Attached files
| file | filename |
|---|---|
| 8-K - INSMED INC. 8-K 12-3-2012 - INSMED Inc | form8-k.htm |

© 2012, Insmed, Inc. All rights reserved.
Corporate Overview
Improving the Lives of Patients
Battling Serious Orphan Lung
Diseases Using Targeted
Inhalation Therapies
Battling Serious Orphan Lung
Diseases Using Targeted
Inhalation Therapies
December, 2012
2
Safe Harbor Statement
This presentation contains forward-looking statements which are made pursuant to provisions of Section 21E
of the Securities Exchange Act of 1934. Words, and variations of words, such as “intend”, “expect”, “will”,
“anticipate”, “believe”, “continue”, “propose” and similar expressions are intended to identify forward-looking
statements. Investors are cautioned that such statements in this presentation, including statements relating to
our financial position, results of operations, the status, results and timing of results of pre-clinical studies and
clinical trials and pre-clinical and clinical data described herein, the timing of and costs associated with pre-
clinical studies and clinical trials, the development of our products, our estimates of the size of the potential
markets for our product candidates, and the business strategies, plans and objectives of management, constitute
forward-looking statements which involve risks and uncertainties that could cause actual results to differ
materially from those in the forward-looking statements. Such risks and uncertainties include, without limitation,
failure or delay of U.S. Food and Drug Administration and other regulatory reviews and approvals, competitive
developments affecting our product development, delays in product development or clinical trials, patent
disputes, unexpected regulatory actions, delays or requests, the failure of future clinical trials, inability to
successfully develop our product candidates or receive necessary regulatory approvals, inability to make
product candidates commercially successful, changes in anticipated expenses, and other risks and challenges
detailed in our filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form
10-K for the year ended December 31, 2011 and our Quarterly Report on Form 10-Q for the quarter ended
September 30, 2012. Investors are cautioned not to place undue reliance on any forward-looking statements
which speak only as of the date of this presentation. We undertake no obligation to update these forward-
looking statements to reflect events or circumstances or changes in expectations.
of the Securities Exchange Act of 1934. Words, and variations of words, such as “intend”, “expect”, “will”,
“anticipate”, “believe”, “continue”, “propose” and similar expressions are intended to identify forward-looking
statements. Investors are cautioned that such statements in this presentation, including statements relating to
our financial position, results of operations, the status, results and timing of results of pre-clinical studies and
clinical trials and pre-clinical and clinical data described herein, the timing of and costs associated with pre-
clinical studies and clinical trials, the development of our products, our estimates of the size of the potential
markets for our product candidates, and the business strategies, plans and objectives of management, constitute
forward-looking statements which involve risks and uncertainties that could cause actual results to differ
materially from those in the forward-looking statements. Such risks and uncertainties include, without limitation,
failure or delay of U.S. Food and Drug Administration and other regulatory reviews and approvals, competitive
developments affecting our product development, delays in product development or clinical trials, patent
disputes, unexpected regulatory actions, delays or requests, the failure of future clinical trials, inability to
successfully develop our product candidates or receive necessary regulatory approvals, inability to make
product candidates commercially successful, changes in anticipated expenses, and other risks and challenges
detailed in our filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form
10-K for the year ended December 31, 2011 and our Quarterly Report on Form 10-Q for the quarter ended
September 30, 2012. Investors are cautioned not to place undue reliance on any forward-looking statements
which speak only as of the date of this presentation. We undertake no obligation to update these forward-
looking statements to reflect events or circumstances or changes in expectations.
ARIKACE is a registered trademark of Insmed Incorporated in the United States and various other countries.
eFlow® Electronic Nebulizer (eFlow®) is a registered trademark of PARI Pharma GmbH.
TOBI (Tobramycin Inhalation Solution) is a registered trademark of Novartis.
Cayston (aztreonam for inhalation solution) is a registered trademark of Gilead Sciences.
eFlow® Electronic Nebulizer (eFlow®) is a registered trademark of PARI Pharma GmbH.
TOBI (Tobramycin Inhalation Solution) is a registered trademark of Novartis.
Cayston (aztreonam for inhalation solution) is a registered trademark of Gilead Sciences.
All other trademarks and registered trademarks are the property of their respective owners.
Persons shown throughout this presentation are models used for illustrative purposes and are not patients.
3
Near Term Goal: The Approval and Commercial
Launch of ARIKACE® in Europe and the US
Launch of ARIKACE® in Europe and the US
Cystic
Fibrosis
Fibrosis
Non-Tuberculous
Mycobacteria (NTM)
Lung Disease
Mycobacteria (NTM)
Lung Disease
ARIKACE
Being developed to treat rare lung infections
in patients with limited options for treatment.
in patients with limited options for treatment.
§ Life expectancy
37 years1
37 years1
§ 30,000 US2
§ 35,000 EU3
§ Estimated $500M
market4
market4
§ Lengthy, repeat
hospitalizations
hospitalizations
§ 50,000 US5
§ No approved
treatments
treatments
1 Adapted from Cystic Fibrosis Foundation, Patient Registry Annual Data Reports, 2011. 2 Cystic Fibrosis Foundation (http://www.cff.org/AboutCF/
as accessed Nov., 2012) 3 Høiby BMC Medicine 2011, 9:32. 4 Patient-based forecast. 5 Clarity Pharma Research, Patient Chart Study, 2012.
as accessed Nov., 2012) 3 Høiby BMC Medicine 2011, 9:32. 4 Patient-based forecast. 5 Clarity Pharma Research, Patient Chart Study, 2012.

4
Orphan Disease #1
Cystic Fibrosis (CF) Patients with
Pseudomonas Lung Infections
5
Orphan Disease #1:
Cystic Fibrosis - Fatal Genetic Disease
Cystic Fibrosis - Fatal Genetic Disease
§ Life expectancy of 37 years1
§ No existing cure
§ Mucous buildup impairs
lung function
lung function
§ 70% of adults have
chronic infection due to
Pseudomonas aeruginosa1
chronic infection due to
Pseudomonas aeruginosa1
§ Infections lead to further
deterioration in lung
function of 1% to 3%
per year2
deterioration in lung
function of 1% to 3%
per year2
Urgent Need for Therapies With Better Efficacy, Safety and Durability
1 Cystic Fibrosis Foundation, Patient Registry, 2010. 2 Liou et al, Journal of Cystic Fibrosis 9 (2010) 250-256.

6
Orphan Disease #1:
Cystic Fibrosis - Current Treatment Options
Cystic Fibrosis - Current Treatment Options
§ Up to 3 hours per day of
medications and treatments
medications and treatments
§ Current inhaled antibiotics all require
2X or 3X / day dosing
2X or 3X / day dosing
§ Most common is
≈20 minutes each treatment
≈20 minutes each treatment
§ Resistance to TOBI has nearly
doubled since 1999
doubled since 1999
§ 85% increase1
Urgent Need for Therapies With Better Efficacy, Safety and Durability
1 FDA advisory panel US-FDA- AIDAC for TOBI-Podhaler- September 2012. Patient image credit Standard-Examiner, Ogden, Utah.
7
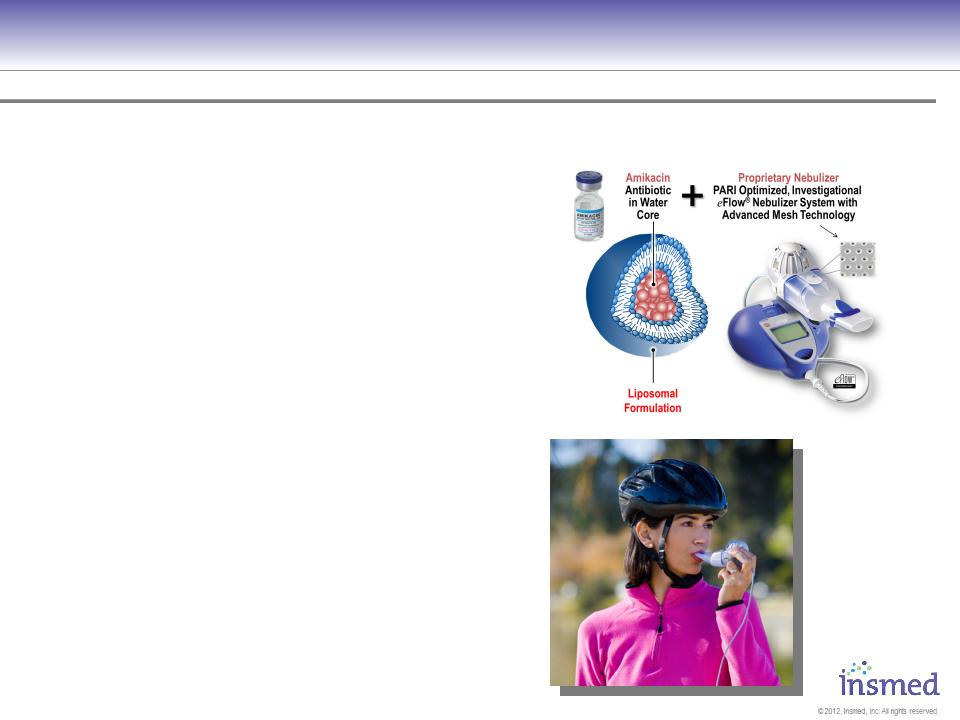
Proprietary Formulation AND Delivery System
concentrates Amikacin antibiotic
near bacteria w/ potential for:
concentrates Amikacin antibiotic
near bacteria w/ potential for:
üImproved Compliance
(first-ever 1X daily
dosing via inhalation)
(first-ever 1X daily
dosing via inhalation)
üIncreased Efficacy
(concentrated at site of bacteria)
(concentrated at site of bacteria)
üIncreased Durability of effect
(benefits when off treatment)
(benefits when off treatment)
üIncreased Safety
(less drug needed to achieve result)
(less drug needed to achieve result)
Insmed Is Developing ARIKACE to Treat Chronic Lung Infections
8
Aerosol
Delivery
(eFlow®
Nebulizer)
Delivery
(eFlow®
Nebulizer)
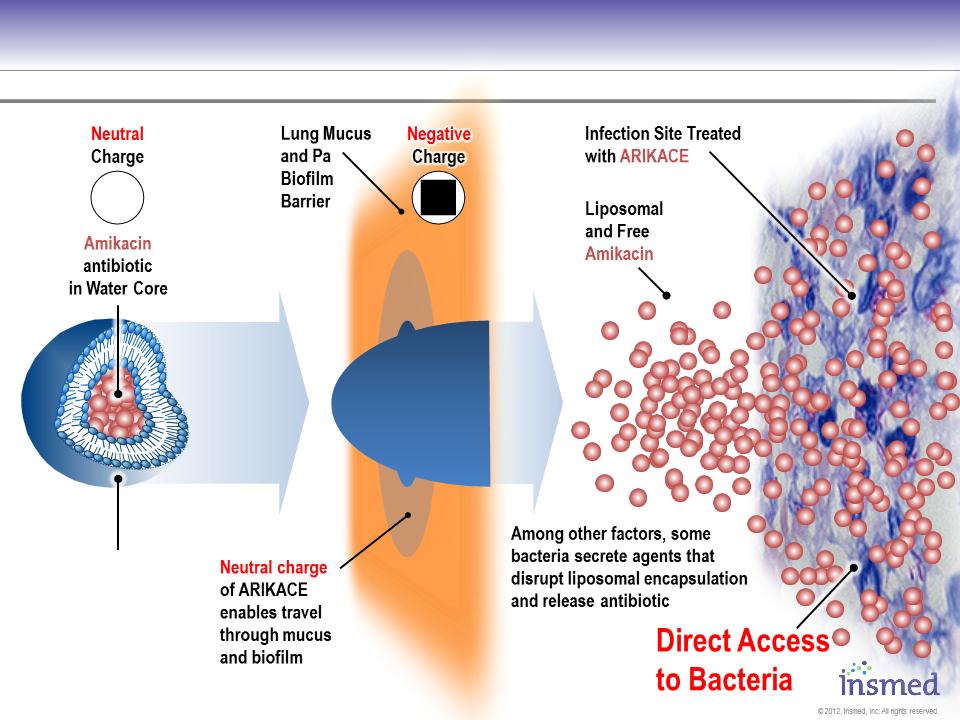
ARIKACE:
Achieves Access to Bacteria in Infected Tissues and Macrophages
Achieves Access to Bacteria in Infected Tissues and Macrophages
Conceptual diagram for illustration purposes only.
Liposomal
Formulation
Formulation
(DPPC and
Cholesterol)
9
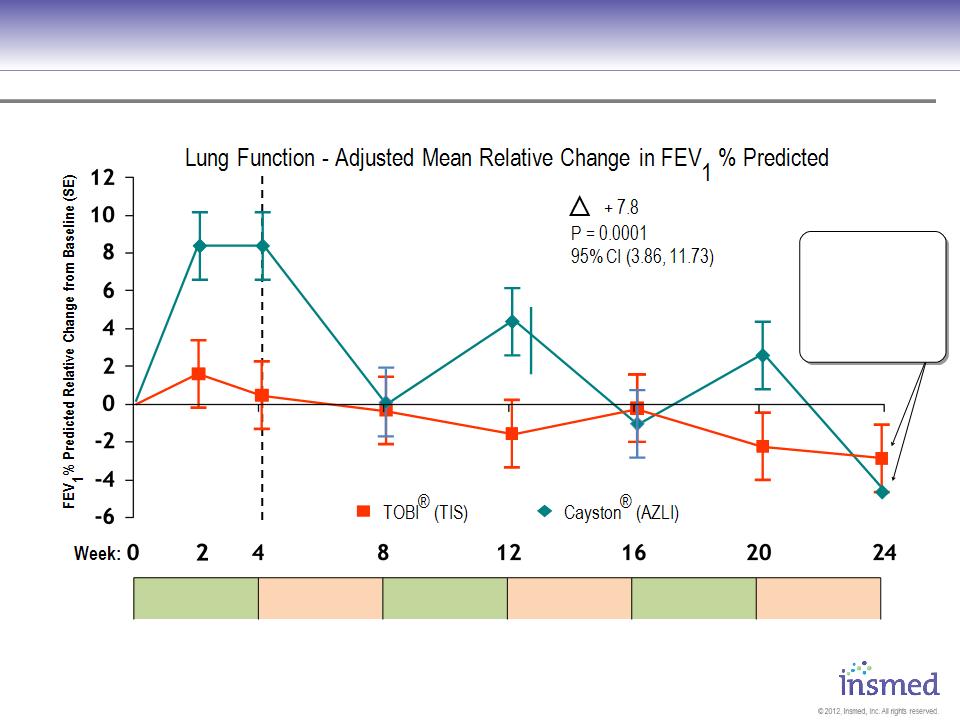
Gilead Cayston vs. Novartis TOBI: Both Show Decline In Lung Function
Source: 2010 North American CF Conference Poster 305 and Slide Presentation, 10/10.
AZLI = Cayston; TIS = TOBI
|
AZLI / TIS
28 Days
|
|
AZLI / TIS
28 Days
|
|
AZLI / TIS
28 Days
|
|
CF Phase 3 Gilead Sponsored Open Label Trial Results
(ON)
(OFF)
(ON)
(OFF)
(ON)
(OFF)
Entry Criteria: ● FEV1 ≤75% ● Age: ≥6 ● N=268 randomized and treated
After initial improvement,
lung function dropped
back to baseline or
lower during each off
treatment period and at
the end of 24 weeks.
lung function dropped
back to baseline or
lower during each off
treatment period and at
the end of 24 weeks.
10
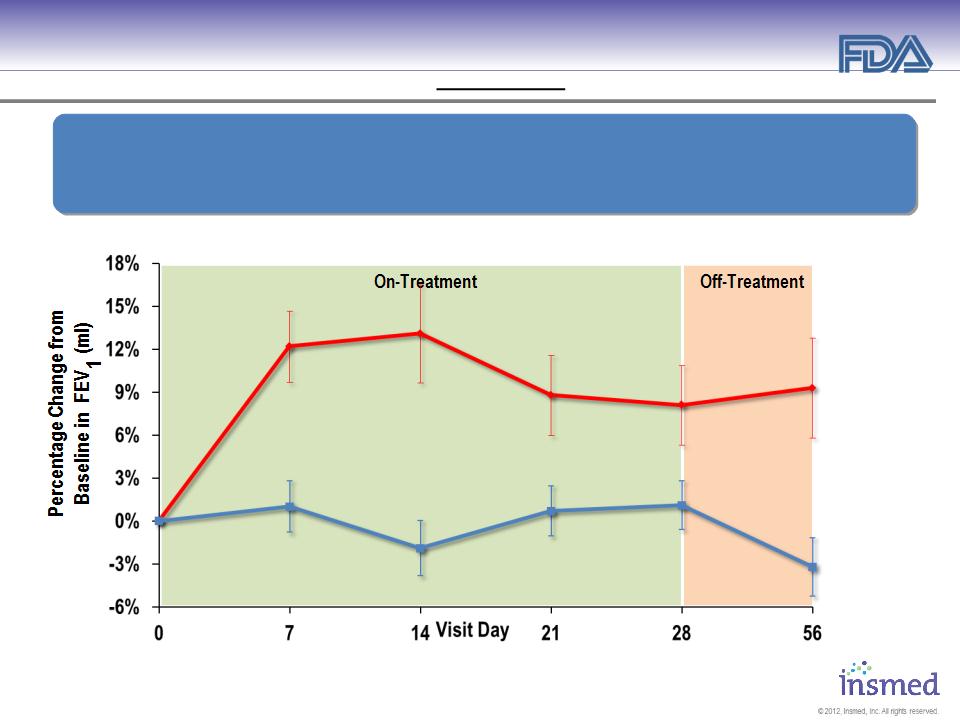
ARIKACE: Treatment of Pseudomonas Lung Infections in CF
Phase 2 Pooled Results (560mg 1X per Day): Pulmonary Function
Phase 2 Pooled Results (560mg 1X per Day): Pulmonary Function
§ ARIKACE demonstrated statistically significant and clinically meaningful
improvement in pulmonary function throughout the 28-day treatment period.
improvement in pulmonary function throughout the 28-day treatment period.
§ Improvement was sustained through the off-treatment period.
Mean (SE)
P = 0.033
P = 0.003
(36/36)
(36/35)
(33/36)
(32/35)
(34/35)
(34/34)
(N)
Lung Function - % Change in FEV1 (ml) vs. Baseline
ARIKACE
560mg
560mg
Placebo
(ARIKACE / Placebo)
Entry Criteria: ● FEV1 >40 % ● Age: >6 ● N= 72 ● Pre-treated w/ inhaled antibiotics ≈27%
11
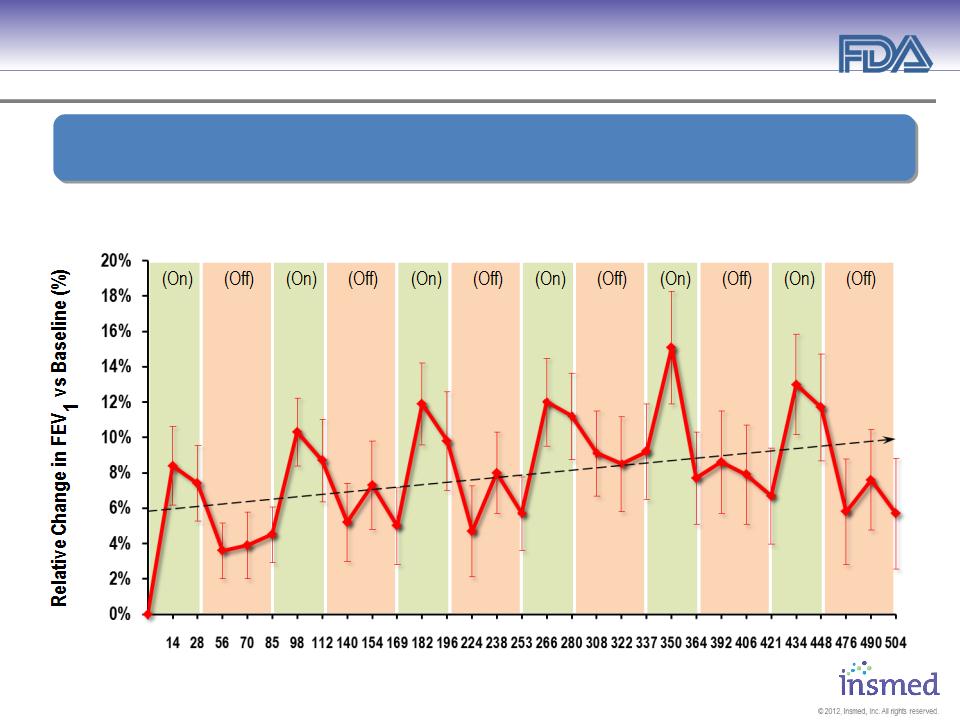
ARIKACE: Treatment of Pseudomonas Lung Infections in CF
Open Label Extension (TR02-105): Durability of Response
Open Label Extension (TR02-105): Durability of Response
* Significance at end of treatment over 6 cycles ** Significance 56 days off-treatment over 6 cycles
Patients Receiving 560 mg ARIKACE Once Daily for 28 Days and Off-Treatment for 56 Days
42
41
42
42
41
41
41
41
41
45
45
47
46
44
45
47
46
46
45
44
44
44
43
43
43
43
44
43
45
p=0.0001**
p<0.0001*
41
47
(N)
1
2
3
4
5
6
Treatment Cycle à
Visit Days à
Demonstrated Sustained Efficacy of ARIKACE
During and Between Multiple Cycles of Therapy
During and Between Multiple Cycles of Therapy
12
ARIKACE: Cystic Fibrosis
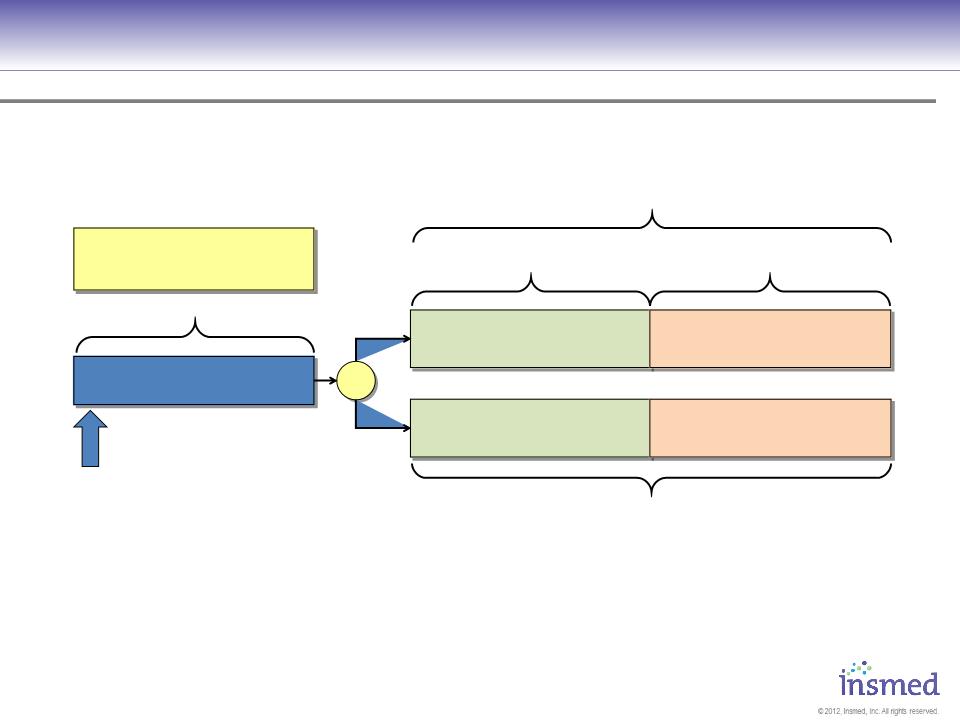
Phase 3 Study Design Europe and Canada
Phase 3 Study Design Europe and Canada
The registration trial for Europe is a comparator study vs. TOBI and will evaluate FEV1
improvement at week 24 as the primary efficacy endpoint.
improvement at week 24 as the primary efficacy endpoint.
R
28 Day Daily Dosing
28 Days Follow-Up
28 Days Off Inhaled Antibiotics
Screening Period
Day -18 to Day -4
TOBI
twice daily by PARI-LC Star®
No inhaled antibiotics
560mg ARIKACE
once daily by eFlow®
Key Inclusion Criteria
§ FEV1 ≥ 25%
§ Age ≥6 years
§ Chronic Pa Infection
§ 28 Days Off Inhaled
Antibiotics
Antibiotics
§ AZI, DNAse, and/or
hypertonic saline continued
hypertonic saline continued
Monthly Efficacy and Safety Evaluation
Assessments of PFT, CFU, Antibiotic Rescue Treatment,
Respiratory Exacerbations, Hospitalizations, PRO/QOL
Respiratory Exacerbations, Hospitalizations, PRO/QOL
Primary End-Point: Relative Change in FEV1 at week 24
Three Cycles of Treatment (24 weeks)
Completers eligible to participate in CLEAR-110, long term open-label extension where patients receive ARIKACE every other month for ≤2yrs.
Abbreviations: AZI - Azithromycin . Pa - Pseudomonas aeruginosa. PFT - Pulmonary Function Testing,
FEV1 - forced expiratory volume in 1 second. CFU - colony forming units. PRO - Patient Reported Outcome.
FEV1 - forced expiratory volume in 1 second. CFU - colony forming units. PRO - Patient Reported Outcome.
Randomization:1:1
Power 80%
N≈300
≈260 patients required to demonstrate non-inferiority
at agreed upon margin with 80% power
at agreed upon margin with 80% power
No inhaled antibiotics

13
Orphan Disease #2
Non-TB Mycobacteria (NTM)
Lung Infections
§ No approved treatments
14
Orphan Disease #2: Non-TB Mycobacteria (NTM) Lung Infections
“Current treatment for NTM
lung disease requires lengthy
multi-drug regimens that can
be poorly tolerated and have
limited efficacy, especially in
patients with severe disease
or in those who have failed
prior treatment attempts.”
lung disease requires lengthy
multi-drug regimens that can
be poorly tolerated and have
limited efficacy, especially in
patients with severe disease
or in those who have failed
prior treatment attempts.”
David E. Griffith, M.D.
§Professor of Medicine at the University
of Texas Health Science Center at Tyler
of Texas Health Science Center at Tyler
§Lead author of the ATS/IDSA's diagnosis
and treatment guidelines for NTM
and treatment guidelines for NTM

15
Orphan Disease #2: Non-TB Mycobacteria (NTM) Lung Infections
- Debilitating and Wide Spread
- Debilitating and Wide Spread
1 Incidence of TB from Center for Disease Control and Prevention Morbidity and Mortality Weekly Report (March, 2012). 2 Clarity Pharma Research, Patient
Chart Study, 2012. 3 Adjemian et al. Prevalence of Pulmonary Nontuberculous Mycobacterial Disease among Medicare Beneficiaries, USA, 1997-2007,
American Journal of Respiratory and Critical Care Medicine. Apr 2012. 4 Mouse macrophage forming two processes to phagocytize two smaller particles,
possibly pathogens (image licensed under Creative Commons Attribution-Share Alike 2.0 Generic).
Chart Study, 2012. 3 Adjemian et al. Prevalence of Pulmonary Nontuberculous Mycobacterial Disease among Medicare Beneficiaries, USA, 1997-2007,
American Journal of Respiratory and Critical Care Medicine. Apr 2012. 4 Mouse macrophage forming two processes to phagocytize two smaller particles,
possibly pathogens (image licensed under Creative Commons Attribution-Share Alike 2.0 Generic).
§ 4X to 5X more common than TB in U.S.1
§ 50,000 cases per year in U.S.2
§ Diagnosis growing ≈8% per yr3
§ Age >65 are 40% more likely
to die from 1997 to 20073
to die from 1997 to 20073
§ NTM taken up by and
multiply inside macrophages
multiply inside macrophages
§ Most antibiotics have poor
macrophage penetration
macrophage penetration
Lung macrophage
reside on respiratory
surfaces and clean off
particles such as dust
or microorganisms.4
reside on respiratory
surfaces and clean off
particles such as dust
or microorganisms.4

16
Orphan Disease #2: Non-TB Mycobacteria (NTM) Lung Infections
- Current Treatment Options
- Current Treatment Options
§ No approved treatment;
all treatments are off label
all treatments are off label
§ Off label treatments carry significant
toxicities and limitations
toxicities and limitations
§ Avg. 7.6 antibiotic courses/yr1
§ Chronic, debilitating, progressive
§ Lengthy, repeat hospitalizations
§ Average stay 10.2 days1
1 SDI Healthcare Database, July 2009; Mycobacterium avium Complex; M. abscessus = Mycobacterium abscessus
17
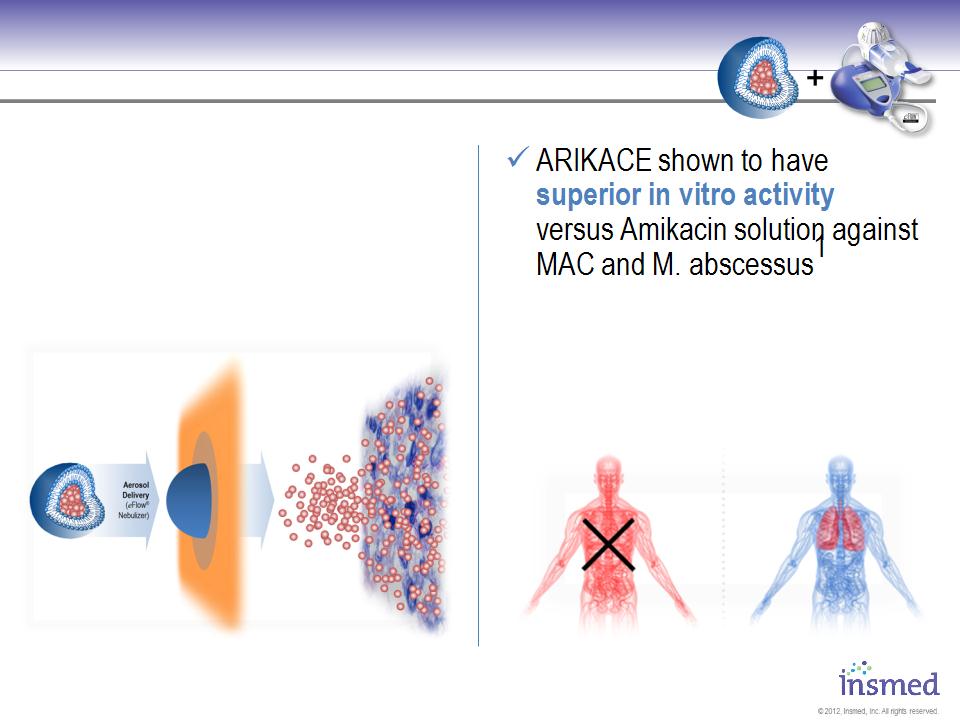
ARIKACE: Positioned to Become the First Drug
Approved for Non-TB Mycobacteria Lung Infections
Approved for Non-TB Mycobacteria Lung Infections
Conceptual diagram for illustration purposes only.
1. Study conducted by L. E. Bermudez at
Oregon State University (data on file). (2010)
Oregon State University (data on file). (2010)
ü ARIKACE once-daily treatment
ü ARIKACE is taken up by lung
macrophages where NTM
infections are located
macrophages where NTM
infections are located
Greatly Reduced Systemic Exposure
Localized Lung Treatment
ü ARIKACE provides localized
lung treatment with greatly
reduced systemic exposure
lung treatment with greatly
reduced systemic exposure
18
ARIKACE: Non-TB Mycobacteria Lung Infections
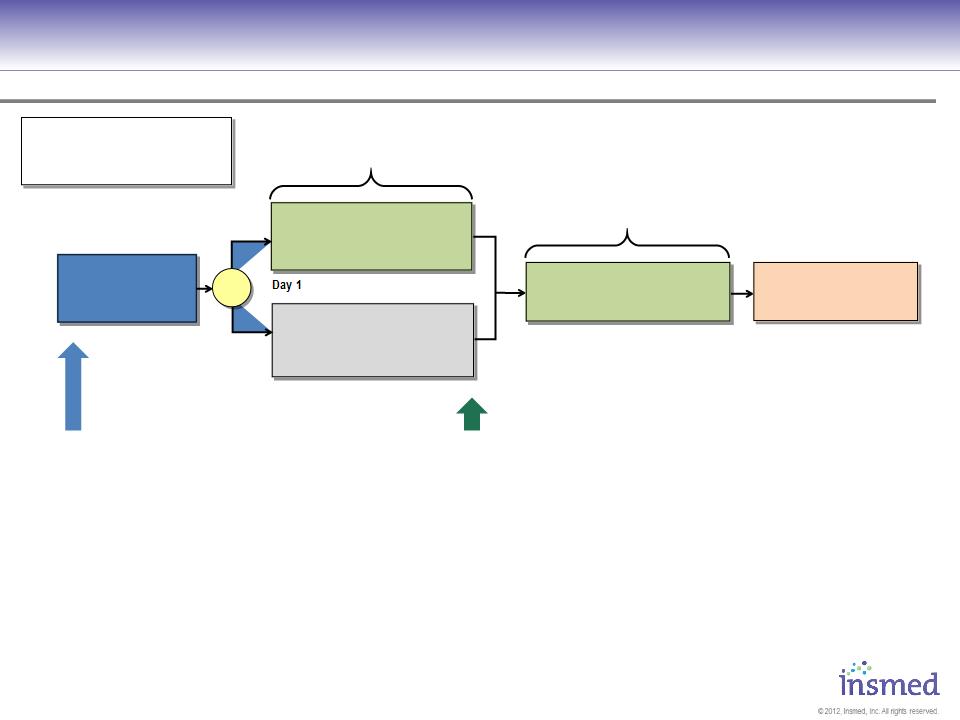
Phase 2 Study Design: TR02-112
Phase 2 Study Design: TR02-112
Abbreviations: CT - computed tomography. FEV1 - forced expiratory volume in 1 second. NTM - non-TB mycobacterium. SGRQ - Saint George’s
respiratory questionnaire.
respiratory questionnaire.
Primary Endpoints: Efficacy
§Reduction in bacterial density at Day 84
Secondary Endpoints:
§Time to sputum conversion
§Time to Pulmonary Exacerbation
§Change in PRO/QOL
§6 minute walk test
§Change in Clinical Signs and Symptoms
§Safety
Screening Period
Day -42
to Day -4
Day -42
to Day -4
84 Days Daily Dosing
Background Therapy
+ 560mg ARIKACE
+ 560mg ARIKACE
once daily by eFlow®
Background Therapy
+ Placebo
+ Placebo
once daily by eFlow®
Day 1
Day 85
Day 197
Day 169
84 Days Daily Dosing,
Open-Label
28 Day Follow-Up
No inhaled antibiotics
No inhaled antibiotics
560mg ARIKACE
once daily by eFlow®
Abbreviations: CT - computed tomography. FEV1 - forced expiratory volume in 1 second. NTM - non-TB mycobacterium.
Randomize N≈100 (1:1)
Stratify: CF vs. Non-CF
MAC vs. M. abscessus
MAC vs. M. abscessus
R
Key Inclusion Criteria
§Age ≥18 years, ≤85 years
§DX of pulmonary NTM lung disease
according to 2007 ATS/IDSA criteria with
evidence of nodular bronchiectasis and/or
cavitary disease by chest CT.
according to 2007 ATS/IDSA criteria with
evidence of nodular bronchiectasis and/or
cavitary disease by chest CT.
§History of chronic Mycobacterium avium
complex or Mycobacterium abscessus (at least
3 documented positive cultures in prior 2
years, and at least one within 6 months).
complex or Mycobacterium abscessus (at least
3 documented positive cultures in prior 2
years, and at least one within 6 months).
§Multi-drug regimen for at least 6 months prior
to screening with persistently positive
mycobacterial culture
to screening with persistently positive
mycobacterial culture
19
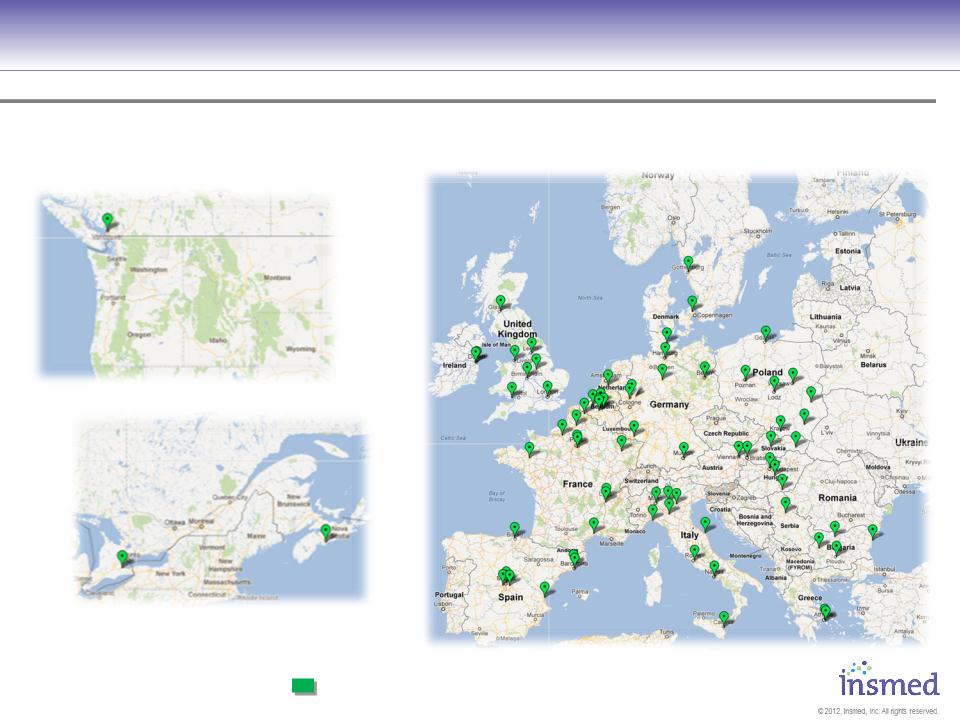
Phase III Pivotal Trial Investigator Sites
Are Laying the Groundwork for Strong Commercial Launch
Are Laying the Groundwork for Strong Commercial Launch
= Activated Investigator with Screened Patients (75)
CLEAR-108 European Sites
CLEAR-108 Canadian Sites

20
Manufacturing
§ CGMP Facility
§ Currently 3-4
batches/month
batches/month
§ 2+ years of stability
on 25l capacity
on 25l capacity
§ 12-month extractables
and leachables (E&L)
study underway
and leachables (E&L)
study underway
§ Planned scale up
program underway
program underway
§ Selection of 2nd source
supplier progressing
supplier progressing
21
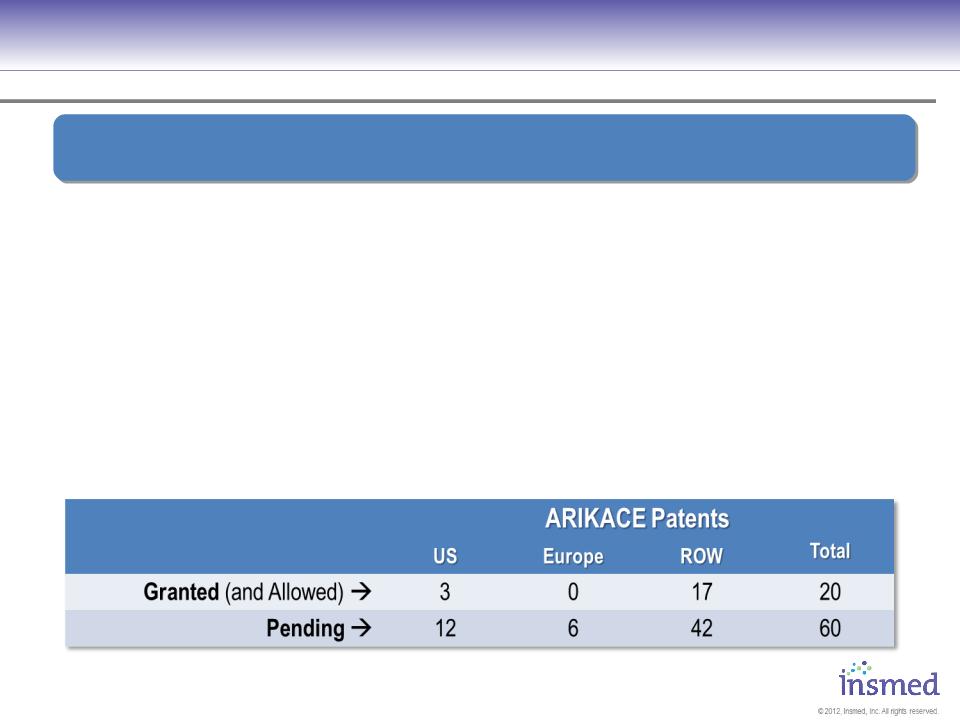
ARIKACE
IP and Exclusivity
IP and Exclusivity
§ US Composition-of-matter patent, with an added 6 months of pediatric
exclusivity, provides coverage through Feb 2029
exclusivity, provides coverage through Feb 2029
§ Additional global applications under review including U.S., EU and
Japanese composition-of-matter applications
Japanese composition-of-matter applications
§ Orphan drug status for CF, pending for NTM
§ Nebulizer device exclusivity for minimum of 15 years
§ Liposomal delivery technology = added regulatory barrier to entry
Insmed Has Extensive Patent Coverage and Other Exclusive
Arrangements Protecting Composition, Use, and Delivery Of ARIKACE
Arrangements Protecting Composition, Use, and Delivery Of ARIKACE

22
2013 Is Focused On Advancing Our European Filing
and Pre-Commercial Preparation
and Pre-Commercial Preparation
ü EU Phase III pivotal underway
ü US Phase II NTM underway
ü Senior Team build out
ü $20M Hercules debt facility
ü $25M+ capital raised
2013
§ Plan to launch compassionate
use program in NTM patients
use program in NTM patients
§ Phase III pivotal data
§ Advance EU filing
§ EU backbone build out
§ Scale up / second source
2012
23
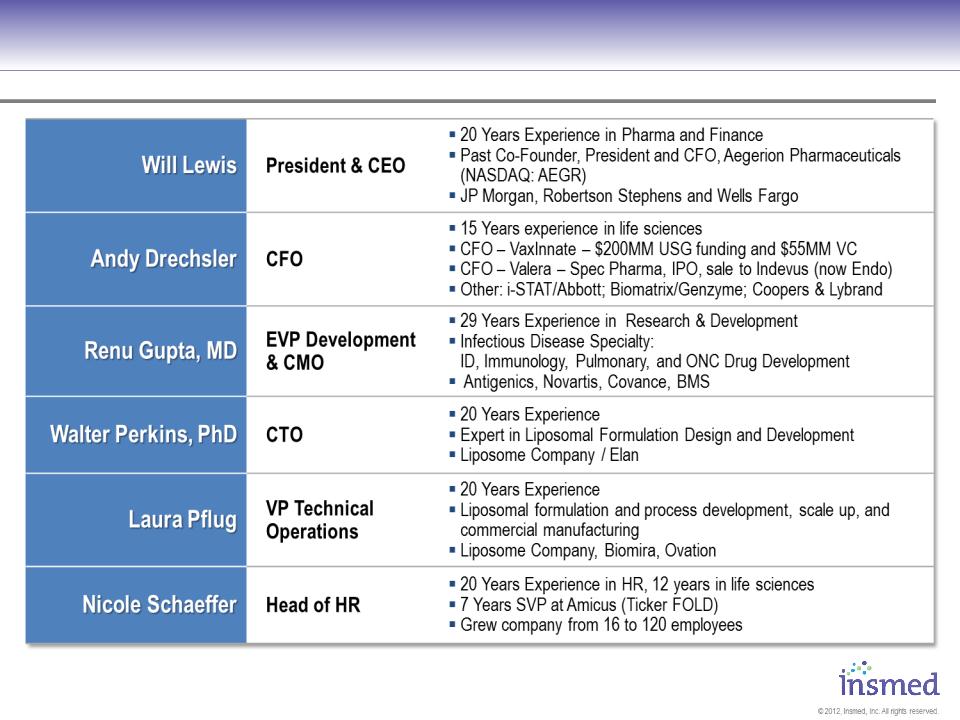
Management Has Extensive Experience with
Anti-Infective Development, Regulatory, and Commercial
Anti-Infective Development, Regulatory, and Commercial
24
Strong Cash Position
Capital Structure and Key Figures
Capital Structure and Key Figures
Present Capital Structure (NASDAQ: INSM)
§33.9 million fully diluted shares:
§ 31.4 million common shares
§ 2.5 million options, restricted
stock units, and warrants
stock units, and warrants
Balance Sheet
§Cash of $91.9 million as of September 30, 2012
§$10M of debt o/s and $10M more available to be drawn by YE 2012
Projected Cash Year End 2012
§Approximately $85 to $90 million currently forecast
§ Consistent with previous guidance and reflective of $25.7M raise in Q3
Employees
§44 current employees
§Planned additions: commercial ops, Europe, regulatory, project management

© 2012, Insmed, Inc. All rights reserved.
Thank You
Improving the Lives of Patients
Battling Serious Orphan Lung
Diseases Using Targeted
Inhalation Therapies
Battling Serious Orphan Lung
Diseases Using Targeted
Inhalation Therapies
www.INSMED.com

26
Appendix
Additional Clinical Data

27
Resistance
“ Most resistance to aminoglycosides is
caused by bacterial inactivation by
intracellular enzymes. Because of
structural differences, amikacin is not
inactivated by the common enzymes
that inactivate gentamicin and
tobramycin. Therefore, a large proportion
of the gram-negative aerobes that are
resistant to gentamicin and tobramycin
are sensitive to amikacin. In addition, with
increased use of amikacin, a lower
incidence of resistance has been
observed compared with increased use of
gentamicin and tobramycin. ”
caused by bacterial inactivation by
intracellular enzymes. Because of
structural differences, amikacin is not
inactivated by the common enzymes
that inactivate gentamicin and
tobramycin. Therefore, a large proportion
of the gram-negative aerobes that are
resistant to gentamicin and tobramycin
are sensitive to amikacin. In addition, with
increased use of amikacin, a lower
incidence of resistance has been
observed compared with increased use of
gentamicin and tobramycin. ”
1 Aminoglycosides: A Practical Review, UIS S. GONZALEZ III, PHARM.D., and JEANNE P. SPENCER, M.D.,
Conemaugh Memorial Medical Center, Johnstown, Pennsylvania, Am Fam Physician. 1998 Nov 15;58(8):1811-1820.
Conemaugh Memorial Medical Center, Johnstown, Pennsylvania, Am Fam Physician. 1998 Nov 15;58(8):1811-1820.
28
ARIKACE: Cystic Fibrosis
Phase 2 Pooled Results
Phase 2 Pooled Results
N = 64
22
Placebo
(1.5% saline)
(1.5% saline)
21
21
ARIKACE 280mg
ARIKACE 560mg
TR02-105* (Europe)
The Phase 2 program was prospectively designed to analyze pooled cohorts from the US and EU.
The following summary presents pooled data for the ARIKACE 560mg and placebo cohorts.
The following summary presents pooled data for the ARIKACE 560mg and placebo cohorts.
N = 41
14
Placebo
(1.5% saline)
(1.5% saline)
12
15
ARIKACE 70 or 140mg
ARIKACE 560mg
TR02-106* (US)
Weekly Safety Evaluation
Assessments of PFT, CFU, Exacerbations,
Time to Rescue Antibiotics, CFQ-R and PK
Time to Rescue Antibiotics, CFQ-R and PK
N = 72
36
Placebo
(1.5% saline)
(1.5% saline)
36
ARIKACE 560mg
TR02-105/106 (Pooled)
Key Inclusion Criteria
•FEV1 ≥ 40%
•Age ≥ 6 years
•Chronic Pa Infection
• 28 Days Off Inhalation Antibiotics
• Azithromycin, DNAse, hypertonic
saline, bronchodilators continued
saline, bronchodilators continued
29
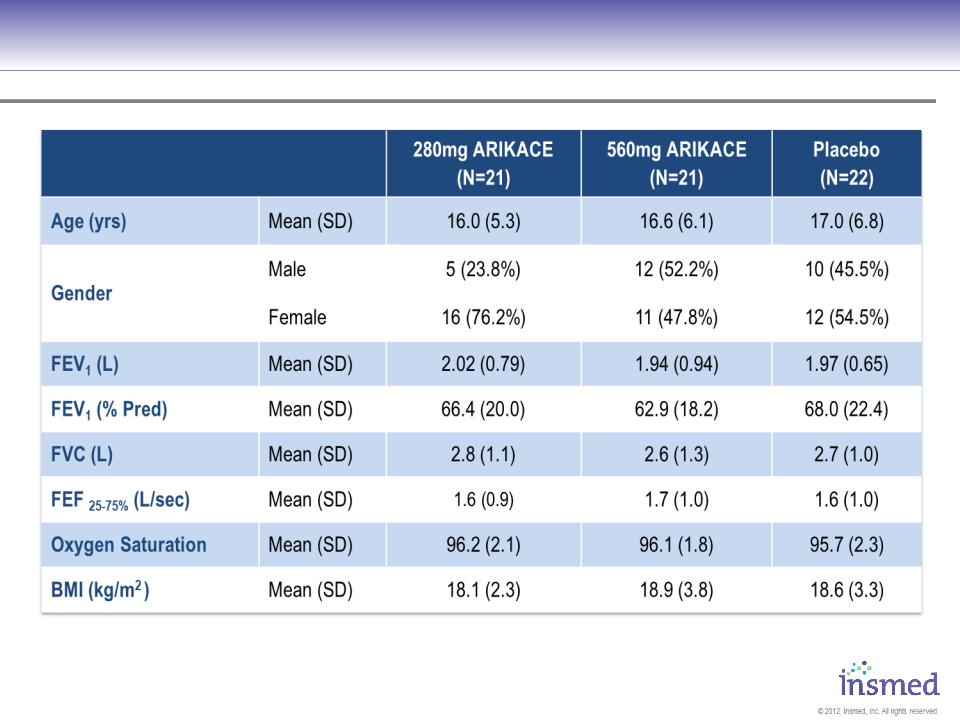
ARIKACE: TR02-105 - Patient Characteristics
No significant differences assessed by ANOVA for continuous variables, chi-square test for categorical variables
29
30
Visit Day
* Mean (SD)
|
ARIKACE 560 *
|
15.4% (16.5)
|
18.4% (21.3)
|
13.2% (15.3)
|
13.2% (16.2)
|
11.5% (16.4)
|
13.2% (24.3)
|
|
ARIKACE 280 *
|
10.9% (10.6)
|
9.4% (12.6)
|
9.6% (12.5)
|
10.1% (12.8)
|
1.7% (9.0)
|
2.0% (8.6)
|
|
Placebo *
|
0.6% (11.7)
|
-3.2% (12.2)
|
1.8% (10.9)
|
2.2% (11.9)
|
-0.3% (12.0)
|
-4.4% (13.0)
|
Percent Change in FEV1 - ITT Phase II Study1
ARIKACE 280
Placebo
ARIKACE 560
1 European Phase 2 study (TR02-105) of ARIKACE in patients with CF and chronic Pseudomonas aeruginosa infection.
31
Visit Day
* Mean (SD)
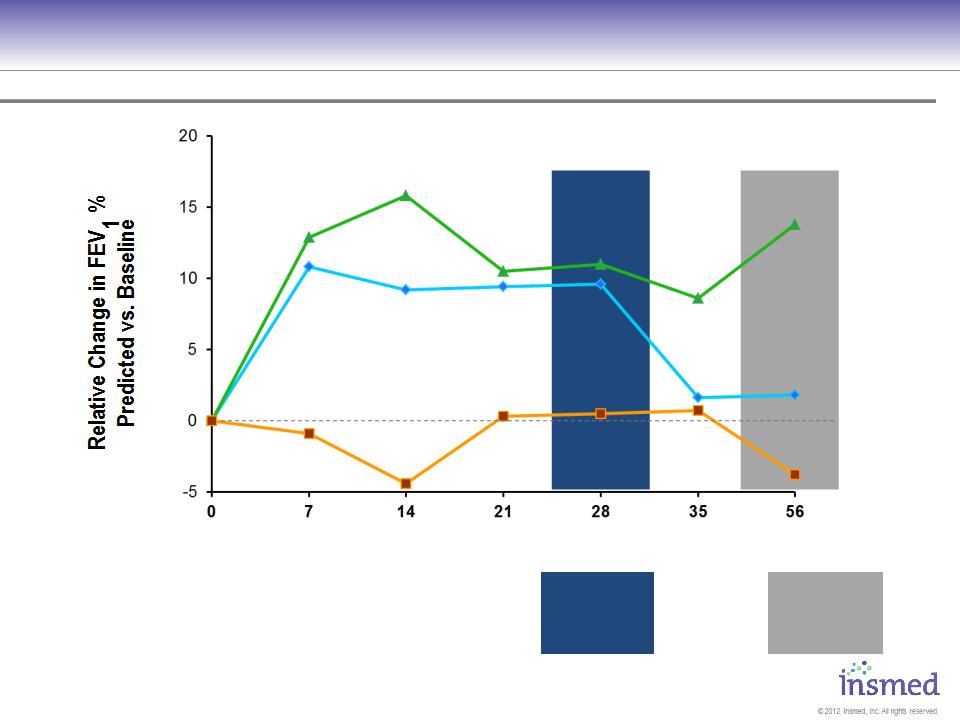
Change in FEV1 (% predicted) - ITT Phase II Study1
P=0.009
P=0.019
P=0.124
P=0.021
|
ARIKACE 560 *
|
12.9% (17.2)
|
15.8% (22.5)
|
10.5% (15.6)
|
11.0% (16.4)
|
8.6% (17.7)
|
13.8% (26.2)
|
|
ARIKACE 280 *
|
10.8% (10.8)
|
9.2% (13.1)
|
9.4% (12.9)
|
9.6% (13.7)
|
1.6% (9.6)
|
1.8% (8.8)
|
|
Placebo *
|
-0.9% (10.7)
|
-4.4% (11.3)
|
0.3% (9.9)
|
0.5% (10.5)
|
0.7% (9.6)
|
-3.8% (13.5)
|
ARIKACE 280
Placebo
ARIKACE 560
1 European Phase 2 study (TR02-105) of ARIKACE in patients with CF and chronic Pseudomonas aeruginosa infection.
32
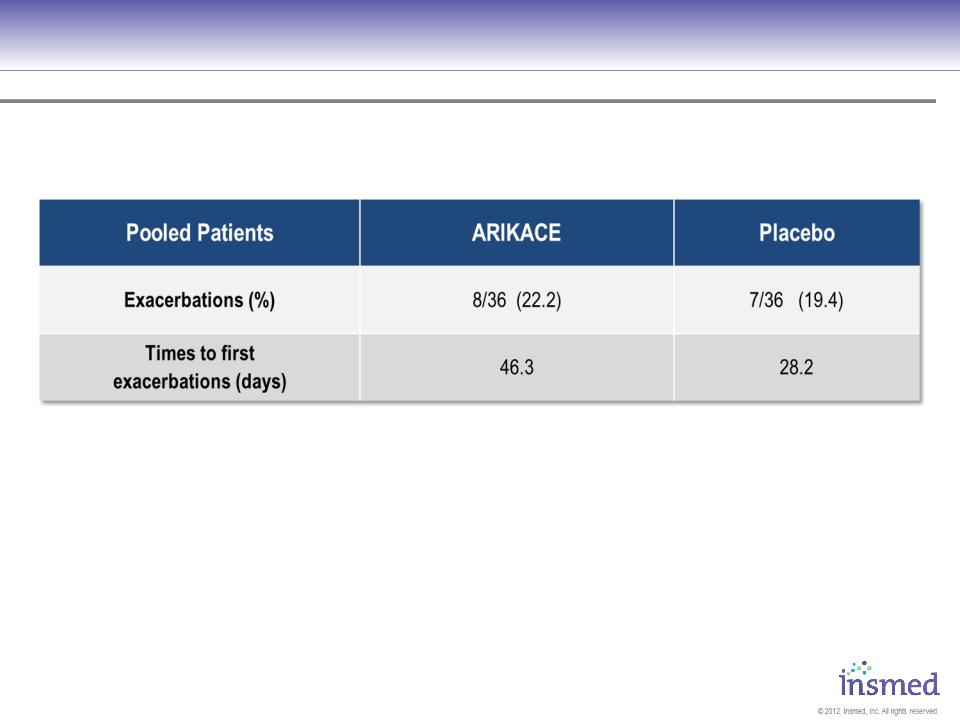
105&106 - Pooled Pulmonary Exacerbations ≈560 mg Cohort
No significant differences assessed by ANOVA for continuous variables, chi-square test for categorical variables
32
33
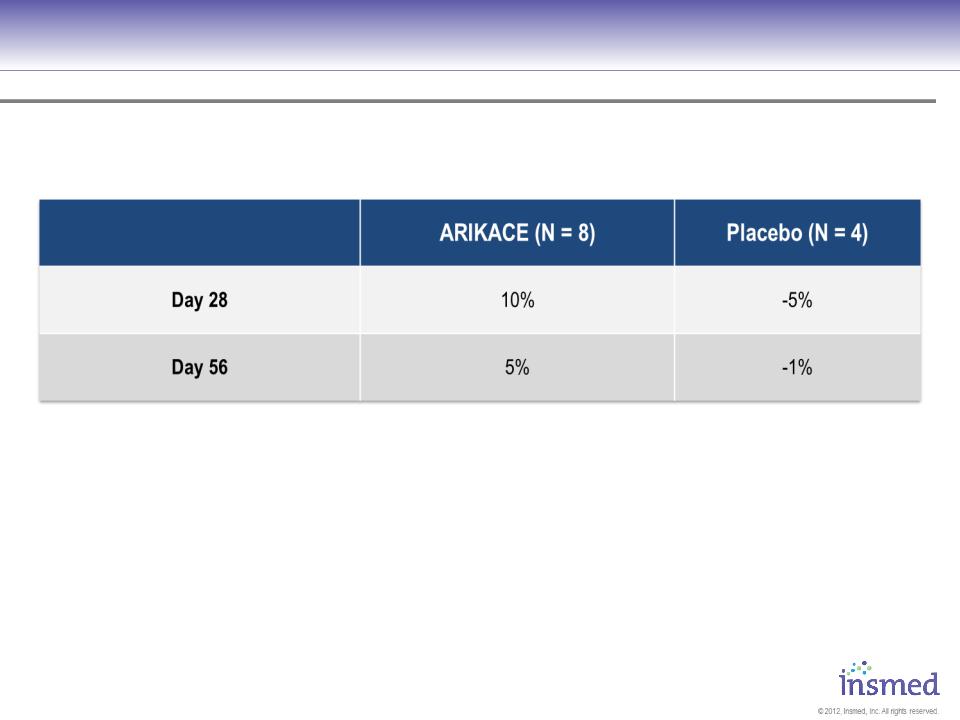
Prior Use of Inhalation Antibiotic
No significant differences assessed by ANOVA for continuous variables, chi-square test for categorical variables
33
Relative Change FEV1 (ml)
34
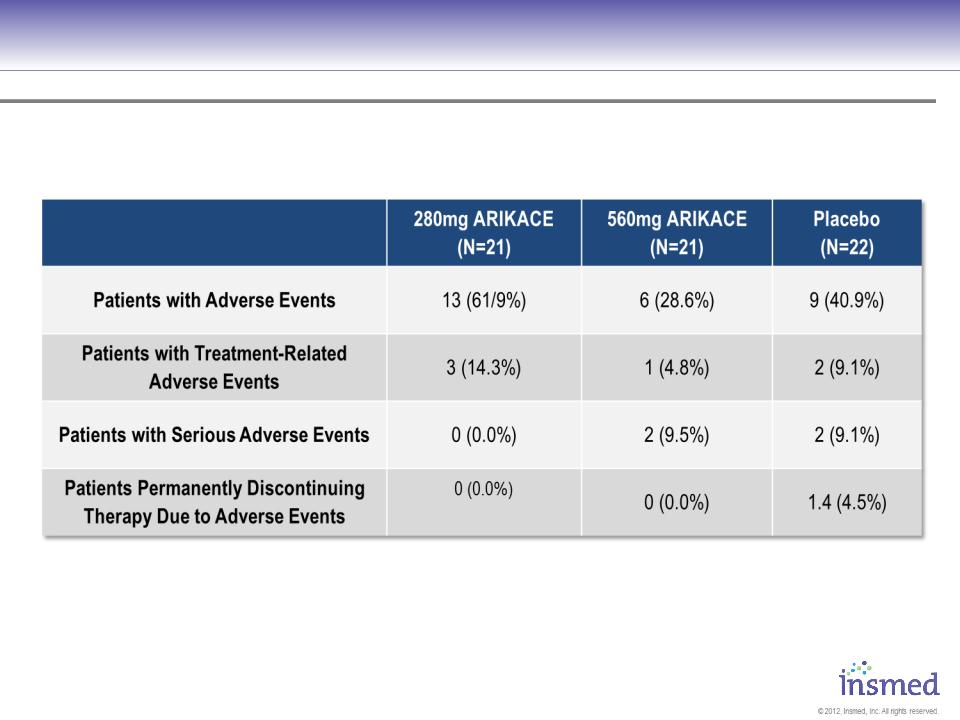
Results - Safety
No significant differences assessed by ANOVA for continuous variables, chi-square test for categorical variables
34
35
ARIKACE: Cystic Fibrosis
Phase 1/2 Clinical Research Program: N=208
Phase 1/2 Clinical Research Program: N=208
§ Scintigraphy study - normal volunteers: N = 6
§ Phase I Single Dose: N = 24
§ 2 Phase1b/2a, 14 Days Dosing Open Label
Studies: N = 24
Studies: N = 24
§ 1 European Phase 2, 28 Days Dosing,
Randomized, Placebo Comparative Study: N=64
Randomized, Placebo Comparative Study: N=64
§ 1 US Phase 2, 28 Days Dosing, Randomized,
Placebo Comparative Study: N= 41
Placebo Comparative Study: N= 41
§ 1 European Open label, Single arm Long-term
Safety and Efficacy: N= 49
Safety and Efficacy: N= 49
36
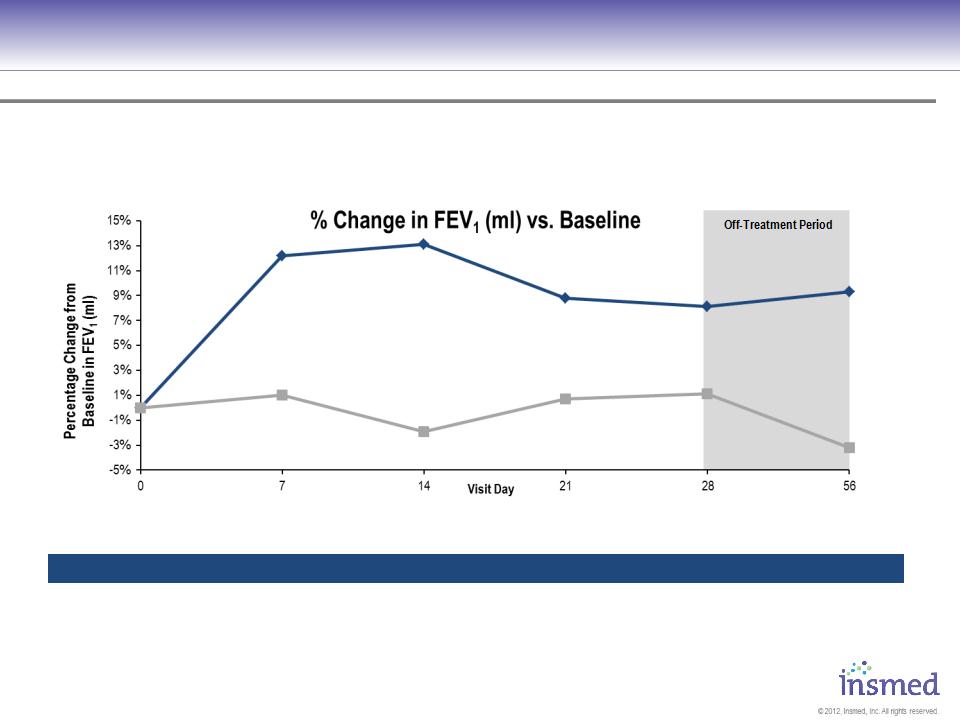
ARIKACE: Cystic Fibrosis
Phase 2 Pooled Results: Pulmonary Function
Phase 2 Pooled Results: Pulmonary Function
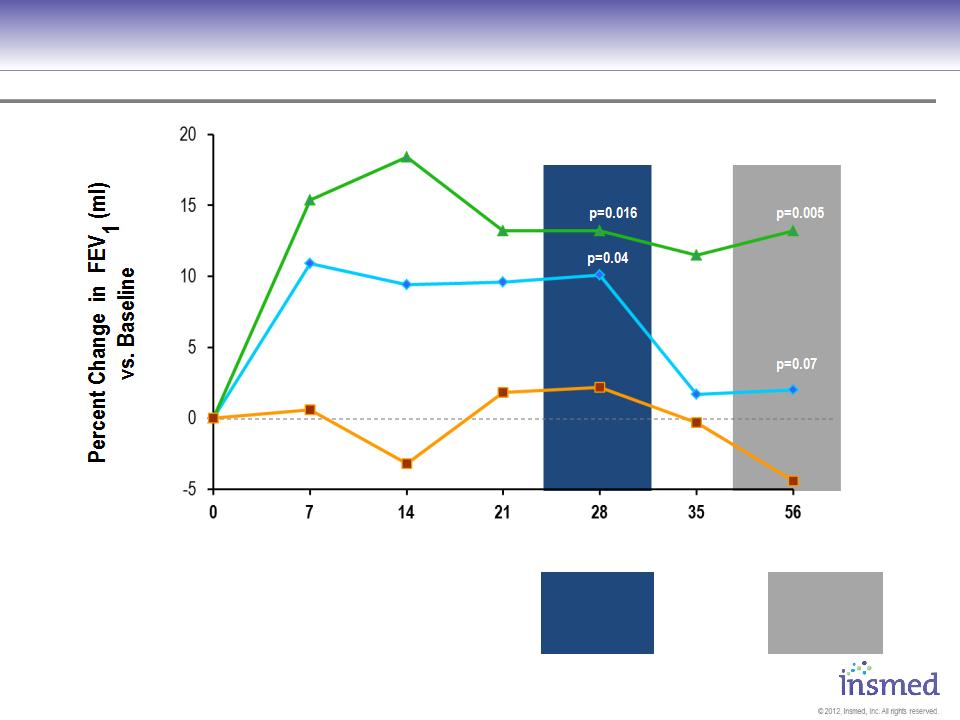
ARIKACE demonstrated statistically significant and clinically meaningful improvement in pulmonary
function throughout the 28-day treatment period that was sustained through the off-treatment period.
function throughout the 28-day treatment period that was sustained through the off-treatment period.
|
|
Day 7
|
Day 14
|
Day 21
|
Day 28
|
Day 56
|
|
ARIKACE 560 - Mean (Std Dev)
|
12.2% (14.9)
|
13.1% (19.7)
|
8.8% (15.8)
|
8.1% (16.1)
|
9.3% (20.3)
|
|
Placebo - Mean (Std Dev)
|
1.0% (10.6)
|
-1.9% (11.5)
|
0.7% (10.3)
|
1.1% (10.1)
|
-3.2% (11.9)
|
P = 0.003
P = 0.033
[N=ARIKACE/Placebo]
[N=36/36]
[N=36/35]
[N=33/36]
[N=32/35]
[N=34/35]
[N=34/34]
Placebo
- Mean (SD)
- Mean (SD)
ARIKACE 560
- Mean (SD)
- Mean (SD)
37
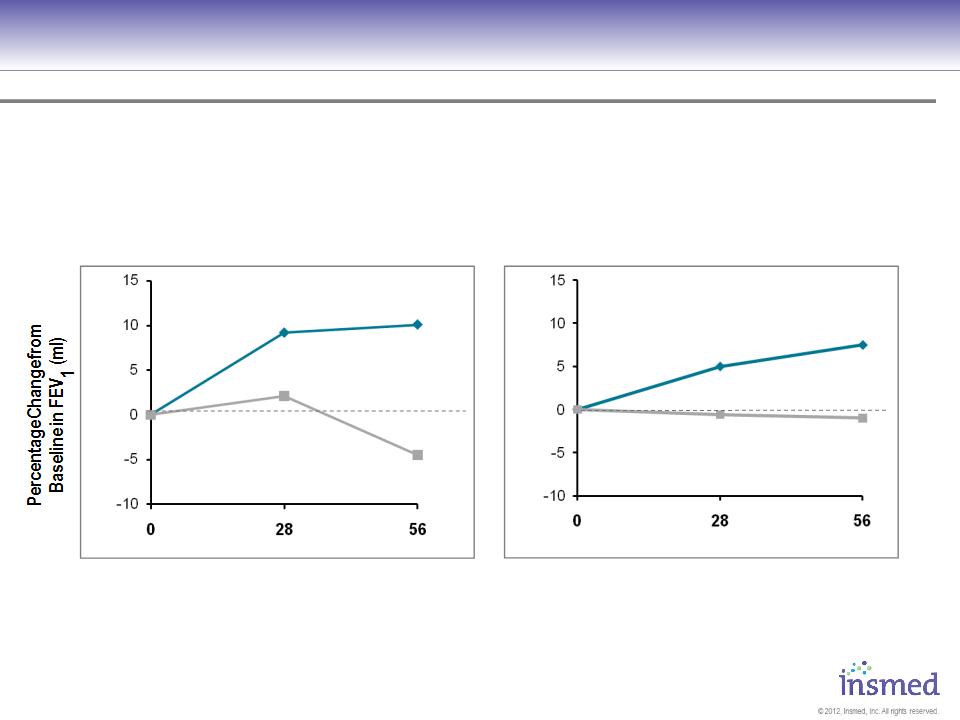
ARIKACE: Cystic Fibrosis
Phase 2 Pooled Results: Pulmonary Function
Phase 2 Pooled Results: Pulmonary Function
* Mean (SD)
ARIKACE 560
Placebo
Visit Day
Visit Day
|
ARIKACE *
|
9.2% (16.3)
|
10.1% (19.3)
|
|
Placebo *
|
2.1% (11.7)
|
-4.5% (13.1)
|
|
ARIKACE *
|
5% (16.2)
|
7.5% (23.5)
|
|
Placebo *
|
-0.6% (6.6)
|
-1% (9.6)
|
9/13
10/12
560mg Cohort
FEV1% >75%
560mg Cohort
FEV1% <=75%
ARIKACE 560
Placebo
25/22
24/22
ARIKACE produced comparable improvements in lung function for patients with baseline FEV1 %
predicted above and below 75%
predicted above and below 75%
38
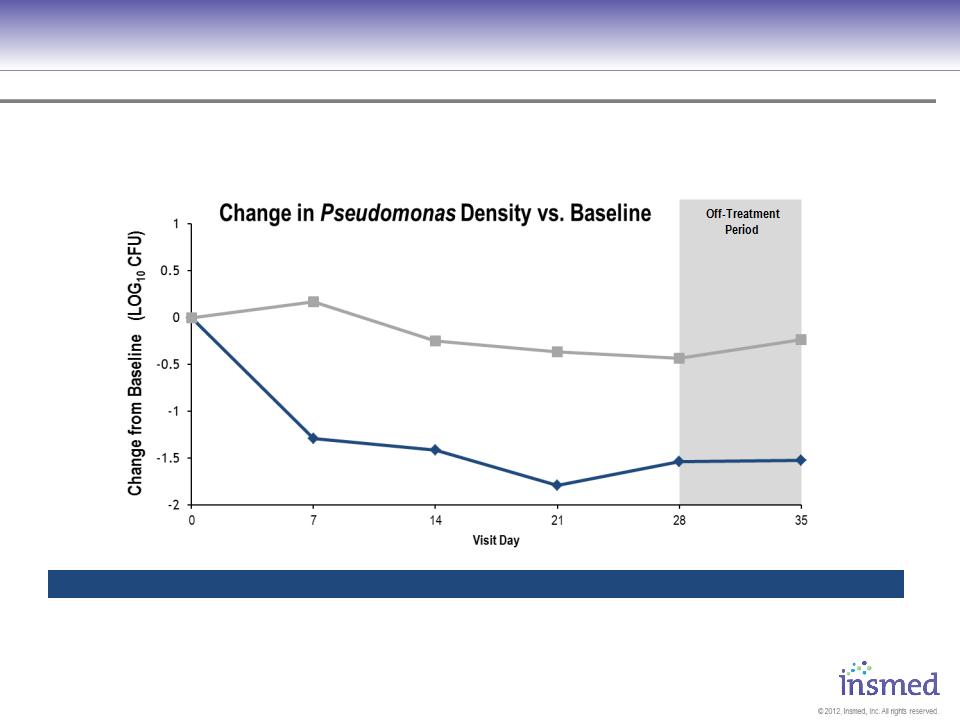
ARIKACE: Cystic Fibrosis
Phase 2 Pooled Results: Microbiology
Phase 2 Pooled Results: Microbiology
[N=36/36]
[N=34/32]
[N=31/32]
[N=31/30]
[N=32/28]
[N=31/29]
P = 0.021
P = 0.007
P = 0.007
P = 0.007
P = <0.001
ARIKACE demonstrated statistically significant reduction in the bacterial burden of CF patients, including a
reduction in the mucoid strains.
reduction in the mucoid strains.
|
|
Day 7
|
Day 14
|
Day 21
|
Day 28
|
Day 35
|
|
ARIKACE 560 - Mean (Std Dev)
|
-1.294 (1.881)
|
-1.413 (1.928)
|
-1.789 (2.512)
|
-1.536 (1.768)
|
-1.523 (2.707)
|
|
Placebo - Mean (Std Dev)
|
0.171 (1.447)
|
-0.248 (1.370)
|
-0.363 (1.183)
|
-0.434 (1.170)
|
-0.238 (1.098)
|
[N=ARIKACE/Placebo]
Placebo
- Mean (SD)
- Mean (SD)
ARIKACE 560
- Mean (SD)
- Mean (SD)
39
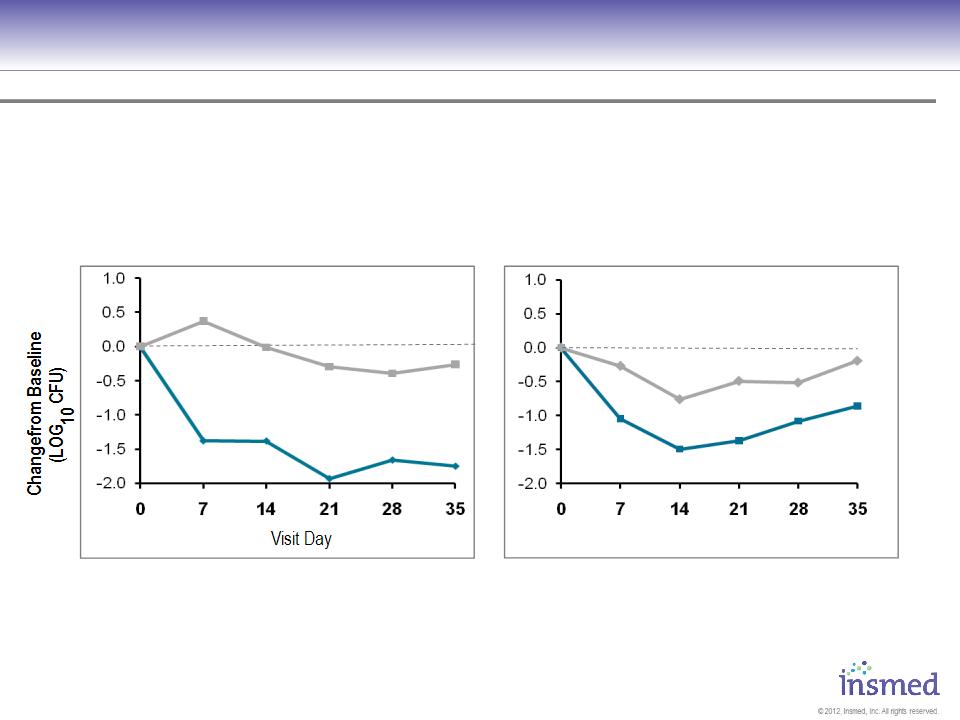
ARIKACE: Cystic Fibrosis
Phase 2 Pooled Results: Microbiology
Phase 2 Pooled Results: Microbiology
560mg Cohort
FEV1% >75%
560mg Cohort
FEV1% <75%
ARIKACE produced comparable reductions in Pseudomonas density for patients with baseline FEV1 %
predicted above and below 75%.
predicted above and below 75%.
|
ARIKACE *
|
-1.38
(1.9) |
-1.38
(2.08) |
-1.93
(2.67) |
-1.66
(1.77) |
-1.75
(2.71) |
|
Placebo *
|
0.37
(1.35) |
-0.01
(1.46) |
-0.3
(1.04) |
-0.4
(1.19) |
-0.26
(0.83) |
|
ARIKACE *
|
-1.05
(1.91) |
-1.49
(1.52) |
-1.37
(2.1) |
-1.08
(1.82) |
-0.86
(2.76) |
|
Placebo *
|
-0.27
(1.63) |
-0.76
(1.04) |
-0.49
(1.48) |
-0.52
(1.18) |
-0.19
(1.54) |
* Mean (SD)
ARIKACE 560
Placebo
Visit Day
9/10
8/10
8/10
8/10
7/9
25/22
23/22
23/20
25/19
23/19
Placebo
ARIKACE 560
40
ARIKACE: Cystic Fibrosis
Phase 2 Pooled Results: Safety Summary
Phase 2 Pooled Results: Safety Summary
Overall, ARIKACE 560 mg, administered once daily for 28 days is well-tolerated
§ No unexpected AEs were observed
§ There were no appreciable changes in acute tolerability
§ There was improvement in oxygen saturation
§ No significant differences between groups in overall rates of AEs
§ AEs were generally consistent with underlying CF disease although a trend towards
mild to moderate dysphonia in the higher dose ARIKACE group
mild to moderate dysphonia in the higher dose ARIKACE group
§ In summary, nebulized ARIKACE is well-tolerated and demonstrates adverse effects
that are consistent with those expected in a population of CF patients receiving
inhalation medicines
that are consistent with those expected in a population of CF patients receiving
inhalation medicines
ARIKACE 560mg dosed once daily for 28 days is safe and well tolerated in CF patients with chronic
Pseudomonas infections.
Pseudomonas infections.
41
In Vitro Bactericidal Effect of ARIKACE
On Mycobacterium Avium Complex1
On Mycobacterium Avium Complex1
Summary
§ARIKACE at 10 µg/ml and 4 µg/ml is active against intracellular Mycobacterium avium
(MAC). Liposome by itself did not show any anti-mycobacterial activity.
(MAC). Liposome by itself did not show any anti-mycobacterial activity.
§ARIKACE at equivalent concentration to free amikacin (10 µg/ml) had greater
activity against MAC.
activity against MAC.
§ARIKACE anti-intracellular MAC activity, compared to historic data obtained with
clarithromycin, achieves greater decrease in the levels of intracellular bacteria.
clarithromycin, achieves greater decrease in the levels of intracellular bacteria.
§ARIKACE did not show any toxicity towards macrophages, both infected and
uninfected.
uninfected.
§This short-term in vitro study was performed to test the hypothesis that ARIKACE
exhibits bactericidal activity against intracellular MAC, and this has been satisfied.
Therefore, ARIKACE may be an effective therapy against pulmonary infections caused
by MAC which persist and grow in alveolar macrophages.
exhibits bactericidal activity against intracellular MAC, and this has been satisfied.
Therefore, ARIKACE may be an effective therapy against pulmonary infections caused
by MAC which persist and grow in alveolar macrophages.
1 Study conducted by L. E. Bermudez at Oregon State University (data on file). (2010)
