Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - INTERMUNE INC | d462967d8k.htm |
 J.P. Morgan 31st Annual
Healthcare Conference
January 7-10, 2013
Exhibit 99.1 |
 2
Forward-looking Statements
This
presentation
contains
forward-looking
statements
made
pursuant
to
the
"safe
harbor"
provisions
of
the
Private
Securities
Litigation Reform Act of 1995. Investors are cautioned that, without limitation,
statements in this presentation regarding InterMune's plans and
expectations; anticipated availability of top-line results from the ASCEND trial; the estimated patient
populations
suffering
from
IPF
and
market
potential
for
Esbriet;
anticipated
timing
of
pricing
and
reimbursement
discussions
and/or initiating commercial launches for Esbriet; intellectual property protection
for Esbriet; and expectations regarding the ASCEND trial and prospects for
success thereof are forward-looking statements. All forward-looking statements included in
this presentation are based on information available to InterMune as of the date
hereof, and InterMune assumes no obligation to update any such
forward-looking statements. Actual results could differ materially from those described in the forward-
looking statements. Factors that could cause or contribute to such differences
include, but are not limited to, those discussed in
detail
under
the
heading
“Risk
Factors”
in
InterMune’s
periodic
reports
filed
with
the
SEC,
including
but
not
limited
to
the
following: (i) the risks related to the uncertain, lengthy and expensive clinical
development process for the company’s product candidates; (ii) risks
related to the regulatory process for Esbriet, including that the results of the ASCEND trial may not be
satisfactory to the FDA to receive regulatory approval; (iii) risks related to
unexpected regulatory actions or delays or government regulation generally;
(iv) risks related to the company’s manufacturing strategy; (v) government, industry and
general public pricing pressures; (vi) risks related to the company’s ability
to successfully launch and commercialize Esbriet; and (vii) the
company’s ability to maintain intellectual property protection. The risks and other factors discussed above should
be considered only in connection with the risks and other factors discussed in
detail in InterMune’s Form 10-K, Form 10-Q and its other
periodic reports filed with the SEC, which are also available at www.intermune.com. |
 3
Our Vision is that in 2015
InterMune is:
•
A biotech company focused on serving patients with
unmet medical needs in specialty pulmonary and
orphan
fibrotic diseases
•
The recognized world-wide leader in IPF
•
Among the Top 20 U.S. small to mid-sized
biopharmaceutical companies in terms of revenue and
market capitalization
•
Successfully marketing Esbriet
®
(pirfenidone) in the EU,
U.S., Canada and other markets of interest, where it is
–
the standard of care and
–
the backbone of combination regimens for treating
IPF patients
•
Advancing an attractive and balanced R&D pipeline
–
Built first
on the Esbriet platform
VISION
2015 |
 4
2012 –
Strong Progress Toward Realizing our Vision
COMMERCIAL
CLINICAL
•
Esbriet launched in 9 EU countries including the two largest:
–
Germany (Sept 2011) and France (November 2012)
–
Esbriet launch in Germany among Top 5 best-ever for orphans
•
Attractive pricing and reimbursement (P&R) of Esbriet
–
Priced in 9 of the Top 15 European countries: $33K-$47K/yr.
–
Strong P&R progress in remaining 6 EU countries
•
Esbriet approved in Canada –
the world’s 9th largest market
•
Closing in on lucrative U.S. IPF market
–
Completion of enrollment in pivotal Phase 3 study –
“ASCEND”
–
Top-Line results in Q2 2014
•
Opportunities in Esbriet LCM and Research programs in fibrosis
|
 Commercial Launch
of Esbriet
®
(pirfenidone) |
 Idiopathic Pulmonary Fibrosis (IPF) –
A Large, Lethal Orphan Disease
•
Progressive scarring of the lungs
with no known cause
–
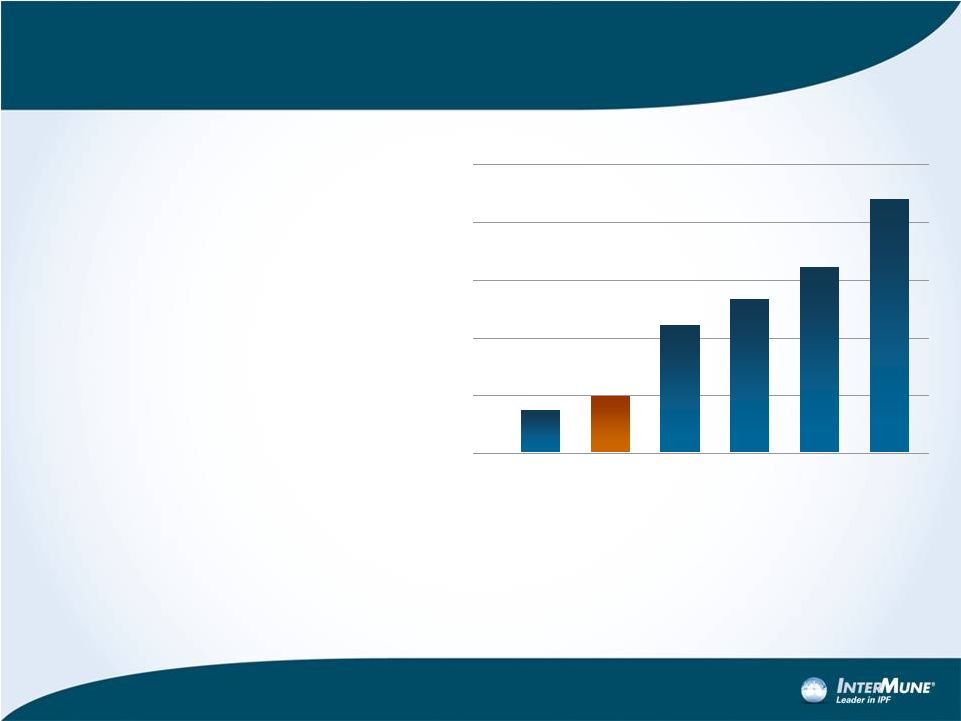
Median survival: 2-5 years
1
•
Large market in NA/EU15
–
118,000-158,000
prevalence
–
28,000-37,000 incidence
•
No EMA-
or Canada-approved
medicines
prior
to
Esbriet
®
•
None approved in U.S.
6
1.Bjoraker JA, Am J Respir Crit Care Med. 1998 Jan; 157(1):199-203.
% Patients Surviving at 5 Years
100%
80
60
40
20
Lung
Cancer
IPF
Ovarian
Cancer
PAH
Colo-
rectal
Cancer
Breast
Cancer |
 7
Esbriet: The One and Only Approved Medicine for IPF |
 8
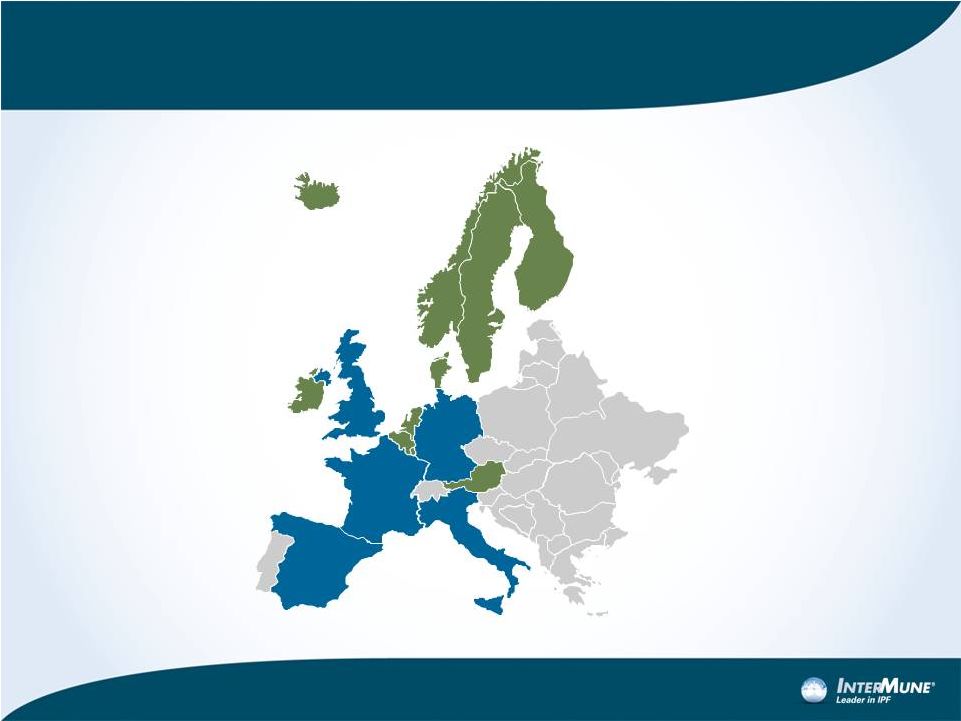
Entry Strategy: Focus on Top 5+ (80-85% of market)
Focus first on
Top 5 countries:
Germany, France, Italy,
Spain, UK
Population of 314 million
~ 70-75% of EU market
value
Invest in 10 Mid-Sized
Countries & Regions:
Austria, Nordics (4),
Netherlands, Belgium
Ireland, Iceland and
Luxembourg
~ 10% of EU market
value |
 9
2012 Esbriet Net Revenue –
Consistent Quarterly Growth Since Launch
* Includes $0.5 million in favorable adjustments to revenue primarily
related to foreign currency exchange procedures ** Includes effect of the
expected 11% price reduction in Germany (effective September 15, 2012) (unaudited)
Net Esbriet Revenue by Quarter ($ in millions)
4.9
5.5
2.7
7.5*
Q3’12
Q2’12
Q1’12
Q4’11
0
2.5
5
7.5
10
8.2**
Q4’12 |
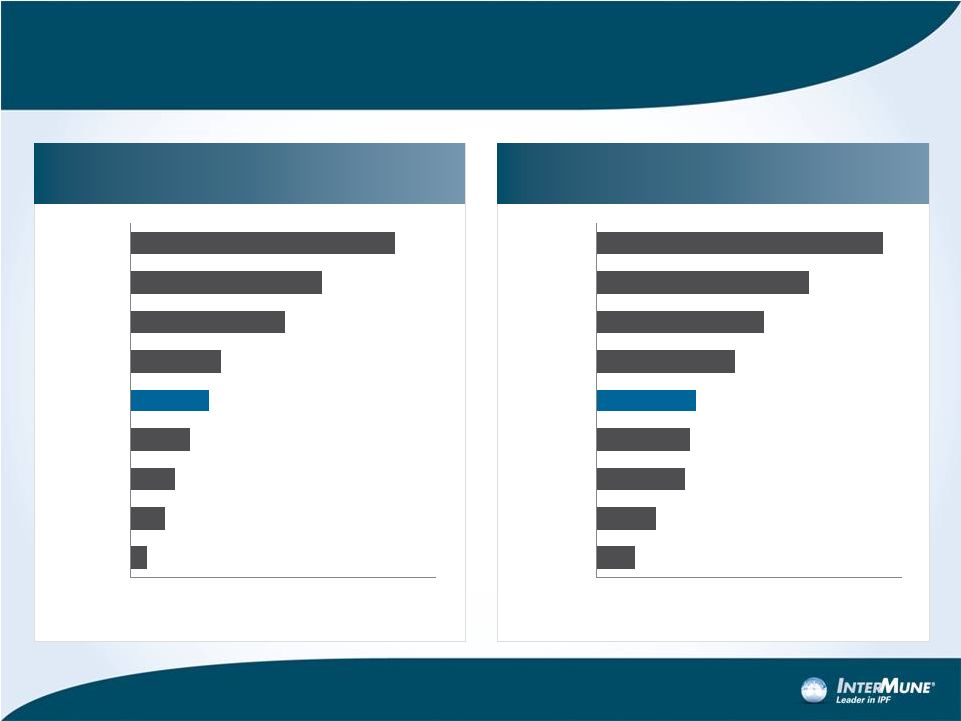 Esbriet Among the Best Orphan Drug Launches in Germany*
15 Full Months from Launch
10
Net Sales ($) months 2-16 Germany
(Euro=$1.30)
Patients (POD) end of 16th month Germany
(80% compliance+15% IMS increase)
Millions
*Analysis of 30 products in diseases with prevalence > 30K pts. in Europe
among all 66 orphan registrations in Europe through 6/10.
5.3
11.3
14.7
19.4
25.8
29.7
50.8
63.0
86.8
0
20
40
60
80
100
Adcirca
Tracleer
Tasigna
Sprycel
Esbriet
Exjade
Nexavar
Glivec
Revlimid
374
585
868
920
950-1,000
1,367
1,656
2,096
2,820
0
1000
2000
3000
Tasigna
Tracleer
Sprycel
Revlimid
Esbriet
Nexavar
Certican
Exjade
Glivec |
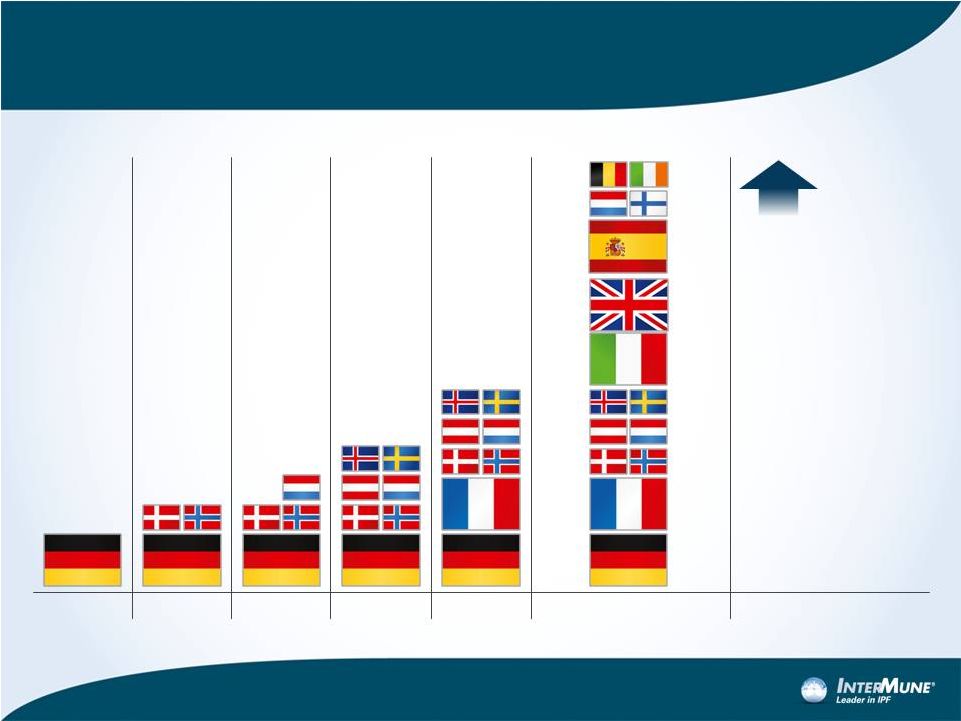 EU
Pricing, Reimbursement and Launch Sequence: Expect launch in up to 15 targeted
markets by mid-2013 11
Q4 2011
Q1 2012
Q2 2012
Q3 2012
Q4 2012
Geographic
expansion
2H 2013, 2014
1H 2013
2H 2013 + |
 12
Esbriet Launched in Canada –
January 2, 2013
•
Health Canada completed Priority Review on October 1, 2012
–
First approved treatment for mild to moderate IPF patients in Canada
–
Expect reimbursement from private insurance plans during H1’13 and
from all public (provincial) plans ~12 to 18 months from marketing approval
•
Canada is an important pharma market
–
3,500 to 5,000 mild to moderate IPF patients
–
Introductory launch price of $35,000 to $42,000 CAD
–
Final price to be based on median public price in five EU reference
countries
•
Germany, France, Sweden, Italy and the UK |
 The U.S. Market &
ASCEND Phase 3
Study Rationale |
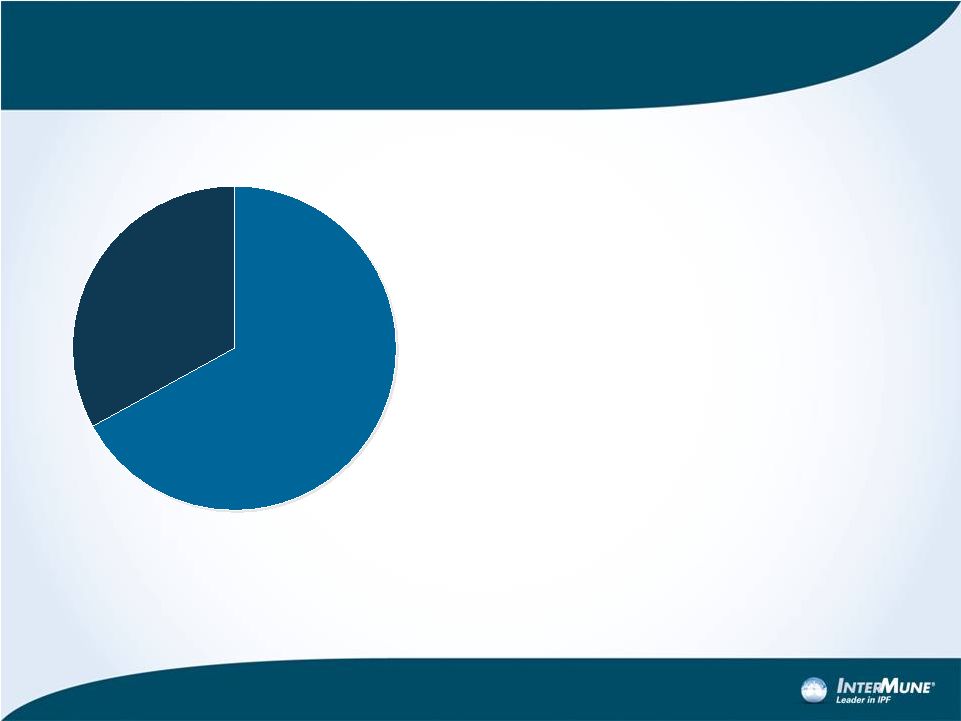 The
U.S. IPF Market is Very Attractive •
High Prevalence and Incidence:
–
31,000 to 48,000 mild to moderate
IPF patients
–
Incidence: 14,000 to 19,000/year
•
Strong Annual Pricing:
–
EU Esbriet pricing: $33K-$47K/yr.
–
U.S. PAH drugs: $70K-$75K/yr.
•
Small Target Prescribing Audience:
–
~3,000 M.D.s
•
Strong Operating Margins
14
U.S. IPF Prevalence
Mild to Moderate
(31,000-48,000)
Severe
(~20,500) |
 Esbriet Has Demonstrated a Consistent Benefit in Multiple
IPF Trials Conducted to Date
15
Standardized treatment effect
Week 48/52
Standardized treatment effect
PIPF-004
PIPF-006
SP3
SP2
Meta Analysis
0.0
0.2
0.4
0.6
0.8
Standardized treatment effect
Week 24/28
Week 36/40
Standardized treatment effect
Week 72
0.0
0.2
0.4
0.6
0.8
0.0
0.2
0.4
0.6
0.8
0.0
0.2
0.4
0.6
0.8
Highly consistent results on FVC/VC
change at one year of treatment |
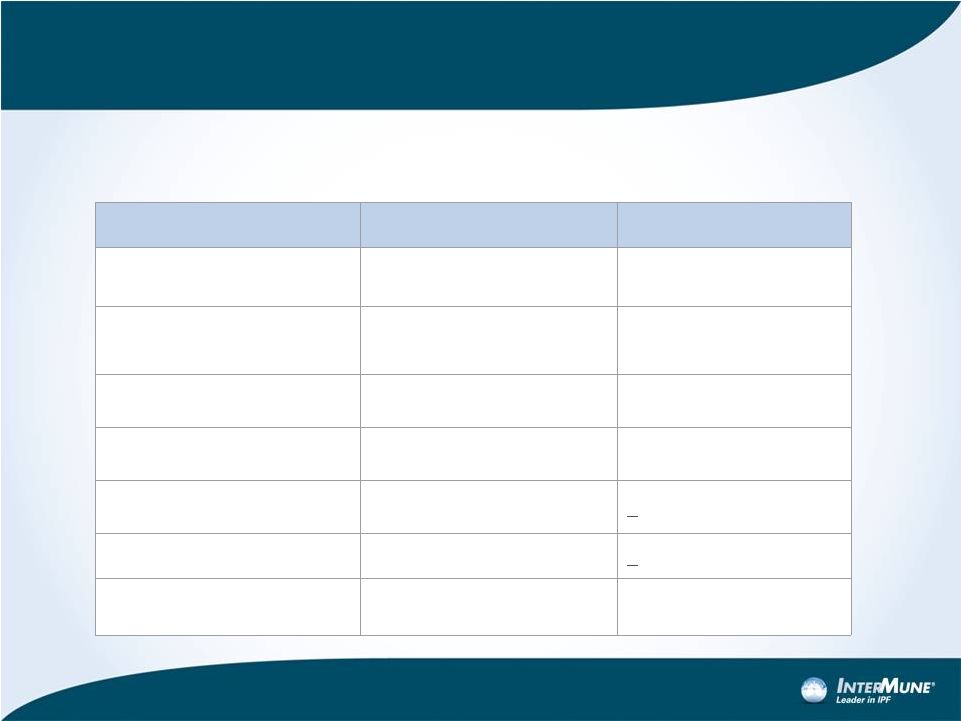 Phase
3 “ASCEND” Design
Eligibility Criteria
ASCEND Trial
CAPACITY Trials
Primary endpoint
FVC Change
FVC Change
Key Secondary Endpoints
6MWT distance, PFS
6MWT distance, PFS
Duration
52 Weeks*
72 Weeks
Patients
~500
779
% Predicted FVC
50% -
90%
>50%
% DLco
30%
-
90%
>35%
FEV1 / FVC Ratio
>0.80
>0.70
•
Randomized, double-blind, placebo-controlled trial
•
Pirfenidone 2403 mg/d vs. placebo with 1:1 randomization
*Red denotes change from Phase 3 CAPACITY program
16 |
 17
Summary of Observations Supporting
ASCEND Eligibility Criteria
•
ASCEND Study enriched for IPF patient more likely to experience
FVC decline and disease progression
–
Eligibility criteria derived from CAPACITY (studies 004 and 006)
and corroborated with other independent data sets, including
INSPIRE Study
•
Analyses of CAPACITY patients meeting “ASCEND Eligibility Criteria”
also show enhanced magnitude of pirfenidone treatment effect
–
FVC and secondary endpoints
–
Independent studies 004 and 006, and pooled CAPACITY
•
Consistent augmentation of disease progression AND pirfenidone
treatment effect across independent endpoints and studies strongly
suggest these observations are at least directionally accurate
|
 18
FVC Change -
ITT Group of CAPACITY vs.
“ASCEND Criteria”
Subgroup
0
-5
-10
12
24
36
48
60
72
Weeks
CAPACITY ITT
Week 48
Week 72
Absolute
3.3%
2.5%
Relative
41.6%
22.8%
P-value
<0.001
0.005
CAPACITY ITT (Pooled Studies 004 and 006)
Pirfenidone
Placebo
-10
60
72
36
48
24
12
-5
0
Weeks
“ASCEND Criteria”
subgroup
“ASCEND”
Week 48
Week 72
Absolute
6.1%
7.9%
Relative
63.3%
57.0%
P-value
<0.001
<0.001 |
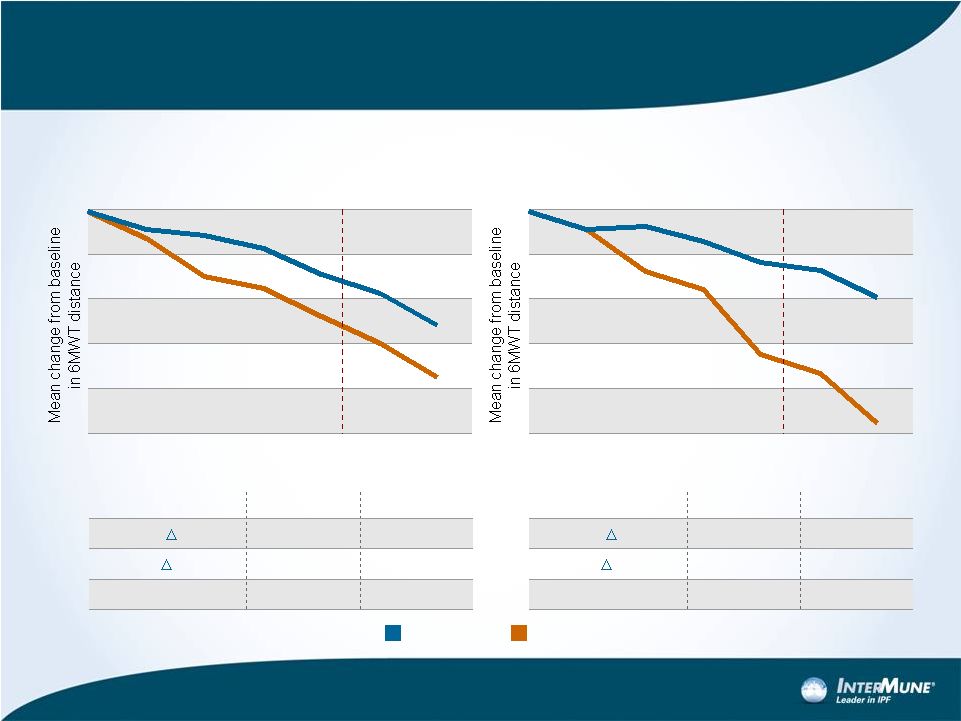 -40
-60
-80
-20
0
“ASCEND Criteria”
Weeks
60
72
36
48
24
12
19
6MWD Change -
ITT Group of CAPACITY vs.
“ASCEND Criteria”
Subgroup
0
-20
-40
-60
-80
12
24
36
48
60
72
CAPACITY ITT (Pooled Studies 004 and 006)
Pirfenidone
Placebo
Weeks
CAPACITY ITT
Week 48
Week 72
Absolute
19.8 m
24.0 m
Relative
40.5%
31.3%
P-value
0.004
<0.001
“ASCEND”
Week 48
Week 72
Absolute
42.4 m
58.7 m
Relative
64.2%
59.5%
P-value
<0.001
<0.001 |
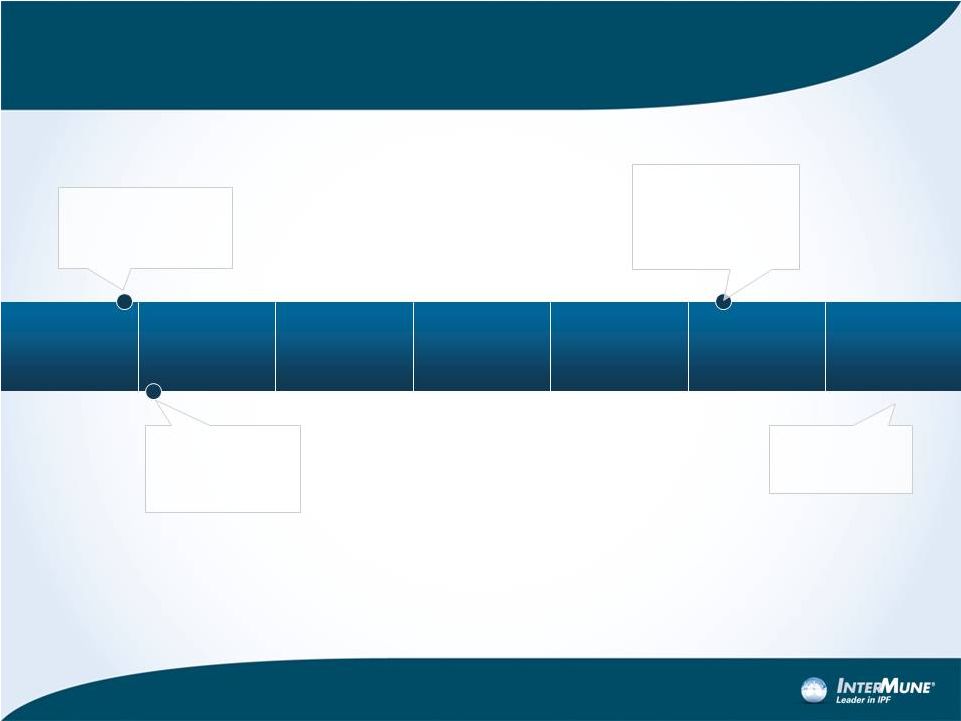 20
ASCEND
Phase
3
Trial
–
Timelines
December 2012:
500
th
patient
randomized
January 9, 2013:
Last patient
randomized
2Q 2014:
Top-line Results
February 2014:
Last patient
completes ~35-day
safety follow-up
4Q
2012
1Q
2013
2Q
2013
3Q
2013
4Q
2013
1Q
2014
2Q
2014 |
 2013 Outlook |
 22
What to Expect from InterMune in 2013 –
Further Progress Toward Our 2015 Vision
•
Consistent,
steady
growth
of
Esbriet
®
in
Europe
–
Growth of Esbriet in the 9 countries already launched
–
Launch of Esbriet in our 6 remaining EU countries by mid-year 2013
•
Three “Top 5”
countries –
Italy, UK and Spain
•
Launch of Esbriet in our first North American market, Canada (January)
•
Pre-launch preparation for Esbriet in the U.S.
–
CME, KOL engagement, publications, HQ staffing etc.
•
Investment in our pipeline
–
Esbriet Life Cycle Management and formulation opportunities
–
Advancing anti-fibrotic research programs |
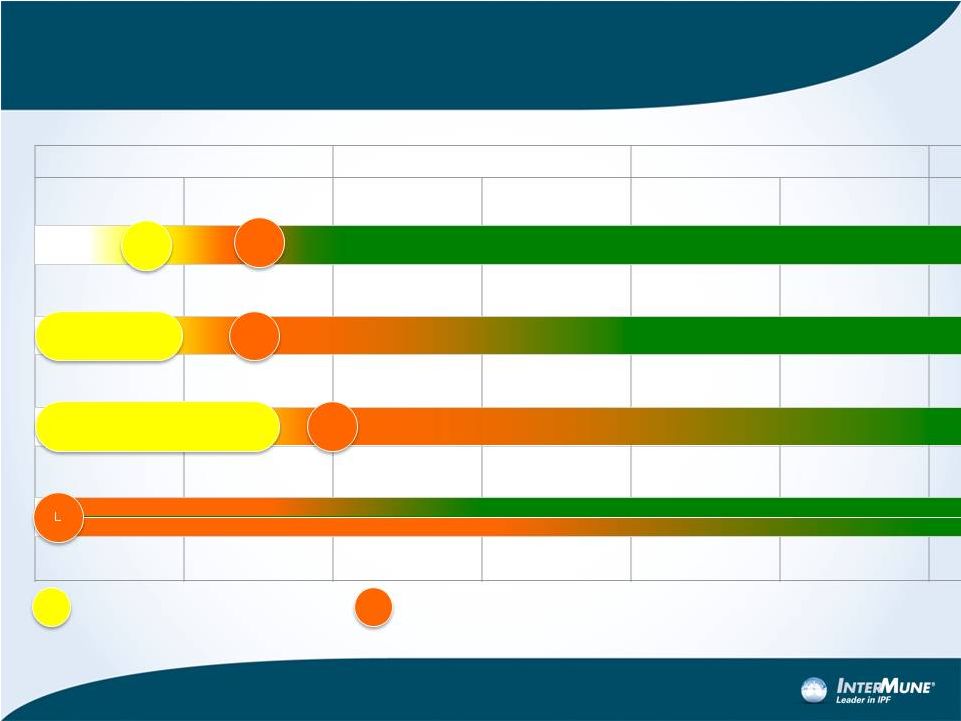 Esbriet Roll-Out: Summary of Current Expectations for
Major Markets Where Esbriet is Not Yet Launched *
23
** England and Wales; SMC (HTA for Scotland) in parallel
P&R
L
* Assumes P&R is granted in each country
L
Pricing and Reimbursement concluded
Launch
Spain
Canada
Italy
UK**
P&R
P&R
1H 2013
2H 2013
1H 2014
P&R
PRIVATE INSURANCE PROCEDURES
PROVINCIAL REIMBURSEMENT PROCEDURES
REGIONAL PROCEDURES
REGIONAL PROCEDURES
L
L |
 Projected Esbriet Revenue in 2013
24
Countries
2012
Revenue*
(in millions)
2013
Projected
Revenue
Range
(in millions)
Launched Countries
(Germany, France, Canada and
7
Mid-Sized EU countries)
$26
$40 -
$55
Not Yet Launched Countries
(Italy,
UK,
Spain
and
3
Mid-Sized
EU Countries)
$0
$0 -
$15
Total: Top 15 European Countries
and Canada
$26
$40 -
$70
* Unaudited |
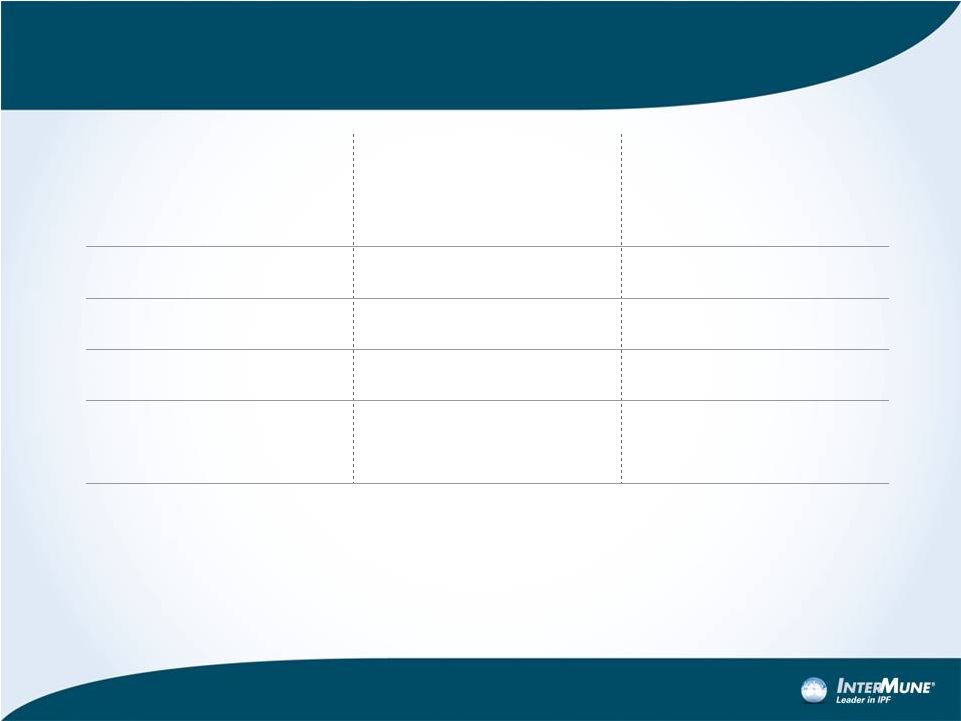 Financial Overview
•
Convertible debt:
–
$85M due 2015: 5.00% coupon, $18.88 conversion price
–
$155M due 2018: 2.50% coupon, $31.80 conversion price
25
Full-Year 2012
Guidance
(1/3/13)
Full-Year 2013
Guidance
Revenue
$26.1M (unaudited)
$40 -
$70M
R&D
$100 -
$105M
$100 -
$120M
SG&A
$105 -
$110M
$145 -
$165M
Total (R&D and
SG&A)
$205 -
$215M
$245 -
$285M |
 26
Summary
•
IPF is a large orphan market with attractive commercial potential
•
Attractive pricing, focused marketing
high operating margins
•
Esbriet has first-mover advantage in this market
•
Esbriet launch in Germany ranks among the best-ever orphan launches
•
Solid execution in 2012 on Esbriet Pricing and Reimbursement in Europe
during one of the most challenging economic periods
•
2013
is
the
“EU
Launch
Year”
–
expect
to
be
in
market
in
all
Top
15
•
North America the next major territory
•
Approval
of
Esbriet
in
Canada
–
Launched
January
2
•
Phase 3 ASCEND study is now fully enrolled and designed to deliver a
positive result and access to the very attractive U.S. market
|
 |
