Attached files
| file | filename |
|---|---|
| EX-23.1 - CONSENT OF ERNST & YOUNG - AUDEO ONCOLOGY, INC. | d365958dex231.htm |
| EX-10.21 - AMENDMENT DEED BETWEEN ALCHEMIA LIMITED AND THE REGISTRANT. - AUDEO ONCOLOGY, INC. | d365958dex1021.htm |
Table of Contents
As filed with the Securities and Exchange Commission on November 8, 2012
Registration No. 333-182568
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 3
to
FORM S-1
REGISTRATION STATEMENT
Under
The Securities Act of 1933
AUDEO ONCOLOGY, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 80-0823961 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
100 Pine Street
Suite 2040
San Francisco, California 94111
(415) 984-0300
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Peter Smith
Chief Executive Officer
Audeo Oncology, Inc.
100 Pine Street
Suite 2040
San Francisco, California 94111
(415) 984-0300
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| John A. Fore Andrew D. Hoffman Wilson Sonsini Goodrich & Rosati, P.C. 650 Page Mill Road Palo Alto, California 94304 (650) 493-9300 |
Eric S. Haueter Istvan A. Hajdu Sidley Austin LLP 555 California Street San Francisco, California 94104 (415) 772-1200 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer | ¨ | ||||
| Non-accelerated filer x (do not check if a smaller reporting company) | Smaller reporting company | ¨ |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED NOVEMBER 8, 2012
PROSPECTUS
3,250,000 Shares

COMMON STOCK
Audeo Oncology, Inc. is offering 3,250,000 shares of common stock. This is our initial public offering and no public market currently exists for our common stock. We estimate that the initial public offering price will be between $14.00 and $16.00 per share.
We have applied to list our common stock on The Nasdaq Global Market under the symbol “AURX.”
We are currently a subsidiary of Alchemia Limited, an Australian company whose ordinary shares are listed on the Australian Securities Exchange under the symbol “ACL.” Approximately two trading days following the completion of this offering, we intend to complete implementation of our demerger from Alchemia Limited pursuant to which Alchemia Limited will distribute one share of our common stock to Alchemia Limited shareholders (or a depositary on their behalf) for every 37 ordinary shares of Alchemia Limited that they hold. All conditions precedent to the demerger, other than those related to completion of this offering, have been satisfied prior to the date of this prospectus. Upon completion of the demerger, we expect that depositary shares, referred to as CHESS Depository Interests, representing shares of our common stock, will be listed on the Australian Securities Exchange under the symbol “AKQ.” This prospectus does not constitute an offer to sell, or the solicitation of an offer to buy, any of these depositary shares or shares of our common stock distributed in the demerger.
We are an “emerging growth company” under the federal securities laws and, as a result, we are subject to reduced disclosure requirements with respect to this prospectus and will be subject to reduced public company reporting requirements.
Investing in our common stock involves risks. See “Risk Factors” beginning on page 17 of this prospectus.
| Per Share | Total |
|||||||
| Price to public |
$ | $ | ||||||
| Underwriting discount |
$ | $ | ||||||
| Proceeds, before expenses, to us |
$ | $ | ||||||
We have granted the underwriters a 30-day option to purchase up to an additional 487,500 shares of our common stock solely to cover over-allotments, if any.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities, or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares of common stock on or about , 2012.
| Leerink Swann |
Oppenheimer & Co. |
The date of this prospectus is , 2012
Table of Contents
HyACT Mechanism of Action
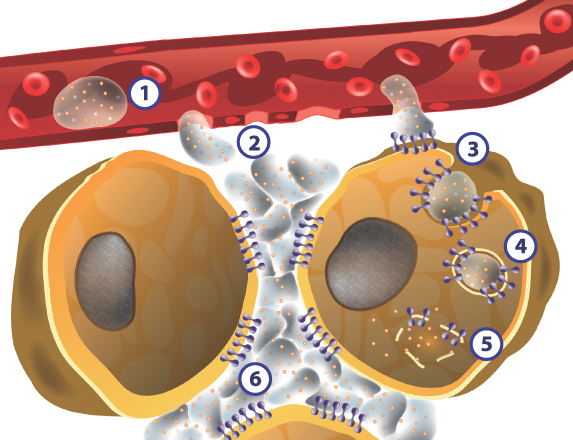
|
|
HyACT-targeted drug delivery vehicle and anti-cancer drug in the bloodstream after infusion | |
|
|
HyACT-targeted drug accesses the tumor environment through leaky vasculature typical of blood vessels that supply blood to tumors. HyACT-targeted drugs form a drug depot in the extracellular space of the tumor | |
|
|
HyACT-targeted drug binds to activated CD44 and then is rapidly internalized, resulting in more drug entering the tumor cell | |
|
|
HyACT-targeted drug is transported into an intracellular vesicle and the hyaluronic acid is degraded | |
|
|
HyACT-targeted drug is released within the cancer cell, increasing the likelihood of cell death and enhanced tumor response | |
|
|
The HyACT-targeted drug depot generally persists for at least 24 hours, resulting in repeated cycles of drug internalization and release inside tumor cells |
The figure above represents our hypothesis regarding the HyACT mechanism of action based on our preclinical data and on published literature.
Table of Contents
| Page | ||||
| 1 | ||||
| 17 | ||||
| 67 | ||||
| 69 | ||||
| 70 | ||||
| 70 | ||||
| 71 | ||||
| 75 | ||||
| 79 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
82 | |||
| 102 | ||||
| 133 | ||||
| 142 | ||||
| 152 | ||||
| Arrangements between Audeo Oncology, Inc. and Alchemia Limited |
154 | |||
| 159 | ||||
| 162 | ||||
| 168 | ||||
| Material U.S. Federal Income Tax Consequences To Non-U.S. Holders |
173 | |||
| 177 | ||||
| 185 | ||||
| 185 | ||||
| Limitation on Independent Registered Public Accounting Firm’s Liability |
185 | |||
| 185 | ||||
| 186 | ||||
| F-1 | ||||
You should rely only on the information contained in this prospectus or in any free writing prospectus prepared by or on behalf of us. Neither we nor the underwriters have authorized anyone to provide you with information different from, or in addition to, that contained in this prospectus or any related free writing prospectus. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We and the underwriters are not offering to sell, or seeking offers to buy, our common stock in any jurisdiction where such offer or sale is not permitted. The information contained in this prospectus is current only as of its date, regardless of the time of delivery of this prospectus or any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
This prospectus, the registration statement of which this prospectus forms a part and the offering have not been, nor will they need to be, lodged with the Australian Securities & Investments Commission. This prospectus and the registration statement of which this prospectus forms a part are not a “Prospectus” under Chapter 6D of the Corporations Act 2001 (Cth) of Australia, or the Australian Corporations Act. Any offer of shares of our common stock in Australia is made only to persons to whom it is lawful to offer shares of our common stock without disclosure under one or more of certain of the exemptions set out in section 708 of the Australian Corporations Act, or an “exempt person.” Further details of the exemptions are set out below in “Underwriting
-i-
Table of Contents
— Selling Restrictions — Australia.” By accepting this prospectus, an offeree in Australia represents that the offeree is an exempt person. No shares of our common stock will be issued or sold in this offering in circumstances that would require the giving of a “Prospectus” under Chapter 6D of the Australian Corporations Act.
Audeo Oncology, Inc., the Audeo Oncology, Inc. logo and other trademarks or service marks of Audeo Oncology, Inc. appearing in this prospectus are the property of Audeo Oncology, Inc. Alchemia Limited, the Alchemia Limited logo and other trademarks or service marks of Alchemia Limited appearing in this prospectus are the property of Alchemia Limited. Trade names, trademarks and service marks of other companies appearing in this prospectus are the property of their respective holders.
-ii-
Table of Contents
The following summary highlights selected information appearing elsewhere in this prospectus and is qualified in its entirety by the more detailed information and financial statements included elsewhere in this prospectus. This summary does not contain all the information you should consider before investing in our common stock. You should carefully read this prospectus in its entirety before investing in our common stock, including the sections entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this prospectus. Our fiscal year ends on June 30. As such, references to fiscal 2009, 2010, 2011, 2012 and 2013 herein refer to the fiscal years ended June 30, 2009, 2010, 2011, 2012 and 2013, respectively.
Unless otherwise expressly stated or the context otherwise requires, references throughout this prospectus to “we,” “us,” “our” or the “company” and similar references refer to Audeo Oncology, Inc. and its consolidated subsidiaries, Alchemia Oncology Pty Limited (formerly known as Meditech Research Ltd.), which we sometimes refer to as Alchemia Oncology, and Audeo Discovery Pty Limited; references to “dollars,” “$,” “U.S. dollars” and “US$” refer to United States dollars; and references to “Australian dollars,” “Australia $,” “AUD dollars” and “AUD$” refer to Australian dollars.
Overview
We are a late stage biopharmaceutical company primarily focused on utilizing our Hyaluronic Acid Chemotransport Technology, or HyACT, to target cancer drugs preferentially to tumor cells to enhance drug activity. HyACT is a flexible platform technology designed to increase the effectiveness of anti-cancer agents without increasing treatment toxicity. We seek to reduce the risks related to drug development by using known anti-cancer drugs, and aim to enhance their commercial value by improving their effectiveness.
Our lead HyACT product candidate, HA-Irinotecan, is currently in a pivotal Phase III clinical trial for metastatic colorectal cancer, or mCRC, meaning that we intend to use the data we collect from the clinical trial to seek regulatory approval from the U.S. Food and Drug Administration, or FDA, and the European Medicines Agency, or EMA, of HA-Irinotecan for the treatment of mCRC. HA-Irinotecan is also in an investigator-sponsored Phase II clinical trial for the treatment of small cell lung cancer, or SCLC. We also have two other HyACT product candidates that have successfully completed Phase I clinical trials. In addition to our current clinical-stage product candidates, we aim to develop a pipeline of product candidates by exploiting our significant know-how in cancer stem cell biology and cancer metabolism combined with the Versatile Assembly on Stable Templates, or VAST, small molecule drug discovery technology that we have in-licensed.
Each of our current product candidates combines HyACT with a known anti-cancer drug. HyACT uses hyaluronic acid, or HA, which delivers additional drug to the tumor and promotes uptake of the drug into the tumor cells. HyACT binds to the activated receptor CD44, a naturally occurring HA receptor, which has been shown in numerous studies to be present in high levels in many prevalent solid tumor cancer types but, more importantly, is generally not activated in healthy tissue. CD44 over-expression is associated with more aggressive, metastatic tumors and is also a marker for treatment-resistant cancer stem cells. “Cancer stem cells,” sometimes referred to as tumor-initiating cells, is a term used to describe a small subset of cells within the tumor that, although not actual stem cells, demonstrate stem cell-like characteristics. Cancer stem cells are generally more resistant to current chemotherapy regimens than cancer cells, and their persistence after therapy is thought to be one of the key reasons for disease progression and treatment failure. We believe, based on preclinical studies, that the CD44 receptor-based mechanism has the potential for improving the effectiveness of drugs combining HyACT with an anti-cancer drug, which we refer to as HyACT-targeted drugs. In preclinical studies, HyACT-targeted drugs delivered at least double the dose of anti-cancer drug to the tumor when compared with the drug alone. Our in vitro studies have shown that HyACT significantly increases drug uptake into cancer cells. Finally, preclinical studies have also shown HyACT-targeted chemotherapies are more potent than the original drug at killing cancer stem cells and other CD44-expressing cells.
-1-
Table of Contents
Despite the considerable research and development efforts of the pharmaceutical industry, cancer remains a significant unmet medical need. Chemotherapeutics remain the backbone of many drug-based anti-cancer treatment regimens. Due to the non-specific nature of current chemotherapeutics, they are typically administered at the maximum tolerated dose, not at the optimal dose required to kill the cancer. As evidenced by the current recurrence and mortality rates associated with cancers, there remains a need to identify new and more effective anti-cancer treatments.
We believe that HyACT is a broadly applicable platform technology that has the potential for enhancing the efficacy of a range of anti-cancer drugs in a number of different cancer indications. In addition to HA-Irinotecan’s Phase II clinical trial data, HyACT-targeted 5-fluorouracil and HyACT-targeted doxorubicin have both demonstrated tolerability and a trend towards efficacy in Phase I / IIa clinical trials. In preclinical studies, we have also had promising results combining HyACT with seven other anti-cancer agents, including other chemotherapies and monoclonal antibodies.
We have considerable know-how, research and preclinical expertise in HA, cancer metabolism, CD44 and cancer stem cells. We have also in-licensed rights to Alchemia Limited’s VAST small molecule drug discovery technology, which provides innovative pyranose compounds for drug discovery. Our strategy is to use these capabilities to investigate new pathways relating to CD44 signaling, HA metabolism and pro-oncogenic pathways that we believe are important to the promotion and proliferation of cancer stem cells. We aim to generate future drugs designed to more effectively target and kill cancer and cancer stem cells.
Our Strategy
Our goal is to build a global biopharmaceutical company focused on the development and commercialization of anti-cancer treatments in the United States and other major markets worldwide. Key elements of our strategy to achieve this objective are:
| • | Leveraging our proprietary HyACT technology to bring improved anti-cancer agents to market. Our objective is to use our HyACT technology to improve the delivery of chemotherapeutic agents to cancer cells, boosting drug efficacy without increasing side-effects, thereby increasing the therapeutic index of current chemotherapeutics. We intend to utilize the FDA’s 505(b)(2) regulatory pathway, which may offer a lower development risk profile and accelerated development compared with completely new cancer drugs. |
| • | Successfully developing our lead product candidate, HA-Irinotecan, for the treatment of metastatic colorectal cancer. After having consultations with the FDA and the EMA, we began recruiting patients for a pivotal Phase III clinical trial testing HA-Irinotecan in patients with mCRC in November 2011. If this clinical trial is successful, we intend to seek regulatory approval from both the FDA and the EMA of HA-Irinotecan for the treatment of second and third line mCRC patients in the United States and the European Union. |
| • | Enhancing the commercial value of HyACT-targeted drugs. Where appropriate, we intend to pursue commercialization relationships with pharmaceutical companies and other strategic partners providing for our products to be distributed through their sales and marketing organizations in order to gain access to global oncology markets for any of our product candidates that receive regulatory approval. Over the longer term, we may ultimately build an internal commercial infrastructure to directly market and sell any HyACT-targeted drugs that may receive regulatory approval. |
| • | Leveraging our expertise and know-how to develop additional anti-cancer agents of high value. We plan to utilize our in-licensed small molecule drug discovery technology, VAST, and extensive preclinical expertise in stem cell biology and oncology drug development to develop new therapies targeted at modulating innovative pathways associated with HA metabolism and cancer stem cells. |
-2-
Table of Contents
HyACT
HyACT-targeted product candidates work by exploiting the unique structure and inherent biological properties of HA, a natural glycosaminoglycan polymer, which is a key component of the extracellular matrix in mammals. The specific form of HA used in our HyACT-targeted product candidates combines the effective drug entrapment and delivery properties of HA with the active transport and internalization characteristics resulting from HA’s ability to bind to activated CD44, a receptor generally over-expressed on cancer cells. HA has been shown in preclinical studies to not only carry the anti-cancer drug preferentially to cancer cells but also increase its uptake by those cancer cells.
Our HyACT-targeted product candidates are designed to overcome some of the limitations of current chemotherapies by delivering anti-cancer drugs preferentially to cancer cells in order to increase the response of cancers to standard chemotherapy. Because our initial HyACT-targeted product candidates consist of two FDA-approved agents (HA and chemotherapeutics with well-characterized toxicity and efficacy profiles), we believe that these HyACT-targeted product candidates, which we expect will utilize the FDA’s 505(b)(2) regulatory approval pathway, may offer a lower development risk profile and accelerated development compared with completely new cancer drugs.
Our Product Candidates
Consistent with our strategy, each of our product candidates is being developed for selected cancer therapies to treat specific therapeutically-relevant cancer indications. We have not licensed rights to any of our product candidates to any third parties. The following table summarizes the status of (a) HA-Irinotecan for mCRC and our other product candidates, (b) an investigator-sponsored trial of HA-Irinotecan for SCLC (we do not currently intend to pursue further clinical trials or commercialization of HA-Irinotecan for this indication) and (c) drug discovery activities using the VAST technology:
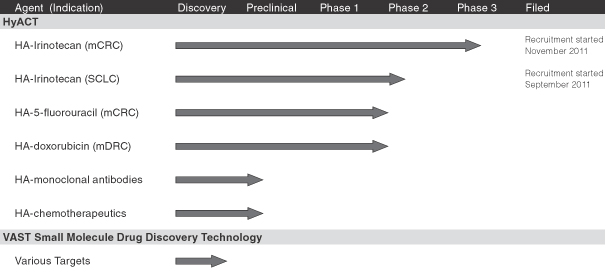
mCRC is metastatic colorectal cancer
SCLC is small cell lung cancer
mDRC is metastatic drug resistant cancer
-3-
Table of Contents
HA-Irinotecan
Our lead product candidate is HA-Irinotecan for the treatment of mCRC. Irinotecan, which is marketed in major markets by Pfizer as Camptosar, is an off-patent chemotherapy widely used in the treatment of mCRC. In a randomized Phase II clinical trial of HA-Irinotecan compared with irinotecan alone, based on 76 patients with mCRC, HA-Irinotecan was able to double the progression free survival, or PFS, in mCRC patients (5.2 months for HA-Irinotecan versus 2.4 months for irinotecan alone). Based on these findings, we began enrollment for a pivotal Phase III clinical trial, testing HA-Irinotecan in patients with mCRC in November 2011.
In addition to its approved use, published studies suggest that irinotecan may have activity in a range of solid tumors such as those of the breast, pancreas, brain and ovaries. According to reports by Pfizer, sales of Camptosar (proprietary irinotecan, which became generic in 2008) were over $950 million in 2007. We believe that, to the extent clinical trials confirm HA-Irinotecan’s safety and demonstrate significantly improved clinical efficacy compared with irinotecan alone, HA-Irinotecan has the potential to replace irinotecan in first to third line irinotecan-containing treatment regimens.
After having consultations with the FDA and the EMA, we commenced our pivotal Phase III clinical trial of HA-Irinotecan for the treatment of mCRC. Our Phase III clinical trial is comparing the effectiveness of HA-Irinotecan with irinotecan, in each case as components of the FOLFIRI chemotherapy regimen (leucovorin, 5-fluorouracil and irinotecan), in 390 irinotecan-naïve, second and third line patients with mCRC. We believe that the FOLFIRI regimen is generally considered the leading standard of care for second and third line treatment for mCRC in the United States and Europe. The primary objective of this study is to demonstrate that HA-Irinotecan provides superior efficacy compared with irinotecan alone, as indicated by a significantly increased period of PFS. As HA-Irinotecan is a formulation of two previously approved products (the cancer drug irinotecan and HA), we intend to utilize an alternative type of New Drug Application, or NDA, commonly referred to as a Section 505(b)(2) NDA, which enables us to rely, in part, on the FDA’s previous approval of a similar product, or on published literature, in support of our application. The 505(b)(2) regulatory approach may enable us to produce an abbreviated preclinical data package, thereby saving development time and costs. Based on our initial consultations with the FDA, we believe that the 505(b)(2) regulatory approach is an appropriate pathway to seek regulatory approval of HA-Irinotecan, as HA-Irinotecan is a formulation of two previously approved products (the cancer drug irinotecan and HA), although there can be no assurance in this regard. Reliance on the 505(b)(2) regulatory pathway requires establishing that such reliance is scientifically appropriate at the time of submission of the NDA. We have sought and have received input from the EMA on the preclinical and clinical development of HA-Irinotecan for the treatment of mCRC and are taking into consideration this input in our European regulatory strategy. If this pivotal trial is successful, we intend to seek regulatory approval from both the FDA and the EMA for HA-Irinotecan for the treatment of second and third line mCRC patients in the United States and the European Union.
In September 2011, recruitment began for an investigator-sponsored Phase II clinical trial of HA-Irinotecan plus carboplatin versus irinotecan plus carboplatin for the treatment of SCLC. Patients will be randomized to receive either HA-Irinotecan or irinotecan, each in combination with carboplatin. The original protocol provided for recruitment of first line chemotherapy-naïve patients, and the first five patients in the safety cohort were all treated with HA-Irinotecan plus carboplatin. In May 2012, a protocol amendment expanded the study to both first and second line irinotecan-naïve SCLC patients. After the first five patient cohort, the first line patients are to be randomized 1:1 into both arms of the trial, while the second line patients will all receive HA-Irinotecan plus carboplatin. The primary endpoints for this clinical trial are (i) incidence of grade 3 and 4 toxicity and (ii) tumor stem cell burden during and at the conclusion of the clinical trial treatment. Secondary endpoints include cumulative dose of irinotecan, PFS at six months, objective response rates, quality of life and survival. A total of approximately 40 patients are to be recruited to the trial, which is being conducted at Monash Cancer Centre, Southern Health and Peninsula Oncology Centre in Victoria, Australia. This trial was initiated under the Australian Therapeutic Goods Administration, Clinical Trials Notification Scheme. Our participation in this
-4-
Table of Contents
investigator-sponsored trial has the potential to further validate the HyACT technology as well as to provide clinical data on HA-Irinotecan’s activity on cancer stem cells. As this is an investigator-sponsored trial, our involvement in the trial has been limited. We currently do not intend to pursue further clinical trials or commercialization of HA-Irinotecan for the treatment of SCLC and, accordingly, references to our “product candidates” do not include HA-Irinotecan for the treatment of SCLC.
Other HyACT Programs
We used HyACT-targeted 5-fluorouracil in a Phase I / IIa clinical trial in 13 patients with mCRC. We also used HyACT-targeted doxorubicin in a Phase I / IIa clinical trial in 16 patients with metastatic cancer with a life expectancy of at least 12 weeks. We have also conducted preclinical studies that have suggested that HyACT has the potential for improving efficacy of a number of other cancer drugs including gemcitabine (Gemzar), methotrexate (Rheumatrex), carboplatin (Paraplatin) and vinorelbine tartrate (Navelbine). We have also tested in preclinical studies a number of monoclonal antibodies, including bevacizumab (Avastin) and cetuximab (Erbitux), and are investigating the potential of running a parallel clinical trial comparing an HA-monoclonal antibody with the monoclonal antibody alone.
VAST Drug Discovery Technology
In addition to the HyACT platform, we have in-licensed worldwide rights to the VAST small molecule drug discovery technology, which we expect will give us the ability to research and develop new anti-cancer drug candidates. This VAST technology has been deployed by Alchemia Limited in several collaborations with pharmaceutical companies, including Amgen Inc. and Genentech, Inc., and research institutes, including the University of Queensland, the Institute of Molecular Biology, Brisbane, the Monash Institute of Pharmaceutical Sciences, Monash University and the Walter and Eliza Hall Institute, exploring its capability and application in non-cancer therapeutic areas. These collaborations are exploiting the chemistry and molecular diversity offered by the VAST technology and diversity scanning array by addressing known targets in an innovative way. We plan to initiate programs to study inflammation pathways associated with cancer stem cells and cancer metabolism with a target involved in HA synthesis. These programs are intended to make use of the selectivity and compound efficiency of the VAST technology. Our VAST programs are run on a business model designed to limit our cash expenditures through the use of partnerships and, if and when available, government grants. These programs include the allosteric modulation of family B G-protein coupled receptors for chronic obstructive pulmonary disease and type II diabetes, as well as opioid receptor agonists and ion channel inhibitors for pain. The collaboration agreements referred to above that are currently in effect are between Alchemia Limited and various collaborators, and any arrangements for government grants relating to the VAST technology are also between Alchemia Limited and the Australian government. We are not a party to any of these collaboration agreements or government grant arrangements, and Alchemia Limited may be required to obtain the consent of its collaborators and the Australian government in order to transfer these collaboration agreements and government grant arrangements to us. If these consents are not granted, it may have a material adverse effect on our ability to exploit the VAST technology. See “Risk Factors—Alchemia Limited may be unable to transfer its rights under collaboration agreements and government grant arrangements relating to the VAST technology to us.” We aim to partner with pharmaceutical and biotechnology companies in areas outside of oncology to validate and commercialize various programs employing the VAST technology in new drug development while progressing certain projects internally.
-5-
Table of Contents
Risk Factors
Our business is subject to numerous risks and uncertainties, including those highlighted here and described in further detail in “Risk Factors” immediately following this Prospectus Summary and elsewhere in this prospectus. You should carefully read “Risk Factors” for a detailed explanation of some of these risks before investing in our common stock. Some of these risks include the following:
| • | we have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future and we may never achieve or sustain profitability and will require additional funding; |
| • | we are a clinical-stage company with no approved products, which makes it difficult to assess our future viability; |
| • | we are highly dependent on the success of our lead product candidate, the cancer drug HA-Irinotecan for the treatment of mCRC, and we cannot give any assurance that we will successfully complete its clinical development, or that it will receive regulatory approval, or if approved, that it will receive adequate labeling that will allow for successful commercialization for the treatment of mCRC; |
| • | our proprietary rights may not adequately protect our technologies and product candidates; |
| • | there is a risk that our development and clinical activities will not result in commercial products, and we will invest in our current development and clinical programs, to the exclusion of others, for several more years before it is known whether one or more of our product candidates will receive regulatory approval, obtain adequate labeling or be commercially introduced; |
| • | our development activities could be delayed or stopped for a number of reasons, many of which are outside our control, including failure to recruit and enroll patients for clinical trials; |
| • | the regulatory approval processes of the FDA and non-U.S. regulatory authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, or if approved, the labeling is not adequate, our business will be substantially harmed; |
| • | if HA-Irinotecan does not demonstrate superior efficacy to generic irinotecan for the treatment of mCRC in our Phase III clinical trial, it is unlikely that we will receive FDA approved labeling allowing us to price HA-Irinotecan at a premium to generic irinotecan, and even if our label contains data adequate to allow us to price HA-Irinotecan at a premium to generic irinotecan, there is no assurance that payors will pay such a premium, either of which could have a material adverse effect on our business; |
| • | we will require substantial additional funding which may not be available to us on acceptable terms, or at all; |
| • | our ability to raise additional capital may be significantly limited by listing rules of the Australian Securities Exchange that limit the amount of common stock that we are permitted to issue without stockholder approval; |
| • | we expect that our stock price will fluctuate significantly, you may not be able to resell your shares at or above the initial public offering price and you may lose all or part of your investment; |
| • | CHESS Depository Interests, or CDIs, which we expect will represent a substantial majority of our shares of common stock outstanding immediately after this offering, will be listed on the Australian |
-6-
Table of Contents
| Securities Exchange and will be freely tradable in the public markets in Australia and we expect that the CDIs will begin trading on such exchange approximately seven trading days following the date of this prospectus, which may have a material adverse effect on the trading price of our common stock on the Nasdaq Global Market; |
| • | investors purchasing shares of our common stock in this offering will not be able to freely sell those shares, or CDIs representing those shares, in Australia during the 12 months after the issue date of those shares in this offering and therefore will not be able to take advantage of any liquidity that may be available for CDIs traded on the Australian Securities Exchange during that period; and |
| • | all of the shares of our common stock and the CDIs representing those shares to be outstanding following the Demerger and this offering will be freely tradable in the public markets and a substantial majority of the shares of our common stock distributed in the Demerger and the CDIs representing those shares will not be subject to lock-up agreements. |
Corporate Information
Audeo Oncology, Inc., or Audeo Oncology, the issuer of the common stock to be sold in this offering, was incorporated in Delaware in June 2012 and acquired by Alchemia Limited as a wholly-owned subsidiary. Our principal executive offices are located at 100 Pine Street, Suite 2040, San Francisco, California 94111, and our telephone number is (415) 984-0300. Our website is www.audeooncology.com. Information contained on, or that can be accessed through, our website or the Alchemia Limited website is not incorporated by reference into this prospectus, and you should not consider information on our website or the Alchemia Limited website to be part of this prospectus.
Corporate Reorganization
Our business was in the past conducted through Alchemia Oncology Pty Limited, an Australian company that we sometimes refer to as Alchemia Oncology. Alchemia Oncology, which was previously known as Meditech Research Ltd., was acquired by Alchemia Limited in 2006 and was a wholly-owned direct subsidiary of Alchemia Limited. In June 2012, Alchemia Limited effected a reorganization, which we sometimes refer to as the Reorganization, pursuant to which we became a wholly-owned subsidiary of Alchemia Limited and Alchemia Oncology became a wholly-owned direct subsidiary of ours and a wholly-owned indirect subsidiary of Alchemia Limited. Accordingly, the description of our business and our consolidated financial statements and other financial information included in this prospectus as of the dates and for the periods prior to the Reorganization reflect the business, results of operations and financial position of Alchemia Oncology, and the description of our business and our consolidated financial statements and other financial information as of the dates and for the periods from and after the date of Reorganization reflect the business, results of operations and financial condition of Audeo Oncology, Inc. and its consolidated subsidiaries, in each case unless otherwise expressly stated or the context otherwise requires.
In the past, our business has been financed by related party loans from Alchemia Limited. As of June 30, 2012, all of these loans were converted into newly-issued ordinary shares of Alchemia Oncology and, as a result, the loans were cancelled. We subsequently issued 7.5 million shares of our common stock to Alchemia Limited and, in return, Alchemia Limited transferred to us all of Alchemia Oncology’s outstanding ordinary shares in the Reorganization. We sometimes refer to the conversion of all of these outstanding related party loans into newly-issued ordinary shares of Alchemia Oncology and the cancellation of these loans as the Loan Conversion. On November 5, 2012, we issued an additional aggregate of 92,000 shares of our common stock to Alchemia Limited in further consideration for our receipt of the shares of Alchemia Oncology and which, together with the 7.5 million shares of our common stock previously issued, will be distributed in the Demerger, as defined below.
-7-
Table of Contents
The Demerger
Audeo Oncology, Inc., the issuer of the common stock being sold in this offering, is a newly formed Delaware corporation and is currently a wholly-owned subsidiary of Alchemia Limited, an Australian company whose common shares are listed on the Australian Securities Exchange. We were formed for the purpose of (a) acquiring Alchemia Oncology and forming our other subsidiary, Audeo Discovery Pty Limited, an Australian company organized to in-license the VAST small molecule drug discovery technology owned by Alchemia Limited, and (b) demerging from Alchemia Limited as an independent public company with shares of our common stock listed on the Nasdaq Global Market and CHESS Depository Interests, or CDIs, representing shares of our common stock to be listed on the Australian Securities Exchange. Our business and operations consist solely of the business and operations of Alchemia Oncology and Audeo Discovery Pty Limited, both of which are our wholly-owned subsidiaries. We will rename Alchemia Oncology following implementation of the Demerger, as defined in the next sentence. Approximately two trading days following the completion of this offering, we intend to complete implementation of our demerger, or the Demerger, from Alchemia Limited pursuant to a Scheme of Arrangement that was approved by the Federal Court of Australia and by more than 75% in voting interest and a majority in number of Alchemia Limited’s shareholders present and voting at a meeting of shareholders. All conditions precedent to the Demerger, other than those related to completion of this offering, have been satisfied prior to the date of this prospectus. Trading of the CDIs on the Australian Securities Exchange is not expected to commence until approximately seven trading days following the date of this prospectus.
Pursuant to the Demerger, Alchemia Limited, which currently owns all of the outstanding shares of our common stock, will distribute one share of our common stock to the shareholders of Alchemia Limited (or a depositary on their behalf) for every 37 ordinary shares of Alchemia Limited that they hold as of the record date for the Demerger, which will be approximately four trading days following the date of this prospectus, with fractional shares of our common stock that would otherwise be distributed to Alchemia Limited shareholders in the Demerger being rounded upwards to the nearest whole share. To the extent that the number of shares of our common stock currently owned by Alchemia Limited is insufficient to permit the distribution of one share of our common stock for every 37 ordinary shares of Alchemia Limited outstanding at the time of the Demerger, we will issue such number of additional shares of our common stock to Alchemia Limited as may be necessary to permit that distribution to be made in full. As our common stock will not be listed on any Australian stock exchange, Alchemia Limited plans to distribute CDIs representing shares of our common stock to Alchemia Limited shareholders in the Demerger, although those shareholders will have the option of receiving the actual shares of common stock instead. Unless otherwise expressly stated or the context otherwise requires, information in this prospectus assumes that 7,592,020 shares of our common stock will be distributed in the Demerger, which number of shares is calculated on the basis of 280,812,962 ordinary shares of Alchemia Limited outstanding as of November 1, 2012. However, the actual number of shares of our common stock distributed and that will be outstanding upon completion of this offering in the Demerger will be greater than the number of shares we have assumed for the purposes of this prospectus, as discussed below under “— The Offering.”
As our common stock will not be listed on any Australian stock exchange, we expect that Alchemia Limited shareholders will generally elect to receive CDIs representing shares of our common stock in the Demerger rather than exercising their option to receive shares of our common stock instead of CDIs. We will deliver the shares of our common stock represented by the CDIs to a depositary, which will hold those shares on behalf of the holders of the CDIs. Holders of CDIs will have substantially the same economic, voting and other rights as holders of our common stock, and the CDIs therefore should be considered as the functional equivalent of shares of our common stock. The CDIs have been conditionally approved for quotation on the Australian Securities Exchange under the symbol “AKQ” and will begin trading on such exchange approximately seven trading days following the date of this prospectus. As our common stock will not be listed on any Australian securities exchange, the purpose of the CDIs is to facilitate trading, settlement and clearance in Australia by shareholders of Alchemia Limited who would otherwise receive shares of our common stock in the Demerger. For additional information concerning our CDIs, see “Description of Capital Stock — CHESS Depositary Interests.” This prospectus does not constitute an offer to sell, or the solicitation of an offer to buy, any CDIs or shares of our common stock distributed in the Demerger.
-8-
Table of Contents
The Offering
| Common stock offered by us |
3,250,000 shares. |
| Over-allotment option |
487,500 shares. |
| Common stock estimated to be outstanding immediately after this offering |
10,842,020 shares (or 11,329,520 shares if the underwriters’ exercise their over-allotment option in full). |
| Use of proceeds |
We currently intend to use the net proceeds we receive from this offering as follows: to complete our pivotal Phase III clinical trial and related regulatory filings for HA-Irinotecan for the treatment of mCRC; to prepare for commercial-scale manufacturing of HA-Irinotecan, if approved for the treatment of mCRC, which would entail procurement of key raw materials, developing and validating a manufacturing process to manufacture commercial-scale quantities of product and preparing and filing regulatory documents; to fund our research and development activities related to our HyACT platform; to fund our research and drug discovery activities related to our in-licensed VAST drug discovery technology; and the balance for general corporate purposes, including working capital and capital expenditures, which may include the acquisition or in-licensing of additional compounds, product candidates and technology, although we have no present commitments or agreements to enter into any such acquisitions. See “Use of Proceeds.” |
| Risk factors |
See the section entitled “Risk Factors” beginning on page 17 and the other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our common stock. |
| Proposed Nasdaq symbol |
“AURX.” |
The number of shares of our common stock to be outstanding immediately after this offering as set forth above assumes that Alchemia Limited will distribute 7,592,020 shares of our common stock that are currently outstanding and held by it to Alchemia Limited shareholders (or a depositary on their behalf) in the Demerger, which number of shares is calculated on the basis of 280,812,962 ordinary shares of Alchemia Limited outstanding as of November 1, 2012 and the distribution of one share of our common stock for every 37 outstanding ordinary shares of Alchemia Limited. However, the actual number of shares of our common stock distributed in the Demerger will depend upon the number of holders and the number of outstanding ordinary shares of Alchemia Limited as of the record date for the Demerger, which will be approximately four trading days following the date of this prospectus, and the terms of the Demerger provide that fractional shares of our common stock that would otherwise be distributed pursuant to the Demerger will be rounded upwards to the nearest whole share. In addition, options to purchase a total of 3,573,000 ordinary shares of Alchemia Limited at exercise prices ranging from AUD$0.280 to AUD$1.040 per share (equivalent to approximately US$0.291 to US$1.079 per share based on the currency exchange rate as of November 1, 2012) were outstanding as of November 1, 2012, and any exercise of these options or any other options that Alchemia Limited may grant prior to the record date for the Demerger would increase the number of ordinary shares of Alchemia Limited outstanding as of such record date. Any increase in the number of holders or the number of outstanding ordinary shares of Alchemia Limited as of the
record date for the Demerger compared with the number of holders or the number of outstanding ordinary shares
-9-
Table of Contents
as of November 1, 2012 would increase, and the fact that fractional shares of our common stock distributed in the Demerger will be rounded upwards to the nearest whole share as described above will increase, the number of shares of our common stock distributed in the Demerger above the number of shares we have assumed for purposes of this prospectus. To the extent that the total number of shares of our common stock distributed in the Demerger exceeds the 7,592,020 shares currently held by Alchemia Limited, we will issue additional shares of common stock for distribution in the Demerger. As a result, both the number of shares of our common stock that will be distributed in the Demerger and that will be outstanding immediately after this offering will be greater than the respective numbers of shares we have assumed for purposes of this prospectus. Each 37 share increase (decrease) in the number of ordinary shares of Alchemia Limited outstanding as of the record date for the Demerger would increase (decrease) the number of shares of our common stock to be distributed in the Demerger and to be outstanding immediately after this offering by one share (before giving effect to the rounding upwards of fractional shares as described above).
The number of shares of our common stock to be outstanding immediately after this offering excludes:
| • | shares of our common stock, or the Warrant Shares, issuable upon the exercise of warrants, or the Warrants, that we will issue after this offering to investors who purchased ordinary shares of Alchemia Limited in a private placement and that will have an exercise price equal to 130% of the volume weighted average price of our common stock for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market (we sometimes refer to such volume weighted average price for such period as the Average Trading Price). The Warrants will be issuable approximately 60 trading days subsequent to completion of this offering and the number of Warrants so issued will be equal to the quotient obtained by dividing (1) AUD$8,073,000 (equivalent to approximately US$8,377,352 based on the currency exchange rate as of November 1, 2012) by (2) 130% of the Average Trading Price. Each Warrant will initially be exercisable for one share of our common stock. Assuming the currency exchange rate mentioned above and assuming an Average Trading Price of US$15.00 per share (the midpoint of the estimated price range set forth on the cover page of this preliminary prospectus), approximately 429,608 Warrants (initially exercisable for a like number of Warrant Shares) would be issuable with an exercise price of approximately US$19.50 per share. However, as the number of Warrants and exercise price both depend upon the actual Average Trading Price following this offering and the number of Warrants also depends upon the actual exchange rate between U.S. dollars and Australian dollars following this offering, the actual number of Warrants that we issue (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the actual exercise price will likely differ from the numbers we have assumed for purposes of this preliminary prospectus. For example, a US$1.00 increase or decrease in the assumed Average Trading Price would decrease and increase, respectively, the number of Warrants (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) by approximately 26,851 and 30,686, respectively, and increase and decrease, respectively, the exercise price per share by approximately US$1.30, while a 10% increase (decrease) in the number of U.S. dollars per Australian dollar compared with the assumed currency exchange rate mentioned above would increase (decrease) the number of Warrants (and therefore the number of Warrant Shares for which the Warrants are exercisable) by approximately 42,961. In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock. For additional information, see “Description of Capital Stock — Warrants;” |
| • | options to purchase 948,000 shares of our common stock granted under our 2012 Stock Plan at an exercise price per share equal to the initial public offering price per share set forth on the cover page of this prospectus (following this offering, we do not expect to make further grants pursuant to our 2012 Stock Plan); and |
-10-
Table of Contents
| • | 1,250,000 shares of our common stock reserved for future issuance under our 2012 Equity Incentive Plan, which will become effective in connection with this offering, and additional shares of our common stock that will become available for issuance under our 2012 Equity Incentive Plan pursuant to provisions thereof that automatically increase the number of shares of our common stock reserved for issuance under the plan each year, beginning with our fiscal year commencing July 1, 2013, as more fully described in “Executive Compensation — 2012 Equity Incentive Plan.” |
Unless otherwise expressly stated or the context otherwise requires, the information in this prospectus, including the number of shares that will be outstanding immediately after this offering, reflects, assumes and gives effect to the following:
| • | the assumed distribution of 7,592,020 shares of our common stock in the Demerger (as described above, the actual number of shares of our common stock that are distributed in the Demerger will be greater); |
| • | the filing of our amended and restated certificate of incorporation and the effectiveness of our amended and restated bylaws, which will occur immediately prior to completion of this offering; |
| • | no exercise of any options to purchase Alchemia Limited ordinary shares during the period from November 1, 2012 through the record date for the Demerger, as such exercise would increase the number of shares of our common stock distributed in the Demerger; |
| • | no purchase of shares of our common stock in this offering by existing Alchemia Limited shareholders; and |
| • | no exercise by the underwriters of their over-allotment option. |
As discussed above under “—Corporate Information—Corporate Reorganization, ” on November 5, 2012, we issued an additional aggregate of 92,000 shares of our common stock to Alchemia Limited in further consideration for our receipt of the shares of Alchemia Oncology and which, together with the shares of our common stock previously owned by Alchemia Limited, will be distributed in the Demerger. Unless otherwise stated or the context otherwise requires, our historical share and per share information appearing in this prospectus has been retroactively adjusted to give effect to this issuance of additional shares of our common stock to Alchemia Limited.
-11-
Table of Contents
Special Note Regarding Currency Exchange Rate
Our functional currency for accounting purposes is the Australian dollar and our reporting currency and the currency in which we prepare our consolidated financial statements is the U.S. dollar. All dollar figures contained in this prospectus are set forth in U.S. dollars, except as otherwise indicated. All Australian dollar amounts translated into U.S. dollars have been translated at the following rates per Australian dollar, except as otherwise indicated. The following currency exchange rates reflect the average buying rates for the 24 hour period ending 22:00 Universal Coordinated Time on the respective prior days, published at www.oanda.com/currency.
| U.S. Dollars per Australian Dollar | ||||||||
| For Revenues
and Expenses(1) |
For Assets
and Liabilities(2) |
|||||||
| Fiscal Year Ended June 30, |
||||||||
| 2011 |
$ | 0.9890 | $ | 1.0595 | ||||
| 2012 |
$ | 1.0323 | $ | 1.0159 | ||||
| Three Months Ended September 30, |
||||||||
| 2011 |
$ | 1.0522 | $ | 0.9791 | ||||
| 2012 |
$ | 1.0391 | $ | 1.0377 | ||||
| (1) | These exchange rates represent average exchange rates during the periods. |
| (2) | These represent the exchange rates as of June 30 or September 30, as applicable. |
In addition, the exchange rate of U.S. dollars per Australian dollar as of November 1, 2012 was $1.0377.
-12-
Table of Contents
Summary Consolidated Financial Data
You should read the following summary consolidated financial data in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and related notes, all included elsewhere in this prospectus. As we are a subsidiary of Alchemia Limited, our consolidated financial statements are derived from the consolidated financial statements and accounting records of Alchemia Limited. Accordingly, our consolidated financial statements include allocations of expenses, primarily salaries and benefits (including stock-based compensation) of officers and other employees of Alchemia Limited, expenses for compensation and reimbursement paid to directors of Alchemia Limited and insurance expenses incurred by Alchemia Limited. These expenses have been allocated based on our estimated share of Alchemia Limited’s expenses using the proportional cost allocation method. Although we believe that these allocation methodologies are reasonable, the summary consolidated financial information set forth below and our consolidated financial statements do not purport to reflect the results of operations, financial position and cash flows that we would have achieved as a separate, publicly traded company during the periods presented or those that we will achieve in the future.
We derived the summary consolidated statement of operations data for the years ended June 30, 2011 and June 30, 2012 from our audited consolidated financial statements included elsewhere in this prospectus. The summary consolidated statement of operations data for the three months ended September 30, 2011 and September 30, 2012 and for the period from inception (May 29, 2006) to September 30, 2012 and the summary consolidated balance sheet data as of September 30, 2012 have been derived from our unaudited interim condensed consolidated financial statements included elsewhere in this prospectus. Our historical results of operations and financial condition do not purport to be indicative of our results of operations or financial condition as of any future date or for any future period.
-13-
Table of Contents
The following pro forma and pro forma as adjusted balance sheet data is based upon our consolidated balance sheet as of September 30, 2012. The pro forma and pro forma as adjusted balance sheet data set forth in the table below is illustrative only, does not purport to reflect what our actual consolidated balance sheet data would have been had the transactions described in the notes to such table occurred as of September 30, 2012, and our actual consolidated balance sheet data following those transactions will be different than the data set forth in such table. You should read this pro forma and pro forma as adjusted information together with “— Corporate Information,” “— The Offering,” and “Arrangements between Audeo Oncology, Inc. and Alchemia Limited.”
| Fiscal Year Ended June 30, |
Three Months Ended September 30, |
For the
period from inception (May 29, 2006) to September 30, 2012(2) | |||||||||||||||||||||||
| 2011 | 2012 | 2011 | 2012 | ||||||||||||||||||||||
| (in thousands, except per share data) | |||||||||||||||||||||||||
| Consolidated Statement of Operations Data: |
|||||||||||||||||||||||||
| Income: |
|||||||||||||||||||||||||
| Grant income |
$ | — | $ | — | $ | — | $ | 61 | $ | 1,570 | |||||||||||||||
| Research funding income |
— | — | — | — | 434 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Total income |
— | — | — | 61 | 2,004 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Operating expenses: |
|||||||||||||||||||||||||
| Research and development(1) |
6,986 | 7,807 | 850 | 4,020 | 42,325 | ||||||||||||||||||||
| General and administrative(1) |
929 | 1,674 | 234 | 850 | 6,629 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Total operating expenses |
7,915 | 9,481 | 1,084 | 4,870 | 48,954 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Loss from operations |
(7,915 | ) | (9,481 | ) | (1,084 | ) | (4,809 | ) | (46,950 | ) | |||||||||||||||
| Other income (expense): |
|||||||||||||||||||||||||
| Interest income |
2 | 3 | — | 10 | 209 | ||||||||||||||||||||
| Other income |
— | 7 | — | — | 434 | ||||||||||||||||||||
| Change in fair value of rights(3) |
— | (481 | ) | — | (336 | ) | (817 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Total other income (expense) |
2 | (471 | ) | — | (326 | ) | (174 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Loss before income taxes |
$ | (7,913 | ) | $ | (9,952 | ) | ($ | 1,084 | ) | $ | (5,135 | ) | $ | (47,124 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Income tax expense |
— | — | — | — | — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Net loss |
$ | (7,913 | ) | $ | (9,952 | ) | $ | (1,084 | ) | $ | (5,135 | ) | $ | (47,124 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Other comprehensive loss, net of tax: |
|||||||||||||||||||||||||
| Foreign currency translation adjustment |
(2,976 | ) | 1,421 | 1,751 | 158 | (2,101 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Total comprehensive loss |
$ | (10,889 | ) | $ | (8,531 | ) | $ | 667 | $ | (4,977 | ) | $ | (49,225 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Net loss per share: |
|||||||||||||||||||||||||
| Basic and diluted |
$ | (4.03) | $ | (4.95) | $ | (0.55) | $ | (0.68) | $ | (21.45) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Weighted-average shares outstanding: |
|||||||||||||||||||||||||
| Basic and diluted |
1,965,641 | 2,011,885 | 1,965,641 | 7,592,020 | |
2,196,429 |
| ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| (1) | Includes stock-based compensation expense as follows: |
| Fiscal Year Ended June 30, |
Three Months Ended September 30, |
For the period from inception (May 29, 2006) to September 30, 2012(2) | |||||||||||||||||||||||
| 2011 | 2012 | 2011 | 2012 | ||||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||||
| Research and development |
$ | 85 | $ | 97 | $ | 23 | $ | 5 | $ | 657 | |||||||||||||||
| General and administrative |
30 | 91 | 6 | 6 | 571 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Total stock-based compensation |
$ | 115 | $ | 188 | $ | 29 | $ | 11 | $ | 1,228 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
-14-
Table of Contents
| (2) | Date of inception refers to date of Alchemia Limited acquired Alchemia Oncology (which was known as Meditech Research Ltd. prior to its acquisition). |
| (3) | As described above under “—The Offering,” if and when this offering is completed and the Average Trading Price of our common stock has been determined for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market, we are required to issue Warrants to purchase shares of our common stock to investors who purchased ordinary shares of Alchemia Limited in a private placement in November and December 2011. The rights of these investors, or the Rights, pursuant to which we are required, contingent upon completion of this offering, to issue the Warrants, are classified as a current liability on our balance sheet in an amount initially equal to their fair value on the date of issuance and the fair value of the Rights is remeasured at the end of each reporting period with any change in the fair value recorded in our statement of operations for such period. One factor impacting the fair value of the Rights is the underlying value of our common stock. Assuming no changes to other factors, the fair value of the Rights increases or decreases in proportion with increases and decreases, respectively, in the value of our common stock. In addition, if and when this offering is completed and the Average Trading Price of our common stock is determined as described above under “—The Offering,” the fair value of the Rights will be remeasured. As the initial exercise price of the Warrants and the number of Warrants will have been fixed when such Average Trading Price is determined, the fair value of the Warrants will at that time be reclassified from current liabilities to stockholders’ equity on our balance sheet and the Warrants will be accounted for as equity awards. Any increase or decrease in the fair value for the then current period compared with the fair value as of the end of our then most recently ended reporting period will be reflected in our statement of operations for the then current period. The remeasurement of the fair value of the Rights at each balance sheet date (prior to such reclassification) and at the time of such reclassification may result in additional expense, and any such expense may have a material adverse effect on our results of operations. The remeasurement of the fair value of the Rights will cease after they have been reclassified to stockholders’ equity. In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Operations Overview—Fair Value of Financial Instruments” and “—Off-Balance Sheet Arrangements—Warrants.” |
| As of September 30, 2012 | ||||||||||||
| Actual | Pro
Forma for Demerger(1)(2) |
Pro Forma As Adjusted for Offering(2)(3) |
||||||||||
| (in thousands) | ||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||
| Cash and cash equivalents |
$ | 4,503 | 4,218 | 46,917 | ||||||||
| Short-term deposit |
21 | 21 | 21 | |||||||||
| Due from related party |
275 | — | — | |||||||||
| Total assets |
7,805 | 7,245 | 47,685 | |||||||||
| Due to related party |
697 | — | — | |||||||||
| Total current liabilities |
5,534 | 4,974 | 2,953 | |||||||||
| Total liabilities |
5,744 | 5,184 | 3,163 | |||||||||
| Total stockholders’ equity |
2,061 | 2,061 | 44,522 | |||||||||
| (1) | Gives effect to the Demerger, including our anticipated assumption of approximately $137,129 of Alchemia Limited’s employee entitlements liabilities in connection with the Demerger and the assumption that all balances due to and/or due from Alchemia Limited (which are reflected on our balance sheet as amounts due from or owed to related party) will be cash settled prior to the Demerger, as if all of those transactions had occurred as of September 30, 2012. This pro forma balance sheet data is illustrative only, is based on the |
-15-
Table of Contents
| amount of the applicable employee entitlements liabilities and amounts due to and/or due from Alchemia Limited as of September 30, 2012, does not purport to reflect what our actual consolidated balance sheet data would have been had the foregoing transactions occurred as of that date and will change based on the actual amount of Alchemia Limited’s employee entitlements liabilities that we assume in the Demerger and the actual amount of balances due to and/or due from Alchemia Limited that are settled in cash. |
| (2) | As described in note (3) to our summary consolidated statement of operations data above, if and when this offering is completed and the Average Trading Price of our common stock has been determined for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market, the fair value of the Rights will be remeasured. As the initial exercise price of the Warrants and the number of Warrants will have been fixed when such Average Trading Price is determined, the fair value of the Warrants will at that time be reclassified from current liabilities to stockholders’ equity on our balance sheet and the Warrants will be accounted for as equity awards. However, because neither the Rights nor the Warrants are directly attributable to this offering and such reclassification will not occur until approximately 60 trading days after our common stock is listed on the Nasdaq Global Market, the pro forma and pro forma as adjusted information set forth above does not give effect to this reclassification. The remeasurement of the fair value of the Rights at each balance sheet date (prior to such reclassification) and at the time of such reclassification may result in additional expense, and any such expense may have a material adverse effect on our results of operations. In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Operations Overview—Fair Value of Financial Instruments” and “—Off-Balance Sheet Arrangements—Warrants.” |
| (3) | Gives effect to the transactions described in note (1) above and the sale of shares of our common stock in this offering at an assumed public offering price of $15.00 per share (the midpoint of the estimated price range set forth on the cover page of this preliminary prospectus), after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us (excluding $237,775 of offering expenses already paid by us through September 30, 2012), as if all of those transactions had occurred as of September 30, 2012. This pro forma as adjusted information is illustrative only, is based on the amount of the applicable employee entitlements liabilities and amounts due to and/or due from Alchemia Limited as of September 30, 2012, does not purport to reflect what our actual consolidated balance sheet data would have been had the forgoing transactions occurred as of that date and will change based on the actual amount of Alchemia Limited’s employee entitlements liabilities that we assume in the Demerger and the actual amount of balances due to and/or due from Alchemia Limited that are settled in cash, the actual initial public offering price and other terms of this offering determined at pricing and the actual offering expenses payable by us. Each $1.00 increase (decrease) in the assumed initial public offering price of $15.00 per share would increase (decrease) cash and cash equivalents, total assets and total stockholders’ equity by approximately $3.0 million, assuming that there is no change in the amount of employee entitlements liabilities assumed or amount of balances due to and/or due from Alchemia Limited that are settled in cash in connection with the Demerger, that the number of shares offered by us, as set forth on the front cover of this prospectus, remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted data assumes that we initially invest all of the estimated net proceeds from this offering in cash and cash equivalents and does not give effect to our use of the proceeds from the sale of shares of common stock in this offering for the other purposes set forth under “Use of Proceeds.” |
-16-
Table of Contents
Investing in our common stock involves a high degree of risk. You should carefully consider the following risks and all other information contained in this prospectus, including our consolidated financial statements and the related notes, before investing in our common stock. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, also may become important factors that affect us. If any of the following risks materialize, our business, financial condition, results of operations and prospects could be materially adversely affected. In that case, the trading price of our common stock could decline, and you may lose some or all of your investment.
Risks Related to Our Business and Industry
We have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future. We may never achieve or sustain profitability and will require additional funding.
We are a clinical-stage biopharmaceutical company with a limited operating history. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. We are not profitable and have incurred losses in each year since our inception and we expect to have substantial losses and negative cash flow from operations for the foreseeable future. We have not generated any revenue from product sales to date. We continue to incur substantial research and development and other expenses related to our ongoing operations. Our net loss for the years ended June 30, 2011 and 2012 was approximately $7.9 million and $10.0 million, respectively, and for the three months ended September 30, 2011 and 2012 was approximately $1.1 million and $5.1 million, respectively. As of September 30, 2012, we had an accumulated deficit of approximately $47.1 million. We expect to continue to incur significant losses and negative cash flow from operations for fiscal 2013 and the foreseeable future, and we expect these losses and negative cash flow from operations to increase as we continue to pursue development of, and seek regulatory approvals for, oncology drugs using our HyACT technology, including HA-Irinotecan for the treatment of metastatic colorectal cancer, or mCRC, and begin to commercialize any of those product candidates that may receive regulatory approval, and as we pursue discovery of new drugs using our VAST technology. As a result, we are subject to all of the risks incident to the creation of new drugs and oncology treatments, and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. If any of our product candidates fails in clinical trials or does not gain regulatory approval, or if any of our product candidates receives regulatory approval but does not receive adequate labeling or thereafter fails to achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. In addition, we will require additional funding in the future. See the risk factor below entitled “— We will require substantial additional funding which may not be available to us on acceptable terms, or at all.”
We are a clinical-stage company with no approved products, which makes it difficult to assess our future viability.
We are a clinical-stage company, do not have any approved products or products pending approval and therefore have not derived any revenue from the sales of products. Our operations to date have been limited to organizing and staffing our company, acquiring, developing and securing our technology, undertaking preclinical studies and clinical trials of our product candidates and engaging in research. We have not yet demonstrated an ability to obtain regulatory approval, formulate and manufacture commercial-scale products, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, there is limited information about us for investors to use as a basis for assessing our future viability and whether we will be successful in developing our product candidates, conducting clinical trials, obtaining regulatory approvals or commercializing any product candidates for which we may receive regulatory approval.
-17-
Table of Contents
We are highly dependent on the success of our lead product candidate, the cancer drug HA-Irinotecan for the treatment of mCRC, and we cannot give any assurance that we will successfully complete its clinical development, or that it will receive regulatory approval, or if approved, that it will receive adequate labeling that will allow for successful commercialization for the treatment of mCRC.
Our lead product candidate, HA-Irinotecan for the treatment of mCRC, has been evaluated in a randomized Phase II clinical trial for the treatment of mCRC, and we recently began enrollment for a Phase III clinical trial. Although enrollment also recently began for an investigator-sponsored Phase II clinical trial for HA-Irinotecan for the treatment of small cell lung cancer, or SCLC, and our participation in this investigator-sponsored trial has the potential to further validate the HyACT technology as well as to provide clinical data on HA-Irinotecan’s activity on cancer stem cells, we currently do not intend to pursue further clinical trials or commercialization of HA-Irinotecan for the treatment of SCLC. Our current and future trials may not be successful, and HA-Irinotecan may never receive regulatory approval, or if approved, may not receive adequate labeling that allows for successful commercialization for the treatment of mCRC. We may fail to obtain necessary marketing approvals for HA-Irinotecan for the treatment of mCRC from the U.S. Food and Drug Administration, or FDA, or similar non-U.S. regulatory authorities if our clinical development program for HA-Irinotecan for the treatment of mCRC fails to demonstrate with substantial evidence that it is safe and effective to the satisfaction of such authorities, or if we have inadequate financial or other resources to advance HA-Irinotecan for the treatment of mCRC through clinical trials. Even if HA-Irinotecan for the treatment of mCRC receives regulatory approval, we may not receive adequate labeling for the product or be successful in marketing it for a number of reasons, including the introduction by our competitors of more clinically-effective or cost-effective alternatives or failure of our sales and marketing efforts. Any failure to obtain regulatory approval of HA-Irinotecan for the treatment of mCRC and successfully commercialize it would have a material and adverse impact on our business.
The results of our previous clinical trials may not be predictive of future results, and our current and planned clinical trials may not satisfy the requirements of the FDA or other non-U.S. regulatory authorities.
The clinical trials of our product candidates are, and the manufacturing and marketing of any of our product candidates that may receive regulatory approval will be, subject to extensive and rigorous review and regulation by numerous government authorities in the United States and in other countries where we intend to apply for registration and, if approved, market our product candidates. Before obtaining regulatory approvals for the commercial sale of any product candidate, we must demonstrate through preclinical testing and clinical trials that the product candidate is safe and effective for use in each indication for which we intend to market such product candidate. This process can take many years and requires the expenditure of substantial financial and human resources, may include post-marketing trials and surveillance and ultimately may not prove successful. To date, we have not completed any Phase III clinical trials. We have completed one Phase II randomized clinical trial with HA-Irinotecan for the treatment of patients with mCRC. In addition, we have other product candidates in the discovery and preclinical testing stages.
Positive results from preclinical studies and early-stage clinical trials should not be relied upon as evidence that later-stage or large-scale clinical trials will succeed. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, even after promising results in earlier trials. We will be required to demonstrate with substantial evidence through adequate and well-controlled clinical trials, including our on-going Phase III trial of HA-Irinotecan for the treatment of mCRC, that our product candidates are safe and effective for use in the target population before we can seek regulatory approvals for their commercial sale.
Our Phase II clinical trial of HA-Irinotecan did not meet its primary endpoint and our Phase III clinical trial of HA-Irinotecan may not be approved even if it achieves its primary endpoint.
Our product candidates may not be approved even if they achieve the primary endpoints in Phase III clinical trials or registration trials. The FDA or non-U.S. regulatory authorities may disagree with our trial design,
-18-
Table of Contents
clinical end points or our interpretation of data from preclinical studies and clinical trials or may change requirements for the approval of our product candidates even after reviewing and providing comment on a protocol for a pivotal Phase III clinical trial that has the potential to result in FDA approval.
Our Phase II clinical trial of HA-Irinotecan for the treatment of mCRC did not meet its primary endpoint of a significant reduction in the incidence of diarrhea and our Phase III clinical trial of HA-Irinotecan for the treatment of mCRC may not be approved even if it achieves its primary endpoint of progression free survival, or PFS. After having consultations with the FDA and the EMA, we commenced our Phase III clinical trial of HA-Irinotecan for the treatment of mCRC. Based on our initial consultations with the FDA, we believe that the 505(b)(2) regulatory approach is an appropriate pathway to seek regulatory approval of HA-Irinotecan, as HA-Irinotecan is a formulation of two previously approved products (the cancer drug irinotecan and HA), although there can be no assurance in this regard. Reliance on the 505(b)(2) regulatory pathway requires establishing that such reliance is scientifically appropriate at the time of submission of a New Drug Application, or NDA. If the FDA does not agree with our position that we may rely on the 505(b)(2) regulatory pathway, we will not be permitted to reference approval of similar products or published literature and will be required to develop our own data to pursue regulatory approval, which would substantially increase both the time and cost of the regulatory process. We have sought and have received input from the EMA on the preclinical and clinical development of HA-Irinotecan for the treatment of mCRC and are taking into consideration this input in our European regulatory strategy.
Because of the difficulty in reliably determining cancer progression based on imaging studies in cancer, the FDA’s policy is to recommend that the primary endpoint for a cancer registration trial be overall survival, or OS, whereas our Phase III clinical trial has PFS as its primary endpoint. If our pivotal Phase III clinical trial is successful, we intend to seek regulatory approval from the FDA of HA-Irinotecan for the treatment of mCRC in the United States; however, even if such approval is granted, it may not allow us to charge a premium over a generic version of irinotecan if the data from our Phase III clinical trial is not sufficient to support FDA approval of a label containing data that HA-Irinotecan is superior to a generic version of irinotecan, and even if our label contains data adequate to allow us to price HA-Irinotecan at a premium to generic irinotecan, there is no assurance that payors will pay such a premium. If we are unable to charge, or if payors are unwilling to pay, a premium price for HA-Irinotecan, we may not be able to successfully commercialize this product candidate, which could have a material adverse effect on our business.
We have proceeded with our Phase III trial of HA-Irinotecan for the treatment of mCRC even though our Phase II trial did not meet its primary endpoint of a significant reduction in the incidence of diarrhea. Our Phase III trial of HA-Irinotecan has PFS as its primary endpoint and, even if this trial meets that endpoint, there can be no assurance that the FDA will grant approval based on this trial. The FDA may require an additional trial with OS as a primary endpoint before or after approval. For example, even if our Phase III clinical trial meets its PFS primary endpoint, an additional trial regarding OS could be required as either a pre-approval requirement or as a post-marketing commitment should approval be granted based on PFS. If the FDA were to require a second Phase III clinical trial as a pre-approval requirement, it would substantially increase the time and cost of our development activities and, in any event, may not result in regulatory approval. In addition, if the FDA approves HA-Irinotecan for mCRC based on meeting our PFS primary endpoint but requires post-marketing clinical trials or analyses, the approval could be withdrawn if any required post-marketing trials or analyses do not meet FDA requirements. Any requirement for additional clinical trials following completion of our Phase III trial of HA-Irinotecan would substantially increase our costs and could have a material adverse effect on our business, results of operations and prospects.
-19-
Table of Contents
There is a risk that our development and clinical activities will not result in commercial products, and we will invest in our current development and clinical programs, to the exclusion of others, for several more years before it is known whether one or more of our product candidates will receive regulatory approval, obtain adequate labeling or be commercially introduced.
Our product candidates are in various stages of development and are prone to the risks of failure inherent in biopharmaceutical development. We will need to complete significant additional clinical trials demonstrating that our product candidates are safe and effective to the satisfaction of the FDA and other non-U.S. regulatory authorities. Pursuit of our drug discovery activities and clinical programs are costly, time consuming and of uncertain outcome. Failure can occur at any stage of the process. If such programs are not successful, we may invest substantial amounts of time and money without developing revenue-producing products. As we enter a more extensive clinical program in several different diseases, the data generated in these studies may not be as compelling as the earlier results. Further, even if our product candidates receive required regulatory approvals, we cannot assure you that they will be successful commercially. In addition, we have a number of product candidates in our development pipeline, and while we invest in the technology and indications that we believe are most promising, financial and resource constraints may require us to forego or delay opportunities that may ultimately have greater commercial potential than those programs we are currently actively developing or choose to actively develop in the future. There is no assurance that product candidates developed using our technology will prove to be safe and efficacious in clinical trials, or that the regulatory approval to manufacture and market our product candidates will be received, or if received, that such regulatory approval will result in a commercially viable product. Product candidates utilizing our technology may be shown to be unsafe, non-efficacious, difficult or impossible to manufacture on a large scale, uneconomical to market, compete with superior products marketed by third parties or not be as attractive as alternative treatments.
Our development activities could be delayed or stopped for a number of reasons, many of which are outside our control, including failure to recruit and enroll patients for clinical trials.
Clinical trials of promising product candidates can take years, the duration depending on, among other factors, type, complexity, novelty and intended use of the product candidate. We do not know whether our current clinical trials will be completed on schedule, or at all, and we cannot guarantee that our future planned clinical trials will begin on time, or at all. Our current and planned clinical trials could be substantially delayed or prevented by several factors, including:
| • | limited number of, and competition for, suitable sites to conduct our clinical trials; |
| • | government or regulatory delays and changes in regulatory requirements, policy and guidelines; |
| • | delay or failure to obtain sufficient supplies of the product candidate for our clinical trials as a result of non-compliance with regulatory requirements, including current Good Manufacturing Practice, or GMP, by our suppliers or for other reasons; |
| • | delay or failure to reach agreement on acceptable clinical trial agreement terms with prospective sites or investigators; |
| • | failure to obtain required regulatory approvals; |
| • | delay or failure to obtain institutional review board, or IRB, approval to conduct a clinical trial at a prospective site; |
| • | slower than expected rates of patient recruitment and enrollment; |
| • | variability in the number and types of patients available for each study; |
| • | unforeseen safety issues or side effects; |
-20-
Table of Contents
| • | lack of efficacy evidenced during clinical trials, which risk may be heightened given the advanced state of disease and lack of response to prior therapies of patients in our clinical trials for HA-Irinotecan; |
| • | termination or delays of our clinical trials by an IRB at one or more institutions assisting us with our clinical trials; |
| • | adding new clinical trial sites; |
| • | inability or unwillingness of patients or medical investigators to follow our clinical trial protocols, or medical investigators and their staff to comply with Good Clinical Practices, or GCPs; |
| • | difficulty in maintaining contact with patients after treatment, resulting in incomplete data; and |
| • | inability to monitor patients adequately during or after treatment or high patient dropout rates. |
Patient enrollment, a significant factor in the timing of clinical trials, is affected by many factors including the size and nature of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating. We began recruiting patients for our Phase III clinical trial testing HA-Irinotecan in patients with mCRC in November 2011. Recruitment and enrollment of patients for this clinical trial was initially slow, with our first patient dosed in January 2012, and we cannot guarantee that patient enrollment for this or any future clinical trials will be completed by the dates we anticipate or will not be adversely affected by the factors described above. Furthermore, we rely on contract research organizations and clinical trial sites to ensure the proper and timely conduct of our clinical trials and while we typically enter into agreements governing the activities that they conduct for us, we have limited influence over their actual performance.
We could encounter delays if a clinical trial is suspended or terminated by us, by the IRBs of the institutions in which such trials are being conducted, by the Data Safety Monitoring Board for such trial or by the FDA or other regulatory authorities. Such authorities may suspend or terminate a clinical trial due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If we experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate revenues from any of these product candidates will be delayed or terminated. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that could cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
The regulatory approval processes of the FDA and non-U.S. regulatory authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, or if approved, the labeling is not adequate, our business will be substantially harmed.
The research, development, manufacture, marketing and sale of product candidates using our technology are subject to extensive regulation by a number of government authorities, particularly the FDA. The time required to obtain approval by the FDA and non-U.S. regulatory authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the
-21-
Table of Contents
substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not submitted for nor obtained regulatory approval for any product candidate and it is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain regulatory approval.
The regulatory approval process is inherently subject to risks, including that approval will not be granted, approval may not support commercial launch or approval may be subject to unforeseen delay or may be subject to onerous conditions which may adversely impact our ability to achieve our objectives. Our product candidates could fail to receive regulatory approval for many reasons, including the following:
| • | the FDA or non-U.S. regulatory authorities may disagree with the design or implementation of our clinical trials or study endpoints; |
| • | we may be unable to demonstrate to the satisfaction of the FDA or non-U.S. regulatory authorities that a product candidate is safe and effective for its proposed indication or that our product candidates provide significant clinical benefits; |
| • | the results of clinical trials may not meet the level of statistical significance required by the FDA or non-U.S. regulatory authorities in order to demonstrate that our product candidate has superior efficacy to a generic version of the applicable cancer drug and allow approval of a label allowing us to price at a premium compared with the generic product; |
| • | we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| • | the FDA or non-U.S. regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| • | the data collected from clinical trials of our product candidates may not be sufficient to support the submission of an NDA or other submission or to obtain regulatory approval in the United States or elsewhere; |
| • | the FDA or non-U.S. regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
| • | the approval policies or regulations of the FDA or non-U.S. regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
We may be unable to secure necessary approvals from regulatory agencies and institutional bodies (including clinics and hospitals) to conduct clinical trials. The lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval to market HA-Irinotecan for the treatment of mCRC or our other product candidates, which would materially harm our business, results of operations and prospects. To date, we have not filed for marketing approval for any of our product candidates and may not receive the approvals necessary to successfully commercialize our product candidates in any market. The failure to obtain regulatory approval, or approval of a label stating that our product candidate has demonstrated superior efficacy of statistical significance over a generic version of the applicable cancer drug and allowing us to price at a premium to the generic drug in any jurisdiction could materially harm our business and may have a negative effect on the regulatory approval process in other jurisdictions.
In addition, even if we were to obtain approval, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may not approve the price we intend to charge for our product candidates, may grant approval contingent on the performance of costly post-marketing clinical
-22-
Table of Contents
trials, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Any of the foregoing could materially harm the commercial prospects for our product candidates.
If HA-Irinotecan does not demonstrate superior efficacy to generic irinotecan for the treatment of mCRC in our Phase III clinical trial, it is unlikely that we will receive FDA approved labeling allowing us to price HA-Irinotecan at a premium to generic irinotecan, and even if our label contains data adequate to allow us to price HA-Irinotecan at a premium to generic irinotecan, there is no assurance that payors will pay such a premium, either of which could have a material adverse effect on our business.
We believe that HA-Irinotecan for the treatment of mCRC, if it receives regulatory approval, has the potential of commanding pricing consistent with proprietary drugs. However, such pricing is dependent upon a number of factors, including HA-Irinotecan demonstrating superior efficacy over generic irinotecan for the treatment of mCRC in clinical trials and the FDA approving a label containing data that HA-Irinotecan has demonstrated superior efficacy to a generic version of irinotecan, and we cannot assure you that this will occur. If our clinical trials do not demonstrate superior efficacy, then we may not receive FDA approval, or if approved, our label may not include data to support that HA-Irinotecan has superior efficacy to generic irinotecan, and the pricing for HA-Irinotecan for the treatment of mCRC may be substantially lower than we anticipate, which could have a material adverse effect on our business, operating results and financial condition. Moreover, even if our label contains data adequate to allow us to price our product candidate at a premium to generic irinotecan, there is no assurance that payors will pay such a premium, which could also have a material adverse effect on our business, operating results and financial condition.
We may not obtain approval to market our product candidates outside the United States or negotiate satisfactory pricing for our product candidates in foreign jurisdictions, which could adversely impact our future profitability.
We intend to seek approval to market certain of our product candidates in both the United States and in non-U.S. jurisdictions. In order to market our product candidates in the European Union and many other non-U.S. jurisdictions, we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. To date, we have not filed for marketing approval for any of our product candidates and may not receive the approvals necessary to successfully commercialize our product candidates in any market. The approval procedure varies among countries and can involve additional testing and data review. The time and safety and efficacy data required to obtain non-U.S. regulatory approval may differ from that required to obtain FDA approval. The non-U.S. regulatory approval process will include all of the risks associated with obtaining FDA approval. We may not obtain non-U.S. regulatory approvals on a timely basis, or at all. Approval by the FDA does not ensure approval by regulatory agencies in other countries, and approval by one non-U.S. regulatory authority does not ensure approval by regulatory agencies in other countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in other jurisdictions, including approval by the FDA. The failure to obtain regulatory approval in any jurisdiction could materially harm our business.
Even if we receive regulatory approval for any of our product candidates, we will be subject to ongoing obligations and continued regulatory review, which will result in significant additional expense. Additionally, our product candidates, if approved, could be subject to labeling and other restrictions and market withdrawal and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with any of our product candidates that receive regulatory approval.
Any regulatory approvals that we receive for our product candidates may also be subject to limitations on the approved indicated uses or comparative statements for which the product candidates may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including post-marketing clinical trials, and surveillance to monitor the safety and efficacy of the product candidate. In addition,
-23-
Table of Contents
if the FDA or a non-U.S. regulatory authority approves any of our product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the product candidate will be subject to extensive and ongoing regulatory requirements. These requirements will include submissions of safety and other post-marketing information and reports and registration, as well as continued compliance with current Good Manufacturing Practices, or cGMPs, and GCPs, for any clinical trials that we conduct post-approval. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| • | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| • | fines, warning letters, adverse publicity or holds on clinical trials; |
| • | refusal by the FDA or similar non-U.S. regulatory authorities to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals; |
| • | product seizure or detention, or refusal to permit the import or export of products; and |
| • | injunctions or the imposition of civil or criminal penalties. |
The policies of the FDA or similar non-U.S. regulatory authorities may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
The coverage and reimbursement status of newly approved pharmaceuticals is uncertain, and failure to obtain adequate coverage and adequate reimbursement of HA-Irinotecan or other product candidates could limit our ability to generate revenue.
There is significant uncertainty related to the third-party coverage and reimbursement of newly approved drugs. The commercial success of our product candidates, including HA-Irinotecan, in both domestic and international markets will depend in large part on the availability of coverage and adequate reimbursement from third-party payors, including government payors such as the Medicare and Medicaid programs and managed care organizations, and may be affected by existing and future health care reform measures. Government and other third-party payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for new drugs and, as a result, they may not cover or provide adequate payment for our product candidates. Obtaining coverage and reimbursement approval for a product from a government or other third-party payor is a time consuming and costly process that could require us to provide supporting scientific, clinical and cost-effectiveness data for the use of our products to the payor. We may not be able to provide data sufficient to gain acceptance with respect to adequate coverage and reimbursement. These payors may conclude that our product candidates are less safe, less effective or less cost-effective than existing or later introduced products, and third-party payors may not approve our product candidates for coverage and reimbursement or may cease providing or provide inadequate coverage and reimbursement for these product candidates. As there is a large number of both governmental and third-party payors, obtaining coverage reimbursement in the United States and internationally will likely take significant time and cause us to spend significant resources. We also anticipate that we will need to provide substantial evidence to payors that HA-Irinotecan for the treatment of mCRC, if approved, provides superior efficacy compared with generic irinotecan before these payors will be willing to pay a price for our drug that is greater than the price for generic irinotecan. There can be no assurance that HA-Irinotecan will demonstrate superior efficacy over generic irinotecan for the treatment of mCRC in our Phase III clinical trial, or that the FDA
-24-
Table of Contents
will approve a label for HA-Irinotecan for the treatment of mCRC containing data that HA-Irinotecan has demonstrated superior efficacy to a generic version of irinotecan and allowing us to price HA-Irinotecan at a premium to generic irinotecan, and even if our label contains data adequate to allow us to price HA-Irinotecan at a premium to generic irinotecan, there is no assurance that payors will pay such a premium. The failure to obtain coverage and adequate reimbursement for our product candidates or healthcare cost containment initiatives that limit or deny reimbursement for our product candidates may materially and adversely affect our business.
In the United States and in other countries, there have been and we expect there will continue to be a number of legislative and regulatory proposals to change the healthcare system in ways that could significantly and adversely affect our business. International, federal and state lawmakers regularly propose and, at times, enact legislation that would result in significant changes to the healthcare system, some of which are intended to contain or reduce the costs of medical products and services. The U.S. government and other governments have shown significant interest in pursuing healthcare reform, as evidenced by the passing of the Patient Protection and Affordable Care Act and its amendment, the Health Care and Education Reconciliation Act, or the Health Care Reform Law. Such government-adopted reform measures may adversely impact the pricing of healthcare products and services in the United States or internationally and the amount of reimbursement available from governmental agencies or other third-party payors. In addition, in some foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our product candidates, if approved, profitably. The continuing efforts of U.S. and other governments, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce healthcare costs may adversely affect the prices we are able to charge for our product candidates, if approved, and our ability to generate revenues and achieve and maintain profitability.
In addition, the Health Care Reform Law mandates that manufacturers of branded prescription drugs pay an annual fee based on the sales activity of the manufacturer. Federal agencies will monitor and report the sales of pharmaceutical manufacturers in different sectors of the industry and the annual fees for each manufacturer will be based, in part, on the industry sector as well as their market share within that sector. We cannot predict how this annual fee may impact our future operating results or financial condition in the event that any of our product candidates receives regulatory approval.
In some countries, particularly in the European Union, prescription drug pricing is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct additional clinical trials that compare the cost-effectiveness of our product candidates to other available therapies. If reimbursement of our product candidates is unavailable or limited in scope or amount in a particular country, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability of our product candidates in such country.
Even if we are able to obtain regulatory approval of HA-Irinotecan in the treatment of mCRC, marketing will be limited by the intended indication and the potential size of the market.
Even if we are able to obtain regulatory approval of HA-Irinotecan based on our Phase III clinical trial for mCRC and formulate and manufacture a commercial-scale product, our marketing of HA-Irinotecan will be limited to our initial intended indication of mCRC and not cancer generally, or any other type of cancer. Our marketing claims may also be limited in the comparison statements we can make to irinotecan.
According to the American Cancer Society, colorectal cancer is the third most common form of cancer diagnosed in the United States, excluding skin cancers. According to the American Cancer Society, approximately 143,000 new cases of colorectal cancer are expected to be reported in the United States in 2012, with approximately 51,000 patients in the United States expected to die from colorectal cancer in 2012. Surgery is often the first line treatment for early stage colorectal cancer. When colorectal cancer metastasizes (spreads to other parts of the body such as the liver), chemotherapy is commonly used. Marketing of HA-Irinotecan, if
-25-
Table of Contents
approved for our intended indication, will be limited to those people with mCRC. Marketing efforts for HA-Irinotecan outside of our approved indications will require additional regulatory approvals, which we may never pursue or receive. In addition, we cannot guarantee that we will recover the cost of developing HA-Irinotecan or any of our other product candidates from any future commercial sales.
Our product candidates may cause undesirable side effects that could delay or prevent their regulatory approval or commercialization.
Our product candidates combining HyACT with a cancer drug have been observed to cause side effects, including, among others, nausea, vomiting, diarrhea and fatigue, that are consistent with the side effects observed to have been caused by the applicable drugs alone. As our HyACT-targeted product candidates have been tested in relatively small patient populations and for limited durations to date, additional side effects may be observed as their development progresses. The drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims.
Undesirable side effects caused by any of our product candidates could cause us or regulatory authorities to interrupt, delay or discontinue clinical trials and could result in the denial of regulatory approval by the FDA or non-U.S. regulatory authorities for any or all targeted indications. This, in turn, could prevent us from commercializing our product candidates and generating revenues from their sale. In addition, if one of our product candidates receives marketing approval and we or others later identify undesirable side effects caused by this product candidate:
| • | regulatory authorities may withdraw their approval of this product; |
| • | we may be required to recall this product, change the way this product is administered, conduct additional clinical trials or change the labeling of this product in a way that significantly narrows the indications for use; |
| • | we could be sued and held liable for harm caused to patients; |
| • | this product may be rendered less competitive and sales may decrease; or |
| • | our reputation may suffer generally both among clinicians and patients. |
Any one or a combination of these events could prevent us from achieving or maintaining market acceptance of the affected product candidate or could substantially increase the costs and expenses of commercializing the product candidate, which in turn could delay or prevent us from generating significant revenues from the sale of the product candidate.
We will require substantial additional funding which may not be available to us on acceptable terms, or at all.
We are advancing multiple product candidates through clinical development. As of September 30, 2012, our cash and cash equivalents were only approximately $4.5 million, our working capital was only approximately $1.8 million and we had an accumulated deficit of approximately $47.1 million. We expect to continue to incur substantial losses and negative cash flow from operations for fiscal 2013 and the forseeable future, we have no credit facility or other committed source of external financing and our cash, cash equivalents and working capital are limited. We estimate that as of November 1, 2012, we will need approximately $16.3 million to complete the current Phase III clinical trial of HA-Irinotecan for the treatment of mCRC and approximately $0.3 million to complete the current investigator-sponsored Phase II clinical trial of HA-Irinotecan for the treatment of SCLC, although these estimates are subject to assumptions and uncertainties and there can be no assurance that the actual costs will not be greater. Accordingly, we are and will be entirely dependent upon external financing to fund our cash needs and, even after giving effect to our receipt of the estimated net proceeds from this offering, we will need to raise substantial additional capital to continue our clinical development and preparing for
-26-
Table of Contents
commercialization activities. In particular, if our Phase III clinical trial is successful, we will need to raise substantial additional capital to fund the costs, which we expect will be significant, of seeking regulatory approval for HA-Irinotecan for the treatment of mCRC (if the proceeds from this offering are insufficient to cover those costs), and will also need to raise substantial additional capital to fund the costs, which we also expect to be significant, associated with commercializing HA-Irinotecan in the absence of a licensing partner, or if the FDA requires further clinical trials with respect to HA-Irinotecan for the treatment of mCRC. We will also need to raise substantial additional capital in order to proceed with the clinical development of, and seek regulatory approval for, any of our other product candidates. In addition, our VAST small molecule drug discovery technology currently relies on partnerships with pharmaceutical and biotechnology companies and on collaborations with academic groups and in the past has relied and in the future may rely on government grants, if available, and if Alchemia Limited is unable to assign these collaboration agreements and grant arrangements to us as described below or if those partnerships or collaborations terminate or if we are unsuccessful in obtaining future grants, we may need to find alternative sources of funding to further our drug discovery initiatives. There is no guarantee that we would be successful in obtaining any such funding on terms that we deem acceptable, or at all. In addition, limitations on new share issuances under Australian Securities Exchange listing rules may limit or prevent us from raising additional capital by issuing and selling shares of common stock or other securities when such additional capital is required. See “Our ability to raise additional capital may be significantly limited by listing rules of the Australian Securities Exchange that limit the amount of common stock that we are permitted to issue without stockholder approval.” Furthermore, as discussed below under the risk factor titled “Alchemia Limited may be unable to transfer its rights under collaboration agreements and government grant arrangements relating to the VAST technology to us,” there is also no guarantee that Alchemia Limited will be able to transfer its collaboration agreements and current grant funding arrangements relating to the VAST small molecule drug discovery technology to us, which may have an adverse affect on our funding.
Moreover, we have to date relied entirely upon Alchemia Limited, our parent corporation, for all of the funds necessary to conduct our research and development activities and operate our business. Alchemia Limited has provided these funds to us through loans, all of which Alchemia Limited converted to newly-issued ordinary shares of Alchemia Oncology prior to the date of this preliminary prospectus. Alchemia Limited has no obligation to provide us with financing following this offering and we understand that Alchemia Limited has no intention of doing so. We have no experience raising debt or equity financing from third parties and there can be no assurance that we will be able to do so on terms we deem acceptable, or at all.
The timing and amount of any future funding requirements will depend on many factors, including but not limited to:
| • | our need to expand our research and development activities; |
| • | the rate of progress and cost of our clinical trials and the need to conduct clinical trials beyond those currently planned; |
| • | the costs associated with establishing a sales force and commercialization capabilities, if any of our product candidates receives regulatory approval; |
| • | the costs of acquiring, licensing or investing in businesses, product candidates and technologies; |
| • | the costs and timing of seeking and obtaining approval from the FDA and non-U.S. regulatory authorities; |
| • | our ability to maintain, defend and expand the scope of our intellectual property portfolio; |
| • | our need and ability to hire additional management and scientific and medical personnel; |
| • | the effect of competing technological and market developments; |
-27-
Table of Contents
| • | our need to implement additional internal systems and infrastructure, including financial and reporting systems appropriate for a public company; |
| • | the economic and other terms and timing of collaboration, licensing or other arrangements into which we may enter; and |
| • | uncertainties regarding our ability to continue and maintain collaborations with pharmaceutical and biotechnology companies and collaborations with academic groups. |
Until we can generate a sufficient amount of revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs primarily through public or private equity or debt financings, any strategic collaborations we may enter into and any future government grants we may receive. We do not know whether additional funding, collaborations or grants will be available on acceptable terms, or at all, and we may not be able to raise funds as and when they are required. If we are not able to secure additional funding when needed, we may have to:
| • | delay, reduce the scope of or eliminate one or more of our clinical trials or research and development programs, including our VAST small molecule drug discovery technology; |
| • | enter into collaboration or other arrangements with other companies to provide such funding in exchange for our affording such partner commercialization or other rights to our product candidates, any products of ours that may receive regulatory approval, or other intellectual property or other assets; |
| • | license or sell our technologies on unfavorable terms; or |
| • | scale down or cease operations. |
Our ability to raise additional capital may be significantly limited by listing rules of the Australian Securities Exchange that limit the amount of common stock that we are permitted to issue without stockholder approval.
Limitations on new share issuances under Australian Securities Exchange listing rules may significantly limit or prevent us from raising additional capital by issuing and selling shares of our common stock or other securities when such additional capital is required. In particular, the Australian Securities Exchange listing rules will prohibit us from issuing, during any 12-month period, shares of our common stock in an amount greater than 15% of the total number of shares of our common stock then outstanding without the affirmative vote of the holders of a majority of the outstanding shares of our common stock. We have received the approval of Alchemia Limited’s shareholders to permit us to issue up to 25% of the total number of shares of our common stock then outstanding during the 12-month period commencing on the date we are admitted to the official list of the Australian Securities Exchange; however, this increase is only effective for that 12-month period and may still limit or prevent us from raising additional capital when such additional capital is required. As discussed elsewhere in this prospectus, we will require substantial additional financing to develop and commercialize our product candidates and execute our strategy and, because we do not have any revenues from product sales and would likely be unable to raise capital by borrowing funds, we will be dependent primarily upon issuing and selling additional shares of common stock to obtain such financing. The foregoing listing rule of the Australian Securities Exchange is substantially more restrictive than the comparable Nasdaq rule and, even with the approval of Alchemia Limited’s shareholders to permit us to issue up to 25% of the total number of shares of our common stock then outstanding during the 12-month period commencing on the date we are admitted to the official list of the Australian Securities Exchange, this rule may significantly limit or prevent us from raising funds by issuing and selling shares of our common stock, which may have a material adverse effect on our results of operations, financial condition and the development of our business. Moreover, seeking shareholder approval to issue common stock is likely to take considerable time and expense and there can be no assurance that any such approval will be given in the future.
-28-
Table of Contents
Alchemia Limited may be unable to transfer its rights under collaboration agreements and government grant arrangements relating to the VAST technology to us.
In connection with this offering, we have in-licensed the VAST small molecule drug discovery technology from Alchemia Limited. The VAST technology currently relies on Alchemia Limited’s collaborations with pharmaceutical companies and research institutes and has relied and in the future may rely on grants from the Australian government. We are not a party to any of these collaboration agreements or government grant arrangements and Alchemia Limited may be required to obtain the consent of its collaborators and the Australian government in order to transfer these collaboration agreements and government grant arrangements to us. There can be no assurance that these consents will be granted and, if they are not, it may have a material adverse effect on our ability to exploit the VAST technology and we may not receive the support provided by these collaborations and the additional funding potentially available under these government grant programs. Moreover, the license agreement pursuant to which we have in-licensed the VAST technology requires that we make certain minimum annual expenditures to exploit the VAST technology. If we do not receive the benefit of these collaborations and government grant arrangements, it may lessen our incentive to meet these annual expenditure requirements, which could result in a revocation of the VAST in-license by Alchemia Limited.
Raising additional capital may cause dilution to existing stockholders, restrict our operations, encumber our intellectual property or other assets or require us to relinquish rights.
We may seek the additional capital necessary to fund our operations through public or private equity or debt financings, and collaborative and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted and the terms of those securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures, or declaring dividends, or which impose financial covenants on us that limit our operating flexibility to achieve our business objectives. Likewise, we may be required to pledge some or all of our intellectual property or other assets to secure debt financing and, in the event we fail to comply with financial or other covenants in debt agreements or certain specified events of default occur, the lenders will typically be permitted to demand immediate repayment of all amounts that we have borrowed and, in the case of secured indebtedness, to seize and sell the collateral to repay that indebtedness. If we raise additional funds through collaboration and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us. In addition, we cannot assure you that additional funds will be available to us on favorable terms or at all.
We may be required to repay our government grant from the Australian government or any future grants that we receive.
We received one research grant from the Australian government under the Commercial Ready Grant for our HA-Irinotecan program to fund 50% of our research and development expenditures for a term of 3 years and six months, which ended on February 28, 2009. The total amount of grants received since the commencement of the Commercial Ready Program is approximately $1.7 million. The Australian government may require us to repay some or all of the grants we have received from them in the past or that we may receive from them in the future, together with interest, under specified circumstances. For example, we may be required to repay our past grants if we fail to use our best endeavors to commercialize the relevant grant project within a reasonable time of completion of the project, upon termination of the grant due to our breach of the agreement or insolvency, there is a change in control or ownership of our company that the Australian government reasonably considers to have an adverse effect on our ability to comply with any of our obligations in the grant agreement or we cease to carry on a substantial part of our business.
-29-
Table of Contents
We rely on third parties to manufacture and supply both the active pharmaceutical ingredients used in our HyACT product candidates and finished drug products, and our commercialization of any of our product candidates could be stopped, delayed or made less profitable if those third parties fail to obtain or maintain the approval of the FDA or non-U.S. regulatory authorities, fail to provide us with sufficient quantities of active pharmaceutical ingredients or drug product or fail to do so at acceptable quality levels or prices.
We do not manufacture, formulate or package any of our product candidates, except for small quantities used in preclinical studies and clinical trials that are manufactured for us by third parties. We do not currently own or operate manufacturing facilities for clinical or commercial production of our product candidates, and we are reliant on third parties for such activities. We have limited experience in drug formulation or manufacturing, and we lack the resources and the capability to manufacture any of our product candidates on a clinical or commercial scale. The facilities used by our contract manufacturers to manufacture both the active pharmaceutical ingredients, or API, used in our HyACT product candidates and finished drug products must be inspected and approved by the FDA pursuant to inspections that will be conducted after we submit our NDA to the FDA. Likewise, similar approvals are required by non-U.S. regulatory authorities. We do not control the manufacturing process of, and are completely dependent on, our contract manufacturing partners for compliance with the regulatory requirements, known as cGMPs, for manufacture of both API and finished drug products. If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or others, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. In addition, we have no control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or a non-U.S. regulatory authority does not approve these facilities for the manufacture of both the API and finished drug products or if it withdraws any such approval in the future, we would need to find alternative manufacturing facilities, which must be approved by regulatory authorities before we can use them, and which would significantly limit our ability to develop, obtain regulatory approval for or market our product candidates, if approved. Likewise, we may suffer significant delays and expenses in transitioning manufacturing to another facility or supplier. If any of our product candidates receives regulatory approval and we seek to manufacture commercial quantities of that product candidate, the ability of our contract manufacturers to produce commercial quantities will be subject to risks similar to those described above.
If for some reason our contract manufacturers cannot perform as agreed, we may be unable to replace them in a timely manner and the production of our product candidates would be interrupted, resulting in delays in clinical trials and additional costs. Switching manufacturers may be difficult because the number of potential manufacturers is limited and the FDA and similar non-U.S. regulatory authorities, if applicable, must approve any replacement manufacturer prior to manufacturing our product candidates. Such approval would require new testing and compliance inspections. In addition, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our product candidates and the API used in those product candidates after receipt of FDA or non-U.S. regulatory approval to manufacture any of our product candidates. It may be difficult or impossible for us to find a replacement manufacturer on acceptable terms quickly, or at all.
To date, our product candidates and the API used in those product candidates have been manufactured in small quantities for preclinical studies and clinical trials by third-party manufacturers, including, for HA-Irinotecan, Camptosar branded irinotecan hydrochloride, manufactured by, and sourced from, Pfizer Australia Pty Ltd., 1% sodium in water for injection, manufactured by Gland Pharma Ltd (India) using sodium hyaluronate from htl Biotechnology S.A.S. (France), and 5% dextrose solution commercially sourced from Baxter Healthcare Ltd (UK). If the FDA or other regulatory authorities approve any of our product candidates for commercial sale, we expect that we would continue to rely on third-party manufacturers to produce commercial quantities of our approved product candidates and the API used in those product candidates. These manufacturers (including the manufacturer that has agreed to act as our exclusive supplier of commercial quantities of HA as described below) may not be able to successfully build or increase the manufacturing capacity for any of our approved product candidates or the API used in those product candidates in a timely or economic manner, or at all. Newly constructed manufacturing facilities must be approved by the FDA and applicable non-U.S. regulatory authorities, and significant scale-up of manufacturing may
-30-
Table of Contents
require additional validation studies, which the FDA and any other applicable regulatory authorities must review and approve. Additionally, any third-party manufacturer we retain to manufacture our product candidates on a commercial scale must pass an FDA pre-approval inspection for conformance to the cGMPs before we can obtain approval of our product candidates and similar approvals are required by various non-U.S. regulatory authorities. If our third party manufacturers are unable to successfully increase the manufacturing capacity for a product candidate in conformance with cGMPs and similar requirements of applicable non-U.S. regulatory authorities, the regulatory approval or commercial launch of such product candidates may be delayed or there may be a shortage in supply.
We have entered into a supply agreement with Novozymes Biopharma DK A/S, or Novozymes, whereby we agree to use Novozymes as our exclusive supplier of HA if our HA-Irinotecan product candidate receives regulatory approval. Novozymes does not have the manufacturing capacity to supply the quantities of HA called for by the agreement and, although it has agreed to use commercially reasonable endeavors to build and maintain sufficient manufacturing capacity to supply us with HA pursuant to this agreement, there can be no assurance that it will be able to do so. Likewise, even if Novozymes builds sufficient manufacturing capacity, its ability to supply us with HA in commercial quantities is subject to approval of those facilities by the FDA and non-U.S. regulatory authorities and the other risks described in this risk factor. Either party may terminate the supply agreement upon a breach of the agreement by the other party, upon certain insolvency events relating to the other party or if it becomes unlawful for the other party to perform under the agreement. To the extent that Novozymes is unable to supply commercial quantities of HA for our HA-Irinotecan product candidate, if it receives regulatory approval, or if the agreement were terminated, it may have a material adverse effect on our business, results of operations and financial condition. For additional information about this supply agreement, see “Business—Manufacturing.”
Our product candidates require precise, high quality manufacturing. Failure by our contract manufacturers to achieve and maintain high manufacturing standards could result in patient injury or death, product recalls or withdrawals, adverse publicity, delays or failures in testing or delivery, cost overruns, or other problems that could seriously harm our business. Contract manufacturers may encounter difficulties involving production yields, quality control and quality assurance.
We rely on our manufacturers to purchase from third-party suppliers the materials necessary to produce our product candidates for our clinical trials. There are a limited number of suppliers for raw materials that are used to manufacture our drugs and there may be a need to assess alternate suppliers to prevent a possible disruption in supply of the materials necessary to produce our product candidates for our clinical trials, and if approved, ultimately for commercial sale. We do not have any control over the process or timing of the acquisition of these raw materials by our manufacturers. Although we generally do not begin a clinical trial unless we believe we have a sufficient supply of a product candidate to complete the clinical trial, any significant delay in the supply of a product candidate, or the raw material components thereof, for an ongoing clinical trial could considerably delay completion of our clinical trials, product testing and potential regulatory approval of our product candidates. If our manufacturers or we are unable to purchase these raw materials after regulatory approval has been obtained for our product candidates, the commercial launch of our product candidates would be delayed or there would be a shortage in supply, which would impair our ability to generate revenues from the sale of our product candidates.
We expect to continue to depend on third-party contract manufacturers for the foreseeable future. Apart from our agreement with Novozymes, we have not entered into long-term agreements with any contract manufacturers or with any alternate suppliers, and, although we intend to do so prior to commercial launch if any of our product candidates is approved, we may be unable to enter into such an agreement or do so on commercially reasonable terms, which could have a material adverse impact upon our business. We currently obtain our supplies of finished drug product through individual purchase orders. There can be no guarantee that such third parties will continue to supply us with our product candidates and the API used in those product candidates, or that their manufacturing facilities will remain at the appropriate standard. Any performance failure on the part of current or future third party manufacturers could delay clinical development, marketing approval or, if any of our product candidates are approved, commercialization of our product candidates. Our current and
-31-
Table of Contents
anticipated dependence on others for the manufacture of our product candidates or products may adversely affect our results of operations and our ability to commercialize any product candidates that receive market approval.
A shortage in the supply of the anti-cancer drugs used in our product candidates could increase our costs and adversely affect our ability to commercialize any of our product candidates.
The anti-cancer drugs used in our product candidates have from time to time been in short supply and, because many or all of these anti-cancer drugs are also widely used in cancer treatment currently, we will compete with a broad range of healthcare providers and other companies for the supply of those drugs. Any shortage of the drugs used in our product candidates could adversely affect our ability to timely conduct preclinical studies and clinical trials and, if any of those product candidates receives regulatory approval, to commercialize those products and also may result in an increase, which could be significant, in our costs of procuring those drugs.
If we do not establish development or commercialization collaborations, we may have to alter our development and marketing plans.
Our development programs and potential commercialization of our product candidates, if approved, will require substantial additional cash. We may need to enter into collaborative agreements to develop, and, if approved, we will likely seek to enter into collaborations to market, HA-Irinotecan product candidates from our HyACT platform. These collaborations may require us or our partners to undertake or fund certain research and development activities and may require our partners to make payments to us on achievement of certain milestones and pay royalties or make profit-sharing payments to us when and if a product candidate is marketed. As of August 2012, Alchemia Limited had collaborations with pharmaceutical companies, including Amgen Inc. and Genentech, Inc., and research institutes, including the University of Queensland, the Monash Institute of Pharmaceutical Sciences, Monash University, the Walter and Eliza Hall Institute and the Institute of Molecular Biology, Brisbane, for pursuit of our VAST small molecule drug discovery technology and we expect to seek additional collaborations with other research institutions and pharmaceutical and biotechnology companies to assist us in furthering development and potential commercialization of some of our product candidates in the United States or internationally. The collaboration agreements referred to above that are currently in effect are between Alchemia Limited and various collaborators. We are not a party to any of these collaboration agreements, and Alchemia Limited may be required to obtain the consent of its collaborators in order to transfer these collaboration agreements to us. If these consents are not granted, it may have a material adverse effect on our ability to exploit the VAST technology. See “Alchemia Limited may be unable to transfer its rights under collaboration agreements and government grant arrangements relating to the VAST technology to us.”
The collaborations described above include gross-revenue sharing based on the financial contribution of each party at the point of sub-licensing and any future research collaborations may include similar revenue sharing arrangements, while we expect that any collaborations we enter into with a pharmaceutical or biotechnology company may provide for us to receive upfront, research and development, milestone or royalty payments or some combination thereof. We may seek to enter into collaborations in the future, especially for target indications in which the potential collaborator has particular therapeutic expertise or for markets outside of the United States.
The success of our collaborations may depend on the resources devoted to them by us or our partners. There is no certainty that we will attract appropriate strategic partners or collaborators, that we will be able to enter into agreements with strategic partners or collaborators on terms we find acceptable, or at all, or that any goals, arrangements or payments contemplated by any agreements we do enter into will be achieved or made. We face significant competition in seeking appropriate collaborators and these collaborations are complex and time-consuming to negotiate and document. Collaborative agreements may be terminable by our industry partners, and we may not be able to negotiate collaborations on acceptable terms, or at all. If that were to occur, we may have to curtail the development of a product candidate, reduce or delay its development or one or more of our other
-32-
Table of Contents
development programs or, in the case of any product candidate for which regulatory approval is received, delay its potential commercialization, reduce the scope of our sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we will likely need to obtain additional capital, which may not be available to us on acceptable terms, or at all. If we do not have sufficient funds, we will not be able to continue our clinical trials, preclinical studies and VAST small molecule drug discovery projects or bring to market any of our product candidates that receive regulatory approval and generate revenue. In addition, we cannot assure you that the objectives of our existing or future development or commercialization arrangements will be realized or that the arrangement will not be terminated or expire. Suspension or termination of collaborative agreements, or the failure to enter into appropriate collaborative arrangements, may have a material adverse impact on our business, financial condition and results of operations.
If our competitors develop and market products that are more effective, safer or less expensive than our product candidates, our commercial opportunities will be negatively impacted. In addition, all of our current product candidates are combinations of HA and existing cancer drugs and will likely face continued competition from those existing drugs used alone and in alternative treatment regimens.
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technology, clinical development experience and scientific knowledge provide us with competitive advantages, we face competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with existing treatment regimens and new treatment regimens that may become available in the future.
There are a number of companies that carry out some form of competitive activities. Competitive activities could include developing reformulations of irinotecan and other anti-cancer drugs for use in oncology applications, exploiting HA as a therapeutic, researching and developing product candidates from applied research on cancer stem cells, and conducting late stage clinical development for application in colorectal cancer. Our lead product candidate is HA-Irinotecan for the treatment of mCRC and the following table shows potential competitors with drugs approved or in late stage clinical development for mCRC as of November 1, 2012:
| Company | Drug Name | Stage of Development | ||
| Bayer-Schering |
regorafenib |
Approved | ||
| Sanofi/Regeneron |
aflibercept | Approved | ||
| Light Sciences Oncology |
talaporfin sodium | Phase III | ||
| Nektar Therapeutics |
PEG-irinotecan | Phase II |
We are aware of a potential competitor, Nektar Therapeutics, or Nektar, which is developing a suite of new chemical entities with modified pharmacokinetic profiles through the pegylation of known therapeutic agents. For instance, Nektar’s product candidate is a pegylated version of irinotecan that is being assessed in a variety of cancers, including a Phase III clinical trial in metastatic breast cancer and two Phase II clinical trials in mCRC and platinum resistant ovarian cancer. In addition, Merrimack Pharmaceuticals, Inc., or Merrimack, is developing a new formulation of irinotecan for application in pancreatic cancer. Having completed a Phase II clinical trial in May 2011, Merrimack recently initiated a Phase III clinical trial for the same indication. Merrimack also has a Phase II clinical trial and two Phase I clinical trials in oncology indications ongoing. Companies working on cancer stem cells include Boston Biomedical, Inc. (acquired by Dainippon Sumitomo Pharma Co., Ltd.), OncoMed Pharmaceuticals, Inc., Stemline Therapeutics, Inc. and Verastem, Inc.
Other companies are or may be developing, and in the future may develop, drugs for the treatment of indications targeted by HA-Irinotecan for the treatment of mCRC and our other product candidates.
-33-
Table of Contents
It is possible that competitors may develop new products in our target market before we are able to develop such products. It is also possible that our competitors may establish licensing agreements with large multinational pharmaceutical companies in our target markets before we are able to establish such licensing arrangements. Furthermore, it is possible that one or many competing products will achieve FDA approval before HA-Irinotecan for the treatment of mCRC or any of our other product candidates, which may limit our ability to successfully commercialize HA-Irinotecan for the treatment of mCRC, if approved, or any of our other product candidates which may receive regulatory approval.
The VAST technology is a chemistry platform that provides innovative pyranose compounds for drug discovery, with competition anticipated from other drug discovery companies with novel chemistries. For instance, we are aware of companies, including Ensemble Therapeutics, Summit PLC and Tranzyme Pharma, that all have innovative chemistry platforms. Summit PLC has a small carbohydrate library based on imino sugar scaffolds which offer unique activities predominantly in carbohydrate modification pathways. Ensemble Therapeutics and Tranzyme Pharma use macrocyclic technology to position functionality on larger molecules accessing new chemical space between small molecules, such as VAST compounds, and biologics, such as antibodies. Other competitors include companies with an interest in cancer stem cells, mentioned above, and other HA-based companies.
Many of our competitors have significantly greater financial and other resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Large pharmaceutical and biotechnology companies, in particular, have extensive experience in clinical testing and in obtaining regulatory approvals for drugs and in marketing and distributing drugs. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. It is possible that competitors may develop new products in our target market before we are able to develop such products.
It is also possible that our competitors may establish licensing agreements with large pharmaceutical and biotechnology companies in our target markets before we are able to establish such licensing arrangements or we may be unable to establish such licensing arrangements at all. Furthermore, it is possible that one or more of the competing products will achieve FDA approval before HA-Irinotecan for the treatment of mCRC or any of our other product candidates, which may limit our ability to successfully commercialize HA-Irinotecan for the treatment of mCRC, if approved, or any of our other product candidates which may have received regulatory approval.
Competition may increase further as a result of advances in the commercial applicability of technologies currently being developed and a greater availability of capital investment in those fields. Some of the companies developing products which may compete with HA-Irinotecan for the treatment of mCRC include Sanofi/Regeneron, Bayer-Schering, AstraZeneca and Bristol-Myers Squibb. These companies also have significantly greater research and marketing capabilities than we do. In addition, many universities and U.S. private and public research institutes are active in cancer research, the results of which may result in direct competition with HA-Irinotecan for the treatment of mCRC or other of our product candidates. These companies and institutions may seek intellectual property protection with respect to potentially competitive products or technologies. These organizations may also establish exclusive collaborative or licensing relationships with our competitors. Our competitors may succeed in developing, acquiring or licensing on an exclusive basis drug products that are more effective or less costly than any drug candidate that we are currently developing or that we may develop.
In certain instances, the drugs which will compete with our product candidates are widely available or have established, existing standards of care or may be subject to new standards of care. In particular, all of our product candidates are combinations of HA and existing cancer drugs and therefore will likely compete with those
-34-
Table of Contents
existing cancer drugs used alone and combinations of existing drugs used in existing treatment regimens and would compete with combinations of existing drugs used with future standards of care. To compete effectively with these drugs, our product candidates will need to demonstrate advantages that lead to improved clinical safety or efficacy compared with these competitive products, particularly as we expect that our products candidates, if approved, will cost more than the cancer drugs alone. We cannot assure you that we will be able to achieve competitive advantages versus alternative drugs or treatment regimens. If our competitors market products that are more effective, safer or less expensive than our product candidates, or that reach the market sooner than our product candidates, if approved, we may not achieve commercial success.
We believe that our ability to successfully compete will depend on, among other things:
| • | our ability to design and successfully execute appropriate clinical trials; |
| • | our ability to recruit and enroll patients for our clinical trials; |
| • | the results of our clinical trials and the efficacy and safety of our product candidates; |
| • | the speed at which we develop our product candidates; |
| • | achieving and maintaining compliance with regulatory requirements applicable to our business; |
| • | the timing and scope of regulatory approvals, including labeling; |
| • | adequate levels of reimbursement under private and governmental health insurance plans, including Medicare; |
| • | our ability to protect intellectual property rights related to our product candidates; |
| • | our ability to commercialize and market any of our product candidates that may receive regulatory approval; |
| • | our ability to have our partners manufacture and sell commercial quantities of any approved product candidates to the market; |
| • | acceptance of our product candidates by physicians, other healthcare providers and patients; and |
| • | the cost of treatment in relation to alternative treatment regimens. |
In addition, the biopharmaceutical industry is characterized by rapid technological change. Our future will depend in large part on our ability to maintain a competitive position with respect to these technologies. Our competitors may render our technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages in our drug discovery process that we believe we derive from our research approach and proprietary technologies. Also, as our research approach integrates many technologies, it may be difficult for us to stay abreast of the rapid changes in each technology. If we fail to stay at the forefront of technological change, we may be unable to compete effectively.
We will not have the right to sub-license the VAST small molecule drug discovery technology without Alchemia Limited’s consent, which may prevent us from commercializing that technology.
Under the agreement pursuant to which we have in-licensed the VAST small molecule drug discovery technology from Alchemia Limited, we are prohibited from sub-licensing any of that technology without the
-35-
Table of Contents
prior written consent of Alchemia Limited, which may not be unreasonably withheld. Accordingly, we will not be able to sub-license this technology to third parties or enter into collaborations with third parties pursuant to which we give them rights to the technology without Alchemia Limited’s consent. To the extent that Alchemia Limited does not give this consent, it will prevent us from realizing revenues from that technology through sub-licenses and may prevent us from commercializing that technology through other arrangements.
If we fail to attract and retain key management and scientific personnel, we may be unable to successfully develop or commercialize our product candidates.
Companies in our industry have experienced a high rate of turnover of management personnel in recent years. We will need to expand and effectively manage our managerial, operational, financial, development and other resources in order to successfully pursue our research, development and commercialization efforts for our product candidates. Because of the specialized nature of our business, we are highly dependent on qualified, scientific, technical and managerial personnel. Our success depends on our continued ability to attract, retain and motivate highly qualified personnel. There is significant competition for qualified personnel in our business. The loss of the services of any of our senior management, including Peter Smith, our President and Chief Executive Officer, Charles Walker, our Chief Financial Officer, and Tracey Brown, our Chief Scientific Officer, could delay or prevent the commercialization of our product candidates. We will maintain “key man” insurance policies on the lives of these officers; however, we generally do not expect the proceeds from any of these policies to adequately compensate us for the loss of any of these individuals. We employ these individuals on an at-will basis and their employment can be terminated by us or them at any time, for any reason. In order to induce valuable employees to continue their employment with us, we will provide stock options that vest over time. The value to employees of stock options that vest over time is significantly affected by movements in our stock price that are beyond our control, and may at any time be insufficient to counteract more lucrative offers from other companies. We will need to hire additional personnel as we continue to expand our research and development activities and if we ultimately proceed with building a sales and marketing function.
We have scientific and clinical advisors who assist us in formulating our research, development and clinical strategies. These advisors are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. In addition, our advisors may have arrangements with other companies to assist those companies in developing products or technologies that may compete with ours.
We may not be able to attract or retain qualified management, technical and scientific personnel in the future due to the intense competition for a limited number of qualified personnel among biotechnology, pharmaceutical and other businesses. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that will impede the achievement of our research and development objectives, our ability to raise additional capital and our ability to implement our business strategy. In particular, if we lose any members of our senior management team, we may not be able to find suitable replacements in a timely fashion, or at all, and our business may be harmed as a result. The loss of the services of existing personnel, as well as the failure to recruit and retain additional key scientific, technical, managerial and other personnel in a timely manner could harm our research and development programs and our business.
If our product candidates receive regulatory approval and we begin efforts to commercialize those products, we may encounter difficulties in managing our growth and expanding our operations successfully.
As we pursue our drug discovery projects and advance our product candidates through clinical trials, we will need to expand our development, regulatory, manufacturing, marketing and sales capabilities or contract with third parties to provide these capabilities for us. To the extent that our operations expand, we expect that we will need to manage additional relationships with such third parties, as well as additional collaborators and suppliers. Maintaining these relationships and managing our future growth will impose significant added responsibilities on
-36-
Table of Contents
members of our management and other personnel. We must be able to: manage our development efforts effectively; manage our clinical trials effectively; hire, train and integrate additional management, development, administrative and, if our products receive regulatory approval, sales and marketing personnel; improve our managerial, development, operational and finance systems; and expand our facilities, all of which may impose a strain on our administrative and operational infrastructure.
We currently have no marketing, sales or distribution capabilities. If our product candidates receive regulatory approval, we intend to pursue relationships with pharmaceutical companies and other strategic partners to assist us in the commercialization and distribution of our product candidates. We expect that we will initially rely upon these third parties to provide marketing, sales and distribution capabilities for any of our product candidates that receive regulatory approval. In addition, to the extent we are successful in obtaining regulatory approval for our product candidates, we may over the longer term elect to establish an internal sales and marketing organization to supplement these sales and marketing arrangements with third parties. Establishing an internal sales and marketing organization is expensive and time consuming and ultimately may not prove to be successful. Any failure or delay in the development of sales, marketing and distribution capabilities, whether through collaborations with third parties or otherwise, would adversely impact the commercialization of any product candidates that receive regulatory approval. To the extent that we enter into agreements with third parties providing for them to market and distribute our products, any revenue we generate from sales of those products is likely to be lower than if we directly marketed or sold those product, and any revenue we receive will depend in whole or in part upon the efforts of such third parties, which may not be successful and are generally not within our control. Moreover, if we are not successful in developing sales and marketing arrangements with third parties on acceptable terms or, should we choose to do so over the longer term, developing our own sales and marketing organization, we may not be able to commercialize any of our product candidates that receive regulatory approval. Any failure to successfully commercialize our product candidates would have a material adverse effect on our business.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA or other regulations, provide accurate information to the FDA or other regulatory authorities, comply with manufacturing standards we have established, comply with federal, state and foreign health-care fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing, promotion, sales commission, customer incentive and other business arrangements and programs. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We have adopted a Code of Business Ethics in connection with this offering, but there can be no assurance that we will be able to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective. Employee misconduct may result in governmental investigations or other actions or lawsuits that may result in substantial liabilities or other sanctions and substantial diversion of our management’s attention. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a material adverse impact on our business and results of operations, including the imposition of significant fines or other sanctions.
Our commercial success depends upon attaining significant market acceptance of our product candidates, if approved, among physicians, patients, healthcare payors and major operators of cancer clinics.
Even if we obtain regulatory approval for our product candidates, the product candidate may not gain market acceptance among physicians, health care payors, patients and the medical community, which is critical
-37-
Table of Contents
to commercial success. Market acceptance of any product candidate for which we receive approval depends on a number of factors, including:
| • | the efficacy and safety of such product candidate, as demonstrated in clinical trials; |
| • | the timing of market introduction of such product candidate as well as competitive products; |
| • | the clinical indications for which the drug is approved; |
| • | acceptance of the drug by physicians, major operators of cancer clinics and patients as a safe and effective treatment; |
| • | the potential and perceived advantages of such product candidate over alternative treatments; |
| • | the cost of treatment in relation to alternative treatments; |
| • | the availability of adequate reimbursement and pricing by third-party payors and government authorities; |
| • | relative convenience and ease of administration; |
| • | the prevalence and severity of adverse side effects; and |
| • | the effectiveness of our sales and marketing efforts. |
If our product candidates are approved but fail to achieve an adequate level of acceptance by physicians, health care payors, patients and the medical community, we will not be able to generate significant revenues, and we may not become or remain profitable.
We rely on third parties to conduct clinical trials for our product candidates and research and development on the VAST small molecule drug discovery projects, and we plan to rely on third parties to conduct future clinical trials, research and development. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be unable to obtain regulatory approval for or commercialize our product candidates.
We do not have the ability to independently conduct Phase II or Phase III clinical trials for any of our product candidates. We rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to conduct all of our clinical, nonclinical and preclinical studies of our product candidates; however, we remain responsible to regulatory authorities and other parties for ensuring that each of our clinical trials is conducted in accordance with its investigational plan and protocol and legal requirements. Moreover, the FDA and other non-U.S. regulatory authorities require us to comply with regulations and standards, commonly referred to as GCPs, for conducting, monitoring, recording and reporting the results of clinical trials to ensure that the data and results are scientifically credible and accurate and that the trial subjects are adequately informed of the potential risks of participating in clinical trials. Our reliance on third parties does not relieve us of these responsibilities and requirements. If the third parties conducting our clinical trials do not perform their contractual duties or obligations, do not meet expected deadlines or need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to lack of compliance with GCPs or our clinical protocols, regulatory requirements or for any other reason, we may need to enter into new arrangements with alternative third parties and our clinical trials may be extended, delayed or terminated and the cost of those clinical trials may increase substantially, and such lack of compliance with GCPs or protocols may render any such clinical data potentially worthless to us and jeopardize the integrity and viability of the affected clinical trials. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
-38-
Table of Contents
If any of our relationships with these third-party contract research organizations terminate, we may not be able to enter into arrangements with alternative contract research organizations or to do so on commercially reasonable terms. In addition, our contract research organizations are not our employees, and except for remedies available to us under our agreements with such organizations, we cannot control whether or not they devote sufficient time and resources to our on-going clinical, nonclinical and preclinical programs.
Switching or adding additional contract research organizations involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new contract research organization commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines and can increase our costs significantly. Though we seek to manage our relationships with our contract research organizations, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
We plan to undertake drug discovery projects internally and through collaborations with research institutes and pharmaceutical and biotechnology companies. We cannot guarantee that our strategic partners or collaborators involved in VAST small molecule drug discovery projects will deliver the results necessary for commercialization. Strategic partners and collaborators undertaking research may not perform their contractual duties or obligations, may not meet expected deadlines or may need to be replaced. We may need to renegotiate agreements or find alternative partners, which may extend, delay or terminate projects, potentially increasing costs.
If a successful clinical trial or product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, we could be forced to pay substantial damage awards.
The use of any of our product candidates in clinical trials, and the sale of any approved product candidates, may expose us to clinical trial and product liability claims. We currently maintain life science liability insurance coverage in an amount of up to $20 million per occurrence, or up to $20 million in the aggregate, and do not currently maintain product liability insurance. However, we cannot assure you that such insurance coverage will protect us against some or all of the claims to which we might become subject. Moreover, in the future, we might not be able to maintain adequate insurance coverage at a reasonable cost or in sufficient amounts or scope to protect us against potential losses. In the event a claim is brought against us and our insurance is either inadequate to cover or does not cover that claim or we do not have insurance at all, we would be required to pay legal and other expenses to defend the claim, as well as uncovered damages awards resulting from a claim brought successfully against us or any uncovered amounts that we may be required to pay to settle such claim. Furthermore, whether or not we are ultimately successful in defending any such claims, we might be required to direct substantial financial and managerial resources to such defense and adverse publicity could result, all of which could materially harm our business.
If we violate the rules and regulations governing our business, including regulations pertaining to promotion and advertising of our clinical candidates or any approved product candidates, we may be subject to disciplinary action by the FDA’s Office of Prescription Drug Promotion or other regulatory authorities.
Our business is subject to significant regulation both in the United States and internationally. The FDA’s Office of Prescription Drug Promotion, or OPDP, is responsible for reviewing prescription drug advertising and promotional labeling to ensure that the information contained in these materials is not false or misleading. There are specific disclosure requirements and the applicable regulations mandate that advertisements cannot be false or misleading or omit material facts about the product. Prescription drug promotional materials must present a fair balance between the drug’s effectiveness and the risks associated with its use. Most warning letters from OPDP cite inadequate disclosure of risk information or promotion of off-label use.
OPDP prioritizes its actions based on the degree of risk to the public health and often focuses on newly introduced drugs and those associated with significant health risks. There are two types of letters that OPDP
-39-
Table of Contents
typically sends to companies that violate its drug advertising and promotional guidelines: notice of violation letters, or untitled letters, and warning letters. In the case of an untitled letter, OPDP typically alerts the company of the violation and issues a directive to refrain from future violations but does not typically demand other corrective action. A warning letter is typically issued in cases that are more serious or where the company is a repeat offender. We may violate OPDP’s guidelines in the future and be subject to an OPDP untitled letter or warning letter, which may require restrictions on our ability to market our product candidates, if approved, or even require full market withdrawal, either of which will have a negative impact on our business. We may also in the future violate similar laws and regulations of non-U.S. governmental authorities, which may also have a negative impact on our business.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
If we obtain FDA approval for any of our product candidates and begin commercializing those product candidates in the United States, our operations will be directly, or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act. These laws will impact, among other things, our proposed sales, marketing and education programs. In addition, we will be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
| • | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
| • | HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
To the extent that our product candidates receive regulatory approvals from countries outside of the United States, we may also be subject to similar laws and regulations in those countries.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from having our products reimbursed by federal healthcare programs and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
-40-
Table of Contents
We deal with hazardous materials and must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our activities involve the controlled storage, use, and disposal of hazardous materials, including corrosive, explosive and flammable chemicals, biologic waste and various radioactive compounds. We are subject to federal, state, and local laws and regulations governing the use, manufacture, storage, handling, and disposal of these hazardous materials. Although we believe that our safety procedures for the handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of contamination or injury from these materials.
In the event of an accident, state or federal authorities may curtail our use of these materials, and we could be liable for any civil damages, which may exceed our financial resources and may seriously harm our business. Additionally, an accident could damage, or force us to temporarily shut down, our operations. In addition, if we develop a manufacturing capacity, we may incur substantial costs to comply with environmental regulations and would be subject to the risk of accidental contamination or injury from the use of hazardous materials in our manufacturing process.
Our business and operations would suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems and those of our contract research organizations and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we have not experienced any such system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from completed or ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach was to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed.
Changes in foreign currency exchange rates could materially adversely affect our business, results of operations or financial condition.
In our operations, there are transactions and balances denominated in currencies other than the U.S. dollar (which is the currency used to report our results of operations and financial condition in our financial statements), consisting primarily of the Australian dollar. Our functional currency for accounting purposes is the Australian dollar. We recorded a net loss from foreign currency translation of approximately $3.0 million for the fiscal year ended June 30, 2011, and recorded a net gain from foreign currency translation of approximately $1.4 million, approximately $0.2 million and approximately $1.8 million for the fiscal year ended June 30, 2012, the three months ended September 30, 2012 and the three months ended September 30, 2011, respectively. To the extent our assets and liabilities denominated in Australian dollars as of September 30, 2012 are not hedged, we estimate that a 10% change in the exchange rate versus the U.S. dollar would expose us to foreign currency gains or losses of approximately U.S. $0.7 million for the Australian dollar.
In addition, all of our facilities (except for a small amount of executive office space in the United States) are located in Australia, all of our officers and employees have their permanent residences in Australia and substantially all of our expenses (except for costs of clinical trials, which are typically payable into U.S. dollars) are payable in Australian dollars. In the event that the U.S. dollar weakens compared with the Australian dollar, our results of operations or financial condition may be adversely affected, perhaps substantially.
-41-
Table of Contents
The requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract and retain executive management and qualified board members, particularly after we are no longer an “emerging growth company.”
As a public company, we will be subject to the reporting requirements of the Securities Exchange Act of 1934, or the Exchange Act, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The Nasdaq Stock Market and other applicable securities rules and regulations. Compliance with these rules and regulations will substantially increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly after we are no longer an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, as described in the second succeeding risk factor. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. In order to remediate an existing material weakness in our internal control over financial reporting, and to maintain and improve our disclosure controls and procedures and internal control over financial reporting to meet this standard, significant resources and management oversight will be required. As a result, management’s attention may be diverted from other business concerns and these efforts will require us to hire additional personnel and incur significant expenses, which could adversely affect our business and operating results. None of our current employees has the requisite knowledge with regard to U.S. GAAP accounting or U.S. public company reporting requirements. We plan to hire a controller and an outside firm proficient in these areas to help us comply with these requirements and we may need to hire more employees in the future or engage additional outside experts, which will increase our costs and expenses. In addition, Section 404 of the Sarbanes-Oxley Act requires that our independent public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting at such time as we have ceased to be an “emerging growth company” as defined in the JOBS Act, which will result in substantial additional expense. Although the JOBS Act discussed in the second succeeding risk factor may for a limited period of time somewhat lessen the cost of complying with some of these additional regulatory and other requirements, we nonetheless expect a substantial increase in legal, accounting, insurance and certain other expenses in the future, which will adversely impact our results of operations and financial condition.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing authorities. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from other activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing authorities due to ambiguities related to practice, regulatory authorities may initiate legal or administrative proceedings against us and our business may be adversely affected.
We also expect that being a public company, together with these new rules and regulations, will make it more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our board of directors, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
As a result of disclosure of information in this prospectus and in filings required of a public company, our business and financial condition will become more visible, which we believe may result in threatened or actual litigation, including by competitors and other third parties. If such claims are successful, our business and
-42-
Table of Contents
operating results could be adversely affected, and even if the claims do not result in litigation or are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and adversely affect our business and operating results.
Our independent public accounting firm identified a material weakness in our internal control over financial reporting. If we fail to remediate this material weakness or are unable to achieve and maintain effective internal control over financial reporting in the future, the accuracy and timeliness of our financial reporting may be adversely affected.
Effective internal controls are necessary for us to provide reliable financial reports. Our independent public accounting firm has identified a material weakness in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected in a timely basis. The material weakness relates to our lack of sufficient accounting personnel with an appropriate level of knowledge, experience and training in U.S. GAAP accounting and U.S. public company reporting requirements to properly identify, analyze and conclude on accounting issues and to prepare financial statements in accordance with U.S. GAAP and U.S. public company reporting requirements. Our independent public accountants also noted that we lack the formal policies and procedures to ensure that U.S. GAAP accounting practices are appropriate and consistently applied. Examples of this material weakness included the following: for our 2006 acquisition of Meditech Research Ltd (currently known as Alchemia Oncology Pty Limited, or Alchemia Oncology), management was unable to identify that, under U.S. GAAP, the transaction was to be accounted for as an asset acquisition, and in addition, management failed to properly consider that, under U.S. GAAP, the patents acquired should be classified as in-process research and development (which is required to be written off immediately if there is no alternative future use). Further, our management failed to properly consider other intangibles that are potentially acquired in an acquisition in its U.S. GAAP analysis of the acquisition. Likewise, our independent public accountant noted that disclosure adjustments were required in connection with our future commitments under research and development agreements. All of our financial reporting is carried out by our Chief Financial Officer and a limited number of other employees, all of whom lack expertise in U.S. GAAP accounting and public company reporting requirements.
We have not yet been able to remediate this material weakness. However, we plan to take steps intended to address the underlying causes of the material weakness in the immediate future, primarily through the hiring of a third party firm proficient in U.S. GAAP to review our financial statements and a controller with knowledge, experience and training in U.S. GAAP accounting and U.S. public company reporting, as well as the development and implementation of formal policies and improved processes and procedures, and by having our Chief Financial Officer and our other employees responsible for the preparation of the financial statements attend U.S. GAAP accounting and public company reporting training. We do not know the specific timeframe needed to remediate the internal control deficiency underlying this material weakness. In addition, we expect to incur significant costs associated with this remediation, primarily due to the hiring of this controller, the retention of this third-party firm, and the enhancement of our accounting and financial reporting systems. If we fail to enhance our internal controls to remediate this material weakness or to meet the demands that will be placed upon us as a public company, including the requirements of the Sarbanes-Oxley Act, we may be unable to accurately report our financial results, or report them within the timeframes required by law or stock exchange regulations. Failure to comply with Section 404 of the Sarbanes-Oxley Act could also potentially subject us to sanctions or investigations by the SEC or other regulatory authorities. We cannot assure you that we will be able to remediate this material weakness in a timely manner, or at all, or that in the future additional material weaknesses or significant deficiencies will not exist or otherwise be discovered, a risk that is significantly increased in light of our multi-national operations. If our efforts to remediate the material weakness identified are not successful, or if other material weaknesses or other deficiencies occur, our ability to accurately and timely report our financial position could be impaired, which could result in late filings of our annual and quarterly reports under the Exchange Act, restatements of our consolidated financial statements, a decline in our stock price, suspension or delisting of our common stock from the Nasdaq Global Market, and could adversely affect our reputation, results of operations and financial condition.
-43-
Table of Contents
The JOBS Act allows us to postpone the date by which we must comply with certain laws and regulations intended to protect investors and allows us to reduce the amount of information we have provided to you in this prospectus and will provide in our reports filed with the SEC subsequent to this offering, which may have an adverse effect on the trading price of our common stock.
The JOBS Act is intended to reduce the regulatory burden on “emerging growth companies.” As defined in the JOBS Act, a public company whose initial public offering of common equity securities occurred after December 8, 2011 and whose annual gross revenues are less than $1.0 billion will, in general, qualify as an “emerging growth company” until the earliest of:
| • | the last day of its fiscal year following the fifth anniversary of the date of its initial public offering of common equity securities; |
| • | the last day of its fiscal year in which it has annual gross revenue of $1.0 billion or more; |
| • | the date on which it has, during the previous three-year period, issued more than $1.0 billion in non-convertible debt; and |
| • | the date on which it is deemed to be a “large accelerated filer,” which will occur at such time as the company (a) has an aggregate worldwide market value of common equity securities held by non-affiliates of $700 million or more as of the last business day of its most recently completed second fiscal quarter, (b) has been required to file annual and quarterly reports under the Exchange Act for a period of at least 12 months, and (c) has filed at least one annual report pursuant to the Exchange Act. |
Under this definition, we will be an “emerging growth company” upon completion of this offering and could remain an emerging growth company until as late as June 30, 2018.
We have chosen to take advantage of certain provisions of the JOBS Act that allow us to reduce the amount of information we have provided to you in this prospectus. As a result, this prospectus includes less information about us than would otherwise be required if we were not an “emerging growth company,” which may make it more difficult for you to evaluate an investment in our company. Likewise, although we are still evaluating the JOBS Act, we currently intend to take advantage of some or all of the reduced regulatory and reporting requirements applicable to reports and proxy statements that we file with the SEC in the future, which will be available to us so long as we qualify as an “emerging growth company,” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. Among other things, this means that our independent registered public accounting firm will not be required to provide an attestation report on the effectiveness of our internal control over financial reporting so long as we qualify as an emerging growth company, which may increase the risk that material weaknesses or other deficiencies in our internal control over financial reporting go undetected. So long as we qualify as an “emerging growth company,” we may elect not to provide you with certain information, including certain financial information and certain information regarding compensation of our executive officers, that we would otherwise have been required to provide in filings we make with the SEC, which may make it more difficult for investors and securities analysts to evaluate our company. As a result, investor confidence in our company and the market price of our common stock may be adversely affected.
We may be unsuccessful in managing our international operations which could adversely affect our ability to grow.
As noted above, all of our facilities (except for a small amount of executive office space) and employees are located in Australia and, in the future, we may add employees and facilities in Australia or other foreign
-44-
Table of Contents
countries. Operating in international markets requires significant resources and management attention and subjects us to regulatory, economic and political risks and competition that are different from those in the United States. We cannot assure you that we will be successful in managing our international operations.
Our international operations may not succeed due to other risks inherent in operating businesses internationally, including:
| • | our lack of familiarity with commercial and social norms and customs in international countries, which may harm our ability to operate in these countries; |
| • | difficulties and costs associated with managing foreign operations; |
| • | the potential diversion of management’s attention to oversee and direct operations that are geographically distant; |
| • | compliance with multiple, conflicting and changing governmental laws and regulations; |
| • | legal systems in which our ability to enforce and protect our rights may be different or less effective than in the United States and in which the ultimate result of dispute resolution is more difficult to predict; |
| • | laws and business practices favoring local competitors and new and different sources of competition; |
| • | more limited protection for intellectual property rights in some countries; |
| • | fluctuations in currency exchange rates; and |
| • | restrictions on the transfer of funds. |
Our failure to manage any of these risks successfully could harm our existing and future international operations and impair our overall business.
We may be adversely affected by seismic activity or other natural disasters, and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster.
Our executive offices are located in the San Francisco Bay Area and our research and development facilities are located in Australia. The San Francisco Bay Area is known to experience earthquakes from time to time, some of which have been severe. In addition, severe weather systems frequently affect Australia. Earthquakes in the San Francisco Bay Area or Asia Pacific region may also result in tsunamis. We do not carry earthquake insurance. If a disaster, power outage or other event occurred that prevented us from using all or a significant portion of our facilities, that damaged critical infrastructure, or that otherwise disrupted our operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. The disaster recovery and business continuity planning that we have done is limited and is unlikely to prove adequate in the event of a serious disaster or similar event. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans which, particularly when taken together with our lack of earthquake insurance, could have a material adverse effect on our business.
Our ability to use net operating losses to offset future taxable income may be subject to certain limitations.
Audeo Oncology, Inc. is a newly incorporated company in the United States and currently has no net operating loss carryforwards, or NOLs, for U.S. or other tax purposes. Alchemia Oncology, a wholly-owned subsidiary of Audeo Oncology, Inc., has historically been subject to taxation in Australia and not the United
-45-
Table of Contents
States. As of September 30, 2012, Alchemia Oncology had NOLs for Australian tax purposes of approximately $79.6 million that are available to offset any future taxable income we may generate in Australia and which can be carried forward indefinitely. A lack of future taxable income in Australia would adversely affect our ability to utilize these NOLs. In addition, these NOLs may be adjusted if we are unable to comply with the recognition criteria for NOLs under Australian tax laws. Future events such as capital injections and changes in our business or capital structure require evaluation as to the impact on continued availability of historical tax losses to offset future income, if any. Pursuant to Australian tax law, NOLs may not be available and would thus be derecognized (i.e. lost) where there has been a greater than 50 percent change in the cumulative ownership of Alchemia Oncology combined with a change in our business or capital structure. Accordingly, certain years’ tax losses may fail the continuity of ownership test, or COT, and later years’ tax losses may pass. If a company does not satisfy the COT in respect of a particular loss year, it must instead satisfy the same business test, or SBT, to be eligible to utilize prior year losses. The SBT broadly requires the company to carry on the same business for the entire year in which it seeks to recoup losses as it did when it failed COT. Future changes in our stock ownership, many of the causes of which are outside of our control, could result in an ownership change. As a result of these limitations, we may not be able to utilize any or a material portion of the NOLs. In addition, these NOLs would not be available to offset taxable income, if any, that we may generate in tax jurisdictions outside of Australia.
Risks Related to Intellectual Property
Our proprietary rights may not adequately protect our technologies and product candidates.
Our commercial success will depend in part on our ability to obtain additional patents and protect our existing patent position as well as our ability to maintain adequate protection of other intellectual property for our technologies, product candidates, and any future product candidates in the United States and other countries. We pursue a policy of seeking to obtain patent protection for our inventions in Australia, the U.S., Europe, Japan and other selected countries. We also rely on trade secrets, know-how, continuing technology innovations and licensing opportunities to develop and maintain our competitive position. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability. The laws of some foreign countries do not protect our proprietary rights to the same extent or in the same manner as U.S. laws, and we may encounter significant problems in protecting and defending our proprietary rights in these countries. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies are covered by valid and enforceable patents or are effectively maintained as trade secrets. In addition, protecting patents and other intellectual property from alleged infringement may require expensive and time-consuming legal proceedings, and our ability to defend our intellectual property will therefore also depend upon the sufficiency of our cash resources.
We maintain an active practice of filing patent applications, and we apply for patents covering our technologies if we believe that the cost of obtaining patent protection for the technology is justified by its commercial potential, but typically seek patent protection only in those countries that we believe present significant commercial opportunities. However, we may fail to apply for patents on important technologies in a timely fashion, or at all. Our existing patents and any future patents we obtain may not be sufficiently broad to prevent others from practicing our technologies or from developing competing products and technologies. Likewise, obtaining patent protection in any specific country may be expensive and time-consuming and we have elected, and in the future likely will elect, not to pursue patent protection in certain countries, meaning that we will not be able to prevent third parties from using our technologies in those countries. We cannot be certain that our patent applications, or the applications of parties that have licensed their inventions to us, will be approved or that any patents owned by or licensed to us will adequately protect our intellectual property or product candidates. Issued patents may not provide significant proprietary protection or commercial advantage or may be infringed or designed around by others. In addition, we generally do not have the right to control the defense of patents and other intellectual property that we license from others. Accordingly, we are unable to exercise the
-46-
Table of Contents
same degree of control over this intellectual property as we would over our own and would need to involve the third party licensors in legal proceedings or obtain their permission, which may not be granted, to enforce these intellectual property rights on their behalf. Moreover, the patent positions of biopharmaceutical companies are often highly uncertain and involve complex legal and factual questions for which important legal principles are often evolving and remain unresolved. As a result, the validity and enforceability of patents cannot be predicted with certainty. In addition, we do not know whether:
| • | we or our licensors were the first to make the inventions covered by each of the issued patents and patent applications that we own or that are licensed to us; |
| • | we or our licensors were the first to file patent applications for these inventions; |
| • | any of our product candidates will be eligible for listing in the publication Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book, a resource that identifies drug products approved by the FDA on the basis of safety and effectiveness; |
| • | others will independently develop similar or alternative technologies or duplicate any of our technologies; |
| • | any of our or our licensors’ pending patent applications will result in issued patents; |
| • | any of our or our licensors’ patents will be valid or enforceable; |
| • | any patents issued to us or our licensors and collaboration partners will provide us with any competitive advantages, or will be challenged by third parties; |
| • | we will develop additional proprietary technologies that are patentable; |
| • | the U.S. government will exercise any of its statutory rights to our intellectual property that was developed with government funding; or |
| • | our business may infringe the patents or other proprietary rights of others. |
The actual protection afforded by a patent varies based on products or processes, from country to country and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory related extensions, the availability of legal remedies in a particular country, the validity and enforceability of the patents and our financial ability to enforce our patents and other intellectual property. Our ability to maintain and solidify our proprietary position for our technology will depend on our success in obtaining effective claims and enforcing those claims once granted. Our issued patents and those that may be issued in the future, or those licensed to us, may be challenged, narrowed, invalidated or circumvented, and the rights granted under any issued patents may not provide us with proprietary protection or competitive advantages against competitors with similar products or technologies. Due to the extensive amount of time required for the development, testing and regulatory review of a potential product, it is possible that, before any of our product candidates can be commercialized, any patent on the technology relevant to that product candidate may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent.
We also rely on trade secrets to protect some of our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to maintain. While we use reasonable efforts to protect our trade secrets, our or any of our collaboration partners’ employees, consultants, contractors or scientific and other advisors may unintentionally or willfully disclose our proprietary information to competitors and we may not have adequate remedies in respect of that disclosure. Enforcement of claims that a third party has illegally obtained and is using trade secrets is expensive, time consuming and uncertain. In
-47-
Table of Contents
addition, non-U.S. courts are sometimes less willing than U.S. courts to protect trade secrets. If our competitors independently develop equivalent knowledge, methods and know-how, we would not be able to assert our trade secrets against them and our business could be harmed. No assurance can be given that others will not independently develop substantially equivalent proprietary technology and techniques, or otherwise gain access to our proprietary technology or disclose such technology, or that we can meaningfully protect our rights to our unpatented proprietary technology, trade secrets and know-how.
We are aware of two patents that could potentially impede our ability to commercialize HA-Irinotecan for the treatment of mCRC and certain other product candidate and we may need to in-license those patents in order to commercialize those product candidates.
We are aware of two U.S. patents, both held by Pfizer, that could potentially impede our ability to commercialize HA-Irinotecan for the treatment of mCRC if it receives regulatory approval. These patents could also potentially impede our ability to commercialize certain of our other product candidates that may receive regulatory approval. We may need to obtain a non-exclusive license to these two patents, both of which cover methods of treating cancer, including mCRC, involving administration of compounds used in the FOLFIRI chemotherapy regimen. There can be no assurance that we will be successful in in-licensing these patents or, if we are successful in doing so, as to the amount of royalties or other amounts we will be required to pay. If we fail to in-license these patents, it may limit our commercialization of our lead product candidate, HA-Irinotecan, if approved, which will likely have a material adverse effect on our business, results of operations and financial condition. Likewise, even if we are able to in-license these patents, we may be required to pay substantial royalties or other amounts, the term of the in-license may be limited and therefore require periodic renewals, and the other terms of the license may be unfavorable to us, any of which could have a material adverse effect on our business, results of operations and financial condition.
These patents also could potentially impede our ability to commercialize certain of our current product candidates (if approved), including HA-5-fluorouracil, and may impede our ability to commercialize future product candidates which we may develop and which may receive regulatory approval. Accordingly, we also may be required to obtain a non-exclusive in-license to the rights to these two patents in order to pursue our efforts to commercialize our other product candidates and any failure to do so, or any terms of any in-license that are unfavorable to us, could also have a material adverse effect on our results of operations, financial condition and business.
The protection for any intellectual property we may in-license in the future may be dependent on third parties.
We may in the future in-license certain patents and other intellectual property relevant to our product candidates. The right and/or obligation to prosecute and maintain patents, patent applications and other intellectual property that we may in-license in the future may be retained by the applicable licensor and, in those cases, we will not have the right to prosecute and maintain such patents, patent applications or other intellectual property without the consent of the applicable licensor or in other limited circumstances specified by the applicable license agreement. If any licensor fails to appropriately prosecute and maintain patent protection for any of the patents, patent applications or other intellectual property that they have licensed to us, our ability to develop and commercialize our product candidates may be adversely affected and we may not be able to prevent competitors from making, using and selling competing products.
If we breach any of the agreements under which we license rights to patents or other intellectual property from third parties, we could lose license rights that are important to our business.
We have in-licensed the rights to the technology for the VAST small molecule drug discovery technology from Alchemia Limited through our Australian subsidiary, Audeo Discovery Pty Limited, and we may in the future in-license patents or other intellectual property relevant to our product candidates. Under the license
-48-
Table of Contents
agreement with Alchemia Limited, we are subject to development obligations, reporting obligations, sublicense revenue sharing requirements, royalty payments and other obligations and in the future we may be subject to similar obligations with respect to any patents or other intellectual property we in-license. If we fail to comply with any of our obligations under, or otherwise breach our license agreement with Alchemia Limited or any other future in-licenses, the applicable licensor may have the right to terminate the license in whole or in part or, in some cases, to terminate the exclusive nature of the license. Generally, the loss of any current or future licenses or the exclusivity rights provided therein could materially harm our financial condition and operating results.
The patent protection for the technologies relevant to our product candidates and the VAST small molecule drug discovery technology may expire before we are able to derive any commercial value from them, which may subject us to increased competition and reduce or eliminate our opportunity to generate product revenue.
The patents for the technologies relevant to our product candidates and the VAST small molecule drug discovery technology have varying expiration dates and, if these patents expire, we may be subject to increased competition and we may not be able to recover our development costs or market any of our approved product candidates profitably. Our patents relating to the HyACT technologies will expire, excluding possible patent extensions under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, on various dates between 2014 and 2026. The material patents relating to the VAST technology that we have in-licensed will expire, excluding possible patent extensions under the Hatch-Waxman Act, on various dates between 2017 and 2028. In some of the larger potential markets, such as the United States and Europe, patent term extension or restoration may be available to compensate for time taken during aspects of the product’s development and regulatory review. However, we cannot be certain that such an extension will be granted, or if granted, what the applicable time period or the scope of patent protection afforded during any extension period will be. In addition, even though some regulatory authorities may provide some other exclusivity for a product under their own laws and regulations, we may not be able to qualify any of our product candidates for this treatment. If we are unable to obtain patent term extension/restoration or some other exclusivity, we could be subject to increased competition and our opportunity to establish or maintain product revenue could be substantially reduced or eliminated. Furthermore, we may not have sufficient time to recover our development costs prior to the expiration of our owned and in-licensed U.S. and non-U.S. patents.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on the intellectual property relevant to our product candidates throughout the world would be prohibitively expensive. Accordingly, we plan to pursue patent protection for intellectual property relevant to our product candidates only in major markets, such as the United States, Australia, the European Union and Japan, and only for those technologies where we believe that the costs of obtaining patent protection are justified by their commercial potential. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection but where enforcement is not as strong as that in the United States. These products may compete with our product candidates or the VAST small molecule drug discovery technology in jurisdictions where we do not have any patent protection and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to pharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
-49-
Table of Contents
We may be subject to claims that we have wrongfully hired an employee from a competitor or that we or our employees have wrongfully used or disclosed alleged confidential information or trade secrets of their former employers.
As is commonplace in our industry, we employ individuals who were previously employed at other biopharmaceutical companies, including our competitors or potential competitors. Although we have not received any claim to date, we may be subject in the future to claims that our employees or prospective employees are subject to a continuing obligation to their former employers, such as non-competition or non-solicitation obligations, or claims that our employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to our management.
If we are sued for infringing intellectual property rights of third parties, litigation will be costly and time-consuming and could prevent us from developing or commercializing our product candidates.
Our commercial success depends, in part, on our not infringing the patents and proprietary rights of other parties and not breaching any collaboration or other agreements we have entered into with regard to our technologies and product candidates. Numerous third-party U.S. and non-U.S. issued patents and pending applications exist in the areas relevant to our technologies and product candidates.
As patent applications can take several years to issue, if they are issued at all, there may currently be pending applications, unknown to us, that may result in issued patents that cover our technologies or product candidates. As publication of inventions or discoveries in scientific or patent literature often lags behind actual invention or discovery, it is possible that the inventions covered by each of our pending patent applications may not have dominant status in terms of date of invention. It is uncertain whether the issuance of any third-party patent would require us to alter our product candidates or processes, obtain licenses or cease activities related to the development or commercialization of our product candidates. If we wish to use the technology or compound claimed in issued and unexpired patents owned by others, we may need to obtain a license from, and pay royalties to, the owner, enter into litigation to challenge the validity of the patents or incur the risk of litigation in the event that the owner asserts that any of our product candidates infringe its patents. The failure to obtain a license to technology or the failure to challenge an issued patent that we may require to discover, develop or commercialize our product candidates may have a material adverse impact on us.
There is a substantial amount of litigation involving intellectual property in the biopharmaceutical industry generally. If a third party asserts that our product candidates or technologies infringe its patents or other proprietary rights, we could face a number of risks that could seriously harm our results of operations, financial condition and competitive position, including:
| • | infringement and other intellectual property claims, which would be costly and time-consuming to defend, whether or not the claims have merit, and which could delay the regulatory approval process for our product candidates and divert management’s attention from our business; |
| • | substantial damages for past infringement, which we may have to pay if a court determines that our product candidates or technologies infringe a competitor’s patent or other proprietary rights or in order to settle similar claims; |
| • | a court prohibiting us from selling or licensing our technologies or our product candidates unless the third-party licenses its patents or other proprietary rights to us on commercially reasonable terms, which it is not required to do; |
| • | if a license is available from a third party, we may have to pay substantial royalties or lump sum payments or grant cross-licenses to our patents or other proprietary rights to obtain that license; and |
-50-
Table of Contents
| • | redesigning our product candidates so they do not infringe, which may not be possible or may require substantial monetary expenditure and time. |
To our knowledge, as of November 1, 2012, no third party had threatened or instituted proceedings against us or against Alchemia Limited or any of its subsidiaries relating to any of the intellectual property that we own or that we license from Alchemia Limited. Although, as of November 1, 2012, neither we nor Alchemia Limited, nor any of its subsidiaries, was a party to any legal proceedings relating to any such intellectual property, in the future, third parties may file claims asserting that our technologies or product candidates infringe on their intellectual property. We cannot predict whether third parties will assert these claims against us, against Alchemia Limited or against any other current or future licensors of technology licensed to us, or whether those claims will harm our business. If we are forced to defend against these claims, whether they are with or without any merit and whether they are resolved in favor of or against us or our licensors, we may face costly litigation and diversion of management’s attention and resources. Moreover, in the case of infringement claims relating to intellectual property that has been licensed to us, we may not be able to control or even participate in the defense of those claims and therefore may be entirely dependent on the licensor or other owner of that intellectual property for that defense. As a result of these disputes, we may have to develop costly non-infringing technology, or enter into licensing agreements requiring us to make substantial payments to the licensors. These agreements, if necessary, may be unavailable on terms acceptable to us, or at all, which could seriously harm our business or financial condition.
One or more third-party patents or patent applications may conflict with patent applications to which we have rights. Any such conflict may substantially reduce the coverage of any rights that may issue from the patent applications to which we have rights. If third parties file patent applications in technologies that also claim technology to which we have rights, we may have to participate in interference proceedings with the U.S. Patent and Trademark Office, or USPTO, or non-U.S. patent regulatory authorities, as applicable, to determine priority of invention.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property rights, which could be expensive, time-consuming and unsuccessful.
As of November 1, 2012, neither we nor Alchemia Limited, nor any of its subsidiaries, had threatened or instituted proceedings against any third party on patent or other proprietary rights. However, competitors may infringe our patents or other intellectual property rights. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming. To the extent such claims relate to patents licensed to us by Alchemia Limited or any other third party, either Alchemia Limited or the applicable licensor, as the case may be, would have to file such an infringement lawsuit since we do not have the independent right to enforce their intellectual property. In addition, in an infringement proceeding, a court may decide that a patent that we own or that is licensed to us is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of the patents that we own or in-license at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
Interference or derivation proceedings brought by the USPTO may be necessary to determine the priority of inventions with respect to our patents and patent applications or those of our current or future licensors and collaborators. Our patents and patent applications may also be the subject of other contested proceedings before the USPTO or other patent offices. An unfavorable outcome could require us to cease using the technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if a prevailing party does not offer us a license on terms that are acceptable to us. Litigation, interference or derivation proceedings or other contested proceedings may have unfavorable outcomes and, even if successful, may result in substantial costs and distraction of our management and other employees. We may not be able to prevent, alone or with our
-51-
Table of Contents
collaborators, misappropriation of our proprietary rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential and proprietary information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
Risks Related to Our Demerger from Alchemia Limited
We have no operating history as a separate public company, and our historical and pro forma financial information is not necessarily representative of the results that we would have achieved as a separate, publicly traded company and may not be a reliable indicator of our future results.
Prior to the consummation of this offering and the Demerger, our business has been operated as part of the business of Alchemia Limited. As a result, our consolidated financial statements and other historical and pro forma financial information included in this prospectus are derived from the consolidated financial statements and accounting records of Alchemia Limited and our consolidated financial statements include allocations of expenses, primarily salaries and benefits (including stock-based compensation) of officers and other employees of Alchemia Limited, expenses for compensation and reimbursement paid to directors of Alchemia Limited and insurance expenses incurred by Alchemia Limited. These expenses have been allocated based on our estimated share of Alchemia Limited’s expenses using the proportional cost allocation method. Although we believe that these allocation methodologies are reasonable, these methodologies are nonetheless subject to a number of uncertainties and assumptions. Likewise, our expenses are expected to increase substantially when we begin operating as a separate public company and there are likely to be other significant differences in our operations compared with our operations while we were part of Alchemia Limited. Accordingly, the consolidated financial statements and other historical and pro forma financial information included here do not purport to reflect the results of operations, financial position and cash flows that we would have achieved as a separate, publicly traded company during the periods presented or those that we will achieve in the future. In addition, the following factors, among others, may cause differences between our results of operations and financial condition prior to and after this offering:
| • | To date, all funds necessary for our research and development, working capital and other requirements, including costs of preclinical and clinical trials, research and development, general and administrative and capital expenditures, have been provided by Alchemia Limited. Following the completion of the Demerger and this offering, we will need to obtain substantial additional external financing in order to complete clinical trials, commercialize any product candidates that may receive regulatory approval and meet other cash needs, and neither Alchemia Limited nor any other third party will have any obligation to provide us financing in the future. Accordingly, we will be wholly dependent on our ability to raise additional capital through public offerings or private placements of debt or equity securities, strategic relationships or other arrangements, and there can be no assurance that we will be able to do so on acceptable terms or at all. |
| • | Subsequent to the completion of the Demerger, the cost of capital for our business may be higher than Alchemia Limited’s cost of capital prior to the separation. |
Other significant changes may occur in our cost structure, management, financing and business operations as a result of operating as a public company separate from Alchemia Limited. The adjustments and allocations we have made in preparing our consolidated financial statements and other historical and pro forma financial information included herein do not purport to reflect our operations during those periods as if we had in fact operated as a stand-alone entity, or what the actual effect of our Demerger from Alchemia Limited will be.
-52-
Table of Contents
The Demerger may adversely affect our business, and we may not achieve some or all of the expected benefits of the Demerger.
We may not be able to achieve the benefits expected to result from the Demerger, or such benefits may be delayed or not occur at all. As a result of the Demerger, we anticipate benefits that may include improved strategic planning, increased management focus and streamlined decision-making for the HyACT and VAST platforms. We may not achieve these anticipated benefits for a variety of reasons. For example, we will not receive loans or other financial support from Alchemia Limited and will not have the financial support of the cash flow generated from Alchemia Limited’s business after the Demerger and will require substantial additional funding, which may not be available to us on acceptable terms or at all. We will also be a smaller, less diversified company as a result of the Demerger, which could result in greater volatility in the trading price of our common stock compared with that of Alchemia Limited and may also make obtaining additional funding more difficult or expensive. It is not possible to predict the market value of our shares of common stock or the CDIs following implementation of the Demerger. Moreover, our corporate and operating costs will be greater than the comparable costs of Alchemia Limited if the Demerger were not consummated. In addition, as described below under “Certain of our officers and directors will hold positions at Alchemia Limited for a short time after the Demerger, which may cause them to have actual or potential conflicts of interest and will limit the attention they devote to our business. In addition, all of our officers and some of our employees own Alchemia Limited ordinary shares and/or options to purchase Alchemia Limited ordinary shares, which may also result in actual or potential conflicts of interest,” certain of our officers and directors may have actual or potential conflicts of interest because of their positions with Alchemia Limited. There can be no assurance that the Demerger will not adversely affect our business.
Our inability to resolve any disputes that arise between us and Alchemia Limited with respect to our past and ongoing relationships may adversely affect our business.
Disputes may arise between Alchemia Limited and us in a number of areas relating to our past and ongoing relationships, including
| • | tax, indemnification and other matters arising from the Demerger; |
| • | pricing for and provision of transitional services; and |
| • | licensing arrangements relating to VAST technology. |
We may not be able to resolve any potential conflicts, and even if we do, the resolution may be less favorable than if we were dealing with an unaffiliated party. The ultimate outcome of any disputes or disagreements with Alchemia Limited, if unfavorable to us, could have an adverse effect on our business, which could be material.
Certain of our officers and directors will hold positions at Alchemia Limited for a short time after the Demerger, which may cause them to have actual or potential conflicts of interest and will limit the attention they devote to our business. In addition, all of our officers and some of our employees own Alchemia Limited ordinary shares and/or options to purchase Alchemia Limited ordinary shares, which may also result in actual or potential conflicts of interest.
Certain of our officers and directors may have actual or potential conflicts of interest because of their positions with Alchemia Limited. For example, we currently expect that our Chief Executive Officer and Chief Financial Officer will each continue to serve as the chief executive officer and chief financial officer of Alchemia Limited for up to six months after the date of the implementation of the Demerger as part of the transition services agreement between us and Alchemia Limited. In addition, such officers own, and all of our other officers and some of our employees own, Alchemia Limited ordinary shares and/or options to purchase Alchemia Limited ordinary shares, and these holdings of Alchemia Limited ordinary shares and/or options to purchase
-53-
Table of Contents
ordinary shares of Alchemia Limited may be significant for some of these persons compared with these persons’ total assets. These positions at Alchemia Limited and the ownership of any Alchemia Limited shares or equity awards creates, or may create the appearance of, conflicts of interest when these officers, directors and employees are faced with decisions that could have different implications for Alchemia Limited than the decisions have for us. In addition, during a period of up to six months following the date of implementation of the Demerger, our Chief Executive Officer and Chief Financial Officer will, so long as they are also serving as officers of Alchemia Limited, divide their attentions between Alchemia Limited and our company, providing a total of up to 26 days each to Alchemia Limited, which will limit the attention they devote to our business and may adversely affect our business and prospects.
Third parties may seek to hold us responsible for liabilities of Alchemia Limited or raise claims or institute litigation against us for actions undertaken in connection with the Demerger.
Until the Demerger, our business will have been operated as a part of Alchemia Limited. Accordingly, third parties may seek to hold us responsible for Alchemia Limited’s liabilities, whether incurred prior to or after the Demerger. In addition, we may be required to indemnify Alchemia Limited for those claims and any related losses pursuant to the agreement described in the next risk factor. Third parties may also seek to raise claims or institute litigation against us for actions undertaken in connection with or relating to the Demerger. Such claims could have a material adverse effect on our business, results of operations and financial condition.
We will be subject to risks resulting from cross-indemnification in connection with the Demerger.
In connection with the Demerger, Alchemia Limited will agree to indemnify us for any claim or loss suffered by us where the claim or loss relates to the business of Alchemia Limited (excluding the business of Alchemia Oncology). (We will be responsible for any income tax payable by Alchemia Oncology for the period prior to and after the Demerger.) If third parties bring claims against us for liabilities relating to Alchemia Limited’s business that would entitle us to that indemnity and if Alchemia Limited fails to indemnify us, it could have a material adverse effect on our results of operations and financial condition. Likewise, we have agreed to indemnify Alchemia Limited for any claim or loss relating to the business of our subsidiary Alchemia Oncology. Any requirement that we indemnify Alchemia Limited under these circumstances could also have a material adverse effect on our results of operations and financial condition.
Risks Related to Ownership of Our Common Stock and this Offering
We expect that our stock price will fluctuate significantly, you may not be able to resell your shares at or above the initial public offering price and you may lose all or part of your investment.
Prior to this offering, there has been no public market for shares of our common stock. We cannot predict the extent to which investor interest in our company will lead to the development of an active trading market for our common stock on The Nasdaq Global Market or otherwise or how liquid that market might become. If an active trading market does not develop, you may have difficulty selling any of our shares of common stock that you buy. We and the underwriters will determine the initial public offering price of our common stock through negotiation. This price will not necessarily reflect the price at which investors in the market will be willing to buy and sell our shares following this offering. In addition, the trading price of our common stock following this offering may be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, and you may lose all or part of your investment. These factors include:
| • | actual or anticipated quarterly variations in our results of operations or the results of our competitors; |
| • | announcements by us or our competitors of new commercial products, significant contracts, commercial relationships or capital commitments; |
-54-
Table of Contents
| • | issuance of new or changed securities analysts’ reports or recommendations for our stock; |
| • | developments or disputes concerning our intellectual property or other proprietary rights; |
| • | commencement of, or our involvement in, litigation; |
| • | market conditions in the life sciences sectors; |
| • | failure to complete clinical trials successfully, or at all, or to obtain regulatory approvals for our product candidates; |
| • | manufacturing or supply disruptions at our contract manufacturers, or failure by our contract manufacturers to obtain or maintain approval of the FDA or comparable regulatory authorities; |
| • | any future sales of our common stock or other securities; |
| • | any major change in our board of directors or management; |
| • | adverse results in preclinical studies or clinical trials by us or other companies; |
| • | prohibitions under applicable Australian legal requirements that prevent the underwriters from creating a naked short position in connection with this offering, despite naked short positions being common in offerings in the United States, which will limit their ability to combat downward pressure on the market price of our common stock; |
| • | general economic conditions and slow or negative growth of our markets; and |
| • | other events or factors, including those resulting from war, incidents of terrorism or responses to these events. |
In addition, the CDIs representing shares of our common stock distributed in the Demerger will be listed and will trade on the Australian Securities Exchange and, as a result, there will be an independent trading market for our CDIs in Australia. The CDIs are, in general, the economic equivalent of shares of our common stock and, as a result, the trading price of the CDIs on the Australian Securities Exchange will likely affect the trading price of our common stock on the Nasdaq Global Market, and vice versa. The trading price of the CDIs may be influenced by factors different from those that affect the trading price of our common stock on the Nasdaq Global Market and, as discussed below in these risk factors, may be influenced by arbitrage activities. In addition, holders of shares of our common stock, including shares sold in this offering, may deliver those shares to the depositary for the CDIs in exchange for CDIs. If a significant number of the shares of our common stock that are sold in the offering are exchanged for CDIs, it may have an adverse effect, which could be material, on the liquidity and trading price of our common stock on the Nasdaq Global Market.
The stock market in general, and market prices for the securities of biopharmaceutical companies like ours in particular, have from time to time experienced extreme volatility that often has been unrelated to the operating performance of the underlying companies. A certain degree of stock price volatility can also be attributed to being a newly public company. These broad market and industry fluctuations may adversely affect the market price of our common stock, regardless of our operating performance. In several recent situations where the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders were to bring a lawsuit against us, the defense and disposition of the lawsuit could be costly and divert the time and attention of our management and harm our operating results.
-55-
Table of Contents
If securities or industry analysts do not publish research or reports about our business, publish negative reports about our business or cease coverage of our company, our share price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business, our market and our competitors. We do not have any control over these analysts. If one or more of the analysts who cover us were to downgrade our shares, change their opinion of our shares or cease coverage of our company, our share price would likely decline. If one or more of these analysts ceased coverage of our company or failed to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
We may invest or spend the proceeds of this offering in ways with which you may not agree or in ways which may not yield a return.
We currently intend to use the net proceeds we receive from this offering as follows: to complete our pivotal Phase III clinical trial and related regulatory filings for HA-Irinotecan for the treatment of mCRC; to prepare for commercial-scale manufacturing of HA-Irinotecan, if approved for the treatment of mCRC, which would entail procurement of key raw materials, developing and validating a manufacturing process to manufacture commercial-scale quantities of product and preparing and filing regulatory documents; to fund our research and development activities related to our HyACT platform; to fund our research and drug discovery activities related to our in-licensed VAST drug discovery technology; and the balance for general corporate purposes, including working capital and capital expenditures, which may include the acquisition or in-licensing of additional compounds, product candidates and technology, although we have no present commitments or agreements to enter into any such acquisitions. Our management will have considerable discretion in the application of the net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. In addition, it is possible that the net proceeds we receive from this offering will not be sufficient for the purposes discussed under “Use of Proceeds.” The net proceeds may be used for purposes that do not increase the value of our business, which could cause our stock price to decline. Until these funds are used, they may be placed in investments that produce only limited income or do not produce income at all or that lose value.
Future sales of shares of our common stock could cause the market price of our common stock to decline. This risk is heightened by the fact that, in addition to the shares sold in this offering, shares of our common stock distributed in the Demerger and CDIs representing those shares will be freely tradable in the public markets immediately upon completion of this offering, and a substantial majority of the shares of our common stock distributed in the Demerger and the CDIs representing those shares will not be subject to lock-up agreements.
The market price of shares of our common stock could decline as a result of sales of our common stock or CDIs representing those shares following this offering, particularly sales by Alchemia Limited shareholders that receive CDIs or shares of our common stock in the Demerger, or the perception that these sales could occur. Immediately after this offering, we estimate that we will have approximately 10,982,020 outstanding shares of our common stock, calculated as of the date, in the manner and subject to the assumptions set forth under “Prospectus Summary — The Offering” (as described under that caption, the actual number of shares that will be outstanding immediately after this offering will be greater than the foregoing estimated number of shares). All of the shares of our common stock outstanding immediately after this offering (which will consist of the shares sold in this offering and the shares distributed in the Demerger), as well as CDIs representing shares distributed in the Demerger, will be freely tradable in the public markets except for shares and CDIs that are held by our “affiliates” (as defined for purposes of the Securities Act of 1933), but, as of November 7, 2012, only 941,922 shares, or approximately 8.7% of the number of shares of our common stock that we have estimated will be outstanding immediately after this offering and the Demerger, will be subject to lock-up agreements described under “Underwriting — Lock-Up Agreements.” This means that the approximately 9.9 million remaining shares, or approximately 91.3% of the estimated number of shares of common stock to be outstanding immediately after this offering (and, in the case of shares distributed in the Demerger, CDIs representing those shares), may be sold
-56-
Table of Contents
in the public markets immediately after this offering. Because of the substantial number of shares of common stock and CDIs representing those shares that will be freely tradable in the public markets but will not be subject to lock-up agreements, there is a substantial risk that sales of these shares or CDIs may cause the market price of our common stock to decline, perhaps significantly, and that these declines may occur immediately after our common stock and the CDIs begin to trade on the Nasdaq Global Market and the Australian Securities Exchange, respectively, or at any time thereafter.
Except for the shares sold in this offering, all of the shares of our common stock outstanding immediately after this offering will be shares distributed in the Demerger to Alchemia Limited shareholders (either in the form of CDIs representing shares of our common stock or, at their option, as shares of our common stock), all of which will be freely tradable in the public markets except for shares and CDIs that are held by our “affiliates” (as defined for purposes of the Securities Act of 1933), subject in certain cases to the lock-up agreements described under “Underwriting — Lock-Up Agreements.” Although we are unable to predict whether or not shareholders of Alchemia Limited will seek to dispose of CDIs or shares of our common stock received in the Demerger, there is a risk that they may be more likely to dispose of CDIs and shares of our common stock than investors who purchase our shares in this offering and that those dispositions may begin to occur as soon as our common stock or the CDIs, as the case maybe, begin to trade on the Nasdaq Global Market and the Australian Securities Exchange, respectively.
In addition, Alchemia Limited’s stock records indicate that certain Alchemia Limited shareholders reside or are located in jurisdictions where, as a result of local securities laws, they will not be permitted to receive CDIs or shares of our common stock distributed in the Demerger (we sometimes refer to these shareholders as ineligible foreign shareholders). Accordingly, CDIs or shares of our common stock that would otherwise be distributed in the Demerger to these ineligible foreign shareholders will instead be delivered to a sales facility agent, who will then sell those CDIs or shares of our common stock on the Australian Securities Exchange or the Nasdaq Global Market, as applicable, during the approximately 10 trading days following the completion of this offering (or such longer period of time, being no more than approximately three months after the completion of this offering, which the sales facility agent and Alchemia Limited determine is reasonable, having regard to the demand for shares of our common stock and the CDIs) at such prices as the sales facility agent determines, and remit the proceeds to the ineligible foreign shareholders. These sales may result in a decline, which could be substantial, in the market price of our common stock.
In addition, options to purchase 948,000 shares of our common stock were granted under our 2012 Stock Plan at an exercise price equal to the initial public offering price per share set forth on the cover page of this prospectus (following this offering, we do not expect to make further grants pursuant to our 2012 Stock Plan), and concurrently with the closing of this offering we plan to establish a 2012 Equity Incentive Plan that will provide for the issuance of up to 1,250,000 shares of our common stock, plus annual increases, beginning with our fiscal year commencing July 1, 2013, in the number of shares available for issuance under such plan, as described under “Executive Compensation — 2012 Stock Plan” and “ — 2012 Equity Incentive Plan.” We also intend to file a registration statement on Form S-8 to register shares of our common stock that may be issued upon exercise of these options or that we may otherwise issue under our 2012 Equity Incentive Plan. Once we register these shares, they will be freely tradable in the public market upon issuance. Significant sales of our common stock pursuant to options and equity incentive plans could also harm the prevailing market price for our common stock.
As of November 7, 2012, our officers, directors and employees and certain Alchemia Limited shareholders who we expect will receive CDIs or shares of our common stock in the Demerger (assuming they do not sell or otherwise transfer their ordinary shares of Alchemia Limited prior to the record date for the Demerger) had entered into lock-up agreements described under “Underwriting — Lock-Up Agreements” that will cover approximately 8.7% of the number of shares of our common stock that we have estimated will be outstanding immediately after this offering and the Demerger. However, if any of the Alchemia Limited shareholders who have signed lock-up agreements sell or otherwise transfer their ordinary shares of Alchemia Limited prior to the record date for the Demerger to a person who has not signed a lock-up agreement, then the CDIs or shares of our
-57-
Table of Contents
common stock distributed in respect of those shares in the Demerger will no longer be subject to a lock-up agreement. As described above under “Prospectus Summary — The Offering,” the actual number of shares of our common stock that will be outstanding after this offering and the Demerger will be greater than the number we have assumed for purposes of computing the foregoing percentage. Leerink Swann LLC and Oppenheimer & Co. Inc. may, in their sole discretion and without notice to the public, permit our officers and directors and these shareholders to sell shares of our common stock and CDIs prior to the expiration of the lock-up agreements. Sales of our common stock or CDIs following any such waivers or upon expiration of such lock-up agreements could adversely affect the market price of our common stock.
As described under “Description of Capital Stock — Warrants,” as required by the terms of a private placement of Alchemia Limited’s ordinary shares to investors in 2011, following this offering we will issue Warrants to purchase shares of our common stock to investors that purchased ordinary shares in that placement. Each of the Warrants will be issuable approximately 60 trading days subsequent to completion of this offering and will initially be exercisable, commencing six months after the date of this offering, for one share of our common stock. Assuming that the volume weighted average price of our common stock for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market (we sometimes refer to such volume weighted average price over such period as the Average Trading Price) is US$15.00 per share (the midpoint of the estimated price range set forth in the cover page of this preliminary prospectus) and utilizing the currency exchange rate as of November 1, 2012, approximately 429,608 Warrants (initially exercisable for a like number of Warrant Shares) would be issuable with an exercise price of approximately US$19.50 per share. Any exercise of these Warrants and sale of the shares of our common stock issued on exercise of these Warrants could have an adverse effect on the market price of our trading stock. Please note that, because the number of Warrants and the exercise price both depend upon the actual Average Trading Price following this offering and the number of Warrants also depends upon the actual exchange rate between U.S. dollars and Australian dollars following this offering, the actual number of Warrants that we issue (and, therefore, the number of Warrant Shares for which the Warrants are exercisable), and the actual exercise price will likely differ from the numbers we have assumed for purposes of this preliminary prospectus. For information on how the number of Warrants and exercise price will be calculated, and for an analysis showing how changes in the assumed Average Trading Price affect the number of Warrants (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the exercise price of the warrants and how changes in the assumed currency exchange rate change the number of Warrants (and therefore, the number of Warrants Shares for which the Warrants are exercisable), see “Prospectus Summary — The Offering” and “Description of Capital Stock — Warrants.”
Purchasers in this offering will experience immediate and substantial dilution in the book value of their investment.
The initial public offering price per share in this offering will be substantially higher than the pro forma as adjusted net tangible book value per share of our common stock prior to this offering. As a result, investors purchasing common stock in this offering will experience immediate dilution of $10.89 per share in pro forma as adjusted net tangible book value per share from the initial public offering price, assuming an initial public offering price of $15.00 per share (which is the midpoint of the estimated price range appearing on the cover page of this preliminary prospectus), calculated as described below under “Dilution.” This dilution is due in large part to the fact that the deemed consideration we received for certain of the shares of our common stock that Alchemia Limited will distribute in the Demerger is substantially less than the initial public offering price of our common stock and the fact that we will not receive any payment for the other shares of our common stock distributed in the Demerger. Furthermore, if the underwriters exercise their option to purchase additional shares from us, you will experience, and if we issue additional equity securities, you may experience, additional dilution. In addition, as described below under “We will have outstanding options and warrants, and in the future we expect to issue additional options, that have the potential to dilute stockholder value,” we have or will have options and warrants that have the potential to dilute stockholder value further.
-58-
Table of Contents
We will have outstanding options and warrants, and in the future we expect to issue additional options, that have the potential to dilute stockholder value.
Options to purchase 948,000 shares of our common stock were granted under our 2012 Stock Plan at an exercise price equal to the initial public offering price per share set forth on the cover page of this prospectus, and concurrently with the closing of this offering we plan to establish a 2012 Equity Incentive Plan that will provide for the issuance of up to 1,250,000 shares of our common stock, plus annual increases in the number of shares available for issuance under that plan, beginning with our fiscal year commencing July 1, 2013, as described under “Executive Compensation — 2012 Stock Plan” and “ — 2012 Equity Incentive Plan.” In the future, we do not expect to make further grants pursuant to our 2012 Stock Plan, but we expect to issue stock options or other forms of stock-based compensation to our directors, officers and employees pursuant to our 2012 Equity Incentive Plan or subsequent equity incentive plans. In addition, as described above under “Future sales of shares of our common stock could cause the market price of our common stock to decline. This risk is heightened by the fact that, in addition to the shares sold in this offering, shares of our common stock distributed in the Demerger and CDIs representing those shares will be freely tradable in the public markets immediately upon completion of this offering, and a substantial majority of the shares of our common stock distributed in the Demerger and the CDIs representing those shares will not be subject to lock-up agreements,” following this offering we will issue Warrants to purchase shares of our common stock at an exercise price equal to 130% of the Average Trading Price of our common stock. The exercise of any of those options or warrants, and the grant or exercise of other stock-based awards we may make under our equity incentive plan, may dilute the voting, economic and other interests of holders of common stock purchased in this offering.
As a result of becoming a public company, we will be obligated to develop and maintain proper and effective internal control over financial reporting. We may not complete our analysis of our internal control over financial reporting in a timely manner, or this internal control may not be determined to be effective, which may adversely affect investor confidence in our company and, as a result, the value of our common stock.
We will be required, pursuant to Section 404 of the Sarbanes-Oxley Act, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting beginning with our second filing of an Annual Report on Form 10-K with the Securities and Exchange Commission, or the SEC, after we become a public company. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting, as well as a statement that our independent registered public accounting firm has issued an opinion on our internal control over financial reporting. Likewise, our independent registered public accounting firm will be required to provide an attestation report on the effectiveness of our internal control over financial reporting at such time as we cease to be an “emerging growth company,” as defined in the JOBS Act, although, as described in the risk factor above entitled “The JOBS Act allows us to postpone the date by which we must comply with certain laws and regulations intended to protect investors and allows us to reduce the amount of information we have provided to you in this prospectus and will provide in our reports filed with the SEC subsequent to this offering, which may have an adverse effect on the trading price of our common stock,” we could potentially qualify as an “emerging growth company” until June 30, 2018.
We have not yet begun the costly and challenging process of compiling the system and processing documentation necessary to perform the evaluation needed to comply with Section 404. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion, or to remediate the existing material weakness in our internal control. During the evaluation and testing process, if we are unable to remediate the existing material weakness in our internal control, or if we identify one or more additional material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control is effective. In addition, the fact that our independent registered public accounting firm will not be required to provide an attestation report on the effectiveness of our internal control over financial reporting so long as we qualify as an “emerging growth company” may increase the risk that material weaknesses or deficiencies in our internal control over financial reporting go undetected.
-59-
Table of Contents
If we are unable to certify that our internal control over financial reporting is effective, or if our independent registered public accounting firm is unable (at such time as it is required to do so) to express an opinion on the effectiveness of our internal control, or if we fail to remediate material weaknesses in our internal control, we could be subject to regulatory scrutiny and the loss of public confidence, which could harm our reputation and the market price of our common stock.
To comply with the requirements of being a public company, we will need to undertake various actions, such as implementing new internal controls and procedures and hiring accounting or internal audit staff, to a level necessary to comply with our obligations as a public company, manage our growth and to remediate the existing material weakness in our internal control, and we may discover additional material weaknesses or other deficiencies in existing systems and controls that we may not be able to remediate in an efficient or timely manner. None of our current employees has the requisite knowledge with regard to U.S. GAAP accounting or U.S. public company reporting requirements. If we do not maintain adequate financial and management personnel, processes and controls, we may not be able to manage our business effectively or accurately report our financial performance on a timely basis, which could cause a decline in our common stock price and adversely affect our results of operations and financial condition.
We are an “emerging growth company” and the reduced disclosure requirements applicable to emerging growth companies may make it more difficult for you to evaluate an investment in our company and may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we currently intend to take advantage of some of the exemptions from various reporting requirements and other obligations that are applicable to public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Compliance with new or revised accounting standards may adversely impact our reported financial performance. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
As an “emerging growth company” we have also chosen to take advantage of certain provisions of the JOBS Act that allow us to provide you with less information in this prospectus than would otherwise be required if we are not an “emerging growth company.” As a result, this prospectus includes less information about us than would otherwise be required if we were not an “emerging growth company” within the meaning of the JOBS Act, which may make it more difficult for you to evaluate an investment in our company.
For additional information, see the risk factor above entitled “The JOBS Act allows us to postpone the date by which we must comply with certain laws and regulations intended to protect investors and allows us to reduce the amount of information we have provided to you in this prospectus and will provide in our reports filed with the SEC subsequent to this offering, which may have an adverse effect on the trading price of our common stock.”
-60-
Table of Contents
The liability of our independent registered public accounting firm is limited with respect to claims arising out of its audit report.
Our registered public accounting firm, Ernst & Young, is an Australian partnership and a member of the global network of Ernst & Young firms. The liability of Ernst & Young with respect to claims arising out of its audit report included in this prospectus is subject to the limitations set forth in the Institute of Chartered Accountants in Australia Scheme (Queensland), or ICAA Scheme, approved under the Professional Standards Act 2004 of Queensland, Australia, as amended, which we refer to as the Professional Standards Act. The ICAA Scheme, which commenced on February 1, 2008, may limit the liability of Ernst & Young for damages with respect to certain civil claims arising in, or governed by the laws of, Queensland directly or vicariously from anything done or omitted in the performance of its professional services for us, including, without limitation, its audits of our financial statements included in this prospectus, to the lesser of (in the case of audit services) ten times the reasonable charge for services provided over AUD$100,000 and up to a maximum liability of AUD$75 million (equivalent to approximately US$77.8 million, based on currency exchange rates as of November 1, 2012).
In addition, there is equivalent professional standards legislation in place in other states and territories in Australia and amendments have been made to a number of Australian federal statutes to limit liability under those statutes to the same extent as liability is limited under state and territory laws by professional standards legislation.
These limitations of liability may limit recovery upon the enforcement in Australian courts of any judgment under U.S. or foreign laws rendered against Ernst & Young based on or related to its audit report on our financial statements. However, to our knowledge the Professional Standards Act has not been subject to judicial consideration in Australia and therefore how the limitation will be applied by the courts and the effect of the limitation on the enforcement of foreign judgments are untested.
CHESS Depository Interests, or CDIs, which we expect will represent a substantial majority of our shares of common stock outstanding immediately after this offering, will be listed on the Australian Securities Exchange and will be freely tradable in the public markets in Australia and we expect that the CDIs will begin trading on such exchange approximately seven trading days following the date of this prospectus, which may have a material adverse effect on the trading price of our common stock on the Nasdaq Global Market.
In the Demerger, Alchemia Limited’s shareholders will receive CHESS Depository Interests, or CDIs, representing shares of our common stock or, at their option, shares of our common stock. As most of Alchemia Limited shareholders are located in Australia and only the CDIs (and not our common stock) will be listed on the Australian Securities Exchange, we expect that those shareholders will generally elect to receive CDIs in the Demerger. If that occurs, we expect that those CDIs will represent a substantial majority of our outstanding shares of common stock immediately after this offering.
Alchemia Limited will deliver the shares of our common stock represented by the CDIs distributed in the Demerger to a depositary (which will be CHESS Depositary Nominees Pty Ltd, a subsidiary of the Australian Securities Exchange), which will hold such shares on behalf of the holders of the CDIs. The holders of the CDIs will be the beneficial owners of, and will be entitled to exchange their CDIs for, the underlying shares of our common stock held by the depositary. In addition, holders of CDIs will generally be entitled to exercise the same voting and other rights as holders of our common stock, although they will be required to exercise those rights indirectly through the depositary unless they request the depositary to grant them a proxy to vote directly or exchange their CDIs for the underlying shares of our common stock. Nonetheless, holders of CDIs will have substantially the same economic, voting and other rights as holders of our common stock and the CDIs therefore should be considered as the functional equivalents of shares of our common stock. In addition, holders of shares of our common stock will be permitted to deliver those shares to the depositary in exchange for CDIs.
The purpose of the CDIs is to facilitate trading, settlement and clearance in Australia by shareholders of Alchemia Limited who would otherwise receive shares of our common stock in the Demerger. As our common
-61-
Table of Contents
stock will not be listed on a securities exchange in Australia, Australian residents wishing to trade our common stock would be required to do so through The Nasdaq Global Market in transactions settled in U.S. dollars, which may be inconvenient due to time zone and currency differences, among other factors. However, because we plan to list the CDIs on the Australian Securities Exchange, Alchemia Limited shareholders who receive CDIs in the demerger will be able to trade those CDIs on a local Australian market in transactions settled in Australian dollars. Trading of the CDIs on the Australian Securities Exchange is not expected to commence until approximately seven trading days following the date of this prospectus.
We estimate that approximately 7,592,020 shares of our common stock, or approximately 70% of the estimated total number of shares of our common stock that will be outstanding immediately after this offering and the Demerger, calculated as of the date, in the manner and subject to the assumptions set forth under “Prospectus Summary — The Offering,” will be distributed to Alchemia Limited shareholders in the Demerger (as described under that caption, the actual number of shares that will be distributed in the Demerger will be greater than the foregoing amount). All of these shares, and the CDIs evidencing these shares, will, in general, be freely tradable in the public markets in Australia, subject in some case to the lock-up agreements described under “Underwriting — Lock-Up Agreements.” However, as of November 7, 2012, we expect that approximately 6.7 million shares of our common stock distributed in the Demerger (and any CDIs representing those shares), or approximately 61.3% of the estimated total number of shares of our common stock that will be outstanding immediately after this offering and the Demerger, will not be subject to these lock-up agreements and therefore may be traded in the public markets immediately.
The large number of shares of our common stock distributed in the Demerger that will be freely tradable in the public markets, the fact that many of those shares (and CDIs representing those shares) will not be subject to lock-up agreements, and the existence of a trading market in Australia for CDIs representing those shares, may have a material adverse effect on the market price of, and the trading market for, our common stock on The Nasdaq Global Market. Among other things:
| • | shares of our common stock and CDIs representing those shares that are not to be subject to lock-up agreements may be sold in the public markets in Australia beginning approximately seven trading days following the date of this prospectus, which may have a material adverse effect on the trading price of our common stock on the Nasdaq Global Market; |
| • | holders of CDIs will have the right to surrender their CDIs in exchange for the underlying shares of our common stock and sell that stock on the Nasdaq Global Market, which may result in a decline in the trading price of our common stock on the Nasdaq Global Market; |
| • | holders of our common stock will have the right to surrender their shares to the depositary in exchange for CDIs, which may result in a decline in the trading volume and liquidity of our common stock on the Nasdaq Global Market; |
| • | market prices of the CDIs will be quoted in Australian dollars on the Australian Securities Exchange whereas market prices of our common stock will be quoted in U.S. dollars on the Nasdaq Global Market. Fluctuations in the exchange rate between U.S. dollars and Australian dollars and other changes in the Australian economy affecting investors in securities traded on Australian markets may result in a decrease in demand for and a downward pressure on the trading price of CDIs, which in turn may have a material adverse effect on the trading price of our common stock on the Nasdaq Global Market; and |
| • | as described in the second succeeding risk factor, we may be subject to arbitrage risks that could adversely affect the market price of our common stock. |
As a result, the issuance of the CDIs and the existence of an independent trading market for the CDIs may have a material adverse impact on the trading price of the common stock on the Nasdaq Global Market. Moreover, we expect that a substantial number of CDIs will be outstanding for the indefinite future, so the potential for such a material adverse impact will also continue into the indefinite future.
-62-
Table of Contents
Investors purchasing shares of our common stock in this offering will not be able to freely sell those shares, or CDIs representing those shares, in Australia during the 12 months after the issue date of those shares in this offering and therefore will not be able to take advantage of any liquidity that may be available for CDIs traded on the Australian Securities Exchange during that period.
The shares sold in this offering will not be freely tradable in Australia during the 12 months after their issue date in this offering. In general, shares purchased in this offering may be resold in Australia during that period only to certain “sophisticated investors” and “professional investors” (as defined in the Australian Corporations Act) and certain persons associated with us under Section 708(12) of the Australian Corporations Act, and any subsequent resale of those shares will also be subject to the same restrictions during the 12 months after their issue date in this offering. See “Underwriting — Selling Restrictions — Australia.” So long as those restrictions are in effect, to the extent that investors who purchase shares in this offering are able to resell those shares in Australia, the price they receive will likely be substantially below the market price of our common stock. Likewise, while investors purchasing shares in this offering will be entitled to exchange those shares for CDIs, which are listed on the Australian Securities Exchange, sales of those CDIs in Australia will be subject to the same restrictions that are applicable to the underlying shares of common stock as described above. As a result, any sale of those CDIs in Australia while those restrictions are in effect is also likely to be at a substantial discount to their market price. Although we expect that the majority of the shares of common stock outstanding immediately after this offering will be represented by CDIs that are traded on the Australian Securities Exchange, investors purchasing shares in this offering will not be able to freely sell those shares, or CDIs representing those shares, in Australia during the 12 months after the issue date of those shares in this offering and therefore will not be able to take advantage of any liquidity which may be available for CDIs traded on the Australian Securities Exchange during that period, and any amounts received upon the resale by investors purchasing in this offering while those restrictions are in effect will likely be at a substantial discount to the market price. We intend to apply to the Australian Securities and Investments Commission for waivers from the application of these resale restrictions; however, there is no certainty that all or any of the requested waivers will be granted or, if granted, that such waivers will provide relief from all of these resale restrictions.
As a result of listing CDIs on the Australian Securities Exchange, we will be subject to the listing rules of the Australian Securities Exchange, which may strain our resources, divert management’s attention and affect our ability to manage our business or raise additional capital.
As a result of listing CDIs on the Australian Securities Exchange, we will be subject to the listing rules of the Australian Securities Exchange, which may strain our resources, divert management’s attention and affect our ability to manage our business or raise additional capital. The listing rules of the Australian Securities Exchange differ from, and in some cases are more restrictive than, the rules and requirements of the Nasdaq Global Market, including restrictions that:
| • | limit non-executive director compensation to a maximum amount approved by shareholders at a general meeting; |
| • | require that the terms of every class of our securities, including any preferred stock, be approved by the Australian Securities Exchange; |
| • | prohibit us from removing or changing the voting rights or dividend rights (if any) of our securities, except in certain circumstances; |
| • | specify certain terms and conditions of options and rights plans; |
| • | prohibit issuing equity securities without shareholder approval in the three months after we receive any notice in writing that a person proposes to make a takeover bid; |
-63-
Table of Contents
| • | limit the issuance of restricted (escrowed) securities; and |
| • | prohibit “golden parachutes” or other termination benefits for officers upon a change in ownership or control of us. |
These listing rules may, in some cases, limit our ability to take certain actions that would otherwise be permitted by Nasdaq rules and may affect our ability to manage our business and to attract and retain key management and scientific personnel. In addition, the listing rules of the Australian Securities Exchange include approval and reporting requirements that differ from the requirements under the Nasdaq rules, such as requirements to:
| • | comply with required timetables for issuance of equity securities; |
| • | deliver notice to the Australian Securities Exchange prior to the release of restricted (escrowed) securities; |
| • | file quarterly, half-yearly and annual periodic reports that include specific disclosure required by the listing rules of the Australian Securities Exchange; |
| • | obtain stockholder approval for certain related-party transactions and for securities issuances to directors; |
| • | deliver drafts to the Australian Securities Exchange of charter documents, debt and convertible securities documents, certain meeting notices and documents sent to certain holders of securities; and |
| • | prior to release to any other person, release announcements through the Australian Securities Exchange as the central collection point for market sensitive information. |
Compliance with these additional rules will increase our legal and financial compliance costs, make some activities or transactions more difficult, time-consuming or costly, may limit or prevent us from raising additional capital by issuing and selling shares of our common stock or other securities and increase demand on our systems and resources. We applied to the Australian Securities Exchange for, and received, certain waivers from the application of some of its listing rules; however, such waivers will not afford us relief from all of the increased restrictions and requirements imposed by such listing rules. Increases in our costs and expenses associated with compliance with the Australian Securities Exchange listing rules will adversely impact our results of operations and financial condition.
In addition, limitations on new share issuances under Australian Securities Exchange listing rules may limit or prevent us from raising additional capital by issuing and selling shares of our common stock or other securities when such additional capital is required, which may have a material adverse effect on our results of operations, financial condition and the development of our business. See “— Our ability to raise additional capital may be significantly limited by listing rules of the Australian Securities Exchange that limit the amount of common stock that we are permitted to issue without stockholder approval.”
The market price of our common stock may be adversely affected by arbitrage activities.
Investors may seek to profit by exploiting the difference, if any, in the price of our shares of common stock as reflected by the trading price of our CDIs, which will represent shares of our common stock, on the Australian Securities Exchange and the trading price of our shares of common stock on the Nasdaq Global Market. Such arbitrage activities could cause the price of our common stock or the CDIs representing our common stock, as the case may be, in the market with the higher value to decrease to the price set by the market with the lower value or could otherwise adversely affect the market price of our common stock. These arbitrage risks may be increased by the fact that our common stock will be quoted in U.S. dollars on the Nasdaq Global Market while our CDIs will be quoted in Australian dollars on the Australian Securities Exchange, which may also give investors the opportunity to exploit the impact of fluctuations in currency exchange rates on the market price of our common stock and the CDIs.
-64-
Table of Contents
We do not intend to pay cash dividends for the foreseeable future.
We have never declared or paid any cash dividends on our common stock and do not intend to pay any cash dividends in the foreseeable future. We anticipate that we will retain all of our cash for use in the development of our business and for general corporate purposes. Accordingly, investors seeking or expecting cash dividends should not purchase our common stock.
Since our subsidiaries, Alchemia Oncology Pty Limited and Audeo Discovery Pty Limited are both companies incorporated in Australia, substantially all of our assets are located in Australia, all of our officers, our independent accountants and two of our directors reside in Australia, and all or a substantial portion of their respective assets is located outside the United States, there is a risk that service of process, enforcement of judgments and bringing of original actions against any of them will be more difficult.
Audeo Oncology, Inc., the issuer of the common stock in this offering, is a newly formed Delaware corporation whose assets consist of primarily the capital stock of its two subsidiaries. Those subsidiaries, Alchemia Oncology and Audeo Discovery Pty Limited, which holds the in-license to the VAST technology, are both companies incorporated in Australia. Apart from a small amount of office space in San Francisco, all of our facilities and all or substantially all of our other assets are located in Australia and are owned by our Australian subsidiaries. In addition, all of our officers and two of our directors reside in Australia, and the independent accountants named herein are located in Australia, and all or substantially all of their respective assets are or may be located outside the United States. As a result, it may not be possible to effect service of process within the United States upon our subsidiaries or such persons. It is highly unlikely that an Australian court would be able to enforce proceedings in relation to federal securities laws of the United States that had not first proceeded to a final and conclusive judgment in the United States. In addition, a judgment of a U.S. court (whether or not such a judgment relates to U.S. federal securities laws) against us or our subsidiaries, directors or employees, may not be enforceable in Australia in certain circumstances. See “Enforceability of Foreign Judgments in Australia.” In addition, our independent accountants are entitled to the protections described above under the risk factor entitled “The liability of our independent registered public accounting firm is limited with respect to claims arising out of its audit report.”
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us more difficult, limit attempts by our stockholders to replace or remove our current management and limit the market price of our common stock.
Provisions in our certificate of incorporation and bylaws, as amended and restated in connection with this offering, may have the effect of delaying or preventing a change of control or other takeover of us or changes in our management. Our amended and restated certificate of incorporation and bylaws include provisions that:
| • | authorize our board of directors to issue, without further action by the stockholders, shares of preferred stock with terms, rights and preferences determined by our board of directors, which may include dividend and liquidation rights and preferences, conversion rights and voting rights; |
| • | require that any action to be taken by our stockholders be effected at a duly called annual or special meeting and not by written consent; |
| • | specify that special meetings of our stockholders can be called only by our board of directors, the chairman of our board of directors, our chief executive officer or the president (in the absence of a chief executive officer) and not by our stockholders or any other persons; |
| • | establish an advance notice procedure for stockholder proposals to be brought before a stockholder meeting, including proposed nominations of persons for election to our board of directors; |
-65-
Table of Contents
| • | establish that our board of directors is divided into three classes, with each class serving three-year staggered terms; |
| • | prohibit cumulative voting in the election of directors, which means that the holders of a majority of our outstanding shares of common stock can elect all directors standing for election; |
| • | provide that our directors may be removed only for cause; |
| • | provide that vacancies on our board of directors or newly created directorships resulting from an increase in the number of our directors may be filled only by a majority of directors then in office, even though less than a quorum; |
| • | require the approval of our board of directors or the holders of two-thirds of our outstanding shares of common stock to amend our bylaws and certain provisions of our certificate of incorporation; and |
| • | provide that a state or federal court located within the State of Delaware will be the exclusive forum for any derivative action brought on our behalf, any action asserting a breach of fiduciary duty, any action asserting a claim against us arising under the Delaware General Corporation Law and certain other claims. |
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with any “interested” stockholder for a period of three years following the date on which the stockholder became an “interested” stockholder.
The foregoing provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management. The foregoing provisions may also make it more difficult for a third party to take control of our company or our board of directors, which could limit the opportunity for our stockholders to receive a premium for their shares of common stock and could also adversely affect the price that some investors are willing to pay for our common stock.
-66-
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve substantial risks, assumptions and uncertainties. The forward-looking statements are contained principally in the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” but are also contained elsewhere in this prospectus. Forward-looking statements include information concerning:
| • | our business and scientific strategies; |
| • | the progress of and our expectations regarding our product development programs, including our clinical trials; |
| • | our expectations with respect to regulatory submissions and approvals; |
| • | our expectations with respect to collaborations and government grants; |
| • | our estimates regarding our research and development expenses; |
| • | our financial performance, including our operating expenses and the length of time before which we will require additional funding; |
| • | our anticipated cash needs and our estimates regarding our capital requirements and our need for additional financing; |
| • | our ability to effectively manage our growth and development; |
| • | our ability to manage our international operations, including the impact of changes in currency exchange rates; |
| • | our ability to adapt to changing market conditions; |
| • | the effects of competition in our market; |
| • | our ability to maintain, protect and enhance our intellectual property; |
| • | our ability to attract and retain key management and scientific personnel; |
| • | existing and future regulations that affect our business; |
| • | additional costs and expenses that we will incur as the result of being a public company; |
| • | the number of shares of our common stock that will be distributed to Alchemia Limited’s shareholders in the Demerger, the number of Warrants that will be issuable after this offering and the exercise price for those Warrants; and |
| • | other risk factors included under “Risk Factors” in this prospectus. |
Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “anticipates,” “believes,” “could,” “continue,” “envisage,” “estimates,” “expects,” “intends,” “may,” “might,” “objective,” “plans,” “potential,” “predicts, “projects,” “seeks,” “should,” “pro forma,” “will,” “would” or similar expressions intended to identify statements about the future and the negatives of those terms.
-67-
Table of Contents
Forward-looking statements involve known and unknown risks, uncertainties, assumptions and other factors that may cause our actual results, performance or achievements to be materially different from information expressed or implied by the forward-looking statements. We discuss some of these risks in greater detail in “Risk Factors” and elsewhere in this prospectus. Given these uncertainties, you should not place undue reliance on these forward-looking statements and we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate. Also, forward-looking statements represent our management’s beliefs and assumptions only as of the date of this prospectus. You should read this prospectus and the documents that we have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
We assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements.
-68-
Table of Contents
This prospectus contains estimates, projections and other information concerning our industry, our business, the regulatory approval processes of the FDA and non-U.S. regulatory authorities and the markets for our product candidates, including data regarding the estimated size of those markets, the number of cases of and the mortality rates for certain types of cancer, the incidence of certain other medical conditions, sales of existing anti-cancer drugs, statements that certain drugs, treatment regimens or other therapies are the leading or among the leading drugs, treatment regimens or other therapies and that certain drugs, classes of drugs or dosages are the most widely prescribed in the United States or other markets, as well as data regarding market research, estimates and forecasts prepared by our management and market research firms. Information of this nature is typically based upon a number of significant assumptions and estimates and is inherently subject to uncertainties, and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated or the context otherwise requires, we obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources. In some cases, we do not expressly refer to the sources from which this data is derived. In those cases, when we refer to one or more sources of this type of data in any paragraph, you should assume that other data of this type appearing in the same paragraph is derived from the same sources, unless otherwise expressly stated or the context otherwise requires.
-69-
Table of Contents
We estimate that the net proceeds from our issuance and sale of common stock in this offering will be approximately $42.5 million, or $49.3 million if the underwriters exercise their over-allotment option in full, based on an assumed initial public offering price of $15.00 per share, the midpoint of the estimated price range set forth on the front cover of this preliminary prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us (including $237,775 of offering expenses already paid by us through September 30, 2012). Each $1.00 increase (decrease) in the assumed initial public offering price would increase (decrease) the net proceeds to us from this offering by approximately $3.0 million, assuming the number of shares offered by us, as set forth on the front cover of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions.
The principal purposes of this offering are to obtain additional capital, facilitate our future access to the public equity markets, create a public market for our common stock and generally increase the awareness of our company.
We currently intend to use the net proceeds we receive from this offering as follows:
| • | approximately $21.0 million to complete our pivotal Phase III clinical trial and related regulatory filings for HA-Irinotecan for the treatment of mCRC; |
| • | approximately $6.6 million to prepare for commercial-scale manufacturing of HA-Irinotecan, if approved for the treatment of mCRC, which would entail procurement of key raw materials, developing and validating a manufacturing process to manufacture commercial-scale quantities of product and preparing and filing regulatory documents; |
| • | approximately $3.9 million to fund our research and development activities related to our HyACT platform; |
| • | approximately $3.8 million to fund our research and drug discovery activities related to our in-licensed VAST drug discovery technology; and |
| • | the balance for general corporate purposes, including working capital and capital expenditures, which may include the acquisition or in-licensing of additional compounds, product candidates and technology, although we have no present commitments or agreements to enter into any such acquisitions. |
The amounts set forth in the first bullet point above reflect our estimate, as of November 1, 2012, of the cost of completing our pivotal Phase III clinical trial and related regulatory filings for HA-Irinotecan for the treatment of mCRC, and this estimate is subject to assumptions and uncertainties, and there can be no assurance that the actual costs will not be greater.
The expected uses of the net proceeds we receive from this offering represent our intentions based upon our current plans and business conditions. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors. Accordingly, we will have broad discretion over the uses of the net proceeds in this offering and investors will be relying on the judgment of our management regarding the application of the net proceeds. In addition, it is possible that the amount set forth above will not be sufficient for the purposes described above.
Pending these uses, we intend to invest the net proceeds from this offering in interest bearing and similar investments with terms of 18 months or less, which may include interest-bearing bank accounts, money market funds, certificates of deposit and government securities.
We have never declared or paid cash dividends on our common stock. We currently intend to retain all available funds for use in the operation of our business and do not intend to pay any cash dividends on our common stock in the foreseeable future. Any future determination to declare cash dividends will be made at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions, relevant legal requirements, any limitations on dividends set forth in agreements to which we may become a party in the future and other factors that our board of directors may deem relevant.
-70-
Table of Contents
The following table sets forth our consolidated cash and cash equivalents, short-term deposit and our consolidated capitalization as of September 30, 2012:
| • | on an actual basis; |
| • | on a pro forma basis to reflect the Demerger and certain related transactions described in note (1) to the following table, as if all of the foregoing transactions had occurred as of September 30, 2012 (as indicated in note (1) to the following table, the actual number of shares of our common stock distributed in the Demerger will be greater than the number of shares we have assumed for purposes of this pro forma data); |
| • | on a pro forma as adjusted basis to reflect (i) the transactions described in the immediately preceding bullet point, (ii) our receipt of the net proceeds from our sale of common stock in this offering at an assumed initial public offering price of $15.00 per share, the midpoint of the estimated price range set forth on the front cover of this preliminary prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us (excluding $237,775 of offering expenses already paid by us through September 30, 2012) and (iii) the filing of our amended and restated certificate of incorporation and the effectiveness of our amended and restated bylaws, which will occur immediately prior to completion of this offering. |
The pro forma and pro forma as adjusted information set forth below is based upon our consolidated balance sheet as of September 30, 2012. The following pro forma and pro forma as adjusted information is illustrative only, is based on the amount of the applicable employee entitlements liabilities and amounts due to and/or due from Alchemia Limited as of September 30, 2012, does not purport to reflect what our actual cash and cash equivalents, short-term deposit, issued and outstanding shares of common stock or actual capitalization would have been had the transactions described above and in the notes to the following table occurred as of September 30, 2012, and our actual cash and cash equivalents, short-term deposit, issued and outstanding shares of common stock and capitalization following those transactions will be different from the amounts reflected below. You should read this table together with “Prospectus Summary — The Offering,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes appearing elsewhere in this prospectus.
| As of September 30, 2012 | ||||||||||||
| Actual | Pro
Forma for Demerger(1)(2) |
Pro Forma As Adjusted for Offering(2)(3) |
||||||||||
| (in thousands, except per share data) | ||||||||||||
| Cash and cash equivalents |
$ | 4,503 | $ | 4,218 | $ | 46,917 | ||||||
|
|
|
|
|
|
|
|||||||
| Short-term deposit |
$ | 21 | $ | 21 | $ | 21 | ||||||
|
|
|
|
|
|
|
|||||||
| Stockholders’ equity: |
||||||||||||
| Common stock, $0.001 par value; 30,000,000 shares authorized, 7,592,020 shares issued and outstanding, actual; 75,000,000 shares authorized, 7,592,020 shares issued and outstanding, pro forma for Demerger; and 75,000,000 shares authorized, 10,842,020 shares issued and outstanding, pro forma as adjusted for offering |
$ | 8 | $ | 8 | $ | 11 | ||||||
| Preferred stock, $0.001 par value; no shares authorized, issued or outstanding, actual or pro forma for Demerger; 25,000,000 shares authorized, no shares issued or outstanding, pro forma as adjusted for offering |
— | — | — | |||||||||
| Additional paid-in capital |
51,279 | 51,279 | 93,737 | |||||||||
| Accumulated other comprehensive loss |
(2,102) | (2,102) | (2,102) | |||||||||
| Deficit accumulated during development stage |
(47,124) | (47,124) | (47,124) | |||||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ equity |
2,061 | 2,061 | 44,522 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | 2,061 | $ | 2,061 | $ | 44,522 | ||||||
|
|
|
|
|
|
|
|||||||
-71-
Table of Contents
| (1) | Gives effect to the Demerger, including our anticipated assumption of approximately $137,129 of Alchemia Limited’s employee entitlements liabilities in connection with the Demerger, the assumption that all balances due to and/or due from Alchemia Limited (which are reflected on our balance sheet as amounts due from or owed to related party) will be cash settled prior to the Demerger, and the assumption that Alchemia Limited will distribute 7,592,020 shares of our common stock that are currently outstanding and held by it (or CDIs representing those shares) to Alchemia Limited shareholders (or a depositary on their behalf) in the Demerger, which number of shares is calculated on the basis of 280,812,962 ordinary shares of Alchemia Limited outstanding as of November 1, 2012, and the distribution of one share of our common stock for every 37 outstanding ordinary shares of Alchemia Limited, as if all of the foregoing transactions had occurred as of September 30, 2012. This pro forma data is illustrative only, is based on the amount of the applicable employee entitlements liabilities and amounts due to and/or due from Alchemia Limited as of September 30, 2012, does not purport to reflect what our actual cash and cash equivalents, short-term deposit, issued and outstanding shares of common stock or capitalization would have been had the foregoing transactions occurred as of that date and will change based on the actual amount of Alchemia Limited’s employee entitlements liabilities that we assume in the Demerger, the actual amount of balances due to and/or due from Alchemia Limited that are settled in cash and the actual number of shares of our common stock distributed in the Demerger. As noted above under “Prospectus Summary — The Offering,” the actual number of shares of our common stock distributed in the Demerger will depend upon the number of holders of ordinary shares of Alchemia Limited, and the number of ordinary shares of Alchemia Limited outstanding, as of the record date for the Demerger, which will be approximately four trading days following the date of this prospectus, and will in any event be greater than the number we have assumed for purposes of this prospectus because the terms of the Demerger provide that fractional shares of our common stock that would otherwise be distributed pursuant to the Demerger will be rounded upwards to the nearest whole share. In addition, options to purchase a total of 3,573,000 ordinary shares of Alchemia Limited at exercise prices ranging from AUD$0.280 to AUD$1.040 per share (equivalent to approximately US$0.291 to US$1.079 per share based on the currency exchange rate as of November 1, 2012) were outstanding as of September 30, 2012 and any exercise of these options or any other options that Alchemia Limited may grant prior to the record date for the Demerger would increase the number of ordinary shares of Alchemia Limited outstanding as of such record date. Any increase in the number of holders of ordinary shares of Alchemia Limited, or the number of ordinary shares of Alchemia Limited outstanding, as of the record date for the Demerger compared with the number of holders or the number of ordinary shares outstanding as of November 1, 2012 would increase, and the fact that fractional shares of our common stock distributed in the Demerger will be rounded upwards to the nearest whole share as described above will increase, the number of shares of our common stock distributed in the Demerger above the number of shares we have assumed for purposes of this pro forma data. To the extent that the total number of shares of our common stock distributed in the Demerger exceeds the number of shares currently held by Alchemia Limited, we will issue additional shares of common stock for distribution in the Demerger (as of November 5, 2012, Alchemia Limited held 7,592,020 shares of our common stock). As a result, both the number of shares of our common stock that will be distributed in the Demerger and that will be outstanding immediately after this offering will be greater than the respective numbers of shares we have assumed for purposes of this pro forma data. Each 37 share increase (decrease) in the number of ordinary shares of Alchemia Limited outstanding as of the record date for the Demerger would increase (decrease) the number of shares of our common stock issued and outstanding, pro forma for the Demerger, as reflected in the common stock line item in the table above, by one share (before giving effect to the rounding upwards of fractional shares as described above). |
| (2) | As described above under “Prospectus Summary–the Offering,” if and when this offering is completed and the Average Trading Price of our common stock has been determined for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market, the fair value of the Rights will be remeasured. As the initial exercise price of the Warrants and the number of Warrants will have been fixed when such Average Trading Price is determined, the fair value of the Warrants will at that time be reclassified from current liabilities to stockholders’ equity on our balance sheet and the Warrants will be accounted for as equity awards. However, because neither the Rights nor the Warrants are directly attributable to this offering and such reclassification will not occur until approximately 60 trading days after our common stock is listed on the Nasdaq Global Market, the pro forma and pro forma as adjusted information set forth above does not give effect to this reclassification. The remeasurement of the fair value of the Rights at each balance sheet date (prior to such reclassification) and at the time of such |
-72-
Table of Contents
| reclassification may result in additional expense, and any such expense may have a material adverse effect on our results of operations. In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Operations Overview—Fair Value of Financial Instruments” and “—Off-Balance Sheet Arrangements—Warrants.” |
| (3) | Gives effect to the transactions described in note (1) above and the sale of shares of common stock in this offering at an assumed public offering price of $15.00 per share (the midpoint of the estimated price range set forth on the cover page of this preliminary prospectus), after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us (excluding $237,775 of offering expenses already paid by us through September 30, 2012), as if those transactions had occurred as of September 30, 2012. This pro forma as adjusted information is illustrative only, is based on the amount of the applicable employee entitlements liabilities and amounts due to and/or due from Alchemia Limited as of September 30, 2012, does not purport to reflect what our actual cash and cash equivalents, short-term deposit, issued and outstanding shares of common stock or capitalization would have been had the foregoing transactions occurred as of that date and will change based on the actual amount of Alchemia Limited’s employee entitlements liabilities that we assume in the Demerger, the actual amount of balances due to and/or from Alchemia Limited that are settled in cash, the actual number of shares of our common stock distributed in the Demerger, the actual initial public offering price and other terms of this offering determined at pricing and the actual offering expenses payable by us. Each $1.00 increase (decrease) in the assumed initial public offering price of $15.00 per share, the midpoint of the estimated price range set forth on the front cover of this preliminary prospectus, would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $3.0 million, assuming that there is no change in the amount of employee entitlements liabilities assumed or amount of balances due to and/or due from Alchemia Limited that are settled in cash in connection with the Demerger, that 7,592,020 shares of our common stock are distributed to Alchemia Limited shareholders in the Demerger, that the number of shares offered by us, as set forth on the front cover of this prospectus, remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. As discussed in note (1) above, the actual number of shares of our common stock distributed in the Demerger will be greater than the number of shares we have assumed for purposes of this pro forma as adjusted data. Each 37 share increase (decrease) in the estimated number of ordinary shares of Alchemia Limited outstanding as of the record date for the Demerger would increase (decrease) the number of shares of our common stock issued and outstanding, pro forma as adjusted for this offering, as reflected in the common stock line item in the table above, by one share (before giving effect to the rounding upwards of fractional shares as described above). The pro forma as adjusted data assumes that we initially invest all of the estimated net proceeds from this offering in cash and cash equivalents and does not give effect to our use of proceeds from the sale of shares of common stock in this offering for the other purposes set forth under “Use of Proceeds.” |
The actual, pro forma and pro forma as adjusted amounts in the above table exclude:
| • | shares of our common stock, or the Warrant Shares, issuable upon the exercise of the Warrants that we will issue after this offering to investors who purchased ordinary shares of Alchemia Limited in a private placement and that will have an exercise price equal to 130% of the volume weighted average price of our common stock for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market (we sometimes refer to such volume weighted average price for such period as the Average Trading Price). The Warrants will be issuable approximately 60 trading days subsequent to completion of this offering and the number of Warrants so issued will be calculated as described in “Prospectus Summary — The Offering.” Each Warrant will initially be exercisable for one share of our common stock. However, as the number of Warrants and exercise price both depend upon the actual Average Trading Price following this offering and the number of Warrants also depends upon the actual exchange rate between U.S. dollars and Australian dollars following this offering, the actual number of Warrants that we issue (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the actual exercise price will likely differ from the numbers we have assumed for purposes of this preliminary prospectus. See “Prospectus Summary — The |
-73-
Table of Contents
| Offering” for an analysis showing how changes in the assumed Average Trading Price change the number of Warrants (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the exercise price of the Warrants and how changes in the assumed currency exchange rate change the number of Warrants (and therefore, the number of Warrants Shares for which the Warrants are exercisable). In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock; |
| • | options to purchase 948,000 shares of our common stock granted under our 2012 Stock Plan at an exercise price per share equal to the initial public offering price per share set forth on the cover page of this prospectus (following this offering, we do not expect to make further grants pursuant to our 2012 Stock Plan); and |
| • | 1,250,000 shares of our common stock reserved for future issuance under our 2012 Equity Incentive Plan, which will become effective in connection with this offering, and additional shares of our common stock that will become available for issuance under our 2012 Equity Incentive Plan pursuant to provisions thereof that automatically increase the number of shares of our common stock reserved for issuance under the plan each year, beginning with our fiscal year commencing July 1, 2013, as more fully described in “Executive Compensation — 2012 Equity Incentive Plan.” |
-74-
Table of Contents
If you invest in our common stock, your interest in our company will be diluted to the extent of the difference between the amount per share paid by purchasers of shares of common stock in this initial public offering and the pro forma as adjusted net tangible book value per share of common stock immediately after this offering.
Dilution results from the fact that the per share offering price of our common stock is substantially in excess of the book value per share attributable to the existing stockholders (for purposes of this discussion of dilution, we are including Alchemia Limited shareholders who receive shares of our common stock or CDIs representing those shares in the Demerger as “existing stockholders”). Net tangible book value per share represents the amount of our total tangible assets less our total liabilities, divided by the shares of common stock outstanding. As of September 30, 2012, we had a net tangible book value of approximately $2.1 million, or approximately $0.27 per share.
After giving effect to the Demerger, including our anticipated assumption of approximately $137,129 of Alchemia Limited’s employee entitlements liabilities in connection with the Demerger, the assumed cash settlement of balances due to and/or due from Alchemia Limited prior to the Demerger, the assumption that Alchemia Limited will distribute 7,592,020 shares of our common stock that are currently outstanding and held by it to Alchemia Limited shareholders (or a depositary on their behalf) in the Demerger, which number of shares is calculated on the basis of 280,812,962 ordinary shares of Alchemia Limited outstanding as of November 1, 2012 and the distribution of one share of our common stock for every 37 outstanding ordinary shares of Alchemia Limited (before giving effect to the fact that fractional shares of our common stock that would otherwise be distributed in the Demerger will be rounded upwards to the nearest whole share), as if those transactions had occurred as of September 30, 2012, our pro forma net tangible book value as of that date would have been approximately $2.1 million, or approximately $0.27 per share of our outstanding common stock. Likewise, after giving effect to the transactions described in the immediately preceding sentence and the sale of shares of our common stock in this offering at an assumed initial public offering price of $15.00 per share, the midpoint of the estimated price range set forth on the cover page of this preliminary prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, as if those transactions had occurred as of September 30, 2012, our pro forma as adjusted net tangible book value as of that date would have been approximately $44.5 million, or approximately $4.11 per share of our common stock. This represents an immediate increase in pro forma as adjusted net tangible book value of $3.84 per share to existing stockholders and an immediate dilution of $10.89 per share to new investors purchasing shares in this offering. As noted above under “Prospectus Summary — The Offering,” the actual number of shares of our common stock distributed in the Demerger will depend upon the number of holders and the number of outstanding ordinary shares of Alchemia Limited as of the record date for the Demerger, which will be approximately four trading days following the date of this prospectus, and will in any event be greater than the number we have assumed for purposes of this prospectus because the terms of the Demerger provide that fractional shares of our common stock that would otherwise be distributed pursuant to the Demerger will be rounded upwards to the nearest whole share. In addition, options to purchase a total of 3,573,000 ordinary shares of Alchemia Limited at exercise prices ranging from AUD$0.280 to AUD$1.040 per share (equivalent to approximately US$0.291 to US$1.079 per share based on the currency exchange rate as of November 1, 2012) were outstanding as of September 30, 2012 and any exercise of these options or any other options that Alchemia Limited may grant prior to the record date for the Demerger would increase the number of ordinary shares of Alchemia Limited outstanding as of such record date. Any increase in the number of holders or the number of outstanding ordinary shares of Alchemia Limited as of the record date for the Demerger compared with the number of holders or the number of outstanding ordinary shares as of November 1, 2012 would increase, and the fact that fractional shares of our common stock distributed in the Demerger will be rounded upwards to the nearest whole share as described above will increase, the number of shares of our common stock distributed in the Demerger above the number of shares we have assumed for purposes of the pro forma and pro forma as adjusted data set forth in this section. To the extent that the total number of shares of our common stock distributed in the Demerger exceeds the number of shares currently held by Alchemia Limited, we will issue
-75-
Table of Contents
additional shares of common stock for distribution in the Demerger (as of November 5, 2012, Alchemia Limited held 7,592,020 shares of our common stock). As a result, both the number of shares of our common stock that will be distributed in the Demerger and that will be outstanding immediately after this offering will be greater than the respective numbers of shares we have assumed for purposes of the pro forma and pro forma as adjusted data set forth in this section and our actual pro forma and pro forma as adjusted net tangible book value per share, pro forma as adjusted dilution per share and the other information in this section that gives effect to the Demerger will be different from the amounts and other information set forth in this section.
The following table illustrates this dilution:
| Assumed initial public offering price per share |
$ | 15.00 | ||||||
| Actual net tangible book value per share as of September 30, 2012 |
$ | 0.27 | ||||||
| Net decrease in net tangible book value per share of common stock attributable to the Demerger and the distribution of shares of our common stock to Alchemia Limited shareholders in the Demerger |
— | |||||||
|
|
|
|||||||
| Pro forma net tangible book value per share after giving effect to the transactions described in the preceding line item |
0.27 | |||||||
| Net increase in net tangible book value per share of common stock attributable to this offering |
3.84 | |||||||
|
|
|
|||||||
| Pro forma as adjusted net tangible book value per share after the Demerger and this offering |
4.11 | |||||||
|
|
|
|||||||
| Pro forma as adjusted dilution per share to new investors in this offering |
$ | 10.89 | ||||||
|
|
|
The dilution information discussed above is illustrative only, is based on the amount of the applicable employee entitlements liabilities and amounts due to and/or due from Alchemia Limited as of September 30, 2012, and will change based on the actual amount of employee entitlements liabilities assumed by us and the actual amount of balances due to and/or due from Alchemia Limited that are settled in cash in connection with the Demerger, the actual initial public offering price and other terms of this offering determined at pricing, the actual offering expenses payable by us and the actual number of shares of our common stock distributed in the Demerger. Each $1.00 increase (decrease) in the assumed initial public offering price of $15.00 per share, the midpoint of the estimated price range set forth on the front cover of this preliminary prospectus, would increase (decrease) our pro forma as adjusted net tangible book value by approximately $0.28 per share and increase (decrease) the dilution in pro forma as adjusted net tangible book value per share to new investors in this offering by approximately $0.72 per share, assuming that there is no change in the amount of employee entitlements liabilities assumed or the amount of balances due to and/or due from Alchemia Limited that are cash settled in connection with the Demerger, that 7,592,020 shares of our common stock are distributed to Alchemia Limited shareholders in the Demerger and that the number of shares offered by us, as set forth on the front cover of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Each 100,000 share increase (decrease) in the number of shares of our common stock distributed in the Demerger would decrease (increase) our pro forma as adjusted net tangible book value per share by approximately $0.04 per share and would increase (decrease) the dilution in pro forma as adjusted net tangible book value per share to new investors in the offering by approximately $0.04 per share, assuming an initial public offering price of $15.00 per share, the midpoint of the estimated price range set forth on the front cover of this preliminary prospectus, that there is no change in the amount of employee entitlements liabilities assumed or the amount of balances due to and/or due from Alchemia Limited that are cash settled in the Demerger and that the number of shares offered by us, as set forth on the front cover of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters exercise their over-allotment option in full, the pro forma as adjusted net tangible book value per share of our common stock after this offering would be approximately $4.53 per share of common
-76-
Table of Contents
stock and the pro forma as adjusted dilution per share to new investors in this offering would be approximately $10.47 per share of our common stock, in each case calculated as described above.
The following table summarizes, on a pro forma as adjusted basis as of September 30, 2012, the total number of shares of common stock purchased from us, the total consideration paid to us, and the average price per share paid to us by existing stockholders and by new investors purchasing shares in this offering, after giving effect the assumption that Alchemia Limited will distribute 7,592,020 shares of our common stock that are currently outstanding and held by it to Alchemia Limited shareholders (or a depositary on their behalf) in the Demerger, which number of shares is calculated on the basis of 280,812,962 ordinary shares of Alchemia Limited outstanding as of November 1, 2012 and the distribution to Alchemia Limited shareholders of one share of our common stock for every 37 outstanding ordinary shares of Alchemia Limited (but without giving effect to the rounding upwards of fractional shares of our common stock as described above), and our sale of common stock in this offering at an assumed initial public offering price of $15.00 per share, the midpoint of the estimated price range set forth on the front cover of this preliminary prospectus, before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, as if those transactions had occurred as of September 30, 2012. As noted above, the actual number of shares of our common stock that are distributed in the Demerger will be greater than the number of shares of our common stock we have assumed for purposes of the following data. For purposes of the following table, we have included shares distributed pursuant to the Demerger as shares purchased by existing stockholders at an assumed average purchase price of approximately $6.76 per share; such average purchase price reflects the fact that the deemed consideration we received for shares distributed in the Demerger was approximately $6.76 per share.
| Shares Purchased | Total Consideration | Average Price Per Share |
||||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||||
| Existing stockholders |
7,592,020 | 70 | % | $ | 51,286,876 | 51 | % | $ | 6.76 | |||||||||||
| New investors |
3,250,000 | 30 | 48,750,000 | 49 | 15.00 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total for all stockholders |
10,842,020 | 100 | % | $ | 100,036,876 | 100 | % | 9.23 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
Each $1.00 increase (decrease) in the assumed public offering price of $15.00 per share, the midpoint of the estimated price range set forth on the front cover of this preliminary prospectus, would increase (decrease) total consideration paid by new investors, total consideration paid by all stockholders and the average price per share paid by all stockholders by $3.3 million, $3.3 million and $0.30, respectively, assuming the number of shares offered by us, as set forth on the front cover of this prospectus, remains the same and that 7,592,020 shares of our common stock are distributed in the Demerger and before deducting estimated underwriting discounts and commissions and estimated offering expenses. Each 100,000 share increase (decrease) in the assumed number of shares of our common stock distributed in the Demerger would decrease (increase) the average price per share paid by existing stockholders and average price per share paid by all stockholders by $0.09 and $0.08, respectively, assuming an initial public offering price of $15.00 per share and that the number of shares offered by us, as set forth on the front cover of this prospectus, remains the same and before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters exercise their over-allotment option in full, the total consideration paid by new investors, total consideration paid by all stockholders and the average price per share paid by all stockholders would be $56.1 million, $107.3 million and $9.48, respectively, in each case calculated as described above.
The foregoing discussion and tables exclude:
| • | shares of our common stock, or the Warrant Shares, issuable upon the exercise of the Warrants that we will issue after this offering to investors who purchased ordinary shares of Alchemia Limited in a |
-77-
Table of Contents
| private placement and that will have an exercise price equal to 130% of the volume weighted average price of our common stock for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market (we sometimes refer to such volume weighted average price for such period as the Average Trading Price). The Warrants will be issuable approximately 60 trading days subsequent to completion of this offering and the number of Warrants so issued will be calculated as described in “Prospectus Summary — The Offering.” Each Warrant will initially be exercisable for one share of our common stock. However, as the number of Warrants and exercise price both depend upon the actual Average Trading Price following this offering and the number of Warrants also depends upon the actual exchange rate between U.S. dollars and Australian dollars following this offering, the actual number of Warrants that we issue (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the actual exercise price will likely differ from the numbers we have assumed for purposes of this preliminary prospectus. See “Prospectus Summary — The Offering” for an analysis showing how changes in the assumed Average Trading Price change the number of Warrants (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the exercise price of the Warrants and how changes in the assumed currency exchange rate change the number of Warrants (and therefore, the number of Warrants Shares for which the Warrants are exercisable). In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock; |
| • | options to purchase 948,000 shares of our common stock granted under our 2012 Stock Plan at an exercise price per share equal to the initial public offering price per share set forth on the cover page of this prospectus (following this offering, we do not expect to make further grants pursuant to our 2012 Stock Plan); and |
| • | 1,250,000 shares of our common stock reserved for future issuance under our 2012 Equity Incentive Plan, which will become effective in connection with this offering, and additional shares of our common stock that will become available for issuance under our 2012 Equity Incentive Plan pursuant to provisions thereof that automatically increase the number of shares of our common stock reserved for issuance under the plan each year, beginning with our fiscal year commencing on July 1, 2013, as more fully described in “Executive Compensation — 2012 Equity Incentive Plan.” |
If any of the options or warrants referred to above are exercised or if any options we grant in the future are exercised, there may be further dilution to investors participating in this offering. In addition, if we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
The foregoing discussion gives pro forma effect to the issuance of 92,000 shares of common stock that we issued to Alchemia Limited on November 5, 2012, as if this issuance had occurred prior to September 30, 2012.
-78-
Table of Contents
SELECTED CONSOLIDATED FINANCIAL DATA
You should read the following selected consolidated financial data in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and related notes, all included elsewhere in this prospectus. As we are a subsidiary of Alchemia Limited, our consolidated financial statements are derived from the consolidated financial statements and accounting records of Alchemia Limited. Accordingly, our consolidated financial statements include allocations of expenses, primarily salaries and benefits (including stock-based compensation) of officers and other employees of Alchemia Limited, expenses for compensation and reimbursement paid to directors of Alchemia Limited and insurance expenses incurred by Alchemia Limited. These expenses have been allocated based on our estimated share of Alchemia Limited’s expenses using the proportional cost allocation method. Although we believe that these allocation methodologies are reasonable, the selected consolidated financial information set forth below and our consolidated financial statements do not purport to reflect the results of operations, financial position and cash flows that we would have achieved as a separate, publicly traded company during the periods presented or those that we will achieve in the future.
We derived the selected consolidated statement of operations data for the years ended June 30, 2011 and June 30, 2012 and the selected consolidated balance sheet data as of June 30, 2011 and June 30, 2012 from our audited consolidated financial statements included elsewhere in this prospectus. The selected consolidated statement of operations data for the three months ended September 30, 2011 and September 30, 2012 and for the period from inception (May 29, 2006) to September 30, 2012 and the selected consolidated balance sheet data as of September 30, 2012 have been derived from our unaudited interim condensed consolidated financial statements included elsewhere in this prospectus. Our historical results of operations and financial condition do not purport to be indicative of our results of operations or financial condition as of any future date or for any future period.
| Fiscal Year Ended June 30, |
Three Months Ended September 30 |
For the period from inception (May 29, 2006) to September 30, 2012(2) |
||||||||||||||||||
| 2011 | 2012 | 2011 | 2012 | |||||||||||||||||
| (in thousands, except per share data) |
||||||||||||||||||||
| Consolidated Statement of Operations Data: |
||||||||||||||||||||
| Income: |
||||||||||||||||||||
| Grant income |
$ | — | $ | — | $ | — | $ | 61 | $ | 1,570 | ||||||||||
| Research funding income |
— | — | $ | — | $ | — | $ | 434 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total income |
— | — | — | 61 | 2,004 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating expenses: |
||||||||||||||||||||
| Research and development(1) |
$ | 6,986 | $ | 7,807 | $ | 850 | $ | 4,020 | $ | 42,325 | ||||||||||
| General and administrative(1) |
929 | 1,674 | 234 | 850 | 6,629 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
7,915 | 9,481 | 1,084 | 4,870 | 48,954 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(7,915 | ) | (9,481 | ) | (1,084 | ) | (4,809 | ) | (46,950 | ) | ||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest income |
2 | 3 | — | 10 | 209 | |||||||||||||||
| Other income |
— | 7 | — | — | 434 | |||||||||||||||
| Change in fair value of rights(3) |
— | (481 | ) | — | (336 | ) | (817 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total other income (expense) |
2 | (471 | ) | — | (326 | ) | (174 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss before income taxes |
$ | (7,913 | ) | $ | (9,952 | ) | $ | (1,084 | ) | $ | (5,135 | ) | $ | (47,124 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income tax expense |
— | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (7,913 | ) | $ | (9,952 | ) | $ | (1,084 | ) | $ | (5,135 | ) | $ | (47,124 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Other comprehensive loss, net of tax: |
||||||||||||||||||||
| Foreign currency translation adjustment |
(2,976 | ) | 1,421 | 1,751 | 158 | (2,101 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
-79-
Table of Contents
| Fiscal Year Ended June 30, | Three Months Ended September 30, |
For the period from inception (May 29, 2006) to September 30, 2012(2) |
||||||||||||||||||
| 2011 | 2012 | 2011 | 2012 | |||||||||||||||||
| (in thousands, except per share data) |
||||||||||||||||||||
| Total comprehensive loss |
$ | (10,889 | ) | $ | (8,531 | ) | $ | 667 | $ | (4,977 | ) | $ | (49,225 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per share: |
||||||||||||||||||||
| Basic and diluted |
$ | (4.03) | $ | (4.95) | $ | (0.55) | $ | (0.68) | $ | (21.45) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted-average shares outstanding: |
||||||||||||||||||||
| Basic and diluted |
1,965,641 | 2,011,885 | 1,965,641 | 7,592,020 | 2,196,429 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Includes stock-based compensation expense as follows: |
| Fiscal Year Ended June 30, |
Three Months Ended September 30, |
For the period from inception (May 29, 2006) to September 30, 2012(2) |
||||||||||||||||||
| 2011 | 2012 | 2011 | 2012 | |||||||||||||||||
| (in thousands) |
||||||||||||||||||||
| Research and development |
$ | 85 | $ | 97 | $ | 23 | $ | 5 | $ | 657 | ||||||||||
| General and administrative |
30 | 91 | 6 | 6 | 571 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total stock-based compensation |
$ | 115 | $ | 188 | $ | 29 | $ | 11 | $ | 1,228 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (2) | Date of inception refers to date of Alchemia Limited acquired Alchemia Oncology (which was known as Meditech Research Ltd. prior to its acquisition). |
| (3) | As described above under “Prospectus Summary—The Offering,” if and when this offering is completed and the Average Trading Price of our common stock has been determined for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market, we are required to issue Warrants to purchase shares of our common stock to investors who purchased ordinary shares of Alchemia Limited in a private placement in November and December 2011. The rights of these investors, or the Rights, pursuant to which we are required, contingent upon completion of this offering, to issue the Warrants, are classified as a current liability on our balance sheet in an amount initially equal to their fair value on the date of issuance and the fair value of the Rights is remeasured at the end of each reporting period with any change in the fair value recorded in our statement of operations for such period. One factor impacting the fair value of the Rights is the underlying value of our common stock. Assuming no changes to other factors, the fair value of the Rights increases or decreases in proportion with increases and decreases, respectively, in the value of our common stock. In addition, if and when this offering is completed and the Average Trading Price of our common stock is determined as described under “Prospectus Summary—The Offering,” the fair value of the Rights will be remeasured. As the initial exercise price of the Warrants and the number of Warrants will have been fixed when such Average Trading Price is determined, the fair value of the Warrants will at that time be reclassified from current liabilities to stockholders’ equity on our balance sheet and the Warrants will be accounted for as equity awards. Any increase or decrease in the fair value for the then current period compared with the fair value as of the end of our then most recently ended reporting period will be reflected in our statement of operations for the then current period. The remeasurement of the fair value of the Rights at each balance sheet date (prior to such reclassification) and at the time of such reclassification may result in additional expense, and any such expense may have a material adverse effect on our results of operations. The remeasurement of the fair value of the Rights will cease after they have been reclassified to stockholders’ equity. In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Operations Overview—Fair Value of Financial Instruments” and “—Off-Balance Sheet Arrangements—Warrants.” |
-80-
Table of Contents
| June 30, | September 30, | |||||||||||
| 2011 | 2012 | 2012 | ||||||||||
| (in thousands) | ||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||
| Cash and cash equivalents |
$ | 159 | $ | 7,636 | $ | 4,503 | ||||||
| Short-term deposit |
21 | 20 | 21 | |||||||||
| Due from related party |
— | 68 | 275 | |||||||||
| Total assets |
912 | 9,712 | 7,805 | |||||||||
| Due to related party |
— | 167 | 697 | |||||||||
| Total current liabilities |
22,968 | (1) | 2,604 | 5,534 | ||||||||
| Total liabilities |
23,030 | 2,680 | 5,744 | |||||||||
| Total stockholders’ equity (deficit) |
$ | (22,119 | ) | $ | 7,033 | $ | 2,061 | |||||
| (1) | Includes approximately $22.3 million of related party loans consisting of unsecured, non-interest bearing loans with no maturity date made to us by Alchemia Limited. Pursuant to the Loan Conversion, as of June 30, 2012, all of these loans were converted into newly-issued ordinary shares of Alchemia Oncology, and we subsequently acquired such ordinary shares from Alchemia Limited. See “Prospectus Summary—Corporate Information.” |
-81-
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with the consolidated financial statements and related notes that are included elsewhere in this prospectus. This discussion contains forward looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward looking statements as a result of various factors, including those set forth under “Risk Factors” and “Special Note Regarding Forward-Looking Statements” or in other parts of this prospectus. The last day of our fiscal year is June 30. Our fiscal quarters end on September 30, December 31, March 31, and June 30. Fiscal 2013, our current fiscal year, will end on June 30, 2013.
Our consolidated financial statements are derived from the consolidated financial statements and accounting records of Alchemia Limited. Our consolidated financial statements include allocations of expenses, primarily salaries and benefits (including stock-based compensation) of officers and other employees of Alchemia Limited, expenses for compensation and reimbursement paid to directors of Alchemia Limited and insurance expenses incurred by Alchemia Limited. These expenses have been allocated based on our estimated share of Alchemia Limited’s expenses using the proportional cost allocation method. Although we believe that these allocation methodologies are reasonable, the financial information included in this discussion and our consolidated financial statements do not purport to reflect the results of operations, financial position and cash flows that we would have achieved as a separate, publicly traded company during the periods presented or those that we will achieve in the future.
Overview
We are a late stage biopharmaceutical company primarily focused on utilizing our Hyaluronic Acid Chemotransport Technology, or HyACT, to target cancer drugs preferentially to tumor cells to enhance drug activity. HyACT is a flexible platform technology designed to increase the effectiveness of anti-cancer agents without increasing treatment toxicity. We seek to reduce the risks related to drug development by using known anti-cancer drugs, and aim to enhance their commercial value by improving their effectiveness. Our lead HyACT product candidate, HA-Irinotecan, is currently in a pivotal Phase III clinical trial for metastatic colorectal cancer, or mCRC, meaning that we intend to use the data we collect from the clinical trial to seek regulatory approval from the U.S. Food and Drug Administration, or FDA, and the European Medicines Agency, or EMA, of HA-Irinotecan for the treatment of mCRC. HA-Irinotecan is also in an investigator-sponsored Phase II clinical trial for small cell lung cancer, or SCLC. We also have two other HyACT product candidates that have successfully completed Phase I clinical trials. In addition to our current clinical-stage product candidates, we aim to develop a pipeline of product candidates by exploiting our significant know-how in cancer stem cell biology and cancer metabolism combined with the Versatile Assembly on Stable Templates, or VAST, small molecule drug discovery technology that we have in-licensed.
We believe that HyACT is a broadly applicable platform technology that has the potential for enhancing the efficacy of a range of anti-cancer drugs in a number of different cancer indications. In addition to HA-Irinotecan’s Phase II clinical trial data, HyACT-targeted 5-fluorouracil and HyACT-targeted doxorubicin have both demonstrated tolerability and a trend towards efficacy in Phase I / IIa clinical trials. In preclinical studies, we have also had promising results combining HyACT with seven other anti-cancer agents, including other chemotherapies and monoclonal antibodies.
We have considerable know-how, research and preclinical expertise in HA, cancer metabolism, CD44 and cancer stem cells and we have in-licensed rights to Alchemia Limited’s VAST small molecule drug discovery technology, which provides innovative pyranose compounds for drug discovery. Our strategy is to use these capabilities to investigate new pathways relating to CD44 signaling, HA metabolism and pro-oncogenic
-82-
Table of Contents
pathways that we believe are important to the promotion and proliferation of cancer stem cells. We aim to generate future drugs designed to more effectively target and kill cancer and cancer stem cells.
Our business was in the past conducted through Alchemia Oncology Pty Limited, an Australian company that we sometimes refer to as Alchemia Oncology. Alchemia Oncology, which was previously known as Meditech Research Ltd., was acquired by Alchemia Limited in 2006 and was a wholly-owned direct subsidiary of Alchemia Limited. In June 2012, Alchemia Limited effected a reorganization, which we sometimes refer to as the Reorganization, pursuant to which we became a wholly-owned subsidiary of Alchemia Limited and Alchemia Oncology became a wholly-owned direct subsidiary of ours and a wholly-owned indirect subsidiary of Alchemia Limited. Accordingly, the description of our business and our consolidated financial statements and other financial information included in this prospectus as of the dates and for the periods prior to the Reorganization reflect the business, results of operations and financial position of Alchemia Oncology, and the description of our business and our consolidated financial statements and other financial information as of the dates and for the periods from and after the date of Reorganization reflect the business, results of operations and financial condition of Audeo Oncology, Inc. and its consolidated subsidiaries, in each case unless otherwise expressly stated or the context otherwise requires.
In the past, our business has been financed by related party loans from Alchemia Limited. As of June 30, 2012, all of these loans were converted into newly-issued ordinary shares of Alchemia Oncology and, as a result, the loans were cancelled. We subsequently issued 7.5 million shares of our common stock to Alchemia Limited and, in return, Alchemia Limited transferred to us all of Alchemia Oncology’s outstanding ordinary shares in the Reorganization. We sometimes refer to the conversion of all of these outstanding related party loans into newly-issued ordinary shares of Alchemia Oncology and the cancellation of these loans as the Loan Conversion. On November 5, 2012, we issued an additional aggregate of 92,000 shares of our common stock to Alchemia Limited in further consideration for our receipt of the shares of Alchemia Oncology and which, together with the 7.5 million shares of our common stock previously issued, will be distributed in the Demerger, as defined below. Unless otherwise stated or the context otherwise requires, our historical share and per share information appearing in this prospectus has been retroactively adjusted to give effect to this issuance of 92,000 additional shares of our common stock to Alchemia Limited.
We were formed for the purpose of (a) acquiring Alchemia Oncology and forming our other subsidiary, Audeo Discovery Pty Limited, an Australian company organized to in-license the VAST small molecule drug discovery technology owned by Alchemia Limited, and (b) demerging from Alchemia Limited as an independent public company with shares of our common stock listed on the Nasdaq Global Market and CHESS Depository Interests, or CDIs, representing shares of our common stock to be listed on the Australian Securities Exchange. Our business and operations consist solely of the business and operations of Alchemia Oncology and Audeo Discovery Pty Limited, both of which are our wholly-owned subsidiaries. We will rename Alchemia Oncology following implementation of the Demerger, as defined in the next sentence. Approximately two trading days following the completion of this offering, we intend to complete implementation of our demerger, or the Demerger, from Alchemia Limited pursuant to a Scheme of Arrangement that was approved by the Federal Court of Australia and by more than 75% in voting interest and a majority in number of Alchemia Limited’s shareholders present and voting at a meeting of shareholders. All conditions precedent to the Demerger, other than those related to completion of this offering, have been satisfied prior to the date of this prospectus.
Pursuant to the Demerger, Alchemia Limited, which currently owns all of the outstanding shares of our common stock, will distribute one share of our common stock to the shareholders of Alchemia Limited (or a depositary on their behalf) for every 37 ordinary shares of Alchemia Limited that they hold as of the record date for the Demerger, which will be approximately four trading days following the date of this prospectus, with fractional shares of our common stock that would otherwise be distributed to Alchemia Limited shareholders in the Demerger being rounded upwards to the nearest whole share. To the extent that the number of shares of our common stock currently owned by Alchemia Limited is insufficient to permit the distribution of one share of our common stock for every 37 ordinary shares of Alchemia Limited outstanding at the time of the Demerger, we will issue such number of additional shares of our
-83-
Table of Contents
common stock to Alchemia Limited as may be necessary to permit that distribution to be made in full. In connection with the Demerger, we anticipate that we will assume approximately $137,129 of Alchemia Limited’s employee entitlements liabilities and that all balances due to and/or due from Alchemia Limited (which are reflected on our balance sheet as amounts due from or owed to related party) will be cash settled prior to the Demerger.
None of our product candidates has received regulatory approval and, to date, we have not generated any revenues from the sale of products, and our parent company, Alchemia Limited, has provided all of the funds necessary for our operations. We have experienced net losses in each year since our inception. We have never been profitable and, as of September 30, 2012 we had an accumulated deficit of $47,124,258. We expect our net losses to continue and to increase as the continued development of our HyACT product candidates will require significant additional expenditure for a variety of activities, including continued preclinical studies, clinical trials, research and development, manufacturing development and regulatory approvals and because of the significant additional expenses we will incur as a result of being a public company. We do not expect to generate revenue from the sale of our products until one or more of our product candidates is approved for sale by the FDA and successfully commercialized and, even if a product candidate receives regulatory approval, there can be no assurance that it will generate revenue. We cannot assure you that any of our product candidates will receive FDA approval in a timely manner or at all or, if approved, will be successfully commercialized. We may not obtain regulatory approval for many reasons, including among others:
| • | our inability to complete our ongoing and planned clinical trials in a timely manner |
| • | the results of our clinical trials may not effectively demonstrate the safety and efficacy of our product candidates; |
| • | the data from our clinical trials may not support a New Drug Application; |
| • | the FDA or non-U.S. regulatory authorities may disagree with the results of our clinical trials; or |
| • | the FDA or non-U.S. regulatory authorities may change their approval policies and procedures. |
Even if one of our product candidates receives regulatory approval, the labeling for that product candidate may not allow us to charge a price in excess of the price of generic cancer drugs if we do not demonstrate through our clinical trials that the product candidate has superior efficacy to a generic version and if the approved labeling for the product candidate does not include data supporting its superior efficacy to a generic version. Moreover, even if our label contains data adequate to allow us to price our product candidate at a premium to generics, there is no assurance that payors will pay such a premium.
If we are unable to obtain regulatory approval of any of our product candidates, we will be unable to generate revenue and may never become profitable and, even if regulatory approval is granted for one or more of our product candidates, we may be unable to successfully commercialize those product candidates.
Critical Accounting Policies
This management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities in our financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those related to research and development costs and stock-based compensation. We base our estimates on historical experience, known trends and events and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
-84-
Table of Contents
While our significant accounting policies are described in more detail in Note 2 to our consolidated financial statements appearing elsewhere in this prospectus, we believe the following accounting policies involve a greater degree of judgment and complexity. Accordingly, we believe these are the most critical accounting policies to the process of making significant judgments and estimates in the preparation of our consolidated financial statements.
Fair value of financial instruments
The carrying amounts of financial assets and liabilities, such as cash and cash equivalents, short-term deposit, amounts due to and from related party, trade and other payables and related party loans approximate their fair value due to their short term nature. The fair value of the rights of certain investors, or the Rights, pursuant to which we are required, approximately 60 trading days subsequent to completion of this offering, to issue Warrants was initially measured at the date of issuance and is subsequently remeasured at the end of each reporting period with any change in the fair value recorded in our statement of operations for such period. We determined the estimated fair value of the Rights that are recognized in our consolidated financial statements.
Government Grant Income
Government grants are recognized as income when earned and any remaining obligations on our part are determined to be inconsequential or perfunctory. When we receive grants before they are earned, such grants are recognized as a liability in the balance sheet.
When the grant relates to an expense item, we match the grant with the costs that the grant is intended to compensate and recognize the grant as income over the periods in which the related cost is incurred.
When the grant relates to an asset, the grant is released to the income statement over the expected useful life of the relevant asset by equal annual installments.
We received one Australian government research grant under the Commercial Ready Grant for our HA-Irinotecan program to fund 50% of our research and development expenditures for a term of three years and six months, which ended on February 28, 2009. We have the obligation to report the progress of the project five years post completion date and have done so to date. The Australian Government may require us to repay all or some of the amount of the grant together with interest in any of the following circumstances:
| • | we fail to use our best endeavors to commercialize the relevant grant project within a reasonable time of completion of the project; |
| • | upon termination of a grant due to our breach of the agreement or insolvency; |
| • | there is a change in control or ownership of our company that the Australian Government reasonably considers to have an adverse effect on our ability to comply with any of our obligations in the grant agreement; or |
| • | we cease to carry on a substantial part of our business. |
Technical failure of the grant funded project does not, in and of itself, constitute failure to use best endeavors to commercialize the relevant grant project and we believe that we have complied with our obligation to commercialize the project funded by the grant program. The obligation to report progress of the project for periods subsequent to February 28, 2009 requires minimal effort and cost on our part, and we determined such obligation to be inconsequential and perfunctory. The total amount received since the commencement of the Commercial Ready Program is $1,675,201, which includes $166,345 received prior to our inception.
-85-
Table of Contents
By virtue of Alchemia Limited’s two existing grants for the development of the VAST technology, we recognized a total of $61,205 in grant income in the three months ended September 30, 2012. Alchemia Limited obtained permission to subcontract research to be performed under these grants to us from the Queensland Government, and we received such funding under this authority.
Research Funding Income
Prior to entering into the supply agreement and royalty agreement with Novozymes Biopharma DK A/S, or Novozymes, described below under “—Contractual Obligations and Commitments,” we had two agreements with Novozymes pursuant to which we received $433,733 of funding. Subsequent to the achievement of the substantive milestones specified in these agreements relating to our Phase II clinical trials of HA-Irinotecan, $253,069 and $180,664 were recognized as research and development income in the years ended June 30, 2007 and 2009, respectively. After the achievement of the last milestone of our Phase II clinical trials, on July 1, 2009, these agreements were terminated by mutual agreement of the parties. On the same date, the royalty agreement and supply agreement, described below under “—Contractual Obligations and Commitments,” were entered into. See “—Contractual Obligations and Commitments.”
Research and Development Expenses
Research and development expenses are expensed when incurred. Such costs include, but are not limited to, direct salaries and benefits, stock-based compensation expense, consultant costs, patient recruitment fees, contract research costs, laboratory expenses, rent and utilities. Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations, or information provided to us by our vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the financial statements as prepaid research and development or other payables.
Stock-Based Compensation
Equity awards granted to our employees and non-employees (i.e., independent contractors) by Alchemia Limited under its stock option plan consist of ordinary shares of Alchemia Limited and options to purchase Alchemia Limited’s ordinary shares and are accounted for under Accounting Standards Codification, or ASC, 718-10, “Share-Based Payment” and ASC 505-50, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services,” respectively. The stock-based compensation expenses for employees and non-employees (i.e., independent contractors) employed by our subsidiary, Alchemia Oncology, relating to these equity awards are recognized in our financial statements with a corresponding entry to additional paid-in capital. A portion of stock-based compensation expenses for employees of Alchemia Limited that have a percentage of their salary allocated to us in accordance with our allocation of general and administrative expenses accounting policy described under the caption “Allocation of general and administrative expenses” in note (2) to our audited financial statements included elsewhere herein, has been recognized in our financial statements as general and administrative expense with a corresponding entry to related party loan and due to related party for periods prior and subsequent to the cancellation of related party loans on June 28, 2012, respectively.
All grants of stock options and ordinary shares to employees are recognized in our financial statements based on their grant date fair values. We have elected to recognize stock-based compensation expense using the straight-line method over the period during which the employee is required to perform service in exchange for the award. For the awards granted to non-employees, we record stock-based compensation expense equal to the fair value of the stock options and ordinary shares at the measurement date, which is determined to be the earlier
-86-
Table of Contents
of the performance commitment date or the service completion date and is recognized using the accelerated method. We use the Black-Scholes option pricing model in determining the fair value of the equity awards and ordinary shares granted.
ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from initial estimates. Forfeiture rate is estimated based on historical and future expectation of employee turnover rate and is adjusted to reflect future change in circumstances and facts, if any. Stock-based compensation expense is recorded net of estimated forfeitures such that expense is recorded only for those stock-based awards that are expected to vest. To the extent we revise this estimate in the future, stock-based compensation expense could be materially impacted in the period of revision, as well as in following periods. For the periods presented, we estimated that the forfeiture rate for both our management and non-management employees was zero.
We currently have our 2012 Stock Plan and have adopted our 2012 Equity Incentive Plan, which will become effective in connection with this offering. See “Prospectus Summary — The Offering.”
On September 28, 2012, we granted options to purchase 948,000 shares of common stock to our employees and directors at an exercise price equal to the initial public offering price per share in this offering. 25% of these options will vest one year following the completion of this offering with the remaining 75% vesting in equal monthly installments over a three-year period beginning one year following completion of this offering. As the exercise price of the share options is not known until the initial public offering price is established, the grant date will not be established until completion of this offering. In addition, given that the inability of the optionees to vest in these share options until the completion of this offering constitutes a performance condition that is not considered probable until completion of this offering, we will not recognize any compensation expense until completion of this offering. Upon completion of this offering, we will measure total compensation expense to be recognized and recognize expense associated with the service period between the award date and the date of completion of this offering and amortize the remaining compensation expenses over the remaining vesting period using the accelerated method.
Assuming an initial public offering price of $15.00 per share (which is the midpoint of the estimated price range set forth on the cover page of this preliminary prospectus), total stock-based compensation to be recognized in respect of the options to purchase 948,000 shares mentioned above, after giving effect to estimated forfeitures, would be approximately $10,745,021, with approximately $974,346 recognized at completion of this offering and reflected in our financial statements for the fiscal quarter in which this offering is completed, approximately $2,696,135 recognized in the remainder of the fiscal year ending June 30, 2013 and approximately $7,074,540 recognized in future periods.
Income Taxes
We use the liability method of accounting for income taxes as set forth in the authoritative guidance for income taxes. Under this method, we recognize deferred tax liabilities and assets for the expected future tax consequences of temporary differences between the respective carrying amounts and tax bases of our assets and liabilities.
We continue to assess the realizability of our deferred tax assets, which primarily consist of net operating loss carry-forwards for Australia income tax purposes. In assessing the realizability of these deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. We establish valuation allowances when necessary to reduce deferred tax assets to the amounts expected to be realized. The factors used to assess the likelihood of realization include our latest forecast of future operating results and available tax planning strategies that could be implemented to realize the net deferred tax assets.
-87-
Table of Contents
Audeo Oncology, Inc. is a newly incorporated company in the United States and currently has no carry-forward tax losses for U.S. or other tax purposes. Alchemia Oncology, our wholly-owned subsidiary, had net operating tax loss carry-forwards for Australian tax purposes of approximately $79.6 million, $64.7 million and $75.6 million as of September 30, 2012, June 30, 2011 and June 30, 2012, respectively, which can be carried forward indefinitely. These also may be adjusted if we are unable to comply with the recognition criteria for carry forward tax losses under Australian tax laws. Future events such as capital injections and changes in our business or capital structure require evaluation as to the impact on continued availability of historical tax losses to offset future income, if any. Pursuant to Australian tax law, tax losses may not be available and would thus be derecognized (i.e. lost) where there has been a greater than 50% change in the cumulative ownership of Alchemia Oncology combined with a change in our business or capital structure. Accordingly, certain years’ tax losses may fail the continuity of ownership test, or COT, and later years’ tax losses may pass. If a company does not satisfy the COT in respect of a particular loss year, it must instead satisfy the same business test, or SBT, to be eligible to utilize prior year losses. The SBT broadly requires the company to carry on the same business for the entire year in which it seeks to recoup losses as it did when it failed COT. We have analyzed these requirements with our Australian tax adviser and believe that, presently, none of Alchemia Oncology’s net operating tax loss carry-forwards will be de-recognized based on past cumulative changes in ownership or as a result of the distribution of shares of our common stock in the Demerger and the issuance of shares of our common stock in this offering, although there can be no assurance in this regard. As a result of these tax loss carry-forwards, we have significant deferred tax assets; however, due to our lack of earnings history, realization of these deferred tax assets is not more likely than not; therefore the deferred tax assets have been fully offset by a valuation allowance.
We have a history of net losses and there can be no assurance that we will ever achieve or sustain profitability. However, if any of our product candidates is approved for commercial sale and we start to generate revenue, we may determine that there is sufficient positive evidence to support a reversal of, or decrease in, the valuation allowance. If we were to reverse all or some part of our valuation allowance, our financial statements in the period of reversal would likely reflect an increase in assets on our balance sheet and a corresponding tax benefit to our statement of operations in the amount of the reversal.
Because Audeo Oncology, Inc. is a Delaware corporation with its executive offices in California, we became subject to U.S. and California income taxes in calendar year 2012.
We adopted Accounting Standards Codification (ASC) 740-10, Accounting for Uncertainty in Income Tax — an Interpretation of FASB Statement No. 109. We analyzed our tax position in all jurisdictions where we are required to file an income tax return and concluded that we do not have any material unrecognized tax benefits. We intend to file a U.S. income tax return as well as returns for any state jurisdiction in which we are authorized to conduct business. Our policy is to recognize interest and penalties accrued on any unrecognized tax benefit within the provision for income taxes on the statement of operations. We have no interest or penalties accrued for any unrecognized tax benefits for any periods presented in our consolidated financial statements included elsewhere herein.
Our annual provision for income taxes and the determination of the resulting deferred tax assets and liabilities involve a significant amount of management judgment. Management’s judgments, assumptions and estimates relative to the current provision for income taxes take into account current tax laws, our interpretation of current tax laws and possible outcomes of current and future audits conducted by foreign and domestic tax authorities. We operate within federal, state and international taxing jurisdictions and are subject to audit in these jurisdictions. These audits can involve complex issues that may require an extended period of time to resolve.
Financial Operations Overview
Research and Development Expense
Research and development activities are the cornerstone of our business, and research and development expenses consist of in-process research and development that we expensed in 2006 in connection with our
-88-
Table of Contents
acquisition of Meditech and costs associated with our research activities, including our drug discovery efforts. These costs include, but are not limited to, direct salaries and benefits, stock-based compensation expense, consultant costs, patient recruitment fees, contract research costs, laboratory expenses, rent and utilities. We expense research and development expenses as incurred. From inception through September 30, 2012 we have incurred $42,324,867 for research and development expense.
General and Administrative Expense
General and administrative expenses mainly consist of compensation for our executive and administrative personnel, including stock-based compensation, and, in the future, will also include compensation expense, including stock-based compensation expense, for our board of directors. Other general and administrative expenses also include facility costs not otherwise included in research and development expenses, patent related costs, insurance, travel and professional fees for legal and accounting services. From inception through September 30, 2012 we have incurred $6,628,876 for general and administrative expense.
Government Grant Income
Grant income consists of grants received from the Government of Australia and the Queensland Government. From inception to September 30, 2012, we received $1,570,061 of grant income. In the three months ended September 30, 2012, grant income of $61,205 from the Queensland Government is receivable for two VAST projects where research under grants held by Alchemia Limited was subcontracted to us.
Research Funding Income
Research funding income received from inception through September 30, 2012 consists of $433,733 of income related to a Research and Development and Clinical Trial Agreement, which we refer to as the RD&CT agreement, with Novozymes to fund Phase II clinical trial expenses for metastatic colorectal cancer. However, the RD&CT agreement has been terminated, we have not received any research funding income subsequent to fiscal 2009 and there is no certainty that we will receive any future research funding income.
Loan Conversion
In the past, our business has been financed by related party loans from Alchemia Limited. As of June 30, 2012, all of these loans were converted into newly-issued ordinary shares of Alchemia Oncology and, as a result, the loans were cancelled. We subsequently issued 7.5 million shares of our common stock to Alchemia Limited on June 28, 2012 and, in return, Alchemia Limited transferred all of Alchemia Oncology’s outstanding ordinary shares to us.
Total Other Income
Total other income includes interest income and other income as discussed below.
Interest income consists of interest earned on our cash and cash equivalents.
Other income received from inception through September 30, 2012 includes $314,698 of other income related to an amount received from Jagotec AG for the relinquishment of rights for the sale of Solarase in Australia and New Zealand in 2007. From inception to September 30, 2012, we also received $119,070 as royalties from a licensing agreement with Jagotec AG entered into in connection with the sale of Solarase in 2002. This agreement expires in August 2012.
As described under “Arrangements between Audeo Oncology, Inc. and Alchemia Limited,” we will provide certain accounting, treasury, management, tax support, investor relations, information technology and other
-89-
Table of Contents
administrative services to Alchemia Limited for six months after the implementation of the Demerger pursuant to a transition services agreement. Under the agreement, Alchemia Limited must pay us a monthly service charge and secondment fee and reimburse our out-of-pocket expenses in providing the services. If the cost of providing these services to Alchemia Limited under this agreement exceeds the amounts we are paid, it could adversely affect our results of operations and financial condition.
Fair Value of Financial Instruments
The fair value of the Rights, pursuant to which we are required, contingent upon completion of this offering, to issue Warrants following the listing of our common stock on the Nasdaq Global Market was initially measured at the date of issuance and is subsequently remeasured at the end of each reporting period, with any change in the fair value recorded in our statement of operations for such period. We determined the estimated fair value of the Rights that are recognized in our consolidated financial statements.
The Rights are classified as a current liability on our balance sheet in an amount initially equal to their fair value on the date of issuance and the fair value of the Rights is remeasured at the end of each reporting period with any change in the fair value recorded in our statement of operations for such period. One factor impacting the fair value of the Rights is the underlying value of our common stock. Assuming no changes to other factors, the fair value of the Rights increases or decreases in proportion with increases and decreases, respectively, in the value of our common stock. In addition, if and when this offering is completed and the Average Trading Price of our common stock is determined as described under “Prospectus Summary—The Offering,” the fair value of the Rights will be remeasured. As the initial exercise price of the Warrants and the number of Warrants will have been fixed when such Average Trading Price is determined, the fair value of the Warrants will be reclassified from current liabilities to stockholders’ equity on our balance sheet and the Warrants will at that time be accounted for as equity awards. Any increase or decrease in the fair value for the then current period compared with the fair value as of the end of our then most recently ended reporting period will be reflected in our statement of operations for the then current period. The remeasurement of the fair value of the Rights at each balance sheet date (prior to such reclassification) and at the time of such reclassification may result in additional expense, and any such expense may have a material adverse effect on our results of operations. Following such reclassification, we will account for the Warrants as equity awards (as both the initial number of shares issuable on exercise and the initial exercise price shall have been determined as of the date of the remeasurement of the fair value of the Rights in connection with such reclassification) and the remeasurement of the fair value of the Rights will cease. In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock.
In November and December 2011, Alchemia Limited issued and sold its ordinary shares to investors in a private placement, or the Placement, for total net proceeds of approximately AUD$16.1 million. Pursuant to the terms of the Placement, the investors were given Rights pursuant to which we are required, contingent on completion of this offering by December 31, 2012, to issue Warrants to the investors entitling them to acquire shares of our common stock. The initial fair value of these Rights upon issuance was determined to be insignificant as (i) we had not commenced planning for this offering and (ii) the obligation to issue the Warrants terminates on December 31, 2012, giving us a period of only approximately one year from the date of issuance to complete this offering. As of September 30, 2012, we determined that the fair value of the Rights amounted to $819,280. The key assumptions are disclosed in Note 13 to our consolidated financial statements appearing elsewhere in this prospectus.
Recent Health Care Legislation
Recent legislation mandates that manufacturers of branded prescription drugs pay an annual fee based on the sales activity of the manufacturer. Federal agencies will monitor and report the sales of pharmaceutical
-90-
Table of Contents
manufacturers in different sectors of the industry and the annual fees for each manufacturer will be based, in part, on the industry sector as well as their market share within that sector. We cannot predict how this annual fee may impact our future operating results or financial condition in the event that any of our product candidates receives regulatory approval.
VAST License Agreement
In August 2012, we entered into a technology license agreement with Alchemia Limited, which provides us with a non-transferable, worldwide license to the VAST technology. This license agreement requires us to deploy staff with relevant scientific qualifications occupying a minimum of four full-time equivalent positions (see “—Demerger” below) to develop and exploit the VAST technology and to expend a minimum of AUD$500,000 per annum (with a target of expending AUD$1.5 million per annum) on developing, marketing and exploiting the VAST technology. Failure to meet the minimum expenditure requirement may allow Alchemia Limited to terminate the technology license agreement. The expenditure requirements under the technology license agreement, as well as the costs of maintaining these four full-time equivalent positions, will substantially increase our expenses in future periods.
Demerger
Four employees of Alchemia Limited who work on the VAST technology transferred to us in August 2012 and became our employees. As we did not carry out any operations with respect to the VAST technology prior to the in-licensing of that technology in August 2012, salaries and benefits relating to these employees have not been allocated to us or recorded in our consolidated financial statements prior to the date they became our employees. We have assumed the legal liability of leave entitlements for these employees, which at September 30, 2012, totaled approximately $87,154.
Five employees of Alchemia Limited (in addition to the four employees noted in the preceding paragraph) transferred to us in August 2012 and became our employees. Our Chief Executive Officer and Chief Financial Officer will begin receiving compensation and other benefits from us upon implementation of the Demerger. Salaries and benefits relating to these employees have been allocated to us based on the proportional allocation method and have been recorded in our consolidated financial statements. We have assumed the legal liability of leave entitlements for the five employees, which at September 30, 2012 totaled $110,957, and will assume the legal liability of leave entitlements for our Chief Executive Officer and Chief Financial Officer, which at September 30, 2012, together totaled approximately $137,129.
Any remaining net amounts due to or from Alchemia Limited will be settled in cash prior to the Demerger.
Results of Operations
Our functional currency for accounting purposes is the Australian dollar; however our reporting currency is the U.S. dollar. As a result, in preparing our consolidated financial statements for purposes of this prospectus and going forward in our annual and quarterly reports, we must convert amounts recorded in our functional currency to our reporting currency. For revenues and expenses reported in any period, we use the average currency exchange rate between U.S. dollars and Australian dollars, based on the average buying rates for the 24 hour period ending 22:00 Universal Coordinated Time on the respective prior days, published at www.oanda.com/currency. For assets and liabilities, we use the current currency exchange rate as at the end of the period, based on the same source. Given the fluctuations in currency exchange rates, we may experience changes in reported amounts from period to period that occur primarily as a result of these fluctuations and that are not reflective of actual changes in our business or operations.
Currently, we are exposed to foreign exchange risk, particularly with the U.S. dollar and Australian dollar, as a result of certain research and development activities that are undertaken internationally. It is our policy to limit the
-91-
Table of Contents
use of financial derivatives and seek risk mitigation through natural hedges. These natural hedges include the maintenance of US dollar and Australian bank accounts and deposits to primarily facilitate the payment of research and development activities. Because our functional currency is the Australian dollar, our reported financial results are subject to fluctuation resulting from changes in the Australian dollar to U.S. dollar exchange rate.
As discussed below, our research and development expense increased substantially in the three months ended September 30, 2012 compared with the three months ended September 30, 2011, primarily as a result of the cost of our pivotal Phase III clinical trial of HA- Irinotecan. We expect that our research and development costs will increase substantially in future periods, both as a result of the costs of clinical trials and other research and development activities.
As of September 30, 2012, we had seventeen full-time employees, nine of whom joined us in August 2012. As a result of this and the fact that, upon implementation of the Demerger, we will begin paying compensation and other benefits to our Chief Executive Officer and Chief Financial Officer, our employee compensation expenses will substantially increase in future periods.
As described above under “— Critical Accounting Policies — Stock-Based Compensation” and “Prospectus Summary — The Offering,” we granted options to purchase 948,000 shares of our common stock at an exercise price equal to the initial public offering price per share set forth on the cover page of this prospectus. Because these options will not begin vesting until the completion of this offering, we will not recognize any compensation expense in respect of these options until this offering is completed. As a result, stock-based compensation expense related to these options will be recognized at completion of this offering and in subsequent periods and reflected in our financial statements commencing with the fiscal quarter in which this offering is completed. For additional information, see “— Critical Accounting Policies — Stock-Based Compensation” above and note 5(b) to our unaudited interim condensed consolidated financial statements included elsewhere in this prospectus.
Comparison of Three Months Ended September 30, 2012 and 2011
Research and Development Expense
Research and development expense increased by $3,169,617, or 372.8%, to $4,019,917 for the three months ended September 30, 2012 as compared with $850,300 for the three months ended September 30, 2011. This increase was primarily the result of an increase in clinical trial costs, consultant costs, drug supply-related costs and travel costs as we advanced our pivotal Phase III clinical trial in Europe and other sites.
Research and development expenses represented 82.5% of our total operating expenses for the three months ended September 30, 2012 compared with 78.4% for the three months ended September 30, 2011. We expect research and development expenses (which includes costs of clinical trials) to continue to increase, both in absolute dollars and as a percentage of our total operating expenses, as we devote substantial resources to research and development to support the continued development of our product candidates. We also expect to incur additional research and development costs advancing our oncology drug discovery efforts and the VAST small molecule drug discovery technology that we have in-licensed.
As disclosed in “— Critical Accounting Policies — Stock-Based Compensation,” we also expect to recognize additional stock-based compensation expense at completion of this offering and in subsequent periods.
General and Administrative Expense
General and administrative expense increased by $617,033, or 264.1%, to $850,693 for the three months ended September 30, 2012 as compared with $233,660 for the three months ended September 30, 2011. This
-92-
Table of Contents
increase was largely due to an increase in salary and wages resulting in part from the addition of nine full-time employees in August 2012 and to increased costs associated with this offering and the Demerger.
We expect that our general and administrative expenses will increase substantially following the Demerger and the commencement of operations as an independent corporation, particularly as a result of expenses associated with our obligations as a public company and as a result of the nine full-time employees we added in August 2012 and the fact that we will begin paying compensation and other benefits to our Chief Executive Officer and Chief Financial Officer upon implementation of the Demerger. Further, we intend to continue to expand our infrastructure to support our growing research and development.
As disclosed in “— Critical Accounting Policies — Stock-Based Compensation,” we also expect to recognize additional stock-based compensation expense at completion of this offering and in subsequent periods.
Changes in Fair Value of Rights
For the three months ended September 30, 2012, we determined that the change in fair value of the Rights amounted to $335,858, as compared with $473,709 as of June 30, 2012. If and when this offering is completed and the Average Trading Price of our common stock has been determined for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market, the fair value of the Rights will be remeasured. As the initial exercise price of the Warrants and the number of Warrants will have been fixed when such Average Trading Price is determined, the fair value of the Warrants will at that time be reclassified from current liabilities to stockholders’ equity on our balance sheet. The remeasurment of the fair value of the Rights at each balance sheet date (prior to such reclassification) and at the time of such reclassification may result in additional expense, and any such expense may have a material adverse effect on our results of operations.
Comparison of Years Ended June 30, 2012 and 2011
Research and Development Expense
Research and development expense increased by $820,472, or 11.8%, to $7,806,351 for the year ended June 30, 2012 as compared with $6,985,879 for the year ended June 30, 2011. This increase was primarily the result of an increase in clinical trial costs, consultant costs, drug supply-related costs and travel costs as we advanced our pivotal Phase III clinical trials in Europe and other sites. Research and development expenses represented 82.3% of our total operating expenses for the year ended June 30, 2012 compared with 88.3% for the year ended June 30, 2011.
General and Administrative Expense
General and administrative expense increased by $745,164, or 80.2%, to $1,674,235 for the year ended June 30, 2012 as compared with $929,071 for the year ended June 30, 2011. This increase was largely due to an increase in salary and wages and to increased costs associated with this offering and the Demerger.
Changes in Fair Value of Rights
For the year ended June 30, 2012, we determined, with the assistance of an independent third party valuation firm, that the change in fair value of the Rights amounted to $481,356, as compared with $0 as of June 30, 2011, as the Rights were given subsequent to such date. If and when this offering is completed and the Average Trading Price of our common stock has been determined for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market, the fair value of the Rights will be remeasured. As the initial exercise price of the Warrants and the number of Warrants will have been fixed when such Average
-93-
Table of Contents
Trading Price is determined, the fair value of the Warrants will at that time be reclassified from current liabilities to stockholders’ equity on our balance sheet. The remeasurement of the fair value of the Rights at each balance sheet date (prior to such reclassification) and at the time of such reclassification may result in additional expense, and any such expense may have a material adverse effect on our results of operations.
Comparison of Years Ended June 30, 2011 and 2010
Research and Development Expense
Research and development expense increased by $3,848,579, or 122.7%, to $6,985,879 for the year ended June 30, 2011 as compared with $3,137,300 for the year ended June 30, 2010. This increase was mainly a result of an increase in consultant and drug-supply related costs as we prepared for our pivotal Phase III clinical trial, which commenced patient enrollment in fiscal 2012. Research and development expenses represented 88.3% of our total operating expenses for the year ended June 30, 2011 compared with 78.4% for the year ended June 30, 2010.
General and Administrative Expense
General and administrative expenses increased by $63,982, or 7.4%, to $929,071 for the year ended June 30, 2011 as compared with $865,089 for the year ended June 30, 2010. This increase was largely due to an increase in salary and wages.
Liquidity and Capital Resources
Sources of funding
Since inception, our cash needs have been funded entirely by our parent company, Alchemia Limited. As of June 28, 2012, we had approximately $37.6 million, including effects of foreign currency translation of approximately $0.9 million, of outstanding related party loans owing to Alchemia Limited. These loans were non-interest bearing with no maturity date and no repayment terms. On June 28, 2012, Alchemia Limited converted all of these loans into newly-issued ordinary shares of Alchemia Oncology and, as a result, the loans were cancelled. We sometimes refer to the conversion of all of these outstanding related party loans into newly-issued ordinary shares of Alchemia Oncology and the cancellation of these loans as the Loan Conversion.
Alchemia Limited will not have any direct or indirect obligation to provide us with loans or other funds subsequent to the date of the Demerger and we understand that Alchemia Limited has no intention of doing so. Accordingly, we will be entirely dependent upon our own capital resources independent of Alchemia Limited in order to finance our business going forward. In addition, we have experienced net losses since inception and, as of September 30, 2012, we had an accumulated deficit of $47,124,258, and expect to continue to incur substantial net losses and negative cash flow from operations for the foreseeable future.
The following table sets forth our cash and cash equivalents as of June 30, 2011 and 2012 and September 30, 2012:
| As of June 30, | As of September 30, |
|||||||||||
| 2011 | 2012 | 2012 | ||||||||||
| (in thousands) | ||||||||||||
| Cash and cash equivalents |
$ | 159 | $ | 7,636 | $ | 4,503 | ||||||
-94-
Table of Contents
The following table sets forth our cash flows for the fiscal years ended June 30, 2011 and 2012 and for the three months ended September 30, 2011 and 2012:
| Fiscal Year Ended June 30, | Three Months Ended September 30, |
|||||||||||||||
| 2011 | 2012 | 2011 | 2012 | |||||||||||||
| (in thousands) | ||||||||||||||||
| Net cash provided by (used in) operating activities(1) |
$ | 644 | $ | 7,630 | $ | (157 | ) | $ | (3,285 | ) | ||||||
| Net cash provided by (used in) investing activities |
(539 | ) | (27 | ) | — | (16 | ) | |||||||||
| Effect of exchange rate changes on cash and cash equivalents |
16 | (127 | ) | (1 | ) | 168 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Increase (decrease) in cash and cash equivalents |
$ | 121 | $ | 7,476 | $ | (158 | ) | $ | (3,133 | ) | ||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Net cash provided by (used in) operating activities includes the impact of $8,404,955 and $16,722,100 of related party loans from Alchemia Limited in the fiscal years ended June 30, 2011 and 2012, respectively. |
Cash Flows
Operating Activities
The cash flows provided by (used in) operating activities primarily resulted from our net loss, adjusted for non-cash items, and changes in our operating assets and liabilities.
Operating activities used $3,285,499 of cash during the three months ended September 30, 2012. We had a net loss of $5,135,262, which included non-cash stock-based compensation expenses of $6,158, non-cash stock-based compensation expenses of Alchemia Limited employees allocated to us of $5,225, depreciation and expenses of $63,520 and change in fair value of Warrants of $335,858. Changes in our operating assets and liabilities provided $1,438,972 of cash during the three months ended September 30, 2012, primarily due to an increase of $521,810 in the net amount payable by us to Alchemia Limited, increases in our trade and other payables of $1,915,323, offset in part by an increase in deferred initial public offering costs of $1,489,097, an increase in our employee provision of $232,012 and a decrease in our prepaid research and development costs of $464,182.
Operating activities used $157,155 of cash during the three months ended September 30, 2011. We had a net loss of $1,083,960, which included non-cash stock-based compensation expenses of $6,235, non-cash stock-based compensation expenses of Alchemia Limited employees allocated to us of $22,539, and depreciation and expenses of $61,049. Changes in our operating assets and liabilities provided $836,982 of cash during the three months ended September 30, 2011, primarily due to increases of $999,454 in our related party loan from Alchemia Limited, partially offset by an increase in prepaid research and development costs of $3,071 and a decrease in our trade and other payables of $162,024.
Operating activities provided $7,630,475 of cash during the year ended June 30, 2012. We had a net loss of $9,952,440, which included non-cash stock-based compensation expenses of $96,894, non-cash stock-based compensation expenses of Alchemia Limited employees allocated to us of $91,079, depreciation expenses of $243,644 and change in fair value of Warrants of $481,356. Changes in our operating assets and liabilities provided $16,669,942 of cash during the year, primarily due to increases of $16,772,100 in our related party loan from Alchemia Limited, partially offset by an increase in prepaid research and development costs of $706,106, an increase in our trade and other payables of $1,291,206 and an increase in deferred initial public offering costs of $818,490.
Operating activities provided $644,341 of cash in the year ended June 30, 2011. We had a net loss of $7,913,268, which included non-cash stock-based compensation expenses of $84,837, non-cash stock-based compensation expenses of Alchemia Limited employees allocated to us of $30,625, and depreciation expenses of
-95-
Table of Contents
$97,626. Changes in our operating assets and liabilities provided $8,344,521 of cash during the year, primarily due to increases of $8,404,955 in our related party loan from Alchemia Limited, partially offset by an increase in prepaid research and development costs of $173,590.
Investing Activities
Cash used in investing activities was for the purchase of property and equipment. We used $15,597 in the three months ended September 30, 2012 to purchase laboratory and computer equipment, $0 in the three months ended September 30, 2011, $26,941 in the year ended June 30, 2012 to purchase computer hardware and software and $539,378 in the year ended June 30, 2011 to purchase laboratory equipment and computer hardware.
Operating and Capital Expenditure Requirements
From our inception in 2006 to September 30, 2012, we spent $42,324,867 on research and development, $6,628,876 on general and administrative expenses and $692,311 on capital equipment. We believe that the net proceeds raised from this offering and our cash and cash equivalents will be sufficient to satisfy our anticipated cash needs for working capital and capital expenditures for at least the next 12 months. However, this estimate is based on numerous assumptions and uncertainties that may be proven wrong and as such, we could use our available financial resources sooner than we currently expect. Our forecast of the period of time that our financial resources will be adequate to support operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed under “Risk Factors” and “Special Note Regarding Forward-Looking Statements.” In light of the numerous risks and uncertainties associated with the development and, if any of our product candidates receive regulatory approval, commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated pre-clinical studies, clinical trials or other research and development activities. Our future funding requirements will depend on a number of factors including:
| • | the scope, results, rate of progress, timing and costs of pre-clinical studies and clinical trials and other development activities; |
| • | the costs and timing of seeking and obtaining regulatory approvals; |
| • | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
| • | the costs of securing coverage, payment and reimbursement of our product candidates, if any of our product candidates receive regulatory approval; |
| • | the effects of completing clinical, technological and market developments; |
| • | the costs of hiring additional employees as we develop our business; |
| • | additional costs as a result of our being a public company; |
| • | the costs of our obligations pursuant to our license agreement for the VAST drug discovery technology; |
| • | the costs of remediating a material weakness in our internal system of financial control, including costs of hiring a controller and an outside firm proficient in U.S. GAAP; |
| • | if any product candidates receive regulatory approval, the costs of developing any sales and marketing organization that we may seek to establish to commercialize those product candidates; and |
-96-
Table of Contents
| • | the terms, timing and cash requirements of any future acquisition, collaborative arrangements, licensing of product candidates or investing in businesses, product candidates and technologies. |
We expect that our cash requirements will increase substantially in the future, both as a result of the significant costs associated with clinical trials and preclinical studies, preparing for commercial manufacturing, the increase in the number of our full-time employees, and our obligations as a public company. In addition, although we are currently not a party to any agreement or letter of intent with respect to potential investments in or acquisitions of other businesses or technologies, we may enter into these types of agreements in the future, which could require us to seek additional financing. Finally, as discussed above, we believe that the net proceeds raised from this offering together with our cash and cash equivalents will be sufficient to satisfy our anticipated cash needs for working capital and capital expenditures for only a limited period of time, and we therefore will require substantial external financing in order to complete our current clinical trials and otherwise continue our business. We will therefore need to raise substantial additional capital in the future, and there can be no assurance that we will be able to do so on terms we consider acceptable, or at all. In addition, limitations on new share issuances under Australian Securities Exchange listing rules may limit or prevent us from raising additional capital by issuing and selling shares of common stock or other securities when such additional capital is required. See “Risk Factors — Our ability to raise additional capital may be significantly limited by listing rules of the Australian Securities Exchange that limit the amount of common stock that we are permitted to issue without stockholder approval.” If we are unable to raise additional funds when needed, we may not be able to continue development of our product candidates or we could be required to delay, scale back or eliminate some or all of our development programs and other operations. We may seek to raise additional funds through public or private equity or debt financing, strategic partnerships or other arrangements. Any additional equity, convertible debt or other equity-related securities that we issue could materially dilute the ownership interests of holders of our common stock. Debt financing may be unavailable (particularly as we do not have a reliable stream of revenue available to pay principal and interest) and would require that we use a portion of our cash, which could be material, to fund debt service payments and also could require that we comply with financial and other covenants that limit our flexibility and operations or that we pledge assets, including intellectual property, to secure payment of the principal of and interest on borrowings. If we raise funds through collaborative or licensing arrangements we may be required to relinquish, on terms that are not favorable to us, rights to some of our technologies or product candidates that we would otherwise seek to develop or commercialize ourselves, and we may be required to share any revenues generated by any of our product candidates that may receive regulatory approval and are successfully commercialized with our collaborators or licensing partners. In addition, our VAST small molecule drug discovery technology currently relies on partnerships with pharmaceutical and biotechnology companies and on collaborations with academic groups and in the past has relied and in the future may rely on government grants, if available. If Alchemia Limited is unable to assign these collaboration agreements and grant arrangements to us as described below or if those partnerships or collaborations terminate or if we are unsuccessful in obtaining future grants, we may need to find alternative sources of funding to further our drug discovery initiatives. There is no guarantee that we would be successful in obtaining any such funding on terms that we deem acceptable, or at all. In addition, as noted above, limitations on new share issuances under Australian Securities Exchange listing rules may limit or prevent us from raising additional capital by issuing and selling shares of common stock or other securities when such additional capital is required. Furthermore, as described above under “Risk Factors — Alchemia Limited may be unable to transfer its rights under collaboration agreements and government grant arrangements relating to the VAST technology to us,” if Alchemia Limited is unable to obtain necessary consents to assign collaboration agreements and government grant arrangements relating to the VAST technology to us, it will deprive us of the support and, if applicable, any funding potentially available under those agreements and arrangements, which would increase our need for external financing. Our failure to raise capital when needed could have a material adverse impact on our research and development activities, results of operations, financial condition and business generally.
-97-
Table of Contents
Contractual Obligations and Commitments
The following table summarizes our contractual obligations as at September 30, 2012:
| Total | Less than 1 Year |
1-3 Years | 3-5 Years | More than 5 Years |
||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Obligations to Monash University |
$ | 176 | $ | 176 | $ | — | $ | — | $ | — | ||||||||||
| Obligations to BNY Trust Company of Australia |
1,667 | 417 | 872 | 378 | — | |||||||||||||||
| Total |
$ | 1,843 | $ | 593 | $ | 872 | $ | 378 | $ | — | ||||||||||
We have a research and development agreement with Monash University whereby the University provides staff, consumables and infrastructure facilities to carry out the ongoing HyACT projects on their Clayton campus in Melbourne, Australia. The annual fee for use of these facilities for the calendar year 2012 is $689,101. Either party may terminate this agreement upon a breach of the agreement by the other party or upon certain insolvency events relating to the other party, and we may terminate the agreement if we consider that it is not commercially viable to continue. This is an annually renewable contract and the annual fee is therefore renegotiated in connection with each renewal.
Our RD&CT agreement with Novozymes was superseded by two other agreements we entered into in July 2009. The first agreement is a supply agreement whereby we agree to use Novozymes as our exclusive supplier of HA for our HA-Irinotecan-based product and Novozymes agrees to supply us with HA and to use commercially reasonable endeavors to build and maintain sufficient manufacturing capacity to reliably supply us with HA, including the construction of a new HA production facility. Novozymes does not currently have the manufacturing capacity to supply HA in accordance with the agreement, and although the agreement provides that Novozymes use commercially reasonable endeavors to achieve such sufficient manufacturing capacity by no later than January 1, 2014, we cannot assure you that this will occur. Assuming that Novozymes is able to construct a manufacturing facility as contemplated by the agreement, the agreement calls for us to provide Novozymes with a rolling three year estimate of our annual need of HA. We are not obligated to forecast any minimum estimate. Upon acceptance of our forecast by Novozymes, we and Novozymes will each be obligated to purchase and supply, respectively, 100% of the total forecasted quantities for the first year based on an agreed scheme adjusted for price index changes. The forecast for the two remaining years is non-binding. Novozymes agrees that the price at which it supplies HA to us is to be the best price it offers to any of its customers for HA of a reasonably similar quality and volume. The supply agreement will remain in effect for the later of seven years from its commencement or five years after the date when Novozymes begins supplying us with HA. At the end of such term and any further term, the supply agreement will automatically extend for a further five years unless either party has given notice not to extend it. Either party may terminate the supply agreement upon a breach of the agreement by the other party, upon certain insolvency events relating to the other party or if it becomes unlawful for the other party to perform under the agreement.
In the related royalty agreement between us and Novozymes, for as long as the relevant product remains covered by a HyACT patent or we receive any royalties from a licensee of the product, we are committed to pay Novozymes a 1.0% royalty on the net sales from any HA-Irinotecan product and a 0.5% royalty on net sales of any other product containing HA developed under the HyACT patents, regardless of whether Novozymes is able to supply HA to us. If Novozymes is capable of supplying HA to our specifications and we do not use them as our supplier for HA, we have to pay a 2.0% royalty on our net sales from any HA-Irinotecan product and a 1.0% royalty on our net sales of any other product containing HA, provided that such royalties will be reduced to 1.0% for HA-Irinotecan product and 0.5% for any other products containing HA if Novozymes is the defaulting party under the supply agreement or if it fails to complete the new HA production facility contemplated in the supply agreement. In the event that we license any other product containing HA to a licensee and the royalty we receive from the licensee is less than 10.0% of the net sales of such product and development for such product has not commenced a Phase II trial at the time the license is entered into, the royalty payable to Novozymes under the royalty agreement will be 5.0% of the percentage of
-98-
Table of Contents
the net sales we receive. We must comply with the license agreement whether or not Novozymes is able to supply HA to us under the supply agreement. Subject to certain termination events, including breach of the agreement by the other party, certain insolvency events relating to the other party or if it becomes unlawful for the other party to perform under the agreement, this agreement will remain in effect until the expiry of our last HyACT patent, which, as of November 1, 2012, is in 2026.
We have a Clinical Research Services Agreement with PSI CRO AG, or PSI, an international clinical trial management company. Pursuant to this agreement, PSI is managing and coordinating the HA-Irinotecan Phase III clinical trial. The amount payable to PSI is milestone driven with a total estimated cost of approximately $9.3 million if those milestones are achieved, of which an amount of $4,096,970 had been paid as of September 30, 2012. In addition, we are obliged to reimburse PSI for out-of-pocket expenses and third party vendor costs. This contract is cancellable at any time at our sole discretion.
We have also entered into a General Services Agreement with BioClinica Inc, an international medical image management provider for clinical trials, to provide medical imaging services for the Phase III clinical trial. The overall project cost is estimated at approximately $1.7 million, of which an amount of $716,267 has been paid as of September 30, 2012. This contract is cancellable at any time at our sole discretion.
As described under “Business—Facilities,” we lease approximately 25,000 square feet of office and lab space in Brisbane, Australia under a lease that will expire in July 2016. The base rent for the first year of this lease is AUD$400,000, and that annual base rent will increase at a rate of 3.0% in each subsequent year of the lease. In addition to the base rent, we must reimburse the lessor a portion of its related expenses, such as utilities, taxes and other levies, etc. on a monthly basis. The estimated amount for this reimbursement for fiscal 2013 is $74,000.
Off-Balance Sheet Arrangements
As at the date of this filing, other than the issuance of the Rights noted below, we did not have any off-balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on the results of operations or our financial condition, including, and without limitation, such considerations as liquidity and capital resources.
Warrants
In November and December 2011, Alchemia Limited issued and sold its ordinary shares to investors in the Placement for total net proceeds of approximately AUD$16.1 million. Pursuant to the terms of the Placement, the investors were given Rights pursuant to which we are required, contingent on completion of this offering by December 31, 2012, to issue Warrants to the investors entitling them to acquire shares of our common stock. The exercise price of the Warrants will be equal to 130% of the volume weighted average price of our common stock for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market (we sometimes refer to such volume weighted average price for such period as the Average Trading Price). The Warrants will be issuable approximately 60 trading days subsequent to completion of this offering and the number of Warrants so issued will be equal to the quotient obtained by dividing (1) AUD$8,073,000 (equivalent to approximately US$8,377,352 based on the currency exchange rate as of November 1, 2012) by (2) 130% of the Average Trading Price. Each Warrant will initially be exercisable for one share of our common stock. Assuming the currency exchange rate mentioned above and assuming an Average Trading Price of US$15.00 per share (the midpoint of the estimated price range set forth on the cover page of this preliminary prospectus), approximately 429,608 Warrants (initially exercisable for a like number of Warrant Shares) would be issuable with an exercise price of approximately US$19.50 per share. However, because the number of Warrants and exercise price both depend upon the actual Average Trading Price following this offering and the number of Warrants also depends upon the actual exchange rate between U.S. dollars and Australian dollars following this offering, the actual number of Warrants that we issue (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the actual exercise price will likely differ from the numbers we have assumed
-99-
Table of Contents
for purposes of this preliminary prospectus. See “Prospectus Summary — The Offering” for an analysis showing how changes in the assumed Average Trading Price change the number of Warrants (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the exercise price of the Warrants and how changes in the currency exchange rate change the number of Warrants (and therefore, the number of Warrants Shares for which the Warrants are exercisable). In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock.
The Warrants will vest and be exercisable from the date that is six months after the date of this offering, or the IPO Date, until the date that is the later to occur of the following, or the Expiry Date:
| • | three years from the IPO Date; and |
| • | the earlier of (A) six months from the date that we announce to the market the results for the primary endpoint of the Phase III clinical trial period for HA-Irinotecan in colorectal cancer and (B) five years from the IPO Date. |
Any Warrant that is not exercised prior to the Expiry Date will automatically expire on the Expiry Date.
The initial fair value of these Rights upon issuance was determined to be insignificant as (i) we had not commenced planning for this offering and (ii) the obligation to issue the Warrants terminates on December 31, 2012, giving us a period of only approximately one year from the date of issuance to complete this offering. As of September 30, 2012, we determined that the fair value of the Rights amounted to $819,280. The key assumptions are disclosed in Note 8 to our unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus.
The Rights are classified as a current liability on our balance sheet in an amount initially equal to their fair value on the date of issuance and the fair value of the Rights is remeasured at the end of each reporting period with any change in the fair value recorded in our statement of operations for such period. One factor impacting the fair value of the Rights is the underlying value of our common stock. Assuming no changes to other factors, the fair value of the Rights increases or decreases in proportion with increases and decreases, respectively, in the value of our common stock. In addition, if and when this offering is completed and the Average Trading Price of our common stock is determined as described under “Prospectus Summary—The Offering,” the fair value of the Rights will be remeasured. As the initial exercise price of the Warrants and the number of Warrants will have been fixed when such Average Trading Price is determined, the fair value of the Warrants will at that time be reclassified from current liabilities to stockholders’ equity on our balance sheet and the Warrants will be accounted for as equity awards. Any increase or decrease in the fair value for the then current period compared with the fair value as of the end of our then most recently ended reporting period will be reflected in our statement of operations for the then current period. The remeasurement of the fair value of the Rights at each balance sheet date (prior to such reclassification) and at the time of such reclassification may result in additional expense, and any such expense may have a material adverse effect on our results of operations. Following such reclassification, we will account for the Warrants as equity awards (as both the initial number of shares issuable on exercise and the initial exercise price shall have been determined as of the date of the remeasurement of the fair value of the Rights in connection with such reclassification) and the remeasurement of the fair value of the Rights will cease. In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock.
-100-
Table of Contents
Quantitative and Qualitative Disclosures about Market Risk
Our exposure to market risk is confined to our cash and cash equivalents which have maturities of three months or less. The primary objective of our investment activities is to preserve our capital to fund operations. We also seek to increase income from our investments without assuming significant risk. Historically we have not held any significant cash or cash equivalent or short-term deposit amounts, but with approximately $7.5 million of additional cash loaned to us by Alchemia Limited on June 28, 2012 (which loan was cancelled as described above under “—Financial Operations Overview—Loan Conversion”) and the net proceeds we receive from this offering, the amount we hold in cash, cash equivalents and short-term deposit will increase. We intend to invest the net proceeds from this offering in interest bearing and similar investments with terms of 18 months or less, which may include interest-bearing bank accounts, money market funds, certificates of deposit and government securities.
As our functional currency is the Australian dollar, we are exposed to foreign exchange risk, mainly with the U.S. dollar, which is the currency in which we are invoiced by most of our contract research organizations for the costs of conducting our clinical trials. Our policy is to minimize the use of financial derivatives and we endeavor to achieve risk mitigation through natural hedges. These natural hedges may include the maintenance of a U.S. dollar bank account and bank accounts in any other currency to which we may have significant exposure in the future to facilitate the settlement of invoices in these currencies.
Emerging Growth Company Status
Section 107 of the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933 for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
-101-
Table of Contents
Overview
We are a late stage biopharmaceutical company primarily focused on utilizing our Hyaluronic Acid Chemotransport Technology, or HyACT, to target cancer drugs preferentially to tumor cells to enhance drug activity. HyACT is a flexible platform technology designed to increase the effectiveness of anti-cancer agents without increasing treatment toxicity. We seek to reduce the risks related to drug development by using known anti-cancer drugs, and aim to enhance their commercial value by improving their effectiveness. Our lead HyACT product candidate, HA-Irinotecan, is currently in a pivotal Phase III clinical trial for metastatic colorectal cancer, or mCRC, meaning that we intend to use the data we collect from the clinical trial to seek regulatory approval from the U.S. Food and Drug Administration, or FDA, and the European Medicines Agency , or EMA, of HA-Irinotecan for the treatment of mCRC. HA-Irinotecan is also in an investigator-sponsored Phase II clinical trial for the treatment of small cell lung cancer, or SCLC. We also have two other HyACT product candidates that have successfully completed Phase I clinical trials. In addition to our current clinical-stage product candidates, we aim to develop a pipeline of product candidates by exploiting our significant know-how in cancer stem cell biology and cancer metabolism combined with the Versatile Assembly on Stable Templates, or VAST, molecule drug discovery technology that we have in-licensed.
Each of our current product candidates combines HyACT with a known anti-cancer drug. HyACT uses hyaluronic acid, or HA, which delivers additional drug to the tumor and promotes uptake of the drug into the tumor cells. HyACT binds to the activated receptor CD44, a naturally occurring HA receptor, which has been shown in numerous studies to be present in high levels in many prevalent solid tumor cancer types but, more importantly, is generally not activated in healthy tissue. CD44 over-expression is associated with more aggressive, metastatic tumors and is also a marker for treatment-resistant cancer stem cells. “Cancer stem cells,” sometimes referred to as tumor-initiating cells, is a term used to describe a small subset of cells within the tumor that, although not actual stem cells, demonstrate stem cell-like characteristics. Cancer stem cells are generally more resistant to current chemotherapy regimens than cancer cells, and their persistence after therapy is thought to be one of the key reasons for disease progression and treatment failure. We believe, based on preclinical studies, that the CD44 receptor-based mechanism has the potential for improving the effectiveness of drugs combining HyACT with an anti-cancer drug, which we refer to as HyACT-targeted drugs. In preclinical studies, HyACT-targeted drugs delivered at least double the dose of anti-cancer drug to the tumor when compared with the drug alone. Our in vitro studies have shown that HyACT significantly increases drug uptake into cancer cells. Finally, preclinical studies have also shown HyACT-targeted chemotherapies are more potent than the original drug at killing cancer stem cells and other CD44-expressing cells.
Our
lead product candidate is HyACT-targeted irinotecan, or HA-Irinotecan, for the treatment of mCRC. Irinotecan, which is marketed in major markets by Pfizer as Camptosar, is an off-patent chemotherapy drug widely used in the treatment of mCRC. In a 76
patient, randomized Phase II clinical trial of HA-Irinotecan compared with irinotecan alone, HA-Irinotecan was able to double the time it took for patients’ tumors to grow (also known as progression free survival, or PFS) in mCRC patients.
After having consultations with the FDA and the EMA, we commenced a pivotal Phase III clinical trial testing HA-Irinotecan in patients with mCRC in November 2011. Recruitment and enrollment of patients for the clinical trial was initially slow, with
our first patient dosed in January 2012, but enrollment reached over 72% as of October 30, 2012 and is currently on track, and we have increased the number of trial sites. This trial is designed to recruit 390 irinotecan-naïve, second and
third line patients and will directly compare
HA-Irinotecan with irinotecan alone, in each case as components of the FOLFIRI chemotherapy regimen (leucovorin, 5-fluorouracil and irinotecan). The primary endpoint for the trial is PFS, and if the
trial is successful, we intend to seek regulatory approval from both the FDA (using the 505(b)(2) regulatory approval pathway) and the EMA of HA-Irinotecan for the treatment of mCRC in the United States and the European Union. Based on our initial
consultations with the FDA, we believe that the 505(b)(2) regulatory approach is an appropriate pathway to seek regulatory approval of HA-Irinotecan, as HA-Irinotecan is a formulation of two previously approved products (the cancer drug irinotecan
and HA), although there can be no
-102-
Table of Contents
assurance in this regard. Reliance on the 505(b)(2) regulatory pathway requires establishing that such reliance is scientifically appropriate at the time of submission of the NDA. We have sought and have received input from the EMA on the preclinical and clinical development of HA-Irinotecan for the treatment of mCRC and are taking into consideration this input in our European regulatory strategy. In addition, a Phase II clinical trial of HA-Irinotecan for the treatment of SCLC started recruitment in September 2011. This investigator-sponsored trial will examine the clinical benefits of HA-Irinotecan compared with irinotecan alone, as well as the direct effect of HA-Irinotecan on cancer stem cells and other aggressive cancer cell populations through analysis of tumor biopsies. The objective of this Phase II trial is to demonstrate that, by targeting the CD44 receptor on cancer stem cells, HA-Irinotecan may enhance the killing of the cancer stem cell and cancer cell populations, which may ultimately translate into increased patient survival. Our participation in this investigator-sponsored trial has the potential to further validate the HyACT technology as well as to provide clinical data on HA-Irinotecan’s activity on cancer stem cells. We currently do not intend to pursue further clinical trials or commercialization of HA-Irinotecan for the treatment of SCLC and, accordingly, references to our “product candidates” do not include HA-Irinotecan for the treatment of SCLC.
Despite the considerable research and development efforts of the pharmaceutical industry, cancer remains a significant unmet medical need. Chemotherapeutics remain the backbone of many drug-based anti-cancer treatment regimens. Due to the non-specific nature of current chemotherapeutics, they are typically administered at the maximum tolerated dose, not at the optimal dose required to kill the cancer. As evidenced by the current recurrence and mortality rates associated with cancers, there remains a need to identify new and more effective anti-cancer treatments.
We believe that HyACT is a broadly applicable platform technology that has the potential for enhancing the efficacy of a range of anti-cancer drugs in a number of different cancer indications. In addition to HA-Irinotecan’s Phase II clinical trial data, HyACT-targeted 5-fluorouracil and HyACT-targeted doxorubicin have both demonstrated tolerability and a trend towards efficacy in Phase I / IIa clinical trials. In preclinical studies, we have also had promising results combining HyACT with seven other anti-cancer agents, including other chemotherapies and monoclonal antibodies.
We have considerable know-how, research and preclinical expertise in HA, cancer metabolism, CD44 and cancer stem cells. We have also in-licensed rights to Alchemia Limited’s Versatile Assembly on Stable Templates, or VAST, small molecule drug discovery technology, which provides innovative pyranose compounds for drug discovery. Our strategy is to use these capabilities to investigate new pathways relating to CD44 signaling, HA metabolism and pro-oncogenic pathways that we believe are important to the promotion and proliferation of cancer stem cells. We aim to generate future drugs designed to more effectively target and kill cancer and cancer stem cells.
The Demerger
Audeo Oncology, Inc., the issuer of the common stock being sold in this offering, is a newly formed Delaware corporation and is currently a wholly-owned subsidiary of Alchemia Limited, an Australian company whose common shares are listed on the Australian Securities Exchange. We were formed for the purpose of (a) acquiring Alchemia Oncology Pty Limited, or Alchemia Oncology, and forming our other subsidiary, Audeo Discovery Pty Limited, an Australian company organized to in-license the VAST small molecule drug discovery technology owned by Alchemia Limited, and (b) demerging from Alchemia Limited as an independent public company with shares of our common stock listed on the Nasdaq Global Market and CHESS Depository Interests, or CDIs, representing shares of our common stock to be listed on the Australian Securities Exchange. Our business and operations consist solely of the business and operations of Alchemia Oncology and Audeo Discovery Pty Limited, both of which are our wholly-owned subsidiaries. We will rename Alchemia Oncology following implementation of the Demerger, as defined in the next sentence. Approximately two trading days following the completion of this offering, we intend to complete implementation of our demerger, or the Demerger, from Alchemia Limited. All conditions precedent to the Demerger, other than those related to completion of this offering, have been satisfied prior to the date of this prospectus. For more details regarding the
-103-
Table of Contents
Demerger, see “Prospectus Summary — Corporate Information — Corporate Reorganization,” “Prospectus Summary — Corporate Information — The Demerger” and “Arrangements between Audeo Oncology, Inc. and Alchemia Limited.”
Our Strategy
Our goal is to build a global biopharmaceutical company focused on the development and commercialization of anti-cancer treatments in the United States and other major markets worldwide. Key elements of our strategy to achieve this objective are:
| • | Leveraging our proprietary HyACT technology to bring improved anti-cancer agents to market. Our objective is to use our HyACT technology to improve the delivery of chemotherapeutic agents to cancer cells, boosting drug efficacy without increasing side-effects, thereby increasing the therapeutic index of current chemotherapeutics. We intend to utilize the FDA’s 505(b)(2) regulatory pathway, which may offer a lower development risk profile and accelerated development compared with completely new cancer drugs. |
| • | Successfully developing our lead product candidate, HA-Irinotecan, for the treatment of metastatic colorectal cancer. After having consultations with the FDA and the EMA, we began recruiting patients for a pivotal Phase III clinical trial testing HA-Irinotecan in patients with mCRC in November 2011. If this clinical trial is successful, we intend to seek regulatory approval from both the FDA and the EMA of HA-Irinotecan for the treatment of second and third line mCRC patients in the United States and the European Union. |
| • | Enhancing the commercial value of HyACT-targeted drugs. Where appropriate, we intend to pursue commercialization relationships with pharmaceutical companies and other strategic partners providing for our products to be distributed through their sales and marketing organizations in order to gain access to global oncology markets for any of our product candidates that receive regulatory approval. Over the longer term, we may ultimately build an internal commercial infrastructure to directly market and sell any HyACT-targeted drugs that may receive regulatory approval. |
| • | Leveraging our expertise and know-how to develop additional anti-cancer agents of high value. We plan to utilize our in-licensed small molecule drug discovery technology, VAST, and extensive preclinical expertise in stem cell biology and oncology drug development to develop new therapies targeted at modulating innovative pathways associated with HA metabolism and cancer stem cells. |
Our Technologies
HyACT
HyACT-targeted product candidates work by exploiting the unique structure and inherent biological properties of HA, a natural glycosaminoglycan polymer, which is a key component of the extracellular matrix in mammals. The specific form of HA used in our HyACT-targeted product candidates combines the effective drug entrapment and delivery properties of HA with the active transport and internalization characteristics resulting from HA’s ability to bind to activated CD44, a receptor generally over-expressed on cancer cells. HA has been shown in preclinical studies to not only carry the anti-cancer drug preferentially to cancer cells but also increase its uptake by those cancer cells.
Our HyACT-targeted product candidates are designed to overcome some of the limitations of current chemotherapies by delivering anti-cancer drugs preferentially to cancer cells in order to increase the response of cancers to standard chemotherapy. Because our initial HyACT-targeted product candidates consist of two FDA-approved agents (HA and chemotherapeutics with well-characterized toxicity and efficacy profiles), we believe
-104-
Table of Contents
that these HyACT-targeted product candidates, which we expect will utilize the FDA’s 505(b)(2) regulatory approval pathway, may offer a lower development risk profile and accelerated development compared with completely new cancer drugs.
HyACT as a Drug Delivery Vehicle
HA’s physiochemical structure allows it to form a “meshwork” well-suited for entrapping smaller molecules and proteins such as chemotherapeutic agents and monoclonal antibodies. Specifically, in aqueous solution, the linear, unbranched HA polymer exhibits a tertiary coiled structure that allows multiple hydrogen bonds to form between adjacent saccharides. The dynamic nature of these multiple hydrogen bonds creates a stiffened but flexible polymer with a large hydrodynamic domain sufficient to entrap a number of drugs.
HyACT as a Tumor Targeting Agent
HyACT has a high avidity for the activated form of its native receptor, CD44. Upon binding to CD44, HyACT-targeted drugs are readily internalized into the tumor cell. CD44 is a broadly distributed transmembrane glycoprotein that plays a key role in the behavior of malignant cells, including adhesion, migration, invasion, and survival. In its activated, high-affinity state, CD44 is capable of binding to HA. In contrast to normal cells, tumor-derived cells predominantly express activated CD44. HyACT-targeted drugs bind to activated CD44, which triggers internalization of the anti-cancer drug into the cell, resulting in increased intracellular drug concentrations that we believe may translate into superior efficacy. Preclinical biodistribution studies have demonstrated that within 30 minutes after intravenous injection, HA is internalized within the microvasculature of the tumor. This has been shown in preclinical studies to result in up to 400% more drug in the tumor and lymph nodes for HyACT-targeted anti-cancer drugs compared with the same anti-cancer drugs administered alone. The following table, which reflects data that we obtained from various published sources, shows the approximate percentage of activated CD44 receptors found in certain types of healthy and malignant tissue:
| Approximate Percentage of Activated CD44 Receptors | ||||
| Cancer Type |
Healthy Tissue | Diseased Tissue | ||
| Bladder |
0% | 100% | ||
| Breast |
0% | 50-100% | ||
| Colorectal |
0% | 70-80% | ||
| Endometrial |
0% | 50-80% | ||
| Malignant mesothelioma |
0% | 100% | ||
| Ovarian |
0% | 100% | ||
| Pancreatic |
0% | 70-100% | ||
| Prostate |
0% | 60-80% | ||
| Renal |
0% | 50% | ||
Based on preclinical studies, we believe that some of the key characteristics of HyACT include (i) HyACT’s drug entrapment properties, (ii) HyACT’s ability to carry anti-cancer drugs to the tumor, (iii) HyACT’s formation of cancer-localized drug depots, and (iv) the internalization of HA in an active CD44 receptor-based mechanism. The result is that HyACT-targeted drugs are maintained in the tumor environment for longer with enhanced uptake by the cancer cells through activated CD44. In addition, we have seen no significant increase in toxicity in our preclinical studies and clinical trials of HyACT-targeted drugs compared with the drugs alone. Specifically, the incidence and severity of the side-effects observed to have been caused by our product candidates combining HyACT with a cancer drug, including, among others, nausea, vomiting, diarrhea and fatigue, are consistent with the side effects observed to have been caused by the applicable drugs alone.
-105-
Table of Contents
The figure below depicts the HyACT mechanism of action, from the bloodstream into a cancer cell, based on preclinical testing:
HyACT Mechanism of Action
|
|
HyACT-targeted drug delivery vehicle and anti-cancer drug in the bloodstream after infusion | |
|
|
HyACT-targeted drug accesses the tumor environment through leaky vasculature typical of blood vessels that supply blood to tumors. HyACT-targeted drugs form a drug depot in the extracellular space of the tumor | |
|
|
HyACT-targeted drug binds to activated CD44 and then is rapidly internalized, resulting in more drug entering the tumor cell | |
|
|
HyACT-targeted drug is transported into an intracellular vesicle and the hyaluronic acid is degraded | |
|
|
HyACT-targeted drug is released within the cancer cell, increasing the likelihood of cell death and enhanced tumor response | |
|
|
The HyACT-targeted drug depot generally persists for at least 24 hours, resulting in repeated cycles of drug internalization and release inside tumor cells | |
The figure above represents our hypothesis regarding the HyACT mechanism of action based on our preclinical data and on published literature.
-106-
Table of Contents
HyACT and Cancer Stem Cells
“Cancer stem cells,” sometimes referred to as tumor-initiating cells, is a term used to describe a small subset of cells within the tumor that, although not actual stem cells, demonstrate stem cell-like characteristics, such as self-renewal and the ability to form multiple lineages. Cancer stem cells have been identified in many types of cancer, including colon, breast, pancreatic, brain, lung and leukemia. Following cancer treatment, the residual tumor mass can still contain a high percentage of cancer stem cells, which are generally resistant to further treatment. In addition, patients who relapse can develop metastatic disease in which the cancer spreads to other sites in the body. Scientific literature suggests that the expression of high levels of CD44 is a distinguishing feature of the more resistant and aggressive cancer cell populations, including cancer stem cells.
Although there is no universal cancer stem cell marker, we believe that CD44, CD133, EpCAM and aldehyde dehydrogenase, or ALDH, are generally accepted as some of the better phenotypic markers of cancer stem cells. The consistent identification of CD44 on cancer stem cells led us to investigate whether the HyACT-targeted technology also preferentially bound to cancer stem cells, delivering increased concentrations of chemotherapy into those cells. Preclinical investigations in colon and breast cancer stem cells demonstrated the anti-cancer properties of HyACT-targeted irinotecan, HyACT-targeted doxorubicin and HyACT-targeted gemcitabine on cancer stem cells. This can be seen in the following photographs, which show the punctuate fluorescence (indicated by the arrow on the left-hand bottom panel) associated with the increased intracellular uptake of doxorubicin and HA-doxorubicin in breast cancer stem cells with activated CD44 as well as in other breast cancer cells with and without activated CD44 (referred to as activated CD44 positive and activated CD44 negative, respectively, in the captions above these photographs):
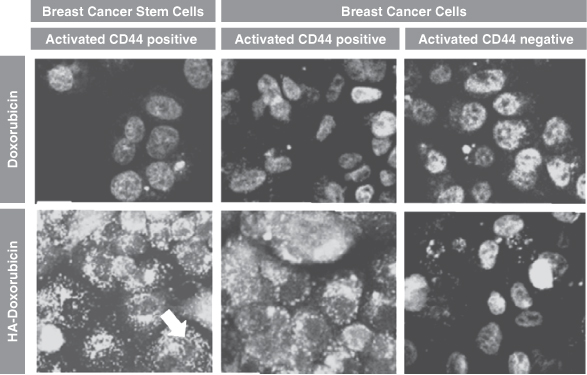
VAST
We have in-licensed worldwide rights to the VAST technology from our parent, Alchemia Limited. This technology is designed to enable the discovery of new, small molecule drugs through the use of molecular
-107-
Table of Contents
“probes.” The VAST technology is based on high throughput, synthetic chemistry on pyranose scaffolds and comprises an array of compounds, which due to their rigidity and high chirality (compound shape), can enable the systematic exploration of new drugs. In 2009, we completed the synthesis of a diversity scanning array, or DSA, a suite of approximately 14,000 pyranose-based compounds that systematically arrange typical binding groups in a broad range of possible three-dimensional orientations. This array has the ability to identify the shape and functional requirements of molecules that modulate a target.
Our Product Candidates
Consistent with our strategy, each of our product candidates is being developed for selected cancer therapies to treat specific therapeutically-relevant cancer indications. We have not licensed rights to any of our product candidates to any third parties. The following table summarizes the status of (a) HA-Irinotecan for mCRC and our other product candidates, (b) an investigator-sponsored trial of HA-Irinotecan for SCLC (we do not currently intend to pursue further clinical trials or commercialization of HA-Irinotecan for this indication) and (c) drug discovery activities using the VAST technology:

mCRC is metastatic colorectal cancer
SCLC is small cell lung cancer
mDRC is metastatic drug resistant cancer
HA-Irinotecan
Overview
Our lead product candidate is HA-Irinotecan for the treatment of metastatic colorectal cancer, or mCRC. Irinotecan, which is marketed in major markets by Pfizer as Camptosar, is an off-patent chemotherapy widely used in the treatment of mCRC. In a randomized Phase II clinical trial of HA-Irinotecan compared with irinotecan alone, based on 76 patients with mCRC, HA-Irinotecan was able to double the progression free survival, or PFS, in mCRC patients (5.2 months for HA-Irinotecan versus 2.4 months for irinotecan alone). Based on these findings, we began enrollment for a pivotal Phase III clinical trial, testing HA-Irinotecan in patients with mCRC in November 2011.
In addition to its approved use, published studies suggest that irinotecan may have activity in a range of solid tumors such as those of the breast, pancreas, brain and ovaries. According to reports by Pfizer, sales of
-108-
Table of Contents
Camptosar (proprietary irinotecan, which became generic in 2008) were over $950 million in 2007. We believe that, to the extent clinical trials confirm HA-Irinotecan’s safety and demonstrate significantly improved clinical efficacy compared with irinotecan alone, HA-Irinotecan has the potential to replace irinotecan in first to third line irinotecan-containing treatment regimens. See “Risk Factors — If HA-Irinotecan does not demonstrate superior efficacy to generic irinotecan for the treatment of mCRC in our Phase III clinical trial, it is unlikely that we will receive FDA approved labeling allowing us to price HA-Irinotecan at a premium to generic irinotecan, and even if our label contains data adequate to allow us to price HA-Irinotecan at a premium to generic irinotecan, there is no assurance that payors will pay such a premium, either of which could have a material adverse effect on our business.”
In fiscal 2011, fiscal 2012, the three months ended September 30, 2012 and for the period from inception to
September 30, 2012, we have invested approximately $5.5 million, $5.9 million, $3.2 million and $16.2 million, respectively, in initiating and conducting our pivotal Phase III clinical trial of HA-Irinotecan for the treatment of mCRC. The
Phase III clinical trial is expected to continue through fiscal 2013, although it may take longer than expected and in any event may not be successful. If our Phase III clinical trial is successful, we will need to raise substantial additional
capital to fund the costs, which we expect to be significant, of seeking regulatory approval of
HA-Irinotecan for the treatment of mCRC (if the proceeds from this offering are insufficient to cover those costs) and will also need to raise
substantial additional capital to fund the costs, which we also expect to be significant, associated with commercialization of HA-Irinotecan. See “Risk Factors—We will require substantial additional funding which may not be
available to us on acceptable terms, or at all.”
Metastatic Colorectal Cancer Market
According to the American Cancer Society, colorectal cancer is the third most common form of cancer diagnosed in the United States, excluding skin cancers. According to the American Cancer Society, approximately 143,000 new cases of colorectal cancer are expected to be reported in the United States in 2012, with approximately 51,000 patients in the United States expected to die from colorectal cancer in 2012. Surgery is often the first line treatment for early stage colorectal cancer. When colorectal cancer metastasizes (spreads to other parts of the body such as the liver), chemotherapy is commonly used. Chemotherapy regimens (i.e., FOLFOX (leucovorin, 5-fluorouracil and oxaliplatin) or FOLFIRI (leucovorin, 5-fluorouracil and irinotecan), either with or without bevacizumab) have been shown to increase survival rates in patients with metastatic/advanced colorectal cancer and are among the leading first and second line treatments in the United States and Europe. Currently, there are nine FDA approved drugs for patients with mCRC: 5-fluorouracil, aflibercept (Zaltrap), bevacizumab (Avastin), capecitabine (Xeloda), cetuximab (Erbitux), irinotecan, leucovorin, oxaliplatin and panitumumab (Vectibix). Depending on the stage of the cancer, two or more of these drugs may be combined at the same time or used after one another. Bevacizumab, a vascular endothelial growth factor, or VEGF, monoclonal antibody, is commonly administered with chemotherapy. Typically, patients who fail 5-fluorouracil, oxaliplatin, irinotecan, and bevacizumab-containing therapies, and who have wild-type KRAS status, receive epidermal growth factor receptor, or EGFR, monoclonal antibody therapy with either cetuximab or panitumumab, together with chemotherapy. We believe that if approved, HA-Irinotecan could replace irinotecan currently in use in the FOLFIRI treatment regimen.
-109-
Table of Contents
HA-Irinotecan Preclinical Results
In preclinical studies on human cancer xenografts, we demonstrated a significant increase in the concentration of HA-Irinotecan within tumor cells compared with irinotecan alone. The following figure shows HA-Irinotecan versus irinotecan uptake in human colorectal cancer xenografts in one of those studies:
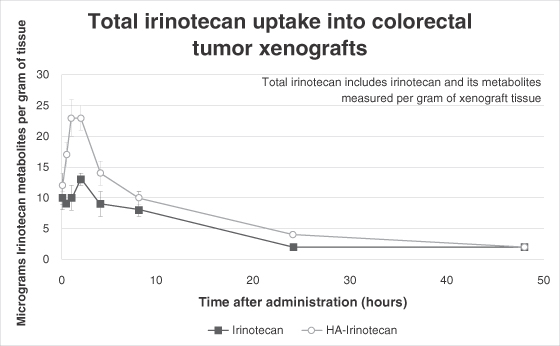
As seen in the figures below, our subsequent preclinical studies on human colorectal cancer xenografts showed HA-Irinotecan reduced the size of tumor xenografts when compared with irinotecan alone in irinotecan-resistant and irinotecan-sensitive tumors, providing our first indication of the potential efficacy improvements in tumor shrinkage by HA-Irinotecan:
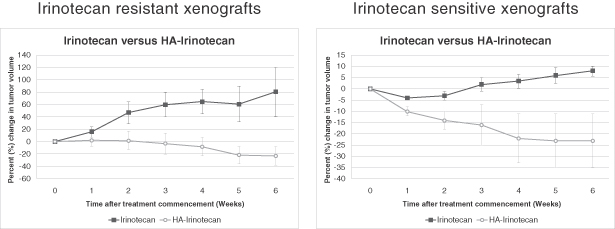
-110-
Table of Contents
HA-Irinotecan Clinical Development
In our completed Phase I and Phase II clinical trials, we have tested HA-Irinotecan in 88 patients, demonstrating no significant increase in toxicity or plasma pharmacokinetic characteristics, with significant improvements in certain measures of efficacy versus irinotecan alone in the Phase II clinical trial.
Phase I Clinical Trial for Metastatic Colorectal Cancer
In our open-label Phase I dose escalating trial, we tested 12 patients who had previously failed treatment with 5-flourouracil. Patients were given an initial dose of HA-Irinotecan containing 300 mg/m2 irinotecan, followed by dose escalation to 350 mg/m2 irinotecan, where these therapies were administered on a 21 day cycle. In this trial, 17% of patients showed an overall response (comprised of both partial and complete responders) and 50% of patients demonstrated stable disease. There was no evidence of increased toxicity, such as grade 3 or grade 4 diarrhea, vomiting and neutropenia, compared with historic studies of single agent irinotecan when administered under the same schedule. We achieved our primary objective of demonstrating safety and tolerability of HA-Irinotecan in this Phase I trial.
Randomized Phase II Clinical Trial for Metastatic Colorectal Cancer
The Phase II clinical trial was conducted at 10 clinical trial sites in Australia. This randomized study tested HA-Irinotecan versus irinotecan alone in 76 patients who had previously failed treatment for mCRC with a 5-fluorouracil-containing regimen. Patients were randomized to receive up to eight cycles of treatment with either irinotecan alone or HA-Irinotecan, each containing 350 mg/m2 irinotecan administered every 21 days.
While we did not achieve the primary endpoint of a reduction in diarrhea in our Phase II clinical trial, we did observe statistically significant improvements in progression free survival, or PFS, and other efficacy endpoints for HA-Irinotecan compared with irinotecan alone. Key data from this Phase II clinical trial showed:
| • | patients receiving HA-Irinotecan experienced statistically significant increases in the period of PFS (5.2 months versus 2.4 months, + 116%; p=0.017), with a hazard ratio of 0.46 (p=0.011) after adjustment for 13 prognostic factors; |
| • | patients receiving HA-Irinotecan experienced a significantly longer time to treatment failure, or TTF (4.0 months versus 1.8 months, + 122%; p=0.007); |
| • | patients receiving HA-Irinotecan experienced a trend where overall survival, or OS, increased by about two months (10.1 versus 8.0 months; p=0.196), with a hazard ratio of 0.75 (p=0.376) after adjustment for 13 prognostic factors; |
| • | HA-Irinotecan exhibited a superior anti-cancer effect, with more patients experiencing anti-tumor responses or stable disease, with a final disease control rate of 76% in the HA-Irinotecan arm of the trial versus 46% in the irinotecan-alone arm (p=0.053); |
| • | HA-Irinotecan patients were able to be treated for significantly more cycles: the median number of completed cycles was six compared with two for irinotecan alone patients (p = 0.005). Within the study cohort, the total number of cycles administered was 213 for HA-Irinotecan-treated patients compared with 123 for irinotecan alone patients; |
| • | 14 (34%) HA-Irinotecan-treated patients and five (14%) irinotecan-alone-treated patients completed eight treatment cycles (p = 0.062); |
-111-
Table of Contents
| • | dose intensity per cycle was almost identical for both treatment arms, with the total cumulative dose being attributable to the delay in tumor progression (median 2.50 times higher for HA-Irinotecan; mean 1.46 times higher for HA-Irinotecan); and |
| • | no significant change in safety and no significant increase in toxicity were observed; a similar frequency and spectrum of toxicity were observed in both arms of the clinical trial. |
The following figure shows the statistical significance in the period of PFS for patients receiving HA-irinotecan versus irinotecan alone:
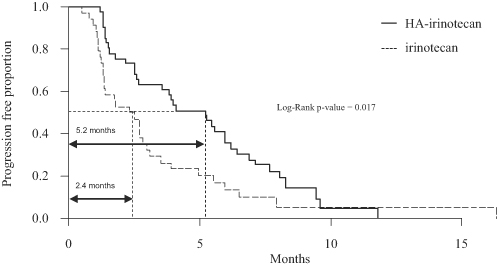
On-Going, Randomized, Double-Blind Phase III Clinical Trial of HA-Irinotecan in 2nd/3rd Line Metastatic Colorectal Cancer
After having consultations with the FDA and the EMA, we commenced our pivotal Phase III clinical trial of HA-Irinotecan for the treatment of mCRC. Our pivotal Phase III clinical trial is comparing the effectiveness of HA-Irinotecan with irinotecan, in each case as components of the FOLFIRI chemotherapy regimen (leucovorin, 5-fluorouracil and irinotecan), in 390 irinotecan-naïve, second and third-line patients with mCRC. We believe that the FOLFIRI regimen is generally considered the leading standard of care for second and third line treatment for mCRC in the United States and Europe. The primary objective of this study is to demonstrate that HA-Irinotecan provides superior efficacy compared with irinotecan alone, as indicated by a significantly increased period of PFS.
The randomized, multi-centered, double-blind trial is designed to detect a six week or greater difference in PFS (primary endpoint is PFS, 85% power) with monitoring for up to 18 months following randomization. HA-Irinotecan is being used as an absolute replacement for irinotecan alone within the FOLFIRI regimen, where it is used at the same dose and administered in a substantially identical manner to that currently used for irinotecan in the clinic. Recruitment commenced in November 2011 and, while initially slow, with our first patient dosed in January 2012, enrollment reached over 72% as of October 30, 2012 and is currently on track. We have increased the number of trial sites and the trial is being conducted at sites in Australia, Bulgaria, Russia, Poland, Serbia, Ukraine and the United Kingdom. Trial sites were selected on the basis of, among other factors, the availability of patients, quality of patient management and experience in data handling and clinical trials. PSI, our contracted research organization for this trial, is an international clinical research company that conducts its business in North and South America, Asia, Australia, Europe and Russia. According to PSI, oncology is the largest therapeutic area in which it works and over the past 15 years, it has run over 150 oncology studies. Also
-112-
Table of Contents
according to PSI, the following drugs were approved by the FDA and/or EMA with pivotal data coming from PSI: Abraxane, Halaven, Femara, Firmagon, Thalomid, Aloxi, Zarzio and Neulasta. For information on our clinical research services agreement with PSI, see “Management’s Discussion of Financial Condition and Results of Operations—Contractual Obligations and Commitments.”
In addition to the primary endpoint of PFS, we are conducting a small sub-study looking at QTc prolongation and the pharmacokinetics of HA-Irinotecan within the FOLFIRI regimen. If this clinical trial is successful, we intend to seek regulatory approval from both the FDA and the EMA for HA-Irinotecan for the treatment of second and third line mCRC patients in the United States and the European Union, although we cannot assure you that this will occur. See “Risk Factors — We are highly dependent on the success of our lead product candidate, the cancer drug HA-Irinotecan for the treatment of metastatic colorectal cancer, and we cannot give any assurance that we will successfully complete its clinical development, or that it will receive regulatory approval, or if approved, that it will receive adequate labeling that will allow for successful commercialization for the treatment of mCRC” and “— Regulatory Strategy” below.
Because of the difficulty in reliably determining cancer progression based on imaging studies in cancer, the FDA’s policy is to recommend that the primary endpoint for a cancer registration trial be overall survival, or OS, whereas our Phase III clinical trial has PFS as its primary endpoint. However, certain drugs have been approved by the FDA based on primary endpoints other than OS, including Erbitux for mCRC and Abraxane for metastatic breast cancer. As our trial has PFS as its primary endpoint, it is essential that the tumor responses are stringently monitored to enable the FDA and EMA to accurately determine the efficacy of HA-Irinotecan versus irinotecan alone. We believe that we have developed a rigorous protocol designed to produce consistent tumor measurements. The process we designed and adopted involves tumor scans being independently analyzed by separate onsite and offsite evaluations. Each evaluation of a scan occurs in a double blinded fashion, with none of the physician, the patient or the radiologist knowing which treatment the patient is receiving. Each patient has a tumor scan prior to commencing treatment. Then, commencing six weeks after the first treatment and generally every six weeks thereafter, patients present themselves at a medical facility for tumor scans. An onsite evaluation of the scan is then performed by the lead investigator or a radiologist who is experienced in using the Response Evaluation Criteria in Solid Tumors version 1.1, or RECIST, criteria. In the event that the evaluator believes that a tumor scan documents progressive disease based on the RECIST criteria, a second onsite evaluator (either the lead investigator or a radiologist) must concur in the evaluation. In the event of a disagreement between the two onsite evaluators, the patient must continue as part of the trial and on trial chemotherapy as tolerated until the next scheduled tumor assessment. These onsite evaluations are performed for patient management purposes in order to determine whether the patient should continue as part of the trial. The blinded tumor scans are subsequently sent to BioClinica, an international medical image management provider for clinical trials, for offsite evaluation. BioClinica reviews each scan and applies the RECIST criteria. Two BioClinica-contracted radiologists must independently arrive at the same conclusion on a patient’s tumor response for that response to be confirmed; otherwise a third BioClinica-contracted radiologist is brought into the assessment process to make the decision regarding when the patient progressed. The BioClinica assessment (and not the onsite evaluation) is used in the analysis of results from the trial. In addition, we have engaged an independent statistical team, Pharmanet (USA), to verify, approximately every four weeks, that the onsite interpretation of the data from the scans concurs with Pharmanet (USA)’s assessment according to the RECIST criteria. For information on our general services agreement with BioClinica, see “Management’s Discussion of Financial Condition and Results of Operations—Contractual Obligations and Commitments.”
The primary endpoint will be reached when 350 patients have died or experienced disease progression. We have secured an independent Data Safety and Monitoring Board, or DSMB, which, as of October 30, 2012, had assessed data from the initial 220 patients and found no unexpected toxicities and therefore approved the continuation of the trial. As of October 30, 2012, 281 patients had been recruited and randomized to receive treatment and there had been no unexpected toxicities and no suspected unexpected serious adverse reactions in the patients treated.
-113-
Table of Contents
Regulatory Strategy
As HA-Irinotecan is a formulation of two previously approved products (the cancer drug irinotecan and HA), we intend to utilize an alternative type of New Drug Application, or NDA, commonly referred to as a Section 505(b)(2) NDA, which enables us to rely, in part, on the FDA’s previous approval of a similar product, or on published literature, in support of our application. Reliance on the 505(b)(2) regulatory pathway requires establishing that such reliance is scientifically appropriate at the time of submission of the NDA. Irinotecan was developed and marketed by Pfizer as Camptosar, while HA formulations have been approved by the FDA for use in ocular surgery, dermal fillers and cosmetics. The 505(b)(2) regulatory approach may enable us to produce an abbreviated preclinical data package, thereby saving development time and costs.
A fixed dose combination product is one in which two or more separate drug ingredients are combined in a single dosage form. In order for a fixed dose combination product to be approved by the FDA, the applicant must demonstrate that each drug makes a contribution to the claimed effects of the combination, and that the dosage of each drug is such that the combination is safe and effective for a significant patient population requiring concurrent therapy with the drugs. One example of a fixed dose combination product is where one drug is combined with another drug in order to enhance the second drug’s safety or effectiveness. The FDA views our HA-Irinotecan product candidate, and may also view each of our other HyACT-containing product candidates, as such a combination and has indicated that we must demonstrate through a clinical trial that HyACT contributes to the efficacy and/or safety of the combination.
After having consultations with the FDA and the EMA, we commenced our pivotal Phase III clinical trial of HA-Irinotecan for mCRC. If this pivotal trial is successful, we intend to seek regulatory approval from both the FDA and the EMA of HA-Irinotecan for the treatment of mCRC in the United States and the European Union. However, even if our pivotal Phase III trial meets its PFS primary endpoint, an additional trial regarding OS could be required as either a pre-approval requirement or as a post-marketing commitment should approval be granted based on PFS. If the FDA were to require a second Phase III clinical trial as a pre-approval requirement, it would substantially increase the time and cost of our development activities and, in any event, may not ultimately result in regulatory approval. In addition, if the FDA approves HA-Irinotecan for mCRC based on meeting our PFS primary endpoint but requires post-marketing clinical trials or analyses, the approval could be withdrawn if any required post-marketing trials or analyses do not meet FDA requirements. Moreover, even if HA-Irinotecan receives regulatory approval, such approval may not allow us to charge a premium over a generic version of irinotecan if our clinical trial data does not support FDA approval of a label stating the superior efficacy of HA-Irinotecan over a generic version of irinotecan, and even if our label contains data adequate to allow us to price HA-Irinotecan at a premium to generic irinotecan, there is no assurance that payors will pay such a premium; if we are unable to charge, or if payors are unwilling to pay, a premium price for HA-Irinotecan, we may not be able to successfully commercialize this product candidate, which could have a material adverse effect on our business. See “Risk Factors — We are highly dependent on the success of our lead product candidate, the cancer drug HA-Irinotecan for the treatment of mCRC, and we cannot give any assurance that we will successfully complete its clinical development, or that it will receive regulatory approval, or if approved, that it will receive adequate labeling that will allow for successful commercialization for the treatment of mCRC.” and “Business — Government Regulations.”
Investigator-Sponsored Phase II Trial of Small Cell Lung Cancer
In September 2011, recruitment began for an investigator-sponsored Phase II clinical trial of HA-Irinotecan plus carboplatin versus irinotecan plus carboplatin in small cell lung cancer, or SCLC. Patients will be randomized to receive either HA-Irinotecan or irinotecan, each in combination with carboplatin. The original protocol provided for recruitment of first line chemotherapy-naïve patients, and the first five patients in the safety cohort were all treated with HA-Irinotecan plus carboplatin. In May 2012, a protocol amendment expanded the study to both first and second line irinotecan-naïve SCLC patients. After the first five patient cohort, the first line
-114-
Table of Contents
patients are to be randomized 1:1 into both arms of the trial, while the second line patients will all receive HA-Irinotecan plus carboplatin. The primary endpoints for this clinical trial are (i) incidence of grade 3 and 4 toxicity and (ii) tumor stem cell burden during and at the conclusion of the clinical trial treatment. Secondary endpoints include cumulative dose of irinotecan, PFS at six months, objective response rates, quality of life and survival. A total of approximately 40 patients are to be recruited to the trial, which is being conducted at Monash Cancer Centre, Southern Health and Peninsula Oncology Centre in Victoria, Australia. This trial was initiated under the Australian Therapeutic Goods Administration, Clinical Trials Notification Scheme. Our participation in this investigator-sponsored trial has the potential to further validate the HyACT technology as well as to provide clinical data on cancer stem cells. As this is an investigator-sponsored trial, our involvement in the trial has been limited. We currently do not intend to pursue further clinical trials or commercialization of HA-Irinotecan for the treatment of SCLC and, accordingly, references to our “product candidates” do not include HA-Irinotecan for the treatment of SCLC.
Preclinical studies have demonstrated that HA-Irinotecan, either in combination with other chemotherapeutics or alone, can target and kill the SCLC stem cells, which are believed to often contribute to recurring disease and ultimately death of the patient; therefore, if the preclinical findings translate into the clinical setting, it is possible that durable clinical responses may be observed. If this trial provides clinical data demonstrating that HA-Irinotecan is more effective at removing cancer stem cells than irinotecan alone, this finding could support our research on mode of action and could significantly expand the types of cancers where HyACT may provide a superior benefit.
Other HyACT Programs
We used HyACT-targeted 5-fluorouracil in a Phase I / IIa clinical trial in 13 patients with mCRC. Nine patients received HyACT-targeted 5-fluorouracil, with three of these patients recording stable disease. Another five patients received leucovorin and HyACT-targeted 5-fluorouracil, with two patients recording stable disease. In this study, no unexpected toxicity was observed, suggesting an ability to safely combine HA with a clinically active dose of 5-fluorouracil.
We used HyACT-targeted doxorubicin in a Phase I / IIa clinical trial in 16 patients with metastatic cancer with a life expectancy of at least 12 weeks. Four of 16 patients treated with HA-doxorubicin had a partial tumor response, while another three recorded stable disease. In the trial, no unexpected toxicity was observed, suggesting an ability to safely combine HA with a clinically active dose of doxorubicin.
Preclinical Programs
We have also conducted preclinical studies that have suggested that HyACT has the potential for improving the efficacy of a number of other cancer drugs including gemcitabine (Gemzar), methotrexate (Rheumatrex), carboplatin (Paraplatin) and vinorelbine tartrate (Navelbine). We have also tested in preclinical studies a number of monoclonal antibodies, including bevacizumab (Avastin) and cetuximab (Erbitux), and are investigating the potential of running a parallel clinical trial comparing an HA-monoclonal antibody with the monoclonal antibody alone.
-115-
Table of Contents
The following charts provide additional details of some of the preclinical work we have conducted so far, suggesting the efficacy of various HyACT-targeted anti-cancer drugs compared with the anti-cancer drugs alone, including doxorubicin, methotrexate, 5-fluorouracil, gemcitabine, irinotecan and cetuximab:
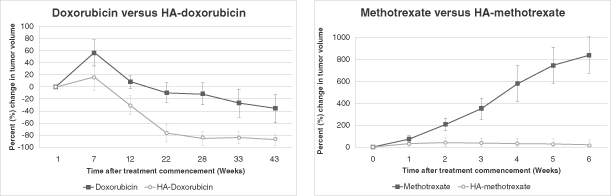
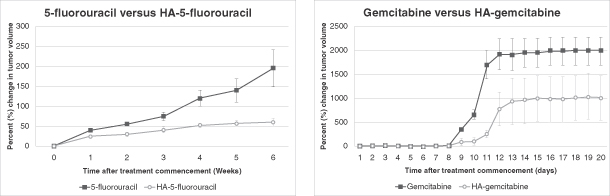
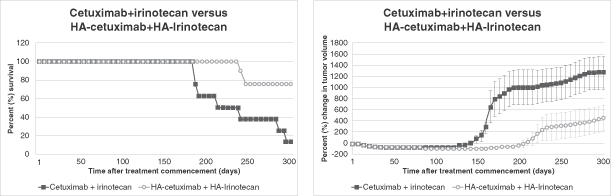
In fiscal 2011, fiscal 2012, the three months ended September 30, 2012 and for the period from inception to September 30, 2012, we have invested approximately $1.5 million, $1.9 million, $0.7 million and $26.1 million, respectively in the research and development of our HyACT technology. Such expenditures have included researching drugs which could be targeted utilizing the HyACT technology and preparing for commercial manufacture of HyACT. We will need to raise substantial additional capital, in addition to the proceeds from this offering, to continue development of our HyACT technology and identification of additional drug candidates. See “Risk Factors — We will require substantial additional funding which may not be available to us on acceptable terms, or at all.”
-116-
Table of Contents
VAST Drug Discovery Technology
In addition to the HyACT platform, we have in-licensed worldwide rights to the VAST small molecule drug discovery technology, which we expect will give us the ability to research and develop new anti-cancer drug candidates. This VAST technology has been deployed by Alchemia Limited in several collaborations with pharmaceutical companies, including Amgen Inc. and Genentech, Inc., and research institutes, including the University of Queensland, the Institute of Molecular Biology, Brisbane, the Monash Institute of Pharmaceutical Sciences, Monash University and the Walter and Eliza Hall Institute, exploring its capability and application in non-cancer therapeutic areas. These collaborations are exploiting the chemistry and molecular diversity offered by the VAST technology and diversity scanning array, or DSA, by addressing known targets in an innovative way. We plan to initiate programs to study inflammation pathways associated with cancer stem cells and cancer metabolism with a target involved in HA synthesis. These programs are intended to make use of the selectivity and compound efficiency of the VAST technology. Our VAST programs are run on a business model designed to limit our cash expenditures through the use of partnerships and, if and when available, government grants. These programs include the allosteric modulation of family B G-protein coupled receptors for chronic obstructive pulmonary disease and type II diabetes, as well as opioid receptor agonists and ion channel inhibitors for pain. The collaboration agreements referred to above that are currently in effect are between Alchemia Limited and various collaborators, and any arrangements for government grants relating to the VAST technology are also between Alchemia Limited and the Australian government. We are not a party to any of these collaboration agreements or government grant arrangements, and Alchemia Limited may be required to obtain the consent of its collaborators and the Australian government in order to transfer these collaboration agreements and government grant arrangements to us. If these consents are not granted, it may have a material adverse effect on our ability to exploit the VAST technology. See “Risk Factors — Alchemia Limited may be unable to transfer its rights under collaboration agreements and government grant arrangements relating to the VAST technology to us.”
We aim to partner with pharmaceutical and biotechnology companies in areas outside of oncology to validate and commercialize various programs employing the VAST technology in new drug development while progressing certain projects internally. However, the terms of our license agreement with Alchemia Limited provide that we may not sub-license this technology without Alchemia Limited’s consent, not to be unreasonably withheld. This limitation on sub-licensing may prevent us from entering into collaborations or similar arrangements or otherwise commercializing VAST technology.
We have in-licensed rights to the VAST drug discovery technology from our parent, Alchemia Limited. In fiscal 2011 and fiscal 2012, Alchemia Limited invested approximately $0.5 million and $1.1 million, respectively, and in the three months ended September 30, 2012, we invested approximately $0.3 million, in the VAST drug discovery technology, mainly for proprietary programs screening potential drug candidates. We will need to raise substantial additional capital, in addition to the proceeds from this offering, to validate the VAST drug discovery technology through the screening of proprietary and third party targets. See “Risk Factors — We will require substantial additional funding which may not be available to us on acceptable terms, or at all.”
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technology, clinical development experience and scientific knowledge provide us with competitive advantages, we face competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with existing treatment regimens and new treatment regimens that may become available in the future.
Many of our competitors have significantly greater financial and other resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals
-117-
Table of Contents
and marketing approved products than we do. It is possible that competitors may develop new products in our target market before we are able to develop such products. There are a number of companies that carry out some form of competitive activities. Competitive activities could include developing reformulations of irinotecan and other anti-cancer drugs for use in oncology applications, exploiting HA as a therapeutic, researching and developing product candidates from applied research on cancer stem cells, and conducting late stage clinical development for application in colorectal cancer. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Our lead product candidate is HA-Irinotecan for the treatment of metastatic colorectal cancer, or mCRC, and the following table shows potential competitors with drugs approved or in late stage clinical development for mCRC as of November 1, 2012:
| Company |
Drug Name |
Stage of Development | ||
| Bayer-Schering |
regorafenib |
Approved | ||
| Sanofi/Regeneron |
aflibercept | Approved | ||
| Light Sciences Oncology |
talaporfin sodium | Phase III | ||
| Nektar Therapeutics |
PEG-irinotecan | Phase II |
We are aware of a potential competitor, Nektar Therapeutics, or Nektar, which is developing a suite of new chemical entities with modified pharmacokinetic profiles through the pegylation of known therapeutic agents. For instance, Nektar’s product candidate is a pegylated version of irinotecan that is being assessed in a variety of cancers, including a Phase III clinical trial in metastatic breast cancer and two Phase II clinical trials in metastatic colorectal cancer and platinum resistant ovarian cancer. In addition, Merrimack Pharmaceuticals, Inc., or Merrimack, is developing a new formulation of irinotecan for application in pancreatic cancer. Having completed a Phase II clinical trial in May 2011, Merrimack recently initiated a Phase III clinical trial for the same indication. Merrimack also has a Phase II clinical trial and two Phase I clinical trials in oncology indications ongoing. Companies working on cancer stem cells include Boston Biomedical, Inc. (acquired by Dainippon Sumitomo Pharma Co., Ltd.), OncoMed Pharmaceuticals, Inc., Stemline Therapeutics, Inc. and Verastem, Inc.
The VAST technology is a chemistry platform that provides innovative pyranose compounds for drug discovery, with competition anticipated from other drug discovery companies with novel chemistries. For instance, we are aware of companies, including Ensemble Therapeutics, Summit PLC and Tranzyme Pharma, that all have innovative chemistry platforms. Summit PLC has a small carbohydrate library based on imino sugar scaffolds which offer unique activities predominantly in carbohydrate modification pathways. Ensemble Therapeutics and Tranzyme Pharma use macrocyclic technology to position functionality on larger molecules accessing new chemical space between small molecules, such as VAST compounds, and biologics, such as antibodies. Other competitors include companies with an interest in cancer stem cells, mentioned above, and other HA-based companies.
For additional information on competition that we currently face and may face in the future, see “Risk Factors — If our competitors develop and market products that are more effective, safer or less expensive than our product candidates, our commercial opportunities will be negatively impacted. In addition, all of our current product candidates are combinations of HA and existing cancer drugs and will likely face continued competition from those existing drugs used alone and in alternative treatment regimens.”
VAST License Agreement
In August 2012, our Australian subsidiary, Audeo Discovery Pty Limited, entered into a Technology License Agreement with Alchemia Limited. The license agreement provides us with a non-transferable, worldwide license to use Alchemia Limited’s VAST technology, including related patents, patent applications and know-how. The license is exclusive except in relation to one family of patents in respect of which there is an
-118-
Table of Contents
existing non-exclusive third party license. Under the Technology License Agreement, we are subject to development obligations, reporting obligations, sublicense revenue sharing requirements, royalty payments and other obligations. In particular, we are obligated to pay Alchemia Limited quarterly royalty payments equal to 5% of the net revenues received by us or on our behalf in each fiscal quarter in respect of sales of any products or services based on, incorporating or made utilizing the VAST technology, or sub-licensing the VAST technology. In that regard, the license agreement does not permit us to sub-license any of the VAST technology without Alchemia Limited’s prior consent, which may not be unreasonably withheld.
The agreement requires us to deploy staff with relevant scientific qualifications occupying a minimum of four full-time equivalent positions to develop and exploit the VAST technology and to expend a minimum of AUD$500,000 per annum (with a target of expending AUD$1.5 million per annum) on developing, marketing and exploiting the VAST technology and otherwise using our best endeavors to exploit the technology. Failure to meet the minimum expenditure requirement may give rise to a termination right on the part of Alchemia Limited, as discussed below. If we fail to meet the targeted amount of expenditure of AUD$1.5 million per annum, Alchemia Limited will have the right to require that we consult with them on changes to our program for developing, marketing and exploiting the VAST technology to reach this annual target amount and, in that case, we will be required to comply with any commercially reasonable requests of Alchemia Limited regarding any required changes to that program. The agreement will be subject to termination by either party in the event of: (a) bankruptcy, insolvency, receivership or similar events relating to the other party; (b) failure by the other party to pay any amount due under the agreement for 20 business days after notice requiring payment; (c) a material breach of the agreement by the other party which is not capable of remedy; or (d) a breach of the agreement by the other party which is capable of remedy but is not remedied within 20 business days after notice requiring the breach to be remedied. Alchemia Limited may also terminate the agreement if it is prevented from continuing to license the VAST technology to us due to a third party claim of intellectual property infringement (after legal advice, investigation and consultation with us). The license agreement will continue until terminated in accordance with its terms.
Under the license agreement, Alchemia Limited, after consultation with us, may initially determine whether or not to take any action against a third party that is or may be infringing the VAST technology or to defend the validity of the VAST technology. However, Alchemia Limited is under no obligation to take any such action and, if it chooses to take no action, we will have a right, at our own expense, to take action on behalf of Alchemia Limited. In addition, we are obligated to file, prosecute and maintain the patents and patent applications relating to the VAST technology at our own cost and expense, and we are entitled to make decisions relating to the prosecution and maintenance of the patents and patent applications, subject to consultation with Alchemia Limited.
See “Risk Factors — If we breach any of the agreements under which we license rights to patents or other intellectual property from third parties, we could lose license rights that are important to our business” and “We will not have the right to sub-license the VAST small molecule drug discovery technology without Alchemia Limited’s consent, which may prevent us from commercializing that technology.”
Government Regulations
Government authorities in the United States (including federal, state and local authorities) and in other countries extensively regulate the manufacture, research, clinical development, labeling, packaging, distribution, post-approval monitoring and reporting, advertising, promotion, export and import of pharmaceutical products, such as those we are developing. The process of obtaining and maintaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources.
United States
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FFDCA, and related regulations. Drugs are also subject to other federal, state and local statutes and regulations. Failure to
-119-
Table of Contents
comply with the applicable U.S. regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an Institutional Review Board, or IRB, of a clinical hold on trials, the FDA’s refusal to approve pending applications or supplemental applications, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of product or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
The Investigational New Drug Process
An Investigational New Drug application, or an IND, is a request for authorization from the FDA to administer an investigational drug to humans. Such authorization must be secured before commencing clinical trials of any new drug candidate in humans.
The central focus of the initial IND submission is on the general investigational plan and the protocol(s) for human studies. The IND also includes results of laboratory and animal studies, as well as manufacturing information, analytical data and any available clinical data or literature to support the use of the investigational new drug in humans. An IND must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after the receipt by the FDA, unless before that time the FDA raises concerns or questions related to the proposed clinical trials as outlined in the IND. In such a case, the IND is placed on clinical hold until any outstanding concerns or questions are resolved.
Clinical trials involve the administration of the investigational drug to subjects or patients under the supervision of qualified investigators in accordance with Good Clinical Practices, or GCPs. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trials and the parameters to be used in monitoring safety and efficacy criteria. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. Additionally, approval must also be obtained from each clinical site’s IRB before the trials may be initiated. All participants in clinical trials must provide their informed consent in writing prior to their enrollment in the trial.
The clinical investigation of an investigational drug is generally divided into three phases. Although the phases are usually conducted sequentially, they may overlap or be combined. The three phases of an investigation are as follows:
| • | Phase I: Phase I involves the initial introduction of an investigational drug into humans. Phase I clinical trials are typically closely monitored and may be conducted in patients with the target disease or condition or healthy volunteers. These studies are designed to evaluate the safety, dosage tolerance, metabolism and pharmacological actions of the investigational drug in humans, the side effects associated with increasing doses, and if possible, to gain early evidence of effectiveness. During Phase I clinical trials, sufficient information about the investigational drug’s pharmacokinetic and pharmacologic effects may be obtained to permit the design of well-controlled and scientifically valid Phase II clinical trials. |
| • | Phase II: Phase II clinical trials are conducted to preliminarily or further evaluate the effectiveness of the investigational drug for a particular indication in patients with the disease or condition under study, to determine dosage tolerance and optimal dosage, and to identify possible adverse side effects and safety risks associated with the drug. Phase II clinical trials are typically well controlled, closely monitored and conducted in a limited patient population, usually involving no more than several hundred participants. |
| • | Phase III: Phase III clinical trials are conducted in an expanded patient population generally at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting effectiveness of the drug has been obtained and are intended to further evaluate dosage, effectiveness |
-120-
Table of Contents
| and safety, to establish the overall benefit-risk relationship of the investigational drug, and to provide a scientific and statistical basis for product approval by the FDA. |
The decision to terminate a study of an investigational drug may be made by us, the FDA or an Institutional Review Board. Additionally, some trials are overseen by an independent group of qualified experts organized by the trial sponsor, known as a data safety monitoring board or committee. This group provides a recommendation of whether or not a trial may move forward at pre-specified check points based on access that only the group maintains to available data from the trial. Suspension or termination of development during any phase of clinical trials can occur if it is determined that the participants or patients are being exposed to an unacceptable health risk. We may decide to suspend or terminate development based on evolving business objectives and/or the competitive climate.
In addition, there are regulations that require the posting of ongoing clinical trials on public registries and the disclosure of designated clinical trial results and related payments to healthcare professionals.
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational drug information is submitted to the FDA in the form of a New Drug Application, or NDA, requesting approval to market the product for one or more indications.
In most cases, the FDA requires at least two adequate and well-controlled Phase III clinical trials to support marketing approval. However, in certain cases such as oncology where there is a significant unmet medical need, evidence from a single Phase III clinical trial may be sufficient. A single trial may also be sufficient in cases where a randomized, double-blind, multicenter study provides highly reliable and statistically strong evidence of an important clinical benefit, and where confirmation of the result in a second trial would be practically or ethically impossible.
The NDA Approval Process
In order to obtain approval to market a drug in the United States, a marketing application must be submitted to the FDA that provides data establishing substantial evidence of the safety and effectiveness of the investigational drug for the proposed indication to the FDA’s satisfaction. The application includes all relevant data available from pertinent preclinical and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or from a number of alternative sources, including studies initiated by investigators.
The FDA will initially review the NDA for completeness before it accepts the NDA for filing. The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. After the NDA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with current Good Manufacturing Practices, or cGMP, to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Based on Phase III clinical trial results submitted in an NDA, the FDA may assign the application a priority review designation, which sets the target date for FDA action on the application at six months. Priority review is given where preliminary estimates indicate that a product, if approved, has the potential to provide a safe and
-121-
Table of Contents
effective therapy where no satisfactory alternative therapy exists, or a significant improvement compared with marketed products is possible. If criteria are not met for priority review, the standard FDA review period is ten months. Priority review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval.
Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCPs. If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
Section 505(b)(2) New Drug Applications
Section 505(b)(2) of the FFDCA permits the filing of an NDA where at least some of the information required for approval comes from prior studies used to support approval of a currently marketed drug which were not conducted by or for the applicant and for which the applicant has not obtained a right of reference. If the 505(b)(2) applicant can establish that reliance on the FDA’s previous approval of a drug (the reference drug) is scientifically appropriate, it may eliminate the need to conduct certain preclinical or clinical studies of a new formulation of that reference drug. The FDA will generally require a company to perform additional studies to support approval of the new formulation or change from the reference drug. The FDA may then approve the new formulated drug candidate for all or some of the label indications for which the referenced drug has been approved, as well as for new indications sought by the 505(b)(2) applicant. We intend to utilize the 505(b)(2) regulatory pathway in seeking FDA approval of our HA-Irinotecan product candidate for the treatment of metastatic colorectal cancer, or mCRC, and for all of our other HyACT-targeted cancer compounds in development.
The testing and approval process for a drug requires substantial time, effort and financial resources and this process may take several years to complete. Data obtained from the clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to develop our HA-Irinotecan for the treatment of mCRC or any other HyACT-targeted cancer compound and secure necessary governmental approvals, which could delay or preclude us from marketing our products. Even if the FDA approves HA-Irinotecan, it may limit the approved indications for use or place other conditions on approval that could restrict commercial application, such as a requirement that we implement special risk management measures through a Risk Evaluation and Mitigation Strategy.
Available Special Regulatory Procedures
Fast-Track Approval
Congress created a pathway to expedite the availability of a drug for seriously ill patients by establishing fast-track authority to FDA. Fast-track designation is available for drugs or biologics that demonstrate the potential to address unmet medical needs for serious or life-threatening conditions.
The sponsor must first request that the FDA designate its drug as a fast-track product. If the FDA concurs, approval may be based on the drug’s effect on a clinical endpoint or on a “surrogate endpoint” that reasonably suggests clinical benefit. Approval may be conditioned on the completion of postmarketing, or Phase IV, clinical studies to verify and describe the drug’s clinical benefit and to resolve remaining uncertainty about the endpoint
-122-
Table of Contents
on the basis of which the product was approved. Approval may also be subject to predissemination review requirements for promotional labeling and advertising, and to a streamlined procedure for withdrawal of approval if, among other reasons, a postmarketing clinical study fails to verify clinical benefit. The FDA may review marketing applications for fast-track products on a rolling basis, i.e., may begin review based on partial submissions.
Benefits of Fast-Track designation include scheduled meetings to seek FDA input into development plans, the option of submitting the NDA in sections rather than all components simultaneously, and the option of requesting evaluation of studies using surrogate endpoints.
Orphan Drug Designation
The FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition that affects fewer than 200,000 individuals in the United States, or if it affects more than 200,000 individuals in the United States and there is no reasonable expectation that the cost of developing and making the drug for this type of disease or condition will be recovered from sales in the United States. In the European Union, the Committee for Orphan Medicinal Products grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than 5 in 10,000 persons in the European Union community. Additionally, designation is granted for products intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in the European Union would be sufficient to justify the necessary investment in developing the drug or biological product.
In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. In addition, if a product and its active ingredients receive the first FDA approval for the indication for which it has orphan designation, the product is entitled to orphan drug exclusivity, which means the FDA may not approve any other application to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan exclusivity.
In the European Union, orphan drug designation also entitles a party to financial incentives such as reduction of fees or fee waivers and 10 years of market exclusivity is granted following drug or biological product approval. This period may be reduced to six years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity or a safer, more effective or otherwise clinically superior product is available.
An application for marketing authorization can be submitted after the application for orphan drug designation has been submitted, while the designation is still pending, but should be submitted prior to the designation application in order to obtain a fee reduction. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
Pediatric Development
In the United States, the FFDCA provides for an additional six months of marketing exclusivity for a drug if reports are filed of investigations studying the use of the drug product in a pediatric population in response to a written request from the FDA. Separate from this potential exclusivity benefit, NDAs must contain data (or a proposal for the collection of such data post-approval) to assess the safety and effectiveness of an investigational drug product for the claimed indications in all relevant pediatric populations in order to support dosing and administration for each pediatric subpopulation for which the drug is safe and effective. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults or full or partial waivers if certain criteria are met. Discussions about pediatric development plans can be discussed with the FDA at any time, but usually occur between the end-of-Phase II meeting and submission of the NDA.
-123-
Table of Contents
For the EMA, a Pediatric Investigation Plan, and/or a request for waiver or deferral, is required for submission prior to submitting a marketing authorization application.
Post-Approval Regulation
After regulatory approval of a drug product is obtained, we would be required to comply with a number of post-approval requirements. For example, as a condition of approval of an NDA, the FDA may require post-marketing testing and clinical trials and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization. Regulatory approval of oncology products often requires that patients in clinical trials be followed for long periods to determine the overall survival benefit of the drug. In addition, if any of our HyACT-targeted cancer product candidates are approved, we would be required to report, among other things, certain adverse reactions and production problems to the FDA, to provide updated safety and efficacy information, and to comply with regulations concerning advertising and promotional labeling for any of our products. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval to assure and preserve the long-term stability of the drug product. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes extensive procedural, substantive and record-keeping requirements. In addition, changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require FDA approval before being implemented. FDA regulations would also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use if our product candidates are approved. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our HyACT-targeted product candidates. Future FDA inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of previously unknown problems with a product or the failure to comply with applicable regulations may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial action that could delay or prohibit further marketing. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures. Also new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our product candidates under development.
Europe/Rest of World Government Regulation
In addition to regulations in the United States, we are and will be subject, either directly or through our distribution partners, to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products, if approved.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have a process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In Europe, for example, a clinical trial application, or CTA, must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed.
-124-
Table of Contents
The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with GCP and other applicable regulatory requirements.
To obtain regulatory approval of an investigational drug under European Union regulatory systems, we must submit a marketing authorization application. This application is similar to the NDA in the United States, with the exception of, among other things, country-specific document requirements. Medicines can be authorized in the European Union by using either the centralized authorization procedure or national authorization procedures. We intend to use the centralized authorization procedure for our HyACT-targeted product candidates.
The EMA implemented the centralized procedure for the approval of human medicines to facilitate marketing authorizations that are valid throughout the European Union. This procedure results in a single marketing authorization granted by the European Commission that is valid across the European Union, as well as Iceland, Liechtenstein and Norway. The centralized procedure is compulsory for human medicines that are: derived from biotechnology processes, such as genetic engineering; contain a new active substance indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative disorders or autoimmune diseases and other immune dysfunctions; and officially designated orphan medicines. The centralized procedure may, subject to certain conditions, also be used for all products at the request of the applicant.
Under the centralized procedure in the European Union, the maximum timeframe for the evaluation of a marketing authorization application is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the Committee for Medicinal Products for Human Use, or CHMP), with adoption of the actual marketing authorization by the European Commission thereafter. Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest from the point of view of therapeutic innovation, defined by three cumulative criteria: the seriousness of the disease to be treated; the absence or insufficiency of an appropriate alternative therapeutic approach; and anticipation of high therapeutic benefit. In this circumstance, EMA ensures that the evaluation for the opinion of the CHMP is completed within 150 days and the opinion issued thereafter.
For other countries outside of the European Union, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, again, the clinical trials are conducted in accordance with GCP and the other applicable regulatory requirements.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension of clinical trials, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Compliance
During all phases of development and after product approval, failure to comply with the applicable regulatory requirements may result in administrative or judicial sanctions. These sanctions could include the following actions by the FDA or other regulatory authorities: imposition of a clinical hold on trials; refusal to approve pending applications; withdrawal of an approval; adverse publicity; warning letters; product recalls; product seizures; total or partial suspension of production or distribution; product detention or refusal to permit the import or export of products; injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
-125-
Table of Contents
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we may obtain regulatory approval. In the United States and other countries, sales of any products for which we may receive regulatory approval for commercial sale will depend in part on the availability of reimbursement from third-party payors. Third-party payors include government health administrative authorities, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product. Third-party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drugs for a particular indication. Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of our products, in addition to the costs required to obtain FDA and other approvals. Our HyACT-targeted products may not be considered medically necessary or cost-effective. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
In 2003, the United States government enacted legislation providing a partial prescription drug benefit for Medicare recipients, which became effective at the beginning of 2006. Government payment for some of the costs of prescription drugs may increase demand for any products for which we receive marketing approval. However, to obtain payments under this program, we would be required to sell products to Medicare recipients through prescription drug plans operating pursuant to this legislation. These plans will likely negotiate discounted prices for our products. Federal, state and local governments, in the United States continue to consider legislation to contain healthcare costs, including the cost of prescription drugs. Future legislation could limit payments for pharmaceuticals such as the drug candidates that we are developing.
Different pricing and reimbursement schemes exist in other countries. In the European Union, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national healthcare systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost-effectiveness of a particular HyACT-targeted product to currently available therapies. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. In the European Union, the downward pressure on healthcare costs in general, particularly prescription drugs, has become very intense. As a result, it is becoming increasingly difficult to introduce and commercialize new products. In addition, in some countries, cross-border imports from low-priced markets exert a commercial pressure on pricing within a country.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate, or decline to provide, coverage and reimbursement. In addition, an increasing emphasis on managed care in the United States has increased and we expect will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more product candidates for which we may receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Other Healthcare Laws and Compliance Requirements
In the United States, our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services (formerly the
-126-
Table of Contents
Health Care Financing Administration), or CMS, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, and state and local governments. For example, sales, marketing and scientific/educational grant programs must comply with the anti-fraud and abuse provisions of the Social Security Act, the False Claims Act, the privacy provisions of the Health Insurance Portability and Accountability Act, or HIPAA, and similar state laws, each as amended. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990 and the Veterans Health Care Act of 1992, each as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. Under the Veterans Health Care Act, or VHCA, drug companies are required to offer certain drugs at a reduced price to a number of federal agencies including U.S. Department of Veteran Affairs and U.S. Department of Defense, the Public Health Service and certain private Public Health Service designated entities in order to participate in other federal funding programs including Medicare and Medicaid. Recent legislative changes purport to require that discounted prices be offered for certain U.S. Department of Defense purchases for its TRICARE program via a rebate system. Participation under the VHCA requires submission of pricing data and calculation of discounts and rebates pursuant to complex statutory formulas, as well as the entry into government procurement contracts governed by the Federal Acquisition Regulations.
In order to distribute products commercially, we must comply with state laws that require the registration of manufacturers and wholesale distributors of pharmaceutical products in a state, including, in certain states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities or register their sales representatives, as well as prohibiting pharmacies and other healthcare entities from providing certain physician prescribing data to pharmaceutical companies for use in sales and marketing, and prohibiting certain other sales and marketing practices. All of our activities are potentially subject to federal and state consumer protection and unfair competition laws.
New Legislation and Regulations
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the testing, approval, manufacturing and marketing of products regulated by the FDA. Recent legislation mandates that manufacturers of branded prescription drugs pay an annual fee based on the sales activity of the manufacturer. Federal agencies will monitor and report the sales of pharmaceutical manufacturers in different sectors of the industry and the annual fees for each manufacturer will be based, in part, on the industry sector as well as their market share within that sector. We cannot predict how this annual fee may impact our future operating results or financial condition in the event that any of our product candidates receives regulatory approval. In addition to new legislation, FDA regulations and policies are often revised or interpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether further legislative changes will be enacted or whether FDA regulations, guidance, policies or interpretations will be changed or what the effect of such changes, if any, may be.
Patents and Proprietary Rights
The proprietary nature of, and protection for, our HyACT and in-licensed VAST technologies, product candidates, processes and know-how are vital to our business. Our success depends in significant part on our ability to protect the proprietary nature of our product candidates, technology, in-licensed technology and know-how, to operate without infringing on the proprietary rights of others, and to prevent others from infringing our
-127-
Table of Contents
proprietary rights. We have sought and plan to continue to seek patent protection in the United States and select foreign countries for our proprietary and in-licensed technologies. As of November 6, 2012, we own or have in-licensed approximately 46 pending patent applications, 107 issued patents and one allowed patent, including 6 pending patent applications, 18 issued patents and one allowed patent in the United States, and 40 pending patent applications and 89 issued patents outside of the United States. Our policy is to patent the technology, inventions and improvements that we consider important to the development of our business, but only in those cases where we believe that the costs of obtaining patent protection are justified by the commercial potential of the technology, and typically only in those jurisdictions that we believe present significant commercial opportunities. We also rely on trade secrets, know-how, our VAST in-license and continuing innovation to develop and maintain our competitive position. We cannot be sure that patents will be granted with respect to any of our pending patent applications, any patent applications filed by us in the future or any pending or future patent applications that have been or may be filed with respect to the VAST technology, nor can we be sure that any of our existing patents, any patents granted to us in the future or any patents in-licensed to us or that may be in-licensed to us in the future will be commercially useful in protecting our technology. In addition, we have patents and pending patent applications relating to the HyACT technology in the United States and a limited number of other jurisdictions, and we have in-licensed patents and pending patent applications relating to the VAST technology in the United States and a limited number of other jurisdictions, and, as a result, there are a large number of jurisdictions, including certain major markets, where we do not own or in-license any patents or pending patent applications.
Our owned and in-licensed patents extend for varying periods according to the date of patent filing or grant and the legal term of patents in the various countries where patent protection is obtained. Our material owned and in-licensed patents relate to the VAST and HyACT technologies. Our patents relating to the HyACT technologies will expire, excluding possible patent extensions under the Hatch-Waxman Act (as defined below), on various dates between 2014 and 2026. The material patents relating to the VAST technology that we have in-licensed will expire, excluding possible patent extensions under the Hatch-Waxman Act, on various dates between 2017 and 2028. Several of these patents will have potential patent term adjustments and extensions, depending on the length of time required to conduct clinical trials and obtain regulatory approval.
The patent positions of biotechnology firms like us are generally uncertain and involve complex legal, scientific and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued. Consequently, we do not know whether any of the technologies covered by the patent applications that we own or in-license will gain patent protection or, if any patents are issued, whether they will provide significant proprietary protection or will be challenged, circumvented or invalidated. Because patent applications in the United States and certain other jurisdictions are maintained in secrecy for 18 months,
and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications that we own or in-license. Moreover, we may have to participate in interference proceedings called by the U.S. Patent and Trademark Office, or PTO, or a foreign patent office to determine priority of invention, or in opposition proceedings in a foreign patent office, either of which could result in substantial cost to us, even if the eventual outcome is favorable to us, or may result in an unfavorable outcome. There can be no assurance that current or, if issued, new patents would be held valid by a court of competent jurisdiction. An adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties for significant royalty payments or require us to cease using specific compounds or technology.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we or, in the case of technology we have in-licensed, our licensor has filed, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent’s term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the PTO in granting a patent, or may be shortened if a patent is terminally disclaimed over another patent.
-128-
Table of Contents
The patent term of a patent that covers an FDA-approved drug may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and other non-U.S. jurisdictions to extend the term of a patent that covers an approved drug. In the future, if and when our pharmaceutical product candidates receive FDA approval, we may apply for patent term extensions on patents covering those products.
To protect our rights to any of our issued patents and proprietary information, we may need to litigate against infringing third parties, or avail ourselves of the courts or participate in hearings to determine the scope and validity of those patents or other proprietary rights. In the case of any patents and proprietary information that we have in-licensed, we may not have the right to litigate against infringing parties except under limited circumstances specified in the applicable license agreement (which may include, for example, failure by the licensor to litigate against infringing parties), and we therefore may be entirely or primarily dependent upon proceedings instituted by the licensor to protect the relevant intellectual property. In those cases, the licensor may elect not to pursue infringement proceedings, which means that we may be unable to prevent third parties from utilizing the technology that we have in-licensed; likewise, a licensor may not devote its full resources to the defense of the relevant intellectual property, which may result in an adverse decision in any infringement proceedings. In addition, these types of proceedings are often costly and could be very time-consuming to us, and we cannot assure you that the deciding authorities will rule in our favor. An unfavorable decision could allow third parties to use technology that we own or in-license without being required to pay us licensing fees, may compel us to pay substantial royalties to license needed technologies to avoid infringing third-party patent and proprietary rights or may require that we cease using certain products or technology, which may prevent us from using or producing certain product candidates or products for which we may receive regulatory approval. Such a decision could even result in the invalidation or a limitation in the scope of patents that we own or in-license or forfeiture of the rights associated with those patents or pending patent applications.
Under the license agreement pursuant to which we in-license the VAST technology from Alchemia Limited, Alchemia Limited, after consultation with us, may initially determine whether or not to take any action against a third party that is or may be infringing the VAST technology or to defend the validity of the VAST technology. However, Alchemia Limited is under no obligation to take any such action and, if it chooses to take no action, we will have a right, at our own expense, to take action on behalf of Alchemia Limited. In addition, we are obligated to file, prosecute and maintain the patents and patent applications relating to the VAST technology at our own cost and expense, and we are entitled to make decisions relating to the prosecution and maintenance of the patents and patent applications, subject to consultation with Alchemia Limited.
We also rely on trade secret protection for our confidential and proprietary information. No assurance can be given that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technology, or that we can meaningfully protect our trade secrets.
It is our policy to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. There can be no assurance, however, that these agreements will provide meaningful protection for our trade secrets or adequate remedies in the event of unauthorized use or disclosure of such information.
For additional information, see “Risk Factors — Risks Related to Intellectual Property.”
-129-
Table of Contents
Manufacturing
We do not own or operate any manufacturing facilities. We currently contract with third parties for the manufacture of our product candidates for preclinical studies and clinical trials and intend to do so in the future. The HA-Irinotecan for our Phase III and Phase II clinical trials is in the form of a kit, which is assembled by Almac Clinical Services Limited and distributed to trial sites as required. The kit contains the following components:
| • | Camptosar-branded irinotecan hydrochloride, manufactured by, and sourced from, Pfizer Australia Pty Ltd.; |
| • | 1% sodium hyaluronate solution in water for injection. This product is manufactured for us by Gland Pharma Ltd (India) using sodium hyaluronate from htl Biotechnology S.A.S. (France); |
| • | 5% dextrose solution commercially sourced from Baxter Healthcare Ltd (UK); and |
| • | Consumables for preparation of solutions for infusion and conduct of the trial. |
The hospitals utilize the kits to prepare bags containing HA-Irinotecan or irinotecan alone, which are used by healthcare staff for the pivotal Phase III clinical trial.
Except for the agreement with Novozymes referred to below, we have not entered into long-term agreements with contract manufacturers. We currently obtain our supplies of the components of our product candidates and the finished drug product through individual purchase orders. We currently have no plans to build our own clinical or commercial-scale manufacturing capabilities. If HA-Irinotecan for treatment of mCRC receives regulatory approval following completion of our Phase III clinical trial, it is anticipated that the irinotecan hydrochloride, sodium hyaluronate and 5% dextrose solution will be centrally compounded by alternate suppliers, and presented to hospitals and prescribers ready for use, with no requirement for compounding at hospitals.
We entered into two agreements with Novozymes Biopharma DK A/S, or Novozymes, in July 2009. The first agreement is a supply agreement whereby we agree to use Novozymes as our exclusive supplier of HA for our HA-Irinotecan-based product and Novozymes agrees to supply us with HA and to use commercially reasonable endeavors to build and maintain sufficient manufacturing capacity to reliably supply us with HA, including the construction of a new HA production facility. Novozymes does not currently have the manufacturing capacity to supply HA in accordance with the agreement, and although the agreement provides that Novozymes use commercially reasonable endeavors to achieve such sufficient manufacturing capacity by no later than January 1, 2014, we cannot assure you that this will occur. Assuming that Novozymes is able to construct a manufacturing facility as contemplated by the agreement, the agreement calls for us to provide Novozymes with a rolling three year estimate of our annual need of HA. We are not obligated to forecast any minimum estimate. Upon acceptance of our forecast by Novozymes, we and Novozymes will each be obligated to purchase and supply, respectively, 100% of the total forecasted quantities for the first year based on an agreed scheme adjusted for price index changes. The forecast for the two remaining years is non-binding. Novozymes agrees that the price at which it supplies HA to us is to be the best price it offers to any of its customers for HA of a reasonably similar quality and volume. The supply agreement will remain in effect for the later of seven years from its commencement or five years after the date when Novozymes begins supplying us with HA. At the end of such term and any further term, the supply agreement will automatically extend for a further five years unless either party has given notice not to extend it. Either party may terminate the supply agreement upon a breach of the agreement by the other party, upon certain insolvency events relating to the other party or if it becomes unlawful for the other party to perform under the agreement.
In the related royalty agreement between us and Novozymes, for as long as the relevant product remains covered by a HyACT patent or we receive any royalties from a licensee of the product, we are committed to pay
-130-
Table of Contents
Novozymes a 1.0% royalty on the net sales from any HA-Irinotecan product and a 0.5% royalty on net sales of any other product containing HA developed under the HyACT patents, regardless of whether Novozymes is able to supply HA to us. If Novozymes is capable of supplying HA to our specifications and we do not use them as our supplier for HA, we have to pay a 2.0% royalty on our net sales from any HA-Irinotecan product and a 1.0% royalty on our net sales of any other product containing HA, provided that such royalties will be reduced to 1.0% for HA-Irinotecan product and 0.5% for any other products containing HA if Novozymes is the defaulting party under the supply agreement or if it fails to complete the new HA production facility contemplated in the supply agreement. In the event that we license any other product containing HA to a licensee and the royalty we receive from the licensee is less than 10.0% of the net sales of such product and development for such product has not commenced a Phase II trial at the time the license is entered into, the royalty payable to Novozymes under the royalty agreement will be 5.0% of the percentage of the net sales we receive. We must comply with the license agreement whether or not Novozymes is able to supply HA to us under the supply agreement. Subject to certain termination events, including breach of the agreement by the other party, certain insolvency events relating to the other party or if it becomes unlawful for the other party to perform under the agreement, this agreement will remain in effect until the expiry of our last HyACT patent, which, as of November 1, 2012, is in 2026.
To date, our third-party manufacturers have met our manufacturing requirements. We expect third-party manufacturers to be capable of providing sufficient quantities of our product candidates to meet anticipated commercial demands, if any of our product candidates is approved. We believe that there are alternate sources of supply that can satisfy our clinical and, if applicable, commercial requirements, although we cannot be certain that identifying and establishing relationships with such sources, if necessary, would not result in significant delay or material additional costs. If we are unable to arrange for such a third-party manufacturing source, or fail to do so on commercially reasonable terms, we may not be able to continue clinical trials or, if any of our product candidates receives regulatory approval, successfully produce and market our products.
Commercialization
Our operations to date have been primarily limited to organizing and staffing our company, taking steps to effect the Demerger and related transactions, acquiring, developing and securing our technology, undertaking preclinical studies and clinical trials of our product candidates and engaging in research and development. We have not yet demonstrated an ability to obtain regulatory approval, formulate and manufacture commercial-scale products, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, it is difficult to predict our future success and the viability of any commercial programs that we may choose to take forward if any of our product candidates receives regulatory approval.
We do not have any internal sales, marketing or distribution infrastructure. In the event any of our product candidates receives regulatory approval, we intend, where appropriate, to pursue commercialization relationships with pharmaceutical companies and other strategic partners providing for our products to be distributed through their sales and marketing organizations in order to gain access to global oncology markets. Over the longer term, we may ultimately build an internal commercial infrastructure to directly market and sell any HyACT-targeted drugs that may receive regulatory approval.
Research and Development
A significant portion of our operating expenses is related to research and development and we intend to maintain our strong commitment to research and development. Our research and development expenses totaled approximately $7.0 million in fiscal 2011, approximately $7.8 million in fiscal 2012 and approximately $4.0 million for the three months ended September 30, 2012.
-131-
Table of Contents
Employees
As of September 30, 2012, we had seventeen full-time employees. We also make use of independent contractors on a regular basis. None of our employees is represented by labor unions or covered by collective bargaining agreements. We consider our relationship with our employees to be good.
Facilities
Our principal executive offices are located in San Francisco, California, where we lease approximately 300 square feet of office space under a lease that expires in June 2013 and that is subject to a three month renewal at our option. We also lease approximately 25,000 square feet of office and lab space in Brisbane, Australia under a lease that will expire in July 2016 and we have access to approximately 3,500 square feet of lab space in Melbourne, Australia under a research and development agreement with Monash University that we first entered into in April 2004 and that is subject to annual renewal by both parties; the current term of this agreement expires in December 2012. We believe that our facilities are sufficient to meet our current needs and that suitable additional space will be available as and when needed.
Legal Proceedings
We are not currently a party to any material legal proceedings.
-132-
Table of Contents
Executive Officers and Directors
The following table sets forth the names, ages and positions of our executive officers and directors as of November 1, 2012:
| Name |
Age | Position | ||
| Executive Officers |
||||
| Peter Smith, PhD. |
49 | President, Chief Executive Officer and Director | ||
| Charles Walker, MBA |
41 | Chief Financial Officer and Secretary | ||
| Tracey Brown, PhD |
49 | Chief Scientific Officer, Vice President of Oncology | ||
| Wim Meutermans, PhD |
49 | Vice President of Discovery | ||
| Key Employees |
||||
| Michael West, PhD |
49 | Vice President of Intellectual Property & Technology Transfer | ||
| Non-Employee Directors |
||||
| Stephen Hill, BMBCh, FRCS(1)(2)(3) |
54 | Chairman of the Board | ||
| Leigh Bonney, MBA(1)(2)(3) |
54 | Director | ||
| Susan Kelley, MD(1)(2)(3) |
58 | Director | ||
| Tracie Ramsdale, PhD |
50 | Director | ||
| David U’Prichard, PhD(1)(2)(3) |
64 | Director | ||
| (1) | Member of the Audit Committee. |
| (2) | Member of the Compensation Committee. |
| (3) | Member of the Nominating and Corporate Governance Committee. |
Executive Officers
Peter Smith, PhD, President, Chief Executive Officer and Director: Peter Smith has served as our President and Chief Executive Officer and as a director since June 2012. Dr. Smith has served as Chief Executive Officer of Alchemia Limited since April 2007 and will continue in that position for up to six months after completion of this offering. Dr. Smith previously served as Head of Commercialisation and Business Development at Alchemia Limited from 2006 to April 2007. Prior to joining Alchemia Limited, Dr. Smith was Chief Executive Officer and Managing Director of Amrad Limited, a biotechnology company, from 2003 to 2005 (renamed Zenyth Therapeutics Limited and subsequently acquired by CSL Limited in 2006) and created the spin-out anti-infectives company Avexa in 2004. In 1997, Dr. Smith founded UK biotech company Onyvax and served as the Chief Financial Officer of Onyvax from 1997 through 2001. Prior to that, he was a pharmaceutical research analyst at HSBC and UBS from 1988 to 1996. Dr. Smith holds a PhD in Biochemistry and a MA in Natural Sciences from Cambridge University in the UK.
Dr. Smith was selected to serve on our board of directors based on his historic knowledge of our company, the continuity he provides on our board of directors, his strategic vision for our company and his background in the biopharmaceutical industry.
Charles Walker, MBA, Chief Financial Officer and Secretary: Charles Walker has served as our Chief Financial Officer and Secretary since June 2012. Mr. Walker has served as Chief Financial Officer of Alchemia Limited since March 2011 and will continue in that position for up to six months after completion of this offering. Mr. Walker joined Alchemia Limited from RedFlow Limited, where he worked first as a consultant and then as Head of Corporate Development between July 2010 and March 2011. Prior to that, Mr. Walker spent over 10 years as an investment banker at WestLB Panmure Limited, Code Securities Limited and Nomura Code
-133-
Table of Contents
Securities Limited. Mr. Walker co-founded Code Securities Limited in London in 2003, which was subsequently sold to Nomura International PLC in 2005. Prior to investment banking, Mr. Walker consulted for CombiChem, Inc., a combinatorial chemistry company, from September 1998 to September 1999 and began his career working for an international pharmaceutical consultancy, CONNECT Pharma Limited, where he worked from 1994 to 1997. Mr. Walker has a BSc (Hons) in Pharmacology from the University of Bristol and an MBA from Warwick University.
Tracey Brown, PhD, Chief Scientific Officer, Vice President of Oncology: Associate Professor Tracey Brown has served as our Chief Scientific Officer and Vice President of Oncology since June 2012. Associate Professor Brown previously served as Chief Scientific Officer and Vice President of Oncology of Alchemia Oncology from 2010 to 2012 and as Vice President of Development of Alchemia Oncology from 2006 to 2010, having joined Alchemia Limited as a result of its acquisition of Meditech Research Ltd, where she served as Chief Scientific Officer from 2001 until its acquisition in 2006. Prior to this, Associate Professor Brown worked as the Director of the Hyaluronan Laboratory, Department of Biochemistry and Molecular Biology, Monash University from 1996 to 2001. Associate Professor Brown holds an adjunct position as an Associate Professor in the Department of Biochemistry and Molecular Biology at Monash University. Associate Professor Brown holds a PhD in Medicine and Biochemistry from Monash University, a BSc in Biochemistry and Cell Biology from the Royal Melbourne Institute of Technology, or RMIT, and a DpSc in Biology from RMIT.
Wim Meutermans, PhD, Vice President of Discovery: Dr. Wim Meutermans has served as our Vice President of Discovery since June 2012. Dr. Meutermans joined Alchemia Limited in 2000, where he previously served as Vice President of Drug Discovery from 2007 to 2012, served as Research Director from 2006 to 2007, served Director of Chemistry from 2002 to 2006, and served as Senior Combinatorial Chemist from 2000 to 2002. Prior to this, Dr. Meutermans was employed by The University of Queensland, Australia as Senior Research Officer at the Centre for Drug Design and Development from 1997 to 2000 and was employed as Research Officer at the University of Queensland from 1993 to 1997. Dr. Meutermans obtained a PhD from the Katholieke Universiteit Leuven in Belgium and held postdoctoral positions at Griffith University in 1992, the Spanish National Research Council, known as CSIC, from 1990 to 1991, and the University of Queensland from 1988 to 1990.
Key Employees
Michael West, PhD, Vice President of Intellectual Property & Technology Transfer: Michael West has served as our Vice President of Intellectual Property & Technology Transfer since June 2012. Dr. West joined Alchemia Limited, in December 1997 as its first employee and held positions in research and development as well as intellectual property management until 2012. From 1995 to 1997, Dr. West consulted to a number of companies and organizations and served on the Pharmaceutical Subcommittee, a committee advising the Australian Evaluation Committee to the Australian Therapeutic Goods Administration. Dr. West started his career at GlaxoSmithKline, where he worked from March 1991 to January 1993, and he subsequently worked as a senior postdoctoral fellow at the Centre for Drug Design and Development at the University of Queensland from February 1993 to December 1997. Dr. West holds a PhD in Chemistry from James Cook University, a Masters in Industrial Property from University of Technology, Sydney and a BSc (Hons) in Chemistry from James Cook University.
Non-Employee Directors
Stephen Hill, BMBCh, FRCS, Chairman of the Board: Stephen Hill has served as our chairman of the board of directors since June 2012. Dr. Hill was the President and CEO of 21CB, a nonprofit initiative of UPMC designed to provide the United States government with a domestic solution for its biodefense and infectious disease biologics portfolio, from March 2011 until December 2011. Dr. Hill served as President of Solvay Pharmaceuticals, Inc. from April 2008 until its acquisition by Abbott Laboratories in 2010. Dr. Hill served as President and Chief Executive Officer of biotechnology firm ArQule, Inc. from 1999 to 2008. Prior to his position at ArQule, Dr. Hill was the Head of Global Drug Development at F. Hoffmann-La Roche Ltd. from
-134-
Table of Contents
1997 to 1999. He joined Roche in 1989 as Medical Adviser to Roche Products in the United Kingdom and held several senior positions there that included U.K. Medical Director and Head of International Drug Regulatory Affairs at Roche headquarters in Basel, Switzerland. Dr. Hill has served as chairman of the board of directors of Novelos Therapeutics since September 2007. Dr. Hill is a Fellow of the Royal College of Surgeons of England and holds his scientific and medical degrees from St. Catherine’s College at Oxford University.
Dr. Hill was selected to serve on our board of directors based on his significant scientific expertise in biopharmaceuticals and his extensive experience in a broad range of senior management positions with companies in the life sciences sector.
Leigh Bonney, MBA, Director: Leigh Bonney has served as a member of our board of directors since September 2012. Ms. Bonney has been the Chief Financial Officer at The Leona M. and Harry B. Helmsley Charitable Trust since March 2012. Prior to joining the trust, Ms. Bonney spent 15 years at Pfizer in senior positions in Strategy and Finance before retiring in early 2011. Among the positions she held at Pfizer, Ms. Bonney served as Chief Financial Officer of Pfizer Global Research and Development from 1999 to 2003. Ms. Bonney has also served as Principal at Maple Rock Advisors, LLC since 2011, where she provided strategic advice to multi-national corporations in the healthcare sector. She has been an independent board member for the Eliassen Group, a technology staffing and consulting service firm since 2011. Ms. Bonney has also served on the Board of the Nature Conservancy of Connecticut since 2006 and was Chair of the Board of the Nature Conservancy of Connecticut from 2008 to 2011. As of October 2012, she became the Chair of the Trustee Council of the Nature Conservancy, which represents trustees from all of the Conservancy’s US and international chapters. Ms. Bonney served on the Board of the Big Apple Circus, a non-profit performing arts organization with extensive community programs, from 1999 to 2011; she was president of the board from 2006 to 2011. She was a board member of Phillips Exeter Academy for 14 years, from 1994 to 1998 and from 2000 to 2010, serving as Chair of the Finance Committee and a member of the Executive Committee. She also chaired the search committee for the 14th Principal of Exeter in 2008. Ms. Bonney holds a BA in Latin American Studies from Yale University, an MA in Latin American Studies from Stanford University, and an MBA from Harvard.
Ms. Bonney was selected to serve on our board of directors based on her extensive background in the biopharmaceutical industry and her extensive experience in a broad range of advisory and director positions.
Susan Kelley, MD, Director: Dr. Susan Kelley has served as a member of our board of directors since September 2012. Dr. Kelley has also served on the board of directors of ArQule, Inc. since April 2011. From 2001 to 2008, Dr. Kelley was employed by Bayer Healthcare Pharmaceuticals and Bayer-Schering Pharma in Germany and the United States, serving as Vice President, Global Strategic Drug Development, Cancer and Metabolics from April 2001 to May 2002 and from May 2002 until June 2008 as Vice President, Global Clinical Development and Therapeutic Area Head-Oncology. From July 2008 to March 2011, she was Chief Medical Officer of The Multiple Myeloma Research Foundation/Consortium. Most recently, she has been an independent consultant to the pharmaceutical and biotechnology industries in the field of oncology drug development and strategy. Dr. Kelley has an AB in Biology, magna cum laude from Colgate University and an MD from Duke University School of Medicine. She was a fellow in Medical Oncology and Clinical Fellow in Medicine at Dana-Farber Cancer Institute, Harvard Medical School and a Fellow in Medical Oncology and Pharmacology at Yale University School of Medicine where she also served as a Clinical Assistant Professor of Medicine.
Dr. Kelley was selected to serve on our board of directors based on her significant scientific expertise in biopharmaceuticals and biotechnology and her extensive experience in a broad range of senior management positions with companies in the life sciences sector.
Tracie Ramsdale, PhD, Director: Tracie Ramsdale has served as a member of our board of directors since June 2012. Dr. Ramsdale founded Anandabio, a company pursuing research into the treatment of chronic pain, in 2011. Dr. Ramsdale co-founded Alchemia Limited in 1995 and led Alchemia Limited’s development as its General Manager and Chief Executive Officer from 1998 to 2007. From 2007 to 2011, Dr. Ramsdale worked as a biotechnology consultant and advisor to government, academia and industry. Before establishing Alchemia Limited, Dr. Ramsdale
-135-
Table of Contents
was a Principal Investigator and Commercial Manager of the Centre for Drug Design and Development at the University of Queensland (Institute for Molecular Bioscience) from 1994 to 1998. Dr. Ramsdale served as a Non-Executive Director of Incitive Limited, an Australian biotechnology company, from 2007 to 2008 and is a Non-Executive Director of Alchemia Limited, Chairman of its Remuneration Committee and Chairman of its Scientific Advisory Board. Dr. Ramsdale is an adjunct Professor at the School of Chemical and Molecular Biosciences, University of Queensland, a member of the Australian Advisory Council on Intellectual Property and is a Fellow of the Australian Academy of Technological Sciences and Engineering. Dr. Ramsdale holds a PhD in Biochemistry from the University of Queensland, a Master of Pharmacy from The Victorian College of Pharmacy and a Bachelor of Applied Science (Chemistry) from the Royal Melbourne Institute of Technology.
Dr. Ramsdale was selected to serve on our board of directors based on her historic knowledge of our company, the continuity she provides on our board of directors, her strategic vision for our company and her background in the biopharmaceutical industry.
David U’Prichard, PhD, Director: Dr. David U’Prichard has served as a member of our board of directors since September 2012. Dr. U’Prichard has also served on the boards of directors of Cyclacel Pharmaceuticals, Inc., as Chairman, and Life Technologies, Inc. since May 2004 and joined the board of Iroko Pharmaceuticals in 2010. He has been President of Druid Consulting LLC, a pharmaceutical and biotechnology-consulting firm, providing customized services to life sciences clients in the United States and Europe, since 2003, and a founding partner of Druid Bio Ventures LLP since 2009. Dr. U’Prichard is also part-time Chief Scientific Officer of BioMotiv, the operating company arm of The Harrington Discovery and Development Project (Cleveland, OH), a national initiative to accelerate new drug development inspired by physician-scientists, where he has served since 2012. Previously, he was Chief Executive Officer of 3-Dimensional Pharmaceuticals, Inc. from 1999 to 2003. In addition, he held a variety of positions within the pharmaceutical and biotechnology industries, including, President and Chairman of Research and Development for SmithKline Beecham Pharmaceuticals from 1997 to 1999; Executive Vice President and International Research Director, and a Member of the Board of Management of Zeneca Pharmaceuticals from 1994 to 1997; General Manger, Research Department, ICI Pharmaceuticals, from 1991 to 1994 and Vice President Biomedical Research, ICI Pharmaceuticals, from 1986 to 1991; and Senior Vice President and Scientific Director for Nova Pharmaceutical Corporation from 1983 to 1986. He was a director of Alpharma Inc. from 2007 to 2008, Guilford Pharmaceuticals Inc. from 2003 to 2005, Silence Therapeutics plc from 2004 to 2011, Lynx Therapeutics, Inc. from 2001 to 2006 and non-executive Chairman of Oxagen Ltd. from 2004 to 2011. He was a Venture Partner with Red Abbey Venture Partners, private equity providers, from 2005 to 2010. Dr. U’Prichard was Chairman of the Pennsylvania Biotechnology Association from 2004 to 2005, and from 1992 to 1997, he was a member of the board of directors of the Biotechnology Industry Organization (BIO). He received a BSc in Pharmacology from The University of Glasgow and a PhD in Pharmacology from The University of Kansas in 1975.
Dr. U’Prichard was selected to serve on our board of directors based on his significant expertise in biopharmaceuticals and his extensive experience in a broad range of senior management and director positions with companies in the life sciences sector.
Board Composition; Classified Board
Our business affairs are managed under the direction of our board of directors, which is currently composed of six members. Four of our directors are independent within the meaning of the independent director guidelines of The Nasdaq Stock Market. In accordance with our amended and restated certificate of incorporation, which will be in effect upon completion of this offering, our board of directors will be divided into three staggered classes of directors. At each annual meeting of stockholders, a class of directors will be elected for a three-year term to succeed the same class whose terms are then expiring. The terms of the current directors will expire upon the election and qualification of successor directors at the annual meeting of stockholders to be held during the year 2013 for the Class I directors, 2014 for the Class II directors and 2015 for the Class III directors.
| • | Our Class I directors will be Ms. Bonney and Dr. Hill. |
-136-
Table of Contents
| • | Our Class II directors will be Drs. Kelley and Ramsdale. |
| • | Our Class III directors will be Drs. Smith and U’Prichard. |
Our amended and restated certificate of incorporation and bylaws will provide that the number of our directors shall be fixed from time to time by a resolution of our board of directors. Each of our executive officers serves at the discretion of our board of directors and holds office until his or her successor is duly appointed and qualified or until his or her earlier resignation or removal. There are no family relationships among any of our directors or executive officers.
The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change of control. See “Description of Capital Stock — Anti-Takeover Effects of Delaware Law and Our Certificate of Incorporation and Bylaws” and “Risk Factors — Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us more difficult, limit attempts by our stockholders to replace or remove our current management and limit the market price of our common stock” for a discussion of these and other anti-takeover provisions found in our amended and restated certificate of incorporation and bylaws.
Director Independence
In connection with this offering, we intend to list our common stock on The Nasdaq Global Market. Under the rules of The Nasdaq Stock Market, independent directors must comprise a majority of a listed company’s board of directors within a specified period of the completion of an initial public offering. In addition, the rules of The Nasdaq Stock Market require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and corporate governance committees must be independent. Under the rules of The Nasdaq Stock Market, a director is independent only if our board of directors makes an affirmative determination that the director has no material relationship with us.
In September 2012, our board of directors undertook a review of its composition, the composition of its committees and the independence of each director. The determination of independence of members of our board of directors was based upon information requested from and provided by each director concerning his background, employment and affiliations, including family relationships. In making this determination, our board of directors considered the relationships that each non-employee director has with us and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director. Our board of directors has determined that Drs. Hill, Kelley and U’Prichard and Ms. Bonney, representing four of our six directors, are “independent” as that term is defined under the rules of The Nasdaq Stock Market for purposes of serving on our board of directors.
Board Committees
Our board of directors has the authority to appoint committees to perform certain management and administrative functions. Our board of directors has an audit committee, a compensation committee and a nominating and corporate governance committee, each of which has the composition and the responsibilities described below.
Audit Committee
Our audit committee will oversee our accounting and financial reporting process and the audit of our financial statements and assist our board of directors in monitoring our financial systems and our legal and regulatory compliance. Our audit committee will be responsible for, among other things:
| • | appointing, compensating, retaining, overseeing and, where appropriate, replacing our registered public accounting firm, including reviewing and resolving any disagreements between management and the |
-137-
Table of Contents
| independent registered public accounting firm regarding internal control over financial reporting or regarding financial reporting; |
| • | reviewing and approving engagements of the independent registered public accounting firm to render any audit or permissible non-audit services and any associated fees; |
| • | reviewing the qualifications and independence of the independent registered public accounting firm; |
| • | reviewing our financial statements and related disclosures and reviewing our critical accounting policies and practices; |
| • | reviewing and discussing with management the adequacy and effectiveness of our internal control over financial reporting; |
| • | establishing procedures for the receipt, retention and treatment of accounting and auditing related complaints and concerns; |
| • | preparing the audit committee report required by SEC rules to be included in our annual proxy statement; |
| • | reviewing and discussing with management and the independent registered public accounting firm the results of our annual audit, our quarterly financial statements and our publicly filed reports; and |
| • | reviewing and overseeing transactions between us and a related person. |
We intend that the functioning of our audit committee will comply with the applicable requirements of The Nasdaq Stock Market and SEC rules and regulations.
The members of our audit committee are Drs. Hill, Kelley and U’Prichard and Ms. Bonney. Our board of directors has determined that Ms. Bonney is a financial expert as contemplated by the rules of the SEC implementing Section 407 of the Sarbanes Oxley Act of 2002. Ms. Bonney has also been appointed to serve as our audit committee chairman.
In September 2012, our board of directors considered the independence and other characteristics of each member of our audit committee and has concluded that the composition of our audit committee meets the requirements for independence under the current requirements of The Nasdaq Stock Market and SEC rules and regulations. Audit committee members must satisfy additional independence criteria set forth under Rule 10A-3 of the Securities Exchange Act of 1934, as amended. In order to be considered independent for purposes of Rule 10A-3, an audit committee member may not, other than in his capacity as a member of the audit committee, our board of directors or another committee of our board of directors, accept consulting, advisory or other fees from us or be an affiliated person of us. Each of the members of our audit committee is expected to qualify as an independent director pursuant to Rule 10A-3.
Compensation Committee
Our compensation committee will oversee our compensation policies, plans and programs. The compensation committee will be responsible for, among other things:
| • | reviewing and recommending policies, plans and programs relating to compensation and benefits of our directors, officers and employees; |
| • | reviewing and approving compensation and the corporate goals and objectives relevant to compensation of our Chief Executive Officer; |
-138-
Table of Contents
| • | reviewing and approving compensation and corporate goals and objectives relevant to compensation for executive officers other than our Chief Executive Officer; |
| • | evaluating the performance of our Chief Executive Officer and other executive officers in light of established goals and objectives; and |
| • | administering our equity incentive plans for our employees and directors. |
The members of our compensation committee are Drs. Hill, Kelley and U’Prichard and Ms. Bonney. Dr. U’Prichard is the chairman of our compensation committee. In September 2012, our board of directors determined that each member of our compensation committee is independent within the meaning of the independent director guidelines of The Nasdaq Stock Market and it is our intention that the composition and functioning of our compensation committee comply with the applicable requirements of The Nasdaq Stock Market and SEC rules and regulations, as well as Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code.
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee will oversee and assist our board of directors in reviewing and recommending corporate governance policies and nominees for election to our board of directors and its committees. The nominating and corporate governance committee will be responsible for, among other things:
| • | evaluating and making recommendations regarding the organization and governance of our board of directors and its committees and changes to our certificate of incorporation and bylaws and stockholder communications; |
| • | reviewing succession planning for our Chief Executive Officer and other executive officers and evaluating potential successors; |
| • | assessing the performance of board members and making recommendations regarding committee and chair assignments and composition and the size of our board of directors and its committees; |
| • | recommending desired qualifications for board and committee membership and conducting searches for potential members of our board of directors; |
| • | determining the manner in which stockholders may send communications to the board, as well as what the board’s response, if any, should be; |
| • | evaluating and making recommendations regarding the creation of additional committees or the change in mandate or dissolution of committees; |
| • | reviewing and making recommendations with regard to our corporate governance guidelines and compliance with laws and regulations; and |
| • | reviewing and approving actual and potential conflicts of interest of our directors and corporate officers, other than related person transactions reviewed by the audit committee. |
The members of our nominating and corporate governance committee are Drs. Hill, Kelley and U’Prichard and Ms. Bonney. Dr. Kelley is the chairman of our nominating and corporate governance committee. In September 2012, our board of directors determined that each member of our nominating and corporate governance committee is independent within the meaning of the independent director guidelines of The Nasdaq Stock Market.
Our board of directors may from time to time establish other committees.
-139-
Table of Contents
Code of Business Conduct and Ethics
Our code of business conduct and ethics is applicable to all of our employees, officers and directors, including our chief executive and senior financial officers. The code of business conduct and ethics will be available on our website at www.audeooncology.com. We expect that any amendment to the code, or any waivers of its requirements, will be disclosed on our website. Information contained on, or that can be accessed through, our website or the Alchemia Limited website is not incorporated by reference into this prospectus, and you should not consider information on our website or the Alchemia Limited website to be part of this prospectus.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee is an officer or employee of our company. With the exception of Peter Smith, none of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or compensation committee. See “Arrangements between Audeo Oncology, Inc. and Alchemia Limited — Transition Services Agreement” for a discussion of Dr. Smith’s secondment to Alchemia Limited.
Non-Employee Director Compensation
We expect to reimburse our non-employee directors for expenses incurred in connection with attending board and committee meetings. We have adopted a cash and equity compensation program for non-employee directors that will be effective immediately upon the closing of this offering. Pursuant to this program, each member of our board of directors who is not our employee will receive the following cash compensation for board services, as applicable:
| • | $50,000 per year for service as a board member; |
| • | $10,000 per year for service as chair of the board of directors; and |
| • | $10,000 per year for service as chair of the audit committee. |
In addition, after this offering we will continue to reimburse our non-employee directors for expenses incurred in attending board and committee meetings.
Each non-employee director on our board of directors on the date that we enter into the underwriting agreement for this offering will receive options to purchase 30,000 shares our common stock pursuant to our 2012 Stock Plan at an exercise price equal to the initial public offering price per share set forth on the cover page of this prospectus in connection with the closing of this offering. 25% of these options will vest one year following the closing date of this offering, or the vesting commencement date, with the remaining 75% vesting in equal monthly installments over a three-year period beginning one year following the vesting commencement date. Each non-employee director who joins our board of directors after the completion of this offering will be granted a non-statutory stock option under our 2012 Equity Incentive Plan, which will become effective in connection with this offering, to purchase 30,000 shares of our common stock, with an exercise price equal to the then fair market value of our common stock; these stock options are expected to vest one-third on the date of grant and an additional one-third on each anniversary of the date of grant thereafter, provided that the recipient continues to serve as a director through each such date. On the date of each annual meeting of our stockholders beginning after completion of this offering, each non-employee director who has served on our board of directors for at least the preceding six months will be granted a non-statutory stock option under our 2012 Equity Incentive Plan to purchase 10,000 shares of our common stock. Annual stock option grants are expected to vest one-third on the date of grant and an additional one-third on each anniversary of the date of grant thereafter, provided that the recipient continues to serve as a director through each such date. All director stock options granted under our 2012 Stock Plan or 2012 Equity Incentive Plan are expected to have a term of ten years. Each option granted to a non-employee director is expected to vest in full immediately prior to a change in control (as defined in our 2012 Stock Plan or 2012 Equity Incentive Plan, as applicable) of us.
-140-
Table of Contents
The following table shows, for fiscal 2012, certain information with respect to the compensation of all of our non-employee directors.
Fiscal 2012 Director Compensation Table(1)
| Name |
Fees Earned or Paid in Cash ($) |
Stock Awards ($) |
Option Awards ($)(2) |
Non-Equity Incentive Plan Compensation ($) |
All Other Compensation ($) |
Total ($) |
||||||||||||||||||
| Stephen Hill, BMBCh, FRCS |
0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Leigh Bonney, MBA |
0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Susan Kelley, MD |
0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Tracie Ramsdale, PhD |
0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| David U’Prichard, PhD |
0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| (1) | Dr. Hill and Dr. Ramsdale joined our board of directors near the end of the fiscal year ended June 30, 2012 and did not receive any compensation for that fiscal year. Ms. Bonney, Dr. Kelley and Dr. U’Prichard joined our board of directors subsequent to the fiscal year ended June 30, 2012. See “—Non-Employee Director Compensation” above for information regarding option grants made to these directors subsequent to June 30, 2012. |
| (2) | The dollar amounts in this column represent the compensation cost for the fiscal year ended June 30, 2012 of stock option awards granted in fiscal year 2012. |
Stock Ownership of Directors and Executive Officers in Alchemia Limited
The following table sets forth the number of ordinary shares of Alchemia Limited beneficially owned and the number of shares of Alchemia Limited issuable upon the exercise of options beneficially owned on September 30, 2012 by each director, each of the executive officers named in the Summary Compensation Table in the “Executive Compensation” section below, and all of our directors and executive officers as a group. Except as otherwise noted, the individual directors or executive officers or their family members have sole voting and investment power with respect to such securities. The total number of ordinary shares of Alchemia Limited outstanding as of September 30, 2012 was 280,812,962 ordinary shares.
| Name |
Number of Ordinary Shares of Alchemia Limited Beneficially Owned(1) |
Percentage of Ordinary Shares of Alchemia Limited Beneficially Owned |
Number of Alchemia Limited Options Owned |
|||||||
| Peter Smith, PhD.(2) |
1,285,503 | * | 1,000,000 | |||||||
| Charles Walker, MBA |
99,946 | * | 680,000 | |||||||
| Tracey Brown, PhD |
460,509 | * | 300,000 | |||||||
| Stephen Hill, BMBCh, FRCS |
0 | * | 0 | |||||||
| Leigh Bonney, MBA |
0 | * | 0 | |||||||
| Susan Kelley, MD |
0 | * | 0 | |||||||
| Tracie Ramsdale, PhD(3) |
1,350,012 | * | 0 | |||||||
| David U’Prichard, PhD |
0 | * | 0 | |||||||
| All current executive officers and directors as a group (8 persons) |
3,195,970 | 1.14% | 1,980,000 | |||||||
| * | Represents beneficial ownership of less than 1% of Alchemia Limited’s ordinary shares. |
| (1) | Excludes ordinary shares issuable upon exercise of options. |
| (2) | Includes (a) 612,604 shares held by Dr. Smith and (b) 672,899 shares held by Jill M. Ewing, Dr. Smith’s spouse. |
| (3) | Includes (a) 1,103,705 shares held by Dr. Ramsdale, (b) 169,443 shares held by Merlinium Pty Ltd, for which Dr. Ramsdale is the sole director and has sole voting and investment power, and (c) 76,864 shares held by the Leilah Ramsdale Child Maintenance Trust dated June 18, 2007, for which Dr. Ramsdale is the sole trustee and has sole voting and investment power. |
-141-
Table of Contents
Fiscal 2012 and Fiscal 2011 Summary Compensation Table
The following table summarizes information regarding the compensation awarded to, earned by or paid to our Chief Executive Officer, our Chief Financial Officer and our one other most highly compensated executive officer during fiscal 2012 and fiscal 2011 while employed at Alchemia Limited. We refer to these individuals in this prospectus as our named executive officers.
| Name and Principal Position(1) |
Year | Salary ($) |
Bonus ($)(2) |
Stock Awards ($)(3) |
Option Awards ($)(4) |
Non-Equity Incentive Plan Compensation ($)(5) |
Total ($) | |||||||||||||||||||||
| Peter Smith, PhD. |
2012 | 421,905 | — | 34,647 | 47,235 | 8,662 | 512,449 | |||||||||||||||||||||
| President and Chief Executive Officer(6) |
2011 | 391,090 | — | 32,162 | — | 8,040 | 431,292 | |||||||||||||||||||||
| Charles Walker, MBA(7) |
2012 | 325,466 | — | 26,846 | 120,526 | 6,712 | 479,550 | |||||||||||||||||||||
| Chief Financial Officer and Secretary |
2011 | 89,385 | — | 14,953 | 61,250 | 3,738 | 169,326 | |||||||||||||||||||||
| Tracey Brown, PhD |
2012 | 246,619 | — | 20,210 | 17,996 | 5,053 | 289,878 | |||||||||||||||||||||
| Chief Scientific Officer |
2011 | 228,184 | — | 37,522 | — | 9,381 | 275,087 | |||||||||||||||||||||
| (1) | The compensation set forth in the table above was provided by Alchemia Limited to the following individuals for their services while employed at Alchemia Limited. |
| (2) | Represents discretionary cash bonus amounts paid in excess of the goals achieved by us to such individuals pursuant to Alchemia Limited’s bonus entitlement program. |
| (3) | Represents incentive compensation payments paid to such individuals in ordinary shares of Alchemia Limited for performance against target corporate and individual goals for the performance period pursuant to Alchemia Limited’s bonus entitlement program. |
| (4) | The dollar amounts in this column represent the compensation cost for the fiscal year ended June 30, 2011 of stock option awards granted in fiscal year 2011 and for the fiscal year ended June 30, 2012 of stock option awards granted in fiscal year 2012. These amounts have been calculated in accordance with FASB ASC Topic 718, “Compensation — Stock Compensation,” using the Black-Scholes option-pricing model. Pursuant to SEC rules, the amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. For a discussion of valuation assumptions, see the notes to our financial statements included elsewhere in this prospectus. See “—Outstanding Equity Awards as of June 30, 2012” below for information regarding option grants made by us to these officers subsequent to June 30, 2012. |
| (5) | Represents cash incentive compensation payments paid to such individuals for performance against target corporate and individual goals for the performance period pursuant to Alchemia Limited’s bonus entitlement program. |
| (6) | Dr. Smith is also a member of our board of directors and the board of directors of Alchemia Limited but does not receive any additional compensation in his capacity as a director. |
| (7) | Mr. Walker commenced employment with Alchemia Limited on March 17, 2011. |
Narrative to Summary Compensation Table
To date, our officers have received compensation from Alchemia Limited for their services while employed at Alchemia Limited. Contemporaneously with implementation of the Demerger, our officers will begin receiving compensation for their services to our company and will thereupon cease receiving compensation from Alchemia Limited. The following is a description of the employment arrangements with our named executive officers that will be effective upon completion of this offering.
-142-
Table of Contents
Employment Agreements
Peter Smith
On September 28, 2012, our subsidiary, Alchemia Oncology, entered into an employment agreement with Peter Smith, our President and Chief Executive Officer and the Chief Executive Officer and Managing Director of Alchemia Oncology. The agreement, which will be effective upon implementation of the Demerger, provides for employment for no specific duration and constitutes at-will employment. The employment agreement provides for an initial base salary of AUD$377,392, plus annual superannuation (retirement benefits) of 9% of the base salary and any additional amounts up to 100% of the base salary as determined by the compensation committee of our board of directors based on reasonable key performance indicators determined by the compensation committee of our board of directors.
Upon termination of Dr. Smith’s employment for any reason, Dr. Smith’s employment agreement provides for payment in lieu of accrued annual leave and any entitlement to long service leave up to the date of termination, calculated by reference to his salary. If Dr. Smith’s position becomes redundant and Alchemia Oncology or a related corporation does not offer alternative employment on similar terms, Dr. Smith is entitled to an additional six months’ salary. If Dr. Smith’s employment is terminated by him or Alchemia Oncology upon the giving of six months’ notice, he is entitled to his current salary and any superannuation benefits for the balance of the notice period remaining after termination of his employment. Alchemia Oncology may terminate Dr. Smith’s employment without notice or payment in lieu of notice in certain circumstances as described in his employment agreement.
Dr. Smith’s employment agreement provides that for a period of up to twelve months following termination of his employment with Alchemia Oncology and within Australia, he shall not induce any director, manager, officer, employee, servant or contractor of Alchemia Oncology, a related corporation or Alchemia Limited, to end their relationship with Alchemia Oncology, a related corporation or Alchemia Limited or engage in other competitive acts more fully described in his employment agreement.
Charles Walker
On September 28, 2012, our subsidiary, Alchemia Oncology, entered into an employment agreement with Charles Walker, our Chief Financial Officer and Secretary and the Chief Financial Officer of Alchemia Oncology. The agreement, which will be effective upon implementation of the Demerger, provides for employment for no specific duration and constitutes at-will employment. The employment agreement provides for an initial base salary of AUD$292,428, plus annual superannuation of 9% of the base salary and any additional amounts up to 100% of the base salary as determined by the compensation committee of our board of directors based on reasonable key performance indicators determined by the compensation committee of our board of directors.
Upon termination of Mr. Walker’s employment for any reason, Mr. Walker’s employment agreement provides for payment in lieu of accrued annual leave and any entitlement to long service leave up to the date of termination, calculated by reference to his salary. If Mr. Walker’s position becomes redundant and Alchemia Oncology or a related corporation does not offer similar alternative employment on similar terms, Mr. Walker is entitled to an additional six months’ salary. If Mr. Walker’s employment is terminated by him or Alchemia Oncology upon the giving of six months’ notice, he is entitled to his current salary and any superannuation benefits for the balance of the notice period remaining after termination of his employment. Alchemia Oncology may terminate Mr. Walker’s employment without notice or payment in lieu of notice in certain circumstances as described in his employment agreement.
Mr. Walker’s employment agreement provides that for a period of up to twelve months following termination of his employment with Alchemia Oncology, within Australia, he shall not induce any director, manager, officer, employee, servant or contractor of Alchemia Oncology, a related corporation or Alchemia
-143-
Table of Contents
Limited, to end their relationship with Alchemia Oncology, a related corporation or Alchemia Limited or engage in other competitive acts more fully described in his employment agreement.
Tracey Brown
On September 28, 2012, our subsidiary, Alchemia Oncology, entered into an employment agreement with Tracey Brown, our Chief Scientific Officer and the VP-Oncology and Chief Scientific Officer of Alchemia Oncology. The agreement provides for employment for no specific duration and constitutes at-will employment. The employment agreement provides for an initial base salary of AUD$220,146, plus annual superannuation of 9% of the base salary and any additional amounts of up to 100% of the base salary as determined by the compensation committee of our board of directors based on reasonable key performance indicators determined by the compensation committee of our board of directors.
Upon termination of Associate Professor Brown’s employment for any reason, Associate Professor Brown’s employment agreement provides for payment in lieu of accrued annual leave and any entitlement to long service leave up to the date of termination, calculated by reference to her salary. If Associate Professor Brown’s position becomes redundant and Alchemia Oncology or a related corporation does not offer similar alternative employment on similar terms, Associate Professor Brown is entitled to an additional six months’ salary. If Associate Professor Brown’s employment is terminated by her or Alchemia Oncology upon the giving of six months’ notice, she is entitled to her current salary and any superannuation benefits for the balance of the notice period remaining after termination of her employment. Alchemia Oncology may terminate Associate Professor Brown’s employment without notice or payment in lieu of notice in certain circumstances as described in her employment agreement.
Associate Professor Brown’s employment agreement permits her to continue to pursue certain business and academic interests and commitments, including pursuit of scientific developments and ownership of certain intellectual property unrelated to the business of Alchemia Oncology. Associate Professor Brown’s employment agreement provides that for a period of up to twelve months following termination of her employment with Alchemia Oncology, within Australia, she shall not induce any director, manager, officer, employee, servant or contractor of Alchemia Oncology or a related corporation, to end their relationship with Alchemia Oncology or a related corporation or engage in other competitive acts more fully described in her employment agreement.
Outstanding Equity Awards as of June 30, 2012
The following table provides information about outstanding options to purchase shares of our common stock held by each of our named executive officers at June 30, 2012. Options were subsequently granted to certain of our officers and employees, including our named executive officers, pursuant to our 2012 Stock Plan, as described below.
| Name(1) |
Number of Securities Underlying Unexercised Options Exercisable(2) |
Number of Securities Underlying Unexercised Options Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
||||||||||||
| Peter Smith, PhD. |
0 | 0 | 0 | N/A | ||||||||||||
| Charles Walker, MBA |
0 | 0 | 0 | N/A | ||||||||||||
| Tracey Brown, PhD |
0 | 0 | 0 | N/A | ||||||||||||
| (1) | Our named executive officers have not received any equity awards in our company although, as described below, they will receive options to purchase our common stock in connection with the completion of this offering. |
-144-
Table of Contents
| (2) | Unless otherwise specified, all option awards were granted under our 2012 Stock Plan and 25% of the options vest one year following the vesting commencement date with the remaining 75% vesting in equal monthly installments over a three-year period beginning one year following the vesting commencement date, and all options have a ten-year term. |
Our named executive officers also will receive options to purchase our common stock pursuant to our 2012 Stock Plan at an exercise price equal to the initial public offering price per share set forth on the cover page of this prospectus in connection with the closing of this offering as follows: Dr. Smith will receive options to purchase 250,000 shares of our common stock, Mr. Walker will receive options to purchase 125,000 shares of our common stock and Associate Professor Brown will receive options to purchase 75,000 shares of our common stock. 25% of these options will vest one year following the vesting commencement date with the remaining 75% vesting in equal monthly installments over a three-year period beginning one year following the vesting commencement date, and all of these options will have a ten-year term.
We believe that our ability to grant equity-based awards is a valuable and necessary compensation tool that aligns the long-term financial interests of our employees and directors with the financial interests of our stockholders. In addition, we believe that our ability to grant stock options and other equity-based awards helps us to attract, retain and motivate qualified employees, and encourages them to devote their best efforts to our business and financial success. The following is a description of some of the terms of our current 2012 Stock Plan and our 2012 Equity Incentive Plan, which we expect will become effective in connection with this offering. These summaries do not purport to be complete and are qualified in their entirety by the provisions of the 2012 Stock Plan and the 2012 Equity Incentive Plan. For more complete information, you should review the forms of our 2012 Stock Plan and our 2012 Equity Incentive Plan, which have been filed as exhibits to the registration statement of which this prospectus forms a part.
2012 Stock Plan
In August 2012, our board of directors and our stockholders adopted our 2012 Stock Plan. Our 2012 Stock Plan permits the grant of incentive stock options, within the meaning of Section 422 of the Code, to our employees and the grant of nonstatutory stock options to our employees, directors, and consultants.
Authorized Shares
The maximum aggregate number of shares issuable under the 2012 Stock Plan is 948,000 shares of our common stock. Options were granted under our 2012 Stock Plan to purchase shares of our common stock at an exercise price equal to the initial public offering price per share set forth on the cover page of this prospectus to officers, directors and employees, as described above under “Prospectus Summary—The Offering.” We do not expect to make further grants pursuant to our 2012 Stock Plan but we expect to issue stock options or other forms of stock-based compensation pursuant to our 2012 Equity Incentive Plan.
Plan Administration
Our board of directors or one or more committees appointed by our board of directors will administer the 2012 Stock Plan. It is anticipated that the compensation committee of the board of directors will administer the 2012 Stock Plan.
Subject to the provisions of our 2012 Stock Plan, the plan administrator has the power to: determine the terms of awards, including the recipients, the exercise price, the number of shares covering each award and the fair market value of a share of our common stock; select the individuals to whom awards may be granted; approve the forms of agreements; determine when awards may be exercised and any vesting acceleration or
-145-
Table of Contents
waiver forfeiture restrictions; institute an exchange program by which outstanding awards may be surrendered in exchange for awards that may have different exercise prices and terms; prescribe, amend and rescind rules and regulations relating to the 2012 Stock Plan; allow participants to satisfy tax withholding obligations; modify or amend each award; and construe and interpret the terms of the 2012 Stock Plan and awards granted under the 2012 Stock Plan.
Stock Options
The plan administrator may grant incentive and/or nonstatutory stock options under our 2012 Stock Plan, provided that incentive stock options are granted only to employees. The exercise price of such options must equal at least the fair market value of our common stock on the date of grant. The term of an incentive or nonstatutory stock option may not exceed 10 years. However, an incentive stock option held by a participant who owns more than 10% of the total combined voting power of all classes of our stock (or any parent or subsidiary), may not have a term in excess of five years and must have an exercise price of at least 110% of the fair market value of our common stock on the grant date. Options may be granted under the 2012 Stock Plan at a price less than 100% of fair market value pursuant to a transaction described in, and in a manner consistent with, Section 424(a) of the Code.
The plan administrator will determine the methods of payment of the exercise price of an option, which may include cash, shares, or other property acceptable to the plan administrator, as well as other types of consideration permitted by applicable law. Subject to the provisions of our 2012 Stock Plan, the plan administrator determines the remaining terms of the options (e.g., vesting). After the termination of service of an employee, director, or consultant, other than upon the individual’s death or disability, the participant may exercise his or her option, to the extent vested as of such date of termination, within 30 days of termination or a longer period of time stated in the participant’s award agreement. After the termination of service of an employee, director, or consultant on account of the individual’s disability, the participant may exercise his or her option, to the extent vested as of such date of termination, within six months of termination, or such longer period as specified in the participant’s award agreement. After the termination of service of an employee, director, or consultant, on account of the individual’s death, the participant’s options may be exercised, to the extent vested as of such date of termination, within six months of termination, or such longer period as specified in the participant’s award agreement, by the participant’s beneficiary. However, in no event may an option be exercised later than the expiration of its term.
Transferability of Awards
Unless the plan administrator provides otherwise, our 2012 Stock Plan generally does not allow for the transfer of awards and only the recipient of an award may exercise such an award during his or her lifetime.
Certain Adjustments
In the event of certain changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under the 2012 Stock Plan, the plan administrator will make adjustments to one or more of the number and class of shares that may be delivered under the plan and/or the number, class, exercise price and price of shares covered by each outstanding award granted under the 2012 Stock Plan. In the event of our proposed liquidation or dissolution, the plan administrator will notify participants as soon as practicable and all awards will terminate immediately prior to the consummation of such proposed transaction.
Merger or Change in Control
Our 2012 Stock Plan provides that in the event of our merger or change in control, as defined under the 2012 Stock Plan, each outstanding award will be treated as the plan administrator determines, except that if a
-146-
Table of Contents
successor corporation or its parent or subsidiary does not assume or substitute an equivalent award for any outstanding award, then such award will fully vest, all restrictions on such award will lapse, all performance goals or other vesting criteria applicable to such award will be deemed achieved at 100% of target levels and such award will become fully exercisable, if applicable, for a specified period prior to the transaction. The award will then terminate upon the expiration of the specified period of time.
Plan Amendment, Termination
Our board of directors has the authority to amend, suspend or terminate the 2012 Stock Plan provided such action does not impair the existing rights of any participant, unless mutually agreed upon by the participant and the plan administrator. Our 2012 Stock Plan will automatically terminate in 2022, unless we terminate it sooner.
2012 Equity Incentive Plan
In August 2012, our board of directors and our stockholders adopted our 2012 Equity Incentive Plan, which will become effective in connection with the completion of this offering. Our 2012 Equity Incentive Plan permits the grant of incentive stock options, within the meaning of Section 422 of the Code, to our employees and any of our subsidiary corporations’ employees, and the grant of nonstatutory stock options, stock appreciation rights, restricted stock, restricted stock units, performance units and performance shares to our employees, directors and consultants and our subsidiaries’ employees and consultants.
Authorized Shares
The maximum aggregate number of shares initially issuable under the 2012 Equity Incentive Plan is 1,250,000 shares of our common stock. In addition, the number of shares available for issuance under the 2012 Equity Incentive Plan will be annually increased on the first day of each of our fiscal years beginning with the fiscal year commencing July 1, 2013, provided, that our board of directors specifically approves the increase, by an amount equal to the least of:
| • | 250,000 shares; |
| • | 2.0% of the outstanding shares of our common stock as of the last day of our immediately preceding fiscal year; or |
| • | such other amount as our board of directors may determine. |
Shares issued pursuant to awards under the 2012 Equity Incentive Plan that we repurchase due to their failure to vest, shares subject to awards that expire, become unexercisable or are forfeited, shares subject to awards that are surrendered pursuant to an exchange program in exchange for awards that have different exercise prices and terms, as well as shares used to pay the exercise price of an award or to satisfy the tax withholding obligations related to an award, will become available for future grant under the 2012 Equity Incentive Plan. In addition, to the extent that an award is paid out in cash rather than shares, such cash payment will not reduce the number of shares available for issuance under the 2012 Equity Incentive Plan. With respect to stock appreciation rights, only shares actually issued pursuant to the award cease to be available under the 2012 Equity Incentive Plan.
Plan Administration
Our board of directors or one or more committees appointed by our board of directors will administer the 2012 Equity Incentive Plan. It is anticipated that the compensation committee of the board of directors will administer the 2012 Equity Incentive Plan.
-147-
Table of Contents
Subject to the provisions of our 2012 Equity Incentive Plan, the plan administrator has the power to determine the terms of awards, including the recipients, the exercise price, if any, the number of shares covering each award, the fair market value of a share of our common stock, the vesting schedule applicable to the awards, together with any vesting acceleration, the form of consideration, if any, payable upon exercise of the award, and the terms of the award agreement for use under the 2012 Equity Incentive Plan. The plan administrator also has the authority, subject to the terms of the 2012 Equity Incentive Plan, to amend existing awards to reduce or increase their exercise price, to allow participants the opportunity to transfer outstanding awards to a financial institution or other person or entity selected by the plan administrator, to institute an exchange program by which outstanding awards may be surrendered in exchange for awards that may have different exercise prices and terms, to prescribe rules and to construe and interpret the 2012 Equity Incentive Plan and awards granted under the 2012 Equity Incentive Plan.
Stock Options
The plan administrator may grant incentive and/or nonstatutory stock options under our 2012 Equity Incentive Plan, provided that incentive stock options are granted only to employees. The exercise price of such options must equal at least the fair market value of our common stock on the date of grant. The term of an incentive or nonstatutory stock option may not exceed 10 years; provided, however, that an incentive stock option held by a participant who owns more than 10% of the total combined voting power of all classes of our stock (or any parent or subsidiary), may not have a term in excess of five years and must have an exercise price of at least 110% of the fair market value of our common stock on the grant date. Options may be granted under the 2012 Equity Incentive Plan at a price less than 100% of fair market value pursuant to a transaction described in, and in a manner consistent with, Section 424(a) of the Code.
The plan administrator will determine the methods of payment of the exercise price of an option, which may include cash, shares or other property acceptable to the plan administrator, as well as other types of consideration permitted by applicable law. Subject to the provisions of our 2012 Equity Incentive Plan, the plan administrator determines the remaining terms of the options (e.g., vesting). After the termination of service of an employee, director or consultant, other than upon the individual’s death or disability, the participant may exercise his or her option, to the extent vested as of such date of termination, for the period of time stated in the participant’s option agreement (or within three months if no period is stated). After the termination of service of an employee, director, or consultant on account of the individual’s disability, the participant may exercise his or her option, to the extent vested as of such date of termination, within the period of time stated in the participant’s option agreement (or within 12 months of termination if no period is stated). After the termination of service of an employee, director, or consultant, on account of the individual’s death, the participant’s options may be exercised, to the extent vested as of such date of termination, within the period of time stated in the participant’s option agreement (or within 12 months of termination if no period is stated), by the participant’s beneficiary. However, in no event may an option be exercised later than the expiration of its term.
Stock Appreciation Rights
Stock appreciation rights may be granted under our 2012 Equity Incentive Plan. Stock appreciation rights allow the recipient to receive the appreciation in the fair market value of our common stock between the exercise date and the date of grant. Subject to the provisions of our 2012 Equity Incentive Plan, the plan administrator determines the terms of stock appreciation rights, including when such rights vest and become exercisable and whether to settle such awards in cash or with shares of our common stock, or a combination thereof, except that the per share exercise price for the shares subject to a stock appreciation right will be no less than 100% of the fair market value per share on the date of grant. The specific terms of any grant of stock appreciation rights will be set forth in an award agreement between us and the recipient.
-148-
Table of Contents
Restricted Stock
Restricted stock may be granted under our 2012 Equity Incentive Plan. Restricted stock awards are grants of shares of our common stock that are subject to various restrictions, including restrictions on transferability and forfeiture provisions. Shares of restricted stock will vest and the restrictions on such shares will lapse, in accordance with terms and conditions established by the plan administrator. Such terms may include, among other things, vesting upon the achievement of specific performance goals determined by the plan administrator and/or continued service to us. The plan administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed. Recipients of restricted stock awards generally will have voting and dividend rights with respect to such shares upon grant without regard to vesting, unless the plan administrator provides otherwise. Shares of restricted stock that do not vest for any reason will be forfeited by the recipient and will revert to us. The specific terms of any grant of restricted stock will be set forth in an award agreement between us and the recipient.
Restricted Stock Units
Restricted stock units may be granted under our 2012 Equity Incentive Plan. Each restricted stock unit granted is a bookkeeping entry representing an amount equal to the fair market value of one share of our common stock. The plan administrator determines the terms and conditions of restricted stock units including the vesting criteria, which may include achievement of specified performance criteria or continued service to us, and the form and timing of payment. The plan administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed. The plan administrator determines in its sole discretion whether an award will be settled in stock, cash or a combination of both. The specific terms of any grant of restricted stock units will be set forth in an award agreement between us and the recipient.
Performance Units/Performance Shares
Performance units and performance shares may be granted under our 2012 Equity Incentive Plan. Performance units and performance shares are awards that will result in a payment to a participant only if performance goals established by the plan administrator are achieved or the awards otherwise vest. The plan administrator will establish organizational or individual performance goals in its discretion, which, depending on the extent to which they are met, will determine the number and/or the value of performance units and performance shares to be paid out to participants. After the grant of a performance unit or performance share, the plan administrator, in its sole discretion, may reduce or waive any performance objectives or other vesting provisions for such performance units or performance shares. Performance units shall have an initial dollar value established by the plan administrator prior to the grant date. Performance shares will have an initial value equal to the fair market value of our common stock on the grant date. The plan administrator, in its sole discretion, may pay earned performance units or performance shares in the form of cash, in shares or in some combination thereof. The specific terms of any grant of performance units and performance shares will be set forth in an award agreement between us and the recipient.
Transferability of Awards
Unless the plan administrator provides otherwise, our 2012 Equity Incentive Plan generally does not allow for the transfer of awards and only the recipient of an award may exercise such an award during his or her lifetime.
Certain Adjustments
In the event of certain changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under the 2012 Equity Incentive Plan, the plan administrator will make adjustments to one or more of the number and class of shares that may be delivered under the plan and/or the number, class,
-149-
Table of Contents
exercise price and price of shares covered by each outstanding award and the numerical share limits on the annual increase in shares available under the 2012 Equity Incentive Plan. In the event of our proposed liquidation or dissolution, the plan administrator will notify participants as soon as practicable and all awards will terminate immediately prior to the consummation of such proposed transaction.
Merger or Change in Control
Our 2012 Equity Incentive Plan provides that in the event of our merger or change in control, as defined under the 2012 Equity Incentive Plan, each outstanding award will be treated as the plan administrator determines, except that if a successor corporation or its parent or subsidiary does not assume or substitute an equivalent award for any outstanding award of ours, then such award will fully vest, all restrictions on such award will lapse, all performance goals or other vesting criteria applicable to such award will be deemed achieved at 100% of target levels and such award will become fully exercisable, if applicable, for a specified period prior to the transaction. The award will then terminate upon the expiration of the specified period of time. If the service of an outside director is terminated on or following a change in control, other than pursuant to a voluntary resignation, his or her awards will become fully vested and exercisable, and all performance goals or other vesting requirements will be deemed achieved at 100% of target levels.
Plan Amendment; Termination
Our board of directors has the authority to amend, suspend or terminate the 2012 Equity Incentive Plan provided such action does not impair the existing rights of any participant, unless mutually agreed upon by the participant and the plan administrator. Our 2012 Equity Incentive Plan will automatically terminate in 2022, unless we terminate it sooner.
401(k) Plan
We currently do not have any U.S. employees. If we have any U.S. employees, we may elect to establish a tax-qualified retirement plan that provides eligible U.S. employees with an opportunity to save for retirement on a tax advantaged basis. Eligible employees would be able to defer eligible compensation subject to applicable annual Code limits. Pre-tax contributions would be allocated to each participant’s individual account and would then be invested in selected investment alternatives according to the participants’ directions. Employees would be immediately and fully vested in their contributions. We would intend the 401(k) plan to qualify under Sections 401(a) and 501(a) of the Code. As a tax-qualified retirement plan, contributions to the 401(k) plan and earnings on those contributions would not be taxable to the employees until distributed from the 401(k) plan.
Limitations on Liability and Indemnification Matters
Upon the closing of this offering, our amended and restated certificate of incorporation will contain provisions that limit the liability of our current and former directors for monetary damages to the fullest extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for any breach of fiduciary duties as directors, except liability for:
| • | any breach of the director’s duty of loyalty to the corporation or its stockholders; |
| • | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | any unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
| • | any transaction from which the director derived an improper personal benefit. |
-150-
Table of Contents
This limitation of liability does not apply to liabilities arising under federal securities laws and does not affect the availability of equitable remedies such as injunctive relief or rescission.
Our amended and restated certificate of incorporation and our amended and restated bylaws will provide that we are required to indemnify our directors to the fullest extent permitted by Delaware law. Our amended and restated bylaws will also provide that, upon satisfaction of specified conditions, we are required to advance expenses incurred by a director in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of Delaware law. Our amended and restated bylaws will also provide our board of directors with discretion to indemnify our officers and employees when determined appropriate by the board of directors. We expect to enter into agreements to indemnify our directors and executive officers, if applicable, as determined by the board of directors. With specified exceptions, these agreements will provide for indemnification for related expenses including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding. We believe that these amended and restated certificate of incorporation and bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. We also will maintain customary directors’ and officers’ liability insurance.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers as required by these indemnification provisions. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought and we are not aware of any threatened litigation that may result in claims for indemnification.
Rule 10b5-1 Sales Plan
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, in which they will contract with a broker to buy or sell shares of our common stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or officer when entering into the plan, without further direction from them. The director or officer may amend a Rule 10b5-1 plan in some circumstances and may terminate a plan at any time. Our directors and executive officers also may buy or sell additional shares outside of a Rule 10b5-1 plan when they are not in possession of material nonpublic information, subject to compliance with the terms of our insider trading policy. Our directors or executive officers may not establish any such plan prior to the expiration of the lock-ups described under “Underwriting—Lock-Up Agreements.”
-151-
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than compensation arrangements, we describe below transactions and series of similar transactions during our last three fiscal years, to which we or Alchemia Oncology, now our wholly-owned subsidiary, were a party or will be a party, in which:
| • | the amounts involved exceeded or will exceed $120,000; and |
| • | any of our directors, executive officers or beneficial holders of more than 5% of any class of our capital stock, or any immediate family member of such related person, had or will have a direct or indirect material interest. |
Reorganization and Demerger
In connection with the Reorganization and Demerger, we have entered into and will enter into agreements with Alchemia Limited. See “Prospectus Summary — Corporate Information — Corporate Reorganization” and “— Demerger” and “Arrangements between Audeo Oncology, Inc. and Alchemia Limited.”
Director and Named Executive Officer Compensation
Please see “Management — Non-Employee Director Compensation” and “Executive Compensation” for a discussion of information regarding the compensation of our non-employee directors and our named executive officers.
Employment Agreements
We have entered into employment agreements with our executive officers that, among other things, provide for certain severance and change in control benefits. For more information regarding these agreements, see “Executive Compensation — Narrative to Summary Compensation Table — Employment Agreements.”
Stock Option Grants to Named Executive Officers and Directors
We have granted and expect to grant stock options to our directors and named executive officers. For more information regarding these stock options, see “Executive Compensation — 2012 Stock Plan” and “— 2012 Equity Incentive Plan.”
Indemnification Agreements
Our amended and restated certificate of incorporation and bylaws will provide that we will indemnify our directors and executive officers to the fullest extent permitted by Delaware law. In addition, we have entered into indemnification agreements with each of our directors and executive officers that may be broader in scope than the specific indemnification provisions contained in the Delaware General Corporation Law. For more information regarding these agreements, see “Executive Compensation — Limitations on Liability and Indemnification Matters.”
Policies and Procedures for Related Party Transactions
Our related person transaction policy sets forth our procedures for the identification, review, consideration and approval or ratification of related person transactions. For purposes of our policy only, a related person
-152-
Table of Contents
transaction is a transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we and any related person are, were or will be participants in which the amount involved exceeds $120,000. Transactions involving compensation for services provided to us as an employee or director are not covered by this policy. A related person is any executive officer, director or beneficial owner of more than 5% of any class of our voting securities, including any of their immediate family members and any entity owned or controlled by such persons.
Under the policy, if a transaction has been identified as a related person transaction, including any transaction that was not a related person transaction when originally consummated or any transaction that was not initially identified as a related person transaction prior to consummation, our management must present information regarding the related person transaction to our audit committee, or, if audit committee approval would be inappropriate, to another independent body of our board of directors, for review, consideration and approval or ratification. The presentation must include a description of, among other things, the material facts, the interests, direct and indirect, of the related persons, the benefits to us of the transaction and whether the transaction is on terms that are comparable to the terms available to or from, as the case may be, an unrelated third-party or to or from employees generally. Under the policy, we will collect information that we deem reasonably necessary from each director, executive officer and, to the extent feasible, significant stockholder to enable us to identify any existing or potential related person transactions and to effectuate the terms of the policy. In addition, under our Code of Business Conduct and Ethics, which we will adopt in connection with this offering our employees and directors have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest.
-153-
Table of Contents
ARRANGEMENTS BETWEEN AUDEO ONCOLOGY, INC. AND ALCHEMIA LIMITED
We have provided below a description of the material terms of the Intra-group Transfer Agreement between us and Alchemia Limited, the Technology License Agreement between our subsidiary, Audeo Discovery Pty Limited, and Alchemia Limited and the other material agreements that relate to the Demerger. This description is not complete and is qualified in its entirety by the provisions of those agreements. You should read the full text of the agreements, which have been or will be filed with the SEC as exhibits to the registration statement of which this prospectus is a part.
Intra-group Transfer Agreement
On June 28, 2012, we and Alchemia Limited entered into an Intra-group Transfer Agreement pursuant to which on June 28, 2012:
| • | Alchemia Limited transferred to us its shares in Alchemia Oncology; and |
| • | we issued 7.5 million shares of our common stock to Alchemia Limited. |
In addition, we agreed to issue to Alchemia Limited such number of additional shares of our common stock as is required to ensure that Alchemia Limited has a sufficient number of shares of our common stock to distribute to holders of ordinary shares of Alchemia Limited on the record date for the Demerger, as required by the terms of the Demerger. On November 5, 2012, we issued an additional aggregate of 92,000 shares of our common stock to Alchemia Limited in further consideration for our receipt of the shares of Alchemia Oncology and which, together with the 7.5 million shares of our common stock previously issued, will be distributed in the Demerger.
The agreement provides for us to assume Alchemia Limited’s obligation to issue the Warrants described in “Prospectus Summary — The Offering,” contingent upon completion of this offering by December 31, 2012. We agreed to indemnify Alchemia Limited in respect of any liability or loss suffered by Alchemia Limited if we fail to issue the Warrants.
Technology License Agreement
On August 22, 2012, our subsidiary, Audeo Discovery Pty Limited, entered into a Technology License Agreement with Alchemia Limited. The license agreement provides Audeo Discovery Pty Limited with a non-transferable, worldwide license to use Alchemia Limited’s VAST technology, including related patents, patent applications and know-how. The license is exclusive except in relation to one family of patents in respect of which there is an existing non-exclusive third party license. Under the Technology License Agreement, Audeo Discovery Pty Limited is subject to development obligations, reporting obligations, sublicense revenue sharing requirements, royalty payments and other obligations. In particular, Audeo Discovery Pty Limited must pay Alchemia Limited quarterly royalty payments equal to 5% of the net revenue received by Audeo Discovery Pty Limited in each fiscal quarter in respect of sales of any products or services based on, incorporating or made utilizing the VAST technology, or sub-licensing of the VAST technology. In that regard, the license agreement does not permit us to sub-license any of the VAST technology without Alchemia Limited’s prior consent, which may not be unreasonably withheld.
The Technology License Agreement requires Audeo Discovery Pty Limited to deploy staff with relevant scientific qualifications occupying a minimum of four full-time equivalent positions to develop and exploit the VAST technology and to expend a minimum of AUD$500,000 per annum (with a target of expending AUD$1.5 million per annum) on developing, marketing and exploiting the VAST technology and otherwise using its best endeavors to exploit the technology. Failure to meet the minimum expenditure requirement may give rise to a termination right on the part of Alchemia Limited, as discussed below. If Audeo Discovery Pty Limited fails to meet the targeted amount of expenditure of AUD$1.5 million per annum, Alchemia Limited will have the right to require that Audeo Discovery Pty Limited consult with it on changes to the program for developing, marketing and exploiting the VAST technology to reach this annual target amount and, in that case, Audeo Discovery Pty Limited will be required to
-154-
Table of Contents
comply with any commercially reasonable requests of Alchemia Limited regarding any required changes to that program. The agreement will be subject to termination by either party in the event of: (a) the bankruptcy, insolvency, receivership or similar events relating to the other party; (b) failure by the other party to pay any amount due under the agreement for 20 business days after notice requiring payment; (c) a material breach of the agreement by the other party which is not capable of remedy; or (d) a breach of the agreement by the other party which is capable of remedy but is not remedied within 20 business days after notice requiring the breach to be remedied. Alchemia Limited may also terminate the agreement if it is prevented from continuing to license the VAST technology to us due to a third party claim of intellectual property infringement (after legal advice, investigation and consultation with us). The license agreement will continue until terminated in accordance with its terms.
Under the license agreement, Alchemia Limited, after consultation with us, may initially determine whether or not to take any action against a third party that is or may be infringing the VAST technology or to defend the validity of the VAST technology. However, Alchemia Limited is under no obligation to take any such action and, if it chooses to take no action, we will have a right, at our own expense, to take action on behalf of Alchemia Limited. In addition, we are obligated to file, prosecute and maintain the patents and patent applications relating to the VAST technology at our own cost and expense, and we are entitled to make decisions relating to the prosecution and maintenance of the patents and patent applications, subject to consultation with Alchemia Limited.
See “Risk Factors — If we breach any of the agreements under which we license rights to patents or other intellectual property from third parties, we could lose license rights that are important to our business” and “We will not have the right to sub-license the VAST small molecule drug discovery technology without Alchemia Limited’s consent, which may prevent us from commercializing that technology.”
Transition Services Agreement
On August 22, 2012, we entered into a Transition Services Agreement with Alchemia Limited pursuant to which Alchemia Limited may request that we provide certain accounting, payroll, management, investor relations, office facilities, intellectual property management and administrative services to Alchemia Limited following the Demerger. Under the agreement, Alchemia Limited must pay us a monthly service charge and secondment fee and reimburse our out-of-pocket expenses in providing the services. If the cost of providing these services to Alchemia Limited under this agreement exceeds the amounts we are paid, it could adversely affect our results of operations and financial condition. The services are to be provided until six months after the implementation of the Demerger, although Alchemia Limited may terminate the agreement on five business days notice. Either party may terminate the agreement for an uncured breach by the other or if an insolvency event occurs in relation to the other party.
In addition, pursuant to the terms of the Transition Services Agreement, Peter Smith, our Chief Executive Officer and President, and Charles Walker, our Chief Financial Officer, will be seconded, on a part-time basis, to Alchemia Limited as its Chief Executive Officer and Chief Financial Officer respectively for up to six months after the date of the implementation of the Demerger for a total of up to 26 days each over such period. Dr. Smith and Mr. Walker will not receive any additional compensation from us or from Alchemia Limited for their services to Alchemia Limited, although Alchemia Limited will pay us for their services pursuant to the Transition Services Agreement.
Alchemia Limited has agreed not to offer to employ any of our employees until at least six months after the services are last provided by us under the Transition Services Agreement. We have agreed to use all reasonable endeavors to provide the services although we will not be liable for any failure to provide the services in accordance with the terms of the agreement unless such failure is caused by our negligence or wilful act or omission. The parties have agreed to certain financial caps and time limits on any claims under the agreement. Neither party is liable for loss of profits or indirect or other consequential loss in relation to the provision of the services.
On November 6, 2012, Alchemia Oncology entered into a sub-lease in relation to a part of the office space leased in Brisbane, Australia which allows Alchemia Limited to use part of these facilities. This sub-lease provides for Alchemia Limited to pay us rent calculated on a cost recovery basis. The term of the sub-lease is four years.
-155-
Table of Contents
Demerger Deed
On August 22, 2012, we entered into a Demerger Deed with Alchemia Limited which addresses certain commercial, legal and transitional issues arising in connection with our legal separation from Alchemia Limited.
Pursuant to the Demerger Deed, except as specifically provided in the agreements described above and below, following the Demerger, we will have:
| • | the entire economic and commercial benefit of the businesses of Alchemia Oncology; |
| • | the entire economic and commercial risk and liabilities of the businesses of Alchemia Oncology; and |
| • | none of the economic or commercial benefit, risk or liabilities (whenever arising) of the remaining business of Alchemia Limited. |
Alchemia Limited has agreed to indemnify us for any claim or loss suffered by us relating to or against the business of Alchemia Limited (other than the business of Alchemia Oncology). We have agreed to indemnify Alchemia Limited for any claim or loss suffered by Alchemia Limited relating to the business of Alchemia Oncology.
In the Demerger Deed, we and Alchemia Limited acknowledge that once the Demerger is completed, we will not have any rights against Alchemia Limited, and Alchemia Limited will not have any rights against us, except in specified agreed circumstances. Neither we nor Alchemia Limited will have any right to make a claim against the other for liability or loss arising directly or indirectly in relation to the Intra-group Transfer Agreement, the Demerger, the Demerger scheme booklet or the operation of the businesses of Alchemia Oncology prior to the Demerger date (in our case) or the operation of the business of Alchemia Limited prior to the Demerger date (in the case of Alchemia Limited).
If an asset which belongs to the businesses of Alchemia Oncology is not transferred to us within 24 months of the Demerger date, Alchemia Limited must transfer that asset. If an asset which belongs to the business of Alchemia Limited after the Demerger is not transferred to Alchemia Limited within 24 months of the Demerger date, we must transfer that asset.
We agree to use our best endeavors to refund or release any guarantees, indemnities or financial support which is provided by Alchemia Limited in favor of a third party in relation to the businesses of Alchemia Oncology. Alchemia Limited agrees to use its best endeavors to refund or release any guarantees, indemnities or financial support which is provided by us in favor of a third party in relation to the business of Alchemia Limited.
Except as permitted under the Transition Services Agreement, the Technology License Agreement or any other Demerger document described above or below, Alchemia Limited must cease using any intellectual property relating to the businesses of Alchemia Oncology and we must cease using any intellectual property relating to the remaining business of Alchemia Limited (excluding any intellectual property that Alchemia Limited has licensed to us) and the name “Alchemia.” We will rename of our subsidiary, Alchemia Oncology, following implementation of the Demerger.
We have agreed to use our best endeavors to novate or assign any contract which relates to the business of Alchemia Limited after the Demerger. Alchemia Limited agrees to use its best endeavours to novate or assign any contract which relates to the businesses of Alchemia Oncology or Audeo Discovery Pty Limited after the Demerger.
We agreed to cause our subsidiary, Audeo Discovery Pty Limited, to perform, observe and assume full liability for all of the obligations of Alchemia Limited related to certain VAST-related commercial contracts and government grants.
-156-
Table of Contents
We and Alchemia Limited have agreed to assist each other in relation to the management of litigation matters. We have agreed to indemnify Alchemia Limited in respect of liability or loss suffered by Alchemia Limited in connection with a claim arising from such litigation matters commenced prior to the Demerger where that liability relates to the business of Alchemia Oncology. Alchemia Limited has agreed to indemnify us in respect of liability or loss suffered by us in connection with a claim arising from such litigation matters commenced prior to the Demerger where that liability relates to the business of Alchemia Limited (other than the business of Alchemia Oncology).
We may offer employment to each employee of Alchemia Limited (other than certain agreed employees) prior to the Demerger. Alchemia Limited has agreed to indemnify us for any liability arising from any Alchemia Limited current or past employee’s employment with or termination of employment by Alchemia Limited.
From the implementation date of the Demerger, Alchemia Limited has agreed to maintain a directors’ and officers’ insurance policy for the directors of Alchemia Limited for a period of seven years. Alchemia Limited has agreed to either, at its election, assign the benefit of all the insurance policies held by Alchemia Limited that are primarily in respect of the business of Alchemia Oncology to us or cancel them.
Alchemia Limited will remain liable for all of its income tax, and will be entitled to any tax refunds payable to it, for the period prior to and after the Demerger. We will be responsible for all income tax payable by, and will be entitled to any tax refunds payable to, Alchemia Oncology for the period prior to and after the Demerger. We and Alchemia Limited will assist each other in relation to the preparation of our respective tax returns and in the event of any tax audit by a relevant authority. The Demerger Deed also contains provisions as to the handling of any tax audit or review conducted by a governmental agency.
Demerger Implementation Agreement
On August 22, 2012, we entered into the Demerger Implementation Agreement with Alchemia Limited, which was subsequently amended on October 17, 2012 and which sets out the steps required to be taken by each of Alchemia Limited and us to give effect to the Demerger and other steps necessary to give effect to the Demerger.
Under the Demerger Implementation Agreement, we have agreed that we will take certain steps necessary or required to implement the Demerger, including:
| • | applying for admission to NASDAQ, preparing the registration statement of which this prospectus forms a part, and using our best endeavors to ensure that the SEC grants an order of effectiveness for such registration statement and that trading in our shares of common stock commences as soon as possible prior to the date of the Demerger; |
| • | applying for admission to the Australian Securities Exchange, preparing a listing memorandum and using our best endeavours to ensure that the Australian Securities Exchange grants approvals for our application for admission and trading in our CDIs commences as soon as possible following the date of the Demerger; |
| • | doing all things necessary to enable our shares of common stock and the CDIs to be issued; |
| • | if the Demerger scheme becomes effective, executing the underwriting agreement for this offering (if agreed to by Alchemia Limited) and using our best endeavours to ensure this offering proceeds to closing; |
| • | if the scheme becomes effective, registering the transfer of our shares or CDIs required to be transferred under the terms of the scheme. |
| • | issuing or causing to be issued, in the case of our shares, transaction confirmations and, in the case of CDIs, holding statements, within the time required by the Australian Securities Exchange operating rules and U.S. securities laws (as appropriate); and |
| • | issuing the Warrants in accordance with the terms of the Intra-group Transfer Agreement. |
-157-
Table of Contents
Alchemia Limited agrees that it will take certain steps necessary or required to implement the Demerger, including:
| • | promptly preparing and, subject to court approval, dispatching the Demerger scheme booklet; |
| • | promptly appointing an independent expert in connection with the Demerger scheme booklet; |
| • | convening and holding a general meeting of its shareholders; |
| • | applying to the Federal Court of Australia for an order to convene a meeting of its shareholders to approve the Demerger and holding such meeting; |
| • | applying to the Federal Court of Australia for an order approving the Demerger scheme and lodging a copy of the order with the Australian Securities and Investments Commission; |
| • | on or prior to the implementation date of the Demerger, ensuring that its board of directors resolves to pay the dividend, if any, due to Alchemia Limited shareholders if the price of our common stock in this offering multiplied by the number of our shares of common stock distributed to Alchemia Limited shareholders exceeds our book value; |
| • | on the date of the Demerger, applying the entitlement of each Alchemia Limited shareholder as consideration for the transfer by Alchemia Limited to each such shareholder (or to a sale facility agent as nominee for and on behalf of any ineligible foreign shareholders) of our shares of common stock or the CDIs under the terms of the Demerger scheme; |
| • | procuring the sale of our shares of common stock and the CDIs by a sale facility agent in respect of any ineligible foreign shareholders; and |
| • | using its best endeavors to transition away from using our services under the Transition Services Agreement. |
The Demerger Implementation Agreement also contains details of the order in which the obligations of the parties as mentioned are to be performed, and certain other steps to be completed by Alchemia Limited and us in order to give effect to the implementation of the Demerger.
It is a condition to the scheme that we appoint a sales facility agent to sell the CDIs or shares of our common stock on behalf of ineligible foreign shareholders in the Demerger. Under a scheme of arrangement entered into between Alchemia Limited and Alchemia Limited’s shareholders, Alchemia Limited will agree to effect the Demerger (including distributing shares of our common stock or CDIs to Alchemia Limited’s shareholders and the sales facility agent) as contemplated by the Demerger scheme booklet.
It is a condition to implementation of the Demerger that this offering closes by December 31, 2012.
Demerger Deed Poll
On August 22, 2012, we entered into a Demerger Deed Poll under which we covenant, in favor of each Alchemia Limited shareholder who is eligible to participate in the Demerger scheme and persons and entities who have a right to receive Warrants, to:
| • | perform our obligations under the Demerger and do all things necessary or expedient on our part to implement the Demerger; and |
| • | contingent upon completion of this offering by December 31, 2012 and the determination of the Average Trading Price, issue the Warrants to persons and entities who have the right to receive the Warrants in accordance with their terms. |
-158-
Table of Contents
The following table sets forth the beneficial ownership of our common stock as of November 1, 2012, and as adjusted to reflect the distribution of shares of our common stock to Alchemia Limited shareholders in the Demerger and sale of common stock offered by us in this offering, for:
| • | each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common stock; |
| • | each of our named executive officers; |
| • | each of our directors; and |
| • | all of our executive officers and directors as a group; |
Beneficial ownership is determined in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and include shares of common stock issuable upon the exercise of stock options that are immediately exercisable or exercisable within 60 days. Common stock subject to stock options exercisable immediately or within 60 days of November 1, 2012, is deemed to be outstanding for computing the percentage ownership of the person holding these options and the percentage ownership of any group of which the holder is a member but are not deemed outstanding for computing the percentage ownership of any other person. Except as otherwise indicated, all of the shares reflected in the table are shares of common stock and all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. The information is not necessarily indicative of beneficial ownership for any other purpose.
Information regarding any person or group that will beneficially own more than 5% of our outstanding common stock following the Demerger and this offering is based upon Alchemia Limited’s stock records as of November 1, 2012.
Percentage ownership calculations prior to the Demerger and this offering are based on 7,592,020 shares of our common stock outstanding as of November 1, 2012 on a pro forma basis after giving effect to the issuance of an additional aggregate of 92,000 shares of our common stock that we issued to Alchemia Limited on November 5, 2012 as if those shares had been issued as of November 1, 2012. Percentage ownership calculations after the Demerger and this offering are based on the assumption that (i) we will issue 3,250,000 shares of our common stock in this offering (assuming the underwriters do not exercise their overallotment option) and (ii) Alchemia Limited will distribute 7,592,020 shares of our common stock that are currently outstanding and held by it to Alchemia Limited shareholders (or a depositary on their behalf) in the Demerger, which number of shares is calculated on the basis of 280,812,962 ordinary shares of Alchemia Limited outstanding as of November 1, 2012 and the distribution to Alchemia Limited’s shareholders of one share of our common stock for every 37 outstanding ordinary shares of Alchemia Limited. However, the actual number of shares of our common stock distributed in the Demerger will depend upon the number of holders and the number of outstanding ordinary shares of Alchemia Limited as of the record date for the Demerger, which will be approximately four trading days following the date of this prospectus, and will in any event be greater than the number we have assumed for purposes of this prospectus because the terms of the Demerger provide that fractional shares of our common stock that would otherwise be distributed pursuant to the Demerger will be rounded upwards to the nearest whole share. In addition, options to purchase a total of 3,573,000 ordinary shares of Alchemia Limited at exercise prices ranging from AUD$0.280 to AUD$1.040 per share (equivalent to approximately US$0.291 to US$1.079 per share based on the currency exchange rate as of November 1, 2012) were outstanding as of November 1, 2012 and any exercise of these options or any other options that Alchemia Limited may grant prior to the record date for the Demerger would increase the number of ordinary shares of Alchemia Limited outstanding as of such record date. Any increase in the number of holders or the number of outstanding ordinary
-159-
Table of Contents
shares of Alchemia Limited as of the record date for the Demerger compared with the number of holders or the number of outstanding ordinary shares as of November 1, 2012 would increase, and the fact that fractional shares of our common stock distributed in the Demerger will be rounded upwards to the nearest whole share as described above will increase, the number of shares of our common stock distributed in the Demerger above the number of shares we have assumed for the purposes of the following table. To the extent that the total number of shares of our common stock distributed in the Demerger exceeds the 7,592,020 shares currently held by Alchemia Limited, we will issue additional shares of common stock for distribution in the Demerger. As a result, both the number of shares of our common stock that will be distributed in the Demerger and that will be outstanding immediately after this offering will be greater than the respective numbers of shares we have assumed for purposes of the following table. Information in the following table and the notes thereto that includes or reflects shares owned after the Demerger includes all shares of our common stock distributed in the Demerger, regardless of whether the recipients elect to receive them in the form of shares of our common stock or CDIs representing shares of our common stock.
Except as otherwise indicated in the table below, addresses of named beneficial owners are in care of Audeo Oncology, Inc., 100 Pine Street, Suite 2040, San Francisco, California 94111.
| Shares Beneficially Owned Prior to the Demerger and this Offering |
Shares Beneficially Owned After the Demerger and this Offering(1)(2) |
|||||||||||||||
| Name of Beneficial Owner |
Number | Percentage | Number | Percentage | ||||||||||||
| Greater than 5% Stockholders |
||||||||||||||||
| Alchemia Limited(3) |
7,592,020 | 100 | % | 0 | * | |||||||||||
| Entities managed by Allan Gray Australia Pty Ltd and Orbis Investment Management Limited(4) |
0 | * | 1,443,043 | 13.3% | ||||||||||||
| Named Executive Officers and Directors |
||||||||||||||||
| Peter Smith, PhD(5) |
0 | * | 34,746 | * | ||||||||||||
| Charles Walker, MBA |
0 | * | 2,702 | * | ||||||||||||
| Tracey Brown, PhD |
0 | * | 12,447 | * | ||||||||||||
| Wim Meutermans, PhD |
0 | * | 9,003 | * | ||||||||||||
| Stephen Hill, BMBCh, FRCS |
0 | * | 0 | * | ||||||||||||
| Leigh Bonney, MBA |
0 | * | 0 | * | ||||||||||||
| Susan Kelley, MD |
0 | * | 0 | * | ||||||||||||
| Tracie Ramsdale PhD(6) |
0 | * | 36,490 | * | ||||||||||||
| David U’Prichard, PhD |
0 | * | 0 | * | ||||||||||||
| All executive officers and directors as a group (9 persons) |
0 | * | 95,388 | * | ||||||||||||
| * | Represents beneficial ownership of less than 1% of our common stock. |
| (1) | Assumes no purchase of shares of our common stock in this offering by existing Alchemia Limited shareholders. |
| (2) | Reflects distribution by Alchemia Limited to its greater than 5% stockholders and our named executive officers and directors of one share of our common stock for every 37 ordinary shares of Alchemia Limited that they hold as of the record date for the Demerger, with fractional shares of our common stock that would otherwise be distributed in the Demerger being rounded up to the nearest whole share. |
| (3) | Alchemia Limited is located at 3 Hi-Tech Court, Eight Mile Plains, Brisbane, QLD 4113 Australia. |
| (4) | Entities managed by Allan Gray Australia Pty Ltd, or Allan Gray, and Orbis Investment Management Limited, or OIML, an entity affiliated with Allan Gray, are shareholders of Alchemia Limited and are expected to receive shares of our common stock distributed by Alchemia Limited in the Demerger. The shares beneficially owned after the Demerger and this offering are based on the number of ordinary shares of Alchemia Limited held by entities affiliated with OIML as of November 1, 2012. Based on information |
-160-
Table of Contents
| set forth in a Form 604 filed under the Australian Corporations Act with the Australian Securities Exchange on June 1, 2007, and subsequent correspondence with Allan Gray, this includes: (a) 23,976,837 ordinary shares held by various Australian domiciled pooled vehicles, which includes Australian-based pension funds, for which Allan Gray is an investment manager and has been granted voting and investment power; and (b) 29,415,489 ordinary shares held by various pooled vehicles, consisting of Orbis Global Equity Fund (Australia Registered), Orbis Global Equity Fund Limited, Orbis Optimal SA Fund Limited and Orbis SICAV—Global Equity Fund, for which OIML is investment manager and has been granted voting and investment power. According to information provided by Allan Gray, Simon Marais, Chief Executive Officer and director of Allan Gray, has the power to vote and/or provide voting recommendations with respect to the shares held by entities managed by Allan Gray, and William Gray, President and director of OIML, has voting and investment power with respect to the shares held by entities managed by OIML. Each of these individuals disclaims beneficial ownership of such ordinary shares. The principal address of each of these entities is c/o Allan Gray, Level 2, Challis House, 4–10 Martin Place, Sydney, NSW 2000 Australia. |
| (5) | Includes (a) 16,559 shares held by Dr. Smith and (b) 18,187 shares held by Jill M. Ewing, Dr. Smith’s spouse. |
| (6) | Includes (a) 29,831 shares held by Dr. Ramsdale, (b) 4,581 shares held by Merlinium Pty Ltd, for which Dr. Ramsdale is the sole director and has sole voting and investment power, and (c) 2,078 shares held by the Leilah Ramsdale Child Maintenance Trust dated June 18, 2007, for which Dr. Ramsdale is the sole trustee and has sole voting and investment power. |
-161-
Table of Contents
General
The following is a description of some of the terms of our common stock and preferred stock, certain warrants that we will issue following this offering, certain provisions of our amended and restated certificate of incorporation and bylaws, as they will be in effect upon the completion of this offering, and certain provisions of the Delaware General Corporation Law, or the DGCL. This summary does not purport to be complete and is qualified in its entirety by the provisions of our amended and restated certificate of incorporation and bylaws, as they will be in effect upon completion of this offering and the relevant provisions of the DGCL. For more complete information, you should review the forms of our amended and restated certificate of incorporation and bylaws, which are filed as exhibits to the registration statement of which this prospectus is a part.
Immediately following the completion of this offering, our authorized capital stock will consist of 100,000,000 shares, with a par value of $0.001 per share, of which:
| • | 75,000,000 shares are designated as common stock; and |
| • | 25,000,000 shares are designated as preferred stock. |
As of November 5, 2012, we had 7,592,020 shares of our common stock outstanding (all of which were held by Alchemia Limited) and no shares of preferred stock outstanding. As of November 1, 2012, Alchemia Limited had 280,812,962 ordinary shares outstanding and 5,654 shareholders. This prospectus assumes that 7,592,020 shares of our common stock will be distributed by Alchemia Limited to its shareholders in the Demerger. As noted above under “Prospectus Summary — The Offering,” the actual number of shares of our common stock distributed in the Demerger will depend upon the number of holders and the number of outstanding ordinary shares of Alchemia Limited as of the record date for the Demerger, which will be approximately four trading days following the date of this prospectus, and the foregoing assumed number of shares of our common stock to be distributed in the Demerger does not give effect to the fact that the terms of the Demerger provide that fractional shares of our common stock that would otherwise be distributed pursuant to the Demerger will be rounded upwards to the nearest whole share. In addition, options to purchase a total of 3,573,000 ordinary shares of Alchemia Limited at exercise prices ranging from AUD$0.280 to AUD$1.040 per share (equivalent to approximately US$0.291 to US$1.079 per share based on the currency exchange rate as of November 1, 2012) were outstanding as of November 1, 2012 and any exercise of these options or any other options that Alchemia Limited may grant prior to the record date for the Demerger would increase the number of ordinary shares of Alchemia Limited outstanding as of such record date. Any increase in the number of holders or the number of outstanding ordinary shares of Alchemia Limited as of the record date for the Demerger compared with the number of holders or the number of outstanding ordinary shares as of November 1, 2012 would increase, and the fact that fractional shares of our common stock distributed in the Demerger will be rounded upwards to the nearest whole share as described above will increase, the number of shares of our common stock distributed in the Demerger above the number of shares we have assumed for purposes of this prospectus, which will in turn increase the number of shares of our common stock outstanding after this offering. To the extent that the total number of shares of our common stock distributed in the Demerger exceeds the 7,592,020 shares currently held by Alchemia Limited, we will issue additional shares of common stock for distribution in the Demerger. As a result, the actual number of shares of our common stock distributed in the Demerger will be greater than the number of shares set forth above. For an analysis showing how changes in the number of ordinary shares of Alchemia Limited outstanding as of the record date for the Demerger would change the estimated number of shares of our common stock distributed in the Demerger and that will be outstanding immediately upon completion of this offering, see “Prospectus Summary — The Offering.” In addition, Alchemia Limited shareholders will receive CDIs in the Demerger, unless they elect to receive shares of our common stock, which will reduce the number of shareholders of record of our common stock following the Demerger.
-162-
Table of Contents
Common Stock
The holders of common stock are entitled to one vote per share on all matters submitted to a vote of our stockholders on which holders of common stock are entitled to vote and do not have cumulative voting rights. Accordingly, subject to the rights, if any, of any preferred stock outstanding at any time to vote in the election of directors, holders of a majority of the outstanding shares of our common stock may elect all of the directors standing for election. Subject to preferences that may be applicable to any preferred stock outstanding at the time, the holders of outstanding shares of common stock are entitled to receive ratably any dividends declared by our board of directors out of assets legally available. It is our present intention not to pay any cash dividends on our common stock for the foreseeable future. See the section above entitled “Dividend Policy.” Upon our liquidation, dissolution or winding up, holders of our common stock are entitled to share ratably in all assets remaining after payment of or provision for our liabilities and the liquidation preference of any then outstanding shares of preferred stock. Holders of common stock have no preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock.
Preferred Stock
Pursuant to our certificate of incorporation, our board of directors will have the authority, without further action by the stockholders, to issue from time to time up to 25,000,000 shares of preferred stock in one or more series. Our board of directors may designate the rights, preferences, privileges and restrictions of the preferred stock of any series, which may include dividend rights, conversion rights, voting rights, redemption rights, liquidation preference, sinking fund provisions and the number of shares constituting such series. The issuance of preferred stock could have the effect of restricting or preventing the payment of dividends on our common stock (if we were in the future to elect to pay dividends on our common stock) or the amounts, if any, paid to holders of our common stock in the event of our liquidation, dissolution or winding up or diluting the voting power of the common stock. Such issuance could have the effect of decreasing the market price of the common stock. The issuance of preferred stock or even the ability to issue preferred stock could also have the effect of delaying, deterring or preventing a change in control of our company. We currently have no plans to issue any shares of preferred stock.
Warrants
In November and December 2011, Alchemia Limited issued and sold its ordinary shares to investors in a private placement, or the Placement, for total net proceeds of approximately AUD$16.1 million. Pursuant to the terms of the Placement, the investors were given rights, or the Rights, pursuant to which we are required, contingent on completion of this offering by December 31, 2012, to issue Warrants to the investors entitling them to acquire shares of our common stock. The exercise price of the Warrants will be equal to 130% of the volume weighted average price of our common stock for the 60 trading days immediately following the listing of our common stock on the Nasdaq Global Market (we sometimes refer to such volume weighted average price for such period as the Average Trading Price). The Warrants will be issuable approximately 60 trading days subsequent to completion of this offering and the number of Warrants so issued will be equal to the quotient obtained by dividing (1) AUD$8,073,000 (equivalent to approximately US$8,377,352 based on the currency exchange rate as of November 1, 2012) by (2) 130% of the Average Trading Price. Each Warrant will initially be exercisable for one share of our common stock. Assuming the currency exchange rate mentioned above and assuming an Average Trading Price of US$15.00 per share (the midpoint of the estimated price range set forth on the cover page of this preliminary prospectus), approximately 429,608 Warrants (initially exercisable for a like number of Warrant Shares) would be issuable with an exercise price of approximately US$19.50 per share. However, because the number of Warrants and exercise price both depend upon the actual Average Trading Price following this offering and the number of Warrants also depends upon the actual exchange rate between U.S. dollars and Australian dollars following this offering, the actual number of Warrants that we issue (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the actual exercise price will likely differ from the numbers we have
-163-
Table of Contents
assumed for purposes of this preliminary prospectus. See “Prospectus Summary — The Offering” for an analysis that shows how changes in the assumed Average Trading Price change the number of Warrants (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the exercise price of the Warrants and how changes in the assumed currency exchange rate change the number of Warrants (and therefore, the number of Warrants Shares for which the Warrants are exercisable). In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock.
The Warrants will vest and be exercisable from the date that is six months after the date of this offering, or the IPO Date, until the date that is the later to occur of the following, or the Expiry Date:
| • | three years from the IPO Date; and |
| • | the earlier of (A) six months from the date that we announce to the market the results for the primary endpoint of the Phase III clinical trial period for HA-Irinotecan in colorectal cancer and (B) five years from the IPO Date. |
Any Warrant that is not exercised prior to the Expiry Date will automatically expire on the Expiry Date.
CHESS Depositary Interests
In the Demerger, Alchemia Limited’s shareholders will receive CHESS Depository Interests, or CDIs, representing shares of our common stock or, at their option, shares of our common stock. Alchemia Limited will deliver the shares of our common stock represented by the CDIs to a depositary (which will be CHESS Depositary Nominees Pty Ltd, a subsidiary of the Australian Securities Exchange), which will hold such shares on behalf of the holders of the CDIs. The holders of the CDIs will be the beneficial owners of, and will be entitled to exchange their CDIs for, the underlying shares of our common stock held by the depositary. In addition, holders of CDIs will generally be entitled to exercise the same voting and other rights as holders of our common stock, although they will be required to exercise those rights indirectly through the depositary unless they request the depositary to grant them a proxy to vote directly or exchange their CDIs for the underlying shares of our common stock. Nonetheless, holders of CDIs will have substantially the same economic, voting and other rights as holders of our common stock and the CDIs therefore should be considered as the functional equivalents of shares of our common stock. In addition, holders of shares of our common stock, including shares sold in this offering, will be permitted to deliver those shares to the depositary in exchange for CDIs.
The purpose of the CDIs is to facilitate trading, settlement and clearance in Australia by shareholders of Alchemia Limited who would otherwise receive shares of our common stock in the Demerger. Because our common stock will not be listed on a securities exchange in Australia, Australian residents wishing to trade our common stock would be required to do so through The Nasdaq Global Market in transactions settled in U.S. dollars, which may be inconvenient due to time zone and currency differences, among other factors. However, because we plan to list the CDIs on the Australian Securities Exchange, Alchemia Limited shareholders who receive CDIs in the Demerger will be able to trade those CDIs on a local Australian market in transactions settled in Australian dollars. Trading of the CDIs on the Australian Securities Exchange is not expected to commence until approximately seven trading days following the date of this prospectus.
Holders of CDIs will generally be entitled to surrender those CDIs to the depositary in exchange for the underlying shares of common stock, and holders of our common stock will generally be entitled to deliver those shares to the depositary in exchange for CDIs representing those shares. However, investors purchasing shares of our common stock in this offering will not be able to freely resell those shares, or CDIs representing those shares, in Australia during the 12 months after the issue date of those shares in this offering and therefore will not be
-164-
Table of Contents
able to take advantage of any liquidity that may be available for CDIs traded on the Australian Securities Exchange during that period. We intend to apply to the Australian Securities and Investments Commission for waivers from the application of these resale restrictions; however, there is no certainty that all or any of the requested waivers will be granted or, if granted, that such waivers will provide relief from all of these resale restrictions. See “Risk Factors — Risks Relating to our Common Stock — Investors purchasing shares of our common stock in this offering will not be able to freely sell those shares, or CDIs representing those shares, in Australia during the 12 months after the issue date of those shares in this offering and therefore will not be able to take advantage of any liquidity that may be available for CDIs traded on the Australian Securities Exchange during that period.”
Anti-Takeover Effects of Delaware Law and Our Certificate of Incorporation and Bylaws
Provisions in our certificate of incorporation and bylaws, as amended and restated in connection with this offering, may have the effect of delaying, deferring or discouraging another party from acquiring control of us. These provisions and certain provisions of Delaware law, which are described below, could discourage takeovers, coercive or otherwise. These provisions are also designed, in part, to encourage persons seeking to acquire control of us to negotiate first with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us. However, these anti-takeover provisions may prevent a change in control or other takeover of our company that our stockholders consider to be in our best interest, including transactions that might result in a premium being paid over the market price of our common stock, and may also limit the price that investors are willing to pay for our common stock. These provisions may also have the effect of preventing changes in our management.
Undesignated Preferred Stock
As discussed above, our board of directors has the ability, without further action by the stockholders, to designate and issue preferred stock with voting or other rights or preferences that could deter hostile takeovers or delay changes in our control or management.
Limits on Ability of Stockholders to Act by Written Consent or Call a Special Meeting
Our amended and restated certificate of incorporation will provide that our stockholders may not act by written consent. This limit on the ability of stockholders to act by written consent means that any action taken by our stockholders may only be effected at a duly called annual or special meeting, which may lengthen the amount of time required to take stockholder actions.
In addition, our amended and restated bylaws will provide that special meetings of the stockholders may be called only by our board of directors, the chairman of the board of directors, our chief executive officer or our president (in the absence of our chief executive officer). A stockholder may not call a special meeting, which may delay the ability of our stockholders to force consideration of a proposal or for holders controlling a majority of our capital stock to take any action, including the removal of directors.
Requirements for Advance Notification of Stockholder Nominations and Proposals
Our amended and restated bylaws will establish advance notice procedures in order for stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of our board of directors or a committee of the board of directors, to be brought before a stockholder meeting. These procedures may have the effect of preventing a stockholder proposal or the election of a director nominated by a stockholder from being voted upon at a stockholder meeting if the proper procedures are not followed, and may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors or otherwise attempt to obtain control of our company.
-165-
Table of Contents
Board Classification
Our board of directors will be divided into three classes. The directors in each class will serve for a three-year term, one class being elected each year by our stockholders. This system of electing directors may tend to discourage a third party from making a tender offer or otherwise attempting to obtain control of us, because it generally makes it more difficult for stockholders to replace a majority of the directors. In addition, cumulative voting in the election of directors is prohibited, which means that the holders of a majority of our outstanding shares of common stock can elect all directors standing for election.
Limitation on Removal and Appointment of Directors
Our amended and restated bylaws will provide that our directors may be removed only for cause and our amended and restated certificate of incorporation and bylaws will provide that vacancies on our board of directors or newly created directorships resulting from an increase in the number of our directors may be filed only by a majority of the directors then in office, even though less than a quorum, or by the sole remaining director. These provisions will make it more difficult for both our stockholders and a potential acquirer to replace members of our board of directors or appoint additional members to our board of directors.
Super-Majority Requirements to Change Anti-Takeover Provisions
Our amended and restated certificate of incorporation and bylaws will require the approval of the holders of two-thirds of the outstanding shares of our common stock or, in the case of our amended and restated bylaws, the approval of our board of directors to amend our bylaws and certain provisions of our amended and restated certificate of incorporation, as applicable including certain of the anti-takeover provisions described above.
Choice of Forum
Our amended and restated certificate of incorporation will provide that a state or federal court located within the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a breach of fiduciary duty by any director, officer or other employee, any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, or any action asserting a claim against us that is governed by the internal affairs doctrine.
Delaware Anti-Takeover Statute
Upon the closing of this offering, we will be subject to the provisions of Section 203 of the Delaware General Corporation Law regulating corporate takeovers. In general, Section 203 prohibits a publicly held Delaware corporation from engaging, under certain circumstances, in a “business combination” with an “interested stockholder,” as those terms are defined in the DGCL, for a period of three years following the date the person became an interested stockholder unless:
| • | prior to the date of the transaction, our board of directors approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| • | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of our voting stock outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, but not the outstanding voting stock owned by the interested stockholder, (1) shares owned by persons who are directors and also officers and (2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| • | at or subsequent to the date of the transaction, the business combination is approved by our board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by |
-166-
Table of Contents
| the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
Generally, a business combination includes, among other things, a merger, sale of at least 10% of our assets, or other transaction resulting in a financial benefit to the interested stockholder. An interested stockholder is a person who, together with affiliates and associates, owns or, within three years prior to the determination of interested stockholder status, did own 15% or more of our outstanding voting stock. We expect the existence of this provision to have an anti-takeover effect with respect to transactions our board of directors does not approve in advance. We also anticipate that Section 203 may discourage attempts that might result in a premium over the market price for the shares of common stock held by stockholders.
The provisions of Delaware law and the provisions of our amended and restated certificate of incorporation and bylaws described above could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they might also inhibit temporary fluctuations in the market price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions might also have the effect of preventing changes in our management. It is also possible that these provisions could make it more difficult to accomplish transactions that stockholders might otherwise deem to be in their best interests.
Limitation on Liability of Officers and Directors; Indemnification of Directors and Officers
As permitted by the DGCL, our amended and restated certificate of incorporation will include a provision that eliminates the personal liability of a director for monetary damages to us or our stockholders for breach of his or her fiduciary duty as a director, except for liability for:
| • | any breach of the director’s duty of loyalty to us or our stockholders; |
| • | any acts or omissions not in good faith or involving intentional misconduct or a knowing violation of law; |
| • | any unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the DGCL; or |
| • | any transactions from which the director derived an improper personal benefit. |
Our amended and restated certificate of incorporation and bylaws will collectively require us to indemnify our directors and executive officers to the fullest extent permitted by the DGCL or any other applicable law and allow us to indemnify other employees and other agents to the extent permitted by the DGCL or any other applicable law.
We believe that these limitation of liability and indemnification provisions are useful to attract and retain qualified directors and officers.
Transfer Agent and Registrar
Upon the completion of this offering, the transfer agent and registrar for our common stock will be . American Stock Transfer and Trust Company, LLC. The transfer agent’s address is 6201 15th Avenue, Brooklyn, New York 11219.
Market Listing
We have applied to list our common stock on The Nasdaq Global Market under the symbol “AURX.”
Following the demerger, CDIs representing our common stock will be listed on the Australian Securities Exchange under the symbol “AKQ.” The CDIs have been conditionally approved for quotation on the Australian Securities Exchange.
-167-
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for shares of our common stock. Future sales of substantial amounts of shares of common stock, including shares distributed in the Demerger (or CDIs representing those shares) and shares issued upon the exercise of options and warrants, in the public market after this offering, or the possibility or perception of these sales occurring, could adversely affect the prevailing market price for our common stock or impair our ability to raise equity capital.
We estimate that upon the completion of this offering and the Demerger, a total of 10,842,020 shares of our common stock will be outstanding, assuming that Alchemia Limited will distribute 7,592,020 shares of our common stock that are currently outstanding and held by it to Alchemia Limited shareholders (or a depositary on their behalf) in the Demerger, which number of shares is calculated on the basis of 280,812,962 ordinary shares of Alchemia Limited outstanding as of November 1, 2012 and the distribution to Alchemia Limited shareholders of one share of our common stock for every 37 outstanding ordinary shares of Alchemia Limited. However, the actual number of shares of our common stock distributed in the Demerger will depend upon the number of holders and the number of outstanding ordinary shares of Alchemia Limited as of the record date for the Demerger, which will be approximately four trading days following the date of this prospectus, and the foregoing pro forma number of outstanding shares of our common stock to be outstanding after this offering does not give effect to the fact that the terms of the Demerger provide that fractional shares of our common stock that would otherwise be distributed pursuant to the Demerger will be rounded upwards to the nearest whole share. In addition, options to purchase a total of 3,573,000 ordinary shares of Alchemia Limited at exercise prices ranging from AUD$0.280 to AUD$1.040 per share (equivalent to approximately US$0.291 to US$1.079 per share based on the currency exchange rate as of November 1, 2012) were outstanding as of November 1, 2012 and any exercise of these options or any other options that Alchemia Limited may grant prior to the record date for the Demerger would increase the number of ordinary shares of Alchemia Limited outstanding, as of such record date. Any increase in the number of holders or the number of outstanding ordinary shares of Alchemia Limited as of the record date for the Demerger compared with the number of holders or the number of outstanding ordinary shares as of November 1, 2012 would increase, and the fact that fractional shares of our common stock distributed in the Demerger will be rounded upwards to the nearest whole share as described above will increase, the number of shares of our common stock distributed in the Demerger above the number of shares we have assumed for purposes of the discussion in this section. To the extent that the total number of shares of our common stock distributed in the Demerger exceeds the 7,592,020 shares currently held by Alchemia Limited, we will issue additional shares of common stock for distribution in the Demerger. As a result, both the number of shares of our common stock that will be distributed in the Demerger and that will be outstanding immediately after this offering will be greater than the respective numbers of shares we have assumed for purposes of the discussion in this section Each 37 share increase (decrease) in the estimated number of ordinary shares of Alchemia Limited outstanding as of the record date for the Demerger would increase (decrease) estimated the number of shares of our common stock to be distributed in the Demerger and to be outstanding immediately after this offering by one share (before giving effect to the rounding upwards of fractional shares as described above).
-168-
Table of Contents
Of the shares of our common stock to be outstanding upon completion of this offering, all shares of common stock sold in this offering by us, including any shares sold upon exercise of the underwriters’ over-allotment option, will be freely tradable in the public market without restriction or further registration under the Securities Act, unless these shares are held by our “affiliates,” as that term is defined in Rule 144 under the Securities Act, and subject in certain cases to the lock-up agreements referred to below. In addition, 7,592,020 additional shares of our common stock (being the estimated number of shares of our common stock, including shares represented by CDIs, to be distributed in the Demerger) will be freely tradable in the public markets without restriction or further registration under the Securities Act pursuant to Section 3(a)(10) under the Securities Act, unless those shares are held by our “affiliates” as defined above, and subject in certain cases to the lock-up agreements referred to below. As a result of the lock-up agreements described below, the shares of our common stock that we estimate will be outstanding upon completion of this offering and the Demerger will be available for sale in the public markets as follows:
| Date |
Number of Shares Eligible for Sale in Public Market / Percent of Outstanding Stock |
Comment | ||||
| At the date of this prospectus |
9,900,098/91.3 | % | Shares sold in this offering or distributed in the Demerger | |||
| Between 90 and 180 days after the date of this prospectus |
9,900,098/91.3 | % | Shares eligible for sale under Rule 144 | |||
| After 180 days after the date of this prospectus and various times thereafter |
10,842,020/100 | % | Shares eligible for sale under Rules 144 or upon expiration of lock-up agreements | |||
As described under “Underwriting — Lock-Up Agreements,” the lock-up agreements referred to below prohibit, in general and subject to exceptions, sales of shares of our common stock for a period of 180 days after the date of this prospectus without the prior written consent of each of Leerink Swann LLC and Oppenheimer & Co. Inc. Information in the table above is based on lock-up agreements that have been signed through November 5, 2012.
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to public company reporting requirements of the Securities Act for at least 90 days, a person who is not deemed to have been one of our affiliates for purposes of Rule 144 under the Securities Act at any time during the three months preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than our affiliates, is entitled to sell such shares in the public markets (subject to the lock-up agreements referred to below, if applicable) without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then such person is entitled to sell such shares without complying with any of the requirements of Rule 144 (subject to the lock-up agreements referred to below, if applicable).
In general, under Rule 144, as currently in effect, our affiliates or persons selling shares on behalf of our affiliates who have beneficially owned the shares proposed to be sold for at least six months are entitled to sell upon expiration of the lock-up agreements described below (if applicable), within any three-month period beginning 90 days after the effective date of the registration statement of which this prospectus is part, a number of shares that does not exceed the greater of:
| • | 1% of the number of shares of our common stock then outstanding, which we estimate will equal approximately 108,420 shares immediately after this offering, assuming that Alchemia Limited will |
-169-
Table of Contents
| distribute 7,592,020 shares of our common stock that are currently outstanding and held by it to Alchemia Limited shareholders (or a depositary on their behalf) in the Demerger, which number of shares is calculated on the basis of 280,812,962 ordinary shares of Alchemia Limited outstanding as of November 1, 2012 and the distribution to Alchemia Limited shareholders of one share of our common stock for every 37 outstanding ordinary shares of Alchemia Limited. However, as discussed above, the actual number of shares of our common stock distributed in the Demerger and that will be outstanding upon completion of this offering will be greater than the number of shares we have assumed for the purposes of this prospectus. Each 37 share increase (decrease) in the estimated number of ordinary shares of Alchemia Limited outstanding as of the record date for the Demerger would increase (decrease) the estimated number of shares of our common stock to be distributed in the Demerger and to be outstanding immediately after this offering by one share (before giving effect to the rounding upwards of fractional shares as described above); or |
| • | the average weekly trading volume of the common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale. |
Sales under Rule 144 by our “affiliates” or persons selling shares on behalf of our “affiliates” are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
Section 3(a)(10)
Section 3(a)(10) of the Securities Act is an exemption from registration for offers and sales of securities in specified exchange transactions, subject to certain requirements, including obtaining approval from a court or authorized governmental entity that the exchange is fair to the security holders participating in the exchange. Section 3(a)(10) generally allows a stockholder who received shares of our common stock or CDIs pursuant to the Demerger and who is not deemed to be an “affiliate” (as defined above) of our company and who has not been such an “affiliate” of ours within 90 days of the Demerger, to sell these shares and CDIs without regard to Rule 144. Section 3(a)(10) also permits stockholders who are affiliates of our company to sell their shares and CDIs received in the Demerger under Rule 144. We expect that the shares of our common stock and CDIs distributed in the Demerger will be entitled to the exemption provided by Section 3(a)(10), subject to the foregoing limitation applicable to affiliates. Lock-up agreements referred to below limit the ability of certain holders to sell these shares and CDIs.
Lock-Up Agreements
We and our officers, directors, and certain Alchemia Limited shareholders who will receive CDIs or shares of our common stock in the Demerger have entered into lock-up agreements that, in general and subject to exceptions, prohibit us from issuing or selling shares of our common stock and prohibit those persons from selling or otherwise transferring their shares of common stock or CDIs for a period of 180 days after the date of this prospectus, subject to specified exceptions, without the prior written consent of Leerink Swann LLC and Oppenheimer & Co. Inc. For more information see “Underwriting — Lock-Up Agreements” below.
Warrants
In connection with the Placement in November and December of 2011 of ordinary shares to investors of Alchemia Limited, we are required, contingent on completion of this offering by December 31, 2012, to issue Warrants to purchase shares of our common stock to investors who purchased Alchemia Limited ordinary shares in that Placement. The exercise price of the Warrants will be equal to 130% of the volume weighted average price of our common stock for the 60 trading days immediately following the listing of our common stock on the
-170-
Table of Contents
Nasdaq Global Market (we sometimes refer to such volume weighted average price for such period as the Average Trading Price). The Warrants will be issuable approximately 60 trading days subsequent to completion of this offering and the number of Warrants so issued will be equal to the quotient obtained by dividing (1) AUD$8,073,000 (equivalent to approximately US$8,377,352 based on the currency exchange rate as of November 1, 2012) by (2) 130% of the Average Trading Price. Each Warrant will initially be exercisable for one share of our common stock. Assuming the currency exchange rate mentioned above and assuming an Average Trading Price of US$15.00 per share (the midpoint of the estimated price range set forth on the cover page of this preliminary prospectus), approximately 429,608 Warrants (initially exercisable for a like number of Warrant Shares) would be issuable with an exercise price of approximately US$19.50 per share. However, because the number of Warrants and exercise price both depend upon the actual Average Trading Price following this offering and the number of Warrants also depends upon the actual exchange rate between U.S. dollars and Australian dollars following this offering, the actual number of Warrants that we issue (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the actual exercise price will likely differ from the numbers we have assumed for purposes of this preliminary prospectus. See “Prospectus Summary — The Offering “ for an analysis that shows how changes in the assumed Average Trading Price change the number of Warrants (and, therefore, the number of Warrant Shares for which the Warrants are exercisable) and the exercise price of the Warrant and how changes in the assumed currency exchange rate change the number of Warrants (and therefore, the number of Warrants Shares for which the Warrants are exercisable). In addition, the number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on our shares of common stock. The Warrants and the shares of our common stock issued upon exercise of the Warrants will be restricted securities under the Securities Act and may be sold pursuant to Rule 144 or another exemption from registration.
Registration Statements on Form S-8
In connection with this offering, options to purchase 948,000 shares of our common stock were granted under our 2012 Stock Plan at an exercise price equal to the initial public offering price per share of our common stock sold in this offering. For additional information, see “Executive Compensation — 2012 Stock Plan” above. In addition, 1,250,000 shares of our common stock will be available for future award under our 2012 Equity Incentive Plan, plus automatic annual increases in the number of shares available for future award under that plan. For additional information, see “Executive Compensation — 2012 Equity Incentive Plan” above. Shares of common stock issuable upon exercise of those options granted pursuant to our 2012 Stock Plan, and shares of common stock granted, or issuable upon exercise of options or other awards granted, pursuant to our 2012 Equity Incentive Plan will be eligible for resale under the provisions of Rule 144, subject to compliance with the terms of that rule and any applicable lock-up agreements.
In addition, we intend to file a registration statement on Form S-8 under the Securities Act to register the shares of common stock issuable or reserved for issuance under our 2012 Stock Plan and 2012 Equity Incentive Plan. We expect to file this registration statement shortly after this offering. Shares covered by this registration statement will be eligible for sale in the public market upon the expiration or release from the terms of the lock-up agreements, if applicable, and subject to vesting of such shares.
Sales on Behalf of Ineligible Foreign Shareholders
Based on Alchemia Limited’s stock records, we believe that certain Alchemia Limited shareholders reside or are located in jurisdictions where, as a result of local securities laws, they will not be permitted to receive shares of our common stock or CDIs distributed in the Demerger (we sometimes refer to these shareholders as ineligible foreign shareholders). Accordingly, CDIs or shares of our common stock that would otherwise be distributed in the Demerger to these ineligible foreign shareholders will instead be delivered to a sales facility agent, who will then sell those CDIs or shares of our common stock on the Australian Securities Exchange or the Nasdaq Global Market, as applicable, during the approximately 10 trading days following the completion of this
-171-
Table of Contents
offering (or such longer period of time, being no more than approximately three months after the completion of this offering, which the sales facility agent and Alchemia Limited determine is reasonable having regard to the demand for shares of our common stock and the CDIs) at such prices as the sales facility agent determines, and remit the proceeds to the ineligible foreign shareholders. See “Risk Factors — Future sales of shares of our common stock could cause the market price of our common stock to decline. This risk is heightened by the fact that the shares sold in this offering, and the shares of our common stock distributed in the Demerger and CDIs representing those shares will be freely tradable in the public markets immediately upon completion of this offering, and a substantial majority of those shares of our common stock and the CDIs will not be subject to lock-up agreements.”
-172-
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following discussion is a summary of the material U.S. federal income tax consequences applicable to non-U.S. holders (as defined below) of the acquisition, ownership and disposition of our common stock sold pursuant to this offering. This discussion is not a complete analysis of all the potential U.S. federal income tax consequences relating thereto, nor does it address any tax consequences arising under any state, local or non-U.S. tax laws, the U.S. federal estate tax or gift tax rules or any other U.S. federal tax laws. This discussion is based on the Code, Treasury Regulations promulgated thereunder, judicial decisions and published rulings and administrative pronouncements of the Internal Revenue Service, or IRS, all as in effect as of the date of this prospectus. These authorities may change, possibly retroactively, resulting in U.S. federal income tax consequences different from those discussed below.
This discussion is limited to non-U.S. holders who purchase our common stock pursuant to this offering and who hold our common stock as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax considerations that may be relevant to a particular holder in light of that holder’s particular circumstances. This discussion also does not consider any specific facts or circumstances that may be relevant to holders subject to special rules under the U.S. federal income tax laws, including, without limitation, persons that own, or are deemed to own, more than 5% of our capital stock (except to the extent specifically set forth below), certain former citizens or long-term residents of the United States, an integral part or controlled entity of a foreign sovereign, partnerships and other pass-through entities, real estate investment trusts, regulated investment companies, “controlled foreign corporations,” “passive foreign investment companies,” corporations that accumulate earnings to avoid U.S. federal income tax, banks, financial institutions, insurance companies, brokers, dealers or traders in securities, commodities or currencies, tax-exempt organizations, tax-qualified retirement plans, persons subject to the alternative minimum tax, persons holding our common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment or persons deemed to sell our common stock under the constructive sale provisions of the Code.
If a partnership (or other entity taxed as a partnership for U.S. federal income tax purposes) holds our common stock, the U.S. federal income tax treatment of a partner in the partnership generally will depend on the status of the partner and upon the activities of the partnership. Accordingly, partnerships that hold our common stock and partners in such partnerships are urged to consult their tax advisors regarding the specific U.S. federal income tax consequences to them of acquiring, owning or disposing of our common stock.
PROSPECTIVE INVESTORS ARE URGED TO CONSULT THEIR TAX ADVISORS REGARDING THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES TO THEM OF ACQUIRING, OWNING AND DISPOSING OF OUR COMMON STOCK WITH RESPECT TO THEIR PARTICULAR SITUATIONS, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL OR NON-U.S. TAX LAWS, THE U.S. FEDERAL ESTATE OR GIFT TAX RULES, ANY OTHER U.S. FEDERAL TAX LAWS AND ANY APPLICABLE TAX TREATY.
Definition of Non-U.S. Holder
For purposes of this discussion, a non-U.S. holder is any beneficial owner of our common stock that is not a “U.S. person” or a partnership for U.S. federal income tax purposes. A U.S. person is any of the following:
| • | an individual citizen or resident of the United States; |
| • | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
-173-
Table of Contents
| • | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust (i) if a court within the United States is able to exercise primary supervision over the administration of such trust and one or more U.S. persons have the authority to control all substantial decisions of such trust or (ii) that has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person for U.S. federal income tax purposes. |
Distributions on Our Common Stock
As described in the section entitled “Dividend Policy,” we do not anticipate paying cash dividends on our common stock. If, however, we make distributions of cash or property (other than certain pro rata distributions of shares of our common stock) on our common stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a return of capital and will first be applied against and reduce a non-U.S. holder’s adjusted tax basis in its common stock, but not below zero. Any excess will be treated as gain from the sale of stock and will be treated as described under the section entitled “Gain on Sale or Disposition of Our Common Stock” below.
Dividends paid to a non-U.S. holder of our common stock that are not effectively connected with a U.S. trade or business conducted by such holder generally will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends, or such lower rate specified by an applicable tax treaty. To receive the benefit of a reduced treaty rate, a non-U.S. holder must timely furnish to us or our paying agent a valid IRS Form W-8BEN (or applicable successor form) certifying such holder’s qualification for the reduced rate. This certification must be provided to us or our paying agent prior to the payment of dividends and must be updated periodically. If the non-U.S. holder holds the stock through a financial institution or other agent acting on the non-U.S. holder’s behalf, the non-U.S. holder will be required to provide appropriate documentation to the agent, who then will be required to provide certification to us or our paying agent, either directly or through other intermediaries. Non-U.S. holders that do not timely provide us or our paying agent with the required certification, but that qualify for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. holders should consult their tax advisors regarding possible entitlement to benefits under a tax treaty.
If a non-U.S. holder holds our common stock in connection with the conduct of a trade or business in the United States, and dividends paid on the common stock are effectively connected with such non-U.S. holder’s U.S. trade or business (and, if required by an applicable tax treaty, attributable to a permanent establishment maintained by the non-U.S. holder in the United States), dividends paid to the non-U.S. holder will be exempt from the U.S. federal withholding tax described above. To claim the exemption, the non-U.S. holder must furnish to us or our paying agent a valid IRS Form W-8ECI (or applicable successor form), certifying that the dividends are effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States.
Any dividends paid on our common stock that are effectively connected with a non-U.S. holder’s U.S. trade or business (and, if required by an applicable tax treaty, attributable to a permanent establishment maintained by the non-U.S. holder in the United States) generally will be subject to graduated U.S. federal income tax rates, net of deductions and credits, in the same manner as if such holder were a U.S. person. Dividends that are effectively connected with the conduct of a U.S. trade or business and paid to a non-U.S. holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable tax treaty). Non-U.S. holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
-174-
Table of Contents
Gain on Sale or Disposition of Our Common Stock
Subject to the discussions below regarding backup withholding and legislation relating to foreign accounts, a non-U.S. holder generally will not be subject to U.S. federal income tax on any gain realized upon the sale or disposition of our common stock unless:
| • | the gain is effectively connected with the non-U.S. holder’s conduct of a trade or business in the United States and, if required by an applicable tax treaty, attributable to a permanent establishment maintained by the non-U.S. holder in the United States; |
| • | the non-U.S. holder is a nonresident alien individual present in the United States for a period or periods of 183 days or more in the aggregate during the taxable year of the sale or disposition and certain other requirements are met; or |
| • | our common stock constitutes a U.S. real property interest by reason of our status as a U.S. real property holding corporation, or USRPHC, for U.S. federal income tax purposes during the relevant statutory period. |
The gain described in the first bullet point above generally will be subject to U.S. federal income tax at graduated tax rates on a net income basis in the same manner as if such non-U.S. holder were a U.S. person. A non-U.S. holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable tax treaty). Non-U.S. holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
Gain described in the second bullet point above generally will be subject to U.S. federal income tax at a flat 30% rate (or such a lower rate specified by an applicable income tax treaty), but may be offset by U.S. source capital losses of the non-U.S. holder (even though the individual is not considered a resident of the United States), provided that the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, we would be a USRPHC if the fair market value of our interests in U.S. real property equals or exceeds 50% of the aggregate fair market value of our worldwide real property interests and our other assets used or held for use in a trade or business. We believe that we currently are not, and we do not anticipate becoming, a USRPHC for U.S. federal income tax purposes. Because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property interests relative to the fair market value of our non-U.S. real property interests and other trade or business assets, however, there can be no assurance that we will not become a USRPHC in the future. In the event we do become a USRPHC, as long as our common stock is “regularly traded,” as defined by applicable Treasury Regulations, on an established securities market, our common stock will be treated as a U.S. real property interest only with respect to a non-U.S. holder that actually or constructively holds more than 5% of our common stock at any time during the shorter of the five-year period preceding the date of disposition or the non-U.S. holder’s holding period. We expect our common stock to be “regularly traded” on an established securities market, although there can be no assurance that it will be so traded. If gain on the sale or other taxable disposition of our stock were subject to taxation under the third bullet point above, the non-U.S. holder would be subject to regular U.S. federal income tax with respect to such gain in generally the same manner as a U.S. person.
Information Reporting and Backup Withholding
Generally, we must report annually to the IRS and to each non-U.S. holder the amount of dividends paid to such non-U.S. holder, the name and address of the non-U.S. holder, and the amount of any tax withheld with respect to those dividends. This information also may be made available under a specific treaty or agreement with the tax authorities of the country in which the non-U.S. holder resides or is established. Under certain
-175-
Table of Contents
circumstances, the Code imposes an information reporting and a backup withholding obligation (currently at a rate of 28% but scheduled to increase to 31% for payments made after December 31, 2012) on certain reportable payments such as dividends paid on or the gross proceeds from disposition of our common stock. Backup withholding generally will not, however, apply to payments of dividends to a non-U.S. holder of our common stock provided the non-U.S. holder furnishes to us or our paying agent the required certification as to its non-U.S. status, such as by providing a valid IRS Form W-8BEN or W-8ECI, or otherwise establishes an exemption. Notwithstanding the foregoing, backup withholding may apply if either we or our paying agent has actual knowledge, or reason to know, that the holder is a U.S. person that is not an exempt recipient.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
Legislation Relating to Foreign Accounts
Legislation enacted in 2010 may impose withholding taxes on certain types of payments made to “foreign financial institutions” (as specially defined under these rules) and certain other non–U.S. entities. Under this legislation, the failure to comply with additional certification, information reporting and other specified requirements could result in withholding tax being imposed on payments of dividends and sales proceeds to foreign intermediaries and certain non-U.S. holders. The legislation imposes a 30% withholding tax on dividends on, or gross proceeds from the sale or other disposition of, our common stock paid to a foreign financial institution or to a foreign non-financial entity, unless (i) the foreign financial institution undertakes certain diligence and reporting obligations or (ii) the foreign non-financial entity either certifies it does not have any substantial U.S. owners or furnishes identifying information regarding each substantial U.S. owner. If the payee is a foreign financial institution, it must enter into an agreement with the U.S. Treasury requiring, among other things, that it undertake to identify accounts held by certain U.S. persons or U.S.-owned foreign entities, annually report certain information about such accounts, and withhold 30% on payments to account holders whose actions prevent it from complying with these reporting and other requirements. Under certain transition rules, any obligation to withhold under the legislation with respect to dividends on our common stock will not begin until January 1, 2014 and with respect to the gross proceeds of a sale or other disposition of our common stock will not begin until January 1, 2017. Under certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. Prospective investors should consult their tax advisors regarding the possible implications of this legislation on their investment in our common stock.
-176-
Table of Contents
Subject to the terms and conditions set forth in an underwriting agreement dated the date of this prospectus among us, our subsidiary, Alchemia Oncology Pty Limited, an Australian company which we sometimes refer to as Alchemia Oncology, and the underwriters named below, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase from us, the number of shares of common stock listed next to its name in the following table. Leerink Swann LLC and Oppenheimer & Co. Inc. are acting as joint book-running managers for the offering and as representatives of the underwriters.
| Number of Name Shares |
||||
| Leerink Swann LLC |
||||
| Oppenheimer & Co. Inc. |
||||
|
|
|
|||
| Total |
3,250,000 | |||
|
|
|
|||
The underwriters are committed to purchase all the shares of common stock offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated. The underwriters are not obligated to purchase the shares of common stock covered by the underwriters’ over-allotment option described below.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of customary closing documents. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Over-Allotment Option
We have granted the underwriters an option to purchase up to additional 487,500 shares of common stock at the public offering price appearing on the cover page of this prospectus, less underwriting discounts and commissions. The underwriters may exercise this option for 30 days from the date of this prospectus solely to cover sales of shares of common stock by the underwriters in excess of the total number of shares set forth in the table above. If any shares are purchased pursuant to this over-allotment option, the underwriters will purchase their pro rata portions of the additional shares in approximately the same respective proportions as shown in the table above. If any of these additional shares are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares reflected in the table above are being offered.
Discounts and Commissions
The underwriters propose initially to offer the shares to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. The underwriters may allow, and the dealers may reallow, a discount not in excess of $ per share to other dealers. After the initial offering of the shares, the public offering price and other selling terms may be changed by the representatives.
The following table shows the public offering price, underwriting discounts and commissions and proceeds before expenses to us. The information is shown on a per share basis and also is shown in the aggregate, assuming either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Share | Without Option | With Option | ||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discounts and commissions |
$ | $ | $ | |||||||||
| Proceeds, before expenses, to us |
$ | $ | $ | |||||||||
-177-
Table of Contents
The expenses of the offering that are payable by us are estimated to be approximately $2.9 million (excluding underwriting discounts and commissions), of which approximately $0.2 million had been paid through September 30, 2012.
Initial Public Offering Pricing
Prior to this offering, there has been no public market for our common stock. The initial public offering price will be negotiated between us and the representatives. The factors considered in these negotiations may include, among others:
| • | the prospects for our company and the industry in which we operate; |
| • | our past and present financial and operating performance; |
| • | financial and operating information and market valuations of publicly traded companies engaged in activities similar to ours; |
| • | an assessment of our management; |
| • | the prevailing conditions of U.S. securities markets at the time of this offering; and |
| • | other factors deemed relevant. |
The estimated initial public offering price range set forth on the cover of this preliminary prospectus is subject to change as a result of market conditions and other factors.
Lock-Up Agreements
We and our officers, directors, Alchemia Limited, and certain Alchemia Limited shareholders who will receive CDIs or shares of our common stock in the Demerger have agreed with the underwriters, subject to certain exceptions, not to dispose of any of our shares of common stock, CDIs or securities convertible into or exercisable or exchangeable for shares of our common stock or CDIs during the period from the date of this prospectus continuing through the date 180 days after the date of this prospectus, except with the prior written consent of Leerink Swann LLC and Oppenheimer & Co. Inc.
In the case of the lock-up agreements applicable to our officers, directors, Alchemia Limited and certain Alchemia Limited shareholders, the restrictions described in the immediately preceding paragraph do not apply to, among other things:
| • | transactions relating to shares of our common stock, CDIs or other securities acquired in open market transactions after the closing date of this offering, provided that no filing under Section 16(a) of the Exchange Act, or other public notice, filing or announcement, is required or voluntarily made in connection with subsequent sales or transfers of our common stock, CDIs or other securities acquired in such open market transactions; |
| • | transfers or contributions of shares of our common stock, CDIs or any security convertible into or exercisable or exchangeable for shares of our common stock or CDIs (i) as a bona fide gift, in connection with estate planning or upon death by will or intestate succession; (ii) to an immediate family member (as defined) of the applicable security holder or to any trust for the direct or indirect benefit of the security holder or the immediate family of the security holder; (iii) if the applicable security holder is a trust, to a settlor, grantor or beneficiary of the trust; or (iv) if the applicable security holder is a company, corporation, partnership, limited liability company or similar entity, to limited |
-178-
Table of Contents
| partners, members, stockholders or “affiliates” (as defined by Rule 12b-2 under the Exchange Act) of the security holder or to any investment fund or other entity controlled or managed by the security holder or as part of a disposition, transfer or distribution by the security holder to the security holder’s equity holders; provided that in each case, the donee, trustee, settlor, grantor, beneficiary, transferee or distributee, as the case may be, signs and delivers a lock-up agreement and no filing under Section 16(a) of the Exchange Act, or other public notice, filing or announcement, reporting a reduction in beneficial ownership of shares of our common stock, CDIs or any security convertible into or exercisable or exchangeable for shares of our common stock or CDIs is required or voluntarily made during the 180-day restricted period described above; |
| • | sales or transfers of shares of our common stock to us pursuant to the exercise by a security holder on a net-issuance basis of any of the Warrants, provided that such net-issuance does not involve the sale or other disposition of any shares of our common stock issued or issuable upon exercise of such Warrants or any CDIs evidencing or representing such shares; |
| • | transfers of shares of our common stock, CDIs or any securities convertible into or exercisable for shares of our common stock or CDIs if required by a domestic relations order or a negotiated divorce settlement; |
| • | exercise of any options to purchase shares of our common stock granted pursuant to our 2012 Stock Plan or 2012 Equity Incentive Plan and exercise of any Warrants, provided that the shares of our common stock issued upon exercise of any such options or Warrants and any CDIs evidencing or representing such shares will be subject to the lock-up restrictions described above; |
| • | the transfer of up to 625 shares of our common stock (or CDIs representing up to such number of shares of our common stock) from Tracie Ramsdale, a member of our board of directors, provided that such transfer shall not involve disposition for value; or |
| • | the distribution by Alchemia Limited, in connection with the Demerger, of shares of our common stock and CDIs to holders of ordinary shares of Alchemia Limited and to a depositary holding shares of our common stock represented by CDIs and, in the case of certain holders of Alchemia Limited’s ordinary shares who, under applicable local securities or similar laws, are not permitted to receive shares of our common stock or CDIs in the Demerger, to a sales agent on behalf of such shareholders, provided that any shares of our common stock or CDIs not so distributed by Alchemia Limited shall be returned to us. |
In the case of the lock-up agreement applicable to us, the restrictions described above do not apply to, among other things:
| • | the sale of shares of our common stock to the underwriters; |
| • | our issuance of the Warrants and shares of our common stock issued upon exercise of the Warrants; |
| • | the grant by us of stock options or other stock-based awards (and the issuance of shares of our common stock upon exercise of such options or awards or of any options outstanding on the date of this prospectus) to eligible participants pursuant to our 2012 Stock Plan or 2012 Equity Incentive Plan, provided that, prior to the grant of any such stock options or other stock-based awards that vest within the 180-day restricted period described above, each recipient of such grant signs and delivers a lock-up agreement (if such recipient has not previously signed such a lock-up agreement); |
| • | the filing of registration statements on Form S-8 or any successor form with respect to the registration of securities to be offered under our 2012 Stock Plan or 2012 Equity Incentive Plan; or |
| • | the issuance of additional shares of our common stock in the Demerger pursuant to the Demerger scheme. |
-179-
Table of Contents
Leerink Swann LLC and Oppenheimer & Co. Inc., in their sole discretion, may release the common stock and other securities subject to the lock-up agreements described above in whole or in part at any time without notice. When determining whether or not to release common stock or other securities from the lock-up agreements, Leerink Swann LLC and Oppenheimer & Co. Inc. may consider, among other factors, the reasons for requesting the release, the number of shares of common stock and other securities for which the release is being requested and market conditions at the time.
Indemnification
We and Alchemia Oncology have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
The Nasdaq Global Market Listing
We have applied to have our common stock listed on the Nasdaq Global Market under the symbol “AURX.”
Following the Demerger, CHESS Depository Interests representing shares of our common stock will be listed on the Australian Securities Exchange under the symbol “AKQ.” The CDIs have been conditionally approved for quotation on the Australian Securities Exchange.
Price Stabilization, Short Positions and Penalty Bids
In order to facilitate the offering of our common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock. Specifically, the underwriters may sell more shares than they are obligated to purchase under the underwriting agreement, creating a short position. “Covered” short sales are sales made in an amount not greater than the underwriters’ over-allotment option to purchase additional shares of common stock in the offering. The underwriters may close out any covered short position by either exercising their over-allotment option or by engaging in “short covering” transactions, which means the purchase of shares of common stock in the open market. In determining the source of shares of common stock to close out the covered short position, the underwriters may consider, among other things, the price of shares available for purchase in the open market as compared with the price at which they may purchase shares through the over-allotment option. “Naked” short sales, which are common in the United States, are sales made in an amount in excess of the over-allotment option and must be closed out by purchasing shares of common stock in the open market. A naked short position is more likely to be created if there is a concern that there may be downward pressure on the price of the common stock in the open market after pricing that could adversely affect investors who purchase in the offering. In order to comply with applicable Australian legal requirements, the underwriters will not be permitted to create a naked short position in connection with this offering, which will limit their ability to combat downward pressure on the market price of our common stock, which may adversely affect the market price of our common stock. The underwriters may also engage in stabilizing transactions, which consist of various bids for or purchases of shares of common stock made by the underwriters in the open market prior to the completion of this offering for the purpose of preventing or retarding a decline in the market price of common stock.
The underwriters may also impose penalty bids on members of the underwriting syndicate. Penalty bids permit the representatives of the underwriters to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transactions to cover syndicate short positions.
These syndicate covering transactions, stabilizing transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market
-180-
Table of Contents
price of our common stock. As a result, the market price of our common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on the Nasdaq Global Market or otherwise. Although the underwriters will not undertake any of these transactions with respect to the CDIs representing shares of our common stock, which CDIs will be listed on the Australian Securities Exchange, these syndicate covering transactions, stabilizing transactions and penalty bids with respect to our common stock may also have the effect of raising or maintaining the market price of our CDIs or preventing or retarding a decline in the market price of our CDIs and, as a result, the market price of our CDIs may be higher than the price that would otherwise exist in the open market.
Neither we nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above, if commenced, may have on the price of our common stock. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Offer, Sale and Distribution of Shares
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares of common stock to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on the websites of the underwriters and any selling group members and any information contained in any other website maintained by any of the underwriters and any selling group members is not part of this prospectus or the registration statement of which this prospectus forms a part.
Discretionary Sales
The underwriters have informed us that they do not intend to confirm sales to discretionary accounts that exceed 5% of the total number of shares offered by them.
Stamp Taxes
If you purchase shares of common stock offered in this prospectus, you may be required to pay stamp taxes and other charges under the laws and practices of the country or other jurisdiction of purchase, in addition to the offering price listed on the cover page of this prospectus.
Other Relationships
The underwriters and their affiliates may in the future provide investment banking, commercial banking and advisory services to us from time to time for which they expect to receive customary compensation and expense reimbursement.
Selling Restrictions
European Economic Area
In relation to each member state of the European Economic Area that has implemented the Prospectus Directive (each, a relevant member state), with effect from and including the date on which the Prospectus Directive is implemented in that relevant member state (the relevant implementation date), an offer of shares of common stock described in this prospectus may not be made to the public in that relevant member state other than:
| • | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
-181-
Table of Contents
| • | to fewer than 100 or, if the relevant member state has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive subject to obtaining the prior consent of the representatives of the underwriters for any such offer; or |
| • | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of shares of common stock shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
For purposes of this provision, the expression an “offer of shares of common stock to the public” in any relevant member state means the communication in any form and by any means of sufficient information on the terms of the offer and the shares of common stock to be offered so as to enable an investor to decide to purchase or subscribe the shares of common stock, as the expression may be varied in that relevant member state by any measure implementing the Prospectus Directive in that relevant member state, and the expression “Prospectus Directive” means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the relevant member state), and includes any relevant implementing measure in each relevant member state and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
We have not authorized and do not authorize the making of any offer of shares of common stock through any financial intermediary on our behalf, other than offers made by the underwriters with a view to the final placement of the shares of common stock as contemplated in this prospectus. Accordingly, no purchaser of the shares of common stock, other than the underwriters, is authorized to make any further offer of the shares of common stock on behalf of us or the underwriters.
United Kingdom
This prospectus is only being distributed to, and is only directed at, persons who are also (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”), (ii) high net worth entities, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order, or (iii) are persons to whom an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000, as amended (the “FSMA”) (all such persons together being referred to as “relevant persons”). This prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom that is not a relevant person should not act or rely on this prospectus or any of its contents.
The applicable provisions of the FSMA must be complied with in respect of anything done in relation to the offering of the shares of common stock in, from or otherwise involving the United Kingdom.
Australia
No prospectus or any other disclosure document (as defined in the Corporations Act 2001 (Cth) of Australia, or the Australian Corporations Act) in relation to the offer contained in this prospectus and the registration statement of which this prospectus forms a part has been, nor will they need to be, lodged with the Australian Securities & Investments Commission, or ASIC. This prospectus and the registration statement of which this prospectus forms a part is not a “Prospectus” under Chapter 6D of the Australian Corporations Act. Any offer of shares of our common stock in Australia is made only to persons to whom it is lawful to offer shares of our
-182-
Table of Contents
common stock without disclosure under one or more of certain of the exemptions set out in section 708 of the Australian Corporations Act, or an “exempt person.” This document has not been lodged with ASIC and is only directed to certain categories of exempt persons. Accordingly, if you receive this document in Australia:
| (a) | you confirm and warrant that you are either: |
| (i) | a “sophisticated investor” under section 708(8)(a) or (b) of the Australian Corporations Act; |
| (ii) | a “sophisticated investor” under section 708(8)(c) or (d) of the Australian Corporations Act and that you have provided an accountant’s certificate to us which complies with the requirements of section 708(8)(c)(i) or (ii) of the Australian Corporations Act and related regulations before the offer has been made; |
| (iii) | a person associated with us under section 708(12) of the Australian Corporations Act (an “associated person”); or |
| (iv) | a “professional investor” within the meaning of section 708(11)(a) or (b) of the Australian Corporations Act; |
and to the extent that you are unable to confirm or warrant that you are an exempt sophisticated investor, associated person or professional investor under the Australian Corporations Act, any offer made to you under this document is void and incapable of acceptance; and
| (b) | you warrant and agree that you will not offer any of the shares of our common stock purchased in this offering (or any CDIs representing these shares) for resale in Australia within 12 months of those shares of common stock being issued unless any such resale offer is exempt from the requirement to issue a disclosure document under section 708 or 708A of the Australian Corporations Act. |
Hong Kong
The shares of our common stock may not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance, or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the shares of our common stock may be issued, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares of our common stock which are or are intended to be disposed of only to persons outside of Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
Israel
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, and has not been filed with or approved by the Israel Securities Authority. In Israel, this prospectus is being distributed only to, and is directed only at, investors listed in the first addendum, or the Addendum, to the Israeli Securities Law, consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters purchasing for their own account, venture capital funds, entities with equity in excess of NIS 50 million and “qualified individuals”, each as defined in the Addendum (as it may be amended from time to time, collectively referred to as qualified investors). Qualified investors may be required to submit written confirmation that they fall within the scope of the Addendum.
-183-
Table of Contents
New Zealand
This prospectus has not been registered with the office of the Registrar of Companies in New Zealand and is not a registered prospectus or investment statement for the purposes of New Zealand law.
The provision of this prospectus to any person in New Zealand does not constitute an offer of the shares of our common stock to that person or an invitation to that person to subscribe for the shares of our common stock other than (i) to any or all of the following persons only (A) to persons whose principal business is the investment of money or who, in the course of and for the purposes of their business, habitually invest money, and/or (B) persons who are each required to pay a minimum subscription price of at least NZ$500,000 for the shares of our common stock, and/or (C) any other person who in all the circumstances can properly be regarded as having been selected other than as members of the public; or (ii) to eligible persons only in accordance with section 5(2CB) of the Securities Act 1978 (New Zealand).
No investor shall subscribe for, offer, sell or deliver any shares of our common stock or distribute this prospectus or any advertisement relating to the shares of our common stock in breach of the Securities Act 1978 and, in particular, no investor shall offer for sale shares of our common stock to any member of the public in New Zealand in breach of the Securities Act 1978. By subscribing for the shares of our common stock, each investor: (a) warrants it is a person described in paragraph (i) or (ii) above and (b) undertakes to comply with the above selling restrictions.
Singapore
This prospectus has not been registered with the Monetary Authority of Singapore. Accordingly, the underwriters have not offered or sold any shares of our common stock or caused the shares of our common stock to be made the subject of an invitation for subscription or purchase and may not offer or sell any shares of our common stock or cause the shares of our common stock to be made the subject of an invitation for subscription or purchase, and have not circulated or distributed, nor will they circulate or distribute, the prospectus or any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares of our common stock, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the SFA, (ii) to a relevant person, or any person pursuant to Section 257(1A), and in accordance with the conditions, specified in Section 275 of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
The underwriters will notify (whether through the distribution of the prospectus or otherwise) each of the following relevant persons specified in Section 275 of the SFA which has subscribed or purchased shares of our common stock from or through that underwriter, namely a person which is:
| • | a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| • | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor. |
Shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for six months after that corporation or that trust has acquired the shares of our common stock under Section 275 except:
| • | to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; |
| • | where no consideration is given for the transfer; or |
| • | by operation of law. |
-184-
Table of Contents
The validity of the shares of common stock offered hereby will be passed upon for us by Wilson Sonsini Goodrich & Rosati, Professional Corporation, Palo Alto, California. Certain matters of Australian law will be passed upon for us by Corrs Chambers Westgarth, Brisbane, Australia. Sidley Austin LLP, San Francisco, California, will act as counsel to the underwriters.
Our consolidated financial statements as of June 30, 2011 and 2012, and for each of the two years in the period ended June 30, 2012, appearing in this prospectus and registration statement have been audited by Ernst & Young, an independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given the authority of such firm as experts in accounting and auditing. The liability of Ernst & Young with respect to claims arising out of its audit report is subject to limitations pursuant to Australian law. See the section below entitled “Limitation on Independent Registered Public Accounting Firm’s Liability.”
LIMITATION ON INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM’S LIABILITY
Our registered public accounting firm, Ernst & Young, is an Australian partnership and a member of the global network of Ernst & Young firms. The liability of Ernst & Young with respect to claims arising out of its audit report included in this prospectus is subject to the limitations set forth in the Institute of Chartered Accountants in Australia Scheme (Queensland), or ICAA Scheme, approved under the Professional Standards Act 2004 of Queensland, Australia, as amended, which we refer to as the Professional Standards Act. The ICAA Scheme, which commenced on February 1, 2008, may limit the liability of Ernst & Young for damages with respect to certain civil claims arising in, or governed by the laws of, Queensland directly or vicariously from anything done or omitted in the performance of its professional services for us, including, without limitation, its audits of our financial statements included in this prospectus, to the lesser of (in the case of audit services) ten times the reasonable charge for services provided over AUD$100,000 and up to a maximum liability of AUD$75 million (equivalent to approximately US$77.8 million, based on currency exchange rates as of November 1, 2012).
In addition, there is equivalent professional standards legislation in place in other states and territories in Australia and amendments have been made to a number of Australian federal statutes to limit liability under those statutes to the same extent as liability is limited under state and territory laws by professional standards legislation.
These limitations of liability may limit recovery upon the enforcement in Australian courts of any judgment under U.S. or foreign laws rendered against Ernst & Young based on or related to its audit report on our financial statements. However, to our knowledge the Professional Standards Act has not been subject to judicial consideration in Australia and therefore how the limitation will be applied by the courts and the effect of the limitation on the enforcement of foreign judgments are untested.
ENFORCEABILITY OF FOREIGN JUDGMENTS IN AUSTRALIA
Audeo Oncology, Inc., the issuer of the common stock in this offering, is a newly formed Delaware corporation whose assets consist of primarily the capital stock of its two subsidiaries. Those subsidiaries, Alchemia Oncology and Audeo Discovery Pty Limited, which holds the in-license to the VAST technology, are both companies incorporated in Australia. Apart from a small amount of office space in San Francisco, all of our
-185-
Table of Contents
facilities and all or substantially all of our other assets are located in Australia and are owned by our Australian subsidiaries. In addition, all of our officers and two of our directors reside in Australia, and the independent accountants named herein are located in Australia, and all or substantially all of their respective assets are or may be located outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon our subsidiaries or such persons. It is highly unlikely that an Australian court would be able to enforce proceedings in relation to federal securities laws of the United States that had not first proceeded to a final and conclusive judgment in the United States. In addition, a judgment of a U.S. court (whether or not such judgment relates to U.S. federal securities laws) against us or our subsidiaries, directors or employees may not be enforceable in Australia in certain circumstances, including where the judgment contravenes local public policy, was obtained by fraud or breach of natural justice, is not final or conclusive, is subject to a declaration under the Foreign Proceedings (Excess of Jurisdiction) Act 1984 (Cth), is not for a fixed or readily ascertainable sum, or is of a penal nature. Further, our independent accountants are entitled to protections described above under “Limitation on Independent Registered Public Accounting Firm’s Liability.”
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered by this prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement, some of which is contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and our common stock, we refer you to the registration statement, including the exhibits filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract or any other document are not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please see the copy of the contract or document that has been filed. Each statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit. You may obtain copies of this information at the Public Reference Room of the SEC, 100 F Street, N.E., Room 1580, Washington, D.C. 20549, at prescribed rates. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy statements and other information about issuers, like us, that file electronically with the SEC and you may review and obtain a copy of the registration statement and the exhibits on that website. The address of that website is www.sec.gov.
As a result of this offering, we will become subject to the information and reporting requirements of the Securities Exchange Act of 1934 and, in accordance with this law, we will file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information will be available for inspection and copying at the SEC’s Public Reference Room and the website of the SEC referred to above. We also maintain a website at www.audeooncology.com. Upon completion of this offering, you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on, or that can be accessed through, our website or the Alchemia Limited website is not incorporated by reference into this prospectus, and you should not consider information on our website or the Alchemia Limited website to be part of this prospectus.
-186-
Table of Contents
(a development stage company)
Index to Consolidated Financial Statements
| Page | ||||
| F-2 | ||||
| Consolidated Financial Statements as of and for the Years Ended June 30, 2011 and 2012, and the Period from May 29, 2006 (Inception) to June 30, 2012 |
||||
| F-3 | ||||
| Consolidated Statements of Operations and Comprehensive Loss |
F-4 | |||
| Consolidated Statements of Changes in Stockholders’ Equity (Deficit) |
F-5 | |||
| F-6 | ||||
| F-7 | ||||
| Unaudited Interim Condensed Consolidated Financial Statements as of and for the Three Months Ended September 30, 2011 and 2012 |
||||
| F-27 | ||||
| Unaudited Interim Condensed Consolidated Statements of Operations and Comprehensive Loss |
F-28 | |||
| Unaudited Interim Condensed Consolidated Statements of Changes in Stockholders’ Equity (Deficit) |
F-29 | |||
| Unaudited Interim Condensed Consolidated Statements of Cash Flows |
F-30 | |||
| Notes to the Unaudited Interim Condensed Consolidated Financial Statements |
F-31 | |||
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Audeo Oncology, Inc.
We have audited the accompanying consolidated balance sheets of Audeo Oncology, Inc. (a development stage company) (the “Company”) as of June 30, 2011 and 2012, and the related consolidated statements of operations and comprehensive loss, changes in stockholders’ equity/(deficit), and cash flows for each of the two years in the period ended June 30, 2012 and the period from May 29, 2006 (date of inception) to June 30, 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Audeo Oncology, Inc. (a development stage company) at June 30, 2011 and 2012, and the consolidated results of its operations and comprehensive loss and its cash flows for each of the two years in the period ended June 30, 2012 and the period from May 29, 2006 (date of inception) to June 30, 2012, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young
Brisbane, Australia
August 22, 2012, except for Note 14(e), as to which the date is November 8, 2012.
F-2
Table of Contents
(a development stage company)
CONSOLIDATED BALANCE SHEETS
(U.S. DOLLARS)
| June 30, | ||||||||
| 2011 | 2012 | |||||||
| Assets: |
||||||||
| Current Assets: |
||||||||
| Cash and cash equivalents |
$ | 159,494 | $ | 7,635,670 | ||||
| Short-term deposit |
21,190 | 20,318 | ||||||
| Due from related party |
— | 68,453 | ||||||
| Prepaid research and development expenses |
32,539 | 691,924 | ||||||
| Deferred initial public offering costs |
— | 805,487 | ||||||
|
|
|
|
|
|||||
| Total current assets |
213,223 | 9,221,852 | ||||||
| Non-current assets: |
||||||||
| Property and equipment, net |
484,542 | 251,342 | ||||||
| Non-current prepaid research and development expenses |
213,879 | 239,241 | ||||||
|
|
|
|
|
|||||
| Total non-current assets |
698,421 | 490,583 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 911,644 | $ | 9,712,435 | ||||
|
|
|
|
|
|||||
| Liabilities and stockholders’ equity (deficit): |
||||||||
| Current liabilities: |
||||||||
| Trade and other payables |
$ | 614,096 | $ | 1,859,516 | ||||
| Due to related party |
— | 167,086 | ||||||
| Related party loans |
22,260,705 | — | ||||||
| Employee provisions |
93,483 | 104,071 | ||||||
| Rights |
— | 473,709 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
$ | 22,968,284 | $ | 2,604,382 | ||||
| Non-current liabilities: |
||||||||
| Employee provisions |
62,010 | 75,538 | ||||||
|
|
|
|
|
|||||
| Total non-current liabilities |
62,010 | 75,538 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
23,030,294 | 2,679,920 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity (deficit): |
||||||||
| Common Stock, $0.001 par value: 30,000,000 shares authorized; 1,965,641 shares and 7,592,020 shares issued and outstanding at June 30, 2011 and June 30, 2012, respectively |
1,966 | 7,592 | ||||||
| Additional paid-in capital |
13,596,727 | 51,273,126 | ||||||
| Accumulated other comprehensive loss |
(3,680,787 | ) | (2,259,207 | ) | ||||
| Deficit accumulated during development stage |
(32,036,556 | ) | (41,988,996 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity (deficit) |
(22,118,650 | ) | 7,032,515 | |||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity (deficit) |
$ | 911,644 | $ | 9,712,435 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these financial statements
F-3
Table of Contents
(a development stage company)
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(U.S. DOLLARS)
| Year Ended June 30, | For the period from date of inception (May 29, 2006) to June 30, 2012 |
|||||||||||
| 2011 | 2012 | |||||||||||
| Income: |
||||||||||||
| Grant income |
$ | — | $ | — | $ | 1,508,856 | ||||||
| Research funding income |
— | — | 433,733 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total income |
— | — | 1,942,589 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Research and development |
6,985,879 | 7,806,351 | 38,304,950 | |||||||||
| General and administrative |
929,071 | 1,674,235 | 5,778,183 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
7,914,950 | 9,480,586 | 44,083,133 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(7,914,950 | ) | (9,480,586 | ) | (42,140,544 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other income (expense): |
||||||||||||
| Interest income |
1,682 | 2,913 | 199,136 | |||||||||
| Other income |
— | 6,589 | 433,768 | |||||||||
| Changes in fair value of rights |
— | (481,356 | ) | (481,356 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense) |
1,682 | (471,854 | ) | $ | 151,548 | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
$ | (7,913,268 | ) | $ | (9,952,440 | ) | $ | (41,988,996 | ) | |||
|
|
|
|
|
|
|
|||||||
| Income tax expense |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (7,913,268 | ) | $ | (9,952,440 | ) | $ | (41,988,996 | ) | |||
|
|
|
|
|
|
|
|||||||
| Other comprehensive loss, net of tax: |
||||||||||||
| Foreign currency translation adjustment |
(2,975,795 | ) | 1,421,580 | (2,259,207 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total comprehensive loss |
$ | (10,889,063 | ) | $ | (8,530,860 | ) | $ | (44,248,203 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share — basic and diluted |
$ | (4.03 | ) | $ | (4.95 | ) | $ | (21.28 | ) | |||
|
|
|
|
|
|
|
|||||||
| Basic and diluted weighted average common shares outstanding |
1,965,641 | 2,011,885 | 1,973,231 | |||||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these financial statements
F-4
Table of Contents
(a development stage company)
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
(U.S. DOLLARS)
|
Common stock |
Additional Paid-in Capital |
Accumulated Other Comprehensive Income (Loss) |
Deficit Accumulated During Development Stage |
Total Stockholders’ Equity (Deficit) |
||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
| Balance at inception (May 29, 2006) |
||||||||||||||||||||||||
| Deemed issuance of common stock (Note 1) |
1,965,641 | $ | 1,966 | $ | 13,128,416 | — | — | $ | 13,130,382 | |||||||||||||||
| Net loss (from inception to June 30, 2010) |
— | — | — | — | (24,123,288 | ) | (24,123,288 | ) | ||||||||||||||||
| Foreign translation adjustments (from inception to June 30, 2010) |
— | — | — | (704,992 | ) | — | (704,992 | ) | ||||||||||||||||
| Stock-based compensation (from inception to June 30, 2010) |
— | — | 383,474 | — | — | 383,474 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at June 30, 2010 |
|
1,965,641 |
|
$ | 1,966 | $ | 13,511,890 | $ | (704,992 | ) | $ | (24,123,288 | ) | $ | (11,314,424 | ) | ||||||||
| Net loss |
— | — | — | — | (7,913,268 | ) | (7,913,268 | ) | ||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | (2,975,795 | ) | — | (2,975,795 | ) | ||||||||||||||||
| Stock-based compensation |
— | — | 84,837 | — | — | 84,837 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at June 30, 2011 |
1,965,641 | $ | 1,966 | $ | 13,596,727 | $ | (3,680,787 | ) | $ | (32,036,556 | ) | $ | (22,118,650 | ) | ||||||||||
| Issuance of common stock (Note 6) |
|
5,626,379 |
|
5,626 | 37,577,539 | — | — | 37,583,165 | ||||||||||||||||
| Additional paid-in capital upon the Reorganization (Note 1) |
— | — | 1,966 | — | — | 1,966 | ||||||||||||||||||
| Net loss |
— | — | — | — | (9,952,440 | ) | (9,952,440 | ) | ||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | 1,421,580 | — | 1,421,580 | ||||||||||||||||||
| Stock-based compensation |
— | — | 96,894 | — | — | 96,894 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at June 30, 2012 |
|
7,592,020 |
|
$ | 7,592 | $ | 51,273,126 | $ | (2,259,207 | ) | $ | (41,988,996 | ) | $ | 7,032,515 | |||||||||
The accompanying notes are an integral part of these financial statements
F-5
Table of Contents
(a development stage company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. DOLLARS)
|
Years Ended June 30, |
For the Period from May 29, 2006 (date of inception) to June 30, 2012 |
|||||||||||
| 2011 | 2012 | |||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (7,913,268 | ) | $ | (9,952,440 | ) | $ | (41,988,996 | ) | |||
| Adjustments to reconcile net loss to net cash provided by operating activities: |
||||||||||||
| Depreciation and amortization |
97,626 | 243,644 | 495,269 | |||||||||
| Acquired in-process research and development |
— | — | 14,604,511 | |||||||||
| Stock-based compensation expense |
84,837 | 96,894 | 565,205 | |||||||||
| Stock-based compensation allocated from Alchemia Limited |
30,625 | 91,079 | 651,745 | |||||||||
| Changes in fair value of rights |
— | 481,356 | 481,356 | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Prepaid research and development expenses |
(173,590 | ) | (706,106 | ) | (896,416 | ) | ||||||
| Deferred initial public offering costs |
— | (818,490 | ) | (818,490 | ) | |||||||
| Due from related party |
— | (69,558 | ) | (69,558 | ) | |||||||
| Trade and other payable |
64,353 | 1,291,206 | 1,698,548 | |||||||||
| Due to related party |
— | 169,783 | 169,783 | |||||||||
| Employee provisions |
48,803 | 31,007 | 100,356 | |||||||||
| Short term deposit |
— | — | (20,770 | ) | ||||||||
| Related party loans |
8,404,955 | 16,772,100 | 35,997,321 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by operating activities |
644,341 | 7,630,475 | 10,969,864 | |||||||||
| Cash flows from investing activities |
||||||||||||
| Cash acquired in acquisition of Meditech Research |
— | — | 703,996 | |||||||||
| Purchase of property and equipment |
(539,378 | ) | (26,941 | ) | (676,714 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
(539,378 | ) | (26,941 | ) | 27,282 | |||||||
| Effect of exchange rate changes on cash and cash equivalents |
15,771 | (127,358 | ) | 3,361,476 | ||||||||
| Increase in cash and cash equivalents |
120,734 | 7,476,176 | 7,635,670 | |||||||||
| Cash and cash equivalents at beginning of period |
38,760 | 159,494 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of period |
$ | 159,494 | $ | 7,635,670 | $ | 7,635,670 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplementary disclosure of cash flow information: |
||||||||||||
| Interest received |
$ | 1,682 | $ | 2,913 | $ | 199,136 | ||||||
| Non-cash investing activities |
||||||||||||
| Deemed issuance of common stock issued upon inception to purchase Meditech Research (Note 1) |
— | — | $ | (13,130,382 | ) | |||||||
| Loans from Alchemia Limited to purchase Meditech Research |
— | — | $ | (503,468 | ) | |||||||
| Non-cash financing activities |
||||||||||||
| Deemed issuance of common stock upon conversion of related party loan (Note 6) |
— | $ | (37,585,131 | ) | $ | (37,585,131 | ) | |||||
The accompanying notes are an integral part of these financial statements
F-6
Table of Contents
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 1. | Nature of Business |
Organization and nature of operations
Audeo Oncology, Inc (the “Company”) was incorporated in Delaware, United States of America on June 1, 2012, as a wholly-owned subsidiary of Alchemia Limited (“ACL”). As used herein, the term “Company” means Audeo Oncology, Inc. and its wholly-owned subsidiaries, unless otherwise expressly stated or the context otherwise requires.
The Company does not conduct any substantive operations on its own but instead conducts its principal activities through its wholly-owned subsidiary, Alchemia Oncology Pty Limited (“AOL”), which is located in Australia. It also wholly-owns Audeo Discovery Pty Limited (“Audeo Discovery”), an Australian shell company incorporated on June 21, 2012. The principal activity of the Company is the development of product candidates focused on the utilization of its Hyaluronic Acid Chemotransport Technology platform (“HyACT”). The most advanced product candidate derived from this technology is HA-Irinotecan pursuant for which a pivotal Phase III trial in metastatic colorectal cancer and a Phase II trial in small cell lung cancer are currently in progress.
The Company’s activities to date have included technology development, obtaining research and funding grants, securing patents and intellectual property rights, undertaking pre-clinical studies and clinical trials and securing finance for working capital and capital expenditures. The Company has not yet commenced its planned principal operations and has not generated any significant revenue stream from these activities. Accordingly, the Company is considered to be in the development stage as defined in the Financial Accounting Standards Boards Accounting Standards Codification (“ASC”) Topic 915, “Development Stage Entities”.
In preparation for the planned initial public offering of the Company (“IPO”), on June 28, 2012, ACL, a company listed on the Australian Securities Exchange, transferred its entire equity interest in AOL to the Company in exchange for 7.6 million shares of common stock of the Company (the “Reorganization”). This was accounted for as a reorganization of entities under common control in a manner similar to a pooling-of-interests, with assets and liabilities carried over at their historical amounts. Accordingly, the accompanying consolidated financial statements have been prepared as if the current corporate structure had been in existence throughout the periods presented.
On May 29, 2006 (date of inception), ACL acquired AOL, formerly Meditech Research Limited (“Meditech Research”), in exchange for 13,979,427 shares of ACL. Such acquisition was accounted for as an asset acquisition as Meditech Research was a development stage company that had not yet commenced its planned principal operations and had no significant revenue streams. Meditech Research did not possess the necessary elements of a business because it did not have completed products and, therefore, no ability to access customers. The aggregate purchase consideration of $13,633,850 was primarily allocated to in-process research and development (“IPR&D”), which was pushed down to AOL and expensed immediately as Meditech Research’s research and development programs that had not yet reached technological feasibility and had no alternative future use as of the acquisition date.
The Company has incurred significant net losses since inception and has relied on related party loans provided by ACL, the ultimate parent of the Company (Note 6). The Company is subject to a number of risks including, but not limited to, the need to obtain adequate additional funding, possible failure of its clinical trials, failure of its third party vendors to meet the Company’s requirements and competition from other biotechnological companies. The Company’s success is dependent on its ability to obtain regulatory approval of its product
F-7
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
candidates and, if that occurs, successfully commercialize its product pipeline. Until and unless it does this, the Company will need to raise additional capital. ACL will continue to financially support the Company’s operations until the date additional capital is raised by the Company through an IPO of its common stock or other financing transactions, at which point it is contemplated that ACL will terminate its financial support of the Company. The Company may also seek additional capital through one or more private and public equity offerings, debt financings and collaborations and strategic licensing arrangements. If adequate funds are not available to the Company on a timely basis, or at all, the Company may be required to terminate or delay clinical trials or other development activities.
| 2. | Summary of significant accounting policies |
Basis of presentation
The financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) and are presented in U.S. dollars.
Principles of consolidation
The consolidated financial statements include the accounts of the Company and its subsidiaries. All significant inter-company transactions and balances between the Company and its subsidiaries are eliminated upon consolidation.
Use of estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, expenses and disclosures of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the period. Areas where management uses subjective judgment include, but not limited to, estimated useful lives for property and equipment, accounting for stock-based compensation and deferred income taxes. The valuation and accounting of the Rights (Note 10) also requires significant estimates and judgments by management. Changes in facts and circumstances may result in revised estimates. Actual results could differ from those estimates, and as such, those differences may be material to the consolidated financial statements.
Allocation of general and administrative expenses
The accompanying financial statements include allocations of costs, primarily salaries and benefits (including stock-based compensation expense) of officers and other employees of ACL, expenses for compensation and reimbursement paid to directors of ACL, and insurance costs incurred by ACL. These costs have been allocated based on the Company’s estimated share of ACL expenses using the proportional cost allocation method. In management’s estimation, the allocation methodologies used are reasonable and result in an allocation of the cost of doing business borne by ACL on behalf of the Company; however, these allocations may not be indicative of the cost of future operations or the amount of future allocations.
F-8
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Foreign currency
The functional currency of the Company is the Australian dollar (“AUD$”) as determined based on the criteria of ASC 830, Foreign Currency Matters. The Company uses the United States dollar (“US$”) as its reporting currency.
Foreign currency transactions are initially remeasured to the functional currency at the rate of exchange at the date of the transaction. At the balance sheet date, amounts payable and receivable in foreign currencies are remeasured to the functional currency at rates of exchange current at that date. An insignificant amount of exchange gains and losses are recorded in the periods presented.
Assets and liabilities of the Company are translated into US$ using the fiscal year end rate of exchange. Income and expense items are translated into US$ using the average rate of exchange during the fiscal year. The resulting translation adjustments are included as a component of comprehensive loss.
Cash and cash equivalents and short-term deposit
Cash and cash equivalents comprise cash on hand and cash held with Australian banks, which are unrestricted as to withdrawal and use and with an original maturity of three months or less. Short-term deposit is a liquid investment with a stated maturity of greater than three months but less than 12 months comprised of an interest-bearing money market fund placed with an Australian bank.
Property and equipment
Property and equipment is stated at cost, net of accumulated depreciation. Property and equipment are depreciated on a straight-line basis the estimated useful live of the assets, as follows.
| Furniture, fixtures and office equipment |
2.5 – 10 years | |||
| Lab equipment |
2 – 7 years | |||
| Computer software |
2.5 years |
Repair and maintenance costs are charged to expense as incurred, whereas the costs of betterments that extend the useful life of property and equipment are capitalized as additions to the related assets. Retirements, sale and disposals of assets are recorded by removing the cost and accumulated depreciation with any resulting gain or loss reflected in the consolidated statements of operations.
Impairment of Long-Lived Assets
The Company evaluates if long lived assets are impaired whenever indicators of impairment are present. Management considers assets to be impaired if the carrying value exceeds the future undiscounted net cash flows from related operations. If the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, the Company would recognize an impairment loss based on the excess of the carrying amount of the asset over its fair value. Fair value is determined based on an estimate of discounted future cash flows, when the market prices are not readily available for the long-lived assets. Management also re-evaluates the periods of depreciation or amortization to determine whether subsequent events and circumstances warrant revised estimates of useful lives. The Company did not record any impairment charges in any of the periods presented.
F-9
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Accruals for employee entitlements
The Company accrues compensated absences and related benefits as current charges when the following criteria are met: (i) the employee’s right to receive compensation for the future absences is attributable to services already performed by the employee; (ii) the employee’s right to receive the compensation for the future absences is vested, or accumulates; (iii) it is probable that the compensation will be paid; and (iv) the amount of compensation is reasonably estimable.
Fair value of financial instruments
The carrying amounts of financial assets and liabilities, such as cash and cash equivalents, short-term deposit, amounts due to and from related parties, trade and other payable and related party loans approximate their fair value due to their short term nature. The fair value of the rights (the “Rights”), pursuant to which the Company is required approximately 60 trading days subsequent to completion of an initial public offering, to issue Warrants (Note 10) was initially measured at the date of issuance and is subsequently remeasured at the end of each reporting period with any change in fair value recorded in the Company’s statement of operations for such period. The Company, with the assistance of an independent third party valuation firm, determined the estimated fair value of the Rights that are recognized in its consolidated financial statements.
Government grant income
Government grants are recognized as income when earned and any remaining obligations on the Company’s part are determined to be inconsequential or perfunctory. When grants are received prior to being earned, they are recognized as a liability in the balance sheet.
When the grant relates to an expense item, it is recognized as income over the periods necessary to match the grant on a systematic basis to the costs that it is intended to compensate.
When the grant relates to an asset, the grant is released to the statement of operations over the expected useful life of the relevant asset by equal annual installments.
Research funding income
On December 18, 2003, Meditech Research entered into an arrangement with Novozymes Biopharma DK A/S (“Novozymes”) whereby (i) each party agreed to license certain patents each party owned to the other in return for future royalties upon successful development and commercialization of products stemming from the use of such patents (“Technology License Agreement”) and (ii) Novozymes agreed to provide funding for Phase II clinical trials of HA-Irinotecan, one of the products that utilizes the HyACT platform, for a period of 20 years or when the last of the patents which are used in the Phase II clinical trials expire, in return for future royalties upon successful development and commercialization of products stemming from the use of HA-Irinotecan (“Research Development and Clinical Trial Agreement”). The total arrangement consideration payable by Novozymes consisted of an upfront non-refundable payment and milestone payments upon achievement of certain milestones related to the Phase II clinical trials of HA-Irinotecan. The payments, once received, are non-refundable under any condition. On March 1, 2005, an amendment was entered to increase the funding, without changing any other terms of the arrangement.
F-10
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of May 29, 2006 (date of inception), this contract arrangement was novated from Meditech Research to AOL, without amendment, when ACL acquired Meditech Research (Note 1). As of the acquisition date, there remained funding of $433,733 under the arrangement which was contingent upon substantive milestones to be achieved. The Company determined that this arrangement was not a financing transaction, as the funding is non-refundable regardless of the outcome of the research and development. The Company then evaluated whether the two deliverables in the arrangement, namely the licensing of AOL’s patents and research and development activities, can be separated or whether they must be accounted for as a single unit of accounting, in accordance with ASC 605-25, “Multi-Element Arrangements”. Based on that evaluation, the Company determined that although two separate elements existed, no value was allocable to the licensing element given that the remaining consideration consisted of contingent milestone payments, which are not considered fixed or determinable, and the technology to which the license relates was in an early stage of development. In addition, no deferred revenue was recognized on the acquisition date as there was no legal obligation on behalf of the Company to perform under the arrangement. Subsequent to the acquisition date, and pursuant to the achievement of the remaining substantive milestones, $253,069 and $180,664 were recognized as research and development income in the year ended June 30, 2007 and 2009, respectively.
After the achievement of the last milestone of Phase II clinical trials of HA-Irinotecan pursuant to the Research Development and Clinical Trial Agreement, on July 1, 2009, the Technology License Agreement was also terminated by mutual agreement of the two parties. On the same date, the Royalty Agreement and Supply Agreements were entered into, whereby the Company agreed to appoint Novozymes as its exclusive supplier of hyaluronic acid (“HA”), a substance used in the research and development, and any future manufacturing, of products utilizing HA-Irinotecan, in return for reduced future royalty payments stemming from sales of products using HA-Irinotecan. Pursuant to the Supply Agreement, the Company is required to provide a rolling forecast of its demand of HA, with a binding commitment to purchase only the forecasted quantity in the subsequent year at comparable market prices.
Other income
Other income pertains to certain royalty payments and payments received for license relinquishment that are recognised as income as the amounts are received.
Royalty payments received of $0, $6,589 and $119,070 for the years ended June 30, 2011 and 2012, and for the period from May 29, 2006 (date of inception) to June 30, 2012, respectively, are related to a license agreement for patents unrelated to HyACT. In addition to the royalty payment, AOL received $314,698 for relinquishing its rights previously granted by the counterparty to license the counterparty’s product in certain jurisdictions.
Research and development expenses
Research and development expenses are expensed when incurred. Such costs include, but are not limited to, direct salaries and benefits, stock-based compensation expense, consultant costs, patient recruitment fees, contract research costs, laboratory expenses, rent and utilities. Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations, or information provided to the Company by its vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the financial statements as prepaid research and development or other payables.
F-11
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Income taxes
Deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases, as well as net operating loss and capital loss carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the financial statements in the period that includes the legislative enactment date. The Company recognizes the financial statement effects of a tax position only when it is more likely than not that the position will be sustained upon examination and recognizes any interest and penalties accrued in relation to unrecognized tax benefits in income tax expense. The Company establishes valuation allowances against deferred tax assets when it is more likely than not that the realization of those deferred tax assets will not occur.
Comprehensive loss
Comprehensive loss is defined as the changes in equity of the Company during a period from transactions and other events and circumstances excluding transactions resulting from investments by owners and distributions to owners. In accordance with ASC 220, “Comprehensive Income”, the Company presents items of net loss and other comprehensive loss in one continuous statement, the Consolidated Statements of Operations and Comprehensive Loss. For each of the periods presented, the Company’s comprehensive loss includes net loss and cumulative foreign currency translation adjustments.
Net loss per share
Net loss per share is calculated in accordance with ASC 260-10, “Earnings Per Share.” Basic loss per share is calculated by dividing net loss applicable to common stockholders by the weighted average number of common stock outstanding during the periods. Diluted loss per share is computed by dividing net loss applicable to common stockholders by the weighted average number of shares and dilutive equivalent shares outstanding (if any) during the period. Dilutive equivalent shares are excluded from the computation of diluted loss per share if their effects would be anti-dilutive.
Stock-based compensation
Equity awards granted to the Company’s employees and non-employees (i.e., independent contractors) by ACL under ACL’s stock option plan consist of ordinary shares of ACL and options to purchase ACL’s ordinary shares and are accounted for under ASC 718-10, “Share-Based Payment” and ASC 505-50, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services,” respectively. The stock-based compensation expenses for employees and non-employees (i.e., independent contractors) employed by AOL relating to these equity awards are recognized in the Company’s financial statements with a corresponding entry to additional paid-in capital. A portion of stock-based compensation expenses for employees of ACL that have a percentage of their salary allocated to the Company, in accordance with the Company’s allocation of general and administrative expenses accounting policy described above in this Note (2) under “Allocation of general and administrative expenses,” has been recognized in the Company’s financial statements as general and administrative expense with a corresponding entry to related party loan and due to related party for periods prior and subsequent to the cancellation of related party loans on June 28, 2012 (Note 6), respectively.
F-12
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
All grants of stock options and ordinary shares to employees are recognized in the financial statements based on their grant date fair values. The Company has elected to recognize stock-based compensation expense using the straight-line method over the period during which the employee is required to perform service in exchange for the award. For the awards granted to non-employees, the Company records stock-based compensation expense equal to the fair value of the stock options and ordinary shares at the measurement date, which is determined to be the earlier of the performance commitment date or the service completion date and is recognized using the accelerated method. The Company uses the Black-Scholes option pricing model in determining the fair value of the equity awards and ordinary shares granted.
ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from initial estimates. Forfeiture rate is estimated based on historical and future expectation of employee turnover rate and is adjusted to reflect future change in circumstances and facts, if any. Stock-based compensation expense is recorded net of estimated forfeitures such that expense was recorded only for those stock-based awards that are expected to vest. To the extent the Company revises this estimate in the future, the stock-based compensation expense could be materially impacted in the period of revision, as well as in following periods. For the periods presented, the Company estimated that the forfeiture rate for both the management and non-management employees of the Company was zero.
Segment reporting
The Company’s chief operating decision-maker, who has been identified as the Chief Executive Officer, reviews the consolidated results when making decisions about allocating resources and assessing performance of the Company as a whole and hence, the Company has only one operating and reportable segment. The Company operates and manages its operations as a single segment, which is the development of the therapeutic products for the treatment of cancers. As the Company’s long-lived assets are substantially all located in Australia and no revenues from product sales have been earned yet, no geographical segments are presented.
| 3. | Concentration of risks |
Concentration of credit risk
Cash and cash equivalents and short-term deposit held with financial institutions in Australia consist of financial instruments that potentially subject the Company to concentration of credit risk to the extent of the amount recorded on the balance sheet. The Company is exposed to credit risk in the event of default by the financial institutions holding the cash and cash equivalents and short-term deposit to the extent of the amount recorded on the balance sheets. Management monitors the credit worthiness of these financial institutions.
| 4. | Deferred initial public offering costs |
Direct costs incurred by the Company attributable to its planned initial public offering of shares of common stock in the United States have been deferred and will be charged against the gross proceeds received from such offering. Such costs include legal and other professional fees related to the offering.
F-13
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 5. | Property and equipment |
The components of property and equipment are as follows:
| Year Ended June 30, | ||||||||
| 2011 | 2012 | |||||||
| Furniture, fixtures and office equipment |
$ | 188,000 | $ | 185,625 | ||||
| Lab equipment |
575,280 | 551,606 | ||||||
| Computer software |
12,900 | 33,521 | ||||||
|
|
|
|
|
|||||
| Total property and equipment |
776,180 | 770,752 | ||||||
| Accumulated depreciation |
(291,638 | ) | (519,410 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 484,542 | $ | 251,342 | ||||
|
|
|
|
|
|||||
Depreciation expense related to property and equipment amounted to $97,626, $243,644 and $495,269 for the years ended June 30, 2011 and 2012, and for the period from May 29, 2006 (date of inception) to June 30, 2012, respectively.
| 6. | Related party loans |
| Year Ended June 30, | ||||||||
| 2011 | 2012 | |||||||
| Related party loans due to ACL |
$ | 22,260,705 | $ | — | ||||
The related party loans are comprised of advances made by ACL to AOL using net proceeds from equity offerings made by ACL. The related party loans were unsecured, non-interesting bearing and were callable on demand.
On June 28, 2012, ACL loaned an additional AUD$7.5 million to AOL and increased the related party loan outstanding balance to $37.6 million. On the same date, ACL converted 100% of this outstanding related party loan amount with AOL into ordinary shares of AOL, whereupon all of the related party loans were cancelled. After the conversion transaction, the Company then issued 7,592,000 shares of its common stock to ACL in return for all outstanding shares of AOL held by ACL.
The weighted-average related party loans balances due to ACL are as follows:
| Year Ended June 30, | ||||||||
| 2011 | 2012 | |||||||
| Weighted-average related party loans due to ACL |
$ | 14,522,587 | $ | 21,887,918 | ||||
| 7. | Due from and to related party |
| Year Ended June 30, | ||||||||
| 2011 | 2012 | |||||||
| Due from related party |
$ | — | $ | 68,453 | ||||
|
|
|
|
|
|||||
| Due to related party |
$ | — | $ | 167,086 | ||||
|
|
|
|
|
|||||
F-14
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Due to and from related party represents receivables and payables, respectively, between the Company and ACL, the ultimate parent company to pay for things such as professional fees and other business related expenses. Due from and to related party balances are unsecured and interest-free, and the outstanding balances will be settled in cash prior to the Demerger (Note 14 (c)).
| 8. | Stock-based compensation |
(a) ACL options granted to Company employees
The stock options to purchase ordinary shares of ACL granted by ACL to the Company’s employees are classified as equity awards which are measured based on the grant date fair value. Since the options were granted by ACL to the Company’s employees for their provision of service to the Company, the compensation cost is recognized in the Company’s financial statements with a corresponding credit to additional paid-in capital, representing ACL’s capital contribution. The options are exercisable any time immediately to three years after the grant date and expire three to five years after the grant date.
ACL granted options to purchase 61,404 ordinary shares of ACL to employees of the Company on August 21, 2006, which vested over 3 years after grant. The weighted average exercise price of each option is AUD$1.59. As of June 30, 2012, none of these options were outstanding and exercisable.
ACL granted options to purchase 138,890 ordinary shares of ACL to employees of the Company on January 25, 2007, which vested immediately after grant. The weighted average exercise price of each option is AUD$5.73. As of June 30, 2012, none of these options were outstanding and exercisable.
ACL granted options to purchase 10,000 ordinary shares of ACL to employees of the Company on May 23, 2007, which vested over 3 years after grant. The weighted average exercise price of each option is AUD$1.73. As of June 30, 2012, none of these options were outstanding and exercisable.
ACL granted options to purchase 150,000 ordinary shares of ACL to employees of the Company on July 27, 2007, which vested over 2 years after grant. The weighted average exercise price of each option is AUD$0.86. As of June 30, 2012, there were 150,000 of these options outstanding and exercisable.
ACL granted options to purchase 10,000 ordinary shares of ACL to employees of the Company on December 14, 2007, which vested over 3 years after grant. The weighted average exercise price of each option is AUD$1.02. As of June 30, 2012, there were 10,000 of these options outstanding and exercisable.
ACL granted options to purchase 320,000 ordinary shares of ACL to employees of the Company on June 30, 2008, which vested over 1 year after grant. The weighted average exercise price of each option is AUD$0.47. As of June 30, 2012, none of these options were outstanding and exercisable.
ACL granted options to purchase 320,000 ordinary shares of ACL to employees of the Company on June 30, 2009, which vested over 1 year after grant. The weighted average exercise price of each option is AUD$0.34. As of June 30, 2012, there were 320,000 of these options outstanding and exercisable.
ACL granted options to purchase 210,000 ordinary shares of ACL to employees of the Company on August 18, 2011, which vest over 1 year after grant. The weighted average exercise price of each option is AUD$0.33. As of June 30, 2012, there were 210,000 of these options outstanding and none of these options were exercisable.
F-15
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Stock-based compensation expenses for options to purchase ordinary shares of ACL granted by ACL to the Company’s employees amounted to $779, $37,793 and $258,433 for the years ended June 30, 2011 and 2012, and for the period from May 29, 2006 (date of inception) to June 30, 2012, respectively.
The following table summarizes the share options granted by ACL to the Company’s employees between May 29, 2006 (date of inception) and June 30, 2012 (dollar amounts reflect AUD$):
| # of shares | Weighted-average exercise price per share |
Weighted-average grant-date fair value per share |
Weighted-average remaining option term (in years) |
|||||||||||||
| AUD$ | AUD$ | Year | ||||||||||||||
| Options to purchase ACL ordinary shares granted to Company employees: |
||||||||||||||||
| Outstanding, Inception (May 29, 2006) |
— | $ | — | $ | — | — | ||||||||||
| Granted |
1,010,294 | 1.29 | 0.21 | — | ||||||||||||
| Expired |
(138,890 | ) | 5.73 | 0.06 | — | |||||||||||
| Exercised |
— | — | — | — | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding, June 30, 2011 |
871,404 | 0.58 | 0.24 | 1.32 | ||||||||||||
| Granted |
210,000 | 0.33 | 0.20 | 5.13 | ||||||||||||
| Expired |
(391,404 | ) | 0.67 | 0.16 | — | |||||||||||
| Exercised |
— | — | — | — | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding, June 30, 2012 |
690,000 | $ | 0.46 | $ | 0.06 | 1.56 | ||||||||||
|
|
|
|||||||||||||||
| Stock options vested and expected to vest as of June 30, 2012 |
480,000 | $ | 0.51 | $ | 0.30 | 0.48 | ||||||||||
|
|
|
|||||||||||||||
| Stock options exercisable as of June 30, 2012 |
480,000 | $ | 0.51 | $ | 0.30 | 0.48 | ||||||||||
|
|
|
|||||||||||||||
The aggregate intrinsic value is calculated as the difference between the exercise price of the awards and the estimated fair value of the underlying stock at each reporting date for the those awards that have an exercise price below the estimated fair value of ACL’s ordinary shares. As of June 30, 2012, ACL has options outstanding to purchase an aggregate of 320,000 shares with an exercise price below the estimated fair value of ACL’s shares, resulting in an aggregate intrinsic value of $33,660.
The fair value of each stock-based award is estimated on the grant date using the Black-Scholes option pricing model using the weighted-average assumptions provided in the following table:
| For year ended June 30, 2012 |
For the period from inception (May 29, 2006) to June 30, 2012 |
|||||||
| Historic ACL volatility(a) |
68.32 | 62.92 | ||||||
| Risk free interest rate (%)(b) |
3.6 | % | 5.4 | % | ||||
| Expected term from grant date (in years)(c) |
2.5 | 2.61 | ||||||
| Dividend yield (%) |
— | — | ||||||
| (a) | Historic ACL volatility: The historic ACL volatility was estimated using the historical prices of ACL’s ordinary shares. |
F-16
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| (b) | Risk-free interest rate: The rate is based on the yield on the grant date of a zero-coupon Australian Government bond whose maturity period approximates the options’ expected term. |
| (c) | Expected term from grant date: The expected life of the award was estimated based on the midpoint between the vesting date and the end of the option term. |
There were no options to purchase ACL ordinary shares granted to the Company’s employees during the year ended June 30 2011.
(b) ACL shares granted to Company employees
The ordinary shares of ACL granted by ACL to the Company’s employees are classified as equity awards which are measured based on the grant date fair value. Since the shares were granted by ACL to the Company’s employees for their provision of service to the Company, the compensation cost is recognized in the Company’s financial statements with a corresponding credit to additional paid-in capital, representing ACL’s capital contribution.
ACL granted 132,846, 161,402, 217,947 and 176,746 ordinary shares of ACL to the Company’s employees on July 14, 2008, September 1, 2009, August 10, 2010 and November 10, 2011 respectively, which are measured based on the market prices at grant date of AUD$0.32, AUD$0.47, AUD $0.39 and AUD$0.33 per share, respectively.
Stock-based compensation expenses for ordinary shares of ACL granted to the Company’s employees as bonuses amounted to $84,058, $53,702 and $209,281 for the years ended June 30, 2011 and 2012, and for the period from May 29, 2006 (date of inception) to June 30, 2012, respectively.
(c) ACL options granted to non-employees (i.e., independent contractors) of the Company
ACL granted options to purchase 622,224 ordinary shares of ACL to non-employees of the Company on January 25, 2007, which were immediately vested upon grant. The weighted average exercise price of each option is AUD$4.46. As of June 30, 2012, none of these options were outstanding and exercisable.
ACL granted options to purchase 50,000 ordinary shares of ACL to non-employees of the Company on July 27, 2007, which vested over 2 years after grant. The weighted average exercise price of each option is AUD$0.86. As of June 30, 2012, there were 50,000 of these options outstanding and exercisable.
ACL granted options to purchase 20,000 ordinary shares of ACL to non-employees of the Company on November 26, 2007, which vested over 3 years after grant. The weighted average exercise price of each option is AUD$0.95. As of June 30, 2012, there were 20,000 of these options outstanding and exercisable.
ACL granted options to purchase 100,000 ordinary shares of ACL to non-employees of the Company on June 30, 2008, which vested over 1 year after grant. The weighted average exercise price of each option is AUD$0.47. As of June 30, 2012, none of these options were outstanding and exercisable.
ACL granted options to purchase 40,000 ordinary shares of ACL to non-employees of the Company on June 30, 2009, which vested over 1 year after grant. The weighted average exercise price of each option is AUD$0.34. As of June 30, 2012, there were 40,000 of these options outstanding and exercisable.
F-17
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
ACL granted options to purchase 30,000 ordinary shares of ACL to non-employees of the Company on August 18, 2011, which vest over 1 year after grant. The weighted average exercise price of each option is AUD$0.33. As of June 30, 2012, there were 30,000 of these options outstanding and none of these options were exercisable.
Stock-based compensation expenses for options to purchase ACL ordinary shares issued to non-employees of the Company amounted to $0, $5,399 and $97,491 for the years ended June 30, 2011 and 2012, and for the period from May 29, 2006 (date of inception) to June 30, 2012, respectively.
The stock-based compensation expense relating to ACL shares and share options granted to the Company’s employees and non-employees for the years ended June 30, 2011 and 2012, and for the period from May 29, 2006 (date of inception) to June 30, 2012 is as follows:
| Year Ended June 30, | Period from May 29, 2006 (inception) to June 30, |
|||||||||||
| 2011 | 2012 | 2012 | ||||||||||
| General and administrative(1) |
$ | 30,625 | $ | 91,079 | $ | 651,745 | ||||||
| Research and development |
84,837 | 96,894 | 565,205 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total stock based compensation expense |
$ | 115,462 | $ | 187,973 | $ | 1,216,950 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | General and administrative stock-based compensation expenses for these periods consist entirely of the portion of the stock-based compensation expense incurred by ACL attributable to employees of ACL that have a percentage of their salary allocated to the Company in accordance with the Company’s allocation of general and administrative expense accounting policy described in note (2) above under “Allocation of general and administrative expenses.” |
| 9. | Employee defined contributions |
As required by Australian law, the Company contributes annually to a defined contribution superannuation fund on behalf of all Australian employees at an amount up to 9% of an employee’s salary. The Company contributed $98,762, $121,625 and $538,983 to superannuation funds for the years ended June 30, 2011 and 2012 and for the period from May 29, 2006 (date of inception) to June 30, 2012, respectively. A portion of those contributions consist of contributions made by ACL on behalf of its Australian employees that have a percentage of their salary allocated to the Company in accordance with the Company’s allocation of general and administrative expense accounting policy described in note (2) above under “Allocation of general and administrative expenses.”
| 10. | Issuance of Rights |
In November and December 2011, ACL issued and sold its ordinary shares to investors in a private placement (the “Placement”) for total net proceeds of approximately AUD$16.1 million. Pursuant to the terms of the Placement, the investors were given rights (the “Rights”), pursuant to which the Company is required, contingent upon consummation of an initial public offering by the Company on an internationally recognized stock exchange by December 31, 2012, to issue options (which are hereinafter referred to as the “Warrants”) to the investors entitling them to purchase shares of the Company’s common stock (the “Warrant Shares”). The exercise price of the Warrants will be 130% of the volume weighted average price of the Company’s common
F-18
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
stock for the 60 trading days immediately following the listing of the common stock on an internationally recognized stock exchange (the “Average Trading Price”). The Warrants will be issuable approximately 60 trading days subsequent to completion of the initial public offering and the number of Warrants so issued will be equal to the quotient obtained by dividing (a) AUD$8,073,000 (equivalent to approximately US$8,066,542 based on the average of the currency exchange rates for November 11, 2011 and December 21, 2011, the dates on which the Placement closed) by (b) 130% of the Average Trading Price. Each Warrant will initially be exercisable for one share of the Company’s common stock. The number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on shares of the Company’s common stock.
The Warrants will vest and be exercisable from the date that is six months after the date of the Company’s initial public offering (the “IPO Date”), until the date that is the later to occur of the following (the “Expiry Date”):
| • | three years from the IPO Date; |
and
| • | the earlier of (A) six months from the date the Company announces to the market the results for the primary endpoint of the Phase III clinical trial for HA-Irinotecan in colorectal cancer and (B) five years from the IPO Date. |
Any Warrant that is not exercised prior to the Expiry Date will automatically expire on the Expiry Date.
The Rights are a liability classified in accordance with ASC 480, “Distinguishing Liabilities from Equity” as (i) the ordinary shares of ACL in the Placement and the Rights are legally detachable instruments meaning that there is no requirement that they be transferred as a unit and (ii) the Rights can only be settled with a variable number of the Company’s shares of common stock with a fixed monetary amount of approximately AUD$8.1 million. Accordingly, such Rights are also recorded as a pushdown liability incurred by the Company, given its obligation to issue such Warrants to purchase its shares approximately 60 trading days after following completion of its initial public offering. Such Rights were initially recognized at fair value on the date of issuance and are remeasured at the end of each reporting period with any change in the fair value recorded in the Company’s statement of operations for such period.
The initial fair value of these Rights upon issuance was determined to be insignificant as (i) the Company has not commenced planning for initial public offering and (ii) the obligation to issue the Warrants terminates on December 31, 2012, giving the Company a period of only approximately one year from the date of issuance to complete its initial public offering. As of June 30, 2012, the Company determined that the fair value of the Rights amounted to $473,709, with the assistance of an independent third party valuation firm. The key assumptions are disclosed in Note 13.
If and when the Company’s initial public offering is completed and the Average Trading Price of the Company’s common stock is determined, the fair value of the Rights will be remeasured. As the initial exercise price of the Warrants and the number of Warrants will have been fixed when such Average Trading Price is determined, the fair value of the Warrants will at that time be reclassified from current liabilities to stockholders’ equity on the Company’s balance sheet and the Warrants will be accounted for as equity awards. Any increase or decrease in the fair value for the then current period compared with the fair value as of the end of the Company’s then most recently ended reporting period will be reflected in the Company’s statement of operations for the then
F-19
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
current period. The remeasurement of the fair value of the Rights at each balance sheet date (prior to such reclassification) and at the time of such reclassification may result in additional expense, and any such expense may have a material adverse effect on the Company’s results of operations.
| 11. | Income taxes |
The Company’s subsidiary AOL has been operating under Australian tax law that has a corporate rate of 30% for all periods presented. There are no operations under US tax laws by the Company for the periods presented.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the corresponding amount used for income tax purposes. Significant components of the Company’s deferred tax items, which are predominantly long term in nature, are as follows:
Deferred income tax
Deferred income tax relates to the following:
| Years Ended June 30, | ||||||||
| 2011 | 2012 | |||||||
| Deferred tax liabilities |
||||||||
| Unrealized foreign currency exchanges |
$ | (42,005 | ) | $ | (9,030 | ) | ||
|
|
|
|
|
|||||
| Gross deferred tax liabilities |
$ | (42,005 | ) | $ | (9,030 | ) | ||
|
|
|
|
|
|||||
| Deferred tax assets |
||||||||
| Employee benefits |
$ | 46,648 | $ | 53,883 | ||||
| Amortisation of patent costs |
395,755 | 388,958 | ||||||
| Losses available for offset against future taxable income |
19,402,474 | 22,682,199 | ||||||
|
|
|
|
|
|||||
| Gross deferred tax assets |
$ | 19,844,877 | $ | 23,125,040 | ||||
|
|
|
|
|
|||||
| Valuation allowance |
(19,802,872 | ) | (23,116,010 | ) | ||||
| Deferred tax assets |
$ | 42,005 | $ | 9,030 | ||||
| Net deferred tax assets |
$ | — | $ | — | ||||
| Comprising: |
||||||||
| US |
— | — | ||||||
| Foreign |
19,844,877 | 23,125,040 | ||||||
|
|
|
|
|
|||||
| 19,844,877 | 23,125,040 | |||||||
In assessing the realizability of deferred tax assets, the Company considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. The Company also considers the scheduled reversal of deferred tax liabilities, projected future results or operations, and tax planning strategies in making this assessment. Based upon its history of tax losses, the Company does not believe realization of these tax assets is more likely than not. As such, full valuation allowances for the deferred tax assets were established.
At June 30, 2012 the Company had net operating tax loss carry forwards in Australia of approximately $75.6 million (June 30, 2011: $64.7 million), which can be carried forward indefinitely. These also may be adjusted if the Company is unable to comply with the recognition criteria for carry forward tax losses under Australian tax laws. Future events such as capital injections and changes in the Company’s business or capital structure require
F-20
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
evaluation as to the impact on continued availability of historical tax losses to offset future income, if any. Pursuant to Australian tax law, tax losses may not be available and would thus be derecognized (i.e. lost) where there has been a greater than 50% change in the cumulative ownership of the Company combined with a change in the Company’s business or capital structure. Accordingly certain years’ tax losses may fail the continuity of ownership test (“COT”) and later years’ tax losses may pass. If a company does not satisfy the COT in respect of a particular loss year, it must instead satisfy the same business test (“SBT”) to be eligible to utilize prior year losses. The SBT broadly requires the company to carry on the same business for the entire year in which it seeks to recoup losses as it did when it failed COT. Presently, the Company does not believe that any of its net operating tax loss carry forwards will be de-recognized based on past cumulative changes in ownership or the proposed issuance of its shares of common stock in the proposed demerger from ACL and initial public offering. As a result of these tax loss carry forwards, the Company has significant deferred tax assets; however due to the Company’s lack of earnings history, realization of these deferred tax assets is not more likely than not, therefore the deferred tax assets have been fully offset by a valuation allowance.
Reconciliation of income tax expense attributable to continuing operations and income tax expense resulting from applying the statutory tax rate to pre-tax loss from continuing operations:
| Years Ended June 30, | ||||||||
| 2011 | 2012 | |||||||
| Accounting loss before income tax |
$ | (7,913,268 | ) | $ | (9,952,440 | ) | ||
| Tax at the applicable rates(1) |
(2,373,980 | ) | (2,985,732 | ) | ||||
| Tax effect of allocation and other non tax adjustments |
248,965 | (1,240,622 | ) | |||||
| Prior year true up of tax return |
(12,877 | ) | (146,882 | ) | ||||
| Permanent differences – non deductible items |
26,156 | 178,541 | ||||||
|
|
|
|
|
|||||
| Net taxable loss |
(2,111,736 | ) | (4,194,695 | ) | ||||
| Valuation allowance |
2,111,736 | 4,194,695 | ||||||
|
|
|
|
|
|||||
| Tax expense (benefit) |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
| (1) | The statutory tax rate is based on the Australian rate of 30%. |
The Company’s deferred income tax balance and the difference between income tax computed at the Australian statutory income rate and income tax expense is primarily the result of tax losses carried forward.
No jurisdiction tax rate adjustment is calculated to account for the difference between the Australian and US federal statutory tax rates as the Company had no operations under US tax laws for the periods presented.
The change in the valuation allowance and the corresponding change in deferred tax asset for the years ended June 30, 2011 and 2012 is as follows:
| Additions | ||||||||||||||||
| Description at |
Balance at Beginning of Period |
Charged to Costs and Expenses |
Effect of Foreign Currency Translation |
Balance at End of Period |
||||||||||||
| Year ended June 30, 2011 |
||||||||||||||||
| Deferred income tax valuation allowance |
$ | 14,452,850 | $ | 2,111,736 | $ | 3,238,288 | $ | 19,802,874 | ||||||||
| Year ended June 30, 2012 |
||||||||||||||||
| Deferred income tax valuation allowance |
$ | 19,802,874 | $ | 4,194,695 | ($ | 881,559 | ) | $ | 23,116,010 | |||||||
F-21
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Interest and penalties related to gross unrecognized tax benefits are recorded within the provision for income taxes. To the extent accrued interest and penalties do not ultimately become payable, amounts accrued will be reduced in the period that such determination is made, and reflected as a reduction of the overall income tax provision, to the extent that the interest expense had been provided through the tax provision. There were no interest or penalties recorded as for the years then ended.
The tax years remaining open to tax examination by the taxing authorities for the Company are 2004 to 2012.
| 12. | Commitments and contingencies |
(a) Research and development expenditure
The Company had future research and development commitments due in fiscal 2013 of $344,552 at June 30, 2012. These costs are included in research and development expense as incurred.
In February 2000, the Company entered into a research and development agreement with Monash University, which is renewable on an annual basis. Quarterly payment is made to the university to provide staff, consumables and infrastructure facilities to carry out the Company’s ongoing HyACT projects on the university’s campus in Melbourne, Australia. The annual fee for the calendar year 2012 is $689,101, which includes, but is not limited to, research costs, laboratory expenses, rent and utilities. The annual fee under this agreement is renegotiated annually.
(b) Government research grants
The Company received one Australian Government research grant under the Commercial Ready Grant for the HA-Irinotecan program to fund 50% of its research and development expenditures for a term of 3 years and six months, which ended on February 28, 2009. The Company has the obligation to report the progress of the project for five years post completion date and has done so to date. The Australian Government may require the Company to repay all or some of the amount of the grant together with interest in any of the following circumstances:
| • | the Company fails to use its best endeavors to commercialize the relevant grant project within a reasonable time of completion of the project; |
| • | upon termination of a grant due to the Company’s breach of agreement or insolvency; |
| • | there is a change in control or ownership of the Company which the Australian Government reasonably considers has an adverse effect on the Company’s ability to comply with any of its obligations in the grant agreement; or |
| • | the Company ceases to carry on business, or a substantial part of its business. |
Technical failure of the grant funded project does not, in and of itself, constitute failure to use best endeavors to commercialize the relevant grant project. The Company believes that it has complied, and will continue to comply with its obligations to commercialize the project funded by the grant program. The obligation
F-22
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
to report progress of the project for periods subsequent to February 28, 2009 requires minimal effort and cost on the part of the Company, and it determined such obligation to be inconsequential and perfunctory. The total amount received since the commencement of the Commercial Ready Program until the end of the project was $1,675,201, which includes $166,345 received prior to the Company’s inception.
(c) Novozymes Biopharmaa DK royalty agreement
The Company entered into a royalty agreement with Novozymes in July 2009 whereby the Company is committed to pay Novozymes a 1.0% royalty on the net sales from any HA-Irinotecan product and a 0.5% royalty on net sales of any other product containing HA developed under the HyACT patents in return for Novozymes having funded a portion of the Phase II clinical trials of HA-Irinotecan (see Note 2). If Novozymes is capable of supplying HA to the Company’s specifications and the Company do not use them as its supplier for HA, the Company is committed to pay a 2.0% royalty on the net sales from any HA-Irinotecan product and a 1.0% royalty on net sales of any other product containing HA. Subject to certain termination events, including breach of the agreement by the other party, certain insolvency events relating to the other party or if it becomes unlawful for the other party to perform under the agreement, this agreement will remain in effect until the expiry of the last of Company’s HyACT patents. As of August 15, 2012, the latest date on which any of the Company’s HyACT patents expires was in 2026.
(d) PSI CRO AG clinical research services agreement
The Company entered into a Clinical Research Services Agreement with PSI CRO AG, or PSI, an international clinical trial management company. Pursuant to this agreement, PSI is managing and coordinating the HA-Irinotecan Phase III clinical trial. The amount payable to PSI is milestone driven with a total estimated cost of approximately $8.9 million if those milestones are achieved, of which an amount of $3,462,494 had been paid as of June 30, 2012. In addition, the Company is obliged to reimburse PSI for out-of-pocket expenses and third party vendor costs. This contract is cancellable at any time at the Company’s sole discretion.
(e) BioClinica Inc general services agreement
The Company entered into a general services agreement with BioClinica Inc, an international medical image management provider for clinical trials, to provide medical imaging services for the Phase III clinical trial. The overall project cost is estimated at approximately $1.7 million of which an amount of $712,651 had been paid as of June 30, 2012. This contract is cancellable at any time at the Company’s sole discretion.
| 13. | Fair value measurement |
The Company applies ASC 820, “Fair Value Measurement and Disclosures”. ASC 820 defines fair value, establishes a framework for measuring fair value and expands disclosures about fair value measurements. ASC 820 requires disclosures to be provided on fair value measurement.
ASC 820 establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1 — Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets.
F-23
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Level 2 — Include other inputs that are directly or indirectly observable in the market place.
Level 3 — Unobservable inputs which are supported by little or no market activity.
ASC 820 describes three main approaches to measuring the fair value of assets and liabilities: (1) market approach; (2) income approach and (3) cost approach. The market approach uses prices and other relevant information generated from market transactions involving identical or comparable assets or liabilities. The income approach uses valuation techniques to convert future amounts to a single present value amount. The measurement is based on the value indicated by current market expectations about those future amounts. The cost approach is based on the amount that would currently be required to replace an asset.
In accordance with ASC 820, the Company measures cash equivalents and short-term deposit and the Rights (Note 10) at fair value. Cash equivalents and short-term deposit are classified within Level 1 as they are valued using quoted market prices. The Rights are classified within Level 3 as they are valued using the Black Scholes model that utilizes the following unobservable key inputs:
| For year ended June 30, 2012 |
||||
| Expected volatility (%)(a) |
90.0 | % | ||
| Risk free interest rate(b) |
.333 | % | ||
| Expected term from grant date (in years)(c) |
3 | |||
| Expected dividend yield |
0 | |||
| Probability of IPO |
15 | % | ||
| (a) | Volatility: Since the Company is private and does not have any trading history for its common stock, the expected stock price volatility was calculated on the average volatility for comparable publicly traded biopharmaceutical companies using daily price observations over a period equal to the expected term of the Warrants. The comparable companies were chosen based on their similar size, stage in the life cycle, and financial leverage to the Company. |
| (b) | Risk-free interest rate: The rate is based on the yield on June 30, 2012 of a zero-coupon Australian Government bond whose maturity period approximates the Warrants expected term. |
| (c) | Expected term from grant date: The Company estimated the expected term based on the terms of the Warrants. |
The following table presents a reconciliation of all liabilities measured at fair value on a recurring basis using significant unobservable inputs (level 3):
| Fair Value | ||||
| Fair value at July 1, 2011 |
— | |||
| Issuance of Rights (Note 10) |
— | |||
| Changes in the fair value |
481,356 | |||
| Fair Value at June 30, 2012 |
473,709 | |||
Changes in the fair value of the Rights will be recorded in the Company’s statement of operations.
F-24
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 14. | Subsequent events |
In accordance with ASC 855, “Subsequent Event”, the Company evaluated subsequent events through August 22, 2012.
(a) Adoption of Company equity incentive plans
In August 2012, the Board of Directors and stockholders of the Company adopted the 2012 Stock Plan and the 2012 Equity Incentive Plan. The 2012 Stock Plan provides for the award of grants of incentive stock options and nonstatutory stock options and the 2012 Equity Incentive Plan provides for the award of grants of incentive stock options, nonstatutory stock options, restricted stock, restricted stock units, stock appreciation rights, performance units, and performance shares. Awards under each plan can be made to employees, outside directors and consultants of the Company and will be subject to terms of each award’s award agreement. 948,000 shares of the Company’s common stock have been reserved for future issuances under the 2012 Stock Plan, which is currently effective. In addition, 1,250,000 shares of the Company’s common stock have been reserved for future issuances under the 2012 Equity Incentive Plan, which will become effective in connection with the initial public offering, plus additional shares of common stock that will become available for issuance under the 2012 Equity Incentive Plan pursuant to the provisions thereof that automatically increase the number of shares of common stock reserved for issuance under the plan each year, beginning with the Company’s fiscal year commencing July 1, 2013.
(b) VAST technology agreement
In August 2012, the Company’s subsidiary, Audeo Discovery, entered into a technology license agreement with ACL, which provides Audeo Discovery with a non-transferable, worldwide license to use ACL’s versatile assembly on stable templates (VAST) technology, including related patents, patent applications and know-how. VAST is a molecule drug discovery technology of ACL, which provides innovative pyranose compounds for drug discovery. This agreement requires Audeo Discovery to deploy staff with relevant scientific qualifications occupying a minimum of four full-time equivalent positions to develop and exploit the VAST technology and to expend a minimum of AUD$500,000 per annum (with a target of expending AUD$1.5 million per annum) on developing, marketing and exploiting the VAST technology. Audeo Discovery must pay ACL quarterly royalty payments equal to 5% of the net revenue received by Audeo Discovery in each fiscal quarter in respect of sales of any products or services based on, incorporating or made utilizing the VAST technology, or sub-licensing the VAST technology. Because the obligation to pay any such royalties is neither probable nor reasonably estimable, the Company has not recorded a liability on its balance sheet for any such contingencies. The timing and amount of royalty payments (if any) in the future are not certain. The agreement may be immediately terminated by either party in the event of, among other things, the bankruptcy or insolvency or similar event relating to the other party or certain breaches of the agreement.
As the in-licensing of the VAST technology is transacted between parties under common control with no initial upfront payment, the VAST license will be recorded by the Company at the historical carrying value in ACL’s books, which is $0, as all research and development costs incurred on the VAST technology has been fully expensed in ACL’s books.
Four employees of ACL who worked on the VAST technology transferred to the Company in August 2012 and became its employees. As the Company did not carry out any operations with respect to the VAST
F-25
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
technology prior to the in-licensing of that technology in August 2012, salaries and benefits relating to these employees have not been allocated to the Company or recorded in the Company’s consolidated financial statements prior to the date they became the Company’s employees. The Company has assumed the legal liability of leave entitlements for these employees, which at June 30, 2012 totaled approximately US$87,954.
(c) Demerger from ACL
On completion of the Demerger, shares of the Company’s common stock will be distributed by ACL to ACL’s shareholders in the same proportions as their respective shareholdings in ACL, with such shareholders receiving one share of the Company’s common stock for every 37 ordinary shares of ACL they hold on the record date for the Demerger. The following transactions have been or will also be consummated in connection with implementation of the Demerger:
| (i) | Five employees of ACL (in addition to the four employees discussed in Note 14(b)) transferred to the Company in August 2012 and became its employees. The Company’s chief executive officer and chief financial officer will begin receiving compensation and other benefits from the Company upon implementation of the Demerger. Salaries and benefits relating to these employees have been allocated to the Company based on a proportional cost allocation method as disclosed in Note (2) above under, “Allocation of general and administrative expenses” and have been recorded in the Company’s consolidated financial statements. The Company has assumed the legal liability of leave entitlements for the five employees, and will assume the legal liability of leave entitlements for its chief executive officer and chief financial officer, which at June 30, 2012 together totaled approximately US$226,612. |
| (ii) | Any remaining net amounts due to or from ACL will be settled in cash prior to the Demerger. |
(d) Adoption of transition services agreement
In August 2012, the Board of Directors of the Company adopted the transition services agreement. Under the agreement, ACL may request that the Company provide certain accounting, treasury, management, tax support, investor relations, information technology and other administrative services to ACL for six months after implementation of the Demerger. Under the agreement, ACL must pay the Company a monthly service charge and secondment fee and reimburse the Company’s out-of-pocket expenses in providing the services.
(e) Issuance of Additional Common Stock to ACL
On November 5, 2012, the Company issued an additional aggregate of 92,000 shares of its common stock to ACL in further consideration for the Company’s receipt of the shares of AOL and which, together with 7,500,000 shares of the Company’s common stock previously issued to ACL (Note 6), will be distributed in the Demerger.
The Company’s historical share and per share information has been retroactively adjusted to give effect to this issuance of additional shares of the Company’s common stock to ACL.
F-26
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
UNAUDITED INTERIM CONDENSED CONSOLIDATED BALANCE SHEETS
(U.S. DOLLARS)
| June 30, | September 30, | |||||||
| 2012 | 2012 | |||||||
| (unaudited) | ||||||||
| Assets: |
||||||||
| Current Assets: |
||||||||
| Cash and cash equivalents |
$ | 7,635,670 | $ | 4,502,876 | ||||
| Short-term deposit |
20,318 | 20,754 | ||||||
| Due from related party |
68,453 | 274,903 | ||||||
| Prepaid research and development expenses |
691,924 | 243,218 | ||||||
| Deferred initial public offering costs |
805,487 | 2,309,862 | ||||||
|
|
|
|
|
|||||
| Total current assets |
$ | 9,221,852 | $ | 7,351,613 | ||||
| Non-current assets: |
||||||||
| Property and equipment, net |
251,342 | 208,876 | ||||||
| Non-current prepaid research and development expenses |
239,241 | 244,375 | ||||||
|
|
|
|
|
|||||
| Total non-current assets |
490,583 | 453,251 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 9,712,435 | $ | 7,804,864 | ||||
|
|
|
|
|
|||||
| Liabilities and stockholders’ equity: |
||||||||
| Current liabilities: |
||||||||
| Trade and other payables |
$ | 1,859,516 | $ | 3,812,161 | ||||
| Due to related party |
167,086 | 697,027 | ||||||
| Employee provisions |
104,071 | 205,596 | ||||||
| Rights |
473,709 | 819,280 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
$ | 2,604,382 | $ | 5,534,064 | ||||
| Non-current liabilities: |
||||||||
| Employee provisions |
75,538 | 209,567 | ||||||
|
|
|
|
|
|||||
| Total non-current liabilities |
75,538 | 209,567 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
2,679,920 | 5,743,631 | ||||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Common Stock, $0.001 par value: 30,000,000 shares authorized; 7,592,020 shares and 7,592,020 shares issued and outstanding at June 30, 2012, and September 30, 2012, respectively |
7,592 | 7,592 | ||||||
| Additional paid-in capital |
51,273,126 | 51,279,284 | ||||||
| Accumulated other comprehensive loss |
(2,259,207 | ) | (2,101,385 | ) | ||||
| Deficit accumulated during development stage |
(41,988,996 | ) | (47,124,258 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity: |
7,032,515 | 2,061,233 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity: |
$ | 9,712,435 | $ | 7,804,864 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these financial statements
F-27
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF
OPERATIONS AND COMPREHENSIVE LOSS
(U.S. DOLLARS)
| For the period from date of inception (May 29th, 2006) to September 30, 2012 |
||||||||||||
| Three Months
Ended September 30, |
||||||||||||
| 2011 | 2012 | |||||||||||
| Income: |
||||||||||||
| Grant income |
$ | — | $ | 61,205 | $ | 1,570,061 | ||||||
| Research funding income |
— | — | 433,733 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total income |
— | 61,205 | 2,003,794 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Research and development |
850,300 | 4,019,917 | 42,324,867 | |||||||||
| General and administrative |
233,660 | 850,693 | 6,628,876 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
1,083,960 | 4,870,610 | 48,953,743 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(1,083,960 | ) | (4,809,405 | ) | (46,949,949 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other income (expense): |
||||||||||||
| Interest income |
— | 10,001 | 209,137 | |||||||||
| Other income |
— | — | 433,768 | |||||||||
| Changes in fair value of rights |
— | (335,858 | ) | (817,214 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense) |
— | (325,857 | ) | (174,309 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(1,083,960 | ) | (5,135,262 | ) | (47,124,258 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Income tax expense |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (1,083,960 | ) | $ | (5,135,262 | ) | $ | (47,124,258 | ) | |||
|
|
|
|
|
|
|
|||||||
| Other comprehensive loss, net of tax: |
||||||||||||
| Foreign currency translation adjustment |
1,751,402 | 157,822 | (2,101,385 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total comprehensive loss |
$ | 667,442 | $ | (4,977,440 | ) | $ | (49,225,643 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Net loss per share – basic and diluted |
$ | (0.55 | ) | $ | (0.68 | ) | $ | (21.45 | ) | |||
|
|
|
|
|
|
|
|||||||
| Basic and diluted weighted average common shares outstanding |
1,965,641 | 7,592,020 | 2,196,429 | |||||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these financial statements
F-28
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
(U.S. DOLLARS)
| Additional Paid-in Capital |
Accumulated Other Comprehensive Income (Loss) |
Deficit Accumulated During Development Stage |
Total Stockholders’ Equity/ (Deficit) |
|||||||||||||||||||||
| Common Stock | ||||||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
| Balance at Inception (May 29, 2006) |
||||||||||||||||||||||||
| Deemed issuance of common stock |
1,965,641 | $ | 1,966 | $ | 13,128,416 | $ | — | $ | — | $ | 13,130,382 | |||||||||||||
| Net loss (from inception to June 30, 2011) |
— | — | — | — | (32,036,556 | ) | (32,036,556 | ) | ||||||||||||||||
| Foreign translation adjustments (from inception to June 30, 2011) |
— | — | — | (3,680,787 | ) | — | (3,680,787 | ) | ||||||||||||||||
| Stock-based compensation (from inception to June 30, 2011) |
— | — | 468,311 | — | — | 468,311 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at June 30, 2011 |
1,965,641 | $ | 1,966 | $ | 13,596,727 | $ | (3,680,787 | ) | $ | (32,036,556 | ) | $ | (22,118,650 | ) | ||||||||||
| Net loss |
— | — | — | — | (1,083,960 | ) | (1,083,960 | ) | ||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | 1,751,402 | — | 1,751,402 | ||||||||||||||||||
| Stock-based compensation |
— | — | 6,235 | — | — | 6,235 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at September 30, 2011 |
1,965,641 | $ | 1,966 | $ | 13,602,962 | $ | (1,929,385 | ) | $ | (33,120,516 | ) | $ | (21,444,973 | ) | ||||||||||
| Issuance of common stock |
5,626,379 | 5,626 | 37,577,539 | — | — | 37,583,165 | ||||||||||||||||||
| Additional paid-in capital upon the Reorganization (Note 1) |
— | — | 1,966 | — | — | 1,966 | ||||||||||||||||||
| Net loss |
— | — | — | — | (8,868,480 | ) | (8,868,480 | ) | ||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | (329,822 | ) | — | (329,822 | ) | ||||||||||||||||
| Stock-based compensation |
— | — | 90,659 | — | — | 90,659 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at June 30, 2012 |
7,592,020 | $ | 7,592 | $ | 51,273,126 | $ | (2,259,207 | ) | $ | (41,988,996 | ) | $ | 7,032,515 | |||||||||||
| Net loss |
— | — | — | — | (5,135,262 | ) | (5,135,262 | ) | ||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | 157,822 | — | 157,822 | ||||||||||||||||||
| Stock-based compensation |
— | — | 6,158 | — | — | 6,158 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at September 30, 2012 |
7,592,020 | $ | 7,592 | $ | 51,279,284 | $ | (2,101,385 | ) | $ | (47,124,258 | ) | $ | 2,061,233 | |||||||||||
The accompanying notes are an integral part of these financial statements
F-29
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. DOLLARS)
| Three Months Ended September 30, |
For the period from (May 29th, |
|||||||||||
| 2011 | 2012 | 2012 | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (1,083,960 | ) | $ | (5,135,262 | ) | $ | (47,124,258 | ) | |||
| Adjustments to reconcile net loss to net cash provided by operating activities: |
||||||||||||
| Depreciation and amortization |
61,049 | 63,520 | 558,789 | |||||||||
| Acquired in-process research and development |
— | — | 14,604,511 | |||||||||
| Stock-based compensation expense |
6,235 | 6,158 | 571,363 | |||||||||
| Stock-based compensation allocated from Alchemia Limited |
22,539 | 5,225 | 657,000 | |||||||||
| Changes in fair value of rights |
— | 335,858 | 817,214 | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Prepaid research and development expenses |
(3,071 | ) | 464,182 | (432,234 | ) | |||||||
| Deferred initial public offering costs |
— | (1,489,097 | ) | (2,307,587 | ) | |||||||
| Due from related party |
— | (205,258 | ) | (274,816 | ) | |||||||
| Trade and other payable |
(162,024 | ) | 1,915,323 | 3,613,871 | ||||||||
| Due to related party |
— | 521,810 | 691,593 | |||||||||
| Employee provisions |
2,623 | 232,012 | 332,368 | |||||||||
| Short-term deposit |
— | — | (20,770 | ) | ||||||||
| Related party loans |
999,454 | — | 35,997,321 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) operating activities |
(157,155 | ) | (3,285,499 | ) | 7,684,365 | |||||||
| Cash flows from investing activities |
||||||||||||
| Cash acquired in acquisition of Meditech Research |
— | — | 703,996 | |||||||||
| Purchase of property and equipment |
— | (15,597 | ) | (692,311 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
— | (15,597 | ) | 11,685 | ||||||||
| Effect of exchange rate changes on cash and cash equivalents |
(1,184 | ) | 168,302 | (3,193,174 | ) | |||||||
| Increase (decrease) in cash and cash equivalents |
(158,339 | ) | (3,132,794 | ) | 4,502,876 | |||||||
| Cash and cash equivalents at beginning of period |
159,494 | 7,635,670 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of period |
$ | 1,155 | $ | 4,502,876 | $ | 4,502,876 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplementary disclosure of cash flow information: |
||||||||||||
| Interest received |
$ | 1,682 | $ | 8,021 | $ | 204,244 | ||||||
| Non-cash investing activities |
||||||||||||
| Deemed issuance of common stock issued upon inception to purchase Meditech Research |
— | — | $ | (13,130,382 | ) | |||||||
| Loans from Alchemia Limited to purchase Meditech Research |
— | — | $ | (503,468 | ) | |||||||
| Non-cash financing activities |
||||||||||||
| Deemed issuance of common stock upon conversion of related party loan |
— | — | $ | (37,585,131 | ) | |||||||
The accompanying notes are an integral part of these financial statements
F-30
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
1. Basis of presentation
These unaudited interim condensed consolidated financial statements of Audeo Oncology, Inc. (“the Company”) and its subsidiaries, have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) for interim financial information using accounting policies consistent with those used in the preparation of the Company’s audited consolidated financial statements for the year ended June 30, 2012. Accordingly, these unaudited interim condensed consolidated financial statements do not include all of the information and footnotes required by U.S. GAAP for annual financial statements.
In the opinion of the Company’s management, the accompanying unaudited interim condensed consolidated financial statements contain all adjustments, consisting of normal and recurring adjustments, necessary to present fairly the financial position, results of operations and cash flows for each of the periods presented. The results of operations for the three months ended September 30, 2012 are not necessarily indicative of the operating results for any other interim period or the year ending June 30, 2013. The June 30, 2012 consolidated balance sheet was derived from audited financial statements at that date, but does not include all disclosures required by U.S. GAAP for annual financial statements. These unaudited condensed consolidated financial statements should be read in conjunction with the Company’s consolidated financial statements for the year ended June 30, 2012.
Demerger from Alchemia Limited (“ACL”)
On completion of the demerger of the Company from ACL (the “Demerger”), shares of the Company’s common stock will be distributed by ACL to ACL’s shareholders in the same proportions as their respective shareholdings in ACL, with such shareholders receiving one share of the Company’s common stock for every 37 ordinary shares of ACL they hold on the record date for the Demerger. The following transactions have been or will also be consummated in connection with implementation of the Demerger:
| (i) | Five employees of ACL (in addition to the four employees discussed in Note 7(f)) transferred to the Company in August 2012 and became its employees. The Company’s chief executive officer and chief financial officer will begin receiving compensation and other benefits from the Company upon implementation of the Demerger. Salaries and benefits relating to these employees have been allocated to the Company based on a proportional cost allocation in accordance with the Company’s allocation of general and administrative expense accounting policy and have been recorded in the Company’s consolidated financial statements. The Company has assumed the legal liability of leave entitlements for the five employees, which totalled $110,957, and will assume the legal liability of leave entitlements for its chief executive officer and chief financial officer, which at September 30, 2012 together totalled approximately $137,129. |
| (ii) | Any remaining net amounts due to or from ACL will be settled in cash prior to the Demerger. |
2. Summary of significant accounting policies
Use of estimates
The preparation of unaudited interim condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, expenses and disclosures of contingent assets and liabilities at the date of the unaudited interim condensed consolidated financial statements and the reported amounts of revenues and expenses during the
F-31
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE UNAUDITED INTERIM CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
period. Areas where management uses subjective judgment include, but not limited to, estimated useful lives for property and equipment, accounting for stock-based compensation and deferred income taxes. The valuation and accounting of the Rights (Note 6) also requires significant estimates and judgments by management. Changes in facts and circumstances may result in revised estimates. Actual results could differ from those estimates, and as such, those differences may be material to the unaudited condensed consolidated financial statements.
3. Property and equipment
The components of property and equipment are as follows:
| June 30, | September 30, | |||||||
| 2012 | 2012 | |||||||
| Furniture, fixtures and office equipment |
$ | 185,625 | $ | 195,134 | ||||
| Lab equipment |
551,606 | 573,493 | ||||||
| Computer software |
33,521 | 34,240 | ||||||
|
|
|
|
|
|||||
| Total property and equipment |
770,752 | 802,867 | ||||||
| Accumulated depreciation |
(519,410 | ) | (593,991 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 251,342 | $ | 208,876 | ||||
|
|
|
|
|
|||||
Depreciation expense related to property and equipment amounted to $61,049, $63,520 and $558,789 for the three months ended September 30, 2011 and 2012, and for the period from May 29, 2006 (date of inception) to September 30, 2012, respectively.
4. Due from and to related party
| June 30, | September 30, | |||||||
| 2012 | 2012 | |||||||
| Due from related party |
$ | 68,453 | $ | 274,903 | ||||
|
|
|
|
|
|||||
| Due to related party |
$ | 167,086 | $ | 697,027 | ||||
|
|
|
|
|
|||||
Due to and from related party represents receivables and payables, respectively, between the Company and ACL, the ultimate parent company, such as professional fees, employee entitlement expenses and other business related expenses. Due from and to related party balances are unsecured and interest-free, and the outstanding balances will be settled in cash prior to the Demerger.
5. Stock-based compensation
(a) ACL shares granted to Company employees and non-employees
Stock-based compensation expenses for options to purchase ordinary shares of ACL granted by ACL to the Company’s employees amounted to $5,422, $5,354 and $263,787 for the three months ended September 30, 2011 and 2012, and for the period from May 29, 2006 (date of inception) to September 30, 2012, respectively.
F-32
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE UNAUDITED INTERIM CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
The following table summarizes the share options granted by ACL to the Company’s employees between June 30, 2012 and September 30, 2012 (dollar amounts reflect AUD$):
| Options to purchase ACL ordinary shares |
# of shares |
Weighted-average exercise price per share |
Weighted- average grant- date fair value per share |
Weighted- average remaining option term (in years) |
||||||||||||
| AUD$ | AUD$ | Year | ||||||||||||||
| Outstanding, June 30, 2012 |
690,000 | $ | 0.46 | $ | 0.06 | 1.56 | ||||||||||
|
|
|
|||||||||||||||
| Granted |
— | — | — | — | ||||||||||||
| Expired |
(150,000 | ) | 0.86 | 0.48 | — | |||||||||||
| Exercised |
— | — | — | — | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding, September 30, 2012 |
540,000 | $ | 0.35 | $ | 0.02 | — | ||||||||||
|
|
|
|||||||||||||||
| Stock options exercisable as of September 30, 2012 |
540,000 | $ | 0.35 | $ | 0.55 | 2.60 | ||||||||||
|
|
|
|||||||||||||||
| Stock options vested and expected to vest as of September 30, 2012 |
540,000 | $ | 0.35 | $ | 0.55 | 2.60 | ||||||||||
|
|
|
|||||||||||||||
The aggregate intrinsic value is calculated as the difference between the exercise price of the awards and the estimated fair value of the underlying stock at each reporting date for the those awards that have an exercise price below the estimated fair value of ACL’s ordinary shares. As of September 30, 2012, ACL has options outstanding to purchase an aggregate of 530,000 shares with an exercise price below the estimated fair value of ACL’s shares, resulting in an aggregate intrinsic value of $103,141.
The stock-based compensation expense relating to ACL shares and share options granted to the Company’s employees and non-employees for the three month period ended September 30, 2011 and 2012, and for the period from May 29, 2006 (date of inception) to September 30, 2012 is as follows:
| Three Month Period Ended September 30, |
Period from May 29, 2006 (inception) to September 30, |
|||||||||||
| 2011 | 2012 | 2012 | ||||||||||
| General and administrative(1) |
$ | 22,539 | $ | 5,255 | $ | 657,000 | ||||||
| Research and development |
6,235 | 6,158 | 571,363 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total stock based compensation expense |
$ | 28,774 | $ | 11,413 | $ | 1,228,363 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | General and administrative stock-based compensation expenses for these periods consist entirely of the portion of the stock-based compensation expense incurred by ACL attributable to employees of ACL that have a percentage of their salary allocated to the Company in accordance with the Company’s allocation of general and administrative expense accounting policy. |
F-33
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE UNAUDITED INTERIM CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
(b) Options granted to employees of the Company
In August 2012, the Board of Directors and stockholders of the Company adopted the 2012 Stock Plan and the 2012 Equity Incentive Plan. The 2012 Stock Plan provides for the grants of incentive stock options and nonstatutory stock options and the 2012 Equity Incentive Plan provides for grants of incentive stock options, nonstatutory stock options, restricted stock, restricted stock units, stock appreciation rights, performance units, and performance shares. Awards under each plan can be made to employees, outside directors and consultants of the Company and will be subject to the terms of each award’s award agreement.
On September 28, 2012, the Company granted options to purchase 948,000 shares of common stock to employees and directors at an exercise price equal to the initial public offering price per share in the IPO (as defined below). 25% of these options will vest one year following the completion of the planned initial public offering of the Company (“IPO”) with the remaining 75% vesting in equal monthly installments over a three-year period beginning one year following the IPO completion date. As the exercise price of the share options is not known until the initial public offering price is established, the grant date will not be established until the IPO completion date. In addition, given that the inability of the optionees to vest in these share options until the completion of the IPO constitutes a performance condition that is not considered probable until the IPO completion date, the Company will not recognize any compensation expense until an IPO occurs. Upon the IPO completion date, the Company will measure total compensation expense to be recognized and recognize expense associated with the service period between the award date and the IPO completion date and amortize the remaining compensation expenses over the remaining vesting period using the accelerated method.
In addition, 1,250,000 shares of the Company’s common stock have been reserved for future issuances under the 2012 Equity Incentive Plan, which will become effective in connection with the IPO, plus additional shares of common stock that will become available for issuance under the 2012 Equity Incentive Plan pursuant to the provisions thereof that automatically increase the number of shares of common stock reserved for issuance under the plan each year, beginning with the Company’s fiscal year commencing July 1, 2013.
6. Issuance of Rights
In November and December 2011, ACL issued and sold its ordinary shares to investors in a private placement (the “Placement”) for total net proceeds of approximately AUD$16.1 million. Pursuant to the terms of the Placement, the investors were given rights (the “Rights”), pursuant to which the Company is required, contingent upon consummation of an IPO by the Company on an internationally recognized stock exchange by December 31, 2012, to issue options (which are hereinafter referred to as the “Warrants”) to the investors entitling them to purchase shares of the Company’s common stock (the “Warrant Shares”). The exercise price of the Warrants will be 130% of the volume weighted average price of the Company’s common stock for the 60 trading days immediately following the listing of the common stock on an internationally recognized stock exchange (the “Average Trading Price”). The Warrants will be issuable approximately 60 trading days subsequent to completion of the IPO and the number of Warrants so issued will be equal to the quotient obtained by dividing (a) AUD$8,073,000 (equivalent to approximately US$8,377,352 based on the currency exchange rate as of September 30, 2012) by (b) 130% of the Average Trading Price. Each Warrant will initially be exercisable for one share of the Company’s common stock. The number of Warrant Shares for which the Warrants are exercisable and/or the exercise price are subject to adjustment pursuant to the terms of the Warrants, including in the event of stock splits, stock combinations and similar events as well as in the event of dividends or other distributions on shares of the Company’s common stock.
F-34
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE UNAUDITED INTERIM CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
The Warrants will vest and be exercisable from the date that is six months after the date of the Company’s initial public offering (the “IPO Date”), until the date that is the later to occur of the following (the “Expiry Date”):
| • | three years from the IPO Date; and |
| • | the earlier of (A) six months from the date the Company announces to the market the results for the primary endpoint of the Phase III clinical trial for HA-Irinotecan in colorectal cancer and (B) five years from the IPO Date. |
Any Warrant that is not exercised prior to the Expiry Date will automatically expire on the Expiry Date.
The Rights are a liability classified in accordance with ASC 480, “Distinguishing Liabilities from Equity”. Accordingly, such Rights are also recorded as a pushdown liability incurred by the Company, given its obligation to issue such Warrants to purchase its shares approximately 60 trading days after following completion of its initial public offering. Such Rights were initially recognized at fair value on the date of issuance and are remeasured at the end of each reporting period with any change in the fair value recorded in the Company’s statement of operations for such period.
As of June 30 and September 30, 2012, the Company determined that the fair value of the Rights amounted to $473,709 and $819,280. The key assumptions are disclosed in Note 8.
If and when the Company’s initial public offering is completed and the Average Trading Price of the Company’s common stock is determined, the fair value of the Rights will be remeasured. As the initial exercise price of the Warrants and the number of Warrants will have been fixed when such Average Trading Price is determined, the fair value of the Warrants will at that time be reclassified from current liabilities to stockholders’ equity on the Company’s balance sheet and the Warrants will be accounted for as equity awards. Any increase or decrease in the fair value for the then current period compared with the fair value as of the end of the Company’s then most recently ended reporting period will be reflected in the Company’s statement of operations for the then current period. The remeasurement of the fair value of the Rights at each balance sheet date (prior to such reclassification) and at the time of such reclassification may result in additional expense, and any such expense may have a material adverse effect on the Company’s results of operations.
7. Commitments and contingencies
(a) Research and development expenditure
The Company had future research and development commitments due in fiscal 2013 of $175,973 at September 30, 2012. These costs are included in research and development expense as incurred.
In February 2000, the Company entered into a research and development agreement with Monash University, which is renewable on an annual basis. Quarterly payment is made to the university to provide staff, consumables and infrastructure facilities to carry out the Company’s ongoing HyACT projects on the university’s campus in Melbourne, Australia. The annual fee for the calendar year 2012 is $689,101, which includes, but is not limited to, research costs, laboratory expenses, rent and utilities. The annual fee under this agreement is renegotiated annually.
F-35
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE UNAUDITED INTERIM CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
(b) Government research grants
The Company received one Australian Government research grant under the Commercial Ready Grant for the HA-Irinotecan program to fund 50% of its research and development expenditures for a term of 3 years and six months, which ended on February 28, 2009. The Company has the obligation to report the progress of the project for five years post completion date and has done so to date. The Australian Government may require the Company to repay all or some of the amount of the grant together with interest in any of the following circumstances:
| • | the Company fails to use its best endeavours to commercialize the relevant grant project within a reasonable time of completion of the project; |
| • | upon termination of a grant due to the Company’s breach of agreement or insolvency; |
| • | there is change in control or ownership of the Company which the Australian Government reasonably considers has an adverse effect on the Company’s ability to comply with any of its obligations in the grant agreement; or |
| • | the Company ceases to carry on business, or a substantial part of its business. |
Technical failure of the grant funded project does not, in and of itself, constitute failure to use best endeavours to commercialize the relevant grant project. The Company believes that it has complied, and will continue to comply with its obligations to commercialize the project funded by the grant program. The obligation to report progress of the project for periods subsequent to February 29, 2009 requires minimal effort and cost on the part of the Company, and it determined such obligation to be inconsequential and perfunctory. The total amount received since the commencement of the Commercial Ready Program until the end of the project was $1,675,201, which includes $166,345 received prior to the Company’s inception.
(c) Novozymes Biopharmaa DK royalty agreement
The Company entered into a royalty agreement with Novozymes in July 2009 whereby the Company is committed to pay Novozymes a 1.0% royalty on the net sales from any HA-Irinotecan product and a 0.5% royalty on net sales of any other product containing HA developed under the HyACT patents in return for Novozymes having funded a portion of the Phase II clinical trials of HA-Irinotecan (see Note 2 of the audited consolidated financial statements). If Novozymes is capable of supplying HA to the Company’s specifications and the Company do not use them as its supplier for HA, the Company is committed to pay a 2.0% royalty on the net sales from any HA-Irinotecan product and a 1.0% royalty on net sales of any other product containing HA. Subject to certain termination events, including breach of the agreement by the other party, certain insolvency events relating to the other party or if it becomes unlawful for the other party to perform under the agreement, this agreement will remain in effect until the expiry of the last of Company’s HyACT patents. As of September 30, 2012, the latest date on which any of the Company’s HyACT patent expires was in 2026.
(d) PSI CRO AG clinical research services agreement
The Company entered into a Clinical Research Services Agreement with PSI CRO AG, or PSI, an international clinical trial management company. Pursuant to this agreement, PSI is managing and coordinating the HA-Irinotecan Phase III clinical trial. The amount payable to PSI is milestone driven with a total estimated cost of approximately $9.3 million if those milestones are achieved, of which an amount of $4,096,970 had been paid as of September 30, 2012. In addition, the Company is obliged to reimburse PSI for out-of-pocket expenses and third party vendor costs. This contract is cancellable at any time at the Company’s sole discretion.
F-36
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE UNAUDITED INTERIM CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
(e) BioClinica Inc general services agreement
The Company entered into a general services agreement with BioClinica Inc, an international medical image management provider for clinical trials, to provide medical imaging services for the Phase III clinical trial. The overall project cost is estimated at approximately $1.7 million of which an amount of $716,267 had been paid as of September 30, 2012. This contract is cancellable at any time at the Company’s sole discretion.
(f) VAST technology agreement
In August 2012, the Company’s subsidiary, Audeo Discovery Pty Limited (“Audeo Discovery”), entered into a technology license agreement with ACL, which provides Audeo Discovery with a non-transferable, worldwide license to use ACL’s versatile assembly on stable templates (VAST) technology, including related patents, patent applications and know-how. VAST is a small molecule drug discovery technology of ACL, which provides innovative pyranose compounds for drug discovery. This agreement requires Audeo Discovery to deploy staff with relevant scientific qualifications occupying a minimum of four full-time equivalent positions to develop and exploit the VAST technology and to expend a minimum of AUD$500,000 per annum (with a target of expending AUD$1.5 million per annum) on developing, marketing and exploiting the VAST technology. Audeo Discovery must pay ACL quarterly royalty payments equal to 5% of the net revenue received by Audeo Discovery in each fiscal quarter in respect of sales of any products or services based on, incorporating or made utilizing the VAST technology, or sub-licensing the VAST technology. Because the obligation to pay any such royalties is neither probable nor reasonably estimable, the Company has not recorded a liability on its balance sheet for any such contingencies. The timing and amount of royalty payments (if any) in the future are not certain. The agreement may be terminated by either party in the event of, among other things, the bankruptcy or insolvency or similar event relating to the other party or certain breaches of the agreement.
As the in-licensing of the VAST technology is transacted between parties under common control with no initial upfront payment, the VAST license will be recorded by the Company at the historical carrying value in ACL’s books, which is $0, as all research and development costs incurred on the VAST technology has been fully expensed in ACL’s books.
Four employees of ACL who worked on the VAST technology transferred to the Company in August 2012 and became its employees. As the Company did not carry out any operations with respect to the VAST technology prior to the in-licensing of that technology in August 2012, salaries and benefits relating to these employees have not been allocated to the Company or recorded in the Company’s consolidated financial statements prior to the date they became the Company’s employees. The Company has assumed the legal liability of leave entitlements for these employees, which totalled approximately US$87,154.
(g) Leases
The Company entered into an operating lease agreement for its office and lab space in Brisbane, Australia with the original lease period expiring in 2016. This arrangement has an escalating rent payment provision, and the Company recognizes rent expense on a straight-line basis.
F-37
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE UNAUDITED INTERIM CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
The following is a schedule, by fiscal years, of the future minimum lease payments required under the non-cancellable operating lease as of September 30, 2012:
| Operating Lease |
||||
| 2013 |
$ | 311,310 | ||
| 2014 |
426,495 | |||
| 2015 |
439,290 | |||
| 2016 |
452,468 | |||
| 2017 |
37,797 | |||
|
|
|
|||
| Total minimum lease payments |
$ | 1,667,360 | ||
|
|
|
|||
In addition to the above base rent, the Company has to reimburse the lessor a portion of its related expenses, such as utilities, taxes and other levies etc. on a monthly basis. The estimated amount of this reimbursement for fiscal 2013 is $74,000.
Operating lease expenses totaled $0, $69,564 and $69,564 for the quarters ended September 30, 2011 and 2012 and for the period from May 29, 2006 (date of inception) to September 30, 2012, respectively.
8. Fair value measurement
The Company applies ASC 820, “Fair Value Measurement and Disclosures”. ASC 820 defines fair value, establishes a framework for measuring fair value and expands disclosures about fair value measurements. ASC 820 requires disclosures to be provided on fair value measurement.
ASC 820 establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
| Level 1 — |
Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets. | |
| Level 2 — |
Include other inputs that are directly or indirectly observable in the market place. | |
| Level 3 — |
Unobservable inputs which are supported by little or no market activity. |
ASC 820 describes three main approaches to measuring the fair value of assets and liabilities: (1) market approach; (2) income approach and (3) cost approach. The market approach uses prices and other relevant information generated from market transactions involving identical or comparable assets or liabilities. The income approach uses valuation techniques to convert future amounts to a single present value amount. The measurement is based on the value indicated by current market expectations about those future amounts. The cost approach is based on the amount that would currently be required to replace an asset.
F-38
Table of Contents
AUDEO ONCOLOGY, INC.
(a development stage company)
NOTES TO THE UNAUDITED INTERIM CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
In accordance with ASC 820, the Company measures cash equivalents and short-term deposit and the Rights (Note 6) at fair value. Cash equivalents and short-term deposit are classified within Level 1 as they are valued using quoted market prices. The Rights are classified within Level 3 as they are valued using the Black Scholes model that utilizes the following unobservable key inputs:
| For the year ended June 30, 2012 |
For the three months ended September 30, 2012 |
|||||||
| Expected volatility (%)(a) |
90.0 | % | 90.0 | % | ||||
| Risk free interest rate(b) |
0.333 | % | 0.310 | % | ||||
| Expected term from grant date (in years)(c) |
3 | 3 | ||||||
| Expected dividend yield |
0 | 0 | ||||||
| Probability of IPO |
15.0 | % | 25.0 | % | ||||
| (a) | Volatility: Since the Company is private and does not have any trading history for its common stock, the expected stock price volatility was calculated on the average volatility for comparable publicly traded biopharmaceutical companies using daily price observations over a period equal to the expected term of the Warrants. The comparable companies were chosen based on their similar size, stage in the life cycle, and financial leverage to the Company. |
| (b) | Risk-free interest rate: The rate is based on the U.S. Treasury yield curve in effect at the time of grant with maturities equal to the Warrants’ expected term. |
| (c) | Expected term from grant date: The Company estimated the expected term based on the terms of the Warrants. |
The changes in fair value of the Company’s Level 3 Rights liability during the three months ended September 30, 2012 were as follows:
| Fair value at June 30, 2012 |
$ | 473,709 | ||
| Fair value adjustment to Rights liability |
335,858 | |||
| Foreign exchange translation adjustment |
9,713 | |||
|
|
|
|||
| Fair value at September 30, 2012 |
$ | 819,280 | ||
|
|
|
Changes in the fair value of the Rights will be recorded in the Company’s statement of operations.
9. Subsequent events
(a) Issuance of Additional Common Stock to ACL
On November 5, 2012, the Company issued an additional aggregate of 92,000 shares of its common stock to ACL in further consideration for the Company’s receipt of the shares of AOL and which, together with 7,500,000 shares of the Company’s common stock previously issued to ACL, will be distributed in the Demerger (Note 1).
The Company’s historical share and per share information has been retroactively adjusted to give effect to this issuance of additional shares of the Company’s common stock to ACL.
(b) Office Space Sub-lease
On November 6, 2012, AOL entered into a sub-lease in relation to a part of the office space leased in Brisbane, Australia which allows ACL to use part of these facilities. This sub-lease provides for ACL to pay the Company rent calculated on a cost recovery basis. The term of the sub-lease is four years.
F-39
Table of Contents
Through and including, , 2012 (which is the 25th day after the date of this prospectus), all dealers effecting transactions in our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to unsold allotments or subscriptions.
3,250,000 Shares

Audeo Oncology, Inc.
Common Stock
PROSPECTUS
Leerink Swann Oppenheimer & Co.
Table of Contents
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth costs and expenses, other than underwriting discounts and commissions, payable by the Registrant in connection with the sale of the common stock being registered under this registration statement. All amounts shown are estimated except for the SEC registration fee, the FINRA filing fee and the initial listing fee.
| SEC registration fee |
$ | 6,876 | ||
| FINRA filing fee |
9,500 | |||
| Initial Nasdaq listing fee |
125,000 | |||
| Printing expenses |
400,000 | |||
| Legal fees and expenses |
1,278,000 | |||
| Accounting fees and expenses |
751,000 | |||
| Transfer agent and registrar fees and expenses |
6,000 | |||
| Miscellaneous |
300,000 | |||
|
|
|
|||
| Total |
2,876,376 | |||
|
|
|
Item 14. Indemnification of Directors and Officers.
On completion of this offering, the Registrant’s amended and restated certificate of incorporation will contain provisions that eliminate, to the maximum extent permitted by the General Corporation Law of the State of Delaware, the personal liability of the Registrant’s directors and executive officers for monetary damages for breach of their fiduciary duties as directors or officers. The Registrant’s amended and restated certificate of incorporation and bylaws will provide that the Registrant must indemnify its directors and executive officers and may indemnify its employees and other agents to the fullest extent permitted by the General Corporation Law of the State of Delaware.
Sections 145 and 102(b)(7) of the General Corporation Law of the State of Delaware provide that a corporation may indemnify any person made a party to an action by reason of the fact that he or she was a director, executive officer, employee or agent of the corporation or is or was serving at the request of a corporation against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with such action if he or she acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of an action by or in right of the corporation, no indemnification may generally be made in respect of any claim as to which such person is adjudged to be liable to the corporation.
The Registrant has entered into indemnification agreements with its directors and executive officers, in addition to the indemnification provided for in its amended and restated certificate of incorporation and bylaws, and intends to enter into indemnification agreements with any new directors and executive officers in the future.
The Registrant has purchased and intends to maintain insurance on behalf of each and any person who is or was a director or officer of the Registrant against any loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions.
II-1
Table of Contents
The Underwriting Agreement (Exhibit 1.1 hereto) provides for indemnification by the underwriters of the Registrant and its executive officers and directors, and by the Registrant of the underwriters, for certain liabilities, including liabilities arising under the Securities Act.
See also the undertakings set out in response to Item 17 herein.
Item 15. Recent Sales of Unregistered Securities.
Seth forth below is information regarding unregistered shares of our common stock issued and options granted by us since July 1, 2009 through the date of this prospectus. Also included is the consideration, if any, received by us for such shares and options and information relating to the section of the Securities Act, or rules of the SEC, under which exemption from registration was claimed. No underwriters were involved in any of such sales or grants. Some of the transactions described below involved directors, officers and five-percent stockholders.
Common Stock Issuances
In June 2012, we sold an aggregate of 20 shares of our common stock to our President and Chief Executive Officer, Peter Smith, and our Chief Financial Officer and Secretary, Charles Walker, in connection with our initial incorporation in Delaware, at a purchase price of $0.001, for an aggregate purchase price of $0.02.
In June 2012, we issued an aggregate of 7,500,000 shares of our common stock to our parent, Alchemia Limited in consideration for receipt of all outstanding shares of Alchemia Oncology Pty Limited. On November 5, 2012, we issued an additional aggregate of 92,000 shares of our common stock to Alchemia Limited in further consideration for our receipt of the shares of Alchemia Oncology Pty Limited and which, together with the 7,500,000 shares of our common stock previously issued, will be distributed in the Demerger.
Pursuant to the Demerger, one share of our common stock will be distributed to the shareholders of Alchemia Limited for every 37 ordinary shares of Alchemia Limited that they hold, with fractional shares of our common stock that would otherwise be distributed to Alchemia Limited shareholders in the Demerger being rounded upwards to the nearest whole share. Information in this prospectus assumes that Alchemia Limited will distribute 7,592,020 shares of our common stock that are currently outstanding and held by it to Alchemia Limited shareholders (or a depositary on their behalf) in the Demerger, which number of shares is calculated on the basis of 280,812,962 ordinary shares of Alchemia Limited outstanding as of November 1, 2012. However, the actual number of shares of our common stock distributed in the Demerger will depend upon the number of holders and the number of outstanding ordinary shares of Alchemia Limited as of the record date for the Demerger, which will be approximately four trading days following the date of this prospectus, and the terms of the Demerger provide that fractional shares of our common stock that would otherwise be distributed pursuant to the Demerger will be rounded upwards to the nearest whole share. In addition, options to purchase a total of 3,573,000 ordinary shares of Alchemia Limited at exercise prices ranging from AUD$0.280 to AUD$1.040 per share (equivalent to approximately US$0.291 to US$1.079 per share based on the currency exchange rate as of November 1, 2012) were outstanding as of November 1, 2012, and any exercise of these options or any other options that Alchemia Limited may grant prior to the record date for the Demerger would increase the number of ordinary shares of Alchemia Limited outstanding as of such record date and the number of shares of our common stock distributed in the Demerger and to be outstanding immediately after this offering. As a result, the actual number of shares of our common stock distributed in the Demerger and that will be outstanding upon completion of this offering will be greater than the number of shares we have assumed for the purposes of this prospectus.
Option Grants
Options to purchase 948,000 shares of our common stock were granted under our 2012 Stock Plan at an exercise price equal to the initial public offering price per share of our common stock sold in this offering.
II-2
Table of Contents
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions or any public offering, and the Registrant believes that each transaction was exempt from the registration requirements of the Securities Act in reliance upon Section 4(2) of the Securities Act, Regulation D or Regulation S promulgated thereunder, or Section 3(a)(10) of the Securities Act. The recipients of the securities in each of these transactions (except the Demerger) represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed upon the stock certificates issued in these transactions. All recipients had adequate information about the Registrant, or had adequate access through their relationship with the Registrant, to information about the Registrant.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits:
We have filed the exhibits listed on the accompanying Exhibit Index of this Registration Statement.
(b) Financial Statement Schedules.
All other schedules have been omitted because the information required to be presented in them is not applicable or is shown in the consolidated financials statements or related notes.
Item 17. Undertakings.
The Registrant hereby undertakes to provide to the underwriters at the closing as specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933, as amended, and will be governed by the final adjudication of such issue.
The Registrant hereby undertakes that:
| • | For purposes of determining any liability under the Securities Act of 1933, as amended, the information omitted from a form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in the form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933, as amended, shall be deemed to be part of this registration statement as of the time it was declared effective. |
| • | For the purpose of determining any liability under the Securities Act of 1933, as amended, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-3
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this amendment to the registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Francisco, State of California, on November 8, 2012.
| AUDEO ONCOLOGY, INC. | ||||
| By: |
/S/ PETER SMITH | |||
| Name: | Peter Smith | |||
| Title: | President and Chief Executive Officer | |||
Pursuant to the requirements of the Securities Act of 1933, this amendment to the registration statement has been signed by the following persons in the capacities indicated below:
| Signature |
Title |
Date | ||
| /S/ PETER SMITH Peter Smith |
President, Chief Executive Officer and Director (Principal Executive Officer) | November 8, 2012 | ||
| /S/ CHARLES WALKER Charles Walker |
Chief Financial Officer (Principal Financial and Accounting Officer) | November 8, 2012 | ||
| * Leigh Bonney |
Director | November 8, 2012 | ||
| * Stephen Hill |
Director | November 8, 2012 | ||
| * Susan Kelley |
Director | November 8, 2012 | ||
| * Tracie Ramsdale |
Director | November 8, 2012 | ||
| * David U’Prichard |
Director | November 8, 2012 | ||
| *By: | /S/ CHARLES WALKER | |
| Charles Walker Attorney-in-fact | ||
II-4
Table of Contents
EXHIBIT INDEX
| Exhibit Number |
Description | |
| 1.1* | Form of Underwriting Agreement. | |
| 3.1+ | Form of Amended and Restated Certificate of Incorporation of the Registrant, to be in effect upon the completion of this offering. | |
| 3.2+ | Form of Amended and Restated Bylaws of the Registrant, to be in effect upon the completion of this offering. | |
| 3.3+ | Certificate of Incorporation of the Registrant, as currently in effect. | |
| 3.4+ | Bylaws of the Registrant, as currently in effect. | |
| 4.1+ | Specimen common stock certificate of the Registrant. | |
| 5.1* | Opinion of Wilson Sonsini Goodrich & Rosati, Professional Corporation. | |
| 10.1+† | Supply Agreement, dated July 13, 2009, between Alchemia Oncology Pty Limited and Novozymes Biopharma DK A/S. | |
| 10.2+ | Royalty Agreement, dated July 13, 2009, between Alchemia Oncology Pty Limited and Novozymes Biopharma DK A/S. | |
| 10.3+ | Intra-group Transfer Agreement, dated June 28, 2012, between Alchemia Limited and the Registrant. | |
| 10.4+ | Sublease Agreement, dated June 28, 2012, between Blueprint Life Science Group, LLC and the Registrant. | |
| 10.5+† | General Services Agreement, dated May 18, 2010, between BioClinica, Inc. and Alchemia Oncology Pty Limited. | |
| 10.6+† | Addendum to General Services Agreement, dated December 29, 2010, between BioClinica, Inc. and Alchemia Oncology Pty Limited. | |
| 10.7+† | Addendum to General Services Agreement, dated December 19, 2011, between BioClinica, Inc. and Alchemia Oncology Pty Limited. | |
| 10.8+† | Clinical Research Services Agreement, dated June 25, 2010, between PSI CRO AG and Alchemia Oncology Pty Limited. | |
| 10.9+† | Amendment No. 1 to Clinical Research Services Agreement, dated February 17, 2012, between PSI CRO AG and Alchemia Oncology Pty Limited. | |
| 10.10+ | Technology License Agreement, dated August 22, 2012, between Alchemia Limited and Audeo Discovery Pty Limited. | |
| 10.11+ | Demerger Deed, dated August 22, 2012, between Alchemia Limited and the Registrant. | |
| 10.12+ | Demerger Implementation Agreement, dated August 22, 2012, between Alchemia Limited and the Registrant. | |
| 10.13+ | Transition Services Agreement, dated August 22, 2012, between Alchemia Limited and the Registrant. | |
| 10.14+ | Demerger Deed Poll, dated August 22, 2012, by the Registrant. | |
| 10.15+# | Form of Indemnification Agreement between the Registrant and its directors and officers. | |
| 10.16+# | 2012 Stock Plan. | |
| 10.17+# | 2012 Equity Incentive Plan. | |
| 10.18+# | Employment Agreement, dated September 28, 2012, between Peter Smith and Alchemia Oncology Pty Limited. | |
Table of Contents
| 10.19+# | Employment Agreement, dated September 28, 2012, between Charles Walker and Alchemia Oncology Pty Limited. | |
| 10.20+# | Employment Agreement, dated September 28, 2012, between Tracey Brown and Alchemia Oncology Pty Limited. | |
| 10.21 | Amendment Deed, dated October 17, 2012, between Alchemia Limited and the Registrant. | |
| 21.1+ | List of subsidiaries of the Registrant. | |
| 23.1 | Consent of Ernst & Young, Independent Registered Public Accounting Firm. | |
| 23.2* | Consent of Wilson Sonsini Goodrich & Rosati, Professional Corporation (included in Exhibit 5.1). | |
| 24.1+ | Power of Attorney (included on signature pages to this registration statement). | |
| * | To be filed by amendment. |
| † | Portions of this exhibit have been omitted pending a determination by the Securities and Exchange Commission as to whether these portions should be granted confidential treatment. |
| # | Indicates management contract or compensatory plan. |
| + | Previously filed. |












