Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a13-2264_18k.htm |
| EX-99.2 - EX-99.2 - AMICUS THERAPEUTICS, INC. | a13-2264_1ex99d2.htm |
Exhibit 99.1
|
|
At the Forefront of Therapies for Rare and Orphan Diseases™ January 2013 |
|
|
Safe Harbor This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business, operations and financial conditions of Amicus including but not limited to preclinical and clinical development of Amicus’ candidate drug products, the timing and reporting of results from clinical trials evaluating Amicus’ candidate drug products. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Although Amicus believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in Amicus’ forward-looking statements due to numerous known and unknown risks and uncertainties, including the “Risk Factors” described in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2012. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. Slide 2 |
|
|
Company Mission Amicus Therapeutics is a biopharmaceutical company at the forefront of developing next-generation medicines to treat a range of rare and orphan diseases, with a focus on improved therapies for Lysosomal Storage Disorders Slide 3 |
|
|
JPMorgan Healthcare Conference 2013 Key Messages Phase 3 Fabry monotherapy study (Study 011) ongoing: Top-line 6-month (Stage 1) results encouraging and consistent with Phase 2 experience FDA to consider “entirety of data” from both 6 and 12 months Pompe Chaperone-ERT co-administration repeat-dose clinical study to begin 3Q13 Fabry Chaperone-ERT co-formulated product advancing towards clinic Proprietary Pompe next-generation ERT in preclinical development ~$100M cash at 12/31/12 Slide 4 |
|
|
Core Technology and Focus Potential to Transform LSD Treatments Oral Monotherapy Orally administered Binds to and stabilizes patient’s own natural enzyme Replaces need for ERT for patients with amenable genetic mutations Slide 5 Oral Co-Administration Binds to and stabilizes currently marketed ERTs Increases tissue uptake Potentially reduces immunogenicity of ERT Chaperone Co-Formulation Proprietary next-generation ERTs All benefits of co-administration…plus stability from infusion bag to target tissue Small Molecule Pharmacological Chaperones Chaperone Chaperone ERT Next Gen ERTs |
|
|
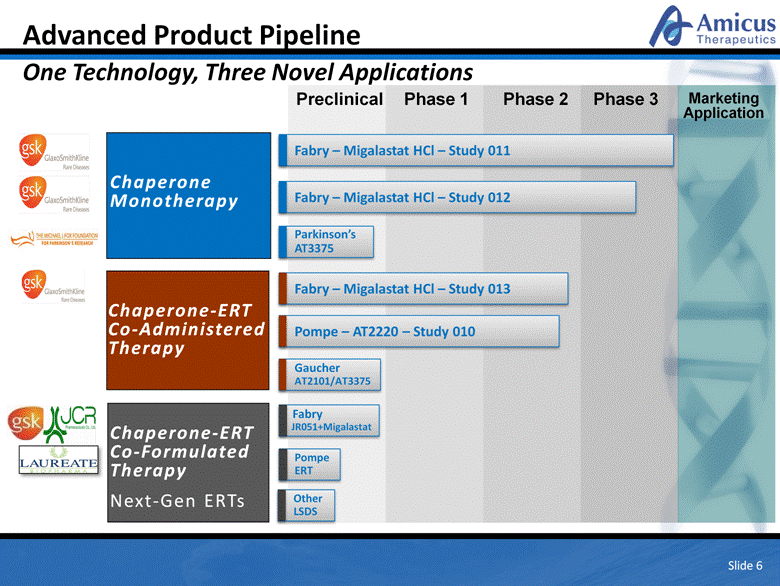
Fabry – Migalastat HCl – Study 013 Pompe – AT2220 – Study 010 Fabry JR051+Migalastat Fabry – Migalastat HCl – Study 011 Fabry – Migalastat HCl – Study 012 Parkinson’s AT3375 Advanced Product Pipeline One Technology, Three Novel Applications Slide 6 Chaperone-ERT Co-Administered Therapy Preclinical Phase 1 Phase 2 Phase 3 Marketing Application Other LSDS Gaucher AT2101/AT3375 Chaperone-ERT Co-Formulated Therapy Next-Gen ERTs Pompe ERT Chaperone Monotherapy |
|
|
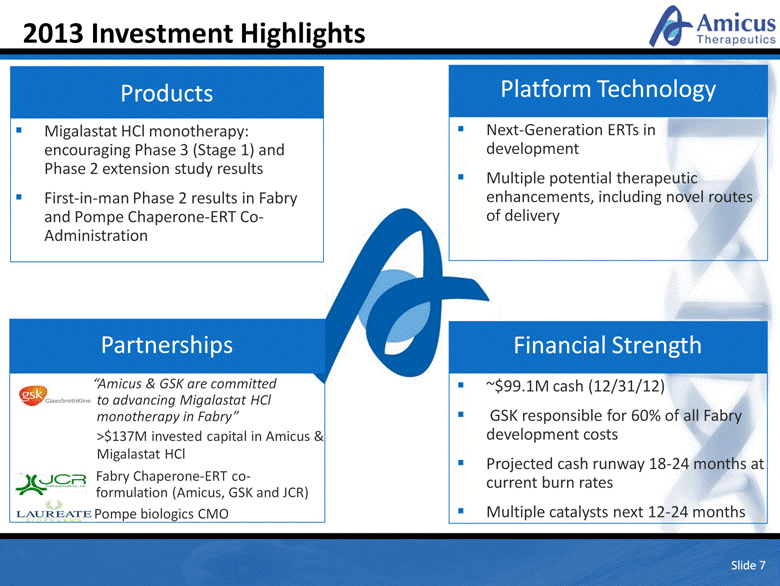
2013 Investment Highlights Slide 7 Migalastat HCl monotherapy: encouraging Phase 3 (Stage 1) and Phase 2 extension study results First-in-man Phase 2 results in Fabry and Pompe Chaperone-ERT Co-Administration Products Next-Generation ERTs in development Multiple potential therapeutic enhancements, including novel routes of delivery Platform Technology ~$99.1M cash (12/31/12) GSK responsible for 60% of all Fabry development costs Projected cash runway 18-24 months at current burn rates Multiple catalysts next 12-24 months Financial Strength “Amicus & GSK are committed to advancing Migalastat HCl monotherapy in Fabry” >$137M invested capital in Amicus & Migalastat HCl Fabry Chaperone-ERT co-formulation (Amicus, GSK and JCR) Pompe biologics CMO Partnerships |
|
|
Slide 8 PHARMACOLOGICAL CHAPERONES MONOTHERAPY DEVELOPMENT IN FABRY DISEASE |
|
|
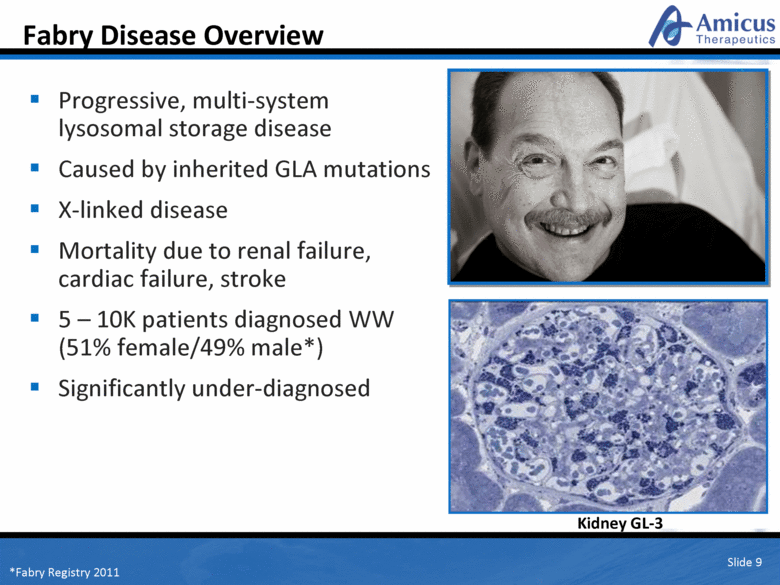
Fabry Disease Overview Kidney GL-3 Slide 9 Progressive, multi-system lysosomal storage disease Caused by inherited GLA mutations X-linked disease Mortality due to renal failure, cardiac failure, stroke 5 – 10K patients diagnosed WW (51% female/49% male*) Significantly under-diagnosed *Fabry Registry 2011 |
|
|
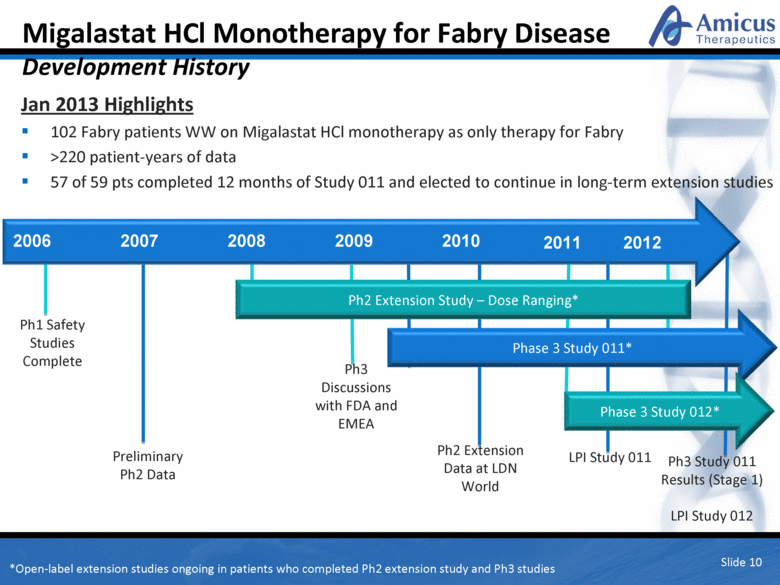
Migalastat HCl Monotherapy for Fabry Disease Development History Slide 10 Jan 2013 Highlights 102 Fabry patients WW on Migalastat HCl monotherapy as only therapy for Fabry >220 patient-years of data 57 of 59 pts completed 12 months of Study 011 and elected to continue in long-term extension studies 2006 2007 2008 2009 2010 2011 2012 Ph1 Safety Studies Complete Preliminary Ph2 Data Ph2 Extension Study – Dose Ranging* Ph3 Discussions with FDA and EMEA Ph2 Extension Data at LDN World Phase 3 Study 011* LPI Study 011 Ph3 Study 011 Results (Stage 1) LPI Study 012 Phase 3 Study 012* *Open-label extension studies ongoing in patients who completed Ph2 extension study and Ph3 studies |
|
|
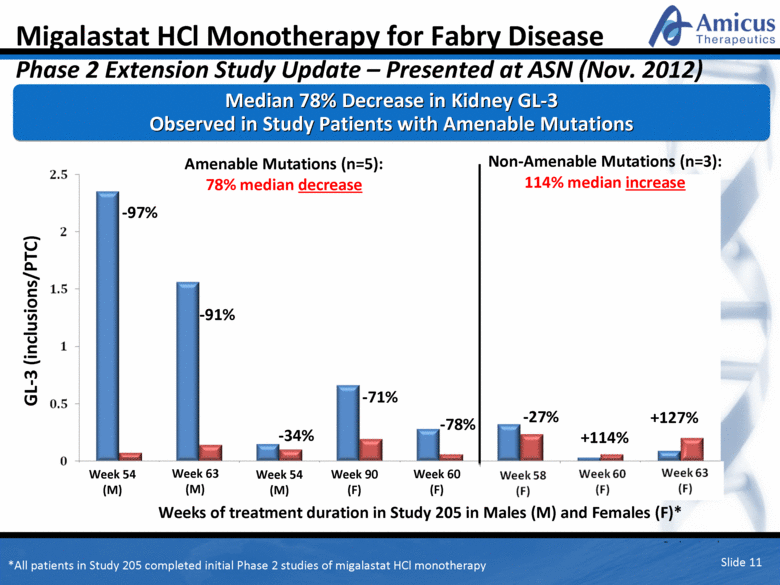
Amenable Mutations (n=5): 78% median decrease Non-Amenable Mutations (n=3): 114% median increase -71% -34% -91% -97% -78% +127% +114% -27% Week 54 (M) Weeks of treatment duration in Study 205 in Males (M) and Females (F)* Week 54 (M) Week 63 (M) Week 60 (F) Week 90 (F) Median 78% Decrease in Kidney GL-3 Observed in Study Patients with Amenable Mutations Migalastat HCl Monotherapy for Fabry Disease Phase 2 Extension Study Update – Presented at ASN (Nov. 2012) GL-3 (inclusions/PTC) Slide 11 *All patients in Study 205 completed initial Phase 2 studies of migalastat HCl monotherapy |
|
|
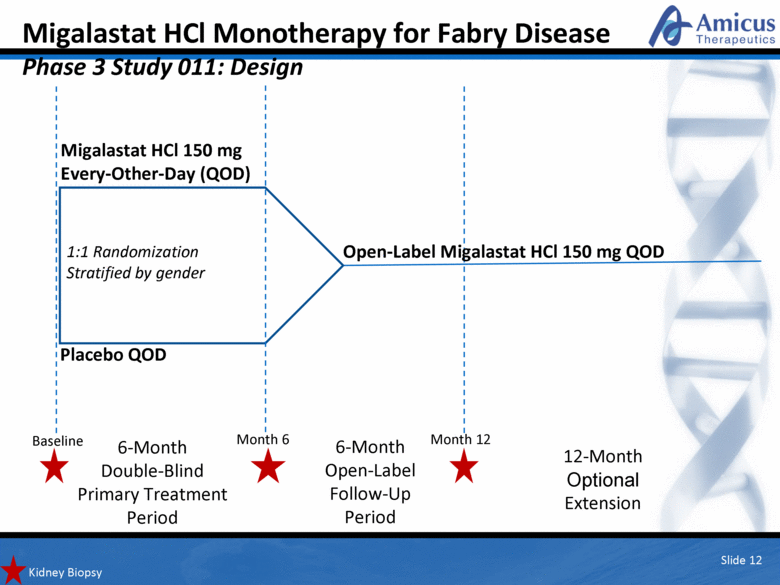
Migalastat HCl Monotherapy for Fabry Disease Phase 3 Study 011: Design Kidney Biopsy 1:1 Randomization Stratified by gender Baseline 6-Month Open-Label Follow-Up Period 12-Month Optional Extension Placebo QOD Migalastat HCl 150 mg Every-Other-Day (QOD) Open-Label Migalastat HCl 150 mg QOD Slide 12 6-Month Double-Blind Primary Treatment Period Month 12 Month 6 |
|
|
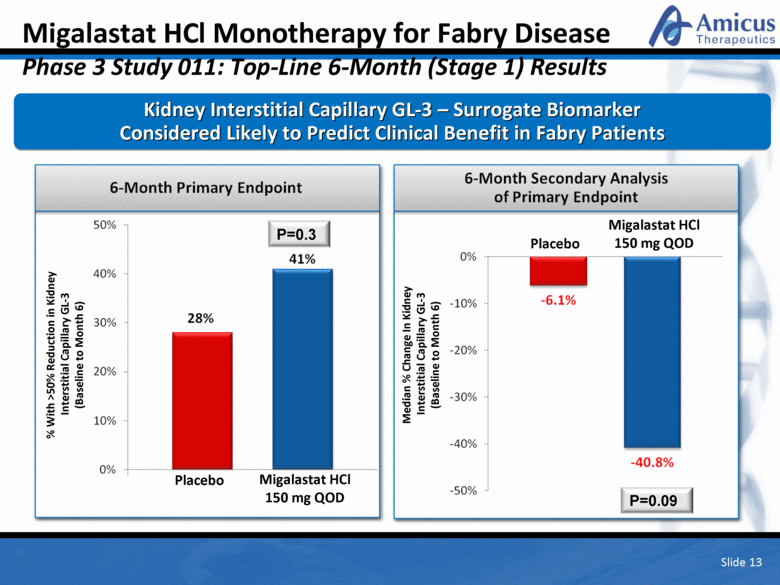
Migalastat HCl Monotherapy for Fabry Disease Phase 3 Study 011: Top-Line 6-Month (Stage 1) Results Kidney Interstitial Capillary GL-3 – Surrogate Biomarker Considered Likely to Predict Clinical Benefit in Fabry Patients P=0.3 Placebo Migalastat HCl 150 mg QOD 9/32 13/32 Median % Change In Kidney Interstitial Capillary GL-3 (Baseline to Month 6) Placebo Migalastat HCl 150 mg QOD P=0.09 Slide 13 % With >50% Reduction in Kidney Interstitial Capillary GL-3 (Baseline to Month 6) |
|
|
FDA Responsiveness and Flexibility in Rare Diseases PDUFA V Impact “FDA assesses the benefit-risk of new drugs on a case-by-case basis, considering the degree of unmet need and the severity and morbidity of the condition the drug is intended to treat. This approach has been critical to increasing patient access to new drugs for cancer and rare and other serious diseases, where existing therapies have been few and limited in their effectiveness.” Janet Woodcock, M.D. Director, CDER U.S. FDA Slide 14 Statement on FDA User Fee Agreements: Strengthening FDA and the Medical Products Industry for the Benefit of Patients (March 29, 2012) |
|
|
Migalastat HCl Monotherapy for Fabry Disease Regulatory Guidance and Path Forward Study 011 is an Ongoing 12-Month Pivotal Study of Migalastat HCl in Patients with Fabry Disease with Amenable Mutations Slide 15 U.S. FDA Feedback 6-month analysis is Stage 1 FDA to consider Stage 1 and Stage 2 (12-month) data for NDA submission FDA will evaluate efficacy and safety based on entirety of Study 011 data (no single endpoint will be determinitive) FDA meeting anticipated in mid-2013 to discuss US approval pathway |
|
|
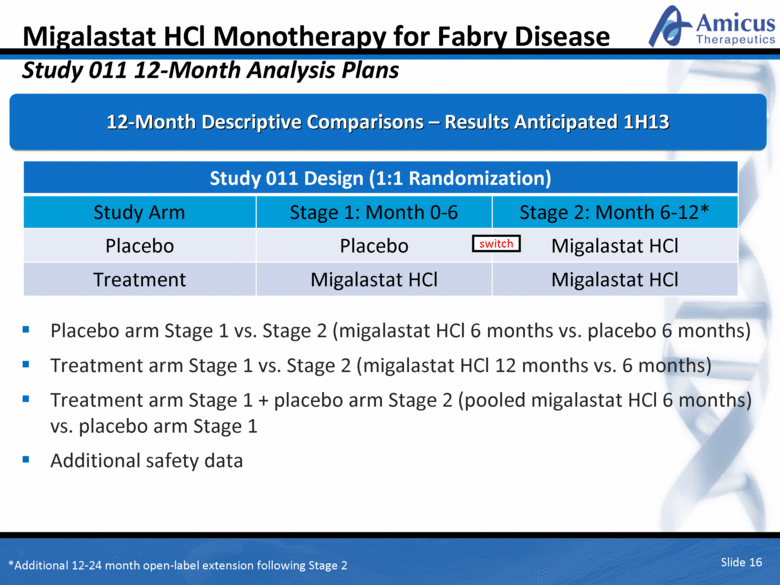
Migalastat HCl Monotherapy for Fabry Disease Study 011 12-Month Analysis Plans Placebo arm Stage 1 vs. Stage 2 (migalastat HCl 6 months vs. placebo 6 months) Treatment arm Stage 1 vs. Stage 2 (migalastat HCl 12 months vs. 6 months) Treatment arm Stage 1 + placebo arm Stage 2 (pooled migalastat HCl 6 months) vs. placebo arm Stage 1 Additional safety data Slide 16 Study 011 Design (1:1 Randomization) Study Arm Stage 1: Month 0-6 Stage 2: Month 6-12* Placebo Placebo Migalastat HCl Treatment Migalastat HCl Migalastat HCl switch *Additional 12-24 month open-label extension following Stage 2 12-Month Descriptive Comparisons – Results Anticipated 1H13 |
|
|
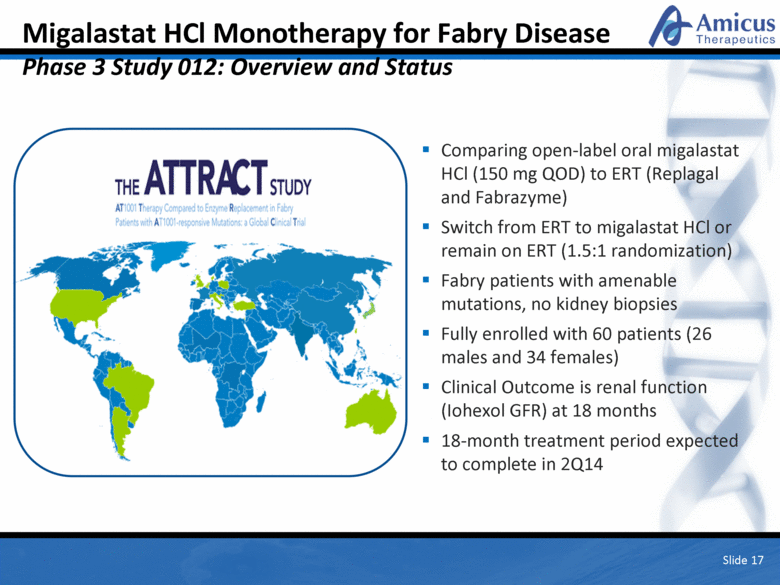
Migalastat HCl Monotherapy for Fabry Disease Phase 3 Study 012: Overview and Status Comparing open-label oral migalastat HCl (150 mg QOD) to ERT (Replagal and Fabrazyme) Switch from ERT to migalastat HCl or remain on ERT (1.5:1 randomization) Fabry patients with amenable mutations, no kidney biopsies Fully enrolled with 60 patients (26 males and 34 females) Clinical Outcome is renal function (Iohexol GFR) at 18 months 18-month treatment period expected to complete in 2Q14 Slide 17 |
|
|
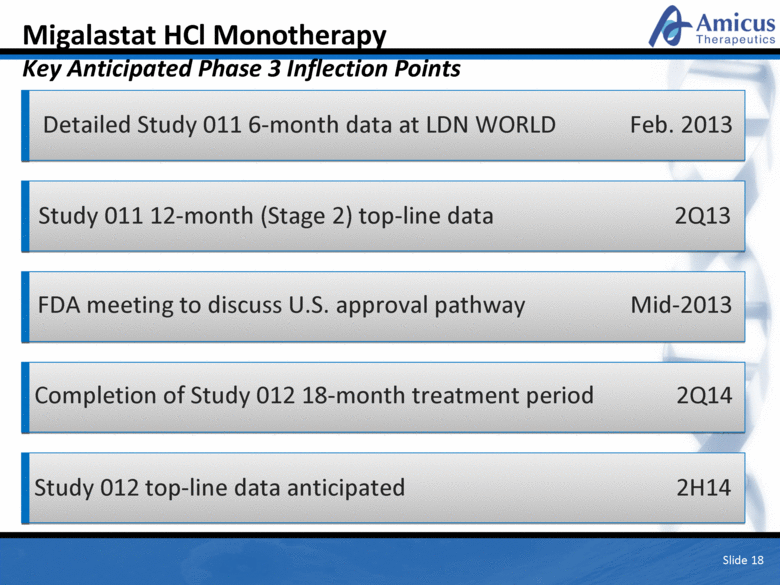
Migalastat HCl Monotherapy Key Anticipated Phase 3 Inflection Points Detailed Study 011 6-month data at LDN WORLD Feb. 2013 Study 011 12-month (Stage 2) top-line data 2Q13 FDA meeting to discuss U.S. approval pathway Mid-2013 Completion of Study 012 18-month treatment period 2Q14 Study 012 top-line data anticipated 2H14 Slide 18 |
|
|
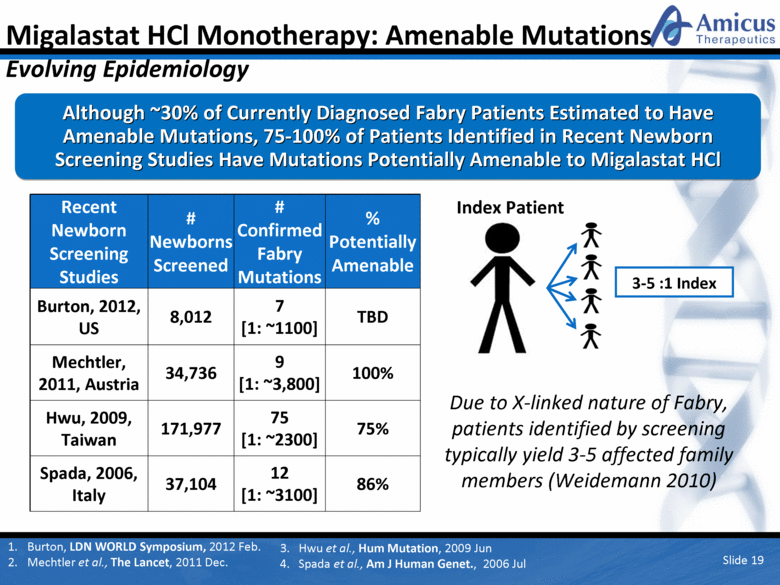
Migalastat HCl Monotherapy: Amenable Mutations Evolving Epidemiology Although ~30% of Currently Diagnosed Fabry Patients Estimated to Have Amenable Mutations, 75-100% of Patients Identified in Recent Newborn Screening Studies Have Mutations Potentially Amenable to Migalastat HCl Recent Newborn Screening Studies # Newborns Screened # Confirmed Fabry Mutations % Potentially Amenable Burton, 2012, US 8,012 7 [1: ~1100] TBD Mechtler, 2011, Austria 34,736 9 [1: ~3,800] 100% Hwu, 2009, Taiwan 171,977 75 [1: ~2300] 75% Spada, 2006, Italy 37,104 12 [1: ~3100] 86% Index Patient 3-5 :1 Index Due to X-linked nature of Fabry, patients identified by screening typically yield 3-5 affected family members (Weidemann 2010) Slide 19 Burton, LDN WORLD Symposium, 2012 Feb. Mechtler et al., The Lancet, 2011 Dec. 3. Hwu et al., Hum Mutation, 2009 Jun 4. Spada et al., Am J Human Genet., 2006 Jul |
|
|
Slide 20 PHARMACOLOGICAL CHAPERONES CO-ADMINISTERED WITH MARKETED ERTS IMPROVING CURRENT ERTS FOR LYSOSOMAL STORAGE DISORDERS |
|
|
Photo by J Pastor/Univ of Florida Protein Instability Blood & Infusion Bag LSD Products Today Potential Issues Slide 21 Immunogenicity Dosing Limitations Duration of Infusion |
|
|
Chaperone-ERT Combination Therapy Goals Potential to Improve Safety and Efficacy of ERT While Reducing Patient Burden Enhance ERT pharmacokinetics (greater overall exposure) Increase enzyme uptake in tissues, possibly into cell types and tissues not well-served by ERT alone (e.g., podocytes, cardiomyocytes, brain) Improve substrate reduction in disease-relevant cell types and tissues Mitigate ERT-mediated immunogenicity Reduce burden of therapy for patients (e.g., more convenient ERT) Slide 22 |
|
|
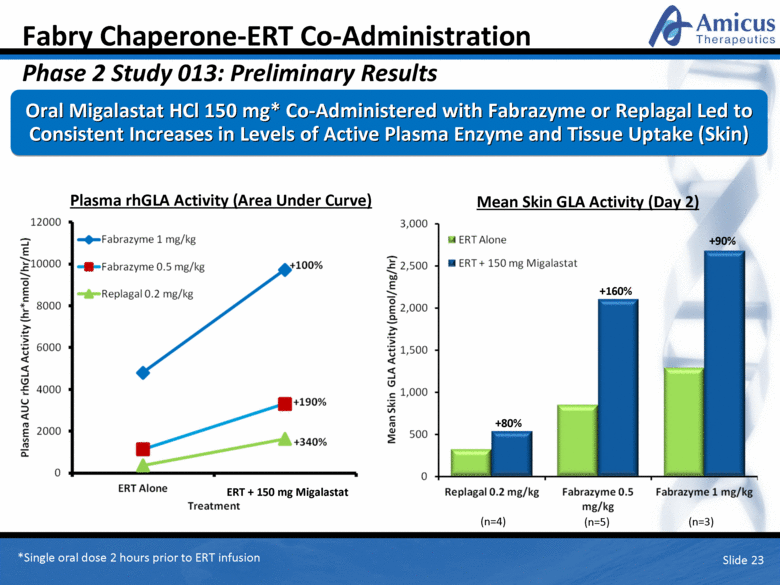
Fabry Chaperone-ERT Co-Administration Phase 2 Study 013: Preliminary Results Oral Migalastat HCl 150 mg* Co-Administered with Fabrazyme or Replagal Led to Consistent Increases in Levels of Active Plasma Enzyme and Tissue Uptake (Skin) Plasma rhGLA Activity (Area Under Curve) Slide 23 Mean Skin GLA Activity (Day 2) +90% +160% +80% *Single oral dose 2 hours prior to ERT infusion (n=4) (n=5) (n=3) ERT + 150 mg Migalastat |
|
|
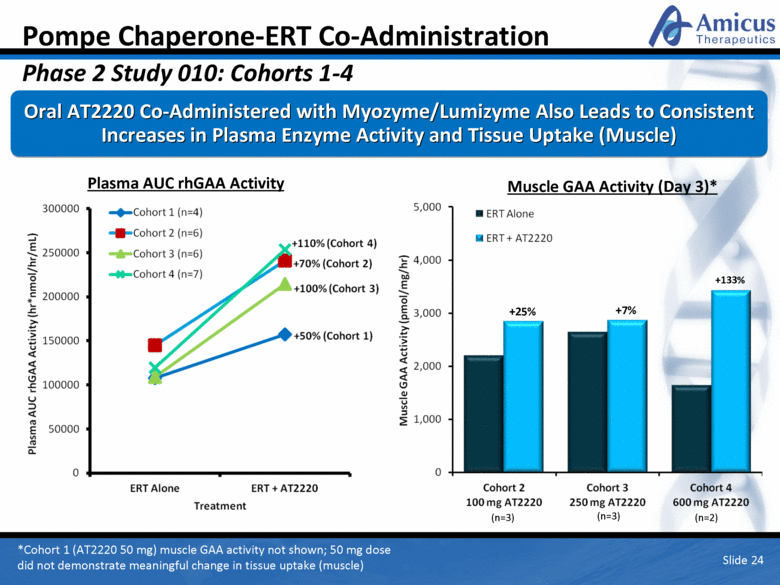
Pompe Chaperone-ERT Co-Administration Phase 2 Study 010: Cohorts 1-4 Oral AT2220 Co-Administered with Myozyme/Lumizyme Also Leads to Consistent Increases in Plasma Enzyme Activity and Tissue Uptake (Muscle) *Cohort 1 (AT2220 50 mg) muscle GAA activity not shown; 50 mg dose did not demonstrate meaningful change in tissue uptake (muscle) Plasma AUC rhGAA Activity Muscle GAA Activity (Day 3)* Slide 24 +133% +7% +25% (n=3) (n=3) (n=2) |
|
|
Pompe Chaperone-ERT Co-Administration ERT-Related Immunogenicity Problem “…. identification of patients at risk for developing high sustained antibody titer is critical.”1 “…. approximately 40% of the administered alglucosidase alfa was captured by circulating antibodies.”2 “…. infusion-associated reactions (IARs) [occur] in ~50% of patients receiving alglucosidase alfa infusions.”3 Slide 25 Banugaria et al., Gen. Med., 2011 Aug. de Vries et al., Mol Genet Metab., 2010 Dec. Banati et al., Muscle Nerve, 2011 Dec. |
|
|
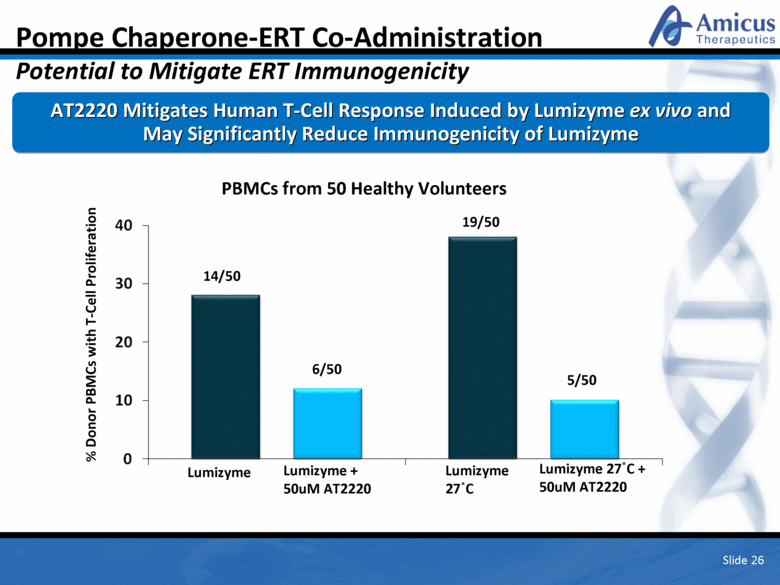
Pompe Chaperone-ERT Co-Administration Potential to Mitigate ERT Immunogenicity AT2220 Mitigates Human T-Cell Response Induced by Lumizyme ex vivo and May Significantly Reduce Immunogenicity of Lumizyme % Donor PBMCs with T-Cell Proliferation PBMCs from 50 Healthy Volunteers Lumizyme Lumizyme + 50uM AT2220 14/50 6/50 5/50 19/50 Lumizyme 27oC Lumizyme 27oC + 50uM AT2220 Slide 26 |
|
|
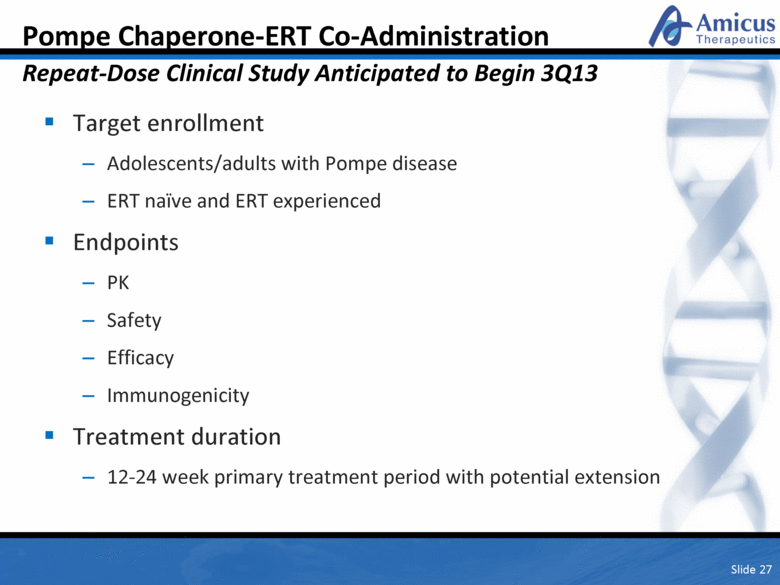
Slide 27 Pompe Chaperone-ERT Co-Administration Repeat-Dose Clinical Study Anticipated to Begin 3Q13 Target enrollment Adolescents/adults with Pompe disease ERT naïve and ERT experienced Endpoints PK Safety Efficacy Immunogenicity Treatment duration 12-24 week primary treatment period with potential extension |
|
|
Slide 28 PHARMACOLOGICAL CHAPERONES CO-FORMULATED WITH RECOMBINANT ERTS TOWARD THE NEXT-GENERATION OF PROPRIETARY ERTS FOR LYSOSOMAL STORAGE DISORDERS |
|
|
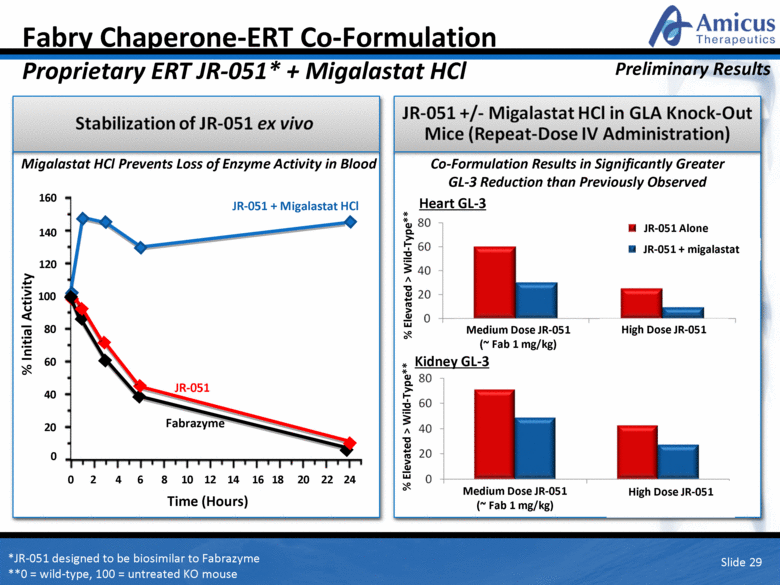
Fabry Chaperone-ERT Co-Formulation Proprietary ERT JR-051* + Migalastat HCl Slide 29 JR-051 + Migalastat HCl Fabrazyme Co-Formulation Results in Significantly Greater GL-3 Reduction than Previously Observed Time (Hours) Heart GL-3 Kidney GL-3 Medium Dose JR-051 (~ Fab 1 mg/kg) *JR-051 designed to be biosimilar to Fabrazyme **0 = wild-type, 100 = untreated KO mouse % Elevated > Wild-Type** % Elevated > Wild-Type** Preliminary Results JR-051 High Dose JR-051 Medium Dose JR-051 (~ Fab 1 mg/kg) High Dose JR-051 JR-051 Alone JR-051 + migalastat Migalastat HCl Prevents Loss of Enzyme Activity in Blood JR-051 + Migalastat HCl Fabrazyme 160 140 120 80 60 40 20 100 8 6 4 2 16 24 18 0 12 10 0 14 20 22 JR-051 % Initial Activity |
|
|
Fabry Chaperone-ERT Co-Formulation Development Status and Anticipated Milestones Slide 30 Now manufacturing at 2,000 L scale IND-enabling studies underway Potential to enter clinic 4Q13/1Q14 Advancing JR-051 + Migalastat HCl Toward Clinic |
|
|
Pompe Chaperone-ERT Co-Formulation Next-Generation ERT for Pompe Slide 31 Combining Core Pharmacological Chaperone Technology with Advanced Biologics Capabilities to Create a Next-Generation Pompe ERT Potential Improvements Optimized glycosylation (e.g., M6-P) De-immunization Recombinant Human GAA Next-Generation Pompe ERT P M C P C C C N H O H O H H O O H H C l . ATT2220 Small Molecule Stabilizer Increased exposure & tissue uptake Reduced immunogenicity Formulation for SQ route of administration |
|
|
Slide 32 2013 KEY MILESTONES AND CATALYSTS |
|
|
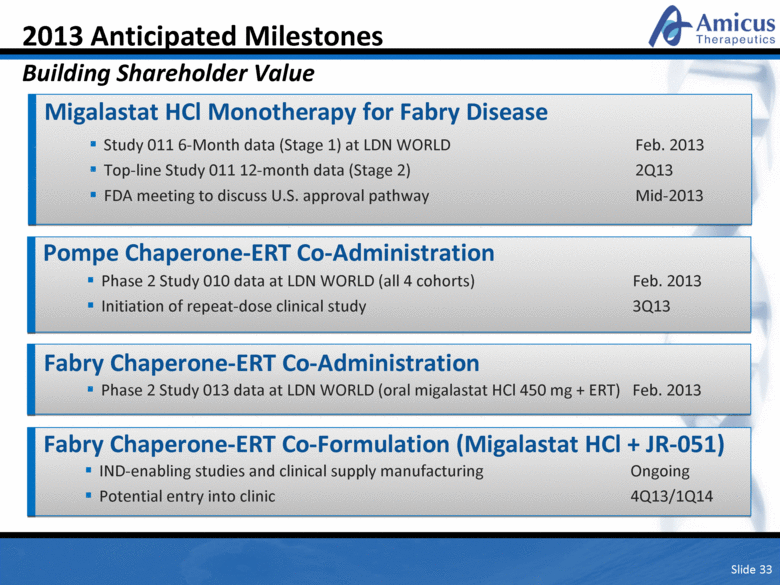
Slide 33 2013 Anticipated Milestones Building Shareholder Value Migalastat HCl Monotherapy for Fabry Disease Study 011 6-Month data (Stage 1) at LDN WORLD Feb. 2013 Top-line Study 011 12-month data (Stage 2) 2Q13 FDA meeting to discuss U.S. approval pathway Mid-2013 Pompe Chaperone-ERT Co-Administration IND-enabling studies and clinical supply manufacturing Ongoing Potential entry into clinic 4Q13/1Q14 Phase 2 Study 010 data at LDN WORLD (all 4 cohorts) Feb. 2013 Initiation of repeat-dose clinical study 3Q13 Fabry Chaperone-ERT Co-Formulation (Migalastat HCl + JR-051) Fabry Chaperone-ERT Co-Administration Phase 2 Study 013 data at LDN WORLD (oral migalastat HCl 450 mg + ERT) Feb. 2013 |