Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PROTALEX INC | v327634_8k.htm |

Edward Bernton MD, 1 Eduard Krantz MD, 2 William Gannon MD 1 1 Protalex Inc., Summit, NJ, USA 2 Parexel Clinical Pharmacology, Bloemfontein, South Africa A Phase 1, Randomized, Double - blind, Placebo - controlled Multiple - dose Study of Intravenous Staphylococcal Protein A in Patients with Active Rheumatoid Arthritis on Methotrexate: Pharmacokinetics, Safety and Tolerability

FORWARD LOOKING STATEMENTS INFORMATION Various statements made in this presentation are forward - looking within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward - looking statements include those which express plan, anticipation, intent, contingency, goals, targets or future development and/or otherwise are not statements of historical fact. We have based these forward - looking statements on our current expectations and projections about future events and they are subject to risks and uncertainties known and unknown which could cause actual results and developments to differ materially from those expressed or implied in such statements. In some cases, you can identify forward - looking statements by terminology such as “may”, “will”, “should”, “could”, “would”, “expect”, “plan”, “anticipate”, “believe”, “estimate”, “target”, “goal”, “continue”, or the negative of such terms or other similar expressions. Factors that might cause or contribute to differences include, but are not limited to, those discussed in Item 1A. Risk Factors of our Annual Report on Form 10 - K for year ended May 31, 2012 and discussed in our other Securities and Exchange Commission (“SEC”) filings, which discloses all material factors known to us that we believe could cause actual results to differ materially from those expressed or implied by forward - looking statements.

Disclosures Study funded by Protalex Inc., Summit, NJ Drs Bernton and Gannon serve as paid consultants to Protalex Inc. Dr Krantz, country Principal Investigator for the study, is an employee of Parexel Clinical Pharmacology, contracted by Protalex Inc. to conduct the study
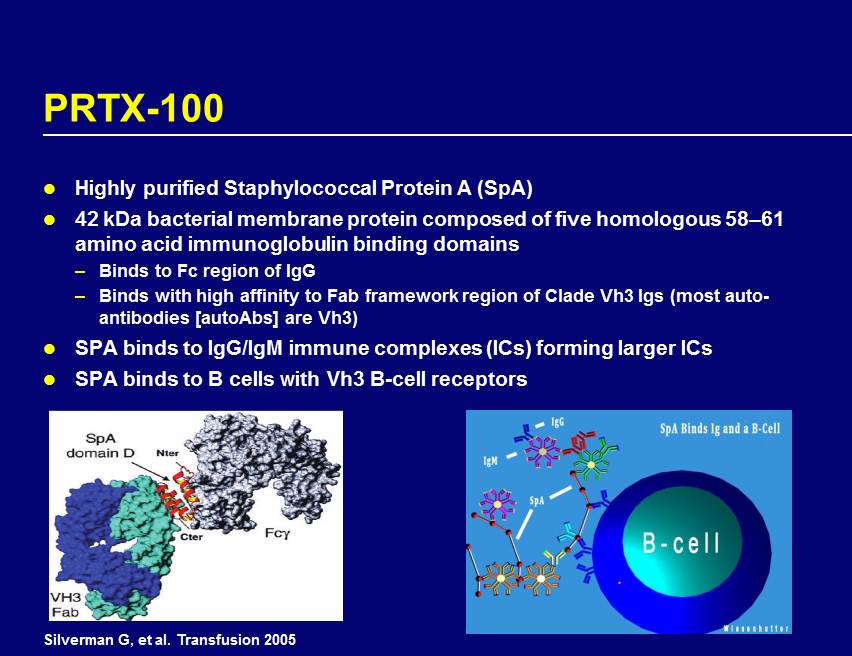
PRTX - 100 Highly purified Staphylococcal Protein A (SpA) 42 kDa bacterial membrane protein composed of five homologous 58 – 61 amino acid immunoglobulin binding domains – Binds to Fc region of IgG – Binds with high affinity to Fab framework region of Clade Vh3 Igs (most auto - antibodies [autoAbs] are Vh3) SPA binds to IgG/IgM immune complexes (ICs) forming larger ICs SPA binds to B cells with Vh3 B - cell receptors Silverman G, et al. Transfusion 2005 Figure 3. Following infusion into the mouse, SpA rapidly binds to immunoglobulin and B-Cells as depicted. Initially rather large doses of SpA were used in these mouse models, but subsequent studies used doses of < 2 ug, which are doses of SpA that are comparable to those being used in the upcoming Protalex studies treating rheumatoid arthritis in humans (PRTX-100 IB). PATHOGNESIS OF RHEUMATOID ARTHRITIS: One of the most widely accepted current theories for the pathogenesis of rheumatoid arthritis (RA) has to do with the spontaneous production and subsequent dissemination of small pathogenic circulating immune complexes (spCIC). The steps involved in the formation of these spCIC are as follows: Step #1. A B-Cell, by an unlucky chance event, makes an antibody in high affinity to the Fc portion of another antibody, i.e. a rheumatoid factor (RF) is produced as shown in Figure 4 [22]. This chance event most likely occurs in the bone marrow, peripheral lymph nodes, liver, or spleen. Some of the immune complexes formed will also consist of an antibody directed to another antibody of the same clone, i.e. these two antibodies will be structurally identical antibodies as shown in Figure 5 for an IgG rheumatoid factor. Step #2. These pathogenic immune complexes (spCIC) are very small, enter the vasculature, and from there readily enter the extravascular space where they can then penetrate into other areas, such as the joint space, pericardium etc.

PRTX - 100 SpA immuno - adsorption column approved in 1999 as medical device for treatment of refractory RA – Responses after 6 – 12 weekly treatments – Often maintained for ≥10 months MOA not adsorption of autoAbs but exposure to leached SpA – 400 – 1200 μ g per Rx in returned plasma ≈5 – 15 μ g/kg dose Protalex Phase I studies characterized safety and PK of single IV doses of SpA up to 0.45 µg/kg in healthy volunteers (HVs)
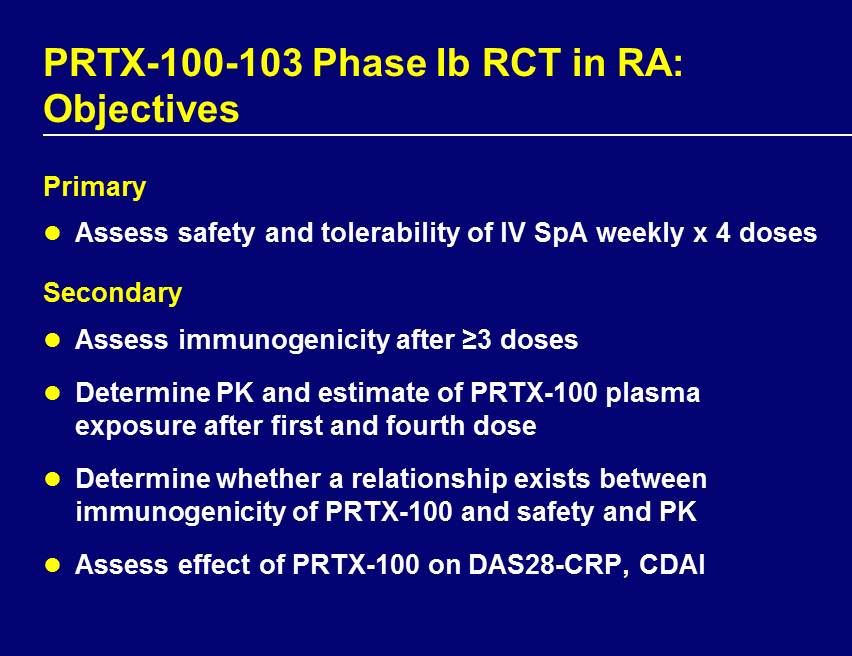
PRTX - 100 - 103 Phase Ib RCT in RA: Objectives Primary Assess safety and tolerability of IV SpA weekly x 4 doses Secondary Assess immunogenicity after ≥3 doses Determine PK and estimate of PRTX - 100 plasma exposure after first and fourth dose Determine whether a relationship exists between immunogenicity of PRTX - 100 and safety and PK Assess effect of PRTX - 100 on DAS28 - CRP, CDAI
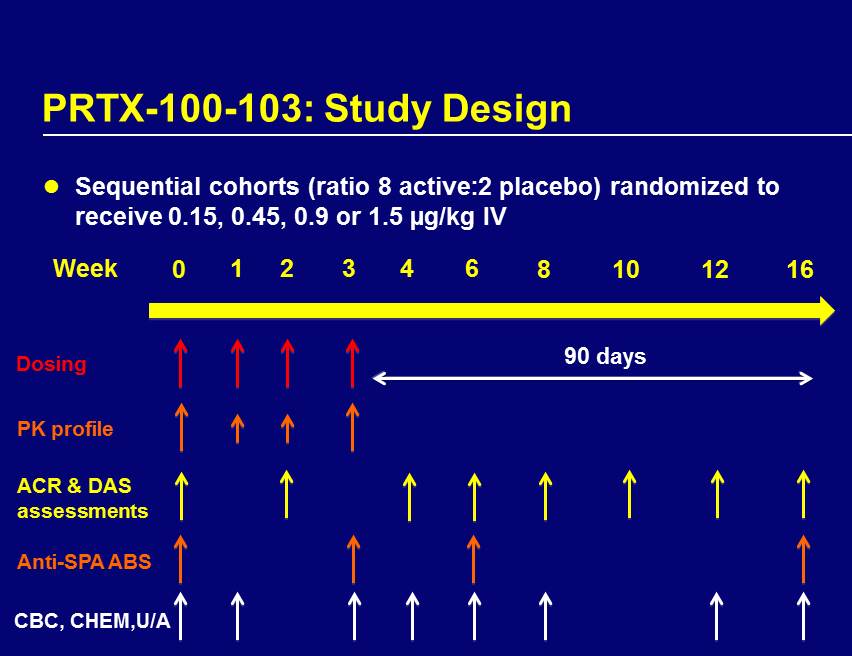
PRTX - 100 - 103: Study Design Sequential cohorts (ratio 8 active:2 placebo) randomized to receive 0.15, 0.45, 0.9 or 1.5 µg/kg IV Week 0 1 2 3 6 4 10 8 12 16 Dosing ACR & DAS assessments Anti - SPA ABS 90 days PK profile CBC, CHEM,U/A
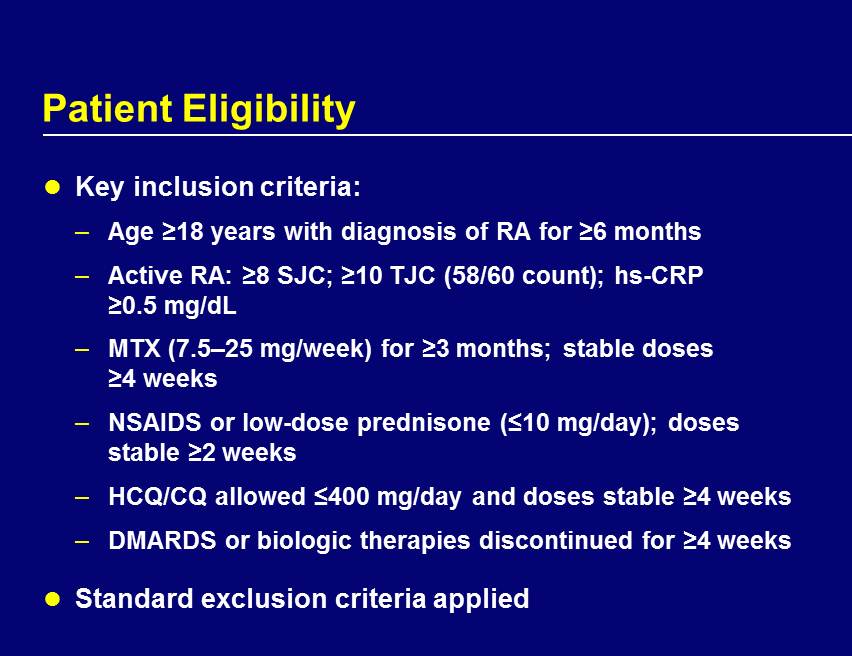
Patient Eligibility Key inclusion criteria: – Age ≥18 years with diagnosis of RA for ≥6 months – Active RA: ≥8 SJC; ≥10 TJC (58/60 count); hs - CRP ≥0.5 mg/dL – MTX (7.5 – 25 mg/week) for ≥3 months; stable doses ≥4 weeks – NSAIDS or low - dose prednisone (≤10 mg/day); doses stable ≥2 weeks – HCQ/CQ allowed ≤400 mg/day and doses stable ≥4 weeks – DMARDS or biologic therapies discontinued for ≥4 weeks Standard exclusion criteria applied
Disease Activity Endpoints Primary outcome measure Number (%) of patients attaining DAS28 - CRP <3.2 = low disease activity by Week 6 Other outcome measures Absolute and percentage change from baseline of DAS28 - CRP across study timepoints (through 16 weeks) Change from baseline in Clinical Disease Activity Index (CDAI)
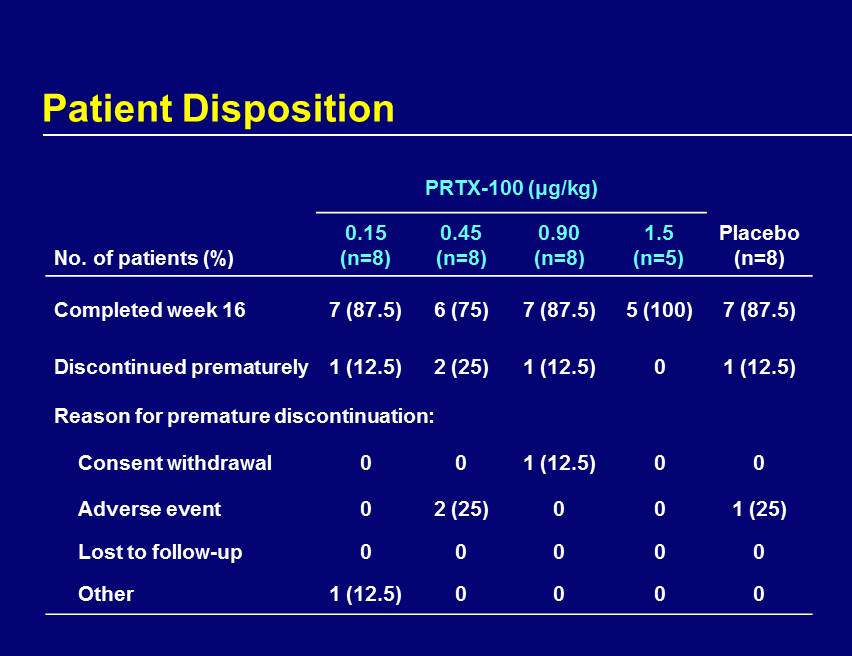
Patient Disposition PRTX - 100 (μg/kg) No. of patients (%) 0.15 (n=8) 0.45 (n=8) 0.90 (n=8) 1.5 (n=5) Placebo (n=8) Completed week 16 7 (87.5) 6 (75) 7 (87.5) 5 (100) 7 (87.5) Discontinued prematurely 1 (12.5) 2 (25) 1 (12.5) 0 1 (12.5) Reason for premature discontinuation: Consent withdrawal 0 0 1 (12.5) 0 0 Adverse event 0 2 (25) 0 0 1 (25) Lost to follow - up 0 0 0 0 0 Other 1 (12.5) 0 0 0 0
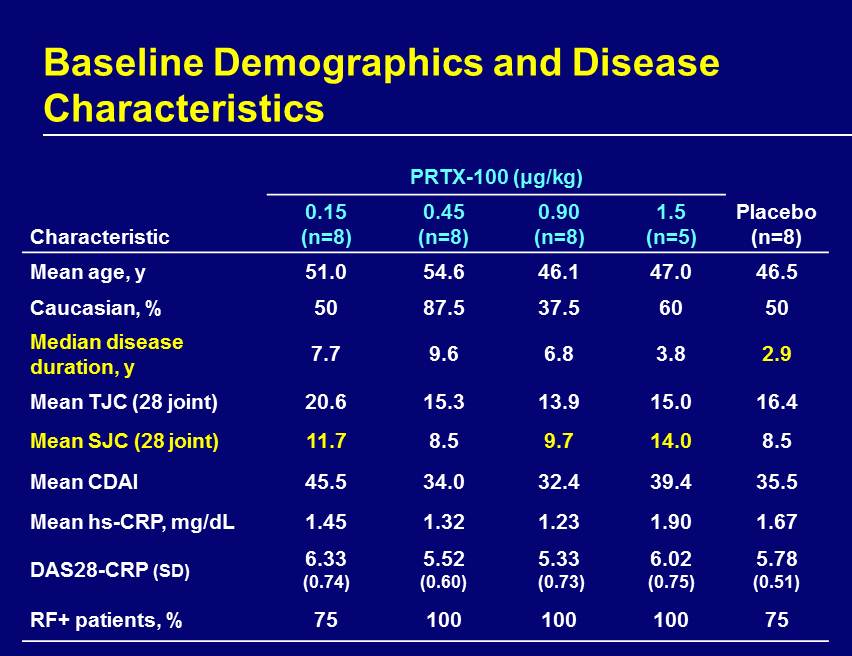
Baseline Demographics and Disease Characteristics PRTX - 100 (μg/kg) Characteristic 0.15 (n=8) 0.45 (n=8) 0.90 (n=8) 1.5 (n=5) Placebo (n=8) Mean age, y 51.0 54.6 46.1 47.0 46.5 Caucasian, % 50 87.5 37.5 60 50 Median disease duration, y 7.7 9.6 6.8 3.8 2.9 Mean TJC (28 joint) 20.6 15.3 13.9 15.0 16.4 Mean SJC (28 joint) 11.7 8.5 9.7 14.0 8.5 Mean CDAI 45.5 34.0 32.4 39.4 35.5 Mean hs - CRP, mg/dL 1.45 1.32 1.23 1.90 1.67 DAS28 - CRP (SD) 6.33 (0.74) 5.52 (0.60) 5.33 (0.73) 6.02 (0.75) 5.78 (0.51) RF+ patients, % 75 100 100 100 75

Safety: Serious Adverse Events SAEs: – 0.45 μ g/kg – fracture of L humerus – 0.45 μ g/kg – acute gastritis and colitis, resolved after discontinuation of meloxicam and treatment with PPI and 5 - ASA , no acute colonoscopy, no biopsy patient withdrew from study during follow - up phase – 0.9 μ g/kg – diverticulitis or infectious colitis, treated with antibiotics, laxatives – Placebo – worsening of RA, requiring hospitalization (onset 6 weeks after last dose ) patient withdrew from study during follow - up phase No SAEs considered related to study drug No SAEs in highest dose group (1.50 μg /kg )
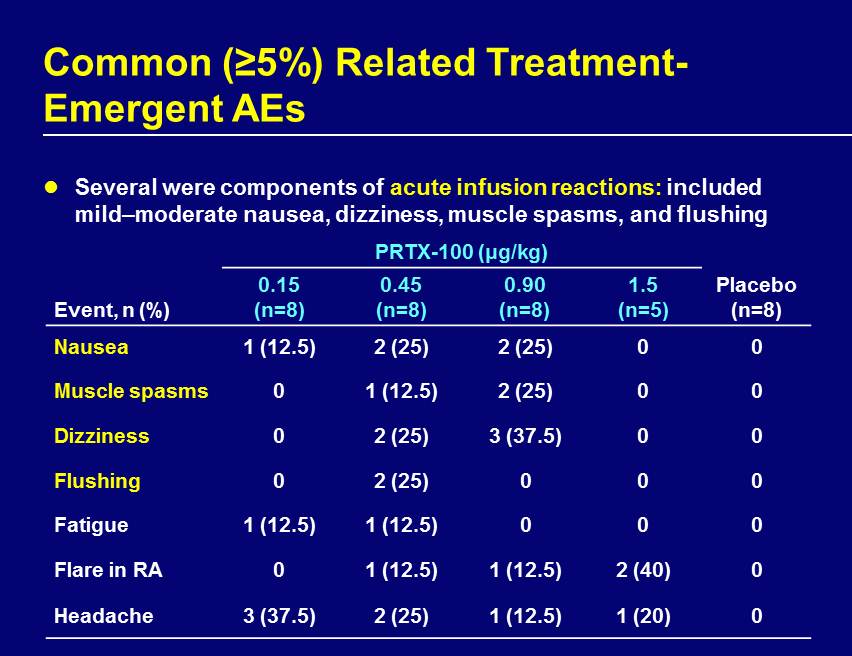
Common (≥5%) Related Treatment - Emergent AEs Several were components of acute infusion reactions: included mild – moderate nausea, dizziness, muscle spasms, and flushing PRTX - 100 (μg/kg) Event, n (%) 0.15 (n=8) 0.45 (n=8) 0.90 (n=8) 1.5 (n=5) Placebo (n=8) Nausea 1 (12.5) 2 (25) 2 (25) 0 0 Muscle spasms 0 1 (12.5) 2 (25) 0 0 Dizziness 0 2 (25) 3 (37.5) 0 0 Flushing 0 2 (25) 0 0 0 Fatigue 1 (12.5) 1 (12.5) 0 0 0 Flare in RA 0 1 (12.5) 1 (12.5) 2 (40) 0 Headache 3 (37.5) 2 (25) 1 (12.5) 1 (20) 0
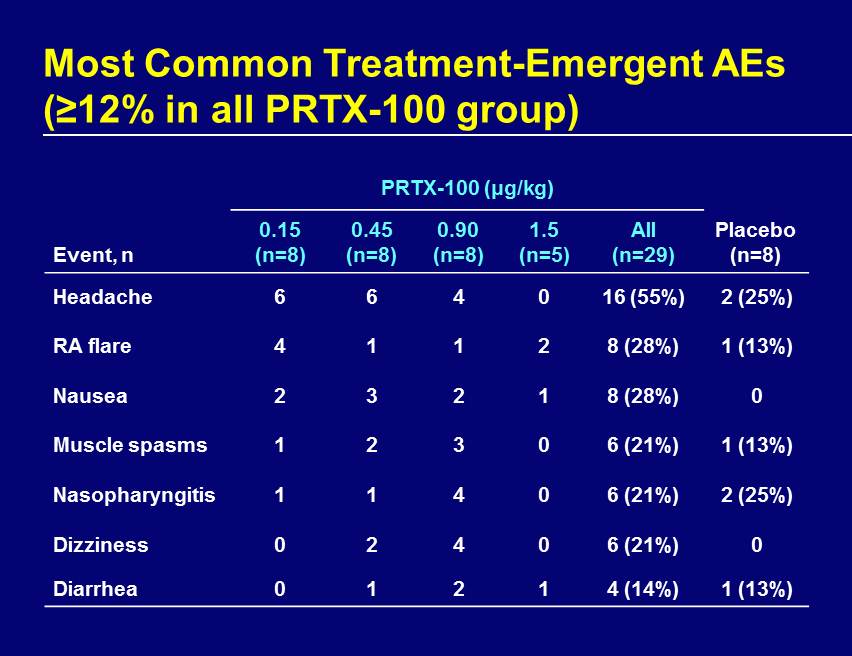
Most Common Treatment - Emergent AEs (≥12% in all PRTX - 100 group) PRTX - 100 (μg/kg) Event, n 0.15 (n=8) 0.45 (n=8) 0.90 (n=8) 1.5 (n=5) All (n=29) Placebo (n=8) Headache 6 6 4 0 16 (55%) 2 (25%) RA flare 4 1 1 2 8 (28%) 1 (13%) Nausea 2 3 2 1 8 (28%) 0 Muscle spasms 1 2 3 0 6 (21%) 1 (13%) Nasopharyngitis 1 1 4 0 6 (21%) 2 (25%) Dizziness 0 2 4 0 6 (21%) 0 Diarrhea 0 1 2 1 4 (14%) 1 (13%)

Laboratory AEs Laboratory findings: – No trends seen in changes from baseline observed in any laboratory parameters in any treatment group – Individual clinically significant lab values included anemia, hyponatremia, hypokalemia, and lymphopenia not felt to be related to PRTX - 100
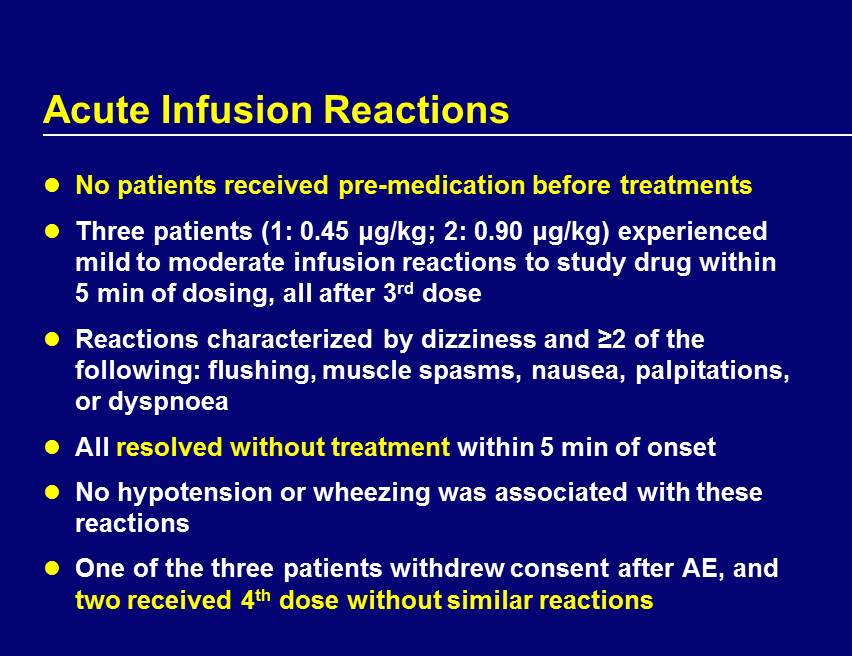
Acute Infusion Reactions No patients received pre - medication before treatments Three patients (1: 0.45 μg/kg; 2: 0.90 μg/kg) experienced mild to moderate infusion reactions to study drug within 5 min of dosing, all after 3 rd dose Reactions characterized by dizziness and ≥2 of the following: flushing, muscle spasms, nausea, palpitations, or dyspnoea All resolved without treatment within 5 min of onset No hypotension or wheezing was associated with these reactions One of the three patients withdrew consent after AE, and two received 4 th dose without similar reactions
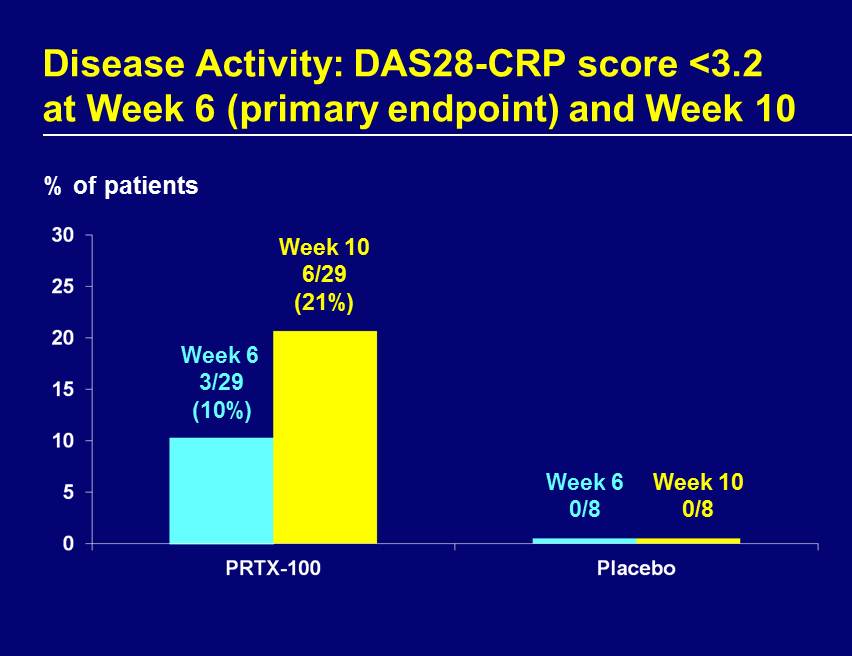
Disease Activity: DAS28 - CRP score <3.2 at Week 6 (primary endpoint) and Week 10 % of patients Week 6 3/29 (10%) Week 6 0/8 Week 10 6/29 (21%) Week 10 0/8
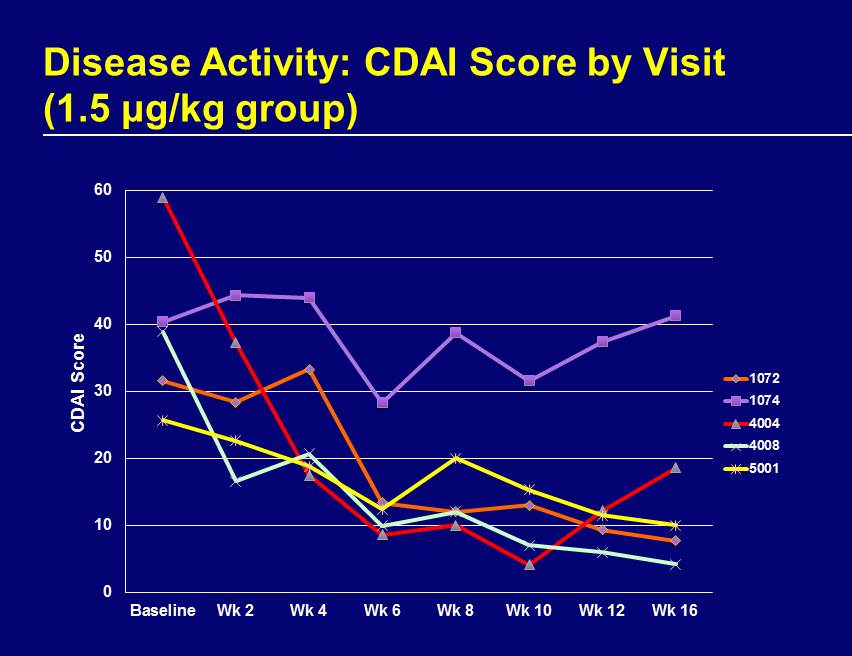
0 10 20 30 40 50 60 Baseline Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Wk 16 CDAI Score 1072 1074 4004 4008 5001 Disease Activity: CDAI Score by Visit (1.5 μ g/kg group)
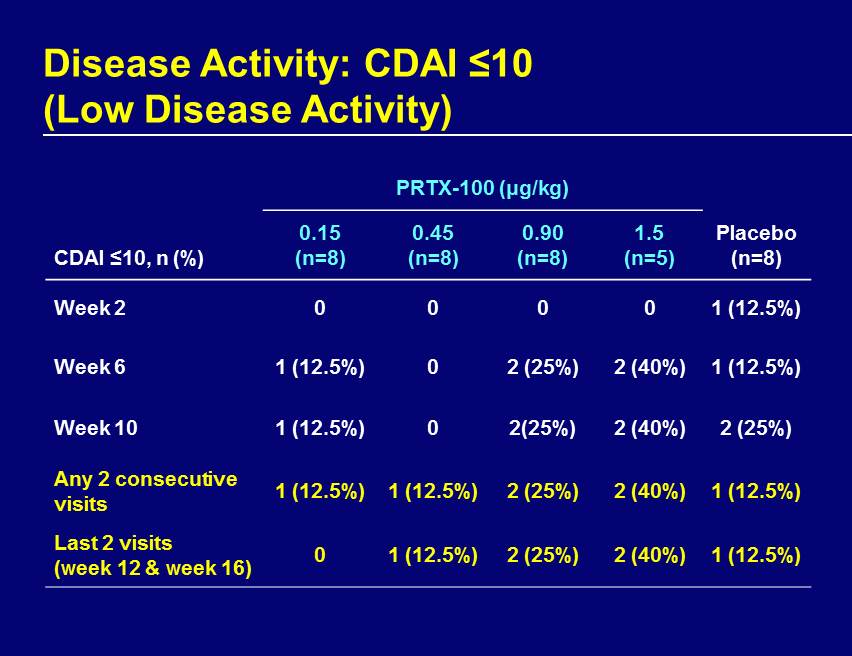
Disease Activity: CDAI ≤10 (Low Disease Activity) PRTX - 100 (μg/kg) CDAI ≤10, n (%) 0.15 (n=8) 0.45 (n=8) 0.90 (n=8) 1.5 (n=5) Placebo (n=8) Week 2 0 0 0 0 1 (12.5%) Week 6 1 (12.5%) 0 2 (25%) 2 (40%) 1 (12.5%) Week 10 1 (12.5%) 0 2(25%) 2 (40%) 2 (25%) Any 2 consecutive visits 1 (12.5%) 1 (12.5%) 2 (25%) 2 (40%) 1 (12.5%) Last 2 visits (week 12 & week 16) 0 1 (12.5%) 2 (25%) 2 (40%) 1 (12.5%)
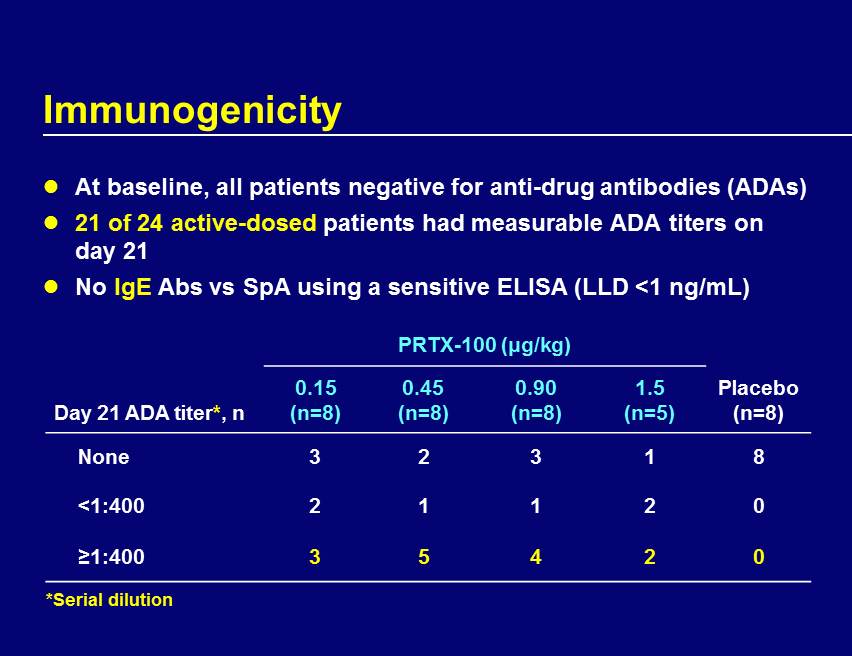
Immunogenicity At baseline, all patients negative for anti - drug antibodies (ADAs) 21 of 24 active - dosed patients had measurable ADA titers on day 21 No IgE Abs vs SpA using a sensitive ELISA (LLD <1 ng/mL) PRTX - 100 (μg/kg) Day 21 ADA titer * , n 0.15 (n=8) 0.45 (n=8) 0.90 (n=8) 1.5 (n=5) Placebo (n=8) None 3 2 3 1 8 <1:400 2 1 1 2 0 ≥1:400 3 5 4 2 0 *Serial dilution
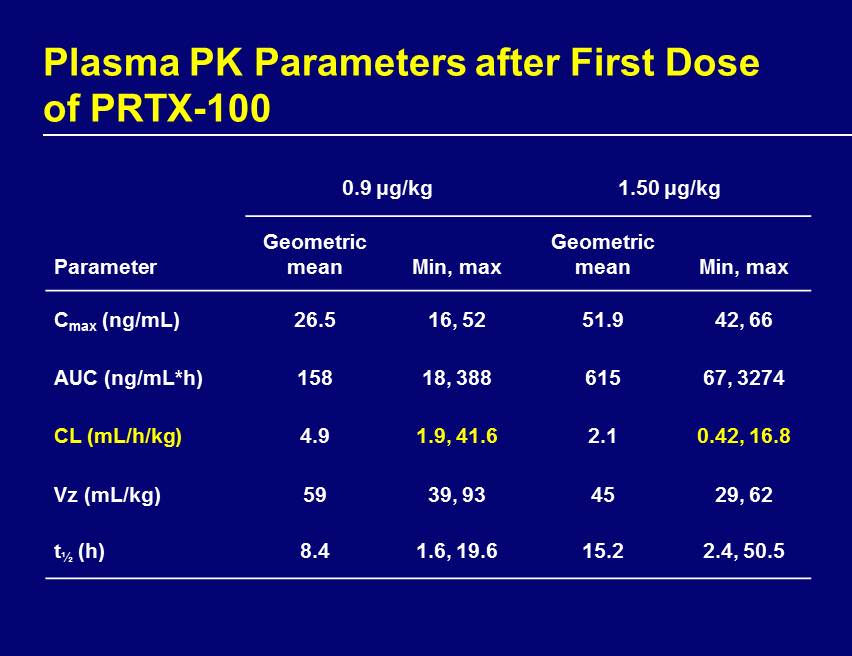
Plasma PK Parameters after First Dose of PRTX - 100 0.9 μg/kg 1.50 μg/kg Parameter Geometric mean Min, max Geometric mean Min, max C max (ng/mL) 26.5 16, 52 51.9 42, 66 AUC (ng/mL*h) 158 18, 388 615 67, 3274 CL (mL/h/kg) 4.9 1.9, 41.6 2.1 0.42, 16.8 Vz (mL/kg) 59 39, 93 45 29, 62 t ½ (h) 8.4 1.6, 19.6 15.2 2.4, 50.5
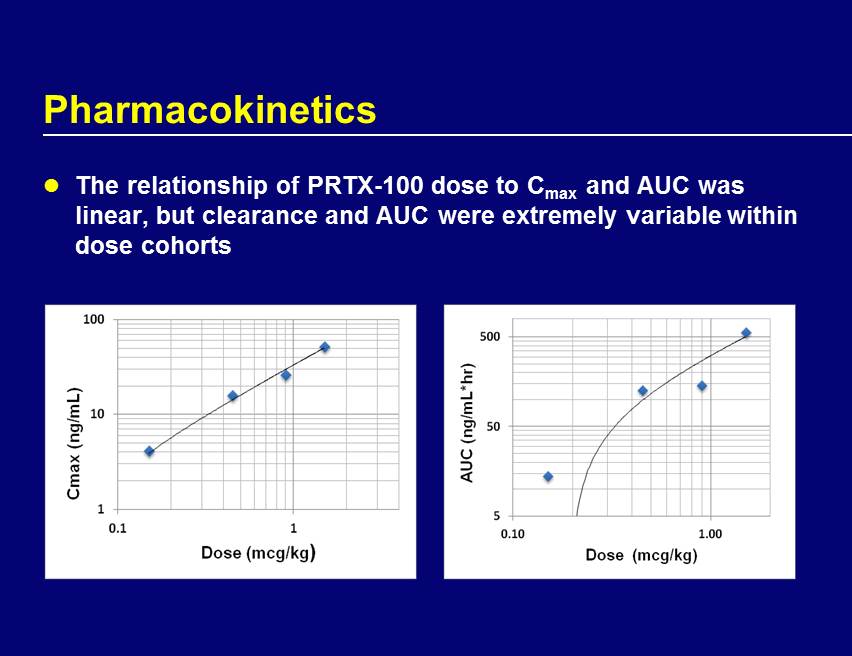
Pharmacokinetics The relationship of PRTX - 100 dose to C max and AUC was linear, but clearance and AUC were extremely variable within dose cohorts
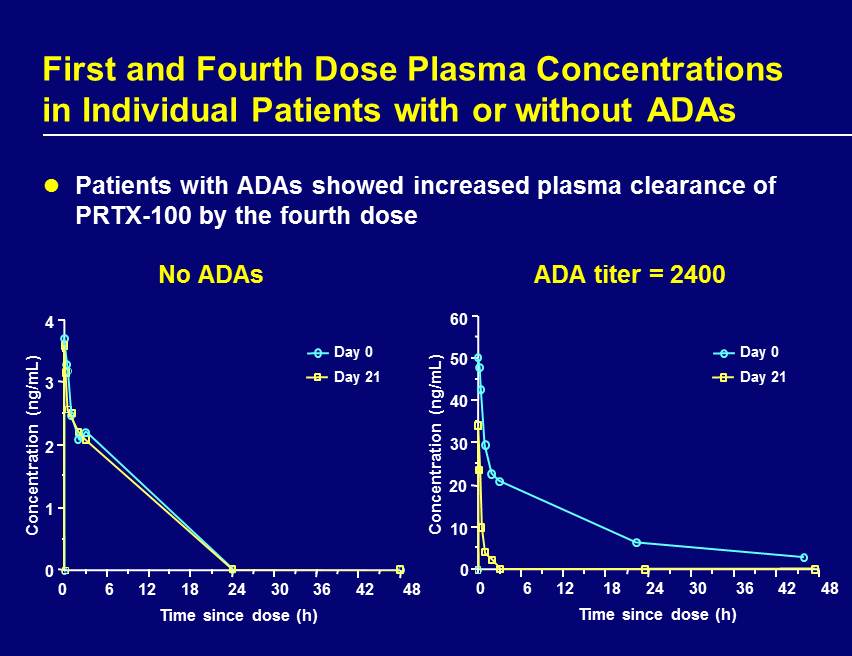
Patients with ADAs showed increased plasma clearance of PRTX - 100 by the fourth dose First and Fourth Dose Plasma Concentrations in Individual Patients with or without ADAs No ADAs ADA titer = 2400 0 6 12 18 24 30 36 42 48 0 4 3 2 1 Time since dose (h) Concentration (ng/mL) Day 0 Day 21 Concentration (ng/mL) Time since dose (h) 0 60 50 40 10 30 20 0 6 12 18 24 30 36 42 48 Day 0 Day 21
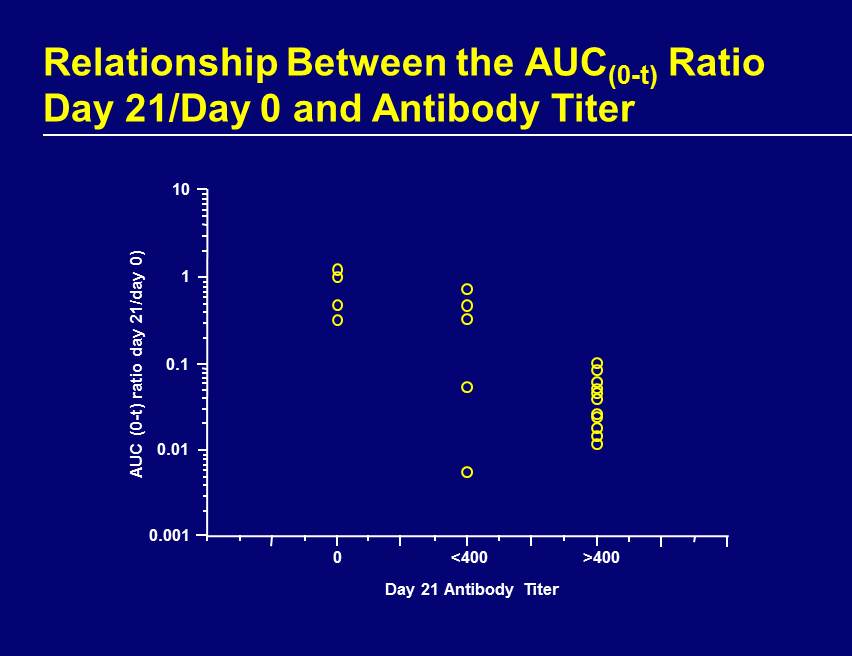
Relationship Between the AUC (0 - t) Ratio Day 21/Day 0 and Antibody Titer Day 21 Antibody Titer AUC (0 - t) ratio day 21/day 0) 0.001 10 0.01 1 0.1 0 <400 >400
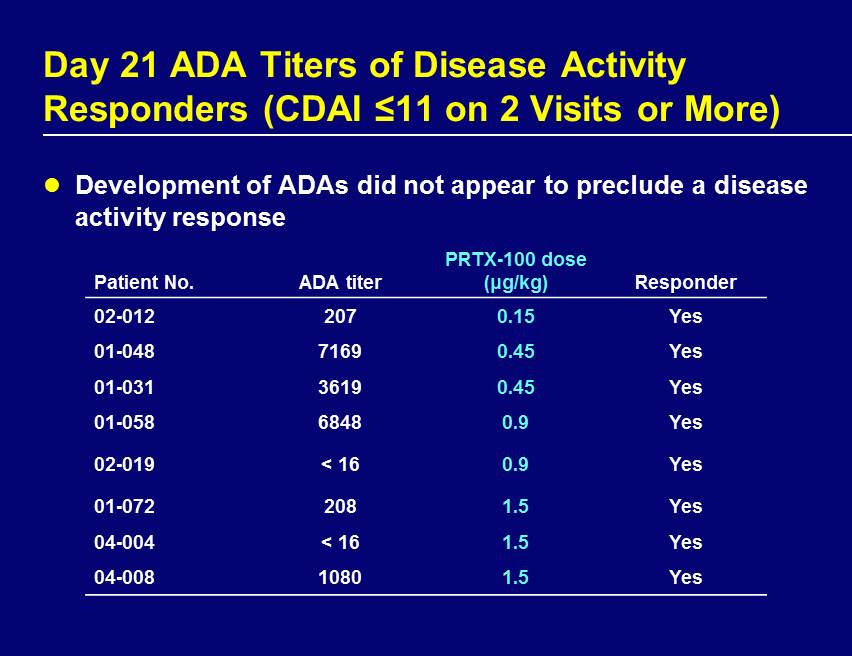
Day 21 ADA Titers of Disease Activity Responders (CDAI ≤11 on 2 Visits or More) Development of ADAs did not appear to preclude a disease activity response Patient No. ADA titer PRTX - 100 dose ( μ g/kg) Responder 02 - 012 207 0.15 Yes 01 - 048 7169 0.45 Yes 01 - 031 3619 0.45 Yes 01 - 058 6848 0.9 Yes 02 - 019 < 16 0.9 Yes 01 - 072 208 1.5 Yes 04 - 004 < 16 1.5 Yes 04 - 008 1080 1.5 Yes
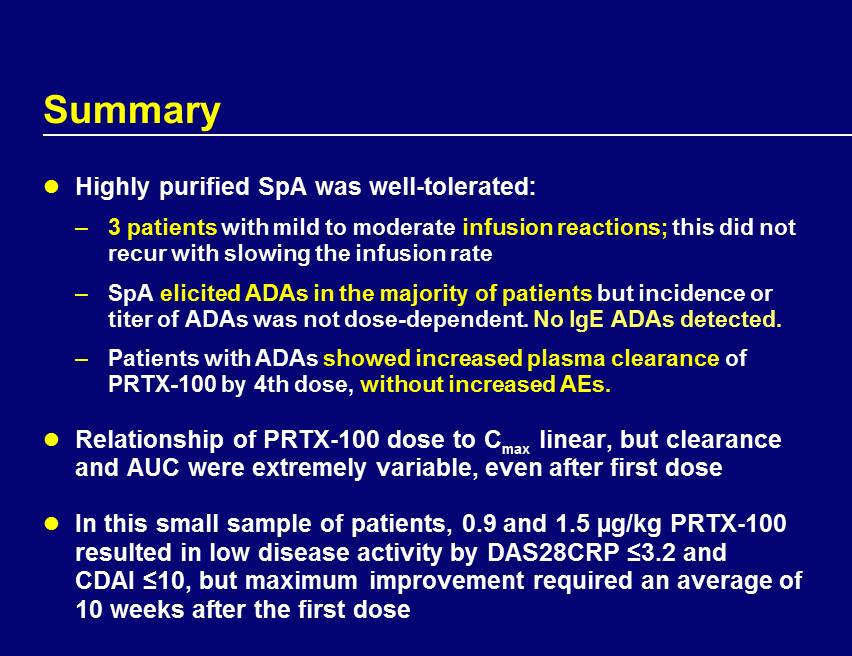
Summary Highly purified SpA was well - tolerated: – 3 patients with mild to moderate infusion reactions; this did not recur with slowing the infusion rate – SpA elicited ADAs in the majority of patients but incidence or titer of ADAs was not dose - dependent. No IgE ADAs detected. – Patients with ADAs showed increased plasma clearance of PRTX - 100 by 4th dose, without increased AEs. Relationship of PRTX - 100 dose to C max linear, but clearance and AUC were extremely variable, even after first dose In this small sample of patients, 0.9 and 1.5 µg/kg PRTX - 100 resulted in low disease activity by DAS28CRP ≤3.2 and CDAI ≤10, but maximum improvement required an average of 10 weeks after the first dose

Conclusions This pilot study demonstrated acceptable tolerability and toxicity of four weekly IV doses of PRTX - 100 up to 1.5 µg/kg There is a suggestion that PRTX - 100 has an effect on disease activity, even in patients with ADAs, which would require confirmation in a larger study. These findings support further studies in patients with active RA, using PRTX - 100 at 1.5 µg/kg and higher

Investigators Dr Eduard Krantz: Parexel International, Bloemfontein, South Africa Dr Dawie Kruger: Parexel International, George, South Africa Dr Francios Malan, Dr Reinard McPherson: Parexel International, Port Elizabeth, South Africa Dr Margare du Toit: Synexus Clinical Research Centre, Pretoria, South Africa Dr Haylene Nell: Karl Bremer Hospital, Bellville, South Africa
