Attached files
| file | filename |
|---|---|
| 8-K - SUCAMPO PHARMACEUTICALS, INC. 8-K - Sucampo Pharmaceuticals, Inc. | a50522335.htm |
| EX-99.6 - EXHIBIT 99.6 - Sucampo Pharmaceuticals, Inc. | a50522335ex99_6.htm |
| EX-99.3 - EXHIBIT 99.3 - Sucampo Pharmaceuticals, Inc. | a50522335ex99_3.htm |
| EX-99.2 - EXHIBIT 99.2 - Sucampo Pharmaceuticals, Inc. | a50522335ex99_2.htm |
| EX-99.4 - EXHIBIT 99.4 - Sucampo Pharmaceuticals, Inc. | a50522335ex99_4.htm |
| EX-99.5 - EXHIBIT 99.5 - Sucampo Pharmaceuticals, Inc. | a50522335ex99_5.htm |
| EX-99.1 - EXHIBIT 99.1 - Sucampo Pharmaceuticals, Inc. | a50522335ex99_1.htm |
Exhibit 99.7

SUCAMPO PHARMACEUTICALS, INC. Corporate Update January 2013

2 Ryuji Ueno, M.D., Ph.D., Ph.D. Chairman, Chief Executive Officer, and Chief Scientific Officer Cary J. Claiborne Chief Financial Officer Peter Lichtlen, M.D., Ph.D. Senior Medical Officer and Vice President of European Operations Stanley G. Miele Senior Vice President, Sales and Marketing, Sucampo Pharmaceuticals, Inc. / President, Sucampo Pharma Americas, LLC Silvia Taylor Senior Vice President, Investor Relations, Public Relations, and Corporate Communications Erika Trahan Financial Reporting and Communications Manager

3• This press release contains “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements are based on management’s current expectations and involve risks and uncertainties, which may cause results to differ materially from those set forth in the statements. The forward-looking statements may include statements regarding product development, product potential, future financial and operating results, and other statements that are not historical facts. The following factors, among others, could cause actual results to differ from those set forth in the forward-looking statements: the impact of pharmaceutical industry regulation and health care legislation; Sucampo’s ability to accurately predict future market conditions; dependence on the effectiveness of Sucampo’s patents and othe protections for innovative products; the risk of new and changing regulation and health policies in the US and internationally and the exposure to litigation and/or regulatory actions. • No forward-looking statement can be guaranteed and actual results may differ materially from those projected. Sucampo undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise. Forward-looking statements in this presentation should be evaluated together with the many uncertainties that affect Sucampo’s business, particularly those mentioned in the risk factors and cautionary statements in Sucampo’s Form 10-K for the year ended Dec. 31, 2011 and in Sucampo’s Form 10-Qs for the first three quarters of 2012, which the Company incorporates by reference.
4 Sucampo Snapshot: Prostone Pioneers Commercial-stage, global biopharmaceutical company • 2 FDA-approved drugs based on our proprietary prostone technology – AMITIZA® (lubiprostone) in gastroenterology – RESCULA® (unoprostone isopropyl) in ophthalmics Prostone pioneers • Therapeutic potential 1st identified by Sucampo’s founders, Drs Ryuji Ueno and Sachiko Kuno Sucampo Mission To develop and commercialize prostone-based medicines to meet the major unmet medical needs of patients on a global basis ® Registered trademark of Sucampo
5 Sucampo Has Pioneered the Field of Prostones • Prostones: – Functional fatty acids naturally occuring in the human body – Selective ion-channel activators – Physiological mediators of restoration of cellular homeostasis and tissue regeneration • Clinical safety profile of prostones is excellent, as demonstrated by the clinical safety record of AMITIZA and RESCULA • Clinical potential of prostones is broad and applicable to various therapeutic fields beyond those already established Sucampo is the only company developing and commercializing prostone compounds globally See Reference 1
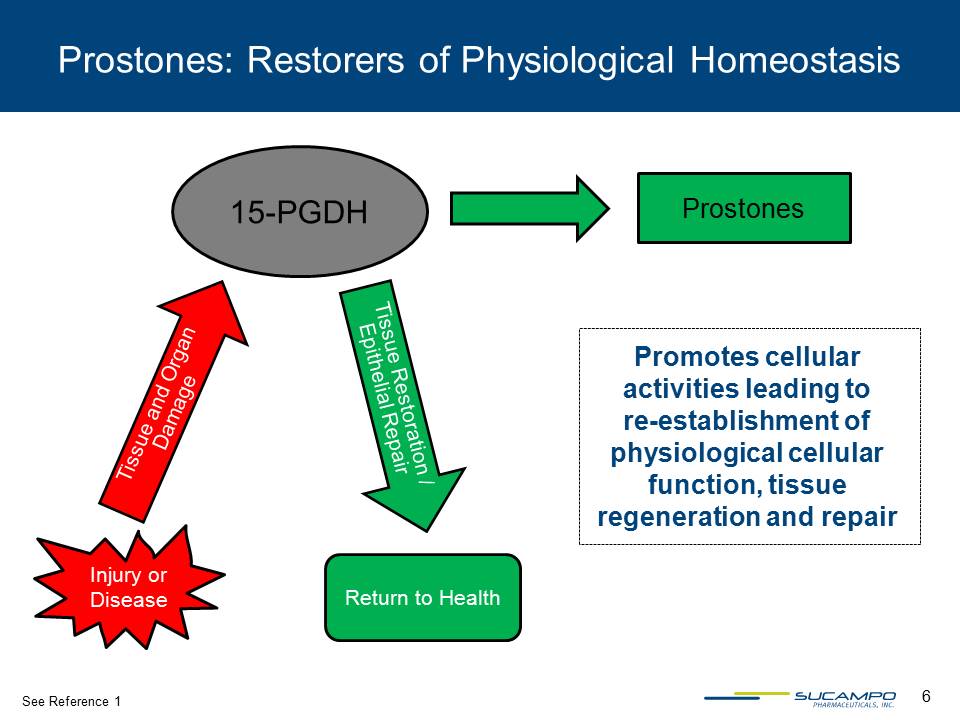
6 See Reference 1 Promotes cellular activities leading to re-establishment of physiological cellular function, tissue regeneration and repair 15-PGDH Prostones
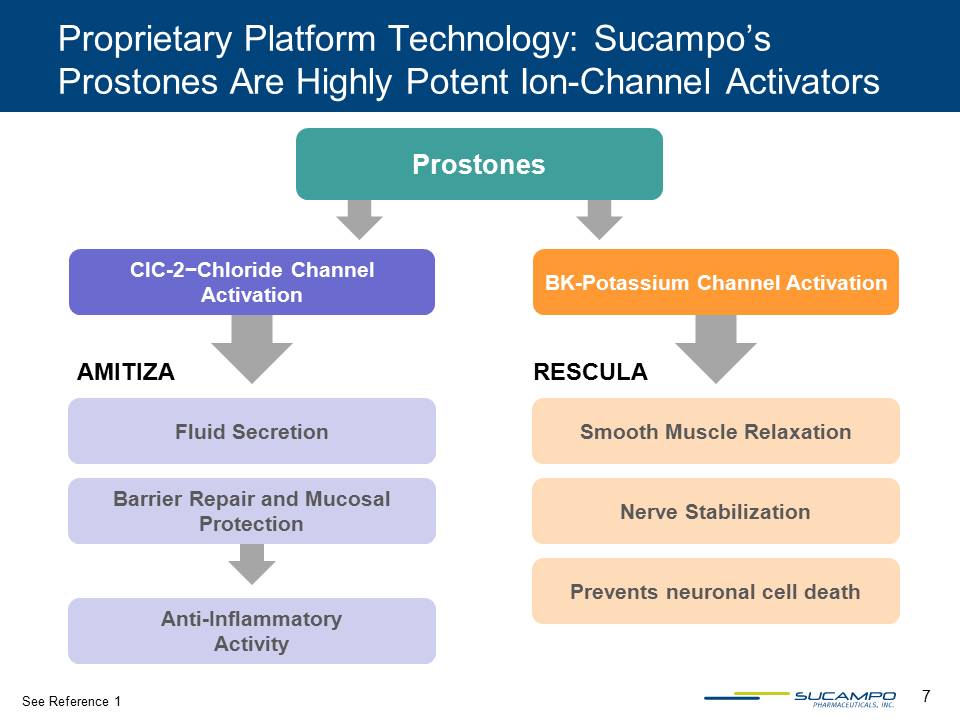
7 Proprietary Platform Technology: Sucampo’s Prostones Are Highly Potent Ion-Channel Activators AMITIZA RESCULA Fluid Secretion Barrier Repair and Mucosal Protection Anti-Inflammatory Activity ClC-2−Chloride Channel Activation BK-Potassium Channel Activation Prevents neuronal cell death Smooth Muscle Relaxation Nerve Stabilization Prostones See Reference 1
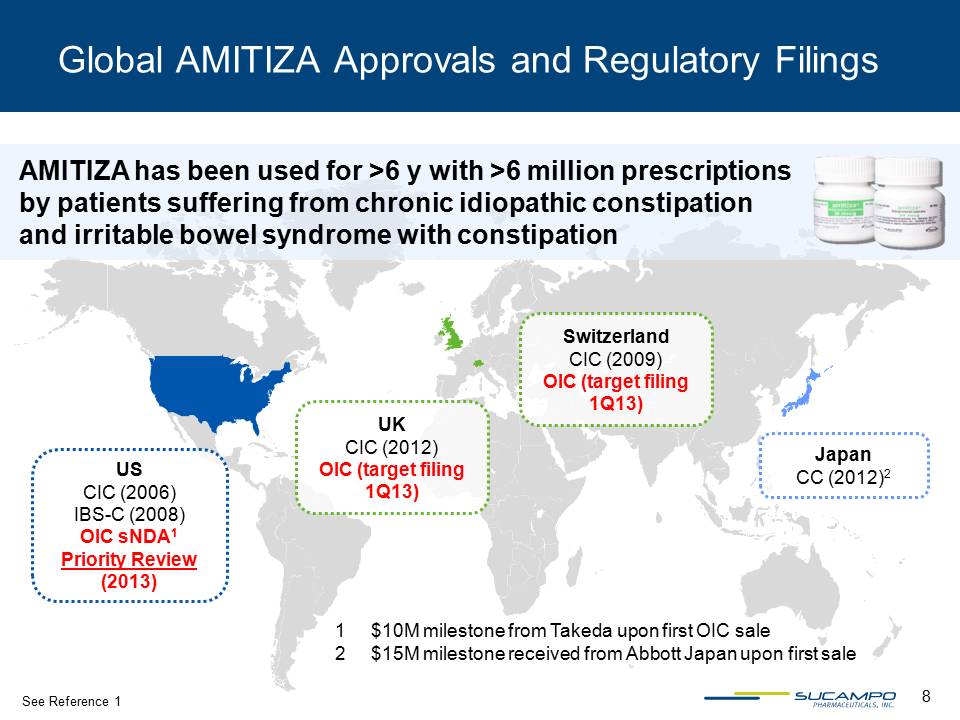
8 Global AMITIZA Approvals and Regulatory Filings Japan CC (2012)2 UK CIC (2012) OIC (target filing 1Q13) US CIC (2006) IBS-C (2008) OIC sNDA1 Priority Review (2013) Switzerland CIC (2009) OIC (target filing 1Q13) AMITIZA has been used for >6 y with >6 million prescriptions by patients suffering from chronic idiopathic constipation and irritable bowel syndrome with constipation 1 $10M milestone from Takeda upon first OIC sale 2 $15M milestone received from Abbott Japan upon first sale See Reference 1

9 AMITIZA Label Update • December 7, 2012 Updates to AMITIZA Label – All pregnancy-related warnings and precautions have been removed, including the removal of a requirement for a negative pregnancy test prior to beginning therapy – Product labeling was updated to include additional animal data and a clinical consideration section, with the pregnancy category remaining unchanged – Previous labeling statements regarding the potential for serious adverse reactions in nursing infants have been removed, although caution should be exercised when AMITIZA is administered to a nursing mother and Nursing infants should be monitored for diarrhea – The mechanism of action section of the label was updated to include that lubiprostone has been shown to reduce intestinal permeability
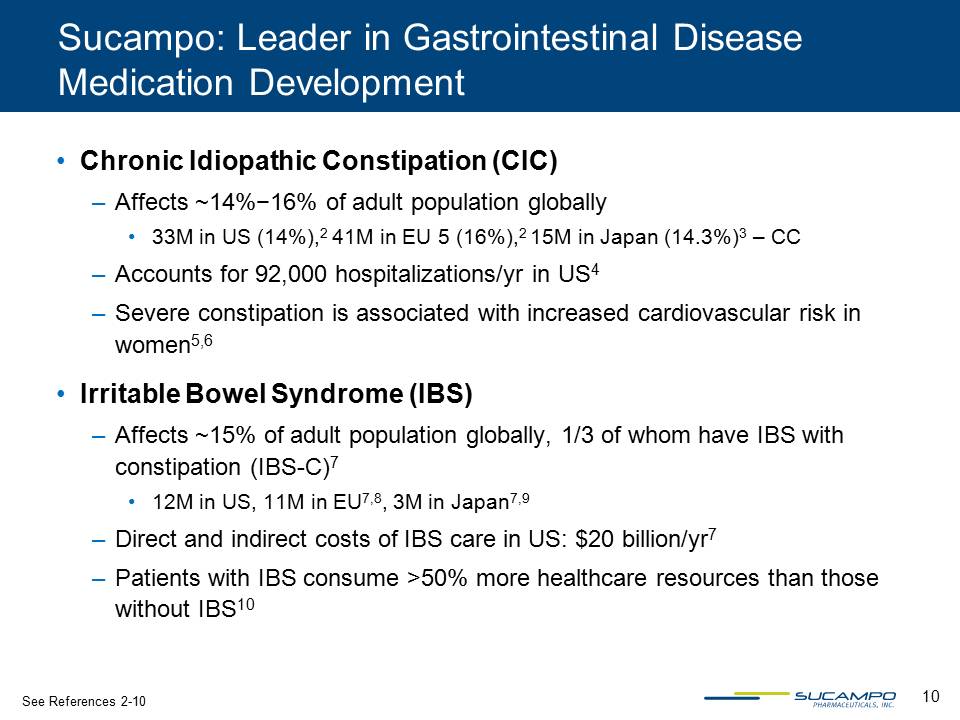
10 Sucampo: Leader in Gastrointestinal Disease Medication Development • Chronic Idiopathic Constipation (CIC) – Affects ~14%−16% of adult population globally • 33M in US (14%),2 41M in EU 5 (16%),2 15M in Japan (14.3%)3 – CC – Accounts for 92,000 hospitalizations/yr in US4 – Severe constipation is associated with increased cardiovascular risk in women5,6 • Irritable Bowel Syndrome (IBS) – Affects ~15% of adult population globally, 1/3 of whom have IBS with constipation (IBS-C)7 • 12M in US, 11M in EU7,8, 3M in Japan7,9 – Direct and indirect costs of IBS care in US: $20 billion/yr7 – Patients with IBS consume >50% more healthcare resources than those without IBS10 See References 2-10
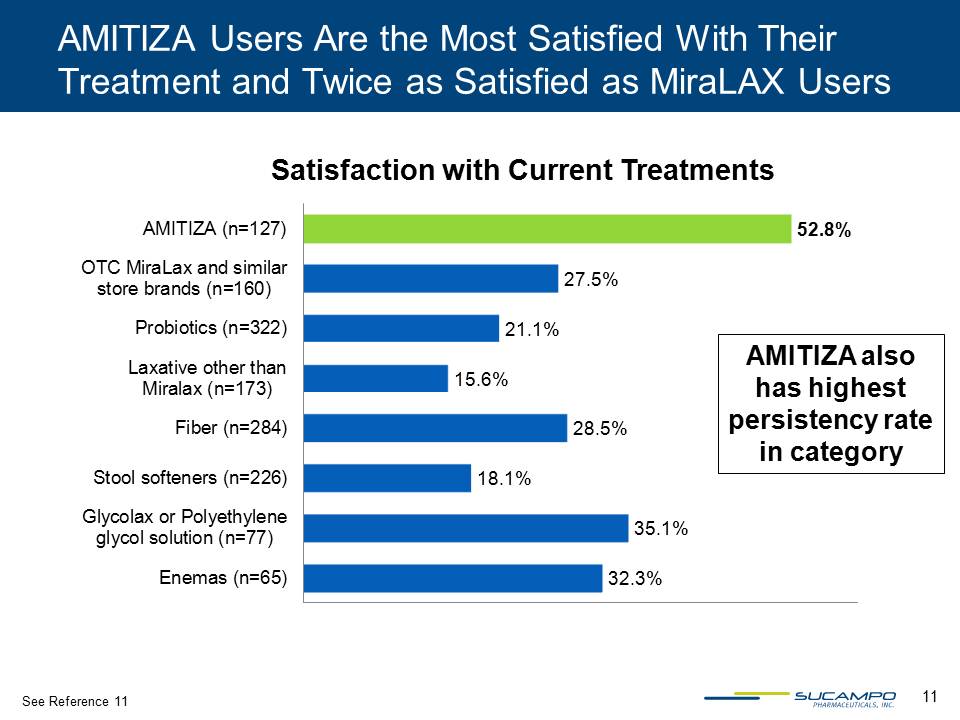
11 AMITIZA Users Are the Most Satisfied With Their Treatment and Twice as Satisfied as MiraLAX Users 32.3% 35.1% 18.1% 28.5% 15.6% 21.1% 27.5% 52.8% Enemas (n=65) Glycolax or Polyethylene glycol solution (n=77) Stool softeners (n=226) Fiber (n=284) Laxative other than Miralax (n=173) Probiotics (n=322) OTC MiraLax and similar store brands (n=160) AMITIZA (n=127) Satisfaction with Current Treatments AMITIZA also has highest persistency rate in category See Reference 11
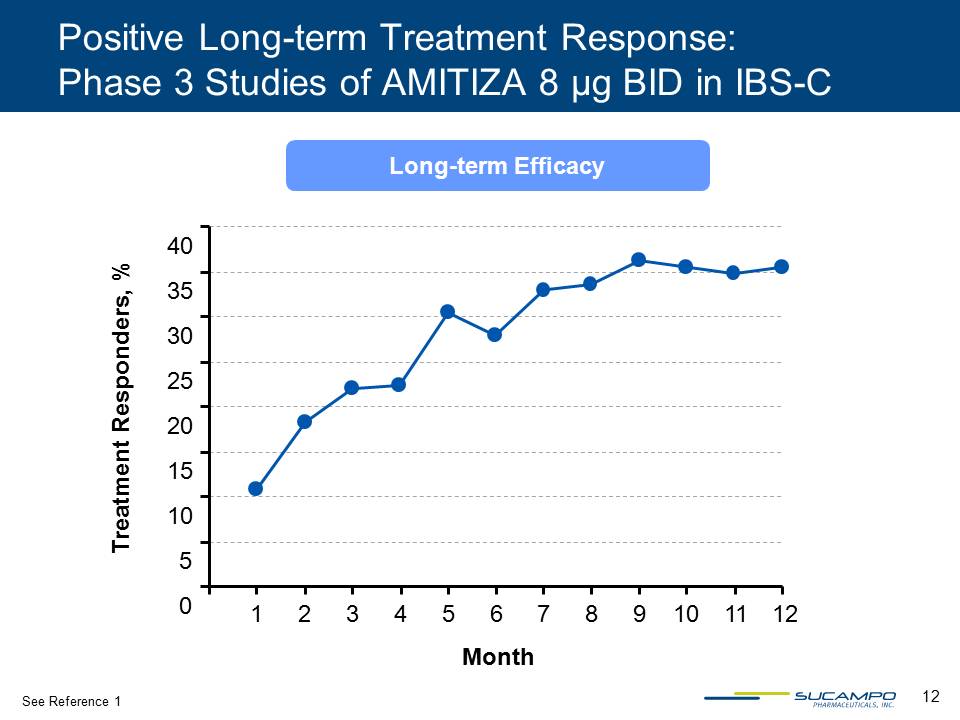
12 Long-term Efficacy Positive Long-term Treatment Response: Phase 3 Studies of AMITIZA 8 µg BID in IBS-C Month 0 5 10 15 20 25 30 35 40 Treatment Responders, % 1 2 3 4 5 6 7 8 9 10 11 12 See Reference 1
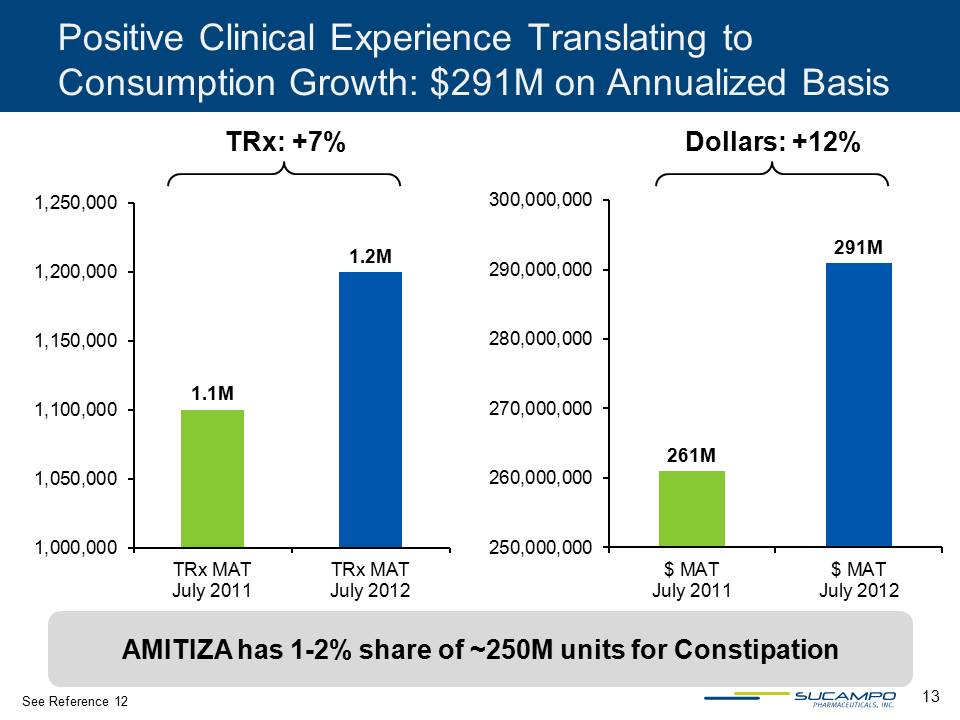
13 1.1M 1.2M 1,000,000 1,050,000 1,100,000 1,150,000 1,200,000 1,250,000 TRx MAT July 2011 TRx MAT July 2012 261M 291M 250,000,000 260,000,000 270,000,000 280,000,000 290,000,000 300,000,000 $ MAT July 2011 $ MAT July 2012 TRx: +7% Dollars: +12% Positive Clinical Experience Translating to Consumption Growth: $291M on Annualized Basis See Reference 12 AMITIZA has 1-2% share of ~250M units for Constipation
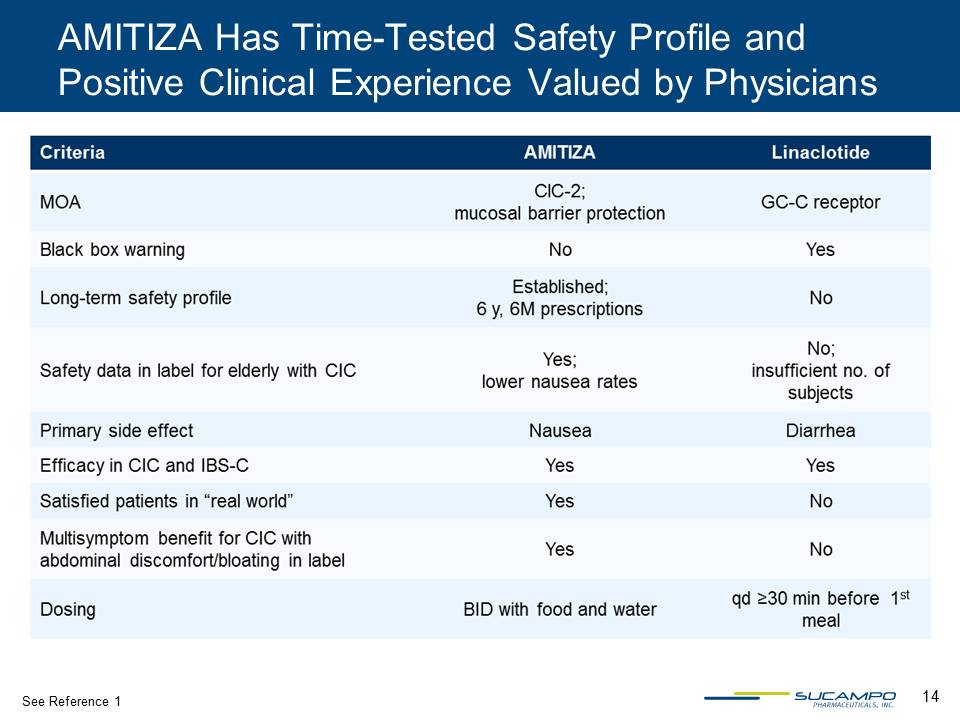
14 AMITIZA Has Time-Tested Safety Profile and Positive Clinical Experience Valued by Physicians See Reference 1
15 Opioid-Induced Constipation: Increase Potential Population for AMITIZA and Strengthen Efficacy Positioning • Moderate−severe OIC affects ~2.0M−2.5M patients – Currently no approved oral product for OIC – Most common reason for discontinuation of opioid therapy – OIC patients are viewed as “difficult to treat” and are dissatisfied – PCPs welcome 1 medicine indicated for multiple causes of constipation • AMITIZA does not act on opiate receptors or inhibit analgesic activity of opioid therapy • Mu-opioid−receptor agonist compounds under development may have cardiac safety concerns See References 13-16 FDA priority review action date: late April 2013
16 AMITIZA Intellectual Property • Paragraph IV certification notice letter to Sucampo received on January 2, 2013 regarding ANDA submitted to FDA by Anchen Pharmaceuticals • AMITIZA has a robust patent estate – Latest patents expire in 2027 • Notice letter alleges AMITIZA’s composition, method of use, and/or formulation patents are invalid, unenforceable, and/or will not be infringed by Anchen’s manufacture, use or sale of the product described in its ANDA. • Sucampo intends to vigorously enforce our intellectual property Hatch-Waxman Act provides that a federal court lawsuit will preclude FDA from approving ANDA until the earlier of 30 months or a district court decision finding AMITIZA’s patents invalid
17 Summary and Outlook for AMITIZA • Continue growth in US: over 6M prescriptions used over 6+ years, with favorable benefit-risk profile • Near-term goals – Leverage new US product label, which removed pregnancy warnings and precautions (including removal of requirement for negative pregnancy test prior to therapy initiation), clarified information regarding use in pregnant and/or nursing women, and expanded labeling text of the Mechanism of Action – Seek approval for OIC indication in US and submit labeling applications for OIC abroad – Expand global approvals and launches for AMITIZA worldwide – Develop and seek approval for AMITIZA in pediatric constipation • Currently unmet medical need; no approved prescription medications – Develop liquid formulation of AMITIZA for pediatric and long-term care markets – Evaluate potential of AMITIZA for new indications, such as mixed irritable bowel syndrome
18 Sucampo Is an Emerging Player in Ophthalmics: RESCULA • Glaucoma is a group of ocular diseases with various causes that ultimately are associated with a progressive optic neuropathy leading to loss of vision • Age-related disease: − Second leading cause of bilateral blindness worldwide − Will affect an estimated 79.6 million people worldwide by 202018 • Reduction in intra-ocular pressure (IOP) is currently the only modifiable risk factor for patients with glaucoma and ocular\ hypertension See References 17-18
19 US Glaucoma Market Overview • The US glaucoma market is 29.2M TRx’s22 4-5M potential patients21,22,24 ̶ 67% of the market is generic22 ̶ 80% of TRx’s are by eye specialists22 – ~$3B: US sales volume (2012) – ~$1B: Japan sales volume (2011) • Compliance and adherence are unmet needs – 50% of new patients drop off therapy within one year of initiation • Prostaglandins are inflammatory agents which depolarize cell\ membranes – #1 reason for discontinuation of prostaglandins is hyperemia20,23,24 See References 19-24
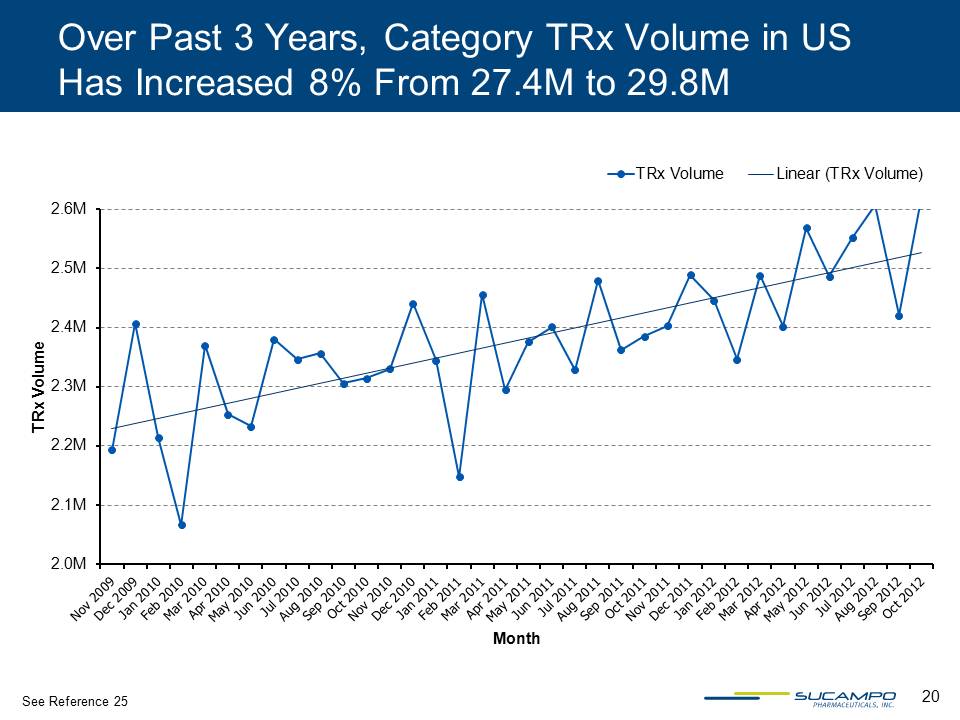
20 Over Past 3 Years, Category TRx Volume in US Has Increased 8% From 27.4M to 29.8M 2.0M 2.1M 2.2M 2.3M 2.4M 2.5M 2.6M TRx Volume Month TRx Volume Linear (TRx Volume) See Reference 25
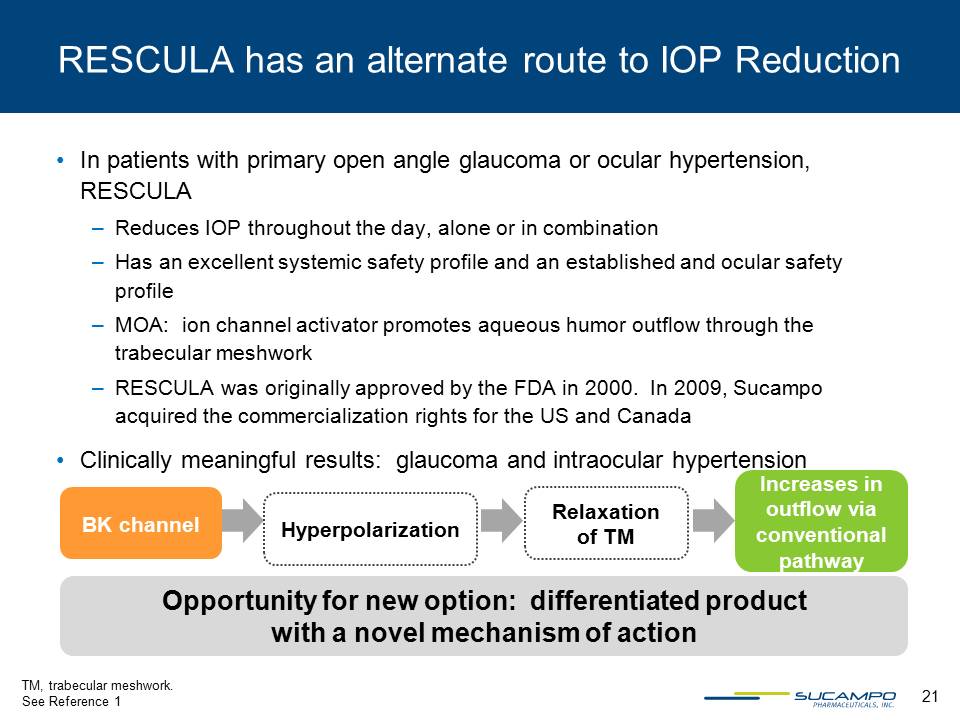
21 RESCULA has an alternate route to IOP Reduction • In patients with primary open angle glaucoma or ocular hypertension, RESCULA – Reduces IOP throughout the day, alone or in combination – Has an excellent systemic safety profile and an established and ocular safety profile – MOA: ion channel activator promotes aqueous humor outflow through the trabecular meshwork – RESCULA was originally approved by the FDA in 2000. In 2009, Sucampo acquired the commercialization rights for the US and Canada • Clinically meaningful results: glaucoma and intraocular hypertension BK channel Hyperpolarization Relaxation of TM Increases in outflow via conventional pathway TM, trabecular meshwork. See Reference 1 Opportunity for new option: differentiated product with a novel mechanism of action
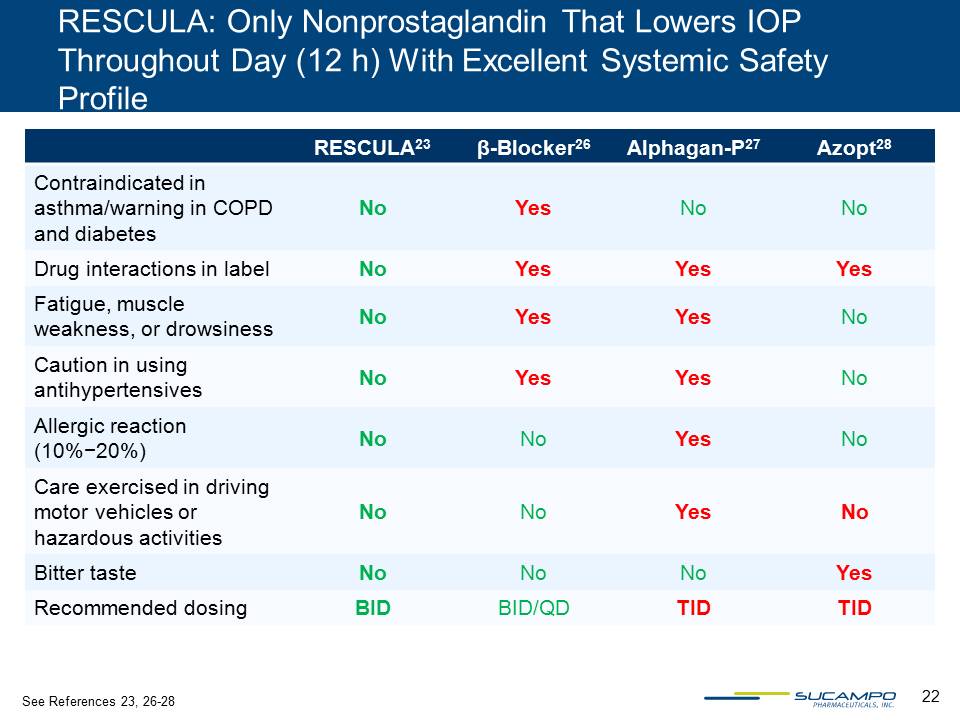
22 RESCULA: Only Nonprostaglandin That Lowers IOP Throughout Day (12 h) With Excellent Systemic Safety Profile RESCULA23 β-Blocker26 Alphagan-P27 Azopt28 Contraindicated in asthma/warning in COPD and diabetes No Yes No No Drug interactions in label No Yes Yes Yes Fatigue, muscle weakness, or drowsiness No Yes Yes No Caution in using antihypertensives No Yes Yes No Allergic reaction (10%−20%) No No Yes No Care exercised in driving motor vehicles or hazardous activities No No Yes No Bitter taste No No No Yes Recommended dosing BID BID/QD TID TID See References 23, 26-28

23 RESCULA US Launch Overview • sNDA approved December 2012 – RESCULA may be used as a first-line agent or concomitantly with other topical ophthalmic drug products to lower intraocular pressure – RESCULA is a BK (Big Potassium) channel activator, which is different from other intraocular pressure lowering agents. – RESCULA is believed to reduce elevated IOP by increasing the outflow of aqueous humor through the trabecular meshwork • Sucampo plans to launch RESCULA in US Q1 2013
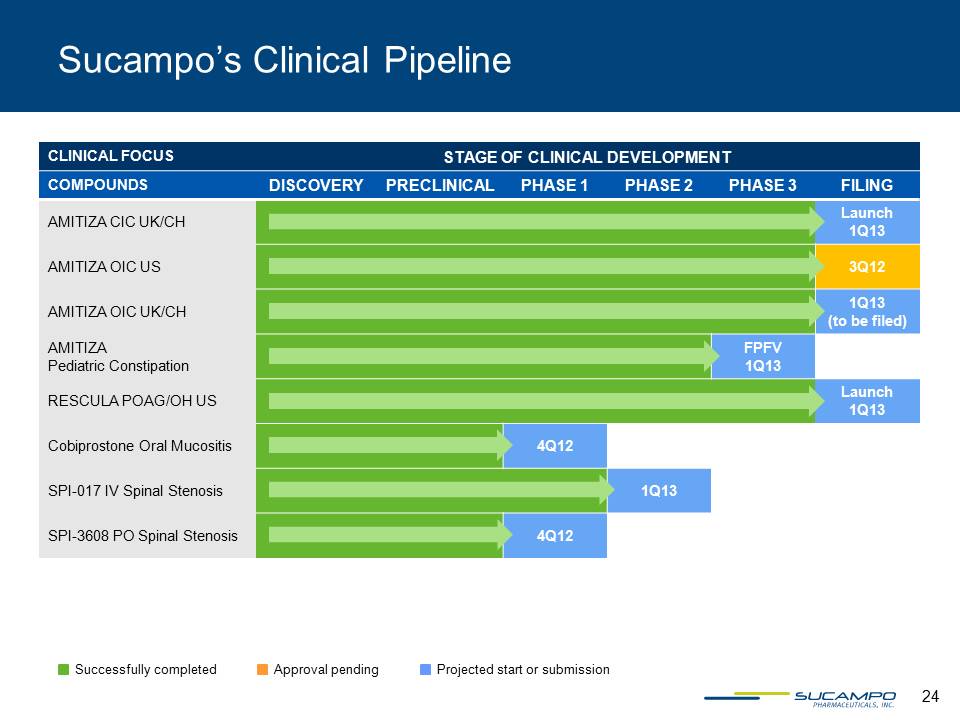
24 Sucampo’s Clinical Pipeline CLINICAL FOCUS STAGE OF CLINICAL DEVELOPMENT COMPOUNDS DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 FILING AMITIZA CIC UK/CH Launch 1Q13 AMITIZA OIC US 3Q12 AMITIZA OIC UK/CH 1Q13 (to be filed) AMITIZA Pediatric Constipation FPFV 1Q13 RESCULA POAG/OH US Launch 1Q13 Cobiprostone Oral Mucositis 4Q12 SPI-017 IV Spinal Stenosis 1Q13 SPI-3608 PO Spinal Stenosis 4Q12 Successfully completed Approval pending Projected start or submission
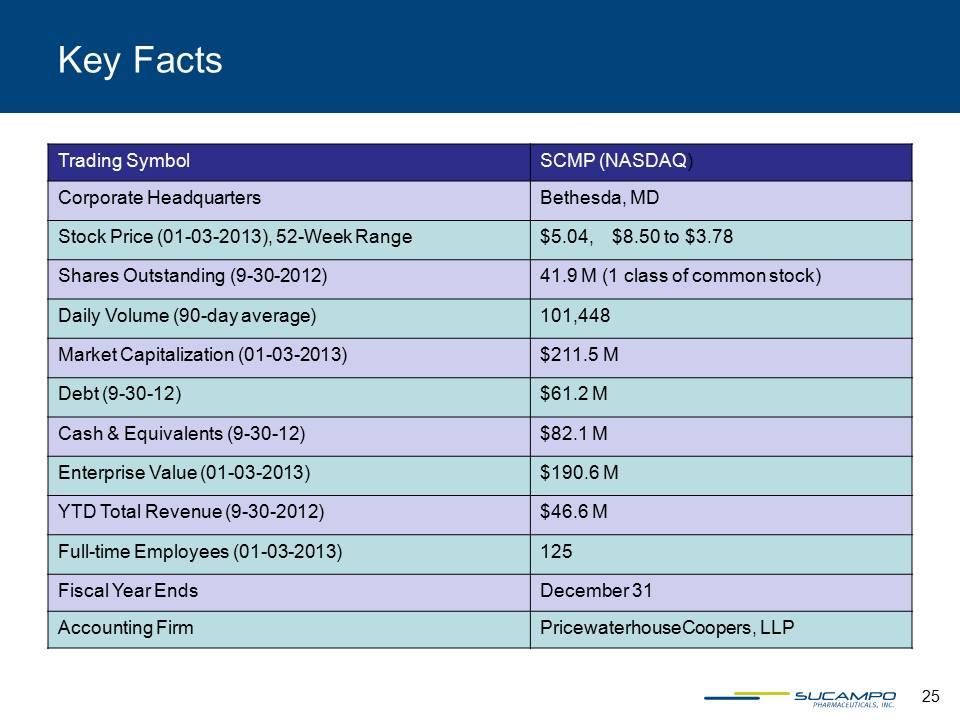
25 Trading Symbol SCMP (NASDAQ) Corporate Headquarters Bethesda, MD Stock Price (01-03-2013), 52-Week Range $5.04, $8.50 to $3.78 Shares Outstanding (9-30-2012) 41.9 M (1 class of common stock) Daily Volume (90-day average) 101,448 Market Capitalization (01-03-2013) $211.5 M Debt (9-30-12) $61.2 M Cash & Equivalents (9-30-12) $82.1 M Enterprise Value (01-03-2013) $190.6 M YTD Total Revenue (9-30-2012) $46.6 M Full-time Employees (01-03-2013) 125 Fiscal Year Ends December 31 Accounting Firm PricewaterhouseCoopers, LLP
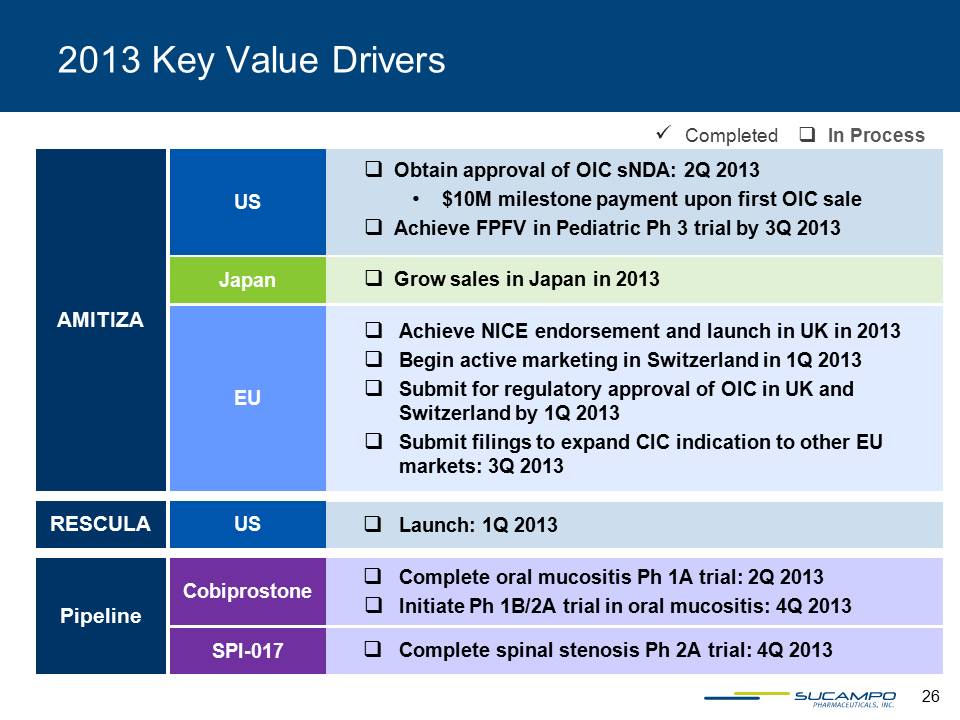
26 2013 Key Value Drivers Completed In Process Obtain approval of OIC sNDA: 2Q 2013 • $10M milestone payment upon first OIC sale Achieve FPFV in Pediatric Ph 3 trial by 3Q 2013 US AMITIZA Grow sales in Japan in 2013 Japan Achieve NICE endorsement and launch in UK in 2013 Begin active marketing in Switzerland in 1Q 2013 Submit for regulatory approval of OIC in UK and Switzerland by 1Q 2013 Submit filings to expand CIC indication to other EU markets: 3Q 2013 EU RESCULA Launch: 1Q 2013 US Pipeline Complete oral mucositis Ph 1A trial: 2Q 2013 Initiate Ph 1B/2A trial in oral mucositis: 4Q 2013 Cobiprostone Complete spinal stenosis Ph 2A trial: 4Q 2013

Appendix SUCAMPO PHARMACEUTICALS, INC.
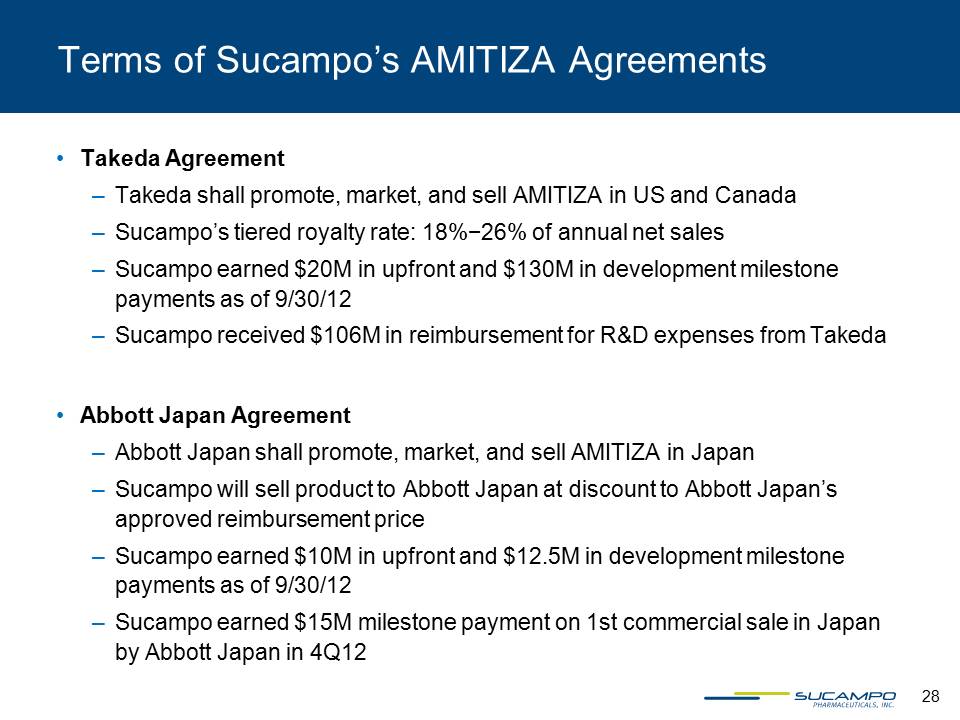
28 Terms of Sucampo’s AMITIZA Agreements • Takeda Agreement – Takeda shall promote, market, and sell AMITIZA in US and Canada – Sucampo’s tiered royalty rate: 18%−26% of annual net sales – Sucampo earned $20M in upfront and $130M in development milestone payments as of 9/30/12 – Sucampo received $106M in reimbursement for R&D expenses from Takeda • Abbott Japan Agreement – Abbott Japan shall promote, market, and sell AMITIZA in Japan – Sucampo will sell product to Abbott Japan at discount to Abbott Japan’s approved reimbursement price – Sucampo earned $10M in upfront and $12.5M in development milestone payments as of 9/30/12 – Sucampo earned $15M milestone payment on 1st commercial sale in Japan by Abbott Japan in 4Q12
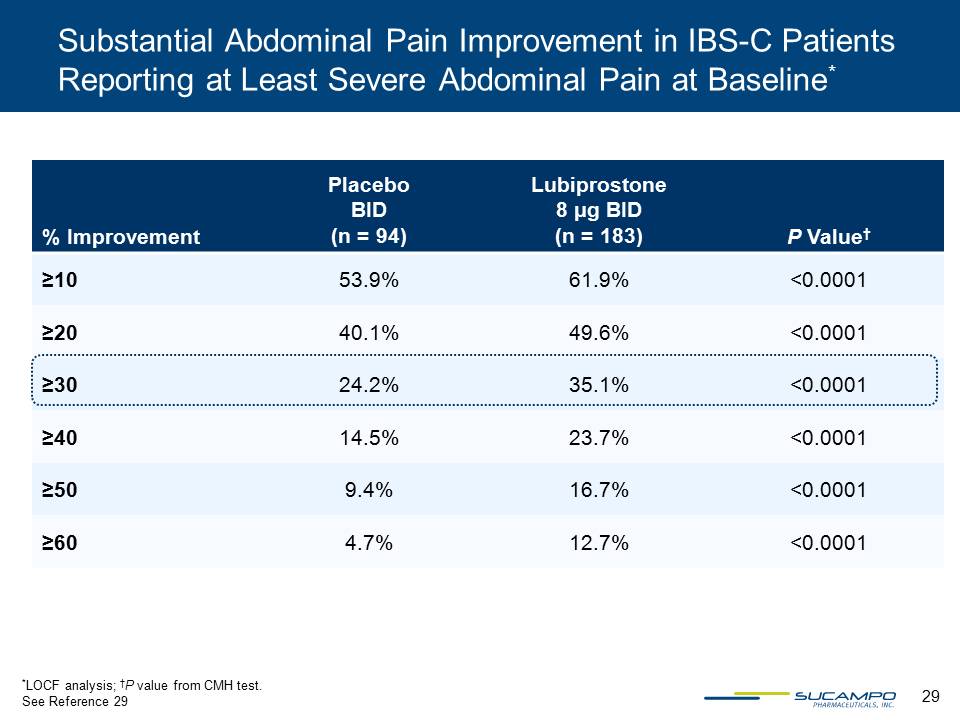
29 Substantial Abdominal Pain Improvement in IBS-C Patients Reporting at Least Severe Abdominal Pain at Baseline* % Improvement Placebo BID (n = 94) Lubiprostone 8 µg BID (n = 183) P Value† ≥10 53.9% 61.9% <0.0001 ≥20 40.1% 49.6% <0.0001 ≥30 24.2% 35.1% <0.0001 ≥40 14.5% 23.7% <0.0001 ≥50 9.4% 16.7% <0.0001 ≥60 4.7% 12.7% <0.0001 *LOCF analysis; †P value from CMH test. See Reference 29
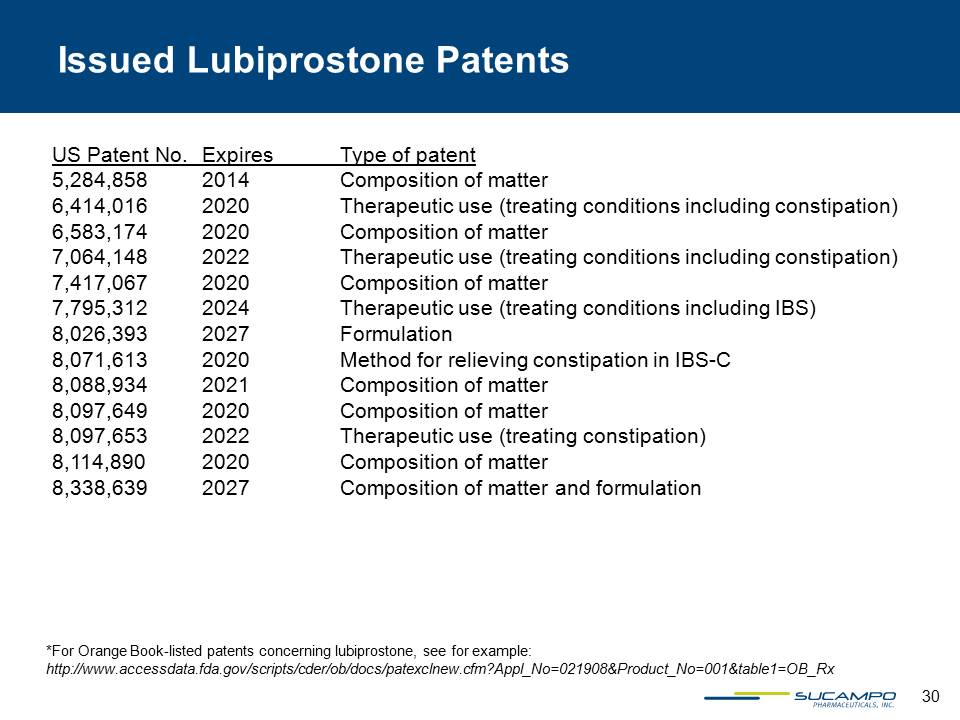
30 US Patent No. Expires Type of patent 5,284,858 2014 Composition of matter 6,414,016 2020 Therapeutic use (treating conditions including constipation) 6,583,174 2020 Composition of matter 7,064,148 2022 Therapeutic use (treating conditions including constipation) 7,417,067 2020 Composition of matter 7,795,312 2024 Therapeutic use (treating conditions including IBS) 8,026,393 2027 Formulation 8,071,613 2020 Method for relieving constipation in IBS-C 8,088,934 2021 Composition of matter 8,097,649 2020 Composition of matter 8,097,653 2022 Therapeutic use (treating constipation) 8,114,890 2020 Composition of matter 8,338,639 2027 Composition of matter and formulation Issued Lubiprostone Patents *For Orange Book-listed patents concerning lubiprostone, see for example: http://www.accessdata.fda.gov/scripts/cder/ob/docs/patexclnew.cfm?Appl_No=021908&Product_No=001&table1=OB_Rx
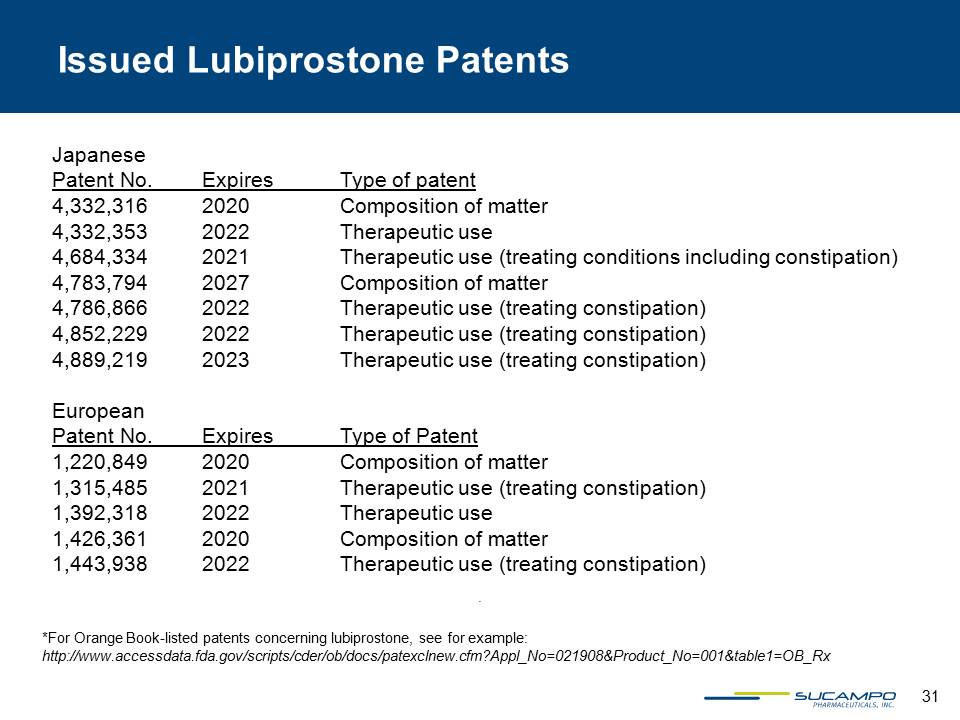
31 Japanese Patent No. Expires Type of patent 4,332,316 2020 Composition of matter 4,332,353 2022 Therapeutic use 4,684,334 2021 Therapeutic use (treating conditions including constipation) 4,783,794 2027 Composition of matter 4,786,866 2022 Therapeutic use (treating constipation) 4,852,229 2022 Therapeutic use (treating constipation) 4,889,219 2023 Therapeutic use (treating constipation) European Patent No. Expires Type of Patent 1,220,849 2020 Composition of matter 1,315,485 2021 Therapeutic use (treating constipation) 1,392,318 2022 Therapeutic use 1,426,361 2020 Composition of matter 1,443,938 2022 Therapeutic use (treating constipation) *For Orange Book-listed patents concerning lubiprostone, see for example:http://www.accessdata.fda.gov/scripts/cder/ob/docs/patexclnew.cfm?Appl_No=021908&Product_No=001&table1=OB_Rx
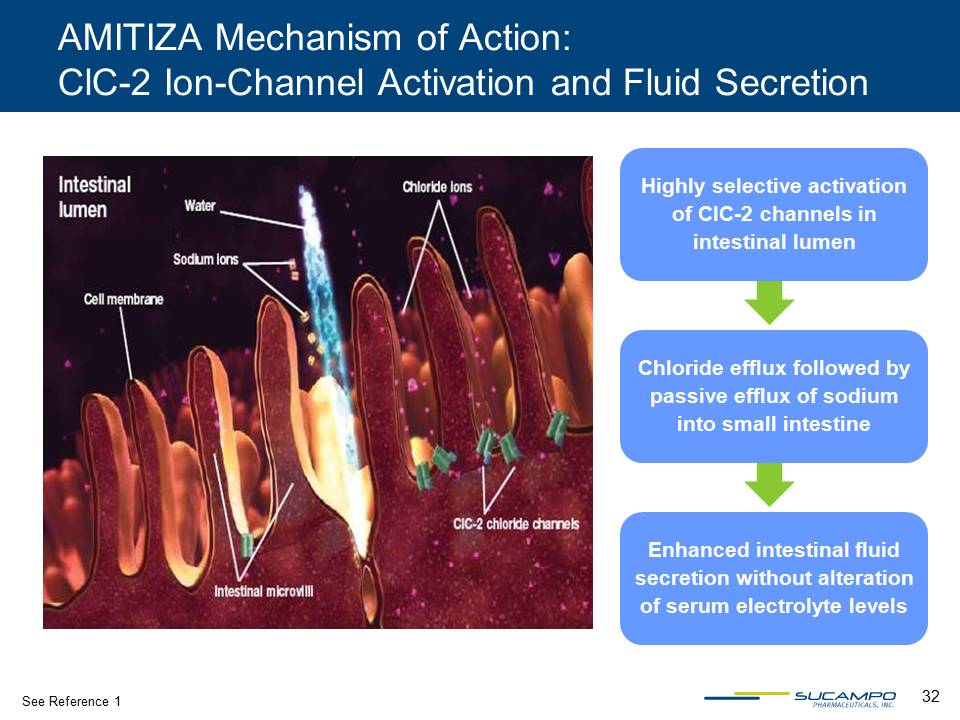
32 AMITIZA Mechanism of Action: ClC-2 Ion-Channel Activation and Fluid Secretion Highly selective activation of ClC-2 channels in intestinal lumen Chloride efflux followed by passive efflux of sodium into small intestine Enhanced intestinal fluid secretion without alteration of serum electrolyte levels See Reference 1
33 References 1. Sucampo data on file. 2. Suares et al. Am J Gastroenterol. 2011 3. Kantar Health Epi database http://epidb.khapps.jp 4. Lembo et al. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 2010 5. Salmoirago-Blotcher et al. Am J Med. 2011 6. Talley et al. Am J Gastroenterol. 2001 7. Saito et al. Am J Gastroenterol. 2002 8. Muller-Lissner S et al. Digestion. 2001 9. Kubo et al. Neurogastroenterol Motil. 2011 10. Hulisz D. J Manag Care Pharm. 2004 11. Sucampo data on file – Physician ATU 12. IMS MAT July 2012 compared with MAT July 2011 13. IMS Health 14. Verispan PDDA 15. Physician Interviews 16. ClearView Analysis 17. RESCULA Package Insert 18. Quigley et al. Br J Ophthalmol 2006 Mar;90(3):252-7

34 References Cont.19. American Academy of Ophthalmology 20. Friedman et al. Prevalence of Open-Angle Glaucoma Among Adults in the United States. Arch Ophthalmol. 2004 Apr;122(4):532-8 21. July 2011-June 2012 MATTY IMS NPS Data 22. July 2011-June 2012 MATTY IMS NPA Data 23. Catalina Presentation 2011 24. Input from KOLs 25. IMS NPA data, MATTY June 2009 to MATTY June 2012 26. Timoptic Prescribing Information; 2005. Merck & Co. Inc., Whitehouse Station, NJ 27. Alphagan-P Prescribing Information. 2005. Allergan Inc, Irvine, CA 28. Azopt Prescribing information. 2000–2009. Alcon Laboratories Inc, Fort Worth, TX 29. Joswick et al. Digestive Disease Week, 2012

January 2013 SUCAMPO PHARMACEUTICALS, INC.
Corporate Update
