 (n=80) PR 12/80 (15%); 10 ovarian, 2 breast SD 24/80 (30%); ovarian, breast, 3/3 lung, other SD > 12 weeks 18/80 (22.5%) Clinical activity in ovarian patients (n=39 evaluable) PR 10/39 (26%); SD 13/39 (33%) BRCA+ patients: PR 7/19 (37%); SD 5/19 (26%); DCR1 63% Ovarian Cancer Patients (ASCO Poster) 1Disease control rate BRCA Status Total Efficacy Assessment PD n (%) SD x 12 weeks n (%) PR n (%) Clinical Benefit Unknown 20 15 7 (47) 4 (27) 3 (20) 47% Negative 6 5 2 (40) 0 0 0% Positive 21 19 7 (37) 4 (21) 7 (37) 58% Combined 47 39 16 (41) 8 (21) 10 (26) 46% 20 |
|
|
NIRAPARIB DEVELOPMENT PLAN Design optimal development plan based upon: Phase 1 trial results Collective experience review of PARP inhibitor clinical studies Discussions with experienced clinical investigators and scientists Biomarker/patient selection strategy Potential cancer targets include breast, gastric, lung, ovarian and others Niraparib has the potential to be Best and First-in-Class Disease control rate of ~ 60% as monotherapy in a selected patient population Demonstrated reduction of PARP activity in humans Dose responsive PK Formulation amenable to commercial application 21 |
|
|
TSR-011 (ALK INHIBITOR) NSCLC AND OTHER CANCERS TESARO, INC. |
|
|
TSR-011 OVERVIEW Global rights to AMGEN’s Anaplastic Lymphoma Kinase (ALK) Program acquired in March 2011 A potentially Best-in-Class molecularly targeted approach to treating certain cancer sub-populations TSR-011 is a highly selective and potent oral compound that possess desirable pharmaceutical properties Clinical trials of Pfizer’s crizotinib (cMET / ALK Inhibitor) demonstrated impressive efficacy in a NSCLC sub-population1 Commercially available diagnostic2 may allow for rapid and efficient development Intend to develop TSR-011 for oncology indications, including the treatment of ALK+ NSCLC Ongoing Phase 1/2 trial to assess activity in each setting in which ALK is altered; initiated third dose level. Potential for rapid and efficient development 23 1Pfizer XALKORI® (crizotinib) approved US, Europe 2Abbott Vysis ALK Break Apart Fish Probe test approved US, Roche VENTANA ALK IHC approved Europe |
|
|
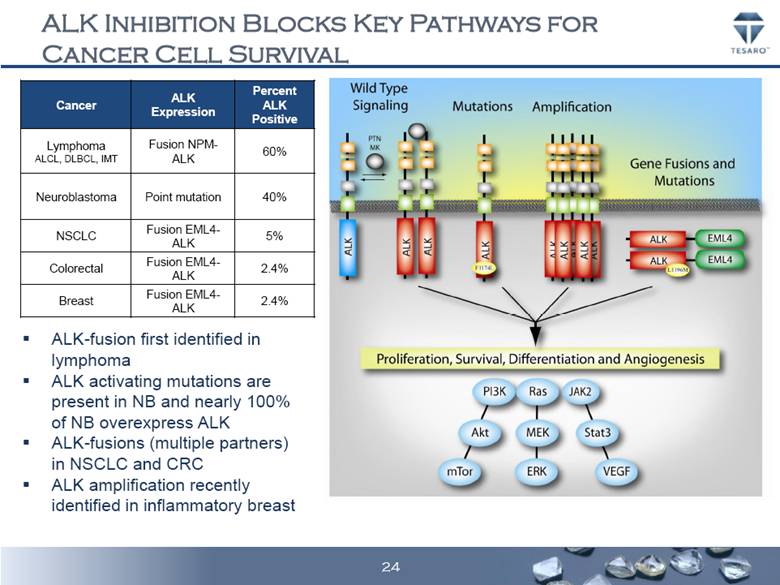
ALK INHIBITION BLOCKS KEY PATHWAYS FOR CANCER CELL SURVIVAL ALK-fusion first identified in lymphoma ALK activating mutations are present in NB and nearly 100% of NB overexpress ALK ALK-fusions (multiple partners) in NSCLC and CRC ALK amplification recently identified in inflammatory breast Cancer ALK Expression Percent ALK Positive Lymphoma ALCL, DLBCL, IMT Fusion NPM- ALK 60% Neuroblastoma Point mutation 40% NSCLC Fusion EML4- ALK 5% Colorectal Fusion EML4- ALK 2.4% Breast Fusion EML4- ALK 2.4% 24 |
|
|
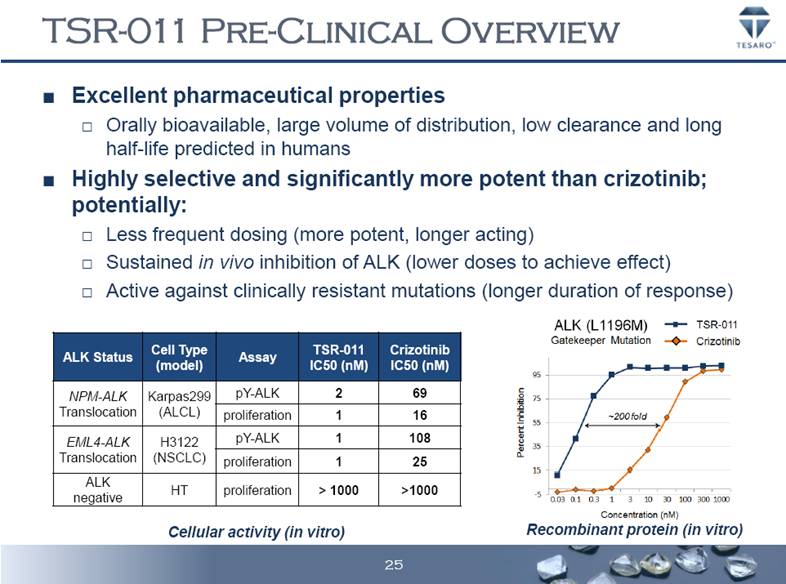
TSR-011 PRE-CLINICAL OVERVIEW Excellent pharmaceutical properties Orally bioavailable, large volume of distribution, low clearance and long half-life predicted in humans Highly selective and significantly more potent than crizotinib; potentially: Less frequent dosing (more potent, longer acting) Sustained in vivo inhibition of ALK (lower doses to achieve effect) Active against clinically resistant mutations (longer duration of response) ALK Status Cell Type (model) Assay TSR-011 IC50 (nM) Crizotinib IC50 (nM) NPM-ALK Translocation Karpas299 (ALCL) pY-ALK 2 69 proliferation 1 16 EML4-ALK Translocation H3122 (NSCLC) pY-ALK 1 108 proliferation 1 25 ALK negative HT proliferation > 1000 >1000 Recombinant protein (in vitro) Cellular activity (in vitro) 25 |
|
|
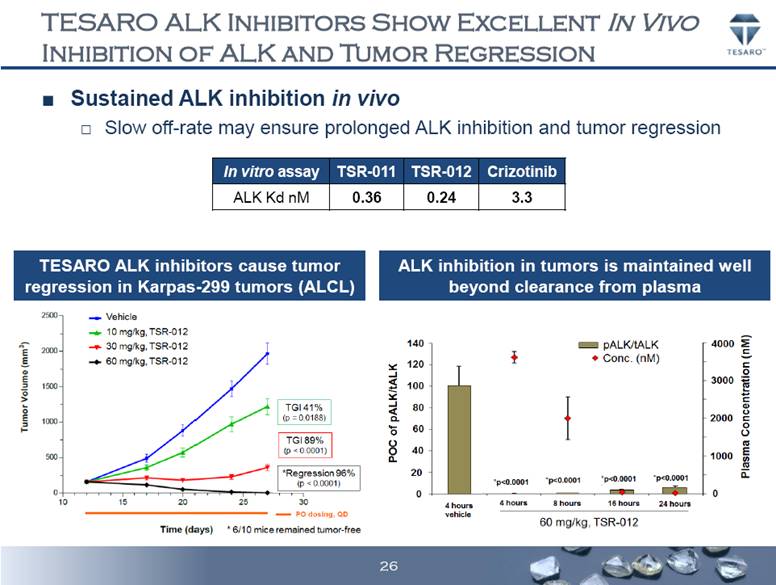
TESARO ALK INHIBITORS SHOW EXCELLENT IN VIVO INHIBITION OF ALK AND TUMOR REGRESSION Sustained ALK inhibition in vivo Slow off-rate may ensure prolonged ALK inhibition and tumor regression TESARO ALK inhibitors cause tumor regression in Karpas-299 tumors (ALCL) ALK inhibition in tumors is maintained well beyond clearance from plasma In vitro assay TSR-011 TSR-012 Crizotinib ALK Kd nM 0.36 0.24 3.3 26 |
|
|
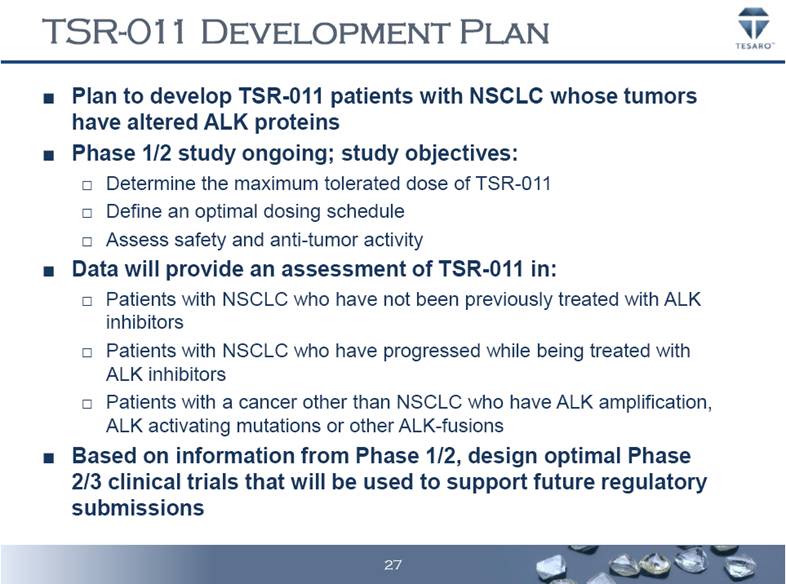
TSR-011 DEVELOPMENT PLAN Plan to develop TSR-011 patients with NSCLC whose tumors have altered ALK proteins Phase 1/2 study ongoing; study objectives: Determine the maximum tolerated dose of TSR-011 Define an optimal dosing schedule Assess safety and anti-tumor activity Data will provide an assessment of TSR-011 in: Patients with NSCLC who have not been previously treated with ALK inhibitors Patients with NSCLC who have progressed while being treated with ALK inhibitors Patients with a cancer other than NSCLC who have ALK amplification, ALK activating mutations or other ALK-fusions Based on information from Phase 1/2, design optimal Phase 2/3 clinical trials that will be used to support future regulatory submissions 27 |
|
|
SUMMARY TESARO, INC. |
|
|
SUMMARY Late stage oncology supportive care product Differentiated product profile Phase 3 data expected 2H:13 Significant market potential Rapidly advancing a development-stage pipeline of cancer therapeutics Potentially First and Best-in-Class PARP inhibitor Potentially Best-in-Class ALK inhibitor Proven management team with highly relevant experience Well capitalized company with strong investor backing 29 |
|
|
An oncology-focused biopharmaceutical company dedicated to improving the lives of cancer patients by developing and commercializing safer and more effective supportive care agents and therapeutics. MISSION STATEMENT 30 |