Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Anthera Pharmaceuticals Inc | a12-26912_1ex99d2.htm |
| 8-K - 8-K - Anthera Pharmaceuticals Inc | a12-26912_18k.htm |
Exhibit 99.1
Blisibimod, an Inhibitor of B cell Activating Factor, in Patients with Moderate-to-Severe Systemic Lupus Erythematosus
Richard A Furie, MD(1); Morton A Scheinberg, MD(2); Gustavo Leon, MD(3); Edgar B Ramiterre, MD(4); Matthew Thomas, MD (5); Alvina Chu, MD(6); Renee S Martin, PhD(6); Michelle A Petri, MD(7); for the PEARL-SC Study.
(1)North Shore—Long Island Jewish Health System, Lake Success, New York, USA, (2)Rheumatology Hospital Abreu Sodre Pesquisa Clínica, São Paulo, Brazil, (3)Rheumatology Gynecology & Reproduction Institute, Lima, PERU, (4)Brokenshire Memorial Hospital, Davao City, Philippines, (5)Health and Research Centre, Trivandrum, Kerala India, (6)Anthera Pharmaceuticals, Hayward, California USA, (7)Hopkins University School of Medicine, Baltimore, Maryland, USA
Introduction
Bliisibimod is a potent and selective inhibitor of soluble and membrane-bound forms of BAFF (KD = 1 pM, Hsu 2012). It is a peptibody dimer, comprised of 4 high-affinity binding domains fused to a fully-human IgG1 Fc domain. As with other Fc-continaing molecules, it has a long serum half life of ~ 10 days (Stohl 2008).
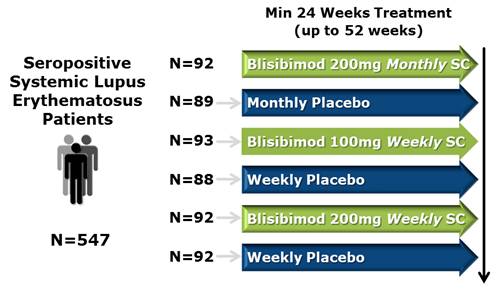
The PEARl-SC study evaluated the efficacy and safety of subcutaneously-administered blisibimod on top of standard-of-care medication in patients with moderate-to-severe, seropositive SLE.
Methods and Materials
Key Inclusion Criteria
· Fulfill at least 4 of the criteria for SLE defined by the ACR.
· Receiving stable SLE treatment.
· Seropositive for ANA or anti-dsDNA antibodies.
Key Exclusion Criteria
· Severe vasculitis, CNS lupus, lupus nephritis.
· Anemia, neutropenia, or thrombocytopenia.
· Malignancy within past 5 years
· Exposure to B cell depleting therapy in the past 18 months.
The primary efficacy endpoint compared responder rates in the pooled active and pooled placebo groups using the SLE Responder Index (SRI-5) at week 24, defined as:
· >5 point improvement in the SELENA-SLEDAI AND
· No new BILAG 1A or 2B organ domain flares AND
· No worsening in Physician’s Global Assessment (PGA) (< 0.3 increase)
· No new or increased doses of steroids or immunosuppressives beyond protocol-mandated limits.
Secondary end point analyses included:
· SRI response using more stringent forms of the SRI requiring improvements in SELENA-SLEDAI score of >6, >7, >8 and >9
· SRI response in subgroups of subjects with more severe disease, SELENA-SLEDAI >10 and receiving corticosteroids
· Time to, and incidence of SLE flare
· Changes in SLE biomarkers
· Safety and tolerability
Subjects were invited to participate in an open-label extension study after completion of this trial
Results
Summary of Demographics
|
Demographics |
|
|
|
|
Age (years) |
|
37.5 |
|
|
Weight (kg) |
|
65.6 |
|
|
Gender, % |
|
|
|
|
Female |
|
94.0 |
|
|
Male |
|
6.0 |
|
|
Race, % |
|
|
|
|
White |
|
25.0 |
|
|
Asian |
|
19.7 |
|
|
Black or African |
|
8.4 |
|
|
Other |
|
46.8 |
|
|
Region, % |
|
|
|
|
Asia/Pacific |
|
19.0 |
|
|
Latin America |
|
71.1 |
|
|
North America |
|
9.9 |
|
|
SLE Duration (years) |
|
6.1 |
|
|
Baseline Disease Characteristics |
|
|
|
|
SELENA-SLEDAI (mean, SD) |
|
10.1 (3.6) |
|
|
BILAG 1A or 2B, % |
|
50.3 |
|
|
PGA (mean score, SD) |
|
1.4 (0.4) |
|
|
Proteinuria > 2g/24h, % |
|
4.4 |
|
|
ANA > 1:80, % |
|
78.8 |
|
|
Anti-dsDNA >30 IU, % |
|
68.4 |
|
|
Low C3 (< 900 mg/L), % |
|
42.4 |
|
|
Low C4 (<16 mg/dL), % |
|
50.2 |
|
|
Prednisone dose (mg/day) |
|
12.0 |
|
|
Steroid >7.5 mg prednisone/day, % |
|
60.1 |
|
|
Immunosuppressive use, % |
|
45.0 |
|
|
Anti-malarial use, % |
|
70.9 |
|
Baseline Disease Characteristics
SRI-5 Response at Week 24 in the mITT population
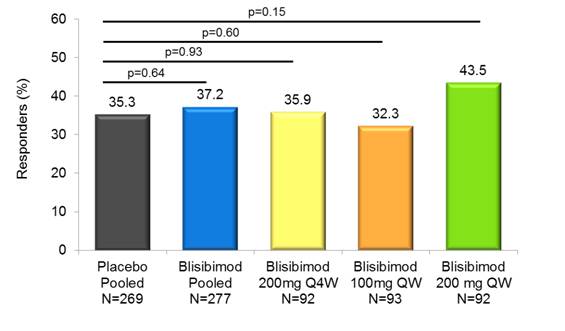
|
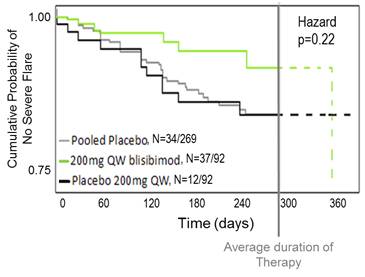
Kaplan Meier Analysis of Time to First Severe Flare (SLE Flare Index) |
Decrease in Proteinuria Subgroup: baseline proteinuria=1-6 g/24h
|
|
|
|
Increases in SRI Response with more Stringent
SELENA-SLEDAI Criteria in mITT Population
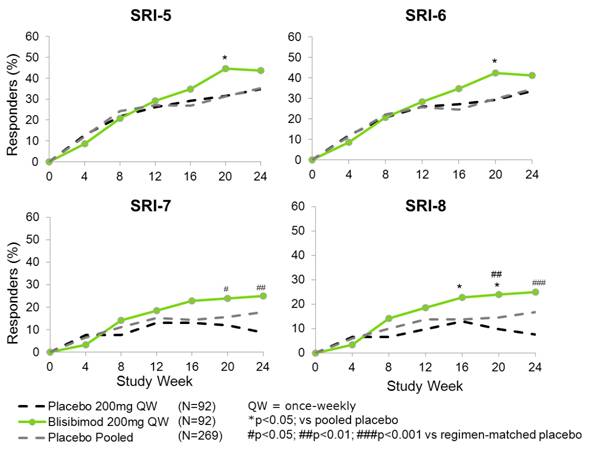
SRI Responses in mITT Population
|
|
|
Placebo |
|
Blisibimod |
|
|
| ||||||||||||
|
|
|
Pooled |
|
200mg Q4W |
|
100mg QW |
|
200mg QW |
|
Pooled |
|
200mg Q4W |
|
100mg QW |
|
200mg QW |
|
∆SRI |
|
|
Endpoint |
|
N=269 |
|
N=89 |
|
N=88 |
|
N=92 |
|
N=277 |
|
N=92 |
|
N=93 |
|
N=92 |
|
200mg QW |
|
|
|
|
95 |
|
31 |
|
32 |
|
32 |
|
103 |
|
33 |
|
30 |
|
40 |
|
|
|
|
|
|
35.3% |
|
34.8% |
|
36.4% |
|
34.8% |
|
37.2% |
|
35.9% |
|
32.3% |
|
43.5% |
|
8.7% |
|
|
SRI-5 |
|
|
|
|
|
|
|
|
|
*p=0.635 |
|
*p=0.925 |
|
*p=0.603 |
|
*p=0.154 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#p=0.884 |
|
#p=0.561 |
|
#p=0.227 |
|
|
|
|
|
|
93 |
|
30 |
|
32 |
|
31 |
|
97 |
|
29 |
|
30 |
|
38 |
|
|
|
|
|
|
34.6% |
|
33.7% |
|
36.4% |
|
33.7% |
|
35.0% |
|
31.5% |
|
32.3% |
|
41.3% |
|
7.6% |
|
|
SRI-6 |
|
|
|
|
|
|
|
|
|
*p=0.901 |
|
*p=0.588 |
|
*p=0.695 |
|
*p=0.237 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#p=0.754 |
|
#p=0.561 |
|
#p=0.286 |
|
|
|
|
|
|
48 |
|
16 |
|
24 |
|
8 |
|
55 |
|
15 |
|
17 |
|
23 |
|
|
|
|
|
|
17.8% |
|
18.0% |
|
27.3% |
|
8.7% |
|
19.9% |
|
16.3% |
|
18.3% |
|
25.0% |
|
16.3% |
|
|
SRI-7 |
|
|
|
|
|
|
|
|
|
*p=0.521 |
|
*p=0.711 |
|
*p=0.887 |
|
*p=0.119 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#p=0.765 |
|
#p=0.149 |
|
#p=0.003 |
|
|
|
|
|
|
45 |
|
16 |
|
22 |
|
7 |
|
55 |
|
15 |
|
17 |
|
23 |
|
|
|
|
|
|
16.7% |
|
18.0% |
|
25.0% |
|
7.6% |
|
19.9% |
|
16.3% |
|
18.3% |
|
25.0% |
|
17.4% |
|
|
SRI-8 |
|
|
|
|
|
|
|
|
|
*p=0.321 |
|
*p=0.905 |
|
*p=0.692 |
|
*p=0.069 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#p=0.765 |
|
#p=0.272 |
|
#p=0.001 |
|
|
|
∆SRI calculated for 200mgQW blisibimod - 200mgQW placebo; #vs regimen-matched placebo, *vs pooled placebo
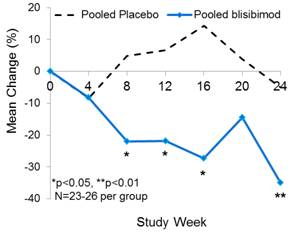
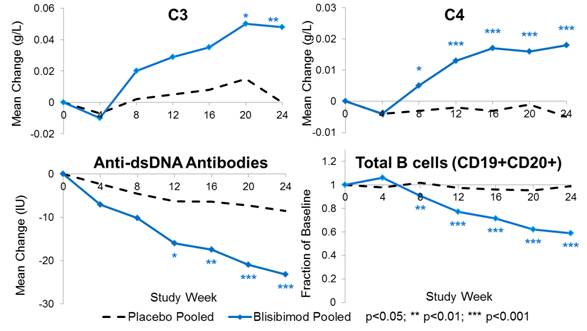
Change in SLE Biomarkers in the mITT Population
Increases in SRI Response in Subjects with Baseline
SELENA-SLEDAI >10 and Receiving Steroid
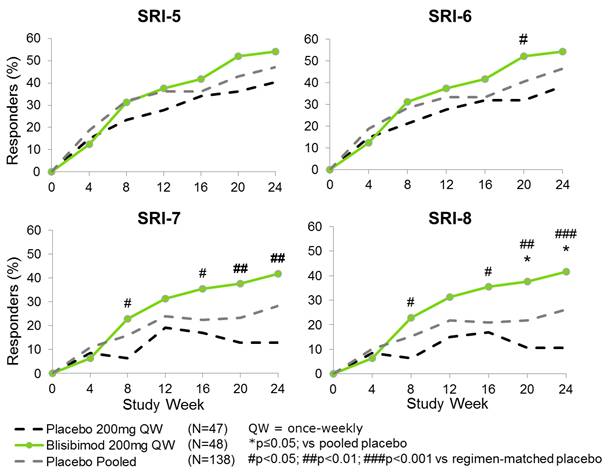
SRI Responses in Subjects with SELENA-SLEDAI>10, Receiving Steroid
|
|
|
Placebo |
|
Blisibimod |
|
|
| ||||||||||||
|
|
|
Pooled |
|
200mg Q4W |
|
100mg QW |
|
200mg QW |
|
Pooled |
|
200mg Q4W |
|
100mg QW |
|
200mg QW |
|
∆SRI |
|
|
Endpoint |
|
N=138 |
|
N=44 |
|
N=47 |
|
N=47 |
|
N=140 |
|
N=42 |
|
N=50 |
|
N=48 |
|
200mg QW |
|
|
|
|
65 |
|
21 |
|
25 |
|
19 |
|
64 |
|
21 |
|
17 |
|
26 |
|
|
|
|
|
|
47.1% |
|
47.7% |
|
53.2% |
|
40.4% |
|
45.7% |
|
50.0% |
|
34.0% |
|
54.2% |
|
13.8% |
|
|
SRI-5 |
|
|
|
|
|
|
|
|
|
*p=0.682 |
|
*p=0.819 |
|
*p=0.084 |
|
*p=0.482 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#p=0.833 |
|
#p=0.057 |
|
#p=0.18 |
|
|
|
|
|
|
64 |
|
21 |
|
25 |
|
18 |
|
63 |
|
20 |
|
17 |
|
26 |
|
|
|
|
|
|
46.4% |
|
47.7% |
|
53.2% |
|
38.3% |
|
45.0% |
|
47.6% |
|
34.0% |
|
54.2% |
|
15.9% |
|
|
SRI-6 |
|
|
|
|
|
|
|
|
|
*p=0.682 |
|
*p=0.971 |
|
*p=0.100 |
|
*p=0.428 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#p=0.992 |
|
#p=0.057 |
|
#p=0.121 |
|
|
|
|
|
|
39 |
|
13 |
|
20 |
|
6 |
|
45 |
|
12 |
|
13 |
|
20 |
|
|
|
|
|
|
28.3% |
|
29.5% |
|
42.6% |
|
12.8% |
|
32.1% |
|
28.6% |
|
26.0% |
|
41.7% |
|
28.9% |
|
|
SRI-7 |
|
|
|
|
|
|
|
|
|
*p=0.517 |
|
*p=0.983 |
|
*p=0.773 |
|
*p=0.105 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#p=0.921 |
|
#p=0.085 |
|
#p=0.002 |
|
|
|
|
|
|
36 |
|
13 |
|
18 |
|
5 |
|
45 |
|
12 |
|
13 |
|
20 |
|
|
|
|
|
|
26.1% |
|
29.5% |
|
38.3% |
|
10.6% |
|
32.1% |
|
28.6% |
|
26.0% |
|
41.7% |
|
31.1% |
|
|
SRI-8 |
|
|
|
|
|
|
|
|
|
*p=0.291 |
|
*p=0.795 |
|
*p=0.996 |
|
*p=0.054 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
#p=0.921 |
|
#p=0.194 |
|
#p<0.001 |
|
|
|
∆SRI calculated for 200mgQW blisibimod - 200mgQW placebo; #vs regimen-matched placebo, *vs pooled placebo
SRI-8 Response is Driven by Clinical Improvement
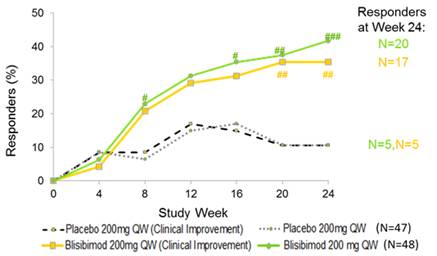
Clinical Improvement meets SRI-8 criteria without counting low complement, increased DNA binding, thrombocytopenia, leukopenia
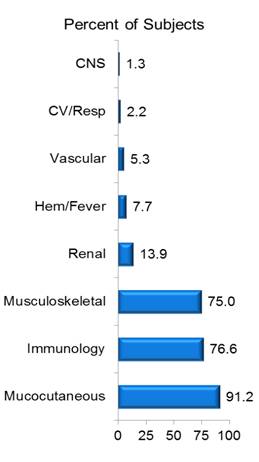
Summary of Adverse Events
|
|
|
Pooled Placebo |
|
Pooled Blisibimod |
|
200mg QW Blisibimod |
|
|
Overview (% incidence) |
|
|
|
|
|
|
|
|
AE |
|
85.0 |
|
82.5 |
|
83.7 |
|
|
Serious AE |
|
15.8 |
|
11.1 |
|
7.6 |
|
|
AEs related to study drug |
|
37.2 |
|
40.0 |
|
48.9 |
|
|
AEs leading to withdrawal |
|
7.9 |
|
5.7 |
|
6.5 |
|
|
AEs leading to death |
|
1.1 |
|
1.4 |
|
1.1 |
|
|
Severe Infection AEs |
|
1.1 |
|
1.4 |
|
2.2 |
|
|
Severe Injection site reactions |
|
0 |
|
0 |
|
0 |
|
|
Serious Adverse Events, n(%) |
|
|
|
|
|
|
|
|
Herpes zoster |
|
2 (0.8) |
|
2 (0.7) |
|
0 |
|
|
Pneumonia |
|
4 (1.5) |
|
3 (1.1) |
|
2 (2.2) |
|
|
Urinary tract infections |
|
2 (0.8) |
|
2 (0.7) |
|
0 |
|
|
SLE |
|
3 (1.1) |
|
2 (0.7) |
|
0 |
|
|
Deep vein thrombosis |
|
2 (0.8) |
|
3 (1.1) |
|
1 (1.1) |
|
· 2 malignancies were reported (1 blisibimod, 1 placebo)
· 6 subjects withdrew due to pregnancy (3 blisibimod, 3 placebo)
Conclusions
· Blisibimod was safe and well-tolerated at all dose levels with no meaningful imbalances in serious adverse events or infections compared with placebo.
· While the primary endpoint was not achieved, analyses identified patient populations and endpoints with superior responses to regimen-matched placebo:
· Subjects treated with 200mg QW achieving SRI-7 and SRI-8 response criteria.
· Subjects with baseline SELENA-SLEDAI>10 and receiving steroids treated with 200mg QW and meeting SRI-7 and SRI-8 response criteria.
· Early onset of response was observed across all SRI analyses in the mITT population and in subjects with SELENA-SLEDAI>10 who were receiving steroid therapy.
· Significant improvements in proteinuria, C3, C4 and anti-dsDNA were observed in subjects treated with blisibimod.
· The data support further evaluation of 200mg QW blisibimod using more stringent thresholds of the SRI in patients with severe seropositive SLE.
· Phase 3 clinical studies with blisibimod are anticipated to commence in Q1 2013.
References
Stohl W et al. Phase 1a Single- and Phase 1b Multiple-Dose Studies of AMG 623 (an Anti-BAFF Peptibody) in SLE. ACR; October, 2008.
Hsu H et al. A novel modality of BAFF-specific inhibitor AMG623 peptibody reduces B-cell number and improves outcomes in murine models of autoimmune disease. Clin Exp Rheumatol. 2012;30(2) 197.
Presented at the American College of Rheumatology Annual Meeting, November 10-14 2012, Washington DC, USA