Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - INFINITY PHARMACEUTICALS, INC. | d462841d8k.htm |
| EX-99.2 - PRESS RELEASE - INFINITY PHARMACEUTICALS, INC. | d462841dex992.htm |
 Building a Fully Integrated
Biopharmaceutical Company
January 2013
Exhibit 99.1 |
 Forward-Looking Statements
2
•
This presentation contains forward-looking statements within the meaning of The
Private Securities Litigation Reform Act of 1995. •
Such forward-looking statements include those regarding the Company’s
expectations about: the timing of data from clinical trials of its PI3K and
Hsp90 programs; its ability to execute on its strategic plans; the therapeutic potential of its PI3K inhibitors and
Retaspimycin HCl; its 2013 research and development goals, including without
limitation clinical development plans, for its PI3K program and its Hsp90
program; its current cash position; and its financial guidance for 2013 with respect to operating expenses,
net loss and cash and investments.
•
Such
statements
are
subject
to
numerous
important
factors,
risks
and
uncertainties
that
may
cause
actual
events
or
results
to
differ
materially from the company’s current expectations. For example, there can be
no guarantee that Infinity will report data in the time frames it has
estimated, that any product candidate Infinity is developing will successfully complete necessary preclinical and clinical
development phases or that development of any of Infinity’s product candidates
will continue. Further, there can be no guarantee that any positive
developments in Infinity’s product portfolio will result in stock price appreciation. Management’s expectations and,
therefore, any forward-looking statements in this presentation could also be
affected by risks and uncertainties relating to a number of other factors,
including the following: Infinity’s results of clinical trials and preclinical studies, including subsequent analysis of
existing data and new data received from ongoing and future studies; the content
and timing of decisions made by the U.S. FDA and other regulatory
authorities, investigational review boards at clinical trial sites and publication review bodies; Infinity’s ability to obtain
and maintain requisite regulatory approvals and to enroll patients in its clinical
trials; unplanned cash requirements and expenditures; development of agents
by Infinity’s competitors for diseases in which Infinity is currently developing its product
candidates; and Infinity’s ability to obtain, maintain and enforce patent and
other intellectual property protection for any product candidates it is
developing. •
These
and
other
risks
which
may
impact
management’s
expectations
are
described
in
greater
detail
under
the
caption
“Risk
Factors”
included
in
Infinity’s
current
report
on
Form
8-K
filed
with
the
Securities
and
Exchange
Commission
(SEC)
on
December
12,
2012,
and
other
filings
filed
by
Infinity
with
the
SEC.
•
Any forward-looking statements contained in this presentation speak only as of
the date hereof, and Infinity expressly disclaims any obligation to update
any forward-looking statements, whether as a result of new information, future events or otherwise.
•
Infinity’s website is http://www.infi.com. Infinity regularly uses its website
to post information regarding its business, product development programs and
governance. Infinity encourages investors to use www.infi.com, particularly the information in the
section
entitled
“Investors/Media,”
as
a
source
of
information
about
Infinity.
References
to
www.infi.com
in
this
presentation
are
not
intended to, nor shall they be deemed to, incorporate information on www.infi.com
into this presentation by reference. |
 Infinity Today: Solid Foundation for Building a Fully
Integrated Biopharmaceutical Company
Encouraging clinical activity
Worldwide rights to entire portfolio of product candidates
Strong intellectual property
Experienced team
Financial strength
3 |
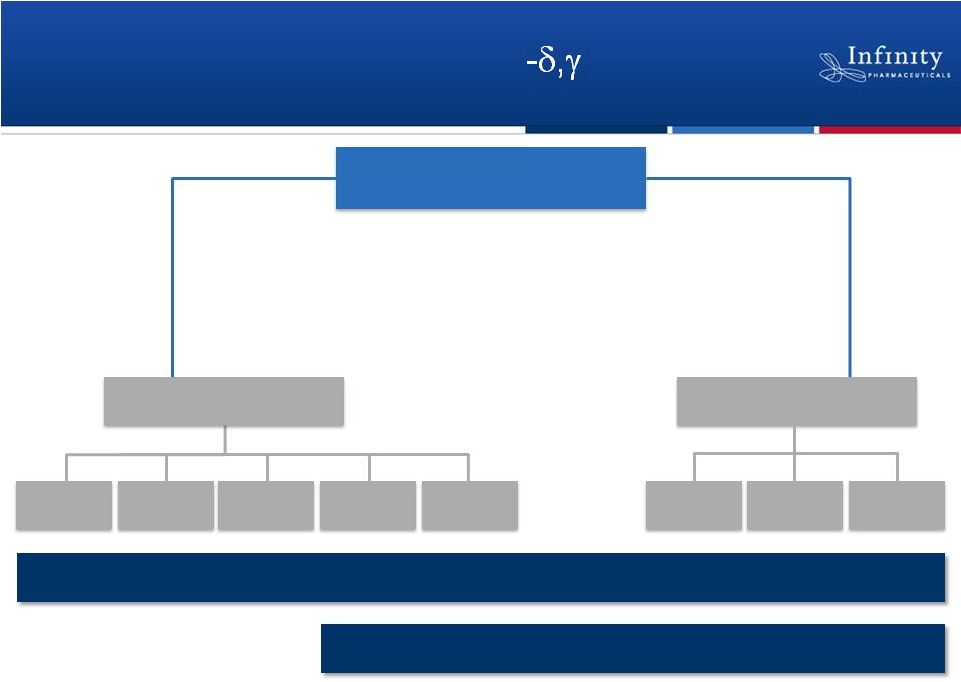 Advancing
a
Portfolio
of
PI3K
Inhibitors
Potential First-in-Class
IPI-145
Potential Best-in-Class
Heme Malignancies
Inflammation
CLL
iNHL
T-Cell
Asthma
RA
Others
MCL
4
Others |
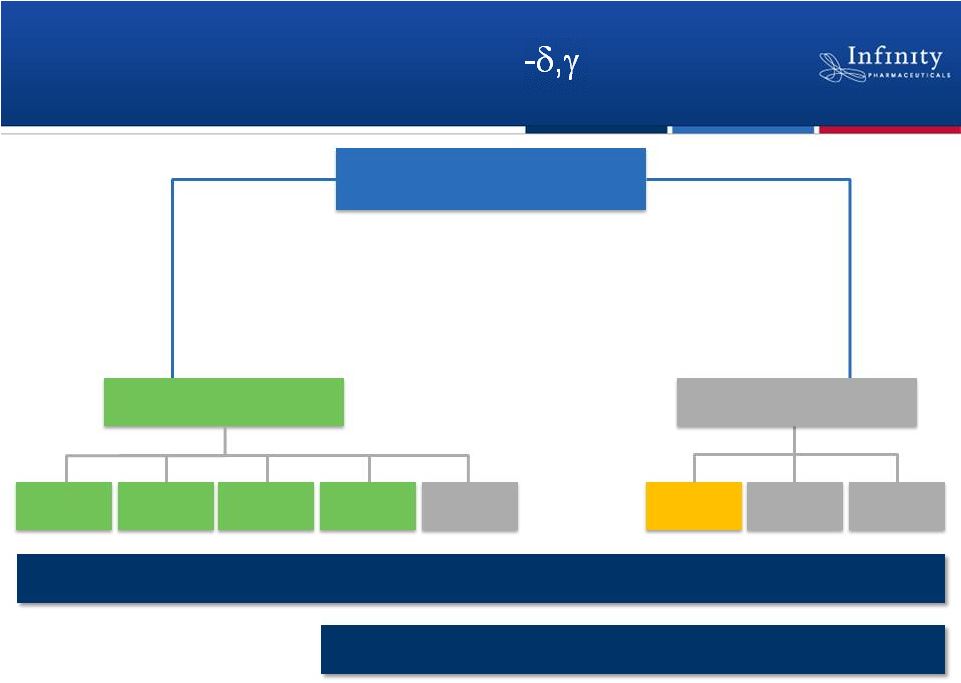 Advancing a Portfolio of PI3K Inhibitors
IPI-145
CLL
iNHL
T-Cell
Asthma
RA
Others
MCL
5
Others
Heme Malignancies
Inflammation
Potential First-in-Class
Potential Best-in-Class |
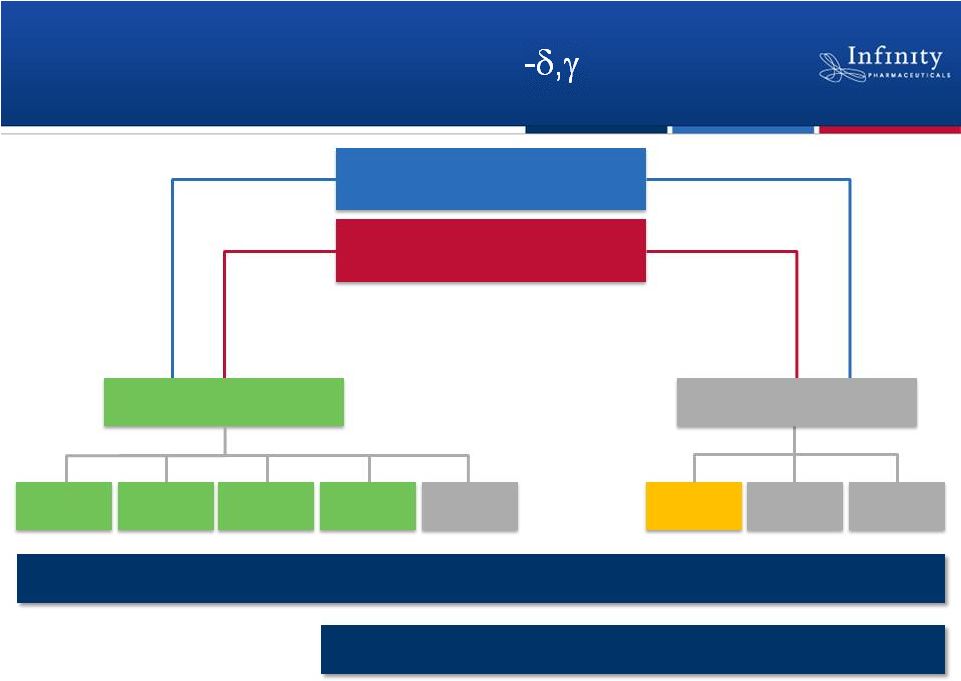 Advancing a Portfolio of PI3K
Inhibitors
IPI-145
CLL
iNHL
T-Cell
Asthma
RA
Others
MCL
6
Others
IPI-443
Heme Malignancies
Inflammation
Potential First-in-Class
Potential Best-in-Class |
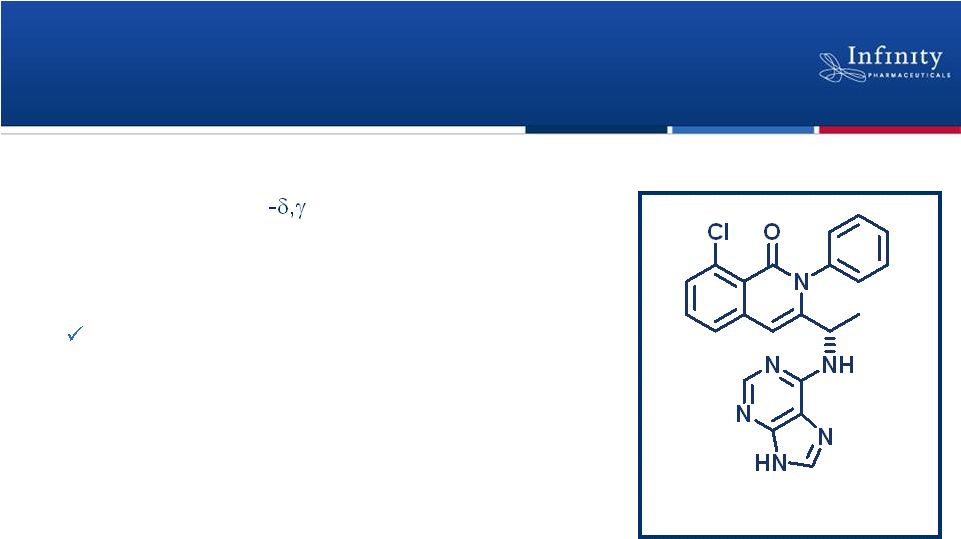 IPI-145: Potential Best-in-Class PI3K Inhibitor
Profile
Clinical Development Status
Intellectual Property
7
IPI-145
•
Potent, oral PI3K inhibitor
•
Selective for PI3K
Completed Phase 1 trial in healthy subjects
•
Enrolling
–
Phase 1 trial in advanced hematologic malignancies
–
Phase 2a trial in mild, allergic asthma
•
Planning Phase 2 trial in RA
•
Issued U.S. composition patent expiry: 2030
•
Numerous additional U.S. and ex-U.S. patents
pending |
 IPI-145
Targeting B-Cell and T-Cell
Malignancies |
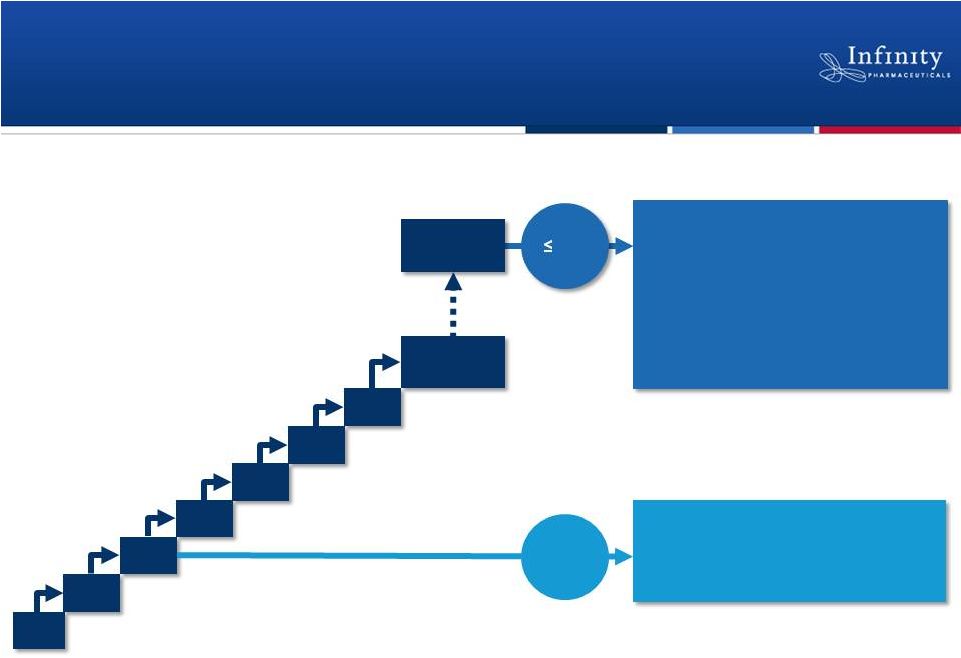 Phase 1 Trial in
Hematologic Malignancies 8 mg
BID
15 mg
BID
25 mg
BID
50 mg
BID
Ongoing
escalation
25 mg BID expansion (n=30)
•
•
•
9
Potential cohort expansions
•
•
•
•
•
35 mg
BID
75 mg
BID
60 mg
BID
100 mg BID
(enrolling)
Pre-MTD
MTD
Diffuse large B-cell lymphoma
T-cell lymphomas
Acute lymphoblastic leukemia
Myeloproliferative neoplasms
CLL, iNHL, MCL
Chronic lymphocytic leukemia
Indolent non-Hodgkin’s lymphoma
Mantle cell lymphoma |
 Well Tolerated at Doses Through 75 mg BID
Phase 1 Trial in Heme Malignancies
•
No dose-related trends in adverse events
–
Serious adverse events were infrequent and consistent with
co-morbidities seen in patients with advanced hematologic malignancies
–
Most common Grade 3,4 events: cytopenias and transaminase elevations,
with a minority of patients requiring dose reductions or interruptions
•
67% of all patients remain on study
–
90% of patients who did not experience progressive disease after
two cycles remain on study
•
Maximum tolerated dose not yet reached
–
100 mg BID dose escalation cohort now enrolling
10
Flinn et al. ASH 2012. |
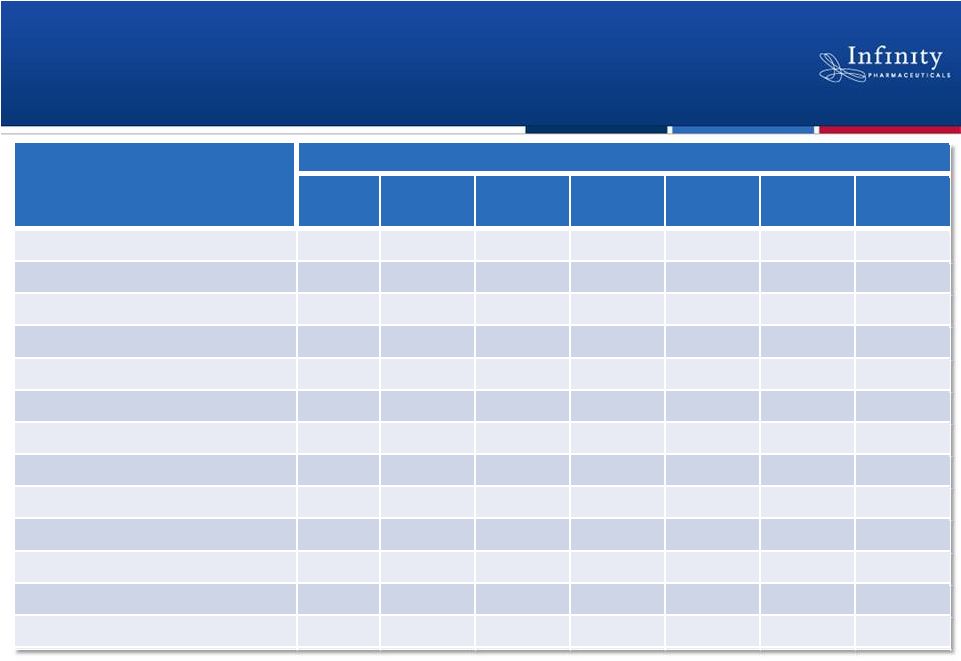 No
Dose-Related Trends in Adverse Events Phase 1 Trial in Heme
Malignancies 11
Flinn et al. ASH 2012.
Grade 3 and 4
Related AEs
IPI-145
BID Dose (n)
8 mg
(n =
1)
15 mg
(n = 6)
25 mg
(n = 34)
35 mg
(n
= 3)
50 mg
(n = 3)
60 mg
(n = 3)
75 mg
(n
= 5)
Neutropenia
1
3
3
1
0
0
0
Febrile neutropenia
1
0
0
0
0
0
0
Anemia
0
1
0
0
0
0
0
Thrombocytopenia
0
1
0
0
0
0
0
ALT/AST increased
0
1
3
0
0
1
1
Rash (general)
0
0
0
0
1
0
1
Cellulitis
0
0
0
0
0
0
1
Pneumonitis
0
1
0
0
0
0
0
Tumor
lysis
/ Hyperkalemia
0
0
1
0
0
0
0
Nausea
0
1
0
0
0
0
0
Dehydration
0
0
1
0
0
0
0
Mucosal inflammation
0
0
1
0
0
0
0
Hypophosphatemia
0
0
1
0
0
0
0 |
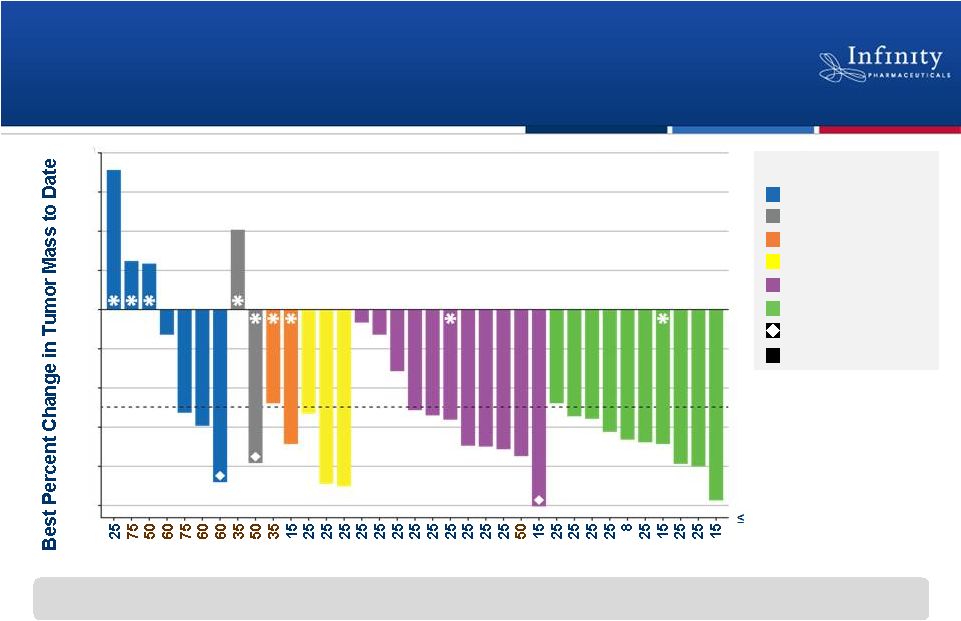 25
mg > 25 mg
Dose (mg BID)
Clinical Activity Observed Across a Broad
Range of Hematologic Malignancies
12
Reduction in tumor mass was observed in all indications and at all dose levels
evaluated T-Cell Lymphoma
HL
aNHL
Cancer Diagnosis
MCL
iNHL
CLL/SLL
Off study
CR by PET
*
-50
Flinn et al. ASH 2012.
80
60
40
20
0
-20
-40
-60
-80
-100
1 |
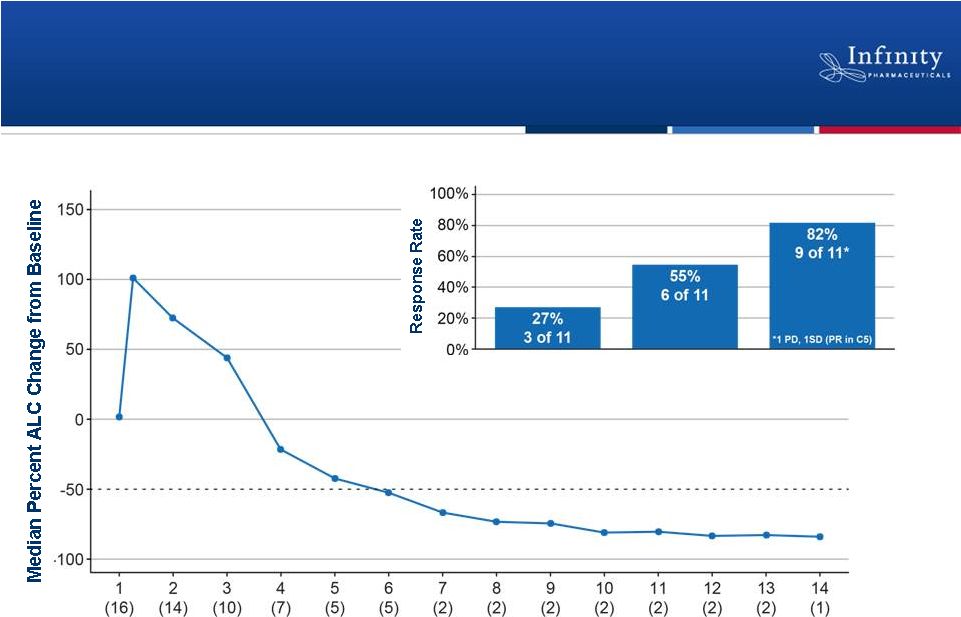 Rapid Onset of Clinical Activity
by IPI-145 in CLL/SLL
13
Cycle (N patients)
Partial Response
Total Response
(Partial + Nodal)
Nodal Response
Flinn et al. ASH 2012.
Response Post 2 Cycles of IPI-145 (n = 11) |
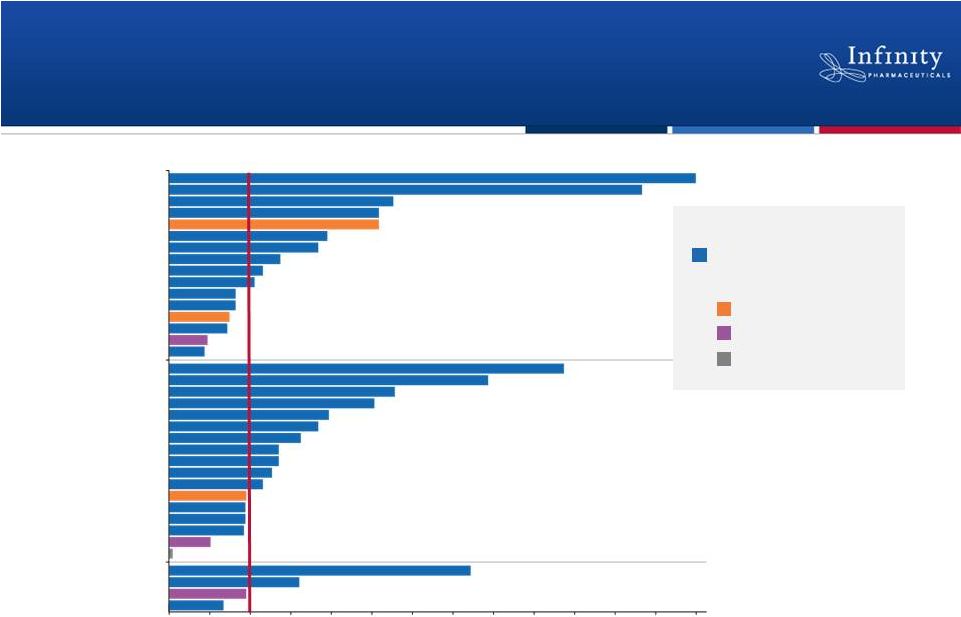 Many CLL, iNHL and MCL Patients
Continuing on Study
14
Months on Treatment by Subject and Diagnosis
CLL
MCL
iNHL
On Study
Off Study:
Adverse Event
Progressive Disease
Disposition
Ineligible
Flinn et al. ASH 2012.
13
12
11
10
9
8
7
6
5
4
3
2
1
0
Months on Study |
 Early Signs of Clinical Activity of IPI-145
in T-Cell Lymphoma
15
25 mg BID
50 mg BID
60 mg BID
75 mg BID
Dose
C1 = Cycle 1
C3 = Cycle 3
SD
TBD
PR
CR
Flinn et al. ASH 2012.
PD
PD
PD
•
First response assessment after 2 cycles of IPI-145: 1 CR, 1 PR, 1 SD, 1TBD, 3
PD •
4 patients remain on study |
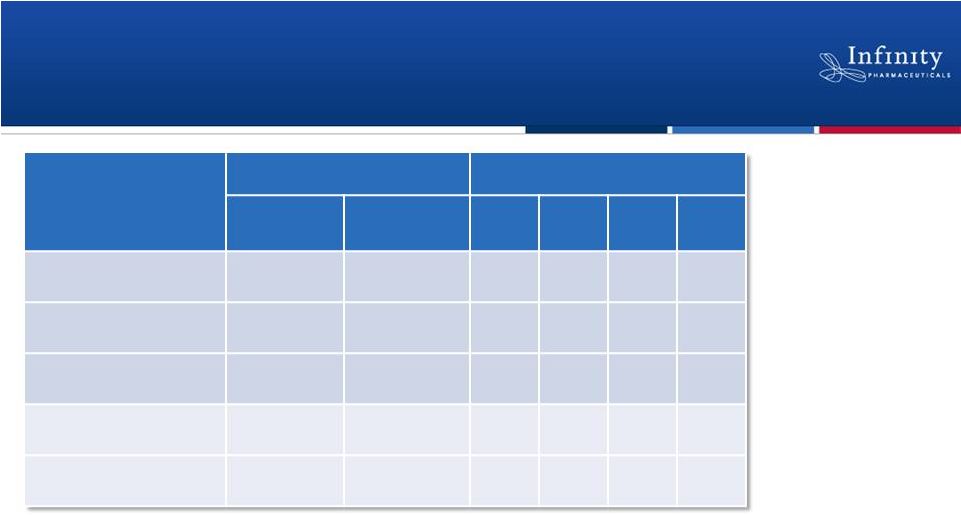 Clinical Responses Observed Across a Broad
Range of Malignancies
Population
Patients (n)
Best Observed Response (n)
Treated
Evaluable*
CR
PR
SD
PD
iNHL
17
13
1
7
4
1
CLL
16
11
0
6
4
#
1
MCL
4
3
0
2
0
1
T-Cell Lymphoma
7
6
1
1
1
3
HL
3
2
1
0
0
1
16
Responses not observed in multiple myeloma or aggressive non-Hodgkin’s
lymphoma as of 11/20/2012 data cutoff. *At least 1 response assessment or
PD Complete Response (CR), Partial Response (PR), Stable Disease (SD),
Progressive Disease (PD) #4 Nodal Responses
Flinn et al. ASH 2012. |
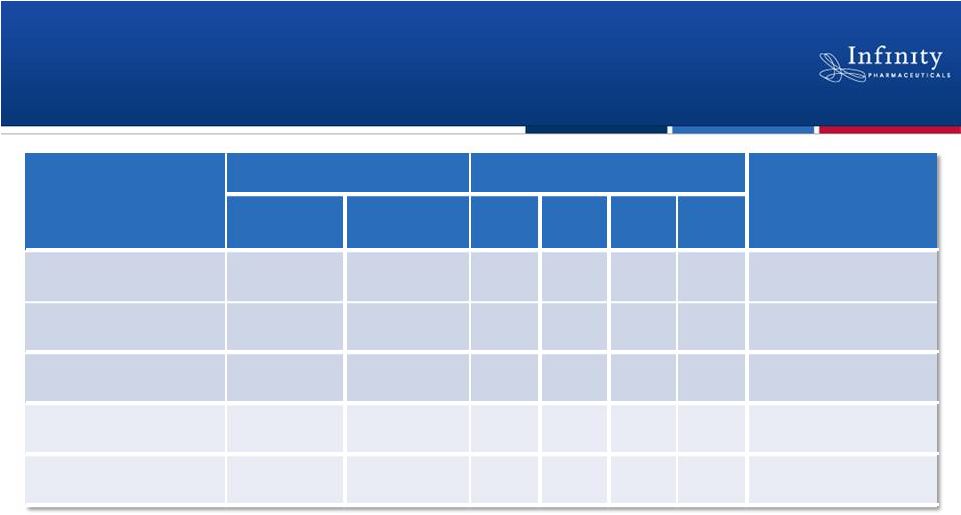 Clinical Responses Observed Across a Broad
Range of Malignancies
17
Responses not observed in multiple myeloma or aggressive non-Hodgkin’s
lymphoma as of 11/20/2012 data cutoff. *At least 1 response assessment or
PD Complete Response (CR), Partial Response (PR), Stable Disease (SD),
Progressive Disease (PD) #4 Nodal Responses
Flinn et al. ASH 2012.
Population
Patients (n)
Best Observed Response (n)
Median Time to
Response
in
months
Treated
Evaluable*
CR
PR
SD
PD
iNHL
17
13
1
7
4
1
1.8
CLL
16
11
0
6
4
#
1
2.9
MCL
4
3
0
2
0
1
1.9
T-Cell Lymphoma
7
6
1
1
1
3
2.4
HL
3
2
1
0
0
1
1.7 |
 Summary of Phase 1 Trial of IPI-145 in
Advanced Hematologic Malignancies
•
Well tolerated up to 75 mg BID
•
Active across a broad range of B-cell malignancies as well
as T-cell lymphoma
•
Rapid responses observed, as evidenced by median time to
response
18
Flinn et al. ASH 2012. |
 IPI-145
First-in-Class Opportunity in
Inflammatory Diseases |
 Phase 1 Trial in Healthy Subjects
Study Design
•
Objectives: Tolerability, pharmacokinetics (PK) and
pharmacodynamics (PD) following single and repeat doses
•
84 subjects evaluated
•
Data reported at ACR 2012
Part 1: Single Ascending Dose
•
Randomized, double-blind, placebo-controlled
•
Doses evaluated: 1, 2, 5, 10, 25 and 30 mg
Part 1: Single Ascending Dose
•
Randomized, double-blind, placebo-controlled
•
Doses evaluated: 1, 2, 5, 10, 25 and 30 mg
Part
2:
Multiple
Ascending
Dose
(14
days
dosing)
•
Randomized, double-blind, placebo-controlled
•
Doses evaluated: 1, 2 and 5 mg BID; 10 mg QD
Part
2:
Multiple
Ascending
Dose
(14
days
dosing)
•
Randomized, double-blind, placebo-controlled
•
Doses evaluated: 1, 2 and 5 mg BID; 10 mg QD
20 |
 IPI-145 Phase 1 Trial in Healthy Subjects
Safety Findings
•
IPI-145 well tolerated at doses up to 10 mg daily for 14 days
•
No signs or symptoms attributable to treatment (e.g., headache,
common cold symptoms, fatigue, dizziness) at any dose level
•
No unusual or opportunistic viral or other infections
•
No safety related laboratory findings attributable to treatment at
any dose level
21
Porter et al. ACR 2012. |
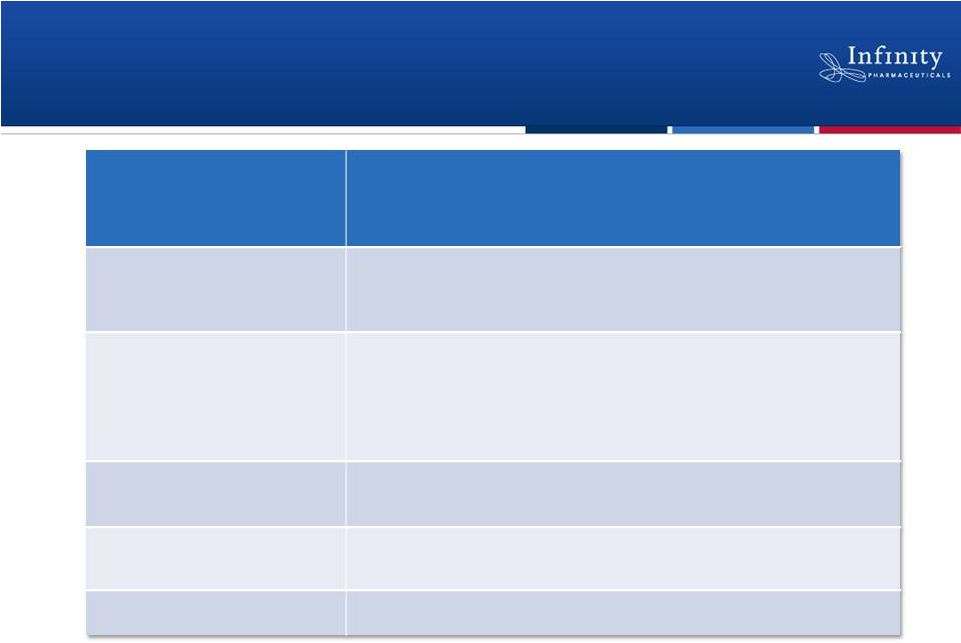 IPI-145 Is Active in All Inflammation
Models Evaluated
22
Palombella. New York Academy of Sciences 2012.
Disease
Rodent Inflammation Model
Asthma
•
Ovalbumin challenge
Rheumatoid Arthritis
•
Adjuvant Induced (AIA)
•
Collagen Induced (CIA)
•
Peptidoglycan Polysaccharide Induced (PG-PS)
•
Collagen Antibody Induced (CAIA)
Crohn’s
Disease
•
CD45RB
high
transfer
Lupus
•
NZB/W f1
Multiple Sclerosis
•
Experimental
Autoimmune Encephalopathy (EAE) |
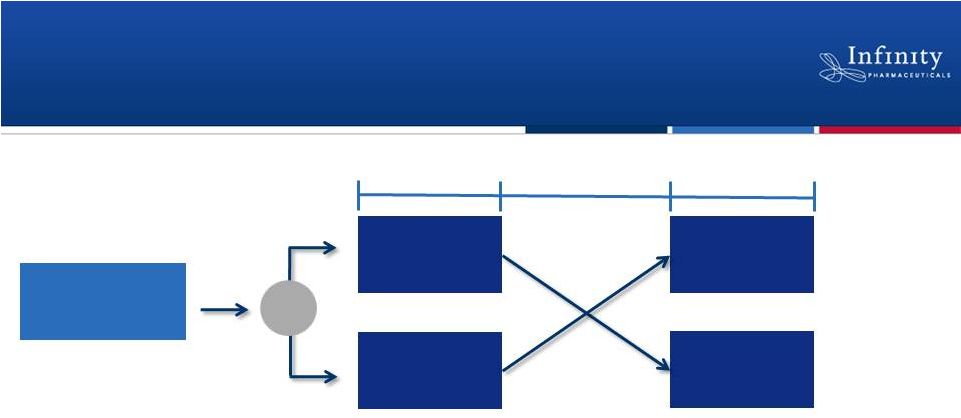 Initiated Phase 2a Trial in Asthma
•
Double-blind, randomized, placebo-controlled, crossover study
•
Approximately 30 subjects with mild, allergic asthma
•
Multiple-dose study designed to evaluate safety, PK, and activity
–
Efficacy endpoints include FEV
1
, markers of inflammation
and airway hyperresponsiveness
23
~30 subjects
IPI-145
Placebo
IPI-145
Placebo
14 Days
14 Days
7-12
Day
Washout
ClinicalTrials.gov NCT01653756.
R |
 Planning Phase 2 Trial in RA
•
Double-blind, randomized, placebo-controlled
•
Designed to evaluate safety and activity of multiple doses
24
Patients with moderate
to severe RA
Multiple doses of IPI-145
Placebo
R |
 IPI-443
Second PI3K-Delta,Gamma Inhibitor |
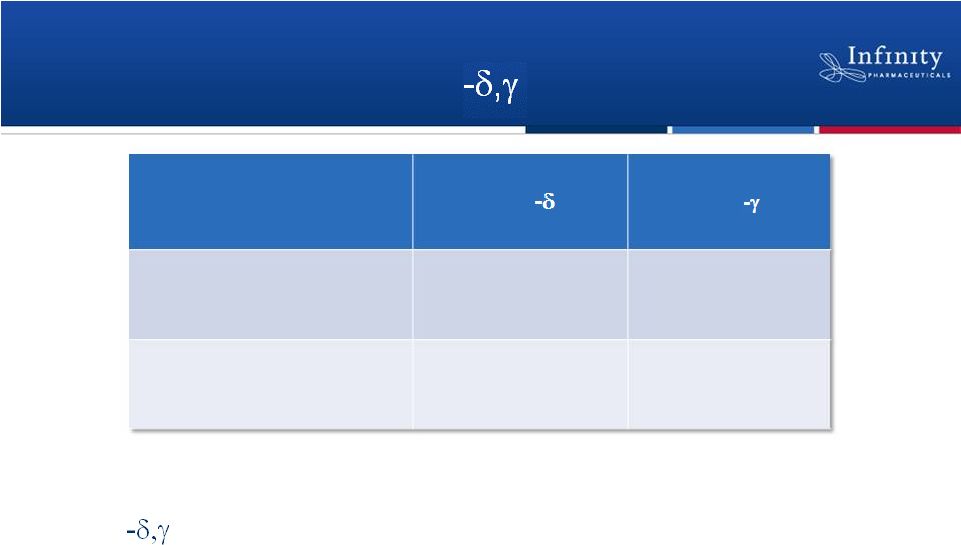 IPI-443:
Maximizing Value of PI3K Franchise
•
PI3K portfolio enables the evaluation of relative attributes of
PI3K inhibition
•
Nonclinical studies of IPI-443 under way to enable
Phase 1 development
26
Product Candidate (K
d
)
PI3K
PI3K
IPI-145
IPI-443
23 pM
21 pM
243 pM
73 pM |
 Retaspimycin HCl (IPI-504)
Potent, Selective Inhibitor of Heat
Shock Protein 90 (Hsp90) |
 Hsp90 Plays an Important Role in Cancer
Cell Survival
Function of Hsp90 in Cancer Cells
•
Chaperone necessary for stability and
function, to maintain protein
homeostasis
•
Hsp90 is elevated in cancer cells and
buffers cancer specific stress
Profile of Retaspimycin HCl
•
Selective and potent Hsp90 inhibitor
Intellectual Property
•
Composition and methods patents
issued broadly worldwide; earliest
expiry 2024
ATP
Retaspimycin HCl
Oncoprotein
Hsp90
28 |
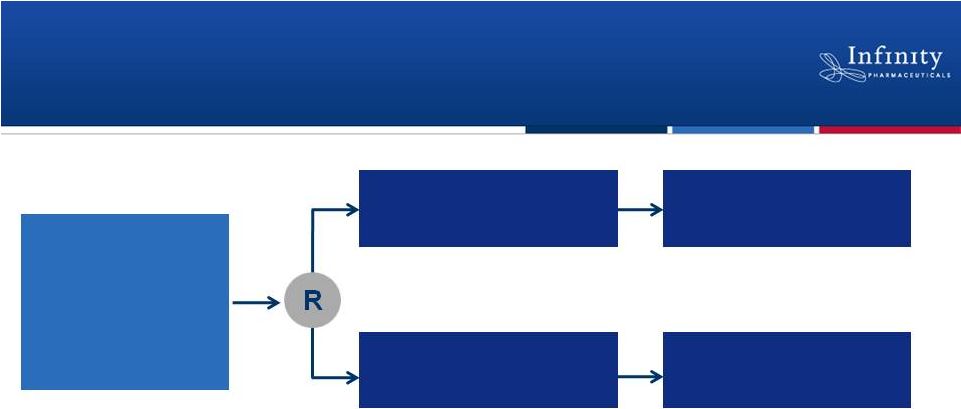 Phase 2 Trial in NSCLC Patients with a
Smoking History
•
Randomized, double-blind, placebo-controlled trial
•
Enrollment complete with topline overall survival data expected in 1H’13
29
Smokers
w/ 2
nd
-
or 3
rd
-line
NSCLC
(docetaxel naïve)
N = 226
Follow-up for OS
Follow-up for OS
Docetaxel +
Retaspimycin HCl
(N = ~105)
Docetaxel +
placebo
(N = ~105)
ClinicalTrials.gov NCT01362400.
–
Co-primary endpoints: Overall survival in total population and squamous
cell –
Secondary endpoints: Predictive biomarkers, progression free survival,
overall response rate |
 Financial Highlights |
 2013 Financial Guidance*
•
Approximately $327M in cash and investments at 12/31/12 (unaudited)
•
Operating expenses: $115M to $125M
•
Net loss: $115M to $125M
•
Year-end 2013 cash and investments: $210M to $220M
–
Cash runway into 2015
31
*Financial guidance provided as of January 7, 2013 and is based on Infinity’s
then-current operating plan and exclusive of any potential business
development activities. |
 Anticipated 2013 Program Milestones
IPI-145 in Hematologic Malignancies
1H13:
Begin
up
to
five
cohort
expansions
in
Phase
1
trial
1H13:
Define
recommended
Phase
2
dose
2013:
Initiate
at
least
two
additional
trials
IPI-145 in Inflammation
1H13:
Initiate
Phase
2
trial
in
rheumatoid
arthritis
2H13:
Provide
update
on
Phase
2a
trial
in
asthma
PI3K Pipeline Expansion
2H13:
Complete
nonclinical
studies
to
enable
initiation
of
Phase
1
development
of
IPI-443
Retaspimycin HCl in NSCLC
1H13:
Report
topline
OS
data
from
Phase
2
trial
in
combination
with
docetaxel
1H13:
Provide
update
on
Phase
1b/2
trial
in
combination
with
everolimus
32 |
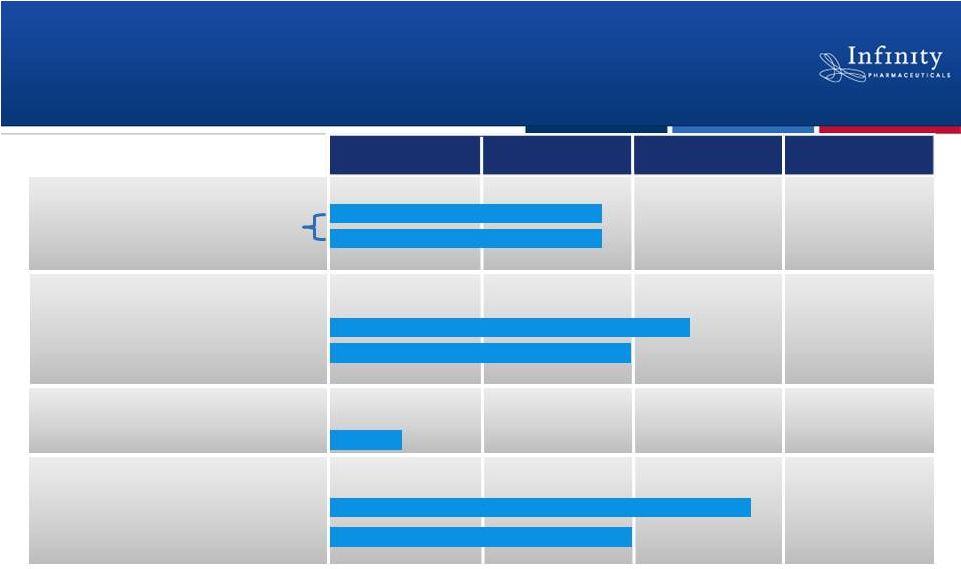 Pipeline Today
33
IPI-145 in Heme
IPI-443
Adv. Hematologic Malignancies
Phase 1
Phase 2
Phase 3
PI3K Pipeline Development
Preclinical
Asthma
Rheumatoid Arthritis
IPI-145 in Inflammation
NSCLC (Heavy smokers)
NSCLC (mKRAS)
Hsp90: Retaspimycin HCl
Dose-Escalation
25 mg BID Expansion
Docetaxel Combo
Everolimus Combo |
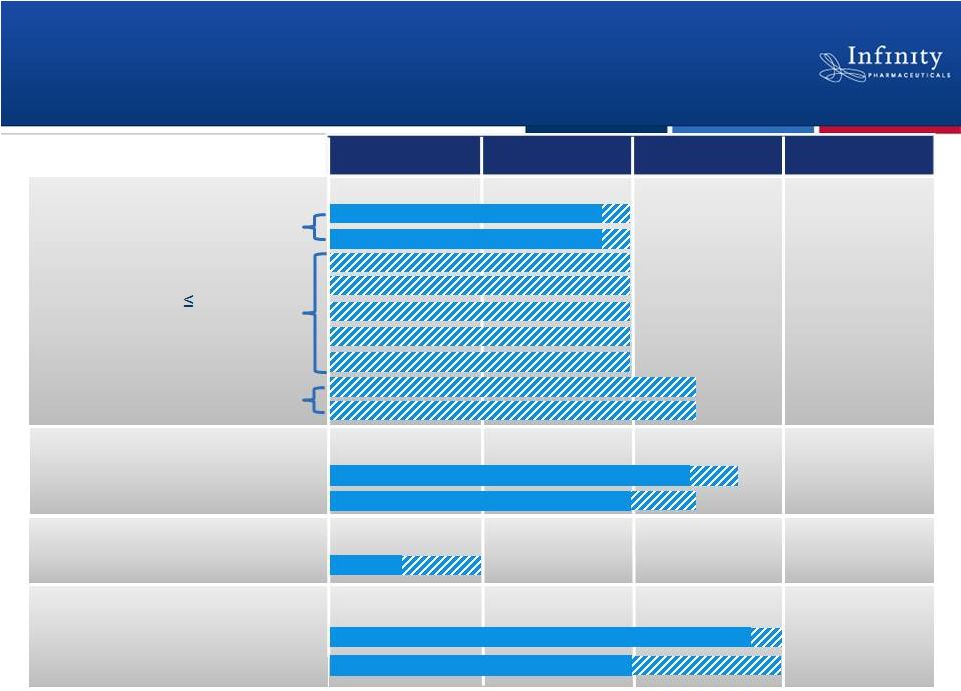 Phase 1
Phase 2
Phase 3
Preclinical
Potential Pipeline at Year-End 2013*
34
IPI-145 in Heme
IPI-443
PI3K Pipeline Development
IPI-145 in Inflammation
Asthma
Rheumatoid Arthritis
5 Additional
Cohort Expansions
NSCLC (Heavy smokers)
NSCLC (mKRAS)
Hsp90: Retaspimycin HCl
Additional Trials
*If data are positive.
Adv. Hematologic Malignancies
Dose-Escalation
25 mg BID Expansion
Docetaxel Combo
Everolimus Combo |
 Building a Fully Integrated
Biopharmaceutical Company
January 2013 |
