Attached files
| file | filename |
|---|---|
| EX-99.1 - PRESS RELEASE - AtriCure, Inc. | d462665dex991.htm |
| 8-K - CURRENT REPORT - AtriCure, Inc. | d462665d8k.htm |
 Investor
Presentation January 2013
Exhibit 99.2 |
 1
Forward Looking Statements
Non-GAAP Measures
This
presentation
contains
“forward-looking
statements”
within
the
meaning
of
the
Private
Securities
Litigation
Reform Act of 1995. Forward-looking statements include statements that address activities,
events or developments
that
AtriCure
expects,
believes
or
anticipates
will
or
may
occur
in
the
future,
such
as
earnings
estimates, other predictions of financial performance, launches by AtriCure of new products and
market acceptance of AtriCure’s products. Forward-looking statements are based
on AtriCure’s experience and perception of current conditions, trends, expected
future developments and other factors it believes are appropriate under the circumstances
and are subject to numerous risks and uncertainties, many of which are beyond
AtriCure’s control. These risks and uncertainties include the rate and degree of market acceptance of
AtriCure’s products in the United States, AtriCure’s ability to develop and market new
and enhanced products, the timing of and ability to obtain and maintain regulatory
clearances and approvals for its products and the impact of failure to obtain such
clearances and approvals on its ability to promote its products and train doctors in the use
of its products, the timing of and ability to obtain reimbursement of procedures utilizing
AtriCure’s products and the potential impact of current healthcare reform
initiatives thereon, competition from existing and new products and procedures or
AtriCure’s ability to effectively react to other risks and uncertainties described from time to
time
in
AtriCure’s
SEC
filings,
such
as
fluctuation
of
quarterly
financial
results,
reliance
on
third
party
manufacturers and suppliers, litigation or other proceedings (including by the FDA), government
regulation, negative publicity, current worldwide economic conditions and stock price
volatility. AtriCure does not guarantee any forward-looking statements, and actual
results may differ materially from those projected. Unless required by
law,
AtriCure
undertakes
no
obligation
to
publicly
update
any
forward-looking
statement,
whether
as
a
result
of new information, future events or otherwise. A list and description of risks, uncertainties
and other matters can be found in AtriCure’s Annual Report on Form 10-K for 2010
and in AtriCure’s reports on Forms 10-Q and 8-K. This presentation includes
the use of non-GAAP measures, which are noted with a *. Reference AtriCure’s 8-K’s
filings which include the furnishing of our earnings releases for a reconciliation to the
related GAAP measure. |
 2
AtriCure Highlights
New leadership November 2012
–
Focus on accelerating growth, commercial execution, R&D innovation
Large underpenetrated market with few sustainable treatment options
Undisputed market leader in all types of surgical ablation
–
Broad and deep product portfolio
–
Strong IP in the field of AF
–
Strongest brand –
recognized for high-quality and innovative products
–
Leading KOL support and enthusiasm
Accelerating
revenue
growth
see
path
to
15%+
growth
–
Open
only
AF
label
for
surgical
ablation
–
Minimally
Invasive
Solutions
(MIS)
Largest
long-term
market
–
Left
Atrial
Appendage
(LAA)
Management
Solution
Danger
Zone….Opportunities
–
International Expansion
Improve share and enter new markets
Opportunity
for
expanding
gross
margins
see
path
to
75%+ |
 3
AtriCure at a Glance –
Strong Track Record
Surgical ablation leader for the treatment of atrial fibrillation (AF)
–
Over 100,000 Open procedures in >700 medical centers
–
Over 10,000 MIS procedures in ~130 medical centers
Leader in implants designed to exclude left atrial appendage (LAA)
–
Over 14,000 safely and effectively implanted
100+ peer-reviewed publications highlighting product results
Significant key opinion leader (KOL) support
Global innovator with comprehensive product line
–
AtriCure
Synergy
Ablation
System
–
bipolar
ablation
clamp
system
–
Cryoablation –
reusable and disposable cryoablation devices
–
AtriClip –
designed to safely and effectively exclude the LAA
Experienced Sales Force
–
Over 40 US territories led by eight managers
Preliminary
2012
Revenue
-
$70.2
million
–
Expected Growth of ~9% y/y |
 4
AF Population: Large, Growing & Undertreated
(1)Miyasaka Y, et al. Circulation. 2006;114(2):119-125.
(2)Lloyd-Jones D, et al. [published online ahead of print December 17, 2009].
Circulation. doi:10.1161/CIRCULATIONAHA.109.192667.
(3)Lloyd-Jones DM, et al. Circulation. 2004;110(9):1042-1046.
(4) Fuster V, et al. J Am Coll Cardiol. 2001;38(4):1231-12665
(5)
Benjamin EJ, et al. Circulation. 1998;98(10):946-952.
(6)
Kim M, et al. Circ Cardiovasc Qual Outcomes. 2011;
4:313-320
AF affects over 5
million in the U.S.
(1)
Significant costs to
healthcare system
•
U.S. prevalence projected to grow to 12-15 million by 2050
•
International prevalence is comparable to the U.S.
•
Most
common
sustained
cardiac
arrhythmia
(2)
•
Lifetime
risk
of
AF:
~1
in
4
for
adults
40
years
of
age
(3)
AF increases 5-fold
the
risk
of
stroke
(4,5)
•
AF
is
leading
cause
of
stroke
–
over
15%
in
US
linked
to
AF
(5)
•
AF
results
in
early
mortality
and
cause
of
stroke
in
elderly
(4)
•
AF-related strokes are more severe
(5)
Issues with non-
surgical treatment
of AF
•
Warfarin drug therapy has complications
•
Anti-arrhythmic drugs often not well-tolerated and ineffective
•
< 3% of AF patients are treated with catheter or surgical
ablation
•
Direct
medical
costs
are
~73%
higher
in
AF
patients
(6)
•
Net
incremental
cost
of
$8,705
per
patient
per
annum
(6)
•
U.S.
annual
incremental
cost
of
AF
is
~$26.0
billion
(6) |
 5
Atrial Fibrillation U.S. Market Opportunity
•
Labeling advantage in US
•
Best products
•
High gross margins
•
Education key
•
Competition is large device
•
High reimbursement
•
High gross margins
•
Growing internationally
•
Development needed
•
Competition is start-ups
AtriCure is the only
on-label product
Developing Market
Market
Opportunity,
$370M
Current
Market,
$130M
Market
Opportunity,
$3.9 billion
Current
Market,
$40M
Open
Surgical
Patient
Opportunity,
100,000
Untreated
or
Managed
with
Drugs,
2.3
Million
MIS/Stand-
Alone
Patient
Opportunity,
600,000
MIS Market
Open Surgical Market
3-5 Million Patients in U.S. with AF |
 Left Atrial
Appendage U.S. Market Opportunity •
Mechanical, Electrical,
Atraumatic; Solution
•
Competition includes Tiger
Paw, staple, suture,
ligature (endloop) –
NO
Complete Solution
•
Attractive sole therapy
reimbursement
•
Competition includes
implants and EP closure
(w/o FDA approval)
AtriCure has more
implants than
competition (14K+)
~1M U.S, LAA Patients
LAA (Concomitant)
LAA
(Concomitant)
100,000
patients
LAA
(Sole Therapy)
~750,000
patients
Market
Opportunity,
$100M
Current
Market,
$10M
Current
Market,
$32M
Market
Opportunity,
$1.5B
LAA ( Sole Therapy)
6 |
 7
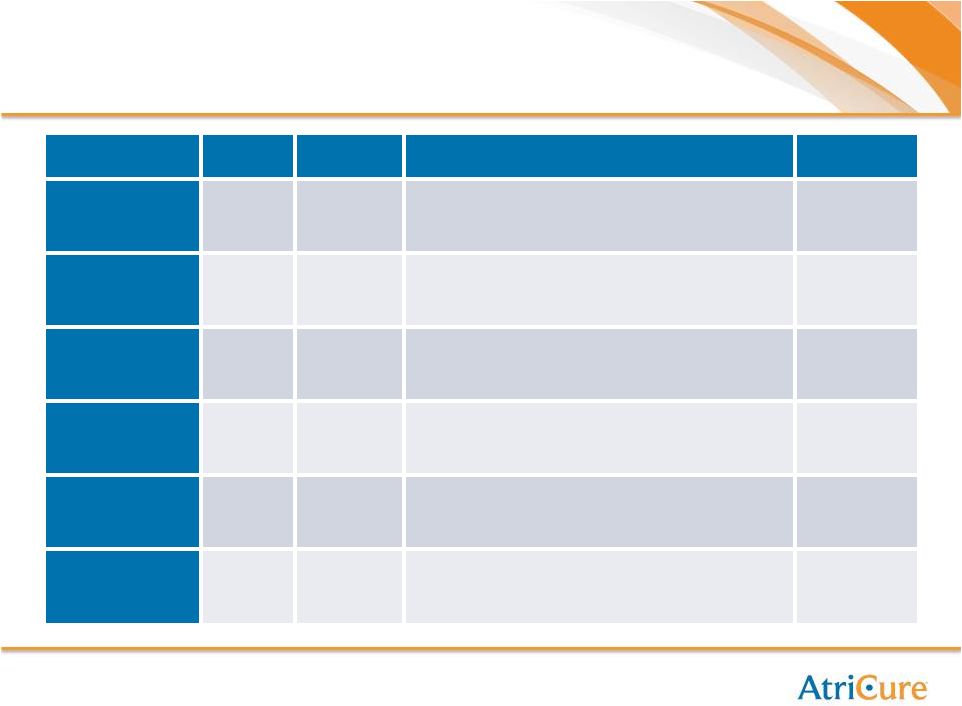
AtriCure Business Overview
Focus Areas
2012
Growth
Market
Size ($M)
Keys to Success
Current
Trials
Open AF
(Concomitant)
+13%
$500M
Education; referral dev.; Reimbursement;
awareness; conversions; add-on sales
PAS
MIS AF Sole
Therapy
-11%
$4,000M
Referral development; new products; hybrid;
surgeon proctoring; development of less invasive
approach (products and procedures)
Staged DEEP
Open Clip
(Concomitant)
$100M
Market awareness; open growth; overcoming
cost argument; stroke RCT
Papers
Clip Sole
Therapy
New ’13
$1,500M
Market awareness; international; eliminating
complications; supporting early adopters; referral
development; prominent KOLs; stroke RCT
Papers
International
+14%
Same as US
Market development; reimbursement; coverage;
marketing; customer service
Involvement
Above
Overall
+9%
$12,000M
Investments in marketing, international, R&D
pipeline
+26% |
 8
Growth Strategy: Overview
Expand Open-Heart Sales
Penetrate LAA Opportunity
Build MIS Platform
International Expansion
•
Leverage recent AF indication
•
Increased training and education
•
Capitalize on sales force realignment
and AF Strategic Marketing Team
•
Capitalize on cross-sale opportunities
resulting from AF labeling
•
Capitalize on increased investments in
direct sales team
•
Geographic expansion and new products
•
Increase support for distributors
•
Penetrate open-heart ablation centers
•
Featured in our training and education
for open-heart
•
FDA approved for use in DEEP AF trial
•
Longer-term: commercialization of sole-
therapy platform
•
Support existing MIS surgeons
•
Support staged DEEP AF trial
AtriCure
is
the
only
company
with
FDA
approval
to
treat
the
AF
disease
state
and
is a leader in the emerging market of LAA exclusion |
 9
Growth Strategy: Expand Open-Heart Sales
•
FDA approved the AtriCure Synergy
Ablation System for treatment of non-
paroxysmal Atrial Fibrillation in
patients undergoing other structural
heart procedures (December 2011)
•
Only surgical ablation system that
has received FDA indication
•
AtriCure can partner with surgical AF
leaders to provide comprehensive
training and support
•
Support “on-label”
surgeon training
and education
–
Improves safety and efficacy
–
Increases surgeon confidence
–
Reduces operative time
•
Actively increase market awareness
•
Competitive account conversions
FDA Approval
Leveraging FDA Approval |
 10
Growth Strategy: Expand Open-Heart Sales
•
Significant
progress
in
both
surgeon
and
site
certification
–
all
achieved
in
a
9
month
period (18 month program)
•
Expectation that revenue for all accounts trained will be consistent with these results
•
Significant competitor conversions during 2012
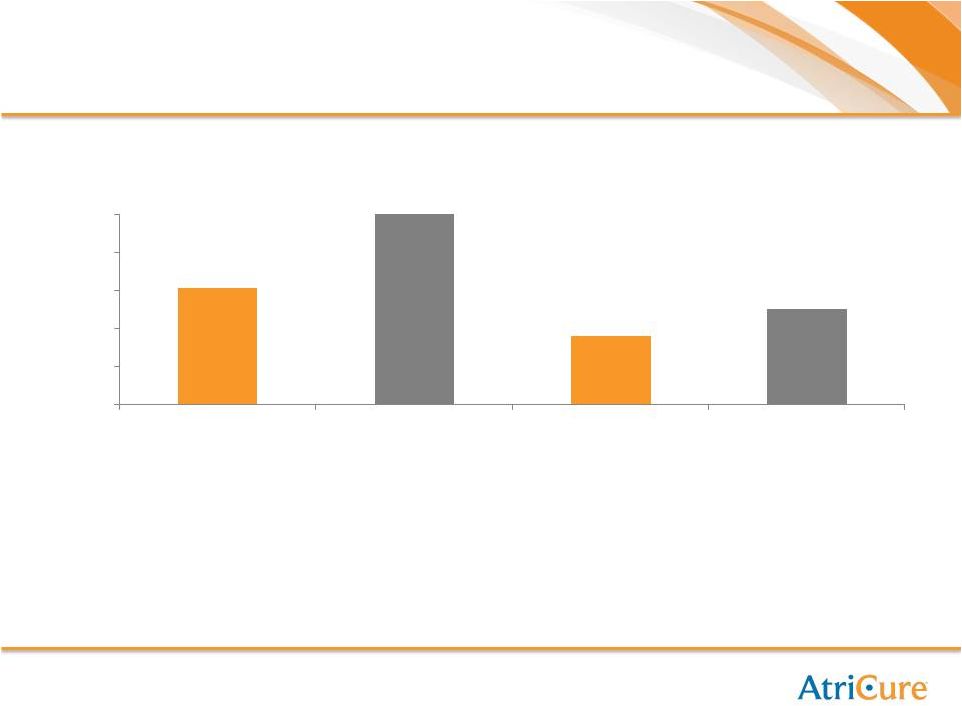
Surgeons Certified
Sites Certified
609
1,000
359
500
-
200
400
600
800
1,000
9 Month Progress
18 Month Goal
9 Month Progress
18 Month Goal |
 11
Growth Strategy: Build MIS Platform
•
Leverage HRS consensus statement
•
AF Centers of Excellence
•
AtriCure Maze IV surgeon post training
marketing programs
•
AF Awareness Campaign focused on
surgical options
•
Sole therapy clinical trials spur interest
in surgical AF
•
Product launches
–
Coolrail
re-launch
–
October
2012
–
AtriClip
Pro
launch
–
October
2012
•
DEEP AF / Staged DEEP AF IDE
Feasibility Trial
•
Randomized CA vs. MIS PCORI Study
Submission (Ellenbogen)
•
Support academic papers
Build Clinical Evidence
Strategic Initiatives |
 12
Growth Strategy: Penetrate LAA Opportunity
•
14,000+ AtriClips implanted:
–
No reported erosions
–
No clip migration
–
No leakage
•
Mechanical closure reduces nidus for AF-
related emboli
•
Electrically isolates the appendage,
reduces source for ectopic firing
•
AtriCure has first 510(k) device clearance
for LAA specific exclusion device
EXCLUDE 510(k) trial demonstrated 98% of patients with complete closure at three
month endpoint and zero device-related serious adverse effects
Efficacy
Safety
Safety & Efficacy of Open and MIS AtriClip products is Well-Established
|
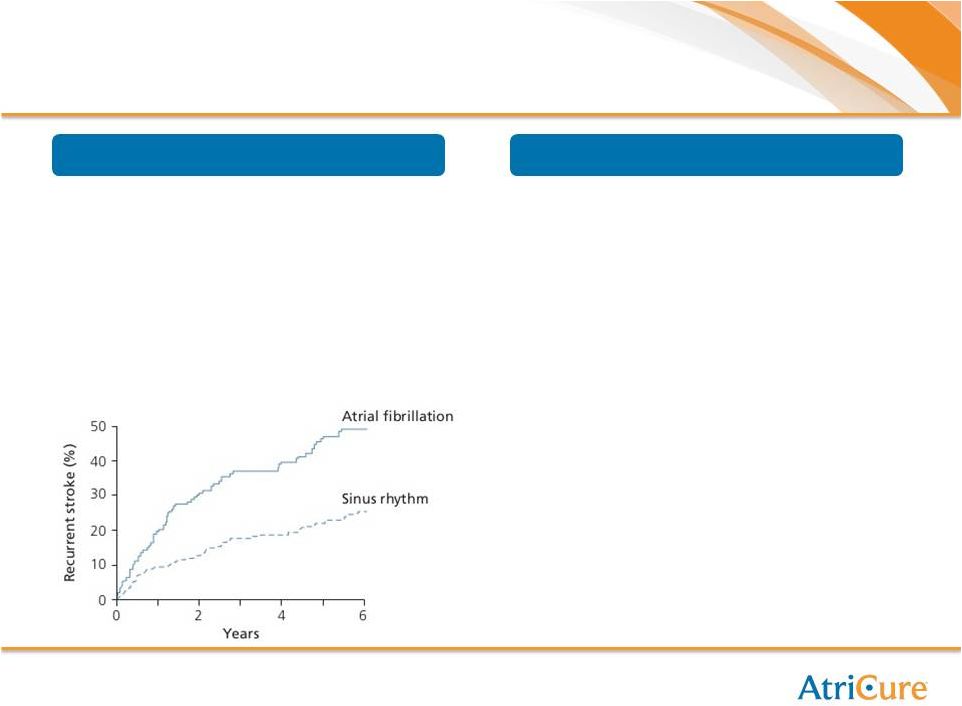 13
Growth Strategy: Penetrate LAA Opportunity
•
Most prevalent and life-threatening
complication of AF is stroke
•
LAA is the site of more than 90% of
detected thrombi in AF patients
(1)
•
Atritech Watchman PROTECT AF clinical
trial results confirms LAA exclusion
reduces AF related stroke
•
The 2006 AHA/ACC/ESC guidelines:
–
“The LAA should be removed from
circulation when possible during
cardiac surgery in patients at risk of
developing postoperative AF”
•
Conventional surgical techniques for
exclusion are suboptimal
(3)
–
60% of closures were unsuccessful
–
41% with unsuccessful LAA exclusion
had thrombus in LAA
–
15% with unsuccessful closure had
evidence of stroke or TIA
•
Growing patient awareness created by Big
Pharma
•
Reimbursement code established for sole
therapy LAA exclusion
(1)
Blackshear
(2)
Am J Med, vol. 114, Penado et al. 2003
(3)
(1) Kanderian, MD, Anne S., A. Marc Gillinov, MD, Gosta B. Pettersson, MD, PHD, Eugene
Blackstone, MD, and Allan L. Klein, MD, FACC. "Success of Surgical Left Atrial
Appendage Closure." JACC. 52.11 (2008): 924-9. Print.
Cumulative
Stroke
Recurrence
Rate
(2)
LAA Exclusion Market Drivers
Significant Risks Associated with LAA |
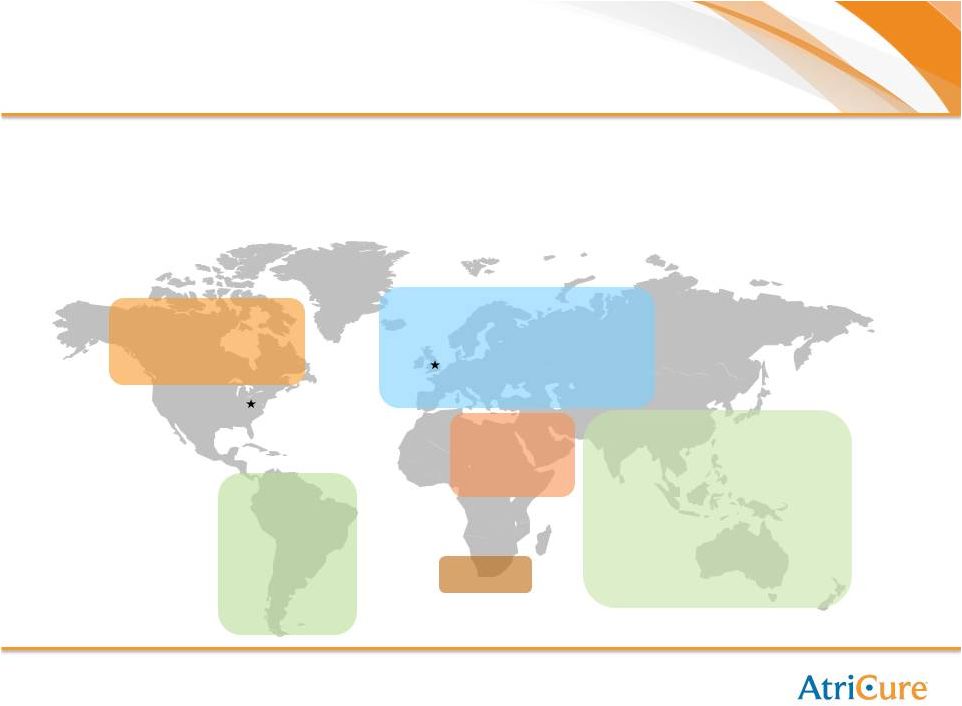 14
Growth Strategy: International Expansion
•
EMEA office based in Amsterdam with infrastructure to support commercial activities
•
Direct sales in Germany, Switzerland
(1)
, Austria, Benelux and the UK in 2013
•
Exclusive country distributors in other regions
(1)
Sales agent
Amsterdam
Cincinnati
South America
Distributor
relationships:
Canada
Asia Pacific
Distributor relationships:
Europe
China
Hong Kong
Japan
Korea
Thailand
Malaysia
Singapore
India
Australia /
New Zealand
Taiwan
Brazil
Colombia
Chile
Mexico
Argentina
Costa Rica
Distributor relationships:
Italy
Spain
Denmark
Poland
Portugal
Sweden
Turkey
Czech
Republic
Greece
Norway
Russia
South Africa
Middle East
Distributor
relationships:
Pakistan
Saudi Arabia |
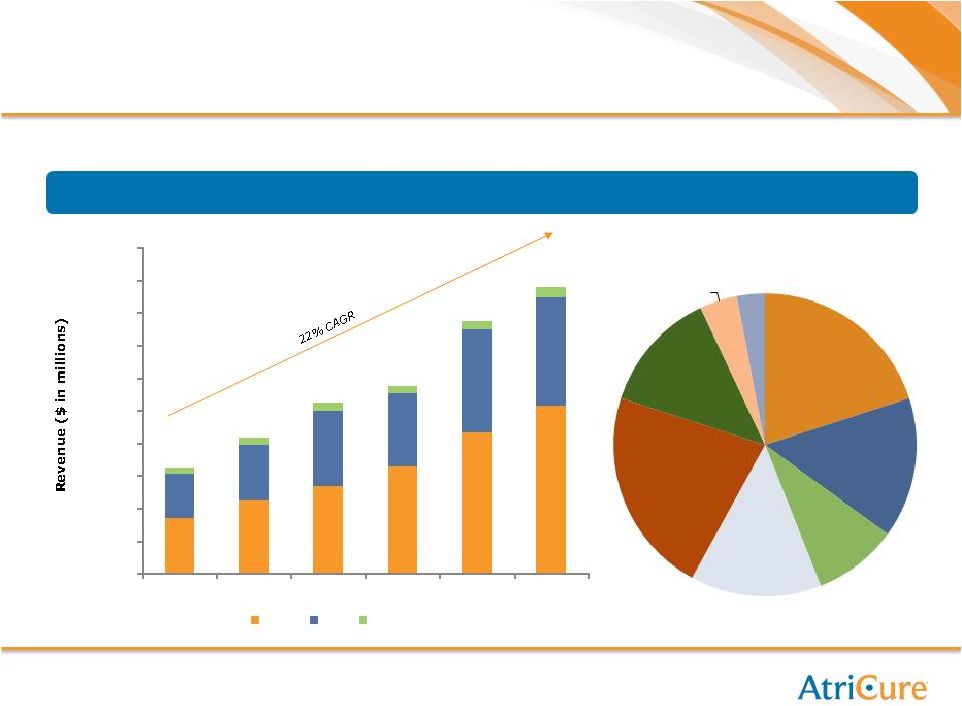 15
Growth Strategy: International Expansion
$6.4
$8.3
$10.5
$11.5
$15.5
$17.6
International
Revenue
By
Region
(2007
–
2012)
$3.4
$4.5
$5.4
$6.6
$8.7
$10.3
$2.7
$3.4
$4.6
$4.5
$6.3
$6.7
$0.4
$0.4
$0.5
$0.4
$0.5
$0.6
-
$2.0
$4.0
$6.0
$8.0
$10.0
$12.0
$14.0
$16.0
$18.0
$20.0
2007
2008
2009
2010
2011
2012
EMEA
Asia
ROW
$
Benelux,
20%
Germany,
15%
UK, 9%
Other
EMEA,
14%
Japan,
22%
China,
13%
Other
Asia, 4%
ROW, 3%
International sales growing rapidly – 25% of total revenue |
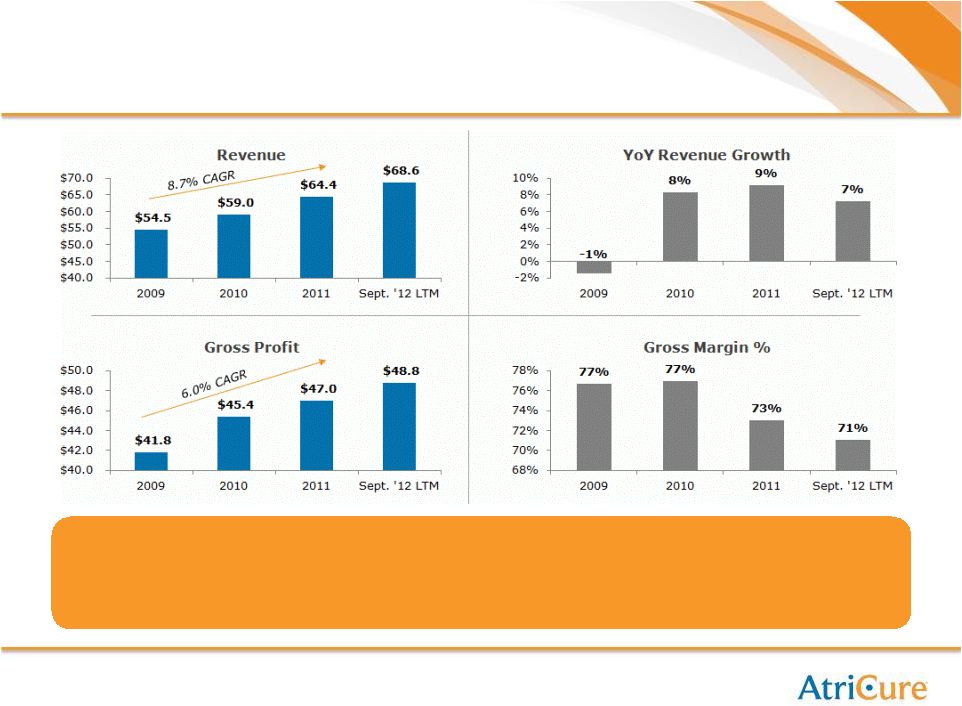 16
Key Financial Highlights
Opportunity for…
Accelerating revenue growth to 15%+ (8% 5yr. CAGR today)
Expanding Gross Margins to over 75% (71% today) |
 17
AtriCure Highlights
New leadership since November 2012
–
Focus on accelerating growth, commercial execution, R&D innovation
Large underpenetrated market with few sustainable treatment options
Undisputed market leader in all types of surgical ablation
–
Broad and deep product portfolio
–
Strong IP in the field of AF
–
Strongest brand –
recognized for high-quality and innovative products
–
Leading KOL support and enthusiasm
Accelerating
revenue
growth
see
path
to
15%+
growth
–
Open
only
AF
label
for
surgical
ablation
–
Minimally
Invasive
Solutions
(MIS)
Largest
long-term
market
–
Left
Atrial
Appendage
(LAA)
Management
Solution
Danger
Zone….Opportunities
–
International
Expansion
Improve
share
and
enter
new
markets
Opportunity
for
Expanding
Gross
Margins
see
path
to
75%+ |
 Appendix
|
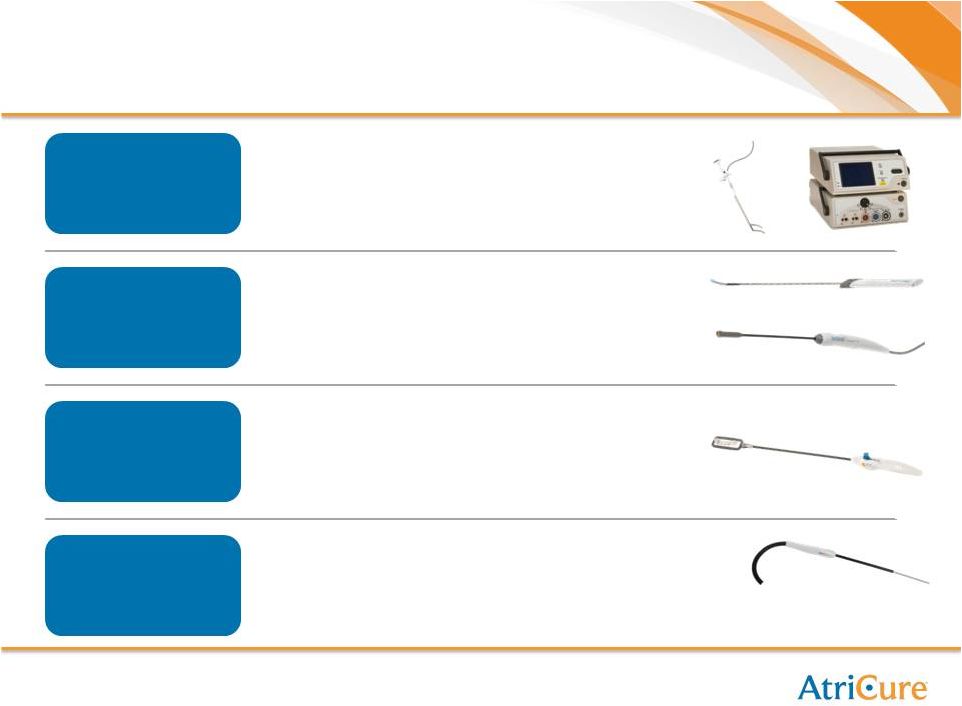 19
Broadest Product Portfolio
Probes/Pens
Synergy
Cryoablation
AtriClip
•
Workhorse ablation system
•
Completely automated
•
Rapid: 10-30 second ablations
•
Used in Open and MIS
•
Deepest unidirectional lesion formation
•
Totally thoracoscopic design
•
Simple user interface
•
Clip
easily
be
repositioned
–
MIS
and
Open
•
Fabric prevents slippage, promotes in-growth
•
Appendage atrophies away
•
Used for LAA
•
Faster and More consistent to achieve lethal
probe temperature
•
Only probe with Defrost feature
•
Greatest work capacity in cryo probe market |
 20
Robust Product Pipeline
AtriClip
RF Ablation
Cryoablation
Long Linear Ablation
•
One-sided approach to perform
Pulmonary Vein Isolation
•
Improved safety and efficacy
over competitive devices
Next
Generation
AtriClip
-
BOA2
•
Tailored to most difficult and
challenging access
•
Launched 3Q 2012
Reusable Clip Deployment
•
Reusable Platform
•
Lower cost clip option
Next Generation AtriClip and
AtriClip –
ACH2
•
Improved access via malleable
shaft and in-line design
•
Open-ended clip design
Cryo Console Adapter Box-AAM
Next Generation Cryo-
CRYO2A
•
Increased Malleability
Next Generation RF & Cryo
Generators
•
Improved usability and branding
•
Increased capabilities to support
future platforms
•
Ability to use re-usable cryo
platform with automated
cryo module (ACM)
Current status:
94 active product versions and 153 active product codes |
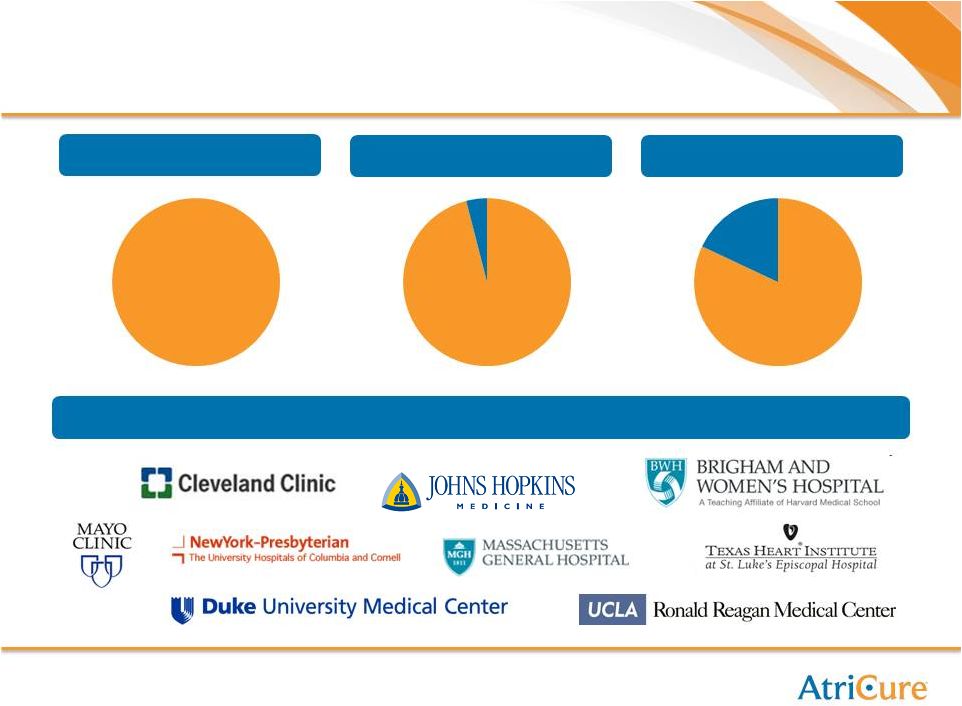 21
Blue Chip Customer Base
Top 10 “Heart Hospitals”
U.S. News & World Report
Top 25 “Heart Hospitals”
U.S. News & World Report
Top 50 “Heart Hospitals”
U.S. News & World Report
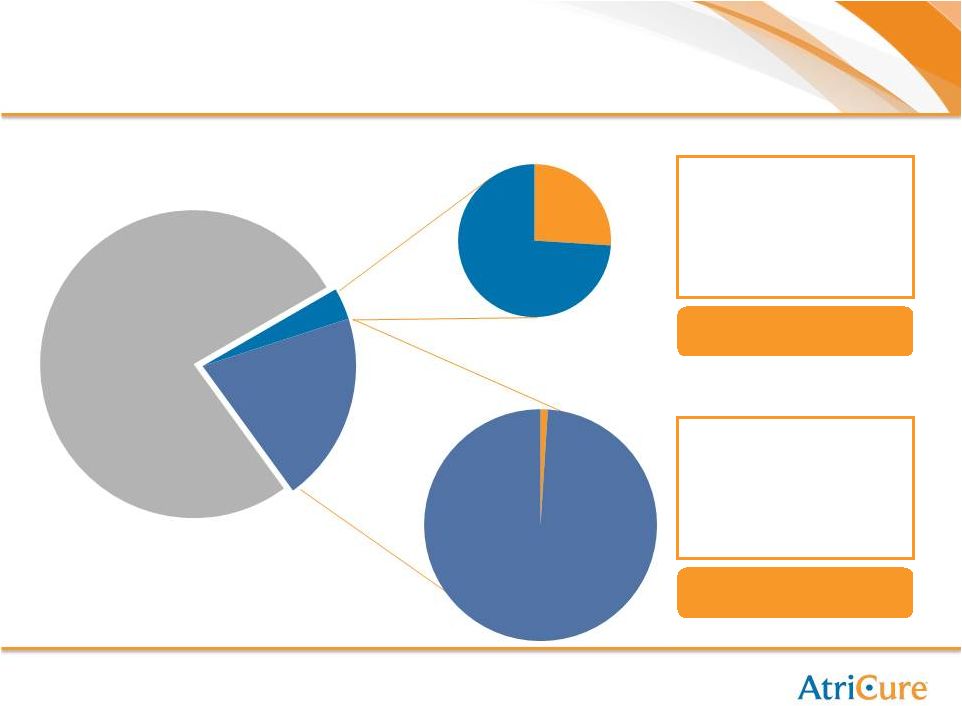
Representative Blue Chip Customers
AtriCure
Hospitals,
100%
Non-
AtriCure
Hospitals,
4%
AtriCure
Hospitals,
96%
Non-
AtriCure
Hospitals,
18%
AtriCure
Hospitals,
82% |
