Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - BIOCRYST PHARMACEUTICALS INC | d450707d8k.htm |
| EX-99.1 - PRESS RELEASE - BIOCRYST PHARMACEUTICALS INC | d450707dex991.htm |
 BioCryst
Strategy & Restructuring Update December 7, 2012
Exhibit 99.2 |
 2
Forward-looking statement
BioCryst’s presentation may contain forward-looking statements, including statements
regarding future results, unaudited and forward-looking financial information and
company performance or achievements. These statements are subject to known and
unknown risks and uncertainties which may cause our actual results, performance or
achievements to be materially different from any future results or performances
expressed or implied in this presentation.
You should not place undue reliance on the
forward-looking statements.
For additional information, including important risk
factors, please refer to BioCryst’s documents filed with the SEC and located at
http://investor.shareholder.com/biocryst/sec.cfm |
 3
Path forward to rebuild shareholder value
Restructure & focus
Invest primarily in three core programs
Oral hereditary angioedema treatments/BCX4161 and next generation molecule
Hemorrhagic fevers/broad spectrum antiviral BCX4430
HCV antiviral/BCX5191
Restructuring goals:
Scale organization appropriately
Preserve cash and extend runway
Reach near-term value creating milestones |
 4
BioCryst’s pipeline
1. Peramivir is approved in Japan & Korea
No additional clinical investment planned
Inflammatory Disease
Preclinical
Phase 1
Phase 2
Pivotal
Filed
Approved
Ulodesine
(Gout)
BCX4161
(Hereditary angioedema)
Infectious Disease
Preclinical
Phase 1
Phase 2
Pivotal
Filed
Approved
Peramivir Outpatient Flu¹
(Seasonal influenza / i.v.)
Peramivir Inpatient Flu
(Acute influenza / i.v.)
BCX5191
(Hepatitis C)
BCX4430
(Broad spectrum antiviral)
Enrollment suspended |
 Financial impact
of corporate restructuring Recent setbacks in the peramivir & BCX5191 programs, as
well as the delay in the BCX4161 program, necessitate a significant reduction in
BioCryst’s cost structure Significant operating expense
reductions provide a 38-45%
decrease in 2013 cash use vs.
$40 million guidance for 2012
Reduction of 38 positions (50%) in the
employee base lowers infrastructure
costs
The restructuring significantly reduces BioCryst’s cost base, extends its cash
runway into 2014 and enables the achievement of additional value
creating events
5 |
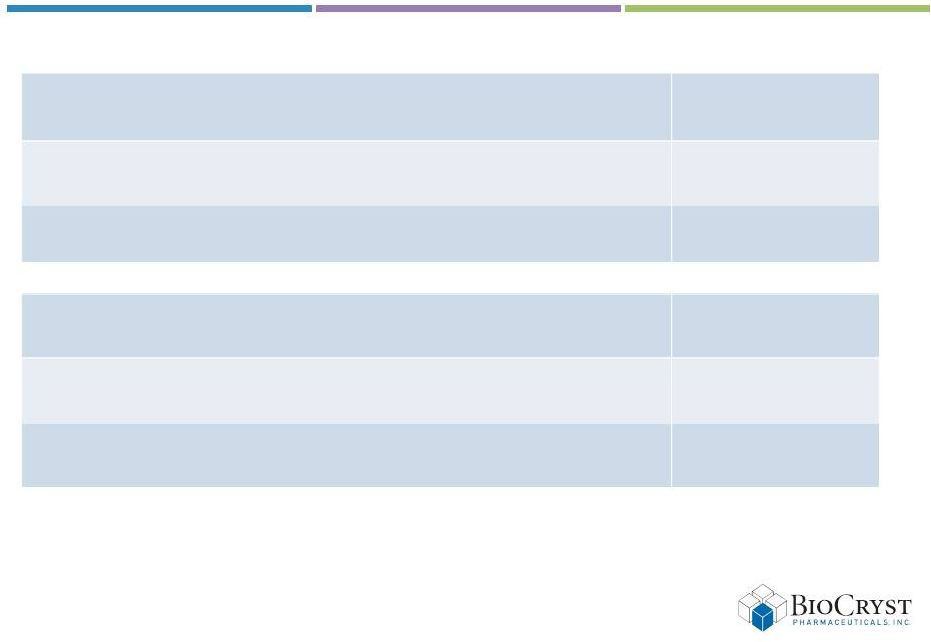 Implications of
restructuring & preliminary guidance* *Preliminary Guidance will be updated with
formal 2013 guidance during fiscal 2012 results reporting in February 2013 +Excludes
restructuring & deal charges Cash & investments as of December 31,
2012 $35 –
37
Restructuring charge (2012)
$2 –
4
Deal charge (2012)
$1 –
2
2012 cash utilization
+
$40
2013 cash utilization
+
$22 –
25
Months of cash runway from January 1, 2013
15 –
18
6
(in millions) |
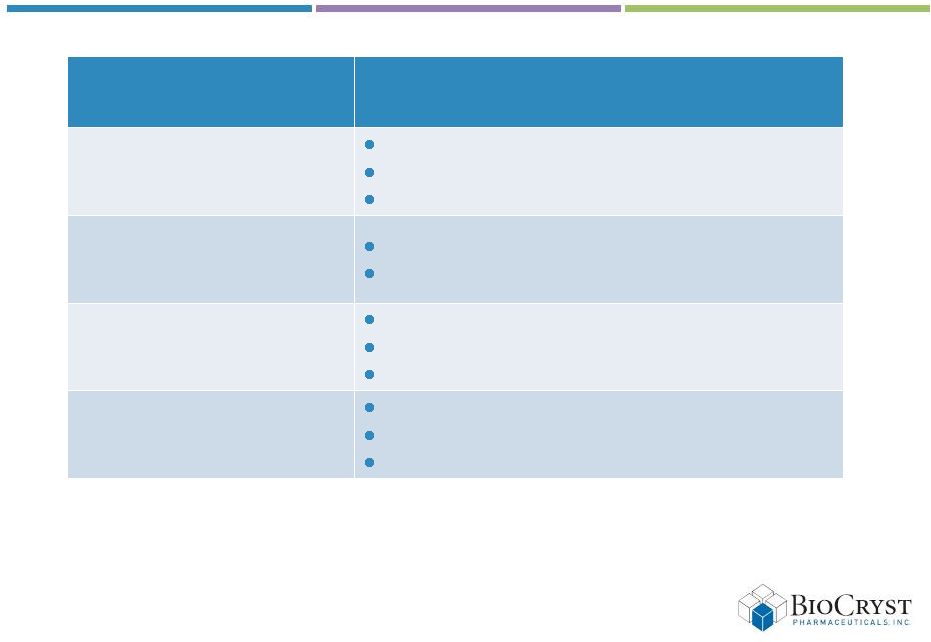 7
Milestones though mid-2014
Program
Milestones
BCX4161
(Hereditary angioedema)
Begin Phase 1 trial
Complete Phase 1
Start trial in HAE patients
Next gen HAE compound
Begin preclinical development
File IND
BCX4430
(Broad spectrum antiviral)
Publish filovirus animal proof of concept data
Secure government funding for development
File IND
BCX5191
(Hepatitis C antiviral)
Complete chimpanzee study
File new IND
Begin Phase 1 trial |
