Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HERON THERAPEUTICS, INC. /DE/ | d462570d8k.htm |
 Company Overview
OTCBB: APPA
January 2013
Exhibit 99.1 |
 Legal
Disclaimer This presentation contains "forward-looking statements"
as defined by the Private Securities Litigation Reform Act of 1995.
These forward-looking statements involve risks and uncertainties,
including uncertainties associated with timely development, approval, launch
and acceptance of new products, satisfactory completion of clinical studies,
establishment of new corporate alliances, progress in research and
development programs and other risks and uncertainties identified in the
Company's filings with the Securities and Exchange Commission. Actual
results may differ materially from the results expected in our forward looking
statements. We caution investors that forward-looking statements
reflect our analysis only on their stated date. We do not intend to
update them except as required by law.
2
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 Company:
A.P. Pharma, Inc.
Ticker:
OTCBB: APPA.OB
Stock Price:
$0.60 (1/4/13)
Market Capitalization:
$280.3
million
Cash:
$60.0
million
Debt:
$4.7
million
Stock Summary
1
Based on 499.0 million fully diluted, as-converted common shares assuming the
full conversion of convertible debt outstanding and 80 million warrants
using treasury stock method; not including options
2
As of September 30, 2012
3
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
1
2
2 |
 John
B. Whelan President & CEO
Raven Biotechnologies
Eos Biotechnology
Hewlett Packard/Agilent
Michael A. Adam, Ph.D.
Senior Vice President &
Chief Operating Officer
Spectrum Pharmaceuticals
Pfizer/Agouron
Bristol-Myers Squibb
Mark Gelder, M.D.
Senior Vice President &
Chief Medical Officer
GE Healthcare
Bayer Healthcare
Wyeth
Robert Rosen
Senior Vice President &
Chief Commercial Officer
Bayer Healthcare
Sanofi-Synthèlabo
Imclone
Senior Management
4
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 A.P.
Pharma Highlights January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
5
Lead product candidate, APF530, is long-acting, injectable
product for chemotherapy-induced nausea and vomiting (CINV)
Incorporates widely
used
5-HT3
antagonist
-
granisetron
(Kytril
®
)
5-day delivery profile
Reduces both acute-
and delayed-onset CINV with single injection
Patent coverage into 2024
APF530
shown
to
be
non-inferior
to
market
leader
Aloxi
®
1,341-patient, randomized, controlled, Phase 3 study
FDA PDUFA Action Date of March 27, 2013
Resubmitted NDA for APF530 in September 2012
Addressed issues raised in Complete Response Letter
Product launch planned for 2H 2013
APF530 targets a $900 million market opportunity in US alone*
Recent competitive setbacks could enhance commercial uptake
Could be second, long-acting, injectable product on market
A.P. Pharma has the potential to leverage its Biochronomer™
drug delivery technology into other opportunities
*Branded market estimate using 2011 units based on Wolters Kluwer and Aloxi ASP
|
 Important APF530 Milestones
Milestone
Timing
Status
Successful End-of-Review Meetings with
FDA
1Q 2011
Successful Completion of Thorough QT
and Metabolism Studies
1Q 2012
Completed $53.6MM PIPE Financing
3Q 2012
Successful Completion of Human Factors
Validation Study
3Q 2012
Successful Completion of CMC Activities
3Q 2012
Resubmitted NDA
Sept 2012
Key Commercial Organization Hires
4Q 2012
FDA PDUFA Action Date
March 27, 2013
Target Product Launch
2H 2013*
* Indicates expected milestone timing
6
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 Clinical Summary
7
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 APF530
Pivotal Phase 3 Study Overview Randomized, controlled, multi-center
study 1,341 patients in primary efficacy population
Two doses of APF530 (5 mg and 10 mg granisetron)
compared to the approved dose of Aloxi
Patients stratified by type of chemotherapy regimen
(moderately or highly emetogenic)
Primary end point compared complete response between
groups in both the acute (day 1) and delayed (days 2-5)
phase
Complete response defined as no emesis and no rescue medications
A ±15% margin was used to establish non-inferiority
8
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
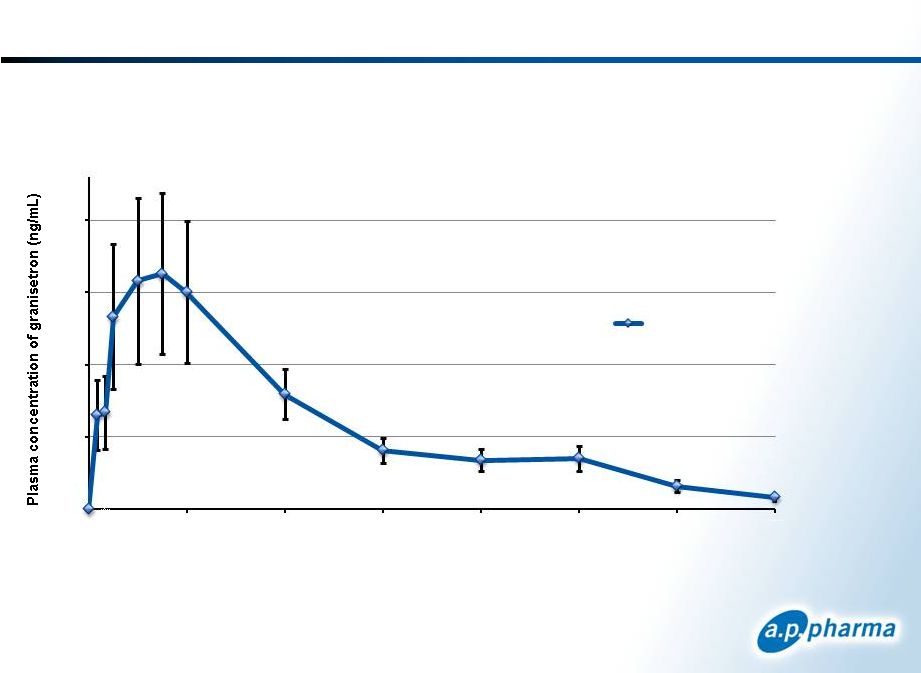 5-Day Profile: APF530 Pharmacokinetics
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
9
Granisetron is released rapidly following injection of APF530 and continues to be
released over a 5-day period.
0
5
10
15
20
0
24
48
72
96
120
144
168
Time after Dosing (h)
All subjects (n= 18)
mean ±
SEM |
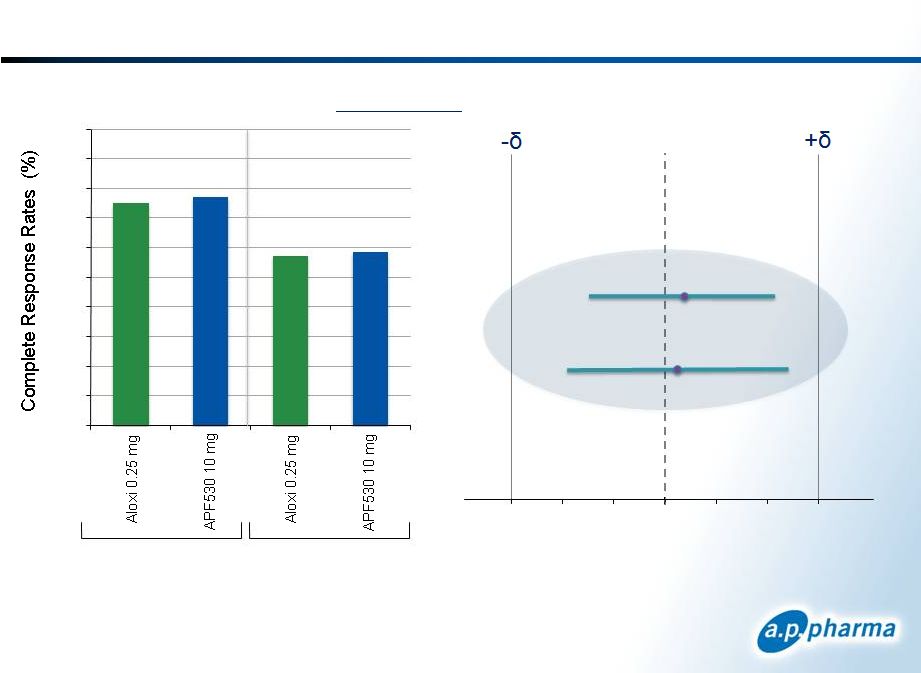 Primary Efficacy Results: Complete Response
Patients Receiving Moderately
Emetogenic Chemotherapy
Acute
APF530 10mg
Acute
Delayed
Difference in Complete Response
APF530-Aloxi (97.5% CI)
-15
-10
-5
0
5
10
15
10
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
Delayed
75.0
76.9
57.2
58.5
0.0
10.0
20.0
30.0
40.0
50.0
60.0
70.0
80.0
90.0
100.0 |
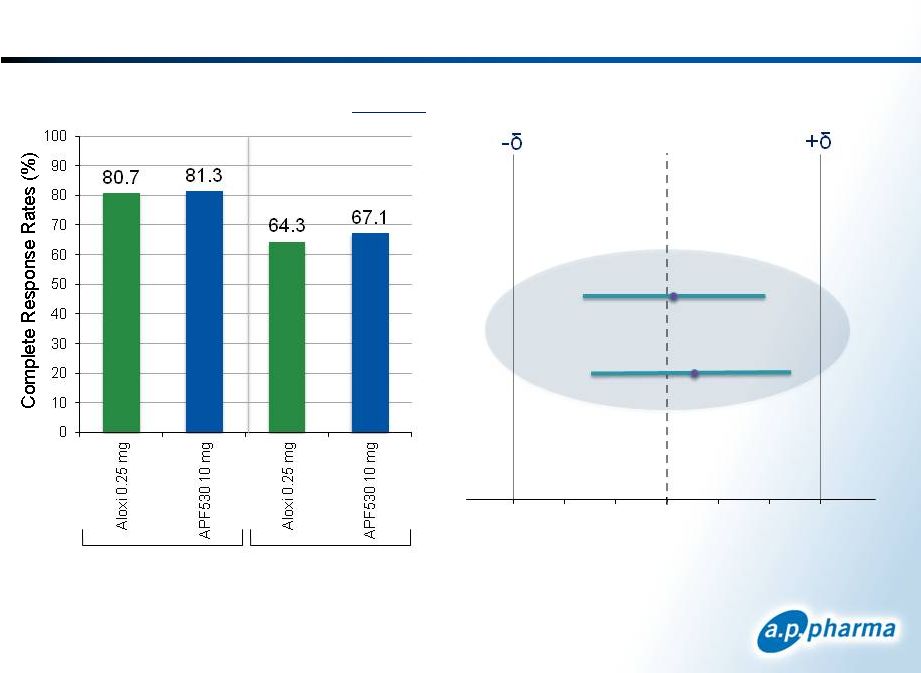 Primary Efficacy Results: Complete Response
Difference in Complete Response
APF530-Aloxi (98.33% CI)
-15
-10
-5
0
5
10
15
Patients Receiving Highly
Emetogenic Chemotherapy
Acute
Delayed
11
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
Acute
Delayed
APF530 10mg |
 Safety
Summary 1
APF530 5 mg dose studied in separate arm of the phase 3 study; one pulmonary
embolism in morbidly obese patient on day 16 (0.2%); one dyspepsia event
(0.2%) 2
>90% of injection site reactions were reported as mild; one patient
discontinued due to injection site reaction
Reported in Cycle 1
12
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
APF530 10 mg
Aloxi 0.25 mg
N
%
N
%
Drug
Related
Serious
Adverse
Events
0
0
0
0
Discontinued
Due
to
Adverse
Event
1
0.2
0
0
Frequent Adverse Events
Gastrointestinal Disorders
Constipation
Diarrhea
Abdominal pain
72
44
13
15.4
9.4
2.8
62
39
28
13.4
8.4
6.0
Nervous System
Headache
47
10.0
45
9.7
Injection Site
Placebo (NaCl)
Bruising
Erythema (redness)
Nodule (lump)
Pain
93
51
50
33
19.9
10.9
10.7
7.1
41
14
3
5
8.9
3.0
0.6
1.1
1
1
2 |
 Efficacy through Multiple Chemotherapy Cycles
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
13
1
Overall Complete Response defined as no emesis and no rescue medications during 0
to 120 hours following chemotherapy
Moderately Emetogenic
Chemotherapy
Highly Emetogenic
Chemotherapy
Overall
Complete
Response
Rates
for
APF530
10
mg
53.8
58.8
61.7
0
10
20
30
40
50
60
70
80
90
100
Cycle 1
Cycle 2
Cycle 3
Cycle 4
63.3
73.7
76.9
82.1
0
10
20
30
40
50
60
70
80
90
100
Cycle 1
Cycle 2
Cycle 3
Cycle 4
62.0
1 |
 January
2013 ©
2013. A.P. Pharma, Inc. All rights reserved.
14
1
Overall Complete Response defined as no emesis and no rescue medications during 0
to 120 hours following chemotherapy. Protocol criteria may vary across
studies. MEC
HEC
Ondansetron
Ondansetron
Granisetron
Gralla
Eisenberg
Aapro
Emend Label Studies
Emend
Label
Saito
50%
34%
25%
43%
52%
43%
33%
40%
MEC
HEC
Eisenberg
Gralla
Grunberg
Hajdenberg
APPA Ph 3
Aapro
APPA Ph 3
46%
69%
59%
59%
52%
41%
62%
Aloxi –
Second Generation
First Generation 5-HT3 Antagonists
1
Overall
Complete
Response
Rates |
 APF530’s Efficacy with Difficult Chemo Regimens
Treatment
Chemotherapeutic Regimen
APF530 10 mg
Aloxi 0.25 mg
Moderately
Emetogenic
Acute
Cyclophosphamide/Doxorubicin
70.7%
65.7%
All other regimens
84.4%
85.0%
Delayed
Cyclophosphamide/Doxorubicin
47.4%
46.3%
All other regimens
72.9%
70.0%
Highly
Emetogenic
Acute
Cisplatin regimens
81.1%
75.5%
Carboplatin/Paclitaxel
85.4%
89.8%
All other regimens
75.4%
67.6%
Delayed
Cisplatin regimens
66.0%
60.4%
Carboplatin/Paclitaxel
70.8%
71.4%
All other regimens
65.2%
57.4%
1
Data from post-hoc analysis. Not statistically significant.
15
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
1 |
 Summary of APF530 Phase 3 Results
One of the largest, randomized, controlled clinical studies
conducted in the CINV setting
Bioerodible polymer technology releases granisetron to
prevent CINV over 5 days
Non-inferiority to Aloxi was demonstrated at 10 mg
For both acute-
and delayed-onset CINV
With both moderately and highly emetogenic chemotherapy
APF530 was well-tolerated
Incidence of adverse events comparable to Aloxi
Good response rates were observed in difficult
chemotherapy regimens
Efficacy was maintained through multiple cycles of
chemotherapy
16
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 Regulatory Status
17
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 APF530
NDA Status Submitted NDA in May 2009 under 505(b)(2) filing pathway
Received Complete Response Letter in March 2010
FDA raised issues in three main areas:
Dosing system
Two-syringe system
Chemistry, Manufacturing, and Controls (CMC)
Sterilization
Characterization
Clinical/statistical
Specific studies
Presentation of data
Held end-of-review meetings with FDA in 1Q 2011
No additional clinical efficacy studies requested
Resubmitted NDA in September 2012
PDUFA Action Date of March 27, 2013
18
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 Addressed Complete Response Letter Issues
Dosing System
Change to single-syringe system
Enhanced dosing instructions
Successfully evaluated in a Human Factors study
Chemistry, Manufacturing, and Controls
Change from bulk to terminal irradiation
Additional specifications and assays for raw materials, polymer and drug
product
Three registration lots completed implementing these changes
Clinical/Statistical
Thorough QT study
Metabolism study
Phase 3 clinical data presentation revision
19
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
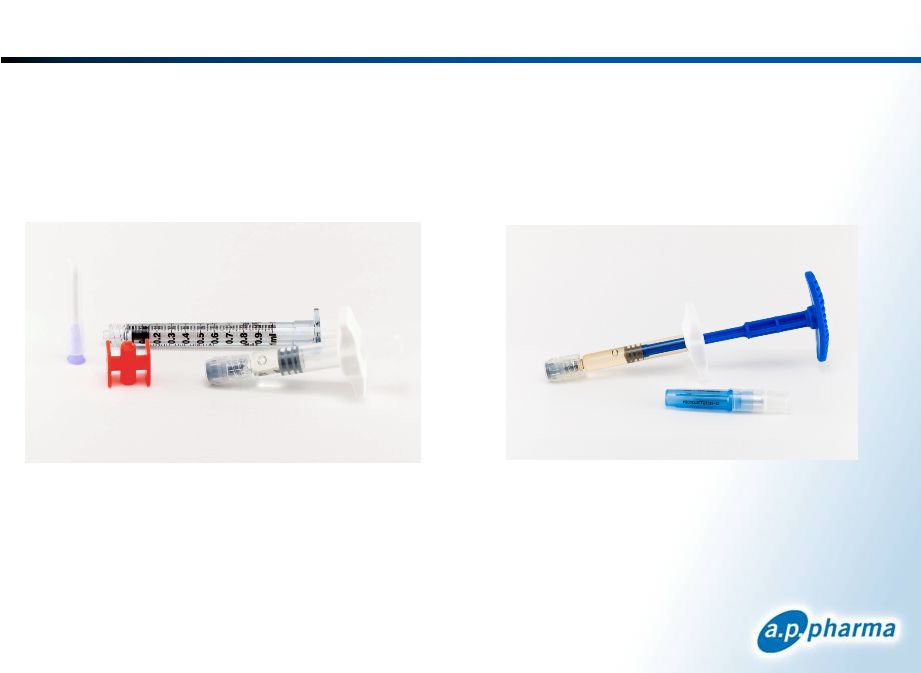 Improved Dosing System
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
20
New Single-syringe System
Original Two-syringe System |
 Thorough QT Study Background
Prolongation of the QT/QTc interval is associated with increased
susceptibility to fatal cardiac tachyarrhythmias
Thorough
QT
studies
are
intended
to
determine
whether
a
drug
has
a
threshold pharmacologic effect on cardiac repolarization
Thorough QT studies are now routinely required by the FDA prior to
drug approval
FDA has raised QT cardiac safety concerns with 5-HT3 antagonists
The QT interval represents
the amount of time the
heart’s electrical system
takes to repolarize after
each beat
21
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 Zofran
Use in CINV Restricted Most widely used generic 5-HT3 now
restricted FDA issued a Drug Safety Communication June 29, 2012
“The
use
of
a
single
32
mg
intravenous
dose
of
ondansetron
should
be
avoided. New information indicates that QT prolongation occurs in a
dose-dependent manner, and specifically at a single intravenous dose of
32 mg.”
“No single intravenous dose of ondansetron should exceed 16 mg due to
the risk of QT prolongation.”
“The lower dose intravenous regimen of 0.15 mg/kg every 4 hours for
three doses may be used in adults with chemotherapy-induced nausea
and vomiting.”
Results of Zofran tQT study
32 mg IV dose causes 20 ms increase in QTcF
8 mg IV dose causes 6 ms increase in QTcF
FDA removed 32 mg dose from market December 4, 2012
Impact on sales may be significant
22
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 Anzemet in CINV Previously Removed
FDA issued a Drug Safety Communication Dec. 17, 2010
“Anzemet causes a dose-dependent prolongation in the QT, PR, and QRS
intervals on an electrocardiogram (ECG) …”
“Anzemet injection should no longer be used to prevent nausea and
vomiting associated with initial and repeat courses of emetogenic cancer
chemotherapy.”
Anzemet label changed to remove CINV indication
IV Anzemet sales fell to near zero in one quarter
23
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 APF530
Thorough QT Study Design Double-blind, single-site
Four-way crossover
56 healthy male and female subjects
Study Arms
SC APF530 1 g (granisetron 20 mg) –
2x therapeutic dose
IV Granisetron 50 µg/kg over 3 minutes –
5x therapeutic dose
Oral Moxifloxacin 400 mg (Avelox®) –
positive control
Placebo 0.9% Normal Saline 0.84 mL
Primary endpoint: the upper bound of the one-sided 95%
confidence interval for placebo-adjusted, baseline-
subtracted QTcF being less than 10 milliseconds at all
time points
24
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 APF530
Thorough QT Study Results Primary Endpoint Achieved in Both Granisetron Dose
Groups
Both APF530 and IV granisetron dose groups did not approach or exceed
the upperbound of 10 ms at any time point
The primary end point was met irrespective of heart-rate correction
methodology –
QTcF, QTcI, QTcB
PK/PD relationship was flat –
also showing no QTc signal
Valid Study
Moxifloxacin positive control group showed expected change –
assay
sensitivity reached
25
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
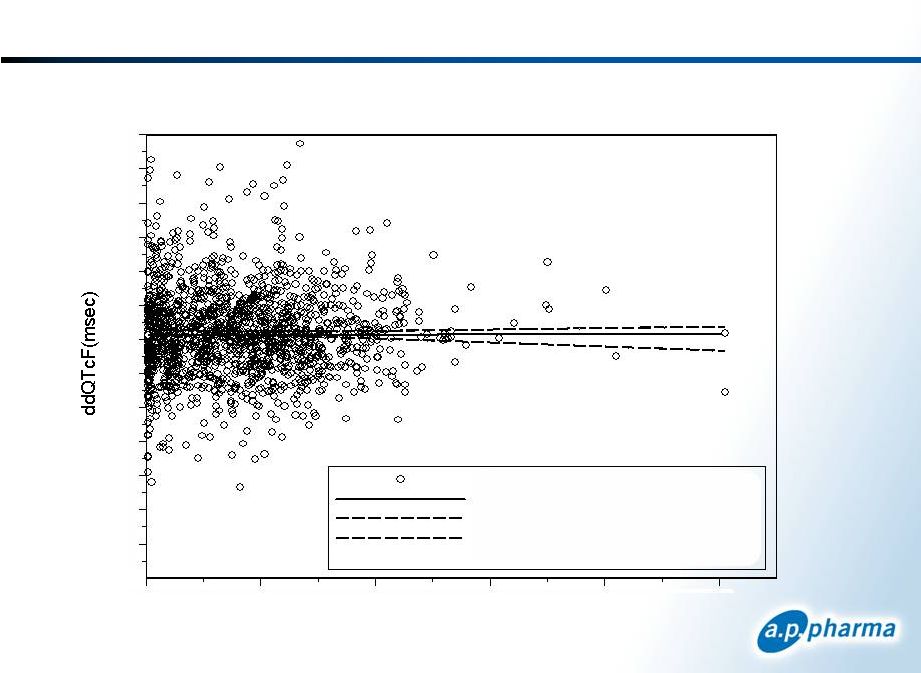 Granisetron Thorough QT PK/PD Results
ddQTcF vs. Granisetron Plasma Concentration
Slope = -0.019
Plasma Concentration (ng/ml)
26
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
ddQTcF vs PlCon
LME Regression Line
90% Upper Confidence Interval
90% Lower Confidence Interval
Corr = 0.02 (p>0.32)
0
20
40
60
80
100
-60
-50
-40
-30
-20
-10
0
10
20
30
40
50
60 |
 Metabolism/Fate of Polymer Study
27
FDA requested study at end-of-review meeting in 1Q 2011
Purpose of study is to demonstrate fate of polymer in
human subjects
Confirm polymer breaks down into same hydrolytic end-products as seen
in animals
Confirm lack of other detectable polymer-related metabolites
Protocol reviewed by FDA prior to initiating study
Single blind, single site
14 healthy male and female subjects
Gather and analyze plasma and urine samples for metabolic products
Study objectives achieved
Confirmed polymer breaks down into same hydrolytic end-products as in
animals
Confirmed lack of other detectable polymer-related metabolites
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 Human
Factors Study January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
28
Assess the Instructions-for-Use and usability of APF530 in
simulated oncology setting
Follows June 2011 FDA guidance: “Applying Human Factors and Usability
Engineering to Optimize Medical Device Design”
Initial risk assessment followed by iterative process of formative studies
Validation study completed following formative studies
New single-syringe design improves overall usability
All subjects successfully followed instructions
Average injection time reduced by 45%
Previous Syringe
Current Syringe
Ease
3.1
8.3
Comfort
5.2
9.4
Control
7.7
9.5
1
0
–
very
difficult,
10
–
very
easy
Mean Usability Scores
1 |
 Commercial Opportunity
29
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 Continuing Unmet Need in CINV
Need for long-acting antiemetic
therapies
Delayed CINV (days 2-5) remains
particularly challenging to manage
Significant portion of patients fail to
respond to Aloxi
Need for antiemetic therapies with
sustained efficacy
CINV risk increases over multiple
chemotherapy cycles
1
Available at:
http://www.cancer.gov/cancertopics/pdq/supportivecare/nausea/HealthProfessional/page6#Section_183
“Despite the use of both first-generation
and second-generation 5-HT
3
receptor
antagonists, the control of acute CINV, and
especially delayed nausea and vomiting, is
suboptimal,
and
there
is
considerable
opportunity for improvement
with either
the addition or substitution of new agents in
current regimens.”
NCI Statement On The Existing
Unmet
Need
in
CINV
1
30
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
 Addressing Debilitating Effects of CINV
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
31
More than 7 million cycles of
chemotherapy administered each
year
~27% are highly emetogenic
~46% are moderately emetogenic
Most chemotherapy patients will
undergo 4-15 cycles of
chemotherapy
5-HT3 antagonists are standard-
of-
care for CINV
Recommended in ASCO, NCCN
and ONS guidelines
NK-1 antagonists are only indicated
in combination with 5-HT3
antagonists
An Injectable 5-HT3 antagonist is
co-administered with more than
90% of MEC and HEC regimens
If initial regimen is non-effective,
drugs are added or changed to
address CINV in subsequent cycles |
 5-HT3 Antagonists Have Made a Substantial Impact
The use of high-dose
metoclopramide
Introduction of first-generation
5-HT3 antagonists (short-acting)
Approval of second-generation
5-HT3 antagonist (long-acting)
Approval of first
NK-1 antagonist
Adapted from Hawkins et al, Clinical Journal
of Oncology Nursing 2009, Volume 13, Number 1
FDA withdrawal of Anzemet and 32 mg dose of
ondansetron (Zofran) due to QT prolongation risk
5-HT3 receptor antagonists approved for CINV
32
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
2003
2006
2011
1990s
1980s
Chemotherapy
Acute CINV
Delayed CINV
Highly
Emetogenic
granisetron
(Kytril)
ondansetron
(Zofran)
Aloxi
None
Moderately
Emetogenic
granisetron
(Kytril)
ondansetron
(Zofran)
Aloxi
Aloxi |
 U.S.
CINV Market Dynamics Source: Wolters Kluwer
Usage in CINV estimated based on vial size
33
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
~85% drop following tQT
FDA Safety Letter
~40% drop following FDA
32 mg dose warning
~35% drop and rebound
in 6-month period
0
100,000
200,000
300,000
400,000
500,000
600,000
700,000
800,000
Q2'06
Q4'06
Q2'07
Q4'07
Q2'08
Q4'08
Q2'09
Q4'09
Q2'10
Q4'10
Q2'11
Q4'11
Q2'12
Injectable Drugs for the Prevention of CINV
Number of Package Units Sold by Quarter
ALOXI
ANZEMET
KYTRIL
KYTRIL Generic (GRANISETRON)
ZOFRAN
ZOFRAN Generic (ONDANSETRON)
EMEND
* US Oncology data added starting 1/2009. |
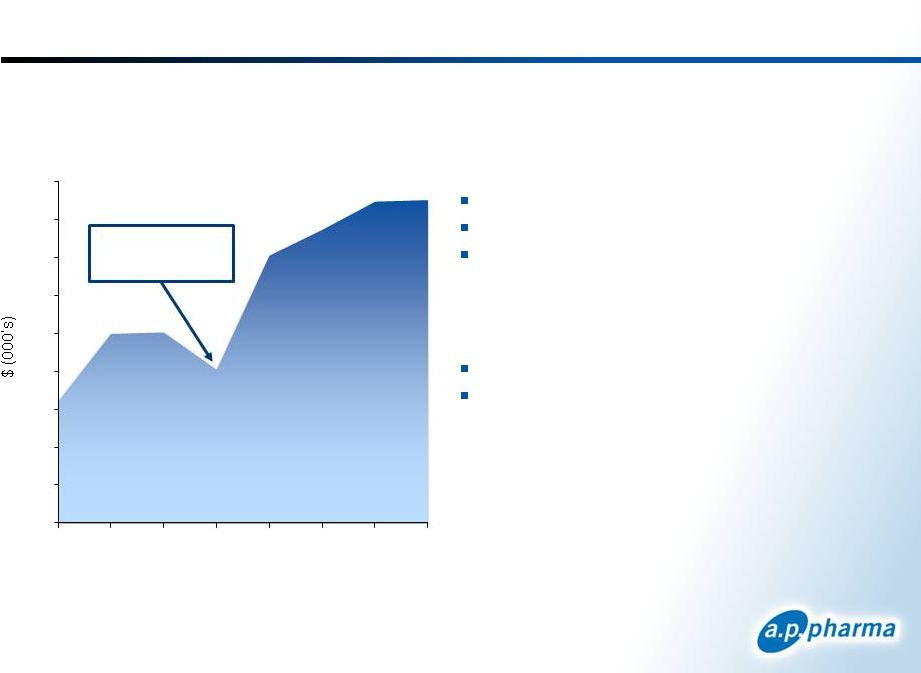 Aloxi Market
Performance Pricing
Average Selling Price = $175
Medicare Reimbursement = $186
Wholesale Acquisition Cost ~ $380
Orange Book Patent Exclusivity
One patent expires April 2015
Three patents expire January 2024
34
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
0
50
100
150
200
250
300
350
400
450
2004
2005
2006
2007
2008
2009
2010
2011
Zofran went
generic
Aloxi Sales |
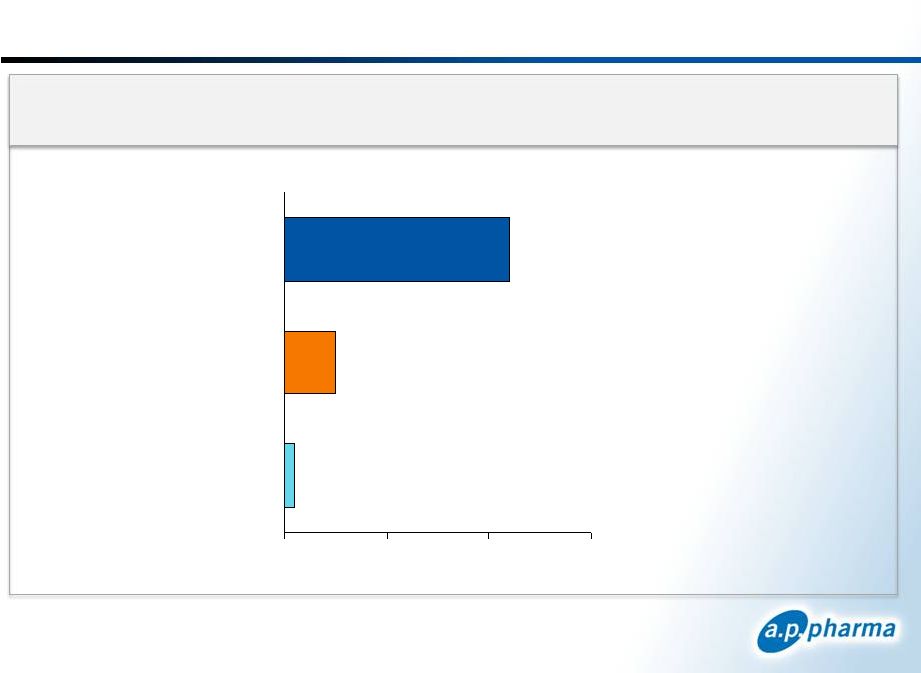 ~80%
of Aloxi Is Used in Clinics IMS Health and Source Healthcare Analytics (WKH)
data and Eisai Co., Ltd. published sales figures 35
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
U.S.
Aloxi
Units
by
Class
of
Trade
–
12
Months
Ending
June
30
th
2012
Hospital
Other
Clinics
1
2.2 million units
2
0.5 million units
0
3
0.1 million units |
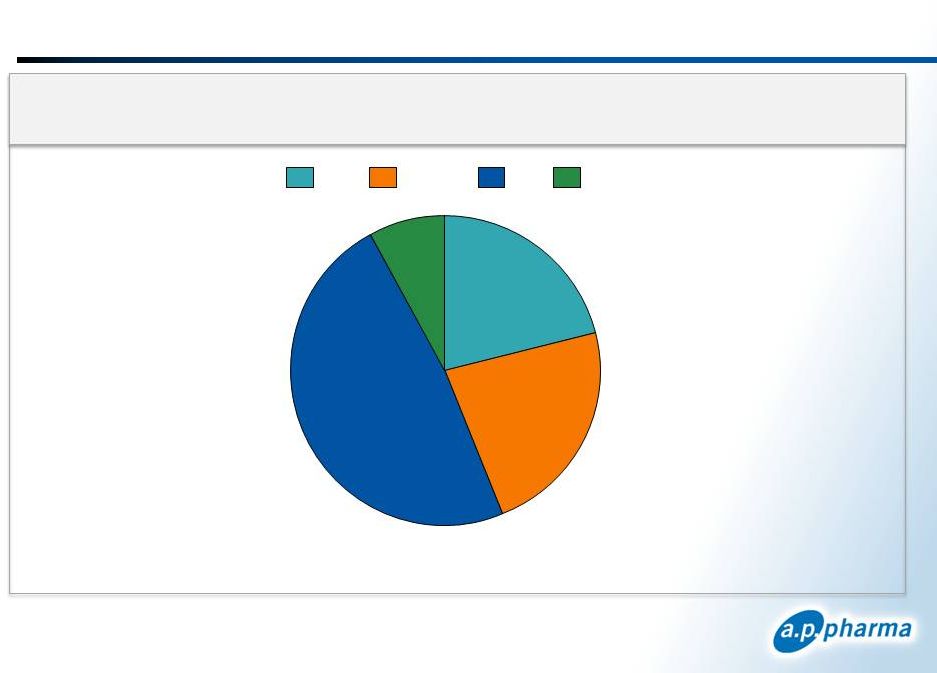 >90% of Aloxi Units Contracted Through GPOs
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
36
2.2 Million Clinic Units
8%
23%
48%
21%
Misc
Onmark
ION
USO
IMS Health and Source Healthcare Analytics (WKH) data and Eisai Co., Ltd. published
sales figures Clinic
Units
by
GPO
–
12
Months
Ending
June
30
th
2012 |
 WKH Data Oct. 2012 –
Clinic Analysis
Aloxi Clinical Use Is Largely Concentrated
Cumulative Number of Accounts
32
1% accts
87
4% accts
161
7% accts
252
11% accts
500
22% accts
674
29% accts
904
39% accts
1246
54% accts
2305
100% accts
362
15% accts
80% of units (1.7M) comes from 39% of accounts (904)
50% of units (1.1M) comes from 15% of accounts (362)
37
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
0
200
400
600
800
1,000
1,200
1,400
1,600
1,800
2,000
2,200 |
 No New
Injectable 5-HT3 Drug on Horizon 38
*Company reports, Leerink Swann; **Clinical Trials.gov NCT01339260 FDC = Fixed Dose Combo;
PDUFA date expected 12-15 months post P3 data EUR-1025 development program
uncertain (once-a-day oral modified-release formulation of ondansetron)
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
APF530 FDA PDUFA Date
2012
2013
2014
2015
Rolapitant Oral
FDA Approval
Expected Q4 2014
Rolapitant IV
Study Data 1H 2014
FDC P3 Oral
Data Expected Q1 2013
FDC Oral
FDA Approval 1H 2014
Rolapitant P3
Oral Data
Expected 2H 2013
Rolapitant IV
FDA Approval
Expected 2H 2015
Candidate
Class
Indication
Sponsor
Possible Launch
APF530
5-HT3 Extended
release
Prevention
of CINV in MEC/HEC
A.P. Pharma
2H 2013
FDC**
FDC combines netupitant
(NK-1) with
palonosetron
(Aloxi) in a single oral tablet
Prevention of CINV in MEC/HEC
Eisai / Helsinn
Oral 2H 2014
Rolapitant*
Long-acting NK-1
Prevention
of CINV in MEC/HEC only
in combination with 5-HT3
Tesaro
Oral 2H 2014
IV
2H 2015 |
 APF530
Proposed Label January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
39
Proposed indication submitted in NDA
INDICATIONS AND USAGE
1.1 Chemotherapy-Induced Nausea and Vomiting
APF530 is indicated for:
• Moderately emetogenic
cancer chemotherapy (MEC)-
prevention of acute and delayed nausea and vomiting
associated with initial and repeat courses
• Highly emetogenic
cancer chemotherapy (HEC)-
prevention of
acute nausea and vomiting associated with initial and repeat
courses |
 APF530
Planned Market Positioning January 2013
40
©
2013. A.P. Pharma, Inc. All rights reserved.
Acute Onset
24 Hours)
Delayed Onset
120 Hours)
Moderately
Emetogenic
Highly
Emetogenic
Moderately
Emetogenic
Highly
Emetogenic
(0-
(24-
APF530
Aloxi
Kytril
Zofran
If approved by FDA, APF530 and Aloxi would be the only
“long acting”
5-HT3 antagonists approved for the
prevention of acute and delayed-onset CINV |
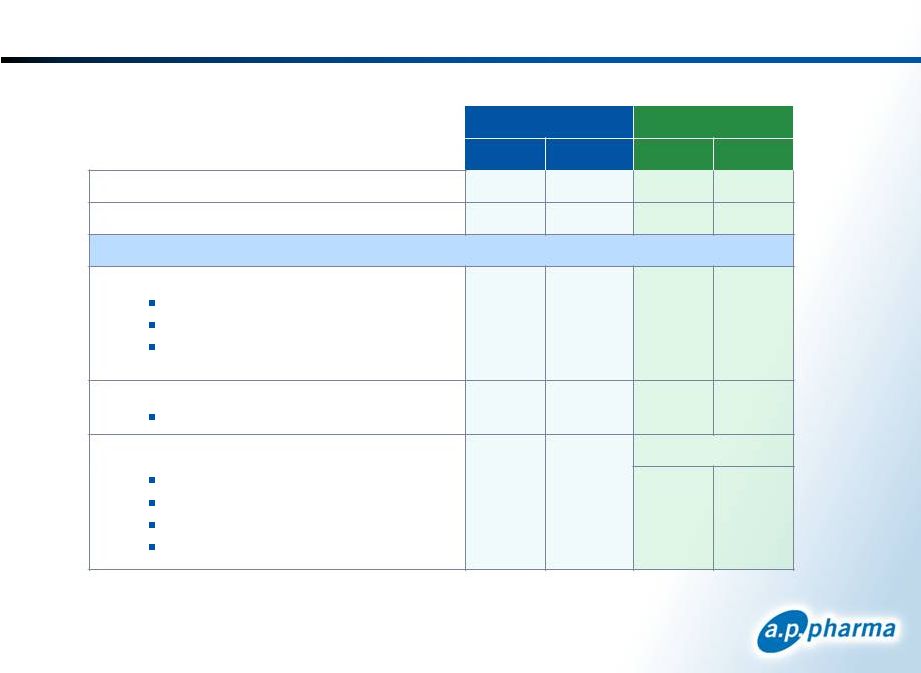
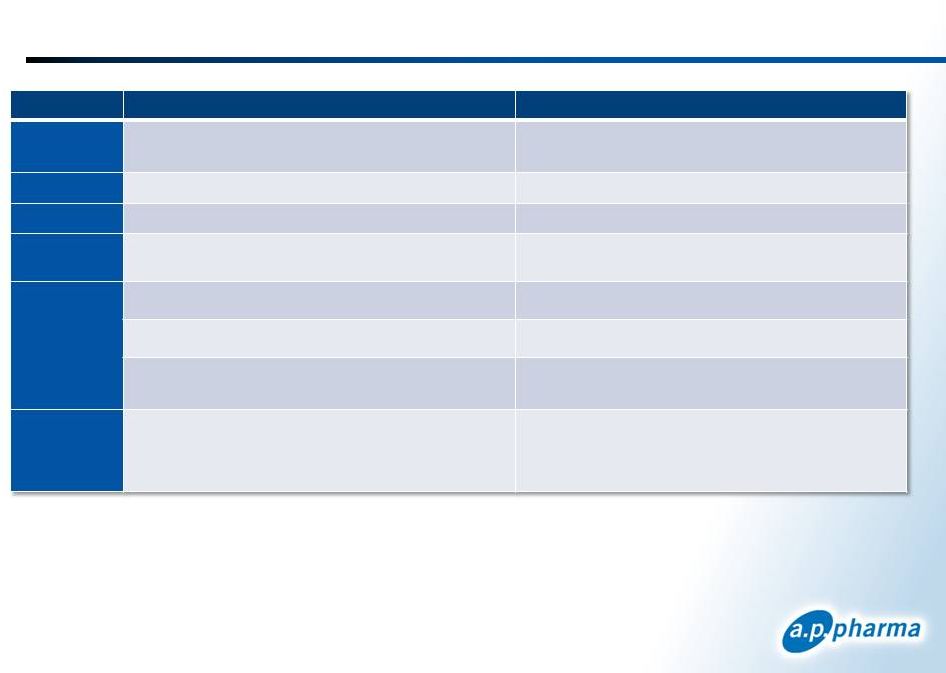 APF530
and Aloxi Profiles 41
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
Attribute
APF530
Aloxi
Indication
MEC –
acute and delayed CINV
HEC –
acute CINV
MEC –
acute and delayed CINV
HEC –
acute CINV
MOA
5-HT3 receptor antagonist
5-HT3 receptor antagonist
Dosing
SC injection once per cycle
IV once
per cycle
Long acting
agent
Bioerodible polymer technology releases granisetron
over 5 days
~ 40 hour half life
Efficacy
Non
-inferiority to Aloxi
Effective in difficult chemotherapy regimens
Effective in difficult chemotherapy regimens
Demonstrated efficacy through multiple cycles in
MEC and HEC
Safety
Headache, constipation,
injection site bruising and
pain
Clean QT profile
Headache
and constipation
Clean QT profile |
 Summary
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
42
$900 million market potential for injectable 5-HT3 antagonists*
APF530 demonstrated non-inferiority to the market share leader
Aloxi
APF530 product profile
5-day release PK profile
Good response in difficult chemotherapy regimens
Efficacy through multiple cycles of chemotherapy
Clean QT results
Current market dynamics are stale with minimal investment
providing opportunities to become the market leader
Competitive landscape creates opportunity with the removal of
Anzemet and Zofran 32mg
Greater than 80% of Aloxi sales are in the community setting
and highly concentrated consistent with other supportive care
products
*Branded market estimate using 2011 units based on Wolters Kluwer and Aloxi ASP
|
 A.P.
Pharma Product Lifecycle Considerations 43
APF530 covered by multiple patents
2 patents covering combination of polymer, excipients and drug expire in
2021
3 patents covering APF530 expire in 2024
Polymer-based injectable products are difficult to copy
independent of IP
ANDA FDA requirements for injectable products
Must have same inactive ingredients in the same concentration as
the
reference listed drug
Polymers are complex mixtures of varying-length molecules, making
characterization for “sameness”
very challenging
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
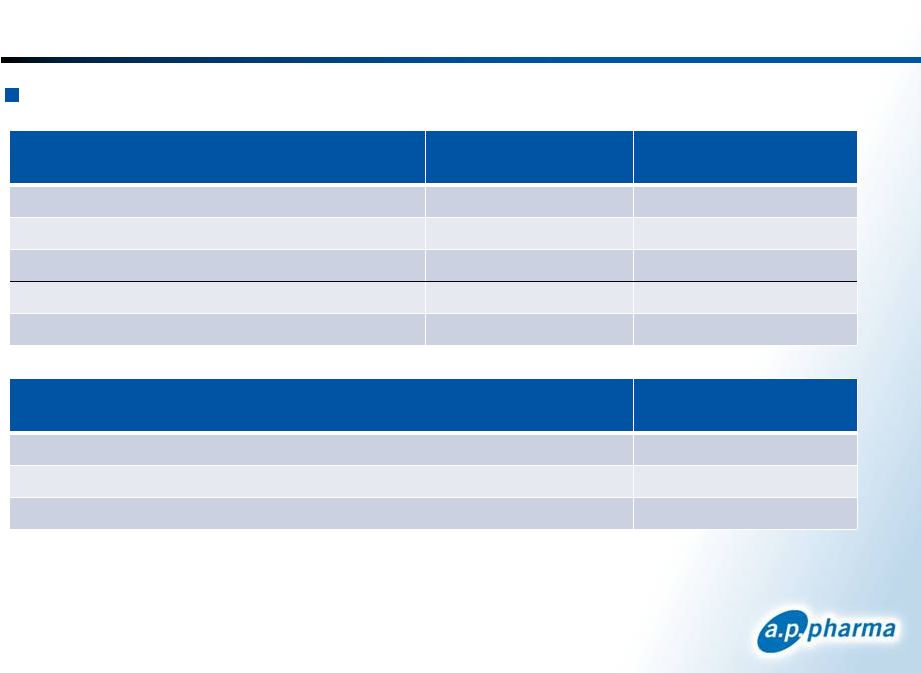 Financial Summary
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
44
Expect cash sufficient to fund commercial launch of APF530
Summary Statement of Operations
(In thousands, except per share data)
Year Ended
December 31, 2011
Nine Months Ended
September 30, 2012
Revenue
$ 646
$ –
Operating expenses
11,708
15,203
Other income (expenses)
(752)
(408)
Net loss
$ (11,814)
$ (15,611)
Net loss per share
1
$ (0.10)
$ (0.07)
Condensed Balance Sheet Data
(In thousands)
September 30, 2012
Cash and cash equivalents
$ 60,048
Total assets
$ 61,761
Total stockholders’
equity
$ 57,517
1
Based on 120.3 and 225.1 million weighted average common shares outstanding for the
periods ended December 31, 2011 and September 30, 2012, respectively
|
 A.P.
Pharma Highlights January 2013
©
2013. A.P. Pharma, Inc. All rights reserved.
45
Lead product candidate, APF530, is long-acting, injectable
product for chemotherapy-induced nausea and vomiting (CINV)
Incorporates
widely
used
5-HT3
antagonist
-
granisetron
(Kytril)
5-day delivery profile
Reduces both acute-
and delayed-onset CINV with single injection
Patent coverage into 2024
APF530 shown to be non-inferior to market leader Aloxi
1,341-patient, randomized, controlled, Phase 3 study
FDA PDUFA Action Date of March 27, 2013
Resubmitted NDA for APF530 in September 2012
Addressed issues raised in Complete Response Letter
Product launch planned for 2H 2013
APF530 targets a $900 million market opportunity in US alone*
Recent competitive setbacks could enhance commercial uptake
Could be second, long-acting, injectable product on market
A.P. Pharma has the potential to leverage its Biochronomer
drug delivery technology into other opportunities
*Branded market estimate using 2011 units based on Wolters Kluwer and Aloxi ASP
|
 Thank You
A.P. Pharma, Inc.
OTCBB: APPA
January 2013
46
January 2013
©
2013. A.P. Pharma, Inc. All rights reserved. |
